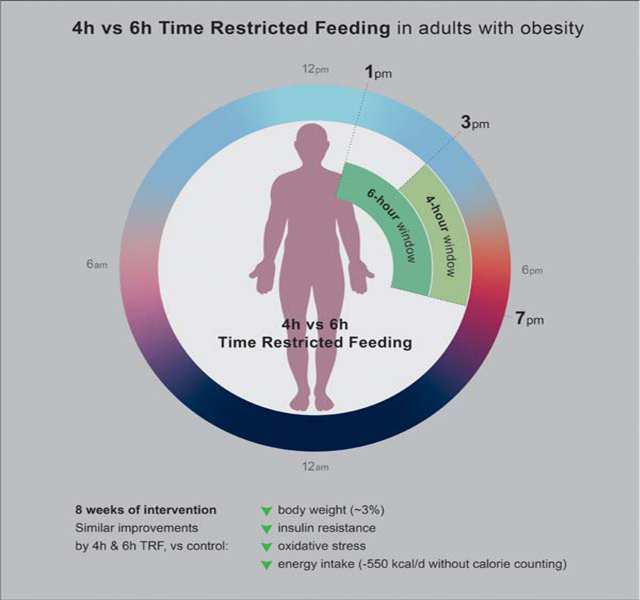
Summary
Time-restricted feeding (TRF) regimens have grown in popularity; however, very few studies have examined their weight loss efficacy. We conducted the first human trial (Clinicaltrials.gov - NCT03867773) to compare the effects of two popular forms of TRF (4-h and 6-h) on body weight and cardiometabolic risk factors. Adults with obesity were randomized to 4-h TRF (eating only between 3 to 7 pm), 6-h TRF (eating only between 1 to 7 pm) or a control group (no meal timing restrictions). After 8 weeks, 4-h and 6-h TRF produced comparable reductions in body weight (∼3%), insulin resistance and oxidative stress, versus controls. Energy intake was reduced by ∼550 kcal/d in both TRF groups, without calorie counting. These findings suggest that 4-h and 6-h TRF induce mild reductions in body weight over 8 weeks, and show promise as interventions for weight loss. These diets may also improve some aspects of cardiometabolic health.
Keywords: Time-restricted feeding, intermittent fasting, body weight, insulin resistance, obesity
IN BRIEF
Cienfuegos et al. compared the effect of two popular forms of time-restricted feeding (4-h and 6-h) on body weight and cardiometabolic risk factors in adults with obesity. Both diets produced comparable reductions in body weight, energy intake, insulin resistance and oxidative stress.
INTRODUCTION
Intermittent fasting has greatly increased in popularity over the past few years owing to its ability to produce clinically significant weight loss and confer protection against metabolic disease (de Cabo and Mattson, 2019; Patterson and Sears, 2017). Intermittent fasting is an umbrella term for three different types of diets: alternate-day fasting, the 5:2 diet and time-restricted feeding (TRF). Alternate day fasting involves a “fast day”, where energy is severely restricted (e.g. 0–800 kcal/0–3350 kJ consumed), alternated with an ad libitum intake “feast day”. The 5:2 diet, is a modified version of alternate-day fasting, and includes only two fast days per week followed by five ad libitum feast days. TRF, on the other hand, differs from these two other approaches in that it involves deliberately restricting the times during which energy is ingested. This diet involves confining the eating window to a specified number of hours per day, and fasting (with zero-calorie beverages allowed) for the remaining hours of the day. During the eating window, individuals are not required to count calories or monitor food intake in any way. Two of the most popular forms of TRF followed by the general public are 4-h TRF (a.k.a. “The Warrior Diet”) and 6-h TRF (a.k.a. “The 18:6 Diet”). Despite their growing popularity, no clinical trial to date has examined whether these regimens are actually effective for producing clinically significant weight loss.
The effects of alternate-day fasting and the 5:2 diet on metabolic disease risk have been studied in dozens of human trials to date. Accumulating evidence suggests that alternate-day fasting produces 5–7% weight loss over short durations (<6 months) (Bhutani et al., 2013; Bowen et al., 2018; Catenacci et al., 2016; Eshghinia and Mohammadzadeh, 2013; Hoddy et al., 2014; Johnson et al., 2007; Kalam et al., 2019; Klempel et al., 2013; Trepanowski et al., 2017; Varady et al., 2009; Varady et al., 2013). Alternate-day fasting also produces several cardiometabolic benefits, such as reducing blood pressure, LDL cholesterol levels, triglycerides, fasting insulin, insulin resistance, inflammation and oxidative stress (Bhutani et al., 2013; Bowen et al., 2018; Catenacci et al., 2016; Eshghinia and Mohammadzadeh, 2013; Heilbronn et al., 2005; Hoddy et al., 2014; Johnson et al., 2007; Kalam et al., 2019; Klempel et al., 2013; Trepanowski et al., 2017; Varady et al., 2009; Varady et al., 2013). As for the 5:2 diet, findings from human trials suggest that this regimen produces very similar reductions in body weight and metabolic disease risk parameters as alternate-day fasting (Antoni et al., 2018; Harvie et al., 2011; Schubel et al., 2018; Sundfor et al., 2018).
The effects of TRF have been studied much less extensively. To date, only six human trials of TRF have been performed (Gabel et al., 2018; Gill and Panda, 2015; Moro et al., 2016; Sutton et al., 2018; Tinsley et al., 2019; Wilkinson et al., 2020), and only three of these have examined the effects of this diet on weight loss (Gabel et al., 2018; Gill and Panda, 2015; Wilkinson et al., 2020). Initially, a single-arm 16-week study of 10-h TRF demonstrated that adults who were overweight lost 3.6% of body weight and reduced energy intake by ∼20%, without calorie counting (Gill and Panda, 2015). The next study examined the weight-loss efficacy of 8-h TRF (Gabel et al., 2018). After 12 weeks, adults with obesity lost 2.6% of body weight and reduced energy intake by ∼20% from baseline. Most recently, another single-arm trial of 10-h TRF demonstrated 3.0% weight loss and an 8% reduction in caloric intake after 12 weeks in participants with metabolic syndrome (Wilkinson et al., 2020). Each of these studies report excellent compliance with the prescribed eating windows, i.e. subjects ate within their prescribed eating windows 80–90% of the time over 12–16 weeks.
In addition to weight loss, TRF may also benefit cardiometabolic health. After 8-weeks of 8-h TRF, pronounced reductions in fasting glucose, insulin and insulin resistance were observed (Moro et al., 2016). Improvements in insulin sensitivity and beta-cell function were also demonstrated when food intake was limited to a 6-h window in men with pre-diabetes (Sutton et al., 2018). Blood pressure is regularly decreased with this diet, (Gabel et al., 2018; Wilkinson et al., 2020), even in the absence weight loss (Sutton et al., 2018). The effects of TRF on plasma lipids levels, however, is less clear. While some studies show improvements in triglycerides (Moro et al., 2016) and LDL cholesterol levels (Wilkinson et al., 2020), most report no effect, versus controls, on any lipid parameter (Gabel et al., 2018; Sutton et al., 2018; Tinsley et al., 2019).
Whether TRF exerts its metabolic benefits by improving markers of oxidative stress and inflammation is an important issue that remains unresolved. The effects of TRF on oxidative stress have only been evaluated in one human trial to date (Sutton et al., 2018). After 5 weeks, early 6-h TRF (i.e. eating all food before 3 pm in a 6-h window) lowered 8-isoprostane levels (a marker of oxidative stress to lipids) by 14% (Sutton et al., 2018). As for inflammatory markers, the limited data available suggests that TRF has no effect on circulating TNF-alpha and IL-6 in human subjects (Moro et al., 2016; Sutton et al., 2018).
Very few adverse events have been reported during TRF. After 12 weeks of 8-h or 10-h TRF, occurrences of nausea, constipation, diarrhea, headaches, fatigue and irritability did not change from baseline to post-treatment (Gabel et al., 2019b; Wilkinson et al., 2020). Complete blood count and disordered eating behaviors were also unaltered after 12 weeks of 8-h TRF (Gabel et al., 2019b). In contrast, early 6-h TRF resulted in a few minor cases of vomiting, headaches, increased thirst and diarrhea (Sutton et al., 2018). When 8-h TRF was combined with resistance training, reductions in the thyroid hormone, total triiodothyronine (T3), slightly below the normal level, were reported (Moro et al., 2016). As for sleep, no negative effects on sleep quantity or quality have been observed with either 8-h or 10-h regimens (Gabel et al., 2019a; Gill and Panda, 2015).
Whether the timing of the eating window (early versus late in the day) during TRF impacts weight loss and metabolic disease risk is still largely unknown due to the paucity of data in this area. Accumulating evidence suggests that the body is optimized for food intake in the morning (Morris et al., 2015a; Poggiogalle et al., 2018; Scheer et al., 2009). That is, insulin sensitivity, beta-cell responsiveness and thermic effect of food are all higher in the morning than in the afternoon or evening (Morris et al., 2015a; Poggiogalle et al., 2018; Scheer et al., 2009). As such, it has been postulated that earlier eating windows during TRF may produce superior metabolic benefits than later eating windows. In a recent study (Sutton et al., 2018), insulin sensitivity and beta-cell function were improved by early 6-h TRF (eating all food before 3 pm) when compared to controls (eating all food between 7 am to 7 pm). While the results of this highly-controlled trial are valuable to the field, this study is limited in that it did not directly compare the effects of early versus late TRF. The effect of meal timing on body weight and glycemic control has also been evaluated in human trials of breakfast skipping. While some studies suggest that skipping breakfast (fasting until 11 am) has negative effects on weight management and glycemic control (Geliebter et al., 2014; Levitsky and Pacanowski, 2013; Nas et al., 2017), others show no deleterious effects on these parameters (Chowdhury et al., 2019; Dhurandhar et al., 2014; Sievert et al., 2019). Thus, whether extended morning fasts negatively impact body weight and glucose homeostasis is still unclear. In designing a TRF intervention, it is also important to consider the social aspects of eating. The majority of social eating and drinking events occur in the evening (e.g. eating dinner with one’s family). Allowing participants to continue to engage in their habitual social eating patterns could play an important role in diet adherence and tolerability (Antoni, 2018). In the present study, we chose to shift the eating window to the afternoon and evening as later eating occasions allow individuals to engage in more family meals and social eating, which may improve overall compliance.
This study is the first randomized controlled trial to compare the weight loss efficacy of 4-h versus 6-h TRF in adults with obesity. The specific objective of this trial was to evaluate the impact of 4-h TRF (ad libitum intake from 3 to 7 pm) versus 6-h TRF (ad libitum intake from 1 to 7 pm) on body weight and metabolic disease risk parameters, versus a control group that had no meal timing restrictions. Compliance with the diets and occurrence of adverse events was also examined. We hypothesized that the 4-h TRF group would produce greater weight loss, when compared to the 6-h TRF group and controls. We also hypothesized that the 4-h group would yield greater blood pressure reductions, better glycemic control and more pronounced improvements in oxidative stress, due to larger decreases in body weight.
RESULTS AND DISCUSSION
We conducted a 10-week randomized parallel-arm trial to compare the effects of 4-h and 6-h TRF versus controls on body weight in adults with obesity (BMI 30–50 kg/m2). Participants were randomized by a stratified random sample (based on age, sex, and BMI) into 1 of 3 groups: 4-h TRF, 6-h TRF or a no-intervention control group. Briefly, the trial consisted of a 2-week baseline weight stabilization period followed by an 8-week TRF intervention period. During the 8-week intervention, the 4-h TRF group was instructed to eat ad libitum from 3 to 7 pm daily, and fast from 7 to 3 pm (20-h fast) (Figure 1). The 6-h TRF group was instructed to eat ad libitum from 1 to 7 pm daily, and fast from 7 to 1 pm (18-h fast). During the feeding windows, TRF participants were not required to monitor caloric intake and there were no restrictions on types or quantities of foods consumed. During the fasting window, TRF participants were encouraged to drink plenty of water and were permitted to consume energy-free beverages, such as black tea, coffee and diet sodas. Controls were instructed to maintain their weight throughout the trial, and not to change their eating or physical activity habits. Controls did not receive any dietary advice, but they visited the research center at the same frequency as the intervention groups for clinical measurements. The primary outcome measure was change in body weight. Secondary outcome measures were insulin resistance, blood pressure, plasma lipids, inflammatory cytokines, oxidative stress and diet adherence.
Figure 1. Time restricted feeding interventions.
During the 8-week intervention period, the 4-h TRF group ate ad libitum (Ad lib) from 3:00–7:00 pm daily (20-h fast). The 6-h TRF group ate ad libitum from 1:00–7:00 pm daily (18-h fast). During the feeding window, there were no restrictions on types or quantities of foods consumed and participants were not required to monitor caloric intake. Controls were instructed to continue their usual diet pattern and did not have any meal timing restrictions.
Participants
As shown in Figure 2, 82 individuals expressed interest in the study. Of these participants, 24 were excluded as they did not meet one or more inclusion criteria. Inclusion and exclusion criteria are listed in the STAR methods section. A total of 58 participants were randomized into the 4-h TRF group (n = 19), 6-h TRF group (n = 20) or the control group (n = 19). At the conclusion of the 10-week trial, there were 16 completers in the 4-h TRF group, 19 completers in the 6-h TRF group and 14 completers in the control group. The main reason for participant attrition was scheduling conflicts. Notably, no one dropped out of the study due to dislike of the TRF intervention. Table 1 displays the baseline characteristics of the completers, dropouts and a pooled analysis of all participants. At baseline, there were no significant differences between the 4-h TRF, 6-h TRF or control groups for the primary outcome measure (body weight) or any secondary outcome measure upon analysis of all participants. Participants were primarily middle-aged females with obesity who were normotensive and normocholesterolemic but insulin resistant (defined as HOMA-IR ≥ 2.7 (Gayoso-Diz et al., 2013; Sumner and Cowie, 2008)).
Figure 2. CONSORT diagram showing participant flow through the trial.
A total of 82 individuals were screened and 24 were excluded as they did not meet one or more inclusion criteria. 58 participants were randomized into 1 of 3 groups: 4-h TRF, 6-h TRF, or control. Baseline measures were assessed after randomization. At the conclusion of the 8-week intervention period, there were 16 completers in the 4-h TRF group, 19 completers in the 6-h TRF group, and 14 completers in the control group.
Table 1.
Baseline characteristics
| Variable | Completers (n = 49) | Dropouts (n = 9) | All participants (n = 58) | P-value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 4-h TRF | 6-h TRF | Control | 4-h TRF | 6-h TRF | Control | 4-h TRF | 6-h TRF | Control | ||
| n | 16 | 19 | 14 | 3 | 1 | 5 | 19 | 20 | 19 | |
| Age (y) | 49 ± 2 | 46 ± 3 | 45 ± 2 | 41 ± 8 | 62 ± 0 | 47 ± 4 | 47 ± 2 | 47 ± 3 | 45 ± 2 | 0.82 |
| Sex | ||||||||||
| Female | 14 (88%) | 18 (95%) | 12 (86%) | 3 (100%) | 1 (100%) | 5 (100%) | 17 (89%) | 19 (95%) | 17 (89%) | 0.38 |
| Male | 2 (12%) | 1 (5%) | 2 (14%) | 0 (0%) | 0 (0%) | 0 (0%) | 2 (11%) | 1 (0%) | 2 (11%) | |
| Race or ethnic group | ||||||||||
| White | 1 (6%) | 3 (16%) | 2 (14%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (5%) | 3 (15%) | 2 (11%) | 0.55 |
| Black | 12 (75%) | 12 (63%) | 6 (43%) | 2 (67%) | 1 (100%) | 4 (80%) | 14 (74%) | 13 (65%) | 10 (53%) | |
| Asian | 0 (0%) | 3 (16%) | 2 (14%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 3 (15%) | 2 (11%) | |
| Hispanic | 2 (13%) | 1 (5%) | 4 (29%) | 1 (33%) | 0 (0%) | 1 (20%) | 3 (16%) | 1 (5%) | 5 (26%) | |
| Other | 1 (6%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (5%) | 0 (0%) | 0 (0%) | |
| Body composition | ||||||||||
| Body weight (kg) | 101 ± 5 | 99 ± 5 | 93 ± 5 | 92 ± 5 | 92 ± 0 | 97 ± 4 | 100 ± 4 | 99 ± 4 | 94 ± 3 | 0.53 |
| Fat mass (kg) | 48 ± 3 | 48 ± 3 | 43 ± 3 | -- | -- | -- | 48 ± 3 | 48 ± 3 | 43 ± 3 | 0.24 |
| Lean mass (kg) | 52 ± 2 | 50 ± 3 | 48 ± 3 | -- | -- | -- | 52 ± 2 | 50 ± 3 | 48 ± 3 | 0.10 |
| Visceral fat mass (kg) | 1.4 ± 0.2 | 1.3 ± 0.1 | 1.1 ± 0.2 | -- | -- | -- | 1.4 ± 0.2 | 1.3 ± 0.1 | 1.1 ± 0.2 | 0.28 |
| Height (cm) | 167 ± 2 | 163 ± 2 | 160 ± 2 | 159 ± 2 | 152 ± 0 | 160 ± 3 | 166 ± 2 | 162 ± 2 | 160 ± 2 | 0.15 |
| BMI (kg/m2) | 36 ± 1 | 37 ± 1 | 36 ± 1 | 37 ± 3 | 40 ± 0 | 38 ± 2 | 37 ± 1 | 37 ± 1 | 36 ± 1 | 0.84 |
| Glucoregulatory factors | ||||||||||
| Fasting glucose (mg/dl) | 88 ± 2 | 94 ± 2 | 96 ± 5 | 87 ± 0 | 87 ± 0 | 95 ± 5 | 88 ± 2 | 93 ± 2 | 96 ± 3 | 0.23 |
| Fasting insulin (μIU/mL) | 12 ± 2 | 16 ± 3 | 12 ± 2 | 13 ± 0 | 23 ± 0 | 15 ± 6 | 12 ± 2 | 18 ± 3 | 13 ± 2 | 0.38 |
| HOMA-IR | 2.7 ± 0.4 | 3.7 ± 0.8 | 2.9 ± 0.4 | 2.7 ± 0 | 4.9 ± 0 | 3.6 ± 1.2 | 2.7 ± 0.4 | 4.1 ± 0.9 | 3.1 ± 0.4 | 0.34 |
| HbAlc(%) | 5.9 ± 0.2 | 5.9 ± 0.1 | 5.9 ± 0.2 | 6.0 ± 0 | 5.4 ± 0 | 6.2 ± 0.5 | 5.9 ± 0.2 | 5.9 ± 0.1 | 6.0 ± 0.2 | 0.88 |
| BP and heart rate | ||||||||||
| Systolic BP (mm Hg) | 135 ± 5 | 128 ± 4 | 122 ± 5 | 124 ± 0 | 153 ± 0 | 150 ± 23 | 134 ± 4 | 130 ± 4 | 127 ± 6 | 0.59 |
| Diastolic BP (mm Hg) | 88 ± 2 | 84 ± 2 | 81 ± 3 | 82 ± 0 | 98 ± 0 | 94 ± 9 | 88 ± 2 | 84 ± 2 | 84 ± 3 | 0.54 |
| Heart rate (bpm) | 73 ± 2 | 68 ± 2 | 70 ± 3 | 72 ± 0 | 66 ± 0 | 70 ± 9 | 73 ± 2 | 68 ± 2 | 70 ± 3 | 0.37 |
| Plasma lipids | ||||||||||
| LDL cholesterol (mg/dl) | 95 ± 6 | 104 ± 8 | 108 ± 6 | 104 ± 0 | 143 ± 0 | 96 ± 18 | 96 ± 6 | 107 ± 8 | 104 ± 6 | 0.44 |
| HDL cholesterol (mg/dl) | 57 ± 5 | 54 ± 3 | 56 ± 4 | 49 ± 0 | 52 ± 0 | 63 ± 10 | 57 ± 4 | 54 ± 3 | 58 ± 4 | 0.71 |
| Triglycerides (mg/dl) | 91 ± 11 | 95 ± 7 | 84 ± 11 | 110 ± 0 | 76 ± 0 | 94 ± 27 | 92 ± 10 | 94 ± 7 | 87 ± 10 | 0.85 |
| Inflammation/ ox stress | ||||||||||
| TNF-alpha (pg/ml) | 8.3 ± 1.7 | 14.2 ± 2.7 | 11.9 ± 2.6 | -- | -- | -- | 8.3 ± 1.7 | 14.2 ± 2.7 | 11.9 ± 2.6 | 0.37 |
| IL-6 (pg/ml) | 2.4 ± 0.7 | 5.2 ± 1.6 | 4.5 ± 1.3 | -- | -- | -- | 2.4 ± 0.7 | 5.2 ± 1.6 | 4.5 ± 1.3 | 0.63 |
| 8-isoprostane (pg/ml) | 34.1 ± 4.6 | 33.8 ± 3.6 | 32.6 ± 4.8 | -- | -- | -- | 34.1 ± 4.6 | 33.8 ± 3.6 | 32.6 ± 4.8 | 0.76 |
BMI: Body-mass index, BP: Blood pressure. HOMA-IR: Homeostasis Model Assessment-Insulin resistance. -- Data not available. Dropout data: All dropouts occurred during week 1 or 2 of the study. Baseline body weight, height and BMI was collected for all dropouts. None of the dropouts attended the baseline DXA scan visit, so no body composition data are available for these participants. Only a few dropouts attended the baseline blood draw/blood pressure visit (4-h TRF: n = 1, 6-h TRF: n = 1, Control: n = 3), so only data for these subjects are included. TNF-alpha, IL- 6, and 8-isoprostane data are not available for dropouts as not enough blood could be collected from these participants. Values are expressed as mean ± SEM. P-value between groups for “All participants”: One-way ANOVA with Tukey post-hoc (continuous variables) and McNemar test (categorical variables).
4-h TRF does not produce superior changes in body weight compared to 6-h TRF
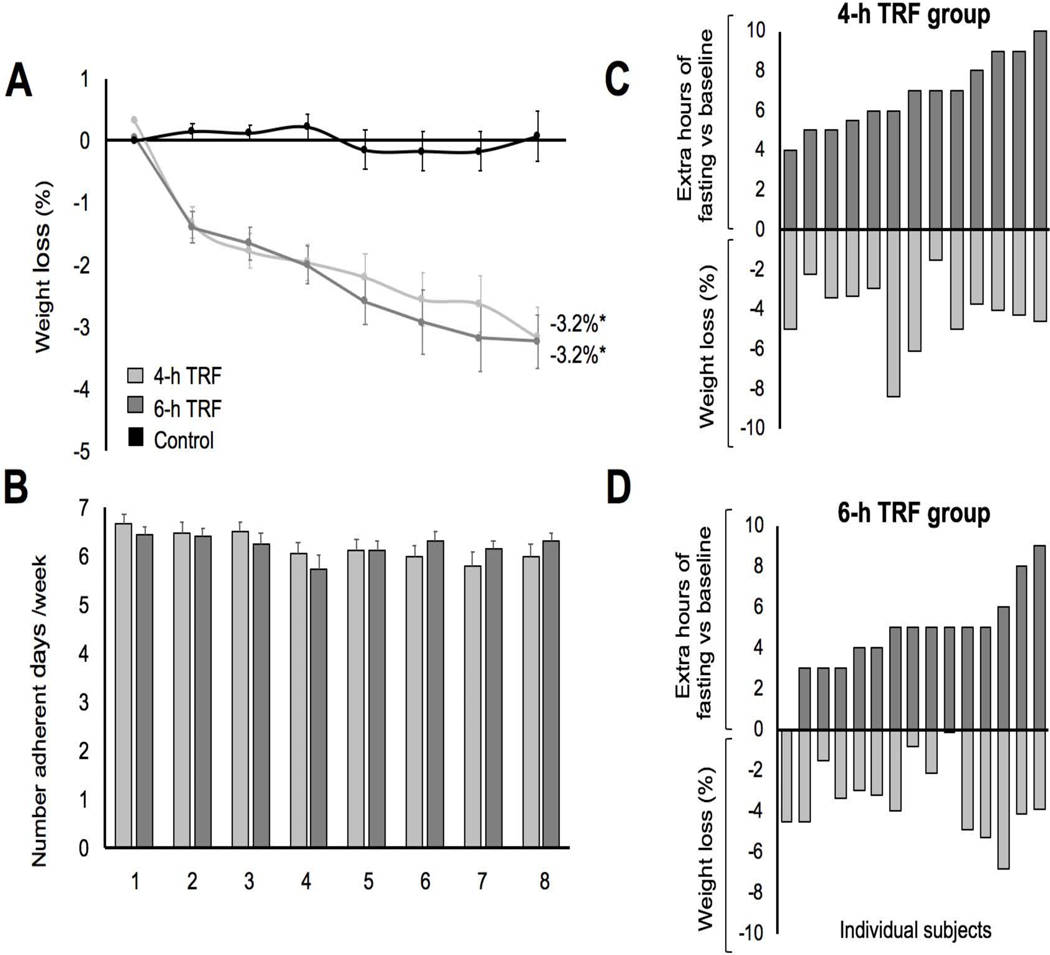
As shown in Figure 3A, weight loss by week 8 was significantly different across the three groups (P < 0.001), with the 4-h TRF group (Δ = −3.2 ± 0.4%) and 6-h TRF group (Δ = −3.2 ± 0.4%) losing significantly more weight than controls (Δ = 0.1 ± 0.4%, P < 0.001 and P < 0.001, respectively), with no significant difference between intervention groups. Compliance with the TRF interventions was excellent (Figure 3B). On average, participants in the 4-h TRF and 6-h TRF group reported being compliant with their feeding window on 6.2 ± 0.2 d/week and 6.2 ± 0.1 d/week, respectively, and this level of adherence did not change over the course of the trial (P = 0.76). Thus, 4-h TRF does not produce superior changes in body weight compared to 6-h TRF, as we had hypothesized.
Figure 3. Weight loss and diet compliance.
(A) Percentage weight loss vs baseline for the 4-h TRF, 6-h TRF group and Ad lib control group during the 8-week intervention period of the study. Each data point represents each week of the 8-week period, starting at week 1.
(B) The number of adherent days of the dietary regimen for the 4-h (light grey) and 8- hr (dark grey) TRF groups over the 8-week intervention period of the study.
(C) The extent of the fasting window and the percentage weight loss in the 4-h TRF group. Completers only analysis.
(D) The extent of the fasting window and the percentage weight loss in the 6-h TRF group. Completers only analysis.
Values reported as mean ± SEM, where appropriate. Data were included for 58 participants; means were estimated by an intention-to-treat analysis using last observation carried forward. *P < 0.001, change score between baseline to week 8 by ANCOVA.
However, it is possible that the higher dropout rate in the control group could bias the results towards finding an effect (when using the intention-to-treat analysis with last observation carried forward). As such, we performed a secondary sensitivity analysis in those who completed the study to ensure that the by-definition lack of weight loss in the control group is not due to the 5 dropouts. The results for this completers analysis are as follows: Weight loss by week 8 was significantly different across the three groups (P < 0.001), with the 4-h TRF group (Δ = −3.9 ± 0.4%) and 6-h TRF group (Δ = −3.4 ± 0.4%) losing significantly more weight than controls (Δ = 0.1 ± 0.6%, P < 0.001 and P < 0.001, respectively), with no significant difference between intervention groups. Thus, it appears as though the lack of weight loss in the control group is not due to the 5 dropouts.
Very few studies have examined the weight-loss efficacy of TRF in individuals with obesity (Gabel et al., 2018; Gill and Panda, 2015; Wilkinson et al., 2020). In a recent trial of 8-h TRF, body weight was reduced by 2.6% after 12 weeks in men and women with obesity (Gabel et al., 2018). Likewise, 10-h TRF produced 3.6% weight loss after 16 weeks (Gill and Panda, 2015) and 3.0% weight loss after 12 weeks (Wilkinson et al., 2020). To our knowledge, no other study has examined the effect of 4-h or 6-h TRF as a weight-loss regimen; thus, there is no data to which to compare our findings. In comparison with other forms of intermittent fasting, the degree of weight loss achieved with 4-h and 6-h TRF may be on par with that observed during short-term alternate day fasting (Bhutani et al., 2013; Catenacci et al., 2016; Hutchison et al., 2019; Klempel et al., 2013; Trepanowski et al., 2017; Varady et al., 2013) and the 5:2 diet (Harvie et al., 2011; Schubel et al., 2018; Sundfor et al., 2018).
At baseline (pre-intervention), the average fasting window was not significantly different (P = 0.55) between the 4-h TRF (10.8 ± 0.5 h) and 6-h TRF (10.3 ± 0.6 h) group. However, the duration of the baseline fasting window varied vastly between individual participants. For instance, in the 4-h TRF group, baseline fasting window ranged from 10 to 16 h/d. While in the 6-h TRF group, baseline fasting window ranged from 9 to 19 h/d. As such, we were interested in determining if participants who experienced the greatest increase in their fasting windows, would lose the greatest amount of weight. Results reveal (Figure 3C and 3D) that greater extensions in fasting were not related to weight loss in either the 4-h TRF (r = −0.06, P = 0.86) or 6-h TRF group (r = −0.09, P = 0.76). Thus, baseline eating patterns may not predict weight loss success with TRF.
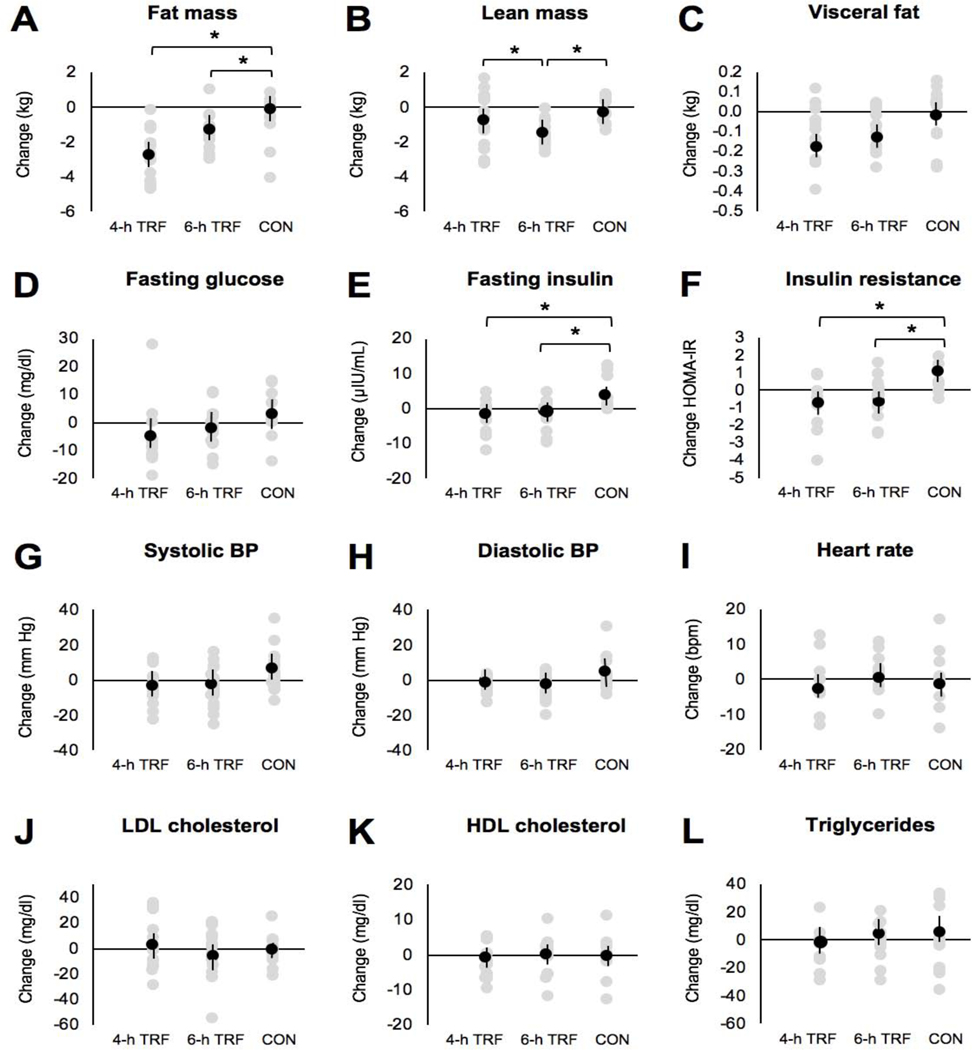
Fat mass by week 8 was significantly different across the three groups (P = 0.002), with the 4-h TRF group (Δ = −2.8 ± 0.4 kg) and 6-h TRF group (Δ = −1.4 ± 0.3 kg) losing significantly more fat mass than controls (Δ = −0.6 ± 0.4 kg, P = 0.001 and P = 0.03, respectively), with no significant difference between intervention groups (Figure 4A). Lean mass by week 8 was significantly different across the three groups (P = 0.04), with the 6-h TRF group (Δ = −1.5 ± 0.2 kg) losing significantly more lean mass than the 4-h TRF group (Δ = −0.8 ± 0.4 kg, P = 0.04) and controls (Δ = −0.3 ± 0.2 kg, P = 0.03), with no difference between the 4-h TRF group and controls (Figure 4B). Visceral fat mass change by week 8 was not significantly different (P = 0.43) across the 4-h TRF group (Δ = −0.18 ± 0.07 kg), 6-h TRF group (Δ = −0.14 ± 0.06 kg) or controls (Δ = −0.02 ± 0.05 kg) (Figure 4C).
Figure 4. Body composition and metabolic risk markers.
A) Change in fat mass after 8-weeks of intervention for the 4-h TRF, 6-h TRF and Ad lib control group.
B) Change in lean mass after 8-weeks of intervention for the 4-h TRF, 6-h TRF and Ad lib control group.
C) Change in visceral fat mass after 8-weeks of intervention for the 4-h TRF, 6-h TRF and Ad lib control group.
D) Change in fasting glucose levels after 8-weeks of intervention for the 4-h TRF, 6-h TRF and Ad lib control group.
E) Change in fasting insulin levels after 8-weeks of intervention for the 4-h TRF, 6-h TRF and Ad lib control group.
F) Change in insulin resistance (measured by HOMA-IR) after 8-weeks of intervention for the 4-h TRF, 6-h TRF and Ad lib control group.
G) Change in systolic blood pressure after 8-weeks of intervention for the 4-h TRF, 6-h TRF and Ad lib control group.
H) Change in diastolic blood pressure after 8-weeks of intervention for the 4-h TRF, 6-h TRF and Ad lib control group.
I) Change in heart rate after 8-weeks of intervention for the 4-h TRF, 6-h TRF and Ad lib control group.
J) Change in LDL cholesterol levels after 8-weeks of intervention for the 4-h TRF, 6-h TRF and Ad lib control group.
K) Change in HDL cholesterol levels after 8-weeks of intervention for the 4-h TRF, 6-h TRF and Ad lib control group.
L) Change in triglycerides after 8-weeks of intervention for the 4-h TRF, 6-h TRF and Ad lib control group.
Values reported as mean ± SEM. Data were included for 58 participants; means were estimated by an intention-to-treat analysis using last observation carried forward. Each data point represents an individual participant. Mean change is indicated by a black data point. *P < 0.05, change score between baseline to week 8 by ANCOVA.
4-h and 6-h TRF produce similar reductions in fasting insulin and insulin resistance
Change in fasting glucose by week 8 was not significantly different (P = 0.15) across the 4-h TRF (Δ = −5.0 ± 3.8 mg/dl), 6-h TRF (Δ = −2.3 ± 2.0 mg/dl), or control groups (Δ = 2.6 ± 2.6 mg/dl) (Figure 4D). Change in fasting insulin by week 8 was significantly different across the three groups (P = 0.03), with the 4-h TRF group (Δ = −2.3 ± 1.5 μIU/mL) and 6-h TRF group (Δ = −1.9 ± 1.1 μIU/mL) experiencing greater decreases than controls (Δ = 3.5 ± 1.4 μIU/mL, P = 0.02 and P = 0.04, respectively), with no significant difference between intervention groups (Figure 4E). Likewise, change in insulin resistance by week 8 was significantly different across the three groups (P = 0.04), with the 4-h TRF group (Δ = −0.8 ± 0.4, 29% reduction) and 6-h TRF group (Δ = −0.5 ± 0.3, 12% reduction) experiencing greater decreases than controls (Δ = 1.0 ± 0.4, P = 0.03 and P = 0.04, respectively), with no significant difference between intervention groups (Figure 4F). The change in circulating %HbA1c by week 8 was not significantly different (P = 0.55) across the 4-h TRF (Δ = −0.2 ± 0.1%), 6-h TRF (Δ = −0.2 ± 0.1%), or control groups (Δ = −0.1 ± 0.1%) (data not shown).
This trial is the first to compare the effects of 4-h versus 6-h TRF on glucoregulatory factors. No change in glucose was noted, which is similar to what has been reported previously by other intermittent fasting studies (Antoni et al., 2018; Gabel et al., 2019c; Harvie et al., 2011; Heilbronn et al., 2005; Hoddy et al., 2016; Sutton et al., 2018). In contrast, insulin and insulin resistance are routinely improved by TRF, alternate-day fasting and 5:2 (Antoni et al., 2018; Gabel et al., 2019c; Harvie et al., 2011; Heilbronn et al., 2005; Hoddy et al., 2016; Sutton et al., 2018). TRF has also been shown to improve beta-cell responsiveness in participants with prediabetes (Sutton et al., 2018). More recently, it was shown that intermittent fasting lowered insulin resistance twice as much as daily calorie restriction (CR), despite similar weight loss between the two intervention groups (Gabel et al., 2019c). It should be noted, however, that the reductions in insulin and insulin resistance noted here are partly driven by a worsening in the control arm. It is questionable whether these improvements by TRF would have been noted in the absence of this. Our results are also limited in that we measured these glucoregulatory parameters only in the morning. Insulin sensitivity and glucose tolerance peak shortly after waking (Poggiogalle et al., 2018). As such, future studies should measure these endpoints over a 24-h period (instead of the morning only) to see these regimens truly only impact insulin and insulin resistance, without concomitant changes in glucose. One proposed mechanism by which fasting may improve glycemic control involves the metabolic switch. The metabolic switch, which occurs when changing from fed to fasted state, induces hepatocyte production of ketone bodies, increasing insulin sensitivity and decreasing fat accumulation. Insulin sensitivity of muscle cells is also enhanced in response to the metabolic switch (de Cabo and Mattson, 2019).
Another outstanding question in the field is whether the metabolic benefits of intermittent fasting are due to fasting (i.e., long periods of food abstinence during the day) or merely just weight loss. To determine whether fasting has benefits independent of weight loss, some TRF studies have required subjects to stay weight stable by consuming isocaloric and/or eucaloric diets. In a 6-h TRF trial, several metabolic indicators, including insulin sensitivity, beta-cell responsiveness and oxidative stress improved after 5 weeks, despite no weight loss (Sutton et al., 2018). Contrary to these findings, two other studies show impaired glucose tolerance in conjunction with elevations in LDL cholesterol and blood pressure when subjects consumed all of their energy needs in a single meal over a 8-week period (Carlson et al., 2007; Stote et al., 2007). As the data in this area are still limited, it is difficult to draw meaningful conclusions. Nevertheless, these preliminary findings suggest that fasting produces metabolic benefits independent of weight loss, just as long as subjects are not required to gorge (consume all their energy needs for the day) within a 1-h time frame. It is apparent that much more research will be needed before solid conclusions can be reached.
4-h and 6-h TRF do not affect blood pressure, LDL cholesterol, HDL cholesterol or triglycerides
The change in systolic blood pressure by week 8 was not significantly different (P = 0.06) across the 4-h TRF group (Δ = −5.0 ± 2.2 mm Hg), 6-h TRF group (Δ = −4.4 ± 2.3 mm Hg) or controls (Δ = 3.7 ± 2.8 mm Hg) (Figure 4G). Likewise, the change in diastolic blood pressure by week 8 was not significantly different (P = 0.11) across the 4-h TRF group (Δ = −2.8 ± 1.0 mm Hg), 6-h TRF group (Δ = −3.2 ± 1.5 mm Hg) or controls (Δ = 2.4 ± 2.2 mm Hg) (Figure 4H). In addition, the change in heart rate by week 8 was not significantly different (P = 0.59) across the 4-h TRF (Δ = −2.8 ± 1.7 bpm), 6-h TRF (Δ = 0.6 ± 2.0 bpm), or control groups (Δ = −1.6 ± 2.0 bpm) (Figure 4I). These findings are contrary to what has been reported previously. For instance, after 2–3 months of alternate-day fasting or the 5:2 diet, systolic blood pressure is typically lowered by 5–8 mm Hg, while diastolic blood pressure is reduced by 3–5 mm Hg (Bowen et al., 2018; Eshghinia and Mohammadzadeh, 2013; Harvie et al., 2011; Hoddy et al., 2014; Varady et al., 2009). As for TRF, 6-h early TRF produced dramatic decreases in both systolic and diastolic blood pressure (−10–11 mm Hg) (Sutton et al., 2018), while 8-h TRF has been shown to reduce systolic blood pressure (−7 mm Hg) (Gabel et al., 2018), but not always (Tinsley et al., 2019). It is unclear why blood pressure was not reduced by TRF in the present study. However, our study was not properly powered to see an effect in this secondary outcome variable.
Neither intervention had any effect on plasma lipid levels. For example, the change in LDL cholesterol by week 8 was not significantly different (P = 0.76) across the 4-h TRF (Δ = 2.6 ± 5.7 mg/dl), 6-h TRF (Δ = −4.8 ± 5.1 mg/dl), or control groups (Δ = −2.0 ± 3.7 mg/dl) (Figure 4J). Likewise, the change in HDL cholesterol by week 8 was not significantly different (P = 0.93) across the 4-h TRF (Δ = −2.4 ± 1.3 mg/dl), 6-h TRF (Δ = −0.8 ± 1.4 mg/dl), or control groups (Δ = −0.7 ± 1.0 mg/dl) (Figure 4K). Further, the change in triglycerides by week 8 was not significantly different (P = 0.96) across the 4-h TRF (Δ = −1.9 ± 6.7 mg/dl), 6-h TRF (Δ = 2.6 ± 5.7 mg/dl), or control group (Δ = 4.5 ± 3.2 mg/dl) (Figure 4L). The effects of intermittent fasting on plasma lipids are highly variable. While some studies report decreases in triglycerides and LDL cholesterol (Bowen et al., 2018; Harvie et al., 2011; Johnson et al., 2007; Varady et al., 2009), most show no effect on these lipid parameters (Bhutani et al., 2013; Eshghinia and Mohammadzadeh, 2013; Gabel et al., 2018; Hoddy et al., 2014; Moro et al., 2016; Sutton et al., 2018). HDL also generally remains unaffected by these diets, though one study observed minor increases (Trepanowski et al., 2017). It should be noted, however, that the participants in the present study (and most previous studies) were not hypercholesterolemic. As their baseline levels of LDL cholesterol and triglycerides were already in the normal range, it is not surprising that further reductions were not observed.
4-h and 6-h TRF produce comparable reductions in oxidative stress but do not affect inflammatory markers
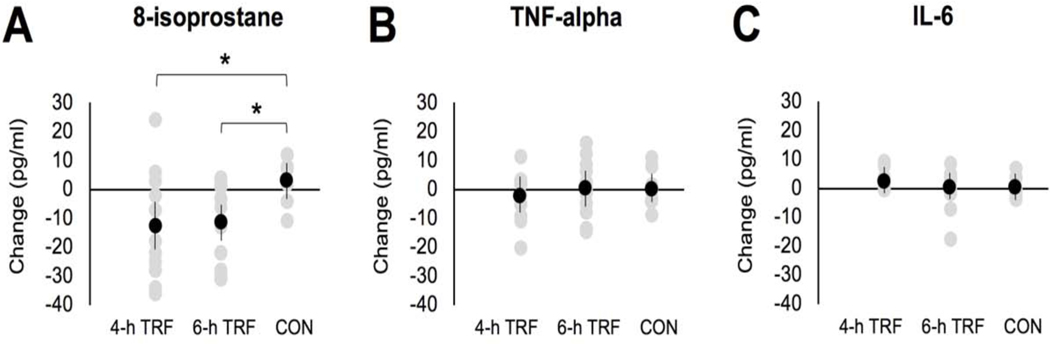
8-isoprostane is a marker of oxidative stress to lipids. The change in plasma levels of 8-isoprostane by week 8 was significantly different across the three groups (P = 0.02), with the 4-h TRF group (Δ = −13 ± 6 pg/ml, 37% reduction) and 6-h TRF group (Δ = −12 ± 4 pg/ml, 34% reduction) experiencing greater decreases than controls (Δ = 3 ± 3 pg/ml, P = 0.02 and P = 0.03, respectively), with no significant difference between intervention groups (Figure 5A). In contrast, neither intervention had any impact on inflammatory markers. For instance, the changes in plasma levels of TNF-alpha by week 8 were not significantly different (P = 0.21) across the 4-h TRF (Δ = −2.4 ± 2.6 pg/ml), 6-h TRF (Δ = 0.4 ± 2.4 pg/ml), or control groups (Δ = 2 ± 1.8 pg/ml) (Figure 5B). Similarly, the changes in plasma levels of IL-6 by week 8 were not significantly different (P = 0.92) across the 4-h TRF (Δ = 2.4 ± 1.0 pg/ml), 6-h TRF (Δ = 0.4 ± 1.7 pg/ml), or control groups (Δ = 0.5 ± 1.1 pg/ml) (Figure 5C).
Figure 5. Oxidative stress and inflammatory markers.
A) Change in 8-isoprostane levels (marker of oxidative stress to lipids) after 8-weeks of intervention for the 4-h TRF, 6-h TRF and Ad lib control group.
B) Change in plasma TNF-alpha levels after 8-weeks of intervention for the 4-h TRF, 6-h TRF and Ad lib control group.
C) Change in plasma IL-6 levels after 8-weeks of intervention for the 4-h TRF, 6-h TRF and Ad lib control group.
Values reported as mean ± SEM. Data were included for 58 participants; means were estimated by an intention-to-treat analysis using last observation carried forward. Each data point represents an individual participant. Mean change is indicated by a black data point. *P < 0.05, change score between baseline to week 8 by ANCOVA.
These reductions in oxidative stress are consistent with other human trials of intermittent fasting. In a 5-week trial of 6-h TRF, circulating 8-isoprostane was reduced by 14% in men with obesity and prediabetes, even without weight loss (Sutton et al., 2018). Correspondingly, 8-weeks of alternate-day fasting decreased several markers of oxidative stress, including 8-isoprostane, 4-hydroxynonenal adducts, protein carbonyls and nitrotyrosine (Johnson et al., 2007). As for inflammatory markers, human trials of intermittent fasting report no change in I L-6, TNF-alpha or CRP (Bhutani et al., 2013; Harvie et al., 2011; Moro et al., 2016; Sutton et al., 2018; Trepanowski et al., 2018). Taken together, TRF along with other forms of fasting, have little effect on inflammation but have potent effects on oxidative stress. It is also likely that the decrease in oxidative stress noted here, is related to improvements in insulin resistance. Studies have demonstrated a clear link between insulin resistance and oxidative stress. Under oxidative conditions, insulin signaling is impaired, resulting in insulin resistance of the cell (Houstis et al., 2006; Rains and Jain, 2011). Other studies have shown improvement in insulin sensitivity when administering antioxidants, such as vitamin E (Zaulkffali et al., 2019). Therefore, we could speculate that one of the mechanisms by which intermittent fasting improves insulin resistance is by decreasing oxidative stress.
Adverse events
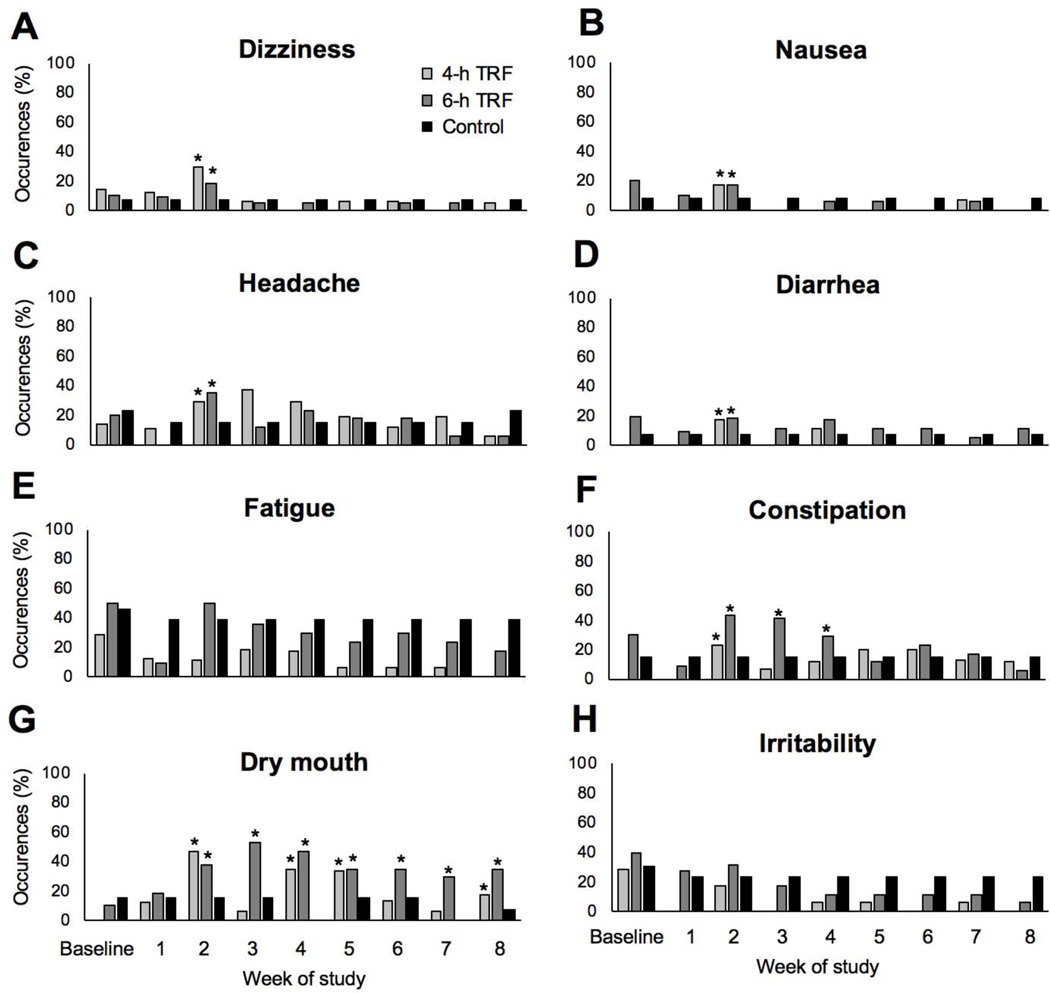
No serious adverse events were reported. Mild adverse events such as dizziness, nausea, headaches and diarrhea peaked at week 2 in both TRF interventions, relative to controls, but disappeared by week 3 and did not reoccur during the trial (Figure 6A-D). Levels of fatigue did not change over the course of the trial relative to controls (Figure 6E). Constipation (Figure 6F) and dry mouth (Figure 6G) were observed in both the 4-h and 6-h TRF groups at week 2 relative to controls, and these issues persisted throughout some or most of the study. Occurrences of irritability remained low throughout the trial and were not significantly different from controls (Figure 6H). These findings suggest that mild adverse effects, such as dizziness, nausea, headaches and diarrhea may occur at the onset of TRF, but they disappear when the participant becomes adjusted to the diet.
Figure 6. Adverse events.
A) Percent occurrences of dizziness at each week of the study.
B) Percent occurrences of nausea at each week of the study.
C) Percent occurrences of headache at each week of the study.
D) Percent occurrences of diarrhea at each week of the study.
E) Percent occurrences of fatigue at each week of the study.
F) Percent occurrences of constipation at each week of the study.
G) Percent occurrences of dry mouth at each week of the study.
H) Percent occurrences of irritability at each week of the study.
All values reported as percent occurrences at each week of the study. * P < 0.05, percent occurrences significantly different from controls at each time point by ANCOVA.
4-h and 6-h TRF produce similar reductions in energy intake without calorie counting
The change in energy intake by week 8 was significantly different across the three groups (P = 0.008), with the 4-h TRF group (Δ = −528 ± 102 kcal/d, −2209 ± 427 kJ/d, 30% reduction) and 6-h TRF group (Δ = −566 ± 142 kcal/d, −2368 ± 594 kJ/d, 29% reduction) experiencing greater reductions than controls (Δ = −105 ± 52 kcal/d, 439 ± 217 kJ/d, P = 0.02 and P = 0.01, respectively), with no significant difference between intervention groups (Table S1). TRF is a unique weight loss regimen in that it does not require calorie counting. Participants are simply asked to consume all their food for the day within a specified time frame, and water fast for the remaining hours of the day. We show here that by simply limiting the eating window to 4-h or 6-h, participants with obesity naturally decrease energy intake by ∼550 kcal/d (2300 kJ/d). From a clinical standpoint, these findings are paramount. One of the main reasons for participant attrition during daily CR and alternate day fasting trials is frustration with having to vigilantly monitor energy intake on a regular basis (Dansinger et al., 2005; Das et al., 2007; Trepanowski et al., 2017). TRF regimens are able to avoid this requirement by allowing participants to simply watch the clock instead of monitoring calories, while still producing weight loss. Human trials of TRF with longer durations (>12 month) will be needed to test if these changes in energy intake persist long-term.
TRF appears to produce comparable reductions in energy intake (20–30%) as daily CR. Interestingly, despite similar degrees of energy restriction, TRF produces less weight loss compared to daily CR over the same duration of time. For instance, recent controlled trials of CR report 4–7% weight loss over 2–3 months in adults with obesity (Jimenez Jaime et al., 2015; Ravussin et al., 2015; Redman et al., 2007; Trepanowski et al., 2017), while TRF trials report 3–4% weight loss (Gabel et al., 2018; Gill and Panda, 2015; Wilkinson et al., 2020). The reason for this discrepancy is unclear. It is possible however, that estimates of energy restriction in the TRF trials are inaccurate since this data was quantified via food records. This is evident in the present trial as well – our participants report ∼550 kcal/d reductions in energy intake, which would equate to ∼4 kg weight loss over 8 weeks, but only ∼3 kg was actually lost. These issues are also evident in the control group. Controls reported ∼100 kcal/d reductions in energy intake, yet body weight slightly increased in this group. It is well known that participants with obesity underreport energy intake by 20–40% in food diaries (Goris et al., 2000; Kretsch et al., 1999). Many CR trials, in contrast, use doubly-labeled water to assess energy restriction, which is the gold standard method (Ravussin et al., 2015; Redman et al., 2007; Trepanowski et al., 2017). In order to ascertain whether TRF truly produces 20–30% energy restriction, future trials should implement the doubly-labeled water technique. Studies that directly compare the effects of TRF to CR are also undoubtedly needed, as none have been performed to date.
We also assessed changes in diet quality during TRF. It is conceivable that limiting the eating window to 4 or 6 hours per day could lead to the increased consumption of energy dense foods and compensatory drinking (i.e. increased diet soda and caffeine intake). As such, we examined whether key diet quality indicators, such as sugar, saturated fat, cholesterol, fiber and sodium intake, changed from baseline to week 8. Results reveal that changes in these parameters of diet quality by week 8 were not significantly different between the 4-h TRF, 6-h TRF, or control groups (Table S1). Intakes of sugar, saturated fat, cholesterol, fiber and sodium were similar to what is typically consumed by the average American at baseline and post-treatment (Powell et al., 2016; Rehm et al., 2016). In addition, changes in diet soda, sugar sweetened soda, caffeinated beverages excluding sodas (caffeinated coffee, caffeinated tea, energy drinks) or alcohol intake by week 8 were not significantly different between the 4-h TRF, 6-h TRF, or control groups (Table S1). Although the present short-term (8-week) trial shows no change in these key indicators of diet quality, these findings will need confirmation by a well-powered study that specifically examines the impact of 4-h and 6-h TRF on these parameters.
Our study, to the best of our knowledge, is the first randomized controlled trial to compare the weight loss efficacy of 4-h versus 6-h TRF in adults with obesity. Findings from this trial suggest that 4-h TRF does not produce superior weight loss versus 6-h TRF. Both fasting regimens induce mild reductions in body weight over 8 weeks (∼3%), and show promise as interventions for weight loss. Reductions in insulin resistance and oxidative stress were also noted, which bode well for the use of these regimens in preventing cardiometabolic disease. Compliance was similar for 4-h and 6-h TRF, and both regimens reduced daily energy intake by ∼550 kcal/d, 2300 kJ/d (30% reduction), without calorie counting. Though these findings are promising, future trials will be needed to examine the feasibility of TRF long-term and also examine whether the weight loss and cardiometabolic benefits observed here can be sustained over longer periods of time.
Limitations of Study
This study has several limitations. First, our sample size was small and we were underpowered to detect differences between groups for certain secondary outcome measures. A larger clinical trial powered for these secondary outcomes is warranted. Second, we did not evaluate the effects of the 4-h or 6-h regimens at different times in the day (early TRF versus late TRF). Insulin sensitivity has been purported to be higher in the morning than in the evening (Morris et al., 2015b; Poggiogalle et al., 2018). Thus, it is possible that if these regimens were shifted to earlier in the day, more pronounced reductions in insulin resistance would have been noted. Third, we only measured one indicator of oxidative stress, 8-isoprostane. It would have been useful to determine if other measures of oxidative stress (i.e. 4-hydroxynonenal adducts, protein carbonyls, and nitrotyrosine) are also improved with this diet. Fourth, these TRF interventions failed to produce clinically significant weight loss, i e. 5% from baseline (Williamson et al., 2015), over 8-weeks. Longer-term trials will be required to see if these diets can indeed be implemented to produce the 5% weight loss necessary to observe lasting benefits to overall health. Lastly, our study did not implement a cross-over design. A cross-over trial could help to reduce the influence of confounding covariates, as each participant would act as their own control.
STAR METHODS
RESOURCE AVAILABILITY
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Krista Varady (varady@uic.edu).
Materials availability
This study did not generate new unique reagents.
Data and code availability
The datasets supporting the current study have not been deposited in a public repository but are available from the corresponding author on request.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Human subjects
Participants were recruited from the Chicago area via advertisements placed around the University of Illinois Chicago campus. Participants were screened via a questionnaire, BMI assessment and pregnancy test. A total of 82 participants were consented and were assessed for eligibility (Figure 2). Of these 82 participants, 24 were excluded because they did not meet one or more inclusion criteria. Inclusion criteria was as follows: male; female; body mass index (BMI) between 30.0 and 49.9 kg/m2; age between 18 and 65 years; sedentary (light exercise less than 1 h per week) or moderately active (moderate exercise 1 to 2 h per week); weight stable for 3 months prior to the beginning of the study (gain or loss <4 kg); and able to give written informed consent. Exclusion criteria: diabetes mellitus; use of medications that could affect study outcomes; night shift workers; perimenopausal or otherwise irregular menstrual cycle; pregnant or trying to become pregnant; and current smokers. The experimental protocol was approved by the University of Illinois Chicago Office for the Protection of Research Subjects, and all research participants gave their written informed consent to participate in the trial. The trial was conducted between February 2019 to October 2019.
Experimental design
A 10-week randomized parallel-arm trial was implemented to compare the effects of 4-h and 6-h TRF versus controls on body weight and secondary outcome measures. The trial consisted of a 2-week baseline period followed by an 8-week TRF intervention period. Before commencing the study, all subjects participated in a 2-week baseline weight stabilization period. During this period, participants were requested to remain weight stable by consuming their usual diet and not changing their physical activity habits. During the 8-week intervention period, the 4-h TRF group was instructed to eat ad libitum from 3 to 7 pm daily, and fast from 7 to 3 pm (20-h fast). The 6-h TRF group was instructed to eat ad libitum from 1 to 7 pm daily, and fast from 7 to 1 pm (18-h fast). During the 4-h and 6-h feeding windows, there were no restrictions on types or quantities of foods consumed. Moreover, participants were not required to monitor caloric intake during this ad libitum feeding period. During the fasting period, participants were encouraged to drink plenty of water and were permitted to consume energy-free beverages, such as black tea, coffee, and diet sodas. Controls were instructed to maintain their weight throughout the trial, and not to change their eating or physical activity habits. Controls received no diet advice but visited the research center at the same frequency as the intervention groups to alleviate any investigator-interaction bias. Controls who completed the 10-week trial received 4 sessions of free weight loss counseling at the end of the study.
METHOD DETAILS
Body weight and body composition
The primary outcome of the study was change in body weight. Body weight was assessed to the nearest 0.25 kg every week at the research center without shoes and in light clothing using a digital scale (HealthOMeter, Boca Raton, FL). Height was assessed during the screening visit using a wall-mounted stadiometer (HealthOMeter, Boca Raton, FL) to the nearest 0.1 cm. Body composition (fat mass, lean mass, visceral fat mass) was measured at baseline and week 8 using dual x-ray absorptiometry (DXA; iDXA, General Electric Inc).
Adherence to the TRF protocols
Adherence to the 4-h and 6-h TRF windows was measured using a daily adherence log, which recorded the times each participant started and stopped eating each day. If the log indicated that the participant ate within the prescribed 4-h or 6-h window, that day was labeled “adherent”. If the log indicated that the participant consumed food outside of the prescribed 6-h or 4-h feeding windows, that day was labeled as “non-adherent”. Adherence to the TRF diet was assessed as the number of adherent days per week. Throughout the trial, TRF participants met with the study coordinator on a weekly basis (after the weigh in) to review the adherence log. At each of these meetings, the study coordinator emphasized the importance of eating within the prescribed window. Participants were also encouraged to discuss any issues they had with adhering to the diet during these meetings.
Metabolic disease risk factors
Blood samples were collected after a 12-h fast at week 1 (before starting the intervention) and at week 8, between 6:00–9:00 am. All blood draws were performed at the Human Nutrition Research Unit at the University of Illinois at Chicago. Blood was centrifuged for 20 min at 520 x g and 4°C to separate plasma from red cells and stored at −80°C until analyzed. Fasting plasma total cholesterol, direct LDL cholesterol, HDL-cholesterol, triglycerides, glucose and insulin concentrations were measured by a commercial lab (Medstar, Chicago, IL). Insulin resistance (IR) was calculated using the HOMA (Homeostasis Model Assessment) method, by applying the following formula: [HOMA-IR = Fasting insulin (μlU/ml) × Fasting glucose (mg/dL) / 405]. Blood pressure and heart rate were measured in triplicate using a digital automatic blood pressure/heart rate monitor (Omron HEM 705 LP, Kyoto, Japan) with the participant in a seated position after a 10-min rest.
Inflammatory markers and oxidative stress
Plasma levels of the inflammatory cytokines, TNF-alpha and IL-6, and the oxidative stress marker, 8-isoprostane, were measured by ELISA (R&D Systems, Minneapolis, MN; Cayman Chemical Company; Ann Arbor, MI, respectively) on a Bio Rad Microplate reader (Bio-Rad Laboratories; Hercules, CA).
Adverse events
Neurological issues (dizziness, headache, fatigue, and irritability) and gastrointestinal issues (nausea, diarrhea, constipation, and dry mouth) were assessed by an adverse events questionnaire at baseline and during each week of the intervention period.
Dietary intake and physical activity
TRF and control participants completed a 7-d food record during the baseline period and at week 8. A dietitian provided 15 min of instruction to each participant on how to complete the food records. These instructions included information and reference guides on how to estimate portion sizes and record food items in sufficient detail to obtain accurate estimates of dietary intake. Participants were not required to weigh foods but were asked to measure the volume of foods consumed with household measures (i.e. measuring cups and measuring spoons). The timing of food intake (for each beverage or food item) was also recorded in the food record. The records were collected at the weigh-in at baseline and week 8 and were reviewed by the dietitian for accuracy and completeness. The food analysis program, Nutritionist Pro (Axxya Systems, Stafford, TX) was used to calculate the total daily intake of energy, total fat, saturated fat, monounsaturated fat, polyunsaturated fat, protein, carbohydrate, total sugar, cholesterol, fiber, sodium, and alcohol. Consumption of diet sodas, sugar-sweetened sodas, and caffeinated beverages excluding sodas (caffeinated coffee, caffeinated tea, energy drinks) are presented as mean intakes by volume (ml/d). All participants were asked to maintain their level of physical activity throughout the entire trial. Step counts were measured over 7-d during the baseline period and at week 8 by Fitbit Alta HR (Fitbit, San Francisco, CA). Participants were instructed to wear the device all day and night (except while showering).
QUANTIFICATION AND STATISTICAL ANALYSIS
Power and sample size
For the sample size calculation, we estimated that the 4-h TRF group would lose 5% and the 6-h TRF group would lose 2% of body weight over 8 weeks (Gabel et al., 2018). We calculated that n = 16 participants per group would provide 80% power to detect a significant difference of 3% in body weight between the 4-h and 6-h TRF groups by week 8, using an independent samples t-test with a = 0.05. We anticipated a dropout rate of 20%. Thus, we initially aimed to recruit 57 participants (n = 19 per group), assuming that 48 participants (n = 16 per group) would complete the trial.
Randomization
Participants were randomized in a 1:1:1 ratio to a 4-h TRF group, a 6-h TRF group, or a nointervention control group (before all baseline assessments and the 2-week baseline window). Randomization was performed by a stratified random sampling procedure by sex, age (18–42 y/ 43–65 y), and BMI (30.0–39.9 kg/m2 / 40.0–49.9 kg/m2).
Statistical analyses
Statistical analyses were performed using SPSS v.25.0 for Mac (SPSS Inc.). A two-tailed P value of less than 0.05 was considered statistically significant. All data are presented as mean ± standard error of the mean (SEM). Tests for normality were included in the model, and all data were found to be normally distributed. At baseline, differences between treatment arms (4-h TRF, 6-h TRF, and control) were tested by a one-way ANOVA with a Tukey post-hoc test (continuous variables) or McNemar test (categorical variables). At week 8, differences across treatment arms (4-h TRF, 6-h TRF, and control) were evaluated as change scores (from baseline to week 8) using ANCOVA with baseline as a covariate. If the overall ANCOVA across the three arms was significant, pairwise comparisons were performed to evaluate differences between arms. Bonferroni post-hoc tests were used for these comparisons. Change scores are represented by “Δ” in the results text. Pearson correlations were performed to assess the relationship between weight loss and change from baseline in the duration of the fasting window.
Data were included for 58 participants, and means were estimated using an intention-to- treat analysis using last observation carried forward. All dropouts occurred during week 1 or 2 of the study. Baseline body weight, height and BMI were collected for all dropouts (4-h TRF: n = 3, 6-h TRF: n = 1, Control: n = 5). None of the dropouts attended the baseline DXA scan visit, thus, no body composition data are available for these participants. Moreover, none the dropouts returned food records or adherence logs, so there is no dietary intake or compliance data for the subjects. Only a few dropouts attended the baseline blood draw/blood pressure visit (4-h TRF: n = 1, 6-h TRF: n = 1, Control: n = 3), so data for these parameters are limited. TNF-alpha, IL-6, and 8-isoprostane data are not available for dropouts as not enough blood could be collected from these participants.
ADDITIONAL RESOURCES
Prior to enrolling participants, the trial was preregistered on clinicaltrials.gov (NCT03867773).
Supplementary Material
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Critical Commercial Assays | ||
| Human TNF-alpha Quantikine ELISA kit | R&D Systems | DTA00D |
| Human IL-6 Quantikine ELISA kit | R&D Systems | D6050 |
| 8-Isoprostane ELISA kit | Cayman Chemical | 516351 |
| Software and Algorithms | ||
| SPSS v.25.0 | IBM | www.ibm.com |
CONTEXT AND SIGNIFICANCE
Time-restricted feeding (TRF) has become a popular weight-loss regimen. The approach involves confining the eating window to a specified number of hours per day, while fasting (though zero-calorie beverages are allowed) for the remaining hours of the day. Here, Krista Varady and her colleagues at the University of Illinois study for the first time two popular forms of TRF (specifically, 4-hour and 6-hour eating windows) over the course of two months. They find these versions of TRF are effective for reducing body weight in adults with obesity, as both regimens resulted in the subjects eating ∼550 fewer calories per day, compared to their baseline intake, without calorie counting. These findings suggest that this form of severe TRF is achievable and can help adults with obesity lose weight, without having to count calories.
HIGHLIGHTS
4-h and 6-h time-restricted feeding regimens were tested in adults with obesity
Both regimens produce similar weight loss over the 2 months of the study
Both regimens reduce energy intake by ∼550 kcal per day without calorie counting
Both regimens produce similar reductions in insulin resistance and oxidative stress
Acknowledgments
The authors would like to thank the study participants for their time and effort in participating in the trial. We would also like to thank Nicolas Côté for his assistance in preparing the graphical abstract. This study was supported by R01DK119783 from the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Funding:
National Institutes of Health, NIDDK, R01DK119783
Footnotes
Declarations of interest
KAV received author fees from Hachette Book Group for the book, “The Every Other Day Diet”. The other authors declare no competing interests.
Trial registration: Clinicaltrials.gov NCT03867773
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Antoni R, Johnston KL, Collins AL, and Robertson MD (2018). Intermittent v. continuous energy restriction: differential effects on postprandial glucose and lipid metabolism following matched weight loss in overweight/obese participants. Br J Nutr 119, 507–516. [DOI] [PubMed] [Google Scholar]
- Antoni R, Robertson TM, Robertson MD, Johnston JD (2018). A pilot feasibility study exploring the effects of a moderate time-restricted feeding intervention on energy intake, adiposity and metabolic physiology in free-living human subjects. J Nutr Sci 7, 22. [Google Scholar]
- Bhutani S, Klempel MC, Kroeger CM, Trepanowski JF, and Varady KA (2013). Alternate day fasting and endurance exercise combine to reduce body weight and favorably alter plasma lipids in obese humans. Obesity (Silver Spring) 21, 1370–1379. [DOI] [PubMed] [Google Scholar]
- Bowen J, Brindal E, James-Martin G, and Noakes M (2018). Randomized Trial of a High Protein, Partial Meal Replacement Program with or without Alternate Day Fasting: Similar Effects on Weight Loss, Retention Status, Nutritional, Metabolic, and Behavioral Outcomes. Nutrients 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson O, Martin B, Stote KS, Golden E, Maudsley S, Najjar SS, Ferrucci L, Ingram DK, Longo DL, Rumpler WV, et al. (2007). Impact of reduced meal frequency without caloric restriction on glucose regulation in healthy, normal-weight middle- aged men and women. Metabolism 56, 1729–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catenacci VA, Pan Z, Ostendorf D, Brannon S, Gozansky WS, Mattson MP, Martin B, MacLean PS, Melanson EL, and Troy Donahoo W (2016). A randomized pilot study comparing zero-calorie alternate-day fasting to daily caloric restriction in adults with obesity. Obesity (Silver Spring) 24, 1874–1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury EA, Richardson JD, Gonzalez JT, Tsintzas K, Thompson D, and Betts JA (2019). Six Weeks of Morning Fasting Causes Little Adaptation of Metabolic or Appetite Responses to Feeding in Adults with Obesity. Obesity (Silver Spring) 27, 813–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dansinger ML, Gleason JA, Griffith JL, Selker HP, and Schaefer EJ (2005). Comparison of the Atkins, Ornish, Weight Watchers, and Zone diets for weight loss and heart disease risk reduction: a randomized trial. JAMA 293, 43–53. [DOI] [PubMed] [Google Scholar]
- Das SK, Gilhooly CH, Golden JK, Pittas AG, Fuss PJ, Cheatham RA, Tyler S, Tsay M, McCrory MA, Lichtenstein AH, et al. (2007). Long-term effects of 2 energy- restricted diets differing in glycemic load on dietary adherence, body composition, and metabolism in CALERIE: a 1-y randomized controlled trial. Am J Clin Nutr 85, 1023–1030. [DOI] [PubMed] [Google Scholar]
- de Cabo R, and Mattson MP (2019). Effects of Intermittent Fasting on Health, Aging, and Disease. N Engl J Med 381, 2541–2551. [DOI] [PubMed] [Google Scholar]
- Dhurandhar EJ, Dawson J, Alcorn A, Larsen LH, Thomas EA, Cardel M, Bourland C, Astrup A, St-Onge MP, Hill JO, et al. (2014). The effectiveness of breakfast recommendations on weight loss: a randomized controlled trial. Am J Clin Nutr 100, 507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshghinia S, and Mohammadzadeh F (2013). The effects of modified alternate-day fasting diet on weight loss and CAD risk factors in overweight and obese women. J Diabetes Metab Disord 12, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabel K, Hoddy KK, Burgess HJ, and Varady KA (2019a). Effect of 8-h time-restricted feeding on sleep quality and duration in adults with obesity. Appl Physiol Nutr Metab 44, 903–906. [DOI] [PubMed] [Google Scholar]
- Gabel K, Hoddy KK, Haggerty N, Song J, Kroeger CM, Trepanowski JF, Panda S, and Varady KA (2018). Effects of 8-hour time restricted feeding on body weight and metabolic disease risk factors in obese adults: A pilot study. Nutr Healthy Aging 4, 345–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabel K, Hoddy KK, and Varady KA (2019b). Safety of 8-h time restricted feeding in adults with obesity. Appl Physiol Nutr Metab 44, 107–109. [DOI] [PubMed] [Google Scholar]
- Gabel K, Kroeger CM, Trepanowski JF, Hoddy KK, Cienfuegos S, Kalam F, and Varady KA (2019c). Differential Effects of Alternate-Day Fasting Versus Daily Calorie Restriction on Insulin Resistance. Obesity (Silver Spring) 27, 1443–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gayoso-Diz P, Otero-Gonzalez A, Rodriguez-Alvarez MX, Gude F, Garcia F, De Francisco A, and Quintela AG (2013). Insulin resistance (HOMA-IR) cut-off values and the metabolic syndrome in a general adult population: effect of gender and age: EPIRCE cross-sectional study. BMC Endocr Disord 13, 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geliebter A, Astbury NM, Aviram-Friedman R, Yahav E, and Hashim S (2014). Skipping breakfast leads to weight loss but also elevated cholesterol compared with consuming daily breakfasts of oat porridge or frosted cornflakes in overweight individuals: a randomised controlled trial. J Nutr Sci 3, e56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill S, and Panda S (2015). A Smartphone App Reveals Erratic Diurnal Eating Patterns in Humans that Can Be Modulated for Health Benefits. Cell Metab 22, 789–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goris AH, Westerterp-Plantenga MS, and Westerterp KR (2000). Undereating and underrecording of habitual food intake in obese men: selective underreporting of fat intake. Am J Clin Nutr 71, 130–134. [DOI] [PubMed] [Google Scholar]
- Harvie MN, Pegington M, Mattson MP, Frystyk J, Dillon B, Evans G, Cuzick J, Jebb SA, Martin B, Cutler RG, et al. (2011). The effects of intermittent or continuous energy restriction on weight loss and metabolic disease risk markers: a randomized trial in young overweight women. Int J Obes (Lond) 35, 714–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilbronn LK, Civitarese AE, Bogacka I, Smith SR, Hulver M, and Ravussin E (2005). Glucose tolerance and skeletal muscle gene expression in response to alternate day fasting. Obes Res 13, 574–581. [DOI] [PubMed] [Google Scholar]
- Hoddy KK, Bhutani S, Phillips SA, and Varady KA (2016). Effects of different degrees of insulin resistance on endothelial function in obese adults undergoing alternate day fasting. Nutr Healthy Aging 4, 63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoddy KK, Kroeger CM, Trepanowski JF, Barnosky A, Bhutani S, and Varady KA (2014). Meal timing during alternate day fasting: Impact on body weight and cardiovascular disease risk in obese adults. Obesity (Silver Spring) 22, 2524–2531. [DOI] [PubMed] [Google Scholar]
- Houstis N, Rosen ED, and Lander ES (2006). Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature 440, 944–948. [DOI] [PubMed] [Google Scholar]
- Hutchison AT, Liu B, Wood RE, Vincent AD, Thompson CH, O’Callaghan NJ, Wittert GA, and Heilbronn LK (2019). Effects of Intermittent Versus Continuous Energy Intakes on Insulin Sensitivity and Metabolic Risk in Women with Overweight. Obesity (Silver Spring) 27, 50–58. [DOI] [PubMed] [Google Scholar]
- Jimenez Jaime T, Leiva Balich L, Barrera Acevedo G, de la Maza Cave MP, Hirsch Birn S, Henriquez Parada S, Rodriguez Silva J, and Bunout Barnett D (2015). Effect of calorie restriction on energy expenditure in overweight and obese adult women. Nutr Hosp 31, 2428–2436. [DOI] [PubMed] [Google Scholar]
- Johnson JB, Summer W, Cutler RG, Martin B, Hyun DH, Dixit VD, Pearson M, Nassar M, Telljohann R, Maudsley S, et al. (2007). Alternate day calorie restriction improves clinical findings and reduces markers of oxidative stress and inflammation in overweight adults with moderate asthma. Free Radic Biol Med 42, 665–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalam F, Gabel K, Cienfuegos S, Wiseman E, Ezpeleta M, Steward M, Pavlou V, and Varady KA (2019). Alternate day fasting combined with a low-carbohydrate diet for weight loss, weight maintenance, and metabolic disease risk reduction. Obes Sci Pract 5, 531–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klempel MC, Kroeger CM, and Varady KA (2013). Alternate day fasting (ADF) with a high-fat diet produces similar weight loss and cardio-protection as ADF with a low-fat diet. Metabolism 62, 137–143. [DOI] [PubMed] [Google Scholar]
- Kretsch MJ, Fong AK, and Green MW (1999). Behavioral and body size correlates of energy intake underreporting by obese and normal-weight women. J Am Diet Assoc 99, 300–306; quiz 307–308. [DOI] [PubMed] [Google Scholar]
- Levitsky DA, and Pacanowski CR (2013). Effect of skipping breakfast on subsequent energy intake. Physiol Behav 119, 9–16. [DOI] [PubMed] [Google Scholar]
- Moro T, Tinsley G, Bianco A, Marcolin G, Pacelli QF, Battaglia G, Palma A, Gentil P, Neri M, and Paoli A (2016). Effects of eight weeks of time-restricted feeding (16/8) on basal metabolism, maximal strength, body composition, inflammation, and cardiovascular risk factors in resistance-trained males. J Transl Med 14, 290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris CJ, Garcia JI, Myers S, Yang JN, Trienekens N, and Scheer FA (2015a). The Human Circadian System Has a Dominating Role in Causing the Morning/Evening Difference in Diet-Induced Thermogenesis. Obesity (Silver Spring) 23, 2053–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris CJ, Yang JN, Garcia JI, Myers S, Bozzi I, Wang W, Buxton OM, Shea SA, and Scheer FA (2015b). Endogenous circadian system and circadian misalignment impact glucose tolerance via separate mechanisms in humans. Proc Natl Acad Sci U S A 112, E2225–2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nas A, Mirza N, Hagele F, Kahlhofer J, Keller J, Rising R, Kufer TA, and Bosy-Westphal A (2017). Impact of breakfast skipping compared with dinner skipping on regulation of energy balance and metabolic risk. Am J Clin Nutr 105, 1351–1361. [DOI] [PubMed] [Google Scholar]
- Patterson RE, and Sears DD (2017). Metabolic Effects of Intermittent Fasting. Annu Rev Nutr 37, 371–393. [DOI] [PubMed] [Google Scholar]
- Poggiogalle E, Jamshed H, and Peterson CM (2018). Circadian regulation of glucose, lipid, and energy metabolism in humans. Metabolism 84, 11–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell ES, Smith-Taillie LP, and Popkin BM (2016). Added Sugars Intake Across the Distribution of US Children and Adult Consumers: 1977–2012. J Acad Nutr Diet 116, 1543–1550 e1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rains JL, and Jain SK (2011). Oxidative stress, insulin signaling, and diabetes. Free Radic Biol Med 50, 567–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravussin E, Redman LM, Rochon J, Das SK, Fontana L, Kraus WE, Romashkan S, Williamson DA, Meydani SN, Villareal DT, et al. (2015). A 2-Year Randomized Controlled Trial of Human Caloric Restriction: Feasibility and Effects on Predictors of Health Span and Longevity. J Gerontol A Biol Sci Med Sci 70, 1097–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman LM, Heilbronn LK, Martin CK, Alfonso A, Smith SR, Ravussin E, and Pennington CT (2007). Effect of calorie restriction with or without exercise on body composition and fat distribution. J Clin Endocrinol Metab 92, 865–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehm CD, Penalvo JL, Afshin A, and Mozaffarian D (2016). Dietary Intake Among US Adults, 1999–2012. JAMA 315, 2542–2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheer FA, Hilton MF, Mantzoros CS, and Shea SA (2009). Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci U S A 106, 4453–4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubel R, Nattenmuller J, Sookthai D, Nonnenmacher T, Graf ME, Riedl L, Schlett L, von Stackelberg O, Johnson T, Nabers D, et al. (2018). Effects of intermittent and continuous calorie restriction on body weight and metabolism over 50 wk: a randomized controlled trial. Am J Clin Nutr 108, 933–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sievert K, Hussain SM, Page MJ, Wang Y, Hughes HJ, Malek M, and Cicuttini FM (2019). Effect of breakfast on weight and energy intake: systematic review and meta-analysis of randomised controlled trials. BMJ 364, l42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stote KS, Baer DJ, Spears K, Paul DR, Harris GK, Rumpler WV, Strycula P, Najjar SS, Ferrucci L, Ingram DK, et al. (2007). A controlled trial of reduced meal frequency without caloric restriction in healthy, normal-weight, middle-aged adults. Am J Clin Nutr 85, 981–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner AE, and Cowie CC (2008). Ethnic differences in the ability of triglyceride levels to identify insulin resistance. Atherosclerosis 196, 696–703. [DOI] [PubMed] [Google Scholar]
- Sundfor TM, Svendsen M, and Tonstad S (2018). Effect of intermittent versus continuous energy restriction on weight loss, maintenance and cardiometabolic risk: A randomized 1-year trial. Nutr Metab Cardiovasc Dis 28, 698–706. [DOI] [PubMed] [Google Scholar]
- Sutton EF, Beyl R, Early KS, Cefalu WT, Ravussin E, and Peterson CM (2018). Early Time-Restricted Feeding Improves Insulin Sensitivity, Blood Pressure, and Oxidative Stress Even without Weight Loss in Men with Prediabetes. Cell Metab 27, 1212–1221 e1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinsley GM, Moore ML, Graybeal AJ, Paoli A, Kim Y, Gonzales JU, Harry JR, VanDusseldorp TA, Kennedy DN, and Cruz MR (2019). Time-restricted feeding plus resistance training in active females: a randomized trial. Am J Clin Nutr 110, 628–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trepanowski JF, Kroeger CM, Barnosky A, Klempel M, Bhutani S, Hoddy KK, Rood J, Ravussin E, and Varady KA (2018). Effects of alternate-day fasting or daily calorie restriction on body composition, fat distribution, and circulating adipokines: Secondary analysis of a randomized controlled trial. Clin Nutr 37, 1871–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trepanowski JF, Kroeger CM, Barnosky A, Klempel MC, Bhutani S, Hoddy KK, Gabel K, Freels S, Rigdon J, Rood J, et al. (2017). Effect of Alternate-Day Fasting on Weight Loss, Weight Maintenance, and Cardioprotection Among Metabolically Healthy Obese Adults: A Randomized Clinical Trial. JAMA Intern Med 177, 930–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varady KA, Bhutani S, Church EC, and Klempel MC (2009). Short-term modified alternate-day fasting: a novel dietary strategy for weight loss and cardioprotection in obese adults. Am J Clin Nutr 90, 1138–1143. [DOI] [PubMed] [Google Scholar]
- Varady KA, Bhutani S, Klempel MC, Kroeger CM, Trepanowski JF, Haus JM, Hoddy KK, and Calvo Y (2013). Alternate day fasting for weight loss in normal weight and overweight subjects: a randomized controlled trial. Nutr J 12, 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson MJ, Manoogian ENC, Zadourian A, Lo H, Fakhouri S, Shoghi A, Wang X, Fleischer JG, Navlakha S, Panda S, et al. (2020). Ten-Hour Time-Restricted Eating Reduces Weight, Blood Pressure, and Atherogenic Lipids in Patients with Metabolic Syndrome. Cell Metab 31, 92–104 e105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson DA, Bray GA, and Ryan DH (2015). Is 5% weight loss a satisfactory criterion to define clinically significant weight loss? Obesity (Silver Spring) 23, 2319–2320. [DOI] [PubMed] [Google Scholar]
- Zaulkffali AS, Md Razip NN, Syed Alwi SS, Abd Jalil A, Abd Mutalib MS, Gopalsamy B, Chang SK, Zainal Z, Ibrahim NN, Zakaria ZA, et al. (2019). Vitamins D and E Stimulate the PI3K-AKT Signalling Pathway in Insulin-Resistant SK-N-SH Neuronal Cells. Nutrients 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting the current study have not been deposited in a public repository but are available from the corresponding author on request.