Abstract
Osteoarthritis is among the most prevalent of musculoskeletal disorders in the world that causes joint pain, deformity, and limited range of movement. The resulting osteochondral defect can significantly decrease the patient's quality of life, but current treatment options have not demonstrated the capacity to fully regenerate the entire osteochondral microenvironment. Structurally, the osteochondral unit is a composite system composed of three layers—articular cartilage, calcified cartilage, and subchondral bone. Collectively these distinct layers contribute to the distinct biomechanical properties that maintain the health and aid in load transfer during joint articulation. The purpose of this review was to examine the role of the osteochondral interface in tissue engineering. Topics of discussion include the biomechanics of the osteochondral unit and an overview of various strategies for osteochondral interface tissue engineering, with a specific focus on three-dimensional bioprinting. The goal of this review was to elucidate the importance of the osteochondral interface and overview some strategies of developing an interface layer within tissue engineered scaffolds.
Impact Statement
This review provides an overview of interface tissue engineering for osteochondral regeneration. It offers a detailed investigation into the biomechanics of the osteochondral unit as it relates to tissue engineering, and highlights the strategies that have been utilized to develop the osteochondral interface within tissue engineering scaffolds.
Keywords: osteochondral regeneration, tissue engineering, calcified cartilage, interface scaffold, 3D bioprinting
Introduction
Osteoarthritis is the most common joint disorder in the United States.1 The resulting osteochondral defects can cause joint pain, impaired function, deformity, and limited range of motion that can be debilitating and significantly influence the patient's quality of life.2 Full thickness osteochondral lesions can also result from traumatic injuries in young individuals, which can lead to joint pain and swelling in the absence of diffuse osteoarthritis. Although palliative and surgical treatments exist to mend osteochondral defects, these options have not demonstrated the capacity to fully regenerate healthy hyaline cartilage within the articulating joint.3 As a result, tissue engineering strategies have been explored to fully regenerate osteochondral defects.4
Structurally, the articular cartilage, calcified cartilage, and subchondral bone make up the osteochondral unit. This composite system possesses distinct biomechanical properties that facilitate the load transfer during joint articulation.5 However, when the osteochondral unit is compromised by trauma or pathology, the cartilage and subchondral bone experience structural changes that lead to further mechanical instability within the complex.6 Irreversible deterioration of this complex limits joint mobility and will perpetuate the onset of osteoarthritis that affects all structures within the region, which is characterized by structural changes such as joint space narrowing, osteophyte formation, and subchondral sclerosis.7 In addition to the alterations to the chondrocytes and cartilage matrix, the subchondral bone undergoes changes early in the disease process, although the exact mechanism involved in the process is unknown.8 Ultimately, the intricate balance between bone and cartilage influences biochemical and biomechanical changes experienced within the osteochondral unit.9
Therefore, to design a biomimetic osteochondral scaffold, the layer-specific structural and biomechanical properties have to be recapitulated within the scaffold. Three-dimensional (3D) printing is an additive manufacturing strategy that has been available since the 1980s, and it has been increasingly utilized for osteochondral tissue engineering.10 This fabrication strategy can replicate complex macroscale geometries using patient defect-specific scanning techniques with consistent microscale geometry while eliminating sample-to-sample variability.11 Therefore, this approach allows researchers to easily produce scaffolds with the complex geometries without encountering inconsistencies that arise when manufacturing on larger scales. This review aims to investigate the function of the osteochondral interface within tissue engineering scaffolds. We will overview the structure and biomechanics of the osteochondral unit and overview various strategies that have been utilized for interface tissue engineering for osteochondral regeneration. A particular focus of the review will be on recent studies that utilize extrusion-based 3D bioprinting in the development of an interface within osteochondral scaffolds.
Methods
PubMed through NCBI was utilized to gather and examine all cited sources published until July 1, 2021. The keywords utilized in conducting the database searches were 3D printing, calcified cartilage, hybrid composites, mechanical interlocking, osteochondral interface, osteochondral tissue engineering, polymer interface, scaffold, shear strength, and tissue engineering. References included were gathered through primary searches, articles known to the authors, or from citation lists contained in other literature reviews.
Structure and Biomechanics of Osteochondral Defects
The osteochondral unit possesses a distinct ability to transfer loads during joint movement (Fig. 1A, B).5 Because each layer of the osteochondral unit has its own unique hierarchical structure and biological properties, the composition, structure, and function of each layer has to be considered to design a biomimetic osteochondral scaffold.
FIG. 1.
(A) The articular cartilage is a complex structure subdivided into three distinct layers based on the arrangement of chondrocytes and orientation of collagen fibrils within each layer. The calcified cartilage is the critical interface lying between articular cartilage and subchondral bone that facilitates load transmission and limits diffusion of the contents from the subchondral bone to the deep cartilage layers. (B) Biomechanically, the articular cartilage provides a smooth, lubricated surface for articulation and helps transmit the loads with low friction. The calcified cartilage serves as the interface layer that adheres the articular cartilage to the subchondral bone, which enables a gradual stiffness transition and facilitates the stress distribution from cartilage to bone. Finally, the subchondral bone plays a critical role in maintaining healthy articular cartilage by tempering most of the load experienced within the osteochondral unit.
The principal function of articular cartilage is to enable low friction articulation and facilitate load transmission through the osteochondral unit. In addition to the lubricated surface, the articular cartilage also possesses unique viscoelastic properties to withstand high cyclic loads without degenerative change.12 Its overall composition can be thought of as a biphasic medium consisting of a fluid phase and a solid phase owing to its compositional nature.13 Two mechanisms have been identified to describe the viscoelasticity and mechanical behavior of articular cartilage.14,15 First, the flow-independent mechanism of viscoelasticity describes the viscoelastic behavior that arise from the collagen–proteoglycan interactions within the extracellular matrix (ECM).16 Conversely, the flow-dependent mechanism depends on the interstitial fluid and frictional drag resulting from the fluid flow.17
The flow-independent solid phase of articular cartilage consists of the porous and permeable ECM.17 As a viscoelastic structure, the articular cartilage displays a time-dependent behavior upon constant load.18 The boundaries of the osteochondral unit are designed to restrict mechanical deformation at the contact surface. When the articular cartilage is subjected to a constant compressive load, it demonstrates a creep and stress–relaxation response.17 As the cartilage deforms and is held at a constant strain, the stress experienced by the structure reaches a maximum. Upon the removal of strain, the stress relaxes within the structure and reaches an equilibrium. Therefore, articular cartilage cannot be defined by a single Young's modulus as it generally stiffens with increasing strain.19
The fluid phase of articular cartilage is mainly water that makes up to 80% of its wet weight.13 When the diarthrodial joint experiences a load, the interstitial fluid flows out of the ECM because of the rapid buildup of pressure during function, which will eventually flow back in upon the release of the load.20 The low permeability of articular cartilage slows down the interstitial fluid flow owing to the frictional drag resulting from the matrix.21 Ultimately, the fluid pressure plays a significant role in stress reduction and structural support experienced by the entire complex upon load. In addition, in conjunction with the solid phase, negative electrostatic repulsive forces help resist the compressive forces as the proteoglycan aggregates of the matrix interact with the interstitial fluid.22
The calcified cartilage serves as an interface layer to help adhere the articular cartilage to the subchondral bone.23 This layer enables a gradual stiffness transition from cartilage to bone that ultimately facilitates the stress distribution. Because the vertically positioned collagen fibers extend from the calcified cartilage layer into the intermediate region of articular cartilage, delamination owing to horizontal shear stress would occur just above this region.24 This specific matrix organization connects the relatively softer articular cartilage to the stiffer calcified cartilage and aids joint stress transmission throughout the osteochondral unit.25
Finally, the subchondral bone is responsible for the dispersion of axial loads across the osteochondral unit while preserving the articular cartilage above it.26 During regular joint movement, the subchondral bone dissipates the forces with ease because its capacity to deform is tenfold that of long bones.27 When the articular cartilage is under duress, the various layers of the arthrodial joint work together to facilitate support and force distribution. Because the subchondral bone interfaces with the articular cartilage through the calcified cartilage layer, the forces are transferred to the subchondral bone and the shear stresses in the cartilage layer are minimized.28 Accordingly, the trabeculae lying deeper within the subchondral bone helps dissipate the load across the layers of the joint.
Although subtle but detectable changes can be detected early on in subchondral bone, most osteochondral defects arising from the region are caused by pathologies causing significant lesions, such as osteosclerosis and osteochondritis dissecans.29 Ultimately, the functional coordination among the different layers capably offsets the forces imposed on the joint. Because joint motion is anatomically restricted by the joint shape and attachments of various ligaments, the dissipation of the stresses through the subchondral bone follows a distinctive pattern.30 As a muscle or tendon within the osteochondral unit contributes surface loads to the joint, they also proceed to neutralize the torque resulting from the ground reaction force that acts along the lever arm of the limb.27 Because the lever arm associated with the limb is considerably longer than that of the tendon, the tensile force of the tendon is greater than the corresponding ground reaction force that amplifies the contact forces within the joint.
If these joint forces persist over the long term, the joint will adapt to the repetitive stresses by bone remodeling.26 Although physiological loads help maintain joint homeostasis, abnormal joint loading resulting from obesity or trauma can precipitate the formation of osteoarthritis owing to heightened catabolic activity, chondrocyte apoptosis/necrosis, and damage to the surrounding collagen network.29 When the osteochondral unit begins to deteriorate because disease or trauma, the normal process of mechanical load transfer is compromised. Anderson et al. indicated that the shear stress levels increase within the deepest layers of articular cartilage upon degradative change within the osteochondral unit.31 The finite element data from the study suggested that the alterations in the juxtaarticular stress distribution could lead to concomitant cartilage thinning and subchondral bone thickening.
Current Treatment Options for Osteochondral Defects
Currently available treatment options for osteochondral defects are dictated by the severity of symptoms and defect size (Fig. 2). Initial treatment goals are palliative to manage pain, improve function, and control the structural deterioration of the affected joints.3 Patients have traditionally been managed noninvasively with analgesics, nonsteroidal anti-inflammatory drugs (NSAIDs), physical therapy, and weight loss exercise.32 Intra-articular injections of glucocorticoids, hyaluronic acid, and recently platelet-rich plasma (PRP) can also provide symptomatic relief. However, because these palliative treatment methods do not replace large damaged portions of the osteochondral unit, more invasive treatments are often sought to replace symptomatic partial or full-thickness defects.
FIG. 2.
Treatment strategies for osteochondral defects depend on the severity of the defect. The treatment options for early-stage osteochondral defects are generally palliative to manage pain and control the structural deterioration of the affected joints. Noninvasive palliative strategies generally involve physical therapy, analgesics, and NSAIDs. However, late-stage osteochondral defects require reparative treatment options that can replace partial or full-thickness osteochondral defects. Microfracture or mosaicplasty is often the first surgical procedure considered to repair the defect site of <2 cm. Osteochondral allografts are generally attempted as a single-stage technique to address large osteochondral defects involving extensive subchondral bone loss. NSAIDs, nonsteroidal anti-inflammatory drugs.
Marrow stimulation techniques, such as microfracture, are often the first surgical procedures considered to treat smaller defects (<2 cm2).33 Microfracture surgery is often considered for the initial intervention because it is minimally invasive, has a well-documented track record of success, and is relatively inexpensive compared with other surgical options.34 This procedure first involves the removal of the damaged cartilage and calcified cartilage layer. Subsequent drilling into the subchondral bone allows bone marrow and blood to permeate into the osteochondral region, which ultimately promotes repair and regeneration. However, the procedure is contraindicated when there is global cartilage degeneration, an open defect that cannot maintain clot formation, and inability to comply with weight-bearing restrictions after the procedure.35 Marrow stimulation is ideal for small, contained, and isolated osteochondral lesions.
Osteochondral autograft transfer is another well-established surgical technique to repair small to mid-sized cartilage defects.36 The procedure involves transplanting cylindrical autologous grafts extracted from the nonweight-bearing areas of the knee joint.37 Mosaicplasty is a similar surgical technique that implants multiple full-thickness “plugs” into a large-sized osteochondral lesion. As a one-stage procedure, mosaicplasty has demonstrated favorable success rates in treating full-thickness osteochondral defects of <2.5 cm2.38 One 10-year clinical study demonstrated that patients experience a poor outcome 40% of the time, but a subgroup involving younger men with a defect size <3 cm2 experienced a failure rate of only 12.5%.39 Hangody and Fules also observed good to excellent postoperative results in ∼90% of the patients who underwent femoral condyle or tibial plateau mosaicplasty for up to 10 years.36 Nonetheless, the outcomes still varied greatly because of age, gender, and defect size, as the failure rates were higher among women, individuals older than age 40, and those with large defects.39
Autologous chondrocyte implantation (ACI) is a surgical technique that harvests the patient's own chondrocytes to be implanted into osteochondral defects. Although this two-stage procedure is more surgically invasive and highly expensive, the first iteration of this procedure has demonstrated success in younger patients who possess a single defect exceeding 2 cm2.40 One long-term follow-up study demonstrated that treating large, full-thickness cartilage defects with first-generation ACI significantly improved pain relief and functional rehabilitation within patients.41 However, treatment complications, including periosteal hypertrophy, graft delamination, arthrofibrosis, and graft failure have led to a significant rate of reoperation after the initial procedure.42 Advances in various regenerative strategies have prompted the development of enhanced ACI techniques. Unlike first-generation ACI, matrix-assisted autologous chondrocyte implantation (MACI) involves the expansion and placement of autologous chondrocytes on a matrix, which is implanted inside the defect to assist osteochondral regeneration.43 The MACI procedure is technically easier to perform and has mostly replaced ACI in the United States. Although long-term data for MACI is still limited, Kreuz et al. recently demonstrated in a 12-year follow-up study that patients who received MACI exhibited significant increases in clinical outcome and function.44 In addition, >70% of the patients within the study reached near normal values upon radiological evaluation, further suggesting that MACI has great potential as a long-term cartilage repair procedure. However, ACI and MACI cannot address any bone deficiency and may not be the ideal treatment for defects with significant osseous involvement.
Finally, allografts provide surgeons with the flexibility to treat larger defects when the defect size is exceedingly large or previous surgical repair attempts have failed.45 Osteochondral allografts are generally attempted as an one-stage procedure to address large-sized defects and can also address extensive subchondral bone loss.46 Levy et al. achieved a 82% graft survivorship at 10 years in patients who underwent osteochondral allograft transplantation of the femoral condyle.47 However, the study also suggested that individuals younger than 30 years of age with no more than one prior surgical operation were the best candidates for allograft procedures. Old age, osteoarthritis, and bipolar allografts have all been suggested to increase the failure rate of these procedures.48,49 In addition, allograft transplantation have increased the risk of disease transmission from the allograft.50 As allografts become more common as a restorative procedure to treat knee pathologies, more investigations will be needed to better determine their viability of an osteochondral restorative option.
Fabrication of the Interface Layer Within Multiphasic Osteochondral Scaffolds
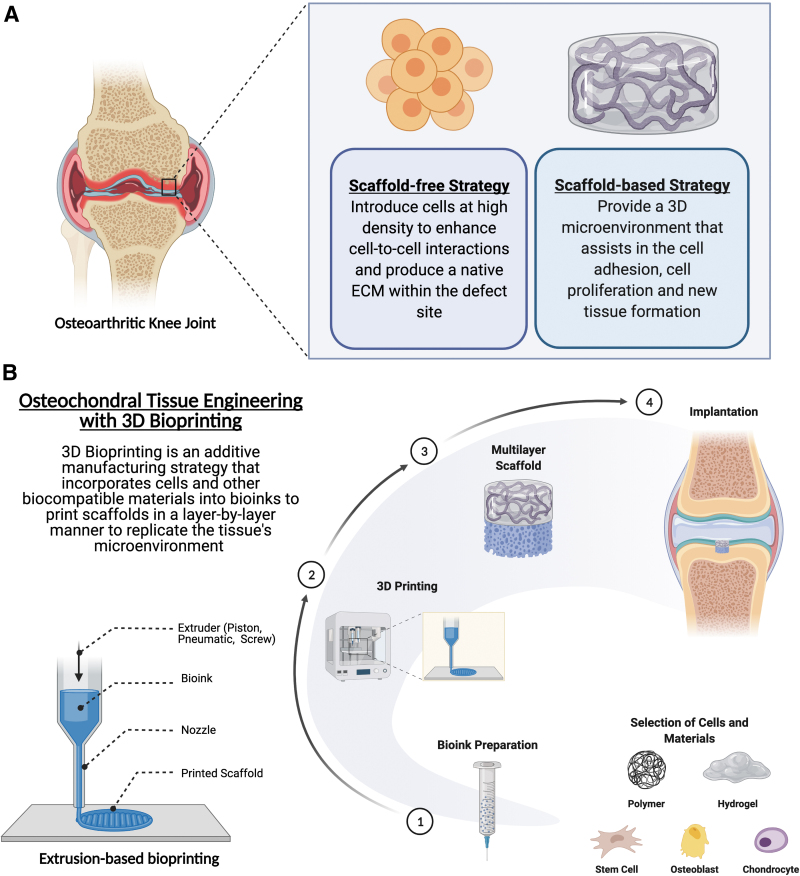
Tissue engineering is a promising strategy that could offer potential solutions to regenerate the osteochondral complex that current treatment solutions cannot accomplish (Fig. 3A). Cells, bioactive signals, and scaffolds generally make up the foundation of most osteochondral tissue engineering approaches.4 In general, most strategies are classified as either a scaffold-free strategy or a scaffold-based strategy.
FIG. 3.
(A) Tissue engineering offers potential solutions to regenerate the osteochondral complex, and most strategies are categorized as either a scaffold-free strategy or a scaffold-based strategy. (B) 3D bioprinting helps combine cells, growth factors, and biocompatible materials within a bioink, which then is precisely laid down layer by layer into a biomimetic structure. Extrusion-based bioprinting, which utilizes pneumatic or mechanical force to precisely deposit material layer by layer, is a promising approach to create scaffolds with the distinct heterogeneous zonal architecture of the osteochondral unit. 3D, three-dimensional.
Scaffold-free osteochondral regeneration is an attractive option owing to minimal preparation procedures, optimized time and cost, and minimal risk of material rejection by the body. The essence of scaffold-free tissue engineering is to introduce cells at a high density as a cell sheet or spheroid to enhance cell-to-cell interactions and produce a native ECM within the defect site.51 Nonetheless, this strategy is susceptible to failure because of decreased mechanical strength and instability. In addition, the inability for scaffold-free strategy to maintain a tight connection with the native tissue highlights the fact that proper fixation is critical to the success and proper load transfers between the cartilage and bone layers.52
A scaffold-based tissue engineering strategy conversely provides a 3D microenvironment that assists in cell attachment and proliferation, cell differentiation, and new tissue development. The scaffold should ideally be porous and have interconnected pore structure for proper cell migration, growth, and nutrient/waste transport.53 Table 1 provides a summary of scaffold-based interface tissue engineering strategies for osteochondral regeneration.54–75 Early design of osteochondral scaffolds was primarily monophasic and homogenous in makeup.76 The porosity and structure within these scaffolds are uniform throughout the construct, but recent studies have demonstrated that these single-phase scaffolds cannot sufficiently regenerate the anisotropic and biomechanical properties of the osteochondral unit.77 Because this complex exhibits depth-dependent mechanical heterogeneity throughout its ECM, the osteochondral scaffolds require up to three distinct layers—the articular cartilage, calcified cartilage, and subchondral bone layers—that guide the infiltrating cells through layer-specific mechanical stimuli. It has been well established in the literature that each tissue has a characteristic stiffness that affects tissue development by directing cell behavior and matrix protein production.29 Furthermore, mesenchymal stem cells (MSCs) have been shown to be highly mechanosensitive and respond to stiffness and mechanical loading to influence signal transduction pathways dictating stem cell fate.78 Therefore, coordinating the interplay of biomaterials and cells by varying the mechanical properties, such as material stiffness, could assist in the regeneration of the osteochondral unit.
Table 1.
Scaffold-Based Interface Tissue Engineering Strategies for Osteochondral Regeneration
| Study type | Objective | Fabrication method | Osteochondral scaffold layers | Scaffold constituents | Cells | Mechanical properties reported | Observations | References |
|---|---|---|---|---|---|---|---|---|
| In vitro | Optimize the effects of chondrocyte density on matrix production and on mechanical properties of the cartilage region. Evaluate the effects of the BG phase of the PLGA-BG composite on chondrocyte mineralization potential and the formation of a calcified cartilage region |
Casting | Hyaline cartilage (cartilage region). Calcified cartilage (interface region). ScB (bone region) |
Agarose 45S5 BG PLGA |
Bovine articular chondrocytes. Bovine osteoblasts |
Compressive modulus | The stratified scaffold supported the region-specific coculture of chondrocytes and osteoblasts that promotes the formation of three distinct yet continuous regions of cartilage, calcified cartilage, and bone-like matrices. Higher cell density improved chondrogenesis and enhanced the scaffold's mechanical properties. PLGA-BG microspheres were found to increase chondrocyte mineralization potential and were required for the formation of a calcified cartilage and bone layers of the scaffold |
Jiang et al.54 |
| In vitro | Compare impact of hydroxyapatite on hypertrophic and non-hypertrophic chondrocytes in a composite scaffold Optimize the particle size and concentration of hydroxyapatite in composite scaffolds |
Casting | Calcified cartilage (monophasic construct) | Agarose. Hydroxyapatite |
Bovine articular (deep zone) chondrocytes | Complex shear modulus. Compressive modulus. Phase angle |
Hypertrophic chondrocytes exhibited higher levels of matrix deposition and mineralization potential with addition of hydroxyapatite, without affecting deep zone chondrocyte biosynthesis and hypertrophy Higher matrix content corresponded to significant increases in both compressive and shear mechanical properties. Matrix deposition was higher only with the addition of micron-sized ceramic particles, while cell hypertrophy was independent of ceramic size |
Khanarian et al.55 |
| In vitro | Determine the response of chondrocytes to a composite alginate-HA scaffold and identify chondrocyte density that optimizes calcified cartilage formation | Casting | Calcified cartilage | Alginate. Hydroxyapatite |
Bovine articular chondrocytes | Complex shear modulus. Compressive modulus |
Composite HA/alginate scaffolds promoted the formation of a proteoglycan and type II collagen rich matrix when seeded with chondrocytes from the deep zone of cartilage. Composite scaffold exhibited increased compressive and shear moduli |
Khanarian et al.56 |
|
In vitro In vivo (rabbit) |
Evaluate the impact of a compact layer in a biphasic scaffold in promoting osteochondral tissue regeneration | Chondral phase: modified temperature gradient-guided TIPS technique. Compact layer: phase separation. Bony phase: rapid prototyping technique |
Hyaline cartilage (chondral phase). Calcified cartilage (compact layer). ScB (bony phase) |
Bovine decellularized cartilage ECM. Poly(lactic-co-glycolic acid) (PLGA) β-TCP Type I collagen |
Rabbit bone marrow MSCs | Maximal shear strength (N/m2). Maximal tensile strength (N/m2) |
Scaffolds with a compact layer displayed superior glycosaminoglycan and collagen content over controls without the compact layer in vivo. Anti-tensile and anti-shear properties were significantly enhanced within scaffolds containing the compact layer |
Da et al.57 |
| In vitro | Design a bilayered scaffold using ACECM and hydroxylapatite. Assess the impact of scaffold permeability on cartilage defect healing and cell behavior |
Casting via liquid-phase cosynthesis technique and TIPS technique | Hyaline cartilage (porous upper layer). Calcified cartilage (dense mineralized lower layer) |
ACECM. Hydroxyapatite |
Rabbit chondrocytes | Compressive modulus. Interface shear stiffness |
Gradual interfacial region was formed with pore sizes varying from 128.2 ± 20.3 μm in the mineralized component and 21.2 ± 3.1 μm in the nonmineralized component. Scaffold permeability decreased with increasing compressive strain and hydroxyapatite content while shear stiffness was higher in scaffolds containing lower concentration of hydroxylapatite. Chondrocytes could not penetrate the interface but were responsible for abundant matrix deposition in the upper layer |
Wang et al.58 |
| In vitro | Engineer hyaline and calcified cartilage layers via scaffold-free engineering | Gravity sintering for calcium polyphosphate disks. Sol-gel processing for hydroxyapatite coating |
Hyaline cartilage. Calcified cartilage |
Calcium polyphosphate. Hydroxyapatite |
Sheep bone marrow stromal cells predifferentiated into chondrocytes | Compressive modulus. Interface shear strength |
The engineered construct consisted of a hyaline cartilage zone rich in proteoglycans and collagen type II, as well as a highly mineralized calcified cartilage zone with type X collagen. Constructs that included the calcified interface had compressive strength on par with native sheep tissue and higher shear strength than uncalcified constructs |
Lee et al.59 |
| In vitro | Investigate the effects of extracellular calcium concentration on hASC chondrogenesis and the potential to use controlled calcium delivery to induce site-specific chondrogenesis and osteogenesis using hASCs in a single osteochondral scaffold | Electrospinning | Hyaline Cartilage layer (three layers). ScB (two layers). Nanofibrous scaffolds stacked together using type I collagen gel |
PLA β-TCP Type I collagen |
hASCs | Not applicable | Elevated extracellular calcium levels induced osteogenesis of hASCs while inhibiting chondrogenesis. The multilayer stacked nanofibrous construct with regional incorporation of TCP nanoparticles enabled layer-specific hASC differentiation within the same structure |
Mellor et al.60 |
| In vitro | Evaluate the efficacy of a dual chambered well system for simultaneously providing osteogenic and chondrogenic stimulation to different layers of an osteochondral scaffold | Freeze-drying | Hyaline cartilage (chondrogenic region). Calcified cartilage (middle region). ScB (osteogenic region) |
RADA self-assembly peptide. Silk fibers (Bombyx mori) |
Rabbit bone marrow stromal cells | Compressive load | The specially designed two-chambered well could successfully provide specific chemical stimulation to BMSCs located in different regions of a single scaffold, leading to the formation of distinct hyaline cartilage, calcified cartilage, and ScB layers. Cells in the intermediate region were found to be hypertrophic chondrocytes that produced a matrix of GAGs and collagen types I, II and X. |
Chen et al.61 |
| In vivo (goat) | Assess the ability of a multilayered collagen-based scaffold to regenerate and repair osteochondral tissue in two surgically created critical sized osteochondral defects within the caprine stifle joint. | Casting. Iterative freeze-drying method |
Hyaline cartilage. Calcified cartilage. ScB |
Hyaluronic acid. Hydroxyapatite. Type I collagen. Type II collagen |
Acellular | Not applicable | Compared with the bilayered synthetic polymer scaffold, the multilayered scaffold improved regeneration upon evaluation of repair up to 12 months, with a zonal architecture comparable to that of native osteochondral tissue. These scaffolds demonstrated increased cartilage thickness and superior levels of ScB formation in the multilayered scaffold group compared with empty and synthetic polymer scaffold groups |
Levingstone et al.62 |
| In vitro | Evaluate the impact of calcium phosphate ratio on deep zone chondrocytes within hydrogel-ceramic hybrid scaffolds | Casting | Calcified cartilage (monophasic construct) | Agarose CDA |
Bovine articular (deep zone) chondrocytes | Complex shear modulus. Compressive modulus. Phase angle |
Higher calcium-phosphorus ratio increased chondrocyte proliferation and glycosaminoglycan production while the group with a lower calcium–phosphorus ratio produced results on par with a ceramic-free control | Boushell et al.63 |
| In vitro | Fabricate and characterize a triphasic, anisotropic scaffold | Freeze casting and lyophilization | Hyaline cartilage (superficial and transition zones). Calcified cartilage (calcified cartilage zone). ScB (osseous zone) |
Collagen. Hyaluronic acid. Hydroxyapatite |
Acellular | Collapse plateau modulus. Compressive modulus. Elastic compressive strength |
Zone-specific localization of hyaluronic acid resembling the depth-dependent increase in glycosaminoglycans of native osteochondral unit. Compressive testing revealed depth-dependent increase in stiffness and that the compressive moduli of the chondral and osseous zones was within the range needed to promote chondrogenic/osteogenic differentiation of MSCs |
Clearfield et al.64 |
| Clinical study (human RCT) | Evaluate the efficacy of a multiphasic collagen-hydroxyapatite-based scaffold in patients suffering from osteochondral knee defects | Physical combination of separately prepared layers. Freeze-drying |
Hyaline cartilage (100% type I collagen with smooth surface). Calcified cartilage (intermediate layer; 40% Mg-HA and 60% type I collagen). ScB (70% Mg-HA and 30% type I collagen) |
Hydroxyapatite. Type I collagen |
Acellular | Not applicable | Two-year follow-up showed that patients with deep osteochondral lesions or sport active patients that received the coll-HA scaffold responded significantly better than the control group patients treated with BMS. Although there was no statistically significant difference between coll-HA scaffolds and BMS for chondral lesions, the procedure demonstrated potential to treat osteochondral lesions with coll-HA scaffolds |
Kon et al.65 |
|
In vitro In vivo (murine) |
Develop a bizonal scaffold that can induce in vivo regeneration of cartilage while preventing mineralization | Casting | Hyaline cartilage. Calcified cartilage |
Heparin StarPEG |
Porcine articular chondrocytes in superficial layer. Porcine bone marrow MSCs in calcified cartilage layer |
Not applicable | Bizonal StarPEG/heparin scaffold supplemented with cell-type mediated spatiotemporal regulation allowed for growth of bizonal cartilage with stable calcified cartilage layer | Kunisch et al.66 |
| In vitro | Assess the impact of different formulations of ScB scaffold degradation parameters including mass loss, change in environmental pH, bioactivity, compressive mechanics. Improve the material homogeneity and compressive mechanics of ScB construct. Construct multilayered osteochondral scaffold by combining the ScB construct with a cartilage analog |
Casting | Hyaline cartilage Calcified cartilage ScB |
Hydroxyapatite PEG PLGA |
Human bone marrow derived stromal cells | Hysteresis Complex, storage, and loss modulus Compressive modulus Phase angle |
ScB construct formed the foundation of a cytocompatible, multi-layered osteochodnral constructed that supported a mechanically competent cartilage layer Optimized ScB construct did not alter pH upon degradation, exhibited bioactivity, and had significantly greater compressive mechanics compared to other constructs |
Marionneaux et al.67 |
|
In vitro In vivo (rabbit) |
Investigate how Ica-HA/Col hydrogel scaffolds respond to the different culture conditions, stimulate the chondrogenic and osteogenic differentiation of BMSCs, and facilitate the deposition of calcified layer matrix in different inductive media | Casting | Monophasic construct | Ica-HA. Type I collagen (Col) |
Rabbit bone marrow MSCs | Compressive modulus | Ica-HA/Col hydrogel enhanced the osteogenic and chondrogenic differentiation of BMSCs in vitro. Ica-HA/Col hydrogel promoted the synthesis of type X collagen and deposition of calcium salt in mixed chondrogenic/osteogenic inductive media. In vivo rabbit model study demonstrated that Ica-HA/Col constructs had a potential to facilitate the reconstruction of the osteochondral interface |
Yang et al.68 |
| In vivo (porcine) | Determine whether hydrogel-filled PCL-constructs with a chondrocyte-seeded upper layer and an MSC-seeded bottom layer deemed to induce calcified cartilage can improve cartilage regeneration of superficial osteochondral defects in vivo | Casting (hydrogel). 3D printing (PCL mesh) |
Hyaline cartilage (upper layer). Calcified cartilage and ScB (bottom layer) |
Heparin PCL StarPEG or StarPEG-MMP-conjugates |
Porcine articular chondrocytes. Porcine bone marrow mesenchymal stromal cells |
Hardness | Grafts showed comparable hardness at implantation and did not cause visible signs of inflammation. After 6 months, μCT analysis revealed significant bone loss in both treatment groups compared with the control. Some parts of the PCL mesh and hydrogel were retained in all defects, but most implants were pressed into the ScB |
Bothe et al.69 |
|
In vitro In vivo (goat) |
Assess the potential of osteochondral tissue-specific ECM-derived scaffolds to spatially direct MSC differentiation in vitro. Determine the mechanics by which the scaffolds can direct joint repair in vivo following their cell-free implantation in critically sized osteochondral defects |
Iterative freeze-drying process | Hyaline cartilage. ScB |
ACECM (cartilage layer). Growth plate ECM (ScB layer) |
Porcine bone marrow derived MSCs (for in vitro evaluation). Acellular (in vivo) |
Not applicable | Scaffolds could spatially direct stem cell differentiation in vitro, promoting the formation of graded cartilage tissue transitioning from calcified cartilage to hyaline cartilage. Over 12 months in vivo, the bilayered ECM derived scaffolds promoted the regeneration of hyaline cartilage with a collagen fiber architecture recapitulating the native tissue compared to commercially available control scaffolds |
Cunniffe et al.70 |
|
In vitro In vivo (rabbit) |
Fabricate and assess regenerative performance of gradient osteochondral scaffold | Electrospinning (single-layer fibrous mesh). Gradient 3D scaffold constructed by stacking different cell/mesh complexes layer-by-layer |
Hyaline cartilage (four layers). Calcified cartilage (interface; four layers). ScB (four layers) |
Gelatin PLA |
Rabbit bone marrow MSCs that were predifferentiated in three different culture conditions (chondrogenic, mixed, and osteogenic) | Not applicable | The multilayered osteochondral scaffolds regenerated distinct osteochondral layers better than homogenous constructs within rabbit knee defect model | Jin et al.71 |
| In vitro | Assess the regenerative potential of triphasic osteochondral scaffolds fabricated via an iterative layering process with biomimetic ratios of natural ECM components | Hyaline and calcified cartilage layers: thermal gelation. ScB layer: freeze-drying method All layers combined by iteratively overlaying each layer on top of the other |
Hyaline cartilage. Calcified cartilage. ScB |
Chitosan. Hydroxyapatite. Type I collagen. Type II collagen |
Murine chondrocytes (ATDC5). Murine preosteoblasts (MC3T3-E1) |
Not applicable | Final stratified scaffold demonstrated compact structure and no separation upon fabrication. Coculture of preosteoblasts and chondrocytes within the scaffold showed high viability and potential for selective maintenance of layer-specific cells without the addition of growth factors. ECM production was enhanced upon 21-day culture |
Korpayev et al.72 |
|
In vitro In vivo (rabbit) |
Investigate the role of a calcified cartilage layer and adipose tissue derived stem cells in promoting osteochondral regeneration in a rabbit model | Paraffin-sphere leaching and modified temperature gradient-guided TIPS technique | Hyaline cartilage. Calcified cartilage. ScB |
Hydroxyapatite. Silk fibroin |
Rabbit adipose tissue-derived MSCs | Mechanical stiffness (N/m) | Scaffolds containing both adipose tissue-derived stem cells and a calcified cartilage layer outperformed other scaffolds in terms of biomechanics and promoting osteochondral regeneration within rabbit osteochondral defects | Zhao et al.73 |
| In vivo (porcine) | Explore the use of natural calcified cartilage zone in trilayer osteochondral scaffolds. Elucidate the role of the calcified cartilage zone in the osteochondral repair process |
Type II collagen sponge grafted onto decellularized scaffolds consisting of ScB with or without native calcified cartilage zone, and then lyophilized | Hyaline cartilage (chondral phase). Calcified cartilage. ScB |
Porcine decellularized calcified cartilage and/or ScB. Type II collagen |
Porcine bone marrow stem cells | Not applicable | Implants that contained natural calcified cartilage layer were superior in promoting osteochondral repair in a minipig model compared with the control group treated with scaffolds without the calcified cartilage layer | Huang et al.74 |
| In vivo (sheep) | Evaluate the healing process of osteochondral defects in the sheep stifle joint supported by an acellular porous PHB/chitosan-based implant | Casting | Single layer | CHIT PHB |
Acellular | Not applicable | The healing osteochondral defect was comparable with the intact cartilage at the surface of the defect upon MRI evaluation 6 months after surgery. CT scan showed low regenerative potential of the implant at the osseous zone of defect compared with chondral zone. Hyaline-like cartilage was observed in most of the treated animals, except for one that healed with fibrocartilage formation |
Petrovova et al.75 |
μCT, micro-computed tomography; β-TCP, β-tricalcium phosphate; 3D, three-dimensional; ACECM, articular cartilage extracellular matrix; BG, bioactive glass; BMS, bone marrow stimulation; BMSCs, bone marrow stromal cells; CDA, calcium-deficient apatite; CHIT, chitosan; ECM, extracellular matrix; GAGs, glycosaminoglycans; hASC, human adipose-derived stem cell; Ica-HA, icariin-conjugated hyaluronic acid; MRI, magnetic resonance imaging; MSC, mesenchymal stem cell; PCL, polycaprolactone; PEG, polyethylene glycol; PHB, polyhydroxybutyrate; PLA, polylactic acid; PLGA, poly(lactic-co-glycolic acid); RCT, randomized clinical trial; ScB, subchondral bone; StarPEG, star-shaped poly(ethylene glycol); TIPS, thermal-induced phase separation.
More recent efforts to regenerate the osteochondral tissue have resorted to biphasic or multiphasic scaffolds. The multiphasic nature of an osteochondral implant has required the use of multiple materials or varied structural organization of the scaffold to replicate the complex architecture of the osteochondral unit. Kunisch et al. designed a bizonal cartilage scaffold utilizing a polyethylene glycol (PEG)/heparin-based hydrogel system.66 The spaciotemporal design of the casted hydrogel system supported articular chondrocytes in the superficial layer and MSCs in the deep cartilage layer, which helped maintain the bizonal organization to match the cellular organization within articular cartilage. Jin et al. demonstrated the potential to regenerate osteochondral tissue utilizing stackable cell sheets precultured on an electrospun fibrous mesh.71 Bone marrow MSCs were precultured on fibrous meshes consisting of poly (l-lactic acid) (PLLA) and gelatin, and differentiated separately to form three distinct layers, which were eventually stacked together to create a multiphasic scaffold. Finally, some multilayered osteochondral scaffolds were able to progress toward clinical trials. Kon et al. conducted a randomized clinical trial involving 100 patients to examine the potential of a nanostructured collagen–hydroxyapatite (HAP) bilayer scaffold.65 Upon a 2-year follow-up period, the patients who received the bilayer scaffold demonstrated increased tissue regeneration based on efficacy measurements and radiologic evaluation with the magnetic resonance imaging (MRI).
3D Bioprinting the Interface Layer Within Osteochondral Scaffolds
The use of 3D bioprinting has recently received much attention as a potential strategy to fabricate osteochondral scaffolds (Fig. 3B and Table 2).79–87 This technique helps combine cells, growth factors, and biocompatible materials within a bioink, which then can be precisely laid down layer-by-layer into a biomimetic structure.88 Therefore, 3D printing can more readily fabricate the complex zonal organization with distinct biomechanical properties of the osteochondral unit. Because numerous 3D printing strategies are available, the selection of a bioprinting strategy most suitable for osteochondral regeneration depends primarily on the processing conditions, material type, and scaffold design.89
Table 2.
Three-Dimensional Bioprinting Strategies for Osteochondral Interface Tissue Engineering
| Study type | Objectives | Printing method | Layers included | Primary scaffold materials | Cells | Mechanical properties reported | Observations | References |
|---|---|---|---|---|---|---|---|---|
| In vitro | Develop a PEG/β-TCP osteochondral scaffold integrated via biomimetic interface structure design and 3D printing. Determine which interface microstructure enhances interfacial integration |
Casting. Stereolitho-graphy |
Hyaline cartilage (chondral phase). Calcified cartilage (chondral and bone phases). ScB (osseous phase) |
PEG β-TCP |
Acellular | Compressive modulus. Interfacial shear strength |
Interfacial shear strength of 30% pore area group was nearly three folds greater than that of 0% pore area percentage group, and more than 50 folds improved compared with that of traditional integration | Zhang et al.79 |
| In vitro | Development and characterization of a biphasic osteochondral scaffold system constructed using a combination of 3D plotting and gel casting with photopolymerization | Extrusion-based printing | Hyaline cartilage. ScB |
ALG GelMA HAMA Hydroxyapatite |
hAC | Compressive modulus | No cytotoxicity was observed from scaffold components. Incorporation of HAMA into hydrogels improved chondrogenesis. The use of ALG/HAP versus ALG did not encourage the formation of a ZCC |
Bartnikowski et al.80 |
| In vitro | Test the mechanical properties of the microstructure of the multilayer scaffold and evaluate the distribution, proliferation, and morphology of cells within the scaffold to understand the in vitro effects of the physical properties and 3D internal structures of different materials | Extrusion-based printing (thermally induced crystallization). “Dissolved adhesion” process with 1,4-dioxane and freeze drying |
Hyaline cartilage (cartilage scaffold). Calcified cartilage (calcified layer). ScB (bone scaffold) |
Bovine cartilage matrix. Hydroxyapatite. PLGA β-TCP |
Bovine bone marrow MSC | Maximum shear strength. Maximum tensile strength |
Biomechanical tests showed that the scaffolds were significantly stronger than scaffolds without a calcified layer in terms of maximum tensile strength and maximum shear strength. Cell-seeded scaffolds exhibited similar cell adherence and proliferation to traditional scaffolds |
Li et al.81 |
|
In vitro In vivo (rat) |
Evaluate the performance and printability of alginate and hydroxyapatite composite hydrogel with SC as a dispersant. Examine the role of hydroxyapatite in stimulating chondrocytes to secrete a calcified matrix |
Extrusion-based printing | Calcified cartilage | ALG HAP SC |
Chick cartilaginous sternum chondrocytes | Not applicable | The addition of SC allowed homogeneous dispersal of HAP particles within ALG hydrogel Compared with ALG only controls, the ALG scaffolds with HAP promoted collagen type X secretion, ALP activity, and mineral deposition by impregnated chondrocytes. ALG/HAP composite hydrogels could be printed into 3D scaffolds with a porous structure |
You et al.82 |
| In vitro | Evaluate calcified cartilage formation by 3D-printed hydrogel/CSi-Mg10 hybrid seeded with human deep zone chondrocyte | Extrusion-based printing | Single layer | Hydroxyapatite. Mg-doped Wollastonite (CSi-Mg10). Sodium alginate. β-TCP. Type I collagen |
hDZCs | Not applicable | Osteogenic and chondrogenic induction were both upregulated in a dose-dependent manner. Human deep zone chondrocytes (hDZCs) seeded on 6% CSi-Mg10 scaffold exhibited higher mineralization compared to scaffolds containing β-TCP or nHAp of equivalent concentration. The hDZCs in the 6% CSi-Mg10 scaffolds maintained a higher expression of the calcified cartilage zone specific ECM marker and hypertrophic marker, collagen type X |
Yu et al.83 |
|
In vitro In vivo (rat) |
Demonstrate the feasibility of fabricating a heterogeneous scaffold that recapitulates the complex biological gradients with a microfluidic 3D bioprinting approach | Extrusion-based printing (with microfluidic print head) | Hyaline cartilage layer. Calcified cartilage layer |
ALG CS-AEMA GelMA HAMA |
hAC human BM-MSCs |
Compressive modulus | Coculture of hMSC and hAC in ALG+GelMA+CS-AEMA hydrogel yields more hyaline phenotype than the hMSC monoculture. Introduction of HAMA and TCP microparticles into ALG+GelMA+CS-AEMA hydrogel promotes development of hypertrophic chondrocytes with upregulated expression of ALPL and COL10A |
Idaszek et al.84 |
| In vitro | Evaluate the efficacy of extrusion-based bioprinting in fabricating a scaffold resembling calcified cartilage. Investigate the impact of β-TCP in modulating BM-MSCs toward calcified cartilage formation |
Extrusion-based printing (with coaxial needle system) | Calcified cartilage | Alginate. Gelatin methacryloyl (GelMA). β-TCP |
Human bone marrow MSCs | Compressive modulus. Loss modulus. Storage modulus |
Composite bioink formulation based on alginate, GelMA and β-TCP particles can modulate BM-MSCs toward the formation of calcified zone of osteochondral tissue. The incorporation of 0.5% w/v TCP is the optimal concentration to form stable scaffolds with high shape fidelity, mechanics, and biological properties relevant for the development of calcified cartilage |
Kosik-Koziol et al.85 |
|
In vitro
Ex vivo |
Directly form a secure integrated interface layer between two mechanically distinct materials—particularly between cell-laden hydrogels and biologically relevant ceramics and polymers | Extrusion-based printing. MEW |
Hyaline cartilage (chondral compartment). Calcified cartilage (interface). ScB (bony compartment) |
Hydroxyapatite. Poloxamer (Pluronic® F-127 or custom-synthesized with modification with caprolactone oligomers and methacryloyl (P-CL-MA) groups). α-Tricalcium phosphate |
Equine BM-MSCs Equine articular chondroprogenitor cells |
Compressive modulus. Interfacial shear stress |
Interlocking design enhanced the hydrogel-to-ceramic adhesion strength over 6.5-fold compared with the noninterlocking fiber architectures. MEW meshes endowed the chondral compartment with compressive properties approaching those of native cartilage. Osteal and chondral compartments supported osteogenesis and cartilage matrix deposition in vitro, and the synthesized cartilage matrix enhanced mechanical properties at the ceramic–hydrogel interface |
Diloksumpan et al.86 |
| In vitro | Describe the effect of the mineral phase on human primary chondrocyte redifferentiation by applying biphasic constructs in which the CPC and the cell-encapsulating AlgMC hydrogel are combined and in direct contact with each other | Extrusion-based printing | Hyaline cartilage (articular cartilage). Calcified cartilage. ScB |
AlgMC. Calcium phosphate cement |
hAC | Not applicable | The majority of encapsulated hCh survived the printing process and were able to redifferentiate to produce their respective matrix components up in 3 weeks. Presence of a mineralized zone was found to support chondrogenic ECM production by altering the ionic concentrations of calcium and phosphorus in in vitro culture conditions |
Kilian et al.87 |
ALG, alginate; AlgMC, alginate methylcellulose; BM-MSCs, bone marrow mesenchymal stem cells; CS-AEMA, chondroitin sulfate/2-aminoethylmethacrylate; CPC, calcium phosphate cement; GelMA, gelatin methacryloyl; hAC, human articular chondrocytes; HAMA, hyaluronic acid methacrylate; HAP, hydroxyapatite; hDZCs, human deep zone chondrocytes; MEW, melt electrowriting; PLGA, poly(lactic-co-glycolic acid); SC, sodium citrate; ZCC, zone of calcified cartilage.
Extrusion-based bioprinting, which utilizes pneumatic or mechanical force to precisely deposit material layer by layer, has demonstrated promise to create scaffolds with the distinct heterogeneous zonal architecture within the osteochondral unit. Guo et al. demonstrated that a multilayer cartilage scaffold, fabricated with poly(l-lactide-co-caprolactone) (PLCL) and amine end-capped poly(lactic-co-glycolic acid) (aPLGA), can be successfully printed with high resolution through an extrusion-based 3D printer to induce polymer and cell alignment analogous to the zonal arrangement of articular cartilage.90 Although this particular study only focused on the zonal organization of cartilage, other 3D printing studies have explored strategies to venture into the calcified cartilage and subchondral bone regions of the osteochondral unit.
Kosik-Koziol et al. utilized an extrusion-based bioprinter configured with a coaxial needle to engineer the calcified cartilage layer within a hydrogel scaffold system embedded with β-tricalcium phosphate (β-TCP) particles.85 The composite alginate (ALG)/gelatin methacryloyl (GelMA) bioink optimized the gelation process for high-resolution printing and introduced the cell-binding motifs. The optimized hydrogel system consisting of 0.5% of β-TCP particles appeared to demonstrate the potential to modulate the bone marrow mesenchymal stem cells (BM-MSCs) toward the formation of calcified cartilage. Although the multilayered scaffold was not examined in this particular study, another group utilized a similar bioink formulation to recapitulate the different cartilage zones within a single construct.84 The extrusion-based bioprinter was configured with a microfluidic device to help vary composition within each layer and induce heterogeneous differentiation of cells. The results demonstrated that the coculture of MSCs and chondrocytes appeared to yield a more hyaline-like phenotype than culturing MSCs alone.
To fully capitalize on the advantages offered by 3D printing for osteochondral regeneration, several technical challenges still need to be overcome. Some of these challenges include issues with incorporating multiple materials into the bioink, nozzle clogging, optimizing the bioink, and dealing with the sensitivity of bioinks to processing parameters.91 You et al. utilized sodium citrate as a dispersant to evenly distribute HAP within ALG hydrogel.82 This homogenous ALG/HAP composite hydrogel helped promote chondrocytes to exhibit greater amounts of collagen X, which is characteristic of calcified cartilage. The use of dispersant could ultimately improve the mineralization capability and 3D printing outcomes.
In addition, the integration between the osteal and chondral layers could be essential for the success of engineered osteochondral scaffold. Delaminated cartilage grafts have consistently compromised the outcome of the defect site repair on numerous occasions because of inadequate integration.92 When it comes to biphasic osteochondral scaffolds, most have been integrated by suturing, gluing, or crosslinking.93–95 Although these integration strategies fail to fully satisfy the mechanical requirements of the osteochondral scaffold, they are still readily relied on to integrate the bone and cartilage layers. Therefore, more fabrication strategies have to be examined to improve the integration within biomimetic, multilayer osteochondral scaffolds.79
Effectively integrating the cartilage and bone regions with an interface layer is critical for the success of osteochondral scaffolds, because engineered cartilage have been shown undergo delamination and ultimately jeopardize cartilage repair.96 Although suturing, adhesives, and chemical crosslinking have been utilized to augment the integration within biphasic osteochondral scaffolds, these methods still fail to achieve the levels of integration necessary for osteochondral integration.93,97 Therefore, engineering an osteochondral interface requires a strategy that facilitates the load bearing and stress distribution experienced within this region.
Strategies to Enhance Biomechanics of Bioprinted Osteochondral Scaffolds
Adhesion can simply be described as the two-dimensional adherence of two materials to each other, and much of the insight pertaining to it comes from studying adhesives and adhesion bonds over the years. Although the interaction between adhesive and adherent layers within an adhesive system takes place at different levels of scale, most of these interactions act on the molecular or atomic level.98 To date, there is still no consensus on the theories of adhesion or on the mechanisms involved. Conventional adhesion theories postulated that adsorption, diffusion, electrostatic reactions, and mechanical interlocking best described the mechanisms involved during adhesion.99 However, additional theories of adhesion have gained traction in recent years to supplement the previous explanations to include wettability, chemical bonding, acid-base, and weak boundary layer theories.98 All these mechanisms are essential to adequately describe an adhesive system, because no single mechanism can completely describe an adhesive system. In addition, the role each mechanism plays will vary within each adhesive system.
Although there is plenty of evidence that a modified contact surface improves adhesion, there are still questions as to whether mechanical interlocking can create strong bonds between the adhesive and adherent layers. Some have postulated that increasing the contact surface on a substrate can heighten other adhesion mechanisms through the formation of a clean reactive surface and increase in overall surface area.100 These modifications consequently alter the physical and chemical properties of the substrate surface, such as wetting and chemical bonding, to increase the adhesion within the system. In 2006, Romito and Ameer engineered osteochondral scaffolds that could recreate the interfacial structure and biomechanics of articular cartilage.101 The composite scaffold was created from a nonwoven PLLA mesh mechanically interlocked to a solid core at a microscopic level and seeded with bovine articular chondrocytes. The biochemical and histological analyses demonstrated that these constructs supported the development of a cartilage ECM for at least 6 weeks in vitro with improved adhesion strength, whereas a scaffold without the interface achieved <5% of this adhesion strength. These results suggested that mechanical interlocking should be utilized as an adhesive mechanism to engineer a multilayer osteochondral scaffold with synthetic materials.
Zhang et al. developed a biphasic osteochondral scaffold with enhanced interfacial integration by 3D printing PEG hydrogel on top of gel-casted β-TCP ceramic scaffold with a stereolithographic printer.79 Microscopic and scanning electron microscope (SEM) characterization of the interfaces showed that the PEG hydrogels were tightly interlocked within the ceramic scaffold by extending into the pores. The interfacial shear strength of the biphasic scaffolds with a 30% pore area percentage exhibited a threefold improvement compared with that of 0% pore area scaffolds. In an alternative approach, Diloksumpan et al. utilized multiscale printing to integrate a hydrogel-based cartilage layer to a stiff osteo matrix.86 By combining extrusion-based 3D printing with electrowriting, ceramic-integrated melt electrowriting (MEW) meshes were designed to protrude into the interface region of the composite hydrogel–ceramic scaffold. The interlocking features within these composite scaffolds enhanced structural stability during handling and implantation, as the interface adhesion strength and compressive properties were shown to be superior to the noninterlocking counterparts.
Other adhesive strategies for 3D bioprinted osteochondral scaffolds have been minimal to date. Li et al. designed a triphasic composite scaffold that simulated the full-thickness structure of the osteochondral unit.81 The composite scaffold was formed utilizing a “dissolved-adhesion technology,” which involved spraying an organic solvent to the surface of the calcified cartilage and chondral phases. Once both surfaces underwent mild dissolution, the anisotropic chondral phase was gently applied onto the bony phase and eventually lyophilized to complete the osteochondral construct. Although the biomechanical tests demonstrated that these composite scaffolds possessed significantly stronger maximum tensile and shear strengths than scaffolds without the calcified cartilage structure, two different fabrication methodologies were utilized in this study, and the adhesive strategy utilized to conjoin the cartilage and bone layers involved manual intervention. Once again, the existing studies (Table 2) provide us with relatively few insights on how adhesive strategies can be utilized in bioprinted interface scaffolds for osteochondral scaffolds.
Conclusion
Both articular cartilage and subchondral bone contribute to the distinct biomechanical properties that maintain the health and aid in load transfer throughout the osteochondral unit. While preemptive treatment of osteochondral defects is essential to successfully manage their associated pathologies or traumatic injuries, existing nonsurgical treatment modalities are palliative in nature and generally just serve to slow down disease progression. In addition, current surgical treatment options present with an abundance of risks, limitations, and expenses. Tissue engineering offers promising solutions to regenerate the osteochondral complex that current treatment solutions cannot accomplish. 3D bioprinting especially has demonstrated the capability to create multilayered scaffolds with heterogeneous properties that recapitulate the native osteochondral architecture and composition. Most of the literature retrieved for this review demonstrates the potential to develop an osteochondral interface that enhances the biomechanics of the scaffold. Future studies will improve upon the interface layer that can improve the biomechanics within multilayered scaffolds for enhanced osteochondral regeneration.
Acknowledgment
Figures were created with Biorender.com
Disclosure Statement
No competing financial interests exist for all authors.
Funding Information
The authors acknowledge support by the NIBIB/NIH Center for Engineering Complex Tissues (P41 EB023833) and MPowers Graduate Fellowship program.
References
- 1. GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 392, 1789, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Martel-Pelletier, J., Boileau, C., Pelletier, J.P., and Roughley, P.J.. Cartilage in normal and osteoarthritis conditions. Best Pract Res Clin Rheumatol 22, 351, 2008. [DOI] [PubMed] [Google Scholar]
- 3. Walker-Bone, K., Javaid, K., Arden, N., and Cooper, C.. Regular review: medical management of osteoarthritis. BMJ 321, 936, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tamaddon, M., Wang, L., Liu, Z., and Liu, C.. Osteochondral tissue repair in osteoarthritic joints: clinical challenges and opportunities in tissue engineering. Biodes Manuf 1, 101, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Goldring, S.R., and Goldring, M.B.. Changes in the osteochondral unit during osteoarthritis: structure, function and cartilage-bone crosstalk. Nat Rev Rheumatol 12, 632, 2016. [DOI] [PubMed] [Google Scholar]
- 6. Yousefi, A.M., Hoque, M.E., Prasad, R.G., and Uth, N.. Current strategies in multiphasic scaffold design for osteochondral tissue engineering: a review. J Biomed Mater Res A 103, 2460, 2015. [DOI] [PubMed] [Google Scholar]
- 7. Kloppenburg, M., and Berenbaum, F.. Osteoarthritis year in review 2019: epidemiology and therapy. Osteoarthritis Cartilage 28, 242, 2020. [DOI] [PubMed] [Google Scholar]
- 8. Li, G., Yin, J., Gao, J., et al. . Subchondral bone in osteoarthritis: insight into risk factors and microstructural changes. Arthritis Res Ther 15, 223, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hu, W., Chen, Y., Dou, C., and Dong, S.. Microenvironment in subchondral bone: predominant regulator for the treatment of osteoarthritis. Ann Rheum Dis 80, 413, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tang, D., Tare, R.S., Yang, L.Y., et al. . Biofabrication of bone tissue: approaches, challenges and translation for bone regeneration. Biomaterials 83, 363, 2016. [DOI] [PubMed] [Google Scholar]
- 11. Moreno Madrid, A.P., Vrech, S.M., Sanchez, M.A., and Rodriguez, A.P.. Advances in additive manufacturing for bone tissue engineering scaffolds. Mater Sci Eng C Mater Biol Appl 100, 631, 2019. [DOI] [PubMed] [Google Scholar]
- 12. Buckwalter, J.A. Articular cartilage: injuries and potential for healing. J Orthop Sports Phys Ther 28, 192, 1998. [DOI] [PubMed] [Google Scholar]
- 13. Sophia Fox, A.J., Bedi, A., and Rodeo, S.A.. The basic science of articular cartilage: structure, composition, and function. Sports Health 1, 461, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mankin, H.J. The response of articular cartilage to mechanical injury. J Bone Joint Surg Am 64, 460, 1982. [PubMed] [Google Scholar]
- 15. Ateshian, G.A., Warden, W.H., Kim, J.J., Grelsamer, R.P., and Mow, V.C.. Finite deformation biphasic material properties of bovine articular cartilage from confined compression experiments. J Biomech 30, 1157, 1997. [DOI] [PubMed] [Google Scholar]
- 16. Hayes, W.C., and Bodine, A.J.. Flow-independent viscoelastic properties of articular cartilage matrix. J Biomech 11, 407, 1978. [DOI] [PubMed] [Google Scholar]
- 17. Mow, V.C., Holmes, M.H., and Lai, W.M.. Fluid transport and mechanical properties of articular cartilage: a review. J Biomech 17, 377, 1984. [DOI] [PubMed] [Google Scholar]
- 18. Han, L., Frank, E.H., Greene, J.J., et al. . Time-dependent nanomechanics of cartilage. Biophys J 100, 1846, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Barker, M.K., and Seedhom, B.B.. The relationship of the compressive modulus of articular cartilage with its deformation response to cyclic loading: does cartilage optimize its modulus so as to minimize the strains arising in it due to the prevalent loading regime? Rheumatology (Oxford) 40, 274, 2001. [DOI] [PubMed] [Google Scholar]
- 20. Linn, F.C., and Sokoloff, L.. Movement and composition of interstitial fluid of cartilage. Arthritis Rheum 8, 481, 1965. [DOI] [PubMed] [Google Scholar]
- 21. Maroudas, A., and Bullough, P.. Permeability of articular cartilage. Nature 219, 1260, 1968. [DOI] [PubMed] [Google Scholar]
- 22. Frank, E.H., and Grodzinsky, A.J.. Cartilage electromechanics—I. electrokinetic transduction and the effects of electrolyte pH and ionic strength. J Biomech 20, 615, 1987. [DOI] [PubMed] [Google Scholar]
- 23. Daubs, B.M., Markel, M.D., and Manley, P.A.. Histomorphometric analysis of articular cartilage, zone of calcified cartilage, and subchondral bone plate in femoral heads from clinically normal dogs and dogs with moderate or severe osteoarthritis. Am J Vet Res 67, 1719, 2006. [DOI] [PubMed] [Google Scholar]
- 24. Gallo, R.A., and Mosher, T.J.. Imaging of cartilage and osteochondral injuries: a case-based review. Clin Sports Med 32, 477, 2013. [DOI] [PubMed] [Google Scholar]
- 25. Muller-Gerbl, M., Schulte, E., and Putz, R.. The thickness of the calcified layer of articular cartilage: a function of the load supported? J Anat 154, 103, 1987. [PMC free article] [PubMed] [Google Scholar]
- 26. Radin, E.L., and Paul, I.L.. Response of joints to impact loading. I. In vitro wear. Arthritis Rheum 14, 356, 1971. [DOI] [PubMed] [Google Scholar]
- 27. Stewart, H.L., and Kawcak, C.E.. The importance of subchondral bone in the pathophysiology of osteoarthritis. Front Vet Sci 5, 178, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Radin, E.L., and Rose, R.M. Role of subchondral bone in the initiation and progression of cartilage damage. Clin Orthop Relat Res 34, 1986. [PubMed] [Google Scholar]
- 29. Davis, S., Roldo, M., Blunn, G., Tozzi, G., and Roncada, T.. Influence of the mechanical environment on the regeneration of osteochondral defects. Front Bioeng Biotechnol 9, 603408, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Egloff, C., Hugle, T., and Valderrabano, V.. Biomechanics and pathomechanisms of osteoarthritis. Swiss Med Wkly 142, w13583, 2012. [DOI] [PubMed] [Google Scholar]
- 31. Anderson, D.D., Brown, T.D., and Radin, E.L. The influence of basal cartilage calcification on dynamic juxtaarticular stress transmission. Clin Orthop Relat Res 298, 1993. [PubMed] [Google Scholar]
- 32. Zhang, W., Robertson, W.B., Zhao, J., Chen, W., and Xu, J.. Emerging trend in the pharmacotherapy of osteoarthritis. Front Endocrinol (Lausanne) 10, 431, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Medina, J., Garcia-Mansilla, I., Fabricant, P.D., Kremen, T.J., Sherman, S.L., and Jones, K. Microfracture for the treatment of symptomatic cartilage lesions of the knee: a survey of International Cartilage Regeneration & Joint Preservation Society. Cartilage 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yen, Y.M., Cascio, B., O'Brien, L., Stalzer, S., Millett, P.J., and Steadman, J.R.. Treatment of osteoarthritis of the knee with microfracture and rehabilitation. Med Sci Sports Exerc 40, 200, 2008. [DOI] [PubMed] [Google Scholar]
- 35. Krych, A.J., Saris, D.B.F., Stuart, M.J., and Hacken, B.. Cartilage injury in the knee: assessment and treatment options. J Am Acad Orthop Surg 28, 914, 2020. [DOI] [PubMed] [Google Scholar]
- 36. Hangody, L., and Fules, P.. Autologous osteochondral mosaicplasty for the treatment of full-thickness defects of weight-bearing joints: ten years of experimental and clinical experience. J Bone Joint Surg Am 85-A(Suppl 2), 25, 2003. [DOI] [PubMed] [Google Scholar]
- 37. Angele, P., Niemeyer, P., Steinwachs, M., et al. . Chondral and osteochondral operative treatment in early osteoarthritis. Knee Surg Sports Traumatol Arthrosc 24, 1743, 2016. [DOI] [PubMed] [Google Scholar]
- 38. Ozturk, A., Ozdemir, M.R., and Ozkan, Y.. Osteochondral autografting (mosaicplasty) in grade IV cartilage defects in the knee joint: 2- to 7-year results. Int Orthop 30, 200, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Solheim, E., Hegna, J., Oyen, J., Harlem, T., and Strand, T.. Results at 10 to 14 years after osteochondral autografting (mosaicplasty) in articular cartilage defects in the knee. Knee 20, 287, 2013. [DOI] [PubMed] [Google Scholar]
- 40. Peterson, L., Minas, T., Brittberg, M., and Lindahl, A.. Treatment of osteochondritis dissecans of the knee with autologous chondrocyte transplantation: results at two to ten years. J Bone Joint Surg Am 85-A(Suppl 2), 17, 2003. [DOI] [PubMed] [Google Scholar]
- 41. Beris, A.E., Lykissas, M.G., Kostas-Agnantis, I., and Manoudis, G.N.. Treatment of full-thickness chondral defects of the knee with autologous chondrocyte implantation: a functional evaluation with long-term follow-up. Am J Sports Med 40, 562, 2012. [DOI] [PubMed] [Google Scholar]
- 42. Henderson, I., Tuy, B., and Oakes, B.. Reoperation after autologous chondrocyte implantation. Indications and findings. J Bone Joint Surg Br 86, 205, 2004. [DOI] [PubMed] [Google Scholar]
- 43. Marlovits, S., Zeller, P., Singer, P., Resinger, C., and Vecsei, V.. Cartilage repair: generations of autologous chondrocyte transplantation. Eur J Radiol 57, 24, 2006. [DOI] [PubMed] [Google Scholar]
- 44. Kreuz, P.C., Kalkreuth, R.H., Niemeyer, P., Uhl, M., and Erggelet, C.. Long-term clinical and MRI results of matrix-assisted autologous chondrocyte implantation for articular cartilage defects of the knee. Cartilage 10, 305, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gomoll, A.H., Filardo, G., Almqvist, F.K., et al. . Surgical treatment for early osteoarthritis. Part II: allografts and concurrent procedures. Knee Surg Sports Traumatol Arthrosc 20, 468, 2012. [DOI] [PubMed] [Google Scholar]
- 46. Gortz, S., and Bugbee, W.D.. Allografts in articular cartilage repair. Instr Course Lect 56, 469, 2007. [PubMed] [Google Scholar]
- 47. Levy, Y.D., Gortz, S., Pulido, P.A., McCauley, J.C., and Bugbee, W.D.. Do fresh osteochondral allografts successfully treat femoral condyle lesions? Clin Orthop Relat Res 471, 231, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. McDermott, A.G., Langer, F., Pritzker, K.P., and Gross, A.E. Fresh small-fragment osteochondral allografts. Long-term follow-up study on first 100 cases. Clin Orthop Relat Res 96, 1985. [PubMed] [Google Scholar]
- 49. Ghazavi, M.T., Pritzker, K.P., Davis, A.M., and Gross, A.E.. Fresh osteochondral allografts for post-traumatic osteochondral defects of the knee. J Bone Joint Surg Br 79, 1008, 1997. [DOI] [PubMed] [Google Scholar]
- 50. Ng, V.Y. Risk of disease transmission with bone allograft. Orthopedics 35, 679, 2012. [DOI] [PubMed] [Google Scholar]
- 51. Murata, D., Akieda, S., Misumi, K., and Nakayama, K.. Osteochondral regeneration with a scaffold-free three-dimensional construct of adipose tissue-derived mesenchymal stromal cells in pigs. Tissue Eng Regen Med 15, 101, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ishihara, K., Nakayama, K., Akieda, S., Matsuda, S., and Iwamoto, Y.. Simultaneous regeneration of full-thickness cartilage and subchondral bone defects in vivo using a three-dimensional scaffold-free autologous construct derived from high-density bone marrow-derived mesenchymal stem cells. J Orthop Surg Res 9, 98, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chen, G., and Kawazoe, N.. Porous scaffolds for regeneration of cartilage, bone and osteochondral tissue. Adv Exp Med Biol 1058, 171, 2018. [DOI] [PubMed] [Google Scholar]
- 54. Jiang, J., Tang, A., Ateshian, G.A., Guo, X.E., Hung, C.T., and Lu, H.H.. Bioactive stratified polymer ceramic-hydrogel scaffold for integrative osteochondral repair. Ann Biomed Eng 38, 2183, 2010. [DOI] [PubMed] [Google Scholar]
- 55. Khanarian, N.T., Haney, N.M., Burga, R.A., and Lu, H.H.. A functional agarose-hydroxyapatite scaffold for osteochondral interface regeneration. Biomaterials 33, 5247, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Khanarian, N.T., Jiang, J., Wan, L.Q., Mow, V.C., and Lu, H.H.. A hydrogel-mineral composite scaffold for osteochondral interface tissue engineering. Tissue Eng Part A 18, 533, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Da, H., Jia, S.J., Meng, G.L., et al. . The impact of compact layer in biphasic scaffold on osteochondral tissue engineering. PLoS One 8, e54838, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wang, Y., Meng, H., Yuan, X., et al. . Fabrication and in vitro evaluation of an articular cartilage extracellular matrix-hydroxyapatite bilayered scaffold with low permeability for interface tissue engineering. Biomed Eng Online 13, 80, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lee, W.D., Hurtig, M.B., Pilliar, R.M., Stanford, W.L., and Kandel, R.A.. Engineering of hyaline cartilage with a calcified zone using bone marrow stromal cells. Osteoarthritis Cartilage 23, 1307, 2015. [DOI] [PubMed] [Google Scholar]
- 60. Mellor, L.F., Mohiti-Asli, M., Williams, J., et al. . Extracellular calcium modulates chondrogenic and osteogenic differentiation of human adipose-derived stem cells: a novel approach for osteochondral tissue engineering using a single stem cell source. Tissue Eng Part A 21, 2323, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Chen, K., Ng, K.S., Ravi, S., Goh, J.C., and Toh, S.L.. In vitro generation of whole osteochondral constructs using rabbit bone marrow stromal cells, employing a two-chambered co-culture well design. J Tissue Eng Regen Med 10, 294, 2016. [DOI] [PubMed] [Google Scholar]
- 62. Levingstone, T.J., Ramesh, A., Brady, R.T., et al. . Cell-free multi-layered collagen-based scaffolds demonstrate layer specific regeneration of functional osteochondral tissue in caprine joints. Biomaterials 87, 69, 2016. [DOI] [PubMed] [Google Scholar]
- 63. Boushell, M.K., Khanarian, N.T., LeGeros, R.Z., and Lu, H.H.. Effect of ceramic calcium-phosphorus ratio on chondrocyte-mediated biosynthesis and mineralization. J Biomed Mater Res A 105, 2694, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Clearfield, D., Nguyen, A., and Wei, M.. Biomimetic multidirectional scaffolds for zonal osteochondral tissue engineering via a lyophilization bonding approach. J Biomed Mater Res A 106, 948, 2018. [DOI] [PubMed] [Google Scholar]
- 65. Kon, E., Filardo, G., Brittberg, M., et al. . A multilayer biomaterial for osteochondral regeneration shows superiority vs microfractures for the treatment of osteochondral lesions in a multicentre randomized trial at 2 years. Knee Surg Sports Traumatol Arthrosc 26, 2704, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kunisch, E., Knauf, A.K., Hesse, E., et al. . StarPEG/heparin-hydrogel based in vivo engineering of stable bizonal cartilage with a calcified bottom layer. Biofabrication 11, 015001, 2018. [DOI] [PubMed] [Google Scholar]
- 67. Marionneaux, A., Walters, J., Guo, H., and Mercuri, J.. Tailoring the subchondral bone phase of a multi-layered osteochondral construct to support bone healing and a cartilage analog. Acta Biomater 78, 15, 2018. [DOI] [PubMed] [Google Scholar]
- 68. Yang, J., Liu, Y., He, L., Wang, Q., Wang, L., Yuan, T., Xiao, Y., Fan, Y., and Zhang, X.. Icariin conjugated hyaluronic acid/collagen hydrogel for osteochondral interface restoration. Acta Biomater 74, 1, 2018. [DOI] [PubMed] [Google Scholar]
- 69. Bothe, F., Deubel, A.K., Hesse, E., et al. . Treatment of focal cartilage defects in minipigs with zonal chondrocyte/mesenchymal progenitor cell constructs. Int J Mol Sci 20, 653, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Cunniffe, G.M., Diaz-Payno, P.J., Sheehy, E.J., et al. . Tissue-specific extracellular matrix scaffolds for the regeneration of spatially complex musculoskeletal tissues. Biomaterials 188, 63, 2019. [DOI] [PubMed] [Google Scholar]
- 71. Jin, L., Zhao, W., Ren, B., et al. . Osteochondral tissue regenerated via a strategy by stacking pre-differentiated BMSC sheet on fibrous mesh in a gradient. Biomed Mater 14, 065017, 2019. [DOI] [PubMed] [Google Scholar]
- 72. Korpayev, S., Kaygusuz, G., Sen, M., Orhan, K., Oto, C., and Karakecili, A.. Chitosan/collagen based biomimetic osteochondral tissue constructs: a growth factor-free approach. Int J Biol Macromol 156, 681, 2020. [DOI] [PubMed] [Google Scholar]
- 73. Zhao, Y., Ding, X., Dong, Y., et al. . Role of the calcified cartilage layer of an integrated trilayered silk fibroin scaffold used to regenerate osteochondral defects in rabbit knees. ACS Biomater Sci Eng 6, 1208, 2020. [DOI] [PubMed] [Google Scholar]
- 74. Huang, Y., Fan, H., Gong, X., Yang, L., and Wang, F.. Scaffold with natural calcified cartilage zone for osteochondral defect repair in minipigs. Am J Sports Med 49, 1883, 2021. [DOI] [PubMed] [Google Scholar]
- 75. Petrovova, E., Tomco, M., Holovska, K., et al. . PHB/CHIT scaffold as a promising biopolymer in the treatment of osteochondral defects-an experimental animal study. Polymers (Basel) 13, 1232, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Nagura, I., Fujioka, H., Kokubu, T., Makino, T., Sumi, Y., and Kurosaka, M.. Repair of osteochondral defects with a new porous synthetic polymer scaffold. J Bone Joint Surg Br 89, 258, 2007. [DOI] [PubMed] [Google Scholar]
- 77. Chu, C.R., Dounchis, J.S., Yoshioka, M., Sah, R.L., Coutts, R.D., and Amiel, D. Osteochondral repair using perichondrial cells. A 1-year study in rabbits. Clin Orthop Relat Res 220, 1997. [DOI] [PubMed] [Google Scholar]
- 78. Engler, A.J., Sen, S., Sweeney, H.L., and Discher, D.E.. Matrix elasticity directs stem cell lineage specification. Cell 126, 677, 2006. [DOI] [PubMed] [Google Scholar]
- 79. Zhang, W., Lian, Q., Li, D., et al. . The effect of interface microstructure on interfacial shear strength for osteochondral scaffolds based on biomimetic design and 3D printing. Mater Sci Eng C Mater Biol Appl 46, 10, 2015. [DOI] [PubMed] [Google Scholar]
- 80. Bartnikowski, M., Akkineni, A.R., Gelinsky, M., Woodruff, M.A., and Klein, T.J.. A hydrogel model incorporating 3D-plotted hydroxyapatite for osteochondral tissue engineering. Materials (Basel) 9, 285, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Li, Z., Jia, S., Xiong, Z., et al. . 3D-printed scaffolds with calcified layer for osteochondral tissue engineering. J Biosci Bioeng 126, 389, 2018. [DOI] [PubMed] [Google Scholar]
- 82. You, F., Chen, X., Cooper, D.M.L., Chang, T., and Eames, B.F.. Homogeneous hydroxyapatite/alginate composite hydrogel promotes calcified cartilage matrix deposition with potential for three-dimensional bioprinting. Biofabrication 11, 015015, 2018. [DOI] [PubMed] [Google Scholar]
- 83. Yu, X., Zhao, T., Qi, Y., et al. . In vitro chondrocyte responses in Mg-doped wollastonite/hydrogel composite scaffolds for osteochondral interface regeneration. Sci Rep 8, 17911, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Idaszek, J., Costantini, M., Karlsen, T.A., et al. . 3D bioprinting of hydrogel constructs with cell and material gradients for the regeneration of full-thickness chondral defect using a microfluidic printing head. Biofabrication 11, 044101, 2019. [DOI] [PubMed] [Google Scholar]
- 85. Kosik-Koziol, A., Costantini, M., Mroz, A., et al. . 3D bioprinted hydrogel model incorporating beta-tricalcium phosphate for calcified cartilage tissue engineering. Biofabrication 11, 035016, 2019. [DOI] [PubMed] [Google Scholar]
- 86. Diloksumpan, P., de Ruijter, M., Castilho, M., et al. . Combining multi-scale 3D printing technologies to engineer reinforced hydrogel-ceramic interfaces. Biofabrication 12, 025014, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Kilian, D., Ahlfeld, T., Akkineni, A.R., Bernhardt, A., Gelinsky, M., and Lode, A.. 3D bioprinting of osteochondral tissue substitutes—in vitro-chondrogenesis in multi-layered mineralized constructs. Sci Rep 10, 8277, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Ashammakhi, N., Hasan, A., Kaarela, O., et al. . Advancing frontiers in bone bioprinting. Adv Healthc Mater 8, e1801048, 2019. [DOI] [PubMed] [Google Scholar]
- 89. Di Bella, C., Fosang, A., Donati, D.M., Wallace, G.G., and Choong, P.F.. 3D bioprinting of cartilage for orthopedic surgeons: reading between the lines. Front Surg 2, 39, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Guo, T., Ringel, J.P., Lim, C.G., et al. . Three dimensional extrusion printing induces polymer molecule alignment and cell organization within engineered cartilage. J Biomed Mater Res A 106, 2190, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Lin, K., Sheikh, R., Romanazzo, S., and Roohani, I.. 3D printing of bioceramic scaffolds-barriers to the clinical translation: from promise to reality, and future perspectives. Materials (Basel) 12, 2660, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Harris, J.D., Siston, R.A., Brophy, R.H., Lattermann, C., Carey, J.L., and Flanigan, D.C.. Failures, re-operations, and complications after autologous chondrocyte implantation—a systematic review. Osteoarthritis Cartilage 19, 779, 2011. [DOI] [PubMed] [Google Scholar]
- 93. Schaefer, D., Martin, I., Jundt, G., et al. . Tissue-engineered composites for the repair of large osteochondral defects. Arthritis Rheum 46, 2524, 2002. [DOI] [PubMed] [Google Scholar]
- 94. Schek, R.M., Taboas, J.M., Segvich, S.J., Hollister, S.J., and Krebsbach, P.H.. Engineered osteochondral grafts using biphasic composite solid free-form fabricated scaffolds. Tissue Eng 10, 1376, 2004. [DOI] [PubMed] [Google Scholar]
- 95. Harley, B.A., Lynn, A.K., Wissner-Gross, Z., Bonfield, W., Yannas, I.V., and Gibson, L.J.. Design of a multiphase osteochondral scaffold. II. Fabrication of a mineralized collagen-glycosaminoglycan scaffold. J Biomed Mater Res A 92, 1066, 2010. [DOI] [PubMed] [Google Scholar]
- 96. Scotti, C., Wirz, D., Wolf, F., et al. . Engineering human cell-based, functionally integrated osteochondral grafts by biological bonding of engineered cartilage tissues to bony scaffolds. Biomaterials 31, 2252, 2010. [DOI] [PubMed] [Google Scholar]
- 97. Yang, P.J., and Temenoff, J.S.. Engineering orthopedic tissue interfaces. Tissue Eng Part B Rev 15, 127, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Ebnesajjad, S., and Landrock, A.H.. Chapter 1—introduction and adhesion theories. In: Ebnesajjad, S., and Landrock, A.H., eds. Adhesives Technology Handbook (Third Edition). Boston, MA: William Andrew Publishing, 2015, p. 1. [Google Scholar]
- 99. Brockman, W., Geib, P.L., Kligen, J., and Schroder, B. Adhesive bonding. Materials, Applications and Technology. Wiley-Vch Press, Germany. 2009. [Google Scholar]
- 100. Petrie, E.M. Handbook of Adhesives and Sealants, Second Edition. McGraw-Hill Education, New York, United States. 2007. [Google Scholar]
- 101. Romito, L., and Ameer, G.A.. Mechanical interlocking of engineered cartilage to an underlying polymeric substrate: towards a biohybrid tissue equivalent. Ann Biomed Eng 34, 737, 2006. [DOI] [PubMed] [Google Scholar]