Abstract
The heterocyst is the site of nitrogen fixation in aerobically grown cultures of some filamentous cyanobacteria. Heterocyst development in Anabaena sp. strain PCC 7120 is dependent on the global nitrogen regulator NtcA and requires, among others, the products of the hetR and hetC genes. Expression of hetC, tested by RNA- DNA hybridization, was impaired in an ntcA mutant. A nitrogen-regulated, NtcA-dependent putative transcription start point was localized at nucleotide −571 with respect to the hetC translational start. Sequences upstream from this transcription start point exhibit the structure of the canonical cyanobacterial promoter activated by NtcA, and purified NtcA protein specifically bound to a DNA fragment containing this promoter. Activation of expression of hetC during heterocyst development appears thus to be directly operated by NtcA. NtcA-mediated activation of hetR expression was not impaired in a hetC mutant, indicating that HetC is not an NtcA-dependent element required for hetR induction.
Cyanobacteria are phototrophic bacteria that carry out oxygenic photosynthesis and likely represent the phylogenic ancestors of the chloroplasts of eukaryotic algae and higher plants. Cyanobacteria obtain cellular nitrogen mainly from inorganic sources such as nitrate or ammonium, and many can also use atmospheric nitrogen as a nitrogen source (9, 10). The assimilation of nitrogen by cyanobacteria is subjected to tight regulation so that ammonium is assimilated with preference over other good nitrogen sources such as urea, nitrate, nitrite, or N2 when more than one are available (10). At the molecular level, this possibility of choice is based on a nutritional repression exerted by ammonium on the expression of genes involved in the assimilation of alternative nitrogen sources, and ntcA has been identified as a gene that encodes a transcriptional regulator exerting global nitrogen control that appears to be universally distributed in cyanobacteria (11, 13). The NtcA protein belongs to the cyclic AMP receptor protein family of bacterial regulators and bears close to its C-terminal end a helix-turn-helix motif for interaction with DNA (31). NtcA binds to specific sites in the promoter regions of regulated genes involved in nitrogen assimilation, activating their expression in response to ammonium withdrawal (22). The structure of the cyanobacterial NtcA-activated promoter comprises a −10 box in the form TAN3T and an NtcA-binding site containing the sequence signature GTAN8TAC that is located 20 to 23 nucleotides upstream from the −10 box and that appears to substitute for the −35 box that would be present in promoters similar to the canonical Escherichia coli ς70 promoters (11, 22).
Some filamentous cyanobacteria are able to differentiate, under conditions of aerobiosis and combined nitrogen deprivation, cells specialized in nitrogen fixation called heterocysts, which differentiate from vegetative cells located at semiregular intervals in the filament (5, 35). Heterocysts differ from vegetative cells in many structural and functional features that turn them into efficient factories for nitrogen fixation. At the molecular level, the differential physiology of the heterocyst is supported by a pattern of gene expression that largely differs from that taking place in vegetative cells (5, 35).
In recent years, a number of genes of Anabaena sp. strain PCC 7120 whose function is required for heterocyst development have been identified (5, 17, 34, 35). The products of some of them (such as devA [23], hetM [7], and hepA [20]) serve a structural role, and their inactivation leads to the differentiation of aberrant heterocysts, in most cases carrying an imperfect heterocyst envelope. For others, e.g., hetR (3, 4) and hetC (21), the actual function in heterocyst development is still unknown although their inactivation leads to a lack of differentiation. Also, the patS gene, whose inactivation leads to the differentiation of supernumerary heterocysts, has been described (36). HetR has recently been suggested to have protease activity (37). hetC would encode a 1,044-amino-acid protein belonging to the superfamily of the ATP-binding cassette (ABC) transporters, with the greatest similarity to proteins of the HlyB family that facilitate the export of toxic proteins. Since the mutation of hetC prevents heterocyst differentiation, it has been reasoned that HetC could be involved in the export of an inhibitor of differentiation (21). The expression of hetR (3, 4) and hetC (21) increases in response to combined nitrogen deprivation. The expression of devA, hetM, and hepA (7), as well as of the nifHDK operon encoding nitrogenase (8, 16), is also activated upon combined nitrogen deprivation in a HetR-dependent but, to date, unknown manner.
The ntcA gene has been shown to be necessary for N2 fixation and heterocyst development in Anabaena sp. strain PCC 7120 (12, 33). Mutant strains bearing an inactivated version of ntcA are unable to grow on N2, as well as on nitrate, and do not show any sign of heterocyst differentiation upon combined nitrogen deprivation. Moreover, these ntcA mutants do not show the activation of hetR or of nifHDK expression that takes place in the wild-type strain in response to nitrogen stepdown (12).
Elucidation of the direct targets of NtcA during heterocyst differentiation is crucial for understanding the mechanism by which gene expression is regulated for the development and function of the cyanobacterial heterocyst. In this report, we present a study of the expression of the hetC gene and provide evidence for a direct role for NtcA as an activator of the expression of this gene.
MATERIALS AND METHODS
Bacterial strains.
This study was carried out with the heterocyst-forming cyanobacterium Anabaena sp. strain PCC 7120 and two Het− derivatives, strain CSE2 (an insertional mutant of the ntcA gene) (12) and strain DR1653 (an insertional mutant of the hetC gene) (21). They were grown photoautotrophically at 30°C in BG110C medium (BG110 medium [26] supplemented with 0.84 g of NaHCO3 per liter), bubbled with a mixture of CO2 (1% [vol/vol]) and air, and supplemented with 2 μg of streptomycin and 2 μg of spectinomycin · ml−1 for strains CSE2 and DR1653. When indicated, 8 mM NH4Cl (plus 16 mM TES [N-tris{hydroxymethyl}methyl-2-aminoethanesulfonic acid]–NaOH buffer [pH 7.5]) or 17.6 mM NaNO3 was added as a nitrogen source.
For RNA isolation, cells growing exponentially in BG110C medium supplemented with NH4Cl were harvested at room temperature and either used directly or washed with BG110C medium, resuspended in BG110C medium (nitrogen free) supplemented or not with NH4Cl or NaNO3, and further incubated under culture conditions for the number of hours indicated in the figure legends.
E. coli DH5α (Bethesda Research Laboratories) was used for all plasmid constructions, except for pCSAM70 (see below). E. coli BL21(DE3) (28) was used in this case. Both E. coli strains were grown in Luria broth (LB) as described previously (27). E. coli strains containing pCSAM70 were grown in LB medium supplemented with 0.2% glucose in order to reduce basal expression of the ntcA gene.
Plasmids.
Plasmid pCSAM70 contains a ca. 1.6-kb DNA fragment from the ntcA region of Anabaena sp. strain PCC 7120 cloned into BamHI- and HindIII-digested, Klenow fragment-filled expression vector pQE9 (Qiagen). This fragment extends from the start codon of the ntcA gene (cloned in frame with the site of initiation of translation located after the IPTG [isopropyl-β-d-thiogalactopyranoside]-inducible promoter in pQE9) to the first HincII site located downstream of ntcA (13). The NtcA protein encoded in pCSAM70 contains an N-terminal histidine tag (see below). pCSAM70 was maintained in E. coli cells bearing plasmid pREP4 (Qiagen), which express high levels of the LacIq repressor.
Plasmid pCSAM83 contains an 853-bp fragment from the upstream region, and the initial part of the coding sequence, of the hetC gene from Anabaena sp. strain PCC 7120 (GenBank accession no. U55386) (21). This fragment was amplified by PCR with oligonucleotides HC1 (5′-TAGTACATCGGTGAGGGGTG-3′; corresponding to positions −693 to −674 relative to the translation start of hetC) and HC4 (5′-GCCGAACTACCCAGTTTTGG-3′; complementary to positions +160 to +141 relative to the translation start of hetC) and chromosomal DNA from strain PCC 7120 as the template and cloned into plasmid pGEM-T (Promega).
Plasmid pCSAM86 contains a 1,598-bp fragment internal to the hetC gene from Anabaena sp. strain PCC 7120. This fragment was amplified by PCR with oligonucleotides HC5 (5′-AGAGTTGAGCCAAAACTGG-3′; corresponding to positions +132 to +150 relative to the translation start of hetC) and HC6 (5′-GTAAGGGTAACTGCAACG-3′; complementary to positions +1729 to +1712 relative to the translation start of hetC) and chromosomal DNA from strain PCC 7120 as the template and cloned into plasmid pGEM-T (Promega).
DNA and RNA isolation and manipulation.
Total DNA (6) and RNA (14; based on reference 18) from Anabaena sp. strain PCC 7120 and its derivatives were isolated as previously described. Sequencing was carried out by the dideoxy chain termination method with a T7Sequencing kit (Pharmacia Biotech) and α-35S-thio dATP. DNA fragments were purified from agarose gels with the Geneclean II kit (Bio 101, Inc.).
Plasmid isolation from E. coli, transformation of E. coli, digestion of DNA with restriction endonucleases, ligation with T4 ligase, and PCR were performed by standard procedures (1, 27).
Northern blotting and hybridization.
For Northern analysis, 70 μg of RNA was loaded per lane and electrophoresed in 1% agarose denaturing formaldehyde gels. Transfer and fixation to Hybond-N+ membranes (Amersham Pharmacia) were carried out with 0.1 M NaOH. Hybridization was performed at 65°C according to the recommendations of the manufacturer of the membranes. The hetC probe was amplified by PCR with oligonucleotides HC5 and HC6 (see above) and pCSAM86 as the template. The hetR probe was a 703-bp HaeII fragment containing most of the hetR gene (4). Fragments used as probes were labeled with a Ready to Go DNA labeling kit (Pharmacia Biotech) by using [α-32P]dCTP. Images of radioactive filters were obtained and quantified with a Cyclone storage phosphor system and OptiQuant image analysis software (Packard).
Primer extension analysis.
Oligonucleotides used for primer extension analysis of the hetC transcript were HC2 (5′-TGTGAGCAACATCGACATCTG-3′; complementary to positions −411 to −431 relative to the translation start of hetC), HC3 (5′-CGGCATTTTAATGTACTGCC-3′; complementary to positions −85 to −104 relative to the translation start of hetC), HC4 (see above), and HC7 (5′-GGAAAAGGTTCTCTATGAAC-3′; complementary to positions −348 to −367 relative to the translational start of hetC). Plasmid pCSAM83, which contains the upstream region of the hetC gene, was used to generate dideoxy-sequencing ladders with the same primers.
Oligonucleotides were end-labeled with T4 polynucleotide kinase (Boehringer) and [γ-32P]dATP as described previously (1) and mixed with 25 μg of total RNA in the presence of 10 mM Tris-HCl (pH 8.0)–150 mM KCl–1 mM EDTA. The mixtures were incubated first at 85°C for 10 min for denaturation of RNA and then at 50°C for 1 h for annealing. The extension reactions were carried out at 47°C for 1 h in a final volume of 45 μl containing the whole annealing reaction mixture, 0.25 mM (each) deoxynucleoside triphosphate, 200 U of reverse transcriptase (Superscript II; Gibco-BRL), and the buffer recommended by the transcriptase provider. Reaction mixtures were then treated with RNase A (DNase free; Boehringer) and extracted with phenol. The extended fragments were precipitated with sodium acetate and ethanol, resuspended in formamide loading dye, and loaded onto 6% polyacrylamide-urea sequencing gels next to the corresponding sequencing ladder. Images of radioactive gels were obtained and quantified as described above.
Overproduction and purification of histidine-tagged NtcA.
For purification of histidine-tagged NtcA, saturated cultures of E. coli BL21(DE3) (pREP4, pCSAM70) grown in the presence of 0.2% glucose were centrifuged, diluted 1:50 in LB medium without glucose, and incubated for an additional 2-h period under culture conditions. IPTG was added at 1 mM, and the incubation was continued for three more hours. Cells from 500 ml of cultures were collected, washed with 1 volume of 20 mM sodium phosphate buffer (pH 7.0) containing 200 mM NaCl and 10% glycerol, and resuspended in 5 ml of the same buffer per gram of cells. After the addition of 1 mM phenylmethylsulfonyl fluoride, the cells were disrupted by sonication and the extract was centrifuged at 10,000 × g for 15 min. The resulting crude extract was chromatographed through a 1-ml chelating Sepharose Hitrap column (Pharmacia Biotech) charged with CuSO4, by using a fast protein liquid chromatography system (Pharmacia Biotech). Contaminating proteins were eluted by washing the column with 15 volumes of the same buffer. Bound NtcA was eluted with a linear gradient of imidazole (0 to 0.5 M) in the same buffer as that described above. Pure NtcA, as judged by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), started to elute from the column at about 200 mM imidazole.
Proteins in whole cells or crude extracts were analyzed by standard electrophoresis in SDS-PAGE gels followed by staining with Coomassie brilliant blue R. Protein concentration was estimated by a dye-binding assay (Bio-Rad).
Band-shift assays.
DNA fragments to be used in electrophoretic mobility shift assays were obtained by PCR amplification. Oligonucleotides HC1 and HC2 (see above) and plasmid pCSAM83 were used for the hetC upstream region. For the glnA upstream region, oligonucleotides GA3 (5′-GGATTTTATGTCAAAGTTGACCCC-3′; corresponding to positions −238 to −215 relative to the translation start of glnA) and GA6 (5′-CGAAACAAAGTTGATGAC-3′; complementary to positions −70 to −87 relative to the translation start of glnA) and plasmid pAN503 (29) were used. The same unlabeled DNA fragments were added as competitors in some assays. Alternatively, an unrelated, unlabeled DNA fragment from plasmid pBluescript obtained by PCR amplification with M13 reverse and forward sequencing primers was used. DNA fragments were end-labeled with T4 polynucleotide kinase (Boehringer) and [γ-32P]dATP as described previously (1). Assays were carried out as described previously (22) with 0.05 pmol of labeled fragment and 5 pmol of purified histidine-tagged NtcA. Assays carried out with E. coli crude extracts contained 0.85 μg of total protein carrying, for the extract from IPTG-induced E. coli BL21(DE3) (pREP4, pCSAM70) cells, approximately 1.5 pmol of histidine-tagged NtcA. Unlabeled competitor fragments were added in a 25-fold molar excess. Images of radioactive gels were obtained with a Cyclone storage phosphor system (Packard).
RESULTS
Nitrogen-regulated transcription initiation of the hetC gene.
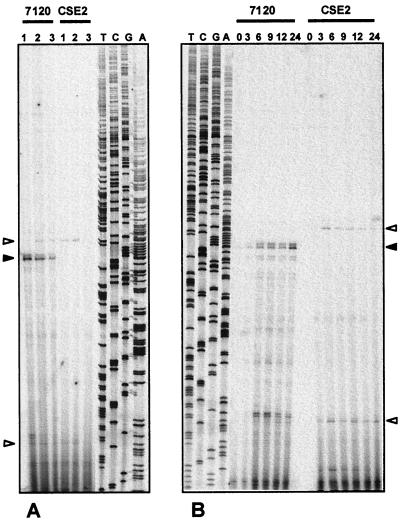
To study transcriptional regulation of hetC, primer extension experiments were carried out with oligonucleotides HC2, HC3, HC4, and HC7 by using RNA isolated from cells grown on ammonium and incubated for 6 h in medium containing no combined nitrogen, nitrate, or ammonium. With oligonucleotide HC2, a major RNA 5′ end that would correspond to a transcription start point (tsp) situated at position −571 with respect to the translational start of the hetC gene was detected (Fig. 1A). The use of this putative tsp was dependent on the nitrogen regime of the cells, being most efficient in the absence of combined nitrogen and more efficient in nitrate- than in ammonium-containing medium. With oligonucleotide HC7 (not shown), the putative tsp located at −571 was confirmed, whereas the bands showing up at position −474/−477 in Fig. 1 were not detected. No other tsp was detected with oligonucleotide HC3 or HC4.
FIG. 1.
Primer extension analysis of expression of the hetC gene in Anabaena sp. strain PCC 7120 and mutant strain CSE2 (mutant ntcA). (A) Primer extension assays were carried out with RNA isolated from cells grown on ammonium and incubated for 6 h in medium lacking combined nitrogen (lanes 1) or containing nitrate (lanes 2) or ammonium (lanes 3). (B) Time course of expression of the hetC gene in Anabaena sp. strain PCC 7120 and mutant strain CSE2 upon combined-nitrogen stepdown. Primer extension assays were carried out with RNA isolated from cultures grown on ammonium (lanes 0) or grown on ammonium and incubated in combined-nitrogen-free medium for 3, 6, 9, 12, or 24 h. Assays were carried out with oligonucleotide HC2 (see Materials and Methods). The sequencing ladders shown were generated with the same oligonucleotide and plasmid pCSAM83. Solid arrowheads point to the putative tsp identified at position −571. Open arrowheads point to the −474/−477 (bottom) and −581 (top) positions (see text).
To determine the time course of activation of this hetC tsp upon combined-nitrogen deprivation, Anabaena sp. strain PCC 7120 cells grown with ammonium were transferred to medium lacking combined nitrogen, and primer extension assays were carried out with RNA extracted from the cultures at several time points after ammonium withdrawal. As shown in Fig. 1B, the abundance of the RNA that would be synthesized from the tsp located at position −571 increased more conspicuously during the first 6 h and then continued to increase up to at least 24 h after the transfer to the combined-nitrogen-free medium, a time at which, under our culture conditions, fully differentiated heterocysts were already present in the cultures.
Regulation by NtcA of hetC expression.
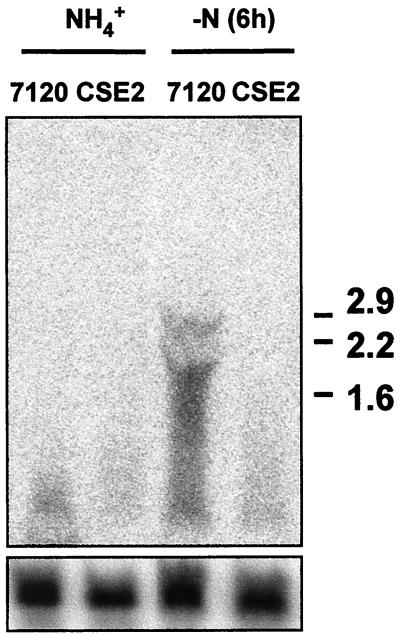
The pattern of expression of hetC in response to the nitrogen regime of the cells is consistent with that expected for an NtcA-regulated gene. To test the involvement of the transcriptional regulator NtcA in the control of hetC expression, Northern and primer extension assays were performed with RNA isolated from cells of mutant strain CSE2, which carries an insertionally inactivated ntcA gene (12), subjected to combined-nitrogen stepdown, and the results obtained were compared to those obtained with RNA from the wild-type strain PCC 7120. No RNA hybridizing to the hetC probe could be detected in strain CSE2 (Fig. 2). Repeated attempts to isolate intact transcripts from the hetC gene were unsuccessful; thus the observed signal in Northern blots corresponds to degradation products of the hetC transcript which, according to the location of the putative tsp and the size of the predicted hetC product (1,044 amino acids), should be at least 3.6 kb long. Additionally, no expression of hetC from the nitrogen-regulated tsp located at position −571 was detected in this mutant (Fig. 1). (The faint band at position −581 that shows up in strain CSE2 [Fig. 1] could be attributable to a putative weak ς70-type promoter whose −35 box, in the form GTAACA, would overlap the NtcA-binding site [see below].)
FIG. 2.
Northern blot analysis of expression of the hetC gene in Anabaena sp. strain PCC 7120 and mutant strain CSE2 (mutant ntcA). RNA was isolated from ammonium-grown cells (NH4+) or from ammonium-grown cells incubated for 6 h in combined-nitrogen-free medium [−N (6 h)]. Hybridization to a probe of the hetC gene (upper panel) was carried out as described in Materials and Methods. Samples contained 70 μg of RNA. Hybridization to rpnB (32) served as a loading and transfer control (lower panel). Size standards in kilobases are indicated on the right.
Binding of purified NtcA to the promoter region of the hetC gene.
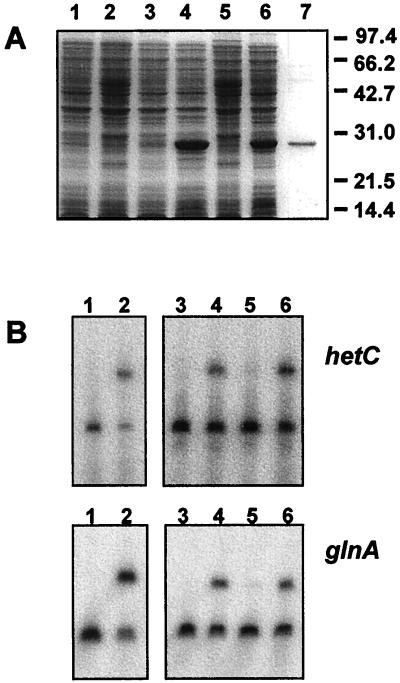
Thirty-three nucleotides upstream from the nitrogen-regulated tsp of hetC determined above, sequence GTAACATGAGATAC is found (21); this sequence conforms to the consensus sequence for NtcA-binding sites on DNA (11, 22). It is located about 605 bp upstream from the putative translation start of the hetC gene. In order to test binding of NtcA to the promoter region of hetC, histidine-tagged NtcA from E. coli cells bearing plasmid pCSAM70 was overproduced and purified. The NtcA protein encoded in pCSAM70 contains a 12-amino-acid N-terminal extension (MetArgGlySer[His]6GlySer-) preceding the complete native Anabaena sp. strain PCC 7120 NtcA sequence (with the only exception that Ile-2 is replaced by Val) and can thus be purified by immobilized metal ion affinity chromatography (see Materials and Methods for details). Figure 3A shows electrophoretic profiles of whole cells of the E. coli strains used for overproduction of NtcA, crude extracts from cells treated with IPTG, and a purified NtcA preparation.
FIG. 3.
Overproduction and purification of histidine-tagged NtcA and band-shift assays of a DNA fragment from the hetC promoter with purified histidine-tagged NtcA. (A) SDS-PAGE of samples of cultures of E. coli BL21(DE3) containing plasmid pREP4 and either vector pQE9 (lanes 1 and 2; 70 μl of culture) or NtcA expression plasmid pCSAM70 (lanes 3 and 4; 100 μl of culture), noninduced (lanes 1 and 3) or induced with IPTG (lanes 2 and 4). Lanes 5 and 6, crude extracts (100 μg of protein) from cultures shown in lanes 2 and 4, respectively; lane 7, 2.4 μg of purified histidine-tagged NtcA. Size standards in kilodaltons are indicated on the right. (B) Band-shift assays with histidine-tagged NtcA. Assays were carried out as described in Materials and Methods with fragments from the upstream regions of hetC or glnA. Left panels correspond to assays carried out with extracts from E. coli BL21(DE3) containing plasmid pREP4 and either vector pQE9 (lanes 1) or NtcA expression plasmid pCSAM70 (lanes 2). Right panels correspond to assays carried out without (lanes 3) or with (lanes 4 to 6) purified histidine-tagged NtcA (5 pmol) without competitor DNA (lanes 4) or with a 25-fold molar excess of the corresponding unlabeled fragment (lanes 5) or an unrelated, unlabeled fragment (lanes 6).
Mobility shift assays were carried out with a 283-bp, 32P-labeled DNA fragment from the promoter region of hetC containing the nitrogen-regulated tsp and sequences around it. Electrophoretic retardation of this DNA fragment was effected by the E. coli crude extract obtained after induction of the expression of the cloned ntcA gene but not by the extract from cells that did not carry a cloned ntcA gene (Fig. 3B, upper panel, lanes 1 and 2). Band retardation was also effected by the purified NtcA protein (Fig. 3B, upper panel, lane 4). Retardation of the labeled fragment was effectively competed by the same unlabeled DNA fragment (Fig. 3B, upper panel, lane 5) but not by an unrelated, unlabeled DNA fragment (Fig. 3B, upper panel, lane 6). For the sake of comparison, parallel experiments were carried out with a 171-bp DNA fragment from upstream of the Anabaena sp. strain PCC 7120 glnA gene, encoding glutamine synthetase, which comprises the PI promoter and which has previously been shown (12, 24) to bear an NtcA-binding site. Figure 3B, lower panel, shows that this fragment is indeed specifically retarded by NtcA. These experiments indicate a specific binding of NtcA to DNA sequences between positions −692 and −411 relative to the start of the hetC gene coding region.
Expression of hetR is independent of hetC.
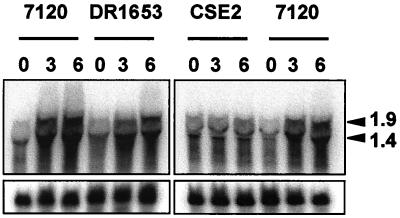
The expression of hetR was investigated by means of RNA-DNA hybridization in strain DR1653, which bears an insertionally inactivated hetC gene (21). Induction of hetR after nitrogen stepdown took place in the hetC mutant in a way similar to that observed in the wild-type strain (Fig. 4), showing that activation of hetR expression is independent of hetC. The observed hetR transcripts, of ca. 1.4 and 1.9 kb, were like those previously reported (4). As a control, expression of hetR was also tested with strain CSE2, in which, according to previously reported results (12), no induction of hetR was observed.
FIG. 4.
Northern blot analysis of expression of the hetR gene in Anabaena sp. strain PCC 7120 and mutant strains DR1653 (mutant hetC) and CSE2 (mutant ntcA). RNA was isolated from ammonium-grown cells (lanes 0) or from ammonium-grown cells incubated for 3 or 6 h in combined-nitrogen-free medium (lanes 3 and 6, respectively). Hybridization to a probe of the hetR gene was carried out as described in Materials and Methods. Samples contained 70 μg of RNA. Hybridization to rpnB (32) served as a loading and transfer control (lower panel). Arrowheads, two hetR transcripts of 1.4 and 1.9 kb (4).
DISCUSSION
In this work, we have determined a nitrogen-regulated tsp of hetC that is located at position −571 with respect to the putative translational start of the gene. This localized the putative NtcA-binding site previously noted upstream from hetC (21) in the position characteristic of transcription activator NtcA sites on DNA (Fig. 5). We have additionally shown that purified NtcA specifically binds to a DNA fragment containing the NtcA-binding site in the putative nitrogen-regulated promoter of hetC. Moreover, we have found that the hetC transcript is barely detectable in an ntcA mutant (Fig. 2) and that no transcription from the nitrogen-regulated promoter in that mutant took place (Fig. 1). These results demonstrate a direct transcriptional activation by NtcA of hetC. Previous studies of complementation of a hetC mutant with hetC-containing plasmids had indicated that the region located upstream from position −532 with respect to the hetC translational start might be required for stimulation of transcription of hetC under nitrogen-deprived conditions (21). This is consistent with the presence of the nitrogen-regulated hetC tsp at position −571.
FIG. 5.
Nucleotide sequences of the DNA regions upstream of the nitrogen-regulated tsp of three genes of Anabaena sp. strain PCC 7120 that have been shown to bear an NtcA-activated promoter. The consensus sequence for NtcA-activated promoters (11, 22) is also shown. The NtcA-binding site and −10 hexamer are indicated by gray boxes. The nucleotide corresponding to the tsp is underlined in each case.
As mentioned above, NtcA is required for activation of the expression of hetR (12); HetR is a key regulatory element that acts very early in heterocyst development and that is required for the expression of some other heterocyst development genes (7). However, no DNA sequence similar to the consensus sequence of the NtcA-binding site is present at the hetR promoters (cited in reference 19); thus, NtcA may have its induction effect on hetR via the activation of another gene(s). We have observed that hetR induction after nitrogen stepdown takes place normally in a mutant hetC background (Fig. 4), indicating that HetC is not the NtcA-dependent element required for hetR induction. Therefore, the NtcA-dependent direct induction of hetC described in this work indicates that more than one NtcA-dependent activation event is required for heterocyst development. Even more, NtcA-dependent gene expression appears to take place also in the mature heterocyst. Specific binding of NtcA to the promoter of the nifHDK operon has been described previously (24, 30), and we have recently observed that a promoter for the petH gene (encoding ferredoxin-NADP+ reductase), used in the heterocysts, is NtcA dependent and binds NtcA in vitro (30). Additionally, the xisA gene, which is activated late in the course of heterocyst development (15), seems also to be regulated somehow by NtcA, since the xisA upstream sequences bear three NtcA-binding sites (24). All of these data are consistent with the observations that (i) the ntcA gene is expressed both during heterocyst development and in mature heterocysts (2, 25) and (ii) heterocyst extracts appear to contain NtcA protein, as detected by means of mobility shift assays (24).
Our data showing a direct activation of the hetC promoter by NtcA suggest that expression of hetC responds to the environmental cue of nitrogen deficiency and represent the first determination of the mechanism by which regulation of expression of a gene involved in the differentiation of the cyanobacterial heterocyst is operated at the molecular level.
ACKNOWLEDGMENTS
We thank J. E. Frías for helpful technical advice and unpublished plasmids and C. P. Wolk for strain DR1653.
This work was supported by grant no. PB94-0074 from DGICYT and PB97-1137 from DGES (Spain). A.M.M.-P. was the recipient of a postdoctoral contract, and A.V. was the recipient of a fellowship from MEC (Spain).
REFERENCES
- 1.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: Greene Publishing and Wiley-Interscience; 1998. [Google Scholar]
- 2.Bauer C C, Haselkorn R. Vectors for determining the differential expression of genes in heterocysts and vegetative cells of Anabaena sp. strain PCC 7120. J Bacteriol. 1995;177:3332–3336. doi: 10.1128/jb.177.11.3332-3336.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Black T A, Cai Y, Wolk C P. Spatial expression and autoregulation of hetR, a gene involved in the control of heterocyst development in Anabaena. Mol Microbiol. 1993;9:77–84. doi: 10.1111/j.1365-2958.1993.tb01670.x. [DOI] [PubMed] [Google Scholar]
- 4.Buikema W J, Haselkorn R. Characterization of a gene controlling heterocyst differentiation in the cyanobacterium Anabaena 7120. Genes Dev. 1991;5:321–330. doi: 10.1101/gad.5.2.321. [DOI] [PubMed] [Google Scholar]
- 5.Buikema W J, Haselkorn R. Molecular genetics of cyanobacterial development. Annu Rev Plant Physiol Plant Mol Biol. 1993;44:33–52. [Google Scholar]
- 6.Cai Y, Wolk C P. Use of a conditionally lethal gene in Anabaena sp. strain PCC 7120 to select for double recombinants and to entrap insertion sequences. J Bacteriol. 1990;172:3138–3145. doi: 10.1128/jb.172.6.3138-3145.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cai Y, Wolk C P. Anabaena sp. strain PCC 7120 responds to nitrogen deprivation with a cascade-like sequence of transcriptional activations. J Bacteriol. 1997;179:267–271. doi: 10.1128/jb.179.1.267-271.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elhai J, Wolk C P. Developmental regulation and spatial pattern of expression of the structural genes for nitrogenase in the cyanobacterium Anabaena. EMBO J. 1990;9:3379–3388. doi: 10.1002/j.1460-2075.1990.tb07539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fay P. Oxygen relations of nitrogen fixation in cyanobacteria. Microbiol Rev. 1992;56:340–373. doi: 10.1128/mr.56.2.340-373.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flores E, Herrero A. Assimilatory nitrogen metabolism and its regulation. In: Bryant D A, editor. The molecular biology of cyanobacteria. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1994. pp. 487–517. [Google Scholar]
- 11.Flores E, Muro-Pastor A M, Herrero A. Cyanobacterial nitrogen assimilation genes and NtcA-dependent control of gene expression. In: Peschek G A, Loffelhardt W, Schmetterer G, editors. The phototrophic prokaryotes. New York, N.Y: Plenum Publishing Corp.; 1999. pp. 463–477. [Google Scholar]
- 12.Frías J E, Flores E, Herrero A. Requirement of the regulatory protein NtcA for the expression of nitrogen assimilation and heterocyst development genes in the cyanobacterium Anabaena sp. PCC 7120. Mol Microbiol. 1994;14:823–832. doi: 10.1111/j.1365-2958.1994.tb01318.x. [DOI] [PubMed] [Google Scholar]
- 13.Frías J E, Mérida A, Herrero A, Martín-Nieto J, Flores E. General distribution of the nitrogen control gene ntcA in cyanobacteria. J Bacteriol. 1993;175:5710–5713. doi: 10.1128/jb.175.17.5710-5713.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.García-Domínguez M, Florencio F J. Nitrogen availability and electron transport control the expression of glnB gene (encoding PII protein) in the cyanobacterium Synechocystis sp. PCC 6803. Plant Mol Biol. 1997;35:723–734. doi: 10.1023/a:1005846626187. [DOI] [PubMed] [Google Scholar]
- 15.Golden J W, Robinson S J, Haselkorn R. Rearrangement of nitrogen fixation genes during heterocyst differentiation in the cyanobacterium Anabaena. Nature. 1985;314:419–423. doi: 10.1038/314419a0. [DOI] [PubMed] [Google Scholar]
- 16.Golden J W, Whorff L L, Wiest D R. Independent regulation of nifHDK operon transcription and DNA rearrangement during heterocyst differentiation in the cyanobacterium Anabaena sp. strain PCC 7120. J Bacteriol. 1991;173:7098–7105. doi: 10.1128/jb.173.22.7098-7105.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Golden J W, Yoon H-S. Heterocyst formation in Anabaena. Curr Opin Microbiol. 1998;1:623–629. doi: 10.1016/s1369-5274(98)80106-9. [DOI] [PubMed] [Google Scholar]
- 18.Golden S S, Brusslan J, Haselkorn R. Genetic engineering of the cyanobacterial chromosome. Methods Enzymol. 1987;153:215–231. doi: 10.1016/0076-6879(87)53055-5. [DOI] [PubMed] [Google Scholar]
- 19.Haselkorn R, Schlictman D, Jones K, Buikema W J. Heterocyst differentiation and nitrogen fixation in cyanobacteria. In: Elmerich C, Kondorosi A, Newton W E, editors. Biological nitrogen fixation for the 21st century. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1998. pp. 93–96. [Google Scholar]
- 20.Holland D, Wolk C P. Identification and characterization of hetA, a gene that acts early in the process of morphological differentiation of heterocysts. J Bacteriol. 1990;172:3131–3137. doi: 10.1128/jb.172.6.3131-3137.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khudyakov I, Wolk C P. hetC, a gene coding for a protein similar to bacterial ABC protein exporters, is involved in early regulation of heterocyst differentiation in Anabaena sp. strain PCC 7120. J Bacteriol. 1997;179:6971–6978. doi: 10.1128/jb.179.22.6971-6978.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luque I, Flores E, Herrero A. Molecular mechanism for the operation of nitrogen control in cyanobacteria. EMBO J. 1994;13:2862–2869. doi: 10.1002/j.1460-2075.1994.tb06580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maldener I, Fiedler G, Ernst A, Fernandez-Piñas F, Wolk C P. Characterization of devA, a gene required for the maturation of proheterocysts in the cyanobacterium Anabaena sp. strain PCC 7120. J Bacteriol. 1994;176:7543–7549. doi: 10.1128/jb.176.24.7543-7549.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramasubramanian T S, Wei T-F, Golden J W. Two Anabaena sp. strain PCC 7120 DNA-binding factors interact with vegetative cell- and heterocyst-specific genes. J Bacteriol. 1994;176:1214–1223. doi: 10.1128/jb.176.5.1214-1223.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramasubramanian T S, Wei T-F, Oldham A K, Golden J W. Transcription of the Anabaena sp. strain PCC 7120 ntcA gene: multiple transcripts and NtcA binding. J Bacteriol. 1996;178:922–926. doi: 10.1128/jb.178.3.922-926.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rippka R, Deruelles J, Waterbury J B, Herdman M, Stanier R Y. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J Gen Microbiol. 1979;111:1–61. [Google Scholar]
- 27.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 28.Studier F W, Rosenberg A H, Dunn J J, Dubendorff J W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- 29.Tumer N E, Robinson S J, Haselkorn R. Different promoters for the Anabaena glutamine synthetase gene during growth using molecular or fixed nitrogen. Nature. 1983;306:337–342. [Google Scholar]
- 30.Valladares A, Muro-Pastor A M, Fillat M F, Herrero A, Flores E. Constitutive and nitrogen-regulated promoters of the petH gene encoding ferredoxin:NADP+ reductase in the heterocyst-forming cyanobacterium Anabaena sp. FEBS Lett. 1999;449:159–164. doi: 10.1016/s0014-5793(99)00404-4. [DOI] [PubMed] [Google Scholar]
- 31.Vega-Palas M A, Flores E, Herrero A. NtcA, a global nitrogen regulator from the cyanobacterium Synechococcus that belongs to the Crp family of transcriptional regulators. Mol Microbiol. 1992;6:1853–1859. doi: 10.1111/j.1365-2958.1992.tb01357.x. [DOI] [PubMed] [Google Scholar]
- 32.Vioque A. The RNase P from cyanobacteria: short tandemly repeated repetitive (STRR) sequences are present within the RNase P RNA gene in heterocyst-forming cyanobacteria. Nucleic Acids Res. 1997;25:3471–3477. doi: 10.1093/nar/25.17.3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wei T-F, Ramasubramanian T S, Golden J W. Anabaena sp. strain PCC 7120 ntcA gene required for growth on nitrate and heterocyst development. J Bacteriol. 1994;176:4473–4482. doi: 10.1128/jb.176.15.4473-4482.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wolk C P. Heterocyst formation. Annu Rev Genet. 1996;30:59–78. doi: 10.1146/annurev.genet.30.1.59. [DOI] [PubMed] [Google Scholar]
- 35.Wolk C P, Ernst A, Elhai J. Heterocyst metabolism and development. In: Bryant D A, editor. The molecular biology of cyanobacteria. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1994. pp. 769–823. [Google Scholar]
- 36.Yoon H-S, Golden J W. Heterocyst pattern formation controlled by a diffusible peptide. Science. 1998;282:935–938. doi: 10.1126/science.282.5390.935. [DOI] [PubMed] [Google Scholar]
- 37.Zhou R, Wei X, Jiang N, Li H, Dong Y, Hsi K-L, Zhao J. Evidence that HetR protein is an unusual serine-type protease. Proc Natl Acad Sci USA. 1998;95:4959–4963. doi: 10.1073/pnas.95.9.4959. [DOI] [PMC free article] [PubMed] [Google Scholar]