Abstract
Background and Objectives
Alzheimer disease (AD) and other forms of dementia represent a rising global public health crisis. Because effective treatments to prevent, cure, or slow progression of dementia are unavailable, identification of treatable risk factors that increase dementia risk such as obstructive sleep apnea (OSA) could offer promising means to modify dementia occurrence or severity. Here, we systematically reviewed the impact of positive airway pressure (PAP) therapy on the incidence of cognitive disorders and cognitive decline among middle-aged and older adults with OSA.
Methods
We performed a systematic search of MEDLINE, EMBASE, Scopus, and CINAHL before May 2021 to identify articles that focused on associations between PAP therapy use and cognitive disorders. We included studies that examined the effects of PAP treatment on (1) the incidence of cognitive disorders among individuals ≥40 years of age diagnosed with OSA and (2) the progression of cognitive decline among people with preexisting cognitive disorders and OSA.
Results
We identified 11 studies (3 clinical trials and 8 observational studies). In these studies, 96% participants had OSA (n = 60,840) and 9% had baseline cognitive impairment (mild cognitive impairment [MCI] or AD) (n=5,826). Of all study participants, 43,970 obtained PAP therapy, and 16,400 were untreated or in a placebo group. Nine out of 11 studies reported a protective effect of PAP therapy on MCI and AD incidence, e.g., delayed age at MCI onset, reduced MCI or AD incidence, slower cognitive decline, or progression to AD.
Discussion
These findings suggest a role for OSA as a modifiable risk factor for cognitive decline. Identification of modifiable risk factors is imperative for alleviating the impact of cognitive disorders on aging adults and their family members. Future research should build on this review and focus on PAP interventions as a potential means to alleviate the incidence of cognitive disorders and cognitive decline, particularly among ethnoracial groups who have been underrepresented and underinvestigated in the extant literature.
Approximately 6.2 million older adults in the United States are currently living with dementia,1 a term that describes a group of progressive, incurable neurologic syndromes associated with irreversible decline in cognitive functions and behavioral changes. Alzheimer disease (AD) is the most common type of dementia and is characterized by progressive neurodegeneration, while vascular dementia, the second most common type, results from reduced blood supply to the brain due to acute or chronic cerebrovascular diseases.2 Age is the single greatest risk factor for AD, and the number of older Americans is projected to increase by 30 million in 2050.3 This demographic shift will double the annual incidence of dementia cases by 2050, a threat to public health that disproportionally burdens women and ethnoracial groups underrepresented in medicine.4
Mild cognitive impairment (MCI) is a heterogeneous condition of cognitive impairment that does not meet the threshold for dementia.5 Nearly 15% to 20% of adults >60 years of age present with MCI in various cognitive domains.6 Furthermore, pathways to MCI are complex, and the annual conversion rate to dementia is estimated to be between 8% and 15%. However, not all individuals with MCI develop dementia; some revert to normal cognitive status,7 suggesting that MCI may be modifiable.
Although lifestyle factors and medications, including cholinesterase inhibitors and a new anti–β-amyloid monoclonal antibody, may offer modest protective effects or symptomatic benefit,8 the lack of effective treatments to prevent or substantially slow cognitive decline has motivated investigations to identify modifiable risk factors for cognitive disorders. A potential risk factor, obstructive sleep apnea (OSA), is a condition marked by hypoxia, sleep deprivation, and sleep fragmentation. Common in middle-aged and older adults, OSA frequently remains undiagnosed.9 Although recent work has identified OSA as a potentially modifiable risk factor for cognitive decline and dementia,10 relationships between positive airway pressure (PAP) therapy—the gold standard OSA therapy11—and neurocognitive disorders are poorly understood. Only a few studies have examined the influence of PAP therapy on cognitive function,12 MCI, and dementia risk.13,14 While these emerging data show promise for a protective role of PAP on dementia risk in older adults with OSA, a systematic review would provide an integration of existing studies and identify gaps in knowledge.9 We therefore conducted a systematic search of the literature to examine the associations between PAP therapy, MCI, and dementia (with an emphasis on AD) in adults with OSA. In this review, we evaluate whether PAP therapy is associated with the incidence of MCI and dementia, as well as its influence on the progression of cognitive decline among those with MCI or dementia at baseline.
Methods
Standard Protocol Approvals, Registrations, and Patient Consents
This review was deemed nonregulated by the Medical School Institutional Review Board at the University of Michigan because only publicly available and aggregate data were used.
An experienced health science librarian (C.S.) conducted a systematic search of MEDLINE (PubMed), EMBASE, Scopus, and CINAHL Complete (EBSCOhost) to identify articles related to how interventions to treat OSA affect cognitive health. Five sentinel articles were used to harvest search terms, including Medical Subject Headings, Emtree, and key words (tagged as title/abstract) terms. Search terms included obstructive sleep apnea, central sleep apnea, sleep disordered breathing, continuous positive airway pressure, Alzheimer's disease, cognition disorders, and dementia (a comprehensive term that included vascular dementia; eMethods, links.lww.com/WNL/C17, provides the complete search strategy). The searches were completed by May 10, 2021, and included all articles published by this date.
The original PubMed search strategy was translated and adapted to other databases with the SR Accelerator15 and at the searcher's discretion. To reduce bias, no filters (including publication date or language) were used, and both published peer-reviewed articles and unpublished abstracts were considered through searches in EMBASE and Scopus. Findings are reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement16 elaboration, and explanation17 and Statement for Reporting Literature Searches for Systematic Reviews.18
This review included empirical studies that examined, among adults with OSA, the potential influence of PAP therapy on MCI or dementia incidence or progression of cognitive decline in middle-aged adults (≥40 years of age) who were diagnosed with MCI or dementia. We excluded letters, abstracts, case studies, reviews, or commentaries and publications in non-English languages.
Three reviewers (M.M.S., A.B.Z., and G.L.D.) screened abstracts and titles and read the full articles that met inclusion criteria. Any disagreement between reviewers over inclusion of studies was resolved with discussion and consensus. Bias assessment was performed by 1 reviewer (M.M.S.). For clinical trials, the reviewer used the Cochrane Risk of Bias for Randomized Trials19 (with risk of bias rated as low, some concerns, or high on the basis of 5 domains: randomization process, deviations from intended interventions, missing outcome data, measurement of the outcome, and selection of the reported result). For observational studies, the reviewer used the Cochrane Risk of Bias in Non-Randomized Studies of Interventions tool20 (with risk of bias rated as low, moderate, serious, or critical on the basis of 7 domains: confounding, selection of participants, classification of interventions, deviations from intended interventions, missing data, measurement of the outcome, and selection of the reported result). Citations were imported into EndNote (Thomson Reuters, New York, NY) for deduplication and exported to Excel (Microsoft, Bellingham, WA) for analysis.
The heterogeneity of study designs, exposure, and outcomes assessments of the included studies precluded meta-analyses of PAP therapy utility for cognitive disroders incidence and progression.
Data Availability
The search strategy and data extraction sheets are available on request to the first author.
Results
Search Strategy
The initial search identified 3,155 articles. After the exclusion of 985 duplicates, 2,170 titles and abstracts were screened, and 84 articles received a full-text review. Seventy-three articles were removed after full-text review for the following reasons: written in a non-English language; were letters, abstracts, case studies, reviews, or commentaries; or included no examination of the association of interest (PAP treatment, MCI, dementia or cognitive decline outcomes). While age <40 years was an exclusion criterion, none of the studies were excluded on the basis of age <40 years alone (Figure).
Figure. PRISMA Flow Diagram: Number of Articles Included and Excluded.
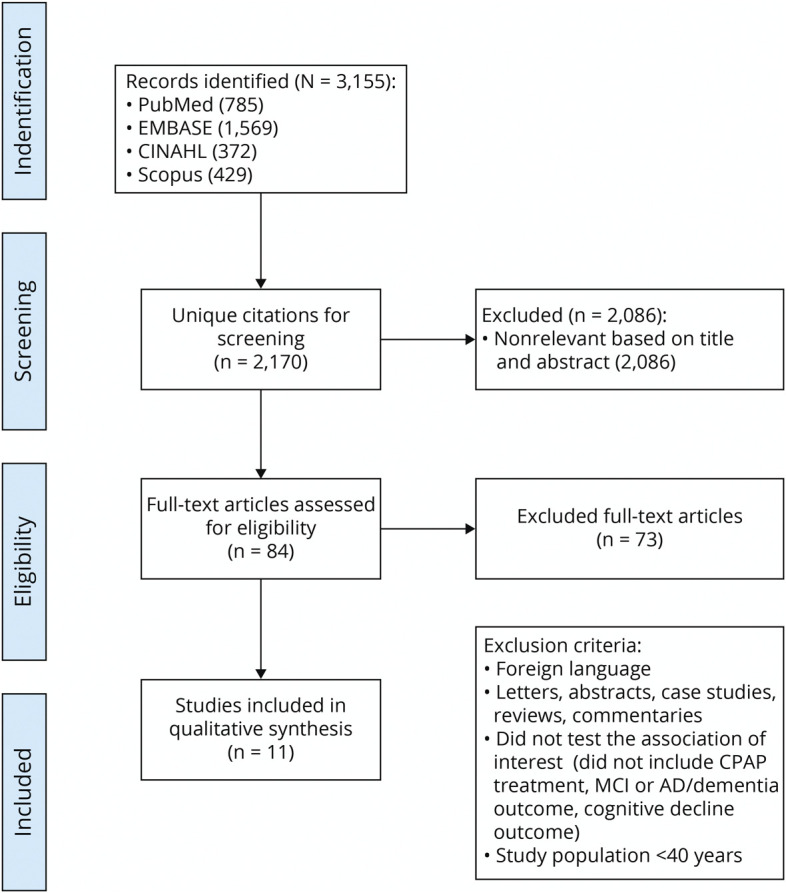
AD = Alzheimer disease; CPAP = continuous positive airway pressure; MCI = mild cognitive impairment; PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Study Characteristics
We identified 11 studies that examined PAP therapy in relation to cognitive disorders: 1 randomized controlled trial (RCT), 2 quasi-experimental clinical trials, 2 prospective observational studies, 5 retrospective observational studies, and 1 cross-sectional study. The majority of studies (n = 8) were conducted in the United States13,14,21-26; 2 were conducted in Europe27,28; and 1 was conducted in Asia.29 These studies used diverse cohorts, including nationwide databases (National Health Insurance Research Database of Taiwan,29 a 5% sample of US Medicare beneficiaries),14,22 a cohort from the Alzheimer's Disease Neuroimaging Initiative,23 a cohort from the Alzheimer's Disease Research Center, and clinical samples of adults who obtained care at neurology, sleep, and memory clinics.13,21,24-28 In US-based studies, participants were predominantly White (range 59%–98%), and across all studies, the proportion of women ranged between 25% and 53%. Six studies included both middle-aged and older participants,13,23-25,27,29 while 5 focused on older adults (≥65 years of age).14,21,22,26,28
Most study participants carried a diagnosis of OSA (96%, n = 60,840), and most (70%, n = 43,970) received PAP therapy. Of the 11 identified studies, 6 considered apnea-hypopnea index (AHI) a measure for OSA severity (AHI ≥10, mild to severe21,24,26; AHI 10–14, mild25; and AHI ≥30, severe28) and all levels of OSA severity.13 Baseline cognitive impairment (MCI or AD) was present among 9% (n = 5,826) of study participants. Data extraction summaries are reported in Tables 1 and 2.
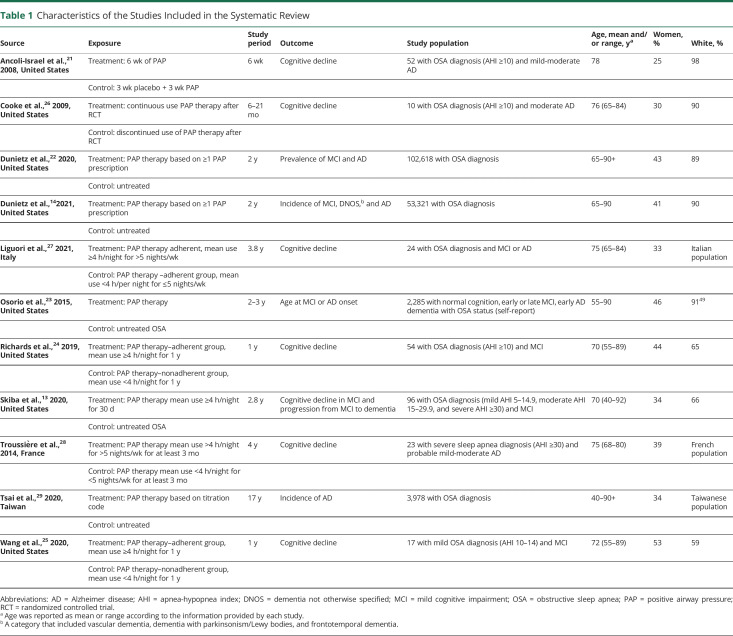
Table 1.
Characteristics of the Studies Included in the Systematic Review
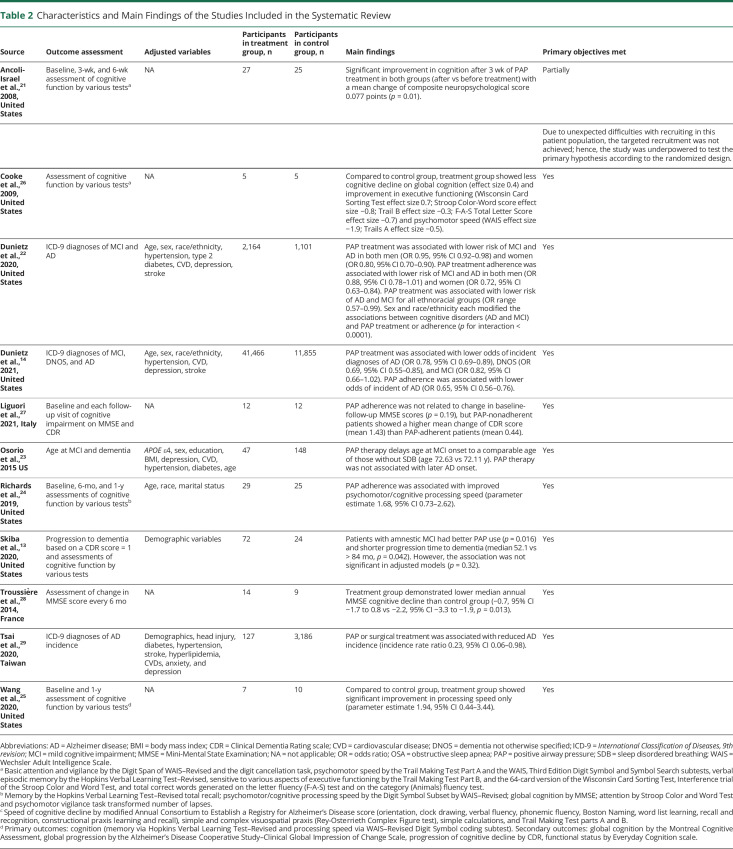
Table 2.
Characteristics and Main Findings of the Studies Included in the Systematic Review
Bias Assessment
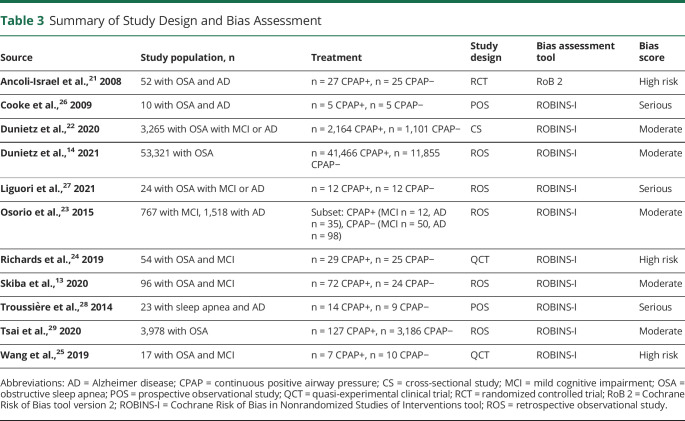
Table 3 presents a summary of study designs and bias assessment of all studies included in this review. High risk of bias was found in all 3 clinical trials: 2 studies used a quasi-experimental design without randomization and limited controlling of confounders,24,25 and 1 study modified the analytical strategy due to unachieved targeted recruitment.21 Three observational studies26-28 were considered to have a serious risk of bias due to limited controlling of confounders. The remaining 5 observational studies were categorized as having moderate risk of bias,13,14,22,23,29 and while not comparable to a well-performed randomized trial, they provided sound evidence.
Table 3.
Summary of Study Design and Bias Assessment
Exposure or Intervention Assessment
Assessment of PAP therapy varied by study. In 1 clinical trial, standard PAP therapy was randomly assigned to a treatment group for 6 weeks, while the control group wore placebo masks with a large air leak and pressure reducer for 3 weeks, followed by 3 additional weeks of standard PAP therapy. The standard PAP therapy provided new masks determined by a formal PAP titration polysomnography to establish the therapeutic pressure.21 No significant difference in adherence to PAP therapy was found in treatment vs control groups, 5.8 h/night for 73% of nights vs 6.4 h/night for 67% of the nights in the first 3 weeks and 4.9 h/night for 62% of the nights in the standard PAP therapy period.
In 2 quasi-experimental studies, participants in the treatment group used standard PAP therapy for ≥4 h/night for 1 year, while controls used PAP therapy <4 h/night for 1 year.24,25
In all observational studies, PAP therapy was defined either by Healthcare Common Procedure Coding System code on ≥1 PAP prescription and titration Current Procedural Terminology codes from claims data14,22,29 or by mean PAP use duration (ranging from 1 week–1 year).13,23,26-28
Outcome Assessment
Of the 11 studies included, 4 examined the incidence, prevalence, or age at diagnosis of MCI or AD, and 7 examined progression of cognitive decline in adults with existing MCI or AD. Most studies assessed the progression of cognitive decline, MCI, or dementia by using comprehensive neuropsychological batteries, brief cognitive screening tools, or clinical diagnosis.
Incidence of MCI or Dementia
Several studies used clinical diagnosis codes from administrative claims data to assess cognitive disorders. In US Medicare beneficiaries claims data, 2 studies used Healthcare Common Procedure Coding System, Current Procedural Terminology, and ICD codes to examine associations between PAP therapy, PAP adherence, and prevalence and incidence of MCI, AD, and dementia not otherwise specified (DNOS).14,22 Similarly, a large retrospective study in Taiwan compared AD incidence, captured by ICD-9 codes in a health insurance database, among adults diagnosed with OSA and sociodemographically matched controls over 16 years.29 Last, a multisite US study of cognitively intact adults with OSA examined the age at incident MCI or AD among those who reported PAP therapy vs those who did not.23 All participants completed a comprehensive neuropsychological evaluation, including functional measures, and those who met National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association Alzheimer's Criteria were assigned an AD diagnosis by study clinicians.
Progression of Cognitive Decline
One RCT conducted in a US-based clinical research center examined improvement in cognitive functioning among adults with AD after PAP therapy over 3 to 6 weeks.21 Cognitive change was assessed with a comprehensive neuropsychological battery, including a brief screening measure of global cognitive functioning; measures of learning and memory, executive functions, and attention and vigilance; and measures of processing speed. A follow-up study 6 to 21 months later assessed a subset of participants from the initial RCT with the same neuropsychological battery to evaluate differences in cognitive decline between those who continued PAP therapy and those who discontinued.26
Subjective measures of cognitive status were used in another pilot clinical trial conducted in a US-based sleep and geriatrics clinic24 and its follow-up study.25 Both studies used brief cognitive screening tools and a battery of neuropsychological tests to assess verbal memory, attention and vigilance, and processing speed. Change in cognition—improvement or decline—was compared between adults with MCI who were PAP adherent and those who were nonadherent over a 1-year period.
A brief cognitive screener and a single cognitive test to assess attention and executive functioning abilities were used in a retrospective chart review conducted in a large urban health center in the United States.13 This study followed up adults with MCI and OSA with a 2.8-year follow-up on average and evaluated cognitive change among those who were PAP adherent, those who were nonadherent, and those who did not use PAP therapy. Brief cognitive screening tools were also used in 2 independent studies: a retrospective multicenter study of Italian adults with MCI and AD and a prospective clinic-based study of French adults with AD. These studies examined change in cognition over ≈3 years.27,28
Analytic Strategy
Various statistical approaches were used to examine the associations between PAP therapy, incident MCI or AD, and cognitive decline among adults with MCI or AD. All studies conducted descriptive statistics procedures to estimate effect sizes of PAP therapy on progression of cognitive decline in MCI or AD. Due to limited sample size, only 6 studies pursued association analyses with regression methods. Logistic regression procedures were applied to quantify the impact of PAP therapy on the prevalence of MCI or AD.22 When time-to-event data were available, Cox proportional hazard regression was used. Two studies adjusted for demographic variables in their analysis,13,24 while others further adjusted for additional potential confounders, including comorbid conditions (hypertension, diabetes, cardiovascular disease, anxiety, depression) and APOE ε4 allele status.14,22,23,29 However, some comorbid conditions, i.e., hypertension, diabetes, and cardiovascular disease, may be alleviated by PAP therapy and thus are potential mediators on the pathway between PAP therapy and cognitive disorders. Indeed, a primary risk factor for vascular dementia, cerebral small vessel disease, has been associated with moderate to severe OSA in cohorts of older adults and patients seeking sleep evaluation.30,31 These studies highlight the mediating role of vascular morbid conditions in the pathophysiology of dementia. Similarly, impaired glucose and insulin function has been linked to dementia.32 To avoid overadjustment bias that would reduce the effect estimates,33 adjustment for mediators is discouraged. Two studies examined the role of effect modification in the association between PAP therapy and cognitive impairment. In one study, sex and race/ethnicity were examined as potential modifiers of the relationships between PAP therapy and MCI or AD,22 while another study evaluated whether MCI type (nonamnestic/amnestic) and OSA severity modified the association between PAP therapy and rate cognitive decline.13
Impact of PAP Therapy on Cognition: Summary of Findings
Occurrence of MCI or Dementia
Most studies included in this review reported beneficial effects of PAP therapy on the occurrence MCI or AD, i.e., delayed age at MCI onset,23 reduced MCI or AD prevalence,22 and reduced incidence.14,29
Age at Onset of MCI or AD
PAP therapy was associated with delayed age at MCI onset among older adults with OSA compared to those who were untreated (age at onset 80.10 years vs 72.63 years).23 However, PAP therapy was not associated with later AD onset.23
Prevalence of MCI or AD
Overall, PAP therapy and adherence were associated with lower odds of MCI and AD prevalence. However, sex and racial/ethnic disparities were noted in the associations between PAP therapy, PAP adherence, and MCI or AD (p for interaction < 0.0001).22 Specifically, older women and racial/ethnic minorities with OSA were less likely to obtain and adhere to PAP therapy.
Incidence of MCI or AD
Lower odds of incident AD and DNOS were observed among Medicare beneficiaries who were prescribed PAP therapy for their OSA vs those who were untreated (odds ratio [OR] 0.78, 95% CI 0.69–0.89; and OR 0.69, 95% CI 0.55–0.85 for AD and DNOS, respectively). Associations between PAP therapy, PAP adherence, and incident MCI approached statistical significance (OR 0.82, 95% CI 0.66–1.02).14 Last, a large study from Taiwan demonstrated reduced AD incidence among adults with OSA who received PAP therapy or underwent a surgery for their OSA compared to those without treatment (incidence rate ratio 0.23, 95% CI 0.06–0.98).29
Time to Dementia
A study of 96 adults with MCI and OSA diagnosis found no difference in time to dementia between adherent PAP therapy users, nonadherent users, or untreated individuals (p = 0.94).13
Progression of Cognitive Decline
In most studies, slower rate of cognitive decline was observed among PAP therapy users. Only 2 studies found no protective effect of PAP therapy in slowing cognitive decline,13,27 while others reported improvement in some cognitive domains among PAP users.21,24-26,28 Specifically, among adults with both MCI and OSA, there was no significant difference in cognitive decline (i.e., Trail Making Tests parts A and B) in the untreated, PAP-nonadherent, and PAP-adherent groups.13 In addition, PAP adherence was not associated with change in baseline to follow-up Mini-Mental State Examination scores (p = 0.19).27 Nonetheless, the study showed a greater mean change of Clinical Dementia Rating scale score (mean 1.43) in PAP-nonadherent adults with OSA compared with PAP-adherent adults (mean 0.44).27
A 6-week RCT of 52 adults with OSA reported a significant improvement in cognition after 3 weeks of PAP therapy in mean change of composite neuropsychological score (p = 0.01).21 Moreover, PAP adherence was associated with improved psychomotor/cognitive processing speed (Digit Symbol subtest from the Wechsler Adult Intelligence age-adjusted total scaled score) (β = 1.68, 95% CI 0.73–2.62).24 Similarly, a study of 17 adults with mild OSA found significant improvement in processing speed among treated vs untreated controls (β = 1.94, 95% CI 0.44–3.44).25 Slower rate of decline in global cognition and improved executive functioning and psychomotor speed were also apparent among adults with OSA treated with PAP compared to untreated controls.26 Slower rate of cognitive decline (median annual Mini-Mental State Examination score) was also associated with PAP therapy in adults with AD and OSA (median [treated] −0.7, 95% CI −1.7 to 0.8 vs untreated −2.2, 95% CI −3.3 to −1.9, p = 0.013).28
Summary of Findings in Light of Study Design and Quality
Of the 5 studies with moderate risk of bias, 4 examined associations between PAP therapy and incidence of MCI or AD. These 4 studies reported significant associations between PAP therapy and incidence of MCI or AD. Six studies with serious or high risk of bias have examined mainly the role of PAP therapy in cognitive decline. Five of these 6 studies linked PAP therapy to slower rate of cognitive decline.
Discussion
Despite the high prevalence and consequences of OSA in older adults, the potential benefit of PAP therapy on cognitive function in older adults has rarely been examined. This systematic review identified 11 studies that investigated associations between PAP therapy and incidence or progression of cognitive decline in adults with OSA, providing information to suggest a protective effect of PAP therapy on incidence of MCI or AD. However, data on the rate of cognitive decline in relation to PAP therapy are mixed. Nonetheless, emerging evidence suggests that intervention with PAP therapy may alleviate the burden of cognitive impairment in older adults with OSA. These findings align with reports on the physiologic benefits of PAP.
OSA could contribute to cognitive impairment among middle-aged and older adults through cerebrovascular and neurodegenerative pathways.34,35 Indicators of small vessel cerebrovascular disease such as white matter hyperintensities (WMH), asymptomatic lacunar infarctions, cerebral microbleeds, and enlarged perivascular spaces are nearly 4 times more prevalent among individuals with moderate to severe OSA than those without OSA.30,31 Given the existing literature on WMH as a predictor of dementia,36 the robust association between moderate to severe OSA and elevated WMH in the literature37 may reflect a modifiable pathway underlying the association between OSA and cognitive impairment or dementia. Moreover, chronic intermittent hypoxia associated with uncontrolled OSA has been linked to neuronal damage and loss in the hippocampus, as well as CNS regions involved in executive functions, language, and perception such as the frontal, temporal, and parietal cortices.38 Hypoxia and sleep fragmentation associated with OSA may also increase the risk for AD by increasing amyloid burden through either increased amyloid production or decreased clearance. Associations between hypoxia and CSF biomarkers of AD may vary according to genotypic predispositions such as APOE allele status, although inconsistent findings have been reported.39,40 In a recent study of adults with mild to moderate AD, APOE status was not associated with the presence or severity of OSA.41 In addition, elevated levels of tau and biomarkers of inflammation such as interleukin-6 observed in the blood plasma of younger adults with moderate to severe OSA may increase risk for the development of neurodegenerative diseases such as AD in later life.42
Emerging research suggests that PAP treatment may have restorative effects on white matter structural integrity and concomitant improvement in neurocognitive function.37 Growing evidence also suggests that PAP may alleviate the neurodegenerative and cognitive effects of OSA by reducing inflammation and increasing cerebral blood flow.43 PAP therapy may also enhance slow wave activity, which is critical for memory consolidation. In a case-matched study of adults with newly diagnosed OSA, those who completed 3 months of PAP treatment demonstrated improved slow wave sleep and performance on a declarative memory task consistent with healthy controls; however, those in the non-PAP treatment group did not show this level of improvement.44 Moreover, prior studies have shown improved cognitive functioning and partial reversal of OSA-associated brain damage, including increased brain volume and neuroplasticity in affected regions after both short-term45 and long-term PAP treatment.46-48
Although this review suggests a protective role of PAP therapy against cognitive impairment, current findings must be interpreted with caution given the high degree of bias in most of the reviewed studies, including RCTs. Furthermore, given the variability in populations (heterogeneity in cognitive status at baseline, pathophysiology, duration of follow-up, assessment of PAP exposure, and assessment of cognitive outcomes), clinical recommendations regarding who is most likely to benefit cognitively from PAP treatment, the duration of PAP therapy for observed benefit, and expected benefits cannot be ascertained from available data. That said, this systematic review, which summarizes the state of the evidence on the role of PAP therapy in cognitive impairment, highlights these key gaps in knowledge to inform high-quality, prospective studies on the effects of PAP for specific populations.
This systematic review included studies with diverse designs, populations, cognitive measures, and statistical approaches, features that influence their quality and the generalizability of their findings. For example, cross-sectional examinations may lack temporal associations between PAP therapy and cognitive impairment, and their findings could lead to information bias.22 However, except for 1 study, most studies in this review used a prospective or retrospective design.
Heterogeneous sample sizes across studies influenced their statistical power. Seven studies that examined progression to MCI or AD relied on a relatively smaller sample sizes, ranging from 10 to 96 participants, and limited assessment of cognitive function. Of the 7 studies, 5 identified a protective role of PAP therapy against cognitive decline, while 2 reported no cognitive benefit of PAP therapy. In addition to limited statistical power to detect differences between study groups, the use of brief measures of cognition may have contributed to the mixed results.13,27 Conversely, consistent associations between PAP therapy and delayed incidence of MCI or AD were observed in studies with larger cohorts. Specifically, a protective effect of PAP therapy was suggested for reduced prevalence MCI or AD,22 delayed age at MCI onset,23 and reduced MCI or AD incidence.14,29
Beyond sample size, duration of follow-up studies could also lead to mixed results. For example, in 1 clinical trial, the duration of follow-up period was 6 weeks,21 perhaps insufficient to capture the long-term beneficial effect of PAP therapy. Last, heterogeneity among study groups in demographic and health profiles and differences in severity of OSA or cognitive disorders may confound or dilute the impact of PAP therapy on cognitive function.
This review also evaluated the statistical methods used in relation to their respective research questions. Of the 11 studies included in this review, some studies considered a wide range of potential confounders, including sociodemographic variables (age, sex, race/ethnicity, socioeconomic status), access to health care, and baseline anthropometric measurements (body mass index). Furthermore, adjustment for potential mediators, for example, depression, chronic kidney disease, cardiovascular morbidity, hypertension, and diabetes, could lead to biased and attenuated effects estimates. Misclassification of continuous PAP therapy and incidence of cognitive disorders could arise from heavy reliance on self-report and claims data. Last, the majority of persons with OSA remain undiagnosed and untreated.e1 Therefore, the participants included in these studies may not be fully representative of the population at large.
Disparities in diagnosis of AD have been shown in women and ethnoracial underrepresented in medicine groups. The disproportional burden of AD among women—nearly 1.6 times as high relative to men—has been reported in several European and US cohorts and becomes apparent as early as 75 years of age.e2 While these sex differences have been attributed primarily to the longer life span of women,e3 sex-specific etiologies involving biological and environmental determinants and lower thresholds of disease pathology for women are also plausible.e2
A disproportional burden of AD has also been shown among ethnoracial groups underrepresented in medicine, with 2-fold and 1.5-fold higher prevalence among non-Hispanic Black and Hispanic individuals, respectively, compared to White individuals.e4 These ethnoracial differences in AD burden have biological, social, and cultural roots. Genetic factors and a higher prevalence of cardiovascular morbidity among ethnoracial groups underrepresented in medicine have been suggested as determinants of AD disparities.e5 However, beyond disparities in these risk factors, greater symptom severity at first AD onset has been reported in Hispanic patients, but they have longer survival with the disease.e5 A meta-synthesis of studies that evaluated barriers and facilitators to dementia care among ethnoracial groups underrepresented in medicine has indicated gaps in education on brain health and symptoms of cognitive decline, in addition to disparities in health care access, that jointly affect the timing of diagnosis and treatment of cognitive disorders.e6,e7 Last, the use of universal assessments for neurocognitive disorders rather than cultural-specific tools adds further assessment challenges because daily living experiences and meanings associated with dementia vary across ethnoracial groups underrepresented in medicine.e8
As a potential risk factor for AD, OSA identification and treatment could alleviate cognitive decline and AD incidence. However, gaps in OSA evaluation, treatment, and treatment adherence in women, ethnoracial groups underrepresented in medicine, and older adults have been reported.9,22 Addressing sex and ethnoracial inequities in both OSA and dementia has the potential to alleviate the development and progression of dementia and to decrease gaps in care and quality of life among older adults with dementia.
The current review has several limitations. First, the heterogeneity of study designs, exposure, and outcomes assessments of included reports precludes meta-analyses of PAP therapy utility for cognitive disorders incidence and progression. Second, across all studies, participants were predominately White, which limits the generalizability of the findings to other ethnoracial groups. This limitation calls for further investigations to address the impact of PAP therapy on the rate of neurodegeneration among ethnoracial groups underrepresented in medicine and potential disparities emerging from differential access to health care. Third, MCI may be associated with differential loss of cognitive abilities or influenced by sleep and mood.e9 Challenges in MCI assessment, inherent in claims-based analyses, could result in misclassification of cognitive outcomes and generate biased effect estimates.
This systematic review provides a comprehensive summary of the current literature on the role of PAP therapy in cognition and highlights the need for rigorous, longitudinal studies with diverse cohorts. Overall, we found a protective effect of PAP therapy on incidence of MCI or AD. Findings regarding the impact of PAP therapy on cognitive decline were promising. Longer follow-up periods and in-depth cognitive testing are warranted. PAP therapy may serve as an effective intervention for cognitive health particularly among White older adults. Although preliminary evidence also holds promise for ethnoracial minorities, potential benefits of PAP therapy among non-Whites require further investigation.
Glossary
- AD
Alzheimer disease
- AHI
apnea-hypopnea index
- DNOS
dementia not otherwise specified
- ICD
International Classification of Diseases
- MCI
mild cognitive impairment
- OR
odds ratio
- OSA
obstructive sleep apnea
- PAP
positive airway pressure
- RCT
randomized controlled trial
- WMH
white matter hyperintensities
Appendix. Authors

Footnotes
Podcast: NPub.org/Podcast9828
Study Funding
No targeted funding reported.
Disclosure
M.M. Shieu is supported by a T32 grant from National Heart, Lung, and Blood Institute (T32HL110952). A. Zaheed is supported by a F31 predoctoral fellowship from the National Institute on Aging (1F31AG067717-01). T.J. Braley and G.L. Dunietz report funding from the National Institute on Aging, award R01AG074342. T.J. Braley also receives support from the NIH/National Center for Complementary and Integrative Health (1R01AT011341) and Patient-Centered Outcomes Research Institute (1610–36980) and is named in a patent concerning treatment for OSA held by the University of Michigan. H.L. Paulson reports funding from NIH/National Institute on Aging for the Michigan Alzheimer Disease Research Center, award P30AG 072931. R.D. Chervin reports grant support from the NIH (U01NS099043, T32HL110952). He is a member of the Board of Directors and treasurer for the International Pediatric Sleep Association and is a member of the Advisory Board for the nonprofit Pajama Program. He is named in patents, patents pending, and copyrighted material owned by the University of Michigan and designed to facilitate diagnosis or treatment of sleep disorders, including OSA. He has received royalties for a questionnaire licensed to Zansors. He is an editor and author for UpToDate. Go to Neurology.org/N for full disclosures.
References
- 1.2021 Alzheimer's disease facts and figures. Alzheimers Dement. 2021;17(3):327-406. [DOI] [PubMed] [Google Scholar]
- 2.O'Brien JT, Thomas A. Vascular dementia. Lancet. 2015;386(10004):1698-1706. [DOI] [PubMed] [Google Scholar]
- 3.He W, Goodkind D, Kowal PR. An Aging World: 2015. US Census Bureau; 2016. [Google Scholar]
- 4.Power MC, Bennett EE, Turner RW, et al. Trends in relative incidence and prevalence of dementia across non-Hispanic Black and White individuals in the United States, 2000-2016. JAMA Neurol. 2021;78(3):275-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Portet F, Ousset PJ, Visser PJ, et al. Mild cognitive impairment (MCI) in medical practice: a critical review of the concept and new diagnostic procedure: report of the MCI Working Group of the European Consortium on Alzheimer's Disease. J Neurol Neurosurg Psychiatry. 2006;77(6):714-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petersen RC. Mild cognitive impairment. Continuum. 2016;22(2 Dementia):404-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tokuchi R, Hishikawa N, Kurata T, et al. Clinical and demographic predictors of mild cognitive impairment for converting to Alzheimer's disease and reverting to normal cognition. J Neurol Sci. 2014;346(1-2):288-292. [DOI] [PubMed] [Google Scholar]
- 8.Se Thoe E, Fauzi A, Tang YQ, Chamyuang S, Chia AYY. A review on advances of treatment modalities for Alzheimer's disease. Life Sci. 2021;276:119129. [DOI] [PubMed] [Google Scholar]
- 9.Braley TJ, Dunietz GL, Chervin RD, Lisabeth LD, Skolarus LE, Burke JF. Recognition and diagnosis of obstructive sleep apnea in older Americans. J Am Geriatr Soc. 2018;66(7):1296-1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shieu MM, Dunietz GL, Paulson HL, Chervin RD, Braley TJ. The association between obstructive sleep apnea risk and cognitive disorders: a population-based study. J Clin Sleep Med. 2022;18(4):1177-1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cao MT, Sternbach JM, Guilleminault C. Continuous positive airway pressure therapy in obstructive sleep apnea: benefits and alternatives. Expert Rev Respir Med. 2017;11(4):259-272. [DOI] [PubMed] [Google Scholar]
- 12.Kim H, Im S, Park JI, Kim Y, Sohn MK, Jee S. Improvement of cognitive function after continuous positive airway pressure treatment for subacute stroke patients with obstructive sleep apnea: a randomized controlled trial. Brain Sci. 2019;9(10):252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Skiba V, Novikova M, Suneja A, McLellan B, Schultz L. Use of positive airway pressure in mild cognitive impairment to delay progression to dementia. J Clin Sleep Med. 2020;16(6):863-870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dunietz GL, Chervin RD, Burke JF, Conceicao AS, Braley TJ. Obstructive sleep apnea treatment and dementia risk in older adults. Sleep. 2021;44(9):zsab076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clark JM, Sanders S, Carter M, et al. Improving the translation of search strategies using the Polyglot Search Translator: a randomized controlled trial. J Med Libr Assoc. 2020;108(2):195-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rethlefsen ML, Kirtley S, Waffenschmidt S, et al. PRISMA-S: an extension to the PRISMA statement for reporting literature searches in systematic reviews. Syst Rev. 2021;10(1):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
- 20.Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ancoli-Israel S, Palmer BW, Cooke JR, et al. Cognitive effects of treating obstructive sleep apnea in Alzheimer's disease: a randomized controlled study. J Am Geriatr Soc. 2008;56(11):2076-2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dunietz GL, Chervin RD, Burke JF, Braley TJ. Obstructive sleep apnea treatment disparities among older adults with neurological disorders. Sleep Health. 2020;6(4):534-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Osorio RS, Gumb T, Pirraglia E, et al. Sleep-disordered breathing advances cognitive decline in the elderly. Neurology. 2015;84(19):1964-1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Richards KC, Gooneratne N, Dicicco B, et al. CPAP adherence may slow 1-year cognitive decline in older adults with mild cognitive impairment and apnea. J Am Geriatr Soc. 2019;67(3):558-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y, Cheng C, Moelter S, et al. One year of continuous positive airway pressure adherence improves cognition in older adults with mild apnea and mild cognitive impairment. Nurs Res. 2020;69(2):157-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cooke JR, Ayalon L, Palmer BW, et al. Sustained use of CPAP slows deterioration of cognition, sleep, and mood in patients with Alzheimer's disease and obstructive sleep apnea: a preliminary study. J Clin Sleep Med. 2009;5(4):305-309. [PMC free article] [PubMed] [Google Scholar]
- 27.Liguori C, Cremascoli R, Maestri M, et al. Obstructive sleep apnea syndrome and Alzheimer's disease pathology: may continuous positive airway pressure treatment delay cognitive deterioration? Sleep Breath. 2021:1-5. [DOI] [PubMed] [Google Scholar]
- 28.Troussière A-C, Charley CM, Salleron J, et al. Treatment of sleep apnoea syndrome decreases cognitive decline in patients with Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2014;85(12):1405-1408. [DOI] [PubMed] [Google Scholar]
- 29.Tsai MS, Li HY, Huang CG, et al. Risk of Alzheimer's disease in obstructive sleep apnea patients with or without treatment: real-world evidence. Laryngoscope. 2020;130(9):2292-2298. [DOI] [PubMed] [Google Scholar]
- 30.Song T-J, Park J-H, Choi KH, et al. Moderate-to-severe obstructive sleep apnea is associated with cerebral small vessel disease. Sleep Med. 2017;30:36-42. [DOI] [PubMed] [Google Scholar]
- 31.Del Brutto OH, Mera RM, Zambrano M, Castillo PR. Relationship between obstructive sleep apnea and neuroimaging signatures of cerebral small vessel disease in community-dwelling older adults. The Atahualpa Project. Sleep Med. 2017;37:10-12. [DOI] [PubMed] [Google Scholar]
- 32.Biessels GJ, Staekenborg S, Brunner E, Brayne C, Scheltens P. Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol. 2006;5(1):64-74. [DOI] [PubMed] [Google Scholar]
- 33.Schisterman EF, Cole SR, Platt RW. Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiology. 2009;20(4):488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferini-Strambi L, Marelli S, Galbiati A, Castronovo C. Effects of continuous positive airway pressure on cognition and neuroimaging data in sleep apnea. Int J Psychophysiol. 2013;89(2):203-212. [DOI] [PubMed] [Google Scholar]
- 35.Kim H, Yun CH, Thomas RJ, et al. Obstructive sleep apnea as a risk factor for cerebral white matter change in a middle-aged and older general population. Sleep. 2013;36(5):709-715b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prins ND, Scheltens P. White matter hyperintensities, cognitive impairment and dementia: an update. Nat Rev Neurol. 2015;11(3):157-165. [DOI] [PubMed] [Google Scholar]
- 37.Salsone M, Caligiuri ME, Castronovo V, et al. Microstructural changes in normal-appearing white matter in male sleep apnea patients are reversible after treatment: a pilot study. J Neurosci Res. 2021;99(10):2646-2656. [DOI] [PubMed] [Google Scholar]
- 38.Zimmerman ME, Aloia MS. A review of neuroimaging in obstructive sleep apnea. J Clin Sleep Med. 2006;2(4):461-471. [PubMed] [Google Scholar]
- 39.Bubu OM, Andrade AG, Umasabor-Bubu OQ, et al. Obstructive sleep apnea, cognition and Alzheimer's disease: a systematic review integrating three decades of multidisciplinary research. Sleep Med Rev. 2020;50:101250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O'Hara R, Schröder CM, Kraemer HC, et al. Nocturnal sleep apnea/hypopnea is associated with lower memory performance in APOE epsilon4 carriers. Neurology. 2005;65(4):642-644. [DOI] [PubMed] [Google Scholar]
- 41.Gaeta AM, Benítez ID, Jorge C, et al. Prevalence of obstructive sleep apnea in Alzheimer's disease patients. J Neurol. 2020;267(4):1012-1022. [DOI] [PubMed] [Google Scholar]
- 42.Motamedi V, Kanefsky R, Matsangas P, et al. Elevated tau and interleukin-6 concentrations in adults with obstructive sleep apnea. Sleep Med. 2018;43:71-76. [DOI] [PubMed] [Google Scholar]
- 43.Foster GE, Hanly PJ, Ostrowski M, Poulin MJ. Effects of continuous positive airway pressure on cerebral vascular response to hypoxia in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 2007;175(7):720-725. [DOI] [PubMed] [Google Scholar]
- 44.Djonlagic IE, Guo M, Igue M, Kishore D, Stickgold R, Malhotra A. Continuous positive airway pressure restores declarative memory deficit in obstructive sleep apnea. Am J Respir Crit Care Med. 2021;203(9):1188-1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rosenzweig I, Glasser M, Crum WR, et al. Changes in neurocognitive architecture in patients with obstructive sleep apnea treated with continuous positive airway pressure. EBioMedicine. 2016;7:221-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim H, Joo E, Suh S, Kim JH, Kim ST, Hong SB. Effects of long-term treatment on brain volume in patients with obstructive sleep apnea syndrome. Hum Brain Mapp. 2016;37(1):395-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Canessa N, Castronovo V, Cappa SF, et al. Obstructive sleep apnea: brain structural changes and neurocognitive function before and after treatment. Am J Respir Crit Care Med. 2011;183(10):1419-1426. [DOI] [PubMed] [Google Scholar]
- 48.Castronovo V, Scifo P, Castellano A, et al. White matter integrity in obstructive sleep apnea before and after treatment. Sleep. 2014;37(9):1465-1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weiner MW, Veitch DP, Aisen PS, Beckett LA, Cairns NJ, Cedarbaum J, et al. on behalf of the Alzheimer's Disease Neuroimaging Initiative. 2014 Update of the Alzheimer's Disease Neuroimaging Initiative: A review of papers published since its inception. Alzheimers Dement. 2015;11(6):e1-120. doi: 10.1016/j.jalz.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The search strategy and data extraction sheets are available on request to the first author.