Summary
Slow oxidative muscle, most notably the soleus, is inherently well equipped with the molecular machinery for regulating blood-borne substrates. However, the entire human musculature accounts for only ∼15% of the body’s oxidative metabolism of glucose at the resting energy expenditure, despite being the body’s largest lean tissue mass. We found the human soleus muscle could raise local oxidative metabolism to high levels for hours without fatigue, during a type of soleus-dominant activity while sitting, even in unfit volunteers. Muscle biopsies revealed there was minimal glycogen use. Magnifying the otherwise negligible local energy expenditure with isolated contractions improved systemic VLDL-triglyceride and glucose homeostasis by a large magnitude, e.g., 52% less postprandial glucose excursion (∼50 mg/dL less between ∼1 and 2 h) with 60% less hyperinsulinemia. Targeting a small oxidative muscle mass (∼1% body mass) with local contractile activity is a potent method for improving systemic metabolic regulation while prolonging the benefits of oxidative metabolism.
Subject area: Health sciences, Physiology, Human metabolism
Graphical abstract
Hamilton, MT. et al. (2022) iScience. A potent physiological method to magnify and sustain soleus oxidative metabolism improves glucose and lipid regulation
Highlights
-
•
We developed a method to capitalize upon the unique phenotype of the soleus
-
•
“A high quality versus large quantity perspective” for muscle activation
-
•
Singular movement targeting the 1 kg soleus easily sustains oxidative metabolism
-
•
This method provides a distinct muscular activity stimulus for metabolic control
Health sciences; Physiology; Human metabolism.
Introduction
By ∼2010 over half of American adults and 80% of those >65 years old had either prediabetes or diabetes (Menke et al., 2015; Xia et al., 2022). There is also currently a high prevalence of prolonged sitting between 9 and 11 h/day (Craft et al., 2012; Healy et al., 2015; Matthews et al., 2018; van der Berg et al., 2016) at a low metabolic rate during seated behaviors (Newton et al., 2013), especially in people who are at high risk for age-associated metabolic diseases such as metabolic syndrome and type 2 diabetes (van der Berg et al., 2016). Even in nondiabetics, postprandial glucose concentration in the 60–120 min range of an oral glucose tolerance test (OGTT) has often been described as one of the strongest independent metabolic risk factors for chronic disease because of linkages to Alzheimer disease (Kakehi et al., 2018; Ohara et al., 2011), neuropathies (Buysschaert et al., 2015; Papanas et al., 2011), dyslipidemia (DeFronzo and Abdul-Ghani, 2011; Festa et al., 2004), and cardiovascular conditions (DeFronzo and Abdul-Ghani, 2011; Succurro et al., 2009). Of concern, glucose tolerance is relatively difficult to improve by a meaningful amount during most therapies, including after substantial amounts of weight loss or exercise (Jansen et al., 2022; King et al., 1995; Knudsen et al., 2014; Magkos et al., 2016; Rose et al., 2001; Ross et al., 2000, 2015; Slentz et al., 2016).
There is no doubt that inactive muscle fibers require little energy (Dela et al., 2019; Kelley et al., 1994; Rolfe and Brown, 1997) and that the whole-body oxidative metabolism is low throughout many hours of the day when sitting with inactive muscles (Newton et al., 2013); this may be one of the most fundamental yet overlooked issues guiding the way toward discovering metabolic solutions to assist in preventing some age-associated chronic diseases. During periods of inactivity, skeletal muscle accounts for only ∼15% of the whole-body postprandial glucose oxidation in nondiabetic controls of similar age and BMI as in the present studies (Kelley et al., 1994), despite being the body’s largest lean tissue mass (∼21–31 kg in women and men) (Heymsfield et al., 2022). Consistent with this, multiple studies using the arteriovenous balance method of the lower limb have calculated that the oxygen consumption (VO2) of inactive muscle is ∼1–2 mL/min/kg Dela et al. (2019); Kelley et al. (1994); (Rolfe and Brown, 1997). Therefore, during acute inactivity, the muscle-mass-specific VO2 (in units of mL/min/kg muscle) is even less than the modest value of ∼3.0–3.5 mL/min/kg body weight for the basal metabolic rate lying down or during prolonged sitting throughout the day in a whole room calorimeter (Newton et al., 2013). Thus, contrary to a common notion, even though skeletal muscle is the body’s largest lean tissue mass, it is unlikely the dominant contributor to the oxidative metabolism of either glucose or lipids when sitting at resting energy expenditure. The prevailing perspective (mostly from epidemiology) has been that there is a whole-body metabolic rate threshold that must be exceeded to induce a robust gain in metabolic health responses. Furthermore, the specific muscles recruited and types of contractile activity have largely been disregarded in human research. Taking a step back, we took a more physiological perspective in the current experiments. Herein, we tested the direct and immediate effects of sustaining a high duration of elevated oxidative muscle metabolism when sitting. We used 2 guiding principles in our approach.
First and foremost, as described earlier, the energy demand is minimal in resting muscle fibers. Therefore, mitochondrial oxidative phosphorylation is capped at a relatively low ceiling during inactivity (Dela et al., 2019; Kelley et al., 1994; Rolfe and Brown, 1997). Related to this, the elevated energy demands and fuel requirements for carbohydrate oxidation quickly come to an end when an exercise bout ends (Horton et al., 1998; Wasserman et al., 1991). For these reasons, there is a need for understanding the biochemical effects of sustaining an elevated rate of oxidative metabolism by skeletal muscle, but with a subtle rate of whole-body energy expenditure.
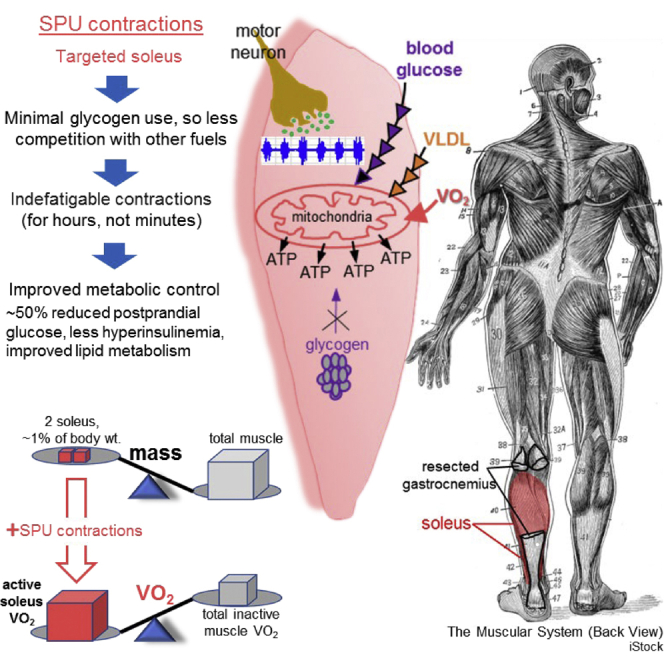
Second, slow oxidative muscle has multiple intrinsic molecular and phenotypic features favoring specialization in prolonged contractile activity, in part because of the capacity for using more blood-borne fuels and hypothetically less glycogen in some physiological conditions; this is supported by animal (Bey and Hamilton, 2003; Cartee et al., 2016; Deshmukh et al., 2021; Halseth et al., 1998; James et al., 1985; Mackie et al., 1980; McDonough et al., 2005; Terry et al., 2018) and human (Deshmukh et al., 2021; Gollnick et al., 1974a, 1974b; Jensen et al., 2012; Johnson et al., 1973; Murgia et al., 2021) studies that have long described the heterogeneous qualities between different fiber types within a muscle and between different muscles. The soleus has a greater predominance of slow-oxidative fibers (∼88% of the soleus mass is type I slow-twitch fibers) than 36 other human muscles that have also been fiber typed (Johnson et al., 1973). The soleus is a slow-twitch postural muscle that has motor neurons and other features favoring a lower threshold of effort needed to recruit it for more time and intensity than other limb muscles (Hodgson et al., 2005; Monster et al., 1978). Compared with other leg muscles, highly controlled studies in rodents have found the soleus has a phenotype favoring more uptake of both plasma TG (Bey and Hamilton, 2003; Mackie et al., 1980) and blood glucose (Halseth et al., 1998; James et al., 1985). It has distinctive vascular features enhancing delivery of blood-borne fuels and oxygen (McDonough et al., 2005), relatively high levels of hexokinase II and GLUT4 (Jensen et al., 2012), and a relatively low concentration of glycolytic enzymes and glycogen phosphorylase (Gollnick et al., 1974a, 1974b). However, walking can cause rapid rates of glycogen depletion in the soleus as in other muscles (Jensen et al., 2012). Therefore, it is far from certain whether there is an effective physiological approach to capitalize on the phenotype of this slow oxidative muscle to improve systemic lipid and glucose metabolism.
The scientific challenges and potential impact of developing a method for raising oxidative metabolism locally by a small tissue is perhaps best understood in light of the already much more established scientific interest (Chen et al., 2020) in activating another small tissue with an oxidative phenotype, brown adipose tissue (BAT). The soleus (Bey and Hamilton, 2003; Halseth et al., 1998; James et al., 1985; Jensen et al., 2012; Mackie et al., 1980; Petersen et al., 2003; Song et al., 1999) and BAT (Chondronikola et al., 2014; McNeill et al., 2020) are both tissues making up too small a percentage of total body mass to alter energy expenditure unless methods are developed to cause an intense local metabolic activation. Yet under some conditions, both might possibly be equipped with a phenotype favoring exceptional metabolic rates over prolonged periods of time. The specific questions we posed are analogous to the hurdles already faced in BAT research; how can people consistently activate tissue specific oxidative metabolism at a meaningful rate to increase whole-body oxygen consumption and then sustain it for hours at a time? Even if methods were developed to make that possible, would raising the local metabolic rate by a small mass of tissue be sufficient to impact systemic metabolic parameters as complex as very-low-density lipoprotein (VLDL)-TG concentration and postprandial glucose tolerance?
This work was part of an effort to develop a method of muscular contractile activity specifically geared for sustaining the possible distinct benefits of oxidative metabolism for prolonged periods, instead of sitting with inactive muscle at a low metabolic rate. The present experiments were designed to test the potential physiological influence of the human soleus muscle during hours of prolonged contractile activity.
Results
Overview of participants and experimental approach to raise muscle metabolism
As outlined below and described in more detail in the STAR Methods and supplemental information, participants included an equal number of male and female volunteers with a wide range of BMI, age, sedentary time, and habitual daily steps (Table S1). With regard to free-living sedentary time and activity profiling (Table S1), the volunteers were representative of the populations we and others studied with objective wearable tracking devices (Barreira et al., 2016; Matthews et al., 2018; van der Berg et al., 2016). Free-living activity assessment showed an average of 10.7 ± 2.1 h/day sitting time (mean ± SD) with a range of 6–14 h/day.
These studies focused on understanding the responses from local contractile activity of slow oxidative muscle when the total energy expenditure was relatively close to resting metabolic rate (∼0.5–1.5 kcals/min above rest, or ∼1.3–2.0 metabolic equivalents [METs, 1 MET = 3.5 mL oxygen/kg/min]; Figure S1 and Tables 1 and 2). This was accomplished by developing and testing a special type of isolated plantarflexion activity targeting the soleus when sitting (Figure S2), to increase the oxygen consumption from local contractile activity as described in the STAR Methods and supplemental information (Figures S3 and S4). For clarity and brevity, we use the term SPU, or “soleus push up,” for this specific type of plantarflexion because the relatively high soleus electromyography (EMG) on-time (i.e., soleus activation) coincided with upward angular motion of the ankle (Figures S2, S4, and S5).
Table 1.
Metabolic rate and glycogen use during local contractile activity with SPU contractions
| Experiment I | Sedentary control | SPU contractions | p-value |
|---|---|---|---|
| Energy expenditure during SPU contractions | |||
| METs | 0.92 ± 0.04 | 2.03 ± 0.08 | 8 × 10−8 |
| AEE (Δ kcal/min during contractions) | — | 1.51 ± 0.15 | 4 × 10−6 |
| % increase whole-body energy expenditure during muscle contractions | — | 124 ± 9 | 3 × 10−7 |
| Muscle glycogen concentration (mmol/kg) | |||
| Vastus lateralis at the final biopsy | 96 ± 6 | 92 ± 6 | 0.601 |
| Soleus at the first biopsy (130 min contractions) | 91 ± 5 | 76 ± 5 | 0.183 |
| Soleus at the final biopsy (270 min contractions) | 90 ± 5 | 68 ± 5 | 0.007 |
| % of AEE contributed by soleus glycogen | — | 4.1 ± 1.0 | 0.003 |
Mean ± SEM. Glycogen contribution to activity energy expenditure (AEE) was based on 3.75 kcal per gram of monomeric glucose units derived from glycogen if completely oxidized. The soleus mass averaged 1.07 ± 0.25 kg (combined mass in both legs) in these 10 participants. The calculation of the % of the total AEE contributed by soleus glycogen during 270 min of contractions was calculated as described in the STAR Methods. The full aerobic combustion of 22 mmol/kg (90–68 mmol/kg) of glycogen would provide about 16 kcal for the combined 1.07 kg soleus muscles. To determine if the energetics of SPU contractions were different than when sitting inactive (control), the results were analyzed with paired t tests. To determine the effect of SPU contractions on soleus glycogen, a mixed effects model with Tukey’s multiple comparison tests was used, because comparisons of control versus contractions were performed at two time points (130 and 270 min). See also Experiment I results in Figures S1 and S8 and Table S2.
Table 2.
Metabolic rate and carbohydrate oxidation during the 3-h oral glucose tolerance test
| Experiment II | Sedentary control | SPU1 | SPU2 | p-value |
||
|---|---|---|---|---|---|---|
| SED vs SPU1 | SED vs SPU2 | SPU1 vs SPU2 | ||||
| METs | 0.86 ± 0.05 | 1.31 ± 0.07 | 1.69 ± 0.12 | 5 × 10−8 | 3 × 10−6 | 9 × 10−6 |
| AEE (Δ kcal/min) | --- | 0.60 ± 0.05 | 1.12 ± 0.11 | 2 × 10−8 | 3 × 10−6 | 4 × 10−6 |
| Energy exp. (kcal/3 h) | 207 ± 11 | 315 ± 16 | 402 ± 24 | 2 × 10−8 | 4 × 10−6 | 8 × 10−6 |
| Total AEE (Δ kcal/3 h) | --- | 108 ± 9 (+36 kcal/h) | 201 ± 19 (+67 kcal/h) | 2 × 10−8 | 3 × 10−6 | 4 × 10−6 |
| RER (VCO2/VO2) | 0.84 ± 0.02 | 0.88 ± 0.01 | 0.90 ± 0.02 | 0.019 | 0.003 | 0.112 |
| Total carbohydrate oxidation (mg/min) | 135 ± 18 | 276 ± 14 | 392 ± 25 | 1 × 10−6 | 4 × 10−6 | 0.0002 |
| Δ mg/min above control | --- | 141 ± 16 | 253 ± 26 | 1 × 10−6 | 5 × 10−6 | 0.0002 |
| Total in 3 h (g) | 24.2 ± 3.2 | 49.7 ± 2.5 | 70.6 ± 4.6 | 1 × 10−6 | 4 × 10−6 | 0.0002 |
| Δ above control (g) | --- | 25.5 ± 2.9 (2.1 fold) | 45.5 ± 4.6 (2.9 fold) | 1 × 10−6 | 5 × 10−6 | 0.0002 |
| Soleus carbohydrate oxidation (Δ mg/min) | --- | 113 ± 13 | 202 ± 21 | 1 × 10−6 | 5 × 10−6 | 0.0002 |
Mean ± SEM. Metabolic rate and carbohydrate oxidation during the postprandial period following the ingestion of 75 g glucose. Each individual performed a sedentary control and one or both of the levels of local contractile activity. The delta (Δ) is the difference between sedentary control and activity. Differences between conditions were determined by a mixed effects model followed by Tukey’s multiple comparison tests. N = 15 for SPU1 effects and N = 10 for SPU2. See also Experiment II results in Figure S1 and Table S2.
Figure S6 provides a schematic overview to summarize the tests performed in two related experiments, each examining effects caused by raising the local energy expenditure with this type of soleus dominant plantarflexion. In the first experiment, we obtained 60 muscle biopsies from the soleus and vastus lateralis (VL) for measuring glycogen, which led us to determine in the second experiment the effects of sustaining this type of muscle metabolism in a 13-point OGTT after ingesting a 75-gram glucose load in a diverse group of 15 men and women.
SPU contractions induce minimal soleus glycogen depletion
Volunteers all responded well to the prolonged contractile activity and did not experience fatigue or other adverse responses to the prolonged contractile activity, such as cramps, joint pain, or muscle soreness. It is important to note that volunteers in Experiment I (Table S1) were typically sedentary (verified with an objective tracking device), and none of them had a high aerobic cardiorespiratory fitness (determined by treadmill VO2max or the maximal oxygen consumption test). In Experiment I, the SPU contractions increased the rate of total body energy expenditure from an average of 0.93 ± 0.04 METs to 2.03 ± 0.08 METs during the acute activity (Table 1). A more detailed analysis of the muscle mass and analysis of the rate of oxygen consumption (VO2) by the working muscle mass is provided in another section below.
Glycogen was measured in the VL on each test day as a control for an inactive muscle during this local activity (Table 1). Moreover, when sitting inactive, the average soleus muscle glycogen was steady between the first and final biopsy (91 versus 90 mmol/kg). Together these findings show there was not significant uncontrolled day-to-day variation, and as expected, glycogen was stable in an inactive muscle during SPU contractions.
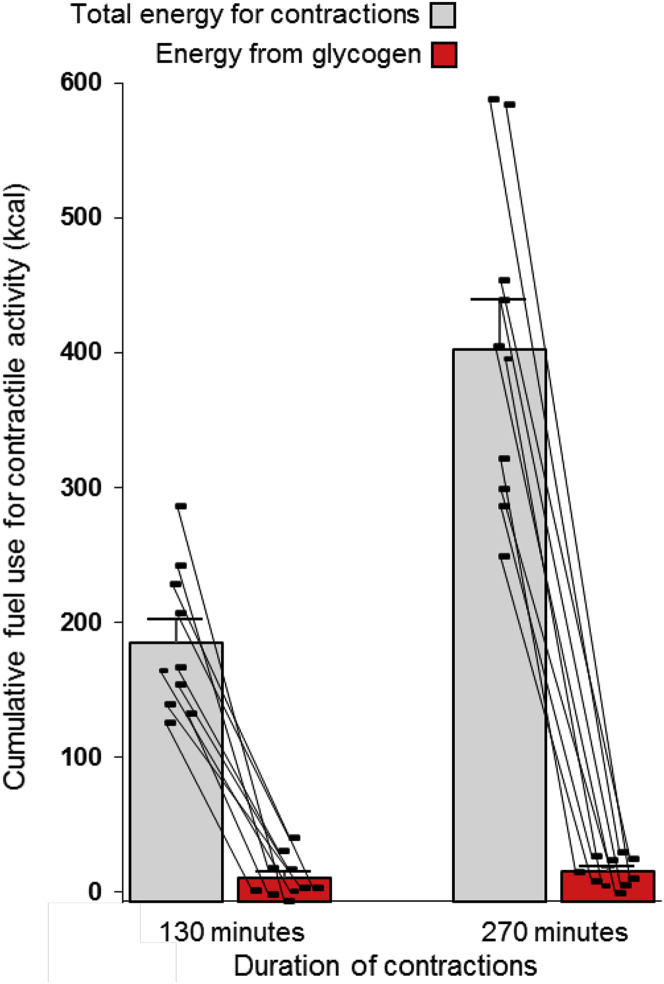
The soleus glycogen concentration was reduced an average of 22 mmol/kg (90 to 68 mmol/kg) because of the 270 min of SPU contractions, which corresponded to a net rate of glycogen use of 0.080 ± 0.017 mmol/kg/min. The more certain rate between the two biopsies on the active day was similarly low at 0.053 ± 0.032 mmol/kg/min, but it was not significantly different from 0 (p = 0.130). Glycogen had not reached statistical significance after 130 min of contractions (p = 0.183). The concentration difference was significant at 270 min (Table 1). However, the net soleus glycogen reduction was equivalent to 4% of the 403 kcals total AEE (Table 1 and Figure 1). Consistent with that, theoretical calculations show that the high local energy demand could not have been sustainable for long as a result of the potential energy from soleus glycogen. Even in the extreme theoretical scenario of 100% glycogen depletion, aerobic combustion of all soleus glycogen can provide no more than 65 kcals (90 mmol/kg x 1.07 kg soleus x 0.675 kcal/mmol = 65 kcals) of the actual total 403 kcals or ∼5 kcals if the glycogen was broken down for nonoxidative glycolytic ATP production. The lack of a sensation of local fatigue or rising effort over time is consistent with minimal glycogen depletion.
Figure 1.
Minimal contribution from soleus glycogen to the total energy for contractions (the activity energy expenditure) during prolonged local activity of the soleus with SPU contractions
Individual results are shown with the mean ± SEM. N = 10 in Experiment I. The glycogen contribution was negligible at both 130 and 270 min and significantly less than the total energy demand for contractions at both time points (both p < 0.0001, mixed effects model followed by Tukey’s multiple comparison tests). See also Figures S7 and S8, and Table 1 for more details.
SPU contractions reduce VLDL-TG while raising fat and carbohydrate oxidation
We measured the increase in total body fat and carbohydrate oxidation during the SPU contractions and compared this to when sitting inactive on the sedentary control test day. Furthermore, we determined if this method of local contractile activity would decrease VLDL-TG (and the total number of VLDL particles in plasma). There is strong prior evidence that acute activity/inactivity is a direct determinant of plasma-lipoprotein-derived TG uptake because of local mechanisms in the microcirculation of muscle, as shown with radioactive tracer studies in rats (Bey and Hamilton, 2003; Hamilton et al., 1998; Mackie et al., 1980). Fat and carbohydrate oxidation were increased in each individual during the SPU contractions (Figure S7); this was when the average respiratory exchange ratio (RER) was 0.78 ± 0.01 during the inactive test day and 0.80 ± 0.01 during SPU contractions (not statistically different). SPU contractions caused a significant VLDL-TG decrease (Figure S8). The triglyceride content (Figure S8) and number of VLDL particles (Figure S8 inset) within all 3 sizes of VLDL in the circulation were responsive. Most of the TG was contained in the large particles (Figure S8), even though most of the particles were small (Figure S8 inset).
SPU contractions are a method to induce and maintain a relatively high local rate of oxygen consumption (VO2/min/kg muscle) during prolonged contractile activity
A fundamental principle in exercise physiology is that a small muscle mass working in isolation can achieve a higher local oxygen consumption (VO2/min per kg) than when recruiting a large muscle mass (Cardinale et al., 2019). For example, the VO2 in the whole lower limb musculature in young ultra-endurance athletes reached almost 200 mL/min/kg during exhaustive cycling in a VO2max test and a significantly greater maximal local rate of ∼350 mL/min/kg by the quadriceps during intense isolated leg extensions (Cardinale et al., 2019). Thus, to better describe the effects of elevated oxidative metabolism on soleus glycogen, measurements of muscle mass were also obtained in Experiment I. These 10 individuals were also studied during treadmill exercise because that is a large muscle mass modality requiring compound movements across all of the joints and muscle groups in the lower limbs. The muscle mass of the entire lower limb (14.8 ± 1.1 kg) was estimated from the appendicular lean mass (minus bone) from dual-energy X-ray absorptiometry (DEXA) for when comparing energetic calculations described later.
The mass of the soleus and other triceps surae (TS) muscles was directly measured from magnetic resonance imaging (MRI). The soleus was significantly larger than the two gastrocnemius muscles; soleus 1.07 ± 0.08, lateral gastrocnemius (LG) 0.169 ± 0.02, medial gastrocnemius (MG) 0.350 ± 0.02 kg. The soleus was 1.34% of body weight and 67% of the triceps surae, which was similar to previous MRI results of apparently healthy men and women (Kolk et al., 2015). The soleus dominated the recruited mass of the TS muscle group even more when calculated as the product of the anatomical mass and the percentage recruitment (percent EMGmax); the soleus accounted for ∼80% of the recruited mass with SPU contractions (Figure 2B). The estimated 20% contribution by the gastrocnemius is potentially an overestimate because, unlike the soleus, the gastrocnemius is a 2-joint muscle crossing the knee and ankle joints. The energetic contribution from the gastrocnemius is markedly suppressed by bending the knee and thereby demanding a significantly greater energy contribution from the soleus (Niess et al., 2018; Price et al., 2003). The gastrocnemius remains in a flaccid position while the soleus is contracting intensely if the knee is bent during ankle plantarflexion (Kawakami et al., 1998). The soleus also has a highly pennated architecture favoring greater amounts of muscular work during plantarflexion than predicted by mass alone, giving it a physiological cross-sectional area that is exceptionally high (3–8 times more than most of the 20 other limb muscles studied) (Ward et al., 2009). Because of these reasons, the exact contribution by the soleus can be underestimated by estimates from only the anatomical mass. There are other smaller muscles in the lower leg that might offset the gastrocnemius estimates. Therefore, ∼80% of the increased energy demand above rest is the best estimate we have for calculating the local soleus oxidative metabolism of substrates during SPU contractions.
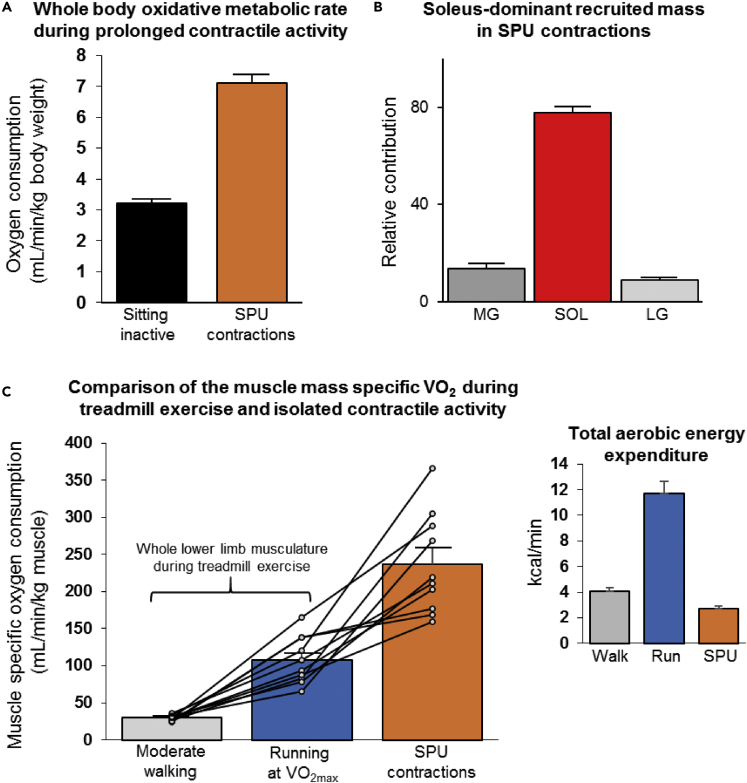
Figure 2.
Whole-body and local oxidative metabolism during SPU contractions when sitting and during treadmill exercise
(A) SPU contractions approximately doubled whole-body VO2 above the normal resting metabolic rate when sitting (p = 8 × 10−8, paired t test, N = 10).
(B) The relative contribution of the medial gastrocnemius (MG), soleus (SOL), and lateral gastrocnemius (LG) to the estimated proportion of the recruited mass as determined with MRI and EMG.
(C) The calculated VO2 per kg soleus muscle during SPU contractions as a mode of isolated plantarflexion was compared with the VO2 per kg of the whole lower limb musculature during walking at a moderate-intensity (p = 0.00001) and high-intensity treadmill exercise (p = 0.001). Statistics were determined with a mixed effects model followed by Tukey’s multiple comparison tests. Individual results of 10 untrained/unfit participants with an average VO2max of 30 mL/min/kg body weight are shown with the mean ± SEM. See also Figure S1.
The estimated soleus VO2 during the prolonged SPU contractions averaged 237 ± 21 mL/min/kg (80% of the delta VO2 divided by the soleus anatomical mass; Figure 2C). Furthermore, because the soleus accounts for most of the TS anatomical mass in these individuals (1.07 of the 1.59 kg), the soleus VO2 would only be slightly reduced to 197 ± 17 mL/min/kg if the oxygen consumption were distributed evenly across the entire TS muscle group. The rate of total body energy expenditure during moderate walking and SPU contractions was obviously much less than running (Figure 2C inset). However, the local soleus VO2/kg during SPUs was greater than the average muscle VO2/kg of the lower limb muscle mass during treadmill exercise (Figure 2C). The estimated VO2 of the lower limb musculature was 108 ± 10 mL/min/kg muscle during the last stage before exhaustion, assuming 75% of the increase in total body oxygen consumption is in the lower limbs at the end of the VO2max test (Cardinale et al., 2019). This same approach calculated that moderate-intensity walking at 3 METs consumed an estimated 30 ± 1 mL/min/kg for the lower limb musculature. From this, VO2/kg in the lower limb during walking is roughly ∼13% of the soleus VO2/kg during the 4.5 h of SPU contractions.
In summary, the rate of total body energy expenditure from aerobic metabolism (kcals/min calculated from total body VO2) was by design lower when working the small muscle mass than using a large muscle mass while briefly running at VO2max (Figure 2C inset). Most importantly, though, these findings demonstrate the human soleus of untrained adults is capable of sustaining a high local rate of oxygen consumption in parallel with a low amount of glycogen depletion during prolonged contractile activity.
SPU contractions improve postprandial glucose tolerance
Table 2 describes the postprandial metabolic rate after ingesting a 75-gram glucose load when sedentary or at two levels of SPU contractions. Before beginning contractions, the fasted glucose values were not different (Table 3). Then, beginning early in the postprandial period and lasting until the final time point of the 180-min test, both levels of contractions resulted in sustained reductions in glucose concentration (Table 3 and Figures S10A and S10B). These effects were evident in both SPU test days. In SPU2, the average glucose concentration was reduced significantly by 19 mg/dL already at 30 min (Table 3) and trending to decrease 10 mg/dL in SPU1. The largest treatment effect over a 60-min period (averaging 5 consecutive measurements of each individual) was 50 ± 6 mg/dL in SPU2. Statistically significant differences lasted until at least 180 min. The separation in glucose concentration between the sedentary control and activity trials expanded up until ∼75–135 min (Table 3); this coincides with about the time frame most commonly studied when relating glucose to clinically meaningful pathologies (Buysschaert et al., 2015; DeFronzo and Abdul-Ghani, 2011; Festa et al., 2004; Kakehi et al., 2018; Ohara et al., 2011; Papanas et al., 2011; Succurro et al., 2009).
Table 3.
Effect of sustaining local muscle metabolism on glucose concentration at each time point
| Time |
SPU1 effect at each time point |
SPU2 effect at each time point |
||||
|---|---|---|---|---|---|---|
| minutes | N = 15 Control |
SPU1 | Δ mg/dL | N = 10 Control |
SPU2 | Δ mg/dL |
| 0 | 99 ± 2 | 101 ± 2 | NS, p = 0.524 | 99 ± 3 | 102 ± 4 | NS, p = 0.416 |
| 15 | 132 ± 5 | 123 ± 4 | NS, p = 0.061 | 132 ± 7 | 124 ± 5 | NS, p = 0.427 |
| 30 | 167 ± 6 | 157 ± 5 | NS, p = 0.092 | 166 ± 7 | 147 ± 7 | −19 ± 6∗ |
| 45 | 190 ± 7 | 167 ± 7 | −22 ± 5∗∗ | 193 ± 6 | 165 ± 7 | −28 ± 8∗ |
| 60 | 201 ± 8 | 169 ± 8 | −33 ± 7∗∗∗ | 206 ± 6 | 163 ± 7 | −43 ± 7∗∗∗ |
| 75 | 198 ± 8 | 164 ± 8 | −34 ± 8∗∗ | 207 ± 4 | 159 ± 7 | −47 ± 6∗∗∗∗ |
| 90 | 192 ± 9 | 157 ± 7 | −34 ± 7∗∗∗ | 198 ± 4 | 151 ± 7 | −48 ± 8∗∗∗ |
| 105 | 186 ± 10 | 155 ± 6 | −31 ± 6∗∗∗ | 191 ± 4 | 142 ± 6 | −49 ± 7∗∗∗∗†† |
| 120 | 175 ± 9 | 146 ± 6 | −29 ± 6∗∗∗ | 182 ± 5 | 136 ± 5 | −46 ± 7∗∗∗†† |
| 135 | 165 ± 10 | 134 ± 8 | −31 ± 6∗∗∗ | 176 ± 6 | 128 ± 5 | −48 ± 6∗∗∗∗†† |
| 150 | 146 ± 11 | 121 ± 8 | −25 ± 9∗ | 154 ± 11 | 116 ± 7 | −38 ± 8∗∗ |
| 165 | 129 ± 11 | 106 ± 7 | −24 ± 10 | 137 ± 13 | 103 ± 9 | −34 ± 9∗∗ |
| 180 | 117 ± 10 | 97 ± 7 | −20 ± 7∗ | 121 ± 13 | 92 ± 9 | −29 ± 8∗∗ |
Mean ± SEM. Effects of SPU contractions were determined by a mixed effects model followed by Tukey’s multiple comparison tests. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001 versus sedentary control; †p < 0.05, ††p < 0.01 for SPU2 versus SPU1. N = 15 for SPU1 effects and N = 10 for SPU2. The zero time point was always taken when in the overnight fasted state and sitting inactive before the 3-h 75-g OGTT. See also Figures S10A and S10B for the graphical depiction of the time course for glucose concentrations for these results in Experiment II.
Significant improvements in the total 3-h glucose incremental area under the curve (iAUC) (Figure 3A) were found in both men and women, and in younger and older adults, and after segregating the subjects by other common ways of categorizing participants such as by BMI categories and if they were more or less habitually active than the average person (Table 4). Some participants in Experiment II had never exercised regularly in their past. Some others had been competitive athletes. However, all had noticeably more hyperglycemia during the acute sitting at a low metabolic rate and improved glucose tolerance by SPU contractions (Figure 3A and Table 4); this demonstrates there is an apparent biochemical robustness in realizing the immediate benefits of this type of muscle metabolism. Although we did not observe a trend between sexes or other groups (Table 4), one should be cautious when interpreting the relative effectiveness in subcategories until follow-up studies with larger sample sizes are performed.
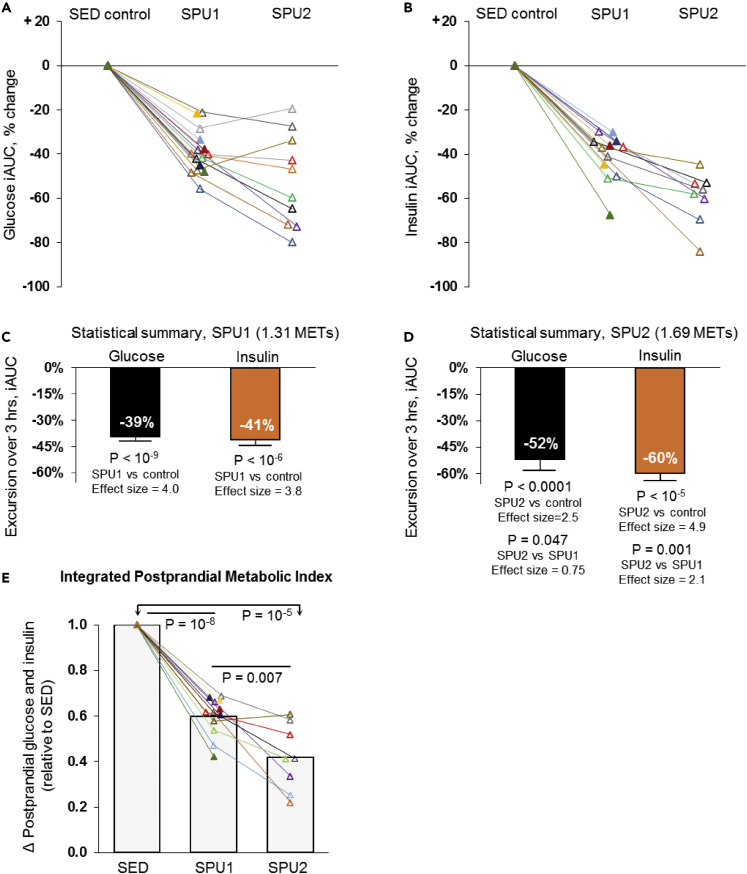
Figure 3.
Sustaining elevated muscle metabolism with soleus contractions is sufficient to cause improved glucose tolerance and reduced postprandial hyperinsulinemia, with up to a 52%–60% reduction in the blood glucose and insulin iAUC
See Table 2 for complete results of the energetics for SPU1 (N = 15) and SPU2 (N = 10). Responses reveal a robust soleus muscle activity-dependent glucose (A) and insulin (B) lowering in each individual during a 3-h 75-g OGTT. Statistical summary (C and D) of the average iAUC responses from 0 to 180 min. Effect sizes are calculated by Cohen’s d test. SPU1 and SPU2 had effect sizes considered to be “huge” (>2.0) (Sawilowsky, 2009) for both glucose and insulin iAUC.
(E) This index is the average of the glucose iAUC and the insulin iAUC for each individual, expressed relative to when sitting inactive (SED). Differences between conditions were determined by mixed effects models followed by Tukey’s multiple comparison tests. Mean ± SEM. The actual glucose concentration differences between conditions at each time point are in Table 3.
Table 4.
Three-hour postprandial glucose tolerance (iAUC) after subdividing participants
| Characteristics | Mean | Range | N | % iAUC | SPU1 Effect p-value |
Interaction p-value |
|---|---|---|---|---|---|---|
| Females | 8 | −39.8 ± 3.9 | 0.00002 | 0.804 | ||
| Males | 7 | −38.5 ± 3.4 | 0.00003 | |||
| Youngest (years) | 38 | 22–51 | 7 | −39.2 ± 3.2 | 0.00002 | 0.986 |
| Oldest (years) | 68 | 56–82 | 8 | −39.2 ± 4.0 | 0.00002 | |
| Lower BMI | 23.3 | 19.7–27.8 | 8 | −37.9 ± 3.4 | 0.00001 | 0.602 |
| Obese BMI | 34.3 | 29.2–42.9 | 7 | −40.7 ± 4.0 | 0.00005 | |
| Lowest habitual sitting time (h/d) | 9.0 | 6.7–10.6 | 7 | −39.4 ± 4.5 | 0.0001 | 0.939 |
| Highest habitual sitting time (h/d) | 12.0 | 10.9–13.9 | 8 | −39.0 ± 3.0 | 0.000004 | |
| Lowest habitual steps (step/day) | 4,365 | 2,061–5,544 | 8 | −37.5 ± 3.3 | 0.000009 | 0.484 |
| Highest habitual steps (step/day) | 7,922 | 5,828–10,843 | 7 | −41.2 ± 4.0 | 0.00005 | |
| Normal fasting glucose (mg/dL) | 94 | 91–99 | 8 | −37.9 ± 3.8 | 0.00002 | 0.595 |
| Impaired fasting glucose (mg/dL) | 108 | 102–115 | 7 | −40.7 ± 3.5 | 0.00002 |
The 15 participants were divided according to the above characteristics to compare the % change in the glucose iAUC caused by SPU1 compared with when sitting inactive in Experiment II. Two-tailed paired t tests were used to determine if the % change in glucose iAUC caused by SPU1 (versus sedentary control) was significant in each subdivision (bolded column). The participant characteristic by activity level interaction was determined with a mixed effects model to determine if there was a difference in the iAUC response to SPU1 within each subdivision pair (far right column). From this analysis, we conclude that there was no evidence to suggest men were different than women for the glucose response to SPU1 nor were there any significant characteristic by activity level interactions for the other 5 characteristics. Mean ± SEM. See also Experiment II demographics in Table S1.
SPU contractions reduce postprandial hyperinsulinemia and insulin secretion
For the insulin as well as the glucose iAUC (Figures 3C and 3D), the Cohen’s d effect size (ES) was in the statistical category of “huge” (Sawilowsky, 2009) for both levels of muscle metabolism (SPU1 mean −41% and 3.8 ES; SPU2 mean −60% and 4.9 ES).
By the 3rd hour, the average insulin concentration differences between the active and inactive state (Figures S10C and S10D) had expanded further to −50% (SPU1) and −71% (SPU2). These results indicate a progressively greater percentage effect of contractions over time on insulin, especially when examining the SPU2 effect. The trend over time indicates the insulin reduction (compared to sedentary control) would last longer than 3 h had the test been extended (Figures S10C and S10D). Therapies impacting postprandial metabolism are difficult to compare without evaluating both hyperglycemia in tandem with hyperinsulinemia because exposure to both impacts glucose uptake by body tissues additively or synergistically. Thus, in a simple index we computed the average of both glucose and insulin iAUC relative to the SED control. There was a large magnitude of effect in both SPU levels studied (Figure 3E).
From these findings below, the reduction in insulin concentration was likely in part due to reduction in pancreatic insulin secretion. Overall, the C-peptide response was strongly correlated with the glucose effects (r = 0.81, p = 0.00003), as were the insulin effects (r = 0.65, p = 0.002). The C-peptide iAUC was reduced 30 ± 3% (p = 0.00002) by SPU1 and 44 ± 6% (p = 0.0003) by SPU2. Thus, there are other systemic responses of this type of sustained muscle metabolism beyond improved glucose concentration per se.
SPU contractions increase carbohydrate oxidation during the OGTT
We calculated the rate of carbohydrate oxidation during the OGTT in each individual (Figure 4A) from VO2 and RER as summarized in Table 2. The individual results illustrate carbohydrate oxidation when inactive in the postprandial period and the consistently greater rate during SPU contractions in everyone (Figure 4A).
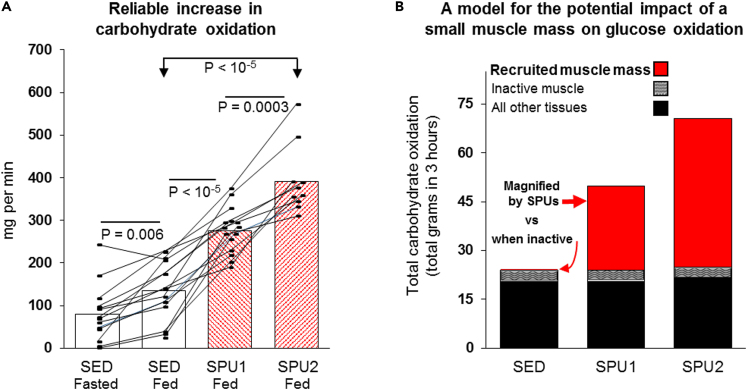
Figure 4.
Recruitment during locally intense activation of a small mass dominated by the soleus consistently has the capability to raise whole-body carbohydrate oxidation above the rest of the body
(A) The rate of carbohydrate oxidation after ingesting a glucose load was consistently increased during both levels of local contractile activity (see Table 2 for more results). The fasted condition was measured sitting at rest prior to the OGTT, and SED was the inactive control condition during the OGTT. SPU1 (N = 15) and SPU2 (N = 10). Differences between conditions were determined by mixed effects models followed by Tukey’s multiple comparison tests.
(B) A model summarizing the influence of a small muscle mass on oxidative metabolism during the 75 g OGTT. Although contributing a negligible amount to systemic metabolism when not contracting, the energy demand of even a relatively small muscle mass has the potential to contribute meaningfully to carbohydrate metabolism when contracting with this single isolated SPU movement. This model is consistent with the findings that the total body skeletal muscle mass at rest accounts for ∼15% of the total systemic glucose oxidation in the postprandial period in nondiabetic controls with similar age and BMI as participants in the present study (Kelley et al., 1994). SPU contractions caused a 2.1- (SPU1) and 2.9-fold (SPU2) increase in the total body carbohydrate oxidation (Figure 4A). As shown in red bars in Figure 4B, the local contractile activity was sufficient to raise glucose oxidation above all inactive muscles and other body tissues combined.
From the averages, the cumulative whole-body carbohydrate oxidation was about 24, 50, and 71 grams within the 3 h after ingesting the 75 g glucose load for the sedentary control, SPU1, and SPU2, respectively (Table 2 and Figure 4B). This local SPU contractile activity was sufficient to more than double carbohydrate oxidation and raise this source of fuel utilization higher than in the rest of all the tissues in the body combined (Figure 4B). Kelley’s study (Kelley et al., 1994) of nondiabetic subjects at a similar age and BMI as in the present study directly measured skeletal muscle carbohydrate oxidation in the whole leg at rest, and the rate by muscle was estimated at ∼0.7 mg/min/kg muscle (Kelley et al., 1994). This rate would be undetectable in a small muscle mass of 1 or even 2 kg, which would obviously be too small to cause a measurable influence on the total carbohydrate oxidation when inactive (see the Figure 4B model). This small muscle mass actively increased the rate 141 mg/min by SPU1; this corresponds to a rate of 113 mg/min by the 1.07 kg soleus, assuming 80% of the increase in carbohydrate oxidation was by the local muscle contraction. Therefore, any potential effect of the soleus muscle phenotype on postprandial carbohydrate oxidation would likely be undetectable at rest and would be magnified substantially by raising the local energy demand and VO2; this is illustrated in a simple model (Figure 4B) of 3 body compartments (a small recruited muscle mass, a much larger whole-body muscle mass, and the rest of the body).
Taken together, these findings clearly demonstrate that the SPU contractions were magnifying the normally low rate of carbohydrate oxidation by a small mass of muscle, to the point that it becomes the most dominant tissue for carbohydrate oxidation in the entire body for the 3 h after ingesting glucose (Figure 4B).
The sustained oxidative muscle metabolism concept: An integrative physiology model for understanding the impact of contractions on carbohydrate oxidation and glucose tolerance
To reiterate, glucose regulation was improved by sustaining the local rate of oxidative metabolism during a relatively small increase in total body energy expenditure (Figure S1). Restraining the rate of total body energy expenditure was a way of minimizing systemic homeostatic disturbances such as an increase in catecholamines, which not only increases heart rate but also may be required for high rates of glycogenolysis during isolated soleus contractions (Richter et al., 1982). The small heart rate and blood pressure responses were a clear indication of a negligible systemic neurohumoral stress response to this type of contractile activity. The respective heart rate in SED, SPU1, and SPU2 was 73 ± 6, 79 ± 8, and 89 ± 7 beats/min; systolic blood pressure was 116 ± 6, 123 ± 4, and 124 ± 6 mmHg; and diastolic blood pressure was 77 ± 4, 78 ± 4, and 77 ± 3 mmHg. To put this in perspective, the total body energy demand of 1.3 METs (Table 2) ensured that the total body rate of energy expenditure was definitely less than the historical 3 MET minimal threshold often historically believed to be required for prevention of prediabetes and diabetes (Colberg et al., 2016). In fact, it is even below the threshold that behaviorists have used to define nonsedentary time (>1.5 METs).
But are these low rates of total body energy expenditure and thereby low rates of carbohydrate oxidation theoretically sufficient to explain the large systemic glucose concentration differences? To address this, we analyzed the glucose lowering using the simplest possible mathematical model. The delta blood glucose was 33 mg/dL lower between SPU1 and control at 60 min (Table 3). Thereafter, this concentration difference between the active and inactive state remained in steady state (Table 3). Therefore, the grams of glucose accumulated in the blood and the rest of the extracellular distribution volume (Vd) can be calculated from a mass balanced equation. The Vd pool size is approximately equal to blood volume and interstitial fluid, or 230 mL/kg body weight (Livesey et al., 1998), which is ∼186 dL. The Vd accumulated 33 mg/dL less glucose during SPU1 in the first hour. On average, the Vd accumulated glucose at a 102 mg/min slower rate because of SPU1 contractions (33 mg/dL x 186 dL in 60 min). Therefore, the observed differences in glucose concentration required a physiological process to slow the rate of glucose accumulation by at least 102 mg/min. The observed increase in whole-body carbohydrate oxidation caused by SPU1 contractions above sedentary control averaged 141 mg/min (Table 2). The observed decrease in the insulin concentration during contractions (Figure 3) would obviously tend to attenuate the blood glucose reductions because of less insulin-dependent glucose uptake in various body tissues. Finally, the contractile-activity-dependent increase in glucose oxidation by the soleus muscle was calculated for SPU1 at 113 mg/min (Table 2). In conditions when intramuscular glycogen is not the predominant fuel for contractile activity, there is less competition with alternative substrates, including blood glucose. Therefore, from the findings we propose a model in which glucose tolerance can be rapidly improved by a large magnitude while sustaining a subtle, yet proportionate increase in carbohydrate oxidation, but this is under specific conditions when the recruited muscle fibers do not rely mostly on intramuscular glycogen to fuel contractions.
Discussion
These results indicate that the human soleus muscle is capable of raising, and sustaining for hours, the local rate of oxidative metabolism to high levels. From a physiological perspective, this kind of contractile activity was effective at improving systemic metabolic regulation quickly and by a biochemically meaningful amount to improve glucose regulation (Figure 3), even at the lowest SPU intensity studied (SPU1; Tables 2, 3, and 4 and Figure 3). Furthermore, the high level of endurance of the soleus during SPU contractions provides a tool for reversing the otherwise slow rate of muscle metabolism during long periods of inactivity (Dela et al., 2019; Kelley et al., 1994; Rolfe and Brown, 1997). We are unaware of any existing or promising pharmaceuticals that come close to raising and sustaining whole-body oxidative metabolism at the magnitude in the current study (Tables 1 and 2), including drugs that may activate BAT (Loh et al., 2019). A beta-3 agonist given at double the Food and Drug Administration (FDA)-approved dose increased energy expenditure in 3 h, but by only an average of ∼8.5 kcal/h (Loh et al., 2019). By comparison, SPU contractions were capable of raising the energy demand 10-fold greater (91 kcal/h above control during the 270 min of activity in our first experiment; Table 1), without any evidence of progressive fatigue or other physiological limitations in the unfit individuals studied (Table 1).
We learned that through this method of contractile activity specifically geared for sustaining oxidative metabolism, only an extra 100–200 mg/min of local carbohydrate oxidation (Table 2) by a small muscle mass was potent enough to improve glucose regulation by a large amount after ingesting a large glucose load (Table 3). As described more later, carbohydrate oxidation increases profoundly during the acute minutes of relatively heavy whole-body exercise. However, there is not compelling evidence to believe that either in humans or animal models, carbohydrate oxidation remains elevated by even a small amount in the hours after ending an acute exercise bout (Horton et al., 1998; Wasserman et al., 1991). There was not an increase in carbohydrate oxidation after large muscle mass exercise within subgroups of people who had a significant excess post-exercise oxygen consumption (EPOC) (Horton et al., 1998). This finding of no increase in post-exercise carbohydrate oxidation above sedentary control levels was also demonstrated when experimentally changing the insulin concentration over a wide range (Wasserman et al., 1991).
There is a competition at the cellular level between various substrates to supply the necessary energy for contractions. During exercise, muscle glycogen is generally the primary carbohydrate to fuel contractions and often accounts for about 72%–95% of the carbohydrate oxidation when it is available in normal concentrations (Bergman et al., 1999; Helge et al., 2007; Horowitz et al., 1999); this is observed even with lower intensity exercises such as cycle ergometry at 20%–30% of VO2 max (Gollnick et al., 1974a, 1974b) or quadricep extensions at 25% of the local VO2 peak (Helge et al., 2007). During isolated knee extensions at a local VO2 of 190 mL/min/kg muscle (when roughly comparable to SPU contractions in Figure 2C), the VL muscle depleted twice as much glycogen in 35 min (Helge et al., 2007) as the soleus did during 270 min of SPU contractions (Table 1); this is consistent with the possibility that with SPU contractions, the soleus may in some conditions deplete glycogen at a rate 10–15 times slower than the VL muscle of the thigh during knee extensions (Helge et al., 2007) or cycling (Bergman et al., 1999; Gollnick et al., 1974a, 1974b). A thorough review by Sylow and Richter concluded that blood glucose accounts for only ∼10%–18% of the energy during the time frame of a typical exercise session (Sylow et al., 2017). By comparison, these types of large muscle mass exercises (Bergman et al., 1999) potentially increase the total carbohydrate oxidation more than 10-fold greater than SPU contractions (Table 2). With cycle ergometry at ∼5 METs for 1 h, the rate of total carbohydrate oxidation was ∼1600 mg/min (Bergman et al., 1999), which was many times greater than SPU contractions could induce; this is also consistent with the carbohydrate oxidation in another study during 60 min of moderate intensity cycling combined with glucose ingestion (Horowitz et al., 1999). However, once glycogen is accounted for, the remaining carbohydrate oxidation due to blood glucose by the entirety of both legs during cycling was ∼180 mg/min (Bergman et al., 1999), which was relatively similar to the estimated rate of carbohydrate oxidation by the working soleus muscle during SPU contractions (Table 2). Prior work has suggested that when recruiting a smaller instead of a larger mass of muscle, there may be a shift in the relative reliance on fuels from glycogen to utilization of more lipids (Helge et al., 2007) and/or more blood glucose (Richter et al., 1988) to fuel contractions. A plausible hypothesis is the intrinsic metabolic phenotype of the soleus (Bey and Hamilton, 2003; Gollnick et al., 1974a, 1974b; Halseth et al., 1998; Hodgson et al., 2005; James et al., 1985; Jensen et al., 2012; Mackie et al., 1980; McDonough et al., 2005; Monster et al., 1978) requires less glycogen to fuel contractions, especially when there is not epinephrine stimulation (Richter et al., 1982). As described below, in addition to low muscle glycogen use, there are also other systemic processes that tend to offset the ability of contractions to attenuate postprandial hyperglycemia during and after large muscle mass exercise (Devlin et al., 1989; Hamilton et al., 1996; Knudsen et al., 2014; Maehlum et al., 1978; Steenberg et al., 2020).
Results from many studies support the concept that it is often difficult to improve postprandial glucose tolerance. Studies have identified specific mechanistic explanations for why oral glucose tolerance is generally not improved (Devlin et al., 1989; Hamilton et al., 1996; Knudsen et al., 2014; Maehlum et al., 1978; Rose et al., 2001) or even made worse (Flockhart et al., 2021; Knudsen et al., 2014; Rose et al., 2001) in the hours following traditional types of exercise that would rely predominantly on muscle glycogen as the source of carbohydrate. Those observations include people with normal glucose tolerance (Flockhart et al., 2021; Knudsen et al., 2014; Rose et al., 2001) to people with diabetes (Knudsen et al., 2014). The mechanistic explanations to date include compelling evidence of increased insulin resistance within the unrecruited muscle fibers after exercise sessions (Devlin et al., 1989; Steenberg et al., 2020), elevated rates of glucose appearance into the bloodstream (Hamilton et al., 1996; Knudsen et al., 2014; Maehlum et al., 1978), and also possible impairment of intrinsic mitochondrial function from intense exercise training (Flockhart et al., 2021). In studying the chronic effects of exercise training, a landmark study reported that there was a modest 13 mg/dL decrease in the 2-h glucose without a significant improvement in the entire AUC; this only occurred when doubling the recommended weekly volume of exercise training combined with a vigorous intensity (7 METs) and nutritional conditions, allowing for a 4.6 cm reduction in waist circumference (Ross et al., 2015). Other carefully controlled studies showed unequivocally no reduction in the 2 h glucose (or AUC) with progressive weight loss of 5%, 11%, or even 16% (Magkos et al., 2016); this was confirmed in another large experimental weight loss study after inducing 15% weight loss (Jansen et al., 2022). Nevertheless, given that carbohydrate oxidation is not elevated after an acute exercise session ends, the post-exercise glucose utilization by muscle is apparently restricted to the nonoxidative pathways. Therefore, as described next, there has been the need to better understand the immediate and direct effects of contractile activity.
The present study tested the effects of sustaining continuous contractile activity, at two levels of energy demand, throughout the entire 3-h postprandial period. The contractions were already attenuating glucose concentration quickly within the first 30 min when glucose was still rising (Table 3). Thereafter, the glucose improvements continued to increase and remained significantly lower than the sedentary control level throughout the entire 3-h postprandial time course. Earlier studies also found that activity involving a large muscle mass (cycle ergometry at 59%–67% HRmax) completed after 45 min of the postprandial period transiently blunted the rise in blood glucose and insulin during the contractions (Aadland and Hostmark, 2008). However, this effect was short-lived and evident only between 30 and 45 min of the postprandial period, followed by significantly greater hyperglycemia than the inactive sitting trial after exercise stopped. Similarly, Kanaley’s group (Holmstrup et al., 2014) found that subjects with low glucose tolerance were able to decrease the hyperglycemia during 60 min of exercise (60%–65% VO2max). After stopping the exercise, the blood glucose increased above the level when they never exercised, and thus the total postprandial iAUC was not improved (Holmstrup et al., 2014). Although an intermittent exercise pattern slightly improved the glucose iAUC compared with the 60 min bolus of exercise, spreading out the contractile activity with brief breaks did not reduce the glucose iAUC compared with sitting inactive in their volunteers who had low glucose tolerance as the present participants (Holmstrup et al., 2014). That said, it is important to understand that as previously emphasized, there are distinct molecular and physiological processes impacting metabolic regulation with specific inactivity/activity approaches (Bey and Hamilton, 2003; Hamilton et al., 1998, 2004, 2007, 2014; Zderic and Hamilton, 2012). Here we have focused on a method of raising slow oxidative muscle metabolism to complement (not replace) existing approaches.
Prolonged sitting has become ubiquitous across the lifespan (Craft et al., 2012; Healy et al., 2015; Matthews et al., 2018; van der Berg et al., 2016) and is not significantly less in regular exercisers (Craft et al., 2012). But it is important to remember that regardless of whether ∼45 min of activity is administered as many separate brief breaks or as a single daily bolus, it mathematically increases the energy demand for muscular work for only ∼5% of the waking day. Thus, currently recommended activity approaches are a not a direct solution for a high amount of time when skeletal muscle has a low muscle metabolism. A large number of studies have focused on “brief breaks” from sitting inactive, e.g., 2–5 min activity breaks each half hour or 6 min once each hour (Henson et al., 2020; Larsen et al., 2015; Loh et al., 2020; Thorsen et al., 2019). What may be missed is that brief breaks are, by definition, brief amounts of contractile activity and thus only brief periods to potentially benefit from oxidative metabolism. A systematic review has reported that lifestyle interventions replacing sitting time with standing and/or brief walking breaks have decreased sitting time on average only 30.4 min/day (Peachey et al., 2020). That said, a promising early study reported that taking 3 brief interruptions each hour to walk at 2 mph (∼3 METs) may reduce the blood glucose by approximately 5, 15, and 6 mg/dL at 1, 2, and 3 h respectively, with the average insulin concentration (based on AUC) reduced by about 12% (Larsen et al., 2015). In a thorough recent study, no differences were observed for plasma glucose or insulin with any of the 3 patterns of walking breaks to interrupt inactive sitting in men with abdominal obesity (Thorsen et al., 2019). Henson et al. (2020) summarized results when combining 4 large postprandial lab studies and concluded that the glucose and insulin were not decreased by standing versus sitting in the postprandial period. Also, the brief walking breaks generally reduced glucose <10 mg/dL and thus were physiologically small and/or nonsignificant in some large categories of people (e.g., no statistical influence in males) (Henson et al., 2020). Physiological studies relevant to this found that muscle glucose uptake appears to have a relatively slow time course at the onset of some types of contractile activity (Bergman et al., 1999; Horowitz et al., 1999; Mossberg et al., 1993). In studying the time course in the human lower limb musculature during cycling, there was no increase in glucose uptake in the leg after either 5 or 15 min of continuous contractions, and significant responses were not evident until after 30 min (Bergman et al., 1999). Taken together, there is a biochemical basis for the need to develop methods to understand and benefit biochemically from prolonged contractile activity.
The literature is understandably filled with language about designing programs to “engage as much skeletal muscle mass as possible” and the necessity of “a sufficient amount of muscle mass” (Ivy et al., 1999; Laughlin, 2016). The findings presented here do not in any way interfere with traditional exercise programs for their own distinct benefits. There are hundreds of skeletal muscles and many microvascular and metabolic exercise training adaptations that are restricted to the recruited muscles (Laughlin, 2016). Thus, it is understandable that the paradigm for glucose management and other cardiometabolic outcomes has focused less on the quality of contractile activity than on the quantity of recruited muscle and raising the total metabolic rate to higher levels for short exercise bouts (e.g., 150 min/week). The Diabetes Prevention Program (DPP) and other recommendations either implied or explicitly stated that health-enhancing metabolic effects may require an energy expenditure in the >3 MET range (Knowler et al., 2002). The current findings obviously are inconsistent with the notion that all contractile activity near ∼1.3 or ∼1.7 METs is a “stepping stone” to the goal of exercising at >3 METs (Dunstan et al., 2021). All of this points to the “specificity principle” that is a key axiom in exercise physiology (Hamilton et al., 2007, 2014). Applied here, it means that the phenotype of the muscles recruited, and the duration of the elevated metabolic rate, will determine the distinct biochemical processes that regulate the effectiveness of physical activity.
There have more recently been national guidelines proposing that people sit less and/or move more in addition to traditional methods of exercise (Dunstan et al., 2021). Unfortunately, this advice is still lacking in specifics about how to reduce sedentary time enough for the most meaningful health gains. The rapid and large decreases in skeletal muscle TG uptake observed in rodent inactivity physiology studies (Bey and Hamilton, 2003; Hamilton et al., 2007) have been heavily cited by epidemiologists and clinical trials specialists to provide biological plausibility to understand the observational associations of diseases related to sedentary time. What has been overlooked is the same publications cited to provide the biological plausibility for why muscular inactivity is unhealthy also alluded to a potential solution; those studies were primarily based upon the large local molecular and biochemical responses in the soleus muscle (in rodents) that are dependent on prolonged contractile activity (Bey and Hamilton, 2003; Bey et al., 2003). The present findings provide evidence that the human soleus muscle has the potential to contribute to systemic metabolic regulation.
Finally, it might prove to be that the most interesting hypothesis raised by these results is that the human soleus, although only ∼1% of body weight, can sustain a sufficient metabolic rate for an impressive duration and improve glucose and lipid metabolism. Others have demonstrated significant beneficial correlations between the slow-red oxidative fiber type and chronic disease states (Gaster et al., 2001; Hickey et al., 1995). There has been interest in applying molecular biology techniques to therapeutically enhance the quality of skeletal muscle by increasing the amount of slow oxidative muscle fibers (Gan et al., 2013). It is important to note that the results were obtained from adults across the lifespan (22–82 years of age) and with a wide BMI range and habitual physical activity levels (Table 4). Findings reveal that the human soleus of these ordinary people was already physiologically capable of producing these responses. Looking back, studies in the 1870–1880s by Ranvier described the soleus remarkably well as a red muscle with curvy capillaries that is relatively slowly contracting and fatigue resistant, even when the muscle was obtained from highly sedentary animals such as the domesticated rabbits and cats (Ranvier, 1873, 1880). The SPU method is specifically geared for sustaining positive effects of prolonging an elevated muscle metabolism for hours (not minutes), but with a very subtle increase in whole-body energy expenditure while sitting (Figure S1). This low effort method by a muscle that is naturally geared toward prolonged contractile activity may avoid the potentially serious deleterious cardiac effects of prolonged endurance training in some people or the impairment of mitochondrial function after training with excessive exercise intensity (Eijsvogels et al., 2016; Flockhart et al., 2021). Many other questions will need to be addressed in order to understand the full translational potential. One understandable viewpoint is that prolonged periods of elevated muscle metabolism is an unrealistic expectation. Another perspective is that it is an opportunity for gaining the distinct health effects of elevating muscle metabolism by a biochemically meaningful amount.
Limitations of the study
(1) This study was not a clinical trial. This was an experimental physiological study, conducted in highly controlled laboratory conditions. This study also did not test effectiveness of a free-living lifestyle intervention. The underlying distinct cellular stimuli and systemic metabolic responses during this approach for local muscular exercise have potential to complement other unique types of activity that typically involve a large muscle mass, different kinds of muscle contractions, and a lower duration of activity. The development of the SPU contraction method provides a unique physiological method that has never been tested before to raise and sustain muscle metabolism (for hours, not minutes). The present study does raise more than one translational hypothesis for the field to test. First, this method provides muscle physiologists with an opportunity to determine the effects of prolonged and locally intense contractile activity on muscle plasticity and response to high duration stimuli. Second, interdisciplinary clinical trials specialists in related fields who study diabetes, resting energy expenditure, skeletal muscle, exercise, and sedentary behavior may find the present results impactful for informing their new research ideas. One should be cautious when interpreting the relative effectiveness in subcategories until follow-up studies with a large sample size are performed. The practicality will also depend on implementation in large parts of the population. The practicality will depend in part on evidence that people are capable of successfully performing SPU contractions outside of a laboratory without EMG feedback. There is a need to test when this could be integrated within the lifestyle without disrupting various seated behaviors.
(2) These studies only identified some of the immediate responses to SPU contractions. There is a need to describe the additional longer-term cumulative effects of an intervention in people living with a higher rate of muscle metabolism.
(3) Muscle glycogen utilization was not measured in Experiment II (during the glucose tolerance test). Therefore, we did not test for the interaction of hyperglycemia and SPU contractions on glycogen use by the soleus. However, it has already been established multiple times that feeding a moderate-to-large glucose load does not increase the reliance on muscle glycogen to fuel contractions, and more expectedly sometimes there is a tendency for glucose ingestion to modestly attenuate contraction-induced muscle glycogen reductions (Akerstrom et al., 2006). Also see Criteria for selection of the metabolic intensity in Experiment II in the STAR Methods.
(4) Interrogating glucose kinetics with tracers and catheterization of an artery and vein in the legs for A-V balance measurements would be insightful to further test the model proposed herein. A catheterization study would also be informative to measure the peak VO2 during SPU contractions. The VO2 measurements we did obtain were always at a submaximal intensity that could be sustained with a low effort in order to avoid biasing the results when using stabilizer muscles while straining during an intense performance test.
(5) We cannot discern from the current findings how SPU contractions are impacting either the endogenous or exogenous (ingested) glucose disposal, the effect of SPU contractions on the rate of appearance of blood glucose relative to the rate of glucose disappearance, and how the parallel decrease in plasma insulin is attenuating glucose uptake by insulin-dependent tissues (e.g., resting skeletal muscle in the arms while the leg muscles are doing SPU contractions).
(6) One may find it tempting to generalize the effects we found to other modes and doses of activity. However, these results were limited to when performing a specialized type of contractile activity. Other types of “low effort” activity do not necessarily activate the soleus muscle metabolism enough to cause the same magnitude as demonstrated in the present experiments (Gao et al., 2017; Pettit-Mee et al., 2021; Thorp et al., 2014). Other types of activity may also rely more heavily on muscle glycogen and/or may stimulate systemic processes that tend to be counteractive to glucose lowering (Helge et al., 2007; Richter et al., 1988). One well-designed study reported that although standing continuously over a 2-h OGTT can raise EMG in the large muscles of the lower body, it did not reduce glucose at all compared with sitting inactive (Gao et al., 2017).
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Biological samples | ||
| Human blood samples | This study | N/A |
| Human skeletal muscle biopsies | This study | N/A |
| Critical commercial assays | ||
| Insulin ELISA | Mercodia | 10-1113-01 |
| C-peptide ELISA | Millipore Sigma | EZHCP-20K |
| Infinity Glucose Hexokinase Reagent | Thermo Scientific | TR15421 |
| EDTA | BD Vacutainer | BD 368857 |
| Software and algorithms | ||
| EMG Works | Delsys | Version 4.5 |
| Prism statistics | GraphPad | Version 8.4.3 |
| Excel | Microsoft | For Microsoft 365 |
| Other | ||
| True One 2400 | Parvo Medics | N/A |
| Biopsy needles with suction (5 mm) | Micrins | INS122-5 |
| Treadmill | Sole | F85 |
| Trigno EMG system | Delsys | N/A |
| Electrogoniometer with two ends connected by composite wire with a series of strain gauges | Biometrics | SG110 |
| activPAL | PAL Technologies | Model 3 |
| Blood pressure monitor | Omron and Tango | HEM-FL31 and M2 |
| 3.0T MRI Scanner | GE Medical Systems | N/A |
| iDXA | GE Healthcare Lunar | N/A |
Resource availability
Lead contact
Further information and requests for resources should be directed to the lead contact, Dr. Marc Hamilton (mhamilton7@uh.edu).
Materials availability
This study did not generate new unique reagents.
Experimental model and subject details
Human subjects
Informed consent was obtained before participation. Research conformed to the standards set by the Declaration of Helsinki and was approved by the appropriate Institutional Review Boards at the Pennington Biomedical Research Center and the University of Houston.
There were in total 25 human volunteers in 2 sequential experiments, each using a randomized cross-over design so that individual participants could serve as their own internal control (Figure S6). Recruitment was aimed at providing an equal distribution of sexes, with a moderately wide range in age, BMI, sedentary time, and free-living activity level. The ranges and means for each descriptive characteristic are provided in Table S1. Details about inclusion/exclusion are included in the Method Details below for each of these experiments.
Method details
Soleus SPU contractions
Both experiments utilized the same type of isolated plantarflexion that was done when sitting comfortably in normal chairs. This specific type of plantarflexion depended predominantly on the soleus muscle with some assistance from the gastrocnemius muscles (Figures S3 and S4). Secondly, unlike isometric plantarflexion contractions more commonly studied, this was an isotonic soleus activation that coincided with the angular motion of the ankle only when the ankle was moving upwards (Figure S4). Thus, to not overgeneralize from this to other types of plantarflexion and for brevity, the movement is described as an SPU, or “soleus push up”. See also the introduction of the Results for a summary of the supplemental figures for and Table 1 describing the participants.
One primary intent was to limit the rise in total body energy expenditure to be well below the lower threshold of 3 METs defining “moderate intensity activity”. To that end, Experiment I tested the SPU contractions corresponding to ∼2 METs. Experiment II tested SPU contractions at ∼1.3 and ∼1.7 METs. The direct comparison of the whole-body energy expenditure of SPU contractions with treadmill exercise is described in Figure S1. Additional rationale and methods for testing this metabolic rate are described in more detail below.
This singular movement by a small mass of muscle was isolated from other muscle groups typically used for large weight bearing compound movements like walking. The effects of this muscular activity were tested only while sitting comfortably. The feet were positioned on the floor and under the knees so that the knees were bent (Figure S2). The starting angle of the ankle was ∼70–90 degrees (90 degrees defined as when the tibia and sole of the foot are at a right angle). This type of plantarflexion movement was performed without adding external resistance beyond the weight of the leg (Figure S2). That avoided the necessity of using a resistance device while also minimizing potential muscle fatigue and tension-induced strain on soft tissues. Furthermore, the soleus contribution was accentuated during the seated plantarflexion because the knee was always bent, such that the metatarsophalangeal joint (MTP joint) in the foot was below the knee. The MTP joint was bending in concert with the plantarflexion of the ankle joint. When the knee is bent, the soleus contributes more to the work of plantarflexion as the gastrocnemius recruitment and energy demand is reduced compared to when the limb is straight (Cresswell et al., 1995; Kawakami et al., 1998; Niess et al., 2018; Price et al., 2003).
Measurement of the soleus EMG provided instantaneous feedback to guide the intensity of soleus contractions and help teach how to effectively raise the local metabolic rate with this type of contractile activity (Figure S3). We had no difficulty teaching volunteers in either Experiment I or II how to do the local contractile activity in generally 1–2 sessions, especially with the assistance of the soleus EMG. Subjects were all consistently able to learn how to sustain a soleus EMG that is markedly greater than possible when doing treadmill walking. We found it was productive to instruct participants to focus mostly on raising the range of motion (ROM) (Figure S5) of the ankle plantarflexion in order to raise the soleus EMG intensity and VO2 (Figure S3B). Raising the rate was a less effective strategy (Figure S5). Notice in the examples of a male and female in Figure S5, when the ROM was doubled from a low level of 15 degrees to a moderate level of 30 degrees, the soleus EMG predictably doubled in these 2 volunteers. However, notice also that when the rate was doubled (from 50 to 100 contractions/min), the soleus EMG increased less than predicted. Furthermore, we found from experience that it could be counterproductive for volunteers to focus too much on using the rate as their guide for soleus activation, because raising the rate often caused an involuntary reduction in the ROM and VO2 response. In summary, we found it effective to provide EMG feedback while instructing participants to select a moderate ROM in order to comfortably maintain their desired level.
A developmental study that proved to be instructive in perfecting the methods revealed a close relationship between soleus EMG and the contractile activity VO2 (Figure S3B). In 10 volunteers, the soleus EMG was incrementally increased in 6–10 min stages while measuring the steady-state VO2 responses within the last 3 min of each stage. The participants were instructed to begin at what was perceived as a very low ROM. The steady-state VO2 responses were measured when they raised the soleus EMG to progressively higher levels. A total of 42 measurements were obtained and the linear relationship between soleus EMG and the contractile activity VO2 response above sitting inactive is illustrated (Figure S3B).
In summary, both experiments involved testing a subtle elevation in whole body metabolic rate above resting by contractile activity while sitting, and EMG feedback with direct measurements of oxygen consumption assisted in guiding the activity.
Experiment I
Participants
Volunteers (Table S1) were recruited from a combination of newspaper and other types of advertising to interest the kind of participants who often avoid participation in a physical activity study. It was explained that this work may add knowledge about raising metabolic rate throughout much of the day, distinct from traditional exercises. Exclusion criteria included orthopedic or cardiovascular limitations prohibiting a safe treadmill VO2 max test, conditions contraindicating biopsies, and an inability to have an MRI.
Protocol and procedures
A total of 60 muscle biopsies were obtained for understanding the soleus glycogen responses of relatively intense local contractile activity from 10 somewhat unfit/untrained individuals (5 men and 5 women). Each subject served as their own control, for both an active and an inactive test day (always while sitting). Except for the activity, the sedentary control test was identical to the active test in all respects including the diet and activity before the testing. In addition to biopsies on each day, a blood sample was also obtained prior to the final biopsy for testing the effect of this small muscle mass activity on VLDL-TG concentration. The purpose of this first experiment was to study substrate metabolism over a prolonged duration of SPU contractions instead of inactive sitting. The habitual free-living sedentary time was measured in the current participants as described in detail below (see ActivPAL device). Most people in modern times have at least 7–8 h per day of sedentary time as determined with objective activity monitors in the USA and other developed nations (Craft et al., 2012; Healy et al., 2015; Matthews et al., 2018; van der Berg et al., 2016). One day participants sat inactive for 7–8 h. During the active SPU trial, subjects never sat inactive for more than 4 min at a time while accumulating 270 min of SPU contractions (Figure S6).
Although these individuals were sedentary, they were instructed not to do any intentional moderate to vigorous exercise for at least 3 days prior to testing in order to avoid potentially glycogen lowering exercise. In order to help reduce possible variability in glycogen concentration leading into each test day, participants walked 30 min on a treadmill at a comfortable 2 mph pace the evening prior to the testing days (under supervision) and then fed the standardized dinner meal (also under supervision). Standardized meals were provided for all 3 meals the day prior to testing in addition to the small breakfast on the test day. The study was timed so that a blood sample for measuring VLDL-TG and then the final biopsy were obtained about 7–8 h after a small, controlled breakfast (7 kcal/kg, 33% carbohydrate, 14% protein, and 53% fat) which was provided 12–14 h after an overnight fast.
Because these were among the first participants studied with this type of prolonged contractile activity and they were generally not accustomed to exercise, we asked them repeatedly to tell us if there was any type of discomfort from the contractions, including cramps, a progressive sense of muscle fatigue, joint pain, etc. and none of those type of adverse events was ever encountered. After enrolling participants, we incorporated time for preliminary testing for them to become familiar with the testing procedures, including how to correctly and reproducibly do the SPU motion with real time EMG feedback. (Figure S3A). In the preliminary testing, the participants walked for several minutes on the treadmill and then practiced until they could confidently sustain an EMG level that was always greater than the soleus EMG when walking (typically about twice the soleus EMG of walking and sometimes more). Experience showed that by raising the ROM, the soleus EMG could be maintained at a markedly greater level than when instead focusing on increasing the rate of contractions (Figure S5).
In the preliminary testing and on the actual test days, oxygen consumption (VO2) and carbon dioxide (VCO2) production were measured at least every 30 min during the SPU contractions. VO2 was always obtained in steady state conditions. Subjects were not allowed to fidget when measuring VO2, under direct observation by at least 2 study staff at all times, in order to ensure an accurate evaluation of AEE. We allowed ample time for using the restroom and other breaks from the activity to remain comfortable. Using the real-time EMG feedback, they maintained the soleus EMG at an individual level determined in the preliminary test day to be above walking, required an energy demand ∼2 METs, and did not cause fatigue. The contractions with VO2 measurements were done in blocks of time up to ∼7 min long (longer if there was instability in VO2 because of a cough or movement), followed by shorter rest intervals to help maintain a resting position and possibly adjust the angle and setback of the chair or height.
Muscle biopsies
In all, a total of 40 soleus and 20 VL biopsies (150–200 mg) were obtained in 10 participants (2 soleus and 1 VL biopsies on each of the 2 days). Biopsies were taken at the midway and endpoint at the same time on the 2 test days for each individual. The 2 soleus biopsies each day were from different legs to completely avoid chances of inflammation or other effects of the first biopsy impacting results of the second biopsy. We used ultrasound to ensure optimal placement of the biopsy needle in the belly of the soleus muscle at the greatest muscle girth, using a Bergstrom needle with suction. Biopsies were obtained after 130 and 270 min of the contractile activity (Table 1 and Figure S6 top panel). The VL of the thigh was also biopsied as an inactive control at only the final time point (after the soleus). The VL biopsy served as an internal control for the glycogen concentration in a muscle that was not recruited to contract.
VO2 and VCO2 gas exchange with indirect calorimetry
VO2 and VCO2 production were determined using a TrueOne 2400 metabolic system from Parvo Medics. The gas analyzers and pneumotach were calibrated according to standard manufacturer procedures using certified calibration gases. Sufficient time to flush out the gas lines and average steady state measurements was always confirmed. The measurement period was extended when it was deemed helpful (such as if there was a fluctuation in VO2 caused by a cough or when taking additional time to confirm the precision of the result). We were careful to ensure participants were positioned when sitting completely relaxed to avoid extraneous movement beyond the intended SPU plantarflexion movement. This included positioning the chair back rest and height for each individual to optimize a restful position.
Glycogen assay
Muscle glycogen was determined from the measurement of glucose after acid hydrolysis with HCl using a standard enzymatic technique. Immediately upon taking the biopsy, any connective tissue or blood was removed and then frozen in liquid nitrogen. Muscle samples were then homogenized and boiled (100°C) in 1 M HCl for 2 h. After neutralization with NaOH, the resultant glucose concentration was determined by incubating for 45 min at room temperature with an Infinity Hexokinase reagent (Thermo-Scientific) that contained hexokinase and G-6-P dehydrogenase. Then the resultant molar concentrations of total glucosyl units were expressed relative to the starting mass of the boiled muscle sample (i.e, mmol glucosyl units/kg muscle).
Magnetic resonance imaging (MRI) of the soleus anatomical mass
The soleus anatomical mass was determined in these 10 individuals from MRI images of the TS muscle group in order to calculate the soleus contribution to the recruited muscle mass during plantarflexion. A 3.0T MRI scanner (Excite HD system, GE Medical Systems) obtained images every 1.0 cm of the entire soleus, medial and lateral gastrocnemius (MG and LG) muscles for determining the muscle volume and then calculating the muscle mass assuming a density of 1.04 g/mL (Kim et al., 2002). The relative recruitment of the soleus muscle and the other 2 TS muscles (MG and LG) was calculated as the product of the anatomical mass and the percentage of the maximal EMG. Maximal EMG was obtained from the highest EMG in at least 2 sets of standing heel lifts when standing on a single leg. When doing this, they stood on 1 leg and did maximal plantarflexion as rapidly and high as possible. The highest 1.5 s RMS signal from a moving average assessed every 0.01 s was taken as the maximum EMG signal.
Dual-energy X-Ray absorptiometry (DEXA) and VO2max
On a separate day from the two primary test days, DEXA (Lunar iDXA GE Healthcare Lunar) was used to calculate body fat as well as muscle mass of the entire lower limb. Additionally, an assessment of maximal oxygen consumption (VO2max) was performed, using an incremental treadmill test to exhaustion. They began by walking on a level grade for a 5-min warm up, and we progressively increased the speed and grade. We confirmed VO2max by heart rate and RER responses. The heart rate at maximum averaged 180 beats per minute and the age predicted heart rate was 182 beats per minute. The RER at the end of the test averaged 1.29, with a range of 1.16–1.51. After the 5-min warm up, the average time to exhaustion was 7.9 min (range of 5–12 min).
VLDL-TG concentration
A blood sample to measure plasma VLDL-TG concentration was obtained from an antecubital vein without a tourniquet before the final biopsy. EDTA treated blood was placed on ice, centrifuged under refrigeration for 15 min at 1000 × g, and stored at −80°C. The plasma VLDL-TG was measured by nuclear magnetic resonance spectroscopy as described previously by our laboratory (Harrison et al., 2012).
Electromyography (EMG)
The raw EMG was collected at 2000 Hz and bandpass filtered (20–450 Hz) with the Trigno EMG system (Delsys). EMGworks software version 4.5 (Delsys) was used to analyze the EMG and goniometry signals. The EMG signal was rectified by taking the root mean square (RMS) after subtraction of the mean microvolts. In preliminary measurements evaluating ROM (Figures S4 and S5), an ankle strain gauge goniometer (Biometrics) sampling at 148 Hz was used. One end of the 75–110 mm goniometer was attached superior to the lateral malleolus and the other block attached to the lateral aspect of the foot.
ActivPAL device for demographic purposes during free-living behavioral monitoring
For demographic purposes of free-living behavior, the ActivPAL device (PAL Technologies) was worn by all participants in both Experiments I and II. Participants were instructed to wear the device taped to the anterior aspect of the mid-thigh whenever awake (but not in water) for at least 4 days (averaging 10 days) during typical free-living conditions to capture sitting, standing, and stepping time; wear time was 16.3 ± 0.7 h/day over 10.3 ± 5.0 days (mean ± SD).
Experiment II
The second experiment determined the ability of 2 levels of this type of local contractile activity to impact postprandial glucose during a 13-point OGTT (N = 15).
Participants
Participants volunteered in part through community outreach at churches and senior centers. For practical and ethical reasons, a VO2max test was not included in this group because there was no exclusion criterion for orthopedic and cardiovascular conditions. However, people were excluded if they reported any recent or planned changes in diet, physical activity, medications, and other potentially confounding lifestyle factors that could be a risk for adherence. They were also excluded if they had a fasting glucose >125 mg/dL or HbA1c >6.4% (the thresholds for Type 2 diabetes), had physician diagnosed diabetes, or took glucoregulatory medications other than metformin. Three participants had been taking metformin for over 1 year. The free-living sedentary and activity time are in Table S1 and had a relatively wide range. By design, this experiment was inclusive of a relatively diverse group of people, including 7 men and 8 women (Table S1). We sought out participants who were at some risk for type 2 diabetes (T2D) based on either the participant (with regards to their own phenotype) or a family member with T2D. These individuals had a HbA1c range of 4.9–6.1 and fasting glucose between 91-115. Inclusion criteria included the goal of an equal number of both sexes/gender and adults age across the lifespan (range of 22 – 82 years). There was a fairly well-balanced proportion of minorities (7 non-Hispanic white, 5 African American, 2 Hispanic, 1 Middle Eastern).
Protocol and procedures
Generally, within one visit we were able to teach each one the proper biomechanical movement for an SPU contraction. All participants were able to learn how to successfully perform SPU contractions. Fifteen participants performed an OGTT while sedentary for 3 h and during at least one activity condition for 3 h. The 75 g OGTTs were performed following a 12–14 h overnight fast. This test was used because it is the standard for assessing the integrative physiology of glucose regulation and for comparing between studies (Knudsen et al., 2014; Magkos et al., 2016). The participants either sat inactive (control) or performed seated SPU contractions throughout the entire 3-h OGTT period using a repeated measures design in which each subject served as their own control. When recruited, participants were given the option of choosing to participate in two slightly different levels of this activity (SPU1 and SPU2 in random order). Steady-state VO2 and RER measurements were recorded at least once during each of the 3 h (on average 5 times). The average energy expenditure each hour is provided in Table S2.
In addition to a thorough explanation of study requirements and demonstration of procedures, time was allotted for ensuring each individual was comfortable with how to weigh and pre-package their food to maintain a consistent diet the day before each test day. Participants were provided with food scales. If the participants felt that replicating their diet would be difficult, we purchased and provided them with food. With this approach, these participants were able to maintain their habitual dietary preferences/requirements.
Criteria for selection of the metabolic intensity in experiment II
Because this second experiment followed the results of the first experiment, the criteria for the setting the AEE (MET intensity) was as follows. The methodological aim was for these participants to perform SPU contractions at an AEE intensity less than that used in the prior Experiment I (see Figure S1 and compare Tables 1 and 2). The first rationale for a lower AEE range was to increase the probability that there would be the same or less demand for soleus muscle glycogen used to fuel contractions than in the first study. This avoided the unnecessary subject burden of the added biopsies. Secondly, we aimed to limit the VO2 to assess glucose tolerance when raising total carbohydrate oxidation by an expected amount of about 100-250 mg/min above the normal sedentary level, with the aim of testing if that range was sufficient to reduce the blood glucose response. The reason for focusing on this rate of carbohydrate oxidation is described in more detail in the Results and Discussion.
Primary outcome in experiment II (postprandial hyperglycemia)
This experiment tested the primary hypothesis that sustaining a low AEE (about 0.6 kcals/min and close to one-half of Experiment I) by SPU contractions would be sufficient to improve postprandial glucose concentration compared to sitting with inactive muscle at the normal resting metabolic rate. From the 180 min after ingesting 75 g glucose, the postprandial glucose responses were assessed by calculating the incremental area under the curve (iAUC) using the trapezoid rule. Furthermore, it was important that the absolute glucose concentration (mg/dL) and the delta glucose concentration were reported at each time point. Given the importance in reporting these results relative to sex and age (and other characteristics of interest to different fields of researchers), the average glucose iAUC responses were calculated in categorically different groups.
Method of measuring blood glucose and insulin
Blood during each OGTT was sampled at 13 timepoints in 3 h (in triplicate 10 min before the glucose ingestion and every 15 min until 180 min after the glucose ingestion) from a warmed hand. It was tested using a Contour Next EZ (Bayer) blood glucose meter. This involved a well-validated approach of skin punctures for obtaining arterialized glucose excursions during an OGTT (Brouns et al., 2005; Förster et al., 1972; Whichelow et al., 1967). Direct comparisons with arterial catheterization have shown that capillary blood from skin punctures on average agrees closely (within 2–3 mg/dL) during OGTTs with catheterizing an artery (Förster et al., 1972; Whichelow et al., 1967), thus accurately reflecting the delivery of glucose to tissues from the systemic circulation. A validation study in our lab was performed. From this, results established that there was linearity in the observed and predicted glucose concentration in the range of 81–335 mg/dL with the Contour Next EZ (Bayer) blood glucose meter. In our lab’s validation study, the average measured values of whole blood samples, were 3 ± 1% higher than predicted, while the coefficient of variation was 1.3% between replicates (Figure S6).
Plasma insulin (Mercodia) and C-peptide (Millipore Sigma) concentrations were measured with commercially available ELISAs. EDTA treated venous blood was obtained every 30 min from an indwelling catheter in an antecubital vein and processed to measure plasma C-peptide in addition to insulin and additional assays (beyond the scope of this paper). The 6 mL blood samples were centrifuged at 1000 × g for 15 min in a refrigerated centrifuge and the resultant plasma stored at −80C. For reasons beyond our control, there was not venous plasma available for insulin assays in 3 of 15 subjects for SPU1 and 2 of 10 for SPU2 comparisons.
Blood pressure and heart rate assessment
Although not a major outcome, we checked heart rate and blood pressure (BP) in Experiment II during the activity and when sitting inactive with an automated cuff and blood pressure monitor (Omron model HEM-FL31, Tango model M2). In one particularly obese individual, we used a manual sphygmomanometer and stethoscope in order to obtain readings. The results were averaged for 8 participants in which we have complete data from all 3 test days.
Quantification and statistical analysis
Calculations
During physical activity, the total energy expenditure (TEE) is the sum of the activity energy expenditure (AEE) and the resting energy expenditure (REE). AEE is therefore calculated by subtracting the REE when sitting inactive from the TEE:
The caloric contribution of soleus muscle glycogen (in kcal units) to fuel the total AEE was calculated from the combustion of muscle glycogen as follows:
where Δ glycogen is the difference between the inactive and active soleus glycogen concentrations (mmol/kg) and the glycogen energy (GE) is 0.675 kcal/mmol which is the amount of energy (kcals) derived from 1 mmol of glycogen when the glucosyl units from glycogen are completely metabolized by oxidative phosphorylation. The soleus muscle mass (kg) was determined in each individual from MRI. This is based on the understanding that each mmol of glucosyl units in glycogen provides 0.675 kcal energy (i.e., 0.675 kcal = 3.75 kcal/g x 180 g/1000 mmol).
The increase in oxygen consumption of the entire lower limb musculature during treadmill exercise was calculated as follows. In each of these individuals, the entire lower limb lean mass was measured with DEXA. Running at VO2max was used as a mode of activity to exercise the muscles of the lower limb intensely. The VO2 per kg of the lower limb musculature during treadmill exercise was estimated with the understanding that skeletal muscle accounts for ∼90% of the leg lean mass (Cardinale et al., 2019), and that 75% of the oxygen consumption during “whole-body” exercise involving weight-bearing activity is from the large muscle mass in the lower limbs (Cardinale et al., 2019). From this, the oxygen consumption of lower limb musculature during treadmill exercise was calculated as follows:
where the lower limb muscle mass = 0.9 × lower limb lean mass, and ΔVO2 is the increase in the measured whole-body VO2 during exercise above resting.
Each statistical test used is reported in the table and figure legends. Paired Student’s t-tests were performed when there was a single repeated measures factor with only 2 levels (e.g., inactive and active). When there were 2 factors (e.g., activity/inactivity and time) or more than 2 levels (e.g., inactive, SPU1, SPU2), results were analyzed with a mixed effects model for repeated measures with Tukey’s multiple comparison tests. Each p-value was adjusted to account for multiple comparisons with a family-wise significance set at a level of 0.05 for each mixed effects model. Effect size (ES) was calculated with Cohen’s d test. ES descriptors were as follows: d (0.01 < 0.2) = very small, d (0.2 < 0.5) = small, d (0.5 < 0.7) = medium, d (0.8 < 1.2) = large, d (1.2 < 2.0) = very large, d (≥2.0) = huge (Sawilowsky, 2009). Normality of the data was tested using the Shapiro-Wilk test. Non-normally distributed variables were log transformed before further analysis. Linear regression was used to examine the relationship between specific variables. Statistical tests were two-sided and significance was set at p < 0.05. GraphPad Prism software version 8.4.3 was used. Results are expressed as mean ± SE unless stated otherwise.
Acknowledgments
We appreciate the financial support of the American Diabetes Association (1-15-TS-14).
Author contributions
M.T.H., D.G.H., and T.W.Z. designed research; M.T.H., D.G.H., and T.W.Z. performed the research; M.T.H., D.G.H., and T.W.Z. analyzed data; and M.T.H., D.G.H., and T.W.Z. wrote the paper. All authors approved the final version of the manuscript.
Declaration of interests
We, the authors and our immediate family members, have no related patents to declare.
The authors have been developing technologies to help instruct people how to do the SPU movement optimally. The University of Houston has the intent to patent potential intellectual property.
Inclusion and diversity
We worked to ensure gender balance in the recruitment of human subjects. We worked to ensure ethnic or other types of diversity in the recruitment of human subjects. We worked to ensure that the study questionnaires were prepared in an inclusive way. One or more of the authors of this paper received support from a program designed to increase minority representation in science.
Published: September 16, 2022
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2022.104869.
Supplemental information
Data and code availability
Any reasonable request for data reported in this paper will be shared by the lead contact upon request. This paper does not report original code. Any reasonable request for additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- Aadland E., Hostmark A.T. Very light physical activity after a meal blunts the rise in blood glucose and insulin. Open Nutr. J. 2008;2:94–99. [Google Scholar]
- Akerstrom T.C.A., Birk J.B., Klein D.K., Erikstrup C., Plomgaard P., Pedersen B.K., Wojtaszewski J. Oral glucose ingestion attenuates exercise-induced activation of 5′-AMP-activated protein kinase in human skeletal muscle. Biochem. Biophys. Res. Commun. 2006;342:949–955. doi: 10.1016/j.bbrc.2006.02.057. [DOI] [PubMed] [Google Scholar]
- Barreira T.V., Hamilton M.T., Craft L.L., Gapstur S.M., Siddique J., Zderic T.W. Intra-individual and inter-individual variability in daily sitting time and MVPA. J. Sci. Med. Sport. 2016;19:476–481. doi: 10.1016/j.jsams.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman B.C., Butterfield G.E., Wolfel E.E., Lopaschuk G.D., Casazza G.A., Horning M.A., Brooks G.A. Muscle net glucose uptake and glucose kinetics after endurance training in men. Am. J. Physiol. 1999;277:81–92. doi: 10.1152/ajpendo.1999.277.1.E81. [DOI] [PubMed] [Google Scholar]
- Bey L., Akunuri N., Zhao P., Hoffman E.P., Hamilton D.G., Hamilton M.T. Patterns of global gene expression in rat skeletal muscle during unloading and low-intensity ambulatory activity. Physiol. Genom. 2003;13:157–167. doi: 10.1152/physiolgenomics.00001.2002. [DOI] [PubMed] [Google Scholar]
- Bey L., Hamilton M.T. Suppression of skeletal muscle lipoprotein lipase activity during physical inactivity: a molecular reason to maintain daily low-intensity activity. J. Physiol. 2003;551:673–682. doi: 10.1113/jphysiol.2003.045591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouns F., Bjorck I., Frayn K.N., Gibbs A.L., Lang V., Slama G., Wolever T.M.S. Glycaemic index methodology. Nutr. Res. Rev. 2005;18:145–171. doi: 10.1079/NRR2005100. [DOI] [PubMed] [Google Scholar]
- Buysschaert M., Medina J.L., Bergman M., Shah A., Lonier J. Prediabetes and associated disorders. Endocrine. 2015;48:371–393. doi: 10.1007/s12020-014-0436-2. [DOI] [PubMed] [Google Scholar]
- Cardinale D.A., Larsen F.J., Jensen-Urstad M., Rullman E., Søndergaard H., Morales-Alamo D., Ekblom B., Calbet J.A.L., Boushel R. Muscle mass and inspired oxygen influence oxygen extraction at maximal exercise: role of mitochondrial oxygen affinity. Acta Physiol. 2019;225 doi: 10.1111/apha.13110. e13110–14. [DOI] [PubMed] [Google Scholar]
- Cartee G.D., Arias E.B., Yu C.S., Pataky M.W. Novel single skeletal muscle fiber analysis reveals a fiber type-selective effect of acute exercise on glucose uptake. Am. J. Physiol. Endocrinol. Metab. 2016;311:E818–E824. doi: 10.1152/ajpendo.00289.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K.Y., Brychta R.J., Abdul Sater Z., Cassimatis T.M., Cero C., Fletcher L.A., Israni N.S., Johnson J.W., Lea H.J., Linderman J.D., et al. Opportunities and challenges in the therapeutic activation of human energy expenditure and thermogenesis to manage obesity. J. Biol. Chem. 2020;295:1926–1942. doi: 10.1074/jbc.REV119.007363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chondronikola M., Volpi E., Børsheim E., Porter C., Annamalai P., Enerbäck S., Lidell M.E., Saraf M.K., Labbe S.M., Hurren N.M., et al. Brown adipose tissue improves whole-body glucose homeostasis and insulin sensitivity in humans. Diabetes. 2014;63:4089–4099. doi: 10.2337/db14-0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colberg S.R., Sigal R.J., Yardley J.E., Riddell M.C., Dunstan D.W., Dempsey P.C., Horton E.S., Castorino K., Tate D.F. Physical activity/exercise and diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2016;39:2065–2079. doi: 10.2337/dc16-1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft L.L., Zderic T.W., Gapstur S.M., VanIterson E.H., Thomas D.M., Siddique J., Hamilton M.T. Evidence that women meeting physical activity guidelines do not sit less: an observational inclinometry study. Int. J. Behav. Nutr. Phys. Act. 2012;9:122–129. doi: 10.1186/1479-5868-9-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cresswell A.G., Löscher W.N., Thorstensson A. Influence of gastrocnemius muscle length on triceps surae torque development and electromyographic activity in man. Exp. Brain Res. 1995;105:283–290. doi: 10.1007/BF00240964. [DOI] [PubMed] [Google Scholar]
- DeFronzo R.A., Abdul-Ghani M. Assessment and treatment of cardiovascular risk in prediabetes: impaired glucose tolerance and impaired fasting glucose. Am. J. Cardiol. 2011;108:3B–24B. doi: 10.1016/j.amjcard.2011.03.013. [DOI] [PubMed] [Google Scholar]
- Dela F., Ingersen A., Andersen N.B., Nielsen M.B., Petersen H.H.H., Hansen C.N., Larsen S., Wojtaszewski J., Helge J.W. Effects of one-legged high-intensity interval training on insulin-mediated skeletal muscle glucose homeostasis in patients with type 2 diabetes. Acta Physiol. 2019;226:e13245. doi: 10.1111/apha.13245. [DOI] [PubMed] [Google Scholar]
- Deshmukh A.S., Steenberg D.E., Hostrup M., Birk J.B., Larsen J.K., Santos A., Kjøbsted R., Hingst J.R., Schéele C.C., Murgia M., et al. Deep muscle-proteomic analysis of freeze-dried human muscle biopsies reveals fiber type-specific adaptations to exercise training. Nat. Commun. 2021;12:1600. doi: 10.1038/s41467-021-22015-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin J.T., Barlow J., Horton E.S. Whole body and regional fuel metabolism during early postexercise recovery. Am. J. Physiol. 1989;256:E167–E172. doi: 10.1152/ajpendo.1989.256.1.E167. [DOI] [PubMed] [Google Scholar]
- Dunstan D.W., Dogra S., Carter S.E., Owen N. Sit less and move more for cardiovascular health: emerging insights and opportunities. Nat. Rev. Cardiol. 2021;18:637–648. doi: 10.1038/s41569-021-00547-y. [DOI] [PubMed] [Google Scholar]
- Eijsvogels T.M.H., Fernandez A.B., Thompson P.D. Are there deleterious cardiac effects of acute and chronic endurance exercise? Physiol. Rev. 2016;96:99–125. doi: 10.1152/physrev.00029.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Festa A., D’Agostino R., Hanley A.J.G., Karter A.J., Saad M.F., Haffner S.M. Differences in insulin resistance in nondiabetic subjects with isolated impaired glucose tolerance or isolated impaired fasting glucose. Diabetes. 2004;53:1549–1555. doi: 10.2337/diabetes.53.6.1549. [DOI] [PubMed] [Google Scholar]
- Flockhart M., Nilsson L.C., Tais S., Ekblom B., Apró W., Larsen F.J. Excessive exercise training causes mitochondrial functional impairment and decreases glucose tolerance in healthy volunteers. Cell Metab. 2021;33:957–970.e6. doi: 10.1016/j.cmet.2021.02.017. [DOI] [PubMed] [Google Scholar]
- Förster H., Haslbeck M., Mehnert H. Metabolic studies following the oral ingestion of different doses of glucose. Diabetes. 1972;21:1102–1108. doi: 10.2337/diab.21.11.1102. [DOI] [PubMed] [Google Scholar]
- Gan Z., Rumsey J., Hazen B.C., Lai L., Leone T.C., Vega R.B., Xie H., Conley K.E., Auwerx J., Smith S.R., et al. Nuclear receptor/microRNA circuitry links muscle fiber type to energy metabolism. J. Clin. Invest. 2013;123:2564–2575. doi: 10.1172/JCI67652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y., Silvennoinen M., Pesola A.J., Kainulainen H., Cronin N.J., Finni T. Acute metabolic response, energy expenditure, and EMG activity in sitting and standing. Med. Sci. Sports Exerc. 2017;49:1927–1934. doi: 10.1249/MSS.0000000000001305. [DOI] [PubMed] [Google Scholar]
- Gaster M., Staehr P., Beck-Nielsen H., Schrøder H.D., Handberg A. GLUT4 is reduced in slow muscle fibers of type 2 diabetic patients: is insulin resistance in type 2 diabetes a slow, type 1 fiber disease? Diabetes. 2001;50:1324–1329. doi: 10.2337/diabetes.50.6.1324. [DOI] [PubMed] [Google Scholar]
- Gollnick P.D., Piehl K., Saltin B. Selective glycogen depletion pattern in human muscle fibres after exercise of varying intensity and at varying pedalling rate. J. Physiol. 1974;241:45–57. doi: 10.1113/jphysiol.1974.sp010639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollnick P.D., Sjödin B., Karlsson J., Jansson E., Saltin B. Human soleus muscle: a comparison of fiber composition and enzyme activities with other leg muscles. Pflugers Arch. 1974;348:247–255. doi: 10.1007/BF00587415. [DOI] [PubMed] [Google Scholar]
- Halseth A.E., Bracy D.P., Wasserman D.H. Limitations to exercise- and maximal insulin-stimulated muscle glucose uptake. J. Appl. Physiol. 1998;85:2305–2313. doi: 10.1152/jappl.1998.85.6.2305. [DOI] [PubMed] [Google Scholar]
- Hamilton K.S., Gibbons F.K., Bracy D.P., Lacy D.B., Cherrington A.D., Wasserman D.H. Effect of prior exercise on the partitioning of an intestinal glucose load between splanchnic bed and skeletal muscle. J. Clin. Invest. 1996;98:125–135. doi: 10.1172/JCI118756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M.T., Etienne J., McClure W.C., Pavey B.S., Holloway A.K. Role of local contractile activity and muscle fiber type on LPL regulation during exercise. Am. J. Physiol. 1998;275:E1016–E1022. doi: 10.1152/ajpendo.1998.275.6.E1016. [DOI] [PubMed] [Google Scholar]
- Hamilton M.T., Hamilton D.G., Zderic T.W. Role of low energy expenditure and sitting in obesity, metabolic syndrome, type 2 diabetes, and cardiovascular disease. Diabetes. 2007;56:2655–2667. doi: 10.2337/db07-0882. [DOI] [PubMed] [Google Scholar]
- Hamilton M.T., Hamilton D.G., Zderic T.W. Sedentary behavior as a mediator of type 2 diabetes. Med. Sport Sci. 2014;60:11–26. doi: 10.1159/000357332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M.T., Hamilton D.G., Zderic T.W. Exercise physiology versus inactivity physiology: an essential concept for understanding lipoprotein lipase regulation. Exerc. Sport Sci. Rev. 2004;32:161–166. doi: 10.1097/00003677-200410000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison M., Moyna N.M., Zderic T.W., Ogorman D.J., McCaffrey N., Carson B.P., Hamilton M.T. Lipoprotein particle distribution and skeletal muscle lipoprotein lipase activity after acute exercise. Lipids Health Dis. 2012;11:64–68. doi: 10.1186/1476-511X-11-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healy G.N., Winkler E.A.H., Owen N., Anuradha S., Dunstan D.W. Replacing sitting time with standing or stepping : associations with cardio-metabolic risk biomarkers. Eur. Heart J. 2015;36:2643–2649. doi: 10.1093/eurheartj/ehv308. [DOI] [PubMed] [Google Scholar]
- Helge J.W., Stallknecht B., Richter E.A., Galbo H., Kiens B. Muscle metabolism during graded quadriceps exercise in man. J. Physiol. 2007;581:1247–1258. doi: 10.1113/jphysiol.2007.128348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson J., Edwardson C.L., Celis-Morales C.A., Davies M.J., Dunstan D.W., Esliger D.W., Gill J.M.R., Kazi A., Khunti K., King J., et al. Predictors of the acute postprandial response to breaking up prolonged sitting. Med. Sci. Sports Exerc. 2020;52:1385–1393. doi: 10.1249/MSS.0000000000002249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heymsfield S.B., Smith B., Chung E.A., Watts K.L., Gonzalez M.C., Yang S., Heo M., Thomas D.M., Turner D., Bosy-Westphal A., Müller M.J. Phenotypic differences between people varying in muscularity. J. Cachexia Sarcopenia Muscle. 2022;13:1100–1112. doi: 10.1002/jcsm.12959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey M.S., Carey J.O., Azevedo J.L., Houmard J.A., Pories W.J., Israel R.G., Dohm G.L. Skeletal muscle fiber composition is related to adiposity and in vitro glucose transport rate in humans. Am. J. Physiol. 1995;268:E453–E457. doi: 10.1152/ajpendo.1995.268.3.E453. [DOI] [PubMed] [Google Scholar]
- Hodgson J.A., Roy R.R., Higuchi N., Monti R.J., Zhong H., Grossman E., Edgerton V.R. Does daily activity level determine muscle phenotype? J. Exp. Biol. 2005;208:3761–3770. doi: 10.1242/jeb.01825. [DOI] [PubMed] [Google Scholar]
- Holmstrup M., Fairchild T., Keslacy S., Weinstock R., Kanaley J. Multiple short bouts of exercise over 12-h period reduce glucose excursions more than an energy-matched single bout of exercise. Metabolism. 2014;63:510–519. doi: 10.1016/j.metabol.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz J.F., Mora-Rodriguez R., Byerley L.O., Coyle E.F. Substrate metabolism when subjects are fed carbohydrate during exercise. Am. J. Physiol. 1999;276:E828–E835. doi: 10.1152/ajpendo.1999.276.5.E828. [DOI] [PubMed] [Google Scholar]
- Horton T.J., Pagliassotti M.J., Hobbs K., Hill J.O. Fuel metabolism in men and women during and after long-duration exercise. J. Appl. Physiol. 1998;85:1823–1832. doi: 10.1152/jappl.1998.85.5.1823. [DOI] [PubMed] [Google Scholar]
- Ivy J.L., Zderic T.W., Fogt D.L. Prevention and treatment of non-insulin-dependent diabetes mellitus. Exerc. Sport Sci. Rev. 1999;27:1–35. [PubMed] [Google Scholar]
- James D.E., Jenkins A.B., Kraegen E.W. Heterogeneity of insulin action in individual muscles in vivo: euglycemic clamp studies in rats. Am. J. Physiol. 1985;248:567–574. doi: 10.1152/ajpendo.1985.248.5.E567. [DOI] [PubMed] [Google Scholar]
- Jansen L.T., Yang N., Wong J.M.W., Mehta T., Allison D.B., Ludwig D.S., Ebbeling C.B. Prolonged glycemic adaptation following transition from a low- to high-carbohydrate diet : a randomized controlled feeding trial. Diabetes Care. 2022;45:576–584. doi: 10.2337/dc21-1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen T.E., Leutert R., Rasmussen S.T., Mouatt J.R., Christiansen M.L.B., Jensen B.R., Richter E.A. EMG-normalised kinase activation during exercise is higher in human gastrocnemius compared to soleus muscle. PLoS One. 2012;7:e31054. doi: 10.1371/journal.pone.0031054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M.A., Johnson M.A., Weightman D., Appleton D. Data on fibre size in thirty-six human muscles. An autopsy study. J. Neurol. Sci. 1973;19:307–318. doi: 10.1016/0022-510x(73)90094-4. [DOI] [PubMed] [Google Scholar]
- Kakehi E., Kotani K., Nakamura T., Takeshima T., Kajii E. Non-diabetic glucose levels and cancer mortality: a literature review. Curr. Diabetes Rev. 2018;14:434–445. doi: 10.2174/1573399813666170711142035. [DOI] [PubMed] [Google Scholar]
- Kawakami Y., Ichinose Y., Fukunaga T. Architectural and functional features of human triceps surae muscles during contraction. J. Appl. Physiol. 1998;85:398–404. doi: 10.1152/jappl.1998.85.2.398. [DOI] [PubMed] [Google Scholar]
- Kelley D., Mokan M., Veneman T. Impaired postprandial glucose utilization in non-insulin-dependent diabetes mellitus. Metabolism. 1994;43:1549–1557. doi: 10.1016/0026-0495(94)90015-9. [DOI] [PubMed] [Google Scholar]
- Kim J., Wang Z., Heymsfield S.B., Baumgartner R.N., Gallagher D. Total-body skeletal muscle mass: estimation by a new dual-energy X-ray absorptiometry method. Am. J. Clin. Nutr. 2002;76:378–383. doi: 10.1093/ajcn/76.2.378. [DOI] [PubMed] [Google Scholar]
- King D.S., Baldus P.J., Sharp R.L., Kesl L.D., Feltmeyer T.L., Riddle M.S. Time course for exercise-induced alterations in insulin action and glucose tolerance in middle-aged people. J. Appl. Physiol. 1995;78:17–22. doi: 10.1152/jappl.1995.78.1.17. [DOI] [PubMed] [Google Scholar]
- Knowler W.C., Barrett-Connor E., Fowler S.E., Hamman R.F., Lachin J.M., Walker E.A., Nathan D.M. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen S.H., Karstoft K., Pedersen B.K., Van Hall G., Solomon T.P.J. The immediate effects of a single bout of aerobic exercise on oral glucose tolerance across the glucose tolerance continuum. Physiol. Rep. 2014;2 doi: 10.14814/phy2.12114. e12114–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolk S., Klawer E.M.E., Schepers J., Weerdesteyn V., Visser E.P., Verdonschot N. Muscle activity during walking measured using 3D MRI segmentations and [18F]-fluorodeoxyglucose in combination with positron emission tomography. Med. Sci. Sports Exerc. 2015;47:1896–1905. doi: 10.1249/MSS.0000000000000607. [DOI] [PubMed] [Google Scholar]
- Larsen R.N., Kingwell B.A., Robinson C., Hammond L., Cerin E., Shaw J.E., Healy G.N., Hamilton M.T., Owen N., Dunstan D.W. Breaking up of prolonged sitting over three days sustains, but does not enhance, lowering of postprandial plasma glucose and insulin in overweight and obese adults. Clin. Sci. 2015;129:117–127. doi: 10.1042/CS20140790. [DOI] [PubMed] [Google Scholar]
- Laughlin M.H. Physical activity-induced remodeling of vasculature in skeletal muscle: role in treatment of type 2 diabetes. J. Appl. Physiol. 2016;120:1–16. doi: 10.1152/japplphysiol.00789.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livesey G., Wilson P.D., Dainty J.R., Brown J.C., Faulks R.M., Roe M.A., Newman T.A., Eagles J., Mellon F.A., Greenwood R.H. Simultaneous time-varying systemic appearance of oral and hepatic glucose in adults monitored with stable isotopes. Am. J. Physiol. 1998;275:E717–E728. doi: 10.1152/ajpendo.1998.275.4.E717. [DOI] [PubMed] [Google Scholar]
- Loh R., Stamatakis E., Folkerts D., Allgrove J.E., Moir H.J. Effects of interrupting prolonged sitting with physical activity breaks on blood glucose, insulin and triacylglycerol measures: a systematic review and meta-analysis. Sports Med. 2020;50:295–330. doi: 10.1007/s40279-019-01183-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh R.K.C., Formosa M.F., La Gerche A., Reutens A.T., Kingwell B.A., Carey A.L. Acute metabolic and cardiovascular effects of mirabegron in healthy individuals. Diabetes Obes. Metab. 2019;21:276–284. doi: 10.1111/dom.13516. [DOI] [PubMed] [Google Scholar]
- Mackie B.G., Dudley G.A., Kaciuba-Uscilko H., Terjung R.L. Uptake of chylomicron triglycerides by contracting skeletal muscle in rats. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1980;49:851–855. doi: 10.1152/jappl.1980.49.5.851. [DOI] [PubMed] [Google Scholar]
- Maehlum S., Felig P., Wahren J. Splanchnic glucose and muscle glycogen metabolism after glucose feeding during postexercise recovery. Am. J. Physiol. 1978;235:E255–E260. doi: 10.1152/ajpendo.1978.235.3.E255. [DOI] [PubMed] [Google Scholar]
- Magkos F., Fraterrigo G., Yoshino J., Luecking C., Kirbach K., Kelly S.C., De Las Fuentes L., He S., Okunade A.L., Patterson B.W., Klein S. Effects of moderate and subsequent progressive weight loss on metabolic function and adipose tissue biology in humans with obesity. Cell Metab. 2016;23:591–601. doi: 10.1016/j.cmet.2016.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews C.E., Kozey Keadle S., Moore S.C., Schoeller D.S., Carroll R.J., Troiano R.P., Sampson J.N. Measurement of active and sedentary behavior in context of large epidemiologic studies. Med. Sci. Sports Exerc. 2018;50:266–276. doi: 10.1249/MSS.0000000000001428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonough P., Behnke B.J., Padilla D.J., Musch T.I., Poole D.C. Control of microvascular oxygen pressures in rat muscles comprised of different fibre types. J. Physiol. 2005;563:903–913. doi: 10.1113/jphysiol.2004.079533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeill B.T., Morton N.M., Stimson R.H. Substrate utilization by brown adipose tissue: what’s hot and what’s not? Front. Endocrinol. 2020;11:1–8. doi: 10.3389/fendo.2020.571659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menke A., Casagrande S., Geiss L., Cowie C.C. Prevalence of and trends in diabetes among adults in the United States, 1988-2012. JAMA. 2015;314:1021–1029. doi: 10.1001/jama.2015.10029. [DOI] [PubMed] [Google Scholar]
- Monster A.W., Chan H., O’Connor D. Activity patterns of human skeletal muscles : relation to muscle fiber type composition. Science. 1978;200:314–317. doi: 10.1126/science.635587. [DOI] [PubMed] [Google Scholar]
- Mossberg K.A., Mommessin J.I., Taegtmeyer H. Skeletal muscle glucose uptake during short-term contractile activity in vivo: effect of prior contractions. Metabolism. 1993;42:1609–1616. doi: 10.1016/0026-0495(93)90158-k. [DOI] [PubMed] [Google Scholar]
- Murgia M., Nogara L., Baraldo M., Reggiani C., Mann M., Schiaffino S. Protein profile of fiber types in human skeletal muscle: a single-fiber proteomics study. Skelet. Muscle. 2021;11:24. doi: 10.1186/s13395-021-00279-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton R.L., Han H., Zderic T., Hamilton M.T., Hamilton M. The energy expenditure of sedentary behavior: a whole room calorimeter study. PLoS One. 2013;8:e63171. doi: 10.1371/journal.pone.0063171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niess F., Fiedler G.B., Schmid A.I., Laistler E., Frass-Kriegl R., Wolzt M., Moser E., Meyerspeer M. Dynamic multivoxel-localized 31P MRS during plantar flexion exercise with variable knee angle. NMR Biomed. 2018;31:e3905. doi: 10.1002/nbm.3905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohara T., Doi Y., Ninomiya T., Hirakawa Y., Hata J., Iwaki T., Kanba S., Kiyohara Y. Glucose tolerance status and risk of dementia in the community: the Hisayama study. Neurology. 2011;77:1126–1134. doi: 10.1212/WNL.0b013e31822f0435. [DOI] [PubMed] [Google Scholar]
- Papanas N., Vinik A.I., Ziegler D. Neuropathy in prediabetes: does the clock start ticking early? Nat. Rev. Endocrinol. 2011;7:682–690. doi: 10.1038/nrendo.2011.113. [DOI] [PubMed] [Google Scholar]
- Peachey M.M., Richardson J., V Tang A., Dal-Bello Haas V., Gravesande J. Environmental, behavioural and multicomponent interventions to reduce adults’ sitting time: a systematic review and meta-analysis. Br. J. Sports Med. 2020;54:315–325. doi: 10.1136/bjsports-2017-098968. [DOI] [PubMed] [Google Scholar]
- Petersen H.A., Fueger P.T., Bracy D.P., Wasserman D.H., Halseth A.E. Fiber type-specific determinants of Vmax for insulin-stimulated muscle glucose uptake in vivo. Am. J. Physiol. Endocrinol. Metab. 2003;284:E541–E548. doi: 10.1152/ajpendo.00323.2002. [DOI] [PubMed] [Google Scholar]
- Pettit-Mee R.J., Ready S.T., Padilla J., Kanaley J.A. Leg fidgeting during prolonged sitting improves postprandial glycemic control in people with obesity. Obesity. 2021;29:1146–1154. doi: 10.1002/oby.23173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price T.B., Kamen G., Damon B.M., Knight C.A., Applegate B., Gore J.C., Eward K., Signorile J.F. Comparison of MRI with EMG to study muscle activity associated with dynamic plantar flexion. Magn. Reson. Imaging. 2003;21:853–861. doi: 10.1016/s0730-725x(03)00183-8. [DOI] [PubMed] [Google Scholar]
- Ranvier L. Au Bureau de la Gazette Medicale; 1873. Comptes rendus des seances et memoires de la societe de biologie; pp. 267–268. [Google Scholar]
- Ranvier L. V.A. Delahaye et Cie; 1880. Lecons d’anatomie generale sur le systeme musculaire; pp. 202–219. [Google Scholar]
- Richter E.A., Ruderman N.B., Gavras H., Belur E.R., Galbo H. Muscle glycogenolysis during exercise: dual control by epinephrine and contractions. Am. J. Physiol. 1982;242:E25–E32. doi: 10.1152/ajpendo.1982.242.1.E25. [DOI] [PubMed] [Google Scholar]
- Richter E.A., Kiens B., Saltin B., Christensen N.J., Savard G. Skeletal muscle glucose uptake during dynamic exercise in humans: role of muscle mass. Am. J. Physiol. 1988;254:E555–E561. doi: 10.1152/ajpendo.1988.254.5.E555. [DOI] [PubMed] [Google Scholar]
- Rolfe D.F.S., Brown G.C. Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol. Rev. 1997;77:731–758. doi: 10.1152/physrev.1997.77.3.731. [DOI] [PubMed] [Google Scholar]
- Rose A.J., Howlett K., King D.S., Hargreaves M. Effect of prior exercise on glucose metabolism in trained men. Am. J. Physiol. Endocrinol. Metab. 2001;281:766–771. doi: 10.1152/ajpendo.2001.281.4.E766. [DOI] [PubMed] [Google Scholar]
- Ross R., Dagnone D., Jones P.J., Smith H., Paddags A., Hudson R., Janssen I. Reduction in obesity and related comorbid conditions after diet-induced weight loss or exercise-induced weight loss in men: a randomized, controlled trial. Ann. Intern. Med. 2000;133:92–103. doi: 10.7326/0003-4819-133-2-200007180-00008. [DOI] [PubMed] [Google Scholar]
- Ross R., Hudson R., Stotz P.J., Lam M. Effects of exercise amount and intensity on abdominal obesity and glucose tolerance in obese adults: a randomized trial. Ann. Intern. Med. 2015;162:325–334. doi: 10.7326/M14-1189. [DOI] [PubMed] [Google Scholar]
- Sawilowsky S.S. New effect size rules of thumb. J. Mod. Appl. Stat. Methods. 2009;8:597–599. [Google Scholar]
- Slentz C.A., Bateman L.A., Willis L.H., Granville E.O., Piner L.W., Samsa G.P., Setji T.L., Muehlbauer M.J., Huffman K.M., Bales C.W., Kraus W.E. Effects of exercise training alone vs a combined exercise and nutritional lifestyle intervention on glucose homeostasis in prediabetic individuals: a randomised controlled trial. Diabetologia. 2016;59:2088–2098. doi: 10.1007/s00125-016-4051-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X.M., Ryder J.W., Kawano Y., Chibalin A.V., Krook A., Zierath J.R. Muscle fiber type specificity in insulin signal transduction. Am. J. Physiol. 1999;277:R1690–R1696. doi: 10.1152/ajpregu.1999.277.6.R1690. [DOI] [PubMed] [Google Scholar]
- Steenberg D.E., Hingst J.R., Birk J.B., Thorup A., Kristensen J.M., Sjøberg K.A., Kiens B., Richter E.A., Wojtaszewski J.F.P. A single bout of one-legged exercise to local exhaustion decreases insulin action in nonexercised muscle leading to decreased whole-body insulin action. Diabetes. 2020;69:578–590. doi: 10.2337/db19-1010. [DOI] [PubMed] [Google Scholar]
- Succurro E., Marini M.A., Arturi F., Grembiale A., Lugarà M., Andreozzi F., Sciacqua A., Lauro R., Hribal M.L., Perticone F., Sesti G. Elevated one-hour post-load plasma glucose levels identifies subjects with normal glucose tolerance but early carotid atherosclerosis. Atherosclerosis. 2009;207:245–249. doi: 10.1016/j.atherosclerosis.2009.04.006. [DOI] [PubMed] [Google Scholar]
- Sylow L., Kleinert M., Richter E.A., Jensen T.E. Exercise-stimulated glucose uptake-regulation and implications for glycaemic control. Nat. Rev. Endocrinol. 2017;13:133–148. doi: 10.1038/nrendo.2016.162. [DOI] [PubMed] [Google Scholar]
- Terry E.E., Zhang X., Hoffmann C., Hughes L.D., Lewis S.A., Li J., Wallace M.J., Riley L.A., Douglas C.M., Gutierrez-Monreal M.A., et al. Transcriptional profiling reveals extraordinary diversity among skeletal muscle tissues. Elife. 2018;7 doi: 10.7554/eLife.34613. e34613–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorp A.A., Kingwell B.A., Sethi P., Hammond L., Owen N., Dunstan D.W. Alternating bouts of sitting and standing attenuate postprandial glucose responses. Med. Sci. Sports Exerc. 2014;46:2053–2061. doi: 10.1249/MSS.0000000000000337. [DOI] [PubMed] [Google Scholar]
- Thorsen I.K., Johansen M.Y., Pilmark N.S., Jespersen N.Z., Brinkløv C.F., Benatti F.B., Dunstan D.W., Karstoft K., Pedersen B.K., Ried-Larsen M. The effect of frequency of activity interruptions in prolonged sitting on postprandial glucose metabolism: a randomized crossover trial. Metabolism. 2019;96:1–7. doi: 10.1016/j.metabol.2019.04.003. [DOI] [PubMed] [Google Scholar]
- van der Berg J.D., Stehouwer C.D.A., Bosma H., van der Velde J.H.P.M., Willems P.J.B., Savelberg H.H.C.M., Schram M.T., Sep S.J.S., van der Kallen C.J.H., Henry R.M.A., et al. Associations of total amount and patterns of sedentary behaviour with type 2 diabetes and the metabolic syndrome: the Maastricht Study. Diabetologia. 2016;59:709–718. doi: 10.1007/s00125-015-3861-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward S.R., Eng C.M., Smallwood L.H., Lieber R.L. Are current measurements of lower extremity muscle architecture accurate? Clin. Orthop. Relat. Res. 2009;467:1074–1082. doi: 10.1007/s11999-008-0594-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman D.H., Geer R.J., Rice D.E., Bracy D., Flakoll P.J., Brown L.L., Hill J.O., Abumrad N.N. Interaction of exercise and insulin action in humans. Am. J. Physiol. 1991;260:E37–E45. doi: 10.1152/ajpendo.1991.260.1.E37. [DOI] [PubMed] [Google Scholar]
- Whichelow M.J., Wigglesworth A., Cox B.D., Butterfield W.J., Abrams M.E. Critical analysis of blood sugar measurements in diabetes detection and diagnosis. Diabetes. 1967;16:219–226. doi: 10.2337/diab.16.4.219. [DOI] [PubMed] [Google Scholar]
- Xia P.F., Tian Y.X., Geng T.T., Li Y., Tu Z.Z., Zhang Y.B., Guo K., Yang K., Liu G., Pan A. Trends in prevalence and awareness of prediabetes among adults in the U.S., 2005–2020. Diabetes Care. 2022;45:e21–e23. doi: 10.2337/dc21-2100. [DOI] [PubMed] [Google Scholar]
- Zderic T.W., Hamilton M.T. Identification of hemostatic genes expressed in human and rat leg muscles and a novel gene (LPP1/PAP2A) suppressed during prolonged physical inactivity (sitting) Lipids Health Dis. 2012;11:137. doi: 10.1186/1476-511X-11-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Any reasonable request for data reported in this paper will be shared by the lead contact upon request. This paper does not report original code. Any reasonable request for additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.