Abstract
In October 2021, the European Commission asked EFSA to provide scientific and technical assistance in accordance with Article 21(2) of Regulation (EC) No 1107/2009 and to deliver a statement on the information submitted by the applicant taking into consideration the assessment of the rapporteur Member State (RMS) in the context of the review of the approval of the active substance ipconazole. The current statement contains a summary of the main findings of the assessment of the risks posed to birds from use of ipconazole and whether the requirements regarding negligible exposure for humans (dietary and non‐dietary exposure) set out in point 3.6.4 of Annex II to Regulation (EC) No 1107/2009 may be considered satisfied. The identified concerns are presented.
Keywords: ipconazole, peer review, risk assessment, pesticide, fungicide, seed treatment, granivorous birds
Summary
Ipconazole has been deemed to be approved on 1 September 2014 under Regulation (EC) No 1107/2009 by Commission Implementing Regulation (EU) No 571/2014, in accordance with Commission Implementing Regulation (EU) No 540/2011, as amended by Commission Implementing Regulation (EU) No 541/2011. It was a specific provision of the approval that the applicant was required to submit to the European Commission further studies on the acceptability of the long‐term risk to granivorous birds, and on the acceptability of the risk to soil macro‐organisms by 31 August 2016.
In accordance with the specific provision, the applicants, Kureha GmbH Germany and UPL (formerly Arysta LifeScience Great Britain Limited), submitted an updated dossier in August 2016, which was evaluated by the designated rapporteur Member State (RMS), the United Kingdom, in the form of an addendum to the draft assessment report. In compliance with guidance document SANCO 5634/2009‐rev.6.1, the RMS distributed the addendum to Member States, the applicant and EFSA for comments on 28 February 2017. The RMS collated all comments in the format of a reporting table, which was submitted to EFSA on 23 May 2017. EFSA added its scientific views on the specific points raised during the commenting phase in column 4 of the reporting table and published a Technical Report in July 2017 in which it concluded that based on the confirmatory information provided by the applicants, the risk to soil macroorganisms was low. However, EFSA concluded that the information ‘did not resolve the long‐term risk to granivorous birds’.
Furthermore, on 9 March 2018, the Committee for Risk Assessment (RAC) of the European Chemicals Agency adopted an Opinion in which it concluded that ipconazole meets the criteria to be classified as toxic for reproduction, category 1B (R1B) and this classification has been included in Annex VI to Regulation (EC) No 1272/2008. Given the classification as R1B and that a risk to granivorous birds can still not be excluded, it appears that ipconazole may no longer satisfy the approval criteria in Article 4 of Regulation (EC) No 1107/2009. Therefore, pursuant to Article 21(1) of Regulation (EC) No 1107/2009, the Commission informed the applicants about the concerns and invited them to provide comments or information. The applicant submitted further information in November 2020, which has been evaluated by the designated rapporteur Member State (RMS) after Brexit, Belgium, and submitted to the Commission and EFSA on 21 October 2021. In its assessment, the RMS took into account a study on dermal absorption submitted by the applicant in the context of an application for authorisation of a plant protection product. In order to ensure that the assessment is complete and takes into account all relevant information, EFSA should also take that study into account.
In accordance with Article 21(2) of Regulation (EC) No 1107/2009, EFSA was requested in October 2021 (amended request in November 2021) to provide scientific and technical assistance and to deliver a statement on the information submitted by the applicant, taking into consideration the assessment of the RMS within 3 months from the receipt of this mandate. Specifically, EFSA should consider the risks posed to birds from the representative uses of ipconazole on wheat and barley and whether the requirements regarding negligible exposure for humans (dietary and non‐dietary exposure) set out in point 3.6.4 of Annex II to Regulation (EC) No 1107/2009 may be considered satisfied.
For the assessment of whether exposure of humans to ipconazole, under realistic conditions of use, can be considered negligible, and taking into account the absence of a final guidance document and ongoing discussions in the Standing Committee on Plants, Animals, Food and Feed (PAFF Committee), the draft guidance document made available for stakeholder consultation and published on Commissions’ website on 25 June 2015 should be considered (draft dated May 2015; SANCO/2014/12096 (European Commission, 2000, 2015)). In the absence of agreed threshold values for assessing negligible exposure, a conclusion regarding such agreed threshold is not possible. However, in order to provide risk managers with the relevant information for decision‐making, EFSA was requested to (a) calculate the actual expected exposure values in absolute values and percentage of the established toxicological reference values (e.g. acceptable operator exposure level (AOEL)); (b) consider potential technical mitigation measures to reduce exposure as those mentioned in the draft guidance, that have been proposed by the applicant and/or by the RMS, or by EFSA, if and when appropriate.
As for the dietary exposure, in the absence of pertinent new residue data, the conclusions of previous EFSA evaluation in the framework of the peer review regarding seed‐treated cereals still apply. For the representative uses, residue concentrations of the parent compound ipconazole in grain and straw of wheat and barley, in potential succeeding crops and in food items of animal origin were determined by data or can be reasonably expected to be less than 0.01 mg/kg. In a consumer dietary risk assessment for parent ipconazole using PRIMo rev. 3.1, chronic dietary exposure was < 1% of the acceptable daily intake (ADI) for ipconazole and the maximum acute dietary exposure was 1% of the ARfD for ipconazole.
For the non‐dietary exposure of operators, workers, residents and bystanders, the assessment has included the newly available field studies performed with the formulation Rancona 450 FS (and not with the representative formulation Rancona 15ME). Other limitations in the studies in addition to the use of a non‐representative formulation trigger some uncertainties with non‐quantifiable impact on the results. For operators, based on the submitted data, the total systemic exposure is predicted to be less than 5% of the (A)AOEL for an operator wearing normal workwear, gloves and FFP2 mask (with a margin of exposure higher than 1,000). ■■■■■
■■■■■
1. Introduction
Ipconazole has been deemed to be approved on 1 September 2014 under Regulation (EC) No 1107/2009 1 by Commission Implementing Regulation (EU) No 571/2014 2 , in accordance with Commission Implementing Regulation (EU) No 540/2011 3 , as amended by Commission Implementing Regulation (EU) No 541/2011 4 . It was a specific provision of the approval that the applicant was required to submit to the European Commission confirmatory information on:
the acceptability of the long‐term risk to granivorous birds;
the acceptability of the risk to soil macro‐organisms;
the risk of enantioselective metabolisation or degradation;
the potential endocrine disrupting properties of ipconazole for birds and fish.
The information under (a) and (b) was required by 31 August 2016, the information under (c) within 2 years after adoption of the pertinent guidance document on evaluation of isomer mixtures and the information under (d) within 2 years after the adoption of the OECD test guidelines on endocrine disruption or, alternatively, of test guidelines agreed at EU level.
In accordance with the specific provision, the applicants, Kureha GmbH Germany and UPL (formerly Arysta LifeScience Great Britain Limited), submitted information to address points (a) and (b) with an updated dossier in August 2016, which was evaluated by the designated rapporteur Member State (RMS), the United Kingdom, in the form of an addendum to the draft assessment report (United Kingdom, 2017). In compliance with guidance document SANCO 5634/2009‐rev.6.1, the RMS distributed the addendum to Member States, the applicant and EFSA for comments on 28 February 2017. The RMS collated all comments in the format of a reporting table, which was submitted to EFSA on 23 May 2017. EFSA added its scientific views on the specific points raised during the commenting phase in column 4 of the reporting table and published a Technical Report in July 2017 (EFSA, 2017) in which it concluded that based on the confirmatory information provided by the applicant the risk to soil macroorganisms was low. However, EFSA concluded that the information ‘did not resolve the long‐term risk to granivorous birds’.
Furthermore, on 9 March 2018, the Committee for Risk Assessment (RAC) of the European Chemicals Agency adopted an Opinion 5 in which it concluded that ipconazole meets the criteria to be classified as toxic for reproduction, category 1B (R1B) and this classification has been included in Annex VI to Regulation (EC) No 1272/2008 6 . Given the harmonised classification as R1B and that a risk to granivorous birds can still not be excluded, it appears that ipconazole may no longer satisfy the approval criteria in Article 4 of Regulation (EC) No 1107/2009. Therefore, pursuant to Article 21(1) of Regulation (EC) No 1107/2009, the European Commission informed the applicant about the concerns and invited it to provide comments or information. The applicant submitted further information in November 2020, which has been evaluated by the designated rapporteur Member State (RMS) after Brexit, Belgium, and submitted to the Commission and EFSA on 21 October 2021. On 26 October 2021, EFSA was requested by the European Commission to provide scientific and technical assistance and to deliver a statement on the information submitted by the applicant, taking into consideration the assessment of the RMS within 3 months from the receipt of this mandate. In its assessment the RMS took into account a study on dermal absorption submitted by the applicant in the context of an application for authorisation of a plant protection product. In order to ensure that the assessment is complete and takes into account all relevant information, the European Commission sent on 18 November 2021 an amended mandate to EFSA to also take that study into account.
Specifically, EFSA was requested to consider the risks posed to birds from the representative uses of ipconazole on wheat and barley and whether the requirements regarding negligible exposure for humans (dietary and non‐dietary exposure) set out in point 3.6.4 of Annex II to Regulation (EC) No 1107/2009 may be considered satisfied.
For the assessment of whether exposure of humans to ipconazole, under realistic conditions of use, can be considered negligible, and taking into account the absence of a final guidance document and on‐going discussions in the Standing Committee on Plants, Animals, Food and Feed (PAFF Committee), the draft guidance document made available for stakeholder consultation and published on Commissions’ website on 25 June 2015 should be considered (draft dated May 2015; SANCO/2014/12096 (European Commission, 2000, 2015)). In the absence of agreed threshold values for assessing negligible exposure, a conclusion regarding such agreed threshold is not possible. However, in order to provide risk managers with the relevant information for decision making, EFSA was requested to (a) calculate the actual expected exposure values in absolute values and percentage of the established toxicological reference values (e.g. acceptable operator exposure level (AOEL)); (b) consider potential technical mitigation measures to reduce exposure as those mentioned in the draft guidance, that have been proposed by the applicant and/or by the RMS, or by EFSA, if and when appropriate.
Based on that mandate, EFSA prepared a draft statement in December 2021 which was circulated to all Member States for commenting via a written procedure. A key supporting document to this statement is the peer review report (EFSA, 2022), which contains the comments received on the draft statement, in which all views including minority views, where applicable, can be found.
Given the importance of the peer review report, this document is considered as background document to this statement and thus is made publicly available.
1.1. Terms of Reference as provided by the requestor
EFSA was mandated in accordance with Article 21(2) of Regulation (EC) No 1107/2009 by the European Commission on 26 October 2021 (amended mandate received on 18 November 2021) to provide scientific and technical assistance and to deliver a statement on the information submitted by the applicant, taking into consideration the assessment of the RMS within 3 months from the receipt of this mandate. Specifically, EFSA was requested to consider the risks posed to birds from use of ipconazole and whether the requirements regarding negligible exposure for humans (dietary and non‐dietary exposure) set out in point 3.6.4 of Annex II to Regulation (EC) No 1107/2009 may be considered satisfied.
For the assessment of whether exposure of humans to ipconazole, under realistic conditions of use, can be considered negligible, and taking into account the absence of a final guidance document and on‐going discussions in the Standing Committee on Plants, Animals, Food and Feed (PAFF Committee), the draft guidance document made available for stakeholder consultation and published on Commissions’ website on 25 June 2015 should be considered (draft dated May 2015; SANCO/2014/12096 (European Commission, 2000, 2015)). In the absence of agreed threshold values for assessing negligible exposure, a conclusion regarding such agreed threshold is not possible. However, in order to provide risk managers with the relevant information for decision‐making, EFSA was requested:
To calculate the actual expected exposure values in absolute values and percentage of the established toxicological reference values (e.g. acceptable operator exposure level (AOEL));
To consider potential technical mitigation measures to reduce exposure as those mentioned in the draft guidance, that have been proposed by the applicant and/or by the RMS, or by EFSA, if and when appropriate.
The Applicant has been requested by the European Commission and submitted to EFSA and all Member States the information it provided to the Commission and the RMS in November 2020.
2. Assessment
2.1. Negligible exposure assessment
For the active substance ipconazole, the assessment of negligible exposure has been triggered by its harmonised classification as Reproductive toxicant category 1B (May damage the unborn child) according to Regulation (EC) No 1272/2008. Under the peer review assessment for approval (EFSA, 2013a), the representative formulated product was ‘Rancona 15ME’, a micro‐emulsion (ME) containing 15 g/L ipconazole, and the representative uses were as a fungicide for seed treatment of wheat and barley. Additional uses do not fall within the scope of the current mandate and could therefore not be considered in the current statement by EFSA.
As agreed during the peer review (EFSA, 2013a), the acceptable daily intake (ADI) is 0.015 mg/kg bw per day based on the 1‐year dog study and applying an uncertainty factor (UF) of 100. The acute reference dose (ARfD) and the acceptable operator exposure level (AOEL) are both 0.015 mg/kg bw per day, based on the rat developmental toxicity study (with a critical developmental no observed adverse effect level (NOAEL) of 3 mg/kg bw per day) and applying an increased UF of 200, justified by the need to have a sufficient margin of safety between the reference value and the dose level where the teratogenic effect was observed. In the absence of derivation of an acute AOEL (AAOEL), the ARfD will be used for the acute non‐dietary risk assessment.
With regard to the dermal absorption, a value of 5% was agreed for both the concentrate and the dilution of the representative formulation Rancona 15ME. Taking into account that the criteria for the use of dermal absorption data obtained with similar formulations (EFSA PPR Panel, 2017) are not fulfilled, the results of the newly submitted study with the formulation Rancona 450FS cannot be used for ‘Rancona 15ME’.
An operator exposure study (Kureha Corporation, 2021) and a worker and bystander exposure (Kureha Corporation, 2021) study were submitted. Analytical methods used in the exposure studies were validated in accordance with the relevant guidance.
2.1.1. Dietary
The following draft guidance document was used for this assessment: European Commission (2000, 2015).
The representative uses in wheat and barley as a seed treatment evaluated during the peer review of ipconazole (EFSA, 2013a) were considered for the consumer dietary risk assessment. EFSA has recently also assessed the existing maximum residue levels for ipconazole according to Article 12 of Regulation (EC) No 396/2005 (EFSA, 2020), including further uses in different cereal crops. In the addendum on confirmatory data (Belgium, 2021), the RMS referred to the Art. 12 reasoned opinion; however, additional uses do not fall within the scope of the current mandate and could therefore not be considered in this statement by EFSA.
For the current mandate, the applicant did not provide any new data in the Residues section. Therefore, the conclusions of the previous EFSA evaluation in the framework of the peer review (EFSA, 2013a) regarding seed‐treated cereals also apply to the current mandate and can be recapped as follows:
When applied as a seed treatment in wheat, ipconazole was extensively metabolised. Ipconazole was not identified in wheat grain. The major components in wheat grain were TA, representing 56–57% TRR, and TAA, representing 25–32% TRR. Triazolyl pyruvate was a minor residue (< 3% TRR).
Ipconazole also did not dominate the residue pattern in wheat forage (6–26% TRR), hay (6–13% TRR) and straw (3–28% TRR). Hydroxylated metabolites of ipconazole (free and conjugated) and triazole derivative metabolites (TDMs) were always present at similar or higher proportions of the TRR compared to ipconazole and varied depending on the investigated radiolabel and the growth stage of the crop. The highest proportions of TRR of individual metabolites were predominantly reported for wheat straw: OH‐IPC‐glycoside 45%, tert‐OH‐isopropyl‐IPC 38% (both for the 14C‐benzyl methylene label); TAA 43% TRR (14C‐triazolyl label). Merely TA and triazolyl pyruvate reached their highest proportions in wheat forage (33% and 17% TRR, respectively).
Considering the application rates and the quantitative findings in the wheat metabolism study with seed treatment, and the application rates of the representative uses, residues of individual metabolites that could reach or exceed concentrations of 0.01 mg/kg in grain or straw would only be expected for TDMs, notably for TA and TAA. Several residue trials in wheat and barley with analysis of grain and straw confirmed this premise. Quantifiable residues of parent ipconazole at or above 0.01 mg/kg were only present in the whole plant at earlier growth stages. However, these findings in the immature plant were not considered relevant for the assessment of the representative uses in the residues section since only good agricultural practices (GAPs) for grain production were requested and none for forage production.
Investigation of residues in rotational crops (carrot, wheat and lettuce) indicated preferential uptake from soil of the triazole ring‐containing metabolites with an observed increase of uptake at each successive plant back interval. TAA and TA were the predominant residues, ipconazole was not detected.
The residue definition for risk assessment previously derived (EFSA, 2013a, 2020) is still applicable to the representative uses under assessment:
(1) Ipconazole and, separately, (2) Triazole alanine (TA) and triazole lactic acid (TLA), (3) Triazole acetic acid (TAA), (4) 1,2,4‐triazole. Parts (2)–(4) of the definition apply in accordance with the harmonised residue definition for triazole pesticide active substances (EFSA, 2018); yet part (4) can be neglected in the assessment of the ipconazole uses as 1,2,4‐triazole 7 was not detected in the available studies.
Separate and different toxicological reference values apply to the components of the above residue definition for risk assessment parts (1)–(3) due to differences in the toxicological profiles of these chemical compounds, with only ipconazole being classified as Reproductive toxicant category 1B according to Regulation (EC) No 1272/2008, and therefore considerations on whether the provisions of negligible exposure are met were only made for ipconazole.
Based on the qualitative and quantitative information on primary and rotational crops to support the representative uses, significant livestock exposure to ipconazole and carry over of ipconazole‐derived residues in animal matrices is not expected.
As for the assessment if the provisions of negligible exposure according to Regulation (EC) 1107/2009 are met, considering the draft technical guidance on assessment of negligible exposure (European Commission, 2000, 2015), the following can be concluded: For the representative uses of ipconazole, residue concentrations of the active substance ipconazole in grain and straw of wheat and barley, in potential succeeding crops and in food items of animal origin were determined by data or can be reasonably expected to be less than 0.01 mg/kg. In a consumer dietary risk assessment for parent ipconazole using PRIMo rev.3.1 (Appendix B), chronic dietary exposure was less than 1% of the ADI for ipconazole and the maximum acute dietary exposure was 1% of the ARfD (wheat, UK, 4–6 years) for ipconazole. Consumer exposure to ipconazole via drinking water is also unlikely for the representative uses, based on the peer‐reviewed groundwater exposure assessments (EFSA, 2013a).
2.1.2. Non‐dietary exposure (NDE)
The following guidance documents were used for this assessment: European Commission (2000, 2015), EFSA (2014), EFSA PPR Panel (2017), OECD (1997).
The supported uses of the product ‘Rancona 15ME’, as evaluated during the peer review of ipconazole (EFSA, 2013a), were considered for the NDE risk assessment. The critical representative use is the application of the undiluted product on barley seeds at a seed loading of 2.0 g ipconazole/100 kg of seeds, and at a sowing rate of 350 kg seed/ha equivalent to 7.0 g ipconazole/ha.
It should be noted that the field studies submitted for this mandate, and assessed by the RMS (Belgium, 2021), were performed with another formulation ‘Rancona 450 FS’ and for another use (see Tables 1 and 7 in Annex). In the following sections, EFSA has provided additional considerations/calculations to align the assessment of these field studies with the critical representative use of ‘Rancona 15ME’.
2.1.2.1. Operator exposure
Operator exposure estimates for the representative uses were already assessed during the peer review of ipconazole using the SeedTropex model. For the current mandate, the applicant provided a new operator exposure study (Kureha Corporation, 2021).
For this study, individual operator exposure estimates are presented due to the low number of operators involved (7). The amounts of ipconazole handled were 594 g in the morning shift (7234.5 kg seed bagged) and 747 g (9100 kg seed bagged) in the afternoon shift. It can be assumed that a static treatment plant with a low level of automation achieves a throughput of 75 tonnes of seed per day (EFSA, 2013a). Although more automated plants would normally achieve higher amount of treated seeds/day and involve the handling of greater amounts of product, levels of exposure during bagging (the task which contributes most to overall exposure together with the equipment cleaning task) would be lower than those estimated for a static treatment plant with a low level of automation. Based on the critical representative use of ‘Rancona 15ME’, the amount of ipconazole that should be handled by the operator is calculated to be 1.5 kg/day (based on a seed loading of 20 g ipconazole/tonne of seed and a throughput of 75 tonnes seed/day) which is significantly higher than the amounts of ipconazole handled by the operators in the study (594 and 747 g). To reflect these uncertainties and in line with the critical representative use (max 1.5 kg ipconazole handled per day), the obtained exposure results for individual operators were scaled up by a factor of 2.53 (morning shift operators) and 2.01 (afternoon shift operators). Scaled exposure results are presented in Table 1 below. The RMS also acknowledged the limitations of the operator exposure study and agreed with the proposed scaling.
■■■■■.
■■■■■
| ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ |
|---|---|---|---|---|---|---|---|
| ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ |
| ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | |||
| ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | |||
| ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | |||
| ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | |||
| ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | |||
| ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | |||
| ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | |||
| ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ |
| ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | |||
| ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | |||
| ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | |||
| ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | |||
| ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | |||
| ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | |||
| ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | |||
| ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ |
| ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | |||
| ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | |||
| ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | |||
| ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | |||
| ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | |||
| ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | |||
| ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ |
■■■■■
■■■■■
■■■■■
■■■■■
■■■■■
■■■■■
■■■■■
■■■■■
■■■■■
■■■■■
■■■■■
The operators in the study wore normal workwear, high visibility waistcoats and gloves, further personal protective equipment (PPE) such as safety caps, face masks and safety googles were used but residue levels were not monitored on these PPEs. The use of certified protective coveralls instead of working clothing, and FFP2 face mask (only applicable to exposure by inhalation since face masks were already worn by operators) has also been considered in a next step in this assessment by applying the agreed protection factors (EFSA, 2014) on the residue values as measured in the study (see Table 1 below).
■■■■■
As second tier approach of the negligible non‐dietary exposure assessment according to European Commission (2000, 2015), the margin of exposure (MoE) between the critical NOAEL (3 mg/kg bw per day for developmental effects) and the total systemic exposure has also been calculated.
■■■■■■■■■■■■■■■
2.1.2.2. Worker exposure
■■■■■
As second tier approach of the negligible non‐dietary exposure assessment according to European Commission (2000, 2015), the margin of exposure (MoE) between the critical NOAEL (3 mg/kg bw per day for developmental effects) and the total scaled systemic exposure has also been calculated.
■■■■■
■■■■■ ■■■■■
| ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ |
|---|---|---|---|---|---|
| ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ |
| ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | |
| ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ |
| ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | |
| ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ |
| ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ |
■■■■■
2.1.2.3. Bystander and resident exposure
Four pathways of bystander and resident exposure are considered: exposure to dust drift, vapour exposure, exposure to surface deposits and entry into treated crops; in addition, for resident longer term exposure, the sum of all pathways (mean) should be considered (EFSA, 2014). The following can be considered for the overall exposure assessment, when taking into account the available exposure study results (Kureha Corporation, 2021):
Exposure to dust drift during seed sowing can be estimated based on the submitted study;
Due to the low vapour pressure of ipconazole (3 × 10−6 Pa, EFSA, 2013a), it can be considered as a low volatile compound, and it can be assumed that vapour exposure is also included in the inhalation exposure data from the study;
Exposure to surface deposits can be calculated using the measured residues deposited in the Petri dishes at the feet of the adult mannequins during the study. In total, three Petri dishes were used and residues were below the limit of detection for two of them and at 0.01 µg/100 cm² in the other one. This corresponds to surface deposits of 10−7 mg/cm2, which leads to very low predicted exposure estimates (see under b) in Results in Annex A.
Entry into treated crops: In the absence of crop foliage, no dermal exposure is foreseen. There might be the case of a child picking up and ingesting treated seeds; however, children are expected to be under the supervision of adults and not left unattended; thus, such a scenario is considered quite unlikely.
Based on the above, exposure to the sum of all pathways is practically exposure to the dust drift as calculated using the study data.
The submitted exposure study for workers included a determination of bystander dermal and inhalation exposure to ipconazole during sowing/drilling of treated seed, using child and adult mannequins placed outside the field during the first day of the trial (at 3 m). As for operators and workers, individual exposure results are considered in this assessment due to the low number of mannequins in the study.
As second tier approach of the negligible non‐dietary exposure assessment according to European Commission (2000, 2015), the margin of exposure (MoE) between the critical NOAEL (3 mg/kg bw per day for developmental effects) and the total scaled systemic exposure has also been calculated.
The overall results of negligible exposure assessment for the residents and bystanders can be found in Table 3, the predicted exposure levels were below 1% of the (A)AOEL with a margin of exposure higher than 100,000. It should also be taken into account that some uncertainties (e.g. limited number of mannequins, lack of data for the dosimeters (see details in Annex), different formulation type and seed type compared to representative use) could not be fully addressed by the current assessment but are not likely to impact significantly on the negligible exposure assessment.
Table 3.
Systemic exposure values (µg/kg bw per day) and margin of exposure (MoE) bystander and residents
| Exposed group | Mannequin No | Systemic exposure based on study data( 1 ) | % (A)AOEL | MoE |
|---|---|---|---|---|
| Adult bystander | Adult 1 | 0.002 | < 1 | 1.3 × 106 |
| Adult 3 | 0.002 | < 1 | 1.3 × 106 | |
| Adult 5 | 0.003 | < 1 | 1.1 × 106 | |
| Adult resident | Adult 1 | 0.002 | < 1 | 1.6 × 106 |
| Adult 3 | 0.002 | < 1 | 1.6 × 106 | |
| Adult 5 | 0.002 | < 1 | 1.5 × 106 | |
| Child bystander | Child 2 | 0.014 | < 1 | 2.2 × 105 |
| Child 4 | 0.015 | < 1 | 2.0 × 105 | |
| Child 6 | 0.014 | < 1 | 2.2 × 105 | |
| Child resident | Child 2 | 0.011 | < 1 | 2.7 × 105 |
| Child 4 | 0.011 | < 1 | 2.6 × 105 | |
| Child 6 | 0.011 | < 1 | 2.7 × 105 |
(A)AOEL: acute acceptable operator exposure level; MoE: margin of exposure.
Calculated assuming light clothing, a dermal absorption value of 5% and default body weight (10 kg child, 60 kg adult) and default inhalation rates (EFSA, 2014).
2.2. Long‐term risk to granivorous birds
2.2.1. Tier 1 risk assessment
The Tier 1 risk assessment for ipconazole assumed that granivorous birds feed exclusively on treated wheat or barley grains. Toxicity:exposure ratio (TER) values for both crops were below the trigger value of 5 (Table 4), resulting in a high risk via long‐term dietary exposure for the representative uses of ipconazole in barley and wheat (EFSA, 2013a).
Table 4.
Tier 1 long‐term risk assessment for small granivorous birds for the representative uses of ipconazole in barley and wheat
| Crop | NAR (mg/kg seed) | FIR/bw | DDD (mg/kg bw/day) | NOEL (mg/kg bw/day) | TERlt | Trigger value |
|---|---|---|---|---|---|---|
| Barley | 20 | 0.3 | 6.0 | 4.3 | 0.72 | 5 |
| Wheat | 15 | 4.5 | 0.96 |
DDD: daily dietary dose; FIR: food intake rate; NAR: nominal application rate; NOEL: no observed effect level; TERlt: long‐term toxicity:exposure ratio.
Values in bold indicate a high risk.
2.2.2. Refined long‐term risk assessment to granivorous birds
To refine the long‐term risk to granivorous birds, the applicant made available data on residue dissipation from grains and plants treated with ipconazole as well as some ecological data for different focal species (i.e. chaffinch, skylark, yellowhammer and woodpigeon). These data are presented in the following sections.
2.2.2.1. Residue dissipation
Residues on grains
The applicant proposed to estimate the 50% degradation time (DT50) of ipconazole on cereal grains based on the results of eight residue decline trials conducted in Northern (three trials) and Southern Europe (five trials) on treated grains. 8
The DT50’s estimated in the trials from both EU regulatory zones were combined since the values from both zones were within the same order of magnitude. Since there was a sufficiently large data set according to EFSA (2019), the geometric mean from all trials was calculated. Overall, a DT50 of 2.67 days in treated cereal grains on the soil surface was estimated for ipconazole. Seasonal variation was not sufficiently demonstrated to derive independent DT50 values for spring and autumn since data from trials in spring (five trials) and autumn (three trials) were conducted in different locations. The locations of the field trials were considered to be representative for the GAP uses of ipconazole.
Regarding the averaging period to calculate the time‐weighted average factor (fTWA). 9 It was considered reasonable to fit the time window to the germination time (EFSA, 2017). Data on germination of cereal grains were available from the residue decline trials in Southern Europe. Since germination time may vary from region to region, averaging intervals of 7 and 10 days were considered realistic. Therefore, to refine the long‐term risk to granivorous birds, two TWA factors were considered: 0.46 and 0.36 (Table 5). As it was not agreed to use seasonal DT50 values, a TWA of 0.30, based on the highest DT50 values from the autumn data set, as proposed by the applicant, was not used in the refined risk assessment.
Table 5.
Time‐weighted average factor (fTWA) considering the 50% degradation time (DT50) values estimated from residue decline trials and 7‐ and 10‐day averaging time
See EFSA (2017) for further information.
Geometric mean calculated from eight residue decline trials in Northern and Southern Europe.
The refined risk assessment for granivorous birds considering a TWA factor of 0.46 or 0.36 based on a DT50 of 2.67 from residue decline trials on treated grains and averaging times of 7 or 10 days, respectively, resulted in a high risk via long‐term dietary exposure for the representative uses of ipconazole in barley and wheat (Table 6).
Table 6.
Refined long‐term risk assessment for granivorous birds for the representative uses of ipconazole on barley and wheat using a time‐weighted average factor of 0.46 and 0.36
| Crop | NAR (mg/kg seed) | FIR/bw | TWA | DDD (mg/kg bw/day) | NOEL (mg/kg bw/day) | TERlt | Trigger value |
|---|---|---|---|---|---|---|---|
| Barley | 20 | 0.3 | 0.46 | 2.77 | 4.3 | 1.6 | 5 |
| Wheat | 20 | 2.07 | 2.1 | ||||
| Barley | 15 | 0.36 | 2.14 | 2.0 | |||
| Wheat | 15 | 1.60 | 2.7 |
DDD: daily dietary dose; FIR: food intake rate; NAR: nominal application rate; NOEL: no observed effect level; TERlt: long‐term toxicity:exposure ratio.
Values in bold indicate a high risk.
Residues on whole plants
It was agreed to use the arithmetic mean of ipconazole residues measured on whole plants at BBCH 07 – 11 (from grains below the soil surface) of 3.52 mg/kg (without considering a fTWA) in the refined risk assessment for newly emerged crop shoots where appropriate (cereal component of the yellowhammer and skylark diet).
2.2.2.2. Drilling loss factor
Based on initial ipconazole residues detected right after drilling from a residue decline study with five trials across the EU, the applicant proposed using a drilling loss factor to the nominal application rates based on the reduction in grain loading of 5.12%. EFSA has agreed to consider a 0.95 drilling loss factor as a further refinement proposed by the applicant. However, taking into account that this correction factor is very close to 1, the weight of such refinement in the overall outcome of the risk assessment has been limited.
2.2.2.3. Exposure through the breeding season
The applicant proposed that, for autumn sown cereals, the exposure of birds in Northern EU Member States would be limited during the breeding season and, therefore, a low long‐term risk to granivorous birds could be concluded for such use. This issue was already discussed at the Pesticides Peer Review Meeting 99 for ipconazole (EFSA, 2013b). 10 In that meeting, some Member States did not agree that this could be readily assumed as it would be possible to have long‐term effects from exposure during the autumn. On that basis, it was not possible to conclude a low risk for the winter applications in cereals.
2.2.2.4. Selection of focal species
Four focal species were selected for demonstrating whether a low long‐term risk could be concluded for granivorous birds foraging in barley and wheat fields treated with ipconazole: the chaffinch, skylark, woodpigeon and yellowhammer. For each of these focal species, several refinements were proposed. In line with the previous assessment (EFSA, 2017), the selected focal species were considered to be a reasonable representation taking into account the representative uses of ipconazole.
Foraging area to find sufficient cereal grains to reach the lethal dose
The applicant proposed to estimate the area that each focal species needs to forage for reaching the lethal dose. This refinement option is proposed in the birds and mammals guidance document (EFSA, 2009). 11 By considering the foraging area indicated in the Northern Zone (2020) guidance document for passerine (35 m2) and non‐passerine (70 m2) granivorous birds, the area that skylarks, chaffinch and yellowhammer need to forage to reach the lethal dose (estimated as the NOEL divided by a safety factor of 5) was not unrealistic. For the woodpigeon (non‐passerine), in contrast, the estimated foraging area to reach the toxic dose was above the proposed area of 70 m2, indicating that a low long‐term risk could be concluded for that focal species. However, since this threshold value has not been agreed at EU level, the outcome of this refinement has only been considered as supportive.
Proportion of the diet obtained from the treated area (PD) and proportion of time spent in the treated field (PT)
To refine the risk for the four focal species, the applicant proposed using PD and PT values. The following considerations have been made:
-
–
For the chaffinch, the proposal to use a PD value of 0.41 for cereal grains for the spring and winter uses in cereals estimated from studies referenced in Buxton et al. (1998) has not been accepted since there were some uncertainties related to the landscape in which the studies were performed. For the spring applications, a PD value for cereal grains of 0.587 has been used to refine the risk. This value is based on a study investigating the diet composition of chaffinches in freshly drilled cereals fields in spring (Kureha Corporation, 2021). For winter cereals, data on the dietary composition were considered more reliable and, on this basis, a cereal grain PD of 0.32 has been used to refine the risk (EFSA, 2017). Regarding the PT value, the proposed value of 0.22 was only accepted for the winter applications. This value corresponds to the maximum individual PT of four individuals that foraged in the target crop (‘consumers only’ group) in a study performed in winter cereal fields (EFSA, 2017). For spring cereals, EFSA agreed with the RMS proposal to use a PT value of 0.63 from Kureha Corporation (2021).
-
–
For the skylark, the proposed PD value of 0.58 for cereal grains for the spring uses based on data from Kureha Corporation (2021) was agreed. For winter applications, the PD value of 0.6 proposed by the applicant was not sufficiently justified and a more conservative value of 0.74 reported in Buxton et al. (1998) and cited in the Northern Zone (2020) guidance document was considered more suitable. Regarding the PT, the proposed value of 0.61 was already considered not acceptable in EFSA (2017) as it is based on observations from only four ‘consumers’. However, EFSA agreed with the RMS proposal to use a PT of 0.86 for the spring uses, based on the 90th percentile resulting from the combined data set used in EFSA (2017) and Kureha Corporation (2021).
-
–
For the woodpigeon, the applicant proposed a PT value of 0.87 and a PT value of 0.53 for the spring and winter applications. However, it was noted that a refined long‐term risk based on PD and PT values was not needed since a low risk could already be concluded for all representative uses of ipconazole when considering a TWA of 0.46 and 0.35 based on a geometric mean DT50 value of 2.67 days (averaging time of 7 and 10 days, respectively).
-
–
For the yellowhammer, the cereal grain PD value of 0.91, based on Prys‐Jones (2000, 2015) (data from March to April) was accepted only for the spring applications. For winter‐grown cereals, a slightly more conservative PD of 0.93 also published in Prys‐Jones (2000, 2015) but considering data from October until November was preferred. Regarding the PT, the proposed values for spring and winter uses in cereals of 0.20 and 0.35, based on data used in EFSA (2017) and Kureha Corporation (2021), respectively, were agreed.
Dehusking
The dehusking behaviour of the chaffinch and the yellowhammer was accepted to refine the long‐term risk to both focal species. Dehusking was considered only in a qualitative way as the dehusking factors proposed by the applicant were not based on specific studies on the relevant focal species.
Furthermore, if dehusking was quantitatively accounted for in the risk assessment it would be necessary to re‐evaluate whether these species were appropriate focal species. It may be the case that a further risk assessment for a species which does not dehusk would be needed.
Accepted refinement options for the relevant focal species
Table 7 summarises all refinement options that have been accepted for the relevant focal species. The applicant and the RMS proposed combining PD and PT values; however, this proposal has not been accepted by EFSA to refine the risk, considering that, within each focal species, the values for both parameters were derived from different studies, the uncertainty related to the determination of both values and that the PT calculation, at least partly, already considers PD and vice versa. Combining of PT and PD value in the risk assessment was not accepted in EFSA (2017). The issue of combining of PT and PD value in the risk assessment was further discussed during the Pesticide Peer Review TC 26 on prothioconazole 12 and it was concluded that for seed treatments, these parameters cannot be combined, as in the refinement of the risk for this type of application, it is assumed that only the cereal seeds are assumed to be contaminated – hence, it is considered to mix PD and PT. Therefore, combining both refinement options would mean to count them double.
Table 7.
Accepted options to refine the long‐term risk to granivorous birds for the representative uses of ipconazole in barley and wheat
| Focal species | Refinement | |||
|---|---|---|---|---|
| fTWA( a ) | PD (cereal grains) | PT | Dehusking behaviour | |
| Chaffinch |
– 0.46 (7‐day averaging time) – 0.36 (10‐day averaging time) |
– Spring: 0.587 – Winter: 0.32 |
– Spring: 0.63 – Winter: 0.22 |
– Yes (qualitatively) |
| Skylark |
– Spring: 0.58 – Winter: 0.74 |
– Spring: 0.86 – Winter: 1 |
– Not proposed | |
| Woodpigeon | – Not needed | – Not needed | – Not proposed | |
| Yellowhammer |
– Spring: 0.91 – Winter: 0.93 |
– Spring: 0.20 – Winter: 0.35 |
– Yes (qualitatively) | |
| Further refinement(s) | ||||
| – Drilling loss factor (0.95) | ||||
PD: Proportion of the diet obtained from treated area; PT: Proportion of time spent in the treated field; TWA: time‐weighted average.
PT and PD were refined separately. The estimated area that needs to be foraged to reach the lethal dose has only been considered as supportive to refine the risk since there are not foraging area values agreed at EU level.
Based on a geomean DT50 of 2.67 days estimated from residue decline trials in Northern (three trials) and Southern Europe (five trials).
2.2.2.5. Refined long‐term toxicity:exposure ratio calculations for the risk to granivorous focal species from ipconazole on seeds
TER values for the different focal species were estimated considering the refinement options listed in Table 7.
Therefore, Table 8 provides the refined chronic risk assessment for birds consuming treated seed in terms of risk from ipconazole. The TER values incorporate the accepted refined PT and PD values separately for an fTWA of 0.46 and 0.36 (7‐ and 10‐day averaging time, respectively).
Table 8.
Refined long‐term TER calculations for granivorous birds consuming treated grains as food items incorporating PD refinements
| ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ||
|---|---|---|---|---|---|---|---|
| ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ||||
| ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ |
| ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | |||
| ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ||
| ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | |||
| ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | |
| ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | |||
| ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ||
| ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | |||
| ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ |
| ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | |||
| ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ||
| ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | |||
| ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | |
| ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | |||
| ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ||
| ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | |||
| ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ |
| ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | |||
| ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ||
| ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | |||
| ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ |
| ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | |||
| ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ||
| ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | |||
| ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | |
| ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | |||
| ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ||
| ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | |||
■■■■■
■■■■■
■■■■■
■■■■■
■■■■■
■■■■■
■■■■■
■■■■■
■■■■■
■■■■■
■■■■■
2.2.3. Conclusions on long‐term risk assessment for granivorous focal species
■■■■■
3. Conclusions
As for the dietary exposure, in the absence of pertinent new residue data, the conclusions of previous EFSA evaluations regarding seed‐treated cereals still apply. For the representative uses, residue concentrations of the parent compound ipconazole in grain and straw of wheat and barley, in potential succeeding crops and in food items of animal origin were determined by data or can be reasonably expected to be < 0.01 mg/kg. In a consumer dietary risk assessment for parent ipconazole using PRIMo rev. 3.1, chronic dietary exposure was less than 1% of the ADI for ipconazole and the maximum acute dietary exposure was 1% of the ARfD for ipconazole.
For the non‐dietary exposure, the assessment has included the newly available field studies for operators, workers and bystanders. For operators, based on the submitted data, the total systemic exposure is predicted to be less than 1% of the (A)AOEL for an operator wearing normal workwear, gloves and FFP2 mask (with a margin of exposure higher than 10,000). It is noted that the study did not include measurements of exposure during the equipment cleaning task, and exposure during bagging was minimised since the process was highly automated. If exposure estimates during bagging and equipment cleaning tasks (using SeedTropex) are included, the total systemic exposure is predicted to be up to 48% of the (A)AOEL (with a margin of exposure between 400 and 500). For workers, the total systemic exposure is predicted below 10% of the (A)AOEL only for one of the two workers (with use of gloves, or gloves and FFP2), with a margin of exposure higher than 2,000. For residents and bystanders, the total systemic exposure is predicted to be lower than 1% of the (A)AOEL and the margin of exposure is higher than 100,000. It should also be taken into account that the impact of some uncertainties, raised by the limitations of the studies (e.g. limited number of subjects with restricted tasks, treated seed type and formulation type different from the representative uses, see also Annex), could not be addressed by the current assessment.
■■■■■
Abbreviations
- µg
microgram
- µm
micrometer (micron)
- AAOEL
acute acceptable operator exposure level
- ADI
acceptable daily intake
- AOEL
acceptable operator exposure level
- ARfD
acute reference dose
- bw
body weight
- CFU
colony forming units
- cm
centimetre
- d
day
- DDD
daily dietary dose
- DNA
deoxyribonucleic acid
- DT50
period required for 50% dissipation (define method of estimation)
- DT90
period required for 90% dissipation (define method of estimation)
- EEC
European Economic Community
- ErC50
effective concentration (growth rate)
- FAO
Food and Agriculture Organization of the United Nations
- FIR
food intake rate
- g
gram
- GAP
Good Agricultural Practice
- GC
gas chromatography
- h
hour(s)
- ha
hectare
- iv
intravenous
- kg
kilogram
- L
litre
- m
metre
- M
mol
- M/L
mixing and loading
- mg
milligram
- mL
millilitre
- mm
millimetre (also used for mean measured concentrations)
- mN
milli‐Newton
- ng
nanogram
- NOAEL
no observed adverse effect level
- NOEC
no observed effect concentration
- NOEL
no observed effect level
- NPD
nitrogen–phosphorus detector
- OECD
Organisation for Economic Co‐operation and Development
- OM
organic matter content
- Pa
Pascal
- PCR
polymerase chain reaction
- PD
proportion of different food types
- PDA
Potato Dextrose Agar
- PPE
personal protective equipment
- ppm
parts per million (10–6)
- PT
proportion of diet obtained in the treated area
- PTT
partial thromboplastin time
- QSAR
quantitative structure–activity relationship
- r2
coefficient of determination
- RAC
regulatory acceptable concentration
- S
svedberg, S (10−13 s)
- SFO
single first‐order
- SMILES
simplified molecular‐input line‐entry system
- t1/2
half‐life (define method of estimation)
- TC
technical material
- tef1
translation elongation factor 1 α gene
- TER
toxicity exposure ratio
- TERA
toxicity exposure ratio for acute exposure
- TERLT
toxicity exposure ratio following chronic exposure
- TERST
toxicity exposure ratio following repeated exposure
- TK
technical concentrate
- TLV
threshold limit value
- TMDI
theoretical maximum daily intake
- TRR
total radioactive residue
- TSH
thyroid‐stimulating hormone (thyrotropin)
- TWA
time‐weighted average
- UDS
unscheduled DNA synthesis
- UF
uncertainty factor
- USDA‐ARS
U.S. Department of Agriculture, Agriculture Research Service
- UV
ultraviolet
- VNTR
variable number tandem repeat
- W/S
water/sediment
- w/v
weight per unit volume
- w/w
weight per unit weight
- WBC
white blood cell
- WHO
World Health Organization
- λ
wavelength
- ε
decadic molar extinction coefficient
Appendix A – Used compound codes
1.
| Code/trivial name( a ) | IUPAC name/SMILES notation/InChiKey( b ) | Structural formula( c ) |
|---|---|---|
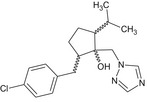
| Ipconazole |
(1RS,2SR,5RS;1RS,2SR,5SR)‐2‐(4‐chlorobenzyl)‐5‐isopropyl‐1‐(1H‐1,2,4‐triazol‐1‐ylmethyl)cyclopentanol Clc1ccc(cc1)CC1CCC(C(C)C)C1(O)Cn1cncn1 QTYCMDBMOLSEAM‐UHFFFAOYSA‐N |
|
| Triazole derivative metabolites | ||
|
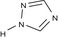
1,2,4‐triazole 1,2,4‐T |
1H‐1,2,4‐triazole c1ncnn1 NSPMIYGKQJPBQR‐UHFFFAOYSA‐N |
|
|
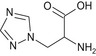
Triazole alanine TA |
3‐(1H‐1,2,4‐triazol‐1‐yl)‐D,L‐alanine or (RS)‐2‐amino‐3‐(1H‐1,2,4 triazol‐1‐yl)propanoic acid NC(Cn1cncn1)C(=O)O XVWFTOJHOHJIMQ‐UHFFFAOYSA‐N |
|
|
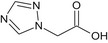
Triazole acetic acid TAA |
1H‐1,2,4‐triazol‐1‐ylacetic acid O = C(O)Cn1cncn1 RXDBSQXFIWBJSR‐UHFFFAOYSA‐N |
|
|
Triazole x`lactic acid or Triazolehydroxy propionic acid TLA |
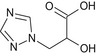
(2RS)‐2‐hydroxy‐3‐(1H‐1,2,4‐triazol‐1‐yl)propanoic acid OC(Cn1cncn1)C(=O)O KJRGHGWETVMENC‐UHFFFAOYSA‐N |
|
| Triazolyl pyruvate |
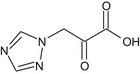
2‐oxo‐3‐(1H‐1,2,4‐triazol‐1‐yl)propanoic acid O = C(Cn1cncn1)C(=O)O HPASHQMJHWVUCD‐UHFFFAOYSA‐N |
|
| OH‐IPC‐glycoside |
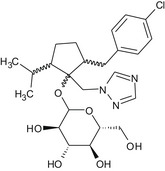
2‐[(4‐chlorophenyl)methyl]‐5‐(propan‐2‐yl)‐1‐[(1H‐1,2,4‐triazol‐1‐yl)methyl]cyclopentyl D‐glucopyranoside Clc1ccc(cc1)CC1CCC(C(C)C)C1(OC1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O)Cn1cncn1 GZNVBOIRVZWFFS‐IYYQGSHMSA‐N |
|
| tert‐OH‐isopropyl‐IPC |
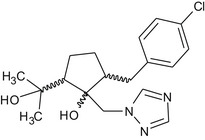
2‐[(4‐chlorophenyl)methyl]‐5‐(2‐hydroxypropan‐2‐yl)‐1‐[(1H‐1,2,4‐triazol‐1‐yl)methyl]cyclopentan‐1‐ol Clc1ccc(cc1)CC1CCC(C1(O)Cn1cncn1)C(C)(C)O YVAQXENVTBBIOH‐UHFFFAOYSA‐N |
|
The metabolite name in bold is the name used in the conclusion.
ACD/Name 2017.2.1 ACD/Labs 2017 Release (File version N40E41, Build 96719, 06 Sep 2017).
ACD/ChemSketch 2017.2.1 ACD/Labs 2017 Release (File version C40H41, Build 99535, 14 Feb 2018).
Appendix B – PRIMo 3.1
1.

Annex A – Assessment of the field studies submitted for non‐dietary negligible exposure of ipconazole
Annex A can be found in the online version of this output (‘Supporting information’ section): https://doi.org/10.2903/j.efsa.2022.7133
Supporting information
Assessment of thefield studies submitted for non‐dietary negligible exposure of ipconazole
EFSA Panel (European Food Safety Authority) , 2022. Statement concerning the review of the approval of the active substance ipconazole. EFSA Journal 2022;20(8):7133, 26 pp. 10.2903/j.efsa.2022.7133
Requestor: European Commission
Question number: EFSA‐Q‐2021‐00742
Declarations of interest: If you wish to access the declaration of interests of any expert contributing to an EFSA scientific assessment, please contact interestmanagement@efsa.europa.eu.
Adopted: 26 January 2022
Notes
Regulation (EC) No 1107/2009 of the European Parliament and of the Council of 21 October 2009 concerning the placing of plant protection products on the market and repealing Council Directives 79/117/EEC and 91/414/EEC. OJ L 309, 24.11.2009, p. 1‐50.
Commission Implementing Regulation (EU) No 571/2014 of 26 May 2014 approving the active substance ipconazole, in accordance with Regulation (EC) No 1107/2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market, and amending the Annex to Commission Implementing Regulation (EU) No 540/2011 OJ L 157, 27.5.2014, p. 96–100.
Commission Implementing Regulation (EU) No 540/2011 of 25 May 2011 implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as regards the list of approved active substances. OJ L 153, 11.6.2011, p. 1‐186.
Commission Implementing Regulation (EU) No 541/2011 of 1 June 2011 amending Implementing Regulation (EU) No 540/2011 implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as regards the list of approved active substances. OJ L 153, 11.6.2011, p. 187‐188.
Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on classification, labelling and packaging of substances and mixtures, amending and repealing Directives 67/548/EEC and 1999/45/EC, and amending Regulation (EC) No 1907/2006. OJ L 353, 31.12.2008, p. 1–1355.
1,2,4‐triazole has recently also been classified as reprotoxic 1B, according to the 17th Amendment to Technical Progress of the CLP Regulation: https://eur‐lex.europa.eu/legal‐content/EN/TXT/HTML/?uri=CELEX:32021R0849&from=EN#d1e549‐27‐1
Three trials were conducted in UK (Report Numbers ESP08/04 and THA/09/01); one in Northern France (<10 days), three in Southern France and one in Italy (Study Number FDD0159/2015‐075).
Calculated using the following equation: TWA = (1−e−ki)/ki; where k = ln(2)/DT50 and i = averaging interval.
Please refer to point 5.1 of the Experts Peer Review Meeting 99 (EFSA, 2013a).
Please refer to Section 5.2.3 of EFSA (2009) for further details.
The peer review process of prothioconazole is currently on ‘long‐term ED clock‐stop’; therefore, the peer review report is not yet publicly available.
References
- Belgium , 2021. Addendum to the confirmatory data on ipconazole, October 2021. Available online: www.efsa.europa.eu
- Buxton JM, Crocker DR and Pascual JA, 1998. Birds and farming: information for risk assessment. Update Contract PN0919 Milestone Report FERA Project No M37. Available online: https://www.hse.gov.uk/pesticides/resources/B/Birdbible.pdf
- EFSA (European Food Safety Authority) , 2009. Guidance on Risk Assessment for Birds and Mammals on request from EFSA. EFSA Journal 2009;7(12):1438, 358 pp. 10.2903/j.efsa.2009.1438 [DOI] [Google Scholar]
- EFSA (European food Safety Authority) , 2013a. Conclusion on the peer review of the pesticide risk assessment of the active substance ipconazole. EFSA Journal 2013;11(4):3181, 76 pp. 10.2903/j.efsa.2013.3181 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , 2013b. Peer Review Report to the conclusion regarding the peer review of the pesticide risk assessment of the active substance ipconazole. Available online: www.efsa.europa.eu
- EFSA (European Food Safety Authority) , 2014. Guidance on the assessment of exposure of operators, workers, residents and bystanders in risk assessment for plant protection products. EFSA Journal 2014;12(10):3874, 55 pp. 10.2903/j.efsa.2014.3874. Available online: www.efsa.europa.eu/efsajournal [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) , 2017. Technical report on the outcome of the consultation with Member States, the applicant and EFSA on the pesticide risk assessment for ipconazole in light of confirmatory data. EFSA Supporting Publication 2017:EN‐1260, 24 pp. 10.2903/sp.efsa.2017.EN-1260 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , 2018. Conclusion on the peer review of the pesticide risk assessment for the triazole derivative metabolites in light of confirmatory data submitted. EFSA Journal 2018;16(7):5376, 20 pp. 10.2903/j.efsa.2018.5376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) , 2019. Technical report on the outcome of the Pesticides Peer Review Meeting on general recurring issues in ecotoxicology. EFSA Supporting Publication 2019:EN‐1673, 117 pp. 10.2903/sp.efsa.2019.EN-1673 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , Anastassiadou M, Bernasconi G, Brancato A, Carrasco Cabrera L, Greco L, Jarrah S, Kazocina A, Leuschner R, Magrans JO, Miron I, Nave S, Pedersen R, Reich H, Rojas A, Sacchi A, Santos M, Stanek A, Theobald A, Vagenende B and Verani A, 2020. Reasoned Opinion on the review of the existing maximum residue levels for ipconazole according to Article 12 of Regulation (EC) No 396/2005. EFSA Journal 2020;18(1):5961, 53 pp. 10.2903/j.efsa.2020.5961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) , 2022. Peer Review Report to the statement regarding the pesticide risk assessment of the active substance ipconazole. Available online: www.efsa.europa.eu
- EFSA (European Food Safety Authority) , Buist H, Craig P, Dewhurst I, Hougaard Bennekou S, Kneuer C, Machera K, Pieper C, Court Marques D, Guillot G, Ruffo F and Chiusolo A, 2017. Guidance on dermal absorption. EFSA Journal 2017;15(6):4873, 60 pp. 10.2903/j.efsa.2017.4873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Commission , 2000. Residues: guidance for generating and reporting methods of analysis in support of pre‐registration data requirements for Annex II (Part A, Section 4) and Annex III (Part A, Section 5) of Directive 91/414. SANCO/3029/99‐rev. 4, 11 July 2000.
- Prys‐Jones RP, 1997. Aspects of reed bunting ecology with comparisons with the yellowhammer. Doctor of Philosophy thesis. University of Oxford. [Google Scholar]
- European Commission , 2015. Draft Technical Guidance Document on assessment of negligible exposure of an active substance in a plant protection product under realistic conditions of use (points 3.6.3 to 3.6.5, and 3.8.2of Annex II of Regulation (EC) No 1107/2009. SANCO/2014/12096), May 2015.
- Kureha Corporation (collaboration partner UPL, previously known as Arysta LifeScience Great Britain, Ltd) , 2021. Additional information on ipconazole submitted by the applicant in accordance with the review of approval under Article 21 of Regulation (EC) 1107/2009. Available online: www.efsa.europa.eu
- Northern Zone , 2020. Pesticide risk assessment for birds and mammals. Selection of relevant species and development of standard scenarios for higher tier risk assessment in the Northern Zone in accordance with Regulation EC 1107/2009. Version 2.1, December 2021. Available online: https://www.kemi.se/download/18.6c26dc74178e406dc61bde/1619513699012/birds‐and‐mammals‐higher‐tier‐risk‐assesment‐northern‐zone‐december‐2020‐ver‐2‐1‐1.docx
- OECD , 1997. Guidance document for the conduct of studies of occupational exposure to pesticides during agricultural application, Series on Testing and Assessment No. 9, 1997.
- United Kingdom , 2017. Addendum to the assessment report on ipconazole, confirmatory data, February 2017, updated in May 2017. Available online: www.efsa.europa.eu
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Assessment of thefield studies submitted for non‐dietary negligible exposure of ipconazole


