Abstract
Despite recovering from the acute phase of coronavirus disease (COVID-19), many patients report continuing symptoms that most commonly include fatigue, cough, neurologic problems, hair loss, headache, and musculoskeletal pain, a condition termed long-COVID syndrome. Neither its etiopathogenesis, nor its clinical presentation or risk factors are fully understood. Therefore, the purpose of this study was to retrospectively evaluate the most common symptoms of long-COVID among patients from the STOP COVID registry of the PoLoCOV study, and to search for risk factors for development of the syndrome. The registry includes patients who presented to the medical center for persistent clinical symptoms following severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. The analysis included data from initial presentation and at three-month follow-up. Of the 2218 patients, 1569 (70.7%) reported having at least one symptom classified as long-COVID syndrome three months after recovery from the initial SARS-CoV-2 infection. The most common symptoms included chronic fatigue (35.6%\), cough (23.0%), and a set of neurological symptoms referred to as brain fog (12.1%). Risk factors for developing long-COVID syndrome included female gender (odds ratio [OR]: 1.48, 95% confidence intervals [CI] [1.19–1.84]), severe COVID-19 (OR: 1.56, CI: 1.00–2.42), dyspnea (OR: 1.31, CI: 1.02–1.69), and chest pain (OR: 1.48, CI: 1.14–1.92). Long-COVID syndrome represents a significant clinical and social problem. The most common clinical manifestations are chronic fatigue, cough, and brain fog. Given the still-limited knowledge of long-COVID syndrome, further research and observation are needed to better understand the mechanisms and risk factors of the disease.
Keywords: long-COVID, brain fog, COVID-19, post-COVID-19 syndrome, fatigue
1. Introduction
The coronavirus disease (COVID-19) pandemic has been ongoing for over two years, and during this time 531,542,973 cases have been reported worldwide, of which 6,310,705 have been fatal and 502,198,989 have recovered [1]. As it has continued, new reports have emerged regarding both severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and the diagnostic and therapeutic recommendations of COVID-19 [2,3]. In addition, as a result of enormous human and financial investment, COVID-19 vaccines were developed in a matter of months and are now the most rapidly invented and deployed formulations [4]. Moreover, in late 2021 and early 2022, the first effective oral antiviral drugs targeting SARS-CoV-2 became available [5,6]. All of this, along with subsequent mutations of the virus, has led to a gradually decreasing mortality rate due to COVID-19 [5,6,7].
Despite this, many patients present with persistent clinical symptoms after the disease has resolved, and this can affect up to 30% of patients [8]. The reported symptoms were very diverse and mainly included: fatigue, hair loss, neurological-cognitive disorders (concentration, memory, and speech disorders), referred to as brain fog, cough or even dyspnea. Patients also complained of chest pains, palpitations, joint pains, headaches, olfactory and taste disorders, and gastrointestinal problems. Psychiatric complications, including anxiety, depression, and even post-traumatic stress disorder have also been recorded. The severity and duration of these symptoms are not uniform. In some patients, symptoms resolve spontaneously, while some patients report persistent symptoms for up to 12 months after acute COVID-19 [9,10,11,12,13].
The literature on persistent symptoms after COVID-19 is still scarce. Moreover, the term used to describe this disease is still not clearly established. The literature contains many terms such as long-COVID, post-COVID-19 syndrome, and post-COVID-19 condition. According to the World Health Organization (WHO) definition, it is a condition in which symptoms persist for at least two months after the disease and cannot be explained by another cause [14]. In contrast, the National Institute for Health and Care Excellence definition of long-COVID includes new or ongoing symptoms 4 to 12 weeks after the acute phase of the illness is diagnosed. Additionally, this definition distinguishes between “ongoing COVID,” which is between 4 and 12 weeks, and “post-COVID-19 syndrome” for symptoms lasting more than 12 weeks [15].
The etiopathogenesis of the disease is also not fully understood. Currently, it is estimated that the cause is complex and consists of persistent inflammation, autoimmunity, and survival of the virus in the body, which may account for the wide variety of symptoms. An important role has also been attributed to the possibility of vascular endothelial damage during infection [16,17].
Interestingly, risk factors have not been clearly established, which potentially include the number of symptoms during COVID-19, old age, female gender, or the presence of chronic conditions such as asthma. However, these factors are not homogeneous for all symptoms classified as long-COVID syndrome. Moreover, most of the available data concern hospitalized patients [11,13,18,19,20]. Taken together, this indicates how much is still unknown and how important it is to delve further into this problem, especially among outpatients.
The aim of this study was to evaluate the most common symptoms of long-COVID among patients of the STOP COVID registry, and to look for risk factors for long-COVID syndrome, including chronic fatigue and brain fog.
2. Materials and Methods
2.1. Methodology
This was a retrospective study based on analysis of data from 2218 patients obtained from the STOP COVID registry (PoLoCOV (ClinicalTrials.gov identifier—NCT05018052)). The STOP COVID registry includes only patients from Poland. This registry contains medical information of patients presenting to health centers for persistent clinical symptoms after COVID-19 and subsequent follow-up visits at 3 and 12 months. The patient registry includes both inpatients and outpatients from 1 September 2020 to 30 September 2021. Data obtained from visit 0 and up to 3 months were used for the purpose of this review. The program is still ongoing, and future publication of the entire follow-up results, including the visit after 12 months, is planned.
Inclusion criteria were consistent throughout the study and included:
-
(a)
diagnosis of COVID-19 (initially by reverse transcription polymerase chain reaction test only, then also by antigen tests—according to the changes to the rules for COVID-19 diagnosis in Poland);
-
(b)
age ≥ 18 years;
-
(c)
written informed consent to participate in the study.
Exclusions included only:
-
(a)
age < 18 years
or
-
(b)
No consent to participate in the study.
Before participating in the study, patients were informed about the study objectives and methodology, after which they gave informed consent to participate in the study.
At individual visits, patients completed health questionnaires regarding persistent symptoms up to three months after completion of isolation, and were physically examined. At the first visit, patients provided their sociodemographic data, details of chronic conditions, clinical symptoms present during COVID-19, sites of isolation, and duration of clinical symptoms. The most common symptoms of COVID-19 were assessed, including, fever, subfebrile state, chills, cough, headache, myalgia, weakness, dyspnea (subjective), olfactory/taste/hearing disturbances, and chest pain. Subsequently, the patient subjectively rated the feeling of severity of COVID-19 course on a four-point scale. Based on all of the above data, the severity of COVID-19 course was rated on a four-point scale.
The criteria for each point included:
- 0 points (asymptomatic/mild course)
-
○asymptomatic course
-
○paroxysmal symptoms lasting no more than 3 days
-
○
- 1 point (mild course)
-
○home isolation
-
○subjective assessment by the patient of a score of ‘1’ on a scale of 0–3
-
○duration of symptoms less than 7 days
-
○
- 2 points (moderate)
-
○subjective assessment by the patient of a score of ‘2’ or ‘3’ on a scale of 03
-
○duration of symptoms lasting between 7 and 14 days
-
○the presence of dyspnea and fever ≥ 38 °C
-
○
3 points (severe course)
one of the following criteria: hospitalization with a diagnosis of pneumonia, respiratory failure, intensive care unit (ICU) treatment, respiratory support, or thromboembolic complications during hospitalization/home isolation
or
in the case of home isolation: symptoms persisting for more than 14 days, temperature above 38 °C, dyspnea, oxygen saturation <94% persisting for at least 3 days, or patient’s subjective assessment of “3” on a scale from 0–3 points
Anthropometric measurements, including height and weight, were also taken, and obese patients with a body mass index ≥30 were singled out. Influenza vaccination was also evaluated, and after the introduction of COVID-19 vaccination in Poland, vaccination status during the course of infection was included. A person was considered vaccinated if he or she had completed the baseline schedule, regardless of the type of formulation used.
The following long-COVID symptoms were assessed: cough, dyspnea, chronic fatigue, hair loss, olfactory disturbances, headache, and osteoarticular pain. In addition, the presence of impaired memory and concentration, difficulty in speaking, inability to focus, and lowered mental acuity were assessed, all of which were defined as brain fog [10].
According to the WHO definition, long-COVID was diagnosed with the presence of at least one symptom in a period of 12 weeks from the end of the illness. The condition may include either new symptoms that appeared during COVID-19, or those that persisted and/or worsened during this period [14].
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Bioethics Committee of the Wroclaw Medical University, Poland (approval number 232/2022).
2.2. Statistical Analysis
The variables analyzed were qualitative, quantitative, and ordinal. Basic descriptive statistics were used to describe the study group and the prevalence of long-COVID.
Logistic regression analysis was used to assess risk factors for the development of long-COVID syndrome. Univariate models were constructed where the dependent variable was the prevalence of long-COVID syndrome and the explanatory variables were, respectively, sociodemographic variables (age, gender), influenza and COVID-19 vaccination status, burden of chronic diseases, and the course of COVID-19 (place of isolation, symptoms during the disease, number and duration of symptoms, and severity of the disease course). Identical univariate models were also constructed for explanatory variables including chronic fatigue and brain fog.
Subsequently, multivariate logistic regression models with the best subset selection were built for the explanatory variables (presence of long-COVID syndrome/brain fog/chronic fatigue) using the Akaike criterion. In these models, the explanatory variables were statistically significant variables obtained by univariate analysis—separately for each explanatory variable.
Results were presented as odds ratio (OR) with 95% confidence intervals (Cl) and p-value, where p < 0.05 was considered significant. Analysis was performed using Statistica 13.0 software from StatSoft (TIBCO Software, Palo Alto, CA, USA).
3. Results
3.1. Characteristics of the Study Group
A total of 2218 patients were included in the analysis, of which 1410 (63.5%) were female, with a mean age of 53.8 ± 13.5 years. At least one chronic condition was recorded in 1569 (70.7%) patients, the most common being hypertension (37.2%), obesity (31.2%), hyperlipidemia (19.8%), and diabetes (10.4%). A detailed description of the study group is shown in Table 1.
Table 1.
Characteristics of the study group.
| Variable | The Whole Group N = 2218 (%) | Female | Male | p | |
|---|---|---|---|---|---|
| Sex | Female | 1410 (63.5) | --- | --- | --- |
| Male | 808 (36.5) | --- | --- | --- | |
| Age (M ± SD) | 53.8 ± 13.5 | 53.84 ± 13.3 | 53.72 ± 13.7 | 0.923 | |
| Weight [kg] (M ± SD) | 79.6 ± 17.2 | 73.3 ± 15.1 | 90.69 ± 14.9 | <0.001 | |
| Height [cm] (M ± SD) | 169.1 ± 9.1 | 164.5 ± 6.2 | 177.1 ± 7.8 | <0.001 | |
| BMI (M ± SD) | 21.3 ± 9.1 | 20.4 ± 9.1 | 22.7 ± 8.9 | <0.001 | |
| Chronic diseases | 1569 (70.7) | 981 (69.6) | 588 (72.7) | 0.123 | |
| Chronic diseases |
Hypertension | 826 (37.2) | 476 (33.8) | 350 (43.3) | <0.001 |
| BMI > 30 | 692 (31.2) | 388 (28.0) | 304 (38.3) | <0.001 | |
| Diabetes | 230 (10.4) | 125 (8.9) | 105 (13.0) | 0.002 | |
| Coronary artery disease | 134 (6.0) | 66 (4.7) | 68 (8.4) | 0.005 | |
| DCM | 17 (0.8) | 5 (0.4) | 12 (1.5) | 0.007 | |
| Heart failure | 26 (1.2) | 12 (0.9) | 14 (1.7) | 0.100 | |
| Venous thromboembolism | 24 (1.1) | 16 (1.1) | 8 (1.0) | 0.917 | |
| Hyperlipidemia | 440 (19.8) | 269 (19.1) | 171 (21.2) | 0.258 | |
| Asthma | 217 (9.8) | 155 (10.9) | 62 (7.7) | 0.014 | |
| COPD | 51 (2.3) | 30 (2.1) | 21 (2.6) | 0.571 | |
| Thyroid disease | 374 (18.9) | 328 (23.3) | 46 (5.7) | <0.001 | |
| Place of Isolation |
Home | 1887 (85.0) | 1229 (86.9) | 658 (79.5) | |
| Hospital—without pneumonia | 33 (1.5) | 17 (1.2) | 16 (1.9) | 0.204 | |
| Hospital—with pneumonia | 279 (12.6) | 140 (9.9) | 139 (17.2) | <0.001 | |
| Hospital—ICU | 19 (0.9) | 11 (0.8) | 8 (1.0) | 0.781 | |
| Flu vaccinations in the previous season | 166 (7.5) | 110 (7.8) | 56 (6.9) | 0.505 | |
| Vaccination against COVID-19 (n = 697) | 494 (70.9) | 341 (72.9) | 153 (66.81) | 0.118 | |
COPD—chronic obstructive pulmonary disease. ICU—intensive care unit M–mean. SD—standard deviation. DCM—dilated cardiomyopathy
3.2. Long-COVID Disease Picture and Its Risk Factors
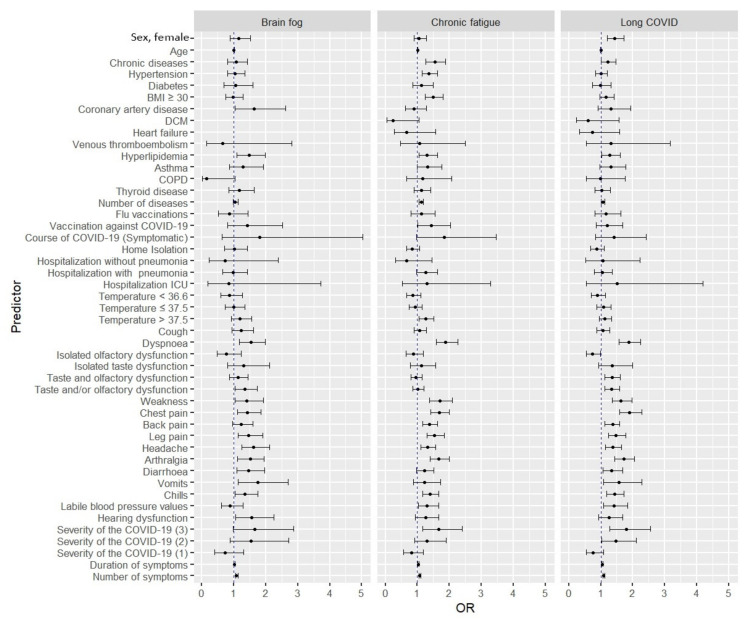
The prevalence of long-COVID-19 syndrome in the study group, detailing the most common clinical manifestations shown in Table 2. In univariate analysis, the risk of developing long-COVID syndrome was shown to be associated with the presence of at least one chronic condition, with the risk increasing with the number of conditions. An analogous relationship was observed for persistent chronic fatigue.
Table 2.
Prevalence of long-COVID-19 syndrome in the study group, detailing the most common clinical manifestations.
| Symptom | N (%) |
|---|---|
| Long-COVID | 1444 (65.1) |
| Chronic fatigue | 789 (35.6) |
| Headache | 661 (29.8) |
| Cought | 510 (23.0) |
| Brain fog | 268 (12.1) |
| Dyspnoea | 137 (6.2) |
| Hair loss | 104 (4.7) |
| Olfactory dysfunction | 98 (4.4) |
| Osteoarticular pain | 91 (4.1) |
Additionally, it was shown that as the severity of COVID-19 increased, the risk of developing long-COVID syndrome increased. In symptom-specific analysis, dyspnea, chest pain, and significant weakness were associated with a higher risk of long-COVID syndrome and persistent chronic fatigue. In both cases, these risks increased with respect to both the number of symptoms manifested and their duration. These factors also increased the risk of developing brain fog (Figure 1). A detailed summary is shown in Table 3.
Figure 1.
Univariate analysis of risk factors for developing long-COVID syndrome, chronic fatigue, and brain fog.
Table 3.
Univariate analysis determining the effect of sociodemographic variables, chronic conditions, influenza and COVID-19 vaccination status, and COVID-19 course on the risk of developing long-COVID syndrome, as well as brain fog symptoms and chronic fatigue.
| Variable | Long-COVID | Brain Fog | Chronic Fatigue | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Percentage (%) | OR (95%Cl) | p | Percentage (%) | OR (95%Cl) |
p | Percentage (%) | OR (95%Cl) |
p | ||
| Sex | Female | 961 (66.6) | 1.44 (1.20–1.72) | <0.001 | 178 (12.6) | 1.15 (0.88–1.51) | 0.301 | 509 (36.1) | 1.06 (0.89–1.28) | 0.493 |
| Male | 483 (59.8) | Ref. | Ref. | 90 (11.1) | Ref. | Ref. | 280 (34.7) | Ref. | Ref. | |
| Age | ----- | 1.00 (0.99–1.01) | 0.261 | ---- | 1.00 (0.99–1.01) | 0.582 | ---- | 1.02 (1.00–1.02) | <0.001 | |
| Chronic diseases | Yes | 1043 (66.5) | 1.23 (1.01–1.48) | 0.035 | 193 (12.3) | 1.07 (0.81–1.43) | 0.624 | 603 (38.4) | 1.55 (1.27–1.89) | <0.001 |
| No | 401 (61.8) | Ref. | Ref. | 75 (11.6) | Ref. | Ref. | 186 (28.7) | Ref. | Ref. | |
| Hypertension | Yes | 538 (61.8) | 1.00 (0.84–1.21) | 0.982 | 102 (12.4) | 1.04 (0.80–1.35) | 0.767 | 331 (40.1) | 1.36 (1.14–1.63) | <0.001 |
| No | 906 (65.1) | Ref. | Ref. | 166 (11.9) | Ref. | Ref. | 458 (32.9) | Ref. | Ref. | |
| Diabetes | Yes | 149 (64.8) | 0.98 (0.74–1.31) | 0.914 | 29 (12.6) | 1.05 (0.69–1.59) | 0.796 | 88 (38.3) | 1.13 (0.86–1.50) | 0.368 |
| No | 1295 (65.1) | Ref. | Ref. | 239 (12.0) | Ref. | Ref. | 701 (35.3) | Ref. | Ref. | |
| Obesity (BMI ≥ 30) | Yes | 468 (67.6) | 1.16 (0.96–1.41) | 0.122 | 82 (11.9) | 0.97 (0.74–1.28) | 0.856 | 291 (42.1) | 1.49 (1.24–1.80) | <0.001 |
| No | 954 (64.2) | Ref. | Ref. | 180 (12.1) | Ref. | Ref. | 486 (32.7) | Ref. | Ref. | |
| Coronary artery disease | Yes | 95 (70.9) | 1.32 (0.91–1.94) | 0.148 | 24 (17.9) | 1.64 (1.03–2.61) | 0.034 | 51 (38.1) | 0.89 (0.62–1.28) | 0.535 |
| No | 1349 (64.7) | Ref. | Ref. | 244 (11.7) | Ref. | Ref. | 738 (35.4) | Ref. | Ref. | |
| DCM | Yes | 9 (52.9) | 0.61 (0.23–1.57) | 0.296 | 0 (0.0) | ---- | --- | 2 (11.8) | 0.24 (0.05–1.05) | 0.058 |
| No | 1435 (65.2) | Ref. | Ref. | 268 (12.2) | Ref. | Ref. | 787 (35.8) | Ref. | Ref. | |
| Heart failure | Yes | 15 (57.7) | 0.73 (0.33–1.59) | 0 (0.0) | ---- | --- | 7 (26.9) | 0.66 (0.27–1.58) | 0.357 | |
| No | 1429 (65.2) | Ref. | Ref. | 268 (12.2) | Ref. | Ref. | 782 (35.7) | Ref. | Ref. | |
| Venous thromboembolism | Yes | 17 (70.8) | 1.31 (0.54–3.17) | 0.555 | 2 (8.3) | 0.66 (0.15–2.81) | 0.574 | 9 (37.5) | 1.08 (0.48–2.50) | 0.842 |
| No | 1427 (65.0) | Ref. | Ref. | 266 (12.1) | Ref. | Ref. | 780 (35.6) | Ref. | Ref. | |
| Hyperlipidemia | Yes | 306 (69.6) | 1.28 (1.03–1.61) | 0.029 | 69 (15.7) | 1.48 (1.10–1.98) | 0.010 | 179 (40.7) | 1.31 (1.06–1.63) | 0.012 |
| No | 1138 (64.0) | Ref. | Ref. | 199 (11.2) | Ref. | Ref. | 610 (34.3) | Ref. | Ref. | |
| Asthma | Yes | 154 (70.5) | 1.31 (0.97–1.79) | 0.079 | 32 (14.8) | 1.29 (0.87–1.93) | 0.206 | 90 (41.5) | 1.32 (0.99–1.76) | 0.056 |
| No | 1291 (64.5) | Ref. | Ref. | 236 (11.8) | Ref. | Ref. | 699 (34.9) | Ref. | Ref. | |
| COPD | Yes | 33 (64.7) | 0.98 (0.55–1.76) | 0.951 | 1 (2.0) | 0.14 (0.01–1.03) | 0.054 | 20 (39.2) | 1.17 (0.66–2.07) | 0.582 |
| No | 1411 (65.1) | Ref. | Ref. | 267 (12.3) | Ref. | Ref. | 769 (35.5) | Ref. | Ref. | |
| Thyroid disease | Yes | 245 (65.5) | 1.02 (0.81–1.29) | 0.857 | 51 (13.6) | 1.18 (0.85–1.64) | 0.312 | 141 (37.7) | 1.12 (0.89–1.41) | 0.346 |
| No | 1199 (65.0) | Ref. | Ref. | 217 (11.8) | Ref. | Ref. | 648 (35.1) | Ref. | Ref. | |
| Number of diseases | --- | 1.06 (1.00–1.12) | 0.049 | ---- | 1.04 (0.97–1.13 | 0.246 | ---- | 1.12 (1.06–1.18) | <0.001 | |
| Flu vaccinations | Yes | 113 (68.1) | 1.16 (0.82–1.62) | 0.404 | 18 (10.8) | 0.87 (0.52–1.45) | 0.610 | 63 (37.9) | 1.12 (0.81–1.55) | 0.505 |
| No | 1331 (64.9) | Ref. | Ref. | 250 (12.2) | Ref. | Ref. | 726 (35.4) | Ref. | Ref. | |
| Vaccination against COVID-19 |
Yes | 326 (66.0) | 1.21 (0.86–1.69) | 0.268 | 57 (11.5) | 1.42 (0.81–2.52) | 0.219 | 197 (39.9) | 1.44 (1.02–2.04) | 0.038 |
| No | 125 (61.6) | Ref. | Ref. | 17 (8.4) | Ref. | Ref. | 64 (31.5) | Ref. | Ref. | |
| Course of COVID-19 | Symptomatic | 1412 (65.1) | 1.41 (0.83–2.42) | 0.207 | 264 (12.2) | 1.81 (0.64–5.04) | 0.257 | 776 (35.9) | 1.85 (0.98–3.46) | 0.054 |
| Asymptomatic | 32 (57.1) | Ref. | Ref. | 4 (7.1) | Ref. | Ref. | 13 (23.2) | Ref. | Ref. | |
| Home Isolation | Yes | 1202 (64.6) | 0.87 (0.68–1.11) | 0.280 | 225 (12.1) | 1.01 (0.71–1.43) | 0.963 | 650 (35.0) | 0.84 (0.67–1.07) | 0.160 |
| No | 242 (67.6) | Ref. | Ref. | 43 (12.0) | Ref. | Ref. | 139 (38.8) | Ref. | Ref. | |
| Hospitalization without pneumonia | Yes | 22 (66.7) | 1.07 (0.52–2.22) | 0.849 | 3 (9.1) | 0.72 (0.22–2.39) | 0.596 | 9 (27.3) | 0.67 (0.31–1.46) | 0.319 |
| No | 1422 (65.1) | Ref. | Ref. | 265 (12.1) | Ref. | Ref. | 780 (35.7) | Ref. | Ref. | |
| Hospitalization with pneumonia | Yes | 184 (66.0) | 1.04 (0.80–1.36) | 0.751 | 33 (11.8) | 0.97 (0.66–1.43) | 0.889 | 113 (40.5) | 1.27 (0.98–1.64) | 0.066 |
| No | 1260 (65.0) | Ref. | Ref. | 235 (12.1) | Ref. | Ref. | 676 (34.9) | Ref. | Ref. | |
| Hospitalization ICU | Yes | 14 (73.7) | 1.51 (0.54–4.2) | 0.434 | 2 (10.5) | 0.85 (0.19–3.72) | 0.834 | 8 (42.12) | 1.31 (0.53–3.30) | 0.552 |
| No | 1430 (65.0) | Ref. | Ref. | 266 (12.1) | Ref. | Ref. | 781 (35.5) | Ref. | Ref. | |
| Temperature< 36.6 | Yes | 198 (63.1) | 0.90 (0.70–1.15) | 0.418 | 34 (10.8) | 0.87 (0.59–1.27) | 0.461 | 102 (32.5) | 0.85 (0.66–1.10) | 0.217 |
| No | 1246 (65.4) | Ref. | Ref. | 234 (12.4) | Ref. | Ref. | 687 (36.1) | Ref. | Ref. | |
| Temperature≤ 37.5 | Yes | 332 (66.4) | 1.08 (0.87–1.32) | 0.486 | 60 (12.0) | 0.99 (0.73–1.34) | 0.948 | 171 (34.2) | 0.93(0.75–1.14) | 0.466 |
| No | 1112 (64.7) | Ref. | Ref. | 208 (12.1) | Ref. | Ref. | 618 (35.9) | Ref. | Ref. | |
| Temperature> 37.5 | Yes | 782 (66.4) | 1.13 (0.95–1.34) | 0.178 | 153 (13.0) | 1.20 (0.93–1.55) | 0.164 | 449 (38.1) | 1.27 (1.06–1.51) | 0.007 |
| No | 662 (63.7) | Ref. | Ref. | 115 (11.1) | Ref. | Ref. | 340 (32.7) | Ref. | Ref. | |
| Cough | Yes | 927 (65.6) | 1.06 (0.88–1.27) | 0.551 | 182 (12.9) | 1.23 (0.94–1.62) | 0.131 | 512 (36.2) | 1.08 (0.90–1.29) | 0.407 |
| No | 517 (64.3) | Ref. | Ref. | 86 (110.7) | Ref. | Ref. | 277 (34.5) | Ref. | Ref. | |
| Dyspnoea | Yes | 767 (74.4) | 1.87 (1.56–2.24) | <0.001 | 153 (14.5) | 1.53 (1.18–1.98) | 0.001 | 457 (43.2) | 1.89 (1.59–2.26) | <0.001 |
| No | 677 (58.4) | Ref. | Ref. | 115 (9.9) | Ref. | Ref. | 332 (28.7) | Ref. | Ref. | |
| Isolated olfactory dysfunction | Yes | 126 (58.6) | 0.74 (0.55–0.98) | 0.035 | 21 (9.8) | 0.77 (0.48–1.23) | 0.274 | 71 (33.0) | 0.88 (0.65–1.19) | 0.411 |
| No | 1318 (65.8) | Ref. | Ref. | 247 (12.3) | Ref. | Ref. | 718 (35.9) | Ref. | Ref. | |
| Isolated taste dysfunction | Yes | 100 (71.4) | 1.36 (0.94–1.99) | 0.105 | 21 (15.0) | 1.31 (0.81–2.12) | 0.275 | 53 (37.9) | 1.12 (0.78–1.58) | 0.559 |
| No | 1344 (64.7) | Ref. | Ref. | 247 (11.9) | Ref. | Ref. | 736 (35.4) | Ref. | Ref. | |
| Taste and olfactory dysfunction | Yes | 684 (68.8) | 1.35 (1.13–1.61) | <0.001 | 127 (12.8) | 1.13 (0.87–1.45) | 0.366 | 349 (35.1) | 0.96 (0.81–1.15) | 0.682 |
| No | 760 (62.1) | Ref. | Ref. | 141 (11.5) | Ref. | Ref. | 440 (36.0) | Ref. | Ref. | |
| Taste and/or olfactory dysfunction | Yes | 717 (68.6) | 1.34 (1.12–1.59) | 0.001 | 144 (13.8) | 1.35 (1.04–1.74) | 0.022 | 374 (35.8) | 1.02 (0.86–1.20) | 0.865 |
| No | 727 (62.0) | Ref. | Ref. | 124 (10.6) | Ref. | Ref. | 415 (35.4) | Ref. | Ref. | |
| Weakness | Yes | 1107 (68.2) | 1.63 (1.35–1.98) | <0.001 | 211 (13.0) | 1.41 (1.03–1.92) | 0.030 | 628 (38.7) | 1.70 (1.38–2.09) | <0.001 |
| No | 337 (56.7) | Ref. | Ref. | 57 (9.6) | Ref. | Ref. | 161 (27.1) | Ref. | Ref. | |
| Chest pain | Yes | 708 (73.1) | 1.90 (1.59–2.28) | <0.001 | 138 (14.3) | 1.43 (1.11–1.85) | 0.005 | 409 (42.3) | 1.68 (1.41–2.00) | <0.001 |
| No | 736 (58.9) | Ref. | Ref. | 130 (10.4) | Ref. | Ref. | 380 (30.4) | Ref. | Ref. | |
| Back pain | Yes | 795 (68.2) | 1.38 (1.13–1.59) | 0.001 | 153 (13.1) | 1.23 (0.95–1.60) | 0.111 | 455 (39.1) | 1.38 (1.16–1.64) | <0.001 |
| No | 649 (61.6) | Ref. | Ref. | 115 (10.9) | Ref. | Ref. | 334 (31.7) | Ref. | Ref. | |
| Leg pain | Yes | 670 (70.2) | 1.48 (1.24–1.78) | <0.001 | 139 (14.5) | 1.47 (1.14–1.90) | 0.003 | 394 (41.3) | 1.54 (1.30–1.84) | <0.001 |
| No | 774 (61.3) | Ref. | Ref. | 130 (10.3) | Ref. | Ref. | 395 (31.3) | Ref. | Ref. | |
| Headache | Yes | 837 (68.3) | 1.37 (1.15–1.64) | <0.001 | 175 (14.3) | 1.61 (1.24–2.11) | <0.001 | 471 (38.5) | 1.33 (1.11–1.58) | 0.002 |
| No | 607 (61.1) | Ref. | Ref. | 93 (9.4) | Ref. | Ref. | 318 (32.0) | Ref. | Ref. | |
| Arthralgia | Yes | 651 (72.3) | 1.72 (1.43–2.06) | <0.001 | 133 (14.8) | 1.52 (1.12–1.95) | 0.001 | 384 (42.6) | 1.67 (1.40–2.00) | <0.001 |
| No | 793 (60.2) | Ref. | Ref. | 135 (10.3) | Ref. | Ref. | 405 (30.8) | Ref. | Ref. | |
| Diarrhoea | Yes | 302 (70.2) | 1.33 (1.06–1.68) | 0.013 | 67 (15.6) | 1.46 (1.09–1.96) | 0.014 | 169 (39.3) | 1.22 (0.98–1.51) | 0.072 |
| No | 1142 (63.9) | Ref. | Ref. | 201 (11.2) | Ref. | Ref. | 620 (34.7) | Ref. | Ref. | |
| Vomits | Yes | 111 (74.0) | 1.57 (108–2.29) | 0.015 | 28 (18.7) | 1.75 (1.13–2.69) | 0.011 | 60 (40.0) | 1.22 (0.87–1.72) | 0.241 |
| No | 1333 (64.5) | Ref. | Ref. | 240 (11.6) | Ref. | Ref. | 729 (35.3) | Ref. | Ref. | |
| Chills | Yes | 559 (70.1) | 1.43 (1.18–1.72) | <0.001 | 113 (14.2) | 1.35 (1.04–1.75) | 0.024 | 323 (40.5) | 1.40 (1.17–1.67) | <0.001 |
| No | 885 (62.3) | Ref. | Ref. | 155 (10.9) | Ref. | Ref. | 466 (32.8) | Ref. | Ref. | |
| Labile blood pressure values | Yes | 234 (71.6) | 1.41 (1.09–1.83) | 0.008 | 36 (11.0) | 0.89 (0.61–1.28) | 0.519 | 134 (41.0) | 1.31 (1.03–1.67) | 0.027 |
| No | 1210 (64.0) | Ref. | Ref. | 232 (12.3) | Ref. | Ref. | 655 (34.6) | Ref. | Ref. | |
| Hearing dysfunction | Yes | 153 (69.6) | 1.25 (0.93–1.69) | 0.146 | 37 (16.8) | 1.55 (1.06–2.26) | 0.024 | 89 (40.5) | 1.26 (0.94–1.67) | 0.111 |
| No | 1291 (64.6) | Ref. | Ref. | 231 (11.6) | Ref. | Ref. | 700 (35.0) | Ref. | Ref. | |
| Severity of the COVID-19 | 3 | 567 (72.9) | 1.80 (1.27–2.55) | <0.001 | 117 (15.1) | 1.65 (0.97–2.87) | 0.075 | 333 (42.9) | 1.67 (1.17–2.40) | 0.004 |
| 2 | 416 (68.8) | 1.47 (1.03–2.10) | 0.011 | 86 (14.2) | 1.54 (0.88–2.71) | 0.131 | 224 (37.0) | 1.31 (0.91–1.90) | 0.146 | |
| 1 | 348 (53.5) | 0.76 (0.54–1.08) | <0.001 | 47 (7.2) | 0.72 (0.39–1.31) | 0.288 | 174 (26.7) | 0.82 (0.56–1.18) | 0.284 | |
| 0 | 99 (60.0) | Ref. | Ref. | 16 (9.7) | Ref. | Ref. | 51 (30.9) | Ref. | Ref. | |
| Duration of symptoms | ---- | 1.05(1.03–1.07) | <0.001 | ---- | 1.02 (1.00-1.04) | 0.037 | ---- | 1.03 (1.01–1.05) | <0.001 | |
| Number of symptoms | ---- | 1.09 (10.7–1.12) | <0.001 | ---- | 1.08 (1.05–1.13) | <0.001 | ---- | 1.08 (1.06–1.11) | <0.001 | |
OR—odds ratio; CI—confidence interval; Ref.—reference; COPD—chronic obstructive pulmonary disease. ICU—intensive care unit; DCM—dilated cardiomyopathy statistically; significant values are in bold with the significance level set at p < 0.05.
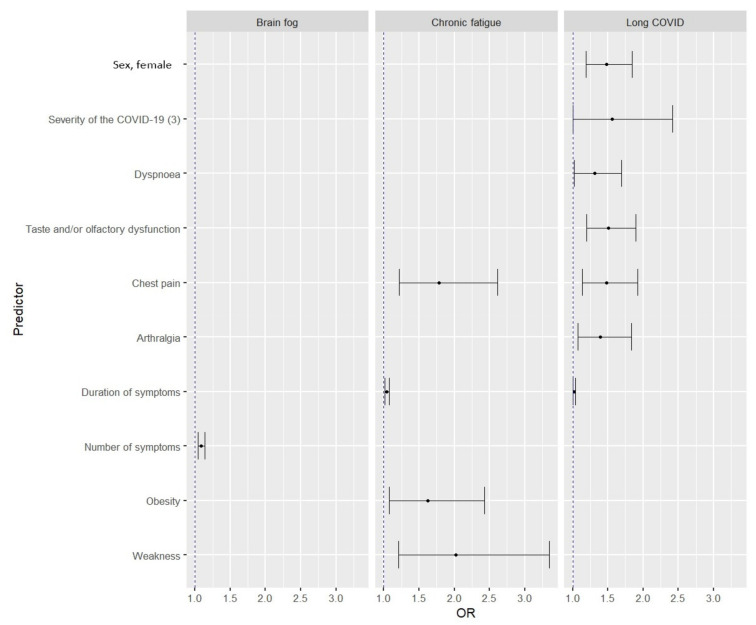
Multivariate models showed that female gender and severe COVID-19 predisposed to the development of long-COVID syndrome. Among clinical symptoms, dyspnea, chest pain, and joint pain were associated with a higher risk of long-COVID. For brain fog, only the number of symptoms with which the risk of the disease increased proved to be statistically significant. In contrast, chest pain, significant weakness, and a history of obesity appeared to be risk factors for developing chronic fatigue (Figure 2). A detailed summary of the multivariate analysis is shown in Table 4.
Figure 2.
Predictors of the risk of developing long-COVID, brain fog, and chronic fatigue in multivariate models.
Table 4.
Multivariate analysis based on a model incorporating statistically significant predictors from univariate analysis on the risk of developing long-COVID syndrome, brain fog, and chronic fatigue.
| Variable | Long-COVID | Brain Fog | Chronic Fatigue | ||||
|---|---|---|---|---|---|---|---|
| OR (95%Cl) | p | OR (95%Cl) |
p | OR (95%Cl) |
p | ||
| Sex, Female | 1.48 (1.19–1.84) | <0.001 | --- | --- | --- | --- | |
| Severity of the COVID-19 | 3 | 1.56 (1.00–2.42) | 0.045 | --- | --- | --- | --- |
| Dyspnoea | 1.31 (1.02–1.69) | 0.035 | --- | --- | --- | --- | |
| Taste and/or olfactory dysfunction | 1.51 (1.20–1.90) | <0.001 | --- | --- | --- | --- | |
| Chest pain | 1.48 (1.14–1.92) | 0.003 | --- | --- | 1.78 (1.22–2.61) | 0.002 | |
| Arthralgia | 1.39 (1.07–1.83) | 0.015 | --- | --- | --- | --- | |
| Duration of symptoms | 1.02 (1.00–1.04) | 0.049 | --- | --- | 1.04 (1.01–1.08) | 0.015 | |
| Number of symptoms | --- | --- | 1.09 (1.04–1.14) | <0.001 | --- | --- | |
| Obesity | --- | --- | --- | --- | 1.62 (1.08–2.43) | 0.019 | |
| Weakness | --- | --- | --- | --- | 2.02 (1.21–3.35) | 0.007 | |
4. Discussion
The purpose of this study was to determine the prevalence of long-COVID syndrome and the main symptoms, among patients affiliated with the STOP-COVID registry, that represent the most common clinical manifestations of long-COVID syndrome. Additionally, a search for the risk factors for developing long-COVID syndrome was undertaken. The registry includes both inpatients and post-hospitalization patients. More than half of the post-COVID-19 patients—1440 (65%)—included in the registry reported the persistence of symptoms up to three months after disease resolution. The most common symptoms were chronic fatigue, cough, headache, and a set of neurological symptoms referred to as brain fog.
Our results appear to be consistent with worldwide reports, where these symptoms are the most common manifestations of long-COVID syndrome. In a large analysis conducted among 21,359 patients, as many as 42.3% of them declared the presence of symptoms 30 days after the end of the disease, and 24.1% after 90 days. The main symptoms included problems with concentration, memory, olfactory and/or taste disorders, fatigue, and cough [13]. However, in a study among patients after hospitalization, as many as 75% declared the occurrence of at least one symptom within three months after discharge, of which the most common were dyspnea, cough, fatigue, and difficulty sleeping [20]. In a Canadian study, this percentage was similar, involving 76% of patients [21]. Interestingly, in studies including only patients who had been treated in the ICU, the most common complaints were cough, dyspnea, and fatigue. The authors pointed to persistent respiratory dysfunction after COVID-19 as the reason for this [22].
In our study, hospitalization was not shown to be a risk factor for disease progression. Interestingly, ICU treatment also did not increase the risk of developing long-COVID syndrome, which has also been confirmed in previous studies [23]. In contrast, in both univariate and multivariate analysis, disease severity significantly increased the risk of developing long-COVID, as did female gender, duration of COVID-19 symptoms, and olfactory and/or taste disturbances, arthralgia, and chest pain. Furthermore, univariate analysis indicated that as the number of symptoms in the acute phase of the disease increased, the likelihood of developing long-COVID syndrome increased. Similar observations were made in a database analysis from the “COVID Symptom Study”, where the presence of at least five symptoms increased the “risk” of long-COVID 3.5-fold [9].
Several studies to date have confirmed that females are more likely to develop long-COVID syndrome, similar to chronic fatigue syndrome (ME/CFS). This syndrome is defined as severe fatigue that does not subside after rest, accompanied by malaise, cognitive impairment, pain, and autonomic dysfunction. According to researchers, these conditions are intertwined and mitochondrial dysfunction and metabolic changes are suspected as the cause [18,23,24,25,26]. It should also be considered that females are far more likely to be predisposed to report symptoms, which may also influence the outcome [26].
Another important aspect worth discussing is the effect of vaccination on the risk of long-COVID. However, the impact is unclear, and the data do not provide a clear answer. Nonetheless, early data indicated that the full vaccination schedule reduces the risk of persistent symptoms beyond 28 days, and data from the United Kingdom even indicated a 41.1% reduction in risk [9,27]. Recent data are consistent with our observations and indicate that vaccination is not effective in the prevention of long-COVID syndrome [28]. However, a significant limitation of our observation is the small population of vaccinated individuals and the lack of knowledge about the type of preparation used, so the result should not be generalized. Undoubtedly, further research in this area is necessary.
Fatigue is described as the most common, or second most common, manifestation of long-COVID syndrome [21,22,23]. Its frequency is also variable, with some authors reporting it in up to 80% of patients, with a higher prevalence in hospitalized patients [11,13]. In univariate analysis, we found that elderly patients, those with comorbidities, and those who had severe COVID-19 were significantly more likely to suffer from persistent fatigue, with an influence of both duration and the number of symptoms. Among individual symptoms, our observations confirm previous reports of a direct effect of severe fatigue during COVID-19 on the risk of its prolongation. Chest pain was also associated with an increased risk. One potential cause may be a history of myocarditis, which may have gone undiagnosed during COVID-19 and resulted in decreased physical performance [29].
The characteristic symptom of long-COVID syndrome is brain fog, which includes a set of neurological symptoms that are a consequence of COVID-19. According to the literature, up to one in seven patients may be affected by this condition [30,31]. Despite observations, its mechanisms are still unknown, though one hypothesis is that damage to the nervous system in response to ongoing inflammation alters brain function, particularly in areas related to cognitive function [32,33]. It would seem that univariate analysis, where headache, olfactory and/or taste disorders, and hearing disorders were associated with a higher risk of developing brain fog, could support the present theses. However, in multivariate analysis, only the number of symptoms significantly increased the risk of brain fog. Undoubtedly, this disease symptomatology requires further research and follow-up.
The authors are aware of the limitations of this work, in particular the selection of the study group. The group included patients who had self-referred to the health center due to persistent symptoms after COVID-19 recover and not all patients presented after COVID-19. Therefore, this group is not representative of COVID-19 survivors. Consequently, the results should not be translated to the entire population of COVID-19 survivors. Furthermore, the methodology of the study is also a limitation, as the retrospective nature of data collection carries the risk of memory error, which may affect the reliability of estimating the frequency of individual symptoms. Moreover, collection of information on all complaints, within a specific time frame, may contribute to both underestimation and overestimation of the frequency of individual symptoms. In addition, in estimating risk factors, the authors did not take into account the impact of chronic medications, or medications used during and after infection, which can undoubtedly have a significant impact on the nature and duration of long-COVID syndrome.
On the other hand, the strength of this study is the fact that, to the best of the authors’ knowledge, it is the first such description of patients from Poland. Moreover, it is one of the largest described patient registries with such a high proportion of patients after outpatient COVID-19 treatment, whereas the literature is rich in post-hospitalization observations. This allowed us to assess the impact of the site of isolation on the risk of developing long-COVID as a whole and its individual clinical manifestations. This is very important given that the vast majority of patients with COVID-19 are treated at home.
Despite the limitations of the present study, it represents an important contribution to the knowledge of long-COVID syndrome. Our observations are largely consistent with existing data indicating that long-COVID is a significant problem that affects millions of people worldwide. Indeed, despite ongoing studies and observations, we are still uncertain about its pathogenesis, risk factors, and therapeutic options. Therefore, it should be one of the health priorities in the near future, particularly as some patients report symptoms even 12 months after the disease. Moreover, its clinical course is highly variable, and both our results and worldwide reports show that different risk factors may predispose to particular symptoms, e.g., fatigue, brain fog, or olfactory and/or taste disturbances.
The diversity of risk factors for individual symptoms indicates to us that, for effective prevention and treatment, the syndrome should be considered not only as a whole clinical picture, but also in the context of individual symptoms. These observations may contribute to the identification of risk groups for long-COVID syndrome, fatigue, and brain fog, and may be used to target early diagnostic and clinical interventions to mitigate the sequelae of COVID-19 and enable early recovery. It is important to remember that a prolonged condition leads to absenteeism from work, impaired daily functioning, and thus quality of life. Looking at the number of people who have contracted or will contract COVID-19, this problem poses a tremendous challenge not only to the individual, but also to society and the economy.
5. Conclusions
During the three months following the acute phase of COVID-19, persistent clinical symptoms were common. The most common clinical manifestations were chronic fatigue, headache, cough, and brain fog. Severe disease course, duration of COVID-19 symptoms, and female gender, increased the risk of long-COVID syndrome. Obesity is an independent risk factor for persistent chronic fatigue, as is significant fatigue during the acute phase of COVID-19. Given the still-limited knowledge of long-COVID syndrome, further research and observations are needed to better understand the mechanisms and risk factors of the disease.
Author Contributions
Conceptualization, M.C.; Data curation, Ż.K.-K. and D.K.; Formal analysis, M.B.; Funding acquisition, M.B.; Investigation, M.C.; Methodology, M.C., M.B. and J.K.; Project administration, M.C. and A.M.-M.; Resources, M.C.; Software, M.C.; Supervision, M.C., P.J. and A.M.-M.; Validation, M.B., Ż.K.-K., D.K. and P.J.; Visualization, M.C., M.B., J.K., Ż.K.-K. and A.M.-M.; Writing—original draft, M.B., J.K., Ż.K.-K. and D.K.; Writing—review & editing, M.C., M.B., J.K., Ż.K.-K., D.K., P.J. and A.M.-M. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Bioethics Committee of the Wroclaw Medical University, Poland (approval number 232/2022).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was founded by Wroclaw Medical University SUBK.C290.22.044.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.COVID Live—Coronavirus Statistics—Worldometer. [(accessed on 11 June 2022)]. Available online: https://www.worldometers.info/coronavirus/
- 2.SeyedAlinaghi S., Mirzapour P., Dadras O., Pashaei Z., Karimi A., MohsseniPour M., Soleymanzadeh M., Barzegary A., Afsahi A.M., Vahedi F., et al. Characterization of SARS-CoV-2 Different Variants and Related Morbidity and Mortality: A Systematic Review. Eur. J. Med. Res. 2021;26:51. doi: 10.1186/s40001-021-00524-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flisiak R., Horban A., Jaroszewicz J., Kozielewicz D., Mastalerz-Migas A., Owczuk R., Parczewski M., Pawłowska M., Piekarska A., Simon K., et al. Management of SARS-CoV-2 Infection: Recommendations of the Polish Association of Epidemiologists and Infectiologists as of February 23, 2022. Pol. Arch. Intern. Med. 2022;132:16230. doi: 10.20452/pamw.16230. [DOI] [PubMed] [Google Scholar]
- 4.Kuter B.J., Offit P.A., Poland G.A. The Development of COVID-19 Vaccines in the United States: Why and How so Fast? Vaccine. 2021;39:2491–2495. doi: 10.1016/j.vaccine.2021.03.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hammond J., Leister-Tebbe H., Gardner A., Abreu P., Bao W., Wisemandle W., Baniecki M., Hendrick V.M., Damle B., Simón-Campos A., et al. Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with COVID-19. N. Engl. J. Med. 2022;386:1397–1408. doi: 10.1056/NEJMoa2118542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jayk Bernal A., Gomes da Silva M.M., Musungaie D.B., Kovalchuk E., Gonzalez A., Delos Reyes V., Martín-Quirós A., Caraco Y., Williams-Diaz A., Brown M.L., et al. Molnupiravir for Oral Treatment of COVID-19 in Nonhospitalized Patients. N. Engl. J. Med. 2022;386:509–520. doi: 10.1056/NEJMoa2116044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sigal A., Milo R., Jassat W. Estimating Disease Severity of Omicron and Delta SARS-CoV-2 Infections. Nat. Rev. Immunol. 2022;22:267–269. doi: 10.1038/s41577-022-00720-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taquet M., Dercon Q., Luciano S., Geddes J.R., Husain M., Harrison P.J. Incidence, Co-Occurrence, and Evolution of Long-COVID Features: A 6-Month Retrospective Cohort Study of 273,618 Survivors of COVID-19. PLoS Med. 2021;18:e1003773. doi: 10.1371/journal.pmed.1003773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Antonelli M., Penfold R.S., Merino J., Sudre C.H., Molteni E., Berry S., Canas L.S., Graham M.S., Klaser K., Modat M., et al. Risk Factors and Disease Profile of Post-Vaccination SARS-CoV-2 Infection in UK Users of the COVID Symptom Study App: A Prospective, Community-Based, Nested, Case-Control Study. Lancet Infect. Dis. 2022;22:43–55. doi: 10.1016/S1473-3099(21)00460-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.CDC Long COVID or Post-COVID Conditions. [(accessed on 11 June 2022)]; Available online: https://www.cdc.gov/coronavirus/2019-ncov/long-term-effects/index.html.
- 11.Yong S.J. Long COVID or Post-COVID-19 Syndrome: Putative Pathophysiology, Risk Factors, and Treatments. Infect. Dis. 2021;53:737–754. doi: 10.1080/23744235.2021.1924397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cirulli E.T., Schiabor Barrett K.M., Riffle S., Bolze A., Neveux I., Dabe S., Grzymski J.J., Lu J.T., Washington N.L. Long-Term COVID-19 Symptoms in a Large Unselected Population. bioRxiv. 2020 [Google Scholar]
- 13.Lu Y., Li X., Geng D., Mei N., Wu P.-Y., Huang C.-C., Jia T., Zhao Y., Wang D., Xiao A., et al. Cerebral Micro-Structural Changes in COVID-19 Patients—An MRI-Based 3-Month Follow-up Study. EClinicalMedicine. 2020;25:100484. doi: 10.1016/j.eclinm.2020.100484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soriano J.B., Murthy S., Marshall J.C., Relan P., Diaz J.V. WHO Clinical Case Definition Working Group on Post-COVID-19 Condition A Clinical Case Definition of Post-COVID-19 Condition by a Delphi Consensus. Lancet Infect. Dis. 2022;22:e102–e107. doi: 10.1016/S1473-3099(21)00703-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Overview—COVID-19 Rapid Guideline: Managing the Long-Term Effects of COVID-19—Guidance—NICE. [(accessed on 5 July 2022)]. Available online: https://www.nice.org.uk/guidance/ng188.
- 16.Mehandru S., Merad M. Pathological Sequelae of Long-Haul COVID. Nat. Immunol. 2022;23:194–202. doi: 10.1038/s41590-021-01104-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Castanares-Zapatero D., Chalon P., Kohn L., Dauvrin M., Detollenaere J., Maertens de Noordhout C., Primus-de Jong C., Cleemput I., van den Heede K. Pathophysiology and Mechanism of Long COVID: A Comprehensive Review. Ann. Med. 2022;54:1473–1487. doi: 10.1080/07853890.2022.2076901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sudre C.H., Murray B., Varsavsky T., Graham M.S., Penfold R.S., Bowyer R.C., Pujol J.C., Klaser K., Antonelli M., Canas L.S., et al. Attributes and Predictors of Long COVID. Nat. Med. 2021;27:626–631. doi: 10.1038/s41591-021-01292-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thompson E.J., Williams D.M., Walker A.J., Mitchell R.E., Niedzwiedz C.L., Yang T.C., Huggins C.F., Kwong A.S.F., Silverwood R.J., Gessa G.D., et al. Risk Factors for Long COVID: Analyses of 10 Longitudinal Studies and Electronic Health Records in the UK. bioRxiv. 2021 [Google Scholar]
- 20.Huang C., Huang L., Wang Y., Li X., Ren L., Gu X., Kang L., Guo L., Liu M., Zhou X., et al. 6-Month Consequences of COVID-19 in Patients Discharged from Hospital: A Cohort Study. Lancet. 2021;397:220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wong A.W., Shah A.S., Johnston J.C., Carlsten C., Ryerson C.J. Patient-Reported Outcome Measures after COVID-19: A Prospective Cohort Study. Eur. Respir. J. 2020;56:2003276. doi: 10.1183/13993003.03276-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Truffaut L., Demey L., Bruyneel A.V., Roman A., Alard S., de Vos N., Bruyneel M. Post-Discharge Critical COVID-19 Lung Function Related to Severity of Radiologic Lung Involvement at Admission. Respir. Res. 2021;22:29. doi: 10.1186/s12931-021-01625-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fernández-de-Las-Peñas C., Martín-Guerrero J.D., Pellicer-Valero Ó.J., Navarro-Pardo E., Gómez-Mayordomo V., Cuadrado M.L., Arias-Navalón J.A., Cigarán-Méndez M., Hernández-Barrera V., Arendt-Nielsen L. Female Sex Is a Risk Factor Associated with Long-Term Post-COVID Related-Symptoms but Not with COVID-19 Symptoms: The LONG-COVID-EXP-CM Multicenter Study. J. Clin. Med. 2022;11:413. doi: 10.3390/jcm11020413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jacobson K.B., Rao M., Bonilla H., Subramanian A., Hack I., Madrigal M., Singh U., Jagannathan P., Grant P. Patients with Uncomplicated Coronavirus Disease 2019 (COVID-19) Have Long-Term Persistent Symptoms and Functional Impairment Similar to Patients with Severe COVID-19: A Cautionary Tale during a Global Pandemic. Clin. Infect. Dis. 2021;73:e826–e829. doi: 10.1093/cid/ciab103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wood E., Hall K.H., Tate W. Role of Mitochondria, Oxidative Stress and the Response to Antioxidants in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: A Possible Approach to SARS-CoV-2 “long-Haulers? Chronic Dis. Transl. Med. 2021;7:14–26. doi: 10.1016/j.cdtm.2020.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tancheva L., Petralia M.C., Miteva S., Dragomanova S., Solak A., Kalfin R., Lazarova M., Yarkov D., Ciurleo R., Cavalli E., et al. Emerging Neurological and Psychobiological Aspects of COVID-19 Infection. Brain Sci. 2020;10:852. doi: 10.3390/brainsci10110852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ayoubkhani D., Bermingham C., Pouwels K.B., Glickman M., Nafilyan V., Zaccardi F., Khunti K., Alwan N.A., Walker A.S. Trajectory of Long COVID Symptoms after COVID-19 Vaccination: Community Based Cohort Study. BMJ. 2022;377:e069676. doi: 10.1136/bmj-2021-069676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Al-Aly Z., Bowe B., Xie Y. Long COVID after Breakthrough SARS-CoV-2 Infection. Nat. Med. 2022;28:1461–1467. doi: 10.1038/s41591-022-01840-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Siripanthong B., Nazarian S., Muser D., Deo R., Santangeli P., Khanji M.Y., Cooper L.T., Jr., Chahal C.A.A. Recognizing COVID-19-Related Myocarditis: The Possible Pathophysiology and Proposed Guideline for Diagnosis and Management. Heart Rhythm. 2020;17:1463–1471. doi: 10.1016/j.hrthm.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Asadi-Pooya A.A., Akbari A., Emami A., Lotfi M., Rostamihosseinkhani M., Nemati H., Barzegar Z., Kabiri M., Zeraatpisheh Z., Farjoud-Kouhanjani M., et al. Long COVID Syndrome-Associated Brain Fog. J. Med. Virol. 2022;94:979–984. doi: 10.1002/jmv.27404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gennaro D., Belati F., Tulone A., Diella O., Bavaro L., Bonica D.F., Genna R., Smith V., Trott L., Bruyere M. Long COVID: A Systematic Review and Meta-Analysis of 120,970 Patients. SSRN Electron. J. 2022 doi: 10.2139/ssrn.4099429. [DOI] [Google Scholar]
- 32.Batabyal R., Freishtat N., Hill E., Rehman M., Freishtat R., Koutroulis I. Metabolic Dysfunction and Immunometabolism in COVID-19 Pathophysiology and Therapeutics. Int. J. Obes. 2021;45:1163–1169. doi: 10.1038/s41366-021-00804-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stefano G.B., Ptacek R., Ptackova H., Martin A., Kream R.M. Selective Neuronal Mitochondrial Targeting in SARS-CoV-2 Infection Affects Cognitive Processes to Induce “Brain Fog” and Results in Behavioral Changes That Favor Viral Survival. Med. Sci. Monit. 2021;27:e930886-1–e930886-4. doi: 10.12659/MSM.930886. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.