Abstract
Cell-based therapy imparts its therapeutic effects via soluble growth factors and vesicular bodies like exosomes. A systematic review with a meta-analysis of pre-clinical studies was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) and the modified Stroke Therapy Academic Industry Roundtable (STAIR) guidelines, to identify exosomes as an archetype biological therapy for dermal wound healing and to provide guidelines for the concentrations to be used in pre-clinical studies. A total of 51 rodent studies were included in the systematic review and meta-analysis (9), respectively. Three independent reviewers cross-screened eligibility and selected studies for quality assessment from 3064 published studies on exosomes and wound healing. The mean quality scores for all studies were 5.08±0.752 and 5.11±1.13 for systematic review and meta-analysis, respectively. Exosome effects were reported to have the highest efficacy at seven days (odds ratio 1.82 with 95% CI [0.69, 2.95]) compared to14 days (odds ratio of 2.29 with 95% CI [0.01, 4.56]) after administration. Exosomes were reported to regulate all phases of skin wound healing, mostly by the actions of circulating microRNA. The outcome of this review may be used to guide pre-clinical and clinical studies on the role of exosomes in wound healing.
Keywords: Exosomes, extracellular vesicles, wound healing, regeneration, angiogenesis, collagen, wound closure
INTRODUCTION
Wound healing is a complex process that comprises three distinct but overlapping phases (inflammation, proliferation, and remodeling), and unique cell populations dominate each phase. These different populations synchronize their presence and activity by secreting a variety of growth factors and other cues, laying the foundation for incoming cells to contribute to the healing process. Stem cell administration is increasingly being used to optimize all three phases of wound healing. During cutaneous wound healing, stem cells are known for their ability to reduce inflammation, accelerate the proliferation phase, and aid in tissue remodeling that ultimately shifts the balance towards better healing outcomes (El Ayadi et al., 2020). Interestingly, while most studies report the disappearance of applied stem cells from the treatment site, these cells were shown to impart therapeutic outcomes even when absent, suggesting that the cells released secretory mediators before being cleared by the host (Eggenhofer et al., 2014, Kurtz, 2008). Corroborating evidence now shows that stem cells exert their therapeutic effects primarily via paracrine mechanisms by releasing growth factors and extracellular vesicles (EVs), including exosomes.
Among EVs, exosomes are biologically active nanoparticles constitutively secreted in the extracellular space by different cell populations as a means of endocrine and paracrine communication. Like other multivesicular bodies, exosomes are either repackaged intracellularly or secreted directly from the plasma membrane. Several studies demonstrated the therapeutic potential of exosomes to affect each wound healing phase. During the inflammation phase, exosomes were reported to affect various immune cells and resident tissue cells, reducing the inflammatory response (Li et al., 2019, Li Xiao et al., 2016, Shi Zhengzhou et al., 2019). During the proliferation phase, exosomes aid with wound closure by activating endothelial cells (Xu et al., 2020, Zhang et al., 2016) and fibroblasts (Ma et al., 2019, Zhao et al., 2018), promoting a pro-angiogenic environment and initiating extracellular matrix deposition, respectively. During the remodeling phase, exosomes alter the ratio of matrix metalloproteinases and tissue inhibitors of matrix metalloproteinases for favorable wound healing outcomes (Yang et al., 2020). Although exosomes undoubtedly affect adipogenesis, immune cells, and other vital physiological and molecular pathways, in the submitted manuscript we are intending to focus on the wound healing aspect of exosomes.
Examining the vast literature archive of nanovesicle research, one encounters significant variations in the isolation (ultracentrifugation vs. others), characterization (size, CD markers), and sources of nanovesicles. The majority of the studies analyzed in this review (>95%) used the term ‘exosome’ to define the nanovesicles used in wound healing studies. Thus, we adopted the term exosome in this review and to define biologically active nanoparticles. Exosomes provide a window into the physiological state of a specific tissue, an individual organ, or even systemically, and can be used as a means of cellular communication (Raposo et al., 1996). Cellular signaling via exosomes is a highly conserved pathway and is observed in almost all living things (Gerlach et al., 2018). The intravesicular compartment of an exosome is enriched with signaling molecules that include nucleic acids, lipids, and proteins, while the outer membrane of the exosome is composed of anchoring molecules, immune eliciting molecules, and lipid rafts. Investigations into the exosome as a nucleic acid (mRNA, miRNA, lncRNA) carrier have grown exponentially over the past decade, and the selective mechanisms involved in loading miRNAs inside exosomes are also being actively investigated (Guduric-Fuchs et al., 2012). An expanding body of literature defines an exosome as having a phospholipid bilayer membrane ranging from 30–150 nm in diameter, loaded with cargo molecules that include lipids, proteins, biologically active receptors, and nucleic acids, all having biological activity (Patel et al., 2019). Exosomes are used as diagnostic tools in various cancers (Sheridan, 2016) and their homing properties make them an exceptional choice for a drug-delivering system (2020, Liu and Su, 2019, Qiao et al., 2020, Walker et al., 2019). Exosomes are stable for long periods, even at room temperature, can be lyophilized (Akers et al., 2016), are immune-privileged, and affected the immune response in several pre-clinical studies (Gallet et al., 2017). Exosomes can also be modified to carry specific cargoes, which decreases the risk of unwanted differentiation. These advantages over cell-based therapies have unequivocally made exosomes an ideal candidate for biological therapy.
Although the revised position statement released by the International Society of Extracellular Vesicles (2018) has attempted to unify the field by providing guidelines for emerging EV and exosome researchers (Thery et al., 2018), a massive disparity remains in defining, isolating, and characterizing exosomes used in wound healing investigations. Furthermore, a dearth of critical systematic assessment of exosome’s role in cutaneous wound healing is present, especially when addressing therapeutic concentrations during efficacy trials. To bridge this knowledge gap, we performed a systematic review and meta-analysis assessing the efficacy of exosome application in rodent wound healing models. The primary objective was to report whether exosomes are a legitimate biological candidate for wound healing, while the secondary objective was to recommend a quantitative concentration of exosomes to be used in a rodent cutaneous wound healing model.
A systematic review was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) and modified Stroke Therapy Academic Industry Roundtable (STAIR) guidelines. The meta-analysis section aimed to corroborate the role of exosomes in cutaneous wound healing and provide guidelines for the concentration of exosomes to be used in future studies. Furthermore, the systematic review aimed to highlight similarities and disparities in experimental design, exosomes characterization, application methods, and outcome analyses among researchers.
RESULTS
Overall study characteristics
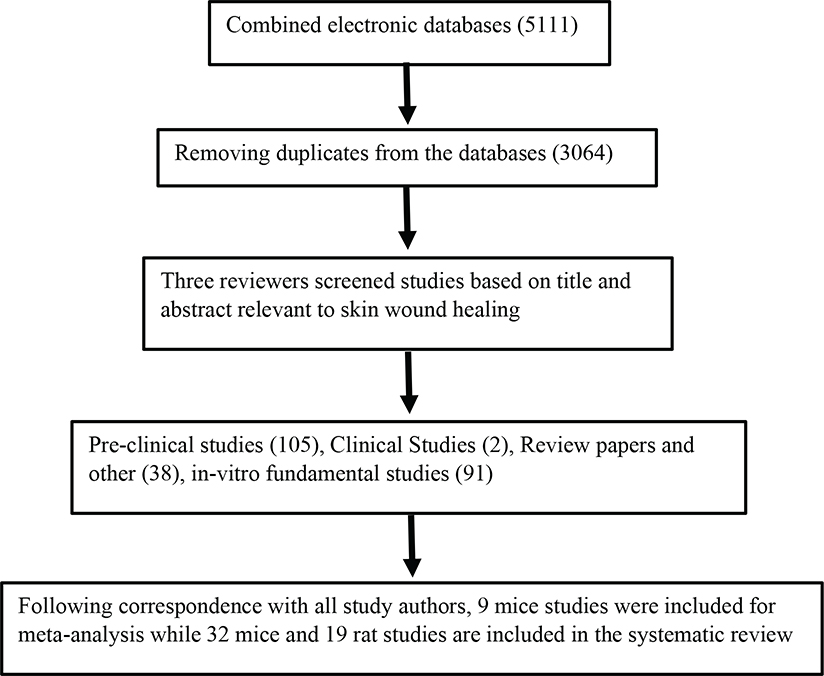
Out of 3,064 studies, a total of 105 pre-clinical studies were identified, of which 51 studies (32 mice and 19 rats) reported the use of exosomes in rodent models of excisional wound healing (Figure 1). We found two clinical studies reporting the use of exosomes to assess various cutaneous pathologies. However, due to extreme heterogeneity between the studies, we chose to focus on the role of exosomes in accelerating wound healing in rodent models. Nearly all studies reported beneficial therapeutic outcomes after exosome administration.
Figure 1.
Flow diagram of the literature search, review, and selection of the studies analyzed for systematic review or meta-analysis. The workflow followed the PRISMA guidelines.
Quality score
The mean Quality Score was 5.08 ± 0.75 for all studies. Studies included in the meta-analysis section had a mean Quality Score of 5.11 ± 1.05. All reported studies were published in a peer-reviewed journal. Most of the studies, 50, reported compliance with animal welfare and avoided the use of anesthetics with intrinsic wound healing properties. Topical anesthetics like bupivacaine are known to affect wound healing outcomes including closure and tensile strength, therefore, avoiding their use is warranted in published animal studies (Kesici et al., 2018). Forty-eight publications stated no conflicts of interest, while 41 studies mentioned randomization of the treatment group. However, only 11 studies specifically described temperature control, three studies reported at least one conflict of interest, and no studies reported calculations of cohort size nor animal numbers excluded from analysis (Supplemental Table 1).
Isolation and characterization of exosomes
The majority of the study was comprised of mice (n=32) and cultured MSCs were the major source of exosomes. Skin excision was the preferred source of injury type followed by the burn procedure (Supplemental Table 2a). Analysis of the reported methods for exosome extraction shows that 64% of the studies used ultracentrifugation, 18% used exosome isolation kits, 7% used a combination of ultracentrifugation and filtration methods, while 5% used of the studies used tangential flow filtration (Supplemental Table 2b).
Visualization and characterization of isolated exosomes used a combination of western blot, flow cytometry, nanoparticle tracking analysis, and transmission electron microscopy (TEM). As shown in Figure 2, 45% of the analyzed studies used western blot in combination with TEM, 30% used a combination of western blot and nanoparticle tracking, 13% used western blot in combination with flow cytometry and TEM, 6% used TEM alone, and 4.5% used TEM in combination with flow cytometry.
Figure 2. Methodologies used to characterize exosomes.
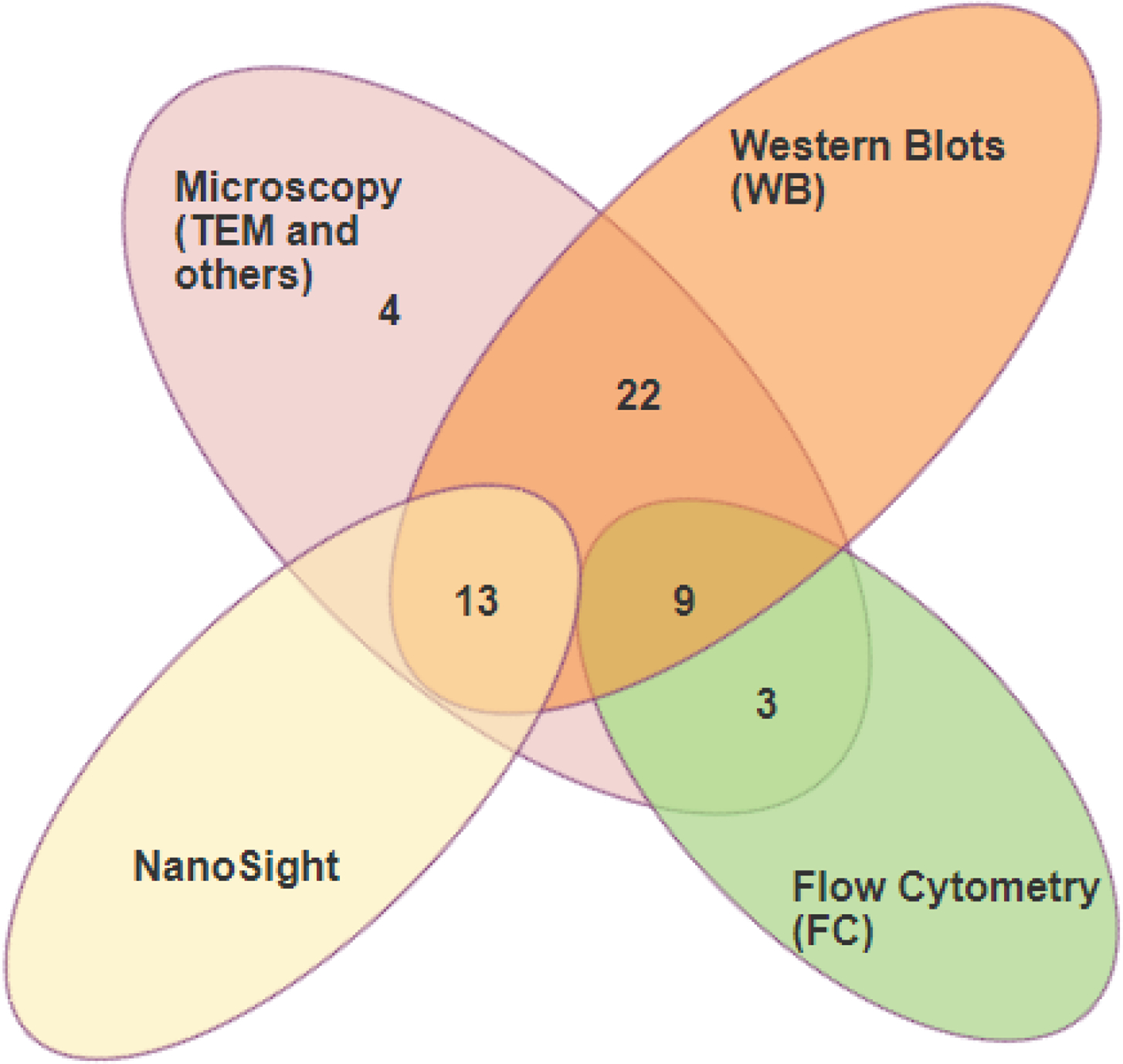
Diagram showing different methods used to visualize and characterize exosomes. Most studies used Western blot in combination with microscopic analysis.
We also analyzed the exosome membrane surface markers utilized in these studies and found that CD63, CD9, CD81, and TSG101 are the most used for exosome characterization (Supplemental Table 2c). Markers like the heat shock proteins HSP70, Grp94−, Flotillin, and Annexin V are less often used for exosome characterization. Most of the exosomes used in wound healing models were obtained from MSC (40%), followed by exosomes from umbilical stem cells (10%) (Supplemental Table 2d). Less than 10% of the studies used exosomes from other sources including embryonic stem cells, human PRP and plasma, HUVEC cells, fibroblasts, keratinocytes, and macrophages. A detailed description of the isolation and characterization techniques used by different studies can be found in Supplemental Table 3.
Extracellular vesicle cargo, wound healing outcomes, and mechanism of action analysis
Analysis of the studies using mouse models of cutaneous injury (Table 1a) show that 53% reported at least one exosome cargo molecule of interest; the remaining studies did not report any exosome cargo molecule. Of the reported exosome cargo molecules, 59% showed significantly upregulated miRNA. The majority of studies reported no overlapping miRNA of interest; however, three independent studies reported the same exosome cargo: miR-21. Notably, these independent studies proposed variable mechanisms by which miR-21 influences wound healing. Other reported cargo molecules of interest included long non-coding RNAs (lncRNA), significantly elevated cytokine concentrations, and alpha-2-macroglobulin. Of reported therapeutic wound healing outcomes, 81% reported that exosome administration significantly improved wound closure rate, while 50% showed significantly increased vascularity, the later outcome owing to exosomes’ proposed mechanism of action in stimulating wound angiogenesis. Other major study outcomes included increased collagen deposition, wound bed cell viability, decreased fibrogenesis, and decrease flap necrosis.
Table 1:
Therapeutic outcome and mechanism of action following exosome administration to mice (A) and rat (B) models of cutaneous wound healing.
| (a) MICE STUDIES | ||||||||
|
| ||||||||
| Study No. | Reference | Mouse (Strain) | Injury Model | Exosome Concentration | Exosome Cargo | Application Method | Major Outcome(s) | Proposed Mechanism |
|
| ||||||||
| 1. | Xiong Y, et al. (Xiong Y. et al., 2020a) | C57BL/6 | Full-thickness, dorsal skin 2.25-cm2 excision | 100 μg/mL | miR-20b | Subcutaneous injection | Exosomal miR-20b decreased wound closure rate Inhibiting miR-20b increased wound closure rate Inhibiting miR-20b increased wound vascularity |
miR-20b overexpression leads to suppression of Wnt9b/β-catenin signaling limiting wound angiogenesis; inhibition of miR-20b restores pathway and rescues angiogenesis |
|
| ||||||||
| 2. | Liu K, et al. (Liu et al., 2019) | Athymic/Nude | Full-thickness, size not reported | Not reported | Not reported | Not reported | Increased wound closure rate Increased wound vascularity |
Molecular mechanism not hypothesized; however, improved wound healing effect of Adipose-derived SC exosomes can be enhanced by hyaluronic acid which acts to immobilize exosomes creating a direct effect in the healing wound bed |
|
| ||||||||
| 3. | Kobayashi H, et al. (Kobayashi et al., 2018) | C57BL/6 db/db | Diabetic, Full-thickness, 8-mm diameter dorsal skin excision | 4 μg/20 μL | Not reported | Subcutaneous injection | Increased wound closure rate Increased wound vascularity |
iPSC-derived exosomes improved wound closure through induction of dermal fibroblast proliferation and migration; angiogenesis mechanism not reported |
|
| ||||||||
| 4. | He X, et al. (He et al., 2019) | C57BL/6 | Full-thickness, dorsal skin 1.2-cm2 excision | 200 μg/200 μL | miR-223 | Intravenous injection | Increased wound closure rate Increased wound vascularity Increased collagen |
Bone marrow MSC-derived exosomes regulate M2 polarization via miR-223; in turn, anti-inflammatory M2 enhance cutaneous wound healing |
|
| ||||||||
| 5. | Su D, et al. (Su et al., 2020) | BALB/c | Full-thickness, 10-mm diameter dorsal skin excision | 0.5 ng/μL in 20% hydrogel | Significantly increased PD-L1 in IFN-Y-treated cells | Topical (hydrogel) | Increased wound closure rate | Exosomal PD-L1 decreased inflammation by directly suppressing T cell activation; improved wound closure by increasing epidermal cell and dermal fibroblast migration |
|
| ||||||||
| 6. | Xu J, et al. (Xu et al., 2020) | C57BL/6 | Streptozotocin-induced diabetic full-thickness, 6-mm diameter dorsal skin excision | 0.1 μg/100 μL | miR-221 | Topical (Directly on wound) | Improved wound closure rate Improved wound vascularity |
miRNA-221-3p expression is significantly elevated in endothelial progenitor-derived exosomes and promoted skin wound healing in diabetic mice via interaction in AGE-RAGE signaling, cell cycle, and the p53 pathway |
|
| ||||||||
| 7. | Zhao G, et al. (Zhao G. et al., 2020) | C57BL/6 | Full-thickness, dorsal skin 0.64-cm2 excision | 100 μL | Not reported | Subcutaneous injection | Increased wound closure rate | hucMSC-derived EVs increase wound epithelialization via increased CK10; upregulated CD31 indicated increased angiogenesis; downregulation of αSMA inhibiting fibrogenesis |
|
| ||||||||
| 8. | Wang L, et al (Wang L. et al., 2019) | BALB/c | Full-thickness, 4-mm dorsal skin excision | 200 μg/200 μL | Increased elastin | Intradermal injection | Increased wound closure rate | Human umbilical cord MSCs derived exosomes increase key ECM protein production in dermal fibroblasts and inhibit MMP-1/MMP-3 expression to improve wound closure |
|
| ||||||||
| 9. | Kim H, et al. (Kim et al., 2019) | BALB/c | Full-thickness, 8-mm diameter dorsal skin punch biopsy | 100 μg/100 μL | Significantly higher cytokines in M2-derived EVs: CCL27, CCL11, CCL24, IL4, CXCL12, bFGF, CCL22, and MFG-E8 | Subcutaneous injection | Increased wound closure rate | M2-derived exosomes convert MΦ to M2; reducing inflammation, enhancing re-epithelialization and angiogenesis |
|
| ||||||||
| 10. | Chen J, et al. (Chen Jialin et al., 2019) | C57BL/6 | Full-thickness, 5-mm diameter dorsal skin punch biopsy | Not reported | Long noncoding RNA-ASLNCS5088 | Subcutaneous injection of exosomes formation inhibitor | Inhibitor GW4869 ameliorated wound fibrogenesis | M2 exosomes induce myofibroblast development and hypertrophic scar formation. Synthetic compound GW4869 suppresses M2 exosomes synthesis, thereby, alleviating scar pathogenesis |
|
| ||||||||
| 11. | Zhao B, et al. (Zhao et al., 2018) | BALB/c | Full-thickness, dorsal skin 1.0-cm2 excision | 50 μg/mL | +/− RNase (RNase A) +/− Protease (Proteinase K) |
Subcutaneous injection | Improved wound closure rate Improved collagen organization |
human amniotic epithelial cell-derived exosomes improved wound healing through RNA transfer and not protein transfer |
|
| ||||||||
| 12. | Chen CY, et al. (Chen et al., 2018) | C57BL/6 | Streptozotocin-induced diabetic full-thickness, 6-mm diameter dorsal skin excision | 200 μg/100 μL | Subcutaneous injection | Increased wound closure rate Increased wound vascularity |
Human urine-derived exosome DMBT1 improved wound angiogenesis by promoting endothelial cell proliferation, migration, and adhesion | |
|
| ||||||||
| 13. | Wang C, et al. (Shi Yu et al., 2019) | ICR | Streptozotocin-induced diabetic full-thickness, 8-mm diameter dorsal skin excision | 10 μg | Not reported | Topical (hydrogel - FHE) | Improved wound closure rate Improved wound vascularity Increased collagen |
FHE hydrogel embedded ADSC exosomes allowed extended wound healing improvement through controlled exosome release |
|
| ||||||||
| 14. | Wang M, et al. (Wang M. et al., 2019) | ICR | Streptozotocin-induced diabetic Full-thickness, 10-mm diameter dorsal skin excision |
1 μg/100 μL hydrogel | Not reported | Topical (hydrogel - FEP | Improved wound closure rate Improved wound vascularity Increased collagen |
FEP hydrogel embedded ADSC exosome allowed extended wound healing improvement through controlled exosome release |
|
| ||||||||
| 15. | Trinh NT, et al. (Trinh et al., 2016) | C57BL/6 | Ischemic/reperfusion via extended dorsal skin flap, 6-cm2 | Not reported | Not reported | Subcutaneous injection | Decreased flap necrosis | Human non-diabetic MSC exosomes can reprogram diabetic MSCs to improve wound healing |
|
| ||||||||
| 16. | Wang X, et al. (Wang Xiao et al., 2019) | BALB/c | Full-thickness, dorsal skin 1-cm2 excision | 200 μg/200 μL | Not reported | Subcutaneous injection | Improved wound closure rate | Fetal dermal mesenchymal cells exosomes may aid in wound healing by activating adult dermal fibroblasts via Notch signaling, enhancing migration and proliferation |
|
| ||||||||
| 17. | Xiong Y, et al. (Xiong Y. et al., 2020b) | C57BL/6 | Full-thickness, dorsal skin 10-mm excision | 200 μg/100 μL | miR-15a | Subcutaneous injection | Improved wound closure rate Improved wound vascularity |
Exosomes containing miR-15a is upregulated in diabetic wounds; miR-15a inhibits NOX5 expression; NOX5 activation increases ROS and activates endothelial cells; inhibiting miR-15a exosomes accelerates wound healing by activating NOX5 activity |
|
| ||||||||
| 18. | Zhang Y., et al. (Zhang et al., 2020) | BALB/c | Full-thickness, dorsal skin 1-cm2excision | 200 μg/mL | Not reported | Subcutaneous injection | Improved wound closure rate | ADSC-exosomes upregulate AKT phosphorylation and HIF-1α expression which promoted migration and proliferation of wound keratinocytes |
|
| ||||||||
| 19. | Chen B, et al. (Chen Bi et al., 2019) | C57BL/6 | Pressure ulcer | 1010/100 μL | miR-200a | Topical (Directly on wound) | Improved wound closure rate Improved wound vascularity Increased collagen |
Embryonic stem cell exosomes are enriched in miR-200a which downregulates Keap1, in turn, negatively regulating Nrf2 expression; Nrf2 can recover age-related angiogenic dysfunction |
|
| ||||||||
| 20. | Hu Y, et al. (Hu et al., 2018) | C57BL/6 | Full-thickness, dorsal skin 12-mm excision | 200 μg/100 μL | miR-21-3 | Subcutaneous injection | Increased wound closure rate Increased wound vascularity |
Umbilical cord blood plasma exosomes are highly enriched in miR-21-3; miR-21-3 inhibits PTEN and PRRY1, thereby enhancing angiogenesis and re-epithelialization |
|
| ||||||||
| 21. | Wang L, et al. (Wang et al., 2017) | BALB/c | Full-thickness, dorsal skin 3.0-cm2 excision | 100 μg/100 μL | Not reported | Intravenous injection | Decreased Col1:Col3 | Adipose-derived SC exosomes modify dermal fibroblasts by reducing fibrotic phenotype to mitigate scarring; decreases fibrotic Col1 and αSMA; increases antifibrotic TGF-β3 |
|
| ||||||||
| 22. | Y ang C, et al. (Yang et al., 2020) | BALB/c | Full-thickness, dorsal skin 1-cm2 excision | Not reported | miR-21 | Subcutaneous injection | Increased wound closure rate | miR-21, highly expressed in ADSCs, inhibits TGF-β1 expression which has an influence on MMP-2 and TIMP-1 protein expression via PI3K/AKT signaling |
|
| ||||||||
| 23. | Bakhtyar N, et al. (Bakhtyar et al., 2018) | C57BL/6 | Full-thickness, dorsal skin 6-mm punch biopsy | 100 μL | alpha-2-macroglobulin | Topical (hydrogel) | Increased cell viability Increased cell migration |
Alpha-2-macroglobulin enhances cell migration, proliferation, and viability |
|
| ||||||||
| 24. | Hu L, et al. (Hu et al., 2016) | BALB/c | Full-thickness, dorsal skin 3.0cm2 excision | 200 μg/200 μL | Not reported | Subcutaneous injection Intravenous injection |
Increased wound closure rate Increased collagen |
ASC exosomes are internalized by wound fibroblasts and stimulate cell migration, proliferation, and collagen synthesis in a dose-dependent manner; gene upregulation of N-cadherin, PCNA, and collagen-I, -III |
|
| ||||||||
| 25. | Dalirfardouei R, et al. (Dalirfardouei et al., 2019) | C57BL/6 | Streptozotocin-induced diabetic Full-thickness, 8-mm diameter dorsal skin excision |
10 μg/100 μL | Not reported | Subcutaneous injection | Increased wound closure rate Increased wound vascularity Increased collagen |
Menstrual-SC exosomes enhance angiogenesis via VEGFα upregulation and aid in re-epithelialization via NF-κB pathway activation; physically manifesting in reduced Col1:Col3 ratio |
|
| ||||||||
| 26. | Geiger A., et al. (2015) (Geiger et al., 2015) | C57BL/6 db/db | Diabetic, full-thickness, 6-mm dorsal skin punch biopsy | 5 μg/200 μL or 50 μg/200 μL | Significantly elevated miR-21, -124a, -125b, -126, -130a and -32 | Subcutaneous injection & Direct wound application | Increased wound closure rate Increased collagen |
Circulating fibrocytes release proangiogenic miRNAs (miR-126, -130a, and -132) that act to induce angiogenesis; miR-21 acts to increase collagen deposition; working in concert to close the wound. |
|
| ||||||||
| 27. | Hu S., et al. (Hu et al., 2019) | Athymic/Nude | UVB Irradiation, cutaneous photoaging (wrinkling) | 1010/mL | Significantly elevated miR-34a, -133a, 199b, -223, 325, 5011 Significantly downregulated miR-196a, -744 |
Subcutaneous injection Topical Jet injection (needle-free) |
Increased dermal thickness Decreased epidermal thickness |
3D-cultured exosomes from human dermal fibroblasts increase procollagen-1 expression and decrease MMP-1 expression by downregulating TNF-α and upregulating TGF-β expression in the cutaneous insult |
|
| ||||||||
| 28. | Y ang K, et al. (Yang et al., 2019) | BALB/c | Deep 2nd-degree burn | 100 μg/200 μL | Increased miR-135, -499a | Subcutaneous | Increased wound closure rate Increased wound vascularity |
Blue light (455nm) enhances the quantity and proangiogenic quality of human umbilical cord MSC-derived exosomes. These exosomes act to downregulate MEF2C signaling, thereby, promoting endothelial cell proliferation and vessel formation |
|
| ||||||||
| 29. | Yin H, et al. (Yin et al., 2019) | C57BL/6 | Full-thickness burn | 100 μg/100 μL | Not reported | Subcutaneous injection | Increased wound closure rate Increase wound vascularity |
S. elongatus exosomes induce IL-6 expression in burn wounds to increase angiogenesis and improve wound healing. |
|
| ||||||||
| 30. | Li B, et al. (Li et al., 2020) | C57BL/6 | Streptozotocin-induced diabetic full-thickness, 10-mm diameter dorsal skin excision | Not reported | lncRNA-H19 | Subcutaneous injection | Increased wound closure rate Increased wound vascularity |
MSC-released exosomal lncRNA H19 inhibits miR152-3p and promotes PTEN expression to enhance proliferation, migration, and suppress apoptosis of diabetic wound fibroblasts |
|
| ||||||||
| 31. | Qiu X, et al. (Qiu et al., 2020) | C57BL/6 | Full-thickness, dorsal skin 1.0-cm2 excision | 100 μg/100 μL | Not reported | Intradermal injection | Increased wound closure rate Increased wound vascularity |
Bone marrow MSCs treated with neonatal serum-derived exosomes had a pro-angiogenic effect by targeting endothelial cells and regulating the AKT/eNOS pathway to improve wound healing |
|
| ||||||||
| 32. | Zhang Y., et al. (Zhang et al., 2019) | BALB/c | Full-thickness, dorsal skin 10-mm excision | Exosomes: not applicable pre-treated MSCs: 5×105MSC/100 μL |
Not reported | Subcutaneous injection | Improved wound closure rate Increased collagen |
Embryonic stem cell exosomes delay the senescence of MSC used in wound healing treatments to enhance the therapeutic effect |
|
| ||||||||
| (b) RAT STUDIES | ||||||||
|
| ||||||||
| Reference | Rat (Strain) | Injury Model | Exosomes Concentration | Exosome Cargo | Application Method | Major Outcome(s) | Proposed Mechanism | |
|
| ||||||||
| Li M, et al. (Li et al., 2019) | Sprague Dawley | Streptozotocin-induced diabetic Full-thickness, 1.5-cm diameter dorsal skin excision |
Low dose: 100 μg/mL High dose: 1 mg/mL |
Not reported | Subcutaneous injection | Increased wound closure rate Increased wound vascularity Decreased inflammation |
Exosomes exerted anti-inflammatory effects by inhibiting the secretion of pro-inflammatory enzymes and cytokines | |
|
| ||||||||
| Zeng T, et al. (Zeng et al., 2019) | Sprague Dawley | Full-thickness, 10-mm diameter dorsal skin excision | 200 μg/100 μL | miR-106b | Subcutaneous injection | Decreased wound closure rate Decreased re-epithelialization |
HUVECs pre-treated with advanced glycation end products produce exosomes enriched in miR-106b that can directly bind ERK1/2 in dermal fibroblasts, activating autophagy and decreasing collagen expression | |
|
| ||||||||
| Li X, et al. (Li Xiao et al., 2016) | Sprague Dawley | Full-thickness 30% TBSA Burn | 800 μg (RNA)/mL | miR-181c | Subcutaneous injection | Decreased inflammation | Administration of miR-181c-expressing hUCMSC-exosomes significantly reduced LPS-induced TLR4 expression by macrophages and subsequently decreased wound inflammation | |
|
| ||||||||
| Zhang J, et al. (Zhang J. et al., 2015) | Sprague Dawley | Full-thickness, 18-mm diameter dorsal skin excision | 40 μg/40 μL | Not reported | Subcutaneous injection | Increased wound closure rate Increased wound vascularity Increased collagen |
Human-induced pluripotent-MSC exosomes promote collagen synthesis and angiogenesis through unknown paracrine mechanisms within the wound | |
|
| ||||||||
| Gao S, et al. (Gao et al., 2020) | Sprague Dawley | Bilateral, full-thickness, 2.25cm2 dorsal skin excision | 40 μg/mL | miR-135a | Topical (in collagen gel vehicle) | Increased wound closure rate | MiR-135a downregulates expression of Large Tumor Suppressor 1 (LAT2) during wound healing which in turn accelerates cell migration and wound closure | |
|
| ||||||||
| Zhang B, et al. (Zhang B. et al., 2015a) | Sprague Dawley | Partial-thickness burn (% TBSA not specified) | 200 μg/200 μL | Not reported | Subcutaneous injection | Increased collagen-1: collagen3 | hucMSC exosomes cause increased activation of Wnt/B-catenin signaling, thereby increasing rate of re-epithelialization | |
|
| ||||||||
| Xiong J, et al. (Xiong J. et al., 2020) | Sprague Dawley | Dermal skin flap | 200 ug/100 μL | miR-760, miR423 | Subcutaneous injection | Increased wound vascularity | ADSC exosomes have upregulated expression of the-miR-760 and downregulated expression of hsa-423-3p that regulate expression of ITGA5 and HDAC5 genes, respectively, in target cell populations within the wound to promote vascularization of skin flaps | |
|
| ||||||||
| Guo SC, et al. (Guo et al., 2017) | Sprague Dawley | Streptozotocin-induced diabetic Full-thickness, 1.8-cm diameter dorsal skin excision |
1% (v/v) exosomes in sodium alignate hydrogel | Not reported | Topical hydrogel | Increased wound closure rate Increased re-epithelialization Increase wound vascularity |
Platelet-rich plasma exosomes increase angiogenesis and fibroblast migration in healing diabetic wounds by directing (yes-associated protein) YAP signaling | |
|
| ||||||||
| Zhang J, et al. (Zhang et al., 2016) | Sprague Dawley | Streptozotocin-induced diabetic Full-thickness, 15-mm diameter dorsal skin excision |
2 × 1010 or 1 × 1011 particles, dissolved in 200 μL of PBS | Not reported | Subcutaneous injection | Increased wound closure rate Increase re-epithelialization Increase wound vascularity |
Endothelial cell progenitor exosomes significantly enhance endothelial cell proliferation and migration through ERK1/2 activation | |
|
| ||||||||
| Zhang., et al. (Zhang B. et al., 2015b) | Male Albino Wistar rats | Skin-deep second-degree burn | 200 μg in 200 μL PBS (3 sites) | Not reported | Subcutaneous injection | exosomes improved the tube-formation ability of Endothelial Cells In Vitro and promoted angiogenesis in vivo | hucMSC-exos mediates Wnt4/beta catenin activation in endothelial cells exerting pro-angiogenic effects | |
|
| ||||||||
| Zhao, et al. (Zhao D. et al., 2020) | Sprague-Dawley | Full-thickness excisional wounds 2× 10 mm × 10 mm square | Scaffold loaded with 1 × 108 particles/mL | Not reported | GelMA loaded with HUVECs-Exos | HUVECs- Exos accelerate wound healing both in vitro and in vivo. Also, increased collagen I expression promoted angiogenesis in the wound sites | HUVECs-Exos could accelerate wound healing and GelMA mediated controlled release of HUVECs-Exos | |
|
| ||||||||
| Ding J, et al. (Ding et al., 2019) | Sprague Dawley | Streptozotocin-induced diabetic excisional wounds (2×) of diameter 20 mm | 100 μg per 100 μL PBS | miR-126 | Subcutaneous injection | Accelerated wound healing, reduced scar with, increased neovascularization, effects more pronounced with Deferoxamine treated cells exosomes | DFO-Exos activate the PI3K/AKT signaling pathway via miR-126 mediated PTEN downregulation to stimulate angiogenesis in vitro | |
|
| ||||||||
| Ismail DI, et al. (Ismail and Aboulkhair, 2018) | Male albino rats | Full-thickness skin excisional 1.5 × 1.5 cm | Unclear whether 200 μL or 200 μg was injected | Not reported | Subcutaneous injection | Increase in SMA, Ki67, epidermal thickness, dermal thickness, % area of (collagen, SMA, KI67, elastic fibers) | No mechanism reported | |
|
| ||||||||
| Jiang T, et al. (Jiang et al., 2020) | Sprague Dawley | Full-thickness skin excisional 10 mm in diameter | Volume unclear 250 μg | Not reported | multi-directional subcutaneous injection | Increased proliferation and migration of fibroblast, accelerated wound closure, SMA and VEGF production | ASC-exosomes downregulated TGF-β1, Smad2, Smad3, and Smad4 expression, and upregulated TGF-β3 and Smad7 expression in the TGF-β/Smad signaling pathway | |
|
| ||||||||
| Li M, et al. (Li M. et al., 2016) | Sprague Dawley | Streptozotocin induced Diabetic / dorsal excision (18mm) wound | Unclear – 200 μL exosome solution was incorporated into the HAP/CS gel | Transfected MSCs over expressing miR-126-3p | Exosomes were embedded into a hydroxyapatite/chitosan gel (HAP/CS) | Improved wound closure, increased blood vessels, and re-epithelialization. | miR-126-3p has significant angiogenic functions, activating MAP/ERK and PI3K/AKT to promote angiogenesis and improve wound healing | |
|
| ||||||||
| Liu J, et al. (Liu et al., 2020) | Sprague Dawley | 20 mm 2nd degree burn (80°C water for 8 s) | 1 mg/200 μL | Not reported | Subcutaneous Injection | Improved wound closure hucMSC-Ex-derived Ang-2 promote angiogenesis and cutaneous wound healing | Angiopoietin-2 improved wound closure rate and hucMSC-Exo expressing short hairpin RNA against Angiopioetin-2 significantly decreased wound closure rate | |
|
| ||||||||
| Lv Q, et al. (Lv et al., 2020) | Sprague Dawley | Full-thickness 15 mm, in a diabetic rat | 12 μg/mL | miR-21 | Topical application | Increased wound Closure, re-epithelialization, angiogenesis, and collagen maturation | miR-21 and/or hASC-exos could increase cell migration aiding in better wound healing outcomes | |
|
| ||||||||
| Sjöqvist S, et al. (Sjoqvist et al., 2019) | Sprague Dawley (part Ex vivo pig) | Full-thickness, 4 × 5 mm punch biopsy | Two treatments (7.6 μg exosomes day 0 and day 1, or 12.5 μg exosomes on day 0) | Not reported | Topical application | Increased wound closure rate | Proof of concept. No mechanism was reported. Conclusion: Exosomes isolated from clinical-grade cell sheet can be utilized as a therapeutic agent to stimulates wound healing | |
|
| ||||||||
| Shi R, et al. (Shi et al., 2020) | Not sure/mice or rat | Streptozotocin-induced diabetic Full-thickness excisional wounds 4 mm punch biopsies |
200 μg/ 100 μl | miR-128-3p | Injections into four sites | Increased autophagy of damaged endothelial cells, increased re-epithelialization, and angiogenesis | Exosomes derived from modified stem cells restore functions of endothelial cells | |
Analysis of studies utilizing rat models of cutaneous injury (Table 1b) showed 42% reported at least one exosome cargo molecule of interest. Of these studies, all cargo reported were miRNA molecules; two studies reported miR-126 as the molecule of interest. These independent studies concluded a similar mechanism of action: miR-126 activates the PI3K/AKT pathway to stimulate angiogenesis and improve wound healing. All other rat studies posited diverse methods of action that improved skin wound healing. Similar to the mice studies, the majority of rat studies reported significantly improved wound closure rate and increased vascularity. Other outcomes included increased fibroblast proliferation, improved collagen maturation, and reduced scarring.
The inter-quartile range of exosome protein used (IQR) of 31.84 μg exosome per cm2) in the studies was reported based on the studies from by meta-analysis section of the paper. The therapeutic concentration of exosomes is reported based on μg of protein used per cm2 of wound area (Table 2). Overall, regardless of exosome cargo, each study reported favorable outcomes with significantly improved or healed wounds with exosome administration.
Table 2:
Concentration of Exosomes and wound area reported in different dermal wound healing studies.
| Study Number | Article Name | Number of Animals | Size of the Wound | Area (cm2) | Concentration | Total Amount (ug) | Standard Conc. (ug/mL) |
|---|---|---|---|---|---|---|---|
|
| |||||||
| 1 | Xiong et al. (Xiong Y. et al., 2020a) | 6 | 1.5 × 1.5 cm full- thickness excision skin wounds | 2.25 | 100 μL PBS, 50 μg mL−1 | 100 | 2 |
| 2 | Liu et al. (Liu et al., 2019) | 6 | 10 mm diameter | 3.14 | 100 ul of PBS, 150 ug of ASC-Exos | 150 | 1.5 |
| 3 | Kobayashi, et al. (Kobayashi et al., 2018) | 6 | 8-mm full-thickness diameter excisional skin wounds | 2.51 | 4 μg/20 μl PBS (4 sites) | 16 | 0.2 |
| 4 | He et al. (He et al., 2019) | 4 | 1.2 cm diameter full-thickness skin excision | 4.52 | 200 ug/ 200 uL | 200 | 1 |
| 5 | Su et al. (Su et al., 2020) | 3 | A 10-mm diameter | 3.14 | 1 μg/μL (50 ul injected) | 50 | 1 |
| 6 | Zhang et al. (Xu et al., 2020) | 5 | 6 mm diameter | 1.13 | 0.1 μg/μL (treated every 3 days for 12 days) | 1.1304 | 0.1 |
| 7 | Zhao et al. (Zhao G. et al., 2020) | 6 | 0.8 cm × 0.8 cm | 0.64 | 100 μg hucMSCs-Ex 100 μL−1 PBS | 100 | 1 |
| 8 | Wang, et al. (Wang L. et al., 2019) | 6 | ∼4 mm diameter | 0.50 | 200 μg of exosomes in 200 μL of PBS | 200 | 1 |
| 9 | Kim, et al. (Kim et al., 2019) | 4 | 8 mm diameter | 2.51 | 100 μg per 100 μL (treated twice to wound site day 1 and day 4 post full- thickness skin excision) | 100 | 1 |
Average μg of exosomes per cm2 of the wound area 83.76 ± 126.4
DISCUSSION
The last two decades saw a significant rise in both pre-clinical and clinical studies utilizing the administration of extracellular vesicles, especially exosomes, to improve cutaneous wound healing outcomes. However, a systematic evaluation and meta-analysis of the pre-clinical studies on the therapeutic efficacy of exosomes and exosomes in wound healing models have not been conducted. High-quality systematic reviews on pre-clinical studies expand scientific understanding, identify emerging trends, and prevent duplication of rodent studies. Thus, these critical reviews uphold the results of efficacious clinical trials and ultimately increase overall patient safety (Pound and Ritskes-Hoitinga, 2020). The primary goal of the current systematic review and meta-analysis of preclinical studies was to assess the role of exosomes in cutaneous wound healing, while the secondary goal was to offer direction for selecting the appropriate concentration of exosomes to be used in further pre-clinical studies. Here we have extracted and compiled data from the existing literature and have provided guidance that can be referenced when designing pre-clinical cutaneous wound healing studies using extracellular vesicles/exosome therapy.
META-ANALYSIS
Although we analyzed the data for 7, 14, and 21 days, due to a limited number of studies with data at 21 days, only the 7- and 14-day data points following the administration of exosomes are reported. Except for a single study (Kim et al., 2019), at day 7, and two studies (Kim et al., 2019, Kobayashi et al., 2018) at day 14, all the reported studies demonstrated the favorability of exosome treatment in the skin excisional model (Figures 3 and 4). According to the meta-analysis, following exosome administration, wound closure at 7 (Figure 3) days post-application was more pronounced compared to that at 14 days (Figure 4). To preserve consistency between exosomes application time and wound healing assessment, two studies (Kim et al., 2019, Xu et al., 2020) that have reported multiple applications were excluded from this analysis. Our meta-analysis results corroborate the current literature – exosomes have a beneficial role in dermal wound healing. Funnel plot analysis showed that there was no significant asymmetry, and the studies were evenly distributed for the wound closure reported at the 7- or 14-day time points, suggesting no inherent bias in our study. However, interestingly, on day 14, most of the studies had lower standard error compared to day 7 indicating that the studies on day 14 had greater precision.
Figure 3. Meta-analysis of the effects of exosomes in wound healing on day 7 after application.
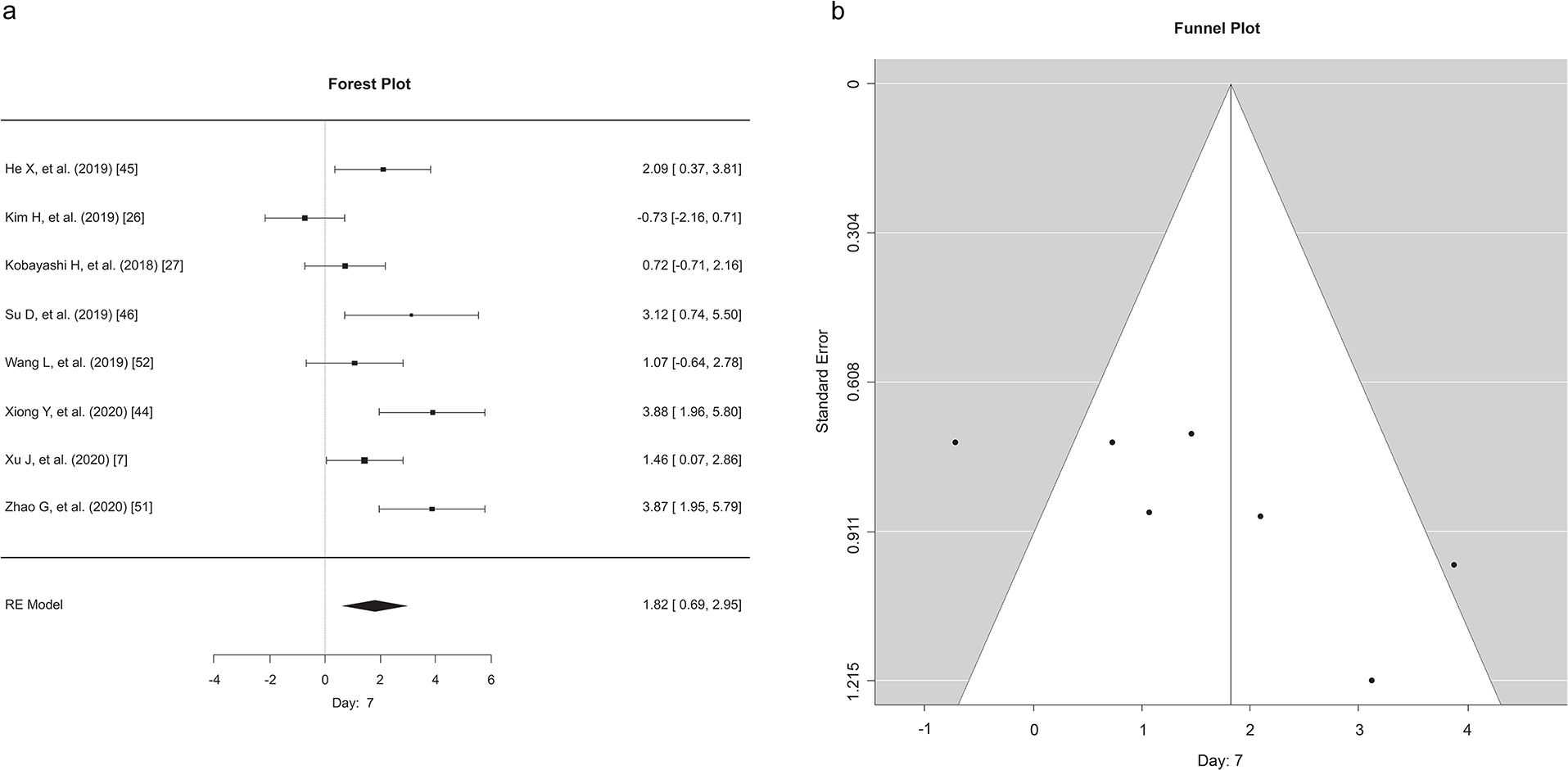
a) Each effect size of EV across studies at 7 days post-application of exosomes. Forest plot showing a median effect size and 95% CI. b) Funnel plots for exosome applications in wound healing models displaying the distribution of researched study outcomes (dots) to estimate potential publication bias in 7 days of post-application studies. Note: data points for Xiong (2020) and Zhao (2020) at 3.88 and 3.87, are overlapping and are depicted as one data point.
Figure 4. Meta-analysis of the effects of exosomes in wound healing on day 14 after application.
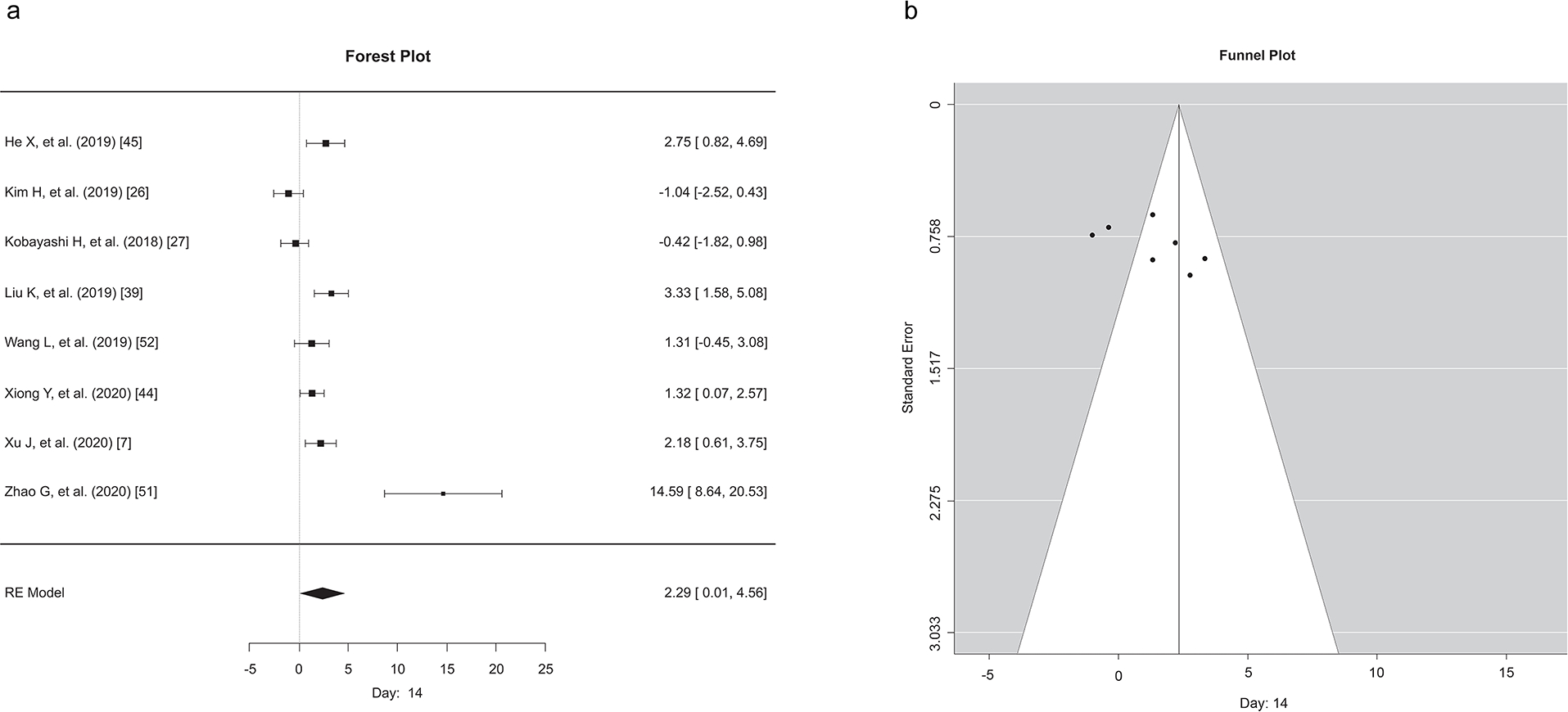
a) Each effect size of EV across studies at 14 days post-application of exosomes. Forest plot showing a median effect size and 95% CI. b) Funnel plots for exosome applications in wound healing models showed the distribution of researched study outcomes (dots) to estimate potential publication bias in 7 days post-application studies.
The systematic review comprised 51 studies (32 mice and 19 rats). The current analysis found that exosome therapy improves wound healing by stabilizing and stimulating a diverse set of mediators activated during each phase. Improved effects were reported higher 14 (Figure 4A) days after exosome administration compared to 7 days (Figure 3A). Findings were consistent across studies: exosomes have therapeutic properties and aid in cutaneous wound healing, regardless of the model, mode of administration, exosome concentration, the number of administrations, or the source of exosomes. These findings further support the use of exosomes in clinical settings.
We observed a quality score of 5.05 ± 0.752, which is comparable to or higher than other preclinical systematic reviews studying the role of exosomes in different disease models (Chen et al., 2016, Lees et al., 2012). However, the quality score is only a semi-quantitative means of evaluating pre-clinical studies. Unlike clinical trials, experimental data and animal studies are reported poorly (Kilkenny et al., 2009), which subsequently affects the quality score data. For instance, few studies specifically mentioned temperature settings during animal housing. In contrast, others mentioned housing in an animal facility without mentioning temperature settings, generating confusion while grading the study for quality score analysis. With the growing number of studies experimenting with the administration of exosomes in different wound healing models, further research and developments are needed to unify the methods for isolation, characterization, application, and most importantly standardized reporting. These measures will enable an accurate comparison among different studies.
We also observed variance in the exosome isolation techniques similar to a recently published exosome-related systematic review (Tan et al., 2020) and the joint statement article released by the International Society for Extracellular Vesicles (Thery et al., 2018). Although ultracentrifugation is regarded as a standard method of isolation, various methods listed in Supplemental Table 2b were used to isolate exosomes. The majority of studies represented in this systematic review reported the use of ultracentrifugation technique (64%), followed using commercial isolation kits (18%), and a combination of different methods like ultracentrifugation plus filtration and tangential flow, among others, for exosome isolation. Recently, an increasing number of studies suggested utilizing Tangential Flow Filtration (TFF) methods for isolation of large volumes and enriching of exosomes in a time-efficient, scalable, and reproducible manner (Busatto et al., 2018, Yoo et al., 2018). Regardless of isolation methods utilized, the consensus reached among researchers was a combination of at least one visualization method and one physical technique should be utilized for quantification and characterization of exosome markers. The most prominent methods are TEM, western blot, flow cytometry, or particle nano-tracking. Almost 94% of the research articles cited in our study used a combination of microscopy along with flow cytometry or western blot analysis to quantify and characterize the isolated exosomes (Supplemental Table 2b). Given the current limitations on size visualization in analyzing nanoparticles via flow cytometry, western blot was the technique of choice in most studies for the identification of exosome surface proteins. The combination of CD9, CD63, and CD81 was the most reported marker for exosome characterization, followed by TSG101 and GM130 (Supplemental Table 2). Other proteins such as EpCam and Grp90 (Sjoqvist et al., 2019) were also used as markers for exosomes. Interestingly, one study also used an immunostaining technique for identifying mesenchymal stem cell markers like CD90, CD105, and CD45 that characterized exosomes, suggesting a mesenchymal origin for the exosomes. However, given the extremely low expression levels of these markers, the positive staining could be an artifact (Trinh et al., 2016). Cell surface protein characterization is a vital step to identify exosomes, and encouragingly, most studies reported the characterization methods used; few studies failed to do this (Ismail and Aboulkhair, 2018, Jiang et al., 2020, Liu et al., 2019, Sinha et al., 2018). Given the novelty of the field and continuous updating of characterization guidelines, applying stringent isolation and characterization conditions is not pertinent to the inclusion and exclusion criteria of the current study.
Most of the studies reported subcutaneous injection to deliver exosomes to both mice and rats. Nine studies included in the meta-analysis section averaged 83.76 ± 126.4 with an inter-quartile range of (IQR) of 31.84 μg exosome per cm2 subcutaneously injected or topically applied to the injured tissue (Table 2). However, it is imperative to understand that extracellular vesicles’ yield is dependent on culture conditions (exosome-depleted serum or regular serum), speed of ultracentrifugation, and type of rotors (sedimentation efficacy, i.e., K-factor); therefore, altering the final therapeutic concentration used (Jeppesen et al., 2014). Although particle count allows for better reproducibility, a handful of studies reported only particle concentration (Chen Bi et al., 2019, Hu et al., 2019, Xiong Y. et al., 2020a, Zhang et al., 2016, Zhao D. et al., 2020).
In terms of sources, most exosomes were reported to originate from human adult stem cell lines. Specifically, MSCs were most often used, closely followed by umbilical cord mesenchymal stem (hUCMSC) cells, as primary cellular sources. Most studies utilizing MSCs reported their isolation from bone marrow as a primary source. Additionally, MSC-derived exosomes were the major source of miRNA. Thirteen unique miRNAs from different sources were cited as being responsible for mediating different phases of wound healing (Figure 5). We found 13 unique proteins and 14 miRNA facilitating wound healing following exosome treatment. Two studies by He et al. (He et al., 2019) and Kim et al. (Kim et al., 2019) reported attenuated inflammation through the process of macrophage polarization; administered exosomes induced the change from a pro-inflammatory M1 phenotype to an anti-inflammatory M2 phenotype, thus affecting the healing cascade and improving therapeutic outcomes. Similarly, a study by Su and colleagues (Su et al., 2020) reported the suppression of T-cell activation during the inflammation phase after administration of exosomes derived from cells overexpressing the Programmed Death Ligand-1 (PD-L1), or IFN-γ stimulated cells. In another study, administration of miR-181c-expressing hUCMSC-exosomes significantly reduced LPS-induced TLR4 expression by macrophages that subsequently decreased wound inflammation in vivo (Li Xiao et al., 2016).
Figure 5.
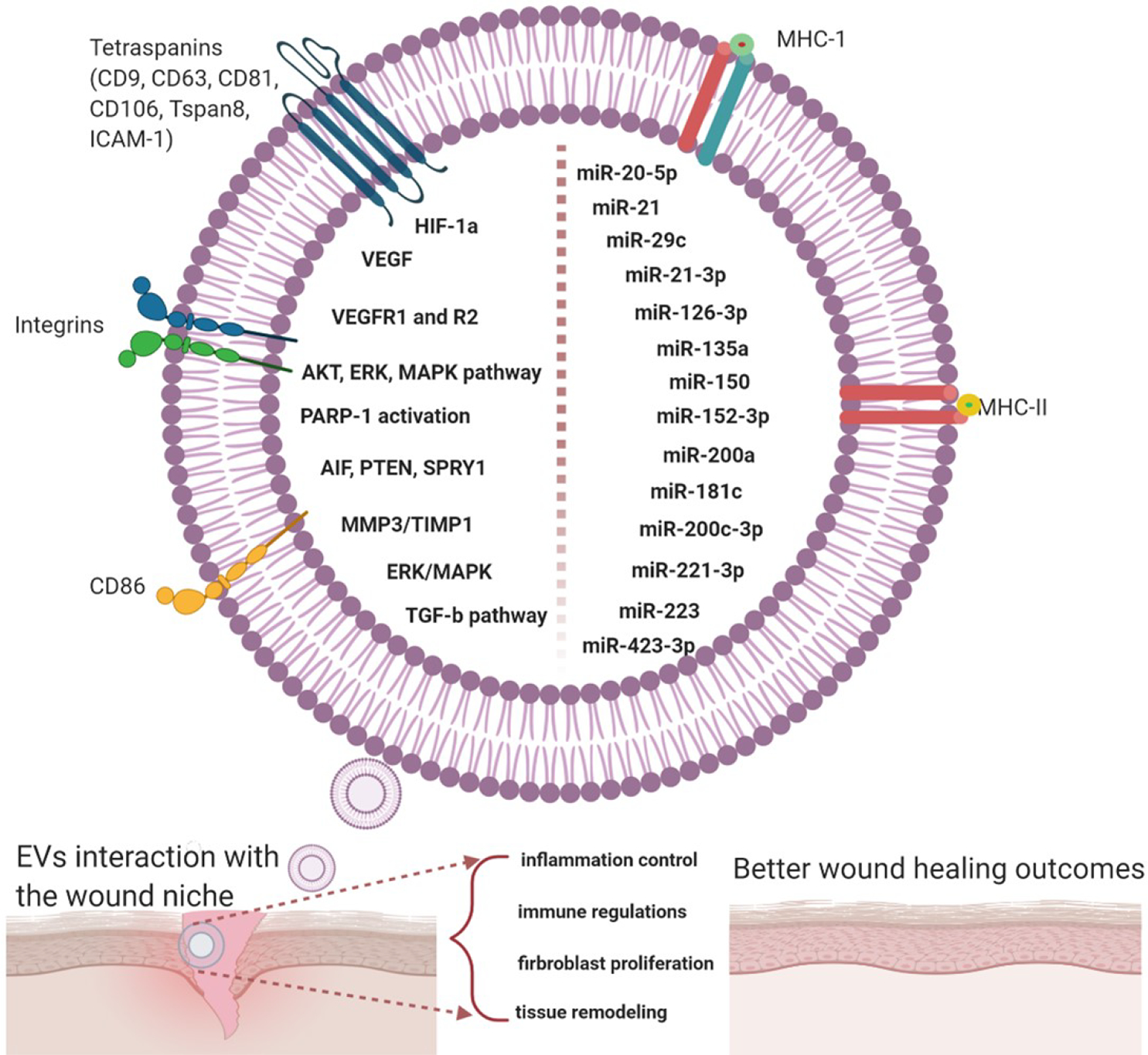
Summary of bioactive contents of exosomes that are facilitating and accelerating wound healing; miRNA was the most common signaling molecule, followed by growth factors.
Exosomes have evident therapeutic benefits during the proliferation phase of wound healing. Exosomes promote fibroblast proliferation and migration by suggested mechanisms such as activating PTEN or stimulating constitutive Notch signaling directly in wound bed fibroblasts (Kobayashi et al., 2018, Li et al., 2020, Wang Xiao et al., 2019). A recent study by Zhang and colleagues (Zhang et al., 2020) demonstrated significantly accelerated keratinocyte proliferation in the exosome-administered group by activating the AKT/HIF1α signaling pathway, thus resulting in faster wound closure. In the diabetic wound model, miR-21 silenced expression of KLF-5 and PTEN, resulting in increased expression of ECM-related genes, driving the conversion of myeloid progenitor cells to fibroblast-like cells (Sinha et al., 2018).
A significant increase in angiogenesis was a primary theme in the analyzed studies. Yin and colleagues (Yin et al., 2019) reported that exosomes enhance endothelial cell proliferation by downregulating MEF2C signaling. Similarly, Xiong and colleagues (Xiong Y. et al., 2020a) showed that overexpression of miR-20b suppressed Wnt9b/β-catenin signaling and limited wound angiogenesis; inhibition of miR-20b rescued angiogenesis. An increase in CD31+ cells, SMA+ cells, blood vessel number, or blood flow are all regarded as indicators of increased angiogenesis. We were unable to systematically assess the correlation between exosome administration and angiogenic potential due to inconsistencies in reporting angiogenesis among the studies.
The final essential phase in the wound healing cascade is termed remodeling - a period in which the ECM undergoes physical and physiologic changes to bring the tissue back to homeostasis. This phase typically begins around 14 days in humans and can last upwards of years in extreme circumstances. Although humans and rodents display key differences in cutaneous wound healing physiology such as differential immunologic activation and differing epidermal layers, the fundamental and temporal features remain similar. Moreover, current wound healing research in rodents, and the studies included in this review, utilize strategies and techniques to overcome these differences and reduce model limitations. Except for two investigations, most studies had endpoints between 7–14 days, not long enough to examine exosomes’ properties in the remodeling phase thoroughly. Although many hurdles exist in extending wound healing studies for longer periods, it is imperative to encourage that studies be extended to evaluate the long-term consequences and efficacy of exosome therapies.
Limitations
Due to the lack of unanimous isolation, characterization, and application techniques, we were not stringent in our inclusion and exclusion criteria. Although diabetic-induced cutaneous wound models are reported to have delayed wound healing, studies using the diabetic model (8 mice and 5 rats) were still included in this systematic review. Comparison of different wound healing models was beyond the scope of the present study. The small number of studies that are included in the meta-analysis section are the main limitations of the study. Moreover, the culture of only publishing efficacious studies may also have significantly resulted in bias during data collection and reporting.
CONCLUSIONS
An overwhelming number of studies demonstrated the efficacy of exosomes to improve cutaneous wound healing by facilitating wound healing cascade. Soon, exosomes may become a powerful clinical tool to deliver treatments to accelerate wound healing and prevent detrimental sequelae such as the increased risk of infection or painful scarring. Our findings corroborate the hypothesis that extracellular vesicles like exosomes can be ideal therapeutic tools for wound healing and regenerative purposes. However as other researchers have resonated, a consensus must be reached defining variables including isolation, quantification, administration, and reporting of exosomes. The unanimous consensus in defining the aforementioned variables will undoubtedly guide the exosome field towards a promising future.
MATERIALS AND METHODS
Search strategy
With a medical librarian’s help, we examined the available databases using Medical Subject Headings terms for exosomes, wound healing, and organ of interest (skin/integument). Details of the literature search are presented in supplemental data. Studies were extracted from Ovid Medline, Scopus, Epub Ahead of Print, Cochrane Library, and Other Non-Indexed Citations from January 01, 1946, to July 10, 2020. The first literature extraction was performed on April 16, 2020, and an updated search was completed on July 10, 2020, amalgamating 3,064 unique peer-reviewed studies on exosomes and wound healing. The template for the electronic search strategy and syntax used in the searches is shown in the supplement resource. Syntaxes were modified according to electronic databases to encompass a wide range of literature. Only full-text articles in English were selected, and only studies investigating the therapeutic effects of exosomes in vivo wound healing models were included. In vitro studies in which exosomes were not administered to animals, and those without preclinical models were excluded.
The citations from different databases were combined using reference managing software Endnote X9 (Clarivate Analytics, Philadelphia, PA, USA). Duplicate articles were removed using the same software. After the initial screen, three independent reviewers (AP, JJ, AE) each manually reviewed the title and abstract from the list of 3,064 studies. Only two studies were found in clinicaltrails.gov and PubMed related to skin wound healing and exosome, which was insufficient for systematic review and meta-analysis, thus those studies are excluded in the current study. Following a consensus, a total of 105 studies underwent full-text review, from which 51 rodent studies (32 mice, 19 rats) were identified. This step was followed by another round of cross-examination between the reviewers. A full-text review was conducted to mine quantitative and qualitative information about the research articles relating to cutaneous wound healing. Any dispute between the two reviewers was resolved by the third reviewer. Corresponding authors were contacted for further information if the publication did not include raw data or inadequately reported studies in the published literature. Six corresponding authors replied with total raw data during a six-week communication effort. A meta-analysis study was performed using the studies with raw data available or was provided by the corresponding authors.
Assessment of quality of studies
We performed a modified quality assessment for each study following the Stroke Therapy Academic Industry Roundtable (STAIR) recommendations (Fisher et al., 2009). We recorded a set of 10 criteria including (1) sample size calculation, (2) publication in a peer-reviewed journal, (3) indication of inclusion and exclusion criteria, (4) randomization, (5) allocation concealment, (6) avoidance of anesthetics with known marked intrinsic wound healing properties, (7) use of animals with relevant comorbidities, (8) reporting of animals excluded from analysis, (9) statement of compliance with animal welfare regulations, and (10) statement reporting declaring conflicts of interest. Zero or one was assigned for each criterion; with one point assigned for each criterion met. Each study’s possible total score ranges from 0 to 10, and higher scores indicate higher-quality studies (Supplemental Table 1).
Statistical analysis
Means and standard deviations of percentage wound closure, and animal numbers within study groups, were either extracted from each paper or obtained following correspondence with the authors. A meta-analysis was carried out using random-effects models where individual effects are taken into consideration and no fixed effects are analyzed as this model helps in controlling for unobserved heterogeneity. Results were summarized with effect size and 95% confidence intervals. Forest and funnel plots were used to graphically present results and visually assess possible publication bias. All analyses were carried out using the “metaphor” package within R (R Core Team 2020). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL (https://www.R-project.org/). Although meta-analysis was conducted for 7, 14, and 21 days, due to the small number of data points for 21 days only days 7 and 14 are reported in the current study.
Supplementary Material
ACKNOWLEDGEMENTS
This research would not have been possible without the outstanding support and direction of Julie Trumble, Head of Reference and Educational Services of the Moody Medical Library at the University of Texas Medical Branch, Galveston, TX. We would also like to acknowledge Eileen Figueroa and David Chavarria of the Surgery Department for assistance with formatting the paper. We are also grateful to all the authors who responded to our request for information regarding different aspects of the paper.
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- No authors listed. Exosomes. Nat Biotechnol 2020;38:1150. [DOI] [PubMed] [Google Scholar]
- Akers JC, Ramakrishnan V, Yang I, Hua W, Mao Y, Carter BS, et al. Optimizing preservation of extracellular vesicular miRNAs derived from clinical cerebrospinal fluid. Cancer Biomark 2016;17:125–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakhtyar N, Jeschke MG, Herer E, Sheikholeslam M, Amini-Nik S. Exosomes from acellular Wharton’s jelly of the human umbilical cord promotes skin wound healing. Stem Cell Res Ther 2018;9:193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busatto S, Vilanilam G, Ticer T, Lin WL, Dickson DW, Shapiro S, et al. Tangential flow filtration for highly efficient concentration of extracellular vesicles from large volumes of fluid. Cells 2018;7:273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B, Sun Y, Zhang J, Zhu Q, Yang Y, Niu X, et al. Human embryonic stem cell-derived exosomes promote pressure ulcer healing in aged mice by rejuvenating senescent endothelial cells. Stem Cell Res Ther 2019;10:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CY, Rao SS, Ren L, Hu XK, Tan YJ, Hu Y, et al. Exosomal DMBT1 from human urine-derived stem cells facilitates diabetic wound repair by promoting angiogenesis. Theranostics 2018;8:1607–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Zhou R, Liang Y, Fu X, Wang D, Wang C. Blockade of lncRNA-ASLNCS5088-enriched exosome generation in M2 macrophages by GW4869 dampens the effect of M2 macrophages on orchestrating fibroblast activation. FASEB J 2019;33:12200–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Zhang G, Gu Y, Guo X. Meta-analysis and systematic review of neural stem cells therapy for experimental ischemia stroke in preclinical studies. Sci Rep 2016;6:32291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalirfardouei R, Jamialahmadi K, Jafarian AH, Mahdipour E. Promising effects of exosomes isolated from menstrual blood-derived mesenchymal stem cell on wound-healing process in diabetic mouse model. J Tissue Eng Regen Med 2019;13:555–68. [DOI] [PubMed] [Google Scholar]
- Ding J, Wang X, Chen B, Zhang J, Xu J. Exosomes derived from human bone marrow mesenchymal stem cells stimulated by deferoxamine accelerate cutaneous wound healing by promoting angiogenesis. Biomed Res Int 2019;2019:9742765. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Eggenhofer E, Luk F, Dahlke MH, Hoogduijn MJ. The life and fate of mesenchymal stem cells. Front Immunol 2014;5:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Ayadi A, Jay JW, Prasai A. Current approaches targeting the wound healing phases to attenuate fibrosis and scarring. Int J Mol Sci 2020;21:1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M, Feuerstein G, Howells DW, Hurn PD, Kent TA, Savitz SI, et al. Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke 2009;40:2244–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallet R, Dawkins J, Valle J, Simsolo E, de Couto G, Middleton R, et al. Exosomes secreted by cardiosphere-derived cells reduce scarring, attenuate adverse remodelling, and improve function in acute and chronic porcine myocardial infarction. Eur Heart J 2017;38:201–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao S, Chen T, Hao Y, Zhang F, Tang X, Wang D, et al. Exosomal miR-135a derived from human amnion mesenchymal stem cells promotes cutaneous wound healing in rats and fibroblast migration by directly inhibiting LATS2 expression. Stem Cell Res Ther 2020;11:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger A, Walker A, Nissen E. Human fibrocyte-derived exosomes accelerate wound healing in genetically diabetic mice. Biochem Biophys Res Commun 2015;467:303–9. [DOI] [PubMed] [Google Scholar]
- Gerlach P, Schuller JM, Bonneau F, Basquin J, Reichelt P, Falk S, et al. Distinct and evolutionary conserved structural features of the human nuclear exosome complex. Elife 2018;7:e38686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guduric-Fuchs J, O’Connor A, Camp B, O’Neill CL, Medina RJ, Simpson DA. Selective extracellular vesicle-mediated export of an overlapping set of microRNAs from multiple cell types. BMC Genomics 2012;13:357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo SC, Tao SC, Yin WJ, Qi X, Yuan T, Zhang CQ. Exosomes derived from platelet-rich plasma promote the re-epithelization of chronic cutaneous wounds via activation of YAP in a diabetic rat model. Theranostics 2017;7:81–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Dong Z, Cao Y, Wang H, Liu S, Liao L, et al. MSC-derived exosome promotes M2 polarization and enhances cutaneous wound healing. Stem Cells Int 2019;2019:7132708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L, Wang J, Zhou X, Xiong Z, Zhao J, Yu R, et al. Exosomes derived from human adipose mensenchymal stem cells accelerates cutaneous wound healing via optimizing the characteristics of fibroblasts. Sci Rep 2016;6:32993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S, Li Z, Cores J, Huang K, Su T, Dinh PU, et al. Needle-free injection of exosomes derived from human dermal fibroblast spheroids ameliorates skin photoaging. ACS Nano 2019;13:11273–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Rao SS, Wang ZX, Cao J, Tan YJ, Luo J, et al. Exosomes from human umbilical cord blood accelerate cutaneous wound healing through miR-21–3p-mediated promotion of angiogenesis and fibroblast function. Theranostics 2018;8:169–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail DI, Aboulkhair AG. Histological evaluation of the emerging role of adipose stem cells-derived exosomes in cutaneous wound healing in albino rats. Egyptian Journal of Histology 2018;41:459–72. [Google Scholar]
- Jeppesen DK, Hvam ML, Primdahl-Bengtson B, Boysen AT, Whitehead B, Dyrskjot L, et al. Comparative analysis of discrete exosome fractions obtained by differential centrifugation. J Extracell Vesicles 2014;3:25011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang T, Wang Z, Sun J. Human bone marrow mesenchymal stem cell-derived exosomes stimulate cutaneous wound healing mediates through TGF-beta/Smad signaling pathway. Stem Cell Res Ther 2020;11:198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesici S, Kesici U, Ulusoy H, Erturkuner P, Turkmen A, Arda O. [Effects of local anesthetics on wound healing]. Braz J Anesthesiol 2018;68:375–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny C, Parsons N, Kadyszewski E, Festing MF, Cuthill IC, Fry D, et al. Survey of the quality of experimental design, statistical analysis and reporting of research using animals. PLoS One 2009;4:e7824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Wang SY, Kwak G, Yang Y, Kwon IC, Kim SH. Exosome-guided phenotypic switch of M1 to M2 macrophages for cutaneous wound healing, 10.1002/advs.201900513; 2019. (accessed 4 January 2020). [DOI] [PMC free article] [PubMed]
- Kobayashi H, Ebisawa K, Kambe M, Kasai T, Suga H, Nakamura K, et al. Editors’ Choice Effects of exosomes derived from the induced pluripotent stem cells on skin wound healing. Nagoya J Med Sci 2018;80:141–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz A Mesenchymal stem cell delivery routes and fate. Int J Stem Cells 2008;1:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees JS, Sena ES, Egan KJ, Antonic A, Koblar SA, Howells DW, et al. Stem cell-based therapy for experimental stroke: a systematic review and meta-analysis. Int J Stroke 2012;7:582–8. [DOI] [PubMed] [Google Scholar]
- Li B, Luan S, Chen J, Zhou Y, Wang T, Li Z, et al. The MSC-derived exosomal lncRNA H19 promotes wound healing in diabetic foot ulcers by upregulating PTEN via MicroRNA-152–3p. Mol Ther Nucleic Acids 2020;19:814–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Ke QF, Tao SC, Guo SC, Rui BY, Guo YP. Fabrication of hydroxyapatite/chitosan composite hydrogels loaded with exosomes derived from miR-126–3p overexpressed synovial mesenchymal stem cells for diabetic chronic wound healing. J Mater Chem B 2016;4:6830–41. [DOI] [PubMed] [Google Scholar]
- Li M, Wang T, Tian H, Wei G, Zhao L, Shi Y. Macrophage-derived exosomes accelerate wound healing through their anti-inflammation effects in a diabetic rat model. Artif Cells Nanomed Biotechnol 2019;47:3793–803. [DOI] [PubMed] [Google Scholar]
- Li X, Liu L, Yang J, Yu Y, Chai J, Wang L, et al. Exosome derived from human umbilical cord mesenchymal stem cell mediates MiR-181c attenuating burn-induced excessive inflammation. EBioMedicine 2016;8:72–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Su C. Design strategies and application progress of therapeutic exosomes. Theranostics 2019;9:1015–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Yan Z, Yang F, Huang Y, Yao Y, Zhou L, et al. Exosomes derived from human umbilical cord mesenchymal stem cells accelerate cutaneous wound healing by enhancing angiogenesis through delivering angiopoietin-2, https://pubmed.ncbi.nlm.nih.gov/32613452/; 2020. (accessed 4 January 2021). [DOI] [PubMed]
- Liu K, Chen C, Zhang H, Chen Y, Zhou S. Adipose stem cell-derived exosomes in combination with hyaluronic acid accelerate wound healing through enhancing re-epithelialization and vascularization. Br J Dermatol 2019;181:854–6. [DOI] [PubMed] [Google Scholar]
- Lv Q, Deng J, Chen Y, Wang Y, Liu B, Liu J. Engineered human adipose stem-cell-derived exosomes loaded with miR-21–5p to promote diabetic cutaneous wound healing. Mol Pharm 2020;17:1723–33. [DOI] [PubMed] [Google Scholar]
- Ma T, Fu B, Yang X, Xiao Y, Pan M. Adipose mesenchymal stem cell-derived exosomes promote cell proliferation, migration, and inhibit cell apoptosis via Wnt/beta-catenin signaling in cutaneous wound healing. J Cell Biochem 2019;120:10847–54. [DOI] [PubMed] [Google Scholar]
- Patel GK, Khan MA, Zubair H, Srivastava SK, Khushman M, Singh S, et al. Comparative analysis of exosome isolation methods using culture supernatant for optimum yield, purity and downstream applications. Sci Rep 2019;9:5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pound P, Ritskes-Hoitinga M. Can prospective systematic reviews of animal studies improve clinical translation? J Transl Med 2020;18:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao L, Hu S, Huang K, Su T, Li Z, Vandergriff A, et al. Tumor cell-derived exosomes home to their cells of origin and can be used as Trojan horses to deliver cancer drugs. Theranostics 2020;10:3474–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu X, Liu J, Zheng C, Su Y, Bao L, Zhu B, et al. Exosomes released from educated mesenchymal stem cells accelerate cutaneous wound healing via promoting angiogenesis. Cell Prolif 2020;53:e12830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, et al. B lymphocytes secrete antigen-presenting vesicles. J Exp Med 1996;183:1161–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan C Exosome cancer diagnostic reaches market. Nat Biotechnol 2016;34:359–60. [DOI] [PubMed] [Google Scholar]
- Shi R, Jin Y, Hu W, Lian W, Cao C, Han S, et al. Exosomes derived from mmu_circ_0000250-modified adipose-derived mesenchymal stem cells promote wound healing in diabetic mice by inducing miR-128–3p/SIRT1-mediated autophagy. Am J Physiol Cell Physiol 2020;318:C848–C56. [DOI] [PubMed] [Google Scholar]
- Shi Y, Yang Y, Guo Q, Gao Q, Ding Y, Wang H, et al. Exosomes derived from human umbilical cord mesenchymal stem cells promote fibroblast-to-myofibroblast differentiation in inflammatory environments and benefit cardioprotective effects. Stem Cells Dev 2019;28:799–811. [DOI] [PubMed] [Google Scholar]
- Shi Z, Wang Q, Jiang D. Extracellular vesicles from bone marrow-derived multipotent mesenchymal stromal cells regulate inflammation and enhance tendon healing. J Transl Med 2019;17:211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha M, Sen CK, Singh K, Das A, Ghatak S, Rhea B, et al. Direct conversion of injury-site myeloid cells to fibroblast-like cells of granulation tissue. Nat Commun 2018;9:936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjoqvist S, Ishikawa T, Shimura D, Kasai Y, Imafuku A, Bou-Ghannam S, et al. Exosomes derived from clinical-grade oral mucosal epithelial cell sheets promote wound healing. J Extracell Vesicles 2019;8:1565264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su D, Tsai H-I, Xu Z, Yan F, Wu Y, Xiao Y, et al. Exosomal PD-L1 functions as an immunosuppressant to promote wound healing. J Extracell Vesicles 2020;9:1709262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan SHS, Wong JRY, Sim SJY, Tjio CKE, Wong KL, Chew JRJ, et al. Mesenchymal stem cell exosomes in bone regenerative strategies-a systematic review of preclinical studies. Mater Today Bio 2020;7:100067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thery C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles 2018;7:1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinh NT, Yamashita T, Tu TC, Kato T, Ohneda K, Sato F, et al. Microvesicles enhance the mobility of human diabetic adipose tissue-derived mesenchymal stem cells in vitro and improve wound healing in vivo. Biochem Biophys Res Commun 2016;473:1111–8. [DOI] [PubMed] [Google Scholar]
- Walker S, Busatto S, Pham A, Tian M, Suh A, Carson K, et al. Extracellular vesicle-based drug delivery systems for cancer treatment. Theranostics 2019;9:8001–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Abhange KK, Wen Y, Chen Y, Xue F, Wang G, et al. Preparation of engineered extracellular vesicles derived from human umbilical cord mesenchymal stem cells with ultrasonication for skin rejuvenation. ACS Omega 2019;4:22638–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Hu L, Zhou X, Xiong Z, Zhang C, Shehada HMA, et al. Exosomes secreted by human adipose mesenchymal stem cells promote scarless cutaneous repair by regulating extracellular matrix remodelling. Sci Rep 2017;7:13321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Wang C, Chen M, Xi Y, Cheng W, Mao C, et al. Efficient angiogenesis-based diabetic wound healing/skin reconstruction through bioactive antibacterial adhesive ultraviolet shielding nanodressing with exosome release. ACS Nano 2019;13:10279–93. [DOI] [PubMed] [Google Scholar]
- Wang X, Jiao Y, Pan Y, Zhang L, Gong H, Qi Y, et al. Fetal dermal mesenchymal stem cell-derived exosomes accelerate cutaneous wound healing by activating notch signaling. Stem Cells Int 2019;2019:2402916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong J, Liu Z, Wu M, Sun M, Xia Y, Wang Y. Comparison of proangiogenic effects of adipose-derived stem cells and foreskin fibroblast exosomes on artificial dermis prefabricated flaps. Stem Cells Int 2020;2020:5293850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y, Chen L, Yan C, Zhou W, Endo Y, Liu J, et al. Circulating exosomal miR-20b-5p inhibition restores wnt9b signaling and reverses diabetes-associated impaired wound healing. Small 2020a;16:e1904044. [DOI] [PubMed] [Google Scholar]
- Xiong Y, Chen L, Yu T, Yan C, Zhou W, Cao F, et al. Inhibition of circulating exosomal microRNA-15a-3p accelerates diabetic wound repair. Aging (Albany NY) 2020b;12:8968–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Bai S, Cao Y, Liu L, Fang Y, Du J, et al. MiRNA-221–3p in endothelial progenitor cell-derived exosomes accelerates skin wound healing in diabetic mice. Diabetes Metab Syndr Obes 2020;13:1259–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Luo L, Bai X, Shen K, Liu K, Wang J, et al. Highly-expressed micoRNA-21 in adipose derived stem cell exosomes can enhance the migration and proliferation of the HaCaT cells by increasing the MMP-9 expression through the PI3K/AKT pathway. Arch Biochem Biophys 2020;681:108259. [DOI] [PubMed] [Google Scholar]
- Yang K, Li D, Wang M, Xu Z, Chen X, Liu Q, et al. Exposure to blue light stimulates the proangiogenic capability of exosomes derived from human umbilical cord mesenchymal stem cells. Stem Cell Res Ther 2019;10:358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H, Chen C-Y, Liu Y-W, Tan Y-J, Deng Z-L, Yang F, et al. Synechococcus elongatus PCC7942 secretes extracellular vesicles to accelerate cutaneous wound healing by promoting angiogenesis. Theranostics 2019;9:2678–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo KW, Li N, Makani V, Singh RN, Atala A, Lu B. Large-scale preparation of extracellular vesicles enriched with specific microRNA. Tissue Eng Part C Methods 2018;24:637–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng T, Wang X, Wang W, Feng Q, Lao G, Liang Y, et al. Endothelial cell-derived small extracellular vesicles suppress cutaneous wound healing through regulating fibroblasts autophagy. Clin Sci (Lond) 2019;133:CS20190008. [DOI] [PubMed] [Google Scholar]
- Zhang B, Wang M, Gong A, Zhang X, Wu X, Zhu Y, et al. HucMSC-exosome mediated-Wnt4 signaling is required for cutaneous wound healing. Stem Cells 2015a;33:2158–68. [DOI] [PubMed] [Google Scholar]
- Zhang B, Wu X, Zhang X, Sun Y, Yan Y, Shi H, et al. Human umbilical cord mesenchymal stem cell exosomes enhance angiogenesis through the Wnt4/beta-catenin pathway. Stem Cells Transl Med 2015b;4:513–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Chen C, Hu B, Niu X, Liu X, Zhang G, et al. Exosomes derived from human endothelial progenitor cells accelerate cutaneous wound healing by promoting angiogenesis through Erk1/2 signaling. Int J Biol Sci 2016;12:1472–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Guan J, Niu X, Hu G, Guo S, Li Q, et al. Exosomes released from human induced pluripotent stem cells-derived MSCs facilitate cutaneous wound healing by promoting collagen synthesis and angiogenesis. J Transl Med 2015;13:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Han F, Gu L, Ji P, Yang X, Liu M, et al. Adipose mesenchymal stem cell exosomes promote wound healing through accelerated keratinocyte migration and proliferation by activating the AKT/HIF-1alpha axis. J Mol Histol 2020;51:375–83. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Xu J, Liu S, Lim M, Zhao S, Cui K, et al. Embryonic stem cell-derived extracellular vesicles enhance the therapeutic effect of mesenchymal stem cells. Theranostics 2019;9:6976–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Li X, Shi X, Shi X, Zhang W, Wu G, et al. Exosomal MicroRNAs derived from human amniotic epithelial cells accelerate wound healing by promoting the proliferation and migration of fibroblasts. Stem Cells Int 2018;2018:5420463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao D, Yu Z, Li Y, Wang Y, Li Q, Han D. GelMA combined with sustained release of HUVECs derived exosomes for promoting cutaneous wound healing and facilitating skin regeneration. J Mol Histol 2020;51:251–63. [DOI] [PubMed] [Google Scholar]
- Zhao G, Liu F, Liu Z, Zuo K, Wang B, Zhang Y, et al. MSC-derived exosomes attenuate cell death through suppressing AIF nucleus translocation and enhance cutaneous wound healing. Stem Cell Res Ther 2020;11:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.