Abstract
Bacillus subtilis has developed sophisticated mechanisms to withstand fluctuations in temperature. Membrane fatty acids are the major determinants for a sufficiently fluid membrane state to ensure the membrane’s function at all temperatures. The fatty acid profile of B. subtilis is characterized by a high content of branched fatty acids irrespective of the growth medium. Here, we report on the importance of isoleucine for B. subtilis to survive cold shock from 37 to 15°C. Cold shock experiments with strain JH642 revealed a cold-protective function for all intermediates of anteiso-branched fatty acid biosynthesis. Metabolites related to iso-branched or straight-chain fatty acid biosynthesis were not protective. Fatty acid profiles of different B. subtilis wild-type strains proved the altered branching pattern by an increase in the anteiso-branched fatty acid content and a concomitant decrease of iso-branched species during cold shock. There were no significant changes in the fatty acid saturation or acyl chain length. The cold-sensitive phenotype of isoleucine-deficient strains in the absence of isoleucine correlated with their inability to synthesize more anteiso-branched fatty acids, as shown by the fatty acid profile. The switch to a fatty acid profile dominated by anteiso-C15:0 and C17:0 at low temperatures and the cold-sensitive phenotype of isoleucine-deficient strains in the absence of isoleucine focused our attention on the critical role of anteiso-branched fatty acids in the growth of B. subtilis in the cold.
Bacillus subtilis is a gram-positive soil bacterium that lives in the upper layers of soil and is therefore subjected to frequent changes within the environment, especially in temperature, nutrient availability, and osmolarity. Strategies and mechanisms to withstand these hostile conditions are crucial for survival. B. subtilis is found mostly in a nongrowing or slow-growing state due to these stress conditions. Temperature shifts are easy to simulate in the laboratory, and the physiological and molecular response to cold stress gained our interest (15).
Bacterial adaptation to low temperature is far from being understood, though this topic has been studied recently through numerous physiological and molecular approaches with several organisms. Temperatures above 0°C are usually associated with an adaptive response, whereas subzero temperatures cause a block of metabolic activity and cell growth for most organisms (37, 38). It is generally accepted that the cold stress response consists of two phases: an immediate, transient shock response and the subsequent delayed acclimatization response. Each of these phases can be characterized by a special set of cold-induced proteins, the cold shock proteins and the cold acclimatization proteins, respectively, with the cold shock proteins thought to play a major, not yet fully understood regulatory role in the mechanism of cold adaptation (12–15, 46).
Handling proper gene expression is not the only challenge for cells growing at low temperature. Another problem is the decreasing membrane fluidity with the decreasing temperature, threatening the membrane’s structure and function. Temperature markedly affects the membrane lipid composition. Changes in lipid composition are thought to occur in order to maintain a liquid-crystalline state. In bacteria, which generally lack cholesterol, the modification of membrane fluidity, called homeoviscous adaptation (40), involves changes in the membrane’s fatty acid composition (5, 39, 43, 44). With decreasing temperature, lower-melting-point fatty acids are incorporated into the lipids in a species-specific mode. The low-temperature adaptation mechanisms of membranes involve a reduction in acyl chain length, the introduction of double bonds by desaturase enzymes, or branching of the acyl chains by methyl groups. The best-characterized low-temperature adaptation of the membrane lipids is the desaturation of the fatty acid moieties (32). Organisms use different numbers and types of desaturase enzymes (for a review, see reference 5), frequently having one housekeeping enzyme and other cold-inducible ones (10, 11, 29).
The B. subtilis membrane is characterized by a fatty acid profile dominated to a large extent (>80%) by the odd-numbered, iso- and anteiso-branched-chain fatty acids, with the major species being iso- and anteiso-C15:0 and -C17:0 (20). So far, a single desaturase gene has been characterized which seems to be the only desaturase homologue within the B. subtilis genome (1). This membrane-integrated enzyme catalyzes the conversion of palmitic acid to cis-5 hexadecanoic acid. Although it seems to be the sole desaturase in B. subtilis and the gene is induced severalfold by cold shock, null mutant strains show no defect in their cold shock response compared to the wild type (1). Therefore, alternative means for adaptation of the lipid composition to low temperature have to exist. Among the factors known to affect membrane lipid fluidity are the acyl chain length of the fatty acid moieties and the introduction, or the type, of branching by addition of methyl groups (3, 44). So far, the branching of fatty acids has been described as being strictly dependent on de novo biosynthesis (25, 43). Branched-chain and straight-chain fatty acids seem to share biosynthetic enzymes, with the major difference lying in the mechanism of primer selection that specifies the final product (20).
Another factor of cold stress adaptation in various organisms is the accumulation of stabilizing organic compounds. The accumulated substances range from sugars and polyols to amino acids and quaternary amines (38). These compounds are usually related to the osmolytes accumulated under hyperosmotic stress; due to the lack of adverse effects on essential cellular functions, they are termed compatible solutes (28). In a recent study, the osmoprotective and cold-protective effects of glycine betaine on Listeria monocytogenes were characterized (24). The precise mode of action by which glycine betaine protects cells under osmotic and cold stress is not known, but the prevention of protein denaturation and cell membrane disruption have been discussed (42, 48).
Our group is interested in understanding the mechanisms that allow B. subtilis to survive cold shock. Here, we report on the importance of exogenic isoleucine sources for B. subtilis to survive cold conditions in minimal medium. The putative role of isoleucine as a precursor in anteiso-branched fatty acid synthesis is discussed, and a model is proposed.
MATERIALS AND METHODS
Growth of bacterial cells.
B. subtilis JH642 (pheA1 trpC2 sfp0) (17), 1A57 (ilvC1 pheA1 trpC2; Bacillus Genetic Stock Center [BGSC]), 1A228 (ilvB2 pheA1; BGSC), ATCC 6051 (wild-type), and the Marburg strain 168 were grown in 2× YT as rich medium (components obtained from Oxoid, Wesel, Germany, and AppliChem, Darmstadt, Germany) or Spizizen’s minimal medium (SMM) (41) supplemented with 0.2% (wt/vol) glucose and with phenylalanine and tryptophan (final concentration, 0.02% [each] [16]) at 37°C with 250-rpm gyration. Other amino acids and ingredients (obtained from Sigma, Dassel, Germany) were added at 100 μM final concentration or as indicated. For standard cold shock experiments, cells of an overnight culture were inoculated in 80 ml of prewarmed medium in a 300-ml Erlenmeyer flask to an optical density at 600 nm (OD600) of 0.05, and the cells were grown at 37°C with 250-rpm gyration for good aeration. At an OD600 of 0.45, the cultures were chilled to 15°C in a water bath incubator with gyration. Additional substrates were added 5 min before the cultures were chilled. At least three independent cold shock experiments were performed for each substrate. For monitoring of long-term growth and sporulation, incubation at 15°C with 250-rpm gyration was continued.
Uptake of [14C]isoleucine and labeling of cells.
Cells were grown in SMM-Phe-Trp-glucose at 37°C as described previously to an OD600 of 0.45, at which point a cold shock was performed. After 5 min at 15°C, [14C]isoleucine (240 mCi mmol−1) at a final concentration of 0.15 μM was added and incubation was continued. At different time points, 500-μl aliquots were filtered (ME25 filter discs; 0.45-μm pore size; Schleicher & Schuell, Dassel, Germany) and washed with 5 ml of fresh SMM, and the amount of incorporated isoleucine was determined by counting the filter discs (Rotiszint Eco Plus; Roth, Karlsruhe, Germany) and normalizing for cell number (23). Plotting the incorporated isoleucine versus time allowed us to calculate the initial uptake rate. The same measurements were done with cells grown at 37°C at corresponding optical density for control.
For measuring the Vmax and Km values, the same concentration of radioactively labeled isoleucine was mixed with increasing amounts of unlabeled isoleucine and the uptake rates were determined. Plotting the uptake rates versus isoleucine concentration by the Lineweaver-Burk method allowed us to calculate the Vmax and Km values by interpolation.
To measure the cellular distribution of the isoleucine incorporated after cold shock, 10-ml cell cultures were labeled by incubation with 0.15 μM [14C]isoleucine. More than 80% of the radioactive label was internalized within 2 h. The cells were harvested, washed once in fresh SMM, and resuspended in a final volume of 400 μl of 50 mM Tris HCl (pH 8.0), 15 mM MgSO4, 50 μg (each) of DNase I and RNase A/ml, 0.5 mg of lysozyme/ml, and 120 μg of chloramphenicol/ml. Cell lysis occurred for 30 min at 37°C. Cell wall and the membrane particles were pelleted by spinning them for 60 min at 36,000 × g and 4°C. The pellet was resuspended in 300 μl of 50 mM Tris HCl, pH 8.0. The supernatant was brought to a final concentration of 10% trichloracetic acid (TCA). Proteins were precipitated overnight at 4°C and pelleted by spinning them for 30 min at 13,000 rpm in a benchtop centrifuge (Heraeus Biofuge pico). The protein pellet was resuspended in a volume of 300 μl of 50 mM Tris HCl, pH 8.0. The percentage of isoleucine label in each subcellular fraction was determined by counting aliquots in a Packard Tri Carb 2100TR scintillation counter. Four independent labeling experiments were performed.
Fatty acid analysis.
B. subtilis cells grown in 300-ml volumes in minimal medium with or without isoleucine as indicated were subjected to cold shock and incubated to a final OD600 of 1.2; cells grown to the same OD600 at 37°C were used to prepare reference samples. In the absence of isoleucine, cells of strain JH642 were harvested when the OD had declined by 20% below preshock values (i.e., an OD600 of 0.35) and after 72 h of incubation, when the cells had recovered to an OD600 of 0.75. Cells of four independent cultures were pooled and harvested by centrifugation at 5,000 × g and 15°C for 5 min, washed once in the same medium, and freeze dried. The total fatty acids were saponized, methylated, and extracted from 20 mg (dry weight) of cells by a standard protocol (27, 31). The fatty acid methyl ester mixtures were separated with a model 5898A microbial identification system (Microbial ID, Newark, Del.) equipped with a Hewlett-Packard model 5980 gas chromatography. A 5% phenyl-methyl silicone capillary column (0.2 mm by 25 m) with a flame ionization detector and auto sampler (Hewlett-Packard models 3392 and 7673) was used for detection. Peaks were automatically integrated, and percentages were calculated. Fatty acid data were compared qualitatively and quantitatively with the microbial identification system library generation software (Microbial ID), and the results were presented as a dendrogram. This analysis was performed at the Deutsche Sammlung von Mikroorganismen und Zellkulturen in Braunschweig, Germany.
RESULTS
Definition of a minimal set of substrates necessary for Bacillus subtilis to survive cold shock conditions.
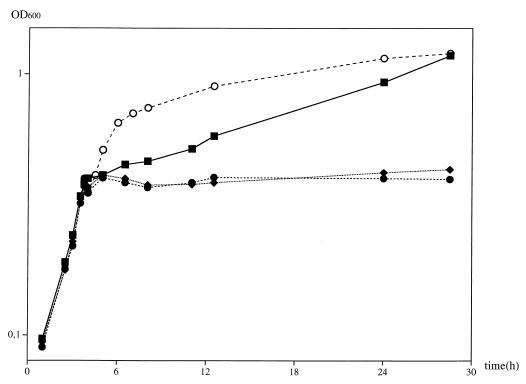
In our laboratory, simulation of cold shock in B. subtilis JH642 is accomplished by chilling exponentially growing cells from 37 to 15°C. In previous work, all cold shock experiments have been performed either in a rich medium or a minimal medium containing 0.01% yeast extract (14). In our attempt to identify small cold-protective molecules in B. subtilis, we tried to define a liquid medium with a minimal set of supplements. SMM (41) with glucose as a carbon source and phenylalanine and tryptophan to overcome the auxotrophic background of B. subtilis JH642 was used as a basic medium that is sufficient for growth at 37°C. In this medium the necessity for various additions for survival after a temperature shift from 37 to 15°C was investigated. Liquid medium as defined above did not permit the growth of cold-shocked cells (Fig. 1) and was therefore used as a negative control in all experiments. However, the addition of 0.02% casamino acids was found to replace the yeast extract, allowing growth to a high OD, and this medium was used as a positive control for subsequent cold shock experiments (Fig. 1). The abilities of single amino acids to confer cold shock tolerance were tested successively. The abilities of the various amino acids to permit growth at a low temperature clustered in three groups, with isoleucine and threonine being as effective as the yeast extract or casamino acids (Fig. 1). In a second class, the acidic amino acids aspartate and glutamate conferred some cold tolerance, as seen by a stable OD for several days of incubation at 15°C. All the other amino acids were not cold protective, and the OD declined, as seen for minimal medium without any supplements (Fig. 1). This reduced absorbance is presumably due to cell death, as the number of viable cells decreased by a factor of 7.5 within the first 18 h of cold shock in the absence of isoleucine [(2.4 ± 0.2) × 108 CFU/ml at the cold shock point versus (3.2 ± 0.4) × 107 CFU/ml after 18 h of cold shock in the absence of isoleucine]. Leucine was found to enhance cold sensitivity. The osmoprotectants glycine betaine and proline did not show any cold-protective effect. Conversely, isoleucine had no osmoprotective effect (data not shown).
FIG. 1.
Adaptation of B. subtilis JH642 to cold shock in minimal medium with different amino acids. After inoculation (OD600, 0.05) from an overnight culture in the same medium, the cells were grown at 37°C to an OD600 of 0.45 and subjected to cold shock (15°C). Amino acids in 100 μM final concentration were added 5 min before the cold shock. Cell density and growth were monitored by measuring the OD600. A cumulative graph of three independent experiments is shown. ■, addition of 0.02% casamino acids; ▴, 100 μM isoleucine; ⧫, 100 μM threonine; ▾, 100 μM leucine; ●, 100 μM aspartate; □, no addition.
Other wild-type B. subtilis strains were tested for their isoleucine requirement in order to survive a cold shock. Unlike strain JH642, ATCC 6051 and the Marburg 168 strain did not show any demand for an exogenous supply of isoleucine for recovery from cold shock (data not shown). Steady-state growth rates were 70 min in minimal medium without added isoleucine and 60 min with added isoleucine (100 μM final concentration) at 37°C. After cold shock, the generation time increased to 12 to 14 h for the Marburg 168 and ATCC 6051 strains irrespective of isoleucine addition as well as for JH642 in the presence of isoleucine. Without isoleucine, JH642 showed an initial decrease in cell density, which after more than 48 h was succeeded by slow growth with a generation time close to 30 h.
Isoleucine and threonine confer a cold resistance phenotype on strain JH642.
The experiments shown in Fig. 1 demonstrated that B. subtilis JH642 has an enhanced requirement for isoleucine and/or threonine in order to resume growth after a cold shock, albeit no auxotrophy for any of these amino acids exists under the normal (i.e., 37°C) growth conditions. Either, the biosynthetic pathways were shut down by the cold shock conditions, conferring a cold-induced auxotrophy, or the requirement for isoleucine and/or threonine at low temperature was enhanced and therefore exceeded the biosynthetic capacity of this strain. As threonine is the precursor of isoleucine in the biosynthetic pathway (7), we wanted to distinguish between a true threonine requirement and an isoleucine requirement. Recovery from cold shock by the strains 1A228 (ilvB) and 1A57 (ilvC), both blocked in the pathway leading from threonine to isoleucine, was tested with both amino acids. With 20 μM (each) isoleucine and valine, B. subtilis 1A57 and 1A228 showed reasonable growth at 37°C and an arrest of growth at 15°C (see Fig. 3). Only the addition of isoleucine (final concentration, 100 μM) conferred cold resistance. The addition of threonine had no protective effect for these strains. This shows that isoleucine is the protective agent in our assays with B. subtilis JH642. The protective effect of threonine is based on its conversion to isoleucine by the ilv gene products (7). The results further demonstrated that under cold stress, B. subtilis has an enhanced requirement for isoleucine, as the isoleucine concentration, which was able to compensate for auxotrophy at 37°C, became limiting under cold shock conditions.
FIG. 3.
Cold shock response of the ilvB mutant B. subtilis 1A228 from the BGSC. The strain was incubated as described in the legend to Fig. 1, with the addition of 20 μM (each) isoleucine and valine to overcome auxotrophy. As shown for growth at 37°C (○), this concentration allowed growth to an OD600 of more than 1.0. Cold shock in the absence of isoleucine (●) abolished growth. The addition of 100 μM isoleucine prior to cold shock (■) permits growth to high density at 15°C, whereas the addition of 100 μM threonine (⧫) had no protective effect in this strain. Experiments with another isoleucine-auxotrophic strain, 1A57 (ilvC), revealed similar results (data not shown).
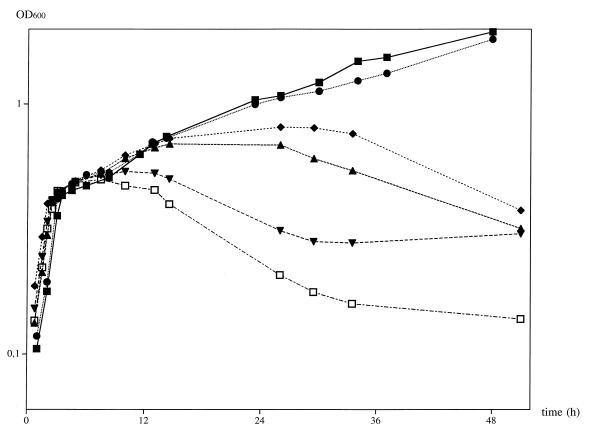
Further experiments proved that the addition of isoleucine (final concentration, 100 μM) up to 1 h after cold shock was sufficient for the recovery of JH642 from cold stress (data not shown). In a series of experiments, we tried to determine the minimal isoleucine concentration that is protective for B. subtilis JH642 in our standard cold shock. Isoleucine in final concentrations ranging from 2 to 100 μM was added 2 min after cold shock (Fig. 2). Isoleucine at 20 μM was found to be sufficient for complete survival and growth to ODs of more than 1.5 OD600 units. Concentrations below 10 μM allowed only an initial recovery: after a slight increase in turbidity (correlating with the isoleucine concentration), the OD declined (Fig. 2).
FIG. 2.
Cold-protective effect of various isoleucine concentrations. Cells were grown under conditions identical to those for Fig. 1. Different final concentrations of isoleucine, added 2 min after cold shock, were tested for their cold-protective efficiency. ■, 50 μM isoleucine; ●, 20 μM isoleucine; ⧫, 10 μM isoleucine; ▴, 8 μM isoleucine; ▾, 4 μM isoleucine; □, 2 μM isoleucine.
Kinetic parameters of isoleucine transport.
Since an exogenous supply of isoleucine is essential for survival of B. subtilis JH642 after cold shock, an effective uptake system has to exist. To determine the mechanism of isoleucine transport in cold-stressed B. subtilis cells, the uptake of [14C]isoleucine was measured. Kinetic analysis of the isoleucine uptake of cold-shocked cells and cells grown at 37°C yielded Michaelis-Menten constants of 6.0 ± 0.5 and 3.2 ± 0.3 μM isoleucine, respectively. The maximum velocities determined were 72.5 ± 0.7 pmol min−1 per 5 × 108 cells versus 650 ± 2.5 pmol min−1 per 5 × 108 cells at 15 and 37°C. At both temperatures, the Km values were within the same micromolar range, suggesting that the same system is responsible for isoleucine uptake under low- and normal-temperature conditions. The lower maximum velocity of cold-shocked cells might be due to the strongly reduced activity of the transport system under these conditions, as our assays were performed 5 min after the shock, when the cell membrane had not yet adapted. Our observations showed that low temperature reduced the maximum velocity of isoleucine uptake, and the difference in the uptake rates does not necessarily imply that different systems are active at different temperatures. Even though isoleucine was a prerequisite for the survival of cold-shocked JH642 cells, the responsible uptake system seems to be neither induced nor activated by these conditions.
Intracellular distribution of labeled isoleucine.
The fate and metabolic destination of the internalized isoleucine was determined by identifying its subcellular localization with [14C]isoleucine as a tracer. The rationale was that a cryoprotective substance, as seen for osmoprotectants like glycine betaine, should accumulate in the cytoplasmic fraction. On the other hand, some evidence exists that cells under cold stress shift their amino acid usage, i.e., the amino acid patterns of proteins predominantly synthesized under cold conditions differ markedly from those of housekeeping or heat stress proteins (6). To investigate the cellular distribution of isoleucine under cold shock, cells were labeled with [14C]isoleucine at 15°C and fractionated as described in Materials and Methods. The amount of [14C]isoleucine in each fraction, i.e., the cell wall and membrane fraction, the protein fraction, and the TCA extract, was determined by scintillation counting. The results presented in Table 1 show that about two-thirds of the radioactivity was found in the membrane fraction and one-third was found in the high-speed supernatant. Only a minor amount was found in the TCA extract, implying that the cryoprotective effect of isoleucine is quite different from the cryoprotective effects of glycine betaine in L. monocytogenes (24). A comparative labeling of cold-shocked JH642 cells with [14C]isoleucine or [35S]methionine and subsequent fractionation showed no difference in the labeling patterns of the proteins, as seen by analysis on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (data not shown). This is in line with the investigations of cold shock regulation by two-dimensional polyacrylamide gel electrophoresis analysis. More than 40 proteins with diverse cellular functions induced by cold shock were identified, but this proteomic approach gave no evidence of a small isoleucine-rich protein (14).
TABLE 1.
Subcellular distribution of internalized [14C]isoleucinea
| Subcellular fraction | % Internalized radioactivity recovered |
|---|---|
| Whole cells | 100 |
| Cell wall pellet | 65 ± 5 |
| High-speed supernatant | 37 ± 4 |
| TCA extract | 3 ± 0.8 |
| TCA precipitate | 19 ± 5 |
Cells were labeled for 2 h at 15°C at a concentration of 0.15 μM [14C]isoleucine. During this time, more than 80% of the radioactivity accumulated in the cells. This value was set as 100% for the whole-cell fraction. Fractionation was performed as described in Materials and Methods, and radioactivity was determined by counting duplicate aliquots of each subcellular fraction. The reported errors are standard errors of four independent labeling reactions.
Cold-protective effects of branched-chain fatty acid precursors that are related to isoleucine.
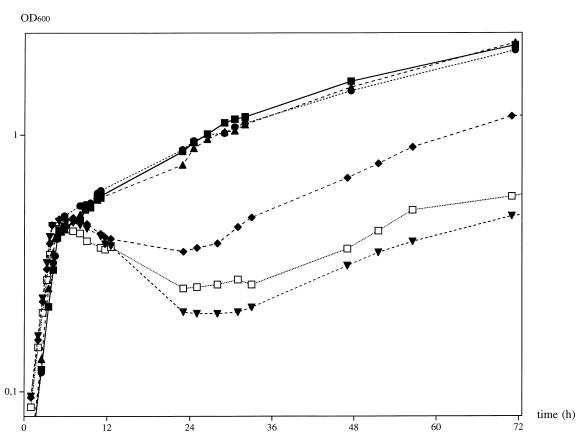
Two main metabolic destinations for isoleucine are known: incorporation into proteins and peptides and serving as a primer for branched-chain fatty acids of the anteiso-branching type (8, 25). As seen in the experiments, most of the labeled isoleucine was retained within the membrane fraction (Table 1). We therefore speculated that isoleucine was a necessary precursor for cold-stressed B. subtilis cells in the adaptation of the fatty acid profile to adjust membrane fluidity. The abilities of several precursors of the branched-chain fatty acid synthesis of isoleucine and nonisoleucine (i.e., valine and leucine) origin to confer cold stress resistance were tested. The different substrates were added 5 min before cold shock in 100 μM final concentration. As shown in Fig. 4, only the isoleucine-related fatty acid precursors α-keto-β-methyl-valerate and 2-methyl-butyrate showed a cold-protective effect comparable to the one seen for isoleucine and threonine, restoring the growth rate and resulting in the same final culture density. The valine derivative α-keto-iso-valerate showed a stabilizing effect; this might be due to impurities in the substance. However, α-keto-iso-valerate did not permit growth of the isoleucine-auxotrophic strains 1A57 and 1A228 at 37°C (data not shown). As seen for leucine, its derivative α-keto-iso-caproate displayed no cold protection. From these results we conclude that the requirement of B. subtilis JH642 cells for isoleucine is due to the adaptation of membrane fluidity and the mechanism responsible for this adaptation.
FIG. 4.
Adaptation of B. subtilis JH642 to cold shock in minimal medium supplemented with different precursors of branched-chain fatty acid biosynthesis. The cold-protective effect of precursors for different branched-chain fatty acids was tested by performing cold shock experiments as described in Materials and Methods. The substrates for fatty acid synthesis were added to a final concentration of 100 μM 5 min prior to chilling. Cellular adaptation was measured by determining cell density at 600 nm. ■, 100 μM isoleucine; ●, 100 μM α-keto-β-methyl-valerate; ▴, 100 μM 2-methyl-butyrate; ▾, 100 μM α-keto-iso-caproate; ⧫, 100 μM α-keto-iso-valerate; □, no addition.
Changes in the fatty acid profile of B. subtilis cells after cold shock.
To verify our model of the cold adaptation of the cell membrane fluidity in B. subtilis, we performed large-scale cold shock experiments to harvest enough cellular material for a fatty acid analysis. Freeze-dried cell pellets were subject to fatty acid preparation and analysis. As described in Materials and Methods, cold-shocked cells were incubated overnight at 15°C and grown to an OD600 of 1.2. Cells grown at 37°C to the same density were used as a reference. The fatty acid composition of strain JH642 after cold shock in the absence of isoleucine was measured after 18 h and when the cells had recovered from shock to an OD600 of 0.75 (i.e., about 84 h after cold shock).
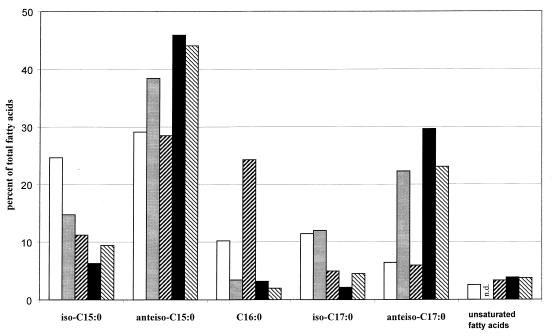
The data presented in Fig. 5 show a drastic change in the fatty acid branching pattern in response to cold shock. The major changes were the decrease in iso-C15:0 and iso-C17:0 and the concomitant increase of the respective anteiso-branched forms. While the frequency of the iso-branched fatty acid species was decreased from 44 to 11% of total cellular fatty acids by cold shock, the anteiso-branched species became the majority, with an increase from 35 to 76% for JH642 in the presence of exogenous isoleucine. When performing the cold shock in minimal medium lacking an isoleucine supply, the amount of anteiso-branched fatty acids did not change, whereas the frequency of the iso-branched species decreased to less than 50% of the corresponding cells cultured at 37°C. This reduction in the iso-branched fatty acid species shifts the iso-anteiso ratio in the same direction as seen for the cells shocked in the presence of isoleucine. The failure of this strain to recover from cold stress in the absence of isoleucine (Fig. 1) indicates that a shift in the iso-anteiso ratio by a reduction of the iso-branched fatty acids alone is not sufficient to permit growth under cold conditions. These results highlight the importance of an exogenous source of isoleucine for the increase in anteiso-branched fatty acids, as the endogenous biosynthetic capacity seems to be limiting in strain JH642. The shift in the fatty acid pattern after isoleucine was provided to strain JH642 grown at 37°C was comparable to earlier results reported by Willecke and Pardee (47).
FIG. 5.
Fatty acid profiles of B. subtilis strains after growth at 37°C or under cold shock conditions. Strain JH642, subjected to cold shock with a supply of isoleucine, was harvested at an OD600 of 1.2 (■). In the absence of isoleucine, JH642 cells were harvested after 18 h of cold shock (▨) or after recovery (that is, growth to an OD600 of 0.75; about 84 h after cold shock) (data not shown). Reference cultures of JH642 were grown at 37°C in the same medium without (□) and with (░⃞) isoleucine supplementation to an identical cell density. To represent other B. subtilis wild-type strains tested, the Marburg 168 strain after cold shock in the absence of isoleucine is depicted (▧). From a complete fatty acid profile recorded for each sample, the frequency of the most prominent species is shown. n.d., concentrations below detection level.
Other B. subtilis wild-type strains displayed the same bias in the iso-to-anteiso ratio when cold shocked. Their iso-anteiso branching pattern shifts from a nearly balanced one at 37°C (data not shown) to a 4.4-fold anteiso-dominated one (15% iso versus 67% anteiso species [Fig. 5]) by the same mechanism: reduction of the amount of iso-fatty acids and increase of anteiso-fatty acid species.
Unsaturated fatty acid species play only a minor role in the cold stress adaptation of B. subtilis, as their overall low abundance did not change significantly with cold shock for all strains and growth conditions we tested (Fig. 5). This fact supports earlier data of Aguilar and coworkers (1), who showed that the desaturase of B. subtilis is not necessary for cold stress adaptation, even though its gene is induced by cold shock. Our study clearly indicates that the presence or absence of isoleucine has no influence on the degree of saturation. Desaturation therefore is not able to counterbalance the inability to synthesize a suitable amount of anteiso-branched fatty acids. The data provided show that, under given conditions, B. subtilis adjusts membrane fluidity to low temperature exclusively by switching from iso- towards anteiso-branched-chain fatty acids.
DISCUSSION
Performing cold shock experiments with B. subtilis JH642 cells, we have observed that isoleucine plays a critical role in the cold shock adaptation of this strain. The mode of cold stress adaptation by isoleucine seems to be based on its function as a precursor in anteiso-branched-chain fatty acid biosynthesis as revealed by the change in the fatty acid profile (Fig. 5) and its replacement by related metabolites of the anteiso-branched fatty acid biosynthesis pathway (Fig. 4 and 6). After uptake, the branched-chain amino acids leucine, isoleucine, and valine participate in oxidative desamination and subsequent decarboxylation reactions where they are converted to coenzyme A (CoA)-activated precursors for fatty acid biosynthesis. Depending on the amino acid, different intermediate substrates are formed, which result in the different fatty acid species (18, 19). From isoleucine, α-keto-β-methyl-valerate and subsequently 2-methyl-butyryl-CoA are formed, yielding the anteiso-branched C15:0 and C17:0 fatty acids. Leucine is converted via α-keto-iso-caproate and isovaleryl-CoA to the iso-branched C15:0 and C17:0 fatty acids, whereas valine, via α-keto-iso-valerate and isobutyryl-CoA, forms the even-numbered iso-C14:0 and -C16:0 species (3). Our results with the isoleucine prerequisite and the fatty acid profile of cold-shocked cells are in agreement with this biosynthetic pathway (Fig. 6).
FIG. 6.
Schematic representation of branched-chain fatty acid biosynthesis with leucine, isoleucine, and valine leading to iso- and anteiso-branched fatty acid products, respectively. a, a soluble branched-chain amino acid amino transferase; b, NAD- and CoA-dependent branched-chain α-keto-acid decarboxylase. The underlined metabolites were supplied externally, as indicated in the text. This scheme is adapted from the one presented by Kaneda (20).
Cold shock of JH642 cells in the absence of an isoleucine supply showed a decrease in viable cell number that corresponds to the decrease in OD. These cultures show an increasing absorption after more than 48 h of cold shock, with a concomitant increase in cell number (data not shown). Repeating cold shock with these cultures (i.e., diluting the cells in fresh medium, growing them at 37°C, and performing a new cold shock experiment) revealed the same growth pattern as seen before with a cold-sensitive phenotype (data not shown). Therefore the occurrence of spontaneous mutation as a putative reason for this phenomenon is not likely. As the decrease in OD and viable cell number after 18 h of cold shock indicate, a large number of cells might lyse, delivering a reasonable amount of free isoleucine and threonine into the medium that is beneficial for viable cells to adapt and restart slow growth. This scenario was corroborated by a fatty acid analysis, where these cell cultures revealed a fatty acid pattern similar to the one seen for cultures of JH642 incubated in the presence of isoleucine (data not shown). However, as the experiments with a second cold shock showed, we have no evidence for any spontaneous mutation leading to improved isoleucine biosynthesis.
As shown in this report, the frequently used B. subtilis strain JH642 and its derivatives are cold shock sensitive in minimal medium. For threonine, isoleucine, and a number of metabolites related to the anteiso-branched fatty acid biosynthesis, a cold-protective function has been shown (Fig. 1 and 4). Threonine as a precursor in isoleucine biosynthesis has the same protective activity as isoleucine itself (Fig. 1). Mutants with defects in isoleucine biosynthesis show that, for the protective effect of threonine, a functional biosynthetic pathway leading from threonine to isoleucine is necessary (Fig. 3). These data show that with threonine, a sufficient supply of metabolites for the synthesis of the anteiso-branched fatty acids is possible. Therefore we conclude that the bottleneck in the biosynthesis of isoleucine under cold stress is below the threonine level, i.e., in the pathway leading from oxal acetate to threonine (7). This correlates with earlier observations that at least some of the isoleucine biosynthetic proteins are induced twofold by cold shock (14). The exact limitation point in the endogenous threonine synthesis has not been discovered, as with our mutant analysis we are getting closer to central metabolic events that make growth under cold conditions even harder and require a supply of multiple amino acids. Other B. subtilis strains do not require an exogenous supply of isoleucine or related metabolites for survival of cold shock. As these strains show similar fatty acid profiles but do not require a supply of precursors for the increased synthesis of anteiso-branched fatty acids, they seem to have a higher biosynthetic capacity for threonine than strain JH642. We presume a genetic difference in the threonine biosynthesis genes of JH642 versus those of other strains, whose nature has not been investigated. The cold shock-sensitive phenotype described here might be a tool to gain an understanding of this interesting genetic diversity.
Supplementation of JH642 cultures with 100 μM α-keto-β-methyl-valerate and 2-methyl-butyrate improves growth of the cold-shocked cultures in the same way as isoleucine does (Fig. 4). Metabolites related to the other branched-chain amino acids, valine and leucine, do not stimulate cold shock adaptation in a similar manner. The small effect of α-keto-iso-valerate seen in our growth measurements might be due to some minor impurities of the substrate or a conversion to α-keto-β-methyl-valerate by an as-yet-unidentified mechanism. However, α-keto-iso-valerate in concentrations up to 1 mM did not compensate for isoleucine auxotrophy (data not shown). The results are consistent with the model of the branched-chain fatty acid biosynthesis pathway (20, 25, 36). In this model, the nature of the precursor defines the branching pattern of the fatty acid species, as the subsequent steps in biosynthesis are repeated additions of C2 units. Therefore the primer-selecting activity of the first steps in fatty acid biosynthesis is critical in defining the final product. It is not yet known how a change in temperature alters the primer specificity in these first steps of fatty acid biosynthesis.
It is suggested that in B. subtilis, the anteiso-branched fatty acids, coupled with small amounts of the straight-chain saturated fatty acids, play a critical role in providing an appropriate degree of membrane fluidity for growth at low temperatures. The invariantly low content of the unsaturated fatty acids is thought to have only minor effects on the adjustment of membrane fluidity (Fig. 5). Switching from iso- to anteiso-branched fatty acids as seen in the fatty acid profile requires a switch in the utilization of fatty acyl primers. Temperature appears to alter the primer selection specificity of the first enzyme(s) of the fatty acid synthase multienzyme complex in some way. Nichols and Russell (33) have described such changes in the fatty acid primer selection in an Arctic psychrophilic bacterium in response to environmental stress. Another factor contributing to this primer selection might be changes in the cellular primer pools (22). Support for this model might come from the fact that leucine and α-keto-iso-caproate, precursors of the iso-branched fatty acids with a higher phase transition temperature, have an intensifying effect on cold shock, as is evident from the data presented in Fig. 1 and 5.
In the absence of exogenous precursors for the anteiso-branched fatty acids, cold-shocked cells of strain JH642 display a frequency of the anteiso-branched species that is close to the values for cells grown at 37°C, indicating that the limiting factor for the synthesis of these fatty acid species is the endogenous supply of the precursors under these conditions. Under such unfavorable conditions, the sole change in the anteiso-iso relation is a reduction in the frequency of the iso-branched fatty acids. Under limiting isoleucine supply, the anteiso/iso ratio becomes 1.4:1, whereas with an exogenous supply permitting growth this ratio is close to 7:1. As the cold shock-sensitive phenotype of strain JH642 (Fig. 1) shows, a switch by reduction of the iso-branched species alone is not sufficient for growth.
Under isoleucine-limiting conditions, JH642 displays a remarkable increase in the straight-chain C16:0 species (Fig. 5). The production of this even-numbered fatty acid species seems to compensate for the reduced amount of odd-numbered iso-branched species. Even with an insufficient supply of precursors for the synthesis of anteiso-branched fatty acids, the synthesizing machinery retains its specificity, as shown by the reduced synthesis of iso-branched fatty acids, and does not compensate for other precursor species (21).
We were unable to detect a role for a fatty acid desaturase activity in any B. subtilis strains tested in this investigation. As our fatty acid profiles show, there is no significant cold shock-dependent increase in desaturation (Fig. 5), although other studies have shown some effect (5, 9, 44). Investigations with a desaturase mutant strain of B. subtilis failed to show a cold-sensitive phenotype, even though an induction of the transcription of the desaturase gene, named yocE by the genome project and renamed to des, has been demonstrated (1). One reason for this cold-resistant phenotype might be the use of a complex medium by the authors, as Luria-Bertani medium contains sufficiently large amounts of amino acids and peptides to permit the synthesis of the anteiso-branched fatty acids under cold shock. Furthermore, the authors used 20°C as the cold shock temperature, which might be less effective in triggering a switch from iso to anteiso fatty acids. Studies with a new desaturase null mutant strain showed recovery from cold shock in minimal medium with isoleucine, whereas the absence of isoleucine leads to rapid cell death by cold shock (46a).
The experiments described here focus on anteiso-C15:0 and C17:0 as playing the critical role in the growth of B. subtilis at low temperature. These two fatty acids become the major species and therefore shift the branching pattern from iso-dominated to very anteiso. The physical basis for this transition is due to a reduced phase transition temperature, based on the significantly larger cross-sectional area occupied by the anteiso-fatty acids compared to that occupied by the respective iso-species. Thus, they disrupt the close packing of the fatty acyl chains and provide a greater flexibility to the membrane even at a low temperature due to reduced Van der Waals interactions (10). This is demonstrated by measuring the phase transition temperatures of phosphatidylcholine containing anteiso-C15:0 and that containing C17:0 (−16.2 and 7.6°C, respectively), which are significantly lower than those of the corresponding isoforms (6.5°C with iso-C15:0 and 27.0°C with iso-C17:0) (20, 43).
Willecke and Pardee (47) isolated a mutant of B. subtilis lacking branched-chain keto acid dehydrogenase activity by mutagenesis of a strain already lacking pyruvate dehydrogenase. This mutant requires short branched-chain fatty acids for conversion to primers in fatty acid biosynthesis and growth. By providing different precursor molecules, Akamatsu et al. manipulated the fatty acid composition of the mutant (2). However, the authors do not report any effect of low growth temperature on the fatty acid profile. Our fatty acid profiles of JH642 grown at 37°C corroborate the influence of fatty acid precursors on the pattern of the cell membrane (Fig. 5).
B. subtilis is closely related to L. monocytogenes, a food-borne pathogen growing at even lower temperature. Both organisms are characterized by a large amount of branched-chain fatty acids in the membrane, with L. monocytogenes having a lower straight-chain fatty acid content that might be a preadaptation to a low-temperature preference. Investigations of the cold shock adaptation in L. monocytogenes show a simultaneous shift to an anteiso-dominated fatty acid profile and a mean acyl chain length reduced by 2 carbon units irrespective of the medium used (3). The experiments were performed at 5°C, a temperature lower than we have used in our investigations. This lower temperature requires a mechanism exceeding the effect of the iso-anteiso switch for adaptation of the membrane fluidity, as seen by experiments with the phase transition temperature (20).
We do not have evidence that the B. subtilis transport system for isoleucine is stimulated by cold stress, as has been seen for the glycine betaine transporter in L. monocytogenes (24). Our preliminary results indicate that the same uptake system is active under normal growth and cold shock conditions, as shown by the Km values being within the same micromolar range. Transport activity at 15°C is reduced because the cell membrane is not yet fully adjusted in its fluidity. This reduced uptake rate under cold conditions is unlikely to be caused by a contrary efflux, as our initial uptake rate experiments to determine the kinetic parameters were initiated by adding labeled isoleucine. The reaction therefore contains no significant contribution from efflux, because there is essentially no labeled internal isoleucine during the initial rate measurements.
Even though the complete genome of B. subtilis has been sequenced (26) and several putative uptake systems for amino acids have been identified, the one which could be responsible for isoleucine and other branched amino acids is not yet characterized. The kinetic data reported here make a high-affinity transporter of the binding-protein-dependent type likely (4). In Escherichia coli and other bacterial species, isoleucine uptake by such systems has been shown (30, 45). In Salmonella, two periplasmic binding proteins displaying different substrate specificities and binding affinities and which are likely to use the same transmembrane components have been characterized and sequenced (34, 35). Investigations of the genes and gene products of B. subtilis responsible for isoleucine uptake are in progress. Isolation of the components should be facilitated by the results reported in this work, as mutations in the isoleucine transporter should confer a cold-sensitive phenotype in a strain JH642 background.
ACKNOWLEDGMENTS
This work was supported by the Deutsche Forschungsgemeinschaft (SFB 395), the Human Frontier in Science Program, and the Fonds der Chemischen Industrie.
We gratefully acknowledge stimulating discussions and careful reading of the manuscript by Thomas Wendrich and Beatrice van Saan-Klein, help in the fatty acid analysis by Angela Smirnova and Matthias Ulrich, and excellent technical assistance by Inge Schüler.
Footnotes
This is dedicated to Rudolf K. Thauer for his 60th birthday.
REFERENCES
- 1.Aguilar P S, Cronan J E, Jr, de Mendoza D. A Bacillus subtilisgene induced by cold shock encodes a membrane phospholipid desaturase. J Bacteriol. 1998;180:2194–2200. doi: 10.1128/jb.180.8.2194-2200.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akamatsu T, Moriyama H, Kaneda T. Genetic mapping of bfmA mutation causing fatty acid deficiency in Bacillus subtilis. Can J Microbiol. 1993;39:629–633. doi: 10.1139/m93-091. [DOI] [PubMed] [Google Scholar]
- 3.Annous B A, Becker L A, Bayles D O, Labeda D P, Wilkinson B J. Critical role of anteiso-C15:0 fatty acid in the growth of Listeria monocytogenesat low temperatures. Appl Environ Microbiol. 1997;63:3887–3894. doi: 10.1128/aem.63.10.3887-3894.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boos W, Lucht J M. Periplasmic binding protein-dependent ABC transporters. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Vol. 1. Washington, D.C: ASM Press; 1996. pp. 1175–1209. [Google Scholar]
- 5.de Mendoza D, Cronan J E. Thermal regulation of membrane lipid fluidity in bacteria. Trends Biochem Sci. 1983;8:49–52. [Google Scholar]
- 6.Feller G, Narinx E, Arpigny J L, Baise A M E, Genicot S, Gerday C. Enzymes from psychrophilic organisms. FEMS Microbiol Rev. 1996;18:189–202. [Google Scholar]
- 7.Fink P S. Biosynthesis of branched-chain amino acids. In: Sonenshein A L, Hoch J A, Losick R, editors. Bacillus subtilis and other gram-positive bacteria: biochemistry, physiology, and molecular genetics. Washington, D.C: American Society for Microbiology; 1993. pp. 307–318. [Google Scholar]
- 8.Fisher S H. Utilization of amino acids and other nitrogen-containing compounds. In: Sonenshein A L, Hoch J A, Losick R, editors. Bacillus subtilis and other gram-positive bacteria: biochemistry, physiology, and molecular genetics. Washington, D.C: American Society for Microbiology; 1993. pp. 221–228. [Google Scholar]
- 9.Fulco A J. Fatty acid metabolism in bacteria. Prog Lipid Res. 1983;22:133–160. doi: 10.1016/0163-7827(83)90005-x. [DOI] [PubMed] [Google Scholar]
- 10.Gombos Z, Wada H, Murata N. Unsaturation of fatty acids in membrane lipids enhances tolerance of the cyanobacterium SynechocystisPCC 6803 to low-temperature photoinhibition. Proc Natl Acad Sci USA. 1992;89:9959–9963. doi: 10.1073/pnas.89.20.9959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gombos Z, Wada H, Varkonyi Z, Los D A, Murata N. Characterization of the Fad12 mutant of Synechocystisthat is defective in delta 12 acyl-lipid desaturase activity. Biochim Biophys Acta. 1996;1299:117–123. doi: 10.1016/0005-2760(95)00204-9. [DOI] [PubMed] [Google Scholar]
- 12.Grau R, de Mendoza D. Regulation of the synthesis of unsaturated fatty acids by growth temperature in Bacillus subtilis. Mol Microbiol. 1993;8:535–542. doi: 10.1111/j.1365-2958.1993.tb01598.x. [DOI] [PubMed] [Google Scholar]
- 13.Grau R, Gardiol D, Glikin G C, de Mendoza D. DNA supercoiling and thermal regulation of unsaturated fatty acid synthesis in Bacillus subtilis. Mol Microbiol. 1994;11:933–941. doi: 10.1111/j.1365-2958.1994.tb00372.x. [DOI] [PubMed] [Google Scholar]
- 14.Graumann P, Wendrich T M, Weber M H W, Schröder K, Marahiel M A. A family of cold shock proteins in Bacillus subtilisis essential for cellular growth and for efficient protein synthesis at optimal and low temperatures. Mol Microbiol. 1997;25:741–756. doi: 10.1046/j.1365-2958.1997.5121878.x. [DOI] [PubMed] [Google Scholar]
- 15.Graumann P L, Marahiel M A. A superfamily of proteins that contain the cold-shock domain. Trends Biochem Sci. 1998;23:286–290. doi: 10.1016/s0968-0004(98)01255-9. [DOI] [PubMed] [Google Scholar]
- 16.Harwood C R, Cutting S M. Molecular biological methods for Bacillus. Chichester, England: John Wiley & Sons Ltd.; 1990. [Google Scholar]
- 17.Hoch J A, Mathews J L. Chromosomal location of pleiotropic negative sporulation mutations in Bacillus subtilis. Genetics. 1973;73:215–228. doi: 10.1093/genetics/73.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaneda T. Biosynthesis of branched-chain fatty acids. IV. Factors affecting relative abundance of fatty acids produced by Bacillus subtilis. Can J Microbiol. 1966;12:501–514. doi: 10.1139/m66-073. [DOI] [PubMed] [Google Scholar]
- 19.Kaneda T. Biosynthesis of branched-chain fatty acids. V. Microbial stereospecific syntheses of d-12-methyltetradecanoic and d-14-methylhexadecanoic acids. Biochim Biophys Acta. 1966;125:43–54. doi: 10.1016/0005-2760(66)90142-1. [DOI] [PubMed] [Google Scholar]
- 20.Kaneda T. Iso- and anteiso-fatty acids in bacteria: biosynthesis, function, and taxonomic significance. Microbiol Rev. 1991;55:288–302. doi: 10.1128/mr.55.2.288-302.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaneda T, Smith E J. Relationship of primer specificity of fatty acid de novo synthetase to fatty acid composition in 10 species of bacteria and yeasts. Can J Microbiol. 1980;26:893–898. doi: 10.1139/m80-155. [DOI] [PubMed] [Google Scholar]
- 22.Kaneda T, Smith E J, Naik D N. Fatty acid composition and primer specificity of de novo fatty acid synthetase in Bacillus globispores, Bacillus insolitus, and Bacillus psychrophilus. Can J Microbiol. 1983;29:1634–1641. doi: 10.1139/m83-250. [DOI] [PubMed] [Google Scholar]
- 23.Klein W, Boos W. Induction of the lambda receptor is essential for effective uptake of trehalose in Escherichia coli. J Bacteriol. 1993;175:1682–1686. doi: 10.1128/jb.175.6.1682-1686.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ko R, Smith L T, Smith G M. Glycine betaine confers enhanced osmotolerance and cryotolerance on Listeria monocytogenes. J Bacteriol. 1994;176:426–431. doi: 10.1128/jb.176.2.426-431.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kroumova A B, Xie Z, Wagner G J. A pathway for the biosynthesis of straight and branched, odd- and even-length, medium-chain fatty acids in plants. Proc Natl Acad Sci USA. 1994;91:11437–11441. doi: 10.1073/pnas.91.24.11437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kunst F, Ogasawara N, Moszer I, Albertini A M, Alloni G, Azevedo V, Bertero M G, Bessiéres P, Bolotin A, Borchert S, Borriss R, Boursier L, Brans A, Braun M, Brignell S C, Bron S, Brouillet S, Bruschi C V, Caldwell B, Capuano V, Carter N M, Choi S-K, Codani J-J, Connerton I F, Cummings N J, Daniel R A, Denizot F, Devine K M, Düsterhöft A, Ehrlich S D, Emmerson P T, Entian K D, Errington J, Fabret C, Ferrari E, Foulger D, Fritz C, Fujita M, Fujita Y, Fuma S, Galizzi A, Galleron N, Ghim S-Y, Glaser P, Goffeau A, Golightly E J, Grandi G, Guiseppi G, Guy B J, Haga K, Haiech J, Harwood C R, Hẽnaut A, Hilbert H, Holsappel S, Hosono S, Hullo M-F, Itaya M, Jones L, Joris B, Karamata D, Kasahara Y, Klaerr-Blanchard M, Klein C, Kobayashi Y, Koetter P, Koningstein G, Krogh S, Kumano M, Kurita K, Lapidus A, Lardinois S, Lauber J, Lazarevic V, Lee S-M, Levine A, Liu H, Masuda S, Mauël C, Médigue C, Medina N, Mellado R P, Mizuno M, Moestl D, Nakai S, Noback M, Noone D, O’Reilly M, Ogawa K, Ogiwara A, Oudega B, Park S-H, Parro V, Pohl T M, Portetelle D, Porwollik S, Prescott A M, Presecan E, Pujic P, Purnelle B, Rapoport G, Rey M, Reynolds S, Rieger M, Rivolta C, Rocha E, Roche B, Rose M, Sadaie Y, Sato T, Scanlan E, Schleich S, Schroeter R, Scoffone F, Sekiguchi J, Sekowska A, Seror S J, Serror P, Shin B-S, Soldo B, Sorokin A, Tacconi E, Takagi T, Takahashi H, Takemaru K, Takeuchi M, Tamakoshi A, Tanaka T, Terpstra P, Tognoni A, Tosato V, Uchiyama S, Vandenbol M, Vannier F, Vassarotti A, Viari A, Wambutt R, Wedler E, Wedler H, Weitzenegger T, Winters P, Wipat A, Yamamoto H, Yamane K, Yasumoto K, Yata K, Yoshida K, Yoshikawa H-F, Zumstein E, Yoshikawo H, Danchin A. The complete genome sequence of the gram-positive bacterium Bacillus subtilis. Nature. 1997;390:249–256. doi: 10.1038/36786. [DOI] [PubMed] [Google Scholar]
- 27.Kuykendall L D, Roy M A, O’Neill J J, Devine T E. Fatty acids, antibiotic resistance, and desoxyribonucleic acid homology groups of Bradyrhizobium japonicum. Int J Syst Bacteriol. 1988;38:358–361. [Google Scholar]
- 28.Le Rudulier D, Strom A R, Dandekar A M, Smith L T, Valentine R C. Molecular biology of osmoregulation. Science. 1984;224:1064–1068. doi: 10.1126/science.224.4653.1064. [DOI] [PubMed] [Google Scholar]
- 29.Los D A, Ray M K, Murata N. Differences in the control of the temperature-dependent expression of four genes for desaturases in Synechocystissp. PCC 6803. Mol Microbiol. 1997;25:1167–1175. doi: 10.1046/j.1365-2958.1997.5641912.x. [DOI] [PubMed] [Google Scholar]
- 30.Matsubara K, Ohnishi K, Kiritani K. Nucleotide sequences and characterization of liv genes encoding components of the high-affinity branched-chain amino acid transport system in Salmonella typhimurium. J Biochem (Tokyo) 1992;112:93–101. doi: 10.1093/oxfordjournals.jbchem.a123872. [DOI] [PubMed] [Google Scholar]
- 31.Miller L T. Single derivatization method for routine analysis of bacterial whole-cell fatty acid methyl esters, including hydroxy acids. J Clin Microbiol. 1982;16:584–586. doi: 10.1128/jcm.16.3.584-586.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murata N, Wada H. Acyl-lipid desaturases and their importance in the tolerance and acclimatization to cold of cyanobacteria. Biochem J. 1995;308:1–8. doi: 10.1042/bj3080001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nichols D S, Russell N J. Fatty acid adaptation in an Arctic bacterium—changes in primer utilization. Microbiology. 1996;142:747–754. doi: 10.1099/00221287-142-4-747. [DOI] [PubMed] [Google Scholar]
- 34.Ohnishi K, Hasegawa A, Matsubara K, Date T, Okada T, Kiritani K. Cloning and nucleotide sequence of the brnQ gene, the structural gene for a membrane-associated component of the LIV-II transport system for branched-chain amino acids in Salmonella typhimurium. Jpn J Genet. 1988;63:343–357. doi: 10.1266/jjg.63.343. . (Erratum, 63:495.) [DOI] [PubMed] [Google Scholar]
- 35.Ohnishi K, Nakazima A, Matsubara K, Kiritani K. Cloning and nucleotide sequences of livB and livC, the structural genes encoding binding proteins of the high-affinity branched-chain amino acid transport in Salmonella typhimurium. J Biochem (Tokyo) 1990;107:202–208. doi: 10.1093/oxfordjournals.jbchem.a123026. [DOI] [PubMed] [Google Scholar]
- 36.Oku H, Onotogi M, Nagata J, Wada K, Chinen I. Selective use of l-valine and l-isoleucine for the biosynthesis of branched-chain fatty acids in rat skin. Biosci Biotechnol Biochem. 1995;59:891–895. doi: 10.1271/bbb.59.891. [DOI] [PubMed] [Google Scholar]
- 37.Panoff J M, Corroler D, Thammavongs B, Boutibonnes P. Differentiation between cold shock proteins and cold acclimation proteins in a mesophilic gram-positive bacterium, Enterococcus faecalisJH2-2. J Bacteriol. 1997;179:4451–4454. doi: 10.1128/jb.179.13.4451-4454.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rowbury R. Strategies to beat the cold: antifreeze, ice nucleation, mini-greenhouse and “Follow that sun!”. Sci Prog. 1995;78:267–280. [Google Scholar]
- 39.Russell N J. Mechanisms of thermal adaptation in bacteria: blueprints for survival. Trends Biochem Sci. 1984;9:108–112. [Google Scholar]
- 40.Sinensky M. Homeoviscous adaptation—a homeostatic process that regulates the viscosity of membrane lipids in Escherichia coli. Proc Natl Acad Sci USA. 1974;71:522–525. doi: 10.1073/pnas.71.2.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spizizen J. Transformation of biochemically deficient strains of Bacillus subtilisby deoxyribonucleate. Proc Natl Acad Sci USA. 1958;44:1072–1084. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Suutari M, Laakso S. Changes in fatty acid branching and unsaturation of Streptomyces griseus and Brevibacterium fermentansas a response to growth temperature. Appl Environ Microbiol. 1992;58:2338–2340. doi: 10.1128/aem.58.7.2338-2340.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Suutari M, Laakso S. Microbial fatty acids and thermal adaptation. Crit Rev Microbiol. 1994;20:285–328. doi: 10.3109/10408419409113560. [DOI] [PubMed] [Google Scholar]
- 44.Suutari M, Laakso S. Unsaturated and branched chain-fatty acids in temperature adaptation of Bacillus subtilis and Bacillus megaterium. Biochim Biophys Acta. 1992;1126:119–124. doi: 10.1016/0005-2760(92)90281-y. [DOI] [PubMed] [Google Scholar]
- 45.Tauch A, Hermann T, Burkovski A, Kramer R, Puhler A, Kalinowski J. Isoleucine uptake in Corynebacterium glutamicum ATCC 13032 is directed by the brnQgene product. Arch Microbiol. 1998;169:303–312. doi: 10.1007/s002030050576. [DOI] [PubMed] [Google Scholar]
- 46.Thieringer H A, Jones P G, Inouye M. Cold shock and adaptation. Bioessays. 1998;20:49–57. doi: 10.1002/(SICI)1521-1878(199801)20:1<49::AID-BIES8>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 46a.Weber, M. H. W. Unpublished data.
- 47.Willecke K, Pardee A B. Fatty acid-requiring mutant of Bacillus subtilisdefective in branched chain alpha-keto acid dehydrogenase. J Biol Chem. 1971;246:5264–5272. [PubMed] [Google Scholar]
- 48.Womersley C, Uster P S, Rudolph A S, Crowe J H. Inhibition of dehydration-induced fusion between liposomal membranes by carbohydrates as measured by fluorescence energy transfer. Cryobiology. 1986;23:245–255. doi: 10.1016/0011-2240(86)90050-7. [DOI] [PubMed] [Google Scholar]