Abstract
We investigated limitations in young infants’ in visual short-term memory (VSTM). We used a one-shot change detection task to ask whether 4- and 8.5-month-old infants (N = 59) automatically encode fixated items in VSTM. Our task included trials that consisted of the following sequence: first a brief (500 ms) presentation with a sample array of two items, next a brief (300 ms) delay period with a blank screen, and finally a test array (2000 ms) that is identical to the sample array except that the color of one of the two items is changed. In Experiment 1, we induced infants to fixate one item by rotating it during the sample (the other item remained stationary); In Experiment 2, none of the items rotated. In both experiments, 4-month-old infants looked equally at the fixated item when it did and did not change color, providing no evidence that they encoded in VSTM the fixated item. In contrast, 8.5-month-old infants in Experiment 1 preferred the fixated item when it changed color from sample to test. Thus, 4-month-old infants do not appear to automatically encode fixated items in VSTM.
Visual short-term memory (VSTM) plays a key role in learning about the visual environment. In adults, VSTM is used to maintain information across saccades and eye blinks during the construction of long-term memory representations of complex scenes (Hollingworth & Henderson, 2002; Irwin, 1991; Luck, 2008). However, studies with young infants reveal limitations in VSTM abilities, raising the question of how they manage to learn about the visual world. For example, although 4-month-old infants can store a single, isolated item in VSTM, they do not appear to be able to store any information in VSTM when confronted with arrays of two or more objects (Ross-Sheehy et al., 2003). Given that the visual world rarely contains a single object, how are young infants able to learn about the world?
Here we explore the possibility that, when faced with multiple-element visual arrays, young infants are able to store information in VSTM, but only about items they have overtly attended. This possibility derives from the finding that adults automatically store the properties of a saccade target in VSTM (Hollingworth et al., 2008; Tas et al., 2016). If young infants also automatically store fixated objects in VSTM, this would help them learn about the objects that attract their attention (especially highly salient items). Some evidence already suggests that by 5.5 months of age, infants may automatically encode attended objects into VSTM (Cantrell et al., 2019; Ross-Sheehy et al., 2011). Moreover, prior studies in which young infants failed to exhibit VSTM in the context of multi-element arrays did not involve eye tracking (Ross-Sheehy et al., 2003, 2011), and it is possible that the presence of memory for fixated items could not be detected in these studies.
Previous research of infants’ VSTM used one of two versions of the change detection task. In the continuous stream version (Ross-Sheehy et al., 2003, 2011), infants are presented with a stream of stimulus arrays that appear briefly (e.g., 500 ms), then disappear for a brief retention period (e.g., 300 ms), and then reappear. This on-off-on-off sequence repeats for many seconds. In changing streams, a feature (e.g., color) of one item in the array is changed on each cycle of the streams. In non-changing streams, the array remains the same from cycle to cycle. If infants look longer at changing streams than at non-changing streams, the assumption is that they must be storing some information about the stimuli in VSTM. In the one-shot version of the task (Oakes et al., 2013, similar to the task shown in Figure 1), each trial consists of a single cycle in which a 500-ms sample array is followed by a 300-ms blank delay period and then a single 2000- to 3000-ms test array. One item changes color from sample to test, and an eye tracker is used to determine whether infants tend to fixate the changed item more than the unchanged item(s), indicating that they remembered the color of that item. Studies using both procedures have found that infants 6 months and younger fail to detect changes when arrays contain 2 to 6 items (Oakes et al., 2013, 2009, 2006; Ross-Sheehy et al., 2003), but infants as young as 4 months can detect changes in arrays containing a single item (Ross-Sheehy et al., 2003). Thus, young infants can store information in VSTM and use it to control their looking behavior, but they appear to fail when confronted with arrays of two or more objects.
Figure 1.
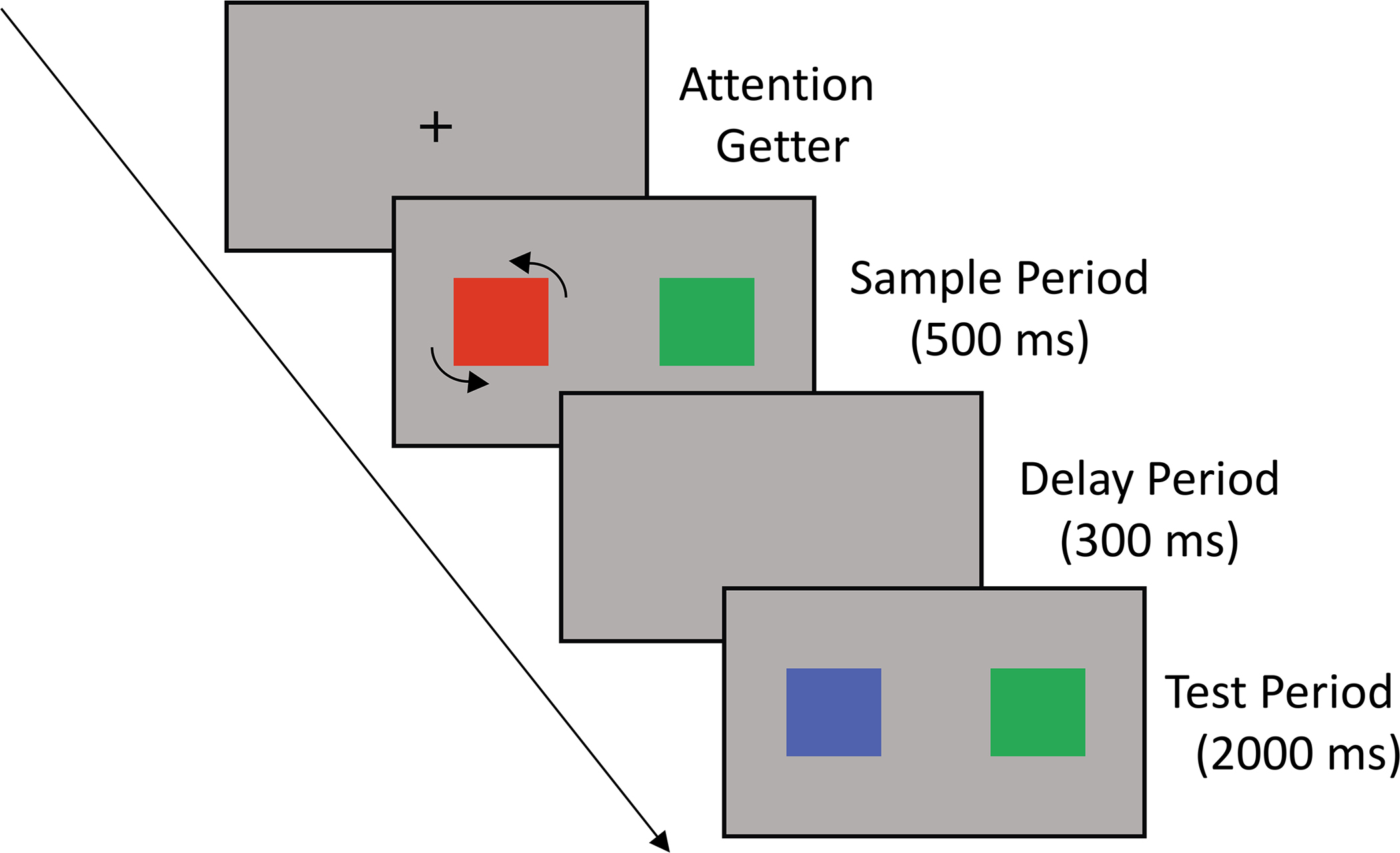
Schematic illustration of experimental stimuli for a single trial in Experiment 1. The depicted trial is a rotation-change trial (because the item that rotates during the sample changes color from sample to test). If this were a rotation-no-change changed trial, the non-rotating (green) item would be a new color during test, and the rotating item would remain red during test.
However, Ross-Sheehy, Oakes, and Luck (2011) found that 5.5-month-old infants did prefer changing streams with 3-item arrays in a continuous streams task if the changing item rotated. Presumably the rotating item attracted gaze, so this result is consistent with the proposal that young infants can successfully encode the items that attract overt attention. Because this previous work did not involve eye tracking, however, we do not know whether infants in this study actually fixated the rotating item.
Using the one-shot change detection task, Cantrell et al. (2019) provided some evidence that 6-month-old infants encode fixated items in VSTM. Specifically, Cantrell et al. showed 6-month-old infants 2-item arrays (similar to the task shown in Figure 1); during the sample period infants fixated one of the two items, and they continued to look at that fixated item longer if it changed color from sample to test than if it did not change color. Other recent work has also shown that infants’ preference for the changed item is influenced by incidental attention to that item during presentation of the initial sample array (Ross-Sheehy & Eschman, 2019).
The goal of the present study was to determine whether very young infants (4 months of age) can encode the objects they fixate in VSTM and use this information to control their subsequent looking behavior. In the natural environment, young infants’ shifts of gaze may be primarily directed toward salient stimuli, so we used a rotating object (paired with a non-rotating object) in Experiment 1 to experimentally induce a shift of gaze (see Figure 1). However, young infants may also need to learn about low-salience objects, so we used two stationary items of approximately equal salience in Experiment 2 and examined spontaneous eye movements (as in Cantrell et al., 2019). Experiment 2 also addressed two other potential shortcomings of the rotation method used in Experiment 1. First, the presence of a rotating item may make the arrays more complex and thus more difficult to process. This issue is avoided by the use of stationary arrays. Second, it is possible that only endogenously controlled shifts of gaze, but not exogenously controlled shifts of gaze, lead to VSTM encoding in young infants. In Experiment 2, infants made spontaneous, endogenously controlled eye movements, just as they would in the natural environment. Thus, Experiment 1 gave us experimental control over the direction of gaze, but Experiment 2 was more natural.
Although this study focused on examining the VSTM abilities of 4-month-old infants, we also tested 8.5-month-old infants in Experiment 1 to verify that older infants would store the salient/fixated item in VSTM even if the younger infants failed to do so. Although any age-related changes in performance may reflect the development of a number of different processes, it was essential to demonstrate that our procedure was effective in older infants.
Experiment 1
In Experiment 1, we used a one-shot change detection task and experimentally induced an attention shift by making one of the two items in the sample array highly salient. Specifically, one of the two sample items rotated for the entire 500 ms sample period, whereas the other item was stationary (see Figure 1). During the post-change test array, both items were stationary. On approximately half of the trials, the item that rotated during the sample changed color from sample to test, and on the other trials the item that was stationary during the sample changed color from sample to test.
We assessed infants’ detection of color change, as is common in the study of VSTM in infancy (Oakes et al., 2013, 2009, 2006; Ross-Sheehy & Eschman, 2019; Ross-Sheehy et al., 2003). Although some evidence suggests that color may not be easily used by young infants in tasks that require object individuation (e.g., Wilcox, 1999), other work suggests that salience is the key factor important for determining whether infants use a given feature dimension (Kaldy & Blaser, 2009). The present experiments extend previous findings that 4-month-old infants detect a color change in the simultaneous stream task if the arrays contain only 1 item, but not if they contain multiple items (Ross-Sheehy et al., 2003).
We expected that the rotating item would automatically capture gaze in both age groups because previous research has shown that highly salient stimuli are effective in capturing overt attention in 4-month-old infants (Kwon et al., 2016; Shaddy & Colombo, 2004). We also expected that the 8.5-month-old infants would store the fixated object in VSTM, as was previously observed in 6-month-old infants (Cantrell et al., 2019). Our primary question was whether 4-month-old infants would store the overtly attended item in VSTM. It seems plausible that even very young infants would store information about the objects they fixate so that they can gain enough knowledge about the world to lay a foundation for future learning. Indeed, adults appear to automatically store saccade targets in working memory, including features of these targets that are task-irrelevant (Hollingworth et al., 2008; Tas et al., 2016). However, because different object features underlie attention-getting and attention-holding in young infants (Cohen, 1972; Kwon et al., 2016), young infants may avoid storing information about everything that captures their attention, and instead only encode information that appears relevant or meaningful. For example, jingling your keys in front of a 4-month-old infant may induce the infant to orient toward the keys (and maybe even smile), but this does not necessarily mean that the infant will store a representation of the keys in VSTM.
Method
Participants.
Our target sample size was 20 to 24 infants in each age group; this target was established by evaluating effect sizes obtained in previous studies using the same basic procedure (Oakes et al., 2013, 2017) and conducting a power analysis using G*Power (Faul et al., 2007). When comparing infants’ responding to chance, previous studies have typically found effect sizes in the range of d = .6 to d = 2.0, and a sample size of 20 to 24 infants provides sufficient (80%) power to detect these effects (24 infants to detect d = .6 and 5 infants to detect d = 2.0). Given our final sample sizes (see below), we have 80% power to detect a difference between age groups of d = .90, and to detect a within-group difference between two means of d = .67.
To achieve this sample size, we tested 31 infants at 4 months and 32 infants at 8.5 months. We excluded from the final analyses infants who failed to contribute sufficient data due to fussiness (n = 2), failure to calibrate (n = 7), or experimenter error/equipment malfunction (n = 4). After data processing, we further excluded five 4-month-old infants and three 8.5-month-old infants for failing to provide sufficient numbers of trials (see data processing section below). Our final sample included 19 4-month-old infants (M = 129.16 days, SD = 5.51; 11 girls) and 23 8.5-month-old infants (M = 254.39 days, SD = 13.98; 8 girls). Of these infants, 23 infants were Caucasian, 7 were mixed race, 1 was African American, 4 were Asian, and race was not reported for 7 infants. Across racial categories, 11 infants were reported to be Hispanic (3 of these infants were Caucasian, 3 were mixed race, and 4 had no race information provided). All mothers had graduated high school, and 23 mothers had earned at least a 4-year degree.
The present study was conducted in accordance with guidelines laid down in the Declaration of Helsinki and written informed consent was obtained from a parent or guardian before data collection. All procedures involving human subjects were approved by the California Committee for the Protection of Human Subjects and the University of California Davis Institutional Review Board. Infants’ names were obtained from the state office of vital records, and information about our program of research and how to participate was sent to all parents who live within a 30-min drive of our laboratory. When infants approached the appropriate age for this study, we contacted parents who volunteered. Only healthy, full-term infants with no history of colorblindness were recruited for this study. Parents were not compensated for their participation, but infants were given a small gift and a certificate of appreciation.
Apparatus
Eye movements were recorded at a rate of 60 Hz using an Applied Science Laboratory (ASL) R6 pan/tilt remote eye tracker. The eye tracker used the reflection of an infrared light source on the cornea and pupil of the right eye to determine the point of gaze (POG). The ASL eye camera was located below a 40-inch (1920 × 1080) Sony Bravia television screen on which stimuli were presented. Infants wore a soft cotton headband with a sensor attached (just above the infants’ right eye), which established the infants’ head position in multidimensional space using an Ascension Mini Bird. When ASL lost track of the infant’s eye, it used information from the Mini Bird to reposition the camera to focus on the right eye. Two Dell computers were used to run the experiment: one computer controlled the ASL eye tracker and the second computer delivered stimuli to the Sony monitor using Paradigm Experiment software (http://www.paradigmexperiments.com/), with the Paradigm Elements add-on for ASL eye trackers.
Stimuli
The main experimental stimuli for the one-shot change detection trials were constructed of colored squares, each 9.46° by 9.46° (14.9 cm by 14.9 cm) at a viewing distance of 90 cm. During the sample and test period (see Figure 1), two colored squares, randomly selected from 8 highly discriminable colors (green, orange, purple, red, cyan, yellow, black, and brown), were presented. During the sample period one (randomly selected) square rotated back and forth, 12 degrees in each direction, at a rate of 72 degrees/per second. Each experimental trial was accompanied by classical music. In addition, we periodically played a series of video clips (e.g., a video of a laughing infant, brief animated segments from children’s television shows, an animated clip of two animals singing “The Lion Sleeps Tonight”, and a short video of randomly moving shapes accompanied by beeping noises) to maintain infants’ interest in the task.
Procedure
Infants sat on their parent’s lap, positioned approximately 75 cm from the eye tracker camera and 90 cm from the stimulus monitor. Parents wore felt-covered glasses to occlude their view of the stimuli on the display and prevent them from inadvertently influencing their infants’ looking behavior. The experiment was conducted by two experimenters who were seated out of sight behind a cloth barrier. The eye tracking experimenter controlled the computer associated with the eye tracker. The stimulus presentation experimenter used a second computer to deliver stimuli and send event codes to the eye tracker.
Infants’ point of gaze (POG) was calibrated to the eye tracking system using a standard procedure in which a looming oval or an animated duck was presented at 5 different locations (in the center of the screen and in four locations 8.07° to the left or right of and 8.07° above or below the center of the monitor). Using the video feed of the infants’ eye, the eye tracking experimenter pressed a key to indicate when the infant looked at each stimulus. After calibration, the two experimenters conducted a visual validation of the calibration (using the cross-hairs superimposed on the stimulus, indicating the infants’ POG) as the looming oval was presented at the calibration points. The calibration procedure was repeated if calibration quality was poor (e.g., the cross-hairs were consistently above or to the side of the stimulus). As described in the participants section, the calibration procedure was unsuccessful for 8 infants. The experimenters could initiate the visual verification of calibration at any point during the experiment, and could repeat the calibration procedure if this verification check revealed calibration drift.
The experimental trials were initiated immediately after calibration was verified. Each experimental trial was preceded by an attention-getting stimulus (a 4.44° by 4.44° fixation cross that flashed at the center of the screen at a rate of approximately 0.7 Hz, accompanied by a sound, i.e., a bell ringing). When the infant fixated the cross (as indicated by the cross-hairs superimposed on the video feed of the stimulus), the stimulus presentation experimenter initiated the experimental trial by pressing a key on the computer keyboard. The fixation cross was immediately replaced with a 500 ms sample array, which consisted of one square on each side of the center, separated by a 9.29° (14.62 cm) edge-to-edge gap. The colors of the two items were selected at random, and one of the two items (also selected at random) rotated at a rate of 72 degrees per second during the entire sample period (see Figure 1). Next, a blank screen was presented for a 300 ms delay period. Thus, there was an 800 ms sample-plus-delay period during which infants could encode information about one or both colored squares into VSTM.
Finally, an array consisting of two stationary squares (the same sizes and locations as the two items in the sample array) was presented for 2000 ms. One item (the unchanged identity item) was the same color as the corresponding sample item, and the other item (the changed identity item) changed to a new randomly selected color. The location of the changed item was selected randomly, independent of the location of the rotation in the preceding sample array. As a result, every test array contained both a previously rotating item and a previously stationary item (i.e., an item that had been rotating or stationary in the sample) and also contained both a changed identity item and an unchanged identity item (i.e., an item that had either changed or not changed colors). For approximately half of the trials, the previously rotating item was also the changed identity item; for the other trials, the previously rotating item was the unchanged identity item. An example of the sequence of events on a series of trials for one infant is available at (https://osf.io/6w5kq/?view_only=e8f67c9fbd4c4244ab3a22f625d7ab47).
Our design allowed us to evaluate the effect of rotation during the sample on infants’ looking during test, the effect of a color change from sample to test on infants’ looking during test, and how the two factors interacted. If rotation is the only factor that influences looking, then infants should look at the rotating item during both the sample and test, regardless of whether it changes color. If an identity (i.e., color) change is the primary factor that influences looking, then infants should look longer at the item that changes color from sample to test, regardless of whether that item rotated during the sample. Finally, if rotating the item induces an overt shift of attention that allows infants to encode identity features of the item, then infants should look longer at the previously rotating item when it changes colors from sample to test than when it does not change color from sample to test.
Trials continued until a maximum of sixty-four trials were presented or until the experimenters and/or the parent agreed that the session should be stopped because of infant fussiness or disinterest (e.g., turning toward the parent, refusal to look at the display).
Data processing
The eye tracker recorded the X and Y coordinates for infants’ POG at a rate of 60 Hz, and stored those coordinates with event codes sent from the stimulus presentation computer. Thus, the data record included information about where the infant was looking and what information was on the display during every 16.7 ms time point. To minimize noise in the data, we used an online filter that calculated a running average of the POG for the current sample and the previous three samples. We also used a custom Matlab toolbox to calculate looking within three areas of interest (AOIs): two 16.46° h by 12.48° w rectangular AOIs (26.03 cm by 19.68 cm) for the individual items presented in the stimulus arrays and a third 12.48° by 5.66°rectangular AOI (8.90 cm by 19.68 cm) in the central region corresponding to the location where the center fixation cross was presented prior to the onset of the sample array. We used AOIs that were larger than the stimulus items to correct for errors in calibration. We then used custom code in R Studio (R Core Team, 2018), custom Matlab routines (MathWorks, Natick, MA), and EyetrackingR (Dink & Ferguson, 2015) to calculate the analysis window and conduct permutation tests.
Data filtering.
Our inclusion criteria for individual trials were based on previous work by Oakes et al. (2013, 2017) and were modified for our analyses. First, we filtered trials on the basis of attention in general. We included trials in which infants (1) accumulated at least 100 ms combined looking towards all three AOIs (the AOIs for each of the objects plus a center AOI where their fixation was at the start of the trial) during the 800 ms sample and delay period, and (2) accumulated at least 200 ms combined looking towards the two AOIs during the post-change analysis window (defined below). We excluded 261 trials that did not meet these criteria. We used the set of included trials to examine infants’ interest in the rotating item before the change.
To examine infants’ attention to the rotating item between the sample and test periods, we further filtered these trials by selecting those trials in which infants directed their overt gaze towards the rotating item for at least 100 ms during an attention capture window (defined below). We excluded an additional 336 trials that failed to meet this criterion.1
Finally, to be included in any of the final analyses, we required that infants contribute at least two trials of each type (rotating item changed, rotating item did not change) after each of the data processing steps described above. As described in the Participants section, 8 infants failed to meet this criterion and were excluded from any of our reported analyses. In the end, the dataset addressing our first question—infants’ looking at the rotating item during the period before the change—included 1689 trials across 42 infants, and the dataset addressing our second question—whether infants encoded in VSTM the identity of the rotating item—included 1372 trials across those same 42 infants.
Analysis windows.
We defined three analyses windows to address different questions using the procedures described in Oakes et al. (2013) (the specifics for how the time courses were determined here can be found in the supplemental materials at https://osf.io/6w5kq/?view_only=e8f67c9fbd4c4244ab3a22f625d7ab47). First we identified an Attention capture analysis window, which we used to evaluate infants’ looking to the rotating item. This window began 200 ms after the onset of the sample array, to account for the amount of time it takes to make a saccade (Hyun et al., 2009; Oakes et al., 2017), and ended 200 ms after the change occurred, which was before infants could execute an eye movement after the onset of the change. Thus, looking during this period would reflect infants’ eye gaze to the items presented during the sample period.
Next, to examine infants’ interest in the rotating item after it did or did not change, we identified a post-change analysis window. This analysis window began 200 ms after the start of the test period (to provide time for infants to make an eye movement) and ended at the end of the 2000 ms test period. Looking during this window was used to determine whether infants’ preferred the rotating item more than expected by chance after it stopped rotating. Finally, we identified a time-course analysis window that spanned both the sample and test periods so that we could evaluate moment-to-moment changes in infants’ looking behavior both before and after the change. This analysis window began at 500 ms after the start of the trial and ended at the end of the trial.
Dependent measures.
During the attention capture window, we determined the total amount of time on each trial that infants were looking at the AOI for the rotating item, the stationary item, or the central item, and we summed the looking time on each trial separately for each of the three AOIs. We also calculated a rotation preference score during this analysis window by dividing the proportion of time spent looking at the rotating item by the total looking time for both the rotating and stationary items (note that we did not include looking to the central item in this calculation). We used the POG data in the post-change analysis window to calculate for each trial the total looking to the previously rotating and previously stationary items, and to calculate a rotation preference by dividing the time spent looking at the AOI for the previously rotating item by the time spent looking at the two AOIs (both items) combined. Finally, during the time-course analysis window, we determined for each 16.67 ms time sample whether infants were looking at the locations of rotating (or previously rotating) item or the stationary item and calculated a rotation preference during this analysis window as described above for the other analysis windows.
Statistical approach for assessing moment-to-moment gaze behavior.
One of the advantages of eye tracking is the ability to assess changes in the direction of overt attention with high temporal resolution (Oakes, 2012). In our case, we have information about infants’ gaze every 16.67 ms. Because conducting t-tests at every datapoint would inflate the family-wise Type I error rate, and because changes in eye movements over time are not independent (i.e., gaze position at one moment is likely to be near the gaze position at the previous moment), we used cluster based permutation tests. This nonparametric approach is commonly used in EEG and MEG research (Maris & Oostenveld, 2007), and has been previously applied to infant eye tracking data (Cantrell et al., 2019; Oakes et al., 2013, 2017). This approach is ideal because it solves the problem of multiple comparisons while preserving the high temporal resolution of the eye tracking data.
This approach involved several steps:
We performed uncorrected t tests at each individual time point,
We identified sets of consecutive significant time points (clusters),
We summed the t values from these points to derive the cluster mass of each cluster.
To determine whether the mass of a particular cluster of significant time points is greater than would be expected by chance, we compared the magnitude of this mass against a null distribution of cluster masses derived from random permutations of the observed data.
We generated our null distribution by randomly shuffling the label corresponding to a particular variable of interest and identifying the largest cluster mass from the randomized (permuted) data. Note that shuffling the way the trial is labeled preserves the sequence of fixations during the trial, and thus preserves the fact that eye gaze at one 16.67 ms time point is not independent from the eye gaze on the adjacent 16.67 ms time point. After shuffling the label, we calculate uncorrected t-tests on the permuted data to identify clusters of successive significant t-tests. We then repeat this procedure 1000 times, saving the mass of the largest cluster for each iteration. This gives us an empirically derived, nonparametric estimate of the null distribution (i.e., the distribution of maximum cluster masses that would be expected if the two types of trials were sampled from equivalent underlying populations). The cluster masses from the observed data are then compared with this null distribution (just as a single t value would be compared with the analytically derived null distribution of the t statistic in a conventional t test). An observed cluster is considered significantly different from chance if is in the bottom or top 2.5 percent of the clusters from the null distribution (corresponding to a two-tailed test with an alpha of .05). We will report the results of several cluster-based permutation tests below.
Results
The processed data used for all the analyses reported here are available at (https://osf.io/6w5kq/?view_only=e8f67c9fbd4c4244ab3a22f625d7ab47). We analyzed our data in three stages. First, we analyzed infants’ general looking behavior to provide information about their level of interest in the task. Second, we determined whether infants overtly attended to the rotating item. Finally, we determined whether infants responded differently when that overtly attended item did and did not change.
General looking behavior
Overall, infants at the two ages spent considerable time looking toward the AOIs during both the 800 ms sample-plus-delay period (total looking to the three AOIs combined for 4-month-old infants M = 641.51 ms, SD = 95.94, and for 8.5-month-old infants, M = 642.95 ms, SD = 68.83), and the 1800 ms test phase analysis window (looking to the two AOIs combined for 4-month-old infants M = 1053.07 ms, SD = 201.18, and for 8.5-month-old infants M = 1116.31 ms, SD = 217.26). During the attention capture window, 4-month-old infants contributed an average of 40.47 trials (SD = 16.08, range 19 to 63), and 8.5-month-old infants contributed an average of 40.00 trials (SD = 17.43, range 8 to 63). During the rotation preference and time course analysis windows, 4-month-old infants contributed an average of 30.47 trials (SD = 14.20, range 8 to 54), and 8.5-month-old infants contributed an average of 34.48 trials (SD = 16.74, range 6 to 62) (recall that these analyses windows did not include trials for which infants failed to fixate the rotating item during the initial attention capture window).
Evidence of overt attention toward rotating item
Next, we asked whether the rotating sample item elicited an overt shift of attention. We calculated a rotation preference score during the attention capture analysis window on each trial for each infant. As is common in this procedure, our primary analyses were conducted on each infant’s median rotation preference score (see Oakes et al., 2013). Figure 2 shows the mean of the medians (height of the bar) and each infant’s median preference (individual dots). During this 800 ms window, both 4- (M = .99, SD = .02) and 8.5-month-old infants (M = 1.0, SD = .00) robustly preferred the rotating item. These median scores indicate that infants directed their gaze to the rotating item on the vast majority of trials; however, they do not mean that infants spent 100% of their time looking at the rotating item. We also calculated mean rotation preferences scores for each individual infant, which provided more information about the variation in infants’ looking to the rotating and non-rotating item. These mean rotation preference scores were, on average, well above chance (.50) for both 4-month-old infants (M = .70, SD = .13) and 8.5-month-old infants (M = .65, SD = .15).
Figure 2.
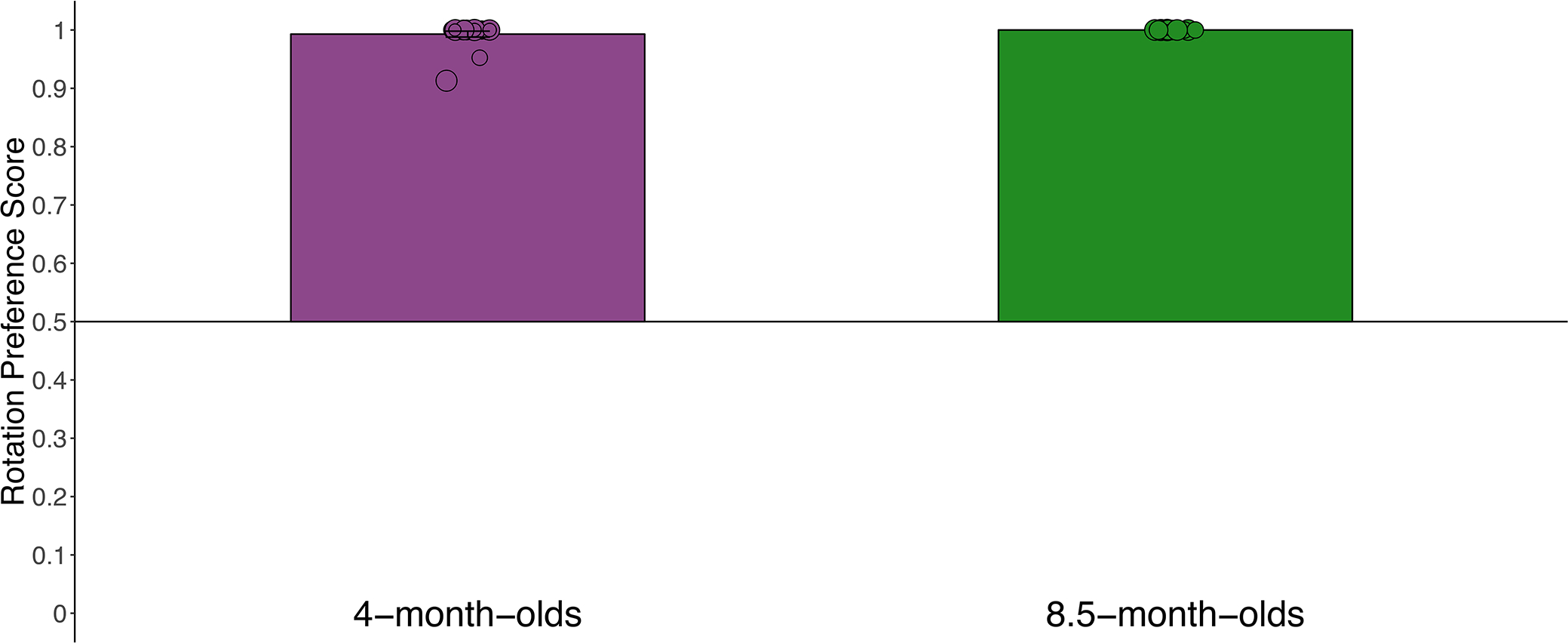
Rotation preference score by age during the attention capture analysis window (200 ms after the onset of the sample array until 200 ms after the onset of the test array) in Experiment 1. The height of the bar represented the mean for each age group, and individual points represent median preference scores for each infant. Error bars—which are difficult to detect due to lack of variability in infants’ responding—represent 95 % confidence intervals and the horizontal line bisecting the y-axis represents chance. Note that all 8.5-month-old infants had a median rotation preference score of 1 so there is no error bar.
Next, we examined moment-to-moment changes in infants’ looking across this period. Figure 3 shows the proportion of trials for which infants fixated the rotating item (blue line) and the stationary item (red line) at each 16.67 ms time point during the attention capture analysis window (i.e., when the item was actually rotating). The proportions are near 0 at the start of the trial, presumably because the infants were fixated on the central location where the fixation cross had been. Similarly, the proportion of trials in which infants’ fixation was on the stationary item remains low, because infants rarely shifted their gaze to this item. The proportion of trials on which infants fixated the rotating item, in contrast, rose sharply between 300 and 400 ms after trial onset as infants overtly attended to that item. Note that averaged across infants, on 60% to 70% of the trials gaze was directed to the rotating item by the time the change occurred; unlike the change preference described for the previous analyses, the proportion of looking at the rotating item at each time point reflects the proportion of the total trials, including trials when the infant was looking at the center location or off the screen.
Figure 3.
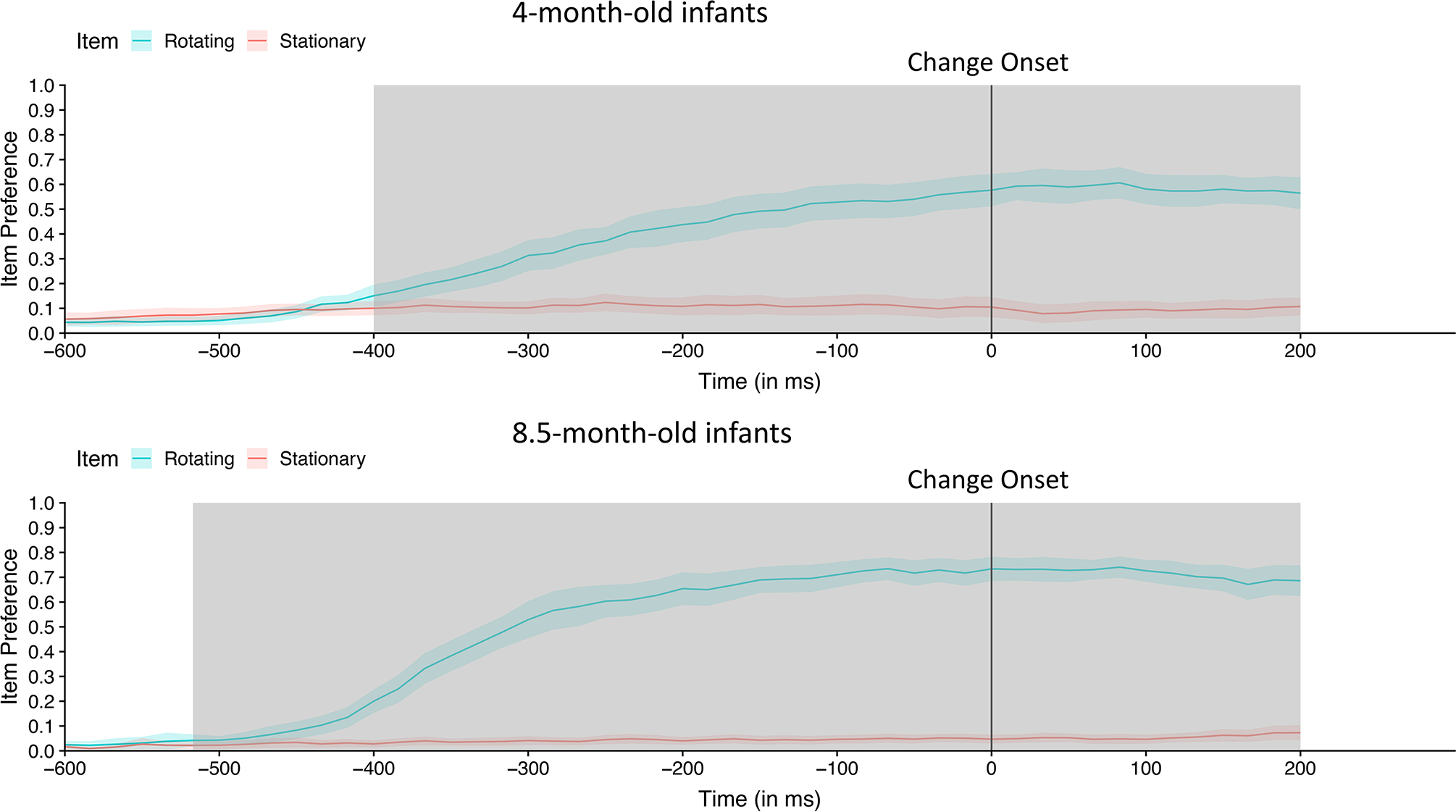
Time-course of looking to the rotating item (blue line) and non-rotating item (red line) during the attention capture analysis window (200 ms after the onset of the sample array to 200 ms after the onset of the test array) for 4-month-old (top) and 8.5-month-old (bottom) infants in Experiment 1. Each point on the line represents the subject-weighted proportion of trials at each 16.67 ms time point that infants looked at the rotating (blue line) and stationary item (red line); the shading around the lines represents 95% confidence interval for each time point. The two proportions do not equal 1 because infants could be fixating neither item (e.g., looking at the center, being off-task). Permutation analyses identified clusters of time points during which infants fixated rotating item on a statistically significant greater proportion of trials than they fixated the stationary item; those clusters are indicated by gray shading.
To determine when infants’ looking shifted preferentially to the rotating item, we computed paired t-tests comparing the probability of gaze for the rotating and stationary locations at each 16.67 ms time point. We then use these t-values to perform the cluster mass permutation-based statistical test described above. In this case, we shuffled (trial-by-trial) which of the two items was labeled as rotating to compute the null distribution (thus, by chance, the rotating item was correctly labeled as rotating on 50% of the iterations in the null distribution). Gaze began to shift significantly away from the central fixation region to the rotating item at 400 ms (relatively to the onset of the sample array) in the 4-month-old infants and at 283 ms in the 8.5-month-old infants (see Table 1). From this point until the end of the attention capture analysis window, gaze was significantly more likely to be directed to the location of the rotating item than toward the location of the stationary item. Thus, infants’ attention was captured by the rotating item, and their gaze was sustained at this location.
Table 1.
Significant clusters for each permutation analysis in Experiment 1.
| Analysis | Age | Trial Type | Clusters | Sum t-statistic | Start Time | End Time | Probability |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Attention Capture | 4 | 1 | 335.8490 | 400 | 1000 | 0.000 | |
| 8.5 | 1 | 732.2192 | 283 | 1000 | 0.000 | ||
|
| |||||||
| Chance Comparison | 4 | Rotation-Change | 1 | 1498.5715 | 500 | 1866 | 0.001 |
| 2 | 18.1130 | 1916 | 2016 | 0.040 | |||
| 3 | 101.9377 | 2200 | 2616 | 0.002 | |||
| Rotation-No-Change | 1 | 1075.9573 | 500 | 1433 | 0.001 | ||
| 2 | 22.9169 | 1933 | 2033 | 0.022 | |||
| 3 | 20.7463 | 2383 | 2383 | 0.025 | |||
| 8.5 | Rotation-Change | 1 | 2642.2029 | 500 | 2083 | 0.001 | |
| 2 | 23.4495 | 2100 | 2233 | 0.018 | |||
| 3 | 39.9936 | 2250 | 2466 | 0.005 | |||
| Rotation-No-Change | 1 | 1122.2224 | 500 | 1250 | 0.001 | ||
|
| |||||||
| Trial Type Comparison | 4 | 1 | 2.1650 | 483 | 500 | 0.936 | |
| 2 | 10.1155 | 1566 | 1633 | 0.327 | |||
| 3 | 16.7238 | 1650 | 1766 | 0.152 | |||
| 4 | 2.4120 | 2433 | 2450 | 0.908 | |||
| 8.5 | 1 | 236.7092 | 1050 | 1933 | 0.001 | ||
|
| |||||||
| Difference Score | 1 | 55.7497 | 1216 | 1567 | 0.012 | ||
Evidence of infants’ preference for the rotating item when it does and does not change
Our primary question was whether infants encoded information about the overtly attended rotating item into VSTM. To answer this question, we examined only trials on which infants directed their gaze to the rotating item, as described earlier. For the test period, we calculated infants’ rotation preference scores (i.e., their preference for the location where the item had rotated during the sample period), and for each infant we calculated separate median rotation preference scores for trials when the rotating item did and did not change color. If infants were more likely to encode the color of the rotating item than the stationary item in the sample display, they should show a stronger preference during the test array when the location of the previously rotating item changed color than when it did not.
Figure 4 shows the results. Individual median rotation preference scores are shown along with the mean of the medians, calculated separately for each age. The 8.5-month-old infants preferred the previously rotating item when it changed color from sample to test and showed no preference when the previously rotating item did not change. The 4-month-old infants, in contrast, preferred the previously rotating item in both trial types.
Figure 4.
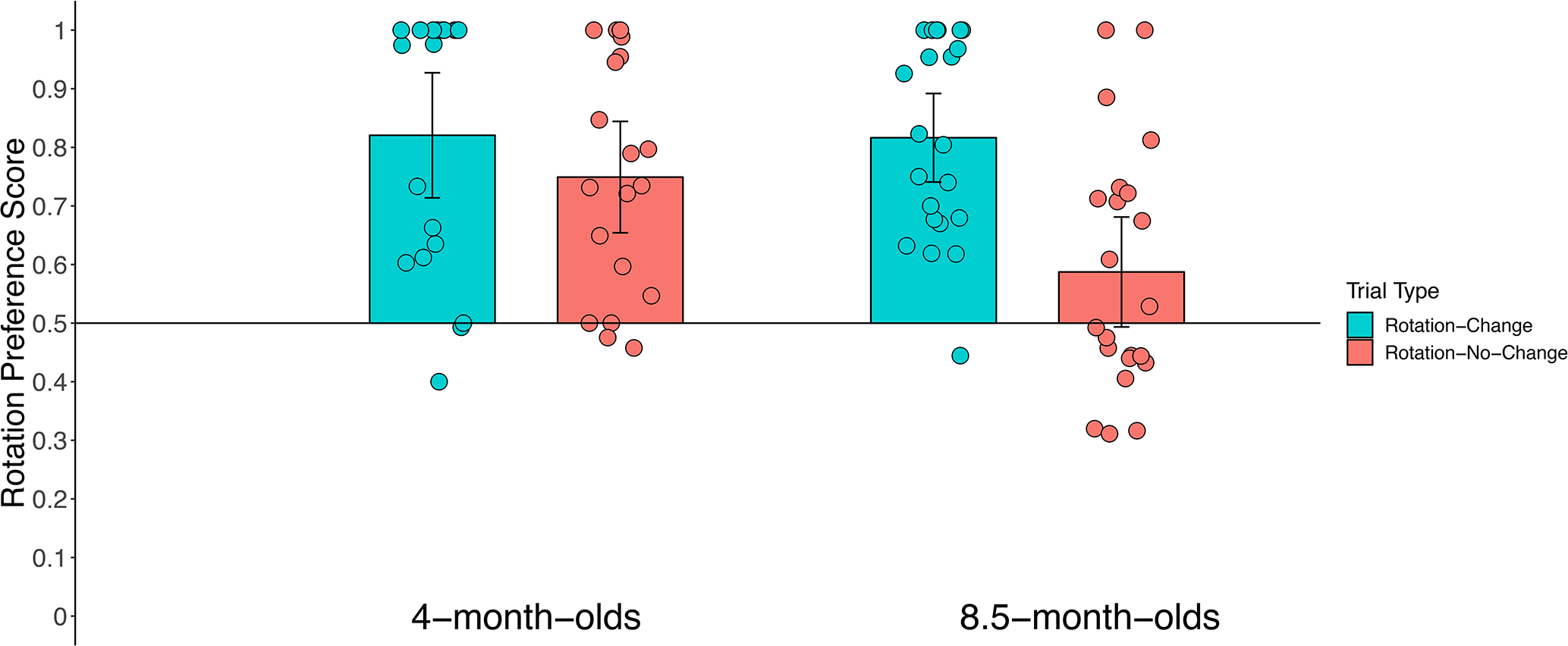
Rotation preference scores for the previously rotating item during the post-change analysis window for trials in which that item changed color (Rotation-Change trials; blue bars) or remained the same color (Rotation-No-Change trials; red bars) for each age group in Experiment 1. The height of the bar represents the mean score for that trial type for that group of infants, and individual circles represent median preference scores for each infant. Error bars represent 95% confidence intervals, and the horizontal line bisecting the y-axis represents chance.
We compared infants’ preference scores to chance (.50) with frequentist hypothesis testing (FHT) using two-tailed one-sample t-tests, and we also computed Bayes factors using the non-informative JZS prior with a scale factor of .707 (Rouder et al., 2009). Four-month-old infants significantly preferred the previously rotating item when that item changed color, t(18) = 6.31, p < .0001, d = 1.45, BF10 > 3,000, and when it did not change color, t(18) = 5.51, p < .0001, d = 1.32, BF10 = 792.47. Thus, these younger infants maintained gaze on the previously rotating item during the test period regardless of whether or not it changed color.
The 8.5-month-old infants exhibited a different pattern. They showed a significant preference for the previously rotating item when it changed color during the test period, t(22) = 8.36, p < .0001, d = 1.72; BF10 > 10,000. However, when the previously rotating item did not change color, 8.5-month-old infants’ preference score did not differ significantly from chance, t(22) = 1.91, p = .07, d = 0.38, and BF10 = 1.02. Thus, these older infants showed a robust preference for the previously rotating item only when it changed color.
To examine how infants’ preferences for the rotating item varied as a function of age and trial type, we conducted an age (4 months vs. 8.5 months) by trial type (previously rotating item changed, previously rotating item no change) repeated measures Analysis of Variance (ANOVA). This analysis revealed a main effect of trial type, F(1,40) = 17.56, p < .001, η2 = .28, no effect of age, F(1,40) = 3.14, p = .08, η2 = .07, and a significant age-by-trial type interaction, F(1,40) = 4.67, p = .04, η2 = .08. We probed this interaction by conducting four follow-up t-tests, using a criterion p-value of .0125 to correct for multiple comparisons. The difference between trial types was nonsignificant at 4 months, t(18) = 2.00, p = .06, d = 0.34, but was significant at 8.5 months, t(22) = 3.95, p < .001, d = 1.18. Bayes factors analyses yielded similar results, BF10 = 1.22 for 4-month-old infants, and BF10 = 49.60 for 8.5-month-old infants. Thus, there was strong evidence of a difference for older infants, and little to no evidence of a difference for younger infants.
Comparisons between 4 and 8.5-month-old infants for each trial type revealed no significant difference between the two ages for rotation preference when the change occurred on the rotating side, t(40) = 0.25, p = .80, d = .08, BF10 = .31. However, compared to the 8.5-month-old infants, 4-month-old infants’ preference for the previously rotating item was significantly greater when that item did not change, t(40) = 2.64, p = .01, d = .84, BF10 = 4.36.
Time course of gaze shifts
To capture how infants’ behavior changes and unfolds over time, we conducted our permutation analyses on the time course data to evaluate looking against chance, looking as a function of trial type, and the effect of age and trial type on infants’ moment-to-moment looking behavior.
Maintenance of Gaze on the Location of the Rotation.
First, we asked whether infants maintained gaze to the location of the rotating item. These data are shown in Figure 5, separated as a function of age group and whether the color change occurred on the rotating side or the stationary side. For each of these four data sets, we calculated uncorrected one-sample t-tests (two-tailed) comparing the observed preference scores against chance (.50) at every 16.67 ms time point. We then conducted the cluster mass permutation-based statistical test described previously, shuffling the labels indicating the side of the rotation when computing the estimated null distributions. We also compared at each age how infants responded to the two different trial types, by conducting a comparison of trials with the change on the rotating side versus the stationary side, using paired t tests at each time point to form the clusters and shuffling the labels for the side of the change to construct the estimated null distributions.
Figure 5.
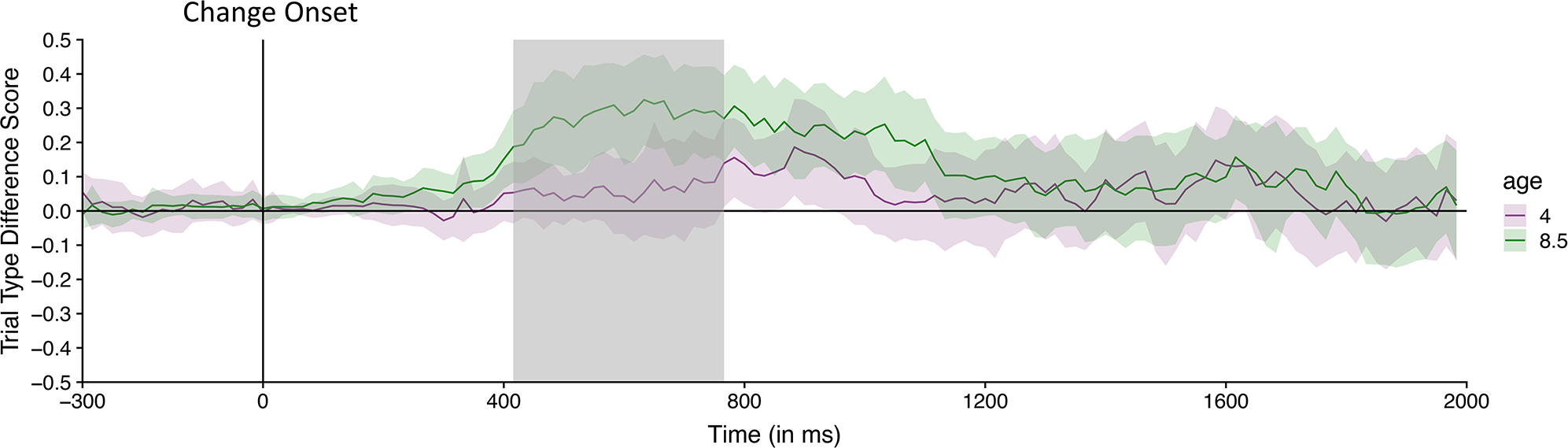
Time-course of looking to the rotating item on trials in which the rotating item changed color (Rotation-Change trials; blue line) and trials in which the item did not change (Rotation-Change No-Change trials; red line) during the time-course analysis window (300 ms before the change onset to the end of the trial) for the 4-month-old (top) and 8.5-month-old (bottom) infants in Experiment 1. The lines represent the mean preference for the location of the rotating item at each 16.67 ms time point before and after the test array appears (indicated by the vertical “Change Onset” line); the shading represents the 95% confidence interval for each time point. Clusters of time points in which infants’ preference was significantly greater than chance (.50; horizontal line bisecting the graph) is indicated by the colored lines at the bottom of each plot; a blue line indicates the time points on Rotation-Change trials in which infants’ preferred the rotating item more than expected by chance, and the red line indicates the time points on Rotation-No-Change trials in which infants preferred the rotating item more than expected by chance. Cluster of time points in which 8.5-month-old infants’ rotation preference preference was significantly greater on Rotation-Change than on Rotation-No-Change trials are indicated by the gray shaded area; at no point did the 4-month-old infants’ preference differ in the two types of trials.
For both age groups and trial types, a strong and statistically significant preference for the side of rotation was present throughout the delay interval and well past the onset of the test array (see Figure 5 and Table 1). Given that these analyses were restricted to trials on which gaze was captured by the rotating item, it is not surprising that a preference for the side of rotation was observed at the beginning of this time window. For the 4-month-old infants, this preference remained significant for most of the duration of the test array, with very little difference in preference between trials with a change on the rotating side and trials with a change on the stationary side. As a result, these young infants demonstrated significant preferences for the previously rotating item for large clusters of time points for both trial types. Indeed, comparison of the two types of trials at each age revealed no significant difference in infants’ preference for the previously rotating item when it did versus did not change at any time point (See Table 1). In other words, the 4-month-old infants’ pattern of gaze did not depend on whether the color changed on the side that had contained the rotation.
The 8.5-month-old infants also had a significant preference for the previously rotating item for the duration of the test period when that item changed from sample to test. However, when the stationary item changed (and the rotating item did not), the preference for the previously rotating item dropped to near-chance levels within 500 ms of the onset of the test (see Figure 5, bottom). Moreover, direct comparison of the two types of trials revealed that 8.5-month-old infants’ preference for the previously rotating item was greater when it changed than when it did not change from 250 to 1150 ms after the onset of the test (indicated by the gray shading in Figure 5). Thus, 8.5-month-old infants’ preference for the side of rotation was greater when it changed color than when it did not change color for a sustained period of time, indicating that they stored information about the color of the rotating sample item in VSTM.
Comparing the difference between the two trials types for 4- and 8.5-month-old infants (age by trial type interaction).
Finally, we examined the interaction between age and trial type on infants’ preference for the side of rotation. For each infant, we calculated a difference score at every time point by subtracting their mean score for trials with versus without a change at the location of the rotation. The time course of this difference score is shown for each age group in Figure 6.
Figure 6.
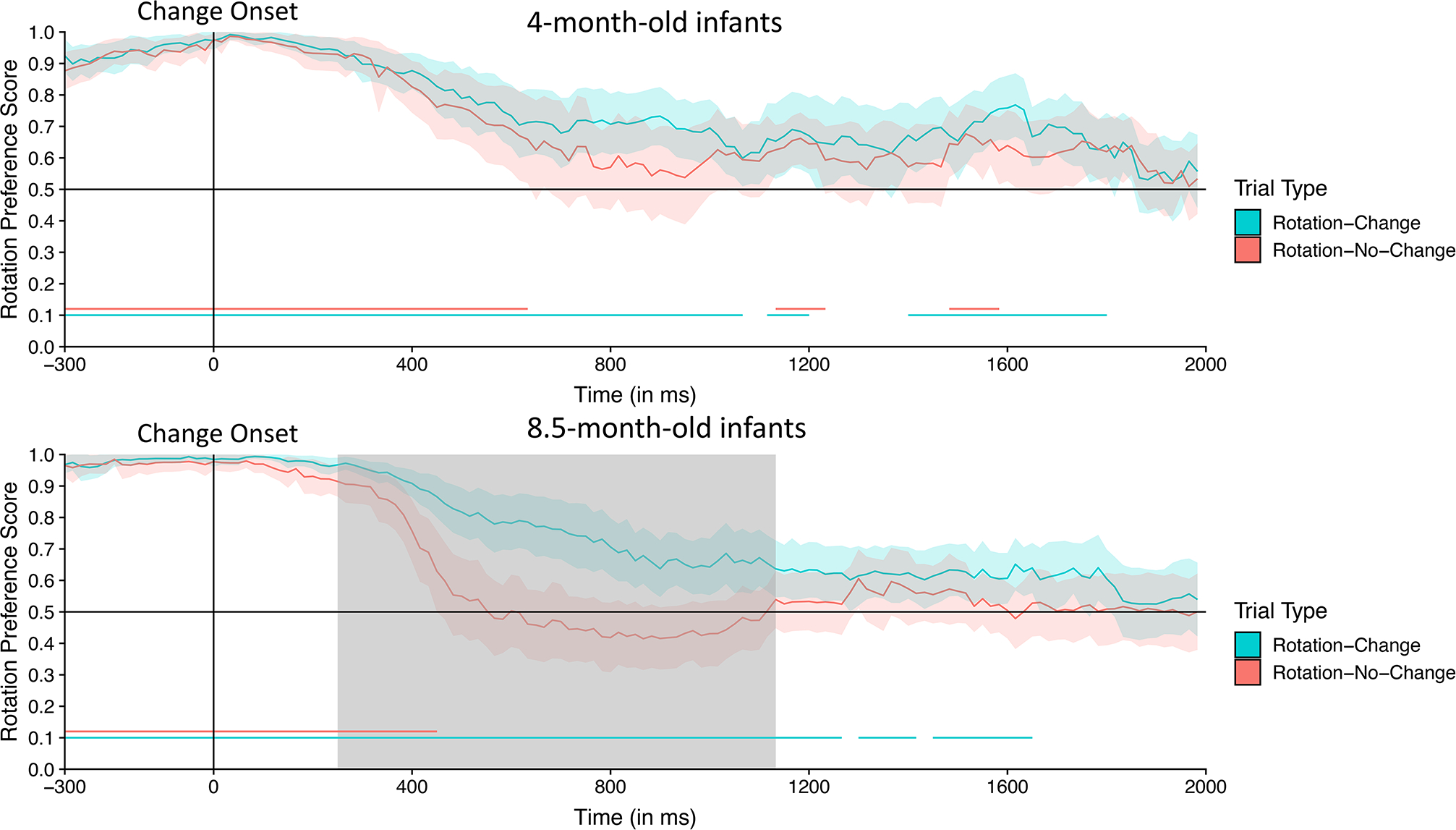
Time-course of the difference in infants’ rotation preference score for Rotation-Change and Rotation-No-Change trials for the 4-month-old infants (purple) and 8.5-month-old infants (green) during the time-course analysis window (300 ms before the change onset to the end of the trial) in Experiment 1. The lines represent the difference in preference at each 16.67 ms time point before and after the test array appears (indicated by the vertical “Change Onset” line); the shading around these lines represents the 95% confidence interval. Chance performance (0, indicating no difference between the two types of trials) is indicated by the horizontal line bisecting the vertical axis. The gray area represents clusters of time points during which the difference score for the 8.5-month-old infants was significantly greater than that for the 4-month-old infants.
Consistent with the raw data shown in Figure 5, the difference scores for the 4-month-old infants remained near zero for the entire analysis window, with only weak evidence of a difference between trials with a color change at the rotating and stationary locations. By contrast, the difference scores for the 8.5-month-old infants rose well above zero (indicating more looking at the rotating location when the color change also occurs at that location) starting at approximately 400 ms after the onset of the test array and continuing for several hundred ms. We then statistically compared the two age groups by conducting independent-samples t tests comparing the difference score for 4- and 8.5-month-old infants at each time point and using these values in a cluster mass analysis, shuffling the age labels to estimate the null distribution. The difference score was significantly greater for the 8.5-month-old infants than for the 4-month-old infants from 416 ms to 766 ms after the onset of the test array (see Table 1). Note that this significant difference is analogous to the significant two-way interaction between age and side of change in the ANOVA described earlier, except that the present analysis indicates the specific time period in which the interaction was significant. Thus, the apparent differences in looking behavior between the younger and older infants shown in Figures 6 are reliable.
Discussion
The results of Experiment 1 suggests that infants younger than 6 months do not automatically encode into VSTM any fixated item. We found that although 8.5-month-old infants preferred a previously rotating item when it changed color compared to when it did not change color, 4-month-old infants demonstrated no such preference. This finding is remarkable given that the rotating item was highly salient and induced an overt attention shift in both younger and older infants.
These findings provide evidence for a developmental shift in the encoding of overtly attended items in VSTM. These conclusions, however, are based upon the observation that 4-month-old infants provided no evidence that they encoded the color or identity of an overtly attended item in VSTM. One possible explanation for this finding is that we artificially induced a shift of attention with a very potent stimulus feature, which may have interfered with the process of encoding other information in VSTM. The stimulus features that capture young infants’ attention are not necessarily the same features that induce information processing and memory (Cohen, 1972). Thus, one possibility is that endogenous, but not exogenous shifts of attention, induce learning about an attended stimulus. Alternatively, the high salience of the rotation may have caused the 4-month-old infants to focus solely on the rotation of the item to the exclusion of other properties of the rotating object, such as its color. That is, the 4-month-old-old infants may have continued to prefer the previously rotating item because this item had changed from rotating to stationary whereas the other item was stationary in both the sample and test displays. The purpose of Experiment 2 was to rule out these possibilities.
Experiment 2
We tested 4-month-old infants in a change detection task in which the sample array items were both stationary. We let infants spontaneously move their eyes to one of two stationary items of approximately equal salience. If young infants’ overt gaze towards an item fails to induce encoding of that item in VSTM, even when both items are approximately equal in their salience, then this would provide additional evidence that they do not automatically encode information about overtly attended items in VSTM. We only tested 4-month-old infants in Experiment 2 because (Cantrell et al., 2019) previously showed that this procedure leads to effective VSTM encoding in 6-month-old infants.
Method
Participants
As in Experiment 1, our target sample size was 20–24. We tested thirty-four 4-month-old infants, and we excluded from the final analysis infants who failed to contribute data due to fussiness (n = 2), failure to calibrate (n = 5), experimenter error/equipment malfunction (n = 2), and poor tracking (n = 1), or failure to provide a sufficient number of trials that met our inclusion criteria (n = 7). Our final sample included 17 infants (M= 129.82, SD= 8.68); 15 were Caucasian, 1 was African American, and 1 was Asian, and across racial categories, 2 infants were Hispanic (both were Caucasian). All mothers had graduated high school and 13 had earned at least a 4-year degree.
Apparatus and procedure.
The apparatus and procedure were identical to those used in Experiment 1.
Stimuli
The experimental stimuli were identical to those of Experiment 1 except for two differences: 1) both items items were stationary during the sample array; 2) the items were rectangles (9.46° w X 13.7° h, 14.9 cm w X 21.5 cm h) rather than squares.
Results and Discussion
The data were analyzed as described in Experiment 1, except as described below. Overall, infants were consistently engaged in the task, looking for a mean of 594.030 ms (SD= 212.82) toward the three AOIs combined during the 800 ms sample-plus-delay period, and a mean of 1085.79 ms (SD= 481.75) toward the two AOIs combined during the 1800 ms test phase analysis window. Infants contributed an average of 32.35 trials (SD = 15.98, range = 7 to 53) during the attention capture analysis window (200 to 1000 ms, determined as described in Experiment 1, see supplemental materials at https://osf.io/6w5kq/?view_only=e8f67c9fbd4c4244ab3a22f625d7ab47) and 25.59 trials (SD= 13.21, range 7 to 44) during the test period and time-course analysis windows (750 ms to 2683 ms and 1000 ms to 2800 ms respectively). Note that the analysis window for Experiment 2 started later than Experiment 1 (see supplemental materials at https://osf.io/6w5kq/?view_only=e8f67c9fbd4c4244ab3a22f625d7ab47 for details).
Because neither of the items rotated, we sorted trials on the basis of whether infants looked more at the location of the changing item or the unchanging item during the sample and delay period (before the change occurred). We made this determination by (1) identifying trials on which infants directed their gaze for at least 100 ms towards at least one of the items, and (2) identifying which of the items infants looked at for longer duration. This preferred item hereafter will be called the target.
We calculated each infant’s median target preference scores during the post-change analysis window for trials in which the target changed color from sample to test (Target-Change trials) and for trials in which the target did not change color (Target-No-Change trials). Figure 7 shows the individual infant medians and the mean of the medians. Comparison of target preference score against chance (.50) revealed that, during the test period, infants significantly preferred the target item both when that item changed color, t(16) = 5.96, p < .0001, d = 1.45, BF10 > 1,000, and when that item remained the same color, t(16) = 4.38, p < .001, d = 1.06, BF10 = 73.62. Thus, infants sustained their attention to the preferred item during the test period regardless of whether or not it changed color, confirming the pattern observed in Experiment 1. Moreover, the preference did not differ significantly between these two trial types, t(16) = 0.87, p = 0.40, d = 0.17, BF10 = 0.35.
Figure 7.
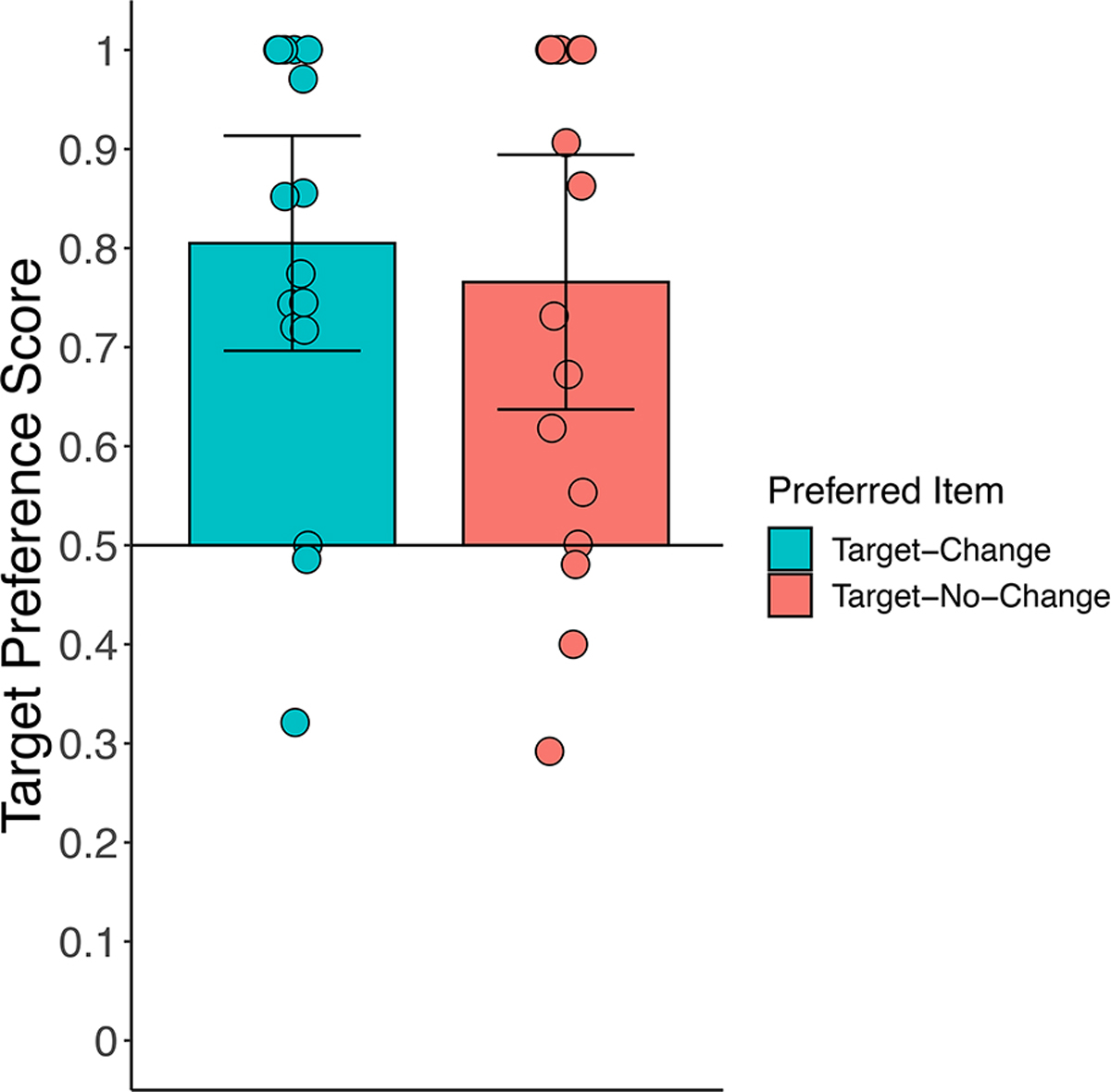
Target preference scores for the preferred item during the post-change analysis window for trials in which that item changed color (Target-Change trials; blue bars) or remained the same color (Target-No-Change trials; red bars) for Experiment 2. The height of the bar represents the mean score for that trial type for that group of infants, and individual circles represent median preference scores for each infant. Error bars represent the 95% confidence intervals, and the horizontal line bisecting the y-axis represents chance.
The time-course data for each type of trial is presented in Figure 8. Permutation analyses on the time course data revealed that, for both trial types, a strong and statistically significant preference for the location of the target (e.g., the preferred item as established before the change) was present during the delay and well past the onset of the test array (Table 2), again confirming the findings in Experiment 1 for 4-month-old infants. Infants’ preference for the target did not differ on trials in which it changed color compared to trials in which it remained the same. Therefore, even when both items were approximately equal in salience, 4-month-old infants failed to show evidence that the overtly attended item was stored in VSTM. Thus, in both Experiments 4-month-old infants did not show evidence of encoding the color of the fixated item. The consistency of the results in the two experiments allows us to rule out the possibility that the failure of younger infants to detect a change in Experiment 1 was due to the presence of rotation, and our artificially inducing infants to shift their gaze. Direct comparison of the 4-month-old infants in the two experiments revealed no significant differences between the two groups of infants (see full details at https://osf.io/6w5kq/?view_only=e8f67c9fbd4c4244ab3a22f625d7ab47). Thus inducing a fixation (by rotating an item) did not change 4-month-old infants’ ability to encode information in VSTM.
Figure 8.
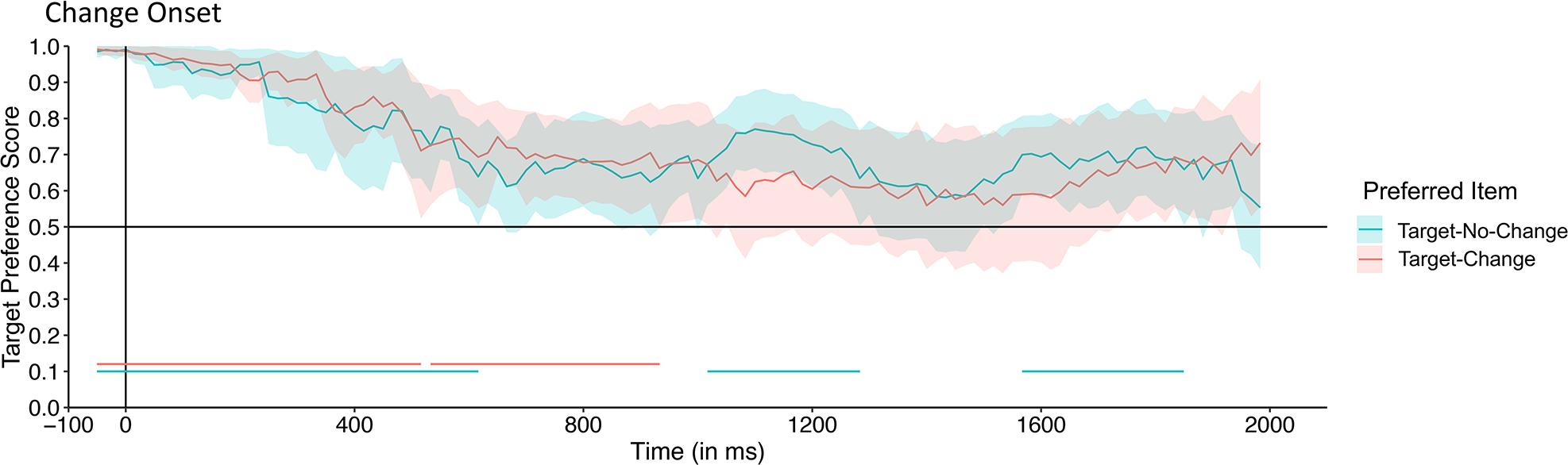
Time-course of looking to the target (as determined by preference during the sample plus delay period) on trials in which the target changed color (Target-Change trials; blue line) and trials in which the target did not change (Target-No-Change trials; red line) during the time-course analysis window (50 ms before the change onset to the end of the trial) for the 4-month-old infants in Experiment 2. The lines represent the mean preference for the target item at each 16.67 ms time point before and after the test array appears (indicated by the vertical “Change Onset” line); the shading represents the 95% confidence interval for each time point. Clusters of time points in which infants’ preference was significantly greater than chance (.50; horizontal line bisecting the graph) is indicated by the colored lines at the bottom the plot; a blue line indicates the time points on Target-Change trials in which infants’ preferred the rotating item more than expected by chance, and the red line indicates the time points on Target-No-Change trials in which infants preferred the rotating item more than expected by chance. At no point did the 4-month-old infants’ preference differ in the two types of trials.
Table 2.
Significant clusters for each permutation analysis in Experiment 2.
| Analysis | Preferred Item | Clusters | Sum t-statistic | Start Time | End Time | Probability |
|---|---|---|---|---|---|---|
|
| ||||||
| Attention Capture | 1 | 94.7819 | 567 | 1000 | 0.003 | |
|
| ||||||
| Chance Comparison | “Target-Change” | 1 | 620.6912 | 750 | 1416 | 0.001 |
| 2 | 21.6980 | 1816 | 2083 | 0.003 | ||
| 3 | 55.8733 | 2367 | 2650 | 0.004 | ||
| “Target-No-Change” | 1 | 888.2265 | 750 | 1316 | 0.001 | |
| 2 | 83.1473 | 1333 | 1733 | 0.002 | ||
|
| ||||||
| Trial Type Comparison | 1 | 2.2293 | 1450 | 1467 | 0.705 | |
| 2 | 2.1640 | 2683 | 2700 | 0.754 | ||
| 3 | 7.2957 | 2750 | 2800 | 0.314 | ||
General Discussion
These experiments were designed to better understand VSTM in young infants, given previous results that infants under 6 months did not encode or store object identity from items in multiple-item arrays in VSTM (Ross-Sheehy et al., 2003). Results reported by Cantrell et al. (2019) indicate that by 6 months, infants encode in VSTM the identity of overtly fixated items in multiple-item arrays. We asked here if this process was in place by 4 months. Our experiments revealed no evidence that overt attention allows younger infants to encode information from multi-item arrays in VSTM. Four-month-old infants failed to demonstrate a stronger preference for a fixated item that changed color than a fixated item that did not change color. This contrasts with our findings that 8.5-month-old infants did encode the the color of an item that they were experimentally induced to fixate, and with previous research showing that adults automatically store the properties of a saccade target in VSTM which then influences their subsequent oculomotor behavior (Hollingworth et al., 2008; Tas et al., 2016). Thus, the correspondence between overt gaze and encoding in VSTM may develop during infancy.
One possible conclusion from these results in combination with the existing literature is that 4-month-old infants are indeed unable to encode information about individual object identity in VSTM from scenes involving multiple objects. That is, when faced with multiple-item arrays, 4-month-old infants may experience a catastrophic failure of the VSTM system, and not encode any features of the individual items (Feigenson & Carey, 2005), for a similar argument for infants’ representation of the number of items in an array). Instead, at this age infants may encode global features of scenes, detecting changes in, for example, overall configuration or number of items.
However, an alternative possibility is that young infants can store information in VSTM from such arrays, but aspects of the experimental procedures used here inhibited their ability to do so. Although beyond the scope of the present work, understanding how features of the task inhibit and facilitate infants’ VSTM will provide deeper insight into the early development of VSTM, and how young infants use VSTM in the natural environment. For example, as described earlier, it is possible that 4-month-old infants cannot store color in VSTM, and we would have observed a different pattern had we examined VSTM for a different feature, such as shape, as has been found for infants’ object individuation (Wilcox, 1999). However, by 4 months infants can detect changes in object color (Bremner et al., 2013). And Ross-Sheehy et al. (2003) found in a task very similar to that used here 4-month-old infants did detect a color change when arrays involved only one item. Thus, rather than infants being insensitive to a specific dimension (such as color), differences in salience and discriminability likely determine whether or not infants use features in any tasks (Kaldy & Blaser, 2009, 2013); for similar arguments with adult change detection in VSTM see (Awh et al., 2007).
Moreover, features other than physical salience might underlie young infants’ selective attention to and encoding of objects in VSTM. It would not be surprising if young infants selected and encoded relevant items (e.g., human faces). Because the studies of VSTM in infancy have used simple shapes (for an exception see Kwon et al., 2014, who used unfamiliar complex objects), it is yet unknown how such aspects of stimuli contribute to young infants’ encoding of item features in VSTM.
In our procedure, young infants may have had difficulty disengaging attention from fixated items. Previous research has documented that young infants do not easily disengage from a stimulus (Frick et al., 1999; Hood & Atkinson, 1993), although typically infants have overcome this type of “sticky fixation” by 4 months (Hood, 1995). Nevertheless, some studies have revealed that even at 7 months infants have difficulty disengaging from some stimuli (Peltola et al., 2008), and it is therefore possible that difficulties with disengagement contributed to infants’ performance in our tasks. Similarly, 4-month-old infants may encode information about overtly attended objects, but our procedure was not sensitive to this ability because they do not use that stored information to guide their subsequent looking behavior. However, a large literature has shown that young infants’ looking time is reliably influenced by whether or not the currently fixated object matches the contents of memory (Roder et al., 2000; Rose et al., 1982; Welch, 1974; Wetherford & Cohen, 1973). Thus, infants of this age can use such information to guide their looking behavior under some conditions.
Finally, differences in speed of processing may have contributed to the development we observed, and younger infants would be better able to show evidence of encoding color of the fixated item in VSTM if they were given more exposure time during the sample period. That is, the timing of our procedure, which estimates natural eye movements, may not have allowed younger infants sufficient time to select, process, and encode the features of one of the items in the array. Our timing was selected carefully based on findings from other research. For example, Catherwood, Skoien, Green, & Hold (1996) found that young infants can encode and remember color with only a 250 ms exposure. Thus, our 500 sample plus the iconic memory representation during the 300-ms delay period should be sufficient for them to encode the color of a fixated item. Moreover, Kwon et al. (2014) found that extending the exposure time for 6-month-old infants in the simultaneous stream task did not enhance their VSTM for complex objects. Nevertheless, it is possible that younger infants’ failure to demonstrate that they encoded item color in VSTM reflected some aspect of the procedure, and future research may reveal that such factors contribute to the developmental differences observed here.
It is important to point out that although 8.5-month-old infants stored information in VSTM in this procedure, our results confirm that 8.5-month-old infants stored only information about the item they fixated. Previous research using both continuous stream and one-shot versions of the change detection task have found that infants of 8 months or older show evidence of VSTM in the context of 2- and even 3-item stimulus arrays. In theory, however, above-chance performance with such arrays could be achieved on average if infants only stored one of the items in VSTM. In the current study, 8.5-month-old infants showed a preference for the rotating item when it changed color but not when it remained the same color from sample to test. Thus, the color of the rotating item was stored in VSTM, but we have no evidence that the color of the stationary sample item also was stored in VSTM. If infants also stored the color of the (non-fixated) stationary item, they should have looked toward that side of the test array when the item on that side changed color (which would have produced a preference score below 0.5 at some point after the change occurred). Instead, the 8.5-month-old infants’ preference scores approached chance on these trials, providing no evidence that they had detected a change on the side of the stationary sample item. The high salience of the rotating item may have prevented infants from encoding information about the (non-fixated) stationary item in VSTM. However, it is also possible that the salience of the (formerly) rotating item at one location and the detection of a color change at the other location competed for infants’ attention. For example, once gaze was directed to the location of the rotating item, any change signal from the location of the stationary item would need to overcome the tendency to keep the eyes on their current location. Thus, the near-chance preference scores may have reflected a mixture of some trials in which gaze shifted to the changed color at the stationary location and other trials in which gaze remained on the location of the rotation owing to simple inertia. Further research will be necessary to distinguish between these possibilities.
This study demonstrates the utility of the one-shot change detection task to uncover the processes of VSTM. The temporal and spatial resolution of the eye tracking task allowed us to draw precise conclusions about the processes of VSTM. Specifically, our results are consistent with those reported by Ross-Sheehy et al. (2011), but by using an eye tracker we know that infants actually fixated the rotating item. If we had used the continuous streams procedure used by Ross-Sheehy et al. and shown that 4-month-old infants failed to prefer streams in which the rotating item changed to streams in which the rotating item did not change, it would be impossible to know whether that failure simply reflected infants’ failure to fixate the rotating item. In the present experiments, we showed that 4-month-old infants failed to show evidence that they detected a change in object color despite the fact they did fixate that item.
The temporal resolution of the eye tracking procedure allowed us to examine how infants’ attention unfolded over the course of the trial. For example, comparison of the time courses for the 4-month-old infants in the two experiments showed that the pattern of fixation was the same both when one item rotated and when both items were stationary. This comparison helps to address the possibility that we disrupted encoding by rotating one item and artificially inducing a fixation. Although rotating an item increased infants’ fixation of that item, infants’ duration of fixation to an item did not differ if they were induced to fixated it (in Experiment 1) or if they spontaneously fixated one of two equally salient items (in Experiment 2).
In summary, these results further our understanding of how overt attention contributes to what information is encoded in VSTM during infancy. First, we demonstrated that a rotating item elicits an overt shift of attention in both younger and older infants, allowing them to prioritize this item. Second, we found age-related changes in what infants encode in VSTM about that overtly attended item. Whereas for older infants, overt attention towards the salient item facilitated their ability to encode identity information about that item in VSTM, such overt attention did not facilitate younger infants’ encoding of identity information. Furthermore, younger infants did not encode information about the overtly attended item, even when both items were approximately equal in their salience.
Supplementary Material
Acknowledgments
This research and preparation of this manuscript were made possible by NIH grant R01EY022525 awarded to LMO. AGB and LMC were supported on NIH training grant T32EY015387. The authors declare no conflicts of interest with regard to the funding source for this study. We thank the students and staff in the Infant Cognition Laboratory at the University of California, Davis, for their help with data collection.
Footnotes
Because an infant might look toward the rotating item for at least 100 ms and yet look longer toward the nonrotating item on that trial, our final data also included a small number (49) of trials in which infants looked longer the stationary item than at the rotating item during the attention capture window. We chose not to exclude these trials for two reasons. First, our primary interest was in assessing whether infants’ overt gaze to the rotating item induced encoding of that item in VSTM. Thus, we predicted that overt gaze would induce infants to encode information about the rotating item in VSTM, regardless of whether they focused their attention solely on the rotating item. Second, as shown in Figure 2, because across all the trials included in this window infants robustly preferred the rotating item during the attention capture analysis window (showing a near ceiling preference for that item across trials), the inclusion of these 49 trials did not strongly impact individual infants’ preference scores for the rotating item.
References
- Awh E, Barton B, & Vogel EK (2007). Visual working memory represents a fixed number of items regardless of complexity. Psychological Science, 18(7), 622–628. [DOI] [PubMed] [Google Scholar]
- Bremner JG, Slater AM, Mason UC, Spring J, & Johnson SP (2013). Trajectory perception and object continuity: effects of shape and color change on 4-month-olds’ perception of object identity. Developmental Psychology, 49(6), 1021–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantrell LM, Kanjlia S, Harrison M, Luck SJ, & Oakes LM (2019). Cues to individuation facilitate 6-month-old infants’ visual short-term memory. In Developmental Psychology (Vol. 55, Issue 5, pp. 905–919). American Psychological Association. 10.1037/dev0000683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catherwood D, Skoien P, Green V, & Holt C (1996). Assessing the primary moments in infant encoding of compound visual stimuli. Infant Behavior & Development, 19(1), 1–11. [Google Scholar]
- Cohen LB (1972). Attention-getting and attention-holding processes of infant visual preferences. Child Development, 43(3), 869–879. [PubMed] [Google Scholar]
- Dink J, & Ferguson B (2015). eyetrackingR: An R Library for Eye-tracking Data Analysis. http://www.eyetracking-r.com/
- Faul F, Erdfelder E, Lang AG, & Buchner A (2007). G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods, 39, 175–191. [DOI] [PubMed] [Google Scholar]
- Feigenson L, & Carey S (2005). On the limits of infants’ quantification of small object arrays. Cognition, 97(3), 295–313. [DOI] [PubMed] [Google Scholar]
- Frick JE, Colombo J, & Saxon TF (1999). Individual and developmental differences in disengagement of fixation in early infancy. Child Development, 70(3), 537–548. [DOI] [PubMed] [Google Scholar]
- Hollingworth A, & Henderson JM (2002). Accurate visual memory for previously attended objects in natural scenes. Journal of Experimental Psychology. Human Perception and Performance, 28(1), 113–136. [Google Scholar]
- Hollingworth A, Richard AM, & Luck SJ (2008). Understanding the function of visual short-term memory: transsaccadic memory, object correspondence, and gaze correction. Journal of Experimental Psychology. General, 137(1), 163–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood BM (1995). Shifts of visual attention in the human infant: A neuroscience approach. In Rovee-Collier C & Lipsitt LP (Eds.), Advances in Infancy Research (Vol. 9, pp. 163–216). Ablex. [Google Scholar]
- Hood BM, & Atkinson J (1993). Disengaging visual attention in the infant and adult. Infant Behavior & Development, 16(4), 405–422. [Google Scholar]
- Hyun J-S, Woodman GF, Vogel EK, Hollingworth A, & Luck SJ (2009). The comparison of visual working memory representations with perceptual inputs. Journal of Experimental Psychology-Human Perception and Performance, 35(4), 1140–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin DE (1991). Information integration across saccadic eye movements. Cognitive Psychology, 23(3), 420–456. [DOI] [PubMed] [Google Scholar]
- Kaldy Z, & Blaser E (2009). How to compare apples and oranges: infants’ object identification tested with equally salient shape, luminance, and color changes. Infancy, 14(2), 222–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaldy Z, & Blaser E (2013). Red to green or fast to slow? Infants’ visual working memory for “just salient differences.” Child Development, 84(6), 1855–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon M-K, Luck SJ, & Oakes LM (2014). Visual short-term memory for complex objects in 6- and 8-month-old infants. Child Development, 85(2), 564–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon M-K, Setoodehnia M, Baek J, Luck SJ, & Oakes LM (2016). The development of visual search in infancy: Attention to faces versus salience. Developmental Psychology, 52(4), 537–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck SJ (2008). Visual Memory. In Luck SJ & Hollingworth A (Eds.), Visual Memory (1st ed., pp. 43–85). Oxford University Press. [Google Scholar]
- Maris E, & Oostenveld R (2007). Nonparametric statistical testing of EEG- and MEG-data. Journal of Neuroscience Methods, 164(1), 177–190. [DOI] [PubMed] [Google Scholar]
- Oakes LM (2012). Advances in eye tracking in infancy research. Infancy: The Official Journal of the International Society on Infant Studies, 17(1), 1–8. [DOI] [PubMed] [Google Scholar]
- Oakes LM, Baumgartner HA, Barrett FS, Messenger IM, & Luck SJ (2013). Developmental changes in visual short-term memory in infancy: Evidence from eye-tracking. Frontiers in Psychology, 4(697), 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakes LM, Baumgartner HA, Kanjlia S, & Luck SJ (2017). An eye tracking investigation of color–location binding in infants’ visual short-term memory. Infancy: The Official Journal of the International Society on Infant Studies, 22(5), 584–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakes LM, Messenger IM, Ross-Sheehy S, & Luck SJ (2009). New evidence for rapid development of color-location binding in infants’ visual short-term memory. Visual Cognition, 17(1–2), 67–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakes LM, Ross-Sheehy S, & Luck SJ (2006). Rapid development of feature binding in visual short-term memory. Psychological Science, 17(9), 781–787. [DOI] [PubMed] [Google Scholar]
- Peltola MJ, Leppänen JM, Palokangas T, & Hietanen JK (2008). Fearful faces modulate looking duration and attention disengagement in 7-month-old infants. Developmental Science, 11(1), 60–68. [DOI] [PubMed] [Google Scholar]
- R Core Team (2018). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/. [Google Scholar]
- Roder BJ, Bushnell EW, & Sasseville AM (2000). Infants’ Preferences for Familiarity and Novelty During the Course of Visual Processing. Infancy: The Official Journal of the International Society on Infant Studies, 1(4), 491–507. [DOI] [PubMed] [Google Scholar]
- Rose SA, Gottfried AW, Melloy-Carminar P, Bridger WH, & Mello-Carmina P (1982). Familiarity and novelty preferences in infant recognition memory: Implications for information processing. Developmental Psychology, 18(5), 704–713. [Google Scholar]
- Ross-Sheehy S, & Eschman B (2019). Assessing visual STM in infants and adults: eye movements and pupil dynamics reflect memory maintenance. Visual Cognition, 27(1), 78–92. [Google Scholar]
- Ross-Sheehy S, Oakes LM, & Luck SJ (2003). The development of visual short-term memory capacity in infants. Child Development, 74(6), 1807–1822. [DOI] [PubMed] [Google Scholar]
- Ross-Sheehy S, Oakes LM, & Luck SJ (2011). Exogenous attention influences visual short-term memory in infants. Developmental Science, 14(3), 490–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouder JN, Speckman PL, Sun D, Morey RD, & Iverson G (2009). Bayesian t tests for accepting and rejecting the null hypothesis. Psychonomic Bulletin & Review, 16(2), 225–237. [DOI] [PubMed] [Google Scholar]
- Shaddy DJ, & Colombo J (2004). Developmental changes in infant attention to dynamic and static stimuli. Infancy: The Official Journal of the International Society on Infant Studies, 5, 355–365. [Google Scholar]
- Tas AC, Luck SJ, & Hollingworth A (2016). The relationship between visual attention and visual working memory encoding: A dissociation between covert and overt orienting. Journal of Experimental Psychology. Human Perception and Performance, 42(8), 1121–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch MJ (1974). Infants’ visual attention to varying degrees of novelty. Child Development, 45(2), 344–350. [PubMed] [Google Scholar]
- Wetherford MJ, & Cohen LB (1973). Developmental changes in infant visual preferences for novelty and familiarity. Child Development, 44(3), 416–424. [PubMed] [Google Scholar]
- Wilcox T (1999). Object individuation: Infants’ use of shape, size, pattern, and color. Cognition, 72(2), 125–166. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


