Abstract
Traumatic brain injury (TBI) is a major cause of death in the young age group and leads to persisting neurological impairment in many of its victims. It may result in permanent functional deficits because of both primary and secondary damages. This review addresses the role of oxidative stress in TBI-mediated secondary damages by affecting the function of the vascular unit, changes in blood-brain barrier (BBB) permeability, posttraumatic edema formation, and modulation of various pathophysiological factors such as inflammatory factors and enzymes associated with trauma. Oxidative stress plays a major role in many pathophysiologic changes that occur after TBI. In fact, oxidative stress occurs when there is an impairment or inability to balance antioxidant production with reactive oxygen species (ROS) and reactive nitrogen species (RNS) levels. ROS directly downregulate proteins of tight junctions and indirectly activate matrix metalloproteinases (MMPs) that contribute to open the BBB. Loosening of the vasculature and perivascular unit by oxidative stress-induced activation of MMPs and fluid channel aquaporins promotes vascular or cellular fluid edema, enhances leakiness of the BBB, and leads to progression of neuroinflammation. Likewise, oxidative stress activates directly the inflammatory cytokines and growth factors such as IL-1β, tumor necrosis factor-α (TNF-α), and transforming growth factor-beta (TGF-β) or indirectly by activating MMPs. In another pathway, oxidative stress-induced degradation of endothelial vascular endothelial growth factor receptor-2 (VEGFR-2) by MMPs leads to a subsequent elevation of cellular/serum VEGF level. The decrease in VEGFR-2 with a subsequent increase in VEGF-A level leads to apoptosis and neuroinflammation via the activation of caspase-1/3 and IL-1β release.
Keywords: Traumatic brain injury, Oxidative stress, Blood-brain barrier, Matrix metalloproteinases, Vascular endothelial growth factor, Neuroinflammation, Edema
Introduction
Traumatic brain injury (TBI) is characterized by physical brain injury that temporarily or permanently impairs cognitive function and is a major cause of death and disability worldwide, particularly among the young population. TBI causes approximately 1.5 million deaths and hospitalizations annually in the USA. TBI is classified based on the origin of mechanical forces as blast, blunt, and ballistic. In ballistic injury, the penetrating object pierces the skull and enters to the brain. Then the penetrating object may be expelled out, depending on the kinetic energy at the time of contact, from the opposite side along with some attached brain tissues or stuck inside the brain [1]. In blunt injury, the head of the victim collides with stationary or moving object. During the process of collision, the head encounters a directional force in a local region. Furthermore, the head and the brain translate or rotate depending on the magnitude and direction of the impacting force. The blast injury is complex and the facets of the injuries include components of both blunt and ballistic injuries. Based on the severity of TBI, patients are typically categorized into mild, moderate, and severe by using the Glasgow Coma Scale, a system used to assess coma and impaired consciousness [2]. The Glasgow Coma Scale is divided into three components—eye opening, verbal response, and motor responses. These are usually added together to produce a total score. A Glasgow Coma Scale score of 13–15 is defined as mild, 9–12 as moderate, and 3–8 as severe [2]. The mechanisms of brain tissue injury associated with TBI have been classified as primary and secondary. Primary injury is the result of mechanical forces applied to the skull and brain at the time of impact, which is believed to be irreversible [3]. The primary injury leads to skull fractures, brain contusions, axonal injuries, rupturing of blood vessels, and intracranial hemorrhages [4]. Secondary injury, on the other hand, evolves over time [5]. These are characterized by a complex cascade of biochemical events that lead to elevated intracranial pressure, blood-brain barrier (BBB) disruption, neuroinflammation, brain edema, cerebral hypoxia, ischemia, and delayed neurodegeneration [6-8]. Furthermore, a series of molecular, neurochemical, cellular, and pathophysiological mechanisms contribute to secondary injury. Secondary brain injury may be reversible; therefore, therapeutic intervention can be targeted. We and some other investigators showed that oxidative stress plays a pivotal role in secondary brain injury [9-11].
Oxidative stress is the biochemical and physiological stress or damage caused by the free radicals and can be neutralized by antioxidants. Free radicals contain unpaired electrons that are formed when oxygen is partially reduced and these free radicals attack cell components. Free radicals can then form long-lasting toxic materials that can make effects even beyond its site of production [12]. These toxic species and free radicals are collectively called reactive oxygen species (ROS) and reactive nitrogen species (RNS). The excitotoxicity and enervation of the endogenous antioxidant system (e.g., superoxide dismutase, glutathione peroxidase, and catalase) favors the production of a high level of ROS/RNS that induces peroxidation of cellular and vascular structures, protein oxidation, cleavage of DNA, and impairment of the mitochondrial electron transport chain [13]. These mechanisms are adequate to contribute to neuroinflammation, early or late apoptotic programs, and immediate cell death. In this review, we discuss about oxidative stress in TBI and its effect on BBB dysfunction, neuroinflammation, neurodegeneration, and impairment of cognitive function. In addition to these, a prior description on the major biomarkers of TBI is essential as they are the primary measures of brain damages.
Biomarkers of Traumatic Brain Injury
The use of markers for tissue damage in the central nervous system (CNS), such as S100 calcium-binding protein β (S100β) and neuron-specific enolase (NSE), has been proposed as potentially useful in order to quantify the severity of the TBI early in the process [14]. S100β is a calcium-binding protein having a molecular mass of 21 kDa. It is mainly released by glial cells, but it is also released by some other cells such as adipocytes, bone marrow, skeletal muscle, and melanocytes [15, 16]. NSE is a glycolytic protein having a molecular mass of 78 kDa. It is mainly released by neurons and some other cells like smooth muscle, adipose tissue, platelets, and red blood cells. NSE is widely used to assess neuronal damage [17]. The release of S100β and NSE from the CNS into peripheral circulation is believed due to disturbed membrane integrity of brain cells and increased permeability of the BBB after a traumatic event [15, 18]. However, the exact mechanism of cellular release is not yet fully studied. The serum measurements for S100β and NSE within 6 h of TBI would give a clear picture of the severity of brain injury and can be correlated with clinical outcome [16]. In our analysis, significantly higher levels of S100β and NSE were found in the blood samples of mild traumatic brain injury (mTBI) shock wave-exposed animals when compared with controls. The maximum level of S100β was found at 6 h, whereas leaking of NSE across the damaged BBB into the bloodstream continued to increase even at 24 h after the primary blasts [9].
Cleaved-tau (C-tau) protein is another important biomarker for TBI. Tau is a microtubule-associated phosphoprotein predominantly expressed in axons of neurons within the CNS. TBI results in the proteolysis of tau, producing a cleaved product called C-tau [19]. Serum C-tau levels are dependent on both compromised BBB as well as the degree of neuronal damage in the brain. A number of investigators reported the elevated levels of tau protein in CSF and serum following TBI [20-22]. A significant increase in the level of C-tau at 6 h of posttrauma in the serum of cortical contusion injury rats has been reported [19]. However, some studies suggested that tau protein is a poor biomarker for mild TBI [23]. Recently, Goldstein et al. [24] reported the high level of phosphorylated tau protein in TBI patients. Phosphorylation of tau protein is developmentally regulated such that fetal tau is more phosphorylated than adult brain tau [25]. Phosphorylation inhibits the ability of tau to bind to microtubules making them less stable. A few other biomarkers have also been reported in TBI, viz. glial fibrillary acidic protein (GFAP), isozyme of creatine kinase, and myelin basic protein (MBP) (see review [26]).
Oxidative Stress in Traumatic Brain Injury
The secondary injury of TBI is primarily due to oxidative stress. It has a significant role in the etiology of progressive neuropathology in TBI. ROS and RNS are the main sources of oxidative stress in brain injury. ROS includes superoxide (O2•−), hydroxyl radical (HO•), hydrogen peroxide (H2O2), and hypochlorous acid (HOCl). RNS refer to various nitric oxide (NO)-derived compounds, such as peroxynitrite (ONOO−) and nitrogen dioxide (NO2). RNS have different reactivities and the half-lives of RNS are generally longer than O2•−and HO• [27]. There are a number of enzymes involved in free radical generation, viz. the NADPH oxidase family, inducible nitric oxide synthase (iNOS), endothelial nitric oxide synthase (eNOS), cytochrome P450 (CYP450), cyclooxygenase (COX), lipoxygenase (LOX), and xanthine oxidase (XO). The role of these enzymes has been reviewed extensively [28-31], and the mechanisms of their activation and inhibition are beyond the scope of this review.
The most common free radical in TBI is superoxide (O2•−). It is produced when oxygen molecules gain an electron from other molecules. This superoxide causes tissue damage by promoting hydroxyl radicals from hydrogen peroxide (H2O2) and peroxynitrite when combined with NO. The power house organelle, mitochondria, is the major source of O2•−in brain injury [32]. In addition, the enzyme NADPH oxidase is a major contributor to posttraumatic cellular ROS production [11, 33]. In the cell, NADPH oxidase produces superoxide by transferring electrons from NADPH across the membrane and combining these to molecular oxygen to produce superoxide anion [34]. Another prooxidant enzyme, iNOS, has a significant role in the production of RNS by catalyzing the generation of NO from l-arginine. Several authors extensively studied the role of NO in generating oxidative stress in TBI [35-37]. NO has both neurodestructive and neuroprotective roles [36, 38]. In TBI, the neurodestructive role of NO is its involvement in the generation of toxic free radicals by lipid peroxidation and protein nitration. RNS are produced by coupling this NO with superoxide to form peroxynitrite (ONOO−). Recently, we have reported that the biochemical damage of the CNS is induced by the upregulation of these free radical-generating enzymes such as NADPH oxidase 1 (NOX1) and iNOS in TBI rats [9]. Induction of these enzymes by shock wave exposure paralleled the signatures of oxidative and nitrosative damage (4-hydroxynonenal (4-HNE)/3-nitrotyrosine (3NT)) [9]. The increased level of NOX activity in the cerebral cortex and hippocampal CA1 regions with an early peak at 1 h has been reported [11]. The significantly high levels of 4-HNE and 3NT at 3 h post-exposure and returned to control levels at 24 h post-exposure have also been reported [10]. In situ localization using oxidized hydroethidine and the neuronal marker, NeuN, reveals that the O2•−induction occurs in neurons at 1 h after TBI. By using the NOX inhibitor, apocyanin, oxidative stress damage can be minimized [11]. Oxidative/nitrosative stresses modify proteins via carbonylation, nitration, and peroxidation. Synaptic proteins also may be affected through these modifications [39].
Hydrogen peroxide (H2O2) is a relatively stable molecule and it is formed by dismutation of superoxide radicals. It is generated from nearly all sources of oxidative stress and can diffuse freely in and out of cells and tissues [40]. It aggravates cell proliferation and induces cell death either via apoptosis or necrosis. Monoamine oxidase is another important source of H2O2 [41]. H2O2 can be activated by transition metals in their reduced states to hydroxyl radical (HO•), which is a potent oxidant and aids to excess tissue damage [40].
Mitochondria are well involved in the generation of ROS. They themselves are targets of oxidative stress and also important in redox signaling from the organelle to the rest of the cell [42]. In 1966, Jensen first reported that ROS produced through mitochondrial respiratory chain [43]. Later, Chance and colleagues showed that the isolated mitochondria produce H2O2 [44-46]. During the Q cycle, it has been shown that electron “leakage” to O2 yields superoxide anion radical (O2•−) and hydrogen peroxide (H2O2). Thus, it appears that the cytochrome bc1 complex may be a significant source of ROS during TBI wave induction. An additional source of mitochondrial ROS generation may be the NADH dehydrogenase complex, in which O2•−is produced during autooxidation of the flavin mononucleotide (FMN) [47]. Therefore, mitochondrial electron transport constitutes the major intracellular source of ROS. Formation of mitochondrial ROS promotes inappropriate activation of the mitochondrial permeability transition, leading the cells to proapoptotic pathways [48].
Biomarkers of Oxidative Stress
ROS can be measured either directly or indirectly following the formation of oxidative by-products of lipids, proteins, or nucleic acids. Different types of oxidative stress biomarkers have been extensively reviewed recently [49, 50]. However, here we discuss about the major biomarkers of oxidative/nitrosative stress briefly.
4-HNE, isoprostanes (IsoPs), and malondialdehyde (MDA) are the major by-products of lipid peroxidation. 4-HNE is one of the most abundant and active lipid peroxides and is derived from the peroxidation of n-6 polyunsaturated fatty acids such as arachidonic and linoleic acids. IsoPs are a family of stable, prostaglandin-like compounds generated from the peroxidation of arachidonic acid [51]. MDA is potentially atherogenic lipid peroxide and generated in vivo via peroxidation of polyunsaturated fatty acids [52]. Another lipid biomarker of oxidative stress is 8-epi-PGF2a. This derivative is produced in vivo by free radical peroxidation of arachidonyl-containing lipids [51].
Nitrosative stress, mainly detected by the presence of 3NT, has been demonstrated in TBI [9]. Protein tyrosine nitration is mediated by RNS such as peroxynitrite (ONOO−) and nitrogen dioxide (NO2) and results in a nitro group adduct on susceptible tyrosine residues [53]. S-glutathionylation is another protein modification marker of oxidative stress. S-glutathionylation is the formation of a disulfide bridge between a reactive cysteine residue and the abundant cellular tripeptide glutathione [54]. Bursell and King have been reported S-glutathionylation of hemoglobin as a potential marker of oxidative stress [55].
Oxidative stress damages nucleic acids either by DNA fragmentation (single- and double-stranded DNA breaks) or modification and loss of bases due to oxidative stress [56]. 8-Hydroxy-2′-deoxyguanosine (8-OHdG) is widely used as an index of DNA oxidative damage.
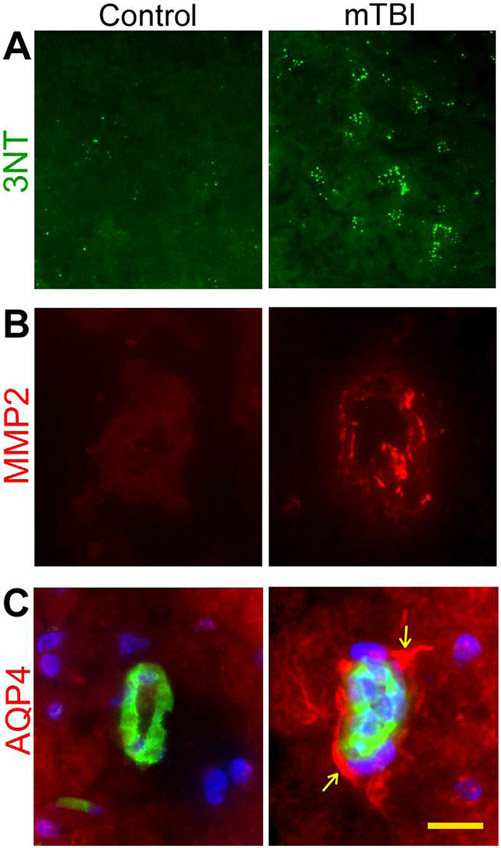
ROS can be detected directly by electron paramagnetic resonance (EPR; or electron spin resonance). Using EPR, the superoxide can be detected with superoxide-specific spin probes [57]. In our previous study, we used 4-HNE and 3NT (Fig. 1a) as major biomarkers of oxidative/nitrosative stress in TBI rats besides the direct measurement of ROS (cumulative detection of both hydroxyl and superoxide free radicals) using EPR [9]. In the next section, we discuss BBB permeability and neuroinflammation in TBI.
Fig. 1.
Induction of oxidative/nitrosative stress and activation of MMP-2 and AQP-4 in primary blast (123-kPa peak overpressure) induced mTBI rat brain microvessels. Immunofluorescent staining of nitrosative stress marker, 3-nitrotyrosine (3NT) in mTBI rat brain cortex (a); matrix metalloproteinases-2 (MMP-2) in mTBI rat in intact brain microvessels (b); aquaporin-4 (AQP-4) (red) and endothelial marker, GLUT1 (green) in brain microvessels of rats exposed to primary blast (c). Cell nuclei were counterstained with DAPI (blue) in c. Scale bar (yellow bar in last panel)=5 μm in all panels. For details, see Abdul-Muneer et al. [9]
Blood-Brain Barrier Impairment in TBI
The blood-brain barrier (BBB) is a regulating interface between the peripheral blood circulation and the CNS. In 1885, Paul Ehrlich first studied the existence of the BBB. The BBB is composed of brain microvascular endothelial cells (BMVEC), astrocytes, basement membrane, and pericytes and neurons and are constituting a “neurovascular unit” [58, 59]. Pericytes and endothelial cells are encircled by the basal lamina; a thin basement membrane supports the abluminal surface of the endothelium and is composed of collagen type IV, heparin sulfate proteoglycans, laminin, fibronectin, and other extracellular matrix proteins [60]. Astrocytes are adjacent to the endothelial cell with astrocytic end feet sharing the basal lamina. In addition to astrocytes, the microglia are also closely associated with the brain endothelium and are involved in the integrity of the BBB. These glial and endothelial cells functionally interact with each other in a paracrine manner.
Under physiological conditions, the BBB ensures constant supply of nutrients (oxygen, glucose, and other substances) for brain cells and guides the inflammatory cells to respond to the changes of the local environment. BMVEC possess unique barrier functional properties and are connected by tight junctions (TJ), which are composed of transmembrane proteins occludin/claudin-5 and intracellular zonula occludens (ZO-1, ZO-2, and ZO-3) [59]. TJ provides structural integrity and low permeability of the monolayer. Several intrinsic signaling pathways are involved in the modulation of expression and subcellular localization of TJ proteins [61].
The BBB compromise or damage leads to several neurodegenerative diseases and other neurological complications. Signs of BBB compromise are seen in neurological disorders including stroke [62], Alzheimer’s disease [63], HIV-1 encephalitis [64], multiple sclerosis [65], and TBI [66]. Besides the pathological conditions or diseases, drug or alcohol abuse compromises the integrity of the BBB [67-69]. This review addresses the neurological complications induced by oxidative stress and other neuroinflammatory agents associated with TBI.
In our recent study, we have shown that oxidative stress induces cerebral vascular injury (BBB damage) and neuroinflammation via activation of matrix metalloproteinases in single (one time only shock wave pressure exposure) or repeated (more than one shock wave pressure exposure on the same animal) exposures to low-intensity blast over pressure [9]. In 123-kPa shock wave pressure-induced mTBI in rats, the immunofluorescence and Western blotting methods showed the diminished expression of tight junction proteins in the brain microvessels of treated rats when compared with controls [9]. The peroxidation of membrane lipids is one of the consequences of oxidative stress in TBI, which would affect the permeability of the BBB [70]. Hydroxyl radicals (•OH) may play an important role in peroxidation of membrane lipids in the formation of 4-HNE that increases the permeability of the BBB [71]. The role of lipid peroxidation has been proven by administrating an inhibitor of lipid peroxidation that blocks posttraumatic damage of the BBB [72]. These pathological processes can contribute to long-term neurological disorders.
Infiltration and accumulation of immune cells into brain parenchyma in acute posttraumatic injury is another consequence of BBB damage [73]. Intercellular adhesion molecule (ICAM-1) helps the migration of immune cells into the damaged tissue by mediating the adhesion of these cells to the endothelium. Upregulation of ICAM-1 has been reported in several experimental TBI models [74, 75]. A number of studies have been addressed to study the BBB disruption by analyzing the level of S100β in CSF [76-78]. The BBB permeability has been proven by injecting intravenously the visible tracers biotin-dextrin-amine 3000 (BDA-3 K, 3 kDa) and horseradish peroxidase (HRP, 44 kDa) at 4 h or 3 or 7 days post-TBI, in which both small and large molecular weight tracers were detected in the contusion area and even in remote regions of the injury. However, the larger tracer molecule (HRP) was not detected at later posttraumatic time periods [6]. In another study, the passive BBB dysfunction has been proven by analyzing the CSF-plasma albumin quotient in TBI patients [79].
Several authors showed evidence of BBB disruption following TBI by using pharmacological inhibitors and some other agents, in which they could attenuate TBI-mediated BBB breakdown, upregulation of matrix metalloproteinases (MMPs), aquaporins (AQPs), and other inflammatory agents. Tail intravenous injection ofpoloxamer 188 (anti-inflammatory drug) in TBI mice could reduce TBI-induced brain edema and restored BBB integrity, suppressed TBI-induced neural cell death, and improved neurological function [80]. Neutrophil depletion with an antipolymorphonuclear leukocyte antibody (anti-PMN) before inducing intracerebral hemorrhage in the rat striatum reduces neutrophil infiltration, BBB breakdown, and expression of MMP-9 [81]. In another study, Lopez et al. [82] showed that treatment with orexigenic hormone ghrelin decreases BBB permeability and the level of perivascular AQP-4 and S100β following TBI in mice.
Role of Matrix Metalloproteinases in BBB Permeability
MMPs are zinc-dependent endopeptidases, collectively called matrixins, that participate mainly in extracellular matrix (ECM) degradation. Under normal physiological conditions, the activities of MMPs are precisely regulated at the level of transcription, activation of the precursor zymogens, interaction with specific ECM components, and inhibition by endogenous inhibitors [83, 84]. The regulation of MMP expression and activation is complex and tightly controlled, and loss of this control may result in diseases such as arthritis, cancer, atherosclerosis, aneurysms, nephritis, tissue ulcers, and fibrosis [85, 86]. MMPs are broadly divided into two general classes: the secreted MMPs and membrane-type MMPs [87]. To date, 24 different vertebrate MMPs have been identified, of which 23 are found in humans. MMPs can be grouped according to their domain structure into collagenases, gelatinases, stromelysins, and matrilysin. The actions of MMPs are strictly controlled by endogenous MMP inhibitors (MMPIs) and tissue inhibitors of MMPs (TIMPs). TIMPs are specific inhibitors of matrixins that participate in controlling the local activities of MMPs in tissues [88]. Overexpression of MMPs results in an imbalance between the activity of MMPs and TIMPs that can lead to a variety of pathological disorders [85]. There are four types of TIMPs: TIMP-1, TIMP-2, TIMP-3, and TIMP-4.
Activation of MMPs due to oxidative stress degrades basement membrane proteins resulting in loss of brain endothelium stability and increases BBB permeability in vivo [89-91] and in vitro [92]. Studies have revealed that activation of MMPs and degradation of microvascular basement membrane play an important role in stroke [93-95]. An increase in the activity of MMPs also degrades basement membrane proteins and disrupt TJ assembly, thus enhancing BBB permeability [96, 97]. Hanumegowda et al. [98] have reported that the loss of TJ function in brain endothelium is associated with the increase in activity of MMP-2 or MMP-9. Moreover, activation of MMPs leads to brain-blood microvessel disruption, featuring leukocyte infiltration and activation of resident brain macrophages (microglia) [94]. Recently, Reijerkerk et al. [99] reported that diapedesis and transendothelial migration of monocytes due to MMP mediated occludin protein degradation in rat brain endothelial cell line (GP8/3.9).
In TBI, several investigators have reported the involvement of MMPs in degrading various types of ECM proteins and TJ proteins of the BBB [100, 101]. MMP has a critical role in the pathophysiology of synaptic loss and BBB breakdown in TBI, alcohol abuse, stroke, and neurodegeneration [68, 102, 103]. We also demonstrated that the induction of free radical-generating enzymes causes oxidative damage, impairment of the BBB, and neuroinflammation via activation of matrix metalloproteinases and fluid channel activator aquaporin-4 in mTBI [9]. As depicted in Fig. 1b for MMP-2, in our study, the level of expression of the three types of MMPs (MMP-2, MMP-3, and MMP-9) was increased in mTBI rats [9]. There are several reports on the upregulation of MMPs, particularly MMP-2, MMP-3, and MMP-9 and their role in causing acute disruption of the BBB, leading to vasogenic edema and following cell death in TBI [104, 105]. Recently, Ranaivo et al. [106] showed the high level of MMP-9 in combination with mild stretch followed by IL-1β treatment in in vitro model of cell stretch to investigate the effects of mild mechanical insult on astrocyte injury. Higashida et al. [107] reported that when inhibiting MMP-9, the permeability of the BBB significantly ameliorated in TBI rats.
Role of Vascular Endothelial Growth Factor in BBB Disruption
Vascular endothelial growth factor (VEGF) is a heparin-binding protein that is considered the most potent proangiogenic growth factor involved in vascular permeability, vascular dilation, endothelial proliferation, and angiogenesis [108, 109]. Three high-affinity tyrosine kinase receptors exist for VEGF, viz. VEGFR-1 (fms-like tyrosine kinase-1), VEGFR-2 (fetal liver kinase-1/kinase domain region), and VEGFR-3 (fms-like tyrosine kinase-4), all of which are expressed almost exclusively on endothelial cells [110]. The vascular endothelial growth factor receptor-2 (VEGFR-2) plays a central role in the proliferation and apoptosis of vascular endothelium [111, 112]. VEGFR-2 (also known as KDR or Flk-1), is a 200–230-kDa high-affinity receptor for VEGF-A, the processed forms of VEGF-C and VEGF-D, and VEGF-E. It is expressed in both vascular endothelial and lymphatic endothelial cells; its expression has also been demonstrated in several other cell types such as megakaryocytes and hematopoietic stem cells [113]. Vegfr-2−/−embryos die by embryonic days 8.5–9.5, exhibiting defects in the development of endothelial and hematopoietic precursors, indicating that the receptor is crucial for vascular development [114].
Previous studies suggested that BBB disruption is due to the proteolytic degradation of the vascular membrane. Among proteases, MMPs, in particular MMP-2 and MMP-9, are able to digest the endothelial basal lamina and, therefore, may play a major role in promoting BBB permeability [115-117]. Valable et al. [118] reported that in ischemic animals, administration of VEGF leads to BBB permeability as to an induction of MMP-9 activity. The elevation of MMP-9 levels in stroke is also reported [119].
There are reports on the cleavage of VEGFR-2 by MMP-7 and MMP-9, where it cleaves VEGFR-2 at multiple positions (e.g., Leu-Ser/Met-Leu, Leu-Ser/Ile-Arg) [120]. Tran et al. [121] reported that the plasma MMP activity causes cleavage of extracellular, but not intracellular, domain of VEGFR-2 on the endothelium. This cleavage reduces the ability of the cell to bind VEGF agonists and may be one of the reasons for the enhanced apoptosis [121]. The inhibition of MMP with doxycycline attenuates VEGFR-2 cleavage as well as endothelial apoptosis [121]. Lee and Agoston [122] reported that a blockade of VEGFR-2 signaling with a selective inhibitor, SU5416, abrogates prosurvival response and induced high activation of caspase-3/7 and leads to cell death in TBI. In our recent study, we found that alcohol-mediated upregulation of MMPs leads to VEGFR-2 degradation in rats. In correlation with this observation, we found profound elevation of VEGF-A in the bloodstream and that in turn activates caspase-1 and causes cell apoptosis. This was evidenced from the treatment of VEGFR-2 kinase inhibitor, ki8751, which induces cell apoptosis [68]. The increase in the level of VEGF after TBI causes edema formation and neutrophilic invasion to the brain [123]. The enhancement of the level of VEGF-A further increases the level of ROS [124]. However, there are reports on the neuroprotective role of VEGF, where VEGF increases neurogenesis and angiogenesis and reduces lesion volume after TBI [125]. To resolve this controversy of whether VEGF damages or protects the brain tissue after TBI will be a field for future study.
Neuroinflammation in TBI
Several studies have shown that cytokines, chemokines, and growth factors have significant roles in the pathophysiology of TBI. Shortly after brain injury, there is mass production of proinflammatory cytokines, such as IL-1β and tumor necrosis factor-α (TNF-α) as well as transforming growth factor-beta (TGF-β), which further exacerbates the trauma condition of the brain with oxidative stress and MMPs to cause delayed recovery [126, 127]. The mRNA and protein concentrations of these cytokines have been shown to increase markedly in the acute posttraumatic period following experimental brain trauma in rats [128-130]. These posttraumatic inflammatory cascades also contribute to BBB dysfunction, which eventually leads to the influx of inflammatory cells from the blood to the brain [73].
In the event of TBI, IL-1β is the most studied cytokine. IL-1β is produced by glial cells and can act on neurons and other brain cells. IL-1β triggers inflammatory reactions and leads to recruitment of immune cells, disruption of the BBB and formation of edema, and loss of neurons [131-134]. The high level of IL-1β has been detected in CSF and brain tissue within early hours of brain injury in humans as well as in experimental animals [135, 136]. The role of IL-1β in the formation of brain edema, neuroinflammation, and neurodegeneration has been evidenced by using IL-1β inhibitor in experimental rats [137, 138]. Intracerebroventricular administration of an IL-1β-neutralizing antibody reduces cerebral edema and tissue loss and improves late cognitive outcome following TBI in mice [137]. Active caspase-1 is essential for the cleavage of pro-IL-1β into its mature, biologically active forms [139-141]. There are several reports on the activation of caspase-1 in TBI [142, 143]. Similarly, there are few reports on the upregulation of other types of interleukins such as IL-6, IL-8, IL-10, IL-12, and IL-18 in post-TBI [128, 144, 145]. Some of these interleukins have neuroprotective actions against injury [146-148].
TNF-α has important roles in neuronal development, cell survival, synaptic plasticity, and ionic homeostasis in the CNS. The dysregulation of TNF-α signaling has been implicated in the pathophysiology of several CNS conditions, specifically relating to immune cells infiltration [149] and increased BBB permeability [150]. TNF-α has been detected in the CSF and serum of patients with TBI [144, 151]. Several authors proved the increased activity of TNF-α in association with the loss of sensorimotor and cognitive functions in TBI models by pharmacologically or genetically inhibiting its activity [129, 152, 153].
Several in vivo studies showed that TGF-β is a major regulator of injury response [154]. This growth factor can be synthesized by nearly all cells of the CNS [155, 156] and its increased level has been detected in several CNS disorders including TBI [157, 158]. The high level of TGF-β in CSF has been detected within initial days of head-injured patients [158]. This high level of TGF-β in CSF than in serum may be as a result of damaged BBB. In 2009, Cacheaux et al. [159] made a striking investigation wherein rats treated with a drug that blocks TGF-β, it prevents epilepsy after brain damage.
Traumatic Brain Injury and Edema Formation
The formation of cerebral edema is one of the major factors leading to high mortality and morbidity in TBI. Cerebral edema may account for up to half of the mortality in all victims of TBI [160]. There are two types of edema: cellular and vasogenic [161]. Cellular edema is characterized by an increase in water content in the intracellular compartment. Vasogenic edema results from the breakdown of the BBB and this allows intravascular proteins and fluid to penetrate into the parenchymal extracellular space [162]. Usually, vasogenic edema forms in the first few hours after TBI, followed by cellular edema that develops more slowly over the next few days and prolongs up to 2 weeks [163]. TBI may lead to swelling of the brain tissue and elevate intracranial pressure (ICP), which may also lead to edema formation and BBB disruption [164].
Several mediators are involved in the formation of edema after TBI. Among them, AQPs are the key player in the development and resolution of cerebral edema [165]. AQPs are integral membrane proteins belonging to a larger family of major intrinsic proteins that form pores in the membranes of mammalian cells [166]. There are 13 known AQPs, and among these, AQP-1, AQP-4, and AQP-9 are highly expressed in the brain [167]. AQP-4 is predominantly expressed in astrocytic end feet. In our study, we reported a high level of AQP-4 in 123 kPa wave pressure-induced mTBI (Fig. 1c) and these AQP-4 were seen to be co-localized with GFAP (astrocytic marker) in close proximity to intracerebral vessels in mTBI brain tissue [9]. Several studies have shown that AQP-1, AQP-4, and AQP-9 expression is clearly altered in both experimental and clinical brain injury [168-170]. Several investigators reported that upregulation of AQPs after brain injury promotes edema formation [171-173] and it is evidenced that pharmacological inhibition of AQP-4 can control the formation of edema in TBI [172, 174, 175]. Blocking of hypoxia-inducible factor-1 alpha (HIF-1α), a transcription factor known to activate the expression of AQP-4 and AQP-9, by 2-methoxyestradiol (2ME2) reduces water content or edema formation in injured animals [172]. Higashida et al. [107] reported that the formation of edema can be reduced after inhibition of AQP-4, MMP-9, or HIF-1α.
Another important mediator of edema formation is MMPs. We have discussed the role of MMPs in the pathogenesis of TBI in the previous section. The role of MMPs in edema formation has been proven by using a pharmacological inhibitor of MMPs such as minocycline or TIMP-1, in which reduced BBB disruption and edema, reduced inflammatory response, and improved cognitive function have been observed [176-178]. Some other vasoactive agents also cause BBB disruption and formation of cerebral edema. These vasoactive agents include kinins, such as bradykinins and tachykinins [162, 179]. Donkin and Vink extensively reviewed about these vasoactive agents that cause edema formation in TBI (reviewed in [162]).
In our recent study, we established that the biochemical cerebral vascular/brain injury occurs within a window of 6–24 h after exposure to low-frequency shock wave pressure. Oxidative/nitrosative stress initiates neurovascular injury via the induction of NOX1 and iNOS by blast wave. The signatures of oxidative/nitrosative damage (4-HNE/3NT) in the microvessels paralleled an induction of NOX1/iNOS. The association of cerebral vascular oxidative injury with the downregulation of TJ proteins and the perivascular units with the subsequently enhanced immune cell infiltration justified the BBB dysfunction. Loosening of this BBB by shock wave exposure is exacerbated by oxidative stress-induced MMPs and fluid channel aquaporin-4. Our findings suggest that impairment of the BBB and perivascular units by MMPs promotes BBB leakiness, vascular fluid cavitation, edema formation, neuroinflammation, and subsequently neurotrauma [9].
Antioxidant Defenses and Clinical Strategies in TBI
The living cell has several defense mechanisms to compete with constant exposure to oxidative stress. The antioxidant defense system, which is one of the major defense mechanisms, assists the living cell to cope directly with oxidative stress and to prevent oxidative damage. The brain cells, which are particularly susceptible to oxidative damage, contain various types of antioxidants in which some of them are exclusive to the brain. There are two major groups of antioxidant system, viz. enzymes and low molecular weight antioxidants [180]. Superoxide dismutase (SOD), catalase, peroxidases, peroxyredoxins, and thioredoxins are several of the major enzymes directly regulating the levels of ROS and RNS. Ascorbic acid, glutathione (GSH), vitamin E, lipoic acid, and coenzyme Q are the low molecular weight antioxidants in the brain. Though the application of antioxidants is promising as therapeutic agents, there are limitations in the use of exogenous antioxidants. They are less amenable to the trafficking across the BBB; moreover, their instability and the fast rate of metabolism and toxicity at a higher dose are the major challenges for their therapeutic use [181]. Despite these limitations, targeted applications of natural and modified antioxidants are promising in developing therapeutic interventions.
Several researchers have been concentrating on neuroprotection in TBI patients or models by inhibiting or reducing oxidative/nitrosative stress and neuroinflammation. The ability of several agents or pharmacological inhibitors to attenuate oxidative stress and secondary cell death has been established in different models of TBI. The superoxide scavengers such as SOD [182], OPC-14117 [183], tempol (4-hydroxy-2,2,6,6-tetramethylpiperidin-1-oxyl) [184], α-phenyl-N-tert-butyl nitrone (PBN) [185], etc. showed to protect the brain from the pathophysiology of TBI. The antioxidant α-tocopherol is hydrophobic and is able to prevent lipid peroxidation [186] and edema [187]. Melatonin is another hydroxyl and superoxide radical scavenger, which reduces brain edema, neuronal death, and memory deficits [188]. Resveratrol, an antioxidant found to be rich in grapes, has been shown to be a promising neuroprotectant in TBI models, possibly by inhibiting lipid peroxidation and minimizing other oxidative damages [189]. Kline et al. examined the neuroprotective effects of bromocriptine (BRO), a dopamine D2 receptor agonist with significant antioxidant properties, with results of reduced lipid peroxidation in a rodent model of focal brain trauma [190]. Tirilazad, a steroid, attenuates cerebral and vasogenic edema by inhibiting lipid peroxidation after TBI [191]. Cannabinoids, a natural psychoactive drug, prevent lipopolysaccharide-induced neurodegeneration in the rat substantia nigra in vivo through inhibition of microglial activation and NOX1 [192]. Likewise, there are myriads of reports on the use of an antioxidant drug against TBI in animals and humans, and several of them give better improvement physically and cognitively.
Similarly, several clinical and basic science trials have been conducted by using different novel treatment strategies to improve the consequences of TBI. The most recent clinical trials have investigated drugs such as steroids (e.g., progesterone) [193], calcium channel inhibitors (e.g., dantrolene) [194], vitamin E [195], amantadine [196], citicholine [197], dexanabinol [198], minocycline [199, 200], and magnesium sulfate [201]. Unfortunately, no interventions have been successful enough in practice to be implemented as standard care [202]. Development of standard care practice by integrating various therapeutic approaches has great significance especially in the scenario of increasing TBI incidents.
Conclusions
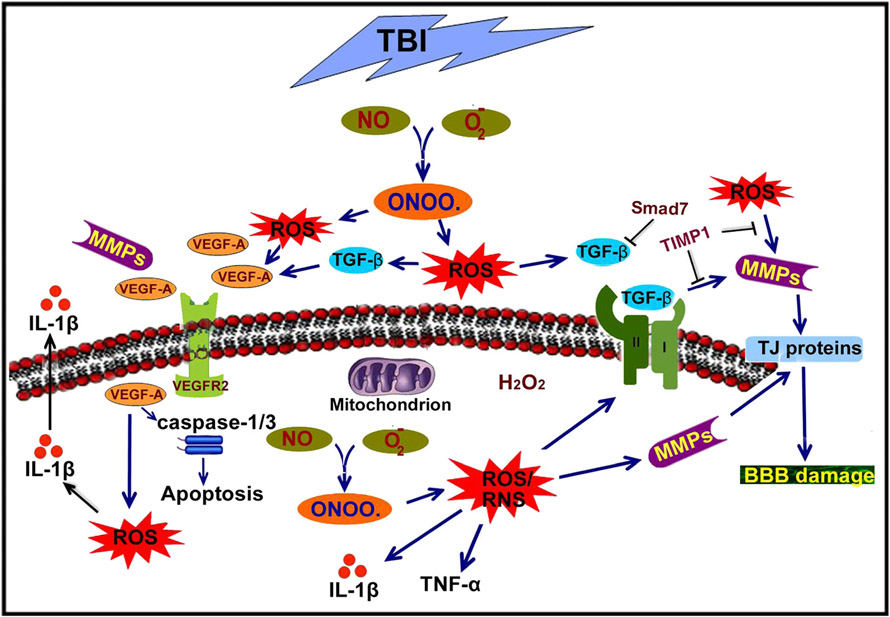
This review has highlighted the role of oxidative stress in the intricacy of BBB pathogenesis and cellular and molecular inflammatory cascades elicited after TBI. Figure 2 depicts the schematic representation of oxidative and inflammatory pathways in TBI. The physiological and biochemical responses to TBI, including MMP activation, VEGF accumulation, and neuroinflammation, have been discussed in this review. Furthermore, the development of cerebral edema and its possible reasons and its contribution to the high mortality and morbidity associated with TBI have been addressed. However, while writing this review, we understood that the exact role of VEGF in trauma formation after BBB impairment and apoptosis is not yet fully elucidated. The therapeutic aspects by attenuating BBB impairment, blocking of MMP activation, and reducing neuroinflammation are other scopes of future study. Resolving the issue of whether VEGF damages or protects the cells or tissues after TBI will also be an area of focus for the field.
Fig. 2.
Schematic representation of oxidative stress-induced BBB disruption and neuroinflammation in traumatic brain injury. Reactive oxygen species (ROS) and reactive nitrogen species (RNS) are the main sources of oxidative stress in brain injury. ROS include superoxide (O2•−), hydroxyl radical (HO•), hydrogen peroxide (H2O2), and hypochlorous acid (HOCl). RNS refer to various nitric oxide (NO)-derived compounds, such as peroxynitrite (−OONO) and nitrogen dioxide (NO2). Superoxide (O2•−) causes tissue damage by promoting hydroxyl radicals from hydrogen peroxide (H2O2) and peroxynitrite (−ONOO) when combined with nitric oxide (NO). ROS activate matrix metalloproteinases (MMPs) that further exacerbate the condition and lead to BBB disruption via degradation of the extracellular matrix and tight junction proteins. Further, MMPs are involved in degradation of vascular endothelial growth factor (VEGFR) and lead to an increase in the level of VEGF that in turn causes ROS and activates caspase-1/3, which leads to cell death. At the same time, ROS or RNS also activate different inflammatory cytokines and growth factors such as IL-1β, TNF-α, and TGF-β, which cause BBB disruption and neuroinflammation
Normal functioning of the BBB is the key factor for brain repair and homeostasis. Attenuation of BBB permeability is a promising approach to control brain edema and associated neuroinflammation. Targeting on restoring BBB function after injury is the main objective for neuroprotection in TBI. It will allow a more reliable delivery of brain-targeted therapeutic agents/drugs. In terms of potential therapeutic strategies and interventions, future treatment of TBI will require a detailed understanding of cerebral physiology and function as well as the pathological and restorative responses toward therapeutic agents. The development of innovative research designs with antioxidants and other anti-inflammatory agents would provide a better therapeutic strategy to treat the pathophysiology of mTBI before advancing toward posttraumatic stress disorder (PTSD).
Acknowledgments
This work was supported by NIH/NIAAA Grant R21AA020370-01A1 to J.H. and by the U.S. Army Research Office project “Army–UNL Center of Trauma Mechanics” (Contract W911NF-08-10483) to N.C.
Footnotes
Conflict of Interest The authors declare that they have no conflict of interest.
Contributor Information
P. M. Abdul-Muneer, Department of Pharmacology and Experimental Neuroscience, University of Nebraska Medical Center, Omaha, NE 68198, USA
Namas Chandra, Department of Biomedical Engineering, New Jersey Institute of Technology, Newark, NJ 07103, USA.
James Haorah, Department of Pharmacology and Experimental Neuroscience, University of Nebraska Medical Center, Omaha, NE 68198, USA.
References
- 1.Lee M, Longoria R, Wilson D (1997) Ballistic waves in high-speed water entry. Fluid Struct 11(7):819–844 [Google Scholar]
- 2.Teasdale G, Jennett B (1974) Assessment of coma and impaired consciousness. A practical scale. Lancet 2(7872):81–84 [DOI] [PubMed] [Google Scholar]
- 3.Gaetz M (2004) The neurophysiology of brain injury. Clin Neurophysiol 115(1):4–18 [DOI] [PubMed] [Google Scholar]
- 4.Maas AI, Stocchetti N, Bullock R (2008) Moderate and severe traumatic brain injury in adults. Lancet Neurol 7(8):728–741 [DOI] [PubMed] [Google Scholar]
- 5.Beauchamp K, Mutlak H, Smith WR, Shohami E, Stahel PF (2008) Pharmacology of traumatic brain injury: where is the “golden bullet”? Mol Med 14(11–12):731–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lotocki G, de Rivero Vaccari JP, Perez ER, Sanchez-Molano J, Furones-Alonso O, Bramlett HM, Dietrich WD (2009) Alterations in blood-brain barrier permeability to large and small molecules and leukocyte accumulation after traumatic brain injury: effects of post-traumatic hypothermia. J Neurotrauma 26(7):1123–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pun PB, Lu J, Moochhala S (2009) Involvement of ROS in BBB dysfunction. Free Radic Res 43(4):348–364 [DOI] [PubMed] [Google Scholar]
- 8.Toklu HZ, Hakan T, Biber N, Solakoğlu S, Oğunç AV, Sener G (2009) The protective effect of alpha lipoic acid against traumatic brain injury in rats. Free Radic Res 43(7):658–667 [DOI] [PubMed] [Google Scholar]
- 9.Abdul-Muneer PM, Schuetz H, Wang F, Skotak M, Jones J, Gorantla S, Zimmerman MC, Chandra N, Haorah J (2013) Induction of oxidative and nitrosative damage leads to cerebrovascular inflammation in an animal model of mild traumatic brain injury induced by primary blast. Free Radic Biol Med 60:282–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Readnower RD, Chavko M, Adeeb S, Conroy MD, Pauly JR, McCarron RM, Sullivan PG (2010) Increase in blood-brain barrier permeability, oxidative stress, and activated microglia in a rat model of blast-induced traumatic brain injury. J Neurosci Res 88(16):3530–3539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang QG, Laird MD, Han D, Nguyen K, Scott E, Dong Y, Dhandapani KM, Brann DW (2012) Critical role of NADPH oxidase in neuronal oxidative damage and microglia activation following traumatic brain injury. PLoS One 7(4):e34504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poon HF, Calabrese V, Scapagnini G, Butterfield DA (2004) Free radicals and brain aging. Clin Geriatr Med 20(2):329–359 [DOI] [PubMed] [Google Scholar]
- 13.Cornelius C, Crupi R, Calabrese V, Graziano A, Milone P, Pennisi G, Radak Z, Calabrese EJ, Cuzzocrea S (2013) Traumatic brain injury: oxidative stress and neuroprotection. Antioxid Redox Signal 19(8):836–853 [DOI] [PubMed] [Google Scholar]
- 14.Herrmann M, Curio N, Jost S, Wunderlich MT, Synowitz H, Wallesch CW (1999) Protein S-100B and neuron specific enolase as early neurobiochemical markers of the severity of traumatic brain injury. Restor Neurol Neurosci 14(2–3):109–114 [PubMed] [Google Scholar]
- 15.Berger RP, Adelson PD, Pierce MC, Dulani T, Cassidy LD, Kochanek PM (2005) Serum neuron-specific enolase, S100B, and myelin basic protein concentrations after inflicted and noninflicted traumatic brain injury in children. J Neurosurg 103(1 Suppl):61–68 [DOI] [PubMed] [Google Scholar]
- 16.Geyer C, Ulrich A, Gräfe G, Stach B, Till H(2009) Diagnostic value of S100B and neuron-specific enolase in mild pediatric traumatic brain injury. J Neurosurg Pediatr 4(4):339–344 [DOI] [PubMed] [Google Scholar]
- 17.de Kruijk JR, Leffers P, Menheere PP, Meerhoff S, Twijnstra A (2001) S-100B and neuron-specific enolase in serum of mild traumatic brain injury patients. A comparison with health controls. Acta Neurol Scand 103(3):175–179 [DOI] [PubMed] [Google Scholar]
- 18.Büttner T, Weyers S, Postert T, Sprengelmeyer R, Kuhn W (1997) S-100 protein: serum marker of focal brain damage after ischemic territorial MCA infarction. Stroke 28(10):1961–1965 [DOI] [PubMed] [Google Scholar]
- 19.Gabbita SP, Scheff SW, Menard RM, Roberts K, Fugaccia I, Zemlan FP (2005) Cleaved-tau: a biomarker of neuronal damage after traumatic brain injury. J Neurotrauma 22:83–94 [DOI] [PubMed] [Google Scholar]
- 20.Marklund N, Blennow K, Zetterberg H, Ronne-Engström E, Enblad P, Hillered L (2009) Monitoring of brain interstitial total tau and beta amyloid proteins by microdialysis in patients with traumatic brain injury. J Neurosurg 110(6):1227–1237 [DOI] [PubMed] [Google Scholar]
- 21.Bulut M, Koksal O, Dogan S, Bolca N, Ozguc H, Korfali E, Ilcol YO, Parklak M (2006) Tau protein as a serum marker of brain damage in mild traumatic brain injury: preliminary results. Adv Ther 23(1):12–22 [DOI] [PubMed] [Google Scholar]
- 22.Zemlan FP, Rosenberg WS, Luebbe PA, Campbell TA, Dean GE, Weiner NE, Cohen JA, Rudick RA, Woo D (1999) Quantification of axonal damage in traumatic brain injury: affinity purification and characterization of cerebrospinal fluid tau proteins. J Neurochem 72(2):741–750 [DOI] [PubMed] [Google Scholar]
- 23.Bazarian JJ, Zemlan FP, Mookerjee S, Stigbrand T (2006) Serum S-100B and cleaved-tau are poor predictors of long-term outcome after mild traumatic brain injury. Brain Inj 20(7):759–765 [DOI] [PubMed] [Google Scholar]
- 24.Goldstein LE, Fisher AM, Tagge CA, Zhang XL, Velisek L, Sullivan JA, Upreti C, Kracht JM, Ericsson M, Wojnarowicz MW et al. (2012) Chronic traumatic encephalopathy in blast-exposed military veterans and a blast neurotrauma mouse model. Sci Transl Med 4(134):134–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goedert M (2001) The significance of tau and alpha-synuclein inclusions in neurodegenerative diseases. Curr Opin Genet Dev 11 (3):343–351 [DOI] [PubMed] [Google Scholar]
- 26.Hergenroeder GW, Redell JB, Moore AN, Dash PK (2008) Biomarkers in the clinical diagnosis and management of traumatic brain injury. Mol Diagn Ther 12(6):345–358 [DOI] [PubMed] [Google Scholar]
- 27.Balazy M, Nigam S (2003) Aging, lipid modifications and phospholipases—new concepts. Ageing Res Rev 2(2):191–209 [DOI] [PubMed] [Google Scholar]
- 28.Brandes RP, Weissmann N, Schröder K (2010) NADPH oxidases in cardiovascular disease. Free Radic Biol Med 49(5):687–706 [DOI] [PubMed] [Google Scholar]
- 29.Uttara B, Singh AV, Zamboni P, Mahajan RT (2009) Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr Neuropharmacol 7(1):65–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bedard K, Krause KH (2007) The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 87(1):245–313 [DOI] [PubMed] [Google Scholar]
- 31.Andrew PJ, Mayer B (1999) Enzymatic function of nitric oxide synthases. Cardiovasc Res 43(3):521–531 [DOI] [PubMed] [Google Scholar]
- 32.Sastre J, Pallardó FV, Viña J (2003) The role of mitochondrial oxidative stress in aging. Free Radic Biol Med 35(1):1–8 [DOI] [PubMed] [Google Scholar]
- 33.Choi BY, Jang BG, Kim JH, Lee BE, Sohn M, Song HK, Suh SW (2012) Prevention of traumatic brain injury-induced neuronal death by inhibition of NADPH oxidase activation. Brain Res 1481:49–58 [DOI] [PubMed] [Google Scholar]
- 34.Shi Q, Gibson GE (2007) Oxidative stress and transcriptional regulation in Alzheimer disease. Alzheimer Dis Assoc Disord 21(4):276–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.DeWitt DS, Prough DS (2009) Blast-induced brain injury and posttraumatic hypotension and hypoxemia. J Neurotrauma 26(6):877–887 [DOI] [PubMed] [Google Scholar]
- 36.Gahm C, Holmin S, Wiklund PN, Brundin L, Mathiesen T (2006) Neuroprotection by selective inhibition of inducible nitric oxide synthase after experimental brain contusion. J Neurotrauma 23(9):1343–1354 [DOI] [PubMed] [Google Scholar]
- 37.Hortobágyi T, Görlach C, Benyó Z, Lacza Z, Hortobágyi S, Wahl M, Harkany T (2003) Inhibition of neuronal nitric oxide synthase-mediated activation of poly(ADP-ribose) polymerase in traumatic brain injury: neuroprotection by 3-aminobenzamide. Neuroscience 121(4):983–990 [DOI] [PubMed] [Google Scholar]
- 38.Sinz EH, Kochanek PM, Dixon CE, Clark RS, Carcillo JA, Schiding JK, Chen M, Wisniewski SR, Carlos TM, Williams D et al. (1999) Inducible nitric oxide synthase is an endogenous neuroprotectant after traumatic brain injury in rats and mice. J Clin Invest 104(5):647–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ansari MA, Roberts KN, Scheff SW (2008) Oxidative stress and modification of synaptic proteins in hippocampus after traumatic brain injury. Free Radic Biol Med 45(4):443–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bayir H, Kochanek PM, Kagan VE (2006) Oxidative stress in immature brain after traumatic brain injury. Dev Neurosci 28(4–5):420–431 [DOI] [PubMed] [Google Scholar]
- 41.Simonson SG, Zhang J, Canada AT, Su YF, Benveniste H, Piantadosi CA (1993) Hydrogen peroxide production by monoamine oxidase during ischemia-reperfusion in the rat brain. J Cereb Blood Flow Metab 13(1):125–134 [DOI] [PubMed] [Google Scholar]
- 42.Balaban RS, Nemoto S, Finkel T (2005) Mitochondria, oxidants, and aging. Cell 120(4):483–495 [DOI] [PubMed] [Google Scholar]
- 43.Jensen PK (1966) Antimycin-insensitive oxidation of succinate and reduced nicotinamide-adenine dinucleotide in electron-transport particles. I. pH dependency and hydrogen peroxide formation. Biochim Biophys Acta 122:157–166 [DOI] [PubMed] [Google Scholar]
- 44.Chance B, Sies H, Boveris A (1979) Hydroperoxide metabolism in mammalian organs. Physiol Rev 59:527–605 [DOI] [PubMed] [Google Scholar]
- 45.Loschen G, Flohé L, Chance B (1971) Respiratory chain linked H2O2 production in pigeon heart mitochondria. FEBS Lett 18(2):261–264 [DOI] [PubMed] [Google Scholar]
- 46.Boveris A, Chance B (1973) The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem J 134(3):707–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Turrens JF, Boveris A (1980) Generation of superoxide anion by the NADH dehydrogenase of bovine heart mitochondria. Biochem J 191(2):421–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hoek JB, Cahill A, Pastorino JG (2002) Alcohol and mitochondria: a dysfunctional relationship. Gastroenterology 122(7):2049–2063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ho E, Galougahi KK, Liu CC, Bhindi R, Figtree GA (2013) Biological markers of oxidative stress: applications to cardiovascular research and practice. Redox Biol 1(1):483–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mendes Arent A, de Souza LF, Walz R, Dafre AL(2014) Perspectives on molecular biomarkers of oxidative stress and antioxidant strategies in traumatic brain injury. Biomed Res Int 2014, 723060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Morrow JD, Awad JA, Boss HJ, Blair IA, Roberts LJ 2nd (1992) Non-cyclooxygenase-derived prostanoids (F2-isoprostanes) are formed in situ on phospholipids. Proc Nat Acad Sci (USA) 89:10721–10725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bevan RJ, Durand MF, Hickenbotham PT, Kitas GD, Patel PR, Podmore ID, Griffiths HR, Waller HL, Lunec J (2003) Validation of a novel ELISA for measurement of MDA-LDL in human plasma. Free Radic Biol Med 35:517–527 [DOI] [PubMed] [Google Scholar]
- 53.Schopfer FJ, Baker PR, Freeman BA (2003) NO-dependent protein nitration: a cell signaling event or an oxidative inflammatory response? Trend Biochem Sci 28:646–654 [DOI] [PubMed] [Google Scholar]
- 54.Rossi R, Dalle-Donne I, Milzani A, Giustarini D (2006) Oxidized forms of glutathione in peripheral blood as biomarkers of oxidative stress. Clin Chem 52:1406–1414 [DOI] [PubMed] [Google Scholar]
- 55.Bursell SE, King GL (2000) The potential use of glutathionyl hemoglobin as a clinical marker of oxidative stress. Clin Chem 46:145–146 [PubMed] [Google Scholar]
- 56.Gavrieli Y, Sherman Y, Ben-Sasson SA (1992) Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol 119(3):493–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Khan N, Swartz H (2002) Measurements in vivo of parameters pertinent to ROS/RNS using EPR spectroscopy. Mol Cell Biochem 234–235(1–2):341–357 [PubMed] [Google Scholar]
- 58.Persidsky Y, Ramirez SH, Haorah J, Kanmogne GD (2006) Blood-brain barrier: structural components and function under physiologic and pathologic conditions. J Neuroimmune Pharmacol 1(3):223–236 [DOI] [PubMed] [Google Scholar]
- 59.Hawkins BT, Davis TP (2005) The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev 57(2):173–185 [DOI] [PubMed] [Google Scholar]
- 60.Farkas E, Luiten PG (2001) Cerebral microvascular pathology in aging and Alzheimer’s disease. Prog Neurobiol 64(6):575–611 [DOI] [PubMed] [Google Scholar]
- 61.de Vries HE, Kuiper J, de Boer AG, Van Berkel TJ, Breimer DD (1997) The blood-brain barrier in neuroinflammatory diseases. Pharmacol Rev 49(2):143–155 [PubMed] [Google Scholar]
- 62.Iłzecka J (1996) The structure and function of blood-brain barrier in ischaemic brain stroke process. Ann Univ Mariae Curie Sklodowska Med 51:123–127 [PubMed] [Google Scholar]
- 63.Fiala M, Liu QN, Sayre J, Pop V, Brahmandam V, Graves MC, Vinters HV (2002) Cyclooxygenase-2-positive macrophages infiltrate the Alzheimer’s disease brain and damage the blood–brain barrier. Eur J Clin Invest 32(5):360–371 [DOI] [PubMed] [Google Scholar]
- 64.Persidsky Y, Heilman D, Haorah J, Zelivyanskaya M, Persidsky R, Weber GA, Shimokawa H, Kaibuchi K, Ikezu T (2006) Rho-mediated regulation of tight junctions during monocyte migration across the blood-brain barrier in HIV-1 encephalitis (HIVE). Blood 107(12):4770–4780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Opdenakker G, Nelissen I, Van Damme J (2003) Functional roles and therapeutic targeting of gelatinase B and chemokines in multiple sclerosis. Lancet Neurol 2(12):747–756 [DOI] [PubMed] [Google Scholar]
- 66.Morganti-Kossmann MC, Hans VH, Lenzlinger PM, Dubs R, Ludwig E, Trentz O, Kossmann T (1999) TGF-beta is elevated in the CSF of patients with severe traumatic brain injuries and parallels blood-brain barrier function. J Neurotrauma 16(7):617–628 [DOI] [PubMed] [Google Scholar]
- 67.Abdul Muneer PM, Alikunju S, Szlachetka AM, Haorah J (2011) Inhibitory effects of alcohol on glucose transport across the blood-brain barrier leads to neurodegeneration: preventive role of acetyl-L-carnitine. Psychopharmacology (Berl) 214(3):707–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Abdul Muneer PM, Alikunju S, Szlachetka AM, Haorah J (2012) The mechanisms of cerebral vascular dysfunction and neuroinflammation by MMP-mediated degradation of VEGFR-2 in alcohol ingestion. Arterioscler Thromb Vasc Biol 32(5):1167–1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Haorah J, Ramirez SH, Schall K, Smith D, Pandya R, Persidsky Y (2007) Oxidative stress activates protein tyrosine kinase and matrix metalloproteinases leading to blood-brain barrier dysfunction. J Neurochem 101(2):566–576 [DOI] [PubMed] [Google Scholar]
- 70.Hall ED, Vaishnav RA, Mustafa AG (2010) Antioxidant therapies for traumatic brain injury. Neurotherapeutics 7(1):51–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mertsch K, Blasig I, Grune T (2001) 4-Hydroxynonenal impairs the permeability of an in vitro rat blood-brain barrier. Neurosci Lett 314(3):135–138 [DOI] [PubMed] [Google Scholar]
- 72.Smith SL, Andrus PK, Zhang JR, Hall ED (1994) Direct measurement of hydroxyl radicals, lipid peroxidation, and blood-brain barrier disruption following unilateral cortical impact head injury in the rat. J Neurotrauma 11(4):393–404 [DOI] [PubMed] [Google Scholar]
- 73.Soares HD, Hicks RR, Smith D, McIntosh TK (1995) Inflammatory leukocytic recruitment and diffuse neuronal degeneration are separate pathological processes resulting from traumatic brain injury. J Neurosci 15(12):8223–8233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Carlos TM, Clark RS, Franicola-Higgins D, Schiding JK, Kochanek PM (1997) Expression of endothelial adhesion molecules and recruitment of neutrophils after traumatic brain injury in rats. J Leukoc Biol 61(3):279–285 [DOI] [PubMed] [Google Scholar]
- 75.Isaksson J, Lewén A, Hillered L, Olsson Y (1997) Up-regulation of intercellular adhesion molecule 1 in cerebral microvessels after cortical contusion trauma in a rat model. Acta Neuropathol 94(1):16–20 [DOI] [PubMed] [Google Scholar]
- 76.Marchi N, Bazarian JJ, Puvenna V, Janigro M, Ghosh C, Zhong J, Zhu T, Blackman E, Stewart D, Ellis J et al. (2013) Consequences of repeated blood-brain barrier disruption in football players. PLoS One 8(3):e56805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kleindienst A, Ross Bullock M (2006) A critical analysis of the role of the neurotrophic protein S100B in acute brain injury. J Neurotrauma 23(8):1185–1200 [DOI] [PubMed] [Google Scholar]
- 78.Kapural M, Krizanac-Bengez L, Barnett G, Perl J, Masaryk T, Apollo D, Rasmussen P, Mayberg MR, Janigro D (2002) Serum S-100beta as a possible marker of blood-brain barrier disruption. Brain Res 940(1–2):102–104 [DOI] [PubMed] [Google Scholar]
- 79.Saw MM, Chamberlain J, Barr M, Morgan MP, Burnett JR, Ho KM (2014) Differential disruption of blood-brain barrier in severe traumatic brain injury. Neurocrit Care 20(2):209–216 [DOI] [PubMed] [Google Scholar]
- 80.Bao HJ, Wang T, Zhang MY, Liu R, Dai DK, Wang YQ, Wang L, Zhang L, Gao YZ, Qin ZH et al. (2012) Poloxamer-188 attenuates TBI-induced blood-brain barrier damage leading to decreased brain edema and reduced cellular death. Neurochem Res 37(12):2856–2867 [DOI] [PubMed] [Google Scholar]
- 81.Moxon-Emre I, Schlichter LC (2011) Neutrophil depletion reduces blood-brain barrier breakdown, axon injury, and inflammation after intracerebral hemorrhage. J Neuropathol Exp Neurol 70(3):218–235 [DOI] [PubMed] [Google Scholar]
- 82.Lopez NE, Krzyzaniak MJ, Blow C, Putnam J, Ortiz-Pomales Y, Hageny AM, Eliceiri B, Coimbra R, Bansal V (2012) Ghrelin prevents disruption of the blood-brain barrier after traumatic brain injury. J Neurotrauma 29(2):385–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sternlicht MD, Werb Z (2001) How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol 17:463–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nagase H, Woessner JF (1999) Matrix metalloproteinases. J Biol Chem 274(31):21491–21494 [DOI] [PubMed] [Google Scholar]
- 85.Engel CK, Pirard B, Schimanski S, Kirsch R, Habermann J, Klingler O, Schlotte V, Weithmann KU, Wendt KU (2005) Structural basis for the highly selective inhibition of MMP-13. Chem Biol 12(2):181–189 [DOI] [PubMed] [Google Scholar]
- 86.Visse R, Nagase H (2003) Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res 92(8):827–839 [DOI] [PubMed] [Google Scholar]
- 87.Nagase H, Visse R, Murphy G (2006) Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res 69(3):562–573 [DOI] [PubMed] [Google Scholar]
- 88.Brew K, Dinakarpandian D, Nagase H (2000) Tissue inhibitors of metalloproteinases: evolution, structure and function. Biochim Biophys Acta 1477(1–2):267–283 [DOI] [PubMed] [Google Scholar]
- 89.Machado LS, Kozak A, Ergul A, Hess DC, Borlongan CV, Fagan SC (2006) Delayed minocycline inhibits ischemia-activated matrix metalloproteinases 2 and 9 after experimental stroke. BMC Neurosci 7:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kim GW, Gasche Y, Grzeschik S, Copin JC, Maier CM, Chan PH (2003) Neurodegeneration in striatum induced by the mitochondrial toxin 3-nitropropionic acid: role of matrix metalloproteinase-9 in early blood-brain barrier disruption? J Neurosci 23(25):8733–8742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gasche Y, Fujimura M, Morita-Fujimura Y, Copin JC, Kawase M, Massengale J, Chan PH (1999) Early appearance of activated matrix metalloproteinase-9 after focal cerebral ischemia in mice: a possible role in blood-brain barrier dysfunction. J Cereb Blood Flow Metab 19(9):1020–1028 [DOI] [PubMed] [Google Scholar]
- 92.Galli A, Svegliati-Baroni G, Ceni E, Milani S, Ridolfi F, Salzano R, Tarocchi M, Grappone C, Pellegrini G, Benedetti A et al. (2005) Oxidative stress stimulates proliferation and invasiveness of hepatic stellate cells via a MMP2-mediated mechanism. Hepatology 41(5):1074–1084 [DOI] [PubMed] [Google Scholar]
- 93.Rosell A, Alvarez-Sabín J, Arenillas JF, Rovira A, Delgado P, Fernández-Cadenas I, Penalba A, Molina CA, Montaner J (2005) A matrix metalloproteinase protein array reveals a strong relation between MMP-9 and MMP-13 with diffusion-weighted image lesion increase in human stroke. Stroke 36(7):1415–1420 [DOI] [PubMed] [Google Scholar]
- 94.Rosell A, Ortega-Aznar A, Alvarez-Sabin J, Fernandez-Cadenas I, Ribo M, Molina CA, Lo EH, Montaner J (2006) Increased brain expression of matrix metalloproteinase-9 after ischemic and hemorrhagic human stroke. Stroke 37:1399–1406 [DOI] [PubMed] [Google Scholar]
- 95.Fukuda S, Fini CA, Mabuchi T, Koziol JA, Eggleston LL, del Zoppo GJ (2004) Focal cerebral ischemia induces active proteases that degrade microvascular matrix. Stroke 35(4):998–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gursoy-Ozdemir Y, Qiu J, Matsuoka N, Bolay H, Bermpohl D, Jin H, Wang X, Rosenberg GA, Lo EH, Moskowitz MA (2004) Cortical spreading depression activates and upregulates MMP-9. J Clin Invest 113(10):1447–1455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sánchez-del-Rio M, Reuter U (2004) Migraine aura: new information on underlying mechanisms. Curr Opin Neurol 17(3):289–293 [DOI] [PubMed] [Google Scholar]
- 98.Hanumegowda UM, Copple BL, Shibuya M, Malle E, Ganey PE, Roth RA (2003) Basement membrane and matrix metalloproteinases in monocrotaline-induced liver injury. Toxicol Sci 76(1):237–246 [DOI] [PubMed] [Google Scholar]
- 99.Reijerkerk A, Kooij G, van der Pol SM, Khazen S, Dijkstra CD, de Vries HE (2006) Diapedesis of monocytes is associated with MMP-mediated occludin disappearance in brain endothelial cells. FASEB J 20(14):2550–2552 [DOI] [PubMed] [Google Scholar]
- 100.Grossetete M, Phelps J, Arko L, Yonas H, Rosenberg GA (2009) Elevation of matrix metalloproteinases 3 and 9 in cerebrospinal fluid and blood in patients with severe traumatic brain injury. Neurosurgery 65(4):702–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jia F, Pan YH, Mao Q, Liang YM, Jiang JY (2010) Matrix metalloproteinase-9 expression and protein levels after fluid percussion injury in rats: the effect of injury severity and brain temperature. J Neurotrauma 27(6):1059–1068 [DOI] [PubMed] [Google Scholar]
- 102.Rosenberg GA (2009) Matrix metalloproteinases and their multiple roles in neurodegenerative diseases. Lancet Neurol 8(2):205–216 [DOI] [PubMed] [Google Scholar]
- 103.Ding JY, Kreipke CW, Schafer P, Schafer S, Speirs SL, Rafols JA (2009) Synapse loss regulated by matrix metalloproteinases in traumatic brain injury is associated with hypoxia inducible factor-1alpha expression. Brain Res 1268:125–134 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 104.Hayashi T, Kaneko Y, Yu S, Bae E, Stahl CE, Kawase T, van Loveren H, Sanberg PR, Borlongan CV (2009) Quantitative analyses of matrix metalloproteinase activity after traumatic brain injury in adult rats. Brain Res 1280:172–177 [DOI] [PubMed] [Google Scholar]
- 105.Tejima E, Guo S, Murata Y, Arai K, Lok J, van Leyen K, Rosell A, Wang X, Lo EH (2009) Neuroprotective effects of overexpressing tissue inhibitor of metalloproteinase TIMP-1. J Neurotrauma 26(11):1935–1941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ranaivo RH, Zunich SM, Choi N, Hodge JN, Wainwright MS (2011) Mild stretch-induced injury increases susceptibility to interleukin-1β-induced release of matrix metalloproteinase-9 from astrocytes. J Neurotrauma 28(9):1757–1766 [DOI] [PubMed] [Google Scholar]
- 107.Higashida T, Kreipke CW, Rafols JA, Peng C, Schafer S, Schafer P, Ding JY, Dornbos D, Li X, Guthikonda M et al. (2011) The role of hypoxia-inducible factor-1α, aquaporin-4, and matrix metalloproteinase-9 in blood-brain barrier disruption and brain edema after traumatic brain injury. J Neurosurg 114(1):92–101 [DOI] [PubMed] [Google Scholar]
- 108.Tonnesen MG, Feng X, Clark RA (2000) Angiogenesis in wound healing. J Invest Dermatol Symp Proc 5:40–46 [DOI] [PubMed] [Google Scholar]
- 109.Singer AJ, Clark RA (1999) Cutaneous wound healing. N Engl J Med 341(10):738–746 [DOI] [PubMed] [Google Scholar]
- 110.Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z (1999) Vascular endothelial growth factor (VEGF) and its receptors. FASEB J 13(1):9–22 [PubMed] [Google Scholar]
- 111.Baffert F, Le T, Sennino B, Thurston G, Kuo CJ, Hu-Lowe D, McDonald DM (2006) Cellular changes in normal blood capillaries undergoing regression after inhibition of VEGF signaling. Am J Physiol Heart Circ Physiol 290(2):H547–H559 [DOI] [PubMed] [Google Scholar]
- 112.Gerber HP, Hillan KJ, Ryan AM, Kowalski J, Keller GA, Rangell L, Wright BD, Radtke F, Aguet M, Ferrara N (1999) VEGF is required for growth and survival in neonatal mice. Development 126(6):1149–1159 [DOI] [PubMed] [Google Scholar]
- 113.Katoh O, Tauchi H, Kawaishi K, Kimura A, Satow Y (1995) Expression of the vascular endothelial growth factor (VEGF) receptor gene, KDR, in hematopoietic cells and inhibitory effect of VEGF on apoptotic cell death caused by ionizing radiation. Cancer Res 55(23):5687–5692 [PubMed] [Google Scholar]
- 114.Shalaby F, Rossant J, Yamaguchi TP, Gertsenstein M, Wu XF, Breitman ML, Schuh AC (1995) Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature 376(6535):62–66 [DOI] [PubMed] [Google Scholar]
- 115.Haorah J, Schall K, Ramirez SH, Persidsky Y (2008) Activation of protein tyrosine kinases and matrix metalloproteinases causes blood-brain barrier injury: novel mechanism for neurodegeneration associated with alcohol abuse. Glia 56(1):78–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cunningham LA, Wetzel M, Rosenberg GA (2005) Multiple roles for MMPs and TIMPs in cerebral ischemia. Glia 50(4):329–339 [DOI] [PubMed] [Google Scholar]
- 117.Yang Y, Estrada EY, Thompson JF, Liu W, Rosenberg GA (2007) Matrix metalloproteinase-mediated disruption of tight junction proteins in cerebral vessels is reversed by synthetic matrix metalloproteinase inhibitor in focal ischemia in rat. J Cereb Blood Flow Metab 27(4):697–709 [DOI] [PubMed] [Google Scholar]
- 118.Valable S, Montaner J, Bellail A, Berezowski V, Brillault J, Cecchelli R, Divoux D, Mackenzie ET, Bernaudin M, Roussel S et al. (2005) VEGF-induced BBB permeability is associated with an MMP-9 activity increase in cerebral ischemia: both effects decreased by Ang-1. J Cereb Blood Flow Metab 25(11):1491–1504 [DOI] [PubMed] [Google Scholar]
- 119.Tsuji K, Aoki T, Tejima E, Arai K, Lee SR, Atochin DN, Huang PL, Wang X, Montaner J, Lo EH (2005) Tissue plasminogen activator promotes matrix metalloproteinase-9 upregulation after focal cerebral ischemia. Stroke 36(9):1954–1959 [DOI] [PubMed] [Google Scholar]
- 120.Turk BE, Huang LL, Piro ET, Cantley LC (2001) Determination of protease cleavage site motifs using mixture-based oriented peptide libraries. Nat Biotechnol 19(7):661–667 [DOI] [PubMed] [Google Scholar]
- 121.Tran ED, DeLano FA, Schmid-Schönbein GW (2010) Enhanced matrix metalloproteinase activity in the spontaneously hypertensive rat: VEGFR-2 cleavage, endothelial apoptosis, and capillary rarefaction. J Vasc Res 47(5):423–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lee C, Agoston DV (2009) Inhibition of VEGF receptor 2 increased cell death of dentate hilar neurons after traumatic brain injury. Exp Neurol 220(2):400–403 [DOI] [PubMed] [Google Scholar]
- 123.Chodobski A, Chung I, Koźniewska E, Ivanenko T, Chang W, Harrington JF, Duncan JA, Szmydynger-Chodobska J (2003) Early neutrophilic expression of vascular endothelial growth factor after traumatic brain injury. Neuroscience 122(4):853–867 [DOI] [PubMed] [Google Scholar]
- 124.Abid MR, Tsai JC, Spokes KC, Deshpande SS, Irani K, Aird WC (2001) Vascular endothelial growth factor induces manganese-superoxide dismutase expression in endothelial cells by a Rac1-regulated NADPH oxidase-dependent mechanism. FASEB J 15(13):2548–2550 [DOI] [PubMed] [Google Scholar]
- 125.Thau-Zuchman O, Shohami E, Alexandrovich AG, Leker RR (2010) Vascular endothelial growth factor increases neurogenesis after traumatic brain injury. J Cereb Blood Flow Metab 30(5):1008–1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Werner C, Engelhard K (2007) Pathophysiology of traumatic brain injury. Br J Anaesth 99(1):4–9 [DOI] [PubMed] [Google Scholar]
- 127.Lucas SM, Rothwell NJ, Gibson RM (2006) The role of inflammation in CNS injury and disease. Br J Pharmacol 147(Suppl 1):S232–S240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hans VH, Kossmann T, Joller H, Otto V, Morganti-Kossmann MC (1999) Interleukin-6 and its soluble receptor in serum and cerebrospinal fluid after cerebral trauma. Neuroreport 10(2):409–412 [DOI] [PubMed] [Google Scholar]
- 129.Shohami E, Bass R, Wallach D, Yamin A, Gallily R (1996) Inhibition of tumor necrosis factor alpha (TNFalpha) activity in rat brain is associated with cerebroprotection after closed head injury. J Cereb Blood Flow Metab 16(3):378–384 [DOI] [PubMed] [Google Scholar]
- 130.Fan L, Young PR, Barone FC, Feuerstein GZ, Smith DH, McIntosh TK (1995) Experimental brain injury induces expression of interleukin-1 beta mRNA in the rat brain. Brain Res Mol Brain Res 30:125–130 [DOI] [PubMed] [Google Scholar]
- 131.Vecil GG, Larsen PH, Corley SM, Herx LM, Besson A, Goodyer CG, Yong VW (2000) Interleukin-1 is a key regulator of matrix metalloproteinase-9 expression in human neurons in culture and following mouse brain trauma in vivo. J Neurosci Res 61(2):212–224 [DOI] [PubMed] [Google Scholar]
- 132.Lawrence CB, Allan SM, Rothwell NJ (1998) Interleukin-1beta and the interleukin-1 receptor antagonist act in the striatum to modify excitotoxic brain damage in the rat. Eur J Neurosci 10(3):1188–1195 [DOI] [PubMed] [Google Scholar]
- 133.Hopkins SJ, Rothwell NJ (1995) Cytokines and the nervous system. I: expression and recognition. Trends Neurosci 18(2):83–88 [PubMed] [Google Scholar]
- 134.Rothwell NJ Hopkins SJ(1995) Cytokines and the nervous system II: actions and mechanisms of action. Trends Neurosci 18(3):130–136 [DOI] [PubMed] [Google Scholar]
- 135.Lu KT, Wang YW, Yang JT, Yang YL, Chen HI (2005) Effect of interleukin-1 on traumatic brain injury-induced damage to hippocampal neurons. J Neurotrauma 22(8):885–895 [DOI] [PubMed] [Google Scholar]
- 136.Knoblach SM, Faden AI (2000) Cortical interleukin-1 beta elevation after traumatic brain injury in the rat: no effect of two selective antagonists on motor recovery. Neurosci Lett 289(1):5–8 [DOI] [PubMed] [Google Scholar]
- 137.Clausen F, Hånell A, Israelsson C, Hedin J, Ebendal T, Mir AK, Gram H, Marklund N (2011) Neutralization of interleukin-1β reduces cerebral edema and tissue loss and improves late cognitive outcome following traumatic brain injury in mice. Eur J Neurosci 34(1):110–123 [DOI] [PubMed] [Google Scholar]
- 138.Loddick SA, Rothwell NJ (1996) Neuroprotective effects of human recombinant interleukin-1 receptor antagonist in focal cerebral ischaemia in the rat. J Cereb Blood Flow Metab 16(5):932–940 [DOI] [PubMed] [Google Scholar]
- 139.Lamkanfi M, Kanneganti TD, Van Damme P, Vanden Berghe T, Vanoverberghe I, Vandekerckhove J, Vandenabeele P, Gevaert K, Núñez G (2008) Targeted peptidecentric proteomics reveals caspase-7 as a substrate of the caspase-1 inflammasomes. Mol Cell Proteomics 7(12):2350–2363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Keller M, Rüegg A, Werner S, Beer HD (2008) Active caspase-1 is a regulator of unconventional protein secretion. Cell 132(5):818–831 [DOI] [PubMed] [Google Scholar]
- 141.Thornberry NA, Bull HG, Calaycay JR, Chapman KT, Howard AD, Kostura MJ, Miller DK, Molineaux SM, Weidner JR, Aunins J (1992) A novel heterodimeric cysteine protease is required for interleukin-1 beta processing in monocytes. Nature 356(6372):768–774 [DOI] [PubMed] [Google Scholar]
- 142.Sifringer M, Stefovska V, Endesfelder S, Stahel PF, Genz K, Dzietko M, Ikonomidou C, Felderhoff-Mueser U (2007) Activation of caspase-1 dependent interleukins in developmental brain trauma. Neurobiol Dis 25(3):614–622 [DOI] [PubMed] [Google Scholar]
- 143.Mejia SRO, Ona VO, Li M, Friedlander RM (2001) Minocycline reduces traumatic brain injury-mediated caspase-1 activation, tissue damage, and neurological dysfunction. Neurosurgery 48(6):1393–1399, discussion 1399-1401 [DOI] [PubMed] [Google Scholar]
- 144.Csuka E, Morganti-Kossmann MC, Lenzlinger PM Joller H, Trentz O, Kossmann T (1999) IL-10 levels in cerebrospinal fluid and serum of patients with severe traumatic brain injury: relationship to IL-6, TNF-alpha, TGF-beta1 and blood-brain barrier function. J Neuroimmunol 101(2):211–221 [DOI] [PubMed] [Google Scholar]
- 145.Bell MJ, Kochanek PM, Doughty LA, Carcillo JA, Adelson PD, Clark RS, Wisniewski SR, Whalen MJ, DeKosky ST (1997) Interleukin-6 and interleukin-10 in cerebrospinal fluid after severe traumatic brain injury in children. J Neurotrauma 14(7):451–457 [DOI] [PubMed] [Google Scholar]
- 146.Krum JM, Mani N, Rosenstein JM (2008) Roles of the endogenous VEGF receptors flt-1 and flk-1 in astroglial and vascular remodeling after brain injury. Exp Neurol 212(1):108–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Penkowa M, Camats J, Hadberg H, Quintana A, Rojas S, Giralt M, Molinero A, Campbell IL, Hidalgo J (2003) Astrocyte-targeted expression of interleukin-6 protects the central nervous system during neuroglial degeneration induced by 6-aminonicotinamide. J Neurosci Res 73(4):481–496 [DOI] [PubMed] [Google Scholar]
- 148.Swartz KR, Liu F, Sewell D, Schochet T, Campbell I, Sandor M, Fabry Z (2001) Interleukin-6 promotes post-traumatic healing in the central nervous system. Brain Res 896(1–2):86–95 [DOI] [PubMed] [Google Scholar]
- 149.Ramilo O, Sáez-Llorens X, Mertsola J, Jafari H, Olsen KD, Hansen EJ, Yoshinaga M, Ohkawara S, Nariuchi H, McCracken GH (1990) Tumor necrosis factor alpha/cachectin and interleukin 1 beta initiate meningeal inflammation. J Exp Med 172(2):497–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kim KS, Wass CA, Cross AS, Opal SM (1992) Modulation of blood-brain barrier permeability by tumor necrosis factor and antibody to tumor necrosis factor in the rat. Lymphokine Cytokine Res 11 (6):293–298 [PubMed] [Google Scholar]
- 151.Ross SA, Halliday MI, Campbell GC, Byrnes DP, Rowlands BJ (1994) The presence of tumour necrosis factor in CSF and plasma after severe head injury. Br J Neurosurg 8(4):419–425 [DOI] [PubMed] [Google Scholar]
- 152.Khuman J, Meehan WP, Zhu X, Qiu J, Hoffmann U, Zhang J, Giovannone E, Lo EH, Whalen MJ (2011) Tumor necrosis factor alpha and Fas receptor contribute to cognitive deficits independent of cell death after concussive traumatic brain injury in mice. J Cereb Blood Flow Metab 31(2):778–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bermpohl D, You Z, Lo EH, Kim HH, Whalen MJ (2007) TNF alpha and Fas mediate tissue damage and functional outcome after traumatic brain injury in mice. J Cereb Blood Flow Metab 27(11):1806–1818 [DOI] [PubMed] [Google Scholar]
- 154.Logan A, Berry M (1993) Transforming growth factor-beta 1 and basic fibroblast growth factor in the injured CNS. Trends Pharmacol Sci 14(9):337–342 [DOI] [PubMed] [Google Scholar]
- 155.Zhu Y, Roth-Eichhorn S, Braun N, Culmsee C, Rami A, Krieglstein J (2000) The expression of transforming growth factor-beta1 (TGF-beta1) in hippocampal neurons: a temporary upregulated protein level after transient forebrain ischemia in the rat. Brain Res 866(1–2):286–298 [DOI] [PubMed] [Google Scholar]
- 156.Lehrmann E, Kiefer R, Christensen T, Toyka KV, Zimmer J, Diemer NH, Hartung HP, Finsen B (1998) Microglia and macrophages are major sources of locally produced transforming growth factor-beta1 after transient middle cerebral artery occlusion in rats. Glia 24(4):437–448 [DOI] [PubMed] [Google Scholar]
- 157.Rimaniol AC, Lekieffre D, Serrano A, Masson A, Benavides J, Zavala F (1995) Biphasic transforming growth factor-beta production flanking the pro-inflammatory cytokine response in cerebral trauma. Neuroreport 7(1):133–136 [PubMed] [Google Scholar]
- 158.Morganti-Kossmann MC, Rancan M, Stahel PF, Kossmann T (2002) Inflammatory response in acute traumatic brain injury: a double-edged sword. Curr Opin Crit Care 8(2):101–105 [DOI] [PubMed] [Google Scholar]
- 159.Cacheaux LP, Ivens S, David Y, Lakhter AJ, Bar-Klein G, Shapira M, Heinemann U, Friedman A, Kaufer D (2009) Transcriptome profiling reveals TGF-beta signaling involvement in epileptogenesis. J Neurosci 29(28):8927–8935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Marmarou A (2003) Pathophysiology of traumatic brain edema: current concepts. Acta Neurochir Suppl 86:7–10 [DOI] [PubMed] [Google Scholar]
- 161.Klatzo I (1987) Pathophysiological aspects of brain edema. Acta Neuropathol 72(3):236–239 [DOI] [PubMed] [Google Scholar]
- 162.Donkin JJ, Vink R (2010) Mechanisms of cerebral edema in traumatic brain injury: therapeutic developments. Curr Opin Neurol 23(3):293–299 [DOI] [PubMed] [Google Scholar]
- 163.Barzó P, Marmarou A, Fatouros P, Hayasaki K, Corwin F (1997) Contribution of vasogenic and cellular edema to traumatic brain swelling measured by diffusion-weighted imaging. J Neurosurg 87(6):900–907 [DOI] [PubMed] [Google Scholar]
- 164.Unterberg AW, Stroop R, Thomale UW, Kiening KL, Päuser S, Vollmann W (1997) Characterisation of brain edema following “controlled cortical impact injury” in rats. Acta Neurochir Suppl 70:106–108 [DOI] [PubMed] [Google Scholar]
- 165.Papadopoulos MC, Verkman AS (2008) Potential utility of aquaporin modulators for therapy of brain disorders. Prog Brain Res 170:589–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Pasantes-Morales H, Cruz-Rangel S (2010) Brain volume regulation: osmolytes and aquaporin perspectives. Neuroscience 168(4):871–884 [DOI] [PubMed] [Google Scholar]
- 167.Badaut J, Lasbennes F, Magistretti PJ, Regli L (2002) Aquaporins in brain: distribution, physiology, and pathophysiology. J Cereb Blood Flow Metab 22(4):367–378 [DOI] [PubMed] [Google Scholar]
- 168.Nag S, Manias JL, Stewart DJ (2009) Pathology and new players in the pathogenesis of brain edema. Acta Neuropathol 118(2):197–217 [DOI] [PubMed] [Google Scholar]
- 169.Tran ND, Kim S, Vincent HK, Rodriguez A, Hinton DR, Bullock MR, Young HF (2010) Aquaporin-1-mediated cerebral edema following traumatic brain injury: effects of acidosis and corticosteroid administration. J Neurosurg 112(5):1095–1104 [DOI] [PubMed] [Google Scholar]
- 170.Aoki K, Uchihara T, Tsuchiya K, Nakamura A, Ikeda K, Wakayama Y (2003) Enhanced expression of aquaporin 4 in human brain with infarction. Acta Neuropathol 106(2):121–124 [DOI] [PubMed] [Google Scholar]
- 171.Qiu B, Li X, Sun X, Wang Y, Jing Z, Zhang X (2014) Overexpression of aquaporin-1 aggravates hippocampal damage in mouse traumatic brain injury models. Mol Med Rep 9(3):916–922 [DOI] [PubMed] [Google Scholar]
- 172.Ding JY, Kreipke CW, Speirs SL, Schafer P, Schafer S, Rafols JA (2009) Hypoxia-inducible factor-1alpha signaling in aquaporin up-regulation after traumatic brain injury. Neurosci Lett 453(1):68–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Manley GT, Fujimura M, Ma T, Noshita N, Filiz F, Bollen AW, Chan P, Verkman AS (2000) Aquaporin-4 deletion in mice reduces brain edema after acute water intoxication and ischemic stroke. Nat Med 6(2):159–163 [DOI] [PubMed] [Google Scholar]
- 174.Fazzina G, Amorini AM, Marmarou CR, Fukui S, Okuno K, Dunbar JG, Glisson R, Marmarou A, Kleindienst A (2010) The protein kinase C activator phorbol myristate acetate decreases brain edema by aquaporin 4 downregulation after middle cerebral artery occlusion in the rat. J Neurotrauma 27(2):453–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Taya K, Gulsen S, Okuno K, Prieto R, Marmarou CR, Marmarou A (2008) Modulation of AQP4 expression by the selective V1a receptor antagonist, SR49059, decreases trauma-induced brain edema. Acta Neurochir Suppl 102:425–429 [DOI] [PubMed] [Google Scholar]
- 176.Candelario-Jalil E, Yang Y, Rosenberg GA (2009) Diverse roles of matrix metalloproteinases and tissue inhibitors of metalloproteinases in neuroinflammation and cerebral ischemia. Neuroscience 158(3):983–994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Asahi M, Wang X, Mori T, Sumii T, Jung JC, Moskowitz MA, Fini ME, Lo EH (2001) Effects of matrix metalloproteinase-9 gene knock-out on the proteolysis of blood-brain barrier and white matter components after cerebral ischemia. J Neurosci 21(19):7724–7732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wang X, Jung J, Asahi M, Chwang W, Russo L, Moskowitz MA, Dixon CE, Fini ME, Lo EH (2000) Effects of matrix metalloproteinase-9 gene knock-out on morphological and motor outcomes after traumatic brain injury. J Neurosci 20(18):7037–7042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Abbott NJ (2000) Inflammatory mediators and modulation of blood-brain barrier permeability. Cell Mol Neurobiol 20(2):131–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Reiter RJ (1995) Oxidative processes and antioxidative defense mechanisms in the aging brain. FASEB J 9:526–533 [PubMed] [Google Scholar]
- 181.Gilgun-Sherki Y, Rosenbaum Z, Melamed E, Offen D (2002) Antioxidant therapy in acute central nervous system injury: current state. Pharmacol Rev 54(2):271–284 [DOI] [PubMed] [Google Scholar]
- 182.Faraci FM, Didion SP (2004) Vascular protection: superoxide dismutase isoforms in the vessel wall. Arterioscler Thromb Vasc Biol 24:1367–1373 [DOI] [PubMed] [Google Scholar]
- 183.Aoyama N, Katayama Y, Kawamata T et al. (2002) Effects of antioxidant, OPC-14117, on secondary cellular damage and behavioral deficits following cortical contusion in the rat. Brain Res 934(2):117–124 [DOI] [PubMed] [Google Scholar]
- 184.Deng-Bryant Y, Singh IN, Carrico KM, Hall ED (2008) Neuroprotective effects of tempol, a catalytic scavenger of peroxynitrite-derived free radicals, in a mouse traumatic brain injury model. J Cereb Blood Flow Metab 28(6):1114–1126 [DOI] [PubMed] [Google Scholar]
- 185.Clausen F, Marklund N, Lewen A, Hillered L (2008) The nitrone free radical scavenger NXY-059 is neuroprotective when administered after traumatic brain injury in the rat. J Neurotrauma 25(12):1449–1457 [DOI] [PubMed] [Google Scholar]
- 186.Inci S, Ozcan OE, Kilinc K (1998) Time-level relationship for lipid peroxidation and the protective effect of α-tocopherol in experimental mild and severe brain injury. Neurosurgery 43(2):330–336 [DOI] [PubMed] [Google Scholar]
- 187.Ikeda Y, Mochizuki Y, Nakamura Y et al. (2000) Protective effect of a novel vitamin E derivative on experimental traumatic brain edema in rats—preliminary study. Acta Neurochir 76(suppl):343–345 [DOI] [PubMed] [Google Scholar]
- 188.Kabadi SV, Maher TJ (2010) Posttreatment with uridine and melatonin following traumatic brain injury reduces edema in various brain regions in rats. Ann N Y Acad Sci 1199:105–113 [DOI] [PubMed] [Google Scholar]
- 189.Singleton RH, Yan HQ, Fellows-Mayle W, Dixon CE (2010) Resveratrol attenuates behavioral impairments and reduces cortical and hippocampal loss in a rat controlled cortical impact model of traumatic brain injury. J Neurotrauma 27(6):1091–1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Kline AE, Massucci JL, Ma X, Zafonte RD, Dixon CE (2004) Bromocriptine reduces lipid peroxidation and enhances spatial learning and hippocampal neuron survival in a rodent model of focal brain trauma. J Neurotrauma 21(12):1712–1722 [DOI] [PubMed] [Google Scholar]
- 191.McIntosh TK, Thomas M, Smith D, Banbury M (1992) The novel 21-aminosteroid U74006F attenuates cerebral edema and improves survival after brain injury in the rat. J Neurotrauma 9:33–46 [DOI] [PubMed] [Google Scholar]
- 192.Chung ES, Bok E, Chung YC, Baik HH, Jin BK(2012) Cannabinoids prevent lipopolysaccharide-induced neurodegeneration in the rat substantia nigra in vivo through inhibition of microglial activation and NADPH oxidase. Brain Res 1451:110–116 [DOI] [PubMed] [Google Scholar]
- 193.Xiao G, Wei J, Yan W, Wang W, Lu Z (2008) Improved out comes from the administration of progesterone for patients with acute severe traumatic brain injury: a randomized controlled trial. Crit Care 12(2), article R61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Muehlschlegel S, Sims JR (2009) Dantrolene: mechanisms of neuroprotection and possible clinical applications in the neurointensive care unit. Neurocrit Care 10:103–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Razmkon A, Ahmad Sadidi A, Sherafat-Kazemzadeh E, Ali Mehrafshan A, Jamali M, Malekpour B, Masoud Saghafinia M (2011) Administration of vitamin C and vitamin E in severe head injury: a randomized double-blind controlled trial. Clin Neurosurg 58:133–137 [DOI] [PubMed] [Google Scholar]
- 196.Giacino JT, Whyte J, Bagiella E, Kalmar K, Childs N, Khademi A, Eifert B, Long D, Katz DI, Cho S, Yablon SA, Luther M, Hammond FM, Nordenbo A, Novak P, Mercer W, Maurer-Karattup P, Sherer M (2012) Placebo-controlled trial of amantadine for severe traumatic brain injury. N Engl J Med 366(9):819–826 [DOI] [PubMed] [Google Scholar]
- 197.Zafonte R, Friedewald WT, Lee SM, Levin B, Diaz-Arrastia R, Ansel B, Eisenberg H, Timmons SD, Temkin N, Novack T, Ricker J, Merchant R, Jallo J (2009) The citicoline brain injury treatment (COBRIT) trial: design and methods. J Neurotrauma 26(12):2207–2216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Maas AIR, Murray G, Henney H 3rd, Kassem N, Legrand V et al. (2006) Efficacy and safety of dexanabinol in severe traumatic brain injury: results of a phase III randomised, placebo-controlled, clinical trial. Lancet Neurol 5:38–45 [DOI] [PubMed] [Google Scholar]
- 199.Homsi S, Federico F, Croci N, Palmier B, Plotkine M et al. (2009) Minocycline effects on cerebral edema: relations with inflammatory and oxidative stress markers following traumatic brain injury in mice. Brain Res 1291:122–132 [DOI] [PubMed] [Google Scholar]
- 200.Viscomi MT, Latini L, Florenzano F, Bernardi G, Molinari M (2008) Minocycline attenuates microglial activation but fails to mitigate degeneration in inferior olive and pontine nuclei after focal cerebellar lesion. Cerebellum 7:401–405 [DOI] [PubMed] [Google Scholar]
- 201.Temkin NR, Anderson GD, Winn HR, Ellenbogen RG, Britz GW, Schuster J, Lucas T, Newell DW, Mansfield PN, Machamer JE, Barber J, Dikmen SS (2007) Magnesium sulfate for neuroprotection after traumatic brain injury: a randomised controlled trial. Lancet Neurol 6(1):29–38 [DOI] [PubMed] [Google Scholar]
- 202.Vink R, Nimmo AJ (2009) Multifunctional drugs for head injury. Neurotherapeutics 6:28–42 [DOI] [PMC free article] [PubMed] [Google Scholar]