Abstract
The ability to selectively disrupt gene function remains a critical element in elucidating information regarding gene essentiality for bacterial growth and/or pathogenesis. In this study, we adapted a tet regulatory expression system for use in Staphylococcus aureus, with the goal of downregulating gene expression via induction of antisense RNA. We demonstrate that this system exhibits a 50- to 100-fold dose-dependent level of induction in bacterial cells grown in culture (i.e., in vitro) and also functions in mice (i.e., in vivo) following oral administration of inducer. To determine whether induced antisense RNA could interfere with chromosomally derived gene expression, we cloned a fragment of the S. aureus alpha-toxin gene (hla) in antisense orientation downstream of the tet promoter system and introduced the construct into S. aureus. Induced antisense hla RNA downregulated chromosomally derived hla gene expression in vitro approximately 14-fold. Similarly, induction of hla antisense RNA in vivo dramatically reduced alpha-toxin expression in two different murine models of S. aureus infection. Most importantly, this reduction completely eliminated the lethality of the infection. These results indicate that the tet regulatory system functions efficiently in S. aureus and induced antisense RNA can effectively downregulate chromosomal gene expression both in vitro and in vivo.
Selective disruption and/or downregulation of gene expression is an important tool for elucidating information on gene essentiality for bacterial growth or pathogenesis. This information is particularly useful for validating appropriate molecular targets for antibiotic discovery. A variety of techniques have been developed in various bacterial systems to achieve functional inactivation of gene products (4, 5, 10, 13). Most of these involve gene knockout methods using point mutation, insertional inactivation, or deletion (e.g. allelic replacement).
A possible refinement over gene knockout technology has been the introduction of controlled gene expression systems that allow particular genes to be regulated and thereby functionally analyzed (7, 23). Information is gained by switching off or on gene expression and monitoring the effect as the product titrates down or up. These systems have the potential to provide more quantitative data on the functional importance of a gene product to either growth or virulence. These regulated systems are best described for Escherichia coli and, more recently, Bacillus subtilis (7). Moreover, they are now beginning to be developed for other pathogens (23).
Another effective means of reducing gene expression has been the use of antisense technology. Antisense methods have been used effectively to downregulate eukaryotic gene expression in a variety of systems (1, 3, 6, 19). These methods have not been routinely adapted for prokaryotes despite the fact that antisense regulation has been shown to be a natural phenomenon in bacteria (25). This may be because of the other options available for bacterial systems. However, recent reports have demonstrated that antisense approaches using various synthetic oligomers can be used effectively in bacteria (8, 14, 22, 26).
The gram-positive bacterium Staphylococcus aureus represents a serious human health threat, causing such varied infections as skin abscesses, osteomyelitis, endocarditis, septicemia, and pneumonia. There exist a variety of genetic tools and approaches for generating and analyzing null mutants in S. aureus (4, 24). However, a facile system for selectively controlling and downregulating specific staphylococcal gene products for analysis both in vitro and in vivo is lacking. The ability to titrate down a gene product either under culture conditions or in an animal model of infection would provide a powerful, additional approach for studying gene essentiality and pathogenesis in this organism.
In this study, we adapted a regulated expression system for use in S. aureus. We show its utility in regulating the expression of an antisense construct of the staphylococcal alpha-toxin gene (hla) and, in turn, regulating the production of the alpha-toxin itself. Most importantly, we demonstrate that induced expression of antisense hla RNA is achieved both in in vitro culture and in two murine models of staphylococcal infection, and downregulation of the toxin reverses its lethal phenotype in an intraperitoneal infection.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The plasmids used in this study were pUC19 (27), pE194 (11), pMH109 (12), and pWH353 (kindly provided by W. Hillen, Institut für Mikrobiologie und Biochemie, Erlangen, Germany). The bacterial strains used in this study were S. aureus RN4220, a derivative of S. aureus 8325-4 that is able to accept transformed DNA (18), and S. aureus WCUH29, a virulent alpha-toxin-producing clinical isolate. S. aureus strains were cultured in tryptic soy broth (TSB; BBL, Sparks, Md.) or TSB plus 1.5% Bacto Agar (TSA; Difco, Detroit, Mich.). To maintain selection of plasmid pYJ90, S. aureus was grown in culture medium containing erythromycin (Erm; 5 μg/ml). Escherichia coli DH5-α, used for construction of shuttle vectors, was grown in Luria-Bertani (LB) broth (BBL) containing chloramphenicol (Cam; 20 μg/ml), Erm (300 μg/ml), or ampicillin (Ap; 100 μg/ml) as appropriate.
Construction of E. coli-S. aureus shuttle vector pYJ90.
To construct a suitable shuttle vector for this study, plasmids pUC19 carrying an Apr marker (27) and pE194 carrying an Ermr marker (11) were each digested with NdeI, gel purified, ligated together, and transformed into E. coli DH5-α by electroporation. Transformants were selected on LB agar containing Ap (100 μg/ml) and Erm (300 μg/ml). Transformants were examined by restriction analysis to identify an appropriate shuttle plasmid containing both pUC19 and pE194 fragments. One recombinant, pYJ90, was confirmed by further restriction enzyme digestion. pYJ90 was then electroporated into S. aureus RN4220 (15, 18). Transformants were selected on TSA containing Erm (5 μg/ml). The stability of plasmid pYJ90 in S. aureus was determined by passaging a culture six times in medium with antibiotics and analyzing plasmid DNA in the bacterial culture.
Construction of a tet regulatory system in plasmid pYJ90.
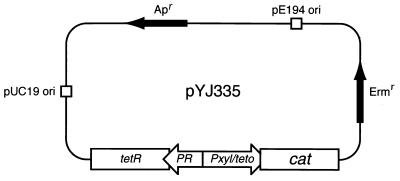
The ClaI-HindIII fragment containing the tetR gene (which encodes the tet repressor), its promoter (PR), and the strong xyl/tet promoter-operator fusion (Pxyl/tetO) was excised from plasmid pWH353 (7) and cloned between the ClaI and HindIII sites in plasmid pBluescript II KS (Stratagene, La Jolla, Calif.). The resulting plasmid, pYJ101, was digested with EcoRI and PstI and an EcoRI-PstI fragment containing a promoterless cat (chloramphenicol acetyltransferase [CAT]-encoding) gene followed by a transcriptional terminator (derived from pMH109 (12), and adapted with the appropriate EcoRI and PstI restriction sites by first moving it into the EcoRI and BamHI sites of pUC19), was inserted. This new construct was named pYJ103. pYJ103 was digested with SalI, and the fragment containing the tetR/PR/Pxyl/tetO-cat region was gel purified and cloned into the SalI site pYJ90. The resulting plasmid, pYJ335, was confirmed by restriction enzyme digestion and DNA sequencing and then electroporated into S. aureus RN4220. One of the transformants, YJSB335, was confirmed and used to make phage lysates by using S. aureus phage φ11 (2).
Construction of plasmid pYJ335 containing antisense hla and sense hla.
A 621-bp hla fragment carrying 354 bp of N-terminal coding region of the hla gene and 268 bp 5′ to this region was generated by PCR amplification using primers hlaFor64 (5′ GGGGGGCCCGGGTATGTCTTTTCCTTGTTTCA 3′) and hlaRev684 (5′GGGGGGCCCGGGATCAGGTAGTTGCAACTG 3′), corresponding to nucleotides 64 to 83 and 684 to 701, respectively (9). Boldface nucleotides correspond to the SmaI restriction enzyme recognition site, and underlined nucleotides correspond to the hla coding sequence. The amplified hla fragment contains the hla promoter region. The PCR product was digested with SmaI, gel purified, and ligated into the EcoRV site of downstream of the xyl/tetO promoter-operator fusion of pYJ335. The orientation of the hla insertion was determined by PCR. As expected, only recombinants containing the hla fragment in the antisense orientation yielded a PCR product of approximately 800 bp, using primers tetRFor1399 and hlaFor64. In contrast, only recombinants containing the hla fragment in the sense orientation produced a PCR product of approximately 800 bp, using primers tetRFor1399 and hlaRev684 (data not shown). The resulting plasmids, pYJ318-7 and pYJ318-16, which contained hla in the antisense and sense orientations, respectively, were electroporated separately into S. aureus. Transformants were selected by Erm resistance, and plasmids were confirmed by restriction enzyme digestion. Transformants YJSB318-7 and YJSB318-16 containing antisense hla and sense hla, respectively, were confirmed and used to make φ11 phage lysates.
S. aureus transductions.
The clinical isolate WCUH29 cannot be transformed directly by electroporation. Therefore, plasmids pYJ335, pYJ318-7, and pJY318-16 were introduced into this strain by phage transduction (2). Phage φ11 was used to make phage lysates by infecting S. aureus YJSB335, YJSB318-7, and YJSB318-16 grown in top agar (TSB containing 0.7% agar and 5 mM CaCl2). The phage lysates were sterilized by passing each through a 0.45-μm-pore-size filter and titered on S. aureus RN4220. Transductions were performed by incubating 5 × 109 CFU of WCUH29 cells with 100 μl of phage lysate (109 to 1010 PFU) and 5 mM CaCl2 at 37°C for 30 min. One milliliter of ice-cold 20 mM sodium citrate was then added to the above mixture to block phage adsorption. The bacterial cells were centrifuged and resuspended in 500 μl of 20 mM sodium citrate. Transductants were selected on TSB-agar containing sodium citrate (500 μg/ml) and Erm (5 μg/ml), and transductants YJ335, YJ318-7, and YJ318-16, containing plasmids pYJ335, pYJ318-7, and pYJ318-16, respectively, were confirmed by restriction enzyme digestion.
PCR, RT-PCR, and DNA sequencing techniques.
The 621-bp hla fragment was generated by PCR using hla-specific primers. The antisense hla and sense hla orientations in plasmids pYJ318-7 and pYJ318-16, respectively, were confirmed by PCR using the plasmid-specific primer tetRFor1399 (5′ CAATACATTGTAGGCTGC 3′), corresponding to nucleotides 1399 to 1416 and hla-specific primers hlaRev684 and hlaFor64. The reaction conditions for all PCRs were 0.2 mM deoxynucleoside triphosphates, 2.5 mM MgCl2, 50 pmol of each primer, 1 ng of template DNA, and 2.5 U of Taq polymerase in buffer supplied by the manufacturer (Gibco-BRL, Rockville, Md.). For the antisense hla and sense hla orientations, the primers were tetRFor1399 plus hlaFor64 and tetRFor1399 plus hlaRev684, respectively, using the same annealing temperature of 48°C. For reverse transcription (RT)-PCR analysis, bacterial RNA was isolated from infected tissue samples by using FastRNA reagents (Bio 101, Vista, Calif.) and treated with RNase-free DNase I (GeneHunter Corporation, Nashville, Tenn.) to remove DNA. Single-stranded cDNA was synthesized by incubating DNase-treated RNA with reverse transcriptase in reaction buffer containing random hexamer primers supplied by the manufacturer (Gibco-BRL). After RNase H treatment, cDNA was used as the template for PCR using the tetR-cat-specific primers tetRFor1399 and catRev768 (5′ GGCAGGTTAGTGACATTAG 3′) and the hla gene-specific primers hlaFor64 and hlaRev684. DNA sequencing was performed to further confirm the tet regulatory elements in pYJ335 and the antisense hla and sense hla orientations in pYJ318-7 and pYJ318-16, respectively.
Specific CAT activity assays.
CAT activity was determined spectrophotometrically as described by Shaw (20), using kinetic SoFTmax PRO II software (Molecular Devices Corporation, Sunnyvale, Calif.) to monitor activity. Briefly, S. aureus YJ335 was grown with shaking in TSB-Erm at 37°C to an A600 of 0.25. The culture was divided, and different doses (0, 2.5, 25, 250, 500, and 1,000 ng/ml) of tetracycline (Tc) were added to the cultures. Two milliliters was removed from each culture 3 h after the addition of Tc for the dose-dependent assay or after 0, 1, 2, 3, and 4 h following the addition of Tc for the time course assay. The bacterial cells were harvested by centrifugation and washed once with 25 mM Tris (pH 7.8)–10 mM EDTA buffer. Crude protein extracts were prepared by centrifugation after the bacterial cells had been suspended in 200 μl of the same buffer containing lysostaphin (0.2 mg/ml; Sigma, St. Louis, Mo.) and incubated at 37°C for 10 min. The total protein concentration was determined by using the Bio-Rad protein microassay (Bio-Rad Laboratories, Hercules, Calif.). Specific CAT activity was calculated as the number of units of CAT activity per milligram of total protein. Experiments were performed in triplicate at least twice, and similar results were obtained.
Northern blot analysis.
S. aureus YJ318-7 and YJ318-16 were grown in TSB-Erm to an A600 of 0.25 with and without Tc (250 ng/ml), and total RNA was extracted by using a Qiagen RNeasy mini protocol kit (Qiagen, Incorporated, Chatsworth, Calif.). Ten-microgram aliquots of total RNA from YJ318-7 and YJ318-16 grown in the presence and absence of Tc (250 ng/ml) were separated by electrophoresis on a 1.2% agarose–1.8% formaldehyde gel and blotted onto a nylon membrane (Boehringer Mannheim Biochemicals, Indianapolis, Ind.). RNA was cross-linked to the membrane by UV irradiation by using a UV Stratalinker (Stratagene). Blots were prehybridized and then hybridized with digoxigenin (DIG)-labeled single-stranded DNA oligonucleotides in high-sodium dodecyl sulfate (SDS) buffer (Boehringer Mannheim Biochemicals) at 50°C for 6 h. Single-stranded DNA oligonucleotides specific hybridization with either sense hla RNA (5′ GGCCAGGCTAAACCACTTTTGTTAGCACCTTCTTCGCTATAAACTCTATA 3′) or antisense hla RNA (5′ TATAGAGTTTATAGCGAAGAAGGTGCTAACAAAAGTGGTTTAGCCTGGCC 3′) were labeled by 3′ tailing DIG-dUTP (Boehringer Mannheim Biochemicals), and 100 pmol of each was used to probe the membranes. The DIG-DNA-RNA hybridization on a nylon membrane was detected with DIG luminescent detection reagents (Boehringer Mannheim Biochemicals) and exposed to X-ray film.
Western blot analysis.
For preparation of extracellular protein, Tc was added to 10-ml cultures of S. aureus WCUH29, YJ318-7, and YJ318-16 to a final concentration of 250 ng/ml and incubated with shaking at 37°C for 8 h. Supernatants were collected after centrifugation, transferred into tubes containing an equal volume of ethanol, and incubated overnight at 4°C. Extracellular proteins were precipitated by centrifugation at 15,000 × g at 4°C for 30 min. SDS-polyacrylamide gel electrophoresis (PAGE) and Western blotting methods were performed as previously described (16). Equal amounts of protein were loaded into each lane of an SDS–12.5% polyacrylamide gel. The primary antiserum used to detect alpha-toxin was provided by M. Burnham, SmithKline Beecham Pharmaceuticals. Standard alpha-toxin and anti-rabbit antibody-alkaline phosphatase conjugate were from Sigma. Western blots were scanned by using Eagle Eye-II software (Stratagene) to quantitate protein bands.
Murine hematogenous pyelonephritis and intraperitoneal infection models.
CD-1 female mice (25 g) obtained from Charles River Laboratories were used for in vivo testing. S. aureus WCUH29, YJ335, YJ318-7, and YJ318-16 were harvested from 1 ml of stationary-phase culture, washed once with 1 ml of phosphate-buffered saline (PBS), and diluted to an A600 of 0.2. These bacterial suspensions were diluted and plated onto TSB-agar plates for determination of viable CFU. A total of six mice per group were infected with approximately 107 CFU of bacteria via an intravenous injection of 0.2 ml of bacterial suspension into the tail vein via a tuberculin syringe. Sublethal doses of Tc (0.5 μg/g) and Erm (5 ng/g) to maintain plasmids were given orally (0.1 ml/mouse) to infected mice on days 1, 2, and 3 after infection. The mice were sacrificed by carbon dioxide overdose 2 h after the last dose of Tc, and kidneys were aseptically removed. The kidneys from three mice in each group were embedded in O.C.T. compound (Tissue-Tek, Torrance, Calif.), frozen in liquid nitrogen, and kept at −80°C for immunostaining. The kidneys from the remaining three mice were cut in half; one half was snap-frozen in cryovials in liquid nitrogen for RT-PCR analysis, and the other half was homogenized in 1 ml of PBS for enumeration of viable bacteria.
For murine intraperitoneal infection, female CD1 mice (10 in each experimental group) were infected as previously described (14). Briefly, 18-h cultures of S. aureus (about 3 × 109 CFU/ml) were collected by centrifugation at 3,000 × g for 10 min, washed three times with PBS, and resuspended in PBS, and 0.5-ml aliquots of the bacterial solutions were injected intraperitoneally. Viable counts were determined by plating dilutions of cultures on TSB-agar Erm plates.
Immunohistochemistry.
Sections of 8 μm were cut from the frozen embedded kidney tissue in O.C.T. compound, mounted onto microslides (selected, precleaned Superfrost Plus; VWR Scientific, West Chester, Pa.), and fixed with cold methanol. The fixed tissue sections were washed in PBS and incubated in 5% dry milk–PBS. The tissue sections were then incubated with specific rabbit anti-alpha-toxin serum at room temperature for 1 h, washed with 0.1% goat serum–PBS, and incubated with biotinylated donkey anti-rabbit antibodies (1:200; Amersham Life Science, Piscataway, N.J.) for 1 h at room temperature. The tissue sections were washed in PBS, processed with Vectastain ABC reagents (Vector Laboratories, Burlingame, Calif.), and washed in PBS again, after which the peroxidase substrate 3,3′-diaminobenzidine (Sigma Fast; Sigma) was added. The sections were washed with tap water, dehydrated, and mounted with coverslips.
RESULTS
Adaptation of the tet regulatory system to S. aureus.
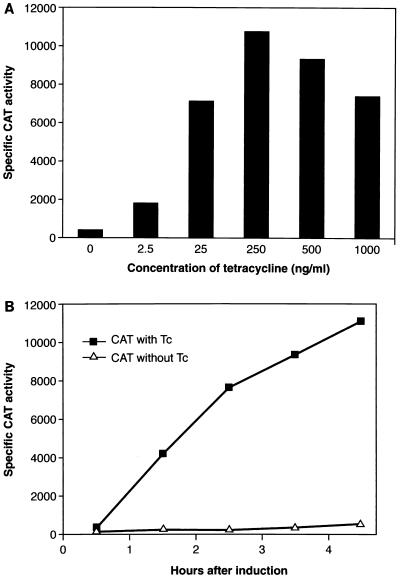
To assess both the function and regulation of the xyl/tet promoter-operator control system, we fused it to a cat gene reporter construct on an E. coli-S. aureus plasmid shuttle vector (Fig. 1) and transferred it into S. aureus (See Materials and Methods for details). The resulting transformant, YJ335, was used to examine the expression of CAT activity in response to Tc induction. The results are shown in Fig. 2A. In the absence of Tc, YJ335 showed low basal levels of CAT activity. In contrast, upon addition of Tc to the culture medium, CAT expression was induced efficiently in a dose-dependent manner, with maximal activity seen 3 h after induction with 250 ng of Tc per ml. Higher Tc doses resulted in decreased levels of CAT expression, presumably due to the effects of the antibiotic on bacterial growth. Our results demonstrate that a strong, dose-dependent induction response can be achieved with this system in S. aureus.
FIG. 1.
Tc-inducible shuttle vector pYJ335 (see Materials and Methods for details). pUC19 ori and pE194 ori, origins of replication from pUC19 and pE194, respectively, allowing plasmid replication in E. coli and S. aureus hosts. A unique EcoRV site is positioned downstream from the xyl/tet promoter and 22 nucleotides upstream of the translation start point of the cat gene.
FIG. 2.
Dependence of CAT activity on Tc concentration (A) and kinetics of Tc induction in S. aureus (B). (A) S. aureus YJ335 was incubated in TSB with 5 ng of Erm per ml to early log phase, and different doses of Tc were added to aliquoted cultures; 2 ml of each culture was transferred into a new tube, and the cells were harvested by centrifugation 3 h after the addition of Tc. (B) Strain YJ335 was incubated to early log phase in TSB, and 250 ng of Tc per ml was added to the culture; 2-ml aliquots of culture were collected 0, 1, 2, 3, and 4 h after addition of Tc. Crude protein preparations were used to analyze CAT activity. Specific CAT activity is defined as units of CAT activity per milligram of total protein.
We also examined the time dependence of CAT expression at 250 ng of Tc per ml. The results (Fig. 2B) indicate that in the absence of Tc, low basal expression was maintained over a 3- to 4-h period. In the presence of Tc, CAT activity increased steadily and achieved a 50- to 100-fold induction between 1 and 4 h. Apparently the tet regulatory system can be used effectively in S. aureus to regulate expression of a gene placed under its control.
Induced antisense RNA downregulates gene expression in vitro.
Next, we wanted to use this plasmid based tet regulation system in S. aureus to induce a specific antisense transcript and measure its effect on the expression of the cognate gene located on the staphylococcal chromosome. We chose for this analysis a 621-bp DNA fragment containing a portion of the staphylococcal alpha-toxin gene (hla). This gene was chosen because it is a known virulence factor (2, 14) and reagents were readily available for monitoring its expression. The hla fragment was inserted in each of its orientations (sense and antisense) downstream from the tet-regulated promoter (Fig. 1). The two plasmids were introduced separately into S. aureus, thereby creating the isogenic transformants YJ318-7 (antisense) and YJ318-16 (sense). These strains were used to characterize the production of the hla fragment transcript in response to Tc and to examine the effects of this induction on alpha-toxin gene expression driven from the normal hla chromosomal locus.
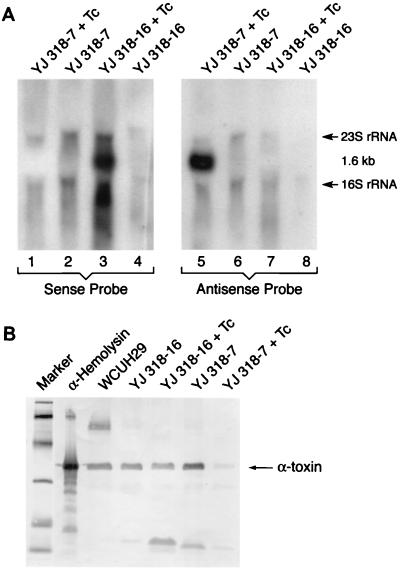
First, we examined, by Northern blot analysis, RNA production from both the sense (YJ318-16) and antisense (YJ318-7) constructs before and after induction. The results (Fig. 3A) show that YJ318-7 selectively produced a major antisense hla RNA product in response to Tc induction (lane 5). Antisense RNA was not detected in the absence of induction (lane 6), nor was any sense RNA produced with or without Tc (lanes 1 and 2). Correspondingly, the control sense construct, YJ318-16, produced sense hla fragment transcript only in response to Tc (lane 3). Again, it appears that the plasmid-based regulation system operated effectively in S. aureus.
FIG. 3.
Northern blot analysis of sense hla and antisense hla transcription (A). DIG-labeled single-stranded DNA oligonucleotide probes hybridized specifically with either sense hla RNA (sense probe) or antisense hla RNA (antisense probe). Hybridization of the 1.6-kb RNA represents the hla-cat cotranscripts. Western blot analysis of alpha-toxin expressed in strain WCUH29 and its isogenic strains with or without Tc induction (B). Lanes: 1, molecular weight markers (biotinylated low-molecular-weight SDS-PAGE standards; Bio-Rad) 2, alpha-hemolysin (Sigma) as positive standard control; 3, proteins from WCUH29 culture supernatant; 4, proteins from YJ318-16 culture supernatant without induction; 5, proteins from YJ318-16 culture supernatant with Tc induction; 6, proteins from YJ318-7 culture supernatant without induction; 7, proteins from YJ318-7 culture supernatant with Tc induction.
Second, we examined the effect of these induced hla RNA segments on the expression of endogenous hla gene product. The culture supernatant of YJ318-7 without Tc induction reacted strongly with anti-alpha-toxin antisera (Fig. 3B), while YJ318-7, following Tc induction, demonstrated a 14-fold-lower reactivity as measured by densitometer scanning. In addition, the amount of alpha-toxin in the culture supernatant of YJ318-16, with or without induction, was similar to that of wild-type WCUH29. These results demonstrate the ability of this tet regulatory system to efficiently inhibit gene expression in S. aureus.
tet-regulated transcription during infection.
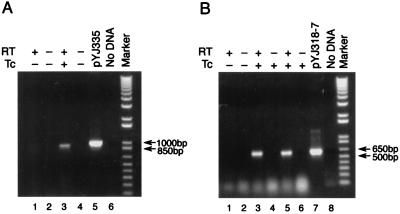
We used a standard murine renal infection model (21) to examine Tc-induced transcription from the tet regulatory system in vivo. S. aureus YJ335 carrying CAT expression vector pYJ335 (Fig. 1) was injected (107 CFU) into the mouse tail vein (see Materials and Methods for details). One day after infection, mice were either not treated or given Tc orally once a day for 3 days. On day 3, the kidneys were removed, and the total RNA was prepared and analyzed by RT-PCR using CAT gene-specific primers. The results (shown in Fig. 4A) demonstrate the selective expression of CAT RNA in the infected mouse kidney (lane 3) in response to Tc induction. No PCR signal was detected in the absence of the RT reaction (lane 4), which demonstrates the lack of any plasmid DNA contamination in the RNA sample. Moreover, no signal was detected in the absence of Tc induction (lanes 1 and 2), thereby confirming that Tc-induced expression of CAT transcript was achieved in vivo.
FIG. 4.
RT-PCR analysis of transcription of cat (A, lane 3) and antisense hla (B, lanes 3 and 5) following in vivo induction with sublethal doses of Tc. The expected sizes of the RT-PCR products are 1 kb for cat and 620 bp for hla. Plasmid DNA template was used as a positive control for the expected PCR product (pYJ335 and pYJ318-7). Negative controls were samples prepared without RT or template DNA. Marker is the 1-Kb Plus DNA ladder from Gibco-BRL.
We repeated this experiment in the same infection model using S. aureus YJ318-7 carrying the antisense construct of hla. The results (Fig. 4B) again demonstrate selective RT-PCR detection of the antisense hla transcript in the infected mouse kidney only in response to Tc induction (lanes 3 and 5).
Induced antisense RNA downregulates gene expression in vivo.
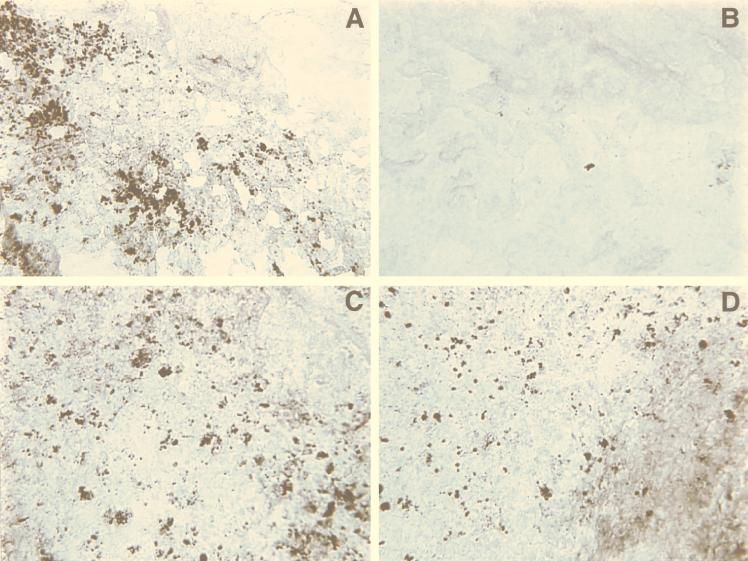
To examine directly the consequences of antisense hla RNA induction in the murine kidney infection model, we monitored the expression of alpha-toxin in infected kidney tissue by using immunohistochemical staining (Fig. 5). As a control, we used wild-type S. aureus carrying the sense fragment hla expression vector, YJ318-16. Kidneys from mice infected with YJ318-16 in the presence and in the absence of Tc induction (Fig. 5C and D, respectively) exhibited strong hla staining, essentially identical to a wild-type staphylococcal infection without plasmid (data not shown). Similarly, kidneys infected with S. aureus carrying antisense hla construct YJ318-7, in the absence of induction, also showed very strong hla staining (Fig. 5A). In contrast, kidneys infected with YJ318-7 following induction of antisense hla RNA showed little to no staining (Fig. 5B). These results clearly indicate that tet regulation of the plasmid vector system functions effectively in vivo and significantly downregulates chromosomally derived hla gene expression.
FIG. 5.
Immunohistochemical detection of expression of alpha-toxin after in vivo induction of antisense hla RNA with sublethal doses of Tc. Sections of the kidneys were from mice infected with YJ318-7 in the absence (A) and presence (B) of Tc induction. Sections of the kidneys were from mice infected with YJ318-16 in the absence (C) and presence (D) of Tc induction. Magnification, ×400.
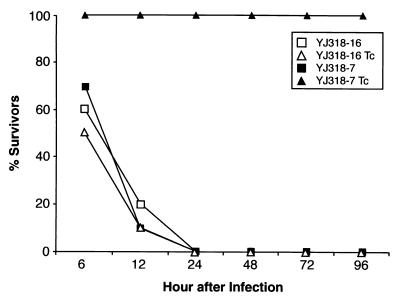
To confirm and extend these results, we examined the effect of antisense hla RNA induction in a second standard mouse model of acute infection. In this case, the mice are infected by the intraperitoneal route (14), which results in death within 12 to 24 h. The results (Fig. 6) indicate that all animals infected with the control carrying the hla sense fragment construct, YJ318-16, died within 24 h of inoculation whether or not they were given the Tc inducer. Similarly, all mice infected with S. aureus carrying the antisense construct, YJ318-7, in the absence of Tc inducer died. In marked contrast, all 10 mice infected with YJ318-7 and subject to oral Tc induction survived throughout the 6-day experiment. Clearly, Tc induction of antisense hla RNA dramatically attenuates the virulence and lethal phenotype of the S. aureus infection.
FIG. 6.
Survival of mice infected intraperitoneally with strains YJ318-7 (1.4 × 109 CFU) and YJ318-16 (1.5 × 109 CFU) followed by oral induction with sublethal doses of Tc (YJ318-7 Tc and YJ318-16 Tc) or in the absence of Tc induction (YJ318-7 and YJ318-16).
DISCUSSION
We have adapted the tet-regulated gene expression system originally developed for controlling expression of heterologous genes in B. subtilis (7) for use in a pathogenic strain of S. aureus. The results demonstrate that the xyl/tet hybrid promoter is functional in S. aureus and can increase expression of a reporter gene (i.e., cat) 50- to 100-fold. The extent of induction is similar to that seen in B. subtilis (7) and, more recently, in S. pneumoniae with an optimized plasmid-based promoter (23). Moreover, we show that in S. aureus, the induction is titrated in a dose-dependent manner, thereby allowing the system to be used for evaluating the level of expression required to achieve gene function and bacterial viability. However, we point out that the plasmid-based system we describe does exhibit some basal expression (albeit low [Fig. 2]) in the absence of induction, and this must be considered when interpreting any titration data obtained.
Because of the strong transcriptional induction achieved with the system, we examined its utility for producing antisense RNA in an efficient, regulated manner in order to selectively downregulate expression of specific chromosomal loci. We used an antisense fragment of the staphylococcal alpha-toxin gene and demonstrated that the plasmid-based tet regulatory system can effectively produce an antisense transcript in response to induction. Importantly, this induction of antisense RNA was shown to effectively inhibit (∼14-fold) hla expression in vitro. Thus, antisense regulation could be achieved in S. aureus and, coupled with a regulated promoter, provides a valuable tool for studying the effects of disrupting gene function.
We were particularly interested in extending the utility of our system to the in vivo analyses of staphylococcal gene function. The ability to examine the effects of downregulating specific bacterial genes in various animal infection models provides tremendous new scope for studying genes that may be essential for growth or virulence during infection. Our data for the murine pyelonephritis model demonstrate that hla expression (i.e., the production of staphylococcal alpha-toxin) can be regulated effectively via antisense RNA production. Direct examination of the infected mouse kidney shows a dramatic reduction in bacterial foci producing alpha-toxin. The few stained foci remaining (Fig. 5B) probably result from plasmid loss during the course of the infection study.
In the murine acute intraperitoneal infection model, we demonstrated that induction of antisense hla RNA abolished the lethal phenotype caused by expression of the toxin. Others have shown alpha-toxin to be a virulence factor in experimental infections such as peritonitis (17) and endocarditis (2). Our data support the importance of this gene product as a pathogene and provide a clear demonstration of the utility of regulated antisense production in vivo for examining virulence factors. By varying the time at which the antisense RNA is induced (i.e., varying the time of oral Tc administration), this methodology should allow examination of the temporal relevance of specific gene products to the early establishment, maintenance, and postsymptomatic periods of the infection process. Thus, the system described should enable a detailed examination of the function of any staphylococcal gene throughout the infection process.
ACKNOWLEDGMENTS
We thank Martin Burnham and Damien McDevitt for many helpful discussions and suggestions, Martin Burnham for the gift of anti-alpha-toxin antiserum, and Wolfgang Hillen for the generous gift of plasmid pWH353. We also thank the SmithKline Beecham Microbiology sequencing group and Microbiology pathogenesis group for skillful technical assistance.
This work was funded by the U.S. Defense Advanced Research Projects Agency (N65236-97-1-5810).
REFERENCES
- 1.Agrawal S, Jiang Z, Zhao Q, Shaw D, Cai Q, Roskey A, Channavajjala L, Saxinger C, Zhang R. Mixed-backbone oligonucleotides as second generation antisense oligonucleotides: in vitro and in vivo studies. Proc Natl Acad Sci USA. 1997;94:2620–2625. doi: 10.1073/pnas.94.6.2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bayerer A S, Ramos M D, Menzies B E, Yeaman M R, Shen A J, Cheung A L. Hyperproduction of alpha-toxin by Staphylococcus aureus results in paradoxically reduced virulence in experimental endocarditis a host defense role for platelet microbicidal proteins. Infect Immun. 1997;65:4652–4660. doi: 10.1128/iai.65.11.4652-4660.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beauregard M C, Pringault E, Robine S, Louvard D. Suppression of villin expression by antisense RNA impairs brush border assembly in polarized epithelial intestinal cells. EMBO J. 1995;14:409–421. doi: 10.1002/j.1460-2075.1995.tb07017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheung A L, Wolz C, Yeaman M R, Bayer A S. Insertional inactivation of a chromosomal locus that modulates expression of potential virulence determinants in Staphylococcus aureus. J Bacteriol. 1995;177:3220–3226. doi: 10.1128/jb.177.11.3220-3226.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Lorenzo V, Timms R N. Analysis and construction of stable phenotypes in gram-negative bacteria with Tn5- and Tn10-derived minitransposons. Methods Enzymol. 1994;235:386–405. doi: 10.1016/0076-6879(94)35157-0. [DOI] [PubMed] [Google Scholar]
- 6.Fire A, Xu S, Montgomery M K, Kostas S A, Driver S E, Mello C C. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–810. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 7.Geissendorfer M, Hillen W. Regulated expression of heterologous genes in Bacillus subtilis using the Tn10 encoded tet regulatory elements. Appl Microbiol Biotechnol. 1990;33:657–663. doi: 10.1007/BF00604933. [DOI] [PubMed] [Google Scholar]
- 8.Good L, Nielsen P E. Antisense inhibition of gene expression in bacteria by PNA targeted to mRNA. Nat Biotechnol. 1998;16:355–358. doi: 10.1038/nbt0498-355. [DOI] [PubMed] [Google Scholar]
- 9.Gray G S, Kehoe M. Primary sequence of the alpha-toxin gene from Staphylococcus aureus Wood 46. Infect Immun. 1984;46:615–618. doi: 10.1128/iai.46.2.615-618.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hensel M J E, Shea, Gleeson C, Jones M D, Dalton E, Holden D W. Simultaneous identification of bacterial virulence genes by negative selection. Science. 1995;269:400–403. doi: 10.1126/science.7618105. [DOI] [PubMed] [Google Scholar]
- 11.Horinouchi S, Weisblum B. Nucleotide sequence and functional map of pE194, a plasmid that specifics inducible resistance to macrolide, lincosamide and streptogramin type B antibiotics. J Bacteriol. 1982;150:804–814. doi: 10.1128/jb.150.2.804-814.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hudson M C, Stewart G C. Differential utilization of Staphylococus aureus promoter sequences by Escherichia coli and Bacillus subtilis. Gene. 1986;48:93–100. doi: 10.1016/0378-1119(86)90355-0. [DOI] [PubMed] [Google Scholar]
- 13.Ji Y L, Mclandsborough, Kondagunta A, Cleary P P. C5a peptidase alters clearance and trafficking of group A streptococci by infected mice. Infect Immun. 1996;64:503–510. doi: 10.1128/iai.64.2.503-510.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kernodle D S, Voladri R K R, Menzies B E, Hager C C, Edwards K M. Expression of an antisense hla fragment in Staphylococcus aureus reduces alpha-toxin production in vitro and attenuates lethal activity in murine model. Infect Immun. 1997;65:179–184. doi: 10.1128/iai.65.1.179-184.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kraemer G R, Iacmdolo J J. High-frequency transformation of Staphylococcus aureus by electroporation. Curr Microbiol. 1990;21:373–376. [Google Scholar]
- 16.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 17.Menzies B E, Kernodle D S. Site-directed mutagenesis of the alpha-toxin gene of Staphylococcus aureus: role of histidines in toxin activity in vitro and in a murine model. Infect Immun. 1994;62:1843–1847. doi: 10.1128/iai.62.5.1843-1847.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Novick R P. The staphylococcus as a molecular genetic system. In: Novick R P, editor. Molecular biology of the staphylococci. New York, N.Y: VCH Publishers; 1990. pp. 1–40. [Google Scholar]
- 19.Ottavio L, Sthandier O, Ricci L, Passananti C, Amati P. Constitutive synthesis of polyoma antisense RNA renders cells immune to virus infection. Virology. 1992;189:812–816. doi: 10.1016/0042-6822(92)90613-t. [DOI] [PubMed] [Google Scholar]
- 20.Shaw W V. Chloramphenicol acetyltransferase from chloramphenicol resistant bacteria. Methods Enzymol. 1975;43:737–755. doi: 10.1016/0076-6879(75)43141-x. [DOI] [PubMed] [Google Scholar]
- 21.Skerrett S J, Schmidt R A, Martin T R. Impaired clearance of aerosolized Legionella pneumophila in corticosteroid-treated rats: a model of legionnaires’ disease in the compromised host. J Infect Dis. 1989;160:261–273. doi: 10.1093/infdis/160.2.261. [DOI] [PubMed] [Google Scholar]
- 22.Soderbom F, Wagner E G H. Degradation pathway of CopA, the antisense RNA that controls replication of plasmid R1. Microbiology. 1998;144:1907–1917. doi: 10.1099/00221287-144-7-1907. [DOI] [PubMed] [Google Scholar]
- 23.Stieger M, Wohlgensinger B, Kamber M, Lutz R, Keck W. Integrational plasmids for the tetracycline-regulated expression of genes in Streptococcus pneumoniae. Gene. 1999;226:243–251. doi: 10.1016/s0378-1119(98)00561-7. [DOI] [PubMed] [Google Scholar]
- 24.von Eiff C, Heilmann C, Proctor R A, Woltz C, Petters G, Gotz F. A site-directed Staphylococcus aureus hemB mutant is a small-colony variant which persists intracellularly. J Bacteriol. 1997;179:4706–4712. doi: 10.1128/jb.179.15.4706-4712.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wagner E G H, Simons R W. Antisense RNA control in bacteria, phages, and plasmids. Annu Rev Microbiol. 1994;48:717–742. doi: 10.1146/annurev.mi.48.100194.003433. [DOI] [PubMed] [Google Scholar]
- 26.Wilson T, deLisle G W, Marcinkeviciene J A, Blanchard J S, Collins D M. Antisense RNA to ahpC, an oxidative stress defence gene involved in isoniazid resistance indicates that AhpC of Mycobacterium bovis has virulence properties. Microbiology. 1998;144:2687–2695. doi: 10.1099/00221287-144-10-2687. [DOI] [PubMed] [Google Scholar]
- 27.Yanisch-Perron C, Vieira J, Messing J. Improved m13 phage cloning vectors and host strains: nucleotide sequences of the m13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]