Abstract
Given that breathing is one of the most fundamental physiological functions, there is an urgent need to broaden our understanding of the fluid dynamics that governs it. There would be many benefits from doing so, including a better assessment of respiratory health, a basis for more precise delivery of pharmaceutical drugs for treatment, and the understanding and potential minimization of respiratory infection transmission. We review the physics of particle generation in the respiratory tract, the fate of these particles in the air on exhalation and the physics of particle inhalation. The main focus is on evidence from experimental studies. We conclude that although there is qualitative understanding of the generation of particles in the respiratory tract, a basic quantitative knowledge of the characteristics of the particles emitted during respiratory activities and their fate after emission, and a theoretical understanding of particle deposition during inhalation, nevertheless the general understanding of the entire process is rudimentary, and many open questions remain.
Subject terms: Applied physics, Physics
In this Perspective on the physics of particle generation in the respiratory tract, fate in the air upon exhalation and the physics of inhalation, the authors conclude that the general understanding of the entire process is rudimentary, and many open questions remain.
Introduction
Inhaling and exhaling air — breathing — is one of the basic physiological functions of the human being. Breathing is essential to nourish the body with oxygen and eliminate the waste generated in the process: carbon dioxide. Because it is a physiological function, it is normally considered in the domain of medical sciences, not physics.
However, physics, and more specifically fluid dynamics, is a critical element of the process1. During inhalation, air enters the respiratory tract and flows down through the upper and lower parts of the tract, finally reaching the alveolar region. During exhalation, when the passages contract, air flows in the opposite direction and is ultimately exhaled. The exhaled stream of air passes at high speed over the surface of the water-based liquid lining the respiratory tract, and aerosolizes the liquid. The particles that are generated contain, in addition to water, many other constituents, including salts, proteins, mucus, and pathogens such as bacteria or viruses. (See Box 1 for a note about terminology.) The process of particle generation during human respiratory activities — which in addition to breathing include speaking, singing or coughing — is, however, more complex than aerosolization from the surface. During exhalation, fluid blockages form in respiratory bronchioles, which burst during subsequent inhalation to produce particles; during vocalization, fluid bathing the larynx is aerosolized owing to vocal cord vibration; and during speech articulation, saliva in the mouth is aerosolized owing to interaction of the tongue, teeth, palate and lips.
After the particles are generated, some are deposited in the respiratory tract, and those that eventually leave the respiratory tract with the airflow are subjected to numerous physical processes, including hygroscopic growth and deposition; both processes change the initial particle size distribution. Once emitted through the nose or mouth, particle characteristics further change in response to the change in temperature and relative humidity between the body and the external environment. The particles are also subject to various forces that affect their fate, which may be deposition and transport within the environment or inhalation by others present there.
In the process of inhalation, particles present in the air enter the respiratory tract and can be deposited there. The particles are not only the aged particles generated from respiratory activities of other people, but also particles of natural or anthropogenic origin that constitute air pollution. Pathogens contained by the particles may cause infections; deposition of any particles on the epithelium of the respiratory tract has numerous other health implications. Therefore, to understand and quantitatively assess possible health implications, physics must provide quantitative information about the process of particle generation during respiratory activities, the fate of the particles in the air, and deposition in the human respiratory tract.
This Perspective gives an overview of the understanding of the physics of particle generation and deposition in the human respiratory tract, and identifies open questions. The aim is not to be exhaustive for experts in this very specific area (of physics or modelling). Instead, this Perspective is mostly intended for a general educated readership who need to understand the general principles of this field to be able to link to other areas such as public health or other scientific fields. Thus, not all aspects are addressed in depth. The focus is on evidence from experimental studies. We consider only human studies and do not include animal studies.
We conducted a literature search to identify experimental studies that investigated particle composition, fate and inhalation; particles emitted from human respiratory activities; and experimental data and models of particle deposition in the lung. The keywords for particle composition, fate and inhalation were: respiratory droplets, bioaerosol, particle size, exhaled breath and expiratory aerosol. The keywords for human respiratory activities were: airflow sampling, particle image velocimetry (PIV), bioaerosol, saliva droplets, biological fluid dynamics and exhaled airflow. The keywords for particle deposition in the lung were: total particle deposition, measurement, human lung, submicrometre particles and ultrafine particles. We identified studies published in English using ScienceDirect, EBSCOhost, Web of Science and Wiley Interscience search engines.
We first discuss the physics of particle generation in the respiratory tract, followed by a short discussion of the fate of these particles in the air, and conclude with physics of particle inhalation, where we consider inhalation of pathogen-laden particles generated by humans and particles that constitute air pollution.
Box 1 A note on terminology.
We use the term particles, rather than aerosols or droplets, to avoid discussions of terminology that has been dividing expert communities166. Briefly, according to aerosol science, an aerosol is an assembly of liquid or solid particles suspended in a gaseous medium long enough to enable observation or measurement167. Although there is no definition of what constitutes ‘long enough’, it is considered that at a particle size of ~100 µm, gravitational deposition removes those particles from the air fast enough that they cannot be considered as suspended in the air. In aerosol science, a droplet is a liquid particle167. By contrast, in medical sciences, an aerosol is a smaller particle, whereas a droplet is a larger particle. A previous paper168 provides additional explanation behind the existing communication difficulties and stresses the need to develop terminology acceptable to and understood by expert communities from all relevant fields.
Particle generation
There are two known physical mechanisms to generate the particles emitted from the human respiratory tract: turbulent aerosolization, and the breakage or burst of a fluid film, filament or bubble (FFBB) (Fig. 1). Turbulent aerosolization is referred to as atomization in fluid mechanics literature and is characterized by turbulent flows stripping particles from a fluid film. This process has also been defined as a shear-induced surface wave instability2, as first described in the work of Lord Rayleigh3, and more recently as turbulent droplet extraction4. The FFBB process generates particles during normal breathing due to clearance of fluid closures in respiratory bronchioles, and during speaking when vocal cords adduct and vibrate in the larynx and when lips open and the tongue separates from the teeth in the mouth5,6. Integral to FFBB particle generation is airway reopening following closure7,8.
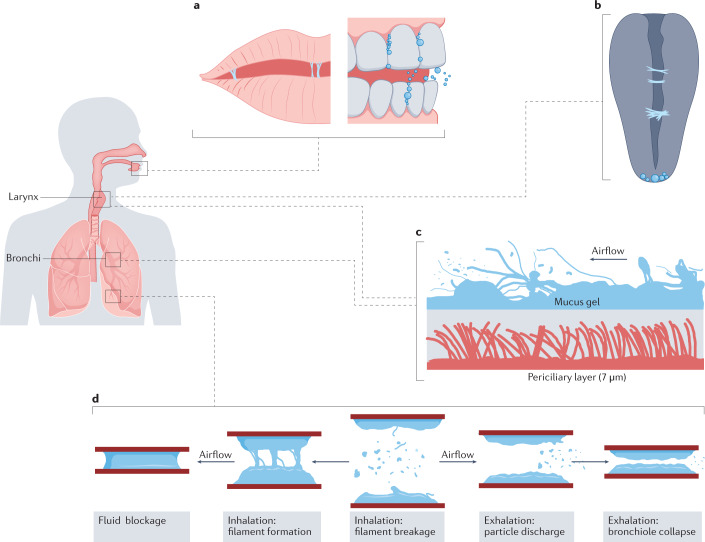
Fig. 1. Sites and mechanisms of particle generation.
a | Fluid film, filament or bubble breakage (FFBB) in the mouth during speech165. b | FFBB due to filament formation at the vocal cords. c | Turbulent aerosolization of viscoelastic mucus from the airway lining in the larynx and large bronchi due to turbulent airflow, based on snapshot of ligament-mediated fragmentation of viscoelastic liquid presented in ref.40. d | FFBB in small airway bronchioles due to clearance of fluid blockages formed during exhalation and airway reopening.
In both turbulent aerosolization and FFBB, particles originate from the airway’s surface liquid film, which is a bilayer with the top layer a mucus gel consisting of water (97%) and a mixture of mucins, non-mucin proteins, salts and cellular debris (3%), and the bottom, low-viscosity periciliary layer containing the cilia9. Turbulent aerosolization in the conventional sense is thought to be most active in large bronchi and the larynx owing to airflows that are partly turbulent even during breathing and with increasing velocity during speaking and coughing owing to partially adducted vocal folds10. In the deepest small airway bronchioles, FFBB is the dominant mechanism for particle generation11.
We discuss the quantity and size distribution of particles generated from these two mechanisms in the next section, but it is important to consider that these characteristics are a function of the thickness of airway lining fluid itself. In particular, it is unlikely that the diameter of generated particles will exceed the thickness of its parent fluid film12. The airway epithelial thickness is greatest, of the order of hundreds of micrometres, in the oral cavity where it also includes an overlying salivary layer13. This thickness decreases on moving deeper into the respiratory tract. In large airways of luminal diameter greater than 2 mm, the airway liquid film can be up to 50 µm thick, whereas in a small airway bronchiole the mucus gel layer is only 0.5–5.0 µm thick9.
Particle quantities and composition
Particles derived from the film of airway lining fluid contain components of the film itself, such as the aforementioned non-volatile material including mucins, non-mucin proteins, salts and cellular debris. Adding to the complexity of the composition, the particle mixture also contains saliva, nasal secretions, serum and blood from oral lesions, and even food debris14. Of public health concern, however, is that the particles may contain pathogens such as bacteria, viruses and fungi. Numerous pathogens have been measured in exhaled breath, including influenza, human rhinovirus and Mycobacterium tuberculosis15. In total, the typical mass or volume proportion of non-water content in a particle generated in the respiratory tract is 1–10%14,16.
The likelihood that a particle contains bacteria or viruses relates to the size of the particle, the pathogenic load in the mucus gel and saliva, and the point of origin of the particle within the respiratory tract. At a viral load of 7 × 106 RNA copies per millilitre oral fluid, the probability that a particle of 50 μm diameter, prior to dehydration, contains at least one virion is ∼37%17–19 The proportionality to the particle volume results in a substantially lower probability of ~0.37% for a 10-μm particle, and ~0.01% for a 3-μm particle. Of course, particles with a diameter less than that of the pathogen itself cannot contain such a pathogen; for SARS-CoV-2 this cut-off is ~0.1 μm (ref.20). This relationship is simplistic in view of the heterogeneity of viral concentrations, but it illustrates the importance of viral load in the quantification of airborne viral emissions.
These calculations can potentially lead one to dismiss particles of the order of 3 μm or less as presenting a negligible risk for secondary transmission once emitted into ambient air, as approximately 104 particles of 3 µm diameter would be needed in order to encounter a single virion at the referenced viral load. However, experiments using laser light scattering methods indicate that the quantities of particles generated during speech and coughing may be orders of magnitude higher than commonly assumed17,21 Indeed, measurements have indicated there are of the order of 105 particles of 2–4 μm and 107 particles of 0.2–0.4 μm for a single average cough21. With respect to the SARS-CoV-2 virus, when considering that viral loads in respiratory fluids can exceed 109 RNA copies per millilitre in certain infected individuals, a single cough can potentially generate thousands of 3-μm particles containing a virion that would be emitted into ambient air. However, whereas coughing is sporadic and characteristic of symptomatic infection, we breathe continuously, which may account for a greater fraction of emitted particles over time even from those with a respiratory tract infection22.
Turbulent aerosolization
Turbulent aerosolization occurs when air sweeps past the liquid film at sufficient velocity to draw a portion of its mass into fine ligaments that shed particles into the airstream upon break-up (also known as fragmentation)23,24. Intuitively, one would expect greater quantities of particles generated by this mechanism from respiratory activities that involve higher air velocities, such as the cough. The physics of gas–liquid aerosolization is best understood in the context of industrial applications, most notably the combustion engine25, and an early pictorial description dating from the 1920s involves water and alcohol in a model of a carburettor throat26. At this time, a simplified atomizing characteristic quantity was defined as the ratio of the static pressure of the airstream to the surface tension of the liquid being aerosolized26. This quantity becomes a Weber number upon incorporating an orifice height, hydraulic diameter or shear boundary layer thickness. Dimensional analysis suggests a drop diameter law quantifying the mean particle diameter generated by turbulent aerosolization:
| 1 |
where σ is the liquid surface tension, ρ is the air density, U is the airstream velocity, and c is a dimensionless quantity related to the viscosity of the media26. Note that other predictions of the mean diameter give greater weight to the larger particles23,27. The drop diameter scaling law is consistent with more recent measurements of drop characteristics in dense sprays28.
Equation (1) suggests that two aspects of turbulent aerosolization are relevant to the particle generation in the human respiratory tract. First, the average size of generated particles decreases with increasing airstream velocity; and second, the average size of generated particles increases with increasing surface tension (cohesion) of the airway liquid film. The phenomena of increased particle size, and diminished quantity of generated particles, by increased surface tension has been experimentally validated29,30, although subsequent work indicates that this may be due to changes in surface viscoelasticity rather than surface tension alone31. Elasticity is also important: because particle size is inversely related to the flow instability mode, it should decrease with increasing surface tension32.
However, equation (1) and the definition of the atomizing characteristic quantity are simplified descriptions, which neglect boundary layers at the fluid interface, secondary aerosolization from ongoing liquid break-up in the airstream, and particle coalescence in the far field33. More importantly, such relationships apply to Newtonian fluids such as water, and airway mucus is a viscoelastic liquid34. A review of the rheology of airway surface liquid35 reports viscosities much higher than water, from 0.058 to >70 Pa s, and influenced by morbidities such as chronic bronchitis and cystic fibrosis. Conversely, the surface tension of airway surface liquid is less variable and lower than water, with a range of 0.01–0.05 N m–1 (ref.36), and a range of 0.001–0.01 N m–1 (ref.37) for alveolar surface tension measured directly in an excised lung of a rat. Unfortunately, none of these rheological properties can be measured directly in the respiratory tract of a living human being, limiting the accuracy of numerical modelling.
In terms of such non-Newtonian surface tension effects, viscoelasticity reduces the duration of ligament stretching as compared with a viscous Newtonian fluid, thus leading to shorter and thicker ligaments that break up into particles that are larger on average38,39 However, viscoelasticity also broadens the size distribution of the aerosolized particles, leading to a greater frequency of both small and large particles that is well described by a gamma distribution40. It then follows that the high viscoelasticity of airway surface liquid probably contributes to the heterogeneous size distribution of emitted particles, spanning several orders of magnitude for coughs, which involve high-velocity turbulent flow21. Furthermore, owing to interpersonal variations in viscoelastic properties that affect airway lining break-up, the overall quantity of particles emitted in exhaled breath can vary by several orders of magnitude between individuals41.
In closing, although the enormous quantities of particles generated from turbulent expirations21 suggest that turbulent flows may cause considerable stripping and/or dislodging of particles from the airway lining film, we note that there is surprisingly limited direct evidence of this mechanism in the literature, and further study is needed10.
Fluid film, filament or bubble breakage
The bronchiole fluid film burst mechanism is a type of FFBB and was introduced to explain the asymmetry of particle generation in the breathing cycle, during which fewer particles are generated during exhalation than inhalation11. This asymmetry is inconsistent with the turbulence-induced aerosolization mechanism described above. The bursting mechanism begins with fluid closures that occur in respiratory bronchioles during the airway collapse following exhalation. During inhalation, a fluid blockage contracts axially as it is drawn radially outward by the expanding bronchiole, ultimately becoming a thin film or bubble. The film or bubble subsequently bursts and fragments into particles, reopening the airway. This mechanism is consistent with the observation that particle generation increases as breathing becomes deeper and faster42, because deeper exhalation results in more blockages that are subsequently reopened upon inhalation11. This explanation is further evidenced by the finding that exhalations that achieved residual volume generated far more particles than shallower exhalations at functional residual capacity8. The presence of biomarkers from alveolar cells in exhaled breath provides further evidence of the importance of this mechanism7.
In addition to the bronchiole fluid film burst, FFBB also occurs in the larynx during speaking, because of fluid films bursting and filaments breaking when the mucus-bathed vocal folds adduct and vibrate43. Furthermore, particle generation rates increase with increasing amplitude of vocalization44, although it is difficult to attribute this solely to enhanced bursting in the larynx, as speaking loudly is likely to require additional airflow, providing additional opportunity for turbulent aerosolization as well as FFBB. Likewise, singing generates more respiratory particles than talking, with the number increasing with song loudness and possibly with higher pitch45.
Although the physics of FFBB is less understood than that of turbulent aerosolization of Newtonian liquids, there is evidence that surface tension rather than gravity drives the collapse of viscous surface bubbles after rupture46. Thus, as with turbulent aerosolization, further study is merited on the surface tension of the viscoelastic film that lines airways, including how it and viscoelasticity can be manipulated to reduce emissions of pathogen-laden particles. Alternatively, rheological properties of the film could be altered to increase the size of generated particles to promote their settling to the ground, as it is unlikely that emissions can be completely eliminated32. However, methods of stabilizing airway lining fluid to suppress pathogenic emissions require much more in-depth research47, including consideration of evaporation in ambient air after emission. Additionally, such methods need not be limited to deep components of the respiratory tract. For example6, it was found that lip balm reduced formation of salivary filaments and subsequent particle generation during speech. It is also of great interest and importance to elucidate the mechanisms responsible for super-emitting individuals who produce substantially more particles than the average person, as has been demonstrated for breathing29, coughing21 and speech44. For example, particle emissions from the respiratory tract seem to increase with increasing viral load of SARS-CoV-2 and body mass index multiplied by age41.
Respiratory particles in the air
To explain the fate in the air of particles generated from human respiratory activities, it is critical to understand what happens to the particles immediately after emission, when the condensed, warm and humid emission plume mixes with and is diluted by ambient air. In the field of aerosol science, the convergence towards developing an understanding of the initial instant of emission of respiratory particles has been long and has not yielded definitive answers7,48, mainly owing to the complexity of physical processes such as evaporation and the difficulty of measuring the particle emission in situ. In addition, different techniques are used to measure somewhat different parameters, often making comparisons of the outcomes difficult. Furthermore, when considering airborne disease transmission, the interaction of the respiratory particles with the airflow is a crucial issue, which makes the process more complex.
Measurement techniques
The exhaled airflow measurement techniques can be divided into two categories49: global flow-field measurements (high-speed photography, schlieren photography and PIV), and pointwise measurements50–53. The global flow-field measurement techniques provide information on the whole flow field and help us to understand the interactions between the exhaled flow, the thermal plume and the room airflow. The pointwise measurements are instead used to measure the initial temperature, initial humidity and velocity. Methods and instrumentation adopted to investigate respiratory particles using global flow-field measurements, and the main findings from the studies, are reported in Table 1 and Supplementary Table 1 reports the corresponding summary for studies of airflow.
Table 1.
Methods and instrumentation adopted to investigate particles emitted from human respiratory activities, and the main findings from the studies
| Year | Methods and instrumentation | Participants | Quantity measured | Particle diameter measurement range (µm) | Main findings | Ref. |
|---|---|---|---|---|---|---|
| 1945 | Bacteria applied to mucous membranes of the throat and nose; emitted particles deposited either on a bacterial growth medium or a glass slide, counted by microscope | 5 | Number of exhaled particles | >20 | 0 particles found from normal mouth breathing; counting loudly resulted in 4–14 times higher particle counts than softly counting; cough results depended on cough performance | 163 |
| 1967 | Mouth swabbed with dye (thus the origin of counted particles was the mouth). Particles settled on paper slips in a box over 30 min were counted | 3 | Size distribution | >1 | Number of particles emitted during coughing is highly variable; particle generation and emission depends on several factors including the amount of secretion; movement of lips, tongue and teeth | 60 |
| 1997 | Several respiratory activities were studied (nose breathing, mouth breathing, coughing and speaking) using real- time analysis by OPC and analysis of dried droplet residues by electron microscopy | 5 | Particle number concentrations | <1 and >1 | Results according to the OPC method showed a prevalent number of particles in the submicrometre range both for mouth breathing and coughing. Conversely, from electron microscopy the size distribution was more heavily weighted towards larger particles. According to the authors, the evaporation and/or losses of large particles in the experimental apparatus may have produced an underestimation in the measure of the original droplet size through the OPC method | 54 |
| 2009 | Participants placed heads in wind tunnel, particles measured using APS | 15 healthy volunteers, age <35 y | Particle number concentration | 0.5–20 | Mouth breathing: 98 particles l–1; unmodulated whisper (speaking): 672 particles l–1; unmodulated vocalization (loudly speaking): 1,088 particles l–1; whispered counting: 100 particles l–1; voiced counting: 130 particles l–1; coughing: 678 particles l–1. Error bars range from 15% to 60% | 56 |
| 2009 | Particle size measured with IMI; air velocity measured by PIV close to mouth during coughing and speaking (loudly counting) | 11 healthy volunteers, age <30 y | Particle size; air velocity | >1 | Measurement of wide size range (2–2,000 μm) with the same measuring system near the point of emission, when the effect of evaporation/condensation was still negligible. Size measurements at 10 mm from the mouth negligibly influenced by evaporation and condensation and can be considered as representative of the ‘original’ emitted size profile | 58 |
| 2009 | Number and size of respiratory droplets produced from the mouth of healthy individuals during talking and coughing, with and without a food dye, were measured using glass slides and a microscope, and an aerosol spectrometer | 25 healthy volunteers | Size distribution and particle number concentration | >1 | Mean size of droplets captured using glass slides and microscope was ~50–100 µm | 164 |
| 2011 | Results from APS and DDA were integrated into a single composite size distribution | 15 healthy volunteers, age <35 y | Size distribution | 0.7–1,000 | The most prominent modes in particle number distribution were identified and linked to distinct sites of origin and mechanisms of generation: one deep in the lower respiratory tract, another in the region of the larynx and a third in the upper respiratory tract including oral cavity | 5 |
| 2012 | Laser diffraction system; participants asked to give best effort to reproduce a ‘real cough’ | 45 healthy non- smokers | Size distribution and particle number concentration | 0.5–20 | Emitted particles 0.1–900 μm. 97% of total number of measured particles had diameter <1 μm. The particle number distribution was not statistically influenced by age, gender, weight, height or corporal mass | 21 |
| 2019 | Emission measured using APS during speaking and breathing | 48 healthy volunteers | Rate of particle emission | 0.5–20 | The rate of particle emission during normal human speech is positively correlated with the loudness (amplitude) of vocalization | 44 |
| 2020 | Real-time visualization of particle emissions speech was conducted with laser light scattering method | – | Airborne lifetime | – | At least 1,000 droplet nuclei that remain airborne for >8 min were estimated for 1 min of loud speaking | 17 |
APS, aerodynamic particle sizer; DDA, droplet deposition analysis; IMI, interferometric Mie imaging; OPC, optical particle counter; PIV, particle image velocimetry.
There has been a variety of instrumentation used in studies of particle size distributions, including a laser diffraction system21, optical particle counter29,42,54, scanning mobility droplet sizer55, aerodynamic particle sizer43,44,55,56, electrical low pressure impactor57, interferometric Mie imaging58, PIV58, laser diffraction system29 and laser light scattering system17.
As a final note, the problem of characterizing particles generated in the respiratory tract is further complicated by the fact that the size distribution of particles at the site of generation within the body is undoubtedly different from the distribution at the moment of emission into the environment, owing to processes such as coalescence, among others. However, the evolution of measurement techniques has also made it possible to get closer and closer to the point of emission (that is, the human face), to reduce as much as possible the effect of evaporation of the water content of the particles before they reach the point of sampling. For instance, PIV and an interferometric Mie imaging (IMI) technique have been used to measure the respiratory air-jet velocities and the size profiles of respiratory particles during speaking and coughing in close proximity (10 mm) to the mouth58, by reducing the effects related to evaporation and condensation.
Size distributions and quantities of particles
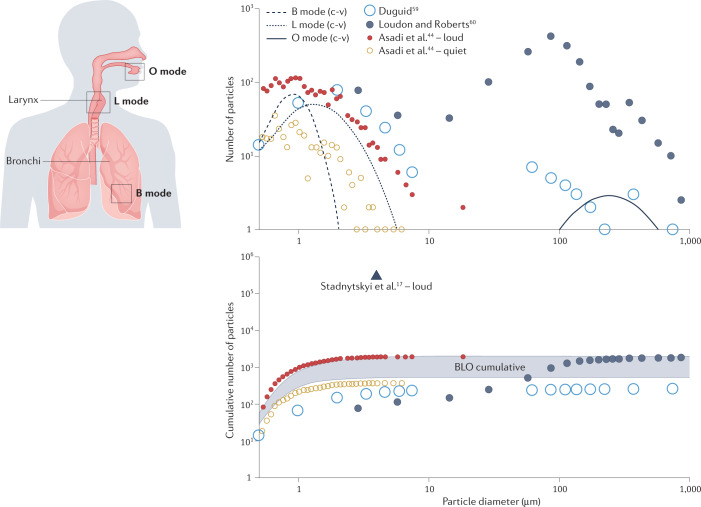
Increasingly accurate measurement techniques have yielded evidence of a trimodal distribution of particles emitted by speaking subjects: the B mode from particles generated in small airway bronchioles during breathing, the L mode from particles generated in the larynx, and the O mode from particles generated in the mouth5. Figure 2 presents graphs updated from a previous comparison, including also a dataset on varying amplitude. One study is the uncorrected BLO individual modes integrated from ref.5 for 2-minute intervals of speaking (c-v), and the cumulative totals for all three c-v modes and for 2 minutes of intermittent, sustained vocalization (aah-v). As described in ref.5, ‘c-v’ represents speech (alternately 10 s of voiced counting and 10 s of naturally paced breathing), while ‘aah-v’ represents sustained vocalization (alternately 10 s of unmodulated vocalization [voiced ‘aah’] and 10 s of naturally paced breathing). A second dataset is the cumulative emission reported in ref.17 which represents the reported rate of 2,600 ~4 µm diameter particles per second extrapolated to 2 minutes of speaking. A third dataset is Table 3 of ref.59, which reports particles emitted from counting loudly to 100. We adjusted the data by dividing the droplet diameter by 6 for measurements below 50 μm to eliminate Duguid’s correction for evaporation and reflect the observation that such particles would have been ~2/3 of their diameter if not for the Congo red dye. A fourth dataset is Table 2 of ref.60, which also reports counting to 100, reflecting the average distribution from the measurements obtained from three subjects (two experiments for each). Finally, we digitized measurements from Figure 3d of ref.44 to depict a representative particle size distribution for one individual speaking for 2 minutes at different amplitudes.
Fig. 2. A trimodal distribution of particles emitted by speaking subjects, and their cumulative emissions.
BLO data (where B represents particles from bronchioles, L larynx and O mouth) are from ref.5. Other data are from refs.44,59,60. The BLO cumulative shaded range spans the c-v to aah-v particle totals from all three modes for uncorrected data (where c-v represents speech and aah-v represents sustained vocalization; see text for details).
Below 10 µm particle diameters, there is remarkable agreement in terms of both size distribution and cumulative quantity between different measurements of the B and L modes from ref.44, the adjusted data from ref.59 and the uncorrected data from ref.5. Specifically, the c-v total is consistent with speaking quietly, whereas the aah-v total is consistent with speaking loudly44.
Although the dataset from ref.60 has been considered an outlier for the substantially larger quantity of O mode particles measured above 10 µm, it is more in line with the recent data of ref.17, which quantified emission rates 2–3 orders of magnitude higher than indicated by prior studies. The substantial increase in particle counts found by laser light scattering is similar to the differences in measured cough emissions between studies21,61 Thus, although there is agreement between the BLO model and more recent particle counter studies, laser light scattering results indicate that the BLO model number and mass concentrations may be inadequate. Considering that the volumetric particle emission rate is an essential component of modelling for airborne transmission risk assessment62, the continued advancement of laser light scattering or diffraction measurement techniques should be seen as a priority.
Particle fates
Once emitted, the fate of the particles depends on complex and interconnected effects of inertia, gravity and evaporation24,63. For isolated respiratory particles (also known as droplets), a critical size of approximately 100 μm was introduced in the 1930s63. Larger particles settle faster than they evaporate by depositing onto close surfaces, whereas smaller particles evaporate faster than they settle and, being small and light, can stay airborne and can be inhaled or may be transported over long distances. The critical size separating these behaviours (~50–150 μm) depends on many physical parameters such as ambient air velocity, ambient air temperature and, above all, relative humidity64.
This approach based on isolated particles represents the benchmark for public health agency guidelines65,66 and is the basis for more recent research67. However, it does not consider the role of the warm and moist air of the turbulent gas puff within which the particle is exhaled and which remains coherent for a short time68–71. The fluid motion of the exhaled jet, supported by the injection of fluid momentum and buoyancy through the orifice (mouth or nose) into the surrounding environment, gradually evolves along its trajectory. It increases its volume for each subsequent respiratory activity with velocity ranging from <1 m s–1 (breathing) to tens of metres per second (sneezing)12. Isothermal jets of the same temperature as the surrounding environment follow a rectilinear trajectory, whereas non-isothermal jets follow a curved trajectory, with the puff evolving into a turbulent cloud64. The ejected particles remain suspended in the cloud even after the puff has lost its coherence because of the perturbations of drag and mass decrease due to evaporation, but their trajectories become dependent on the ambient air currents and turbulence12. However, the fate of larger particles within the jet is different: they move semiballistically with only minimal drag perturbations, and fall quickly down owing to gravity.
The complexity of the composition of the fluid lining airways makes it difficult to accurately estimate transport properties of particles: for example, viscosity of the fluids can be one or two orders of magnitude larger than water, thus reducing the coalescence among the particles12. The puff remains coherent for a longer time and thus greater distance indoors than outdoors. This is because the coherence of the puff is preserved as long as its mean velocity is higher than that of the surrounding air, and outdoor airflow velocity is usually higher than that of indoor air. After the loss of coherence, the cloud is advected by air currents, and the subsequent dynamics is governed by turbulent dispersion67.
As soon as the emitted particles enter the unsaturated air, they begin to evaporate, and their radius contracts over time with a decrease of the water content (except in cold and humid environments), unless the respiratory puff is supersaturated, in which case particles can first experience considerable growth, only later followed by shrinkage72. The rate of mass loss due to evaporation of a particle depends on various physical phenomena, such as the diffusion of the vapour layer away from the surface73–76, or evaporative cooling, in which the high latent heat of evaporation cools the particle surface by decreasing the evaporation rate and the diffusion coefficient77. Other relevant phenomena include Stefan’s flow, that is, induced movement of air away from the particle with increased evaporation rate78; ventilation effects, in which airflow around particles larger than a few tens of micrometres enhances evaporation78,79; and, finally, the presence of non-volatile material (mostly inorganic ions, salt, mucins, proteins, sugars, proteins, lipids, DNA and, potentially, pathogens), which lowers the vapour pressure as determined for an ideal solution through Raoult’s law and the rate of evaporation79,80.
Each particle that remains within the puff evaporates to its stable, smaller, diameter, which depends on the initial amount of non-volatile matter contained within the particle and on the temperature and relative humidity of the air. Historically, this size-stabilized particle has been called the droplet nucleus. The amount of water that remains absorbed within the particle depends on the relative humidity81, even if the relations among the composition, the final size and the influence of the relative humidity are impossible to quantify82. In any case, regardless of whether water evaporates completely leaving only the non-volatile particle content, the important consequence is that the distribution of stable sizes is narrow, on average of the order of 1 µm (ref.12).
An additional complexity that must be considered is that, in reality, particles do not evaporate as if they were independent. Particles dispersed within a room can be considered independent, but this is not the case for particles in a respiratory aerosol jet where the relative humidity remains uniform and close to 100%, leading to reduced evaporation except at the spray boundaries72,83. This high relative humidity makes these particles extremely long-lived, up to 100 times the isolated particle lifetime84–86.
Finally, ventilation-induced airflows play an important role in the fate of particles emitted from respiratory activities in indoor environments: after particles evaporate to their stable size, they can remain suspended in the air for prolonged periods and be transported long distances by indoor airflows87. Therefore, the airflow pattern is the most important factor influencing the spatial concentration of the particles in indoor environments88, but it depends not only on the air distribution system and heat sources but also on the microenvironment around people89. However, this important and complex aspect is outside the scope of this article.
Inhalation of particles
The air that enters the respiratory tract during inhalation contains particles that come from many sources — including combustion sources such as cars and cigarettes, as well as the particles emitted by exhalation — and that vary in size, physicochemical and biological characteristics90. A detailed discussion of the natural and anthropogenic sources and characteristics of particles is outside the scope of this Perspective and can be found, for example, in refs.91–95. A fraction of the particles is deposited in the respiratory tract, and some of them penetrate through the epithelium to the bloodstream and, in turn, to other organs in the body96–98. Because of this, inhalation of airborne particles leads not only to respiratory effects, but many other health impacts, including allergy, effects on the immune system, cancer and effects on reproduction, irritative effects on skin and mucous membranes of eyes, nose and throat, sensory effects on nervous and neurological systems, effects on the cardiovascular system and increased mortality99–103. Airborne particulate matter is considered one of the top ten health risk factors that humans face104 (https://vizhub.healthdata.org/gbd-compare/). In addition, if the particles are pathogens such as viruses or bacteria, or contain pathogens, they can cause infectious diseases, such as common colds, influenza, tuberculosis, COVID-19 and many others105–107.
The severity of the impact due to particles deposited in the respiratory tract depends on the dose received by the exposed persons for specific physicochemical characteristics62,108–110. For a given exposure time, concentration of particles in air and particle size distribution, the dose of particle received is governed by the physics of particle inhalation, including transport and consequent deposition in specific parts of the respiratory tract111,112. Of the particle physical characteristics related to particle deposition in the respiratory tract, the most important are particle numbers, size distribution and particle concentration113,114. Factors that affect transport and deposition of particles in different regions of the respiratory tract during inhalation include the morphometry and thermo-hygrometric conditions of the respiratory tract, breathing patterns and particle characteristics.
Morphometry and thermo-hygrometric conditions of the respiratory system
The functions of the human respiratory system include the supply of oxygen to the alveolar region of the lungs and the exchange of gases (oxygen and carbon dioxide) between the lungs and the bloodstream. To fulfil these tasks, the system has a complex morphometry, as it is made up of a highly efficient airway network from the entry ports (nose or mouth) to the alveoli. A detailed description of the morphometry of the respiratory system is beyond the scope of this Perspective; however, for completeness of this discussion, we summarize its basic components and their roles as reported by the International Commission on Radiological Protection (ICRP)113. According to the ICRP, the human respiratory tract is divided into three main regions: the extrathoracic region (from the nose or the mouth to the entrance of the trachea); the tracheobronchial region (from the trachea to the terminal bronchioles), the role of which is transporting air to and from the alveolar region; and the alveolar region (from respiratory bronchioles to alveoli), the main function of which is to exchange gases.
The airway network is a repeatedly bifurcating three-dimensional asymmetrical network in which small branches of the airways are formed by the division of a larger airway. (The branches are known as generations, with the trachea being generation 0, the mainstream bronchus being generation 1 and the bronchioles being generation 4.) The diameter and length of the airway segment decrease from generation to generation. In addition, the branching angle and inclination change with each bifurcation, making the flow pattern irregular and difficult to model in detail113,115,116. Furthermore, the volume of tracheobronchial and alveolar airways changes substantially during the breathing cycle. In particular, the expansion and contraction that occur during inhalation and exhalation result in different velocity profiles between the two phases of the cycle, allowing for a mixing between the inhaled and reserve air and the consequent migration of particles between them. This effect is known as particle dispersion in the lungs and could partly explain the considerable interpersonal variation in particle deposition fractions115,117. An additional feature of the airways that must be considered in view of understanding the particle deposition process is the characteristic thermo-hygrometric condition: the temperature and relative humidity beyond the first few generations are estimated at approximately 37 °C and 99.5%68,118. This condition allows for substantial growth of inhaled hygroscopic particles and consequently affects their deposition119. Finally, the inner surfaces of the airways are covered by a lining fluid, which acts in part as a protective barrier against foreign particles, but also increases the dissolution of soluble deposited particles. It should be pointed out that the ICRP morphometric model (widely adopted to evaluate particle deposition in the lung) is characteristic of a ‘reference man‘ and uses scale factors based on body height to adjust the dimensions for other subjects, including women and children. Nonetheless, the anatomical variability documented among healthy subjects exceeds what one would assume based on ICRP120–122.
Physical properties of the particles and deposition mechanisms
An accurate prediction of the airflow in the different segments of the respiratory tract is extremely difficult. One reason is that, owing to the size of segments, the flow characteristics vary along their length and the velocity profile is not parabolic, as would be expected for channels115,123,124. Therefore, evaluating the deposition of particles in each airway of the respiratory tract on the basis of analytical equations of airflow is practically impossible. A rough assessment of airflows for a person breathing calmly indicates that turbulent flow typically occurs from the nose and mouth to the trachea, whereas from generation 4 (bronchioles) up to the alveoli the flow is laminar. Between these extremes, from generation 1 to 3 (larger bronchi) the flow is mostly laminar, but turbulent flow may occur because of the instability induced by the larynx and the cartilaginous rings in the trachea115,125.
The properties of the particle that are important in affecting its fate during inhalation and deposition are size (expressed as equivalent diameter), density and shape; these factors influence the aerodynamic and diffusive behaviour of the particles and therefore their transport and deposition. Regarding inhalation, experimental studies carried out considering different orientations of the nose, mouth and head with respect to the airflow showed an inhalability of 100% for particles of a few micrometres and smaller, decreasing to ~50% at 50 µm (ref.113). Regarding deposition, increasing particle sizes and densities increase the inertial forces acting on the particles114,125 and consequently the deposition rate of supermicrometric particles. Because of the abovementioned hygrometric conditions in the lungs, the diameter of particles can more than double after inhalation into the lung68,119.
Measuring deposition
The potential for particles to cause disease depends on the region in which they are deposited. For instance, the deposition of particles in tracheobronchial and alveolar regions is more critical than in the extrathoracic region. However, direct and experimental knowledge of deposition is mainly available only for total deposition in the whole respiratory tract.
Measuring the total deposition of submicrometre particles is not an easy task. The total deposition fraction of submicrometre particles is usually measured by comparing the particle size distributions of air inhaled and exhaled by human volunteers; typically, inhaled and exhaled air are stored in separate chambers in which particle size distribution and total concentration measurements are made continuously using mobility particle sizers. Although only a few studies have made measurements of total deposition of submicrometre particles, these studies have been carried out for different types of aerosols (ambient and combustion aerosols, and aerosol produced by generation systems designed for this purpose), different population groups (adults and children, males and females), different breathing patterns (residence time, tidal volume, breathing frequency), and different groups with respiratory disease125–145.
Measurements of total supermicrometre particle deposition fractions date back to the 1950s. For this range of particles, different methods were applied, including inhalation of particles labelled with a gamma-emitting radionuclide, and comparisons between the concentrations of inhaled and exhaled particles (similarly to those described for submicrometre particles), measured by aerodynamic particle sizers or photometers. The deposition experiments were also carried out for different types of aerosols and different breathing patterns130,134,136,141–145.
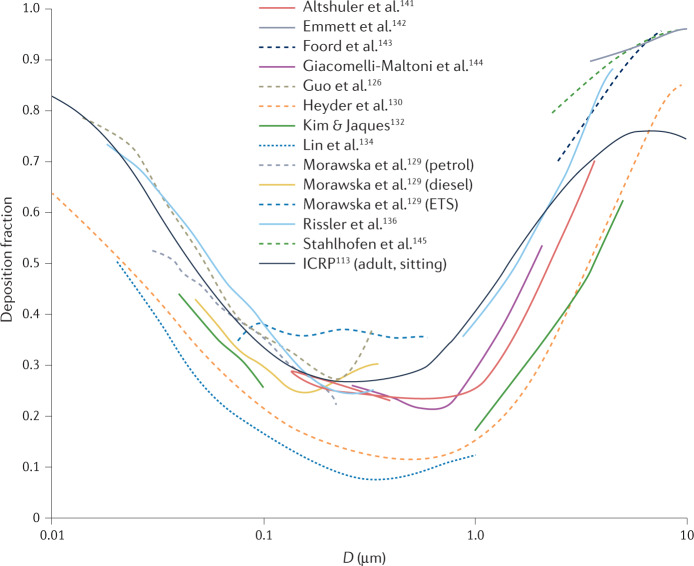
In adults, the total deposition is higher for ultrafine particles (<100 nm), exceeding 50% for diameters <50 nm, as well as for supermicrometre particles, whereas a minimum deposition fraction is seen in the range 100 nm to 1 µm (Fig. 3). The high deposition fractions of ultrafine particles are due to diffusion; the high deposition fractions of supermicrometre particles are due to sedimentation and impaction. Between these two size ranges, diffusion and inertia are less effective.
Fig. 3. Total deposition fraction for healthy adults.
The deposition fraction is shown as a function of the particle size D obtained from reported experimental studies113,126,129,130,132,134,136,141–145 and calculated from the ICRP113 as average values between males and females while sitting (black solid line). ETS, environmental tobacco smoke. The studies are listed in Supplementary Table 2.
Substantial differences between subjects were reported within the studies, and although mean deposition fractions are reported for each study, substantial differences also exist between the studies. In general, such differences are due to the different objectives of the studies, leading to differences in experimental systems and methods used, with all these factors potentially affecting the results, as summarized in a critical review of nanoparticle lung deposition measurement techniques146. In fact, the results are strongly affected by the aerosol source (which affects monodispersity and electrical charge, among other factors), the inhalation system (which affects factors such as particle losses, leaks and breathing patterns), and the particle detector (which affects efficiency and response time, for example). Furthermore, owing to the complexity of the experimental campaigns, the total number of volunteers involved in these studies remains limited, so interpersonal differences, due to the variability of the morphological and breathing pattern of human lungs, also play an important role. As an example, studies imposing different programmed breathing patterns132,136,144,147–151 highlighted that longer residence times and higher tidal volumes increase the efficiency of the deposition, because they enhance the role of diffusion and sedimentation of the particles.
Unlike total deposition, regional deposition fractions cannot be measured directly, so their assessments are less accurate. They are obtained by indirect methods, such by conducting radiolabelled aerosol retention measurements, computed tomography or magnetic resonance imaging scans, and gamma scintigraphy either using hollow cast techniques or human volunteers138,152–159. However, these methods cannot adequately reproduce the complexity of the peripheral airways113,160. Therefore, to estimate regional contributions, measurements have been combined with particle deposition modelling. Detailed reviews of existing models can be found elsewhere125,161.
Outlook
There is an urgent need to broaden our understanding of the physics behind breathing, one of our most fundamental physiological functions. The benefits could include better assessment of respiratory health, more precise delivery of pharmaceutical drugs, and the understanding and potential reduction of respiratory disease transmission. Our understanding of what remains to be done can be summarized as follows.
First, it is critical that new methods and technologies are developed to measure particle formation in situ in the respiratory tract. Although the theoretical understanding of the physics involved in particle generation continues to improve, including through numerical modelling, the relevant rheological properties cannot yet be measured directly in the respiratory tract of a living human being, nor can the quantity and size distribution of generated particles. Advances in nanotechnology may provide a pathway to conduct such measurements, in the form of nanobots capable of collecting and reporting relevant information from inside the respiratory tract.
Second, a much better understanding is needed of the dynamics of the initial moments of the respiratory plume, based on experimental studies and focusing on multiphase jet dynamics. This lack of knowledge has important consequences because the first seconds of the respiratory plume are critical for the airborne transmission of respiratory pathogens, particularly for people in close proximity.
Third, the dynamics of the particles in the lungs is far from being sufficiently understood. The lack of such knowledge has far-reaching consequences, one of them being that the dose–response relationships used to evaluate the risk of infection from exposure to virus-laden particles typically do not explicitly include the deposition fraction, owing to its uncertainty162. Focused efforts are needed experimentally, theoretically and computationally to provide a holistic approach to the physics that drives the elements of the process.
Supplementary information
Author contributions
All authors contributed to the writing of this manuscript.
Peer review
Peer review information
Nature Reviews Physics thanks the anonymous referees for their contributions to the peer review of this work.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s42254-022-00506-7.
References
- 1.Morawska L, Buonanno G. The physics of particle formation and deposition during breathing. Nat. Rev. Phys. 2021;3:300–301. doi: 10.1038/s42254-021-00307-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wei J, Li Y. Airborne spread of infectious agents in the indoor environment. Am. J. Infect. Control. 2016;44:S102–S108. doi: 10.1016/j.ajic.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rayleigh L. On the stability, or instability, of certain fluid motions. Proc. Lond. Math. Soc. 1879;1:57–72. doi: 10.1112/plms/s1-11.1.57. [DOI] [Google Scholar]
- 4.Stadnytskyi V, Anfinrud P, Bax A. Breathing, speaking, coughing or sneezing: what drives transmission of SARS-CoV-2? J. Intern. Med. 2021;290:1010–1027. doi: 10.1111/joim.13326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson G, et al. Modality of human expired aerosol size distributions. J. Aerosol Sci. 2011;42:839–851. doi: 10.1016/j.jaerosci.2011.07.009. [DOI] [Google Scholar]
- 6.Abkarian M, Stone H. Stretching and break-up of saliva filaments during speech: a route for pathogen aerosolization and its potential mitigation. Phys. Rev. Fluids. 2020;5:102301. doi: 10.1103/PhysRevFluids.5.102301. [DOI] [Google Scholar]
- 7.Bake B, Larsson P, Ljungkvist G, Ljungström E, Olin A. Exhaled particles and small airways. Respir. Res. 2019;20:8. doi: 10.1186/s12931-019-0970-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Almstrand A-C, et al. Effect of airway opening on production of exhaled particles. J. Appl. Physiol. 2010;108:584–588. doi: 10.1152/japplphysiol.00873.2009. [DOI] [PubMed] [Google Scholar]
- 9.Fahy JV, Dickey BF. Airway mucus function and dysfunction. N. Engl. J. Med. 2010;363:2233–2247. doi: 10.1056/NEJMra0910061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patterson B, Wood R. Is cough really necessary for TB transmission? Tuberculosis. 2019;117:31–35. doi: 10.1016/j.tube.2019.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson GR, Morawska L. The mechanism of breath aerosol formation. J. Aerosol Med. Pulm. Drug Deliv. 2009;22:229–237. doi: 10.1089/jamp.2008.0720. [DOI] [PubMed] [Google Scholar]
- 12.Balachandar S, Zaleski S, Soldati A, Ahmadi G, Bourouiba L. Host-to-host airborne transmission as a multiphase flow problem for science-based social distance guidelines. Int. J. Multiph. Flow. 2020;132:103439. doi: 10.1016/j.ijmultiphaseflow.2020.103439. [DOI] [Google Scholar]
- 13.Collins L, Dawes C. The surface area of the adult human mouth and thickness of the salivary film covering the teeth and oral mucosa. J. Dent. Res. 1987;66:1300–1302. doi: 10.1177/00220345870660080201. [DOI] [PubMed] [Google Scholar]
- 14.Kaufman E, Lamster IB. The diagnostic applications of saliva — a review. Crit. Rev. Oral. Biol. Med. 2002;13:197–212. doi: 10.1177/154411130201300209. [DOI] [PubMed] [Google Scholar]
- 15.Fennelly KP. Particle sizes of infectious aerosols: implications for infection control. Lancet Respir. Med. 2020;8:914–924. doi: 10.1016/S2213-2600(20)30323-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Effros RM, et al. Dilution of respiratory solutes in exhaled condensates. Am. J. Respir. Crit. Care Med. 2002;165:663–669. doi: 10.1164/ajrccm.165.5.2101018. [DOI] [PubMed] [Google Scholar]
- 17.Stadnytskyi V, Bax CE, Bax A, Anfinrud P. The airborne lifetime of small speech droplets and their potential importance in SARS-CoV-2 transmission. Proc. Natl Acad. Sci. USA. 2020;117:11875–11877. doi: 10.1073/pnas.2006874117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anand S, Mayya Y. Size distribution of virus laden droplets from expiratory ejecta of infected subjects. Sci. Rep. 2020;10:21174. doi: 10.1038/s41598-020-78110-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Basu S. Computational characterization of inhaled droplet transport to the nasopharynx. Sci. Rep. 2021;11:6652. doi: 10.1038/s41598-021-85765-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bar-On YM, Flamholz A, Phillips R, Milo R. Science Forum: SARS-CoV-2 (COVID-19) by the numbers. eLife. 2020;9:e57309. doi: 10.7554/eLife.57309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zayas G, et al. Cough aerosol in healthy participants: fundamental knowledge to optimize droplet-spread infectious respiratory disease management. BMC Pulm. Med. 2012;12:11. doi: 10.1186/1471-2466-12-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lindsley WG, et al. Viable influenza A virus in airborne particles expelled during coughs versus exhalations. Influenza Other Respir. Viruses. 2016;10:404–413. doi: 10.1111/irv.12390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Castleman RA. The mechanism of the atomization of liquids. J. Res. Natl Bur. Stand. 1931;7:269–376. [Google Scholar]
- 24.Wells, W. F. Airborne Contagion and Air Hygiene: An Ecological Study of Droplet Infections (Harvard Univ. Press, 1955).
- 25.Descamps, M., Matas, J.-P. & Cartellier, A. H. Gas–liquid atomisation: gas phase characteristics by PIV measurements and spatial evolution of the spray. In 2nd colloque INCA, Initiative en Combustion Avancée, 1 (HAL Open Science, 2008); https://hal.archives-ouvertes.fr/hal-00704346.
- 26.Scheubel, F. N. On atomization in carburetors. National Advisory Committee for Aeronautics Technical Memorandum 644 (1927); https://ntrs.nasa.gov/api/citations/19930094772/downloads/19930094772.pdf?attachment=true
- 27.Sauter, J. Untersuchung der von Spritzvergasern gelieten Zerstäubung. Forschungsarbeiten auf dem Gebiete des Ingenieurwesens, Vol. 312 (Springer, 1928).
- 28.Hong M, Cartellier A, Hopfinger EJ. Characterization of phase detection optical probes for the measurement of the dispersed phase parameters in sprays. Int. J. Multiph. Flow. 2004;30:615–648. doi: 10.1016/j.ijmultiphaseflow.2004.04.004. [DOI] [Google Scholar]
- 29.Edwards DA, et al. Inhaling to mitigate exhaled bioaerosols. Proc. Natl Acad. Sci. USA. 2004;101:17383–17388. doi: 10.1073/pnas.0408159101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zayas, G., Dimitry, J., Zayas, A., O’Brien, D. & King, M. A new paradigm in respiratory hygiene: increasing the cohesivity of airway secretions to improve cough interaction and reduce aerosol dispersion. BMC Pulm. Med. 5, 10.1186/1471-2466-5-11 (2005). [DOI] [PMC free article] [PubMed]
- 31.Hasan MA, Lange CF, King ML. Effect of artificial mucus properties on the characteristics of airborne bioaerosol droplets generated during simulated coughing. J. Nonnewton. Fluid Mech. 2010;165:1431–1441. doi: 10.1016/j.jnnfm.2010.07.005. [DOI] [Google Scholar]
- 32.Vasudevan M, Lange CF. Surface tension effects on instability in viscoelastic respiratory fluids. Math. Biosci. 2007;205:180–194. doi: 10.1016/j.mbs.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 33.Lasheras J, Villermaux E, Hopfinger E. Break-up and atomization of a round water jet by a high-speed annular air jet. J. Fluid Mech. 1998;357:351–379. doi: 10.1017/S0022112097008070. [DOI] [Google Scholar]
- 34.Villermaux E. Fragmentation versus cohesion. J. Fluid Mech. 2020;898:P1. doi: 10.1017/jfm.2020.366. [DOI] [Google Scholar]
- 35.Chen Z, et al. Determination of rheology and surface tension of airway surface liquid: a review of clinical relevance and measurement techniques. Respir. Res. 2019;20:274. doi: 10.1186/s12931-019-1229-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brown ES, Johnson RP, Clements JA. Pulmonary surface tension. J. Appl. Physiol. 1959;14:717–720. doi: 10.1152/jappl.1959.14.5.717. [DOI] [PubMed] [Google Scholar]
- 37.Schürch S, Bachofen H, Goerke J, Green F. Surface properties of rat pulmonary surfactant studied with the captive bubble method: adsorption, hysteresis, stability. Biochim. Biophys. Acta. 1992;1103:127–136. doi: 10.1016/0005-2736(92)90066-U. [DOI] [PubMed] [Google Scholar]
- 38.Keshavarz B, et al. Studying the effects of elongational properties on atomization of weakly viscoelastic solutions using Rayleigh Ohnesorge jetting extensional rheometry (ROJER) J. Nonnewton. Fluid Mech. 2015;222:171–189. doi: 10.1016/j.jnnfm.2014.11.004. [DOI] [Google Scholar]
- 39.Watanabe W, et al. Why inhaling salt water changes what we exhale. J. Colloid Interface Sci. 2007;307:71–78. doi: 10.1016/j.jcis.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 40.Keshavarz B, Houze EC, Moore JR, Koerner MR, McKinley GH. Ligament mediated fragmentation of viscoelastic liquids. Phys. Rev. Lett. 2016;117:154502. doi: 10.1103/PhysRevLett.117.154502. [DOI] [PubMed] [Google Scholar]
- 41.Edwards DA, et al. Exhaled aerosol increases with COVID-19 infection, age, and obesity. Proc. Natl Acad. Sci. USA. 2021;118:e2021830118. doi: 10.1073/pnas.2021830118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fairchild C, Stampfer J. Particle concentration in exhaled breath. Am. Ind. Hyg. Assoc. J. 1987;48:948–949. doi: 10.1080/15298668791385868. [DOI] [PubMed] [Google Scholar]
- 43.Asadi S, et al. Effect of voicing and articulation manner on aerosol particle emission during human speech. PLoS ONE. 2020;15:e0227699. doi: 10.1371/journal.pone.0227699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Asadi S, et al. Aerosol emission and superemission during human speech increase with voice loudness. Sci. Rep. 2019;9:2348. doi: 10.1038/s41598-019-38808-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alsved M, et al. Exhaled respiratory particles during singing and talking. Aerosol Sci. Technol. 2020;54:1245–1248. doi: 10.1080/02786826.2020.1812502. [DOI] [Google Scholar]
- 46.Oratis AT, Bush JW, Stone HA, Bird JC. A new wrinkle on liquid sheets: turning the mechanism of viscous bubble collapse upside down. Science. 2020;369:685–688. doi: 10.1126/science.aba0593. [DOI] [PubMed] [Google Scholar]
- 47.Fiegel J, Clarke R, Edwards DA. Airborne infectious disease and the suppression of pulmonary bioaerosols. Drug Discov. Today. 2006;11:51–57. doi: 10.1016/S1359-6446(05)03687-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bake B, et al. Exhaled particles after a standardized breathing maneuver. J. Aerosol Med. Pulm. Drug Deliv. 2017;30:267–273. doi: 10.1089/jamp.2016.1330. [DOI] [PubMed] [Google Scholar]
- 49.Merghani KMM, Sagot B, Gehin E, Da G, Motzkus C. A review on the applied techniques of exhaled airflow and droplets characterization. Indoor Air. 2021;31:7–25. doi: 10.1111/ina.12770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gupta J, Lin CH, Chen Q. Flow dynamics and characterization of a cough. Indoor Air. 2009;19:517–525. doi: 10.1111/j.1600-0668.2009.00619.x. [DOI] [PubMed] [Google Scholar]
- 51.Gupta JK, Lin CH, Chen Q. Characterizing exhaled airflow from breathing and talking. Indoor Air. 2010;20:31–39. doi: 10.1111/j.1600-0668.2009.00623.x. [DOI] [PubMed] [Google Scholar]
- 52.Xu C, Nielsen P, Gong G, Liu L, Jensen R. Measuring the exhaled breath of a manikin and human subjects. Indoor Air. 2015;25:188–197. doi: 10.1111/ina.12129. [DOI] [PubMed] [Google Scholar]
- 53.Xu C, Nielsen PV, Liu L, Jensen RL, Gong G. Human exhalation characterization with the aid of schlieren imaging technique. Build. Environ. 2017;112:190–199. doi: 10.1016/j.buildenv.2016.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Papineni RS, Rosenthal FS. The size distribution of droplets in the exhaled breath of healthy human subjects. J. Aerosol Med. 1997;10:105–116. doi: 10.1089/jam.1997.10.105. [DOI] [PubMed] [Google Scholar]
- 55.Yang S, Lee GW, Chen C-M, Wu C-C, Yu K-P. The size and concentration of droplets generated by coughing in human subjects. J. Aerosol Med. 2007;20:484–494. doi: 10.1089/jam.2007.0610. [DOI] [PubMed] [Google Scholar]
- 56.Morawska L, et al. Size distribution and sites of origin of droplets expelled from the human respiratory tract during expiratory activities. J. Aerosol Sci. 2009;40:256–269. doi: 10.1016/j.jaerosci.2008.11.002. [DOI] [Google Scholar]
- 57.Hersen G, et al. Impact of health on particle size of exhaled respiratory aerosols: case-control study. Clean. 2008;36:572–577. doi: 10.1002/clen.200700189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chao CYH, et al. Characterization of expiration air jets and droplet size distributions immediately at the mouth opening. J. Aerosol Sci. 2009;40:122–133. doi: 10.1016/j.jaerosci.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Duguid J. The size and the duration of air-carriage of respiratory droplets and droplet-nuclei. Epidemiol. Infect. 1946;44:471–479. doi: 10.1017/S0022172400019288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Loudon RG, Roberts RM. Droplet expulsion from the respiratory tract. Am. Rev. Respir. Dis. 1967;95:435–442. doi: 10.1164/arrd.1967.95.3.435. [DOI] [PubMed] [Google Scholar]
- 61.Lindsley WG, et al. Quantity and size distribution of cough-generated aerosol particles produced by influenza patients during and after illness. J. Occup. Environ. Hyg. 2012;9:443–449. doi: 10.1080/15459624.2012.684582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Buonanno G, Morawska L, Stabile L. Quantitative assessment of the risk of airborne transmission of SARS-CoV-2 infection: prospective and retrospective applications. Environ. Int. 2020;145:106112. doi: 10.1016/j.envint.2020.106112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wells WF. On air-borne infection. Study II. Droplet and droplet nuclei. Am. J. Epidemiol. 1934;20:611–618. doi: 10.1093/oxfordjournals.aje.a118097. [DOI] [Google Scholar]
- 64.Xie X, Li Y, Chwang A, Ho P, Seto W. How far droplets can move in indoor environments — revisiting the Wells evaporation–falling curve. Indoor Air. 2007;17:211–225. doi: 10.1111/j.1600-0668.2007.00469.x. [DOI] [PubMed] [Google Scholar]
- 65.Scientific Brief, 7 May 2021: SARS-CoV-2 transmission (CDC, 2021).
- 66.Coronavirus disease (COVID-19): How is it transmitted? https://www.who.int/news-room/q-a-detail/coronavirus-disease-covid-19-how-is-it-transmitted (WHO, 2021).
- 67.Bourouiba L, Dehandschoewercker E, Bush JWM. Violent expiratory events: on coughing and sneezing. J. Fluid Mech. 2014;745:537–563. doi: 10.1017/jfm.2014.88. [DOI] [Google Scholar]
- 68.Asgharian B. A model of deposition of hygroscopic particles in the human lung. Aerosol Sci. Technol. 2004;38:938–947. doi: 10.1080/027868290511236. [DOI] [Google Scholar]
- 69.Baturin, V. V. Fundamentals of Industrial Ventilation (Pergamon, 1972).
- 70.Rajaratnam, N. Turbulent Jets (Elsevier, 1976).
- 71.Turner, J. S. Buoyancy Effects in Fluids (Cambridge Univ. Press, 1979).
- 72.Ng CS, et al. Growth of respiratory droplets in cold and humid air. Phys. Rev. Fluids. 2021;6:054303. doi: 10.1103/PhysRevFluids.6.054303. [DOI] [Google Scholar]
- 73.Bradley R, Evans MG, Whytlaw-Gray R. The rate of evaporation of droplets. evaporation and diffusion coefficients, and vapour pressures of dibutyl phthalate and butyl stearate. Proc. R. Soc. A. 1946;186:368–390. doi: 10.1098/rspa.1946.0050. [DOI] [PubMed] [Google Scholar]
- 74.Langmuir I. The evaporation of small spheres. Phys. Rev. 1918;12:368. doi: 10.1103/PhysRev.12.368. [DOI] [Google Scholar]
- 75.Pirhadi M, Sajadi B, Ahmadi G, Malekian D. Phase change and deposition of inhaled droplets in the human nasal cavity under cyclic inspiratory airflow. J. Aerosol Sci. 2018;118:64–81. doi: 10.1016/j.jaerosci.2018.01.010. [DOI] [Google Scholar]
- 76.Sazhin SS. Advanced models of fuel droplet heating and evaporation. Prog. Energy Combust. Sci. 2006;32:162–214. doi: 10.1016/j.pecs.2005.11.001. [DOI] [Google Scholar]
- 77.Božič A, Kanduč M. Relative humidity in droplet and airborne transmission of disease. J. Biol. Phys. 2021;47:1–29. doi: 10.1007/s10867-020-09562-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kukkonen J, Vesala T, Kulmala M. The interdependence of evaporation and settling for airborne freely falling droplets. J. Aerosol Sci. 1989;20:749–763. doi: 10.1016/0021-8502(89)90087-6. [DOI] [Google Scholar]
- 79.Netz RR. Mechanisms of airborne infection via evaporating and sedimenting droplets produced by speaking. J. Phys. Chem. B. 2020;124:7093–7101. doi: 10.1021/acs.jpcb.0c05229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Netz RR, Eaton WA. Physics of virus transmission by speaking droplets. Proc. Natl Acad. Sci. USA. 2020;117:25209–25211. doi: 10.1073/pnas.2011889117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nicas M, Nazaroff WW, Hubbard A. Toward understanding the risk of secondary airborne infection: emission of respirable pathogens. J. Occup. Environ. Hyg. 2005;2:143–154. doi: 10.1080/15459620590918466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu L, Wei J, Li Y, Ooi A. Evaporation and dispersion of respiratory droplets from coughing. Indoor Air. 2017;27:179–190. doi: 10.1111/ina.12297. [DOI] [PubMed] [Google Scholar]
- 83.Villermaux E, Moutte A, Amielh M, Meunier P. Fine structure of the vapor field in evaporating dense sprays. Phys. Rev. Fluids. 2017;2:074501. doi: 10.1103/PhysRevFluids.2.074501. [DOI] [Google Scholar]
- 84.de Oliveira PM, Mesquita LC, Gkantonas S, Giusti A, Mastorakos E. Evolution of spray and aerosol from respiratory releases: theoretical estimates for insight on viral transmission. Proc. R. Soc. A. 2021;477:20200584. doi: 10.1098/rspa.2020.0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chong KL, et al. Extended lifetime of respiratory droplets in a turbulent vapor puff and its implications on airborne disease transmission. Phys. Rev. Lett. 2021;126:034502. doi: 10.1103/PhysRevLett.126.034502. [DOI] [PubMed] [Google Scholar]
- 86.Smith SH, et al. Aerosol persistence in relation to possible transmission of SARS-CoV-2. Phys. Fluids. 2020;32:107108. doi: 10.1063/5.0027844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ai ZT, Melikov AK. Airborne spread of expiratory droplet nuclei between the occupants of indoor environments: a review. Indoor Air. 2018;28:500–524. doi: 10.1111/ina.12465. [DOI] [PubMed] [Google Scholar]
- 88.Mui KW, Wong LT, Wu C, Lai AC. Numerical modeling of exhaled droplet nuclei dispersion and mixing in indoor environments. J. Hazard. Mater. 2009;167:736–744. doi: 10.1016/j.jhazmat.2009.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nielsen PV, Xu C. Multiple airflow patterns in human microenvironment and the influence on short-distance airborne cross-infection — a review. Indoor Built Environ. 2021 doi: 10.1177/1420326X211048539. [DOI] [Google Scholar]
- 90.Riediker M, et al. Particle toxicology and health — where are we? Particle and fibre. Toxicology. 2019;16:19. doi: 10.1186/s12989-019-0302-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hopke PK, Dai Q, Li L, Feng Y. Global review of recent source apportionments for airborne particulate matter. Sci. Total. Environ. 2020;740:140091. doi: 10.1016/j.scitotenv.2020.140091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kerminen V-M, et al. Atmospheric new particle formation and growth: review of field observations. Environ. Res. Lett. 2018;13:103003. doi: 10.1088/1748-9326/aadf3c. [DOI] [Google Scholar]
- 93.Kittelson BD. Engines and nanoparticles: a review. J. Aerosol Sci. 1998;29:575–588. doi: 10.1016/S0021-8502(97)10037-4. [DOI] [Google Scholar]
- 94.Kumar P, Pirjola L, Ketzel M, Harrison RM. Nanoparticle emissions from 11 non-vehicle exhaust sources–A review. Atmos. Environ. 2013;67:252–277. doi: 10.1016/j.atmosenv.2012.11.011. [DOI] [Google Scholar]
- 95.Querol X, et al. Speciation and origin of PM10 and PM2.5 in selected European cities. Atmos. Environ. 2004;38:6547–6555. doi: 10.1016/j.atmosenv.2004.08.037. [DOI] [Google Scholar]
- 96.Peters A, et al. Translocation and potential neurological effects of fine and ultrafine particles a critical update. Part. Fibre Toxicol. 2006;3:13. doi: 10.1186/1743-8977-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kreyling W, et al. Translocation of ultrafine insoluble iridium particles from lung epithelium to extrapulmonary organs is size dependent but very low. J. Toxicol. Environ. Health A. 2002;65:1513–1530. doi: 10.1080/00984100290071649. [DOI] [PubMed] [Google Scholar]
- 98.Oberdörster G, et al. Translocation of inhaled ultrafine particles to the brain. Inhal. Toxicol. 2004;16:437–445. doi: 10.1080/08958370490439597. [DOI] [PubMed] [Google Scholar]
- 99.Beelen R, et al. Effects of long-term exposure to air pollution on natural-cause mortality: an analysis of 22 European cohorts within the multicentre ESCAPE project. Lancet. 2014;383:785–795. doi: 10.1016/S0140-6736(13)62158-3. [DOI] [PubMed] [Google Scholar]
- 100.Weichenthal S. Selected physiological effects of ultrafine particles in acute cardiovascular morbidity. Environ. Res. 2012;115:26–36. doi: 10.1016/j.envres.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 101.Fu P, Guo X, Cheung FMH, Yung KKL. The association between PM2.5 exposure and neurological disorders: a systematic review and meta-analysis. Sci. Total Environ. 2019;655:1240–1248. doi: 10.1016/j.scitotenv.2018.11.218. [DOI] [PubMed] [Google Scholar]
- 102.Raaschou-Nielsen O, et al. Air pollution and lung cancer incidence in 17 European cohorts: prospective analyses from the European study of cohorts for air pollution effects (ESCAPE) Lancet Oncol. 2013;14:813–822. doi: 10.1016/S1470-2045(13)70279-1. [DOI] [PubMed] [Google Scholar]
- 103.Donaldson K, Stone V, Clouter A, Renwick L, MacNee W. Ultrafine particles. Occup. Environ. Med. 2001;58:211–216. doi: 10.1136/oem.58.3.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Global Burden of Disease (GBD) Visualization Hub. https://vizhub.healthdata.org/gbd-compare/ (GBD, 2020).
- 105.Wölfel R, et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 106.Morawska L, Cao J. Airborne transmission of SARS-CoV-2: the world should face the reality. Environ. Int. 2020;139:105730. doi: 10.1016/j.envint.2020.105730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Van Doremalen N, et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Watanabe T, Bartrand TA, Weir MH, Omura T, Haas CN. Development of a dose-response model for SARS coronavirus. Risk Anal. 2010;30:1129–1138. doi: 10.1111/j.1539-6924.2010.01427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Schmid O, Cassee FR. On the pivotal role of dose for particle toxicology and risk assessment: exposure is a poor surrogate for delivered dose. Part. Fibre Toxicol. 2017;14:52. doi: 10.1186/s12989-017-0233-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Schmid O, et al. Dosimetry and toxicology of inhaled ultrafine particles. Biomarkers. 2009;14:67–73. doi: 10.1080/13547500902965617. [DOI] [PubMed] [Google Scholar]
- 111.Licina D, Tian Y, Nazaroff WW. Inhalation intake fraction of particulate matter from localized indoor emissions. Build. Environ. 2017;123:14–22. doi: 10.1016/j.buildenv.2017.06.037. [DOI] [Google Scholar]
- 112.Morawska L, et al. A paradigm shift to combat indoor respiratory infection. Science. 2021;372:689–691. doi: 10.1126/science.abg2025. [DOI] [PubMed] [Google Scholar]
- 113.Human Respiratory Tract Model for Radiological Protection (ICRP, 1994).
- 114.Tsuda A, Henry FS, Butler JP. Particle transport and deposition: basic physics of particle kinetics. Compr. Physiol. 2011;3:1437–1471. doi: 10.1002/cphy.c100085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang, C.-s. Inhaled Particles (Elsevier Academic, 2005).
- 116.Yeh H-C, Schum G. Models of human lung airways and their application to inhaled particle deposition. Bull. Math. Biol. 1980;42:461–480. doi: 10.1016/S0092-8240(80)80060-7. [DOI] [PubMed] [Google Scholar]
- 117.Altshuler B, Palmes E, Yarmus L, Nelson N. Intrapulmonary mixing of gases studied with aerosols. J. Appl. Physiol. 1959;14:321–327. doi: 10.1152/jappl.1959.14.3.321. [DOI] [PubMed] [Google Scholar]
- 118.Ferron G, Haider B, Kreyling W. Inhalation of salt aerosol particles — I. Estimation of the temperature and relative humidity of the air in the human upper airways. J. Aerosol Sci. 1988;19:343–363. doi: 10.1016/0021-8502(88)90274-1. [DOI] [Google Scholar]
- 119.Vu TV, Delgado-Saborit JM, Harrison RM. A review of hygroscopic growth factors of submicron aerosols from different sources and its implication for calculation of lung deposition efficiency of ambient aerosols. Air Qual. Atmos. Health. 2015;8:429–440. doi: 10.1007/s11869-015-0365-0. [DOI] [Google Scholar]
- 120.Christou S, et al. Anatomical variability in the upper tracheobronchial tree: sex-based differences and implications for personalized inhalation therapies. J. Appl. Physiol. 2021;130:678–707. doi: 10.1152/japplphysiol.00144.2020. [DOI] [PubMed] [Google Scholar]
- 121.Hussain M, Renate W-H, Werner H. Effect of intersubject variability of extrathoracic morphometry, lung airways dimensions and respiratory parameters on particle deposition. J. Thorac. Dis. 2011;3:156. doi: 10.3978/j.issn.2072-1439.2011.04.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Poorbahrami K, Vignon-Clementel IE, Shadden SC, Oakes JM. A whole lung in silico model to estimate age dependent particle dosimetry. Sci. Rep. 2021;11:11180. doi: 10.1038/s41598-021-90509-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Martonen T, Zhang Z, Lessmann R. Fluid dynamics of the human larynx and upper tracheobronchial airways. Aerosol Sci. Technol. 1993;19:133–156. doi: 10.1080/02786829308959627. [DOI] [Google Scholar]
- 124.Banko AJ, Coletti F, Elkins CJ, Eaton JK. Oscillatory flow in the human airways from the mouth through several bronchial generations. Int. J. Heat. Fluid Flow. 2016;61:45–57. doi: 10.1016/j.ijheatfluidflow.2016.04.006. [DOI] [Google Scholar]
- 125.Hofmann W. Modelling inhaled particle deposition in the human lung — a review. J. Aerosol Sci. 2011;42:693–724. doi: 10.1016/j.jaerosci.2011.05.007. [DOI] [Google Scholar]
- 126.Guo LL, Johnson GR, Hofmann W, Wang H, Morawska L. Deposition of ambient ultrafine particles in the respiratory tract of children: a novel experimental method and its application. J. Aerosol Sci. 2020;139:105465. doi: 10.1016/j.jaerosci.2019.105465. [DOI] [Google Scholar]
- 127.Guo L, et al. Experimentally determined deposition of ambient urban ultrafine particles in the respiratory tract of children. Environ. Int. 2020;145:106094. doi: 10.1016/j.envint.2020.106094. [DOI] [PubMed] [Google Scholar]
- 128.Londahl J, et al. A set-up for field studies of respiratory tract deposition of fine and ultrafine particles in humans. J. Aerosol Sci. 2006;37:1152–1163. doi: 10.1016/j.jaerosci.2005.11.004. [DOI] [Google Scholar]
- 129.Morawska L, Hofmann W, Hitchins-Loveday J, Swanson C, Mengersen K. Experimental study of the deposition of combustion aerosols in the human respiratory tract. J. Aerosol Sci. 2005;36:939–957. doi: 10.1016/j.jaerosci.2005.03.015. [DOI] [Google Scholar]
- 130.Heyder J, Gebhart J, Rudolf G, Schiller CF, Stahlhofen W. Deposition of particles in the human respiratory tract in the size range 0.005–15 μm. J. Aerosol Sci. 1986;17:811–825. doi: 10.1016/0021-8502(86)90035-2. [DOI] [Google Scholar]
- 131.Jaques PA, Kim CS. Measurement of total lung deposition of inhaled ultrafine particles in healthy men and women. Inhal. Toxicol. 2000;12:715–731. doi: 10.1080/08958370050085156. [DOI] [PubMed] [Google Scholar]
- 132.Kim CS, Jaques PA. Analysis of total respiratory deposition of inhaled ultrafine particles in adult subjects at various breathing patterns. Aerosol Sci. Technol. 2004;38:525–540. doi: 10.1080/02786820490465513. [DOI] [Google Scholar]
- 133.Kim CS, Jaques PA. Total lung deposition of ultrafine particles in elderly subjects during controlled breathing. Inhal. Toxicol. 2005;17:387–399. doi: 10.1080/08958370590929493. [DOI] [PubMed] [Google Scholar]
- 134.Lin C-W, et al. Experimental measurements of regional lung deposition in Taiwanese. Aerosol Air Qual. Res. 2019;19:832–839. doi: 10.4209/aaqr.2018.06.0213. [DOI] [Google Scholar]
- 135.Montoya L, et al. Continuous measurements of ambient particle deposition in human subjects. Aerosol Sci. Technol. 2004;38:980–990. doi: 10.1080/027868290519049. [DOI] [Google Scholar]
- 136.Rissler J, et al. Deposition efficiency of inhaled particles (15–5000 nm) related to breathing pattern and lung function: an experimental study in healthy children and adults. Part. Fibre Toxicol. 2017;14:10. doi: 10.1186/s12989-017-0190-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Jakobsson JK, et al. Altered deposition of inhaled nanoparticles in subjects with chronic obstructive pulmonary disease. BMC Pulm. Med. 2018;18:129. doi: 10.1186/s12890-018-0697-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Möller W, et al. Deposition, retention, and translocation of ultrafine particles from the central airways and lung periphery. Am. J. Respir. Crit. Care Med. 2008;177:426–432. doi: 10.1164/rccm.200602-301OC. [DOI] [PubMed] [Google Scholar]
- 139.Sturm R. Total deposition of ultrafine particles in the lungs of healthy men and women: experimental and theoretical results. Ann. Transl. Med. 2016;4:234. doi: 10.21037/atm.2016.06.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Daigle CC, et al. Ultrafine particle deposition in humans during rest and exercise. Inhal. Toxicol. 2003;15:539–552. doi: 10.1080/08958370304468. [DOI] [PubMed] [Google Scholar]
- 141.Altshuler B, Yarmxus L, Palmes E, Nelson K. Aerosol deposition in the human respiratory tract. I. Experimental procedures and total deposition. Arch. Ind. Health. 1957;15:293–303. [PubMed] [Google Scholar]
- 142.Emmett P, Aitken R, Hannan W. Measurements of the total and regional deposition of inhaled particles in the human respiratory tract. J. Aerosol Sci. 1982;13:549–560. doi: 10.1016/0021-8502(82)90020-9. [DOI] [Google Scholar]
- 143.Foord N, Black A, Walsh M. Regional deposition of 2.5–7.5 μm diameter inhaled particles in healthy male non-smokers. J. Aerosol Sci. 1978;9:343–357. doi: 10.1016/0021-8502(78)90037-X. [DOI] [Google Scholar]
- 144.Giacomelli-Maltoni G, Melandri C, Prodis V, Tarroni G. Deposition efficiency of monodisperse particles in human respiratory tract. Am. Ind. Hyg. Assoc. J. 1972;33:603–610. doi: 10.1080/0002889728506713. [DOI] [PubMed] [Google Scholar]
- 145.Stahlhofen W, Gebhart J, Heyder J. Experimental determination of the regional deposition of aerosol particles in the human respiratory tract. Am. Ind. Hyg. Assoc. J. 1980;41:385–398a. doi: 10.1080/15298668091424933. [DOI] [PubMed] [Google Scholar]
- 146.Löndahl J, et al. Measurement techniques for respiratory tract deposition of airborne nanoparticles: a critical review. J. Aerosol Med. Pulm. Drug Deliv. 2014;27:229–254. doi: 10.1089/jamp.2013.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Dautrebande, L., Beckmann, H. & Walkenhorst, W. in Die Staublungenerkrankungen (eds Jötten, K. W. & Klosterkötter, W.) 297–307 (Springer, 1958).
- 148.Heyder J, Armbruster L, Gebhart J, Grein E, Stahlhofen W. Total deposition of aerosol particles in the human respiratory tract for nose and mouth breathing. J. Aerosol Sci. 1975;6:311–328. doi: 10.1016/0021-8502(75)90020-8. [DOI] [Google Scholar]
- 149.Landahl H, Tracewell T, Lassen W. On the retention of air-borne particulates in the human lung: II. Arch. Ind. Hyg. Occup. Med. 1951;3:359–366. [PubMed] [Google Scholar]
- 150.Landahl H, Tuacewell T, Lassen W. Retention of air-borne particulates in the human lung: III. Arch. Ind. Hyg. Occup. Med. 1952;6:508–511. [PubMed] [Google Scholar]
- 151.Morawska L, Barron W, Hitchins J. Experimental deposition of environmental tobacco smoke submicrometer particulate matter in the human respiratory tract. Am. Ind. Hyg. Assoc. J. 1999;60:334–339. doi: 10.1080/00028899908984450. [DOI] [PubMed] [Google Scholar]
- 152.Geiser M, Kreyling WG. Deposition and biokinetics of inhaled nanoparticles. Part. Fibre Toxicol. 2010;7:2. doi: 10.1186/1743-8977-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Cheng Y-S, Zhou Y, Chen BT. Particle deposition in a cast of human oral airways. Aerosol Sci. Technol. 1999;31:286–300. doi: 10.1080/027868299304165. [DOI] [Google Scholar]
- 154.Carvalho TC, Peters JI, Williams RO., III Influence of particle size on regional lung deposition — what evidence is there? Int. J. Pharm. 2011;406:1–10. doi: 10.1016/j.ijpharm.2010.12.040. [DOI] [PubMed] [Google Scholar]
- 155.Zhou Y, Cheng Y-S. Particle deposition in a cast of human tracheobronchial airways. Aerosol Sci. Technol. 2005;39:492–500. doi: 10.1080/027868291001385. [DOI] [Google Scholar]
- 156.Taylor G, et al. Gamma scintigraphic pulmonary deposition study of glycopyrronium/formoterol metered dose inhaler formulated using co-suspension delivery technology. Eur. J. Pharm. Sci. 2018;111:450–457. doi: 10.1016/j.ejps.2017.10.026. [DOI] [PubMed] [Google Scholar]
- 157.Ghazwani A, Biddiscombe M, Usmani O. Comparing lung geometrical areas of interest in gamma scintigraphy as assessment tool for inhaled drug deposition. Eur. Respir. J. 2018;52:PA1022. [Google Scholar]
- 158.Häussermann S, Sommerer K, Scheuch G. Regional lung deposition: in vivo data. J. Aerosol Med. Pulm. Drug Deliv. 2020;33:291–299. doi: 10.1089/jamp.2020.29032.sh. [DOI] [PubMed] [Google Scholar]
- 159.Dubsky S, Fouras A. Imaging regional lung function: a critical tool for developing inhaled antimicrobial therapies. Adv. Drug Deliv. Rev. 2015;85:100–109. doi: 10.1016/j.addr.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 160.Porra L, et al. Quantitative imaging of regional aerosol deposition, lung ventilation and morphology by synchrotron radiation CT. Sci. Rep. 2018;8:3519. doi: 10.1038/s41598-018-20986-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Phalen R, Raabe O. The evolution of inhaled particle dose modeling: a review. J. Aerosol Sci. 2016;99:7–13. doi: 10.1016/j.jaerosci.2015.12.008. [DOI] [Google Scholar]
- 162.Sze To GN, Chao CYH. Review and comparison between the Wells–Riley and dose–response approaches to risk assessment of infectious respiratory diseases. Indoor Air. 2010;20:2–16. doi: 10.1111/j.1600-0668.2009.00621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Duguid J. The numbers and the sites of origin of the droplets expelled during expiratory activities. Edinb. Med. J. 1945;52:385. [PMC free article] [PubMed] [Google Scholar]
- 164.Xie X, Li Y, Sun H, Liu L. Exhaled droplets due to talking and coughing. J. R. Soc. Interface. 2009;6:S703–S714. doi: 10.1098/rsif.2009.0388.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ehrenstein D. How speaking creates droplets that may spread COVID-19. Physics. 2020;13:157. doi: 10.1103/Physics.13.157. [DOI] [Google Scholar]
- 166.Randall K, Ewing ET, Marr L, Jimenez J, Bourouiba L. How did we get here: what are droplets and aerosols and how far do they go? A historical perspective on the transmission of respiratory infectious diseases. Interface Focus. 2021;11:20210049. doi: 10.1098/rsfs.2021.0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kulkarni, P., Baron, P. A. & Willeke, K. Aerosol Measurement: Principles, Techniques, and Applications (Wiley, 2011).
- 168.Milton DK. A Rosetta stone for understanding infectious drops and aerosols. J. Pediatr. Infect. Dis. Soc. 2020;9:413–415. doi: 10.1093/jpids/piaa079. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.