Abstract
Objective
To determine how early-life risk factors explain socioeconomic inequalities in persistent asthma in adolescence.
Methods
We did a causal mediation analysis using data from 7487 children and young people in the UK Millennium Cohort Study. Persistent asthma was defined as having a diagnosis reported at any two or more time points at 7, 11 or 14 years. The main exposure was maternal education, a measure of early-life socioeconomic circumstances (SECs), used to calculate the relative index of inequality. We assessed how blocks of perinatal (maternal health behaviours, infant characteristics and duration of breastfeeding, measured at 9 months) and environmental risk factors (family housing conditions; potential exposure to infections through childcare type and sibling number, and neighbourhood characteristics, measured at 3 years) mediated the total effect of childhood SECs on persistent asthma risk, calculating the proportion mediated and natural indirect effect (NIE) via blocks of mediators.
Results
At age 14 the overall prevalence of persistent asthma was 15%. Children of mothers with lower educational qualifications were more likely to have persistent asthma, with a clear social gradient (degree plus: 12.8% vs no qualifications: 20.3%). The NIE gives the effect of SECs acting only via the mediators and shows a 31% increased odds of persistent asthma when SECs are fixed at the highest level, and mediators at the level which would naturally occur at the lowest SECs versus highest SECs (NIE OR 1.31, 95% CI 1.04 to 1.65). Overall, 58.9% (95% CI 52.9 to 63.7) of the total effect (OR 1.70, 95% CI 1.20 to 2.40) of SECs on risk of persistent asthma in adolescence was mediated by perinatal and environmental characteristics.
Conclusions
Perinatal characteristics and the home environment in early life are more important in explaining socioeconomic inequalities in persistent asthma in British adolescents than more distal environmental exposures outside the home.
Keywords: asthma, asthma epidemiology
Key messages.
What is the key question?
How does disadvantage during early-life influence inequalities in persistent asthma in adolescence?
What is the bottom line?
Disadvantage in early-life is associated with 70% greater risk of persistent asthma in adolescents in the UK. Almost two-thirds of the excess risk is explained by perinatal and environmental mediators by the age of 3 years.
Why read on?
This study is among the first to test the mediating role of risk factors of social inequality using robust methods for causal inference applied to a contemporary, nationally representative, birth cohort.
Introduction
Asthma is the most common chronic childhood condition in the UK and disproportionately affects health and quality of life in disadvantaged groups. School-aged children in the most deprived areas in England are two and a half times more likely to have an emergency admission for asthma than their most advantaged counterparts in 2015/2016.1 Social gradients in children’s admissions and deaths from asthma have widened in recent years.2
Around 8% of children and young people (CYP) aged 12–17 years are currently receiving treatment for asthma in the UK.3 The UK has among the highest rates of asthma deaths in Europe4 and in 20111/2012 over 25 000 CYP (aged 0–15 years) were hospitalised due to asthma.5
Trajectories of asthma and wheezing are heterogeneous, and various phenotypes have been described.6 7 For example, around 35% of preschool children with recurrent wheeze are diagnosed with asthma by 8 years.8 The other 70% may grow out of recurrent wheeze or be diagnosed with asthma later in life. Even those diagnosed with asthma in childhood have periods of remission.9 In this analysis, we are most interested in the pathway to persistent asthma into adolescence that is likely to track into adulthood.
Life-long lung function is influenced by environmental conditions both before and immediately after birth and in the preschool period.10 11 Previous studies showing socioeconomic differences in wheezing during early-life point to the mediating role of early-life risk factors such as smoking during pregnancy and lower rates of breast feeding among disadvantaged groups.12 However, the relative importance of perinatal and early years environmental characteristics as mediators of the effect of socioeconomic circumstances (SECs) in early-life on persistent asthma in adolescence in the UK remains unclear.13
There are several possible, modifiable, mediating pathways that link SECs in early-life to the development of persistent asthma into adolescence. Maternal smoking,14 low birth weight,15 premature birth,16 not being breast fed,17 poor housing conditions,18 poor indoor and outdoor air quality,19 in varying degrees, have all been found to predispose to asthma and are more common in children growing up in disadvantaged SECs. Bronfenbrenner’s bioecological theory states that the developing child is at the centre of inter-related, hierarchical systems of exposure, moving from the most proximal to the most remote.20 To our knowledge the mediating role of these factors has not been examined in a single analysis across childhood using robust methods for causal mediation analysis, to assess their relative importance in explaining inequalities in persistent asthma in adolescence.
We therefore aimed to use causal mediation analysis to assess the relative importance of early years risk factors associated with social inequalities in persistent adolescent asthma in the UK. We hypothesised that children growing up in more disadvantaged circumstances are at increased risk of persistent asthma due to increased exposure to adverse risk factors in early life. Following Bronfenbrenner’s ecological systems model,20 we also hypothesised that proximal mediators, including perinatal and family housing conditions, would have a greater influence than exposure to infections from outside the home and neighbourhood characteristics on the risk of persistent asthma in adolescence.
Methods
Study design and population
The Millennium Cohort Study (MCS) is a prospective cohort study of 18 818 children born in the UK between September 2000 and January 2002.21 Participating families were randomly sampled from electoral wards, with a stratified cluster sampling design to safeguard representation of all four UK countries, disadvantaged and ethnically diverse areas. The first data collection sweep was when cohort members were approximately 9 months of age and the subsequent five sweeps of data were collected at ages 3, 5, 7, 11 and 14 years. We included all singleton children with complete data provided by the main respondent (usually the mother) who were still in the cohort at 14 years.
Outcome
We derived a longitudinal asthma variable from parental responses to the International Study of Asthma and Allergies in Childhood (ISAAC)22 standardised question, ‘Has your child ever had asthma?’ asked at 7 and 11 years, and parental reports of whether their child currently has asthma at 14 years. We identified three asthma outcomes: (1) has never recorded asthma, (2) transient asthma (recorded once only at 7, 11 or 14 years) or (3) persistent (recorded at any two or more of 7, 11 or 14 years).
Exposure
Our exposure was SECs in early-life captured through highest educational qualification attained by the child’s mother. This was reported at 9 months, but we assume it captures aspects of SEC during pregnancy and at birth. We first identified six groups: (1) higher degree or first-degree qualifications (reference group in regression analysis), (2) diploma in higher education, (3) A levels (exams usually taken around 18 years), (4) General Certificate of Secondary Education (GCSE, exams are usually taken around age 16 years) grades A–C, (5) GCSE grades D–G or (6) none of these qualifications.
In the second step, we calculated the relative index of inequality (RII), which compares the risk of asthma between children with the least and most disadvantaged SECs, taking into account the educational distribution, by ranking the six maternal educational groups from the lowest to the highest and allocating a score (ranging from 0 to 1) that equals the midpoint of the category’s range in the cumulative distribution. The RII is a regression-based index that summarises the relative inequality across the distribution of SECs, considering the size of the population and the relative disadvantage between the different groups.23
Potential mediators
We conducted a review of systematic literature reviews of the social determinants of asthma across the lifecourse,24–27 to identify four blocks of potential early-life mediators of inequalities in adolescent asthma: (1) perinatal period characteristics, including maternal health behaviours, infant characteristics and duration of breast feeding, (2) family housing conditions, (3) potential exposure to infections through childcare type and sibling number and (4) neighbourhood characteristics.
We assigned biological, social and environmental data available from the first and second data collection sweeps of the MCS (9 months and 3 years) to one of these four blocks. We ordered mediators from the most proximal, perinatal period characteristics, to the most distal, neighbourhood characteristics (box 1) in preparation for sequential modelling.
Box 1. Description of blocks of potential mediators.
-
Perinatal period measured at 9 months (first sweep, at birth)
Maternal pre-pregnancy body mass index (BMI): Mothers were asked at first interview, ‘how tall are you (without shoes)’ and ‘Thinking back to just before you became pregnant with [baby], what was your weight then (without clothes)?’. Reported pre-pregnancy weight (in kilos) was then divided by height (in metres) squared to produce pre-pregnancy BMI.
Breastfeeding duration: Mothers were asked at first interview, ‘Did you ever try to breastfeed [baby]?’ and ‘How old was [baby] when [s]he last had breast milk?’ Answers to these questions were then used to make a duration of breastfeeding variable categorised as: (0) <3 months or (1) ≥3 months.
Birth weight: Birth weight of the cohort member in kilograms categorised as (0) low weight, <2.5 kg or (1) healthy weight ≥2.5 kg.
Gestation: Gestation time of the cohort member in days categorised as (0) not preterm, 37 weeks or more or (1) preterm, <37 weeks.
Maternal smoking: Mothers were asked whether they smoked any cigarettes during pregnancy: (0) non-smoker or (1) smoker.
Alcohol use during pregnancy: Mothers were asked about their alcohol consumption during pregnancy which is categorised as (0) never 1–2 units a month, or (1) 1 unit a week or more.
Wheeze: Main respondents (almost always the mother) were asked whether the cohort member had ever seen a health professional for wheeze at 9 months.
-
Housing conditions measured at 3 years
Damp/condensation: Respondents were asked ‘How much of a problem do you have with damp or condensation on the walls in your home, apart from in the kitchen or bathroom?’. Answers were categorised as (0) no (includes ‘no damp’ and ‘not much of a problem’ and (1) yes (includes ‘some problems’ and ‘great problem’).
Tenure: Respondents were asked ‘Do you [or your partner] own or rent your home or have some other arrangement?’. Answers were categorised as (1) own outright, (2) own—mortgage/loan, (3) part rent/part mortgage (shared equity), (4) rent from local authority, (5) rent from housing association, (6) rent privately, (7) living with parents, (8) live rent free and (9) squatting.
Furry pets in household: Respondents were asked ‘Which of the following pets did you keep in your home during [child’s] first year of life?’. Answers were grouped into (0) no pets, (1) not furry and (2) furry pets.
Environmental tobacco smoke: Respondents were asked ‘Does anyone smoke in the same room as [child] nowadays?’ Answers were coded as (0) no or (1) yes.
Access to garden: Respondents were asked ‘do you have access to a garden?’. If they answered yes, ‘Is that for your sole use or shared with anyone else?’. Answers were coded as (0) yes, sole use, (1) yes shared and (2) no.
-
Potential exposure to infection through childcare type and sibling number measured at 3 years
Sibling number in household: Number of siblings in the household was derived from several questions regarding the presence of biological and step siblings.
Informal vs formal childcare: Type of childcare was derived from questions on childcare arrangement.
-
Neighbourhood characteristics measured at 3 years
Volume of traffic: Using a neighbourhood assessment form interviewers were asked ‘How would you rate the volume of traffic on the street?’. Answers were coded as (1) no traffic permitted, (2) light, (3) moderate and (4) heavy.
Housing quality: Using a neighbourhood assessment form interviewers were asked ‘How would you rate the general condition of most of the residences or other buildings in the street?’. Answers were coded as (1) well kept, good repair and exterior surfaces, (2) fair condition, (3) poor condition peeling paint broken windows and (4) badly deteriorated.
Rubbish on pavement: Using a neighbourhood assessment form interviewers were asked ‘Is there any of the following: rubbish, litter, broken glass, drug-related items, beer cans, etc, cigarette ends or discarded packs—in the street or on the pavement?’. Answers were coded as (1) none or almost none, (2) yes some and (3) yes, just about everywhere you look.
Good area: Respondents were asked ‘Is this a good area to bring up children?’. Answers were coded as (1) excellent, (2) good, (3) average, (4) poor and (5) very poor.
Potential confounders
We adjusted for potential confounding associated with SEC in early-life and asthma risk. These included child sex and maternal age at birth (14–24 vs ≥25 years), ethnicity (white; mixed ethnicity; Indian; Pakistani and Bangladeshi; black, or other) and atopy (including allergic predisposition and sensitisation), which was defined as maternal history of asthma or eczema diagnosis (none; either, or both). These are common antecedents of SECs, mediators and asthma.28
Analysis
We analysed the sample characteristics by SECs in early-life, including the proportion of CYP with persistent asthma in each group and tested univariable associations between sample characteristics and persistent asthma using multinomial regression.
Then, the analysis progressed in two stages. First, we used multinomial regression analysis to estimate the strength of association between SEC in early-life and risk for persistent asthma adjusting for blocks of potential mediators in a stepwise manner to assess how the relative risk ratio (RRR) changed on inclusion of each block of potential mediators. We adjusted all models for potential confounding by, child sex and maternal age at birth, ethnicity and atopy, as detailed in the logic model (corresponding to data available in MCS only) in figure 1. To estimate the change in RRR for mothers with the highest educational qualifications compared with those with no qualifications we calculated the difference as 100×(RRR-adjusted RRR)/(RRR-1), whereby adjusted RRRs are the different RRRs following adjustment for different mediating blocks. MCS sampling and response weights were used to account for sampling design and attrition up to the 14-year survey. The analysis was conducted using Stata V.15.
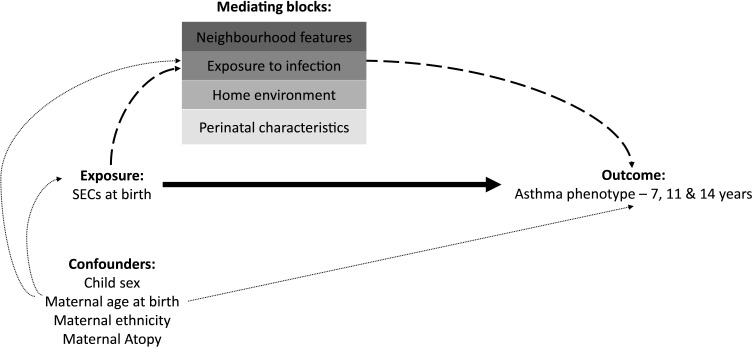
Figure 1.
Logic model of the pathways from SECs at birth (maternal education captured at 9 months) to mid-childhood/adolescent asthma (7, 11 and 14 years), with direct pathway shown in bold, indirect pathways via mediators in dashed lines and baseline confounding pathways in dotted lines. Mediating blocks capture: (1) Perinatal characteristics at 9 months (maternal pre-pregnancy body mass index; breastfeeding duration; birth weight; gestation; maternal smoking; alcohol use during pregnancy; seen a health professional for wheeze?), (2) home environment at 3 years (damp/condensation; tenure; furry pets in household; environmental tobacco smoke; access to garden) (3) exposure to infection at 3 years (sibling number in household and informal vs formal childcare) (4) neighbourhood characteristics at 3 years (volume of traffic; housing quality; rubbish on pavement; good area to bring up children?). This logic model corresponds to data available in Millennium Cohort Study only and is not a complete directed acyclic graph. SECs, socioeconomic circumstances.
As a second step, we used counterfactual mediation analysis to quantify the inequality in persistent asthma (compared with never, transient asthma was excluded) ascribable to each block of mediators using the RII as the exposure. We estimated the ORs and 95% CIs for the natural direct effect (NDE), natural indirect effect (NIE), total effect (TE) and the proportion mediated (formulas and definitions given in online supplemental material S1) for each block of mediators, cumulatively in a stepwise manner, using the Medflex package in the R software environment.29 30
thoraxjnl-2021-217312supp001.pdf (293.7KB, pdf)
Sensitivity analysis
There was variation in the timing and number of times data on mediators were collected (online supplemental table S2). To capture whether the longitudinal nature of the exposure explained the proportion mediated by blocks, we conducted a sensitivity analysis including mediators at all the time points they were available. To examine whether environmental tobacco smoke was the main driver of home environment associations with persistent asthma, we conducted a sensitivity analysis examining environmental tobacco smoke separate from other home environment factors. To examine whether there was heterogeneity between those reporting asthma at two or three times, we conducted sensitivity analyses of the association with SEC in early-life differentiating the persistent asthma group by whether they reported asthma at two or three waves. We also did a sensitivity analysis to explore the association between SECs and current asthma at age 14 years, but not reported at previous waves.
Results
Subjects
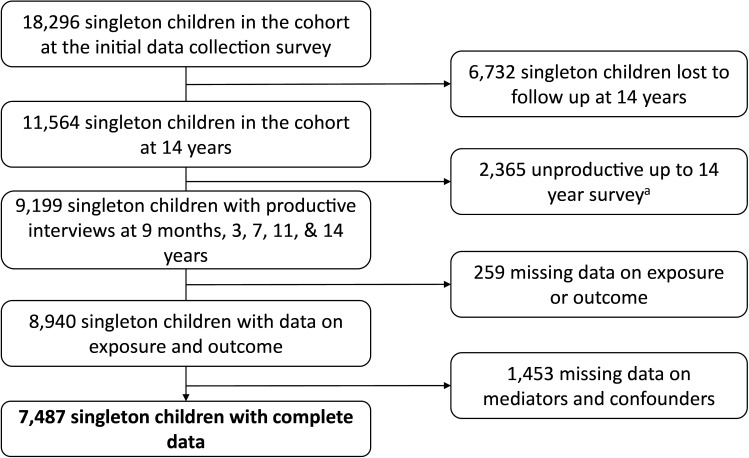
There were 18 296 singleton children included in the first MCS data collection sweep at age 9 months. By age 14 years 9199 singleton children had participated in all subsequent waves. Of these children, 7487 (81%) had complete records for analysis (figure 2). See online supplemental material table S3 for the characteristics of the excluded population.
Figure 2.
Flow diagram of cohort construction a productive cohort members have some data from one of six collection questionnaires at every data collection wave.
Prevalence of transient and persistent asthma by SEC
Table 1 shows that more than one in five children had one or more records of asthma at any wave (23.2%), of whom 8% had transient asthma (reported at one single time point at either 7, 11 or 14 years) and a further 15.2% had persistent asthma (recorded at any two or more of 7, 11 or 14 years). There was a clear social gradient in persistent asthma. Children born to mothers with fewer educational qualifications had a higher risk of persistent asthma by the age of 14 years (degree plus 12.8%; diploma 13.9%; A levels 14.3%; GCSE A–C 15.8%; GCSE D–G 18.9%; none 20.3%). Disadvantaged children were more likely to have a younger mother at birth, belong to an ethnic minority group and have less favourable perinatal, housing and neighbourhood characteristics.
Table 1.
Characteristics (%) of the study population by maternal educational qualification at birth (N=7487)
| % | Degree plus | Diploma | A levels | GCSE A–C | GCSE D–G | None | Total |
| Asthma | |||||||
| None | 79.0 | 78.8 | 77.7 | 75.7 | 70.7 | 72.7 | 76.8 |
| Transient | 8.2 | 7.3 | 8.0 | 8.5 | 10.4 | 6.9 | 8.0 |
| Persistent | 12.8 | 13.9 | 14.3 | 15.8 | 18.9 | 20.3 | 15.2 |
| Child’s sex | |||||||
| Boys | 48.5 | 50.7 | 48.5 | 46.8 | 50.7 | 50.4 | 49.3 |
| Girls | 51.5 | 49.3 | 51.5 | 53.2 | 49.3 | 49.6 | 50.7 |
| Maternal age at birth | |||||||
| ≥25 years | 97.8 | 93.1 | 74.6 | 75.7 | 60.5 | 63.6 | 84.3 |
| 14–24 years | 2.2 | 6.9 | 25.4 | 24.3 | 39.5 | 36.4 | 15.7 |
| Maternal ethnicity | |||||||
| White | 89.0 | 93.4 | 93.5 | 94.0 | 92.2 | 71.9 | 92.2 |
| Mixed | 0.9 | 0.6 | 0.5 | 0.4 | 0.6 | 1.1 | 0.6 |
| Indian | 4.1 | 1.8 | 1.0 | 1.0 | 1.1 | 2.7 | 1.8 |
| Pakistani | 2.0 | 1.2 | 2.9 | 2.8 | 5.4 | 16.6 | 2.7 |
| Black | 2.2 | 1.9 | 1.3 | 1.3 | 0.8 | 4.5 | 1.7 |
| Other | 1.7 | 1.1 | 0.4 | 0.4 | 0.1 | 3.2 | 1.0 |
| Maternal atopy | |||||||
| None | 75.1 | 69.9 | 69.6 | 69.7 | 67.8 | 73.2 | 70.3 |
| Eczema or asthma | 19.8 | 24.5 | 23.0 | 24.1 | 24.2 | 20.9 | 23.5 |
| Both | 4.3 | 5.9 | 7.5 | 7.0 | 11.4 | 5.8 | 6.7 |
| Maternal pre-pregnancy body mass index | |||||||
| Healthy weight | 71.0 | 61.5 | 53.9 | 52.8 | 51.0 | 46.2 | 58.2 |
| Underweight | 2.8 | 2.7 | 3.8 | 3.9 | 5.6 | 7.6 | 3.5 |
| Overweight | 18.8 | 24.8 | 27.4 | 27.4 | 23.8 | 29.9 | 25.4 |
| Obese | 7.4 | 11.1 | 14.9 | 15.9 | 19.5 | 16.3 | 13.0 |
| Breast fed for at least 3 months | |||||||
| No | 59.5 | 62.4 | 74.3 | 78.3 | 91.4 | 87.2 | 69.7 |
| Yes | 40.5 | 37.6 | 25.7 | 21.7 | 8.6 | 12.8 | 30.3 |
| Child’s birth weight | |||||||
| Low weight | 2.8 | 4.8 | 5.7 | 6.5 | 5.4 | 1.2 | 5.5 |
| Healthy weight | 97.2 | 95.2 | 94.3 | 93.5 | 94.6 | 88.2 | 94.5 |
| Gestational age at birth | |||||||
| Not preterm | 95.2 | 93.8 | 93.5 | 92.6 | 94.2 | 90.0 | 93.5 |
| Preterm | 4.8 | 6.2 | 6.5 | 7.4 | 5.8 | 10.1 | 6.5 |
| Maternal smoking in pregnancy | |||||||
| No | 88.0 | 82.3 | 67.3 | 55.5 | 48.0 | 46.6 | 71.7 |
| Yes | 12.0 | 17.7 | 32.7 | 44.5 | 52.0 | 53.4 | 28.3 |
| Alcohol consumption during pregnancy | |||||||
| ≤1 unit per week | 85.4 | 86.1 | 90.9 | 92.1 | 93.1 | 92.7 | 88.7 |
| Any unit per week | 14.6 | 13.9 | 9.1 | 7.9 | 6.9 | 7.3 | 11.3 |
| Wheeze at 9 months | |||||||
| No | 97.6 | 96.8 | 96.9 | 96.5 | 96.0 | 93.3 | 96.7 |
| Yes | 2.4 | 3.2 | 3.1 | 3.5 | 4.0 | 6.7 | 3.3 |
| Damp in the house | |||||||
| No | 89.8 | 89.4 | 87.5 | 86.6 | 79.7 | 75.8 | 87.6 |
| Yes | 10.2 | 10.6 | 12.5 | 13.4 | 20.3 | 24.3 | 12.5 |
| Tenure | |||||||
| Own outright | 8.1 | 5.1 | 4.3 | 3.7 | 5.2 | 6.6 | 5.0 |
| Own mortgage | 87.1 | 84.2 | 69.2 | 59.8 | 34.1 | 21.2 | 72.1 |
| Part rent/mortgage | 0.2 | 0.4 | 1.5 | 0.6 | 0.6 | 0.1 | 5.9 |
| Local authority | 0.5 | 2.5 | 10.4 | 15.7 | 32.3 | 40.1 | 9.2 |
| Housing association | 0.1 | 2.2 | 6.0 | 11.0 | 15.0 | 19.2 | 5.7 |
| Privately rented | 2.3 | 4.1 | 6.0 | 7.0 | 10.8 | 10.9 | 5.4 |
| Living with parents | 1.0 | 1.0 | 2.1 | 1.9 | 1.4 | 1.3 | 1.4 |
| Rent free | 0.8 | 0.6 | 0.5 | 0.3 | 0.6 | 0.7 | 0.5 |
| Furry pets | |||||||
| None | 59.7 | 52.2 | 46.8 | 42.5 | 45.5 | 54.3 | 49.7 |
| Not furry | 7.1 | 9.3 | 9.3 | 10.2 | 7.4 | 11.9 | 9.4 |
| Yes | 33.2 | 38.4 | 43.9 | 47.2 | 47.1 | 33.8 | 40.9 |
| Environmental tobacco smoke | |||||||
| No | 97.6 | 93.3 | 84.8 | 79.3 | 67.8 | 62.8 | 87.0 |
| Yes | 2.4 | 6.7 | 15.2 | 20.7 | 32.3 | 37.2 | 13.0 |
| Garden access | |||||||
| Yes, sole access | 94.3 | 93.7 | 89.0 | 87.1 | 82.3 | 78.3 | 90.4 |
| Yes, shared | 2.6 | 2.2 | 3.0 | 2.4 | 4.3 | 4.0 | 2.6 |
| No | 3.1 | 4.2 | 8.0 | 10.5 | 13.4 | 17.7 | 7.0 |
| Sibling number | |||||||
| 0 | 18.2 | 24.0 | 29.3 | 24.8 | 22.7 | 18.0 | 24.2 |
| 1 | 58.7 | 53.9 | 49.1 | 45.4 | 41.3 | 35.0 | 50.4 |
| 2 or more | 23.1 | 22.2 | 21.6 | 29.8 | 36.1 | 47.0 | 25.4 |
| Childcare | |||||||
| Informal | 62.0 | 76.7 | 88.6 | 91.8 | 96.7 | 99.5 | 82.3 |
| Formal | 38.0 | 23.3 | 11.4 | 8.2 | 3.3 | 0.5 | 17.7 |
| Condition of buildings | |||||||
| Good condition | 78.6 | 75.2 | 59.5 | 52.1 | 35.0 | 22.3 | 64.2 |
| Fair condition | 20.9 | 23.4 | 37.6 | 42.4 | 56.0 | 63.5 | 32.5 |
| Poor condition | 0.5 | 1.3 | 2.9 | 5.1 | 8.8 | 13.4 | 3.1 |
| Bad condition | 0.1 | 0.1 | 0.1 | 0.5 | 0.2 | 0.8 | 0.2 |
| Volume of traffic | |||||||
| None | 3.7 | 4.7 | 5.8 | 4.3 | 5.8 | 5.5 | 4.8 |
| Light | 75.6 | 76.1 | 75.2 | 77.2 | 70.8 | 72.2 | 75.8 |
| Moderate | 15.8 | 14.3 | 14.4 | 13.9 | 17.0 | 16.1 | 14.5 |
| Heavy | 4.9 | 4.9 | 4.6 | 4.6 | 6.3 | 6.2 | 4.9 |
| Litter in the street | |||||||
| None | 92.7 | 90.2 | 81.4 | 73.2 | 58.4 | 45.3 | 82.2 |
| Some | 7.2 | 9.3 | 17.7 | 24.7 | 38.2 | 47.4 | 16.5 |
| Everywhere | 0.1 | 0.6 | 0.9 | 2.1 | 3.4 | 7.3 | 1.3 |
| Good area | |||||||
| Excellent | 50.0 | 44.0 | 31.6 | 27.9 | 17.1 | 16.0 | 36.8 |
| Good | 38.0 | 40.8 | 40.7 | 39.5 | 40.1 | 35.6 | 40.0 |
| Average | 9.9 | 12.8 | 20.5 | 24.0 | 29.1 | 31.5 | 17.6 |
| Poor | 2.1 | 1.9 | 5.1 | 5.6 | 8.0 | 10.1 | 3.8 |
| Very poor | 0.1 | 0.6 | 2.2 | 3.0 | 5.7 | 6.8 | 1.8 |
GCSE, General Certificate of Secondary Education.
Disadvantaged SECs in early-life was associated with a higher RRR of persistent asthma (no maternal qualifications RRR 1.73, 95% CI 1.11 to 2.69), but not transient asthma (online supplemental table S4). As there was no clear social gradient in transient asthma (online supplemental tables S4 and S5), the mediation analysis focused on the results for persistent asthma. Table 2 shows the extent to which the elevated RRR of persistent asthma by SEC in early-life was attenuated when adjusting cumulatively for blocks of mediators in a stepwise manner. Adjusting for perinatal characteristics only (Model 1) led to a 50.7% reduction in the relative risk of persistent asthma in CYP with the lowest SECs (RRR 1.35 (95% CI 0.86 to 2.12); there was no further reduction in relative risk with the addition of housing conditions (RRR 1.35, 95% CI 0.86 to 2.10, Model 2); additionally adjusting for potential infection through childcare type and sibling number (Model 3) led to a 2.8% increase of the relative risk (RRR 1.37 (95% CI 0.87 to 2.16)), and additional adjustment for neighbourhood conditions (Model 4) led to a further 8.5% reduction in relative risk (RRR 1.31 (95% CI 0.82 to 2.09)). In this final model, adjusting for all blocks together, the overall reduction in RRR was 56.3%.
Table 2.
Relative risk ratios (RRR) for transient and persistent asthma compared with never by SEC adjusted for blocks of mediators (N=7487)
| Baseline model | Model 1 | Model 2 | Model 3 | Model 4 | ||||||
| RRR* | 95% CI | RRR | 95% CI | RRR | 95% CI | RRR | 95% CI | RRR | 95% CI | |
| Transient asthma | ||||||||||
| Diploma | 0.86 | 0.62 to 1.19 | 0.81 | 0.58 to 1.12 | 0.81 | 0.58 to 1.12 | 0.79 | 0.56 to 1.10 | 0.78 | 0.55 to 1.09 |
| A levels | 0.93 | 0.65 to 1.34 | 0.83 | 0.57 to 1.21 | 0.82 | 0.57 to 1.19 | 0.79 | 0.54 to 1.15 | 0.76 | 0.52 to 1.12 |
| GCSE A–C | 1.01 | 0.69 to 1.47 | 0.87 | 0.59 to 1.27 | 0.85 | 0.57 to 1.26 | 0.81 | 0.55 to 1.20 | 0.77 | 0.52 to 1.15 |
| GCSE D–G | 1.26 | 0.74 to 2.14 | 1.09 | 0.65 to 1.85 | 1.08 | 0.64 to 1.83 | 1.03 | 0.61 to 1.74 | 0.96 | 0.57 to 1.63 |
| None | 0.92 | 0.56 to 1.51 | 0.73 | 0.44 to 1.23 | 0.71 | 0.42 to 1.21 | 0.68 | 0.40 to 1.15 | 0.63 | 0.38 to 1.05 |
| Persistent asthma | ||||||||||
| Diploma | 1.03 | 0.78 to 1.36 | 0.96 | 0.72 to 1.27 | 0.96 | 0.73 to 1.27 | 0.95 | 0.72 to 1.26 | 0.95 | 0.72 to 1.26 |
| A levels | 1.08 | 0.78 to 1.50 | 0.96 | 0.69 to 1.35 | 0.97 | 0.69 to 1.36 | 0.96 | 0.68 to 1.35 | 0.95 | 0.67 to 1.33 |
| GCSE A–C | 1.24 | 0.91 to 1.70 | 1.09 | 0.79 to 1.51 | 1.10 | 0.80 to 1.52 | 1.10 | 0.79 to 1.53 | 1.08 | 0.78 to 1.49 |
| GCSE D–G | 1.50 | 0.95 to 2.35 | 1.34 | 0.85 to 2.12 | 1.35 | 0.86 to 2.13 | 1.36 | 0.85 to 2.15 | 1.31 | 0.83 to 2.07 |
| None | 1.71 | 1.07 to 2.74 | 1.35 | 0.86 to 2.12 | 1.35 | 0.86 to 2.10 | 1.37 | 0.87 to 2.16 | 1.31 | 0.82 to 2.09 |
| Proportion attenuated (%)† | ||||||||||
| 50.70 | 50.70 | 47.89 | 56.34 | |||||||
Model 1: Baseline model+perinatal characteristics (maternal pre-pregnancy body mass index; breastfeeding duration; birth weight; gestation; maternal smoking during pregnancy; alcohol use during pregnancy; wheeze.
Model 2: Model 1+housing conditions (damp; tenure; furry pets; environmental tobacco smoke; access to garden).
Model 3: Model 2+potential exposure to infection (sibling number; childcare type).
Model 4: Model 3+neighbourhood characteristics (volume of traffic; housing quality; rubbish; area rating).
*All models were adjusted for baseline confounders (child sex, maternal age at birth, maternal ethnicity and maternal atopy). Degree plus is the reference category.
†Proportion of RRR of persistent asthma attenuated by comparison of baseline model with models 1–4 for lowest SECs (100×(RRR-adjusted RRR)/(RRR-1))
GCSE, General Certificate of Secondary Education; SEC, socioeconomic circumstances.
We repeated the regression analysis including mediators available at all time points (9 months, 3 and 5 years, (online supplemental table S2) in supplementary material details the availability of potential mediators by wave). Results were similar to that of the main analysis (online supplemental table S6). An additional analysis examining environmental tobacco smoke (ETS) separate from other home environment factors was conducted. After taking into account the attenuation of risk after adjusting for the perinatal block, there was no additional attenuation after adjusting for ETS exposure (online supplemental table S7). We repeated the regression analysis differentiating the persistent asthma group by whether they reported asthma at two or three waves. Results were also similar to that of the main analysis (online supplemental table S8). Sensitivity analysis to explore the association between SECs and current asthma at age 14 years found no association (online supplemental table S9).
Counterfactual mediation analysis results
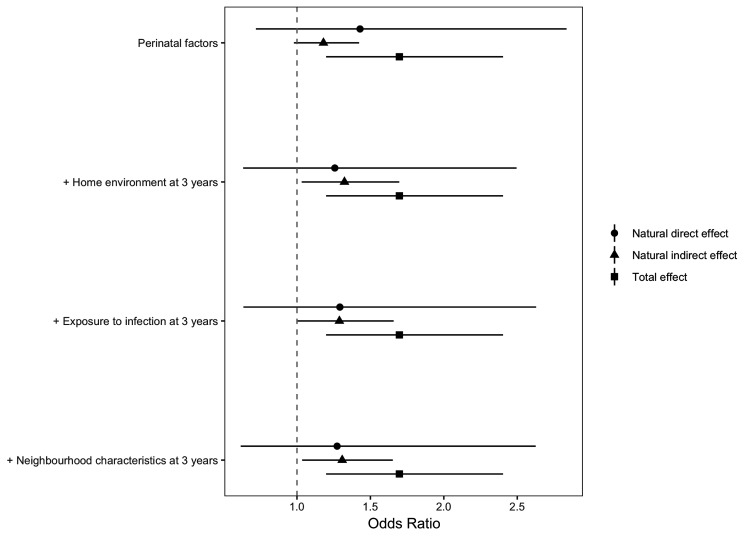
For the second step of the analysis, the counterfactual mediation analysis results are shown in tables 3 and 4 and figure 3. The NDE (OR 1.27, 95% CI 0.62 to 2.63) is the increase in the likelihood of persistent asthma, comparing low-to-high SECs that we would observe if the levels of mediators remained as those for the children in the most advantaged SECs (formulas given in online supplemental material S1). The NIE is the increased likelihood of persistent asthma we would see in the low SEC group if the mediators take on the values they would naturally have if the children had been in the high SEC group (OR 1.31, 95% CI 1.04 to 1.65). The TE, the sum of the NDE and the NIE even in presence of exposure-mediator interaction, had an OR of 1.70 (95% CI 1.20 to 2.40).
Table 3.
Relative risk ratios (RRR) for persistent asthma by relative inequality index adjusted for blocks of mediators (N=7487)
| Baseline model | Model 1 | Model 2 | Model 3 | Model 4 | ||||||
| RRR* | 95% CI | RRR | 95% CI | RRR | 95% CI | RRR | 95% CI | RRR | 95% CI | |
| Transient asthma | ||||||||||
| RII | 1.25 | 0.83 to 1.90 | 1.03 | 0.67 to 1.58 | 1.00 | 0.64 to 1.55 | 0.96 | 0.62 to 1.48 | 0.89 | 0.58 to 1.36 |
| Persistent asthma | ||||||||||
| RII | 1.70 | 1.21 to 2.40 | 1.45 | 1.03 to 2.06 | 1.45 | 1.02 to 2.05 | 1.49 | 1.04 to 2.14 | 1.41 | 0.98 to 2.03 |
Model 1: Baseline model+perinatal characteristics (maternal pre-pregnancy body mass index; breastfeeding duration; birth weight; gestation; maternal smoking during pregnancy; alcohol use during pregnancy; wheeze.
Model 2: Model 1+housing conditions (damp; tenure; furry pets; environmental tobacco smoke; access to garden).
Model 3: Model 2+potential exposure to infection (sibling number; childcare type).
Model 4: Model 3+neighbourhood characteristics (volume of traffic; housing quality; rubbish; area rating).
*All models were adjusted for baseline confounders (child sex, maternal age at birth, maternal ethnicity and maternal atopy).
RII, relative index of inequality.
Table 4.
NDE, NIE, TE and proportion mediated for persistent asthma compared with never by socioeconomic circumstance (N=7487)
| Blocks of mediators | Effects | OR | 95% CI | Proportion mediated % | 95% CI |
| Perinatal characteristics | NDE | 1.43 | 0.72 to 2.84 | 37.5 | 30.6 to 43.3 |
| NIE | 1.18 | 0.98 to 1.42 | |||
| TE | 1.70 | 1.20 to 2.40 | |||
| +Home environment at 3 years | NDE | 1.26 | 0.63 to 2.50 | 61.2 | 56.0 to 65.4 |
| NIE | 1.32 | 1.03 to 1.70 | |||
| TE | 1.70 | 1.20 to 2.40 | |||
| +Exposure to infection at 3 years | NDE | 1.29 | 0.64 to 2.63 | 56.1 | 50.2 to 60.9 |
| NIE | 1.29 | 1.00 to 1.66 | |||
| TE | 1.70 | 1.20 to 2.40 | |||
| +Neighbourhood characteristics at 3 years | NDE | 1.27 | 0.62 to 2.63 | 58.9 | 52.9 to 63.7 |
| NIE | 1.31 | 1.04 to 1.65 | |||
| TE | 1.70 | 1.20 to 2.40 |
NDE, natural direct effect; NIE, natural indirect effect; TE, total effect.
Figure 3.
Mediation analysis with a counterfactual approach by cumulative blocks of mediators (perinatal characteristics, housing conditions, potential exposure to infection and neighbourhood) characteristics in the association between socioeconomic circumstance and persistent asthma.
Findings from the counterfactual mediation analysis show the proportion mediated by each block for persistent asthma compared with never by RII (table 4): Perinatal characteristics alone mediated 37.5% (95% CI 30.6 to 43.3); with the addition of housing conditions 61.2% was mediated (95% CI 56.0 to 65.4); additionally adjusting for potential infection through childcare type and sibling number mediated 56.1% (95% CI 50.2 to 60.9), and after additional adjustment for neighbourhood conditions we found that 58.9% (95% CI 52.9 to 63.7) of the TE of SECs in early-life on the likelihood of persistent asthma in CYP was mediated through collective exposure to all mediating blocks (see table 4).
Discussion
Main findings
The prevalence of persistent asthma in the most disadvantaged British adolescents was 20%, compared with 13% for the most advantaged. Being born into disadvantaged SECs increased the likelihood of developing persistent asthma by 70%. Almost two-thirds (58.9%) of the excess risk attributable to perinatal and environmental exposures by the age of 3 years. Consistent with the Bronfenbrenner model, we found that the proximal environment was somewhat more impactful on persistent asthma risk than the external environment beyond the household, such as potential exposure to infection through childcare type and sibling number and neighbourhood characteristics. In contrast, we did not see a social gradient in transient asthma, which affected 8% of adolescents.
Comparison with other studies
Our findings of a social gradient in persistent asthma are supported by previous studies that reported inequalities in children who have symptoms of severe asthma31–33 and those which lead to hospital admissions.2 For example, Case et al found that disadvantaged children with asthma in the USA were more likely to have symptoms of severe asthma than advantaged counterparts, with the inequality greater for older children.32 Yet, our study is one of the first to assess how early-life risk factors explain the inequality in persistent asthma in adolescence using counterfactual mediation analysis applied to data from birth to adolescence. We have added support to the notion that the perinatal period and initial years of life are a critical period to influence lifelong inequalities in lung development10 11 and explained in part why the asthma epidemic in the UK disproportionately impacts children in disadvantaged SECs.
Previous studies have shown that less favourable perinatal and environmental exposures in early-life, predict later respiratory health.12 14 Our findings add weight to the importance of maternal health even before birth and subsequent perinatal characteristics on later health, including maternal pre-pregnancy body mass index, smoking during pregnancy, preterm birth, birth weight, breast feeding and early wheeze, which accounted for 37.5% of the association between SEC in early-life and persistent asthma by adolescence. Previous studies have shown these characteristics are independent predictors of lung development12 14–16 and in this study, we have formally tested their mediating role in explaining inequalities in persistent asthma. We found that the quality of the home environment is important in the development of inequalities in persistent asthma in adolescence, accounting for a further 23.7%, which supports previous study findings of associations between dampness or ETS and children’s asthma.18
In contrast to studies that have found associations between childcare type,34 sibling number35 and neighbourhood characteristics36 and persistent asthma, additional adjustment of these characteristics did not further mediate the association between SEC in early-life and persistent asthma. This is not to say that more distal environmental conditions are not important in the development of inequalities in persistent asthma, rather that once conditioned on perinatal period characteristics and the home environment there was no additional mediation. This could be because more distal environmental conditions are downstream ancestors in the causal pathway from perinatal characteristics and the home environment.
Strengths and limitations
The significant strengths of our study include the use of data from a large, representative UK cohort, longitudinal follow-up from birth to adolescence and the inclusion of a range of socially patterned risk factors for asthma operating in the early years. A key strength of our analysis is the application of a modern approach to mediation analysis applied to high resolution cohort data to better understand mediating pathways to address health inequalities in asthma. These methods allow us to unpick the mediating pathways linking adverse SECs to outcomes and identify those which appear to be most important. The approach used addresses the limitations of traditional multivariable regression methods for assessing mediation which assumes a linear relationship and no interaction between the exposure and mediator, and, that there is no intermediate confounding. This study is among the first to formally test the mediating role of risk factors of social inequality using counterfactual methods.
However, as with all observational studies, there are several important sources of potential bias to consider. The MCS at baseline had a representative sample of 18 818 children born in the UK between September 2000 and January 2002. Yet, by age 14 years 9199 singleton children had participated in all subsequent waves. To minimise attrition bias, we used response weights to account for the loss of respondents up to age 14 years.37 The attrition weights adjust the sample composition to take account of the selective loss of respondents, for example, low income families who may be less likely to remain in the cohort. More information on the MCS attrition weights can be found on the Centre for Longitudinal Studies website (https://cls.ucl.ac.uk/cls-studies/millennium-cohort-study /). Of the children still in the study at 14 years, 81% had complete data and were included in our analysis. Nevertheless, children with missing data were more likely to be from disadvantaged SECs which may underestimate associations between SECs in early-life and asthma. The MCS oversampled from minority ethnic groups, but our focus was not to understand ethnic health inequalities. In our study sample maternal ethnicity was predominantly white hence we caution against generalising our findings across all ethnic groups and recommend more research to understand ethnic health inequalities in persistent asthma.
Although we used advanced methods for causal mediation analysis and adjusted for a range of potential confounders, the assumption of complete adjustment of confounding is still required for a causal interpretation of our estimates. The total causal effect of SEC in early- life on asthma needs to be interpreted with caution due to likely residual confounding by historic parental SECs. While we partially take genetic predisposition into account in our analysis by adjusting for maternal history of asthma or eczema and ethnicity, we were unable to adjust fully for genetic risk factors for asthma.
Our exposure of SECs in early-life is reported at 9 months, but we assume maternal educational level to be representative of SECs during pregnancy and at birth. We do not expect maternal educational level to have changed much during this period, which is why we can justify looking at mediators during pregnancy. Maternal educational level is linked to general and health-related knowledge, literacy, prestige, earning capacity and self-efficacy. It has been used to measure family SECs in social epidemiological studies,12 38 39 is a more stable measure of SECs than income which may fluctuate over time, and has a greater impact on child health than paternal education.40
The limited data on asthma persistence is a potential area of bias that may underestimate the association between SECs in early-life and persistent asthma into adolescence. We derived a longitudinal asthma variable from parental responses to the standardised ISAAC22 question, ‘Has your child ever had asthma?’ asked at 7 and 11 years, and parental reports of whether their child currently has asthma at 14 years. There was variation between those reporting ever asthma at 7 or 11 years, with almost a third (29%) of those reporting ever asthma at 11 previously reporting no to ever asthma at 7 years. Current asthma at 14 with reported asthma at either 7 or 11 alone did not capture all variations in reporting of persistent asthma between 7 and 14 years, as 10% of those reporting no asthma at 14 years had reported having asthma at 7 or 11 years. Therefore, persistent asthma was defined as any two or more reports of asthma at 7, 11 or 14 years.
As asthma diagnoses are unreliable in early childhood,41 we can be more confident in our outcome of reported asthma diagnosis from 7 years onwards. The prevalence of persistent asthma in our analysis (15%) was higher than that recorded in UK health records for adolescents (8%).3 However, this is in line with previous study findings that parent-reported asthma prevalence is greater than general practitioner (GP) recorded asthma diagnosis.42 Griffiths and colleagues suggest several reasons for why this may arise: parents may not take their child to the GP for asthma symptoms, the GP may record a different diagnosis, or may not record any diagnosis, or parents may report other breathing sounds as asthma. Parent-reported asthma is commonly used in epidemiological studies12 25 31 34 43 and analysis has shown good agreement between parental and offspring asthma reports, making it a useful tool in the absence of GP recorded asthma or objective measurement such as spirometry or peak flow.44
Understanding complex causal pathways is challenging. Mediating blocks are expected to cluster and are difficult to interpret in isolation. We, therefore, took a sequential ‘en-block’ mediation approach and analysed the cumulative effect of blocks, considering proximal to distal mediators.29 Perinatal characteristics were the most influential block of mediators in our analysis, and we can be relatively confident about the proportion mediated through this most proximal block as a whole, while not able to isolate the relative contribution and the temporal sequence of each individual perinatal measure in this block. Furthermore, we can also be confident about the total proportion mediated by all the blocks combined, while the proportion attributable to each block individually is challenging to assess due to the interaction between these pathways.
Some of the measures in mediating blocks are subject to change over early-life. Our data gathered at the age of 3 years provides a snapshot of housing, potential exposure to infection and neighbourhood characteristics. These conditions may change throughout early-life, and potentially more so for disadvantaged families with housing insecurity.45 It is also important to note that the assessment of neighbourhood characteristics is subjective as it includes interviewer observations of volume of traffic, housing quality, littering and area quality on the day of the interview.
The mediating block of potential exposure to infections through childcare type and sibling number does not capture children’s history of infection, rather it is a proxy measure of exposure to children outside the home. Respiratory infections which cause wheeze in early-life are associated with later asthma.46 To partially account for this, we include parental reports of whether children have seen a health professional for wheeze at 9 months in the perinatal mediating block.
Policy implications
Our results support the need for an early prevention strategy to focus on ensuring optimal conditions during pregnancy and childhood to support the healthy development of children and reduce inequalities across the lifecourse. This is currently advocated in Public Health England’s strategy for giving children the best start in life,47 yet, much of the strategy is focused on individual actions and provision of health services for mothers and CYP rather than structural and social determinants of respiratory health.
Through improved understanding of early-life pathways, GPs can better identify children in need of closer monitoring throughout childhood and support families to reduce the risk of persistent asthma into adolescence. However, while quality healthcare is crucial for CYP with asthma, our findings demonstrate the importance of acting to reduce child poverty.
Interventions need to mitigate the effects of disadvantageous SEC in early-life by acting on perinatal and home environment mediating pathways. For the perinatal pathway, this should be through support for smoking cessation in pregnancy and robust systems to support breast feeding delivered through midwifery, children’s centres and ensuring all hospitals are accredited with UNICEF’s baby friendly initiative.48 For the home environment pathway, this should be through improving housing conditions in disadvantaged areas and education on the impacts of damp and ETS on respiratory health.
In conclusion, we found that perinatal characteristics and the home environment in early life are more important in explaining socioeconomic inequalities in persistent asthma in British adolescents than more distal environmental exposures outside the home.
Footnotes
Twitter: @HannaCreese
Contributors: HC, SSax, DT-R, KM and DKS conceived and designed this study. HC prepared the data, wrote and researched the manuscript with critical revisions from all other authors. HC and EL carried out statistical analysis. Specialist input on paediatric respiratory medicine was provided by SSag. All authors contributed to the development of the analysis, interpreting data and preparing the manuscript. HC is the corresponding author and guarantor for the article.
Funding: This project is funded by the National Institute for Health Research (NIHR) School for Public Health Research (SPHR) (Grant Reference Number PD-SPH-2015). SSax is also funded by the NIHR Northwest London Applied Research Collaboration (ARC). The School for Public Health Imperial College London is also grateful for support from the Imperial NIHR Biomedical Research Centre. The NIHR School for Public Health Research is a partnership between the Universities of Sheffield; Bristol; Cambridge; Imperial; and University College London; The London School for Hygiene and Tropical Medicine (LSHTM); LiLaC—a collaboration between the Universities of Liverpool and Lancaster; and Fuse—The Centre for Translational Research in Public Health a collaboration between Newcastle, Durham, Northumbria, Sunderland and Teesside Universities.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available in a public, open access repository. All data relevant to the study are included in the article or uploaded as supplementary information. Millennium Cohort Study data are freely available form the CLS website https://cls.ucl.ac.uk/cls-studies/millennium-cohort-study/
Ethics statements
Patient consent for publication
Not applicable.
References
- 1. Kossarova L CR, Hargreaves D, Keeble E. Admissions of inequality: emergency Hospital use for children and young people. Nuffield Trust, 2017. [Google Scholar]
- 2. Gupta RP, Mukherjee M, Sheikh A, et al. Persistent variations in national asthma mortality, hospital admissions and prevalence by socioeconomic status and region in England. Thorax 2018;73:706–12. 10.1136/thoraxjnl-2017-210714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bloom CI, Saglani S, Feary J, et al. Changing prevalence of current asthma and inhaled corticosteroid treatment in the UK: population-based cohort 2006-2016. Eur Respir J 2019;53:1802130. 10.1183/13993003.02130-2018 [DOI] [PubMed] [Google Scholar]
- 4. RCPCH . State of Child Health 2017 - full report, 2017. Available: https://www.rcpch.ac.uk/sites/default/files/2018-09/soch_2017_uk_web_updated_11.09.18.pdf
- 5. Royal College of Physicians (RCP) . Why asthma still kills: national review of asthma deaths (NRAD) confidential enquiry report; 2014. https://www.rcplondon.ac.uk/projects/outputs/why-asthma-still-kills [Accessed 7 Jul 2020].
- 6. Chung HL. Asthma in childhood: a complex, heterogeneous disease. Korean J Pediatr 2011;54:1–5. 10.3345/kjp.2011.54.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Subbarao P, Mandhane PJ, Sears MR. Asthma: epidemiology, etiology and risk factors. CMAJ 2009;181:E181–90. 10.1503/cmaj.080612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bloom CI, Franklin C, Bush A, et al. Burden of preschool wheeze and progression to asthma in the UK: population-based cohort 2007 to 2017. J Allergy Clin Immunol 2021;147:1949–58. 10.1016/j.jaci.2020.12.643 [DOI] [PubMed] [Google Scholar]
- 9. Trivedi M, Denton E. Asthma in children and Adults-What are the differences and what can they tell us about asthma? Front Pediatr 2019;7:256. 10.3389/fped.2019.00256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bui DS, Lodge CJ, Burgess JA, et al. Childhood predictors of lung function trajectories and future COPD risk: a prospective cohort study from the first to the sixth decade of life. Lancet Respir Med 2018;6:535–44. 10.1016/S2213-2600(18)30100-0 [DOI] [PubMed] [Google Scholar]
- 11. Postma DS, Bush A, van den Berge M. Risk factors and early origins of chronic obstructive pulmonary disease. The Lancet 2015;385:899–909. 10.1016/S0140-6736(14)60446-3 [DOI] [PubMed] [Google Scholar]
- 12. Taylor-Robinson DC, Pearce A, Whitehead M, et al. Social inequalities in wheezing in children: findings from the UK millennium cohort study. Eur Respir J 2016;47:818–28. 10.1183/13993003.01117-2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rona RJ. Asthma and poverty. Thorax 2000;55:239–44. 10.1136/thorax.55.3.239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zacharasiewicz A. Maternal smoking in pregnancy and its influence on childhood asthma. ERJ Open Res 2016;2:00042-2016–2016. 10.1183/23120541.00042-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Xu X-F, Li Y-J, Sheng Y-J, et al. Effect of low birth weight on childhood asthma: a meta-analysis. BMC Pediatr 2014;14:275. 10.1186/1471-2431-14-275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sonnenschein-van der Voort AMM, Arends LR, de Jongste JC, et al. Preterm birth, infant weight gain, and childhood asthma risk: a meta-analysis of 147,000 European children. J Allergy Clin Immunol 2014;133:1317–29. 10.1016/j.jaci.2013.12.1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dogaru CM, Nyffenegger D, Pescatore AM, et al. Breastfeeding and childhood asthma: systematic review and meta-analysis. Am J Epidemiol 2014;179:1153–67. 10.1093/aje/kwu072 [DOI] [PubMed] [Google Scholar]
- 18. Mendell MJ, Mirer AG, Cheung K, et al. Respiratory and allergic health effects of dampness, mold, and dampness-related agents: a review of the epidemiologic evidence. Environ Health Perspect 2011;119:748–56. 10.1289/ehp.1002410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gillespie-Bennett J, Pierse N, Wickens K, et al. The respiratory health effects of nitrogen dioxide in children with asthma. Eur Respir J 2011;38:303–9. 10.1183/09031936.00115409 [DOI] [PubMed] [Google Scholar]
- 20. Bronfenbrenner U. Making human beings human : bioecological perspectives on human development / Urie Bronfenbrenner, editor. Thousand Oaks, Calif, 2005. [Google Scholar]
- 21. Centre for Longitudinal Studies . Millennium cohort study: a guide to the datasets: first, second, third, fourth and fifth surveys. 8th ed. London: Institute of Education, 2014. [Google Scholar]
- 22. Asher MI, Keil U, Anderson HR, et al. International study of asthma and allergies in childhood (Isaac): rationale and methods. Eur Respir J 1995;8:483–91. 10.1183/09031936.95.08030483 [DOI] [PubMed] [Google Scholar]
- 23. Regidor E. Measures of health inequalities: Part 2. J Epidemiol Community Health 2004;58:900–3. 10.1136/jech.2004.023036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Galobardes B, Lynch JW, Davey Smith G. Childhood socioeconomic circumstances and cause-specific mortality in adulthood: systematic review and interpretation. Epidemiol Rev 2004;26:7–21. 10.1093/epirev/mxh008 [DOI] [PubMed] [Google Scholar]
- 25. Propper CR. Understanding socio-economic inequalities in childhood respiratory health, C.f.A.o.S. exclusion, editor. London: School of Economics and Political Science, 2006. [Google Scholar]
- 26. Sullivan K, Thakur N. Structural and social determinants of health in asthma in developed economies: a scoping review of literature published between 2014 and 2019. Curr Allergy Asthma Rep 2020;20:5. 10.1007/s11882-020-0899-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Williams DR, Sternthal M, Wright RJ. Social determinants: taking the social context of asthma seriously. Pediatrics 2009;123 Suppl 3:S174–84. 10.1542/peds.2008-2233H [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. VanderWeele TJ. Principles of confounder selection. Eur J Epidemiol 2019;34:211–9. 10.1007/s10654-019-00494-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Loeys T, Loeys T, Moerkerke B, et al. medflex : An R Package for Flexible Mediation Analysis using Natural Effect Models. J Stat Softw 2017;76. 10.18637/jss.v076.i11 [DOI] [Google Scholar]
- 30. Vansteelandt S, Bekaert M, Lange T. Imputation strategies for the estimation of natural direct and indirect effects. Epidemiol Method 2012;1:131–58. 10.1515/2161-962X.1014 [DOI] [PubMed] [Google Scholar]
- 31. Sherriff A, Peters TJ, Henderson J, et al. Risk factor associations with wheezing patterns in children followed longitudinally from birth to 3(1/2) years. Int J Epidemiol 2001;30:1473–84. 10.1093/ije/30.6.1473 [DOI] [PubMed] [Google Scholar]
- 32. Case A, Lubotsky D, Paxson C. Economic status and health in childhood: the origins of the gradient. Am Econ Rev 2002;92:1308–34. 10.1257/000282802762024520 [DOI] [PubMed] [Google Scholar]
- 33. Cumella A, Haque A. On the edge: how inequality affects people with asthma, 2018. [Google Scholar]
- 34. Svanes C, Jarvis D, Chinn S, et al. Early exposure to children in family and day care as related to adult asthma and hay fever: results from the European community respiratory health survey. Thorax 2002;57:945–50. 10.1136/thorax.57.11.945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Karmaus W, Botezan C. Does a higher number of siblings protect against the development of allergy and asthma? A review. J Epidemiol Community Health 2002;56:209–17. 10.1136/jech.56.3.209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Feng X, Astell-Burt T. Is neighborhood green space protective against associations between child asthma, neighborhood traffic volume and perceived lack of area safety? multilevel analysis of 4447 Australian children. Int J Environ Res Public Health 2017;14. 10.3390/ijerph14050543. [Epub ahead of print: 19 05 2017]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Institute of Education, Fitzsimons E, ed. Millennium cohort study: sixth survey 2015-2016 user guide (first edition. London: Centre for Longitudinal Studies, 2017. [Google Scholar]
- 38. Straatmann VS, Lai ETC, Taylor-Robinson DC. How do adverse childhood experiences explain social inequalities in adolescent health outcomes? findings from the UK millennium cohort study. The Lancet 2018;392: :S83. 10.1016/S0140-6736(18)32093-2 [DOI] [Google Scholar]
- 39. Straatmann VS, Lai E, Lange T, et al. How do early-life factors explain social inequalities in adolescent mental health? findings from the UK millennium cohort study. J Epidemiol Community Health 2019;73): :1049–60. 10.1136/jech-2019-212367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lundborg P, Nilsson A, Rooth D-O. Parental education and offspring outcomes: evidence from the Swedish compulsory school reform. Am Econ J Appl Econ 2014;6:253–78. 10.1257/app.6.1.253 [DOI] [Google Scholar]
- 41. Moral L, Vizmanos G, Torres-Borrego J, et al. Asthma diagnosis in infants and preschool children: a systematic review of clinical guidelines. Allergol Immunopathol 2019;47:107–21. 10.1016/j.aller.2018.05.002 [DOI] [PubMed] [Google Scholar]
- 42. Griffiths LJ, Lyons RA, Bandyopadhyay A, et al. Childhood asthma prevalence: cross-sectional record linkage study comparing parent-reported wheeze with general practitioner-recorded asthma diagnoses from primary care electronic health records in Wales. BMJ Open Respir Res 2018;5:e000260. 10.1136/bmjresp-2017-000260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Panico L, Bartley M, Marmot M, et al. Ethnic variation in childhood asthma and wheezing illnesses: findings from the millennium cohort study. Int J Epidemiol 2007;36:1093–102. 10.1093/ije/dym089 [DOI] [PubMed] [Google Scholar]
- 44. Kuiper IN, Svanes C, Benediktsdottir B, et al. Agreement in reporting of asthma by parents or offspring - the RHINESSA generation study. BMC Pulm Med 2018;18:122. 10.1186/s12890-018-0687-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. The Children’s Society . Moving, always moving: the normalisation of housing insecurity among children in low income households in England. University of Bath, 2020. [Google Scholar]
- 46. Koponen P, Helminen M, Paassilta M, et al. Preschool asthma after bronchiolitis in infancy. Eur Respir J 2012;39:76–80. 10.1183/09031936.00040211 [DOI] [PubMed] [Google Scholar]
- 47. Public Health England . Health matters: giving every child the best start in life, 2016. [Google Scholar]
- 48. Unicef . About baby friendly, 2019. Available: https://www.unicef.org.uk/babyfriendly/about/ [Accessed 14 Dec 2020].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
thoraxjnl-2021-217312supp001.pdf (293.7KB, pdf)
Data Availability Statement
Data are available in a public, open access repository. All data relevant to the study are included in the article or uploaded as supplementary information. Millennium Cohort Study data are freely available form the CLS website https://cls.ucl.ac.uk/cls-studies/millennium-cohort-study/