Abstract
Diabetic cardiomyopathy (DCM) is described as abnormalities of myocardial structure and function in diabetic patients without other well-established cardiovascular factors. Although multiple pathological mechanisms involving in this unique myocardial disorder, mitochondrial dysfunction may play an important role in its development of DCM. Recently, considerable progresses have suggested that mitochondrial biogenesis is a tightly controlled process initiating mitochondrial generation and maintaining mitochondrial function, appears to be associated with DCM. Nonetheless, an outlook on the mechanisms and clinical relevance of dysfunction in mitochondrial biogenesis among patients with DCM is not completely understood. In this review, hence, we will summarize the role of mitochondrial biogenesis dysfunction in the development of DCM, especially the molecular underlying mechanism concerning the signaling pathways beyond the stimulation and inhibition of mitochondrial biogenesis. Additionally, the evaluations and potential therapeutic strategies regarding mitochondrial biogenesis dysfunction in DCM is also presented.
Keywords: Mitochondrial biogenesis, Diabetes, Cardiomyopathy, PGC-1α, Diabetic cardiomyopathy
INTRODUCTION
Diabetes mellitus (DM) is a global public health concern with an overall prevalence of 9.4% (435 million adults) in 2015. China ranks number one with an estimate of 10.9% (109.6 million) adults with DM (GBD 2015 Disease and Injury Incidence and Prevalence Collaborators, 2016). Notably, it has been well recognized that DM is one of the major risk factors for cardiovascular diseases (CVD). Compared to non-diabetic populations, the risk of heart failure (HF) in diabetes increased 5-fold in women and 2.4-fold in men (Kannel and McGee, 1979), even after adjusting for well-established risk factors such as age, blood pressure, weight, and cholesterol (Kannel et al., 1974; Riehle and Bauersachs, 2018).
Diabetic cardiomyopathy (DCM) is a DM-induced abnormalities in myocardial structure and function without coronary artery disease (CAD), valvular disease, hypertension, or other potential etiologies (Lam, 2015). DCM occurs in almost 12% of diabetic patients and affects approximately 22% of subjects over 64 years old (Lorenzo-Almorós et al., 2017). There are four stages of DCM: being at risk of HF (Stage A), asymptomatic with impaired cardiac diastolic function without systolic dysfunction, a condition termed HF with preserved ejection fraction (HFpEF, EF>50%, Stage B). HF with clinical symptoms (Stage C), and refractory HF (Stage D) with reduced ejection fraction, an end-stage condition without specific therapeutic options (Lam, 2015).
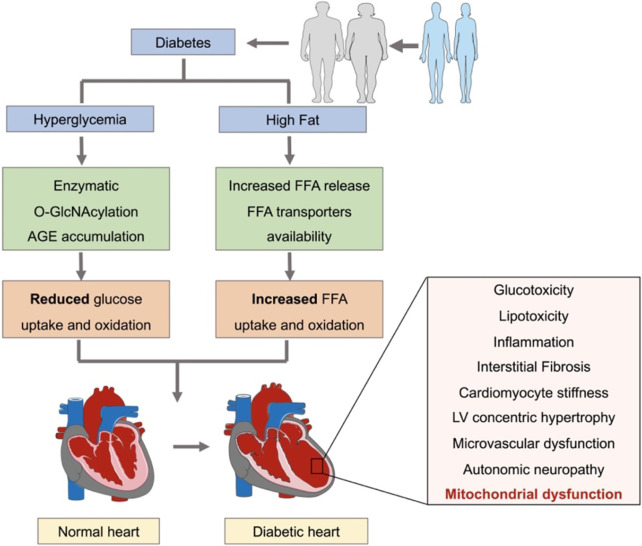
Multiple pathological mechanisms underlying cardiac dysfunction in diabetic patients have been reviewed (Murtaza et al., 2019; Parim et al., 2019; Peterson and Gropler, 2020). They include exposure of the diabetic hearts to both hyperglycemia and high fat levels (Jia et al., 2018b). Hyperglycemia can induce enzymatic O-GlcNAcylation and advanced glycation end-product (AGE) formation, leading to reduced glucose uptake, glycolysis, and glucose oxidation. Besides, exposure to high lipid levels may result in increased free fatty acids (FFAs) release and elevated capacity of myocyte sarcolemma FFA transporters, finally leading to increased FFA uptake and oxidation (Paolillo et al., 2019; Lu et al., 2020). These metabolic remodeling may eventually contribute to cardiomyocyte stiffness, oxidative stress, interstitial fibrosis, cardiomyocyte hypertrophy, inflammation, autonomic neuropathy, microvascular disorder, and mitochondrial dysfunction (Fig. 1) (Knapp et al., 2019; Paolillo et al., 2019). Considerable progresses have been made concerning mitochondrial dysfunction as a characteristic of the diabetic heart (Liang and Kobayashi, 2016). Thus, how to maintain a pool of healthy mitochondria to support energy demand in diabetic patients may be regarded as novel therapeutics. Of note, mitochondrial homeostasis is maintained through tightly control of mitochondrial biogenesis that generates new mitochondrial to replenish the mitochondrial pool (Popov, 2020). Therefore, in this review, we will discuss recent literature shedding light on the functional roles of mitochondrial biogenesis in DCM and explore their potentials for targeted therapeutic manipulations.
Fig. 1.
Cardiac metabolic remodeling in response to diabetes. Diabetic hearts are suffered from both hyperglycemia and high fat levels, leading to reduced glucose uptake and oxidation, but increased FFA uptake and oxidation. These metabolic remodeling eventually contribute to cardiac structural, electrical, and functional disorders. FFA, free fatty acid.
MITOCHONDRIA AND ITS NORMAL FUNCTION IN MYOCYTES
Mitochondria are double-membrane organelles that construct highly dynamic and multifunctional networks (Balaban,1990). The predominant physiological function of mitochondria is the generation of adenosine triphosphate (ATP) by oxidative phosphorylation. Additionally, other functions include generation and detoxification of reactive oxygen species (ROS), involvement in cell death and survival, regulation of cytoplasmic and mitochondrial matrix calcium, production and catabolism of metabolites, and transportation of the organelles themselves to suitable locations within the cell. Any abnormalities of these processes can be defined as mitochondrial dysfunction (Golpich et al., 2017). In fact, mitochondria construct a highly dynamic intracellular network modifying their morphology and content in response to external and internal stimuli.
The homeostasis of mitochondria depends on the co-ordination between two opposite processes, including new mitochondrial generation and damaged mitochondrial removal (Ploumi et al., 2017). Instead of de novo generation, new mitochondria arise from pre-existing ones through a multi-step process involving mitochondrial fusion and fission. Consequently, mitochondrial biogenesis controls replication of mitochondrial DNA (mtDNA) and transcription and translation of mtDNA-related genes (Taherzadeh-Fard et al., 2011; Cameron et al., 2016), as well as synthesis, import, and assembly of mitochondrial proteins encoded by nuclear DNA (nDNA) (Bruggisser et al., 2017). Moreover, a perturbed mitochondrial function may induce cell apoptosis and death, with autophagy being the most predominant process (Vafai and Mootha, 2012).
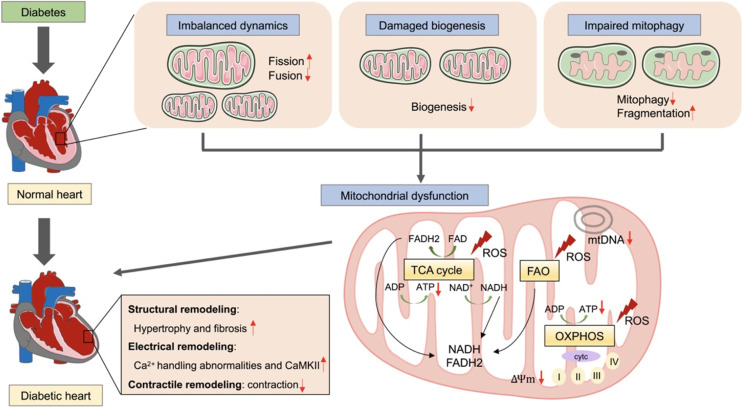
Maintaining mitochondrial integrity and function is critical for cellular physiologies particularly in the heart, which has high energy demands. Normal cardiac contractile function depends on mitochondrial oxidative phosphorylation to generate ATP, 60% of which from FFA oxidation and 40% originating from other fuel substrates including glucose, lactate, ketone bodies, and amino acids (De Jong and Lopaschuk, 2017). In contrast, normal cardiac diastolic function mainly depends on glucose oxidation (Sun et al., 2016). In diabetic heart, mitochondrial stress result in ultrastructural abnormalities, bioenergetic deficiency, impaired mitochondrial biogenesis and imbalanced mitophagy. Myocardium energy consumption shifts from FFA oxidation toward ketone body utilization, a characteristic of the failing heart (Cook et al., 2017). Conversely, mitochondrial dysfunction in diabetic heart may be explained by reduced ATP production and ROS damage, leading to cardiac structural, electrical, and functional disorder (Fig. 2) (Jia et al., 2018a; Berthiaume et al., 2019; Dillmann, 2019). Because disruption of mitochondrial quality control is a predominant mechanism implicated in decreased ATP generation and elevated ROS damage in CVD, therapeutics aimed at maintaining and/or restoring mitochondrial quality and related cellular processes are of significant importance (Li et al., 2021a; Ji et al., 2022). Specifically, pharmacological control of mitochondrial biogenesis is a promising strategy for a wide range of acute and chronic heart diseases characterized by mitochondrial dysfunction.
Fig. 2.
The role of mitochondrial dysfunction in the development of diabetic cardiomyopathy. In diabetic heart, mitochondria are suffered from imbalanced dynamics, damaged biogenesis, and impaired mitophagy. Conversely, mitochondrial dysfunction may be explained by reduced ATP production and ROS damage, leading to cardiac structural, electrical, and functional disorder. ADP, adenosine-diphosphate; ATP, adenosine-triphosphate; TCA, tricarboxylic acid cycle; FAO, Fatty Acid Oxidation; OXPHOS, oxidative phosphorylation; ROS, reactive oxygen species; mtDNA, mitochondrial DNA.
MITOCHONDRIAL BIOGENESIS AND DIABETIC CARDIOMYOPATHY
Mitochondrial biogenesis
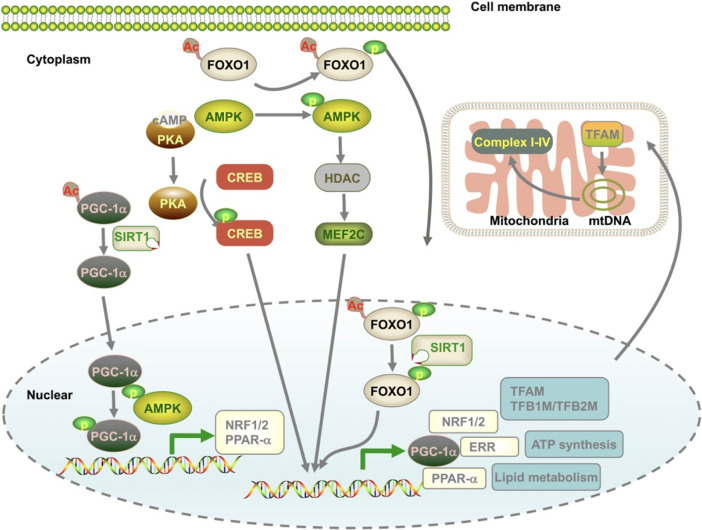
Mitochondrial biogenesis is orchestrated by the growth and division of pre-existing organelles and requires co-ordination between both mitochondrial and nuclear genomes. Interestingly, the two genomes are synthesized by the transcriptional coregulator peroxisome proliferator-activated receptor γ (PPARγ) coactivator 1α (PGC-1α), a master regulator of mitochondrial biogenesis and maturation. PGC-1α expression is regulated by activation of transcription factors acting on mitochondrial genes including myocyte enhancer factor 2 (MEF2) (Moore et al., 2003), forehead box class-O3 (FoxO3) (Nakae et al., 2008), the silent information regulator 1 (SIRT1, Thirupathi and de Souza, 2017), and cAMP response element–binding protein (CREB) (Herzig et al., 2001), and several other signaling inducers including AMPK, AKT-eNOs, calmodulin-dependent protein kinase IV (CaMK IV), calcineurin A, and protein kinase A (Angus et al., 2005; Gleyzer and Scarpulla, 2016; Yao et al., 2016; Popov, 2020). PGC-1α is also regulated by multi-posttranslational modifications including acetylation, methylation, phosphorylation, ubiquitination, and O-linked N-acetylglucosylation (Fig. 3) (Fernandez-Marcos and Auwerx, 2011). For the downstream signaling pathways, PGC-1α binds to a series of nuclear transcription factors, including nuclear respiratory factor 1/2 (NRF1/2), estrogen-related receptor α (ERR-α), and PPARs (Biswas and Chan, 2010; Bruni et al., 2010; Satoh et al., 2013; Yang et al., 2014). Stimulation of NRF1/2 activates the downstream factors involved in mtDNA transcription and replication [(transcription factor A mitochondrial (TFAM)], transcription factor B1 mitochondrial (TFB1M), and transcription factor B2 mitochondrial (TFB2M) (Biswas and Chan, 2010; Satoh et al., 2013; Yang et al., 2014), respiratory chain [cytochrome C oxidase subunit IV (COXIV) and cytochrome c (Bruni et al., 2010)], and the mitochondrial protein import machinery (translocase of outer mitochondrial membrane 34kDa subunit, TOMM34) (Blesa et al., 2008). For ERR-α, in cooperation with PGC-1α, ERR-α regulates mitochondrial energy transduction and adenosine-triphosphate (ATP) synthesis, including fatty acid oxidation (FAO), tricarboxylic acid (TCA) cycle, and the electron transport chain/oxidative phosphorylation (ETC/OXPHOS) (Sakamoto et al., 2020). The PPARs are PGC-1α coactivators and can serve as critical regulators in the biogenesis of mitochondrial FAO and other cellular lipid metabolic pathways (Zhou et al., 2018).
Fig. 3.
Mechanisms that contribute to mitochondrial biogenesis. Mitochondrial biogenesis is a well-controlled process and is coupled with a complex transcriptional network involving mitochondrial DNA and nuclear DNA. PGC-1α is a master regulator and coordinates with several different pathways to meet the metabolic demand of the cells under various conditions. mtDNA, mitochondrial DNA.
Changes of mitochondrial biogenesis in diabetic cardiomyopathy
Cardiac mitochondrial biogenesis is a dynamic process. During physiological conditions, stimulation or inhibition of mitochondrial biogenesis induced by either up- or down- regulation of transcriptional factors are exhibited according to different energy demands. While during pathological conditions, there are two sides regarding the disturbances of mitochondrial biogenesis: i) an impairment condition in which stimulation of mitochondrial biogenesis is required; ii) the abnormal worsen that a removal is necessary. In OVE26 mouse model of type 1 diabetic (T1DM) heart, impaired mitochondrial function and increased oxidative stress were observed as evidenced by increased mitochondrial area and reduced respiratory control ratio. However, these damaged mitochondria might be due to enhanced mitochondrial biogenesis exhibited as elevated mtDNA and mRNA levels for TFAM, cytochrome b, and cytochrome c in T1DM (Shen et al., 2004). Alternatively, in streptozotocin (STZ)-induced T1DM heart mouse model, mitochondrial biogenesis was significantly impaired showed as dysregulated mitochondrial structure, reduced mtDNA, and decreased biogenesis-related mRNAs level (Tao et al., 2020). In ob/ob mouse model of type 2 diabetic (T2DM) heart, significant mitochondrial disorders, reduced mtDNA and impaired mitochondrial biogenesis were identified in cardiomyocytes (Yan et al., 2013). Nevertheless, in fat-enriched regimen and STZ injection-induced T2DM mice model, cardiac contractile dysfunction was associated with decreased mitochondrial oxygen consumption, as well as reduced ATP production, and despite enhanced mitochondrial biogenesis signaling (Marciniak et al., 2014). In patients with T2DM, mitochondrial biogenesis appeared to be decreased in skeletal and heart samples, as shown by reduced mtDNA content and mitochondrial volume (Karamanlidis et al., 2010). The inconsistencies between mitochondrial biogenesis and mitochondrial function may be possibly explained by the evidence that diabetes impaired mitochondrial degradation and accumulation of damaged mitochondria. Furthermore, changes of mitochondrial number in diabetic hearts may affect the rates of mitochondrial fission and/or fusion (Liang and Kobayashi, 2016). Additionally, although up-regulation of related transcriptional factors is a key player in mitochondrial biogenesis, unflavored genes may be equally activated, resulting in detrimental effects depending on ligands specificity (Cameron et al., 2016). Finally, energy demands of different cell types in diabetic hearts may also be one of the reasons. However, the extent and duration of energy demand in different cell types in diabetic hearts are uncertain till now. Hence, further experiments are needed to differentiate these possibilities.
Stimulators and inhibitors of mitochondrial biogenesis in DCM
In diabetic conditions, several stimulators or inhibitors of cardiac mitochondrial biogenesis have been identified within last decades. Examples of drugs or hormone substances are presented as follows (Table 1).
Table 1.
Stimulators and inhibitors of mitochondrial biogenesis in animal DCM models
| Types | DCM models | Mechanisms | References | |
|---|---|---|---|---|
| Stimulators | ||||
| Melatonin | T1DM | STZ injection mice | SIRT3/Mst1 | Yu et al., 2021 |
| T2DM | High-fat diet and STZ injection rats | SIRT6, AMPK-PGC-1α-AKT | Zhang et al., 2017 | |
| Resveratrol | T2DM | High-fat diet and STZ injection rats | SIRT1-PGC-1α deacetylation | Fang et al., 2018 |
| SIRT1 deletion mice | SIRT1-PGC-1α deacetylation | Ma et al., 2017 | ||
| Salidroside | T2DM | High-fat and STZ-injection mice | SIRT3, AMPK/AKT/PGC-1α-TFAM | Li et al., 2021b |
| Pterostillbene | T1DM | High-glucose diet rats | AMPK/NRF2/HO-1/PGC-1α | Kosuru et al., 2018 |
| miR-144 | T1DM | STZ-injection mice | Rac-1/AMPK/PGC-1α | Tao et al., 2020 |
| BH4 | T2DM | OLETF rats | CaMKK2-AMPK/CAMP/CREB/PGC-1α | Kim et al., 2020 |
| Exercise | ||||
| Resistance | T2DM | OLETF rats | UCP2/UCP3 | Ko et al., 2018 |
| Moderate | T2DM | Db/db mice | CXC3 | Veeranki et al., 2016 |
| Ensurance | T2DM | Db/db mice | PGC-1α-AKT | Wang et al., 2015 |
| Inhibitors | ||||
| ADMA | T2DM | High-fat and STZ injection rats | PGC-1α phosphorylation and acetylation | Xiong et al., 2020 |
| T1DM | STZ injection rats | NO/PGC-1α | Xiong et al., 2021 | |
T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus; STZ, streptozotocin; OLETF, Otsuka long-evans Tokushima fatty; BH4, tetrahydrobiopterin; ADMA, asymmetric dimethylarginine.
Melatonin, a hormone known for its antioxidant capacity, that protected against myocardial remodeling by preserving mitochondrial biogenesis in both type 1 and type 2 DCM model. A study indicated that melatonin could improve mitochondrial function through SIRT6 mediated AMPK-PGC-1α-AKT axis in high-fat diet and STZ injection-induced rat model (Yu et al., 2021) or mammalian Ste20-like kinase 1 (Mst1)/SIRT3 signaling in STZ injection-induced mice model (Zhang et al., 2017). Another study showed that resveratrol, a SIRT1 activator, could increase the affinity of SIRT1 for both nicotinamide adenine dinucleotide (NAD+) and the acetylated substrate. Resveratrol treatment significantly alleviated cardiac hypertrophy, improved mitochondrial biogenesis, and decreased cardiomyocyte apoptosis in DCM models induced by SIRT1 deletion. Resveratrol further enhanced PGC-1α deacetylation and activated downstream targets (Ma et al., 2017; Fang et al., 2018).
Some other natural extracts were also assumed to regulate mitochondrial biogenesis. For instance, Li et al. (2021b) have reported that salidroside can protect against diabetic cardiac systolic function, improve insulin resistance, and rescue impaired mitochondrial biogenesis through SIRT3, AMPK/Akt, and PGC-1α/TFAM signaling in high fat and STZ injection-induced mice models. Additionally, pterostilbene, an antioxidant in blueberries, was also reported to rescue myocardial inflammation, oxidative stress, and mitochondrial biogenesis in high glucose rat models through AMPK/NRF2/HO-1/PGC-1α signaling (Kosuru et al., 2018).
Notably, it has been demonstrated that chemical compounds, including miR-144 agonists, could improve mitochondrial biogenesis and suppress cardiomyocyte apoptosis via targeting Rac-1 in STZ-challenged heart samples. This study also found that decreased Rac-1 levels further induced AMPK phosphorylation and PGC-1α deacetylation (Tao et al., 2020). Another compound was tetrahydrobiopterin (BH4), prolonged BH4 supplementation could bind to CAMKK2 and activate AMPK/CaMK IV/CREB/PGC-1α to rescue cardiac dysfunction, improve mitochondrial biogenesis, and correct morphological abnormalities of cardiac muscle in Otsuka long-evans tokushima fatty (OLETF) rat models (Kim et al., 2020).
Interestingly, previous studies found that regular exercise conferred a multitude of cardiovascular benefits during diabetes. Resistance exercise, defined as repeatedly climbing a 1m grid ladder inclined at 85°, 20 times during each session, lasting 5 days/week for 12 weeks, was effective at maintaining diabetic cardiac contractility and function by enhancing mitochondrial biogenesis in OLETF rat models, which were accompanied by elevated superoxide dismutase 2 and reduced uncoupling protein (UCP) 2 and UCP3 levels (Ko et al., 2018). Moderate-intensity exercise covering a daily distance of 330 meters at a speed of 10 m/min for 2 weeks and at a speed of 11 m/min for the rest of the 3 weeks for 5 days/week, could prevent db/db mice cardiac contractile functional deficiencies by improving mitochondrial biogenesis, sustaining trans-membrane potential and reducing excessive mitochondrial fission through the restoration of connexin 43 signaling (Veeranki et al., 2016). Moreover, continuous exercise (1 h running/day at a speed of 10 m/min for 15 weeks) also preserved cardiac systolic function and enhanced mitochondrial biogenesis in the late stage of DCM in a db/db mouse model by activation of PGC-1α and Akt signaling (Wang et al., 2015). These results indicated that exercise with different intensities or durations might affect cardiac mitochondrial biogenesis to varying degrees. More recently, a large sample, community-dwelling study confirmed that an average of 12 min acute exercise elicited widespread metabolic changes central to cardiovascular disease and cardiometabolic health (Nayor et al., 2020). However, whether acute exercise affect cardiac metabolic remodeling by regulating mitochondrial biogenesis is still uncertain, and the longevity of exercise-mediated cardiac benefits in diabetes needs to be further explored.
Previous data have suggested inhibitors of mitochondrial biogenesis can down-regulate the expression level of associated-transcriptional factors or reduce signaling pathways such as AMPK. A study indicated that asymmetric dimethylarginine (ADMA), a nitric oxide (NO) synthase inhibitor, could inhibit myocardial mitochondrial biogenesis in a T2DM rat model induced by high-fat feeding plus STZ injection. The underlying mechanisms might be related to decreased PGC-1α promoter activity and activated PGC-1α protein phosphorylation and acetylation (Xiong et al., 2020). Moreover, ADMA accumulation was also associated with cardiac and mitochondrial dysfunction in parallel with decreased NO content and PGC-1α level in a T1DM induced by STZ rat model (Xiong et al., 2021).
Therefore, as mitochondrial biogenesis is a dynamically controlled process where mitochondrial quality, activity, and maintenance are constantly adapted to the cell’s bioenergetic needs, and the role of the two opposite states (impairment or enhancement) of mitochondrial biogenesis in cardio-protection can give insights into novel therapeutics for DCM.
MITOCHONDRIAL BIOGENESIS AND CLINICAL RELEVANCE IN DIABETIC CARDIOMYOPATHY
Clinical evaluation of mitochondrial biogenesis
Mitochondrial biogenesis plays an essential role in DCM in both in vitro and in vivo, but its potential clinical relevance in DCM has not been fully elucidated. mtDNA synthesis reflects cell energy demands (Malik and Czajka, 2013; Melser et al., 2015) and can be regarded as an early biomarker of mitochondrial biogenesis. In DCM, both mtDNA mutation accumulation and mtDNA copy number (mtDNAcn) disturbance can be used to explain the impaired mtDNA synthesis.
mtDNA mutations are a common cause of mitochondrial dysfunction. They are often present in only a fraction of mtDNA copies and act recessively to induce several diseases, including diabetic cardiac disorders. Compared to ordinary diabetic patients, patients with mtDNA mutation at base pair 3243 had more severe cardiac autonomic nervous dysfunction with sympathovagal imbalance as investigated by heart rate variability. Diabetic patients with mtDNA mutation exhibited smaller standard deviation of all R-R intervals (SDNN) index and smaller total and low-frequency spectra (Momiyama et al., 2002). In addition to heart rate, diabetic patients with tRNA (Leu (UUR)) gene mutation also showed cardiac structural and functional impairments as evidenced by a significantly thicker interventricular septum and lower fractional shortening than those without mutations (Ueno and Shiotani, 1999). Furthermore, variants at 16189 of mtDNA mutation were more likely to develop in diabetic patients with left ventricle hypertrophy (LVH) than that in diabetic patients without LVH (Momiyama et al., 2003).
In addition to mtDNA mutation, mtDNAcn disturbance has been also suggested to be a potential biomarker for cardiovascular disease. In the blood, mtDNAcn was significantly reduced in patients with CAD and was associated with metabolic risk factors, including hypertension and glomerular filtration rate (GFR) (Bordoni et al., 2021). In plasma, cell-free mtDNA was elevated in patients with CAD and DM compared to that in those without DM or healthy controls. Furthermore, mtDNA levels were positively correlated with fasting blood glucose levels in patients with CAD and DM (Liu et al., 2016). Interestingly, recent evidence demonstrated that blood also contained circulating cell-free respiratory competent mitochondria (Al Amir Dache et al., 2020), supporting the novel interest towards mtDNA measurement as an easy-accessible biomarker in CVD.
However, there are some limitations of current studies. First, only a small number of studies have focused on the relationship between circulating mtDNA and DCM in human beings. More large-scale clinical studies are warranted to infer the direct role of mtDNA in DCM. Second, the precise mechanism underlying the link between mtDNA damage and DCM is poorly elucidated. It is well recognized that mtDNA is a major target of ROS (Quan et al., 2020). In diabetic conditions, hyperglycemia and insulin insistence increases oxygen metabolism at complexes I and III, leading to mitochondrial inner membrane hyperpolarization and excessive ROS production (Jia et al., 2018a). Thus, we hypothesized that oxidative stress might be a causal factor in mtDNA damage in DCM, which ultimately resulted in mitochondrial dysfunction. Further research on mtDNA, ROS, DCM, and other implicated mechanisms is required. Third, although a positive link between the number of mitochondria and mtDNAcn has been confirmed (Lee and Wei, 2000), mtDNAcn is not always correlated with mitochondrial gene expression. Thus, mtDNAcn cannot be used as a reliable biomarker of mitochondrial abundance (Cayci et al., 2012; Qiu et al., 2013). Future mechanistic studies are necessary to clarify the real role of mtDNA in DCM.
Glucose-lowering drugs and mitochondrial biogenesis
Various pharmacological and genetic preclinical models of DM have documented the detrimental effects of DM on the myocardium, which extend well beyond hyperglycemia (Murtaza et al., 2019). Veteran Affairs Diabetes Trial (VADT) clinical trials confirmed that there was no evidence of a legacy effect or mortality benefit with intensive glucose control in patients with T2DM (Reaven et al., 2019). Therefore, recent clinical and experimental studies have described the evolving landscape of some glucose-lowering drugs with cardio-protective properties such as sodium-glucose co-transporter-2 inhibitors (SGLT2is), glucagon-like peptide-1 receptor agonists (GLP-1RAs), and dipeptidyl peptidase-4 inhibitors (DPP-4is). These cardio-protective effects may be achieved by “pleiotropic actions” that go beyond glucose control. Currently, emerging evidence showed that one of the “pleiotropic actions” may consist of maintaining mitochondrial biogenesis (Table 2).
Table 2.
Glucose-lowering drugs and mitochondrial biogenesis
| Drugs | Types | Diabetic models | Mechanisms | References |
|---|---|---|---|---|
| Empagliflozin | T2DM | High-fat and STZ injection rats | PGC-1α-NRF1-TFAM | Habibi et al., 2017 |
| Alogliptin | T1DM | Alloxan-induced rabbits | PGC-1α-NRF1-TFAM | Zhang et al., 2018 |
| Metformin | T1DM | High glucose-induced cardiomyocytes | PGC-1α-NRF1-TFAM | Packer, 2020 |
| Pioglitazone | T1DM | Alloxan-induced mice | PGC-1α | Zhang et al., 2021 |
T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus; STZ, streptozotocin.
Sodium-glucose co-transporter-2 inhibitor: SGLT2is are novel glucose-lowering drugs that act via specific renal action by inducing glucosuria, independently of insulin (Li and Zhou, 2020). Large clinical studies such as Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients-Removing Excess Glucose (EMPA-REG OUTCOME) (Zinman et al., 2014), Canagliflozin Cardiovascular Assessment Study (CANVAS) (Neal et al., 2017), and Dapagliflozin Effect on CardiovasculAR Events (DECLARE-TIMI 58) (Wiviott et al., 2019) have confirmed the protective effects of taking SGLT2is in reducing CV mortality and hospitalization for heart failure in T2DM patients. Recent evidence indicated that SGLT2is might perform a cardio-protective function by regulating mitochondrial function in diabetic models (Peng et al., 2020; Yurista et al., 2020). In eleven-week-old female db/db mice, empagliflozin was shown to normalize mitochondrial ultrastructural anomalies, including the disorganized appearance of sarcomeres, decreased matrix electron density, loss and fusion of cristae, and increased mitochondrial fragmentation (Habibi et al., 2017). In high-fat diet-induced obese insulin-resistant rats, administration of dapagliflozin for 4 weeks before cardiac ischemic/reperfusion injury attenuated mitochondrial ROS production, swelling, and depolarization. Furthermore, data also suggested that dapagliflozin also improved mitochondrial ultrastructure by reducing mitochondrial fragmentation and cristae loss (Tanajak et al., 2018). In STZ-induced diabetic rats on a high-fat diet, empagliflozin was demonstrated to ameliorate the atrial structure and electrical remodeling through increasing mitochondrial respiratory function and mitochondrial biogenesis. Consequently, empagliflozin preventd the inducibility of atrial fibrillation (AF) by activating the PGC-1α-NRF1-TFAM signaling pathway (Shao et al., 2019), which played a crucial role in mitochondrial biogenesis. However, the exact roles of mitochondrial biogenesis in the occurrence and progression of ischemic cardiomyopathy or AF in diabetic patients remain unclear. It is still uncertain whether these benefits are from direct cardiac or systemic effects or if they are drug-specific effects of empagliflozin or a class effect of SGLT2is, all of which need further exploration.
Dipeptidyl peptidase-4 inhibitors and glucagon-like peptide-1 receptor agonists (GLP-1RAs): As well known, DPP-4is inhibit the enzyme degrading 2 gut-derived incretin hormones, GLP-1, and glucose-dependent-insulinotropic polypeptide (Scheen, 2018). Functionally, DPP-4i can stimulate insulin section and repress glucagon section in a glucose-dependent manner, contributing to glucose-lowering effects. Nauck et al. (2017) found that in diabetic myocardium DPP-4is had protective effects against cardiovascular (CV) risk factors, including body weight, blood pressure, postprandial lipemia, inflammation, and oxidative stress. Mitochondrial biogenesis has been reported to be involved in the cardioprotective actions of DPP-4i. In alloxan-induced diabetic rabbits, DPP-4i alogliptin alleviated cardiac ventricular hypertrophy, diastolic dysfunction, and interstitial fibrosis, which were associated with increased mitochondrial biogenesis via PGC-1α/NRF1/TFAM signaling pathway and improved mitochondrial function as evidenced by decreased mitochondrial ROS production, sustained mitochondrial membrane depolarization, and elevated mitochondrial swelling (Zhang et al., 2018).
GLP-1 is classically viewed as the primary substrate of DPP-4 and capable of modulating CV function. In a myocardial infarction mouse model study, DPP-4i (MK-0626) and GLP-1A (Exendin-4) were found to exert favorable effects to preserve mitochondrial quality and biogenesis in skeletal muscle, and both were inhibited by GLP-1 antagonist [Exendin-(9-39)], indicating that DPP-4i/GLP-1 receptor signaling could be a potential treatment target for HF patients with exercise intolerance via mitochondrial biogenesis (Takada et al., 2016). Furthermore, GLP-1RAs could reduce established CV risk factors in T2DM, such as hyperglycemia, obesity, dysfunctional lipid profile, and high blood pressure (Marso et al., 2016; Kristensen et al., 2019; Verma et al., 2020). However, whether GLP-1RAs’ potential cardioprotective effects are derived from mitochondrial biogenesis is an area of growing interest.
Other glucose-lowering drugs: In addition to SGLT2is, GLP-1Ras, and DPP-4is, other antihyperglycemic drugs also act on mitochondrial biogenesis, which we describe briefly below. Metformin is a widely prescribed antihyperglycemic drug. In diabetic hearts, metformin promoted mitochondrial autophagy and ameliorates cardiomyocyte dysfunction, whose actions can either be dependent or independent of AMPK (Packer, 2020). Furthermore, in high glucose-induced cardiomyocytes, metformin also stimulated mitochondrial biogenesis as evidenced by increased expression of mitochondrial biogenesis-related transcription factors (PGC-1α, NRF1, and TFAM) (Liu et al., 2020), but the detailed mechanisms of metformin regulating mitochondrial function in DCM remained unclear. PPARγ agonist, pioglitazone, mimicked the effect of PGC-1α, prevented atrial structural remodeling, and lowered the incidence of inducible AF, which were associated with improved mitochondrial structure and elevated mitochondrial biogenesis in diabetic mice induced by alloxan monohydrate. (Zhang et al., 2021).
CONCLUSIONS AND PERSPECTIVES
In conclusion, mounting evidence shows that the dysfunction of mitochondrial biogenesis is a major contributor to the development of DCM. However, it is necessary to determine whether changes in mitochondrial biogenesis are maladaptive or adaptive by using gain- and loss-of-function experiments to target mitochondrial biogenesis for therapeutics in DCM successfully. Furthermore, as mitochondrial homeostasis is controlled by several mechanisms, including mitochondrial dynamics, mitophagy, and biogenesis, identifying relative contributions, molecular underpinnings, and coordination between different processes is crucial. Animal models indicate that regulating mitochondrial biogenesis of the human diabetic hearts may be appropriate targets for future interventions. Finally, more studies are needed to examine the potential role and mechanisms of novel glucose-lowering treatments on mitochondrial biogenesis in patients with DCM.
ACKNOWLEDGMENTS
This research was funded by the National Natural Science Foundation of China (NSFC) grants 82170356, China Postdoctoral Science Foundation grant 2018M642317, Post-Doctoral Foundation of Jiangsu Province grant 2018K095B, Six Talent Peaks Project of Jiangsu Province grants WSN-202 and WSW-183, Changzhou Sci&Tech Program grant CJ20210091, Maternal and Child Health Research Project of Jiangsu Province grant F201803.
Footnotes
CONFLICT OF INTEREST
The authors declare no potential conflicts of interest.
REFERENCES
- Al Amir Dache Z., Otandault A., Tanos R., Pastor B., Meddeb R., Sanchez C., Arena G., Lasorsa L., Bennett A., Grange T., El Messaoudi S., Mazard T., Prevostel C., Thierry A. R. Blood contains circulating cell-free respiratory competent mitochondria. FASEB J. 2020;34:3616–3630. doi: 10.1096/fj.201901917RR. [DOI] [PubMed] [Google Scholar]
- Angus L. M., Chakkalakal J. V., Méjat A., Eibl J. K., Bélanger G., Megeney L. A., Chin E. R., Schaeffer L., Michel R. N., Jasmin B. J. Calcineurin-NFAT signaling, together with GABP and peroxisome PGC-1{alpha}, drives utrophin gene expression at the neuromuscular junction. Am. J. Physiol. Cell Physiol. 2005;289:C908–C917. doi: 10.1152/ajpcell.00196.2005. [DOI] [PubMed] [Google Scholar]
- Balaban R. S. Regulation of oxidative phosphorylation in the mammalian cell. Am. J. Physiol. 1990;258:C377–C389. doi: 10.1152/ajpcell.1990.258.3.C377. [DOI] [PubMed] [Google Scholar]
- Berthiaume J. M., Kurdys J. G., Muntean D. M., Rosca M. G. Mitochondrial NAD(+)/NADH redox state and diabetic cardiomyopathy. Antioxid. Redox. Signal. 2019;30:375–398. doi: 10.1089/ars.2017.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas M., Chan J. Y. Role of Nrf1 in antioxidant response element-mediated gene expression and beyond. Toxicol. Appl. Pharmacol. 2010;244:16–20. doi: 10.1016/j.taap.2009.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blesa J. R., Prieto-Ruiz J. A., Abraham B. A., Harrison B. L., Hegde A. A., Hernández-Yago J. NRF-1 is the major transcription factor regulating the expression of the human TOMM34 gene. Biochem. Cell Biol. 2008;86:46–56. doi: 10.1139/O07-151. [DOI] [PubMed] [Google Scholar]
- Bordoni L., Petracci I., Pelikant-Malecka I., Radulska A., Piangerelli M., Samulak J. J., Lewicki L., Kalinowski L., Gabbianelli R., Olek R. A. Mitochondrial DNA copy number and trimethylamine levels in the blood: new insights on cardiovascular disease biomarkers. FASEB J. 2021;35:e21694. doi: 10.1096/fj.202100056R. [DOI] [PubMed] [Google Scholar]
- Bruggisser J., Käser S., Mani J., Schneider A. Biogenesis of a mitochondrial outer membrane protein in Trypanosoma brucei: targeting signal and dependence on a unique biogenesis factor. J. Biol. Chem. 2017;292:3400–3410. doi: 10.1074/jbc.M116.755983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruni F., Polosa P. L., Gadaleta M. N., Cantatore P., Roberti M. Nuclear respiratory factor 2 induces the expression of many but not all human proteins acting in mitochondrial DNA transcription and replication. J. Biol. Chem. 2010;285:3939–3948. doi: 10.1074/jbc.M109.044305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron R. B., Beeson C. C., Schnellmann R. G. Development of therapeutics that induce mitochondrial biogenesis for the treatment of acute and chronic degenerative diseases. J. Med. Chem. 2016;59:10411–10434. doi: 10.1021/acs.jmedchem.6b00669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cayci T., Kurt Y. G., Akgul E. O., Kurt B. Does mtDNA copy number mean mitochondrial abundance? J. Assist. Reprod. Genet. 2012;29:855. doi: 10.1007/s10815-012-9803-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook G. A., Lavrentyev E. N., Pham K., Park E. A. Streptozotocin diabetes increases mRNA expression of ketogenic enzymes in the rat heart. Biochim. Biophys. Acta Gen. Subj. 2017;1861:307–312. doi: 10.1016/j.bbagen.2016.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jong K. A., Lopaschuk G. D. Complex energy metabolic changes in heart failure with preserved ejection fraction and heart failure with reduced ejection fraction. Can. J. Cardiol. 2017;33:860–871. doi: 10.1016/j.cjca.2017.03.009. [DOI] [PubMed] [Google Scholar]
- Dillmann W. H. Diabetic cardiomyopathy. Circ. Res. 2019;124:1160–1162. doi: 10.1161/CIRCRESAHA.118.314665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang W. J., Wang C. J., He Y., Zhou Y. L., Peng X. D., Liu S. K. Resveratrol alleviates diabetic cardiomyopathy in rats by improving mitochondrial function through PGC-1α deacetylation. Acta Pharmacol. Sin. 2018;39:59–73. doi: 10.1038/aps.2017.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Marcos P. J., Auwerx J. Regulation of PGC-1α, a nodal regulator of mitochondrial biogenesis. Am. J. Clin. Nutr. 2011;93:884s–890s. doi: 10.3945/ajcn.110.001917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GBD 2015 Disease and Injury Incidence and Prevalence Collaborators, author. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1545–1602. doi: 10.1016/S0140-6736(16)31678-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleyzer N., Scarpulla R. C. Concerted action of PGC-1-related coactivator (PRC) and c-MYC in the stress response to mitochondrial dysfunction. J. Biol. Chem. 2016;291:25529–25541. doi: 10.1074/jbc.M116.719682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golpich M., Amini E., Mohamed Z., Azman Ali R., Mohamed Ibrahim N., Ahmadiani A. Mitochondrial dysfunction and biogenesis in neurodegenerative diseases: pathogenesis and treatment. CNS Neurosci. Ther. 2017;23:5–22. doi: 10.1111/cns.12655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habibi J., Aroor A. R., Sowers J. R., Jia G., Hayden M. R., Garro M., Barron B., Mayoux E., Rector R. S., Whaley-Connell A., DeMarco V. G. Sodium glucose transporter 2 (SGLT2) inhibition with empagliflozin improves cardiac diastolic function in a female rodent model of diabetes. Cardiovasc. Diabetol. 2017;16:9. doi: 10.1186/s12933-016-0489-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzig S., Long F., Jhala U. S., Hedrick S., Quinn R., Bauer A., Rudolph D., Schutz G., Yoon C., Puigserver P., Spiegelman B., Montminy M. CREB regulates hepatic gluconeogenesis through the coactivator PGC-1. Nature. 2001;413:179–183. doi: 10.1038/35093131. [DOI] [PubMed] [Google Scholar]
- Ji H., Wang J., Muid D., Song W., Jiang Y., Zhou H. FUNDC1 activates the mitochondrial unfolded protein response to preserve mitochondrial quality control in cardiac ischemia/reperfusion injury. Cell. Signal. 2022;92:110249. doi: 10.1016/j.cellsig.2022.110249. [DOI] [PubMed] [Google Scholar]
- Jia G., Hill M. A., Sowers J. R. Diabetic cardiomyopathy: an update of mechanisms contributing to this clinical entity. Circ. Res. 2018a;122:624–638. doi: 10.1161/CIRCRESAHA.117.311586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia G., Whaley-Connell A., Sowers J. R. Diabetic cardiomyopathy: a hyperglycaemia- and insulin-resistance-induced heart disease. Diabetologia. 2018b;61:21–28. doi: 10.1007/s00125-017-4390-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannel W. B., Hjortland M., Castelli W. P. Role of diabetes in congestive heart failure: the Framingham study. Am. J. Cardiol. 1974;34:29–34. doi: 10.1016/0002-9149(74)90089-7. [DOI] [PubMed] [Google Scholar]
- Kannel W. B., McGee D. L. Diabetes and cardiovascular disease. The Framingham study. JAMA. 1979;241:2035–2038. doi: 10.1001/jama.1979.03290450033020. [DOI] [PubMed] [Google Scholar]
- Karamanlidis G., Nascimben L., Couper G. S., Shekar P. S., del Monte F., Tian R. Defective DNA replication impairs mitochondrial biogenesis in human failing hearts. Circ. Res. 2010;106:1541–1548. doi: 10.1161/CIRCRESAHA.109.212753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. K., Ko T. H., Song I. S., Jeong Y. J., Heo H. J., Jeong S. H., Kim M., Park N. M., Seo D. Y., Kha P. T., Kim S. W., Lee S. R., Cho S. W., Won J. C., Youm J. B., Ko K. S., Rhee B. D., Kim N., Cho K. I., Shimizu I., Minamino T., Ha N. C., Park Y. S., Nilius B., Han J. BH4 activates CaMKK2 and rescues the cardiomyopathic phenotype in rodent models of diabetes. Life Sci. Alliance. 2020;3:e201900619. doi: 10.26508/lsa.201900619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp M., Tu X., Wu R. Vascular endothelial dysfunction, a major mediator in diabetic cardiomyopathy. Acta Pharmacol. Sin. 2019;40:1–8. doi: 10.1038/s41401-018-0042-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko T. H., Marquez J. C., Kim H. K., Jeong S. H., Lee S., Youm J. B., Song I. S., Seo D. Y., Kim H. J., Won D. N., Cho K. I., Choi M. G., Rhee B. D., Ko K. S., Kim N., Won J. C., Han J. Resistance exercise improves cardiac function and mitochondrial efficiency in diabetic rat hearts. Pflugers Arch. 2018;470:263–275. doi: 10.1007/s00424-017-2076-x. [DOI] [PubMed] [Google Scholar]
- Kosuru R., Kandula V., Rai U., Prakash S., Xia Z., Singh S. Pterostilbene decreases cardiac oxidative stress and inflammation via activation of AMPK/Nrf2/HO-1 pathway in fructose-fed diabetic rats. Cardiovasc. Drugs Ther. 2018;32:147–163. doi: 10.1007/s10557-018-6780-3. [DOI] [PubMed] [Google Scholar]
- Kristensen S. L., Rørth R., Jhund P. S., Docherty K. F., Sattar N., Preiss D., Køber L., Petrie M. C., McMurray J. J. V. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2019;7:776–785. doi: 10.1016/S2213-8587(19)30249-9. [DOI] [PubMed] [Google Scholar]
- Lam C. S. Diabetic cardiomyopathy: an expression of stage B heart failure with preserved ejection fraction. Diab. Vasc. Dis. Res. 2015;12:234–238. doi: 10.1177/1479164115579006. [DOI] [PubMed] [Google Scholar]
- Lee H. C., Wei Y. H. Mitochondrial role in life and death of the cell. J. Biomed. Sci. 2000;7:2–15. doi: 10.1007/BF02255913. [DOI] [PubMed] [Google Scholar]
- Li N., Zhou H. SGLT2 Inhibitors: a novel player in the treatment and prevention of diabetic cardiomyopathy. Drug Des. Devel. Ther. 2020;14:4775–4788. doi: 10.2147/DDDT.S269514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Feng Y. F., Liu X. T., Li Y. C., Zhu H. M., Sun M. R., Li P., Liu B., Yang H. Songorine promotes cardiac mitochondrial biogenesis via Nrf2 induction during sepsis. Redox Biol. 2021a;38:101771. doi: 10.1016/j.redox.2020.101771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Wei X., Liu S. L., Zhao Y., Jin S., Yang X. Y. Salidroside protects cardiac function in mice with diabetic cardiomyopathy via activation of mitochondrial biogenesis and SIRT3. Phytother. Res. 2021b;35:4579–4591. doi: 10.1002/ptr.7175. [DOI] [PubMed] [Google Scholar]
- Liang Q., Kobayashi S. Mitochondrial quality control in the diabetic heart. J. Mol. Cell. Cardiol. 2016;95:57–69. doi: 10.1016/j.yjmcc.2015.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Zou Y., Tang Y., Xi M., Xie L., Zhang Q., Gong J. Circulating cell-free mitochondrial deoxyribonucleic acid is increased in coronary heart disease patients with diabetes mellitus. J. Diabetes Investig. 2016;7:109–114. doi: 10.1111/jdi.12366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X. D., Li Y. G., Wang G. Y., Bi Y. G., Zhao Y., Yan M. L., Liu X., Wei M., Wan L. L., Zhang Q. Y. Metformin protects high glucose-cultured cardiomyocytes from oxidative stress by promoting NDUFA13 expression and mitochondrial biogenesis via the AMPK signaling pathway. Mol. Med. Rep. 2020;22:5262–5270. doi: 10.3892/mmr.2020.11599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo-Almorós A., Tuñón J., Orejas M., Cortés M., Egido J., Lorenzo Ó. Diagnostic approaches for diabetic cardiomyopathy. Cardiovasc. Diabetol. 2017;16:28. doi: 10.1186/s12933-017-0506-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu S., Liao Z., Lu X., Katschinski D. M., Mercola M., Chen J., Heller Brown J., Molkentin J. D., Bossuyt J., Bers D. M. Hyperglycemia acutely increases cytosolic reactive oxygen species via O-linked GlcNAcylation and CaMKII activation in mouse ventricular myocytes. Circ. Res. 2020;126:e80–e96. doi: 10.1161/CIRCRESAHA.119.316288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S., Feng J., Zhang R., Chen J., Han D., Li X., Yang B., Li X., Fan M., Li C., Tian Z., Wang Y., Cao F. SIRT1 activation by resveratrol alleviates cardiac dysfunction via mitochondrial regulation in diabetic cardiomyopathy mice. Oxid. Med. Cell. Longev. 2017;2017:4602715. doi: 10.1155/2017/4602715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik A. N., Czajka A. Is mitochondrial DNA content a potential biomarker of mitochondrial dysfunction? Mitochondrion. 2013;13:481–492. doi: 10.1016/j.mito.2012.10.011. [DOI] [PubMed] [Google Scholar]
- Marciniak C., Marechal X., Montaigne D., Neviere R., Lancel S. Cardiac contractile function and mitochondrial respiration in diabetes-related mouse models. Cardiovasc. Diabetol. 2014;13:118. doi: 10.1186/s12933-014-0118-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marso S. P., Daniels G. H., Brown-Frandsen K., Kristensen P., Mann J. F., Nauck M. A., Nissen S. E., Pocock S., Poulter N. R., Ravn L. S., Steinberg W. M., Stockner M., Zinman B., Bergenstal R. M., Buse J. B. Liraglutide and cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 2016;375:311–322. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melser S., Lavie J., Bénard G. Mitochondrial degradation and energy metabolism. Biochim. Biophys. Acta. 2015;1853:2812–2821. doi: 10.1016/j.bbamcr.2015.05.010. [DOI] [PubMed] [Google Scholar]
- Momiyama Y., Furutani M., Suzuki Y., Ohmori R., Imamura S., Mokubo A., Asahina T., Murata C., Kato K., Anazawa S., Hosokawa K., Atsumi Y., Matsuoka K., Kimura M., Kasanuki H., Ohsuzu F., Matsuoka R. A mitochondrial DNA variant associated with left ventricular hypertrophy in diabetes. Biochem. Biophys. Res. Commun. 2003;312:858–864. doi: 10.1016/j.bbrc.2003.10.195. [DOI] [PubMed] [Google Scholar]
- Momiyama Y., Suzuki Y., Ohtomo M., Atsumi Y., Matsuoka K., Ohsuzu F., Kimura M. Cardiac autonomic nervous dysfunction in diabetic patients with a mitochondrial DNA mutation: assessment by heart rate variability. Diabetes Care. 2002;25:2308–2313. doi: 10.2337/diacare.25.12.2308. [DOI] [PubMed] [Google Scholar]
- Moore M. L., Park E. A., McMillin J. B. Upstream stimulatory factor represses the induction of carnitine palmitoyltransferase-Ibeta expression by PGC-1. J. Biol. Chem. 2003;278:17263–17268. doi: 10.1074/jbc.M210486200. [DOI] [PubMed] [Google Scholar]
- Murtaza G., Virk H. U. H., Khalid M., Lavie C. J., Ventura H., Mukherjee D., Ramu V., Bhogal S., Kumar G., Shanmugasundaram M., Paul T. K. Diabetic cardiomyopathy - a comprehensive updated review. Prog. Cardiovasc. Dis. 2019;62:315–326. doi: 10.1016/j.pcad.2019.03.003. [DOI] [PubMed] [Google Scholar]
- Nakae J., Cao Y., Oki M., Orba Y., Sawa H., Kiyonari H., Iskandar K., Suga K., Lombes M., Hayashi Y. Forkhead transcription factor FoxO1 in adipose tissue regulates energy storage and expenditure. Diabetes. 2008;57:563–576. doi: 10.2337/db07-0698. [DOI] [PubMed] [Google Scholar]
- Nauck M. A., Meier J. J., Cavender M. A., Abd El Aziz M., Drucker D. J. Cardiovascular actions and clinical outcomes with glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors. Circulation. 2017;136:849–870. doi: 10.1161/CIRCULATIONAHA.117.028136. [DOI] [PubMed] [Google Scholar]
- Nayor M., Shah R. V., Miller P. E., Blodgett J. B., Tanguay M., Pico A. R., Murthy V. L., Malhotra R., Houstis N. E., Deik A., Pierce K. A., Bullock K., Dailey L., Velagaleti R. S., Moore S. A., Ho J. E., Baggish A. L., Clish C. B., Larson M. G., Vasan R. S., Lewis G. D. Metabolic architecture of acute exercise response in middle-aged adults in the community. Circulation. 2020;142:1905–1924. doi: 10.1161/CIRCULATIONAHA.120.050281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal B., Perkovic V., Matthews D. R., Mahaffey K. W., Fulcher G., Meininger G., Erondu N., Desai M., Shaw W., Vercruysse F., Yee J., Deng H., de Zeeuw D. Rationale, design and baseline characteristics of the CANagliflozin cardioVascular Assessment Study-Renal (CANVAS-R): a randomized, placebo-controlled trial. Diabetes Obes. Metab. 2017;19:387–393. doi: 10.1111/dom.12829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packer M. Autophagy-dependent and -independent modulation of oxidative and organellar stress in the diabetic heart by glucose-lowering drugs. Cardiovasc. Diabetol. 2020;19:62. doi: 10.1186/s12933-020-01041-4.835b15e8dc6e4d04af7eadde6f9be9d4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolillo S., Marsico F., Prastaro M., Renga F., Esposito L., De Martino F., Di Napoli P., Esposito I., Ambrosio A., Ianniruberto M., Mennella R., Paolillo R., Gargiulo P. Diabetic cardiomyopathy: definition, diagnosis, and therapeutic implications. Heart Fail. Clin. 2019;15:341–347. doi: 10.1016/j.hfc.2019.02.003. [DOI] [PubMed] [Google Scholar]
- Parim B., Sathibabu Uddandrao V. V., Saravanan G. Diabetic cardiomyopathy: molecular mechanisms, detrimental effects of conventional treatment, and beneficial effects of natural therapy. Heart Fail. Rev. 2019;24:279–299. doi: 10.1007/s10741-018-9749-1. [DOI] [PubMed] [Google Scholar]
- Peng X., Li L., Zhang M., Zhao Q., Wu K., Bai R., Ruan Y., Liu N. Sodium-glucose cotransporter 2 inhibitors potentially prevent atrial fibrillation by ameliorating ion handling and mitochondrial dysfunction. Front. Physiol. 2020;11:912. doi: 10.3389/fphys.2020.00912.e2b827d93108401a877c5fa82cb572eb [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson L. R., Gropler R. J. Metabolic and molecular imaging of the diabetic cardiomyopathy. Circ. Res. 2020;126:1628–1645. doi: 10.1161/CIRCRESAHA.120.315899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploumi C., Daskalaki I., Tavernarakis N. Mitochondrial biogenesis and clearance: a balancing act. FEBS J. 2017;284:183–195. doi: 10.1111/febs.13820. [DOI] [PubMed] [Google Scholar]
- Popov L. D. Mitochondrial biogenesis: an update. J. Cell. Mol. Med. 2020;24:4892–4899. doi: 10.1111/jcmm.15194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu C., Hevner K., Abetew D., Sedensky M., Morgan P., Enquobahrie D. A., Williams M. A. Mitochondrial DNA copy number and oxidative DNA damage in placental tissues from gestational diabetes and control pregnancies: a pilot study. Clin. Lab. 2013;59:655–660. doi: 10.7754/Clin.Lab.2012.120227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan Y., Xin Y., Tian G., Zhou J., Liu X. Mitochondrial ROS-modulated mtDNA: a potential target for cardiac aging. Oxid. Med. Cell. Longev. 2020;2020:9423593. doi: 10.1155/2020/9423593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaven P. D., Emanuele N. V., Wiitala W. L., Bahn G. D., Reda D. J., McCarren M., Duckworth W. C., Hayward R. A. Intensive glucose control in patients with type 2 diabetes - 15-year follow-up. N. Engl. J. Med. 2019;380:2215–2224. doi: 10.1056/NEJMoa1806802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riehle C., Bauersachs J. Of mice and men: models and mechanisms of diabetic cardiomyopathy. Basic Res. Cardiol. 2018;114:2. doi: 10.1007/s00395-018-0711-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto T., Matsuura T. R., Wan S., Ryba D. M., Kim J. U., Won K. J., Lai L., Petucci C., Petrenko N., Musunuru K., Vega R. B., Kelly D. P. A critical role for estrogen-related receptor signaling in cardiac maturation. Circ. Res. 2020;126:1685–1702. doi: 10.1161/CIRCRESAHA.119.316100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh J., Kawana N., Yamamoto Y. Pathway analysis of ChIP-Seq-based NRF1 target genes suggests a logical hypothesis of their involvement in the pathogenesis of neurodegenerative diseases. Gene Regul. Syst. Bio. 2013;7:139–152. doi: 10.4137/GRSB.S13204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheen A. J. Cardiovascular effects of new oral glucose-lowering agents: DPP-4 and SGLT-2 inhibitors. Circ. Res. 2018;122:1439–1459. doi: 10.1161/CIRCRESAHA.117.311588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Q., Meng L., Lee S., Tse G., Gong M., Zhang Z., Zhao J., Zhao Y., Li G., Liu T. Empagliflozin, a sodium glucose co-transporter-2 inhibitor, alleviates atrial remodeling and improves mitochondrial function in high-fat diet/streptozotocin-induced diabetic rats. Cardiovasc. Diabetol. 2019;18:165. doi: 10.1186/s12933-019-0964-4.c8bcfe7294d740b29b42e41e29df2c01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen X., Zheng S., Thongboonkerd V., Xu M., Pierce W. M., Jr., Klein J. B., Epstein P. N. Cardiac mitochondrial damage and biogenesis in a chronic model of type 1 diabetes. Am. J. Physiol. Endocrinol. Metab. 2004;287:E896–E905. doi: 10.1152/ajpendo.00047.2004. [DOI] [PubMed] [Google Scholar]
- Sun W., Quan N., Wang L., Yang H., Chu D., Liu Q., Zhao X., Leng J., Li J. Cardiac-specific deletion of the Pdha1 gene sensitizes heart to toxicological actions of ischemic stress. Toxicol. Sci. 2016;153:411. doi: 10.1093/toxsci/kfw154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taherzadeh-Fard E., Saft C., Akkad D. A., Wieczorek S., Haghikia A., Chan A., Epplen J. T., Arning L. PGC-1alpha downstream transcription factors NRF-1 and TFAM are genetic modifiers of Huntington disease. Mol. Neurodegener. 2011;6:32. doi: 10.1186/1750-1326-6-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada S., Masaki Y., Kinugawa S., Matsumoto J., Furihata T., Mizushima W., Kadoguchi T., Fukushima A., Homma T., Takahashi M., Harashima S., Matsushima S., Yokota T., Tanaka S., Okita K., Tsutsui H. Dipeptidyl peptidase-4 inhibitor improved exercise capacity and mitochondrial biogenesis in mice with heart failure via activation of glucagon-like peptide-1 receptor signalling. Cardiovasc. Res. 2016;111:338–347. doi: 10.1093/cvr/cvw182. [DOI] [PubMed] [Google Scholar]
- Tanajak P., Sa-Nguanmoo P., Sivasinprasasn S., Thummasorn S., Siri-Angkul N., Chattipakorn S. C., Chattipakorn N. Cardioprotection of dapagliflozin and vildagliptin in rats with cardiac ischemia-reperfusion injury. J. Endocrinol. 2018;236:69–84. doi: 10.1530/JOE-17-0457. [DOI] [PubMed] [Google Scholar]
- Tao L., Huang X., Xu M., Yang L., Hua F. MiR-144 protects the heart from hyperglycemia-induced injury by regulating mitochondrial biogenesis and cardiomyocyte apoptosis. FASEB J. 2020;34:2173–2197. doi: 10.1096/fj.201901838R. [DOI] [PubMed] [Google Scholar]
- Thirupathi A., de Souza C. T. Multi-regulatory network of ROS: the interconnection of ROS, PGC-1 alpha, and AMPK-SIRT1 during exercise. J. Physiol. Biochem. 2017;73:487–494. doi: 10.1007/s13105-017-0576-y. [DOI] [PubMed] [Google Scholar]
- Ueno H., Shiotani H. Cardiac abnormalities in diabetic patients with mutation in the mitochondrial tRNA(Leu(UUR)) gene. Jpn. Circ. J. 1999;63:877–880. doi: 10.1253/jcj.63.877. [DOI] [PubMed] [Google Scholar]
- Vafai S. B., Mootha V. K. Mitochondrial disorders as windows into an ancient organelle. Nature. 2012;491:374–383. doi: 10.1038/nature11707. [DOI] [PubMed] [Google Scholar]
- Veeranki S., Givvimani S., Kundu S., Metreveli N., Pushpakumar S., Tyagi S. C. Moderate intensity exercise prevents diabetic cardiomyopathy associated contractile dysfunction through restoration of mitochondrial function and connexin 43 levels in db/db mice. J. Mol. Cell. Cardiol. 2016;92:163–173. doi: 10.1016/j.yjmcc.2016.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma S., McGuire D. K., Bain S. C., Bhatt D. L., Leiter L. A., Mazer C. D., Monk Fries T., Pratley R. E., Rasmussen S., Vrazic H., Zinman B., Buse J. B. Effects of glucagon-like peptide-1 receptor agonists liraglutide and semaglutide on cardiovascular and renal outcomes across body mass index categories in type 2 diabetes: results of the LEADER and SUSTAIN 6 trials. Diabetes Obes. Metab. 2020;22:2487–2492. doi: 10.1111/dom.14160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Bei Y., Lu Y., Sun W., Liu Q., Wang Y., Cao Y., Chen P., Xiao J., Kong X. Exercise prevents cardiac injury and improves mitochondrial biogenesis in advanced diabetic cardiomyopathy with PGC-1α and Akt activation. Cell. Physiol. Biochem. 2015;35:2159–2168. doi: 10.1159/000374021. [DOI] [PubMed] [Google Scholar]
- Wiviott S. D., Raz I., Bonaca M. P., Mosenzon O., Kato E. T., Cahn A., Silverman M. G., Zelniker T. A., Kuder J. F., Murphy S. A., Bhatt D. L., Leiter L. A., McGuire D. K., Wilding J. P. H., Ruff C. T., Gause-Nilsson I. A. M., Fredriksson M., Johansson P. A., Langkilde A. M., Sabatine M. S. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 2019;380:347–357. doi: 10.1056/NEJMoa1812389. [DOI] [PubMed] [Google Scholar]
- Xiong Y., Hai C. X., Fang W. J., Lei Y. P., Li X. M., Zhou X. K. Endogenous asymmetric dimethylarginine accumulation contributes to the suppression of myocardial mitochondrial biogenesis in type 2 diabetic rats. Nutr. Metab. 2020;17:72. doi: 10.1186/s12986-020-00486-4.03ecf3afb6a74d2b88b66ea03f1f54d8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y., He Y. L., Li X. M., Nie F., Zhou X. K. Endogenous asymmetric dimethylarginine accumulation precipitates the cardiac and mitochondrial dysfunctions in type 1 diabetic rats. Eur. J. Pharmacol. 2021;902:174081. doi: 10.1016/j.ejphar.2021.174081. [DOI] [PubMed] [Google Scholar]
- Yan W., Zhang H., Liu P., Wang H., Liu J., Gao C., Liu Y., Lian K., Yang L., Sun L., Guo Y., Zhang L., Dong L., Lau W. B., Gao E., Gao F., Xiong L., Wang H., Qu Y., Tao L. Impaired mitochondrial biogenesis due to dysfunctional adiponectin-AMPK-PGC-1α signaling contributing to increased vulnerability in diabetic heart. Basic Res. Cardiol. 2013;108:329. doi: 10.1007/s00395-013-0329-1. [DOI] [PubMed] [Google Scholar]
- Yang Z. F., Drumea K., Mott S., Wang J., Rosmarin A. G. GABP transcription factor (nuclear respiratory factor 2) is required for mitochondrial biogenesis. Mol. Cell. Biol. 2014;34:3194–3201. doi: 10.1128/MCB.00492-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao K., Zhang W. W., Yao L., Yang S., Nie W., Huang F. Carvedilol promotes mitochondrial biogenesis by regulating the PGC-1/TFAM pathway in human umbilical vein endothelial cells (HUVECs) Biochem. Biophys. Res. Commun. 2016;470:961–966. doi: 10.1016/j.bbrc.2016.01.089. [DOI] [PubMed] [Google Scholar]
- Yu L. M., Dong X., Xue X. D., Xu S., Zhang X., Xu Y. L., Wang Z. S., Wang Y., Gao H., Liang Y. X., Yang Y., Wang H. S. Melatonin attenuates diabetic cardiomyopathy and reduces myocardial vulnerability to ischemia-reperfusion injury by improving mitochondrial quality control: role of SIRT6. J. Pineal Res. 2021;70:e12698. doi: 10.1111/jpi.12698. [DOI] [PubMed] [Google Scholar]
- Yurista S. R., Silljé H. H. W., Rienstra M., de Boer R. A., Westenbrink B. D. Sodium-glucose co-transporter 2 inhibition as a mitochondrial therapy for atrial fibrillation in patients with diabetes? Cardiovasc. Diabetol. 2020;19:5. doi: 10.1186/s12933-019-0984-0.f5980cffc9ce4acea7ab328783ae6874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M., Lin J., Wang S., Cheng Z., Hu J., Wang T., Man W., Yin T., Guo W., Gao E., Reiter R. J., Wang H., Sun D. Melatonin protects against diabetic cardiomyopathy through Mst1/Sirt3 signaling. J. Pineal Res. 2017;63:e12418. doi: 10.1111/jpi.12418. [DOI] [PubMed] [Google Scholar]
- Zhang X., Zhang Z., Yang Y., Suo Y., Liu R., Qiu J., Zhao Y., Jiang N., Liu C., Tse G., Li G., Liu T. Alogliptin prevents diastolic dysfunction and preserves left ventricular mitochondrial function in diabetic rabbits. Cardiovasc. Diabetol. 2018;17:160. doi: 10.1186/s12933-018-0803-z.f711913d27074c488fea9c0dc687fcc2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Zhang X., Meng L., Gong M., Li J., Shi W., Qiu J., Yang Y., Zhao J., Suo Y., Liang X., Wang X., Tse G., Jiang N., Li G., Zhao Y., Liu T. Pioglitazone inhibits diabetes-induced atrial mitochondrial oxidative stress and improves mitochondrial biogenesis, dynamics, and function through the PPAR-γ/PGC-1α signaling pathway. Front. Pharmacol. 2021;12:658362. doi: 10.3389/fphar.2021.658362.5080a93b70a64425a03ca55c6a2d4b3a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L., Yu M., Arshad M., Wang W., Lu Y., Gong J., Gu Y., Li P., Xu L. Coordination among lipid droplets, peroxisomes, and mitochondria regulates energy expenditure through the CIDE-ATGL-PPARα pathway in adipocytes. Diabetes. 2018;67:1935–1948. doi: 10.2337/db17-1452. [DOI] [PubMed] [Google Scholar]
- Zinman B., Inzucchi S. E., Lachin J. M., Wanner C., Ferrari R., Fitchett D., Bluhmki E., Hantel S., Kempthorne-Rawson J., Newman J., Johansen O. E., Woerle H. J., Broedl U. C. Rationale, design, and baseline characteristics of a randomized, placebo-controlled cardiovascular outcome trial of empagliflozin (EMPA-REG OUTCOMETM) Cardiovasc. Diabetol. 2014;13:102. doi: 10.1186/1475-2840-13-102. [DOI] [PMC free article] [PubMed] [Google Scholar]