Abstract
The search for clinically effective antivirals against the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) is ongoing. Repurposing of drugs licensed for non–coronavirus disease 2019 (COVID-19) indications has been extensively investigated in laboratory models and in clinical studies with mixed results. Nafamostat mesylate (nafamostat) is a drug licensed in Japan and Korea for indications including acute pancreatitis and disseminated intravascular coagulation. It is available only for continuous intravenous infusion. In vitro human lung cell line studies with nafamostat demonstrate high antiviral potency against SARS-CoV-2 (half maximal inhibitory concentration [IC50] of 0.0022 µM [compared to remdesivir 1.3 µM]), ostensibly via inhibition of the cellular enzyme transmembrane protease serine 2 (TMPRSS2) preventing viral entry into human cells. In addition, the established antithrombotic activity is hypothesised to be advantageous given thrombosis-associated sequelae of COVID-19. Clinical reports to date are limited, but indicate a potential benefit of nafamostat in patients with moderate to severe COVID-19. In this review, we will explore the pre-clinical, pharmacokinetic and clinical outcome data presently available for nafamostat as a treatment for COVID-19. The recruitment to ongoing clinical trials is a priority to provide more robust data on the safety and efficacy of nafamostat as a treatment for COVID-19.
Key Points
| In vitro studies have demonstrated that nafamostat mesylate has antiviral activity and appears to be one of the most potent drugs against severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). |
| Clinical trials to date are limited but suggest a potential benefit of nafamostat in patients with severe coronavirus disease 2019 (COVID-19). |
| Current evidence from case reports and observational studies provides guidance for potential adverse effects. |
Introduction
There is an urgent need for effective antivirals to combat the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), responsible for the globally disruptive coronavirus disease 2019 (COVID-19) pandemic. Since December 2019, there have been hundreds of millions of cases worldwide and millions of deaths. Therefore, establishing effective treatments for patients with COVID-19 is a priority for clinicians and researchers, and would also provide much hope for the broader community.
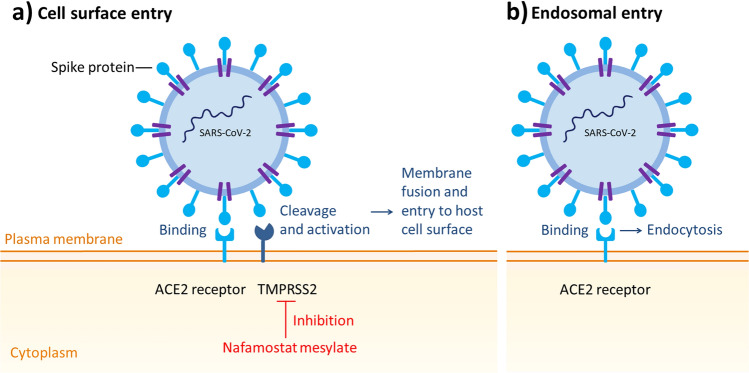
Like other coronaviruses, SARS-CoV-2 is an enveloped single-strand RNA virus that contains four main structural proteins: the spike (S), nucleocapsid (N), membrane (M) and envelope (E) proteins [1]. Early SARS-CoV-2 in vitro studies showed that the S protein promotes entry into human lung epithelium-derived Calu-3 cells [2] and binds to the angiotensin-converting enzyme 2 (ACE2) receptor on the host cell, and employs the cellular enzyme transmembrane protease serine 2 (TMPRSS2) for S protein priming [3]. In respiratory epithelia, the S protein is cleaved by TMPRSS2, which facilitates membrane fusion and entry to the host cell surface [4]. Targeting host proteins instead of viral proteins could prevent treatment failure due to target mutations [5]. Therefore, TMPRSS2 represents a promising drug target.
Nafamostat mesylate (nafamostat) is a broad-spectrum synthetic serine protease inhibitor that has been widely used in Japan and Korea for non-infection indications such as pancreatitis and disseminated intravascular coagulation. It has demonstrated nanomolar potency in in vitro SARS-CoV-2 studies [6]. In studies combining antiviral potency with achievable plasma concentrations, nafamostat is consistently one of the most potent drugs against SARS-CoV-2 [7]. However, its limited global licensing as well as its pharmacokinetic characteristics mean that it has not been extensively studied to date as a potential COVID-19 antiviral treatment.
In this review, we describe nafamostat’s pharmacology, potency against SARS-CoV-2 and available clinical outcome data.
Methods
We conducted a literature search in PubMed and Cochrane databases between October 2021 and February 2022, for keywords nafamostat and COVID-19. The medRxiv Health Sciences source was searched to identify preprints of preliminary reports not yet peer reviewed or published. Additionally, registers of ongoing clinical studies were screened in the US National Library of Medicine (ClinicalTrials.gov), International Clinical Trials Registry Platform (ICTRP – trialsearch.who.int), International Standard Randomised Controlled Trial Number registry (ISRCTN – isrctn.com) and the European Clinical Trials Register (clinicaltrialsregister.eu).
Pharmacology
Nafamostat mesylate ((6-carbamimidoylnaphthalen-2-yl) 4-(diaminomethylideneamino) benzoate) [8] (Fig. 1) is a broad-spectrum synthetic serine protease inhibitor that has been widely used in Japan and Korea for the treatment of acute pancreatitis and disseminated intravascular coagulation [9]. The dosing for nafamostat varies based on the indication. For acute pancreatitis, the approved mode of administration is 10 mg by intermittent intravenous (IV) infusion over 2 h [10], once or twice daily according to the patient’s status. For disseminated intravascular coagulation, a continuous infusion of 0.06–0.20 mg/kg/h is used [9, 11].
Fig. 1.
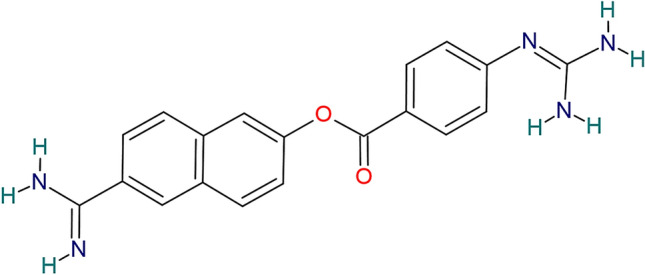
Chemical structure depiction of nafamostat mesylate
Nafamostat is also approved as an anticoagulant therapy for patients with bleeding tendencies undergoing continuous renal replacement therapy or cardiopulmonary bypass. It has actions as an anticoagulant (it inhibits the activity of a variety of serine proteases generated during the coagulation cascade and the inflammatory process, such as the activated factors VIIa and XIIa, kallikrein, thrombin, components of the complement system and trypsin [8, 9]), antifibrinolytic [12] (it inhibits tissue-type and urokinase plasminogen activators) and antiplatelet [13]. The indicated dose of nafamostat when used to prevent blood coagulation during extracorporeal blood circulation is from 20 to 50 mg/h continuously infused [14].
Nafamostat has been identified as a potent inhibitor of the S-mediated membrane fusion [5], and manifests antiviral activity through inhibition of TMPRSS2 [5, 15] (Fig. 2).
Fig. 2.
SARS-CoV-2 entry pathways. ACE2 angiotensin-converting enzyme 2, SARS-CoV-2 severe acute respiratory syndrome coronavirus-2, TMPRSS2 transmembrane protease serine 2
Pharmacokinetics and Pharmacodynamics
In non-COVID-19 patients, a very short half-life between 5 and 23 min has been reported [16–19]. Plasma concentrations of nafamostat in healthy volunteers immediately following the 90-min infusion of single 10, 20 and 40 mg doses were 10–20, 30–60 and 70–90 ng/mL, respectively [9]. The steady-state plasma concentrations of nafamostat when continuously infused to patients with disseminated intravascular coagulation at 0.1 or 0.2 mg/kg/h were 14–130 ng/mL [20]. For use as a COVID-19 treatment, continuous infusion of nafamostat is advised over intermittent infusion because of its short half-life and to ensure consistent concentrations above SARS-CoV-2 90% maximal effective concentration (EC90) values [20].
Nafamostat is rapidly metabolised by esterases in circulating blood into 6-amidino-2-naphthol (AN) and 4-guanidinobenzoic acid (4-GBA), which are inactive protease inhibitors [21]. Hyperkalaemia is a known side effect of nafamostat mesylate administration [22–25]. Renal and extrarenal K+ imbalance have been described as mechanisms of hyperkalaemia related to nafamostat. Muto et al. [26] reported that the two metabolites of nafamostat act on the apical membrane of the collecting duct cell and inhibit the amiloride-sensitive Na+ conductance, causing inhibition of K+ secretion. In addition, Ookawara et al. [27] reported that nafamostat and its metabolite AN inhibit erythrocyte potassium influx by suppressing the Na-K ATPase-dependent pathway. The findings of the previous investigations suggest that hyperkalaemia is caused by these metabolites rather than nafamostat.
There is only one report of nafamostat pharmacokinetics in COVID-19 patients, which was a small (n = 42) phase Ib/IIa, open-label, randomised, controlled trial exploring the safety and tolerability of nafamostat in patients with COVID-19 pneumonia, the DEFINE trial [28]. Patients randomised to the nafamostat arm (n = 21) received the drug as a continuous IV infusion at a dosage of 0.2 mg/kg/h, with nafamostat and its inactive metabolite 4-GBA measured in blood samples prior to starting drug administration and at 50 min, 2 h and 6 h after commencing the infusion. The authors observed plasma concentrations of nafamostat were almost undetectable, while plasma concentrations of its inactive metabolite were elevated, which suggests rapid breakdown. The authors attempted to determine why their pharmacokinetic results were significantly different from that reported in previous non-COVID-19 studies, using in vitro experiments, but could not identify an underlying rationale. Other than speculation about COVID-19 disease and drug co-therapy, it remains unclear why nafamostat was not measurable in this study, when it has been measurable in non-COVID-19 patient populations [16, 19, 29].
Potency Against SARS-CoV-2
In vitro studies of the anti-SARS-CoV-2 activity of nafamostat have been performed in human lung epithelium-derived Calu-3 cells [7, 30] and H3255 cells [30]. Relative to other drugs with SARS-CoV-2 potency data from human cell lines, nafamostat appears to be a highly potent antiviral drug [7]. In Calu-3 cells, nafamostat has a SARS-CoV-2 50% maximal effective concentration (EC50) ranging from 1–10 nM [30, 31]. In a human lung cell model (Calu-3 cells), nafamostat was reported to have a half maximal inhibitory concentration (IC50) of 2.2 nM [7], which is significantly lower than other COVID-19 antivirals, including remdesivir (IC50 = 1300 nM) [7], molnupiravir (IC50 = 1965 nM) [32] and nirmatrelvir (IC50 = 176.5 nM) [32]. These nafamostat potency values are well below the steady-state blood concentrations of nafamostat, 30–240 nM, achieved with typical continuous infusion dosages in non-COVID-19 patients, 0.1–0.2 mg/kg/h [30]. Combination antivirals may be useful for further improving outcomes. There is no clinical data to support the use of combination therapy, but further studies could explore this possibility. Nafamostat has been administered concomitantly with other antiviral agents such as favipiravir [33] and lopinavir [34] in the reports described below.
SARS-CoV-2 mutations and the emergence of new variants have the potential to alter antiviral potency. The Delta and Omicron variants are reported to have 11 [35] and 32 [36] mutations in the spike, respectively. There is variability in the finding of studies that address whether the potency of drugs that block TMPRSS2 entry of SARS-CoV-2 is modified against Delta and Omicron strains. In a pre-print from Meng and colleagues [37] comparing potency of various antivirals against both Delta and Omicron strains using lung cells and Calu-3 cell models, Omicron was shown to be more likely to enter human cells via endosomes rather than TMPRSS2 receptors (Fig. 2). The authors found that camostat, another serine protease inhibitor, had reduced potency against Omicron compared with Delta in a lung cell line [38]. This suggests that nafamostat potency may be different with the Omicron variant. However, Bojkova and collaborators [39] tested the effect of nafamostat on the replication of two SARS-CoV-2 Omicron isolates and one Delta isolate in Calu-3 cells. The authors did not detect differences between the sensitivity of Omicron and Delta isolates to nafamostat (Delta IC50 0.037 µM; Omicron 1 IC50 0.035 µM; Omicron 2 IC50 0.043 µM). Thus, there is no conclusive evidence that nafamostat has significantly lost potency for Omicron.
IC50 data of drugs that exhibit antiviral effect against SARS-CoV-2 in Calu-3 human lung cells are shown in Table 1.
Table 1.
Potency of drugs that exhibit antiviral effect against SARS-CoV-2 in Calu-3 human lung cells
| Drug | Half maximal inhibitory concentration [IC50] (µM) | References |
|---|---|---|
| Nafamostat | 0.002 | Ko et al. [7] |
| Nirmatrelvir | 0.177 | Li et al. [32] |
| Ivermectin | 0.2 | Jitobaom et al. [54] |
| Camostat | ~1 | Ellinger et al. [55] |
| Remdesivir | 1.3 | Ko et al. [7] |
| Molnupiravir | 1.965 | Li et al. [32] |
| Lopinavir | 21.7 | Ko et al. [7] |
| Favipiravir | 913.4 | Jitobaom et al. [54] |
SARS-CoV-2 severe acute respiratory syndrome coronavirus-2
Clinical Outcome Studies of Nafamostat
The available clinical data regarding the use of nafamostat in the treatment of COVID-19 patients is summarised in Table 2.
Table 2.
Clinical data available on the use of nafamostat mesylate in the treatment of COVID-19 patients
| Authors | Location | Time when study was conducted | Type of study | Nafamostat dosing regimen | Participants | Outcomes | Adverse events reported |
|---|---|---|---|---|---|---|---|
| Iwasaka et al. [41] | Japan | February, 2020 | Case series | 0.2–0.4 mg/kg/h, in combination with hydroxychloroquine | 1 elderly patient | Respiratory status and imaging results improved, and the patient was discharged from hospital | None reported |
| Jang and Rhee [39] | South Korea | February–March, 2020 | Case series | 200 mg for 24 h by continuous infusion in combination with lopinavir/ritonavir and hydroxychloroquine | 3 elderly patients | All the patients showed radiological improvement and were discharged from the hospital | None reported |
| Hifumi et al. [42] | Japan | March–April, 2020 | Case series | 200 mg/day | 1 adult patient | The patient recovered well | Diffuse microbleeding |
| Doi et al. [38] | Japan | April, 2020 | Case series | 0.2 mg/kg/h by continuous infusion, in combination with favipiravir | 11 adult patients | 7 (64%) successfully weaned from mechanical ventilation, 9 (82%) discharged from the ICU, 7 (64%) discharged from the hospital, 1 (9%) died | Hyperkalaemia 1 (9%) |
| Okajima et al. [25] | Japan | April, 2020 | Case series | 0.13–0.16 mg/kg/h | 4 adult patients | 4 (100%) developed hyperkalaemia immediately after nafamostat mesylate administration | Hyperkalaemia 4 (100%) |
| Takahashi et al. [40] | Japan | Not reported | Case series | 200 mg/24 h, in combination with unfractioned heparin | 1 elderly patient | The respiratory condition of the patient improved. Nafamostat-induced hyperkalaemia | Hyperkalaemia 1 (100%) |
| Koriyama et al. [43] | Japan | Not reported | Case series | 100 mg daily in combination with dexamethasone | 1 elderly patient | Fever reduction, decrease in D-dimer levers and oxygen administration discontinued. Patient was discharged | Not reported |
| Doi et al. [20] | Japan | October, 2020 | Observational study | Doses according to disease severity (not specified) by continuous or intermittent infusion | 515 adult patients | 299 (58.1%) discharged alive, 52 (10.5%) transferred for de-escalation of care, 38 (7.4%) still hospitalised, 35 (6.8%) transferred for escalation of care, 89 (17.3%) died in hospital | Not reported |
| Inokuchi et al. [44] | Japan | January–December, 2020 | Observational study | Not specified | 15,859 adult patients (only 121 received nafamostat) | No difference in in-hospital mortality between the groups with nafamostat | Not reported |
| Zhuravel et al. [45] | Russia | September, November, 2020 |
Phase II, open-label, multicentre, randomised, controlled trial |
4.8 mg/kg/day via 24-h intravenous infusion plus standard of care vs standard of care alone | 102 adult patients |
No significant difference in time to clinical improvement between the groups. No significant difference observed between the groups in the time of recovery. The observed benefits of nafamostat were more evident in patients with baseline NEWS ≥7. Secondary endpoints such as change in clinical status, shorter length of hospital stay, and 28-day mortality improved in the nafamostat group |
Catheter site phlebitis 9 (8.8%) Hyponatremia 4 (3.9%) Respiratory failure 4 (3.9%) |
| Quinn et al. [28] | United Kingdom | September 2020–February 2021 |
Phase Ib/IIa, open-label, multicentre, platform, randomised, controlled trial ISRCTN14212905 NCT04473053 DEFINE trial |
0.2 mg/kg/h by continuous infusion plus standard of care vs standard of care alone | 42 adult patients | The nafamostat group had an average longer hospital stay and were on oxygen for a median of 2 days more than patients in the standard of care group. Nafamostat did not improve either the clinical biomarkers that show disease severity or the rate of change in any immune parameters | Hyperkalaemia 6 (14.3%) |
COVID-19 coronavirus disease 2019, ICU intensive care unit, NEWS National Early Warning Score
Case Series
The clinical effects of nafamostat have been reported in small case series of patients hospitalised with COVID-19 pneumonia. In the first case series by Doi et al. [33], 11 adult patients with SARS-CoV-2 infection were admitted to the intensive care unit (ICU) at the University of Tokyo Hospital between April 6 and April 21, 2020. All patients needed high-level oxygen support: eight patients (73%) invasive mechanical ventilation and three patients (27%) venovenous extracorporeal membrane oxygenation. Nafamostat was administered by continuous IV infusion at a dosage of 0.2 mg/kg/h, with favipiravir as combination therapy for a median treatment duration of 14 days. Seven patients (64%) were successfully weaned from mechanical ventilation, nine patients (82%) were discharged from the ICU, seven patients (64%) were discharged from the hospital, and one patient (9%) died. Only one patient had treatment interruption caused by hyperkalaemia associated with nafamostat (severity not defined by the authors).
Jang and Rhee [34] described three elderly patients with COVID-19 pneumonia and a supplementary oxygen requirement, with underlying diseases including hypertension and diabetes mellitus, in South Korea between February and March 2020. Each patient was receiving lopinavir/ritonavir and hydroxychloroquine and was administered nafamostat continuously at a dose of 200 mg for 24 h. The patients experienced clinical and radiological improvement after nafamostat administration and were discharged. The authors reported no adverse events associated with nafamostat.
Okajima et al. [25] described the time course of four critically ill mechanically ventilated patients with severe COVID-19 pneumonia. Importantly, hyperkalaemia (˃ 6 mEq/L in two patients) was observed immediately after the administration of nafamostat at a dosage of 0.13–0.16 mg/kg/h. The serum potassium concentrations of all the patients normalised after cessation of the nafamostat infusion.
Case reports of continuously infused nafamostat from Takahashi et al. [40], Iwasaka et al. [41] and Hifumi et al. [42] are also available. In each case, the patient was discharged alive, but each detailed important considerations. Takahashi et al. [40] reported a case of hyperkalaemia associated with nafamostat (20 mg/day) that resolved with cessation of drug, prompting the authors to hypothesise that a 4-h infusion discontinuation during a 24-h period may mitigate against hyperkalaemia. Iwasaka et al. [41] reported the use of nafamostat as an anticoagulant during continuous haemodiafiltration (0.2–0.4 mg/kg/h) in a critically ill COVID-19 patient. Finally, Hifumi et al. [42] reported the use of nafamostat (200 mg/day) in a critically ill COVID-19 patient receiving venovenous extracorporeal membrane oxygenation for severe hypoxia. Even though the patient recovered, the clinical course was complicated, including acute cognitive dysfunction associated with microbleeding in the subcortical area. Although cerebral haemorrhage causes remain unknown and this effect has not been widely reported, the importance of considering bleeding complications with nafamostat is highlighted.
Koriyama et al. [43] reported the case of a 70-year-old man hospitalised with COVID-19 with many risk factors for disease aggravation in whom early multidrug therapy was effective. Laboratory findings showed coagulation abnormalities such as fibrinogen degradation products and high fibrinogen and D-dimer levels (518 mg/dL and 1.68 µg/mL, respectively), along with typical findings of early mild COVID-19 pneumonia. The patient was started with favipiravir (3600 mg daily), which was discontinued due to side effects (somnolence and weakness). Oxygen saturation (SpO2) decreased, and 1 L/min oxygen administration by nasal cannula was started. Then remdesivir was administered (200 mg, with 100 mg daily for the following 5 days). However, a tendency of increasing D-dimer levels was observed, and SpO2 continued to decrease, requiring an increase in oxygen flow rate to 4 L/min. Thus, a combination of nafamostat (100 mg daily by continuous IV infusion) and dexamethasone (6 mg daily) was administered for 4 days. The authors state that the treatment was remarkably effective, resulting in fever reduction and decrease in D-dimer levels. After the fourth day of nafamostat administration, oxygen administration could be discontinued. Although it took some more time for SpO2 to stabilise, it eventually improved, and the patient was discharged.
Observational Studies
An observational study from Japan reported the outcomes of 699 COVID-19 patients from 171 hospitals treated with nafamostat up until the end of October 2020 [20]. Patients had respiratory failure and were mostly > 60 years of age with underlying diseases. Forty-three per cent of patients did not require supplemental oxygen, 42.5% did require supplemental oxygen, and 14.5% required mechanical ventilation or extracorporeal membrane oxygenation. Prior to administration of nafamostat, patients may have been treated with a variety of drugs including favipiravir, ciclesonide, dexamethasone, methylprednisolone and/or remdesivir. Nafamostat was administered by continuous infusion in 76% of the patients and intermittent infusion in 24% of the patients at different doses (not specified) according to the severity of the disease, for a median treatment duration of 6 days. The clinical outcome was assessed at approximately 1 month after hospital admission. Of the 515 patients whose clinical outcome was recorded, 299 (58.1%) were discharged alive, 52 (10.5%) were transferred for de-escalation of care, 38 (7.4%) were still hospitalised, 35 (6.8%) were transferred for escalation of care, and 89 (17.3%) died in hospital. No data on nafamostat adverse effects were recorded.
A retrospective study from Japan used propensity score matching from electronic health record data of patients admitted for COVID-19 to evaluate in-hospital mortality [44]. Patients who received nafamostat within 2-days of admission were compared to a control group that did not receive nafamostat. A large number of patients were eligible for analysis (n = 15,859), although only 121 patients received nafamostat. Unmatched groups were dramatically different in terms of age, comorbidities, mechanical ventilation and other treatments used such as antibiotics, heparin, vasopressor therapy and steroids. In the unmatched groups, the in-hospital mortality rates were 13.2% for nafamostat and 5.0% for the control group. After imputing data for missing patient parameters (e.g. body mass index and smoking status), propensity score matching using a logistic regression model predicting nafamostat use was undertaken. The authors found no difference in in-hospital mortality between the groups treated with nafamostat (odds ratio 1.27, 95% confidence interval 0.62–2.64; p = 0.52), where 1 is the reference value for no nafamostat administration.
Randomised Clinical Trials
To date, only two published randomised controlled trials of nafamostat for treatment of COVID-19 are available. The first was a phase II, open-label, randomised, controlled trial of nafamostat plus standard of care versus standard of care alone in hospitalised patients with COVID-19 pneumonia [45] (NCT04623021). This study was conducted in 13 sites across Russia between September 25 and November 14 2020, and included 102 adult COVID-19 patients requiring supplemental oxygen treatment, nasal high-flow oxygen or non-invasive mechanical ventilation. Patients were randomly assigned to receive nafamostat at 4.8 mg/kg/day via 24-h continuous IV infusion for 10 days or until hospital discharge. Glucocorticoid use was not permitted. The primary outcome was time to clinical improvement, defined by the authors as ‘time from randomisation to either discharge from hospital or improvement of two points on the 7-category ordinal scale (National Early Warning Score [NEWS] as recommended by World Health Organisation), which ever came first’. The time to recovery was defined by the authors as either discharge from hospital or hospitalisation for infection-control purposes only and was included as a secondary outcome. Other secondary outcomes included the proportion of patients with recovery, rate of clinical improvement, time to NEWS ≤ 2, duration of hospitalisation and 28-day mortality, among others. No participants received remdesivir, and few received baricitinib (n = 13) or tocilizumab (n = 2).
In the overall cohort, the authors found no significant difference in time to clinical improvement between the group that received nafamostat (n = 52) versus standard of care alone (n = 50) (median 11 vs 11 days, p = 0.953). In sub-analyses, in the most unwell patients (baseline NEWS ≥7), time to clinical improvement was shortened by 3 days in the nafamostat group compared to the standard of care group (median 11 vs 14 days, p = 0.012). Furthermore, the time to clinical improvement was numerically shorter in the nafamostat group than in the standard of care group in patients aged ≥ 65 years (median 11 vs 14 days, p = 0.083) as well as in the patients with baseline oxygen saturations < 90% (median 11 vs 14 days, p = 0.190). No significant difference was observed between the nafamostat group and the standard of care group in the time to recovery (median 11 vs 11 days, p = 0.968) as well as in the response rates of recovery (88.5% vs 80.0%). However, in patients with baseline NEWS ≥ 7, the time to recovery was reduced by 4 days in the nafamostat group compared to the standard of care group (10 vs 1 4 days, p = 0.012), which was associated with a trend to more rapid viral elimination. Additionally, time to NEWS ≤ 2, which was maintained for 24 h, was three times faster in the nafamostat group (p = 0.007). Additional secondary endpoints that improved in the nafamostat group include change in clinical status, shorter length of hospital stay and 28-day mortality (1.9% [1/52] for nafamostat and 8% [4/50] for standard of care, p = 0.155). The observed benefits of nafamostat treatment appeared strongly related to disease severity and having a baseline NEWS ≥ 7. Most adverse events in the nafamostat group were mild in severity, and no worsening of pneumonia symptoms or fatal outcomes were reported. The most common adverse events associated with nafamostat were catheter site phlebitis (n = 7 [13.5%] vs 2 [3.9%] in the standard of care group), hyponatraemia (n = 4 [7.7%] vs 0) and respiratory failure (n = 3 [5.8%] vs 1 [2%]). The authors concluded that in the most unwell patients (baseline NEWS ≥ 7), nafamostat added to the standard of care was superior to standard of care alone in accelerating the clinical improvement and recovery of COVID-19 patients.
As described above (see the ‘Pharmacokinetics and Pharmacodynamics’ section), the DEFINE trial was a small, phase Ib/IIa, open-label, multicentre, platform, randomised, controlled trial exploring the safety and tolerability of nafamostat in patients with COVID-19 pneumonia [28] in the United Kingdom. Participants were assigned to different treatment groups: nafamostat plus standard of care (n = 21), standard of care alone (n = 21), or an ‘alternative’ therapy (no data available). The primary endpoint was the safety and tolerability of IV nafamostat as add on therapy for patients hospitalised with COVID-19 pneumonia. Secondary endpoints included biomarker and SARS-CoV-2 kinetics. Patients randomised to the nafamostat arm received the intervention for 7 days, or until discharge or withdrawal. The treatment group that received nafamostat had an average longer hospital stay, and were on oxygen for a median of 2 days more than patients in the standard of care group. There was no difference observed in the viral load between the nafamostat and the standard of care groups. The nafamostat group experienced more adverse events compared to the standard of care group, although there were no serious adverse events reported in either group. However, the treatment course was discontinued early in six patients (29%) due to moderate hyperkalaemia (authors did not define potassium levels), remarking that this effect is driven by the metabolites and not the parent nafamostat drug [21]. While little to no anticoagulant effect (measured as clotting time) was evident in most patients receiving nafamostat, an antifibrinolytic effect (by means of the lysis time) was observed. The authors concluded that their study does not support the use of nafamostat in hospitalised patients with COVID-19.
Discussion
Nafamostat has a number of key characteristics that suggest it may be a promising drug candidate for the treatment of COVID-19. Clinical studies are limited. In vitro data demonstrates that nafamostat is a highly potent inhibitor of viral replication by preventing the fusion of the envelope of the SARS-CoV-2 with the host cell. In addition to the reported antiviral activity, nafamostat anticoagulant, antiplatelet and antifibrinolytic [9] activity may be advantageous in severe COVID-19 patients where thrombotic complications are common. Nafamostat has been shown to be a safe and effective anticoagulant [17, 46, 47]. However, one must be aware of potential interactions with other anticoagulant therapy that may be administered concomitantly, which could result in bleeding.
The safety profile of nafamostat is well established since it has been used therapeutically in Japan and Korea for over 30 years. Hyperkalaemia appears common and may warrant cessation of nafamostat infusions. Yet, collection of further safety and pharmacokinetic data for nafamostat should be prioritised in COVID-19 patients. Nafamostat dosing for treatment of COVID-19 has been derived from non-infectious indications, and is yet to be validated in COVID-19 patients as achieving plasma concentrations exceeding target EC50/EC90 values. Adequately powered viral kinetics and immunology studies to characterise the mechanisms of any observed clinical outcomes compared to the standard of care are also warranted.
While case series and case reports are available, these are small in sample size and do not offer a high level of evidence. However, the observational studies demonstrate nafamostat can be given to hospitalised COVID-19 patients and provide some guidance for potential adverse effects. The available randomised clinical trials are limited in sample size to detect differences in patient-centred clinical outcomes but suggest the most unwell hospitalised patients would be more likely to benefit. Therefore, larger trials that provide evidence of the efficacy of nafamostat mesylate in the treatment of COVID-19 are urgently needed. However, the unascertained viral evolution, the impediments procuring nafamostat for study given the limited number of manufacturers around the world, the logistics of administering a 24-h continuous IV infusion in COVID-19 patients as well as the broader organisational challenges with researching during a global pandemic [48, 49] ensure that performing such larger trials for nafamostat is difficult.
More clinical trial data are expected for nafamostat soon, with studies in progress around the globe [50–53] (Table 3). The results of these trials have not been reported, and most are only looking at surrogate outcomes with limited sample sizes < 300 patients. Assessment of the trial results should be performed through a meta-analysis.
Table 3.
Ongoing clinical trials of nafamostat mesylate in the treatment of COVID-19 patients
| Trial registration, date posted, and location | Study title | Intervention model description | Treatment | Primary outcome measures | Participants | Status |
|---|---|---|---|---|---|---|
|
Posted April 2020 Italy |
Efficacy of Nafamostat in COVID-19 Patients (RACONA Study) | Prospective, double blind, randomised, placebo-controlled parallel group, on top of best standard of care | Nafamostat administered by continuous infusion, placebo | Time to clinical improvement | 256 | Recruiting |
|
Posted May 2020 Senegal |
Efficacy and Safety Evaluation of Treatment Regimens in Adult COVID-19 Patients in Senegal | Open-label, multicentre, randomised, parallel assignment | Nafamostat (0.1–0.2 mg/kg/h), standard of care | SARS-CoV-2 viral load level | 186 | Recruiting |
|
JPRN-jRCTs031200026 Posted May 2020 Japan |
Combination therapy of Favipiravir and Nafamostat Mesylate in Patients with COVID-19 pneumonia | Randomised controlled trial | Nafamostat, favipiravir, standard of care | Change in patient condition on a 10-point scale from baseline to day 7 | 160 | Recruiting |
|
KCT0005003 Posted June 2020 South Korea |
Clinical Efficacy of Nafamostat Mesylate for COVID-19 Pneumonia | Open-label, randomised, parallel assignment | Nafamostat (0.1–0.2 mg/kg/h by continuous infusion), conventional therapy | Proportion of patients with clinical improvement | 84 | Not yet recruiting |
|
ACTRN12620000445976 Posted July 2020 Australia |
Australasian COVID-19 Trial (ASCOT) ADAptive Platform Trial (ASCOT ADAPT) | Open-label, international multicentre, randomised | Nafamostat (0.2 mg/kg/h by continuous infusion), hyperimmune globulin, enoxaparin, dalteparin, tinzaparin | Death or requirement of new intensive respiratory support (invasive or non-invasive ventilation) or vasopressor/inotropic support | 2400 (estimated) | Recruiting |
|
CTRI/2020/06/026220 Posted July 2020 India |
A study to evaluate the efficacy and safety of Nafamostat Mesylate in treatment of Coronavirus infection | Open-label, multicentre, randomised, parallel/crossover assignment | Nafamostat (0.1 mg/kg/h for 24 h by continuous infusion for 10 days), standard of care | Proportion of patients showing clinical improvement by day 14 | 40 | Completed |
|
Posted November 2020 South Korea |
A Study Evaluating the Efficacy and Safety of CKD-314 in Hospitalized Adult Patients Diagnosed With COVID-19 Pneumonia | Open-label, multicentre, randomised, parallel assignment | Nafamostat administered by continuous infusion, standard of care | Time to clinical improvement | 13 | Completed |
|
JPRN-jRCTs031210183 Posted July 2021 Japan |
Exploratory research of nafamostat mesylate in early mild COVID-19 patients | Open-label, randomised, parallel assignment | Nafamostat (0.1 or 0.2 mg/kg/h for 6 days), standard treatment | AUC of SARS-CoV-2 virus reduction in the nasopharynx from before the start of treatment to the 6th day of treatment | 30 | Recruiting |
COVID-19 coronavirus disease 2019, SARS-CoV-2 severe acute respiratory syndrome coronavirus-2
Conclusion
The COVID-19 pandemic continues to be associated with high numbers of patients requiring hospitalisation and high-level health care. Improving treatments, including antivirals for mild, moderate and severely ill patients is essential. Nafamostat has preliminary data that supports evaluation in large clinical studies, but this may prove challenging.
Declarations
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. Jason A. Roberts would like to acknowledge funding from the Australian National Health and Medical Research Council for a Centre of Research Excellence (APP2007007) and an Investigator Grant (APP2009736) as well as an Advancing Queensland Clinical Fellowship. Asha C. Bowen received salary support from the Australian National Health and Medical Research Council Investigator Award (GNT1175509).
Conflict of interest
The following authors Steven Y.C. Tong, Justin T. Denholm, Gregory J. Dore, Asha C. Bowen, Sharon R. Lewin, Balasubramanian Venkatesh, Thomas E. Hills, Zoe McQuilten, David L. Paterson, Susan C. Morpeth and Jason A. Roberts are members of the Australasian COVID-19 Trial (ASCOT), which is studying nafamostat as an antiviral treatment for non-critically ill, hospitalised COVID-19 patients. ASCOT has received nafamostat drug and an unrestricted investigator-initiated grant from manufacturer Chong Kun Dang Pharmaceuticals (Seoul, Korea) and Institute Pasteur Korea. ASCOT is supported by the Australian Partnership for Preparedness Research on Infectious Disease Emergencies (APPRISE), The BHP Foundation, Health Research Council of New Zealand, Hospital Research Foundation, The Macquarie Group Foundation, The Minderoo Foundation, The Pratt Foundation, Royal Brisbane and Women’s Hospital Foundation, The Common Good (The Prince Charles Hospital Foundation), Wesley Medical Research, Chong Kun Dang Pharmaceutical Corporation, NSW Office for Health and Medical Research, Medical Research Future Fund (grant MRF2002132) and the Russell and Womersley Foundation.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Authors’ contributions
All authors contributed to the work presented in this manuscript.
References
- 1.Fehr AR, Perlman S. Coronaviruses: methods and protocols. In: Maier HJ (ed) Methods Mol Biol. Springer Science; 2015. p. 1–282.
- 2.Yamamoto M, Kiso M, Sakai-Tagawa Y, Iwatsuki-Horimoto K, Imai M, Takeda M, et al. The anticoagulant nafamostat potently inhibits SARS-CoV-2 infection in vitro: an existing drug with multiple possible therapeutic effects. bioRxiv. 2020;1–19. [DOI] [PMC free article] [PubMed]
- 3.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li K, Meyerholz DK, Bartlett JA, McCray PB. The TMPRSS2 inhibitor nafamostat reduces SARS-CoV-2 pulmonary infection in mouse models of COVID-19. Am Soc Microbiol. 2021;12:1–11. doi: 10.1128/mBio.00970-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamamoto M, Matsuyama S, Li X, Takeda M, Kawaguchi Y, Inoue JI, et al. Identification of Nafamostat as a potent inhibitor of middle east respiratory syndrome coronavirus S protein-mediated membrane fusion using the split-protein-based cell-cell fusion assay. Antimicrob Agents Chemother. 2016;60:6532–6539. doi: 10.1128/AAC.01043-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoffmann M, Schroeder S, Kleine-Weber H, Müller MA, Drosten C, Pöhlmann S. Nafamostat mesylate blocks activation of SARS-CoV-2: new treatment option for COVID-19. Antimicrob Agents Chemother. 2020;64:1–3. doi: 10.1128/AAC.00754-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ko M, Jeon S, Ryu W-S, Kim S. Comparative analysis of antiviral efficacy of FDA-approved drugs against SARS-CoV-2 in human lung cells: Nafamostat is the most potent antiviral drug candidate. J Med Virol. 2020;93:1403–1408. doi: 10.1002/jmv.26397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Center for Biotechnology Information. PubChem Compound Summary for CID 4413, Nafamostat [Internet]. https://pubchem.ncbi.nlm.nih.gov/compound/Nafamostat. Accessed 15 Nov 2021
- 9.Okajima K, Uchiba M, Murakami K. Nafamostat Mesilate. Cardiovasc Drug Rev. 1995;13:51–65. doi: 10.1111/j.1527-3466.1995.tb00213.x. [DOI] [Google Scholar]
- 10.Nichi-Iko Pharmaceutical Co. Ltd. Nafamostat mesylate Pharmaceutical Interview Form [Internet]. Ja; 2019. https://www.nichiiko.co.jp/medicine/file/31050/interview
- 11.Minakata D, Fujiwara SI, Ikeda T, Kawaguchi SI, Toda Y, Ito S, et al. Comparison of gabexate mesilate and nafamostat mesilate for disseminated intravascular coagulation associated with hematological malignancies. Int J Hematol. 2019;109:141–146. doi: 10.1007/s12185-018-02567-w. [DOI] [PubMed] [Google Scholar]
- 12.Asakura H, Ogawa H. COVID-19-associated coagulopathy and disseminated intravascular coagulation. Int J Hematol [Internet]. Springer Singapore; 2020. 10.1007/s12185-020-03029-y [DOI] [PMC free article] [PubMed]
- 13.McFadyen JD, Stevens H, Peter K. The emerging threat of (Micro)thrombosis in COVID-19 and its therapeutic implications. Circ Res. 2020;127:571–587. doi: 10.1161/CIRCRESAHA.120.317447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nichi-Iko Pharmaceutical Co. L. Pharmaceutical interview form for FUTHAN 10 INJ., FUTHAN 50 INJ. 6th Edition [Internet]. 2019. https://www.nichiiko.co.jp/medicine/file/31050/interview
- 15.Hempel T, Raich L, Olsson S, Azouz NP, Klingler AM, Hoffmann M, et al. Molecular mechanism of inhibiting the SARS-CoV-2 cell entry facilitator TMPRSS2 with camostat and nafamostat. Chem Sci. 2021;12:983–992. doi: 10.1039/D0SC05064D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ohtake Y, Hirasawa H, Sugai T, Oda S, Shiga H, Matsuda K, et al. Nafamostat mesylate as anticoagulant in continuous hemofiltration and continuous hemodiafiltration. Contrib Nephrol. 1991;93:215–217. doi: 10.1159/000420222. [DOI] [PubMed] [Google Scholar]
- 17.Choi J-Y, Kang Y-J, Jang HM, Jung H-Y, Cho J-H, Park S-H, et al. Nafamostat mesilate as an anticoagulant during continuous renal replacement therapy in patients with high bleeding risk. A randomized clinical trial. Medicine (Baltimore) 2015;94:1–7. doi: 10.1097/MD.0000000000002392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsukagoshi S. Pharmacokinetics studies of nafamostat mesilate (FUT), a synthetic protease inhibitor, which has been used for the treatments of DIC and acute pancreatitis, and as an anticoagulant in extracorporeal circulation. Jpn J Cancer Chemother. 2000;27:767–774. [PubMed] [Google Scholar]
- 19.Hirayama T, Nosaka N, Okawa Y, Ushio S, Kitamura Y, Sendo T, et al. AN69ST membranes adsorb nafamostat mesylate and affect the management of anticoagulant therapy: a retrospective study. J Intensive Care. 2017;5:1–7. doi: 10.1186/s40560-017-0244-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doi Y, Kondo M, Ando M, Kuwatsuka Y, Ishihara T. COVID-19 Nafamostat Observational Study in Japan: Preliminary Report. Japan; 2020.
- 21.Muto S, Imai M, Asano Y. Mechanisms of hyperkalemia caused by nafamostat mesilate. Gen Pharmacol. 1995;26:1627–1632. doi: 10.1016/0306-3623(95)00072-0. [DOI] [PubMed] [Google Scholar]
- 22.Kitagawa H, Chang H, Fujita T. Hyperkalemia due to nafamostat mesylate. N Engl J Med. 1995;332:687. doi: 10.1056/NEJM199503093321018. [DOI] [PubMed] [Google Scholar]
- 23.Park J-H, Her C, Min H-K, Kim D-K, Park S-H, Jang H-J. Nafamostat mesilate as a regional anticoagulant in patients with bleeding complications during extracorporeal membrane oxygenation. Int J Artif Organs. 2015;38:595–599. doi: 10.5301/ijao.5000451. [DOI] [PubMed] [Google Scholar]
- 24.Ookawara S, Saitoh M, Yahagi T, Tabei K, Asano Y. Two cases of nafamostat mesilate-induced hyperkalemia. Jpn Soc Dial Ther. 1995;28.
- 25.Okajima M, Takahashi Y, Kaji T, Ogawa N, Mouri H. Nafamostat mesylate-induced hyperkalemia in critically ill patients with COVID-19: four case reports. World J Clin Cases. 2020;8:5320–5325. doi: 10.12998/wjcc.v8.i21.5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muto S, Imai M, Asano Y. Mechanisms of the hyperkalaemia caused by nafamostat mesilate: effects of its two metabolites on Na+ and K+ transport properties in the rabbit cortical collecting duct. Br J Pharmacol. 1994;111:173–178. doi: 10.1111/j.1476-5381.1994.tb14040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ookawara S, Tabei K, Sakurai T, Sakairi Y, Furuya H, Asano Y. Additional mechanisms of nafamostat mesilate-associated hyperkalaemia. Eur J Clin Pharmacol. 1996;51:149–151. doi: 10.1007/s002280050176. [DOI] [PubMed] [Google Scholar]
- 28.Quinn TM, Gaughan EE, Bruce A, Antonelli J, O’Connor R, Li F, et al. Randomised controlled trial of intravenous nafamostat mesylate in COVID pneumonitis: Phase 1b/2a experimental study to investigate safety, Pharmacokinetics and Pharmacodynamics. eBioMedicine [Internet]. 2022;76:103856. http://medrxiv.org/content/early/2021/10/07/2021.10.06.21264648.abstract [DOI] [PMC free article] [PubMed]
- 29.Cao Y-G, Chen Y-C, Hao K, Zhang M, Liu X-Q. An in vivo approach for globally estimating the drug flow between blood and tissue for Nafamostat Mesilate: the main hydrolysis site determination in human. Biol Pharm Bull. 2008;31:1985–1989. doi: 10.1248/bpb.31.1985. [DOI] [PubMed] [Google Scholar]
- 30.Yamamoto M, Kiso M, Sakai-Tagawa Y, Iwatsuki-Horimoto K, Imai M, Takeda M, et al. The anticoagulant nafamostat potently inhibits SARS-CoV-2 S protein-mediated fusion in a cell fusion assay system and viral infection in vitro in a cell-type-dependent manner. Viruses. 2020;12. [DOI] [PMC free article] [PubMed]
- 31.Hoffmann M, Schroeder S, Kleine-Weber H, Müller MA, Drosten C, Pöhlmann S. Nafamostat mesylate blocks activation of SARS-CoV-2: new treatment option for COVID-19. Antimicrob Agents Chemother. 2020;64:19–21. doi: 10.1128/AAC.00754-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li P, Wang Y, Lavrijsen M, Lamers MM, de Vries AC, Rottier RJ, et al. SARS-CoV-2 Omicron variant is highly sensitive to molnupiravir, nirmatrelvir, and the combination. Cell Res. 2022;32:322–324. doi: 10.1038/s41422-022-00618-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Doi K, Ikeda M, Hayase N, Moriya K, Morimura N, Maehara H, et al. Nafamostat mesylate treatment in combination with favipiravir for patients critically ill with Covid-19: a case series. Crit Care. 2020;24. [DOI] [PMC free article] [PubMed]
- 34.Jang S, Rhee J. Three cases of treatment with nafamostat in elderly patients with COVID-19 pneumonia who need oxygen therapy. Int J Infect Dis. 2020;96:500–502. doi: 10.1016/j.ijid.2020.05.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mittal A, Khattri A, Verma V. Structural and antigenic variations in the spike protein of emerging SARS-CoV-2 variants. PLoS Pathog. 2022;18:1–25. doi: 10.1371/journal.ppat.1010260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Planas D, Saunders N, Maes P, Guivel-Benhassine F, Planchais C, Buchrieser J, et al. Considerable escape of SARS-CoV-2 Omicron to antibody neutralization. Nature. Springer US; 2021;602:671–5. [DOI] [PubMed]
- 37.Meng B, Ferreira IAT., Abdullahi A, Saito A, Kimura I, Yamasoba D, et al. SARS-CoV-2 Omicron spike mediated immune escape, infectivity and cell-cell fusion. bioRxiv [Internet]. 2021; https://www.biorxiv.org/content/10.1101/2021.12.17.473248v2
- 38.Meng B, Abdullahi A, Ferreira IATM, Goonawardane N, Saito A, Kimura I, et al. Altered TMPRSS2 usage by SARS-CoV-2 Omicron impacts tropism and fusogenicity. Nature. 2022; [DOI] [PMC free article] [PubMed]
- 39.Bojkova D, Widera M, Ciesek S, Wass MN, Michaelis M, Cinatl J. Reduced interferon antagonism but similar drug sensitivity in Omicron variant compared to Delta variant of SARS-CoV-2 isolates. Cell Res. 2022;32:319–321. doi: 10.1038/s41422-022-00619-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takahashi W, Yoneda T, Koba H, Ueda T, Tsuji N, Ogawa H, et al. Potential mechanisms of nafamostat therapy for severe COVID-19 pneumonia with disseminated intravascular coagulation. Int J Infect Dis [Internet]. 2021;102:529–531. doi: 10.1016/j.ijid.2020.10.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iwasaka S, Shono Y, Tokuda K, Nakashima K, Yamamoto Y, Maki J, et al. Clinical improvement in a patient with severe coronavirus disease 2019 after administration of hydroxychloroquine and continuous hemodiafiltlation with nafamostat mesylate. J Infect Chemother [Internet]. 2020;26:1319–1323. doi: 10.1016/j.jiac.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hifumi T, Isokawa S, Otani N, Ishimatsu S. Adverse events associated with nafamostat mesylate and favipiravir treatment in COVID-19 patients. Crit Care 2020;24. [DOI] [PMC free article] [PubMed]
- 43.Koriyama N, Moriuchi A, Higashi K, Kataoka T, Arimizu T, Takaguchi G, et al. COVID-19 with rapid progression to hypoxemia likely due to imbalance between ventilation and blood flow: a case report. Clin Med Insights Circ Respir Pulm Med. 2022;16:1–7. doi: 10.1177/11795484211073273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Inokuchi R, Kuno T, Komiyama J, Uda K, Miyamoto Y, Taniguchi Y, et al. Association between Nafamostat Mesylate and In-Hospital Mortality in Patients with Coronavirus Disease 2019: A Multicenter Observational Study. J Clin Med. 2022;11 [DOI] [PMC free article] [PubMed]
- 45.Zhuravel SV, Khmelnitskiy OK, Burlaka OO, Gritsan AI, Goloshchekin BM, Kim S, et al. Nafamostat in hospitalized patients with moderate to severe COVID-19 pneumonia: a randomised Phase II clinical trial. EClinicalMedicine. 2021;41:101169. doi: 10.1016/j.eclinm.2021.101169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hwang SD, Hyun YK, Moon SJ, Lee SC, Yoon SY. Nafamostat mesilate for anticoagulation in continuous renal replacement therapy. Int J Artif Organs. 2013;36:208–216. doi: 10.5301/ijao.5000191. [DOI] [PubMed] [Google Scholar]
- 47.Lee YK, Lee HW, Choi KH, Kim BS. Ability of Nafamostat mesilate to prolong filter patency during continuous renal replacement therapy in patients at high risk of bleeding: a randomized controlled study. PLoS ONE. 2014;9:1–8. doi: 10.1371/journal.pone.0108737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bowen AC, Tong SYC, Davis JS. Australia needs a prioritised national research strategy for clinical trials in a pandemic: lessons learned from COVID-19. Med J Aust. 2021;215:56–58.e1. doi: 10.5694/mja2.51143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ramanan M, Tong SYC, Kumar A, Venkatesh B. Geographical representation of low- and middle-income countries in randomized clinical trials for COVID-19. JAMA Netw Open. 2022;32:319–321. doi: 10.1001/jamanetworkopen.2022.0444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moon K, Hong K, Bae I. Treatment effect of nafamostat mesylate in patients with COVID-19 pneumonia : study protocol for a randomized controlled trial. Trials. 2021;22:832. doi: 10.1186/s13063-021-05760-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rhee J. A review of the possibility of Nafamostat Mesylate in COVID-19 treatment. J Cell Immunol. 2021;3:1–7. [Google Scholar]
- 52.EUnetHTA Rolling Collaborative Review (RCR05) Authoring Team. Nafamostat for the treatment of COVID-19. Report No: RCR05, v. 7.0 [Internet]. Diemen (The Netherlands); 2021. https://www.eunethta.eu/wp-content/uploads/2021/05/EUnetHTA-Covid-19_RCR05_Nafamostat_V7.0.pdf
- 53.Denholm JT, Venkatesh B, Davis J, Bowen AC, Hammond NE, Jha V, et al. ASCOT ADAPT study of COVID-19 therapeutics in hospitalised patients: an international multicentre adaptive platform trial. Res Sq. 2022;1–20. 10.21203/rs.3.rs-1045085/v1 [DOI] [PMC free article] [PubMed]
- 54.Jitobaom K, Boonarkart C, Manopwisedjaroen S, Punyadee N, Borwornpinyo S, Thitithanyanont A, et al. Favipiravir and Ivermectin Showed in Vitro Synergistic Antiviral Activity against SARS-CoV-2. Res Sq. 2021
- 55.Ellinger B, Bojkova D, Zaliani A, Cinatl J, Claussen C, Westhaus S, et al. A SARS-CoV-2 cytopathicity dataset generated by high-content screening of a large drug repurposing collection. Sci Data [Internet]. Springer US; 2021;8:1–11. 10.1038/s41597-021-00848-4 [DOI] [PMC free article] [PubMed]