Abstract
Background
The COVID-19 pandemic profoundly impacted the delivery of care and timing of elective surgical procedures. Most endocrine-related operations were considered elective and safe to postpone, providing a unique opportunity to assess clinical outcomes under protracted treatment plans.
Methods
American Association of Endocrine Surgeon members were surveyed for participation. A Research Electronic Data Capture survey was developed and distributed to 27 institutions to assess the impact of COVID-19-related delays. The information collected included patient demographics, primary diagnosis, resumption of care, and assessment of disease progression by the surgeon.
Results
Twelve out of 27 institutions completed the survey (44.4%). Of 850 patients, 74.8% (636) were female; median age was 56 (interquartile range, 44–66) years. Forty percent (34) of patients had not been seen since their original surgical appointment was delayed; 86.2% (733) of patients had a delay in care with women more likely to have a delay (87.6% vs 82.2% of men, = 3.84, P = .05). Median duration of delay was 70 (interquartile range, 42–118) days. Among patients with a delay in care, primary disease site included thyroid (54.2%), parathyroid (37.2%), adrenal (6.5%), and pancreatic/gastrointestinal neuroendocrine tumors (1.3%). In addition, 4.0% (26) of patients experienced disease progression and 4.1% (24) had a change from the initial operative plan. The duration of delay was not associated with disease progression (P = .96) or a change in operative plan (P = .66).
Conclusion
Although some patients experienced disease progression during COVID-19 delays to endocrine disease-related care, most patients with follow-up did not. Our analysis indicated that temporary delay may be an acceptable course of action in extreme circumstances for most endocrine-related surgical disease.
Introduction
The COVID-19 (SARS-CoV-2) pandemic has profoundly impacted the delivery of health care, particularly the timing of elective surgical procedures. Due to scarce resources resulting from the overwhelming burden on health care systems during the first wave of the epidemic, hospitals opted to conserve critical personal protective equipment and manage intensive care unit and surgical resources by triaging clinical and surgical care. Consequently, there were global disruptions to elective and nonurgent procedures. In March 2020, the Centers for Disease Control and Prevention and the American College of Surgeons recommended physicians consider postponement or cancellation of elective procedures.1, 2, 3 Following suit, 35 states published guidance in the form of either a mandate or recommendation for management of elective surgeries. Similar recommendations were made by governments across the world; however, initial guidelines were limited in specifying which patients should be prioritized for surgery.4 , 5
To provide further guidance, several surgical societies published proposed guidelines of a hierarchy of surgical care recommending which procedures can be safely delayed, and when immediate surgical intervention is necessary.6, 7, 8, 9, 10, 11, 12 Institutions also created committees of clinical peers among surgical subspecialties and devised their own triage guidelines.7 , 9 , 13 Following these recommendations, most endocrine-related (eg, thyroid, parathyroid, adrenal, and neuroendocrine) surgeries were considered elective and subsequently postponed. This aligned with the 2020 American Association of Endocrine Surgeons (AAES) management guidelines as well as recent literature showing comparable outcomes with more conservative management of thyroid disease for many benign endocrinopathies and malignant disease, particularly papillary thyroid carcinoma.14, 15, 16, 17, 18 The ongoing COVID-19 pandemic has limited surgical options for patients, providing a unique opportunity to monitor clinical outcomes under protracted treatment plans, which would have been potentially unethical in standard clinical scenarios.
A multi-institutional international database of patients was established to assess impacts of delay in diagnosis, delay in treatment, use of alternative treatment, and delay of surveillance. In this study, we aimed to assess whether diagnostic delays and delayed treatment of benign and malignant endocrine diseases impacted daily practice and cancer outcomes to provide guidance for ongoing and future pandemics and inform standard care.
Methods
Study population
All AAES members were surveyed for participation in the study via e-mail. Twenty-seven institutions were involved in the development of a Research Electronic Data Capture survey to assess the impact of COVID-19-related delays to care during the first wave of the pandemic. Institutions were excluded if there was no mandated delay of care at that site. Institutional Review Board approval and Data Use Agreements were obtained independently for all study sites. Data at each participating institution was obtained retrospectively on patients with any delay to diagnosis or treatment of an endocrine disease due to COVID-19. All adult patients with diseases of the thyroid, parathyroid, adrenal gland, or pancreatic/gastrointestinal neuroendocrine tumors with diagnostic or treatment delays stemming from the COVID-19 pandemic were included in this study. Prior to the COVID-19 pandemic, some of these disease processes would not have been considered elective (eg, medullary thyroid carcinoma, parathyroid carcinoma, etc); however, at most institutions included in this study, all endocrine-related procedures were considered elective when mandated institutional delays were enforced. Due to varying dates of mandatory restrictions on elective procedures and in-person care across the country, the inclusion date range for the participants is site-specific (Supplementary Table S1).
Data acquisition
A 70-item survey was distributed to each study site to collect patient demographics and comorbidities, primary diagnosis, reason for delay, duration of delay, clinical staging, resumption of care (either in-person or virtual), use of virtual visits, COVID-19 status, type of insurance, factors impacting rescheduled surgeries, change in planned operation, and assessment of disease progression. The number of items per patient was dependent on the response to conditional questions (ie, if a patient was indicated to have a thyroid related diagnosis, then subsequent questions would ask about subtype, tumor size, etc). The Research Electronic Data Capture survey, with all items, is available as Supplementary Document S1. All data were extracted from clinical charts. The duration of delay was defined as time from the original scheduled visit (eg, diagnostic work-up or surgery) that was cancelled to resumption of clinical care. However, at some institutions, patients were put on a waitlist before an appointment being scheduled. In these instances, the institutional COVID-19 entry date (ie, the date restrictions were imposed across the institution) was used as the original appointment date to calculate the duration of delay. Disease progression was assessed by the site’s surgeon and defined as intraoperative findings or other evidence of progression of clinical disease that occurred during the delay. For patients that underwent surgery after the resumption of in-person care, change in operative plan was defined as a change in the type of surgery performed on re-entry from the original planned surgery. Patients were considered lost to follow-up if they were not seen in person following their delay and there was no record of a virtual visit.
Statistical analysis
All analyses were conducted using R version 4.1.1 (R Foundation for Statistical Computing, Vienna, Austria). The continuous variables are reported as median (IQR); the categorical variables are reported as frequency and percentage. The differences in delay by age, sex ethnicity, race, and primary disease site were assessed using Wilcoxon rank-sum test, χ2 analysis, and Fisher exact test, as appropriate (Table I ). Patients with unknown or missing demographic data were excluded from percentage calculations and statistical tests for demographic data. The differences in the duration of delay, change in operative plan, and disease progression by disease site were evaluated using Kruskal-Wallis rank sum test and Fisher exact test, as appropriate (Table II ). The duration of delay analysis included all patients with a reported date of delay and date of resumed in-person care after the delay (n = 733). Patients were included in the analysis for a change in operative plan if they had undergone surgery after their delay (n = 580). Patients were included in the analysis for disease progression if they had either been seen in person or had a virtual visit for follow-up evaluation after their delay (n = 653). Wilcoxon rank-sum test was used to assess mean differences in delay based on evidence of disease progression and changes to operative plan. Fisher exact test was used to assess disease progression by thyroid diagnosis and thyroid cancer type. For factors impacting the decision to proceed with care without delay, missing values were due to nonresponse and excluded from the reported results (Table III ). For patients with a delay, the type of care that was delayed and the reason(s) for the delay are summarized as counts since the survey allowed for multiple responses to each item (Figures 1 and 2 ).
Table I.
Patient characteristics
| Characteristic | Overall, N = 850∗ | Delay, N = 733∗ | No delay, n = 117∗ | P value |
|---|---|---|---|---|
| Age | 56 (44–66) | 57 (45–67) | 52 (35–64) | .001 |
| Sex | .050 | |||
| Female | 636 (74.82%) | 557 (75.99%) | 79 (67.52%) | |
| Male | 214 (25.18%) | 176 (24.01%) | 38 (32.48%) | |
| Ethnicity† | > .900 | |||
| Not Hispanic or Latino | 727 (89.09%) | 622 (88.98%) | 105 (89.74%) | |
| Hispanic or Latino | 89 (10.91%) | 77 (11.02%) | 12 (10.26%) | |
| Race‡ | .120 | |||
| Asian | 42 (5.51%) | 32 (4.90%) | 10 (9.17%) | |
| Native Hawaiian or other Pacific Islander | 1 (0.13%) | 1 (0.15%) | 0 (0.00%) | |
| Black or African American | 119 (15.62%) | 103 (15.77%) | 16 (14.68%) | |
| White | 567 (74.41%) | 485 (74.27%) | 82 (75.23%) | |
| >1 race | 33 (4.33%) | 32 (4.90%) | 1 (0.92%) | |
| Primary disease site§ | < .001 | |||
| Thyroid | 458 (54.20%) | 378 (51.85%) | 80 (68.97%) | |
| Parathyroid | 314 (37.16%) | 291 (39.92%) | 23 (19.83%) | |
| Adrenal | 55 (6.51%) | 43 (5.90%) | 12 (10.34%) | |
| Pancreatic/GI neuroendocrine tumors | 11 (1.30%) | 11 (1.51%) | 0 (0.00%) | |
| Other | 7 (0.83%) | 6 (0.82%) | 1 (0.86%) |
GI, gastrointestinal.
Median (IQR); n (%).
34 patients with unknown ethnicity were excluded from percentage calculations.
88 patients with unknown race were excluded from percentage calculations.
5 patients with unknown primary disease site; these were excluded from percentage calculations.
Table II.
Disease site and outcomes
| Characteristic | Overall, N = 729∗,† | Thyroid, N = 378∗ | Parathyroid, N = 291∗ | Adrenal, N = 43∗ | Pancreatic/GI neuroendocrine tumors, N = 11∗ | Other, N = 6∗ | P value |
|---|---|---|---|---|---|---|---|
| Duration of delay, d | 70 (42–118) | 62 (34–99) | 80 (49–132) | 69 (48–126) | 99 (91–146) | 84 (69–105) | < .001 |
| Change in operative plan‡ | 24/580 (4.1%) | 15/290 (5.2%) | 6/240 (2.5%) | 1/35 (2.9%) | 2/10 (20%) | 0/5 (0%) | .091 |
| Disease progression during delay§ | 26/653 (4.0%) | 9/334 (2.7%) | 9/265 (3.4%) | 3/38 (7.9%) | 5/10 (50%) | 0/6 (0%) | < .001 |
GI, gastrointestinal.
Median (IQR); n/N (%).
5 patients with unknown delays were excluded.
All patients who underwent surgery following delay.
Missing patients have not been seen in person or virtually since their delay.
Table III.
Factors impacting decision to proceed without delay
| Factor | N (n/N, %) |
|---|---|
| Review of case with colleagues (N = 82) | |
| Not at all | 52 (63.4%) |
| A little | 8 (9.8%) |
| A lot | 22 (26.8%) |
| Patient preference or anxiety (N = 82) | |
| Not at all | 62 (75.6%) |
| A little | 16 (19.5%) |
| A lot | 4 (4.9%) |
| Belief that it was not safe for patient to delay care (N = 83) | |
| Not at all | 37 (44.6%) |
| A little | 20 (24.1%) |
| A lot | 26 (31.3%) |
| Change in clinical status (N = 80) | |
| Not at all | 66 (82.5%) |
| A little | 7 (8.8%) |
| A lot | 7 (8.8%) |
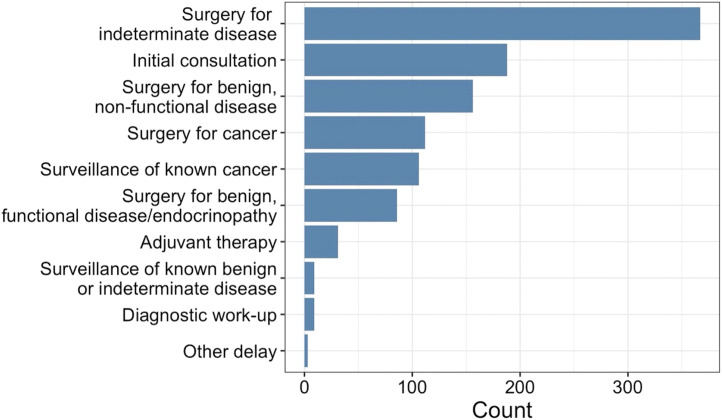
Figure 1.
Phase of the patient’s care that was delayed.
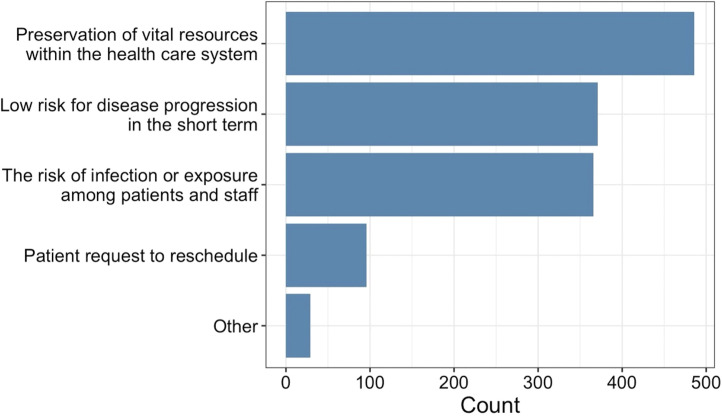
Figure 2.
Reason for delay in care.
Results
Study population characteristics
Twelve out of 25 institutions completed the survey. Of 850 patients (Table I), 74.8% (n = 636) were female with a median age of 56 (IQR, 44–66) years. There were 86.1% (732) from US study sites, whereas 7.7% (65) were from Canada, and 6.2% (53) were from Saudi Arabia. The primary disease sites included thyroid (54.2%), parathyroid (37.2%), adrenal (6.5%), pancreatic/gastrointestinal neuroendocrine tumors (1.3%), and other (0.8%). In addition, 86.2% (733) of patients had a delay in care, primarily for surgery for indeterminate disease, initial consultation, and surgery for benign, nonfunctional disease (Figure 1). The proportion of women with a delay was higher than the proportion of men (87.6% vs 82.2% of men, =3.84, P = .05). Patients with a delay were older (57 vs 52 years old, P = .001); however, there was no significant difference among ethnic or racial groups (P = .90 and P = .12, respectively). Furthermore, 4.0% (34) of patients have not been seen since their original appointment was delayed and thus were considered lost to follow-up. For study sites in the United States, there was no evidence that health insurance status (eg, private insurance, Medicare/Medicaid, and uninsured) impacted delays in care (P = .70).
Duration of delay and outcomes
The median duration of delay was 70 (IQR, 42–118) days (Table II). When evaluated by disease site, patients with parathyroid and pancreatic/gastrointestinal neuroendocrine related disease experienced the longest delays (80 and 99 days, respectively; P < .001). Among patients with a thyroid-related diagnosis, a majority were for a thyroid nodule/multinodular goiter (59%) or thyroid cancer (28%; Table IV ). Of patients with a thyroid-related diagnosis that had evidence of disease progression (n = 9), there was a higher percentage of patients with thyroid cancer with disease progression (67%, n = 6) compared with patients with thyroid nodules or multinodular goiters (33%, n = 3; P = .13). Most patients with thyroid cancer had a preoperative diagnosis of papillary thyroid carcinoma (84%, n = 74). All patients with thyroid cancer with evidence of disease progression had a diagnosis of papillary thyroid carcinoma.
Table IV.
Disease site and disease progression
| Characteristic | Overall N = 657 | No disease progression N = 631 | Disease progression N = 26 | P value |
|---|---|---|---|---|
| Primary thyroid diagnosis (at COVID entry) | .130 | |||
| Thyroid nodule (s), multi nodular goiter | 195 (59%) | 192 (59%) | 3 (33%) | |
| Thyroid cancer | 92 (28%) | 86 (27%) | 6 (67%) | |
| Hyperthyroidism | 30 (9.0%) | 30 (9.3%) | 0 (0%) | |
| Other | 15 (4.5%) | 15 (4.6%) | 0 (0%) | |
| Thyroid cancer type (if known at COVID entry) | > .900 | |||
| Papillary | 74 (84%) | 68 (83%) | 6 (100%) | |
| Follicular | 2 (2.3%) | 2 (2.4%) | 0 (0%) | |
| Hurthle cell | 3 (3.4%) | 3 (3.7%) | 0 (0%) | |
| Medullary | 6 (6.8%) | 6 (7.3%) | 0 (0%) | |
| Other | 3 (3.4%) | 3 (3.7%) | 0 (0%) | |
| Parathyroid diagnosis | < .001 | |||
| Primary hyperparathyroidism | 241 (91%) | 237 (93%) | 4 (44%) | |
| Secondary hyperparathyroidism | 8 (3.0%) | 7 (2.7%) | 1 (11%) | |
| Tertiary hyperparathyroidism | 7 (2.6%) | 4 (1.6%) | 3 (33%) | |
| Recurrent/persistent hyperparathyroidism | 8 (3.0%) | 8 (3.1%) | 0 (0%) | |
| Parathyroid carcinoma | 1 (0.4%) | 0 (0%) | 1 (11%) | |
The most common parathyroid diagnosis was primary hyperparathyroidism (91%, n = 241). Of patients with a parathyroid diagnosis with evidence of disease progression (n = 9), most had primary hyperparathyroidism (44%, n = 4) or tertiary hyperparathyroidism (33%, n = 3; P < .001). One patient with primary hyperparathyroidism was admitted with hypercalcemic crisis. Progression of disease in the few tertiary hyperparathyroid patients included worsening bone loss evidenced as significant hungry bone disease after parathyroidectomy and cardiovascular disease.
Among the 580 patients who underwent surgery after their delay, 24 (4.1%) had a change to their initial operative plan. There was no evidence of a difference in change in operative plan based on disease site (P = .09). Among the 653 patients with follow-up assessment (in-person or virtual) after their delay, 26 (4.0%) experienced progression of their disease during their delay. Notably, there was a higher percentage of patients with pancreatic/gastrointestinal neuroendocrine tumors with evidence of disease progression (50%, n = 5) compared with the other disease sites. The duration of delay was not associated with disease progression (P = .96) or a change in operative plan (P = .66).
The decision to delay care was most affected by the need for preservation of vital resources within the health care system (66.3%), perceived low risk for disease progression in the short term (50.6%), and the risk of infection or exposure to COVID-19 among patients and staff (49.9%; Figure 2). For patients who proceeded without a delay in care, a review of the case with colleagues impacted the decision by “a little” or “a lot” in 36.6% of cases; patient preference and/or anxiety impacted the decision in 24.4% of cases and change in clinical status impacted the decision in 17.6% of cases (Table III). In 55.4% of cases, the belief that it was not safe for a patient to delay care impacted the decision.
Discussion
To characterize the impact of COVID-19-related delays in care for endocrine patients, we analyzed patient outcomes data across 12 institutions in 3 countries that implemented resource triage policies that resulted in delays to patient care. We found that few patients experienced a progression in their disease or a change in operative plan after their delay. Furthermore, few patients were considered lost to follow-up after their initial procedure or appointment was delayed. These findings indicated a successful response with regards to endocrine care in the face of a difficult resource allocation problem experienced by the health care system.
The burden of COVID-19 on health care workers and resource allocation necessitated the development of triage recommendations to guide decisions on proceeding with surgery in the case of procedures deemed “urgent” or delaying care in the case of “elective” procedures. According to guidelines developed by clinical experts, national, and international surgical societies, most endocrine related surgeries were considered “elective” and thus could be safely delayed.6, 7, 8, 9, 10 , 19 In the United States, most states imposed mandatory restrictions on elective procedures in March 2020, although timing and stratification of delayed care varied by region and health care system.1 , 2 The American College of Surgeons along with other national surgical societies, including the AAES and Society of Surgical Oncology who provided endocrine-specific guidance, supported this recommendation stating that physicians should only provide time-sensitive or emergency care.3 , 11 , 12 Similar recommendations were made in Canada, where provinces began cancelling all nonurgent surgeries and procedures in mid-March.5 In Saudi Arabia, the Saudi General Surgery Society in collaboration with the Saudi Patient Safety Center categorized surgeries into 4 priority-based groups.4 Most endocrine-related procedures fell within Priority 4, indicating the procedure could be delayed for >30 days. Although there was a consensus that endocrine-related procedures could be safely delayed, this was an unprecedented situation requiring protracted treatment plans without knowledge on the impact of those delays.
Several studies have evaluated changes to endocrine surgical volumes and the increased use of telemedicine during the COVID-19 pandemic.20, 21, 22, 23, 24, 25 However, the literature is limited in studies assessing outcomes of endocrine surgery patients who had delays to their care due to COVID-19.26 An international, multicenter, prospective cohort study evaluated outcomes of 380 emergency and elective endocrine surgery patients using data from PanSurg-PREDICT.26 Although 97% of the surgeries captured by the database were considered elective, only 8.1% of patients had any delay in care due to COVID-19. Of those with delays, a majority underwent surgery within 3 months and only 1 patient was delayed for >6 months. Generally, there were low morbidity and mortality rates; however, these were not subgrouped based on delay status. With a comparatively large cohort of patients with delays to care, we were able to assess outcomes as they related to delays, and we reported similarly low rates of adverse events (assessed by the surgeon as disease progression and change in operative plan). Whereas Van Den Heede et al evaluated general outcomes and characteristics of patients undergoing endocrine surgery undergoing procedures during COVID-19, our study was the first to assess the impact of delays on endocrine-specific outcomes.
Our findings of minimal oncological disease progression or changes to operative plan due to delays in care aligned with recent studies that have demonstrated the value of active surveillance over immediate surgical intervention in certain malignant endocrine pathologies. The marked increase in incidence in thyroid cancer diagnosis over the last few decades27 , 28 may have partially resulted from increased detection due to the sensitivity of diagnostic techniques. This may have led to overdiagnosis and potential overtreatment of patients with indolent thyroid cancer. It is likely that incidence, or rather detection, of thyroid cancer has decreased during the epidemic. Recently, there has been a shift in the endocrine surgical community toward less aggressive surgical management in lieu of active surveillance for certain indolent, endocrine pathologies, including certain thyroid cancers. The most recent American Thyroid Association guidelines recommended the consideration of hemithyroidectomy in patients with low-risk differentiated thyroid carcinoma between 1 to 4 cm and active-surveillance for differentiated thyroid carcinoma <1 cm.29 Similarly, several studies reported the low risk of adverse outcomes in opting for active surveillance, rather than immediate surgery, particularly in low-risk papillary microcarcinomas.15, 16, 17 , 30 The COVID-19 pandemic allowed an opportunity for the evaluation of short-term forced delays to care instead of surgery for most patients. In this study, few thyroid cancer patients had evidence of disease progression during their delay in care. When evaluated in conjunction with the recent literature, it is plausible that many procedures could be safely delayed in the short term with appropriate follow-up. When evaluating longer delays, other studies have shown increased mortality in thyroid cancer,31 and increased predicted risk of dying in head and neck cancer patients associated with longer delays to care.32 Thus, although few of our patients showed evidence of disease progression in the short-term, it is possible that more adverse outcomes would have been observed given longer delays in care. When faced with an unprecedented situation requiring delays to endocrine surgery care, implementation of evidence-based guidelines developed across many countries allowed for necessary preservation of hospital resources for COVID-19-related and emergency procedures without a significant burden on patient outcomes. Moreover, these findings underscored the importance of the surgeon’s risk stratification and triage of which patients for delay versus immediate treatment. However, future considerations should be given to examining ways to improve the outcomes for the patients who did ultimately experience disease progression, change in operative plan, or who were lost to follow-up.
Similarly positive outcomes were seen in the functional endocrinopathies, including adrenal (eg, Conn Syndrome, Cushing Syndrome, pheochromocytoma) and parathyroid-related (eg, hyperparathyroidism) disease, with relatively low rates of disease progression and changes in their initial operative plan. However, it is important to note that due to the nature of these disease processes, data on physical disease progression may overlook possible physiologic impacts of delays. Unless patients presented with physical findings of progression (eg, nephrolithiasis or hypertensive crisis), any changes in their disease status likely would not have been captured in our study. Although most of these patients did not show overt physical progress in their disease, physiologic impacts were not assessed. Although there are clear indications for surgical intervention (based on laboratory tests, bone mineral density, and symptoms) in mild asymptomatic primary hyperparathyroidism, as well as for secondary and tertiary hyperparathyroidism, the literature is unclear on how long these patients may be observed with non-operative management before they experience progression of disease.33, 34, 35, 36 Due to the forced delays to operative management that the COVID-19 pandemic posed to these patients, the multidisciplinary care team played an essential role in observing medical changes and selecting necessary patients for surgical management of patients with functional endocrinopathies.
This study was limited by its retrospective nature. Although it was a multi-institutional and international cohort, all patients were from large, academic centers, and a large proportion of the data comes from institutions in the United States. Furthermore, due to the large number of items included in the survey, there may have been an inherent difference that survey respondents had more resources to follow-up and provide care to patients who were delayed compared with nonrespondents. Thus, these results may not be broadly generalizable. Additionally, the timing of delays varied from institution to institution. It is also worth noting the potential for underreporting patients lost to follow-up given that this was a select group of patients that were planned for surgery (ie, workup complete). Given that it was not usual practice to interview patients virtually at the time of the study inception, we did not evaluate whether some virtual appointments could have been at the time of initial delay and not representative of the patient receiving follow-up care. Additionally, the assessment of disease progression was subjective, given it was assessed by the surgeon. For certain disease states, particularly primary and tertiary hyperparathyroidism, disease progression was difficult to quantify, and it is possible that progression may have happened even if there was no delay. At some of the participating institutions, additional information was provided that detailed progression of disease; however, this was not routinely obtained for all patients in the survey. Finally, although few patients had oncological or physical disease progression due to their delay, patients may have undergone alternative treatment outside of standard of care (ie, radioactive iodine or neoadjuvant chemotherapy). Despite these limitations, necessary treatment delays provided an opportunity to assess the impact of delays in endocrine disease-related care when it would otherwise have been unethical.
In summary, although some patients experienced overt disease progression during COVID-19 delays to endocrine disease-related care, most patients with follow-up did not. Our analysis indicated that temporary delay may be an acceptable course of action in extreme circumstances for most endocrine-related surgical disease, but the psychological impact on patients is unknown. Few patients during the initial waves of the COVID-19 pandemic had experienced disease progression, indicating that surgeons were able to differentiate patients for whom delay in care was appropriate versus those who required immediate surgery.
Funding/Support
This work was supported by NIH NCI R37 231957 (CCL).
Conflict of interest/Disclosure
The authors have no conflicts of interests or disclosures to report.
Footnotes
Findings have been accepted for podium presentation at the American Association of Endocrine Surgeons Annual Meeting, Cleveland, Ohio, May 22-24, 2022.
Supplementary material associated with this article can be found, in the online version, at https://doi.org/10.1016/j.surg.2022.06.043.
Supplementary Materials
References
- 1.State Guidance on Elective Surgeries. 2020. https://www.ascassociation.org/asca/resourcecenter/latestnewsresourcecenter/covid-19/covid-19-state. Accessed July 1, 2021.
- 2.Factsheet . American Medical Association; 2020. State action related to delay and resumption of “elective” procedures during COVID-19 pandemic.https://www.ama-assn.org/system/files/2020-06/state-elective-procedure-chart.pdf Accessed August 1, 2021. [Google Scholar]
- 3.COVID-19: Elective Case Triage Guidelines for Surgical Care. American College of Surgeons; 2020. https://www.facs.org/covid-19/clinical-guidance/elective-case Accessed August 1, 2021. [Google Scholar]
- 4.Alsofyani M.A., Malaekah H.M., Bashawyah A., et al. Safety measures for COVID-19: a review of surgical preparedness at four major medical centres in Saudi Arabia. Patient Saf Surg. 2020;14:1–14. doi: 10.1186/s13037-020-00259-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.A Measured Approach to Planning for Surgeries and Procedures During the COVID-19 Pandemic. Ontario Health; 2020. https://www.ontariohealth.ca/sites/ontariohealth/files/2021-07/A%20Measured%20Approach%20to%20Planning%20for%20Surgeries%20and%20Procedures%20During%20the%20COVID-19%20Pandemic.pdf Accessed January 1, 2022. [Google Scholar]
- 6.Baud G., Brunaud L., Lifante J.C., et al. Endocrine surgery during and after the COVID-19 epidemic: expert guidelines from AFCE. J Visc Surg. 2020;157:S43–S49. doi: 10.1016/j.jviscsurg.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jozaghi Y., Zafereo M.E., Perrier N.D., et al. Endocrine surgery in the Coronavirus disease 2019 pandemic: surgical triage guidelines. Head Neck. 2020;42:1325–1328. doi: 10.1002/hed.26169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raghavan D., Tan A.R., Story E.S., et al. Management changes for patients with endocrine-related cancers in the COVID-19 pandemic. Endocr Relat Cancer. 2020;27:R357–R374. doi: 10.1530/ERC-20-0229. [DOI] [PubMed] [Google Scholar]
- 9.Topf M.C., Shenson J.A., Holsinger F.C., et al. Framework for prioritizing head and neck surgery during the COVID-19 pandemic. Head Neck. 2020;42:1159–1167. doi: 10.1002/hed.26184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsang V.H.M., Gild M., Glover A., Clifton-Bligh R., Robinson B.G. Thyroid cancer in the age of COVID-19. Endocr Relat Cancer. 2020;27:R407–R416. doi: 10.1530/ERC-20-0279. [DOI] [PubMed] [Google Scholar]
- 11.Bartlett D.L., Howe J.R., Chang G., et al. Management of cancer surgery cases during the COVID-19 pandemic: considerations. Ann Surg Oncol. 2020;27:1717–1720. doi: 10.1245/s10434-020-08461-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elective Endocrine Surgery. American Association of Endocrine Surgeons; 2020. https://www.endocrinesurgery.org/assets/COVID-19/AAES-Elective-Endocrine-Surgery.pdf Accessed January 1, 2022. [Google Scholar]
- 13.Prachand V.N., Milner R., Angelos P., et al. Medically necessary, time-sensitive procedures: scoring system to ethically and efficiently manage resource scarcity and provider risk during the COVID-19 pandemic. J Am Coll Surg. 2020;231:281–288. doi: 10.1016/j.jamcollsurg.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amit M., Rudnicki Y., Binenbaum Y., Trejo-Leider L., Cohen J.T., Gil Z. Defining the outcome of patients with delayed diagnosis of differentiated thyroid cancer. Laryngoscope. 2014;124:2837–2840. doi: 10.1002/lary.24744. [DOI] [PubMed] [Google Scholar]
- 15.Ito Y., Miyauchi A., Oda H. Low-risk papillary microcarcinoma of the thyroid: a review of active surveillance trials. Eur J Surg Oncol. 2018;44:307–315. doi: 10.1016/j.ejso.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 16.Jeon M.J., Kim W.G., Kwon H., Kim M., Park S., Oh H.S., et al. Clinical outcomes after delayed thyroid surgery in patients with papillary thyroid microcarcinoma. Eur J Endocrinol. 2017;177:25–31. doi: 10.1530/EJE-17-0160. [DOI] [PubMed] [Google Scholar]
- 17.Miyauchi A. Clinical trials of active surveillance of papillary microcarcinoma of the thyroid. World J Surg. 2016;40:516–522. doi: 10.1007/s00268-015-3392-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patel K.N., Yip L., Lubitz C.C. The American Association of Endocrine Surgeons guidelines for the definitive surgical management of thyroid disease in adults. Ann Surg. 2020;271:e21–e93. doi: 10.1097/SLA.0000000000003580. [DOI] [PubMed] [Google Scholar]
- 19.Givi B., Schiff B.A., Chinn S.B., et al. Safety recommendations for evaluation and surgery of the head and neck during the COVID-19 pandemic. JAMA Otolaryngol Head Neck Surg. 2020;146:579–584. doi: 10.1001/jamaoto.2020.0780. [DOI] [PubMed] [Google Scholar]
- 20.Schumm M.A., Pyo H.Q., Ohev-Shalom R., Tseng C.H., et al. Patient experience with electronic health record-integrated postoperative telemedicine visits in an academic endocrine surgery program. Surgery. 2021;169:1139–1144. doi: 10.1016/j.surg.2020.11.019. [DOI] [PubMed] [Google Scholar]
- 21.Zheng H., Rosen J.E., Bader N.A., Lai V. Endocrine surgery patients' and providers' perceptions of telemedicine in the COVID era. J Surg Res. 2022;269:76–82. doi: 10.1016/j.jss.2021.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Medas F., Ansaldo G.L., Avenia N., et al. The THYCOVIT (Thyroid Surgery during COVID-19 pandemic in Italy) study: results from a nationwide, multicentric, case-controlled study. Updates Surg. 2021;73:1467–1475. doi: 10.1007/s13304-021-01051-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tunca F., Iscan Y., Sormaz I.C., Aksakal N., Senyurek Y. Impact of the coronavirus disease pandemic on the annual thyroid, parathyroid, and adrenal surgery volume in a tertiary referral endocrine surgery center in 2020. Sisli Etfal Hastan Tip Bul. 2021;55:286–293. doi: 10.14744/SEMB.2021.64920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ermer J.P., Ballester J.M.S., Go B.C., et al. Endocrine surgical procedures during COVID-19: patient prioritization and time to surgery. J Surg Res. 2021;268:459–464. doi: 10.1016/j.jss.2021.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beninato T., Laird A.M., Graves C.E., et al. Impact of the COVID-19 pandemic on the practice of endocrine surgery. Am J Surg. 2022;223:670–675. doi: 10.1016/j.amjsurg.2021.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Den Heede K., Chidambaram S., Winter Beatty J., et al. The PanSurg-PREDICT study: endocrine surgery during the COVID-19 pandemic. World J Surg. 2021;45:2315–2324. doi: 10.1007/s00268-021-06099-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vaccarella S., Dal Maso L., Laversanne M., Bray F., Plummer M., Franceschi S. The impact of diagnostic changes on the rise in thyroid cancer incidence: a population-based study in selected high-resource countries. Thyroid. 2015;25:1127–1136. doi: 10.1089/thy.2015.0116. [DOI] [PubMed] [Google Scholar]
- 28.Ahn H.S., Kim H.J., Welch H.G. Korea's thyroid-cancer "epidemic:" screening and overdiagnosis. N Engl J Med. 2014;371:1765–1767. doi: 10.1056/NEJMp1409841. [DOI] [PubMed] [Google Scholar]
- 29.Haugen B.R., Alexander E.K., Bible K.C., et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26:1–133. doi: 10.1089/thy.2015.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tuttle R.M., Fagin J.A., Minkowitz G., et al. Natural history and tumor volume kinetics of papillary thyroid cancers during active surveillance. JAMA Otolaryngol Head Neck Surg. 2017;143:1015–1020. doi: 10.1001/jamaoto.2017.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fligor S.C., Lopez B., Uppal N., Lubitz C.C., James B.C. Time to surgery and thyroid cancer survival in the United States. Ann Surg Oncol. 2021;28:3556–3565. doi: 10.1245/s10434-021-09797-z. [DOI] [PubMed] [Google Scholar]
- 32.Matos L.L., Forster C.H.Q., Marta G.N., et al. The hidden curve behind COVID-19 outbreak: the impact of delay in treatment initiation in cancer patients and how to mitigate the additional risk of dying—the head and neck cancer model. Cancer Causes & Control. 2021;32:459–471. doi: 10.1007/s10552-021-01411-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pitt S.C., Sippel R.S., Chen H. Secondary and tertiary hyperparathyroidism, state of the art surgical management. Surgical Clinics. 2009;89:1227–1239. doi: 10.1016/j.suc.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Niederle B., Wemeau J.-L. Is surgery necessary for ‘mild’ or ‘asymptomatic’ hyperparathyroidism? Eur J Endocrinol. 2015;173:D13–D20. doi: 10.1530/EJE-15-0277. [DOI] [PubMed] [Google Scholar]
- 35.Anagnostis P., Vaitsi K., Veneti S., et al. Efficacy of parathyroidectomy compared with active surveillance in patients with mild asymptomatic primary hyperparathyroidism: a systematic review and meta-analysis of randomized-controlled studies. J Endocrinol Invest. 2021;44:1127–1137. doi: 10.1007/s40618-020-01447-7. [DOI] [PubMed] [Google Scholar]
- 36.Ambrogini E., Cetani F., Cianferotti L., et al. Surgery or surveillance for mild asymptomatic primary hyperparathyroidism: a prospective, randomized clinical trial. J Clin Endocrinol Metab. 2007;92:3114–3121. doi: 10.1210/jc.2007-0219. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.