Abstract
Surgery has been the primary treatment for breast cancer. However, instant postoperative complications, such as sleep disorder and pain, dramatically impair early postoperative quality of recovery, resulting in more extended hospital stays and higher costs. Recent clinical trials indicated that stellate ganglion block (SGB) could prolong sleep time and improve sleep quality in breast cancer survivors. Moreover, during the perioperative period, SGB enhanced the recovery of gastrointestinal functions in patients with laparoscopic colorectal cancer surgery and thoracolumbar spinal surgery. Furthermore, perioperative SGB decreased intraoperative requirements for anesthetics and analgesics in patients with complex regional pain syndrome. However, information is scarce regarding the effects of SGB on postoperative quality recovery in patients with breast cancer surgery. Therefore, we investigated the effects of SGB on the postoperative quality of recovery of patients undergoing breast cancer surgery. Sixty patients who underwent an elective unilateral modified radical mastectomy were randomized into two 30-patient groups that received either an ultrasound-guided right-sided SGB with 6 ml 0.25% ropivacaine (SGB group) or no block (control group). The primary outcome was the quality of postoperative recovery 24 hours after surgery, assessed with a Chinese version of the 40-item Quality of Recovery (QoR-40) questionnaire. Secondary outcomes were intraoperative requirements of propofol and opioids, rest pain at two, four, eight, and 24 hours after surgery, patient satisfaction score, and the incidence of postoperative abdominal distension. At 24 hours after surgery, global QoR-40 scores were higher in the SGB group than in the control group. Besides, in the SGB group, patients needed less propofol, had a lower incidence of postoperative abdominal bloating, and had higher satisfaction scores. Ultrasound-guided SGB could improve the quality of postoperative recovery in patients undergoing breast cancer surgery by less intraoperatively need for propofol and better postoperative recovery of sleep and gastrointestinal function.
1. Introduction
Rapid development of machine learning, practically deep learning, improves the accuracy of breast cancer detection [1], making it possible for breast cancer patients to receive early and effective treatments. Surgery has been the primary treatment for breast cancer [2], and modified radical mastectomy is acknowledged as a standard surgical treatment. However, early postoperative complications, such as pain [3, 4], general discomfort, fatigue, and sleep disturbances [5], significantly impede postoperative recovery, leading to extended hospital stays and high costs. Several clinical trials focused on breast cancer survivors, indicating drug administration, such as melatonin [6] and paroxetine [7], and stellate ganglion block (SGB) [7, 8] alleviated sleep disturbances and improved sleep quality. However, daily drug administration usually lasts several weeks or months, making it difficult for breast cancer patients to follow the prescription strictly. Furthermore, the effects of SGB were generally investigated during the rehabilitation period rather than during the perioperative period.
“Blind,” traditional SGB solely relies on anatomical landmarks, resulting in a high incidence of serious complications, including the unsatisfied level of block and direct puncture damage to the nerve structures. However, ultrasound-guided SGB is easy and safe to perform, improving the block quality and avoiding severe complications due to direct visualization of the needle position and the distribution of local anesthetics [9, 10]. SGB, a well-established anesthesia technique, is commonly used to treat sympathetically related pain in the upper limbs, head, and neck. Besides, ultrasound-guided SGB preceding general anesthesia enhanced the recovery of gastrointestinal functions in patients with laparoscopic colorectal cancer surgery [11] and thoracolumbar spinal surgery [12]. Furthermore, SGB could also relieve postoperative pain and decrease intraoperative requirements for anesthetics and analgesics in patients with complex regional pain syndrome [13].
However, little is known about the effects of ultrasound-guided SGB on the postoperative quality of recovery of patients with breast cancer surgery. Therefore, we planned to investigate the effects of ultrasound-guided SGB on the postoperative quality of recovery in patients undergoing breast cancer surgery. We hypothesized that patients who received an ultrasound-guided SGB before general anesthesia would have better postoperative recovery quality than those who did not.
2. Methods and Materials
This prospective, single-center, randomized controlled trial was conducted at the First Affiliated Hospital of Anhui Medical University in China. This study was approved by the Ethics Committee of the First Affiliated Hospital of Anhui Medical University (protocol number: PJ2020-05-09) and registered with the Chinese Clinical Trial Registry (Register number: chiCTR2000032658) on 5th May 2020, following the Helsinki Declaration and its revisions. Patients provided written informed consent before inclusion.
2.1. Patient Selection
Women with breast cancer who were scheduled for elective, unilateral, modified radical mastectomy were screened and recruited during preoperative assessment. The inclusion criteria were the following factors: aged 18–70 years and class I or II ranking based on the physical status evaluation system of the American Society of Anesthesiologists. The exclusion criteria were the following features: BMI greater than 30 kg m−2, a history of allergy to local anesthetics, infection near the puncture site, patients unable to communicate, and patients with chronic use of opioids.
2.2. Randomization and Masking
A list of randomized sequences was generated by an online random generator and sealed in opaque envelopes by staff not involved in the research. All participants were assigned randomly in a 1 : 1 ratio of a control group (ultrasound scanning only) and a group of patients who received ultrasound-guided SGB with 6 ml 0.25% ropivacaine (Astra Zeneca AB, Sodertalje, Sweden) (Figure 1). All patients, anesthetists, and outcome assessors were blinded to the study group allocation.
Figure 1.
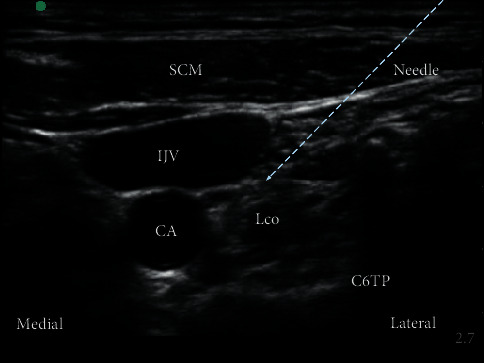
Horizontal ultrasound imaging of the stellate ganglion block. SCM, sternocleidomastoid muscle; IJV, internal jungle vein; CA, carotid artery; Lco, longus colli muscle, C6TP, transverse processes of the sixth cervical vertebra.
2.3. Intervention
Ultrasound-guided SGB was administered before general anesthesia in the preoperative area. Specifically, a right-sided single injection SGB was performed with real-time ultrasound (SonoSite Inc, Bothell, Washington, USA) by an attending anesthesiologist familiar with the ultrasound-guided nerve block. First, the patient was placed in the supine position with slight neck extension under appropriate monitoring. A 5–12 MHz linear transducer (SonoSite Inc, Bothell, Washington, USA) was placed at the sixth cervical vertebra (C6) level. After determining the location of the longus colli muscle, a 22 Gauge needle (KDL Medical Company, Zhejiang, China) tip was advanced posterior to the carotid artery, anterior to the longus colli muscle. Then, 6 ml of 0.25% ropivacaine was injected with the in-plane technique (Figure 1). Ten minutes later, patients were transferred to the operating room to induce general anesthesia. The presence of Horner's syndrome, including the decreased pupil size and ptosis (drooping of the upper eyelid), indicated the effectiveness of SGB.
2.4. Anesthesia Procedure
Standard monitoring and intravenous access were available. Standard monitoring included noninvasive blood pressure measurement, electrocardiography, peripheral pulse oximetry, partial pressure of carbon dioxide at the end of exhalation, temperature measurement, and bispectral index. Before anesthesia, all patients were given 10 mg dexamethasone for nausea prophylaxis. Total intravenous general anesthesia was sequentially induced with 0.1 mg kg−1 midazolam, 2.0 mg kg−1 propofol, and 0.5 μg kg−1 sufentanil. Once the bispectral index value was less than 60, 0.2 mg kg−1 cisatracurium was intravenously administered to facilitate the insertion of a laryngeal airway mask (LMA). During the surgery, anesthesia was maintained with intravenous propofol infusion to maintain the bispectral index value between 40 and 50. Moreover, remifentanil was administered to keep the heart rate and blood pressure within 20% of the baseline. Additional sufentanil (0.2 μg kg−1) and cisatracurium (0.05 mg kg−1) were intravenously administered as needed. After removing LMA, all patients were transported to the postanesthesia care unit (PACU) and then transferred to general wards if the postoperative steward score reached 6.
2.5. Outcome Measurements
The primary outcome was the global score of the quality of recovery, assessed with the Chinese version of the QoR-40 questionnaire. The questionnaire contains 40 questions that evaluate recovery in five aspects: nine items for emotional status, twelve items for physical comfort, seven items for psychological support, five items for physical independence, and seven items for pain. The total score is from 40, representing the poor quality of recovery, to 200, indicating excellent recovery. Each item is rated on a five-point scale: one (none of the time), two (some of the time), three (usually), four (most of the time), and five (all the time).
The secondary outcomes included intraoperative doses of propofol and opioids during general anesthesia, postoperative gastrointestinal function, and patient satisfaction scores measured with a ten-point scale (one = highly dissatisfied, ten = highly satisfied).
Intraoperative propofol and opioid doses, anesthesia time, surgery time, and recovery time were recorded, respectively. One team member who was blind to grouping recorded the scores of the QoR-40 questionnaire before and 24 hours after surgery. The visual analog scale score (zero = no pain and ten = worst pain imaginable) was used to evaluate at-rest patient postoperative pain intensity at discharge from the postanesthesia care unit, two hours, four hours, eight hours, and 24 hours after surgery. Two dimensions, namely, the first time for flatus and the incidence of abdominal distension, were used to evaluate the postoperative recovery of gastrointestinal function.
2.6. Sample Size
Power analysis of the two-tailed testing was based on the primary endpoint of the global QoR-40 score. A 10-point difference in the QoR-40 score after surgery was considered a clinically relevant enhancement in the quality of recovery [14, 15]. In our preliminary study, the global QoR-40 score, mean ± standard deviation (SD), was 160.7 ± 14.5 at 24 hours after the operation. Considering a 10% dropout rate, we enrolled 60 patients. The participant number was calculated using PASS version 15 (NCSS Statistical Software, LLC, Kaysville, Utah, USA) with α = 0.05 and β = 0.2.
2.7. Statistical Analysis
SPSS 21.0 software (SPSS, Chicago, Illinois, USA) was used for statistical analysis. The Shapiro–Wilk test was used to test the normal distribution of data. Parametric variables were reported as mean ± SD and analyzed between the groups using the independent-samples t-test. Nonparametric variables were reported as median (interquartile range (IQR)), and the Mann–Whitney U test was used to compare the two groups. Proportions were analyzed using χ2 analysis or Fisher's exact test as appropriate. All statistical tests were two-sided, and significance was accepted at P < 0.05.
3. Results
From May 2020 to December 2020, we conducted a single-center clinical trial at the First Affiliated Hospital of Anhui Medical University to investigate the effects of SGB on postoperative recovery in female patients with breast cancer surgery. Initially, 77 patients were assessed for study eligibility. Ten patients failed the inclusion criteria, and seven patients declined to enroll. Finally, 60 patients were enrolled and completed the study, with 30 in the ultrasound-guided SGB group and 30 in the control group (Figure 2).
Figure 2.
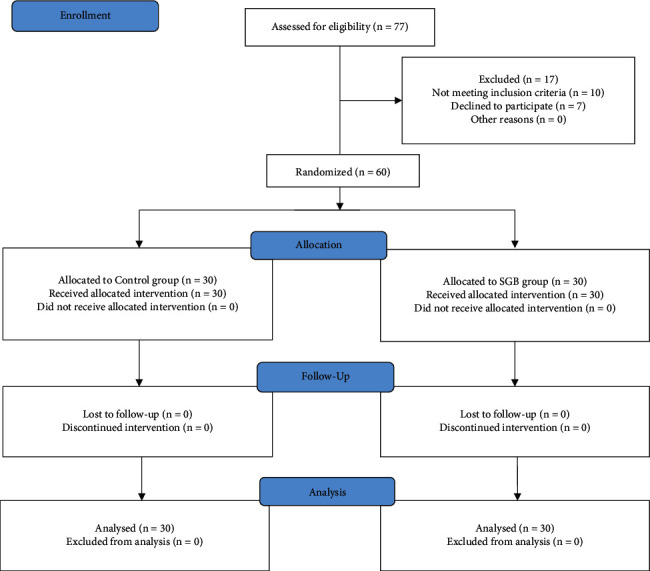
CONSORT flow of clinical procedures for the study. SGB, stellate ganglion block.
Two groups were compared regarding demographics and clinical characteristics (Table 1). Global scores and each item score of the QoR-40 questionnaire before and after surgery are depicted in Table 2. Global QoR-40 scores at 24 hours after surgery were significantly greater in the ultrasound-guided SGB group (170.0 (166.8–173.0)) compared with the control group (160.0 (153.7–164.0)). Among the five dimensions of the QoR-40 questionnaire, only the scores of emotional status and physical comfort were significantly higher in the ultrasound-guided SGB group than in the control group (40.0 (39.0–42.0) vs. 37.0 (35.0–39.0), 49.5 (49.0–52.0) vs. 44.5 (41.0–47.3), respectively).
Table 1.
Patient demographic and operative characteristics.
| Variables | Control group (n = 30) | SGB group (n = 30) | P value |
|---|---|---|---|
| Age (years) | 50.3 ± 6.8 | 51.4 ± 6.5 | 0.501 |
| Height (cm) | 161.2 ± 3.5 | 160.5 ± 4.0 | 0.476 |
| Weight (kg) | 59.5 ± 8.2 | 59.4 ± 5.9 | 0.943 |
| Site of surgery (left/right) | 11/19 | 13/17 | 0.598 |
| ASA I/II | 13/17 | 12/18 | 0.793 |
| Duration of anesthesia (min) | 106.7 ± 12.7 | 103.2 ± 12.6 | 0.271 |
| Duration of surgery (min) | 85.4 ± 13.7 | 82.2 ± 13.6 | 0.370 |
The variables are presented as mean ± SD. SGB, stellate ganglion block; ASA, American Society of Anesthesiologists; SD, standard deviation.
Table 2.
Quality of recovery 40-item scores before and 24 hours after breast cancer surgery.
| Variables | Control group (n = 30) | SGB group (n = 30) | P value |
|---|---|---|---|
| Before the surgery | |||
| Global QoR-40 scores | 184.0 (174.0–189.3) | 184.5 (175.0–191.2) | 0.739 |
|
| |||
| 24 hours after surgery | 160.0 (153.7–164.0) | 170.0 (166.8–173.0) | <0.001 |
|
| |||
| Global QoR-40 scores | |||
| Emotional status | 37.0 (35.0–39.0) | 40.0 (39.0–42.0) | <0.001 |
| Physical comfort | 44.5 (41.0–47.3) | 49.5 (49.0–52.0) | <0.001 |
| Psychological support | 31.0 (29.7–31.3) | 32.0 (30.0–33.1) | 0.268 |
| Physical independence | 18.0 (16.0–19.0) | 19.0 (17.0–20.3) | 0.105 |
| Pain | 29.0 (27.7–31.3) | 29.0 (28.0–30.2) | 0.857 |
The variables are presented as median (IQR). SGB, stellate ganglion block; QoR-40, 40-item quality of recovery questionnaire; IQR, interquartile range.
Intraoperative propofol, opioid consumption, and recovery time are depicted in Table 3. In the ultrasound-guided SGB group, the intraoperative need for propofol was considerably lower (P=0.006), and the recovery time was shorter in the ultrasound-guided SGB group (P < 0.001) as compared with the control group. However, the two groups had no significant differences in sufentanil or remifentanil consumption. Postoperative pain intensity at rest also did not significantly differ between the two groups (Table 4). Furthermore, fewer patients experienced postoperative abdominal bloating (P=0.024), and patient satisfaction scores were higher in the ultrasound-guided SGB group compared with the control group (P=0.016) (Table 5).
Table 3.
Intraoperative propofol, opioid consumption, and recovery time.
| Variables | Control group (n = 30) | SGB group (n = 30) | P value |
|---|---|---|---|
| Propofol consumption (mg) | 592.6 ± 87.8 | 531.8 ± 77.1 | 0.006 |
| Sufentanil consumption (μg) | 37.6 ± 4.2 | 38.1 ± 4.8 | 0.651 |
| Remifentanil consumption (μg) | 705.0 ± 96.1 | 680.3 ± 79.6 | 0.283 |
| Recovery time (min) | 23.1 ± 5.1 | 18.4 ± 3.9 | <0.001 |
The variables are presented as mean ± SD. SGB, stellate ganglion block; SD, standard deviation.
Table 4.
Postoperative visual analog score at rest.
| Time points | Control group (n = 30) | SGB group (n = 30) | P value |
|---|---|---|---|
| PACU discharge | 2.0 (1.0–3.2) | 2.0 (1.0–3.0) | 0.885 |
| 2 hours postoperatively | 2.0 (1.0–3.0) | 2.0 (1.0–3.0) | 0.867 |
| 4 hours postoperatively | 1.0 (0–2.0) | 2.0 (1.0–2.2) | 0.152 |
| 8 hours postoperatively | 1.0 (0.7–2.0) | 1.0 (0–2.0) | 0.542 |
| 24 hours postoperatively | 1.5 (1.0–2.0) | 1.0 (0–2.0) | 0.346 |
The variables are presented as median (IQR). SGB, stellate ganglion block; IQR, interquartile range; PACU, postanesthesia care unit.
Table 5.
Postoperative gastrointestinal function and patient satisfaction score at 24 hours after breast cancer surgery.
| Variables | Control group (n = 30) | SGB group (n = 30) | P value |
|---|---|---|---|
| First-time flatus time (hour) | 8.0 (6.7–9.0) | 7.0 (6.0–9.0) | 0.380 |
| Postoperative abdominal bloating | 13 (43.3%) | 5 (16.7%) | 0.024 |
| Patient satisfaction score | 7.0 (5.7–8.0) | 8.0 (7.0–9.0) | 0.016 |
The variables are presented as median (IQR) or proportions. SGB, stellate ganglion block; IQR, interquartile range.
4. Discussion
This study demonstrated that after receiving a single ultrasound-guided SGB with 6 ml of 0.25% ropivacaine, significant improvements were found in the quality of recovery of patients who had an elective, unilateral, modified radical mastectomy. Specifically, patients in the ultrasound-guided SGB group had higher global QoR-40 questionnaire scores, greater satisfaction levels, and faster recovery of gastrointestinal function up to 24 hours after surgery.
The QoR-40 questionnaire is considered a reliable, multidimensional assessment tool [16]. Myles et al. considered that no less than a ten-point difference strongly indicated a clinically related improvement or deterioration [17]. Later, a 6.3-point difference was considered to represent a clinically associated change in the quality of recovery [18]. In our study, the postoperative global QoR-40 score was higher than ten points in the ultrasound-guided SGB group compared with the control group, which indicated that preoperative ultrasound-guided SGB was beneficial to the postoperative recovery of patients who had breast cancer surgery.
We did not find any complications resulting from SGB, mainly because ultrasound provided the direct monitoring of the needle advancement and the spread of local anesthetics. Left-sided SGB is typically used to manage refractory ventricular arrhythmia [19, 20]. In contrast, right-sided SGB exhibits a more effective antioxidative effect, reducing the catecholamine concentration in blood [21]. Therefore, we performed right-sided SGB in this study.
In our study, we found that patients in the ultrasound-guided SGB group had high emotional status and physical comfort scores, similar to earlier clinical findings. In a randomized controlled clinical trial, Wu et al. reported that SGB improved postoperative sleep by prolonging sleep time and improving sleep efficiency in patients with thoracoscopic surgery [22]. Similarly, Lipov et al. reported that SGB led to lower postoperative Pittsburgh Sleep Quality Index scores [7] and decreased night awakenings [8]. The autonomic nervous system is closely associated with sleep regulation. During different sleep stages, a dynamic balance is maintained between the sympathetic nervous system and the vagal nervous system [23, 24]. Patients with breast cancer have a higher extension of the sympathetic nervous system with a greater risk of experiencing sleep disturbances before and after surgery [25]. Sleep disturbance can negatively impact postoperative recovery [26, 27]. Although possible mechanisms of sleep disturbance are multifactorial, including severe anxiety and depression, immune response disorder, and circadian rhythm disorder [28], preoperative SGB can alleviate sleep disturbances by reducing sympathetic nervous activity, which benefits the postoperative quality of recovery in patients with breast cancer surgery.
In our study, we also found that the intraoperative need for propofol was dramatically decreased, probably due to the sedative effect of SGB. In an experimental study, the rats with SGB showed declined electroencephalogram activities [29], generally known as the depth of anesthesia. Besides, in the clinical trial with healthy volunteers, participants with SGB indicated decreased Observer's Assessment of Alertness/Sedation scores and bispectral index values [30]. The reduced intraoperative need for propofol could partially explain why the recovery time was shorter in patients with SGB. However, our study did not find the analgesic effect of SGB, contradicting the traditional notion that SGB could reduce sympathetic nervous system-related pain. The main reason is that perioperative pain in our study was mainly caused by surgery rather than being involved with high intensity of the sympathetic nervous system.
The postoperative period is associated with the disturbance of gastrointestinal function, such as bowel irritation and abdominal bloating. These problems occur mainly due to the imbalance between the sympathetic nervous system and the vagal nerve system attributed to operation stress, immobilization, and perioperative administration of opioids and narcotics [12]. Gastrointestinal morbidity can cause delayed feeding, anxiety, and sleep disturbance, leading to impaired patient recovery, prolonged hospital stay, and higher healthcare costs [31]. Early return of gastrointestinal function facilitates postoperative recovery of patients under general anesthesia. SGB can inhibit the excitation of the sympathetic nervous system, thereby restoring the balance of the autonomic nervous system and rebuilding the homeostasis of the neuroendocrine-immune system [32]. Furthermore, Zhao et al. reported that SGB relieved symptoms of chronic ulcerative colitis [33]. In our study, we found that patients in the ultrasound-guided SGB group had a lower incidence of abdominal bloating, indicating that SGB promoted the postoperative recovery of gastrointestinal function. Postoperative sleep and gastrointestinal function are governed by the autonomic nervous system and influence each other. Both have effects on the postoperative quality of recovery. Since the SGB inhibited the sympathetic system, the intraoperative stress was reduced, and then sleep and gastrointestinal function improved in the early postoperative period, which partially explained why the patient in the SGB group had a better quality of postoperative recovery.
Our study had some limitations. First, we only investigated the effects of ultrasound-guided SGB on female patients who had breast cancer surgery. Gender bias may influence the results. In addition, we only studied unilateral modified radical mastectomy; therefore, our findings cannot be generalized to other types of breast cancer surgery, like simple mastectomy or breast reconstruction. To design and more fully evaluate the effectiveness of ultrasound-guided SGB, we recommend additional multicenter studies that include larger numbers of participants and men who have other types of breast cancer surgery.
5. Conclusion
Ultrasound-guided SGB is likely to be associated with improved postoperative quality of recovery in patients with breast cancer surgery by less intraoperatively need for propofol and better postoperative recovery of sleep and gastrointestinal function.
Acknowledgments
The authors thank AiMi Academic Services (https://www.aimieditor.com) for the English language editing and review services. This study was supported by the National Natural Science Foundation of China (81970542) to Lijian Chen.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
Xiuli Yang conceptualized the study and wrote, reviewed, and edited the article. Qixing Wu involved in data curation and developed software. Huan Wang administered the project aand wrote, reviewed, and edited the article. Yuwen Zhang and Xiaohui Peng investigated the study and wrote, reviewed, and edited the article. Lijian Chen conceptualized and supervised the study, acquired the fund, and wrote, reviewed, and edited the article.
References
- 1.Gupta M., Mahajan S., Bhardwaj A. K., Pandit A. K. Advances in Intelligent Computing and Communication . Singapore: Springer; 2022. Improvement in breast cancer detection using deep learning. [DOI] [Google Scholar]
- 2.McDonald E. S., Clark A. S., Tchou J., Zhang P., Freedman G. M. Clinical diagnosis and management of breast cancer. Journal of Nuclear Medicine . 2016;57:9S–16S. doi: 10.2967/jnumed.115.157834. [DOI] [PubMed] [Google Scholar]
- 3.Kamiya Y., Hasegawa M., Yoshida T., Takamatsu M., Koyama Y. Impact of pectoral nerve block on postoperative pain and quality of recovery in patients undergoing breast cancer surgery: a randomised controlled trial. European Journal of Anaesthesiology . 2018;35(3):215–223. doi: 10.1097/eja.0000000000000762. [DOI] [PubMed] [Google Scholar]
- 4.Yao Y., Li J., Hu H., Xu T., Chen Y. Ultrasound-guided serratus plane block enhances pain relief and quality of recovery after breast cancer surgery: a randomised controlled trial. European Journal of Anaesthesiology . 2019;36(6):436–441. doi: 10.1097/eja.0000000000001004. [DOI] [PubMed] [Google Scholar]
- 5.Schreier A. M., Johnson L. A., Vohra N. A., Muzaffar M., Kyle B. Post-treatment symptoms of pain, anxiety, sleep disturbance, and fatigue in breast cancer survivors. Pain Management Nursing . 2019;20(2):146–151. doi: 10.1016/j.pmn.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 6.Innominato P. F., Lim A. S., Palesh O., et al. The effect of melatonin on sleep and quality of life in patients with advanced breast cancer. Supportive Care in Cancer . 2016;24(3):1097–1105. doi: 10.1007/s00520-015-2883-6. [DOI] [PubMed] [Google Scholar]
- 7.Rahimzadeh P., Imani F., Nafissi N., Ebrahimi B., Faiz S. H. R. Comparison of the effects of stellate ganglion block and paroxetine on hot flashes and sleep disturbance in breast cancer survivors. Cancer Management and Research . 2018;10:4831–4837. doi: 10.2147/cmar.s173511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lipov E. G., Joshi J. R., Sanders S., et al. Effects of stellate-ganglion block on hot flushes and night awakenings in survivors of breast cancer: a pilot study. The Lancet Oncology . 2008;9(6):523–532. doi: 10.1016/s1470-2045(08)70131-1. [DOI] [PubMed] [Google Scholar]
- 9.Narouze S. Ultrasound-guided stellate ganglion block: safety and efficacy. Current Pain and Headache Reports . 2014;18(6):p. 424. doi: 10.1007/s11916-014-0424-5. [DOI] [PubMed] [Google Scholar]
- 10.Imani F., Hemati K., Rahimzadeh P., Kazemi M. R., Hejazian K. Effectiveness of stellate ganglion block under fuoroscopy or ultrasound guidance in upper extremity CRPS. Journal of Clinical and Diagnostic Research . 2016;10(1):Uc09–12. doi: 10.7860/jcdr/2016/14476.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu G., Kang Z., Chen Y., Zeng J., Su C., Li S. Ultrasound-guided stellate ganglion block alleviates stress responses and promotes recovery of gastrointestinal function in patients. Digestive and Liver Disease . 2021;53(5):581–586. doi: 10.1016/j.dld.2020.11.028. [DOI] [PubMed] [Google Scholar]
- 12.Peng K., Zhang J., Chen W. R., Liu Hy, Ji Fh. Ultrasound-guided stellate ganglion block improves gastrointestinal function after thoracolumbar spinal surgery. Clinical Therapeutics . 2017;39(11):2322–2330. doi: 10.1016/j.clinthera.2017.09.008. [DOI] [PubMed] [Google Scholar]
- 13.Rath G., Rajagopalan V., Chouhan R., Pandia M., Lamsal R., Bithal P. Effect of stellate ganglion block on intraoperative propofol and fentanyl consumption in patients with complex regional pain syndrome undergoing surgical repair of brachial plexus injury: a randomized, double-blind, placebo-controlled trial. Neurology India . 2020;68(3):617–623. doi: 10.4103/0028-3886.288992. [DOI] [PubMed] [Google Scholar]
- 14.Kim M. H., Kim M. S., Lee J. H., Kim S. T., Lee J. R. Intravenously administered lidocaine and magnesium during thyroid surgery in female patients for better quality of recovery after anesthesia. Anesthesia & Analgesia . 2018;127(3):635–641. doi: 10.1213/ane.0000000000002797. [DOI] [PubMed] [Google Scholar]
- 15.Myles P., Hunt J., Fletcher H., Solly R., Woodward D., Kelly S. Relation between quality of recovery in hospital and quality of life at 3 months after cardiac surgery. Anesthesiology . 2001;95(4):862–867. doi: 10.1097/00000542-200110000-00013. [DOI] [PubMed] [Google Scholar]
- 16.Gornall B. F., Myles P. S., Smith C. L., et al. Measurement of quality of recovery using the QoR-40: a quantitative systematic review. British Journal of Anaesthesia . 2013;111(2):161–169. doi: 10.1093/bja/aet014. [DOI] [PubMed] [Google Scholar]
- 17.Myles P. S., Hunt J. O., Nightingale C. E., et al. Development and psychometric testing of a quality of recovery score after general anesthesia and surgery in adults. Anesthesia & Analgesia . 1999;88(1):83–90. doi: 10.1097/00000539-199901000-00016. [DOI] [PubMed] [Google Scholar]
- 18.Myles P. S., Myles D. B., Galagher W., Chew C., MacDonald N., Dennis A. Minimal clinically important difference for three quality of recovery scales. Anesthesiology . 2016;125(1):39–45. doi: 10.1097/aln.0000000000001158. [DOI] [PubMed] [Google Scholar]
- 19.Savastano S., Pugliese L., Baldi E., Dusi V., Tavazzi G., De Ferrari G. M. Percutaneous continuous left stellate ganglion block as an effective bridge to bilateral cardiac sympathetic denervation. EP Europace . 2020;22(4):p. 606. doi: 10.1093/europace/euaa007. [DOI] [PubMed] [Google Scholar]
- 20.Yang S. C., Wu C. C., Hsieh Y. J. Left stellate ganglion block, a rescue treatment for ventricular arrhythmia refractory to radiofrequency catheter ablation: a care-compliant case report. Medicine (Baltimore) . 2019;98(44):p. e17790. doi: 10.1097/md.0000000000017790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wei N., Chi M., Deng L., Wang G. Antioxidation role of different lateral stellate ganglion block in isoproterenol-induced acute myocardial ischemia in rats. Regional Anesthesia and Pain Medicine . 2017;42(5):588–599. doi: 10.1097/aap.0000000000000647. [DOI] [PubMed] [Google Scholar]
- 22.Wu C. N., Wu X. H., Yu D. N., Ma W. H., Shen C. H., Cao Y. A single-dose of stellate ganglion block for the prevention of postoperative dysrhythmias in patients undergoing thoracoscopic surgery for cancer: a randomised controlled double-blind trial. European Journal of Anaesthesiology . 2020;37(4):323–331. doi: 10.1097/eja.0000000000001137. [DOI] [PubMed] [Google Scholar]
- 23.de Zambotti M., Trinder J., Silvani A., Colrain I. M., Baker F. C. Dynamic coupling between the central and autonomic nervous systems during sleep: a review. Neuroscience & Biobehavioral Reviews . 2018;90:84–103. doi: 10.1016/j.neubiorev.2018.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trinder J., Kleiman J., Carrington M., et al. Autonomic activity during human sleep as a function of time and sleep stage. Journal of Sleep Research . 2001;10(4):253–264. doi: 10.1046/j.1365-2869.2001.00263.x. [DOI] [PubMed] [Google Scholar]
- 25.Colagiuri B., Christensen S., Jensen A. B., Price M. A., Butow P. N., Zachariae R. Prevalence and predictors of sleep difficulty in a national cohort of women with primary breast cancer three to four months postsurgery. Journal of Pain and Symptom Management . 2011;42(5):710–720. doi: 10.1016/j.jpainsymman.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 26.Rampes S., Ma K., Divecha Y. A., Alam A., Ma D. Postoperative sleep disorders and their potential impacts on surgical outcomes. J Biomed Res . 2019;34(4):271–280. doi: 10.7555/JBR.33.20190054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Q., Yu C. Negative role of sleep disturbance in the recovery of gastrointestinal postoperative patients. Expert Review of Gastroenterology & Hepatology . 2020;14(4):229–230. doi: 10.1080/17474124.2020.1738925. [DOI] [PubMed] [Google Scholar]
- 28.Luo M., Song B., Zhu J. Sleep disturbances after general anesthesia: current perspectives. Frontiers in Neurology . 2020;11:p. 629. doi: 10.3389/fneur.2020.00629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jeong S., Jeon Y., Yeo J., Baek W. The effects of stellate ganglion block on the electroencephalogram in rats. Journal of Anesthesia . 2014;28(4):601–605. doi: 10.1007/s00540-013-1780-8. [DOI] [PubMed] [Google Scholar]
- 30.Jeon Y., Jeon Y. Effects of stellate ganglion block on sedation as assessed by bispectral index in normal healthy volunteers. Pain Physician . 2015;2;18(2;3):173–178. doi: 10.36076/ppj/2015.18.173. [DOI] [PubMed] [Google Scholar]
- 31.Schulze T., Heidecke C. D. Treatment of postoperative impairment of gastrointestinal motility, cholangitis and pancreatitis. Chirurg . 2015;86(6):540–546. doi: 10.1007/s00104-015-0004-1. [DOI] [PubMed] [Google Scholar]
- 32.Wen B., Wang Y., Zhang C., Fu Z. Effect of stellate ganglion block on postoperative recovery of gastrointestinal function in patients undergoing surgery with general anaesthesia: a meta-analysis. BMC Surgery . 2020;20(1):p. 284. doi: 10.1186/s12893-020-00943-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao H. Y., Yang G. T., Sun N. N., Kong Y., Liu Y. F. Efficacy and safety of stellate ganglion block in chronic ulcerative colitis. World Journal of Gastroenterology . 2017;23(3):533–539. doi: 10.3748/wjg.v23.i3.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon request.


