Abstract
“Sarcopenic obesity” refers to a condition of low muscle mass in the context of obesity, though may be difficult to assess in patients with cirrhosis who are acutely ill. We aimed to define sarcopenic visceral obesity (SVO) using CT-based skeletal muscle index (SMI) and visceral-to-subcutaneous adipose tissue ratio (VSR) to examine its association with post-transplant mortality. We analyzed 116 adult inpatients with cirrhosis who were urgently listed and transplanted between 1/2005 and 12/2017 at 4 North American transplant centers. SVO was defined as patients with sarcopenia (SMI <50 cm2/m2 in men and <39 cm2/m2 in women) and visceral obesity (VSR ≥1.54 in men and ≥1.37 in women). The percentage who met criteria for sarcopenia, visceral obesity, and SVO were 45%, 42%, and 20%, respectively. Cumulative rates of post-transplant mortality were higher in patients with SVO compared to patients with sarcopenia or visceral obesity alone at 36 months (39% vs 14% vs 8%) [logrank p=0.01]. In univariable regression, SVO was associated with post-transplant mortality (HR 2.92, 95%CI 1.04–8.23) and remained significant after adjusting for age, sex, diabetes, encephalopathy, hepatocellular carcinoma, and MELD-Na (HR 3.50, 95%CI 1.10–11.15). In conclusion, SVO is associated with increased post-transplant mortality in acutely ill patients with cirrhosis.
Keywords: body composition, visceral adipose tissue, visceral-to-subcutaneous adipose tissue ratio, subcutaneous adipose tissue, skeletal muscle mass
INTRODUCTION
The prevalence of obesity among patients with cirrhosis undergoing liver transplantation is currently 35–40% and is anticipated to rise.1 While obesity is generally considered a risk factor for poor outcomes in the general population, in patients with cirrhosis undergoing liver transplantation, results are conflicting with some studies demonstrating that obesity is a risk factor for post-transplant mortality2–4 and others demonstrating no association5–7. Several reasons may explain these conflicting results. Obesity has traditionally been defined by thresholds of body mass index (BMI), but body weight can be unreliable in patients with cirrhosis due to dynamic shifts in volume retention. Cirrhosis is characterized by muscle wasting and cachexia, so in select patients with decompensated cirrhosis, obesity may be a surrogate marker for relative “health”. On the other hand, approximately 20–35% of patients with cirrhosis have been shown to experience substantial muscle wasting in addition to an accumulation of body fat, a condition known as sarcopenic obesity, which is associated with higher rates of transplant morbidity and mortality compared to either sarcopenia or obesity alone.8–12 Clearly, more reliable metrics of “obesity” that are associated with adverse outcomes are needed.
This problem of inaccurate risk assessment is particularly concerning among patients with cirrhosis who are hospitalized with acute on chronic hepatic decompensation undergoing urgent evaluation for liver transplantation. Decisions regarding whether to proceed with liver transplantation in those patients with cirrhosis who are not previously known to the transplant center but may appear quite ill due to their acute decompensating event must be made based on objective and accurate data that can be obtained in the hospital despite severe fluid retention, hepatic encephalopathy, and relative immobility.
Substantial evidence, to date, has demonstrated the prognostic value of computed tomography (CT)-based measures of muscle loss (e.g., sarcopenia) and adipose tissue distribution (e.g., visceral-to-subcutaneous adipose tissue ratio, or VSR) in ambulatory patients with cirrhosis undergoing liver transplantation.13–16 Based on these data, we hypothesized that the combination of CT-based measures of muscle with adipose tissue—which we used to define “sarcopenic visceral obesity (SVO)”—could better identify obesity-related morphometric characteristics associated with post-transplant outcomes. Specifically, we aimed to define SVO in acutely ill patients with cirrhosis and to quantify its association with outcomes after liver transplantation.
MATERIALS AND METHODS
Study Design and Patient Selection
This retrospective study included adult patients with cirrhosis who were non-electively hospitalized and underwent urgent evaluation and liver transplantation within 30 days of listing during the same hospitalization between January 1, 2005 and December 31, 2017, and had available abdominal CT within 90 days prior to transplant. Data were obtained from four academic transplant centers in North America: University of California, San Francisco (n=40), University of Pittsburgh (n=46), University of Alberta (n=20), and Mayo Clinic Scottsdale (n=16). Patients were excluded if they underwent liver transplantation for fulminant hepatic failure.
Demographic and clinical data, including primary liver disease etiology, comorbid conditions, laboratory, and outcome data were manually chart reviewed and abstracted from the electronic health records at each site by trained study personnel who were blinded to all body composition data. Clinical evidence of ascites and hepatic encephalopathy were ascertained through a manual chart review of clinical physical exam and note at the time of listing.
Patients’ outcomes were obtained retrospectively. The primary outcome was all-cause mortality after liver transplantation. Other post-transplant clinical outcomes ascertained included total intensive care unit and total hospital length of stay, and events within 6 months from transplant such as re-hospitalization, infection (with positive microbial culture), biopsy-proven acute cellular rejection, hepatic artery thrombosis, portal vein thrombosis, biliary complication, and re-operation (other than re-transplantation). All patients were followed until death or the last known date of clinical follow-up through August 31, 2020.
Measurement of Body Compositions
Body composition was assessed using secondary analysis of abdominal CT scans as part of the liver transplant evaluation. CT-based measures of skeletal muscle (psoas, erector spinae, multifidus, quadratus lumborum, rectus abdominis, transverse abdominis, and internal/external oblique), visceral adipose tissue (VAT), and subcutaneous adipose tissue (SAT), were quantified (cm2) at the lumbar (L3) vertebral level using a post-processing workstation (General Electric Advanced Workstation 4.6, Volume Viewer software, GE Healthcare, Waukesha, WI, USA), which enabled specific tissue demarcation using standard Hounsfield Unit thresholds of −29 to 150 for skeletal muscle17, −150 to −50 for VAT18, and −190 to −30 for SAT19. As reported in prior studies using these specific Hounsfield Unit thresholds, tissue areas are outlined on an individual CT section/slice by individuals trained in musculoskeletal anatomy resulting in a semiautomatic computed total cross-sectional area (cm2) by summing tissue pixels and multiplying by pixel surface area.20 All CT images were analyzed by trained personnel who were blinded to all clinical data. All values were normalized by height (m2), resulting in a skeletal muscle index (SMI, cm2/m2), visceral adipose tissue index (VATI, cm2/m2), and subcutaneous adipose tissue index (SATI, cm2/m2). VSR was calculated by dividing VATI and SATI.
Sarcopenia was defined by previously established cutpoints of SMI <50 cm2/m2 for men and <39 cm2/m2 for women which have been shown to be associated with pre-transplant mortality independent of age and MELD score.21,22
Statistical Analysis
Data were presented as numbers and percentages (%) for categorical variables or medians and interquartile ranges (IQR) for continuous variables. Variables were compared by using Wilcoxon rank-sum and Pearson’s chi-square tests, as appropriate. Survival time was defined as the time from liver transplantation to death due to any causes and was censored at the last known date of clinical follow-up if death was not observed during the study period. The survival outcome was summarized using the Kaplan-Meier method and compared between groups using the log-rank test. Time-dependent receiver operating characteristic (ROC) method was used to identify optimal VSR cutoffs to predict post-transplant mortality, where the concordance probability function (defined as the product of sensitivity and specificity) at month 36 is maximized to determine the cutoffs.23,24 The effect of SVO on survival was assessed by univariate and multivariate Cox regression analyses. Established clinical prognostic factors of mortality and candidate predictors with a p-value <0.10 in the univariable analysis were included in multivariable Cox regression analysis. A two-sided p-value <0.05 was considered statistical significance. Analyses were performed using STATA, version 15.1 (StataCorp, College Station, TX, USA).
The study was approved by the Institutional Review Boards at each institution before data collection. De-identified clinical and radiologic data were shared under the provisions of a multicenter data use agreement among the participating institutions.
RESULTS
Baseline Patient Characteristics
Baseline clinical characteristics of the 116 patients from the 4 transplant centers are shown in Table 1. The majority were male (66%) and non-Hispanic white (69%), with a median age of 53 years and BMI of 27.5 kg/m2. Common etiologies of cirrhosis were chronic hepatitis C (31%) and alcohol-related liver disease (27%), followed by autoimmune or cholestatic liver disease (14%), nonalcoholic fatty liver disease (NAFLD) or non-alcoholic steatohepatitis (NASH) (10%), and chronic hepatitis B (9%). Rates of hypertension were 28% and of diabetes were 18%. The majority had evidence of ascites (80%) and hepatic encephalopathy (63%) with a median Model for End-Stage Liver Disease sodium (MELD) of 30 at admission and 32 at transplant. Median MELD sodium (MELD-Na) was 33 at admission and 32 at transplant. Hepatocellular carcinoma (HCC) was present in 13% of patients. Nine patients (8%) underwent simultaneous liver-kidney transplantation.
Table 1.
Clinical demographic and laboratory characteristics according to sarcopenic visceral obesity
| Overall n=116 |
Non-sarcopenic visceral obesity n=93 |
Sarcopenic visceral obesity n=23 |
p-value | |
|---|---|---|---|---|
| Age (years) | 53 (46–58) | 53 (46–58) | 54 (42–58) | 0.87 |
| Sex (male) | 76 (66) | 58 (62) | 18 (78) | 0.15 |
| Race | 0.86 | |||
| Non-Hispanic white | 80 (69) | 62 (67) | 18 (79) | |
| Hispanic white | 16 (14) | 14 (15) | 2 (9) | |
| Asian | 7 (6) | 6 (6) | 1 (4) | |
| Black | 5 (4) | 4 (4) | 1 (4) | |
| Other | 8 (7) | 7 (8) | 1 (4) | |
| Body mass index (kg/m2) | 27.5 (23.6–32.9) | 27.6 (23.5–33.0) | 27.4 (23.9–32.7) | 0.64 |
| Weight (kg) | 79.7 (69.1–95.1) | 79.3 (69.0–95.0) | 85.1 (69.2–99.1) | 0.63 |
| Etiology | ||||
| Hepatitis C infection | 35 (31) | 27 (29) | 8 (35) | 0.59 |
| Hepatitis B infection | 11 (9) | 10 (11) | 1 (4) | 0.35 |
| Alcohol | 31 (27) | 27 (29) | 4 (17) | 0.26 |
| NAFLD/NASH | 12 (10) | 10 (11) | 2 (9) | 0.77 |
| AIH/PBC/PSC | 16 (14) | 13 (14) | 3 (13) | 0.91 |
| Other | 11 (9) | 6 (6) | 5 (22) | 0.03 |
| Hypertension | 33 (28) | 25 (27) | 8 (35) | 0.45 |
| Diabetes mellitus | 21 (18) | 15 (16) | 6 (26) | 0.28 |
| Coronary artery disease | 5 (4) | 2 (2) | 3 (14) | 0.02 |
| Ascites | 0.23 | |||
| None | 23 (20) | 21 (23) | 2 (9) | |
| Mild/Moderate | 50 (43) | 37 (40) | 13 (57) | |
| Severe/refractory | 42 (37) | 34 (37) | 8 (35) | |
| Hepatic encephalopathy | 0.11 | |||
| None | 42 (37) | 36 (39) | 6 (26) | |
| Altered mood/confusion | 43 (37) | 30 (33) | 13 (57) | |
| Marked confusion | 30 (26) | 26 (28) | 4 (18) | |
| Hepatocellular carcinoma | 15 (13) | 11 (12) | 4 (17) | 0.48 |
| MELD | ||||
| Admission | 30 (21–37) | 30 (22–37) | 29 (19–39) | 0.58 |
| Transplant | 32 (25–37) | 31 (25–37) | 32 (29–36) | 0.52 |
| MELD-Na | ||||
| Admission | 33 (25–39) | 33 (26–38) | 33 (25–40) | 0.92 |
| Transplant | 32 (26–37) | 32 (26–38) | 34 (30–36) | 0.45 |
| Body composition | ||||
| SMI (cm2/m2) | 47 (42–53) | 48 (44–55) | 42 (37–47) | <0.001 |
| VSR | 1.24 (0.46–2.16) | 0.76 (0.41–1.54) | 2.26 (1.73–3.13) | <0.001 |
| Simultaneous liver kidney transplant | 9 (8) | 7 (8) | 2 (9) | 0.85 |
| Follow-up duration (month) | 67 (39–132) | 72 (44–127) | 64 (4–144) | 0.44 |
Values reported in median (IQR) or number (percentage). Abbreviations: AIH/PBC/PSC, autoimmune hepatitis/primary biliary cholangitis/primary sclerosing cholangitis; MELD-Na, model for end-stage liver disease-sodium; NAFLD/NASH, nonalcoholic fatty liver disease/nonalcoholic steatohepatitis; SMI, skeletal muscle index; VSR, visceral-to-subcutaneous adipose tissue ratio.
Association of Body Composition and Post-Transplant Mortality
The median (IQR) time from CT imaging to transplant was 13 (7–29) days. Overall median VATI and SATI were 41 and 32 cm2/m2, respectively, with a median VSR of 1.24. A higher proportion of men (55%) met criterion for sarcopenia with a median SMI of 49 cm2/m2 compared to women (25%) with a median SMI of 44 cm2/m2. Baseline clinical characteristics by sex are shown in Supplementary Table 1. At a median follow-up duration of 67 months, a total of 34 (29%) patients died after liver transplantation. Men who died had lower median SMI (46 vs 50 cm2/m2), higher median VATI (41 vs 37 cm2/m2), lower median SATI (30 vs 32 cm2/m2), and higher median VSR (1.60 vs 1.25) compared to those alive, with none reaching statistical significance. A similar relationship of body composition was also observed in women who died compared to those alive, with a lower median SMI (44 vs 45 cm2/m2), higher median VATI (46 vs 36 cm2/m2), lower median SATI (27 vs 36 cm2/m2), and higher median VSR (1.37 vs 0.89), with none reaching statistical significance.
Identifying Optimal Cutoff Values for Visceral Obesity Associated with Post-Transplant Mortality
To identify an optimal cutoff of VSR to define visceral obesity, we started by assessing the relationship between post-transplant mortality and VSR stratified by sex based on the different visceral and subcutaneous adiposity observed in our cohort among men and women. Using the time-dependent ROC method to identify a cutoff that could be used in clinical practice, an optimal (highest concordance probability function at month 36) VSR cutoff of ≥1.54 for men and ≥1.37 for women were used to define visceral obesity in our cohort.
Association of Sarcopenic Visceral Obesity and Post-Transplant Outcomes
We then defined SVO as the combination of sarcopenia (SMI <50 cm2/m2 for men and <39 cm2/m2 for women) and visceral obesity (VSR ≥1.54 for men and ≥1.37 for women). Among the 116 patients in our cohort, 38 (33%) were non-sarcopenic and non-visceral obese, 26 (22%) were non-sarcopenic and visceral obese, 29 (25%) were sarcopenic and non-visceral obese, and 23 (20%) were sarcopenic visceral obese. Baseline clinical characteristics in patients with and without SVO were similar (Table 1).
As shown in Figure 1, patients with SVO had a significantly higher cumulative incidence of post-transplant mortality at 12 months (30% vs 8%) and 36 months (39% vs 13%). The presence of SVO remained significantly associated with cumulative incidence of post-transplant mortality stratified by sex: 60% vs 20% (logrank p=0.01) in women and 33% vs 9% (logrank p=0.001) in men. Among various body composition groups, the cumulative incidence of post-transplant mortality was higher in patients with both sarcopenia and visceral obesity compared to patients with sarcopenia without visceral obesity or visceral obesity without sarcopenia at 12 months (30% vs 7% vs 8%) and 36 months (39% vs 14% vs 8%), respectively (Figure 2).
Figure 1.
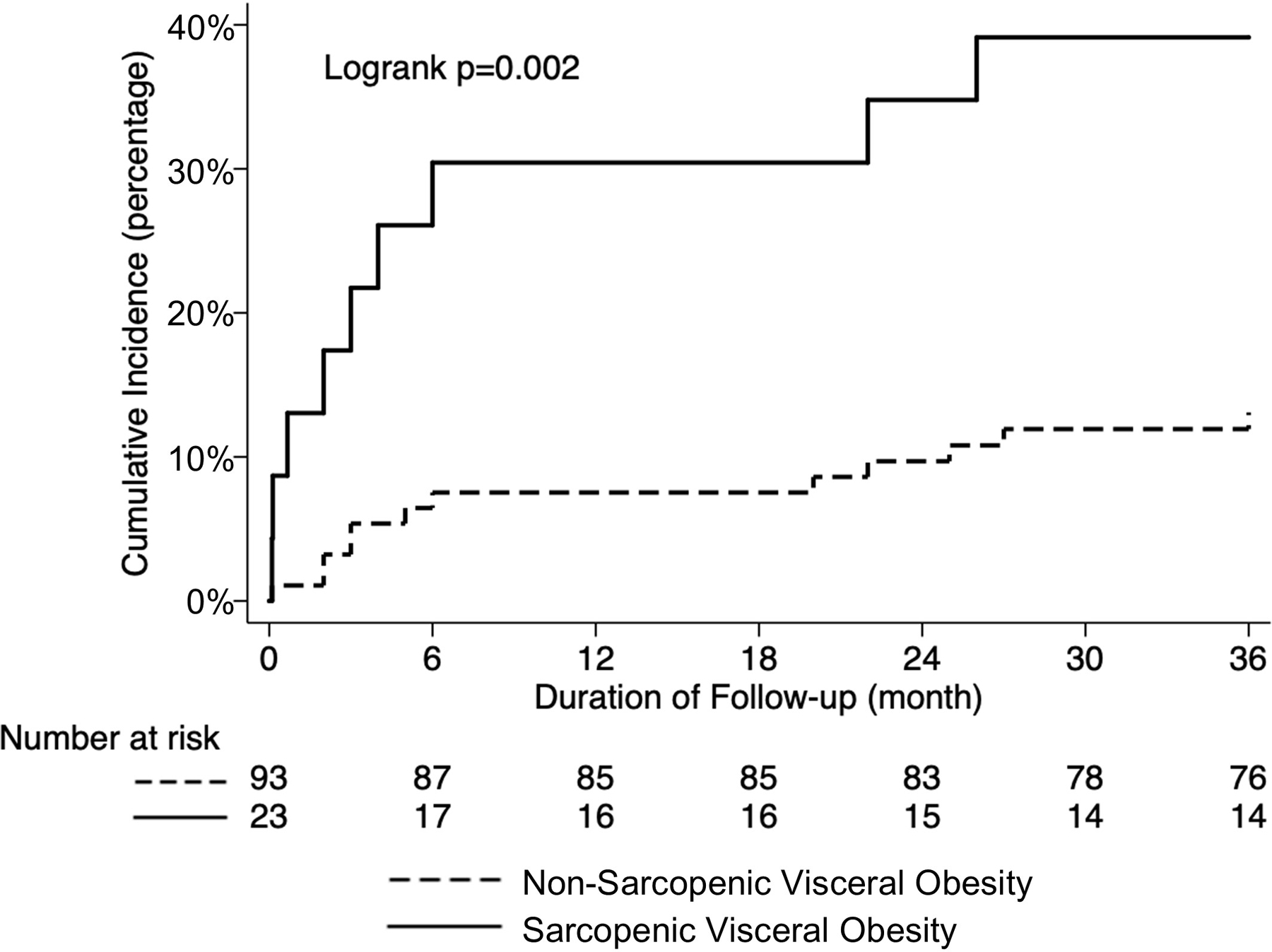
Cumulative incidence of post-transplant mortality according to sarcopenic visceral obesity
Figure 2.
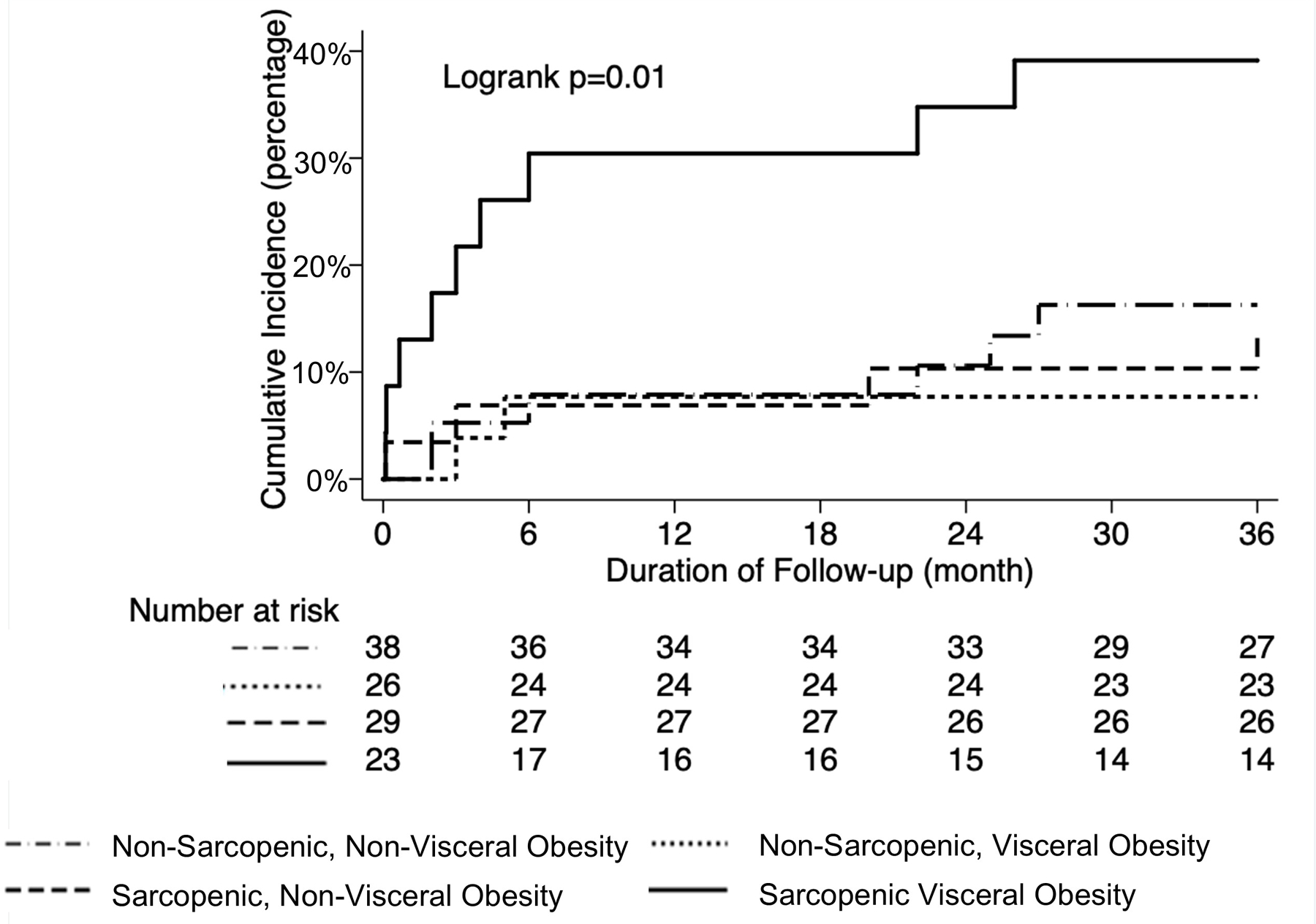
Cumulative incidence of post-transplant mortality according to different subgroups of sarcopenia and visceral obesity
In univariable Cox regression analysis, only SVO (HR 2.92, 95% CI 1.04–8.23, p=0.04) was significantly associated with post-transplant mortality. In multivariable Cox regression, after adjusting for age, sex, diabetes, hepatic encephalopathy, HCC, and MELD-Na at transplant, SVO remained independently associated with post-transplant mortality (HR 3.50, 95% CI 1.10–11.15, p=0.03) (Table 2). When the above univariable and multivariable Cox regression model was analyzed using MELD at transplant while also adjusting for age, sex, diabetes, hepatic encephalopathy, and HCC, SVO remained independently associated with post-transplant mortality (HR 3.52, 95% CI 1.11–11.19, p=0.03).
Table 2.
Univariable and multivariable Cox regression for predictors of post-transplant mortality
| Univariable analysis | Multivariable analysis | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Age (year) | 1.02 | 0.97–1.06 | 0.46 | 1.01 | 0.97–1.06 | 0.59 |
| Sex (male) | 0.54 | 0.23–1.28 | 0.16 | 0.37 | 0.14–1.02 | 0.054 |
| Diabetes | 2.03 | 0.79–5.24 | 0.14 | 0.98 | 0.31–3.09 | 0.97 |
| Hepatic encephalopathy | 2.66 | 0.90–7.92 | 0.08 | 1.97 | 0.59–6.58 | 0.27 |
| Hepatocellular carcinoma | 1.64 | 0.55–4.88 | 0.37 | 2.27 | 0.55–9.38 | 0.26 |
| MELD-Na (per 1 point) | 1.04 | 0.99–1.10 | 0.11 | 1.06 | 0.99–1.13 | 0.08 |
| Body composition | ||||||
| Non-sarcopenic & non-visceral obese | Ref | - | - | Ref | - | - |
| Non-sarcopenic & visceral obese | 0.47 | 0.09–2.32 | 0.35 | 0.45 | 0.09–2.30 | 0.34 |
| Sarcopenic & non-visceral obese | 0.86 | 0.24–3.03 | 0.81 | 1.25 | 0.33–4.80 | 0.74 |
| Sarcopenic & visceral obese | 2.92 | 1.04–8.23 | 0.04 | 3.50 | 1.10–11.15 | 0.03 |
Abbreviations: AIH/PBC/PSC, autoimmune hepatitis/primary biliary cholangitis/primary sclerosing cholangitis; MELD-Na, model for end-stage liver disease-sodium; NAFLD/NASH, nonalcoholic fatty liver disease/nonalcoholic steatohepatitis
Other post-transplant clinical outcomes in patients with and without SVO are shown in Supplementary Figure 1. Although none reached statistical significance, a higher proportion of patients with SVO required total hospital length of stay >14 days (65% vs 48%) with a median duration of 19 vs 14 days (p=0.13), intensive care unit length of stay >7 days (48% vs 29%) with a median duration of 7 vs 4 days (p=0.17), acute infection (57% vs 52%), biopsy-proven acute cellular rejection (17% vs 15%), hepatic artery thrombosis (13% vs 6%), and portal vein thrombosis (9% vs 2%).
Comparison of Different Visceral Obesity Criteria: VSR, VAT area, and BMI
We then evaluated the degree of improvement in predicting post-transplant mortality when VSR was used to define visceral obesity compared to other criteria used in prior studies: BMI ≥25.0 kg/m2 10,12,25, BMI ≥30.0 kg/m2 8,and/or VAT area ≥100 cm2 9–11. If SVO was defined by either VAT area ≥100 cm2 (HR 2.04, 95% CI 0.73–5.68, p=0.17) or BMI ≥25.0 kg/m2 (HR 2.39, 95% CI 0.89–6.40, p=0.08), there was no statistically significant association with post-transplant mortality (adjusting for age, sex, diabetes, hepatic encephalopathy, HCC, and MELD-Na at transplant). Furthermore, although the cumulative mortality rate through 36 months was significantly higher in patients with SVO defined by VSR compared to those without (Figure 1), there were no statistically significant differences in mortality rates between patients with and without SVO when it was defined by either VAT area ≥100 cm2 (Supplementary Figure 2) or BMI ≥25.0 kg/m2 (Supplementary Figure 3).
DISCUSSION
The association between BMI and outcomes in patients with cirrhosis remains controversial due to significant fluid retention (e.g., ascites and systemic edema), sex and racial differences in body composition (e.g., visceral vs subcutaneous fat and skeletal muscle), and various criteria for classifying obesity (e.g., BMI ≥25 kg/m2, ≥30 kg/m2, or ≥27 kg/m2 in Asian). Moreover, BMI is unable to account for weight loss due to loss of muscle mass in sarcopenia and/or malnutrition as it is likely masked by fluid retention in the majority of these patients. Given these limitations, cross-sectional imaging with CT is emerging as a key tool to non-invasively assess body composition, particularly muscle and fat mass.21,26 This may be particularly clinically useful for acutely ill patients in whom performance-based metrics (e.g., hand grip strength, chair stands) or laboratory tests (e.g., albumin) may be transiently impaired during acute illness in ways that are not necessarily prognostic.26
In this multicenter study of acutely ill patients with cirrhosis who underwent urgent listing and liver transplantation, we observed that sarcopenia and visceral obesity when defined by CT-quantified body compositions, were present in nearly one-half and two-fifth of patients, respectively, with 20% categorized as SVO based on the combination of both sarcopenia and visceral obesity. We further observed that the presence of SVO was independently associated with higher rates of post-transplant mortality, resulting in 1- and 3-year mortality rates of 30% and 39%, respectively, compared to patients with either sarcopenia or visceral obesity alone.
It is worth noting the sex differences that we observed in body composition. Sarcopenia was less prevalent in women compared to men (25% vs 55%) and was not significantly associated with post-transplant mortality when defined as SMI <39 cm2/m2, which we have reported previously13 and may suggest a disproportionate association between sarcopenia and increase mortality in men as compared to women with cirrhosis.27,28 Visceral obesity defined by our VSR cutoffs, however, was similar between women and men, with no significant differences in rates of SVO. Unlike sarcopenia, which we previously reported to have no significant association with post-transplant mortality in women, SVO was associated with an increased risk of post-transplant mortality in both women and men. While the precise pathogenesis of these sex differences in associations between sarcopenia and mortality is unclear, our findings raise the possibility that SVO, rather than sarcopenia alone, may be a more appropriate metric for use in clinical practice to identify both acutely ill women and men with decompensated cirrhosis who are at increased risk for poor post-transplant outcomes. Given the increasing ease with which body composition can be measured on cross-sectional images in a semi-automated—and likely, in the future, automated—fashion, this new metric combining both muscle and fat quantification advances our understanding of pragmatic risk assessments in this population.
Additionally, body fat distribution may be more important than overall adiposity given the direct and inverse relationship of VAT and SAT, respectively, and its association with poor clinical outcomes in cirrhosis. Prior studies have used CT-quantified VAT area of ≥100 cm2 to define visceral obesity in sarcopenic patients with cirrhosis, though they did not adjust for sex and/or stature.9–11,29 In the present study, medium BMI was observed to be similar in both patients with and without SVO (27.4 vs 27.6 kg/m2), which is not unexpected given that ascites was present in the majority of our cohort. Furthermore, only SVO defined by VSR was significantly associated with cumulative rates of post-transplant mortality through 36 months and independently associated with post-transplant mortality even after adjusting for age, sex, diabetes, hepatic encephalopathy, HCC, and MELD-Na at transplant, which was neither observed when SVO was defined by other criteria used in prior studies: VAT area ≥100 cm2 9–11 or BMI ≥25.0 kg/m2 10,12,25.
Furthermore, there were relatively few patients with NAFLD or NASH in our cohort (10%). Our cohort included patients transplanted back in 2005 when the diagnosis of NAFLD/NASH was less frequent. Additionally, we only included acutely ill inpatients with cirrhosis, whereas patients with cirrhosis secondary to NASH may more commonly present as outpatients. Given the reported associations between sarcopenia and NAFLD/NASH, in particularly its shared physiological pathways 30–32– muscle-liver-adipose axis – future studies with a larger cohort of patients with NAFLD/NASH should examine the potential interaction between NAFLD/NASH and sarcopenia and visceral obesity on post-transplant outcomes.
The findings of this study should be considered in light of several limitations. Given our interest in identifying an objective metric to evaluate sarcopenia and visceral obesity in a selective population of acutely ill patients with cirrhosis undergoing urgent evaluation and liver transplantation, our relatively small sample size may not reflect the broader population of patients with cirrhosis and limits the generalizability of our findings. However, the prevalence of sarcopenia, visceral obesity, and SVO in our population were similar to rates reported in previous studies that included a more heterogeneous cohort of patients with cirrhosis awaiting liver transplantation using similar CT-quantification of body compositions.12,22,33,34 Additionally, the median SMI in our cohort was similar to that reported in our other study (47.0 vs 47.6 cm2/m2) that included a population of outpatients with cirrhosis awaiting liver transplantation with a median MELD of 15, supporting the generalizability of our findings. Furthermore, the inclusion of patients from four transplant centers improves the generalizability of these findings to the broader high-MELD liver transplant population as a whole. Only patients with available CT imaging within 90 days prior to transplant were included, subjecting to further selection bias; however, routine pre-transplant CT imaging is standard of care for all sites, making this less likely. Another limitation was the timing and availability of CT-quantified adipose tissue and skeletal muscle mass, which was done at a single time point. Although the median (IQR) time from body composition imaging to transplant was 13 (7–29) days, there were 35 patients with imaging completed within 7 days from transplant, in which both skeletal muscle wasting and loss of VAT has the potential to occur in the setting of critical illness; however, there was no statistically significant difference in timing of imaging on quantified SMI, VSR, and post-transplant mortality. Lastly, the small cohort size limited our ability to significantly evaluate the association between sarcopenic visceral obesity and other post-transplant outcomes besides mortality. Larger studies including a heterogeneous cohort of patients with cirrhosis undergoing transplant evaluation are necessary to validate our proposed VSR cutoffs in defining visceral obesity and its association along with sarcopenia resulting in suboptimal clinical outcomes.
In conclusion, our data demonstrate that the relative distribution of adipose tissue with CT-quantified VSR rather than the absolute value of its different depots or anthropometric measurements may more comprehensively and objectively define visceral obesity. Importantly, the concordance of sarcopenia and visceral obesity was associated with worst post-transplant mortality than seen with either condition alone. Our data offer the transplant community a tool to assess body composition in both women and men with cirrhosis who are acutely ill, in whom other metrics of nutritional status and physical function may not be as reliable.
Supplementary Material
Supplementary Figure 3. Cumulative incidence of post-transplant mortality according to sarcopenic visceral obesity by BMI ≥25 kg/m2
Supplementary Figure 1. Percentage of post-transplant outcomes according to sarcopenic visceral obesity
Supplementary Figure 2. Cumulative incidence of post-transplant mortality according to sarcopenic visceral obesity by VATI ≥100 cm2
Financial Support:
This study was funded by NIH R01AG059183 (Lai), NIH P30DK026743 (Huang, Lai, Shui), NIH R21AG067554 (Lai), and NIH 5T32DK060414–18 (Ha). These funding agencies played no role in the analysis of the data or the preparation of this manuscript.
List of Abbreviations:
- BMI
body mass index
- CT
computed tomography
- HCC
hepatocellular carcinoma
- IQR
interquartile range
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- MELD-Na
model for end-stage liver disease sodium
- SMI
skeletal muscle index
- SAT
subcutaneous adipose tissue
- SATI
subcutaneous adipose tissue index
- VAT
visceral adipose tissue
- VATI
visceral adipose tissue index
- VSR
visceral-to-subcutaneous adipose tissue ratio
Footnotes
Conflict of Interest: The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Data Availability Statement:
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1.Kwong AJ, Kim WR, Lake JR, et al. OPTN/SRTR 2019 Annual Data Report: Liver. Am J Transplant 2021;21 Suppl 2:208–315. [DOI] [PubMed] [Google Scholar]
- 2.Hakeem AR, Cockbain AJ, Raza SS, et al. Increased morbidity in overweight and obese liver transplant recipients: a single-center experience of 1325 patients from the United Kingdom. Liver Transpl 2013;19(5):551–562. [DOI] [PubMed] [Google Scholar]
- 3.LaMattina JC, Foley DP, Fernandez LA, et al. Complications associated with liver transplantation in the obese recipient. Clin Transplant 2012;26(6):910–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singhal A, Wilson GC, Wima K, et al. Impact of recipient morbid obesity on outcomes after liver transplantation. Transpl Int 2015;28(2):148–155. [DOI] [PubMed] [Google Scholar]
- 5.Fujikawa T, Fujita S, Mizuno S, et al. Clinical and financial impact of obesity on the outcome of liver transplantation. Transplant Proc 2006;38(10):3612–3614. [DOI] [PubMed] [Google Scholar]
- 6.Nair S, Cohen DB, Cohen MP, Tan H, Maley W, Thuluvath PJ. Postoperative morbidity, mortality, costs, and long-term survival in severely obese patients undergoing orthotopic liver transplantation. Am J Gastroenterol 2001;96(3):842–845. [DOI] [PubMed] [Google Scholar]
- 7.Spengler EK, O’Leary JG, Te HS, et al. Liver Transplantation in the Obese Cirrhotic Patient. Transplantation 2017;101(10):2288–2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carias S, Castellanos AL, Vilchez V, et al. Nonalcoholic steatohepatitis is strongly associated with sarcopenic obesity in patients with cirrhosis undergoing liver transplant evaluation. J Gastroenterol Hepatol 2016;31(3):628–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hara N, Iwasa M, Sugimoto R, et al. Sarcopenia and Sarcopenic Obesity Are Prognostic Factors for Overall Survival in Patients with Cirrhosis. Intern Med 2016;55(8):863–870. [DOI] [PubMed] [Google Scholar]
- 10.Kamo N, Kaido T, Hamaguchi Y, et al. Impact of sarcopenic obesity on outcomes in patients undergoing living donor liver transplantation. Clin Nutr 2019;38(5):2202–2209. [DOI] [PubMed] [Google Scholar]
- 11.Kobayashi A, Kaido T, Hamaguchi Y, et al. Impact of Sarcopenic Obesity on Outcomes in Patients Undergoing Hepatectomy for Hepatocellular Carcinoma. Ann Surg 2019;269(5):924–931. [DOI] [PubMed] [Google Scholar]
- 12.Montano-Loza AJ, Angulo P, Meza-Junco J, et al. Sarcopenic obesity and myosteatosis are associated with higher mortality in patients with cirrhosis. J Cachexia Sarcopenia Muscle 2016;7(2):126–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuo SZ, Ahmad M, Dunn MA, et al. Sarcopenia Predicts Post-transplant Mortality in Acutely Ill Men Undergoing Urgent Evaluation and Liver Transplantation. Transplantation 2019;103(11):2312–2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Montano-Loza AJ, Meza-Junco J, Baracos VE, et al. Severe muscle depletion predicts postoperative length of stay but is not associated with survival after liver transplantation. Liver Transpl 2014;20(6):640–648. [DOI] [PubMed] [Google Scholar]
- 15.Terjimanian MN, Harbaugh CM, Hussain A, et al. Abdominal adiposity, body composition and survival after liver transplantation. Clin Transplant 2016;30(3):289–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsien C, Garber A, Narayanan A, et al. Post-liver transplantation sarcopenia in cirrhosis: a prospective evaluation. J Gastroenterol Hepatol 2014;29(6):1250–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mitsiopoulos N, Baumgartner RN, Heymsfield SB, Lyons W, Gallagher D, Ross R. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol (1985) 1998;85(1):115–122. [DOI] [PubMed] [Google Scholar]
- 18.Vehmas T, Kairemo KJ, Taavitsainen MJ. Measuring visceral adipose tissue content from contrast enhanced computed tomography. Int J Obes Relat Metab Disord 1996;20(6):570–573. [PubMed] [Google Scholar]
- 19.Kvist H, Sjostrom L, Tylen U. Adipose tissue volume determinations in women by computed tomography: technical considerations. Int J Obes 1986;10(1):53–67. [PubMed] [Google Scholar]
- 20.Meza-Junco J, Montano-Loza AJ, Baracos VE, et al. Sarcopenia as a prognostic index of nutritional status in concurrent cirrhosis and hepatocellular carcinoma. J Clin Gastroenterol 2013;47(10):861–870. [DOI] [PubMed] [Google Scholar]
- 21.Carey EJ, Lai JC, Sonnenday C, et al. A North American Expert Opinion Statement on Sarcopenia in Liver Transplantation. Hepatology 2019;70(5):1816–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carey EJ, Lai JC, Wang CW, et al. A multicenter study to define sarcopenia in patients with end-stage liver disease. Liver Transpl 2017;23(5):625–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cattaneo M, Malighetti P, Spinelli D. Estimating receiver operative characteristic curves for time-dependent outcomes: The stroccurve package. The Stata Journal 2017;17(4):1015–1023. [Google Scholar]
- 24.Liu X Classification accuracy and cut point selection. Stat Med 2012;31(23):2676–2686. [DOI] [PubMed] [Google Scholar]
- 25.Hammad A, Kaido T, Hamaguchi Y, et al. Impact of sarcopenic overweight on the outcomes after living donor liver transplantation. Hepatobiliary Surg Nutr 2017;6(6):367–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lai JC, Tandon P, Bernal W, et al. Malnutrition, Frailty, and Sarcopenia in Patients With Cirrhosis: 2021 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2021. [DOI] [PMC free article] [PubMed]
- 27.DiMartini A, Cruz RJ Jr., Dew MA, et al. Muscle mass predicts outcomes following liver transplantation. Liver Transpl 2013;19(11):1172–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ebadi M, Tandon P, Moctezuma-Velazquez C, et al. Low subcutaneous adiposity associates with higher mortality in female patients with cirrhosis. J Hepatol 2018;69(3):608–616. [DOI] [PubMed] [Google Scholar]
- 29.Examination Committee of Criteria for ‘Obesity Disease’ in J, Japan Society for the Study of O. New criteria for ‘obesity disease’ in Japan. Circ J 2002;66(11):987–992. [DOI] [PubMed] [Google Scholar]
- 30.Bhanji RA, Narayanan P, Allen AM, Malhi H, Watt KD. Sarcopenia in hiding: The risk and consequence of underestimating muscle dysfunction in nonalcoholic steatohepatitis. Hepatology 2017;66(6):2055–2065. [DOI] [PubMed] [Google Scholar]
- 31.Lee YH, Jung KS, Kim SU, et al. Sarcopaenia is associated with NAFLD independently of obesity and insulin resistance: Nationwide surveys (KNHANES 2008–2011). J Hepatol 2015;63(2):486–493. [DOI] [PubMed] [Google Scholar]
- 32.Lee YH, Kim SU, Song K, et al. Sarcopenia is associated with significant liver fibrosis independently of obesity and insulin resistance in nonalcoholic fatty liver disease: Nationwide surveys (KNHANES 2008–2011). Hepatology 2016;63(3):776–786. [DOI] [PubMed] [Google Scholar]
- 33.Fazel Y, Koenig AB, Sayiner M, Goodman ZD, Younossi ZM. Epidemiology and natural history of non-alcoholic fatty liver disease. Metabolism 2016;65(8):1017–1025. [DOI] [PubMed] [Google Scholar]
- 34.Montano-Loza AJ, Duarte-Rojo A, Meza-Junco J, et al. Inclusion of Sarcopenia Within MELD (MELD-Sarcopenia) and the Prediction of Mortality in Patients With Cirrhosis. Clin Transl Gastroenterol 2015;6:e102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 3. Cumulative incidence of post-transplant mortality according to sarcopenic visceral obesity by BMI ≥25 kg/m2
Supplementary Figure 1. Percentage of post-transplant outcomes according to sarcopenic visceral obesity
Supplementary Figure 2. Cumulative incidence of post-transplant mortality according to sarcopenic visceral obesity by VATI ≥100 cm2
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


