Abstract
Cardoon (C. cardunculus var. altilis DC) is commonly cultivated in the Mediterranean area to produce stalks that are consumed once cooked. Before cooking, stalks are usually subjected to blanching, which means they are exposed to darkness for a few weeks. The present work analyzed the effect of field blanching carried out for 40 days in different ways (burying the stalks under soil or covering them with plastic sheet) on the total phenolic content (TPC), phenolic profile, cynaropicrin content (a bitter compound), and antioxidant activity (AA) of two cardoon cultivars. The nutraceutical quality of blanched cardoons was also investigated following boiling. The phenolic profile revealed a higher number of compounds in blanched stalks than in raw ones. The cynaropicrin content decreased in both cultivars after blanching, indicating a sensitivity to dark conditions and the effectiveness of blanching method in reducing its bitterness. The data presented contribute to improving the knowledge about the effect of blanching and boiling on the quality of cardoon stalks.
Keywords: antioxidant activity, blanching, Cynara cardunculus, cynaropicrin, heat treatment, phenolics
1. Introduction
Cynara cardunculus L. is a species belonging to the Asteraceae family and includes artichoke (C. cardunculus var. scolymus (L.) Fiori), cultivated cardoon (C. cardunculus var. altilis DC), and wild cardoon (C. cardunculus var. sylvestris (Lamk) Fiori) [1]. These species have a perennial biological cycle with a period of vegetative growth occurring from autumn to spring, the period with the most abundant rain in the Mediterranean area, while the ripening of seeds occurs in the summer [2].
Cultivated cardoon is popular in the Mediterranean region, mainly in Spain, France, Italy, Greece, and southern Portugal [3,4]. This crop is used to produce fleshy stalks that are consumed as a traditional dish once boiled, fried, sautéed, or baked [4,5]. Cardoon stalks are usually subjected to blanching, which exposes stalks to dark conditions for about a few weeks in the field. This process is performed in several different ways according to the labor requirement and cost-effectiveness. Blanching is carried out to reduce the bitterness and to accentuate the flavor and tenderness of cardoon stalks [5]. Indeed, this vegetable is characterized by a strong bitter taste due to the high content of cynaropicrin (56–83%), followed by geosheimin (2–18%) and cuanaratriol (4–22%) [6]. Cynaropicrin is a sesquiterpene with marked antioxidant and medicinal properties such as antiproliferative, antifeedant, and anti-inflammatory properties [7,8]. Moreover, the antioxidant activity (AA) of cultivated cardoon stalks is also due to the high content of flavonoids and phenolic acids. However, few works have reported the phenolic profile of cardoon stalks, underlining their richness in caffeoylquinic acid derivatives and the glycosidic forms of apigenin and luteolin [3,4,5,9].
Moreover, to the best of our knowledge, only Pinelli et al. [5] has studied the effect of blanching on the content of bioactive compounds in cardoon stalks, revealing a decrease in polyphenols after blanching, without any specification of the blanching method [5]. Likewise, only two recent articles analyzed the effect of cooking and, consequently, of heat treatment, on the bioactive compounds and AA of cardoon stalks [4,10]. In addition, Huarte et al. [4] revealed that a blanching treatment (98 °C for 30 s) increased the content of caffeoylquinic acids in cardoon stalks without any significant effect on the AA when determined using a 2,2′-diphenyl-1-picrylhydrazyl radical (DPPH) assay. In comparison, the frying treatment decreased the AA, notwithstanding an increase in the flavonoid content [4]. Similarly, Juániz et al. [10] found an increase in the concentration of phenolic compounds in cardoon stalks once fried or grilled.
The aim of the present work was the assessment of the effect of different blanching methods in the field and boiling on the phenolic and cynaropicrin content, the phenolic profile, and the AA of cardoon stalks grown in a field in the Mediterranean area. For the first time, both blanching and heat treatment on cardoon were analyzed in the same paper, presenting some ideas of the nutraceutical quality of the product utilized by the consumer.
2. Materials and Methods
2.1. Plant Material
Two experiments were carried out with different cultivars of C. cardunculus var. altilis, Pieno Inerme Lucchese (PIL) and Plain Blanc Inerme (PBI), cultivated in a field in Lucca (Italy) and in Peccioli (Pisa, Italy), respectively. These were either subjected or not subjected to stalk blanching, which was performed using two different methods: stalks were either buried under loose soil (PIL) or wrapped with a 0.5 mm-thick white and black plastic sheet made of low-density polyethylene (LDPE; Jolly Plastic, Larciano, PT) (PBI). In both experiments, blanching was applied for 40 days before the harvest, which took place on 29 January 2021.
Three samples, each consisting of blanched or unblanched stalks collected from separate plants, were collected from both fields, and three sub-samples were immediately frozen in liquid nitrogen and stored at –80 °C until the biochemical analysis. Another three subsamples were frozen in liquid nitrogen and then lyophilized and stored at –80 °C for the chromatographic identification and quantification of cynaropicrin and phenolic compounds. Three sub-samples were also taken and dried in a ventilated oven (Memmert GmbH Co., KG Universal Oven UN30, Schwabach, Germany) at 105 °C until the sub-sample reached a constant weight for the determination of the moisture content.
2.2. Boiling Treatment
Blanched stalks were washed with distilled water and cut into rectangular homogeneous pieces (2 cm × 2 cm approx.), manually mixed and divided into three portions (150 g of each one), and then boiled in 600 mL of distilled water for 30 min. Boiled stalks were sampled for laboratory determinations as previously described.
2.3. Total Phenolic Content (TPC) Assay
The TPC was determined following the method in Dewanto et al. [11]. The oxidation of phenolic compounds along with the reduction in the metals present in the solution of phosphomolybdate–phosphotungstate of the Folin–Ciocalteu reagent and the consequent color blue were measured at 760 nm with a spectrophotometer (Ultrospec 2100 Pro, GE Healthcare Ltd., Chalfont, Buckinghamshire, UK). The TPC was expressed as mg gallic acid equivalents (GAE) per g dry weight (DW).
2.4. Cynaropicrin and Phenolic Compound Extraction
The extraction of cynaropicrin and phenolic compounds was performed according to Zhou et al. [12] using methanol at a ratio of 1:20 (w/v). An amount of lyophilized cardoon stalk was homogenized in liquid nitrogen and in a 75% (v/v) aqueous solution of methanol mixed with 0.1% formic acid. Samples were sonicated twice in a water bath sonicator (VWR, Leuven, Belgium) for 30 min and then agitated for 20 min. The supernatant was filtered at 0.2 μm with a polytetrafluoroethylene filter before UHPLC analysis.
2.5. Cynaropicrin and Phenolic Characterization
Phenolic characterization was performed by an Agilent 1200 Liquid Chromatography system (Agilent Technologies, Palo Alto, CA, USA) equipped with a standard autosampler as reported by Negro et al. [13]. The UHPLC column was an Agilent Extended C18 (1.8 µm, 2.1 × 50 mm). Separation was carried out at 40 °C with a gradient elution program at a flowrate of 0.5 mL min−1. The mobile phases consisted of water plus 0.1% formic acid (A) and acetonitrile (B). The following multistep linear gradient was applied: 0–2 min, 1% B; 13 min, 25% B; 19 min, 40% B; 21 min, 90%B.
The UHPLC system was coupled to an Agilent 6320 TOF mass spectrometer equipped with a dual electrospray ionization (ESI) interface (Agilent Technologies, Palo Alto, CA, USA), and a calibration solution containing the internal reference mass was introduced. The system operated on a negative-ion mode to obtain the phenolic characterization and on a positive-ion mode to obtain cynaropicrin characterization and quantification. Accurate measurements of the mass corresponding to each total ionic current (TIC) peak were obtained with a pump (Agilent G1310B) with a low flow (20 µL min−1) of a calibration solution containing internal reference masses at m/z 112.9856, 301.9981, 601.9790, and 1033.9881 for negative ions and 121.050873, 149.023320, 322.048121, and 922.009798 for positive ions, and using a dual nebulizer ESI source. A cynaropicrin standard was solubilized in a solution of methanol–water at 75:25 (v/v) and diluted with water–formic acid at 99.9:0.1 (v/v) and at a final concentration of 0.5–10 μg mL−1.
2.6. Antioxidant Activity (AA) Assay
The AA was measured as reported by Brand-Williams et al. [14]. An amount (10 μL) of phenolic extract was mixed with 990 μL of a methanolic solution of 3.12 × 10−1 M DPPH (w/v) and left alone for 30 min for the DPPH radical to react with the antioxidant compounds present in the samples. The change in color (from violet to pink) of DPPH due to the reduction in this reagent by the antioxidant compounds in the extract solution was measured at 515 nm against a blank solution (with no extracts) with the same spectrophotometer used for the determination of the TPC. The AA was expressed as mg Trolox equivalents (TE) per g DW.
2.7. Statistical Analysis
Data from each experiment were subjected to a one-way ANOVA, and the means (± standard error) were separated using Tukey’s test. All statistical analyses were conducted using GraphPad (GraphPad, La Jolla, CA, USA).
3. Results and Discussion
3.1. Effect of Blanching
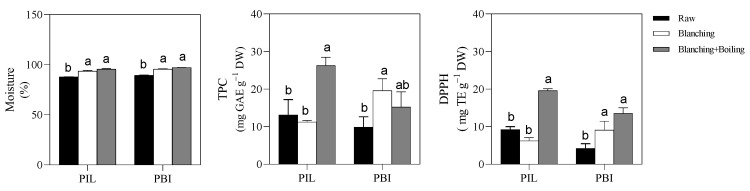
Figure 1 shows the moisture content, the TPC, and AA of unblanched (control), blanched, and boiled and blanched stalks of PIL and PBI.
Figure 1.
Moisture, total phenolic content (TPC), and antioxidant activity (AA) of stalks of two cardoon cultivars (Cynara cardunculus var. altilis DC cv. Pieno Inerme Lucchese, PIL, and Cynara cardunculus var. altilis DC cv. Plain Blanc Inerme; PBI) grown in the field. Raw, blanched, and blanched+boiled cardoon stalks are represented by closed, open, and gray bars, respectively. For each cultivar separately, means (±SE; n = 3) indicated by different letters differ significantly (p ≤ 0.05). GAE: gallic acid equivalents; TE: Trolox equivalents; DW: dry weight.
In both cultivars, blanching significantly increased the moisture content of stalks. In PBI, the TPC and AA of blanched stalks were greater compared with the controls, whilst in PIL, no significant differences were found in the TPC and AA. The TPC values of both PIL and PBI yielded very similar results to those reported by Huarte et al. [4] for raw C. cardunculus var. altilis stalks, and the AA values found by these authors yielded similar results to the PIL results (36 μmol TE g−1 DW) but were higher than those reported for raw PBI stalks (17 μmol TE g−1 DW). Indeed, Huarte et al. [4] reported a TPC value of 8.28 ± 0.03 mg GAE g−1 DW and an AA value of 39.5 ± 4.72 μmol TE g−1 DW. The AA values of PBI accorded with the results of Huarte et al. [4] once subjected to the blanching process (about 36 μmol TE g−1 DW), likely because the Spanish authors analyzed cardoon stalks that were purchased in a local market, which had surely already been subjected to the blanching process. We could hypothesize that the cardoon stalks utilized in Huarte et al.’s [4] experiment were subjected to blanching with a plastic sheet, given the similarity of the TPC and AA results with our results for blanched PBI stalks. Although no information about different blanching processes is present in the literature, this increase in the nutraceutical quality of PBI after the blanching process could likely be due to the increase in temperature and relative humidity caused by the plastic wrapping [15,16], as suggested by the results of the UHPLC/DAD/TOF analysis reported in Table 1. Indeed, blanched PBI stalks yielded the richest content in different phenolic compounds; in these stalks, 20 phenolic compounds were identified, 11 of which were not found in the controls.
Table 1.
Identification of phenolic compounds, [M-H]-, by UHPLC/DAD/TOF analysis of stalks of cardoon (Cynara cardunculus var. altilis DC cv. Pieno Inerme Lucchese; PIL and Cynara cardunculus var. altilis DC cv. Plain Blanc Inerme; PBI) cultivated in the field. The symbol (+) indicates the presence of that phenolic compound in the sample. MW: molecular weight.
| No | Name | Formula [M-H]- |
Exp. MW [M-H]- |
Calculated MW [M-H]- |
∆, ppm | Score | Unbl. PIL |
Bl. PIL |
Boil. PIL | Unbl. PBI |
Bl. PBI |
Boil. PBI | Refs |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Quinic acid I | C7H12O6 | 191.0575 | 191.0561 | −7.15 | 87.28 | + | + | + | + | + | [17] | |
| 2 | Unknown | C13H17O12 | 365.0737 | 365.0725 | −3.15 | 73.66 | + | - | |||||
| 3 | Poly-galacturonic acid methyl ester | C20H27O18 | 555.1198 | 555.1203 | 0.96 | 83.66 | + | [18] | |||||
| 4 | Poly-galacturonic acid | C27H37O24 | 745.1655 | 745.1680 | 3.44 | 74.5 | + | [18] | |||||
| 5 | Dihydroxybenzene | C6H6O2 | 109.0291 | 109.295 | 3.47 | 86.14 | + | [4] | |||||
| 6 | Quinic acid II | C7H11O6 | 191.0574 | 191.0561 | −6.52 | 88.32 | + | + | + | [17] | |||
| 7 | Caffeic acid I * | C9H8O4 | 179.0361 | 179.0350 | −6.09 | 87.24 | + | + | + | [4,17] | |||
| 8 | Chlorogenic acid * | C16H18O9 | 353.0907 | 353.0878 | −8.34 | 78.95 | + | + | [4] | ||||
| 9 | Unknown | C8H8O2 | 135.0463 | 135.0452 | −8.61 | 85.41 | - | ||||||
| 10 | Unknown | C8H7O2 | 135.458 | 135.452 | −4.56 | 93.19 | + | - | |||||
| 11 | Caffeic acid II | C9H8O4 | 179.0373 | 179.0350 | −12.9 | 70.1 | + | [4,17] | |||||
| 12 | Caffeoyl-di(dihydro)caffeoylquinic acid methyl ester | C35H35O15 | 695.1865 | 695.1888 | 3.22 | 85.2 | + | [19] | |||||
| 13 | Caffeoyl-Hexoside | C15H17O9 | 341.0876 | 341.0878 | 0.71 | 86.67 | + | + | [4] | ||||
| 14 | Chlorogenic acid hexoside | C22H27O14 | 515.1441 | 515.1465 | 4.71 | 81.76 | + | [20,21] | |||||
| 15 | Monocaffeoylquinic acid I | C16H18O9 | 353.0899 | 353.0878 | −6.04 | 81.99 | + | + | [20] | ||||
| 16 | Monocaffeoylquinic acid dimer I | C16H18O9 | 707.1885 | 707.1829 | −7.92 | 70.32 | + | + | + | + | [20] | ||
| 17 | Quinic acid III | C7H12O6 | 191.0541 | 191.0561 | −10.31 | 65.63 | + | + | + | [17] | |||
| 18 | Caffeic acid III | C9H8O4 | 179.0362 | 179.0350 | −6.95 | 85.63 | + | + | [17,20] | ||||
| 19 | Unknown | C18H25O11 | 417.1445 | 417.1402 | −10.31 | 56.95 | + | - | |||||
| 20 | Monocaffeoylquinic acid I | C16H18O9 | 353.0895 | 353.0878 | −4.68 | 81.04 | + | [4] | |||||
| 21 | Quinic acid IV | C7H12O6 | 191.0578 | 191.0561 | −8.99 | 80.7 | + | + | + | [4,17] | |||
| 22 | Monocaffeoylquinic acid dimer I | C16H18O9 | 707.1885 | 707.1829 | −7.92 | 70.32 | + | + | [20] | ||||
| 23 | Unknown | C19H30O8 | 385.1883 | 385.1868. | −3.82 | 84.23 | + | - | |||||
| 24 | Shikimic acid * | C7H10O5 | 173.0467 | 173.0455 | −6.74 | 87.81 | + | + | - | ||||
| 25 | p-Coumaroylquinic acid | C22H31O11 | 471.1911 | 471.1872 | −8.39 | 70.15 | + | + | + | + | [19] | ||
| 26 | Cynaroside | C21H19O11 | 447.1034 | 447.0992 | −9.51 | 81.23 | + | + | [4,17,21] | ||||
| 27 | Monocaffeoylquinic acid III | C16H18O9 | 353.0895 | 353.0878 | −4.68 | 81.04 | + | + | [20] | ||||
| 28 | Monocaffeoylquinic acid IV | C16H18O9 | 353.0904 | 353.0878 | −7.42 | 66.29 | + | [20] | |||||
| 29 | Monocaffeoylquinic acid V | C16H18O9 | 353.0899 | 353.0878 | −5.79 | 72.14 | + | [20] | |||||
| 30 | Dicaffeoylquinic acid hexoside | C31H34O17 | 677.1726 | 677.1723 | −0.46 | 80.3 | + | [20] | |||||
| 31 | Luteolin-7-O-glucoside * | C21H19O11 | 447.0943 | 447.0933 | −2.38 | 78.41 | + | + | + | [4,17,21] | |||
| 32 | Dicaffeoylquinic acid I | C25H24O12 | 515.1223 | 515.1195 | −5.37 | 79.06 | + | + | + | + | + | [4] | |
| 33 | Dicaffeoylquinic acid III | C18H28O17 | 515.1225 | 515.1254 | 5.54 | 80.21 | + | + | [20] | ||||
| 34 | Unknown | C25H26O13 | 533.1317 | 533.1301 | −2.97 | 75.99 | + | - | |||||
| 35 | Myricetin galloylhexoside I | C29H28O16 | 631.1309 | 631.1305 | −0.77 | 79.27 | + | + | [20] | ||||
| 36 | Myricetin galloylhexoside II | C29H28O16 | 631.1309 | 631.1305 | −0.77 | 79.27 | + | [20] | |||||
| 37 | Myricetin galloylhexoside III | C29H28O16 | 631.1308 | 631.1305 | −0.54 | 79.09 | + | [20] | |||||
| 38 | Dicaffeoylquinic acid II | C18H28O17 | 515.1225 | 515.1254 | 5.54 | 80.21 | + | + | + | + | [4] | ||
| 39 | Caffeoylquinic acid II | C18H28O17 | 515.1225 | 515.1195 | −5.73 | 79.89 | + | + | [20] | ||||
| 40 | Quinic acid V | C7H12O6 | 191.0575 | 191.0561 | −7.15 | 88.03 | + | + | + | + | [17] | ||
| 41 | Monocaffeoylquinic acid II | C16H18O9 | 353.0904 | 353.0878 | −7.42 | 66.29 | + | + | + | [4] | |||
| 42 | Apigenin glucuronide | C21H17O11 | 445.0771 | 445.0776 | 1.2 | 79.34 | + | + | [9] | ||||
| 43 | Caffeoylquinic acid I | C18H28O17 | 515.1225 | 515.1195 | −5.73 | 79.89 | + | + | + | + | [4] | ||
| 44 | Apigeninacetyl glucoside | C23H22O12 | 489.1032 | 489.1038 | 1.31 | 77.72 | + | + | [9] | ||||
| 45 | 4-O-Caffeoyl-5-O-[3-methoxy-3-(3,4-dihydroxyphenyl)-propionyl] quinic acid methyl ester I | C27H29O13 | 561.1613 | 561.1614 | 0.07 | 82.21 | + | [22] | |||||
| 46 | 4-O-Caffeoyl-5-O-[3-methoxy-3-(3,4-dihydroxyphenyl) -propionyl] quinic acid methyl ester II |
C27H29O13 | 561.1613 | 561.1614 | 0.05 | 82.45 | + | [22] | |||||
| 47 | Dicaffeoylquinic acid IV | C18H28O17 | 515.1209 | 515.1195 | −2.74 | 76.53 | + | [20] | |||||
| 48 | Caffeoyl-dihydrocaffeoyl-sinapoyl quinic acid II | C36H35O16 | 723.1901 | 723.1931 | 4.08 | 74.8 | + | [19] | |||||
| 49 | Caffeoyl-dihydrocaffeoyl-sinapoyl quinic acid II | C36H35O16 | 723.1901 | 723.1931 | 4.08 | 74.8 | + | [19] | |||||
| 50 | Apigenin * | C15H9O5 | 269.0459 | 269.0455 | −1.45 | 87.78 | + | [21] | |||||
| 51 | Kaempferol * | C15H9O6 | 285.0417 | 285.0405 | −4.37 | 88.32 | + | + | - | ||||
| 52 | Unknown | C35H55O13 | 683.3629 | 683.3648 | 2.79 | 73.81 | + | + | - | ||||
| 53 | Trihydroxy-octradecenoic acid | C18H33O5 | 329.2324 | 329.2333 | 2.91 | 85.72 | + | [19] | |||||
| 54 | Dihydroxy-octradecenoic acid | C18H31O4 | 311.2220 | 311.2228 | 2.47 | 74.33 | + | + | [21] | ||||
| 55 | Unknown | C18H29O4 | 313.2422 | 313.2384 | −11.88 | 55.84 | + | - | |||||
| 56 | Unknown | C29H45O7 | 564.3321 | 564.3093 | −0.87 | 74.37 | + | - | |||||
| 57 | Unknown | C15H22O3 | 249.1522 | 249.1496 | −10.25 | 67.16 | + | - |
* Confirmed by authentic chemical standards.
Twenty-nine phenolic compounds belonging to the phenolic acid, catechol, and flavonoid classes were found in C. carduculus var. altilis samples, and twenty-five of these were also identified. Only the quinic acid isomer was found in all samples (independently of blanching). Five phenolic compounds were found in the control PIL stalks and six in the blanched stalks, while only one compound was found in both unblanched and blanched samples. The presence of flavonoids and caffeoylquinic acids in C. cardunculus was confirmed in other species or/and varieties of the genus Cynara by other authors [4,5,9].
Dihydroxybenzene, quinic acid II, p-cumaroylquinic acid, and kaempferol in PIL samples, and monocaffeoylquinic acid II and p-cumaroylquinic acid in PBI samples, were no longer detectable after blanching (Table 1). These findings agree with those of Pinelli et al. [5], who reported the disappearance of some phenolic compounds in cardoon stalks after blanching.
In PIL, the content of the main phenolics was under the detection limit or higher in control samples than in blanched samples (Table 2). At the same time, new phenolic compounds were found in blanched samples compared with the compounds identified in controls.
Table 2.
Content of some phenolic compounds in stalks of cardoon (Cynara cardunculus var. altilis DC cv. Pieno Inerme Lucchese; PIL and Cynara cardunculus var. altilis DC cv. Plain Blanc Inerme; PBI) cultivated in the field. Mean values, mg g−1 DW, (±SE; n = 3) followed by different letters differ significantly (p ≤ 0.05).
| Cardoon Cultivar | Treatment | 1 Monocaffeoylquinic Acid | Caffeic Acid | 1 Monocaffeoylquinic Acid Dimer | 1 Dicaffeoylquinic Acid | Luteolin Glucoside |
|---|---|---|---|---|---|---|
| Pieno Inerme Lucchese (PIL) | Control | <LOD | <LOD | <LOD | <LOD | <LOD |
| Blanching | <LOD | <LOD | <LOD | <LOD | <LOD | |
| Blanching+Boiling | 11.54 ± 0.85 | 3.12 ± 0.36 | 43.54 ± 1.08 | 29.67 ± 1.15 | 0.05 ± 0.09 | |
| Plain Blanc Inerme (PBI) | Control | <LOD | 0.09 ± 0.05 | 42.20 ± 0.76 a | 13.30 ± 0.61 a | 0.035 ± 0.06 |
| Blanching | <LOD | <LOD | 19.12 ± 0.48 b | 5.01 ± 0.50 b | <LOD | |
| Blanching+Boiling | 0.77 ± 0.31 | 0.14 ± 0.08 | 3.26 ± 0.86 c | 11.91 ± 1.39 a | 0.02 ± 0.08 | |
| Significance (ANOVA) | ns | *** | *** | ns |
<LOD: under the limit of detection; 1 determined with caffeic acid equivalent; significance level: *** p ≤ 0.001, ns: non-significant.
We hypothesized that the influence of the different environmental conditions underground in PIL and, especially, under a plastic sheet in PBI, could accelerate the plant’s defense responses, resulting in an accumulation of new phenolic compounds. This hypothesis was confirmed by Dawidowicz and Typek [23], although they analyzed a different matrix (coffee beans). Indeed, they observed the degradation of chlorogenic acid isomers by heat treatment that caused their transformation to quinic acid and chlorogenic acid lactones, changing the ratio between chlorogenic acid isomers and mono- and di-caffeoylquinic acid isomers.
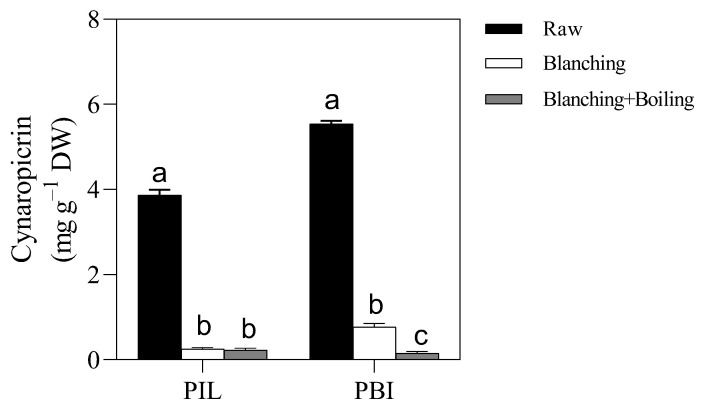
Moreover, we found a lower cynaropicrin content in blanched stalks than in controls in both PLI and PBI (Figure 2). Scavo et al. [24] found a lower cynaropicrin content in wild cardoon stalks grown under shade in winter (about 25 mg L−1) as compared with cardoon stalks grown under full light in winter (about 45 mg L−1). These authors hypothesized that this was due to a reduction in the terpenoid biosynthesis. Taking into account the darkness induced by the blanching, this explanation could also explain our results.
Figure 2.
Content of cynaropicrin in stalks of cardoon (Cynara cardunculus var. altilis DC cv. Pieno Inerme Lucchese; PIL and Cynara cardunculus var. altilis DC cv. Plain Blanc Inerme; PBI). Raw, blanched, and blanched+boiled cardoon stalks are represented by closed, open, and gray bars, respectively. For each cultivar separately, means (±SE; n = 3) indicated by different letters differ significantly (p ≤ 0.05).
3.2. Effect of Boiling
The boiling treatment did not affect the TPC of the stalks of either PBI, whilst it increased the TPC of PIL stalks and the AA of PIL and PBI stalks compared with the controls (Figure 1).
The pattern of the AA in boiled PIL and PBI stalks agreed with the findings of Juániz et al. [10]. These authors reported a high increase in AA when cardoon was subjected to 150 °C for 10 min and then fried in oil at 110 °C for 5 min (90 μmol TE g−1 DW) compared to raw samples (about 30 μmol TE g−1 DW), which showed a small but non-significant increase in AA when cardoon was fried in sunflower oil (about 35 μmol TE g−1 DW), and a decrease when they were fried in olive oil (about 20 μmol TE g−1 DW). These authors ascribed the high increase in the AA of the cardoon stalks subjected to temperatures between 110 and 150 °C to the destruction of cell walls and subcellular compartments, which favored the release of bioactive compounds from internal compartments of plant cells at the cooking mean, independently of the plant matrix under investigation [10]. Conversely, Martini et al. [25] suggested that the food matrix played an important role in the retention of phenolic compounds after heat treatment. Nevertheless, no studies on the use of boiling for cardoon stalks have been reported in the literature.
The UHPLC/DAD/TOF analysis showed the presence of a higher number of phenolic compounds in boiled stalks when compared to the raw ones (Table 1). Forty-three phenolic compounds belonging mainly to the class of phenolic acids were identified, whilst seven compounds remained unknown. The most representative compounds were the quinic and caffeoylquinic acids and their derivatives. Among the latter, the monocaffeoylquinic acid, dicaffeoylquinic acid, and monocaffeoylquinic acid dimer were the most abundant in boiled PIL stalks, whilst the dicaffeoylquinic acid was the most representative compound in PBI stalks (Table 2). Other authors showed the retention of caffeic acid derivatives after heat treatment in cardoon stalks but also in different plant matrices, such as eggplant, confirming the heat resistance of this class of compounds [10,25].
The blanching and boiling of cardoon stalks are necessary to reduce their bitterness, which is due to the content of some sesquiterpenes, especially cynaropicrin, and to increase their tenderness.
No significant effect of the boiling treatment was found on the cynaropicrin content of blanched, raw PIL stalks, whilst a lower cynaropicrin content was observed in the boiled PBI stalks, likely due to a higher starting content of cynaropicrin in PBI stalks than in PIL stalks (Figure 2).
4. Conclusions
The present work analyzed the effect of field blanching and boiling on some biochemical attributes of cardoon stalks grown in the Mediterranean area. As expected, blanching led to a decrease in the cynaropicrin content while inducing the synthesis of some phenolic compounds that were not detected in unblanched stalks. Similarly, the boiling treatment for blanched stalks reduced their cynaropicrin content and increased the antioxidant capacity and the diversity of phenolic acids as compared to non-boiled blanched stalks. This study was a first step towards the assessment of the nutraceutical quality of processed cardoon stalks, as there was little information in the literature about this vegetable. However, more investigations are needed for a deeper understanding of the effect of field blanching and boiling on the biochemical characteristics of cardoon stalks.
Author Contributions
Conceptualization, investigation, data curation, and writing—original draft preparation, C.C.; supervision, validation, visualization, and writing—review and editing, L.D.B.; supervision, validation, writing—review and editing, L.G.; methodology, investigation, data curation, and writing—original draft preparation, C.N., supervision, validation, visualization, and writing—review and editing, A.P., funding acquisition, project administration, resources, and writing—review and editing, L.I. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by the “AMARCORT- Old fruit and vegetable germoplasm of Tuscany” project (Rural Development Policy 2014–2020-Measure 16.1: Support to the Operational Groups of Agricultural European Innovation Partnership (EIP-AGRI)).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rottenberg A., Zohary D. The wild ancestry of the cultivated artichoke. Genet. Resour. Crop Evol. 1996;43:53–58. doi: 10.1007/BF00126940. [DOI] [Google Scholar]
- 2.Francaviglia R., Bruno A., Falcucci M., Farina R., Renzi G., Russo D.E., Sepe L., Neri U. Yields and quality of Cynara cardunculus L. wild and cultivated cardoon genotypes. A case study from a marginal land in Central Italy. Eur. J. Agron. 2016;72:10–19. doi: 10.1016/j.eja.2015.09.014. [DOI] [Google Scholar]
- 3.Ferioli F., D’Antuono L.F. Phenolic compounds in local Italian types of cultivated cardoon (Cynara cardunculus L. var. altilis DC) stalks and artichoke (Cynara cardunculus L. var. scolymus L.) edible sprouts. J. Food Compos. Anal. 2021;106:104342. doi: 10.1016/j.jfca.2021.104342. [DOI] [Google Scholar]
- 4.Huarte E., Juániz I., Cid C., de Peña M.P. Impact of blanching and frying heating rate/time on the antioxidant capacity and (poly) phenols of cardoon stalks (Cynara cardunculus L. var. altilis DC) Int. J. Gastron. Food Sci. 2021;26:100415. doi: 10.1016/j.ijgfs.2021.100415. [DOI] [Google Scholar]
- 5.Pinelli P., Agostini F., Comino C., Lanteri S., Portis E., Romani A. Simultaneous quantification of caffeoyl esters and flavonoids in wild and cultivated cardoon leaves. Food Chem. 2007;105:1695–1701. doi: 10.1016/j.foodchem.2007.05.014. [DOI] [Google Scholar]
- 6.Belitz H.D., Wieser H. Bitter compounds: Occurrence and structure-activity relationships. Food Rev. Int. 1985;1:271–354. doi: 10.1080/87559128509540773. [DOI] [Google Scholar]
- 7.Ramos P.A., Guerra Â.R., Guerreiro O., Santos S.A., Oliveira H., Freire C.S., Silvestre A.J.D., Duarte M.F. Antiproliferative effects of Cynara cardunculus L. var. altilis (DC) lipophilic extracts. Int. J. Mol. Sci. 2017;18:63. doi: 10.3390/ijms18010063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brás T., Neves L.A., Crespo J.G., Duarte M.F. Effect of extraction methodologies and solvent selection upon cynaropicrin extraction from Cynara cardunculus leaves. Sep. Purif. Technol. 2020;236:116283. doi: 10.1016/j.seppur.2019.116283. [DOI] [Google Scholar]
- 9.Pandino G., Lombardo S., Mauromicale G., Williamson G. Profile of polyphenols and phenolic acids in bracts and receptacles of globe artichoke (Cynara cardunculus var. scolymus) germplasm. J. Food Compos. Anal. 2011;24:148–153. doi: 10.1016/j.jfca.2010.04.010. [DOI] [Google Scholar]
- 10.Juániz I., Ludwig I.A., Huarte E., Pereira-Caro G., Moreno-Rojas J.M., Cid C., De Peña M.P. Influence of heat treatment on antioxidant capacity and (poly) phenolic compounds of selected vegetables. Food Chem. 2016;197:466–473. doi: 10.1016/j.foodchem.2015.10.139. [DOI] [PubMed] [Google Scholar]
- 11.Dewanto V., Wu X., Adom K.K., Liu R.H. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J. Agric. Food Chem. 2002;50:3010–3014. doi: 10.1021/jf0115589. [DOI] [PubMed] [Google Scholar]
- 12.Zhou X., Chan S.W., Tseng H.L., Deng Y., Hoi P.M., Choi P.S., Or P.M.Y., Yang J., Lam F.F.Y., Lee S.M.Y., et al. Danshensu is the major marker for the antioxidant and vasorelaxation effects of Danshen (Salvia miltiorrhiza) water-extracts produced by different heat water-extractions. Phytomedicine. 2012;19:1263–1269. doi: 10.1016/j.phymed.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 13.Negro C., Aprile A., Luvisi A., De Bellis L., Miceli A. Antioxidant Activity and Polyphenols Characterization of Four Monovarietal Grape Pomaces from Salento (Apulia, Italy) Antioxidants. 2021;10:1406. doi: 10.3390/antiox10091406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brand-Williams W., Cuvelier M.E., Berset C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995;28:25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- 15.Laddomada B., Blanco A., Mita G., D’Amico L., Singh R.P., Ammar K., Crossa J., Guzmán C. Drought and heat stress impacts on phenolic acids accumulation in durum wheat cultivars. Foods. 2021;10:2142. doi: 10.3390/foods10092142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rivero R.M., Ruiz J.M., Garcıa P.C., Lopez-Lefebre L.R., Sánchez E., Romero L. Resistance to cold and heat stress: Accumulation of phenolic compounds in tomato and watermelon plants. Plant Sci. 2001;160:315–321. doi: 10.1016/S0168-9452(00)00395-2. [DOI] [PubMed] [Google Scholar]
- 17.Garcìa L.M., Ceccanti C., Negro C., De Bellis L., Incrocci L., Pardossi A., Guidi L. Effect of drying methods on phenolic compounds and antioxidant activity of Urtica dioica L. leaves. Horticulturae. 2021;7:10. doi: 10.3390/horticulturae7010010. [DOI] [Google Scholar]
- 18.Sun J., Deering R.W., Peng Z., Najia L., Khoo C., Cohen P., Seeram N., Rowley D. Pectic oligosaccharides from cranberry prevent quiescence and persistence in the uropathogenic Escherichia coli CFT073. Sci. Rep. 2019;9:1959. doi: 10.1038/s41598-019-56005-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Costa F.N., Borges R.M., Leitão G.G., Jerz G. Preparative mass-spectrometry profiling of minor concentrated metabolites in Salicornia gaudichaudiana Moq by high-speed countercurrent chromatography and off-line electrospray mass-spectrometry injection. J. Sep. Sci. 2019;42:1528–1541. doi: 10.1002/jssc.201801195. [DOI] [PubMed] [Google Scholar]
- 20.Abu-Reidah I.M., Arráez-Román D., Segura-Carretero A., Fernández-Gutiérrez A. Extensive characterisation of bioactive phenolic constituents from globe artichoke (Cynara scolymus L.) by HPLC–DAD-ESI-QTOF-MS. Food Chem. 2013;141:2269–2277. doi: 10.1016/j.foodchem.2013.04.066. [DOI] [PubMed] [Google Scholar]
- 21.Farag M.A., El-Ahmady S.H., Elian F.S., Wessjohann L.A. Metabolomics driven analysis of artichoke leaf and its commercial products via UHPLC–q-TOF-MS and chemometrics. Phytochemistry. 2013;95:177–187. doi: 10.1016/j.phytochem.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 22.Ge L., Wan H., Tang S., Chen H., Li J., Zhang K., Zhou B., Fei J., Wu S., Zeng X. Novel caffeoylquinic acid derivatives from Lonicera japonica Thunb. flower buds exert pronounced anti-HBV activities. RSC Adv. 2018;8:35374–35385. doi: 10.1039/C8RA07549B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dawidowicz A.L., Typek R. Transformation of chlorogenic acids during the coffee beans roasting process. Eur. Food Res. Technol. 2017;243:379–390. doi: 10.1007/s00217-016-2751-8. [DOI] [Google Scholar]
- 24.Scavo A., Rial C., Molinillo J.M., Varela R.M., Mauromicale G., Macías F.A. Effect of shading on the sesquiterpene lactone content and phytotoxicity of cultivated cardoon leaf extracts. J. Agric. Food Chem. 2020;68:11946–11953. doi: 10.1021/acs.jafc.0c03527. [DOI] [PubMed] [Google Scholar]
- 25.Martini S., Conte A., Cattivelli A., Tagliazucchi D. Domestic cooking methods affect the stability and bioaccessibility of dark purple eggplant (Solanum melongena) phenolic compounds. Food Chem. 2021;341:128298. doi: 10.1016/j.foodchem.2020.128298. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable.