Abstract
Helicobacter pylori is naturally competent for DNA transformation, but the mechanism by which transformation occurs is not known. For Haemophilus influenzae, dprA is required for transformation by chromosomal but not plasmid DNA, and the complete genomic sequence of H. pylori 26695 revealed a dprA homolog (HP0333). Examination of genetic databases indicates that DprA homologs are present in a wide variety of bacterial species. To examine whether HP0333 has a function similar to dprA of H. influenzae, HP0333, present in each of 11 strains studied, was disrupted in two H. pylori isolates. For both mutants, the frequency of transformation by H. pylori chromosomal DNA was markedly reduced, but not eliminated, compared to their wild-type parental strains. Mutation of HP0333 also resulted in a marked decrease in transformation frequency by a shuttle plasmid (pHP1), which differs from the phenotype described in H. influenzae. Complementation of the mutant with HP0333 inserted in trans in the chromosomal ureAB locus completely restored the frequency of transformation to that of the wild-type strain. Thus, while dprA is required for high-frequency transformation, transformation also may occur independently of DprA. The presence of DprA homologs in bacteria known not to be naturally competent suggests a broad function in DNA processing.
Helicobacter pylori, a microaerophilic, gram-negative bacterium that colonizes the human stomach, has been recognized as the major causative agent of chronic gastritis and as playing a role in peptic ulcer disease and gastric cancer (8). H. pylori strains have a high degree of diversity at the genetic level (2, 19, 34), including a high rate of point mutations in conserved genes such as ureB (28) and flaA (45), mosaicism within genes such as vacA (5), and the presence of nonconserved DNA fragments, in particular, the cag pathogenicity island (2, 10, 13, 50). Other factors that lead to further diversity among H. pylori strains are the presence of insertion sequences (10, 23) or plasmids (31), variation in gene order (25), and the complement of putative restriction endonucleases (2, 48).
The genetic diversity of H. pylori has clinical significance, since markers for strains with enhanced virulence have been identified (5, 13, 39, 50). Additionally, techniques such as restriction fragment length polymorphism and randomly amplified polymorphic DNA PCR have been used to exploit this heterogeneity for epidemiologic purpose (2, 3, 41, 51).
Horizontal DNA transfer within the reservoir for H. pylori, i.e., the stomach of primates, would contribute to the development of genetic diversity (33). There is substantial evidence that recombination among H. pylori strains has been an important feature of their evolution (5, 23, 46). Natural transformation in bacteria is a complex process involving DNA binding, uptake/translocation, and recombination. Many H. pylori strains are known to be naturally competent for transformation in vitro (32, 37, 43, 49, 55). However, the mechanisms for transformation of DNA have not been closely studied for H. pylori. Thus far, only recA (44, 47) and the comB locus (22) have been identified as having a role in H. pylori transformation. Recognition of the mechanisms involved in genetic exchange may help us to understand the adaptation of H. pylori to changing environments and could shed light on clinically important issues of virulence and development of antibiotic resistance (7, 21, 52). The complete genomic sequence of H. pylori 26695 (48) revealed a 810-bp open reading frame (ORF) (HP0333) encoding a deduced protein of 270 amino acids (30,470-Da molecular mass) with homology to DprA (encoded by dprA) of Haemophilus influenzae. In H. influenzae, dprA is required for transformation by chromosomal DNA (29). We therefore sought to determine whether HP0333 is required for natural transformation in H. pylori.
MATERIALS AND METHODS
Strains and growth conditions.
Strains and plasmids used in this study are listed in Table 1. Escherichia coli was routinely grown at 37°C in Luria-Bertani broth or agar supplemented with ampicillin (100 μg/ml), kanamycin (25 μg/ml), and/or chloramphenicol (60 μg/ml), when appropriate. H. pylori strains were grown on Trypticase soy agar (TSA) with 5% sheep blood (BBL) or Brucella-serum (BS;BBL) agar with 10% newborn calf serum (Intergen) plates at 37°C in a 5% CO2 atmosphere. Antibiotic-resistant H. pylori transformants were selected with kanamycin (25 μg/ml), chloramphenicol (10 μg/ml), streptomycin (20 μg/ml), or spectinomycin (20 μg/ml). We selected H. pylori strains that were spontaneously streptomycin or spectinomycin resistant (Strr or Spcr) by plating large numbers (approximately 1010) of bacteria on TSA medium containing streptomycin (10 μg/ml) or spectinomycin (10 μg/ml), respectively.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain (alternate name) or plasmid | Genotype or characteristics | Reference or source |
|---|---|---|
| H. pylori | ||
| 26695 (88-21) | Wild type | 48 |
| 84-183 | Wild type | 50 |
| HPK5 (98-716) | Wild type | 49 |
| 98-712 | 84-183, StrrureB::aphA | This study |
| 98-706 | 84-183, Spcr | This study |
| 98-707 | HPK5, Strr, pHP1 (Kanr, Ampr) | This study |
| 98-708 | HPK1, Spcr | This study |
| 98-700 | 84-183/0333::aphA | This study |
| 98-701 | HPK5/0333::aphA | This study |
| 98-709 | HPK5/0333::aphA, pANDO1 (Ampr, Camr) | This study |
| C5 (98-710) | HPK5, Pure-HP0333-cat-′ureAB | This study |
| C5G9 (98-702) | HPK5/0333::aphA, Pure-HP0333-cat-′ureAB | This study |
| C5G10 (98-711) | HPK5, Pure-HP0333::aphA-cat-′ureAB | This study |
| E. coli DH5α | endA1 hsdR17 (rK− mK+) supE44 thi-1 recA gyrA (Nalr) relA1 Δ(argF-lacZYA)U169 deoR [φ80dlacΔ(lacZ)M15] | |
| Plasmids | ||
| pGEM-T Easy | ColE1, Ampr, PCR cloning vector | Promega |
| pDAI12 | pGEM-T Easy containing HP0333 | This study |
| pUK4K | ColE1 (Kanr, Ampr) | Pharmacia |
| pDPR2 | pDAI12, HP0333::aphA | This study |
| pHP1 | H. pylori-E. coli shuttle vector (Ampr, Kanr) | 31 |
| pUC19 | ColE1, Ampr | New England Biolabs |
| pBSC103 | Ampr, Camr | 54 |
| pANDO2 | pUC19, Pure-HP0333-cat-′ureAB | This study |
Construction of plasmids and strains.
The HP0333 ORF of strain 26695 (48) was amplified by PCR using primers A9853 and A9854. The product was ligated into pGEM-T Easy and transformed into E. coli DH5α. A unique BamHI site was created by inverse PCR with primers C3581 and C3582. Plasmid pUC4K was digested with BamHI, after which the kanamycin resistance (Kanr; aphA) cassette was isolated by agarose gel electrophoresis and ligated into the inverse PCR product to disrupt the HP0333 ORF, creating pDPR2. H. pylori 84-183 and HPK5 were transformed to Kanr with pDPR2, to create 84-183/0333::aphA and HPK5/0333::aphA, respectively. Chromosomal DNA was isolated from strains 84-183, HPK5, 84-183/0333::aphA, and HPK5/0333::aphA, and the insertion of the aphA cassette within HP0333 in the transformants was confirmed by PCR using primer pairs C3635-C3636, C3635-C3632, and C3634-C3636. PCR oligonucleotide primers specific for HP0333 or aphA were used, and the sizes of the PCR products were evaluated by agarose gel electrophoresis (Table 2).
TABLE 2.
Oligonucleotide primers used in this study
| Primer designation | Site | Location in genea | Orientationb | Primer sequence (5′→3′)c |
|---|---|---|---|---|
| A9853 | HP0333 | 22 to 43 | F | CACTTCCAATACAGCCACGCTAG |
| A9854 | HP0333 | 694 to 671 | R | CTAAAAATTCATCTTCCATTTCAG |
| C3581 | HP0333 | 369 to 388 | F | CATAGGATCCTTGATTTTAAGCGAATATG |
| C3582 | HP0333 | 357 to 336 | R | GATAGGATCCGATTTCTTGGATCACTTTATG |
| C3635 | minE | 196 to 206 | F | GTGGAAACGATTGAAGTAGAG |
| C3636 | HP0334 | 92 to 72 | R | TGGCGTAAGATCGCTTCTAAG |
| C3632 | aphA | 83 to 64 | R | CTTGTGCAATGTAACATCAG |
| C3634 | aphA | 1146 to 1165 | F | CAAAGCTCTCATCAACCGTG |
| AN586 (a) | nadE | 41 to 22 | F | TCGCATAAATAAACGATGAG |
| AN587 (b) | ilvC | 63 to 43 | R | CACTTGTAAGGATTGGATAAC |
| AN588 (c) | ilvC | 744 to 765 | F | CTTAAGGAAACACATTTCTAAC |
| AN589 (d) | minD | 30 to 13 | R | TACCTGAAGTGATAGTAAC |
| AN590 (e) | minD | 570 to 586 | F | GATGATTTCTATAGAAGAAG |
| AN591 (f) | minE | 70 to 50 | R | GAATCAATTTCAATCGGTCTG |
| AN860 (g3) | minE | 108 to 126 | F | GAAATCATTGCCGTCATTC |
| AN862 (h2) | HP0333 | 314 to 295 | R | TCCAAACTGCAAGGCGAAAG |
| AN594 (I) | HP0333 | 564 to 582 | F | GAGCGATGGCACTAATGAG |
| AN595 (j) | HP0334 | 92 to 72 | R | TGGCGTAAGATCGCTTCTAAG |
| AN863 (k2) | HP0334 | 320 to 338 | F | CGCTATCAAAGACGGTCGG |
| AN864 (12) | HP0335 | −16 to −35 | R | TAAACGCACAACCCAAGCAC |
| B9135 (1) | minE | 50 to 70 | F | CAGCTGCAGCAGACCGATTGAAATTGATTC |
| B9136 (2) | HP0334 | 92 to 72 | R | TAGCCCGGGCTGCAGTGGCGTAAGATCGCTTCTAAG |
| B9138 (4) | ureA | −5 to −23 | F | CAGCTGCAGTCTAGATTCTCCTATTCTTAAAGTG |
| B9140 (6) | ureA | −318 to −299 | R | GAGGTACCTAGCTCAGTTGGTAGAGCAC |
| B9141 (8) | ureA | 347 to 367 | R | GCTCTAGACCCGGGAAGACATCACTATCAACGAAG |
| B9142 (9) | ureB | 213 to 194 | F | CCAATGCATGTGATGATTAGATCCAATTC |
| B9139 (5) | HP0333 | 2 to 29 | F | GCTCTAGATGAATCAACGAATGAAAAGCC |
| A1747 | lspA | 389 to 408 | R | ATCTTGCATGTCTTTGTTAC |
| A1746 | ureB | 319 to 300 | F | CTGATGTCATGATAGATGTG |
For HP0333, nadE, ilvC, minD, minE, HP0344, HP0337, ureA, and ureB, locations refer to positions within ORFs in strain 26695 based on the first nucleotide of the initiation codon (48).
F, forward; R, reverse. For aphA, orientation in relation to ORF location refers to Kanr cassette of pUC4K.
Added restriction sites underlined: GGATCC (BamHI), CTGCAG (PstI), CCCGGG (SmaI), TCTAGA (XbaI), GGTACC (KpnI), and ATGCAT (NsiI).
Electroporation.
HPK5 was transformed with pHP1 by electroporation as described elsewhere (32), resulting in strain 98-716 (Table 1).
DNA techniques and sequence analysis.
Standard molecular techniques were used (42). H. pylori chromosomal DNA was prepared from cells of each strain after 48 h of growth on two agar plates as described previously (56). Plasmid DNA was prepared from H. pylori after 48 h of growth or from E. coli after overnight cultures, using a midi-prep protocol (Qiagen Inc., Valencia, Calif.) according to the manufacturer’s instructions. Database similarity searches of GenBank and Unfinished Microbial Genome sequences were done with the BLAST algorithms (4). Sequence comparisons was performed with Genetics Computer Group analysis programs (5). Secondary structure analysis was done by the Chou-Fasman algorithm (12), using the program Peptidestructure (24). Further analysis of predicted protein sequences was done with PAUP 3.1 (Smithsonian Institution, Washington, D.C.), MacClade version 3 (35), Phylip (17), and TreeView (38).
Natural transformation.
Recipient H. pylori cells were harvested from 48-h growth on one agar plate into 1 ml of phosphate-buffered saline (PBS) and then centrifuged at 8,500 g for 5 min. The pellet was resuspended in 300 μl of PBS. Each transformation mixture, consisting of 25 μl of recipient cells and 30 ng of donor DNA, was spotted onto a TSA plate (approximately 20 ng of DNA/25 μl of cells is a saturating amount of DNA [data not shown]). Plates were incubated overnight at 37°C in a 5% CO2 atmosphere. After 18 h of incubation, the transformation mixture was harvested into 1 ml of PBS, and 100-μl aliquots of appropriate serial dilutions were plated on BS-streptomycin or BS-spectinomycin plates and on TSA plates. All plates were incubated for 4 days at 37°C in a 5% CO2 atmosphere, after which transformants and total viable cells were counted. The transformation frequency was determined by the number of Strr or Spcr colonies per microgram of DNA per recipient CFU.
Analyses of complementation.
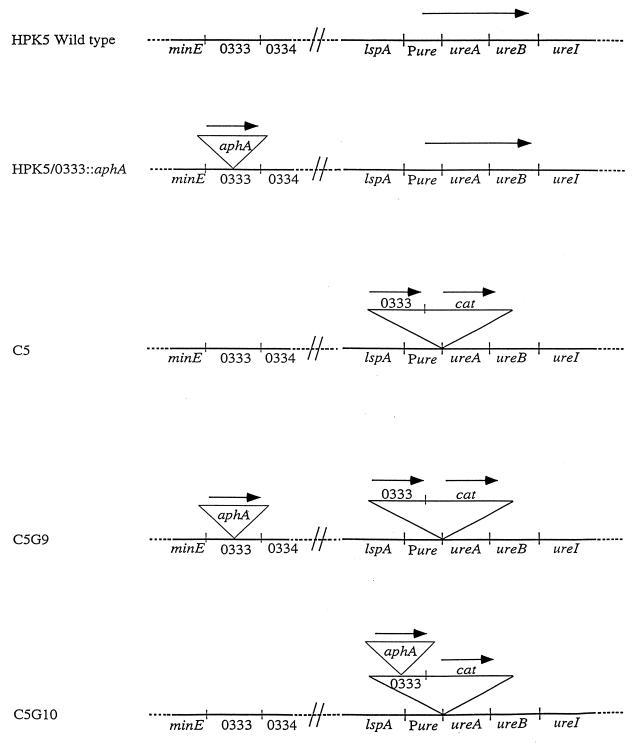
Portions of the H. pylori urease operon (ureAB) and its promoter region (Pure) were amplified from strain 26695 by PCR using primers described in Table 2, and these products were ligated into pUC19. HP0333 was amplified from chromosomal DNA from strain 26695 and ligated between Pure and ureAB. The cat cassette was isolated from pBSC103 by digestion with SmaI and EcoRV, purified, and ligated between HP0333 and ureAB to create pANDO2. HPK5 was transformed with pANDO2, resulting in C5. C5 was transformed by pDPR2 to create C5G9 and C5G10. The frequency of natural transformation was compared for the wild-type strain HPK5 and its isogenic mutants, HPK5/0333::aphA, C5, C5G9, and C5G10 (see Fig. 5).
FIG. 5.
Construction of H. pylori mutants involving HP0333. For each strain, the loci surrounding HP0333 and ureAB are shown. A Kanr (aphA) cassette was introduced into HP0333 of HPK5 to create HPK5/0333::aphA. The insert of pANDO2 was introduced into the ureAB locus of HPK5 downstream of the ureAB promoter (Pure) to create C5. An aphA cassette was introduced into strain C5 to create either C5G9 or C5G10.
RESULTS
Comparison of H. pylori HP0333 with other bacterial sequences.
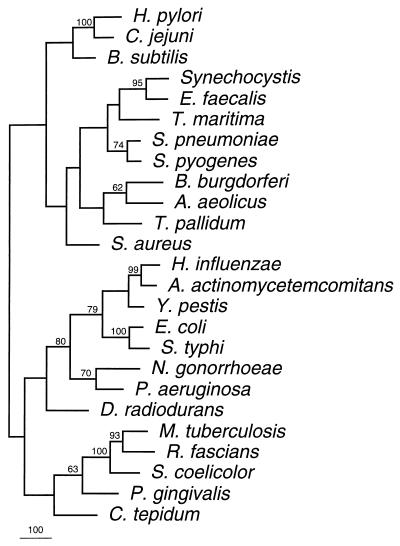
Comparison of the predicted product of HP0333 from strain 26695 with the genomic sequence of strain J99 (3) revealed a homolog (JHP316) with 93.7% nucleotide identity and 95.9% amino acid identity. HP0333 is predicted to encode a protein with 270 amino acids, while JHP316 is predicted to encode a protein of 266 amino acids. We next sought to determine whether the predicted product of HP0333 had homology to proteins other than DprA of H. influenzae. A BLAST search of GenBank and Unfinished Microbial Genome sequences with the HP0333 predicted protein sequence revealed strong homology (P = 4 × 10−53 to 3 × 10−5) with predicted proteins from 24 different bacterial species, including H. influenzae (P = 5 × 10−15). Of the 24 completed and annotated genomes searched (from 22 species), homologs were found in 15 (13 species) (Fig. 1). These species included both gram-negative and gram-positive bacteria, as well as an archaeal organism, Pyrococcus furiosus (data not shown). Of these organisms, only six, H. pylori, Campylobacter jejuni, Bacillus subtilis, Streptococcus pneumoniae, H. influenzae, and Neisseria gonorrhoeae, are known to be naturally competent (Fig. 1). No homologs were identified in Archaeoglobus fulgidus, Rickettsia prowazekii, Chlamydia pneumoniae, Chlamydia trachomatis, Methanobacterium thermoautotrophicum, Methanococcus jannaschii, or Pyrococcus horikoshii, for which the complete genome sequences are available. This indicates that DprA is not universally present in bacteria. In addition, homologs were identified in 14 strains (14 species) for which partial genome data are available. The proteins for which complete sequences are available are predicted to vary in size from 240 (C. jejuni) to 398 amino acids (Synechocystis and N. gonorrhoeae). The strongest homology was to a predicted protein from C. jejuni (Cj0634; P = 4 × 10−53), and phylogenetic analysis also indicates that these sequences are closely related (Fig. 1). As expected, other homologs that are closely related to one another are from S. pneumoniae and Streptococcus pyogenes, from two members of the family Enterobacteriaceae (E. coli and Salmonella typhi), and from Mycobacterium tuberculosis, Rhodococcus fascians, and Streptomyces coelicolor.
FIG. 1.
Phylogram showing relatedness of putative proteins with homology to HP0333. Organisms in which related putative proteins were found are indicated. GenBank accession numbers for complete DNA sequence submissions are as follows: H. influenzae, U18657; Synchocystis, D90905; B. subtilis, Z99112; M. tuberculosis, Z74024; S. coelicolor, AL023797; B. burgdorferi, AE001137; E. coli, X65946; T. pallidum, AE001217; S. aureus AB015195; and A. aeolicus AE000728. Sequences from unfinished microbial genomes are from the following databases: C. jejuni, The Sanger Centre (42a); C. tepidum, The Institute for Genomic Research (TIGR) (23a); Y. pestis, The Sanger Centre; N. gonorrhoeae, University of Oklahoma’s Advanced Center for Genome Technology (OU-AGCT) (1); P. gingivalis, TIGR; D. radiodurans, TIGR; A. actinomycetemcomitans, OU-AGCT; S. pneumoniae, TIGR; P. aeruginosa, Pseudomonas Genome Project (40a); S. pyogenes, OU-AGCT; S. typhi, The Sanger Centre; E. faecalis, TIGR; T. maritima, TIGR. The accession number for the R. fascians homolog is AF001836.
When the sequences of these predicted proteins were aligned, a region of 193 residues including amino acids 17 to 201 of HP0333 was found to be highly similar among all of the proteins (Fig. 2). Of the 210 positions, 80 (38.1%) were conserved in at least 60% of the sequences and an additional 22 (10.5%) positions had conservative substitutions, for a total of 48.6% similar residues. Within this region, there are three areas of high relatedness. Amino acids 47 to 60 (relative to the consensus sequence) have 53.8% identity (76.9% similarity), amino acids 75 to 120 have 61.0% identity (68.3% similarity), and amino acids 152 to 200 have 59.6% identity (78.7% similarity). As expected, secondary structure predictions of the putative proteins from H. pylori, H. influenzae, and C. jejuni show similarity within the region of amino acid sequence homology, including many potential turns (data not shown). In total, these data indicate that HP0333 belongs to a family of genes conserved among many, but not all, known bacterial genera.
FIG. 2.
Multiple sequence alignment of predicted H. pylori HP0333 product and 24 homologs. Alignment includes region of conservation between HP0333 product and proteins identified by BLAST search. Consensus amino acids, i.e., those which are conserved among greater than 60% of the sequences, are indicated by black boxes (and the corresponding amino acids on the consensus line), whereas gray boxes (and a + on the consensus line) indicate similar amino acids. Alignment was performed with the Genetics Computer Group program Pileup and shaded with MacBoxshade.
Construction of HP0333 H. pylori mutants.
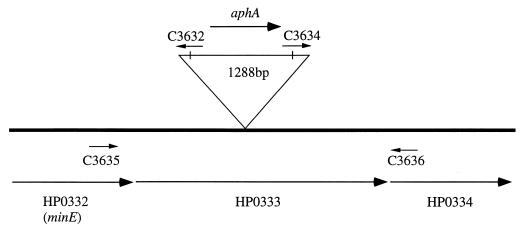
To study the function of HP0333, which we hypothesized to be involved in the natural competence of H. pylori, we constructed mutants of strains HPK5 and 84-183 in which there is an aphA insertion in the HP0333 ORF. First, HP0333 was PCR amplified by using primers based on HP0333 from strain 26695 and then cloned in pGEM-T Easy. A unique BamHI site within HP0333 was created by inverse PCR, and the aphA cassette was ligated into this product, disrupting the HP0333 ORF, resulting in pDPR2. When wild-type H. pylori strains were transformed to Kanr with pDPR2, the frequencies of transformation were 2.2 × 10−10 transformants/μg of DNA/CFU for 84-183 and 1.1 × 10−6 transformants/μg of DNA/CFU for HPK5. PCR with HP0333 and aphA-specific primers confirmed the correct replacement of HP0333 with HP0333::aphA by allelic exchange (Fig. 3). The colony morphology and growth on TSA plates of the HP0333::aphA mutants appeared unchanged from their wild-type parental strains.
FIG. 3.
Map of the region of HP0332 to HP0334 showing PCR primers in relation to HP0333 and the inserted aphA cassette.
Ability of wild-type H. pylori and HP0333 mutants to be transformed by H. pylori chromosomal DNA.
To examine whether disruption of HP0333 affected the ability of H. pylori cells to be transformed by H. pylori chromosomal DNA, we used chromosomal DNA from Strr or Spcr strains as the donor DNA to determine the effect of HP0333 disruption on transformation frequency (Table 3). Neither the source of the donor DNA nor the antibiotic resistance marker used had any significant effect on transformation frequency (Table 3). The median frequencies of transformation of the wild-type H. pylori strains to Strr or Spcr were 4.1 × 10−4 transformants/μg of DNA/CFU for 84-183 and 5.4 × 10−4 transformants/μg of DNA/recipient CFU for HPK5. In contrast, the median frequencies of transformation of the mutant H. pylori strains were 2.0 × 10−7/μg of DNA/CFU for 84-183/0333::aphA and 6.7 × 10−6/μg of DNA/CFU for HPK5/0333::aphA (Table 3). Thus, disruption of HP0333 substantially reduced (medians of 3.3 log10 for 84-183 and 1.9 log10 for HPK5) but did not eliminate H. pylori transformation for either strain.
TABLE 3.
Transformation frequencies of wild-type and HP0333 mutant H. pylori strains by chromosomal DNA
| DNA source | Phenotypic marker | Transformation frequencya for indicated recipient H. pylori strain
|
|||
|---|---|---|---|---|---|
| 84-183 | 84-183/0333::aphA | HPK5 | HPK5/0333::aphA | ||
| H. pylori 84-183 | Strr | 0.3 × 10−4 | 4.3 × 10−6 | 6.4 × 10−4 | 2.3 × 10−6 |
| Spcr | 3.5 × 10−4 | 1.6 × 10−7 | 2.1 × 10−4 | 1.1 × 10−6 | |
| H. pylori HPK5 | Strr | 1.0 × 10−4 | 2.4 × 10−7 | 4.4 × 10−4 | 1.0 × 10−5 |
| Spcr | 4.6 × 10−4 | <3.5 × 10−7 | 7.5 × 10−4 | 2.0 × 10−5 | |
Determined on the basis of antibiotic-resistant colonies per microgram of DNA per recipient CFU. Data represent the means of two experiments. Negative controls with no donor DNA resulted in no Strr or Specr colonies.
Genetic characterization of the HP0333 locus.
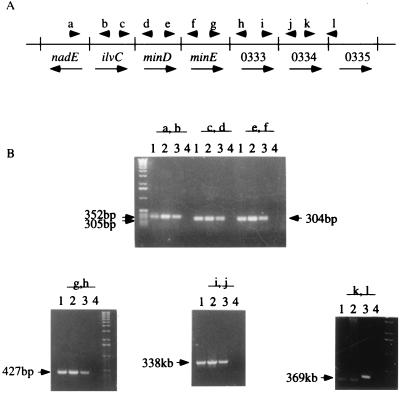
In H. pylori 26695, HP0333 is flanked by three predicted genes upstream (ilvC, minD, and minE homologs) and several ORFs downstream that could represent an operon. We examined 11 H. pylori strains to determine whether HP0333 was universally present. By PCR using primers C3635 and C3636, all 11 strains had the expected 939-bp product, indicating that HP0333 was conserved (data not shown). We next examined whether the locus of HP0333 in the wild-type strains that we studied was similar (Fig. 4). A series of PCRs involving HP0333 and its flanking genes indicated essentially identical organization of this locus in three strains studied. Only the PCR involving HP0334 and HP0335 showed any heterogeneity. Sequence analysis of the PCR products from strains 26695 and 84-183 revealed a 53-bp deletion in strain 84-183 between ORFs HP0334 and HP0335 relative to strain 26695 (data not shown). In strain J99, ilvC, minD, and minE homologs also are present upstream of JHP316, the dprA homolog. Because of this conservation of gene order suggestive of a polycistronic operon, we sought to examine the function of HP0333 in isolation from its flanking genes.
FIG. 4.
Conservation of chromosomal organization flanking HP0333 in three H. pylori strains. (A) Map of 4.7-kb region from nadE (HP0329) through HP0335 in strain 26695. Arrowheads refer to PCR primers (Table 2); arrows reflect the direction of transcription. (B) Products of PCR using these primer pairs for strains 84-183 (lanes 1), HPK5 (lanes 2), and 26695 (lanes 3). Lanes 4 are no-DNA controls.
Complementation analyses.
To confirm that the decreased transformation frequency of the mutants is due to loss of HP0333 and not due to a polar effect on downstream genes, we sought to complement the mutation. To restore HPK5/0333::aphA to a wild-type phenotype, we developed a strategy to integrate HP0333 into the H. pylori chromosome downstream of the ureAB promoter (Fig. 5). First, we cloned a fragment of the ureAB locus into pUC19 and then sequentially introduced HP0333 and cat to create pANDO2. Then, H. pylori HPK5 was transformed with pANDO2, resulting in strain C5, in which HP0333 is expressed from the native ureAB promoter (Table 1; Fig. 5). Next we transformed strain C5 with pDPR2, which disrupted either the ORF of HP0333 or the copy of HP0333 in the ureAB locus, to create strain C5G9 or C5G10, respectively (Table 1; Fig. 5). The identity of each of these mutants was confirmed by specific PCRs (data not shown). We then tested the wild-type strain HPK5 and its derivatives, HPK5/0333::aphA, C5, C5G9, and C5G10, for the ability to be transformed by homologous chromosomal DNA from a Strr strain (Table 4). In strain C5G9, with HP0333 in the ureAB locus, the frequency of transformation was completely restored to that of the wild-type strain HPK5. That the decrease in transformability by an insertion within HP0333 could be complemented by HP0333 in trans indicates that the loss of phenotype in HPK5/0333::aphA was not due to a polar effect on downstream genes. Additionally, the transformation frequency was not increased compared to the wild-type strain when two intact copies of HP0333 were present in strain C5 (Table 4).
TABLE 4.
Relationship between intact copies of HP0333 and H. pylori transformation frequencies
| Recipient strain | No. of intact copies of HP0333 | Location of intact HP0333 | Transformation frequencya |
|---|---|---|---|
| HPK5 | 1 | Native | 2.6 × 10−4 |
| HPK5/0333::aphA | 0 | 3.3 × 10−6 | |
| C5 | 2 | Native and ureAB locus | 1.6 × 10−4 |
| C5G9 | 1 | ureAB locus | 2.0 × 10−4 |
| C5G10b | 1 | Native | 2.9 × 10−4 |
DNA used for transformation was chromosomal DNA from Strr HPK5. The transformation frequency was determined on the basis of Strr colonies per microgram of DNA per recipient CFU. Data represent the means of two experiments. Negative controls with no DNA resulted in no Strr colonies.
A disrupted HP0333 in the ureAB locus also is present.
Efficiency of transformation of the wild-type H. pylori strains and mutants by plasmid DNA.
In H. influenzae, dprA is necessary for uptake of chromosomal but not plasmid DNA (29). Using HPK5 and its mutant derivatives, we sought to examine the role of HP0333 in uptake of a plasmid that does not integrate into the bacterial chromosome. The strains were examined for competence by natural transformation with plasmid pHP1HPK5, which carries an ampicillin resistance (Ampr; bla) marker. The frequency of transformation of the wild-type H. pylori strain HPK5 to Ampr was 4.9 × 10−5/μg of DNA/CFU, and that of HPK5/0333::aphA strain was less than 5.1 × 10−7/μg of DNA/recipient CFU (Table 5). Complementation with HP0333 in trans in strain C5G9 restored the wild-type phenotype. Thus, mutation in HP0333 also was shown to result in a marked decrease (>1.9 log10) in transformation by plasmid DNA.
TABLE 5.
Transformation frequencies of wild-type and HP0333 mutant H. pylori strains by plasmid DNA
| Recipient strain | No. of intact copies of HP0333 | Location of intact HP0333 | Transformation frequencya |
|---|---|---|---|
| HPK5 | 1 | Native | 4.9 × 10−5 |
| HPK5/0333::aphA | 0 | <5.1 × 10−7 | |
| C5G9 | 1 | ureAB locus | 4.0 × 10−5 |
DNA used for transformation was plasmid DNA from pHP1HPK5. The transformation frequency was determined as Ampr colonies per microgram of DNA per recipient CFU. Data represent the means of two experiments. Negative controls with no DNA resulted in no Ampr colonies.
DISCUSSION
From the strong homology of the HP0333 product with predicted proteins from diverse bacterial genera, it is clear that the HP0333 product belongs to a family of bacterial proteins that, although their exact biochemical function is not known, has been shown to be involved in DNA transformation in two naturally competent organisms: H. influenzae (29) and now H. pylori. The first member of this family described was smf of E. coli; however, no function was identified (36). Although these proteins are conserved among many gram-negative and gram-positive organisms, homologs are not present in a number of archaea, including A. fulgidus, M. thermoautotrophicum, and P. horikoshii. However, the presence of a homolog in P. furiosus indicates that it is not exclusively a eubacterial protein. Additionally, obligate intracellular organisms with small genomes, such as C. trachomatis, M. genitalium, M. pneumoniae, and R. prowazekii, do not appear to have homologs, suggesting that this protein is not a part of the minimal complement required by bacteria. Analysis of the predicted proteins indicates close relationships, as expected, between organisms known to have similar phylogenies (Fig. 1). The wide distribution, conserved sequence, and this phylogenetic pattern imply an ancient origin and an important function. Based on the first protein for which a function was ascribed (29), we provisionally call this the DprA family. Similarity of the predicted secondary structures for the dprA homologs from H. pylori, C. jejuni, and H. influenzae also suggest a close functional relationship between these proteins.
Many H. pylori strains are highly competent for transformation by chromosomal DNA (32, 37, 49, 55). The presence of colonization of humans by more than one strain is common (11, 26, 53), and the strongly recombinational population structure of H. pylori (20, 46) suggests that horizontal gene transfer is a frequent event. In wild-type strains, transformation by homologous DNA was no more frequent than by DNA from a heterologous H. pylori strain. This observation implies that there are not substantial restriction barriers for H. pylori uptake of native chromosomal DNA. Thus, the existence of specialized mechanisms to permit DNA uptake and incorporation by H. pylori may be postulated. In H. influenzae and S. pneumoniae, two other naturally competent bacteria, dprA and cilB (dprA homolog), respectively, are required for transformation by chromosomal DNA (9, 29). Since the genomes of H. pylori strains 26695 and J99 contain a dprA homolog (ORFs HP0333 and JHP316, respectively) (3, 48), we sought to examine its role in the transformation of H. pylori.
The presence of HP0333 in all 11 strains studied and its invariant relationship to flanking genes suggest a conserved function within H. pylori. That disruption of HP0333 markedly reduced the transformation of H. pylori strains by chromosomal DNA supports our hypothesis. These findings are not due to a polar effect, since complementation with HP0333 in trans restored the wild-type phenotype. Thus, HP0333 has a dprA function and might appropriately be called H. pylori dprA. Additionally, the complementation studies showed that having two functional copies of dprA does not enhance transformation; therefore, other steps in DNA transformation must be rate limiting.
Natural transformation is believed to involve three sequential steps: binding, uptake/translocation, and recombination. For H. influenzae dprA, mutational analysis (29) showed that its role occurred after binding, being involved in DNA translocation into the cytoplasm and/or recombination. Based on the strong homology of the central regions of the dprA from H. influenzae and H. pylori, we speculate that a similar step may be involved, but this has not yet been studied directly.
Although H. pylori and H. influenzae dprA have similar functions, there are important differences. As with H. influenzae, dprA disruption in H. pylori substantially reduced, but did not eliminate, transformation. However, dprA disruption in H. influenzae reduced the transformation frequency 10,000-fold (29), whereas in H. pylori the disruption of HP0333 resulted in only a 100-fold decrease in transformation frequency. Thus, dprA-independent mechanisms of transformation probably exist in H. pylori. In H. influenzae, dprA, while necessary for transformation by chromosomal DNA, is not necessary for plasmid DNA (29). In contrast, disruption of HP0333 completely eliminated H. pylori transformation by plasmid DNA. Considering the three-step model presented above, transformation by chromosomal or plasmid DNA potentially share the first two steps, but recombination is not required for transformation by plasmid DNA in all organisms. Our data therefore suggest that the shared H. pylori dprA function occurs during the first two steps. Since binding to the bacterial cell may not be involved, the remaining step is uptake/translocation. For H. influenzae, both chromosomal and plasmid DNA are initially taken up into the transformasome as double-stranded DNA (6, 16, 27, 40). Although we do not know the mechanism by which H. pylori takes up chromosomal or plasmid DNA, it is possible that the mechanism is different from the H. influenzae model.
The genes upstream of dprA in H. pylori, homologs of minD, minE, and ilvC, are not present in the same locus in H. influenzae. In H. influenzae dprA (HI0985) and ilvC (HI0682) are located far apart on the chromosome, and minD and minE are not present. minD and minE are involved in cell division in E. coli (14), while ilvC is required for isoleucine and valine synthesis (1). The function of the putative operon containing dprA, homologs of these three genes (ilvC, minD, and minE), and 13 other ORFs with unknown homologs is not yet apparent. The organization of dprA in relation to its flanking genes in 26695 (48) and J99 (3) appears conserved in the two other strains tested, suggesting that in all those strains dprA may be part of a polycistronic operon. Like dprA in H. influenzae, the genes flanking H. pylori dprA are homologous to metabolic genes or those for which no function is known. For H. influenzae, it is unclear whether the genes downstream of dprA are required for transformation. Karudapuram and Barcak showed the dprA and the downstream dprB and dprC are transcriptionally coregulated and competence inducible (30). However, our complementation studies show H. pylori dprA, by itself, is sufficient to restore the full wild-type phenotype. Therefore, the flanking genes, even if they are cotranscribed, are not required for the full transformation event.
Although H. influenzae, S. pneumoniae, and H. pylori are naturally competent for DNA transformation, and their DprA homologs play a role in uptake, the function of other DprA family members, which are present in species that are not naturally competent, is unknown. Based on its role in H. influenzae, S. pneumoniae, and H. pylori, we speculate that DprA may be a DNA-processing protein.
ACKNOWLEDGMENTS
Takafumi Ando and Dawn Israel contributed equally to this report.
This work was supported in part by grant R01-DK53707 from the National Institutes of Health and by the Medical Research Service of the Department of Veterans Affairs.
REFERENCES
- 1.Advanced Center for Genome Technology, University of Oklahoma. 23 July 1999, revision date. [Online.] http://www.genome.ou.edu/. [30 July 1999, last date accessed.]
- 1a.Aguilar O M, Grasso D H. The product of the Rhizobium meliloti ilvC gene is required for isoleucine and valine synthesis and nodulation of alfalfa. J Bacteriol. 1991;173:7756–7764. doi: 10.1128/jb.173.24.7756-7764.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akopyanz N, Bukanov N O, Westblom T U, Kresovich S, Berg D E. DNA diversity among clinical isolates of Helicobacter pylori detected by PCR-based RAPD fingerprinting. Nucleic Acids Res. 1992;20:5137–5142. doi: 10.1093/nar/20.19.5137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alm R A, Ling L L, Moir D T, King B L, Brown E D, Doig P C, Smith D R, Noonan B, Guild B C, deJonge B L, Carmel G, Tummino P J, Caruso A, Nickelsen M U, Mills D M, Ives C, Gibson R, Merberg D, Mills S D, Jiang Q, Taylor D E, Vovis G F, Trust T J. Genomic-sequence comparison of two unrelated isolates of the human gastric pathogen Helicobacter pylori. Nature. 1999;397:176–180. doi: 10.1038/16495. [DOI] [PubMed] [Google Scholar]
- 4.Altschul S F, Madden Y L, Schaffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atherton J C, Cao P, Peek R M, Tummuru M K R, Blaser M J. Mosaicism in vacuolating cytotoxin alleles of Helicobacter pylori. J Biol Chem. 1995;270:17771–17777. doi: 10.1074/jbc.270.30.17771. [DOI] [PubMed] [Google Scholar]
- 6.Barney F, Kahn M E, Smith H O. Directional transport and integration of donor DNA in Haemophilus influenzae transformation. Proc Natl Acad Sci USA. 1983;80:7274–7278. doi: 10.1073/pnas.80.23.7274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berg D E, Gilman R H, Lelwala-Guruge J, Srivastava K, Valdez Y, Watanabe J, Miyagi J, Akopyants N S, Ramirez-Ramos A, Yoshiwara T H, Recavarren S, Leon-Barua R. Helicobacter pylori populations in Peruvian patients. Clin Infect Dis. 1997;25:996–1002. doi: 10.1086/516081. [DOI] [PubMed] [Google Scholar]
- 8.Blaser M J, Parsonnet J. Parasitism by the “slow” bacterium Helicobacter pylori leads to altered gastric homeostasis and neoplasia. J Clin Investig. 1994;94:4–8. doi: 10.1172/JCI117336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campbell E A, Choi S Y, Masure H R. A competence regulon in Streptococcus pneumoniae revealed by genomic analysis. Mol Microbiol. 1998;27:929–939. doi: 10.1046/j.1365-2958.1998.00737.x. [DOI] [PubMed] [Google Scholar]
- 10.Censini S, Lange C, Xiang Z, Crabtree J E, Ghiara P, Borodovsky M, Rappuoli R, Covacci A. cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proc Natl Acad Sci USA. 1996;93:14648–14653. doi: 10.1073/pnas.93.25.14648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chalkauskas H, Kersulyte D, Cepuliene I, Urbonas V, Ruzeviciene D, Barakauskiene A, Raudonikiene A, Berg D E. Genotypes of Helicobacter pylori in Lithuanian families. Helicobacter. 1998;4:296–302. [PubMed] [Google Scholar]
- 12.Chou P Y, Fasman G D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- 13.Covacci A, Censini S, Bugnoli M, Petracca R, Burroni D, Macchia G, Massone A, Papini E, Xiang Z, Figura N, et al. Molecular characterization of the 128-kDa immunodominant antigen of Helicobacter pylori associated with cytotoxicity and duodenal ulcer. Proc Natl Acad Sci USA. 1993;90:5791–5795. doi: 10.1073/pnas.90.12.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Boer P A J, Crossley R E, Rothfield L I. Role of MinC and MinD in the site-specific septation block mediated by the MinCDE system of Escherichia coli. J Bacteriol. 1992;174:63–70. doi: 10.1128/jb.174.1.63-70.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Devereux J, Haeberli P, Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984;12:387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dreiseikelmann B. Translocation of DNA across bacterial membranes. Microbiol Rev. 1994;58:293–316. doi: 10.1128/mr.58.3.293-316.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Felsenstein J. PHYLIP—Phylogeny Inference Package (version 3.2) Cladistics. 1989;5:164–166. [Google Scholar]
- 18.Fraser C M, Gocayne J D, White O, Adams M D, Clayton R A, Fleischmann R D, Bult C J, Kerlavage A R, Sutton G, Kelley J M, Fritchman J L, Nguyen D T, Utterback T R, Saudek D M, Phillips C A, Merrick J M, Tomb J-F, Dougherty B A, Bott K F, Hu P-C, Lucier T S, Peterson S N, Smith H O, Hutchison C A I, Venter J C. The minimal gene complement of Mycoplasma genitalium. Science. 1995;270:397–403. doi: 10.1126/science.270.5235.397. [DOI] [PubMed] [Google Scholar]
- 19.Fujimoto S, Marshall B, Blaser M J. PCR-based restriction fragment polymorphism typing of Helicobacter pylori. J Clin Microbiol. 1994;32:331–334. doi: 10.1128/jcm.32.2.331-334.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Go M F, Kapur V, Graham D Y, Musser J M. Population genetic analysis of Helicobacter pylori by multilocus enzyme electrophoresis: extensive allelic diversity and recombinational population structure. J Bacteriol. 1996;178:3934–3938. doi: 10.1128/jb.178.13.3934-3938.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoffman P S, Goodwin A, Johnsen J, Magee K, Veldhuyzen van Zanten S J. Metabolic activities of metronidazole-sensitive and -resistant strains of Helicobacter pylori: repression of pyruvate oxidoreductase and expression of isocitrate lyase activity correlate with resistance. J Bacteriol. 1996;178:4822–4829. doi: 10.1128/jb.178.16.4822-4829.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hofreuter D, Odenbreit S, Henke G, Haas R. Natural competence for DNA transformation in Helicobacter pylori: identification and genetic characterization of the comB locus. Mol Microbiol. 1998;28:1027–1038. doi: 10.1046/j.1365-2958.1998.00879.x. [DOI] [PubMed] [Google Scholar]
- 23.Hook-Nikanne J, Berg D E, Peek R M, Kersulyte D, Tummuru M K, Blaser M J. DNA sequence conservation and diversity in transposable element IS605 of Helicobacter pylori. Helicobacter. 1998;3:79–85. doi: 10.1111/j.1523-5378.1998.08011.x. [DOI] [PubMed] [Google Scholar]
- 23a.The Institute for Genomic Research. 7 April 1999, revision date. [Online.] http://www.tigr.org. [30 July 1999, last date accessed.]
- 24.Jameson B A, Wolf H. The antigenic index: a novel algorithm for predicting antigenic determinants. Comput Appl Biosci. 1988;4:181–186. doi: 10.1093/bioinformatics/4.1.181. [DOI] [PubMed] [Google Scholar]
- 25.Jiang Q, Hiratsuka K, Taylor D E. Variability of gene order in different Helicobacter pylori strains contributes to genome diversity. Mol Microbiol. 1996;20:833–842. doi: 10.1111/j.1365-2958.1996.tb02521.x. [DOI] [PubMed] [Google Scholar]
- 26.Jorgensen M, Daskalopoulos G, Warburton V, Mitchell H M, Hazell S L. Multiple strain colonization and metronidazole resistance in Helicobacter pylori-infected patients: identification from sequential and multiple biopsy specimens. J Infect Dis. 1996;174:631–635. doi: 10.1093/infdis/174.3.631. [DOI] [PubMed] [Google Scholar]
- 27.Kahn M E, Barney F, Smith H O. Transformasomes: specialized membranous structures that protect DNA during Haemophilus transformation. Proc Natl Acad Sci USA. 1983;80:6927–6931. doi: 10.1073/pnas.80.22.6927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kansau I, Raymond J, Bingen E, Courcoux P, Kalach N, Bergeret M, Braimi N, Dupont C, Labigne A. Genotyping of Helicobacter pylori isolates by sequencing of PCR products and comparison with the RAPD technique. Res Microbiol. 1996;147:661–669. doi: 10.1016/0923-2508(96)84023-x. [DOI] [PubMed] [Google Scholar]
- 29.Karudapuram S, Zhao X, Barcak G J. DNA sequence and characterization of Haemophilus influenzae dprA+, a gene required for chromosomal but not plasmid DNA transformation. J Bacteriol. 1995;177:3235–3240. doi: 10.1128/jb.177.11.3235-3240.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karudapuram S, Barcak G J. The Haemophilus influenzae dprABC genes constitute a competence-inducible operon that requires the product of the tfoX (sxy) gene for transcriptional activation. J Bacteriol. 1997;179:4815–4820. doi: 10.1128/jb.179.15.4815-4820.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kleanthous H, Clayton C L, Tabaqchali S. Characterization of a plasmid from Helicobacter pylori encoding a replication protein common to plasmids in gram-positive bacteria. Mol Microbiol. 1991;5:2377–2389. doi: 10.1111/j.1365-2958.1991.tb02084.x. [DOI] [PubMed] [Google Scholar]
- 32.Kuipers E J, Israel D A, Blaser M J. Evidence for a conjugation-like mechanism of DNA transfer in Helicobacter pylori. J Bacteriol. 1998;180:2901–2905. doi: 10.1128/jb.180.11.2901-2905.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lawrence J G, Roth J R. Selfish operons: horizontal gene transfer may drive the evolution of gene clusters. Genetics. 1996;143:1843–1860. doi: 10.1093/genetics/143.4.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Logan R P H, Berg D E. Genetic diversity of Helicobacter pylori. Lancet. 1996;348:1462–1463. doi: 10.1016/s0140-6736(05)65885-0. [DOI] [PubMed] [Google Scholar]
- 35.Maddison W P, Maddison D R. MacClade version 3. Sunderland, Mass: Sinauer Associates; 1992. [Google Scholar]
- 36.Meinnel T, Guillon J M, Mechulam Y, Blanquet S. The Escherichia coli fmt gene, encoding methionyl-tRNA (fMet) formyltransferase, escapes metabolic control. J Bacteriol. 1993;175:993–1000. doi: 10.1128/jb.175.4.993-1000.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nedenskov P, Sorensen, Bukholm G, Bovre K. Natural competence for genetic transformation in Campylobacter pylori. J Infect Dis. 1990;161:365–366. doi: 10.1093/infdis/161.2.365. [DOI] [PubMed] [Google Scholar]
- 38.Page R D M. TREEVIEW: an application to display phylogenetic trees on personal computers. Comput Appl the Biosci. 1996;12:357–358. doi: 10.1093/bioinformatics/12.4.357. [DOI] [PubMed] [Google Scholar]
- 39.Peek R M, Jr, Thompson S A, Donahue J P, Tham K T, Atherton J C, Blaser M J, Miller G G. Adherence to gastric epithelial cells induces expression of a Helicobacter pylori gene, iceA, that is associated with clinical outcome. Proc Assoc Am Phys. 1998;110:531–544. [PubMed] [Google Scholar]
- 40.Pifer M L. Plasmid establishment in competent Haemophilus influenzae occurs by illegitimate transformation. J Bacteriol. 1986;168:683–687. doi: 10.1128/jb.168.2.683-687.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40a.Pseudomonas Genome Project. 15 July 1999, revision date. [Online.] http://www.pseudomonas.com. [30 July 1999, last date accessed.]
- 41.Riccardi V M, Rotter J I. Familial Helicobacter pylori infection. Societal factors, human genetics, and bacterial genetics. Ann Intern Med. 1994;120:1043–1045. doi: 10.7326/0003-4819-120-12-199406150-00012. [DOI] [PubMed] [Google Scholar]
- 42.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 42a.Sanger Centre. 21 July 1999, revision date. [Online.] http://www.sanger.ac.uk. [30 July 1999, last date accessed.]
- 43.Segal E D, Tompkins L S. Transformation of Helicobacter pylori by electroporation. BioTechniques. 1993;14:225–226. [PubMed] [Google Scholar]
- 44.Schmitt W, Odenbreit S, Heuermann D, Haas R. Cloning of the Helicobacter pylori recA gene and functional characterization of its product. Mol Gen Genet. 1995;248:563–572. doi: 10.1007/BF02423452. [DOI] [PubMed] [Google Scholar]
- 45.Suerbaum S, Josenhans C, Labigne A. Cloning and characterization of the Helicobacter pylori and Helicobacter mustelae flaB flagellin genes and construction of H. pylori flaA- and flaB-negative mutants by electroporation-mediated allelic exchange. J Bacteriol. 1993;175:3278–3288. doi: 10.1128/jb.175.11.3278-3288.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Suerbaum S, Smith J M, Bapumia K, Morelli G, Smith N H, Kunstmann E, Dyrek I, Achtman M. Free recombination within Helicobacter pylori. Proc Natl Acad Sci USA. 1998;95:12619–12624. doi: 10.1073/pnas.95.21.12619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thompson S A, Blaser M J. Isolation of the Helicobacter pylori recA gene and involvement of the recA region in resistance to low pH. Infect Immun. 1995;181:3144–3154. doi: 10.1128/iai.63.6.2185-2193.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tomb J-F, White O, Kerlavage A R, Clayton R A, Sutton G G, Fleischmann R D, Ketchum K A, Klenk H P, Gill S, Dougherty B A, Nelson K, Quackenbush J, Zhou L, Kirkness E F, Peterson S, Loftus B, Richardson D, Dodson R, Khalak H G, Glodek A, McKenney K, Fitzegerald L M, Lee N, Adams M D, Hickey E K, Berg D E, Gocayne J D, Utterback T R, Peterson J D, Kelley J M, Cotton M D, Weidman J M, Fujii C, Bowman C, Watthey L, Wallin E, Hayes W S, Borodovsky M, Karp P D, Smith H O, Fraser C M, Venter J C. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature. 1997;388:539–547. doi: 10.1038/41483. [DOI] [PubMed] [Google Scholar]
- 49.Tsuda M, Karita M, Nakazawa T. Genetic transformation in Helicobacter pylori. Microbiol Immunol. 1993;37:85–89. doi: 10.1111/j.1348-0421.1993.tb03184.x. [DOI] [PubMed] [Google Scholar]
- 50.Tummuru M K, Cover T L, Blaser M J. Cloning and expression of a high-molecular-mass major antigen of Helicobacter pylori: evidence of linkage to cytotoxin production. Infect Immun. 1993;61:1799–1809. doi: 10.1128/iai.61.5.1799-1809.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van der Hulst R W M, Rauws E A J, Koycu B, Keller J J, ten Kate F J W, Dankert J, Tytgat G N J, van der Ende A. Helicobacter pylori reinfection is virtually absent after successful eradication. J Infect Dis. 1997;176:196–200. doi: 10.1086/514023. [DOI] [PubMed] [Google Scholar]
- 52.Versalovic J, Shortridge D, Kibler K, Griffy M V, Beyer J, Flamm R K, Tanaka S K, Graham D Y, Go M F. Mutations in 23S rRNA are associated with clarithromycin resistance in Helicobacter pylori. Antimicrob Agents Chemother. 1996;40:477–480. doi: 10.1128/aac.40.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang G E, Jiang Q, Taylor D E. Genotypic characterization of clarithromycin-resistant and susceptible Helicobacter pylori strains from the same patient demonstrates existence of two unrelated isolates. J Clin Microbiol. 1998;36:2730–2731. doi: 10.1128/jcm.36.9.2730-2731.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang Y, Taylor D E. Chloramphenicol resistance in Campylobacter coli. Gene. 1990;94:23–28. doi: 10.1016/0378-1119(90)90463-2. [DOI] [PubMed] [Google Scholar]
- 55.Wang Y, Roos K P, Taylor D E. Transformation of Helicobacter pylori by chromosomal metronidazole resistance and by a plasmid with a selectable chloramphenicol resistance marker. J Gen Microbiol. 1993;139:2485–2493. doi: 10.1099/00221287-139-10-2485. [DOI] [PubMed] [Google Scholar]
- 56.Wilson K. Preparation of genomic DNA from bacteria. In: Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. current protocols in molecular biology. Vol. 1. New York, N.Y: John Wiley & Sons; 1995. pp. 2.4.1–2.4.5. [DOI] [PubMed] [Google Scholar]