Background:
Experimental evidence suggests a key role of SIRT1 (silent information regulator 1) in age- and metabolic-related vascular dysfunction. Whether these effects hold true in the human microvasculature is unknown. We aimed to investigate the SIRT1 role in very early stages of age- and obesity-related microvascular dysfunction in humans.
Methods:
Ninety-five subjects undergoing elective laparoscopic surgery were recruited and stratified based on their body mass index status (above or below 30 kg/m2) and age (above or below 40 years) in 4 groups: Young Nonobese, Young Obese, Old Nonobese, and Old Obese. We measured small resistance arteries’ endothelial function by pressurized micromyography before and after incubation with a SIRT1 agonist (SRT1720) and a mitochondria reactive oxygen species (mtROS) scavenger (MitoTEMPO). We assessed vascular levels of mtROS and nitric oxide availability by confocal microscopy and vascular gene expression of SIRT1 and mitochondrial proteins by qPCR. Chromatin immunoprecipitation assay was employed to investigate SIRT1-dependent epigenetic regulation of mitochondrial proteins.
Results:
Compared with Young Nonobese, obese and older patients showed lower vascular expression of SIRT1 and antioxidant proteins (FOXO3 [forkhead box protein O3] and SOD2) and higher expression of pro-oxidant and aging mitochondria proteins p66Shc and Arginase II. Old Obese, Young Obese and Old Nonobese groups endothelial dysfunction was rescued by SRT1720. The restoration was comparable to the one obtained with mitoTEMPO. These effects were explained by SIRT1-dependent chromatin changes leading to reduced p66Shc expression and upregulation of proteins involved in mitochondria respiratory chain.
Conclusions:
SIRT1 is a novel central modulator of the earliest microvascular damage induced by age and obesity. Through a complex epigenetic control mainly involving p66Shc and Arginase II, it influences mtROS levels, NO availability, and the expression of proteins of the mitochondria respiratory chain. Therapeutic modulation of SIRT1 restores obesity- and age-related endothelial dysfunction. Early targeting of SIRT1 might represent a crucial strategy to prevent age- and obesity-related microvascular dysfunction.
Keywords: aging, endothelial cells, microcirculation, mitochondria, obesity, sirtuins
Novelty and Significance.
What Is Known?
The increasing prevalence of ageing and obesity fuels cardiovascular mortality and morbidity.
Microcirculation is one of the earliest sites on which age and metabolic diseases exert their damage.
SIRT1 (silent information regulator 1) mediates the benefits of calorie restriction in models of impaired ageing or metabolism.
What New Information Does This Article Contribute?
SIRT1 is a central modulator of the earliest microvascular damage induced by age and obesity.
Targeting SIRT1 restores endothelial dysfunction to a degree directly proportional to the age- and obesity-related damage.
SIRT1 modulation of endothelial function is mediated by epigenetic changes mainly involving p66Shc and Arginase II and promoting an improved mitochondrial redox state.
The steadily increasing cardiovascular disease prevalence requires novel, effective therapeutic strategies. Research must observe cardiovascular risk from a new perspective: age and metabolic-related damage. Consistent with this premise, we focused on the earliest impairment site: microcirculation. To the best of our knowledge, this is the first time that the direct contribution of vascular SIRT1 to the restoration of microvascular endothelial function in both ageing and obesity has been explored in humans. We demonstrate a novel and crucial role in rescuing the human microcirculatory endothelial dysfunction, consistent across the whole spectrum of age- and metabolism-related impairment. Our findings depict SIRT1 as a novel central regulator of a homeostatic axis that connects vascular phenotype to systemic metabolism and ageing and to exposure to external adverse stimuli. SIRT1 capacity to protect and reverse the harmful epigenetic signature inflicted by ageing and obesity required further dedicated research. The vascular-specific protective effect described in humans might be translated into therapeutic strategies to preserve or restore SIRT1 in the human microcirculation, ultimately relieving healthcare systems.
In This Issue, see p 473
Meet the First Author, see p 474
Cardiovascular morbidity and mortality burden health care systems worldwide and are deemed to worsen in the following years.1 Two are the primary culprits: age and obesity. Increased lifespan often leads to unhealthy aging,2 while obesity, whose prevalence also is expected to increase,3 ignites the cardiovascular risk,4 inflicting permanent damage which persists despite the optimal disease control.5 It is becoming clear how, to challenge the age- and obesity-related cardiovascular risk, we must target the earliest site of damage: microvascular endothelial dysfunction.6–8
Sirtuins have raised substantial attention.9 The mammalian SIRT1 (silent information regulator 1) is a histone deacetylase overexpressed during calorie restriction.10 It promotes beneficial effects on lifespan and cardiometabolic homeostasis. In vitro and in vivo observations have demonstrated the downregulation of SIRT1 during aging9 and obesity11 and that, in murine models, restoring the activity of SIRT1 might improve endothelial function.12 This evidence might suggest a relevant role for this histone deacetylase in linking the aging process and the metabolic damage to vascular health.9,11 Mitochondria have been, at least in part, appointed as putative mediators of these effects.13 Indeed, mitochondria dysfunction has been identified as a hallmark of several age-related14 and metabolic diseases,15,16 being in a tight relationship with endothelial function.17 SIRT1 influences the activity of mitochondria by reducing the levels of mitochondrial reactive oxygen species (mtROS),18 potentially preserving their oxidative phosphorylation efficiency and dynamics.19–21
In humans, some indirect observation with resveratrol (a SIRT1 activator)22 shows encouraging findings, and some trials involving SIRT1 agonists are currently ongoing.9 However, evidence is limited and, in part, controversial.9 No study by far has assessed the effect of SIRT1 across aging and metabolic disease directly on the vascular phenotype.
We aimed to explore the contribution of SIRT1 to obesity- and age-related microvascular dysfunction in humans, focusing on its very early stages. To conduct a comprehensive and exhaustive exploration, we also investigated the mechanisms of the potential influence of SIRT1 on microvascular dysfunction, focusing on mitochondria oxidative stress signaling.
Methods
Data Availability
All data are available to qualified investigators upon reasonable request.
Population
In a case-control design, 47 obese patients and 48 nonobese were consecutively recruited among patients referring to the Department of Clinical and Experimental Medicine of the University of Pisa for laparoscopic bariatric surgery and among consecutive patients referring to the Department of Surgery of the University of Pisa to undergo elective inguinal hernia repair. The population inclusion and exclusion criteria are reported in the Supplemental Material.
Microvessels (150–300 µm) were isolated from subcutaneous adipose tissue and mounted on a pressurized myograph to assess the vascular phenotype. Vessels were also processed for gene expression profiling, in situ mtROS and NO, Western blot, mitochondrial swelling and chromatin immunoprecipitation assays (Supplemental Material). The procedures of the study were in accordance with the institutional guidelines. The protocol was approved by the local Ethical Committee (protocol n. 12589), and each participant gave written informed consent to the study. All human studies and handling of human material were in accordance with the declaration of Helsinki.
Statistical Analysis
Continuous variables were tested for normality by the Shapiro-Wilk test. Non-normal variables were natural log-transformed when used in regression models or parametric tests. Data were presented as mean±SD for continuous parametric variables, as median (IQR) for continuous nonparametric variables and as percentages for binary variables. Participants were stratified based on their body mass index (BMI) status (≤ or >30 kg/m2) and age (≤ or >40 years) into 4 groups: Young Nonobese, Young Obese, Old Nonobese and Old Obese. Pearson correlation was used to assess association, as appropriate. Independent samples Student t test or 2-way ANOVA using age and BMI categories as fixed factors and Holm-Sidak as a post hoc test was used to determine significant differences between groups, as appropriate. ANOVA for repeated measures, followed by a post hoc test with Holm-Sidak correction, was used to evaluate differences in the vasodilation obtained after incubation of the vessel with different drugs. When nonparametric tests were needed, the Kruskal-Wallis test with Dwass-Steel-Critchlow-Flinger post hoc test or Scheirer-Hare-Ray test using age and BMI categories as fixed factors followed by Dunn post hoc correction were used to compare independent groups. Within the same group, data were compared by Friedman test with Durbin-Conover as a post hoc test. No experiment-wide/across-tests multiple test correction was applied. A value of P<0.05 was considered statistically significant. All analyses were performed using SPSS software (IBM, version 20.0), GraphPad Prism (GraphPad Software, version 7.04), and jamovi (The Jamovi project 2022, version 2.3).
Results
Biochemical Characterization and Vascular Phenotype
Table reports the studied population’s clinical characteristics, stratified by age and BMI groups. Sex distribution was not statistically different across the 4 groups. There was no significant difference in age between Young Obese and Young Nonobese groups, while the age of the Old Nonobese was higher than the Old Obese (P<0.001). BMI was not different between Young Obese and Old Obese and between Young Nonobese and Old Nonobese. As expected, fasting insulin and the homeostatic model assessment for insulin resistance index were higher in Old Obese and Young Obese than in Old Nonobese and Young Nonobese. Also, eGFR was different between the groups, influenced by age-related decline and obesity-related hyperfiltration.23
Table.
Characteristics of the Study Population
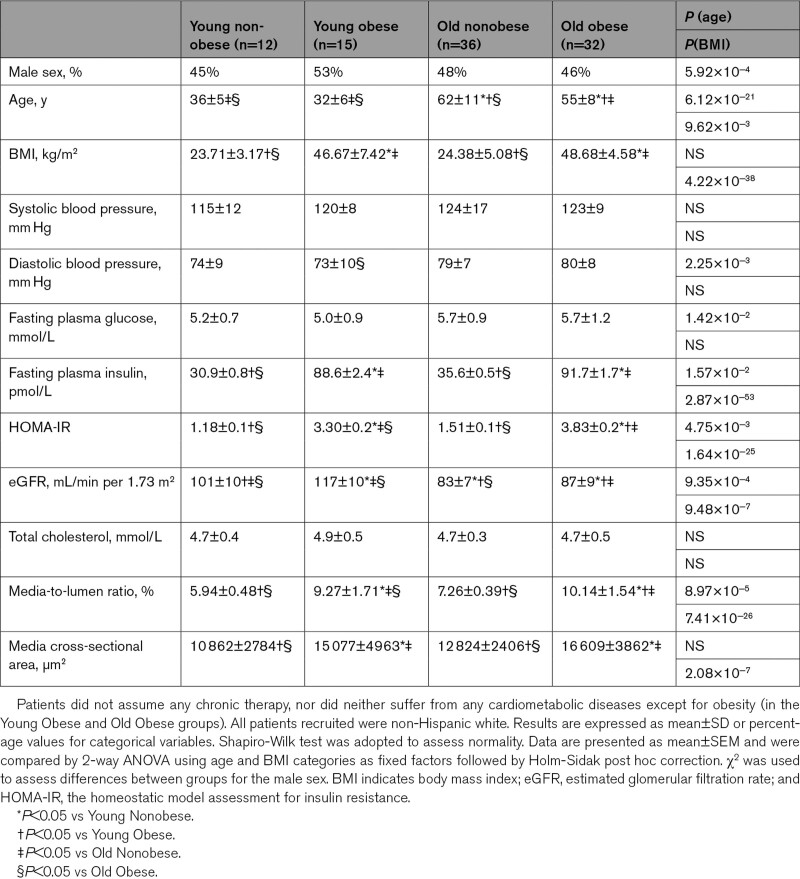
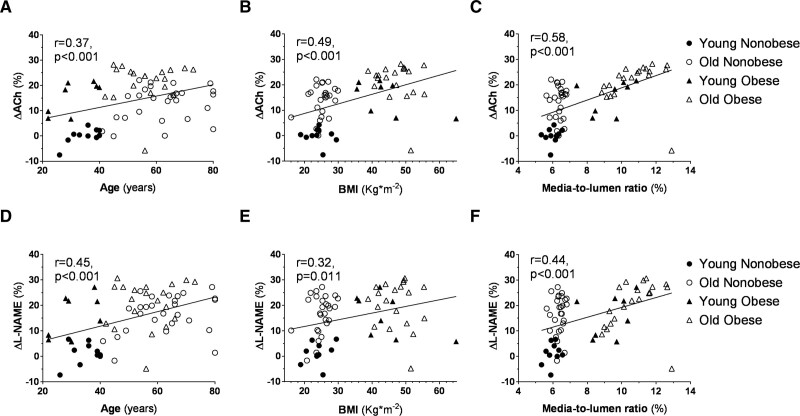
Vessel structural parameters changed across the 4 groups. Figure 1A shows the predicted media-to-lumen ratio by age in obese versus nonobese. A 5-fold steeper slope was observed in the obese group, and a significant age*BMI group interaction (P<0.01) was shown. Endothelium-dependent vasodilation showed a graded decline from the Young Nonobese to the Old Obese groups. The Old Nonobese and Young Obese groups presented a similar vasodilatory response to acetylcholine (Figure 1B), while Old Nonobese and Old Obese showed a similar inhibition to L-NAME (Figure 1C). The nonendothelial dependent vasodilatory response was not different between groups (Figure 1D). The impaired endothelial function observed across the 4 groups was associated with substantial changes in intracellular mtROS and NO levels. There was a graded increase in the levels of mtROS with age and obesity (Figure 1E), also confirmed by the use of more specific fluorescent probes in a small number of patients (n=3 for each group; Figure S1). NO availability followed a reverse pattern across the 4 groups (Figure 1F; Figure S1). These features are in keeping with previously reported data from our group8 and confirm the role of obesity in promoting early small vessel aging.24
Figure 1.
Microvascular characterization of the study population. A, Media-to-Lumen (M/L) ratio per year of age in nonobese (white circle; n=42) and obese (black triangle; n=47) subjects. Regression lines for each group are shown. M/L ratio is expressed as percentage (%). Age and M/L are tightly related in both groups (obese: r=0.487, P<0.01; nonobese: r=0.555, P=0.001). The slope was 5-fold steeper in the obese group. In detail, the regression coefficients (x=0.05 in obese, x=0.01 in nonobese) depict how M/L increase of 0.5%/10 years in obese vs 0.1%/10 years in nonobese. Shapiro-Wilk test was adopted to assess normality. Linear regression analysis was conducted by adopting the M/L ratio as dependent variable, age as a covariate and BMI group (nonobese vs obese) as a factor. Interaction between terms was tested (P<0.01). B, Vasorelaxation to cumulative concentration of ACh in vessels precontracted with norepinephrine in the 4 groups (Young Nonobese: n=12; Old Nonobese: n=31; Young Obese: n=15; Old Obese: n=32). Vasodilatory response is expressed as % of the maximal diameter (Young Nonobese: black circle, Old Nonobese: white circle, Young Obese: black triangle, Old Obese: white triangle). C, Inhibition of ACh dilation by L-NAME expressed as the difference between the maximal vasodilatory response to ACh alone vs co-incubated with L-NAME. D, Vasorelaxation to cumulative concentration of SNP in vessels precontracted with norepinephrine in the 4 groups (young nonobese: n=12; old nonobese: n=31; young obese: n=15; old obese: n=32). Vasodilatory response is expressed as % of the maximal diameter (young nonobese: black circle, old nonobese: white circle, young obese: black triangle, old obese: white triangle). E and F, Differences in mtROS (E) and NO levels (F) assessed by mitoSOX and DAF-FM fluorescence in the 4 groups (n=5 for each group). Fluorescence is calculated as mean fluorescence intensity (MFI) and expressed as % of the Young Nonobese group. Original magnification is 40×. Shapiro-Wilk test was adopted to assess normality. Data are presented as mean±SEM and were compared by compared by 2-way ANOVA using age and BMI categories as fixed factors followed by Holm-Sidak post hoc correction (B–D) and Scheirer-Hare-Ray test using age and BMI categories as fixed factors followed by Dunn post hoc correction (E and F). A P<0.05 was considered significant. *P<0.05. **P<0.01. Ach indicates acetylcholine; M/L, media-to-lumen ratio; MFI, mean fluorescence intensity; ON, old nonobese; OO, old obese; SNP, sodium nitroprusside; YN, young nonobese; and YO, young obese.
SIRT1 Expression and Modulation of Genes Involved in the Control of Intracellular mtROS
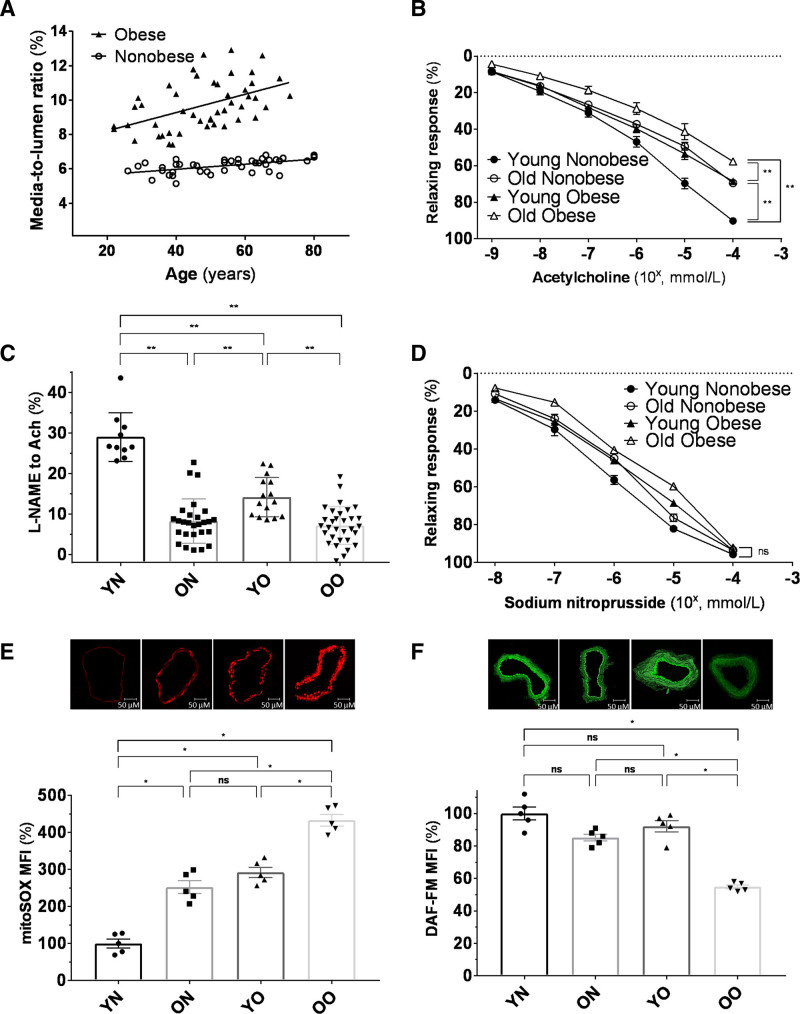
Gene expression analysis revealed substantial changes in the expression of SIRT1 and several mitochondrial genes regulating NO production/degradation across the 4 groups. SIRT1 was significantly lower in Young Obese and Old Obese groups than in Young Nonobese and Old Nonobese. Old Nonobese also showed reduced SIRT1 expression when confronted with Young Nonobese (Figure 2A). Pro-oxidant enzyme p66Shc, a mitochondria respiratory chain uncoupling factor,25 followed a reverse trend (Figure 2B). This pattern was consistent for all the explored genes. FOXO3 (Forkhead box protein O3) and superoxide dismutase-2 (SOD2), mitochondrial antioxidant enzymes,26 were downregulated in Old Obese, Young Obese and Old Nonobese (Figure 2C and 2D). Arginase II, known to impair NO availability during aging through competition with the eNOS for its substrate arginine,8 was increasingly expressed moving from Young Nonobese to Old Obese (Figure 2E).
Figure 2.
Expression of SIRT1 (silent information regulator 1) and SIRT1-related genes in the vascular wall of the 4 groups. A, Expression of SIRT1. B, Expression of the pro-oxidant enzyme p66Shc. C, Expression of the antioxidant FOXO3 (Forkhead box protein O3). D, Expression of the SOD2 (superoxide dismutase-2). E, Expression of Arginase II. Results are expressed as % with respect to the Young Nonobese group. Shapiro-Wilk test was adopted to assess normality. FOXO3 and SOD2 were natural log-transformed for the means of the analyses. Young Nonobese: n=10, Old Nonobese: n=12, Young Obese: n=10, Old Obese: n=8. Data are presented as mean±SD and were compared by 2-way ANOVA followed by Holm-Sidak post hoc correction. A P<0.05 was considered significant. *P<0.05.**P<0.01. ArgII indicates Arginase II; FOXO3, Forkhead box protein O3; ON, old nonobese; OO, old obese; SOD-2, superoxide dismutase-2; YN, young nonobese; and YO, young obese.
SRT1720 Rescues Age- and Obesity-Related Endothelial Dysfunction
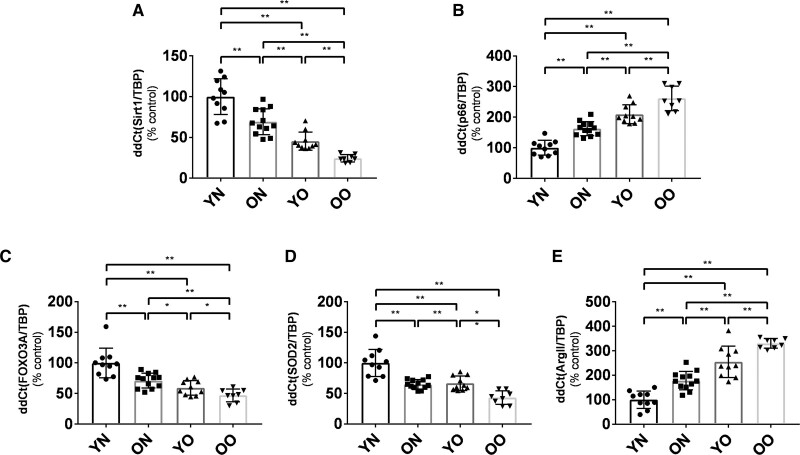
Incubation of the small arteries with SRT1720 improved endothelial function in Old Obese, Young Obese and Old Nonobese groups but not in the Young Nonobese group (Figure 3A through 3D). This improvement was more remarkable in the group with the most impaired phenotype (Old Obese) and of a similar magnitude between Old Nonobese and Young Obese; no statistical difference was reached between Young Obese and Old Obese (Figure 3E and 3F). Restoration of L-NAME inhibition to ACh response followed a similar trend across the 4 groups. In this case, Old Obese improvement was not different to the one observed for neither Young Obese nor Old Nonobese. The restoration of both ACh and L-NAME responses were related to age, BMI, and vascular remodelling (for Ach: P<0.001 for each; for L-NAME: P<0.001 for age, P=0.0107 for BMI, P<0.001 for media-to-lumen ratio; Figure 4). This relationship was also confirmed after adjustment for age, BMI, sex, mean blood pressure, creatinine and homeostatic model assessment for insulin resistance (for Ach: P<0.001 for age, P=0.028 for BMI, P<0.001 for media-to-lumen ratio; for L-NAME: P<0.001 for age, P=0.003 for media-to-lumen ratio; BMI was nonsignificant). Notably, sex was not related to the rescue of endothelial function in unadjusted or adjusted models.
Figure 3.
SRT1720 rescues relaxing response to Ach and L-NAME inhibition in the 4 groups. A–D, Relaxing response to cumulative concentration to Ach in vessels precontracted with norepinephrine in the 4 groups (young nonobese: n=10; old nonobese: n=27; young obese: n=8; old obese: n=20). Vasodilatory response is expressed as % of the maximal diameter. The experiment was repeated 4 times for each patient by incubating the vessel with saline (black circle), L-NAME (white circle), SRT1720 (black triangle), SRT1720+L-NAME (white triangle). E and F, AUC (E) and maximal (F) vasodilatory response to Ach expressed as % of the maximal diameter in the 4 groups (young nonobese: n=10; old nonobese: n=27; young obese: n=8; old obese: n=20) in vessel precontracted with norepinephrine in the 4 groups. Vessels were incubated with saline (blue bars), SRT1720 (green bars), L-NAME (red bars) and SRT1720+L-NAME (orange bars). Baseline AUC and maximal vasodilatory response (blue bars) differ between the 4 groups (P<0.01), as expected. G, Maximal improvement in vasodilatory response to ACh (ΔACh, blue bars) or inhibitory response to L-NAME (ΔL-NAME, red bars) after SRT1720 incubation in the 4 groups (young nonobese: n=10; old nonobese: n=27; young obese: n=8; old obese: n=20). Shapiro-Wilk test was adopted to assess normality. Data are presented as mean±SEM and were compared by ANOVA for repeated measures followed by a post hoc test with Holm-Sidak post hoc correction. Baseline AUC and maximal vasodilatory response and maximal improvement in vasodilatory response to ACh (ΔACh) or inhibitory response to L-NAME in the 4 groups were compared by 2-way ANOVA followed by Holm-Sidak post hoc correction. A P<0.05 was considered significant. *P<0.05.**P<0.01. In the last panel (G): **P=0.01 or less for ΔACh response, °°P=0.01 or less for ΔL-NAME response. Ach indicates acetylcholine; ON, old nonobese; OO, old obese; YN, young nonobese; and YO, young obese.
Figure 4.
Relationship between the improvement observed with SRT1720 in ACh and L-NAME response and age, body mass index (BMI), and vascular remodeling. SRT1720 induced improvement in vasodilatory response to ACh (A–C) and inhibitory response to L-NAME (D–F) shows a direct relationship with age, BMI and M/L in the 4 groups (young nonobese: black circle, old nonobese: white circle, young obese: black triangle, old obese: white triangle; young nonobese: n=10; old nonobese: n=27; young obese: n=8; old obese: n=20). The improved response is expressed as the improvement in maximal vasodilatory response to ACh (ΔACh) or inhibitory response to L-NAME (ΔL-NAME) after SRT1720 incubation. Shapiro-Wilk test was adopted to assess normality. Linear regression was used to model the relationship between the factors. Age, BMI and M/L were used as continuous variables in the model. Data are shown unadjusted. A P<0.05 was considered significant. Ach indicates acetylcholine; and M/L, media-to-lumen ratio.
Selective SIRT1 Inhibition in Young Nonobese Subjects Blunts Microvascular Endothelial Function
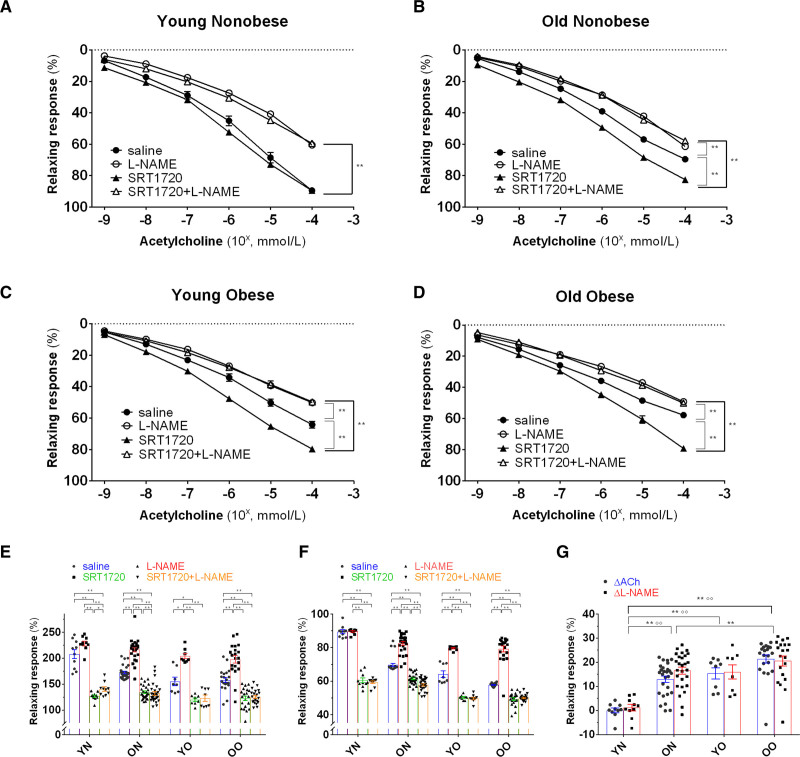
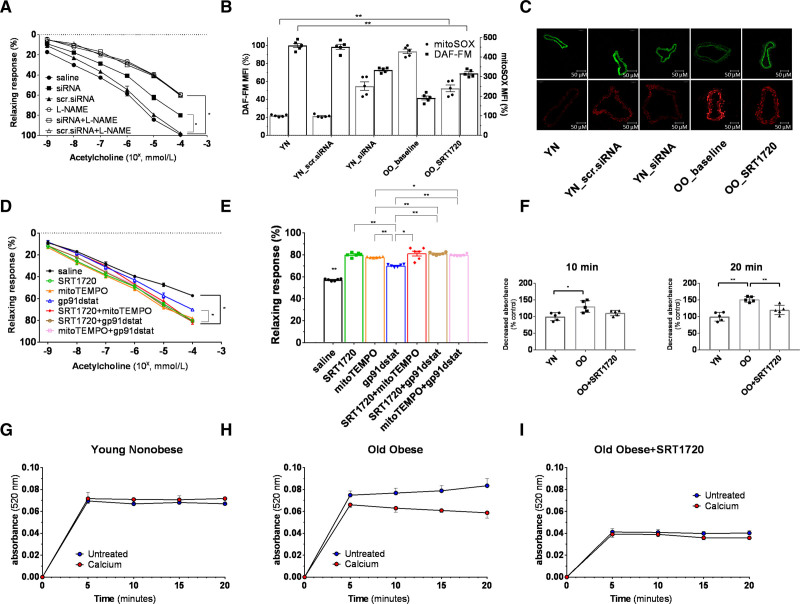
To explore the mechanisms underpinning SIRT1 restored endothelial function, we first aimed to assess selective SIRT1 effects on the microvascular phenotype. Thus, we tested selective SIRT1 inhibition on Young Nonobese vessels (n=5). The Young Nonobese group, that is, individuals with the most preserved vascular phenotype, was chosen for both the expected more significant difference in effect magnitude and the avoidance of underlying confounding impairment. Vasodilatory response to ACh and L-NAME of Young Nonobese small vessel transfected with SIRT1 siRNA sc-40986 was similar to the one observed at baseline for the Young Obese group (Figure 5A). mtROS and NO availability were as well impaired (Figure 5B and 5C). Western blot and qPCR assays were used to confirm the abrogation of SIRT1 levels in siRNA-treated vessels (Figure S2).
Figure 5.
Exploration of the effect and mechanism of the SRT1720-induced rescuing of age- and obesity-related endothelial dysfunction. A, Vasorelaxation to cumulative concentration of ACh in vessels precontracted with norepinephrine. Vasodilatory response is expressed as % of the maximal diameter. The experiment was conducted on vessels from Young Nonobese (n=5) at baseline (saline group), after overnight resting in transfection culture medium with scrambled siRNA (scr.siRNA group), and after overnight transfection with SIRT1 siRNA sc-40986 (siRNA group). The curves were repeated 2 times for each vessel, the first with ACh alone and the second after co-incubation with L-NAME (white triangle). Data are presented as mean±SEM and were compared by the Kruskal-Wallis test with Dwass-Steel-Critchlow-Flinger post hoc test. Within the same group, data were compared by the Friedman test. B and C, Differences in mtROS (red staining) and NO (green staining) levels assessed by mitoSOX and DAF-FM fluorescence between Young Nonobese vessels (n=5) at baseline (Young Nonobese), Young Nonobese vessels (n=5) after overnight resting in transfection culture medium with scrambled siRNA (Young Nonobese_scr.siRNA), Young Nonobese vessels (n=5) after overnight transfection with SIRT1 siRNA sc-40986 (Young Nonobese_siRNA) and between Young Nonobese vessels (n=5) at baseline (Young Nonobese), Old Obese vessels (n=5) before overnight incubation with SRT1720 (Old Obese_baseline), Old Obese vessels (n=5) after overnight incubation with SRT1720 (Old Obese_SRT1720). Data are presented as mean±SEM and were compared by the Kruskal-Wallis test with Dwass-Steel-Critchlow-Flinger post hoc test. Fluorescence is calculated as mean fluorescence intensity (MFI) and expressed as % of the Young Nonobese group. Original magnification is 40×. D, Relaxing response to cumulative concentration to Ach in vessels from Old Obese group (n=6) precontracted with norepinephrine. Vasodilatory response is expressed as % of the maximal diameter. The experiment was repeated 7 times for each patient, respectively by incubating the vessel with saline alone (black line), SRT1720 (green line), mitoTEMPO (orange line), gp91dstat (blue line), SRT1720+mitoTEMPO (red line), SRT1720+gp91dstat (brown line), mitoTEMPO+gp91dstat (pink line). Shapiro-Wilk test was adopted to assess normality. Data are presented as mean±SEM and were compared by ANOVA for repeated measures followed by a post hoc test with Holm-Sidak post hoc correction. E, Maximal vasodilatory response to Ach expressed as % of the maximal diameter in vessels from the Old Obese group (n=6) precontracted with norepinephrine. The experiment was repeated 7 times for each patient, respectively by incubating the vessel with saline alone (black bar), SRT1720 (green bar), mitoTEMPO (orange bar), gp91dstat (blue bar), SRT1720+mitoTEMPO (red bar), SRT1720+gp91dstat (brown bar), mitoTEMPO+gp91dstat (pink bar). Data are presented as mean±SEM and were compared by ANOVA for repeated measures followed by a post hoc test with Holm-Sidak post hoc correction. F–I, Mitochondria swelling assay of small resistance arteries of Young Nonobese (n=5) and Old Obese group (n=5). Mitochondria were tested before (blue dots) and after (red dots) the calcium load at baseline for Young Nonobese (G) and Old Obese groups (H) and, for the Old Obese group, after 24-hour incubation with SRT1720 (I). After the incubation with SRT1720, the reduction of absorbance was attenuated both at 10 and 20 minutes (F) and expressed as % of the Young Nonobese group, used as a control. Data are presented as mean±SEM and were compared by the Kruskal-Wallis test with Dwass-Steel-Critchlow-Flinger post hoc test. *P<0.05.**P<0.01. OO indicates old obese; and YN, young nonobese.
SIRT1 Inhibition on p66Shc and Arginase II Is Attenuated in Small Visceral Arteries of Old Obese
To explore SIRT1 epigenetic control on p66Shc and Arginase II, we performed a chromatin immunoprecipitation assay in the Old Obese and Young Nonobese groups, reflecting the most deranged and preserved phenotypes, respectively. We observed that the binding of SIRT1 on p66Shc and Arginase II promoters is markedly reduced in Old Obese (Figure S3). This marked difference shows that in Old Obese the downregulated SIRT1 cannot prevent, by epigenetic modulation of p66Shc and Arginase II promoters, the overexpression of these 2 pro-oxidant and pro-aging factors, as shown in Figure 2B and 2E.
SRT1720 Overnight Incubation Reduces mtROS Levels and Increases NO Availability in Small Visceral Arteries of Old Obese
To support the potential short-term influence of SIRT1 on the control of mtROS in obesity and to gather further information on the possible mechanisms underlying these effects, segments of small resistance arteries of Old Obese patients (n=5) were studied before or after incubation for 24 hours with SRT1720. Baseline Young Nonobese vessels used for the siRNA experiments were used as controls to compare fluorescence intensity. The segments studied after incubation with SRT1720 showed a significant reduction in the levels of mtROS and a substantial improvement in the NO availability compared with the segments studied immediately after isolation though inferior to the Young Nonobese (Figure 5B and 5C). These findings were also confirmed in a smaller sample of patients (n=3) by adopting ENZ-53013 and mitoPY1, more specific fluorescent probes for mtROS and NO availability, respectively (Figure S1).
Comparison of SRT1720 With mtROS and NADPH-Oxidase ROS Scavengers
The independent and additive contribution of mtROS and NADPH-oxidase ROS scavenging was tested in small resistance arteries of Old Obese patients (n=6). We explored the impact of these factors on vessels from the Old Obese as this group represents an example of an extreme dysfunctional phenotype, therefore, ensuring enough power to observe significant potential improvement. As expected by the other experiments showing the SRT1720-mediated reduction of mtROS, SRT1720 and mitoTEMPO effects are superimposable. Instead, gp91dstat alone impact is smaller (Figure 5D and 5E). These experiments highlight how SIRT1 modulation ameliorates endothelial functions in markedly deranged subjects (Old Obese group) with no additive contribution from the mitochondrial- and NADPH-derived ROS scavenging.
SRT1720 Restores Old Obese Small Vessel-Derived Mitochondria Integrity and Respiratory Chain Genes Expression
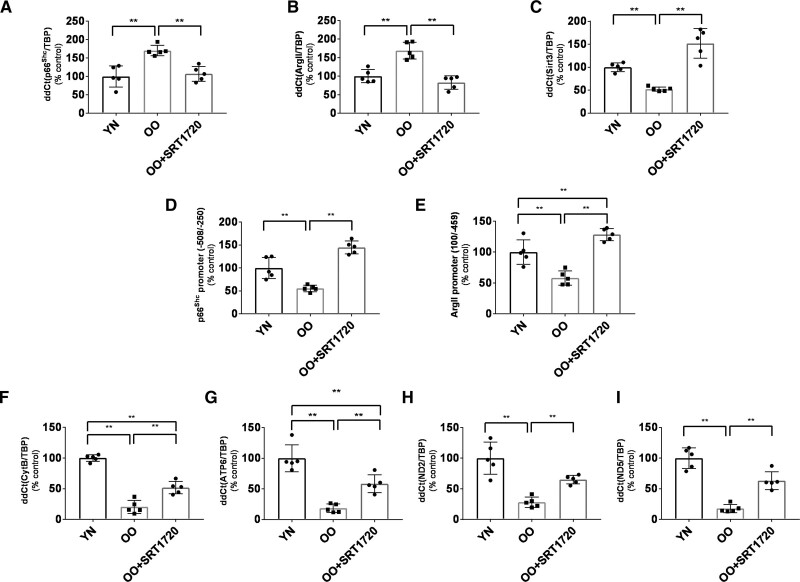
Mitochondria swelling assay was performed to characterize further the protection induced by a restored SIRT1 function on the mitochondria functional and structural integrity. Mitochondria extracted from segments of small resistance arteries from Old Obese patients (n=5) were studied before and after incubation for 24 hours with SRT1720. The rationale for the choice of the Old Obese group was the same as above. The incubation with SRT1720 improved the resistance of the mitochondria to the calcium load, suggesting an increased resistance of their membrane to fragmentation mainly related to oxidative damage (Figure 5F through 5I). Vessels from the same Old Obese patients (n=5) before and after overnight incubation with SRT1720 were processed to analyze the changes in gene expression and epigenetic patterns related to mitochondria ROS levels. Incubation with SRT1720 caused a downregulation in the expression of the p66Shc (P=0.02 versus baseline, Figure 6A) and Arginase II (P=0.02 versus baseline, Figure 6B) associated with increased binding of SIRT1 to p66Shc and Arginase II promoters (Figure 6D and 6E). SIRT3 expression was also increased after SRT1720 incubation (P=0.02 versus baseline, Figure 6C); however, chromatin immunoprecipitation assays did not show an interaction of SIRT1 with SIRT3 promoter. Mitochondria respiratory chain genes, namely ATP6 (ATP synthase 6), cytochrome b (Cytb), ND2 (NADH dehydrogenase 2) and ND5 (NADH dehydrogenase 5; Figure 6F through 6I), were found upregulated. These results suggest that SIRT1 activity can protect against mitochondria’ increased ROS generation.
Figure 6.
Genic profile of several proteins in the vessel wall of Old Obese subjects (n=5) before and after incubation with SRT1720. A, Expression of p66Shc. B, Expression of Arginase II. C, Expression of Sirt3. Notably, the ChIP assay did not detect the binding of SIRT1 (silent information regulator 1) on the Sirt3 promoter. D, qPCR after ChIP assay showing binding of SIRT1 on the promoter region of p66Shc before and after incubation with SRT1720. E, qPCR after ChIP assay showing binding of SIRT1 on the promoter region of Arginase II before and after incubation with SRT1720. F–I Expression of mitochondria respiratory chain enzymes: ATP synthase 6 (F), Cytochrome b (G), NADH dehydrogenase 2 (H) and NADH dehydrogenase 5 (I). Data are presented as mean±SD and were compared by the Kruskal-Wallis test with Dwass-Steel-Critchlow-Flinger post hoc test. A P<0.05 was considered significant. **P<0.01. ArgII indicates arginase II; ATP6, ATP synthase 6; Cytb, Cytochrome b; ND2, NADH dehydrogenase 2; OO, old obese; and YN, young nonobese.
SRT1720 and mitoTEMPO Effect Is Consistent Across Age- and Obesity-Related Endothelial Dysfunction
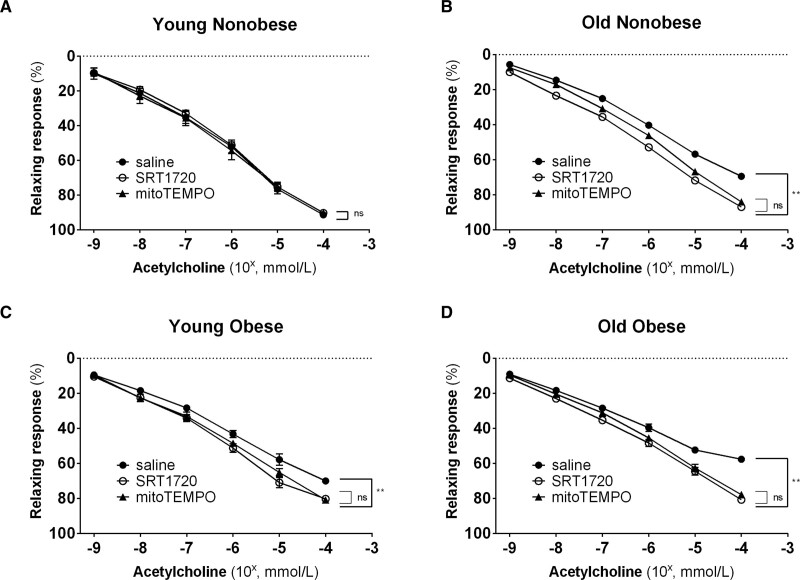
MitoTEMPO induced a significant increase in the endothelial-dependent vasodilatory response to the Ach in the Young Obese, Old Obese and Old Nonobese groups (P<0.001) but not in the Young Nonobese group (Figure 7A through 7D). Intriguingly, the extent to which the endothelial-dependent vasodilation was rescued with MitoTEMPO was comparable to the effect obtained with SRT1720. In a small subpopulation (n=12, 3 subjects for each group), we compared the impact of SRT1720 on the vasodilatory response also with low-dose rotenone, an electron transport chain complex I inhibitor,27 and tempol, a nontargeted SOD mimetic, confirming the superimposable effect of SIRT1 and an inhibitor of mtROS generation (Figure S4). These finding indirectly confirms that SRT1720 acts on pathways involving mtROS mitigation to control endothelial function across the whole spectrum of aging and obesity.
Figure 7.
Comparison of SRT1720 and mitoTEMPO rescuing microcirculatory dysfunction in the 4 groups. A–D, Relaxing response to cumulative concentration to Ach in vessels precontracted with norepinephrine in the 4 groups (young nonobese: n=5; old nonobese: n=16; young obese: n=8; old obese: n=22). Vasodilatory response is expressed as % of the maximal diameter. The experiment was repeated 3 times for each patient by incubating the vessel with saline (black circle), SRT1720 (white circle), and mitoTEMPO (black triangle). Shapiro-Wilk test was adopted to assess normality. Data are presented as mean±SEM and were compared by the Friedman test followed by Durbin-Conover as a post hoc test (A) and ANOVA for repeated measures followed by a post hoc test with Holm-Sidak correction (B–D). A P<0.05 was considered significant. **P<0.01.
Discussion
We report for the first time that (1) human microvascular age- and obesity-related endothelial dysfunction are similarly characterized by a progressive reduction in vascular SIRT1 expression; (2) this decrease in microvascular SIRT1 expression is paired with reduced NO availability and increased vascular p66Shc and mtROS levels; (3) specific SIRT1 modulation significantly restores endothelial dysfunction in Old Obese, Young Obese and Old Nonobese individuals; (4) SRT1720 restored endothelial function by epigenetic changes, mainly involving p66Shc and Arginase II, leading to a marked reduction of mtROS levels with a consistent effect across the whole age- and obesity spectrum. This is the first study to document a central involvement of SIRT1 in human aging- and obesity-related microvascular dysfunction underpinned by its significant modulation of mtROS levels.
Several observations have linked SIRT1 to aging and obesity.9,11 Lower SIRT1 expression and activity are found in obese mice28,29 and within the peripheral circulation and adipose tissue of obese subjects.30,31 The tight regulation of SIRT1 by the availability of its metabolic cofactor, NAD+, and the related intermediates, NADH and nicotinamide, might account for the negative effects of obesity on SIRT1 activity/expression.10,32,33 Similarly, SIRT1 is downregulated in tissues of older mice34 and peripheral T-cells of older human donors.34 To our knowledge, this is the first evidence of a reduced expression of SIRT1 directly on the human microvasculature. Only a previous study explored the expression of SIRT1 in endothelial cells35 but was limited to the aging phenotype and adopted cells from a large vessel (brachial artery). We investigated SIRT1 relationship with the microvascular phenotype, an acknowledged driver of age- and metabolic-related disease.6,24 In mice, SIRT1 protects from age-related endothelial dysfunction.36 In gain/loss-of-function murine models, SIRT1 exerts a protective effect on metabolic-related endothelial dysfunction.9 Selective endothelial SIRT1 deficiency rarefies glycocalyx in mice.37 In humans, systemic lower circulating SIRT1 in childhood is associated with microvascular dysfunction during adulthood.38 Our findings confirm the tight relationship between vascular SIRT1 and age- and metabolic-related microvascular endothelial function in humans. They promote the hypothesis of SIRT1 as a crucial biological link between cellular energy availability, metabolism, and aging.9 We describe how in obesity, a condition of excessive nutrient availability ultimately accelerating aging,39 the downregulation of SIRT1 is associated with the acquisition of a vascular aging phenotype. This is characterized by reduced epigenetic control on p66Shc,40 reduced NO availability, increased mtROS levels and the consequent onset of endothelial dysfunction. In the long term, these alterations might lead to faster arteriolar remodeling with aging41 (Figure 1A). Obesity considerably accelerates alterations related to attenuation of SIRT1 signaling, as these are already present in the Young Obese group.
Exogenous stimulation of SIRT1 activity has been proposed as a promising cardiovascular therapeutic target.42,43 In vitro, SRT1720 protects microvascular endothelial cells from pro-inflammatory stimuli.44 In mice, SRT1720 reverses vascular endothelial dysfunction, excessive superoxide production, inflammation,12 and arterial stiffness28 related to aging. In humans, results on SIRT1 agonists are scarce, and their therapeutic potential is yet to be explored.9 Resveratrol improves endothelial dysfunction in patients with obesity.22,45 SRT2104 showed a trend in improving the estimated mitochondrial oxidative capacity in humans.46 However, evidence is poor, and both resveratrol and SRT1720 partially fail to achieve cardiometabolic relevant effect due to their demonstrated lack of vascular selectivity, instead exhibiting an organ preference for liver and white adipose tissue.47 Here, we report for the first time a direct effect of SRT1720 on the human microcirculatory function, accurately measured through a complex ex vivo setting. The magnitude of the impact is consistent on different vascular phenotypes. It leverages a significant contribution of NO availability, as witnessed by the concordant improvement of L-NAME inhibition to ACh. The direct and strong relationship with age, BMI and vascular remodeling points out the crucial contribution of SIRT1 in rescuing metabolic-related endothelial dysfunction. Our choice to investigate the role of SIRT1 in a nondiabetic microvascular phenotype stresses the relevance of our findings, placing SIRT1 signaling-related impairment at the beginning of the health-to-disease phenotyping translation.
The control of SIRT1 on endothelial cell function involves several mechanisms. Besides the well-established capacity of SIRT1 to influence the expression of the eNOS48 through modulation of the FOXO factor axis,49 we found that vessels from obese patients had a reduced binding of SIRT1 on the promoter region of Arginase II, accompanied by an increased expression of this enzyme. We recently showed that arginase II contributes to obesity- and aging-related endothelial dysfunction by competing with the eNOS for the arginine substrate.8 However, the influence of SIRT1 on NO availability is not limited to its activity on the eNOS. Indeed, it affects the expression of other enzymes with essential roles in regulating the mitochondria oxidative stress balance. First, we document an epigenetic control of SIRT1 on the expression of the p66Shc. The lifespan determinant p66Shc is involved in obesity-induced oxidative stress, mitochondrial dysfunction and vascular inflammation.50,51 p66Shc is responsible for vascular detrimental epigenetic signature related to excessive availability of metabolic substrates.25,52 While subsequent reports confirmed the role of p66Shc in promoting endothelial dysfunction in human obesity,53 no studies explored, by far, its control by SIRT1 nor the similar alteration of the SIRT1/p66Shc axis in aging and obesity in humans. The SIRT1 binding on the p66Shc promoter was paired to higher p66Shc gene expression. It should be noted that some studies report that only phosphorylated p66Shc levels, not total p66Shc, are higher in the aging vasculature.54 However, other observations in vitro and in vivo showed that total, mitochondrial-specific and phosphorylated p66Shc were all found upregulated in different models of metabolic damage.25,40,55 Although this is the first study to explore the SIRT1/p66Shc axis in aging and obesity microvasculature in humans, an increase of p66Shc mRNA expression has also been found in humans in peripheral blood in other models of aging56 and metabolic damage.53,57 This provides further consistency to our results and supports the hypothesis that both total and phosphorylated p66Shc affect microvascular homeostasis.
The documented effect of SIRT1 on Arginase II and p66Shc might be crucial in mediating its beneficial impact on vascular homeostasis. Our findings also did not exclude a possible post-translational role of SIRT1 in regulating Arginase II and p66Shc functions. Second, we document that a restored SIRT1 activity can increase the production of mitochondria enzymes with key antioxidant capacities, such as SIRT3 and SOD2. We did not find evidence of direct binding of SIRT1 on the promoter region of SIRT3. However, SIRT3 higher levels after vessel exposure to SRT1720 suggest the involvement of post-translational SIRT1-mediated regulation. This is consistent with previous experiments reporting that endothelial cells exposed to a short incubation with resveratrol show upregulation of the AMPK-PGC-1α-ERRα pathway, indirectly increasing SIRT3.58
The upregulation of several mitochondrial antioxidant genes paired with the increased expression of genes involved in the respiratory chain might be one of the mechanisms explaining the positive modulation of SIRT1 on mitochondria ROS levels. An increased expression of the various complexes involved in oxidative phosphorylation in the electron transport chain improves the ATP synthesis derived from oxidative metabolism.59 The mitigation of ROS levels by SIRT1 is acknowledged, repeatedly described in vitro,18 also in human microvascular endothelial cells.44 However, it is still debated60 whether its primary target is NADPH-oxidase61 or mtROS.62 We compared the endothelial function-specific impact of NADPH-oxidase inhibition and mtROS scavenging to SRT1720. The different magnitude of the effect and several indirect proofs of improved mitochondria homeostasis, as the restored resistance to mitochondria swelling assays, support a predominant mtROS-driven SIRT1 targeting action. This has been proposed but never reported in human vasculature.63,64 We here describe it in the human small vessel arteries and shows its consistence across the whole range of age- and obesity-related microvascular impairment.
Our study has some weaknesses. The myograph-microvessel system is an ex vivo technique to assess microvascular function and structure on isolated vessels. Consequently, differences in local flow and mechanical forces imposed by the remodeling of large vessels in obesity might attenuate in vivo the benefits obtained through restoring the SIRT1 activity described in our experiments. However, micromyography remains the gold standard method for assessing endothelial microvascular function and structure in humans.65 We focused on mitochondrial pathways that influence mitochondria and endothelial function, potentially connected with SIRT1 as documented in animal or in vitro experiments.22,50 Therefore, we cannot exclude the presence of other epigenetic mechanisms through which SIRT1 might influence mitochondria oxidative stress and endothelial functions in humans. It should be noted that qPCR assays were performed on isolated microvessels composed of smooth muscle and endothelial cells. Thus, we did not investigate the contribution of SIRT1 to smooth muscle cell (SMC) homeostasis. Indeed, a recent observation in mice reported a positive effect of endothelial SIRT1 to SMC vasodilator response. However, this held true only in eNOS knockout mice.36 We focused on endothelium because of its very early involvement during age- and obesity-related microvascular dysfunction, being acknowledged as one of the first actors involved in the healthy-to-disease transition.6,7,24,41 Moreover, the preserved vasodilation to sodium nitroprusside indirectly excludes a nonendothelial substantial contribution to the phenotype. Also, the specificity of the fluorescent probes DAF-FM and mitoSOX is partial. However, both are still widely adopted for investigating tissue mtROS and NO levels.66 Moreover, we have confirmed our results with more specific probes,27,67–69 even though in a small subpopulation (Figure S1). The potential metabolic implications of our findings remain to be elucidated. Indeed, we have demonstrated the influence of SIRT1 on the expression of mitochondria proteins involved in the electron transport system. Still, we have not directly measured the mitochondria respiratory chain efficiency nor potential changes induced by a restored SIRT1 activity. Nonetheless, some observations described the potential benefits on the mitochondria oxidative phosphorylation induced by the changes in protein expression described in our study.70–74 We have also to report that we did not measure protein levels, as we focused on mRNA. However, it should be noted that mRNA levels are acknowledged as informative of protein expression and are currently widely employed, especially in in-human studies.53,56,57 Finally, it should be noted that the recruited obese population reflect a population of patients with a mild degree of disease, as we intended to investigate metabolic microvascular dysfunction from its very early stages. Although this is a strength in terms of pathophysiologic exploration of the health-to-disease transition, it might limit the generalization of the results to the standard population of patients with obesity. Finally, it should be clarified that even if SRT1720 can modulate the activity of SIRT3, this effect is dosage-dependent as SRT1720 EC50 is 0.16 µmol/L for SIRT1 instead of >300 µmol/L for SIRT3.75 Thus, the dosage adopted in our study did not directly modulate SIRT3 activity.
In conclusion, SIRT1 represents a significant pathway influencing obesity- and aging-related microvascular dysfunction since its earliest stages through a complex epigenetic control on mtROS (Figure S5). Given the centrality of the microvascular system in response to metabolic demand,6,7 SIRT1 endothelial-specific regulation influences the vascular phenotype and impacts systemic metabolism and aging, placing SIRT1 at the center of the crosstalk between substrates availability, cellular metabolism, and vascular phenotype. The microcirculatory endothelium is the earliest organ damaged by cardiovascular risk factors6 which, since childhood,76 inflict a permanent vascular-specific epigenetic wound.7 Our findings strongly support early treatments to preserve the SIRT1 expression/activity. For instance, a recent long-term observation showed how high dietary intake of NAD+ reduces the incidence of heart failure with preserved ejection fraction,77 that is, the metabolic phenotype of heart failure.78 Similarly, ketogenic states mitigate cardiometabolic damage79 and preserve microvascular aging.80 SIRT1 targeting intervention might thus represent crucial strategies to alleviate health care systems from the currently unbearable cardiovascular burden.
Article Information
Sources of Funding
F. Paneni is the recipient of a H.H. Sheikh Khalifa bin Hamad Al Thani Foundation Assistant Professorship at the Faculty of Medicine, University of Zurich. This work was supported by the Swiss National Science Foundation, the Zürich Heart House, the Swiss Heart Foundation, the Swiss Life Foundation, the Kurt und Senta-Hermann Stiftung, the EMDO Stiftung and the Schweizerische Diabetes-Stiftung to F. Paneni; the Holcim Foundation and the Swiss Heart Foundation (to S. Costantino).
Disclosures
None.
Supplemental Material
Detailed Methods
Figures S1 through S5
Table S1
Supplementary Material
Nonstandard Abbreviations and Acronyms
- ATP6
- ATP synthase 6
- BMI
- body mass index
- Cytb
- cytochrome b
- FOXO3
- forkhead box protein O3
- mtROS
- mitochondria reactive oxygen species
- ND2
- NADH dehydrogenase 2
- ND5
- NADH dehydrogenase 5
- SIRT1
- silent information regulator 1
- SOD2
- superoxide dismutase-2
A. Mengozzi, S. Costantino, A. Virdis, and S. Masi contributed equally.
For Sources of Funding and Disclosures, see page 489.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCRESAHA.122.320888.
References
- 1.Costantino S, Paneni F, Cosentino F. Ageing, metabolism and cardiovascular disease. J Physiol. 2016;594:2061–2073. doi: 10.1113/JP270538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Franceschi C, Garagnani P, Parini P, Giuliani C, Santoro A. Inflammaging: a new immune-metabolic viewpoint for age-related diseases. Nat Rev Endocrinol. 2018;14:576–590. doi: 10.1038/s41574-018-0059-4 [DOI] [PubMed] [Google Scholar]
- 3.Ward ZJ, Bleich SN, Cradock AL, Barrett JL, Giles CM, Flax C, Long MW, Gortmaker SL. Projected U.S. State-level prevalence of adult obesity and severe obesity. N Engl J Med. 2019;381:2440–2450. doi: 10.1056/NEJMsa1909301 [DOI] [PubMed] [Google Scholar]
- 4.Eckel N, Li Y, Kuxhaus O, Stefan N, Hu FB, Schulze MB. Transition from metabolic healthy to unhealthy phenotypes and association with cardiovascular disease risk across BMI categories in 90 257 women (the Nurses’ Health Study): 30 year follow-up from a prospective cohort study. Lancet Diabetes Endocrinol. 2018;6:714–724. doi: 10.1016/S2213-8587(18)30137-2 [DOI] [PubMed] [Google Scholar]
- 5.Reddy MA, Zhang E, Natarajan R. Epigenetic mechanisms in diabetic complications and metabolic memory. Diabetologia. 2015;58:443–455. doi: 10.1007/s00125-014-3462-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooke JP. Endotheliopathy of Obesity. Circulation. 2020;142:380–383. doi: 10.1161/CIRCULATIONAHA.120.047574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gutterman DD, Chabowski DS, Kadlec AO, Durand MJ, Freed JK, Ait-Aissa K, Beyer AM. The Human Microcirculation: Regulation of Flow and Beyond. Circ Res. 2016;118:157–172. doi: 10.1161/CIRCRESAHA.115.305364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Masi S, Colucci R, Duranti E, Nannipieri M, Anselmino M, Ippolito C, Tirotta E, Georgiopoulos G, Garelli F, Nericcio A, et al. Aging Modulates the Influence of Arginase on Endothelial Dysfunction in Obesity. Arterioscler Thromb Vasc Biol. 2018;38:2474–2483. doi: 10.1161/ATVBAHA.118.311074 [DOI] [PubMed] [Google Scholar]
- 9.Kane AE, Sinclair DA. Sirtuins and NAD+ in the development and treatment of metabolic and cardiovascular diseases. Circ Res. 2018;123:868–885. doi: 10.1161/CIRCRESAHA.118.312498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen HY, Miller C, Bitterman KJ, Wall NR, Hekking B, Kessler B, Howitz KT, Gorospe M, de Cabo R, Sinclair DA. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science. 2004;305:390–392. doi: 10.1126/science.1099196 [DOI] [PubMed] [Google Scholar]
- 11.Pardo PS, Boriek AM. SIRT1 regulation in ageing and obesity. Mech Ageing Dev. 2020;188:111249. doi: 10.1016/j.mad.2020.111249 [DOI] [PubMed] [Google Scholar]
- 12.Gano LB, Donato AJ, Pasha HM, Hearon CM, Jr, Sindler AL, Seals DR. The SIRT1 activator SRT1720 reverses vascular endothelial dysfunction, excessive superoxide production, and inflammation with aging in mice. Am J Physiol Heart Circ Physiol. 2014;307:H1754–H1763. doi: 10.1152/ajpheart.00377.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Imai S, Guarente L. NAD+ and sirtuins in aging and disease. Trends Cell Biol. 2014;24:464–471. doi: 10.1016/j.tcb.2014.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kudryavtseva AV, Lipatova AV, Zaretsky AR, Moskalev AA, Fedorova MS, Rasskazova AS, Shibukhova GA, Snezhkina AV, Kaprin AD, Alekseev BY, et al. Important molecular genetic markers of colorectal cancer. Oncotarget. 2016;7:53959–53983. doi: 10.18632/oncotarget.9796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Mello AH, Costa AB, Engel JDG, Rezin GT. Mitochondrial dysfunction in obesity. Life Sci. 2018;192:26–32. doi: 10.1016/j.lfs.2017.11.019 [DOI] [PubMed] [Google Scholar]
- 16.Zhou B, Tian R. Mitochondrial dysfunction in pathophysiology of heart failure. J Clin Invest. 2018;128:3716–3726. doi: 10.1172/JCI120849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kluge MA, Fetterman JL, Vita JA. Mitochondria and endothelial function. Circ Res. 2013;112:1171–1188. doi: 10.1161/CIRCRESAHA.111.300233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singh CK, Chhabra G, Ndiaye MA, Garcia-Peterson LM, Mack NJ, Ahmad N. The role of sirtuins in antioxidant and redox signaling. Antioxid Redox Signal. 2018;28:643–661. doi: 10.1089/ars.2017.7290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang X, Liu Q, Li Y, Tang Q, Wu T, Chen L, Pu S, Zhao Y, Zhang G, Huang C, et al. The diabetes medication canagliflozin promotes mitochondrial remodelling of adipocyte via the AMPK-Sirt1-Pgc-1α signalling pathway. Adipocyte. 2020;9:484–494. doi: 10.1080/21623945.2020.1807850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Price NL, Gomes AP, Ling AJ, Duarte FV, Martin-Montalvo A, North BJ, Agarwal B, Ye L, Ramadori G, Teodoro JS, et al. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab. 2012;15:675–690. doi: 10.1016/j.cmet.2012.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng Y, Zeng X, Mai Q, Bai X, Jiang Y, Li J, Fan S, Ding H. Insulin injections inhibits PTZ-induced mitochondrial dysfunction, oxidative stress and neurological deficits via the SIRT1/PGC-1α/SIRT3 pathway. Biochim Biophys Acta Mol Basis Dis. 2021;1867:166124. doi: 10.1016/j.bbadis.2021.166124 [DOI] [PubMed] [Google Scholar]
- 22.Masi S, Ambrosini S, Mohammed SA, Sciarretta S, Lüscher TF, Paneni F, Costantino S. Epigenetic remodeling in obesity-related vascular disease. Antioxid Redox Signal. 2021;34:1165–1199. doi: 10.1089/ars.2020.8040 [DOI] [PubMed] [Google Scholar]
- 23.Sasson AN, Cherney DZ. Renal hyperfiltration related to diabetes mellitus and obesity in human disease. World J Diabetes. 2012;3:1–6. doi: 10.4239/wjd.v3.i1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mengozzi A, Pugliese NR, Chiriacò M, Masi S, Virdis A, Taddei S. Microvascular ageing links metabolic disease to age-related disorders: the role of oxidative stress and inflammation in promoting microvascular dysfunction. J Cardiovasc Pharmacol. 2021;78:S78–S87. doi: 10.1097/FJC.0000000000001109 [DOI] [PubMed] [Google Scholar]
- 25.Paneni F, Mocharla P, Akhmedov A, Costantino S, Osto E, Volpe M, Lüscher TF, Cosentino F. Gene silencing of the mitochondrial adaptor p66(Shc) suppresses vascular hyperglycemic memory in diabetes. Circ Res. 2012;111:278–289. doi: 10.1161/CIRCRESAHA.112.266593 [DOI] [PubMed] [Google Scholar]
- 26.Hagenbuchner J, Ausserlechner MJ. Mitochondria and FOXO3: breath or die. Front Physiol. 2013;4:147. doi: 10.3389/fphys.2013.00147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Freed JK, Beyer AM, LoGiudice JA, Hockenberry JC, Gutterman DD. Ceramide changes the mediator of flow-induced vasodilation from nitric oxide to hydrogen peroxide in the human microcirculation. Circ Res. 2014;115:525–532. doi: 10.1161/CIRCRESAHA.115.303881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fry JL, Al Sayah L, Weisbrod RM, Van Roy I, Weng X, Cohen RA, Bachschmid MM, Seta F. Vascular smooth muscle sirtuin-1 protects against diet-induced aortic stiffness. Hypertension. 2016;68:775–784. doi: 10.1161/HYPERTENSIONAHA.116.07622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nawaz A, Mehmood A, Kanatani Y, Kado T, Igarashi Y, Takikawa A, Yamamoto S, Okabe K, Nakagawa T, Yagi K, et al. Publisher Correction: Sirt1 activator induces proangiogenic genes in preadipocytes to rescue insulin resistance in diet-induced obese mice. Sci Rep. 2018;8:14597. doi: 10.1038/s41598-018-32600-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Costa Cdos S, Hammes TO, Rohden F, Margis R, Bortolotto JW, Padoin AV, Mottin CC, Guaragna RM. SIRT1 transcription is decreased in visceral adipose tissue of morbidly obese patients with severe hepatic steatosis. Obes Surg. 2010;20:633–639. doi: 10.1007/s11695-009-0052-z [DOI] [PubMed] [Google Scholar]
- 31.Mariani S, di Giorgio MR, Martini P, Persichetti A, Barbaro G, Basciani S, Contini S, Poggiogalle E, Sarnicola A, Genco A, et al. Inverse association of circulating SIRT1 and adiposity: a study on underweight, normal weight, and obese patients. Front Endocrinol (Lausanne). 2018;9:449. doi: 10.3389/fendo.2018.00449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chang HC, Guarente L. SIRT1 and other sirtuins in metabolism. Trends Endocrinol Metab. 2014;25:138–145. doi: 10.1016/j.tem.2013.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jukarainen S, Heinonen S, Rämö JT, Rinnankoski-Tuikka R, Rappou E, Tummers M, Muniandy M, Hakkarainen A, Lundbom J, Lundbom N, et al. Obesity is associated with low NAD(+)/SIRT pathway expression in adipose tissue of BMI-discordant monozygotic twins. J Clin Endocrinol Metab. 2016;101:275–283. doi: 10.1210/jc.2015-3095 [DOI] [PubMed] [Google Scholar]
- 34.Xu C, Wang L, Fozouni P, Evjen G, Chandra V, Jiang J, Lu C, Nicastri M, Bretz C, Winkler JD, et al. SIRT1 is downregulated by autophagy in senescence and ageing. Nat Cell Biol. 2020;22:1170–1179. doi: 10.1038/s41556-020-00579-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Donato AJ, Magerko KA, Lawson BR, Durrant JR, Lesniewski LA, Seals DR. SIRT-1 and vascular endothelial dysfunction with ageing in mice and humans. J Physiol. 2011;589:4545–4554. doi: 10.1113/jphysiol.2011.211219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guo Y, Xu C, Man AWC, Bai B, Luo C, Huang Y, Xu A, Vanhoutte PM, Wang Y. Endothelial SIRT1 prevents age-induced impairment of vasodilator responses by enhancing the expression and activity of soluble guanylyl cyclase in smooth muscle cells. Cardiovasc Res. 2019;115:678–690. doi: 10.1093/cvr/cvy212 [DOI] [PubMed] [Google Scholar]
- 37.Lipphardt M, Song JW, Goligorsky MS. Sirtuin 1 and endothelial glycocalyx. Pflugers Arch. 2020;472:991–1002. doi: 10.1007/s00424-020-02407-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rodriguez-Miguelez P, Looney J, Thomas J, Harshfield G, Pollock JS, Harris RA. Sirt1 during childhood is associated with microvascular function later in life. Am J Physiol Heart Circ Physiol. 2020;318:H1371–H1378. doi: 10.1152/ajpheart.00024.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tam BT, Morais JA, Santosa S. Obesity and ageing: two sides of the same coin. Obes Rev. 2020;21:e12991. doi: 10.1111/obr.12991 [DOI] [PubMed] [Google Scholar]
- 40.Zhou S, Chen HZ, Wan YZ, Zhang QJ, Wei YS, Huang S, Liu JJ, Lu YB, Zhang ZQ, Yang RF, et al. Repression of P66Shc expression by SIRT1 contributes to the prevention of hyperglycemia-induced endothelial dysfunction. Circ Res. 2011;109:639–648. doi: 10.1161/CIRCRESAHA.111.243592 [DOI] [PubMed] [Google Scholar]
- 41.Masi S, Georgiopoulos G, Chiriacò M, Grassi G, Seravalle G, Savoia C, Volpe M, Taddei S, Rizzoni D, Virdis A. The importance of endothelial dysfunction in resistance artery remodelling and cardiovascular risk. Cardiovasc Res. 2020;116:429–437. doi: 10.1093/cvr/cvz096 [DOI] [PubMed] [Google Scholar]
- 42.Winnik S, Auwerx J, Sinclair DA, Matter CM. Protective effects of sirtuins in cardiovascular diseases: from bench to bedside. Eur Heart J. 2015;36:3404–3412. doi: 10.1093/eurheartj/ehv290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guo Y, Xu A, Wang Y. SIRT1 in endothelial cells as a novel target for the prevention of early vascular aging. J Cardiovasc Pharmacol. 2016;67:465–473. doi: 10.1097/FJC.0000000000000344 [DOI] [PubMed] [Google Scholar]
- 44.Fiorentino TV, Procopio T, Mancuso E, Arcidiacono GP, Andreozzi F, Arturi F, Sciacqua A, Perticone F, Hribal ML, Sesti G. SRT1720 counteracts glucosamine-induced endoplasmic reticulum stress and endothelial dysfunction. Cardiovasc Res. 2015;107:295–306. doi: 10.1093/cvr/cvv169 [DOI] [PubMed] [Google Scholar]
- 45.Akbari M, Tamtaji OR, Lankarani KB, Tabrizi R, Dadgostar E, Kolahdooz F, Jamilian M, Mirzaei H, Asemi Z. The effects of resveratrol supplementation on endothelial function and blood pressures among patients with metabolic syndrome and related disorders: a systematic review and meta-analysis of randomized controlled trials. High Blood Press Cardiovasc Prev. 2019;26:305–319. doi: 10.1007/s40292-019-00324-6 [DOI] [PubMed] [Google Scholar]
- 46.Libri V, Brown AP, Gambarota G, Haddad J, Shields GS, Dawes H, Pinato DJ, Hoffman E, Elliot PJ, Vlasuk GP, et al. A pilot randomized, placebo controlled, double blind phase I trial of the novel SIRT1 activator SRT2104 in elderly volunteers. PLoS One. 2012;7:e51395. doi: 10.1371/journal.pone.0051395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Svensson K, Schnyder S, Albert V, Cardel B, Quagliata L, Terracciano LM, Handschin C. Resveratrol and SRT1720 elicit differential effects in metabolic organs and modulate systemic parameters independently of skeletal muscle peroxisome proliferator-activated receptor γ Co-activator 1α (PGC-1α). J Biol Chem. 2015;290:16059–16076. doi: 10.1074/jbc.M114.590653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang QJ, Wang Z, Chen HZ, Zhou S, Zheng W, Liu G, Wei YS, Cai H, Liu DP, Liang CC. Endothelium-specific overexpression of class III deacetylase SIRT1 decreases atherosclerosis in apolipoprotein E-deficient mice. Cardiovasc Res. 2008;80:191–199. doi: 10.1093/cvr/cvn224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xia N, Strand S, Schlufter F, Siuda D, Reifenberg G, Kleinert H, Förstermann U, Li H. Role of SIRT1 and FOXO factors in eNOS transcriptional activation by resveratrol. Nitric Oxide. 2013;32:29–35. doi: 10.1016/j.niox.2013.04.001 [DOI] [PubMed] [Google Scholar]
- 50.Paneni F, Volpe M, Lüscher TF, Cosentino F. SIRT1, p66(Shc), and Set7/9 in vascular hyperglycemic memory: bringing all the strands together. Diabetes. 2013;62:1800–1807. doi: 10.2337/db12-1648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Paneni F, Costantino S, Kränkel N, Cosentino F, Lüscher TF. Reprogramming ageing and longevity genes restores paracrine angiogenic properties of early outgrowth cells. Eur Heart J. 2016;37:1733–1737. doi: 10.1093/eurheartj/ehw073 [DOI] [PubMed] [Google Scholar]
- 52.Kumar S, Kim YR, Vikram A, Naqvi A, Li Q, Kassan M, Kumar V, Bachschmid MM, Jacobs JS, Kumar A, et al. Sirtuin1-regulated lysine acetylation of p66Shc governs diabetes-induced vascular oxidative stress and endothelial dysfunction. Proc Natl Acad Sci U S A. 2017;114:1714–1719. doi: 10.1073/pnas.1614112114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Costantino S, Akhmedov A, Melina G, Mohammed SA, Othman A, Ambrosini S, Wijnen WJ, Sada L, Ciavarella GM, Liberale L, et al. Obesity-induced activation of JunD promotes myocardial lipid accumulation and metabolic cardiomyopathy. Eur Heart J. 2019;40:997–1008. doi: 10.1093/eurheartj/ehy903 [DOI] [PubMed] [Google Scholar]
- 54.Kumar S. P66Shc and vascular endothelial function. Biosci Rep. 2019;39:BSR20182134. doi: 10.1042/BSR20182134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Camici GG, Schiavoni M, Francia P, Bachschmid M, Martin-Padura I, Hersberger M, Tanner FC, Pelicci P, Volpe M, Anversa P, et al. Genetic deletion of p66(Shc) adaptor protein prevents hyperglycemia-induced endothelial dysfunction and oxidative stress. Proc Natl Acad Sci U S A. 2007;104:5217–5222. doi: 10.1073/pnas.0609656104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Streese L, Khan AW, Deiseroth A, Hussain S, Suades R, Tiaden A, Kyburz D, Hanssen H, Cosentino F. Physical activity may drive healthy microvascular ageing via downregulation of p66Shc. Eur J Prev Cardiol. 2020;27:168–176. doi: 10.1177/2047487319880367 [DOI] [PubMed] [Google Scholar]
- 57.Costantino S, Paneni F, Battista R, Castello L, Capretti G, Chiandotto S, Tanese L, Russo G, Pitocco D, Lanza GA, et al. Impact of glycemic variability on chromatin remodeling, oxidative stress, and endothelial dysfunction in patients with type 2 diabetes and with target HbA1c levels. Diabetes. 2017;66:2472–2482. doi: 10.2337/db17-0294 [DOI] [PubMed] [Google Scholar]
- 58.Zhou X, Chen M, Zeng X, Yang J, Deng H, Yi L, Mi MT. Resveratrol regulates mitochondrial reactive oxygen species homeostasis through Sirt3 signaling pathway in human vascular endothelial cells. Cell Death Dis. 2014;5:e1576. doi: 10.1038/cddis.2014.530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nemoto S, Combs CA, French S, Ahn BH, Fergusson MM, Balaban RS, Finkel T. The mammalian longevity-associated gene product p66shc regulates mitochondrial metabolism. J Biol Chem. 2006;281:10555–10560. doi: 10.1074/jbc.M511626200 [DOI] [PubMed] [Google Scholar]
- 60.Xia N, Daiber A, Förstermann U, Li H. Antioxidant effects of resveratrol in the cardiovascular system. Br J Pharmacol. 2017;174:1633–1643. doi: 10.1111/bph.13492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zarzuelo MJ, López-Sepúlveda R, Sánchez M, Romero M, Gómez-Guzmán M, Ungvary Z, Pérez-Vizcaíno F, Jiménez R, Duarte J. SIRT1 inhibits NADPH oxidase activation and protects endothelial function in the rat aorta: implications for vascular aging. Biochem Pharmacol. 2013;85:1288–1296. doi: 10.1016/j.bcp.2013.02.015 [DOI] [PubMed] [Google Scholar]
- 62.Ungvari Z, Labinskyy N, Mukhopadhyay P, Pinto JT, Bagi Z, Ballabh P, Zhang C, Pacher P, Csiszar A. Resveratrol attenuates mitochondrial oxidative stress in coronary arterial endothelial cells. Am J Physiol Heart Circ Physiol. 2009;297:H1876–H1881. doi: 10.1152/ajpheart.00375.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Man AWC, Xia N, Li H. Circadian rhythm in adipose tissue: novel antioxidant target for metabolic and cardiovascular diseases. Antioxidants (Basel). 2020;9:E968. doi: 10.3390/antiox9100968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kong X, Guan J, Li J, Wei J, Wang R. P66Shc-SIRT1 regulation of oxidative stress protects against cardio-cerebral vascular disease. Mol Neurobiol. 2017;54:5277–5285. doi: 10.1007/s12035-016-0073-2 [DOI] [PubMed] [Google Scholar]
- 65.Masi S, Rizzoni D, Taddei S, Widmer RJ, Montezano AC, Lüscher TF, Schiffrin EL, Touyz RM, Paneni F, Lerman A, et al. Assessment and pathophysiology of microvascular disease: recent progress and clinical implications. Eur Heart J. 2020:1–15. doi: 10.1093/eurheartj/ehaa857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–225. doi: 10.1038/nature09663 [DOI] [PubMed] [Google Scholar]
- 67.Mailloux RJ. An update on methods and approaches for interrogating mitochondrial reactive oxygen species production. Redox Biol. 2021;45:102044. doi: 10.1016/j.redox.2021.102044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Beyer AM, Zinkevich N, Miller B, Liu Y, Wittenburg AL, Mitchell M, Galdieri R, Sorokin A, Gutterman DD. Transition in the mechanism of flow-mediated dilation with aging and development of coronary artery disease. Basic Res Cardiol. 2017;112:5. doi: 10.1007/s00395-016-0594-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mahmoud AM, Hwang CL, Szczurek MR, Bian JT, Ranieri C, Gutterman DD, Phillips SA. Low-fat diet designed for weight loss but not weight maintenance improves nitric oxide-dependent arteriolar vasodilation in obese adults. Nutrients. 2019;11:1339. doi: 10.3390/nu11061339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bonnet C, Kaltimbacher V, Ellouze S, Augustin S, Bénit P, Forster V, Rustin P, Sahel JA, Corral-Debrinski M. Allotopic mRNA localization to the mitochondrial surface rescues respiratory chain defects in fibroblasts harboring mitochondrial DNA mutations affecting complex I or v subunits. Rejuvenation Res. 2007;10:127–144. doi: 10.1089/rej.2006.0526 [DOI] [PubMed] [Google Scholar]
- 71.Eya JC, Ukwuaba VO, Yossa R, Gannam AL. Interactive effects of dietary lipid and phenotypic feed efficiency on the expression of nuclear and mitochondrial genes involved in the mitochondrial electron transport chain in rainbow trout. Int J Mol Sci. 2015;16:7682–7706. doi: 10.3390/ijms16047682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Srivastava S, Barrett JN, Moraes CT. PGC-1alpha/beta upregulation is associated with improved oxidative phosphorylation in cells harboring nonsense mtDNA mutations. Hum Mol Genet. 2007;16:993–1005. doi: 10.1093/hmg/ddm045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hallows WC, Yu W, Denu JM. Regulation of glycolytic enzyme phosphoglycerate mutase-1 by Sirt1 protein-mediated deacetylation. J Biol Chem. 2012;287:3850–3858. doi: 10.1074/jbc.M111.317404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Burman JL, Itsara LS, Kayser EB, Suthammarak W, Wang AM, Kaeberlein M, Sedensky MM, Morgan PG, Pallanck LJ. A Drosophila model of mitochondrial disease caused by a complex I mutation that uncouples proton pumping from electron transfer. Dis Model Mech. 2014;7:1165–1174. doi: 10.1242/dmm.015321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Milne JC, Lambert PD, Schenk S, Carney DP, Smith JJ, Gagne DJ, Jin L, Boss O, Perni RB, Vu CB, et al. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature. 2007;450:712–716. doi: 10.1038/nature06261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jenkins NDM, Rogers EM, Banks NF, Tomko PM, Sciarrillo CM, Emerson SR, Taylor A, Teague TK. Childhood psychosocial stress is linked with impaired vascular endothelial function, lower SIRT1, and oxidative stress in young adulthood. Am J Physiol Heart Circ Physiol. 2021;321:H532–541. doi: 10.1152/ajpheart.00123.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Abdellatif M, Trummer-Herbst V, Koser F, Durand S, Adão R, Vasques-Nóvoa F, Freundt JK, Voglhuber J, Pricolo MR, Kasa M, et al. Nicotinamide for the treatment of heart failure with preserved ejection fraction. Sci Transl Med. 2021;13:eabd7064. doi: 10.1126/scitranslmed.abd7064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pugliese NR, Paneni F, Mazzola M, De Biase N, Del Punta L, Gargani L, Mengozzi A, Virdis A, Nesti L, Taddei S, et al. Impact of epicardial adipose tissue on cardiovascular hemodynamics, metabolic profile and prognosis in heart failure. Eur J Heart Fail. 2021;23:1858–1871. doi: 10.1002/ejhf.2337 [DOI] [PubMed] [Google Scholar]
- 79.Deng Y, Xie M, Li Q, Xu X, Ou W, Zhang Y, Xiao H, Yu H, Zheng Y, Liang Y, et al. Targeting mitochondria-inflammation circuit by β-hydroxybutyrate mitigates HFpEF. Circ Res. 2021;128:232–245. doi: 10.1161/CIRCRESAHA.120.317933 [DOI] [PubMed] [Google Scholar]
- 80.McCarthy CG, Chakraborty S, Singh G, Yeoh BS, Schreckenberger ZJ, Singh A, Mell B, Bearss NR, Yang T, Cheng X, et al. Ketone body β-hydroxybutyrate is an autophagy-dependent vasodilator. JCI Insight. 2021;6:e149037. doi: 10.1172/jci.insight.149037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Virdis A, Santini F, Colucci R, Duranti E, Salvetti G, Rugani I, Segnani C, Anselmino M, Bernardini N, Blandizzi C, et al. Vascular generation of tumor necrosis factor-α reduces nitric oxide availability in small arteries from visceral fat of obese patients. J Am Coll Cardiol. 2011;58:238–247. doi: 10.1016/j.jacc.2011.01.050 [DOI] [PubMed] [Google Scholar]
- 82.De Ciuceis C, Porteri E, Rizzoni D, Corbellini C, La Boria E, Boari GE, Pilu A, Mittempergher F, Di Betta E, Casella C, et al. Effects of weight loss on structural and functional alterations of subcutaneous small arteries in obese patients. Hypertension. 2011;58:29–36. doi: 10.1161/HYPERTENSIONAHA.111.171082 [DOI] [PubMed] [Google Scholar]
- 83.Di Pietro P, Carrizzo A, Sommella E, Oliveti M, Iacoviello L, Di Castelnuovo A, Acernese F, Damato A, De Lucia M, Merciai F, et al. Targeting the ASMase/S1P pathway protects from sortilin-evoked vascular damage in hypertension. J Clin Invest. 2022;132:e146343. doi: 10.1172/JCI146343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.De Stefano A, Tesauro M, Di Daniele N, Vizioli G, Schinzari F, Cardillo C. Mechanisms of SGLT2 (Sodium-Glucose Transporter Type 2) inhibition-induced relaxation in arteries from human visceral adipose tissue. Hypertension. 2021;77:729–738. doi: 10.1161/HYPERTENSIONAHA.120.16466 [DOI] [PubMed] [Google Scholar]
- 85.Good ME, Musante L, La Salvia S, Howell NL, Carey RM, Le TH, Isakson BE, Erdbrügger U. Circulating extracellular vesicles in normotension restrain vasodilation in resistance arteries. Hypertension. 2020;75:218–228. doi: 10.1161/HYPERTENSIONAHA.119.13363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wallis SJ, Firth J, Dunn WR. Pressure-induced myogenic responses in human isolated cerebral resistance arteries. Stroke. 1996;27:2287–90; discussion 2291. doi: 10.1161/01.str.27.12.2287 [DOI] [PubMed] [Google Scholar]
- 87.Tian XY, Wong WT, Xu A, Lu Y, Zhang Y, Wang L, Cheang WS, Wang Y, Yao X, Huang Y. Uncoupling protein-2 protects endothelial function in diet-induced obese mice. Circ Res. 2012;110:1211–1216. doi: 10.1161/CIRCRESAHA.111.262170 [DOI] [PubMed] [Google Scholar]
- 88.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 89.Endemann DH, Pu Q, De Ciuceis C, Savoia C, Virdis A, Neves MF, Touyz RM, Schiffrin EL. Persistent remodeling of resistance arteries in type 2 diabetic patients on antihypertensive treatment. Hypertension. 2004;43:399–404. doi: 10.1161/01.HYP.0000112029.03691.e7 [DOI] [PubMed] [Google Scholar]
- 90.Virdis A, Duranti E, Rossi C, Dell’Agnello U, Santini E, Anselmino M, Chiarugi M, Taddei S, Solini A. Tumour necrosis factor-alpha participates on the endothelin-1/nitric oxide imbalance in small arteries from obese patients: role of perivascular adipose tissue. Eur Heart J. 2015;36:784–794. doi: 10.1093/eurheartj/ehu072 [DOI] [PubMed] [Google Scholar]
- 91.Bruno RM, Duranti E, Ippolito C, Segnani C, Bernardini N, Di Candio G, Chiarugi M, Taddei S, Virdis A. Different impact of essential hypertension on structural and functional age-related vascular changes. Hypertension. 2017;69:71–78. doi: 10.1161/HYPERTENSIONAHA.116.08041 [DOI] [PubMed] [Google Scholar]
- 92.Paneni F, Osto E, Costantino S, Mateescu B, Briand S, Coppolino G, Perna E, Mocharla P, Akhmedov A, Kubant R, et al. Deletion of the activated protein-1 transcription factor JunD induces oxidative stress and accelerates age-related endothelial dysfunction. Circulation. 2013;127:1229–40, e1. doi: 10.1161/CIRCULATIONAHA.112.000826 [DOI] [PubMed] [Google Scholar]
- 93.Li W, Zhang C, Sun X. Mitochondrial Ca2+ retention capacity assay and Ca2+-triggered mitochondrial swelling assay. J Vis Exp. 2018:56236. doi: 10.3791/56236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Richardson AP, Halestrap AP. Quantification of active mitochondrial permeability transition pores using GNX-4975 inhibitor titrations provides insights into molecular identity. Biochem J. 2016;473:1129–1140. doi: 10.1042/BCJ20160070 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available to qualified investigators upon reasonable request.