Abstract
The production and use of plastics has constantly increased over the last 30 years. Over one third of the plastics is used in disposables, which are discarded within three years of their production. Despite efforts towards recycling, a substantial volume of debris has accumulated in the environment and is slowly degraded to micro- and nanoplastics by weathering and aging. It has recently been discovered that these small particles can enter the food chain, as for example demonstrated by the detection of microplastic particles in honey, beer, salt, sea food and recently in mineral water. Human exposure has further been documented by the detection of plastic microparticles in human feces. Potential toxic consequences of oral exposure to small plastic particles are discussed. Due to lacking data concerning exposure, biodistribution and related effects, the risk assessment of micro- and nanoplastics is still not possible. This review focuses on the oral uptake of plastic and polymer micro- and nanoparticles. Oral exposure, particle fate, changes of particle properties during ingestion and gastrointestinal digestion, and uptake and transport at the intestinal epithelium are reviewed in detail. Moreover, the interaction with intestinal and liver cells and possibly resulting toxicity are highlighted.
This review focuses on micro- and nanoplastic particles with the main focus on oral uptake and transport at the intestinal epithelium and potential toxic consequences.
Introduction
The production of plastics has constantly increased over the last 30 years. In 2018, over 360 million tons of plastic materials were produced, 62 million tons in Europe. Plastic polymers are used for a wide variety of applications and have become an essential material in our daily life. The most demanded polymers in Europe are (in decreasing order) polypropylene (PP), polyethylene (PE), polyvinyl chloride (PVC), polyurethane (PU), polyethylene terephthalate (PET) and polystyrene (PS).1 Over one-third of the plastics in Europe as well as in the United States is used in disposables which are discarded within three years of their production.2 Despite efforts towards recycling, a substantial volume of debris has accumulated in the environment, especially in the oceans with multiple routes of entry. The Worldwatch Institute estimated that 10 to 20 million tons of plastic end up in the oceans each year.3 This global problem affects probably all ecosystems and therefore the complete food chain. Besides, also contamination during food production should be considered. The most studies are done for the marine ecosystem. Plastic polymers in the ocean are susceptible to degradation via biotic and/or abiotic processes.2,4 Most plastics start to degrade at their accessible polymer surface. The degradation process yields smaller fragments, which in turn have a higher fragmentation rate due to their increasingly higher surface-to-volume ratio.2 Such small fragments, so-called microplastic particles, have gained public attention in the last years. There is no officially published definition of microplastics, but in general they are considered to vary in size ranging from 0.1–5000 μm.5 The smaller group of nanoplastics can be specified based on current definitions for nanomaterials6 as particles that range from 1–100 nm in size. Microplastics are divided into primary and secondary particles: primary microplastics are industrially produced as such, whereas secondary microplastics result from plastic waste via degradation processes such as UV light and physical abrasion.5 Micro- and nanoplastics may enter the food chain by different paths, resulting in exposure of consumers via the diet. Microplastics have been detected in some food products such as honey, table salt, milk, mineral water and seafood, giving at least some impression of human exposure via the food chain.7–10 Although evidence on the presence of plastic particles in food is increasing, quantitative human exposure data via the diet are not yet available, although first estimations have been made (Toussaint et al. 2019155). Indeed, there is still no legislation for micro- and nanoscaled plastics in foodstuff. Similar to nanoparticles, where toxicological concerns were based e.g. on the higher surface-to-volume ratio and surface reactivity as compared to larger particles, nanoplastics might be more prone to pose health risks to humans. In addition, the possibility of toxic effects due to additives and adsorbed contaminants is discussed. Moreover, influences and changes that occur to the particles during digestion just came into focus. Risk assessment for micro- and especially nanoplastics is challenging, amongst others because of the diversity of polymer particles as well as a lack of reference materials and validated detection methods, particularly in complex biological matrices. Therefore, the aim of this review is to give an overview of the current knowledge on human oral uptake, with a special focus on digestion and possible effects of micro- and nanoplastics in the digestive system.
Definition, detection methods and available research materials
Before talking about plastic particles of any size, terminology should be explained. In principle, general definitions of nanomaterials can be adapted to plastic particles in the respective size range. Several relevant definitions are available that apply to different regulations and scope of applications. The most important definitions can be found in the norm ISO/TS 80004-1:2015, the regulation (EC) no 1223/2009 on cosmetic products, the regulation (EU) 2015/2283 on novel foods, and the regulation (EU) no 1169/2011 on the provision of food information to consumers. They have in common that the term “nano” refers to structures that possess at least one dimension in a size range between 1 and 100 nm. Applying these definitions for polymer-based particles, it can be used as definition for nanoplastics.6
In contrast to nanoparticles, no consensual scientific or regulatory definition is available for microplastics. Most work uses the term microplastics for small solid particles made of a synthetic polymer. In some definitions of microplastics, biodegradable plastics are excluded.11 In 2019, the European Chemicals Agency (ECHA) proposed a regulatory definition for microplastics under the REACH (Registration, Evaluation and Authorisation and Restriction of CHemicals) legislation. It describes microplastics as (i) solid polymer-containing particles where all dimensions are between 1 nm and 5 mm and (ii) fibers with a length of 3 nm to 15 mm (length-to-diameter ratio greater than 3). This definition would also include nanoplastics. A group of chief scientific advisors of the EU also defined an upper size limit of 5 mm.12 Sometimes also the term submicroplastics can be found, which aims to describe particles smaller than 1 μm but bigger than the upper size limit of the definition of nanoparticles of 100 nm.
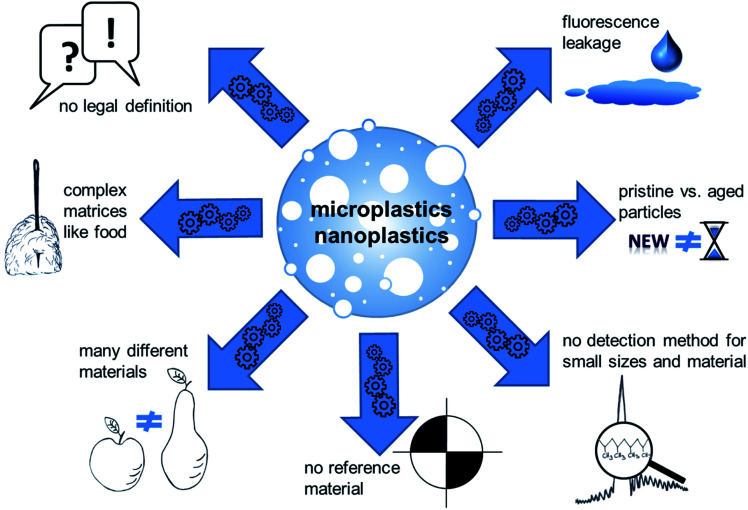
The following paragraph aims to give an overview on current analytical detection methods and available materials that can be used for research purposes. Micro- and nanoplastic analytics have already been reviewed in detail, for example in “Methods for the analysis of submicrometer- and nanoplastic particles in the environment”,13,14 “Finding Microplastics in Soils: A Review of Analytical Methods”15 or “Separation and Analysis of Microplastics and Nanoplastics in Complex Environmental Samples”.16 Even if detailed methodological considerations of micro- and nanoplastic analytics do not fall within the scope of this work, a number of critical points are worth being mentioned (Fig. 1). As required to fulfill the definition, analytical detection of plastic particles require to obtain information on both the chemical composition and the morphological structure of the particles. Even though a number of techniques are available for particle separation and size determination, the methods available for material characterization are rather limited and it is therefore challenging to find suitable combinations. So far, micro-Raman spectroscopy and micro-FTIR spectroscopy are the most frequently used methods for identification of the chemical composition of plastic particles. Particle size, however, limits their applicability. Micro-Raman spectroscopy enables analyses of particles down to a size of about 1 μm.17 The size limitation has lately been pushed down to 200 nm by optical tweezers.18 For micro-FTIR spectroscopy the actual size limit is 20 μm.14 It is obvious that the applicability of these methods do not comprise the size range of nanoparticles according to the current legal definitions. Apart from these examples, there are a few more methods with different limitations. For more details refer to other reviews on this topic like “Identification methods in microplastic analysis: a review”,19 “Microplastics in the environment: Challenges in analytical chemistry – A review”20 or others.
Fig. 1. Challenges and pitfalls in the field of micro- and nanoplastics research.
In addition to the analysis step itself, preceding sample preparation is a critical step. Firstly, unintended sample contamination with plastic material during procession and analytics is hard to avoid due to its ubiquitous presence. Secondly, analytical investigation of plastic particles is challenging for complex matrices. Recent approaches try to circumvent this problem e.g. by using near-infrared (NIR) process-spectroscopic methods to enable a high-throughput analysis with minimal sample preparation, as shown for soil samples.21 Nevertheless, most analytical approaches for complex matrices like food still comprise several steps: destruction of the matrix, filtration or separation of the particles, enrichment and the analysis of size and material. Since micro- and nanoplastics are very complex mixtures of materials and established analytical methods are not sufficiently adapted and validated, harmonized structured methodological recommendations are still missing. Although, the analytic of plastic particles is already reviewed in detail.22 The term “plastics” summarizes a variety of materials with different properties. This implies that a certain detection method or the result of a toxicological test cannot be easily transferred to other particles.23 Available analytical methods have often been demonstrated using one specific material, particularly polystyrene (PS). This polymer has mostly been used for toxicological studies as well, because this polymer has a density which allows easy suspension in aqueous media, which makes it easy to use in in vitro assays but limits their relevance as a model for “environmental” microplastics. PS particles are frequently used because of the ease to generate particles with a precise size distribution and because of the ease to attach other molecules, for example fluorescent dyes to facilitate detection. However, a possible leakage of the fluorescent marker cannot be ruled out during toxicological studies, which may lead to false results. Necessary controls for fluorescent dye leachate and cellular autofluorescence are often missing.24 A biased material selection impedes the transfer of scientific findings to other polymers, because particles do not only differ in their chemical structure, but also in properties such as size, surface, density, and other parameters. Methods to produce non-PS particles are just emerging.25–27 Moreover, the materials produced for testing do not necessarily reflect the properties of real-life particles, for example aged materials from the environment.28
Changes of plastics in the environment and the food chain
Micro- and nanoplastics contaminating food and drinking water undergo various steps prior to their ingestion by humans. During their life cycle, plastic materials and particles can change due to different environmental conditions. Still, most research on the uptake and effects of microplastics and nanoplastics has been done with pristine, intentionally manufactured particles. Therefore, the various aspects that can change the properties of plastic particles during their life cycle from production to food must be taken into account. These aspects comprise aging and degradation, formation of a protein corona and biofilms, leaching of additives, and sorption of compounds.
Aging of plastics in the environment
Plastics are mainly categorized into two groups, namely non-biodegradable and biodegradable plastic types.29 Non-biodegradable plastic polymers mostly have a very stable structure with long saturated organic chains. Those chains contain balanced charges along their length, making the molecule chemically inert.30 Plastic materials, which are circulating in flows of goods or are released into the environment undergo either aging or weathering during their life cycle. This can generate micro- and finally nanoplastics. Aging is a term used when the properties of the polymer change over a period of time.31 These changes can affect polymer composition, physical integrity of the particle, and its surface properties. In a natural environment, this process is termed weathering. In general, weathering and aging lead to polymer transformation and degradation through a number of complex processes: photo-oxidation, hydrolysis, mechanical abrasion, swelling, release of additives, biodegradation, organic matter coating of the surface including protein corona formation, pollutant adsorption, and colonization by microorganisms. Surface coverage by a complex mixture of organic and inorganic molecules is termed eco-corona formation. Weathering in the marine environment has been studied most extensively.32 Both abiotic degradation and biodegradation of plastics is considered a very slow process in the marine environment, leading to plastic fragmentation on a time scale spanning decades.2,33 In the marine environment, abiotic degradation through sunlight, oxidants and physical stress34 is generally recognized as a starting point of plastic degradation, breaking down the plastic structures and allowing a progressive decomposition prior to the occurrence of biotic effects.35 The smaller polymer fragments formed can be biodegraded by the action of microbes present in the marine environment.2,30 Despite a sometimes unclear ecological relevance, most studies have been carried out under laboratory-controlled settings, with a special emphasis on the effects of UV light exposure or shearing forces, and do not consider all details of real-life conditions, such as water salinity, mixed microbiological populations and natural cycles of temperature and light.4 Brandon et al. investigated the influence of water type and light on the degradation of plastics to predict the age of environmental samples. They found that aging or weathering induce a very complex physicochemical change of polymeric materials, depending on the type of polymer and environmental conditions.36 In fact, even very robust and inert polymers like PE can be severely damaged during weathering in the open ocean, with a shortening of the polymer chains.37 For more details on plastic aging in the marine environment, please refer to one of the reviews available on that topic, for example “The chemical behaviors of microplastics in marine environment: A review”38 or “Interactions of microplastic debris throughout the marine ecosystem”.39
Besides environmental factors such as light, water and mechanical stress, the formation of a protein corona and biofilms also accounts to the aging of plastic materials. Molecules from biological samples, especially proteins, can associate with biopolymers, forming a so-called “protein corona” that is associated with the particle and continuously exchanging with proteins from the surrounding environment. Hence, it gives a new biological identity of the particles and may affect their biological responses.40 The binding forces that are responsible for the interactions between proteins and particle surface include van-der-Waals interactions, hydrogen bonds, hydrophobic interactions, as well as electrostatic forces.40 This has been extensively studied for diverse nanoparticles,41 with some noteworthy studies focusing on plastic and polymer particles. Often in older protein corona studies, plastic materials are imprecisely referred to as “latex”,42 “resin”43 or “polymer” particles or beads. These expressions include materials like PS,44 polylactic acid,45 polyacrylonitrile46 or metacrylate.47 Binding studies have been performed for investigating these interactions, with single proteins such as BSA48 but also with more complex systems, for example with blood, serum and plasma from humans or other mammalian species, or with cell culture media.49–55 An inter-species comparison was performed by Muller et al. using functionalized polystyrene particles with human, rat, sheep and rabbit blood plasma. They showed that the composition of protein corona depends on the plasma source.56 Walczak et al. determined the composition of protein coronae in artificial chyme to simulate the digestion process.57 Food components can also affect the interaction between particles and intestinal cells. Sinnecker et al. reported for PS nanoparticles treated with casein, BSA or meat extract (a mixture of peptides and amino acids representative for an alimentary peptide source) that the meat extract appeared to have only a minor effect on the adsorption of plastic particles on the cells. Moreover, individual peptides and amino acids of the meat extract did not modify the surface of the nanoparticles, as seen with BSA.58 The resulting protein coronae are different depending on particle sizes, surface charges,59 surface functionalization60,61 or variations in exposure time.45 In many studies, the protein compositions are identified by electrophoresis and mass spectrometric analysis techniques after an in vitro isolation of the corona proteins.49
When the environment of the particles also contains microorganisms, the formation of biofilms is likely62 and should also be considered. Although, this topic is not in focus of this review and can be found in detail elsewhere.63
Plastic additives and contaminants
Plastics can contain additives to enhance their physical properties. They are intentionally added during production as softeners, flame retardants, UV-stabilizers and production agents. ECHA lists about 400 additives.64 Plastics additives of concern to human health are for example phthalates, bisphenols, brominated flame retardants, triclosan, and organotins.65 These chemicals can leach from the polymer surface to the surroundings. Some basic principles about parameters that affect the migration potential of an additive from plastic particles are described below. Polymers have a three-dimensional porous structure in which the additives are distributed. The pore diameter and the size of the additive correlate in a way that smaller (lower molecular weight) additives move more easily through a polymer with a bigger pore size. Temperature and the physico–chemical properties of the additive and the environment also constitute important parameters. In contrast to non-covalently bound additives, the release of a reactively bonded compound from a polymer requires cleavage of the covalent bond(s) before migration can take place. The loss of polymer constituents from a polymer is thus probably due to the release of unreacted (non-bonded) constituents left over from polymer synthesis.66 Some studies have described the leaching of additives (bisphenol A and nonylphenol) from plastics in the intestinal tracts of worms and fish.67 Leaching as well as degradation of plasticizers and polymers are complex phenomena dependent on the environmental conditions and the chemical properties of each additive. The extent and rate of chemical (de)sorption are influenced by factors including the sorbent (plastic polymer) properties, sorbate (additive) properties, dissolved organic compounds in the aqueous phase, pH and temperature.66 Temperature and UV-light, e.g. plastics exposed to sunlight for longer periods, can affect the polymer structure and make additive migration more likely. This would, on the one hand, allow additives to leach into the environment, and, on the other hand, allow chemicals to adsorb to the surface. This is dependent on the types of plastics and their individual transition temperatures, i.e. the temperatures that change the structure of the plastics such as melting transition or glass transition. EFSA, however, estimates the contribution of microplastic-bound additives as minor to the overall exposure.5
Similar to the situation with inorganic nanoparticles potentially acting as Trojan horses for intracellularly released metal ions,68 it was hypothesized that plastic particles might function as Trojan horses for contaminants, due to their hydrophobicity and large surface. Many persistent organic pollutants can bind to organic materials due to their hydrophobic surface. The hydrophobicity of polymers, in combination with the high surface area, causes micrometer- and nanometer-sized plastic particles to be efficient sorbents for e.g. hydrophobic organic chemicals such as polychlorinated biphenyls (PCBs) or polycyclic aromatic hydrocarbons (PAHs), leading to high plastic–water distribution coefficients.69 Dependent on the chemical properties of these substances, a release into biological compartments appears possible. Binding of chemicals to plastic surfaces has been widely studied, even though often without specific regard to the material size. However, for the basic chemical processes, it does not generally matter if macroscopic material or microparticles are regarded.70 The binding process can be distinguished into adsorption and absorption.71 Adsorption is a fast and strong binding to a surface, which occurs mainly with robust vitreous materials such as PS or PVC and strongly hydrophobic substances without a deeper penetration into the materials. In contrast, absorption is characterized by a certain penetration of chemicals into the outer layers of a porous material like rubber, or into flexible thermoplasts such as PE or PP, which allows certain diffusion into and out of the material, triggered by weaker forces such as van-der-Waals interactions. For example, HDPE, LDPE and PP consistently sorb higher amounts of PAHs and PCBs than PET and PVC. Rubbery polymers such as HDPE, LDPE and PP are expected to demonstrate larger diffusion than the glassy polymers PET and PVC, which may explain these trends.72 The ad- and absorption of hydrophobic chemicals to plastics polymers has been previously studied for PAHs to plastic pellets,72–74 and for PCBs to micro- and nanoplastics.75 Further studies have analyzed the adsorption of the heavy metals like copper, cadmium and zinc to different kinds of microplastics.76–78 The physicochemical interrelations underlying these processes have been extensively described,79 for example on surfaces or packaging.
For orally ingested plastic particles present in foodstuff, it remains to be elucidated whether the increased surface area and a possibly enhanced cellular uptake of small particles might influence the release of ad- or absorbed chemicals. Available studies with aquatic organisms demonstrate that microplastic particles can act as carriers for contaminants, for example PCBs, leading to an increased body burden of the contaminant.80 This does, however, not automatically lead to a higher exposure of cells to the unbound chemicals and thus to enhanced biological effects, because particles can function as sink. Likewise, no direct conclusions can be drawn about the situation in higher mammals. In contrast to the above scenario, plastic particles may also act as a sink for some contaminants: the binding of the chemical to the particles can lead to smaller bioavailable amounts and then lower biological effects, for example in the case of PAHs,81 even though there is a carrier effect.82 Particles may thus act as carriers of pollutants in a way that they either increase or reduce their availability as a free compound in the body, depending on their material and particle size. It is conceivable that a very strong binding of pollutants to plastic materials could allow only a minor release, which in turn leads to a negligible exposure to the unbound contaminants.70 A calculation by EFSA determined the contribution of plastic-bound pollutants to the overall exposure as very low.5 Additionally, the sorption of contaminants on aged particles appears to be lower, as compared to the pristine particles mainly used for toxicological research. This was shown by Hüffer et al. who performed UV-aging of PS particles. It had little effect on the surface area, whereas it significantly increased surface functionalities such as carbonyl and hydroxyl groups on the sorbent. The surface oxidation and localized micro-crack formation led to a decrease in the sorption coefficients of organic compounds by PS microplastics, which turned out to be up to one order of magnitude lower than for the pristine particles.83 Nevertheless, also other factors relevant for pollutant release, such as local or chronic effects, could be relevant in the discussion about health risks of micro- and nanoplastics.84
Human oral exposure
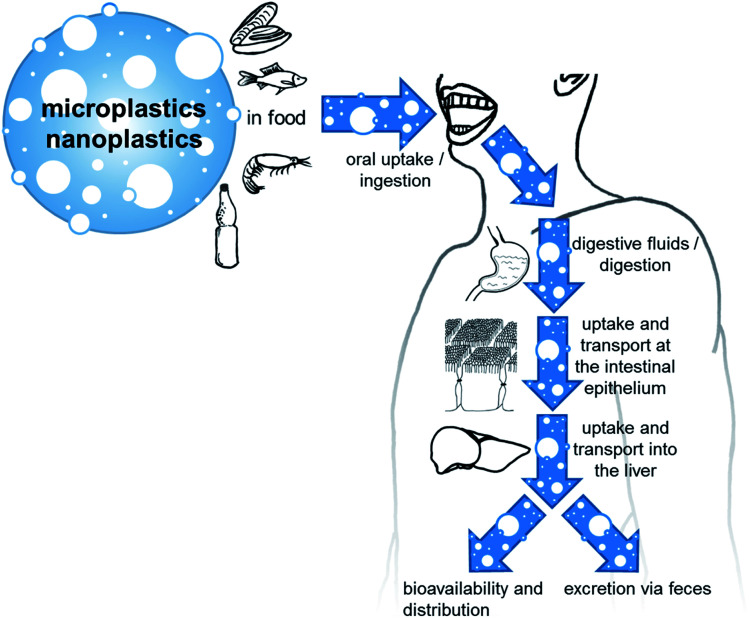
Micro- and nanoplastics can reach the human body amongst others via the oral route. Oral ingestion is followed by a number of steps that influence the particles and therefore their interactions, like the contact with digestive fluids, the contact to intestinal cells, uptake and transport in the intestine and liver, and excretion. This is illustrated in Fig. 2.
Fig. 2. Human exposure and the path of micro- and nanoplastic particles in the human body.
Due to the already discussed difficulties to find suitable analytical methods for detecting microplastics and especially nanoplastics in complex matrices, the number of studies performed and data published on the amount of plastic particles in food is still limited. Nevertheless, data on plastic particle contents of some types of foodstuff are available and the number of publications is constantly increasing with the evolution of analytical methods and the growing public interest. Studies are available from environmental research that investigate microplastic particles in aquatic organisms. With respect to the organs and tissues analyzed and the knowledge of the parts of the animals we consume as food, some results can be used for exposure assessment.85 Generally, fishes accumulate microplastics in their gills, liver and gut, which may not be relevant to human consumption since these tissues are not usually consumed.86 Some studies proposed an annual intake from shellfish consumption for a European top consumer as high as 11 000 microplastic particles.87 There are also studies dealing with the microplastic content of food products other than seafood. Namely, honey and beer have been some of the first food products where microplastics contamination was investigated.88,89 In these initial studies, the measurements were performed in a qualitative way by microscopy without using additional analytical techniques. Other studies investigated the occurrence of microplastics in table salt.90–92 The first contamination of drinking water was reported in 2017 (ref. 93) with data provided from 14 countries on the presence of plastic particles in tap water. The highest mean concentrations of particles ranging from 0.1-5 mm occurred in the US (9.24 ± 11.8 particles per L) and the lowest in Germany (0.91 ± 1.29 particles per L). About 98% of the particles were fibers. Furthermore, some studies have been conducted up to now to collect data from bottled mineral waters.94–96 Different size limitations (from 1 μm to 500 μm) were used to detect microplastics. PET was the main polymer detected from the plastic bottles probably due to degradation of packaging, but microplastic particles were detected also in mineral waters from glass bottles. Recently human exposure through water has been pointed out both from bottled water and from ground waters,93,97 even though the latter contains only very low amounts of microplastics. In a recent study 57 beverages such as soft drinks, energy drinks, cold tea and beer were analyzed for the presence of microplastics. The most common polymers identified were PA and polyesteracetal (PEA), while PET and acrylonitrile butadiene styrene (ABS) were present only in small amounts in beer and soft drinks. Beer contained the highest total amount of microplastics (152 ± 50.97 particles per L), followed by energy drinks (14 ± 5.79 particles per L), soft drinks (40 ± 24.53 particles per L) and cold tea (11 ± 5.26 particles per L). Here the possible routes of microplastic contamination may be the water source used during the production processes, and/or the process of beverage packaging.98 Another study suggests contamination of dairy milk products by the addition of water during production and processing. In this study, 23 milk samples (8 different brands from Mexico) were analyzed. Results showed polyethersulfone (PES) as the most common type of plastic particles found in the samples. Particles had different sizes (0.1 to 5 mm), shape (fibers and fragments) and colors (blue, brown, red and pink). The most common microplastic shape was fiber (97.5%), while the most predominant color was blue (72%). The levels of microparticle concentrations varied between 3 ± 2 to 11 ± 3.54 particles per L with an overall average of 6.5 ± 2.3 particles per L.10 Kedzierski et al. analyzed the surface of packaged meat products (white chicken breast and turkey escalope) and found PS microplastics ranging from 4.0 to 18.7 particles per kg of packaged meat and size between 130 to 450 μm. These microplastics were found to stick to the meat surface, before and after washing, hence increasing the possibility of human consumption. The authors suggested that the contamination with plastic particles was due to polystyrene dust suspended in the air of the production facility.99 Another study was done on fruits and vegetables. Therein, different sizes (1.51 to 2.52 μm) of microplastics were detected in carrots, lettuces, broccoli, potatoes, apples, and pears. Fruits, in particular apples, contained higher level of microplastics (223 000 p per g; range 52 600 to 307 750) than vegetables like carrots 97 800 particles per g (72 175 to 130500), while lettuce samples contained the lowest 52 050 p per g (26 375 to 75 425).100
Complementing these data of plastic particles in the human diet, analyses of human stool samples display another proof for human exposure to microplastics through the food chain.101 There are also first attempts to use the scarce data to estimate human exposure. Therefore, Cox et al. grouped the available literature data on microplastic occurrence into categories and used dietary recommended intake data of each food group to calculate an average intake. Extrapolations of the overall exposure range between 39 000 and 52 000 microplastic particles per year. A worst case scenario was not calculated.9 This estimation does not provide information about the particle size, does not include nanoplastic particles, and concedes a severe variation due to people's consumer behavior. As microplastics are found nearly everywhere, the problem of assay contamination can be crucial and blank samples are required to obtain relevant data.97 Therefore, exposure estimations based on data from studies not using blank samples could constitute an overestimation of real exposure. The size range of the plastic particles detected in experimental studies, methodically limited to around 20 μm, likely underestimates the real contamination. Human exposure is still not well elucidated.102
Ingestion and digestion
This paragraph will focus on the behavior of micro- and nanoplastics during the digestion process. Before reaching the intestinal epithelium, particles have to pass through different compartments of the gastrointestinal tract that may affect their physicochemical properties and surface parameters. While most publications focused on the environmental degradation of plastic particles,2,4 the knowledge on their fate along the mammalian gastrointestinal tract is rather sparse. Most of the non-plastic biodegradable substances contain some mixture of carbon and heteroatoms like oxygen, nitrogen, sulfur, and phosphorus, which create charge imbalances that digestion enzymes can exploit. By contrast, the stability of the plastic materials diminishes the possibilities of enzymatic or chemical degradation. The harshest chemical condition, i.e. the low gastric pH, will be in contact with the ingested particles for about two hours, and specialized enzymes for plastic degradation are lacking in the mammalian intestine. This suggests that most likely no major degradation of plastic particles will occur during digestion. Some bacteria have been shown to break down plastics through enzymes called oxygenases, which can add oxygen to long carbon chains. Such a modification destabilizes the local electric charge, and provokes plastic breakdown. However, these oxygenase enzymes are not widely distributed, because they may also destroy molecules in the bacteria that carry them.103 Using neutral, carboxylated and aminated PS particles of 20 and 200 nm,104 showed that particles formed aggregates when in contact with human saliva. Mucins (MUC7 and MUC5B) from saliva can bind to the surface of PS particles (more to the 200 nm than to the 20 nm particles). Diffusion in the saliva was higher for aminated or carboxylated 20 nm PS, as compared to larger 200 nm particles, whereas no size-dependent difference was observed with neutral PS.104 Stock et al. showed a strong attachment of gastro-intestinal biological compounds, which however did not affect particle structure and size.105 Probably the most important parameter that digestion could affect is the formation of the protein corona: Walczak et al. found that in vitro digestion affected the composition of protein corona and induced a shift towards proteins with lower molecular weight.106 This may substantially affect biological interactions and intestinal uptake of particles. In vitro analyses not considering digestion may therefore over- or underestimate particle uptake under real-life conditions.
Beside their participation in the formation of a protein corona on the surface of the particles, the intestinal mucins also form the first barrier on the top of the epithelial cells of the gastrointestinal tract. The pore size of mucus gels is around 100 to 200 nm.107 Lai et al. showed that 500 and 200 nm PS particles coated with polyethylene glycol, diffused through physiological, even though not gastrointestinal, human mucus.108 The impact of the mucin layer along the gastrointestinal tract has been evaluated by Norris and Sinko. They concluded that larger PS microspheres (>0.5 μm) have a limited ability to diffuse through the mucin layer and that surface ionization and hydrophobicity are important for microsphere translocation through a mucin layer. However, due to differences of viscosity between young and adult organisms (piglets compared to pigs), it was shown that negatively charged, carboxy-functionalized, PS model particles diffused more into the intestinal mucus of piglets than of pigs.109 According to the results of Ensign et al. the mucus from the mouse small intestine is permeable to larger nanoparticles than mucus from the colon (100 nm compared to 200 nm).110 Additionally, also the surface properties of the polymer particles determine the interaction with the mucus as well as the underlying cells.111
Uptake and transport at the gastrointestinal epithelium
This section reviews the available information about the uptake of plastic particles into gastrointestinal epithelial cells, transport via the intestinal barrier, and bioavailability. Although most studies ignore the aspect of protein corona formation, it could be assumed that orally ingested particles have a corona consisting of proteins and other surrounding molecules that attached to the surface during their path though the environment and first contact with digestive fluids. For particles used in in vitro models this corona probably consists of proteins from the cell culture media and therefore may be different from environmental samples.
Prior to reaching the epithelium of the intestine, which is considered the main region of nutrition absorption, orally ingested plastic particles pass the epithelium of the stomach. For this type of cells there is at least one in vitro study available showing a size-dependent uptake of PS particles into gastric adenocarcinoma cells.112
The intestinal tissue functions as a biological barrier in order to prevent systemic distribution of harmful substances. In general, the crossing of the intestinal barrier by plastic or other particles occurs in a size-dependent manner. For the fate of plastic particles entering the intestine, different scenarios can be proposed:
(1) The particles stay in the lumen. In this case, a possible health impact of such particles can thus still occur by mechanically disrupting the cellular layer, by local irritation of the intestinal tissue, or by acting as a local depot to release toxic pollutants.
(2) The particles cross the epithelium through the paracellular route (through the tight junctions or via persorption, which is the passage of the intestinal epithelial by using gaps between the intestinal epithelial cells).
(3) The particles are taken up into the enterocytes of the intestinal epithelium and potentially cross the intestinal barrier by leaving the cells on the basolateral side.
(4) The particles can be taken up by cell types of the intestinal epithelium other than enterocytes and thereby reach the basolateral side.
(5) It can be also expected that some particles will not cross into the bloodstream but stay inside the intestinal cells. Such particles are likely to be released into the gut lumen at the end of the life cycle of the intestinal cell (around 72 h).
In principle, the uptake and transport of particles up to a size of maximum 5 to 10 μm into intestinal cells appears possible. Keeping in mind that previous contact with intestinal fluids can cause agglomeration of particles, like discussed in the previous section, this may influence the uptake compared to the pristine single particles. An intracellular uptake of larger particles would be incompatible with the size of intestinal epithelial cells of about 10 μm. EFSA is considering that microplastics have a very limited bioavailability of less than 0.3%, and only plastic particles smaller than 150 μm in size might in principle cross the intestinal epithelium. They are occasionally found in tissue but are likely unreactive and get deposited without being systemically bioavailable. Only much smaller particles up to 1.5 μm could be distributed systemically. Studies with differently sized PS particles show systemic bioavailability in rats with a presence in liver and spleen, with an inverse correlation between uptake and particle size.113 The smallest particles in the submicron range showed the highest uptake and transport, while particles with a size of 3 μm were present in the liver but not in the spleen or the bloodstream. A systemic distribution of nano-scaled plastic particles is likely.113–115 Cellular uptake of particles with the size of 10 μm or more could occur in specialized cells, such as macrophages.116,117 Notably the uptake of particles into cells of the intestinal tissue without subsequent transport into the bloodstream does not lead to systemic distribution. Nevertheless, a paracellular transport of particles can be imagined. The gut can exhibit leakages in the intestinal cell monolayer, mainly at the villi tips, and by using these gaps in the epithelium a transport of bigger particles to the portal blood vessels is possible. This process is called persorption118 and can lead to a crossing of the barrier by particles that are much bigger than the uptake limits of the cells. Several studies indicate a transport of bigger particles, as documented by the occurrence of bigger plastic particles in the liver and further remote organs like lymph nodes and spleen.113,117 Avio et al. found plastic particles with sizes between 200 and 600 μm in the gut and, to a lower extent, in the liver of fishes.85 Collard and colleagues detected micron-sized particles up to 438 μm in the livers of anchovies.119 By contrast, many other studies detected plastic particles only in the stomach and intestinal tissue of wild fishes.120–122
So far, there are a few studies published using in vitro systems to investigate the uptake of plastic particles. Most experiments have been performed with PS and on the cell line Caco-2 and thereof-derived co-cultures. Caco-2 is a well-established model for human enterocytes, which was developed in the 1970s.123 These cells spontaneously differentiate and form a monolayer suitable for transport studies when cultured on permeable membranes.124–126 Kulkarni & Feng showed a clear cellular uptake of fluorescent PS of different sizes (from 25 to 500 nm) by Caco-2 cells, except for 500 nm where only few particles were detected. The highest uptake efficiency was observed for 100 nm PS particles. Moreover, TPGS (d-α-tocopheryl polyethylene glycol 1000 succinate, emulsifier from medical use) coating increased the uptake efficiency for all sizes. Longer incubation time also led to higher cellular uptake efficiency.127 Abdelkhaliq et al. exposed Caco-2 cells for 24 h to negatively charged PS particles (50 and 200 nm), functionalized with sulfone or carboxyl groups at concentrations up to 250 μg mL−1. The nanoparticles were partially internalized into the lysosomes of Caco-2 cells. The largest extent of transport occurred with the 50 nm and sulfonated particles (13.9%), while it was low with the 50 nm and carboxylated particles (2.82%), and nearly undetectable (<1%) with the other particles.128 Magri et al. also investigated the cellular uptake of nanoparticles in Caco-2 cells, but used PET particles that where approximately 100 nm in size produced by laser ablation. Monitoring of the particle uptake into Caco-2 cells with 30 μg mL−1 PET for up to 24 h revealed a linear increase of cellular uptake. Additionally, a small amount of the particles was transported through the Caco-2 monolayer after 24 h of incubation, and the transported amount increased during nine days of exposure. In summary, the results indicate a small translocation of PET nanoparticles through the intestinal barrier.129 The uptake of carboxylated PS particles of different sizes (20, 100 and 200 nm) in Caco-2 cell monolayers was decreased when particles were incubated in the presence of bovine serum albumin (BSA) and casein, irrespective of the nanoparticle size. It was suggested that the contact of the nanoparticles with the cell surface was diminished due to the enhanced stability of the nanoparticle colloidal solution. With meat extract, the uptake was slightly increased only for 20 nm nanoparticles. Additionally, the smaller (20 and 100 nm) nanoparticles incubated with intestinal fluid showed a more pronounced attachment to the cells, while the 200 nm particles exhibited a slightly reduced binding.58 After contact with saliva, differently coated PS particles were not equally internalized by non-keratinized buccal cells: the uptake of 20 nm PS and carboxy- or amino-functionalized 200 nm PS was low (less than 1% after 1 h), while the non-functionalized 200 nm PS were more effectively taken up (around 3% after 1 h).104 Irrespective of the charge of the particle coating, the translocation of 50 and 100 nm PS particles was increased after contact of particles with an in vitro digestion system.106 Comparing Caco-2, Caco-2/HT29-MTX (mucus-producing) and Caco-2/HT29-MTX/Raji B cultures (mucus-producing and M-cell-building), Walczak et al. (2015) highlighted that the translocation of neutral, positively and negatively-charged 50 and 100 nm PS particles differs depending on the model, but did never exceed 10%. The translocation was lower with bigger particles (100 nm) irrespective of the coating of PS and the cell model. In both Caco-2 monocultures and Caco-2/HT29-MTX/Raji B triple-cultures, neutral 50 nm PS nanoparticles were translocated to a higher extent than negatively charged amine-PS and negatively charged carboxylated PS. No or very low translocation with another negatively charged carboxylated PS was observed.130 A similar co-culture was used by Domenech et al. for pristine PS particles in the range of 0.05 to 0.1 μm in size. They found a translocation as well as an uptake into the cells as well as into the nucleus.131 Similarly, Stock et al. investigated the uptake of fluorescent PS microparticles in Caco-2 alone or in co-culture with HT29-MTX or Raji B incubated for 24 h with either 1 μm, 4 μm particles (each 1 × 108 mL) or 10 μm particles (3 × 106 mL). The highest uptake into Caco-2 cells was seen for 4 μm particles. Small differences in intracellular uptake between the co-culture models were observed within the smallest particle size. Uptake was, surprisingly, significantly higher in the mucus model.132 Hesler et al. reported the translocation of 50 nm and 500 nm carboxylated PS particles in a mucus-producing model (Caco-2 and HT29-MTX co-culture), but found no significant transport of nanoparticles through the intestinal barrier. Using this in vitro model, the cells were exposed to 10 and 100 μg mL−1 of PS particles for 24 h. No transport was detected, but internalization of some particles was visible using confocal microscopy.133
Several years before the issue of microplastics reached the public focus, in vivo uptake studies with PS particles ranging from 50 nm to 3 μm showed systemic bioavailability of these particles, especially in the submicron diameter range.113 More recently, the bioaccumulation of PS microparticles in different organs of mice exposed during 4 weeks lead to a very different conclusion. Results of a study by Deng et al. published in 2017, where mice were fed with 5 μm and 20 μm diameter PS particles at daily concentrations of 0.01, 0.1 and 0.5 mg per day for up to 28 days proclaimed high uptake and systemic distribution. Deng and colleagues detected both particle types in the liver, gut and kidney, which reached a steady-state after 14 days of oral treatment.134 However, these results are scientifically disputed, as they imply an oral bioavailability of 100% of the cumulative applied dose.135,136 In contrast, Stock et al. conducted a 28 day mice feeding study with oral gavage of microplastics three times per week, consisting of a mixture of 1 μm (4.55 × 107 particles), 4 μm (4.55 × 107 particles) and 10 μm (1.49 × 106 particles) carboxylated polystyrene particles. Different organs were examined three days after the last dosage. Only a very small amount of plastic particles was detected in the jejunum and duodenum, but not in other organs such as kidney, spleen or liver. Further in vitro experiments confirmed the plausibility of these findings.132 Studies with animals suggest a low oral bioavailability of microplastic particles, thus implying substantial fecal excretion. In fact, excretion of ingested micro- and nanoplastics was estimated to more than 90% through feces, depending on some physicochemical characteristics including size, shape, and chemical composition,5,137 comparable to what is known from non-soluble nanoparticles of non-plastic origin.138 Recently, the detection of microplastics in human feces (on average 2 microplastic particles per g feces) has been reported.139 Up to nine different types of plastics, sized between 50 and 500 μm, were found in human feces, with PP and PET being most common. It needs to be noted that the detection of particles in feces just indicates the oral uptake of plastic particles but does not justify conclusions about the ingested dose or the oral bioavailability.
Effect of diseases on the intestinal absorption of microplastics
Diseases can alter the intestinal barrier and thereby affect the crossing of particles, their systemic bioavailability and potential toxicity. Using mutant Caenorhabditis elegans, Qu et al. have shown that PS nanoparticles (around 100 nm) can be translocated to systemic organs. In wild-type nematodes, the particles did not affect the functional state of intestinal barrier.140 Induction of diabetes in rats did not affect the uptake of 2 μm microplastic particles in epithelial cells, but clearly reduced their transepithelial passage across the GI tract and the translocation to secondary organs.141 On the contrary,142 did not detect differences of 2 μm size PS particle uptake between normal and immunodeficient mice with disorganized mucosa-associated lymphoid tissue (MALT). This is plausible, as the passage of the particles occurred through the villous epithelium not affected in the mouse model used. Other findings highlight that nano- and microparticles can have a different behavior at the inflamed intestinal mucosa (e.g. in Crohn's disease and ulcerative colitis): Poly(l-lactide-co-glycolide) (PLGA) microparticles, which are biodegradable polymers used to produce pharmaceuticals with depot function, distinctively accumulated in ulcerous lesions, while nanoparticles were only present in traces.143,144 However, contrary to microparticles, the translocation of nanoparticles to the serosal compartment was significantly increased in ulcerative colitis mucosae.143 Therefore, the behavior of nano- and microplastics in connection with specific diseases deserves further attention.
Toxicity of micro- and nanoplastics in the intestine
Usually plastic polymers are considered to be inert and therefore of possess only low chemical reactivity. Toxicological studies often relate to the toxicity of additives or remnant monomers.145 conducted a comprehensive hazard ranking of plastic polymers based on their chemical composition. They studied 55 of the most widely used polymer types with global production volumes > 10 000 tons per year. A model for ranking the hazard of each polymer was developed according to the monomer chemicals that form the polymer. The polymers classified as most hazardous were those produced from monomers classified as carcinogenic, mutagenic or both. Hazard classification data was mainly obtained from Annex VI of the EU classification, labelling and packaging (CLP) regulation which is based on the UN Globally Harmonized System (GHS). This approach led to a high ranking for polyurethanes, polyvinylchloride, epoxy resins and styrene polymers. However, the lack of safety data for many of the listed substances was pointed out by the authors. In particular, no hazard classification was available for chemicals suspected of being endocrine disruptors, including bisphenol A, phthalates, and epichlorohydrin. This toxicity endpoint was therefore not included in the hazard assessment.65,145 The toxic effects of microplastics have been investigated in numerous aquatic species and inflammation, genotoxicity and oxidative stress responses have been pointed out.146 However, data on effects in mammalian systems are limited.5
Except from work with PS particles, only few in vitro toxicity studies with micro- or nanoplastics have been conducted so far and are summarized in Table 1. In 2018, Abdelkhaliq et al. reported effects of PS particles varying in size (50 nm and 200 nm) and in surface (carboxylated or sulfonated) on Caco-2 cells. No cytotoxicity (WST-1 assay, 24 h treatment) of PS particles was observed at a concentration of 250 mg mL−1.128 Similarly, no impact on Caco-2 cell viability was measured with 1 to 30 μg mL−1 laser-ablated approximately 100 nm PET particles.129 Moreover, the authors did not show indication of inflammation up to 24 h of incubation. In accordance with the findings above, Hesler et al. (2019) did also report the absence of toxicity at concentrations below 100 μg mL−1 PS particles after 24 h of incubation.133 When treating Caco-2 cells and Caco-2-based co-cultures with 1, 4 and 10 μm PS particles, functionalized with carboxy- or sulfone-groups, a significant decrease of Caco-2 cell viability was only measured at very high concentrations of 1 μm PS particles (1 × 108 particles per mL) in a combined CTB/MTT-assay after 48 h of incubation. Furthermore, the macrophage cell-line THP-1 was used to investigate macrophage polarization after particle exposure. No impact on cell polarization or differentiation into macrophages was observed.132 Another study with an inverted cell culture system showed cytotoxicity of low-density PE microparticles on HepG2 cells only in overload situations above 25 mg mL−1.147 After 12 h of incubation with 0.1 and 5 μm PS particles up to 200 μg mL−1, no impact on Caco-2 cell viability and only weak toxic effects with respect to oxidative stress and membrane integrity were observed. Disruption of the mitochondrial membrane potential (stronger with 5 μm particles) and inhibition of plasma membrane-located ATP binding cassette (ABC) transporter activity (stronger with 0.1 μm particles) were reported.148 The most complex coculture model was used by Lehner et al., combining Caco-2/HT29-MTX cells with human blood monocyte-derived macrophages and dendritic cells. This model was used to investigate different polymers like PP, tire rubber, polyamide (PA) and PU by incubation via dry powder insufflator system to aerosolize the particles directly on the intestinal model's surface. They did not find any cytotoxicity, release of inflammatory cytokines or changes of the barrier integrity.149
Summary of the reviewed literature using in vitro models to study the effect of micro- and nanoplastics.
| Reference | Cell model & particles used | Results |
|---|---|---|
| Toxic effects | ||
| Wu et al., 2019 (ref. 148) | - Caco-2 cell model | - No cytotoxicity |
| - PS: 100 & 500 nm | - Weak toxic effects on oxidative stress and membrane integrity | |
| - 1–200 μg mL−1 | - Disruption of mitochondrial membrane potential, especially with 500 nm PS | |
| - Inhibition of plasma membrane-located ABC transporter in 100 nm PS | ||
| No toxic effects | ||
| Magri et al., 2018 (ref. 129) | - Caco-2 cell model | - No cytotoxicity, no LDH release |
| - PET: 100 nm, laser ablated, different characteristics | - Cellular uptake near lysosomes | |
| - 1-30 μg mL−1 | - After 24 h transport across Caco-2 layer was visible, 3% transported | |
| - No toxic effects after 24 h | ||
| Stock et al., 2019 (ref. 132) | - Caco-2 monoculture | - Toxicity only in unphysiological concentrations with 1 μm PS(−) |
| - Caco-2/Raji B M-cell model | ||
| - Caco-2/HT29-MTX mucus model | - Minimal uptake, highest with 4 μm (max. 3%), small differences between models | |
| - PS: 1 μm, carboxylated | ||
| - PS: 4 & 10 μm, sulfonated | - Macrophage polarization → no impacts on polarization or differentiation | |
| - 1 × 103 to 1 × 1010 μm2 surface particles per mL | ||
| Hesler et al., 2019 (ref. 133) | - Caco-2/HT29-MTX mucus model | - No cytotoxicity |
| - PS: 50 & 500 nm | - No significant transport across barrier | |
| - Carboxylated | - Intercellular distribution of particles | |
| - 0.01-100 μg mL−1 | - Cellular uptake: Internalized cells were visible with electron microscopy | |
| Abdelkhaliq et al., 2018 (ref. 128) | - Caco-2 cell model | - No cytotoxicity |
| - PS: 50 nm & 200 nm | - Minimal transport, ranging from 2.82% (50 nm (carboxylated)) to 13.9% (50 nm (sulfonated)) | |
| - Carboxylated or sulfonated | - Composition of protein corona & surface of PS influence cellular uptake and transport | |
| - 15–250 μg mL−1 | ||
| Stock et al., 2020 (ref. 147) | - Inversed cell culture model for low-density particles with HepG2 | - Cytotoxicity only in overload situations |
| - PE: polydisperse | ||
| - 25-100 μg mL−1 | ||
| Lehner et al., 2020 (ref. 149) | - Caco-2/HT29-MTX co-culture with human blood monocyte-derived macrophages and dendritic cells | - No cytotoxicity |
| - PP, PU, PA, tire rubber polydisperse | - No release of inflammatory cytokines | |
| - No changes in barrier integrity | ||
The in vivo data are also limited with some scientifically disputed results. To get an overview, in vivo studies are depicted in Table 2. In a 28 day study, mice were fed134 with 5 and 20 μm polystyrene particles at daily doses of 0.01, 0.1 and 0.5 mg. The mice showed a decrease of relative liver weight with liver inflammation and accumulation of lipid droplets. Dose-dependent effects (from 0.01 to 0.5 mg per day) were noticed on several markers related to energy metabolism, lipid metabolism and oxidative stress although not much difference between the effects of the different particle sizes were detected. Decreasing ATP levels and a rising release of LDH were measured as parameters indicating liver toxicity. Alterations in metabolic profiles were also reported. The authors suggested that ingested microplastics could affect food absorption, inhibit food digestion and may affect neurotransmission. However, the implausibility of plastic particle uptake data in this study strongly questions the validity of the results.135 In the study by Stock et al., heme oxygenase-1 triple transgenic (HOTT) reporter mice were treated orally three times per week for a total period of 4 weeks with a mixture of 1, 4 and 10 μm PS particles. These mice express the LacZ gene that is under control of the Hmox1 (heme oxygenase 1) promoter thereby providing a reporter system potentially detecting oxidative stress and inflammation. The intestine (duodenum, jejunum, ileum, colon), kidney, spleen, liver, testes, lung and heart were examined, but no positive responses were observed.132 In contrast, Lu et al. reported effects on hepatic lipid metabolism in mice after exposure to 0.5 and 50 μm PS microplastics through drinking water for 5 weeks at concentrations of 100 and 1000 μg L−1. They observed a decrease of body weight, liver weight, serum triglycerides and total cholesterol as well as expression of key genes involved in triglyceride synthesis. Furthermore, in intestine, mucus secretion was decreased and gut microbiota composition was impaired (at the phylum and genus levels). Similar results were published by the same group (Jin et al. in 2019) with mice exposed to 1000 μg L−1 5 μm PS microplastics for 6 weeks via drinking water. Accumulation of particles in the gut, decrease of mucus secretion, dysfunction of the intestinal barrier, gut microbiota dysbiosis and impairment of bile acid metabolism were reported. Unfortunately, in these two studies, the individual exposure could not be established as the volume of water intake was not documented or controlled.150,151 The effects of PS particles were also investigated in pregnant mice.152 The animals were exposed via drinking water to 100 and 1000 μg L−1 PS particles with 0.5 and 5 μm in size during gestation and serum and liver samples were analyzed in F1 offspring. Changes in serum levels and hepatic markers (serum and total cholesterol, serum and total triglycerides, HDL and LDL levels) as well as fatty acid and amino acid metabolism changes were observed. The bigger particles caused stronger effects. Average body weights of the animals were not altered.152 In a second study performed by the same group, the dams as well as the F1 and F2 offspring were analyzed after maternal exposure of mice to 5 μm PS particles during gestation and lactation. Again, changes in liver metabolism, gut microbiota composition and gut barrier function were observed. Further analyses in the offspring also showed metabolic disorders in the liver and changes in serum biochemistry in the F1 generation as well as long-term effects on the metabolism of lipids (after 280 days). They also found some effects in F2 offspring and concluded long-lasting consequences of maternal PS exposure.153 In both studies, the individual consumption of PS particles was not reported or controlled. Other studies showing no effect should not be misinterpreted, because the absence of evidence is not evidence of absence, like emphasized by Leslie et al.154 Furthermore, dose–response relationships need to be taken into account when trying to translate the findings from high-dose animal studies to humans which are likely to be exposed to considerably lower levels of such particles. Moreover, more detailed controls are necessary to exclude the possibility that the effect could be based on substances from the solvent used for the particle dispersions, monomers or contaminants. Currently, most studies use particles as delivered or even treat them with ultrasounds.
Summary of the reviewed literature using in vivo models to study the effect of micro- and nanoplastics.
| Reference | Study & particles used | Main findings | Comments |
|---|---|---|---|
| Toxic effects | |||
| Deng et al., 2018 (ref. 134) | - 28 days mice study | - Tissue accumulation in liver, kidney, gut | - For critical comments refer to 135 and 136 |
| - PS 5 & 20 μm | - Inflammation and lipid accumulation in liver | ||
| - 0.01, 0.1, 0.5 mg per day | |||
| - Daily oral gavage | - Oxidative stress in liver | ||
| Lu et al., 2018 (ref. 150) | - 5 week mice study | - Hepatic metabolism & gut microbiota disorder | |
| - PS: 0.5 & 50 μm | - Decrease of colonic mucus secretion & serum triglycerides | - Partly from the same authors like Luo et al., 2019 | |
| - 100 & 1000 μg L−1 (about 1.456 × 1010 particles per L for 0.5 μm and 1.456 × 104 particles per L for 50 μm) | |||
| - Direct drinking with continuous exposure for 5 weeks | |||
| - Particles were used as received | - Water input not detected, unknown amount of PS intake | ||
| - Basic diet | |||
| Jin et al., 2019 (ref. 151) | - 6 week mice study | - Increase of bile acids and their metabolites in liver | |
| - PS: 5 μm | - Decrease of mucus secretion in colon | ||
| - 100 μg L−1 (approximately 1.456 × 106 particles per L) 1000 μg L−1 (approximately 1.456 × 107 particles per L) | - Control group received water | ||
| - Direct drinking with continuous exposure for 6 weeks | |||
| - Particles were used as received, stock solutions were treated with ultrasound for 30 min | |||
| - Basic diet | |||
| Luo et al., 2019 (ref. 152) | - Mice study with maternal & offspring (F1, F2) mice | - Changes in serum and hepatic markers | - Partly from the same authors like Lu et al., 2018 and Jin et al., 2019 |
| - PS: 0.5 & 5 μm | - Fatty acid and metabolic disorders in F1 offsprings | - Water input not detected, unknown amount of PS intake | |
| - 100 & 1000 μg L−1 | - Gut microbiota dysbiosis and barrier dysfunction | ||
| - Maternal exposure during gestation (from GD 0 to production day) through drinking water, F1 & F2: No gavage | - Unknown feed supply | ||
| - Particles were used as received, stock solutions were treated with ultrasound for 30 min | |||
| Luo et al., 2019 (ref. 153) | - PS: 5 μm | - Maternal metabolic disorder associated with gut microbiota dysbiosis and gut barrier dysfunction | - Control group received water |
| - 100 & 1000 μg L−1 | |||
| - Maternal exposure during pregnancy and lactation (∼6 weeks) through drinking water, F1 & F2: No gavage | |||
| - Particles were used as received, stock solutions were treated with ultrasound for 30 min | - Long-term metabolic consequences in the F1 and F2 generations | ||
| No toxic effects | |||
| Stock et al., 2019 (ref. 132) | - 28 days HOTT mice study | - Minor uptake in intestinal cells | - Different type of particle administration |
| - PS mixture: 1, 4 & 10 μm | - Mice had altered genetic background | ||
| - 4.55 × 107 & 1.49 × 106 particles, 10 mL per kg per bw | - No bioaccumulation in different tissues | ||
| - Oral gavage 3× per week | - No inflammation, oxidative stress or toxic effects | ||
Conclusion
In summary, risk assessment of micro- and nanoplastics is still not possible, due to various data gaps in terms of exposure, biodistribution and related effects. Nevertheless, some perspectives to target these open questions have been opened in the recent years. For exposure assessment, existing analytical methods, which are already used in nanotoxicological research, need to be adapted and validated for micro- and especially nanoplastics, particularly with respect to a quantification in complex matrices such as food products. In principle, oral exposure of consumers to microplastics is considered as certain. A passage of a low percentage of ingested particles through the gastrointestinal barrier appears possible, especially for smaller and smallest particles. Cellular toxicity-related effects have rarely been detected, mainly in overload situations and with rather unspecific endpoints. The overall number of studies is still very limited. In vitro studies with model particles often used extremely high concentrations and in vivo studies provided weak results, which do not allow an evaluation that withstands critical considerations. Neither dose–response relationships can be established, nor can molecular initiating events be proclaimed which would allow generating adverse outcome pathways as a helpful tool to integrate mechanistic data into risk assessment. Long-term and chronic studies are missing, too. Continuous efforts are required to obtain relevant data to address gaps in the risk assessment of micro- and nanoplastics found in human diets.
Abbreviations
- ABS
Acrylonitrile butadiene styrene
- BSA
Bovine serum albumin
- CLP
Classification, labelling and packaging of chemicals, EU
- CTB
Cell titer blue
- ECHA
European chemicals agency
- EFSA
European food safety authority
- FTIR
Fourier-transform infrared spectroscopy
- GHS
UN globally harmonized system
- HDL
High-density lipoprotein
- HOTT
Heme oxygenase-1 triple transgenic
- LDH
Lactate dehydrogenase
- LDL
Low-density lipoprotein
- MALT
Mucosa-associated lymphoid tissue
- MTT
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- NIR
Near-infrared
- PA
Polyamide
- PE
Polyethylene
- PEA
Polyesteracetal
- PET
Polyethylene terephthalate
- PLGA
Poly(l-lactide-co-glycolide)
- PP
Polypropylene
- PS
Polystyrene
- PU
Polyurethane
- PVC
Polyvinyl chloride
- REACH
Registration, evaluation and authorization of chemicals
Conflicts of interest
There are no conflicts to declare.
Supplementary Material
Acknowledgments
The authors thank Pr Alfonso Lampen for helpful discussions on the topic of microplastics. This work was supported by a Maria-Sibylla-Merian fellowship of the German Federal Institute for Risk Assessment awarded to V. Fessard (2018–2020), by European Food Safety Authority (EU-FORA fellowship program) and by the German Federal Institute for Risk Assessment (Project 1323-102).
References
- PlasticsEurope, www.plasticseurope.org/de/resources/publications/1804-plastics-facts-20192019
- Gewert B. Plassmann M. M. MacLeod M. Pathways for degradation of plastic polymers floating in the marine environment. Environ. Sci.: Processes Impacts. 2015;17(9):1513–1521. doi: 10.1039/C5EM00207A. [DOI] [PubMed] [Google Scholar]
- Worldwatch-Institute, Plastic Pollution, The Impact on our oceans and what we can do about it, Slo ACtive, 2018, https://sloactive.com/plastic-pollution/#uvembed60837
- da Costa J. P. Santos P. S. Duarte A. C. Rocha-Santos T. (Nano) plastics in the environment–sources, fates and effects. Sci. Total Environ. 2016;566:15–26. doi: 10.1016/j.scitotenv.2016.05.041. [DOI] [PubMed] [Google Scholar]
- EFSA Presence of microplastics and nanoplastics in food, with particular focus on seafood. EFSA Journal. 2016;14(6):e04501. [Google Scholar]
- Hardy A. Benford D. Halldorsson T. Jeger M. J. Knutsen H. K. More S. et al., Guidance on risk assessment of the application of nanoscience and nanotechnologies in the food and feed chain: Part 1, human and animal health. EFSA Journal. 2018;16(7):e05327. doi: 10.2903/j.efsa.2018.5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auta H. Emenike C. Fauziah S. Distribution and importance of microplastics in the marine environment: a review of the sources, fate, effects, and potential solutions. Environ. Int. 2017;102:165–176. doi: 10.1016/j.envint.2017.02.013. [DOI] [PubMed] [Google Scholar]
- Horton A. A. Walton A. Spurgeon D. J. Lahive E. Svendsen C. Microplastics in freshwater and terrestrial environments: evaluating the current understanding to identify the knowledge gaps and future research priorities. Sci. Total Environ. 2017;586:127–141. doi: 10.1016/j.scitotenv.2017.01.190. [DOI] [PubMed] [Google Scholar]
- Cox K. D. Covernton G. A. Davies H. L. Dower J. F. Juanes F. Dudas S. E. Human Consumption of Microplastics. Environ Sci Technol. 2019;53(12):7068–7074. doi: 10.1021/acs.est.9b01517. [DOI] [PubMed] [Google Scholar]
- Kutralam-Muniasamy G. Pérez-Guevara F. Elizalde-Martínez I. Shruti V. Branded milks–Are they immune from microplastics contamination? Sci. Total Environ. 2020;714:136823. doi: 10.1016/j.scitotenv.2020.136823. [DOI] [PubMed] [Google Scholar]
- RIVM, Factsheet over microplastics in Nederlandse wateren, https://wwwrivmnl/en/microplastics, 2019
- Bujnicki J., Dykstra P., Fortunato E., Grobert N., Heuer R. and Keskitalo C., et al., Environmental and health risks of microplastic pollution, Directorate-General for Research and Innovation, European Commission, Group of Chief Scientific Advisors, 2019, vol. 6 [Google Scholar]
- Schwaferts C. Niessner R. Elsner M. Ivleva N. P. Methods for the analysis of submicrometer-and nanoplastic particles in the environment. TrAC Trends in Analytical Chemistry. 2019;112:52–65. doi: 10.1016/j.trac.2018.12.014. [DOI] [Google Scholar]
- Braun U., Jekel M., Gerdts G., Ileva N. and Reiber J., Mikroplastik-Analytik: Probenahme, Probenaufbereitung und Detektionsverfahren, https://bmbf-plastikde/sites/default/files/2018-10/Diskussionspapier%20Mikroplastik-Analytikpdf, 2018
- Möller J. N. Löder M. G. Laforsch C. Finding Microplastics in Soils: A Review of Analytical Methods. Environ. Sci. Technol. 2020;54(4):2078–2090. doi: 10.1021/acs.est.9b04618. [DOI] [PubMed] [Google Scholar]
- Nguyen B. Claveau-Mallet D. Hernandez L. M. Xu E. G. Farner J. M. Tufenkji N. Separation and analysis of microplastics and nanoplastics in complex environmental samples. Acc. Chem. Res. 2019;52(4):858–866. doi: 10.1021/acs.accounts.8b00602. [DOI] [PubMed] [Google Scholar]
- Oßmann B. E. Sarau G. Schmitt S. W. Holtmannspötter H. Christiansen S. H. Dicke W. Development of an optimal filter substrate for the identification of small microplastic particles in food by micro-Raman spectroscopy. Anal. Bioanal. Chem. 2017;409(16):4099–4109. doi: 10.1007/s00216-017-0358-y. [DOI] [PubMed] [Google Scholar]
- Schwaferts C. Sogne V. Welz R. Meier F. Klein T. Niessner R. et al., Nanoplastic Analysis by Online Coupling of Raman Microscopy and Field-Flow Fractionation Enabled by Optical Tweezers. Anal. Chem. 2020;92(8):5813–5820. doi: 10.1021/acs.analchem.9b05336. [DOI] [PubMed] [Google Scholar]
- Shim W. J. Hong S. H. Eo S. E. Identification methods in microplastic analysis: a review. Analytical Methods. 2017;9(9):1384–1391. doi: 10.1039/C6AY02558G. [DOI] [Google Scholar]
- Silva A. B. Bastos A. S. Justino C. I. da Costa J. P. Duarte A. C. Rocha-Santos T. A. Microplastics in the environment: Challenges in analytical chemistry-A review. Anal. Chim. Acta. 2018;1017:1–19. doi: 10.1016/j.aca.2018.02.043. [DOI] [PubMed] [Google Scholar]
- Paul A. Wander L. Becker R. Goedecke C. Braun U. High-throughput NIR spectroscopic (NIRS) detection of microplastics in soil. Environmental Science and Pollution Research. 2019;26(8):7364–7374. doi: 10.1007/s11356-018-2180-2. [DOI] [PubMed] [Google Scholar]
- Dehaut A. Hermabessiere L. Duflos G. Current frontiers and recommendations for the study of microplastics in seafood. TrAC Trends in Analytical Chemistry. 2019;116:346–359. doi: 10.1016/j.trac.2018.11.011. [DOI] [Google Scholar]
- Correia M. Loeschner K. Detection of nanoplastics in food by asymmetric flow field-flow fractionation coupled to multi-angle light scattering: possibilities, challenges and analytical limitations. Anal. Bioanal. Chem. 2018;410(22):5603–5615. doi: 10.1007/s00216-018-0919-8. [DOI] [PubMed] [Google Scholar]
- Catarino A. I. Frutos A. Henry T. B. Use of fluorescent-labelled nanoplastics (NPs) to demonstrate NP absorption is inconclusive without adequate controls. Sci. Total Environ. 2019;670:915–920. doi: 10.1016/j.scitotenv.2019.03.194. [DOI] [PubMed] [Google Scholar]
- Balakrishnan G. Déniel M. Nicolai T. Chassenieux C. Lagarde F. Towards more realistic reference microplastics and nanoplastics: preparation of polyethylene micro/nanoparticles with a biosurfactant. Environmental Science: Nano. 2019;6(1):315–324. [Google Scholar]
- von der Esch E. Lanzinger M. Kohles A. J. Schwaferts C. Weisser J. Hofmann T. et al., Simple Generation of Suspensible Secondary Microplastic Reference Particles via Ultrasound Treatment. Frontiers in Chemistry. 2020;8(169):1–15. doi: 10.3389/fchem.2020.00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandl F. Thünemann A. F. Beuermann S. Poly(meth)acrylate-PVDF core–shell particles from emulsion polymerization: preferential formation of the PVDF β crystal phase. Polymer Chemistry. 2018;9(44):5359–5369. doi: 10.1039/C8PY01236A. [DOI] [Google Scholar]
- Hurley R. R. Nizzetto L. Fate and occurrence of micro(nano)plastics in soils: Knowledge gaps and possible risks. Current Opinion in Environmental Science & Health. 2018;1:6–11. [Google Scholar]
- Landva A. O. and Clark J. I., Geotechnics of waste fill, Geotechnics of waste fills—Theory and practice, ASTM International, 1990 [Google Scholar]
- Tokiwa Y. Calabia B. P. Ugwu C. U. Aiba S. Biodegradability of plastics. Int. J. Mol. Sci. 2009;10(9):3722–3742. doi: 10.3390/ijms10093722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J. R. Polymer ageing: physics, chemistry or engineering? Time to reflect. Comptes Rendus Chimie. 2006;9(11–12):1396–1408. doi: 10.1016/j.crci.2006.07.008. [DOI] [Google Scholar]
- Galgani L. Beiras R. Galgani F. Panti C. Borja A. Editorial: Impacts of Marine Litter. Frontiers in Marine Science. 2019;6(208):1–4. [Google Scholar]
- Albertsson A.-C. Andersson S. O. Karlsson S. The mechanism of biodegradation of polyethylene. Polymer degradation and stability. 1987;18(1):73–87. doi: 10.1016/0141-3910(87)90084-X. [DOI] [Google Scholar]
- Highlights in chemistry and physics of polymer stabilization, ed. Pospíšil J and Nešpůrek S, Macromolecular Symposia, Wiley Online Library, 1997 [Google Scholar]
- Lambert S., Sinclair C. and Boxall A., Occurrence, degradation, and effect of polymer-based materials in the environment, Reviews of Environmental Contamination and Toxicology, Springer, 2014, vol. 227, pp. 1–53 [DOI] [PubMed] [Google Scholar]
- Brandon J. Goldstein M. Ohman M. D. Long-term aging and degradation of microplastic particles: Comparing in situ oceanic and experimental weathering patterns. Marine Pollution Bulletin. 2016;110(1):299–308. doi: 10.1016/j.marpolbul.2016.06.048. [DOI] [PubMed] [Google Scholar]
- Ter Halle A. Ladirat L. Martignac M. Mingotaud A. F. Boyron O. Perez E. To what extent are microplastics from the open ocean weathered? Environmental Pollution. 2017;227:167–174. doi: 10.1016/j.envpol.2017.04.051. [DOI] [PubMed] [Google Scholar]
- Guo X. Wang J. The chemical behaviors of microplastics in marine environment: A review. Marine Pollution Bulletin. 2019;142:1–14. doi: 10.1016/j.marpolbul.2019.03.019. [DOI] [PubMed] [Google Scholar]
- Galloway T. S. Cole M. Lewis C. Interactions of microplastic debris throughout the marine ecosystem. Nature Ecology & Evolution. 2017;1(5):1–8. doi: 10.1038/s41559-017-0116. [DOI] [PubMed] [Google Scholar]
- Wolfram J. Yang Y. Shen J. Moten A. Chen C. Shen H. et al., The nano-plasma interface: Implications of the protein corona. Colloids Surf. B Biointerfaces. 2014;124:17–24. doi: 10.1016/j.colsurfb.2014.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundqvist M. Stigler J. Elia G. Lynch I. Cedervall T. Dawson K. A. Nanoparticle size and surface properties determine the protein corona with possible implications for biological impacts. Proc. Natl. Acad. Sci. U.S.A. 2008;105(38):14265–14270. doi: 10.1073/pnas.0805135105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peula J. M. Hidalgo-Alvarez R. de las Nieves FJ. Coadsorption of IgG and BSA onto sulfonated polystyrene latex: I. Sequential and competitive coadsorption isotherms. J Biomater Sci Polym Ed. 1995;7(3):231–240. doi: 10.1163/156856295X00274. [DOI] [PubMed] [Google Scholar]
- Tripisciano C. Eichhorn T. Harm S. Weber V. Adsorption of the inflammatory mediator high-mobility group box 1 by polymers with different charge and porosity. Biomed Res Int. 2014;2014:238160. doi: 10.1155/2014/238160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gessner A. Lieske A. Paulke B. Müller R. Influence of surface charge density on protein adsorption on polymeric nanoparticles: analysis by two-dimensional electrophoresis. Eur J Pharm Biopharm. 2002;54(2):165–170. doi: 10.1016/S0939-6411(02)00081-4. [DOI] [PubMed] [Google Scholar]
- Allémann E. Gravel P. Leroux J. C. Balant L. Gurny R. Kinetics of blood component adsorption on poly(D,L-lactic acid) nanoparticles: evidence of complement C3 component involvement. J Biomed Mater Res. 1997;37(2):229–234. doi: 10.1002/(SICI)1097-4636(199711)37:2<229::AID-JBM12>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Staufenbiel S. Merino M. Li W. Huang M. D. Baudis S. Lendlein A. et al., Surface characterization and protein interaction of a series of model poly[acrylonitrile-co-(N-vinyl pyrrolidone)] nanocarriers for drug targeting. Int J Pharm. 2015;485(1–2):87–96. doi: 10.1016/j.ijpharm.2015.02.072. [DOI] [PubMed] [Google Scholar]
- Dai Q. Guo J. Yan Y. Ang C. S. Bertleff-Zieschang N. Caruso F. Cell-Conditioned Protein Coronas on Engineered Particles Influence Immune Responses. Biomacromolecules. 2017;18(2):431–439. doi: 10.1021/acs.biomac.6b01545. [DOI] [PubMed] [Google Scholar]
- Buyukserin F. Camli S. T. Yavuz M. S. Budak G. G. Novel antifouling oligo(ethylene glycol) methacrylate particles via surfactant-free emulsion polymerization. Journal of Colloid and Interface Science. 2011;355(1):76–80. doi: 10.1016/j.jcis.2010.11.081. [DOI] [PubMed] [Google Scholar]
- Böhmert L. Voß L. Stock V. Braeuning A. Lampen A. Sieg H. Isolation methods for particle protein corona complexes from protein-rich matrices. Nanoscale Advances. 2020;2(2):563–582. doi: 10.1039/C9NA00537D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira C. L. Veiga F. Varela C. Roleira F. Tavares E. Silveira I. et al., Characterization of polymeric nanoparticles for intravenous delivery: Focus on stability. Colloids Surf B Biointerfaces. 2017;150:326–333. doi: 10.1016/j.colsurfb.2016.10.046. [DOI] [PubMed] [Google Scholar]
- Nagayama S. Ogawara K. Fukuoka Y. Higaki K. Kimura T. Time-dependent changes in opsonin amount associated on nanoparticles alter their hepatic uptake characteristics. Int J Pharm. 2007;342(1–2):215–221. doi: 10.1016/j.ijpharm.2007.04.036. [DOI] [PubMed] [Google Scholar]
- Stolnik S. Daudali B. Arien A. Whetstone J. Heald C. R. Garnett M. C. et al., The effect of surface coverage and conformation of poly(ethylene oxide) (PEO) chains of poloxamer 407 on the biological fate of model colloidal drug carriers. Biochimica et Biophysica Acta (BBA) - Biomembranes. 2001;1514(2):261–279. doi: 10.1016/S0005-2736(01)00376-5. [DOI] [PubMed] [Google Scholar]
- Zhang H. Burnum K. E. Luna M. L. Petritis B. O. Kim J. S. Qian W. J. et al., Quantitative proteomics analysis of adsorbed plasma proteins classifies nanoparticles with different surface properties and size. Proteomics. 2011;11(23):4569–4577. doi: 10.1002/pmic.201100037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H. Wu T. Yu W. Ruan S. He Q. Gao H. Ligand Size and Conformation Affect the Behavior of Nanoparticles Coated with in Vitro and in Vivo Protein Corona. ACS Appl Mater Interfaces. 2018;10(10):9094–9103. doi: 10.1021/acsami.7b16096. [DOI] [PubMed] [Google Scholar]
- Bertrand N. Grenier P. Mahmoudi M. Lima E. M. Appel E. A. Dormont F. et al., Mechanistic understanding of in vivo protein corona formation on polymeric nanoparticles and impact on pharmacokinetics. Nat Commun. 2017;8(1):777. doi: 10.1038/s41467-017-00600-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller L. K. Simon J. Rosenauer C. Mailänder V. Morsbach S. Landfester K. The Transferability from Animal Models to Humans: Challenges Regarding Aggregation and Protein Corona Formation of Nanoparticles. Biomacromolecules. 2018;19(2):374–385. doi: 10.1021/acs.biomac.7b01472. [DOI] [PubMed] [Google Scholar]
- Walczak A. P. Kramer E. Hendriksen P. J. Helsdingen R. van der Zande M. Rietjens I. M. et al., In vitro gastrointestinal digestion increases the translocation of polystyrene nanoparticles in an in vitro intestinal co-culture model. Nanotoxicology. 2015;9(7):886–894. doi: 10.3109/17435390.2014.988664. [DOI] [PubMed] [Google Scholar]
- Sinnecker H. Ramaker K. Frey A. Coating with luminal gut-constituents alters adherence of nanoparticles to intestinal epithelial cells. Beilstein journal of nanotechnology. 2014;5(1):2308–2315. doi: 10.3762/bjnano.5.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lück M. Paulke B.-R. Schröder W. Blunk T. Müller R. H. Analysis of plasma protein adsorption on polymeric nanoparticles with different surface characteristics. Journal of Biomedical Materials Research. 1998;39(3):478–485. doi: 10.1002/(SICI)1097-4636(19980305)39:3<478::AID-JBM19>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Cho W. S. Thielbeer F. Duffin R. Johansson E. M. Megson I. L. MacNee W. et al., Surface functionalization affects the zeta potential, coronal stability and membranolytic activity of polymeric nanoparticles. Nanotoxicology. 2014;8(2):202–211. doi: 10.3109/17435390.2013.773465. [DOI] [PubMed] [Google Scholar]
- Lundqvist M. Stigler J. Cedervall T. Berggård T. Flanagan M. B. Lynch I. et al., The evolution of the protein corona around nanoparticles: a test study. ACS Nano. 2011;5(9):7503–7509. doi: 10.1021/nn202458g. [DOI] [PubMed] [Google Scholar]
- Vroom R. J. Koelmans A. A. Besseling E. Halsband C. Aging of microplastics promotes their ingestion by marine zooplankton. Environmental Pollution. 2017;231:987–996. doi: 10.1016/j.envpol.2017.08.088. [DOI] [PubMed] [Google Scholar]
- Oberbeckmann S. Labrenz M. Marine Microbial Assemblages on Microplastics: Diversity, Adaptation, and Role in Degradation. Ann Rev Mar Sci. 2020;12:209–232. doi: 10.1146/annurev-marine-010419-010633. [DOI] [PubMed] [Google Scholar]
- ECHA, Plastic Additives Initiative, https://echaeuropaeu/de/mapping-exercise-plastic-additives-initiative, 2016
- Galloway T. S.. Micro-and nano-plastics and human health, Marine anthropogenic litter, Springer, Cham, 2015, pp. 343–66 [Google Scholar]
- Teuten E. L. Saquing J. M. Knappe D. R. Barlaz M. A. Jonsson S. Björn A. et al., Transport and release of chemicals from plastics to the environment and to wildlife. Philosophical Transactions of the Royal Society B: Biological Sciences. 2009;364(1526):2027–2045. doi: 10.1098/rstb.2008.0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koelmans A. A. Besseling E. Foekema E. M. Leaching of plastic additives to marine organisms. Environmental Pollution. 2014;187:49–54. doi: 10.1016/j.envpol.2013.12.013. [DOI] [PubMed] [Google Scholar]
- Hsiao I. L. Hsieh Y. K. Wang C. F. Chen I. C. Huang Y. J. Trojan-horse mechanism in the cellular uptake of silver nanoparticles verified by direct intra- and extracellular silver speciation analysis. Environ Sci Technol. 2015;49(6):3813–3821. doi: 10.1021/es504705p. [DOI] [PubMed] [Google Scholar]
- Liu L. Fokkink R. Koelmans A. A. Sorption of polycyclic aromatic hydrocarbons to polystyrene nanoplastic. Environmental toxicology and chemistry. 2016;35(7):1650–1655. doi: 10.1002/etc.3311. [DOI] [PubMed] [Google Scholar]
- Gouin T. Roche N. Lohmann R. Hodges G. A thermodynamic approach for assessing the environmental exposure of chemicals absorbed to microplastic. Environmental Science & Technology. 2011;45(4):1466–1472. doi: 10.1021/es1032025. [DOI] [PubMed] [Google Scholar]
- Hartmann N. B. Rist S. Bodin J. Jensen L. H. Schmidt S. N. Mayer P. et al., Microplastics as vectors for environmental contaminants: exploring sorption, desorption, and transfer to biota. Integrated environmental assessment and management. 2017;13(3):488–493. doi: 10.1002/ieam.1904. [DOI] [PubMed] [Google Scholar]
- Rochman C. M. Hoh E. Hentschel B. T. Kaye S. Long-term field measurement of sorption of organic contaminants to five types of plastic pellets: implications for plastic marine debris. Environmental Science & Technology. 2013;47(3):1646–1654. doi: 10.1021/es303700s. [DOI] [PubMed] [Google Scholar]
- Fries E. Zarfl C. Sorption of polycyclic aromatic hydrocarbons (PAHs) to low and high density polyethylene (PE) Environmental Science and Pollution Research. 2012;19(4):1296–1304. doi: 10.1007/s11356-011-0655-5. [DOI] [PubMed] [Google Scholar]
- Lee H. Shim W. J. Kwon J.-H. Sorption capacity of plastic debris for hydrophobic organic chemicals. Science of the Total Environment. 2014;470:1545–1552. doi: 10.1016/j.scitotenv.2013.08.023. [DOI] [PubMed] [Google Scholar]
- Velzeboer I. Kwadijk C. Koelmans A. Strong sorption of PCBs to nanoplastics, microplastics, carbon nanotubes, and fullerenes. Environmental Science & Technology. 2014;48(9):4869–4876. doi: 10.1021/es405721v. [DOI] [PubMed] [Google Scholar]
- Brennecke D. Duarte B. Paiva F. Caçador I. Canning-Clode J. Microplastics as vector for heavy metal contamination from the marine environment. Estuarine, Coastal and Shelf Science. 2016;178:189–195. doi: 10.1016/j.ecss.2015.12.003. [DOI] [Google Scholar]
- Almeida C. M. R. Manjate E. Ramos S. Adsorption of Cd and Cu to different types of microplastics in estuarine salt marsh medium. Marine Pollution Bulletin. 2020;151:110797. doi: 10.1016/j.marpolbul.2019.110797. [DOI] [PubMed] [Google Scholar]
- Zou J. Liu X. Zhang D. Yuan X. Adsorption of three bivalent metals by four chemical distinct microplastics. Chemosphere. 2020;248:126064. doi: 10.1016/j.chemosphere.2020.126064. [DOI] [PubMed] [Google Scholar]
- Fang X. Vitrac O. Predicting diffusion coefficients of chemicals in and through packaging materials. Critical Reviews in Food Science and Nutrition. 2017;57(2):275–312. doi: 10.1080/10408398.2013.849654. [DOI] [PubMed] [Google Scholar]
- Gerdes Z. Ogonowski M. Nybom I. Ek C. Adolfsson-Erici M. Barth A. et al., Microplastic-mediated transport of PCBs? A depuration study with Daphnia magna. PloS one. 2019:14. doi: 10.1371/journal.pone.0205378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinteich J. Seidensticker S. Marggrander N. Zarfl C. Microplastics reduce short-term effects of environmental contaminants. Part II: polyethylene particles decrease the effect of polycyclic aromatic hydrocarbons on microorganisms. International Journal of Environmental Research and Public Health. 2018;15(2):287. doi: 10.3390/ijerph15020287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batel A. Linti F. Scherer M. Erdinger L. Braunbeck T. Transfer of benzo [a] pyrene from microplastics to Artemia nauplii and further to zebrafish via a trophic food web experiment: CYP1A induction and visual tracking of persistent organic pollutants. Environmental Toxicology and Chemistry. 2016;35(7):1656–1666. doi: 10.1002/etc.3361. [DOI] [PubMed] [Google Scholar]
- Hüffer T. Weniger A.-K. Hofmann T. Sorption of organic compounds by aged polystyrene microplastic particles. Environmental Pollution. 2018;236:218–225. doi: 10.1016/j.envpol.2018.01.022. [DOI] [PubMed] [Google Scholar]
- Koelmans A. A. Bakir A. Burton G. A. Janssen C. R. Microplastic as a vector for chemicals in the aquatic environment: critical review and model-supported reinterpretation of empirical studies. Environmental Science & Technology. 2016;50(7):3315–3326. doi: 10.1021/acs.est.5b06069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avio C. G. Gorbi S. Regoli F. Experimental development of a new protocol for extraction and characterization of microplastics in fish tissues: first observations in commercial species from Adriatic Sea. Marine Environmental Research. 2015;111:18–26. doi: 10.1016/j.marenvres.2015.06.014. [DOI] [PubMed] [Google Scholar]
- Waring R. H. Harris R. M. Mitchell S. C. Plastic contamination of the food chain: A threat to human health? Maturitas. 2018;115:64–68. doi: 10.1016/j.maturitas.2018.06.010. [DOI] [PubMed] [Google Scholar]
- Van Cauwenberghe L. Janssen C. R. Microplastics in bivalves cultured for human consumption. Environmental Pollution. 2014;193:65–70. doi: 10.1016/j.envpol.2014.06.010. [DOI] [PubMed] [Google Scholar]
- Liebezeit G. Liebezeit E. Non-pollen particulates in honey and sugar. Food Addit. Contam., Part A: Chem., Anal., Control, Exposure Risk Assess. 2013;30(12):2136–2140. doi: 10.1080/19440049.2013.843025. [DOI] [PubMed] [Google Scholar]
- Liebezeit G. Liebezeit E. Synthetic particles as contaminants in German beers. Food Addit. Contam., Part A: Chem., Anal., Control, Exposure Risk Assess. 2014;31(9):1574–1578. doi: 10.1080/19440049.2014.945099. [DOI] [PubMed] [Google Scholar]
- Karami A. Golieskardi A. Keong Choo C. Larat V. Galloway T. S. Salamatinia B. The presence of microplastics in commercial salts from different countries. Sci Rep. 2017;7:46173. doi: 10.1038/srep46173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D. Shi H. Li L. Li J. Jabeen K. Kolandhasamy P. Microplastic Pollution in Table Salts from China. Environ Sci Technol. 2015;49(22):13622–13627. doi: 10.1021/acs.est.5b03163. [DOI] [PubMed] [Google Scholar]
- Gündoğdu S. Contamination of table salts from Turkey with microplastics. Food Addit. Contam., Part A: Chem., Anal., Control, Exposure Risk Assess. 2018;35(5):1006–1014. doi: 10.1080/19440049.2018.1447694. [DOI] [PubMed] [Google Scholar]
- Kosuth M. Mason S. A. Wattenberg E. V. Anthropogenic contamination of tap water, beer, and sea salt. PloS One. 2018;13(4):e0194970. doi: 10.1371/journal.pone.0194970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schymanski D. Goldbeck C. Humpf H. U. Fürst P. Analysis of microplastics in water by micro-Raman spectroscopy: Release of plastic particles from different packaging into mineral water. Water Res. 2018;129:154–162. doi: 10.1016/j.watres.2017.11.011. [DOI] [PubMed] [Google Scholar]
- Oßmann B. E. Sarau G. Holtmannspötter H. Pischetsrieder M. Christiansen S. H. Dicke W. Small-sized microplastics and pigmented particles in bottled mineral water. Water Res. 2018;141:307–316. doi: 10.1016/j.watres.2018.05.027. [DOI] [PubMed] [Google Scholar]
- Mason S. A. Welch V. G. Neratko J. Synthetic Polymer Contamination in Bottled Water. Front Chem. 2018;6:407. doi: 10.3389/fchem.2018.00407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintenig S. M. Löder M. G. J. Primpke S. Gerdts G. Low numbers of microplastics detected in drinking water from ground water sources. Sci. Total Environ. 2019;648:631–635. doi: 10.1016/j.scitotenv.2018.08.178. [DOI] [PubMed] [Google Scholar]
- Shruti V. Pérez-Guevara F. Elizalde-Martínez I. Kutralam-Muniasamy G. First study of its kind on the microplastic contamination of soft drinks, cold tea and energy drinks-Future research and environmental considerations. Sci. Total Environ. 2020:138580. doi: 10.1016/j.scitotenv.2020.138580. [DOI] [PubMed] [Google Scholar]
- Kedzierski M. Lechat B. Sire O. Le Maguer G. Le Tilly V. Bruzaud S. Microplastic contamination of packaged meat: Occurrence and associated risks. Food Packaging and Shelf Life. 2020;24:100489. doi: 10.1016/j.fpsl.2020.100489. [DOI] [Google Scholar]
- Conti G. O. Ferrante M. Banni M. Favara C. Nicolosi I. Cristaldi A. et al., Micro-and nano-plastics in edible fruit and vegetables. The first diet risks assessment for the general population. Environmental Research. 2020:109677. doi: 10.1016/j.envres.2020.109677. [DOI] [PubMed] [Google Scholar]
- Schwabl P. Köppel S. Königshofer P. Bucsics T. Trauner M. Reiberger T. et al., Detection of Various Microplastics in Human Stool: A Prospective Case Series. Ann Intern Med. 2019;171(7):453–457. doi: 10.7326/M19-0618. [DOI] [PubMed] [Google Scholar]
- Smith M. Love D. C. Rochman C. M. Neff R. A. Microplastics in Seafood and the Implications for Human Health. Curr. Environ. Health Rep. 2018;5(3):375–386. doi: 10.1007/s40572-018-0206-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S. K. Pal S. Ray S. Study of microbes having potentiality for biodegradation of plastics. Environ. Sci. Pollut. Res. 2013;20(7):4339–4355. doi: 10.1007/s11356-013-1706-x. [DOI] [PubMed] [Google Scholar]
- Teubl B. J. Stojkovic B. Docter D. Pritz E. Leitinger G. Poberaj I. et al., The effect of saliva on the fate of nanoparticles. Clin. Oral Invest. 2018;22(2):929–940. doi: 10.1007/s00784-017-2172-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock V. Fahrenson C. Thuenemann A. Donmez M. H. Voss L. Bohmert L. et al., Impact of artificial digestion on the sizes and shapes of microplastic particles. Food Chem. Toxicol. 2020;135:111010. doi: 10.1016/j.fct.2019.111010. [DOI] [PubMed] [Google Scholar]
- Walczak A. P. Kramer E. Hendriksen P. J. Helsdingen R. van der Zande M. Rietjens I. M. et al., In vitro gastrointestinal digestion increases the translocation of polystyrene nanoparticles in an in vitro intestinal co-culture model. Nanotoxicology. 2015;9(7):886–894. doi: 10.3109/17435390.2014.988664. [DOI] [PubMed] [Google Scholar]
- Pearson J. P. Chater P. I. Wilcox M. D. The properties of the mucus barrier, a unique gel–how can nanoparticles cross it? Therapeutic Delivery. 2016;7(4):229–244. doi: 10.4155/tde-2015-0002. [DOI] [PubMed] [Google Scholar]
- Lai S. K. O'Hanlon D. E. Harrold S. Man S. T. Wang Y.-Y. Cone R. et al., Rapid transport of large polymeric nanoparticles in fresh undiluted human mucus. Proc. Natl. Acad. Sci. U.S.A. 2007;104(5):1482–1487. doi: 10.1073/pnas.0608611104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris D. A. Sinko P. J. Effect of size, surface charge, and hydrophobicity on the translocation of polystyrene microspheres through gastrointestinal mucin. J. Appl. Polym. Sci. 1997;63(11):1481–1492. doi: 10.1002/(SICI)1097-4628(19970314)63:11<1481::AID-APP10>3.0.CO;2-5. [DOI] [Google Scholar]
- Ensign L. M. Henning A. Schneider C. S. Maisel K. Wang Y. Y. Porosoff M. D. et al., Ex vivo characterization of particle transport in mucus secretions coating freshly excised mucosal tissues. Mol Pharm. 2013;10(6):2176–2182. doi: 10.1021/mp400087y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inkielewicz-Stepniak I. Tajber L. Behan G. Zhang H. Radomski M. W. Medina C. et al., The role of mucin in the toxicological impact of polystyrene nanoparticles. Materials. 2018;11(5):724. doi: 10.3390/ma11050724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forte M. Iachetta G. Tussellino M. Carotenuto R. Prisco M. De Falco M. et al., Polystyrene nanoparticles internalization in human gastric adenocarcinoma cells. Toxicology in Vitro. 2016;31:126–136. doi: 10.1016/j.tiv.2015.11.006. [DOI] [PubMed] [Google Scholar]
- Jani P. Halbert G. W. Langridge J. Florence A. T. Nanoparticle uptake by the rat gastrointestinal mucosa: quantitation and particle size dependency. J. Pharm. Pharmacol. 1990;42(12):821–826. doi: 10.1111/j.2042-7158.1990.tb07033.x. [DOI] [PubMed] [Google Scholar]
- Walczak A. P. Hendriksen P. J. Woutersen R. A. van der Zande M. Undas A. K. Helsdingen R. et al., Bioavailability and biodistribution of differently charged polystyrene nanoparticles upon oral exposure in rats. J. Nanoparticle Res. 2015;17(5):231. doi: 10.1007/s11051-015-3029-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarlo K. Blackburn K. L. Clark E. D. Grothaus J. Chaney J. Neu S. et al., Tissue distribution of 20 nm, 100 nm and 1000 nm fluorescent polystyrene latex nanospheres following acute systemic or acute and repeat airway exposure in the rat. Toxicology. 2009;263(2–3):117–126. doi: 10.1016/j.tox.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Hasegawa T. Hirota K. Tomoda K. Ito F. Inagawa H. Kochi C. et al., Phagocytic activity of alveolar macrophages toward polystyrene latex microspheres and PLGA microspheres loaded with anti-tuberculosis agent. Colloids Surf. B Biointerfaces. 2007;60(2):221–228. doi: 10.1016/j.colsurfb.2007.06.017. [DOI] [PubMed] [Google Scholar]
- Tomazic-Jezic V. J. Merritt K. Umbreit T. H. Significance of the type and the size of biomaterial particles on phagocytosis and tissue distribution. J. Biomed. Mater. Res. 2001;55(4):523–529. doi: 10.1002/1097-4636(20010615)55:4<523::AID-JBM1045>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Volkheimer G. Passage of particles through the wall of the gastrointestinal tract. Environ. Health Perspect. 1974;9:215–225. doi: 10.1289/ehp.749215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collard F. Gilbert B. Compère P. Eppe G. Das K. Jauniaux T. et al., Microplastics in livers of European anchovies (Engraulis encrasicolus, L.) Environ. Pollut. 2017;229:1000–1005. doi: 10.1016/j.envpol.2017.07.089. [DOI] [PubMed] [Google Scholar]
- Choy C. A. Drazen J. C. Plastic for dinner? Observations of frequent debris ingestion by pelagic predatory fishes from the central North Pacific. Mar. Ecol.: Prog. Ser. 2013;485:155–163. doi: 10.3354/meps10342. [DOI] [Google Scholar]
- Davison P. Asch R. G. Plastic ingestion by mesopelagic fishes in the North Pacific Subtropical Gyre. Mar. Ecol.: Prog. Ser. 2011;432:173–180. doi: 10.3354/meps09142. [DOI] [Google Scholar]
- Boerger C. M. Lattin G. L. Moore S. L. Moore C. J. Plastic ingestion by planktivorous fishes in the North Pacific Central Gyre. Mar. Pollut. Bull. 2010;60(12):2275–2278. doi: 10.1016/j.marpolbul.2010.08.007. [DOI] [PubMed] [Google Scholar]
- Fogh J. Fogh J. M. Orfeo T. One Hundred and Twenty-Seven Cultured Human Tumor Cell Lines Producing Tumores in Nude Mice. J. Natl. Cancer Inst. 1977;59(1):221–226. doi: 10.1093/jnci/59.1.221. [DOI] [PubMed] [Google Scholar]
- Pinto M. S. Robineleon S. Appay M. D. Kedinger N. Triadou N. Dussaulx E. et al., Enterocyte like differentiation and polarization of the human colon carcinoma cell line Caco-2 in culture. Biol Cell. 1983;(47):323–330. [Google Scholar]
- Hilgers A. R. Conradi R. A. Burton P. S. Caco-2 cell monolayers as a model for drug transport across the intestinal mucosa. Pharmaceut. Res. 1990;7(9):902–910. doi: 10.1023/A:1015937605100. [DOI] [PubMed] [Google Scholar]
- Buhrke T. Lengler I. Lampen A. Analysis of proteomic changes induced upon cellular differentiation of the human intestinal cell line Caco-2. Dev. Growth Differ. 2011;53(3):411–426. doi: 10.1111/j.1440-169X.2011.01258.x. [DOI] [PubMed] [Google Scholar]
- Kulkarni S. A. Feng S.-S. Effects of particle size and surface modification on cellular uptake and biodistribution of polymeric nanoparticles for drug delivery. Pharmaceut. Res. 2013;30(10):2512–2522. doi: 10.1007/s11095-012-0958-3. [DOI] [PubMed] [Google Scholar]
- Abdelkhaliq A. Van Der Zande M. Punt A. Helsdingen R. Boeren S. Vervoort J. J. et al., Impact of nanoparticle surface functionalization on the protein corona and cellular adhesion, uptake and transport. J. Nanobiotechnol. 2018;16(1):1–13. doi: 10.1186/s12951-018-0394-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magrì D. Sánchez-Moreno P. Caputo G. Gatto F. Veronesi M. Bardi G. et al., Laser ablation as a versatile tool to mimic polyethylene terephthalate nanoplastic pollutants: characterization and toxicology assessment. ACS Nano. 2018;12(8):7690–7700. doi: 10.1021/acsnano.8b01331. [DOI] [PubMed] [Google Scholar]
- Walczak A. P. Kramer E. Hendriksen P. J. Tromp P. Helsper J. P. van der Zande M. et al., Translocation of differently sized and charged polystyrene nanoparticles in in vitro intestinal cell models of increasing complexity. Nanotoxicology. 2015;9(4):453–461. doi: 10.3109/17435390.2014.944599. [DOI] [PubMed] [Google Scholar]
- Domenech J. Hernández A. Rubio L. Marcos R. Cortés C. Interactions of polystyrene nanoplastics with in vitro models of the human intestinal barrier. Archives of Toxicology. 2020;94:2997–3012. doi: 10.1007/s00204-020-02805-3. [DOI] [PubMed] [Google Scholar]
- Stock V. Böhmert L. Lisicki E. Block R. Cara-Carmona J. Pack L. K. et al., Uptake and effects of orally ingested polystyrene microplastic particles in vitro and in vivo. Archives of toxicology. 2019;93(7):1817–1833. doi: 10.1007/s00204-019-02478-7. [DOI] [PubMed] [Google Scholar]
- Hesler M. Aengenheister L. Ellinger B. Drexel R. Straskraba S. Jost C. et al., Multi-endpoint toxicological assessment of polystyrene nano-and microparticles in different biological models in vitro. Toxicology in Vitro. 2019;61:104610. doi: 10.1016/j.tiv.2019.104610. [DOI] [PubMed] [Google Scholar]
- Deng Y. Zhang Y. Lemos B. Ren H. Tissue accumulation of microplastics in mice and biomarker responses suggest widespread health risks of exposure. Sci. Rep. 2017;7:46687. doi: 10.1038/srep46687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braeuning A. Uptake of microplastics and related health effects: a critical discussion of Deng et al., Scientific reports 7: 46687, 2017. Arch. Toxicol. 2019;93(1):219–220. doi: 10.1007/s00204-018-2367-9. [DOI] [PubMed] [Google Scholar]
- Böhmert L. Stock V. Braeuning A. Plausibility of microplastic uptake in a paper by Deng et al., Scientific reports 7: 46687, 2017. Arch. Toxicol. 2019;93(1):217–218. doi: 10.1007/s00204-018-2383-9. [DOI] [PubMed] [Google Scholar]
- Wright S. L. Kelly F. J. Plastic and human health: a micro issue? Environ. Sci. Technol. 2017;51(12):6634–6647. doi: 10.1021/acs.est.7b00423. [DOI] [PubMed] [Google Scholar]
- Bouwmeester H. Hollman P. C. Peters R. J. Potential Health Impact of Environmentally Released Micro- and Nanoplastics in the Human Food Production Chain: Experiences from Nanotoxicology. Environ. Sci. Technol. 2015;49(15):8932–8947. doi: 10.1021/acs.est.5b01090. [DOI] [PubMed] [Google Scholar]
- Schwabl P. Köppel S. Königshofer P. Bucsics T. Trauner M. Reiberger T. et al., Detection of various microplastics in human stool: a prospective case series. Ann. Intern. Med. 2019;171(7):453–457. doi: 10.7326/M19-0618. [DOI] [PubMed] [Google Scholar]
- Qu M. Xu K. Li Y. Wong G. Wang D. Using acs-22 mutant Caenorhabditis elegans to detect the toxicity of nanopolystyrene particles. Sci. Total Environ. 2018;643:119–126. doi: 10.1016/j.scitotenv.2018.06.173. [DOI] [PubMed] [Google Scholar]
- McMinn L. H. Hodges G. M. Carr K. E. Gastrointestinal uptake and translocation of microparticles in the streptozotocin-diabetic rat. J. Anat. 1996;189(3):553–559. [PMC free article] [PubMed] [Google Scholar]
- Smyth S. H. Feldhaus S. Schumacher U. Carr K. E. Uptake of inert microparticles in normal and immune deficient mice. Int. J. Pharm. 2008;346(1):109–118. doi: 10.1016/j.ijpharm.2007.06.049. [DOI] [PubMed] [Google Scholar]
- Schmidt C. Lautenschlaeger C. Collnot E. M. Schumann M. Bojarski C. Schulzke J. D. et al., Nano- and microscaled particles for drug targeting to inflamed intestinal mucosa: a first in vivo study in human patients. J. Contr. Release. 2013;165(2):139–145. doi: 10.1016/j.jconrel.2012.10.019. [DOI] [PubMed] [Google Scholar]
- Makadia H. K. Siegel S. J. Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers. 2011;3(3):1377–1397. doi: 10.3390/polym3031377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lithner D. Larsson Å. Dave G. Environmental and health hazard ranking and assessment of plastic polymers based on chemical composition. Sci. Total Environ. 2011;409(18):3309–3324. doi: 10.1016/j.scitotenv.2011.04.038. [DOI] [PubMed] [Google Scholar]
- de Sá L. C. Oliveira M. Ribeiro F. Rocha T. L. Futter M. N. Studies of the effects of microplastics on aquatic organisms: What do we know and where should we focus our efforts in the future? Sci. Total Environ. 2018;645:1029–1039. doi: 10.1016/j.scitotenv.2018.07.207. [DOI] [PubMed] [Google Scholar]
- Stock V. Böhmert L. Dönmez M. H. Lampen A. Sieg H. An inverse cell culture model for floating plastic particles. Anal. Biochem. 2020;591:113545. doi: 10.1016/j.ab.2019.113545. [DOI] [PubMed] [Google Scholar]
- Wu B. Wu X. Liu S. Wang Z. Chen L. Size-dependent effects of polystyrene microplastics on cytotoxicity and efflux pump inhibition in human Caco-2cells. Chemosphere. 2019;221:333–341. doi: 10.1016/j.chemosphere.2019.01.056. [DOI] [PubMed] [Google Scholar]
- Lehner R. Wohlleben W. Septiadi D. Landsiedel R. Petri-Fink A. Rothen-Rutishauser B. A novel 3D intestine barrier model to study the immune response upon exposure to microplastics. Archives of Toxicology. 2020;94(7):2463–2479. doi: 10.1007/s00204-020-02750-1. [DOI] [PubMed] [Google Scholar]
- Lu L. Wan Z. Luo T. Fu Z. Jin Y. Polystyrene microplastics induce gut microbiota dysbiosis and hepatic lipid metabolism disorder in mice. Sci. Total Environ. 2018;631:449–458. doi: 10.1016/j.scitotenv.2018.03.051. [DOI] [PubMed] [Google Scholar]
- Jin Y. Lu L. Tu W. Luo T. Fu Z. Impacts of polystyrene microplastic on the gut barrier, microbiota and metabolism of mice. Sci. Total Environ. 2019;649:308–317. doi: 10.1016/j.scitotenv.2018.08.353. [DOI] [PubMed] [Google Scholar]
- Luo T. Zhang Y. Wang C. Wang X. Zhou J. Shen M. et al., Maternal exposure to different sizes of polystyrene microplastics during gestation causes metabolic disorders in their offspring. Environ. Pollut. 2019;255:113122. doi: 10.1016/j.envpol.2019.113122. [DOI] [PubMed] [Google Scholar]
- Luo T. Wang C. Pan Z. Jin C. Fu Z. Jin Y. Maternal Polystyrene Microplastic Exposure during Gestation and Lactation Altered Metabolic Homeostasis in the Dams and Their F1 and F2 Offspring. Environ. Sci. Technol. 2019;53(18):10978–10992. doi: 10.1021/acs.est.9b03191. [DOI] [PubMed] [Google Scholar]
- Leslie H. A. Depledge M. H. Where is the evidence that human exposure to microplastics is safe? Environ. Int. 2020;142:105807. doi: 10.1016/j.envint.2020.105807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toussaint B. Raffael B. Angers-Loustau A. Gilliland D. Kestens V. Petrillo M. et al., Review of micro- and nanoplastic contamination in the food chain. Food Additives & Contaminants Part A, Chemistry, Analysis, Control, Exposure & Risk Assessment. 2019;36(5):639–673. doi: 10.1080/19440049.2019.1583381. [DOI] [PubMed] [Google Scholar]