Highlights
-
•
Regional Glu reduction found in adults with persistent post-concussive symptoms.
-
•
Higher GSH associated with greater functional impact of headache.
-
•
Enhanced GSH synthesis and energy shortage may contribute to Glu deficit.
-
•
Higher GABA linked to history of previous mTBI.
Keywords: Magnetic resonance spectroscopy, Hadamard encoding and reconstruction of MEGA-edited spectroscopy (HERMES), Mild traumatic brain injury (mTBI), Glutamate, GABA, Glutathione
Abbreviations: ACC, anterior cingulate cortex; DHI, Dizziness Handicap Inventory; ESS, Epworth Sleepiness Scale; GABA, γ-aminobutyric acid; GAD-7, Generalized Anxiety Disorder-7 scale; Glu, glutamate; Glx, glutamate + glutamine; GSH, glutathione; HERMES, Hadamard Encoding and Reconstruction of MEGA-Edited Spectroscopy; HIT-6, Headache Impact Test-6; mIns, myo-inositol; MoCA, Montreal Cognitive Assessment; MRS, magnetic resonance spectroscopy; mTBI, mild traumatic brain injury; PCSC, Postconcussion Syndrome Checklist; PPCS, persistent post-concussive symptoms; PRESS, Point Resolved Spectroscopy; QOLIBRI, Quality of Life After Brain Injury scale; RPQ, Rivermead Post Concussion Symptoms Questionnaire; RSM, right sensorimotor cortex; tCho, choline + glycerophosphocholine + phosphocholine; tCr, creatine + phosphocreatine; tNAA, N-acetyl-aspartate + N-acetyl-aspartylglutamate
Abstract
Persistent post-concussive symptoms (PPCS) are debilitating and endure beyond the usual recovery period after mild traumatic brain injury (mTBI). Altered neurotransmission, impaired energy metabolism and oxidative stress have been examined acutely post-injury but have not been explored extensively in those with persistent symptoms. Specifically, the antioxidant glutathione (GSH) and the excitatory and inhibitory metabolites, glutamate (Glu) and γ-aminobutyric acid (GABA), are seldom studied together in the clinical mTBI literature. While Glu can be measured using conventional magnetic resonance spectroscopy (MRS) methods at 3 Tesla, GABA and GSH require the use of advanced MRS methods. Here, we used the recently established Hadamard Encoding and Reconstruction of MEGA-Edited Spectroscopy (HERMES) to simultaneously measure GSH and GABA and short-echo time point resolved spectroscopy (PRESS) to measure Glu to gain new insight into the pathophysiology of PPCS. Twenty-nine adults with PPCS (mean age: 45.69 years, s.d.: 10.73, 22 females, 7 males) and 29 age- and sex-matched controls (mean age: 43.69 years, s.d.: 11.00) completed magnetic resonance spectroscopy scans with voxels placed in the anterior cingulate and right sensorimotor cortex. Relative to controls, anterior cingulate Glu was significantly reduced in PPCS. Higher anterior cingulate GABA was significantly associated with a higher number of lifetime mTBIs, suggesting GABA may be upregulated with repeated incidence of mTBI. Furthermore, GSH in both regions of interest was positively associated with symptoms of sleepiness and headache burden. Collectively, our findings suggest that the antioxidant defense system is active in participants with PPCS, however this may be at the expense of other glutamatergic functions such as cortical excitation and energy metabolism.
1. Introduction
While most individuals recover within days and weeks following concussion, up to 30% of adults will go on to experience persistent post-concussive symptoms (PPCS) (Sigurdardottir et al., 2009, Hou et al., 2012). Presentation of PPCS after concussion, a term often used interchangeably with mild traumatic brain injury (mTBI), is highly variable. Symptoms often include a combination of headache, dizziness, blurred or double vision, noise and light sensitivity, memory problems, poor concentration, low mood, depression, anxiety, fatigue, sleep difficulties and irritability (Marshall et al., 2012, Polinder et al., 2018, Permenter and Sherman, 2020). Post-traumatic headache is one of the most common symptoms following mTBI and has been described as the most prevalent type of pain after mTBI (Defrin, 2014).
Although the existence of PPCS is well-established, current understanding of the pathophysiology of PPCS is limited. Brain damage following mTBI is seldom identified with conventional neuroimaging. However, advanced neuroimaging methods, notably diffusion tensor imaging, functional magnetic resonance imaging, susceptibility-weighted imaging and magnetic resonance spectroscopy (MRS) have revealed subtle alterations with mTBI (Koerte et al., 2016). MRS is a non-invasive method that measures metabolites in vivo and has shown to be a valuable tool to probe the neurochemistry of a variety of neurological and psychiatric conditions (Lee et al., 2012).
In the acute phase following mTBI, altered cortical neurotransmission, impaired energy metabolism and oxidative stress are widely described (Giza and Hovda, 2014, Guerriero et al., 2015). Thus, the primary excitatory and inhibitory neurotransmitters, glutamate (Glu) and γ-aminobutyric acid (GABA), respectively, as well as the antioxidant glutathione (GSH) are of great interest when studying mTBI, and can be measured using MRS. Immediately following mTBI, excess Glu release creates a hyperexcitable state and elevates levels of cortical oxidative stress (Giza and Hovda, 2014). An increase in GABA is thought to be compensatory against Glu-induced cortical hyperexcitability (Guerriero et al., 2015). In addition to being primary excitatory and inhibitory neurotransmitters, Glu and GABA also have important metabolic roles in the brain. Both metabolites are linked to the citric acid cycle, a key metabolic pathway for cellular energy production. Glu exists in dynamic equilibrium with α-ketoglutarate (intermediate of the citric acid cycle) (Rae, 2014). GABA can contribute to the citric acid cycle via the GABA shunt (Rae, 2014).
Oxidative stress in the weeks and months following mTBI has not been well studied, though it is suggested that long-term mTBI sequelae are linked to increased oxidative stress (Cruz-Haces et al., 2017). Directly measuring oxidative stress in the brain is challenging as reactive oxygen species have very short lifespans (on the order of µs) (Berkowitz, 2018). Oxidative stress can, however, be studied indirectly by measuring GSH levels. In response to mild elevation in reactive oxygen species levels, GSH synthesis increases for antioxidant defense (Xiao and Loscalzo, 2020). In cases of severe oxidative stress, as seen in Parkinson’s disease (Mischley et al., 2016), schizophrenia (Do et al., 2000) and multiple sclerosis (Choi et al., 2010), high oxidative stress can overwhelm the brain’s endogenous antioxidant defense system and lead to depletion of GSH stores (Lu, 2009, Rae and Williams, 2017). Together, this makes GSH a desirable research target in the investigation of PPCS pathophysiology.
Glu, GABA and GSH are linked through metabolic pathways in the brain (Rae, 2014). Glu is converted to GABA via action of the enzyme glutamic acid decarboxylase. Additionally, Glu is one of the three amino acids that form the GSH tripeptide. Due to their metabolic linkage, the concentrations of these metabolites are related but not wholly determined by each other. For these reasons, it is advantageous to simultaneously investigate differences in Glu, GABA and GSH in patients with PPCS relative to non-head injured individuals.
GABA and GSH are present in low concentrations in the brain and their peaks in the 1H MRS spectrum are obscured by the more prominent signals, namely creatine. As such, they cannot be quantified reliably using conventional MRS methods at 3 T. Therefore, these metabolites are seldom studied in mTBI despite their biological relevance. J-difference editing enables quantification of GABA and GSH by resolving signals of these metabolites from overlapping signal peaks. Individual edited-MRS acquisitions have previously been required for each metabolite of interest (i.e., one for GABA, one for GSH). This results in long scan times, particularly when measuring more than one region. The recently developed Hadamard Encoding and Reconstruction of MEGA-Edited Spectroscopy (HERMES) editing scheme allows for detection of multiple metabolites that require editing within a single acquisition, enabling simultaneous quantification of GABA and GSH (Saleh et al., 2016).
Concussion is considered a diffuse injury, affecting widespread brain regions (McKee and Daneshvar, 2015). However, certain brain regions are functionally relevant to the symptoms of concussion and are thus of particular interest. In this study, metabolites were quantified in the anterior cingulate cortex (ACC) and right sensorimotor cortex (RSM) as these regions are functionally relevant to mTBI symptoms given their respective roles in executive function/emotional regulation and sensory processing (Stevens et al., 2011, Borich et al., 2015). Additionally, the ACC and RSM are relevant target regions as they have both been linked to headache (Obermann et al., 2009, Qin et al., 2020, Peek et al., 2021, Messina et al., 2022, Nahman‐Averbuch et al., 2022), one of the most common sequelae of mTBI (Ashina et al., 2019).
To date, only a few studies have used HERMES to study GABA and GSH in clinical populations (Flores-Ramos et al., 2019, Weerasekera et al., 2019, Gong et al., 2020, Gong et al., 2021), and this is the first study where it has been applied in traumatic brain injury. Short-echo time point resolved spectroscopy (PRESS) (Bottomley, 1987) was used to quantify Glu and the combined Glu + glutamine (Glx) signal to investigate relationships between levels of these interrelated metabolites. Our objectives were (1) to determine whether cortical metabolite concentrations differed between PPCS participants and age- and sex-matched non-head injured controls, and (2) to examine metabolite relationships with symptoms, mTBI history and time since injury.
2. Methods
2.1. Study design
This cross-sectional study was part of the Aerobic Exercise for Chronic Symptoms following mild Traumatic Brain Injury (ACTBI) trial. The ACTBI trial was registered at clinicaltrials.gov (NCT03895450) with ethics approval from the University of Calgary Research Ethics Board (REB18-1329). All participants provided written informed consent. ACTBI trial methods can be found in Mercier et al. (2020) and relevant details are described below.
2.2. Participants
PPCS participants were recruited from the Calgary Brain Injury Program and the Calgary Pain Centre between May 2019 and December 2021. Eligibility criteria included: (1) age 18–65 years; (2) diagnosis of mTBI based on the American Congress of Rehabilitative Medicine criteria (Kay et al., 1993); (3) diagnosis of PPCS based on the ICD-10 post-concussional syndrome criteria (World Health Organization, 1993); (4) minimum of 3 months to maximum of 5 years since most recent mTBI; (5) exercise intolerance defined as an acute exacerbation of post-concussive symptoms with exercise (Mercier et al., 2020); (6) maintenance of a stable pharmacological regimen for a minimum of one month prior to study participation. As per American Congress of Rehabilitative Medicine criteria for mTBI (concussion), no PPCS participants sustained loss of consciousness for >30 mins, after 30 min had an initial Glasgow Coma Scale score <13 or experienced post-traumatic amnesia for >24 h post-injury (Kay et al., 1993). No participants with PPCS reported sustaining a complicated mTBI (mTBI with intracranial abnormalities on structural neuroimaging post-injury, (Iverson et al., 2012)). Furthermore, no intracranial abnormalities were identified on T1-weighted imaging acquired for this research study. PPCS participants were excluded if they presented with a history of cardiopulmonary, or musculoskeletal condition. Age (±5 years) and sex-matched controls were recruited from the community using posters and word of mouth. All participants were screened to rule out any previous neurological conditions including moderate-to-severe TBI or major psychiatric history and MRI contraindications. Controls with any previous brain injury history (including mild TBI) were excluded. Self-reported demographic and injury characteristic data were collected from all participants.
2.3. Magnetic resonance spectroscopy acquisition
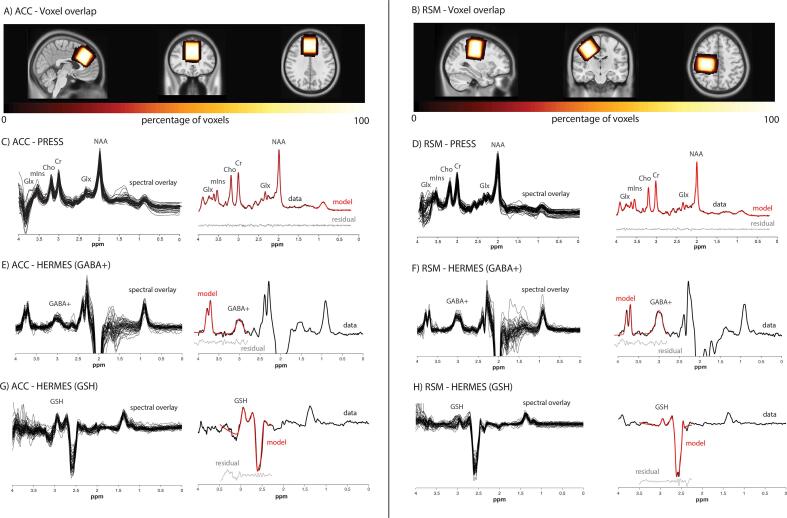
All imaging data were acquired on a 3 T MRI scanner (750w, General Electric) equipped with a 32-channel head coil. T1-weighted structural images (BRAVO; TR/TE/TI = 7.30 ms/2.70 ms/600 ms, 1 mm3 isotropic voxels, flip angle = 10°, acquisition time 4 min 10 s) were acquired for MRS voxel placement and tissue segmentation. MRS data were acquired from two 3 × 3 × 3 cm3 voxels. The RSM voxel was centered on the central sulcus posterior to the motor hand knob (Yousry et al., 1997) of the right precentral gyrus in the axial plane. It was rotated such that the sagittal and coronal planes aligned with the cortical surface. The ACC voxel was placed along the midline, parallel to the dorsal aspect of the genu of the corpus callosum and perpendicular to the anterior portion of the corpus callosum. Voxel placements are shown in Fig. 1.
Fig. 1.
Voxel placement in the A) ACC and B) RSM. The voxels shown are the overlay of all participants’ voxels normalized to standard space (MNI152 template brain). Lighter colors indicate a greater degree of overlap. C-H) Left column: Overlay of all included PRESS spectra and HERMES difference spectra in the ACC and RSM. Right column: A single representative spectrum for each region/sequence. Raw data is shown in black with the model fit in red and the data residual in gray. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
A short-echo time PRESS sequence (TR/TE = 2000/35 ms, 64 water-suppressed scan averages, 8 water unsuppressed scan averages, number of data points = 4096, spectral width = 5000 Hz, acquisition time 2 min 48 s) with chemical shift selective (CHESS) water suppression to quantify Glu and Glx as well as other MRS metabolites including tNAA (N-acetyl-aspartate + N-acetyl-aspartylglutamate), tCho (choline + glycerophosphocholine + phosphocholine), tCr (creatine + phosphocreatine) and mIns (myo-inositol). As the Glu and glutamine signals are highly overlapped, both Glu and Glx were examined and reported to assess for consistency of results. GABA+ (GABA + macromolecules) and GSH data were acquired using a HERMES sequence (TR/TE = 2000/80 ms, 320 water-suppressed scan averages, 16 unsuppressed scan averages, number of data points = 4096, spectral width = 5000 Hz, acquisition time 11 min 20 s). Twenty millisecond editing pulses were executed in a four step Hadamard encoding scheme, in an interleaved fashion as previously described by Saleh et al. (2016). The editing pulses were applied at 1.9 ppm and 4.56 ppm to refocus the coupling of the 3 ppm GABA peak and the 2.9 ppm GSH peak, respectively.
2.4. Magnetic resonance spectroscopy analysis
PRESS data were preprocessed with the FID-A toolbox including the following preprocessing steps: coil combination, removal of motion-corrupted averages, and retrospective frequency and phase correction (Simpson et al., 2017). PRESS data were then analyzed in LCModel (version 6.3–1 J) (Provencher, 1993) using a basis set also developed from the FID-A toolkit, including alanine, aspartate, β-hydroxybutyrate, choline, citrate, creatine, GABA, glucose, glutamine, Glu, glycine, glycerophosphocholine, GSH, lactate, myo-inositol, NAA, N-acetyl-aspartyl-glutamate, phosphocholine, phosphocreatine, phosphoethanolamine, scyllo-inositol and taurine using scanner specific pulse and sequence timings (Simpson et al., 2017). HERMES data were analyzed using Gannet (version 3.1) (Edden et al., 2014) in MATLAB 2020a (Mathworks, Natick, MA, USA). The Gannet3.1 software package includes the following preprocessing steps: coil combination, down-weighting of motion-corrupted averages and retrospective frequency and phase correction (Edden et al., 2014). Additionally, Gannet3.1 generates normalized fit errors by combining the residuals from GABA+ and water data. A detailed description of fit error calculations can be found in Edden et al. (2014).
Visual inspection of spectral data was performed to detect artifacts such as baseline distortions, broadened linewidths of metabolite peaks, frequency and phase errors and assessing the overall appearance of the spectrum. HERMES data were also evaluated for subtraction artifacts. MRS data were excluded if spectra had unresolvable subtraction artifact, GABA+ or GSH fit error > 20 % or the calculated concentration values were > 1.5 times the interquartile range outside of the first quartile or third quartile.
Voxel masks were co-registered to each individual T1-weighted image using Gannet 3.1 to determine voxel fractions of gray matter (GM), white matter (WM) and CSF. Data were tissue-corrected following current recommendations (Near et al., 2021). Briefly, this tissue-correction approach for metabolite quantification uses literature values to account for tissue specific water T1 and T2 relaxation and water density as well as metabolite specific T1 and T2 relaxation constants (Gasparovic et al., 2018). For detailed derivations on this tissue correction approach, readers are directed to Near et al., 2021, Gasparovic et al., 2018. Relaxation and water density values used in correction can be found in Supplemental Table 1. All metabolite concentrations were corrected for the negligible metabolite content in CSF. GABA+ levels have been shown to be approximately twice as high in GM than WM (Harris et al., 2015a). To reduce dependency of the GABA+ measure on tissue fraction, we applied the α-correction method by Harris et al. (2015a):
where cmeas is the measured concentration, ccorr is the corrected concentration, fGM and fWM are the GM and WM fractions and the α factor is the ratio of GABA in WM to GM (i.e., 1:2 = 0.5). This correction adjusts GABA+ estimates to account for heterogeneous tissue composition in the voxel. While Glu and GSH are also likely different between WM and GM, a comparable correction factor has yet to be established in the literature so one has not been applied (i.e., the concentration ratio between WM and GM is assumed to be 1). Additionally, ratios to tCr were calculated for all metabolites.
2.5. Symptom questionnaires
To characterize our sample, all participants were administered the following clinical questionnaires:
-
°
Patient Health Questionnaire-9 (PHQ-9) – Depressive symptoms
-
°
Generalized Anxiety Disorder-7 Scale (GAD-7) – Anxiety
-
°
Fatigue Severity Scale (FSS) – Fatigue
-
°
Epworth Sleepiness Scale (ESS) – Daytime sleepiness
To examine mTBI symptom severity and burden, PPCS participants (but not controls) completed the following measures:
-
°
Rivermead Post Concussion Symptoms Questionnaire (RPQ) – mTBI symptom burden
-
°
Postconcussion Syndrome Checklist (PCSC) – mTBI symptom frequency, intensity and duration
-
°
Headache Impact Test-6 (HIT-6) – Functional impact of headache
-
°
Montreal Cognitive Assessment (MoCA) – Screen for mild cognitive impairment
-
°
Dizziness Handicap Inventory (DHI) – Dizziness handicap
-
°
Quality of Life after Brain Injury scale (QOLIBRI) – Quality of life
Detailed descriptions and citations for all questionnaires can be found in Mercier et al. (2020).
2.6. Statistical analysis
All statistical analyses were performed using SPSS Statistics 28 (IBM, Armonk, NY, USA). Demographic data of the two groups (controls, PPCS) were compared using independent samples t-tests (questionnaire scores) and Chi-squared tests (education, handedness, work status). Descriptive statistics were used to report injury characteristics and questionnaire scores in PPCS participants. Independent samples t-tests were conducted to compare metabolite levels (concentrations and ratios to tCr), measures of MRS data quality (signal-to-noise ratio, linewidth, fit error) and voxel tissue composition between PPCS and control participants. Levene’s test was used to assess for equality of variances for independent samples t-test analyses. As this is the first study using the HERMES method in traumatic brain injury, no informative a priori sample size calculation could be performed; G*Power (Faul et al., 2007) was used for post-hoc power calculations of significant group differences. Correlation analyses were carried out solely in the PPCS group. For correlation analyses examining the relationships between metabolites and mTBI history, time since injury and symptom scores (RPQ, HIT-6, FSS, ESS, PHQ-9, GAD-7, DHI), Shapiro-Wilk tests were used to assess data for normality and determine the appropriate correlation, either Pearson’s correlation (parametric data) or Spearman’s correlation (non-parametric data). The relationship between age, metabolites, symptom scores, time since injury and number of lifetime mTBI were additionally investigated using Pearson’s correlations. Given the sample size and high number of questionnaires, a Holm correction was applied to metabolite associations with symptom scores.
3. Results
3.1. Demographics and injury characteristics
Twenty-nine PPCS participants completed this study (mean age: 45.69 years, s.d.: 10.73, 22 females, 7 males). Twenty-nine age- (±5 years on individual basis) and sex-matched non-head injured control participants were recruited for comparison (mean age: 43.69 years, s.d.: 11.00, 22 females, 7 males). The two groups did not differ in education or self-reported handedness. Demographic information and injury characteristics are summarized in Table 1 and Table 2, respectively.
Table 1.
Demographic characteristics of PPCS and control participants. Scoring ranges for PHQ-9, GAD-7, FSS and ESS are reported. ESS: Epworth Sleepiness Scale; FSS: Fatigue Severity Scale; GAD-7: Generalized Anxiety Disorder-7; PHQ-9: Patient Health Questionnaire-9.
| Demographics | Controls (n = 29) | PPCS (n = 29) | p value |
|---|---|---|---|
| Age, mean (s.d.) | 43.69 (11.00) | 45.83 (10.73) | |
| Age, median [range] | 43.00 [24–62] | 45.00 [23–63] | |
| Sex, M/F | 7M/22F | 7M/22F | |
| Education, n (%) | 0.164 | ||
| PhD/Medical Doctorate | 5 (17 %) | 0 (0 %) | |
| Master’s Degree | 4 (14 %) | 5 (17 %) | |
| Bachelor’s Degree | 13 (45 %) | 12 (41 %) | |
| Trades School/Vocational Education | 5 (17 %) | 8 (28 %) | |
| Grade 12 | 2 (7 %) | 4 (14 %) | |
| Current work status, n (%) | <0.001 | ||
| Full-time (employed or student) | 23 (79 %) | 10 (34 %) | |
| Part-time (employed or student) | 4 (14 %) | 6 (21 %) | |
| Not currently working (employed or student prior to accident) | n/a | 12 (41 %) | |
| Not employed or student | 2 (7 %) | 1 (3 %) | |
| Questionnaires, mean (s.d.) | |||
| Depression, PHQ-9 [scoring range: 0–27] | 3 (5) | 12 (6) | <0.001 |
| Anxiety, GAD-7 [scoring range: 0–21] | 3 (3) | 7 (5) | <0.001 |
| Fatigue, FSS [scoring range: 9–63] | 22 (9) | 45 (15) | <0.001 |
| Daytime sleepiness, ESS [scoring range: 0–24] | 5 (3) | 8 (5) | 0.003 |
| Handedness, R/L | 26R/3L | 28R/1L | 0.300 |
| History of drug or alcohol use disorder | None reported | None reported |
Table 2.
Descriptive summary of PPCS participant characteristics. Clinical questionnaire (RPQ, PCSC, HIT-6, MoCA, DHI and QOLIBRI) scoring ranges and total scores are reported. DHI: Dizziness Handicap Inventory; HIT-6: Headache Impact Test; MoCA: Montreal Cognitive Assessment; NSAID: non-steroidal anti-inflammatory drug; PCSC: Postconcussional Syndrome Checklist; QOLIBRI: Quality of Life after Brain Injury scale; RPQ: Rivermead Post Concussion Symptoms Questionnaire; SNRI: serotonin-norepinephrine reuptake inhibitors; SSRI: selective serotonin reuptake inhibitor.
| Injury characteristics and clinical questionnaire scores | |
|---|---|
| Months since injury, mean (range) | 23 (3–60) |
| Mechanism of injury, n (%) | |
| Sports/recreational play | 12 (41 %) |
| Motor vehicle collision | 7 (24 %) |
| Fall | 6 (21 %) |
| Assault | 1 (3 %) |
| Other | 3 (10 %) |
| Loss of consciousness, n (%) | |
| Yes | 8 (28 %) |
| No | 16 (55 %) |
| Unknown | 5 (17 %) |
| Number of lifetime mTBI, mean (range) | 3 (1–6) |
| Medications, n (%) | |
| Amitriptyline | 6 (21 %) |
| Botox | 4 (14 %) |
| Cholesterol and/or BP-related | 3 (10 %) |
| Levothyroxine | 1 (3 %) |
| Lyrica | 2 (7 %) |
| Menopausal hormone therapy | 2 (7 %) |
| NSAID | 4 (14 %) |
| SNRI | 2 (7 %) |
| SSRI | 4 (14 %) |
| Synthroid | 1 (3 %) |
| Triptan | 3 (10 %) |
| Vyvanse | 1 (3 %) |
| Zopiclone | 2 (7 %) |
| Questionnaires, mean (s.d.) | |
| RPQ [scoring range: 0–64] | 36 (11) |
| Subscales: | |
| RPQ-3 [scoring range: 0–12] | 6 (3) |
| RPQ-13 [scoring range: 0–54] | 29 (10) |
| PCSC [scoring range: 30–150] | 92 (18) |
| HIT-6 [scoring range: 36–78] | 61 (6) |
| MoCA [scoring range: 0–30] | 27 (2) |
| DHI [scoring range: 0–100] | 38 (24) |
| Subscales: | |
| Physical [scoring range: 0–28] | 13 (7) |
| Emotional [scoring range: 0–36] | 10 (9) |
| Functional [scoring range: 0–36] | 15 (9) |
| QOLIBRI [scoring range: 0–100] | 49 (15) |
| Subscales: | |
| Cognition [scoring range: 0–100] | 48 (18) |
| Self [scoring range: 0–100] | 39 (19) |
| Daily life & autonomy [scoring range: 0–100] | 51 (21) |
| Social relationships [scoring range: 0–100] | 59 (19) |
| Emotions [scoring range: 0–100] | 63 (22) |
| Physical problems [scoring range: 0–100] | 39 (22) |
3.2. Data quality and voxel tissue composition
The number of datasets included in statistical analyses together with quantitative quality metrics (signal-to-noise ratio, linewidth, fit error) are reported in Table 3. Due to technical error, PRESS data for four participants were acquired at a TR = 1800 ms and was addressed using appropriate tissue correction parameters. Comparisons were conducted with and without these four participants which yielded equivalent findings. Therefore, results are reported with these data included. The PPCS participants and matched control voxels did not differ in fraction of GM, WM or CSF in the ACC or RSM (see Table 3). Voxel placement and spectral data from both the ACC and RSM can be seen in Fig. 1.
Table 3.
Number of datasets included in final analysis, quantitative quality metrics and voxel tissue fractions. HERMES data quality for GABA+ and GSH subspectra was assessed by SNR, linewidth and fit error. PRESS data quality assessed using the tNAA signal for SNR, linewidth and CRLBs. Data quality and voxel tissue composition did not differ significantly between groups with the exception of ACC GABA+ SNR (t(49) = 2.21, p = 0.032) which was higher in PPCS participants than controls. SNR: signal-to-noise ratio. fGM/fWM/fCSF are the voxel fractions of GM, WM and CSF.
| Anterior cingulate cortex |
Right sensorimotor cortex |
|||
|---|---|---|---|---|
| Control | PPCS | Control | PPCS | |
| scanned | 29 | 28 | 29 | 28 |
| retained | 26 | 25 | 27 | 28 |
| GABA+ SNR | 10.73 ± 1.77 | 11.90 ± 2.02 | 15.28 ± 2.63 | 15.55 ± 2.80 |
| GABA+ FWHM | 23.93 ± 3.58 | 24.14 ± 3.31 | 21.96 ± 1.90 | 21.68 ± 1.14 |
| GABA+ fit error | 9.86 ± 3.71 | 8.90 ± 3.22 | 5.83 ± 1.87 | 5.88 ± 2.10 |
| scanned | 29 | 28 | 29 | 28 |
| retained | 25 | 27 | 26 | 27 |
| GSH SNR | 17.61 ± 4.21 | 18.22 ± 3.66 | 9.07 ± 2.81 | 8.06 ± 1.64 |
| GSH FWHM | 14.61 ± 2.51 | 14.21 ± 3.04 | 11.72 ± 3.99 | 11.63 ± 3.59 |
| GSH fit error | 9.40 ± 2.85 | 10.49 ± 3.51 | 10.92 ± 3.42 | 10.45 ± 2.92 |
| scanned | 29 | 29 | 29 | 29 |
| retained | 29 | 29 | 29 | 29 |
| tNAA SNR | 134.15 ± 19.29 | 134.37 ± 18.83 | 158.28 ± 21.31 | 156.55 ± 24.72 |
| tNAA FWHM | 12.50 ± 3.06 | 12.41 ± 3.11 | 8.74 ± 3.76 | 9.96 ± 3.21 |
| tNAA CRLB | 2.14 ± 0.58 | 2.10 ± 0.31 | 1.24 ± 0.44 | 1.31 ± 0.47 |
| fGM | 0.477 ± 0.033 | 0.469 ± 0.030 | 0.324 ± 0.032 | 0.331 ± 0.036 |
| fWM | 0.347 ± 0.051 | 0.362 ± 0.046 | 0.598 ± 0.042 | 0.591 ± 0.046 |
| fCSF | 0.176 ± 0.043 | 0.169 ± 0.048 | 0.078 ± 0.027 | 0.079 ± 0.031 |
3.3. Group comparisons
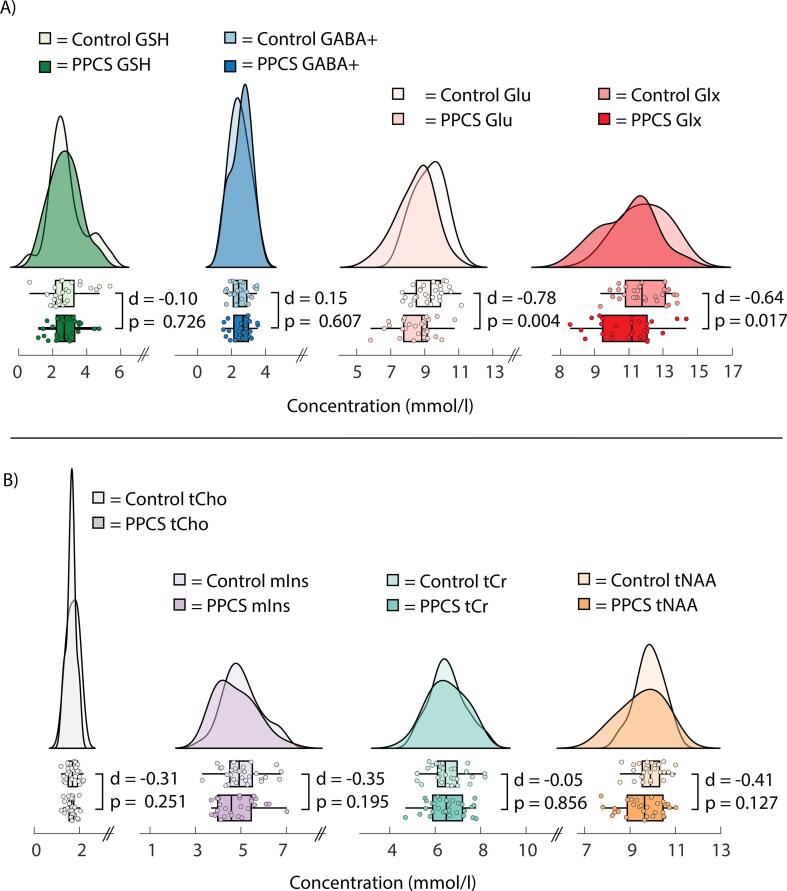
PPCS participants had lower levels of ACC Glu (t(56) = −2.98, p = 0.004, d = −0.78, power (1-β) = 0.83), and the Glx quantification was consistent with this finding (t(56) = −2.45, p = 0.017, d = −0.64, power (1-β) = 0.67) relative to controls. No other metabolite levels (GABA+, GSH, tNAA, tCho, tCr and mIns) differed between groups in the ACC (Fig. 2) and no metabolites differed in the RSM. However, Levene’s test indicated unequal variances in ACC tNAA levels (F = 5.92, p = 0.018). No other metabolite concentrations exhibited unequal variances between groups. These findings were consistent when referencing to tCr. All data are summarized in Supplemental Table 2a and 2b.
Fig. 2.
Raincloud plots depicting anterior cingulate A) GSH, GABA+, Glu, Glx and B) tCho, mIns, tCr and tNAA concentration (mmol/l) in controls and PPCS participants. Independent samples t-tests were used for group comparisons. Raincloud plots were created with script modified from Allen et al. (2021).
3.4. Symptom scores
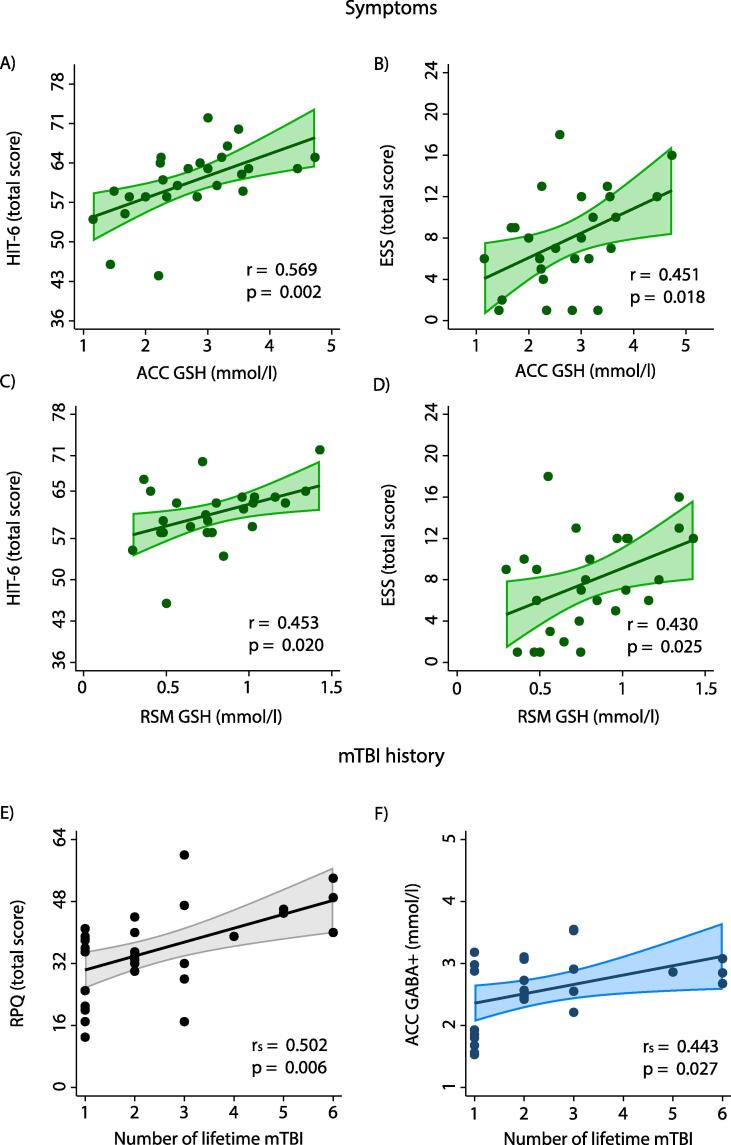
Higher GSH was associated with worse scores on the HIT-6 and the ESS in both regions of interest (HIT-6ACC: r(26) = 0.569, p = 0.002; HIT-6RSM: r(26) = 0.453, p = 0.020; ESSACC: r(27) = 0.451, p = 0.018; ESSRSM: r(27) = 0.430, p = 0.025) (Fig. 3). After applying the Holm correction for multiple comparisons, only the HIT-6 relationship with GSH in the ACC remained statistically significant. Holm-corrected thresholds of statistical significance for the HIT-6 and ESS in both regions of interest were p < 0.007 and p < 0.008 respectively.
Fig. 3.
A-D) Associations between symptom scores and metabolite concentrations (mmol/l). Only the HIT-6 association with ACC GSH survived the Holm correction for multiple comparisons. One HIT-6 score was excluded as an outlier (>1.5 * IQR above Q3). Also depicted are relationships between number of mTBI sustained over lifetime and E) symptom burden as assessed by the RPQ as well as F) ACC GABA+ (mmol/l). ESS: Epworth Sleepiness Scale (scoring range 0–24); HIT-6: Headache Impact Test (scoring range 36–78); IQR: interquartile range; Q3: third quartile. RPQ: Rivermead Post Concussion Symptoms Questionnaire. Shading indicates the 95 % confidence interval.
3.5. mTBI history
ACC GABA+ levels were positively associated with number of mTBIs sustained by PPCS participants (GABA+ACC: rs(25) = 0.443, p = 0.027). No other associations were found between mTBI history and GABA+, Glu, Glx and GSH. Furthermore, the association between number of mTBIs and PPCS symptom burden as assessed by the RPQ was statistically significant, rs(29) = 0.502, p = 0.006 (Fig. 3).
3.6. Time since injury
Of all metabolites assessed, only GSH in the RSM was significantly associated with time since injury, r(27) = 0.413, p = 0.032 (Supplemental Fig. 1).
3.7. Age
No significant relationships between age and metabolites, symptom scores, time since injury and number of lifetime mTBI were detected. Please see Supplemental Table 3a and 3b for the full results.
4. Discussion
This study used the HERMES method to simultaneously investigate GABA+ and GSH in conjunction with Glu/Glx in individuals with PPCS. Compared to controls, the PPCS group showed a regional reduction of Glu and Glx in the ACC. No other significant group differences in metabolite concentrations were found. Relationships between GSH levels and symptoms of daytime sleepiness as well as functional impact of headache were identified in both the ACC and RSM. Higher ACC GABA+ was related to number of previous concussions.
Alterations in Glu (or Glx) levels are often interpreted as a change in cortical excitation. While Glu is an important excitatory neurotransmitter, it also has other roles in the brain. For example, Glu can be oxidized to α-ketoglutarate, a key citric acid cycle substrate (McKenna, 2007). As Glu and α-ketoglutarate are in fast exchange, Glu serves as an index of α-ketoglutarate levels and, by extension, citric acid cycle activity (Andersen et al., 2021). Following the acute post-mTBI energy crisis, energy metabolism is expected to normalize with recovery in days to weeks (Giza and Hovda, 2014). The lower ACC Glu observed here may reflect ongoing energy shortage in PPCS.
Glu is also one of three metabolites (Glu, cysteine, glycine) that form the GSH tripeptide (Rae and Williams, 2017). Low Glu in PPCS may be a result of increased GSH synthesis to maintain sufficient antioxidant levels to mitigate oxidative stress. GSH is upregulated in response to mild stress (Maher, 2005). While GSH levels did not differ between PPCS and controls, GSH showed significant positive association with functional impact of headache and daytime sleepiness, although the relationship with sleepiness was not significant after correction for multiple comparisons. Oxidative stress has been linked to various headache disorders, including migraine, that share features with post-traumatic headache (Geyik et al., 2016, Ashina et al., 2019, Capi et al., 2020) and is often implicated in pre-clinical models of sleep disruption (Villafuerte et al., 2015, Hill et al., 2018, Grubač et al., 2021). We suggest that PPCS symptoms such as headache are related to oxidative stress such that more symptomatic PPCS patients have higher GSH while noting that oxidative stress is not unique to mTBI (Ghezzi et al., 2018) thus levels are still within normal GSH ranges.
It may be positive that GSH levels in PPCS are not different from controls. Low GSH seen chronically in conditions such as Parkinson’s disease (Mischley et al., 2016) and multiple sclerosis (Choi et al., 2010) indicates an inability to mount an antioxidant response to oxidative stress. A lack of GSH group differences between PPCS and controls suggests that the antioxidant defense system is still active in participants with PPCS. However, the maintenance of increased GSH levels may reduce the amount of Glu available for other functions.
In this study, regional differences in GSH were evident; GSH was higher in the ACC than RSM in both PPCS participants and controls. Neuronal morphology and molecular composition vary throughout the brain and contributes to the selective vulnerability of certain brain regions to oxidative stress (Wang et al., 2005, Wang and Michaelis, 2010). The RSM may be inherently less vulnerable to oxidative stress than the ACC and thus maintains lower levels of GSH. Since GSH levels in the RSM are relatively low, Glu stores are not taxed as extensively for GSH synthesis which may explain why reductions in Glu were not found in the RSM.
There was a broad range in time since injury (3 – 42 months) in this study. GSH in the RSM was the only metabolite found to be associated with time since injury. Higher GSH levels seen with greater time since injury is consistent with the self-perpetuating nature of oxidative stress and may indicate the on-going nature of PPCS. No other associations were found between metabolite levels and the time since injury, suggesting that overall metabolite levels are stable in >3 months post-concussion.
While GABA+ did not differ between PPCS participants and controls, PPCS participants who had sustained a greater number of lifetime mTBIs had higher ACC GABA+ concentrations. Wilke et al. have also shown that a history of mTBI is linked to higher GABA+ (Wilke et al., 2017) which may be an adaptive response to repeated incidence of mTBI-induced glutamatergic hyperexcitability (Guerriero et al., 2015). A responsive GABAergic system is important for neuroplasticity as GABAergic tone decreases in long-term potentiation-like plasticity (Hess et al., 1996) and motor learning (Stagg et al., 2011). Reducing GABA levels are thought to act as a gaiting mechanism for neuroplasticity. As PPCS participants with a higher number of previous mTBI also exhibited the greatest overall mTBI symptom burden, high GABA may impede recovery.
GABA+ and Glu/Glx have previously been studied in patients with PPCS, although definitive conclusions have yet to be made in the existing literature (Yasen et al., 2020). Comparing and contrasting different types and stages of recovery from brain injury as well as regions studied can also assist to interpret results. For example, repetitive TBI, as seen in boxers, appears to decrease GABA+ (Kim et al., 2019). Years of repetitive TBI may cause irreversible tissue and cell damage resulting in metabolite decreases whereas in the current study recovery, while prolonged since injury, may still occur. Conversely, with recovery from concussion, data at 2 months (Yasen et al., 2018) and 3 years (Tremblay et al., 2014) following injury suggests no difference in GABA+ from controls, consistent with our suggestion that increased GABA+ in PPCS (3 months – 5 years post-injury) is related to the persistence of symptoms.
To our knowledge only one study has examined GSH in mTBI, and found that increased GSH concentrations were associated with poor gait performance acutely following mTBI (Charney et al., 2020). GSH has not yet been studied in PPCS and this is the first use of editing for GSH in mTBI, which is likely more reliable than GSH measurements acquired from short-echo time PRESS acquisitions at 3 T (Sanaei Nezhad et al., 2017). The spectral editing sequence used here (HERMES) enables simultaneous editing and quantification of GABA+ and GSH, eliminating the need for separate acquisitions, thus reducing overall scan time while maintaining comparable data quality to single metabolite edited MRS experiments (Saleh et al., 2016). Measurement of Glu does not require spectral editing, however, Glu shares similar chemical composition with glutamine and these two metabolites have similar spectra. It is unclear how well Glu can be resolved from glutamine at 3 T and as such they are often reported as the combined ‘Glx’ signal. While the hypothesis of this study was focused on Glu, we report Glx to indicate the consistency between these measures. Another strength of this study was the measurement of metabolites in multiple brain regions. The differing findings in the two regions highlight the importance of avoiding generalizations to the whole brain from a single brain region.
There are several limitations to this study. Editing pulses applied to target GABA spins at 1.9 ppm are not perfectly selective and are known to co-edit macromolecule spins at 1.7 ppm (Choi et al., 2007). Advanced MRS methods have been developed to suppress macromolecule contributions to the GABA signal (Edden et al., 2012). However, macromolecule-suppressed GABA has an even lower signal to noise ratio than typical spectral-edited GABA+ measurements which are already inherently low (Harris et al., 2015b). Secondly, water suppression is an on-going challenge in MRS. We used the well-established and clinically available CHESS method, though more advanced research methods exist. While established, water suppression can be imperfect and can therefore impact quantification. For this study, we have no reason to expect it would have a biased impact between groups or across concussion severities or symptoms. Similarly, general data analysis for advanced MRS methods such as HERMES continue to evolve, including modeling the baseline and data fitting. The tissue correction methods carried out in this study include an α-correction to account for higher GABA in GM than WM as this method is widely used in studies examining GABA+ in the literature (Ip et al., 2021, Bernardino et al., 2022, Heise et al., 2022, Stoby et al., 2022, Trujillo et al., 2022). Relative GM vs WM levels of other metabolites, such as GSH and Glu are not defined (Srinivasan et al., 2010, An et al., 2015, Chan et al., 2019). As such, the lack of correction for GM/WM differences for metabolites other than GABA+ is a limitation. Given fractions of GM, WM and CSF did not differ between PPCS participants and controls, it is unlikely that tissue variation is driving group differences. Additionally, these data and analyses were cross-sectional and as such, causation cannot be determined. Furthermore, there is potential for sampling bias in our PPCS cohort. PPCS participants were recruited from brain injury and pain clinics where they were actively seeking treatment. As such, they may represent a more extreme end of the PPCS severity spectrum and may have a higher number of comorbidities than individuals in the community with persistent symptoms who are not actively seeking treatment. However, PPCS patients with high symptom burden are also those who most urgently need their condition to be better understood so that effective treatments can be developed. PPCS participants were recruited as part of a larger trial where exercise intolerance was an eligibility criterion. As the prevalence of exercise intolerance in PPCS patients has not been established, this may limit the generalizability of our findings to the broader PPCS population. Our sample was largely female. Incidence of PPCS is higher in females than males (Cnossen et al., 2018) and participants were age- and sex-matched, so sex is not driving group differences. However, the unequal sex distribution of our overall sample may influence the detection of metabolite differences between groups. Lastly, medication usage was not exclusionary to avoid disruption of recommended care. Participants were required to maintain a stable pharmacological regimen in the month prior to their MRS scan; however, medication usage remains a possible confound for this study.
5. Conclusion
This study used the recently-developed HERMES spectral editing method to measure GABA+ and GSH and short-echo time PRESS to measure Glu in adults with PPCS. It provides new insight to PPCS and provides rationale for further investigation into Glu, GABA and GSH with larger samples as well as different brain regions. Higher GSH was associated with greater daytime sleepiness and functional impact of headache in both brain regions of interest. Lower regional Glu levels were found in PPCS participants. Together, we suggest that in PPCS the antioxidant defense system may be functioning at the expense of decreasing Glu thus impacting other important brain processes such as cortical excitation and energy metabolism. A history of greater incidence of lifetime mTBI was related to greater PPCS symptom burden and higher ACC GABA+. While mTBI is known to affect widespread brain regions, our data suggests that certain brain regions may be more susceptible to metabolite alteration in symptomatic patients in the chronic phase post-injury.
CRediT authorship contribution statement
Julie M. Joyce: Conceptualization, Investigation, Data curation, Formal analysis, Writing – original draft, Writing – review & editing, Visualization. Leah J. Mercier: Conceptualization, Investigation, Data curation, Writing – review & editing. Mehak Stokoe: Investigation, Writing – review & editing. Parker L. La: Investigation, Writing – review & editing. Tiffany Bell: Investigation, Writing – review & editing. Julia M. Batycky: Investigation, Writing – review & editing. Chantel T. Debert: Conceptualization, Funding acquisition, Resources, Supervision, Writing – review & editing. Ashley D. Harris: Conceptualization, Funding acquisition, Resources, Supervision, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
The authors acknowledge Amanda Ip, Lydia Cho, and Raven Yip for their help with data collection. We thank the support of the Integrative Concussion Research Program (ICRP) at the University of Calgary.
Funding
This work was supported by a New Frontiers in Research Fund Exploration Grant, Foundations of Physical Medicine and Rehabilitation, an Hotchkiss Brain Institute PFUND Award and a Canada Foundation for Innovation John R. Evans Leaders Fund award. ADH holds a Canada Research Chair in Magnetic Resonance Spectroscopy in Brain Injury.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2022.103152.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Allen M., Poggiali D., Whitaker K., Marshall T., van Langen J., Kievit R. Raincloud plots: a multi-platform tool for robust data visualization. Wellcome Open Res. 2021;4(63) doi: 10.12688/wellcomeopenres.15191.12681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An L., Li S., Murdoch J.B., Araneta M.F., Johnson C., Shen J. Detection of glutamate, glutamine, and glutathione by radiofrequency suppression and echo time optimization at 7 tesla. Magn. Reson. Med. 2015;73(2):451–458. doi: 10.1002/mrm.25150. [DOI] [PubMed] [Google Scholar]
- Andersen J.V., Markussen K.H., Jakobsen E., Schousboe A., Waagepetersen H.S., Rosenberg P.A., Aldana B.I. Glutamate metabolism and recycling at the excitatory synapse in health and neurodegeneration. Neuropharmacology. 2021;196:108719. doi: 10.1016/j.neuropharm.2021.108719. [DOI] [PubMed] [Google Scholar]
- Ashina H., Porreca F., Anderson T., Amin F.M., Ashina M., Schytz H.W., Dodick D.W. Post-traumatic headache: epidemiology and pathophysiological insights. Nat. Rev. Neurol. 2019;15(10):607–617. doi: 10.1038/s41582-019-0243-8. [DOI] [PubMed] [Google Scholar]
- Berkowitz B.A. Oxidative stress measured in vivo without an exogenous contrast agent using QUEST MRI. J. Magn. Reson. 2018;291:94–100. doi: 10.1016/j.jmr.2018.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardino I., Dionísio A., Violante I.R., Monteiro R., Castelo-Branco M. Motor cortex excitation/inhibition imbalance in young adults with Autism Spectrum Disorder: A MRS-TMS approach. Front. Psychiatry. 2022;13 doi: 10.3389/fpsyt.2022.860448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borich M.R., Brodie S.M., Gray W.A., Ionta S., Boyd L.A. Understanding the role of the primary somatosensory cortex: Opportunities for rehabilitation. Neuropsychologia. 2015;79(Pt B):246–255. doi: 10.1016/j.neuropsychologia.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottomley P.A. Spatial localization in NMR spectroscopy in vivo. Ann. N. Y. Acad. Sci. 1987;508:333–348. doi: 10.1111/j.1749-6632.1987.tb32915.x. [DOI] [PubMed] [Google Scholar]
- Capi M., Pomes L.M., Andolina G., Curto M., Martelletti P., Lionetto L. Persistent post-traumatic headache and migraine: Pre-clinical comparisons. Int. J. Environ. Res. Public Health. 2020;17(7):2585. doi: 10.3390/ijerph17072585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan K.L., Oeltzschner G., Saleh M.G., Edden R.A.E., Barker P.B. Simultaneous editing of GABA and GSH with Hadamard-encoded MR spectroscopic imaging. Magn. Reson. Med. 2019;82(1):21–32. doi: 10.1002/mrm.27702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charney M.F., Howell D.R., Lanois C., Starr T.C., Liao H., Coello E., Breedlove K.M., Meehan W.P., Koerte I., Lin A.P. Associations between neurochemistry and gait performance following concussion in collegiate athletes. J. Head Trauma Rehabil. 2020;35(5):342–353. doi: 10.1097/HTR.0000000000000616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi C., Bhardwaj P.P., Kalra S., Casault C.A., Yasmin U.S., Allen P.S., Coupland N.J. Measurement of GABA and contaminants in gray and white matter in human brain in vivo. Magn. Reson. Med. 2007;58(1):27–33. doi: 10.1002/mrm.21275. [DOI] [PubMed] [Google Scholar]
- Choi I.Y., Lee S.P., Denney D.R., Lynch S.G. Lower levels of glutathione in the brains of secondary progressive multiple sclerosis patients measured by 1H magnetic resonance chemical shift imaging at 3 T. Mult. Scler. 2010;17(3):289–296. doi: 10.1177/1352458510384010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cnossen M.C., van der Naalt J., Spikman J.M., Nieboer D., Yue J.K., Winkler E.A., Manley G.T., von Steinbuechel N., Polinder S., Steyerberg E.W., Lingsma H.F. Prediction of persistent post-concussion symptoms after mild traumatic brain injury. J. Neurotrauma. 2018;35(22):2691–2698. doi: 10.1089/neu.2017.5486. [DOI] [PubMed] [Google Scholar]
- Cruz-Haces M., Tang J., Acosta G., Fernandez J., Shi R. Pathological correlations between traumatic brain injury and chronic neurodegenerative diseases. Transl. Neurodegener. 2017;6(1) doi: 10.1186/s40035-017-0088-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defrin R. Chronic post-traumatic headache: clinical findings and possible mechanisms. J. Man Manip. Ther. 2014;22(1):36–44. doi: 10.1179/2042618613Y.0000000053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do K.Q., Trabesinger A.H., Kirsten-Krüger M., Lauer C.J., Dydak U., Hell D., Holsboer F., Boesiger P., Cuénod M. Schizophrenia: glutathione deficit in cerebrospinal fluid and prefrontal cortex in vivo. Eur. J. Neurosci. 2000;12(10):3721–3728. doi: 10.1046/j.1460-9568.2000.00229.x. [DOI] [PubMed] [Google Scholar]
- Edden R.A., Puts N.A., Barker P.B. Macromolecule-suppressed GABA-edited magnetic resonance spectroscopy at 3T. Magn. Reson. Med. 2012;68(3):657–661. doi: 10.1002/mrm.24391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edden R.A., Puts N.A., Harris A.D., Barker P.B., Evans C.J. Gannet: A batch-processing tool for the quantitative analysis of gamma-aminobutyric acid-edited MR spectroscopy spectra. J. Magn. Reson. Imaging. 2014;40(6):1445–1452. doi: 10.1002/jmri.24478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul F., Erdfelder E., Lang A.-G., Buchner A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods. 2007;39(2):175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- Flores-Ramos M., Alcauter S., Lopez-Titla M., Bernal-Santamaria N., Calva-Coraza E., Edden R.A.E. Testosterone is related to GABA+ levels in the posterior-cingulate in unmedicated depressed women during reproductive life. J. Affect. Disord. 2019;242:143–149. doi: 10.1016/j.jad.2018.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparovic C., Chen H., Mullins P.G. Errors in 1H-MRS estimates of brain metabolite concentrations caused by failing to take into account tissue-specific signal relaxation. NMR Biomed. 2018;31(6):e3914. doi: 10.1002/nbm.3914. [DOI] [PubMed] [Google Scholar]
- Geyik S., Altunısık E., Neyal A.M., Taysi S. Oxidative stress and DNA damage in patients with migraine. J. Headache Pain. 2016;17(1) doi: 10.1186/s10194-016-0606-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghezzi P., Floridi L., Boraschi D., Cuadrado A., Manda G., Levic S., D'Acquisto F., Hamilton A., Athersuch T.J., Selley L. Oxidative stress and inflammation induced by environmental and psychological stressors: A biomarker perspective. Antioxid. Redox Signal. 2018;28(9):852–872. doi: 10.1089/ars.2017.7147. [DOI] [PubMed] [Google Scholar]
- Giza C.C., Hovda D.A. The new neurometabolic cascade of concussion. Neurosurgery. 2014;75:S24–S33. doi: 10.1227/NEU.0000000000000505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong T., Zhang X., Wei X., Yuan S., Saleh M.G., Song Y., Edden R.A., Wang G. GSH and GABA decreases in IDH1-mutated low-grade gliomas detected by HERMES spectral editing at 3 T in vivo. Neurochem. Int. 2020;141:104889. doi: 10.1016/j.neuint.2020.104889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong T., Liu Y., Chen Y., Lin L., Lin Y., Wang G. Focal cortical dysplasia in epilepsy is associated with GABA increase. NeuroImage Clin. 2021;31 doi: 10.1016/j.nicl.2021.102763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubač Ž., Šutulović N., Šuvakov S., Jerotić D., Puškaš N., Macut D., Rašić-Marković A., Simić T., Stanojlović O., Hrnčić D., Bravo L. Anxiogenic potential of experimental sleep fragmentation Is duration-dependent and mediated via oxidative stress state. Oxid. Med. Cell Longev. 2021;2021:1–14. doi: 10.1155/2021/2262913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerriero R.M., Giza C.C., Rotenberg A. Glutamate and GABA imbalance following traumatic brain injury. Curr. Neurol. Neurosci. Rep. 2015;15(5):27. doi: 10.1007/s11910-015-0545-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris A.D., Puts N.A., Edden R.A. Tissue correction for GABA-edited MRS: Considerations of voxel composition, tissue segmentation, and tissue relaxations. J. Magn. Reson. Imaging. 2015;42(5):1431–1440. doi: 10.1002/jmri.24903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris A.D., Puts N.A.J., Barker P.B., Edden R.A.E. Spectral-editing measurements of GABA in the human brain with and without macromolecule suppression. Magn. Reson. Med. 2015;74(6):1523–1529. doi: 10.1002/mrm.25549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heise K.-F., Rueda-Delgado L., Chalavi S., King B.R., Monteiro T.S., Edden R.A.E., Mantini D., Swinnen S.P. The interaction between endogenous GABA, functional connectivity, and behavioral flexibility is critically altered with advanced age. Commun. Biol. 2022;5(1) doi: 10.1038/s42003-022-03378-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess G., Aizenman C.D., Donoghue J.P. Conditions for the induction of long-term potentiation in layer II/III horizontal connections of the rat motor cortex. J. Neurophysiol. 1996;75(5):1765–1778. doi: 10.1152/jn.1996.75.5.1765. [DOI] [PubMed] [Google Scholar]
- Hill V.M., O’Connor R.M., Sissoko G.B., Irobunda I.S., Leong S., Canman J.C., Stavropoulos N., Shirasu-Hiza M., Taghert P. A bidirectional relationship between sleep and oxidative stress in Drosophila. PLoS Biol. 2018;16(7):e2005206. doi: 10.1371/journal.pbio.2005206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou R., Moss-Morris R., Peveler R., Mogg K., Bradley B.P., Belli A. When a minor head injury results in enduring symptoms: a prospective investigation of risk factors for postconcussional syndrome after mild traumatic brain injury. J. Neurol. Neurosurg. Psychiatry. 2012;83(2):217–223. doi: 10.1136/jnnp-2011-300767. [DOI] [PubMed] [Google Scholar]
- Ip I.B., Emir U.E., Lunghi C., Parker A.J., Bridge H. GABAergic inhibition in the human visual cortex relates to eye dominance. Sci. Rep. 2021;11(1) doi: 10.1038/s41598-021-95685-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iverson G.L., Lange R.T., Wäljas M., Liimatainen S., Dastidar P., Hartikainen K.M., et al. Outcome from complicated versus uncomplicated mild traumatic brain injury. Rehabil. Res. Pract. 2012:415740. doi: 10.1155/2012/415740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay T., Harrington G.E., Adams R., Anderson T., Berrol S., Cicerone K., et al. Definition of mild traumatic brain injury. J. Head Trauma Rehabil. 1993;8(3):86–87. [Google Scholar]
- Kim G.H., Kang I., Jeong H., Park S., Hong H., Kim J., Kim J.Y., Edden R.A.E., Lyoo I.K., Yoon S. Low prefrontal GABA levels are associated with poor cognitive functions in professional boxers. Front. Hum. Neurosci. 2019;13 doi: 10.3389/fnhum.2019.00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koerte I.K., Hufschmidt J., Muehlmann M., Lin A.P., Shenton M.E. Translational research in traumatic brain injury. CRC Press/Taylor & Francis Group; Boca Raton, Florida: 2016. Advanced Neuroimaging of Mild Traumatic Brain Injury. [PubMed] [Google Scholar]
- Lee M.R., Denic A., Hinton D.J., Mishra P.K., Choi D.-S., Pirko I., Rodriguez M., Macura S.I. Preclinical 1H-MRS neurochemical profiling in neurological and psychiatric disorders. Bioanalysis. 2012;4(14):1787–1804. doi: 10.4155/bio.12.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu S.C. Regulation of glutathione synthesis. Mol. Aspects Med. 2009;30(1–2):42–59. doi: 10.1016/j.mam.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher P. The effects of stress and aging on glutathione metabolism. Ageing Res. Rev. 2005;4(2):288–314. doi: 10.1016/j.arr.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Marshall S., Bayley M.M., Berrigan S.L. Clinical practice guidelines for mild traumatic brain injury and persistent symptoms. Can. Fam. Physician. 2012;58(3):257–267. [PMC free article] [PubMed] [Google Scholar]
- McKee A.C., Daneshvar D.H. The neuropathology of traumatic brain injury. Handb. Clin. Neurol. 2015;127:45–66. doi: 10.1016/B978-0-444-52892-6.00004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna M.C. The glutamate-glutamine cycle is not stoichiometric: fates of glutamate in brain. J. Neurosci. Res. 2007;85(15):3347–3358. doi: 10.1002/jnr.21444. [DOI] [PubMed] [Google Scholar]
- Mercier L.J., Fung T.S., Harris A.D., Dukelow S.P., Debert C.T. Improving symptom burden in adults with persistent post-concussive symptoms: a randomized aerobic exercise trial protocol. BMC Neurol. 2020;20(1):46. doi: 10.1186/s12883-020-1622-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messina R., Gollion C., Christensen R.H., Amin F.M. Functional MRI in migraine. Curr. Opin. Neurol. 2022;35(3):328–335. doi: 10.1097/wco.0000000000001060. [DOI] [PubMed] [Google Scholar]
- Mischley L.K., Conley K.E., Shankland E.G., Kavanagh T.J., Rosenfeld M.E., Duda J.E., White C.C., Wilbur T.K., De La Torre P.U., Padowski J.M. Central nervous system uptake of intranasal glutathione in Parkinson's disease. NPJ. Parkinsons Dis. 2016;2(1) doi: 10.1038/npjparkd.2016.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahman‐Averbuch H., Schneider V.J., Lee G.R., Peugh J.L., Hershey A.D., Powers S.W., de Zambotti M., Coghill R.C., King C.D. New insight into the neural mechanisms of migraine in adolescents: Relationships with sleep. Headache. 2022;62(6):668–680. doi: 10.1111/head.14299. [DOI] [PubMed] [Google Scholar]
- Near J., Harris A.D., Juchem C., Kreis R., Marjańska M., Öz G., Slotboom J., Wilson M., Gasparovic C. Preprocessing, analysis and quantification in single-voxel magnetic resonance spectroscopy: experts' consensus recommendations. NMR Biomed. 2021;34(5) doi: 10.1002/nbm.4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obermann M., Nebel K., Schumann C., Holle D., Gizewski E.R., Maschke M., Goadsby P.J., Diener H.-C., Katsarava Z. Gray matter changes related to chronic posttraumatic headache. Neurology. 2009;73(12):978–983. doi: 10.1212/WNL.0b013e3181b8791a. [DOI] [PubMed] [Google Scholar]
- Peek A.L., Leaver A.M., Foster S., Puts N.A., Oeltzschner G., Henderson L., Galloway G., Ng K., Refshauge K., Rebbeck T. Increase in ACC GABA+ levels correlate with decrease in migraine frequency, intensity and disability over time. J. Headache Pain. 2021;22(1) doi: 10.1186/s10194-021-01352-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Permenter C.M., Sherman A.l. StatPearls Publishing; Treasure Island (FL): 2020. Postconcussive syndrome. [PubMed] [Google Scholar]
- Polinder S., Cnossen M.C., Real R.G.L., Covic A., Gorbunova A., Voormolen D.C., Master C.L., Haagsma J.A., Diaz-Arrastia R., von Steinbuechel N. A multidimensional approach to post-concussion symptoms in mild traumatic brain injury. Front. Neurol. 2018;9 doi: 10.3389/fneur.2018.01113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provencher S.W. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn. Reson. Med. 1993;30(6):672–679. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- Qin Z., Su J., He X.-W., Ban S., Zhu Q., Cui Y., Zhang J., Hu Y., Liu Y.-S., Zhao R., Qiao Y., Li J., Liu J.-R., Du X. Disrupted functional connectivity between sub-regions in the sensorimotor areas and cortex in migraine without aura. J. Headache Pain. 2020;21(1) doi: 10.1186/s10194-020-01118-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae C.D. A guide to the metabolic pathways and function of metabolites observed in human brain 1H magnetic resonance spectra. Neurochem. Res. 2014;39(1):1–36. doi: 10.1007/s11064-013-1199-5. [DOI] [PubMed] [Google Scholar]
- Rae C.D., Williams S.R. Glutathione in the human brain: Review of its roles and measurement by magnetic resonance spectroscopy. Anal. Biochem. 2017;529:127–143. doi: 10.1016/j.ab.2016.12.022. [DOI] [PubMed] [Google Scholar]
- Saleh M.G., Oeltzschner G., Chan K.L., Puts N.A.J., Mikkelsen M., Schär M., Harris A.D., Edden R.A.E. Simultaneous edited MRS of GABA and glutathione. NeuroImage. 2016;142:576–582. doi: 10.1016/j.neuroimage.2016.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanaei Nezhad F., Anton A., Parkes L.M., Deakin B., Williams S.R. Quantification of glutathione in the human brain by MR spectroscopy at 3 Tesla: Comparison of PRESS and MEGA-PRESS. Magn. Reson. Med. 2017;78(4):1257–1266. doi: 10.1002/mrm.26532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigurdardottir S., Andelic N., Roe C., Jerstad T., Schanke A.K. Post-concussion symptoms after traumatic brain injury at 3 and 12 months post-injury: a prospective study. Brain Inj. 2009;23(6):489–497. doi: 10.1080/02699050902926309. [DOI] [PubMed] [Google Scholar]
- Simpson R., Devenyi G.A., Jezzard P., Hennessy T.J., Near J. Advanced processing and simulation of MRS data using the FID appliance (FID-A)—An open source, MATLAB-based toolkit. Magn. Reson. Med. 2017;77(1):23–33. doi: 10.1002/mrm.26091. [DOI] [PubMed] [Google Scholar]
- Srinivasan R., Ratiney H., Hammond-Rosenbluth K.E., Pelletier D., Nelson S.J. MR spectroscopic imaging of glutathione in the white and gray matter at 7 T with an application to multiple sclerosis. Magn. Reson. Imaging. 2010;28(2):163–170. doi: 10.1016/j.mri.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Stagg C.J., Bachtiar V., Johansen-Berg H. The role of GABA in human motor learning. Curr. Biol. 2011;21(6):480–484. doi: 10.1016/j.cub.2011.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens F.L., Hurley R.A., Taber K.H., Hurley R.A., Hayman L.A., Taber K.H. Anterior cingulate cortex: Unique role in cognition and emotion. J. Neuropsychiatry Clin. Neurosci. 2011;23(2):121–125. doi: 10.1176/jnp.23.2.jnp121. [DOI] [PubMed] [Google Scholar]
- Stoby K.S., Rafique S.A., Oeltzschner G., Steeves J.K.E. Continuous and intermittent theta burst stimulation to the visual cortex do not alter GABA and glutamate concentrations measured by magnetic resonance spectroscopy. Brain Behav. 2022;12(2):e2478. doi: 10.1002/brb3.2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay S., Beaulé V., Proulx S., Tremblay S., Marjańska M., Doyon J., Lassonde M., Théoret H. Multimodal assessment of primary motor cortex integrity following sport concussion in asymptomatic athletes. Clin. Neurophysiol. 2014;125(7):1371–1379. doi: 10.1016/j.clinph.2013.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trujillo P., Song A.K., Hay K.R., Aumann M., Yan Y., Kang H., Donahue M.J., Claassen D.O. Dopamine-induced changes to thalamic GABA concentration in impulsive Parkinson disease patients. npj Parkinson's Dis. 2022;8(1) doi: 10.1038/s41531-022-00298-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villafuerte G., Miguel-Puga A., Rodríguez E.M., Machado S., Manjarrez E., Arias-Carrión O. Sleep deprivation and oxidative stress in animal models: a systematic review. Oxid. Med. Cell Longev. 2015:234952. doi: 10.1155/2015/234952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Michaelis E.K. Selective neuronal vulnerability to oxidative stress in the brain. Front. Aging Neurosci. 2010;2:12. doi: 10.3389/fnagi.2010.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Pal R., Chen X.W., Limpeanchob N., Kumar K.N., Michaelis E.K. High intrinsic oxidative stress may underlie selective vulnerability of the hippocampal CA1 region. Brain Res. Mol. Brain Res. 2005;140(1–2):120–126. doi: 10.1016/j.molbrainres.2005.07.018. [DOI] [PubMed] [Google Scholar]
- Weerasekera A., Peeters R., Sima D., Dresselaers T., Sunaert S., De Vocht J., Claeys K., Van Huffel S., Van Damme P., Himmelreich U. Motor cortex metabolite alterations in amyotrophic lateral sclerosis assessed in vivo using edited and non-edited magnetic resonance spectroscopy. Brain Res. 2019;1718:22–31. doi: 10.1016/j.brainres.2019.04.018. [DOI] [PubMed] [Google Scholar]
- Wilke S., List J., Mekle R., Lindenberg R., Bukowski M., Ott S., Schubert F., Ittermann B., Flöel A. No effect of anodal transcranial direct current stimulation on gamma-aminobutyric acid levels in patients with recurrent mild traumatic brain injury. J. Neurotrauma. 2017;34(2):281–290. doi: 10.1089/neu.2016.4399. [DOI] [PubMed] [Google Scholar]
- World Health Organization, 1993. “The ICD-10 classification of mental and behavioural disorders: Diagnostic criteria for research.”. 10th ed ed. (Geneva, Switzerland: World Health Organization).
- Xiao W., Loscalzo J. Metabolic responses to reductive stress. Antioxid. Redox Signal. 2020;32(18):1330–1347. doi: 10.1089/ars.2019.7803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasen A.L., Smith J., Christie A.D. Glutamate and GABA concentrations following mild traumatic brain injury: a pilot study. J. Neurophysiol. 2018;120(3):1318–1322. doi: 10.1152/jn.00896.2017. [DOI] [PubMed] [Google Scholar]
- Yasen A.L., Lim M.M., Weymann K.B., Christie A.D. Excitability, inhibition, and neurotransmitter levels in the motor cortex of symptomatic and asymptomatic individuals following mild traumatic brain injury. Front. Neurol. 2020;11:683. doi: 10.3389/fneur.2020.00683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousry T.A., Schmid U.D., Alkhadi H., Schmidt D., Peraud A., Buettner A., et al. Localization of the motor hand area to a knob on the precentral gyrus. Brain. 1997;120(1):141–157. doi: 10.1093/brain/120.1.141. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.