Abstract
Paragangliomas are rare, non-epithelial neuroendocrine neoplasms originating in paraganglia, for instance the adrenal medulla, or at extra-adrenal locations. The aim of this study was to review the literature regarding abdominal extra-adrenal paragangliomas diagnosed pre-operatively with fine-needle biopsy (FNA and/or FNB). The PubMed database was searched to identify such cases, using a specific algorithm and inclusion/exclusion criteria. An unpublished case from our practice was also added to the rest of the data, resulting in a total of 36 cases for analysis. Overall, 24 (67%) lesions were found in females, whereas 12 (33%) in males. Most (21/36; 58.33%) were identified around and/or within the pancreatic parenchyma. FNA and/or FNB reached or suggested a paraganglioma diagnosis in 17/36 cases (47.22%). Of the preoperative misdiagnoses, the most common was an epithelial neuroendocrine tumor (NET). Regarding follow-up, most patients were alive with no reported recurrence; however, 5/36 patients exhibited a recurrence or a widespread disease, whereas one patient died 48 months following her diagnosis. In two patients, transient hypertension was reported during the EUS-FNA procedure. In conclusion, this study showed that the preoperative diagnosis of these lesions is feasible and, while diagnostic pitfalls exist, they could significantly be avoided with the application of immunochemistry.
Keywords: endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA), pancreatic neuroendocrine tumor (PanNET), cytopathology, neoplasm, molecular pathology, pancreas, metastasis, cancer prognosis, immunohistochemistry, paraganglioma
1. Introduction
Paragangliomas are rare, non-epithelial neuroendocrine neoplasms derived from the neural crest, yet their incidence has increased over recent years. Pheochromocytomas and paragangliomas (often referred together as PPGLs) are histologically similar. Notably, according to the latest WHO guidelines, pheochromocytomas are now officially considered as “intra-adrenal paragangliomas” [1,2]. PPGLs originate in structures called the paraganglia—found in close association with the autonomic nervous system—for instance in the adrenal medulla (where the term pheochromocytoma is traditionally used) or in paraganglia located at extra-adrenal locations. The latter are most often found in the head and neck area (e.g., the carotid body) or the retroperitoneum (e.g., the organ of Zuckerkandl). Head and neck paragangliomas are most likely parasympathetic, whereas retroperitoneal paragangliomas are sympathetic [1,3,4]. These neoplasms could be either functional or non-functional; the former could be detected by using a meta-iodobenzylguanidine (MIBG) scan, which results in high MIBG uptake from the lesion, or by measuring the catecholamine or their metabolites (metanephrine) levels in the plasma and/or urine of the patients [5,6]. Of interest, paragangliomas exhibit the highest genetic predisposition among all human neoplasms, and more than 40% of PPGL patients carry germline mutations; in addition, sporadic mutations are prevalent as well and, when existing alongside germline mutations, they may exhibit a synergistic effect. Examples of PPGLA mutations include the RET gene in multiple endocrine neoplasia (MEN) syndromes, the VHL gene in von Hippel–Lindau disease, the NF1 gene in Neurofibromatosis type 1, the MAX gene, or the genes of the succinate dehydrogenase complex (SDHB, SDHD, SDHA, SDHC, SDHAF2) [1,3,7,8,9].
PPGL patients could present with a clinical picture related to catecholamine hypersecretion, such as headaches, palpitation, and high blood pressure, coupled with high levels of metanephrines in their plasma or urine. In such cases, clinicians could suspect, readily identify, and subsequently manage the lesion [5,10,11]. However, this clinical presentation is often absent, and abdominal masses subsequently diagnosed as PPGLs are either identified incidentally in asymptomatic patients using modalities, such as CT or MRI, or present with non-specific symptomatology (e.g., abdominal pain). In such scenarios, clinicians do not often suspect a paraganglioma, and preoperative diagnosis largely relies on the pathologic interpretation. Preoperative diagnosis of such masses is most often performed with fine-needle aspiration (FNA) and/or fine-needle biopsy (FNB), while these procedures are typically guided with imaging, such as endoscopic ultrasound (EUS) [4,5,11]. Due to the possibility of paroxysmal hypertension, biopsies of PPGL masses are often contra-indicated, for instance when they are located in the adrenal glands; this is a main reason why pheochromocytoma biopsies are seldom performed, compared to the neoplasm’s incidence [12]. However, some authors reported that biopsy-related complications were not common in their practice, while, when they occurred, they could be controlled with the administration of adrenergic blocking [13,14].
The aim of this study was to review the literature regarding the reported cases of abdominal extra-adrenal paragangliomas diagnosed pre-operatively with FNA and/or FNB, with the goal to reveal their main demographics, diagnostic pitfalls, and clinical behavior. In addition, we also present an unpublished case of a retroperitoneal paraganglioma from our practice, diagnosed with EUS-FNB.
2. Materials and Methods
2.1. Search Strategy
The PubMed database was searched for cases of abdominal extra-adrenal paragangliomas diagnosed with FNA and/or FNB from 1 January 2000, until 26 May 2022, using the following algorithm: (paraganglioma OR pheochromocytoma) AND (FNA OR “fine-needle aspiration” OR FNB OR EUS-FNA OR EUS-FNB).
2.2. Study Selection
Inclusion criteria involved any studies (e.g., cohort, case series, case reports) reporting abdominal extra-adrenal paragangliomas diagnosed with FNA and/or FNB, where the final diagnosis was reached either preoperatively from the FNA/FNB material or by examining the subsequent surgical excision specimen. Exclusion criteria involved studies reporting solely PPGLs found in the adrenals (pheochromocytomas), outside the abdomen, or diagnosed with modalities other than FNA/FNB. Articles where individual data of the paraganglioma cases described could not be extracted were also excluded at a subsequent step. Three authors (I.P.N., A.I. and M.M.A.) first performed a title–abstract selection, which was followed by a full-text evaluation of all eligible articles. Any discrepancies were resolved by reaching a consensus.
2.3. Data Extraction
The following data regarding individual paraganglioma cases were extracted in an Excel® file: first author, year of publication, gender, age, location of the mass in the abdomen, diameter of the mass assessed with endoscopy or radiology (e.g., EUS or CT), clinical presentation, biopsy type (FNA and/or FNB), preoperative diagnosis derived from the FNA and/or FNB pathologic interpretation, immunochemistry performed on the FNA or FNB material, whether or not the mass was excised with surgery, and follow-up information.
3. Results
3.1. Literature Search
The flowchart of our study is shown in Figure 1. The initial search in the PubMed database identified 201 articles, which were subsequently screened in a title-abstract fashion. In total, 28 studies were eligible for full-text evaluation; of them, 2 could not retrieved, whereas 6 were excluded due to an inability to extract individual data concerning the paraganglioma cases reported. Lastly, 20 studies, reporting 35 abdominal extra-adrenal paragangliomas, were included in the review. An unpublished abdominal extra-adrenal paraganglioma case from our practice was also added to the rest of the data, resulting in a total of 36 cases for analysis.
Figure 1.
Flowchart of our study.
3.2. Characteristics of the Included Studies
The extracted data from the 36 eligible paraganglioma cases are shown in Table 1. Overall, 24 (67%) were females, whereas 12 (33%) were males. Mean and median ages of diagnosis were 54.22 and 54.5 years, respectively. Mean age was 57.04 for females, whereas 48.58 for males. In total, 21 of the 36 abdominal paraganglioma cases (21/36; 58.33%) accessed with a fine-needle biopsy were found to be located around and/or within the pancreatic parenchyma (peripancreatic), most often in association with the pancreatic head. The diameter of the lesions ranged from 19 to 170 mm. Although a few of the cases were symptomatic at diagnosis (e.g., presence of abdominal pain), 13/36 (36%) were detected incidentally with a radiologic modality. Most lesions were retroperitoneal, while one was intraperitoneal [15]. In two patients, transient hypertension was reported during the EUS-FNA procedure [16,17]. FNA was solely used for 31/36 cases, whereas a combination of FNA and FNB for 4/36 and solely FNB for 1/36 cases, respectively. FNA and/or FNB reached or suggested a diagnosis of PPGL in 17/36 cases (47.22%). In the rest of the cases, complete pathologic examination of the surgical specimen was necessary for the final diagnosis. Of the preoperative misdiagnoses, the most common one was an epithelial neuroendocrine tumor (NET), for example a pancreatic NET (PanNET). Application of immunochemistry on cell blocks, cytology slides, or the FNB material was reported in 14/36 cases, while surgical excision of the abdominal mass in 28/36 cases. Regarding follow-up, most patients were alive with no reported recurrence; however, 5/36 patients (13.9%) were reported to have a recurrence or a widespread disease, whereas one patient (1/36; 2.8%) died 48 months following her diagnosis [18].
Table 1.
Literature review (2000–2022) of abdominal extra-adrenal paragangliomas diagnosed with FNA or FNB.
| First Author, Year | Gender, Age | Location | Diameter (Radiology) | Clinical Presentation | Biopsy Type | Preoperative Diagnosis | Immunochemistry on FNA/FNB Material | Surgery Performed | Follow-Up |
|---|---|---|---|---|---|---|---|---|---|
| Radulovic, 2022 [19] | F, 48 | Peripancreatic (Tail) | 35 mm (EUS) | Incidental mass; diarrhea and metrorrhagia | EUS-FNA | PPGL | Chr (+), Syn (+), GATA-3 (+), S100 (+), Ker (−), Inhibin (−), PAX8 (−), WT-1 (−), Ki-67 (<3%) |
Yes | No alterations found in subsequent genetic testing |
| Lanke, 2021 [5] | F, 73 | Peripancreatic (Head) |
19 mm (EUS) | Asymptomatic, incidental mass; metanephrine levels normal | EUS-FNA | PPGL | Chr (+), Syn (+), GATA-3 (+), Ker (−), Ki-67 (<1%) |
No | Alive, no recurrence (12 mo) |
| Thakur, 2021 [20] | M, 58 | Peripancreatic (Head and uncinate process) | 78 mm (EUS) | Asymptomatic, incidental mass; metanephrine levels normal | EUS-FNA | PPGL | Chr (+), Syn (+), GATA-3 (+), Ker (−) |
N/A | Under MIGB therapy, to be followed by surgery |
| Naito, 2021 [21] | F, 61 | Peripancreatic (Head/greater omentum) | 21 mm (CT) | Asymptomatic, incidental mass | EUS-FNA | NET | Chr (+), Syn (+), CD56 (+) |
Yes | Alive, no recurrence (6 mo) |
| Abbasi, 2020 [22] | F, 61 | Peripancreatic (Head) | 64 mm (EUS) | Asymptomatic, incidental mass | EUS-FNA | PanNET | Chr (+), Syn (+) | Yes | Alive, no recurrence (12 mo) |
| Yang, 2019 [23] | F, 67 | Peripancreatic (Head) | 50 mm (CT) | Abdominal pain, weight loss, nausea, vomiting | EUS-FNA | PPGL | Chr (+), Syn (+), CD56 (+), Ker (−), Ki-67 (<1%) |
No | N/A |
| Nguyen, 2018 [24] | F, 70 | Peripancreatic (Tail) |
58 mm (EUS) | Constipation, satiety | EUS-FNA | Suggestive of PPGL | Chr (+), Syn (+), GATA-3 (+), S100 (+), Ker (−), ER (−), CDX2 (−) |
Yes | N/A |
| Fite, 2018 [14] | M, 55 | Retroperitoneal | 97 mm | Discomfort | FNA | Consistent with PPGL | N/A | Yes | Recurrence after 9 years (same location) |
| Fite, 2018 [14] | M, 35 | Retroperitoneal | 83 mm | na | FNA | PPGL | N/A | Yes | Widespread bone and lung metastatic lesions at 5-year follow-up |
| Fite, 2018 [14] | M, 40 | Peripancreatic | 51 mm | Pain and hematuria; plasma metanephrine levels high | FNA | Suggestive of PanNET | N/A | Yes | Alive, no recurrence |
| Fite, 2018 [14] | F, 23 | Peripancreatic | 70 mm | Tachycardia; plasma chromogranin A levels high | FNA | NET | N/A | Yes | Alive, no recurrence |
| Zeng, 2017 [25] | F, 58 | Peripancreatic (Head) | 65 mm (MRI) | Abdominal pain | EUS-FNA | PPGL vs. PanNET | Chr (+), Syn (+), CD117 (−) |
Yes | N/A |
| Zeng, 2017 [25] | F, 53 | Peripancreatic | 25 mm (CT) | Pelvic pain | EUS-FNA | NET | Chr (+), Syn (+) | Yes | N/A |
| Tumuluru, 2016 [26] | F, 62 | Peripancreatic (Body) | 32 mm (EUS) | Asymptomatic; incidental mass | EUS-FNA | Atypical epithelial cells | N/A | Yes | Alive, no recurrence (18 mo) |
| Zhang, 2014 [18] | F, 50 | Widespread (pancreatic head; multiple liver lesions) | 60 mm (CT) for the peripancreatic lesion | Headache, palpitation, sweating, hypertension | FNA | Suggestive of PPGL | Chr (+), Syn (+) | Yes | Died (48 mo after diagnosis) |
| Handa, 2014 [27] | M, 32 | Paraaortic | N/A | Headache | FNA under US guidance | PPGL | N/A | N/A | N/A |
| Handa, 2014 [27] | F, 50 | Paraaortic | N/A | Abdominal mass | FNA under US guidance | PPGL | N/A | N/A | N/A |
| Moslemi, 2012 [15] | F, 55 | Perirenal (Intraperitoneal) | 150 mm (CT) | Abdominal pain, anorexia, weight loss | FNA under US guidance | Undifferentiated carcinoma | N/A | Yes | Alive, no recurrence (12 mo) |
| Ganc, 2012 [28] | F, 37 | Peripancreatic (Head) | 35 mm | Asymptomatic; incidental mass | EUS-FNA | NET | Chr (+), Syn (+) | Yes | N/A |
| Laforga, 2012 [29] | M, 85 | Paragastric | N/A | Abdominal pain | EUS-FNA | N/A | N/A | Yes | Alive, no recurrence (22 mo) |
| Singhi, 2011 [4] | F, 61 | Peripancreatic (Tail) | 140 mm | Abdominal pain | EUS-FNA | Pseudocyst | N/A | Yes | Alive, no recurrence (140 mo) |
| Singhi, 2011 [4] | F, 52 | Peripancreatic (Body) | 140 mm | Abdominal pain | EUS-FNA and FNB | PPGL | N/A | No | Widespread metastatic lesions, DOD (34 mo) |
| Singhi, 2011 [4] | F, 54 | Peripancreatic (Head) | 65 mm | Abdominal pain | EUS-FNA and FNB | PPGL | N/A | Yes | Alive, no recurrence (8 mo) |
| Singhi, 2011 [4] | M, 40 | Peripancreatic (Body) | 51 mm | Asymptomatic; incidental mass in radiology | EUS-FNA | PanNET | N/A | Yes | Alive, no recurrence (4 mo) |
| Singhi, 2011 [4] | F, 78 | Peripancreatic (Body) | 170 mm | Abdominal pain | EUS-FNA | Spindle cell neoplasm | N/A | Yes | Alive, no recurrence (2 mo) |
| Singhi, 2011 [4] | M, 44 | Peripancreatic (Head) | 55 mm | Asymptomatic; incidental mass | EUS-FNA and FNB | PPGL | N/A | Yes | Alive, no recurrence (2 mo) |
| Sangster, 2010 [30] | M, 50 | Peripancreatic (Head) | N/A | Abdominal pain; hypertension | FNA | Poorly differentiated carcinoma | N/A | No (a surgical biopsy was though performed, providing the final diagnosis) | Alive, no recurrence (37 mo) |
| Rangaswamy, 2010 [31] | M, 45 | Perirenal | 120 mm (CT) | Asymptomatic; incidental mass; hypertension (metanephrine levels high) | FNA under CT guidance | Suggestive of PPGL | N/A | Yes | N/A |
| Kubota, 2010 [16] | F, 58 | Paraduodenal | 70 mm (CT) | Asymptomatic; incidental mass; transient hypertension during the EUS-FNA procedure; metanephrine levels high | EUS-FNA | Suggestive of PPGL | N/A | Yes | N/A |
| Jiménez-Heffernan, 2006 [13] | F, 58 | Retroperitoneal | N/A | N/A | FNA | NET | Chr (+) | Yes | N/A |
| Jiménez-Heffernan, 2006 [13] | M, 47 | Retroperitoneal | N/A | N/A | FNA | NET | NP | Yes | N/A |
| Akdamar, 2004 [17] | F, 62 | Paraduodenal | 66 mm (EUS) | Abdominal pain; transient hypertension during the EUS-FNA procedure | EUS-FNA | Suggestive of a neoplasm | N/A | Yes | N/A |
| Gong, 2003 [32] | F, 69 | Organ of Zuckerkandl | 50 mm (CT) | Asymptomatic; incidental mass | FNA under CT guidance | Anaplastic carcinoma of the pancreas | NP | Yes | N/A |
| Gong, 2003 [32] | F, 74 | Retroperitoneal soft tissue | N/A | Large abdominal mass | FNA under US guidance | Pancreatic adenocarcinoma | NP | Yes | Recurrent PPGL lesion in the liver (60 mo) |
| Absher, 2001 [33] | M, 52 | Retrocrunal, paracaval, and paraaortic lesions; also, bone (rib, vertebral) lesions | 70 mm (CT) | Chest wall and back pain | FNA and FNB | PPGL | Chr (+), vim (+), Ker (−), EMA (−), CEA (−), desmin (−) | N/A | Widespread metastatic bone lesions |
| Our case | F, 35 | Paraduodenal | 65 mm (EUS) | Asymptomatic; incidental mass | EUS-FNB | PPGL | Chr (+), Syn (+), CD56 (+) GATA-3 (+), S100(+), Ker (−), PAX8 (−), Melan A (−), DOG1 (−) |
Yes | Alive, no recurrence (10 mo) |
Note: The term “peripancreatic” is used to describe the location of a lesion found around and/or within the pancreas. Abbreviations: PPGL, pheochromocytoma and paraganglioma; NET; neuroendocrine tumor; PanNET; pancreatic neuroendocrine tumor; EUS, endoscopic ultrasound; Chr, chromogranin; Syn, synaptophysin; Ker, Keratin; N/A, information not available; NP; not performed; mo, months.
3.3. Case Report
Our case was about a 35-year-old female patient who underwent an EUS-FNB of a retroperitoneal mass, which had previously been detected incidentally. EUS revealed this mass was located in the paraduodenal area and had a diameter of 65 mm, while it was significantly vascular. Two passes with a 22G FNB needle through the duodenum were performed; cores were put directly into formalin, whereas the remaining material was used for conventional and liquid-based cytology.
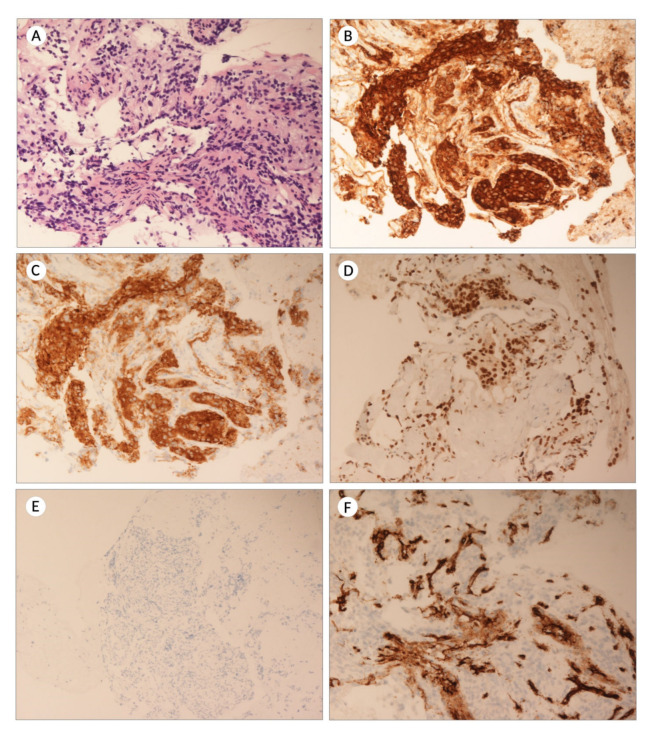
Microscopic examination of the FNB material revealed the presence of neoplastic cells, mostly arranged in syncytial groups (Figure 2). Immunohistochemistry was performed; the neoplastic cells were positive for chromogranin, synaptophysin, CD56, and GATA-3, whereas they were negative for Keratin, Melan-A, PAX8, and DOG-1. CD34 staining revealed the rich network of transgressing blood vessels, while S100 the population of spindle, “sustentacular” cells within the neoplasm. Preoperative diagnosis was paraganglioma.
Figure 2.
Representative images of a paraduodenal paraganglioma from our practice diagnosed with EUS-FNB, with a combination of H&E histomorphology and immunohistochemistry. (A), H&E ×200; (B), Chromogranin ×200; (C), Synaptophysin ×200; (D), GATA-3 ×200; (E), Keratin ×100; and (F), CD34 ×400.
The mass was subsequently excised and surgical pathology confirmed the abovementioned preoperative diagnosis. Immunohistochemistry against SDHA, SDHB, and ATRX was additionally performed, and the neoplastic cells within the mass were found to retain their staining.
4. Discussion
To our knowledge, this is the first literature review summarizing the published data of abdominal extra-adrenal PPGLs diagnosed with FNA and/or FNB. This study showed that PPGLs preoperative diagnosis using the abovementioned modalities is feasible, especially when pathologists are familiar with the cytomorphologic features and immunochemistry is additionally applied on the cytologic or histologic material. As Table 1 shows, the main reason for misdiagnosis was the absence of a complete immunochemical panel. Possible reasons for this could be either that the physicians involved did not suspect a PPGL diagnosis or the pathologic material was inadequate for ancillary studies. The vast majority of the 36 included paragangliomas were retroperitoneal and most appeared in females (67%), in accordance with the literature [25], while FNA (31/36 cases) was more often used to sample them compared to FNB. In 2/36 patients, transient hypertension was reported during the EUS-FNA procedure [16,17]. Where as a few of these cases were symptomatic at diagnosis (e.g., presence of abdominal pain), they often appeared in asymptomatic patients, being detected incidentally with radiology (Table 1). Most patients exhibited a favorable clinical behavior, yet 5/36 patients (13.9%) exhibited a recurrence or widespread disease and one patient died 48 months following her diagnosis.
According to the literature, abdominal extra-adrenal paragangliomas are most often found in the periaortic and pericaval regions [34,35]. Notably, our analysis revealed that, when focusing on the subgroup undergoing biopsy with FNA or FNB, extra-adrenal PPGLs were most often reported to be located around and/or within the pancreas (21/36; 58.33%). In such cases, the first impression of clinicians is a more common primary pancreatic neoplasm rather than the rare paraganglioma, especially in asymptomatic patients devoid of clinical presentation related to the hypersecretion of catecholamines [4,14,25]. Of the pancreatic neoplasms, the most likely differential was found to be an epithelial NET (Table 1). Both PanNETs and paragangliomas are neuroendocrine neoplasms exhibiting similar morphologic characteristics, such as the presence of loosely clustered and isolated epithelioid or polygonal cells, various degrees of pleomorphism, granular cytoplasm, and nuclei with stippled, “neuroendocrine-like” chromatin. In addition, both PanNETs and PPGLs could contain binucleated or even multinucleated giant cells, and both are positive for neuroendocrine markers with immunochemistry, including chromogranin and synaptophysin. In contrast to the PanNETs though, paragangliomas are negative for Keratins (e.g., AE1/AE3 or CAM5.2) or CEA with immunochemistry (as they are non-epithelial neoplasms), whereas they exhibit immunopositivity for GATA-3 and tyrosine hydroxylase (TH) [5,36,37,38,39]. Additionally, in contrast to the peripancreatic paragangliomas, PanNETs are typically located inside the pancreatic parenchyma. However, this is not always straightforward during the radiologic or endoscopic examination, and neoplasm initially thought to be intra-pancreatic are subsequently found to be extra-pancreatic during surgery [4,5,32]. Apart from epithelial NETs, other erroneous preoperative interpretations of peripancreatic paragangliomas revealed in our study included the diagnoses of spindle cell neoplasm, pancreatic pseudocyst, pancreatic adenocarcinoma, or anaplastic carcinoma, particularly when immunochemistry was not performed [4,32].
As shown in our study, neoplasms other than epithelial NETs could also enter the main differential diagnosis, depending on the retroperitoneal location of extra-adrenal PPGLs. For instance, in para-gastric or para-duodenal lesions, a gastrointestinal stromal tumor (GIST) needs to be ruled out; GISTs are positive for DOG1 and CD117 with immunochemistry whereas they are negative for neuroendocrine markers, in contrast to paragangliomas. Furthermore, when found in the perirenal space or the retroperitoneal soft tissue, a renal cell carcinoma or a soft tissue tumor, such as a schwannoma or sarcoma, should also be excluded. In addition, various metastatic carcinomas or a melanoma could often be considered as a potential diagnosis. Metastatic carcinomas would be Keratin positive, while melanomas positive for Melan-A, HMB-45, and diffusely positive for S100 with immunochemistry (in contrast to the paragangliomas, where only the sustentacular cells are highlighted with the S100 immunostaining) [14,40,41]. Lastly, when PPGLs are found in the head and neck area rather than the retroperitoneum, other lesions enter their differential diagnosis, for instance the medullary thyroid carcinoma [42].
The accurate diagnosis of a retroperitoneal PPGL is clinically important, especially its distinction from epithelial NETs. First, paragangliomas could be associated with a distinct clinical picture related to the activation of the sympathetic nervous system, while the metanephrines are regarded as biomarkers to monitor their response to therapy and potential recurrence. Furthermore, as paragangliomas often carry germline (more that 40% of the cases) and/or somatic mutations, genetic testing is offered to the affected patients and its results could impact patients’ management, follow-up, and prognosis [8,10,43,44]. For instance, the presence of SDHB mutations, ATRX mutations, and telomerase inactivation have been correlated with the neoplasm’s metastatic potential or multifocal primary disease [45,46]. Notably, the loss of SDHB expression detected with immunohistochemistry is considered a surrogate marker of a SDHB mutation, while PPGLs with the aforementioned immunohistochemical profile are classified as “SDH-deficient PPGLs”; similarly, the loss of SDHA expression implies SDHA-related disease, while a VHL-related pathogenesis could be featured by using the carbonic anhydrase IX (CAIX) immunostaining [1,3,47,48,49,50]. The latest WHO classification emphasizes that paragangliomas should not be classified as benign or malignant, as all of them have a metastatic potential, while there are no clear-cut criteria to foresee their behavior. In addition, this is not certain whether the presence of a new paraganglioma mass in a patient represents a metastasis or an asynchronous multifocal primary disease, especially in patients with germline susceptibility. Exceptions include the bones and lymph nodes, which do not normally contain chromaffin tissue elements, thus a paraganglioma found in these sites could be considered a metastasis [1,3,51]. To predict the metastatic potential of PPGLs, a few histopathologic scoring systems have been established, for instance the PASS and the GAPP systems [52,53].
This study has some important limitations. Data were extracted from case reports or small case series, which are generally considered of low-quality evidence. This could not be avoided though, as abdominal paragangliomas are rare lesions, while we additionally focused only on the ones diagnosed with FNA and/or FNB. Furthermore, we need to consider the reporting bias, as PPGLs suspected clinically as such were most likely not biopsied. Lastly, in six of the initially eligible studies reporting abdominal extra-adrenal paragangliomas, individual data could not be extracted; thus, these reports were excluded from the review (Figure 1).
5. Conclusions
This study showed that the preoperative diagnosis of abdominal extra-adrenal paragangliomas with FNA or FNB is feasible. Diagnostic pitfalls exist, but could significantly be avoided within a multidisciplinary setting and the application of immunochemistry on the cytologic or histologic material. The retroperitoneal paragangliomas most often aspirated with FNA or FNB were reported to be located around or within the pancreas, and a PanNET was the main differential diagnosis.
Author Contributions
Conceptualization, I.P.N.; investigation, I.P.N., A.I. and M.M.A.; writing—original draft preparation, I.P.N.; writing—review and editing, I.P.N., A.I., M.M.A., E.K., A.S., G.N.T., D.P. (Dimitrios Panagiotakopoulos), D.P. (Dimitrios Papaioannou), and C.S.; visualization, I.P.N., E.K. and C.S.; supervision, I.P.N. and C.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Written informed consent has been obtained from the patient to publish this article.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mete O., Asa S.L., Gill A.J., Kimura N., de Krijger R.R., Tischler A. Overview of the 2022 WHO Classification of Paragangliomas and Pheochromocytomas. Endocr. Pathol. 2022;33:90–114. doi: 10.1007/s12022-022-09704-6. [DOI] [PubMed] [Google Scholar]
- 2.Berends A.M.A., Buitenwerf E., de Krijger R.R., Veeger N.J.G.M., van der Horst-Schrivers A.N.A., Links T.P., Kerstens M.N. Incidence of Pheochromocytoma and Sympathetic Paraganglioma in the Netherlands: A Nationwide Study and Systematic Review. Eur. J. Intern. Med. 2018;51:68–73. doi: 10.1016/j.ejim.2018.01.015. [DOI] [PubMed] [Google Scholar]
- 3.Asa S.L., Ezzat S., Mete O. The Diagnosis and Clinical Significance of Paragangliomas in Unusual Locations. J. Clin. Med. Res. 2018;7:280. doi: 10.3390/jcm7090280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singhi A.D., Hruban R.H., Fabre M., Imura J., Schulick R., Wolfgang C., Ali S.Z. Peripancreatic Paraganglioma: A Potential Diagnostic Challenge in Cytopathology and Surgical Pathology. Am. J. Surg. Pathol. 2011;35:1498–1504. doi: 10.1097/PAS.0b013e3182281767. [DOI] [PubMed] [Google Scholar]
- 5.Lanke G., Stewart J.M., Lee J.H. Pancreatic Paraganglioma Diagnosed by Endoscopic Ultrasound-Guided Fine Needle Aspiration: A Case Report and Review of Literature. World J. Gastroenterol. 2021;27:6322–6331. doi: 10.3748/wjg.v27.i37.6322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taïeb D., Hicks R.J., Hindié E., Guillet B.A., Avram A., Ghedini P., Timmers H.J., Scott A.T., Elojeimy S., Rubello D., et al. European Association of Nuclear Medicine Practice Guideline/Society of Nuclear Medicine and Molecular Imaging Procedure Standard 2019 for Radionuclide Imaging of Phaeochromocytoma and Paraganglioma. Eur. J. Nucl. Med. Mol. Imaging. 2019;46:2112–2137. doi: 10.1007/s00259-019-04398-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burnichon N., Cascón A., Schiavi F., Morales N.P., Comino-Méndez I., Abermil N., Inglada-Pérez L., de Cubas A.A., Amar L., Barontini M., et al. MAX Mutations Cause Hereditary and Sporadic Pheochromocytoma and Paraganglioma. Clin. Cancer Res. 2012;18:2828–2837. doi: 10.1158/1078-0432.CCR-12-0160. [DOI] [PubMed] [Google Scholar]
- 8.Crona J., Taïeb D., Pacak K. New Perspectives on Pheochromocytoma and Paraganglioma: Toward a Molecular Classification. Endocr. Rev. 2017;38:489–515. doi: 10.1210/er.2017-00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nölting S., Bechmann N., Taieb D., Beuschlein F., Fassnacht M., Kroiss M., Eisenhofer G., Grossman A., Pacak K. Personalized Management of Pheochromocytoma and Paraganglioma. Endocr. Rev. 2022;43:199–239. doi: 10.1210/endrev/bnab019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fishbein L., Del Rivero J., Else T., Howe J.R., Asa S.L., Cohen D.L., Dahia P.L.M., Fraker D.L., Goodman K.A., Hope T.A., et al. The North American Neuroendocrine Tumor Society Consensus Guidelines for Surveillance and Management of Metastatic And/or Unresectable Pheochromocytoma and Paraganglioma. Pancreas. 2021;50:469–493. doi: 10.1097/MPA.0000000000001792. [DOI] [PubMed] [Google Scholar]
- 11.Lenders J.W.M., Kerstens M.N., Amar L., Prejbisz A., Robledo M., Taieb D., Pacak K., Crona J., Zelinka T., Mannelli M., et al. Genetics, Diagnosis, Management and Future Directions of Research of Phaeochromocytoma and Paraganglioma: A Position Statement and Consensus of the Working Group on Endocrine Hypertension of the European Society of Hypertension. J. Hypertens. 2020;38:1443–1456. doi: 10.1097/HJH.0000000000002438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trabzonlu L., Jager L., Tabibi S., Compton M.L., Weiss V.L., Kanber Y., Robila V., Antic T., Maleki Z., Morency E., et al. Adrenal Gland Cytology Reporting: A Multi-Institutional Proposal for a Standardized Reporting System. Cancer Cytopathol. 2022;130:423–432. doi: 10.1002/cncy.22564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiménez-Heffernan J.A., Vicandi B., López-Ferrer P., González-Peramato P., Pérez-Campos A., Viguer J.M. Cytologic Features of Pheochromocytoma and Retroperitoneal Paraganglioma: A Morphologic and Immunohistochemical Study of 13 Cases. Acta Cytol. 2006;50:372–378. doi: 10.1159/000325975. [DOI] [PubMed] [Google Scholar]
- 14.Fite J.J., Maleki Z. Paraganglioma: Cytomorphologic Features, Radiologic and Clinical Findings in 12 Cases. Diagn. Cytopathol. 2018;46:473–481. doi: 10.1002/dc.23928. [DOI] [PubMed] [Google Scholar]
- 15.Moslemi M.K., Abolhasani M., Vafaeimanesh J. Malignant Abdominal Paraganglioma Presenting as a Giant Intra-Peritoneal Mass. Int. J. Surg. Case Rep. 2012;3:537–540. doi: 10.1016/j.ijscr.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kubota K., Kato S., Mawatari H., Iida H., Akiyama T., Fujita K., Yoneda M., Takahashi H., Inamori M., Abe Y., et al. Risky Endoscopic Ultrasonography-Guided Fine-Needle Aspiration for Asymptomatic Retroperitoneal Tumors. Dig. Endosc. 2010;22:144–146. doi: 10.1111/j.1443-1661.2010.00939.x. [DOI] [PubMed] [Google Scholar]
- 17.Akdamar M.K., Eltoum I., Eloubeidi M.A. Retroperitoneal Paraganglioma: EUS Appearance and Risk Associated with EUS-Guided FNA. Gastrointest. Endosc. 2004;60:1018–1021. doi: 10.1016/S0016-5107(04)02218-7. [DOI] [PubMed] [Google Scholar]
- 18.Zhang L., Liao Q., Hu Y., Zhao Y. Paraganglioma of the Pancreas: A Potentially Functional and Malignant Tumor. World J. Surg. Oncol. 2014;12:218. doi: 10.1186/1477-7819-12-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kebe Radulović M., Strojan Fležar M. Pheochromocytoma Presenting as a Pancreatic Mass: Avoiding a Dangerous Pitfall in Endoscopic Ultrasound-Guided Fine Needle Aspiration Biopsy Using GATA3 Immunostain. Cytopathology. 2022;33:127–131. doi: 10.1111/cyt.13060. [DOI] [PubMed] [Google Scholar]
- 20.Thakur A., Choudhary N.S., Sarin H., Srivastava S., Puri R. Peripancreatic Paraganglioma: A Diagnostic Dilemma Resolved on Endoscopic Ultrasound Guided Fine Needle Aspiration Cytology. Diagn. Cytopathol. 2021;49:1213–1216. doi: 10.1002/dc.24870. [DOI] [PubMed] [Google Scholar]
- 21.Naito Y., Matsumura M., Horiguchi S.-I., Suzuki M., Kikuyama M., Seyama Y. Laparoscopic Resection of a Paraganglioma in the Greater Omentum Mimicking a Peripancreatic Neoplasm: A Case Report. Clin. J. Gastroenterol. 2021;14:1364–1370. doi: 10.1007/s12328-021-01452-0. [DOI] [PubMed] [Google Scholar]
- 22.Abbasi A., Wakeman K.M., Pillarisetty V.G. Pancreatic Paraganglioma Mimicking Pancreatic Neuroendocrine Tumor. Rare Tumors. 2020;12:2036361320982799. doi: 10.1177/2036361320982799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang S.X., Dogra V.S., Kothari T.H., Reyes M.C.D. Metastatic Pheochromocytoma to the Pancreas Diagnosed by Endoscopic Ultrasound-Guided Fine Needle Aspiration: A Case Report and Review of Literature. Diagn. Cytopathol. 2019;48:217–221. doi: 10.1002/dc.24326. [DOI] [PubMed] [Google Scholar]
- 24.Nguyen E., Nakasaki M., Lee T.K., Lu D. Diagnosis of Paraganglioma as a Pancreatic Mass: A Case Report. Diagn. Cytopathol. 2018;46:804–806. doi: 10.1002/dc.23974. [DOI] [PubMed] [Google Scholar]
- 25.Zeng J., Simsir A., Oweity T., Hajdu C., Cohen S., Shi Y. Peripancreatic Paraganglioma Mimics Pancreatic/gastrointestinal Neuroendocrine Tumor on Fine Needle Aspiration: Report of Two Cases and Review of the Literature. Diagn. Cytopathol. 2017;45:947–952. doi: 10.1002/dc.23761. [DOI] [PubMed] [Google Scholar]
- 26.Tumuluru S., Mellnick V., Doyle M., Goyal B. Pancreatic Paraganglioma: A Case Report. Case Rep. Pancreat. Cancer. 2016;2:79–83. doi: 10.1089/crpc.2016.0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Handa U., Kundu R., Mohan H. Cytomorphologic Spectrum in Aspirates of Extra-Adrenal Paraganglioma. J. Cytol. 2014;31:79–82. doi: 10.4103/0970-9371.138667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ganc R.L., Castro A.C.F., Colaiacovo R., Vigil R., Rossini L.G.B., Altenfelder R. Endoscopic Ultrasound-Guided Fine Needle Aspiration for the Diagnosis of Nonfunctional Paragangliomas: A Case Report and Review of the Literature. Endosc. Ultrasound. 2012;1:108–109. doi: 10.7178/eus.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laforga J.B., Vaquero M., Juanpere N. Paragastric Paraganglioma: A Case Report with Unusual Alveolar Pattern and Myxoid Component. Diagn. Cytopathol. 2012;40:815–819. doi: 10.1002/dc.21665. [DOI] [PubMed] [Google Scholar]
- 30.Sangster G., Do D., Previgliano C., Li B., LaFrance D., Heldmann M. Primary Retroperitoneal Paraganglioma Simulating a Pancreatic Mass: A Case Report and Review of the Literature. HPB Surg. 2010;2010:645728. doi: 10.1155/2010/645728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rangaswamy M., Kumar S.P., Asha M., Manjunath G. CT-Guided Fine Needle Aspiration Cytology Diagnosis of Extra-Adrenal Pheochromocytoma. J. Cytol. 2010;27:26–28. doi: 10.4103/0970-9371.66689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gong Y., DeFrias D.V., Nayar R. Pitfalls in Fine Needle Aspiration Cytology of Extraadrenal Paraganglioma. A Report of 2 Cases. Acta Cytol. 2003;47:1082–1086. doi: 10.1159/000326652. [DOI] [PubMed] [Google Scholar]
- 33.Absher K.J., Witte D.A., Truong L.D., Ramzy I., Mody D.R., Ostrowski M.L. Aspiration Biopsy of Osseous Metastasis of Retroperitoneal Paraganglioma. Report of a Case with Cytologic Features and Differential Diagnostic Considerations. Acta Cytol. 2001;45:249–253. doi: 10.1159/000327284. [DOI] [PubMed] [Google Scholar]
- 34.Cunningham S.C., Suh H.S., Winter J.M., Montgomery E., Schulick R.D., Cameron J.L., Yeo C.J. Retroperitoneal Paraganglioma: Single-Institution Experience and Review of the Literature. J. Gastrointest. Surg. 2006;10:1156–1163. doi: 10.1016/j.gassur.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 35.Erickson D., Kudva Y.C., Ebersold M.J., Thompson G.B., Grant C.S., van Heerden J.A., Young W.F., Jr. Benign Paragangliomas: Clinical Presentation and Treatment Outcomes in 236 Patients. J. Clin. Endocrinol. Metab. 2001;86:5210–5216. doi: 10.1210/jcem.86.11.8034. [DOI] [PubMed] [Google Scholar]
- 36.Mamilla D., Manukyan I., Fetsch P.A., Pacak K., Miettinen M. Immunohistochemical Distinction of Paragangliomas from Epithelial Neuroendocrine Tumors-Gangliocytic Duodenal and Cauda Equina Paragangliomas Align with Epithelial Neuroendocrine Tumors. Hum. Pathol. 2020;103:72–82. doi: 10.1016/j.humpath.2020.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dermawan J.K., Mukhopadhyay S., Shah A.A. Frequency and Extent of Cytokeratin Expression in Paraganglioma: An Immunohistochemical Study of 60 Cases from 5 Anatomic Sites and Review of the Literature. Hum. Pathol. 2019;93:16–22. doi: 10.1016/j.humpath.2019.08.013. [DOI] [PubMed] [Google Scholar]
- 38.So J.S., Epstein J.I. GATA3 Expression in Paragangliomas: A Pitfall Potentially Leading to Misdiagnosis of Urothelial Carcinoma. Mod. Pathol. 2013;26:1365–1370. doi: 10.1038/modpathol.2013.76. [DOI] [PubMed] [Google Scholar]
- 39.Kimura N., Shiga K., Kaneko K., Sugisawa C., Katabami T., Naruse M. The Diagnostic Dilemma of GATA3 Immunohistochemistry in Pheochromocytoma and Paraganglioma. Endocr. Pathol. 2020;31:95–100. doi: 10.1007/s12022-020-09618-1. [DOI] [PubMed] [Google Scholar]
- 40.Cazzato G., Colagrande A., Cimmino A., Demarco A., Lospalluti L., Arezzo F., Resta L., Ingravallo G. The Great Mime: Three Cases of Melanoma with Carcinoid-Like and Paraganglioma-Like Pattern with Emphasis on Differential Diagnosis. Dermatopathology. 2021;8:130–134. doi: 10.3390/dermatopathology8020019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ohsie S.J., Sarantopoulos G.P., Cochran A.J., Binder S.W. Immunohistochemical Characteristics of Melanoma. J. Cutan. Pathol. 2008;35:433–444. doi: 10.1111/j.1600-0560.2007.00891.x. [DOI] [PubMed] [Google Scholar]
- 42.Nikas I.P., Kazamias G., Vrontaki M., Rapti A.S., Mastorakis E. Medullary Thyroid Carcinoma Diagnosed with Liquid-Based Cytology and Immunocytochemistry. J. Immunoass. Immunochem. 2022:1–14. doi: 10.1080/15321819.2022.2070025. ahead-of-print . [DOI] [PubMed] [Google Scholar]
- 43.Buffet A., Burnichon N., Favier J., Gimenez-Roqueplo A.-P. An Overview of 20 Years of Genetic Studies in Pheochromocytoma and Paraganglioma. Best Pract. Res. Clin. Endocrinol. Metab. 2020;34:101416. doi: 10.1016/j.beem.2020.101416. [DOI] [PubMed] [Google Scholar]
- 44.Muth A., Crona J., Gimm O., Elmgren A., Filipsson K., Stenmark Askmalm M., Sandstedt J., Tengvar M., Tham E. Genetic Testing and Surveillance Guidelines in Hereditary Pheochromocytoma and Paraganglioma. J. Intern. Med. 2019;285:187–204. doi: 10.1111/joim.12869. [DOI] [PubMed] [Google Scholar]
- 45.Amar L., Baudin E., Burnichon N., Peyrard S., Silvera S., Bertherat J., Bertagna X., Schlumberger M., Jeunemaitre X., Gimenez-Roqueplo A.-P., et al. Succinate Dehydrogenase B Gene Mutations Predict Survival in Patients with Malignant Pheochromocytomas or Paragangliomas. J. Clin. Endocrinol. Metab. 2007;92:3822–3828. doi: 10.1210/jc.2007-0709. [DOI] [PubMed] [Google Scholar]
- 46.Job S., Draskovic I., Burnichon N., Buffet A., Cros J., Lépine C., Venisse A., Robidel E., Verkarre V., Meatchi T., et al. Telomerase Activation and ATRX Mutations Are Independent Risk Factors for Metastatic Pheochromocytoma and Paraganglioma. Clin. Cancer Res. 2019;25:760–770. doi: 10.1158/1078-0432.CCR-18-0139. [DOI] [PubMed] [Google Scholar]
- 47.Oudijk L., Gaal J., de Krijger R.R. The Role of Immunohistochemistry and Molecular Analysis of Succinate Dehydrogenase in the Diagnosis of Endocrine and Non-Endocrine Tumors and Related Syndromes. Endocr. Pathol. 2019;30:64–73. doi: 10.1007/s12022-018-9555-2. [DOI] [PubMed] [Google Scholar]
- 48.Van Nederveen F.H., Korpershoek E., Lenders J.W.M., de Krijger R.R., Dinjens W.N.M. Somatic SDHB Mutation in an Extraadrenal Pheochromocytoma. N. Engl. J. Med. 2007;357:306–308. doi: 10.1056/NEJMc070010. [DOI] [PubMed] [Google Scholar]
- 49.Favier J., Meatchi T., Robidel E., Badoual C., Sibony M., Nguyen A.T., Gimenez-Roqueplo A.-P., Burnichon N. Carbonic Anhydrase 9 Immunohistochemistry as a Tool to Predict or Validate Germline and Somatic VHL Mutations in Pheochromocytoma and Paraganglioma-a Retrospective and Prospective Study. Mod. Pathol. 2020;33:57–64. doi: 10.1038/s41379-019-0343-4. [DOI] [PubMed] [Google Scholar]
- 50.Van Nederveen F.H., Gaal J., Favier J., Korpershoek E., Oldenburg R.A., de Bruyn E.M.C.A., Sleddens H.F.B.M., Derkx P., Rivière J., Dannenberg H., et al. An Immunohistochemical Procedure to Detect Patients with Paraganglioma and Phaeochromocytoma with Germline SDHB, SDHC, or SDHD Gene Mutations: A Retrospective and Prospective Analysis. Lancet Oncol. 2009;10:764–771. doi: 10.1016/S1470-2045(09)70164-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thompson L.D.R., Gill A.J., Asa S.L., Clifton-Bligh R.J., de Krijger R.R., Kimura N., Komminoth P., Lack E.E., Lenders J.W.M., Lloyd R.V., et al. Data Set for the Reporting of Pheochromocytoma and Paraganglioma: Explanations and Recommendations of the Guidelines from the International Collaboration on Cancer Reporting. Hum. Pathol. 2021;110:83–97. doi: 10.1016/j.humpath.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wachtel H., Hutchens T., Baraban E., Schwartz L.E., Montone K., Baloch Z., LiVolsi V., Krumeich L., Fraker D.L., Nathanson K.L., et al. Predicting Metastatic Potential in Pheochromocytoma and Paraganglioma: A Comparison of PASS and GAPP Scoring Systems. J. Clin. Endocrinol. Metab. 2020;105:e4661–e4670. doi: 10.1210/clinem/dgaa608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stenman A., Zedenius J., Juhlin C.C. The Value of Histological Algorithms to Predict the Malignancy Potential of Pheochromocytomas and Abdominal Paragangliomas-A Meta-Analysis and Systematic Review of the Literature. Cancers. 2019;11:225. doi: 10.3390/cancers11020225. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are contained within the article.