Abstract
Cone photoreceptors provide the foundation of most of human visual experience, but because they are smaller and less numerous than rods in most mammalian retinas, much less is known about their physiology. We describe new techniques and approaches which are helping to provide a better understanding of cone function. We focus on several outstanding issues, including the identification of the features of the phototransduction cascade that are responsible for the more rapid kinetics and decreased sensitivity of the cone response, the roles of inner-segment voltage-gated and Ca2+-activated channels, the means by which cones remain responsive even in the brightest illumination, mechanisms of cone visual pigment regeneration in constant light, and energy consumption of cones in comparison to that of rods.
Keywords: Photoreceptor, Retina, Vision, Cone, Transduction, Channels
Although as physiologists we have been fascinated by the ability of rod photoreceptors to signal the absorption of single photons of light, cones are much more important to our visual behavior. Patients with congenital stationary night blindness, who have decreased rod function, have difficulty driving at night; but patients with age-related macular degeneration and decreased cone function may not be able to drive at all. Cones are less sensitive than rods but respond with much more rapid kinetics and are necessary for detecting changes in illumination, which are critical for the precise determination of object motion and removal of blur. Those of us who live in Southern California know very well that it is dangerous to run in the desert on a moon-lit night, even when visibility seems quite good. Cones are much better than rods at providing the signals we need to process the sudden appearance of a cactus in our path.
It is therefore disappointing to realize that we know much less about cones than about rods, especially for mammals. The reasons are largely technical: cones are smaller and, in most mammalian species, much less abundant than rods. As a consequence, measurements of cone physiology and biochemistry are far more difficult. Although some progress has been made in the study of cones in lower vertebrates [for example 14, 16, 48, 70, 90] and primates [3, 13, 20, 81, 105], this work has yet to answer many fundamental questions about the molecular mechanisms underlying cone function.
It therefore seems appropriate to begin this review by asking, what are the unsolved questions of cone function? Although opinions may differ, we submit that any list would include the following: (1) What features of the phototransduction cascade are responsible for the more rapid kinetics and decreased sensitivity of the cone response compared to rods? (2) What role do inner-segment channels have facilitating the rapid rise and fall of the cone response in changing illumination? (3) Why do cones continue to provide robust responses even in the brightest illumination? (4) How do cones renew visual pigment in constant light? We now know that regeneration of 11-cis retinal by the retinal pigment epithelium is only part of the story. And finally (5), why do cones eventually degenerate after the loss of rods in genetically inherited diseases such as retinitis pigmentosa and Leber congenital amaurosis? Many forms of these diseases are caused by mutations in rod proteins and first produce rod degeneration, but it is the subsequent and seemingly inevitable death of cones that cripples the life of affected patients.
These questions are all profound and fundamental, and we do not presume to be able to answer even one of them in its entirety. We do hope, however, that in this brief survey we can indicate new approaches and present recent data, which may provide a novel perspective on some of the outstanding problems of cone function.
What features of the transduction cascade are responsible for the more rapid kinetics and decreased sensitivity of the cone response?
From many studies of visual pigment and transduction genes, we can be fairly confident that both rods and cones evolved from a cone-like ancestor [61, 91]. We know from the example of lamprey that this evolution was complete already in the late Cambrian [see 35]. Lampreys are agnathans or jaw-less vertebrates, whose ancestors diverged from all of the other vertebrates about 500 million years ago [60]. Nevertheless, the responses of lamprey rods and cones are remarkably similar to those of other vertebrates—in the dark, in background light, and after pigment bleaching [5, 73, 74]. A physiologist, given responses from lamprey photoreceptors placed on one side and salamander photoreceptors on the other, would be hard-pressed to say which was which. Already in lamprey, the cones are nearly 100 times less sensitive than rods. With time constants of response decay at least 10 times shorter, cones have the faster responses required to resolve rapid changes in light intensity.
Recordings from lamprey photoreceptors have also resolved a long-standing question of outer-segment anatomy. The outer segments of rods in most vertebrates have enclosed membranous disks, but cones over most of the length of their outer segments have open membranous lamellae. In lamprey, however, the morphologies of the outer segments of rods and cones are identical: both are cone-like with membranous lamellae [33, 43, 54, 83, 84]. It would therefore appear that the disks of rods in jawed vertebrates evolved after the line leading to lamprey diverged from other vertebrates. Rod disks can therefore have little role in determining photoreceptor kinetics or sensitivity but may contribute to some other cellular function such as membrane renewal [73].
If, then, we put anatomy to one side and ask what molecular differences between the two kinds of photoreceptors might be responsible for their physiological differences, we are presented with an embarrassment of choice. Nearly every important transduction protein is in some way different, either because the rods and cones express different protein isoforms (photopigments, transducin, phosphodiesterase subunits, cyclic-nucleotide-gated channels, Na+/Ca2+-K+ transporter) or because expression levels of proteins are different (guanylyl cyclase, GTPase-activating proteins or GAPs, and probably also phosphodiesterase).
In an attempt to elucidate which of these molecular differences are responsible for the differences in sensitivity and response kinetics, many research groups have expressed cone proteins in rods or have altered protein expression levels [reviewed in 51]. Some of these experiments have revealed differences, for example for transducin [24], phosphodiesterase [67], and increases in GAP expression [23, 59], but many attempts of this kind have resulted in little or no change, and in no case was photoreceptor sensitivity or time course of response decay altered by more than a factor of 2 or 3. We can then conclude that no one molecular change can explain the entire rod/cone difference, but that many differences in protein isoform and expression level must contribute. This conclusion is perfectly consistent with the way we think evolution often works, by the slow and gradual accretion of many small differences to produce a significant change in physiology or behavior.
The question then becomes, which of these differences are the most important? Because further progress with purely experimental approaches may become increasingly complex and technically problematic, we decided to combine experiment with theory. We began with an accepted model of the mouse rod photoreceptor response incorporating the principal features of the phototransduction cascade [26, 45, 46, 93, 96, 98, 99]. We then asked, what changes have to be made in this model to fit responses to cones? To make this approach feasible, we first needed accurate measurements of the light-induced changes in cone outer-segment conductance. Moreover, we needed measurements from cones with alternative genetic backgrounds, so that we could test our model by altering the expression of transduction enzymes [as in 46]. In short, we needed recordings from mouse cones, and we needed voltage-clamp recordings because suction-electrode recordings [79, 80], membrane-potential recordings [4], and pharmacology-isolated cone responses from whole-retina electroretinograms (ERG) [101, 102] are low-pass filtered by the high capacitance of the cone membrane and do not provide unambiguous measurements of changes in membrane conductance [90].
We were therefore very fortunate to have had a talented graduate student, Norianne Ingram, who discovered that she could use the position and appearance of photoreceptor somata to direct her pipettes almost unerringly to the cones [52], even though in mouse they constitute only 3% of the total photoreceptor population [86]. In this way, whole-cell recordings from single cones in retinal slices could be made routinely to examine their physiology.
Examples of such recordings are given in Fig. 1 [52]. Figure 1 a and b are voltage-clamp current responses and current-clamp voltage responses to a series of brief flashes of increasing intensity for a wild-type (WT) mouse cone. Responses like these were shown to originate from cones by including in the patch pipette the dye Alexa Fluor 750 (Fig. 1c, d). The WT cell in Fig. 1 was exceptional because it had little rod input. In other recordings, rod input to the cone could be nearly as large as the intrinsic response of the cone itself. To study cone responses without rod interference, recordings were made from retinas lacking rod transducin (Gnat1−/−), which almost entirely eliminates rod activity [19].
Fig. 1.
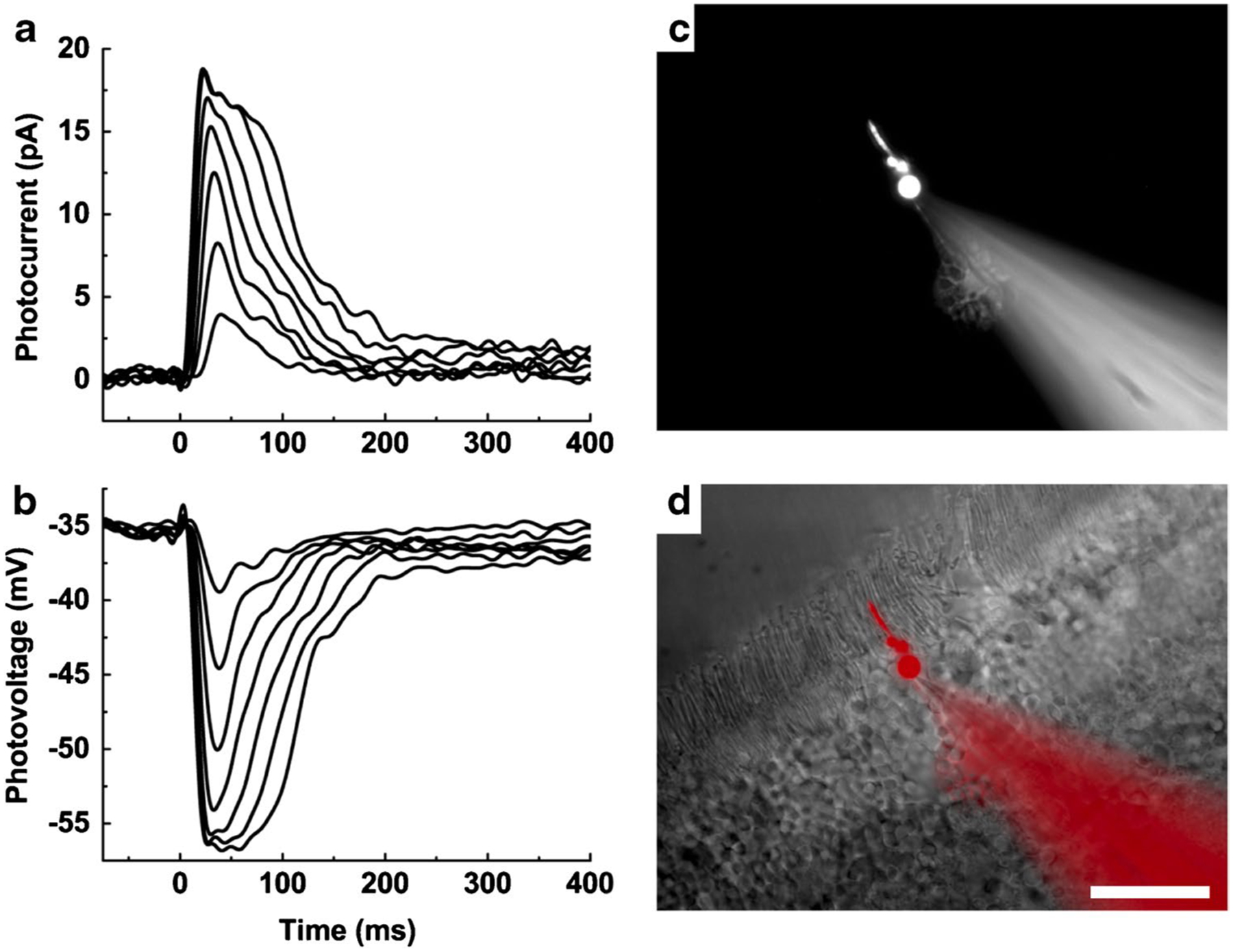
Photoresponses of mouse cones recorded with whole-cell patch clamp. a Current responses from a WT mouse cone. The soma of the cone was voltage clamped and held at − 50 mV. Brief flashes of between 22 and 2100 pigment photoisomerizations (P*) per flash were presented at time = 0 ms. b Voltage responses from the same cone as a in current clamp, stimulated with the same light flashes. Data traces in a and b are averages of seven presentations. c An individual cone visualized with the dye Alexa Fluor 750 dialyzed into the cell from the recording pipette. d Merged image of c (pseudo-colored red) and wide-field image of the same retinal slice. Scale bar, 20 μm. From [52]
Once we had voltage-clamp measurements of changes in cone light–dependent conductance, we worked with Jürgen Reingruber of the École Normale Supérieure in Paris to model the cone responses [97]. We first produced a model of the rods, based both on recordings of WT rods and rods lacking the guanylyl cyclase–activating proteins (GCAPs−/−), with literature values of most of the rod parameters. We used GCAPs−/− rods and cones because they lack Ca2+-dependent regulation of guanylyl cyclase, providing considerable simplification of the model for our initial calculations. We then attempted to fit WT and GCAPs−/− cone responses with the rod model by changing only a single kinetic parameter. In agreement with the molecular biology, we discovered that no one parameter alteration could let us fit the cone light response. We could however get satisfactory fits to both WT (Fig. 2a) and GCAPs−/− cones (Fig. 2b) when we altered the rod model to produce (1) decreased gain of the response, reflecting a reduction in amplification of the transduction cascade probably at least in part due a decrease in transducin activation by excited visual pigment; (2) increased rate of turnover of cGMP in darkness, perhaps due to an increase in cyclase expression and phosphodiesterase (PDE) activity; and (3) increased rate of decay of activated PDE, likely caused by increased GAP expression in cones and more rapid deactivation of transducin. The root-mean-squared error was nearly the same for models with and without an additional acceleration of decay of light-activated photopigment. We were therefore unable to exclude the possibility that light-activated pigment decays more rapidly in cones than in rods. But since rods and cones in mouse use the same rhodopsin kinase (GRK1), the rates of pigment decay may not greatly differ.
Fig. 2.
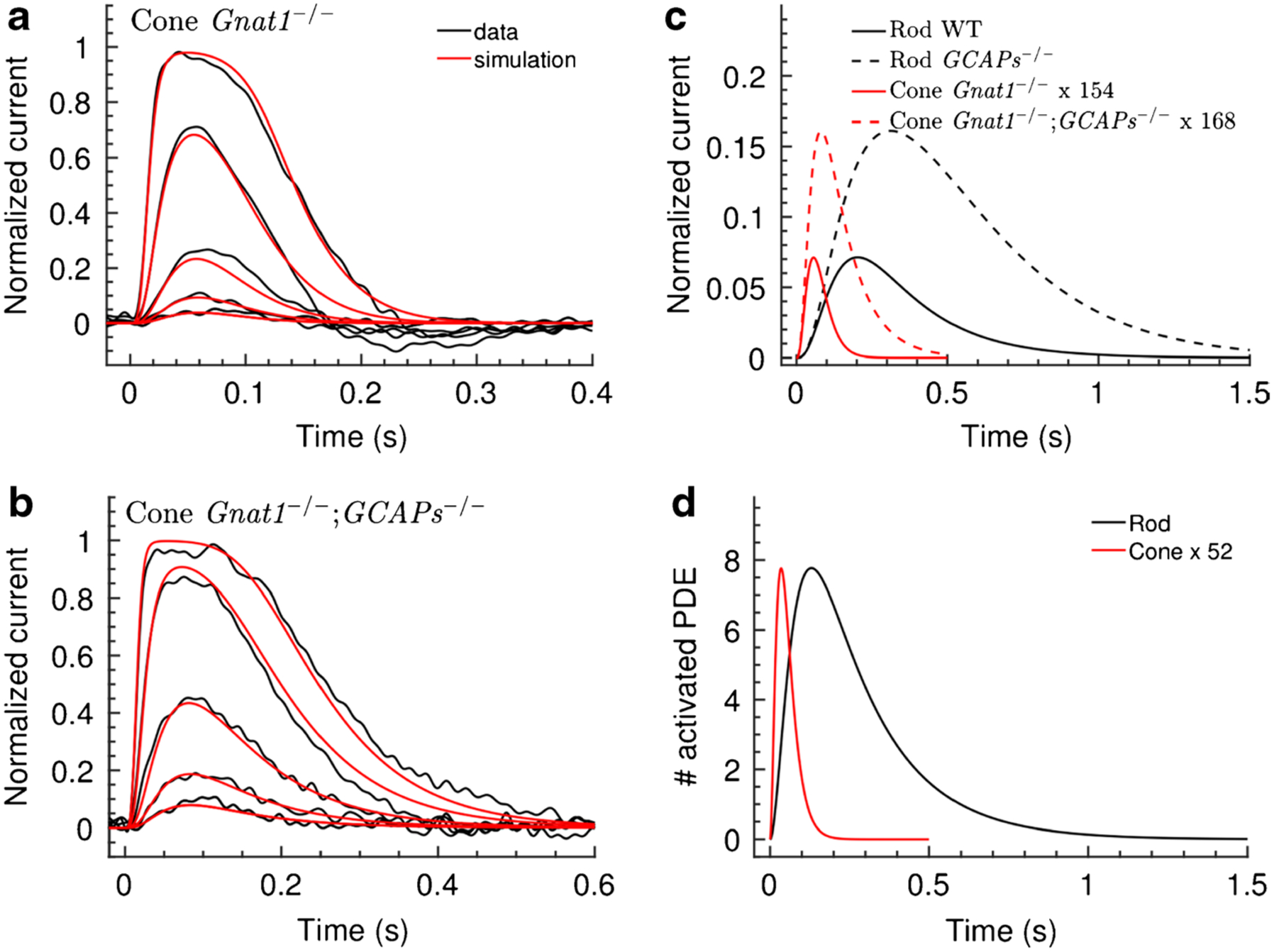
Model of mouse cone response. Black traces are responses from Gnat1−/− cones (a) and Gnat1−/−;GCAPs−/− cones (b). Recordings were made on a Gnat1−/− background to eliminate rod input. Thus, Gnat1−/− cones are essentially WT cones without rod input. Both sets of recordings were fitted concurrently by altering the parameters of the rod model, and red traces are the best fits obtained by decreasing transduction gain, increasing dark cGMP turnover, and accelerating decay of light-activated PDE. c Calculated single-photon responses of WT and GCAPs−/− rods and cones. d Calculated number of PDEs activated by light as a function of time. PDE activation amplitude and kinetics are unaffected by the presence or absence of the GCAPs and are therefore the same for both Gnat1−/− and Gnat1−/−;GCAPs−/− cones. The indications x154 and x168 in c, and x52 in d, mean that displayed responses have been multiplied by the specified factor. From [97]
In Fig. 2, we also compare for rods and cones the single-photon responses (Fig. 2c) and number of PDE molecules activated per photon (Fig. 2d) as calculated from our model. The amplitude and integration times for the various single-photon responses are similar to those actually recorded from rods [for example 8, 26, 71, 104] and cones [80, 103]. We estimated the peak number of PDE molecules activated at any one time during the single-photon response in both WT and GCAPs−/− photoreceptors as a factor of 52 less in a cone than in a rod.
Our study was confined to dark-adapted cones in response to brief flashes and is only a beginning. We think it very likely that other mechanisms may be required to explain the adaptation of cone responses during prolonged stimulation. A different approach may be required because, at present, we lack an adequate model of adaptation in rods. We believe however that we have already been able to identify some of the principal alterations in gain and kinetics of specific steps in phototransduction that distinguish rods from cones, which might have been responsible during evolution for the emergence of the duplex retina.
What role do inner-segment channels have facilitating the rapid rise and fall of the cone response in changing illumination?
Cones in mammals, like rods and cones in all vertebrates, have a variety of ion channels on the membrane of their inner segments (Fig. 3), including voltage-gated Ca2+ channels at the synapse, Ca2+-activated channels permeable to K+ and Cl−, voltage-gated K+ channels, and hyperpolarization-activated and cyclic-nucleotide-gated (HCN) channels permeable to both Na+ and K+ [see 110]. Beginning with our own work many years ago [36, 38–40], many subsequent experiments particularly in lower vertebrates have characterized these conductances [6, 9, 10, 12, 15, 49, 65, 68, 117], and similar experiments have also been done on mammalian rods [30, 31, 88, 107] and cones [109, 118]. Although several attempts have been made to quantify the effects of these conductances on the waveform of the photoreceptor light response, these efforts have largely employed current injection to mimic the voltage-response waveform rather than the voltage responses themselves [for example 12]. No previous study has utilized voltage clamp and current clamp to characterize the properties of cone inner-segment channels and explain how their opening and closing contribute to the transformation of a change of conductance in the outer segment into a voltage response at the synapse. This approach is however the only certain way to quantify the influence of inner-segment conductances on the kinetics of the voltage signal.
Fig. 3.
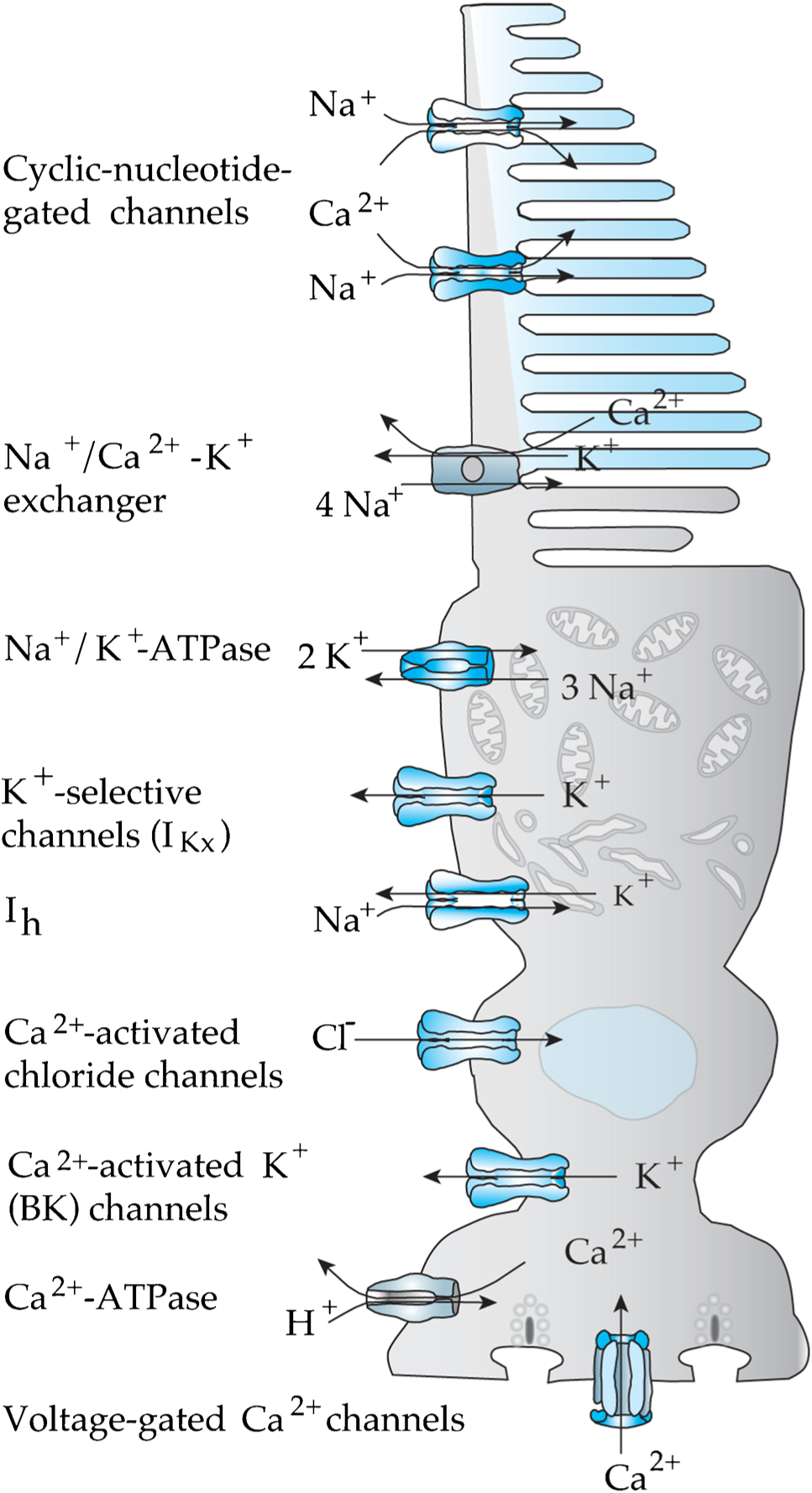
Cone channels, pumps, and transporters
With this end in view, we have used the techniques we developed for recording from mouse cones to characterize their inner-segment conductances. The great asset of mouse is its genetics and the large number of transgenic animals presently available. We exploited this advantage by doing our experiments exclusively on animals lacking connexin-36 gap junctions, which are known to mediate electrical synapses between mouse photoreceptors [55, see 41] and particularly between rods and cones [4]. By this means, we could eliminate rod input into cones and also remove current flow between photoreceptors, improving the space-clamp of our recordings.
From a series of experiments employing voltage steps and cocktails of pharmacological agents [see 110], we were able to identify in mouse cones all of the inner-segment conductances that have been previously described for other vertebrate photoreceptors [53]. Among these channels was one producing an outward current blocked by iberiotoxin, which is diagnostic for Ca2+-activated BK K+ channels. BK channels had been previously described in lower vertebrates [117] but not in mammals [118].
Of the channels we observed, we were able to do the most thorough analysis for the ih current produced by HCN channels (Fig. 4). This current is activated by hyperpolarization, with time constants of activation increasing from 25 ms near rest to less than 10 ms for stronger hyperpolarization and with no perceptible inactivation. It had a reversal potential of −28 ± 1.2 mV (n = 10), and an estimated relative permeability ratio of sodium to potassium PNa/PK of about 0.4, similar to that measured in other species [10, 29, 32, 49, 118]. This current is activated during the hyperpolarizing voltage response and may have a large effect on the kinetics of the response [39]. Experiments on the other channel types are continuing, with the hope of assembling enough information about channel kinetics and voltage and/or Ca2+ dependence to begin modeling the cone voltage waveform, which is the direct driver of changes in glutamate release at the photoreceptor synapse.
Fig. 4.
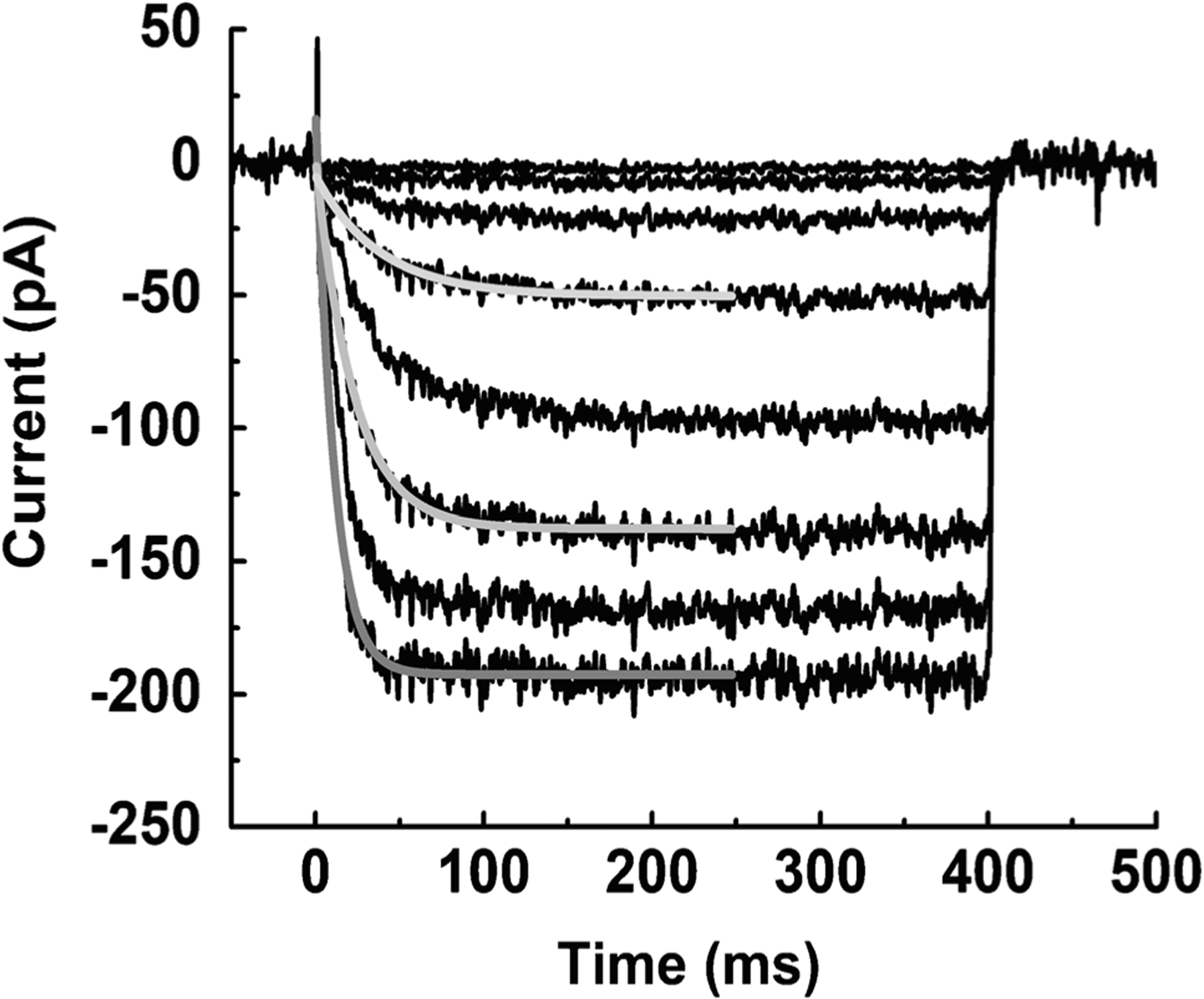
HCN currents of mouse cones, measured with voltage-clamp. Leak-subtracted currents from cone held at − 50 mV and stepped for 400 ms to potentials of − 25 to − 135 mV in increments of 10 mV. Gray lines are fits to single exponential functions to give activation time constants, which varied from 25 ms near rest to less than 10 ms for strong hyperpolarizing steps. We observed no significant inactivation. From [53]
Why do cones continue to respond even in bright illumination?
Many studies have shown that cones give robust responses even in the most intense background light, in part by adaptation of the phototransduction cascade [16, 70]. Some mechanism may allow a recovery of the cGMP concentration in the presence of a maintained stimulus, presumably at least in part from a decrease in the rate of the PDE and/or an increase in the rate of the cyclase. As the cGMP concentration increases, the channels reopen and permit the cone to continue to respond to superimposed increments or decrements of light.
Figure 5a [from 52] illustrates this phenomenon in a voltage-clamped cone in the presence of a bright background light initially bleaching about 0.5% of the photopigment per second. The cone was recorded in a slice of a Gnat1−/− retina to avoid any contribution from rods. Recovery occurred in two phases, one rapid with a time constant of a fraction of a second, and the other much slower occurring over many seconds. As the cone circulating current recovered, flashes of increasing intensity were superimposed on top of the background, and the brightest of these flashes briefly saturated the cone photocurrent. These and other measurements [16, 70] show that recovery from saturation during prolonged stimulation is not the result of a change in the number of outer-segment channels available to be closed but rather a consequence probably at least in part of some alteration in the rate of synthesis or hydrolysis of cGMP.
Fig. 5.
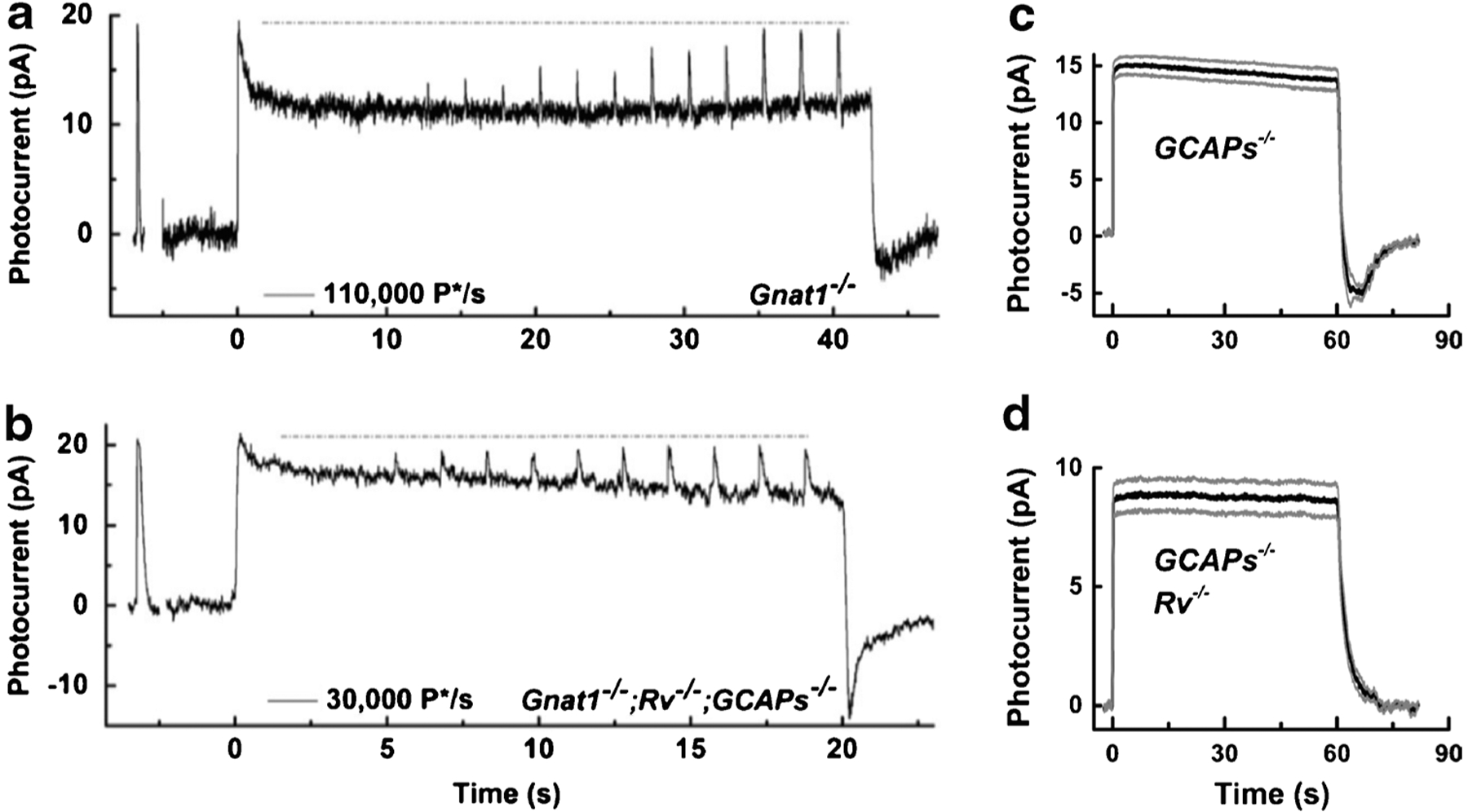
Responses of mouse photoreceptors to steps of illumination. Current responses from a Gnat1−/− cone (a) and a Gnat1−/−;Rv−/−;GCAPs−/− cone (b) from [52]. Gnat1−/− cones lacked rod input, and Gnat1−/−;Rv−/−;GCAPs−/− cones also had genes for both recoverin and the GCAPs deleted. Recordings show first the response to a brief, saturating flash (~5000 P*/flash) followed by exposure to steady light in (a) of 110,000 P*/s and in (b) of 30,000 P*/s. Five seconds after the steady light was turned on, a graded series of stimuli were given from 1800 to 29,000 P* per flash. The dashed line indicates the initial peak-current response. Mean responses of 16 GCAPs−/− (c) and 19 GCAPs−/−;Rv−/− rods (d) from [76] to 60-s steps of light at an intensity of 950 photons μm−2 s−1. Gray traces give ± standard error of the mean (S.E.M.) calculated point by point. Deletion of recoverin eliminated both the slow decline of current during illumination and the undershoot at stimulus cessation
In an attempt to discover the nature of this process, a similar (though somewhat less intense) background light was given to a Gnat1−/− cone which also lacked the genes for both the GCAPs and the small molecular weight, Ca2+-binding protein recoverin (Fig. 5b, [52]). Both the fast and the slow phases of recovery could still be observed, though both were smaller and slower. Once again, the total number of channels available to be closed seemed not to be affected. Responses from cones lacking only the GCAPs were similar. Moreover, responses from cones lacking only recoverin resembled WT cones. It therefore seemed that removal of recoverin by itself had little effect on response waveform and adaptation to background light, whereas the effect of removal of the GCAPs was significant.
These results contrast with our recordings from rods [76]. In rods lacking the GCAPs (Fig. 5c), responses still show some adaptation with a slow recovery of current as in cones (Fig. 5b), followed by a marked undershoot as we [26] and others [17] had previously observed. This undershoot is of some interest, since it cannot be caused by modulation of Rh* or by Ca2+-calmodulin-dependent regulation of the cyclic-nucleotide-gated (CNG) channels [26]. In rods, both the undershoot and current recovery are completely eliminated by deletion of the gene for recoverin (Fig. 5d). Although the explanation of this result is still not entirely clear, it is possible that in rods recoverin regulates the rate of the PDE [25, 76].
What process then mediates the recovery of current in a cone? The results in Fig. 5 a and b indicate that GCAP-dependent activation of the cyclase is part of the story, since when this mechanism is removed the cones recover less completely and more slowly [see also 101, 102]. As our modeling of cone flash responses has shown, light-activated PDE in a cone decays more rapidly than in a rod from a higher concentration of GAP proteins [120]. There may also be other mechanisms of transduction modulation such as channel regulation [58, 95] which may contribute to the decay of the response and prevent cone CNG channels from ever completely closing during maintained illumination.
There is, however, one more trick the cone has up its sleeve. Mechanisms of sensitivity modulation like those mediated by the GCAPs only have to function over a restricted range of background light intensities in a cone, perhaps just 2–3 log units. Once the light intensity becomes sufficiently bright, sensitivity can decline by virtue of the bleaching of photopigment and the consequent decrease in quantum catch.
To illustrate the role of pigment bleaching, we begin with a simple equation. In the presence of a continuous bright background light, cones come to steady state with a fraction of unbleached pigment equal to
| (1) |
where I is the intensity of the steady light in photons μm−2 s−1 (at the λmax of the photopigment), P is the photosensitivity [5.7 × 10−9 μm2, see 82, 116], and τ is the time constant of regeneration. The value of τ is unknown for mouse cones, but it is 120 s for human cones [2] and 470 s for turtle cones [16, but see also 44]. Since pigment regeneration in mouse is rather slow [see for example 66], we will take τ for mouse cones to be 250 s. As we will see, the exact value is not going to matter very much.
We can then infer from Eq. (1) that once the background intensity exceeds about 106 incident photons μm−2, IPτ will begin to be sufficiently greater than 1 so the fraction of bleached pigment will decline in almost inverse proportion to the background light intensity. Provided the sensitivity of the cone is linearly proportional to the pigment concentration (which should be nearly the case at these low pigment concentrations), and provided there still remains enough pigment to trigger phototransduction, cones from this point onward will obey Weber’s law just from the reduction in pigment concentration [16, 70]. Since cones begin to adapt to background light at an intensity of about 104 incident photons μm−2 [101], they need only modulate the transduction cascade from 104 to between 106 and 107 incident photons μm−2 until pigment bleaching takes over. This 2–3 log-unit span is about the range over which rods adapt to background light [37]. We therefore suggest that cones do not require any special mechanism of modulation in addition to those used by rods to prevent saturation, though that is not to say that the two kinds of photoreceptors adapt identically (see Fig. 5). It is sometimes thought that the ability of cones to avoid saturation is a special feature of cone transduction and something remarkable [64]. It is quite possible, however, that cones adapt much like rods but can continue to give robust responses even in very bright light only because they are less sensitive.
How do cones renew visual pigment in constant light?
The retinal pigment epithelium has long been known to convert all-trans retinal to 11-cis retinal to support the formation of the visual pigment, but this mechanism may not be able to regenerate pigment at a rapid enough rate to maintain cone vision in bright light [16, 69]. Early experiments demonstrated that cone but not rod pigment can be regenerated in frog retina in the absence of the pigment epithelium [42, 50], and Vladimir Kefalov and his colleagues have examined this phenomenon in detail. Their work has delineated a mechanism of pigment regeneration specifically for cones mediated by the retinal Müller glial cells [113], which is widespread among vertebrates and present in mammals including primates [112]. How the Müller cells isomerize retinal has however remained unclear.
In collaboration with the laboratory of Gabriel Travis, we have recently shown [75] that Müller cells convert all-trans retinal to 11-cis retinal with a protein called retinal G protein–coupled receptor (RGR) opsin [89]. This protein is related to the opsin photopigments but lacks motifs required for interaction with G proteins and is probably not a signaling molecule. Instead, as Hao and Fong [47] showed, RGR opsin can bind all-trans retinal in the dark and convert it to 11-cis in the light.
We investigated the role of RGR opsin with pharmacologically isolated photoreceptor ERG responses from whole, isolated retinas of Gnat1−/− mice. Because these mice have no functional rods, we could record cone responses without rod-signal contamination. We showed that normal cones in the isolated retina can continue to respond in continuous bright light, with only slow declines in peak amplitude and sensitivity, but that responses of cones in RGR−/− retinas (whose gene for RGR opsin had been deleted) decayed much more rapidly. The effect of deletion of the RGR gene could be mimicked by treating RGR +/+ retinas with the compound α-aminoadipic acid, a drug that poisons Müller cells and blocks the Müller cell mechanism of pigment regeneration [112]. It would therefore appear that the light-dependent regeneration of visual pigment via RGR opsin is responsible for Müller cell regeneration of cone pigment in the isolated retina. This pathway uses a retinol dehydrogenase (probably RDH10) to convert 11-cis retinal into 11-cis retinol [75], and since cones but not rods can utilize 11-cis retinol [56], the RGR opsin together with the dehydrogenase can provide a cone-specific pathway for pigment regeneration within the neural retina.
RGR opsin is also present in the retinal pigment epithelium (RPE) [75], where it has been shown also to produce a light-dependent regeneration of visual pigment [119]. Because the expression of RGR opsin is higher in the RPE than in Müller cells, it would be reasonable to expect that the RPE would make a bigger contribution to light-dependent regeneration than the Müller cells, though the relative contributions of these cell types is not presently known. In addition to RGR opsin, the retina has a second light-dependent pathway utilizing N-retinylidene-phosphatidylethanolamine (N-ret-PE) [57]. The all-trans retinal released from opsin after bleaching condenses with phosphatidylethanolamine to form a retinal-lipid intermediate. Illumination of N-ret-PE can then photoisomerize the retinal to the 11-cis isomer, which can rebind to opsin and regenerate visual pigment. This pathway also contributes to cone sensitivity [57, 75].
We have long appreciated that many invertebrates including insects can use light to regenerate photopigment, and we now know that vertebrates can do this feat as well. At present, we have little information about the relative contributions of N-ret-PE, RGR opsin, and the classic pigment-epithelium visual cycle to the regeneration of cone pigment. The contribution of different mechanisms may be dependent on the intensity of background illumination, perhaps with the N-ret-PE and RGR-opsin pathways only important in very bright light. Future research will need to specify the relative roles of Müller cells and RPE in providing 11-cis retinol to cones. These experiments will be challenging, because recordings will have to be made either from intact animals or whole eyecups, and biochemical measurements of cone pigment in most mammals are problematic because cones are present in such low numbers. We now have an abundance of possible pathways but need much more information about their function and relative importance.
Why do cones eventually degenerate after the loss of rods in genetically inherited rod diseases?
One of the unsolved mysteries of retinal disease is why mutations in rod proteins cause the eventual death of cones [see 78]. Cone death begins to occur at about the time that rod degeneration is mostly complete [94]. Cones first lose their outer segments, then axon and synaptic pedicle, and finally most of the inner segment to form rounded cell bodies [11, 18, 63]. These “dormant” cones can remain viable for a prolonged period of at least a year in mouse but several years in pig [114], often sprouting telodendria throughout the remnant of the outer nuclear layer as if in search of synaptic partners [63] before completely degenerating. Although many possible explanations have been given for why cones die after the death of rods, including oxidative stress and microglial activation, recent work has indicated that cones may die from a lack of metabolic energy [115]. Cone degeneration is preceded by changes in the insulin/mTOR pathway and can be delayed by treatment with insulin [94] or activation of mTOR [111]. Rods may enhance the viability of cones through the trophic factor RdCVF [62] by promoting glucose transport [1], and degeneration can be delayed by increasing glucose uptake [114].
Given the possible importance of glucose utilization in degeneration, we thought it might be useful to estimate the rate of energy expenditure in cones. We have previously shown that a physiological characterization of rod responses can be used to estimate rod ATP utilization, in darkness and in background light [85], but similar calculations have not been made for cones. With this goal in mind, we used patch-clamp recordings from Cx36−/− cones to measure photocur-rents to steps of light to estimate the steady-state rate of entry of Na+ into the outer segment as a function of light intensity. We also recorded voltage responses to steps of light, and we combined these voltages with measurements of the voltage dependence of the major inner-segment conductances (as in Fig. 4). To these numbers, we added estimates of ATP required for the synthesis of cGMP by guanylyl cyclase, on the assumption that the light dependence of the Ca2+ concentration and cyclase activity are similar in cones and rods. Slight differences would have little effect on our estimates, because ion transport is by far the largest utilizer of ATP in photoreceptors [85] as in neurons in the brain [7, 108]. The rates of ATP utilization for synaptic transmitter synthesis and vesicle formation, pigment phosphorylation and regeneration, and protein synthesis and outer-segment renewal are all much smaller by comparison.
The results of our calculations are given in Fig. 6. For rods (Fig. 6a), the largest expenditure of ATP is that required to pump out Na+ entering through the CNG channels of the outer segment. The total energy requirement drops precipitously as the light intensity increases and the rods approach saturation. For cones (Fig. 6b), the largest expenditure is that required to remove Ca2+ entering voltage-gated channels at the synapse. Because cones make many more synapses than rods onto second-order horizontal and bipolar cells, they require more ATP to maintain the Ca+ concentration constant in steady light. The rate of ATP expenditure of cones remains high even in bright light because cones continue to give robust responses. This difference can be more clearly appreciated in Fig. 6c, where we compare the rates of total ATP utilization in rods and cones. Cones are clearly a lot more metabolically expensive.
Fig. 6.
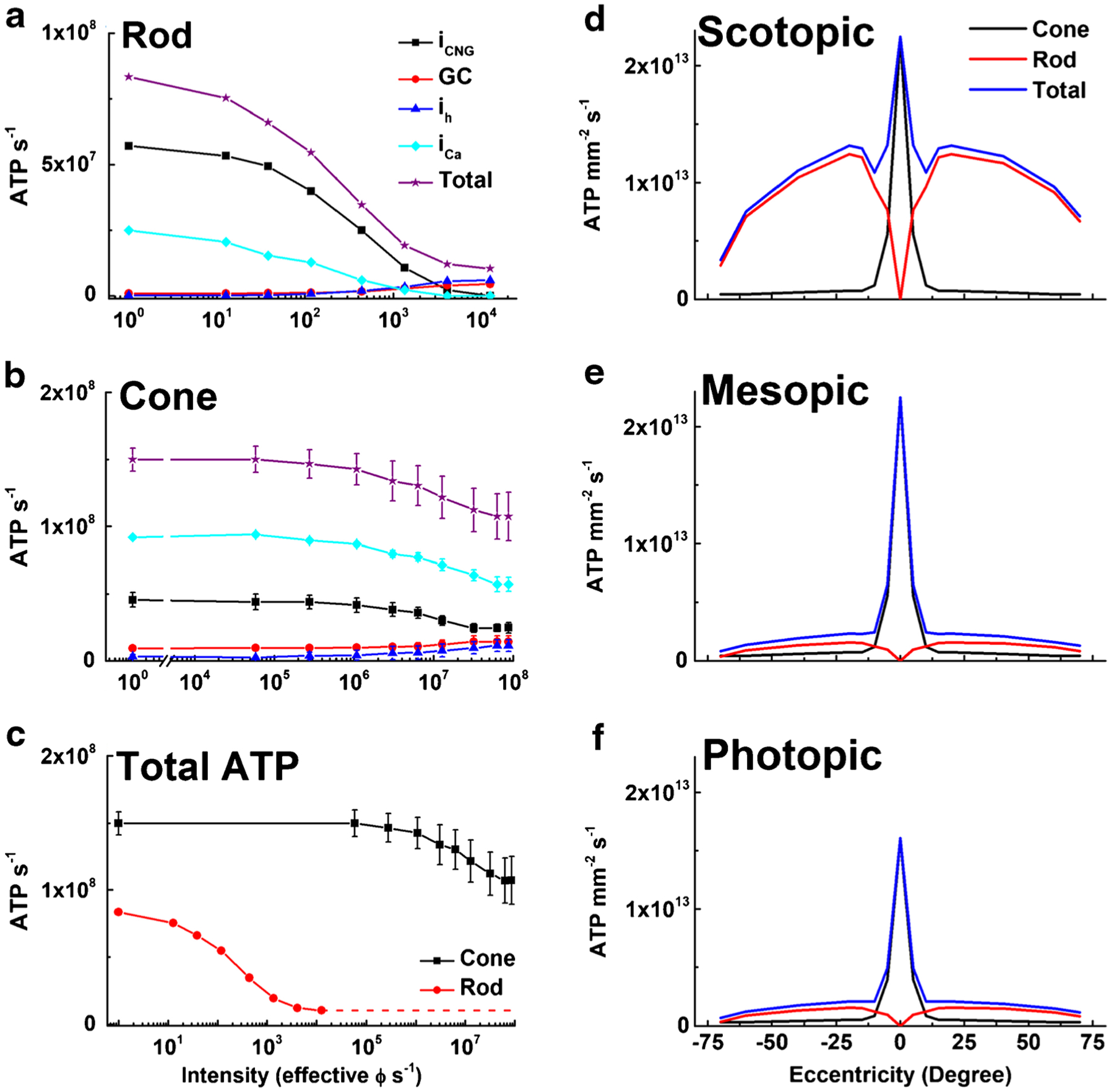
ATP consumption in rods and cones in steady light. Net ATP consumption for rods (a) and cones (b) as a function of light intensity in photons s−1, effective at the λmax of the rod or cone photopigments. ATP consumption was calculated from physiological measurements (see text) and is given as ATP consumed s−1 for CNG channels (iCNG, black squares), HCN channels (ih, blue triangles), Ca2+ influx at synaptic terminals (iCa, cyan diamonds), guanylyl cyclase (GC, red circles), and the sum of all four (Total, purple stars). c Total ATP consumed for rods (red) and cones (black). Data are means ± S.E.M. d–f Total ATP consumption for rods and cones as a function of retinal eccentricity for human retina, calculated from the data in part (c) and the density of photoreceptors [87]. Energy expenditure was calculated for three ambient light intensities: scotopic (d, darkness, 0 effective ϕ cell−1 s−1); mesopic (e, 12,600 effective ϕ rod−1 s−1 or 12,500 effective ϕ cone−1 s−1); and photopic (f, 8.8 × 107 effective ϕ rod−1 s−1 or 8.7 × 107 effective ϕ cone−1 s−1). We used mouse values for cone light–dependent currents, even though foveal cone outer segments are three times longer than mouse cones [21, 34] and likely have larger circulating currents. No attempt was made to take into consideration the difference in the number of synaptic ribbons in the pedicles between foveal and peripheral cones [28]
In most mammals, rods are much more abundant than cones, and this has the consequence that total energy expenditure across the whole of the retina continues to be greater for rods than for cones even in bright light. In the primate fovea, however, the high concentration of cones produces a sharp peak in ATP expenditure, which is present in darkness (Fig. 6d) and becomes more prominent in mesopic background light (Fig. 6e). This peak remains large under photopic conditions during daylight (Fig. 6f), with the result that the fovea acts as an important sink of ATP in primate (including human) retina, which could make this part of the retina especially at risk when glucose transport or availability is decreased [1, 27, 94, 114].
The high metabolic cost of cones may explain why, in diseased retinas, the cones first lose their outer segments [11, 63] and remain viable as rounded cell bodies [94]. It is sometimes thought that cones lose their outer segments to avoid the cost of having to renew them. We think it more likely that outer segments are lost to prevent Na+ entry through CNG channels. An increase in intracellular Na+ concentration can produce an increase in Ca2+ by decreasing Ca2+ export via NCKX exchange [22], and an increase in Ca2+ in photoreceptors as in other cells can trigger apoptosis [see 92].
Future perspectives
Although our understanding of cone physiology has progressed more slowly than for rods, we think the future is bright. Since it is now possible to make routine, reliable recordings from mouse cones, we will be able to exploit mouse genetics to understand cone function and dysfunction in greater detail. We are particularly interested in two avenues of research. As Fig. 3 illustrates, there are at least 5 different voltage-gated or Ca2+-activated channels in the cone inner segment, which are likely to have significant effects on the shaping of the cone voltage response and the mechanism of transmission at the photoreceptor synapse. It would be interesting to explore the function of these channels by recording from cones in which the gene for one or more of the conductance mechanisms had been deleted. It is quite fortunate that animals with deletions in each of the inner-segment channels have been made and are available. One of these animals has already been exploited by two groups [30, 107], who recorded from animals lacking HCN1, a gene for the ih current known to be expressed in mouse photoreceptors [72, 77]. Both groups examined effects only on rods or rod-driven signals. In preliminary experiments before the pandemic closed down our lab, we were able to show that the ih current of Fig. 4 is eliminated in cones lacking the HCN1 gene. We are anxious to continue these experiments to try to understand the role of this current in shaping the waveform of the cone voltage response.
We can also use our new techniques to record from cones in degenerating retina. Very little is known about the changes in photoreceptor physiology during degeneration. Because several mouse lines are available showing progressive but not overly rapid degeneration (such as the retinal degeneration 10 or rd10 line), it should be possible to record from cones as they lose their outer segments, send out processes, and round up into isolated cell bodies. We are particularly curious about “dormant” cones, which have elicited considerable interest because they have been considered to be a target for cone reanimation [see 100]. They maintain a surprisingly depolarized resting potential but are reported to have no light responses with presumably no CNG channels [18]. It is therefore unclear which channel type is keeping the membrane potential depolarized. It is intriguing that cone degeneration is accelerated in animals lacking the HCN1 gene [106]. We are hopeful that recordings from cones at various stages during degeneration will provide new insights into the role of inner-segment channels and the mechanisms of cell death, which may help design treatments to rescue and restore cone function.
Acknowledgements
We are grateful to Margery J. Fain for drawing Figure 3 and for changing fonts in some of the figures from uppercase to lowercase to meet the requirements of the journal, and to Simon Laughlin for his thorough and helpful reading of an earlier draft of the manuscript.
Funding
This work was supported by NEI R01 EY01844 to G.L.F., and NEI R01 EY29817 to A.P.S., an unrestricted grant from Research to Prevent Blindness USA to the UCLA Department of Ophthalmology, and NEI Core Grant EY00311 to the Stein Eye Institute.
Footnotes
Conflict of interest The authors declare no conflicts of interest.
References
- 1.Ait-Ali N, Fridlich R, Millet-Puel G, Clerin E, Delalande F, Jail-lard C, Blond F, Perrocheau L, Reichman S, Byrne LC, Olivier-Bandini A, Bellalou J, Moyse E, Bouillaud F, Nicol X, Dalkara D, van Dorsselaer A, Sahel JA, Leveillard T (2015) Rod-derived cone viability factor promotes cone survival by stimulating aerobic glycolysis. Cell 161:817–832. 10.1016/j.cell.2015.03.023 [DOI] [PubMed] [Google Scholar]
- 2.Alpern M, Maaseidvaag F, Ohba N (1971) The kinetics of cone visual pigments in man. Vision Res 11:539–549. 10.1016/0042-6989(71)90075-7 [DOI] [PubMed] [Google Scholar]
- 3.Angueyra JM, Rieke F (2013) Origin and effect of phototransduction noise in primate cone photoreceptors. Nat Neurosci 16:1692–1700. 10.1038/nn.3534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asteriti S, Gargini C, Cangiano L (2017) Connexin 36 expression is required for electrical coupling between mouse rods and cones. Vis Neurosci 34:e006. 10.1017/S0952523817000037 [DOI] [PubMed] [Google Scholar]
- 5.Asteriti S, Grillner S, Cangiano L (2015) A Cambrian origin for vertebrate rods. eLife 4. 10.7554/eLife.07166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Attwell D (1986) The Sharpey-Schafer lecture. Ion channels and signal processing in the outer retina. Q J Exp Physiol 71:497–536 [PubMed] [Google Scholar]
- 7.Attwell D, Laughlin SB (2001) An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metabol 21. 10.1097/00004647-200110000-00001 [DOI] [PubMed] [Google Scholar]
- 8.Azevedo AW, Rieke F (2011) Experimental protocols alter phototransduction: the implications for retinal processing at visual threshold. J Neurosci 31:3670–3682. 10.1523/JNEUROSCI.4750-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bader CR, Bertrand D, Schwartz EA (1982) Voltage-activated and calcium-activated currents studied in solitary rod inner segments from the salamander retina. J Physiol 331:253–284. 10.1113/jphysiol.1982.sp014372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barnes S, Hille B (1989) Ionic channels of the inner segment of tiger salamander cone photoreceptors. J Gen Physiol 94:719–743. 10.1085/jgp.94.4.719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barone I, Novelli E, Strettoi E (2014) Long-term preservation of cone photoreceptors and visual acuity in rd10 mutant mice exposed to continuous environmental enrichment. Mol Vis 20:1545–1556 [PMC free article] [PubMed] [Google Scholar]
- 12.Barrow AJ, Wu SM (2009) Low-conductance HCN1 ion channels augment the frequency response of rod and cone photoreceptors. J Neurosci 29:5841–5853. 10.1523/JNEUROSCI.5746-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baudin J, Angueyra JM, Sinha R, Rieke F (2019) S-cone photoreceptors in the primate retina are functionally distinct from L and M cones. eLife 8. 10.7554/eLife.39166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baylor DA, Fuortes MG (1970) Electrical responses of single cones in the retina of the turtle. J Physiol (Lond) 207:77–92. 10.1113/jphysiol.1970.sp009049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beech DJ, Barnes S (1989) Characterization of a voltage-gated K+ channel that accelerates the rod response to dim light. Neuron 3:573–581. 10.1016/0896-6273(89)90267-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burkhardt DA (1994) Light adaptation and photopigment bleaching in cone photoreceptors in situ in the retina of the turtle. J Neurosci 14:1091–1105. 10.1523/JNEUROSCI.14-03-01091.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burns ME, Mendez A, Chen J, Baylor DA (2002) Dynamics of cyclic GMP synthesis in retinal rods. Neuron 36:81–91. 10.1016/s0896-6273(02)00911-x [DOI] [PubMed] [Google Scholar]
- 18.Busskamp V, Duebel J, Balya D, Fradot M, Viney TJ, Siegert S, Groner AC, Cabuy E, Forster V, Seeliger M, Biel M, Humphries P, Paques M, Mohand-Said S, Trono D, Deisseroth K, Sahel JA, Picaud S, Roska B (2010) Genetic reactivation of cone photoreceptors restores visual responses in retinitis pigmentosa. Science 329:413–417. 10.1126/science.1190897 [DOI] [PubMed] [Google Scholar]
- 19.Calvert PD, Krasnoperova NV, Lyubarsky AL, Isayama T, Nicolo M, Kosaras B, Wong G, Gannon KS, Margolskee RF, Sidman RL, Pugh EN Jr, Makino CL, Lem J (2000) Phototransduction in transgenic mice after targeted deletion of the rod transducin alpha-subunit. Proc Natl Acad Sci U S A 97:13913–13918. 10.1073/pnas.250478897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cao LH, Luo DG, Yau KW (2014) Light responses of primate and other mammalian cones. Proc Natl Acad Sci U S A 111:2752–2757. 10.1073/pnas.1400268111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carter-Dawson LD, LaVail MM (1979) Rods and cones in the mouse retina. I. Structural analysis using light and electron microscopy. J Comp Neurol 188:245–262. 10.1002/cne.901880204 [DOI] [PubMed] [Google Scholar]
- 22.Cervetto L, Lagnado L, Perry RJ, Robinson DW, McNaughton PA (1989) Extrusion of calcium from rod outer segments is driven by both sodium and potassium gradients. Nature 337:740–743. 10.1038/337740a0 [DOI] [PubMed] [Google Scholar]
- 23.Chen CK, Woodruff ML, Chen FS, Chen D, Fain GL (2010) Background light produces a recoverin-dependent modulation of activated-rhodopsin lifetime in mouse rods. J Neurosci 30:1213–1220. 10.1523/JNEUROSCI.4353-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen CK, Woodruff ML, Chen FS, Shim H, Cilluffo MC, Fain G (2010) Replacing the rod with the cone transducin alpha subunit decreases sensitivity and accelerates response decay. J Physiol 588:3231–3241. 10.1113/jphysiol.2010.191221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen CK, Woodruff ML, Fain GL (2015) Rhodopsin kinase and recoverin modulate phosphodiesterase during mouse photoreceptor light adaptation. J Gen Physiol 145:213–224. 10.1085/jgp.201411273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen J, Woodruff ML, Wang T, Concepcion F, Tranchina D, Fain GL (2010) Channel modulation and the mechanism of light adaptation in mouse rods. J Neurosci 30:16232–16240. 10.1523/JNEUROSCI.2868-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng SY, Cipi J, Ma S, Hafler BP, Kanadia RN, Brush RS, Agbaga MP, Punzo C (2020) Altered photoreceptor metabolism in mouse causes late stage age-related macular degeneration-like pathologies. Proc Natl Acad Sci U S A. 10.1073/pnas.2000339117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chun MH, Grunert U, Martin PR, Wassle H (1996) The synaptic complex of cones in the fovea and in the periphery of the macaque monkey retina. Vision Res 36:3383–3395. 10.1016/0042-6989(95)00334-7 [DOI] [PubMed] [Google Scholar]
- 29.Cia D, Bordais A, Varela C, Forster V, JSahel JA, Rendon A, Picaud S, (2005) Voltage-gated channels and calcium homeostasis in mammalian rod photoreceptors. J Neurophysiol 93:1468–1475. 10.1152/jn.00874.2004 [DOI] [PubMed] [Google Scholar]
- 30.Della Santina L, Piano I, Cangiano L, Caputo A, Ludwig A, Cervetto L, Gargini C (2012) Processing of retinal signals in normal and HCN deficient mice. PLoS ONE 7:e29812. 10.1371/journal.pone.0029812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Demontis GC, Longoni B, Barcaro U, Cervetto L (1999) Properties and functional roles of hyperpolarization-gated currents in guinea-pig retinal rods. J Physiol 515:813–828. 10.1111/j.1469-7793.1999.813ab.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Demontis GC, Moroni A, Gravante B, Altomare C, Longoni B, Cervetto L, DiFrancesco D (2002) Functional characterisation and subcellular localisation of HCN1 channels in rabbit retinal rod photoreceptors. J Physiol 542:89–97. 10.1113/jphysiol.2002.017640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dickson DH, Graves DA (1979) Fine structure of the lamprey photoreceptors and retinal pigment epithelium (Petromyzon marinus L.). Exp Eye Res 29:45–60. 10.1016/0014-4835(79)90165-9 [DOI] [PubMed] [Google Scholar]
- 34.Dowling JE (1965) Foveal receptors of the monkey retina: fine structure. Science 147:57–59. 10.1126/science.147.3653.57 [DOI] [PubMed] [Google Scholar]
- 35.Fain GL (2019) Lamprey vision: photoreceptors and organization of the retina. Semin Cell Dev Biol:2019 Nov 2019. pii: S1084–9521(2019)30258–30257. doi: 30210.31016/j.semcdb.32019.30210.30008. [DOI] [PubMed] [Google Scholar]
- 36.Fain GL, Gerschenfeld HM, Quandt FN (1980) Calcium spikes in toad rods. J Physiol (Lond) 303:495–513. 10.1113/jphysiol.1980.sp013300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fain GL, Matthews HR, Cornwall MC, Koutalos Y (2001) Adaptation in vertebrate photoreceptors. Physiol Rev 81:117–151. 10.1152/physrev.2001.81.1.117 [DOI] [PubMed] [Google Scholar]
- 38.Fain GL, Quandt FN (1980) The effects of tetraethylammonium and cobalt ions on responses to extrinsic current in toad rods. J Physiol (Lond) 303:515–533. 10.1113/jphysiol.1980.sp013301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fain GL, Quandt FN, Bastian BL, Gerschenfeld HM (1978) Contribution of a caesium-sensitive conductance increase to the rod photoresponse. Nature 272:466–469. 10.1038/272467a0 [DOI] [PubMed] [Google Scholar]
- 40.Fain GL, Quandt FN, Gerschenfeld HM (1977) Calcium-dependent regenerative responses in rods. Nature 269:707–710. 10.1038/269707a0 [DOI] [PubMed] [Google Scholar]
- 41.Fain GL, Sampath AP (2018) Rod and cone interactions in the retina. F1000Research 7(F1000 Faculty Rev):657. 10.12688/f1000research.14412.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goldstein EB (1970) Cone pigment regeneration in the isolated frog retina. Vision Res 10:1065–1068. 10.1016/0042-6989(70)90082-9 [DOI] [PubMed] [Google Scholar]
- 43.Govardovskii VI, Lychakov DV (1984) Visual cells and visual pigments of the lamprey, Lampetra fluviatilis. J Comp Physiology A 154:279–286. 10.1007/BF00604994 [DOI] [Google Scholar]
- 44.Granda AM, Maxwell JH, Zwick H (1972) The temporal course of dark adaptation in the turtle, Pseudemys, using a behavioral avoidance paradigm. Vision Res 12:653–672. 10.1016/0042-6989(72)90160-5 [DOI] [PubMed] [Google Scholar]
- 45.Gross OP, Pugh EN Jr, Burns ME (2012) Calcium feedback to cGMP synthesis strongly attenuates single-photon responses driven by long rhodopsin lifetimes. Neuron 76:370–382. 10.1016/j.neuron.2012.07.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hamer RD, Nicholas SC, Tranchina D, Lamb TD, Jarvinen JL (2005) Toward a unified model of vertebrate rod phototransduction. Vis Neurosci 22:417–436. 10.1017/S0952523805224045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hao W, Fong HK (1999) The endogenous chromophore of retinal G protein-coupled receptor opsin from the pigment epithelium. J Biol Chem 274:6085–6090. 10.1074/jbc.274.10.6085 [DOI] [PubMed] [Google Scholar]
- 48.Haynes LW, Kay AR, Yau KW (1986) Single cyclic GMP-activated channel activity in excised patches of rod outer segment membrane. Nature 321:66–70. 10.1038/321066a0 [DOI] [PubMed] [Google Scholar]
- 49.Hestrin S (1987) The properties and function of inward rectification in rod photoreceptors of the tiger salamander. J Physiol 390:319–333. 10.1113/jphysiol.1987.sp016703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hood DC, Hock PA (1973) Recovery of cone receptor activity in the frog’s isolated retina. Vision Res 13:1943–1951. 10.1016/0042-6989(73)90065-5 [DOI] [PubMed] [Google Scholar]
- 51.Ingram NT, Sampath AP, Fain GL (2016) Why are rods more sensitive than cones? J Physiol 594:5415–5426. 10.1113/JP272556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ingram NT, Sampath AP, Fain GL (2019) Voltage-clamp recordings of light responses from wild-type and mutant mouse cone photoreceptors. J Gen Physiol 151:1287–1299. 10.1085/jgp.201912419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ingram NT, Sampath AP, Fain GL (2020) Membrane conductances of mouse cone photoreceptors. J Gen Physiol 152(3):e201912520. 10.1085/jgp.201912520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ishikawa M, Takao M, Washioka H, Tokunaga F, Watanabe H, Tonosaki A (1987) Demonstration of rod and cone photoreceptors in the lamprey retina by freeze-replication and immunofluorescence. Cell Tissue Res 249:241–246. 10.1007/BF00215506 [DOI] [PubMed] [Google Scholar]
- 55.Jin N, Zhang Z, Keung J, Youn SB, Ishibashi M, Tian L-M, Marshak DW, Solessio E, Umino Y, Fahrenfort I, Kiyama T, Mao E-A, You Y, Wei H, Wu J, Postma F, Paul DL, Massey SC, Ribelayga CP (2020) Molecular and functional architecture of the mouse photoreceptor network. Sci Adv 6:eaba7232. 10.1126/sciadv.aba7232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jones GJ, Crouch RK, Wiggert B, Cornwall MC, Chader GJ (1989) Retinoid requirements for recovery of sensitivity after visual-pigment bleaching in isolated photoreceptors. Proc Natl Acad Sci U S A 86:9606–9610. 10.1073/pnas.86.23.9606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kaylor JJ, Xu T, Ingram NT, Tsan A, Hakobyan H, Fain GL, Travis GH (2017) Blue light regenerates functional visual pigments in mammals through a retinyl-phospholipid intermediate. Nat Commun 8:16. 10.1038/s41467-017-00018-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Korenbrot JI, Mehta M, Tserentsoodol N, Postlethwait JH, Rebrik TI (2013) EML1 (CNG-modulin) controls light sensitivity in darkness and under continuous illumination in zebrafish retinal cone photoreceptors. J Neurosci 33:17763–17776. 10.1523/JNEUROSCI.2659-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Krispel CM, Chen D, Melling N, Chen YJ, Martemyanov KA, Quillinan N, Arshavsky VY, Wensel TG, Chen CK, Burns ME (2006) RGS expression rate-limits recovery of rod photoresponses. Neuron 51:409–416. 10.1016/j.neuron.2006.07.010 [DOI] [PubMed] [Google Scholar]
- 60.Kuraku S, Kuratani S (2006) Time scale for cyclostome evolution inferred with a phylogenetic diagnosis of hagfish and lamprey cDNA sequences. Zoolog Sci 23:1053–1064. 10.2108/zsj.23.1053 [DOI] [PubMed] [Google Scholar]
- 61.Lamb TD (2013) Evolution of phototransduction, vertebrate photoreceptors and retina. Prog Retin Eye Res 36:52–119. 10.1016/j.preteyeres.2013.06.001 [DOI] [PubMed] [Google Scholar]
- 62.Léveillard T, Mohand-Said S, Lorentz O, Hicks D, Fintz AC, Clerin E, Simonutti M, Forster V, Cavusoglu N, Chalmel F, Dolle P, Poch O, Lambrou G, Sahel JA (2004) Identification and characterization of rod-derived cone viability factor. Nat Genet 36:755–759. 10.1038/ng1386 [DOI] [PubMed] [Google Scholar]
- 63.Lin B, Masland RH, Strettoi E (2009) Remodeling of cone photoreceptor cells after rod degeneration in rd mice. Exp Eye Res 88:589–599. 10.1016/j.exer.2008.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lobanova ES, Herrmann R, Finkelstein S, Reidel B, Skiba NP, Deng WT, Jo R, Weiss ER, Hauswirth WW, Arshavsky VY (2010) Mechanistic basis for the failure of cone transducin to translocate: why cones are never blinded by light. J Neurosci 30:6815–6824. 10.1523/JNEUROSCI.0613-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.MacLeish PR, Nurse CA (2007) Ion channel compartments in photoreceptors: evidence from salamander rods with intact and ablated terminals. J Neurophysiol 98:86–95. 10.1152/jn.00775.2006 [DOI] [PubMed] [Google Scholar]
- 66.Majumder A, Pahlberg J, Boyd KK, Kerov V, Kolandaivelu S, Ramamurthy V, Sampath AP, Artemyev NO (2013) Transducin translocation contributes to rod survival and enhances synaptic transmission from rods to rod bipolar cells. Proc Natl Acad Sci U S A. 10.1073/pnas.1222666110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Majumder A, Pahlberg J, Muradov H, Boyd KK, Sampath AP, Artemyev NO (2015) Exchange of cone for rod phosphodiesterase 6 catalytic subunits in rod photoreceptors mimics in part features of light adaptation. J Neurosci 35:9225–9235. 10.1523/JNEUROSCI.3563-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mao BQ, MacLeish PR, Victor JD (2003) Role of hyperpolarization-activated currents for the intrinsic dynamics of isolated retinal neurons. Biophys J 84:2756–2767. 10.1016/S0006-3495(03)75080-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mata NL, Radu RA, Clemmons RC, Travis GH (2002) Isomerization and oxidation of vitamin a in cone-dominant retinas: a novel pathway for visual-pigment regeneration in daylight. Neuron 36:69–80. 10.1016/s0896-6273(02)00912-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Matthews HR, Fain GL, Murphy RL, Lamb TD (1990) Light adaptation in cone photoreceptors of the salamander: a role for cytoplasmic calcium. J Physiol (Lond) 420:447–469. 10.1113/jphysiol.1990.sp017922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mendez A, Burns ME, Sokal I, Dizhoor AM, Baehr W, Palczewski K, Baylor DA, Chen J (2001) Role of guanylate cyclase-activating proteins (GCAPs) in setting the flash sensitivity of rod photoreceptors. Proc Natl Acad Sci U S A 98:9948–9953. 10.1073/pnas.171308998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Moosmang S, Stieber J, Zong X, Biel M, Hofmann F, Ludwig A (2001) Cellular expression and functional characterization of four hyperpolarization-activated pacemaker channels in cardiac and neuronal tissues. Eur J Biochem 268:1646–1652. 10.1046/j.1432-1327.2001.02036.x [DOI] [PubMed] [Google Scholar]
- 73.Morshedian A, Fain GL (2015) Single-photon sensitivity of lamprey rods with cone-like outer segments. Curr Biol 25:484–487. 10.1016/j.cub.2014.12.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Morshedian A, Fain GL (2017) Light adaptation and the evolution of vertebrate photoreceptors. J Physiol 595:4947–4960. 10.1113/JP274211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Morshedian A, Kaylor JJ, Ng SY, Tsan A, Frederiksen R, Xu T, Yuan L, Sampath AP, Radu RA, Fain GL, Travis GH (2019) Light-driven regeneration of cone visual pigments through a mechanism involving RGR opsin in Muller glial cells. Neuron 102(1172–1183):e1175. 10.1016/j.neuron.2019.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Morshedian A, Woodruff ML, Fain GL (2018) Role of recoverin in rod photoreceptor light adaptation. J Physiol 596:1513–1526. 10.1113/JP275779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Müller F, Scholten A, Ivanova E, Haverkamp S, Kremmer E, Kaupp UB (2003) HCN channels are expressed differentially in retinal bipolar cells and concentrated at synaptic terminals. Eur J Neurosci 17:2084–2096. 10.1046/j.1460-9568.2003.02634.x [DOI] [PubMed] [Google Scholar]
- 78.Narayan DS, Wood JP, Chidlow G, Casson RJ (2016) A review of the mechanisms of cone degeneration in retinitis pigmentosa. Acta Ophthalmol 94:748–754. 10.1111/aos.13141 [DOI] [PubMed] [Google Scholar]
- 79.Nikonov SS, Daniele LL, Zhu X, Craft CM, Swaroop A, Pugh EN Jr (2005) Photoreceptors of Nrl −/− mice coexpress functional S- and M-cone opsins having distinct inactivation mechanisms. J Gen Physiol 125:287–304. 10.1085/jgp.200409208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nikonov SS, Kholodenko R, Lem J, Pugh EN Jr (2006) Physiological features of the S- and M-cone photoreceptors of wild-type mice from single-cell recordings. J Gen Physiol 127:359–374. 10.1085/jgp.200609490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nunn BJ, Schnapf JL, Baylor DA (1984) Spectral sensitivity of single cones in the retina of Macaca fascicularis. Nature 309:264–266. 10.1038/309264a0 [DOI] [PubMed] [Google Scholar]
- 82.Nymark S, Frederiksen R, Woodruff ML, Cornwall MC, Fain GL (2012) Bleaching of mouse rods: microspectrophotometry and suction-electrode recording. J Physiol 590:2353–2364. 10.1113/jphysiol.2012.228627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Öhman P (1971) The photoreceptor outer segments of the river lamprey (Lampreta fluviatilis). An electron- fluorescence- and light microscopic study. Acta Zoologica 52:287–297. No doi. [Google Scholar]
- 84.Öhman P (1976) Fine structure of photoreceptors and associated neurons in the retina of Lampetra fluviatilis (Cyclostomi). Vision Res 16:659–662. 10.1016/0042-6989(76)90014-6 [DOI] [PubMed] [Google Scholar]
- 85.Okawa H, Sampath AP, Laughlin SB, Fain GL (2008) ATP consumption by mammalian rod photoreceptors in darkness and in light. Curr Biol 18:1917–1921. 10.1016/j.cub.2008.10.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ortin-Martinez A, Nadal-Nicolas FM, Jimenez-Lopez M, Alburquerque-Bejar JJ, Nieto-Lopez L, Garcia-Ayuso D, Villegas-Perez MP, Vidal-Sanz M, Agudo-Barriuso M (2014) Number and distribution of mouse retinal cone photoreceptors: differences between an albino (Swiss) and a pigmented (C57/BL6) strain. PLoS ONE 9:e102392. 10.1371/journal.pone.0102392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Østerberg G (1935) Topography of the layer of rods and cones in the human retina. Acta Opthalmologica (Copenhagen) Supplement 6:1–103 [Google Scholar]
- 88.Pahlberg J, Frederiksen R, Pollock GE, Miyagishima KJ, Sampath AP, Cornwall MC (2017) Voltage-sensitive conductances increase the sensitivity of rod photoresponses following pigment bleaching. J Physiol 595:3459–3469. 10.1113/JP273398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pandey S, Blanks JC, Spee C, Jiang M, Fong HK (1994) Cytoplasmic retinal localization of an evolutionary homolog of the visual pigments. Exp Eye Res 58:605–613. 10.1006/exer.1994.1055 [DOI] [PubMed] [Google Scholar]
- 90.Perry RJ, McNaughton PA (1991) Response properties of cones from the retina of the tiger salamander. J Physiol (Lond) 433:561–587. 10.1113/jphysiol.1991.sp018444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Porter ML, Blasic JR, Bok MJ, Cameron EG, Pringle T, Cronin TW, Robinson PR (2012) Shedding new light on opsin evolution. Proc Biol Sci 279:3–14. 10.1098/rspb.2011.1819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Power M, Das S, Schütze K, Marigo V, Ekström P, Paquet-Durand F (2020) Cellular mechanisms of hereditary photo-receptor degeneration – focus on cGMP. Prog Retin Eye Res 74:100772. 10.1016/j.preteyeres.2019.07.005 [DOI] [PubMed] [Google Scholar]
- 93.Pugh EN Jr, Lamb TD (1993) Amplification and kinetics of the activation steps in phototransduction. Biochim Biophys Acta 1141:111–149. 10.1016/0005-2728(93)90038-h [DOI] [PubMed] [Google Scholar]
- 94.Punzo C, Kornacker K, Cepko CL (2009) Stimulation of the insulin/mTOR pathway delays cone death in a mouse model of retinitis pigmentosa. Nat Neurosci 12:44–52. 10.1038/nn.2234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rebrik TI, Botchkina I, Arshavsky VY, Craft CM, Korenbrot JI (2012) CNG-modulin: a novel Ca-dependent modulator of ligand sensitivity in cone photoreceptor cGMP-gated ion channels. J Neurosci 32:3142–3153. 10.1523/JNEUROSCI.5518-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Reingruber J, Holcman D (2008) The dynamics of phosphodiesterase activation in rods and cones. Biophys J 94:1954–1970. 10.1529/biophysj.107.116202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Reingruber J, Ingram NT, Griffis C, Fain GL (2020) A kinetic analysis of mouse rod and cone photoreceptor responses. J Physiol. 10.1113/JP279524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Reingruber J, Pahlberg J, Woodruff ML, Sampath AP, Fain GL, Holcman D (2013) Detection of single photons by toad and mouse rods. Proc Natl Acad Sci U S A 110:19378–19383. 10.1073/pnas.1314030110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rieke F, Baylor D (1996) Molecular origin of continuous dark noise in rod photoreceptors. Biophys J 71:2553–2572. 10.1016/S0006-3495(96)79448-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sahel JA, Leveillard T, Picaud S, Dalkara D, Marazova K, Safran A, Paques M, Duebel J, Roska B, Mohand-Said S (2013) Functional rescue of cone photoreceptors in retinitis pigmentosa. Graefes Arch Clin Exp Ophthalmol 251:1669–1677. 10.1007/s00417-013-2314-7 [DOI] [PubMed] [Google Scholar]
- 101.Sakurai K, Chen J, Kefalov VJ (2011) Role of guanylyl cyclase modulation in mouse cone phototransduction. J Neurosci 31:7991–8000. 10.1523/JNEUROSCI.6650-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sakurai K, Chen J, Khani SC, Kefalov VJ (2015) Regulation of mammalian cone phototransduction by recoverin and rhodopsin kinase. J Biol Chem 290:9239–9250. 10.1074/jbc.M115.639591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sakurai K, Young JE, Kefalov VJ, Khani SC (2011) Variation in rhodopsin kinase expression alters the dim flash response shut off and the light adaptation in rod photoreceptors. Invest Ophthalmol Vis Sci 52:6793–6800. 10.1167/iovs.11-7158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sampath AP, Strissel KJ, Elias R, Arshavsky VY, McGinnis JF, Chen J, Kawamura S, Rieke F, Hurley JB (2005) Recoverin improves rod-mediated vision by enhancing signal transmission in the mouse retina. Neuron 46:413–420. 10.1016/j.neuron.2005.04.006 [DOI] [PubMed] [Google Scholar]
- 105.Schneeweis DM, Schnapf JL (1999) The photovoltage of macaque cone photoreceptors: adaptation, noise, and kinetics. J Neurosci 19:1203–1216. 10.1523/JNEUROSCI.19-04-01203.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Schön C, Asteriti S, Koch S, Sothilingam V, Garcia Garrido M, Tanimoto N, Herms J, Seeliger MW, Cangiano L, Biel M, Michalakis S (2016) Loss of HCN1 enhances disease progression in mouse models of CNG channel-linked retinitis pigmentosa and achromatopsia. Hum Mol Genet 25:1165–1175. 10.1093/hmg/ddv639 [DOI] [PubMed] [Google Scholar]
- 107.Seeliger MW, Brombas A, Weiler R, Humphries P, Knop G, Tanimoto N, Muller F (2011) Modulation of rod photoreceptor output by HCN1 channels is essential for regular mesopic cone vision. Nat Commun 2:532. 10.1038/ncomms1540 [DOI] [PubMed] [Google Scholar]
- 108.Sterling P, Laughlin S (2015) Principles of neural design. MIT Press, Cambridge [Google Scholar]
- 109.Taylor WR, Morgans C (1998) Localization and properties of voltage-gated calcium channels in cone photoreceptors of Tupaia belangeri. Vis Neurosci 15:541–552. 10.1017/s0952523898153142 [DOI] [PubMed] [Google Scholar]
- 110.Van Hook MJ, Nawy S, Thoreson WB (2019) Voltage- and calcium-gated ion channels of neurons in the vertebrate retina. Prog Retin Eye Res. 10.1016/j.preteyeres.2019.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Venkatesh A, Ma S, Le YZ, Hall MN, Ruegg MA, Punzo C (2015) Activated mTORC1 promotes long-term cone survival in retinitis pigmentosa mice. J Clin Invest 125:1446–1458. 10.1172/JCI79766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang JS, Kefalov VJ (2009) An alternative pathway mediates the mouse and human cone visual cycle. Curr Biol 19:1665–1669. 10.1016/j.cub.2009.07.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wang JS, Kefalov VJ (2011) The cone-specific visual cycle. Prog Retin Eye Res 30:115–128. 10.1016/j.preteyeres.2010.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang W, Lee SJ, Scott PA, Lu X, Emery D, Liu Y, Ezashi T, Roberts MR, Ross JW, Kaplan HJ, Dean DC (2016) Two-step reactivation of dormant cones in retinitis pigmentosa. Cell Rep 15:372–385. 10.1016/j.celrep.2016.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wong F, Kwok SY (2016) The survival of cone photoreceptors in retinitis pigmentosa. JAMA Ophthalmol 134:249–250. 10.1001/jamaophthalmol.2015.5490 [DOI] [PubMed] [Google Scholar]
- 116.Woodruff ML, Lem J, Fain GL (2004) Early receptor current of wild-type and transducin knockout mice: photosensitivity and light-induced Ca2+ release. J Physiol 557:821–828. 10.1113/jphysiol.2004.064014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Xu JW, Slaughter MM (2005) Large-conductance calcium-activated potassium channels facilitate transmitter release in salamander rod synapse. J Neurosci 25:7660–7668. 10.1523/JNEUROSCI.1572-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yagi T, MacLeish PR (1994) Ionic conductances of monkey solitary cone inner segments. J Neurophysiol 71:656–665. 10.1152/jn.1994.71.2.656 [DOI] [PubMed] [Google Scholar]
- 119.Zhang J, Choi EH, Tworak A, Salom D, Leinonen H, Sander CL, Hoang TV, Handa JT, Blackshaw S, Palczewska G, Kiser PD, Palczewski K (2019) Photic generation of 11-cis-retinal in bovine retinal pigment epithelium. J Biol Chem 294:19137–19154. 10.1074/jbc.RA119.011169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhang X, Wensel TG, Kraft TW (2003) GTPase regulators and photoresponses in cones of the eastern chipmunk. J Neurosci 23:1287–1297. 10.1523/JNEUROSCI.23-04-01287.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]


