Key Points
Question
Do patients with atopic dermatitis (AD), particularly those receiving treatment with Janus kinase (JAK) inhibitors, have an increased risk for venous thromboembolism (VTE)?
Findings
This systematic review and meta-analysis included 2 cohort studies and 15 randomized clinical trials with 466 993 participants. The analysis found no significant association of AD with incident VTE nor an increased risk of incident VTE among participants with AD who were receiving JAK inhibitors.
Meaning
The study results indicate that the evidence to date is insufficient to determine a significant association of AD with VTE and support the current warning of VTE being associated with JAK inhibitors in treating AD; future real-world long-term data are warranted.
Abstract
Importance
The risk of venous thromboembolism (VTE) among patients with atopic dermatitis (AD), especially when receiving treatment with Janus kinase (JAK) inhibitors, is unclear.
Objective
To determine the association of AD with incident VTE and evaluate the risk of incident VTE among patients with AD who were receiving treatment with JAK inhibitors.
Data Sources
The MEDLINE, Embase, Cochrane Library, and Web of Science databases were searched with no restrictions on language nor geographic locations from their respective inception to February 5, 2022.
Study Selection
Cohort studies examining the association of AD with incident VTE and randomized clinical trials (RCTs) reporting VTE events in participants with AD receiving JAK inhibitors were included. Around 0.7% of initially identified articles met the selection criteria.
Data Extraction and Synthesis
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline was followed. The risk of bias of included cohort studies and RCTs was assessed by the Newcastle-Ottawa Scale and the Cochrane Risk of Bias Tool 2, respectively. A random-effects model meta-analysis was conducted to calculate the pooled hazard ratio (HR) and risk difference for incident VTE.
Main Outcomes and Measures
The HRs for incident VTE associated with AD and risk difference for incident VTE between participants with AD who were receiving treatment with JAK inhibitors and controls receiving placebo or dupilumab.
Results
Two cohort studies and 15 RCTs with a total of 466 993 participants were included. The meta-analysis found no significant association of AD with incident VTE (HR, 0.95; 95% CI 0.62-1.45; incidence rate of VTE, 0.23 events/100 patient-years). Overall, 3 of 5722 patients with AD (0.05%) who were receiving treatment with JAK inhibitors experienced VTE compared with 1 of 3065 patients with AD (0.03%) receiving placebo or dupilumab (Mantel-Haenszel risk difference, 0; 95% CI, 0-0). The incidence rate of VTE was 0.15 and 0.12 events per 100 patient-years in participants with AD receiving JAK inhibitors and placebo, respectively. The findings were similar in 4 unique JAK inhibitors (abrocitinib, baricitinib, upadacitinib, and SHR0302).
Conclusions and Relevance
The results of this systematic review and meta-analysis suggest that the currently available evidence does not detect an increased risk of VTE associated with AD or treatment with JAK inhibitors. These findings may provide a reference for clinicians in prescribing JAK inhibitors for patients with AD.
This systematic review and meta-analysis examines the risk of incident venous thromboembolism among patients with atopic dermatitis who were receiving treatment with Janus kinase inhibitors.
Introduction
Atopic dermatitis (AD) is a prevalent inflammatory dermatosis with increasing worldwide prevalence.1,2 Symptoms, such as relapsing pruritus and visible lesions, may last for a few years and even persist throughout life, necessitating effective treatments.3,4 Janus kinase (JAK) inhibitors have become a promising treatment option for patients with AD because they have been associated with favorable clinical outcomes in clinical trials.5
Venous thromboembolism (VTE), including deep vein thrombosis (DVT) and pulmonary embolism (PE), is a potentially life-threatening illness associated with high recurrence rates and mortality.6,7 While several studies have investigated the potential associations of AD with VTE, the results were inconsistent.8,9 Because the US Food and Drug Administration issued a black box warning about an increased risk of blood clots for tofacitinib, baricitinib, and upadacitinib in treating arthritis and other chronic inflammatory conditions, concerns have been raised regarding the risk of VTE being associated with JAK inhibitor therapy.10 Previous meta-analyses regarding several immune-mediated inflammatory diseases, such as psoriasis, rheumatoid arthritis, and inflammatory bowel disease, have found no significant increased risk of VTE in patients receiving treatment with JAK inhibitors.11,12 However, a recent randomized clinical trial reported a 3.52-fold increased risk of VTE associated with treatment with tofacitinib, 10 mg, twice daily, for rheumatoid arthritis among patients 50 years or older who had at least 1 additional cardiovascular risk factor.13 Although JAK inhibitors have been approved in treating AD over recent years, to our knowledge, there has been no systematic evaluation of the risk of VTE among patients with AD and the corresponding safety profile of JAK inhibitors in treating AD. To address this knowledge gap, this systematic review and meta-analysis aimed to evaluate the current evidence on the association of AD with incident VTE and the risk of incident VTE in patients with AD who were receiving treatment with JAK inhibitors.
Methods
This systematic review and meta-analysis followed the Meta-analysis of Observational Studies in Epidemiology (MOOSE)14 and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.15 The prespecified study protocol was registered with PROSPERO (CRD42022307784). The study was granted an exemption from institutional review board approval from the Chang Gung Medical Foundation.
Data Sources and Evidence Search
A comprehensive literature search was performed on electronic databases (including MEDLINE, Embase, Cochrane Library, and Web of Science) from their respective inception to February 5, 2022. The search strategy was designed with the assistance of an experienced librarian (L.C.) (eTable in the Supplement). There were neither language nor geographic limitations. The references of eligible studies and relevant reviews were also manually scrutinized for additional studies.
Eligibility Criteria
To investigate the association of AD with incident VTE, we first included studies that met the following criteria: (1) cohort studies examining the association of AD with incident VTE; (2) an exposure group comprising individuals with AD and a nonexposure control group comprising people without AD; (3) reporting the risk estimates of incident VTE. We only included cohort studies to reduce recall bias and examine the temporal association between AD and incident VTE.
To further evaluate the risk of VTE in patients with AD who were treated with JAK inhibitors, we included studies that fulfilled the following criteria: (1) phase 2 and phase 3 randomized clinical trials (RCTs) investigating the safety of JAK inhibitors for patients with AD; (2) an intervention group comprising participants with AD receiving treatment with JAK inhibitors and a control group comprising participants with AD receiving either placebo or dupilumab; (3) reporting the number of VTE events. Open-label or long-term extension studies without a control arm were excluded. Studies with patients using topical JAK inhibitors (eg, delgocitinib, ruxolitinib, and tofacitinib) were also excluded.
When multiple studies presented results from the same database or RCT, we only included the one with the most comprehensive data. We only included studies with confirmed diagnoses of AD and VTE by validated diagnostic codes or clinical criteria. Review articles, editorials, case reports, cross-sectional studies, case-control studies, and studies with nonhuman participants were excluded. Two independent authors (T.C. and L.L.) selected studies by screening the titles and abstracts of the initial literature search. The full text of potentially relevant publications was retrieved for confirming eligibility. Disagreements were resolved through discussion with 2 senior authors (H.H. and C.C.) until reaching consensus.
Data Extraction and Risk of Bias Assessment
Two authors (T.C. and L.L.) independently collated the following data using a standardized data sheet: first author, year of publication, country, database or clinical trial identifier, study period, patient characteristics (sample size, age, and sex), definition of AD, and outcomes of interest (risk estimates or the number of VTE events). For the included cohort studies, we extracted the adjusted hazard ratio (HR) with 95% CIs. The risk estimates with the most appropriate adjustment for confounders, such as age, sex, and comorbidities, were used for data synthesis. For the included RCTs, we extracted the number of VTE events. Both DVT and PE were considered VTE events. For studies that did not report sufficient information for meta-analysis, we contacted the corresponding authors for additional data.
The risk of bias of included cohort studies was assessed using the Newcastle-Ottawa Scale,16 whereas that of RCTs was appraised using the Cochrane risk of bias tool (RoB 2).17 Two authors (T.C. and L.L.) independently performed the risk of bias assessment. Any discrepancy in the extracted data and risk of bias assessment was resolved by discussion with senior authors (H.H. and C.C.).
Statistical Analysis
We used Review Manager, version 5.4.1 (The Cochrane Collaboration, 2020) to conduct meta-analyses.18 Two meta-analyses were separately performed for cohort studies (the association of AD with incident VTE) and RCTs (the risk of incident VTE among patients with AD who were receiving treatment with JAK inhibitors). A P value of <.05 was deemed significant. The pooled HRs and corresponding CIs were synthesized to determine the association of AD with incident VTE. Absolute risk differences were used to measure the risk of incident VTE in patients receiving treatment with various JAK inhibitors when compared with those using placebo or dupilumab.19 The random-effects model was applied for meta-analyses based on the assumption of considerable clinical heterogeneity.20 For RCTs with 0 events of VTE reported in both arms, a fixed value of 0.5 was added to correct for computational errors.18 Heterogeneity between individual studies was quantified using the I2 statistics, with an I2 of greater than 50% indicating at least moderate heterogeneity. Publication bias was evaluated by inspecting funnel plots if there were 10 or more studies for an outcome.
Results
Selection of Studies
The PRISMA study flow diagrams for the cohort study and RCT selection are illustrated in Figure 1. A total of 2370 citations were obtained from the initial database search. After removal of duplicates and screening of titles and abstracts, the full text of 135 records was assessed for eligibility (25 studies [18.5%] and 110 studies [81.5%] in the selection of cohort studies and RCTs, respectively). Eventually, we included 2 cohort studies21,22 and 15 RCTs23,24,25,26,27,28,29,30,31,32,33,34,35,36,37 with a total of 466 993 participants. Only 0.7% of initially identified studies fulfilled the selection criteria.
Figure 1. PRISMA Flow Diagrams for the Selection of Cohort Studies and Randomized Clinical Trials.
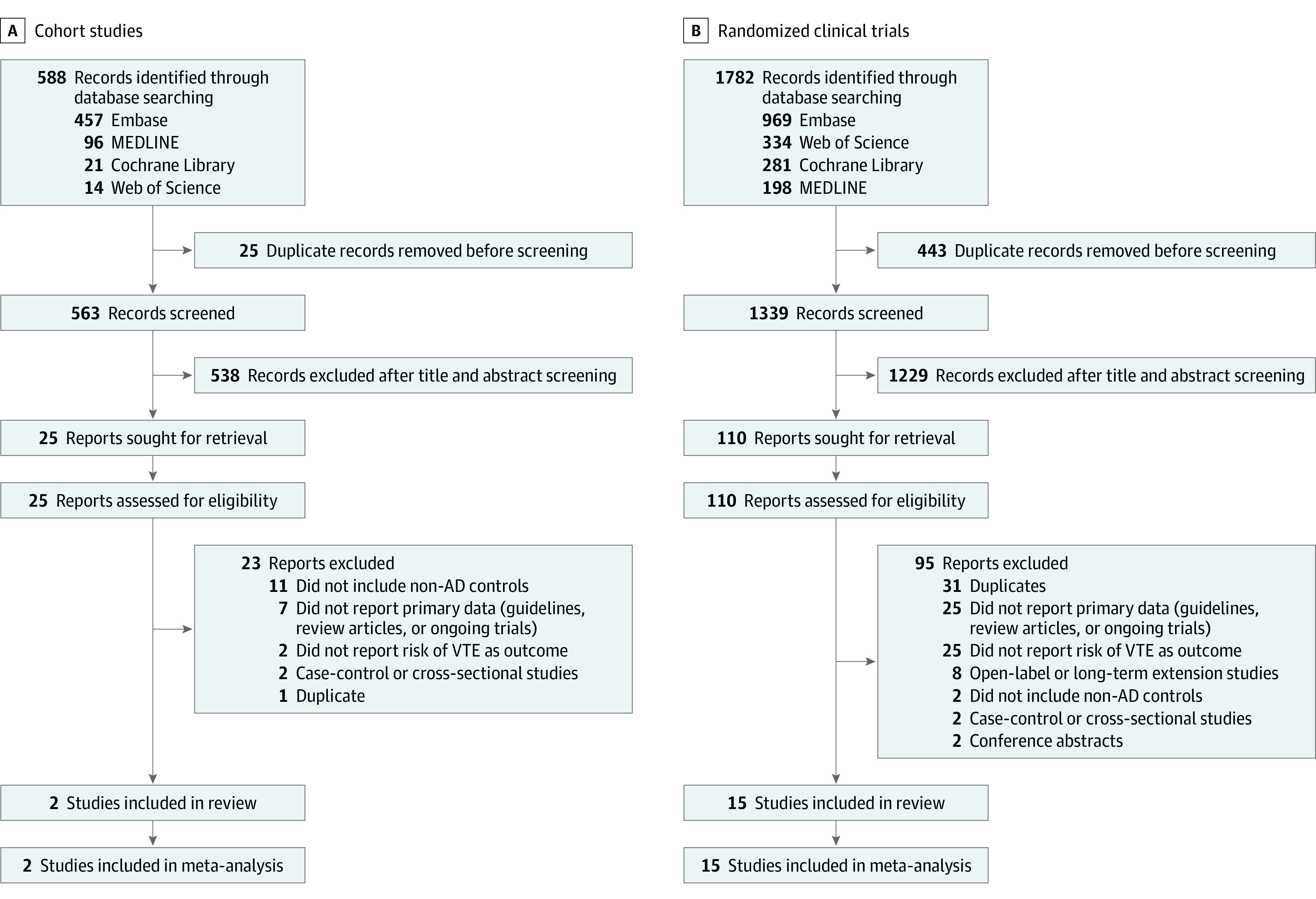
AD indicates atopic dermatitis; VTE, venous thromboembolism.
Characteristics of Included Studies
Table 121,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37 outlines the characteristics of the included studies. The two cohort studies were conducted in the US, whereas most RCTs were conducted across multiple countries. The definition of AD in cohort studies was based on validated International Classification of Diseases, Ninth Revision (ICD-9) and ICD-10 codes.38 The diagnosis of AD in RCTs was based on the Hanifin and Rajka criteria,39 diagnostic criteria proposed by the American Academy of Dermatology,40 and Chinese criteria.41 The participants in the included RCTs were primarily young and middle-aged adults; however, the study by Eichenfield et al26 included mainly adolescents. Four JAK inhibitors (abrocitinib, baricitinib, upadacitinib, and SHR0302) were investigated.
Table 1. Characteristics of Included Studies.
| Source | Cohort studies | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Database | Study period | No. of participants, AD/non-AD | Age, mean (SD), y | Sex, female % | Definition of AD | VTE, HRs (95% CI) | VTE incidence rates per 100 patient-years | |||||||
| Meyers et al,21 2021 | IBM MarketScan Commercial Claims and Encounters, Medicare Supplemental, and Medicaid databases | 2012-2017 | 198 685/198 685 | 46.0 (17.0) | 65.2 | ICD-9 code 691.8 or ICD-10 code L20.0, L20.8x, L20.9 | 0.77 (0.69-0.85) | 0.24 | ||||||
| Schneeweiss et al,22 2021 | Optum’s deidentified Clinformatics Data Mart Database | 2004-2019 | 30 418/30 418 | 47.5 (18.0) | 60.8 | ICD-9 code 691.8 or ICD-10 code L20, L20.82, L20.84 | 1.19 (0.95-1.48) | 0.18 | ||||||
| Source | Randomized clinical trials | |||||||||||||
| Clinical trial identifier | JAK inhibitor | No. of patients with AD, intervention group/control group | Age, mean (SD), y | Sex, female % | Definition of AD | Placebo-controlled phase, weeks | ||||||||
| Bieber et al,23 2021 | NCT03720470 (JADE COMPARE) | Abrocitinib | 464/131 | 38.0 (14.7) | 51.7 | Hanifin and Rajka criteria | 12 | |||||||
| Blauvelt et al,24 2021 | NCT03738397 (Heads up) | Upadacitinib | 348/344 | 36.6 (14.6) | 47.4 | Hanifin and Rajka criteria | 24 | |||||||
| Blauvelt et al,24 2022 | NCT03627767 (JADE REGIMEN) | Abrocitinib | 531/267 | Median (IQR), 29.0 (20-41) | 43.9 | Hanifin and Rajka criteria | 40 | |||||||
| Eichenfield et al,26 2021 | NCT03796676 (JADE TEEN) | Abrocitinib | 189/96 | Median (IQR), 16.0 (14-17) | 46.6 | Hanifin and Rajka criteria | 12 | |||||||
| Gooderham et al,27 2019 | NCT02780167 | Abrocitinib | 211/56 | 40.4 (16.3) | 51.7 | Hanifin and Rajka criteria | 12 | |||||||
| Guttman-Yassky et al,28 2020 | NCT02925117 | Upadacitinib | 126/40 | 40.0 (15.3) | 36.5 | Hanifin and Rajka criteria | 16 | |||||||
| Guttman-Yassky et al,29 2021 | NCT03569293 (Measure Up 1) and NCT03607422 (Measure Up 2) | Upadacitinib | 1124/559 | Median (IQR), 33.8 (12-75) | 44.0 | Hanifin and Rajka criteria | 16 | |||||||
| Katoh et al,30 2022 | NCT03661138 (Rising Up) | Upadacitinib | 182/90 | 35.5 (13.0) | 24.7 | Hanifin and Rajka criteria | 24 | |||||||
| Reich et al,31 2020 | NCT03733301 (BREEZE-AD7) | Baricitinib | 220/108 | 33.9 (12.1) | 34.1 | Diagnostic criteria proposed by AAD guideline | 16 | |||||||
| Reich et al,32 2021 | NCT03568318 (AD Up) | Upadacitinib | 597/303 | Median (IQR), 34.0 (12-74) | 38.2 | Hanifin and Rajka criteria | 16 | |||||||
| Silverberg et al,33 2020 | NCT03575871 (JADE MONO-2) | Abrocitinib | 313/78 | 35.5 (15.3) | 41.9 | Hanifin and Rajka criteria | 12 | |||||||
| Simpson et al,34 2020 | NCT03349060 (JADE MONO-1) | Abrocitinib | 310/77 | 32.8 (16.4) | 44.8 | Hanifin and Rajka criteria | 12 | |||||||
| Simpson et al,35 2020 (Simpson 2020a) | NCT03334396 (BREEZE-AD1) and NCT03334422 (BREEZE-AD2) | Baricitinib | 745/493 | 35.0 (12.8) | 37.0 | Diagnostic criteria proposed by AAD guideline | 16 | |||||||
| Simpson et al,36 2021 (Simpson 2020b) | NCT03435081 (BREEZE-AD5) | Baricitinib | 292/146 | 40.0 (16.0) | 50.9 | Diagnostic criteria proposed by AAD guideline | 16 | |||||||
| Zhao et al,37 2021 | NCT04162899 | SHR0302 | 70/35 | 36.9 (14.8) | 38.6 | Chinese criteria | 12 | |||||||
Abbreviations: AAD, American Academy of Dermatology; AD, atopic dermatitis; HR, hazard ratio; ICD, International Classification of Diseases; VTE, venous thromboembolism.
Risk of Bias Assessment
The risk of bias assessment is summarized in Table 2.21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37 The 2 cohort studies were considered of high quality on the Newcastle-Ottawa Scale.21,22 As to bias in outcome measurement, 5 RCTs were rated as some concerns because there was no information about whether the outcome assessors were masked to the treatments when adjudicating VTE events.
Table 2. Summary of Risk of Bias Assessment.
| Source | Cohort studiesa | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Representativeness of exposed group | Selection of nonexposed group | Ascertainment of exposure | Outcome of interest not present at start of study | Comparability of cohort | Assessment of outcome | Was follow-up long enough for outcomes to occur? | Adequacy of follow-up of cohorts | |||||
| Meyers et al,21 2021 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||||
| Schneeweiss et al,22 2021 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||||
| Source | Randomized clinical trialsb | |||||||||||
| Bias arising from the randomization process | Bias because of deviations from intended interventions | Bias because of missing outcome data | Bias in measurement of the outcome | Bias in selection of the reported result | ||||||||
| Bieber et al,23 2021 | Low | Low | Low | Low | Low | |||||||
| Blauvelt et al,24 2021 | Low | Low | Low | Low | Low | |||||||
| Blauvelt et al,25 2022 | Low | Low | Low | Some concerns | Low | |||||||
| Eichenfield et al,26 2021 | Low | Low | Low | Low | Low | |||||||
| Gooderham et al,27 2019 | Low | Low | Low | Some concerns | Low | |||||||
| Guttman-Yassky et al,28 2020 | Low | Low | Low | Some concerns | Low | |||||||
| Guttman-Yassky et al,29 2021 | Low | Low | Low | Low | Low | |||||||
| Katoh et al,30 2022 | Low | Low | Low | Some concerns | Low | |||||||
| Reich et al,31 2020 | Low | Low | Low | Low | Low | |||||||
| Reich et al,32 2021 | Low | Low | Low | Low | Low | |||||||
| Silverberg et al,33 2020 | Low | Low | Low | Low | Low | |||||||
| Simpson et al,34 2020 (Simpson 2020a) | Low | Low | Low | Low | Low | |||||||
| Simpson et al,35 2020 (Simpson 2020b) | Low | Low | Low | Low | Low | |||||||
| Simpson et al,36 2021 | Low | Low | Low | Low | Low | |||||||
| Zhao et al,37 2021 | Low | Low | Low | Some concerns | Low | |||||||
Risk of bias assessed by the Newcastle-Ottawa Scale. In each domain, 1 corresponds to low risk of bias and 0 corresponds to high risk of bias. Studies with a total score (namely the sum score of all domains) of 7 or more points were considered high-quality studies.
Risk of bias assessed by the Cochrane Risk of Bias Tool (RoB, version 2.0).
Association of AD With Incident VTE
Two studies with 458 206 participants (397 370 and 60 836 participants in Meyers et al21 and Schneeweiss et al,22 respectively) examined the association of AD with incident VTE. The crude incidence rate of VTE among patients with AD was 0.24 and 0.18 events per 100 patient-years in Meyers et al21 and Schneeweiss et al,22 respectively. As illustrated in Figure 2, the risk for incident VTE did not significantly increase among patients with AD compared with non-AD controls (pooled HR, 0.95; 95% CI, 0.62-1.45; I2 = 92%). The overall incidence rate of VTE for patients with AD was 0.23 events per 100 patient-years.
Figure 2. Association of Atopic Dermatitis (AD) With Incident Venous Thromboembolism (VTE).
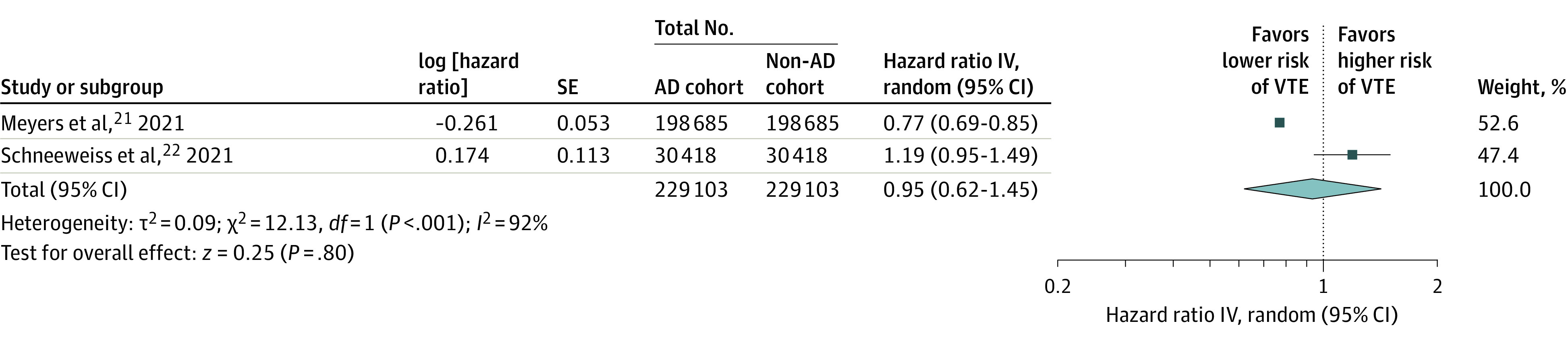
The meta-analysis found no significant association of AD with incident VTE (hazard ratio [HR], 0.95; 95% CI, 0.62-1.45). IV indicates inverse variance.
Risk of Incident VTE in Participants With AD Receiving Treatment With Janus Kinase Inhibitors
Fifteen studies with 8787 participants provided data on VTE events in patients with AD who were receiving treatment with JAK inhibitors.23,24,25,26,27,28,29,30,31,32,33,34,35,36,37 Overall, 3 of 5722 participants (0.05%) with AD receiving JAK inhibitors experienced VTE events compared with 1 of 3065 participants (0.03%) with AD receiving placebo or dupilumab. As demonstrated in eFigure 1 in the Supplement, the meta-analysis found no significant difference in the risk of incident VTE between participants with AD receiving JAK inhibitors and controls with AD receiving placebo or dupilumab (Mantel-Haenszel risk difference, 0; 95% CI, 0-0; I2 = 0%). The findings were similar across all 4 JAK inhibitors. During the placebo-controlled phase across all RCTs, the overall incidence rate of VTE was 0.15 events per 100 patient-years among participants with AD who were receiving JAK inhibitors, while the incidence rate of VTE in participants with AD receiving placebo was 0.12 events per 100 patient-years. No VTE events were recorded in the trials comparing JAK inhibitors with dupilumab. No publication bias was identified by inspecting the funnel plot (eFigure 2 in the Supplement).
Discussion
To our knowledge, this study is the first meta-analysis to investigate the risk of incident VTE in patients with AD. The evidence from cohort studies revealed no statistically significant association between AD and incident VTE. The overall incidence rate of VTE was 0.23 events per 100 patient-years among patients with AD. Meanwhile, the current evidence from RCTs demonstrated no significant differences in the risk of incident VTE between patients with AD who were receiving treatment with JAK inhibitors and patients with AD receiving placebo or dupilumab. The incidence rate of VTE was 0.15 events per 100 patient-years in patients with AD receiving JAK inhibitors.
According to a recently published guideline,42 AD has been associated with few cardiovascular comorbidities.43 The associations are not as strong as those observed with psoriasis,44,45 which do not prompt cardiovascular screening or treatment for individuals with AD. In the case of VTE, the results of the present meta-analysis suggested that AD is not a risk factor for incident VTE. By contrast, a cross-sectional survey conducted by Shaheen et al9 reported a positive association between AD and prevalent VTE (adjusted odds ratio, 1.22; 95% CI, 1.17-1.27). In a case-control study carried out by Undas et al,46 atopic diseases were more prevalent in patients with VTE (38%) than non-VTE controls (23%). Nevertheless, these studies could not assess causality and did not adjust important confounders, such as hormone therapies and smoking status.47,48 Another study using an administrative claims database from the US presented subgroup data of VTE and showed a trend of lower risks of DVT and PE in patients with AD.8
The changes in the coagulation and fibrinolysis system among patients with AD are still unclear.49,50 Elevated plasma levels of platelet activation factors have been observed in patients with AD.51 However, these coagulation markers were not associated with AD severity.52,53 Inflammatory cytokines in AD, such as interleukin (IL)–4 and IL-13, may interact with coagulation cascades in animal models.54,55 Studies have also found that proinflammatory mast cells and tryptases in AD could modulate fibrinolysis, in which is associated with a decreased risk of thrombi formation.56 More research is needed to understand whether the laboratory findings in patients with AD are clinically relevant.
Among the included RCTs, 3 VTE events occurred in participants who were receiving treatment with JAK inhibitors during the randomized placebo-controlled periods. In the JADE REGIMEN trial, a patient receiving abrocitinib, 100 mg, once daily, experienced sudden vision loss and received a diagnosis of retinal vein thrombosis.25,30 Another participant receiving abrocitinib, 200 mg, once daily, was also documented as having PE, which was not considered treatment-related by Gooderham et al.27 The other event of PE was reported with a patient receiving treatment with baricitinib, 4 mg, once daily, in the BREEZE-AD7 trial.31 However, recent studies have provided additional data on VTE in patients with AD who were receiving treatment with JAK inhibitors during the open-label extension periods. A safety analysis of abrocitinib that combined the results of 5 RCTs and a long-term extension study (JADE EXTEND) reported 5 adjudicated VTE events, all in the 200-mg group.57 In a post hoc analysis focusing on 2-mg baricitinib exclusively, no VTE events were recorded.58 During the 52-week follow-up of AD Up trials, 1 patient using upadacitinib received an incidental diagnosis of PE.59 In the present meta-analysis of JAK inhibitors, comparable risks of VTE were indicated in patients receiving JAK inhibitors and those receiving placebo or dupilumab. These results were consistent with previous meta-analyses of JAK inhibitors regarding multiple indications.11,12 Controlling inflammation via inhibition of the JAK pathways may reduce platelet activity and lower VTE events. Dose-response decreases in platelet counts have been observed in several RCTs among patients with AD treated with JAK inhibitors.25,26,60However, some of the included trials did not outline VTE risk factors of the enrollees at baseline (eg, prior VTE or recent surgical history),61 which might have introduced selection bias in reporting VTE events. With the increasing applications of JAK inhibitors in AD, more clinical data are needed to identify patients at high risk for VTE.
Strengths and Limitations
The major strength of this systematic review and meta-analysis is the inclusion of population-based cohort studies and high-quality RCTs to provide up-to-date evidence. The existing evidence from cohort studies suggests that AD is not associated with incident VTE. The evidence from RCTs indicates retention of a null hypothesis of no difference in the risk of VTE between patients with AD who were receiving treatment with JAK inhibitors and controls receiving placebo or dupilumab. Nevertheless, the results of this study need to be considered with some limitations. First, the generalizability may be limited because the included studies were mainly conducted in Western countries. Second, statistical heterogeneity was present in the meta-analysis on the association between AD and VTE. A subgroup analysis or meta-regression to identify potential effect modifiers was infeasible because of the limited number of studies (n = 2). While the crude incidence rate was similar between the 2 included cohort studies, the adjusted HRs were different. Imbalances in health care utilization and differences in confounding adjustments might explain the different risk estimates. Third, the included studies did not report the VTE risk among different patterns of AD (ie, persistent, relapsing, or adulthood-onset forms). Because age is an important risk factor for VTE,61 a subanalysis according to the onset of AD is encouraged. Fourth, the included RCTs may be underpowered or of too short duration to detect rare or long-term adverse events, such as VTE. Given the 5722 patients with AD in the JAK inhibitor group and 3065 patients in the control group, the number of participants in both arms may still be too low for inferences about safety. Hence, the results from the trial-based meta-analysis must be interpreted with caution.
Conclusions
The results of this systematic review and meta-analysis suggest that evidence from cohort studies does not detect an increased risk of incident VTE among patients with AD, nor does the evidence from RCTs detect significant differences in the risk of incident VTE between patients with AD who were receiving treatment with JAK inhibitors and controls receiving placebo or dupilumab. These findings may provide a reference for clinicians in prescribing JAK inhibitors for patients with AD. Further evidence from real-world data on longer-term safety are warranted.
eTable. Search Strategies for (a) MEDLINE, (b) Embase, (c) Cochrane Library, and (d) Web of Science
eFigure 1. Risk Differences in Venous Thromboembolism Between Participants With Atopic Dermatitis Receiving Janus Kinase Inhibitors and Controls Receiving Placebo or Dupilumab
eFigure 2. Funnel Plot of Randomized Controlled Trials
References
- 1.Langan SM, Irvine AD, Weidinger S. Atopic dermatitis. Lancet. 2020;396(10247):345-360. doi: 10.1016/S0140-6736(20)31286-1 [DOI] [PubMed] [Google Scholar]
- 2.Wang CH, Fu Y, Chi CC. Association of atopic dermatitis with inflammatory bowel disease: a systematic review and meta-analysis. Dermatol Sin. 2020;38(3):159-165. doi: 10.4103/ds.ds_20_20 [DOI] [Google Scholar]
- 3.Sidbury R, Davis DM, Cohen DE, et al. ; American Academy of Dermatology . Guidelines of care for the management of atopic dermatitis: section 3: management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71(2):327-349. doi: 10.1016/j.jaad.2014.03.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ee S, Tay YK, Chu CY, Hon KL, Leong KF, Wananukul S. A study on the knowledge, attitudes, and practices of Asian dermatologists in the management of atopic dermatitis. Dermatol Sin. 2020;38(2):67-80. doi: 10.4103/ds.ds_31_19 [DOI] [Google Scholar]
- 5.Chovatiya R, Paller AS. JAK inhibitors in the treatment of atopic dermatitis. J Allergy Clin Immunol. 2021;148(4):927-940. doi: 10.1016/j.jaci.2021.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arshad N, Bjøri E, Hindberg K, Isaksen T, Hansen JB, Braekkan SK. Recurrence and mortality after first venous thromboembolism in a large population-based cohort. J Thromb Haemost. 2017;15(2):295-303. doi: 10.1111/jth.13587 [DOI] [PubMed] [Google Scholar]
- 7.Søgaard KK, Schmidt M, Pedersen L, Horváth-Puhó E, Sørensen HT. 30-year mortality after venous thromboembolism: a population-based cohort study. Circulation. 2014;130(10):829-836. doi: 10.1161/CIRCULATIONAHA.114.009107 [DOI] [PubMed] [Google Scholar]
- 8.Setyawan J, Mu F, Yarur A, et al. Risk of thromboembolic events and associated risk factors, including treatments, in patients with immune-mediated diseases. Clin Ther. 2021;43(8):1392-1407.e1. doi: 10.1016/j.clinthera.2021.06.008 [DOI] [PubMed] [Google Scholar]
- 9.Shaheen MS, Silverberg JI. Association of inflammatory skin diseases with venous thromboembolism in US adults. Arch Dermatol Res. 2021;313(4):281-289. doi: 10.1007/s00403-020-02099-6 [DOI] [PubMed] [Google Scholar]
- 10.US Food and Drug Administration . FDA requires warnings about increased risk of serious heart-related events, cancer, blood clots, and death for JAK inhibitors that treat certain chronic inflammatory conditions. Accessed January 9, 2022. https://www.fda.gov/drugs/drug-safety-and-availability/fda-requires-warnings-about-increased-risk-serious-heart-related-events-cancer-blood-clots-and-death
- 11.Yates M, Mootoo A, Adas M, et al. Venous thromboembolism risk with JAK inhibitors: a meta-analysis. Arthritis Rheumatol. 2021;73(5):779-788. doi: 10.1002/art.41580 [DOI] [PubMed] [Google Scholar]
- 12.Bilal J, Riaz IB, Naqvi SAA, et al. Janus kinase inhibitors and risk of venous thromboembolism: a systematic review and meta-analysis. Mayo Clin Proc. 2021;96(7):1861-1873. doi: 10.1016/j.mayocp.2020.12.035 [DOI] [PubMed] [Google Scholar]
- 13.Ytterberg SR, Bhatt DL, Mikuls TR, et al. ; ORAL Surveillance Investigators . Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med. 2022;386(4):316-326. doi: 10.1056/NEJMoa2109927 [DOI] [PubMed] [Google Scholar]
- 14.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA. 2000;283(15):2008-2012. doi: 10.1001/jama.283.15.2008 [DOI] [PubMed] [Google Scholar]
- 15.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372(71):n71. doi: 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analysis. Accessed November 23, 2006. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 17.Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 18.Higgins JPT, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions Version 6.2). Cochrane; 2021. [Google Scholar]
- 19.Ryan C, Leonardi CL, Krueger JG, et al. Association between biologic therapies for chronic plaque psoriasis and cardiovascular events: a meta-analysis of randomized controlled trials. JAMA. 2011;306(8):864-871. doi: 10.1001/jama.2011.1211 [DOI] [PubMed] [Google Scholar]
- 20.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557-560. doi: 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meyers KJ, Silverberg JI, Rueda MJ, et al. Risk of venous thromboembolism among patients with atopic dermatitis: a cohort study in a US administrative claims database. Dermatol Ther (Heidelb). 2021;11(3):1041-1052. doi: 10.1007/s13555-021-00538-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schneeweiss MC, Kim SC, Wyss R, et al. Incidence of venous thromboembolism in patients with dermatologist-diagnosed chronic inflammatory skin diseases. JAMA Dermatol. 2021;157(7):805-816. doi: 10.1001/jamadermatol.2021.1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bieber T, Simpson EL, Silverberg JI, et al. ; JADE COMPARE Investigators . Abrocitinib versus placebo or dupilumab for atopic dermatitis. N Engl J Med. 2021;384(12):1101-1112. doi: 10.1056/NEJMoa2019380 [DOI] [PubMed] [Google Scholar]
- 24.Blauvelt A, Teixeira HD, Simpson EL, et al. Efficacy and safety of upadacitinib vs dupilumab in adults with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2021;157(9):1047-1055. doi: 10.1001/jamadermatol.2021.3023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blauvelt A, Silverberg JI, Lynde CW, et al. Abrocitinib induction, randomized withdrawal, and retreatment in patients with moderate-to-severe atopic dermatitis: results from the JAK1 Atopic Dermatitis Efficacy and Safety (JADE) REGIMEN phase 3 trial. J Am Acad Dermatol. 2022;86(1):104-112. doi: 10.1016/j.jaad.2021.05.075 [DOI] [PubMed] [Google Scholar]
- 26.Eichenfield LF, Flohr C, Sidbury R, et al. Efficacy and safety of abrocitinib in combination with topical therapy in adolescents with moderate-to-severe atopic dermatitis: the JADE TEEN randomized clinical trial. JAMA Dermatol. 2021;157(10):1165-1173. doi: 10.1001/jamadermatol.2021.2830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gooderham MJ, Forman SB, Bissonnette R, et al. Efficacy and safety of oral Janus kinase 1 inhibitor abrocitinib for patients with atopic dermatitis: a phase 2 randomized clinical trial. JAMA Dermatol. 2019;155(12):1371-1379. doi: 10.1001/jamadermatol.2019.2855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guttman-Yassky E, Thaçi D, Pangan AL, et al. Upadacitinib in adults with moderate to severe atopic dermatitis: 16-week results from a randomized, placebo-controlled trial. J Allergy Clin Immunol. 2020;145(3):877-884. doi: 10.1016/j.jaci.2019.11.025 [DOI] [PubMed] [Google Scholar]
- 29.Guttman-Yassky E, Teixeira HD, Simpson EL, et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): results from two replicate double-blind, randomised controlled phase 3 trials. Lancet. 2021;397(10290):2151-2168. doi: 10.1016/S0140-6736(21)00588-2 [DOI] [PubMed] [Google Scholar]
- 30.Katoh N, Ohya Y, Murota H, et al. A phase 3 randomized, multicenter, double-blind study to evaluate the safety of upadacitinib in combination with topical corticosteroids in adolescent and adult patients with moderate-to-severe atopic dermatitis in Japan (Rising Up): An interim 24-week analysis. JAAD Int. 2021;6:27-36. doi: 10.1016/j.jdin.2021.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reich K, Kabashima K, Peris K, et al. Efficacy and safety of baricitinib combined with topical corticosteroids for treatment of moderate to severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156(12):1333-1343. doi: 10.1001/jamadermatol.2020.3260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reich K, Teixeira HD, de Bruin-Weller M, et al. Safety and efficacy of upadacitinib in combination with topical corticosteroids in adolescents and adults with moderate-to-severe atopic dermatitis (AD Up): results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2021;397(10290):2169-2181. doi: 10.1016/S0140-6736(21)00589-4 [DOI] [PubMed] [Google Scholar]
- 33.Silverberg JI, Simpson EL, Thyssen JP, et al. Efficacy and safety of abrocitinib in patients with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156(8):863-873. doi: 10.1001/jamadermatol.2020.1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simpson EL, Sinclair R, Forman S, et al. Efficacy and safety of abrocitinib in adults and adolescents with moderate-to-severe atopic dermatitis (JADE MONO-1): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet. 2020;396(10246):255-266. doi: 10.1016/S0140-6736(20)30732-7 [DOI] [PubMed] [Google Scholar]
- 35.Simpson EL, Lacour JP, Spelman L, et al. Baricitinib in patients with moderate-to-severe atopic dermatitis and inadequate response to topical corticosteroids: results from two randomized monotherapy phase III trials. Br J Dermatol. 2020;183(2):242-255. doi: 10.1111/bjd.18898 [DOI] [PubMed] [Google Scholar]
- 36.Simpson EL, Forman S, Silverberg JI, et al. Baricitinib in patients with moderate-to-severe atopic dermatitis: results from a randomized monotherapy phase 3 trial in the United States and Canada (BREEZE-AD5). J Am Acad Dermatol. 2021;85(1):62-70. doi: 10.1016/j.jaad.2021.02.028 [DOI] [PubMed] [Google Scholar]
- 37.Zhao Y, Zhang L, Ding Y, et al. Efficacy and safety of SHR0302, a highly selective Janus kinase 1 inhibitor, in patients with moderate to severe atopic dermatitis: a phase II randomized clinical trial. Am J Clin Dermatol. 2021;22(6):877-889. doi: 10.1007/s40257-021-00627-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hsu DY, Dalal P, Sable KA, et al. Validation of International Classification of Disease Ninth Revision codes for atopic dermatitis. Allergy. 2017;72(7):1091-1095. doi: 10.1111/all.13113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol Suppl (Stockh). 1980;92:44-47. https://www.medicaljournals.se/acta/content_files/files/pdf/60/92/924447.pdf [Google Scholar]
- 40.Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: section 1: diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70(2):338-351. doi: 10.1016/j.jaad.2013.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu P, Zhao Y, Mu ZL, et al. Clinical features of adult/adolescent atopic dermatitis and Chinese criteria for atopic dermatitis. Chin Med J (Engl). 2016;129(7):757-762. doi: 10.4103/0366-6999.178960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Davis DMR, Drucker AM, Alikhan A, et al. American Academy of Dermatology guidelines: awareness of comorbidities associated with atopic dermatitis in adults. J Am Acad Dermatol. 2022;86(6):1335-1336.e18. doi: 10.1016/j.jaad.2022.01.009 [DOI] [PubMed] [Google Scholar]
- 43.Ascott A, Mulick A, Yu AM, et al. Atopic eczema and major cardiovascular outcomes: a systematic review and meta-analysis of population-based studies. J Allergy Clin Immunol. 2019;143(5):1821-1829. doi: 10.1016/j.jaci.2018.11.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Armstrong EJ, Harskamp CT, Armstrong AW. Psoriasis and major adverse cardiovascular events: a systematic review and meta-analysis of observational studies. J Am Heart Assoc. 2013;2(2):e000062. doi: 10.1161/JAHA.113.000062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen TL, Lee LL, Huang HK, et al. Association of psoriasis with incident venous thromboembolism and peripheral vascular disease: a systematic review and meta-analysis. JAMA Dermatol. 2022;158(1):59-67. doi: 10.1001/jamadermatol.2021.4918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Undas A, Cieśla-Dul M, Drążkiewicz T, Potaczek DP, Sadowski J. Association between atopic diseases and venous thromboembolism: a case-control study in patients aged 45 years or less. J Thromb Haemost. 2011;9(4):870-873. doi: 10.1111/j.1538-7836.2011.04198.x [DOI] [PubMed] [Google Scholar]
- 47.Vinogradova Y, Coupland C, Hippisley-Cox J. Use of hormone replacement therapy and risk of venous thromboembolism: nested case-control studies using the QResearch and CPRD databases. BMJ. 2019;364:k4810. doi: 10.1136/bmj.k4810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mahmoodi BK, Cushman M, Anne Næss I, et al. Association of traditional cardiovascular risk factors with venous thromboembolism: an individual participant data meta-analysis of prospective studies. Circulation. 2017;135(1):7-16. doi: 10.1161/CIRCULATIONAHA.116.024507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pandher K, Ghamrawi RI, Heron CE, Feldman SR. Controversial cardiovascular and hematologic comorbidities in atopic dermatitis. Arch Dermatol Res. 2022;314(4):317-324. doi: 10.1007/s00403-021-02240-z [DOI] [PubMed] [Google Scholar]
- 50.Potaczek DP. Links between allergy and cardiovascular or hemostatic system. Int J Cardiol. 2014;170(3):278-285. doi: 10.1016/j.ijcard.2013.11.029 [DOI] [PubMed] [Google Scholar]
- 51.Tamagawa-Mineoka R, Katoh N, Ueda E, Masuda K, Kishimoto S. Elevated platelet activation in patients with atopic dermatitis and psoriasis: increased plasma levels of beta-thromboglobulin and platelet factor 4. Allergol Int. 2008;57(4):391-396. doi: 10.2332/allergolint.O-08-537 [DOI] [PubMed] [Google Scholar]
- 52.Koczy-Baron E, Jochem J, Kasperska-Zajac A. Increased plasma concentration of vascular endothelial growth factor in patients with atopic dermatitis and its relation to disease severity and platelet activation. Inflamm Res. 2012;61(12):1405-1409. doi: 10.1007/s00011-012-0543-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nastałek M, Potaczek DP, Wojas-Pelc A, Undas A. Plasma platelet activation markers in patients with atopic dermatitis and concomitant allergic diseases. J Dermatol Sci. 2011;64(1):79-82. doi: 10.1016/j.jdermsci.2011.07.001 [DOI] [PubMed] [Google Scholar]
- 54.Alshehri FSM, Whyte CS, Tuncay A, Williams ML, Wilson HM, Mutch NJ. Monocytes expose factor XIII-A and stabilize thrombi against fibrinolytic degradation. Int J Mol Sci. 2021;22(12):6591. doi: 10.3390/ijms22126591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smiley ST, Boyer SN, Heeb MJ, Griffin JH, Grusby MJ. Protein S is inducible by interleukin 4 in T cells and inhibits lymphoid cell procoagulant activity. Proc Natl Acad Sci U S A. 1997;94(21):11484-11489. doi: 10.1073/pnas.94.21.11484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Caughey GH. Tryptase genetics and anaphylaxis. J Allergy Clin Immunol. 2006;117(6):1411-1414. doi: 10.1016/j.jaci.2006.02.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Simpson EL, Silverberg JI, Nosbaum A, et al. Integrated safety analysis of abrocitinib for the treatment of moderate-to-severe atopic dermatitis from the phase II and phase III clinical trial program. Am J Clin Dermatol. 2021;22(5):693-707. doi: 10.1007/s40257-021-00618-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.King B, Maari C, Lain E, et al. Extended safety analysis of baricitinib 2 mg in adult patients with atopic dermatitis: an integrated analysis from eight randomized clinical trials. Am J Clin Dermatol. 2021;22(3):395-405. doi: 10.1007/s40257-021-00602-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Silverberg JI, de Bruin-Weller M, Bieber T, et al. Upadacitinib plus topical corticosteroids in atopic dermatitis: Week 52 AD Up study results. J Allergy Clin Immunol. 2022;149(3):977-987.e14. doi: 10.1016/j.jaci.2021.07.036 [DOI] [PubMed] [Google Scholar]
- 60.Soto E, Banfield C, Gupta P, Peterson MC. Kinetic-pharmacodynamic model of platelet time course in patients with moderate-to-severe atopic dermatitis treated with oral Janus kinase 1 inhibitor abrocitinib. CPT Pharmacometrics Syst Pharmacol. 2020;9(10):553-560. doi: 10.1002/psp4.12548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Anderson FA Jr, Spencer FA. Risk factors for venous thromboembolism. Circulation. 2003;107(23)(suppl 1):I9-I16. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Search Strategies for (a) MEDLINE, (b) Embase, (c) Cochrane Library, and (d) Web of Science
eFigure 1. Risk Differences in Venous Thromboembolism Between Participants With Atopic Dermatitis Receiving Janus Kinase Inhibitors and Controls Receiving Placebo or Dupilumab
eFigure 2. Funnel Plot of Randomized Controlled Trials


