Abstract
Glioblastoma (GBM) is the most common and highly lethal type of brain tumor, with poor survival despite advances in understanding its complexity. After current standard therapeutic treatment, including tumor resection, radiotherapy and concomitant chemotherapy with temozolomide, the median overall survival of patients with this type of tumor is less than 15 months. Thus, there is an urgent need for new insights into GBM molecular characteristics and progress in targeted therapy in order to improve clinical outcomes. The literature data revealed that a number of different signaling pathways are dysregulated in GBM. In this review, we intended to summarize and discuss current literature data and therapeutic modalities focused on targeting dysregulated signaling pathways in GBM. A better understanding of opportunities for targeting signaling pathways that influences malignant behavior of GBM cells might open the way for the development of novel GBM-targeted therapies.
Keywords: glioblastoma, GBM subtypes, SHH signaling, Wnt/β-catenin signaling, Notch signaling, TGFβ signaling, BMP signaling, Hippo signaling, RA signaling
1. Glioblastoma
Glioblastoma (GBM), a grade IV glioma [1], represents the most aggressive and the deadliest malignant brain tumor, characterized by fast spreading, infiltrative growth and high level of molecular/cytological heterogeneity [2,3,4,5]. Despite decades of research, patients have poor clinical prognosis, with less than 5% surviving 5 years after diagnosis and a median survival time of less than 15 months [6]. The standard treatment for these tumors is radical surgical resection followed by radiotherapy and chemotherapy using temozolomide (TMZ) as currently the main chemotherapeutic agent [7,8,9]. Unfortunately, rapid recurrence after therapy is detected in virtually all patients, which worsens the prognosis (reviewed in [10]). Major contributors of the aggressive course of the disease include the highly mutated genome of GBM and dysregulated signaling pathways involved in cell proliferation, growth, and survival (reviewed in [11,12,13]).
GBM consists of heterogeneous populations of cells in different phases of differentiation (reviewed in [4,5,14,15]). At the apex of GBM cellular hierarchy is a population of undifferentiated, self-renewing, and highly proliferative stem cells named glioblastoma stem cells (GSCs) (reviewed in [16]). These cells are characterized by high plasticity and the ability to give rise to heterogeneous cancer cells within the GBM (reviewed in [17,18]). Multiple studies demonstrated that, besides heterogeneity, GSCs play an indispensable role in the formation, growth, and progression as well as in therapeutic resistance and recurrence of GBM [19,20,21], indicating that these cells could be a crucial target for treatment (reviewed in [18,22]).
Our knowledge of the molecular biology of GBM has increased in recent decades. There are several cellular events during gliomagenesis. They include genetic instability, loss of cell cycle control, overexpression of growth factors and their receptors, angiogenesis, migration, invasion, and aberrant apoptosis (reviewed in [23]). All these processes are regulated by various signaling pathways, and accordingly, targeting key molecules in signaling pathways holds promise for developing novel therapeutic approaches. Targeting of key signaling pathways found deregulated in GBM, such as EGFR, PDGFR, PI3K–PTEN-Akt-mTOR, cell cycle-associated pathways (CDK4/6, CDKN2A/B), P53, pRB, RAS/MAPK, and STAT3, has been previously described in detail (reviewed in [11,24,25,26]).
Strong evidence in the last decade has suggested that GBMs may actually arise from neural stem cells (NSCs) residing in the lining of lateral ventricles that undergo malignant transformation (reviewed in [27,28]). Furthermore, the same studies underscored the parallels between neural development and gliomagenesis [27,28]. A comparison of the lineage hierarchy of the developing human brain to the transcriptome of GBM cells and GSCs derived from IDH-mutant astrocytoma, WHO grade 4, revealed that this type of brain tumor develops along neurodevelopmental gene programs encompassing a rapidly dividing progenitor population [29]. IDH-mutant astrocytoma, WHO grade 4, is hierarchically organized into three cell lineages that correspond to three normal neural lineages, astrocytic, neuronal, and oligodendrocytic, with progenitor cancer cells at its apex [29]. The results of genetic studies from more than 500 GBMs indicated that during tumor growth and invasion, these cells employ the same signaling networks as NSCs during neurogenesis and/or gliogenesis (reviewed in [13]). Signaling pathways involved in the regulation of nervous system development are dysregulated in CNS malignancies including GBM. Having in mind the aforementioned, in the present review, we focus on current strategies for targeting major neurodevelopmental signaling pathways that have been described to promote GBM growth and invasion (reviewed in [13,30]). These signaling pathways include Sonic Hedgehog (SHH), Bone Morphogenetic Protein (BMPs), Transforming Growth Factor β (TGFβ), Wnt, Notch, Hippo and retinoic acid (RA) pathway (reviewed in [30]). Further, these signaling pathways are active in GSCs, a subpopulation of cells within the tumor responsible for increased resistance to chemotherapy and radiotherapy [31,32]. In GSCs, these signaling pathways are mainly responsible for regulation of self-renewal or differentiation [31,32,33,34,35]. In addition to their role in the maintenance of GSCs, they play important roles in the regulation of proliferative, migratory and invasive abilities of GBM cells, contributing to the growth and progression of GBM.
Having in mind the inter- and intra-heterogeneity of GBMs (reviewed in [14]), stratifications of GBMs into molecular subtypes, including profiling of signaling pathway status, is important for clinical management of patients with GBMs.
2. Molecular GBM Subtypes
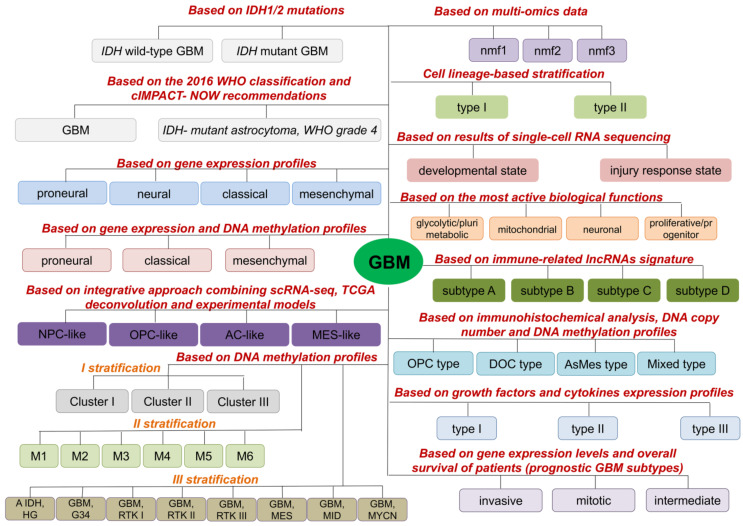
Many different classifications of GBM into subtypes have emerged over the years. Through these classifications, different GBM subtypes are characterized by different features and gene alterations, and they should be considered for different targeted treatments for fighting GBM (Figure 1).
Figure 1.
Stratification of GBM into subtypes. This summary is based on the previously reported publications listed in the main text and in Supplementary Table S1.
One of the first molecular stratifications of GBM is based on the presence/absence of mutations in isocitrate dehydrogenase 1 (IDH1) or 2 (IDH2) genes (Figure 1, Supplementary Table S1). Based on this, GBM is subdivided into two subtypes: IDH wild-type GBM and IDH mutant GBM (reviewed in [36]). IDH wild-type GBM is found in more than 90% of all patients (mostly in elderly patients), and the vast majority of IDH wild-type GBM are primary GBM that develop de novo (reviewed in [36]). IDH mutant GBM is detected in less than 10% of patients with GBM (almost all in young adults) and the vast majority of IDH mutant GBM represent secondary GBM, developed from diffuse or anaplastic astrocytoma (reviewed in [36]). Based on the progress in the diagnosis and management of gliomas from 2016, the term IDH-mutant GBM is discontinued and replaced with IDH-mutant astrocytoma, WHO grade 4 (Figure 1, Supplementary Table S1) [37].
Based on gene expression profile, Verhaak and co-workers classified GBM into four subtypes: proneural, neural, classical, and mesenchymal [38] (Figure 1, Supplementary Table S1). These subtypes differ in mutations, genes expression, as well as responses to chemo- and radiotherapy (reviewed in [39]). Results obtained by Wang and co-workers, analyzing IDH wild-type GBMs, indicated neural subtype as normal neural lineage contamination [40]. Previously, neural subtype has been related to the margin of the tumor where normal neural tissue is likely to be detected [41,42]. The mesenchymal subtype is associated with poor overall survival and poor response to radiotherapy, while the proneural is related to a more favorable outcome [43,44,45,46]. Furthermore, it was detected that a GBM tumor from one patient can harbor cells having characteristics of different GBM molecular subtypes [47]. Additionally, switching from one GBM subtype to another within one GBM tumor has been demonstrated (reviewed in [39]).
Ensenyat-Mendez and co-workers constructed the iGlioSub classifier using machine learning, computational biology algorithms and prioritization of highly informative transcriptomic and epigenomic features which, based on gene expression and DNA methylation profiles, classified GBMs into classical, mesenchymal and proneural subtypes (Figure 1, Supplementary Table S1). This classifier showed better performance in stratifying patients compared to stratification based on gene expression profiles only [48].
An integrative approach applied by Neftel and co-workers revealed that tumor cells in GBM exist in four cellular states that recapitulate neural progenitor-like (NPC-like), oligodendrocyte-progenitor-like (OPC-like), astrocyte-like (AC-like) and mesenchymal-like (MES-like) states (Figure 1, Supplementary Table S1). MES-like cells are further divided into hypoxia-independent (MES1) and hypoxia-dependent (MES2) sub-groups, while NPC-like cells are divided into NPC1 and NPC2 sub-groups that were distinguished by inclusion of OPC-related genes in NPC1 and neuronal lineage genes in NPC2 (Figure 1, Supplementary Table S1) [49].
Three stratifications of GBM into subtypes are proposed based on DNA methylation profiles (Figure 1). In stratification I, Ma and co-workers identified three different GBM prognosis subgroups (Cluster 1–3) with Cluster 3 associated with poor prognosis and relatively lower sample methylation level, and Cluster 2 associated with the best prognosis [50]. On the other side, Brennan and co-workers stratified GBM patients into six methylation classes (M1, M2, M3, M4, M5 (G-CIMP), M6) [51]. Based on DNA methylation in CNS tumors, Capper and co-workers grouped most of the IDH-mutant astrocytoma, WHO grade 4, into methylation subclass “A IDH, HG”, while IDH wild-type GBM were stratified into seven methylation classes (“GBM, G34“, “GBM, RTK I”, “GBM, RTK II”, “GBM, RTK III”, “GBM, MES”, “GBM, MID”, “GBM, MYCN”) [52].
Based on multi-omics data (CNVs, gene expression, protein and phosphoprotein abundances), Wang and co-workers defined three clusters in IDH wild-type GBMs-nmf1 (proneural-like), nmf2 (mesenchymal-like) and nmf3 (classical-like) (Figure 1) enriched in neuron activity-related pathways, immune response pathways and cell cycle pathways, respectively [53]. Furthermore, classification of GBM into lineage-specific subtypes which possess diverse functional properties and therapeutic vulnerabilities was described (Figure 1, Supplementary Table S1) [54]. Based on the results of single-cell RNA sequencing (scRNA-seq) of GSCs and malignant cells from primary GBM tumors, Richards and co-workers stratified cells along a transcriptional gradient from a ‘Developmental’ state to an ‘Injury Response’ state [55,56]. On the other hand, Garofano and co-workers identified four tumor cell states in GBM (proliferative/progenitor, neuronal, mitochondrial and glycolytic/plurimetabolic) converging on two biological axes-neurodevelopmental and metabolic (Figure 1) [57].
Since long noncoding RNAs (lncRNAs) have important roles in development and progression of GBM, and tumor-associated immune processes, Yu and co-workers used immune-related lncRNAs signature for classification of GBM into four subtypes (A-D) (Figure 1), with GBM subtype A showing the most favorable prognosis [58]. Additionally, based on immunohistochemical results in combination with DNA copy number and DNA methylation profiles, Motomura and co-workers stratified GBM into four subtypes: oligodendrocyte precursor type (OPC), differentiated oligodendrocyte type (DOC), astrocytic mesenchymal type (AsMes) and mixed type (Figure 1, Supplementary Table S1) with OPC type showing the most favorable outcome [59]. Based on growth factors and cytokines expression profiles of GBM patients, Hu and co-workers identified three GBM subtypes (type I-III) (Figure 1, Supplementary Table S1) [60]. Prognosis is poorer for patients with GBM Type III compared to patients with Types I and II [60].
According to gene expression levels and overall survival of patients, three prognostic GBM subtypes were identified: invasive (poor), mitotic (favorable), and intermediate (Figure 1) [61].
Results of microarray analysis revealed the presence of two subtypes of GSCs, proneural and mesenchymal [43,62]. These two subtypes are phenotypically different; mesenchymal GSCs are more invasive, angiogenic and more resistant to radiotherapy compared to the proneural subtype [43,62]. Furthermore, a proneural subtype of GSCs might be switched to the mesenchymal upon certain conditions, such as radiation treatment [62], anti-angiogenic therapy [63], increased intracellular levels of reactive oxygen [64], upregulation of transglutaminase 2 (TGM2) [65], upregulation of N-Myc downstream regulated gene 1 (NDRG1) [66], activation of NFE2L2 transcriptional network [64], upregulation of TAZ expression [67], and activation of nuclear factor kappa B (NF-κB) [43].
Stratification of GBMs into different molecular subtypes enables the identification of markers significant for diagnosis, prognosis, and more effective treatment. Precise identification of GBM molecular subtypes is important for improving clinical management of GBM and might lead to targeted molecular therapy for GBM patients.
Molecular GBM stratification revealed that multiple signaling pathways are dysregulated in GBM. Thus, the development of therapies that are focused on targeting dysregulated signaling pathways in GBM provides a new avenue for improving the clinical management of GBM patients. Among others, dysregulated signaling pathways in GBM include SHH, Wnt/β-catenin, Notch, BMP, TGFβ, Hippo and RA signaling pathways. In this review, we have summarized the current data concerning the approaches for targeting these signaling pathways in GBM.
3. Targeting Sonic Hedgehog Signaling Pathway in GBM
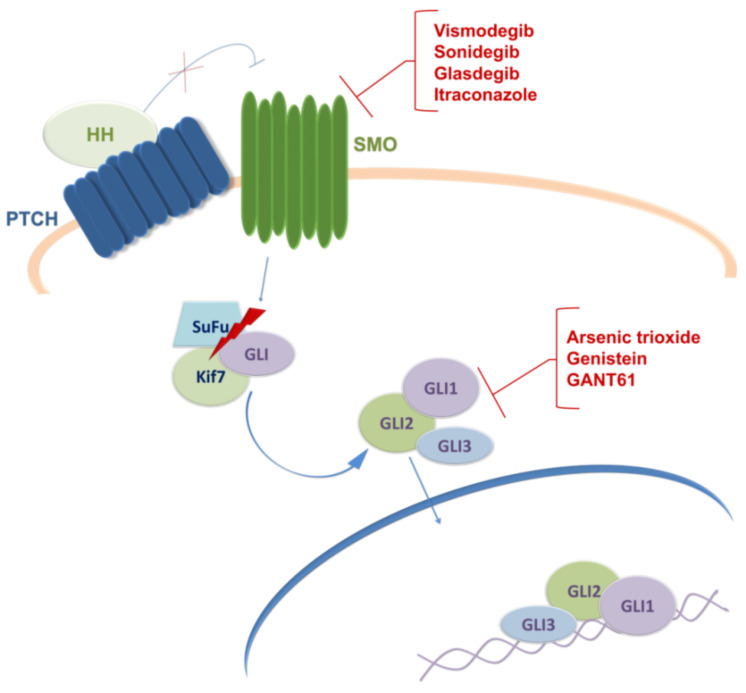
The Hedgehog (HH) signaling is a well-conserved pathway in animals that plays a pivotal role during embryonic development, tissue homeostasis, maintenance of adult stem cells and regeneration [68,69,70]. Its role is critical for the development of brain and spinal cord including midbrain and ventral forebrain neuronal differentiation and proliferation [71,72,73]. There are three mammalian HH ligand proteins, Sonic Hedgehog (SHH), Indian Hedgehog (IHH), and Desert Hedgehog (DHH). Canonical HH signaling occurs by binding HH ligand protein to a transmembrane receptor protein patched (PTCH) [74]. In the absence of HH ligands, PTCH functions as an inhibitor of another transmembrane protein smoothened (SMO). Binding of HH ligands to PTCH receptor relieves the suppression of SMO, resulting in downstream activation of final effectors, GLI transcription factors (GLI1, GLI2, and GLI3) responsible for the transmission of HH signaling to downstream target genes (Figure 2) [75,76].
Figure 2.
The canonical activation of HH pathway and its pharmaceutical inhibitors. The activation of pathway occurs when HH ligand binds to PTCH at the cell membrane. In response to this binding, PTCH no longer inhibits SMO and initiates the downstream signaling, causing rapid dissociation of the SuFu–GLI complex and thus allowing GLI to enter the nucleus and regulate transcription of target genes. Proven pharmacological inhibitors that target SHH signaling components (SMO receptor or GLI transcription factors) are presented in red. References are included in the main text.
Accumulating evidence suggests that aberrant activation of the HH signaling pathway by deregulation of any of its components may be involved in the development and progression of cancers and diseases. Accordingly, dysregulation of SHH signaling has been implicated in the initiation and/or maintenance of different tumor types including GBM [77,78]. The transcriptomics data on 149 clinical cases of The Cancer Genome Atlas-Glioblastoma database showed a strong correlation between PTCH1 and GLI1 upregulated expression in GBM indicating that activation of the canonical SHH pathway might be associated with this malignancy [79]. Furthermore, it was demonstrated that the endothelial cells within the tumor microenvironment (TME) provide the SHH ligand that activates the HH signaling pathway in GBM cells and promotes the appearance of GSCs, as demonstrated by increases in tumorigenicity and expression of stemness genes [80]. It has been revealed that GSCs highly express SHH and that targeting this signaling pathway may overcome chemoresistance and provide a novel therapeutic strategy [22,80,81].
Currently, there are two approaches to inhibiting SHH signaling: ligand-dependent approach, by antagonizing the SMO receptor, and ligand-independent approach, by inhibiting its final effectors, GLI transcription factors [77]. In preclinical studies, various SMO inhibitors exerted antiproliferative effect against tumor cells including those originating from GBM. Cyclopamine, isolated from Veratrum californicum plant, is a natural inhibitor of SMO that consequently blocks the SHH signaling pathway [77,82]. Carballo and co-workers presented data showing that SHH pathway inhibition with cyclopamine interferes with GBM cell viability and also suggested that cyclopamine inhibition of the SHH pathway prior to TMZ treatment could reduce the aggressiveness of the tumor cells by sensitizing the GSCs to TMZ [83]. Among SMO inhibitors, there are two inhibitors, vismodegib, and sonidegib, approved by the FDA for the treatment of locally advanced and metastatic basocellular carcinoma [84]. Bureta and co-workers presented data on the antiproliferative effect of vismodegib on GBM cell lines alone or in combination with arsenic trioxide (ATO) and TMZ [85].
Presently, there are several ongoing clinical trials evaluating SMO inhibitors for the treatment of different types of brain tumors. Initially, SMO was the principal target for the development of SHH inhibitors, and the first clinical trial performed using an SMO inhibitor to treat a brain tumor was conducted in 2008 in a male patient with metastatic medulloblastoma who was treated with a novel HH pathway inhibitor, GDC-0449 (vismodegib) [86]. Treatment led to rapid regression of the tumor and reduction in symptoms, but only transiently [86]. It was observed that the HH pathway inhibition resulted in an incomplete response that led to tumor re-growth, and unfortunately, the patient died after five months of treatment [86]. Vismodegib was evaluated in clinical trials against GBM, but so far, it has not demonstrated clinical benefit as a single agent. Patients with recurrent GBM, in a phase 2 trial, were randomized to a pre-operative and post-operative vismodegib group (group I) versus only the post-operative vismodegib group (group II), with the idea that HH pathway inhibitors selectively target GSCs. Although a significant decrease in the number of CD133+ neurospheres was observed in group I, vismodegib was not efficacious as a single agent in recurrent GBM [87]. It is important to highlight that the efficiency of vismodegib is still being evaluated in one clinical trial for medulloblastoma (NCT01878617) and one for GBM (NCT03158389). Another FDA-approved SMO inhibitor sonidegib was also evaluated in relapsed medulloblastoma in a phase 1/2 trial, with both adult and pediatric patients. Fifty percent of the patients with activated HH pathway in their tumor had a response to sonidegib, which was translated to longer disease-free survival [88]. Currently, two additional SMO inhibitors are under evaluation in clinical trials for the treatment of GBM, and they both belong to drugs approved for other diseases (NCT03466450; NCT02770378). The first is glasdegib, a small molecule inhibitor of SMO and FDA-approved medication for acute myeloid leukemia [89]. The other one is itraconazole, an antifungal drug used for the treatment of fungal infections, which is currently in phase I clinical trial in combination with TMZ (CUSP9v3 Treatment Protocol) for recurrent GBM. Previously, itraconazole has been explored as an anticancer agent for patients with basal cell carcinoma, non-small cell lung cancer, and prostate cancer [90,91,92,93].
Most HH inhibitors that have entered clinical trials targeted SMO, although several mechanisms of resistance to SMO inhibitors have been identified. This is why alternative SHH antagonists that act directly on GLI transcription factors are already being tested in the brain and central nervous system tumors as co-adjuvant therapy with TMZ (reviewed in [77]). ATO is a drug that is being tested for gliomas in phase I and II clinical trials. ATO is an FDA-approved drug which was first used for the treatment of patients with acute promyelocytic leukemia. It has been shown that ATO inhibits GLI-dependent growth in a medulloblastoma mouse model [94]. A recent study demonstrated that treatment of patients in combination with ATO, TMZ, and radiation apparently does not improve the overall outcome in GBM patients [95]. Genistein is an isoflavone found in legumes which is able to inhibit GLI1, and there was a clinical study of its potential against brain malignancies (NCT02624388); unfortunately, the study was terminated due to poor enrolment. However, clinical trials of genistein in cancer therapy have been conducted for other malignancies, including breast, bladder, and prostate cancer. Currently, its clinical applications are still limited due to its poor solubility and bioavailability [96]. Honorato and co-workers reported that selective ligand-independent inhibition of SHH by GANT-61 through targeting GLI1 increased the oxidative stress damage potentiating TMZ effect and inducing cell death in GBM cell lines [97]. Another preclinical study also showed that GANT61 sensitizes glioma cells to TMZ treatment [98]. However, currently, there are no data regarding its clinical evaluation.
Nowadays, the SMO receptor is the primary target used for the development of SHH pathway inhibitors, and there are several ongoing clinical trials for different types of brain tumors. On the other hand, several reports demonstrated that SHH could signal through a noncanonical route, and while it is still unclear how the cells select between canonical and non-canonical routes, it is understood that efficient targeting of downstream effectors (GLIs) could lead to promising results in clinical trials. In conclusion, it is considered that the best way to control the tumor recurrence is to evaluate and establish novel protocols combining SHH signaling inhibition with conventional therapies.
4. Targeting Canonical Wnt/β-Catenin Signaling Pathway in GBM
Wnt/β-catenin signaling pathway plays essential roles in embryonic development and the maintenance of homeostasis and regeneration of adult tissues [99,100]. Canonical Wnt/β-catenin signaling pathway is the most studied part of the complex and evolutionarily conserved Wnt signaling [101].
β-catenin is a central player in the canonical Wnt signaling pathway [102,103]. When Wnt signaling is inactive, the level of β-catenin is kept low due to the activity of the destruction complex in the cytoplasm [104]. The complex is consisting of APC (adenomatous polyposis coli), AXIN1/2 (axis inhibition proteins 1/2), CK1 (casein kinase 1), and GSK3β (glycogen synthase kinase 3β) [104]. Two scaffold proteins, APC and AXIN, bring β-catenin in the position for CK1/GSK3β-mediated phosphorylation which prime it for subsequent ubiquitination and proteasomal degradation [104,105]. Decreased concentration of β-catenin in a cytoplasm prevents its nuclear translocation. In the absence of β-catenin, members of TCF/LEF (T-cell factor/lymphoid enhancer-binding factor) families of transcription factors remain in complexes with corepressors, the Groucho and TLE (transducin-like enhancer), bound to Wnt responsive elements thus repressing transcription of Wnt target genes [106].
Activation of canonical Wnt signaling starts by binding of WNT ligands (family of 19 secreted glycoproteins in mammals) to the Fzd seven-transmembrane receptor (Frizzled) and LRP5/6 (co-receptor low-density lipoprotein receptor-related protein 5, 6) [107,108]. Upon activation, Fzd interacts with Dsh (Disheveled) cytoplasmic protein. Dsh also interacts with Axin, and LRP5/6, via Axin interactions with the coreceptor. These interactions inactivate β-catenin destruction complex [107,108]. Increased concentration of stabilized β-catenin in cytoplasm leads to its nuclear translocation. In the nucleus, β-catenin forms complexes with the members of the TCF/LEF family of transcription factors and co-activators, e.g., CBP (CREB-binding protein) and p300, and enhances transcription of Wnt target genes [106].
Aberrant activity of Wnt/β-catenin signaling is associated with diverse human diseases including cancer [99,100]. Aberrant activation of the Wnt/β-catenin signaling pathway is involved in GBM pathogenesis and progression, maintaining of GSC stemness and acquisition of radio- and chemotherapy resistance [15,109,110,111].
Mutations in key components of Wnt signaling e.g., APC, β-catenin, and AXIN are not hallmarks of GBM, although several studies linked mutations in Wnt signaling proteins to gliomagenesis. In a study on seven patients, Tang and co-workers showed that APC gene mutations occurred in 13% of cases, with a mutation frequency of 14.5% [112]. A study by Morris correlated homozygous deletion in tumor suppressor FAT1 (atypical cadherin 1), negative regulator of the Wnt pathway, with the activation of Wnt signaling. Homozygous deletion of FAT1 occurred in 57% of GBM and was associated with a prolonged survival [113,114]. A study by Sareddy and co-workers showed overexpression of PELP1 (proline-, glutamic acid-, and leucine-rich protein 1) in 100% of the GBM samples. PELP1 is co-regulator of several nuclear receptors, and a potent activator of β-catenin and Wnt signaling [115]. MicroRNA and lncRNA profiling of GBM versus the normal brain revealed the role of epigenetic mechanisms in the activation of Wnt signaling in GBM [116,117].
In search of anticancer therapeutics, numerous natural and synthetic antagonists and agonists were isolated or designed to target major cascades in Wnt/β-catenin signaling pathway, i.e., inhibitors targeting Wnt ligand/FZD receptor complex, inhibitors and agonists targeting the β-catenin-destruction complex and inhibitors targeting β-catenin/TCF complex [108]. Although the Wnt pathway is a validated target in cancer, and drugs targeting this pathway in various cancer types have entered the clinical trials, there are still no FDA-approved drugs targeting this pathway [118]. Unfortunately, only a few candidates have reached early phase clinical trials as therapies targeting GBM [24,119].
Extensive search and chemical screening for Wnt inhibitors identified numerous small molecules and biologics that targeted the Wnt signaling cascade [120]. Small molecules that act as Wnt agonists or antagonists, modulation of Wnt signaling activity induced by these molecules, and their effects on the properties of GBM cells and GSCs are listed in Table 1, Section A [121,122,123,124,125,126,127,128,129]. A schematic representation of their action on the Wnt signaling cascade is given in Figure 3.
Table 1.
Molecules targeting Wnt/β-catenin cascade, mechanisms of action and effects on GBM cells and GSCs: A: Small molecules; B: Natural agents; and C: Repurposed drugs.
| Molecule | Modulation of Wnt Signaling Activity |
Effects on GBM Cells and GSCs Properties | Reference |
|---|---|---|---|
| A: Small molecules | |||
| ONC201 | inhibits expression of components of Wnt pathway and Wnt targets | induces apoptosis in GBM cells induces cytotoxicity in chemo- and radiation-resistant GBM patient samples inhibits the growth of GSCs in 3D neurospheres established from human GBM tumors inhibits tumor growth in GBM mouse models |
[121,127] |
| SEN461 | induces AXIN stabilization | inhibits anchorage-independent growth of human GBM cell lines and patient-derived primary tumor cells reduces tumor growth in a mouse GBM xenograft model |
[122] |
| XAV939 | antagonist of tankyrase-enzymes involved in the degradation of AXIN | decreases the survival and clonogenicity of GBM cells reduces GSC population increases radiosensitization in in vivo radiation model derived from a single human GBM specimen |
[125] |
| LiCl SB216763 |
inhibits GSK3β | induces the expression of differentiation markers in GBM cells depletes GSCs population reduces colony formation and induces cell death in GBM cell-lines |
[126] |
| G007-LK | inhibitor of tankyrase-enzymes involved in the degradation of AXIN | decreases in vitro proliferation and sphere formation in primary GSC cultures reduction in GSC sphere formation in cotreatment with TMZ |
[124] |
| IC261 | inhibitor of CK1 | inhibits growth of GBM cells and GSCs in vitro and induces growth inhibition of human GBM xenografts in mice | [129] |
| LGK974 | inhibitor of porcupine proteins that modulate Wnt ligands | acts synergistically with TMZ to inhibit growth of GBM cells | [128] |
| ICG-001 | CBP antagonist | reduces proliferation and survival of GBM cells | [123] |
| AZD2858 | inhibits GSK-3β | reduces proliferation and survival of GBM cells inhibits the invasion and migration of GBM cells |
[123] |
| B: Natural agents | |||
| shikonin | inhibits β-catenin phosphorylation | inhibits proliferation, migration and invasion of GBM cells | [131] |
| Trichosanthin | inhibits expression of Wnt components | inhibits proliferation, invasion and migration and induces apoptosis of GBM cells | [132] |
| R. crenulata root extract | decreases nuclear localization of β-catenin | inhibits proliferation and tumorsphere formation and promotes differentiation of GBM cells | [133] |
| resveratrol | decreases expression of Wnt signaling components and Wnt targets | inhibits proliferation, motility and invasion of GSCs | [134] |
| carnosic acid | decreases expression of WISP1 | reduces GSC viability suppresses GSC tumorsphere formation inhibits the growth of GSC-derived xenografts |
[135] |
| Indirubin | inhibitor of GSK-3β | reduces invasion of GBM and GSC-enriched neurospheres both in vitro and in vivo improves survival of intracranial glioma-bearing mice |
[136] |
| DATS | decreases nuclear β-catenin level | inhibits cell growth, induces apoptosis and decreases migration and invasion in GBM cells | [137] |
| Sulforaphane | inhibits Wnt/β-catenin signaling | enhances TMZ-induced apoptosis | [138] |
| C: Repurposed drugs | |||
| NSAIDs diclofenac celecoxib aspirin |
reduces phosphorylation of GSK3β inhibits expression of Wnt targets |
inhibits proliferation, colony formation and migration of GBM cells inhibits proliferation and invasion and induces apoptosis of GBM cells |
[139] [140] |
| Niclosamide | decreases concentration of β-catenin in the nucleus | decreases cell viability, exerts antimigratory effects and inhibits the malignant potential of primary GBMs combined treatment with TMZ inhibits viability, stemness, and invasive properties of human GBM tumorspheres and decreases tumor growth in mouse xenograft models |
[141] [142] |
| QUE | decreases phosphorylation of GSK3β | suppresses GSCs-initiated tumor growth in mouse models of gliomas acts synergistically with TMZ to suppress growth of TMZ-resistant tumors originated from GSCs |
[143] |
| Pioglitazone | inhibits β-catenin expression | reduces cell viability, suppresses invasion and induces apoptosis of GBM cells induces decrease in cell viability and proliferation of GSC lines isolated from GBM patients |
[144] [145] |
LiCl, lithium chloride; DATS, garlic-derived diallyl trisulfide; NSAID, nonsteroidal anti-inflammatory drugs; QUE, Quetiapine.
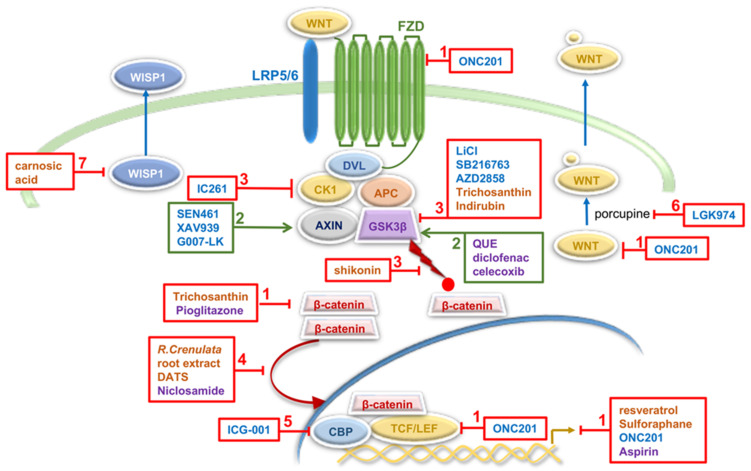
Figure 3.
Overview of the Wnt/β-catenin signaling cascade, targets for potential therapeutic intervention in GBM and molecules investigated in preclinical and clinical studies (based on the data listed in Table 1). Mechanisms of modulation of Wnt/β-catenin activity in GBM (described in detail in Table 1) include (1) down-regulation of expression of Wnt components and Wnt targets, (2) promotion of β-catenin degradation, (3) increasing β-catenin stability, (4) inhibition of β-catenin nuclear translocation, (5) inhibition of β-catenin/CBP transcription complex, (6) down-regulation of WNT secretion and (7) inhibition of Wnt/β-catenin-WISP1 signaling. Small molecules are presented in blue letters, natural agents in orange and repurposed drugs in purple. Molecules in red boxes inhibit expression and activity of denoted Wnt component or processes within the Wnt cascade. Molecules in green increase activity of denoted components. Red circle represents phosphate group; yellow circle represents palmitoyl groups. Based on [120,130] and references included in the main text and the Table 1.
The most promising agent is ONC201, a member of the imipridone class of small molecules. This dopamine receptor D2 antagonist is a widely studied inhibitor of GSC markers expression and suppressor of signaling pathways associated with GSC self-renewal, glioma-initiation and progression, and therapy resistance including Wnt ligands, receptors, and effectors (WNT16, FZD2, FZD4, TCF7L2) [127,146,147]. Additionally, ONC201 showed promising results in phase II clinical trial in patients with recurrent GBM [148]. ONC201 is currently in phase II clinical trial for patients with recurrent GBM and distinct subtypes of glial tumors H3 K27M mutant and Midline Glioma (NCT02525692).
Several biologics targeting Wnt signaling were investigated in various cancer types [120]. Fusion protein Ipafricept (OMP54F28; IPA), which competes with WNT ligands for binding to FZD8 receptor [149,150,151], and Vantictumab (OMP-18R5), a monoclonal antibody targeting FZD1, FZD2, FZD5, FZD7, and FZD8 receptors [150,151], act as Wnt/FZD antagonists and reached clinical I/II phase for breast, pancreatic, hepatocellular and ovarian cancer [120]. However, there are no available data about ongoing clinical trials or plans for further investigations of these biologics in GBM treatments.
Numerous natural products act as inhibitors of the canonical Wnt signaling and show significant therapeutic relevance in various cancer model systems [130]. They mainly act via β-catenin by regulating different steps involved in its stability and transcriptional activity, i.e., expression of β-catenin and its phosphorylation, degradation and nuclear translocation [130]. It is important to point out that about 33% of FDA-approved anticancer drugs are natural products and their derivatives [152] and that natural products represent a major source for drug discovery and development [153,154]. Natural agents acting through Wnt signaling in GBM are presented in Table 1, Section B [131,132,133,134,135,136,137,138]. They affect different processes in the Wnt cascade: β-catenin phosphorylation (shikonin), expression of key proteins in the Wnt/β-catenin signaling pathway (trichosanthin), nuclear translocation of β-catenin (R. crenulata root extract, DATS), expression of Wnt signaling components and Wnt targets (resveratrol, sulforaphane), activity of GSK-3β (indirubin), and Wnt/β-catenin-WISP1 signaling (carnosic acid) (Table 1, Section B and Figure 3) [131,132,133,134,135,136,137,138]. WISP1 (Wnt-induced signaling protein 1) is secreted by GSCs to facilitate a pro-tumor microenvironment and promote survival of both GSCs and tumor-associated macrophages [135].
One of the promising strategies to develop Wnt-targeting anti-GBM therapies is drug repurposing. Several studies revealed tumor-suppressive effects of various drug types: nonsteroidal anti-inflammatory drugs (NSAIDs); niclosamide, an anthelmintic drug for treating intestinal parasite infections; quetiapine (QUE), an atypical antipsychotic drug and pioglitazone, an anti-diabetic drug used to treat type 2 diabetes (Table 1, Section C and Figure 3) [139,140,141,142,143,144,145]. The most promising repurposed drug targeting Wnt signaling in GBM is celecoxib. This NSAID was tested in GBM patients in several phase I and II clinical trials in combination with other drugs. Some trials are still ongoing but preliminary results are not encouraging [24,155]. Tested in phase II trial in newly diagnosed GBMs, celecoxib had no survival benefit when combined with TMZ (NCT00112502) [24].
Several studies point to the translational potential of therapeutic approaches that combined Wnt pathway inhibitors and chemotherapy. For example, Huang and co-workers discovered that canonical Wnt signaling plays a crucial role in stemness activation and chemoresistance in GBM-associated vascular endothelial cells through the HGF/c-Met/β-catenin axis [156]. In endothelial cells under glioma conditions, HGF (hepatocyte growth factor) induces direct β-catenin phosphorylation at Ser675 by HGF receptor kinase c-Met, leading to the β-catenin nuclear translocation and LEF1-mediated activation of Wnt target genes [156]. RNA seq data revealed activation of genes associated with stemness that govern endothelial cells transformation into mesenchymal stem cell-like cells. In addition, they detected increased expression of ABCC1 (multidrug resistance-associated protein 1 (MRP-1)), which is responsible for drug efflux and chemoresistance of these cells. In further investigation of the potential therapeutic relevance of these findings, the authors examined the effects of treatment with Wnt inhibitor XAV939 and TMZ. The combined treatment of XAV939 and TMZ extended mouse survival and inhibited tumor growth, suggesting that Wnt inhibition with XAV939 sensitizes GBM to TMZ chemotherapy [156]. The authors also revealed that Wnt-mediated chemoresistance is potentially endothelial cells-selective mechanism in GBM [156].
In addition, canonical Wnt signaling is involved in one of the molecular pathways of the GBM cells vessel co-option [157], a mechanism of the tumor cells movement towards and along the pre-existing vasculature [158]. Griveau and co-workers described Olig2-Wnt7 signaling axis responsible for single-cell vessel co-option and consequent increased infiltration of patient-derived oligodendrocyte precursors-like cells with preservation of the blood–brain barrier (BBB) [159]. In contrast, Olig2- and Wnt7-negative astrocyte-like GBM cells show collective clusters vessel co-option, which leads to the destruction of the BBB and consequent inflammation [159,160]. It has been also shown that vessel co-option is invasion strategy for orthotopically implanted mouse, rat and human glioma cells and GSCs [161].
In vivo and ex vivo inhibition of Wnt signaling with porcupine inhibitor LGK974 reduced vessel co-option and improved survival in combination with TMZ treatment [159]. Additionally, in patient-derived proneural cell lines, LGK974 treatment down-regulated OLIG2 and WNT7A expression and up-regulated VEGFA, while treatment with VEGF inhibitor B20 increased expression of OLIG2. In vivo, LGK974 treatment increased VEGF expression, whereas B20 treatment increased Wnt activity [159]. In addition, up-regulation of both Wnt7a and Wnt7b were observed in U87 GBM cells resistant to bevacizumab compared to bevacizumab-sensitive U87 cells [159]. This is in concordance with the clinical studies that showed that vessel co-option occurs in some GBM subtypes intrinsically resistant to anti-angiogenic treatment, but it is also a mechanism of resistance to anti-angiogenic treatments (bevacizumab) in the innately angiogenic tumors [158,162]. The ability of GBM cells to switch between co-opting and angiogenic phenotype has great therapeutic relevance. Computational modeling of the inhibition of both mechanisms suggests that sequential inhibition (vessel co-option inhibition followed by anti-angiogenesis therapy) is more efficient than simultaneous blockage [163]. These findings also highlight the importance of classification of the GBM patients based on vessel co-option occurrence.
Inhibition of Wnt in GBM is also challenging due to the essential roles of this pathway in CNS vascularization and BBB integrity [100]. During CNS development, neural progenitors-derived Wnt7a and Wnt7b ligands activate canonical Wnt signaling in vascular endothelium, thus promoting vascularization of CNS and formation of BBB [164]. The same mechanism maintains integrity of BBB in adult brain. In brain endothelial cells, Wnt7 binds to the co-receptor complex RECK-GPR124, consisting of RECK (reversion-inducing cysteine-rich protein with Kazal motifs) and GPR124 (G protein-coupled receptor 124), to activate canonical Wnt signaling through Fz receptor [165,166]. Martin and co-workers took advantage of the selective binding of linker domain of Wnt7a to Fz receptor only in the presence of RECK-GPR124 complex to restrict Wnt activation to the endothelial cells of CNS and avoid their pleiotropic Fz signaling [167]. The authors engineered the Wnt7a mutant ligand, a Gpr124/Reck agonist that showed no off-target activity in the brain [167]. Delivered in the circulation of mouse model of brain tumor, by adeno-associated virus injection, the Gpr124/Reck agonist restored Wnt signaling in endothelial cells, normalized BBB function and reduced GBM expansion [167].
This approach also showed improved BBB function in cerebral artery occlusion model of stroke [167], which is in concordance with previous studies that highlighted the role of Wnt signaling activation in CNS vascularization during development and in hypoxic brain injury. Chavali and co-workers revealed that crosstalk between oligodendrocyte precursor cells (OPCs) and endothelial cells regulates vascular development in neonatal white matter in a Wnt-dependent manner [168]. The authors showed that in hypoxic brain injury, OPCs expressed the Wnt7A ligand, which resulted in paracrine activation of the canonical Wnt signaling in endothelial cells in their proximity and expression of Wnt targets Apcdd1 and Axin2 [168]. They also showed that loss of Wnt ligand production led to the decreased proliferation of endothelial cells and disrupted angiogenic sprouting [168]. The authors concluded that Wnt activity is essential for the maintenance of white matter integrity and that expression of Wnt7 in OPCs is an indicator of the white matter susceptibility to a hypoxic injury [168].
Other Wnt ligands also control brain vasculature in pathological conditions. Reis and co-workers showed that forced activation of canonical Wnt signaling in GBM endothelia, up-regulated by glioma-derived Wnt1, increased Dll4 (Delta-like 4) expression and induced Notch signaling, leading to an angiogenic blockage and quiescence of endothelial cells [169]. Additionally, transcriptional activity of β-catenin induced expression of PDGF-B (platelet derived growth factor B) and consequent recruitment of mural cells [169]. Both cascades led to the vessel stabilization and normalization of BBB function [169].
Although selective targeting of Wnt cascade in neuroendothelia seemed unachievable due to its pleiotropic mode of action, a study by Martin and co-workers provides a promising strategy for BBB-focused therapy approach in GBM [167].
5. Targeting Canonical Notch Signaling Pathway in GBM
Notch signaling is an ancient and evolutionarily conserved signaling pathway that plays a critical role in multiple cellular processes throughout life, including stem cell maintenance, cell fate decisions, cell proliferation and differentiation (reviewed in [170,171]). Accordingly, it was shown to be essential for neural stem cell maintenance and proper control of neurogenesis in both embryonic and adult central nervous system (CNS) [172]. Activation of Notch signaling can inhibit neurogenesis, maintain neural progenitor identity and, in certain settings, promote gliogenesis and drive binary fate choices, leading to various neuronal cell types (reviewed in [173]).
The canonical Notch signaling pathway involves activation of Notch receptor through series of proteolytic cleavages, resulting in translocation of Notch intracellular domain (NICD) to the nucleus, where it activates transcription of target genes through association with DNA-binding proteins and transcriptional co-activators (reviewed in [174,175]). Activation of Notch signaling is accomplished through physical interactions between the Notch receptor of the signal-receiving cell and the membrane-bound ligands of its neighbor, a signal-sending cell. There are four mammalian Notch receptor paralogs (Notch1, Notch2, Notch3 and Notch4), representing large single-pass type I transmembrane proteins that display redundant as well as unique functions [176,177]. Notch receptors in mammals are activated by five type I transmembrane, three Delta-like (Dll1, Dll3 and Dll4) and two Serrate/Jagged (Jag1 and Jag2) [175] ligands. After translation, Notch polypeptide undergoes a series of glycosylations in the endoplasmic reticulum (ER) [178,179] and then translocates into the Golgi apparatus, where it is cleaved by furin-like convertase (S1 cleavage) into a heterodimer in which Notch extracellular domain (NECD) and Notch transmembrane and intracellular domain are non-covalently bonded and transported to the cell membrane (Figure 4) [180,181]. Ligand binding triggers conformational change in the Notch receptor facilitating a second NECD cleavage (S2 cleavage) by ADAM (a disintegrin and metalloprotease) proteases [182,183]. This process detaches NECD from the cell surface leaving the membrane-bound Notch extracellular truncation (NEXT) fragment, which is then cleaved by γ-secretase complex to release the Notch intracellular domain (NICD) (Figure 4) [184,185]. NICD translocates to the nucleus, where it interacts with the DNA-binding protein CSL [186,187,188] and Mastermind-like transcriptional coactivators (MAML) [189]. The stable ternary complex that is formed [190,191,192] further recruits coactivators, such as histone acetyltransferases (CBP/p300) and chromatin remodeling complexes [193,194] to activate transcription of Notch target genes. On the other hand, when NICD is not present, CSL associates with ubiquitous corepressor (Co-R) proteins and histone deacetylases (HDACs) to repress transcription of Notch target genes. The first Notch target genes to be discovered included Hairy/Enhancer of split (HES) and HES-related repressor protein (HERP) gene families (also known as HES-related with YRPW motif- HEY) that encode basic helix–loop–helix transcriptional repressors that play important roles in lineage-commitment decisions [195,196].
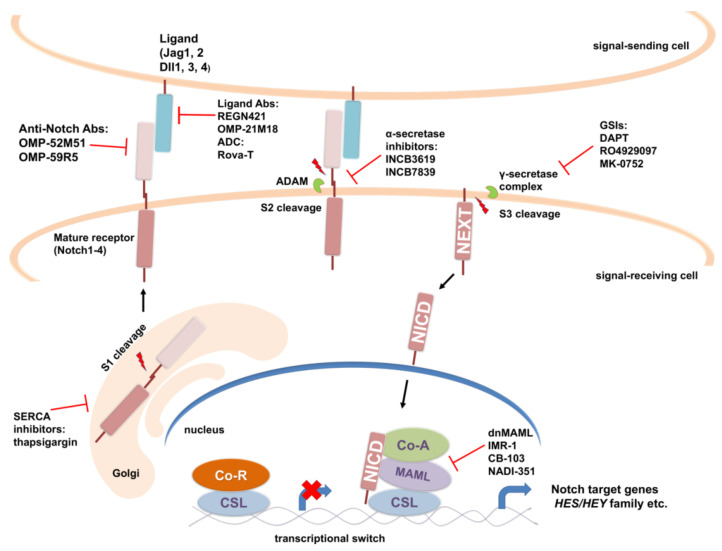
Figure 4.
Scheme of a canonical Notch signaling pathway and therapeutic targets. During transport through endoplasmic reticulum and Golgi apparatus, Notch precursor is glycosylated, cleaved into a heterodimer (S1 cleavage) and transported to the cell membrane. Binding with Notch ligand induces second cleavage (S2 cleavage) by a member of ADAM family of proteases, leaving membrane-bound Notch extracellular truncation (NEXT) fragment. NEXT is subsequently cleaved by γ-secretase complex (S3 cleavage) releasing the active form of the Notch receptor, Notch intracellular domain (NICD), which can translocate to the nucleus, where it activates transcription of Notch target genes by forming transcriptional complex with DNA-binding protein CSL (also known as CBF1/in mammals, Suppressor of Hairless in Drosophila, and LAG-1 in C. elegans) and MAML, which further recruits other transcriptional coactivators (Co-A). Classes of inhibitors and antibodies (Abs, ADC) that target Notch pathway components are indicated. References are included in the main text.
Notch signaling pathway is often deregulated in different cancers including gliomas. Various studies have demonstrated increased expression of distinct components of Notch signaling pathway (such as Notch1, Notch2, Dll1, Dll4 and Jag1) and Notch target genes (Hey1, Hey2, Hes1) in glioma cell lines or primary human glioma samples including GBM [197,198,199].
Depending on the context, Notch signaling pathway may exhibit oncogenic or tumor-suppressive functions in glioma (reviewed in [200]). It has been reported that increased Notch activity is correlated with improved patient survival in defined subsets of glioma [201]. Numerous studies support oncogenic function of Notch signaling in brain tumors. Downregulation of Notch1 receptor or its ligands through RNA interference inhibited proliferation and induced apoptosis of a variety of glioma cell lines and prolonged survival in a mouse orthotopic brain tumor model [198]. Activation of the Notch signaling pathway in GBM-derived neurosphere lines having stem cell-like properties is crucial for their growth in vitro and in vivo [202]. Hu and co-workers have shown that, as in the case with neural stem cells, Notch signaling plays a critical role in the maintenance of the patient-derived glioma stem cells by promoting their self-renewal and inhibiting their differentiation [33]. Endothelial cells in GBM actively participate in this process by providing Notch ligands to Notch receptors expressed in GBM cancer stem-like cells, thereby generating a stem cell niche that enables their self-renewal [203]. Notch signaling also plays a critical role in promoting radioresistance of GSCs via activation of the PI3K/Akt pathway [204] as well as in chemoprotection and repopulation of TMZ-treated gliomas [205].
To date, several approaches for targeting Notch signaling in vitro and in vivo have been developed, with two major classes of Notch inhibitors emerging as promising for the clinical development (Figure 4). In the treatment of cancer, the most utilized are γ-secretase inhibitors (GSIs), which block S3 cleavage and the release of the active form of Notch receptor (NICD) by the γ-secretase complex. The other major approach is usage of monoclonal antibodies (mAbs) against specific Notch ligands or receptors to interfere with their interaction or activation. GSIs were originally developed for the treatment of Alzheimer’s disease because they inhibit cleavage of the amyloid precursor protein that leads to generation of neurotoxic amyloid β-protein. Numerous reports provide evidence of the effectiveness of GSIs against GBM alone or in combination with other therapeutic approaches [206]. One of the most widely used GSIs—DAPT (also known as GSI-IX)—augmented the effect of radiation and reduced proliferation and self-renewal of tumor cells as well as proliferation of endothelial cells, thereby hampering the perivascular niche in GBM explants [207]. Other studies have also shown that Notch blockage by GSIs (DAPT and GSI-I) enhance radiosensitivity of CD133+ GSCs [204,208]. One of the important findings was that the subset of GBM-derived tumor-initiating cells sensitive to three structurally distinct GSIs (DAPT, RO4929097 and BMS-708163) are characterized by a signature enriched in proneural genes and high Notch activity [209]. Notch inhibition by GSIs led to reduced proliferation and self-renewal of these responder GBM-derived tumor-initiating cells and their neuronal and astrocytic differentiation. However, clinical application of these findings is not straightforward because of the intratumoral heterogeneity of GBM. Namely, only about 50% of proneural GBMs also have Notch pathway signature [209].
Although numerous in vitro and in vivo preclinical studies of anticancer effect of GSIs in GBM have shown promising results, to date, only two GSIs, RO4929097and MK-0752, have been tested in clinical trials in that respect. Phase 0/I trial was conducted to evaluate the effect of chemo-radiotherapy in combination with RO4929097 in patients with newly diagnosed GBM or anaplastic astrocytoma [210] (NCT01119599). The combination of RO4929097 with TMZ and radiotherapy was well tolerated, and no dose-limiting toxicities were observed. A substantial reduction in proliferation and NICD expression by tumor cells and blood vessels was detected. Treatment with RO4929097 resulted in specific reduction in the CD133+ cancer-initiating cells population in patient-derived tumor explant cultures. There was a modulation of glioma vasculature during RO4929097 treatment. However, in about one-third of the patients, there was tumor recurrence, which might be the result of tumors switching to a Notch-independent angiogenic profile, underscoring the need of targeting multiple signaling pathways simultaneously in gliomas. Another phase I clinical trial has addressed the issue of angiogenesis, where combined effect of RO4929097 and bevacizumab (Vascular endothelial growth factor inhibitor) in patients with recurrent malignant glioma was evaluated [211] (NCT01189240). The combination of RO4929097 and bevacizumab was well tolerated, but definitive conclusions regarding clinical activity of the drug combination could not be made because of the small number of patients enrolled in the study. A phase II trial of RO4929097 for patients with recurrent and progressive GBM (NCT01122901) demonstrated the lack of activity of RO4929097 against recurrent GBM with minimal inhibition of neurosphere formation in fresh tissue samples [212]. Poor clinical activity of RO4929097 could be explained by autoinduction of RO4909097 metabolism, since it increases CYP3A4 activity in vivo, which might result in a decrease in steady-state drug levels [213]. Another selective GSI, MK-0752, has undergone a phase I trial in children with refractory or recurrent CNS malignancies including GBM [214] (NCT00572182). MK-0752 was well tolerated; however, most patients experienced progression of their disease except for two patients: one patient with ependymoma and one patient with GBM that experienced prolonged stable disease. A phase I pharmacologic and pharmacodynamic study of the GSI MK-0752 in adult patients with advanced solid tumors did not show any clinical activity of MK-0752 in extracranial solid tumors, but a modest level of activity of this drug was observed in patients with various types of glioma [215] (NCT00106145). One patient with an anaplastic astrocytoma had a complete response, and ten patients with various gliomas including GBM had prolonged stable disease.
GSIs cause severe intestinal toxicity on account of inhibiting overall Notch pathway [216,217]. Besides processing Notch receptors, γ-secretase complex cleaves a multitude of other membrane proteins affecting other signaling pathways and likely contributing to the GSIs toxicity [218,219]. In order to inhibit Notch signaling pathway more specifically than GSIs, the researchers have been developing antibodies specifically targeting different receptors and ligands of the Notch signaling pathway (Figure 4). There are two classes of antibodies against Notch receptors: (i) antibodies that target Notch negative regulatory region (NRR) preventing the S2 cleavage by ADAMs necessary for activation of receptor; (ii) antibodies that block interactions between Notch receptor and ligand by targeting epidermal growth factor (EGF)-like repeats of the receptor necessary for ligand binding [220,221]. Antibodies targeting Notch1 (Brontictuzumab, OMP-52M51; NCT01778439), Notch2/Notch3 (Tarextumab, OMP-59R5; NCT01277146) and DLL4 (Enoticumab, REGN421, NCT00187159; Demcizumab, OMP-21M18, NCT00744563) have been tested in phase I trials in patients with solid tumors; however, no GBM patients were included.
Antibodies directed at two or three targets/pathways at the same time (bi- or trispecific IgG-like molecules) have been an attractive and promising strategy in anticancer therapy. In preclinical studies, dual-variable domain immunoglobulin targeting simultaneously DLL4 and VEGF (ABT-165, dilpacimab), significantly inhibited tumor growth and decreased functional tumor angiogenesis in U87-MG human GBM xenograft model [222]. Additionally, in combination with TMZ, ABT-165 substantially increased tumor growth delay compared with either monotherapy alone.
Another approach for more specific targeting of Notch in cancer cells involves usage of antibody–drug conjugates (ADC) that target specific antigens (Notch receptor or ligand) highly expressed in tumor cells to deliver a cytotoxic drug (known as the “payload”) to them [223]. In that context, Notch ligand DLL3 represents an attractive therapeutic target, since it is highly and homogenously expressed in IDH mutant gliomas and rarely detected in non-tumor brain tissue [224]. In vitro studies have shown that patient-derived IDH mutant glioma tumorspheres could be effectively targeted by the anti-DLL3-ADC, rovalpituzumab tesirine (Rova-T) [224]. This first-in-class anti-DLL3-ADC showed promising results in a phase I study of antitumor activity in patients with recurrent small-cell lung carcinoma (SCLC) that exhibit high DLL3 expression (NCT01901653) [225]. However, further work on Rova-T was terminated because later phase III studies showed a lack of survival benefit for patients with SCLC [226,227]. Nevertheless, DLL3 remains an attractive target for the development of new, improved antibody-based biologics.
Another strategy for inhibiting Notch signaling pathway that has emerged over the years is to block the Notch transcriptional complex using dominant-negative form of the MAML coactivator, stapled peptides or small molecules that interfere with protein–protein interactions between the components of the NICD-CSL-MAML transcriptional complex (Figure 4). The lentivirally expressed dominant negative form of Notch coactivator MAML1 (dnMAML1) has been shown to significantly inhibit Notch signaling and reduce GBM cell growth in vitro and in vivo through induction of G0/G1 cell cycle arrest and apoptosis in GBM cell lines [228]. Opačak-Bernardi and co-workers have developed conjugate of dnMAML peptide, thermo-responsive elastin-like polypeptide (for targeted delivery to tumor cells) and cell penetrating peptide (for enhanced cellular uptake and BBB penetration) which was efficiently delivered to GBM cells, causing cell cycle arrest, apoptosis and downregulation of Notch target genes Hes-1 and Hey-L [229].
Small-molecule inhibitors of the Notch transcriptional complex, such as IMR-1 (prevents MAML1 recruitment to the transcription complex), CB-103 (interferes with assembly of the Notch transcription complex), and NADI-351 (first specific inhibitor of Notch1 transcriptional complex) have exhibited antitumor activity in different xenograft models [230,231,232]. Additionally, antitumor activity of recently discovered small organic molecules (JI051 and JI130) that impair the ability of Hes1 to repress transcription have been demonstrated [233]. These molecules represent an attractive avenue to pursue in the treatment of GBM.
Attempts have been made to inhibit ADAMs, a family of α-secretases that cleave Notch extracellular domain upon ligand binding (Figure 4). Floyd and co-workers reported that an α-secretase inhibitor, INCB3619, decreased proliferation of GBM cell lines as well as GBM stem cell lines mainly through Notch inhibition [234]. Moreover, INCB3619 inhibited tumor growth and prolonged the survival in a human GBM stem cell xenograft model in mice. There is an ongoing phase I trial of INCB7839 (aderbasib), inhibitor of the ADAM 10 and 17 proteases, for children with recurrent or progressive high-grade gliomas (NCT04295759). A group of natural compounds (e.g., thapsigargin) that inhibit sarco-endoplasmic reticulum Ca2+-ATPase (SERCA) and affect intracellular trafficking of the Notch receptor causing accumulation of unprocessed Notch1 in the ER/Golgi compartment have emerged as potential therapeutics in cancers associated with NOTCH1 mutations [235]. In a phase II trial of mipsagargin (a thapsigargin prodrug) in patients with recurrent or progressive GBM (NCT02067156), drug treatment led to disease stabilization in 22% of patients [236].
A number of FDA-approved drugs used to treat other diseases/cancers are able to modulate Notch signaling in different cancer cell lines and could potentially be considered for treatment of GBM. These encompass histone deacetylase (HDAC) inhibitors (e.g., vorinostat) [237], enhancer of zeste homolog 2 (EZH2) inhibitors (which now include FDA-approved drug tazemetostat) [238], a thalidomide derivative lenalidomide [239], and antibiotic quinomycin A [240], most of which have been shown to have an antitumor effect on GBM cell lines in vitro or in vivo [241,242,243]. In addition to blockage of the SHH signaling pathway, ATO downregulates Notch signaling by decreasing the levels of Notch1 and Hes1 proteins having an inhibitory effect on GBM cancer stem-like cells in vitro and in vivo [244]. The other study showed that ATO depletes cancer stem-like cells in GBM and inhibits neurosphere recovery and secondary neurosphere formation by inhibition of the phosphorylation and activation of Akt and STAT3 through blockade of Notch signaling [245]. N-acetylcysteine (NAC) is a sulfhydryl-containing compound, with antioxidant, anti-inflammatory and mucolytic properties, initially used for treating cystic fibrosis, acetaminophen overdose and chronic obstructive lung disease [246]. Recently, Deng and co-workers revealed that NAC could efficiently inhibit Notch2 and its downstream signaling by facilitating Notch 2 degradation through lysosome pathway in GBM cell lines [247]. Moreover, NAC was able to reduce proliferation and induce apoptosis in vitro, and to suppress tumor growth of GBM cells in vivo.
Over the years, a great amount of data regarding the ability of different natural products and their derivatives to modulate Notch signaling and to alter the malignant properties of various cancer cells types have been accumulated (reviewed in [248,249]). They include compounds such as quercetin [250], curcumin [251], butein and its derivative chalcone 8 [252], honokiol [253], resveratrol [254], xanthohumol [255], ginsenoside [256], DATS [257], artemisinin [258], luteolin [259] juglone [260], withaferin A [261] and cucurbitacin B [262]. Most of them display antitumor effect on GBM cells in vitro or in vivo through various mechanisms and by affecting multiple signaling pathways, thereby representing promising alternative or adjuvant therapeutics that need to be further explored to improve outcomes of GBM patients.
The list of developed Notch-targeting approaches for combatting cancer is quite remarkable and is constantly expanding. However, a lot of challenges have to be overcome for successful translation of preclinical results into the clinical setting, including a reduction in drug toxicity associated with Notch inhibition, identification of reliable biomarkers of Notch activity for stratification of patients that would benefit from Notch-targeting therapy, and use of Notch therapies in combination with other agents or conventional chemotherapy or radiotherapy to affect multiple targets and cancer-associated processes simultaneously.
6. Targeting TGFβ Signaling Pathway in GBM
TGFβ (Transforming growth factor) is a multifunctional cytokine that plays important roles in the regulation of development and differentiation as well as adult tissue homeostasis (reviewed in [263,264]). In particular, TGFβ is crucial for every step of neural development and is expressed in neurons, astrocytes, and microglia (reviewed in [265]). It is shown that TGFβ and WNT signaling crosstalk controls the growth and size of the developing brain by regulating neural stem cell maintenance and differentiation [266].
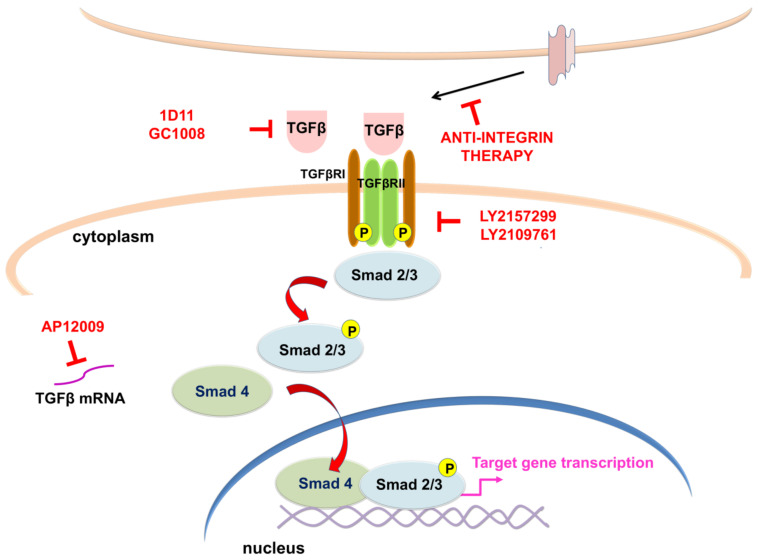
The TGFβ family comprises proteins divided into the following: (I) the TGFβ subfamily, which contains TGFβ, activin beta chains, and the protein Nodal, and (II) the bone morphogenetic protein (BMP) subfamily that comprises BMPs, growth differentiation factors (GDFs), and mullerian inhibitory factor (MIF) (reviewed in [267]). In mammals, there are three isoforms of TGFβ (TGFβ1, 2 and 3) [268,269,270]. After activation, TGFβ ligands bind to TGFβ receptor II (TGFβRII), which is a constitutive active kinase that phosphorylates TGFβ receptor I (TGFβRI), thus enabling the transduction of extracellular signal into the cell (Figure 5). In the canonical (Smad-dependent) pathway, TGFβRI activates receptor-regulated (R-) Smad proteins (Smad 2/3), which form transcription regulatory complexes with the Co-Smad (Smad 4) and translocate into the nucleus where they bind to target DNA sequences to regulate the transcription of numerous genes (reviewed in [264,271]). Smad proteins which have an inhibitory function (I-Smads- Smad 6 and 7) suppress phosphorylation and nuclear translocation of Smad 2/3, thus regulating TGFβ through negative feedback mechanism [272] (Figure 5). In addition to the canonical pathway, TGFβ has the ability to activate various signaling pathways. In the non-canonical (Smad-independent) pathway, TGFβ triggers mitogen-activated protein kinases (MAPK) pathways, such as ERK1/ERK2, Jun-N terminal kinase (JNK) and p38 and PI3K kinases (reviewed in [273]).
Figure 5.
Schematic representation of the canonical (Smad-dependent) TGFβ pathway and TGFβ signaling therapies which have undergone clinical trials in GBM. TGFβ binding to TGFβ receptors II (TGFβRII) results in phosphorylation and activation of TGFβ receptors I (TGFβRI), phosphorylation of Smad 2/3, which interact with Smad 4 and form a complex that translocates into the nucleus to activate target genes. Antisense oligonucleotides (AP12009), anti-integrins, kinase inhibitors (LY2157299, LY2109761) or neutralizing antibodies (1D11, GC1008) used for targeting TGFβ signaling pathway are presented in red. Based on [274] and references included in the main text.
Under pathological conditions such as neurodegenerative diseases, injury, and cancer, a significant increase in the expression of TGFβ was noticed (reviewed in [275,276,277]). Increased mRNA levels of the TGFβ isoforms were detected in GBM, which correlated with the degree of malignancy and prognosis [278]. TGFβ promotes proliferation of gliomas [279,280], invasion [281], angiogenesis (reviewed in [282]), and maintenance of stemness of patient-derived GSCs via the TGFβ-Sox4-Sox2 pathway [34]. Depending on the stage of malignancy, TGFβ has a dual function. At an early stage of the tumor, TGFβ reduces cell proliferation, promotes cell cycle arrest, induces apoptosis, and induces DNA damage in malignant cells acting as a tumor suppressor (reviewed in [277,283,284]). Later, in an advanced stage of malignancy, TGFβ acts as a tumor promoter directly inducing epithelial–mesenchymal transition (EMT), invasion, and metastasis of malignant cells, or indirectly, by promoting angiogenesis (reviewed in [277,285,286]). TGFβ-Smad activity is elevated in aggressive, highly proliferative gliomas and is related to poor patient prognosis [279].
Recently, many studies highlighted the significance of TGFβ in actively shaping and developing the glioma TME. The TME consists of different cells and extracellular materials surrounding the tumor which are responsible for promoting tumorigenesis through processes such as invasion, metastasis, and resistance to therapy. Studies revealed that various cell types, non-immune and immune cells, are associated with TGFβ activation and secretion during cancer progression. Accumulating evidence support the fact TGFβ has a dual role in glioma (reviewed in [287]). TGFβ secreted by glioma cells maintains tumor growth through inducing immune cells to become immunosuppressive, leading to the lack of an effective immune response against gliomas and formation of a permissive microenvironment. On the other hand, TGFβ produced by immune cells upregulates TGFβRI and TGFβRII on glioma cells and supports tumor progression (reviewed in [288]). Further, TGFβ controls differentiation, angiogenesis, and metabolic reprogramming of stromal cells of the TME during tumorigenesis (reviewed in [289]).
Growing evidence suggests that inhibition of TGFβ signaling could provide novel therapeutic options for treating GBM. Knock-down of TGFβ or TGFβ receptors has been shown to limit migration, invasion, and tumorigenicity of glioma cells [281,290]. Numerous different strategies to target TGFβ signaling pathway in GBM have been established and tested in clinical trials including antisense oligonucleotides, neutralizing antibodies, and kinase inhibitors. One of the approaches tested in clinical trials is usage of antisense oligonucleotides (ASOs). ASOs are specifically designed to bind TGFβ mRNA and inhibit its translation thus decreasing its expression. AP 12009 (Trabedersen) is a synthetic phosphorothioate oligodeoxynucleotide that targets one of the TGFβ isoforms, TGFβ2 mRNA (reviewed in [291,292]). Hau and co-workers performed in vitro experiments that demonstrated the specificity and efficacy of AP 12009 in patient-derived malignant glioma cells [293]. They also showed that AP 12009 treatment reversed the immunosuppressive effects of tumor-derived TGFβ. In an autologous cytotoxicity assay, where peripheral blood mononuclear cells isolated from glioma patients were activated by IL-2 and co-incubated with the glioma cells from the same patient, treatment with AP 12009 restored their cytotoxic activity against glioma cells. Additionally, three phase I/II studies confirmed that AP 12009 achieved safety and tolerability in patients with recurrent or refractory malignant (high-grade) glioma, anaplastic astrocytoma (WHO grade III) or GBM. Most importantly, in two patients, complete tumor remission was detected, showing promising efficacy of AP 12009 [293]. Lastly, the phase III clinical trial (NCT00761280) using AP 12009 in the treatment of recurrent or refractory anaplastic astrocytoma or secondary GBM, has been terminated due to insufficient recruitment of patients.
Anti-integrin therapy is considered a promising strategy for inhibition of processes involved in the GBM progression [294]. Roth and co-workers demonstrated that cilengitide, a selective integrin inhibitor, reduced phosphorylation of Smad 2 in vivo, confirming that integrins control TGFβ pathway in GBM [294]. A phase III clinical trial CENTRIC (NCT00689221) was conducted to determine overall survival as well as efficacy and safety of cilengitide in combination with standard chemoradiotherapy, compared to standard treatment alone, in newly diagnosed GBM patients with methylated O6-methylguanine-DNA-methyltransferase (MGMT) gene promoter. Unfortunately, the clinical trial revealed no improvement in overall survival or progression-free survival of patients [295].
Kinase inhibitors reduce TGFβ kinase activity, thus modulating downstream signaling transduction. In various tumors, including GBM, it is well documented that kinase inhibitors can decrease tumor growth and metastasis, and prevent recurrence and angiogenesis in mouse models [296,297,298,299]. In particular, LY2109761, a TGFβRI kinase inhibitor, reduced the survival of U87 and T98 glioma cell lines, and inhibited migration and angiogenesis. Furthermore, LY2109761 delayed tumor growth in vivo alone or in combination with radiation and TMZ [299]. Galunisertib (LY2157299), a small-molecule inhibitor, reduces a kinase activity of TGFβRI in Smad 2/3 phosphorylation. In a preclinical study, Yingling and co-workers confirmed anti-tumor activity of galunisertib in vitro and in vivo [300]. However, a phase I/II clinical trial showed that galunisertib treatment with TMZ-based chemoradiation had no clinical benefit compared to standard TMZ-based chemoradiation [301]. Further, in another phase II study, treatment by galunisertib in combination with lomustine in patients with recurrent glioma did not improve overall survival relative to placebo with lomustine [302]. Spender and co-workers reported preclinical study regarding antitumor effect of two TGFβRI inhibitors, AZ12601011 and AZ12799734. They demonstrated that AZ12601011 and AZ12799734 were more effective in inhibiting TGFβRI-induced transcription and migration than galunisertib. Additionally, the authors confirmed inhibition of tumor growth and metastasis in vivo using a murine model of breast cancer [303].
Preventing TGFβ signaling transduction is possible by administration of antibodies against ligand or its receptors. To accomplish this objective, several antibodies against TGFβ are developed. Literature data revealed that inhibition of TGFβ signaling by applying TGFβ-neutralizing monoclonal antibody 1D11 increased glioma-associated antigen peptide vaccines efficiency in mice [304]. Further, Hulper and co-workers have demonstrated that 1D11 TGFβ neutralizing antibody can be detected in subcutaneous and intracranial gliomas after intravenous injection [305]. Nevertheless, 1D11 treatment of gliomas had diverse effects on the gliomas in immunocompetent and immunodeficient mice. 1D11 treatment of immunocompetent mice bearing subcutaneous glioma resulted in tumor remission, while the same treatment in immunodeficient mice increased tumor size. Additionally, 1D11 treatment of intracranially implanted gliomas impaired glioma cell invasion in normal brain tissue but did not reduce tumor size [305]. One of the important aspects of treating glioma is the permeability of BBB that often prevents the drugs to reach the target site. Radiolabeled human IgG4 monoclonal antibody that recognizes and neutralize TGFβ, 89Zr-GC1008, showed excellent uptake in patients with recurrent high-grade gliomas with no observed toxicity (NCT01472731). However, 89Zr-GC1008 did not demonstrate an antitumor effect [306].
GSCs, which cause GBM tumor recurrences, are able to inhibit natural killer (NK) cell activity via releasing and activation of TGFβ, thus evading immune attack. It was shown that genetic disruption of TGFβR2 in NK cells results in antitumor activity in vivo [307]. There is an ongoing phase I trial (NCT04991870) conducted to evaluate the best dose, possible benefits and side effects of engineered NK cells, with deleted TGFβRII and glucocorticoid receptor NR3C1, for the treatment of recurrent GBM (reviewed in [308]). Additionally, the trial is aimed to determine overall survival, duration of clinical response, progression-free survival and time to progression.
Using bioactive compounds in combination therapy could be a promising strategy for targeting TGFβ signaling pathways. It was shown that resveratrol, a polyphenolic compound obtained from plants, modulates TGFβ signaling (reviewed in [309]) and exhibits the antitumor effect in various tumors [310,311]. In particular, resveratrol suppresses EMT, migration, invasion, and EMT-generated stem cell-like properties in GBM in vitro via canonical TGFβ signaling, and also inhibits the EMT process in vivo [312].
Taken together, clinical trials data presented above display no sufficient benefit of targeting TGFβ signaling in patients with GBM. The complexity of TGFβ signaling itself as well as interactions with various signaling pathways could explain the discouraging results of clinical studies regarding inhibition of TGFβ signaling in GBM.
7. Targeting BMP Signaling Pathway in GBM
The bone morphogenetic proteins (BMPs), the largest part of the TGFβ family, are important regulators of a multitude of processes during embryonic development and homeostasis (reviewed in [313,314,315,316,317]). The body of literature indicates that in the CNS, these proteins have a pleiotropic role during neural stem cell (NSC) proliferation, apoptosis, cell fate decisions and maturation. Furthermore, in the adult neurogenic niches, subventricular and subgranular zones, BMPs are critical for proliferation, maintenance, and survival of NSCs as well as terminal differentiation of newborn neurons (reviewed in [314,315,318,319]), thus profoundly affecting the homeostasis in the adult brain. Interestingly, in contrast to early development, NSCs derived from older animals undergo astrocytic differentiation in response to BMPs (reviewed in [320]).
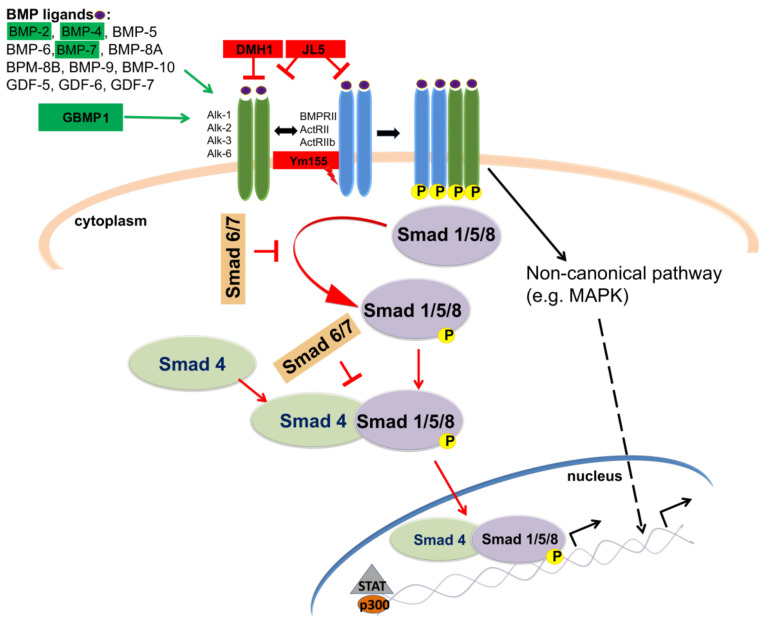
BMP ligands exert their activities in the cells through both canonical and non-canonical pathways (Figure 6) (reviewed in [317,321]). In the canonical signaling pathway, BMP ligands bind to the cell surface receptors to form a heterotetrameric complex, comprising two dimers of type I and type II serine/threonine kinase receptors. There are three “type I” receptors, type 1A BMP (BMPR-1A or ALK3), type 1B BMP (BMPR-1B or ALK6), and type 1A activin receptor (ActR-1A or ALK2) and three “type II” receptors, type 2 BMP (BMPR-2), type 2 activin (ActR-2A), and type 2B activin receptor (ActR-2B). Following the formation of a heterotetrameric complex, the constitutively active type II receptor trans-phosphorylates the type I receptor and allows phosphorylation of the immediately downstream substrate proteins known as the receptor-regulated Smads (R-Smads): Smad 1, 5, and 8 (Figure 6). R-Smads then associate with the Smad 4, and this complex translocates to the nucleus, where it functions as a transcription factor with coactivators and corepressors to regulate gene expression, as already mentioned in the previous section. In addition, non-canonical (Smad-independent) pathways for BMPs can also lead to regulation of gene expression [321]. It has been found to affect different components including TAK-1, a serine–threonine kinase of the MAPK family, PI3K/Akt, P/kc, Rho-GTPases.
Figure 6.
Overview of the canonical BMP cell signaling pathway and molecules investigated for potential therapeutic intervention in GBM. In canonical pathway, various BMP ligands binds to two receptor types (type I and type II) to form a heterotetrameric complex, which then binds to and phosphorylates the receptor-activated Smad 1, Smad 5 and Smad 8. Extracellular inhibitors of BMPs including Noggin, Chordin, and Gremlin inhibit activity of this signaling pathway. Activated Smads (Smad 1, 5, and 8) form complexes with Smad 4, enter the cell nucleus and in combination with co-binding partners, such as p300 or STAT, act as transcription factors and activate multiple gene expression. BMP ligands that activate BMP signaling in the GBM and GSCs are in the green boxes. Receptor inhibitors that suppress BMP signaling in the GBM and GSCs are in the red boxes. DMH1 targets BMP type 1 receptors, JL5 inhibits both the type 1 and type 2 BMP receptors. Ym155 does not bind to the BMP receptors but induces the degradation of BMPR2. References are included in the main text.
Growing data demonstrate that some BMPs are implicated in different pathologies, including processes related to carcinogenesis, such as angiogenesis and EMT, and driving cancer stem cell resistance to treatments (reviewed in [322,323,324,325,326]). Functional studies revealed that depending on the type of cell, TME, epigenetic background of the patient, or stage of tumor growth, some BMPs can be linked to tumor progression, while others can serve as tumor suppressors (reviewed in [325,327,328]). In glioma, expression analysis of molecular components of the BMP pathway revealed significant differences in the level of expression in tumor tissues that were associated with tumor grade and prognosis pointing to them as novel biomarker with potential important therapeutic implications [329,330,331].
Results of previous in vitro and in vivo studies indicate that BMPs are among the most potent therapeutics in preventing the growth and recurrence of GBM. Exposure of stem-like brain tumor cells to neurosphere-derived BMP7 induced differentiation, reduced stem-like marker expression, self-renewal, and the ability for tumor initiation in mice [332]. Similarly, the BMP7 variant decreased proliferation and induced differentiation of GBM stem-like cells and inhibited angiogenic endothelial cord formation [333]. In the same study, the mouse models with subcutaneously or orthotopically implanted GBM stem-like cells reflected in vitro results [333]. In the study where BMP7 was encapsulated in microspheres in the form which provided an effective release for several weeks, primary tumors showed a growth delay. This effect was correlated with the activation of the BMP canonical pathway [334]. BMP4 is another cytokine whose expression is significantly lower in glioma tumor tissue compared to adjacent normal tissue [330]. Previous studies also showed that BMP4 inhibited cell proliferation and induced apoptosis in GSCs [335] and GBM [336] and initiated GBM-derived stem cell astrocyte differentiation [35]. Liu and co-workers detected decreased expression of BMP4 in the multi-drug resistant (MDR) U251 cell line (U251/TMZ) compared to the parental U251 cells. In line with this result, the overexpression of BMP4 in U251/TMZ cells abolished the MDR both in vitro and in vivo through modulation of Bcl-2 and GDNF [337]. BMP2 also makes GSCs more susceptible to TMZ treatment through destabilization of HIF-1 [338], further suggesting BMPs are promising candidates for GSC-targeting GBM therapy. However, the main obstacle could be that glioma stem cells have mechanisms to avoid BMP-induced differentiation as they express a BMP antagonist Gremlin1 [339].
A recent study on patient-derived GSCs showed that treatment with BMP4 caused downregulation of stem cell marker CD133 expression, induced asymmetric cell division and suppressed self-renewal ability of cells [340]. An extensive study using 40 different human GBM-initiating cell cultures demonstrated an extensive diversity in the inhibitory effect of BMP4 on the tumor growth between cell lines [341]. Responsiveness to BMP4 was correlated with a level of SOX2 and proneural mRNA expression in cells [341].
In the contrast to the aforementioned therapeutic strategy to induce BMP signaling to initiate differentiation of GSCs, most recently, Kaye and co-workers showed that inhibition of BMP signaling could also have potential therapeutic relevance for fighting GBM [342]. In this study, BMP receptor inhibitors, DMH1, JL5, and Ym155 significantly decreased the expression of the inhibitor of differentiation protein 1 (ID1) in GBM cell lines [342]. The members of the ID family of protein, are co-expressed in diverse cell populations of GBM and conditional deletion of ID1, ID2 and ID3 alleles suppressed the GBM tumor growth and diminished the population of GSCs (reviewed in [343]). Furthermore, Kaye and co-workers demonstrated that spheres formed from GBM cell lines after BMP4 treatment were smaller in size and number. However, treatment with JL5 completely prevented the formation of spheres, suggesting that inhibition of BMP signaling suppressed self-renewal more than its activation [342].
In addition to activation of direct effectors of BMP signaling, such as BMP4 and BMP7, simulation of the BMP pathway is performed using mimic effectors. Recently, the BMP2 protein mimicking peptide GBMP1 has been synthesized by Rampazzo and co-workers and demonstrated to enhance osteogenic differentiation of mesenchymal stem cells and astroglial differentiation of GSCs in vitro [344]. However, further studies are necessary to prove the usage of this drug in clinical studies.
To this day, numerous strategies that enable modulation of the BMP pathway in vivo (starting from upstream of receptors to effector activity) in different cancer and non-cancer-related pathologies have been described in the literature (reviewed in [24,326,345]). However, to the best of our knowledge, there is only one active phase I clinical trial for the treatment of adults with progressive and/or multiple recurrent GBM, in which human recombinant BMP4 is being administered through intratumoral and interstitial convection-enhanced delivery (CED) (NCT02869243) (reviewed in [24]). The reasons for a low number of clinical trials may come from the fact that these tumors are highly heterogeneous, and that BMP signaling regulates a large network of genes and pathways, and only a small portion of those directly relates to the disease pathology or may even have the opposite effect on the clinical outcome.
8. Targeting Hippo Signaling Pathway in GBM
The Hippo pathway is an evolutionarily well-conserved signaling pathway involved in tissue development and regeneration that controls organ size by regulating cell proliferation and apoptosis [346]. In physiological conditions, the Hippo pathway suppresses growth, mediates stress-induced apoptosis or regulates cell fate decisions [347]. The Hippo pathway consists of a complex cascade of serine/threonine-protein kinases and its core kinases MST1/2 (Mammalian STE-like protein kinase 1/2) and LATS1/2 (Large tumor suppressor 1/2) [348]. Their roles are to inhibit the transcription cofactors YAP (Yes-Associated Protein 1) and its ortholog TAZ (Transcriptional co-activator with PDZ-binding motif). When the pathway is initiated by activation of MST1/2 associated with SAV1 (Salvatore) they further phosphorylate LATS1/2 and its cofactor MOB1, which in turn phosphorylates the transcription cofactors YAP/TAZ, leading to inhibition of their nuclear translocation [349] (Figure 7).
Figure 7.
The canonical Hippo pathway and its pharmaceutical inhibitors. Various extracellular signals including mechanical stress, cellular contact, hormones and growth factors activates Hippo signaling cascades that through serial phosphorylations involving block of kinases inhibits nuclear translocation of transcriptional co-activator YAP/TAZ and consequently their involvement in the regulation of gene transcription. When Hippo pathway is inactive, YAP/TAZ translocates to the nucleus, associates with TEAD family of transcription factors and participates in the regulation of target genes expression. Inhibitors that target important pathway components are indicated in red. References are included in the main text.
Phosphorylated YAP/TAZ is sequestrated in the cytoplasm or degraded by the proteasome [350]. When the Hippo pathway is inactivated, the unphosphorylated YAP/TAZ complex is translocated to the nucleus, where it binds the TEAD (TEA domain) transcription factor family. Indeed, YAP/TAZ association with TEAD transcription factors is essential to the control of several targeted genes, such as MYC (MYC proto-oncogene bHLH transcription factor), BIRC5 (baculoviral IAP repeat containing 5), AXL (AXL receptor tyrosine kinase), CTGF (Connective Tissue Growth Factor) and CYR61 (Cysteine Rich Angiogenic Inducer 61) involved in cell proliferation and survival [351]. This pathway is regulated by cell–cell contact, cell polarity, and actin cytoskeleton, as well as a wide range of signals, including stress signals, cellular energy status, and mechanical and hormonal signals that act through G-protein-coupled receptors (GPCR) [352].
Since MST1/2 and LATS1/2 core constitute a regulatory part of the Hippo signaling associated with a tumor suppressor effect, the transcriptional cofactors YAP/TAZ associated with TEAD transcription factors represent the terminal effectors of this pathway and play a pro-oncogenic role. YAP/TAZ activation is involved in cell proliferation, mesenchymal transition, invasion, and metastasis, as well as in cancer stem cell maintenance and chemoresistance [353]. Elevated levels and nuclear localization of YAP and in some cases TAZ have been reported in a majority of solid cancers, suggesting widespread deregulation of Hippo signaling in human neoplasia [354]. High levels of nuclear YAP1 immunoreactivity were detected in GBM tissues, and it was shown that silencing of YAP1 in glioma cell lines led to a significant reduction in cell growth [355]. In line with this, a comparison of single-cell RNA-seq datasets from patients with GBM revealed that YAP and TAZ drive a regulatory network associated with the GSC state [356]. It has been shown that YAP/TAZ are required to establish GSC properties in primary cells and required for tumor initiation and maintenance in vivo in different mouse and human GBM models [356]. Further, Bhat and co-workers showed elevated expression of TAZ in GBM tissues and associated TAZ expression with mesenchymal gene signature showing a direct role of TAZ and TEAD in driving the mesenchymal differentiation in malignant glioma [67]. Various studies have also found that hyperactivation of YAP/TAZ is associated with resistance to canonical chemotherapies, radiotherapies, and targeted therapies [357,358]. Current evidence suggests that multiple mechanisms contribute to the deregulation of YAP and TAZ in human cancers, including promoter hypermethylation, mutation, and amplification [354]. Reasonably, YAP/TAZ represents one of the most attractive targets in anticancer therapy as final effectors of the Hippo signaling pathway.
High-throughput screening of approximately 3300 FDA-approved drugs for inhibitors of the transcriptional activity of YAP identified 71 hits including several porphyrin compounds, protoporphyrin IX (PPIX), hematoporphyrin (HP), and verteporfin (VP), which stood out among the top candidates [359]. These compounds disrupt physical interactions between YAP and TEAD, thereby inhibiting YAP transcriptional activity, but more importantly, they are able to cross BBB and accumulate in the brain [360]. Vigneswaran and co-workers showed that VP acts as an inducer of apoptosis of patient-derived GBM cells that successfully suppressed expression of YAP/TAZ transcriptional target genes, and had significant survival benefit in an orthotopic xenograft GBM model [361]. Further, a phase 0 clinical trial reported that liposomal VP was effectively absorbed by GBM cells in patients’ tumor tissue, and VP-treated participants preliminarily showed lower YAP/TAZ protein levels compared with a representative untreated control patient [361]. Further studies are needed to elucidate their efficiency in therapeutic protocols, alone or more likely in combination with established treatment regiments.
Another approved drug that emerged as an interesting therapeutic in GBM is valproic acid (VPA). It has been demonstrated that VPA inhibited glioma cell proliferation, migration, and invasion [362]. Experiments on human GBM cell lines have shown that VPA had a strong antiproliferative effect and also led to a reduction in CD44 expression (a cancer stem cell marker) [363]. Further, it has been shown that CD44 is upregulated in GBM tissue samples and that its depletion blocked glioma cells growth in vitro and in vivo and sensitized GBM cells to cytotoxic drugs in vivo [364]. CD44 functions upstream of the mammalian Hippo signaling pathway, and when CD44 is silenced, it is followed by sustained phosphorylation of MST1/2 and LATS1/2 and, consequently, phosphorylation/inactivation of YAP [364]. There have been several trials of VPA in combination with TZM and radiation for the treatment of brain tumors. Results of a single-group study where newly diagnosed GBM patients started VPA treatment following maximal safe resection (all of them were treated with chemoradiation and TMZ) demonstrated no significant treatment-related toxicity; younger patients (age ≤ 45 years) showed a significantly better overall survival (25 months) versus older patients (8 months) [365]. Limitations of this study include the small sample size, no randomization, and the lack of information regarding other potential prognostic factors. Yuan and co-workers performed a meta-analysis using EMBASE, MEDLINE, ClinicalTrials.gov, and Cochrane Central Register of the Controlled Trials databases to assess the effects of VPA on survival times and GBM recurrence [366]. A total of 1634 patients with a confirmed GBM diagnosis were examined in this meta-analysis, and the authors concluded that GBM patients using VPA showed a relatively better outcome when compared to patients using no antiepileptic drugs or other-antiepileptic drugs [366]. This and similar retrospective studies can be useful as a guide in planning other clinical trials investigating the impact of co-medication with VPA in GBM patients.
Amlodipine, a calcium channel blocker medication used to treat high blood pressure and coronary artery disease, has been shown to provoke actin cytoskeleton remodeling and new assembly of F-actin that, in turn, starts kinase cascade and phosphorylates YAP, leading to its degradation [367]. The authors showed that amlodipine inhibits the survival of GBM cell line LN229 by suppressing YAP/TAZ activities and preventing their accumulation in the cell nucleus [367]. Amlodipine, along with several other antihypertensives, has demonstrated promising preclinical results, but unfortunately, it has not gone into clinical trial yet.
Bazedoxifene (BZA) is a small molecule inhibitor currently used in the clinic as a third-generation selective estrogen receptor modulator to treat postmenopausal osteoporosis [368]. BZA preferentially disrupts the gp130-IL6 receptor complex [369], and a recent study displayed that BZA treatment also accelerated YAP phosphorylation, hypothesizing that there is a cross-talk between IL-6-gp130 and YAP [370]. Wightman and co-workers identified BZA as a candidate therapeutic for GBM, showing its ability to penetrate the BBB and increase the time of survival in an orthotopic syngeneic mouse model [371]. Additionally, as presented by Fu and co-workers, BZA combined with paclitaxel had a stronger ability to suppress YAP signaling and inhibit GBM tumor growth in the orthotopic GBM mouse model [370].
It has been already reported that statins impair the viability of GBM cell lines through TGFβ inhibition [372]. Importantly there is evidence that statins, in particular fluvastatin, could inhibit nuclear localization of YAP and TAZ and, consequently, YAP/TAZ-TEAD-dependent reporter activity [373]. A meta-analysis performed by Xie and co-workers included a total of 2519 patients with GBM, including 430 statin users and 2089 nonstatin users [374]. Analyzed data regarding progression-free survival and overall survival revealed that the use of statins was not associated with prolonged survival of patients with GBM [374]. However, a subgroup analysis showed that the use of statins before diagnosis favors the overall survival of GBM patients, while statin usage after diagnosis might be harmful [374]. Currently, there are two clinical trials evaluating the use of statins: a phase I trial evaluating the safety of fluvastatin and celecoxib (Celebrex) association in gliomas (NCT02115074), and a phase II study assessing the efficacy and safety of atorvastatin in combination with radiotherapy and TMZ in GBM (NCT02029573).
In summary, there are many approved drugs that are considered for repurposed use in GMB, and novel randomized trials are needed for proving their therapeutic efficacy. The general opinion is that repurposed agents are more likely to be combined with current standard regimens, suggesting that they should be considered when planning future trials.
9. Targeting Retinoic Acid Signaling Pathway in GBM
Retinoic acid signaling has key roles in vertebrate development [375]. Functions of retinoic acid (RA), a morphogen synthesized from vitamin A [376], in the regulation of different processes, such as fate specification, differentiation, proliferation, apoptosis, immune response, homeostasis, regeneration and maintenance of circadian rhythms have been reported (reviewed in [377,378,379]).
Postnatally, retinoids are derived from carotenoids and retinyl esters. Following ingestion, carotenoids are cleaved into retinal and then reduced to retinol, while retinyl esters are hydrolyzed to retinol. Upon entering into the bloodstream, retinol is bound to retinol-binding protein 4 (RBP4) and this complex enters cells via stimulated-by-retinoic-acid 6 (STRA6) (reviewed in [380]) or by membrane diffusion [381] (Figure 8). Intracellularly retinol is metabolized to RA through a series of oxidation steps (reviewed in [377]). Namely, within cells, retinol is converted to retinaldehyde by cytosolic alcohol dehydrogenases (ADHs) and microsomal retinol dehydrogenases (RDHs). After that, retinaldehyde is oxidized to RA by three aldehyde dehydrogenases ALDH1A1-A3 (reviewed in [380,382]). There are three naturally occurring RA stereoisomers: all-trans retinoic acid (ATRA), 13-cis retinoic acid (13-cis RA) and 9-cis retinoic acid (9-cis RA) (reviewed in [383]). Acting on the producing (autocrine signaling) or the receiving (paracrine signaling) cells, RA enters the nucleus via cellular RA-binding protein 2 (CRABP2) (reviewed in [384]) and interacts with retinoic acid receptor (RAR-RARα, RARβ, RARγ)/retinoid X receptor (RXR-RXRα, RXRβ, RXRγ) heterodimer. Subsequently, this complex interacts with RA-response element (RARE) in the promotor region of RA target genes influencing the transcription of over 500 genes (reviewed in [377]). RA is inactivated by cytochrome P450 family 26 (CYP26) oxidase (reviewed in [382]).
Figure 8.
Overview of RA signaling pathway and RAR/RXR agonists and RAMBAs. In the bloodstream, retinol forms complex with RBP4 and enters the cells via STRA6 or by membrane diffusion. Within cells, retinol is converted to retinaldehyde by ADHs and RDHs and subsequently oxidized to RA by ALDH1A1-3. Three naturally occurring RA stereoisomers are ATRA, 13-cis RA and 9-cis RA. RA enters the nucleus via CRABP2, where it interacts with RAR/RXR heterodimer forming a complex that binds to RARE in the promoter regions of RA target genes. RA is inactivated by CYP26 oxidase (modified based on [380]). Summary of inhibitors of the CYP26A1 enzyme (RAMBAs) and RARs/RXRs agonists is made based on [383] and https://resources.tocris.com/pdfs/literature/reviews/retinoid-receptors-review-2019-web.pdf (accessed on 23 May 2022), respectively. RAR and RXR agonists and RAMBAs used for treatment of GBM cells in vitro or in vivo are presented in green letters, and RAR and RXR agonists and RAMBAs used in clinical trials for GBMs are presented in blue letters, while RAR and RXR agonists and RAMBAs not yet used in treatment of GBM are presented in black letters (based on results of previously reported publications included in the main text and results obtained by [385,386,387,388,389,390]).
Dysregulation of the RA signaling pathway underlies the etiology of different malignancies, such as leukemias, neuroblastoma, lung, skin, breast, ovarian, prostate, head/neck, pancreatic, liver and bladder cancers, renal cell carcinoma and GBM (reviewed in [391]). On the other hand, RA and its derivatives are used for the treatment of cancers. ATRA is a very effective and curative therapy for patients with acute promyelocytic leukemia and a potential therapeutic agent against oral squamous cell carcinoma and oral dysplasia (reviewed in [383]). Preclinical studies demonstrated the usefulness of 9-cis RA in the prevention of prostate and breast cancers and randomized trial of 13-cis RA showed promising results in the treatment of children with high-risk neuroblastoma (reviewed in [383]). It has been reported that RA inhibits the proliferation of tumor cells, promotes their differentiation, induces apoptosis, and inhibits angiogenesis and metastasis [392].
A series of alterations in the RA signaling pathway was reported in GBMs. Methylation in the RBP1 gene was identified in IDH1 and IDH2 mutant tumors. GBM patients with RBP1-unmethylated tumors had decreased median overall survival compared with the patients with RBP1-methylated GBMs [393]. Additionally, CRABP2 was suppressed in GBM through epigenetic silencing [394]. Sanders and co-workers demonstrated increased expression of ALDH1A2 in GBM compared to the expression detected in low-grade gliomas and upregulation of ALDH1A2 expression upon GBM recurrence [395]. Campos and co-workers revealed that the expression of ALDH1A1-3 was decreased in GBMs compared to expression detected in non-tumorous brain tissue [396]. Additionally, CRABP2 expression was reduced in high-grade gliomas [394] and when comparing primary GBM tissues of short-term and long-term survivors Barbus and co-workers detected that RBP1 and CRABP2 expression was higher in GBMs of short-term survivors [397]. Methylation of RARβ was detected in about 70% of GBMs [398]. Literature data showed that glioma cell lines express RARγ [399], while primary cultures of biopsy material from human GBMs expressed RARγ and RXRα [399]. Furthermore, stem-like glioma cells, isolated from primary GBM, expressed RARα. ATRA induced differentiation of these cells, causing antitumorigenic, anti-invasive, antimigratory, antiangiogenic, growth-inhibiting and proapoptotic effects [400]. A block in the retinoid receptor proteasomal degradation pathway and accumulation of sumoylated and high-molecular-weight forms of retinoid receptors that lack transcriptional activity were revealed in glioma stem-like cells [401].
The literature data demonstrated that retinoids might affect the malignant behavior of glioma cells. Bouterfa and co-workers treated glioma cell lines and primary cultures of GBMs with different retinoids (ATRA, 9-cis-RA, 13-cis-RA) and demonstrated that glioma cell lines are generally unresponsive to retinoids, while treatment of primary cultures of GBMs reduced the proliferation and migration of these cells [399]. It has been shown that RA increases the proliferation of GL-15 GBM cells at low concentrations and inhibits proliferation of these cells at high concentration [402]. Additionally, the proliferation of GL-15 GBM cells was inhibited by two structurally related RARγ agonists, CD-437 and CD-2325 [402]. In vitro experiments using GBM cell lines demonstrated that ATRA might induce morphological changes, differentiation, apoptosis, change in the mode of cell migration and reduction in growth, proliferation, invasiveness and adhesion of these cells [403,404,405,406]. Additionally, ATRA increased the asymmetric cell division of GSCs isolated from the U87 GBM cell line [407], induced differentiation and decreased proliferation and invasiveness of U87 cancer stem-like cells [408], induced morphology changes, growth arrest and differentiation of GBM stem-like cells [409] and induced differentiation and apoptosis and reduced proliferation and self-renewal of neurospheres of GBM therapy-resistant cancer stem cells [410]. On the other hand, ATRA treatment of brain tumor stem cells, isolated from the fresh GBM specimen, promoted proliferation and induced differentiation of these cells [411]. Additionally, ATRA has a pro-proliferative and pro-survival effect on stem-like glioma cells mediated by RARα and RARγ [412]. It has been demonstrated that cis-RA treatment of glioma cells may inhibit proliferation and invasiveness and induce differentiation of these cells [399,413,414], while treatment of GSCs with 9-cis RA and 13-cis RA inhibited proliferation and induced their differentiation into neurons, astrocytes and oligodendrocytes [409,415,416]. 13-cis RA showed a significant inhibitory effect on proliferation and clonogenicity of U343 GBM cells [417]. Bexarotene, a RXR agonist, induced morphological changes and differentiation of cultured primary GBM cells and inhibited their neurospheroidal colony formation and migration [418]. Bexarotene or ATRA, alone, reduced tumor size; on the other hand, when treatment was discontinued, the tumor size started to increase [418].
There are literature data about the combined effects of retinoids and other drugs/compounds on the malignant behavior of GBM cells and GSCs. Namely, ATRA in combination with TMZ enhanced TMZ effects on malignant behavior of GBM cells [419], in combination with interferon-gamma (IFN-γ), it induced apoptosis of GBM cell lines [404], and in combination with taxol, paclitaxel or IFN-γ, it induced differentiation, apoptosis and reduction in tumor volume of xenografts of GBM cell lines [420,421,422]. Combined treatment of metformin and 9-cis RA reduced the proliferation rate and increased apoptosis in C6 glioma stem-like cells [423], while 13-cis RA combined with thalidomide delayed the growth of GBM xenografts [424]. Reduced proliferation, invasion and migration of U87 cells, decreased number of colonies of these cells, increased number of apoptotic cells and reduced tumor volumes have been found upon treatment of cells with 6-OH-11-O-hydroxyfenantrene (IIF), an RXR agonist, and pioglitazone, a synthetic PPARγ agonist [425]. Systemic administration of TMZ combined with convection-enhanced delivery (direct intracranial drug infusion technique) of polymeric micellar Am80, a synthetic agonist with high affinity to nuclear RAR, provided longer survival of rats with GBM xenografts compared to controls [390].
The therapeutic benefit of retinoids for therapy of GBM is largely contradictory. Results obtained by Pitz and co-workers demonstrated that there are no significant differences between the survival of GBM patients who were treated with TMZ and cis RA for up to 24 months after surgery and radiation therapy and GBM patients treated for 6 months with TMZ [426]. Additionally, the results of a phase II clinical trial demonstrated that a retinoid combined with TMZ did not increase progression-free survival of GBM patients [155]. Additionally, a phase II trial was conducted to evaluate the effect of concurrent treatment with TMZ and 13-cis RA in combination with conventional radiation therapy in adults with supratentorial GBM, which did not show a survival advantage compared with studies using radiation therapy with TMZ [427]. Furthermore, the phase II trial of fenretinide (4-hydroxyphenyl-retinamide) (NSC 374551), a synthetic derivative of ATRA, in adults with recurrent GBM did not demonstrate clinical efficacy [428]. On the other hand, the combination of 13-cis RA (Accutane) and celecoxib, a COX-2 inhibitor, demonstrated a modest effect on progression-free survival of patients with progressive GBM, but this combination was not more effective than 13-cis RA alone [429]. A phase II evaluation of TMZ and 13-cis RA (NABTC 98–03) revealed a 6-month progression-free survival rate of 32% for patients with GBM [430].
On ClinicalTrial.gov, six studies were found by searching with keywords “retinoid” and “GBM”. Among them, the only one active is focused on evaluating the effects of combined therapy of vorinostat, isotretinoin and TMZ in patients with GBM (NCT00555399). The results of the study NCT00112502 indicated that adding isotretinoin to dose-dense TMZ may be detrimental [155]. Furthermore, there are two on-going studies analyzing the effects of ketoconazole, an inhibitor of the CYP26A1 enzyme. The first study (NCT04869449) analyzes if ketoconazole can enter brain tumors (GBM) at a high enough amount to stop the tumor cells from dividing, while the second (NCT03796273) studies the side effects and how well ketoconazole works before surgery in treating patients with glioma that has come back.
The main limitations of pharmacological applications of RA include poor solubility in aqueous solutions, photosensitivity, rapid metabolism of RA upon intravenous administration, which reduces its efficiency, and side effects after systemic delivery (reviewed in [431]). The results obtained by using ATRA-encapsulated polymeric micelles of a chitosan graft copolymer indicated that encapsulated ATRA is more effective at inhibiting U87 cell migration than free ATRA [432]. Additionally, to stabilize ATRA, Jones and co-workers used a porous poly(1,8-octanediol-co-citrate; POC) wafer which enabled slow release of ATRA leading to differentiation, apoptosis, and inhibition of proliferation of U87 GBM cells [433]. Generally, several strategies for the delivery of RA were used, and the most common strategy used is liposomal or polymeric nanoparticles formed by polyesters, polyimines, polysaccharides and proteins (reviewed in [431]). In conclusions, the use of drug delivery systems that enhance RA solubility, prolong its presence in circulation, and decreased its toxicity might improve its efficiency in cancer treatment, including GBM (reviewed in [431]).
10. Conclusions and Future Perspectives
Common genetic alterations in GBM include the loss of the chromosome arm 10q, alterations in tumor suppressor TP53 and tumor suppressor retinoblastoma RB, amplifications of EGFR and PDGFR, and aberrations in RTK/Ras/PI3K signaling pathways, all of which are major known drivers of GBM pathology (reviewed in [11]). Other frequent mutations include alterations in NF1, PTEN, and MDM2 [38,434]. On the other hand, multiple signaling pathways dysregulated in GBM are involved in the promotion of malignant behavior of GBM cells [12]. Their altered activities in GBM are mostly due to changes in the expression rather than mutations in key pathway components. Mutations in components of signaling pathways presented in this paper are not hallmarks of GBM, although some studies linked several mutations to gliomagenesis. In particular, one study detected mutation in APC gene, a WNT signaling component, in GBM patient samples [112]. Further, mutations in TGFβ receptor that inactivate the receptor are detected in early stages of malignant glioma tumorigenesis [435]. Additionally, studies have reported mutations of Notch pathway genes in grade II and III gliomas [436]. Unlike low-grade gliomas, Notch mutations in GBM are very scarce. Since no other driver mutations in signaling pathways have been identified so far in GBM, evidently, regulation of these pathways activity depends of other mechanisms, including epigenetic alterations. Sakthikumar and co-workers provided results of whole genome sequencing showing evidence for enrichment of non-coding constraint mutations in GBM-associated genes, as well as in more than 1776 other genes that have not been previously linked to GBM, but may have a functional impact on the disease [437]. This could offer an explanation for differences in the regulation of gene expression and, consequently, changes in signaling pathways activity. Importantly, GBMs frequently evolve and, within a single patient, could display numerous subtypes, various gene expression profiles, transcriptome patterns, and methylation statuses, all features that favor subclonal selection and direct response to therapy. There is always a risk that some important players are not identified, but with the current knowledge, it becomes obvious that the combination of inhibitors of multiple pathways and other therapies should be considered as a future direction for GBM treatment. Here, we summarized recent findings of the progress made in targeting these signaling pathways in GBM. Although numerous studies of the anti-GBM effects of modulators of various signaling pathways gave promising results in in vitro and in vivo models, only a small fraction of them reached the first phases of clinical trials. Additionally, most of the agents that entered clinical trials as monotherapy or in combination with chemo- and radiotherapy displayed poor clinical activity or lack of survival benefit for patients.
Several reasons may explain these disappointing results. One of the reasons lies in the fact that adequate patients’ molecular stratifications are often lacking. It has been demonstrated that individual prognostic factors of each patient, including MGMT methylation status, presence of mutant epidermal growth factor variant III (EGFRvIII), the status of signaling pathways activities, baseline performance status, tumor location, and age could influence the success of the trial and final results [438]. The identification of subtype-specific alternations in genes and their expression and related signaling pathways is a crucial step in the discovery and development of new prognostic and therapeutic strategies to target GBM. For example, in search of the subtype-specific prognostic core genes, Park and co-workers showed that in the mesenchymal subtype of GBM, genes were enriched with Wnt/β-catenin-related genes, suggesting that targeting Wnt signaling would be more effective in this subtype of GBM [439]. El-Sehemy and co-workers showed that Norrin, a Wnt ligand that binds FZD4 and activates canonical Wnt/β-catenin signaling, in GSCs with low expression of proneural factor ASCL1 (Achaete-scute homolog 1), exerts tumor-suppressive effects via Wnt signaling, while in GSCs with high ASCL1 expression, Norrin acts as an oncogene by promoting Notch signaling in Wnt-independent manner [440]. These results suggest that Wnt should be considered as a therapeutic target exclusively in GBM with low expression of ASCL1, while in the GBM subtype with high expression of ASCL1, the inhibition of Notch could be a strategy of choice for therapy of GBM. Thus, stratifications of patients based on molecular profiling of their GBMs, including the activity of signaling pathways, would enable the identification of the most relevant targets for each patient.
Several issues need to be addressed for achieving desirable results in GBM therapy, including GBM heterogeneity and plasticity, as well as TME, which contributes to the tumorigenesis and progression of GBM. Today it is accepted that due to the GBM complexity, it is not likely that a single molecular agent could provide a final therapeutic strategy, and combination therapy approaches are likely to perform better in designing novel clinical trials. Based on this, agents that simultaneously target multiple dysregulated pathways might be involved in the development of future therapeutic strategies for GBM. A number of studies have been aimed to identify or develop small-molecule compounds, natural or synthetic, that are able to target multiple signaling pathways simultaneously to combat cancer. Natural compounds with such properties include resveratrol (affects Wnt/β-catenin, Notch and Smad-dependent TGFβ signaling) [134,254,312], DATS (decreases Wnt/β-catenin and Notch) [137,257], honokiol (downregulates Notch and PI3K/Akt/mTOR) [253,441] and garlic-derived Z-ajoene (affects Notch, Wnt and HH) [442]. In our previous work, we found that extracts from Phlomis fruticosa L., Ononis spinosa L. and Anthriscus cerefolium L. plants and bis-Bibenzyls from the Liverwort Pellia endiviifolia have anti-GBM activity in vitro [443,444,445,446], and future research of these extracts and compounds should decipher whether the mechanism underlying their antitumor effect involves targeting dysregulated signaling pathways in GBM. It is important to point out that targeting the same signaling pathway in GSCs and GBM tumor cells can affect diverse sets of target genes and, consequently, different cellular processes. Kaye and co-workers recently demonstrated that both activation and suppression of the BMP signaling pathway had a negative effect on tumor sphere growth by affecting different targets [342]. Thus, there is a constant requirement for further laboratory and clinical research to investigate the mechanisms of receptor signaling downstream pathways in GSCs and GBM tumor cells.
In recent years, 3D and 4D model systems for investigation of different aspects of GBM biology have emerged, holding great potential for the assessment of therapeutic responses and personalized drug screening (reviewed in [447]). They include 3D human brain organoids grafted with patient-derived GSCs or GSC spheres [448] and a 4D platform of GBM patient-derived organoids that self-transforms from 3D cell-culture inserts into histological cassettes [449]. Additionally, different in vitro models of the BBB have been developed (reviewed in [450]). These models can be employed to test the antitumor effect of the inhibitors/modulators of signaling pathways and their ability to cross BBB before considering their inclusion in in vivo or clinical studies. Additionally, significant progress has been made in the development of systems for drug delivery, selective disruption of the BBB using high intensity focused ultrasound, and different systems for intratumoral drug delivery in order to achieve therapeutic drug concentration at the site of the tumor (reviewed in [451]). Having in mind that signaling pathways have key roles in the regulation of cell activity, it is important to develop controlled release systems for targeting signaling pathways in tumor cells in order to avoid targeting normal cells and prevent undesirable toxicity. By controlled release systems, fluctuation of drug concentration might be decreased and side effects might be minimized (reviewed in [452]). It has been shown that hydrogels of natural and synthetic polymers enable controlled release of drugs and targeting, as well as protection of drugs from degradation and metabolism, thereby enhancing treatment efficacy and decreasing toxic effects on normal cells (reviewed in [453]). Nanotechnology enables the delivery of agents into tumor tissues, and the development of stimuli-responsive nanocarriers represents a promising strategy for the delivery of agents that target signaling pathways. Researchers have developed stimuli-responsive nanocarriers that can release drugs into tumor tissue in response to different stimuli, such as temperature, pH, and redox [454,455].
Despite successful outcomes of treatment of other aggressive cancers [456,457]), immunotherapy in GBM faced challenges due to the numerous mechanisms of resistance, including the location of the tumor within the brain and the nature of the BBB, as well as the tumor heterogeneity and its immunosuppressive microenvironment (reviewed in [458]). Numerous immunotherapy strategies for GBM treatment have been employed, including antibodies that reeducate tumor macrophages, vaccinations that introduce tumor-specific dendritic cells (DCs), checkpoint molecule inhibition, and modified T-cells and proteins that help T-cells engage directly with tumor cells (reviewed in [459,460,461]). Even though these strategies applied as monotherapy had only limited benefits for patient survival, preclinical studies showed encouraging results. Strategies that might deliver the real medical benefit for GBM patients include concurrent stimulation of the immune response and inhibition of immunosuppressive components or a combination of immune checkpoint blockade (ICB) with chemotherapy, radiotherapy inducing immunogenic cell death or vaccines (reviewed in [459,460,461]). Currently, there are ongoing clinical trials studying combinations of multiple ICBs and combination of ICBs with radiotherapy or vaccination (reviewed in [460]).
Over the years, single-cell RNA sequencing (scRNA-seq) along with other single-cell profiling techniques have become a powerful tool for examining glioma tumors at a resolution of individual cells, providing a comprehensive insight into the glioma biology, intratumoral genetic heterogeneity, cellular lineages, cancer stem cell programs, TME composition, glioma classification, and response to therapies (reviewed in [462,463]). Unraveling the multiple layers of complexity that characterize GBM will ultimately lead to the identification of novel, more efficient targeted therapies. For example, pathway-based classification by using multiple datasets from GBM scRNA-seq and bulk tumors identified four tumor cell states and GBM subtypes, of which the mitochondrial GBM subtype exhibited significant sensitivity to inhibitors of oxidative phosphorylation, suggesting that GBM patients with this subtype could benefit from targeted metabolic therapy [57]. scRNA-seq profiling of individual GBMs by Neftel and co-workers uncovered that four different malignant cellular states, NPC-like, OPC-like, AC-like, and MES-like, are shared across the GBM subtypes, and that each GBM subtype displays an abundance of distinct cellular states [49,464]. All four GBM cellular states display a proliferation signature, and three of them are able to propagate tumors, with the AC-like state showing decreased potential for tumor initiation. GSCs that may exhibit multiple cellular states, which may also interconvert, pose a great challenge for targeted eradication of GSCs [464]. Suva and Tirosh proposed induction of an AC-like state as a potentially relevant approach in differentiation therapy of IDH-mutant and H3K27M gliomas [464].
In conclusion, although myriad agents targeting signaling pathways dysregulated in cancer have been identified, there is always a need for finding novel drugs or improving the existing drugs in terms of their efficacy and safety in anticancer therapy. Investigating novel or better predictive biomarkers, improving patient stratification, developing of computational methods to accurately predict the response of different parts of the tumor to a given therapy, and decreasing drug toxicities by designing more selective drugs and combinatory regimens that affect multiple targets and processes including proliferation, tumor angiogenesis, and invasiveness, are required to overcome the challenges of signaling pathways-targeting therapies in cancer and in GBM in particular. In the light of current knowledge, the optimal approach to target GBM and control tumor recurrence might be to combine modulators of dysregulated signaling pathways with conventional therapies and immunotherapies.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cells11162530/s1, Table S1: Stratification of GBM into molecular subtypes and characteristics of subtypes.
Author Contributions
Conceptualization, D.D. and M.S. (Milena Stevanovic); literature analyses, all authors; writing—original draft preparation, D.D., M.S. (Marija Schwirtlich), I.P., M.M. (Marija Mojsin), M.M. (Milena Milivojevic), N.K.-G. and M.S. (Milena Stevanovic); writing—review and editing, D.D., M.S. (Marija Schwirtlich), I.P., M.M. (Marija Mojsin), M.M. (Milena Milivojevic), N.K.-G. and M.S. (Milena Stevanovic); designing the figures, D.D., M.S. (Marija Schwirtlich), I.P., M.M. (Marija Mojsin), M.M. (Milena Milivojevic), N.K.-G. and M.S. (Milena Stevanovic); supervision, D.D.; funding acquisition, M.S. (Milena Stevanovic). All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by the Ministry of Education, Science and Technological Development of the Republic of Serbia (Agreement no. 451-03-68/2022-14/200042) and the Serbian Academy of Sciences and Arts (Grant number F24).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Louis D.N., Ohgaki H., Wiestler O.D., Cavenee W.K., Burger P.C., Jouvet A., Scheithauer B.W., Kleihues P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parker N.R., Khong P., Parkinson J.F., Howell V.M., Wheeler H.R. Molecular heterogeneity in glioblastoma: Potential clinical implications. Front. Oncol. 2015;5:55. doi: 10.3389/fonc.2015.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wick W., Platten M. Understanding and Treating Glioblastoma. Neurol. Clin. 2018;36:485–499. doi: 10.1016/j.ncl.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 4.Soeda A., Hara A., Kunisada T., Yoshimura S., Iwama T., Park D.M. The evidence of glioblastoma heterogeneity. Sci. Rep. 2015;5:7979. doi: 10.1038/srep07979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Becker A.P., Sells B.E., Haque S.J., Chakravarti A. Tumor Heterogeneity in Glioblastomas: From Light Microscopy to Molecular Pathology. Cancers. 2021;13:761. doi: 10.3390/cancers13040761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ostrom Q.T., Gittleman H., Fulop J., Liu M., Blanda R., Kromer C., Wolinsky Y., Kruchko C., Barnholtz-Sloan J.S. CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2008–2012. Neuro-Oncology. 2015;17((Suppl. 4)):iv1–iv62. doi: 10.1093/neuonc/nov189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stupp R., Mason W.P., van den Bent M.J., Weller M., Fisher B., Taphoorn M.J., Belanger K., Brandes A.A., Marosi C., Bogdahn U., et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 8.Johnson B.E., Mazor T., Hong C., Barnes M., Aihara K., McLean C.Y., Fouse S.D., Yamamoto S., Ueda H., Tatsuno K., et al. Mutational analysis reveals the origin and therapy-driven evolution of recurrent glioma. Science. 2014;343:189–193. doi: 10.1126/science.1239947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oronsky B., Reid T.R., Oronsky A., Sandhu N., Knox S.J. A Review of Newly Diagnosed Glioblastoma. Front. Oncol. 2020;10:574012. doi: 10.3389/fonc.2020.574012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Birzu C., French P., Caccese M., Cerretti G., Idbaih A., Zagonel V., Lombardi G. Recurrent Glioblastoma: From Molecular Landscape to New Treatment Perspectives. Cancers. 2020;13:47. doi: 10.3390/cancers13010047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mao H., Lebrun D.G., Yang J., Zhu V.F., Li M. Deregulated signaling pathways in glioblastoma multiforme: Molecular mechanisms and therapeutic targets. Cancer Investig. 2012;30:48–56. doi: 10.3109/07357907.2011.630050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khabibov M., Garifullin A., Boumber Y., Khaddour K., Fernandez M., Khamitov F., Khalikova L., Kuznetsova N., Kit O., Kharin L. Signaling pathways and therapeutic approaches in glioblastoma multiforme (Review) Int. J. Oncol. 2022;60:69. doi: 10.3892/ijo.2022.5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mehta S., Lo Cascio C. Developmentally regulated signaling pathways in glioma invasion. Cell. Mol. Life Sci. 2018;75:385–402. doi: 10.1007/s00018-017-2608-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lauko A., Lo A., Ahluwalia M.S., Lathia J.D. Cancer cell heterogeneity & plasticity in glioblastoma and brain tumors. Semin Cancer Biol. 2022;82:162–175. doi: 10.1016/j.semcancer.2021.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ryskalin L., Gaglione A., Limanaqi F., Biagioni F., Familiari P., Frati A., Esposito V., Fornai F. The Autophagy Status of Cancer Stem Cells in Gliobastoma Multiforme: From Cancer Promotion to Therapeutic Strategies. Int. J. Mol. Sci. 2019;20:3824. doi: 10.3390/ijms20153824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lathia J.D., Mack S.C., Mulkearns-Hubert E.E., Valentim C.L., Rich J.N. Cancer stem cells in glioblastoma. Genes Dev. 2015;29:1203–1217. doi: 10.1101/gad.261982.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stevanovic M., Kovacevic-Grujicic N., Mojsin M., Milivojevic M., Drakulic D. SOX transcription factors and glioma stem cells: Choosing between stemness and differentiation. World J. Stem Cells. 2021;13:1417–1445. doi: 10.4252/wjsc.v13.i10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prager B.C., Bhargava S., Mahadev V., Hubert C.G., Rich J.N. Glioblastoma Stem Cells: Driving Resilience through Chaos. Trends Cancer. 2020;6:223–235. doi: 10.1016/j.trecan.2020.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng L., Wu Q., Guryanova O.A., Huang Z., Huang Q., Rich J.N., Bao S. Elevated invasive potential of glioblastoma stem cells. Biochem. Biophys. Res. Commun. 2011;406:643–648. doi: 10.1016/j.bbrc.2011.02.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen J., Li Y., Yu T.S., McKay R.M., Burns D.K., Kernie S.G., Parada L.F. A restricted cell population propagates glioblastoma growth after chemotherapy. Nature. 2012;488:522–526. doi: 10.1038/nature11287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Satterlee A.B., Dunn D.E., Lo D.C., Khagi S., Hingtgen S. Tumoricidal stem cell therapy enables killing in novel hybrid models of heterogeneous glioblastoma. Neuro-Oncology. 2019;21:1552–1564. doi: 10.1093/neuonc/noz138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang X., Zuo C., Fang P., Liu G., Qiu Y., Huang Y., Tang R. Targeting Glioblastoma Stem Cells: A Review on Biomarkers, Signal Pathways and Targeted Therapy. Front. Oncol. 2021;11:701291. doi: 10.3389/fonc.2021.701291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakada M., Kita D., Watanabe T., Hayashi Y., Teng L., Pyko I.V., Hamada J. Aberrant signaling pathways in glioma. Cancers. 2011;3:3242–3278. doi: 10.3390/cancers3033242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cruz Da Silva E., Mercier M.C., Etienne-Selloum N., Dontenwill M., Choulier L. A Systematic Review of Glioblastoma-Targeted Therapies in Phases II, III, IV Clinical Trials. Cancers. 2021;13:1795. doi: 10.3390/cancers13081795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fabro F., Lamfers M.L.M., Leenstra S. Advancements, Challenges, and Future Directions in Tackling Glioblastoma Resistance to Small Kinase Inhibitors. Cancers. 2022;14:600. doi: 10.3390/cancers14030600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu H., Qiu W., Sun T., Wang L., Du C., Hu Y., Liu W., Feng F., Chen Y., Sun H. Therapeutic strategies of glioblastoma (GBM): The current advances in the molecular targets and bioactive small molecule compounds. Acta Pharm. Sin. B. 2022;12:1781–1804. doi: 10.1016/j.apsb.2021.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang G.L., Wang C.F., Qian C., Ji Y.X., Wang Y.Z. Role and mechanism of neural stem cells of the subventricular zone in glioblastoma. World J. Stem Cells. 2021;13:877–893. doi: 10.4252/wjsc.v13.i7.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matarredona E.R., Pastor A.M. Neural Stem Cells of the Subventricular Zone as the Origin of Human Glioblastoma Stem Cells. Therapeutic Implications. Front. Oncol. 2019;9:779. doi: 10.3389/fonc.2019.00779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Couturier C.P., Ayyadhury S., Le P.U., Nadaf J., Monlong J., Riva G., Allache R., Baig S., Yan X., Bourgey M., et al. Single-cell RNA-seq reveals that glioblastoma recapitulates a normal neurodevelopmental hierarchy. Nat. Commun. 2020;11:3406. doi: 10.1038/s41467-020-17186-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Curry R.N., Glasgow S.M. The Role of Neurodevelopmental Pathways in Brain Tumors. Front. Cell Dev. Biol. 2021;9:659055. doi: 10.3389/fcell.2021.659055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bar E.E., Chaudhry A., Lin A., Fan X., Schreck K., Matsui W., Piccirillo S., Vescovi A.L., DiMeco F., Olivi A., et al. Cyclopamine-mediated hedgehog pathway inhibition depletes stem-like cancer cells in glioblastoma. Stem Cells. 2007;25:2524–2533. doi: 10.1634/stemcells.2007-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clement V., Sanchez P., de Tribolet N., Radovanovic I., Ruiz i Altaba A. HEDGEHOG-GLI1 signaling regulates human glioma growth, cancer stem cell self-renewal, and tumorigenicity. Curr. Biol. 2007;17:165–172. doi: 10.1016/j.cub.2006.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hu Y.Y., Zheng M.H., Cheng G., Li L., Liang L., Gao F., Wei Y.N., Fu L.A., Han H. Notch signaling contributes to the maintenance of both normal neural stem cells and patient-derived glioma stem cells. BMC Cancer. 2011;11:82. doi: 10.1186/1471-2407-11-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ikushima H., Todo T., Ino Y., Takahashi M., Miyazawa K., Miyazono K. Autocrine TGF-beta signaling maintains tumorigenicity of glioma-initiating cells through Sry-related HMG-box factors. Cell Stem Cell. 2009;5:504–514. doi: 10.1016/j.stem.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 35.Piccirillo S.G., Reynolds B.A., Zanetti N., Lamorte G., Binda E., Broggi G., Brem H., Olivi A., Dimeco F., Vescovi A.L. Bone morphogenetic proteins inhibit the tumorigenic potential of human brain tumour-initiating cells. Nature. 2006;444:761–765. doi: 10.1038/nature05349. [DOI] [PubMed] [Google Scholar]
- 36.Masui K., Mischel P.S., Reifenberger G. Molecular classification of gliomas. Handb. Clin. Neurol. 2016;134:97–120. doi: 10.1016/B978-0-12-802997-8.00006-2. [DOI] [PubMed] [Google Scholar]
- 37.Weller M., van den Bent M., Preusser M., Le Rhun E., Tonn J.C., Minniti G., Bendszus M., Balana C., Chinot O., Dirven L., et al. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat. Rev. Clin. Oncol. 2021;18:170–186. doi: 10.1038/s41571-020-00447-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Verhaak R.G., Hoadley K.A., Purdom E., Wang V., Qi Y., Wilkerson M.D., Miller C.R., Ding L., Golub T., Mesirov J.P., et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17:98–110. doi: 10.1016/j.ccr.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Agnihotri S., Aldape K.D., Zadeh G. Isocitrate dehydrogenase status and molecular subclasses of glioma and glioblastoma. Neurosurg. Focus. 2014;37:E13. doi: 10.3171/2014.9.FOCUS14505. [DOI] [PubMed] [Google Scholar]
- 40.Wang Q., Hu B., Hu X., Kim H., Squatrito M., Scarpace L., de Carvalho A.C., Lyu S., Li P., Li Y., et al. Tumor Evolution of Glioma-Intrinsic Gene Expression Subtypes Associates with Immunological Changes in the Microenvironment. Cancer Cell. 2017;32:42–56.e46. doi: 10.1016/j.ccell.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gill B.J., Pisapia D.J., Malone H.R., Goldstein H., Lei L., Sonabend A., Yun J., Samanamud J., Sims J.S., Banu M., et al. MRI-localized biopsies reveal subtype-specific differences in molecular and cellular composition at the margins of glioblastoma. Proc. Natl. Acad. Sci. USA. 2014;111:12550–12555. doi: 10.1073/pnas.1405839111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sturm D., Witt H., Hovestadt V., Khuong-Quang D.A., Jones D.T., Konermann C., Pfaff E., Tonjes M., Sill M., Bender S., et al. Hotspot mutations in H3F3A and IDH1 define distinct epigenetic and biological subgroups of glioblastoma. Cancer Cell. 2012;22:425–437. doi: 10.1016/j.ccr.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 43.Bhat K.P.L., Balasubramaniyan V., Vaillant B., Ezhilarasan R., Hummelink K., Hollingsworth F., Wani K., Heathcock L., James J.D., Goodman L.D., et al. Mesenchymal differentiation mediated by NF-kappaB promotes radiation resistance in glioblastoma. Cancer Cell. 2013;24:331–346. doi: 10.1016/j.ccr.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huse J.T., Phillips H.S., Brennan C.W. Molecular subclassification of diffuse gliomas: Seeing order in the chaos. Glia. 2011;59:1190–1199. doi: 10.1002/glia.21165. [DOI] [PubMed] [Google Scholar]
- 45.Phillips H.S., Kharbanda S., Chen R., Forrest W.F., Soriano R.H., Wu T.D., Misra A., Nigro J.M., Colman H., Soroceanu L., et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell. 2006;9:157–173. doi: 10.1016/j.ccr.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 46.Zheng S., Chheda M.G., Verhaak R.G. Studying a complex tumor: Potential and pitfalls. Cancer J. 2012;18:107–114. doi: 10.1097/PPO.0b013e3182431c57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sottoriva A., Spiteri I., Piccirillo S.G., Touloumis A., Collins V.P., Marioni J.C., Curtis C., Watts C., Tavare S. Intratumor heterogeneity in human glioblastoma reflects cancer evolutionary dynamics. Proc. Natl. Acad. Sci. USA. 2013;110:4009–4014. doi: 10.1073/pnas.1219747110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ensenyat-Mendez M., Iniguez-Munoz S., Sese B., Marzese D.M. iGlioSub: An integrative transcriptomic and epigenomic classifier for glioblastoma molecular subtypes. BioData Min. 2021;14:42. doi: 10.1186/s13040-021-00273-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Neftel C., Laffy J., Filbin M.G., Hara T., Shore M.E., Rahme G.J., Richman A.R., Silverbush D., Shaw M.L., Hebert C.M., et al. An Integrative Model of Cellular States, Plasticity, and Genetics for Glioblastoma. Cell. 2019;178:835–849.e821. doi: 10.1016/j.cell.2019.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ma H., Zhao C., Zhao Z., Hu L., Ye F., Wang H., Fang Z., Wu Y., Chen X. Specific glioblastoma multiforme prognostic-subtype distinctions based on DNA methylation patterns. Cancer Gene. 2020;27:702–714. doi: 10.1038/s41417-019-0142-6. [DOI] [PubMed] [Google Scholar]
- 51.Brennan C.W., Verhaak R.G., McKenna A., Campos B., Noushmehr H., Salama S.R., Zheng S., Chakravarty D., Sanborn J.Z., Berman S.H., et al. The somatic genomic landscape of glioblastoma. Cell. 2013;155:462–477. doi: 10.1016/j.cell.2013.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Capper D., Stichel D., Sahm F., Jones D.T.W., Schrimpf D., Sill M., Schmid S., Hovestadt V., Reuss D.E., Koelsche C., et al. Practical implementation of DNA methylation and copy-number-based CNS tumor diagnostics: The Heidelberg experience. Acta Neuropathol. 2018;136:181–210. doi: 10.1007/s00401-018-1879-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang L.B., Karpova A., Gritsenko M.A., Kyle J.E., Cao S., Li Y., Rykunov D., Colaprico A., Rothstein J.H., Hong R., et al. Proteogenomic and metabolomic characterization of human glioblastoma. Cancer Cell. 2021;39:509–528.e520. doi: 10.1016/j.ccell.2021.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang Z., Sun D., Chen Y.J., Xie X., Shi Y., Tabar V., Brennan C.W., Bale T.A., Jayewickreme C.D., Laks D.R., et al. Cell Lineage-Based Stratification for Glioblastoma. Cancer Cell. 2020;38:366–379. doi: 10.1016/j.ccell.2020.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hubert C.G., Lathia J.D. Seeing the GBM diversity spectrum. Nat. Cancer. 2021;2:135–137. doi: 10.1038/s43018-021-00176-x. [DOI] [PubMed] [Google Scholar]
- 56.Richards L.M., Whitley O.K.N., MacLeod G., Cavalli F.M.G., Coutinho F.J., Jaramillo J.E., Svergun N., Riverin M., Croucher D.C., Kushida M., et al. Gradient of Developmental and Injury Response transcriptional states defines functional vulnerabilities underpinning glioblastoma heterogeneity. Nat. Cancer. 2021;2:157–173. doi: 10.1038/s43018-020-00154-9. [DOI] [PubMed] [Google Scholar]
- 57.Garofano L., Migliozzi S., Oh Y.T., D’Angelo F., Najac R.D., Ko A., Frangaj B., Caruso F.P., Yu K., Yuan J., et al. Pathway-based classification of glioblastoma uncovers a mitochondrial subtype with therapeutic vulnerabilities. Nat. Cancer. 2021;2:141–156. doi: 10.1038/s43018-020-00159-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yu W., Ma Y., Hou W., Wang F., Cheng W., Qiu F., Wu P., Zhang G. Identification of Immune-Related lncRNA Prognostic Signature and Molecular Subtypes for Glioblastoma. Front. Immunol. 2021;12:706936. doi: 10.3389/fimmu.2021.706936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Motomura K., Natsume A., Watanabe R., Ito I., Kato Y., Momota H., Nishikawa R., Mishima K., Nakasu Y., Abe T., et al. Immunohistochemical analysis-based proteomic subclassification of newly diagnosed glioblastomas. Cancer Sci. 2012;103:1871–1879. doi: 10.1111/j.1349-7006.2012.02377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hu B., Ruan Y., Wei F., Qin G., Mo X., Wang X., Zou D. Identification of three glioblastoma subtypes and a six-gene prognostic risk index based on the expression of growth factors and cytokines. Am. J. Transl. Res. 2020;12:4669–4682. [PMC free article] [PubMed] [Google Scholar]
- 61.Park J., Shim J.K., Yoon S.J., Kim S.H., Chang J.H., Kang S.G. Transcriptome profiling-based identification of prognostic subtypes and multi-omics signatures of glioblastoma. Sci. Rep. 2019;9:10555. doi: 10.1038/s41598-019-47066-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mao P., Joshi K., Li J., Kim S.H., Li P., Santana-Santos L., Luthra S., Chandran U.R., Benos P.V., Smith L., et al. Mesenchymal glioma stem cells are maintained by activated glycolytic metabolism involving aldehyde dehydrogenase 1A3. Proc. Natl. Acad. Sci. USA. 2013;110:8644–8649. doi: 10.1073/pnas.1221478110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Piao Y., Liang J., Holmes L., Henry V., Sulman E., de Groot J.F. Acquired resistance to anti-VEGF therapy in glioblastoma is associated with a mesenchymal transition. Clin. Cancer Res. 2013;19:4392–4403. doi: 10.1158/1078-0432.CCR-12-1557. [DOI] [PubMed] [Google Scholar]
- 64.Singer E., Judkins J., Salomonis N., Matlaf L., Soteropoulos P., McAllister S., Soroceanu L. Reactive oxygen species-mediated therapeutic response and resistance in glioblastoma. Cell Death Dis. 2015;6:e1601. doi: 10.1038/cddis.2014.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yin J., Oh Y.T., Kim J.Y., Kim S.S., Choi E., Kim T.H., Hong J.H., Chang N., Cho H.J., Sa J.K., et al. Transglutaminase 2 Inhibition Reverses Mesenchymal Transdifferentiation of Glioma Stem Cells by Regulating C/EBPbeta Signaling. Cancer Res. 2017;77:4973–4984. doi: 10.1158/0008-5472.CAN-17-0388. [DOI] [PubMed] [Google Scholar]
- 66.Narayanan A., Gagliardi F., Gallotti A.L., Mazzoleni S., Cominelli M., Fagnocchi L., Pala M., Piras I.S., Zordan P., Moretta N., et al. The proneural gene ASCL1 governs the transcriptional subgroup affiliation in glioblastoma stem cells by directly repressing the mesenchymal gene NDRG1. Cell Death Differ. 2019;26:1813–1831. doi: 10.1038/s41418-018-0248-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bhat K.P., Salazar K.L., Balasubramaniyan V., Wani K., Heathcock L., Hollingsworth F., James J.D., Gumin J., Diefes K.L., Kim S.H., et al. The transcriptional coactivator TAZ regulates mesenchymal differentiation in malignant glioma. Genes Dev. 2011;25:2594–2609. doi: 10.1101/gad.176800.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Beachy P.A., Karhadkar S.S., Berman D.M. Tissue repair and stem cell renewal in carcinogenesis. Nature. 2004;432:324–331. doi: 10.1038/nature03100. [DOI] [PubMed] [Google Scholar]
- 69.Huangfu D., Anderson K.V. Signaling from Smo to Ci/Gli: Conservation and divergence of Hedgehog pathways from Drosophila to vertebrates. Development. 2006;133:3–14. doi: 10.1242/dev.02169. [DOI] [PubMed] [Google Scholar]
- 70.Ingham P.W., McMahon A.P. Hedgehog signaling in animal development: Paradigms and principles. Genes Dev. 2001;15:3059–3087. doi: 10.1101/gad.938601. [DOI] [PubMed] [Google Scholar]
- 71.Ericson J., Morton S., Kawakami A., Roelink H., Jessell T.M. Two critical periods of Sonic Hedgehog signaling required for the specification of motor neuron identity. Cell. 1996;87:661–673. doi: 10.1016/S0092-8674(00)81386-0. [DOI] [PubMed] [Google Scholar]
- 72.Ruiz i Altaba A. Gli proteins and Hedgehog signaling: Development and cancer. Trends Genet. 1999;15:418–425. doi: 10.1016/S0168-9525(99)01840-5. [DOI] [PubMed] [Google Scholar]
- 73.Wechsler-Reya R.J., Scott M.P. Control of neuronal precursor proliferation in the cerebellum by Sonic Hedgehog. Neuron. 1999;22:103–114. doi: 10.1016/S0896-6273(00)80682-0. [DOI] [PubMed] [Google Scholar]
- 74.Stone D.M., Hynes M., Armanini M., Swanson T.A., Gu Q., Johnson R.L., Scott M.P., Pennica D., Goddard A., Phillips H., et al. The tumour-suppressor gene patched encodes a candidate receptor for Sonic hedgehog. Nature. 1996;384:129–134. doi: 10.1038/384129a0. [DOI] [PubMed] [Google Scholar]
- 75.Hooper J.E., Scott M.P. Communicating with Hedgehogs. Nat. Rev. Mol. Cell Biol. 2005;6:306–317. doi: 10.1038/nrm1622. [DOI] [PubMed] [Google Scholar]
- 76.Villavicencio E.H., Walterhouse D.O., Iannaccone P.M. The sonic hedgehog-patched-gli pathway in human development and disease. Am. J. Hum. Genet. 2000;67:1047–1054. doi: 10.1016/S0002-9297(07)62934-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Carballo G.B., Honorato J.R., de Lopes G.P.F., Spohr T. A highlight on Sonic hedgehog pathway. Cell Commun. Signal. 2018;16:11. doi: 10.1186/s12964-018-0220-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gupta S., Takebe N., Lorusso P. Targeting the Hedgehog pathway in cancer. Adv. Med. Oncol. 2010;2:237–250. doi: 10.1177/1758834010366430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chandra V., Das T., Gulati P., Biswas N.K., Rote S., Chatterjee U., Ghosh S.N., Deb S., Saha S.K., Chowdhury A.K., et al. Hedgehog signaling pathway is active in GBM with GLI1 mRNA expression showing a single continuous distribution rather than discrete high/low clusters. PLoS ONE. 2015;10:e0116390. doi: 10.1371/journal.pone.0116390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yan G.N., Yang L., Lv Y.F., Shi Y., Shen L.L., Yao X.H., Guo Q.N., Zhang P., Cui Y.H., Zhang X., et al. Endothelial cells promote stem-like phenotype of glioma cells through activating the Hedgehog pathway. J. Pathol. 2014;234:11–22. doi: 10.1002/path.4349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hung H.C., Liu C.C., Chuang J.Y., Su C.L., Gean P.W. Inhibition of Sonic Hedgehog Signaling Suppresses Glioma Stem-Like Cells Likely Through Inducing Autophagic Cell Death. Front. Oncol. 2020;10:1233. doi: 10.3389/fonc.2020.01233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lee S.T., Welch K.D., Panter K.E., Gardner D.R., Garrossian M., Chang C.W. Cyclopamine: From cyclops lambs to cancer treatment. J. Agric. Food Chem. 2014;62:7355–7362. doi: 10.1021/jf5005622. [DOI] [PubMed] [Google Scholar]
- 83.Carballo G.B., Matias D., Ribeiro J.H., Pessoa L.S., Arrais-Neto A.M., Spohr T. Cyclopamine sensitizes glioblastoma cells to temozolomide treatment through Sonic hedgehog pathway. Life Sci. 2020;257:118027. doi: 10.1016/j.lfs.2020.118027. [DOI] [PubMed] [Google Scholar]
- 84.Xie H., Paradise B.D., Ma W.W., Fernandez-Zapico M.E. Recent Advances in the Clinical Targeting of Hedgehog/GLI Signaling in Cancer. Cells. 2019;8:394. doi: 10.3390/cells8050394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bureta C., Saitoh Y., Tokumoto H., Sasaki H., Maeda S., Nagano S., Komiya S., Taniguchi N., Setoguchi T. Synergistic effect of arsenic trioxide, vismodegib and temozolomide on glioblastoma. Oncol. Rep. 2019;41:3404–3412. doi: 10.3892/or.2019.7100. [DOI] [PubMed] [Google Scholar]
- 86.Rudin C.M., Hann C.L., Laterra J., Yauch R.L., Callahan C.A., Fu L., Holcomb T., Stinson J., Gould S.E., Coleman B., et al. Treatment of medulloblastoma with hedgehog pathway inhibitor GDC-0449. N. Engl. J. Med. 2009;361:1173–1178. doi: 10.1056/NEJMoa0902903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sloan A.E., Nock C.J., Kerstetter A., Supko J., Ye X., Barnholtz-Sloan J.S., Miller R., Rich J., Takebe N., Prados M., et al. Targeting glioma stem cells (GSC): A biomarker and phase ii study of gdc-0449 in patients with recurrent glioblastoma multiforme (GBM) Neuro-Oncology. 2012;14:vi101–vi105. doi: 10.1093/neuonc/nos232. [DOI] [Google Scholar]
- 88.Kieran M.W., Chisholm J., Casanova M., Brandes A.A., Aerts I., Bouffet E., Bailey S., Leary S., MacDonald T.J., Mechinaud F., et al. Phase I study of oral sonidegib (LDE225) in pediatric brain and solid tumors and a phase II study in children and adults with relapsed medulloblastoma. Neuro-Oncology. 2017;19:1542–1552. doi: 10.1093/neuonc/nox109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Thomas X., Heiblig M. An evaluation of glasdegib for the treatment of acute myelogenous leukemia. Expert Opin. Pharm. 2020;21:523–530. doi: 10.1080/14656566.2020.1713094. [DOI] [PubMed] [Google Scholar]
- 90.Aftab B.T., Dobromilskaya I., Liu J.O., Rudin C.M. Itraconazole inhibits angiogenesis and tumor growth in non-small cell lung cancer. Cancer Res. 2011;71:6764–6772. doi: 10.1158/0008-5472.CAN-11-0691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Antonarakis E.S., Heath E.I., Smith D.C., Rathkopf D., Blackford A.L., Danila D.C., King S., Frost A., Ajiboye A.S., Zhao M., et al. Repurposing itraconazole as a treatment for advanced prostate cancer: A noncomparative randomized phase II trial in men with metastatic castration-resistant prostate cancer. Oncologist. 2013;18:163–173. doi: 10.1634/theoncologist.2012-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ip K.H., McKerrow K. Itraconazole in the treatment of basal cell carcinoma: A case-based review of the literature. Australas J. Derm. 2021;62:394–397. doi: 10.1111/ajd.13655. [DOI] [PubMed] [Google Scholar]
- 93.Rudin C.M., Brahmer J.R., Juergens R.A., Hann C.L., Ettinger D.S., Sebree R., Smith R., Aftab B.T., Huang P., Liu J.O. Phase 2 study of pemetrexed and itraconazole as second-line therapy for metastatic nonsquamous non-small-cell lung cancer. J. Thorac. Oncol. 2013;8:619–623. doi: 10.1097/JTO.0b013e31828c3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Beauchamp E.M., Ringer L., Bulut G., Sajwan K.P., Hall M.D., Lee Y.C., Peaceman D., Ozdemirli M., Rodriguez O., Macdonald T.J., et al. Arsenic trioxide inhibits human cancer cell growth and tumor development in mice by blocking Hedgehog/GLI pathway. J. Clin. Investig. 2011;121:148–160. doi: 10.1172/JCI42874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kumthekar P., Grimm S., Chandler J., Mehta M., Marymont M., Levy R., Muro K., Helenowski I., McCarthy K., Fountas L., et al. A phase II trial of arsenic trioxide and temozolomide in combination with radiation therapy for patients with malignant gliomas. J. Neurooncol. 2017;133:589–594. doi: 10.1007/s11060-017-2469-x. [DOI] [PubMed] [Google Scholar]
- 96.Shen H., He D., Wang S., Ding P., Wang J., Ju J. Preparation, characterization, and pharmacokinetics study of a novel genistein-loaded mixed micelles system. Drug Dev. Ind. Pharm. 2018;44:1536–1542. doi: 10.1080/03639045.2018.1483384. [DOI] [PubMed] [Google Scholar]
- 97.Honorato J.R., Hauser-Davis R.A., Saggioro E.M., Correia F.V., Sales-Junior S.F., Soares L.O.S., Lima L.D.R., Moura-Neto V., Lopes G.P.F., Spohr T. Role of Sonic hedgehog signaling in cell cycle, oxidative stress, and autophagy of temozolomide resistant glioblastoma. J. Cell Physiol. 2020;235:3798–3814. doi: 10.1002/jcp.29274. [DOI] [PubMed] [Google Scholar]
- 98.Li J., Cai J., Zhao S., Yao K., Sun Y., Li Y., Chen L., Li R., Zhai X., Zhang J., et al. GANT61, a GLI inhibitor, sensitizes glioma cells to the temozolomide treatment. J. Exp. Clin. Cancer Res. 2016;35:184. doi: 10.1186/s13046-016-0463-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Krishnamurthy N., Kurzrock R. Targeting the Wnt/beta-catenin pathway in cancer: Update on effectors and inhibitors. Cancer Treat. Rev. 2018;62:50–60. doi: 10.1016/j.ctrv.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nusse R., Clevers H. Wnt/beta-Catenin Signaling, Disease, and Emerging Therapeutic Modalities. Cell. 2017;169:985–999. doi: 10.1016/j.cell.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 101.Croce J.C., McClay D.R. Evolution of the Wnt pathways. Methods Mol. Biol. 2008;469:3–18. doi: 10.1007/978-1-60327-469-2_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gordon M.D., Nusse R. Wnt signaling: Multiple pathways, multiple receptors, and multiple transcription factors. J. Biol. Chem. 2006;281:22429–22433. doi: 10.1074/jbc.R600015200. [DOI] [PubMed] [Google Scholar]
- 103.Logan C.Y., Nusse R. The Wnt signaling pathway in development and disease. Annu. Rev. Cell Dev. Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 104.Bilic J., Huang Y.L., Davidson G., Zimmermann T., Cruciat C.M., Bienz M., Niehrs C. Wnt induces LRP6 signalosomes and promotes dishevelled-dependent LRP6 phosphorylation. Science. 2007;316:1619–1622. doi: 10.1126/science.1137065. [DOI] [PubMed] [Google Scholar]
- 105.He X., Semenov M., Tamai K., Zeng X. LDL receptor-related proteins 5 and 6 in Wnt/beta-catenin signaling: Arrows point the way. Development. 2004;131:1663–1677. doi: 10.1242/dev.01117. [DOI] [PubMed] [Google Scholar]
- 106.MacDonald B.T., Tamai K., He X. Wnt/beta-catenin signaling: Components, mechanisms, and diseases. Dev. Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cheng X., Xu X., Chen D., Zhao F., Wang W. Therapeutic potential of targeting the Wnt/beta-catenin signaling pathway in colorectal cancer. Biomed. Pharm. 2019;110:473–481. doi: 10.1016/j.biopha.2018.11.082. [DOI] [PubMed] [Google Scholar]
- 108.Zhang X., Wang L., Qu Y. Targeting the beta-catenin signaling for cancer therapy. Pharm. Res. 2020;160:104794. doi: 10.1016/j.phrs.2020.104794. [DOI] [PubMed] [Google Scholar]
- 109.Latour M., Her N.G., Kesari S., Nurmemmedov E. WNT Signaling as a Therapeutic Target for Glioblastoma. Int. J. Mol. Sci. 2021;22:8428. doi: 10.3390/ijms22168428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Patel A.P., Tirosh I., Trombetta J.J., Shalek A.K., Gillespie S.M., Wakimoto H., Cahill D.P., Nahed B.V., Curry W.T., Martuza R.L., et al. Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science. 2014;344:1396–1401. doi: 10.1126/science.1254257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pulvirenti T., Van Der Heijden M., Droms L.A., Huse J.T., Tabar V., Hall A. Dishevelled 2 signaling promotes self-renewal and tumorigenicity in human gliomas. Cancer Res. 2011;71:7280–7290. doi: 10.1158/0008-5472.CAN-11-1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tang C., Guo J., Chen H., Yao C.J., Zhuang D.X., Wang Y., Tang W.J., Ren G., Yao Y., Wu J.S., et al. Gene mutation profiling of primary glioblastoma through multiple tumor biopsy guided by 1H-magnetic resonance spectroscopy. Int. J. Clin. Exp. Pathol. 2015;8:5327–5335. [PMC free article] [PubMed] [Google Scholar]
- 113.Lorzadeh S., Kohan L., Ghavami S., Azarpira N. Autophagy and the Wnt signaling pathway: A focus on Wnt/beta-catenin signaling. Biochim. Biophys. Acta Mol. Cell Res. 2021;1868:118926. doi: 10.1016/j.bbamcr.2020.118926. [DOI] [PubMed] [Google Scholar]
- 114.Morris L.G., Kaufman A.M., Gong Y., Ramaswami D., Walsh L.A., Turcan S., Eng S., Kannan K., Zou Y., Peng L., et al. Recurrent somatic mutation of FAT1 in multiple human cancers leads to aberrant Wnt activation. Nat. Genet. 2013;45:253–261. doi: 10.1038/ng.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sareddy G.R., Pratap U.P., Viswanadhapalli S., Venkata P.P., Nair B.C., Krishnan S.R., Zheng S., Gilbert A.R., Brenner A.J., Brann D.W., et al. PELP1 promotes glioblastoma progression by enhancing Wnt/beta-catenin signaling. Neurooncol. Adv. 2019;1:vdz042. doi: 10.1093/noajnl/vdz042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Shaji S.K., Sunilkumar D., Mahalakshmi N.V., Kumar G.B., Nair B.G. Analysis of microarray data for identification of key microRNA signatures in glioblastoma multiforme. Oncol. Lett. 2019;18:1938–1948. doi: 10.3892/ol.2019.10521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Vecera M., Sana J., Lipina R., Smrcka M., Slaby O. Long Non-Coding RNAs in Gliomas: From Molecular Pathology to Diagnostic Biomarkers and Therapeutic Targets. Int. J. Mol. Sci. 2018;19:2754. doi: 10.3390/ijms19092754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wang Y.N., Chen W.Q., Shi Y.X., Yan C.R., Kong Z.R., Wang Y.K., Wang Y., Ma W.B. Imposing Phase II and Phase III Clinical Trials of Targeted Drugs for Glioblastoma: Current Status and Progress. Front. Oncol. 2021;11:3611. doi: 10.3389/fonc.2021.719623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.He L., Zhou H., Zeng Z., Yao H., Jiang W., Qu H. Wnt/beta-catenin signaling cascade: A promising target for glioma therapy. J. Cell Physiol. 2019;234:2217–2228. doi: 10.1002/jcp.27186. [DOI] [PubMed] [Google Scholar]
- 120.Zhang Y., Wang X. Targeting the Wnt/beta-catenin signaling pathway in cancer. J. Hematol. Oncol. 2020;13:165. doi: 10.1186/s13045-020-00990-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Allen J.E., Krigsfeld G., Mayes P.A., Patel L., Dicker D.T., Patel A.S., Dolloff N.G., Messaris E., Scata K.A., Wang W., et al. Dual inactivation of Akt and ERK by TIC10 signals Foxo3a nuclear translocation, TRAIL gene induction, and potent antitumor effects. Sci. Transl. Med. 2013;5:171ra117. doi: 10.1126/scitranslmed.3004828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.De Robertis A., Valensin S., Rossi M., Tunici P., Verani M., De Rosa A., Giordano C., Varrone M., Nencini A., Pratelli C., et al. Identification and characterization of a small-molecule inhibitor of Wnt signaling in glioblastoma cells. Mol. Cancer Ther. 2013;12:1180–1189. doi: 10.1158/1535-7163.MCT-12-1176-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gao L., Chen B., Li J., Yang F., Cen X., Liao Z., Long X. Wnt/beta-catenin signaling pathway inhibits the proliferation and apoptosis of U87 glioma cells via different mechanisms. PLoS ONE. 2017;12:e0181346. doi: 10.1371/journal.pone.0181346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kierulf-Vieira K.S., Sandberg C.J., Waaler J., Lund K., Skaga E., Saberniak B.M., Panagopoulos I., Brandal P., Krauss S., Langmoen I.A., et al. A Small-Molecule Tankyrase Inhibitor Reduces Glioma Stem Cell Proliferation and Sphere Formation. Cancers. 2020;12:1630. doi: 10.3390/cancers12061630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kim Y., Kim K.H., Lee J., Lee Y.A., Kim M., Lee S.J., Park K., Yang H., Jin J., Joo K.M., et al. Wnt activation is implicated in glioblastoma radioresistance. Lab. Investig. 2012;92:466–473. doi: 10.1038/labinvest.2011.161. [DOI] [PubMed] [Google Scholar]
- 126.Korur S., Huber R.M., Sivasankaran B., Petrich M., Morin P., Jr., Hemmings B.A., Merlo A., Lino M.M. GSK3beta regulates differentiation and growth arrest in glioblastoma. PLoS ONE. 2009;4:e7443. doi: 10.1371/journal.pone.0007443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Prabhu V.V., Lulla A.R., Madhukar N.S., Ralff M.D., Zhao D., Kline C.L.B., Van den Heuvel A.P.J., Lev A., Garnett M.J., McDermott U., et al. Cancer stem cell-related gene expression as a potential biomarker of response for first-in-class imipridone ONC201 in solid tumors. PLoS ONE. 2017;12:e0180541. doi: 10.1371/journal.pone.0180541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Suwala A.K., Koch K., Rios D.H., Aretz P., Uhlmann C., Ogorek I., Felsberg J., Reifenberger G., Kohrer K., Deenen R., et al. Inhibition of Wnt/beta-catenin signaling downregulates expression of aldehyde dehydrogenase isoform 3A1 (ALDH3A1) to reduce resistance against temozolomide in glioblastoma in vitro. Oncotarget. 2018;9:22703–22716. doi: 10.18632/oncotarget.25210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Varghese R.T., Young S., Pham L., Liang Y., Pridham K.J., Guo S., Murphy S., Kelly D.F., Sheng Z. Casein Kinase 1 Epsilon Regulates Glioblastoma Cell Survival. Sci. Rep. 2018;8:13621. doi: 10.1038/s41598-018-31864-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yu W.K., Xu Z.Y., Yuan L., Mo S., Xu B., Cheng X.D., Qin J.J. Targeting beta-Catenin Signaling by Natural Products for Cancer Prevention and Therapy. Front. Pharm. 2020;11:984. doi: 10.3389/fphar.2020.00984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhang F.Y., Hu Y., Que Z.Y., Wang P., Liu Y.H., Wang Z.H., Xue Y.X. Shikonin Inhibits the Migration and Invasion of Human Glioblastoma Cells by Targeting Phosphorylated beta-Catenin and Phosphorylated PI3K/Akt: A Potential Mechanism for the Anti-Glioma Efficacy of a Traditional Chinese Herbal Medicine. Int. J. Mol. Sci. 2015;16:23823–23848. doi: 10.3390/ijms161023823. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 132.Miao J., Jiang Y., Wang D., Zhou J., Fan C., Jiao F., Liu B., Zhang J., Wang Y., Zhang Q. Trichosanthin suppresses the proliferation of glioma cells by inhibiting LGR5 expression and the Wnt/beta-catenin signaling pathway. Oncol. Rep. 2015;34:2845–2852. doi: 10.3892/or.2015.4290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mora M.C., Bassa L.M., Wong K.E., Tirabassi M.V., Arenas R.B., Schneider S.S. Rhodiola crenulata inhibits Wnt/beta-catenin signaling in glioblastoma. J. Surg. Res. 2015;197:247–255. doi: 10.1016/j.jss.2015.02.074. [DOI] [PubMed] [Google Scholar]
- 134.Cilibrasi C., Riva G., Romano G., Cadamuro M., Bazzoni R., Butta V., Paoletta L., Dalpra L., Strazzabosco M., Lavitrano M., et al. Resveratrol Impairs Glioma Stem Cells Proliferation and Motility by Modulating the Wnt Signaling Pathway. PLoS ONE. 2017;12:e0169854. doi: 10.1371/journal.pone.0169854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tao W., Chu C., Zhou W., Huang Z., Zhai K., Fang X., Huang Q., Zhang A., Wang X., Yu X., et al. Dual Role of WISP1 in maintaining glioma stem cells and tumor-supportive macrophages in glioblastoma. Nat. Commun. 2020;11:3015. doi: 10.1038/s41467-020-16827-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Williams S.P., Nowicki M.O., Liu F., Press R., Godlewski J., Abdel-Rasoul M., Kaur B., Fernandez S.A., Chiocca E.A., Lawler S.E. Indirubins decrease glioma invasion by blocking migratory phenotypes in both the tumor and stromal endothelial cell compartments. Cancer Res. 2011;71:5374–5380. doi: 10.1158/0008-5472.CAN-10-3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tao Q., Wu C., Xu R., Niu L., Qin J., Liu N., Zhang P., Wang C. Diallyl trisulfide inhibits proliferation, invasion and angiogenesis of glioma cells by inactivating Wnt/beta-catenin signaling. Cell Tissue Res. 2017;370:379–390. doi: 10.1007/s00441-017-2678-9. [DOI] [PubMed] [Google Scholar]
- 138.Lan F., Pan Q., Yu H., Yue X. Sulforaphane enhances temozolomide-induced apoptosis because of down-regulation of miR-21 via Wnt/beta-catenin signaling in glioblastoma. J. Neurochem. 2015;134:811–818. doi: 10.1111/jnc.13174. [DOI] [PubMed] [Google Scholar]
- 139.Sareddy G.R., Kesanakurti D., Kirti P.B., Babu P.P. Nonsteroidal anti-inflammatory drugs diclofenac and celecoxib attenuates Wnt/beta-catenin/Tcf signaling pathway in human glioblastoma cells. Neurochem. Res. 2013;38:2313–2322. doi: 10.1007/s11064-013-1142-9. [DOI] [PubMed] [Google Scholar]
- 140.Lan F., Yue X., Han L., Yuan X., Shi Z., Huang K., Yang Y., Zou J., Zhang J., Jiang T., et al. Antitumor effect of aspirin in glioblastoma cells by modulation of beta-catenin/T-cell factor-mediated transcriptional activity. J. Neurosurg. 2011;115:780–788. doi: 10.3171/2011.5.JNS113. [DOI] [PubMed] [Google Scholar]
- 141.Wieland A., Trageser D., Gogolok S., Reinartz R., Hofer H., Keller M., Leinhaas A., Schelle R., Normann S., Klaas L., et al. Anticancer effects of niclosamide in human glioblastoma. Clin. Cancer Res. 2013;19:4124–4136. doi: 10.1158/1078-0432.CCR-12-2895. [DOI] [PubMed] [Google Scholar]
- 142.Oh H.C., Shim J.K., Park J., Lee J.H., Choi R.J., Kim N.H., Kim H.S., Moon J.H., Kim E.H., Chang J.H., et al. Combined effects of niclosamide and temozolomide against human glioblastoma tumorspheres. J. Cancer Res. Clin. Oncol. 2020;146:2817–2828. doi: 10.1007/s00432-020-03330-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wang Y., Huang N., Li H., Liu S., Chen X., Yu S., Wu N., Bian X.W., Shen H.Y., Li C., et al. Promoting oligodendroglial-oriented differentiation of glioma stem cell: A repurposing of quetiapine for the treatment of malignant glioma. Oncotarget. 2017;8:37511–37524. doi: 10.18632/oncotarget.16400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wan Z., Shi W., Shao B., Shi J., Shen A., Ma Y., Chen J., Lan Q. Peroxisome proliferator-activated receptor gamma agonist pioglitazone inhibits beta-catenin-mediated glioma cell growth and invasion. Mol. Cell Biochem. 2011;349:1–10. doi: 10.1007/s11010-010-0637-9. [DOI] [PubMed] [Google Scholar]
- 145.Cilibrasi C., Butta V., Riva G., Bentivegna A. Pioglitazone Effect on Glioma Stem Cell Lines: Really a Promising Drug Therapy for Glioblastoma? PPAR Res. 2016;2016:7175067. doi: 10.1155/2016/7175067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Jin X., Jeon H.Y., Joo K.M., Kim J.K., Jin J., Kim S.H., Kang B.G., Beck S., Lee S.J., Kim J.K., et al. Frizzled 4 regulates stemness and invasiveness of migrating glioma cells established by serial intracranial transplantation. Cancer Res. 2011;71:3066–3075. doi: 10.1158/0008-5472.CAN-10-1495. [DOI] [PubMed] [Google Scholar]
- 147.Zeppernick F., Ahmadi R., Campos B., Dictus C., Helmke B.M., Becker N., Lichter P., Unterberg A., Radlwimmer B., Herold-Mende C.C. Stem cell marker CD133 affects clinical outcome in glioma patients. Clin. Cancer Res. 2008;14:123–129. doi: 10.1158/1078-0432.CCR-07-0932. [DOI] [PubMed] [Google Scholar]
- 148.Arrillaga-Romany I., Kurz S., Tarapore R.S., Sumrall A., Butowski N., Harrison R., de Groot J., Chi A., Shonka N., Umemura Y., et al. Efficacy of Onc201 in Patients with Onc201 for Recurrent H3 K27m-Mutant Diffuse Midline Glioma. Neuro-Oncology. 2020;22:50–51. doi: 10.1093/neuonc/noaa215.204. [DOI] [Google Scholar]
- 149.Le P.N., McDermott J.D., Jimeno A. Targeting the Wnt pathway in human cancers: Therapeutic targeting with a focus on OMP-54F28. Pharmacol. Ther. 2015;146:1–11. doi: 10.1016/j.pharmthera.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Gurney A., Axelrod F., Bond C.J., Cain J., Chartier C., Donigan L., Fischer M., Chaudhari A., Ji M., Kapoun A.M., et al. Wnt pathway inhibition via the targeting of Frizzled receptors results in decreased growth and tumorigenicity of human tumors. Proc. Natl. Acad. Sci. USA. 2012;109:11717–11722. doi: 10.1073/pnas.1120068109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Pavlovic Z., Adams J.J., Blazer L.L., Gakhal A.K., Jarvik N., Steinhart Z., Robitaille M., Mascall K., Pan J., Angers S., et al. A synthetic anti-Frizzled antibody engineered for broadened specificity exhibits enhanced anti-tumor properties. Mabs. 2018;10:1157–1167. doi: 10.1080/19420862.2018.1515565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Newman D.J., Cragg G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020;83:770–803. doi: 10.1021/acs.jnatprod.9b01285. [DOI] [PubMed] [Google Scholar]
- 153.Qian B., Nag S.A., Su Y., Voruganti S., Qin J.J., Zhang R., Cho W.C. miRNAs in cancer prevention and treatment and as molecular targets for natural product anticancer agents. Curr. Cancer Drug Targets. 2013;13:519–541. doi: 10.2174/15680096113139990031. [DOI] [PubMed] [Google Scholar]
- 154.Qin J., Wang W., Zhang R. Novel natural product therapeutics targeting both inflammation and cancer. Chin. J. Nat. Med. 2017;15:401–416. doi: 10.1016/S1875-5364(17)30062-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Penas-Prado M., Hess K.R., Fisch M.J., Lagrone L.W., Groves M.D., Levin V.A., De Groot J.F., Puduvalli V.K., Colman H., Volas-Redd G., et al. Randomized phase II adjuvant factorial study of dose-dense temozolomide alone and in combination with isotretinoin, celecoxib, and/or thalidomide for glioblastoma. Neuro-Oncology. 2015;17:266–273. doi: 10.1093/neuonc/nou155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Huang M., Zhang D., Wu J.Y., Xing K., Yeo E., Li C., Zhang L., Holland E., Yao L., Qin L., et al. Wnt-mediated endothelial transformation into mesenchymal stem cell-like cells induces chemoresistance in glioblastoma. Sci. Transl. Med. 2020;12:eaay7522. doi: 10.1126/scitranslmed.aay7522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Seano G., Jain R.K. Vessel co-option in glioblastoma: Emerging insights and opportunities. Angiogenesis. 2020;23:9–16. doi: 10.1007/s10456-019-09691-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Jain R.K. Antiangiogenesis strategies revisited: From starving tumors to alleviating hypoxia. Cancer Cell. 2014;26:605–622. doi: 10.1016/j.ccell.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Griveau A., Seano G., Shelton S.J., Kupp R., Jahangiri A., Obernier K., Krishnan S., Lindberg O.R., Yuen T.J., Tien A.C., et al. A Glial Signature and Wnt7 Signaling Regulate Glioma-Vascular Interactions and Tumor Microenvironment. Cancer Cell. 2018;33:874–889.e877. doi: 10.1016/j.ccell.2018.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Watkins S., Robel S., Kimbrough I.F., Robert S.M., Ellis-Davies G., Sontheimer H. Disruption of astrocyte-vascular coupling and the blood-brain barrier by invading glioma cells. Nat. Commun. 2014;5:4196. doi: 10.1038/ncomms5196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Baker G.J., Yadav V.N., Motsch S., Koschmann C., Calinescu A.A., Mineharu Y., Camelo-Piragua S.I., Orringer D., Bannykh S., Nichols W.S., et al. Mechanisms of glioma formation: Iterative perivascular glioma growth and invasion leads to tumor progression, VEGF-independent vascularization, and resistance to antiangiogenic therapy. Neoplasia. 2014;16:543–561. doi: 10.1016/j.neo.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Di Tomaso E., Snuderl M., Kamoun W.S., Duda D.G., Auluck P.K., Fazlollahi L., Andronesi O.C., Frosch M.P., Wen P.Y., Plotkin S.R., et al. Glioblastoma recurrence after cediranib therapy in patients: Lack of “rebound” revascularization as mode of escape. Cancer Res. 2011;71:19–28. doi: 10.1158/0008-5472.CAN-10-2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Voutouri C., Kirkpatrick N.D., Chung E., Mpekris F., Baish J.W., Munn L.L., Fukumura D., Stylianopoulos T., Jain R.K. Experimental and computational analyses reveal dynamics of tumor vessel cooption and optimal treatment strategies. Proc. Natl. Acad. Sci. USA. 2019;116:2662–2671. doi: 10.1073/pnas.1818322116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Whelan R., Hargaden G.C., Knox A.J.S. Modulating the Blood-Brain Barrier: A Comprehensive Review. Pharmaceutics. 2021;13:1980. doi: 10.3390/pharmaceutics13111980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Cho C., Smallwood P.M., Nathans J. Reck and Gpr124 Are Essential Receptor Cofactors for Wnt7a/Wnt7b-Specific Signaling in Mammalian CNS Angiogenesis and Blood-Brain Barrier Regulation. Neuron. 2017;95:1056–1073.e1055. doi: 10.1016/j.neuron.2017.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Vanhollebeke B., Stone O.A., Bostaille N., Cho C., Zhou Y., Maquet E., Gauquier A., Cabochette P., Fukuhara S., Mochizuki N., et al. Tip cell-specific requirement for an atypical Gpr124- and Reck-dependent Wnt/beta-catenin pathway during brain angiogenesis. eLife. 2015;4:e06489. doi: 10.7554/eLife.06489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Martin M., Vermeiren S., Bostaille N., Eubelen M., Spitzer D., Vermeersch M., Profaci C.P., Pozuelo E., Toussay X., Raman-Nair J., et al. Engineered Wnt ligands enable blood-brain barrier repair in neurological disorders. Science. 2022;375:eabm4459. doi: 10.1126/science.abm4459. [DOI] [PubMed] [Google Scholar]
- 168.Chavali M., Ulloa-Navas M.J., Perez-Borreda P., Garcia-Verdugo J.M., McQuillen P.S., Huang E.J., Rowitch D.H. Wnt-Dependent Oligodendroglial-Endothelial Interactions Regulate White Matter Vascularization and Attenuate Injury. Neuron. 2020;108:1130–1145.e1135. doi: 10.1016/j.neuron.2020.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Reis M., Czupalla C.J., Ziegler N., Devraj K., Zinke J., Seidel S., Heck R., Thom S., Macas J., Bockamp E., et al. Endothelial Wnt/beta-catenin signaling inhibits glioma angiogenesis and normalizes tumor blood vessels by inducing PDGF-B expression. J. Exp. Med. 2012;209:1611–1627. doi: 10.1084/jem.20111580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Andersson E.R., Sandberg R., Lendahl U. Notch signaling: Simplicity in design, versatility in function. Development. 2011;138:3593–3612. doi: 10.1242/dev.063610. [DOI] [PubMed] [Google Scholar]
- 171.Liu J., Sato C., Cerletti M., Wagers A. Notch signaling in the regulation of stem cell self-renewal and differentiation. Curr. Top. Dev. Biol. 2010;92:367–409. doi: 10.1016/S0070-2153(10)92012-7. [DOI] [PubMed] [Google Scholar]
- 172.Imayoshi I., Sakamoto M., Yamaguchi M., Mori K., Kageyama R. Essential roles of Notch signaling in maintenance of neural stem cells in developing and adult brains. J. Neurosci. 2010;30:3489–3498. doi: 10.1523/JNEUROSCI.4987-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Pierfelice T., Alberi L., Gaiano N. Notch in the vertebrate nervous system: An old dog with new tricks. Neuron. 2011;69:840–855. doi: 10.1016/j.neuron.2011.02.031. [DOI] [PubMed] [Google Scholar]
- 174.Bray S.J. Notch signalling: A simple pathway becomes complex. Nat. Rev. Mol. Cell Biol. 2006;7:678–689. doi: 10.1038/nrm2009. [DOI] [PubMed] [Google Scholar]
- 175.Kopan R., Ilagan M.X. The canonical Notch signaling pathway: Unfolding the activation mechanism. Cell. 2009;137:216–233. doi: 10.1016/j.cell.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Cheng H.T., Kim M., Valerius M.T., Surendran K., Schuster-Gossler K., Gossler A., McMahon A.P., Kopan R. Notch2, but not Notch1, is required for proximal fate acquisition in the mammalian nephron. Development. 2007;134:801–811. doi: 10.1242/dev.02773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Krebs L.T., Iwai N., Nonaka S., Welsh I.C., Lan Y., Jiang R., Saijoh Y., O’Brien T.P., Hamada H., Gridley T. Notch signaling regulates left-right asymmetry determination by inducing Nodal expression. Genes Dev. 2003;17:1207–1212. doi: 10.1101/gad.1084703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Matsuura A., Ito M., Sakaidani Y., Kondo T., Murakami K., Furukawa K., Nadano D., Matsuda T., Okajima T. O-linked N-acetylglucosamine is present on the extracellular domain of notch receptors. J. Biol. Chem. 2008;283:35486–35495. doi: 10.1074/jbc.M806202200. [DOI] [PubMed] [Google Scholar]
- 179.Moloney D.J., Shair L.H., Lu F.M., Xia J., Locke R., Matta K.L., Haltiwanger R.S. Mammalian Notch1 is modified with two unusual forms of O-linked glycosylation found on epidermal growth factor-like modules. J. Biol. Chem. 2000;275:9604–9611. doi: 10.1074/jbc.275.13.9604. [DOI] [PubMed] [Google Scholar]
- 180.Blaumueller C.M., Qi H., Zagouras P., Artavanis-Tsakonas S. Intracellular cleavage of Notch leads to a heterodimeric receptor on the plasma membrane. Cell. 1997;90:281–291. doi: 10.1016/S0092-8674(00)80336-0. [DOI] [PubMed] [Google Scholar]
- 181.Sanchez-Irizarry C., Carpenter A.C., Weng A.P., Pear W.S., Aster J.C., Blacklow S.C. Notch subunit heterodimerization and prevention of ligand-independent proteolytic activation depend, respectively, on a novel domain and the LNR repeats. Mol. Cell Biol. 2004;24:9265–9273. doi: 10.1128/MCB.24.21.9265-9273.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Brou C., Logeat F., Gupta N., Bessia C., LeBail O., Doedens J.R., Cumano A., Roux P., Black R.A., Israel A. A novel proteolytic cleavage involved in Notch signaling: The role of the disintegrin-metalloprotease TACE. Mol. Cell. 2000;5:207–216. doi: 10.1016/S1097-2765(00)80417-7. [DOI] [PubMed] [Google Scholar]
- 183.Van Tetering G., van Diest P., Verlaan I., van der Wall E., Kopan R., Vooijs M. Metalloprotease ADAM10 is required for Notch1 site 2 cleavage. J. Biol. Chem. 2009;284:31018–31027. doi: 10.1074/jbc.M109.006775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.De Strooper B., Annaert W., Cupers P., Saftig P., Craessaerts K., Mumm J.S., Schroeter E.H., Schrijvers V., Wolfe M.S., Ray W.J., et al. A presenilin-1-dependent gamma-secretase-like protease mediates release of Notch intracellular domain. Nature. 1999;398:518–522. doi: 10.1038/19083. [DOI] [PubMed] [Google Scholar]
- 185.Mumm J.S., Schroeter E.H., Saxena M.T., Griesemer A., Tian X., Pan D.J., Ray W.J., Kopan R. A ligand-induced extracellular cleavage regulates gamma-secretase-like proteolytic activation of Notch1. Mol. Cell. 2000;5:197–206. doi: 10.1016/S1097-2765(00)80416-5. [DOI] [PubMed] [Google Scholar]
- 186.Christensen S., Kodoyianni V., Bosenberg M., Friedman L., Kimble J. lag-1, a gene required for lin-12 and glp-1 signaling in Caenorhabditis elegans, is homologous to human CBF1 and Drosophila Su(H) Development. 1996;122:1373–1383. doi: 10.1242/dev.122.5.1373. [DOI] [PubMed] [Google Scholar]
- 187.Fortini M.E., Artavanis-Tsakonas S. The suppressor of hairless protein participates in notch receptor signaling. Cell. 1994;79:273–282. doi: 10.1016/0092-8674(94)90196-1. [DOI] [PubMed] [Google Scholar]
- 188.Tamura K., Taniguchi Y., Minoguchi S., Sakai T., Tun T., Furukawa T., Honjo T. Physical interaction between a novel domain of the receptor Notch and the transcription factor RBP-J kappa/Su(H) Curr. Biol. 1995;5:1416–1423. doi: 10.1016/S0960-9822(95)00279-X. [DOI] [PubMed] [Google Scholar]
- 189.Wu L., Sun T., Kobayashi K., Gao P., Griffin J.D. Identification of a family of mastermind-like transcriptional coactivators for mammalian notch receptors. Mol. Cell Biol. 2002;22:7688–7700. doi: 10.1128/MCB.22.21.7688-7700.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Kovall R.A. More complicated than it looks: Assembly of Notch pathway transcription complexes. Oncogene. 2008;27:5099–5109. doi: 10.1038/onc.2008.223. [DOI] [PubMed] [Google Scholar]
- 191.Nam Y., Sliz P., Song L., Aster J.C., Blacklow S.C. Structural basis for cooperativity in recruitment of MAML coactivators to Notch transcription complexes. Cell. 2006;124:973–983. doi: 10.1016/j.cell.2005.12.037. [DOI] [PubMed] [Google Scholar]
- 192.Wilson J.J., Kovall R.A. Crystal structure of the CSL-Notch-Mastermind ternary complex bound to DNA. Cell. 2006;124:985–996. doi: 10.1016/j.cell.2006.01.035. [DOI] [PubMed] [Google Scholar]
- 193.Kadam S., Emerson B.M. Transcriptional specificity of human SWI/SNF BRG1 and BRM chromatin remodeling complexes. Mol. Cell. 2003;11:377–389. doi: 10.1016/S1097-2765(03)00034-0. [DOI] [PubMed] [Google Scholar]
- 194.Wallberg A.E., Pedersen K., Lendahl U., Roeder R.G. p300 and PCAF act cooperatively to mediate transcriptional activation from chromatin templates by notch intracellular domains in vitro. Mol. Cell Biol. 2002;22:7812–7819. doi: 10.1128/MCB.22.22.7812-7819.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Iso T., Sartorelli V., Chung G., Shichinohe T., Kedes L., Hamamori Y. HERP, a new primary target of Notch regulated by ligand binding. Mol. Cell Biol. 2001;21:6071–6079. doi: 10.1128/MCB.21.17.6071-6079.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Jarriault S., Brou C., Logeat F., Schroeter E.H., Kopan R., Israel A. Signalling downstream of activated mammalian Notch. Nature. 1995;377:355–358. doi: 10.1038/377355a0. [DOI] [PubMed] [Google Scholar]
- 197.El Hindy N., Keyvani K., Pagenstecher A., Dammann P., Sandalcioglu I.E., Sure U., Zhu Y. Implications of Dll4-Notch signaling activation in primary glioblastoma multiforme. Neuro-Oncology. 2013;15:1366–1378. doi: 10.1093/neuonc/not071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Purow B.W., Haque R.M., Noel M.W., Su Q., Burdick M.J., Lee J., Sundaresan T., Pastorino S., Park J.K., Mikolaenko I., et al. Expression of Notch-1 and its ligands, Delta-like-1 and Jagged-1, is critical for glioma cell survival and proliferation. Cancer Res. 2005;65:2353–2363. doi: 10.1158/0008-5472.CAN-04-1890. [DOI] [PubMed] [Google Scholar]
- 199.Shih A.H., Holland E.C. Notch signaling enhances nestin expression in gliomas. Neoplasia. 2006;8:1072–1082. doi: 10.1593/neo.06526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Parmigiani E., Taylor V., Giachino C. Oncogenic and Tumor-Suppressive Functions of NOTCH Signaling in Glioma. Cells. 2020;9:2304. doi: 10.3390/cells9102304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Giachino C., Boulay J.L., Ivanek R., Alvarado A., Tostado C., Lugert S., Tchorz J., Coban M., Mariani L., Bettler B., et al. A Tumor Suppressor Function for Notch Signaling in Forebrain Tumor Subtypes. Cancer Cell. 2015;28:730–742. doi: 10.1016/j.ccell.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 202.Fan X., Khaki L., Zhu T.S., Soules M.E., Talsma C.E., Gul N., Koh C., Zhang J., Li Y.M., Maciaczyk J., et al. NOTCH pathway blockade depletes CD133-positive glioblastoma cells and inhibits growth of tumor neurospheres and xenografts. Stem Cells. 2010;28:5–16. doi: 10.1002/stem.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Zhu T.S., Costello M.A., Talsma C.E., Flack C.G., Crowley J.G., Hamm L.L., He X., Hervey-Jumper S.L., Heth J.A., Muraszko K.M., et al. Endothelial cells create a stem cell niche in glioblastoma by providing NOTCH ligands that nurture self-renewal of cancer stem-like cells. Cancer Res. 2011;71:6061–6072. doi: 10.1158/0008-5472.CAN-10-4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Wang J., Wakeman T.P., Lathia J.D., Hjelmeland A.B., Wang X.F., White R.R., Rich J.N., Sullenger B.A. Notch promotes radioresistance of glioma stem cells. Stem Cells. 2010;28:17–28. doi: 10.1002/stem.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Gilbert C.A., Daou M.C., Moser R.P., Ross A.H. Gamma-secretase inhibitors enhance temozolomide treatment of human gliomas by inhibiting neurosphere repopulation and xenograft recurrence. Cancer Res. 2010;70:6870–6879. doi: 10.1158/0008-5472.CAN-10-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Bazzoni R., Bentivegna A. Role of Notch Signaling Pathway in Glioblastoma Pathogenesis. Cancers. 2019;11:292. doi: 10.3390/cancers11030292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Hovinga K.E., Shimizu F., Wang R., Panagiotakos G., Van Der Heijden M., Moayedpardazi H., Correia A.S., Soulet D., Major T., Menon J., et al. Inhibition of notch signaling in glioblastoma targets cancer stem cells via an endothelial cell intermediate. Stem Cells. 2010;28:1019–1029. doi: 10.1002/stem.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Lin J., Zhang X.M., Yang J.C., Ye Y.B., Luo S.Q. gamma-secretase inhibitor-I enhances radiosensitivity of glioblastoma cell lines by depleting CD133+ tumor cells. Arch. Med. Res. 2010;41:519–529. doi: 10.1016/j.arcmed.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 209.Saito N., Fu J., Zheng S., Yao J., Wang S., Liu D.D., Yuan Y., Sulman E.P., Lang F.F., Colman H., et al. A high Notch pathway activation predicts response to gamma secretase inhibitors in proneural subtype of glioma tumor-initiating cells. Stem Cells. 2014;32:301–312. doi: 10.1002/stem.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Xu R., Shimizu F., Hovinga K., Beal K., Karimi S., Droms L., Peck K.K., Gutin P., Iorgulescu J.B., Kaley T., et al. Molecular and Clinical Effects of Notch Inhibition in Glioma Patients: A Phase 0/I Trial. Clin. Cancer Res. 2016;22:4786–4796. doi: 10.1158/1078-0432.CCR-16-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Pan E., Supko J.G., Kaley T.J., Butowski N.A., Cloughesy T., Jung J., Desideri S., Grossman S., Ye X., Park D.M. Phase I study of RO4929097 with bevacizumab in patients with recurrent malignant glioma. J. Neurooncol. 2016;130:571–579. doi: 10.1007/s11060-016-2263-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Peereboom D.M., Ye X., Mikkelsen T., Lesser G.J., Lieberman F.S., Robins H.I., Ahluwalia M.S., Sloan A.E., Grossman S.A. A Phase II and Pharmacodynamic Trial of RO4929097 for Patients With Recurrent/Progressive Glioblastoma. Neurosurgery. 2021;88:246–251. doi: 10.1093/neuros/nyaa412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Tolcher A.W., Messersmith W.A., Mikulski S.M., Papadopoulos K.P., Kwak E.L., Gibbon D.G., Patnaik A., Falchook G.S., Dasari A., Shapiro G.I., et al. Phase I study of RO4929097, a gamma secretase inhibitor of Notch signaling, in patients with refractory metastatic or locally advanced solid tumors. J. Clin. Oncol. 2012;30:2348–2353. doi: 10.1200/JCO.2011.36.8282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Fouladi M., Stewart C.F., Olson J., Wagner L.M., Onar-Thomas A., Kocak M., Packer R.J., Goldman S., Gururangan S., Gajjar A., et al. Phase I trial of MK-0752 in children with refractory CNS malignancies: A pediatric brain tumor consortium study. J. Clin. Oncol. 2011;29:3529–3534. doi: 10.1200/JCO.2011.35.7806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Krop I., Demuth T., Guthrie T., Wen P.Y., Mason W.P., Chinnaiyan P., Butowski N., Groves M.D., Kesari S., Freedman S.J., et al. Phase I pharmacologic and pharmacodynamic study of the gamma secretase (Notch) inhibitor MK-0752 in adult patients with advanced solid tumors. J. Clin. Oncol. 2012;30:2307–2313. doi: 10.1200/JCO.2011.39.1540. [DOI] [PubMed] [Google Scholar]
- 216.Milano J., McKay J., Dagenais C., Foster-Brown L., Pognan F., Gadient R., Jacobs R.T., Zacco A., Greenberg B., Ciaccio P.J. Modulation of notch processing by gamma-secretase inhibitors causes intestinal goblet cell metaplasia and induction of genes known to specify gut secretory lineage differentiation. Toxicol. Sci. 2004;82:341–358. doi: 10.1093/toxsci/kfh254. [DOI] [PubMed] [Google Scholar]
- 217.Riccio O., van Gijn M.E., Bezdek A.C., Pellegrinet L., van Es J.H., Zimber-Strobl U., Strobl L.J., Honjo T., Clevers H., Radtke F. Loss of intestinal crypt progenitor cells owing to inactivation of both Notch1 and Notch2 is accompanied by derepression of CDK inhibitors p27Kip1 and p57Kip2. EMBO Rep. 2008;9:377–383. doi: 10.1038/embor.2008.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Beel A.J., Sanders C.R. Substrate specificity of gamma-secretase and other intramembrane proteases. Cell Mol. Life Sci. 2008;65:1311–1334. doi: 10.1007/s00018-008-7462-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Hemming M.L., Elias J.E., Gygi S.P., Selkoe D.J. Proteomic profiling of gamma-secretase substrates and mapping of substrate requirements. PLoS Biol. 2008;6:e257. doi: 10.1371/journal.pbio.0060257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Aste-Amezaga M., Zhang N., Lineberger J.E., Arnold B.A., Toner T.J., Gu M., Huang L., Vitelli S., Vo K.T., Haytko P., et al. Characterization of Notch1 antibodies that inhibit signaling of both normal and mutated Notch1 receptors. PLoS ONE. 2010;5:e9094. doi: 10.1371/journal.pone.0009094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Wu Y., Cain-Hom C., Choy L., Hagenbeek T.J., de Leon G.P., Chen Y., Finkle D., Venook R., Wu X., Ridgway J., et al. Therapeutic antibody targeting of individual Notch receptors. Nature. 2010;464:1052–1057. doi: 10.1038/nature08878. [DOI] [PubMed] [Google Scholar]
- 222.Li Y., Hickson J.A., Ambrosi D.J., Haasch D.L., Foster-Duke K.D., Eaton L.J., DiGiammarino E.L., Panchal S.C., Jiang F., Mudd S.R., et al. ABT-165, a Dual Variable Domain Immunoglobulin (DVD-Ig) Targeting DLL4 and VEGF, Demonstrates Superior Efficacy and Favorable Safety Profiles in Preclinical Models. Mol. Cancer. 2018;17:1039–1050. doi: 10.1158/1535-7163.MCT-17-0800. [DOI] [PubMed] [Google Scholar]
- 223.Parakh S., Nicolazzo J., Scott A.M., Gan H.K. Antibody Drug Conjugates in Glioblastoma—Is There a Future for Them? Front. Oncol. 2021;11:718590. doi: 10.3389/fonc.2021.718590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Spino M., Kurz S.C., Chiriboga L., Serrano J., Zeck B., Sen N., Patel S., Shen G., Vasudevaraja V., Tsirigos A., et al. Cell Surface Notch Ligand DLL3 is a Therapeutic Target in Isocitrate Dehydrogenase-mutant Glioma. Clin. Cancer Res. 2019;25:1261–1271. doi: 10.1158/1078-0432.CCR-18-2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Rudin C.M., Pietanza M.C., Bauer T.M., Ready N., Morgensztern D., Glisson B.S., Byers L.A., Johnson M.L., Burris H.A., 3rd, Robert F., et al. Rovalpituzumab tesirine, a DLL3-targeted antibody-drug conjugate, in recurrent small-cell lung cancer: A first-in-human, first-in-class, open-label, phase 1 study. Lancet Oncol. 2017;18:42–51. doi: 10.1016/S1470-2045(16)30565-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Blackhall F., Jao K., Greillier L., Cho B.C., Penkov K., Reguart N., Majem M., Nackaerts K., Syrigos K., Hansen K., et al. Efficacy and Safety of Rovalpituzumab Tesirine Compared With Topotecan as Second-Line Therapy in DLL3-High SCLC: Results From the Phase 3 TAHOE Study. J. Thorac. Oncol. 2021;16:1547–1558. doi: 10.1016/j.jtho.2021.02.009. [DOI] [PubMed] [Google Scholar]
- 227.Johnson M.L., Zvirbule Z., Laktionov K., Helland A., Cho B.C., Gutierrez V., Colinet B., Lena H., Wolf M., Gottfried M., et al. Rovalpituzumab Tesirine as a Maintenance Therapy After First-Line Platinum-Based Chemotherapy in Patients With Extensive-Stage-SCLC: Results From the Phase 3 MERU Study. J. Thorac. Oncol. 2021;16:1570–1581. doi: 10.1016/j.jtho.2021.03.012. [DOI] [PubMed] [Google Scholar]
- 228.Chen J., Kesari S., Rooney C., Strack P.R., Chen J., Shen H., Wu L., Griffin J.D. Inhibition of notch signaling blocks growth of glioblastoma cell lines and tumor neurospheres. Genes Cancer. 2010;1:822–835. doi: 10.1177/1947601910383564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Opacak-Bernardi T., Ryu J.S., Raucher D. Effects of cell penetrating Notch inhibitory peptide conjugated to elastin-like polypeptide on glioblastoma cells. J. Drug Target. 2017;25:523–531. doi: 10.1080/1061186X.2017.1289537. [DOI] [PubMed] [Google Scholar]
- 230.Alvarez-Trotta A., Guerrant W., Astudillo L., Lahiry M., Diluvio G., Shersher E., Kaneku H., Robbins D.J., Orton D., Capobianco A.J. Pharmacological Disruption of the Notch1 Transcriptional Complex Inhibits Tumor Growth by Selectively Targeting Cancer Stem Cells. Cancer Res. 2021;81:3347–3357. doi: 10.1158/0008-5472.CAN-20-3611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Astudillo L., Da Silva T.G., Wang Z., Han X., Jin K., VanWye J., Zhu X., Weaver K., Oashi T., Lopes P.E., et al. The Small Molecule IMR-1 Inhibits the Notch Transcriptional Activation Complex to Suppress Tumorigenesis. Cancer Res. 2016;76:3593–3603. doi: 10.1158/0008-5472.CAN-16-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Lehal R., Zaric J., Vigolo M., Urech C., Frismantas V., Zangger N., Cao L., Berger A., Chicote I., Loubery S., et al. Pharmacological disruption of the Notch transcription factor complex. Proc. Natl. Acad. Sci. USA. 2020;117:16292–16301. doi: 10.1073/pnas.1922606117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Perron A., Nishikawa Y., Iwata J., Shimojo H., Takaya J., Kobayashi K., Imayoshi I., Mbenza N.M., Takenoya M., Kageyama R., et al. Small-molecule screening yields a compound that inhibits the cancer-associated transcription factor Hes1 via the PHB2 chaperone. J. Biol. Chem. 2018;293:8285–8294. doi: 10.1074/jbc.RA118.002316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Floyd D.H., Kefas B., Seleverstov O., Mykhaylyk O., Dominguez C., Comeau L., Plank C., Purow B. Alpha-secretase inhibition reduces human glioblastoma stem cell growth in vitro and in vivo by inhibiting Notch. Neuro-Oncology. 2012;14:1215–1226. doi: 10.1093/neuonc/nos157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Roti G., Carlton A., Ross K.N., Markstein M., Pajcini K., Su A.H., Perrimon N., Pear W.S., Kung A.L., Blacklow S.C., et al. Complementary genomic screens identify SERCA as a therapeutic target in NOTCH1 mutated cancer. Cancer Cell. 2013;23:390–405. doi: 10.1016/j.ccr.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Piccioni D., Juarez T., Brown B., Rose L., Allgood V., Kesari S. Atct-18phase Ii Study of Mipsagargin (G-202), a Psma-Activated Prodrug Targeting the Tumor Endothelium, in Adult Patients with Recurrent or Progressive Glioblastoma. Neuro-Oncology. 2015;17:v5. doi: 10.1093/neuonc/nov206.18. [DOI] [Google Scholar]
- 237.Zhang H., Liu L., Liu C., Pan J., Lu G., Zhou Z., Chen Z., Qian C. Notch3 overexpression enhances progression and chemoresistance of urothelial carcinoma. Oncotarget. 2017;8:34362–34373. doi: 10.18632/oncotarget.16156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Granit R.Z., Masury H., Condiotti R., Fixler Y., Gabai Y., Glikman T., Dalin S., Winter E., Nevo Y., Carmon E., et al. Regulation of Cellular Heterogeneity and Rates of Symmetric and Asymmetric Divisions in Triple-Negative Breast Cancer. Cell Rep. 2018;24:3237–3250. doi: 10.1016/j.celrep.2018.08.053. [DOI] [PubMed] [Google Scholar]
- 239.Ding W., Zeng T., Tao W., Ge W., Deng J., Lei H., Xiao Y., Liao F. Effect of lenalidomide on the human gastric cancer cell line SGC7901/vincristine Notch signaling. J. Cancer Res. 2018;14:S237–S242. doi: 10.4103/0973-1482.183181. [DOI] [PubMed] [Google Scholar]
- 240.Ponnurangam S., Dandawate P.R., Dhar A., Tawfik O.W., Parab R.R., Mishra P.D., Ranadive P., Sharma R., Mahajan G., Umar S., et al. Quinomycin A targets Notch signaling pathway in pancreatic cancer stem cells. Oncotarget. 2016;7:3217–3232. doi: 10.18632/oncotarget.6560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Hanashima Y., Sano E., Sumi K., Ozawa Y., Yagi C., Tatsuoka J., Yoshimura S., Yamamuro S., Ueda T., Nakayama T., et al. Antitumor effect of lenalidomide in malignant glioma cell lines. Oncol. Rep. 2020;43:1580–1590. doi: 10.3892/or.2020.7543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Kader B.A., Distefano R., West K.L., West A.G. EZH2 inhibition in glioblastoma stem cells increases the expression of neuronal genes and the neuronal developmental regulators ZIC2, ZNF423 and MAFB. bioRxiv. 2021:20211122469535. doi: 10.1101/2021.11.22.469535. [DOI] [Google Scholar]
- 243.Yin D., Ong J.M., Hu J., Desmond J.C., Kawamata N., Konda B.M., Black K.L., Koeffler H.P. Suberoylanilide hydroxamic acid, a histone deacetylase inhibitor: Effects on gene expression and growth of glioma cells in vitro and in vivo. Clin. Cancer Res. 2007;13:1045–1052. doi: 10.1158/1078-0432.CCR-06-1261. [DOI] [PubMed] [Google Scholar]
- 244.Zhen Y., Zhao S., Li Q., Li Y., Kawamoto K. Arsenic trioxide-mediated Notch pathway inhibition depletes the cancer stem-like cell population in gliomas. Cancer Lett. 2010;292:64–72. doi: 10.1016/j.canlet.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 245.Wu J., Ji Z., Liu H., Liu Y., Han D., Shi C., Shi C., Wang C., Yang G., Chen X., et al. Arsenic trioxide depletes cancer stem-like cells and inhibits repopulation of neurosphere derived from glioblastoma by downregulation of Notch pathway. Toxicol. Lett. 2013;220:61–69. doi: 10.1016/j.toxlet.2013.03.019. [DOI] [PubMed] [Google Scholar]
- 246.Schwalfenberg G.K. N-Acetylcysteine: A Review of Clinical Usefulness (an Old Drug with New Tricks) J. Nutr. Metab. 2021;2021:9949453. doi: 10.1155/2021/9949453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Deng J., Liu A.D., Hou G.Q., Zhang X., Ren K., Chen X.Z., Li S.S.C., Wu Y.S., Cao X. N-acetylcysteine decreases malignant characteristics of glioblastoma cells by inhibiting Notch2 signaling. J. Exp. Clin. Cancer Res. 2019;38:2. doi: 10.1186/s13046-018-1016-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Majumder S., Crabtree J.S., Golde T.E., Minter L.M., Osborne B.A., Miele L. Targeting Notch in oncology: The path forward. Nat. Rev. Drug Discov. 2021;20:125–144. doi: 10.1038/s41573-020-00091-3. [DOI] [PubMed] [Google Scholar]
- 249.Zhdanovskaya N., Firrincieli M., Lazzari S., Pace E., Scribani Rossi P., Felli M.P., Talora C., Screpanti I., Palermo R. Targeting Notch to Maximize Chemotherapeutic Benefits: Rationale, Advanced Strategies, and Future Perspectives. Cancers. 2021;13:5106. doi: 10.3390/cancers13205106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Li Y., Wang Z., Jin J., Zhu S.X., He G.Q., Li S.H., Wang J., Cai Y. Quercetin pretreatment enhances the radiosensitivity of colon cancer cells by targeting Notch-1 pathway. Biochem. Biophys. Res. Commun. 2020;523:947–953. doi: 10.1016/j.bbrc.2020.01.048. [DOI] [PubMed] [Google Scholar]
- 251.Li Y., Zhang J., Ma D., Zhang L., Si M., Yin H., Li J. Curcumin inhibits proliferation and invasion of osteosarcoma cells through inactivation of Notch-1 signaling. FEBS J. 2012;279:2247–2259. doi: 10.1111/j.1742-4658.2012.08607.x. [DOI] [PubMed] [Google Scholar]
- 252.Mori M., Tottone L., Quaglio D., Zhdanovskaya N., Ingallina C., Fusto M., Ghirga F., Peruzzi G., Crestoni M.E., Simeoni F., et al. Identification of a novel chalcone derivative that inhibits Notch signaling in T-cell acute lymphoblastic leukemia. Sci. Rep. 2017;7:2213. doi: 10.1038/s41598-017-02316-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Lai I.C., Shih P.H., Yao C.J., Yeh C.T., Wang-Peng J., Lui T.N., Chuang S.E., Hu T.S., Lai T.Y., Lai G.M. Elimination of cancer stem-like cells and potentiation of temozolomide sensitivity by Honokiol in glioblastoma multiforme cells. PLoS ONE. 2015;10:e0114830. doi: 10.1371/journal.pone.0114830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Lubecka K., Kurzava L., Flower K., Buvala H., Zhang H., Teegarden D., Camarillo I., Suderman M., Kuang S., Andrisani O., et al. Stilbenoids remodel the DNA methylation patterns in breast cancer cells and inhibit oncogenic NOTCH signaling through epigenetic regulation of MAML2 transcriptional activity. Carcinogenesis. 2016;37:656–668. doi: 10.1093/carcin/bgw048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Sun Z., Zhou C., Liu F., Zhang W., Chen J., Pan Y., Ma L., Liu Q., Du Y., Yang J., et al. Inhibition of breast cancer cell survival by Xanthohumol via modulation of the Notch signaling pathway in vivo and in vitro. Oncol. Lett. 2018;15:908–916. doi: 10.3892/ol.2017.7434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Kang M.S., Baek S.H., Chun Y.S., Moore A.Z., Landman N., Berman D., Yang H.O., Morishima-Kawashima M., Osawa S., Funamoto S., et al. Modulation of lipid kinase PI4KIIalpha activity and lipid raft association of presenilin 1 underlies gamma-secretase inhibition by ginsenoside (20S)-Rg3. J. Biol. Chem. 2013;288:20868–20882. doi: 10.1074/jbc.M112.445734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Kiesel V.A., Stan S.D. Diallyl trisulfide, a chemopreventive agent from Allium vegetables, inhibits alpha-secretases in breast cancer cells. Biochem. Biophys. Res. Commun. 2017;484:833–838. doi: 10.1016/j.bbrc.2017.01.184. [DOI] [PubMed] [Google Scholar]
- 258.Ohtaka M., Itoh M., Tohda S. BMI1 Inhibitors Down-regulate NOTCH Signaling and Suppress Proliferation of Acute Leukemia Cells. Anticancer Res. 2017;37:6047–6053. doi: 10.21873/anticanres.12052. [DOI] [PubMed] [Google Scholar]
- 259.Sun D.W., Zhang H.D., Mao L., Mao C.F., Chen W., Cui M., Ma R., Cao H.X., Jing C.W., Wang Z., et al. Luteolin Inhibits Breast Cancer Development and Progression In Vitro and In Vivo by Suppressing Notch Signaling and Regulating MiRNAs. Cell Physiol. Biochem. 2015;37:1693–1711. doi: 10.1159/000438535. [DOI] [PubMed] [Google Scholar]
- 260.Giuli M.V., Diluvio G., Giuliani E., Franciosa G., Di Magno L., Pignataro M.G., Tottone L., Nicoletti C., Besharat Z.M., Peruzzi G., et al. Notch3 contributes to T-cell leukemia growth via regulation of the unfolded protein response. Oncogenesis. 2020;9:93. doi: 10.1038/s41389-020-00279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Koduru S., Kumar R., Srinivasan S., Evers M.B., Damodaran C. Notch-1 inhibition by Withaferin-A: A therapeutic target against colon carcinogenesis. Mol. Cancer. 2010;9:202–210. doi: 10.1158/1535-7163.MCT-09-0771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Dandawate P., Subramaniam D., Panovich P., Standing D., Krishnamachary B., Kaushik G., Thomas S.M., Dhar A., Weir S.J., Jensen R.A., et al. Cucurbitacin B and I inhibits colon cancer growth by targeting the Notch signaling pathway. Sci. Rep. 2020;10:1290. doi: 10.1038/s41598-020-57940-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Kubiczkova L., Sedlarikova L., Hajek R., Sevcikova S. TGF-beta—An excellent servant but a bad master. J. Transl. Med. 2012;10:183. doi: 10.1186/1479-5876-10-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Xu X., Zheng L., Yuan Q., Zhen G., Crane J.L., Zhou X., Cao X. Transforming growth factor-beta in stem cells and tissue homeostasis. Bone Res. 2018;6:2. doi: 10.1038/s41413-017-0005-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Meyers E.A., Kessler J.A. TGF-beta Family Signaling in Neural and Neuronal Differentiation, Development, and Function. Cold Spring Harb. Perspect. Biol. 2017;9:a022244. doi: 10.1101/cshperspect.a022244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Falk S., Wurdak H., Ittner L.M., Ille F., Sumara G., Schmid M.T., Draganova K., Lang K.S., Paratore C., Leveen P., et al. Brain area-specific effect of TGF-beta signaling on Wnt-dependent neural stem cell expansion. Cell Stem Cell. 2008;2:472–483. doi: 10.1016/j.stem.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 267.Baba A.B., Rah B., Bhat G.R., Mushtaq I., Parveen S., Hassan R., Hameed Zargar M., Afroze D. Transforming Growth Factor-Beta (TGF-beta) Signaling in Cancer-A Betrayal Within. Front. Pharm. 2022;13:791272. doi: 10.3389/fphar.2022.791272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Proetzel G., Pawlowski S.A., Wiles M.V., Yin M., Boivin G.P., Howles P.N., Ding J., Ferguson M.W., Doetschman T. Transforming growth factor-beta 3 is required for secondary palate fusion. Nat. Genet. 1995;11:409–414. doi: 10.1038/ng1295-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Letterio J.J., Roberts A.B. Transforming growth factor-beta1-deficient mice: Identification of isoform-specific activities in vivo. J. Leukoc. Biol. 1996;59:769–774. doi: 10.1002/jlb.59.6.769. [DOI] [PubMed] [Google Scholar]
- 270.Sanford L.P., Ormsby I., Gittenberger-de Groot A.C., Sariola H., Friedman R., Boivin G.P., Cardell E.L., Doetschman T. TGFbeta2 knockout mice have multiple developmental defects that are non-overlapping with other TGFbeta knockout phenotypes. Development. 1997;124:2659–2670. doi: 10.1242/dev.124.13.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Han J., Alvarez-Breckenridge C.A., Wang Q.E., Yu J. TGF-beta signaling and its targeting for glioma treatment. Am. J. Cancer Res. 2015;5:945–955. [PMC free article] [PubMed] [Google Scholar]
- 272.Nakao A., Afrakhte M., Moren A., Nakayama T., Christian J.L., Heuchel R., Itoh S., Kawabata M., Heldin N.E., Heldin C.H., et al. Identification of Smad7, a TGFbeta-in.nducible antagonist of TGF-beta signalling. Nature. 1997;389:631–635. doi: 10.1038/39369. [DOI] [PubMed] [Google Scholar]
- 273.Moustakas A., Heldin C.H. Non-Smad TGF-beta signals. J. Cell Sci. 2005;118:3573–3584. doi: 10.1242/jcs.02554. [DOI] [PubMed] [Google Scholar]
- 274.Teixeira A.F., Ten Dijke P., Zhu H.J. On-Target Anti-TGF-beta Therapies Are Not Succeeding in Clinical Cancer Treatments: What Are Remaining Challenges? Front. Cell Dev. Biol. 2020;8:605. doi: 10.3389/fcell.2020.00605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Dugger B.N., Dickson D.W. Pathology of Neurodegenerative Diseases. Cold Spring Harb. Perspect. Biol. 2017;9:a028035. doi: 10.1101/cshperspect.a028035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Lindholm D., Castren E., Kiefer R., Zafra F., Thoenen H. Transforming growth factor-beta 1 in the rat brain: Increase after injury and inhibition of astrocyte proliferation. J. Cell Biol. 1992;117:395–400. doi: 10.1083/jcb.117.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Lebrun J.J. The Dual Role of TGFbeta in Human Cancer: From Tumor Suppression to Cancer Metastasis. ISRN Mol. Biol. 2012;2012:381428. doi: 10.5402/2012/381428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Kjellman C., Olofsson S.P., Hansson O., Von Schantz T., Lindvall M., Nilsson I., Salford L.G., Sjogren H.O., Widegren B. Expression of TGF-beta isoforms, TGF-beta receptors, and SMAD molecules at different stages of human glioma. Int. J. Cancer. 2000;89:251–258. doi: 10.1002/1097-0215(20000520)89:3<251::AID-IJC7>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 279.Bruna A., Darken R.S., Rojo F., Ocana A., Penuelas S., Arias A., Paris R., Tortosa A., Mora J., Baselga J., et al. High TGFbeta-Smad activity confers poor prognosis in glioma patients and promotes cell proliferation depending on the methylation of the PDGF-B gene. Cancer Cell. 2007;11:147–160. doi: 10.1016/j.ccr.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 280.Alexandrow M.G., Moses H.L. Transforming growth factor beta and cell cycle regulation. Cancer Res. 1995;55:1452–1457. [PubMed] [Google Scholar]
- 281.Wesolowska A., Kwiatkowska A., Slomnicki L., Dembinski M., Master A., Sliwa M., Franciszkiewicz K., Chouaib S., Kaminska B. Microglia-derived TGF-beta as an important regulator of glioblastoma invasion--an inhibition of TGF-beta-dependent effects by shRNA against human TGF-beta type II receptor. Oncogene. 2008;27:918–930. doi: 10.1038/sj.onc.1210683. [DOI] [PubMed] [Google Scholar]
- 282.Nana A.W., Yang P.M., Lin H.Y. Overview of Transforming Growth Factor beta Superfamily Involvement in Glioblastoma Initiation and Progression. Asian Pac. J. Cancer Prev. 2015;16:6813–6823. doi: 10.7314/APJCP.2015.16.16.6813. [DOI] [PubMed] [Google Scholar]
- 283.Katz L.H., Li Y., Chen J.S., Munoz N.M., Majumdar A., Chen J., Mishra L. Targeting TGF-beta signaling in cancer. Expert Opin Targets. 2013;17:743–760. doi: 10.1517/14728222.2013.782287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Zhang Y., Alexander P.B., Wang X.F. TGF-beta Family Signaling in the Control of Cell Proliferation and Survival. Cold Spring Harb. Perspect. Biol. 2017;9:a022145. doi: 10.1101/cshperspect.a022145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Batlle E., Massague J. Transforming Growth Factor-beta Signaling in Immunity and Cancer. Immunity. 2019;50:924–940. doi: 10.1016/j.immuni.2019.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Gu S., Feng X.H. TGF-beta signaling in cancer. Acta Biochim. Biophys. Sin. 2018;50:941–949. doi: 10.1093/abbs/gmy092. [DOI] [PubMed] [Google Scholar]
- 287.Gieryng A., Pszczolkowska D., Walentynowicz K.A., Rajan W.D., Kaminska B. Immune microenvironment of gliomas. Lab. Investig. 2017;97:498–518. doi: 10.1038/labinvest.2017.19. [DOI] [PubMed] [Google Scholar]
- 288.Li W., Graeber M.B. The molecular profile of microglia under the influence of glioma. Neuro-Oncology. 2012;14:958–978. doi: 10.1093/neuonc/nos116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Xue V.W., Chung J.Y., Cordoba C.A.G., Cheung A.H., Kang W., Lam E.W., Leung K.T., To K.F., Lan H.Y., Tang P.M. Transforming Growth Factor-beta: A Multifunctional Regulator of Cancer Immunity. Cancers. 2020;12:3099. doi: 10.3390/cancers12113099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Friese M.A., Wischhusen J., Wick W., Weiler M., Eisele G., Steinle A., Weller M. RNA interference targeting transforming growth factor-beta enhances NKG2D-mediated antiglioma immune response, inhibits glioma cell migration and invasiveness, and abrogates tumorigenicity in vivo. Cancer Res. 2004;64:7596–7603. doi: 10.1158/0008-5472.CAN-04-1627. [DOI] [PubMed] [Google Scholar]
- 291.Schlingensiepen K.H., Schlingensiepen R., Steinbrecher A., Hau P., Bogdahn U., Fischer-Blass B., Jachimczak P. Targeted tumor therapy with the TGF-beta 2 antisense compound AP 12009. Cytokine Growth Factor Rev. 2006;17:129–139. doi: 10.1016/j.cytogfr.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 292.Jaschinski F., Rothhammer T., Jachimczak P., Seitz C., Schneider A., Schlingensiepen K.H. The antisense oligonucleotide trabedersen (AP 12009) for the targeted inhibition of TGF-beta2. Curr. Pharm. Biotechnol. 2011;12:2203–2213. doi: 10.2174/138920111798808266. [DOI] [PubMed] [Google Scholar]
- 293.Hau P., Jachimczak P., Schlingensiepen R., Schulmeyer F., Jauch T., Steinbrecher A., Brawanski A., Proescholdt M., Schlaier J., Buchroithner J., et al. Inhibition of TGF-beta2 with AP 12009 in recurrent malignant gliomas: From preclinical to phase I/II studies. Oligonucleotides. 2007;17:201–212. doi: 10.1089/oli.2006.0053. [DOI] [PubMed] [Google Scholar]
- 294.Roth P., Silginer M., Goodman S.L., Hasenbach K., Thies S., Maurer G., Schraml P., Tabatabai G., Moch H., Tritschler I., et al. Integrin control of the transforming growth factor-beta pathway in glioblastoma. Brain. 2013;136:564–576. doi: 10.1093/brain/aws351. [DOI] [PubMed] [Google Scholar]
- 295.Stupp R., Hegi M.E., Gorlia T., Erridge S.C., Perry J., Hong Y.-K., Aldape K.D., Lhermitte B., Pietsch T., Grujicic D., et al. Cilengitide combined with standard treatment for patients with newly diagnosed glioblastoma with methylated MGMT promoter (CENTRIC EORTC 26071-22072 study): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15:1100–1108. doi: 10.1016/S1470-2045(14)70379-1. [DOI] [PubMed] [Google Scholar]
- 296.Suzuki E., Kim S., Cheung H.K., Corbley M.J., Zhang X., Sun L., Shan F., Singh J., Lee W.C., Albelda S.M., et al. A novel small-molecule inhibitor of transforming growth factor beta type I receptor kinase (SM16) inhibits murine mesothelioma tumor growth in vivo and prevents tumor recurrence after surgical resection. Cancer Res. 2007;67:2351–2359. doi: 10.1158/0008-5472.CAN-06-2389. [DOI] [PubMed] [Google Scholar]
- 297.Rausch M.P., Hahn T., Ramanathapuram L., Bradley-Dunlop D., Mahadevan D., Mercado-Pimentel M.E., Runyan R.B., Besselsen D.G., Zhang X., Cheung H.K., et al. An orally active small molecule TGF-beta receptor I antagonist inhibits the growth of metastatic murine breast cancer. Anticancer Res. 2009;29:2099–2109. [PMC free article] [PubMed] [Google Scholar]
- 298.Zhang M., Kleber S., Rohrich M., Timke C., Han N., Tuettenberg J., Martin-Villalba A., Debus J., Peschke P., Wirkner U., et al. Blockade of TGF-beta signaling by the TGFbetaR-I kinase inhibitor LY2109761 enhances radiation response and prolongs survival in glioblastoma. Cancer Res. 2011;71:7155–7167. doi: 10.1158/0008-5472.CAN-11-1212. [DOI] [PubMed] [Google Scholar]
- 299.Zhang M., Herion T.W., Timke C., Han N., Hauser K., Weber K.J., Peschke P., Wirkner U., Lahn M., Huber P.E. Trimodal glioblastoma treatment consisting of concurrent radiotherapy, temozolomide, and the novel TGF-beta receptor I kinase inhibitor LY2109761. Neoplasia. 2011;13:537–549. doi: 10.1593/neo.11258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Yingling J.M., McMillen W.T., Yan L., Huang H., Sawyer J.S., Graff J., Clawson D.K., Britt K.S., Anderson B.D., Beight D.W., et al. Preclinical assessment of galunisertib (LY2157299 monohydrate), a first-in-class transforming growth factor-beta receptor type I inhibitor. Oncotarget. 2018;9:6659–6677. doi: 10.18632/oncotarget.23795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Wick A., Desjardins A., Suarez C., Forsyth P., Gueorguieva I., Burkholder T., Cleverly A.L., Estrem S.T., Wang S., Lahn M.M., et al. Phase 1b/2a study of galunisertib, a small molecule inhibitor of transforming growth factor-beta receptor I, in combination with standard temozolomide-based radiochemotherapy in patients with newly diagnosed malignant glioma. Investig. New Drugs. 2020;38:1570–1579. doi: 10.1007/s10637-020-00910-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Brandes A.A., Carpentier A.F., Kesari S., Sepulveda-Sanchez J.M., Wheeler H.R., Chinot O., Cher L., Steinbach J.P., Capper D., Specenier P., et al. A Phase II randomized study of galunisertib monotherapy or galunisertib plus lomustine compared with lomustine monotherapy in patients with recurrent glioblastoma. Neuro-Oncology. 2016;18:1146–1156. doi: 10.1093/neuonc/now009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Spender L.C., Ferguson G.J., Hughes G.D., Davies B.R., Goldberg F.W., Herrera B., Taylor R.G., Strathearn L.S., Sansom O.J., Barry S.T., et al. Preclinical Evaluation of AZ12601011 and AZ12799734, Inhibitors of Transforming Growth Factor beta Superfamily Type 1 Receptors. Mol. Pharm. 2019;95:222–234. doi: 10.1124/mol.118.112946. [DOI] [PubMed] [Google Scholar]
- 304.Ueda R., Fujita M., Zhu X., Sasaki K., Kastenhuber E.R., Kohanbash G., McDonald H.A., Harper J., Lonning S., Okada H. Systemic inhibition of transforming growth factor-beta in glioma-bearing mice improves the therapeutic efficacy of glioma-associated antigen peptide vaccines. Clin. Cancer Res. 2009;15:6551–6559. doi: 10.1158/1078-0432.CCR-09-1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Hulper P., Schulz-Schaeffer W., Dullin C., Hoffmann P., Harper J., Kurtzberg L., Lonning S., Kugler W., Lakomek M., Erdlenbruch B. Tumor localization of an anti-TGF-beta antibody and its effects on gliomas. Int. J. Oncol. 2011;38:51–59. [PubMed] [Google Scholar]
- 306.Den Hollander M.W., Bensch F., Glaudemans A.W., Oude Munnink T.H., Enting R.H., den Dunnen W.F., Heesters M.A., Kruyt F.A., Lub-de Hooge M.N., Cees de Groot J., et al. TGF-beta Antibody Uptake in Recurrent High-Grade Glioma Imaged with 89Zr-Fresolimumab PET. J. Nucl. Med. 2015;56:1310–1314. doi: 10.2967/jnumed.115.154401. [DOI] [PubMed] [Google Scholar]
- 307.Shaim H., Shanley M., Basar R., Daher M., Gumin J., Zamler D.B., Uprety N., Wang F., Huang Y., Gabrusiewicz K., et al. Targeting the alphav integrin/TGF-beta axis improves natural killer cell function against glioblastoma stem cells. J. Clin. Investig. 2021;131:e142116. doi: 10.1172/JCI142116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Niedbala M., Malarz K., Sharma G., Kramer-Marek G., Kaspera W. Glioblastoma: Pitfalls and Opportunities of Immunotherapeutic Combinations. OncoTargets Ther. 2022;15:437–468. doi: 10.2147/OTT.S215997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Ashrafizadeh M., Najafi M., Orouei S., Zabolian A., Saleki H., Azami N., Sharifi N., Hushmandi K., Zarrabi A., Ahn K.S. Resveratrol Modulates Transforming Growth Factor-Beta (TGF-beta) Signaling Pathway for Disease Therapy: A New Insight into Its Pharmacological Activities. Biomedicines. 2020;8:261. doi: 10.3390/biomedicines8080261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Kabel A.M., Atef A., Estfanous R.S. Ameliorative potential of sitagliptin and/or resveratrol on experimentally-induced clear cell renal cell carcinoma. Biomed. Pharm. 2018;97:667–674. doi: 10.1016/j.biopha.2017.10.149. [DOI] [PubMed] [Google Scholar]
- 311.Zhang Y., Yang S., Yang Y., Liu T. Resveratrol induces immunogenic cell death of human and murine ovarian carcinoma cells. Infect. Agent Cancer. 2019;14:27. doi: 10.1186/s13027-019-0247-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Song Y., Chen Y., Li Y., Lyu X., Cui J., Cheng Y., Zheng T., Zhao L., Zhao G. Resveratrol Suppresses Epithelial-Mesenchymal Transition in GBM by Regulating Smad-Dependent Signaling. Biomed. Res. Int. 2019;2019:1321973. doi: 10.1155/2019/1321973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Nie X., Luukko K., Kettunen P. BMP signalling in craniofacial development. Int. J. Dev. Biol. 2006;50:511–521. doi: 10.1387/ijdb.052101xn. [DOI] [PubMed] [Google Scholar]
- 314.Bond A.M., Bhalala O.G., Kessler J.A. The dynamic role of bone morphogenetic proteins in neural stem cell fate and maturation. Dev. Neurobiol. 2012;72:1068–1084. doi: 10.1002/dneu.22022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Chen H.L., Panchision D.M. Concise review: Bone morphogenetic protein pleiotropism in neural stem cells and their derivatives--alternative pathways, convergent signals. Stem Cells. 2007;25:63–68. doi: 10.1634/stemcells.2006-0339. [DOI] [PubMed] [Google Scholar]
- 316.Gamez B., Rodriguez-Carballo E., Ventura F. BMP signaling in telencephalic neural cell specification and maturation. Front. Cell Neurosci. 2013;7:87. doi: 10.3389/fncel.2013.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Katagiri T., Watabe T. Bone Morphogenetic Proteins. Cold Spring Harb. Perspect. Biol. 2016;8:a021899. doi: 10.1101/cshperspect.a021899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Bond A.M., Peng C.Y., Meyers E.A., McGuire T., Ewaleifoh O., Kessler J.A. BMP signaling regulates the tempo of adult hippocampal progenitor maturation at multiple stages of the lineage. Stem Cells. 2014;32:2201–2214. doi: 10.1002/stem.1688. [DOI] [PubMed] [Google Scholar]
- 319.Choe Y., Pleasure S.J., Mira H. Control of Adult Neurogenesis by Short-Range Morphogenic-Signaling Molecules. Cold Spring Harb. Perspect. Biol. 2015;8:a018887. doi: 10.1101/cshperspect.a018887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Panchision D.M., McKay R.D. The control of neural stem cells by morphogenic signals. Curr. Opin. Genet. Dev. 2002;12:478–487. doi: 10.1016/S0959-437X(02)00329-5. [DOI] [PubMed] [Google Scholar]
- 321.Wang R.N., Green J., Wang Z., Deng Y., Qiao M., Peabody M., Zhang Q., Ye J., Yan Z., Denduluri S., et al. Bone Morphogenetic Protein (BMP) signaling in development and human diseases. Genes Dis. 2014;1:87–105. doi: 10.1016/j.gendis.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Choi S., Yu J., Park A., Dubon M.J., Do J., Kim Y., Nam D., Noh J., Park K.S. BMP-4 enhances epithelial mesenchymal transition and cancer stem cell properties of breast cancer cells via Notch signaling. Sci. Rep. 2019;9:11724. doi: 10.1038/s41598-019-48190-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Ye L., Jiang W.G. Bone morphogenetic proteins in tumour associated angiogenesis and implication in cancer therapies. Cancer Lett. 2016;380:586–597. doi: 10.1016/j.canlet.2015.10.036. [DOI] [PubMed] [Google Scholar]
- 324.Guyot B., Lefort S., Voeltzel T., Pecheur E.I., Maguer-Satta V. Altered BMP2/4 Signaling in Stem Cells and Their Niche: Different Cancers but Similar Mechanisms, the Example of Myeloid Leukemia and Breast Cancer. Front. Cell Dev. Biol. 2021;9:787989. doi: 10.3389/fcell.2021.787989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Kallioniemi A. Bone morphogenetic protein 4-a fascinating regulator of cancer cell behavior. Cancer Genet. 2012;205:267–277. doi: 10.1016/j.cancergen.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 326.Lowery J.W., Brookshire B., Rosen V. A Survey of Strategies to Modulate the Bone Morphogenetic Protein Signaling Pathway: Current and Future Perspectives. Stem Cells Int. 2016;2016:7290686. doi: 10.1155/2016/7290686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Bach D.H., Park H.J., Lee S.K. The Dual Role of Bone Morphogenetic Proteins in Cancer. Mol. Oncolytics. 2018;8:1–13. doi: 10.1016/j.omto.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Zhang L., Ye Y., Long X., Xiao P., Ren X., Yu J. BMP signaling and its paradoxical effects in tumorigenesis and dissemination. Oncotarget. 2016;7:78206–78218. doi: 10.18632/oncotarget.12151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Bao Z., Zhang C., Yan W., Liu Y., Li M., Zhang W., Jiang T. BMP4, a strong better prognosis predictor, has a subtype preference and cell development association in gliomas. J. Transl. Med. 2013;11:100. doi: 10.1186/1479-5876-11-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Wu Q., Yao J. BMP4, a new prognostic factor for glioma. World J. Surg. Oncol. 2013;11:264. doi: 10.1186/1477-7819-11-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Liu C., Tian G., Tu Y., Fu J., Lan C., Wu N. Expression pattern and clinical prognostic relevance of bone morphogenetic protein-2 in human gliomas. Jpn J. Clin. Oncol. 2009;39:625–631. doi: 10.1093/jjco/hyp094. [DOI] [PubMed] [Google Scholar]
- 332.Chirasani S.R., Sternjak A., Wend P., Momma S., Campos B., Herrmann I.M., Graf D., Mitsiadis T., Herold-Mende C., Besser D., et al. Bone morphogenetic protein-7 release from endogenous neural precursor cells suppresses the tumourigenicity of stem-like glioblastoma cells. Brain. 2010;133:1961–1972. doi: 10.1093/brain/awq128. [DOI] [PubMed] [Google Scholar]
- 333.Tate C.M., Pallini R., Ricci-Vitiani L., Dowless M.M., Shiyanova T., D’Alessandris G.Q., Morgante L., Giannetti S., Larocca L.M., di Martino S., et al. A BMP7 variant inhibits the tumorigenic potential of glioblastoma stem-like cells. Cell Death Differ. 2012;19:1644–1654. doi: 10.1038/cdd.2012.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Gonzalez-Gomez P., Crecente-Campo J., Zahonero C., de la Fuente M., Hernandez-Lain A., Mira H., Sanchez-Gomez P., Garcia-Fuentes M. Controlled release microspheres loaded with BMP7 suppress primary tumors from human glioblastoma. Oncotarget. 2015;6:10950–10963. doi: 10.18632/oncotarget.3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Zhou Z., Sun L., Wang Y., Wu Z., Geng J., Miu W., Pu Y., You Y., Yang Z., Liu N. Bone morphogenetic protein 4 inhibits cell proliferation and induces apoptosis in glioma stem cells. Cancer Biother. Radiopharm. 2011;26:77–83. doi: 10.1089/cbr.2010.0857. [DOI] [PubMed] [Google Scholar]
- 336.Liu B., Tian D., Yi W., Wu L., Cai Q., Dong H., Shen H., Ji B., Wang L., Zhang S., et al. Effect of bone morphogenetic protein 4 in the human brain glioma cell line U251. Cell Biochem. Biophys. 2010;58:91–96. doi: 10.1007/s12013-010-9095-y. [DOI] [PubMed] [Google Scholar]
- 337.Liu B., Chen Q., Tian D., Wu L., Dong H., Wang J., Ji B., Zhu X., Cai Q., Wang L., et al. BMP4 reverses multidrug resistance through modulation of BCL-2 and GDNF in glioblastoma. Brain Res. 2013;1507:115–124. doi: 10.1016/j.brainres.2013.02.039. [DOI] [PubMed] [Google Scholar]
- 338.Persano L., Pistollato F., Rampazzo E., Della Puppa A., Abbadi S., Frasson C., Volpin F., Indraccolo S., Scienza R., Basso G. BMP2 sensitizes glioblastoma stem-like cells to Temozolomide by affecting HIF-1alpha stability and MGMT expression. Cell Death Dis. 2012;3:e412. doi: 10.1038/cddis.2012.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Yan K., Wu Q., Yan D.H., Lee C.H., Rahim N., Tritschler I., DeVecchio J., Kalady M.F., Hjelmeland A.B., Rich J.N. Glioma cancer stem cells secrete Gremlin1 to promote their maintenance within the tumor hierarchy. Genes Dev. 2014;28:1085–1100. doi: 10.1101/gad.235515.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Koguchi M., Nakahara Y., Ito H., Wakamiya T., Yoshioka F., Ogata A., Inoue K., Masuoka J., Izumi H., Abe T. BMP4 induces asymmetric cell division in human glioma stem-like cells. Oncol. Lett. 2020;19:1247–1254. doi: 10.3892/ol.2019.11231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Dalmo E., Johansson P., Niklasson M., Gustavsson I., Nelander S., Westermark B. Growth-Inhibitory Activity of Bone Morphogenetic Protein 4 in Human Glioblastoma Cell Lines Is Heterogeneous and Dependent on Reduced SOX2 Expression. Mol. Cancer Res. 2020;18:981–991. doi: 10.1158/1541-7786.MCR-19-0638. [DOI] [PubMed] [Google Scholar]
- 342.Kaye J., Mondal A., Foty R., Jia D., Langenfeld J. Bone morphogenetic protein receptor inhibitors suppress the growth of glioblastoma cells. Mol. Cell Biochem. 2022;477:1583–1595. doi: 10.1007/s11010-022-04383-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Lasorella A., Benezra R., Iavarone A. The ID proteins: Master regulators of cancer stem cells and tumour aggressiveness. Nat. Rev. Cancer. 2014;14:77–91. doi: 10.1038/nrc3638. [DOI] [PubMed] [Google Scholar]
- 344.Rampazzo E., Dettin M., Maule F., Scabello A., Calvanese L., D’Auria G., Falcigno L., Porcu E., Zamuner A., Della Puppa A., et al. A synthetic BMP-2 mimicking peptide induces glioblastoma stem cell differentiation. Biochim. Biophys. Acta Gen. Subj. 2017;1861:2282–2292. doi: 10.1016/j.bbagen.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 345.Lowery J.W., Rosen V. Bone Morphogenetic Protein-Based Therapeutic Approaches. Cold Spring Harb. Perspect. Biol. 2018;10:a022327. doi: 10.1101/cshperspect.a022327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Huang J., Wu S., Barrera J., Matthews K., Pan D. The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila Homolog of YAP. Cell. 2005;122:421–434. doi: 10.1016/j.cell.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 347.Halder G., Johnson R.L. Hippo signaling: Growth control and beyond. Development. 2011;138:9–22. doi: 10.1242/dev.045500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Bae S.J., Luo X. Activation mechanisms of the Hippo kinase signaling cascade. Biosci. Rep. 2018;38:BSR20171469. doi: 10.1042/BSR20171469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Nishio M., Goto H., Suzuki M., Fujimoto A., Mimori K., Suzuki A. The Hippo Signaling Pathway: A Candidate New Drug Target for Malignant Tumors. In: Nakao K., Minato N., Uemoto S., editors. Innovative Medicine. Springer; Tokyo, Japan: 2015. [PubMed] [Google Scholar]
- 350.Zhao B., Wei X., Li W., Udan R.S., Yang Q., Kim J., Xie J., Ikenoue T., Yu J., Li L., et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007;21:2747–2761. doi: 10.1101/gad.1602907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Pobbati A.V., Hong W. Emerging roles of TEAD transcription factors and its coactivators in cancers. Cancer Biol. 2013;14:390–398. doi: 10.4161/cbt.23788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Meng Z., Moroishi T., Guan K.L. Mechanisms of Hippo pathway regulation. Genes Dev. 2016;30:1–17. doi: 10.1101/gad.274027.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Zanconato F., Cordenonsi M., Piccolo S. YAP/TAZ at the Roots of Cancer. Cancer Cell. 2016;29:783–803. doi: 10.1016/j.ccell.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Johnson R., Halder G. The two faces of Hippo: Targeting the Hippo pathway for regenerative medicine and cancer treatment. Nat. Rev. Drug Discov. 2014;13:63–79. doi: 10.1038/nrd4161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Orr B.A., Bai H., Odia Y., Jain D., Anders R.A., Eberhart C.G. Yes-associated protein 1 is widely expressed in human brain tumors and promotes glioblastoma growth. J. Neuropathol. Exp. Neurol. 2011;70:568–577. doi: 10.1097/NEN.0b013e31821ff8d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Castellan M., Guarnieri A., Fujimura A., Zanconato F., Battilana G., Panciera T., Sladitschek H.L., Contessotto P., Citron A., Grilli A., et al. Single-cell analyses reveal YAP/TAZ as regulators of stemness and cell plasticity in Glioblastoma. Nat. Cancer. 2021;2:174–188. doi: 10.1038/s43018-020-00150-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Fernandez L.A., Squatrito M., Northcott P., Awan A., Holland E.C., Taylor M.D., Nahle Z., Kenney A.M. Oncogenic YAP promotes radioresistance and genomic instability in medulloblastoma through IGF2-mediated Akt activation. Oncogene. 2012;31:1923–1937. doi: 10.1038/onc.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Tian T., Li A., Lu H., Luo R., Zhang M., Li Z. TAZ promotes temozolomide resistance by upregulating MCL-1 in human glioma cells. Biochem. Biophys. Res. Commun. 2015;463:638–643. doi: 10.1016/j.bbrc.2015.05.115. [DOI] [PubMed] [Google Scholar]
- 359.Liu-Chittenden Y., Huang B., Shim J.S., Chen Q., Lee S.J., Anders R.A., Liu J.O., Pan D. Genetic and pharmacological disruption of the TEAD-YAP complex suppresses the oncogenic activity of YAP. Genes Dev. 2012;26:1300–1305. doi: 10.1101/gad.192856.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Hill J.S., Kaye A.H., Sawyer W.H., Morstyn G., Megison P.D., Stylli S.S. Selective uptake of hematoporphyrin derivative into human cerebral glioma. Neurosurgery. 1990;26:248–254. doi: 10.1227/00006123-199002000-00011. [DOI] [PubMed] [Google Scholar]
- 361.Vigneswaran K., Boyd N.H., Oh S.Y., Lallani S., Boucher A., Neill S.G., Olson J.J., Read R.D. YAP/TAZ Transcriptional Coactivators Create Therapeutic Vulnerability to Verteporfin in EGFR-mutant Glioblastoma. Clin. Cancer Res. 2021;27:1553–1569. doi: 10.1158/1078-0432.CCR-20-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Han W., Guan W. Valproic Acid: A Promising Therapeutic Agent in Glioma Treatment. Front. Oncol. 2021;11:687362. doi: 10.3389/fonc.2021.687362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Knupfer M.M., Hernaiz-Driever P., Poppenborg H., Wolff J.E., Cinatl J. Valproic acid inhibits proliferation and changes expression of CD44 and CD56 of malignant glioma cells in vitro. Anticancer Res. 1998;18:3585–3589. [PubMed] [Google Scholar]
- 364.Xu Y., Stamenkovic I., Yu Q. CD44 attenuates activation of the hippo signaling pathway and is a prime therapeutic target for glioblastoma. Cancer Res. 2010;70:2455–2464. doi: 10.1158/0008-5472.CAN-09-2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Valiyaveettil D., Malik M., Joseph D.M., Ahmed S.F., Kothwal S.A., Vijayasaradhi M. Effect of valproic acid on survival in glioblastoma: A prospective single-arm study. South. Asian J. Cancer. 2018;7:159–162. doi: 10.4103/sajc.sajc_188_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Yuan Y., Xiang W., Qing M., Yanhui L., Jiewen L., Yunhe M. Survival analysis for valproic acid use in adult glioblastoma multiforme: A meta-analysis of individual patient data and a systematic review. Seizure. 2014;23:830–835. doi: 10.1016/j.seizure.2014.06.015. [DOI] [PubMed] [Google Scholar]
- 367.Liu Z., Wei Y., Zhang L., Yee P.P., Johnson M., Zhang X., Gulley M., Atkinson J.M., Trebak M., Wang H.G., et al. Induction of store-operated calcium entry (SOCE) suppresses glioblastoma growth by inhibiting the Hippo pathway transcriptional coactivators YAP/TAZ. Oncogene. 2019;38:120–139. doi: 10.1038/s41388-018-0425-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Miller P.D., Chines A.A., Christiansen C., Hoeck H.C., Kendler D.L., Lewiecki E.M., Woodson G., Levine A.B., Constantine G., Delmas P.D. Effects of bazedoxifene on BMD and bone turnover in postmenopausal women: 2-yr results of a randomized, double-blind, placebo-, and active-controlled study. J. Bone Min. Res. 2008;23:525–535. doi: 10.1359/jbmr.071206. [DOI] [PubMed] [Google Scholar]
- 369.Wu X., Cao Y., Xiao H., Li C., Lin J. Bazedoxifene as a Novel GP130 Inhibitor for Pancreatic Cancer Therapy. Mol. Cancer. 2016;15:2609–2619. doi: 10.1158/1535-7163.MCT-15-0921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Fu W., Zhao P., Li H., Fu H., Liu X., Liu Y., Wu J., Fu W. Bazedoxifene enhances paclitaxel efficacy to suppress glioblastoma via altering Hippo/YAP pathway. J. Cancer. 2020;11:657–667. doi: 10.7150/jca.38350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Wightman S.M., Alban T.J., Chen X., Lathia J.D., Wang Y., Stark G.R. Bazedoxifene inhibits sustained STAT3 activation and increases survival in GBM. Transl. Oncol. 2021;14:101192. doi: 10.1016/j.tranon.2021.101192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Xiao A., Brenneman B., Floyd D., Comeau L., Spurio K., Olmez I., Lee J., Nakano I., Godlewski J., Bronisz A., et al. Statins affect human glioblastoma and other cancers through TGF-beta inhibition. Oncotarget. 2019;10:1716–1728. doi: 10.18632/oncotarget.26733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Oku Y., Nishiya N., Shito T., Yamamoto R., Yamamoto Y., Oyama C., Uehara Y. Small molecules inhibiting the nuclear localization of YAP/TAZ for chemotherapeutics and chemosensitizers against breast cancers. FEBS Open Bio. 2015;5:542–549. doi: 10.1016/j.fob.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Xie Y., Lu Q., Lenahan C., Yang S., Zhou D., Qi X. Whether statin use improves the survival of patients with glioblastoma?: A meta-analysis. Medicine. 2020;99:e18997. doi: 10.1097/MD.0000000000018997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Cunningham T.J., Duester G. Mechanisms of retinoic acid signalling and its roles in organ and limb development. Nat. Rev. Mol. Cell Biol. 2015;16:110–123. doi: 10.1038/nrm3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Wiesinger A., Boink G.J.J., Christoffels V.M., Devalla H.D. Retinoic acid signaling in heart development: Application in the differentiation of cardiovascular lineages from human pluripotent stem cells. Stem Cell Rep. 2021;16:2589–2606. doi: 10.1016/j.stemcr.2021.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Dubey A., Rose R.E., Jones D.R., Saint-Jeannet J.P. Generating retinoic acid gradients by local degradation during craniofacial development: One cell’s cue is another cell’s poison. Genesis. 2018;56:e23091. doi: 10.1002/dvg.23091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Ni X., Hu G., Cai X. The success and the challenge of all-trans retinoic acid in the treatment of cancer. Crit. Rev. Food Sci. Nutr. 2019;59:S71–S80. doi: 10.1080/10408398.2018.1509201. [DOI] [PubMed] [Google Scholar]
- 379.Ozgun G., Senturk S., Erkek-Ozhan S. Retinoic acid signaling and bladder cancer: Epigenetic deregulation, therapy and beyond. Int. J. Cancer. 2020;148:2364–2374. doi: 10.1002/ijc.33374. [DOI] [PubMed] [Google Scholar]
- 380.Thompson B., Katsanis N., Apostolopoulos N., Thompson D.C., Nebert D.W., Vasiliou V. Genetics and functions of the retinoic acid pathway, with special emphasis on the eye. Hum. Genom. 2019;13:61. doi: 10.1186/s40246-019-0248-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Fex G., Johannesson G. Retinol transfer across and between phospholipid bilayer membranes. Biochim. Biophys. Acta. 1988;944:249–255. doi: 10.1016/0005-2736(88)90438-5. [DOI] [PubMed] [Google Scholar]
- 382.Luo T., Wagner E., Drager U.C. Integrating retinoic acid signaling with brain function. Dev. Psychol. 2009;45:139–150. doi: 10.1037/0012-1649.45.1.139. [DOI] [PubMed] [Google Scholar]
- 383.Hunsu V.O., Facey C.O.B., Fields J.Z., Boman B.M. Retinoids as Chemo-Preventive and Molecular-Targeted Anti-Cancer Therapies. Int. J. Mol. Sci. 2021;22:7731. doi: 10.3390/ijms22147731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Al Tanoury Z., Piskunov A., Rochette-Egly C. Vitamin A and retinoid signaling: Genomic and nongenomic effects. J. Lipid Res. 2013;54:1761–1775. doi: 10.1194/jlr.R030833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Agnihotri S., Mansouri S., Burrell K., Li M., Mamatjan Y., Liu J., Nejad R., Kumar S., Jalali S., Singh S.K., et al. Ketoconazole and Posaconazole Selectively Target HK2-expressing Glioblastoma Cells. Clin. Cancer Res. 2019;25:844–855. doi: 10.1158/1078-0432.CCR-18-1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Chattopadhyay N., Butters R.R., Brown E.M. Agonists of the retinoic acid- and retinoid X-receptors inhibit hepatocyte growth factor secretion and expression in U87 human astrocytoma cells. Brain Res. Mol. Brain Res. 2001;87:100–108. doi: 10.1016/S0165-3806(00)00154-1. [DOI] [PubMed] [Google Scholar]
- 387.Costa S.L., Paillaud E., Fages C., Rochette-Egly C., Plassat J.L., Jouault H., Perzelova A., Tardy M. Effects of a novel synthetic retinoid on malignant glioma in vitro: Inhibition of cell proliferation, induction of apoptosis and differentiation. Eur. J. Cancer. 2001;37:520–530. doi: 10.1016/S0959-8049(00)00430-5. [DOI] [PubMed] [Google Scholar]
- 388.Hacioglu C., Kar F., Kacar S., Sahinturk V., Kanbak G. Bexarotene inhibits cell proliferation by inducing oxidative stress, DNA damage and apoptosis via PPARgamma/NF-kappaB signaling pathway in C6 glioma cells. Med. Oncol. 2021;38:31. doi: 10.1007/s12032-021-01476-z. [DOI] [PubMed] [Google Scholar]
- 389.Lena A., Rechichi M., Salvetti A., Bartoli B., Vecchio D., Scarcelli V., Amoroso R., Benvenuti L., Gagliardi R., Gremigni V., et al. Drugs targeting the mitochondrial pore act as cytotoxic and cytostatic agents in temozolomide-resistant glioma cells. J. Transl. Med. 2009;7:13. doi: 10.1186/1479-5876-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Yokosawa M., Sonoda Y., Sugiyama S., Saito R., Yamashita Y., Nishihara M., Satoh T., Kumabe T., Yokoyama M., Tominaga T. Convection-enhanced delivery of a synthetic retinoid Am80, loaded into polymeric micelles, prolongs the survival of rats bearing intracranial glioblastoma xenografts. Tohoku J. Exp. Med. 2010;221:257–264. doi: 10.1620/tjem.221.257. [DOI] [PubMed] [Google Scholar]
- 391.Di Masi A., Leboffe L., De Marinis E., Pagano F., Cicconi L., Rochette-Egly C., Lo-Coco F., Ascenzi P., Nervi C. Retinoic acid receptors: From molecular mechanisms to cancer therapy. Mol. Asp. Med. 2015;41:1–115. doi: 10.1016/j.mam.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 392.Li M., Sun Y., Guan X., Shu X., Li C. Advanced progress on the relationship between RA and its receptors and malignant tumors. Crit. Rev. Oncol. Hematol. 2014;91:271–282. doi: 10.1016/j.critrevonc.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 393.Chou A.P., Chowdhury R., Li S., Chen W., Kim A.J., Piccioni D.E., Selfridge J.M., Mody R.R., Chang S., Lalezari S., et al. Identification of retinol binding protein 1 promoter hypermethylation in isocitrate dehydrogenase 1 and 2 mutant gliomas. J. Natl. Cancer Inst. 2012;104:1458–1469. doi: 10.1093/jnci/djs357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Campos B., Warta R., Chaisaingmongkol J., Geiselhart L., Popanda O., Hartmann C., von Deimling A., Unterberg A., Plass C., Schmezer P., et al. Epigenetically mediated downregulation of the differentiation-promoting chaperon protein CRABP2 in astrocytic gliomas. Int. J. Cancer. 2012;131:1963–1968. doi: 10.1002/ijc.27446. [DOI] [PubMed] [Google Scholar]
- 395.Sanders S., Herpai D.M., Rodriguez A., Huang Y., Chou J., Hsu F.C., Seals D., Mott R., Miller L.D., Debinski W. The Presence and Potential Role of ALDH1A2 in the Glioblastoma Microenvironment. Cells. 2021;10:2485. doi: 10.3390/cells10092485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Campos B., Weisang S., Osswald F., Ali R., Sedlmeier G., Bageritz J., Mallm J.P., Hartmann C., von Deimling A., Popanda O., et al. Retinoid resistance and multifaceted impairment of retinoic acid synthesis in glioblastoma. Glia. 2015;63:1850–1859. doi: 10.1002/glia.22849. [DOI] [PubMed] [Google Scholar]
- 397.Barbus S., Tews B., Karra D., Hahn M., Radlwimmer B., Delhomme N., Hartmann C., Felsberg J., Krex D., Schackert G., et al. Differential retinoic acid signaling in tumors of long- and short-term glioblastoma survivors. J. Natl. Cancer Inst. 2011;103:598–606. doi: 10.1093/jnci/djr036. [DOI] [PubMed] [Google Scholar]
- 398.Piperi C., Themistocleous M.S., Papavassiliou G.A., Farmaki E., Levidou G., Korkolopoulou P., Adamopoulos C., Papavassiliou A.G. High incidence of MGMT and RARbeta promoter methylation in primary glioblastomas: Association with histopathological characteristics, inflammatory mediators and clinical outcome. Mol. Med. 2010;16:1–9. doi: 10.2119/molmed.2009.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Bouterfa H., Picht T., Kess D., Herbold C., Noll E., Black P.M., Roosen K., Tonn J.C. Retinoids inhibit human glioma cell proliferation and migration in primary cell cultures but not in established cell lines. Neurosurgery. 2000;46:419–430. doi: 10.1097/00006123-200002000-00029. [DOI] [PubMed] [Google Scholar]
- 400.Campos B., Wan F., Farhadi M., Ernst A., Zeppernick F., Tagscherer K.E., Ahmadi R., Lohr J., Dictus C., Gdynia G., et al. Differentiation therapy exerts antitumor effects on stem-like glioma cells. Clin. Cancer Res. 2010;16:2715–2728. doi: 10.1158/1078-0432.CCR-09-1800. [DOI] [PubMed] [Google Scholar]
- 401.Rodriguez V., Bailey R., Larion M., Gilbert M.R. Retinoid receptor turnover mediated by sumoylation, ubiquitination and the valosin-containing protein is disrupted in glioblastoma. Sci. Rep. 2019;9:16250. doi: 10.1038/s41598-019-52696-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Paillaud E., Costa S., Fages C., Plassat J.L., Rochette-Egly C., Monville C., Tardy M. Retinoic acid increases proliferation rate of GL-15 glioma cells, involving activation of STAT-3 transcription factor. J. Neurosci. Res. 2002;67:670–679. doi: 10.1002/jnr.10110. [DOI] [PubMed] [Google Scholar]
- 403.Chen P.H., Shih C.M., Chang W.C., Cheng C.H., Lin C.W., Ho K.H., Su P.C., Chen K.C. MicroRNA-302b-inhibited E2F3 transcription factor is related to all trans retinoic acid-induced glioma cell apoptosis. J. Neurochem. 2014;131:731–742. doi: 10.1111/jnc.12820. [DOI] [PubMed] [Google Scholar]
- 404.Haque A., Das A., Hajiaghamohseni L.M., Younger A., Banik N.L., Ray S.K. Induction of apoptosis and immune response by all-trans retinoic acid plus interferon-gamma in human malignant glioblastoma T98G and U87MG cells. Cancer Immunol. Immunother. 2007;56:615–625. doi: 10.1007/s00262-006-0219-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Marjanović Vićentić J., Schwirtlich M., Kovačević-Grujičić N., Stevanović M., Drakulić D. All-trans retinoic acid influences viability, migration and adhesion of U251 glioblastoma cells. Arch. Biol. Sci. 2017;69:699–706. doi: 10.2298/ABS170327016M. [DOI] [Google Scholar]
- 406.Zeng Y., Yang Z., Xu J.G., Yang M.S., Zeng Z.X., You C. Differentially expressed genes from the glioblastoma cell line SHG-44 treated with all-trans retinoic acid in vitro. J. Clin. Neurosci. 2009;16:285–294. doi: 10.1016/j.jocn.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 407.Wang R., Liu C. All-trans retinoic acid therapy induces asymmetric division of glioma stem cells from the U87MG cell line. Oncol. Lett. 2019;18:3646–3654. doi: 10.3892/ol.2019.10691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Ling G.Q., Liu Y.J., Ke Y.Q., Chen L., Jiang X.D., Jiang C.L., Ye W. All-trans retinoic acid impairs the vasculogenic mimicry formation ability of U87 stem-like cells through promoting differentiation. Mol. Med. Rep. 2015;12:165–172. doi: 10.3892/mmr.2015.3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Ying M., Wang S., Sang Y., Sun P., Lal B., Goodwin C.R., Guerrero-Cazares H., Quinones-Hinojosa A., Laterra J., Xia S. Regulation of glioblastoma stem cells by retinoic acid: Role for Notch pathway inhibition. Oncogene. 2011;30:3454–3467. doi: 10.1038/onc.2011.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Karsy M., Albert L., Tobias M.E., Murali R., Jhanwar-Uniyal M. All-trans retinoic acid modulates cancer stem cells of glioblastoma multiforme in an MAPK-dependent manner. Anticancer Res. 2010;30:4915–4920. [PubMed] [Google Scholar]
- 411.Niu C.S., Li M.W., Ni Y.F., Chen J.M., Mei J.M., Li J., Fu X.M. Effect of all-trans retinoic acid on the proliferation and differentiation of brain tumor stem cells. J. Exp. Clin. Cancer Res. 2010;29:113. doi: 10.1186/1756-9966-29-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Choschzick I., Hirseland E., Cramer H., Schultz S., Leppert J., Tronnier V., Zechel C. Responsiveness of stem-like human glioma cells to all-trans retinoic acid and requirement of retinoic acid receptor isotypes alpha, beta and gamma. Neuroscience. 2014;279:44–64. doi: 10.1016/j.neuroscience.2014.07.078. [DOI] [PubMed] [Google Scholar]
- 413.Reboul P., George P., Louisot P., Broquet P. Study of retinoic acid effect upon retinoic acid receptors beta (RAR-beta) in C6 cultured glioma cells. Biochem. Mol. Biol. Int. 1995;36:1097–1105. [PubMed] [Google Scholar]
- 414.Rodts G.E., Jr., Black K.L. Trans retinoic acid inhibits in vivo tumour growth of C6 glioma in rats: Effect negatively influenced by nerve growth factor. Neurol. Res. 1994;16:184–186. doi: 10.1080/01616412.1994.11740223. [DOI] [PubMed] [Google Scholar]
- 415.See S.J., Levin V.A., Yung W.K., Hess K.R., Groves M.D. 13-cis-retinoic acid in the treatment of recurrent glioblastoma multiforme. Neuro-Oncology. 2004;6:253–258. doi: 10.1215/S1152851703000607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Tang K., Cao L., Fan S.Q., Wu M.H., Huang H., Zhou Y.H., Zhou M., Tang Y.L., Wang R., Zeng F., et al. Effect of all-trans-retinoic acid on C6 glioma cell proliferation and differentiation. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2008;33:892–897. [PubMed] [Google Scholar]
- 417.Milanovic D., Maier P., Lohr F., Wenz F., Herskind C. Inhibition of 13-cis retinoic acid-induced gene expression of homeobox B7 by thalidomide. Int. J. Cancer. 2007;121:1205–1211. doi: 10.1002/ijc.22815. [DOI] [PubMed] [Google Scholar]
- 418.Heo J.C., Jung T.H., Lee S., Kim H.Y., Choi G., Jung M., Jung D., Lee H.K., Lee J.O., Park J.H., et al. Effect of bexarotene on differentiation of glioblastoma multiforme compared with ATRA. Clin. Exp. Metastasis. 2016;33:417–429. doi: 10.1007/s10585-016-9786-x. [DOI] [PubMed] [Google Scholar]
- 419.Shi L., Li H., Zhan Y. All-trans retinoic acid enhances temozolomide-induced autophagy in human glioma cells U251 via targeting Keap1/Nrf2/ARE signaling pathway. Oncol. Lett. 2017;14:2709–2714. doi: 10.3892/ol.2017.6482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Karmakar S., Banik N.L., Patel S.J., Ray S.K. Combination of all-trans retinoic acid and taxol regressed glioblastoma T98G xenografts in nude mice. Apoptosis. 2007;12:2077–2087. doi: 10.1007/s10495-007-0116-2. [DOI] [PubMed] [Google Scholar]
- 421.Karmakar S., Banik N.L., Ray S.K. Combination of all-trans retinoic acid and paclitaxel-induced differentiation and apoptosis in human glioblastoma U87MG xenografts in nude mice. Cancer. 2008;112:596–607. doi: 10.1002/cncr.23223. [DOI] [PubMed] [Google Scholar]
- 422.Karmakar S., Choudhury S.R., Banik N.L., Ray S.K. Activation of Multiple Molecular Mechanisms for Increasing Apoptosis in Human Glioblastoma T98G Xenograft. J. Cancer Sci. 2010;2:107–113. doi: 10.4172/1948-5956.1000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Songthaveesin C., Sa-Nongdej W., Limboonreung T., Chongthammakun S. Combination of metformin and 9-cis retinoic acid increases apoptosis in C6 glioma stem-like cells. Heliyon. 2018;4:e00638. doi: 10.1016/j.heliyon.2018.e00638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Milanovic D., Sticht C., Rohrich M., Maier P., Grosu A.L., Herskind C. Inhibition of 13-cis retinoic acid-induced gene expression of reactive-resistance genes by thalidomide in glioblastoma tumours in vivo. Oncotarget. 2015;6:28938–28948. doi: 10.18632/oncotarget.4727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Papi A., Tatenhorst L., Terwel D., Hermes M., Kummer M.P., Orlandi M., Heneka M.T. PPARgamma and RXRgamma ligands act synergistically as potent antineoplastic agents in vitro and in vivo glioma models. J. Neurochem. 2009;109:1779–1790. doi: 10.1111/j.1471-4159.2009.06111.x. [DOI] [PubMed] [Google Scholar]
- 426.Pitz M.W., Lipson M., Hosseini B., Lambert P., Guilbert K., Lister D., Schroeder G., Jones K., Mihalicioiu C., Eisenstat D.D. Extended adjuvant temozolomide with cis-retinoic acid for adult glioblastoma. Curr. Oncol. 2012;19:308–314. doi: 10.3747/co.19.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Butowski N., Prados M.D., Lamborn K.R., Larson D.A., Sneed P.K., Wara W.M., Malec M., Rabbitt J., Page M., Chang S.M. A phase II study of concurrent temozolomide and cis-retinoic acid with radiation for adult patients with newly diagnosed supratentorial glioblastoma. Int. J. Radiat. Oncol. Biol. Phys. 2005;61:1454–1459. doi: 10.1016/j.ijrobp.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 428.Puduvalli V.K., Yung W.K., Hess K.R., Kuhn J.G., Groves M.D., Levin V.A., Zwiebel J., Chang S.M., Cloughesy T.F., Junck L., et al. Phase II study of fenretinide (NSC 374551) in adults with recurrent malignant gliomas: A North American Brain Tumor Consortium study. J. Clin. Oncol. 2004;22:4282–4289. doi: 10.1200/JCO.2004.09.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Levin V.A., Giglio P., Puduvalli V.K., Jochec J., Groves M.D., Yung W.K., Hess K. Combination chemotherapy with 13-cis-retinoic acid and celecoxib in the treatment of glioblastoma multiforme. J. Neurooncol. 2006;78:85–90. doi: 10.1007/s11060-005-9062-4. [DOI] [PubMed] [Google Scholar]
- 430.Jaeckle K.A., Hess K.R., Yung W.K., Greenberg H., Fine H., Schiff D., Pollack I.F., Kuhn J., Fink K., Mehta M., et al. Phase II evaluation of temozolomide and 13-cis-retinoic acid for the treatment of recurrent and progressive malignant glioma: A North American Brain Tumor Consortium study. J. Clin. Oncol. 2003;21:2305–2311. doi: 10.1200/JCO.2003.12.097. [DOI] [PubMed] [Google Scholar]
- 431.Ferreira R., Napoli J., Enver T., Bernardino L., Ferreira L. Advances and challenges in retinoid delivery systems in regenerative and therapeutic medicine. Nat. Commun. 2020;11:4265. doi: 10.1038/s41467-020-18042-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Jeong Y.I., Kim S.H., Jung T.Y., Kim I.Y., Kang S.S., Jin Y.H., Ryu H.H., Sun H.S., Jin S., Kim K.K., et al. Polyion complex micelles composed of all-trans retinoic acid and poly (ethylene glycol)-grafted-chitosan. J. Pharm. Sci. 2006;95:2348–2360. doi: 10.1002/jps.20586. [DOI] [PubMed] [Google Scholar]
- 433.Jones T., Zhang B., Major S., Webb A. All-trans retinoic acid eluting poly(diol citrate) wafers for treatment of glioblastoma. J. Biomed. Mater. Res. B Appl. Biomater. 2020;108:619–628. doi: 10.1002/jbm.b.34416. [DOI] [PubMed] [Google Scholar]
- 434.Reifenberger G., Liu L., Ichimura K., Schmidt E.E., Collins V.P. Amplification and overexpression of the MDM2 gene in a subset of human malignant gliomas without p53 mutations. Cancer Res. 1993;53:2736–2739. [PubMed] [Google Scholar]
- 435.Izumoto S., Arita N., Ohnishi T., Hiraga S., Taki T., Tomita N., Ohue M., Hayakawa T. Microsatellite instability and mutated type II transforming growth factor-beta receptor gene in gliomas. Cancer Lett. 1997;112:251–256. doi: 10.1016/S0304-3835(96)04583-1. [DOI] [PubMed] [Google Scholar]
- 436.Suzuki H., Aoki K., Chiba K., Sato Y., Shiozawa Y., Shiraishi Y., Shimamura T., Niida A., Motomura K., Ohka F., et al. Mutational landscape and clonal architecture in grade II and III gliomas. Nat. Genet. 2015;47:458–468. doi: 10.1038/ng.3273. [DOI] [PubMed] [Google Scholar]
- 437.Sakthikumar S., Roy A., Haseeb L., Pettersson M.E., Sundstrom E., Marinescu V.D., Lindblad-Toh K., Forsberg-Nilsson K. Whole-genome sequencing of glioblastoma reveals enrichment of non-coding constraint mutations in known and novel genes. Genome Biol. 2020;21:127. doi: 10.1186/s13059-020-02035-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Madsen H., Hellwinkel J.E., Graner M.W. Clinical Trials in Glioblastoma—Designs and Challenges. In: Lichtor T., editor. Molecular Considerations and Evolving Surgical Management Issues in the Treatment of Patients with a Brain Tumor. IntechOpen; Rijeka, Croatia: 2015. [DOI] [Google Scholar]
- 439.Park A.K., Kim P., Ballester L.Y., Esquenazi Y., Zhao Z. Subtype-specific signaling pathways and genomic aberrations associated with prognosis of glioblastoma. Neuro-Oncology. 2019;21:59–70. doi: 10.1093/neuonc/noy120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.El-Sehemy A., Selvadurai H., Ortin-Martinez A., Pokrajac N., Mamatjan Y., Tachibana N., Rowland K., Lee L., Park N., Aldape K., et al. Norrin mediates tumor-promoting and -suppressive effects in glioblastoma via Notch and Wnt. J. Clin. Investig. 2020;130:3069–3086. doi: 10.1172/JCI128994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Lin C.J., Chen T.L., Tseng Y.Y., Wu G.J., Hsieh M.H., Lin Y.W., Chen R.M. Honokiol induces autophagic cell death in malignant glioma through reactive oxygen species-mediated regulation of the p53/PI3K/Akt/mTOR signaling pathway. Toxicol. Appl. Pharm. 2016;304:59–69. doi: 10.1016/j.taap.2016.05.018. [DOI] [PubMed] [Google Scholar]
- 442.Jung Y., Park H., Zhao H.Y., Jeon R., Ryu J.H., Kim W.Y. Systemic approaches identify a garlic-derived chemical, Z-ajoene, as a glioblastoma multiforme cancer stem cell-specific targeting agent. Mol. Cells. 2014;37:547–553. doi: 10.14348/molcells.2014.0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Ivkovic I., Novakovic M., Veljic M., Mojsin M., Stevanovic M., Marin P.D., Bukvicki D. Bis-Bibenzyls from the Liverwort Pellia endiviifolia and Their Biological Activity. Plants. 2021;10:1063. doi: 10.3390/plants10061063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Stojkovic D., Drakulic D., Dias M.I., Zengin G., Barros L., Ivanov M., Gasic U., Rajcevic N., Stevanovic M., Ferreira I., et al. Phlomis fruticosa L. exerts in vitro antineurodegenerative and antioxidant activities and induces prooxidant effect in glioblastoma cell line. EXCLI J. 2022;21:387–399. doi: 10.17179/excli2021-4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Stojkovic D., Drakulic D., Gasic U., Zengin G., Stevanovic M., Rajcevic N., Sokovic M. Ononis spinosa L., an edible and medicinal plant: UHPLC-LTQ-Orbitrap/MS chemical profiling and biological activities of the herbal extract. Food Funct. 2020;11:7138–7151. doi: 10.1039/D0FO01595D. [DOI] [PubMed] [Google Scholar]
- 446.Stojkovic D., Drakulic D., Schwirtlich M., Rajcevic N., Stevanovic M., Sokovic M.D., Gasic U. Extract of Herba Anthrisci cerefolii: Chemical Profiling and Insights into Its Anti-Glioblastoma and Antimicrobial Mechanism of Actions. Pharmaceuticals. 2021;14:55. doi: 10.3390/ph14010055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Mariappan A., Goranci-Buzhala G., Ricci-Vitiani L., Pallini R., Gopalakrishnan J. Trends and challenges in modeling glioma using 3D human brain organoids. Cell Death Differ. 2021;28:15–23. doi: 10.1038/s41418-020-00679-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Goranci-Buzhala G., Mariappan A., Gabriel E., Ramani A., Ricci-Vitiani L., Buccarelli M., D’Alessandris Q.G., Pallini R., Gopalakrishnan J. Rapid and Efficient Invasion Assay of Glioblastoma in Human Brain Organoids. Cell Rep. 2020;31:107738. doi: 10.1016/j.celrep.2020.107738. [DOI] [PubMed] [Google Scholar]
- 449.Chadwick M., Yang C., Liu L., Gamboa C.M., Jara K., Lee H., Sabaawy H.E. Rapid Processing and Drug Evaluation in Glioblastoma Patient-Derived Organoid Models with 4D Bioprinted Arrays. iScience. 2020;23:101365. doi: 10.1016/j.isci.2020.101365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Stankovic T., Randelovic T., Dragoj M., Stojkovic Buric S., Fernandez L., Ochoa I., Perez-Garcia V.M., Pesic M. In vitro biomimetic models for glioblastoma-a promising tool for drug response studies. Drug Resist. Updat. 2021;55:100753. doi: 10.1016/j.drup.2021.100753. [DOI] [PubMed] [Google Scholar]
- 451.Alomari S., Zhang I., Hernandez A., Kraft C.Y., Raj D., Kedda J., Tyler B. Drug Repurposing for Glioblastoma and Current Advances in Drug Delivery-A Comprehensive Review of the Literature. Biomolecules. 2021;11:1870. doi: 10.3390/biom11121870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Aghabegi Moghanjoughi A., Khoshnevis D., Zarrabi A. A concise review on smart polymers for controlled drug release. Drug Deliv. Transl. Res. 2016;6:333–340. doi: 10.1007/s13346-015-0274-7. [DOI] [PubMed] [Google Scholar]
- 453.Nouri Z., Fakhri S., Nouri K., Wallace C.E., Farzaei M.H., Bishayee A. Targeting Multiple Signaling Pathways in Cancer: The Rutin Therapeutic Approach. Cancers. 2020;12:2276. doi: 10.3390/cancers12082276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Fathi M., Sahandi Zangabad P., Majidi S., Barar J., Erfan-Niya H., Omidi Y. Stimuli-responsive chitosan-based nanocarriers for cancer therapy. Bioimpacts. 2017;7:269–277. doi: 10.15171/bi.2017.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Liu M., Du H., Zhang W., Zhai G. Internal stimuli-responsive nanocarriers for drug delivery: Design strategies and applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2017;71:1267–1280. doi: 10.1016/j.msec.2016.11.030. [DOI] [PubMed] [Google Scholar]
- 456.Hodi F.S., O’Day S.J., McDermott D.F., Weber R.W., Sosman J.A., Haanen J.B., Gonzalez R., Robert C., Schadendorf D., Hassel J.C., et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457.Kazandjian D., Suzman D.L., Blumenthal G., Mushti S., He K., Libeg M., Keegan P., Pazdur R. FDA Approval Summary: Nivolumab for the Treatment of Metastatic Non-Small Cell Lung Cancer with Progression on or after Platinum-Based Chemotherapy. Oncologist. 2016;21:634–642. doi: 10.1634/theoncologist.2015-0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.Yu M.W., Quail D.F. Immunotherapy for Glioblastoma: Current Progress and Challenges. Front. Immunol. 2021;12:676301. doi: 10.3389/fimmu.2021.676301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459.Bausart M., Preat V., Malfanti A. Immunotherapy for glioblastoma: The promise of combination strategies. J. Exp. Clin. Cancer Res. 2022;41:35. doi: 10.1186/s13046-022-02251-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Chan H.Y., Choi J., Jackson C., Lim M. Combination immunotherapy strategies for glioblastoma. J. Neurooncol. 2021;151:375–391. doi: 10.1007/s11060-020-03481-0. [DOI] [PubMed] [Google Scholar]
- 461.Yuan B., Wang G., Tang X., Tong A., Zhou L. Immunotherapy of glioblastoma: Recent advances and future prospects. Hum. Vaccines Immunother. 2022;18:2055417. doi: 10.1080/21645515.2022.2055417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462.Gonzalez Castro L.N., Tirosh I., Suva M.L. Decoding Cancer Biology One Cell at a Time. Cancer Discov. 2021;11:960–970. doi: 10.1158/2159-8290.CD-20-1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.Tirosh I., Suva M.L. Dissecting human gliomas by single-cell RNA sequencing. Neuro-Oncology. 2018;20:37–43. doi: 10.1093/neuonc/nox126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Suva M.L., Tirosh I. The Glioma Stem Cell Model in the Era of Single-Cell Genomics. Cancer Cell. 2020;37:630–636. doi: 10.1016/j.ccell.2020.04.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.