Abstract
Simple Summary
Positron emission tomography/computed tomography (PET/CT) has an increasingly relevant role in the management of oncological patients. It consists of the administration of a radioactive molecule (tracer) which localizes in tumour cells due to specific cellular features (e.g., a receptor expressed by a specific cell population). PET/CT has a variety of applications in oncology, including staging, therapeutic response assessment and restaging in various types of tumours through the administration of different tracers. Among them, prostate-specific membrane antigen (PSMA) radioligands are a relatively novel compound widely employed in prostate cancer diagnostics and therapy. Besides their prostate cancer applications, PSMA radioligands have been shown to be a promising instrument to evaluate the stage and the aggressiveness in several types of cancer, since they allow us to study tumour neoangiogenesis. This review provides an overview of the applications of PSMA radioligand PET/CT in various types of tumours other than prostate cancer.
Abstract
Due to its overexpression on the surface of prostate cancer cells, prostate-specific membrane antigen (PSMA) is a relatively novel effective target for molecular imaging and radioligand therapy (RLT) in prostate cancer. Recent studies reported that PSMA is expressed in the neovasculature of various types of cancer and regulates tumour cell invasion as well as tumour angiogenesis. Several authors explored the role of diagnostic and therapeutic PSMA radioligands in various malignancies. In this narrative review, we describe the current status of the literature on PSMA radioligands’ application in solid tumours other than prostate cancer to explore their potential role as diagnostic or therapeutic agents, with particular regard to the relevance of PSMA radioligand uptake as neoangiogenetic biomarker. Hence, a comprehensive review of the literature was performed to find relevant articles on the applications of PSMA radioligands in non-prostate solid tumours. Data on the general, methodological and clinical aspects of all included studies were collected. Forty full-text papers were selected for final review, 8 of which explored PSMA radioligand PET/CT performances in gliomas, 3 in salivary gland malignancies, 6 in thyroid cancer, 2 in breast cancer, 16 in renal cell carcinoma and 5 in hepatocellular carcinoma. In the included studies, PSMA radioligand PET showed promising performance in patients with non-prostate solid tumours. Further studies are needed to better define its potential role in oncological patients management, especially in those undergoing antineoangiogenic therapies, and to assess the efficacy of PSMA-RLT in this clinical context.
Keywords: PSMA, PET, theragnostics, therapy, radioligand, nuclear medicine
1. Introduction
Due to its overexpression on the surface of prostate cancer cells and its extracellular binding pocket for small-molecule ligands, glutamate carboxypeptidase II (GPII), also known as prostate-specific membrane antigen (PSMA), is a relatively novel effective target for molecular imaging and radioligand therapy (RLT) in prostate cancer [1]. These characteristics allow the development of small molecules binding PSMA, which are for all intents and purposes zinc-binding structures that interact with the binuclear zinc active site of GPII [2]. PSMA is a trans membrane protein encoded by the gene FOLH1 and was first discovered in prostate cancer cells in 1987 [3,4]. Conversely to what its name suggests, PSMA is not selective to prostate cancer cells, but is widely expressed in neovascular endothelial cells of various cancers (including brain, thyroid, renal, lung, liver, colo-rectal and breast) [5,6]. Recent pre-clinical studies suggest that GPII regulates tumour cell invasion and tumour angiogenesis by modulating integrin signal transduction in endothelial cells [7,8]. Considering the high expression of GPII on the cell membrane of prostate cancer cells and based on the first urea-based compounds designed by Kozikowski and colleagues in 2001 [9], several low-molecular weight radiolabelled GPII inhibitors have been developed with the purpose of improving the diagnostic performance of nuclear medicine imaging for prostate cancer.
To date, the most widely employed PSMA radioligand in clinical practice is 68Ga-PSMA-11 (also called 68Ga-DKFZ-PSMA-11 or 68Ga-PSMA-HBED-CC), developed by Eder and Haberkorn [10] and subsequently evaluated in clinical practice by Haberkorn and Afshar-Oromieh [11,12] at the German Cancer Research Centre in the University Hospital of Heidelberg, which consists of a Glu-urea-Lys inhibitor motif conjugated with Ga-specific acyclic chelator (HBED-CC) and shows higher affinity and internalization in prostate cancer cells than the corresponding DOTA analogue. Due to the overwhelming results obtained in the initial evaluation of 68Ga-PSMA-11, it was not long before the rapid development of therapeutic analogues and the first treatments with 177Lu-PSMA I&T (imaging and therapy), which employed DOTAGA as a chelator, and 177Lu-PSMA-617, a DOTA-conjugated compound [2]. Furthermore, DOTAGA- and DOTA-conjugated compounds can be labelled with other radionuclides such as 90Y for β--therapy or 225Ac for α-therapy [13,14,15,16]. More recently, 18F-labeled PSMA radioligands were introduced in clinical practice: the main employed radiopharmaceuticals to date are 18F-DCFPyL and 18F-PSMA-1007 [17,18]. It is interesting that, conversely from any other PSMA radioligand, 18F-PSMA-1007 shows higher lipophilicity and, consequently, may not be found in the ureter and bladder since its clearance is liver-dominant [19]. In 2021 U.S. Food and Drug Administration (FDA) approved both 68Ga-PSMA-11 and 18F-DCFPyL administration in patients with suspected prostate cancer metastases who are potentially curable by surgery or radiation therapy and patients with suspected prostate cancer recurrence [20,21].
As reported by the phase III VISION trial [22], radioligand therapy with 177Lu-PSMA-617 was able to prolong progression-free survival (PFS) and overall survival (OS) when added to standard care in patients with advanced metastatic castration-resistant prostate cancer. 177Lu-PSMA-617 received FDA approval in March 2022 and its administration is indicated in patients with PSMA-positive metastatic castration-resistant prostate cancer previously treated with androgen-receptor pathway inhibitors and taxane-based chemotherapy [23]. This accomplishment makes us question whether similar results can be obtained by employing PSMA-RLT in other types of cancer expressing PSMA on tumour cells or the tumour-associated neovasculature. Angiogenesis is defined as a biological process in which new blood vessels are formed from pre-existing ones, leading to the development of the tumour’s blood supply, and it is considered to be a rate-limiting factor in cancer progression [24,25]. These findings were the basis for the development and the introduction into clinical practice of antineoangiogenic treatments [26]. The antineoangiogenic treatment rationale was to induce “tumour starvation”: preventing angiogenesis and nutrient supply, tumour progression should be slowed down. Since clinical studies with bevacizumab monotherapy did not show any benefit in terms of OS, this theory was soon abandoned [26]. This phenomenon might be explained by the concept of “vessel normalization”, in which vascular endothelial growth factor (VEGF) plays a key role, as VEGF-targeted therapies can restore the balance between anti- and pro-angiogenic factors, so the abnormal tumour vessels are remodelled into normal blood vessels which have a higher pO2, improved pericyte coverage, lower macromolecular permeability and improved delivery of therapeutic agents (this concept may be applied both for traditional chemotherapies and immunotherapies) [27,28]. These findings lead to a second question: can PSMA imaging predict which tumours are more eligible for antineoangiogenic treatment or predict its outcome?
The purpose of this narrative review is to assess whether PSMA radioligands, employed as diagnostic or theragnostic factors, might play a role in the management of patients with solid tumours other than prostate cancer, with particular regard to the significance of PSMA radioligand uptake as a neoangiogenesis biomarker.
2. Materials and Methods
2.1. Search Strategy
Three authors (A.R., G.T., S.A.) performed a comprehensive literature search from PubMed/MEDLINE, EMBASE and Cochrane library databases to find relevant published articles on the applications of PSMA radioligands in tumours other than prostate cancer. A search algorithm based on a combination of these terms and adapted to different organs was used: (“PET” OR “positron emission tomography”) AND (“PSMA” OR “DCFPyL”) AND (“cancer” OR “malignancy” OR “tumour”). Only articles in English were selected. No beginning date restriction was used. The literature search was last updated on 14th July 2022. To expand the research, references in the retrieved papers were also screened for additional studies. A literature search according to the Population, Intervention, Comparator, Outcomes (PICO) framework was performed, establishing criteria for study eligibility as follows: adult (≥18 years) patients with non-prostate solid tumour diagnosis (Population), undergoing PSMA radioligand PET or RLT (Intervention), with or without comparative imaging or therapy (Comparator); the considered outcomes were the evaluation of PSMA radioligand uptake in non-prostate tumour lesions, PSMA radioligand PET and therapy performance as well as change in patient management. All studies investigating the role or the application of PSMA radioligands to solid tumours other than prostate cancer were eligible for inclusion. Exclusion criteria were (a) articles not within the field of PET/CT or RLT, and (b) case reports, review articles, editorials, letters, comments, conference proceedings. Three researchers (A.R., G.T., S.A.) independently reviewed the titles and abstracts of the retrieved articles, applying the inclusion and exclusion criteria mentioned above and rejecting articles if they were clearly ineligible. The same three authors then independently reviewed the full-text version of the remaining articles to assess their eligibility for inclusion, resolving disagreements in a consensus meeting.
2.2. Data Extraction
For each eligible article, information was collected concerning the general study data, number of patients, employed radiopharmaceutical, investigated tumour, clinical setting, number of examined lesions, comparator exams. Moreover, the studies were divided into six groups depending on the primary tumour: (1) gliomas; (2) salivary glands malignancies; (3) thyroid cancer; (4) breast cancer; (5) renal cell cancer; and (6) hepatocellular carcinoma. Figure 1 summarizes the study selection process.
Figure 1.
Summary of study selection process for the review.
3. Results
3.1. Gliomas
Eight full-text articles assessing the potential role of PSMA radioligands in diagnostics of gliomas were reviewed [29,30,31,32,33,34,35,36]. Out of the eight included studies, three had a prospective design [30,32,34]. The sample size varied from 6 to 35 patients, and 178 patients with glioma were evaluated.
In all studies but one [29,30,31,32,33,34,36], PET/CT or PET/MRI was performed using 68Ga-PSMA-11, whereas the remaining study used 68Ga-PSMA-617 [35].
The main types of gliomas investigated were high-grade gliomas (e.g., glioblastoma). Regarding the clinical setting, PET using PSMA radioligands was performed in patients with gliomas to differentiate high-grade gliomas from low-grade gliomas (three articles [31,32,35]), to evaluate a suspicious recurrence of high-grade gliomas (three articles [33,34,36]), or for both indications (two articles [29,30]).
All of the examined papers used MRI as an comparator to evaluate PSMA radioligand PET/CT or PET/MRI performance [30,31,32,33,34,35,36], while three used also 18F-FDG PET/CT as a comparator [29,31,35].
Regarding the clinical findings, PET/CT with PSMA radioligands showed high diagnostic accuracy in detecting high-grade gliomas, both in discriminating high-grade gliomas from low-grade gliomas [29,30,31,32,35] and in detecting the recurrence of high-grade gliomas after treatment [29,30,33,34,36]. PSMA radioligand PET/CT showed comparable performances in high-grade gliomas when compared with MRI [32,34,36] and was superior to 18F-FDG PET both in staging and restaging gliomas mainly due to the absence of the high physiologic background in normal brain parenchyma in 18F-FDG PET/CT exams. In one study, immunohistochemistry analysis showed higher PSMA staining in high-grade gliomas [35]. Characteristics of the examined studies are reported in Table 1.
Table 1.
Characteristics of the included studies which employed PSMA radioligands in gliomas.
| First Author and Year | Type | Country | N. Patients | Tracer | Histopathological Subtype (N. Patients) |
Clinical Setting (N. Patients) |
Analysed Lesions | Comparator |
|---|---|---|---|---|---|---|---|---|
| Sasikumar 2017 [29] | Not reported | India | 6 | 68Ga-PSMA-11 (I) | 6 high-grade | Initial diagnosis or restaging | 6 | MRI 18F-FDG PET/CT |
| Sasikumar 2018 [30] | Prospective | India | 15 | 68Ga-PSMA-11 (I) | 1 low-grade; 14 high-grade |
Initial diagnosis or restaging | 15 | MRI |
| Verma 2019 [31] | Not reported | India | 10 | 68Ga-PSMA-11 (I) | 3 low-grade; 7 high-grade |
Initial diagnosis | 10 | MRI 18F-FDG PET/CT |
| Akgun 2020 [32] | Prospective | Turkey | 35 | 68Ga-PSMA-11 (I) | 14 low-grade; 21 high-grade |
Initial diagnosis | 35 | MRI |
| Kunikowska 2020 [33] | Not reported | Poland | 15 | 68Ga-PSMA-11 (I) | 15 high-grade | Restaging | 15 | MRI |
| Kumar 2021 [34] | Prospective | India | 33 | 68Ga-PSMA-11 (I) | 33 high-grade | Restaging | 33 | MRI |
| Liu 2021 [35] | Retrospective | China | 30 | 68Ga-PSMA-617(I) | 14 low-grade; 16 high-grade |
Initial diagnosis | 30 | MRI 18F-FDG PET/CT |
| Kunikowska 2022 [36] | Not reported | Poland | 34 | 68Ga-PSMA-11 (I) | 34 high-grade | Restaging | 34 | MRI |
PET: positron emission tomography, CT: computed tomography, MRI: magnetic resonance imaging, PSMA: prostate-specific membrane antigen, FDG: fluorodeoxyglucose, I: imaging.
3.2. Salivary Glands Malignances
Three full-text papers assessing the potential role of PSMA radioligands in diagnostics of salivary glands cancer (SGC) were reviewed [37,38,39], which evaluated 9, 25 and 6 patients, respectively. Only one study had a prospective design [38]. All studies employed 68Ga-PSMA-11 as a radiopharmaceutical performing PET/CT. In one study, in addition to 68Ga-PSMA-11, 177Lu-PSMA-617 RLT was performed [39].
All of the included studies explored PSMA-targeted PET/CT performance in adenoid cystic carcinoma (AdCC) [37,38,39]. Other studied histotypes included salivary duct carcinoma (SDC) [38] and acinic cell carcinoma [39]. In all studies, PET/CT examination had the purpose of restaging patients with local or distant recurrence [37,38,39]. With regard to performance, in all studies PSMA-targeted PET/CT seemed to be a reliable instrument to detect local recurrences and distant metastases [37,38,39]. Regarding comparisons between different examinations, Klein Nulent and colleagues compared the performances of 68Ga-PSMA-11 PET/CT and 18F-FDG PET/CT, analysing 18 lesions in nine patients, of which 4 were local recurrences and 14 were distant metastases in different sites (meninx, lung, liver, bone), and reported quite similar performances between both tracers [37]; on the other hand, Van Boxtel compared 68Ga-PSMA-11 PET/CT with full-dose CT scan and reported that PSMA radioligand PET/CT had added diagnostic value in 4 AdCC patients out of the 15 included in the study; in particular, PSMA radioligand PET/CT was able to identify bone metastases in two patients, a AdCC local recurrence in the Bartholin gland and additional lymph node metastases not enhanced by CT examination [38].
All three of the included studies performed immunohistochemistry examination on AdCC primary tumours and metastases biopsies to evaluate PSMA staining and reported variable PSMA expression in cancer cells, ranging from <1% to 95% in local recurrences as well as distant metastases [37,38,39]. In this context, Van Boxtel evaluated PSMA staining in SDC and reported that PSMA is mainly expressed by the neovascular endothelium [38]. In one study, 177Lu-PSMA-617-RLT with palliative intent was performed in six patients with metastatic SGC [39]. Among the analysed patients, two completed the four cycles planned in the study protocol, achieving stable disease and partial response, respectively, three interrupted the RLT after two cycles due to disease progression, and the remaining one interrupted the treatment after one cycle due to the occurrence of side effects (fatigue, xerostomia). The characteristics of the included studies are reported in Table 2.
Table 2.
Characteristics of the included studies which employed PSMA radioligands in salivary gland malignancies.
| First Author and Year | Type | Country | N. Patients | Tracer | Histopathological Subtype (N. Patients) |
Clinical Setting (N. Patients) |
Comparator |
|---|---|---|---|---|---|---|---|
| Klein Nulent 2017 [37] | Retrospective | Netherlands | 9 | 68Ga-PSMA-11 (I) | AdCC | Restaging |
18F-FDG PET/CT CT |
| Van Boxtel 2020 [38] | Prospective | Netherlands | 25 | 68Ga-PSMA-11 (I) | 15 AdCC 10 SDC |
Restaging | CT |
| Klein Nulent 2021 [39] | Retrospective | Netherlands | 6 |
68Ga-PSMA-11 (I) 177Lu-PSMA-617 (T) |
4 AdCC 1 unclassified adenocarcoma 1 acinic cell carcinoma |
Palliative RLT | / |
PET: positron emission tomography, CT: computed tomography, PSMA: prostate-specific membrane antigen, FDG: fluorodeoxyglucose, RLT: radioligand therapy, AdCC: adenoid-cystic carcinoma, SDC: salivary duct carcinoma, I: imaging, T: therapy.
3.3. Thyroid Cancer
Six full-text papers assessing the potential role of PSMA radioligands in the diagnostics and therapy of differentiated thyroid cancer (DTC) were reviewed [40,41,42,43,44,45]. Out of the six included studies, four had a prospective design [40,42,43,45]. The sample size varied from 2 to 11 and from 3 to 43 for patient and lesion analyses, respectively.
In all studies but one [40,41,42,43,44], PET/CT was performed using 68Ga-PSMA-11, whereas the remaining study used 18F-DCFPyl [45]. In one study, in addition to 68Ga-PSMA-11 PET/CT as diagnostic compound, 177Lu-PSMA-617 RLT therapy was performed in two out of five patients [41].
The main DTC investigated histotype was papillary thyroid cancer, as overall a total of 27 patients were examined; further DTC subtypes included were follicular (10 patients), Hürtle cell (2 patients) and anaplastic carcinomas (2 patients). All of the available literature concerning the employment of PSMA radioligands in thyroid cancer focuses on radioiodine-refractory DTC patients [40,41,42,43,44,45].
Two out of the examined papers used only 18F-FDG PET/CT as a comparator to evaluate PSMA radioligand PET/CT performance [40,43], one used only an 131I whole post-therapeutic whole body scan in addition to SPECT/CT [44], two combined PSMA imaging with 18F-FDG PET/CT and 123I or 131I whole body scan [42,45] and the remaining paper did not compare PSMA results with any other exam [41]. When compared to 18F-FDG in radioiodine refractory thyroid cancer patients, PSMA radioligands showed conflicting results, since Lawn-Heath and colleagues reported the superior performance of 18F-FDG both in DTC patients and in dedifferentiated thyroid cancer patients [42], but other authors reported similar results between the two examinations or a slight superiority in favour of PSMA radioligand PET/CT [40,43,45]. Moreover, Pitalua-Cortes observed that 68Ga-PSMA-11 PET/CT had a superior capability for metastatic lesion detection when compared to 131I imaging in radioiodine refractory DTC patients [44].
None of the included studies reported immunohistochemistry data about PSMA staining in DTC.
With regard to clinical findings, PSMA radioligand PET/CT was shown to be a valuable instrument to restage DTC patients with thyroglobulin elevation and negative RAI scintigraphy, despites some evidence showing a heterogeneous uptake in different lesions [40,41,42,43]. The only study that evaluated 177Lu-PSMA-617-RLT performance after two cycles demonstrated a slight, temporary response in one patient and disease progression after one month in the other [41]; no side effects due to PSMA-RLT were reported. The characteristics of the examined studies are reported in Table 3.
Table 3.
Characteristics of the included studies which employed PSMA radioligands in thyroid cancer.
| First Author and Year | Type | Country | N. Patients | Tracer | Histopathological Subtype (N. Patients) |
Clinical Setting | Analysed Lesions | Comparator |
|---|---|---|---|---|---|---|---|---|
| Lütje 2017 [40] |
Prospective | Germany | 6 | 68Ga- PSMA-11 (I) | 2 papillary 4 follicular |
Radioiodine refractory TC | 42 | 18F-FDG PET/CT |
| De Vries 2020 [41] | Retrospective | Netherlands | 5 |
68Ga-PSMA-11 (I) 177Lu-PSMA-617 (T) |
4 papillary 1 follicular variant |
Radioiodine refractory TC | / | / |
| Lawhn-Heath 2020 [42] |
Prospective | USA | 11 | 68Ga- PSMA-11 (I) | 3 papillary 2 follicular 2 Hurthle cell 2 poorly differentiated 2 anaplastic |
TC with abnormal uptake on 18F-FDG PET and/or 123I /131I scan |
43 |
18F-FDG PET/CT 123I /131I scan |
| Verma 2021 [43] |
Prospective | India | 9 | 68Ga-PSMA-11 (I) | 7 papillary 2 follicular variant |
Radioiodine refractory TC | 14 | 18F-FDG PET/CT |
| Pitalua-Cortes 2021 [44] |
Retrospective | Mexico | 10 | 68Ga- PSMA-11 (I) | 7 papillary 3 follicular |
Radioiodine refractory TC | 64 | 131I scan |
| Santhanam 2021 [45] |
Prospective | USA | 2 | 18F-DCFPyl (I) | 1 papillary 1 follicular |
Radioiodine refractory TC | 2 |
18F-FDG PET/CT 123I scan |
PET: positron emission tomography, CT: computed tomography, PSMA: prostate-specific membrane antigen, DCFPyL: piflufolastat, FDG: fluorodeoxyglucose, TC: thyroid cancer, TKI: tyrosine-kinase inhibitor, I: imaging, T: therapy.
3.4. Breast Cancer
Two full-text papers assessing the potential role of PSMA radioligands in the diagnostics of breast cancer (BC) were reviewed [46,47]. One of the examined studies was prospective [46], while the second had a retrospective design [47]. The number of evaluated patients was 19 and 15 in the two studies, which analysed 81 and 127 lesions, respectively [46,47]. Both the included studies reported heterogeneous PSMA radioligands uptake in different histopathological subtypes [46,47], with evidence of higher detection rates in luminal B and triple negative histologies [47]. When compared to 18F-FDG PET/CT, PSMA radioligand PET/CT showed similar performance in Her2-positive and triple negative subtypes, whereas poor performance was observed in luminal A and luminal B Her2-negative subtypes in primary tumour evaluation as well as in lymph node and distant metastases [47].
None of the included studies reported immunohistochemistry data about PSMA staining in BC. The characteristics of the included studies are reported in Table 4.
Table 4.
Characteristics of the included studies which employed PSMA radioligands in breast cancer.
| First Author and Year | Type | Country | N. Patients | Tracer | Histopathological SubType (N. Patients) |
Clinical Setting (N. Patients) |
Analysed Lesions | Comparator |
|---|---|---|---|---|---|---|---|---|
| Sathekge 2017 [46] | Prospective | South Africa | 19 | 68Ga-PSMA-11 (I) | 13 ductal 2 lobular 1 neuroendocrine differentiation 3 unknown |
9 staging 5 restaging for local recurrence 5 restaging for distant metastases |
81 | CT bone scan 18F-FDG PET/CT |
| Medina-Ornelas 2020 [47] | Retrospective | Mexico | 21 | 68Ga-PSMA-11 (I) | 4 luminal A 4 luminal B Her2+ 2 luminal B Her2- 5 triple negative |
Staging of locally advanced and metastatic BC | 127 | 18F-FDG PET/CT |
PET: positron emission tomography, CT: computed tomography, PSMA: prostate-specific membrane antigen, FDG: fluorodeoxyglucose, I: imaging.
3.5. Renal Cell Carcinoma
Sixteen full-text papers assessing the potential role of PSMA radioligands in the diagnostics of renal cell carcinoma (RCC) were reviewed [48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63]. Out of the 16 included studies, 6 had a prospective design [48,49,52,53,57,59]. The sample size varied from 5 to 53 and from 22 to 94 for patient and lesion analyses, respectively.
In 10 studies, PET/CT was performed using 68Ga-PSMA-11 [49,50,51,54,55,57,59,60,61,62] while four used 18F-DCFPyl as a diagnostic radiopharmaceutical [48,52,53,56], one used 18F-PSMA-1007 [58], and the remaining study used both 68Ga-PSMA-11 and 18F-PSMA-1007 [63].
The main RCC histotype investigated was clear cell renal cell carcinoma (ccRCC), since a total of 278 patients were examined in 15 of the included papers [48,49,50,51,53,54,55,56,57,58,59,60,61,62,63]. Further RCC subtypes examined in the included studies were papillary (25 patients in 10 studies [49,50,51,52,54,58,59,60,61,62]) and chromophobe (14 patients in seven studies [50,52,54,59,60,61,62]); the number of patients with other subtypes of renal malignancies (e.g., oncocytoma, unclassified RCC) was 21 across nine studies [49,52,54,58,59,60,61,62,63]. With regard to the clinical setting in which PSMA radioligand imaging was employed, in eight studies, PET/CT had the purpose of staging untreated RCC patients [50,52,53,60,61,62], in two it had the purpose of restaging after surgery [56,57], in two it had the purpose of restaging after suspect findings in other radiological exams [48,52], while in one it had the purpose of assessing treatment response [58], and in the remaining three examined papers, both staging and restaging PET/CT exams were included [51,54,63].
Eight out of the examined papers used CT and/or MRI as a comparator to evaluate PSMA radioligand PET/CT performance [48,49,52,53,57,58,61,63], two used 18F-FDG PET/CT [51,56], while the remaining six performed only PSMA radioligand PET/CT as a diagnostic exam [50,54,55,59,60,62].
All studies evaluating PSMA radioligand PET/CT in ccRCC reported a good performance in assessing the presence of pathological findings in different anatomic sites, including the bone, brain, lymph nodes and soft tissues [48,49,50,51,53,54,55,56,57,58,59,60,61,62,63]. When compared to conventional imaging and 18F-FDG, PSMA radioligand PET/CT showed overall better performance in nine of the included studies which analysed ccRCC patients in all the examined clinical contexts [48,49,53,54,56,57,58,61,63]. Moreover, PSMA radioligand PET/CT was able to change management in 22 patients included in three studies [53,54,63]. Nevertheless, poorer performance was observed in non-ccRCC patients while comparing PSMA radioligand PET/CT to conventional imaging [52]. Based on these results, most of the authors warranted a potential role for this examination in all of the investigated clinical settings. In two papers, a correlation between negative prognostic histopathological factors such as the presence of VEGF receptor (VEGFR) 2/platelet derived growth factor receptor (PGDFR) β expression, tumour necrosis, sarcomatoid or rhabdoid features and 68GaPSMA-11 uptake as well as stronger PSMA staining in immunohistochemistry studies was found [55,60]. Conversely from what observed in ccRCC, PSMA-targeted PET/CT showed overall poor performance in other RCC subtypes when compared to conventional imaging [52]; moreover, weaker PSMA staining was found in RCC subtypes other than ccRCC [61]. The characteristics of the included studies are reported in Table 5.
Table 5.
Characteristics of the included studies which employed PSMA radioligands in renal cell carcinoma.
| First Author and Year | Type | Country | N. Patients | Tracer | Histopathological Subtype (N. Patients) |
Clinical Setting (N. Patients) |
Analysed Lesions | Comparator |
|---|---|---|---|---|---|---|---|---|
| Rowe 2015 [48] | Prospective | USA | 5 | 18F-DCFPyL (I) | 5 clear cell | Restaging in metastatic patients naive to systemic therapies | 29 | CT and MRI |
| Rhee 2016 [49] | Prospective | Australia | 10 | 68Ga-PSMA-11 (I) | 8 clear cell 1 papillary 1 unclassified |
Staging in metastatic patients | 86 | CT |
| Sawicki 2016 [50] | Retrospective | Germany | 6 | 68Ga-PSMA-11 (I) | 4 clear cell 1 papillary 1 chromophobe |
Staging in metastatic patients | 22 | / |
| Siva 2017 [51] | Retrospective | Australia | 8 | 68Ga-PSMA-11 (I) | 7clear cell 1 papillary |
2 staging 5 restaging for suspect recurrence 2 staging and restaging after therapy |
18F-FDG PET/CT | |
| Yin 2018 [52] | Prospective | USA | 8 | 18F-DCFPyL (I) | 3 papillary 2 chromophobe 2 unclassified 1 xp11 translocation |
Restaging in metastatic patients previously treated with multiple lines of systemic therapy | 73 | CT MRI |
| Meyer 2019 [53] | Prospective | USA | 14 | 18F-DCFPyL (I) | 14 clear cell | Staging in oligometastatic patients | 47 | CT MRI |
| Raveenthiran 2019 [54] | Retrospective | Australia | 38 | 68Ga-PSMA-11 (I) | 28 clear cell 1 oncocytoma 1 papillary 1 chromophobe 1 TCC 6 unknown |
16 primary staging 32 restaging of suspected recurrent disease |
51 | / |
| Gao 2020 [55] | Retrospective | China | 36 | 68Ga-PSMA-11 (I) | 36 clear cell | Untreated primary renal cell carcinoma | / | / |
| Liu 2020 [56] | Retrospective | China | 15 | 18F-DCFPyL (I) | 15 clear cell | Post-operative restaging | 42 | 18F-FDG PET/CT |
| Gühne 2021 [57] | Prospective | Germany | 8 | 68Ga-PSMA-11 (I) | 8 clear cell |
Post-operative restaging | 12 | CT |
| Mittlemeier 2021 [58] | Retrospective | Germany | 11 | 18F-PSMA-1007 (I) | 8 clear cell 2 papillary 1 undifferentiated |
Response assessment after therapy | / | CT |
| Golan 2021 [59] | Prospective | Israel | 29 | 68Ga-PSMA-11 (I) | 18 clear cell 4 papillary 2 chromophobe 2 oncocytoma 2 angiomyolipoma 1 mixed epithelial and stromal tumour |
Dynamic PET/CT in the evaluation of localized renal masses. | 29 | / |
| Gao 2022 [60] | Retrospective | China | 48 | 68Ga-PSMA-11 (I) | 37 clear cell 4 papillary 3 chromophobe 4 unclassified |
Staging and comparison between PET parameters with VEGFR-2/PDGFR-β expression | 48 | / |
| Li 2022 [61] | Retrospective | China | 31 | 68Ga-PSMA-11 (I) | 40 clear cell 3 papillary 1 chromophobe 1 mucinous 1 poorly differentiated 4 Others |
Staging in metastatic patients | 94 | CT MRI |
| Meng 2022 [62] | Retrospective | China | 53 | 68Ga-PSMA-11 (I) | 40 clear cell 5 papillary 4 chromophobe 4 other |
Staging | 53 | / |
| Tariq 2022 [63] | Retrospective | Australia | 11 |
68Ga-PSMA-11 (I) 18F-PSMA-1007 (I) |
10 clear cell 1 unclassified |
4 staging 7 restaging |
/ | CT MRI |
PET: positron emission tomography, CT: computed tomography, MRI: magnetic resonance imaging, PSMA: prostate-specific membrane antigen, DCFPyL: piflufolastat, FDG: fluorodeoxyglucose, TCC: transitional cell cancer, VEGFR: vascular endothelial growth factor receptor, PDGFR: platelet derived growth factor receptor, I: imaging, T: therapy.
3.6. Hepatocellular Carcinoma
Five full-text papers assessing the potential role of PSMA radioligands in the diagnostics of hepatocellular carcinoma (HCC) were reviewed [64,65,66,67,68]. Out of the five included studies, four had a prospective design [64,65,66,68]. The sample size varied from 7 to 40 and from 37 to 142 for patient and lesion analyses, respectively.
In all studies, PET/CT was performed using 68Ga-PSMA-11 as the diagnostic radiopharmaceutical [64,65,66,67,68].
With regard to the clinical setting in which PSMA radioligand imaging was employed, in all studies PET/CT had the purpose both to stage untreated HCC patients and to restage after at least one line of treatment (transarterial chemoembolization, surgery, radiofrequency or transarterial radioembolization) [64,65,66,67,68].
All the examined papers used CT and/or MRI as comparator to evaluate PSMA radioligand PET/CT performance [64,65,66,67,68] and two added 18F-FDG PET/CT in the analysis [64,66].
All studies evaluating PSMA radioligand PET/CT in HCC reported a good performance in assessing the presence of pathological findings in liver and lymph-nodes, representing a potential novel imaging modality for patients with HCC, especially for extrahepatic disease detection [64,65,66,67,68]. When compared to other examinations, PSMA radioligand PET/CT showed better performance than 18F-FDG PET/CT in two studies, both in liver lesion detection and extrahepatic involvement assessment (lymph nodes, bone, peritoneum) [64,66]; moreover, PSMA radioligand PET/CT showed quite similar performance compared to MRI, especially in liver lesion assessment [64,65,66,67,68].
Three out of the five included studies performed immunohistochemistry to assess PSMA staining in tumour tissue [64,65,68]. Among them, PSMA expression was reported in tumour-associated endothelial cells in all cases with rare concomitant intratumoral weak PSMA staining (5,5%) in canalicular HCC. Characteristics of the included studies are reported in Table 6.
Table 6.
Characteristics of the included studies which employed PSMA radioligands in hepatocellular carcinoma.
| First Author and Year | Type | Country | N. Patients | Tracer | Clinical Setting (N. Patients) |
Analysed Lesions | Comparator |
|---|---|---|---|---|---|---|---|
| Kesler 2019 [64] | Prospective | Israel | 7 | 68Ga-PSMA-11 (I) | 6 staging 1 restaging after TACE |
37 |
18F-FDG PET/CT CT MRI |
| Kunikowska 2021 [65] | Prospective | Poland | 15 | 68Ga-PSMA-11 (I) | 10 staging, 4 restaging after TACE, 1 restaging after hemiepatectomy and TACE | 44 | CT MRI |
| Gündoğan 2021 [66] | Prospective | Turkey | 14 | 68Ga-PSMA-11 (I) | 12 staging 1 restaging after TACE, 1 restaging after radiofrequency ablation + TACE. |
61 |
18F-FDG PET/CT MRI |
| Hirmas 2021 [67] | Retrospective | Germany | 40 | 68Ga-PSMA-11 (I) | 27 Staging 13 restaging after local or systemic treatment. |
142 | CT |
| Thompson 2022 [68] | Prospective | USA | 31 | 68Ga-PSMA-11 (I) | Staging | 39 | MRI |
PET: positron emission tomography, CT: computed tomography, MRI: magnetic resonance imaging, PSMA: prostate-specific membrane antigen, DCFPyL: piflufolastat, FDG: fluorodeoxyglucose, TACE: transarterial chemo-embolization, TARE: transarterial radio-embolization, I: imaging.
4. Main Findings and Discussion
4.1. General
Since the introduction of PSMA radioligands in clinical practice for diagnostic or therapeutic purposes, several studies have evaluated their possible clinical applications in patients with solid tumours other than prostate cancer to improve the diagnostic accuracy or even to predict the outcome after different treatment schemes [29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68]. The in vivo study of biologic processes such as neoangiogenesis and hypoxia in tumour lesions represents a milestone in the history of cancer care, seeing as these features are targets of novel therapies [69]. Despite the rationale is consistent with a potential role for PSMA radioligands in this clinical context, the current status of the literature is still far from fulfilling this purpose, as we found the majority of extracted studies to be methodologically underpowered by sample size limitations. Moreover, a novel diagnostic examination is considered as being able to change patient management only if its employment might bring about an up- or down-staging when compared to conventional imaging and subsequently affects the treatment plan; despite recent evidences made clear that PSMA radioligand PET is able to change management in prostate cancer patients, this aspect needs to be furtherly explored in patients with tumours other than prostate cancer. Even so, this review tried to assess PSMA radioligands performance in patients with non-prostate solid tumours and whether these malignancies are potentially suitable for PSMA-targeted RLT. Regarding general characteristics, a common limitation, undeniable in most of the examined studies, was the limited number of included patients. The validation of a new employment for a radiopharmaceutical in a specific clinical context needs to be extensively consistent with large prospective cohorts and should rely on long-term follow-ups to assess whether the evidenced predictive values of PSMA radioligand PET/CT are confirmed and to assess if an eventual change in patient management due to PSMA-targeted PET/CT results actually brought benefits in terms of PFS and OS.
With regard to the employed radiopharmaceuticals, most of the included studies used 68Ga-PSMA-11 PET/CT as diagnostic compound to explore its potential role in several solid tumours other than prostate cancer [29,30,31,32,33,34,36,37,38,39,40,41,42,43,44,46,47,49,51,54,55,57,59,60,61,62,63,64,65,66,67,68]. To date, an increasing amount of PSMA radioligands is available for nuclear medicine physicians, including novel 18F-labelled radioligands [5]. Despite numerous studies comparing 18F-labeled and 68Ga-labeled PSMA radioligand performance finding similar results [70], using 18F-labelled compounds seems to rely on several advantages compared to using 68Ga-PSMA radioligands, since it can count on large-scale production, reduced costs, and high-quality images through lower positron energy, and a longer half-life [71]. However, since intense unspecific 18F-PSMA-1007 uptake may be observed within healthy bone, this tracer needs to be treated with caution when used for staging [72]. In this context, Alberts and colleagues presented a protocol design to experiment a head-to-head comparison of 68Ga-PSMA-11 and 18F-PSMA-1007 for the detection of recurrent prostate cancer lesions (ClinicalTrials.gov Identifier NCT05079828) [73]. As with prostate cancer, further studies on intra-patient comparisons with different PSMA-targeted tracers are needed for all of the clinical settings in which PSMA radioligands will find an application.
4.2. Gliomas
Gliomas are the most common primary tumours of the central nervous system, originating from the glial cells, and their annual incidence is about 6 per 100,000 cases worldwide [74,75]. Regarding grading, gliomas are most often referred to as low-grade gliomas or high-grade gliomas, based on the growth potential and aggressiveness of the tumours [76]. High-grade gliomas (e.g., glioblastomas) are characterized by a poor prognosis and high mortality, whereas low-grade gliomas present with a better prognosis [77]. Brain MRI is the diagnostic imaging modality of choice in the evaluation of patients with gliomas [75]. Different PET radiopharmaceuticals have been used to evaluate patients with gliomas with good diagnostic performances [78].
An excellent diagnostic performance of PSMA radioligand PET/CT or PET/MRI has been demonstrated before and after therapy in patients with high-grade gliomas (e.g., glioblastomas) [29,30,31,32,33,34,35,36]. These findings can be explained by the high expression of PSMA in the neovasculature of high-grade gliomas compared to low-grade gliomas or post-treatment abnormalities [79]. Notably, no significant uptake of PSMA radioligands was evident in the normal brain parenchyma, facilitating the detection of brain lesions with increased PSMA expression such as high-grade gliomas.
A clear advantage of PET with PSMA-targeting radiopharmaceuticals compared to 18F-FDG PET for detecting high-grade gliomas has been demonstrated [29,35,41]. The main limitation of 18F-FDG compared to PSMA radioligands is the very high physiological tracer uptake in the normal brain.
Overall, MRI remains the gold standard imaging method in the evaluation of gliomas [75], but PET with PSMA radioligands could be promising as a complementary imaging tool when MRI is doubtful. However, further studies should assess the clear diagnostic advantage of PSMA-targeted PET over MRI in high-grade gliomas.
The potential theragnostic role of PSMA radioligands could be the added value of PET with PSMA radioligands compared to PET with radiolabelled amino acids in high-grade gliomas. However, there are currently no studies comparing PET with these radiopharmaceuticals in high-grade gliomas. Furthermore, beyond case reports, the usefulness of PSMA-targeted therapy in patients with high-grade gliomas should be further demonstrated.
4.3. Salivary Gland Malignancies
SGCs are rare malignant head and neck malignancies, accounting for 3 to 10% of all head and neck tumours and exhibiting a varying clinical and biological behaviour; in this context, AdCC is one of the most common malignant SGCs, comprising 20–35% of all cases [80,81]. Its treatment usually consists of surgery and/or external beam radiation therapy in patients with local disease. Patients with locally advanced disease or distant metastases (frequently in bones or lungs) often show disease recurrence. In these patients, the effectiveness of both systemic chemotherapy and targeted immunotherapy is limited for symptomatic recurrent or distant disease, since a limited number of patients might show good response [82,83].
To date, 18F–FDG PET/CT plays a major role in the detection and staging of patients with head and neck cancer, with most studies focusing on squamous cell carcinoma (SCC). However, it is well known that AdCC has different biological characteristics, and its FDG-uptake is lower when compared to SCC [84]. In the recent literature, 18F-FDG PET/CT showed similar sensitivity for primary lesion detection when compared to CT and superior detection rates in lymph nodes and distant metastases [84].
With regard to PSMA radioligands physiologic biodistribution, it is well known that salivary glands show high uptake of PSMA radioligands [85]; nevertheless, our knowledge is poor about the mechanism underlying PSMA radioligand accumulation in salivary glands, since several immunochemistry studies observed the absence of PSMA expression in this district [86,87]. Moreover, PSMA-specific radioimmunoconjugates, including 111In-J591 and 177Lu-J591, did not show significant accumulation in healthy salivary glands [88]. These findings are consistent with the hypothesis supporting that PSMA radioligands uptake in salivary gland tissue is mainly unspecific.
Conversely from what was observed in the majority of cancer types included in this review, intracellular PSMA expression was observed in AdCC cells as well as in the tumour-induced neovasculature endothelium, although it seems not to be correlated with PSMA radioligand uptake in PET/CT images [37].
Concerning the clinical findings enhanced by the evaluated studies, PSMA radioligand PET/CT showed good performance while detecting and visualizing local recurrent and distant metastatic AdCC. Moreover, PSMA radioligand uptake was supported by high PSMA expression on immunohistochemistry studies [38].
With regard to the potential application of α- or β-emitter-radiolabeled PSMA-targeted ligands, only one study reported the employment of 177Lu-PSMA-617 for palliative intent in six patients, revealing partial response or stable disease in one-third of the enrolled patients [39]. Based on these premises and on the detection of PSMA staining in cancer cells, RLT with PSMA-targeted radioligands might be an option in AdCC patients when regular treatment options fail. In this context, more prospective studies are needed to assess whether RLT may improve prognosis in AdCC patients.
4.4. Thyroid Cancer
Thyroid cancer is the most common endocrine malignancy [89]. Despite its increasing incidence worldwide, most cases of DTC show excellent prognosis after surgery, usually concerning total or partial thyroidectomy with or without lymph-node dissection, TSH suppression due to levothyroxine treatment and radioiodine (RAI) therapy when indicated [89]. DTCs include papillary thyroid cancer (PTC) and follicular thyroid carcinoma (FTC); other less frequent subtypes of thyroid cancer are represented by medullary and anaplastic thyroid carcinomas, which usually show poor prognosis [89]. Nevertheless, about one-third of metastatic DTC patients do not demonstrate RAI uptake in their lesions or show progressive disease after RAI treatment and are classified as radioiodine-refractory DTC [89]. Most radioiodine-refractory DTCs show an aggressive histopathological feature and 18F-fluorodeoxyglucose (18F-FDG) uptake on PET/CT images [90,91].
Recent studies observed PSMA expression in the neovasculature of several thyroid cancer subtypes, including PTC and FTC, and revealed that strong PSMA staining tumours were more clinically aggressive than those with weak PSMA staining [92,93]. However, based on their findings, GPII is only expressed by tumour-associated vascular endothelium and not by cancer cells themselves. Moreover, it has been observed that PSMA expression may depend on DTC histotype: moderate- to high-grade PSMA staining has been observed in papillary and follicular subtypes and weak PSMA expression in anaplastic histologies.
As it is the main concern in physicians who deal with DTC, all the included studies enrolled patients with RAI-refractory thyroid cancer [40,41,42,43,44,45]. Since there are no reliable instruments to predict RAI-refractoriness and few lines of therapy are currently available in this clinical setting [94], this topic raised the attention of many authors searching for prognostic factors and new therapeutic agents.
With regard to clinical findings, PSMA radioligand PET/CT in DTC patients showed different uptake characteristics between primary recurrent lesions and distant metastases as well as among the included DTC histopathological subtypes [45].
Angiogenesis is a key step in tumour progression, and RAI-refractory DTC novel therapies with tyrosine kinase inhibitors (TKI), such as lenvatinib, operate by inhibiting the pathways through VEGFR, fibroblast growth factor receptor (FGFR), PGDFRα, rearranged during transfection (RET) and stem cell factor receptor (c-KIT), showing significant prolongation of PFS and improved response rate compared to placebo [94]. Based on these premises, PSMA radioligand PET/CT may have a role in predicting PFS and OS in RAI-refractory DTC patients undergoing TKI treatment.
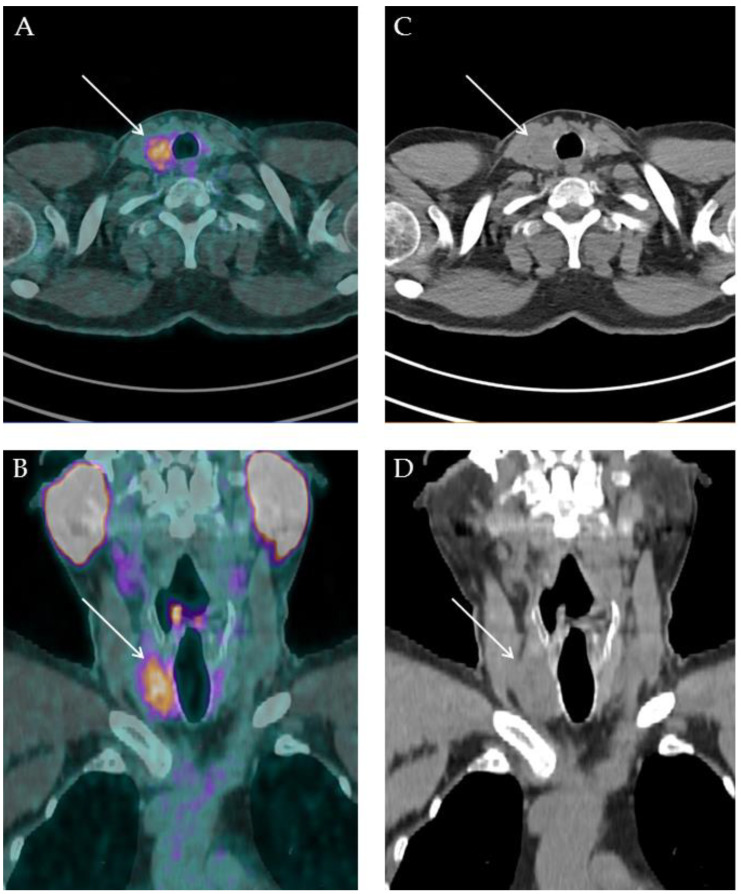
Regarding PSMA-RLT, only one of the examined papers evaluated the employment of 177Lu-PSMA-617 in DTC patients, reporting a slight, temporary response in one patient [41]. The absence of GPII expression on the surface of tumour cells and its variable expression in neovasculature endothelium might explain why RLT showed low effectiveness in RAI-refractory DTC. More prospective studies with larger sample size are needed to correctly evaluate the actual effectiveness of PSMA-targeted RLT. A case of accidental finding of primary DTC is reported in Figure 2.
Figure 2.
A 57-years old man with diagnosis of prostate adenocarcinoma previously treated through external beam radiation therapy in 2019 (Gleason score 4 + 4) came to our attention in December 2021 for biochemical recurrence (PSA value: 0.91 ng/mL) and underwent restaging 18F-PSMA-1007 PET/CT. PET/CT scan was performed 90 min after 270 MBq 18F-PSMA-1007 administration. PET/CT images showed focal uptake in the prostate gland right lobe, attributable to prostate cancer relapse. Furthermore, in the head and neck sections ((A) fused PET/CT axial, (B) fused PET/CT coronal, (C) low-dose CT axial, (D) low-dose CT coronal), an area of abnormal and intense 18F-PSMA-1007 uptake was observed in a thyroid nodule located in the right lobe. After routine diagnostic work up, the patient underwent total thyroidectomy without lymph-node dissection, and histologic examination was consistent with the diagnosis of tall cell variant papillary thyroid cancer.
4.5. Breast Cancer
BC is the most common malignant tumour in women, with an estimated 2.3 million new cases per year and approximately 685,000 deaths per year worldwide [95]. This malignancy comprises various subtypes depending on its stage at presentation, histotype, biomarkers expression including estrogen receptor (ER), progesterone receptor (PgR) and human epidermal growth factor receptor 2 (Her2) as well as proliferation index (Ki67) [96,97,98]. Molecular and histological profiling of breast cancer is fundamental in treatment choice and is classified as: luminal A, characterized by ER and/or PgR expression and a Her2-negative arrangement, luminal B, characterized by ER and/or PgR expression in addition to a Her2-positive arrangement, the Her2-enriched subtype, characterized by the lack of ER and PgR expression but positive Her2 arrangement, and basal-like/triple-negative breast cancer, in which ER, PgR and Her2 are not expressed [96,97,98].
Despite BC demonstrating variable 18F-FDG avidity, 18F-FDG PET/CT plays a major role in BC management, especially in the staging of locally advanced BC, in restaging patients with suspect local disease or distant metastases recurrence, and in evaluating treatment response [99].
Tolkach et al. reported PSMA expression in the tumour-associated neovasculature endothelium in 315 cases of invasive non-special type BC and lobular BC [100]. Moreover, a stronger PSMA staining in higher-grade tumours, in Her2-positive BC and in tumours without ER and PgR expression was observed.
Two studies included in this review explored a possible role for PSMA radioligand PET/CT in staging BC and in restaging patients with suspect disease recurrence. Both studies reported a variable PSMA expression within the investigated lesions and higher uptake in Her2-positive BC subtypes and triple negative BC [46,47].
To date, only one case of 177Lu-PSMA-617 RLT in BC has been reported in a 38-year-old woman with triple negative BC with rapid progressive disease after multiple lines of systemic therapy. Since the patient showed further disease progression after two PSMA-targeted RLT administrations, treatment was dismissed [100]. However, considering the low toxicity of PSMA-targeted RLT, the authors warranted more studies to assess its efficacy in BC, especially in cases of triple negative subtypes.
4.6. Renal Cell Carcinoma
RCC is the most common type of renal tumour. Its incidence has progressively increased in recent decades due to a higher exposure to risk factors (e.g., obesity and alcohol consumption) and the development of more sensitive diagnostic modalities [101]. Based on its histopathology, RCC can be classified in two subtypes: ccRCC and non-ccRCC (which includes at least 15 histotypes, including papillary RCC and chromophobe RCC) [102]. About 20–30% of RCCs are present with distant metastatic lesions at diagnosis.
To date, contrast-enhanced CT and MRI are the main imaging methods employed to stage RCC, while 18F-FDG PET/CT has a limited role in its management, as the physiological renal excretion of 18F-FDG and metabolites hinders the characterization of primary tumours, and the expression of several enzymes such as fructose 1,6-bisphosphatase 1 seems to have an inverse correlation with 18F-FDG avidity in ccRCC [103,104].
Baccala and colleagues evaluated PSMA expression in a kidney tissue microarray including 169 samples of normal parenchyma and several cancer tissues, discovering a significant difference among endothelium of neovasculature in different RCCs [105]. In particular, the subtype with the greatest expression of PSMA was found to be ccRCC. GPII expression was also confirmed in the subtypes of chromophobe RCC and in oncocytoma, but not in papillary RCC, despite it being a neoplasm derived from proximal renal tubular cells, where PSMA is physiologically expressed [105,106]. Conversely from what reported by Baccala et al., a subsequent study from Al-Ahmadie and colleagues demonstrated PSMA expression in 75 RCC samples, including papillary RCC [107]. In particular, a direct relationship was found between the extension of neovascularization and the expression of PSMA, the latter being more strongly expressed in ccRCCa, known to be highly vascularized and expressed with less intensity and with a focal pattern in the papillary RCC, which showed poor vascularization. Moreover, Chang et al. demonstrated the expression of PSMA also in the neovasculature of RCC metastases as well as in the neovasculature endothelium cells of primary RCC [108].
PSMA radioligand PET imaging has been applied to detecting local recurrence and metastatic disease in RCC patients [48,51,52,54,56,63]. Moreover, one study preliminarily revealed that PSMA-based PET imaging demonstrated more precise response assessment and therapy monitoring of metastatic RCC patients to systemic treatment, usually consisting of TKIs or immune check-point inhibitors [58]. Several included studies tried to compare PSMA radioligand uptake characteristics with histopathologic features, reporting a correlation between intense 68Ga-PSMA-11 uptake and VEGFR-2/PDGFR-β expression and hypoxia-inducible factor (HIF) 2α [55,60].
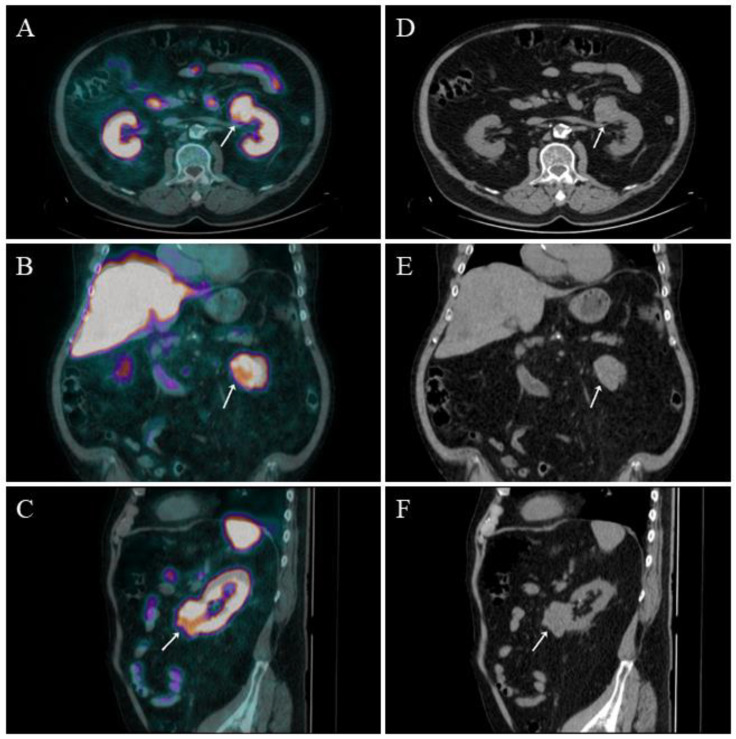
No available studies were found concerning a possible employment of α- or β-emitter-radiolabelled PSMA-targeted ligands. A case of accidental finding of primary ccRCC is reported in Figure 3.
Figure 3.
A 64-years old man with diagnosis of prostate adenocarcinoma previously treated through robot-assisted radical prostatectomy and pelvic lymph-node dissection in 2016 (pT2apN0c; Gleason score 4 + 3) came to our attention in February 2022 for biochemical recurrence (PSA value: 0.64 ng/mL) and underwent 18F-PSMA-1007 PET/CT. PET/CT scan was performed 90 min after 250 MBq 18F-PSMA-1007 administration. PET/CT images did not show any sign of suspect local recurrence nor distant metastases distinctly attributable to prostate cancer. Nevertheless, in the upper abdomen sections ((A) fused PET/CT axial, (B) fused PET/CT coronal, (C) fused PET/CT sagittal, (D) low-dose CT axial, (E) low-dose CT coronal; (F): low-dose CT sagittal), an area of abnormal and intense 18F-PSMA-1007 uptake was observed in an exophitic lesion located at the lower pole of the left kidney (white arrow). After routine diagnostic work up, the patient was submitted to total nephrectomy and histologic examination was consistent with the diagnosis of ccRCC.
4.7. Hepatocellular Carcinoma
HCC is the third most common cause of cancer death worldwide, and its main risk factors are cirrhosis and viral hepatitis infection [109]. Despite several local and systemic treatments currently being available, treatment options for HCC may be limited at presentation due to the patient’s and tumour’s baseline status (lesions size, number and distribution, overall patient performance status), making aggressive management unsafe [110].
To date, HCC is diagnosed by imaging using CT or MRI based on Liver Imaging and Reporting Data System (LI-RADS) criteria [111]. Nevertheless, morphologic imaging is not able to explore tumour biology, usually characterized by marker molecular heterogeneity in this context. 18F-FDG PET/CT has sub-optimal liver imaging kinetics, since only high-grade HCC show significant uptake [112].
As for the majority of the solid tumours investigated in this review, immunohistochemistry studies reported PSMA staining only in the tumour-associated neovasculature endothelium [113]. Consistently with what was reported for other neoplasms, strong PSMA expression was associated with poor prognosis in patients with HCC [113]. However, it is interesting that in one paper, mild PSMA staining in canalicular HCC was described alongside tumour-associated neovasculature PSMA expression [114].
With regard to the clinical setting in which PSMA radioligand PET/CT was employed, the included studies explored potential applications of 68Ga-PSMA-11 both in staging and restaging for local or distant recurrence in patients previously treated with local or systemic therapies [64,65,66,67,68]. Overall, the included studies revealed a superior performance of PSMA-targeted PET/CT compared to 18F-FDG PET/CT and assessed that it might be a potential novel imaging modality supportive to MRI for local disease staging and restaging and to demonstrate extrahepatic involvement [67].
Since first-line therapy in locally advanced and metastatic HCC consists of a combination of immunotherapy and antineaongiogenic therapy (atezolizumab/bevacizumab) [115], the potential role of PSMA radioligands as a prognosis predictor deserves further analyses.
No available studies were found concerning a possible employment of α- or β-emitter-radiolabelled PSMA-targeted ligands.
4.8. Limitations
This review has some limitations. First, the available literature presents a limited amount of studies and most of them suffered from a poor number of enrolled patients undergoing PSMA radioligand administration for PET/CT diagnostics or RLT. Second, due to the heterogeneity of the studies, only a qualitative review with no quantitative meta-analytic data was considered for this literature review.
5. Conclusions
The exploration of PSMA radioligand PET/CT as a possible diagnostic or theragnostic agent in non-prostate solid tumours is in its infancy.
Since PSMA expression in solid tumours other than prostate cancer is primarily observed in the tumour neovasculature, and it showed promising performance compared to standard morphological and functional imaging, further studies are needed to assess whether this novel imaging examination might improve oncological patients’ management in specifical clinical settings such as the monitoring of antineoangiogenic therapies. As several authors pointed out [116], a subset of patients with non-prostate solid tumours show sufficient PSMA radioligand uptake in PET images to be potentially eligible for PSMA-RLT. In this context, further studies are needed to explore RLT feasibility and to assess if the absence of PSMA on cancer cell surface might be a rate-limiting factor for its success aside from the uptake detected in PET/CT images.
Author Contributions
Original Draft Preparation: A.R., G.T., S.A., S.D.; Writing, Review and Editing: L.Z., G.P., V.L., M.R., D.A.P.; Supervision, S.A. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data supporting reported results are available in public bibliographic databases (e.g., PubMed/Medline).
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
Salvatore Annunziata is funded by Ministero della Salute through Ricerca Finalizzata 2019 for a large retrospective oncology research project about PET/CT radiomics in lymphoma (PERL, 2021–2024; grant number: GR-2019-12370372).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Farolfi A., Calderoni L., Mattana F., Mei R., Telo S., Fanti S., Castellucci P. Current and Emerging Clinical Applications of PSMA PET Diagnostic Imaging for Prostate Cancer. J. Nucl. Med. 2021;62:596–604. doi: 10.2967/jnumed.120.257238. [DOI] [PubMed] [Google Scholar]
- 2.Wester H.-J., Schottelius M. PSMA-Targeted Radiopharmaceuticals for Imaging and Therapy. Semin. Nucl. Med. 2019;49:302–312. doi: 10.1053/j.semnuclmed.2019.02.008. [DOI] [PubMed] [Google Scholar]
- 3.Horoszewicz J.S., Kawinski E., Murphy G.P. Monoclonal antibodies to a new antigenic marker in epithelial prostatic cells and serum of prostatic cancer patients. Anticancer Res. 1987;7:927–935. [PubMed] [Google Scholar]
- 4.Israeli R.S., Powell C.T., Fair W.R., Heston W.D. Molecular cloning of a complementary DNA encoding a prostate-specific membrane antigen. Cancer Res. 1993;53:227–230. [PubMed] [Google Scholar]
- 5.Silver D.A., Pellicer I., Fair W.R., Heston W.D., Cordon-Cardo C. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin. Cancer Res. 1997;3:81–85. [PubMed] [Google Scholar]
- 6.Chang S.S., O’Keefe D.S., Bacich D.J., Reuter V.E., Heston W.D., Gaudin P.B. Prostate-specific membrane antigen is pro-duced in tumor-associated neovasculature. Clin. Cancer Res. 1999;5:2674–2681. [PubMed] [Google Scholar]
- 7.Conway R.E., Petrovic N., Li Z., Heston W., Wu D., Shapiro L.H. Prostate-Specific Membrane Antigen Regulates Angiogenesis by Modulating Integrin Signal Transduction. Mol. Cell. Biol. 2006;26:5310–5324. doi: 10.1128/MCB.00084-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conway R.E., Rojas C., Alt J., Nováková Z., Richardson S.M., Rodrick T.C., Fuentes J.L., Richardson N., Attalla J., Stewart S., et al. Prostate-specific membrane antigen (PSMA)-mediated laminin proteolysis generates a pro-angiogenic peptide. Angiogenesis. 2016;19:487–500. doi: 10.1007/s10456-016-9521-x. [DOI] [PubMed] [Google Scholar]
- 9.Kozikowski A.P., Nan F., Conti P., Zhang J., Ramadan E., Bzdega T., Wroblewska B., Neale J.H., Pshenichkin S., Wroblewski J.T. Design of Remarkably Simple, Yet Potent Urea-Based Inhibitors of Glutamate Carboxypeptidase II (NAALADase) J. Med. Chem. 2001;44:298–301. doi: 10.1021/jm000406m. [DOI] [PubMed] [Google Scholar]
- 10.Eder M., Schäfer M., Bauder-Wüst U., Hull W.-E., Wängler C., Mier W., Haberkorn U., Eisenhut M. 68Ga-Complex Lipophilicity and the Targeting Property of a Urea-Based PSMA Inhibitor for PET Imaging. Bioconjug. Chem. 2012;23:688–697. doi: 10.1021/bc200279b. [DOI] [PubMed] [Google Scholar]
- 11.Afshar-Oromieh A., Haberkorn U., Eder M., Eisenhut M., Zechmann C.M. [68Ga]Gallium-labelled PSMA ligand as superior PET tracer for the diagnosis of prostate cancer: Comparison with 18F-FECH. Eur. J. Pediatr. 2012;39:1085–1086. doi: 10.1007/s00259-012-2069-0. [DOI] [PubMed] [Google Scholar]
- 12.Afshar-Oromieh A., Malcher A., Eder M., Eisenhut M., Linhart H.G., Hadaschik B.A., Holland-Letz T., Giesel F.L., Kratochwil C., Haufe S., et al. PET imaging with a [68Ga]gallium-labelled PSMA ligand for the diagnosis of prostate cancer: Biodistribution in humans and first evaluation of tumour lesions. Eur. J. Pediatr. 2013;40:486–495. doi: 10.1007/s00259-012-2298-2. [DOI] [PubMed] [Google Scholar]
- 13.Rathke H., Flechsig P., Mier W., Bronzel M., Mavriopoulou E., Hohenfellner M., Giesel F.L., Haberkorn U.L., Kratochwil C., Roehrich M. Dosimetry Estimate and Initial Clinical Experience with 90Y-PSMA-617. J. Nucl. Med. 2019;60:806–811. doi: 10.2967/jnumed.118.218917. [DOI] [PubMed] [Google Scholar]
- 14.Wang Z., Jacobson O., Tian R., Mease R.C., Kiesewetter D.O., Niu G., Pomper M.G., Chen X. Radioligand Therapy of Prostate Cancer with a Long-Lasting Prostate-Specific Membrane Antigen Targeting Agent 90Y-DOTA-EB-MCG. Bioconjug. Chem. 2018;29:2309–2315. doi: 10.1021/acs.bioconjchem.8b00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kratochwil C., Bruchertseifer F., Giesel F.L., Weis M., Verburg F.A., Mottaghy F., Kopka K., Apostolidis C., Haberkorn U., Morgenstern A. 225Ac-PSMA-617 for PSMA-Targeted α-Radiation Therapy of Metastatic Castration-Resistant Prostate Cancer. J. Nucl. Med. 2016;57:1941–1944. doi: 10.2967/jnumed.116.178673. [DOI] [PubMed] [Google Scholar]
- 16.Kratochwil C., Bruchertseifer F., Rathke H., Bronzel M., Apostolidis C., Weichert W., Haberkorn U., Giesel F.L., Morgenstern A. Targeted α-Therapy of Metastatic Castration-Resistant Prostate Cancer with 225Ac-PSMA-617: Dosimetry Estimate and Empiric Dose Finding. J. Nucl. Med. 2017;58:1624–1631. doi: 10.2967/jnumed.117.191395. [DOI] [PubMed] [Google Scholar]
- 17.Chen Y., Pullambhatla M., Foss C.A., Byun Y., Nimmagadda S., Senthamizhchelvan S., Sgouros G., Mease R.C., Pomper M.G. 2-(3-{1-Carboxy-5-[(6-[18F]Fluoro-Pyridine-3-Carbonyl)-Amino]-Pentyl}-Ureido)-Pentanedioic Acid, [18F]DCFPyL, a PSMA-Based PET Imaging Agent for Prostate Cancer. Clin. Cancer Res. 2011;17:7645–7653. doi: 10.1158/1078-0432.CCR-11-1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cardinale J., Schäfer M., Benešová M., Bauder-Wüst U., Leotta K., Eder M., Neels O.C., Haberkorn U., Giesel F.L., Kopka K. Preclinical Evaluation of 18F-PSMA-1007, a New Prostate-Specific Membrane Antigen Ligand for Prostate Cancer Imaging. J. Nucl. Med. 2017;58:425–431. doi: 10.2967/jnumed.116.181768. [DOI] [PubMed] [Google Scholar]
- 19.Giesel F.L., Will L., Lawal I., Lengana T., Kratochwil C., Vorster M., Neels O., Reyneke F., Haberkon U., Kopka K., et al. Intraindividual Comparison of 18F-PSMA-1007 and 18F-DCFPyL PET/CT in the Prospective Evaluation of Patients with Newly Diagnosed Prostate Carcinoma: A Pilot Study. J. Nucl. Med. 2018;59:1076–1080. doi: 10.2967/jnumed.117.204669. [DOI] [PubMed] [Google Scholar]
- 20.FDA FDA Approves First PSMA-Targeted PET Drug. J. Nucl. Med. 2021;62:11N. [PubMed] [Google Scholar]
- 21.FDA FDA Approves 18F-DCFPyL PET Agent in Prostate Cancer. J. Nucl. Med. 2021;62:11N. [Google Scholar]
- 22.Sartor O., de Bono J., Chi K.N., Fizazi K., Herrmann K., Rahbar K., Tagawa S.T., Nordquist L.T., Vaishampayan N., El-Haddad G., et al. Lutetium-177–PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2021;385:1091–1103. doi: 10.1056/NEJMoa2107322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.FDA FDA Approves Pluvicto/Locametz for Metastatic Castration-Resistant Prostate Cancer. J. Nucl. Med. 2022;63:13N. [PubMed] [Google Scholar]
- 24.Alsultan A.A., Barentsz M.W., Smits M.L., Koopman M., Lam M.G., Rosenbaum C.E. Angiogenesis in 90Y-Radioembolization of Colorectal Liver Metastases. Semin. Nucl. Med. 2019;49:204–210. doi: 10.1053/j.semnuclmed.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 25.Folkman J. Tumor angiogenesis: Therapeutic implications. N. Engl. J. Med. 1971;285:1182–1186. doi: 10.1056/nejm197111182852108. [DOI] [PubMed] [Google Scholar]
- 26.Ronca R., Benkheil M., Mitola S., Struyf S., Liekens S. Tumor angiogenesis revisited: Regulators and clinical implications. Med. Res. Rev. 2017;37:1231–1274. doi: 10.1002/med.21452. [DOI] [PubMed] [Google Scholar]
- 27.Jain R.K. Antiangiogenesis Strategies Revisited: From Starving Tumors to Alleviating Hypoxia. Cancer Cell. 2014;26:605–622. doi: 10.1016/j.ccell.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carmeliet P., Jain R.K. Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat. Rev. Drug Discov. 2011;10:417–427. doi: 10.1038/nrd3455. [DOI] [PubMed] [Google Scholar]
- 29.Sasikumar A., Joy A., Pillai M.R.A., Nanabala R., Jayaprakash P.G., Madhavan J., Nair S. Diagnostic Value of 68Ga PSMA-11 PET/CT Imaging of Brain Tumors—Preliminary Analysis. Clin. Nucl. Med. 2017;42:e41–e48. doi: 10.1097/RLU.0000000000001451. [DOI] [PubMed] [Google Scholar]
- 30.Sasikumar A., Kashyap R., Joy A., Patro K.C., Bhattacharya P., Pilaka V.K.R., Oommen K.E., Pillai M.R.A. Utility of 68Ga-PSMA-11 PET/CT in Imaging of Glioma—A Pilot Study. Clin. Nucl. Med. 2018;43:e304–e309. doi: 10.1097/RLU.0000000000002175. [DOI] [PubMed] [Google Scholar]
- 31.Verma P., Malhotra G., Goel A., Rakshit S., Chandak A., Chedda R., Banerjee S., Asopa R.V. Differential Uptake of 68Ga-PSMA-HBED-CC (PSMA-11) in Low-Grade Versus High-Grade Gliomas in Treatment-Naive Patients. Clin. Nucl. Med. 2019;44:e318–e322. doi: 10.1097/RLU.0000000000002520. [DOI] [PubMed] [Google Scholar]
- 32.Akgun E., Akgun M.Y., Selçuk H.H., Uzan M., Sayman H.B. 68Ga PSMA PET/MR in the differentiation of low and high grade gliomas: Is 68Ga PSMA PET/MRI useful to detect brain gliomas? Eur. J. Radiol. 2020;130:109199. doi: 10.1016/j.ejrad.2020.109199. [DOI] [PubMed] [Google Scholar]
- 33.Kunikowska J., Kuliński R., Muylle K., Koziara H., Królicki L. 68Ga-Prostate-Specific Membrane Antigen-11 PET/CT: A New Imaging Option for Recurrent Glioblastoma Multiforme? Clin. Nucl. Med. 2020;45:11–18. doi: 10.1097/RLU.0000000000002806. [DOI] [PubMed] [Google Scholar]
- 34.Kumar A., ArunRaj S.T., Bhullar K., Haresh K.P., Gupta S., Ballal S., Yadav M., Singh M., Damle N.A., Garg A., et al. Ga-68 PSMA PET/CT in recurrent high-grade gliomas: Evaluating PSMA expression in vivo. Neuroradiology. 2022;64:969–979. doi: 10.1007/s00234-021-02828-2. [DOI] [PubMed] [Google Scholar]
- 35.Liu D., Cheng G., Ma X., Wang S., Zhao X., Zhang W., Yang W., Wang J. PET/CT using 68Ga-PSMA-617 versus 18F-fluorodeoxyglucose to differentiate low- and high-grade gliomas. J. Neuroimaging. 2021;31:733–742. doi: 10.1111/jon.12856. [DOI] [PubMed] [Google Scholar]
- 36.Kunikowska J., Czepczyński R., Pawlak D., Koziara H., Pełka K., Królicki L. Expression of glutamate carboxypeptidase II in the glial tumor recurrence evaluated in vivo using radionuclide imaging. Sci. Rep. 2022;12:652. doi: 10.1038/s41598-021-04613-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nulent T.J.W.K., Van Es R.J.J., Krijger G.C., De Bree R., Willems S.M., de Keizer B. Prostate-specific membrane antigen PET imaging and immunohistochemistry in adenoid cystic carcinoma—A preliminary analysis. Eur. J. Pediatr. 2017;44:1614–1621. doi: 10.1007/s00259-017-3737-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Boxtel W., Lütje S., Grunsven I.C.V.E.-V., Verhaegh G.W., Schalken J.A., Jonker M.A., Nagarajah J., Gotthardt M., van Herpen C.M. 68Ga-PSMA-HBED-CC PET/CT imaging for adenoid cystic carcinoma and salivary duct carcinoma: A phase 2 imaging study. Theranostics. 2020;10:2273–2283. doi: 10.7150/thno.38501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nulent T.J.W.K., van Es R.J.J., Willems S.M., Braat A.J.A.T., Devriese L.A., de Bree R., de Keizer B. First experiences with 177Lu-PSMA-617 therapy for recurrent or metastatic salivary gland cancer. EJNMMI Res. 2021;11:126. doi: 10.1186/s13550-021-00866-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lütje S., Gomez B., Cohnen J., Umutlu L., Gotthardt M., Poeppel T.D., Bockisch A., Rosenbaum-Krumme S. Imaging of Prostate-Specific Membrane Antigen Expression in Metastatic Differentiated Thyroid Cancer Using 68Ga-HBED-CC-PSMA PET/CT. Clin. Nucl. Med. 2017;42:20–25. doi: 10.1097/RLU.0000000000001454. [DOI] [PubMed] [Google Scholar]
- 41.De Vries L.H., Lodewijk L., Braat A.J.A.T., Krijger G.C., Valk G.D., Lam M.G.E.H., Rinkes I.H.M.B., Vriens M.R., De Keizer B. 68Ga-PSMA PET/CT in radioactive iodine-refractory differentiated thyroid cancer and first treatment results with 177Lu-PSMA-617. EJNMMI Res. 2020;10:18. doi: 10.1186/s13550-020-0610-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lawhn-Heath C., Yom S.S., Liu C., Villanueva-Meyer J.E., Aslam M., Smith R., Narwal M., Juarez R., Behr S.C., Pampaloni M.H., et al. Gallium-68 prostate-specific membrane antigen ([68Ga]Ga-PSMA-11) PET for imaging of thyroid cancer: A feasibility study. EJNMMI Res. 2020;10:128. doi: 10.1186/s13550-020-00720-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Verma P., Malhotra G., Meshram V., Chandak A., Sonavane S., Lila A.R., Bandgar T.R., Asopa R.V. Prostate-Specific Membrane Antigen Expression in Patients With Differentiated Thyroid Cancer With Thyroglobulin Elevation and Negative Iodine Scintigraphy Using 68Ga-PSMA-HBED-CC PET/CT. Clin. Nucl. Med. 2021;46:e406–e409. doi: 10.1097/RLU.0000000000003655. [DOI] [PubMed] [Google Scholar]
- 44.Pitalua-Cortes Q., García-Perez F.O., Vargas-Ahumada J., Gonzalez-Rueda S., Gomez-Argumosa E., Ignacio-Alvarez E., Soldevilla-Gallardo I., Torres-Agredo L. Head-to-Head Comparison of 68Ga-PSMA-11 and 131I in the Follow-Up of Well-Differentiated Metastatic Thyroid Cancer: A New Potential Theragnostic Agent. Front. Endocrinol. 2021;12:794759. doi: 10.3389/fendo.2021.794759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Santhanam P., Russell J., Rooper L.M., Ladenson P.W., Pomper M.G., Rowe S.P. The prostate-specific membrane antigen (PSMA)-targeted radiotracer 18F-DCFPyL detects tumor neovasculature in metastatic, advanced, radioiodine-refractory, differentiated thyroid cancer. Med. Oncol. 2020;37:98. doi: 10.1007/s12032-020-01427-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sathekge M., Lengana T., Modiselle M., Vorster M., Zeevaart J., Maes A., Ebenhan T., Van De Wiele C. 68Ga-PSMA-HBED-CC PET imaging in breast carcinoma patients. Eur. J. Pediatr. 2017;44:689–694. doi: 10.1007/s00259-016-3563-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Medina-Ornelas S., García-Perez F., Estrada-Lobato E., Ochoa-Carrillo F. 68Ga-PSMA PET/CT in the Evaluation of Locally Advanced and Metastatic Breast Cancer, a Single Center Experience. Am. J. Nucl. Med. Mol. Imaging. 2020;10:135–142. [PMC free article] [PubMed] [Google Scholar]
- 48.Rowe S.P., Gorin M.A., Hammers H.J., Javadi M.S., Hawasli H., Szabo Z., Cho S.Y., Pomper M.G., Allaf M.E. Imaging of metastatic clear cell renal cell carcinoma with PSMA-targeted 18F-DCFPyL PET/CT. Ann. Nucl. Med. 2015;29:877–882. doi: 10.1007/s12149-015-1017-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rhee H., Blazak J., Tham C.M., Ng K.L., Shepherd B., Lawson M., Preston J., Vela I., Thomas P., Wood S. Pilot study: Use of gallium-68 PSMA PET for detection of metastatic lesions in patients with renal tumour. EJNMMI Res. 2016;6:76. doi: 10.1186/s13550-016-0231-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sawicki L.M., Buchbender C., Boos J., Giessing M., Ermert J., Antke C., Antoch G., Hautzel H. Diagnostic potential of PET/CT using a 68Ga-labelled prostate-specific membrane antigen ligand in whole-body staging of renal cell carcinoma: Initial experience. Eur. J. Pediatr. 2017;44:102–107. doi: 10.1007/s00259-016-3360-2. [DOI] [PubMed] [Google Scholar]
- 51.Siva S., Callahan J., Pryor D., Martin J., Lawrentschuk N., Hofman M.S. Utility of 68Ga prostate specific membrane antigen—Positron emission tomography in diagnosis and response assessment of recurrent renal cell carcinoma. J. Med. Imaging Radiat. Oncol. 2017;61:372–378. doi: 10.1111/1754-9485.12590. [DOI] [PubMed] [Google Scholar]
- 52.Yin Y., Campbell S.P., Markowski M.C., Pierorazio P.M., Pomper M.G., Allaf M.E., Rowe S.P., Gorin M.A. Inconsistent Detection of Sites of Metastatic Non-Clear Cell Renal Cell Carcinoma with PSMA-Targeted [18F]DCFPyL PET/CT. Mol. Imaging Biol. 2019;21:567–573. doi: 10.1007/s11307-018-1271-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Meyer A.R., Carducci M.A., Denmeade S.R., Markowski M.C., Pomper M.G., Pierorazio P.M., Allaf M.E., Rowe S.P., Gorin M.A. Improved identification of patients with oligometastatic clear cell renal cell carcinoma with PSMA-targeted 18F-DCFPyL PET/CT. Ann. Nucl. Med. 2019;33:617–623. doi: 10.1007/s12149-019-01371-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Raveenthiran S., Esler R., Yaxley J., Kyle S. The use of 68Ga-PET/CT PSMA in the staging of primary and suspected recurrent renal cell carcinoma. Eur. J. Nucl. Med. Mol. Imaging. 2019;46:2280–2288. doi: 10.1007/s00259-019-04432-2. [DOI] [PubMed] [Google Scholar]
- 55.Gao J., Xu Q., Fu Y., He K., Zhang C., Zhang Q., Shi J., Zhao X., Wang F., Guo H. Comprehensive evaluation of 68Ga-PSMA-11 PET/CT parameters for discriminating pathological characteristics in primary clear-cell renal cell carcinoma. Eur. J. Pediatr. 2021;48:561–569. doi: 10.1007/s00259-020-04916-6. [DOI] [PubMed] [Google Scholar]
- 56.Liu Y., Wang G., Yu H., Wu Y., Lin M., Gao J., Xu B. Comparison of 18F-DCFPyL and 18F-FDG PET/computed tomography for the restaging of clear cell renal cell carcinoma: Preliminary results of 15 patients. Nucl. Med. Commun. 2020;41:1299–1305. doi: 10.1097/MNM.0000000000001285. [DOI] [PubMed] [Google Scholar]
- 57.Gühne F., Seifert P., Theis B., Steinert M., Freesmeyer M., Drescher R. PSMA-PET/CT in Patients with Recurrent Clear Cell Renal Cell Carcinoma: Histopathological Correlations of Imaging Findings. Diagnostics. 2021;11:1142. doi: 10.3390/diagnostics11071142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mittlmeier L.M., Unterrainer M., Rodler S., Todica A., Albert N.L., Burgard C., Cyran C.C., Kunz W.G., Ricke J., Bartenstein P., et al. 18F-PSMA-1007 PET/CT for response assessment in patients with metastatic renal cell carcinoma undergoing tyrosine kinase or checkpoint inhibitor therapy: Preliminary results. Eur. J. Pediatr. 2021;48:2031–2037. doi: 10.1007/s00259-020-05165-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Golan S., Aviv T., Groshar D., Yakimov M., Zohar Y., Prokocimer Y., Nadu A., Baniel J., Domachevsky L., Bernstine H. Dynamic 68Ga-PSMA-11 PET/CT for the Primary Evaluation of Localized Renal Mass: A Prospective Study. J. Nucl. Med. 2021;62:773–778. doi: 10.2967/jnumed.120.251272. [DOI] [PubMed] [Google Scholar]
- 60.Gao J., Meng L., Xu Q., Zhao X., Deng Y., Fu Y., Guo S., He K., Shi J., Wang F., et al. 68Ga-PSMA-11 PET/CT Parameter Correlates with Pathological VEGFR-2/PDGFR-β Expression in Renal Cell Carcinoma Patients. Mol. Imaging Biol. 2022 doi: 10.1007/s11307-022-01725-1. [DOI] [PubMed] [Google Scholar]
- 61.Li Y., Zheng R., Zhang Y., Huang C., Tian L., Liu R., Liu Y., Zhang Z., Han H., Zhou F., et al. Special issue “the advance of solid tumor research in China”: 68Ga-PSMA-11 PET/CT for evaluating primary and metastatic lesions in different histological subtypes of renal cell carcinoma. Int. J. Cancer. 2022:ijc.34189. doi: 10.1002/ijc.34189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Meng L., Zhang S., Gao J., Xu Q., Fu Y., Zhou Y.-H., Wang F., Guo H. [68Ga]Ga-PSMA-11 PET/CT has potential application in predicting tumor HIF-2α expression and therapeutic response to HIF-2α antagonists in patients with RCC. Eur. Radiol. 2022;32:6545–6553. doi: 10.1007/s00330-022-08738-y. [DOI] [PubMed] [Google Scholar]
- 63.Tariq A., Kwok M., Pearce A., Rhee H., Kyle S., Marsh P., Raveenthiran S., Wong D., McBean R., Westera J., et al. The role of dual tracer PSMA and FDG PET/CT in renal cell carcinoma (RCC) compared to conventional imaging: A multi-institutional case series with intra-individual comparison. Urol. Oncol. Semin. Orig. Investig. 2021;40:66.e1–66.e9. doi: 10.1016/S2666-1683(21)02749-X. [DOI] [PubMed] [Google Scholar]
- 64.Kesler M., Levine C., Hershkovitz D., Mishani E., Menachem Y., Lerman H., Zohar Y., Shibolet O., Even-Sapir E. 68Ga-Labeled Prostate-Specific Membrane Antigen Is a Novel PET/CT Tracer for Imaging of Hepatocellular Carcinoma: A Prospective Pilot Study. J. Nucl. Med. 2019;60:185–191. doi: 10.2967/jnumed.118.214833. [DOI] [PubMed] [Google Scholar]
- 65.Kunikowska J., Cieślak B., Gierej B., Patkowski W., Kraj L., Kotulski M., Zieniewicz K., Królicki L. [68Ga]Ga-Prostate-Specific Membrane Antigen PET/CT: A novel method for imaging patients with hepatocellular carcinoma. Eur. J. Pediatr. 2021;48:883–892. doi: 10.1007/s00259-020-05017-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gündoğan C., Ergül N., Çakır M.S., Kılıçkesmez Ö., Gürsu R.U., Aksoy T., Çermik T.F. 68Ga-PSMA PET/CT Versus 18F-FDG PET/CT for Imaging of Hepatocellular Carcinoma. Mol. Imaging Radionucl. Ther. 2021;30:79–85. doi: 10.4274/mirt.galenos.2021.92053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hirmas N., Leyh C., Sraieb M., Barbato F., Schaarschmidt B.M., Umutlu L., Nader M., Wedemeyer H., Ferdinandus J., Rischpler C., et al. 68Ga-PSMA-11 PET/CT Improves Tumor Detection and Impacts Management in Patients with Hepatocellular Carcinoma. J. Nucl. Med. 2021;62:1235–1241. doi: 10.2967/jnumed.120.257915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Thompson S.M., Suman G., Torbenson M.S., Chen Z.E., Jondal D.E., Patra A., Ehman E.C., Andrews J.C., Fleming C.J., Welch B.T., et al. PSMA as a Theranostic Target in Hepatocellular Carcinoma: Immunohistochemistry and 68Ga-PSMA-11 PET Using Cyclotron-Produced 68Ga. Hepatol. Commun. 2021;6:1172–1185. doi: 10.1002/hep4.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li T., Kang G., Wang T., Huang H. Tumor angiogenesis and anti-angiogenic gene therapy for cancer (Review) Oncol. Lett. 2018;16:687–702. doi: 10.3892/ol.2018.8733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Evangelista L., Maurer T., van der Poel H., Alongi F., Kunikowska J., Laudicella R., Fanti S., Hofman M.S. [68Ga]Ga-PSMA Versus [18F]PSMA Positron Emission Tomography/Computed Tomography in the Staging of Primary and Recurrent Prostate Cancer. A Systematic Review of the Literature. Eur. Urol. Oncol. 2022;5:273–282. doi: 10.1016/j.euo.2022.03.004. [DOI] [PubMed] [Google Scholar]
- 71.Giesel F.L., Hadaschik B., Cardinale J., Radtke J., Vinsensia M., Lehnert W., Kesch C., Tolstov Y., Singer S., Grabe N., et al. F-18 labelled PSMA-1007: Biodistribution, radiation dosimetry and histopathological validation of tumor lesions in prostate cancer patients. Eur. J. Nucl. Med. Mol. Imaging. 2017;44:678–688. doi: 10.1007/s00259-016-3573-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vollnberg B., Alberts I., Genitsch V., Rominger A., Afshar-Oromieh A. Assessment of malignancy and PSMA expression of uncertain bone foci in [18F]PSMA-1007 PET/CT for prostate cancer—A single-centre experience of PET-guided biopsies. Eur. J. Pediatr. 2022 doi: 10.1007/s00259-022-05745-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Alberts I., Bütikofer L., Rominger A., Afshar-Oromieh A. A randomised, prospective and head-to-head comparison of [68Ga]Ga-PSMA-11 and [18F]PSMA-1007 for the detection of recurrent prostate cancer in PSMA-ligand PET/CT—Protocol design and rationale. PLoS ONE. 2022;17:e0270269. doi: 10.1371/journal.pone.0270269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Francis S.S., Ostrom Q.T., Cote D.J., Smith T.R., Claus E., Barnholtz-Sloan J.S. The Epidemiology of Central Nervous System Tumors. Hematol. Clin. N. Am. 2022;36:23–42. doi: 10.1016/j.hoc.2021.08.012. [DOI] [PubMed] [Google Scholar]
- 75.Weller M., van den Bent M., Preusser M., Le Rhun E., Tonn J.C., Minniti G., Bendszus M., Balana C., Chinot O., Dirven L., et al. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat. Rev. Clin. Oncol. 2020;18:170–186. doi: 10.1038/s41571-020-00447-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Komori T. Grading of adult diffuse gliomas according to the 2021 WHO Classification of Tumors of the Central Nervous System. Lab. Investig. 2022;102:126–133. doi: 10.1038/s41374-021-00667-6. [DOI] [PubMed] [Google Scholar]
- 77.Zhou Q., Xue C., Ke X., Zhou J. Treatment Response and Prognosis Evaluation in High-Grade Glioma: An Imaging Review Based on MRI. J. Magn. Reson. Imaging. 2022;56:325–340. doi: 10.1002/jmri.28103. [DOI] [PubMed] [Google Scholar]
- 78.Treglia G., Muoio B., Trevisi G., Mattoli M.V., Albano D., Bertagna F., Giovanella L. Diagnostic Performance and Prognostic Value of PET/CT with Different Tracers for Brain Tumors: A Systematic Review of Published Meta-Analyses. Int. J. Mol. Sci. 2019;20:4669. doi: 10.3390/ijms20194669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Traub-Weidinger T., Poetsch N., Woehrer A., Klebermass E.-M., Bachnik T., Preusser M., Mischkulnig M., Kiesel B., Widhalm G., Mitterhauser M., et al. PSMA Expression in 122 Treatment Naive Glioma Patients Related to Tumor Metabolism in 11C-Methionine PET and Survival. J. Pers. Med. 2021;11:624. doi: 10.3390/jpm11070624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Coca-Pelaz A., Rodrigo J.P., Bradley P.J., Poorten V.V., Triantafyllou A., Hunt J.L., Strojan P., Rinaldo A., Haigentz M., Takes R.P., et al. Adenoid cystic carcinoma of the head and neck—An update. Oral Oncol. 2015;51:652–661. doi: 10.1016/j.oraloncology.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 81.Bjørndal K., Krogdahl A., Therkildsen M.H., Overgaard J., Johansen J., Kristensen C.A., Homøe P., Sørensen C.H., Andersen E., Bundgaard T., et al. Salivary gland carcinoma in Denmark 1990–2005: A national study of incidence, site and histology. Results of the Danish Head and Neck Cancer Group (DAHANCA) Oral Oncol. 2011;47:677–682. doi: 10.1016/j.oraloncology.2011.04.020. [DOI] [PubMed] [Google Scholar]
- 82.Laurie S.A., Ho A.L., Fury M.G., Sherman E., Pfister D.G. Systemic therapy in the management of metastatic or locally recurrent adenoid cystic carcinoma of the salivary glands: A systematic review. Lancet Oncol. 2011;12:815–824. doi: 10.1016/S1470-2045(10)70245-X. [DOI] [PubMed] [Google Scholar]
- 83.Alfieri S., Granata R., Bergamini C., Resteghini C., Bossi P., Licitra L., Locati L.D. Systemic therapy in metastatic salivary gland carcinomas: A pathology-driven paradigm? Oral Oncol. 2017;66:58–63. doi: 10.1016/j.oraloncology.2016.12.016. [DOI] [PubMed] [Google Scholar]
- 84.Freling N., Crippa F., Maroldi R. Staging and follow-up of high-grade malignant salivary gland tumours: The role of traditional versus functional imaging approaches—A review. Oral Oncol. 2016;60:157–166. doi: 10.1016/j.oraloncology.2016.04.016. [DOI] [PubMed] [Google Scholar]
- 85.Prasad V., Steffen I.G., Diederichs G., Makowski M.R., Wust P., Brenner W. Biodistribution of [(68)Ga]PSMA-HBED-CC in Patients with Prostate Cancer: Characterization of Uptake in Normal Organs and Tumour Lesions. Mol. Imaging Biol. 2016;18:428–436. doi: 10.1007/s11307-016-0945-x. [DOI] [PubMed] [Google Scholar]
- 86.Rupp N.J., Umbricht C.A., Pizzuto D.A., Lenggenhager D., Töpfer A., Müller J., Muehlematter U.J., Ferraro D.A., Messerli M., Morand G.B., et al. First Clinicopathologic Evidence of a Non-PSMA-Related Uptake Mechanism for 68Ga-PSMA-11 in Salivary Glands. J. Nucl. Med. 2019;60:1270–1276. doi: 10.2967/jnumed.118.222307. [DOI] [PubMed] [Google Scholar]
- 87.Tönnesmann R., Meyer P.T., Eder M., Baranski A.-C. [177Lu]Lu-PSMA-617 Salivary Gland Uptake Characterized by Quantitative In Vitro Autoradiography. Pharmaceuticals. 2019;12:18. doi: 10.3390/ph12010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pandit-Taskar N., O’Donoghue J.A., Morris M.J., Wills E.A., Schwartz L.H., Gonen M., Scher H.I., Larson S.M., Divgi C.R. Antibody Mass Escalation Study in Patients with Castration-Resistant Prostate Cancer Using 111In-J591: Lesion Detectability and Dosimetric Projections for 90Y Radioimmunotherapy. J. Nucl. Med. 2008;49:1066–1074. doi: 10.2967/jnumed.107.049502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Haugen B.R., Alexander E.K., Bible K.C., Doherty G.M., Mandel S.J., Nikiforov Y.E., Pacini F., Randolph G.W., Sawka A.M., Schlumberger M., et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26:1–133. doi: 10.1089/thy.2015.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Salvatori M., Biondi B., Rufini V. Imaging in Endocrinology: 2-[18F]-fluoro-2-deoxy-D-glucose positron emission tomography/computed tomography in differentiated thyroid carcinoma: Clinical indications and controversies in diagnosis and follow-up. Eur. J. Endocrinol. 2015;173:R115–R130. doi: 10.1530/EJE-15-0066. [DOI] [PubMed] [Google Scholar]
- 91.Rizzo A., Perotti G., Zagaria L., Lanni V., Racca M., Palestini N., Salvatori M. 18F-FDG PET/CT concurrent with first radioiodine post-therapeutic scan in high risk differentiated thyroid cancer: A useful tool or just an expensive diversion? Q. J. Nucl. Med. Mol. Imaging. 2022 doi: 10.23736/S1824-4785.22.03364-7. [DOI] [PubMed] [Google Scholar]
- 92.Moore M., Panjwani S., Mathew R., Crowley M., Liu Y.-F., Aronova A., Finnerty B., Zarnegar R., Fahey T.J., Scognamiglio T. Well-Differentiated Thyroid Cancer Neovasculature Expresses Prostate-Specific Membrane Antigen—A Possible Novel Therapeutic Target. Endocr. Pathol. 2017;28:339–344. doi: 10.1007/s12022-017-9500-9. [DOI] [PubMed] [Google Scholar]
- 93.Sollini M., Di Tommaso L., Kirienko M., Piombo C., Erreni M., Lania A.G., Erba P.A., Antunovic L., Chiti A. PSMA expression level predicts differentiated thyroid cancer aggressiveness and patient outcome. EJNMMI Res. 2019;9:93. doi: 10.1186/s13550-019-0559-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schlumberger M., Tahara M., Wirth L.J., Robinson B., Brose M.S., Elisei R., Habra M.A., Newbold K., Shah M.H., Hoff A.O., et al. Lenvatinib versus Placebo in Radioiodine-Refractory Thyroid Cancer. N. Engl. J. Med. 2015;372:621–630. doi: 10.1056/NEJMoa1406470. [DOI] [PubMed] [Google Scholar]
- 95.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 96.Perou C.M., Sørlie T., Eisen M.B., Van De Rijn M., Jeffrey S.S., Rees C.A., Pollack J.R., Ross D.T., Johnsen H., Akslen L.A., et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 97.Loibl S., Gianni L. HER2-positive breast cancer. Lancet. 2016;389:2415–2429. doi: 10.1016/S0140-6736(16)32417-5. [DOI] [PubMed] [Google Scholar]
- 98.Burguin A., Diorio C., Durocher F. Breast Cancer Treatments: Updates and New Challenges. J. Pers. Med. 2021;11:808. doi: 10.3390/jpm11080808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Paydary K., Seraj S.M., Zadeh M.Z., Emamzadehfard S., Shamchi S.P., Gholami S., Werner T.J., Alavi A. The Evolving Role of FDG-PET/CT in the Diagnosis, Staging, and Treatment of Breast Cancer. Mol. Imaging Biol. 2019;21:1–10. doi: 10.1007/s11307-018-1181-3. [DOI] [PubMed] [Google Scholar]
- 100.Tolkach Y., Gevensleben H., Bundschuh R., Koyun A., Huber D., Kehrer C., Hecking T., Keyver-Paik M.-D., Kaiser C., Ahmadzadehfar H., et al. Prostate-specific membrane antigen in breast cancer: A comprehensive evaluation of expression and a case report of radionuclide therapy. Breast Cancer Res. Treat. 2018;169:447–455. doi: 10.1007/s10549-018-4717-y. [DOI] [PubMed] [Google Scholar]
- 101.Capitanio U., Bensalah K., Bex A., Boorjian S.A., Bray F., Coleman J., Gore J.L., Sun M., Wood C., Russo P. Epidemiology of Renal Cell Carcinoma. Eur. Urol. 2019;75:74–84. doi: 10.1016/j.eururo.2018.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tung I., Sahu A. Immune Checkpoint Inhibitor in First-Line Treatment of Metastatic Renal Cell Carcinoma: A Review of Current Evidence and Future Directions. Front. Oncol. 2021;11:707214. doi: 10.3389/fonc.2021.707214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Urso L., Castello A., Rocca G.C., Lancia F., Panareo S., Cittanti C., Uccelli L., Florimonte L., Castellani M., Ippolito C., et al. Role of PSMA-ligands imaging in Renal Cell Carcinoma management: Current status and future perspectives. J. Cancer Res. Clin. Oncol. 2022;148:1299–1311. doi: 10.1007/s00432-022-03958-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Park J.W., Jo M.K., Lee H.M. Significance of 18F-fluorodeoxyglucose positron-emission tomography/computed tomography for the postoperative surveillance of advanced renal cell carcinoma. Br. J. Urol. 2009;103:615–619. doi: 10.1111/j.1464-410X.2008.08150.x. [DOI] [PubMed] [Google Scholar]
- 105.Baccala A., Sercia L., Li J., Heston W., Zhou M. Expression of Prostate-Specific Membrane Antigen in Tumor-Associated Neovasculature of Renal Neoplasms. Urology. 2007;70:385–390. doi: 10.1016/j.urology.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 106.Ristau B.T., O’Keefe D.S., Bacich D.J. The prostate-specific membrane antigen: Lessons and current clinical implications from 20 years of research. Urol. Oncol. 2014;32:272–279. doi: 10.1016/j.urolonc.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Al-Ahmadie H.A., Olgac S., Gregor P.D., Tickoo S.K., Fine S.W., Kondagunta G.V., Scher H.I., Morris M.J., Russo P., Motzer R.J., et al. Expression of prostate-specific membrane antigen in renal cortical tumors. Mod. Pathol. 2008;21:727–732. doi: 10.1038/modpathol.2008.42. [DOI] [PubMed] [Google Scholar]
- 108.Chang S.S., Reuter V.E., Heston W.D.W., Gaudin P.B. Metastatic renal cell carcinoma neovasculature expresses prostate-specific membrane antigen. Urology. 2001;57:801–805. doi: 10.1016/S0090-4295(00)01094-3. [DOI] [PubMed] [Google Scholar]
- 109.Kulik L., El-Serag H.B. Epidemiology and Management of Hepatocellular Carcinoma. Gastroenterology. 2019;156:477–491.e1. doi: 10.1053/j.gastro.2018.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.El-Serag H.B., Marrero J.A., Rudolph L., Reddy K.R. Diagnosis and Treatment of Hepatocellular Carcinoma. Gastroenterology. 2008;134:1752–1763. doi: 10.1053/j.gastro.2008.02.090. [DOI] [PubMed] [Google Scholar]
- 111.Kanmaniraja D., Dellacerra G., Holder J., Erlichman D., Chernyak V. Liver Imaging Reporting and Data System (LI-RADS) v2018: Review of the CT/MRI Diagnostic Categories. Can. Assoc. Radiol. J. 2021;72:142–149. doi: 10.1177/0846537119888393. [DOI] [PubMed] [Google Scholar]
- 112.Asman Y., Evenson A.R., Even-Sapir E., Shibolet O. [18F]fludeoxyglucose positron emission tomography and computed tomography as a prognostic tool before liver transplantation, resection, and loco-ablative therapies for hepatocellular carcinoma. Liver Transpl. 2015;21:572–580. doi: 10.1002/lt.24083. [DOI] [PubMed] [Google Scholar]
- 113.Jiao D., Li Y., Yang F., Han D., Wu J., Shi S., Tian F., Guo Z., Xi W., Li G., et al. Expression of Prostate-Specific Membrane Antigen in Tumor-Associated Vasculature Predicts Poor Prognosis in Hepatocellular Carcinoma. Clin. Transl. Gastroenterol. 2019;10:1–7. doi: 10.14309/ctg.0000000000000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tolkach Y., Goltz D., Kremer A., Ahmadzadehfar H., Bergheim D., Essler M., Lam M., de Keizer B., Fischer H.-P., Kristiansen G. Prostate-specific membrane antigen expression in hepatocellular carcinoma: Potential use for prognosis and diagnostic imaging. Oncotarget. 2019;10:4149–4160. doi: 10.18632/oncotarget.27024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Llovet J.M., Kelley R.K., Villanueva A., Singal A.G., Pikarsky E., Roayaie S., Lencioni R., Koike K., Zucman-Rossi J., Finn R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Primers. 2021;7:6. doi: 10.1038/s41572-020-00240-3. [DOI] [PubMed] [Google Scholar]
- 116.Uijen M.J.M., Derks Y.H.W., Merkx R.I.J., Schilham M.G.M., Roosen J., Privé B.M., van Lith S.A.M., van Herpen C.M.L., Gotthardt M., Heskamp S., et al. PSMA radioligand therapy for solid tumors other than prostate cancer: Background, opportunities, challenges, and first clinical reports. Eur. J. Nucl. Med. Mol. Imaging. 2021;48:4350–4368. doi: 10.1007/s00259-021-05433-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data supporting reported results are available in public bibliographic databases (e.g., PubMed/Medline).