Abstract
Background
Urinary tract infections (UTIs) are very common, affecting more than 7 million people worldwide. Whilst many people may only experience a single episode in their lifetime and are generally responsive to standard antibiotics, a significant proportion of adults and children (approximately 15% to 25%) are chronic symptomatic UTI sufferers. Certain population groups are at greater risk than others, such as immunosuppressed and people with chronic kidney disease.
D‐mannose is a sugar part of normal human metabolism found within most diets. The mechanism of action is to prevent bacterial adherence to the uroepithelial cells. The D‐mannose‐based inhibitors can block uropathogenic Escherichia coli adhesion and invasion of the uroepithelial cells. The bacteria are then understood to essentially be eliminated by urination.
Early pilot studies on animals and humans have trialled concentrated forms of D‐mannose (tablets or sachets) in doses ranging from 200 mg up to 2 to 3 g and found possible efficacy in reducing UTI symptoms or recurrence.
Although the anti‐adhesive effects of D‐mannose have been well‐established, only recently have we seen a small number of pilot studies and small clinical trials conducted.
Objectives
To assess the benefits and harms of D‐mannose for preventing and treating UTIs in adults and children.
Search methods
We searched the Cochrane Kidney and Transplant Register of Studies up to 22 February 2022 through contact with the Information Specialist using search terms relevant to this review. Studies in the Register are identified through searches of CENTRAL, MEDLINE, and EMBASE, conference proceedings, the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Selection criteria
We included RCTs measuring and reporting the effect of D‐mannose, in any combination and any formulation, to prevent or treat UTIs in adults and children, females and males, in any setting (including perioperative).
Authors independently assessed the retrieved titles and abstracts and, where necessary, the full text to determine which satisfied the inclusion criteria.
Data collection and analysis
Data extraction was independently carried out by two authors using a standard data extraction form. Methodological quality of the included studies was assessed using the Cochrane risk of bias tool. Data entry was carried out by one author and cross‐checked by another author. The certainty of the evidence was assessed using the GRADE approach.
Main results
We included seven RCTs (719 participants) in adult females and males who had either acute cystitis or a history of recurrent (at least two episodes in six months or three episodes in 12 months) UTIs (symptomatic or asymptomatic). Two were prevention studies, four were prevention and treatment studies (two perioperative and one in people with multiple sclerosis), and one was a treatment study. Time periods ranged from 15 days to six months. No two studies were comparable (by dose or treatments), and we could not undertake meta‐analyses.
Individual studies reported no clear evidence to determine whether D‐mannose is more or less effective in preventing or treating UTIs.
D‐mannose (2 g) had uncertain effects on symptomatic and bacteriuria‐confirmed UTIs when compared to no treatment (1 study, 205 participants; very low certainty evidence) and antibiotics (nitrofurantoin 50 mg) (1 study, 206 participants; very low certainty evidence). D‐mannose, in combination with herbal supplements, had uncertain effects on symptomatic and bacteria‐confirmed UTI and pain when compared to no treatment (1 study, 40 participants; very low certainty evidence). D‐mannose 500 mg plus supplements (N‐acetylcysteine and Morinda citrifolia fruit extract) had uncertain effects on symptomatic and bacteriuria‐confirmed UTIs when compared to an antibiotic (prulifloxacin 400 mg) (1 study, 75 participants; very low certainty evidence).
Adverse events were very few and poorly reported; none were serious (mostly diarrhoea and vaginal burning).
Overall, the quality of the evidence is poor. Most studies were judged to have unclear or high risk of bias across most domains. Data was sparse and addressed very few outcomes. The GRADE evaluation was rated as very low certainty evidence due to very serious limitations in the study design or execution (high risk of bias across all studies) and sparse data (single study data and small sample sizes).
Authors' conclusions
There is currently little to no evidence to support or refute the use of D‐mannose to prevent or treat UTIs in all populations.
This review highlights the severe lack of high‐quality RCTs testing the efficacy of D‐mannose for UTIs in any population. Despite UTIs being one of the most common adult infections (affecting 50% of women at least once in their lifetime) and the growing global antimicrobial resistance, we found very few studies that adequately test this alternative treatment.
Future research in this field requires, in the first instance, a single adequately powered RCT comparing D‐mannose with placebo.
Keywords: Adult, Child, Female, Humans, Male, Anti-Bacterial Agents, Anti-Bacterial Agents/therapeutic use, Bacteriuria, Bacteriuria/drug therapy, Kidney, Mannose, Mannose/therapeutic use, Urinary Tract Infections, Urinary Tract Infections/drug therapy, Urinary Tract Infections/prevention & control
Plain language summary
D‐mannose (sugar tablets) for preventing or treating urinary tract infections in adults and children
What is the issue?
Urinary tract infections (UTIs) are very common around the world. At least 50% of females will have a UTI once in their lifetime. Approximately 15% to 25% of adults and children suffer from repeated and long‐term UTIs. In many people, standard antibiotics do not work.
D‐mannose is a sugar which is part of a normal diet and is believed to create a non‐stick surface on the bladder wall, as well as around the bacteria. It is thought that the bacteria is then expelled when urinating, thus preventing the growth of bacteria which leads to an infection inside the bladder or urinary tract.
What did we do?
We reviewed all of the evidence on D‐mannose (tablets or powder) to see whether it can prevent or treat UTIs in adults and children. The evidence is current to 22 February 2022.
What did we find?
We found seven studies enrolling 719 participants, mostly in females who experience recurrent UTIs (at least 2 episodes in 6 months or 3 episodes in 12 months) on a long‐term basis. We could not combine the data because each study investigated different D‐mannose preparations, different populations, and different control groups. We were unable to determine if taking D‐mannose compared to no treatment, other supplements, or antibiotics reduced the number of repeated UTIs. Only a small number of participants experienced diarrhoea or vaginal burning as a side effect.
The quality of the evidence is poor. Studies were conducted using poor‐quality methods and did not enrol enough patients. Only two out of the seven studies blinded the participants to the treatment they receive.
Summary
There is not enough evidence to know whether D‐mannose prevents or treats acute or recurrent UTIs.
Summary of findings
Summary of findings 1. D‐mannose (2 g) versus no treatment for preventing or treating urinary tract infections.
| D‐mannose (2 g) versus no treatment for preventing or treating urinary tract infections | |||||
|
Patient or population: women with acute cystitis or history of recurrent acute cystitis (preventing and treating) Settings: general hospital and general practice Intervention: D‐mannose (2 g) Comparison: no treatment | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (RCTs) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| No treatment | D‐mannose (2 g) | ||||
|
Symptomatic and bacteriuria confirmed UTI (positive culture) Follow‐up at 24 weeks |
608 per 1000 | 146 per 1000 (91 to 237) |
RR 0.24 (0.15 to 0.39) |
205 (1) | ⊕⊝⊝⊝ very low1 |
| Symptomatic‐only UTI | No data | No data | No data | No data | ‐‐ |
| Asymptomatic bacteriuria | No data | No data | No data | No data | ‐‐ |
| Changes to previous treatment regimen | No data | No data | No data | No data | ‐‐ |
| Pain | No data | No data | No data | No data | ‐‐ |
| Cure/complete remission | No data | No data | No data | No data | ‐‐ |
|
Adverse effects Follow‐up at 24 weeks |
No events | 8/103** | RR 16.84 (0.98 to 287.92) |
205 (1) | ⊕⊝⊝⊝ very low1 |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ** Event rate derived from the raw data. A 'per thousand' rate is non‐informative in view of the scarcity of evidence and zero events in the control group CI: Confidence interval; RR: Risk Ratio; UTI: urinary tract infection | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1Downgraded for very serious limitations in the study design or execution (high risk of bias) (‐2), and sparse data (single study data) (‐1)
Summary of findings 2. D‐mannose (2 g) versus nitrofurantoin (50 mg) for preventing or treating urinary tract infections.
| D‐mannose (2 g) versus nitrofurantoin (50 mg) for preventing or treating urinary tract infections | |||||
|
Patient or population: women with acute cystitis or history of recurrent acute cystitis (preventing and treating) Settings: general hospital and general practice Intervention: D‐mannose (2 g) Comparison: nitrofurantoin (50 mg) | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (RCTs) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| nitrofurantoin (50 mg) | D‐mannose (2 g) | ||||
|
Symptomatic and bacteriuria‐confirmed UTI (positive culture) Follow‐up at 24 weeks |
204 per 1000 | 145 per 1000 | RR 0.71 (0.39 to 1.31) |
206 (1) | ⊕⊝⊝⊝ very low1 |
| Symptomatic only UTI | No data | No data | No data | No data | ‐‐ |
| Asymptomatic bacteriuria | No data | No data | No data | No data | ‐‐ |
| Changes to previous treatment regimen | No data | No data | No data | No data | ‐‐ |
| Pain | No data | No data | No data | No data | ‐‐ |
| Cure/complete remission | No data | No data | No data | No data | ‐‐ |
|
Adverse effects Follow‐up at 24 weeks |
282 per 1000 | 79 per 1000 (37 to 160) |
RR 0.28 (0.13 to 0.57) |
206 (1) | ⊕⊝⊝⊝ very low1 |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio; UTI: urinary tract infection | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1Downgraded for very serious limitations in the study design or execution (high risk of bias) (‐2), and sparse data (single study data) (‐1).
Background
Description of the condition
Urinary tract infections (UTIs) are common in the general population globally. Whilst many people may only experience a single episode in their lifetime (at least 50% of females), approximately 15% to 25% of adults (mostly women) and children are chronic symptomatic UTI sufferers under the categories of recurrent (at least 2 episodes in 6 months or 3 episodes in 12 months); persistent (the same pathogen in urine culture); re‐infected (new pathogen in urine culture); or relapsed (initial pathogen in urine culture after it had been eradicated) UTIs. Many cases in clinical practice do not respond to standard antibiotic treatments, creating a significant patient burden and high cost to patients and healthcare systems (Altarac 2014; Rowe 2014).
Symptomatic bacteriuria is the combination of clinical UTI symptoms often called 'cystitis' (including dysuria, urinary frequency, urgency and suprapubic pain, voiding issues, worsening of symptoms) with a positive quantitative urine culture (as confirmed by a catheter specimen of urine, midstream urine specimen if possible, or a clean‐catch specimen and defined as > 10⁵ colony‐forming units (CFU)/mL, or as defined by authors) (Nicolle 2005; Rowe 2014).
Symptomatic UTI is the presence of clinical UTI symptoms 'cystitis' (including dysuria, urinary frequency, urgency and suprapubic pain, voiding issues, worsening of symptoms) without a positive quantitative urine culture (Nicolle 2005; Rowe 2014).
Asymptomatic bacteriuria is the presence of bacteria in the urine without signs or symptoms of a UTI (Foxman 2014; Nicolle 2005). Current guidelines still recommend undertaking treatment because asymptomatic bacteriuria is most common in 1% to 6% of pregnant women, 1% to 25% in elderly women and men (mostly in long‐term care facilities), or in people with diabetes, and is associated with pyelonephritis (US PSTF 2019).
The most common pathogens found in the urogenital tract and bladder which cause UTIs are Enterobacteriaceae: Escherichia coli (E. coli) Proteus, Klebsiella, and Providentia (Rowe 2014).
Currently available prophylactic therapy and treatments range from antibiotics, methenamine hippurate salts, topical oestrogen, urine alkalisers, dietary supplements (cranberry or low acidic foods), and lifestyle and behavioural changes (altering sexual activity, personal hygiene, and clothing). Disadvantages of antibiotics, especially long‐term antibiotic prophylaxis, are the risk of increasing bacterial resistance, high costs to the patient, and repeat visits to the healthcare professional (Altarac 2014). Whilst these therapies are available and recommended by healthcare professionals, not all are efficacious or evidence‐based, hence the constant prevalence of chronic UTIs.
Description of the intervention
D‐mannose is a sugar which is part of normal human metabolism and is found in most diets. It plays an important role in particular in the glycosylation of most secretory proteins and certain glycoproteins in the human body (Hu 2016; Kranjčec 2014). It has been known for many years to impart beneficial effects on intestinal diseases, diabetes, the immune system, metabolic syndrome, and potentially UTI (Hu 2016).
Early pilot studies on animals and humans have trialled concentrated forms of D‐mannose (tablets or sachets) in doses ranging from 200 mg (Lopes De Carvalho 2012a) up to 2 g (Kranjčec 2014; Porru 2014; Salinas‐Casado 2018). These studies investigated D‐mannose in different combinations with other plant extracts or pharmacological agents such as arbutin, berberine, birch, cranberry (Vaccinium macrocarpon), proanthocyanidins, forskolin, nitrofurantoin, noxamicina (propolis extract), nitrofurantoin sulfamethoxazole, trimethoprim antibiotics, and vitamin C. Common treatment regimens appear to be daily doses ranging from three to six months duration. The known half‐life of D‐mannose is approximately four hours as it is known to be metabolised rapidly by the human digestive system (Hu 2016). Interactions with other treatments are currently uncertain.
How the intervention might work
The theoretical mechanism of action is to prevent bacterial adherence to uroepithelial cells (Hu 2016; Kranjčec 2014). D‐Mannose is a simple sugar (monosaccharide) also commonly found in fruits such as grapes, watermelon, cranberries and apples. Once it is eaten, it will be absorbed relatively quickly into the bloodstream and excreted out via the renal tubular cells in the urine, thus reducing bacterial adhesion to the urothelium. The D‐mannose attaches to the bacteria and prevents it from attaching to the urothelial cells. The D‐mannose‐based inhibitors can block uropathogenic E. coli (UPEC) adhesion and invasion of the uroepithelial cells (Kranjčec 2014). The bacteria are then understood to essentially be eliminated by urination.
Why it is important to do this review
D‐mannose has been available on the non‐prescription market in tablet and powder form in most western countries for some time. Although the anti‐adhesive effects of D‐mannose have been well‐established, only recently have we seen a small number of pilot studies and small clinical trials being conducted. It is important to assess and summarise this emerging body of evidence to determine its efficacy (currently unknown) and to ensure high‐quality research is being conducted in this field.
Objectives
To assess the benefits and harms of D‐mannose for preventing and treating UTIs in adults and children.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials (RCTs) and quasi‐RCTs (RCTs in which allocation to treatment was obtained by alternation, use of alternate medical records, date of birth or other predictable methods) were included. Unblinded, single, and double‐blind trials were included.
Cross‐over studies were included, and data from both phases were considered if there was a minimum washout period of seven days. Otherwise, only the results of the first phase were considered for analysis.
Abstracts were included. Unpublished clinical trials with online results available were included.
Studies in any healthcare setting were included, including hospitals.
Excluded study designs: single‐arm studies, commentaries, editorials, and clinical observations.
Types of participants
Inclusion criteria
Adults and children, of any age and sex, in the general population
Pregnant, breastfeeding, and perimenopausal women
Adults in residential and long‐term care facilities
-
Adults and children seeking prophylaxis for UTI:
with an indwelling catheter or requiring intermittent catheterization
with an abnormal urinary tract (e.g. vesicoureteric reflux, urinary obstruction, dysfunctional voiding, pyelonephritis)
with asymptomatic bacteriuria
-
Adults and children seeking treatment for an existing UTI
symptomatic or asymptomatic UTI
upper or lower, complicated or uncomplicated UTI
Studies exclusively involving critically ill, renal abnormalities, diagnosed chronic kidney disease (CKD), kidney transplant, or immunosuppressed patients were to be included but analysed separately as subgroups where possible.
Studies of patients who have co‐morbidities such as diabetes, multiple sclerosis, cardiovascular diseases, neurological disorders, and serious or rare diseases were to be included but analysed separately as subgroups where possible.
Studies of a perioperative nature where UTI prevention or treatment is involved were to be included but analysed separately as subgroups where possible.
Studies of mixed populations and applicable data for patients with our UTI criteria were to be extracted where possible. If this is not possible, the study was to be excluded with the reasons provided.
Exclusion criteria
-
Adults and children receiving concurrent pharmacological medications for co‐morbidities including, but not limited to the following:
Blood glucose medications
Blood pressure medications
Immunosuppressants.
-
Adults and children receiving simultaneous (or in the prior seven days) pharmacological or non‐pharmacological treatments for UTI prevention or treatment which are not of the study criteria including, but not limited to the following:
Antibiotics (either as prophylactic or for treatment of an existing UTI)
Prebiotics, probiotics, or synbiotics
Cranberry‐based treatments (juice, concentrated tablets, fruit)
Diuretics or urinary alkalinization
Natural therapies or Traditional Chinese Medicine
NOTE: these treatments will be accepted as comparison interventions for D‐mannose.
Patients who have signs of systemic illness (such as fever, loin pain, toxicity).
Types of interventions
Studies of prophylaxis and studies of treating existing UTIs were planned to be combined but analysed as subgroups.
Any D‐mannose treatment administered for the prevention or treatment of symptomatic or asymptomatic UTI compared to an active comparator, placebo or no treatment.
Any route of administration, dose, duration, or frequency were accepted.
Formulations such as oral tablets, liquids, and effervescent powders were accepted.
Combination pharmacotherapies (such as D‐mannose plus vitamin or D‐mannose plus cranberry) were accepted and considered as separate treatment arms.
Comparison pairs for analysis
D‐mannose (dose A) versus D‐mannose (dose B)
D‐mannose versus placebo
D‐mannose versus no treatment
D‐mannose versus other pharmacological treatments such as antibiotics, prebiotics, probiotics, synbiotics
D‐mannose versus diuretics or urinary alkalinization
D‐mannose versus non‐pharmacological treatment such as vitamin or herbal supplements, cranberry‐based treatments (juice, concentrated tablets, fruit), Traditional Chinese Medicine (TCM), or natural therapies
D‐mannose versus combination pharmacotherapies (two or more of any of the above in one treatment arm)
D‐mannose in combination with another treatment (two or more of any of the above in one treatment arm) versus any of the above.
Treatment arms where the intervention is in combination with an analgesic were not accepted, such as D‐mannose plus paracetamol, opioids, or an NSAID.
Types of outcome measures
This review did not exclude studies based on non‐reporting of outcomes of interest or availability of data.
Primary outcomes
-
Symptomatic and bacteriuria‐confirmed UTI according to defined clinical symptomatic criteria (including dysuria, urinary frequency, urgency and suprapubic pain, voiding issues, worsening of symptoms), plus a positive quantitative urine culture (as confirmed by a catheter specimen of urine, midstream urine specimen if possible, or a clean catch specimen and defined as > 10⁵ CFU/mL, or as defined by authors), as any of the following measures.
Total number of symptomatic bacteriuria (> 10⁵ CFU/mL) (cystitis or pyelonephritis) in each group following treatment (all time points included)
Recurrent symptomatic bacteriuria (> 10⁵ CFU/mL) (cystitis or pyelonephritis) following treatment (all time points included)
Persistent symptomatic bacteriuria (> 10⁵ CFU/mL) (cystitis or pyelonephritis) following treatment (all time points included)
Re‐infection symptomatic bacteriuria (> 10⁵ CFU/mL) (cystitis or pyelonephritis) following treatment (all time points included)
Relapse symptomatic bacteriuria (> 10⁵ CFU/mL) (cystitis or pyelonephritis) following treatment (all time points included)
Short‐term reduction in symptomatic bacteriuria episodes and bacteriologically confirmed up to two weeks after the start of treatment
Long‐term reduction in symptomatic bacteriuria episodes and bacteriologically confirmed up to eight weeks after the start of treatment.
-
Symptomatic‐only UTI (dysuria, urinary frequency, urgency and suprapubic pain, voiding issues, worsening of symptoms), with negative urine specimen, as any of the following measures.
Total number of patients who develop urinary symptoms following treatment (all time points included)
Recurrent urinary symptoms following treatment (all time points included)
Persistence of urinary symptoms following treatment (all time points included)
Re‐infection of urinary symptoms following treatment (all time points included)
Relapse of urinary symptoms following treatment (all time points included)
Short‐term symptomatic cure: the absence of urinary symptoms up to two weeks after the start of treatment
Long‐term symptomatic cure: the absence of urinary symptoms up to eight weeks after the start of treatment.
Asymptomatic bacteriuria (irrespective of the presence of symptoms suggestive of UTI). "The number of UTI confirmed by appropriate microbiological criteria. Bacteriuria on quantitative urine analysis of more than 100,000 organisms of a single species per mL is the accepted standard ‐ however, the colony count may vary from 100 to 100,000 depending on the clinical setting (Stamm 1988). Therefore in some situations, (such as a clean suprapubic tap) a colony count of less than 100,000 is acceptable." (Nicolle 2005).
Changes to previous treatment regimen prior to study including antibiotic regimen; reduction in analgesics; or the number of return visits to the GP; probiotics; alternative therapies; reduction in the use of acute and prophylactic antibiotics.
Pain (any scale visual analogue scale (VAS)), including neuropathic pain; abdominal or pelvic pain (suprapubic pain, loin pain); other measures of pain.
Definitions
Re‐infection rate: new pathogen in urine culture.
Relapse rate: initial pathogen in urine culture after it had been eradicated.
Cure rates: no clinical signs, bacteriological cure rate defined as eradication of bacteria, combined clinical and bacteriological cure rate defined as no clinical signs and eradication of bacteria.
Secondary outcomes
Cure/complete remission of symptomatic and asymptomatic UTI.
Quality of life using any validated scale, including mental and functional status (e.g. confusion, weakness, falls).
Life participation (lifestyle impact): days absent from work or school; return to normal activities (or ability to do usual activities).
Treatment satisfaction: patient‐reported; healthcare provider‐reported.
Treatment adherence.
Decline in kidney functional measures, including a reduction in estimated glomerular filtration rate (eGFR); proteinuria, and albuminuria.
-
Adverse events: total adverse events, serious adverse events; withdrawals due to adverse events
These include but are not limited to: rash; diarrhoea; gastrointestinal symptoms; pyelonephritis; urosepsis; liver or renal toxicity; worsening of UTI, progression to complicated UTI; any renal parenchymal damage on DMSA, four to six months following UTI; pregnancy‐related outcomes such as preterm birth, stillbirth, small birthweight, or gestational age.
Serious adverse events are considered: fatal, life‐threatening, requiring hospitalisation, intravenous antibiotics, bacteraemia, or fungaemia.
Death (any cause); sepsis‐related deaths.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Kidney and Transplant Register of Studies on 22 February 2022 through contact with the Information Specialist using search terms relevant to this review. The Register contains studies identified from the following sources.
Monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL)
Weekly searches of MEDLINE OVID SP
Searches of kidney and transplant journals and the proceedings and abstracts from major kidney and transplant conferences
Searching the current year of EMBASE OVID SP
Weekly current awareness alerts for selected kidney and transplant journals
Searches of the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Studies contained in the Register are identified through searches of CENTRAL, MEDLINE, and EMBASE based on the scope of Cochrane Kidney and Transplant. Details of search strategies, as well as a list of handsearched journals, conference proceedings and current awareness alerts, are available on the Cochrane Kidney and Transplant website.
See Appendix 1 for search terms used in strategies for this review.
Searching other resources
Reference lists of review articles, relevant studies and clinical practice guidelines.
Contacting relevant individuals/organisations seeking information about unpublished or incomplete studies.
Grey literature sources (e.g. abstracts, dissertations and theses), in addition to those already included in the Cochrane Kidney and Transplant Register of Studies, were not searched.
Data collection and analysis
Selection of studies
The search strategy described was used to obtain titles and abstracts of studies that may be relevant to the review. The titles and abstracts were screened independently by two authors, who discarded studies that were not applicable; however, studies and reviews that may have included relevant data or information on studies were retained initially. Two authors independently assessed the retrieved abstracts and, where necessary, the full text of these studies to determine which studies satisfied the inclusion criteria. Disagreements were planned to be resolved in consultation with a third author. Results of the search are displayed in a PRISMA study flow chart.
Data extraction and management
Data extraction was carried out independently by two authors using standard data extraction forms. Disagreements were to be resolved in consultation with a third author; however, this was not required. Studies reported in non‐English language journals were translated before assessment. Where more than one publication of one study exists, reports were grouped together, and the publication with the most complete data was used in the analyses. Where relevant outcomes were only published in earlier versions, these data were used. Any discrepancy between published versions was planned to be highlighted.
Assessment of risk of bias in included studies
The following items were independently assessed by two authors using the risk of bias assessment tool (Higgins 2020) (see Appendix 2).
Was there adequate sequence generation (selection bias)?
Was allocation adequately concealed (selection bias)?
-
Was knowledge of the allocated interventions adequately prevented during the study?
Participants and personnel (performance bias)
Outcome assessors (detection bias)
Were incomplete outcome data adequately addressed (attrition bias)?
Are reports of the study free of suggestion of selective outcome reporting (reporting bias)?
Was the study apparently free of other problems that could put it at risk of bias?
Measures of treatment effect
For dichotomous outcomes (e.g. death or positive UTI episodes), results were planned to be expressed as risk ratio (RR) to establish a statistical difference, and the number needed to treat for an additional beneficial outcome (NNT) and pooled percentages as absolute measures of effect with 95% confidence intervals (CI).
Where continuous scales of measurement were used to assess the effects of treatment (e.g. pain or decline in kidney function), the mean difference (MD) was planned to be used, or the standardised mean difference (SMD) if different scales had been used.
Where possible, we planned to use the mean change score from baseline. We anticipated that some studies may only report the mean endpoint score of which we planned to use the final time point available and combine these results with the mean change in score, as long as they were of similar scales.
Unit of analysis issues
We only accepted randomisation of the individual participant. For multiple dose studies, we planned to use data for the first dose only. For cross‐over studies, we planned only to use the first phase unless a minimum washout period of seven days had been applied to the study design. The unit of analysis for UTIs was either events or patients analysed separately, depending on what type of data was available.
Dealing with missing data
Any further information required from the original author was requested by written correspondence (e.g. emailing the corresponding author), and any relevant information obtained in this manner was included in the review. Evaluation of important numerical data such as screened, randomised patients, as well as intention‐to‐treat, as‐treated, and per‐protocol population, was carefully performed. Attrition rates, for example, drop‐outs, losses to follow‐up and withdrawals were investigated. Issues of missing data and imputation methods (e.g. last‐observation‐carried‐forward) were critically appraised (Higgins 2020).
Assessment of heterogeneity
We planned to first assess the heterogeneity by visual inspection of the forest plot. We planned to quantify statistical heterogeneity using the I² statistic, which describes the percentage of total variation across studies that is due to heterogeneity rather than sampling error (Higgins 2003). A guide to the interpretation of I² values is as follows.
0% to 40%: might not be important.
30% to 60%: may represent moderate heterogeneity.
50% to 90%: may represent substantial heterogeneity.
75% to 100%: considerable heterogeneity.
The importance of the observed value of I² depends on the magnitude and direction of treatment effects and the strength of evidence for heterogeneity (e.g. P‐value from the Chi² test or a confidence interval for I²) (Higgins 2020).
Assessment of reporting biases
If possible, funnel plots were planned to be used to assess for the potential existence of small study bias (Higgins 2020).
Data synthesis
Data was planned to be pooled using the random‐effects model, but the fixed‐effect model would also be used to ensure the robustness of the model chosen and susceptibility to outliers.
Subgroup analysis and investigation of heterogeneity
Subgroup analysis was planned to be used to explore possible sources of heterogeneity where there were sufficient data. Heterogeneity among participants could be related to age, co‐morbidities, and urological or renal pathologies. Heterogeneity in treatments could be related to the prior agent(s) used and the agent, dose and duration of therapy. Adverse effects were tabulated and assessed with descriptive techniques, as they are likely to be different for the various agents used. Where possible, we planned to use the risk difference with 95% CI to calculate each adverse effect, either compared to no treatment or to another agent.
Planned subgroups where sufficient data are available.
Dose
Time point
Prevention versus treatment of UTI
CKD present
Age.
Sensitivity analysis
We planned to perform sensitivity analyses in order to explore the influence of the following factors on effect size; however, this was not possible.
Repeating the analysis excluding unpublished studies.
Repeating the analysis taking into account the risk of bias, as specified.
Repeating the analysis, excluding any very long or large studies to establish how much they dominate the results.
Repeating the analysis excluding studies using the following filters: diagnostic criteria, the language of publication, source of funding (industry versus other), and country.
Summary of findings and assessment of the certainty of the evidence
We presented the main results of the review in 'Summary of findings' tables. These tables present key information concerning the certainty of the evidence, the magnitude of the effects of the interventions examined, and the sum of the available data for the main outcomes (Schunemann 2020a).
The 'Summary of findings' tables also includes an overall grading of the evidence related to each of the main outcomes using the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) approach (GRADE 2008; GRADE 2011). The GRADE approach defines the certainty of a body of evidence as the extent to which one can be confident that an estimate of effect or association is close to the true quantity of specific interest. This was assessed by two authors. A summary of the assessment process is in Appendix 3. The certainty of a body of evidence involves consideration of the within‐trial risk of bias (methodological quality), directness of evidence, heterogeneity, the precision of effect estimates and risk of publication bias (Schunemann 2020b). We planned to present the following outcomes in the 'Summary of findings' tables.
Symptomatic and bacteria‐confirmed UTI
Symptomatic only UTI
Asymptomatic bacteriuria
Changes to previous treatment regimen
Pain
Cure/complete remission
Adverse effects.
Results
Description of studies
Results of the search
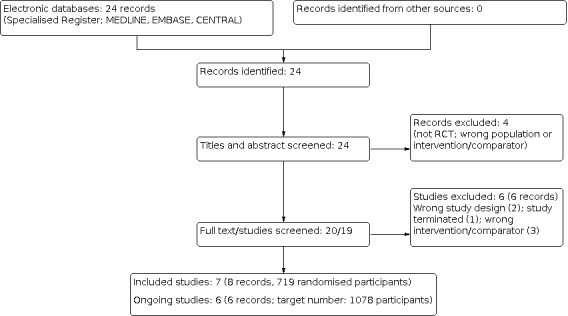
The PRISMA flow diagram is shown in Figure 1.
1.
Study flow diagram.
The search of the register up to 22 February 2022 retrieved 24 records. After title and abstract review, four records were excluded (not RCT, wrong population or intervention/comparator). After full‐text review of the remaining 20 records, seven studies (8 records) were included (De Leo 2017; Kranjcec 2014; Kuzmenko 2019; Lopes de Carvalho 2012; Palleschi 2017; Porru 2014; Russo 2019), six were excluded (Domenici 2016; Genovese 2018; NCT03497598; NCT03996057; Radulescu 2020; Salinas‐Casado 2018), and six studies are ongoing (ACTRN12616001619437; ACTRN12619000183189; DRKS00013240; MERIT 2021; NCT03597152; PROTON 2018) (target number of participants: 1078). We will include these studies in a future update of this review.
Included studies
Seven studies (719 participants) were included See Characteristics of included studies.
One study compared a cross‐over treatment with no washout period (Porru 2014); one study was double‐blind (Lopes de Carvalho 2012), and the remaining five were open‐label studies.
Sample sizes range from 21 (Lopes de Carvalho 2012) to 308 participants (Kranjcec 2014). The studies took place in either primary care/general practitioners or hospital outpatient settings in Croatia, Italy, Russia, and Spain. Two studies investigated both females and males (Lopes de Carvalho 2012; Palleschi 2017), and the remaining four studies investigated females only.
Population
The health status and inclusion criteria for participants varied:
Acute cystitis (single or recurrent) (Kranjcec 2014; Kuzmenko 2019)
Multiple sclerosis (Lopes de Carvalho 2012)
Undergoing a mini‐invasive urological diagnostic procedure (Palleschi 2017)
Acute symptomatic UTI (single or recurrent) (Porru 2014)
Postmenopausal women with symptomatic isolated anterior prolapse POP Q stage ≥ III submitted to native tissue repair for cystocele (Russo 2019;).
Two studies were undertaken in the perioperative period (Palleschi 2017; Russo 2019).
Interventions and comparators
Appendix 4 summarises the comparison pairs and intervention details of the included studies. No studies were similar in drug, dose, comparison, or time point.
Outcomes
All seven studies reported data on the three primary outcomes (symptomatic and bacteriuria‐confirmed UTI, symptomatic only UTI, pain) using different units of analysis, different measurement scales, and a combination of prevention or treatment for UTI. Kuzmenko 2019 did not report suitable quantitative data to report in this review (abstract only).
Prevention of UTI: two studies (both in a perioperative setting) Palleschi 2017; Russo 2019)
Treatment of UTI: one study (Kuzmenko 2019)
Prevention and treatment of UTI: four studies (De Leo 2017; Kranjcec 2014; Lopes de Carvalho 2012; Porru 2014). The baseline population of these studies at enrolment indicated that the patient population had either (a) an acute symptomatic and/or bacteriuria culture‐confirmed UTI or (b) a history of recurrent UTI or symptomatic cystitis as one or more episodes of UTIs documented in the preceding 12 months.
Treatment periods ranged from 15 days (Palleschi 2017) to 24 weeks (Kranjcec 2014).
Excluded studies
See Characteristics of excluded studies.
Domenici 2016: inappropriate and unclear baseline population
Genovese 2018: incomparable treatment arms
NCT03497598: terminated due to insufficient patients
NCT03996057: D‐mannose with or without methenamine for the UTI prevention
Radulescu 2020: inappropriate intervention and study design phases
Salinas‐Casado 2018: inappropriate intervention arms
Ongoing studies
See Characteristics of ongoing studies.
ACTRN12616001619437: D‐mannose for prophylaxis against UTI in spinal cord injury (target number: 40)
ACTRN12619000183189: D‐mannose versus placebo in patients with a high risk of recurrent UTI (target number: 50)
DRKS00013240: D‐mannose plus herbal extracts versus herbal extracts for the dietary management of acute symptomatic uncomplicated UTIs in females (target number: 100)
MERIT 2021: D‐mannose versus placebo to prevent recurrent UTIs in women (target number: 598)
NCT03597152: Nutritional supplementation with D‐mannose versus placebo for recurrent UTIs in women (target number: 250)
PROTON 2018: Winclove CLEAR versus placebo in females with recurrent UTI (target number: 40).
Risk of bias in included studies
See the 'Risk of Bias' section under Characteristics of included studies tables for a detailed assessment of bias within each included study.
See Figure 2 for a graphical summary of the assessment of bias within each included study.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
The majority of studies were characterised by an unclear or high risk of bias across most domains. This was largely due to a lack of detail provided for concealing allocation, open‐label study design, selective reporting, and funding declarations (pharmaceutical or lack of information). Using funnel plots to detect publication bias was not feasible due to the small number of studies.
Allocation
Random sequence generation
Four studies were judged to have unclear risk of bias (De Leo 2017; Kuzmenko 2019; Lopes de Carvalho 2012; Porru 2014). These four studies were reported to be a 'randomised' controlled trial or having 'randomly assigned participants' to treatment groups; however, the methods that were used to carry out the randomisation process were not described in any further or sufficient detail.
Three studies were judged to be at low risk of bias (Kranjcec 2014; Palleschi 2017; Russo 2019). These three studies described adequate details of the methods that were used to carry out the randomisation process (rolling a dice, statistical series based on random sampling, or a uniform allocation ratio (1:1)).
Allocation concealment
Three open‐label studies were judged to be at high risk of bias (De Leo 2017; Kranjcec 2014; Kuzmenko 2019). The remaining four studies did not provide any information concerning allocation concealment and were judged to have unclear risk of bias.
Blinding
Blinding of participants and personnel
Six studies were judged to be at high risk of bias (De Leo 2017; Kranjcec 2014; Kuzmenko 2019; Palleschi 2017; Porru 2014; Russo 2019). All were all open‐label studies and did not blind anyone.
One study was judged to have unclear risk of bias. Lopes de Carvalho 2012 reported that the examining physician and subjects were blinded to the procedure; however, the study states to be blinded, but insufficient details were available.
Blinding of outcome assessors
All seven studies were judged to be at high risk of bias. Six studies were open‐label.
Incomplete outcome data
Four studies did not provide sufficient information and were judged to have unclear risk of bias (De Leo 2017; Kuzmenko 2019; Lopes de Carvalho 2012; Porru 2014.
Three studies were judged to be at low risk of bias. All participants were accounted for from the start to the end of the study, and attrition rates were low (0% to 6.25%) (Kranjcec 2014; Palleschi 2017; Russo 2019).
Selective reporting
Six studies did not provide trial registration numbers or details to an a priori‐published protocol and were judged to have unclear risk of bias.
Porru 2014 provided trial registration numbers and was judged to be at low risk of bias. The methodology, including outcomes planned, matched the study registration details.
Other potential sources of bias
Sources of funding and conflicts of interest
Russo 2019 declared funding from pharmaceutical industry sources and was judged to be at high risk of bias.
Three studies did not declare any information regarding their funding sources and were judged to have unclear risk of other bias (De Leo 2017; Kuzmenko 2019; Palleschi 2017.
Four studies did not declare whether the authors had any conflicts of interest (De Leo 2017; Kuzmenko 2019; Lopes de Carvalho 2012; Palleschi 2017). Kranjcec 2014 and Russo 2019 declared that their authors had no conflicts of interest; however, their funding sources were not reported.
Porru 2014 declared that no funding was received from any commercial source and declared that the authors did not have any conflicts of interest and was judged to be at low risk of bias.
No other potential sources of bias were identified.
Effects of interventions
D‐mannose versus no treatment
Kranjcec 2014 compared D‐mannose (2 g oral powder in 200 mL water) once/day to no treatment in 205 women who have acute or recurrent cystitis over a 24‐week period (prevention and treatment study).
Symptomatic and bacteriuria‐confirmed UTI
Kranjcec 2014 reported D‐mannose reduced the number of symptomatic and bacteriuria‐confirmed UTIs (Analysis 1.1 (1 study, 205 participants): RR 0.24, 95% IC 0.15 to 0.39; very low certainty evidence).
1.1. Analysis.

Comparison 1: D‐mannose (2 g) versus no treatment, Outcome 1: Symptomatic and bacteriuria‐confirmed UTI (positive culture) at 24 weeks
Symptomatic only UTI
Kranjcec 2014 reported median days and IQR time from prophylactic therapy start to cystitis symptoms onset: 43 days (IQR: 15 to 50) for D‐mannose (103 participants) and 28 days (IQR: 20 to 42) for no treatment (102 participants) (very low certainty evidence).
Adverse events
Kranjcec 2014 reported eight adverse events (diarrhoea) in the D‐mannose group and no adverse events in the no treatment group (Analysis 1.2 (1 study, 205 participants): RR 16.84, 95% CI 0.98 to 287.92; very low certainty evidence). See Appendix 5.
1.2. Analysis.

Comparison 1: D‐mannose (2 g) versus no treatment, Outcome 2: Adverse events
Other outcomes
The following outcomes were not reported.
Asymptomatic bacteriuria
Changes to previous treatment regimen
Pain
Cure/complete remission.
D‐mannose versus antibiotics
Kranjcec 2014 compared oral D‐mannose (2 g powder in 200 mL water once/day) to oral nitrofurantoin (50 mg once/day) in 206 women who had acute or recurrent cystitis over a 24‐week period (prevention and treatment study).
Porru 2014 compared oral D‐mannose (3 g tablets/day for 2 weeks, reduced to 2 g/day for 22 weeks) to oral trimethoprim/sulfamethoxazole (160/800 mg twice/day, followed by a single dose at bedtime for 1 week each month in the following 22 weeks), in 120 females who have acute or recurrent symptomatic UTI, over a 24‐week period (prevention and treatment study). Porru 2014 was a cross‐over study with no washout period and no available data from the first phase and was therefore not included in our meta‐analyses (see Appendix 4).
Symptomatic and bacteriuria‐confirmed UTI
Kranjcec 2014 reported no difference between D‐mannose and nitrofurantoin on symptomatic and bacteriuria‐confirmed UTIs (Analysis 2.1 (1 study, 206 participants): RR 0.71, 95% CI 0.39 to 1.31; very low certainty evidence).
2.1. Analysis.

Comparison 2: D‐mannose (2 g) versus nitrofurantoin (50 mg), Outcome 1: Symptomatic and bacteriuria‐confirmed UTI (positive culture) at 24 weeks
Symptomatic only UTI
Kranjcec 2014 reported median days and IQR from prophylactic therapy start to cystitis symptoms onset: 43 days (15 to 50) for D‐mannose (103 participants) and 24 days (15 to 50) for nitrofurantoin (103 participants) (very low certainty evidence).
Adverse events
Kranjcec 2014 reported eight adverse events (diarrhoea) in the D‐mannose group and 29 adverse events (diarrhoea, nausea, headache, skin rash, vaginal burning) in the nitrofurantoin group (Analysis 2.2 (1 study, 206 participants): RR 0.28, 95% CI 0.13 to 0.57; very low certainty evidence). See Appendix 5.
2.2. Analysis.

Comparison 2: D‐mannose (2 g) versus nitrofurantoin (50 mg), Outcome 2: Adverse events
Other outcomes
The following outcomes were not reported.
Asymptomatic bacteriuria
Changes to previous treatment regimen
Pain
Cure/complete remission.
D‐mannose plus supplements versus placebo or no treatment
De Leo 2017 compared a formulation of D‐mannose plus cranberry plus noxamicina (unknown doses, one oral sachet/day) for the first 10 days of the month to no treatment in 150 participants who have recurrent episodes of cystitis over a 12‐week period (prevention and treatment study).
Lopes de Carvalho 2012 compared D‐mannose (100 mg) plus cranberry (40 mg) plus vitamin C (60 mg) (2 doses/day) for 90 days to placebo in 21 females and males who have multiple sclerosis over a 12‐week period (prevention and treatment study).
Russo 2019 compared a formulation of D‐mannose plus cranberry plus Boswellia plus Curcuma plus NoxamicineVR (Kistinox ActVR) oral preparation (doses not reported, 2 doses/day) as a nutritional supplement for two weeks after surgery to no treatment in 40 postmenopausal women who were submitted to native tissue repair to cystocele from symptomatic isolate anterior prolapse stage three or higher, over a four‐week postoperative period (prevention study).
Due to the lack of data on similar outcomes, no meta‐analysis was possible.
Symptomatic and bacteriuria confirmed UTI
Lopes de Carvalho 2012 reported D‐mannose plus cranberry and vitamin C had a "significant reduction in the number of UTIs" compared to placebo (1 study, 21 participants; very low certainty evidence).
Russo 2019 reported 1/20 UTI infections with D‐mannose plus cranberry, Boswellia, Curcuma and NoxamicineVR (Kistinox ActVR) (unknown doses) and 1/20 UTI infections with placebo (Analysis 3.1: 1 study, 40 participants; very low certainty evidence).
3.1. Analysis.

Comparison 3: D‐mannose (no dose provided) plus herbal combination versus no treatment, Outcome 1: Symptomatic and bacteriuria‐confirmed UTI (positive culture) at 2 weeks
Symptomatic only UTI
De Leo 2017 reported a slight decrease in symptomatic cystitis at three months for D‐mannose plus cranberry plus noxamicina (unknown doses) compared to no treatment (Analysis 3.2: 1 study, 150 participants; very low certainty evidence).
3.2. Analysis.

Comparison 3: D‐mannose (no dose provided) plus herbal combination versus no treatment, Outcome 2: Symptomatic only UTI at 3 months
Pain
Russo 2019 reported mean pain on a VAS (scale numbers are not reported, assumed 1 ‐ 10) was (mean ± SD): 1.2 ± 1.1, n = 20 for the D‐mannose formulation and 1.3 ± 0.9, n = 20 for no treatment (Analysis 3.3: 1 study, 40 participants; very low certainty evidence).
3.3. Analysis.

Comparison 3: D‐mannose (no dose provided) plus herbal combination versus no treatment, Outcome 3: Pain (mean score (VAS 1‐10)): average/patient (day 1 postop)
Cure/complete remission of symptomatic and a symptomatic UTI
De Leo 2017 reported "complete remission of symptoms in 92 women overall" but did not stipulate from which intervention arm these results derive (1 study, 150 participants, very low certainty evidence).
Adverse events
De Leo 2017, Lopes de Carvalho 2012 and Russo 2019 reported none of the included participants recorded any adverse events (3 studies, 211 participants; very low certainty evidence). See Appendix 5.
D‐mannose plus supplements versus antibiotics
Palleschi 2017 compared D‐mannose (500 mg) plus N‐acetylcysteine (100 mg) plus Morinda citrifolia fruit extract (300 mg) as 2 vials/day to prulifloxacin (antibiotic) 400 mg/day, in 75 females and males who submitted to mini‐invasive urological diagnostic procedures, over a 15‐day period (prevention study).
Symptomatic and bacteriuria confirmed UTI
Palleschi 2017 reported 2/37 symptomatic and culture‐positive UTIs with the D‐mannose formulation and 3/38 for prulifloxacin 400 mg (Analysis 4.1: 1 study, 75 participants; very low certainty evidence).
4.1. Analysis.

Comparison 4: D‐mannose (500 mg) plus N‐acetylcysteine (100 mg) plus Morinda citrifolia fruit extract (300 mg) versus prulifloxacin (400 mg), Outcome 1: Symptomatic and bacteriuria‐confirmed UTI (positive culture) at 15 days
Adverse events
Palleschi 2017 reported zero adverse events across both treatment arms (1 study, 75 participants; very low certainty evidence). See Appendix 5.
No data were reported on our remaining primary and secondary outcomes.
D‐mannose plus antibiotics and supplements versus antibiotics plus supplements
Kuzmenko 2019 compared D‐mannose (dose unknown) plus inulin (dose unknown) plus E551 (silicon dioxide, dose unknown) plus fosfomycin (3 g, antibiotic) plus prebiotic lactulose (1.5 g) to fosfomycin (3 g, antibiotic) plus prebiotic lactulose (1.5 g) in 60 women with acute uncomplicated cystitis over a 24‐week period (treatment study).
Symptomatic only UTI
Kuzmenko 2019 reported "relief of dysuric phenomena" in both treatment groups (1 study, 60 participants; very low certainty evidence).
Pain
Kuzmenko 2019 reported "relief of pain" in both treatment groups (1 study, 60 participants; very low certainty evidence).
Discussion
Summary of main results
We included seven RCTs (719 participants) in this review; no studies were comparable (by dose or treatment) to enter into a meta‐analysis.
The patient populations varied. All studies were in adults, and four studies were in females only. Studies were a mixture of measuring symptomatic‐only cystitis and/or positive culture‐confirmed bacteriuria. Two studies investigated both the treatment and prevention of UTIs. Two studies investigated the prevention of UTIs in a perioperative setting, and two studies were prevention‐only studies. One study was in adults with multiple sclerosis (no description of the history of UTI). Across all studies, recurrent UTI was defined as recurrent episodes of cystitis with at least one positive urine culture during a six‐month period or two or more episodes over 12 months.
D‐mannose (2 g) compared to no treatment had uncertain effects on symptomatic and bacteriuria‐confirmed UTI (1 study, 205 participants; very low certainty evidence).
D‐mannose (2 g) compared to antibiotics (nitrofurantoin 50 mg) had uncertain effects on symptomatic and bacteriuria‐confirmed UTI (1 study, 206 participants; very low certainty evidence).
D‐mannose in combination with herbal supplements compared to no treatment has uncertain effects on symptomatic and bacteria‐confirmed UTI and pain (1 study, 40 participants, very low certainty evidence).
D‐mannose 500 mg in combination with supplements (N‐acetylcysteine and Morinda citrifolia fruit extract) compared to an antibiotic (prulifloxacin 400 mg) has uncertain effects on symptomatic and bacteriuria‐confirmed UTI (1 study, 75 participants, very low certainty evidence).
Individual studies found no clear results that D‐mannose is more or less effective in preventing or treating UTIs in the participant populations of their own criterion. Adverse events were poorly reported, and of the very few adverse effects reported, none were serious (mostly diarrhoea and vaginal burning).
Overall completeness and applicability of evidence
This review highlights the severe lack of high‐quality RCTs testing the efficacy of D‐mannose for UTIs in any population.
Despite UTIs being one of the most common adult infections (affecting 50% of women at least once in their lifetime) and the growing global antimicrobial resistance, we found very few studies that adequately test this alternative treatment. Four major issues around the completeness of the evidence were:
Limited sample size and insufficient power
Standardised dosing of D‐mannose and comparator treatments
Standardised measuring
Reporting of outcomes.
Three major issues around the applicability of evidence were:
Participant criteria in the included studies: whilst adult women experience the most frequent rates of UTIs, it is important to know the efficacy and harms of D‐mannose in males, as well as in people under 18 years because this is a non‐prescription dietary supplement available over the counter in most western countries.
Outcome measures varied greatly by scale, unit, time point, definitions, and denominators in each group. Two studies did not provide data for the treatment arm from which they expressed a change in symptoms, and therefore, we could not include these studies in our meta‐analysis. Studies reported either rates of UTI or UTI recurrence in a variety of dichotomous and continuous data.
Definitions of UTI varied greatly (recurrence, rate, one or more in a three, six, or 12‐month period). Time points at which the outcomes were measured varied greatly (15 days to six months). Studies mentioned symptomatic cystitis and defined pathologically confirmed UTI as a mixture of 'positive urine cultures'.
We identified significant gaps in the evidence. No two studies were comparable for meta‐analysis due to the different dosing of D‐mannose (500 mg, 1 g, or 3 g titrated down to 2 g) as well as most studies investigated different comparator treatments (placebo, no treatment, antibiotics, herbal supplements).
Quality of the evidence
Overall, the quality of the evidence is poor. Most studies were judged to have unclear or high risk of bias across most domains Figure 2. Data was sparse and addressed very few of the primary and secondary outcomes.
Across all comparisons, GRADE evaluations for all outcomes were judged to be very low certainty evidence. The evidence was downgraded three stages: for very serious limitations in the study design or execution (high risk of bias across all studies) (‐2), and sparse data (single study data and small sample sizes) (‐1) (Table 1, Table 2).
Very low certainty evidence implies we are very uncertain about results (not estimable due to lack of data). We have no evidence to support or refute the use of D‐mannose in preventing or treating UTIs, and the findings should be viewed with caution.
Potential biases in the review process
This review was conducted as per the protocol following pre‐specified inclusion criteria and used comprehensive literature searches to find all relevant studies. We do not believe there are any other potential biases in this review process.
Agreements and disagreements with other studies or reviews
One systematic review has recently been published on this topic (Lenger 2020). The authors report a possible positive effect of D‐mannose for preventing UTIs. However, the findings of our review do not support their assessment due to the lack of available evidence on this topic. The inclusion criteria for Lenger 2020 were restricted to women only, over 18 years, and included observational study designs that were limited to prevention and not treatment. Lenger 2020 included only two of our eight RCTs (Kranjcec 2014; Porru 2014). Lenger 2020 combined studies into a meta‐analysis that included different doses of D‐mannose and comparisons arms that were not similar. In our view, this is an overinterpretation of very sparse and poor‐quality data.
In contrast to Lenger 2020, we feel that the assessments made in Lenger 2020 are not appropriate recommendations on the harms and benefits of D‐mannose based on the available evidence (which is lacking in quantity, precision, and quality). We identified six ongoing studies (ACTRN12616001619437; ACTRN12619000183189; DRKS00013240; MERIT 2021; NCT03597152; PROTON 2018), which we will assess in a future update of this review.
Authors' conclusions
Implications for practice.
Despite UTIs being one of the most common adult infections (affecting 50% of women at least once in their lifetime), and considering the growing global antimicrobial resistance, we found very few studies that adequately test this alternative treatment.
There is currently little to no evidence to support the use of D‐mannose to prevent or treat UTIs. We are not certain whether D‐mannose is effective in preventing or treating UTIs in any population. Adverse events were poorly reported, and of the very few adverse effects reported, none were serious (mostly diarrhoea and vaginal burning).
Implications for research.
We have identified an area of significant uncertainty for the efficacy of D‐mannose in preventing or treating people suffering from UTIs. We are uncertain of the efficacy and harms of D‐mannose in preventing or treating UTIs. Future research in this field requires, in the first instance, a single adequately powered RCT comparing D‐mannose with placebo.
History
Protocol first published: Issue 5, 2020
Acknowledgements
We wish to acknowledge the assistance of the Cochrane Kidney and Transplant Information Specialist, Gail Higgins. The authors are grateful to the following peer reviewers for their time and comments: Gabrielle Williams (Melanoma Institute Australia), and Deirdre Hahn (Department of Nephrology, The Children’s Hospital at Westmead, Australia).
The Methods section of this protocol is based on a standard template used by Cochrane Kidney and Transplant.
We wish to acknowledge the support of Angela James for her contribution to the inception of this review.
Appendices
Appendix 1. Electronic search strategies
| Database | Search terms |
| CENTRAL |
|
| MEDLINE |
|
| EMBASE |
|
Appendix 2. Risk of bias assessment tool
| Potential source of bias | Assessment criteria |
|
Random sequence generation Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence |
Low risk of bias: Random number table; computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimisation (minimisation may be implemented without a random element, and this is considered to be equivalent to being random). |
| High risk of bias: Sequence generated by odd or even date of birth; date (or day) of admission; sequence generated by hospital or clinic record number; allocation by judgement of the clinician; by preference of the participant; based on the results of a laboratory test or a series of tests; by availability of the intervention. | |
| Unclear: Insufficient information about the sequence generation process to permit judgement. | |
|
Allocation concealment Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment |
Low risk of bias: Randomisation method described that would not allow investigator/participant to know or influence intervention group before eligible participant entered in the study (e.g. central allocation, including telephone, web‐based, and pharmacy‐controlled, randomisation; sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes). |
| High risk of bias: Using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure. | |
| Unclear: Randomisation stated but no information on method used is available. | |
|
Blinding of participants and personnel Performance bias due to knowledge of the allocated interventions by participants and personnel during the study |
Low risk of bias: No blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding; blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Blinding of outcome assessment Detection bias due to knowledge of the allocated interventions by outcome assessors. |
Low risk of bias: No blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding; blinding of outcome assessment ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding; blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Incomplete outcome data Attrition bias due to amount, nature or handling of incomplete outcome data. |
Low risk of bias: No missing outcome data; reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; missing data have been imputed using appropriate methods. |
| High risk of bias: Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; ‘as‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation; potentially inappropriate application of simple imputation. | |
| Unclear: Insufficient information to permit judgement | |
|
Selective reporting Reporting bias due to selective outcome reporting |
Low risk of bias: The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way; the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon). |
| High risk of bias: Not all of the study’s pre‐specified primary outcomes have been reported; one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. sub‐scales) that were not pre‐specified; one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; the study report fails to include results for a key outcome that would be expected to have been reported for such a study. | |
| Unclear: Insufficient information to permit judgement | |
|
Other bias Bias due to problems not covered elsewhere in the table |
Low risk of bias: The study appears to be free of other sources of bias. |
| High risk of bias: Had a potential source of bias related to the specific study design used; stopped early due to some data‐dependent process (including a formal‐stopping rule); had extreme baseline imbalance; has been claimed to have been fraudulent; had some other problem. | |
| Unclear: Insufficient information to assess whether an important risk of bias exists; insufficient rationale or evidence that an identified problem will introduce bias. |
Appendix 3. The GRADE approach (Grades of Recommendation, Assessment, Development, and Evaluation)
The GRADE approach assesses the certainty of a body of evidence, rating it into one of four grades (GRADE 2008).
High: we are very confident that the true effect lies close to that of the estimate of the effect.
Moderate: we are moderately confident in the effect estimate; the true effect is likely to be close the estimate of effect, but there is a possibility that it is substantially different.
Low: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect.
Very low: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect.
We decreased the certainty of evidence if there was (Balshem 2011):
serious (‐1) or very serious (‐2) limitation in the study design or execution (risk of bias);
important inconsistency of results (‐1);
some (‐1) or major (‐2) uncertainty about the directness of evidence;
imprecise or sparse data (‐1) or serious imprecision (‐2); or
high probability of publication bias (‐1).
We increased the certainty of evidence if there was (GRADE 2011):
a large magnitude of effect (direct evidence, relative risk (RR) = 2 to 5 or RR = 0.5 to 0.2 with no plausible confounders) (+1); very large with RR > 5 or RR < 0.2 and no serious problems with risk of bias or precision; more likely to rate up if effect is rapid and out of keeping with prior trajectory; usually supported by indirect evidence (+2);
evidence of a dose response gradient (+1); or
all plausible residual confounders or biases would reduce a demonstrated effect, or suggest a spurious effect when results show no effect (+1).
Appendix 4. Extracted outcome data from included studies
| Study ID | Outcome | Arm 1 | Arm 2 | Arm 3 | Comparison pair | |||
| Event or mean (SD) | Total | Event or mean (SD) | Total | Event or mean (SD) | Total | |||
| De Leo 2017 | Cranberry + noxamicina + D‐mannose (unknown doses) | No treatment | ‐ | D‐mannose combined with dietary supplements versus no treatment | ||||
|
Symptomatic‐only UTI (cystitis) Unit: number of patients reporting decrease in symptoms UTI defined as: 'cystitis, with or without bacteriuria'. Time: at 12 weeks Type: "treatment and prevention" |
"Slight decrease in symptoms of UTI in 5 participants". Note: not reported from which arm |
97 | "Slight decrease in symptoms of UTI in 5 participants". Note: not reported from which arm |
50 | ‐ | ‐ | ||
|
Cure/complete remission of symptomatic and asymptomatic UTI Unit: number of patients reporting decrease in symptoms UTI defined as: 'cystitis, with or without bacteriuria' undefined in abstract Time: at 12 weeks Type: "treatment and prevention" |
"Complete remission of symptoms in 92 women overall". Note: not reported from which arm |
100 | "Complete remission of symptoms in 92 women overall". Note: not reported from which arm |
50 | ‐ | ‐ | ||
| Adverse events | 0 | 100 | ||||||
| Kuzmenko 2019 | No appropriate outcome data reported: "relief of dysuric phenomena and pain" in both groups | D‐mannose + antibiotic + prebiotic versus antibiotic + prebiotic | ||||||
| Kranjcec 2014 | D‐mannose 2 g | Nitrofurantoin 50 mg (antibiotic) | No treatment | 1). D‐mannose versus antibiotics 2). D‐mannose versus no treatment |
||||
|
Symptomatic and bacteriuria‐confirmed UTI Unit: number of patients reporting a recurrent UTI UTI defined as: confirmed isolated bacteria in acute cystitis Time: at 24 weeks Type: treatment and preventiona |
15 | 103 | 21 | 103 | 62 | 102 | ||
|
Symptomatic only UTI (cystitis) Unit: time from prophylactic therapy start to cystitis symptoms onset, median (IQR) UTI defined as: 'cystitis' undefined Time: at 24 weeks Type: treatment and preventiona |
43 (15 to 50) | 103 | 24 (15 to 50) | 103 | 28 (20 to 42) | 102 | ||
| Lopes de Carvalho 2012 | D‐mannose 100 mg + cranberry 40 mg + vitamin C 60 mg | Placebo | ‐ | D‐mannose combined with dietary supplements versus placebo | ||||
|
Symptomatic and bacteriuria‐confirmed UTI Unit: not clear from abstract UTI defined as: "urinary infections and urine cultures" Time: at 12 weeks Type: "treatment and prevention" |
"a significant reduction in number of urinary infections and frequency in active group in respect to placebo group". | 11 | "a significant reduction in number of urinary infections and frequency in active group in respect to placebo group". | 10 | ‐ | ‐ | ||
| Palleschi 2017 | D‐mannose 500 mg + N‐acetylcysteine 100 mg + Morinda citrifolia fruit extract 300 mg | Prulifloxacin 400 mg (antibiotic) | ‐ | D‐mannose combined with dietary supplements versus antibiotic | ||||
|
Symptomatic and bacteriuria confirmed UTI Unit: number of patients reporting a UTI UTI defined as: "UTI incidence (symptomatic or asymptomatic) via urine cultures" Time: 15 days from treatment start Type: prevention (perioperative) |
(Female) 1 (Male) 1 |
19 18 |
(Female) 1 (Male) 2 |
17 21 |
‐ | ‐ | ||
| Porru 2014 | D‐mannose 1 g | Trimethoprim 160 mg sulfamethoxazole 800 mg (antibiotics) | ‐ | D‐mannose versus antibiotics | ||||
|
Symptomatic and bacteriuria‐confirmed UTI Unit: number of patients with a UTI UTI defined as: "acute flare of urinary symptoms + positive urine culture with at least 100,000 uropathogens per ml" Time: approximately 52 weeks Type: treatment and preventiona |
No data available for first phase of cross‐over | 60 | No data available for first phase of cross‐over | 60 | ‐ | ‐ | ||
|
Symptomatic and bacteriuria‐confirmed UTI Unit: time to UTI recurrence, mean ± SD UTI defined as: "acute flare of urinary symptoms + positive urine culture with at least 100,000 uropathogens per ml" Time: approximately 52 weeks Type: treatment and preventiona |
No data available for first phase of cross‐over | 60 | No data available for first phase of cross‐over | 60 | ‐ | ‐ | ||
|
Pain Unit: average VAS (1 to 10) pain and urgency scores per patient reported during UTI episodes, mean ± SD Time: approximately 52 weeks Type: treatment and preventiona |
No data available for first phase of cross‐over | 60 | No data available for first phase of cross‐over | 60 | ‐ | ‐ | ||
| Russo 2019 | Cranberry + D‐mannose + Boswellia + Curcuma + NoxamicineVR (Kistinox ActVR ) (doses not reported) | No treatment | ‐ | D‐mannose combined with dietary supplements versus no treatment | ||||
|
Symptomatic and bacteriuria confirmed UTI Unit: Rate of UTI infections, cumulative incidence, unclear whether number of patients reporting or number reported per patient Defined as: symptoms + positive urine culture Time: 2 weeks postop Type: prevention (perioperative) |
1 | 20 | 1 | 20 | ‐ | ‐ | ||
|
Pain Unit: average VAS (scale size unclear) pain scores/patient reported postop, mean ± SD Time: 1 day postop Type: prevention (perioperative) |
1.2 ± 1.1 | 20 | 1.3 ± 0.9 | 20 | ‐ | ‐ | ||
| Salinas‐Casado 2018 | D‐mannose 2 g, 24 hour prolonged release, associated with proanthocyanidin 140 mg + ursolic acid 7.98 mg + vitamin A (unknown dose) + vitamin C (unknown dose) + vitamin D (unknown dose) oligoelement zinc (unknown dose) | Proanthocyanidin 140 mg | ‐ | D‐mannose combined with dietary supplements versus dietary supplement | ||||
|
Symptomatic and bacteriuria‐confirmed UTI Unit: patient Defined as: symptomatic UTI with reactive urine strip and urine culture Time: 24 weeks Type: prevention |
24% | denominator is unclear | 45% | denominator is unclear | ‐ | ‐ | ||
|
Footnotes: a treatment and prevention: indicates that the patient population at enrolment were participants who had both an "acute symptomatic UTI and three or more UTIs documented with culture of midstream urine specimen at inclusion and in the preceding 12 months", or "baseline was women with acute cystitis (isolated bacteria) or history of recurrent cystitis (at least 2 episodes in 6 months or 3 in 12 months)". Therefore possible that participants had UTI at the start of treatment. IQR: interquartile range; SD: standard deviation; UTI: urinary tract infection; VAS: visual analogue scale | ||||||||
Appendix 5. Adverse events for all included studies and all treatments
| Study ID | Treatment, dose, frequency | Number of adverse events | Group total | Percentage |
| De Leo 2017 | D‐mannose + cranberry + noxamicina (unknown doses) | 0 | 100 | 0% |
| No treatment | 0 | 50 | 0% | |
| Kranjcec 2014 | D‐mannose 2 g
|
8 | 103 | 78% |
Nitrofurantoin 50 mg (antibiotic)
|
29 | 103 | 28% | |
| No treatment | 0 | 102 | 0% | |
| Kuzmenko 2019 | Not reported | Not reported | Not reported | Not reported |
| Lopes de Carvalho 2012 | D‐mannose 100 mg + cranberry 40 mg + vitamin C 60 mg | Not reported | Not reported | Not reported |
| Placebo | Not reported | Not reported | Not reported | |
| Palleschi 2017 | D‐mannose 500 mg + N‐acetylcysteine 100 mg + Morinda citrifolia fruit extract 300 mg | 0 | 37 | 0% |
| Prulifloxacin 400 mg (antibiotic) | 0 | 38 | 0% | |
| Porru 2014 | D‐mannose 1 g | Not reported | Not reported | Not reported |
| Trimethoprim 160 mg + sulfamethoxazole 800 mg (antibiotics) | Not reported | Not reported | Not reported | |
| Russo 2019 | Cranberry + D‐mannose + Boswellia + Curcuma + NoxamicineVR (Kistinox ActVR ) oral preparation (doses not reported) | 0 | 20 | 0% |
| No treatment | 0 | 20 | 0% |
Data and analyses
Comparison 1. D‐mannose (2 g) versus no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Symptomatic and bacteriuria‐confirmed UTI (positive culture) at 24 weeks | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.2 Adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 2. D‐mannose (2 g) versus nitrofurantoin (50 mg).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Symptomatic and bacteriuria‐confirmed UTI (positive culture) at 24 weeks | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.2 Adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 3. D‐mannose (no dose provided) plus herbal combination versus no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Symptomatic and bacteriuria‐confirmed UTI (positive culture) at 2 weeks | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.2 Symptomatic only UTI at 3 months | 1 | 150 | Mean Difference (IV, Random, 95% CI) | ‐2.70 [‐3.27, ‐2.13] |
| 3.3 Pain (mean score (VAS 1‐10)): average/patient (day 1 postop) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 4. D‐mannose (500 mg) plus N‐acetylcysteine (100 mg) plus Morinda citrifolia fruit extract (300 mg) versus prulifloxacin (400 mg).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Symptomatic and bacteriuria‐confirmed UTI (positive culture) at 15 days | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.1.1 Females | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.1.2 Males | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
De Leo 2017.
| Study characteristics | ||
| Methods |
Study design
Study duration and follow‐up
Study type
|
|
| Participants |
General information
Baseline characteristics
|
|
| Interventions |
Treatment group
Control group
Co‐interventions or additional treatments
Follow‐up details
|
|
| Outcomes |
All outcomes reported at 3 months
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "the subjects were randomly assigned to two groups" |
| Allocation concealment (selection bias) | High risk | Comment: open‐label study so it is unlikely that allocation to treatment groups was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: open‐label study |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: insufficient information within the abstract |
| Selective reporting (reporting bias) | Unclear risk | Trial registration: not reported A priori published protocol: not reported |
| Other bias | Unclear risk | Funding: not reported Conflicts of interest: not reported |
Kranjcec 2014.
| Study characteristics | ||
| Methods |
Study design
Study duration and follow‐up
Study type
|
|
| Participants |
General information
Baseline characteristics
|
|
| Interventions |
Treatment group 1
Treatment group 2
Control group
Co‐interventions or additional treatments
Follow‐up details
|
|
| Outcomes |
Outcomes reported at 6 months
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients with less than 103 CFU/ml in urine culture and no LUTS were considered cured and were randomly divided by throwing the dice in one of the three groups according to the prophylaxis they would receive during the following 6 months" |
| Allocation concealment (selection bias) | High risk | Comment: appears the patients may have thrown their own dice for randomisation (also the study is open‐label study so unlikely that allocation was concealed) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: open‐label study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 0% lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Trial registration: not reported A priori published protocol: not reported Comment: the outcomes planned in the methods were reported in the results, however, no report or access to a priori methods |
| Other bias | Unclear risk | Funding: not reported Conflicts of interest: "all authors state that they have no conflict of interest" |
Kuzmenko 2019.
| Study characteristics | ||
| Methods |
Study design
Study duration and follow‐up
Study type
|
|
| Participants |
General information
Baseline characteristics
|
|
| Interventions |
Treatment group
Control group
Co‐interventions or additional treatments
Follow‐up details
|
|
| Outcomes |
All outcomes reported at 3 days, 7 days, and 6 months
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "By random sampling, all women were divided into 2 groups". Comment: study states to be randomised but no further details are available within the abstract |
| Allocation concealment (selection bias) | High risk | Comment: no information provided regarding the concealment of allocation to groups within the abstract. However, the study is open‐label study so unlikely that allocation was concealed) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: open‐label study so it is unlikely that allocation to treatment groups was concealed |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: open‐label study so it is unlikely that allocation to treatment groups was concealed |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: insufficient information within the abstract |
| Selective reporting (reporting bias) | Unclear risk | Trial registration: not reported A priori published protocol: not reported |
| Other bias | Unclear risk | Funding: not reported Conflicts of interest: not reported |
Lopes de Carvalho 2012.
| Study characteristics | ||
| Methods |
Study design
Study duration and follow‐up
Study type
|
|
| Participants |
General information
Baseline characteristics
|
|
| Interventions |
Treatment group
Control group
Co‐interventions or additional treatments
Follow‐up details
|
|
| Outcomes |
Outcomes reported at baseline, 30 and 90 days
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "a simple blind randomized design study was used" Comment: study states to be randomised but no further details are available within the abstract |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided regarding the concealment of allocation to groups within the abstract |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "examining physician and subjects were blinded to the procedure" Comment: study states to be blinded but insufficient details available from within the abstract |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "examining physician and subjects were blinded to the procedure" Quote: "a simple blind randomized design study was used..." Comment: does not explicitly state that examining physicians were the outcome assessors, so assumed outcome assessors were not blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: insufficient information within the abstract |
| Selective reporting (reporting bias) | Unclear risk | Trial registration: not reported A priori published protocol: not reported |
| Other bias | Unclear risk | Funding: not reported Conflicts of interest: not reported |
Palleschi 2017.
| Study characteristics | ||
| Methods |
Study design
Study duration and follow‐up
Study type
|
|
| Participants |
General information
Baseline characteristics
|
|
| Interventions |
Treatment group
Control group
Co‐interventions or additional treatments
Follow‐up details
|
|
| Outcomes |
Outcomes reported at 15 days from treatment start
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "a randomized procedure was used for the random allocation of the enrolled patients into two groups of 40 in equal proportions to ensure a uniform allocation ratio (1:1)" |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided regarding the concealment of allocation to groups within the abstract |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: open‐label study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: all participants were accounted for from start to end of study; attrition 6.25% |
| Selective reporting (reporting bias) | Unclear risk | Trial registration: not reported A priori published protocol: not reported |
| Other bias | Unclear risk | Funding: not reported Conflicts of interest: not reported |
Porru 2014.
| Study characteristics | ||
| Methods |
Study design
Study duration and follow‐up
Study type
|
|
| Participants |
General information
Baseline characteristics
|
|
| Interventions |
Treatment group
Control group
Co‐interventions or additional treatments
Follow‐up details
|
|
| Outcomes |
Outcomes reported at 24 weeks
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "each participant entering the trial was assigned to one of the following treatments in a random sequence" Comment: insufficient details provided about the methods used to randomise to treatment groups |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided regarding the concealment of allocation to groups |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: open‐label study |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: attrition and withdrawals not reported, unclear if any participants dropped out and if an intention‐to‐treat analysis was undertaken |
| Selective reporting (reporting bias) | Low risk | Trial registration: NCT01808755 A priori published protocol: not reported Comment: all outcomes planned in the methods were reported in the results, and the methodology matches the trial registration details |
| Other bias | Low risk | Funding: "this research received no specific grant from any funding agency in the public, commercial, or not‐for‐profit sectors" Conflicts of interest: "none declared" |
Russo 2019.
| Study characteristics | ||
| Methods |
Study design
Study duration and follow‐up
Study type
|
|
| Participants |
General information
Baseline characteristics
|
|
| Interventions |
Treatment group
Control group
Co‐interventions or additional treatments
Follow‐up details
|
|
| Outcomes |
Outcomes reported at 2 and 4 weeks postop
Notes
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...randomized as per a simple randomization. Determination of whether a patient would be treated or not was made by reference to a statistical series based on random sampling numbers drawn up by Dr Eleonora Russo." |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided regarding the concealment of allocation to groups |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: open‐label study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: attrition (0%) |
| Selective reporting (reporting bias) | Unclear risk | Trial registration: not reported A priori published protocol: Protocol ID12219 for ethics only |
| Other bias | High risk | Funding: "this work was supported by an unrestricted grant from Laborest and by the University of Pisa funds to Tommaso Simoncini, Pisa, Italy." Conflicts of interest: "the authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported. The authors alone are responsible for the content and writing of the paper." |
CFU: colony forming units; CKD: chronic kidney disease; IQR: interquartile range; LUTS: lower urinary tract symptoms; M/F: male/female; PACs‐A: proanthocyanidins; PVR: post void residual; RCT: randomised controlled trial; SD: standard deviation; UTI: urinary tract infection; VAS: visual analogue scale; WCC: white cell count
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Domenici 2016 | Study design: intervention started as a single arm phase 1 with all participants receiving treatment. Unclear what the phase 2 baseline starting point is for participants. Results for phase 2 not reported separately or clearly |
| Genovese 2018 | Interventions are not comparable. Arm 1 and 2 are same doses of D‐mannose and therefore cannot be compared. To compare arms 2 and 3 against arm 1 is unreliable as the additional combination herbal mixes are complex and too dissimilar to ascertain what would be causing a therapeutic effect |
| NCT03497598 | Study terminated 2 September 2020: not enough patients |
| NCT03996057 | D‐mannose with or without methenamine for the UTI prevention |
| Radulescu 2020 | Two phase trial and data could not be analysed for separate phases for individual doses and regimen |
| Salinas‐Casado 2018 | Interventions: arms are comparing PAC and PAC amongst other supplements |
PAC: proanthocyanidin; UTI: urinary tract infection
Characteristics of ongoing studies [ordered by study ID]
ACTRN12616001619437.
| Study name | D‐mannose for prophylaxis against urinary tract infection in spinal cord injury: pilot randomised control study |
| Methods |
Study design
Study duration and follow‐up
Study dates
|
| Participants |
General information
|
| Interventions |
Treatment group
Control group
Co‐interventions or additional treatments
Follow‐up details
|
| Outcomes |
|
| Starting date | Recruitment completed |
| Contact information | Dr Suresh Subramanian Auckland Spinal rehab unit 30 Bairds Road Otara 1640 Auckland New Zealand +64 9 2709000 Sureshbabu.Subramanian@middlemore.co.nz |
| Notes |
ACTRN12619000183189.
| Study name | The effectiveness of D‐mannose in patients with high risk of recurrent urinary tract infections |
| Methods |
Study design
Study duration and follow‐up
Study dates
|
| Participants |
General information
|
| Interventions |
Treatment group
Control group
Co‐interventions or additional treatments
Follow‐up details
|
| Outcomes |
Outcomes to be assessed at weeks 4, 8, 12, 16, 20 and 24
|
| Starting date | Unknown |
| Contact information | Dr Vicki Patton Sir Charles Gairdner Hospital Level 1 Harry Perkins Research Institute Nedlands WA 6009 Australia +61 861510753 vickip04@gmail.com |
| Notes |
DRKS00013240.
| Study name | Effects of a food for special medical purposes containing D‐mannose, birch extract, vitamin D and vitamin A for the dietary management of acute symptomatic uncomplicated urinary tract infections in females ‐ a randomized, double‐blind, placebo‐controlled, parallel‐design study |
| Methods |
Study design
Study duration and follow‐up
Study dates
|
| Participants |
General information
|
| Interventions |
Treatment group
Control group
|
| Outcomes |
Outcomes to assessed and 24, 48 and 96 hours
|
| Starting date |
|
| Contact information | Hermes Arzneimittel GmbH Mr. Dr. Martin Hellemann Georg‐Kalb‐Straße 5‐8 82049 Pullach Germany Telephone: 0049 89 79 102 0 Fax: 0049 89 79 102 280 E‐mail: info at hermes‐arzneimittel.com URL: http://www.hermes-arzneimittel.com |
| Notes |
MERIT 2021.
| Study name | D‐mannose to prevent recurrent urinary tract infections (MERIT) |
| Methods |
Study design
Study duration and follow‐up
Study dates
|
| Participants |
General information
|
| Interventions |
Treatment group
Control group
Co‐interventions or additional treatments
Follow‐up details
|
| Outcomes |
Primary outcomes
Secondary outcomes
Tertiary outcomes
|
| Starting date | 12/11/2018: ongoing 22/01/2019: no longer recruiting 04/08/2020: last edited |
| Contact information | Jared Robinson Primary Care Clinical Trials Unit Nuffield Department of Primary Care Health Sciences University of Oxford Radcliffe Primary Care Building Radcliffe Observatory Quarter Woodstock Road Oxford OX2 6GG United Kingdom +44 (0)1865 617849 merit@phc.ox.ac.uk Trial website: https://www.phctrials.ox.ac.uk/studies/merit |
| Notes | Funding: National Institute for Health Research (NIHR) (UK) Protocol serial number: 40192 |
NCT03597152.
| Study name | Nutritional supplementation for recurrent urinary tract infections in women |
| Methods |
Study design
Study duration and follow‐up
Study dates
|
| Participants |
General information
|
| Interventions |
Treatment group
Control group
Co‐interventions or additional treatments
Follow‐up details
|
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date | Not yet recruiting, 10 January 2020 |
| Contact information | Contact: Katie O'Brien 501‐219‐8900 ext 2002 katie@arkansasurology.com Contact: Richard Dennis, PhD 501‐960‐8024 protocols@att.net |
| Notes | Contact: Richard Dennis, PhD |
PROTON 2018.
| Study name | A randomized, placebo‐controlled, pilot study to evaluate the effects Winclove CLEAR in female recurrent urinary tract infection patients (PROTON) |
| Methods |
Study design
Study dates
Study duration and follow‐up
|
| Participants |
General information
|
| Interventions |
Treatment group (40 participants)
Control group (20 participants)
Co‐interventions or additional treatments
Follow‐up details
|
| Outcomes |
Primary outcomes
Secondary outcomes
Time points
|
| Starting date | January 2019 |
| Contact information | Dr J. Flach Email: Joost.flach@cr2o.nl Phone: +31 6 40275130 |
| Notes | Funding: This study is performed at the research facility of CR2O by Prof. Dr. Eric Claassen (Principal Investigator) and Drs. Joost Flach (Coordinating Investigator). |
ACSS: Acute Cystitis Symptom Score; BBUSQ: Birmingham Bowel and Urinary Symptoms Questionnaire; BMI: body mass index; CFU: colony forming units; CUTI: catheter‐related urinary tract infection; DDD: defined daily dose; DM: diabetes mellitus; HbA1c: haemoglobin A1c (glycated); HIV: human immunodeficiency virus; IBS: irritable bowel syndrome; PVR: post‐void residual; QoL: quality of life; RCT: randomised controlled trial; SF‐36: 36‐item short form survey (QoL); UTI: urinary tract infection; VAS: visual analogue scale
Differences between protocol and review
There are no methodological variations from the protocol.
Contributions of authors
Drafted the protocol: TC; CT; MH; ATP; AJ; GW
Study selection: TC; CT
Extracted data from studies: TC; CT
Entered data into RevMan: TC; CT
Carried out the analysis: TC; CT
Interpreted the analysis: TC; CT; ATP
Drafted the final review: TC; CT; MH; ATP; AJ; GW
Disagreement resolution: MH; ATP; AJ; GW
Update the review: TC; GW
Sources of support
Internal sources
No sources of support provided
External sources
-
BEAT‐CKD Funding Grant 1092957, Australia
TC and CT are employed under funding from this grant.
Declarations of interest
TC: no relevant interests were disclosed
CT: no relevant interests were disclosed
MH: no relevant interests were disclosed
ATP: no relevant interests were disclosed
AJ: no relevant interests were disclosed
GW: no relevant interests were disclosed
New
References
References to studies included in this review
De Leo 2017 {published data only}
- De Leo V, Cappelli V, Massaro MG, Tosti C, Morgante G. Evaluation of the effects of a natural dietary supplement with cranberry, Noxamicina ® and D-mannose in recurrent urinary infections in perimenopausal women. Minerva Ginecologica 2017;69(4):336-41. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kranjcec 2014 {published data only}
- Altarac S, Papes D. Use of D-mannose in prophylaxis of recurrent urinary tract infections (UTIs) in women. BJU International 2014;113(1):9-10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kranjcec B, Papes D, Altarac S. D-mannose powder for prophylaxis of recurrent urinary tract infections in women: a randomized clinical trial. World Journal of Urology 2014;32(1):79-84. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kuzmenko 2019 {published data only}
- Kuzmenko AV, Kuzmenko VV, Gyaurgiev TA. Efficacy of combined antibacterial-prebiotic therapy in combination with D-mannose in women with uncomplicated lower urinary tract infection. Urologiia 2019;(6):38-43. [MEDLINE: ] [PubMed] [Google Scholar]
Lopes de Carvalho 2012 {published data only}
- Lopes De Carvalho L, Francavilla G, Motta R, Brichetto G. D-mannose, cranberry and Vitamin C are effective in preventing urinary tract infections in multiple sclerosis subjects [abstract no: 108]. Multiple Sclerosis 2012;18(5):S12-3. [EMBASE: 70762266] [Google Scholar]
Palleschi 2017 {published data only}
- Palleschi G, Carbone A, Zanello PP, Mele R, Leto A, Fuschi A, et al. Prospective study to compare antibiosis versus the association of N-acetylcysteine, D-mannose and Morinda citrifolia fruit extract in preventing urinary tract infections in patients submitted to urodynamic investigation. Archivio Italiano di Urologia, Andrologia 2017;89(1):45-50. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Porru 2014 {published data only}
- Porru D, Parmigiani A, Tinelli C, Barletta D, Choussos D, Di Franco C, et al. Oral D-mannose in recurrent urinary tract infections in women: a pilot study. Journal of Clinical Urology 2014;7(3):208-13. [EMBASE: 372993100] [Google Scholar]
Russo 2019 {published data only}
- Russo E, Montt Guevara M, Giannini A, Mannella P, Palla G, Caretto M, et al. Cranberry, D-mannose and anti-inflammatory agents prevent lower urinary tract symptoms in women undergoing prolapse surgery. Climacteric 2019;23(2):201-5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Domenici 2016 {published data only}
- Domenici L, Monti M, Bracchi C, Giorgini M, Colagiovanni V, Muzii L, et al. D-mannose: a promising support for acute urinary tract infections in women. A pilot study. European Review for Medical & Pharmacological Sciences 2016;20(13):2920-5. [MEDLINE: ] [PubMed] [Google Scholar]
Genovese 2018 {published data only}13017713
- Genovese C, Davinelli S, Mangano K, Tempera G, Nicolosi D, Corsello S, et al. Effects of a new combination of plant extracts plus d-mannose for the management of uncomplicated recurrent urinary tract infections. Journal of Chemotherapy 2018;30(2):107-14. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
NCT03497598 {published data only}
- Ryu G. Preventing recurrent urinary tract infections with a-d-mannose (PUTIM) [Preventing recurrent urinary tract infections with α-D-mannose: a prospective, randomized, double-blinded placebo-controlled trial]. www.clinicaltrials.gov/show/NCT03497598 (first received 13 April 2018).
NCT03996057 {published data only}
- Chu C. Methenamine in a non-antibiotic, multimodal approach to UTI prevention [The efficacy and effect of methenamine hippurate in a non-antibiotic, multimodal approach to UTI prevention]. www.clinicaltrials.gov/show/NCT03996057 (first received 24 June 2019).
Radulescu 2020 {published data only}
- Radulescu D, David C, Turcu FL, Spataru DM, Popescu P, Vacaroiu IA. Combination of cranberry extract and D-mannose - possible enhancer of uropathogen sensitivity to antibiotics in acute therapy of urinary tract infections: results of a pilot study. Experimental & Therapeutic Medicine 2020;20(4):3399-406. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Salinas‐Casado 2018 {published data only}
- Salinas-Casado J, Mendez-Rubio S, Esteban-Fuertes M, Gomez-Rodriguez A, Virseda-Chamorro M, Lujan-Galan M, et al. Efficacy and safety of D-mannose (2 g), 24h prolonged release, associated with proanthocyanidin (PAC), versus isolate PAC, in the management of a series of women with recurrent urinary infections [Eficacia y tolerancia terapeutica de la D-manosa (2 g) de liberacion prolongada 24 horas (asociada a proantocianidinas), frente a proantocianidinas aisladas en el manejo de una serie de mujeres con infecciones urinarias recurrentes]. Archivos Espanoles de Urologia 2018;71(2):169-77. [MEDLINE: ] [PubMed] [Google Scholar]
References to ongoing studies
ACTRN12616001619437 {published data only}
- Subramanian S. D-mannose for prophylaxis against urinary tract Infection in spinal cord injury [D-mannose for prophylaxis against urinary tract Infection in SCI: pilot randomised control study]. www.anzctr.org.au/ACTRN12616001619437.aspx (first received 23 November 2016).
ACTRN12619000183189 {published data only}
- Patton V. The effectiveness of D-Mannose in patients with high risk of recurrent urinary tract infections. www.anzctr.org.au/ACTRN12619000183189.aspx (first received 8 February 2019).
DRKS00013240 {published data only}
- Hellemann M. Effects of a food for special medical purposes containing d-mannose, birch extract, vitamin D and vitamin A for the dietary management of acute symptomatic uncomplicated urinary tract infections in females - a randomized, double-blind, placebo-controlled, parallel-design study. www.trialsearch.who.int/Trial2.aspx?TrialID=DRKS00013240 (first received 11 June 2017). [CENTRAL: CN-01890405]
MERIT 2021 {published data only}13283516
- Franssen M, Cook J, Robinson J, Williams N, Glogowska M, Yang Y, et al. D-MannosE to prevent Recurrent urinary tract InfecTions (MERIT): protocol for a randomised controlled trial. BMJ Open 2021;11(1):e037128. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT03597152 {published data only}
- D'Anna R. Nutritional supplementation for recurrent urinary tract infections in women [Nutritional supplementation for extending time between recurrent urinary tract infections in women: a randomized double blind cross-over trial]. www.clinicaltrials.gov/show/NCT03597152 (first received 24 July 2018).
PROTON 2018 {published data only}
- Flach J. Winclove CLEAR for recurrent urinary tract infections in women [A randomized, placebo-controlled, pilot study to evaluate the effects winclove CLEAR in female recurrent urinary tract infection patients]. www.trialregister.nl/trial/6832 (first received 25 January 2018).
Additional references
Altarac 2014
- Altarac S, Papeš D. Use of d-mannose in prophylaxis of recurrent urinary tract infections (UTIs) in women. BJU International 2014;113(1):9-10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Balshem 2011
- Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Foxman 2014
- Foxman B. Urinary tract infection syndromes: occurrence, recurrence, bacteriology, risk factors, and disease burden. Infectious Disease Clinics of North America 2014;28(1):1-13. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
GRADE 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924-6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADE 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383-94. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557-60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2020
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. Available from www.training.cochrane.org/handbook.
Hu 2016
- Hu X, Shi Y, Zhang P, Miao M, Zhang T, Jiang B. D-mannose: properties, production, and applications: an overview. Comprehensive Reviews in Food Science & Food Safety 2016;15(4):773-85. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kranjčec 2014
- Kranjčec B, Papeš D, Altarac S. D-mannose powder for prophylaxis of recurrent urinary tract infections in women: a randomized clinical trial. World Journal of Urology 2014;32(1):79-84. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lenger 2020
- Lenger SM, Bradley MS, Thomas DA, Bertolet MH, Lowder JL, Sutcliffe S. D-mannose vs other agents for recurrent urinary tract infection prevention in adult women: a systematic review and meta-analysis. American Journal of Obstetrics & Gynecology 2020;223(2):265.e1-265.e13. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lopes De Carvalho 2012a
- Lopes De Carvalho L, Francavilla G, Motta R, Brichetto G. D-mannose, cranberry and Vitamin C are effective in preventing urinary tract infections in multiple sclerosis subjects (#108). Multiple Sclerosis 2012;18(5):S12-3. [EMBASE: 70762266] [Google Scholar]
Nicolle 2005
- Nicolle LE, Bradley S, Colgan R, Rice JC, Schaeffer A, Hooton TM, et al. Infectious Diseases Society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults [Erratum in: Clin Infect Dis. 2005 May 15;40(10):1556]. Clinical Infectious Diseases 2005;40(5):643-54. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Rowe 2014
- Rowe TA, Juthani-Mehta M. Diagnosis and management of urinary tract infection in older adults. Infectious Disease Clinics of North America 2014;28(1):75-89. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schunemann 2020a
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. www.training.cochrane.org/handbook.
Schunemann 2020b
- Schünemann HJ, Vist GE, Higgins JP, Santesso N, Deeks JJ, Glasziou P, et al. Chapter 15: Interpreting results and drawing conclusions. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. Available from www.training.cochrane.org/handbook.
US PSTF 2019
- US Preventive Services Task Force, Owens DK, Davidson KW, Krist AH, Barry MJ, Cabana M, et al. Screening for asymptomatic bacteriuria in adults: US Preventive Services Task Force recommendation statement. JAMA 2019;322(12):1188-94. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Cooper 2020
- Cooper TE, Teng C, Howell M, Teixeira-Pinto A, Tong A, Wong G. D‐mannose for preventing and treating urinary tract infections. Cochrane Database of Systematic Reviews 2020, Issue 5. Art. No: CD013608. [DOI: 10.1002/14651858.CD013608] [DOI] [PMC free article] [PubMed] [Google Scholar]


