Abstract
Fusarium head blight (FHB) is a serious wheat disease caused by Fusarium graminearum (Fg) Schwabe. FHB can cause huge loss in wheat yield. In addition, trichothecene mycotoxins produced by Fg are harmful to the environment and humans. In our previous study, we obtained two mutants TPS1− and TPS2−. Neither of these mutants could synthesize trehalose, and they produced fewer mycotoxins. To understand the complex interaction between Fg and wheat, we systematically analyzed the metabolic responses of FHB-susceptible and -resistant wheat to ddH2O, the TPS− mutants and wild type (WT) using NMR combined with multivariate analysis. More than 40 metabolites were identified in wheat extracts including sugars, amino acids, organic acids, choline metabolites and other metabolites. When infected by Fg, FHB-resistant and -susceptible wheat plants showed different metabolic responses. For FHB-resistant wheat, there were clear metabolic differences between inoculation with mutants (TPS1−/TPS2−) and with ddH2O/WT. For the susceptible wheat, there were obvious metabolic differences between inoculation with mutant (TPS1−/TPS2−) and inoculation with ddH2O; however, there were no significant metabolic differences between inoculation with TPS− mutants and with WT. Specifically, compared with ddH2O, resistant wheat increased the levels of Phe, p-hydroxy cinnamic acid (p-HCA), and chlorogenic acid in response to TPS− mutants; however, susceptible wheat did not. Shikimate-mediated secondary metabolism was activated in the FHB-resistant wheat to inhibit the growth of Fg and reduce the production of mycotoxins. These results can be helpful for the development of FHB-resistant wheat varieties, although the molecular relationship between the trehalose biosynthetic pathway in Fg and shikimate-mediated secondary metabolism in wheat remains to be further studied.
Keywords: Fusarium graminearum, resistant and susceptible wheat, trehalose biosynthesis, TPS1 − , TPS2 − , metabonomics, NMR
1. Introduction
Fusarium head blight (FHB) is one of the most devastating diseases of wheat (Triticum aestivum L.) globally. FHB, caused by Fusarium graminearum (Fg) Schwabe (teleomorph Gibberella zeae Petch), not only leads to huge reductions in wheat grain yield, but also harms the environment and humans by producing deteriorated grain quality contaminated with trichothecene mycotoxins [1,2,3,4]. Since 1993, FHB has become a major problem for the agriculture industry in North America [5,6]. Apart from FHB, Fg can also infect other cereals (such as barley, maize, and oats), and cause stalk rot or root rot [7]. Although fungicides have been applied to control Fg, the resulting environmental problems and fungicide resistance are not negligible [7]. Breeding FHB-resistant wheat varieties is considered to be an economical and environmentally friendly approach to managing FHB.
Trehalose is a nonreducing disaccharide formed by two glucose molecules linked with a 1α–1α bond, and is widely found in plants, bacteria, fungi and insects [8]. In recent years, trehalose has drawn considerable attention for its important functions in serving as a carbon source [9], regulating osmotic pressure as a compatible solute in prokaryotes [10], and stabilizing and protecting membranes and proteins [11,12]. In addition, trehalose plays a significant role in the response to various stresses such as oxidative stress, heat, and drought [13,14,15]. More importantly, trehalose may play a role in signaling or regulation [16].
It is reported that there are at least five pathways for trehalose biosynthesis in different organisms [8,16]. The best-known pathway involves two steps: the first step is being catalyzed by trehalose 6-phosphate synthase (TPS1), and the second step is being catalyzed by trehalose 6-phosphate phosphatase (TPS2). TPS1 catalyzes the transfer of combined uridinediphospho (UDP) glucose and glucose 6-phosphate to generate trehalose 6-phosphate (T6P), while TPS2 is responsible for catalyzing the dephosphorylation of T6P to form trehalose [17,18]. Many studies have demonstrated that, apart from involvement in trehalose synthesis, TPS genes also take part in the development, pathogenicity and stress responses in yeast and higher fungi [19]. Blocking trehalose synthesis may be a promising approach for managing fungal diseases [20].
We obtained two mutant strains, TPS1− and TPS2−, from our previous study, carrying a single deletion of TPS1 or TPS2, respectively [21]. The results showed that TPS1 appeared unessential for Fg development and virulence, while TPS2 deletion abolished sporulation and sexual reproduction of Fg. In addition, it was reported that the TPS2− mutant had a more significant reduction in the production of mycotoxins compared with the TPS1− mutant [22].
Metabonomics has emerged as a powerful tool for studying the metabolic responses of plants to both biotic and abiotic stresses [23,24,25,26,27,28,29]. It has been used in understanding the interaction between plants and pathogens [30,31] and between plants and insects [24,32,33]. For example, metabolomics analysis of wheat leaves and stem tissues indicated that the levels of betaine, sucrose, glucose, glutamate, glutamine, alanine, trans-aconitic acid, and some aromatic compounds were positively correlated with FHB resistance [34]. Moreover, Liu et al. [25] found that the activation of γ- amino butyric acid shunt and shikimate-mediated secondary metabolism was vital for rice plants to resist insect infestation. Furthermore, combined transcriptomic and metabolomic analyses revealed that the tryptophan synthesis pathway plays an important role in the resistance of cotton to V. dahlia [35]. So far, the metabolic responses of FHB-resistant and -susceptible wheat to TPS− mutants and WT is unclear. However, this information can afford us metabolites or metabolic pathways related to FHB resistance and can afford help in controlling Fg and developing FHB-resistant wheat varieties.
In this study, we analyzed the metabolomics profiles of FHB-resistant and -susceptible wheat varieties inoculated with ddH2O, WT, and TPS− mutants. Our objectives are to obtain the different metabolic responses of resistant and susceptible wheat to ddH2O, WT, and TPS− mutants, which will offer important information for further cultivating FHB-resistant wheat varieties.
2. Materials and Methods
2.1. Chemicals
Methanol, NaH2PO4.2H2O, and K2HPO4.3H2O were purchased from Guoyao Chemical Co. Ltd. (Shanghai, China), while sodium 3-trimethlysilyl [2, 2, 3, 3-D4] propionate (TSP) and D2O (99.9% D) were obtained from Cambridge Isotope Laboratory (Miami, FL, USA).
2.2. Fungus Material Culture and Plant Materials
Fg strain 5035 (wild type, WT) was isolated from a scabby wheat spike in Wuhan (China). Strain 5035 was highly pathogenic to wheat through producing many mycotoxins [36,37]. TPS1− and TPS2− are two isogenic strains obtained from homologous recombination of Fg strain 5035 through Agrobacterium-mediated transformation [21]. Molecular characterization confirmed that trehalose 6-phosphate synthase gene was deleted in the TPS1− mutant, while trehalose 6-phosphate phosphatase was deleted in the TPS2− mutant [21]. Fg strains were cultured in CMC broth (7.5 g/L of carboxymethyl cellulose, 0.5 g/L of KH2PO4, 0.5 g/L of NH4NO3, 0.25 g/L of MgSO4.7H2O, and 0.5 g/L of yeast extract) [38] at 28 °C for 5 days (200 rpm). Conidiaspores were collected and adjusted to the concentration of about 1 × 106 spores/mL, and 10 μL of the conidia was cultured at 28 °C on potato-dextrose agar (PDA) for 3 days.
In this experiment, the wheat variety Sumai 3, which is resistant to FHB, and Annong 8455, which is susceptible to FHB, were grown in fields at Huazhong Agricultural University, Wuhan, China [39]. At the early anthesis, one spike per wheat was inoculated with 10 μL of ddH2O or the above conidia suspensions (Fg WT, TPS1−, and TPS2−) using a pipette tip for 96 h. Five inoculated spikes were collected as a sample, and a total of 46 samples of the two wheat varieties were obtained to afford five to seven biological replicates (n = 5–7). The wheat spikes were harvested and snap-frozen in liquid nitrogen, then stored at −80 °C until further analysis.
2.3. Metabolite Extraction for Wheat
Metabolites in wheat spikes were extracted using a previously reported method with some improvements [24]. The samples were freeze-dried, ground into powder with a mortar and pestle, and about 25 mg of the powder samples was transferred into a 2 mL Eppendorf tube with the addition of 1.2 mL of methanol/water solution (v/v = 2/1, −40 °C) and one 5 mm tungsten carbide bead (Qiagen, Germany). The mixture was homogenized using a tissuelyser (Qiagen, Germany) after drastically vortexing for 30 s followed by 15 min intermittent sonication (i.e., 30 s sonication with 30 s break) in an ice bath. The supernatant of each sample was transferred into a new 5 mL Eppendorf tube following centrifugation for 10 min (16,099× g, 4 °C). The remaining residues were further extracted twice using the same method, and three supernatants were combined as one sample. After removal of methanol under vacuum, samples were lyophilized. The freeze-dried extracts were redissolved in 600 μL of phosphate buffer (0.1 M K2HPO4-NaH2PO4, pH 7.4) containing 50% D2O (v/v) and 0.02% TSP [40]. After being centrifuged for 10 min (16,099× g, 4°C), a total of 500 μL of supernatant for each sample was transferred into 5 mm NMR tubes for NMR-based metabolite analysis.
2.4. NMR Measurements
All 1H NMR spectra of samples for wheat spikes were acquired at 298 K using an inverse detection cryogenic probe on a Bruker AVIII 600 spectrometer (Bruker Biospin, GmbH, Rheinstetten, Germany). The 1H NMR spectra were acquired using NOESY pulse sequence (RD-90°-t1-90°-tm-90°-acquisition) with 90° pulse length of about 9.4 μs and t1 was set to 2 μs. Water peak was saturated with a weak irradiation during the recycle delay (RD) of 2 s and a mixing time (tm) of 100 ms, and 32 transients were collected into 64 k data points with a spectral width of 20 ppm. A series of 2D NMR spectra including 1H-1H TOCSY, 1H-1H COSY, 1H-JRES, 1H-13C HSQC, and 1H-13C HMBC spectra were acquired using selected samples for metabolite assignments [24].
2.5. Spectral Processing and Multivariate Data Analysis
Following phase and baseline correction using TopSpin (v3.1, Bruker Biospin GmbH, Germany), all NMR spectra were referenced to the internal standard TSP at δ 0.000 ppm. The spectral region between 0.5 and 10.0 ppm was divided into bins with a width of 0.004 ppm (2.4 Hz) using the AMIX software (v 3.8.3, Bruker Biospin GmbH, Germany). The water regions at δ 4.700–5.100 were removed. A total of 2275 bins for all remaining regions were normalized to the dry weight of wheat spikes to give a dataset in the form of signal area (metabolite quantity) per gram dry weight of wheat.
Principal component analysis (PCA) and orthogonal projection to latent structures discriminant analysis (OPLS-DA) [41] were both performed on the normalized NMR data using SIMCA-P+ software (v12.0, Umetrics, Umea, Sweden). In OPLS-DA models, one orthogonal and one predictive component were calculated using the unit-variance (UV) scaled NMR data as X-matrix and the class information as Y-matrix. The model qualities were described by the explained variances for X-matrix (R2X values) and the model predictability (Q2 values) with further assessment with ANOVA of the cross-validated residuals (CV-ANOVA) approach where intergroup differences were considered as significant with p value < 0.05 [42]. Leave-One-Out (LOO) validation was used in the cross-validation of the models. The results were exhibited in both the form of scores plots and loadings plots, in which scores plots and loadings plots showed group clustering and indicated variables (metabolite levels) contributing to inter-group differences, respectively. In such loading plots, variables were color-coded according to absolute values of the correlation coefficients (|r|) [43], and variables (i.e., metabolite contents) with a cool color (e.g., blue) showed less significant contributions to inter-group differences than those with a warm color (e.g., red). In this study, the metabolites showing statistically significant changes were obtained at the level of p < 0.05.
3. Results
3.1. Metabolic Profiles for FHB-Susceptible and -Resistant Wheats
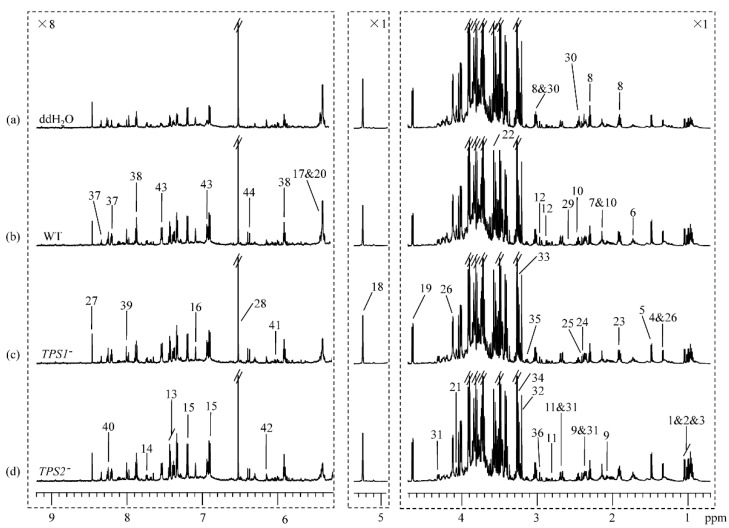
1H NMR spectra of wheat spike extracts showed obvious difference in the metabolic profiles for FHB-resistant wheat (Sumai 3) inoculated with ddH2O, WT, TPS1−, and TPS2− (Figure 1). Similarly, there was also an obvious difference in the metabolic profiles for the FHB-susceptible wheat (Annong 8455) inoculated with ddH2O, WT, TPS1−, and TPS2− (Figure 2). Signals were assigned to individual metabolites (Table S1) based on data in the literature [23,44,45] and in-house databases. A series of 2D NMR spectra were acquired for selected samples to further confirm metabolite identifications. More than 40 metabolites were identified, including 5 sugars (sucrose, glucose, raffinose, fructose, and myo-inositol), 16 amino acids and their metabolites (Val, Ieu, Ile, Thr, Ala, Arg, Met, GABA, Glu, Gln, Asp, Asn, Phe, Trp, Tyr, and His), 9 organic acids (acetate, lactate, pyruvate, succinate, fumarate, citrate, α-ketoglutarate, malate, and formate), 5 choline metabolites (choline, phosphocholine, glycine betaine, ethanolamine, and dimethylglycine), 6 nucleotide metabolites (adenosine, uridine, guanosine, hypoxanthine, inosine, and AMP), and 2 secondary metabolites (p-hydroxy cinnamic acid and chlorogenic acid) (Figure 1 and Figure 2, Table S1).
Figure 1.
1H NMR spectra of extracts for resistant wheat Sumai 3 inoculated with (a) ddH2O, (b) Fg WT 5035, (c) Fg TPS1− mutant, and (d) Fg TPS2− mutant for 96 h. The region δ 5.31–9.21 was vertically expanded 8 times. Keys: 1, isoleucine (Ile); 2, leucine (Leu); 3, valine (Val); 4, threonine (Thr); 5, alanine (Ala); 6, arginine (Arg); 7, methionine (Met); 8, γ-aminobutyrate (GABA); 9, glutamate (Glu); 10, glutamine (Gln); 11, aspartate (Asp); 12, asparagine (Asn); 13, phenylalanine (Phe); 14, tryptophan (Trp); 15, tyrosine (Tyr); 16, histidine (His); 17, sucrose; 18, α-glucose; 19, β-glucose; 20, raffinose; 21, fructose; 22, myo-inositol; 23, acetate; 24, pyruvate; 25, succinate; 26, lactate; 27, formate; 28, fumarate; 29, citrate; 30, α-ketoglutarate (α-KG); 31, malate; 32, choline; 33, phosphocholine (PC); 34, glycine betaine (GB); 35, ethanolamine (EA); 36, dimethylamine; 37, adenosine; 38, uridine; 39, guanosine; 40, hypoxanthine; 41, inosine; 42, deoxy adenosine monophosphate (dAMP); 43, p-hydorxy cinnamic acid; 44, chlorogenic acid; 45, thymidine.
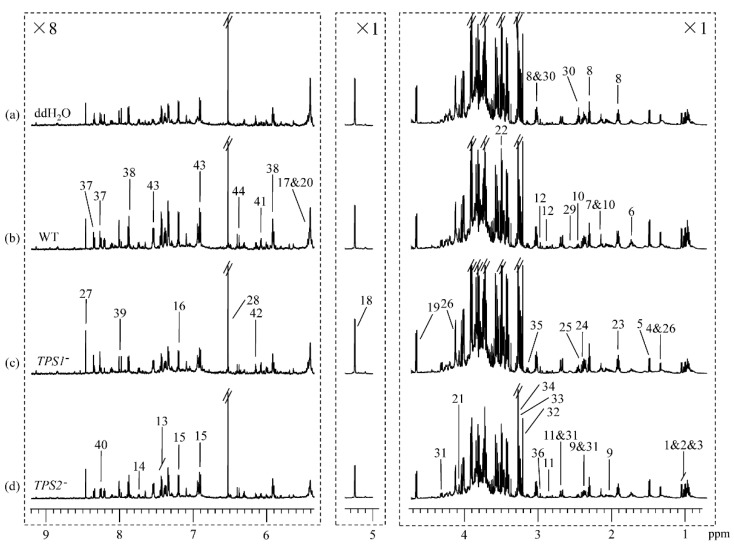
Figure 2.
1H NMR spectra of extracts for susceptible wheat Annong 8455 inoculated with (a) ddH2O, (b) Fg WT 5035, (c) Fg TPS1− mutant, and (d) Fg TPS2− mutant for 96 h. The region δ 5.31–9.21 was vertically expanded 8 times. Keys were indicated in Figure 1 and Table S1.
Visual inspection of Figure 1 suggested that for the resistant wheat Sumai 3, inoculation with the TPS1− mutant for 96 h induced significant elevation in guanosine level along with a decrease in α-ketoglutarate level when compared with inoculation with ddH2O, and it had decreases in myo-inositol and Asp levels when compared with inoculation with WT (Figure 1a–c). In addition, for the resistant wheat Sumai 3, inoculation with the TPS2− mutant induced significantly higher levels of Phe and chlorogenic acid than inoculation with ddH2O, and it had a lower level of sucrose than with inoculation with WT (Figure 1a,b,d).
For the susceptible wheat variety Annong 8455, the level of adenosine was higher in response to inoculation with the TPS1− mutant for 96 h than inoculation with ddH2O, and the levels of sucrose and guanosine were lower after being inoculated with the TPS2− mutant compared with inoculation with ddH2O (Figure 2). Moreover, inoculation with the TPS2− mutant induced a more significant reduction in glucose and malate levels in the susceptible wheat than inoculation with the TPS1− mutant (Figure 2c,d). To obtain more detailed information about metabolic changes in the resistant and susceptible wheats induced by ddH2O, WT, TPS1−, and TPS2−, multivariate data analyses were performed on the NMR data of these wheat spikes.
3.2. Different Metabolic Responses of FHB-Resistant and -Susceptible Wheats to Three Fusarium Strains
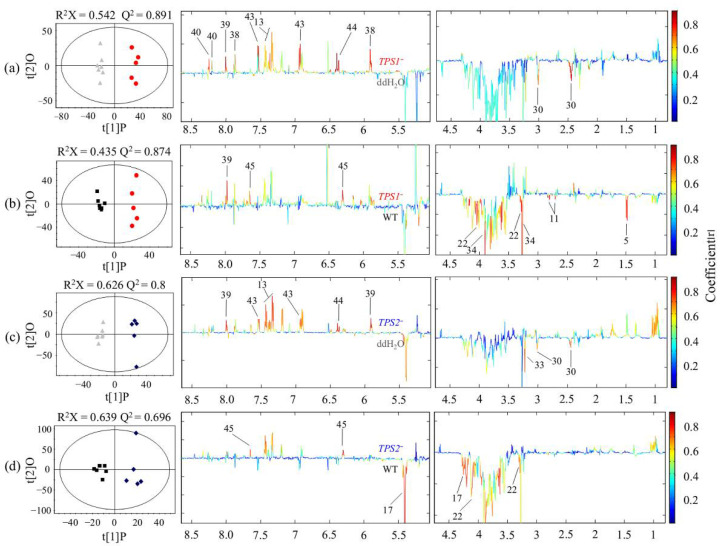
PCA of the NMR data for FHB-resistant and -susceptible wheat spikes showed that there were no obvious metabolic differences between those inoculated with mutants (including TPS1− and TPS2−) and those inoculated with WT/ddH2O, and it was the same between those inoculated with TPS1− and TPS2− (Figure S1). Pairwise OPLS-DA was conducted between the extracts of wheat spikes inoculated with the TPS− mutants and those inoculated with WT/ddH2O for both the resistant and susceptible wheat varieties. In addition, OPLS-DA modeling was also conducted comparing the extracts of wheat spikes inoculated with different TPS- mutants. Significantly different metabolites between these two groups were tabulated in Table 1. OPLS-DA model parameters showed that for the FHB-resistant wheat Sumai 3, there were clear metabolic differences between being inoculated with mutants (including TPS1− and TPS2−) and with ddH2O/WT (Figure 3). Significantly altered metabolites between the two groups were tabulated in Table 1. The loadings plots of OPLS-DA showed that compared with ddH2O, inoculation with TPS1- in the resistant wheat induced increased levels of Phe, uridine, guanosine, hypoxanthine, p-hydorxy cinnamic, acid and chlorogenic acid together with a decreased level of α-ketoglutarate (Figure 3a, Table 1). Inoculation with TPS2− in Sumai 3 led to higher levels for Phe, guanosine, p-hydorxy cinnamic acid, and chlorgenic acid along with a lower level of α-ketoglutarate than with ddH2O, which was similar to inoculation with TPS1−; in addition, inoculation with TPS2− also led to a decrease in phosphocholine level (Figure 3c, Table 1). Compared with WT, inoculation with TPS1− in the FHB-resistant wheat resulted in elevated levels of guanosine and thymidine together with reduced levels of myo-inositiol, Ala, Asp, and glycine betaine (Figure 3b, Table 1). However, inoculation with TPS2− in Sumai 3 led to increased thymidine level together with decreased levels of sucrose and myo-inositol (Figure 3d, Table 1). Furthermore, we compared the metabolic profiles for inoculation with the TPS1− and TPS2− mutants in the resistant wheat. The results indicated that there was no obvious metabolic difference between those inoculated with different TPS− mutants (R2X = 0.636, Q2 = 0.126, CV-ANOVA p = 1).
Table 1.
Significantly changed metabolites in the resistant and susceptible wheat when inoculated with ddH2O (H2O), WT, TPS1−, and TPS2−.
| Coefficient (r) a | |||||||
|---|---|---|---|---|---|---|---|
| Metabolites (No) | Sumai 3 (Resistant) | Annong 8455 (Susceptible) | |||||
| Sugars | TPS1− vs. H2O | TPS1− vs. WT | TPS2− vs. H2O |
TPS2− vs. WT |
TPS1− vs. H2O |
TPS2− vs. H2O |
TPS2− vs. TPS1− |
| sucrose (17) c | −0.849 b | −0.882 | |||||
| α-glucose (18) | −0.826 | ||||||
| β-glucose (19) | −0.816 | ||||||
| fructose (21) | −0.857 | ||||||
| myo-inositol (22) | −0.788 | −0.821 | |||||
| Amino acids | |||||||
| Ala (5) | −0.781 | ||||||
| Gln (10) | −0.762 | ||||||
| Asp (11) | −0.857 | ||||||
| Phe (13) | 0.835 | 0.756 | |||||
| Organic acids | |||||||
| pyruvate (24) | −0.841 | ||||||
| formate (27) | −0.823 | ||||||
| fumarate (28) | 0.794 | −0.904 | |||||
| a-KG (30) | −0.836 | −0.782 | −0.769 | ||||
| malate (31) | −0.963 | ||||||
| Choline metabolites | |||||||
| phosphocholine (33) | −0.859 | −0.877 | −0.871 | ||||
| glycine betaine (34) | −0.837 | 0.814 | −0.892 | ||||
| Nucleotide metabolites | |||||||
| adenosine (37) | 0.824 | −0.805 | −0.917 | ||||
| uridine (38) | 0.825 | ||||||
| guanosine (39) | 0.929 | 0.758 | 0.799 | −0.826 | −0.799 | ||
| hypoxanthine (40) | 0.856 | ||||||
| thymidine (45) | 0.821 | 0.834 | −0.843 | ||||
| Secondary metabolites | |||||||
| p-hydroxy cinnamaic acid (43) | 0.854 | 0.753 | |||||
| chlorogenic acid (44) | 0.922 | 0.826 | |||||
a The coefficients were obtained from OPLS-DA results, and positive and negative signs indicate positive and negative correlation in the concentrations, respectively. b Positive and negative signs indicate the elevation and decrease of the metabolite levels. Values for p ≥ 0.05 were not tabulated. c Metabolite keys are identical to those in Figure 1 and Table S1.
Figure 3.
OPLS-DA scores plots (left) and coefficient-coded loadings plots (right) showing metabolic differences of FHB-resistant wheat Sumai 3 inoculated with (a) TPS1− (red) vs. ddH2O (grey) (CV-ANOVA p = 0.0018), (b) TPS1− (red) vs. WT (black) (CV-ANOVA p = 0.0029), (c) TPS2− (blue) vs. ddH2O (grey) (CV-ANOVA p = 0.014), and (d) TPS2− (blue) vs. WT (black) (CV-ANOVA p = 0.01). Metabolite keys are the same as in Figure 1 and Table S1.
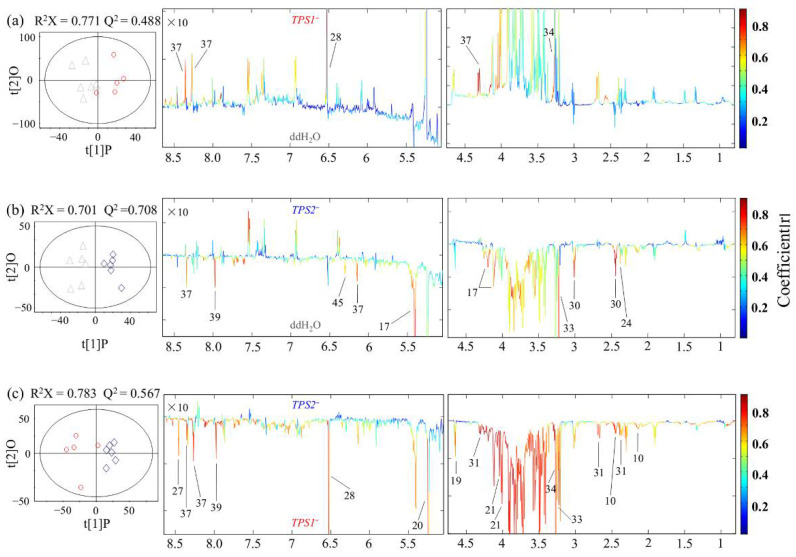
For the susceptible wheat Annong 8455, OPLS-DA model parameters indicated that there were obvious metabolic differences between those inoculated with mutants (including TPS1− and TPS2−) and with ddH2O (Figure 4). The color loading plots of OPLS-DA revealed that compared with ddH2O, infection with the TPS1− mutant in the susceptible wheat induced increased levels of fumarate, glycine betaine, and adenosine (Figure 4a, Table 1). Infection with the TPS2− mutant in Annong 8455 led to lower levels of sucrose, fumarate, α-ketoglutarate, phosphocholine, adenosine, guanosine, and thymidine (Figure 4b, Table 1). However, there were no significant metabolic differences between inoculation with TPS1− and with WT (R2X = 0.868, Q2 = 0.258, CV-ANOVA p = 1) or between inoculation with TPS2− and with WT (R2X = 0.769, Q2 = 0.0965, CV-ANOVA p = 1) in the susceptible wheat. The results also showed that for the susceptible wheat, compared with TPS1−, infection with TPS2− resulted in a reduction in the levels of most metabolites including two sugars (glucose and fructose), Gln, three organic acids (fumarate, malate, and formate), two choline metabolites (glycine betaine and phosphocholine), and two nucleotide metabolites (adenosine and guanosine) (Figure 4c, Table 1).
Figure 4.
OPLS-DA scores plots (left) and coefficient-coded loadings plots (right) showing metabolic differences of FHB-susceptible wheat Annong 8455 inoculated with (a) TPS1− (red) vs. ddH2O (grey) (CV-ANOVA p = 0.005), (b) TPS2− (blue) vs. ddH2O (grey) (CV-ANOVA p = 0.001) and (c) TPS2− (blue) vs. TPS1− (red) (CV-ANOVA p = 0.005). Metabolite keys are the same as in Figure 1 and Table S1.
4. Discussion
FHB induced by Fg is a destructive disease for wheat. In our previous study [21], we obtained two mutants, TPS1− and TPS2−, carrying a single deletion of TPS1 (trehalose 6-phosphate synthase) and TPS2 (trehalose 6-phosphate phosphatase), respectively, both of these two enzymes being involved in trehalose synthesis in Fg. Liu et al. also found that Fg TPS1− and TPS2− both produce fewer mycotoxins than WT (5035), and TPS2− produces fewer mycotoxins than TPS1− [22]. However, the metabolic responses of FHB-resistant and -susceptible wheat to Fg (WT, TPS1−, and TPS2−) inoculation are not clear, but could give us metabolic information related to trehalose synthesis and FHB resistance.
Sumai 3 is a traditional FHB-resistant wheat variety, while Annong 8455 is a known FHB-susceptible wheat variety. The metabolic differences in both FHB-resistant and -susceptible wheat varieties when inoculated with TPS− and with ddH2O could give us metabolic information about Fg pathogenicity, while the metabolic differences between inoculation with TPS− and with WT in both FHB-resistant and -susceptible wheat varieties could give us metabolic information about the difference in pathogenicity induced by TPS1 and TPS2 mutations.
For the resistant wheat Sumai 3, there were higher levels of Phe, guanosine, p-HCA and chlorogenic acid together with a lower level of α-KG after being inoculated with TPS1− or TPS2− when compared with being inoculated with ddH2O (Figure 3 and Figure 5, Table 1). This result indicated that Phe, p-HCA, and chlorogenic acid might be closely related to FHB-resistance. This is not surprising because both p-HCA and chlorogenic acid are secondary metabolites derived from the shikimate pathway for biosynthesis of phenylpropanoid and flavonoid metabolites. It has been reported that Sumai 3 has a fast and strong response primarily through the activation of the shikimate pathway [28]. HCAs have been reported to play roles in plant defense responses to pathogen challenge and wounding as integral components [46,47]. Chlorogenic acid, belonging to phenolic acids, has been reported to be a resistance factor to Fg in maize [48], to Podosphaera in miniature roses [49] and to diatraea saccharalis in sugarcane [50]. Sumai 3 might synthesize HCAs to reduce pathogen advancement through thickening host cell walls together with synthesizing antifungal/antioxidant chlorogenic acid for inhibiting pathogen growth, in turn reducing subsequent mycotoxin biosynthesis in Fg [51]. In a word, shikimate-mediated secondary metabolism was activated in the FHB-resistant wheat to produce HCAs and chlorogenic acid to inhibit the growth of Fg and reduce the production of mycotoxins.
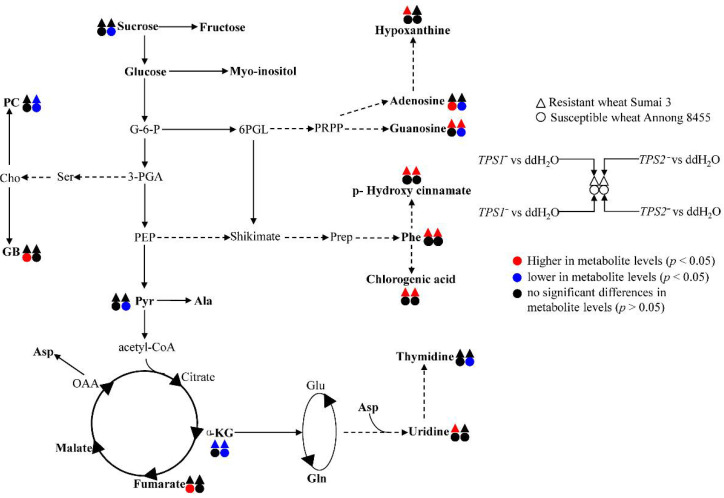
Figure 5.
The metabolic responses of Sumai 3 and Annong 8455 to TPS− mutants and ddH2O inoculation. Red colored symbols indicate significant up-regulations of metabolites (p < 0.05), whereas blue colored symbols represent downregulations of metabolites (p < 0.05). G-6-P, glucose-6-phosphate; 3-PGA, 3-phosphate glycerate; Ser, serine; Cho, choline; PC, phosphocholine; GB, glycine betaine; OAA, oxaloacetic acid; Pyr, pyruvate; PEP, phosphoenolpyruvic acid; 6PGL, 6-phosphogluconate; PRPP, 5-phosphoribosyl diphosphate; Prep, prephenic acid.
In Sumai 3, when compared with that inoculated with WT, only the change trends of thymidine and myo-inositol were similar after being inoculated with TPS1− or TPS2− (Figure 3 and Figure 5, Table 1), which is attributed to the different impacts of TPS1 and TPS2 in Fg on wheat metabolism. The fact that the number of altered metabolites was less in TPS2− vs. WT than in TPS1− vs. WT suggested that the induced resistance response was much stronger in TPS1− than in TPS2−, and the significantly decreased metabolites (Ala, Asp, and GB) might be closely related to FHB resistance in an unknown way (Figure 5).
For FHB-susceptible wheat variety Annong 8455, there were no metabolic differences when inoculated with either TPS1− or TPS2− compared to inoculation with WT. This is not surprising because the metabolic responses of Annong 8455, a highly FHB-susceptible wheat variety, to TPS1−/TPS2− and WT were similar, although the virulence of both TPS1− and TPS2− is lower than that of WT. For Annong 8455, compared to being inoculated with ddH2O, there were significantly higher levels of fumarate, GB and adenosine after being inoculated with TPS1−; however, there were significantly lower levels of sucrose, two organic acids (pyruvate and α-KG), phosphocholine, and three nucleic acids (adenosine, guanosine, and thymidine) after being inoculated with TPS2− (Figure 4 and Figure 5, Table 1). This result indicated that TPS1− infection induced a slight alteration in Annong 8455; nevertheless, TPS2− infection slowed glycolysis, TCA cycle, and nucleotide synthesis. The former might be caused by trehalose deletion, and the latter might be caused by redundancy of T6P in Fg [21,52]. T6P in Fg may affect the global metabolism of wheat either as a metabolic regulator or through interaction with some compounds in wheat to regulate wheat metabolism [20]. However, how the redundancy of T6P in Fg affects wheat metabolism is still to be further studied.
5. Conclusions
In conclusion, when infected by Fg, FHB-susceptible and -resistant wheat showed different metabolic responses. Specifically, compared with ddH2O, resistant wheat increased the levels of Phe, p-HCA, and chlorogenic acid to resist TPS− mutants; however, susceptible wheat did not. The metabolic difference might be caused by trehalose deletion and redundancy of T6P in the susceptible wheat when infected by TPS1− or TPS2− compared with ddH2O. Finally, a hypothesis is proposed that when infected by Fg, shikimate-mediated secondary metabolism was activated in the FHB-resistant wheat to produce HCAs and chlorogenic acid to inhibit the growth of Fg and reduce the production of mycotoxins. However, it should be stressed that the molecular relationships between the trehalose biosynthetic pathway in Fg and shikimate-mediated secondary metabolism in wheat remains to be further studied.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/metabo12080727/s1, Table S1. Assignment of NMR data for metabolites in FHB-resistant (Sumai 3) and -susceptible (Annong 8455) wheat varieties inoculated with ddH2O, wild type (WT), TPS1− and TPS2−; Figure S1. PCA scores plots of the NMR data for FHB-resistant Sumai 3 (a) and -susceptible wheat Annong 8455 (b). The numbers in parentheses indicate the overall variance explained in the first two principal components. Grey (triangle), black (square), red (circle), and blue (diamond) indicate metabolites in both FHB-resistant (solid symbol) and -susceptible wheat (open symbol) inoculated with ddH2O, WT, TPS1−, and TPS2−, respectively.
Author Contributions
Conceptualization, X.Z. and C.L.; methodology, H.L.; software, L.L. and X.F.; validation, X.Z., C.L. and F.C.; formal analysis, X.F.; investigation, X.Z.; resources, X.Z.; data curation, L.L.; writing—original draft preparation, C.L.; writing—review and editing, F.C. and D.Z.; visualization, C.L.; supervision, X.Z.; project administration, X.Z.; funding acquisition, X.Z. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
All authors declared no conflict of interest.
Funding Statement
This research was funded by National Natural Science Foundation of China (32071990, 21991081 and 21735007), the National Key R&D Program of China (2018YFE0202300), and the Interdisciplinary Cultivation Project of the Innovation Academy for Precision Measurement of Science and Technology (S21S1101) and The APC was funded by the National Key R&D Program of China (2018YFE0202300).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Blanco A., Bellomo M., Cenci A., De Giovanni C., D’ovidio R., Iacono E., Laddomada B., Pagnotta M., Porceddu E., Sciancalepore A. A genetic linkage map of durum wheat. Theor. Appl. Genet. 1998;97:721–728. doi: 10.1007/s001220050948. [DOI] [Google Scholar]
- 2.Otto C., Kianian S., Elias E., Stack R., Joppa L. Genetic dissection of a major Fusarium head blight QTL in tetraploid wheat. Plant Mol. Biol. 2002;48:625–632. doi: 10.1023/A:1014821929830. [DOI] [PubMed] [Google Scholar]
- 3.Duffeck M.R., Bandara A.Y., Weerasooriya D.K., Collins A.A., Jensen P.J., Kuldau G.A., Del Ponte E.M., Esker P.D. Fusarium head blight of small grains in pennsylvania: Unravelling species diversity, toxin types, growth, and triazole sensitivity. Phytopathology. 2022;112:794–802. doi: 10.1094/PHYTO-02-21-0070-R. [DOI] [PubMed] [Google Scholar]
- 4.Podgorska-Kryszczuk I., Solarska E., Kordowska-Wiater M. Reduction of the fusarium mycotoxins: Deoxynivalenol, nivalenol and zearalenone by selected non-conventional yeast strains in wheat grains and bread. Molecules. 2022;27:1578. doi: 10.3390/molecules27051578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goswami R.S., Kistler H.C. Heading for disaster: Fusarium graminearum on cereal crops. Mol. Plant Pathol. 2004;5:515–525. doi: 10.1111/j.1364-3703.2004.00252.x. [DOI] [PubMed] [Google Scholar]
- 6.Xu X.-M., Parry D., Nicholson P., Thomsett M., Simpson D., Edwards S., Cooke B., Doohan F., Brennan J., Moretti A. Predominance and association of pathogenic fungi causing Fusarium ear blightin wheat in four European countries. Eur. J. Plant Pathol. 2005;112:143–154. doi: 10.1007/s10658-005-2446-7. [DOI] [Google Scholar]
- 7.Rudd J., Horsley R., McKendry A., Elias E. Host plant resistance genes for Fusarium head blight. Crop Sci. 2001;41:620–627. doi: 10.2135/cropsci2001.413620x. [DOI] [Google Scholar]
- 8.Avonce N., MendozaVargas A., Morett E., Iturriaga G. Insights on the evolution of trehalose biosynthesis. BMC Evol. Biol. 2006;6:109. doi: 10.1186/1471-2148-6-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gancedo C., Flores C.L. The importance of a functional trehalose biosynthetic pathway for the life of yeasts and fungi. FEMS Yeast Res. 2004;4:351–359. doi: 10.1016/S1567-1356(03)00222-8. [DOI] [PubMed] [Google Scholar]
- 10.Purvis J.E., Yomano L., Ingram L. Enhanced trehalose production improves growth of Escherichia coli under osmotic stress. Appl. Environ. Microb. 2005;71:3761–3769. doi: 10.1128/AEM.71.7.3761-3769.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crowe J.H., Crowe L.M., Chapman D. Preservation of membranes in anhydrobiotic organisms: The role of trehalose. Science. 1984;223:701–703. doi: 10.1126/science.223.4637.701. [DOI] [PubMed] [Google Scholar]
- 12.Singer M.A., Lindquist S. Multiple effects of trehalose on protein folding in vitro and in vivo. Mol. Cell. 1998;1:639–648. doi: 10.1016/S1097-2765(00)80064-7. [DOI] [PubMed] [Google Scholar]
- 13.Cao Y., Wang Y., Dai B., Wang B., Zhang H., Zhu Z., Xu Y., Cao Y., Jiang Y., Zhang G. Trehalose is an important mediator of Cap1p oxidative stress response in Candida albicans. Biol. Pharm. Bull. 2008;31:421–425. doi: 10.1248/bpb.31.421. [DOI] [PubMed] [Google Scholar]
- 14.Zakharova K., Tesei D., Marzban G., Dijksterhuis J., Wyatt T., Sterflinger K. Microcolonial fungi on rocks: A life in constant drought? Mycopathologia. 2013;175:537–547. doi: 10.1007/s11046-012-9592-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Z.W., Sun M., Gao Y.M., Luo Y. Exogenous trehalose differently improves photosynthetic carbon assimilation capacities in maize and wheat under heat stress. J. Plant Interact. 2022;17:361–370. doi: 10.1080/17429145.2022.2041119. [DOI] [Google Scholar]
- 16.Elbein A.D., Pan Y., Pastuszak I., Carroll D. New insights on trehalose: A multifunctional molecule. Glycobiology. 2003;13:17–27. doi: 10.1093/glycob/cwg047. [DOI] [PubMed] [Google Scholar]
- 17.Bell W., Klaassen P., Ohnacker M., Boller T., Herweijer M., Schoppink P., Vanderzee P., Wiemken A. Characterization of the 56-kDa subunit of yeast trehalose-6-phosphate synthase and cloning of its gene reveal its identity with the product of CIF1, a regulator of carbon catabolite inactivation. FEBS J. 1992;209:951–959. doi: 10.1111/j.1432-1033.1992.tb17368.x. [DOI] [PubMed] [Google Scholar]
- 18.Vuorio O.E., Kalkkinen N., Londesborough J. Cloning of two related genes encoding the 56-kDa and 123-kDa subunits of trehalose synthase from the yeast Saccharomyces cerevisiae. FEBS J. 1993;216:849–861. doi: 10.1111/j.1432-1033.1993.tb18207.x. [DOI] [PubMed] [Google Scholar]
- 19.Al-Bader N., Vanier G., Liu H., Gravelat F.N., Urb M., Hoareau C.M.-Q., Campoli P., Chabot J., Filler S.G., Sheppard D.C. Role of trehalose biosynthesis in Aspergillus fumigatus development, stress response, and virulence. Infect. Immun. 2010;78:3007–3018. doi: 10.1128/IAI.00813-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Puttikamonkul S., Willger S.D., Grahl N., Perfect J.R., Movahed N., Bothner B., Park S., Paderu P., Perlin D.S., Cramer R.A., Jr. Trehalose 6-phosphate phosphatase is required for cell wall integrity and fungal virulence but not trehalose biosynthesis in the human fungal pathogen Aspergillus fumigatus. Mol. Microbiol. 2010;77:891–911. doi: 10.1111/j.1365-2958.2010.07254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song X.-S., Li H.-P., Zhang J.-B., Song B., Huang T., Du X.-M., Gong A.-D., Liu Y.-K., Feng Y.-N., Agboola R.S. Trehalose 6-phosphate phosphatase is required for development, virulence and mycotoxin biosynthesis apart from trehalose biosynthesis in Fusarium graminearum. Fungal Genet. Biol. 2014;63:24–41. doi: 10.1016/j.fgb.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 22.Liu C., Chen F., Zhang J., Liu L., Lei H., Li H., Wang Y., Liao Y.-C., Tang H. Metabolic changes of Fusarium graminearum induced by TPS gene deletion. J. Proteome Res. 2019;18:3317–3327. doi: 10.1021/acs.jproteome.9b00259. [DOI] [PubMed] [Google Scholar]
- 23.Chen F., Liu C., Zhang J., Lei H., Li H.-P., Liao Y.-C., Tang H. Combined metabonomic and quantitative RT-PCR analyses revealed metabolic reprogramming associated with Fusarium graminearum resistance in transgenic Arabidopsis thaliana. Front. Plant Sci. 2017;8:2177. doi: 10.3389/fpls.2017.02177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu C.X., Ding F., Hao F.H., Yu M., Lei H.H., Wu X.Y., Zhao Z.X., Guo H.X., Yin J., Wang Y.L., et al. Reprogramming of seed metabolism facilitates pre-harvest sprouting resistance of wheat. Sci. Rep. 2016;6:20593. doi: 10.1038/srep20593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu C., Hao F., Hu J., Zhang W., Wan L., Zhu L., Tang H., He G. Revealing different systems responses to brown planthopper infestation for pest susceptible and resistant rice plants with the combined metabonomic and gene-expression analysis. J. Proteome Res. 2010;9:6774–6785. doi: 10.1021/pr100970q. [DOI] [PubMed] [Google Scholar]
- 26.Marti G., Erb M., Boccard J., Glauser G., Doyen G.R., Villard N., Robert C.A.M., Turlings T.C., Rudaz S., Wolfender J.L. Metabolomics reveals herbivore-induced metabolites of resistance and susceptibility in maize leaves and roots. Plant Cell Environ. 2013;36:621–639. doi: 10.1111/pce.12002. [DOI] [PubMed] [Google Scholar]
- 27.Freitas D.D., Carlos E.F., Gil M.C.S.D., Vieira L.G.E., Alcantara G.B. NMR-based metabolomic analysis of huanglongbing-asymptomatic and -symptomatic citrus trees. J. Agric. Food Chem. 2015;63:7582–7588. doi: 10.1021/acs.jafc.5b03598. [DOI] [PubMed] [Google Scholar]
- 28.Cuperlovic-Culf M., Wang L., Forseille L., Boyle K., Merkley N., Burton I., Fobert P.R. Metabolic biomarker panels of response to fusarium head blight infection in different wheat varieties. PLoS ONE. 2016;11:e0153642. doi: 10.1371/journal.pone.0153642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baldaccicresp F., Chang C., Mickaël Maucourt Deborde C., Hopkins J., Lecomte P. (Homo) glutathione deficiency impairs root-knot nematode development in Medicago truncatula. PLoS Pathog. 2012;8:e1002471. doi: 10.1371/journal.ppat.1002471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumar Y., Zhang L., Panigrahi P., Dholakia B.B., Dewangan V., Chavan S.G., Kunjir S.M., Wu X., Li N., Rajmohanan P.R. Fusarium oxysporum mediates systems metabolic reprogramming of chickpea roots as revealed by a combination of proteomics and metabolomics. Plant Biotechnol. J. 2016;14:1589–1603. doi: 10.1111/pbi.12522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marentes-Culma R., Cuellar-Cuestas C., Ardila H.D., Coy-Barrera E. 5- n -alkylresorcinol-based metabolic response of rice to the interaction with burkholderia glumae: A chemical characterization of the temporal and spatial variations depending on environmental conditions. J. Plant Interact. 2022;17:127–139. doi: 10.1080/17429145.2021.2017036. [DOI] [Google Scholar]
- 32.Liu C., Du B., Hao F., Lei H., Wan Q., He G., Wang Y., Tang H. Dynamic metabolic responses of brown planthoppers towards susceptible and resistant rice plants. Plant Biotechnol. J. 2017;15:1346–1357. doi: 10.1111/pbi.12721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Q., Li T., Gao M., Ye M., Lin M., Wu D., Guo J., Guan W., Wang J., Yang K., et al. Transcriptome and metabolome profiling reveal the resistance mechanisms of rice against brown planthopper. Int. J. Mol. Sci. 2022;23:4083. doi: 10.3390/ijms23084083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Browne R.A., Brindle K.M. 1H NMR-based metabolite profiling as a potential selection tool for breeding passive resistance against Fusarium head blight (FHB) in wheat. Mol. Plant Pathol. 2007;8:401–410. doi: 10.1111/j.1364-3703.2007.00400.x. [DOI] [PubMed] [Google Scholar]
- 35.Miao Y., Xu L., He X., Zhang L., Shaban M., Zhang X., Zhu L. Suppression of tryptophan synthase activates cotton immunity by triggering cell death via promoting SA synthesis. Plant J. 2019;98:329–345. doi: 10.1111/tpj.14222. [DOI] [PubMed] [Google Scholar]
- 36.Zhang J.B., Li H.P., Dang F.J., Qu B., Xu Y.B., Zhao C.S., Liao Y.C. Determination of the trichothecene mycotoxin chemotypes and associated geographical distribution and phylogenetic species of the Fusarium graminearum clade from China. Mycol. Res. 2007;111:967–975. doi: 10.1016/j.mycres.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 37.Wu A.B., Li H.P., Zhao C.S., Liao Y.C. Comparative pathogenicity of Fusarium graminearum isolates from China revealed by wheat coleoptile and floret inoculations. Mycopathologia. 2005;160:75–83. doi: 10.1007/s11046-005-1153-4. [DOI] [PubMed] [Google Scholar]
- 38.Duvick J., Rood T., Rao A.G., Marshak D.R. Purification and characterization of a novel antimicrobial peptide from maize (Zea mays L.) kernels. J. Biol. Chem. 1992;267:18814–18820. doi: 10.1016/S0021-9258(19)37034-6. [DOI] [PubMed] [Google Scholar]
- 39.Li X., Zhang J., Song B., Li H., Xu H., Qu B., Dang F., Liao Y. Resistance to Fusarium head blight and seedling blight in wheat is associated with activation of a cytochrome P450 gene. Phytopathology. 2010;100:183–191. doi: 10.1094/PHYTO-100-2-0183. [DOI] [PubMed] [Google Scholar]
- 40.Wu X.Y., Li N., Li H.D., Tang H.R. An optimized method for NMR-based plant seed metabolomic analysis with maximized polar metabolite extraction efficiency, signal-to-noise ratio, and chemical shift consistency. Analyst. 2014;139:1769–1778. doi: 10.1039/C3AN02100A. [DOI] [PubMed] [Google Scholar]
- 41.Trygg J., Wold S. Orthogonal projections to latent structures (O-PLS) J. Chemometr. 2002;16:119–128. doi: 10.1002/cem.695. [DOI] [Google Scholar]
- 42.Eriksson L., Trygg J., Wold S. CV-ANOVA for significance testing of PLS and OPLS® models. J. Chemometr. 2008;22:594–600. doi: 10.1002/cem.1187. [DOI] [Google Scholar]
- 43.Cloarec O., Dumas M.E., Trygg J., Craig A., Barton R.H., Lindon J.C., Nicholson J.K., Holmes E. Evaluation of the orthogonal projection on latent structure model limitations caused by chemical shift variability and improved visualization of biomarker changes in 1H NMR spectroscopic metabonomic studies. Anal. Chem. 2005;77:517–526. doi: 10.1021/ac048803i. [DOI] [PubMed] [Google Scholar]
- 44.Fan T.W.M. Metabolite profiling by one-and two-dimensional NMR analysis of complex mixtures. Prog. Nucl. Mag. Res. Spectrosc. 1996;28:161–219. doi: 10.1016/0079-6565(95)01017-3. [DOI] [Google Scholar]
- 45.Fan T.W.M., Lane A.N. Structure-based profiling of metabolites and isotopomers by NMR. Prog. Nucl. Mag. Res. Spectrosc. 2008;52:69–117. doi: 10.1016/j.pnmrs.2007.03.002. [DOI] [Google Scholar]
- 46.Facchini P.J., Hagel J., Zulak K.G. Hydroxycinnamic acid amide metabolism: Physiology and biochemistry. Can. J. Bot.-Rev. Can. Bot. 2002;80:577–589. doi: 10.1139/b02-065. [DOI] [Google Scholar]
- 47.González-Lamothe R., Mitchell G., Gattuso M., Diarra M.S., Malouin F., Bouarab K. Plant antimicrobial agents and their effects on plant and human pathogens. Int. J. Mol. Sci. 2009;10:3400–3419. doi: 10.3390/ijms10083400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Atanasova-Penichon V., Pons S., Pinson-Gadais L., Picot A., Marchegay G., Bonnin-Verdal M.-N., Ducos C., Barreau C., Roucolle J., Sehabiague P. Chlorogenic acid and maize ear rot resistance: A dynamic study investigating Fusarium graminearum development, deoxynivalenol production, and phenolic acid accumulation. Mol. Plant-Microbe Interact. 2012;25:1605–1616. doi: 10.1094/MPMI-06-12-0153-R. [DOI] [PubMed] [Google Scholar]
- 49.Shetty R., Fretté X., Jensen B., Shetty N.P., Jensen J.D., Jorgensen H.J.L., Newman M.-A., Christensen L.P. Silicon-induced changes in antifungal phenolic acids, flavonoids, and key phenylpropanoid pathway genes during the interaction between Miniature roses and the biotrophic pathogen Podosphaera pannosa. Plant Physiol. 2011;157:2194–2205. doi: 10.1104/pp.111.185215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sabino A.R., Tavares S.S., Riffel A., Li J.V., Oliveira D.J., Feres C.I., Henrique L., Oliveira J.S., Correia G.D., Sabino A.R., et al. 1H NMR metabolomic approach reveals chlorogenic acid as a response of sugarcane induced by exposure to diatraea saccharalis. Ind. Crops Prod. 2019;140:111651. doi: 10.1016/j.indcrop.2019.111651. [DOI] [Google Scholar]
- 51.Gunnaiah R., Kushalappa A.C. Metabolomics deciphers the host resistance mechanisms in wheat cultivar Sumai-3, against trichothecene producing and non-producing isolates of Fusarium graminearum. Plant Physiol. Biochem. 2014;83:40–50. doi: 10.1016/j.plaphy.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 52.Arisan-Atac I., Wolschek M.F., Kubicek C.P. Trehalose-6-phosphate synthase A affects citrate accumulation by Aspergillus niger under conditions of high glycolytic flux. FEMS Microbiol. Lett. 1996;140:77–83. doi: 10.1111/j.1574-6968.1996.tb08318.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.