Abstract
The wastewater generated from textile industries is highly colored and contains dyes including azo dyes, which are toxic to human and water-living organisms. The treatment of these azo dyes using conventional treatment techniques is challenging due to their recalcitrant properties. In the current study, the effect of additional Fe2+ on electrocoagulation (EC) using Fe electrodes has been studied for the removal of methyl orange (MO) azo dye. pH between 4-5 was found to be optimum for EC and treatment efficiency decreased with increasing dye concentrations. With the addition of Fe2+ salt, dye removal for a certain concentration was increased with the increase of current density and Fe2+ up to a certain limit and after that, the removal efficiency decreased. The COD, color and dye removals were 88.5%, 93.1% and 100%, respectively, for EC of 200 mg.L−1 dye solution using only 0.20 mmol.L−1 Fe2+ for 0.40 mA cm−2 current density, whereas for EC, the respective removal efficiencies were 76.7%, 63.4% and 82.4% for 32 min. The respective operating cost for EC was $768 kg−1 removed dye ($0.342 m−3), whereas, for EC with additional Fe2+ salt, it was $350 kg−1 removed dye ($0.189 m−3). The kinetic results revealed that the first-order kinetic model was fitted best for EC, whereas the second-order kinetic model was best fitted for Fe2+ added EC. For real textile wastewater, 57.6% COD removal was obtained for 0.15 mmol.L−1 Fe2+ added EC compared to 27.8% COD removal for EC for 32 min. Based on the study we can conclude that Fe2+ assisted EC can be used for effective treatment of textile wastewater containing toxic compounds like azo dyes.
Keywords: Electrocoagulation, Iron electrodes, Cost estimation, Degradation kinetics, Methyl orange
Graphical abstract
Highlights
-
•
EC represents limiting treatment performance for higher contaminant concentrations.
-
•
0.20 mmol.L−1 Fe2+ salt enhances the EC treatment performance of MO dye to 100%.
-
•
EC followed first-order kinetic model, whereas Fe2+ added EC followed second-order kinetic model.
-
•
Operating cost was reduced to $0.327 m−3 from $0.598 m−3 for EC with additional Fe2+.
-
•
58% COD was removed for 0.15 mmol.L−1 Fe2+ added EC for real textile wastewater.
Electrocoagulation; Iron electrodes; Cost estimation; Degradation kinetics; Methyl orange.
1. Introduction
Textile industries, one of the significant economic contributors in developing countries like China, Vietnam, Bangladesh, India, Sri Lanka, etc., are identified as the largest source of water pollution (Holkar et al., 2016; Lellis et al., 2019). It has been reported that 1 kg of textile product generates 100–200 L of wastewater (Al-Mamun et al., 2021a) which contains both inorganic and organic substances such as metals, dyes, surfactants and microfibers (Yaseen and Scholz, 2019). Approximately 60–70% of the synthetic dyes used in textile processing units are azo dyes that are used due to the stability and fastness of the fabric (Benkhaya et al., 2020; Fernández-Fernández et al., 2013) and 10–15% of these synthetic dyes are lost in the effluent (Al-Mamun et al., 2019). These dyes are toxic to aquatic organisms, have poor biodegradability, and cause changes in the biological cycles of aquatic life adversely when mixed with surface water (Lellis et al., 2019). Due to higher volume, most textile industries discharge the effluent into nearby surface water bodies after inadequate treatment leaving an adverse influence on freshwater supplies and public health.
Physical, chemical and biological treatment technologies have been employed for textile wastewater treatment and a few treatment techniques for textile wastewaters are listed in Table 1. From Table 1, it can be seen that real textile wastewaters contain higher pollutants levels as compared to synthetic textile wastewater. However, physical processes like adsorption and filtration only separate contaminants with higher treatment costs (Couto et al., 2018; Pourrezaei et al., 2010; Roy et al., 2022), biological processes are inefficient (Dogan et al., 2020; Tiwari et al., 2017; Wesley et al., 2011) and chemical processes cause an intensification of dissolved components, costly and ineffective (Panizza and Cerisola, 2009). Advanced oxidation processes (AOPs) such as ozonation (Kurade et al., 2012; Wang et al., 2005), persulfate oxidation (Shuchi et al., 2021), UVH2O2 (Al-Mamun et al., 2021b), solar-TiO2 (Al-Mamun et al., 2022; Hasan Khan Neon and Islam, 2019) have become more popular for the treatment of textile dyes, but these processes cause higher treatment cost. Therefore, an efficient, affordable and environmentally friendly treatment technique is urgently required to treat and manage textile wastewaters for reuse and recycle purposes.
Table 1.
Textile wastewater treatment using different techniques.
| Type of pollutant | Treatment technology | Initial pollutant concentration | Experimental conditions | Results | References |
|---|---|---|---|---|---|
| Real textile wastewater | Sequential Chemical coagulation (CC)-Electro-Oxidation (EO) | pH: 9.3 Cond: 4010 S cm−1 Turbidity (NTU): 161 COD: 720 mg.L−1 TOC: 164 mg.L−1 BOD5: 115 mg.L−1 |
CC Al2(SO4)3 · 18H2O: 600 mg.L−1 pH: 9.3 EO pH: 5.6 Cond: 4.7 mS cm−1 Current density: 15 mA cm−2 Electrode: BDD anode and Fe cathode Time: 45 min |
Color: 100% COD: 93.5% TOC: 75% Cost: 6.91 USD/m3 |
GilPavas et al. (2018) |
| Real textile wastewater | CuO activated carbon (AC) composite with additonal peroxydisulfate (PDS) | COD: 450 mg.L−1 BOD: 190 mg.L−1 TOC: 213 mg.L−1 Color (ADMI): 1209 TDS: 650 mg.L−1 Cl−: 122 mg.L−1 pH: 7.6 Cu: 0.08 mg.L−1 |
PDS: 7 mmol.L−1 CuO-AC: 1 g.L−1 pH: 7 Time: 60 min |
TOC: 61 % Color: 95 % COD: 72 % BOD: 70 % |
Kiani et al. (2020) |
| Real textile wastewater | Combined Chemical coagulation (CC), electrocoagulation (EC), and adsorption | BOD5: 278.54 ± 65.23 mg.L−1 COD: 1,346.17 ± 123.36 mg.L−1 TSS: 178.28 ± 23.82 mg.L−1 |
Poly aluminum chloride (PAC) as coagulant, Al electrodes for EC, Pistachio nut shell ash as adsorbent |
COD: 98% BOD5: 94.2% Dye: 99.9% | Bazrafshan et al. (2016) |
| Real textile wastewater | Advanced oxidation processes (AOPs) based on zero-valent aluminium (ZVAl) | pH: 7.10–7.80 COD: 2155 mg.L−1 BOD: 500 mg.L−1 Ammoniacal nitrogen: 699 mg.L−1 Cl−: 1463 mg.L−1 TDS: 5190 mg.L−1 TSS: 513 mg.L−1 |
ZVAl: 1 g.L−1 Fe3+: 0.5 g.L−1 H2O2: 6.7 g.L−1 Time: 3 h |
COD: 97.9% Color: 94.4% Ammoniacal nitrogen: 58.3% |
Khatri et al. (2018) |
| Real textile wastewater | TiO2/UV | pH: 10.8 Temp: 31.2 Cond: 13.6 mS cm−1 TSS: <3 mg.L−1 DOC: 382 mg.L−1 COD: 1020 mg.L−1 BOD5: 110 mg.L−1 BOD5/COD: 0.11 Cl−: 4578 mg.L−1 Total nitrogen: 32.4 mg.L−1 |
pH: 4.5 TiO2: 200 mg.L−1 Radiation: 190 kJUV.L−1 |
DOC: 36% | Vilar et al. (2011) |
| H2O2+TiO2/UV | pH: 4.5 TiO2: 200 mg.L−1 Radiation: 100 kJUV.L−1 H2O2: 208.1 mmol.L−1 |
DOC: 90% | |||
| Solar-photo-Fenton | Fe2+: 100 mg.L−1 Radiation: 49.1 kJUV.L−1 H2O2: 203.2 mmol.L−1 |
DOC: 89% | |||
| Synthetic wastewater containing Remazol Black B (RBB) | Electrocoagulation (EC) | RBB: 100 mg.L−1 Starch: 1500 mg.L−1 Glucose: 500 mg.L−1 Na2CO3: 1000 mg.L−1 NaHCO3: 1000 mg.L−1 NaOH: 500 mg.L−1 NaCl: 1500 mg.L−1 |
pH: 3 Current density: 10 mA cm−2 Time: 50 min Electrode: SS |
Color: 91% Dye: 86.5% Turbidity: 60.4% COD: 19.8% | Suhan et al. (2020) |
| Real textile wastewater (Pre-treated) | Electrocoagulation (EC) | COD: 287 mg.L−1 TOC: 240 mg.L−1 Turbidity (NTU): 25 Cond: 7.6 ms cm−1 pH: 8 TSS: 34 mg.L−1 Cr: 0.028 mg.L−1 Zn: 0.09 mg.L−1 Cl−: 0.1 mg. L−1 Phenol: 0.355 mg. L−1 |
Time: 120 min pH: 5 Current density: 25 mA cm−2 Electrode: Al |
TOC: 42.5% COD: 18.6% Turbidity: 83.5% SS: 64.7% Color: 92% |
Bener et al. (2019) |
| Textile industry wastewater | EC-Photo-Fenton (PF)- Adsorption | pH: 8.2 Cond: 5.04 mS cm−1 Color (Pt–Co): 1399 BOD: 206 mg.L−1 COD: 970 mg.L−1 TOC: 220 mg.L−1 |
EC pH: 7 Current density: 10 mA cm−2 Stirring: 60 rpm Time: 10 min PF pH: 4.3 Fe2+: 1.1 mmol.L−1 H2O2: 9.7 mmol.L−1 Stirring: 100 rpm Radiation: 365 nm Time: 60 min Adsorption Granular activated carbon bed (Calgon Carbon, Pittsburgh, mesh 10) |
Color: 100% COD: 76% TOC: 78% Toxicity: 100% | GilPavas et al. (2019) |
| Real textile wastewater | EC (Fe)-Nanofiltration | Color (Pt–Co): 2100 COD: 2690 mg.L−1 Turbidity (NTU): 1500 TDS: 7500 mg.L−1 TSS: 280 mg.L−1 Cond: 14.95 mS cm−1 pH: 7.03 |
d: 10 mm Stirring: 200 rpm pH: 7 CD: 20 mA cm−2 Membrane: NP010 NF |
Color: 87.8% COD: 77.6% Turbidity: 99.9% |
Tavangar et al. (2019) |
| EC (Al)-Nanofiltration | Color: 95.2% COD: 81% Turbidity: 99.7% |
||||
| Synthetic wastewater containing reactive dye (RR45) | Adsorption with Symphoricarpus albus modified with sodium diethyldithiocarbamate | 100 mg.L−1 | Continuous flow rate: 1.0 mL min−1 pH: 2 BET surface area: 0.043 m2 g−1 |
Dye: 91.89% Adsorption capacity: 6.22 10−5 mol g−1 |
Kara et al. (2012) |
| Synthetic wastewater containing Acid orange 5 | EC process | 60 mg.L−1 | pH: 7 Current density: 2 mA cm−2 Time: 60 min |
Dye: 99.3 COD: 85.5% |
Shokri (2019) |
| Synthetic wastewater containing Acid red 14 (AR14) | Electro peroxone process | 400 mg.L−1 | Current intensity: 0.7 A pH: 10 Time: 30 min Na2SO4: 0.1mol.L−1 Ozone injection: 0.25 L min−1 Electrode: Pt- carbon-PTFE electrode |
Dye: 100% COD: 69% |
Shokri Karimi (2020) |
∗Cond: conductivity, SS: suspended solid, TDS: total dissolved solid, TSS: total suspended solid, d: inter-electrode distance.
Electrocoagulation (EC) has been proved as a simple, reliable, less cost-oriented and efficient treatment technique for textile effluent (Akter et al., 2022; Khandegar and Saroha, 2013). In this process, on-site in-situ generated metallic ions by the electrolytic dissolution of the sacrificial anodes such as iron (Fe) and aluminum (Al) initiate the coagulation process (Camcioglu et al., 2017; Hakizimana et al., 2017; Shokri, 2018). Hybrid electrodes (Cletus et al., 2022; Sorayyaei et al., 2021), as well as electrodes with different designs (Wu et al., 2021) have also been applied for more efficient treatment of different textile dyes. In the current study, Fe electrodes were used due to their higher treatment efficiency, longer durability, lower cost and non-toxic nature than Al electrodes (Ghalwa et al., 2016; Ghernaout et al., 2011; Moreno et al., 2009). Also, Al electrodes eliminate contaminants only by EC mechanism whereas, Fe electrodes remove contaminants by both EC and electro-oxidation (EO) mechanisms (Benekos et al., 2019; Nasrullah et al., 2018). The anodic electrochemical reactions of Fe follow two mechanisms and generate Fe(OH)3 and Fe(OH)2 (Table 2) (Ghernaout, 2018; Mollah et al., 2001; Zodi et al., 2009). Gomes et al. (2007) observed that both Fe2+ and Fe3+ ions along with their organometallic complexes are present in the solution (Kamaraj and Vasudevan, 2015; Song et al., 2017). Additionally, the generated Fe2+ gets oxidized into Fe3+ in the reaction medium (Sasson et al., 2009). Researchers have also reported that Fe3+ behaves as a stronger coagulant than Fe2+ due to its higher destabilization power (Garcia-Segura et al., 2017; Linares-Hernández et al., 2009).
Table 2.
Reaction mechanisms for the dissolution of Fe electrodes.
| Mechanism 1: | Mechanism 2: |
|---|---|
| Anode: 4Fe (s) 4Fe2+(aq) + 8e− (1) 4Fe2+(aq) + 10 H2O (l) +O2 (g) 4Fe(OH)3 (s) + 8H+(aq) (2) Cathode: 8H+(aq) + 8e− 4H2 (g) (3) Overall: 4Fe (s) + 10H2O (l) + O2 (g) 4Fe(OH)3 (s) + 4H2 (g) (4) |
Anode: Fe (s) Fe2+(aq) + 2e− (5) Fe2+(aq) + 2OH−(aq) Fe(OH)2 (s) (6) Cathode: 2H2O (l) + 2e− H2 (g) + 2OH−(aq) (7) Overall: Fe (s) + 2H2O (l) Fe(OH)2 (s) + H2 (g) (8) |
The generated ions (Fe2+ and Fe3+) in the solution can eliminate the pollutants by (i) involving chemical reaction with organics followed by precipitation, and (ii) producing agglomerates of colloidal particles followed by sequential removal of agglomerated flocs through flotation, which is assisted by the evolution of generated H2 (Sahu et al., 2014; Suhan et al., 2021). For the removal of malathion pesticides by EC, Sankar and Sivasubramanian (2021) observed charge neutralization followed by sweep coagulation with the metal hydroxide to be the main removal mechanism. Elnakar and Buchanan (2020) also recommended that the in-situ generated ferric hydroxide acts as an adsorbent that removes the sCOD through chemisorption. Several phenomena like metal ion formation, electrochemical decomposition, hydrolysis, metal-organic attraction, etc., also happen concurrently during the pollutants' migration through the electric field. These might cause the separation of organic pollutants by altering both the physical and chemical properties of the pollutants (Canizares et al., 2007; Garcia-Segura et al., 2017; Sahu et al., 2014). Researchers recommended that the leading pollutant removal mechanisms depend on pollutant types and the operating principles of EC (Chen, 2004; Islam, 2019).
A comparatively smaller quantity of sludge is generated in EC compared to chemical coagulation (CC) and the typical uses of sludge are (i) manufacturing of fire brick after mixing with clay, (ii) application as fuel in boilers and incinerators, (iii) application of pyrolyzed sludge ash as additives in the clinker for cement manufacturing, and (iv) application of pyrolyzed sludge ash as adsorbents for wastewater treatment (Heikal, 2000; Islam et al., 2021; Kushwaha et al., 2010). Sankar and Sivasubramanian (2021) applied the sludge generated from EC of malathion pesticide for soil amendment and observed an extreme improvement in the quality and the geotechnical properties of the soil, making the sludge a minor threat to the environment.
EC treatment technique has some drawbacks like electrode passivation, higher electric energy consumption and generation of secondary pollutants (Al-Qodah et al., 2020), and lower amount of coagulant generation by the sacrificial anodes (Barrera Díaz and González-Rivas, 2015), which limit its applications and effectiveness. Researchers have recommended that the addition of coagulants into the electrochemical cell can enhance treatment efficiency (El-Ashtoukhy et al., 2017). With this concept, FeSO4.7H2O salt was selected as the source of Fe2+ that works as both a reducing agent (oxidation of Fe2+ into Fe3+) and coagulant.
In this study, degradation of methyl orange (MO) azo dye (anionic) using a customized EC reactor was investigated. MO (C14H14N3NaO3S, molecular weight: 327.34 g mol−1, λmax: 463 nm) (Yan et al., 2010) was selected due to its versatile use in textile industries, paints, cosmetics and laboratories (Ma et al., 2007; Ramírez et al., 2013; Schlichter et al., 2017; Zhang et al., 2012). Although several degradation techniques have been studied for MO, to the best of our knowledge, no previous study has elucidated the influence of additional Fe2+ on the EC technique for MO dye degradation.
The main objective of this study was to investigate the influence of additional Fe2+ on the EC treatment process using Fe electrodes for MO dye removal. The effects of pH, current density and initial dye concentrations as well as concentration of additional Fe2+ were studied. EC process with and without additional Fe2+ was also analyzed and compared using first-order, second-order and pseudo-second-order models to investigate the removal kinetics. Finally, EC with and without additional Fe2+ salt was applied for the treatment of real textile wastewater to represent the applicability of EC in practice.
2. Materials and methods
2.1. Reagents
MO (ACS reagent, 85% purity) dye was purchased from Sigma-Aldrich (USA). Iron (II) sulfate heptahydrate (FeSO4.7H2O), was extra pure and was purchased from Scharlau (Spain). Potassium dichromate (K2Cr2O7), mercuric sulfate (HgSO4) and silver sulfate (Ag2SO4), used for the preparation of digestion solutions for COD measurement, were also purchased from Scharlau (Spain). Dilute (40%) sulfuric acid (H2SO4) and dilute (40%) sodium hydroxide (NaOH) were used for pH adjustment. All MO dye solutions were prepared with distilled water obtained from the laboratory supply.
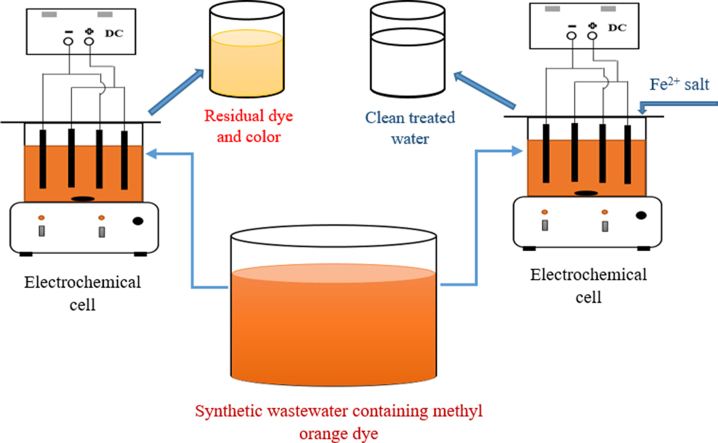
2.2. Electrocoagulation reactor
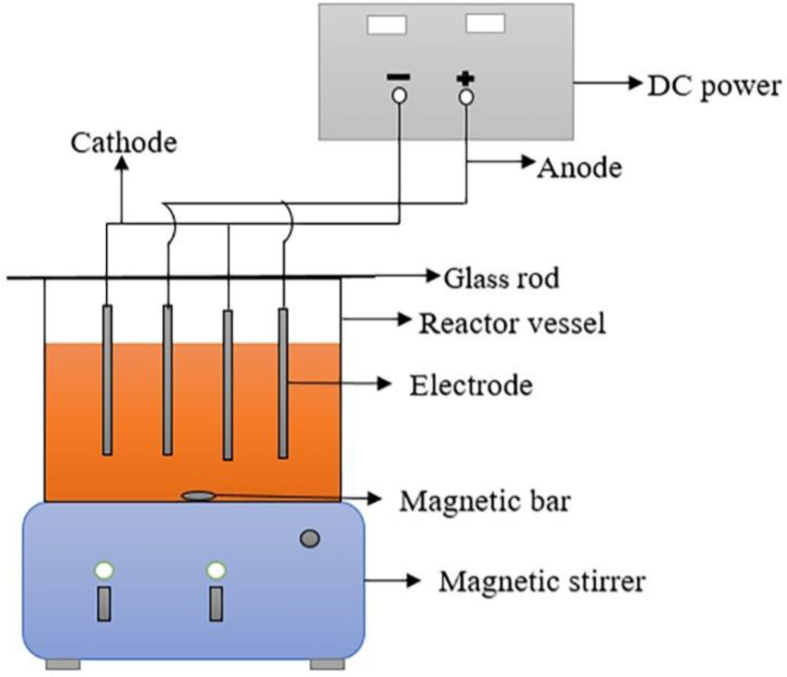
All the EC experiments were conducted in an open, undivided, square cell made of 5 mm PVC transparent sheet having a cross-section of 14.8 × 14.8 cm2 with a volume of 4 L (Figure 1). All the experiments were conducted at ambient temperature (around 30 ) and four Fe electrodes (SS304 sheet) having dimensions of 13 cm × 9 cm × 0.15 cm were used in a monopolar-parallel (MP-P) connection mode with a total surface area of 930 cm2. This is due to the cost-effective nature of monopolar (MP) connection over bipolar (BP) connection for both organic and inorganic pollutants (Alimohammadi et al., 2017; Ghosh et al., 2008; Vasudevan et al., 2013). Before each experiment, all the electrodes were abraded with a steel scrubber followed by sequential washing with tap water and distilled water. The distance between two consecutive electrodes was 3.7 cm and all the experiments were conducted for 2.7 L of sample solution having an immersion of 75% of the total electrode surface area. All the experiments were conducted under a stirred condition of 150 rpm by using a magnetic stirrer and ‘Dazheng DC Power Supply’ converter having a capacity of 5 A and 33 V was used for the power supply.
Figure 1.
Schematic diagram of electrocoagulation reactor.
2.3. Experimental
The influence of pH on EC was observed at first for suitable pH selection as it determines the solution conductivity, pollutant particle distribution, particle-particle interaction, types of hydrolyzed metal species and concentration of gas bubbles (Moussa et al., 2017; Naje et al., 2017; Sahu et al., 2014). pH was optimized from experiments containing freshly prepared 100 mg.L−1 dye solutions having initial pH in between 4-5 (acidic), 6–7 (neutral) and 8–9 (basic) under a constant current density of 0.11 mA cm−2. At the optimum pH, the influence of variable dye concentrations on EC treatment performance was also investigated at 0.11 mA cm−2 current density for 100, 200, and 300 mg.L−1 dye solutions. Additionally, optimum current densities for 100, 200, and 300 mg.L−1 dye solutions were determined at the optimum pH. In literature, it is mentioned that the dye concentration in textile wastewater ranged from 10 to 500 mg.L−1 (Yaseen and Scholz, 2019). Therefore, the experiments were conducted for higher dye concentrations (100–300 mg.L−1 MO dye).
The influence of additional Fe2+ salt was explored for the removal of 200 mg.L−1 MO solution at the optimum pH obtained from EC experiments. Also, the salt solution was added to a previously adjusted acidic solution under rapid mixing conditions to avoid the precipitation of Fe2+ at higher pH (Pétrier et al., 2010). Optimum parameters were also evaluated for EC with additional Fe2+ salt to compare with conventional EC treatment performances. Furthermore, both EC with and without additional Fe2+ salt was investigated for the treatment of real textile wastewater collected from a local textile industry. The raw textile wastewater was highly polluted (pH: 9.5; total suspended solids, TSS: 1320 mg.L−1; chemical oxygen demand, COD: 683 mg.L−1; biological oxygen demand, BOD5: 543 mg.L−1). To evaluate the treatment performance and to compare with synthetic MO solution treatment, % COD removal was considered here.
2.4. Analytical procedures and instruments
For the assessment of treatment performance, samples were collected at a time interval of 8 min during operation and COD (mg.L−1), color (Pt–Co) and dye concentrations (mg.L−1) were measured. The sample solutions were filtered using a 0.2 m nylon syringe filter to eliminate the iron interference in COD measurement. ‘HACH DR 6000’ spectrophotometer was used for the measurement of color and absorbance of the sample solutions and COD of the digested samples. The color was measured based on a visible region of the spectrum and absorbance was measured based on maximum absorbance for each solution pH. Maximum absorbance for each solution pH was measured at first. pH indicator paper was used for pH measurement and hence, solution pH was expressed as pH range. Glass square cuvette was used for color and absorbance (320–1400 nm) measurement. For COD measurement, ‘APHA 5220D’ (closed reflux, colorimetric method) was used for the digestion of sample solutions. Unknown dye concentrations of the solutions were measured by using a calibration curve. % COD removal, % color removal and % dye removal were calculated to evaluate the treatment performance using Eq. (1) (Kader et al., 2022):
| (1) |
where, Co and C are the initial and present values of the COD (mg.L−1), color (Pt–Co) and dye concentrations (mg.L−1), respectively.
The energy consumption per unit of dye removal (E) was calculated to compare the removal performances at different conditions using the following formula (Eq. 2):
| (2) |
where I is the current intensity (A), U is the cell voltage (V), t is the treatment time (h), V is the volume of solution (L), CMO is the initial dye concentration (mg.L−1) and X is the fraction removal for the treatment time.
2.5. Cost analysis
The applicability of a treatment technique depends both on treatment efficiency and cost-effectiveness. The operating cost (OC) of EC and Fe2+ salt-assisted EC was calculated to evaluate the cost-effectiveness. OC of EC includes the cost of electrodes and energy consumption cost mainly (Kobya et al., 2011) and can be expressed by Eq. (3) (Kobya et al., 2016).
| OC = a Cenergy + b Celectrode | (3) |
where, Cenergy is the energy consumption per unit volume (kWh.m−3), Celectrode is the amount of electrode consumption per unit volume (kg.m−3), and a and b are unit cost of energy ($.kW−1.h−1) and electrode material ($.kg−1), respectively. Value of unit cost of energy (a) was taken as $0.114 kW−1 h−1 and unit cost of Fe electrode material (b) was taken as $1.017 kg−1 (Kobya et al., 2016).
Formulae for Cenergy and Celectrode calculation are expressed by Eqs. (4) and (5), respectively (Kobya et al., 2016):
| (4) |
| (5) |
where U is the applied voltage (V), i is the applied current (A), tEC is the EC treatment time (h), v is the solution volume (m3), MFe is the molecular weight of the Fe electrode (55.85 g mol−1), z is the number of electrons involved in the oxidation/reduction reaction (z = 2) and F is the Faraday's constant (96,500 C mol−1).
In the case of EC with additional Fe2+ salt, the cost of added salt should also be added along with the cost of electricity consumption and metal dissolution and can be expressed by Eq. (6)
| OC’ = a Cenergy + b Celectrode + c Csalt | (6) |
where Csalt is the quantity of salt per unit volume (kg.m−3) and c is the unit cost of FeSO4.7H2O that was taken as $8.8 × 10−2 kg−1 (Suhan et al., 2020).
Additionally, the operating cost per unit of dye removal ($.kg−1 dye) was also calculated to evaluate the cost for pollutant removal using Eq. (7):
| (7) |
where TOC is the total operating cost ($), V is the volume of solution (L), CMO is the initial dye concentration (kg.L−1) and X is the fraction removal for the treatment time.
2.6. Degradation kinetics
The degradation kinetics of 200 mg.L−1 MO dye removal by EC and Fe2+ assisted EC around the optimum current densities were studied using first-order, second-order and pseudo-second-order kinetic models for optimum treatment time. Eqs. (8), (9), and (10) represent the linearized first-order (Connors, 1990), second-order (Connors, 1990) and pseudo-second-order (Elkacmi et al., 2017) models, respectively.
| (8) |
| (9) |
| (10) |
where Co is the initial dye concentration (mg.L−1), C is the dye concentration at any time (mg.L−1), Ce is the amount of pollutant removed at equilibrium (mg.L−1) and k is the reaction rate constant.
3. Results and discussions
3.1. Influence of pH on EC
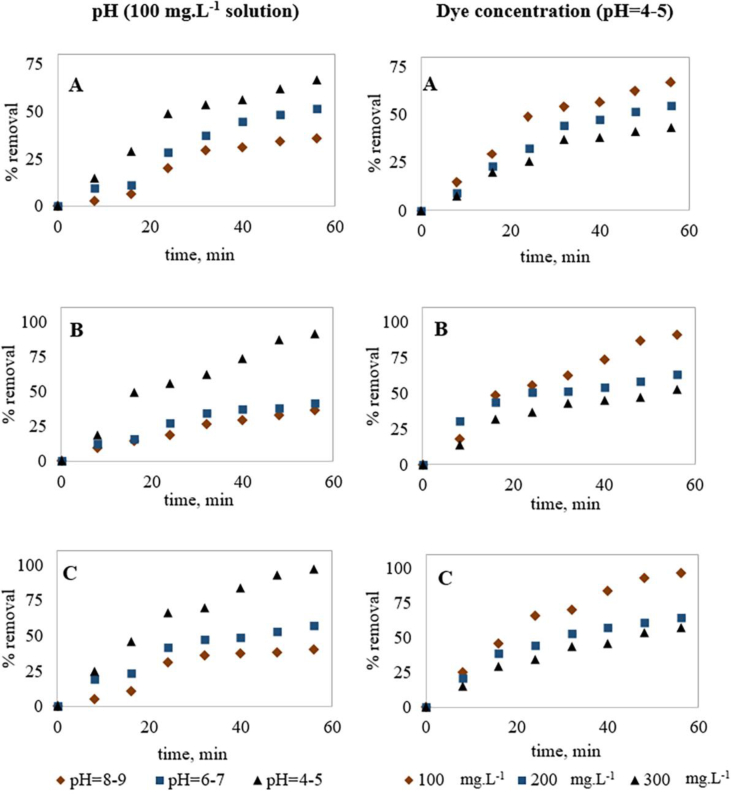
The pH optimization of an EC treatment process depends on electrode material and pollutant types (Koparal et al., 2008). Since solution pH governs the electrode dissolution and formation of hydrolyzed metal species in electrolyte media (Garcia-Segura et al., 2017; Katal and Pahlavanzadeh, 2011), pH was optimized for the EC treatment first. Experimental results are summarized in Table 3 and Figure 2. After 56 min, COD, color and dye removals for the acidic condition were 66.7%, 91.1% and 96.6%, respectively, whereas for the neutral condition these removals were 51.2%, 41.3% and 57.4%, respectively and for basic condition, these were 35.7%, 36.1% and 40.2%, respectively. Therefore, acidic conditions (pH = 4–5) showed the highest treatment performance for EC of MO dye, which supports the previous studies (Li et al., 2014; Sha et al., 2016; Wang et al., 2015; Zhao et al., 2014). This is due to the higher availability of surface charge of generated metal ions at acidic conditions compared to neutral and basic conditions (Mohora et al., 2014), which results in a higher rate of coagulation. Also, the generated Fe ions get precipitated at higher pH (Devi et al., 2009). Pétrier et al. (2010) investigated the easy breakdown of the complex structure of MO under acidic conditions. Devi et al. (2009) also investigated acidic pH to be obvious for MO and observed no color removal at basic conditions. Ma et al. (2007) also obtained 100% color removal and 89.7% COD removal for MO dye by EC at an initial pH of 5.
Table 3.
Treatment performance and cost analysis of EC.
| Dye conc. (mg.L−1) | pH | Current density (mA.cm−2) | % COD removal | % Color removal | % Dye removal | Cost analysis∗ |
||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cenergy (kWh.m−3) 103 | Cmetal (kg.m−3) 103 | OC ($/m3) 103 |
OC/kg removed dye ($/kg dye) | |||||||
| Optimization of pH | 100 | 4–5 | 0.11 | 66.7 | 91.1 | 96.6 | 152.1 | 36.8 | 54.8 | 231.7 |
| 6–7 | 51.2 | 41.3 | 57.4 | 1123.5 | 36.0 | 164.7 | 1062.7 | |||
| 8–9 | 35.7 | 36.1 | 40.2 | 604.9 | 36.0 | 105.6 | 972.8 | |||
| Optimization of current density | 100 | 4–5 | 0.11 | 66.7 | 91.1 | 96.6 | 152.1 | 36.8 | 54.8 | 231.7 |
| 0.25 | 91.7 | 94.8 | 98.3 | 956.4 | 83.7 | 194.2 | 731.6 | |||
| 0.32 | 78.2 | 85.4 | 91.3 | 1409.4 | 107.2 | 269.7 | 1093.9 | |||
| 0.37 | 74.2 | 76.4 | 91.3 | 1932.9 | 123.9 | 346.4 | 1405.1 | |||
| 0.40 | 75.3 | 63.7 | 79.1 | 3253.4 | 134.0 | 507.1 | 2374.5 | |||
| 200 | 4–5 | 0.11 | 54.7 | 63.2 | 64.7 | 162.7 | 36.8 | 56.0 | 160.3 | |
| 0.25 | 61.2 | 85.2 | 83.3 | 1474.8 | 83.7 | 253.3 | 563.1 | |||
| 0.32 | 91.2 | 88.1 | 96.3 | 2520.4 | 107.2 | 396.3 | 762.1 | |||
| 0.37 | 71.3 | 78.5 | 87.8 | 3598.2 | 123.9 | 536.2 | 1131.0 | |||
| 0.40 | 84.6 | 71.2 | 89.6 | 4050.7 | 134.0 | 598.0 | 1236.0 | |||
| 300 | 4–5 | 0.11 | 43.2 | 52.6 | 57.6 | 191.0 | 36.8 | 59.2 | 127.0 | |
| 0.30 | 69.4 | 91.5 | 74.2 | 2227.9 | 100.5 | 356.2 | 592.6 | |||
| 0.35 | 93.9 | 93.1 | 96.1 | 3004.2 | 117.2 | 461.7 | 593.1 | |||
| 0.37 | 77.1 | 84.1 | 91.4 | 3735.0 | 123.9 | 551.8 | 745.3 | |||
| 0.40 | 63.7 | 77.3 | 71.6 | 4243.6 | 134.0 | 620.0 | 1069.1 | |||
Basis: 1 m3 wastewater and 56 min treatment time; unit cost of energy (a): $0.114/kWh, unit cost of Fe electrode material (b): $1.017/kg (Kobya et al., 2016).
Figure 2.
Effect of pH and dye concentrations on EC treatment efficiency; (A) %COD removal, (B) %Color removal and (C) %Dye removal (Note: current density was kept constant at 0.11 mA cm−2).
During the EC experiment, the solution pH changed towards the neutral range (pH = 6–7) supporting the previous studies (Al Aji et al., 2012; Elabbas et al., 2016). This might be the consumption of generated hydroxyl ions that happened during EC, which changed pH to the neutral range (Aswathy et al., 2016; Elabbas et al., 2016). According to Barrera Díaz et al. (2003), Fe(OH)2 is the predominant hydroxide for pH between 4-5 and both Fe(OH)2 and Fe(OH)3 exist in the pH range of 5–7. Also, the organic compounds dissociate into charged ions depending on the solution pH (Ghernaout, 2013).
Additionally, the associated energy consumption per unit of dye removal for acidic, neutral and basic pHs were 0.15, 1.12 and 6.05 kWh.m−3, respectively. This implies the more conductive nature of acidic solution (due to H+) and the least conductive nature at neutral pH. Finally, considering the issues of power requirement, treatment efficiency and higher corrosion rate of the electrodes in a more acidic solution, pH in the range of 4–5 was selected for the rest of the EC experiments.
3.2. Influence of variable dye concentrations on EC
Dye concentration varies extensively in textile wastewater and the treatment efficiency of EC depends on initial pollutant concentration (Yaseen and Scholz, 2019). To investigate the treatment performance for variable dye concentrations, several experiments were conducted for 100, 200 and 300 mg.L−1 MO dye solutions at the optimized pH range (4–5) and at 0.11 mA cm−2 current density. Treatment performances are summarized in Table 3 and removal trends are represented in Figure 2. After 56 min of treatment, COD removals for 100, 200 and 300 mg.L−1 MO dye solutions were 66.7%, 54.6% and 43.2%, respectively; color removals were 91.1%, 63.2% and 52.6%, respectively and dye removals were 96.6%, 64.7% and 57.6%, respectively; indicating the highest removal efficiency for the lowest dye concentration (100 mg.L−1). Additionally, removal efficiencies increased with increasing electrolysis time for 100 mg.L−1 dye solution, whereas higher concentrations (200 and 300 mg.L−1) showed limiting removal efficiencies, which were supported by previous studies (Benekos et al., 2019; El-Ashtoukhy et al., 2017; Zeboudji et al., 2013). Researchers recommended this effect due to the presence of insufficient amounts of flocs and exhaustion of adsorption capability of these limited flocs for higher contaminant concentrations (Ahmadzadeh et al., 2017; An et al., 2017; Kabdaşlı et al., 2012). Additionally, Fe(II) and Fe(III) ions generated for Fe electrodes also impose some color on the treated solution (Cañizares et al., 2007; Zaied et al., 2020), causing lower decolorization efficiency.
Additionally, associated energy consumption for the experiments containing 100, 200 and 300 mg.L−1 MO dye solutions were 0.152, 0.163 and 0.191 kWh.m−3, respectively. This power consumption implies higher energy consumption for solutions having higher contaminant concentrations. The power required and contaminant removal performance varied non-linearly with increasing pollutant concentrations, which is supported by previous studies (Ali et al., 2012; Hamdan and El-Naas, 2014). For the treatment of 25 and 55 mg.L−1 synthetic MO dye in aqueous solutions, Irki et al. (2017) observed 86.89% and 73.81% of color removal was executed by the power consumptions of 31 and 33 kWh.kg−1 MO dye, respectively.
3.3. Optimization of EC for variable dye concentrations
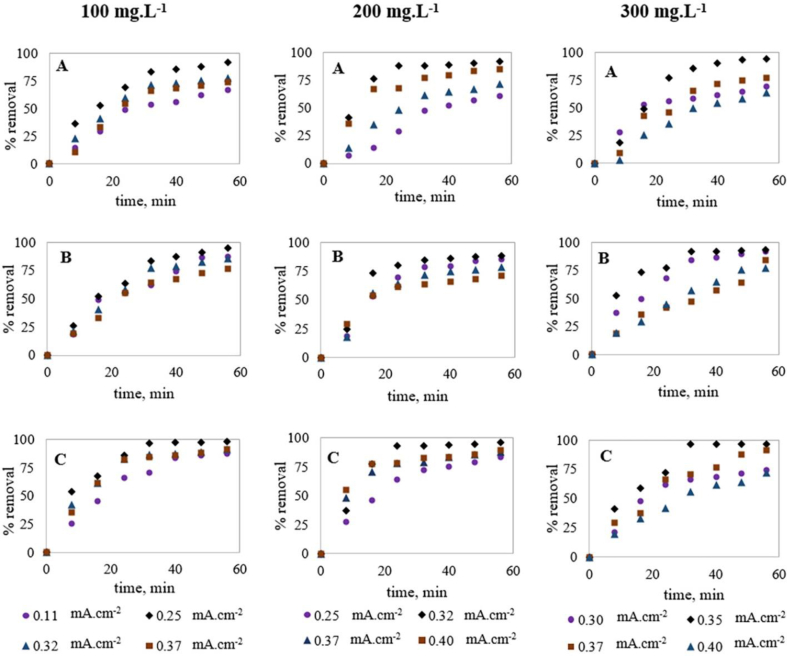
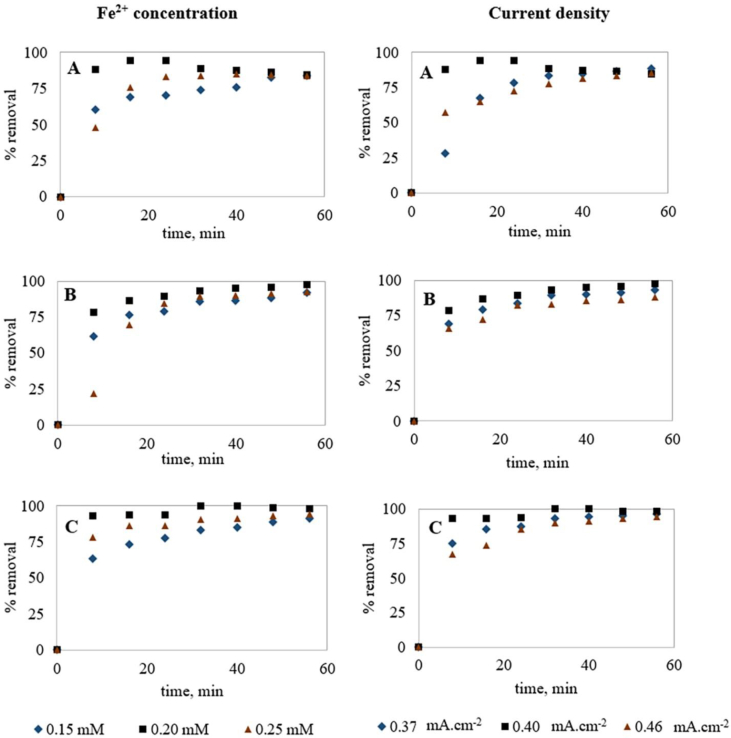
Current density has a collective effect on the EC process efficiency (Islam, 2019; Linares-Hernández et al., 2009) and optimum current density is required for effective treatment performance. Although a higher current density is required for a higher amount of metal coagulant generation for contaminant removal (Bayar et al., 2011; Farhadi et al., 2012), unnecessarily high current values may negatively affect the EC efficiency in addition to higher energy consumption (Farhadi et al., 2012; Hakizimana et al., 2017). Also, Zaied et al. (2020) recommended that there is an optimum treatment time after which an increase in reaction time causes a slight increase in removal efficiency and higher energy consumption. EC for variable dye concentrations (100, 200 and 300 mg.L−1) was optimized to observe the variation of these optimum parameters (current density and treatment time). With the concept of a higher amount of coagulant requirement for a higher amount of contaminant removal, higher current density was applied for higher dye concentrations and Table 3 and Figure 3 represent the results obtained in these experiments.
Figure 3.
Optimization of EC for variable dye concentrations; (A) %COD removal, (B) %Color removal and (C) %Dye removal (Note: pH was kept between 4 to 5).
As seen (Figure 3), among all the experiments conducted for 100 mg.L−1 MO solution, the experiment conducted at 0.25 mA cm−2 current density showed the highest removal efficiency compared to other experiments and after 56 min, 91.7% COD, 94.8% color and 98.3% dye removals were observed. This indicates that the optimum current density for 100 mg.L−1 MO dye solution treatment is 0.25 mA cm−2. For the EC treatment of 200 mg.L−1 MO, Figure 3 shows that the experiment conducted at 0.32 mA cm−2 current density provided the highest removal efficiency compared to other experiments and after 56 min, 91.2% COD, 88.1% color and 96.3% dye removals were obtained. Current density below or above 0.32 mA cm−2 showed lower treatment performance, indicating it as the optimum current density. Furthermore, 24 min was found to be the optimum treatment time for 200 mg.L−1 dye degradation as no noticeable removal was obtained after this time. Similarly, for the EC treatment of 300 mg.L−1 MO, 0.35 mA cm−2 current density was found optimum and after 56 min, 93.9% COD, 93.1% color and 96.1% dye removals were obtained. Also, 30–35 min was observed as the optimum treatment time for 300 mg.L−1 MO dye solution. From the above discussion, it can be concluded that both the optimum current density and optimum treatment time were increased with the increase of initial dye concentration, indicating a more coagulant requirement for higher dye concentrations. For the removal of organic pollutants by EC, researchers also recommended 10–30 min as the effective treatment time for a satisfactory removal (Benhadji et al., 2011; Espinoza-Quiñones et al., 2009; GilPavas et al., 2011).
3.4. Influence of different parameters on EC with additional Fe2+
Although EC removes colloidal particles and suspended solids efficiently, it exhibits some difficulties for wastewaters having a large variety of pollutants (Akter et al., 2022). EC treatment for higher MO concentrations (Figure 2 and Figure 3) also represented limiting treatment performance and higher electrolysis time was required for satisfactory treatment performance. A combination of EC with other treatment techniques is recommended by several authors to achieve satisfactory treatment performance (Kabdaşlı et al., 2012; Shokri and Fard, 2022; Turan, 2020). With the objective to obtain satisfactory treatment performance, EC in combination with chemical coagulation (CC) was applied for MO dye solution treatment following other research studies (Bazrafshan et al., 2016; Shamaei et al., 2018). In this research, Fe salt (FeSO4.7H2O) was used in combination with EC treatment and the influence of salt concentration and current density on treatment performance were observed separately. Here, Fe2+ acted as both a reducing agent (Sasson et al., 2009) and a coagulant while in the Fenton process, Fe2+ acts as a catalyst for the generation of free radicals (de la Plata et al., 2008; Sun et al., 2009). Experimental results are summarized in Table 4 and removal trends are represented in Figure 4.
Table 4.
Treatment performance and cost analysis of EC with additional Fe2+ salt.
| Salt conc. (mM) | Current density (mA.cm−2) | % COD removal | % Color removal | % Dye removal | Cost analysis∗ |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cenergy (kWh.m−3) 103 | Csalt (kg.m−3) 103 | Cmetal (kg.m−3) 103 | OC ($/m3) 103 |
OC/kg removed dye ($/kg dye) | ||||||
| Optimization of Fe2+ salt conc. | 0.15 | 0.40 | 83.6 | 91.8 | 91.3 | 1928.9 | 41.7 | 134.0 | 359.8 | 678.5 |
| 0.20 | 84.5 | 97.5 | 98.2 | 1633.1 | 55.6 | 134.0 | 327.3 | 617.2 | ||
| 0.25 | 83.6 | 92.3 | 94.1 | 1221.6 | 69.5 | 134.0 | 281.6 | 531.1 | ||
| Optimization of current density | 0.20 | 0.37 | 88.5 | 93.4 | 96.4 | 898.1 | 55.6 | 123.9 | 233.3 | 448.2 |
| 0.40 | 84.5 | 97.5 | 98.2 | 1633.1 | 55.6 | 134.0 | 327.3 | 617.2 | ||
| 0.46 | 85.3 | 88.3 | 94.6 | 2218.2 | 55.6 | 154.1 | 414.4 | 811.3 | ||
Basis: 1 m3 wastewater and 56 min treatment time; unit cost of energy (a): $0.114/kWh, unit cost of Fe electrode material (b): $1.017/kg (Kobya et al., 2016), unit cost of FeSO4.7H2O (c): $8.8 × 10−2.kg−1 (Suhan et al., 2020).
Figure 4.
Optimization of EC with additional Fe2+ salt for 200 mg.L−1 dye solution treatment; (A) %COD removal, (B) %Color removal and (C) %Dye removal (Note: pH was kept between 4 to 5).
3.4.1. Influence of salt (Fe2+) concentration
As seen (Figure 4), among three experiments conducted at 0.40 mA cm−2 current density having different salt concentrations (0.15, 0.20, and 0.25 mmol.L−1), the experiment with 0.20 mmol.L−1 Fe2+ showed the highest removal efficiency and after 16 min, 94% COD removal, 85% color removal and 92% dye removal were obtained. Salt concentrations higher and lower than 0.20 mmol.L−1 showed lower treatment performance. Besides, COD removal decreased after 24 min due to the increased concentration of Fe2+ in the solution. This might be due to the overdosage of Fe2+ that reversed the surface charge of particles, reducing the treatment efficiency (Farhadi et al., 2012; Hakizimana et al., 2017). For the degradation of MO by Advanced Fenton Process using zero valent metallic iron, Devi et al. (2009) reported that the excess concentration of Fe2+ form hydroxide compound raising the pH of the solution, which further leads to precipitation, reducing the degradation rate.
Additionally, energy consumptions for the experiments containing 0.25, 0.20 and 0.15 mmol.L−1 Fe2+ were 1.22, 1.63 and 1.93 kWh.m−3, respectively (for 56 min) implying increased solution conductivity with increasing salt concentration. Clearly, 0.20 mmol.L−1 was the optimum Fe2+ salt concentration and 20 min was the optimum treatment time for Fe2+ added EC treatment of 200 mg.L−1 MO dye solution at 0.40 mA cm−2 current density. Previously, for electro-Fenton treatment, limits of Fe2+ concentration were obtained in the range of 0.2–9.0 mmol.L−1 (Pushpalatha and Krishna, 2017).
3.4.2. Influence of current density
As both the electrode material and added salt was of Fe, the influence of current density on Fe2+ salt added EC was observed for solutions containing 0.20 mmol.L−1 Fe2+. As seen (Figure 4), the experiment conducted at 0.40 mA cm−2 current density showed the highest treatment performance and after 24 min, 94.1% COD removal, 88% color removal and 96% dye removal were obtained. Also, removal efficiency decreased after 24 min due to the increased concentration of Fe2+ in the solution. Current density higher or lower than 0.40 mA cm−2 showed lower treatment performance (Figure 4). Additionally, power consumption for the experiments conducted at 0.37, 0.40 and 0.46 mA cm−2 current densities were 0.9, 1.6 and 2.2 kW h.m−3, respectively (for 56 min) implying higher power consumption for higher current density. Clearly, for Fe2+ added EC treatment of 200 mg.L−1 MO dye solution using Fe electrodes, 0.40 mA cm−2 was the optimum current density using 0.20 mmol.L−1 Fe2+ salt and 20 min were observed as the optimum treatment time.
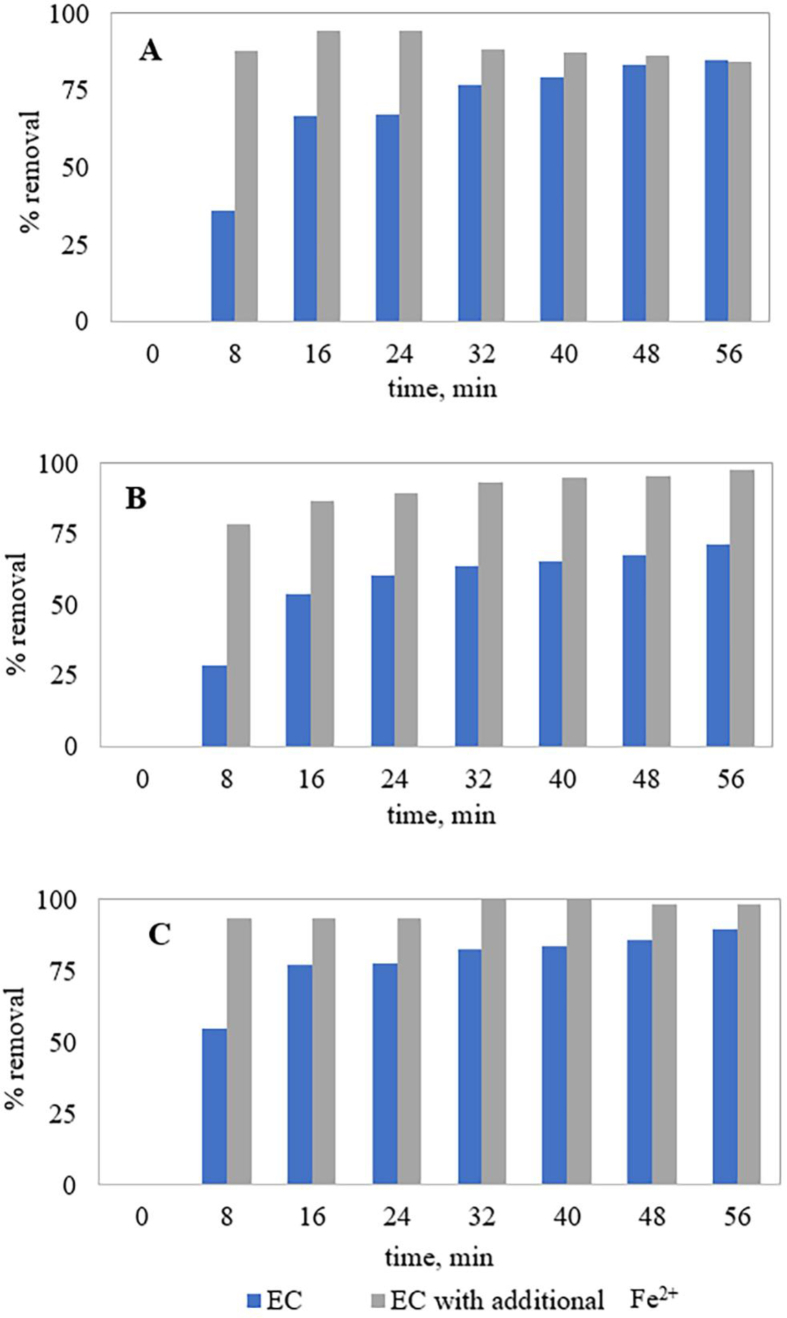
3.5. Competitive treatment performance and power requirements
As the applicability of an EC process depends both on treatment performance and cost-efficiency, a competitive study was performed between EC with and without Fe2+ salt addition for the treatment of 200 mg.L−1 MO solution at 0.40 mA cm−2 current density. As seen in Figure 5, EC with additional Fe2+ (0.20 mmol.L−1) showed higher removal performance compared to EC due to the collective effect of CC and EC. EC with additional Fe2+ provided the highest COD removal efficiency for 24 min, whereas COD removal for EC increased with increasing treatment time. This was due to the over-dosage of Fe2+ ions in solution after 24 min for EC with additional Fe2+. Considering the COD, color and dye removal for Fe2+ salt added EC compared to EC, 32 min could be considered for the highest removal performance. After 32 min, the treatment efficiency of Fe2+ salt added EC (88.5% COD, 93.1% color and 100% dye) was much higher than for EC (76.7% COD, 63.4% color and 82.4% dye). Clearly, treatment performance for EC with additional Fe2+ was more time-dependent compared to EC. Also, the power consumptions for EC and Fe2+ added EC was 2.31 and 0.93 kWh.m−3, respectively for 32 min. This implies that Fe2+ added EC exhibited a lower power requirement with higher removal capability compared to EC only. From this comparison, it can be concluded that only a smaller amount of Fe2+ salt can make the EC treatment process more feasible for industrial applications. Table 5 represents a comparison of MO dye removal from the current study with other research studies. As seen (Table 5), catalytic degradation and oxidation processes provided higher removal efficiencies compared to EC and Fe2+ added EC. According to Pi et al. (2014), periodic reversal of the electrodes or application of AC current in EC or Fe2+ added EC could improve COD removal.
Figure 5.
Competitive degradation of 200 mg.L−1 MO solution by EC and EC with additional Fe2+ salt at 0.40 mA cm−2 current density; (A) %COD removal, (B) %Color removal and (C) %Dye removal.
Table 5.
Different treatment processes for MO dye degradation.
| Treatment process | Initial pollutant levels | Treatment media/system specification | Operating conditions | Treatment efficiency | Reference |
|---|---|---|---|---|---|
| EC | 200 ppm MO | Monopolar SS electrode | pH:4-5 Time: 32 min Volume: 2.7L |
COD: 76.7% Color: 63.4% Dye: 82.4% |
This study |
| Fe2+ added EC | pH:4-5 Time: 24 min Volume: 2.7L Fe2+: 0.20 mmol.L−1 |
COD: 94.1% Color: 93.1% Dye: 100% |
This study | ||
| EC | Real textile wastewater COD: 683 mg.L−1 |
Monopolar SS electrode | pH:4-5 Time: 56 min Volume: 2.7L |
COD: 35.4% | This study |
| Fe2+ added EC | pH:4-5 Time: 32 min Volume: 2.7L Fe2+: 0.15 mmol.L−1 |
COD: 57.6% | This study | ||
| Catalytic degradation of MO | 10 mg.L−1 | MCM-41-NH2 | T: 30 °C pH: 6 Time: 120 min Volume: 150 mL K2S2O8: 30 mg Catalyst: 200 mg |
Color: 35% | Schlichter et al. (2017) |
| Co/MCM-41-NH2 | Color: 96% | ||||
| Mn/MCM-41-NH2 | Color: 60% | ||||
| Cu/MCM-41-NH2 | Color: 98% | ||||
| SBA-15-NH2 | Color: 95% | ||||
| Cu/SBA-15-NH2 | Color: 96% | ||||
| Co/SBA-15-NH2 | Color: 100% | ||||
| MO dye degradation by EC | 125 mg.L−1 | Periodic reversal of the electrodes (PREC) | pH: 7.4 Cond.: 9.4 mS cm−1 Voltage: 4.4 V Current density: 185 mA cm−2 Time: 14 min Cycle of periodic reversal of electrodes: 15 s |
Color: 97% Energy: 44 kWh/kg removed dye |
Pi et al. (2014) |
| Microwave degradation of MO | 50 mg.L−1 | TiO2/AC/MW | pH: 6 Time: 1.5 min Volume: 25 mL Catalyst: 2.0 g.L−1 Radiation: 750 W and 2450 MHz |
Dye: 100% | Zhang et al. (2012) |
| MO degradation using Zn0activated persulfate (PS) | 98 mg.L−1 | pH: 5 T: 25 °C Time: 3 h PS: 71 mg.L−1 Zn0: 1.3 g.L−1 |
COD: 85% TOC: 58% |
Li et al. (2014) | |
| Microbial degradation of MO | 50 mg.L−1 | Kocuria rosea (MTCC 1532) | pH: 6.5 T: 30 °C Time: 75 min |
Color: 95% | Parshetti et al. (2010) |
| MO degradation using Co nanoparticles | 100 mg.L−1 | pH: 2.5 Co nanoparticle: 0.5 g.L−1 Time: 4 min Stirring: 10 rpm |
Color: 99% | Sha et al. (2016) | |
| MO degradation by electro-catalytic oxidation | 400 mg.L−1 | Double anode and single cathode system Anode: Fe plate and graphite plate Cathode: graphite plate Cathode is assisted by Co2O3-CuO-PO43- modified kaolin |
pH: 5 CD: 30 mA cm−2 NaCl: 2.5 g.L−1 d: 0.5 cm Stirring: 100 rpm Time: 60 min |
Coloar: 100% COD: 89.7% |
Ma et al. (2007) |
Note: MCM-41 and SBA-16: metal supports, GO: graphene oxide, SSA: specific surface area, MF: magnetic field, EC: electrocoagulation, EO: electro-oxidation.
Treatment performance of EC with and without Fe2+ could also be improved by supplying air (oxygen) into the EC cell to increase the oxidation of Fe2+ into Fe3+ (Kumar et al., 2018). The limiting performance of this process could also be due to the passivation of Fe electrodes (Shamaei et al., 2018) which could be ignored by using AC current supply (Pi et al., 2014; Yang et al., 2015).
3.6. Cost analysis
The operating cost (OC) of EC experiments at variable conditions was calculated by using Eqs. (3), (4), and (5) and are summarized in Table 3. As seen (Table 3), the lowest OC was obtained for acidic pH ($0.055 m−3, $232 kg−1 removed dye) due to the lower energy consumption. OC for 100 mg.L−1 dye solution at optimum condition (0.25 mA cm−2) was found $0.194 m−3 ($731.6 kg−1 removed dye) that was higher than for the experiment conducted at 0.11 mA cm−2 and lower than other experiments, but it (0.25 mA cm−2) provided highest removal performance. Similarly, OC for 200 and 300 mg.L−1 dye removal at optimum conditions were $0.396 m−3 ($762 kg−1 removed dye) and $0.462 m−3 ($593 kg−1 removed dye), respectively, showing increased OC for increasing dye concentration.
For EC with additional Fe2+ salt, OC was calculated by using Eqs. (4), (5), and (6) and calculated results are summarized in Table 4. As seen, OC decreased with increasing salt concentration irrespective of the cost of added salt. This was due to the higher conductivity of solutions having higher salt concentration, lowering the power consumption. Furthermore, OC increased with increasing current density for a particular salt concentration. OC for the experiment having 0.20 mmol.L−1 Fe2+ and 0.40 mA cm−2 current density was $0.327 m−3 ($617 kg−1 removed dye), whereas, for EC of 200 mg.L−1 dye solution at 0.40 mA cm−2 current density was $0.598 m−3 ($1236 kg−1 removed dye). This implies a higher removal capability of Fe2+ added EC with lower OC as compared to conventional EC.
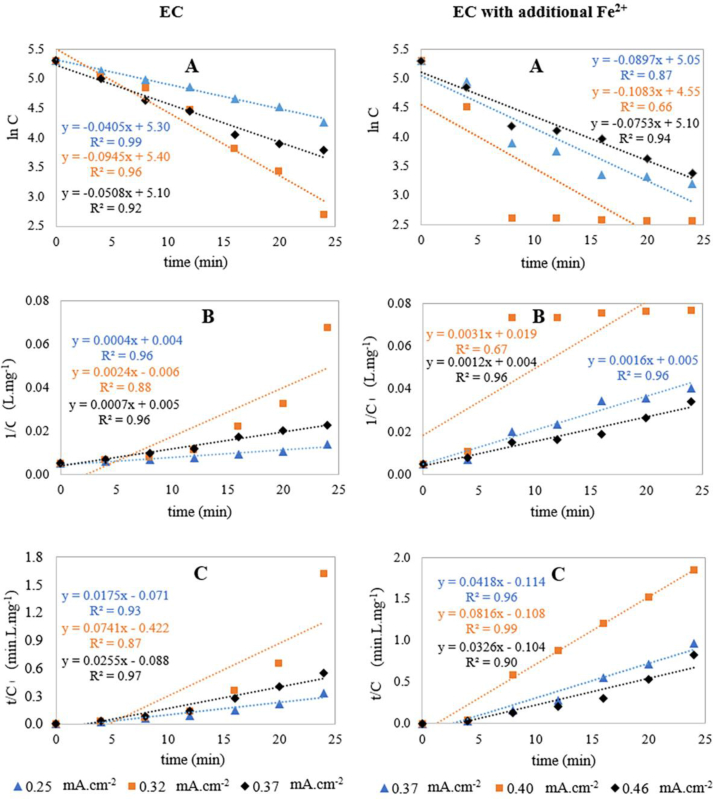
3.7. Kinetics of MO dye degradation
Kinetics of MO dye degradation was evaluated by comparing the values of regression coefficient and reaction rate constant (k). All the experiments were conducted at the same pH range (4–5) and kinetic studies were conducted for 24 min treatment time (optimum treatment time for 200 mg.L−1 dye degradation) around the optimum current density. To represent the kinetic study using a more reliable data series, samples were collected at 4 min intervals. The computed results for first-order, second-order and pseudo-second-order kinetics are summarized in Table 6 and represented by Figure 6.
Table 6.
Modeling parameters for EC and Fe2+ salt added EC of 200 ppm dye solution at different current density (Note: k = reaction rate constant).
| Dye conc. (ppm) | pH | Current density (mA.cm−2) | Parameters | First-order | Second-order | Pseudo second-order | |
|---|---|---|---|---|---|---|---|
| EC | 200 | 4–5 | 0.25 | r2 k |
0.99 0.0405 |
0.96 0.0004 |
0.93 -0.0043 |
| 0.32 | r2 k |
0.96 0.0945 |
0.88 0.0024 |
0.87 -0.013 |
|||
| 0.37 | r2 k |
0.92 0.0508 |
0.96 0.0007 |
0.97 -0.0074 |
|||
| EC with Fe2+ salt | 200 | 4–5 | 0.37 | r2 k |
0.87 0.0897 |
0.96 0.0016 |
0.96 -0.0153 |
| 0.40 | r2 k |
0.66 0.1083 |
0.67 0.0031 |
0.99 -0.0616 |
|||
| 0.46 | r2 k |
0.94 0.0753 |
0.96 0.0012 |
0.90 -0.0102 |
Figure 6.
Kinetic model plots of EC and Fe2+ salt added EC treatment for 200 mg.L−1 MO dye at different current densities; (A) First-order model, (B) Second-order model, (C) Pseudo second-order model.
As seen, the degradation of MO by EC is more fitted to first-order kinetics (r2 = 0.99, 0.96 and 0.92, respectively for 0.25, 0.32 and 0.37 mA cm−2 current density). Also, a decrease in reaction rate constant (k) for current density higher and lower than 0.32 mA cm−2 indicates this as the optimum current density. Degradation of MO by EC with additional Fe2+ is more fitted to second-order kinetics (r2 = 0.96, 0.67 and 0.96, respectively for 0.37, 0.40 and 0.46 mA cm−2 current density). The highest value of reaction rate constant (k) at 0.40 mA cm−2 current density (0.0031 L mg−1.min−1) implies maximum removal performance at this current density that was also found optimum. Poor regression co-efficient for the experiment conducted at 0.40 mA cm−2 might be due to the more instantaneous removal of dyes within the first few minutes (93% dye removal for 8 min) and slower removal of remaining dye molecules after a few minutes. Clearly, EC with and without additional Fe2+ salt followed two different kinetic models for the removal of MO dye.
Pseudo-second-order kinetics was also studied for both EC and EC with additional Fe2+ salt. The negative values of the reaction rate constant indicate a poor pseudo-second-order fit for MO dye removal by both EC and EC with additional Fe2+ salt.
He et al. (2013) observed the degradation of 106 mg.L−1 MO solution by the electro-Fenton (EC + Fe2++H2O2) process and the order of reaction was found 1.4164. For the photo-degradation of MO using a TiO2-mercury lamp, Dai et al. (2007) also investigated that dye degradation followed second-order kinetics having a reaction rate constant of 4.234 mol−1 min−1.
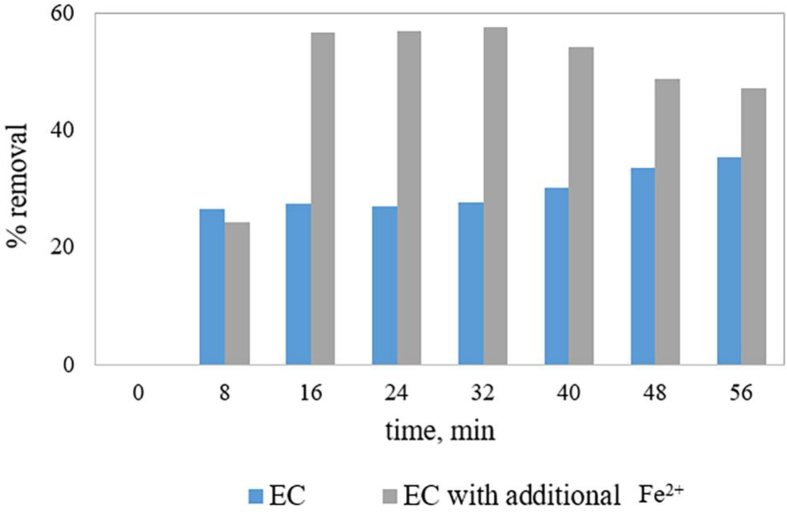
3.8. Experiment with real textile wastewater
An experiment with real textile wastewater was conducted using both EC and Fe2+ salt added EC. Both the experiments were conducted for 56 min. 0.15 mmol.L−1 Fe2+ salt concentration was used to observe the effect of additional Fe2+ salt for real wastewater treatment and experimental results are represented by Figure 7. As seen, for the EC experiment, % COD removal increased with increasing treatment time and 35.4% COD removal was obtained after 56 min. EC with additional Fe2+ showed more instantaneous COD removal performance and 57.6% COD removal was obtained after 32 min. After 32 min, COD removal decreased due to the accumulation of Fe2+ in bulk solution, representing Fe2+ added EC more time-dependent. Vidal et al. (2016) evaluated EC for real textile wastewater treatment and obtained 60% COD removal and 85% TOC removal at a pH of 4 using Al electrodes. From Table 1, it can be said that the combination of electro-oxidation with photo-degradation processes provides satisfactory treatment performance for real textile wastewater treatment. Hence, a combination of any photo-degradation technique with Fe2+ added EC could improve the treatment performance for real textile wastewater.
Figure 7.
EC and Fe2+ added EC for real wastewater treatment.
4. Conclusion
Acidic pH (4–5) was found optimum for the removal of MO by EC. Also, treatment performance decreased with increasing dye concentrations, and the optimum treatment time and current density were increased for higher dye concentrations. EC with additional Fe2+ showed higher and more instantaneous removal efficiency with lower power consumption and lower operating cost (OC) compared to conventional EC. Under optimum conditions, 76.7% COD, 63.4% color and 82.4% dye removals were obtained for EC with an operating cost of $0.342 m−3 ($768 kg−1 removed dye), whereas 88.5% COD, 93.1% color and 100% dye removals were obtained for Fe2+(0.20 mmol.L−1) added EC with an operating cost of $0.189 m−3 ($350 kg−1 removed dye) for 32 min treatment time. Therefore, it can be concluded that EC with additional Fe2+ salt is very efficient in terms of both treatment performance and operating cost.
Declarations
Author contribution statement
Sonia Akter: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Md Shahinoor Islam: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
Dr Md Shahinoor Islam was supported by Bangladesh University of Engineering and Technology [006].
Data availability statement
No data was used for the research described in the article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We are grateful to the Department of Chemical Engineering of Bangladesh University of Engineering and Technology (BUET) for providing laboratory facilities. We are also grateful to K. M. Nazmus Sakib (an undergraduate student, Department of Chemical Engineering, BUET) for assisting us in the laboratory.
References
- Ahmadzadeh S., Asadipour A., Pournamdari M., Behnam B., Rahimi H.R., Dolatabadi M. Removal of ciprofloxacin from hospital wastewater using electrocoagulation technique by aluminum electrode: optimization and modelling through response surface methodology. Process Saf. Environ. 2017;109:538–547. [Google Scholar]
- Akter S., Suhan M.B.K., Islam M.S. Recent advances and perspective of electrocoagulation in the treatment of wastewater: a review. Environ. Nanotechnol. Monit. Manag. 2022;17:100643. [Google Scholar]
- Al Aji B., Yavuz Y., Koparal A.S. Electrocoagulation of heavy metals containing model wastewater using monopolar iron electrodes. Separ. Purif. Technol. 2012;86:248–254. [Google Scholar]
- Al-Mamun M.R., Hossain K.T., Mondal S., Khatun M.A., Islam M.S., Khan M.Z.H. Synthesis, characterization, and photocatalytic performance of methyl orange in aqueous TiO2 suspension under UV and solar light irradiation. S. Afr. J. Chem. Eng. 2022;40:113–125. [Google Scholar]
- Al-Mamun M.R., Kader S., Islam M.S., Khan M.Z.H. Photocatalytic activity improvement and application of UV-TiO2 photocatalysis in textile wastewater treatment: a review. J. Environ. Chem. Eng. 2019;7(5):103248. [Google Scholar]
- Al-Mamun M.,R., Karim M.,N., Afroj N., Kader S., Islam M.,S., Khan M.Z.H. Photocatalytic performance assessment of GO and Ag co-synthesized TiO2 nanocomposite for the removal of methyl orange dye under solar irradiation. Environ. Technol. Innovat. 2021;22:101537. [Google Scholar]
- Al-Mamun M.R., Kader S., Islam M.S. Solar-TiO2 immobilized photocatalytic reactors performance assessment in the degradation of methyl orange dye in aqueous solution. Environ. Nanotechnol. Monit. Manag. 2021;16:100514. [Google Scholar]
- Al-Qodah Z., Tawalbeh M., Al-Shannag M., Al-Anber Z., Bani-Melhem K. Combined electrocoagulation processes as a novel approach for enhanced pollutants removal: a state-of-the-art review. Sci. Total Environ. 2020;744:140806. doi: 10.1016/j.scitotenv.2020.140806. [DOI] [PubMed] [Google Scholar]
- Ali I., Khan T.A., Asim M. Removal of arsenate from groundwater by electrocoagulation method. Environ. Sci. Pollut. Res. 2012;19(5):1668–1676. doi: 10.1007/s11356-011-0681-3. [DOI] [PubMed] [Google Scholar]
- Alimohammadi M., Askari M., Dehghani M.H., Dalvand A., Saeedi R., Yetilmezsoy K., Heibati B., McKay G. Elimination of natural organic matter by electrocoagulation using bipolar and monopolar arrangements of iron and aluminum electrodes. Int. J. Environ. Sci. Te. 2017;14(10):2125–2134. [Google Scholar]
- An C., Huang G., Yao Y., Zhao S. Emerging usage of electrocoagulation technology for oil removal from wastewater: a review. Sci. Total Environ. 2017;579:537–556. doi: 10.1016/j.scitotenv.2016.11.062. [DOI] [PubMed] [Google Scholar]
- Aswathy P., Gandhimathi R., Ramesh S.T., Nidheesh P.V. Removal of organics from bilge water by batch electrocoagulation process. Separ. Purif. Technol. 2016;159:108–115. [Google Scholar]
- Barrera-Dıaz C., Urena-Nunez F., Campos E., Palomar-Pardavé M., Romero-Romo M. A combined electrochemical-irradiation treatment of highly colored and polluted industrial wastewater. Radiat. Phys. Chem. 2003;67(5):657–663. [Google Scholar]
- Barrera Díaz C.E., González-Rivas N. The use of Al, Cu, and Fe in an integrated electrocoagulation-ozonation process. J. Chem. Neuroanat. 2015 [Google Scholar]
- Bayar S., Yıldız Y.Ş., Yılmaz A.E., İrdemez Ş. The effect of stirring speed and current density on removal efficiency of poultry slaughterhouse wastewater by electrocoagulation method. Desalination. 2011;280(1-3):103–107. [Google Scholar]
- Bazrafshan E., Alipour M.R., Mahvi A.H. Textile wastewater treatment by application of combined chemical coagulation, electrocoagulation, and adsorption processes. Desalination Water Treat. 2016;57(20):9203–9215. [Google Scholar]
- Benekos A.K., Zampeta C., Argyriou R., Economou C.N., Triantaphyllidou I.E., Tatoulis T.I., Tekerlekopoulou A.G., Vayenas D.V. Treatment of table olive processing wastewaters using electrocoagulation in laboratory and pilot-scale reactors. Process Saf. Environ. Protect. 2019;131:38–47. [Google Scholar]
- Bener S., Bulca Ö., Palas B., Tekin G., Atalay S., Ersöz G. Electrocoagulation process for the treatment of real textile wastewater: effect of operative conditions on the organic carbon removal and kinetic study. Process Saf. Environ. Protect. 2019;129:47–54. [Google Scholar]
- Benhadji A., Ahmed M.T., Maachi R. Electrocoagulation and effect of cathode materials on the removal of pollutants from tannery wastewater of Rouïba. Desalination. 2011;277(1-3):128–134. [Google Scholar]
- Benkhaya S., M'rabet S., El Harfi A. Classifications, properties, recent synthesis and applications of azo dyes. Heliyon. 2020;6(1):3271. doi: 10.1016/j.heliyon.2020.e03271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camcioglu S., Ozyurt B., Hapoglu H. Effect of process control on optimization of pulp and paper mill wastewater treatment by electrocoagulation. Process Saf. Environ. 2017;111:300–319. [Google Scholar]
- Cañizares P., Jiménez C., Martínez F., Saez C., Rodrigo M.A. Study of the electrocoagulation process using aluminum and iron electrodes. Ind. Eng. Chem. Res. 2007;46(19):6189–6195. [Google Scholar]
- Canizares P., Martínez F., Lobato J., Rodrigo M.A. Break-up of oil-in-water emulsions by electrochemical techniques. J. Hazard Mater. 2007;145(1-2):233–240. doi: 10.1016/j.jhazmat.2006.11.018. [DOI] [PubMed] [Google Scholar]
- Chen G. Electrochemical technologies in wastewater treatment. Separ. Purif. Technol. 2004;38(1):11–41. [Google Scholar]
- Cletus A., Athira S., Ramesh A.G., Priya K.L., Indu M.S. Recent Advancements in Civil Engineering. Springer; Singapore: 2022. Performance evaluation of electrocoagulation with hybrid electrodes in the decolourisation of methyl orange dye; pp. 443–451. [Google Scholar]
- Connors K.A. Wiley-VCH Verlag GmbH; 1990. Chemical Kinetics: the Study of Reaction Rates in Solution. [Google Scholar]
- Couto C.F., Lange L.C., Amaral M.C.S. A critical review on membrane separation processes applied to remove pharmaceutically active compounds from water and wastewater. J. Water Proc. Eng. 2018;26:156–175. [Google Scholar]
- Dai K., Chen H., Peng T., Ke D., Yi H. Photocatalytic degradation of methyl orange in aqueous suspension of mesoporous titania nanoparticles. Chemosphere. 2007;69(9):1361–1367. doi: 10.1016/j.chemosphere.2007.05.021. [DOI] [PubMed] [Google Scholar]
- de la Plata G.B.O., Alfano O.M., Cassano A.E. Optical properties of goethite catalyst for heterogeneous photo-Fenton reactions: comparison with a titanium dioxide catalyst. Chem. Eng. J. 2008;137(2):396–410. [Google Scholar]
- Devi L.G., Kumar S.G., Reddy K.M., Munikrishnappa C. Photo degradation of Methyl Orange an azo dye by Advanced Fenton Process using zero valent metallic iron: influence of various reaction parameters and its degradation mechanism. J. Hazard Mater. 2009;164(2-3):459–467. doi: 10.1016/j.jhazmat.2008.08.017. [DOI] [PubMed] [Google Scholar]
- Dogan A., Płotka-Wasylka J., Kempińska-Kupczyk D., Namieśnik J., Kot-Wasik A. Detection, identification and determination of chiral pharmaceutical residues in wastewater: problems and challenges. TrAC, Trends Anal. Chem. 2020;122:115710. [Google Scholar]
- El-Ashtoukhy E.Z., Amin N.K., Abd El-Latif M.M., Bassyouni D.G., Hamad H.A. New insights into the anodic oxidation and electrocoagulation using a self-gas stirred reactor: a comparative study for synthetic CI Reactive Violet 2 wastewater. J. Clean. Prod. 2017;167:432–446. [Google Scholar]
- Elabbas S., Ouazzani N., Mandi L., Berrekhis F., Perdicakis M., Pontvianne S., Pons M.N., Lapicque F., Leclerc J.P. Treatment of highly concentrated tannery wastewater using electrocoagulation: influence of the quality of aluminium used for the electrode. J. Hazard Mater. 2016;319:69–77. doi: 10.1016/j.jhazmat.2015.12.067. [DOI] [PubMed] [Google Scholar]
- Elkacmi R., Kamil N., Bennajah M. Upgrading of Moroccan olive mill wastewater using electrocoagulation: kinetic study and process performance evaluation. J. Urban Environ. Eng. 2017;11(1):30–41. [Google Scholar]
- Elnakar H., Buchanan I. Soluble chemical oxygen demand removal from bypass wastewater using iron electrocoagulation. Sci. Total Environ. 2020;706:136076. doi: 10.1016/j.scitotenv.2019.136076. [DOI] [PubMed] [Google Scholar]
- Espinoza-Quiñones F.R., Fornari M.M., Módenes A.N., Palácio S.M., Trigueros D.E., Borba F.H., Kroumov A.D. Electrocoagulation efficiency of the tannery effluent treatment using aluminium electrodes. Water Sci. Technol. 2009;60(8):2173–2185. doi: 10.2166/wst.2009.518. [DOI] [PubMed] [Google Scholar]
- Farhadi S., Aminzadeh B., Torabian A., Khatibikamal V., Fard M.A. Comparison of COD removal from pharmaceutical wastewater by electrocoagulation, photoelectrocoagulation, peroxi-electrocoagulation and peroxi-photoelectrocoagulation processes. J. Hazard Mater. 2012;219:35–42. doi: 10.1016/j.jhazmat.2012.03.013. [DOI] [PubMed] [Google Scholar]
- Fernández-Fernández M., Sanromán M.Á., Moldes D. Recent developments and applications of immobilized laccase. Biotechnol. Adv. 2013;31(8):1808–1825. doi: 10.1016/j.biotechadv.2012.02.013. [DOI] [PubMed] [Google Scholar]
- Garcia-Segura S., Eiband M.M.S., de Melo J.V., Martínez-Huitle C.A. Electrocoagulation and advanced electrocoagulation processes: a general review about the fundamentals, emerging applications and its association with other technologies. J. Electroanal. Chem. 2017;801:267–299. [Google Scholar]
- Ghalwa N.M.A., Saqer A.M., Farhat N.B. Removal of Reactive Red 24 dye by clean electrocoagulation process using iron and aluminum electrodes. J. Chem. Eng. Process Technol. 2016 [Google Scholar]
- Ghernaout D. Advanced oxidation phenomena in electrocoagulation process: a myth or a reality? Desalination Water Treat. 2013;51(40-42):7536–7554. [Google Scholar]
- Ghernaout D. Electrocoagulation process: achievements and green perspectives. Colloid Surface Sci. 2018;3:1–5. [Google Scholar]
- Ghernaout D., Ghernaout B., Naceur M.W. Embodying the chemical water treatment in the green chemistry—a review. Desalination. 2011;271(1-3):1–10. [Google Scholar]
- Ghosh D., Medhi C.R., Purkait M.K. Treatment of fluoride containing drinking water by electrocoagulation using monopolar and bipolar electrode connections. Chemosphere. 2008;73(9):1393–1400. doi: 10.1016/j.chemosphere.2008.08.041. [DOI] [PubMed] [Google Scholar]
- GilPavas E., Dobrosz-Gómez I., Gómez-García M.Á. Optimization of sequential chemical coagulation-electro-oxidation process for the treatment of an industrial textile wastewater. J. Water Proc. Eng. 2018;22:73–79. [Google Scholar]
- GilPavas E., Dobrosz-Gómez I., Gómez-García M.Á. The removal of the trivalent chromium from the leather tannery wastewater: the optimisation of the electro-coagulation process parameters. Water Sci. Technol. 2011;63(3):385–394. doi: 10.2166/wst.2011.232. [DOI] [PubMed] [Google Scholar]
- GilPavas E., Dobrosz-Gómez I., Gómez-García M.Á. Optimization and toxicity assessment of a combined electrocoagulation, H2O2/Fe2+/UV and activated carbon adsorption for textile wastewater treatment. Sci. Total Environ. 2019;651:551–560. doi: 10.1016/j.scitotenv.2018.09.125. [DOI] [PubMed] [Google Scholar]
- Gomes J.A., Daida P., Kesmez M., Weir M., Moreno H., Parga J.R., Irwin G., McWhinney H., Grady T., Peterson E., Cocke D.L. Arsenic removal by electrocoagulation using combined Al–Fe electrode system and characterization of products. J. Hazard Mater. 2007;139(2):220–231. doi: 10.1016/j.jhazmat.2005.11.108. [DOI] [PubMed] [Google Scholar]
- Hakizimana J.N., Gourich B., Chafi M., Stiriba Y., Vial C., Drogui P., Naja J. Electrocoagulation process in water treatment: a review of electrocoagulation modeling approaches. Desalination. 2017;404:1–21. [Google Scholar]
- Hamdan S.S., El-Naas M.H. Characterization of the removal of Chromium (VI) from groundwater by electrocoagulation. J. Ind. Eng. Chem. 2014;20(5):2775–2781. [Google Scholar]
- Hasan Khan Neon M., Islam M.S. MoO3 and Ag co-synthesized TiO2 as a novel heterogeneous photocatalyst with enhanced visible-light-driven photocatalytic activity for methyl orange dye degradation. Environ. Nanotechnol. Monit. Manag. 2019;12:100244. [Google Scholar]
- He W., Yan X., Ma H., Yu J., Wang J., Huang X. Degradation of methyl orange by electro-Fenton-like process in the presence of chloride ion. Desalination Water Treat. 2013;51(34-36):6562–6571. [Google Scholar]
- Heikal M. Effect of temperature on the physico-mechanical and mineralogical properties of Homra pozzolanic cement pastes. Cement Concr. Res. 2000;30(11):1835–1839. [Google Scholar]
- Holkar C.R., Jadhav A.J., Pinjari D.V., Mahamuni N.M., Pandit A.B. A critical review on textile wastewater treatments: possible approaches. J. Environ. Manag. 2016;182:351–366. doi: 10.1016/j.jenvman.2016.07.090. [DOI] [PubMed] [Google Scholar]
- Irki S., Ghernaout D., Naceur M.W. Decolourization of Methyl Orange (MO) by electrocoagulation (EC) using iron electrodes under a magnetic field (MF) Desalination Water Treat. 2017;79:368–377. [Google Scholar]
- Islam M.,S., Kwak J.-H., Nzediegwu C., Wang S., Palansuriya K., Kwon E.,E., Anne Naeth M., Gamal El-Din M., Ok Y.,S., Chang S.X. Biochar heavy metal removal in aqueous solution depends on feedstock type and pyrolysis purging gas. Environ. Pollut. 2021;281:117094. doi: 10.1016/j.envpol.2021.117094. [DOI] [PubMed] [Google Scholar]
- Islam S.D.U. Electrocoagulation (EC) technology for wastewater treatment and pollutants removal. Sustain. Water Res. Manage. 2019;5(1):359–380. [Google Scholar]
- Kabdaşlı I., Arslan-Alaton I., Ölmez-Hancı T., Tünay O. Electrocoagulation applications for industrial wastewaters: a critical review. Environ. Technol. 2012;1(1):2–45. [Google Scholar]
- Kader S., Al-Mamun M.R., Suhan M.B.K., Shuchi S.B., Islam M.S. Enhanced photodegradation of methyl orange dye under UV irradiation using MoO3 and Ag doped TiO2 photocatalysts. Environ. Technol. Innovat. 2022;27:102476. [Google Scholar]
- Kamaraj R., Vasudevan S. Evaluation of electrocoagulation process for the removal of strontium and cesium from aqueous solution. Chem. Eng. Res. Des. 2015;93:522–530. [Google Scholar]
- Kara I., Akar S.T., Akar T., Ozcan A. Dithiocarbamated Symphoricarpus albus as a potential biosorbent for a reactive dye. Chem. Eng. J. 2012;211:442–452. [Google Scholar]
- Katal R., Pahlavanzadeh H. Influence of different combinations of aluminum and iron electrode on electrocoagulation efficiency: application to the treatment of paper mill wastewater. Desalination. 2011;265(1-3):199–205. [Google Scholar]
- Khandegar V., Saroha A.K. Electrocoagulation for the treatment of textile industry effluent–a review. J. Environ. Manag. 2013;128:949–963. doi: 10.1016/j.jenvman.2013.06.043. [DOI] [PubMed] [Google Scholar]
- Khatri J., Nidheesh P.V., Singh T.A., Kumar M.S. Advanced oxidation processes based on zero-valent aluminium for treating textile wastewater. Chem. Eng. J. 2018;348:67–73. [Google Scholar]
- Kiani R., Mirzaei F., Ghanbari F., Feizi R., Mehdipour F. Real textile wastewater treatment by a sulfate radicals-Advanced Oxidation Process: peroxydisulfate decomposition using copper oxide (CuO) supported onto activated carbon. J. Water Proc. Eng. 2020;38:101623. [Google Scholar]
- Kobya M., Demirbas E., Ulu F. Evaluation of operating parameters with respect to charge loading on the removal efficiency of arsenic from potable water by electrocoagulation. J. Environ. Chem. Eng. 2016;4(2):1484–1494. [Google Scholar]
- Kobya M., Gebologlu U., Ulu F., Oncel S., Demirbas E. Removal of arsenic from drinking water by the electrocoagulation using Fe and Al electrodes. Electrochim. Acta. 2011;56(14):5060–5070. [Google Scholar]
- Koparal A.S., Yildiz Y.Ş., Keskinler B., Demircioğlu N. Effect of initial pH on the removal of humic substances from wastewater by electrocoagulation. Separ. Purif. Technol. 2008;59(2):175–182. [Google Scholar]
- Kumar A., Nidheesh P.V., Kumar M.S. Composite wastewater treatment by aerated electrocoagulation and modified peroxi-coagulation processes. Chemosphere. 2018;205:587–593. doi: 10.1016/j.chemosphere.2018.04.141. [DOI] [PubMed] [Google Scholar]
- Kurade M.B., Waghmode T.R., Kagalkar A.N., Govindwar S.P. Decolorization of textile industry effluent containing disperse dye Scarlet RR by a newly developed bacterial-yeast consortium BL-GG. Chem. Eng. J. 2012;184:33–41. [Google Scholar]
- Kushwaha J.P., Srivastava V.C., Mall I.D. Organics removal from dairy wastewater by electrochemical treatment and residue disposal. Separ. Purif. Technol. 2010;76(2):198–205. [Google Scholar]
- Lellis B., Fávaro-Polonio C.Z., Pamphile J.A., Polonio J.C. Effects of textile dyes on health and the environment and bioremediation potential of living organisms. Biotechnol. Res. Innov. 2019;3(2):275–290. [Google Scholar]
- Li H., Guo J., Yang L., Lan Y. Degradation of methyl orange by sodium persulfate activated with zero-valent zinc. Separ. Purif. Technol. 2014;132:168–173. [Google Scholar]
- Linares-Hernández I., Barrera-Díaz C., Roa-Morales G., Bilyeu B., Urena-Nunez F. Influence of the anodic material on electrocoagulation performance. Chem. Eng. J. 2009;148(1):97–105. [Google Scholar]
- Ma H., Wang B., Luo X. Studies on degradation of methyl orange wastewater by combined electrochemical process. J. Hazard Mater. 2007;149(2):492–498. doi: 10.1016/j.jhazmat.2007.04.020. [DOI] [PubMed] [Google Scholar]
- Mohora E., Rončević S., Agbaba J., Tubić A., Mitić M., Klašnja M., Dalmacija B. Removal of arsenic from groundwater rich in natural organic matter (NOM) by continuous electrocoagulation/flocculation (ECF) Separ. Purif. Technol. 2014;136:150–156. [Google Scholar]
- Mollah M.Y.A., Schennach R., Parga J.R., Cocke D.L. Electrocoagulation (EC)-science and applications. J. Hazard Mater. 2001;84(1):29–41. doi: 10.1016/s0304-3894(01)00176-5. [DOI] [PubMed] [Google Scholar]
- Moreno C H.A., Cocke D.L., Gomes J.A., Morkovsky P., Parga J.R., Peterson E., Garcia C. Electrochemical reactions for electrocoagulation using iron electrodes. Ind. Eng. Chem. Res. 2009;48(4):2275–2282. [Google Scholar]
- Moussa D.T., El-Naas M.H., Nasser M., Al-Marri M.J. A comprehensive review of electrocoagulation for water treatment: potentials and challenges. J. Environ. Manag. 2017;186:24–41. doi: 10.1016/j.jenvman.2016.10.032. [DOI] [PubMed] [Google Scholar]
- Naje A.S., Chelliapan S., Zakaria Z., Ajeel M.A., Alaba P.A. A review of electrocoagulation technology for the treatment of textile wastewater. Review. Chem. Eng-New York. 2017;33(3):263–292. [Google Scholar]
- Nasrullah M., Singh L., Krishnan S., Sakinah M., Zularisam A.W. Electrode design for electrochemical cell to treat palm oil mill effluent by electrocoagulation process. Environ. Technol. Innovat. 2018;9:323–341. [Google Scholar]
- Panizza M., Cerisola G. Direct and mediated anodic oxidation of organic pollutants. Chem. Rev. 2009;109(12):6541–6569. doi: 10.1021/cr9001319. [DOI] [PubMed] [Google Scholar]
- Parshetti G.K., Telke A.A., Kalyani D.C., Govindwar S.P. Decolorization and detoxification of sulfonated azo dye methyl orange by Kocuria rosea MTCC 1532. J. Hazard Mater. 2010;176(1-3):503–509. doi: 10.1016/j.jhazmat.2009.11.058. [DOI] [PubMed] [Google Scholar]
- Pétrier C., Torres-Palma R., Combet E., Sarantakos G., Baup S., Pulgarin C. Enhanced sonochemical degradation of bisphenol-A by bicarbonate ions. Ultrason. Sonochem. 2010;17(1):111–115. doi: 10.1016/j.ultsonch.2009.05.010. [DOI] [PubMed] [Google Scholar]
- Pi K.W., Xiao Q., Zhang H.Q., Xia M., Gerson A.R. Decolorization of synthetic methyl orange wastewater by electrocoagulation with periodic reversal of electrodes and optimization by RSM. Process Saf. Environ. 2014;92(6):796–806. [Google Scholar]
- Pourrezaei P., Afzal A., Ding N., Islam M.S., Moustafa A., Drzewicz P., Chelme-Ayala P., Gamal El-Din M. Physico-chemical processes. Water environment research. Lit. Rev. 2010;82(10):997–1072. [Google Scholar]
- Pushpalatha M., Krishna B.M. Electro-fenton process for waste water treatment. A review. Int. J. Adv. Res. Ideas Innov. Tech. 2017;3(1):439–451. [Google Scholar]
- Ramírez Cecilia, Saldaña Adriana, Hernández Berenice, Acero Roberto, Guerra Ricardo, Garcia-Segura Sergi, EnricBrillas, Juan M., Peralta-Hernández Electrochemical oxidation of methyl orange azo dye at pilot flow plant using BDD technology. J. Ind. Eng. Chem. 2013;19(2):571–579. [Google Scholar]
- Roy H., Prantika T.R., Riyad M.H., Paul S., Islam M.S. Synthesis, characterizations and RSM analysis of Citrus macroptera peel derived biochar for textile dye treatment. S. Afr. J. Chem. Eng. 2022;41:129–139. [Google Scholar]
- Sahu O., Mazumdar B., Chaudhari P.K. Treatment of wastewater by electrocoagulation: a review. Environ. Sci. Pollut. Res. 2014;21(4):2397–2413. doi: 10.1007/s11356-013-2208-6. [DOI] [PubMed] [Google Scholar]
- Sankar M.R., Sivasubramanian V. Optimization and evaluation of malathion removal by electrocoagulation process and sludge management. J. Environ. Chem. Eng. 2021;9(5):106147. [Google Scholar]
- Sasson M.B., Calmano W., Adin A. Iron-oxidation processes in an electroflocculation (electrocoagulation) cell. J. Hazard Mater. 2009;171(1-3):704–709. doi: 10.1016/j.jhazmat.2009.06.057. [DOI] [PubMed] [Google Scholar]
- Schlichter S., Sapag K., Dennehy M., Alvarez M. Metal-based mesoporous materials and their application as catalysts for the degradation of methyl orange azo dye. J. Environ. Chem. Eng. 2017;5(5):5207–5214. [Google Scholar]
- Sha Y., Mathew I., Cui Q., Clay M., Gao F., Zhang X.J., Gu Z. Rapid degradation of azo dye methyl orange using hollow cobalt nanoparticles. Chemosphere. 2016;144:1530–1535. doi: 10.1016/j.chemosphere.2015.10.040. [DOI] [PubMed] [Google Scholar]
- Shamaei L., Khorshidi B., Perdicakis B., Sadrzadeh M. Treatment of oil sands produced water using combined electrocoagulation and chemical coagulation techniques. Sci. Total Environ. 2018;645:560–572. doi: 10.1016/j.scitotenv.2018.06.387. [DOI] [PubMed] [Google Scholar]
- Shokri A. Employing electrocoagulation for the removal of Acid Red 182 in aqueous environment by using Box-Behenken design method, Desalin. Water Treat. 2018;115:281–287. [Google Scholar]
- Shokri A. Application of electrocoagulation process for the removal of Acid orange 5 in synthetic wastewater. Iran. J. Chem. Chem. Eng. (IJCCE). 2019;38(2):113–119. [Google Scholar]
- Shokri A., Fard M.S. A critical review in electrocoagulation technology applied for oil removal in industrial wastewater. Chemosphere. 2022;288:132355. doi: 10.1016/j.chemosphere.2021.132355. [DOI] [PubMed] [Google Scholar]
- Shokri A., Karimi S. Treatment of aqueous solution containing acid red 14 using an electro peroxone process and a box-Behnken experimental design. Archive. Hygiene Sci. 2020;9(1):48–57. [Google Scholar]
- Shuchi S.B., Suhan M.B.K., Humayun S.B., Haque M.E., Islam M.S. Heat-activated potassium persulfate treatment of Sudan Black B dye: degradation kinetic and thermodynamic studies. J. Water Proc. Eng. 2021;39:101690. [Google Scholar]
- Song P., Yang Z., Zeng G., Yang X., Xu H., Wang L., Xu R., Xiong W., Ahmad K. Electrocoagulation treatment of arsenic in wastewaters: a comprehensive review. Chem. Eng. J. 2017;317:707–725. [Google Scholar]
- Sorayyaei S., Raji F., Rahbar-Kelishami A., Ashrafizadeh S.N. Combination of electrocoagulation and adsorption processes to remove methyl orange from aqueous solution. Environ. Technol. Innovat. 2021;24:102018. [Google Scholar]
- Suhan M.B.K., Mahtab S.T., Aziz W., Akter S., Islam M.S. Sudan black B dye degradation in aqueous solution by Fenton oxidation process: kinetics and cost analysis. Case Studies Chem.Environ. Eng. 2021;4:100126. [Google Scholar]
- Suhan M.B.K., Shuchi S.B., Anis A., Haque Z., Islam M.S. Comparative degradation study of remazol black B dye using electro-coagulation and electro-Fenton process: kinetics and cost analysis. Environ. Nanotechnol. Monit. Manag. 2020;14:100335. [Google Scholar]
- Sun S.P., Li C.J., Sun J.H., Shi S.H., Fan M.H., Zhou Q. Decolorization of an azo dye Orange G in aqueous solution by Fenton oxidation process: effect of system parameters and kinetic study. J. Hazard Mater. 2009;161(2-3):1052–1057. doi: 10.1016/j.jhazmat.2008.04.080. [DOI] [PubMed] [Google Scholar]
- Tavangar T., Jalali K., Shahmirzadi M.A.A., Karimi M. Toward real textile wastewater treatment: membrane fouling control and effective fractionation of dyes/inorganic salts using a hybrid electrocoagulation–nanofiltration process. Separ. Purif. Technol. 2019;216:115–125. [Google Scholar]
- Tiwari B., Sellamuthu B., Ouarda Y., Drogui P., Tyagi R.D., Buelna G. Review on fate and mechanism of removal of pharmaceutical pollutants from wastewater using biological approach. Bioresour. Technol. 2017;224:1–12. doi: 10.1016/j.biortech.2016.11.042. [DOI] [PubMed] [Google Scholar]
- Turan N.B. The application of hybrid electrocoagulation–electrooxidation system for the treatment of dairy wastewater using different electrode connections. Separ. Sci. Technol. 2020;1–14 [Google Scholar]
- Vidal J., Villegas L., Peralta-Hernandez J.M., Salazar Gonzalez R. Removal of Acid Black 194 dye from water by electrocoagulation with aluminum anode. J. Environ. Sci. Health, Part A A. 2016;51(4):289–296. doi: 10.1080/10934529.2015.1109385. [DOI] [PubMed] [Google Scholar]
- Vilar V.J., Pinho L.X., Pintor A.M., Boaventura R.A. Treatment of textile wastewaters by solar-driven advanced oxidation processes. Sol. Energy. 2011;85(9):1927–1934. [Google Scholar]
- Wang A., Qu J., Ru J., Liu H., Ge J. Mineralization of an azo dye Acid Red 14 by electro-Fenton's reagent using an activated carbon fiber cathode. Dyes Pigments. 2005;65(3):227–233. [Google Scholar]
- Wang Y., Xia G., Wu C., Sun J., Song R., Huang W. Porous chitosan doped with graphene oxide as highly effective adsorbent for methyl orange and amido black 10B. Carbohydr. Polym. 2015;115:686–693. doi: 10.1016/j.carbpol.2014.09.041. [DOI] [PubMed] [Google Scholar]
- Wesley M.J., Lerner R.N., Kim E.-S., Islam M.S., Liu Y. Biological fixed film. Water environment research. Lit. Rev. 2011;83(10):1150–1186. [Google Scholar]
- Wu Z., Dong J., Yao Y., Yang Y., Wei F. Continuous flowing electrocoagulation reactor for efficient removal of azo dyes: kinetic and isotherm studies of adsorption. Environ. Technol. Innovat. 2021;22:101448. [Google Scholar]
- Yan S.C., Li Z.S., Zou Z.G. Photodegradation of rhodamine B and methyl orange over boron-doped g-C3N4 under visible light irradiation. Langmuir. 2010;26(6):3894–3901. doi: 10.1021/la904023j. [DOI] [PubMed] [Google Scholar]
- Yang Z.H., Xu H.Y., Zeng G.M., Luo Y.L., Yang X., Huang J., Wang L.K., Song P.P. The behavior of dissolution/passivation and the transformation of passive films during electrocoagulation: influences of initial pH, Cr (VI) concentration, and alternating pulsed current. Electrochim. Acta. 2015;153:149–158. [Google Scholar]
- Yaseen D.A., Scholz M. Impact of pH on the treatment of artificial textile wastewater containing azo dyes using pond systems. Int. J. Environ. Res. 2019;13(2):367–385. [Google Scholar]
- Zaied B.K., Rashid M., Nasrullah M., Zularisam A.W., Pant D., Singh L. A comprehensive review on contaminants removal from pharmaceutical wastewater by electrocoagulation process. Sci. Total Environ. 2020;138095 doi: 10.1016/j.scitotenv.2020.138095. [DOI] [PubMed] [Google Scholar]
- Zeboudji B., Drouiche N., Lounici H., Mameri N., Ghaffour N. The influence of parameters affecting boron removal by electrocoagulation process. Separ. Sci. Technol. 2013;48(8):1280–1288. [Google Scholar]
- Zhang Z., Xu Y., Ma X., Li F., Liu D., Chen Z., Zhang F., Dionysiou D.D. Microwave degradation of methyl orange dye in aqueous solution in the presence of nano-TiO2-supported activated carbon (supported-TiO2/AC/MW) J. Hazard Mater. 2012;209:271–277. doi: 10.1016/j.jhazmat.2012.01.021. [DOI] [PubMed] [Google Scholar]
- Zhao H., Zhang G., Zhang Q. MnO2/CeO2 for catalytic ultrasonic degradation of methyl orange. Ultrason. Sonochem. 2014;21(3):991–996. doi: 10.1016/j.ultsonch.2013.12.002. [DOI] [PubMed] [Google Scholar]
- Zodi S., Potier O., Lapicque F., Leclerc J.P. Treatment of the textile wastewaters by electrocoagulation: effect of operating parameters on the sludge settling characteristics. Separ. Purif. Technol. 2009;69(1):29–36. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.