AKI is a common clinical problem with significant morbidity, mortality, and associated healthcare burden. AKI is caused by a wide range of etiologies, has no current treatment, and increases the risk of developing CKD. Despite the high mortality rate and incidence, no effective therapies to treat AKI have been successfully developed. The current mainstays of clinical management—supportive care and dialysis—inadequately address the complexities of AKI and the AKI to CKD transition. The kidney contains numerous cell types and microenvironments, all of which respond differently to injury. Proximal tubule epithelial cells are often a main site of damage, particularly in ischemia-induced AKI. Other kidney compartments, including endothelial cells of the vasculature, immune cells, podocytes, and interstitial cells, are also involved in the pathogenesis of AKI. Novel therapies that consider all compartments of the kidney and the homeostasis that exists among them are therefore of great interest. Endogenous agents and signaling pathways in the kidney hold great potential to inform targeted therapeutic approaches and many, including Krüppel-like factors (KLFs), have been the subject of preclinical studies. These, like most endogenous agents, have broad and pleiotropic influences on kidney function at baseline and injury. Understanding their molecular regulation in a cell- and injury state-specific manner will improve the development of novel, targeted therapeutics for AKI.
KLFs have emerged in recent decades as critical factors in maintaining kidney homeostasis and influencing injury response. KLFs are a family of zinc-finger transcription factors involved in fundamental processes, including cellular cycling and differentiation, metabolism, and cell morphology. Of the 18 KLFs identified to date, several are found in the kidney. As reviewed by Mallipatu et al. (1), KLFs display cell-specific expression patterns throughout the kidney and serve diverse functions in maintaining homeostasis. For example, KLF6 and KLF15 influence TGF-β signaling and kidney fibrosis. Based on experiments in KLF11 knockout mice, KLF11 mitigates the severity of fibrosis in the unilateral ureteral obstruction model of CKD by inhibiting SMAD3-dependent TGF-β signal transduction (2). KLF2, KLF4, and KLF11 are expressed in kidney endothelial cells at baseline. Expression of KLF2 and KLF4 modulates antithrombotic and anti-inflammatory processes. It has been shown that expression of KLF2 and KLF4 is regulated in part by laminar shear stress in both blood vasculature (1) and lymphatic endothelial cells (3). Within these endothelial populations, KLFs also influence NF-κB-dependent expression of cell adhesion molecules. Unlike KLF2 and KLF4, endothelial-specific KLF11 had not been studied in the context of the kidney until recently.
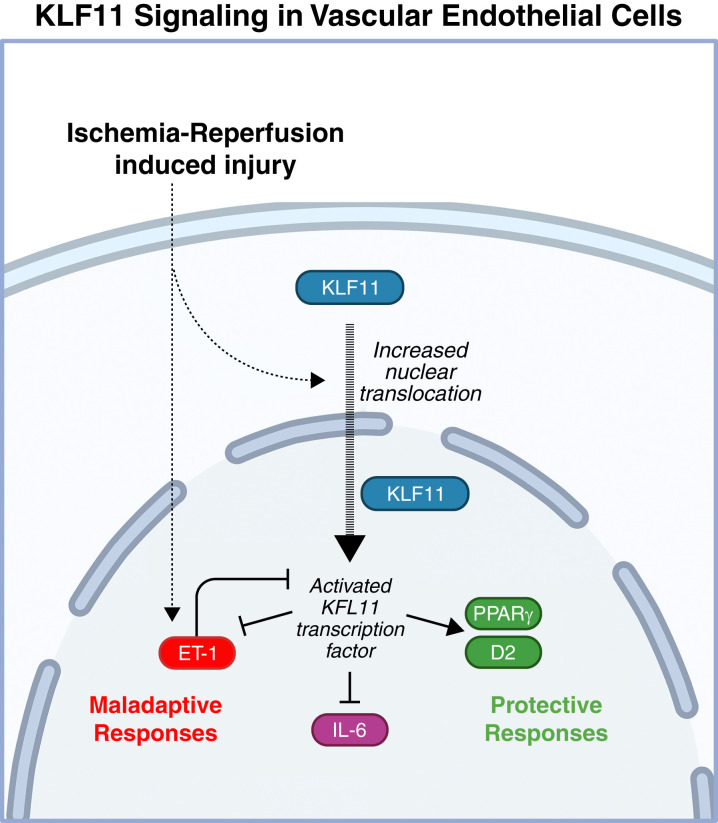
In this issue of Kidney360, Nath et al. (4) identify KLF11 as a novel protective factor with potential for therapeutic targeting in ischemic AKI. To investigate the role of KLF11 in kidney function during AKI, male KLF11 knockout and wild-type control mice were subjected to 22 minutes of bilateral ischemia-reperfusion injury (BIRI). Kidney function and response to AKI were evaluated using several parameters. Elevated serum creatinine and BUN on the day following injury revealed that KLF11 knockout mice are more sensitive to BIRI than wild-type counterparts. Histologic evaluation revealed more extensive and widespread tubular damage in the absence of KLF11. Additionally, knockout mice exhibited more diffuse vascular congestion and exaggerated induction of endothelin-1 and IL-6. The authors postulate the nephroprotective effects of KLF11 may be attributable to the role of KLF11 in regulating endothelial function. The proposed mechanisms of endothelial KLF11 signaling are depicted visually in Figure 1. These findings were predicated on previous in vitro experiments and studies that have investigated the role of KLF11 signaling in endothelial function in response to various insults in tissues outside the kidney as summarized in Table 1.
Figure 1.
The proposed role of KLF11 in kidney endothelial cells. Endothelial cells exhibit cytoplasmic and nuclear KLF11 distribution in basal conditions. Ischemia-reperfusion-induced AKI increases translocation of KLF11 to the nucleus where it acts as a transcription factor, mediating the expression of both maladaptive and protective factors. Pleiotropic IL-6 influences both maladaptive and protective pathways downstream; in ischemic AKI, however, IL-6 is generally associated with an unfavorable functional phenotype. ET-1, endothelin-1; D2, dopamine receptor 2; PPARγ, peroxisome proliferator-activated receptor gamma. Created with BioRender.com.
Table 1.
Summary of key studies of KLF11 in endothelial cell populations
| Endothelial Population/Cell Type of Interest | Key Finding | References |
|---|---|---|
| Human corneal endothelial cell line | KLF11 transcripts are expressed in an established corneal endothelial cell line | Chiambaretta, 2004 (5) |
| Bovine and porcine aortic endothelial cells, ECV304 cell line | KLF11 modulates cholesterol-dependent gene expression through repression of caveolin-1 promoter activity | Cao, 2005 (6) |
| HUVECs, murine aorta (intravital), BAECs | In response to TNF-α, KLF11 regulates leukocyte recruitment through modulation of adhesion molecule expression and interactions with NF-κB | Fan, 20012 (7) |
| Human microvascular endothelial cell line-1 | Finofibrate increases PPARα-mediated KLF11 expression and binding of KLF11 to ET-1 promoter and subsequent downregulation of TGF-β-stimulated ET-1 expression | Glineur, 2013 (8) |
| Murine cerebral vascular endothelial cells | KLF11 cooperates with PPARγ to enhance repression of pro-apoptotic miR-15a and synergistically protect the cerebral vascular endothelium from ischemic stroke | Yin, 2013 (9) |
| HUVECs | KLF11 overexpression decreases TNF-α-induced expression of tissue factor F3 mRNA and protein | Liang, 2019 (10) |
| Murine brain microvascular endothelial cells | KLF11 overexpression can attenuate neuroinflammation and preserve tight junction expression and blood-brain barrier integrity in ischemic brain injury | Zhang, 2020 (11) |
| Murine abdominal aorta, HUVECs (in vitro) | KLF11 expression attenuates development of murine abdominal aortic aneurysm through potential mechanisms in reducing inflammation, expression of MMP9, and production of reactive oxygen species; and maintaining smooth-muscle cell integrity | Zhao, 2021 (12) |
| HUVECs, BAECs, and murine aortic endothelial cells (in vitro) | KLF11 downregulates tissue factor transcription and protects against stasis-induced murine deep vein thrombosis | Liang, 2021 (13) |
| HUVECs, human colon cancer endothelial cells, murine microvascular endothelial cells of the ear | KLF11 expression is upregulated by VEGF-A and required for VEGF-A-induced dopamine D2 receptor expression | Sarkar, 2022 (14) |
BAECs, bovine aortic endothelial cells; ET-1, endothelin-1; HUVECs, human umbilical endothelial cell line; MMP, matrix metallopeptidase 9; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; PPARα, peroxisome proliferator-activated receptor-alpha; PPARγ, peroxisome proliferator-activated receptor-gamma; TGF-β, transforming growth factor-beta; TNF-α, tumor necrosis factor-alpha; VEGF-A, vascular endothelial growth factor A.
The attention to endothelial-specific KLF11 signaling in this study is significant because there is a clear link between endothelial changes that occur following AKI and functional response to injury; yet, modulation of the kidney endothelial systems remains a largely untapped area of therapeutic intervention. The blood and lymphatic vascular endothelial systems work in concert to maintain perfusion and fluid balance, and regulate immune cell trafficking and debris clearance. Both systems respond dynamically to injury. Peritubular blood capillaries undergo a maladaptive process of rarefaction, decrease in density, and become leaky (15–19). Kidney lymphatics, on the other hand, exhibit protective expansion in several animal models of AKI and patients with CKD (20,21). Perturbed hemodynamics and an imbalance between these two systems results in edema in the kidney, which can itself compress vasculature—exacerbating ischemia, impairing complete recovery, and potentially contributing to the AKI to CKD transition (22). Studies designed to improve our understanding of the cellular and molecular control of endothelial homeostasis and injury response will have a significant impact on our ability to design endothelia-modulating therapies.
The protective effects of KLF11 do not appear to be attributable to an induction of KLF11 expression following ischemic injury, but rather normal KLF11 signaling and localization dictate adaptive responses to ischemic injury, and perturbations in normal KLF11 signaling exacerbate injury. Ischemic injury did not significantly alter the total KLF11 protein or mRNA levels in the kidney, but immunofluorescence imaging of wild-type mice revealed that the subcellular localization of KLF11 is altered following BIRI. Increased nuclear localization, and presumed activation of KLF11 transcriptional activity, was seen in endothelial cells, tubular epithelial cells, and smooth-muscle cells. Notably, unlike tubular epithelial cells and smooth-muscle cells, endothelial cells also exhibited nuclear KLF11 prior to ischemic injury, suggesting the importance of basal KLF11 activity in regulating endothelial homeostasis. Quantitative real-time PCR of select genes in KLF11 knockout and wild-type animals in BIRI and sham surgical conditions suggest that basal KLF11 may function to limit expression of monocyte chemoattractant protein-1 (MCP-1/CCL2) to minimize inflammation, SMAD3, which subsequently influences NF-κB signaling, and plasminogen activator inhibitor 1 to attenuate thrombus formation. Although whole kidney lysates did not reveal observable, significant differences in KLF11 mRNA levels between sham and BIRI mice, exploration of publicly available single-cell and single-nuclear transcriptomics datasets of murine kidneys reveals subtle cell-type specific alterations in KLF11 gene expression in response to unilateral ureteral obstruction (23) and BIRI (24) that could be explored in future studies.
The authors are careful not to generalize the protective effects of KLF11 to all etiologies of AKI. As clinical paradigms of AKI management shift toward precision medicine, considerations of cell- and state-specific effects of therapeutic intervention are becoming increasingly important. The role of KLF11 in ischemic injury need not extend to other pathologic states. The authors highlight this important consideration in showing that in response to lipopolysaccharide, KLF11 expression is robustly induced—a drastically different response from what is seen following ischemic injury. Although the complexities of the microenvironments within the kidney and vast range of etiologies of AKI pose challenges for subcellular interrogation of kidney pathophysiology, the growing prevalence and accessibility of single-cell technologies will facilitate detailed and strategic studies. Focused studies such as this one will of course also become increasingly important in validating hypotheses and underlying mechanisms.
Overall, in identifying KLF11 as an important regulator in the response to ischemia-induced AKI, Nath et al. not only signal an appreciation for the role of the endothelium in kidney health but do so while also underpinning the importance of precisely considering molecular determinants of function in relation to specific cell types and disease states.
Disclosures
A. Agarwal serves as a consultant for Dynamed, holds stock options in Goldilocks Therapeutics and Creegh Pharmaceuticals, and is on the advisory boards of Alpha Young, Angion, and Zydus for work outside the scope of this manuscript. The remaining author has nothing to disclose.
Funding
This work was supported by National Institutes of Health grants P30 DK079337, R01 DK59600, and R01 DK118932 (to A. Agarwal) and UAB MSTP T32-GM-008361 (to G. Ghajar-Rahimi).
Acknowledgments
The content of this article reflects the personal experience and views of the authors and should not be considered medical advice or recommendation. The content does not reflect the views or opinions of the American Society of Nephrology (ASN) or Kidney360. Responsibility for the information and views expressed herein lies entirely with the authors.
Footnotes
See related article, “KLF11 Is a Novel Endogenous Protectant against Renal Ischemia-Reperfusion Injury,” on pages 1417–1422.
Author Contributions
G. Ghajar-Rahimi wrote the original draft of the manuscript, and both authors revised the manuscript and endorsed the final manuscript.
References
- 1.Mallipattu SK, Estrada CC, He JC: The critical role of Krüppel-like factors in kidney disease. Am J Physiol Renal Physiol 312: F259–F265, 2017. 10.1152/ajprenal.00550.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Lorenzo SB, Vrieze AM, Johnson RA, Lien KR, Nath KA, Garovic VD, Khazaie K, Grande JP: KLF11 deficiency enhances chemokine generation and fibrosis in murine unilateral ureteral obstruction. PLoS One 17: e0266454, 2022. 10.1371/journal.pone.0266454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choi D, Park E, Jung E, Seong YJ, Hong M, Lee S, Burford J, Gyarmati G, Peti-Peterdi J, Srikanth S, Gwack Y, Koh CJ, Boriushkin E, Hamik A, Wong AK, Hong YK: ORAI1 activates proliferation of lymphatic endothelial cells in response to laminar flow through Krüppel-like factors 2 and 4. Circ Res 120: 1426–1439, 2017. 10.1161/CIRCRESAHA.116.309548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nath K, Singh R, Croatt A, Ackerman A, Grande J, Khazaie K, Chen YE, Zhang J: KLF11 is a novel endogenous protectant against renal ischemia-reperfusion injury [published online ahead of print May 6, 2022]. Kidney360 10.34067/KID.0002272022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiambaretta F, De Graeve F, Turet G, Marceau G, Gain P, Dastugue B, Rigal D, Sapin V: Cell and tissue specific expression of human Krüppel-like transcription factors in human ocular surface. Mol Vis 10: 901–909, 2004 [PubMed] [Google Scholar]
- 6.Cao S, Fernandez-Zapico ME, Jin D, Puri V, Cook TA, Lerman LO, Zhu XY, Urrutia R, Shah V: KLF11-mediated repression antagonizes Sp1/sterol-responsive element-binding protein-induced transcriptional activation of caveolin-1 in response to cholesterol signaling. J Biol Chem 280: 1901–1910, 2005. 10.1074/jbc.M407941200 [DOI] [PubMed] [Google Scholar]
- 7.Fan Y, Guo Y, Zhang J, Subramaniam M, Song CZ, Urrutia R, Chen YE: Krüppel-like factor-11, a transcription factor involved in diabetes mellitus, suppresses endothelial cell activation via the nuclear factor-κB signaling pathway. Arterioscler Thromb Vasc Biol 32: 2981–2988, 2012. 10.1161/ATVBAHA.112.300349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glineur C, Gross B, Neve B, Rommens C, Chew GT, Martin-Nizard F, Rodríguez-Pascual F, Lamas S, Watts GF, Staels B: Fenofibrate inhibits endothelin-1 expression by peroxisome proliferator-activated receptor α-dependent and independent mechanisms in human endothelial cells. Arterioscler Thromb Vasc Biol 33: 621–628, 2013. 10.1161/ATVBAHA.112.300665 [DOI] [PubMed] [Google Scholar]
- 9.Yin KJ, Fan Y, Hamblin M, Zhang J, Zhu T, Li S, Hawse JR, Subramaniam M, Song CZ, Urrutia R, Lin JD, Chen YE: KLF11 mediates PPARγ cerebrovascular protection in ischaemic stroke. Brain 136: 1274–1287, 2013. 10.1093/brain/awt002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liang W, Fan Y, Lu H, Chang Z, Hu W, Sun J, Wang H, Zhu T, Wang J, Adili R, Garcia-Barrio MT, Holinstat M, Eitzman D, Zhang J, Chen YE: KLF11 (Krüppel-like factor 11) inhibits arterial thrombosis via suppression of tissue factor in the vascular wall. Arterioscler Thromb Vasc Biol 39: 402–412, 2019. 10.1161/ATVBAHA.118.311612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang X, Tang X, Ma F, Fan Y, Sun P, Zhu T, Zhang J, Hamblin MH, Chen YE, Yin KJ: Endothelium-targeted overexpression of Krüppel-like factor 11 protects the blood-brain barrier function after ischemic brain injury. Brain Pathol 30: 746–765, 2020. 10.1111/bpa.12831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao G, Chang Z, Zhao Y, Guo Y, Lu H, Liang W, Rom O, Wang H, Sun J, Zhu T, Fan Y, Chang L, Yang B, Garcia-Barrio MT, Chen YE, Zhang J: KLF11 protects against abdominal aortic aneurysm through inhibition of endothelial cell dysfunction. JCI Insight 6: e141673, 2021. 10.1172/jci.insight.141673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liang W, Lu H, Sun J, Zhao G, Wang H, Guo Y, Eitzman D, Chen YE, Fan Y, Zhang J: KLF11 protects against venous thrombosis via suppressing tissue factor expression. Thromb Haemost 122: 777–788, 2021. 10.1055/s-0041-1735191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sarkar C, Chakroborty D, Goswami S, Fan H, Mo X, Basu S: VEGF-A controls the expression of its regulator of angiogenic functions, dopamine D2 receptor, on endothelial cells. J Cell Sci 135: jcs259617, 2022. 10.1242/jcs.259617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Basile DP, Donohoe D, Roethe K, Osborn JL: Renal ischemic injury results in permanent damage to peritubular capillaries and influences long-term function. Am J Physiol Renal Physiol 281: F887–F899, 2001. 10.1152/ajprenal.00050.2001 [DOI] [PubMed] [Google Scholar]
- 16.Basile DP, Donohoe DL, Roethe K, Mattson DL: Chronic renal hypoxia after acute ischemic injury: Effects of L-arginine on hypoxia and secondary damage. Am J Physiol Renal Physiol 284: F338–F348, 2003. 10.1152/ajprenal.00169.2002 [DOI] [PubMed] [Google Scholar]
- 17.Hörbelt M, Lee SY, Mang HE, Knipe NL, Sado Y, Kribben A, Sutton TA: Acute and chronic microvascular alterations in a mouse model of ischemic acute kidney injury. Am J Physiol Renal Physiol 293: F688–F695, 2007. 10.1152/ajprenal.00452.2006 [DOI] [PubMed] [Google Scholar]
- 18.Steegh FM, Gelens MA, Nieman FH, van Hooff JP, Cleutjens JP, van Suylen RJ, Daemen MJ, van Heurn EL, Christiaans MH, Peutz-Kootstra CJ: Early loss of peritubular capillaries after kidney transplantation. J Am Soc Nephrol 22: 1024–1029, 2011. 10.1681/ASN.2010050531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kwon O, Hong SM, Sutton TA, Temm CJ: Preservation of peritubular capillary endothelial integrity and increasing pericytes may be critical to recovery from postischemic acute kidney injury. Am J Physiol Renal Physiol 295: F351–F359, 2008. 10.1152/ajprenal.90276.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim H, Kataru RP, Koh GY: Inflammation-associated lymphangiogenesis: A double-edged sword? J Clin Invest 124: 936–942, 2014. 10.1172/JCI71607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zarjou A, Black LM, Bolisetty S, Traylor AM, Bowhay SA, Zhang MZ, Harris RC, Agarwal A: Dynamic signature of lymphangiogenesis during acute kidney injury and chronic kidney disease. Lab Invest 99: 1376–1388, 2019. 10.1038/s41374-019-0259-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Basile DP, Yoder MC: Renal endothelial dysfunction in acute kidney ischemia reperfusion injury. Cardiovasc Hematol Disord Drug Targets 14: 3–14, 2014. 10.2174/1871529X1401140724093505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu H, Kirita Y, Donnelly EL, Humphreys BD: Advantages of single-nucleus over single-cell RNA sequencing of adult kidney: Rare cell types and novel cell states revealed in fibrosis. J Am Soc Nephrol 30: 23–32, 2019. 10.1681/ASN.2018090912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kirita Y, Wu H, Uchimura K, Wilson PC, Humphreys BD: Cell profiling of mouse acute kidney injury reveals conserved cellular responses to injury. Proc Natl Acad Sci U S A 117: 15874–15883, 2020. 10.1073/pnas.2005477117 [DOI] [PMC free article] [PubMed] [Google Scholar]