Abstract
Purpose
To perform an updated systematic review comparing the clinical outcomes of autograft versus nonirradiated allograft for anterior cruciate ligament reconstruction (ACLR).
Methods
A systematic review was conducted according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines by searching PubMed, the Cochrane Library, and Embase to identify comparative studies directly comparing outcomes of primary ACLR with autograft versus nonirradiated allograft with a minimum 2-year follow-up. The search terms used were: “anterior cruciate ligament” AND autograft AND allograft AND (irradiation OR non-irradiated). Patients were evaluated based on graft failure rates, the Objective International Knee Documentation Committee (IKDC) score, anteroposterior laxity, and patient-reported outcomes (Subjective IKDC score, the visual analog scale [VAS], the Cincinnati Knee Rating System, Lysholm, and Tegner scores). Risk of bias was assessed using the ROBINS-I and Cochrane Collaboration’s risk of bias tool for non-randomized and randomized studies, respectively.
Results
Sixteen studies (3 Level I, 7 Level II, 6 Level III) met inclusion criteria, including a total of 15,502 patients undergoing ACLR with autograft and 1,577 with nonirradiated allograft. The average follow-up ranged from 24.0 to 132.0 months. Graft failure ranged from 0% to 9.4% of patients in the autograft group and 0% to 26.5% in the allograft group. Two studies showed greater failure rates among younger patients in the allograft group. There were no significant differences between the Objective IKDC score, anteroposterior laxity, or patient-reported outcomes between the groups within any of the included studies (P > .05).
Conclusions
Autograft and nonirradiated allograft for primary ACLR demonstrate similar patient-reported outcomes and graft failure rates.
Level of Evidence
III, systematic review of level I-III studies.
Anterior cruciate ligament reconstruction (ACLR) remains one of the most common procedures performed among orthopaedic sports medicine specialists.1,2 When performing ACLR, graft choice is an important factor to consider and may depend on patient age, sports participation, and patient/surgeon preference.3, 4, 5 There are several autograft and allograft options for ACLR, with multiple studies demonstrating increased graft rupture rates with allograft compared with autograft, particularly in younger patients.6, 7, 8, 9
One of the factors thought to be involved in the greater failure rate of allografts is the graft-processing method, namely the use of radiation sterilization due to its detrimental biomechanical effects on allograft tissue.10,11 While studies have repeatedly demonstrated inferior outcomes with irradiated allografts compared with autografts,12, 13, 14, 15 these findings have not been consistently found in studies limited to nonirradiated allografts.16, 17, 18 Advantages of allograft use include smaller incisions, reduced postoperative pain/less donor-site morbidity, larger graft availability, earlier postoperative knee range of motion, and decreased surgical time.19 Disadvantages include risk of immunogenic reaction, bacterial infection, and disease transmission from the graft donor. Another cited disadvantage of allograft use is increased laxity over time, which can result in knee joint instability and failure to return to previous level of activities despite an “intact” graft.19 The purpose of this study was to perform an updated systematic review comparing the clinical outcomes of autograft versus nonirradiated allograft for ACLR. The authors hypothesized that no significant differences would be found between groups in terms of graft rupture rates or patient-reported outcomes (PROs).
Methods
This systematic review was conducted according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines using a PRISMA checklist. Two independent reviewers (J.D., J.W.B.) searched PubMed, Embase, and the Cochrane Library up to August 8, 2021. The electronic search strategy used was as follows: "anterior cruciate ligament" AND autograft AND allograft AND (irradiation OR non-irradiated). A total of 113 studies were reviewed by title and/or abstract to determine study eligibility based on inclusion criteria. In cases of disagreement, a third reviewer (M.J.K.) made the final decision. The inclusion criteria were nonoverlapping human studies directly comparing autograft versus nonirradiated allograft with a minimum 2-year follow-up. Exclusion criteria included noncomparative studies, studies unrelated to the knee, and studies that did not distinguish outcomes between irradiated and nonirradiated allograft. Data extraction from each study was performed independently and then reviewed by a second author (M.J.K.). There was no need for funding or a third party to obtain any of the collected data. Risk of bias for 7 randomized studies9,20, 21, 22, 23, 24, 25 was assessed according to the Cochrane Collaboration’s risk of bias tool,26 which incorporates an assessment of randomization, blinding, completeness of outcomes data, selection of outcomes reported, and other sources of bias. Risk of bias for the 9 remaining nonrandomized studies12,27, 28, 29, 30, 31, 32, 33, 34 was assessed according to the ROBINS-I (i.e., (Risk Of Bias In Non-randomized Studies - of Interventions)) risk of bias tool,35 which incorporates an assessment of bias due to confounding, selection of participants, deviations from intended interventions, completeness of outcomes data, selection of outcomes reported, and other sources of bias. A score of <0.20 indicates poor agreement; 0.21-0.40, fair agreement; 0.41-0.60, moderate agreement; 0.61-0.80, good agreement; and 0.81-1.00, very good agreement.36
Reporting Outcomes
Outcomes assessed included graft failure, PROs, anteroposterior (AP) laxity, and the Objective International Knee Documentation Committee (IKDC) score.37 PROs included the Subjective IKDC score,38 Lysholm score,39 Tegner activity score,40 VAS for pain, and the Cincinnati Knee Rating System.41 An attempt was made to perform a subanalysis of outcomes in younger patients, but this was not possible, as only one study32 reported outcomes based on age.
Study Methodology Assessment
The Modified Coleman Methodology Score (MCMS)42 was used to evaluate study methodology quality. The MCMS has a scaled potential score ranging from 0 to 100. Scores ranging from 85 to 100 are excellent, 70 to 84 are good, 55 to 69 are fair, and less than 55 are poor. The primary outcomes assessed by the MCMS are study size and type, follow-up time, attrition rates, number of interventions per group, and proper description of study methodology.
Results
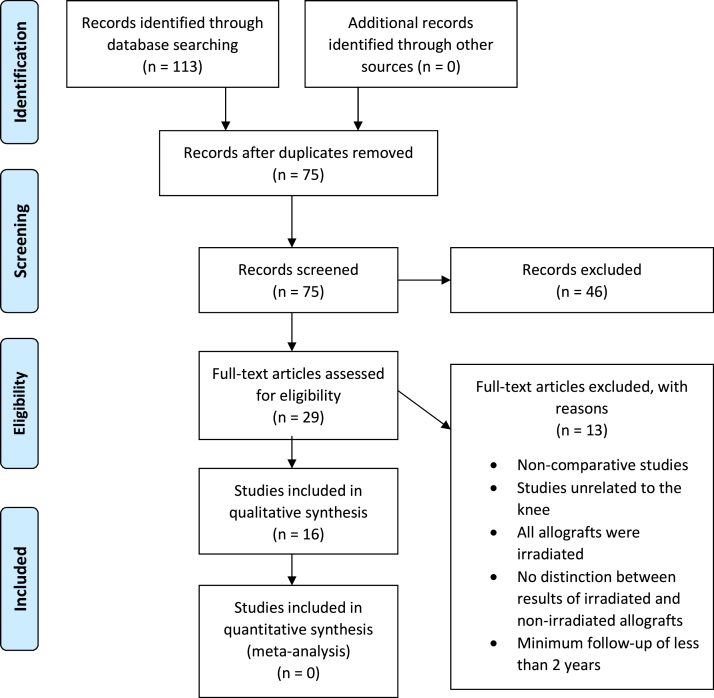
Sixteen studies met inclusion and exclusion criteria (Fig 1). A total of 17,079 patients were included in this systematic review, including 15,502 patients undergoing ACLR with an autograft and 1,577 with a nonirradiated allograft. Patient age ranged from 13.0 to 64.0 years and the mean follow-up time ranged from 24 to 132 months (Table 1). The percentage of male patients ranged from 49.4% to 90.9%. Twelve studies20, 21, 22, 23, 24, 25,27, 28, 29, 30, 31,34 did not report the use of any irradiated allografts. In addition to the nonirradiated allografts analyzed in this review, irradiated allografts were used in the remaining 4 studies in 32 patients,9 68 patients,12 874 patients,32 and 3,022 patients.33
Fig 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram.
Table 1.
Studies Included
| Study | Level of Evidence | n (Auto, Allo) | Patient Age (Auto, Allo), y (Range) | Follow-up, mo (Range) | Sex, % Male |
|---|---|---|---|---|---|
| Bottoni et al., 201520 | I | 48, 49 | 28.9, 29.2 (20.6-42.5) | 126 (120-132) | 86.6 |
| Noh et al., 201122 | I | 33, 32 | 23.0, 22.0 (20-55) | 29.8 (NR) | 86.2 |
| Yoo et al., 201725 | I | 68, 64 | 30.0, 24.0 (13-62) | 33.6 (25.3-59.5) | 90.9 |
| Edgar et al., 200829 | II | 37, 46 | 27.0, 31.0 (15-55) | 49.8 (36.0-74.0) | 55.4 |
| Lawhorn et al., 201221 | II | 74, 73 | 32.0, 33.3 (16-53) | NR | 68.0 |
| Maletis et al., 201732 | II | 4,557; 155 | NR | NR | NR |
| Maletis et al., 201733 | II | 10,264; 729 | NR | NR | NR |
| Sun et al., 20099 | II | 33, 34 | 29.7, 31.8 (19-64) | 30.8 (NR) | 68.7 |
| Sun et al., 201123 | II | 91, 95 | 29.6, 31.2 (18-59) | 94.8 (72-120) | 80.1 |
| Tian et al., 201624 | II | 62, 59 | 30.5, 29.9 (15-56) | 55.2 (48-66) | 79.3 |
| Barber et al., 201427 | III | 53, 28 | 18.6, 20.1 (13-25) | 34.0 (24-132) | 49.4 |
| Barrett et al., 200528 | III | 25, 38 | 44.5, 47.1 (40-58) | NR | 55.6 |
| Guo et al., 201212 | III | 41, 33 | 25.0, 25.3 (16-40) | 80.4 (50.4-98.4) | 63.5 |
| Kane et al., 201630 | III | 60, 59 | 19.8, 20.6 (NR) | NR | NR |
| Kustos et al., 200431 | III | 26, 53 | 24.5, 25.6 (NR) | 38.0 (NR) | 75.9 |
| Peterson et al., 200134 | III | 30, 30 | 25.0, 28.0 (15-55) | 63.6 (55-78) | 55.0 |
NOTE. n refers to the number of knees that underwent ACL reconstruction with either autograft or nonirradiated allograft in each study. Patient age and follow-up are reported as mean (range).
ACL, anterior cruciate ligament; Allo, nonirradiated allograft; Auto, autograft; NR, not reported.
Surgical Technique
Table 2 shows the types of autograft/allografts used for ACLR in the 16 included studies. Nine studies12,20,21,25,27, 28, 29, 30,34 used a transtibial approach for femoral tunnel drilling. Two studies23,24 used an anteromedial portal approach. Five studies9,23,31, 32, 33 did not report their method of femoral tunnel drilling (Table 2).
Table 2.
Surgical Details
| Study | Autograft/Allograft | Method of Femoral Tunnel Drilling | Method of Graft Fixation | Indication for Graft Type |
|---|---|---|---|---|
| Bottoni et al., 201520 | Hamstring/tibialis posterior | TT | Bioabsorbable interference screw | Randomized |
| Noh et al., 201122 | Hamstring/Achilles | TT | Cortical button/bioabsorbable interference screw | Randomized |
| Yoo et al., 201725 | Hamstring/tibialis anterior or posterior | TT | Cortical button/bioabsorbable interference screw | Randomized |
| Edgar et al., 200829 | Hamstring/hamstring | TT | Bioabsorbable interference screw | Randomized/patient choice |
| Lawhorn et al., 201221 | Hamstring/tibialis anterior | TT | Metal interference screw | Randomized |
| Maletis et al., 201732 | BPTB/BPTB | NR | NR | NR |
| Maletis et al., 201733 | BPTB or hamstring/soft tissue | NR | NR | NR |
| Sun et al., 20099 | BPTB/BPTB | NR | Metal/bioabsorbable interference screw | Randomized |
| Sun et al., 201123 | Hamstring/hamstring | AM portal | Cortical button/bioabsorbable interference screw | Randomized |
| Tian et al., 201624 | Hamstring/hamstring | AM portal | Bioabsorbable interference screw | Randomized |
| Barber et al., 201427 | BPTB/BPTB | TT | Bioabsorbable interference screw | Patient choice |
| Barrett et al., 200528 | BPTB/BPTB | TT | Cortical button/bioabsorbable interference screw | NR |
| Guo et al., 201212 | BPTB/BPTB | TT | Bioabsorbable interference screw | Patient choice |
| Kane et al., 201630 | BPTB/BPTB | TT | Bioabsorbable interference screw | Patient choice |
| Kustos et al., 200431 | BPTB/BPTB | NR | Bioabsorbable interference screw | Patient choice |
| Peterson et al., 200134 | BPTB/BPTB | NR | Metal interference screw | Patient choice |
AM, anteromedial; BPTB, bone–patellar tendon–bone; NR, not reported; TT, transtibial.
Seven studies12,20,24,27,29, 30, 31 used bioabsorbable interference screws for graft fixation. Two studies21,34 used a metal interference screw for graft fixation. One study9 used either a metal or bioabsorbable interference screw. Four studies22,23,25,28 used a cortical button (ENDOBUTTON; Smith & Nephew, Andover, MA) to fix the graft on the femoral side and a bioabsorbable interference screw on the tibial side. Two studies32,33 did not detail their method of graft fixation. In 6 of the nonrandomized studies,12,27,29,30,31,34 the graft type was chosen based on patient choice (Table 2).
Graft Failure
Two studies29,31 defined graft failure as graft rupture, whereas 12 studies9,12,20,21,24,25,27,28,30,32, 33, 34 defined graft failure as the need for a revision ACL reconstruction. Overall, graft failure ranged from 0.0% to 9.4% in the autograft group and 0.0% to 26.5% in the allograft group (Table 3). In one study,32 among patients 21 years old and younger, the graft failure rate was 2.9% in the autograft group and 11.4% in the allograft group. In patients 22 years old and older, the graft failure rate was 0.9% in the autograft group and 1.7% in the allograft group. Three studies19,30,32 found a significantly greater graft failure rate in the nonirradiated allograft group at final follow-up, one of which30 limited inclusion to patients aged 25 years or younger.
Table 3.
Graft Failure Rates
| Study | Auto | Allo | Total |
|---|---|---|---|
| Peterson et al., 200134 | 1/30 (3.3%) | 1/30 (3.3%) | 2/60 (3.3%) |
| Kustos et al., 200431 | 1/26 (3.8%) | 2/53 (3.8%) | 3/79 (3.8%) |
| Barrett et al., 200528 | 0/25 (0%) | 1/38 (2.6%) | 1/63 (1.6%) |
| Edgar et al., 200829 | 3/37 (8.1%) | 2/46 (4.3%) | 5/83 (6.0%) |
| Sun et al., 20099 | 2/33 (6.1%) | 3/34 (8.8%) | 5/67 (7.5%) |
| Guo et al., 201212 | 0/41 (0%) | 0/33 (0%) | 0/74 (0%) |
| Lawhorn et al., 201221 | 0/74 (0%) | 0/73 (0%) | 0/147 (0%) |
| Barber et al., 201427 | 5/53 (9.4%) | 2/28 (7.1%) | 7/81 (8.6%) |
| Bottoni et al., 201520 | 4/48 (8.3%) | 13/49 (26.5%) | 17/97 (17.5%) |
| Yoo et al., 201725 | 1/68 (1.5%) | 1/64 (1.6%) | 2/132 (1.5%) |
| Tian et al., 201624 | 0/62 (0%) | 0/59 (0%) | 0/121 (0%) |
| Kane et al., 201630 | 1/60 (1.7%) | 12/59 (20.3%) | 13/119 (10.9%) |
| Maletis et al., 201732 | 85/4,557 (1.9%) | 5/155 (3.2%) | 90/4,712 (1.9%) |
| Maletis et al., 201733 | 217/10,264 (2.1%) | 12/729 (1.6%) | 229/10,993 (2.1%) |
NOTE. Each cell includes the number of graft failures/total number of ACLRs performed (%) within each group.
ACLR, anterior cruciate ligament reconstruction; Allo, nonirradiated allograft; Auto, autograft.
Patient-Reported Outcomes (PROs)
Seven studies8,20,21,23,24,29,30 reported results of the Subjective IKDC score (Table 4). No study found a significant difference in comparison of postoperative scores between the groups.
Table 4.
Subjective IKDC Score
| Study | Auto (Preoperative) | Auto (Postoperative) | Allo (Preoperative) | Allo (Postoperative) | P Value |
|---|---|---|---|---|---|
| Edgar et al., 200829 | 57.5 ± 8.4 | 87.6 ± 10.2 | 54.9 ± 13.1 | 87.0 ± 11.7 | .82 |
| Sun et al., 20098 | NR | 88.0 ± 11 | NR | 89.0 ± 9 | >.05 |
| Sun et al., 201123 | NR | 89.0 ± 12 | NR | 90.0 ± 14 | .548 |
| Lawhorn et al., 201221 | NR | 91.0 | NR | 90.9 | >.05 |
| Bottoni et al., 201520 | NR | 77.2 ± 25.4 | NR | 73.7 ± 25.9 | .51 |
| Tian et al., 201624 | NR | 90.0 ± 11 | NR | 89.0 ± 12 | .63 |
| Kane et al., 201630 | NR | 95.4 | NR | 95.4 | >.05 |
NOTE. Scores are reported as a mean ± SD (when reported) at latest follow-up. Reported P values indicate comparison of postoperative scores between groups.
Allo, nonirradiated allograft; Auto, autograft; IKDC, International Knee Documentation Committee; NR, not reported; SD, standard deviation.
Twelve studies9,12,22, 23, 24, 25,27, 28, 29, 30, 31,34 reported results of the Lysholm score (Table 5). No study found a significant difference in comparison of postoperative scores between the groups.
Table 5.
Lysholm Score
| Study | Auto (Preoperative) | Auto (Postoperative) | Allo (Preoperative) | Allo (Postoperative) | P Value |
|---|---|---|---|---|---|
| Peterson et al., 200134 | NR | 88.6 | NR | 90.0 | >.05 |
| Kustos et al., 200431 | NR | 89.9 ± 8.1 | NR | 84.1 ± 18.6 | >.05 |
| Barrett et al., 200528 | 55.0 | 92.0 | 54.0 | 91.0 | >.05 |
| Edgar et al., 200829 | 71.3 ± 8.6 | 91.0 ± 7.7 | 67.7 ± 17 | 92.7 ± 10 | .75 |
| Sun et al., 20099 | NR | 90.0 ± 9 | NR | 91.0 ± 8 | >.05 |
| Noh et al., 201122 | 54.0 | 98.0 | 56.0 | 99.0 | >.05 |
| Sun et al., 201123 | 60.0 ± 12 | 89.0 ± 9 | 59.0 ± 10 | 90.0 ± 8 | .60 |
| Guo et al., 201212 | 52.1 ± 6.2 | 86.6 ± 9.5 | 43.3 ± 5.7 | 85.6 ± 10.1 | .74 |
| Barber et al., 201427 | 44.8 | 87.0 | 60.3 | 89.9 | .43 |
| Yoo et al., 201725 | NR | 96.0 | NR | 93.0 | >.05 |
| Tian et al., 201624 | 58.0 ± 10 | 90.0 ± 10 | 57.0 ± 8 | 89.0 ± 11 | .6 |
| Kane et al., 201630 | NR | 95.0 | NR | 95.0 | >.05 |
NOTE. Scores are reported as a mean ± SD (when reported) at latest follow-up. Reported P values indicate comparison of postoperative scores between groups.
Allo, nonirradiated allograft; Auto, autograft; NR, not reported; SD, standard deviation.
Ten studies9,12,20,22, 23, 24, 25,28,29,34 reported results of the Tegner score (Table 6). No study found a significant difference in comparison of postoperative scores between the groups.
Table 6.
Tegner Score
| Study | Auto (Preoperative) | Auto (Postoperative) | Allo (Preoperative) | Allo (Postoperative) | P Value |
|---|---|---|---|---|---|
| Peterson et al., 200134 | NR | 6.1 | NR | 5.4 | >.05 |
| Barrett et al., 200528 | 3.9 | 4.3 | 4.3 | 4.1 | >.05 |
| Edgar et al., 200829 | 7.2 ± 1.1 | 6.8 ± 1.2 | 6.8 ± 1.3 | 6.9 ± 1.3 | .08 |
| Sun et al., 20099 | NR | 7.7 ± 1.3 | NR | 7.5 ± 1.5 | >.05 |
| Noh et al., 201122 | 6.0 | 6.0 | 7.0 | 6.0 | >.05 |
| Sun et al., 201123 | 3.0 ± 1.3 | 7.7 ± 1.8 | 3.1 ± 1.5 | 7.6 ± 1.5 | .94 |
| Guo et al., 201212 | 2.1 ± 0.7 | 4.5 ± 1.1 | 2.4 ± 0.9 | 4.1 ± 0.8 | .42 |
| Bottoni et al., 201520 | NR | 4.8 ± 2.3 | NR | 4.5 ± 2.2 | .51 |
| Yoo et al., 201725 | NR | 5.0 | NR | 5.0 | >.05 |
| Tian et al., 201624 | 2.8 ± 0.7 | 7.9 ± 0.8 | 2.9 ± 0.8 | 7.8 ± 1.0 | .54 |
NOTE. Scores are reported as a mean ± SD (when reported) at latest follow-up. Reported P values indicate comparison of postoperative scores between groups.
Allo, nonirradiated allograft; Auto, autograft; NR, not reported; SD, standard deviation.
Three studies9,23,27 reported results for the Cincinnati Knee Rating System. No study found a significant difference in comparison of postoperative scores between the groups.
One study28 reported results of the VAS scale for pain and found no significant difference between groups pre- or postoperatively (P > .05).
AP Knee Laxity
Five studies9,12,23,24,29 measured AP knee laxity, with 3 studies9,12,29 measuring mean side-to-side differences in tibial translation (Table 7). No study found a significant difference in comparison of postoperative measurements between the groups. Two studies12,29 used KT-1000 and one study9 used KT-2000.
Table 7.
AP Knee Laxity
| Study | Auto (Preoperative) | Auto (Postoperative) | Allo (Preoperative) | Allo (Postoperative) | P Value |
|---|---|---|---|---|---|
| Edgar et al., 200829 | 6.0 ± 1.3 | 1.6 ± 1.5 | 5.8 ± 1.5 | 1.4 ± 1.3 | .33 |
| Sun et al., 20099 | NR | 2.4 ± 0.6 | NR | 2.6 ± 0.9 | >.05 |
| Guo et al., 201212 | 5.9 ± 0.7 | 2.3 ± 0.3 | 6.3 ± 0.8 | 2.6 ± 0.4 | .85 |
NOTE. Measurements are reported as a mean ± SD (when reported) side-to-side difference (in millimeters) at latest follow-up. Reported P values indicate comparison of postoperative measurements between groups.
Allo, nonirradiated allograft; AP, anteroposterior; Auto, autograft; NR, not reported; SD, standard deviation.
Objective IKDC
Four studies21,23, 24, 25 reported results for the Objective IKDC score and found no significant difference between the 2 groups at final follow-up (P = .71, P > .05, P > .05, and P > .87, respectively).
Modified Coleman Methodology Score
Table 8 shows the MCMS scores from the 16 included studies. One study23 received an excellent score, 7 studies9,20,22,24,25,29,34 received good scores, and 8 studies12,21,27,28,30, 31, 32, 33 received fair scores.
Table 8.
Modified Coleman Methodology Score (MCMS)
| Study | MCMS |
|---|---|
| Peterson et al., 200134 | 76 |
| Kustos et al., 200431 | 59 |
| Barrett et al., 200528 | 60 |
| Edgar et al., 200829 | 77 |
| Sun et al., 20099 | 79 |
| Noh et al., 201122 | 79 |
| Sun et al., 201123 | 88 |
| Guo et al., 201212 | 69 |
| Lawhorn et al., 201221 | 66 |
| Barber et al., 201427 | 60 |
| Bottoni et al., 201520 | 71 |
| Yoo et al., 201725 | 73 |
| Tian et al., 201624 | 80 |
| Kane et al., 201630 | 63 |
| Maletis et al., 201732 | 68 |
| Maletis et al., 201733 | 68 |
Methodologic Quality Assessment
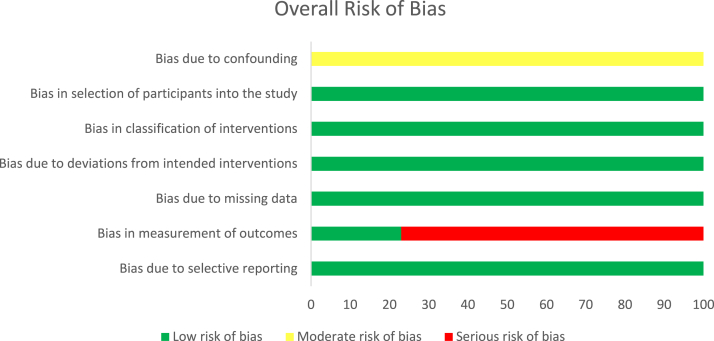
The results of the methodologic quality assessment of the 9 nonrandomized studies using the ROBINS-I risk of bias tool are presented in Figure 2. All 9 studies12,27, 28, 29, 30, 31, 32, 33, 34 showed a moderate risk of bias due to confounding, as there were no prognostic variables that predicted baseline intervention and no patients that switched between interventions during the study period. No studies excluded eligible patients or used variable follow-up times based on intervention (low risk of bias), no studies deviated from the intended intervention (low risk of bias), and all studies clearly classified treatment type (low risk of bias). Two studies29,34 using blinded outcome assessors showed no systematic differences in the care provided between treatment groups (low risk of bias), whereas 7 studies12,27,28,30, 31, 32, 33 used nonblinded but identical postoperative protocols (moderate risk of bias). No studies showed bias due to missing data (low risk of bias). Two studies29,34 demonstrated low risk of bias in measurement of outcomes through use of blinded outcome assessors, whereas 7 studies12,27,28,30, 31, 32, 33 used physicians not blinded to treatment group (serious risk of bias). Finally, no studies showed bias due to selective reporting (low risk of bias). A Cohen’s Kappa score of 0.82 reflected a very good agreement between reviewers.
Fig 2.
Risk of bias graph. Risk of bias is presented as a percentage across all included studies (green, low risk; yellow, unclear; red, high risk).
The remaining 7 randomized studies9,20, 21, 22, 23, 24, 25 were assessed for methodologic quality using the Cochrane Collaboration’s risk of bias tool. Sequence generation and allocation were adequately reported by all studies9,20, 21, 22, 23, 24, 25 (low risk of bias), and 2 studies20,25 were deemed to be at low risk for detection bias because of the blinding of the outcome assessor. Six studies9,21, 22, 23, 24, 25 did not report blinding of either patients or the outcome assessor (high risk of bias). One study20 reported blinding outcome assessors, but not patients (moderate risk of bias). No studies reported significant loss of follow-up (low risk of bias) and no studies was deemed to be at risk of bias for selective reporting or incomplete outcome data (low risk of bias).
Discussion
Based on the findings of this systematic review, there were no statistically significant differences between use of autograft and nonirradiated allograft for primary ACLR with regard to PROs, AP knee laxity, and the Objective IKDC score at a minimum 2-year follow-up. Furthermore, PROs and graft failure rates were similar between groups at final follow-up. Unfortunately, most studies did not report outcomes based on age, and surgeons are likely weary to further investigate this in future studies of young, active patients.
Several previous studies have suggested that the greater failure rates we expect with allografts in general do not necessarily occur with nonirradiated allografts. A meta-analysis published in 201818 with a total of 1,172 patients found no significant differences between autograft and nonirradiated allograft groups for primary ACLR in terms of PROs (Subjective IKDC, Lysholm, Tegner scores), with similar failure rates between the 2 groups (autograft: 2.3%, nonirradiated allograft: 2.8%). Likewise, a systematic review from 201417 with 811 patients found no significant differences in graft failure rate, postoperative knee laxity, or PROs between autograft and nonirradiated allograft tissue for ACLR. Lamblin et al.,16 in a 2013 systematic review of 1,002 patients, found similar outcomes between these 2 groups with regard to graft failure, PROs, and Lachman/pivot shift testing. The current systematic review builds upon these previous reviews with additional studies and a larger sample size included for a more robust and updated set of clinical findings.
Use of autograft tissue for ACLR is not without complications. In terms of hamstring tendon harvesting, there is a high rate of damage to the saphenous nerve, up to 88% in a study by Kjaergaard et al.43 The primary complication for bone–patellar tendon–bone autograft harvesting is anterior knee pain, which is reported in up to 46% of cases.44 This is especially concerning for patients who kneel frequently, such as those who pray daily or workers such as painters or carpenters.8 Other complications can include patellar tendonitis, tendon rupture, or rarely patella fracture.44,45 Another issue with autograft harvesting is the loss of muscle strength postoperatively. A meta-analysis found an extension strength deficit in patients with bone–patellar tendon–bone autograft and flexion strength deficit in patients with hamstring tendon autograft that persisted at 12 months postoperatively.46 Thus, the use of allograft tissue would help eliminate these morbidities as well as reducing the operation time.47
Nonirradiated allografts are not without disadvantages as well. These grafts demonstrate decreased osteoinductive and osteoconductive characteristics, as well as a delayed graft incorporation time in comparison with autografts.48 Another disadvantage is the risk of disease transmission. Although the risk remains low, there is still potential for viral transmission due to human error as well as window periods of infection where detection is missed through serologic tests.49 It is critical to properly sterilize tendon allografts before implantation. A systematic review assessed the different sterilization and disinfection methods and identified gamma or electron beam irradiation, ethylene oxide, supercritical carbon dioxide, and BioCleanse (RTI Surgical, Alachua, FL) as potential methods.50 The authors recommended freezing and gamma or electron beam irradiation at 14.8 to 28.5 kGy as they were effective at maintaining the mechanical properties of the graft, while fully sterilizing the inside and the outside of the tendon. Other sterilization methods (ethylene oxide, supercritical carbon dioxide, BioCleanse) deteriorated the mechanical properties and were not recommended.
Limitations
The limitations of this study should be noted. This systematic review included nonrandomized studies and therefore the individual study indications for use of autograft or allograft tissue may have played a role in the clinical outcomes of these studies. There was heterogeneity in the type of autograft and allograft tissue used, the method of graft fixation, the definition of graft failure between studies, as well as the method of femoral tunnel drilling across studies. The wide heterogeneity of surgical techniques and variety of PROs precluded a meta-analysis. Some studies included patients undergoing concomitant procedures, which may have also affected patient outcomes. Finally, 2 studies32,33 accounted for the majority of the patients included in this systematic review.
Conclusions
Autograft and nonirradiated allograft for primary ACLR demonstrate similar PROs and graft failure rates.
Footnotes
The authors report the following potential conflicts of interest or sources of funding: E.C.M. reports consultant for Biomet; royalties from Zimmer Biomet and Elsevier; and research support from Arthrex, Breg, DJ Orthopaedics, Mitek, Össur, and Smith & Nephew, outside the submitted work. P.C.M. reports consultant for Arthrex and research support from Smith & Nephew, outside the submitted work. A.J.S. reports consultant for Mitek; stock or stock options from Biomet, CONMED Linvatec, Johnson & Johnson, Pfizer, Smith & Nephew, and Stryker; and research support from Isto Biologics, outside the submitted work. Full ICMJE author disclosure forms are available for this article online, as supplementary material.
Supplementary Material
References
- 1.Herzog M.M., Marshall S.W., Lund J.L., Pate V., Mack C.D., Spang J.T. Trends in incidence of ACL reconstruction and concomitant procedures among commercially insured individuals in the United States, 2002-2014. Sports Health. 2018;10:523–531. doi: 10.1177/1941738118803616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Longo U.G., Nagai K., Salvatore G., et al. Epidemiology of anterior cruciate ligament reconstruction surgery in Italy: A 15-year nationwide registry study. J Clin Med. 2021;10:223. doi: 10.3390/jcm10020223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bowman E.N., Limpisvasti O., Cole B.J., ElAttrache N.S. Anterior cruciate ligament reconstruction graft preference most dependent on patient age: A survey of United States surgeons. Arthroscopy. 2021;37:1559–1566. doi: 10.1016/j.arthro.2021.01.042. [DOI] [PubMed] [Google Scholar]
- 4.Houck D.A., Kraeutler M.J., Vidal A.F., et al. Variance in anterior cruciate ligament reconstruction graft selection based on patient demographics and location within the Multicenter Orthopaedic Outcomes Network cohort. J Knee Surg. 2018;31:472–478. doi: 10.1055/s-0037-1604147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salminen M., Kraeutler M.J., Freedman K.B., et al. Choosing a graft for anterior cruciate ligament reconstruction: Surgeon influence reigns supreme. Am J Orthop (Belle Mead NJ) 2016;45:E192–197. [PubMed] [Google Scholar]
- 6.Cruz A.I., Jr., Beck J.J., Ellington M.D., et al. Failure rates of autograft and allograft ACL reconstruction in patients 19 years of age and younger: A systematic review and meta-analysis. JBJS Open Access. 2020;5 doi: 10.2106/JBJS.OA.20.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaeding C.C., Aros B., Pedroza A., et al. Allograft versus autograft anterior cruciate ligament reconstruction: Predictors of failure from a MOON prospective longitudinal cohort. Sports Health. 2011;3:73–81. doi: 10.1177/1941738110386185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kraeutler M.J., Bravman J.T., McCarty E.C. Bone-patellar tendon-bone autograft versus allograft in outcomes of anterior cruciate ligament reconstruction: A meta-analysis of 5182 patients. Am J Sports Med. 2013;41:2439–2448. doi: 10.1177/0363546513484127. [DOI] [PubMed] [Google Scholar]
- 9.Sun K., Tian S., Zhang J., Xia C., Zhang C., Yu T. Anterior cruciate ligament reconstruction with BPTB autograft, irradiated versus non-irradiated allograft: A prospective randomized clinical study. Knee Surg Sports Traumatol Arthrosc. 2009;17:464–474. doi: 10.1007/s00167-008-0714-8. [DOI] [PubMed] [Google Scholar]
- 10.Lansdown D.A., Riff A.J., Meadows M., Yanke A.B., Bach B.R., Jr. What factors influence the biomechanical properties of allograft tissue for ACL reconstruction? A systematic review. Clin Orthop Relat Res. 2017;475:2412–2425. doi: 10.1007/s11999-017-5330-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmidt T., Hoburg A., Broziat C., et al. Sterilization with electronic beam irradiation influences the biomechanical properties and the early remodeling of tendon allografts for reconstruction of the anterior cruciate ligament (ACL) Cell Tissue Bank. 2012;13:387–400. doi: 10.1007/s10561-011-9289-6. [DOI] [PubMed] [Google Scholar]
- 12.Guo L., Yang L., Duan X., et al. Anterior cruciate ligament reconstruction with bone-patellar tendon-bone graft: Comparison of autograft, fresh-frozen allograft, and γ-irradiated allograft. Arthroscopy. 2012;28:211–217. doi: 10.1016/j.arthro.2011.08.314. [DOI] [PubMed] [Google Scholar]
- 13.Li J., Wang J., Li Y., Shao D., You X., Shen Y. A prospective randomized study of anterior cruciate ligament reconstruction with autograft, γ-irradiated allograft, and hybrid graft. Arthroscopy. 2015;31:1296–1302. doi: 10.1016/j.arthro.2015.02.033. [DOI] [PubMed] [Google Scholar]
- 14.Sun K., Zhang J., Wang Y., et al. Arthroscopic anterior cruciate ligament reconstruction with at least 2.5 years’ follow-up comparing hamstring tendon autograft and irradiated allograft. Arthroscopy. 2011;27:1195–1202. doi: 10.1016/j.arthro.2011.03.083. [DOI] [PubMed] [Google Scholar]
- 15.Tian S., Wang B., Liu L., et al. Irradiated hamstring tendon allograft versus autograft for anatomic double-bundle anterior cruciate ligament reconstruction: Midterm clinical outcomes. Am J Sports Med. 2016;44:2579–2588. doi: 10.1177/0363546516655333. [DOI] [PubMed] [Google Scholar]
- 16.Lamblin C.J., Waterman B.R., Lubowitz J.H. Anterior cruciate ligament reconstruction with autografts compared with non-irradiated, non-chemically treated allografts. Arthroscopy. 2013;29:1113–1122. doi: 10.1016/j.arthro.2013.01.022. [DOI] [PubMed] [Google Scholar]
- 17.Mariscalco M.W., Magnussen R.A., Mehta D., Hewett T.E., Flanigan D.C., Kaeding C.C. Autograft versus nonirradiated allograft tissue for anterior cruciate ligament reconstruction: A systematic review. Am J Sports Med. 2014;42:492–499. doi: 10.1177/0363546513497566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang S., Zhang C., Cai Y., Lin X. Autograft or allograft? Irradiated or not? A contrast between autograft and allograft in anterior cruciate ligament reconstruction: A meta-analysis. Arthroscopy. 2018;34:3258–3265. doi: 10.1016/j.arthro.2018.06.053. [DOI] [PubMed] [Google Scholar]
- 19.Dashe J., Parisien R.L., Cusano A., Curry E.J., Bedi A., Li X. Allograft tissue irradiation and failure rate after anterior cruciate ligament reconstruction: A systematic review. World J Orthop. 2016;7:392–400. doi: 10.5312/wjo.v7.i6.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bottoni C.R., Smith E.L., Shaha J., et al. Autograft versus allograft anterior cruciate ligament reconstruction: A prospective, randomized clinical study with a minimum 10-year follow-up. Am J Sports Med. 2015;43:2501–2509. doi: 10.1177/0363546515596406. [DOI] [PubMed] [Google Scholar]
- 21.Lawhorn K.W., Howell S.M., Traina S.M., Gottlieb J.E., Meade T.D., Freedberg H.I. The effect of graft tissue on anterior cruciate ligament outcomes: A multicenter, prospective, randomized controlled trial comparing autograft hamstrings with fresh-frozen anterior tibialis allograft. Arthroscopy. 2012;28:1079–1086. doi: 10.1016/j.arthro.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 22.Noh J.H., Yi S.R., Song S.J., Kim S.W., Kim W. Comparison between hamstring autograft and free tendon Achilles allograft: minimum 2-year follow-up after anterior cruciate ligament reconstruction using EndoButton and Intrafix. Knee Surg Sports Traumatol Arthrosc. 2011;19:816–822. doi: 10.1007/s00167-010-1388-6. [DOI] [PubMed] [Google Scholar]
- 23.Sun K., Zhang J., Wang Y., et al. Arthroscopic reconstruction of the anterior cruciate ligament with hamstring tendon autograft and fresh-frozen allograft: A prospective, randomized controlled study. Am J Sports Med. 2011;39:1430–1438. doi: 10.1177/0363546511400384. [DOI] [PubMed] [Google Scholar]
- 24.Tian S., Wang Y., Wang B., et al. Anatomic double-bundle anterior cruciate ligament reconstruction with a hamstring tendon autograft and fresh-frozen allograft: A prospective, randomized, and controlled study. Arthroscopy. 2016;32:2521–2531. doi: 10.1016/j.arthro.2016.04.013. [DOI] [PubMed] [Google Scholar]
- 25.Yoo S.H., Song E.K., Shin Y.R., Kim S.K., Seon J.K. Comparison of clinical outcomes and second-look arthroscopic findings after ACL reconstruction using a hamstring autograft or a tibialis allograft. Knee Surg Sports Traumatol Arthrosc. 2017;25:1290–1297. doi: 10.1007/s00167-015-3955-3. [DOI] [PubMed] [Google Scholar]
- 26.Higgins J.P., Altman D.G., Gøtzsche P.C., et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barber F.A., Cowden C.H., 3rd, Sanders E.J. Revision rates after anterior cruciate ligament reconstruction using bone-patellar tendon-bone allograft or autograft in a population 25 years old and younger. Arthroscopy. 2014;30:483–491. doi: 10.1016/j.arthro.2013.12.022. [DOI] [PubMed] [Google Scholar]
- 28.Barrett G., Stokes D., White M. Anterior cruciate ligament reconstruction in patients older than 40 years: Allograft versus autograft patellar tendon. Am J Sports Med. 2005;33:1505–1512. doi: 10.1177/0363546504274202. [DOI] [PubMed] [Google Scholar]
- 29.Edgar C.M., Zimmer S., Kakar S., Jones H., Schepsis A.A. Prospective comparison of auto and allograft hamstring tendon constructs for ACL reconstruction. Clin Orthop Relat Res. 2008;466:2238–2246. doi: 10.1007/s11999-008-0305-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kane P.W., Wascher J., Dodson C.C., Hammoud S., Cohen S.B., Ciccotti M.G. Anterior cruciate ligament reconstruction with bone–patellar tendon–bone autograft versus allograft in skeletally mature patients aged 25 years or younger. Knee Surg Sports Traumatol Arthrosc. 2016;24:3627–3633. doi: 10.1007/s00167-016-4213-z. [DOI] [PubMed] [Google Scholar]
- 31.Kustos T., Bálint L., Than P., Bárdos T. Comparative study of autograft or allograft in primary anterior cruciate ligament reconstruction. Int Orthop. 2004;28:290–293. doi: 10.1007/s00264-004-0568-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maletis G.B., Chen J., Inacio M.C.S., Love R.M., Funahashi T.T. Increased risk of revision after anterior cruciate ligament reconstruction with bone–patellar tendon–bone allografts compared with autografts. Am J Sports Med. 2017;45:1333–1340. doi: 10.1177/0363546517690386. [DOI] [PubMed] [Google Scholar]
- 33.Maletis G.B., Chen J., Inacio M.C.S., Love R.M., Funahashi T.T. Increased risk of revision after anterior cruciate ligament reconstruction with soft tissue allografts compared with autografts: Graft processing and time make a difference. Am J Sports Med. 2017;45:1837–1844. doi: 10.1177/0363546517694354. [DOI] [PubMed] [Google Scholar]
- 34.Peterson R.K., Shelton W.R., Bomboy A.L. Allograft versus autograft patellar tendon anterior cruciate ligament reconstruction: A 5-year follow-up. Arthroscopy. 2001;17:9–13. doi: 10.1053/jars.2001.19965. [DOI] [PubMed] [Google Scholar]
- 35.Sterne J.A., Hernan M.A., Reeves B.C., et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McHugh M.L. Interrater reliability: The kappa statistic. Biochem Med (Zagreb) 2012;22:276–282. [PMC free article] [PubMed] [Google Scholar]
- 37.Hefti F., Müller W., Jakob R.P., Stäubli H.U. Evaluation of knee ligament injuries with the IKDC form. Knee Surg Sports Traumatol Arthrosc. 1993;1:226–234. doi: 10.1007/BF01560215. [DOI] [PubMed] [Google Scholar]
- 38.Irrgang J.J., Anderson A.F., Boland A.L., et al. Development and validation of the International Knee Documentation Committee Subjective Knee Form. Am J Sports Med. 2001;29:600–613. doi: 10.1177/03635465010290051301. [DOI] [PubMed] [Google Scholar]
- 39.Lysholm J., Gillquist J. Evaluation of knee ligament surgery results with special emphasis on use of a scoring scale. Am J Sports Med. 1982;10:150–154. doi: 10.1177/036354658201000306. [DOI] [PubMed] [Google Scholar]
- 40.Tegner Y., Lysholm J. Rating systems in the evaluation of knee ligament injuries. Clin Orthop Relat Res. 1985;(198):43–49. [PubMed] [Google Scholar]
- 41.Barber-Westin S.D., Noyes F.R., McCloskey J.W. Rigorous statistical reliability, validity, and responsiveness testing of the Cincinnati Knee Rating System in 350 subjects with uninjured, injured, or anterior cruciate ligament-reconstructed knees. Am J Sports Med. 1999;27:402–416. doi: 10.1177/03635465990270040201. [DOI] [PubMed] [Google Scholar]
- 42.Coleman B.D., Khan H.M., Maffulli N., Cook J.L., Wark J.D. Studies of surgical outcome after patellar tendinopathy: Clinical significance of methodological deficiencies and guidelines for future studies. Victorian Institute of Sport Tendon Study Group. Scand J Med Sci Sports. 2000;10:2–11. doi: 10.1034/j.1600-0838.2000.010001002.x. [DOI] [PubMed] [Google Scholar]
- 43.Kjaergaard J., Faunø L.Z., Faunø P. Sensibility loss after ACL reconstruction with hamstring graft. Int J Sports Med. 2008;29:507–511. doi: 10.1055/s-2008-1038338. [DOI] [PubMed] [Google Scholar]
- 44.Hardy A., Casabianca L., Andrieu K., Baverel L., Noailles T., Junior French Arthroscopy Society Complications following harvesting of patellar tendon or hamstring tendon grafts for anterior cruciate ligament reconstruction: Systematic review of literature. Orthop Traumatol Surg Res. 2017;103:S245–S248. doi: 10.1016/j.otsr.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 45.Rosenberg T.D., Franklin J.L., Baldwin G.N., Nelson K.A. Extensor mechanism function after patellar tendon graft harvest for anterior cruciate ligament reconstruction. Am J Sports Med. 1992;20:519–525. doi: 10.1177/036354659202000506. [DOI] [PubMed] [Google Scholar]
- 46.Xergia S.A., McClelland J.A., Kvist J., Vasiliadis H.S., Georgoulis A.D. The influence of graft choice on isokinetic muscle strength 4-24 months after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2011;19:768–780. doi: 10.1007/s00167-010-1357-0. [DOI] [PubMed] [Google Scholar]
- 47.Mistry H., Metcalfe A., Colquitt J., et al. Autograft or allograft for reconstruction of anterior cruciate ligament: A health economics perspective. Knee Surg Sports Traumatol Arthrosc. 2019;27:1782–1790. doi: 10.1007/s00167-019-05436-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barbour S.A., King W. The safe and effective use of allograft tissue—an update. Am J Sports Med. 2003:31791–31797. doi: 10.1177/03635465030310052801. [DOI] [PubMed] [Google Scholar]
- 49.Tomford W.W. Transmission of disease through transplantation of musculoskeletal allografts. J Bone Joint Surg Am. 1995;77:1742–1754. doi: 10.2106/00004623-199511000-00017. [DOI] [PubMed] [Google Scholar]
- 50.Farago D., Kozma B., Kiss R.M. Different sterilization and disinfection methods used for human tendons—a systematic review using mechanical properties to evaluate tendon allografts. BMC Musculoskelet Disord. 2021;22:404. doi: 10.1186/s12891-021-04296-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.