Abstract
The ability to respond to ambient pH is critical to the growth and virulence of the fungal pathogen Candida albicans. This response entails the differential expression of several genes affecting morphogenesis. To investigate the mechanism of pH-dependent gene expression, the C. albicans homolog of pacC, designated PRR2 (for pH response regulator), was identified and cloned. pacC encodes a zinc finger-containing transcription factor that mediates pH-dependent gene expression in Aspergillus nidulans. Mutants lacking PRR2 can no longer induce the expression of alkaline-expressed genes or repress acid-expressed genes at alkaline pH. Although the mutation did not affect growth of the cells at acid or alkaline pH, the mutants exhibited medium-conditional defects in filamentation. PRR2 was itself expressed in a pH-conditional manner, and its induction at alkaline pH was controlled by PRR1. PRR1 is homologous to palF, a regulator of pacC. Thus, PRR2 expression is controlled by a pH-dependent feedback loop. The results demonstrate that the pH response pathway of Aspergillus is conserved and that this pathway has been adapted to control dimorphism in C. albicans.
Candida albicans is a dimorphic fungus that can cause life-threatening infections in immunocompromised patients. The dimorphic conversion between yeast and hyphal morphologies is thought to be critical for pathogenesis, as mutations that block the transition to either form attenuate virulence (5, 26). The signal(s) that stimulates morphological transition is still not clear, but it is known that external pH and temperature have influential roles, at least in vitro (7, 33). One of the responses of C. albicans to changes in ambient pH is an altered pattern of gene expression. pH-dependent expression has been demonstrated for the genes PHR1, PHR2, and PRA1 (31, 39, 41) and for some of the secretory aspartyl proteinase genes (20, 48). Moreover, this genetic response to external pH is essential to pathogenesis, as indicated by the niche-conditional attenuation of PHR1 and PHR2 mutants (9).
The pathway controlling pH-responsive gene expression has been most extensively dissected for the ascomycete Aspergillus nidulans (8, 12). Central to the pH response is the pH-dependent activation of the zinc finger transcription factor encoded by pacC (46). PacC is synthesized in an inactive form, which is activated at alkaline pH by proteolytic removal of the carboxy terminus (29, 34). Proteolysis is dependent upon six genes, palA, -B, -C, -F, -H, and -I (46). The activated form of PacC induces the expression of alkaline-expressed genes and represses acid-expressed genes (46). This pathway controls diverse characteristics, including conidiation, pigmentation, nitrogen source utilization, penicillin synthesis, and growth at alkaline pH (2, 8, 12, 46).
This regulatory pathway is apparently conserved, as various elements have been identified in other fungi. Homologs of PacC have been identified in Yarrowia lipolytica and Saccharomyces cerevisiae. The Y. lipolytica homolog, YlRIM101, is activated by carboxy-terminal truncation and is required for alkaline-dependent expression of the XPR2-encoded protease but not for acid-dependent expression of AXP2 (22). A null allele of YlRIM101 does not affect growth at acid or alkaline pH but does block mating and sporulation (22). Similarly, the S. cerevisiae homolog, RIM101, was identified as a positive regulator of IME1, which is required for sporulation (43). Rim101p, like PacC, is activated by proteolysis, and this activation is dependent upon RIM8, -9, and -13, with RIM9 encoding a homolog of palI (10, 24). In addition to sporulation, RIM101 also controls invasive growth of haploids and the ability to grow at alkaline pH (15, 24). More recently, partial sequences of the C. albicans homologs of pacC and palA were identified, and homozygous null alleles of these genes compromised filamentous growth on Spider medium (49). It is not known whether these homologs affect pH-dependent gene expression in C. albicans.
In this work, we report the isolation and characterization of the full-length homolog of pacC, which we have designated PRR2 (for pH response regulator). As with other members of this gene family, the protein sequence is highly conserved within the zinc finger domain but shows limited conservation outside this domain. Deletion mutants exhibited defective hyphal development on a number of media but retained the ability to form germ tubes under all but one condition tested. The mutation did not affect growth at either acidic or alkaline pH. Furthermore, the deletion mutants were defective in their expression of both acid- and alkaline-expressed genes. Expression of PRR2 was itself pH dependent and also controlled the alkaline repression of PRR1, the homolog of palF, in an apparent regulatory feedback loop. The results parallel those seen with A. nidulans, suggesting that the pH response pathway is conserved between these two fungi.
MATERIALS AND METHODS
Strains and growth conditions.
The strains used are listed in Table 1. They were routinely cultured on YPD (2% glucose, 1% yeast extract, 2% Bacto Peptone) or YNB (2% glucose, 0.67% Difco yeast nitrogen base) at 30°C. The effects of acid or alkaline growth conditions were tested with medium 199 containing Earle's salts and glutamine but lacking sodium bicarbonate (Gibco-BRL) and containing 150 mM HEPES adjusted to either pH 4.0 or 7.5. Spider medium (25) and the medium of Lee et al. (23) were prepared as described previously. Serum-containing medium was prepared with 10% calf serum (Difco). Media were solidified with 2% agar and supplemented with uridine (25 μg/ml) as needed. Urd− auxotrophs were selected on medium containing 5′-fluoro-orotic acid (5′-FOA) as described previously (4, 14). Germ tube induction was assessed at 37°C following inoculation of stationary-phase cells into prewarmed medium at a density of 6 × 106 cells/ml. Filamentation on agar-solidified media was assessed by spotting 106 cells in 5 μl onto the plates and incubating at 37°C. Invasive growth was examined after washing the agar plates with sterile water to remove surface growth (18).
TABLE 1.
C. albicans strains used in this work
| Strain | Parent | Genotype | Source |
|---|---|---|---|
| SC5314 | Clinical isolate | 17 | |
| CAI4 | Δura3::imm434/Δura3::imm434 | 14 | |
| CAI12 | Δura3::imm434/URA3 | 36 | |
| CAR1 | CAI4 | Δprr2::hisG-URA3-hisG/PRR2 Δura3::imm434/Δura3::imm434 | This work |
| CAR14 | CAR1 | Δprr2::hisG/PRR2 Δura3::imm434/Δura3::imm434 | This work |
| CAR2 | CAR14 | Δprr2::hisG/Δprr2::hisG-URA3-hisG Δura3::imm434/Δura3::imm434 | This work |
| CAR26 | CAR2 | Δprr2::hisG/Δprr2::hisG Δura3::imm434/Δura3::imm434 | This work |
| CAR3 | CAR26 | PRR2-pUC18-URA3-Δprr2::hisG/Δprr2::hisG Δura3::imm434/Δura3::imm434 | This work |
| CAPM3 | Δprr1::hisG-URA3-hisG/Δprrl::hisG Δura3::imm434/Δura3::imm434 | 36 |
Identification, isolation, and sequence analysis of PRR2.
Sequence data for Candida albicans was obtained from the Stanford DNA Sequencing and Technology Center website (42a). A BLASTN (1) search of the C. albicans genome sequence database identified two sequences homologous with the sequence encoding the zinc finger region of PacC. A 182-bp fragment encoding this zinc finger region was amplified from genomic DNA by PCR, and the amplification product was used as a probe for hybridization screening of a λGEM-12 genomic library (3). A 10-kb BamHI insert containing the full-length gene was isolated and subcloned into pUC18 to generate plasmid pARA1.
The nucleotide sequence of the relevant region was determined by cycle sequencing with AmpliTaq DNA polymerase (Perkin-Elmer), the ABI Prism Ready Reaction Kit (Perkin-Elmer), and custom-made oligonucleotide primers. The sequence data was assembled with Lasergene (DNASTAR Inc.) and analyzed with DNA Strider (28). Homology searches were conducted by using the BLAST algorithm (1). Sequence alignments were performed by using LALIGN (35).
Construction of mutant strains.
To construct a PRR2 null mutant, the 3-kb BamHI-NarI fragment from pARA1 was subcloned into the like sites of pUC18. The resulting plasmid, pARA2, was digested with PstI and MscI to remove 1,270 bp encompassing codons 71 to 495 of the PRR2 open reading frame. This region was replaced with a PstI-BglII fragment from plasmid pMB7 (14), which contains the hisG-URA3-hisG cassette. The BglII end was made flush with Klenow DNA polymerase prior to ligation with the blunt-ended MscI-cut site. This plasmid, pARA3, was digested with HindIII and SspBI, releasing the cassette with 407 bp of PRR2 on the 5′ end and 722 bp on the 3′ end. Approximately 7 μg of plasmid DNA was used in a lithium acetate-mediated transformation of C. albicans CAI4 (16). Sequential disruptions of both PRR2 alleles were achieved essentially as previously described (14).
A reconstituted strain was constructed by integration of plasmid pARA4 into the null mutant. This plasmid was constructed by cloning a SacI-SalI fragment, containing URA3 derived from plasmid pSMS44 (39), into the SacI and SmaI sites of pARA2. The SalI end was made flush with Klenow DNA polymerase prior to ligation. The resulting plasmid, pARA4, was linearized at the HindIII site located 196 bp upstream of the PRR2 coding region and used to transform strain CAR26. The occurrence of the desired integration event in each of the strains was verified by Southern blot analysis. Southern blot analysis was conducted as previously described (31) except that the blots were hybridized in 1× phosphate buffer (0.5 M sodium phosphate [pH 7.2], 5% sodium dodecyl sulfate, 10 mM EDTA) and washed in 0.1× phosphate buffer.
Northern blot analyses.
To prepare RNA, a stationary-phase culture grown at 25°C in YPD was used to inoculate 300 ml of medium 199, adjusted to pH 4.0 or pH 7.5, to a density of 6 × 106 cells/ml. The culture was incubated at 25°C for 2 h in an orbital shaker set at 200 rpm. The cells were recovered by centrifugation at 4,000 × g for 10 min and washed in sterile distilled water. The RNA was extracted as described previously (37) except that after washing, the pellet was resuspended in 1.5 ml of LETS buffer (0.1 M LiCl, 10 mM EDTA, 10 mM Tris-HCl [pH 7.4], and 0.2% sodium dodecyl sulfate and mixed with 6 g of sterile acid-washed 0.45-mm-diameter glass beads (Sigma) and 2 ml of phenol (pH 4.3). The concentration of RNA was determined by measuring the absorbance at 260 nm.
Ten micrograms of RNA was fractionated on a 1.2% agarose gel containing 2.2 M formaldehyde in MOPS (morpholinepropanesulfonic acid) buffer. Electrophoresis was carried out essentially as described previously (6) except that 2.2% formaldehyde was included in the running buffer. Blotting and hybridization were conducted as described for the Southern analysis.
Blots were hybridized with one of the following probes: a 942-bp BglII-PstI fragment encompassing the 5′ end of PRR2, a 1,063-bp AatII-NdeI fragment from within the open reading frame of PHR1, a 1,257-bp BamHI-NheI fragment of PHR2, a 2,549-bp BanII-BanII fragment of PRR1 from pAP2 (36), or the 1.9-kb SalI fragment of ACT1. All probes were labeled by random priming with [α-32P]dCTP and Ready-to-Go DNA labeling beads (Amersham Pharmacia Biotech). Hybridization was quantitated by phosphorimaging with a model 445SI PhosphorImager (Molecular Dynamics, Sunnyvale, Calif.) and ImageQuant software) and normalized to ACT1. Northern data were reproducible with at least two independent RNA samples.
RESULTS
Isolation and identification of the pacC homolog.
In previous studies it was demonstrated that the C. albicans homolog of the A. nidulans palF gene was required for pH-dependent gene expression. Assuming that the pH response pathway is conserved between A. nidulans and C. albicans, then the pacC ortholog should also be required for pH-dependent control of gene expression. To test this hypothesis, the C. albicans homolog of pacC was isolated.
A genomic clone of the gene was isolated by hybridization with a PCR-generated fragment complementary to the region of the gene encoding the zinc fingers. This region was identified by a BLASTN search of the genomic database available on the Stanford DNA Sequencing and Technology Center website (42a). A BamHI-BamHI fragment was identified and subcloned in pUC18, generating plasmid pARA1. The nucleotide sequence of a 3,086-bp region centered around the zinc finger region was determined. Analysis of the sequence identified a 1,986-bp open reading frame encoding a putative protein of 661 amino acids with a pI of 6.58 and a theoretical molecular mass of 74.73 kDa. The open reading frame was tentatively designated PRR2 (for pH response regulator) in recognition of its potential role in controlling the pH response.
A BLASTP comparison with the GenBank database (35) revealed that the encoded protein had homology with PacC of A. nidulans, Aspergillus niger, and Penicillum chrysogenum (27, 44, 46), YlRim101p of Y. lipolytica (22), and Rim101p of S. cerevisiae (43). The greatest similarity lay within the zinc finger domain, which encompassed amino acid residues 208 to 300. This region was 68% identical between the C. albicans protein and the PacC and YlRim101p proteins and 60% identical in comparison with Rim101p of S. cerevisiae (Fig. 1). There are three putative zinc fingers of the Cys2His2 class within this domain (Fig. 1). The first finger is the least conserved among all of the homologs, including the C. albicans protein. Unlike the second and third fingers, the first finger of PacC does not contact the DNA, but it appears to stabilize the conformation of the second finger via an essential hydrophobic interaction between the Trp residues located in the Cys knuckle of the first and second zinc fingers (13). These critical Trp residues are conserved in the C. albicans protein as in the other homologs. Site-specific mutagenesis of A. nidulans demonstrated the importance of the histidine in the second finger and the glutamine and lysine residues in the third finger for DNA binding and sequence recognition (13). These critical residues are also conserved in Prr2p. The extensive conservation in this region suggests that the C. albicans protein recognizes the same or a similar DNA binding motif as does PacC.
FIG. 1.
Amino acid sequence alignment of Prr2p from C. albicans and PacC from A. nidulans. Identical residues are boxed.
Little similarity was evident outside the zinc finger domain (Fig. 1). Several potential nuclear localization signals were identified by using PSORT II (32), consistent with the protein's putative role in transcription. These included a bipartite signal, KKHSKTHAEDHPKKLKK, starting at position 290 and two T-antigen-type signals, PKKLKKA, and RKRR, at positions 301 and 361, respectively (19, 38). As noted previously for the other homologs, the candidal protein contained multiple S/TPXX motifs in the amino terminus and contained an acidic carboxy terminus (22, 45).
Subsequent to this sequence determination, the entire sequence became available on the Stanford DNA Sequencing and Technology Center website (42a) as Contig4-3014. These two sequences were identical, and the open reading frame was designated RIM101.
PRR2 is required for pH-dependent gene expression.
pacC of A. nidulans is required to both induce alkaline-expressed genes and repress acid-expressed genes (46). This control is dependent upon the pal genes, and concurrent work demonstrated that the palF homolog of C. albicans is similarly required for the pH-dependent response (36). Thus, if Prr2p is functionally analogous to PacC, then deletion of PRR2 should alter pH-dependent gene expression.
A prr2 null mutant was constructed by replacing a 1,270-bp region from the PRR2 coding region with a hisG-URA3-hisG cassette, as illustrated in Fig. 2. A HindIII-SspBI fragment consisting of the cassette flanked by portions of PRR2 was used to transform strain CAI4 to Urd+. A representative transformant was designed CAR1. Loss of URA3 by intrachromosomal recombination between the hisG repeats was selected for on 5′-FOA. The resulting strain, CAR14, was transformed with the disrupting cassette to replace the remaining allele. The prr2 null mutant strain was called CAR2. An Urd− derivative of CAR2 was isolated by 5′-FOA selection and designated CAR26.
FIG. 2.
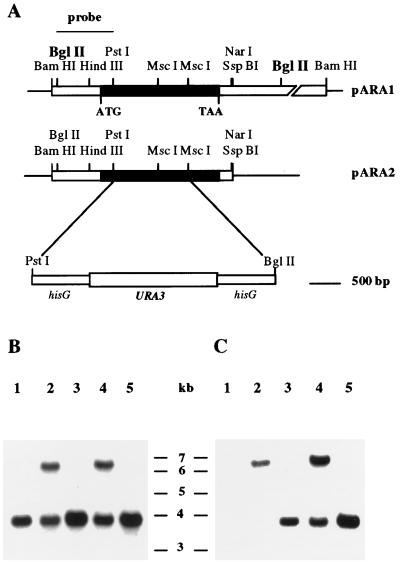
Design and construction of PRR2 mutants. (A) Restriction maps of the locus as cloned in plasmid pARA1 and subcloned in pARA2, as well as the region deleted and replaced with the hisG-URA3-hisG cassette. The open reading frame of PRR2 is indicated in black. The region used as a hybridization probe is overlined. (B and C) Southern blots of genomic DNA digested with BglII (boldface in panel A) were hybridized with either PRR2 (B) or hisG (C) probes. Lanes 1 through 5, DNAs isolated from strains CAI12, CAR1, CAR14, CAR2, and CAR26 respectively.
A Southern blot of BglII-digested genomic DNA from the representative isolates was hybridized with a BglII-PstI fragment of PRR2 (Fig. 2). The parental strain exhibited one hybridization band of approximately 3.8 kb. The first transformant was heterozygous and exhibited one band of 3.8 kb corresponding to the wild-type allele and another band of 6.4 kb corresponding to the replaced allele. The predicted size of the disrupted allele following intrachromosomal recombination was 3.7 kb. Since this fragment was not readily resolved from the native 3.8-kb fragment, a parallel blot was hybridized with hisG DNA to specifically detect the disrupted allele (Fig. 2). The hybridization pattern of the null mutant showed one band of 3.7 kb corresponding to the prr2::hisG allele and another of 6.4 kb representing the new disrupted allele containing the URA3 cassette. Selection on 5′-FOA resulted in loss of the 6.4-kb band and the presence of a single 3.7-kb band that hybridized with both PRR2 and hisG (Fig. 2). A wild-type allele was reintroduced into CAR26 by targeted integration of plasmid pARA4 to generate strain CAR3. The predicted structure of the targeted locus was verified by Southern blot analysis (data not shown).
The effect of the mutations on acid- and alkaline-expressed genes was examined by Northern blot analysis. The control strain CAI12 exhibited the expected pattern of PHR1 expression, i.e., high levels at pH 7.5 and undetectable expression at pH 4.0 (Fig. 3) (39). This alkaline-induced expression was completely abolished in the prr2 null mutant, as no PHR1 transcript was detected at either pH (Fig. 3). Mutants containing a single allele of PRR2 maintained the pH-dependent expression pattern but showed a reproducible reduction in the level of induction, suggestive of a gene dosage effect (Fig. 3).
FIG. 3.
Effect of PRR2 mutations on pH-dependent gene expression. Total RNAs were isolated from the indicated strains cultured at either pH 4.0 or 7.5 and examined by Northern blot analysis. The gene sources of the hybridizing DNA probes are indicated on the left.
The mutations also affected acid-induced gene expression. Normally, the expression of both PHR2 and PRR1 is greatly enhanced at acidic pH (Fig. 3) (31, 36). Deletion of PRR2 resulted in constitutive expression of both genes, indicating that the mutations prevented repression of their expression at alkaline pH. The presence of a single functional allele resulted in an intermediate phenotype for PHR2 gene expression, which was partially derepressed at pH 7.5 (Fig. 3). Whether PRR1 expression was partially affected in the heterozygote was not clear. These results demonstrate that PRR2 is a component of the pH response pathway of C. albicans and that, like pacC, it is required for induction of alkaline-expressed genes and the repression of acid-expressed genes.
Effect of PRR2 mutations on growth and morphological development.
Mutants lacking PRR1 exhibited medium-conditional defects in their ability to form germ tubes and hyphae (31, 36). In addition, a previous report demonstrated that a partial deletion of HRM101/PRR2 compromised the ability of the cells to filament on Spider media (49). To determine if a more extensive deletion had similar effects and to further characterize the extent of the developmental defects, the morphological development of the mutants was examined under a number of conditions.
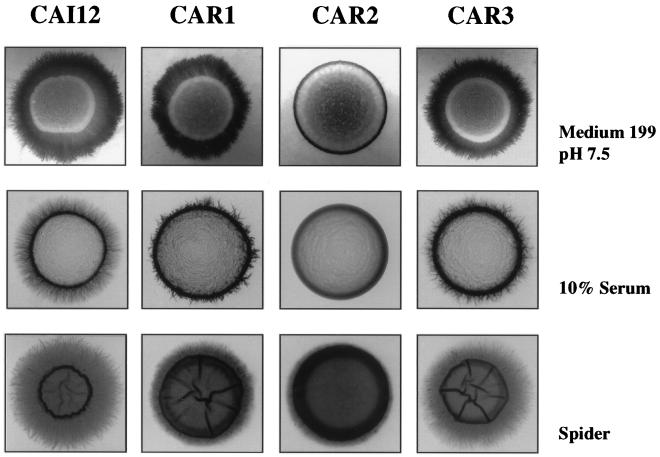
Filamentation ability was examined on agar-solidified medium. Cells were grown to stationary phase in YPD at 25°C and spotted on various media. On medium 199 buffered at pH 7.5, the control strain exhibited extensive filamentation with numerous hyphae extending laterally from the colony dome (Fig. 4). These hyphae were completely absent from the null mutant and noticeably reduced in the heterozygous mutants (Fig. 4). The phenotypes were similar on 10% serum plates (Fig. 4). On Spider medium (Fig. 4) and the medium of Lee et al. (23) (data not shown), the filamentation of the null mutant was greatly attenuated but not eliminated. Despite the absence of peripheral hyphae when the null mutant was grown on medium 199 and 10% serum, the cells were not similarly invasive on these two media. No invasive growth was detected on 10% serum, but invasive growth comparable to that of the control strain was evident on medium 199 (data not shown).
FIG. 4.
Effect of PRR2 mutations on filamentation. The indicated strains were spotted on the indicated media and incubated at 37°C in medium 199 (pH 7.5) for 5 days, in 10% serum for 3 days, and in Spider medium for 6 days.
Medium-conditional effects were also evident in germ tube induction. The null mutant was comparable to the control strain in germ tube formation in 10% serum or the medium of Lee et al. (23). However, in liquid medium 199, the cells failed to develop germ tubes but formed chains of yeast cells (data not shown).
The viability of prr2 mutants showed that PRR2 is not essential, and the growth rate of the mutant was similar to that of the wild type. Growth rates were determined under a number of culture conditions, including medium 199 at pH 7.5 or 4.0, YPD at 25°C, or 2× YPD at 37°C. The doubling time of the mutant was essentially identical to that of strain CAI12 under all of these conditions (data not shown).
All of the phenotypes associated with the null mutant were lost upon reintroduction of the wild-type gene and were reproducible with a set of independently constructed mutants. Thus, PRR2 is required for proper hyphal development. Development is fully PRR2 dependent under some conditions but only partially dependent under others.
Expression of PRR2 is pH dependent and requires PRR1.
Both pacC and its homolog in Y. lipolytica exhibit pH-dependent changes in expression level (22, 46). In A. nidulans this expression pattern is dependent upon palF (46). Therefore, expression of PRR2 was examined to determine if this expression pattern was conserved in C. albicans.
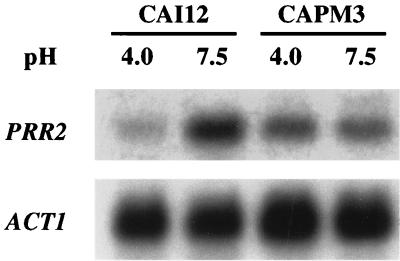
In Northern blot analysis the PRR2 probe hybridized with a 2.3-kb transcript, which is consistent with the predicted open reading frame. The level of this transcript was about threefold higher under alkaline growth conditions than under acidic growth conditions (Fig. 5). This expression pattern was unaffected by the temperature of incubation or morphology of the cells (data not shown). Thus, PRR2 is an alkaline-expressed gene. To test if the pH-dependent expression of PRR2 was dependent upon expression of PRR1, the palF homolog of C. albicans, Northern blot analysis was conducted with RNA from CAPM3, a prr1 null mutant (36). Alkaline induction of PRR2 was not evident in the prr1 mutant, instead, a pattern of low-level constitutive expression was observed (Fig. 5). Thus, expression of PRR2 responds to ambient pH, and PRR1 is a component of the pathway to which it responds.
FIG. 5.
Effect of culture pH and PRR1 mutation on PRR2 expression. Cells of either the control strain CAI12 or the PRR1 null mutant CAPM3 were cultured at either pH 4.0 or 7.5. Total RNA was isolated and examined by Northern blot analysis. The blot was hybridized with either PRR2 or ACT1 DNA.
DISCUSSION
The two major conclusions drawn from this work are that PRR2 defines an additional component of the pH response pathway and that this pathway plays a significant role in morphological development. In terms of pH-dependent gene expression, the phenotype of the prr2 null mutant was identical to that of an A. nidulans pacC null mutant (46). In the absence of PRR2, alkaline-expressed genes were no longer induced at alkaline pH and acid-expressed genes were no longer repressed. The same phenotype was observed in PRR1 mutants, which lack the C. albicans homolog of palF (36). This suggests that PRR2 and PRR1 are components of the same pH response pathway as demonstrated for corresponding genes in A. nidulans. Interestingly, a slightly different response is observed in Y. lipolytica, where deletion of YlRIM101 abrogates alkaline induction of gene expression but does not affect the repression of an acid-expressed gene, AXP (22). Thus, despite the conserved role of these homologs in the pH response, there are likely to be species-specific differences.
These differences are also evident for other phenotypes. Neither the prr2 null mutant nor a ylrim101 null mutant exhibit pH-dependent growth defects (22). However, the A. nidulans pacC mutant is unable to grow at alkaline pH (8). Similarly, an S. cerevisiae CPL1 mutant, lacking the palB homolog, exhibits impaired growth at alkaline pH, and normal growth is restored by an activated allele of RIM101 (15). The downstream targets of the PacC family transcription factors also differ between species. A clear example of this is PHR1 and PHR2. Although expression of these genes responds to ambient pH via PRR2, that of their counterparts in Aspergillus and S. cerevisiae, GEL1 and GAS1, respectively, does not (30, 47).
The regulation of pH-dependent gene expression requires conversion of PacC from an inactive to an active form by proteolytic cleavage (29, 34). Cleavage occurs approximately 85 to 87 residues downstream of the zinc finger domain and removes about 425 C-terminal amino acids (29). Proteolysis does not depend upon specific sequences at the cleavage site but rather is determined by upstream sequences (29). Similarly, Rim101p of S. cerevisiae is activated upon cleavage of the C terminus, but a much smaller region of approximately 70 amino acids is removed (24). Nonetheless, this suggests that proteolytic activation is a common feature of this family of proteins and that Prr2p is likely to be activated in an analogous manner. Because of the limited sequence homology in the C-terminal regions of the PacC homologs and the absence of a sequence-specific proteolytic site, it is not possible to predict whether and where Prr2p might be cleaved.
The greatest degree of conservation between PacC and the other family members, including Prr2p, lies within the zinc finger domain. This high degree of conservation, particularly of known critical residues, suggests that Prr2p likely recognizes the same DNA binding site. The DNA motif recognized by PacC has been well characterized and contains the core consensus 5′-GCCAAG-3′ (11, 13, 46). A thymine preceding the core is optimal for binding, and substitution of G for A at the fifth position is compatible with PacC binding, albeit with a much reduced affinity (13). Multiple consensus sites lie upstream of ipnA, encoding the alkaline-expressed isopenicillin N synthetase of A. nidulans, and binding of activated PacC to these sites appears to be directly responsible for alkaline-induced transcription (13, 46). Indirect evidence suggests that the same model applies to alkaline-induced transcription in Y. lipolytica. Several copies of the consensus PacC binding site are located upstream of the alkaline-induced genes XPR2 and YlRIM101, suggesting that YlRim101p directly activates their transcription. The situation is less clear for C. albicans. Two copies of the PacC recognition site are found upstream of each of the alkaline-induced genes PHR1, PRA1, and PRR2. However, these sites are not required for pH-dependent expression of PHR1 (37a).
PacC not only is required for alkaline-induced gene expression but also mediates the repression of acid-expressed genes (46). This is probably indirect, perhaps through pacM (40). This is suggested first by the fact that a PacC binding site upstream of several acid-expressed genes is absent or present in only one copy and second by the ability of mutations in pacM to suppress pacCc mutations (40). PHR2 and PRR1, acid-expressed genes of C. albicans, contain two consensus sites and one consensus site, respectively, but these do not appear to be required for repression at alkaline pH (36a). That acid repression is indirect is further suggested by the inability of ylrim101 null mutants to relieve alkaline repression of the acid-expressed AXP gene despite the presence of three consensus binding sites upstream of the open reading frame.
Expression of PRR2, that of like pacC and YlRIM101, was induced at alkaline ambient pH (22, 46). Induction was dependent upon PRR1, the C. albicans homolog of palF. This parallels the regulation seen in A. nidulans and Y. lipolytica where PacC/YlRim101p induction is also dependent upon pal functions (22, 46). This pal dependence is an important regulatory feature, assuming that PRR2, like pacC and YlRIM101, is autoregulatory and induces its own expression. In the absence of feedback control, self-induction would lead to an upward spiral in expression, which would likely be detrimental. However, PRR1 expression is simultaneously repressed, providing a balancing mechanism that dampens activation of Prr2p and prevents the runaway expression of PRR2. It is not known if expression of palF responds to ambient pH in a pacC-dependent manner, but this seems likely given the parallels between pH-dependent regulation in C. albicans and A. nidulans. A different control mechanism appears to operate in S. cerevisiae, since expression of RIM101 is not pH responsive and stability of the protein is controlled by CPL1, the homolog of the A. nidulans palB gene (15).
An ultimate consequence of PRR2 function is the control of dimorphism. The effects of PRR2 mutations on morphological development were identical to those seen in PRR1 mutants (36). Medium-conditional defects in filamentation, invasion, and germ tube formation were observed. The null mutant exhibited no filamentation or invasiveness on serum-containing medium, whereas filamentation, but not invasiveness, was absent on medium 199. Conversely, germ tube formation was normal on serum but compromised on medium 199. A partial defect in filamentation on the medium of Lee et al. (23) and on Spider medium was evident, as previously reported (49). An intermediate phenotype was also observed with mutants lacking one copy of PRR2. This apparent gene dosage effect was also evident in the level of induction or repression of pH-dependent gene expression. Dosage effects have been reported for other genes involved in the morphological development of C. albicans (21, 42). Understanding how PRR2 is integrated into the control of dimorphism will provide significant insights into the biology of C. albicans.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grant GM47727 from the National Institutes of Health and the Burroughs Wellcome Fund Scholar Award in Molecular Pathogenic Mycology. Sequencing of Candida albicans was accomplished with the support of the NIDR and the Burroughs Wellcome Fund.
REFERENCES
- 1.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 2.Arst H N, Cove D J. Molybdate metabolism in Aspergillus nidulans II. Mutations affecting phosphatase activity or galactose utilization. Mol Gen Genet. 1970;108:146–153. doi: 10.1007/BF02430520. [DOI] [PubMed] [Google Scholar]
- 3.Birse C E, Irwin M Y, Fonzi W A, Sypherd P S. Cloning and characterization of ECE1, a gene expressed in association with cell elongation of the dimorphic pathogen Candida albicans. Infect Immun. 1993;61:3648–3655. doi: 10.1128/iai.61.9.3648-3655.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boeke J D, LaCroute F, Fink G R. A positive selection for mutants lacking orotidine-5′-phosphate decarboxylase activity in yeast: 5-fluoro-orotic acid resistance. Mol Gen Genet. 1984;197:345–346. doi: 10.1007/BF00330984. [DOI] [PubMed] [Google Scholar]
- 5.Braun B R, Johnson A D. Control of filament formation in Candida albicans by the transcriptional repressor TUP1. Science. 1997;277:105–109. doi: 10.1126/science.277.5322.105. [DOI] [PubMed] [Google Scholar]
- 6.Brown T, Mackey K. Analysis of RNA by Northern and slot blot hybridization. In: Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. Vol. 1. New York, N.Y: Greene Publishing Associates; 1997. pp. 4.9.1–4.9.16. [Google Scholar]
- 7.Buffo J, Herman M A, Soll D R. A characterization of pH-regulated dimorphism in Candida albicans. Mycopathology. 1984;85:21–30. doi: 10.1007/BF00436698. [DOI] [PubMed] [Google Scholar]
- 8.Caddick M X, Brownlee A G, Arst H N J. Regulation of gene expression by pH of the growth medium in Aspergillus nidulans. Mol Gen Genet. 1986;203:346–353. doi: 10.1007/BF00333978. [DOI] [PubMed] [Google Scholar]
- 9.De Bernardis F, Mühlschlegel F A, Cassone A, Fonzi W A. The pH of the host niche controls gene expression in and virulence of Candida albicans. Infect Immun. 1998;66:3317–3325. doi: 10.1128/iai.66.7.3317-3325.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Denison S H, Negrete-Urtasun S, Mingot J M, Tilburn J, Mayer W A, Goel A, Espeso E A, Peñalva M A, Arst H N., Jr Putative membrane components of signal transduction pathways for ambient pH regulation in Aspergillus and meiosis in Saccharomyces are homologous. Mol Microbiol. 1998;30:259–264. doi: 10.1046/j.1365-2958.1998.01058.x. [DOI] [PubMed] [Google Scholar]
- 11.Espeso E A, Peñalva M A. Three binding sites for the Aspergillus nidulans PacC Zinc-finger transcription factor are necessary and sufficient for regulation by ambient pH of the isopenicillin N synthase gene promoter. J Biol Chem. 1996;271:28825–28830. doi: 10.1074/jbc.271.46.28825. [DOI] [PubMed] [Google Scholar]
- 12.Espeso E A, Tilburn J, Arst H N, Peñalva M A. pH regulation is a major determinant in expression of a fungal penicillin biosynthetic gene. EMBO J. 1993;12:3947–3956. doi: 10.1002/j.1460-2075.1993.tb06072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Espeso E A, Tilburn J, Sanchez-Pulido L, Brown C V, Valencia A, Arst H N, Jr, Peñalva M A. Specific DNA recognition by the Aspergillus nidulans three zinc finger transcription factor PacC. J Mol Biol. 1997;274:466–480. doi: 10.1006/jmbi.1997.1428. [DOI] [PubMed] [Google Scholar]
- 14.Fonzi W A, Irwin M Y. Isogenic strain construction and gene mapping in Candida albicans. Genetics. 1993;134:717–728. doi: 10.1093/genetics/134.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Futai E, Maeda T, Sorimachi H, Kitamoto K, Ishiura S, Suzuki K. The protease activity of a calpain-like cysteine protease in Saccharomyces cerevisiae is required for alkaline adaptation and sporulation. Mol Gen Genet. 1999;260:559–568. doi: 10.1007/s004380050929. [DOI] [PubMed] [Google Scholar]
- 16.Gietz D, St. Jean A, Woods R A, Schiestl R H. Improved method for high efficiency transformation of intact yeast cells. Nucleic Acids Res. 1992;20:1425. doi: 10.1093/nar/20.6.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gillum A M, Tsay E Y H, Kirsch D R. Isolation of the Candida albicans gene for orotidine-5′-phosphate decarboxylase by complementation of S. cerevisiae ura3 and E. coli pyrF mutations. Mol Gen Genet. 1984;198:179–182. doi: 10.1007/BF00328721. [DOI] [PubMed] [Google Scholar]
- 18.Gimeno C J, Ljungdahl P O, Styles C A, Fink G R. Unipolar cell division in the yeast S. cerevisiae lead to filamentous growth: regulation by starvation. Cell. 1992;68:1077–1090. doi: 10.1016/0092-8674(92)90079-r. [DOI] [PubMed] [Google Scholar]
- 19.Hicks G R, Raikhel N V. Protein import into the nucleus: an integrated view. Annu Rev Cell Dev Biol. 1995;11:155–188. doi: 10.1146/annurev.cb.11.110195.001103. [DOI] [PubMed] [Google Scholar]
- 20.Hube B, Monod M, Schofield D A, Brown A J, Gow N A. Expression of seven members of the gene family encoding secretory aspartyl proteinases in Candida albicans. Mol Microbiol. 1994;14:87–99. doi: 10.1111/j.1365-2958.1994.tb01269.x. [DOI] [PubMed] [Google Scholar]
- 21.Köhler J R, Fink G R. Candida albicans strains heterozygous and homozygous for mutations in mitogen-activated protein kinase signaling components have defects in hyphal development. Proc Natl Acad Sci USA. 1996;93:13223–13228. doi: 10.1073/pnas.93.23.13223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lambert M, Blanchin-Roland S, Le Louedec F, Lepingle A, Gaillardin C. Genetic analysis of regulatory mutants affecting synthesis of extracellular proteinases in the yeast Yarrowia lipolytica: identification of a RIM101/pacC homolog. Mol Cell Biol. 1997;17:3966–3976. doi: 10.1128/mcb.17.7.3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee K L, Buckley H R, Campbell C C. An amino acid liquid synthetic medium for the development of mycelial and yeast forms of Candida albicans. Sabouraudia. 1975;13:148–153. doi: 10.1080/00362177585190271. [DOI] [PubMed] [Google Scholar]
- 24.Li W, Mitchell A P. Proteolytic activation of Rim1p, a positive regulator of yeast sporulation and invasive growth. Genetics. 1997;145:63–73. doi: 10.1093/genetics/145.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu H, Kohler J, Fink G R. Suppression of hyphal formation in Candida albicans by mutation of a STE12 homolog. Science. 1994;266:1723–1726. doi: 10.1126/science.7992058. [DOI] [PubMed] [Google Scholar]
- 26.Lo H, Köhler J R, DiDomenico B, Loebenberg D, Cacciapuoti A, Fink G R. Nonfilamentous C. albicans mutants are avirulent. Cell. 1997;90:939–949. doi: 10.1016/s0092-8674(00)80358-x. [DOI] [PubMed] [Google Scholar]
- 27.MacCabe A P, Van den Hombergh J P, Tilburn J, Arst H N, Jr, Visser J. Identification, cloning and analysis of the Aspergillus niger gene pacC, a wide domain regulatory gene responsive to ambient pH. Mol Gen Genet. 1996;250:367–374. doi: 10.1007/BF02174395. [DOI] [PubMed] [Google Scholar]
- 28.Marck C. “DNA Strider”: a C program for the fast analysis of DNA and protein sequences on the Apple Macintosh family of computers. Nucleic Acids Res. 1988;16:1829–1836. doi: 10.1093/nar/16.5.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mingot J M, Tilburn J, Diez E, Bignell E, Orejas M, Widdick D A, Sarkar S, Brown C V, Caddick M X, Espeso E A, Arst H N, Jr, Peñalva M A. Specificity determinants of proteolytic processing of Aspergillus PacC transcription factor are remote from the processing site, and processing occurs in yeast if pH signalling is bypassed. Mol Cell Biol. 1999;19:1390–1400. doi: 10.1128/mcb.19.2.1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mouyna, I., T. Fontaine, M. Vai, M. Monod, W. A. Fonzi, M. Diaquin, L. Popolo, B. Henrissat, R. P. Hartland, and J. P. Latgé. The glucanosyltransferase of Aspergillus fumigatus (Gel1p) responsible for the elongation of cell wall β(1-3)glucan is GPI-anchored and homologous to the Gas family of proteins. Submitted for publication.
- 31.Mühlschlegel F A, Fonzi W A. PHR2 of Candida albicans encodes a functional homolog of the pH-regulated gene PHR1 with an inverted pattern of expression. Mol Cell Biol. 1997;17:5960–5967. doi: 10.1128/mcb.17.10.5960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakai K, Kanehisa M. A knowledge base for predicting protein localization sites in eukaryotic cells. Genomics. 1992;14:897–911. doi: 10.1016/S0888-7543(05)80111-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Odds F C. Candida and candidosis. A review and bibliography. 2nd ed. London, United Kingdom: Bailliere Tindal; 1988. [Google Scholar]
- 34.Orejas M, Espeso E A, Tilburn J, Sarkar S, Arst Jr H N, Peñalva M A. Activation of the Aspergillus PacC transcription factor in response to alkaline ambient pH requires proteolysis of the carboxy-terminal moiety. Genes Dev. 1995;9:1622–1632. doi: 10.1101/gad.9.13.1622. [DOI] [PubMed] [Google Scholar]
- 35.Pearson W R, Lipman D J. Improved tools for biological sequence comparison. Proc Natl Acad Sci USA. 1988;85:2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Porta A, Ramon A M, Fonzi W A. PRR1, a homolog of Aspergillus nidulans palF, controls pH-dependent gene expression and filamentation in Candida albicans. J Bacteriol. 1999;181:7516–7523. doi: 10.1128/jb.181.24.7516-7523.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36a.Porta, A., A. M. Ramon, and W. A. Fonzi. Unpublished results.
- 37.Ramon A M, Gil R, Burgal M, Sentandreu R, Valentin E. A novel cell wall protein specific to the mycelial form of Yarrowia lipolytica. Yeast. 1996;12:1535–1548. doi: 10.1002/(SICI)1097-0061(199612)12:15%3C1535::AID-YEA59%3E3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 37a.Ramon, A. M., A. Porta, and W. A. Fonzi. Unpublished results.
- 38.Robbins J, Dilworth S M, Laskey R A, Dingwall C. Two interdependent basic domains in nucleoplasmin nuclear targeting sequence: identification of a class of bipartite nuclear targeting sequence. Cell. 1991;64:615–623. doi: 10.1016/0092-8674(91)90245-t. [DOI] [PubMed] [Google Scholar]
- 39.Saporito-Irwin S M, Birse C E, Sypherd P S, Fonzi W A. PHR1, a pH-regulated gene of Candida albicans, is required for morphogenesis. Mol Cell Biol. 1995;15:601–613. doi: 10.1128/mcb.15.2.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sarkar S, Caddick M X, Bignell E, Tilburn J, Arst H N., Jr Regulation of gene expression by ambient pH in Aspergillus: genes expressed at acid pH. Biochem Soc Trans. 1996;24:360–336. doi: 10.1042/bst0240360. [DOI] [PubMed] [Google Scholar]
- 41.Sentandreu M, Elorza M V, Sentandreu R, Fonzi W A. Cloning and characterization of PRA1, a gene encoding a novel pH-regulated antigen of Candida albicans. J Bacteriol. 1998;180:282–289. doi: 10.1128/jb.180.2.282-289.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sharkey L L, McNemar M D, Saporito-Irwin S M, Sypherd P S, Fonzi W A. HWP1 functions in the morphological development of Candida albicans downstream of EFG1, TUP1, and RBF1. J Bacteriol. 1999;181:5273–5279. doi: 10.1128/jb.181.17.5273-5279.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42a.Stanford DNA Sequencing and Technology Center. July 1999, revision date. [Online.] http://www-sequence.stanford.edu/group/candida. [June 1999, last date accessed.]
- 43.Su S S Y, Mitchell A P. Molecular characterization of the yeast meiotic regulatory gene RIM1. Nucleic Acids Res. 1993;21:3789–3797. doi: 10.1093/nar/21.16.3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suarez T, Peñalva M A. Characterization of a Penicillium chrysogenum gene encoding a PacC transcription factor and its binding sites in the divergent pcbAB-pcbC promoter of the penicillin biosynthetic cluster. Mol Microbiol. 1996;20:529–540. doi: 10.1046/j.1365-2958.1996.5421065.x. [DOI] [PubMed] [Google Scholar]
- 45.Suzuki M. SPXX, a frequent sequence motif in gene regulatory proteins. J Mol Biol. 1989;207:61–84. doi: 10.1016/0022-2836(89)90441-5. [DOI] [PubMed] [Google Scholar]
- 46.Tilburn J, Sarkar E A, Orejas M, Mungroo J, Peñalva M A, Arst H N J. The Aspergillus PacC zinc finger transcription factor mediates regulation of both acid- and alkaline-expressed genes by ambient pH. EMBO J. 1995;14:779–790. doi: 10.1002/j.1460-2075.1995.tb07056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vai M, Orlandi I, Cavadini P, Alberghina L, Popolo L. Candida albicans homologue of GGP1/GAS1 gene is functional in Saccharomyces cerevisiae and contains the determinants for glycosylphosphatidylinositol attachment. Yeast. 1996;12:361–368. doi: 10.1002/(SICI)1097-0061(19960330)12:4%3C361::AID-YEA920%3E3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 48.White T C, Agabian N. Candida albicans secreted aspartyl proteinases: isoenzyme pattern is determined by cell type, and levels are determined by environmental factors. J Bacteriol. 1995;177:5215–5221. doi: 10.1128/jb.177.18.5215-5221.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wilson R B, Davis D, Mitchell A P. Rapid hypothesis testing with Candida albicans through gene disruption with short homology regions. J Bacteriol. 1999;181:1868–1874. doi: 10.1128/jb.181.6.1868-1874.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]