Abstract
IgM monoclonal gammopathy of undetermined significance is a pre-malignant condition for Waldenström macroglobulinemia and other B-cell malignancies, defined by asymptomatic circulating IgM monoclonal protein below 30 g/L with a lymphoplasmacytic bone marrow infiltration of less than 10%. A significant proportion, however, develop unique immunological and biochemical manifestations related to the monoclonal protein itself in the absence of overt malignancy and are termed IgM-related disorders or, more recently, monoclonal gammopathy of clinical significance. The indication for treatment in affected patients is dictated by the pathological characteristics of the circulating IgM rather than the tumor itself. The clinical workup and treatment options vary widely and differ from those for Waldenström macroglobulinemia. The aim of this review is to alert clinicians to IgM monoclonal gammopathy of clinical significance and to provide practical guidance on when to screen for these phenotypes. We discuss clinical characteristics, the underlying clonal profile, diagnostic workup and treatment considerations for five important subtypes: cold agglutinin disease, type I and II cryoglobulinemia, IgM-associated peripheral neuropathy, Schnitzler syndrome and IgM-associated AL amyloidosis. The inhibition of the pathogenic effects of the IgM has led to great success in cold agglutinin disease and Schnitzler syndrome, whereas the other treatments are centered on eradicating the underlying clone. Treatment approaches in cryoglobulinemia and IgM-associated peripheral neuropathy are the least well developed. A multidisciplinary approach is required, particularly for IgM-related neuropathies and Schnitzler syndrome. Future work exploring novel, clone-directed agents and pathogenic IgM-directed therapies is welcomed.
Introduction
IgM monoclonal gammopathy of undetermined significance (MGUS) is defined by asymptomatic circulating IgM monoclonal (M) protein below 30 g/L with a lymphoplasmacytic bone marrow infiltration of less than 10%.1 IgM MGUS is a pre-malignant condition for non-Hodgkin lymphomas, mostly Waldenström macroglobulinemia (WM), chronic lymphocytic lymphoma, and plasma cell neoplasms. Most patients are candidates for observation. However, some develop diverse immunological and biochemical manifestations related to the monoclonal protein itself.2 This may lead to organ damage, even in the absence of overt malignancy. These so-called IgM-related disorders are a distinct clinical entity termed monoclonal gammopathy of clinical significance (MGCS).3 The clinical workup and treatment options vary widely and differ from those for WM, which have been outlined in recent consensus guidelines.4
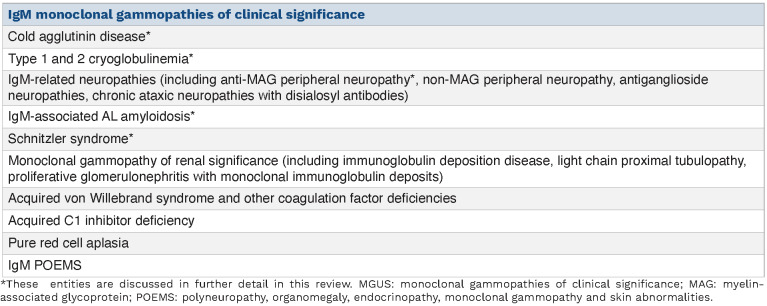
The aim of this review is to alert clinicians to IgM MGCS and to provide practical guidance on when to screen for these phenotypes. We discuss the clinical characteristics, diagnostic workup and treatment considerations for five important subtypes: cold agglutinin disease (CAD), cryoglobulinemia, IgM-associated AL amyloidosis, IgM-related neuropathies and Schnitzler syndrome. A comprehensive list of IgM MGCS is listed below (Table 1).
Clonal characterization
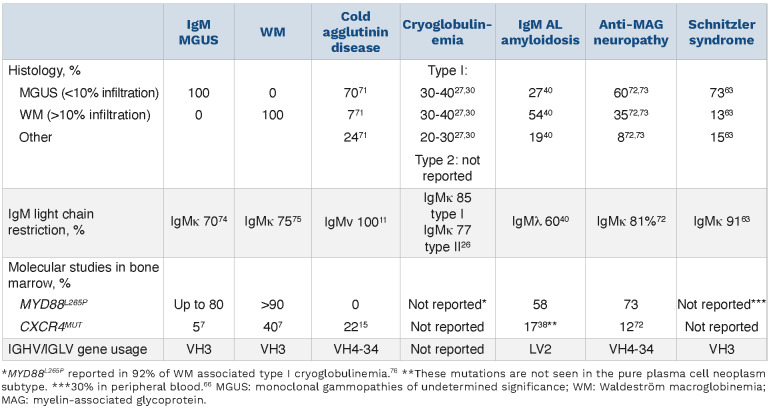
It is important to identify the underlying clone as IgM MGUS may progress to a number of lymphoproliferative disorders or very rarely to myeloma.5 IgM MGUS most commonly arises from a CD20+ lymphoplasmacytic cell without class-switching.1 The risk of progression to lymphoma, chronic lymphocytic leukemia, AL amyloidosis or multiple myeloma is 1.1 event per 100 person-years.5 In the largest series of 210 patients with IgM MGUS with a median follow-up of 29.3 months, no patients progressed to IgM myeloma.5 The incidence and prevalence of IgM MGCS are unknown. Clonal B cells in MGUS have the same genetic and molecular signature as the WM clone. However, MGUS cases have a significantly lower number of mutations than in WM, indicating multiple genetic hits are required for progression. The somatic MYD88L265P mutation constitutively activates nuclear factor kB and triggers B-cell proliferation. It is considered an early acquired mutation and is present in the majority of patients with WM or IgM MGUS.6,7 The gene encoding the chemokine receptor CXCR4, involved in homing of B cells in the bone marrow, is mutated (CXCR4MUT) in a smaller proportion. This is usually a subclonal mutation and likely a late event. IgM myeloma has a distinct cell of origin, a pro-B cell, with frequent t(11;14), an absence of MYD88L265P mutation and high BCL2/BCL2L1 ratio.8 These clonal characteristics may have therapeutic implications. Table 2 summarizes the data on the underlying histology and clonal characteristics of IgM MGCS compared with those seen in WM and IgM MGUS in general.
Table 1.
List of IgM monoclonal gammopathies of clinical significance.
Primary cold agglutinin disease
In primary CAD, autoimmune hemolytic anemia is caused by a cold agglutinin that is a monoclonal IgMk in more than 90% of cases and is produced by clonal lymphocytes in the bone marrow. The antibody binds erythrocyte antigens (typically type I) optimally at 4˚C resulting in agglutination and classical complement pathway activation.9 The thermal amplitude describes the temperature range at which the antibodies are active, and only those with a thermal amplitude reaching higher than 28˚C are considered pathogenic. In most cases, complement activation is incomplete and extravascular hemolysis of C3b-opsonized erythrocytes occurs in the liver. Less frequently there is initiation of the terminal pathway, assembly of the membrane attack complex (C5b-C9) and intravascular hemolysis, which can lead to acute life-threatening anemia. Cold agglutinins in the context of infection, autoimmune disease and overt lymphoma9 (including chronic lymphocytic lymphoma, diffuse large B-cell lymphoma and WM) are referred to as cold agglutinin syndrome. The management of cold agglutinin syndrome is directed at treating the underlying cause and is not further discussed here.
Clinical characteristics
Patients with CAD present with chronic anemia and/or cold-induced circulatory symptoms. Of 232 patients in an international retrospective case series, the median IgM was 3.2 g/L and over 90% had hemolytic anemia and circulatory symptoms. Thirty-eight percent required transfusions at or before diagnosis and 47% during follow-up. Around half had acrocyanosis or Raynaud syndrome affecting daily living. Ulcers or gangrene were rare (<2%).10 In a third of cases, hemoglobin concentration is below 80g/L.11 Circulatory symptoms do not correlate with either the degree of anemia or the bone marrow histology.10,11 There is an increased risk of thrombosis in CAD, likely related to intravascular hemolysis,12,13 which is not correlated with the severity of the anemia.10 CAD is a chronic disease and affected patients have an estimated 16-year survival.10 Clonality has been demonstrated in approximately 80% of cases10,14 and the remainder likely require more sensitive methods to detect the pathogenic clone. The CAD clone has a distinct phenotype that differs from that of WM. MYD88L265P is rarely seen. Recurrent somatic mutations in CXCR4 (20%),10,14,15 KMT2D (69%) and CARD11 (31%) have been described.16 Recurrent chromosomal abnormalities have been identified.17 Based on bone marrow biopsies of 54 cases of CAD, the entity "CAD-associated lymphoproliferative disorder" has been defined, with typical morphology including absent plasmacytoid cells, universally restricted IGHV4-34 gene usage and lack of MYD88L265P.14 Most patients meet the criteria for MGUS and extramedullary disease is rare.10
Table 2.
Clonal characteristics of IgM monoclonal gammopathies of clinical significance, monoclonal gammopathies of undetermined significance and Waldeström macroglobinemia.
Diagnostic workup
Laboratory findings are consistent with hemolysis (and may, therefore, include reticulocytosis, elevated lactate dehydrogenase, unconjugated hyperbilirubinemia and decreased haptoglobin), the monospecific direct antiglobulin test is strongly positive for C3d and there is a cold agglutinin titer of ≥1:64 at 4˚C. Blood samples should be handled warm until separation to prevent agglutination.
Treatment
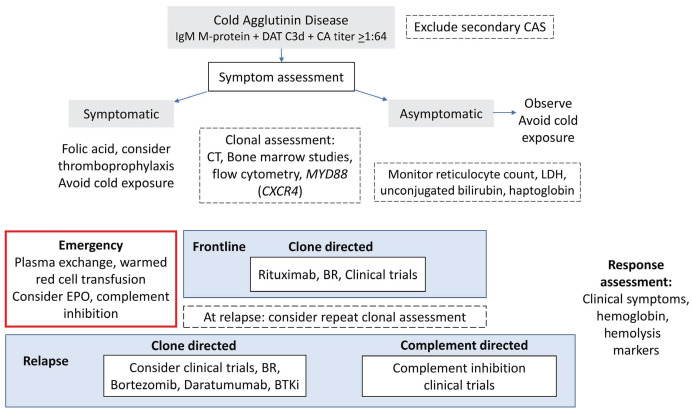
Treatment goals are to alleviate cold-induced symptoms and hemolytic anemia. Response assessment should evaluate hemolytic activity and symptoms as well as clonal response. There are no standard criteria to assess response of cold-induced peripheral symptoms and instead clinicians depend on patient-reported outcomes. A treatment algorithm is provided in Figure 1 and Online Supplementary Table S1. All patients should avoid exposure to cold and be observed, particularly during periods of febrile illness and surgery.10 Red blood cells should be transfused via a blood warmer. Symptomatic patients should commence use of folic acid and be considered for thromboprophylaxis. It is important to note that steroids and splenectomy are not effective in CAD.9,12
We recommend a frontline clone-directed approach (Figure 1), although achieving complete eradication is rare. The most established treatment is rituximab-based therapy. Prospective trials of rituximab monotherapy show a modest response rate of 50% with rare complete responses.18 Real-world data show a 15-month median response duration and repeated responses in over a third of patients.10 Efficacy is greatly improved by the addition of bendamustine. In a prospective study of the bendamustine-rituximab combination with a reduced dose of 70 mg/m2 bendamustine delivered for four cycles to 45 patients, the response rate was 71%, with 40% complete responses and a median increase of hemoglobin of 44 g/L. Grade 4 neutropenia was observed in 20% of the patients and 29% required a dose reduction.19 According to updated data, both the overall and complete response rates improved due to deeper responses over time.10 Rituximab-fludarabine is efficacious (response rate: 62%; complete responses: 38%) but associated with an increased risk of secondary malignancy and is therefore not a preferred option.10,20 Based on a prospective study of 19 patients,21 the response rate to bortezomib-based treatment was 32%, although this was after only a single course of bortezomib. Bruton tyrosine kinase (BTK) inhibitors were effective in all four treated patients with relapsed CAD in a retrospective report.22 There is a case report of the use of daratumumab in CAD.23
Figure 1.
Management of cold agglutinin disease. DAT: direct antiglobulin test; CA: cold agglutinin; CAS: cold agglutinin syndrome; computed tomography; LDH: lactate dehydrogenase; EPO: erythropoietin; BR: bendamustine and rituximab; BTKi: Bruton tyrosine kinase inhibitor.
Clinical trials should be considered in relapsed disease. Promising studies have examined proximal complement inhibition to inhibit extravascular hemolysis. Complement inhibition necessitates indefinite treatment and fails to reduce vascular symptoms. In a phase III study of anti-C1s, sutimlimab, versus best supportive care, it was seen that the complement inhibitor rapidly halted hemolysis, produced transfusion independence in 73% of patients, increased hemoglobin concentration by more than 15 g/L and improved fatigue;24 this drug has now been approved by the Food and Drug Administration.24 The effect of complement inhibition on thrombosis has not been established; however, D-dimer and thrombin-antithrombin complex levels decreased on treatment.13,24 Use of the C5 inhibitor eculizumab rapidly abrogates the terminal complement pathway with a short time to response. However, in a phase II trial there was a marginal hemoglobin rise of 8 g/L.13 Proximal complement inhibition presumably has greater effectiveness because it targets C3-mediated hemolysis via the liver, which is often predominant in CAD. Ongoing clinical trials of complement inhibition in CAD include those studying the C3b inhibitor, pegcetacoplan (phase III, NCT05096403), the complement factor B inhibitor, iptacopan (phase II, NCT05086744), the C1 esterase inhibitor, cinryze (phase II, 2012-003710-13/NL) and the C1s inhibitor BIVV020 (phase Ib, NCT04269551).
Acute life-threatening intravascular hemolysis may necessitate transfusion. Plasma exchange may be employed provided that all priming fluids and the circuit apparatus are pre-warmed and that the replacement products are run through a warmer. Erythropoietin support can be considered as erythropoietin can be inappropriately low in autoimmune hemolytic anemia.25 Complement-directed therapy may act as a bridge for rituximab combinations to target the underlying clone, which can take weeks to have an effect.
Cryoglobulinemia
Cryoglobulinemia is characterized by immunoglobulins that precipitate at temperatures below 37°C and redissolve on warming. Monoclonal IgM can be associated with type I and type II cryoglobulinemia. Type I cryoglobulinemia consists of monoclonal immunoglobulins only. In type II “mixed” cryoglobulinemia there is a monoclonal component possessing avidity for the polyclonal component of a different isotype (most frequently IgM with rheumatoid factor activity, the ability to bind to the Fc portion of IgG). The rheumatoid factor detected in type II cryoglobulinemia is a monoclonal IgMk in over 85% of cases.26 While most cases of type II cryoglobulinemia are related to hepatitis C, here we focus on those related to monoclonal IgM.
Clinical characteristics
Data characterizing patients with monoclonal IgM and cryoglobulinemia are scant. The clinical characteristics have been gleaned from retrospective cohorts grouping together IgG and IgM cases. The largest series reported over 1,600 unselected patients with cryoglobulinemia. Nine percent had type I cryoglobulinemia and 47% had type II.26 The only series characterizing the symptoms of IgM type I cryoglobulinemia included 26 patients; 35% had underlying MGUS, 35% had WM and 31% had non-Hodgkin lymphoma.27 The incidence is likely underestimated as most literature reports are derived from centers that do not routinely screen for cryoglobulinemia upon recognition of an IgM clone.3
A wide spectrum of symptoms may be present (Figure 2). The symptoms of type I cryoglobulinemia are caused by vascular occlusion whereas those of type II are due to small and medium vessel vasculitis. Cutaneous involvement is most frequent in type I cryoglobulinemia. Cutaneous manifestations range from purpura, livedo reticularis, acrocyanosis to cold urticaria, digital ischemia, ulcers and necrosis. Among 26 patients, 46% had skin involvement and less than 10% had peripheral neuropathy (8%), arthralgia (8%) or renal involvement (4%).27 Other studies found peripheral neuropathy in a higher proportion of IgM cases, mainly sensory neuropathy (70%), but sensorimotor polyneuropathy and mononeuritis multiplex were also seen.28 Central nervous system involvement is rare unless due to hyperviscosity.29 No studies have reported specific presenting features of type II cryoglobulinemia in patients with circulating monoclonal IgM. In a mixed cohort of 203 type II patients with an underlying hematologic disorder in 23%, skin manifestations predominated (85%). Compared to type I cryoglobulinemia there was a greater proportion of peripheral neuropathy (56%), joint (41%), renal (38%), gastrointestinal (6%) and pulmonary (2%) involvement. Hyperviscosity is almost never seen.
Diagnostic workup
Laboratory testing is critical as a minimal amount of measurable cryoglobulin may cause symptoms. In one study in which two-thirds of patients were symptomatic, 58% of the IgM type I cryoglobulinemia cases had a cryocrit of <1%, which was a significantly greater proportion than in IgG cryoglobulinemia.27 Symptoms do not correlate with the cryocrit and depend instead on the temperature at which precipitation occurs.29 Accurate detection of cryoglobulins requires samples to be taken into prewarmed tubes which must not be allowed to cool below 37˚C until the serum is separated, as the cryoglobulin may precipitate and not be detected. Similarly, a false-negative M-protein result may result from the same process. In a French study, 9% of cases with negative results were positive on a follow-up test.26 Care must be taken with preanalytical variables; repeat testing of M-protein and cryoglobulins is indicated if the clinical suspicion is high. Increased plasma viscosity in the absence of a high IgM should trigger clinicians to consider cryoglobulinemia.
A tissue biopsy may be indicated to identify renal or nerve involvement and distinguish it from other causes. Intravascular precipitation of IgM triggered by exposure to cold results in thrombotic obstruction and ischemia in small vessels as evidenced on biopsy in type I cryoglobulinemia. Leukocytoclastic vasculitis may be evident in type II cryoglobulinemia.
Treatment
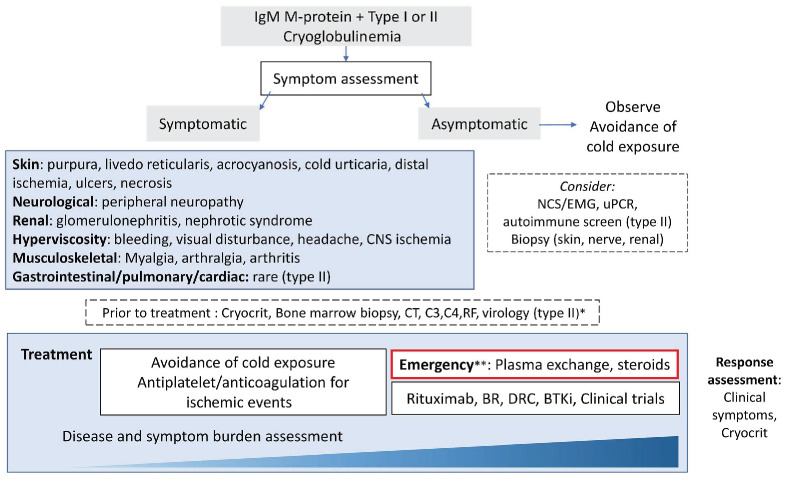
A treatment approach is outlined in Figure 2. There is a paucity of data to guide optimal management. Mild symptoms may abate with cold prevention. Rapidly progressive nephropathy and neuropathy have been reported at various stages of the disease course, so careful monitoring is recommended.28 When cryoglobulinemia is tested for exclusively in symptomatic patients, treatment is commenced for cryoglobulinemia-related symptoms in the majority (80%).30 Response assessment is not standardized and mostly focuses on symptomatic improvement.27 The cryocrit at treatment initiation, change in cryocrit and time to nadir were predictive of symptom improvement in a mixed cohort of patients with IgG and IgM type I cryoglobulinemia. The underlying diagnosis of MGUS or lymphoma did not affect symptom improvement.30
Treatment regimens are heterogeneous and have been used in small series of patients. Plasma exchange may temporize critical symptoms and is used in up to a third of all cases of cryoglobulinemia in mixed cohorts; a warming procedures should be in place.30-32 In the absence of robust evidence, definitive treatment should be directed at the underlying clone. Steroids (1 mg/kg) are used in up to 90% of all cases of cryoglobulinemia, often together with immunosuppression.31,32 Rituximab combinations or bortezomib-based treatment are typically employed27 with symptomatic responses in approximately 80% of cases.27,29,31 Disappearance of cryoglobulin may be seen in half of patients.30 Transient disease exacerbation (an ‘IgM flare’) has been described following the use of rituximab in type I cryoglobulinemia with a low disease burden (<10% infiltrate)33 and in type II cryoglobulinemia.34 Some authors have suggested that a post-rituximab flare in type II cryoglobulinemia may be due to the exogenous IgG from the rituximab infusion which may also be a target of the monoclonal IgM. A study examining plasma exchange prior to rituximab to prevent IgM flares is ongoing (NCT04692363). Currently there are no data on the use of autologous stem cell transplantation or BTK inhibitors in IgM-associated cryoglobulinemia.
Figure 2.
Management of cryoglobulinemia. *Virology testing includes a full hepatitis B profile, hepatitis C, and human immunodeficiency virus. **Emergency indications include symptomatic hyperviscosity, critical ischemia, severe neuropathy, and progressive renal impairment. CNS: central nervous system; NCS/EMG: nerve conduction studies, electromyography; uPCR, urine protein creatinine ratio; CT: computed tomography; BR: bendamustine and rituximab; DRC: dexamethasone, rituximab, cyclophosphamide; BTKi: Bruton tyrosine kinase inhibitor.
IgM-associated AL amyloidosis
AL amyloidosis is a rare disorder caused by extracellular deposition of insoluble misfolded monoclonal light chain fragments, produced by an underlying plasma cell dyscrasia or lymphoma, as amyloid fibrils in tissues. IgM-associated amyloidosis accounts for 5 to 7% of all systemic amyloidoses.35-39 In non-IgM AL amyloidosis, advances in treatment have resulted in marked improvement in survival, although patients with advanced disease have a poor outcome. Data on IgM-associated AL amyloidosis show no improvement over time.40
Clinical characteristics
Due to its rarity, IgM-associated amyloidosis is less well characterized but increasingly recognized as a distinctive entity.40 When compared to non-IgM amyloidosis, patients are older36,37 with a history of MGUS or WM up to 65 months prior to diagnosis.38
Multiple series36,38,41 indicate a smaller proportion of λ light chain involvement compared to that in non-IgM cases. Presenting free light chain levels are lower than in non-IgM AL amyloidosis and in the largest study so far of IgM-associated amyloidosis only two-thirds of the 250 patients had a greater than 50 mg/L difference between involved and uninvolved free light chains.40 The pattern of organ involvement is also different, with a greater propensity for lymph node and soft tissue deposition (35%). Cardiac involvement is less common (45%) and neuropathy more frequent (28%).36,38,40
Diagnostic workup
The exact nature of the clonal dyscrasia in IgM-associated AL amyloidosis remains unclear. The Mayo group has suggested two types, based on morphology; lymphoid predominant (lymphoplasmacytic lymphoma) or plasma cell predominant (pure plasma cell neoplasm).38 Of 75 cases, the lymphoid predominant type (63%) showed a higher tumor infiltrate, MYD88L265P in 84%, CXCR4MUT in 29% but absent t(11;14), similar to WM. By contrast, the cases of pure plasma cell neoplasm (23%) had similar rates of t(11;14) compared to non-IgM-associated amyloidosis and no MYD88L265P/CXCR4MUT, similar to IgM myeloma.38 Patients with the pure plasma cell neoplasm type appear to have poorer outcomes. These findings need independent confirmation to hone treatment approaches.
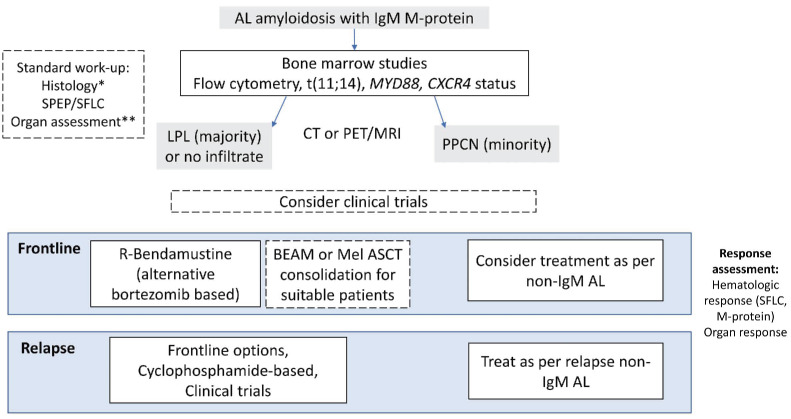
Figure 3.
Management of IgM-associated amyloidosis. *Histological assessment includes targeting affecting organ, consider abdominal fat biopsy. Exclude other acquired and hereditary amyloidoses. **Organ assessment includes comprehensive evaluation of organs including cardiac, renal, neurological, gastrointestinal and soft tissue involvement. M-protein: monoclonal protein; SPEP: serum protein electrophoresis; SFLC: serum free light chains; LPL: lymphoplasmacytic lymphoma; CT: computed tomography; PET: positron emission tomography; MRI: magnetic resonance imaging; PPCN: pure plasma cell neoplasm; R-Bendamustine: rituximab plus bendamustine; BEAM: carmustine, etoposide, cytarabine, melphalan; Mel, melphalan; ASCT: autologous stem cell transplantation; AL: AL amyloidosis.
Treatment
There are no consensus guidelines, approved treatments or prospective clinical trials for IgM amyloidosis. The aims of treatment are to reduce the clonal burden and improve performance status with a view to extending survival. A treatment algorithm is summarized in Figure 3. Evidence is largely limited to retrospective series with heterogeneous regimens. Criteria developed for response assessment in non-IgM AL amyloidosis are applicable to IgM-associated AL amyloidosis with assessment of hematologic response and organ response. Response assessment by both free light chains and M-protein had prognostic significance in retrospective series35,36 alongside age, Mayo stage, cardiac involvement, liver involvement40 and prior WM treatment.38 β2-microglobulin and lactate dehydrogenase levels do not independently affect survival,41 unlike in WM,42 which may be related to the low tumor burden. Despite less cardiac involvement, patients with IgM-associated amyloidosis do not have superior survival compared to those with non-IgM-associated amyloidosis,38 attributable to the inability to achieve deep clonal responses.
Induction of hematologic response is more challenging with a reported 6-month overall response rate of 39% versus 59% (P=0.008), deep responses are seen in only 24%.38
Organ response rates are consequently poor (5% cardiac, 18% renal) and lower than those in a non-IgM-associated cohort.43
Strategies to target the lymphoplasmacytic and plasma cell clones have been employed. The best outcomes have been achieved by autologous stem cell transplantation, with more than 90% achieving a hematologic response.40,41,44 However, up to just 25% of all-comers were eligible for this intense therapy. The largest series of autologous stem cell transplantation in 38 patients44 included 58% who had received prior therapy and the 100-day mortality was 5%. There was, however, a relatively low rate of cardiac involvement (26%), demonstrating the importance of the selection of patients. Induction chemotherapy prior to autologous stem cell transplantation is not universally utilized. Conditioning most commonly involves melphalan, however the BEAM (carmustine, etoposide, cytarabine, melphalan) regimen has also been used.44
As the majority of cases have an underlying lymphoplasmacytic clone, induction therapy with rituximab-based combination chemotherapy is strongly preferred. In 27 cases, the bendamustine-rituximab combination resulted in an intention-to-treat hematologic response rate of 59%, with complete responses in 11%, and a median progression-free survival of 34 months. Sixty percent of patients treated with this combination in second line achieved a very good partial response.45 Bendamustine is neither neurotoxic nor cardiotoxic. Bortezomib in combination with rituximab and dexamethasone may provide rapid disease control. The only prospective trial of this strategy recruited ten patients over 1 year.46 A hematologic response was achieved by 78% with sustained responses at a median of 11 months, after only two cycles. However, there were no complete responses. Treatment had to be interrupted in 30% of patients because of toxicity. Patients with grade 3 sensory and/or grade 1 painful neuropathy were excluded and treatment-related neuropathy is a particular concern in these patients. Responses to frontline alkylating agents have been disappointing. In a series of 46 patients treated after 2003, the hematologic response rate was 37% and there were no complete responses.41 Immunomodulatory drugs alone result in variable response rates, but mostly less than 50%. BTK inhibitors, although promising in WM, have been associated with low response rates in IgM-associated amyloidosis. Of eight patients treated with ibrutinib, only two achieved a hematologic response and the median overall survival was 9 months.47 No studies have examined anti-CD38-bortezomib combinations, which is the standard of care in non-IgM AL amyloidosis.48
We consider upfront bendamustine-rituximab the treatment of choice in IgM-associated amyloidosis, consolidated with autologous stem cell transplantation when the patient’s performance status allows. There is no consensus regarding less fit patients; treatment choices need to be individualized depending on affected organs and tolerance of treatments. Overall in this condition, deep responses remain poor. Future studies are required to address whether regimens based on novel agents (including venetoclax, daratumumab and the newer BTK inhibitors) may lead to improvements in the outcomes of patients with non-IgM AL amyloidosis.
IgM-related neuropathies
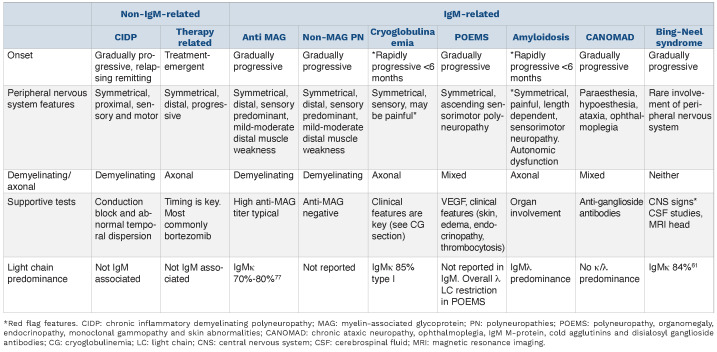
IgM-related peripheral neuropathies encompass an array of entities including immune-mediated neuronal damage, such as that caused by antibodies to myelin-associated glycoprotein (MAG), or direct neurotoxicity with infiltration by lymphoma (neurolymphomatosis), light chains (amyloidosis) or cryoglobulins. Peripheral neuropathy has been found to occur in 15-30% of MGUS and WM cases,49,50 but the prevalence is likely affected by selection bias and variable neurological evaluation in patients as part of a work up of IgM M-protein. The UK registry documented 153 patients with IgM-related neuropathy, comprising antiMAG neuropathy (55%), non-MAG IgM neuropathy (35%) and less frequently (<4% each) AL amyloidosis, cryoglobulinemia, anti-ganglioside neuropathy and CANOMAD syndrome (chronic ataxic neuropathy, ophthalmoplegia, IgM M-protein, cold agglutinins and disialosyl ganglioside antibodies).50
Clinical characteristics
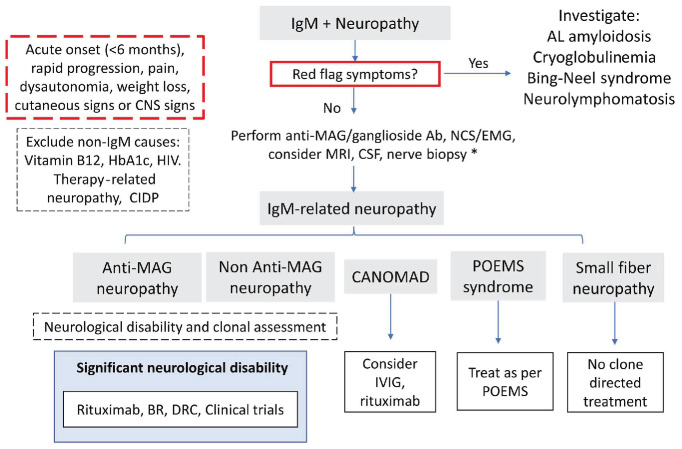
Anti-MAG neuropathy is the most common and best-defined IgM-related neuropathy. Patients typically present with chronic-onset, distal, symmetric neuropathy, sensory ataxia and tremor. Patients may be misdiagnosed as having chronic inflammatory demyelinating polyneuropathy. It is important to correctly classify the neuropathy (Table 3) as this has significant management implications. Atypical “red flag” symptoms not consistent with anti-MAG peripheral neuropathies include acute onset, rapid tempo of symptoms, pain, dysautonomia, weight loss, and cutaneous or central nervous system signs. These should alert the clinician to consider alternate diagnoses (Figure 4). CANOMAD syndrome is a very rare chronic progressive condition associated with antiganglioside antibodies. This syndrome should be considered if there is sensory loss with ophthalmoplegia or ataxia. Bing-Neel syndrome is the term for central nervous system infiltration by lymphoplasmacytic lymphoma; consensus guidelines on its diagnosis, treatment and response criteria have been published.51 Cryoglobulinemia and amyloidosis are discussed in their respective sections.
Diagnostic workup
The majority of patients with IgM-related neuropathy (>90%) have symptoms of the underlying neurological disorder at diagnosis.50 This supports the strong need for careful early evaluation of patients jointly with an expert neurologist. The presence of a peripheral neuropathy alongside a serum monoclonal IgM or anti-MAG antibody does not equal a causal relationship, since gammopathies as well as peripheral neuropathies are both increasingly prevalent with age. Patients should be tested for anti-MAG antibodies, but only high-titer antibodies are clinically relevant in the presence of a characteristic clinical picture in anti-MAG neuropathy.52 A reduction in anti-MAG titers and levels of IgM M-protein with therapy appeared to correlate with improvement in neuropathy in a retrospective analysis of 50 studies.53 Responders also had a younger age of onset.53
Nerve conduction tests and electromyography are warranted and characteristically show demyelination with reduced conduction velocity, disproportionately prolonged distal motor latency and absent sural potentials. Partial motor conduction block is rare. Progressive demyelination may result in secondary axonal loss which affects the likelihood of neural recovery.52 Magnetic resonance imaging of the neuraxis and evaluation of large volumes of cerebrospinal fluid may be required if central nervous system involvement is suspected. A nerve biopsy may be needed if the diagnosis remains elusive despite systematic investigation. Comprehensive consensus guidelines provide further details.52
Table 3.
Features of IgM and non-lgM-related neuropathies.
Figure 4.
Management of IgM-related neuropathies. *Nerve biopsy after consultation with an expert neurologist, in selected cases only. CNS: central nervous system; HIV: human immunodeficiency virus; CIDP: chronic inflammatory demyelinating polyneuropathy; MAG: myelin-associated glycoprotein; Ab: antibody; NCS/EMG: nerve conduction studies/electromyography; MRI: magnetic resonance imaging; CSF: cerebrospinal fluid; CANOMAD: chronic ataxic neuropathy, ophthalmoplegia, IgM M-protein, cold agglutinins and disialosyl ganglioside antibodies; POEMS: polyneuropathy, organomegaly, endocrinopathy, monoclonal gammopathy and skin abnormalities; BR: bendamustine plus rituximab; DRC: dexamethasone, rituximab, cyclophosphamide.
Treatment
In general, in anti-MAG and non-anti-MAG neuropathy, treatment should be initiated only in those with significant or progressive disability.52 The aim of treatment is to halt progression and improve neurological function, although this may potentially take months to years, even after IgM responses. Although many neurological disability scales exist, they are not available outside of specialist neurology clinics and there is no standardized method of response assessment. The use of serial validated patient-reported outcome scores (e.g., the Inflammatory Rasch-Built Overall Disability Scale) is advocated, as this can be easily undertaken in non-specialist clinics.52 An observational trial is currently recruiting with an aim to develop an IgM-specific disability scale (NCT03918421). Patients should be managed in a multidisciplinary fashion with input from neurology, hematology, physiotherapy and occupational therapy.
Rituximab is widely, but inconsistently used in the setting of IgM-related neuropathies. A meta-analysis of rituximab demonstrated improvement in disability scales at 8 to 12 months and long-term efficacy was demonstrated in a third of patients.54 A transient flare of symptoms following the administration of rituximab was observed in 12% in a large series of patients with anti-MAG antitbodies.55 Steroids, intravenous immunoglobulins and plasma exchange alone do not provide long-term clinical benefit in anti-MAG neuropathy56,57 and are resource-intense, respectively. In contrast, intravenous immunoglobulins and rituximab-based regimens are effective in CANOMAD syndrome (producing partial clinical responses or better in 53% and 52% of patients, respectively),58 while chronic inflammatory demyelinating polyneuropathy is responsive to intravenous immunoglobulins,52 highlighting the relevance of correct diagnostic classification.
Although data are largely limited to retrospective series, targeting the underlying clone is feasible in IgM-related neuropathy; the optimum depth of response is unknown. Clinical improvement or stabilization is significantly more likely with rituximab-containing therapy (dexamethasone, rituximab, cyclophosphamide; bendamustine plus rituxi-mab; cyclophosphamide, prednisolone, rituximab, vincris-tine), non-amyloid-related neuropathy and attainment of at least partial haematologic response.49,50
There is an unmet need for reliable biomarkers for diagnosis, appropriate selection of patients for treatment and criteria for monitoring response.59 There is a lack of prospective clinical trials to optimize treatment options. A phase II clinical trial, MAGNAZ, of the oral BTK inhibitor zanubrutinib in anti-MAG peripheral neuropathies is underway.60
Schnitzler syndrome
Schnitzler syndrome is a rare auto-inflammatory disorder characterized by an IgM monoclonal gammopathy and chronic recurrent urticarial rash. The Strasbourg criteria outline additional minor criteria of recurrent fever, abnormal bone remodeling with or without bone pain, neutrophilic dermal infiltrate, leukocytosis and elevated C-reactive protein.61 Around 300 cases have been reported to date. It is underdiagnosed and, despite its rarity, is important to identify as specific treatment can significantly improve quality of life.62
Clinical characteristics
Of 281 cases in the largest case series, fever was present in 72%, anemia in 63%, arthralgia in 68%, bone pain in 55%, lymphadenopathy in 26%, and liver or spleen enlargement and neuropathy in less than 10%.63 In smaller series fatigue and weight loss were documented in up to around 50% of cases.62,64 The urticarial rash can cover any part of the body, but face, palm and sole involvement is infrequent, as is intense pruritis. Skin lesions typically resolve within hours.65 The time from onset of symptoms to diagnosis is long, at a median of 5 years and may be as long as 20 years.62
The monoclonal gammopathy is almost always IgMk. Bone marrow involvement is minimal, being around 4% in one series, and a median M-protein concentration of 6 g/L has been documented.62 In the largest case series, 63% of the 281 bone marrow samples were reported as normal.63 The MYD88L265P mutation was detected in the peripheral blood of 30% of 30 patients.66 The authors suggested that the presence of this mutation may correlate with the risk of WM, although the mutation detection rate may have been underestimated as the sensitivity of detecting peripheral blood B-cell clones may be hampered when the level of disease burden is low. The frequency of the MYD88L265P mutation in bone marrow has not been studied. Chronic inflammation may lead to AA amyloidosis in 2% of cases of Schnitzler syndrome. At a median of 8 years, the rate of evolution to lymphoma is 20%, which is in line with progression in unselected cohorts of patients with IgM MGUS.61,63
Schnitzler syndrome is associated with cytokine dysregulation. It bears close phenotypic resemblance to an inherited disorder, cryopyrin-associated periodic syndrome, caused by gain-of-function mutations in the NLRP3 gene. This results in upregulation of interleukin (IL)-1b production and has informed therapeutic options in Schnitzler syndrome, by targeting IL-1b.
Diagnostic workup
There is no single diagnostic test and the diagnosis is made based on clinical characteristics. Differential diagnoses for the rash and fever include adult-onset Still disease, systemic lupus erythematosus, acquired C1 esterase deficiency, cryopyrinopathies and cryoglobulinemia (coldinduced urticaria). Skin biopsy reveals a neutrophilic urticarial dermatosis without features of vasculitis.
Treatment
Treatment is aimed at reducing the considerable associated morbidity related to rash, fever and joint and bone pain. Symptoms respond poorly to historic first-line agents including antihistamines, nonsteroidal anti-inflammatory drugs, dapsone and colchicine.65 The use of high-dose steroids, although moderately effective, is limited by long-term toxicities.
Without anti-IL treatment, morbidity is high. In a series of 21 patients, all had almost daily symptoms with a profound effect on their quality of life.64 Anti-IL-1 agents, such as anakinra, canakinumab, and rilonacept, have all been used but not directly compared. Anakinra is the agent with which experience is greatest and is the treatment of choice. It is a recombinant IL-1-receptor antagonist and has the greatest efficacy (94% efficacy in 86 cases),63 with durable responses (83% complete responses after a median of 36 months).67 Anakinra has a half-life (t1/2) of 4-6 hours and provides impressive control of all signs within hours, normalization of C-reactive protein levels and abrogation of the risk of AA amyloidosis.64 Nonetheless, patients require continuous daily injections and relapse occurs after treatment discontinuation. Canakinumab, an IL-1b monoclonal antibody, is long-acting (t1/2 21-28 days) and is, therefore, administered less frequently. Data from phase II, placebo-controlled, randomize trials have demonstrated its efficacy. For 17 patients in a long-term study, clinical efficacy was greatest when patients injected canakinumab as needed. A systematic review of 34 patients showed that 59% achieved complete responses.68 Rilonacept, an IL-1 binding and neutralizing fusion protein, achieved near complete responses in 50% of cases.69 To cilizumab, an IL-6 receptor antagonist, has been beneficial in three patients who were refractory to anakinra.70
Cyclophosphamide, rituximab and ibrutinib have achieved responses when treatment was given for overt lymphoma but have been largely ineffective or untested in the absence of lymphoma.65 There is little to support the notion that anti-IL therapy affects the underlying B-cell clone.
Conclusion
We have discussed a range of distinctive entities of IgM MGCS, including their specific clinical characteristics, underlying clonal profile, and diagnostic workup as well as treatment considerations. Careful evaluation of the presenting features and thorough interrogation of the underlying clone are critical. Determining the nature of either a mature B-cell derived clone or plasma cell clone will have management implications. There is an IgMk predominance in all cases except IgM-associated AL amyloidosis. The indication for treatment is dictated by the pathological characteristics of the circulating IgM rather than by the tumor itself. While deep suppression of the pathogenic IgM is typically required for response, achieving long-term clonal eradication is challenging, as demonstrated by low complete response rates. Treatment inhibiting the pathogenic effects of IgM while not directed at the underlying clone has led to great success in CAD (complement inhibitors) and Schnitzler syndrome (cytokine inhibition), whereas the other treatments are centered on eradicating the underlying clone. Treatment approaches in cryoglobulinemia and IgM-related peripheral neuropathies are the least well developed. A multidisciplinary approach is required particularly for IgM-related neuropathies and Schnitzler syndrome.
Due to the rarity of IgM MGCS, data are scant and collaborative research is imperative to aid defining optimal treatment strategies. International registries may better define characteristics and assess treatment outcomes. Future work exploring clone-directed treatment options and pathogenic IgM-directed therapies is welcomed.
Supplementary Material
References
- 1.Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375-2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fermand JP, Bridoux F, Dispenzieri A, et al. Monoclonal gammopathy of clinical significance: a novel concept with therapeutic implications. Blood. 2018;132(14):1478-1485. [DOI] [PubMed] [Google Scholar]
- 3.Owen RG, Treon SP, Al-Katib A, et al. Clinicopathological definition of Waldenstrom's macroglobulinemia: consensus panel recommendations from the Second International Workshop on Waldenstrom's Macroglobulinemia. Semin Oncol. 2003;30(2):110-115. [DOI] [PubMed] [Google Scholar]
- 4.Castillo JJ, Advani RH, Branagan AR, et al. Consensus treatment recommendations from the tenth International Workshop for Waldenström Macroglobulinaemia. Lancet Haematol. 2020;7(11):e827-e837. [DOI] [PubMed] [Google Scholar]
- 5.Kyle RA, Larson DR, Therneau TM, et al. Long-term follow-up of monoclonal gammopathy of undetermined significance. N Engl J Med. 2018;378(3):241-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Treon SP, Xu L, Yang G, et al. MYD88 L265P somatic mutation in Waldenström's macroglobulinemia. N Engl J Med. 2012;367(9):826-833. [DOI] [PubMed] [Google Scholar]
- 7.Varettoni M, Zibellini S, Defrancesco I, et al. Pattern of somatic mutations in patients with Waldenström macroglobulinemia or IgM monoclonal gammopathy of undetermined significance. Haematologica. 2017;102(12):2077-2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bazarbachi AH, Avet-Loiseau H, Szalat R, et al. IgM-MM is predominantly a pre-germinal center disorder and has a distinct genomic and transcriptomic signature from WM. Blood. 2021;138(20):1980-1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jäger U, Barcellini W, Broome CM, et al. Diagnosis and treatment of autoimmune hemolytic anemia in adults: recommendations from the First International Consensus Meeting. Blood Rev. 2020;41:100648. [DOI] [PubMed] [Google Scholar]
- 10.Berentsen S, Barcellini W, D'Sa S, et al. Cold agglutinin disease revisited: a multinational, observational study of 232 patients. Blood. 2020;136(4):480-488. [DOI] [PubMed] [Google Scholar]
- 11.Berentsen S, Ulvestad E, Langholm R, et al. Primary chronic cold agglutinin disease: a population based clinical study of 86 patients. Haematologica. 2006;91(4):460-466. [PubMed] [Google Scholar]
- 12.Barcellini W, Fattizzo B, Zaninoni A, et al. Clinical heterogeneity and predictors of outcome in primary autoimmune hemolytic anemia: a GIMEMA study of 308 patients. Blood. 2014;124(19):2930-2936. [DOI] [PubMed] [Google Scholar]
- 13.Röth A, Bommer M, Hüttmann A, et al. Eculizumab in cold agglutinin disease (DECADE): an open-label, prospective, bicentric, nonrandomized phase 2 trial. Blood Adv. 2018;2(19):2543-2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Randen U, Trøen G, Tierens A, et al. Primary cold agglutinin-associated lymphoproliferative disease: a B-cell lymphoma of the bone marrow distinct from lymphoplasmacytic lymphoma. Haematologica. 2014;99(3):497-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Małecka A, Trøen G, Delabie J, et al. The mutational landscape of cold agglutinin disease: CARD11 and CXCR4 mutations are correlated with lower hemoglobin levels. Am J Hematol. 2021;96(8):E279-E283. [DOI] [PubMed] [Google Scholar]
- 16.Małecka A, Trøen G, Tierens A, et al. Frequent somatic mutations of KMT2D (MLL2) and CARD11 genes in primary cold agglutinin disease. Br J Haematol. 2018;183(5):838-842. [DOI] [PubMed] [Google Scholar]
- 17.Malecka A, Delabie J, Ostlie I, et al. Cold agglutinin disease shows highly recurrent gains of chromosome 3, 12 and 18. Blood. 2019;134(Suppl 1):1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berentsen S, Ulvestad E, Gjertsen BT, et al. Rituximab for primary chronic cold agglutinin disease: a prospective study of 37 courses of therapy in 27 patients. Blood. 2004;103(8):2925-2928. [DOI] [PubMed] [Google Scholar]
- 19.Berentsen S, Randen U, Oksman M, et al. Bendamustine plus ituximab for chronic cold agglutinin disease: results of a Nordic prospective multicenter trial. Blood. 2017;130(4):537-541. [DOI] [PubMed] [Google Scholar]
- 20.Berentsen S, Randen U, Vågan AM, et al. High response rate and durable remissions following fludarabine and rituximab combination therapy for chronic cold agglutinin disease. Blood. 2010;116(17):3180-3184. [DOI] [PubMed] [Google Scholar]
- 21.Rossi G, Gramegna D, Paoloni F, et al. Short course of bortezomib in anemic patients with relapsed cold agglutinin disease: a phase 2 prospective GIMEMA study. Blood. 2018;132(5):547-550. [DOI] [PubMed] [Google Scholar]
- 22.Jalink M, Berentsen S, Castillo JJ, et al. Effect of ibrutinib treatment on hemolytic anemia and acrocyanosis in cold agglutinin disease/cold agglutinin syndrome. Blood. 2021;138(20):2002-2005. [DOI] [PubMed] [Google Scholar]
- 23.Tomkins O, Berentsen S, Arulogun S, Sekhar M, D'Sa S. aratumumab for disabling cold agglutinin disease refractory to B-cell directed therapy. Am J Hematol. 2020. Jul 11. doi: 10.1002/ajh.25932. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 24.Röth A, Barcellini W, D'Sa S, et al. Sutimlimab in cold agglutinin disease. N Engl J Med. 2021;384(14):1323-1334. [DOI] [PubMed] [Google Scholar]
- 25.Fattizzo B, Michel M, Zaninoni A, et al. Efficacy of recombinant erythropoietin in autoimmune hemolytic anemia: a multicenter international study. Haematologica. 2021;132(5):622-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kolopp-Sarda MN, Nombel A, Miossec P. Cryoglobulins today: detection and immunologic characteristics of 1,675 positive samples from 13,439 patients obtained over six years. Arthritis Rheumatol. 2019;71(11):1904-1912. [DOI] [PubMed] [Google Scholar]
- 27.Zhang LL, Cao XX, Shen KN, et al. Clinical characteristics and treatment outcome of type I cryoglobulinemia in Chinese patients: a single-center study of 45 patients. Ann Hematol. 2020;99(8):1735-1740. [DOI] [PubMed] [Google Scholar]
- 28.Néel A, Perrin F, Decaux O, et al. Long-term outcome of monoclonal (type 1) cryoglobulinemia. Am J Hematol. 2014;89(2):156-161. [DOI] [PubMed] [Google Scholar]
- 29.Harel S, Mohr M, Jahn I, et al. Clinico-biological characteristics and treatment of type I monoclonal cryoglobulinaemia: a study of 64 cases. Br J Haematol. 2015;168(5):671-678. [DOI] [PubMed] [Google Scholar]
- 30.Sidana S, Rajkumar SV, Dispenzieri A, et al. Clinical presentation and outcomes of patients with type 1 monoclonal cryoglobulinemia. Am J Hematol. 2017;92(7):668-673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Terrier B, Karras A, Kahn JE, et al. The spectrum of type I cryoglobulinemia vasculitis: new insights based on 64 cases. Medicine (Baltimore). 2013;92(2):61-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Terrier B, Krastinova E, Marie I, et al. Management of noninfectious mixed cryoglobulinemia vasculitis: data from 242 cases included in the CryoVas survey. Blood. 2012;119(25):5996-6004. [DOI] [PubMed] [Google Scholar]
- 33.Nehme-Schuster H, Korganow AS, Pasquali JL, Martin T. Rituximab inefficiency during type I cryoglobulinaemia. Rheumatology (Oxford). 2005;44(3):410-411. [DOI] [PubMed] [Google Scholar]
- 34.Sène D, Ghillani-Dalbin P, Amoura Z, Musset L, Cacoub P. Rituximab may form a complex with IgMkappa mixed cryoglobulin and induce severe systemic reactions in patients with hepatitis C virus-induced vasculitis. Arthritis Rheum. 2009;60(12):3848-3855. [DOI] [PubMed] [Google Scholar]
- 35.Wechalekar AD, Lachmann HJ, Goodman HJ, Bradwell A, Hawkins PN, Gillmore JD. AL amyloidosis associated with IgM paraproteinemia: clinical profile and treatment outcome. Blood. 2008;112(10):4009-4016. [DOI] [PubMed] [Google Scholar]
- 36.Palladini G, Russo P, Bosoni T, et al. AL amyloidosis associated with IgM monoclonal protein: a distinct clinical entity. Clin Lymphoma Myeloma. 2009;9(1):80-83. [DOI] [PubMed] [Google Scholar]
- 37.Gertz MA, Buadi FK, Hayman SR. IgM amyloidosis: clinical features in therapeutic outcomes. Clin Lymphoma Myeloma Leuk. 2011;11(1):146-148. [DOI] [PubMed] [Google Scholar]
- 38.Sidana S, Larson DP, Greipp PT, et al. IgM AL amyloidosis: delineating disease biology and outcomes with clinical, genomic and bone marrow morphological features. Leukemia. 2020;34(5):1373-1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.la Torre A, Reece D, Crump M, et al. Light chain amyloidosis (AL) associated with B cell lymphoma a single center experience. Clin Lymphoma Myeloma Leuk. 2021;21(12):e946-e959. [DOI] [PubMed] [Google Scholar]
- 40.Sachchithanantham S, Roussel M, Palladini G, et al. European collaborative study defining clinical profile outcomes and novel prognostic criteria in monoclonal immunoglobulin M-related light chain amyloidosis. J Clin Oncol. 2016;34(17):2037-2045. [DOI] [PubMed] [Google Scholar]
- 41.Terrier B, Jaccard A, Harousseau JL, et al. The clinical spectrum of IgM-related amyloidosis: a French nationwide retrospective study of 72 patients. Medicine (Baltimore). 2008;87(2):99-109. [DOI] [PubMed] [Google Scholar]
- 42.Kastritis E, Morel P, Duhamel A, et al. A revised international prognostic score system for Waldenström's macroglobulinemia. Leukemia. 2019;33(11):2654-2661. [DOI] [PubMed] [Google Scholar]
- 43.Venner CP, Lane T, Foard D, et al. Cyclophosphamide, bortezomib, and dexamethasone therapy in AL amyloidosis is associated with high clonal response rates and prolonged progression-free survival. Blood. 2012;119(19):4387-4390. [DOI] [PubMed] [Google Scholar]
- 44.Sidiqi MH, Buadi FK, Dispenzieri A, et al. Autologous stem cell transplant for IgM-associated amyloid light-chain amyloidosis. Biol Blood Marrow Transplant. 2019;25(3):e108-e111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Manwani R, Sachchithanantham S, Mahmood S, et al. Treatment of IgM-associated immunoglobulin light-chain amyloidosis with rituximab-bendamustine. Blood. 2018;132(7):761-764. [DOI] [PubMed] [Google Scholar]
- 46.Palladini G, Foli A, Russo P, et al. Treatment of IgM-associated AL amyloidosis with the combination of rituximab, bortezomib, and dexamethasone. Clin Lymphoma Myeloma Leuk. 2011;11(1):143-145. [DOI] [PubMed] [Google Scholar]
- 47.Pika T, Hegenbart U, Flodrova P, Maier B, Kimmich C, Schönland SO. First report of ibrutinib in IgM-related amyloidosis: few responses, poor tolerability, and short survival. Blood. 2018;131(3):368-371. [DOI] [PubMed] [Google Scholar]
- 48.Kastritis E, Palladini G, Minnema MC, et al. Daratumumab-based treatment for immunoglobulin light-chain amyloidosis. N Engl J Med. 2021;385(1):46-58. [DOI] [PubMed] [Google Scholar]
- 49.Treon SP, Hanzis CA, Ioakimidis LI, et al. Clinical characteristics and treatment outcome of disease-related peripheral neuropathy in Waldenstrom's macroglobulinemia (WM). J Clin Oncol. 2010;28(15_suppl):8114. [Google Scholar]
- 50.Tomkins O, Lindsay J, Keddie S, et al. Neuropathy with IgM gammopathy: incidence, characteristics and management, a Rory Morrison W.M.U.K Registry analysis. Blood. 2020;136(Suppl 1):1-2.32430499 [Google Scholar]
- 51.Minnema MC, Kimby E, D'Sa S, et al. Guideline for the diagnosis, treatment and response criteria for Bing-Neel syndrome. Haematologica. 2017;102(1):43-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.D'Sa S, Kersten MJ, Castillo JJ, et al. Investigation and management of IgM and Waldenström-associated peripheral neuropathies: recommendations from the IWWM-8 consensus panel. Br J Haematol. 2017;176(5):728-742. [DOI] [PubMed] [Google Scholar]
- 53.Hänggi P, Aliu B, Martin K, Herrendorff R, Steck AJ. Decrease in serum anti-MAG autoantibodies is associated with therapy response in patients with anti-MAG neuropathy: retrospective study. Neurol Neuroimmunol Neuroinflamm. 2022;9(1):e1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lunn MP, Nobile-Orazio E. Immunotherapy for IgM anti-myelin-associated glycoprotein paraprotein-associated peripheral neuropathies. Cochrane Database Syst Rev. 2016;10(10):CD002827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Svahn J, Petiot P, Antoine JC, et al. Anti-MAG antibodies in 202 patients: clinicopathological and therapeutic features. J Neurol Neurosurg Psychiatry. 2018;89(5):499-505. [DOI] [PubMed] [Google Scholar]
- 56.Dalakas MC. Clinical benefits and immunopathological correlates of intravenous immune globulin in the treatment of inflammatory myopathies. Clin Exp Immunol. 1996;104 (Suppl 1):55-60. [PubMed] [Google Scholar]
- 57.Nobile-Orazio E, Meucci N, Baldini L, Di Troia A, Scarlato G. Long-term prognosis of neuropathy associated with anti-MAG IgM M-proteins and its relationship to immune therapies. Brain. 2000;123(Pt 4):710-717. [DOI] [PubMed] [Google Scholar]
- 58.Le Cann M, Bouhour F, Viala K, et al. CANOMAD: a neurological monoclonal gammopathy of clinical significance that benefits from B-cell-targeted therapies. Blood. 2020;136(21):2428-2436. [DOI] [PubMed] [Google Scholar]
- 59.Amaador K, Wieske L, Koel-Simmelink MJA, et al. Serum neurofilament light chain, contactin-1 and complement activation in anti-MAG IgM paraprotein-related peripheral neuropathy. J Neurol. 2022;269(7):3700-3705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Minnema MC, Vos J, Eftimov F, Vrancken A. P-034: MAGNAZ trial - a prospective phase II study in patients with monoclonal gammopathy of unknown significance (MGUS) and anti-myelin associated glycoprotein (MAG) neuropathy and zanubrutinib treatment. Clin Lymphoma Myeloma Leuk. 2021;21(Suppl 2):S57. [Google Scholar]
- 61.Simon A, Asli B, Braun-Falco M, et al. Schnitzler's syndrome: diagnosis, treatment, and follow-up. Allergy. 2013;68(5):562-568. [DOI] [PubMed] [Google Scholar]
- 62.Jain T, Offord CP, Kyle RA, Dingli D. Schnitzler syndrome: an under-diagnosed clinical entity. Haematologica. 2013;98(10):1581-1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.de Koning HD. Schnitzler's syndrome: lessons from 281 cases. Clin Transl Allergy. 2014;4:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rowczenio DM, Pathak S, Arostegui JI, et al. Molecular genetic investigation, clinical features, and response to treatment in 21 patients with Schnitzler syndrome. Blood. 2018;131(9):974-981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lipsker D. The Schnitzler syndrome. Orphanet J Rare Dis. 2010;5:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pathak S, Rowczenio DM, Owen RG, et al. Exploratory study of MYD88 L265P, rare NLRP3 variants, and clonal hematopoiesis prevalence in patients with Schnitzler syndrome. Arthritis Rheumatol. 2019;71(12):2121-2125. [DOI] [PubMed] [Google Scholar]
- 67.Néel A, Henry B, Barbarot S, et al. Long-term effectiveness and safety of interleukin-1 receptor antagonist (anakinra) in Schnitzler's syndrome: a French multicenter study. Autoimmun Rev. 2014;13(10):1035-1041. [DOI] [PubMed] [Google Scholar]
- 68.Betrains A, Staels F, Vanderschueren S. Efficacy and safety of canakinumab treatment in Schnitzler syndrome: a systematic literature review. Semin Arthritis Rheum. 2020;50(4):636-642. [DOI] [PubMed] [Google Scholar]
- 69.Krause K, Weller K, Stefaniak R, et al. Efficacy and safety of the interleukin-1 antagonist rilonacept in Schnitzler syndrome: an open-label study. Allergy. 2012;67(7):943-950. [DOI] [PubMed] [Google Scholar]
- 70.Krause K, Feist E, Fiene M, Kallinich T, Maurer M. Complete remission in 3 of 3 anti-IL-6-treated patients with Schnitzler syndrome. J Allergy Clin Immunol. 2012;129(3):848-850. [DOI] [PubMed] [Google Scholar]
- 71.Swiecicki PL, Hegerova LT, Gertz MA. Cold agglutinin disease. Blood. 2013;122(7):1114-1121. [DOI] [PubMed] [Google Scholar]
- 72.Allain JS, Thonier F, Pihan M, et al. IGHV segment utilization in immunoglobulin gene rearrangement differentiates patients with anti-myelin-associated glycoprotein neuropathy from others immunoglobulin M-gammopathies. Haematologica. 2018;103(5):e207-e210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vos JM, Notermans NC, D'Sa S, et al. High prevalence of the MYD88 L265P mutation in IgM anti-MAG paraprotein-associated peripheral neuropathy. J Neurol Neurosurg Psychiatry. 2018;89(9):1007-1009. [DOI] [PubMed] [Google Scholar]
- 74.Kyle RA, Benson J, Larson D, et al. IgM monoclonal gammopathy of undetermined significance and smoldering Waldenström's macroglobulinemia. Clin Lymphoma Myeloma. 2009;9(1):17-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kyle RA, Benson JT, Larson DR, et al. Progression in smoldering Waldenstrom macroglobulinemia: long-term results. Blood. 2012;119(19):4462-4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Khwaja J, Patel AS, Arulogun SO, et al. IgM paraprotein-associated type 1 cryoglobulinaemia: clinical characteristics and outcomes. Blood. 2021;138(Suppl 1):4503-4503. [Google Scholar]
- 77.Nobile-Orazio E, Barbieri S, Baldini L, et al. Peripheral neuropathy in monoclonal gammopathy of undetermined significance: prevalence and immunopathogenetic studies. Acta Neurol Scand. 1992;85(6):383-390. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.