Abstract
Secondary lymphoid tissues play a major role in the human immune response to P. falciparum infection. Previous studies have shown that acute falciparum malaria is associated with marked perturbations of the cellular immune system characterized by lowered frequency and absolute number of circulating T cell subsets. A temporary relocation of T cells, possibly by infiltration to secondary lymphoid tissue, or their permanent loss through apoptosis, are two proposed explanations for this observation. We conducted the present study to determine the phenotype of lymphocyte subsets that accumulate in the lymph node and spleen during acute stages of falciparum malaria infection in Malawian children, and to test the hypothesis that lymphocytes are relocated to lymphoid tissues during acute infection. We stained tissue sections from children who had died of the two common clinical forms of severe malaria in Malawi, namely severe malarial anemia (SMA, n = 1) and cerebral malaria (CM, n = 3), and used tissue sections from pediatric patients who had died of non-malaria sepsis (n = 2) as controls. Both lymph node and spleen tissue (red pulp) sections from CM patients had higher percentages of T cells (CD4+ and CD8+) compared to the SMA patient. In the latter, we observed a higher percentage of CD20+ B cells in the lymph nodes compared to CM patients, whereas the opposite was observed in the spleen. Both lymph node and spleen sections from CM patients had increased percentages of CD69+ and CD45RO+ cells compared to tissue sections from the SMA patient. These results support the hypothesis that the relocation of lymphocytes to spleen and lymph node may contribute to the pan-lymphopenia observed in acute CM.
Keywords: P. falciparum malaria, lymphocytes, lymph nodes, spleen
1. Introduction
Although significant gains have been attained in reducing case numbers and mortality over the past decade [1,2] global malaria burden still remains high, with Plasmodium falciparum malaria accounting for an estimated 241 million clinical cases and 627,000 deaths in 2020 [3]. Falciparum malaria can manifest either as uncomplicated (UM) or severe infection. The latter includes cerebral malaria (CM) and severe malarial anemia (SMA) as well as other complications, with a number of possible overlaps [4]. CM is associated with the worst outcome, contributing to the highest number of deaths [5], and to a wide spectrum of neurological sequelae in survivors [6]. Although the pathogenesis of the different clinical types of severe malaria is poorly understood, they result from a combination of host and parasite factors, including high inflammation and sequestration of infected red blood cells (iRBCs) onto the vascular endothelium and a subsequent inflammatory response [7], leading to end-organ damage.
Natural protective immunity against malaria takes years to develop, even in high transmission areas and despite repeated exposure to the parasite. This immunity is not only parasite-specific [8], but also stage-specific [9,10], and is influenced by age, genetics, pregnancy, nutritional status and co-infections [9]. A reduction in exposure can lead to rapid waning of this acquired immunity, potentially increasing the risk of developing more severe forms of the disease [10].
Although vaccines have proven to be the most reliable, cost-effective and efficient method for controlling the burden and spread of many infectious diseases, this has not been the case with malaria. The most promising malaria vaccine candidate to date, RTS,S/AS01, was approved for pilot implementation programme in three African countries in 2015 [11], Malawi being one of the three. The search for a robust and effective malaria vaccine continues and a better understanding of naturally acquired immune responses to the various stages, including the transmissible stages of the parasite, could be crucial in informing effective vaccine designs.
The spleen plays an important role in immunity against malaria, through the mediation of humoral and cellular immune responses, and by physically clearing altered host erythrocytes [12]. Various leucocyte subsets migrate through different zones of the spleen, therefore increasing the chance of detecting P. falciparum antigens [13]. Antigen-presenting cells (APCs) such as monocytes, macrophages and dendritic cells (DCs) phagocytize iRBCs and parasite debris in the marginal zone before migrating to the white pulp, where they activate naïve and memory T cells [14].
Studies in murine models of malaria have shown a depletion of T and B cells from the marginal zone of the spleen with a corresponding increase of the same cells in in the red pulp during the course of a P. chabaudi chabaudi malaria infection [15,16]. This observation was corroborated by a postmortem study of adult Vietnamese patients who died of P. falciparum malaria [17]. Spleen sections in this cohort showed an altered architecture with a distinct dissolution of the splenic marginal zones and substantial loss of B cells [17].
Lymph nodes are also important in the host immune response against malaria, as T and B cells migrate to different segments, where they interact with antigen-carrying APCs and undergo clonal expansion [18,19]. The proliferating cells create germinal centers of activation, proliferation, differentiation and death of B lymphocytes [20].
Our group [21] and others [22] have previously shown that acute falciparum malaria is associated with marked perturbations of the cellular immune system characterized by lowered frequencies and absolute numbers of T cells in the peripheral circulation. A temporary relocation of T cells, possibly by infiltration to secondary lymphoid tissue, and permanent loss of these cells through apoptosis are the two leading explanations suggested for this observation [23,24].
While other groups explored this phenomenon in pediatric UM [23] and in acute P. falciparum or P. vivax malaria [24], we conducted the present study to (i) investigate whether lymphocytes relocate to and accumulate within lymphoid tissues in Malawian pediatric patients with severe P. falciparum malaria (either CM or SMA); and (ii) characterize the phenotypes in both lymph nodes and spleen. We used archived samples from children who died of CM or SMA collected as part of a postmortem study at Queen Elizabeth Central Hospital (QECH) in Blantyre, Malawi [25,26]. Samples from patients who died of non-malarial sepsis were used as controls.
2. Results
2.1. Antibody Titration Results
Tissue labelling using CD56, a marker of natural killer (NK) cells, did not result in any distinguishable positive cells that could clearly be identified as NK cells in any of the three patient groups. Similar negative results were obtained with different antibody concentrations (no dilution, 1:25, 1:50, 1:100 and 1:200) and incubation times (1 h, 2 h, 12 h, 24 h and 48 h). Consequently, we excluded this marker from all subsequent analyses. Staining with CD69 and CD4 only resulted in good outcome when the incubation was done for 24 h; the other primary antibodies resulted in good staining after 1hr incubation (Table 1).
Table 1.
Dilution factors of the primary antibodies (BD Pharmingen, CA, USA) and the incubation duration for staining with the primary antibody and their source.
| Antibody Type | Cell Types and Status | Dilution Factor of Primary Antibody | Incubation Time for Primary Antibody (Hours) |
|---|---|---|---|
| CD3 | T cells | 1:50 | 1 |
| CD4 | CD4+ (Helper) T cells | 1:50 | 24 |
| CD8 | CD8+ (Cytotoxic) T cells | 1:50 | 1 |
| CD20 | B cells | 1:200 | 1 |
| CD45RO | Memory cells | 1:100 | 1 |
| CD69 | Activated cells | 1:25 | 24 |
2.2. Lymphocyte Phenotyping in Lymph Node Sections
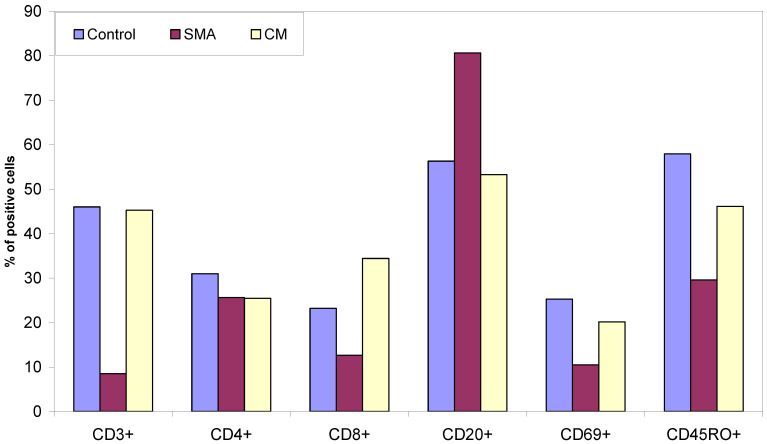
Lymph node tissue sections from CM patients (Figure 1) had a similar percentage of T cells to those observed in sections of control patients who died from non-malaria complications. In contrast, the SMA patient had lower percentage of T cells in lymph node sections compared to both CM and control patients. Lymph node tissue sections from CM and SMA patients had similar percentages of CD4+ T cells, which were marginally lower compared to control tissues. CM patients had the highest, and those of SMA patients had the lowest percentage of CD8+ T cells in their lymph nodes of the three groups. Tissue sections from SMA patients had the highest percentage of CD20+ B cells (Figure 1 and Figure 2).
Figure 1.
Percent of CD3+ T cells, CD4+ and CD8+ T cells, CD20+ B cells, CD69+ and CD45RO+ cells present in lymph node tissue sections from patients who died from non-malaria disease, SMA and CM.
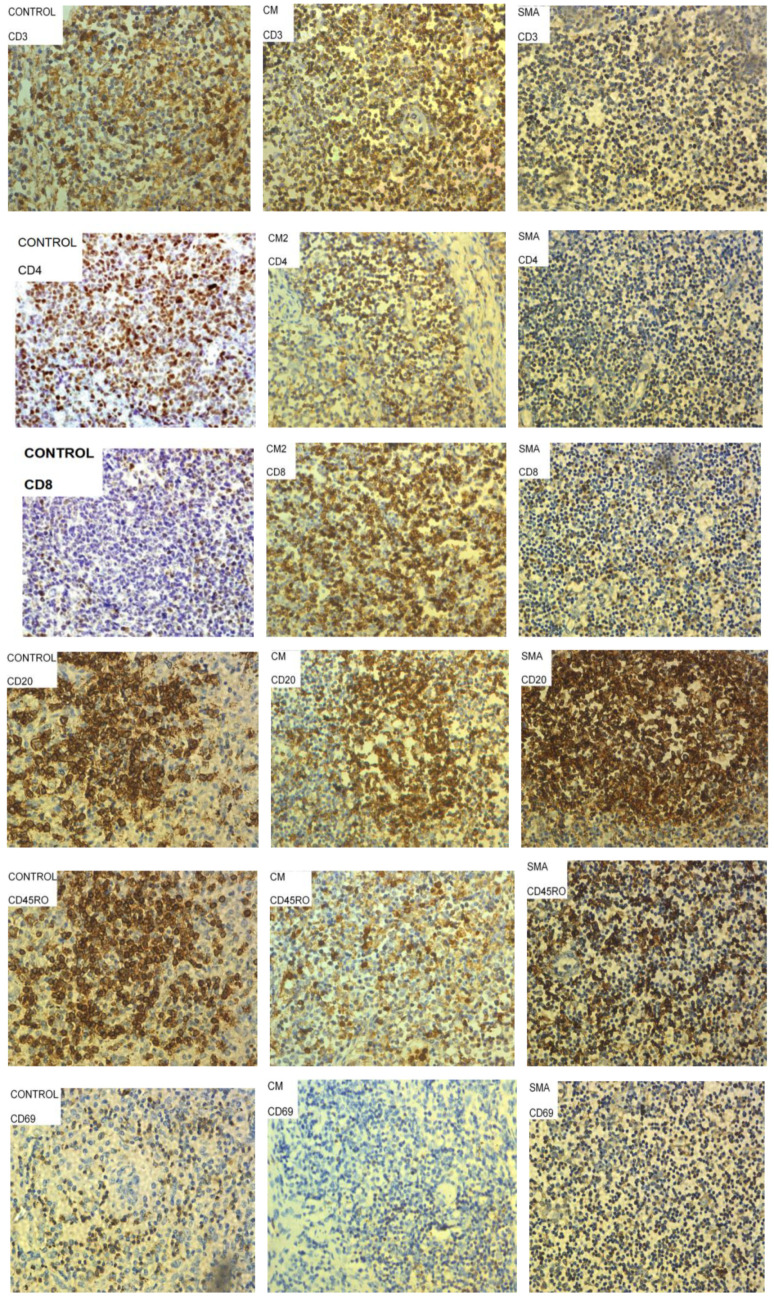
Figure 2.
Representative images of lymph node tissue sections from patients who died of sepsis (controls), Severe Malarial Anemia (SMA) and Cerebral Malaria (CM) stained with antibodies to characterize CD4+ T cells, CD8+ T cells, CD20+ B cells, activated cells (CD69+) and memory cells (CD45RO+). Cells stained brown were considered positive for the marker of interest, and representative examples are circled and highlighted by arrows in some panels above.
Patients who died of non-malaria related complications had higher percentages of CD69+ and CD45RO+ cells in their lymph node sections compared to the percentage of these cells in tissue sections from CM and SMA patients (Figure 1). CM patients had a higher percentage of these two cell types compared to the levels observed in SMA.
2.3. Lymphocyte Phenotyping in Spleen Sections
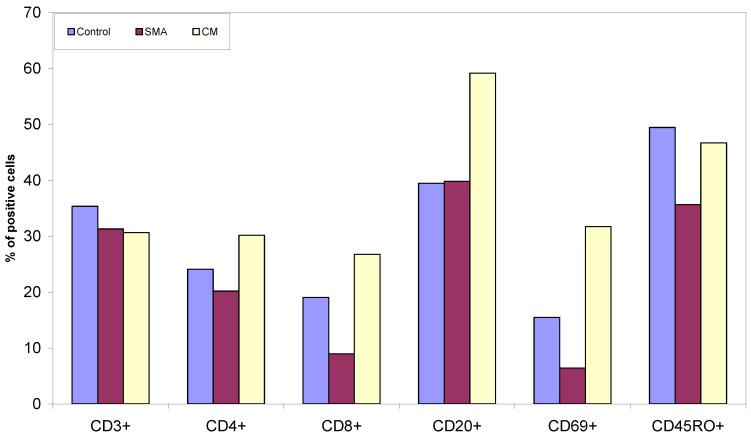
Spleen tissue sections from the control patients had a higher percentage of T cells compared to CM and SMA patients, which showed similar levels across both groups (Figure 3 and Figure 4). CM patients had a higher percentage of CD4+ and CD8+ T cells in their spleen sections compared to controls and SMA patients, and the last group had the lowest percentage of these cells (Figure 3). Tissue sections from CM patients also had a higher percentage of CD20+ B cells and CD69+ cells compared to the other two groups. SMA patients had the lowest percentage of CD69+ and CD45RO+ cells of the three groups (Figure 3).
Figure 3.
Percent CD3+ T cells, CD4+ and CD8+ T cells, CD20+ B cells, CD69+ and CD45RO+ cells present in spleen tissue sections from patients who died from sepsis, SMA and CM.
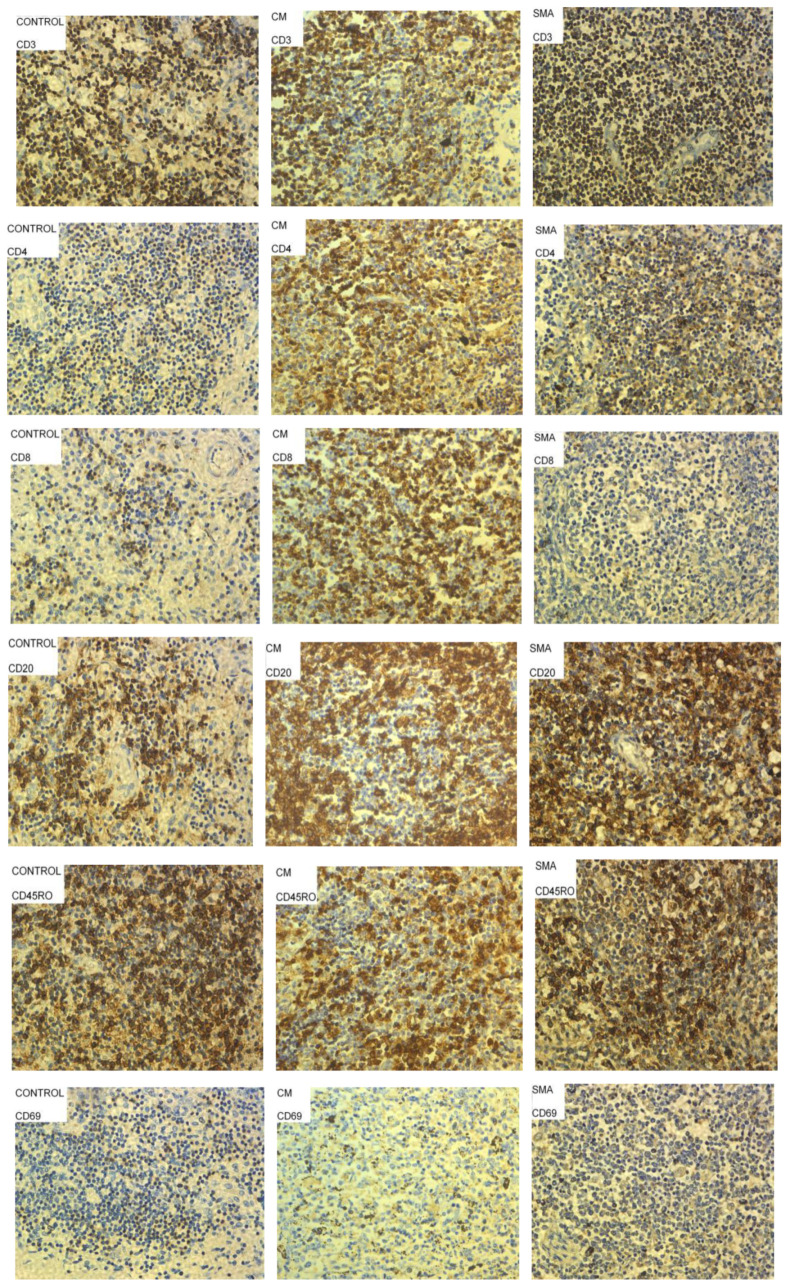
Figure 4.
Images of spleen tissue sections from patients who died of sepsis (Controls), Severe Malarial Malaria Anemia (SMA) and Cerebral Malaria (CM) stained with different antibodies to characterize CD4+ T cells, CD8+ T cells, CD20+ B cells, activated cells (CD69+) and memory cells (CD45RO+). Cells stained brown were considered positive for the marker of interest, and representative examples are circled and highlighted by arrows in some panels above.
3. Discussion
Pan-lymphopenia is a hallmark of severe P. falciparum malaria [21,22,23,24]. This phenomenon is not well understood but may result from a temporary, disease-induced relocation of lymphocytes, especially CD4+ and CD8+ T cells, to secondary lymphoid tissue [24]. Upon treatment and cure, the pan-lymphopenia reverses and a marked increase in lymphocyte counts is observed in the peripheral circulation [21,22]. Here, we show that lymph node and spleen tissue sections from CM patients had higher percentages of CD4+ and CD8+ T cells compared to a patient with SMA; the percentage of B cells in their spleen sections was also higher. In contrast, the SMA patient had a higher percentage of CD20+ B cells in lymph node sections compared to CM patients. The percentages of CD69+ (a marker of early activation) and CD45RO+ (a marker of memory T cells) were higher in lymph node and spleen sections from CM cases than in the SMA patient.
The observation that lymph node and spleen tissue sections from CM patients had higher percentages of CD4+ and CD8+ T cells compared to a patient with SMA might somewhat seem counter-intuitive, since T cells have previously been linked with immunopathology observed in patients presenting with CM [23]. This apparent paradox may be partly explained by the fact that only 5% of lymphocytes are present in peripheral circulation suggesting that various lymphocyte subsets may translocate to secondary lymphoid compartments such as spleen and lymph nodes where they encounter antigens for their subsequent maturation and differentiation. Another possibility is that lymphocyte subsets are lost through apoptosis, which would result in permanent cell loss. The rapid normalization of the observed lymphopenia and our histologic findings seem to support the temporary translocation hypothesis. Lymphoid cells sequestering in the secondary lymphoid tissues of CM patients rapidly rejoin the peripheral circulation once the infection is cleared or controlled.
To date, there is only one study that describes patterns of architectural reorganization in the spleen from adult patients who died from severe malaria in Vietnam [17]. As differences in immunity [27] and clinical presentation [28] have been described between Asian adults and African children with severe falciparum malaria, our study aimed to assess whether similar cellular processes occurred in children from Malawi. Indeed, while the sequestration of iRBCs in secondary lymphoid tissues has been investigated using postmortem tissues [25,26], similar studies aimed at characterizing migration and transient relocation of different leucocyte subsets in secondary lymphoid tissues during malaria infection have mainly been conducted in murine models [16,29,30].
Results of early murine studies showed marked decreases in the numbers of circulating lymphocytes in peripheral circulation 2 to 4 days after infection [28,31]. A similar trend was reported in human infection [22,23]. These numbers then returned to control levels 6 to 8 days post-infection. Subsequent cell trapping experiments showed that peripheral blood lymphocytes were preferentially recruited in the spleen during the initial stages of the infection with Th1 lymphocytes as the main cell type affected [32].
We demonstrate a high percentage of CD4+ and CD8+ T cells in splenic and lymph node sections of CM patients, which is consistent with the reported cellular alterations in the spleen during P. berghei infection [29]. Indeed, the numbers of CD4+ and CD8+ T cells in the spleen of resistant mice strains increase rapidly during infection and are maintained at a high level before migrating into the brain before the onset of experimental CM [29].
In adult patients with severe malaria, the overall number of T cells in the red pulp increased significantly, and there was a profound depletion of B cells from the marginal zone surrounding B cell follicles [17]. Here, we report for the first time similar findings in the spleen and the lymph nodes in fatal pediatric CM, but not SMA. Additional analyses are warranted to confirm potential differences between age groups during fatal SMA. Worth noting is the observation that the lymph node sections from SMA case had on average less than 10% CD3+ (total T) cells as compared to more than 20% CD4+ T cells. This anomaly could have arisen from the possibility that a higher proportion of the T cells were actually CD4+ T cells with very low proportion of CD8+ T cells as indicated in Figure 1.
In addition, the investigators of the Vietnamese study also found that the frequency of germinal centres within lymphoid follicles was significantly reduced in severe malaria, indicating that the differentiation of B cells into plasma and memory cells was compromised [17]. This is consistent with our results, as spleen sections from CM patients had lower percentages of CD20+ B cells compared to the other groups.
In a study on the phenotype and localization of B cells in mice models during infection with P. chabaudi chabaudi, investigators showed that there was a depletion of B cells from the marginal zone and profound disruption of the T-cell areas. Similar to the SMA case we present here, intensive germinal centre formation and extra-follicular B cell proliferation were observed in the red pulp [30].
The predominance of CD69+ cells in the spleen of CM patients is consistent with the results of the Vietnamese study [17], where the expression of the activation marker HLA-DR on sinusoidal lining cells was increased in the red pulp in fatal malaria cases. This too is consistent with the results of our previous work [21], in which severe malaria cases were characterized by highly activated T cells in periphery as shown by a high expression of the activation marker CD69.
In a separate study to determine lymphocyte migration in murine malaria infected with P. chabaudi chabaudi [16] investigators found that although the white pulp of the spleen in infected mice increased in size 11 days after infection, there was a partial lymphocyte depletion which coincided with the occurrence of massive lymphocytosis in the peripheral blood. The investigators also showed a decreased uptake and retention of T and B cells by the spleen of infected mice but an increased retention of T and B cells in the liver and lungs of infected mice [16]. The fact that lymphocyte traffic to the spleen was found to be reduced in murine studies [16,30] but higher numbers of T and B cells were observed both in our cohort and in the Vietnamese one [17] suggests that the relocation of lymphocytes to the spleen could be a feature specific to human CM.
The reduced lymphocyte traffic to the spleen in infected mice coincided with an increased lymphocyte retention in the peripheral circulation, lungs and especially in the liver [16]. This is consistent with the lymphocytosis we described in acute SMA [21]. Immunohistochemical analysis of liver tissue sections from patients may, in the future, provide additional information on lymphocyte trafficking in human SMA. Indeed, studies assessing the importance of the increase in T and B cells traffic to the liver in malaria infection have also provided evidence for a crucial role of the accumulation of leucocytes in the liver to the development of protective immunity during malarial infection [32]. Thus, it is possible that reduced entry of lymphocytes into the spleen may in fact contribute to increase the delivery of committed immunologically competent cells to the liver, where parasite destruction occurs [32].
Our study had several limitations, including a small sample size due to the low availability of these specific tissues in our cohort. In addition, although the staining for some cells such as CD4+ and CD8+ T cells and B cells was not as ideal in the malaria cases, we were still able to distinguish between stained and unstained cells. However, we were unable to optimize the staining for NK cells despite numerous variations of our staining protocol in terms of incubation time and dilution of antibodies. Nevertheless, our findings in pediatric CM are in line with previous ones from Vietnamese adults and contribute to a better understanding of the relocation of lymphocytes to secondary lymphoid organs during P. falciparum infection across different age and geographical groups. Only cervical lymph nodes were used in this study, so additional analyses are warranted to assess whether the proposed malaria-induced relocation of lymphocytes we report also occurs in lymph nodes from other parts of the body. Further studies including liver tissue sections should be considered to investigate the accumulation of leucocytes in the liver, as previously shown in murine malaria [32]. It is also possible that proliferation of tissue-resident cells could have contributed to the lymphocyte counts observed in the tissue sections from CM cases, a hypothesis that should be explored in future work.
Overall, our results are consistent with the hypothesis that the relocation of lymphocytes to spleen and lymph node contribute to the pan-lymphopenia observed in acute CM.
4. Materials and Methods
4.1. Postmortem Tissue Sections
Postmortem spleen and lymph node tissue sections were obtained from the Blantyre Malaria Project Autopsy Study. Cervical lymph node and spleen samples were collected from children who died of severe malaria on the Pediatric Research Ward at Queen Elizabeth Central Hospital (QECH) in Blantyre, Malawi from 1996 to 2009 [25,26]. Sections from three patients diagnosed with CM and one patient with SMA were analyzed for this study, as well as additional samples from two controls cases who died of non-malaria sepsis were analyzed (Table 2).
Table 2.
Diagnosis details of the patients from whom the tissue sections were collected.
| Serial No. | Code Number | Diagnosis Details | Age (Months) | Sex |
|---|---|---|---|---|
| 1 | - | Control | 48 | Male |
| 2 | - | Control | 50 | Female |
| 3 | MP-55 | Cerebral malaria | 52 | Male |
| 4 | MP-64 | Cerebral malaria | 50 | Male |
| 5 | MP-66 | Cerebral malaria | 14 | Female |
| 6 | MP-57 | Severe Malarial Anaemia | 49 | Female |
4.2. Ethical Clearance
The original study was approved by the IRB at Michigan State University and by the College of Medicine Research and Ethics Committee (COMREC) of the former College of Medicine of the University of Malawi, now Kamuzu University of Health Sciences (KUHeS). Informed consent was granted by the families of the deceased, and all agreed to the future use of stored samples in studies of malarial pathogenesis.
4.3. Immunohistochemical Analysis of Post-Mortem Tissue Sections
4.3.1. Reagents
Tris-buffered saline (TBS) was prepared by mixing 6.1 g of Trizma base (Sigma, Darmstadt, Germany) with 9.0 g of sodium chloride (Sigma) in 800 mL of distilled water. The pH was adjusted to 7.6 using concentrated hydrochloric acid (Sigma-Aldrich, Darmstadt, Germany) and the solution made up to 1 L. TBS/Tween 20 1% solution was prepared by mixing 5 mL of Tween 20 (Becton Dickinson, NJ, USA) with 500 mL of TBS.
4.3.2. Antibody Titration and Immunohistochemical Staining Procedure
Antibody titration experiments were performed to establish the optimal dilution for each primary antibody (1:12.5, 1:25, 1:50, 1:100, 1:200 or 1:400). Each concentration was used for staining tissue sections for 1, 2, 4 and 24 h. The staining procedure was carried out as described elsewhere [33]. In summary, formalin-fixed, paraffin-embedded (FFPE) slides were immersed in xylene, a wax removal and antigen retrieval solution, at 96 °C for 30 min using a metal trough. After allowing the W-CAP to cool to room temperature for 20 min, samples were rinsed and rehydrated in cold water for two minutes. Excess water was removed from the slides taking care not to disturb the tissue sections or allow them to dry out, and margins were drawn around the slide sections using a wax pen (ImmEdge Pen, Vector Labs, Burlingame, CA, USA). The slides were laid flat in a humidified chamber and two drops of TBS/Tween 20 1% solution applied to each section for 5 min. The solution was tipped off and excess fluid dried from the slides with tissue paper prior to the addition of the antibodies. Based on the results of these experiments detailed below, specific dilution factors and incubation periods were used (Table 2).
An aliquot of 100 μL of the diluted primary antibody (all from BD Pharmingen, San Diego, CA, USA) was applied to each section and the slides incubated at room temperature for either 1 h (CD3, CD8, CD20, CD45RO) or 24 h (CD4, CD69) (Table 1). For the 24-h-incubation, the container was left at room temperature on an orbital shaker to ensure even coverage of tissue section with antibody solution. At the end of the incubation the primary antibody was removed from the sections, which were then washed with TBS/Tween 20 solution for 5 min and excess fluid dried off. The slides were put back in the humidified chamber and two drops of the secondary biotinylated rabbit anti-goat IgG (DAKO, Glistrup, Denmark) antibody was applied to each section.
The slides were then incubated at room temperature for 30 min before sections were washed with TBS/Tween 20 for 10 min followed by TBS for another 10 min. During the wash an appropriate volume of diaminobenzidine (DAB) solution (DAKO, Glistrup, Denmark) was prepared by mixing one drop of DAB concentrate in 1 mL DAB Buffer. Once washed, the excess TBS was removed from the sections and 100 μL of the diluted DAB solution applied to each section and incubated for five minutes after which the sections were checked microscopically for the correct stain level. The slides were immersed in water for a minute and counterstained with Mayer’s Haemoxylin (DAKO, Glistrup, Denmark) for 20 s before placing them in water for 5 min. The slides were then dehydrated by placing them in 99.9% Ethanol for 5 min twice and then in xylene for 5 min. A drop of distrene, plasticizer, xylene (DPX, Sigma, Darmstadt, Germany) was placed on top of the sample, which was then sealed with a coverslip. Once dry, slides were examined by light microscopy.
4.4. Photography and Quantification of Lymphocyte Subsets
All stained sections were systemically examined using a Phillips CM10 Transmission Electron Microscope under 40× magnification and images were captured using a side mounted Gatan BioScan camera. Sections with optimal staining intensity were selected for image analysis. A graticule fitted into one of the eye pieces of the microscope facilitated cell counting on the tissue sections. A cell was defined as positive for staining if the appropriate marker for that cell type was observed. Two sets of values were obtained, one for the DAB-stained cells and the second for the DAB- and haematoxylin-stained cells. Results were expressed as percentage of positive cells out of the total cells, as described elsewhere [34]. For spleen sections, only stained cells in the red pulp regions were counted and recorded with the counting done in approximately similar regions in all sections used. For consistency’s sake, three fields were counted per tissue slide in order to determine the presented frequencies.
4.5. Statistical Analyses
Means and standard deviations (except for SMA) were then calculated using GraphPad Prism version 6.01 for Windows (GraphPad Software, San Diego, CA, USA).
Acknowledgments
The authors would like to express their gratitude to the parents and relatives of the children whose tissues were used in this study for consenting to full autopsies and tissue sampling.
Author Contributions
T.E.T. prepared the tissue sections, W.L.M. and S.C.W. performed the staining work, W.L.M. and S.W. carried out the microscopy and photography work. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and was approved by the IRB at Michigan State University [IRB No: 06-1012] and by the College of Medicine Research and Ethics Committee (COMREC) of the former College of Medicine of the University of Malawi, now Kamuzu University of Health Sciences (KUHeS) [IRB No: P.11/07/593].
Informed Consent Statement
Informed consent was granted by the families of the deceased, and all agreed to the future use of stored samples in studies of malarial pathogenesis. They also consented to the publication of the data obtained from these future studies.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to ethical reasons considering that the human tissue sections were obtained from postmortem samples.
Conflicts of Interest
The authors express no conflict of interest.
Funding Statement
Funding for this work was sourced under the Gates Malaria Partnership, which was sponsored by the Bill and Melinda Gates Foundation [Grant Number: OPP51941]. The funding for the parent autopsy study was from the US National Institutes of Health [R01AI034969].
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Murray C.J., Ortblad K.F., Guinovart C., Lim S.S., Wolock T.M., Roberts D.A., Dansereau E.A., Graetz N., Barber R.M., Brown J.C., et al. Global, regional, and national incidence and mortality for HIV, tuberculosis, and malaria during 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:1005–1070. doi: 10.1016/S0140-6736(14)60844-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gething P.W., Battle K.E., Bhatt S., Smith D.L., Eisele T.P., Cibulskis R.E., Hay S.I. Declining malaria in Africa: Improving the measurement of progress. Malar. J. 2014;13:39. doi: 10.1186/1475-2875-13-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Malaria Report 2021: World Health Organization. [(accessed on 17 June 2022)]; Available online: https://www.who.int/publications/i/item/9789240040496.
- 4.Wassmer S.C., Taylor T.E., Rathod P.K., Mishra S.K., Mohanty S., Arevalo-Herrera M., Duraisingh M.T., Smith J.D. Investigating the Pathogenesis of Severe Malaria: A Multidisciplinary and Cross-Geographical Approach. Am. J. Trop. Med. Hyg. 2015;93((Suppl. 3)):42–56. doi: 10.4269/ajtmh.14-0841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marsh K., Forster D., Waruiru C., Mwangi I., Winstanley M., Marsh V., Newton C., Winstanley P., Warn P., Peshu N., et al. Indicators of life-threatening malaria in African children. N. Engl. J. Med. 1995;332:1399–1404. doi: 10.1056/NEJM199505253322102. [DOI] [PubMed] [Google Scholar]
- 6.Rosa-Gonçalves P., Ribeiro-Gomes F.L., Daniel-Ribeiro C.T. Malaria Related Neurocognitive Deficits and Behavioral Alterations. Front. Cell Infect. Microbiol. 2022;12:829413. doi: 10.3389/fcimb.2022.829413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Day K.P., Marsh K. Naturally acquired immunity to Plasmodium falciparum. Immunol. Today. 1991;12:A68–A71. doi: 10.1016/S0167-5699(05)80020-9. [DOI] [PubMed] [Google Scholar]
- 8.Plebanski M., Hill A.V. The immunology of malaria infection. Curr. Opin. Immunol. 2000;12:437–441. doi: 10.1016/S0952-7915(00)00117-5. [DOI] [PubMed] [Google Scholar]
- 9.Vermeulen A.N., Ponnudurai T.H.I.V.I., Beckers P.J., Verhave J.P., Smits M.A., Meuwissen J.H. Sequential expression of antigens on sexual stages of Plasmodium falciparum accessible to transmission-blocking antibodies in the mosquito. J. Exp. Med. 1985;162:1460–1476. doi: 10.1084/jem.162.5.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fowkes F.J., Boeuf P., Beeson J.G. Immunity to malaria in an era of declining malaria transmission. Parasitology. 2016;143:139–153. doi: 10.1017/S0031182015001249. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization WHO Recommends Groundbreaking Malaria Vaccine for Children at Risk. [(accessed on 17 June 2022)]; Available online: https://www.who.int/news/item/06-10-2021-who-recommends-groundbreaking-malaria-vaccine-for-children-at-risk.
- 12.Ghosh D., Stumhofer J.S. The spleen: “epicenter” in malaria infection and immunity. J. Leukoc. Biol. 2021;110:753–769. doi: 10.1002/JLB.4RI1020-713R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steiniger B., Barth P., Hellinger A. The perifollicular and marginal zones of the human splenic white pulp: Do fibroblasts guide lymphocyte immigration? Am. J. Pathol. 2001;159:501–512. doi: 10.1016/S0002-9440(10)61722-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Banchereau J., Briere F., Caux C., Davoust J., Lebecque S., Liu Y.J., Pulendran B., Palucka K. Immunobiology of dendritic cells. Annu. Rev. Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 15.Stevenson M.M., Kraal G. Histological changes in the spleen and liver of C57BL/6 and A/J mice during Plasmodium chabaudi AS infection. Exp. Mol. Pathol. 1989;51:80–95. doi: 10.1016/0014-4800(89)90009-9. [DOI] [PubMed] [Google Scholar]
- 16.Kumararatne D.S., Phillips R.S., Sinclair D., Parrott M.V., Forrester J.B. Lymphocyte migration in murine malaria during the primary patent parasitaemia of Plasmodium chabaudi infections. Clin. Exp. Immunol. 1987;68:65–77. [PMC free article] [PubMed] [Google Scholar]
- 17.Urban B.C., Hien T.T., Day N.P., Phu N.H., Roberts R., Pongponratn E., Jones M., Mai N.T., Bethell D., Turner G.D., et al. Fatal Plasmodium falciparum malaria causes specific patterns of splenic architectural disorganization. Infect Immun. 2005;73:1986–1994. doi: 10.1128/IAI.73.4.1986-1994.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Willard-Mack C.L. Normal structure, function, and histology of lymph nodes. Toxicol. Pathol. 2006;34:409–424. doi: 10.1080/01926230600867727. [DOI] [PubMed] [Google Scholar]
- 19.Romani N., Ratzinger G., Pfaller K., Salvenmoser W., Stössel H., Koch F., Stoitzner P. Migration of dendritic cells into lymphatics-the Langerhans cell example: Routes, regulation, and relevance. Int. Rev. Cytol. 2001;207:237–270. doi: 10.1016/s0074-7696(01)07007-3. [DOI] [PubMed] [Google Scholar]
- 20.MacLennan I.C. Germinal centers. Annu. Rev. Immunol. 1994;12:117–139. doi: 10.1146/annurev.iy.12.040194.001001. [DOI] [PubMed] [Google Scholar]
- 21.Mandala W.L., Msefula C.L., Gondwe E.N., Gilchrist J.J., Graham S.M., Pensulo P., Mwimaniwa G., Banda M., Taylor T.E., Molyneux E.E., et al. Lymphocyte Perturbations in Malawian Children with Severe and Uncomplicated Malaria. Clin. Vaccine Immunol. 2016;23:95–103. doi: 10.1128/CVI.00564-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hviid L., Kurtzhals J.A., Goka B.Q., Oliver-Commey J.O., Nkrumah F.K., Theander T.G. Rapid reemergence of T cells into peripheral circulation following treatment of severe and uncomplicated Plasmodium falciparum malaria. Infect. Immun. 1997;65:4090–4093. doi: 10.1128/iai.65.10.4090-4093.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elhassan I.M., Hviid L., Satti G., Akerstrom B., Jakobsen P.H., Jensen J.B., Theander T.G. Evidence of endothelial inflammation, T cell activation, and T cell reallocation in uncomplicated Plasmodium falciparum malaria. Am. J. Trop. Med. Hyg. 1994;51:372–379. doi: 10.4269/ajtmh.1994.51.372. [DOI] [PubMed] [Google Scholar]
- 24.Kassa D., Petros B., Mesele T., Hailu E., Wolday D. Characterization of peripheral blood lymphocyte subsets in patients with acute Plasmodium falciparum and P. vivax malaria infections at Wonji Sugar Estate, Ethiopia. Clin. Vaccine Immunol. 2006;13:376–379. doi: 10.1128/CVI.13.3.376-379.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taylor T.E., Fu W.J., Carr R.A., Whitten R.O., Mueller J.S., Fosiko N.G., Lewallen S., Liomba N.G., Molyneux M.E. Differentiating the pathologies of cerebral malaria by postmortem parasite counts. Nat. Med. 2004;10:143–145. doi: 10.1038/nm986. [DOI] [PubMed] [Google Scholar]
- 26.White V.A. Malaria in Malawi: Inside a Research Autopsy Study of Pediatric Cerebral Malaria. Arch. Pathol. Lab. Med. 2011;135:220–226. doi: 10.5858/135.2.220. [DOI] [PubMed] [Google Scholar]
- 27.Doolan D.L., Dobaño C., Baird J.K. Acquired immunity to malaria. Clin. Microbiol. Rev. 2009;22:13–36. doi: 10.1128/CMR.00025-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ghazanfari N., Mueller S.N., Heath W.R. Cerebral Malaria in Mouse and Man. Front. Immunol. 2018;9:2016. doi: 10.3389/fimmu.2018.02016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ghazanfari N., Gregory J.L., Devi S., Fernandez-Ruiz D., Beattie L., Mueller S.N., Heath W.R. CD8+ and CD4+ T Cells Infiltrate into the Brain during Plasmodium berghei ANKA Infection and Form Long-Term Resident Memory. J. Immunol. 2021;207:1578–1590. doi: 10.4049/jimmunol.2000773. [DOI] [PubMed] [Google Scholar]
- 30.Achtman A.H., Khan M., MacLennan I.C., Langhorne J. Plasmodium chabaudi chabaudi infection in mice induces strong B cell responses and striking but temporary changes in splenic cell distribution. J. Immunol. 2003;171:317–324. doi: 10.4049/jimmunol.171.1.317. [DOI] [PubMed] [Google Scholar]
- 31.Lewis-Hughes P.H., Howell M.J. Early lymphocyte trapping in malaria infections: A particulate antigen mediated phenomenon. J. Parasitol. 1984;70:391–397. doi: 10.2307/3281570. [DOI] [PubMed] [Google Scholar]
- 32.Prommano O., Chaisri U., Turner G.D., Wilairatana P., Ferguson D.J., Viriyavejakul P., White N.J., Pongponratn E. A quantitative ultrastructural study of the liver and the spleen in fatal falciparum malaria. Southeast Asian J. Trop. Med. Public Health. 2005;36:1359–1370. [PubMed] [Google Scholar]
- 33.Helen P., Mark C., Stoler H. Handbook of Immunohistochemistry and in Situ Hybridization of Human Carcinomas. Volume 4. Elsevier; Amsterdam, The Netherlands: 2006. pp. 3–564. [Google Scholar]
- 34.Cathro H.P., Stoler M.H. Manual of Immunohistochemistry Staining and Espinoza, L.A. Counterstaining and Visualization. In: Hayat M.A., editor. Handbook of Immunohistochemistry and in Situ Hybridization of Human Carcinomas. Molecular Genetics Gastrointestinal Carcinoma, and Ovarian Carcinoma. Volume 4. Elsevier; Amsterdam, The Netherlands: 2006. pp. 3–564. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to ethical reasons considering that the human tissue sections were obtained from postmortem samples.