Abstract
Background
There are strong association between remnant cholesterol (RC)/non-high density lipoprotein cholesterol (NHDL-C) and increase cardiovascular (CV) risk. The aim of present study was to investigate the association between target lipid parameters (RC and NHDL-C) and the risk of CV mortality in general population.
Methods
Data set from an open database—National Health and Nutrition Examination Surveys (NHANES) 2003–2014 were extracted (n = 14992). Kaplan-Meier, multivariable COX regression, and restricted cubic spline (RCS) parameters.
Results
Compared to the lowest quartile, RC (adjusted hazard ratio [HR] = 1.63 95%CI 1.05–2.52, P for trend = 0.037) and triglycerides (TG: Model 3: HR = 1.69 95%CI 1.10–2.60, P for trend = 0.049) in the highest quartile were independently associated with the increased cardiovascular mortality, while NHDL-C and apolipoprotein B (ApoB) in adjusted models did not show association (P for trend >0.05). In addition, RCS regression demonstrated that RC (P for nonlinearity = 0.011) and TG (P for nonlinearity = 0.010) levels had a similar J-shape association with CV mortality. Threshold effect analysis showed that when RC ≤ 29.3 mg/dL, the level of RC and CV mortality risk were positively correlated.
Conclusions
Our findings suggest high RC levels are associated with an increased risk of CV mortality, which support that the integration of TG-rich lipoproteins parameters in risk assessment might optimize the identification and management of selected population.
Keywords: Lipid, Remnant cholesterol, Non-high density lipoprotein cholesterol, Cardiovascular mortality, Risk assessment
Lipid; Remnant cholesterol; Non-high density lipoprotein cholesterol; Cardiovascular mortality; Risk assessment.
1. Introduction
Due to the overall social development and population aging, cardiovascular disease (CVD) is the most common cause of death worldwide in the general population. Epidemiological studies demonstrated that CVD accounted for over 17 million deaths worldwide yearly [1, 2, 3]. The prevalence of CVD and disease burden are rising over years [4, 5].
Lipids are essential in the development of atherosclerosis (AS). Serum lipid profile reflects the overall cardiometabolic health and significantly relates to CVDs [6, 7]. The lipid subfractions have gained increasing attention as more valid measures of atherogenicity. As the key factor in the pathogenesis and perpetuation of ASCVD, the treatment toward the atherogenic cholesterol—low-density lipoprotein cholesterol (LDL-C) lowing has been established to reduce certain CVD risks [8, 9, 10]. However, studies showed that LDL-C does not account for the total risk of ASCVD [11], while other forms of dyslipidemia also contribute to the increased risk.
Several corresponding lipid parameters have been shown to further improve management and/or reflect the overlooked residual risk of LDL-C. Previous studies indicated that TRL and their remnants may contribute significantly to residual cardiovascular risk in patients on optimized lipid-lowering therapy [12, 13]. Among, remnant cholesterol (RC) and non-high-density lipoprotein cholesterol (NHDL-C) share overlapped characteristics, and both parameters demonstrated certain associations with TG-rich lipoproteins (TRL) which have a different pathophysiological characteristic to LDL-C.
As the cholesterol content of a subset of the TRL, RC mainly accounts for the very low-density lipoproteins (VLDL) and intermediate-density lipoproteins (IDL) cholesterol. RC is highly correlated with an increased risk of major cardiovascular events regardless of LDL-C levels and statin-treatment [14, 15, 16]. It can be assessed using several methods, including immunoaffinity assays, nuclear magnetic resonance spectroscopy, and calculations-based on plasma triglyceride concentrations.
In addition, NHDL-C specifies the amount of cholesterol associated with TRLs without high-density lipoprotein cholesterol (HDL-C) which being small and dense molecules. While NHDL-C is one of the major targets in assessing CVD risk and is the secondary target of therapy in individuals with hypertriglyceridemia, studies also suggested that NHDL-C has a superior predictive value to LDL-C in estimating the risk of major cardiovascular events [17, 18, 19, 20].
Despite the shared promising values of RC and NHDL-C in risk and/or prognostic evaluation in broad patient cohorts, most of these studies have forced on high-risk populations and the secondary complementary marker to LDL-C [21, 22, 23]. Furthermore, the different roles between RC and NHDL-C in CVD remained unclear.
Given the existing evidence, the current study aimed to examine the association of these lipid markers with the cardiovascular risk and to explore the different effects of NHDL-C and RC in the general US population.
2. Method and material
2.1. Study population and design
This retrospective cohort study used publicly available data from National Health and Nutrition Examination Survey (NHANES) conducted by the US National Center for Health Statistics (Centers for Disease Control and Prevention, Atlanta, GA, USA). Authors did not involve in the collection and production of the database. The detailed survey design, methods, and data are available on the NHANES website [24] and were in accordance with the “Declaration of Helsinki”. The protocols for NHANES were approved by the National Center for Health Statistics of the Centers for Disease Control and Prevention Institutional Review Board, and informed consent was obtained from all participants.
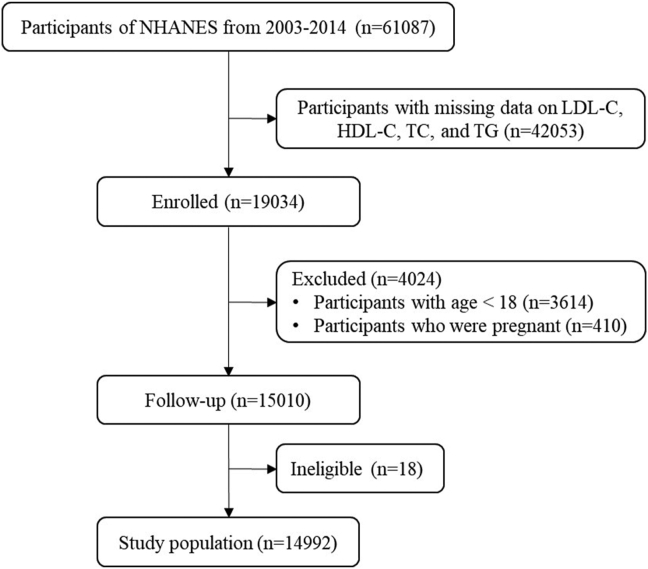
From NHANES 2003–2014 including 6 survey cycles (apolipoprotein B [ApoB] only in the NHANES 2005–2014), there were 14992 participants with available lipid profiles enrolled for the analysis. Inclusion flow chart is shown in Figure 1.
Figure 1.
Inclusion flow chart.
2.2. Demographic characteristics and covariates
Baseline demographic variables including age, gender, education levels (below high school, high school, above high school), race/ethnicity (Mexican American, other Hispanic, non-Hispanic White, non-Hispanic Black, other race), body weight, and height were collected from the household interview. Body mass index (BMI) was calculated as body weight divided by height squared (kg/m2). The prevalence of the comorbidity (hypertension, diabetes, congestive heart failure, coronary heart disease, and stroke) was recorded by a standardized medical condition questionnaire which administered by trained interviewers.
Information on smoking, alcohol use, medication (including lipid-lowering agents), and history of comorbidities had been obtained from the physical examination and associated questionnaire. Smoking habit was identified as someone who smoked ≥100 cigarettes in their lifetime. Alcohol user was defined as those who drank at least 12 alcohol drinks in any one year.
2.3. Lipid profiles
Specimens from subjects who fasted for at least 8.5 h but less than 24 h were assayed for the lipid profile. Total cholesterol (TC), triglyceride (TG), HDL-C, and ApoB level were collected. Across included NHANES cycles, TC and TG were measured using enzymatic assays, and HDL-C and apoB were measured using immunoassays. LDL-C is calculated from measured values of TC, TG, and HDL-C according to the Friedewald calculation. Level of RC was calculated as TC minus LDL-C minus HDL-C [17, 25]. Level of NHDL-C was calculated as the difference between TC and HDL-C [14, 16]. Detailed instructions are discussed in each corresponding NHANES cycle Laboratory Procedures Manual https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/default.aspx.
2.4. Mortality ascertainment
With mean follow-up time of 5.5 years, the primary outcome was cardiovascular (CV) mortality which identified using variable for the leading cause of death. In this study, we defined death resulting from heart disease or cerebrovascular diseases as CV mortality. All-cause mortality and CVD mortality were defined according to the Tenth Version of the International Classification of Diseases (ICD-10). Information on follow-up time and mortality status were ascertained via using person months from the National Death Index to date of death or the end of the mortality period (December 31, 2015) [26]. Secondary outcome of all-cause mortality was acquired accordingly.
2.5. Statistical analysis
Continuous variables accorded with normal distribution are presented as means (standard deviations, SDs); otherwise, they are presented as medians with interquartile ranges (IQRs). Categorical variables are presented as numbers (%). Multiple imputations were applied for the missing cycle of ApoB. RC and NHLD-C were calculated and compared in each of the upper three quartiles to the lowest quartile. The shape of the relationship between lipid parameters and cardiovascular mortality was explored using the restricted cubic spline regression model and used ANOVA to test for nonlinearity. If nonlinearity was detected, we used the segmented regression to fit the piecewise-linear relationship between lipid parameters and CV mortality risk and to calculate the threshold inflection point using a recursive algorithm. Stratified Cox regression models were used to perform subgroup analyses. The significance of interaction (p-interaction) was tested using the likelihood ratio test. Hazard ratios (HR) [95% confidence intervals (CI)] for risk of endpoints were presented.
The cumulative survival rate was calculated by Kaplan-Meier method, and log-rank test was used for comparison between groups. Cox proportional hazards regression was performed, to analyze CV mortality according to the increasing values and quartiles of RC and NHDL-C. We evaluated the associations of lipids with endpoint using multivariate linear regression models analyzed by the following three adjustment models: model 1 was unadjusted; model 2 was adjusted for basic epidemic characteristic including age and sex; model 3 was further adjusted for the significant baseline characteristic of ethnicity, education level, smoker, alcohol user, BMI, lipid-lowering drugs, diabetes mellitus, hypertension, coronary heart disease, congestive heart failure, and stroke.
The statistical analyses were performed by SPSS (version 24.0; IBM) and R software (version 3.6.0; The R Foundation for Statistical Computing). Two-sided P < 0.05 was considered statistically significant.
3. Results
3.1. Baseline characteristics
Table 1 showed the characteristics of the study population. Study included 14992 participants with 49.5% of male and a mean age of 48.1 ± 19.2 years old. There were 16.9% of participants who received lipid-lowering drugs. The mean RC level in the overall population was 24.2 mg/dL, TG was 121.1 mg/dL, NHDL-C was 137.2 mg/dL, and ApoB was 92.1 mg/dL.
Table 1.
Baseline characteristics.
| Variable | All (n = 14992) | Cardiovascular Death (n = 254) | Survival (n = 14738) | P value |
|---|---|---|---|---|
| Age, years | 48.1 (19.2) | 71.8 (12.1) | 47.7 (19.1) | <0.001 |
| Male, % | 7428 (49.5%) | 166 (65.4%) | 7262 (49.3%) | <0.001 |
| Body mass index, kg/m2 | 28.6 (6.8) | 28.3 (6.2) | 28.6 (6.8) | 0.001 |
| Education level, % | <0.001 | |||
| Below high school | 4006 (26.7%) | 103 (40.6%) | 3903 (26.5%) | |
| High school | 3452 (23.0%) | 65 (25.6%) | 3387 (23.0%) | |
| Above high school | 7534 (50.3%) | 86 (33.9%) | 7448 (50.5%) | |
| Race/ethnicity, % | <0.001 | |||
| Mexican American | 2529 (16.9%) | 27 (10.6%) | 2502 (17.0%) | |
| Other Hispanic | 1255 (8.4%) | 11 (4.3%) | 1244 (8.4%) | |
| Non-Hispanic White | 6830 (45.6%) | 164 (64.6%) | 6666 (45.2%) | |
| Non-Hispanic Black | 3140 (20.9%) | 44 (17.3%) | 3096 (21.0%) | |
| Other race | 1238 (8.3%) | 8 (3.1%) | 1230 (8.3%) | |
| Smoker, % | 6789 (45.3%) | 150 (59.1%) | 6639 (45.0%) | <0.001 |
| Alcohol user, % | 10649 (71.0%) | 167 (65.7%) | 10482 (71.1%) | <0.001 |
| Lipid-lowering drugs, % | 2540 (16.9%) | 91 (35.8%) | 2449 (16.6%) | <0.001 |
| TG, mg/dL | 121.1 (66.8) | 145.8 (70.1) | 120.6 (66.7) | <0.001 |
| TC, mg/dL | 191.3 (40.9) | 190.3 (46.2) | 191.1 (40.8) | <0.001 |
| HDL-C, mg/dL | 54.1 (15.6) | 51.9 (15.3) | 54.1 (15.6) | <0.001 |
| LDL-C, mg/dL | 113.0 (35.7) | 111.4 (39.2) | 113.0 (35.6) | <0.001 |
| RC, mg/dL | 24.2 (13.4) | 29.1 (14.0) | 24.1 (13.3) | <0.001 |
| NHDL-C, mg/dL | 137.2 (40.2) | 140.5 (44.5) | 137.1 (40.1) | <0.001 |
| ApoB, mg/dL | 92.1 (25.0) | 98.0 (27.7) | 92.0 (24.9) | <0.001 |
| Comorbid illness, % | ||||
| Diabetes mellitus, % | 1654 (11.0%) | 76 (29.9%) | 1578 (10.7%) | <0.001 |
| Hypertension, % | 5162 (34.4%) | 160 (63.0%) | 5002 (33.9%) | <0.001 |
| Congestive heart failure | 478 (3.2%) | 53 (20.9%) | 425 (2.9%) | <0.001 |
| Coronary heart disease | 594 (4.0%) | 52 (20.5%) | 542 (3.7%) | <0.001 |
| Stroke | 562 (3.7%) | 35 (13.8%) | 527 (3.6%) | <0.001 |
Data are presented as mean (SD) or n (%).
TG, triglyceride; TC, total cholesterol; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; ApoB, apolipoprotein B; RC, remnant cholesterol; NHDL-C, non-high density lipoprotein cholesterol.
Based on the primary endpoint—CV mortality, participants were divided into death group (254, 1.69%) and survival group (14738, 98.31%). Comparing these two groups, significances were shown in age, sex, BMI, education level, ethnicity, cigarette smoke, alcohol use, lipid-lowing drug, lipid profiles (including TG, TC, LDL-C, HDL-C, ApoB, RC, and NHDL-C), and the comorbidity (All P < 0.05).
3.2. Survival analysis
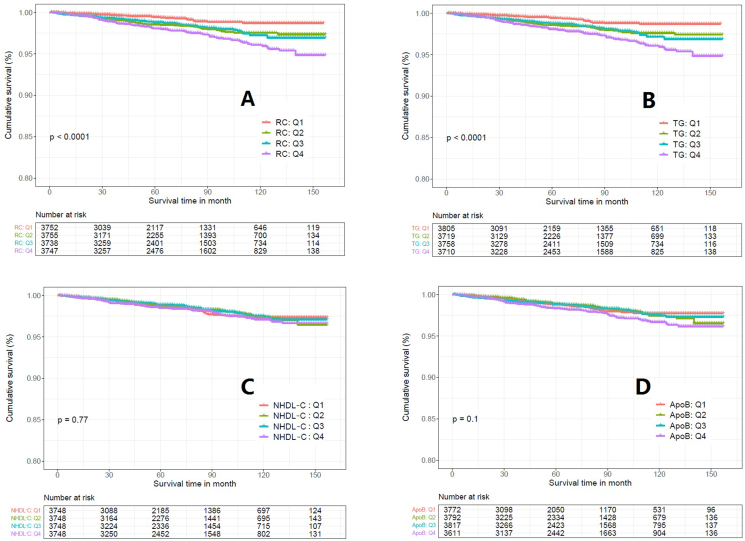
Kaplan-Meier survival curve showed that the survival rate of RC in the highest quartile (Q4) was lower than in the other three quartile groups, and the difference between groups was statistically significant (Log-rank test P < 0.0001, Figure 2A). TG showed a similar result as RC with statistical significance between groups (P < 0.0001, Figure 2B). On the other hand, the survival rate of patients with NHDL-C (P = 0.77, Figure 2C) and ApoB (P = 0.1, Figure 2D) did not show significance between quartiles.
Figure 2.
Kaplan-Meier survival curve between different quartile of the lipid parameters in cardiovascular mortality. A: Remnant Cholesterol; B: Triglyceride; C: Non-High Density Lipoprotein Cholesterol; D: Apolipoprotein B.
3.3. Prognostic value
Furthermore, univariate COX regression analysis showed that the risk of CV mortality of RC was significantly different between quartiles (P < 0.001); compared to Q1, the Q4 group had around 3-fold higher cardiovascular mortality risk (unadjusted HR = 3.23; 95%CI 2.13–4.91, P < 0.001, Table 2); while NHDL-C did not show significance in overall and quartile comparison (All P > 0.05, Table 2). In addition, age, sex, education level, ethnicity, cigarette smoke, alcohol, creatinine, triglyceride, lipid-lowering drugs, and comorbidity (including diabetes mellitus, hypertension, coronary heart disease, congestive heart failure, and stroke) were also associated with the risk of CV mortality (All P < 0.05).
Table 2.
Univariate COX regression analysis for the prediction of cardiovascular mortality.
| HR (95%CI) | P value | |
|---|---|---|
| Age | 1.10 (1.09,1.11) | <0.001 |
| Male | 1.93 (1.49,2.49) | <0.001 |
| Body mass index | 0.99 (0.98,1.01) | 0.571 |
| Education level | <0.001 | |
| Below high school | 1 | |
| High school | 0.73 (0.53,0.99) | 0.042 |
| Above high school | 0.46 (0.34,0.61) | <0.001 |
| Race/ethnicity | <0.001 | |
| Mexican American | 1 | |
| Other Hispanic | 1.04 (0.52,2.10) | 0.914 |
| Non-Hispanic White | 2.47 (1.64,3.71) | <0.001 |
| Non-Hispanic Black | 1.44 (0.89,2.32) | 0.139 |
| Other race | 0.88 (0.40,1.93) | 0.745 |
| Cigarette Smoke | 1.71 (1.33,2.20) | <0.001 |
| Alcohol user | 0.77 (0.59,1.00) | 0.047 |
| Creatinine | 1.00 (1.00,1.01) | <0.001 |
| Lipid-lowering drugs | 3.06 (2.37,3.96) | <0.001 |
| RC | 1.02 (1.01,1.03) | <0.001 |
| Triglyceride | 1.00 (1.00,1.01) | <0.001 |
| NHDL | 1.00 (1.00,1.00) | 0.382 |
| ApoB | 1.01 (1.00,1.01) | 0.007 |
| Comorbidity | ||
| Diabetes mellitus | 3.90 (2.98,5.11) | <0.001 |
| Hypertension | 3.59 (2.78,4.64) | <0.001 |
| Coronary heart disease | 7.10 (5.23,9.63) | <0.001 |
| Congestive heart failure | 10.57 (7.80,14.31) | <0.001 |
| Stroke | 4.87 (3.41,6.96) | <0.001 |
| RC in Quartile | <0.001 | |
| Q1 | 1 (reference) | |
| Q2 | 2.12 (1.36,3.31) | <0.001 |
| Q3 | 2.08 (1.33,3.24) | <0.001 |
| Q4 | 3.23 (2.13,4.91) | <0.001 |
| NHDL-C in Quartile | 0.766 | |
| Q1 | 1 (reference) | |
| Q2 | 0.97 (0.68,1.39) | 0.885 |
| Q3 | 0.93 (0.65,1.33) | 0.688 |
| Q4 | 1,11 (0.79,1.56) | 0.546 |
RC, remnant cholesterol; NHDL-C, non high density lipoprotein Cholesterol; Q, quarter.
RC (mg/dL): Q1 <14.85, Q2 14.85–20.96, Q3 20.96–30.13, Q4 >30.13.
TG (mg/dL): Q1 <73.0, Q2 73.0–104.0, Q3 104.0–152.0, Q4 >152.0.
NHDL (mg/dL): Q1<42.92, Q2 42.92–51.82, Q3 51.82–61.87, Q4 >61.87.
ApoB (mg/dL): Q1 <74.0, Q2 74.0–90.0, Q3 90.0–108.0, Q4 >108.0.
Further analysis using multivariate Cox proportional hazards regression (Table 3), the highest quartile (Q4) of RC remained statistically significant compared to Q1 after adjustment (Model 3: HR = 1.63, 95%CI 1.05–2.52, P = 0.037). Also, across the increasing quartiles of RC showed a significant trend regardless of the adjustment models (All P for trend <0.05).
Table 3.
Multivariate COX regression analysis for the prediction of cardiovascular mortality.
| Model 1 |
Pt | Model 2 |
Pt | Model 3 |
Pt | |
|---|---|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | ||||
| RC | <0.001 | 0.012 | 0.037 | |||
| Q1 | 1 | 1 | 1 | |||
| Q2 | 2.12 (1.36,3.31)∗∗∗ | 1.35 (0.87,2.12) | 1.41 (0.90,2.21) | |||
| Q3 | 2.08 (1.33,3.24)∗∗∗ | 1.12 (0.72,1.75) | 1.11 (0.70,1.74) | |||
| Q4 | 3.23 (2.13,4.91)∗∗∗ | 1.73 (1.14,2.64) ∗ | 1.63 (1.05,2.52)∗ | |||
| TG | <0.001 | 0.016 | 0.049 | |||
| Q1 | 1 | 1 | 1 | |||
| Q2 | 2.04 (1.31,3.17)∗∗ | 1.31 (0.84,2.04) | 1.40 (0.89,2.19) | |||
| Q3 | 2.10 (1.35,3.25)∗∗ | 1.16 (0.75,1.79) | 1.19 (0.76,1.86) | |||
| Q4 | 3.17 (2.09,4.79)∗∗∗ | 1.73 (1.14,2.62)∗ | 1.69 (1.10,2.60)∗ | |||
| NHDL | 0.766 | 0.724 | 0.404 | |||
| Q1 | 1 | 1 | 1 | |||
| Q2 | 0.97 (0.68,1.39) | 0.92 (0.65,1.31) | 1.07 (0.74,1.52) | |||
| Q3 | 0.93 (0.65,1.33) | 0.88 (0.61,1.26) | 1.12 (0.77,1.62) | |||
| Q4 | 1,11 (0.79,1.56) | 1.06 (0.75,1.51) | 1.32 (0.92,1.88) | |||
| ApoB | 0.102 | 0.431 | 0.209 | |||
| Q1 | 1 | 1 | 1 | |||
| Q2 | 1.03 (0.71,1.51) | 0.88 (0.61,1.29) | 0.94 (0.64,1.37) | |||
| Q3 | 1.04 (0.72,1.51) | 0.82 (0.56,1.19) | 1.01 (0.69,1.48) | |||
| Q4 | 1.43 (1.01,2.03)∗ | 1.06 (0.75,1.51) | 1.31 (0.91,1.88) |
HR, Hazard ratio; 95%CI, 95% confidence interval; Pt, P for trend; RC, remnant cholesterol; TG, triglyceride; NHDL-C, non-high density lipoprotein cholesterol; ApoB, apolipoprotein B.
Model 1: unadjusted.
Model 2: adjusted for age and sex.
Model 3: adjusted for age, sex, ethnicity, education level, diabetes mellitus, hypertension, smoker, alcohol user, BMI, coronary heart disease, congestive heart failure and stroke, lipid-lowering drugs.
RC (mg/dL): Q1 <14.85, Q2 14.85–20.96, Q3 20.96–30.13, Q4 >30.13.
TG (mg/dL): Q1 <73.0, Q2 73.0–104.0, Q3 104.0–152.0, Q4 >152.0.
NHDL (mg/dL): Q1<42.92, Q2 42.92–51.82, Q3 51.82–61.87, Q4 >61.87.
ApoB (mg/dL): Q1 <74.0, Q2 74.0–90.0, Q3 90.0–108.0, Q4 >108.0.
TG also demonstrated a similar significant trend for cardiovascular mortality risk across the quartiles (Model 3, Q1 vs Q4: HR = 1.69 95%CI 1.10–2.60, P = 0.049). On the other hand, ApoB only showed significance in Q4 in the unadjusted model. NHDL-C did not show statistically significant in both univariate and multivariate COX regression, P for trend across the quartiles were all >0.05.
Further analysis employing both increasing value and the cut-off values based on previous research [9], the increasing value of RC showed significant association with cardiovascular mortality (Model 3: HR = 1.05, 95%CI 1.01–1.10, P = 0.025, Table 4) but no significance in the cut-off model (≥15.5 mg/dL). NHDL-C did not show significance in CVD mortality, while significantly associated with all-cause mortality after adjustment (Cut-off ≥130 mg/dL, Model 3: HR = 0.79, 95% CI 0.71–0.89, P < 0.001, Table 4).
Table 4.
Multivariate COX regression analysis for the prediction of all-cause mortality and cardiovascular mortality.
| RC per 5 mg/dL increase |
RC ≥ 15.5 mg/dL |
NHDL per 5 mg/dL increase |
NHDL ≥130 mg/dL |
|||||
|---|---|---|---|---|---|---|---|---|
| HR (95%CI) | P | HR (95%CI) | p | HR (95%CI) | P | HR (95%CI) | p | |
| All-cause mortality | ||||||||
| Model 1 | 1.06 (1.04, 1.07) | <0.001 | 1.63 (1.43, 1.87) | <0.001 | 0.99 (0.99, 1.00) | 0.046 | 0.84 (0.75, 0.93) | 0.001 |
| Model 2 | 1.00 (0.98, 1.02) | 0.998 | 0.93 (0.84, 1.07) | 0.355 | 0.99 (0.98, 0.99) | <0.001 | 0.74 (0.67, 0.83) | <0.001 |
| Model 3 | 0.99 (0.97, 1.01) | 0.398 | 0.94 (0.82, 1.08) | 0.400 | 0.99 (0.98, 1.00) | 0.003 | 0.79 (0.71, 0.89) | <0.001 |
| Cardiovascular mortality | ||||||||
| Model 1 | 1.11 (1.07, 1.15) | <0.001 | 2.33 (1.63, 3.33) | <0.001 | 1.01 (0.99, 1.02) | 0.382 | 0.99 (0.77, 1.26) | 0.920 |
| Model 2 | 1.07 (1.02, 1.11) | 0.003 | 1.35 (0.94, 1.93) | 0.103 | 1.00 (0.99, 1.02) | 0.636 | 0.93 (0.73, 1.20) | 0.589 |
| Model 3 | 1.05 (1.01, 1.10) | 0.025 | 1.34 (0.93, 1.94) | 0.114 | 1.01 (1.00, 1.03) | 0.073 | 1.13 (0.87, 1.47) | 0.355 |
Model 1: unadjusted.
Model 2: adjusted for age and sex.
Model 3: adjusted for age, sex, ethnicity, education level, diabetes mellitus, hypertension, smoker, alcohol user, BMI, coronary heart disease, congestive heart failure, stroke, and lipid-lowering drugs.
3.4. Restricted cubic spline
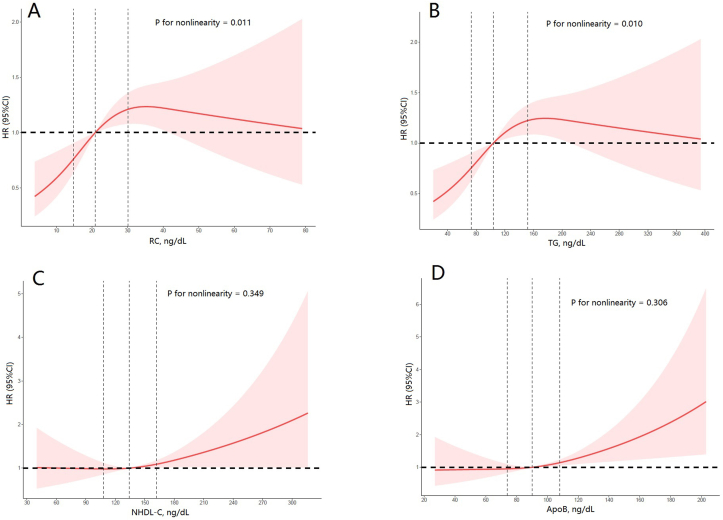
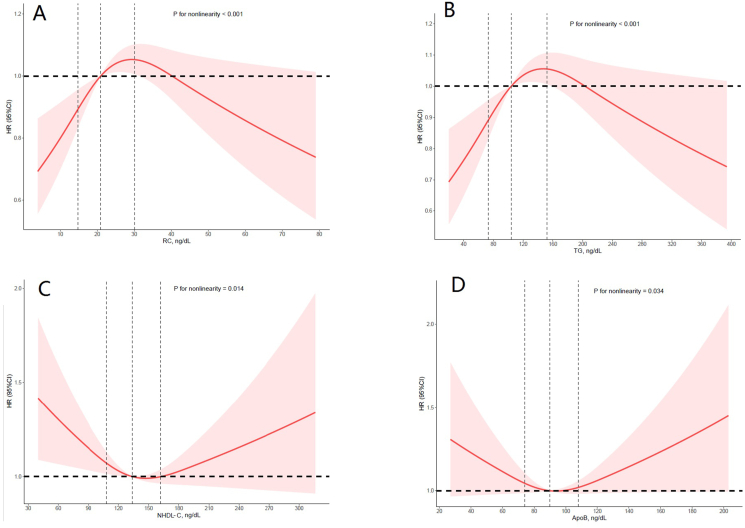
To further evaluate the linearity between the target lipid parameter and the risk of CV mortality, restricted cubic spline model showed a nonlinear J-shaped association in RC (P for non-linearity <0.001, Figure 3A). The inflection point of RC was 29.3 mg/dL (P for log likelihood ratio = 0.003). On the left of the inflection point, the risk of cardiovascular increased with increasing RC (HR = 1.05, 95%CI 1.02–1.08, P < 0.001; Table 5). On the right side of the inflection point, the fluctuation was not significant (HR = 1.00, 95%CI 0.99–1.02, P > 0.05; Table 5). Furthermore, TG also showed a similar nonlinear J-shaped association (P for non-linearity = 0.01, Figure 3B).
Figure 3.
Association between the lipid parameters and cardiovascular (CV) risk. Adjusted hazard ratio of CV mortality from a restricted cubic spline logistic regression model with knots at the 5th, 35th, 65th, and 95th percentiles. Adjusted for age, sex, ethnicity, education level, diabetes mellitus, hypertension, smoker, alcohol user, BMI, coronary heart disease, congestive heart failure and stroke, lipid-lowering drugs. The solid line and marked area represent the log-transformed hazard ratios and corresponding 95% confidence intervals. A: Remnant Cholesterol; B: Triglyceride; C: Non-High Density Lipoprotein Cholesterol; D: Apolipoprotein B.
Table 5.
Threshold effect analysis of the relationship between remnant cholesterol and cardiovascular death using 2 piece-wise linear regression models.
| Remnant Cholesterol Inflection Point | group | HR (95% CI) | P for log likelihood ratio |
|---|---|---|---|
| 29.3 | ≤29.3 | 1.05 (1.02, 1.08)∗∗∗ | 0.003 |
| >29.3 | 1.00 (0.99, 1.02) |
HR: Hazard Ratio.
∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001.
Although ApoB and NHDL-C did not show significant in trend, ApoB (P for non-linearity = 0.001, Figure 3C) and NHDL-C (P for non-linearity = 0.349, Figure 3D) showed a linear association in RCS.
3.5. Result for secondary endpoint of all-cause mortality
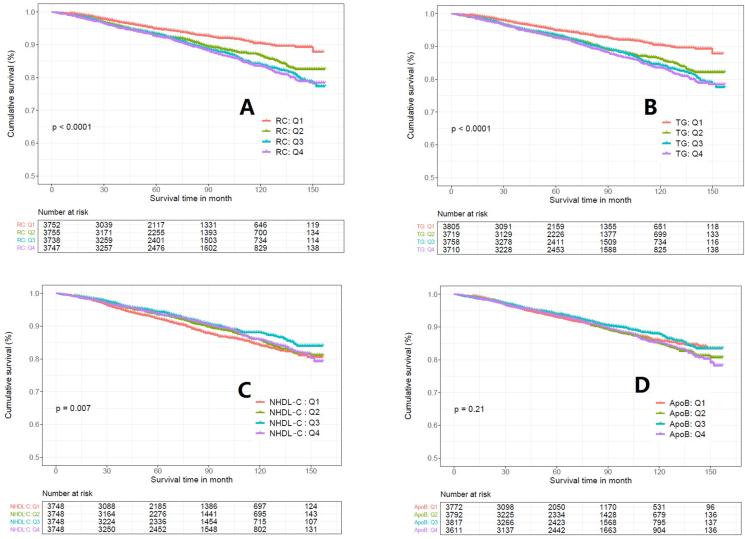
The association of the lipid parameters above with the secondary endpoint—all-cause mortality were also explored (Table 6). RC, TG, NHDL-C, and ApoB were all shown significance in the univariate Cox regression analysis. Results of K-M survival analysis, Cox regression analysis, and RCS suggested that both ApoB and NHDL-C showed a U-shape association with all-cause mortality (Figures 4 and 5, Table 7). To be noted, TG and RC remained a similar trend and pattern in the analysis.
Table 6.
Baseline Characteristics by All-cause death.
| Variable | All (n = 14992) | Death (n = 1411) | Survival (n = 13581) | P value |
|---|---|---|---|---|
| Age, years | 48.1 (19.2) | 70.0 (14.4) | 45.9 (18.2) | <0.001 |
| Male, % | 7428 (49.5%) | 824 (58.4%) | 6604 (48.6%) | <0.001 |
| Body mass index, kg/m2 | 28.6 (6.8) | 28.2 (6.2) | 28.7 (6.8) | 0.001 |
| Education level, % | <0.001 | |||
| Below high school | 4006 (26.7%) | 581 (41.2%) | 3425 (25.2%) | |
| High school | 3452 (23.0%) | 352 (24.9%) | 3100 (22.8%) | |
| Above high school | 7534 (50.3%) | 478 (33.9%) | 7056 (52.0%) | |
| Race/ethnicity, % | <0.001 | |||
| Mexican American | 2529 (16.9%) | 189 (13.4%) | 2340 (17.2%) | |
| Other Hispanic | 1255 (8.4%) | 63 (4.5%) | 1192 (8.8%) | |
| Non-Hispanic White | 6830 (45.6%) | 859 (60.9%) | 5971 (44.0%) | |
| Non-Hispanic Black | 3140 (20.9%) | 248 (17.6%) | 2892 (21.3%) | |
| Other race | 1238 (8.3%) | 52 (3.7%) | 1186 (8.7%) | |
| Smoker, % | 6789 (45.3%) | 816 (57.8%) | 5973 (44.0%) | <0.001 |
| Alcohol user, % | 10649 (71.0%) | 921 (65.3%) | 9728 (71.6%) | <0.001 |
| Lipid-lowering drugs, % | 2540 (16.9%) | 410 (29.1%) | 2130 (15.7%) | <0.001 |
| TC, mg/dL | 191.3 (40.9) | 190.3 (46.2) | 191.4 (40.3) | <0.001 |
| HDL-C, mg/dL | 54.1 (15.6) | 54.0 (16.8) | 54.1 (15.4) | <0.001 |
| LDL-C, mg/dL | 113.0 (35.7) | 109.4 (40.0) | 113.4 (35.2) | <0.001 |
| RC, mg/dL | 24.2 (13.4) | 26.9 (13.7) | 23.9 (13.3) | <0.001 |
| NHDL-C, mg/dL | 137.2 (40.2) | 136.3 (44.2) | 137.3 (39.8) | <0.001 |
| Comorbid illness, % | ||||
| Diabetes mellitus, % | 1654 (11.0%) | 355 (25.2%) | 1299 (9.6%) | <0.001 |
| Hypertension, % | 5162 (34.4%) | 845 (59.9%) | 4317 (31.8%) | <0.001 |
| Coronary heart disease | 478 (3.2%) | 193 (13.7%) | 285 (2.1%) | <0.001 |
| Congestive heart failure | 594 (4.0%) | 201 (14.2%) | 393 (2.9%) | <0.001 |
| Stroke | 562 (3.7%) | 187 (13.3%) | 375 (2.8%) | <0.001 |
Data are presented as mean (SD) or n (%).
TC, total cholesterol; HDL-C, high density lipoprotein cholesterol; LDL, low density lipoprotein cholesterol; RC, remnant cholesterol; NHDL-C, non-high density lipoprotein cholesterol.
Figure 4.
Kaplan-Meier survival curve between different quartile of the lipid parameters in all-cause mortality. A: Remnant Cholesterol; B: Triglyceride; C: Non-High Density Lipoprotein Cholesterol; D: Apolipoprotein B.
Figure 5.
Association between the lipid parameters and all-cause mortality. Adjusted hazard ratio of all-cause mortality from a restricted cubic spline logistic regression model with knots at the 5th, 35th, 65th, and 95th percentiles. Adjusted for age, sex, ethnicity, education level, diabetes mellitus, hypertension, smoker, alcohol user, BMI, coronary heart disease, congestive heart failure and stroke, lipid-lowering drugs. The solid line and marked area represent the log-transformed hazard ratios and corresponding 95% confidence intervals. A: Remnant Cholesterol; B: Triglyceride; C: Non-High Density Lipoprotein Cholesterol; D: Apolipoprotein B.
Table 7.
Multivariate COX regression analysis for the prediction of all-cause mortality.
| Model 1 |
Pt | Model 2 |
Pt | Model 3 |
Pt | |
|---|---|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | ||||
|
RC | ||||||
| Q1 | 1 | <0.001 | 1 | 0.597 | 1 | 0.304 |
| Q2 | 1.46 (1.24,1.73)∗∗∗ | 0.94 (0.80,1.12) | 0.97 (0.82,1.15) | |||
| Q3 | 1.65 (1.40,1.95)∗∗∗ | 0.90 (0.76,1.06) | 0.90 (0.76,1.06) | |||
| Q4 |
1.74 (1.48,2.05)∗∗∗ |
0.93 (0.79,1.09) |
0.88 (0.74,1.04) |
|||
|
TG |
<0.001 |
0.406 |
0.186 |
|||
| Q1 | 1 | 1 | 1 | |||
| Q2 | 1.54 (1.30,1.81)∗∗∗ | 0.99 (0.84,1.18) | 1.04 (0.88,1.23) | |||
| Q3 | 1.63 (1.38,1.91)∗∗∗ | 0.89 (0.76,1.05) | 0.91 (0.77,1.07) | |||
| Q4 |
1.75 (1.49,2.06)∗∗∗ |
0.94 (0.80,1.10) |
0.90 (0.76,1.07) |
|||
|
NHDL |
0.007 |
<0.001 |
0.009 |
|||
| Q1 | 1 | 1 | 1 | |||
| Q2 | 0.88 (0.76,1.02) | 0.79 (0.68,0.91)∗∗ | 0.86 (0.75,1.00) | |||
| Q3 | 0.77 (0.66,0.89) | 0.64 (0.55,0.74)∗∗∗ | 0.77 (0.66,0.90)∗ | |||
| Q4 |
0.87 (0.75,1.00) |
0.69 (0.59,0.80)∗∗∗ |
0.84 (0.72,0.97)∗ |
|||
|
ApoB |
0.213 |
<0.001 |
0.001 |
|||
| Q1 | 1 | 1 | 1 | |||
| Q2 | 1.03 (0.89,1.20) | 0.85 (0.73,0.98)∗ | 0.87 (0.74,1.01) | |||
| Q3 | 0.91 (0.78,1.06) | 0.68 (0.58,0.79)∗∗∗ | 0.75 (0.64,0.88)∗∗∗ | |||
| Q4 | 1.05 (0.91,1.22) | 0.73 (0.63,0.85)∗∗∗ | 0.78 (0.67,0.91)∗∗ | |||
HR, Hazard ratio; 95%CI, 95% confidence interval; Pt, P for trend; RC, remnant cholesterol; TG, triglyceride; NHDL, non-high density lipoprotein cholesterol; ApoB, apolipoprotein B.
Model 1: unadjusted.
Model 2: adjusted for age and sex.
Model 3: adjusted for age, sex, ethnicity, education level, diabetes mellitus, hypertension, smoker, alcohol user, BMI, coronary heart disease, congestive heart failure and stroke, lipid-lowering drugs.
RC (mg/dL): Q1 <14.85, Q2 14.85–20.96, Q3 20.96–30.13, Q4 >30.13.
TG (mg/dL): Q1 <73.0, Q2 73.0–104.0, Q3 104.0–152.0, Q4 >152.0.
NHDL (mg/dL): Q1<42.92, Q2 42.92–51.82, Q3 51.82–61.87, Q4 >61.87.
ApoB (mg/dL): Q1 <74.0, Q2 74.0–90.0, Q3 90.0–108.0, Q4 >108.0.
3.6. Subgroup analysis of CVD mortality risk prediction
While the above results showed the association with RC and cardiovascular mortality and association with NHDL-C and all-cause mortality, subgroup analysis incorporating the Framingham risk score (FRS) did not show added risk prediction value across the increasing quartiles (P > 0.05, Tables 8, 9, and 10).
Table 8.
Discrimination and reclassification after update FRS Model.
| Endpoint level | FRS Model | Update Model | C-index | P value | IDI (95%CI) | P value | NRI (95%CI) | P value |
|---|---|---|---|---|---|---|---|---|
| CV mortality | age, sex, blood pressure, smoking, LDL, and HDL | age, sex, blood pressure, smoking, and RC | 0.863 vs 0.864 | 0.507 | 0.001 (-0.006, 0.006) | 0.931 | 0.057 (-0.048, 0.151) | 0.554 |
| age, sex, blood pressure, smoking, TC, and HDL | age, sex, blood pressure, smoking, and RC | 0.864 vs 0.864 | 0.603 | 0.001 (-0.006, 0.006) | 0.911 | 0.038 (-0.089, 0.145) | 0.653 | |
| All-cause mortality | age, sex, blood pressure, smoking, LDL, and HDL | age, sex, blood pressure, smoking, and NHDL | 0.832 vs 0.831 | 0.200 | 0.002 (-0.002, 0.005) | 0.455 | 0.006 (-0.041, 0.053) | 0.733 |
| age, sex, blood pressure, smoking, TC, and HDL | age, sex, blood pressure, smoking, and NHDL | 0.831 vs 0.831 | 0.386 | 0.002 (-0.001, 0.005) | 0.257 | 0.009 (-0.033, 0.044) | 0.614 |
IDI, integrated discrimination improvement; NRI, net reclassification improvement; CI, confidence interval.
Table 9.
Subgroup analysis of the association with RC and cardiovascular mortality.
| Variables | Subgroups | N | Q1 |
Q2 |
Q3 |
Q4 |
p-t | p-int |
|---|---|---|---|---|---|---|---|---|
| OR | OR (95%CI) | OR (95%CI) | OR (95%CI) | |||||
| Age | <45 | 6995 | 1.00 (Ref.) | 1.30 (0.18, 9.55) | 2.43 (0.41, 14.29) | 1.92 (0.29, 12.65) | 0.770 | 0.708 |
| 45–69 | 5413 | 1.00 (Ref.) | 1.27 (0.51, 3.17) | 1.23 (0.50, 3.00) | 1.60 (0.68, 3.75) | 0.662 | ||
| >69 | 2584 | 1.00 (Ref.) | 1.52 (0.88, 2.61) | 1.07 (0.62, 1.86) | 1.70 (1.00, 2.91) | 0.061 | ||
| Sex | Male | 7428 | 1.00 (Ref.) | 1.60 (0.92, 2.80) | 1.18 (0.67, 2.07) | 1.80 (1.05, 3.08) | 0.067 | 0.856 |
| Female | 7564 | 1.00 (Ref.) | 1.13 (0.53, 2.43) | 1.00 (0.46, 2.14) | 1.49 (0.70, 3.17) | 0.482 | ||
| Lipid-lowering drugs | Yes | 2540 | 1.00 (Ref.) | 2.48 (0.93, 6.62) | 1.66 (0.62, 4.43) | 2.74 (1.06, 7.11) | 0.081 | 0.392 |
| No | 12452 | 1.00 (Ref.) | 1.22 (0.73, 2.04) | 1.04 (0.62, 1.74) | 1.40 (0.85, 2.33) | 0.410 | ||
| Hypertension | Yes | 5162 | 1.00 (Ref.) | 1.20 (0.68, 2.13) | 1.07 (0.61, 1.87) | 1.73 (1.02, 2.93) | 0.051 | 0.574 |
| No | 9830 | 1.00 (Ref.) | 1.74 (0.82, 3.70) | 1.23 (0.57, 2.69) | 1.57 (0.73, 3.39) | 0.391 | ||
| Diabetes | Yes | 1654 | 1.00 (Ref.) | 1.70 (0.61, 4.70) | 1.51 (0.56, 4.09) | 2.15 (0.82, 5.64) | 0.364 | 0.958 |
| No | 13338 | 1.00 (Ref.) | 1.37 (0.83, 2.27) | 1.06 (0.63, 1.77) | 1.56 (0.95, 2.56) | 0.141 | ||
| FRS | Low risk | 11145 | 1.00 (Ref.) | 1.33 (0.69, 2.55) | 0.76 (0.37, 1.54) | 1.23 (0.60, 2.51) | 0.310 | 0.647 |
| Moderate risk | 2682 | 1.00 (Ref.) | 1.28 (0.59, 2.79) | 1.29 (0.60, 2.75) | 1.53 (0.71, 3.29) | 0.745 | ||
| High risk | 1165 | 1.00 (Ref.) | 1.98 (0.57, 6.87) | 1.74 (0.51, 5.92) | 3.00 (0.91, 9.92) | 0.080 |
Analyses were adjusted for covariates age, sex, ethnicity, education level, diabetes mellitus, hypertension, smoker, alcohol user, BMI, coronary heart disease, congestive heart failure, stroke, and lipid-lowering drugs when they were not the strata variables. HR, hazard ratio; CI, confidence interval; Q: quartile; Ref., reference; p-t, p for trend; p-int, p for interaction. FRS, Framingham risk score; Low risk (<10%), moderate risk (10–20%), high risk (>20%). All risks calculated using FRS.
Table 10.
Subgroup analysis of the association with NHDL-C and all-cause mortality.
| Variables | Subgroups | N | Q1 |
Q2 |
Q3 |
Q4 |
p-t | p-int |
|---|---|---|---|---|---|---|---|---|
| OR | OR (95%CI) | OR (95%CI) | OR (95%CI) | |||||
| Age | <45 | 6995 | 1.00 (Ref.) | 0.95 (0.56, 1.63) | 0.97 (0.55, 1.69) | 1.12 (0.65, 1.93) | 0.944 | 0.002 |
| 45–69 | 5413 | 1.00 (Ref.) | 0.73 (0.55, 0.97) | 0.59 (0.44, 0.79) | 0.64 (0.48, 0.84) | 0.002 | ||
| >69 | 2584 | 1.00 (Ref.) | 0.88 (0.73, 1.06) | 0.80 (0.66, 0.97) | 0.81 (0.67, 0.98) | 0.091 | ||
| Sex | Male | 7428 | 1.00 (Ref.) | 0.85 (0.71, 1.03) | 0.71 (0.58, 0.87) | 0.75 (0.61, 0.91) | 0.004 | 0.933 |
| Female | 7564 | 1.00 (Ref.) | 0.87 (0.69, 1.11) | 0.80 (0.63, 1.02) | 0.83 (0.66, 1.05) | 0.304 | ||
| Lipid-lowering drugs | Yes | 2540 | 1.00 (Ref.) | 0.85 (0.66, 1.10) | 0.97 (0.74, 1.28) | 1.16 (0.87, 1.55) | 0.227 | 0.002 |
| No | 12452 | 1.00 (Ref.) | 0.83 (0.69, 0.99) | 0.63 (0.52, 0.76) | 0.67 (0.56, 0.80) | <0.001 | ||
| Hypertension | Yes | 5162 | 1.00 (Ref.) | 0.89 (0.74, 1.08) | 0.86 (0.71, 1.04) | 0.83 (0.68, 1.01) | 0.255 | 0.089 |
| No | 9830 | 1.00 (Ref.) | 0.78 (0.62, 0.99) | 0.57 (0.44, 0.74) | 0.69 (0.55, 0.87) | <0.001 | ||
| Diabetes | Yes | 1654 | 1.00 (Ref.) | 0.88 (0.67, 1.16) | 0.92 (0.68, 1.25) | 0.96 (0.71, 1.30) | 0.819 | 0.152 |
| No | 13338 | 1.00 (Ref.) | 0.84 (0.70, 0.99) | 0.67 (0.56, 0.81) | 0.71 (0.59, 0.84) | <0.001 | ||
| FRS | Low risk | 11145 | 1.00 (Ref.) | 0.73 (0.59, 0.91) | 0.64 (0.50, 0.82) | 0.62 (0.48, 0.80) | <0.001 | 0.055 |
| Moderate risk | 2682 | 1.00 (Ref.) | 0.90 (0.70, 1.16) | 0.71 (0.53, 0.95) | 0.79 (0.59, 1.07) | 0.127 | ||
| High risk | 1165 | 1.00 (Ref.) | 1.29 (0.88, 1.91) | 1.20 (0.81, 1.76) | 1.23 (0.84, 1.82) | 0.630 |
Analyses were adjusted for covariates age, sex, ethnicity, education level, diabetes mellitus, hypertension, smoker, alcohol user, BMI, coronary heart disease, congestive heart failure, stroke, and lipid-lowering drugs when they were not the strata variables. HR, hazard ratio; CI, confidence interval; Q: quartile; Ref., reference; p-t, p for trend; p-int, p for interaction. FRS, Framingham risk score; Low risk (<10%), moderate risk (10–20%), high risk (>20%). All risks calculated using FRS.
Furthermore, association between the lipids and CVD mortality in the subgroup patients who have been taking lipid-lowering therapy were analyzed. Although the overall trend did not show significance, multivariate regression analysis showed that TG (Model 3, Q1 vs Q4: HR = 2.74, 95%CI 1.06–7.11) and RC (Model 3, Q1 vs Q4: HR = 3.01, 95%CI 1.16–7.80) in the highest quartile remained significant among the models (Table 11).
Table 11.
Multivariate COX regression analysis for the prediction of cardiovascular mortality in patients with lipid-lowering therapy.
| Model 1 |
Pt | Model 2 |
Pt | Model 3 |
Pt | |
|---|---|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | ||||
|
RC |
0.128 |
0.016 |
0.081 |
|||
| Q1 | 1 | 1 | 1 | |||
| Q2 | 2.27 (0.85–6.01) | 2.36 (0.89–6.26) | 2.48 (0.93–6.62) | |||
| Q3 | 1.72 (0.65–4.53) | 1.76 (0.66–4.65) | 1.66 (0.62–4.43) | |||
| Q4 |
2.61 (1.03–6.60) ∗ |
3.34 (1.32–8.47) ∗ |
2.74 (1.06–7.11) ∗ |
|||
|
TG |
0.133 |
0.015 |
0.079 |
|||
| Q1 | 1 | 1 | 1 | |||
| Q2 | 2.21 (0.83–5.93) | 2.30 (0.86–6.18) | 2.50 (0.93–6.73) | |||
| Q3 | 1.90 (0.72–4.98) | 1.97 (0.75–5.18) | 1.91 (0.72–5.05) | |||
| Q4 |
2.74 (1.08–6.92) ∗ |
3.52 (1.39–8.93) ∗∗ |
3.01 (1.16–7.80) ∗ |
|||
|
NHDL |
0.681 |
0.186 |
0.166 |
|||
| Q1 | 1 | 1 | 1 | |||
| Q2 | 0.71 (0.40–1.24) | 0.79 (0.45–1.39) | 0.93 (0.52–1.65) | |||
| Q3 | 0.89 (0.50–1.56) | 1.22 (0.69–2.15) | 1.40 (0.79–2.50) | |||
| Q4 |
0.87 (0.49–1.56) |
1.53 (0.84–2.76) |
1.72 (0.94–3.14) |
|||
|
ApoB |
0.713 |
0.265 |
0.251 |
|||
| Q1 | 1 | 1 | 1 | |||
| Q2 | 0.81 (0.45–1.46) | 0.93 (0.51–1.67) | 0.98 (0.54–1.79) | |||
| Q3 | 0.73 (0.40–1.34) | 0.91 (0.49–1.68) | 1.07 (0.58–1.99) | |||
| Q4 | 0.94 (0.53–1.69) | 1.51 (0.83–2.72) | 1.65 (0.90–3.01) |
HR, Hazard ratio; 95%CI, 95% confidence interval; Pt, P for trend; RC, remnant cholesterol; TG, triglyceride; NHDL-C, non-high density lipoprotein cholesterol; ApoB, apolipoprotein B.
Model 1: unadjusted.
Model 2: adjusted for age and sex.
Model 3: adjusted for age, sex, ethnicity, education level, diabetes mellitus, hypertension, smoker, alcohol user, BMI, coronary heart disease, congestive heart failure and stroke, lipid-lowering drugs.
RC (mg/dL): Q1 <14.85, Q2 14.85–20.96, Q3 20.96–30.13, Q4 >30.13.
TG (mg/dL): Q1 <73.0, Q2 73.0–104.0, Q3 104.0–152.0, Q4 >152.0.
NHDL (mg/dL): Q1<42.92, Q2 42.92–51.82, Q3 51.82–61.87, Q4 >61.87.
ApoB (mg/dL): Q1 <74.0, Q2 74.0–90.0, Q3 90.0–108.0, Q4 >108.0.
4. Discussion
Present study demonstrated the association between lipid profiles and cardiovascular mortality in a US national-scale general population. Among, we found that both higher levels of remnant cholesterol and triglyceride were associated with the worse cardiovascular outcome in Kaplan-Meier survival curve and Cox regression analysis. Furthermore, both RC and TG demonstrated a nonlinear J-shaped association with CV mortality. On the other hand, ApoB and NHDL-C did not show a significant association in CV mortality, whereas showed a U-shape association with all-cause mortality.
Currently, management in reducing LDL-C has been recommended as the main strategy for reducing CVD risk. However, there are still wide gaps in the assessment of the total and residual cardiovascular risk. Previous studies showed the levels of TG and RC, but not LDL-C, were associated with cardiovascular outcomes independent of other risk factors [14]. Even after lowering LDL-C to the guideline recommended target, pathogenesis of AS from TRLs remain contribute to residual atherosclerotic CVD risk [27]. TRL is not only a component of residual risk in patients with well-controlled LDL levels on statins, but also a future risk factor for asymptomatic patients with increased risk. Also, study suggested that utilizing ApoB and NHDL-C as secondary targets are superior in identifying statin-treated patients at residual risk of all-cause mortality and myocardial infarction [19]. Therefore, these parameters reflecting the systemic lipo-metabolism such as TG, RC, NHDL-C, and ApoB might be utilized to develop effective and timely strategies to cope with these challenges of CVD epidemics.
Despite the seemingly smaller number of VLDL and IDL parameters, the physical properties of lower density and small molar in nature cause a comparable concentration in plasma. The definition of RC (TC—LDL-C—HDL-C) and NHDL-C (TC—HDL-C) overlaps at a certain level while both parameters contain the class of VLDL/IDL components. In the current study, these two parameters (RC and NHDL-C) demonstrated a distinct prognostic value, while pair of RC and TG/ApoB and NHDL-C demonstrated a similar and significant trend in CVD prognostic evaluation.
Firstly, RC is primarily composed of VLDL, IDL, and chylomicron. In the fasting state, RC is associated with increased risk for CVD [28, 29, 30]. Previous study also showed that the remnant levels were independently correlated with triglyceride levels [31]. RC contributes to the sum of cholesterol carried within the atherogenic ApoB-containing lipoproteins without the level of LDL-C [32]. As a parameter indicating the level of TRL, RC might show a similar trend in CVD prognostic assessment.
On the other hand, NHDL-C includes the assessment of remnant lipoprotein cholesterol and also the additional risk mainly from LDL-C. Therefore, parameters composed with the level of LDL-C might not accurately reflect the lipometabolic state causing the difference in the risk assessment and prognostic values, while RC might be a better marker for patients with the lipid-lowing agents.
In addition, ApoB is a direct measure of circulating numbers of atherogenic lipoproteins containing VLDL, IDL, and LDL. NHDL-C includes but is not limited to these lipid components which could explain the similar results of the current study. Also, previous study showed ApoB is superior to NHDL-C as a secondary target for residual risk control and management in CVD patients. It has been raised that while ApoB is not available, NHDL-C should be used to supplement LDL-C [33]. However, our results regarding NHDL-C only demonstrated significant U-shape association with all-cause mortality similar to a previous study among hypertension population [27], but it did not show positive results in CV mortality. A plausible explanation is that, with the influence of LDL-C, the “harvest” phenomenon occurred wherein the older individuals with hypercholesterolemia are those who are less susceptible to the pathological effects of cholesterol [34, 35].
Moreover, it is worth mentioning that there were J-shaped relationship between TGs (TG and RC) and the cardiovascular risk. There are multiple underlying mechanisms that influenced the TG levels by a combination of environmental and genetic factors that regulate from synthesis to circulation. The pathophysiology of increasing TG overlapped with various metabolic cardiovascular risk conditions [36, 37, 38] such as obesity, diabetes, metabolic syndrome, etc. As in the current study, researchers have focused on the potential role of TG, TRLs, and remnants on cardiovascular risk. The J-shaped association we observed between TGs and CV mortality was consistent with results from some major studies [39].
The mechanisms regulating plasma triglyceride, TRL, and TRL-residue levels involve complex pathways. There is evidence regard to the involvement of triglyceride-rich remnants in development of cardiovascular disease. Because TRL cholesterol (TRL-C) levels change proportionally to residue concentrations, assessing TRL-C provides an approximation of circulating residue levels. Current data emphasizes the distinct value of remnants in CVD and complements a certain knowledge gap for exploring reliable biomarkers in clinical practice. However, it is not an accurate or specific biomarker and must be used with caution. Further prognostic cohort studies should be conducted to determine whether this line of investigation translates into new and effective paradigms for lipid management.
In summary, our findings are consistent with previous studies and support the role of these parameters including TG and RC as risk factors for CVD risk. Thus, measurement of remnant cholesterol levels may be helpful for CVD risk assessment in clinical practice. With the emerging and novel lipid lowering therapies including statins, proprotein convertase subtilisin/kexin type 9 inhibitors, antisense inhibitors of Apolipoprotein (a), microsomal triglyceride transfer protein inhibitors, etc., will likely disturb the precise assessment in these patients. Levels of RC and NHDL-C are relatively accessible parameters. Incorporate these lipid parameters reflecting various aspects of lipometabolism, especially in VLDL and IDL, might provide a better assessment and optimal management target for high CVD risk patients. Results of future prospective cohort studies regarding the measurement of each lipid component and their participating pathway in ASCVD should be further revisited and explored.
4.1. Limitation
There are certain limitations of our study. This study was a community-based retrospective study with a relatively large sample size from representative US general population, but further generalization of the results should be validated in other study settings. Due to the nature of study design, the level of LDL-C was calculated with an indirect method which might lead to a certain level of bias in the analysis of RC. In addition, study included partial participants (16.9%) who received lipid-lowing agents. Although results showed there was significance in the lipid-lowing subgroup which indicated the residual risk, improvement of the methodology and/or selection of population might be needed for precluding bias. Finally, although the regression models included a broad set of covariates, other confounding factors such as eating habits may also have played a role.
5. Conclusion
Our study demonstrated that levels of the target lipid parameters (RC and NHDL-C) are associated with the outcomes in US general population. RC displayed a positive correlation and J-shaped relationship with cardiovascular mortality, while NHDL-C only showed an association in all-cause mortality. Study further supported that considering these TRL parameters in clinical practice could help optimize risk management. Further large-scale prospective studies are required to confirm the prognostic effect and underlying mechanism.
Declarations
Author contribution statement
Iokfai Cheang; Xinli Li: Conceived and designed the experiments; Wrote the paper.
Xu Zhu: Analyzed and interpreted the data; Wrote the paper.
Xinyi Lu; Shi Shi: Analyzed and interpreted the data.
Yuan Tang; Xin Yue; Shengen Liao: Contributed reagents, materials, analysis tools or data.
Wenming Yao; Yanli Zhou; Haifeng Zhang; Yanxiu Li: Conceived and designed the experiments.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Study used publicly available data from National Health and Nutrition Examination Survey (NHANES). Data will be made available on request.
Declaration of interest's statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We appreciate the National Center for Health Statistics (NCHS) of the Centers for Disease Control (CDC) and Prevention and all participants who enrolled in the NHANES.
Contributor Information
Yanxiu Li, Email: liyanx2008@126.com.
Xinli Li, Email: xinli3267@njmu.edu.cn.
References
- 1.Laslett L.J., Alagona P., Jr., Clark B.A., 3rd, Drozda J.P., Jr., Saldivar F., Wilson S.R., et al. The worldwide environment of cardiovascular disease: prevalence, diagnosis, therapy, and policy issues: a report from the American College of Cardiology. J. Am. Coll. Cardiol. 2012 Dec 25;60(25 Suppl):S1–49. doi: 10.1016/j.jacc.2012.11.002. PMID: 23257320. [DOI] [PubMed] [Google Scholar]
- 2.GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015 Jan 10;385(9963):117–171. doi: 10.1016/S0140-6736(14)61682-2. Epub 2014 Dec 18. PMID: 25530442; PMCID: PMC4340604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roth G.A., Huffman M.D., Moran A.E., Feigin V., Mensah G.A., Naghavi M., Murray C.J. Global and regional patterns in cardiovascular mortality from 1990 to 2013. Circulation. 2015 Oct 27;132(17):1667–1678. doi: 10.1161/CIRCULATIONAHA.114.008720. PMID: 26503749. [DOI] [PubMed] [Google Scholar]
- 4.Benjamin E.J., Muntner P., Alonso A., Bittencourt M.S., Callaway C.W., Carson A.P., et al. American heart association council on epidemiology and prevention Statistics committee and stroke Statistics subcommittee. Heart disease and stroke statistics-2019 update: a report from the American heart association. Circulation. 2019 Mar 5;139(10):e56–e528. doi: 10.1161/CIR.0000000000000659. erratum in: Circulation. 2020 Jan 14;141(2):e33. PMID: 30700139. [DOI] [PubMed] [Google Scholar]
- 5.Zhao D., Liu J., Wang M., Zhang X., Zhou M. Epidemiology of cardiovascular disease in China: current features and implications. Nat. Rev. Cardiol. 2019 Apr;16(4):203–212. doi: 10.1038/s41569-018-0119-4. [DOI] [PubMed] [Google Scholar]
- 6.Ruiz-Canela M., Hruby A., Clish C.B., Liang L., Martínez-González M.A., Hu F.B. Comprehensive metabolomic profiling and incident cardiovascular disease: a systematic review. J. Am. Heart Assoc. 2017 Sep 28;6(10) doi: 10.1161/JAHA.117.005705. PMID: 28963102; PMCID: PMC5721826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ference B.A., Graham I., Tokgozoglu L., Catapano A.L. Impact of lipids on cardiovascular health: JACC health promotion series. J. Am. Coll. Cardiol. 2018 Sep 4;72(10):1141–1156. doi: 10.1016/j.jacc.2018.06.046. [DOI] [PubMed] [Google Scholar]
- 8.Navarese E.P., Robinson J.G., Kowalewski M., et al. Association between baseline LDL-C level and total and cardiovascular mortality after LDL-C lowering: a systematic review and meta-analysis [published correction appears in jama. JAMA. 2018;319(15):1566–1579. doi: 10.1001/jama.2018.2525. 2018 oct 2;320(13):1387] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Force Members Authors/Task. ESC Committee for Practice Guidelines (CPG); ESC National Cardiac Societies. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Atherosclerosis. 2019 Nov;290:140–205. doi: 10.1016/j.atherosclerosis.2019.08.014. Epub 2019 Aug 31. Erratum in: Atherosclerosis. 2020 Jan;292:160-162. Erratum in: Atherosclerosis. 2020 Feb;294:80-82. PMID: 31591002. [DOI] [PubMed] [Google Scholar]
- 10.Grundy S.M., Stone N.J., Bailey A.L., et al. AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the management of blood cholesterol: a report of the American college of cardiology/American heart association task force on clinical practice guidelines [published correction appears in J Am coll cardiol. J. Am. Coll. Cardiol. 2019;73(24):e285–e350. doi: 10.1016/j.jacc.2018.11.003. 2019 jun 25;73(24):3237-3241] [DOI] [PubMed] [Google Scholar]
- 11.Ravnskov U., de Lorgeril M., Diamond D.M., Hama R., Hamazaki T., Hammarskjöld B., et al. LDL-C does not cause cardiovascular disease: a comprehensive review of the current literature. Expet Rev. Clin. Pharmacol. 2018 Oct;11(10):959–970. doi: 10.1080/17512433.2018.1519391. PMID: 30198808. [DOI] [PubMed] [Google Scholar]
- 12.Ginsberg H.N., Packard C.J., Chapman M.J., Borén J., Aguilar-Salinas C.A., Averna M., et al. Triglyceride-rich lipoproteins and their remnants: metabolic insights, role in atherosclerotic cardiovascular disease, and emerging therapeutic strategies-a consensus statement from the European Atherosclerosis Society. Eur. Heart J. 2021 Dec 14;42(47):4791–4806. doi: 10.1093/eurheartj/ehab551. PMCID: PMC8670783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nguyen S.V., Nakamura T., Kugiyama K. High remnant lipoprotein predicts recurrent cardiovascular events on statin treatment after acute coronary syndrome. Circ. J. 2014;78(10):2492–2500. doi: 10.1253/circj.cj-14-0380. Epub 2014 Aug 26. PMID: 25168189. [DOI] [PubMed] [Google Scholar]
- 14.Castañer O., Pintó X., Subirana I., Amor A.J., Ros E., Hernáez Á., et al. Remnant cholesterol, not LDL cholesterol, is associated with incident cardiovascular disease. J. Am. Coll. Cardiol. 2020 Dec 8;76(23):2712–2724. doi: 10.1016/j.jacc.2020.10.008. PMID: 33272365. [DOI] [PubMed] [Google Scholar]
- 15.Varbo A., Benn M., Nordestgaard B.G. Remnant cholesterol as a cause of ischemic heart disease: evidence, definition, measurement, atherogenicity, high risk patients, and present and future treatment. Pharmacol. Ther. 2014 Mar;141(3):358–367. doi: 10.1016/j.pharmthera.2013.11.008. Epub 2013 Nov 26. PMID: 24287311. [DOI] [PubMed] [Google Scholar]
- 16.Varbo A., Benn M., Tybjærg-Hansen A., Nordestgaard B.G. Elevated remnant cholesterol causes both low-grade inflammation and ischemic heart disease, whereas elevated low-density lipoprotein cholesterol causes ischemic heart disease without inflammation. Circulation. 2013 Sep 17;128(12):1298–1309. doi: 10.1161/CIRCULATIONAHA.113.003008. Epub 2013 Aug 7. PMID: 23926208. [DOI] [PubMed] [Google Scholar]
- 17.Moriyama K., Takahashi E. Non-HDL cholesterol is a more superior predictor of small-dense LDL cholesterol than LDL cholesterol in Japanese subjects with TG levels < 400 mg/dL. J. Atherosclerosis Thromb. 2016 Sep 1;23(9):1126–1137. doi: 10.5551/jat.33985. Epub 2016 Mar 18. PMID: 27001003; PMCID: PMC5090818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Y., Wu N.Q., Li S., Zhu C.G., Guo Y.L., Qing P., et al. Non-HDL-C is a better predictor for the severity of coronary atherosclerosis compared with LDL-C. Heart Lung Circ. 2016 Oct;25(10):975–981. doi: 10.1016/j.hlc.2016.04.025. [DOI] [PubMed] [Google Scholar]
- 19.Johannesen C.D.L., Mortensen M.B., Langsted A., Nordestgaard B.G. Apolipoprotein B and non-HDL cholesterol better reflect residual risk than LDL cholesterol in statin-treated patients. J. Am. Coll. Cardiol. 2021 Mar 23;77(11):1439–1450. doi: 10.1016/j.jacc.2021.01.027. [DOI] [PubMed] [Google Scholar]
- 20.Harari G., Green M.S., Magid A., Zelber-Sagi S. Usefulness of non-high-density lipoprotein cholesterol as a predictor of cardiovascular disease mortality in men in 22-year follow-up. Am. J. Cardiol. 2017 Apr 15;119(8):1193–1198. doi: 10.1016/j.amjcard.2017.01.008. Epub 2017 Jan 25. PMID: 28267961. [DOI] [PubMed] [Google Scholar]
- 21.Yu D., Wang Z., Zhang X., Qu B., Cai Y., Ma S., et al. Remnant cholesterol and cardiovascular mortality in patients with type 2 diabetes and incident diabetic nephropathy. J. Clin. Endocrinol. Metab. 2021 Nov 19;106(12):3546–3554. doi: 10.1210/clinem/dgab533. [DOI] [PubMed] [Google Scholar]
- 22.Lee E.Y., Yang Y., Kim H.S., Cho J.H., Yoon K.H., Chung W.S., et al. Effect of visit-to-visit LDL-, HDL-, and non-HDL-cholesterol variability on mortality and cardiovascular outcomes after percutaneous coronary intervention. Atherosclerosis. 2018 Dec;279:1–9. doi: 10.1016/j.atherosclerosis.2018.10.012. Epub 2018 Oct 17. PMID: 30359786. [DOI] [PubMed] [Google Scholar]
- 23.Cheng Q., Liu X.C., Chen C.L., Huang Y.Q., Feng Y.Q., Chen J.Y. The U-shaped association of non-high-density lipoprotein cholesterol levels with all-cause and cardiovascular mortality among patients with hypertension. Front Cardiovasc Med. 2021 Jul 14;8 doi: 10.3389/fcvm.2021.707701. PMID: 34336961; PMCID: PMC8316599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.National Center for Health Statistics National Health and Nutrition Examination Survey: NHANES Survey Methods and Analytic Guidelines. https://wwwn.cdc.gov/nchs/nhanes/AnalyticGuidelines.aspx Accessed May 2021.
- 25.de Nijs T., Sniderman A., de Graaf J. ApoB versus non-HDL-cholesterol: diagnosis and cardiovascular risk management. Crit. Rev. Clin. Lab Sci. 2013 Nov;50(6):163–171. doi: 10.3109/10408363.2013.847897. [DOI] [PubMed] [Google Scholar]
- 26.National Center for Health Statistics . March 2019. Office of Analysis and Epidemiology. The Linkage of National Center for Health Statistics Survey Data to the National Death Index—2015 Linked Mortality File (LMF): Methodology Overview and Analytic Considerations.https://www.cdc.gov/nchs/data-linkage/mortality-methods.htm Hyattsville, Maryland. Available at the following address: Accessed October 2021. [Google Scholar]
- 27.Langsted A., Madsen C.M., Nordestgaard B.G. Contribution of remnant cholesterol to cardiovascular risk. J. Intern. Med. 2020 Jul;288(1):116–127. doi: 10.1111/joim.13059. PMID: 32181933. [DOI] [PubMed] [Google Scholar]
- 28.Nakamura T., Takano H., Umetani K., Kawabata K., Obata J.E., Kitta Y., et al. Remnant lipoproteinemia is a risk factor for endothelial vasomotor dysfunction and coronary artery disease in metabolic syndrome. Atherosclerosis. 2005 Aug;181(2):321–327. doi: 10.1016/j.atherosclerosis.2005.01.012. Epub 2005 Feb 16. PMID: 16039286. [DOI] [PubMed] [Google Scholar]
- 29.Kugiyama K., Doi H., Takazoe K., Kawano H., Soejima H., Mizuno Y., et al. Remnant lipoprotein levels in fasting serum predict coronary events in patients with coronary artery disease. Circulation. 1999 Jun 8;99(22):2858–2860. doi: 10.1161/01.cir.99.22.2858. PMID: 10359727. [DOI] [PubMed] [Google Scholar]
- 30.Varbo A., Benn M., Tybjærg-Hansen A., Jørgensen A.B., Frikke-Schmidt R., Nordestgaard B.G. Remnant cholesterol as a causal risk factor for ischemic heart disease. J. Am. Coll. Cardiol. 2013 Jan 29;61(4):427–436. doi: 10.1016/j.jacc.2012.08.1026. Epub 2012 Dec 19. Erratum in: J Am Coll Cardiol. 2019 Mar 5;73(8):987-988. PMID: 23265341. [DOI] [PubMed] [Google Scholar]
- 31.Boekholdt S.M., Hovingh G.K., Mora S., Arsenault B.J., Amarenco P., Pedersen T.R., et al. Very low levels of atherogenic lipoproteins and the risk for cardiovascular events: a meta-analysis of statin trials. J. Am. Coll. Cardiol. 2014 Aug 5;64(5):485–494. doi: 10.1016/j.jacc.2014.02.615. PMCID: PMC4443441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burnett J.R., Hooper A.J., Hegele R.A. Remnant cholesterol and atherosclerotic cardiovascular disease risk. J. Am. Coll. Cardiol. 2020 Dec 8;76(23):2736–2739. doi: 10.1016/j.jacc.2020.10.029. [DOI] [PubMed] [Google Scholar]
- 33.Langlois M.R., Sniderman A.D. Non-HDL cholesterol or apoB: which to prefer as a target for the prevention of atherosclerotic cardiovascular disease? Curr. Cardiol. Rep. 2020 Jun 19;22(8):67. doi: 10.1007/s11886-020-01323-z. [DOI] [PubMed] [Google Scholar]
- 34.Karlamangla A.S., Singer B.H., Reuben D.B., Seeman T.E. Increases in serum non-high-density lipoprotein cholesterol may be beneficial in some high-functioning older adults: MacArthur studies of successful aging. J. Am. Geriatr. Soc. 2004 Apr;52(4):487–494. doi: 10.1111/j.1532-5415.2004.52152.x. [DOI] [PubMed] [Google Scholar]
- 35.Bertolotti M., Mussi C., Pellegrini E., Magni A., Del Puppo M., Ognibene S., et al. Age-associated alterations in cholesterol homeostasis: evidence from a cross-sectional study in a Northern Italy population. Clin. Interv. Aging. 2014 Mar 17;9:425–432. doi: 10.2147/CIA.S57714. PMID: 24669190; PMCID: PMC3962317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang Y.Q., Shen G., Lo K., Huang J.Y., Liu L., Chen C.L., et al. Association of circulating selenium concentration with dyslipidemia: results from the NHANES. J. Trace Elem. Med. Biol. 2020 Mar;58 doi: 10.1016/j.jtemb.2019.126438. PMID: 31760326. [DOI] [PubMed] [Google Scholar]
- 37.So M., Takahashi M., Miyamoto Y., Ishisaka Y., Iwagami M., Tsugawa Y., Egorova N.N., Kuno T. The effect of obesity on in-hospital mortality among patients with COVID-19 receiving corticosteroids. Diabetes Metabol. Syndr. 2021 Dec 29;16(1) doi: 10.1016/j.dsx.2021.102373. Epub ahead of print. PMID: 34979344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Duran E.K., Pradhan A.D. Triglyceride-rich lipoprotein remnants and cardiovascular disease. Clin. Chem. 2021 Jan 8;67(1):183–196. doi: 10.1093/clinchem/hvaa296. [DOI] [PubMed] [Google Scholar]
- 39.Piťha J., Kovář J., Blahová T. Fasting and nonfasting triglycerides in cardiovascular and other diseases. Physiol. Res. 2015;64(Suppl 3):S323–330. doi: 10.33549/physiolres.933196. PMID: 26680665. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Study used publicly available data from National Health and Nutrition Examination Survey (NHANES). Data will be made available on request.