Abstract
Previously, we have shown that the transcription of p35, a lipoprotein gene of Borrelia burgdorferi, is upregulated or initiated during the post-logarithmic bacterial growth phase in vitro. To identify potential regulatory factors, we examined the formation of DNA-protein complexes by electromobility shift assay, using a 157-bp DNA fragment that spans the p35 promoter region and cell-free extracts of spirochetes harvested from both logarithmic and stationary growth phases. The binding properties of the extracts with the promoter region of the flaB gene, a constitutively expressed, growth-phase-independent gene, were also compared. The results from these experiments demonstrate that B. burgdorferi stationary-phase cell-free extracts have a growth-phase-dependent DNA binding protein that interacts specifically with the p35 promoter region. We show, in addition, that a segment from the 157-bp p35 promoter region which contains both a T-rich stretch and an inverted repeat is able to compete off the stationary-phase-specific complex when the segment is present in molar excess.
Borrelia burgdorferi, the spirochete that causes Lyme disease, is able to survive in two very dissimilar host environments: ixodid ticks, which act as its transmission vector, and vertebrates such as rodents, which serve as its most common reservoir host. It has been postulated that B. burgdorferi's adaptation to the tick and mammalian environments likely involves the expression of different surface components (12). In support of this hypothesis, several lipoproteins have been identified whose expression varies depending on the spirochete's milieu (3, 13, 18). Mechanisms of modulation of lipoprotein expression are unknown in B. burgdorferi. An inspection of the B. burgdorferi genome sequence revealed few homologs of other bacterial regulatory proteins, including only two response-regulator two-component systems (4). Only one DNA binding activity which is specific for a region upstream of the ospAB operon has been reported for B. burgdorferi (10). The development of an in vitro model in which regulatory mechanisms of differentially expressed proteins could be easily studied is desirable as a first step toward dissecting B. burgdorferi gene regulation. The observations that B. burgdorferi is able to modulate gene expression in vitro in response to changes in temperature (16, 17) and cell density or growth phase (8, 11) could be further explored to develop such a model. Previously, we demonstrated that the expression of P35, P7.5, OspC, BmpD, and several unidentified B. burgdorferi antigenic proteins is upregulated during post-logarithmic (post-log) spirochetal growth (8, 11). This regulation was shown to occur at the level of transcription for P35, P7.5, OspC, and BmpD (8, 11). P35 was chosen to further investigate this phenomenon. It is possible that the alteration in the rate of transcription or transcriptional initiation is positively or negatively controlled by DNA binding proteins. To identify such potential regulatory factors, we investigated the formation of DNA-protein complexes by electromobility shift assays (EMSA) by using a DNA fragment that encompassed the p35 promoter region and cell-free B. burgdorferi extracts that were harvested from both log and stationary phases.
Low-passage isolates of B. burgdorferi B31 were routinely grown in 1-liter bottles of BSK-H medium (Sigma Chemical Co., St. Louis, Mo.) supplemented with 10% heat-inactivated young rabbit serum (Pel-Freez, Roger, Ark.), 7.5 μg of amphotericin per ml, 48 μg of rifampin per ml, and 192 μg of phosphomycin per ml. Cultures were incubated at 34°C in a trigas incubator set at 3% CO2, 5% O2, and the rest of N2. Spirochetes were counted under a dark-field microscope. Approximately 1010 spirochetes were harvested from log-phase (106 cells/ml) and stationary-phase cultures (108 cells/ml) and used to prepare cell-free extracts for EMSA analysis. Cell-free extracts were prepared as described by Margolis and Samuel (10), and their protein concentrations were determined by the Bradford method (1). EMSA were essentially carried out as described by Chodosh (2). All EMSA presented herein were performed at least twice with the same results. EMSA substrates containing the p35 promoter region were generated by PCR with primer sequences 5′ CGCTCTAGAACTAGTGGATC 3′ and 5′ GTAATTTATATTAATTAATAATTTTAATTACCC 3′ and plasmid DNA from a p35 clone described previously (8). PCRs were performed at an annealing temperature of 45°C for 1 min, an extension temperature of 72°C for 1 min, and a denaturation temperature of 94°C for 1 min for a total of 30 cycles. The DNA fragment that contained the flaB promoter region was generated by PCR as described previously (14). EMSA substrates were digested with appropriate restriction enzymes (New England Biolabs Inc., Beverly, Mass.) and labeled with digoxigenin (DIG)-11-ddUTP and terminal transferase (Boehringer Mannheim, Indianapolis, Ind.) according to the manufacturer's instructions. A typical binding reaction mixture consisted of 23 fmol of EMSA substrate, 2 μg of nonspecific competitor DNA [poly(dI-dC); Sigma], 300 μg of bovine serum albumin per ml (final concentration), 20 mM HEPES (pH 7.9), 40 mM KCl, 1 mM EDTA, 1 mM dithiothreitol, 10% glycerol, and 7 μg of cell-free extract in a final volume of 15 μl. The reaction mixture was incubated for 15 min at 30°C, and the DNA-protein complexes were resolved by electrophoresis through a nondenaturing 6% polyacrylamide gel by using a high-ionic-strength Tris-glycine buffer (250 mM Tris-Cl, 1.9 M glycine, 10 mM EDTA, [pH 8.0]). During competitive binding reactions, cell-free extracts were allowed to preincubate for 5 min at room temperature with the corresponding competitor DNAs added prior to the addition of labeled probe. Double-stranded competitor oligonucleotides were generated by heating a mixture of equal molar amounts of single-stranded complementary oligonucleotides at 95°C in a Perkin-Elmer Cetus 9600 thermal cycler. Double-stranded competitor oligonucleotides were then slowly annealed by allowing the thermal cycler to ramp to 4°C over a 60-min period. Following electrophoresis, resolved components were transferred from the gels to positively charged nylon membranes, probed with an anti-DIG-alkaline phosphatase-labeled antibody, and detected by chemiluminescence as described by the manufacturer (Boehringer). Western blot analysis of B. burgdorferi proteins was performed as described previously (8).
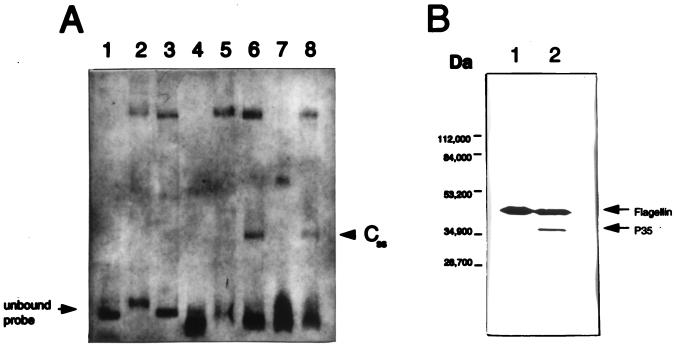
A comparison of the results of the EMSA experiments performed with the p35 promoter region with those of the EMSA experiments with the constitutively expressed, growth-phase-independent flaB promoter region indicates that stationary-phase cell-free extracts contain a DNA binding activity that is specific for the p35 gene. In these experiments, a DIG-labeled 157-bp BamHI-PacI p35 promoter probe (see Fig. 2A) was used as a substrate for protein binding in the EMSA reaction. This fragment includes several potential cis elements which might be involved in DNA-protein interactions, such as a −10/−35 promoter region, a T-rich segment containing an area of dyad symmetry, and a 32-bp AT-rich region. As a negative control for nonspecific binding and growth-phase-independent binding activities, a DIG-labeled 175-bp flaB promoter probe (14) was used as a substrate for protein binding in the EMSA reaction (Fig. 1A, lane 1). The flaB promoter probe in the presence of both log-phase (Fig. 1A, lane 2) and stationary-phase (Fig. 1A, lane 3) cell-free extracts generated a shifted band pattern that consisted predominantly of an intense, slow-migrating complex. The p35 promoter probe (Fig. 1A, lane 4) in the presence of both log-phase (Fig. 1A, lane 5) and stationary-phase (Fig. 1A, lane 6) cell-free extracts generated a similar shifted band pattern as the flaB promoter probe, except for an additional prominent, fast-migrating complex which was contributed only by the stationary-phase cell-free extract. This complex, designated CSS for stationary (-phase)-specific complex, appears to be specific for the p35 promoter region. EMSA extracts were treated with proteinase K to verify that the shifted bands observed were the results of a DNA-protein interaction. In the presence of 80 μg of proteinase K, the shifted band pattern was altered such that CSS and the slow-migrating complex were no longer detectable (Fig. 1A, lane 7). The band observed in lane 7 is believed to be the result of residual binding activity remaining after incomplete proteinase K digestion. To insure that the alteration in banding pattern observed in lane 7 was not due to the interference from the presence of additional protein mass contributed by the proteinase K, the same amount of heat-treated proteinase K (95°C for 10 min) was added to a control EMSA reaction. The result shows that in the presence of heat-treated proteinase K, the stationary-phase complex can be observed (Fig. 1A, lane 8). Western blot analysis of P35 expression was performed with the same log- and stationary-phase cell-free lysates that were used for the EMSA analysis (Fig. 1B, lanes 1 and 2, respectively). The blot was incubated with monoclonal antibodies specific for P35 (8) and flagellin (monoclonal antibody H9724); the latter was included to control for protein mass. The results confirm that the cultured spirochetes used to generate the cell-free extracts were harvested at the appropriate growth phase, i.e., the log-phase cells had not overgrown and begun synthesizing P35. The data from both the EMSA and Western blot analyses suggest that the expression of P35 positively correlates with the appearance of CSS.
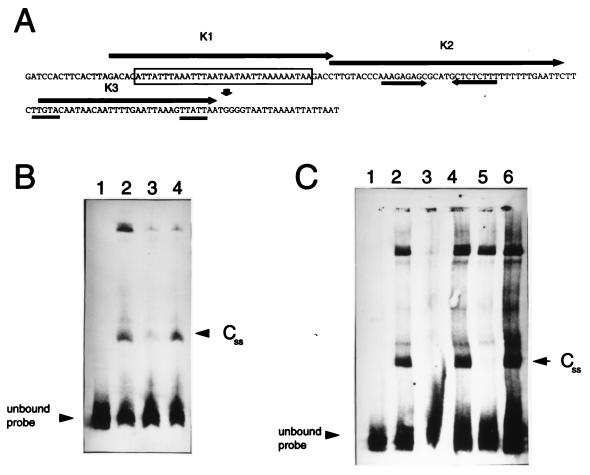
FIG. 2.
(A) Nucleotide sequence of the 157-bp p35 promoter probe used in the EMSA reactions (4). The transcription start site is indicated by the downward pointing arrow. An inverted repeat is indicated by the converging arrows. The putative promoter region is doubly underlined. A 32-nt AT tract is boxed. The arrows designated K1 through K3 encompass each of the oligonucleotides used in the EMSA competition experiments. (B) Competition of DNA binding with the flaB promoter region as competitor. Twenty-three femtomoles of the 157-bp DIG-labeled BamHI-PacI p35 promoter probe was electrophoresed with no cell-free extract added (lane 1) or with 7 μg of extract from spirochetes harvested in stationary phase (lanes 2 to 4), plus a 50 M excess of the unlabeled 157-bp BamHI-PacI p35 probe (lane 3) or a 50 M excess of the unlabeled 175-bp flaB promoter probe (lane 4). (C) Competition of DNA binding with small synthetic double-stranded oligonucleotides. Twenty-three femtomoles of the 157-bp DIG-labeled BamHI-PacI p35 promoter probe was electrophoresed with no cell-free extract added (lane 1) or with 7 μg of extract from spirochetes harvested in stationary phase (lanes 2 to 6), plus a 100 M excess of the unlabeled 157-bp BamHI-PacI probe (lane 3), a 100 M excess of K3 (lane 4), a 100 M excess of K2 (lane 5), or a 100 M excess of K1 (lane 6).
FIG. 1.
(A) EMSA analysis of the B. burgdorferi p35 and flaB promoter regions. Twenty-three femtomoles of the 175-bp DIG-labeled flaB promoter probe was electrophoresed with no cell-free extract added (lane 1) or with 7 μg of extract from spirochetes harvested in log phase (lane 2) or in stationary phase (lane 3). Twenty-three femtomoles of the 157-bp DIG-labeled BamHI-PacI p35 promoter probe was electrophoresed with no cell-free extract added (lane 4), with 7 μg of extract from spirochetes harvested in log phase (lane 5) or in stationary phase (lane 6), or with 7 μg of stationary-phase extract pretreated with 80 μg of proteinase K (lane 7) or heat-inactivated proteinase K (lane 8). (B) Analysis of P35 expression by Western immunoblotting of extracts from cultured spirochetes harvested at log phase (lane 1) and stationary phase (lane 2). The same batches of log- and stationary-phase spirochetes were used to generate cell-free lysates for EMSA analysis. The blot was reacted with a monoclonal antibody specific for P35 (8) and with the flagellin-specific monoclonal antibody H9724. Molecular size markers are indicated on the left.
Additional evidence that CSS is unique to the p35 promoter region was demonstrated by EMSA competition experiments with the flaB promoter sequence as a competitor. The p35 promoter probe, in the presence of 50 M excess cold p35 promoter sequence, is unable to generate the shifted band pattern observed when the competitor is absent from the EMSA reaction (Fig. 2B, lane 3). The uncompeted shifted band pattern is shown in Fig. 2B, lane 2. The p35 promoter probe, in the presence of 50 M excess flaB promoter sequence, is still able to selectively bind CSS (Fig. 2B, lane 4), further indicating that CSS is unique to the p35 promoter region. Lane 1 in Fig. 2B shows the unbound DIG-labeled p35 probe. To further localize the region of DNA sequence responsible for CSS binding, small synthetic double-stranded oligonucleotides reproducing different sections of the p35 promoter region were used as competitors in a series of EMSA experiments (Fig. 2C). The results of these experiments show that a 43-bp segment that contains both a T-rich sequence and an inverted repeat (K2; Fig. 2A) is involved in binding. The K2 fragment (5′ CTTGTACCCAAAGAGAGCGCATGCTCTCTTTTTTTTTGAATTC 3′) was able to selectively outcompete CSS from stationary-phase extracts when the fragment was present in 100 M excess with respect to the labeled probe (Fig. 2C, lane 5). The level of inhibition of CSS binding obtained with K2 is comparable to that observed when a similar molar excess of the 157-bp unlabeled BamHI-PacI p35 promoter probe was used as competitor (Fig. 2C, lane 3). However, the p35 promoter probe inhibited, in addition, the binding of the top band. The inability of K2 to inhibit the top, slower-migrating nonspecific complex further indicates that the formation of CSS is the result of a sequence-specific interaction. The −35/−10 region (K3, 5′ TGTACAATAACAATTTTGAATTAAAGTTATTAA 3′) and the sequence containing an AT-rich region (K1, 5′ GACACATTATTTAAATTTAATAATAATTAAAAAATAAGACC 3′) are not independently involved in the formation of DNA-protein complexes, as evidenced by the probes' failure to effect competition when present in 100 M excess (Fig. 2C, lanes 4 [K3] and 6 [K1]).
In the present study, a growth-phase-dependent DNA binding protein was detected by EMSA by using the p35 promoter region as a binding substrate. Stationary-phase, but not log-phase, B. burgdorferi B31 cell-free extracts contain a DNA binding protein that interacts specifically with the p35 promoter region. This binding activity was selectively inhibited by the presence of a 100 M excess of a 43-bp fragment (K2) which contained two segments that could be incriminated in regulatory activities: a T-rich section and an inverted repeat (Fig. 2A). The K2 fragment is located between positions −39 and −81 relative to the p35 transcriptional start site. The location of the putative protein binding DNA sequence with respect to the p35 −10/−35 promoter region (6) and the positive correlation between induction of p35 expression and detection of CSS binding suggest that the protein(s) interacting with this sequence may activate transcription. The −35 promoter region of the p35 gene is poorly defined, and protein binding near this region could facilitate enhanced RNA polymerase promoter binding. Such a mechanism may require direct contact of the activator with RNA polymerase and/or may require additional protein-protein interactions. Transcriptional activation is a plausible mechanism whereby B. burgdorferi could control gene expression. Thus far, only one other DNA-binding activity has been reported for B. burgdorferi (10).
The identity of the protein(s) that binds to the upstream promoter region of p35 cannot be inferred from our results. However, a T-rich region similar to that described herein was recently identified by Sohaskey et al. as a possible regulatory element in B. burgdorferi (15). Through promoter deletion analyses, these authors identified a T-rich region, located upstream of the ospA gene's −35 promoter region, which positively influenced ospA transcription (15). Presence of this T-rich region may be necessary but is not sufficient for growth-phase-dependent regulation, for ospA is not regulated by growth phase in vitro (11). On the other hand, it is possible that the inverted repeat that is also present on the K2 fragment is, alone or in concert with the adjacent T-rich segment, responsible for the observed DNA binding and the correlated upregulation. Of the three additional genes which are regulated in a growth-phase-dependent fashion whose DNA sequences are known, namely p7.5, bmpD, and ospC, the first two exhibit appropriately located regulatory cis elements, as the one mentioned herein (Indest and Philipp, unpublished). p7.5 and bmpD both contain T-rich sequences upstream from their −35 promoter regions, and bmpD, but not p7.5, also has an inverted repeat in this region. On the other hand, these elements are not easily identifiable in the promoter region of the ospC gene. It is possible that other factors or mechanisms other than the one we suggest here are involved in growth-phase-dependent gene regulation. It is perhaps significant that the 157-bp p35 promoter region contains a portion of a putative integration host factor (IHF) binding site (AAatATAAGACCTTG; consensus IHF binding site, WATCAA-N4-TTR) (7). The latter is located 79 bp upstream from the transcription start site of p35. IHF is a multifunctional DNA binding and bending protein which is found in bacteria and which can mediate protein-protein and DNA-protein interactions. IHF has been shown to positively modulate gene expression in the context of multiple regulatory factors (5, 9). The mechanism by which IHF modulates gene expression is poorly understood. It is assumed that this protein acts as a molecular scaffold of sorts, configuring the DNA in a manner that facilitates the assembly of other protein-DNA complexes (5). AT-rich elements are associated with IHF binding sites and are thought to increase binding of IHF to its target sequence (7). Recently, a gene was isolated from B. burgdorferi which encodes a protein that is homologous to members of the IHF/histone utilization family of E. coli proteins (19).
Currently, we are trying to further characterize the DNA binding activity identified herein. Attempts at cloning this DNA binding activity by screening B. burgdorferi genomic DNA expression libraries with appropriate DNA probes have been unsuccessful. The possibility that this protein(s) may bind to DNA upon acquiring a particular quaternary structure has compelled us to employ DNA-affinity chromatography methods for purification. It is our goal to specifically dissect the mechanism by which the p35 gene is regulated and thus hopefully gain an understanding of how B. burgdorferi regulates gene expression as it shuttles through diverse environments.
Acknowledgments
This work was supported by a National Research Service Award (F32 AI09980-01) to K.J.I. and grant RR00164 (NCRR-NIH).
The photographic skill of Murphy Dowouis is acknowledged with thanks.
REFERENCES
- 1.Bradford M M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 2.Chodosh L A. Mobility shift DNA-binding assay using gel electrophoresis. In: Ausubel F M, et al., editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1994. pp. 12.2.1–12.2.7. [Google Scholar]
- 3.Das S, Barthold S W, Giles S S, Montgomery R R, Telford III S R, Fikrig E. Temporal pattern of Borrelia burgdorferi p21 expression in ticks and the mammalian host. J Clin Investig. 1997;99:987–995. doi: 10.1172/JCI119264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fraser C M, Casjens S, Huang W M, Sutton G G, Clayton R, Lathigra R, White O, Ketchum K A, Dodson R, Hickey E K, et al. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature. 1997;390:580–586. doi: 10.1038/37551. [DOI] [PubMed] [Google Scholar]
- 5.Freundlich M, Ramani N, Mathew E, Sirko A, Tsui P. The role of integration host factor in gene expression in Escherichia coli. Mol Microbiol. 1992;6:2557–2563. doi: 10.1111/j.1365-2958.1992.tb01432.x. [DOI] [PubMed] [Google Scholar]
- 6.Gralla J D, Collado-Vides J. Organization and function of transcription regulatory elements. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. Washington, D.C.: ASM Press; 1996. pp. 1232–1245. [Google Scholar]
- 7.Hales L M, Gumport R I, Gardner J F. Determining the DNA sequence elements required for binding integration host factor to two different target sites. J Bacteriol. 1994;176:2999–3006. doi: 10.1128/jb.176.10.2999-3006.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Indest K J, Ramamoorthy R, Sole M, Gilmore R D, Johnson B J, Philipp M T. Cell-density-dependent expression of Borrelia burgdorferi lipoproteins in vitro. Infect Immun. 1997;65:1165–1171. doi: 10.1128/iai.65.4.1165-1171.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lange R, Barth M, Hengge-Aronis R. Complex transcriptional control of the sigma s-dependent stationary-phase-induced and osmotically regulated osmY (csi-5) gene suggests novel roles for Lrp, cyclic AMP (cAMP) receptor protein-cAMP complex, and integration host factor in the stationary-phase response of Escherichia coli. J Bacteriol. 1993;175:7910–7917. doi: 10.1128/jb.175.24.7910-7917.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Margolis N, Samuel D S. Proteins binding to the promoter of the operon encoding the major surface proteins OspA and OspB of Borrelia burgdorferi. Mol Biol Rep. 1995;21:159–164. doi: 10.1007/BF00997238. [DOI] [PubMed] [Google Scholar]
- 11.Ramamoorthy R, Philipp M T. Differential expression of Borrelia burgdorferi proteins during growth in vitro. Infect Immun. 1998;66:5119–5124. doi: 10.1128/iai.66.11.5119-5124.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwan T G. Ticks and Borrelia: model systems for investigating pathogen-arthropod interaction. Infect Agents Dis. 1996;5:167–181. [PubMed] [Google Scholar]
- 13.Schwan T G, Piesman J, Golde W T, Dolan M C, Rosa P A. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc Natl Acad Sci USA. 1995;92:2909–2913. doi: 10.1073/pnas.92.7.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sohaskey C D, Arnold C, Barbour A G. Analysis of promoters in Borrelia burgdorferi by use of a transiently expressed reporter gene. J Bacteriol. 1997;179:6837–6842. doi: 10.1128/jb.179.21.6837-6842.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sohaskey C D, Zuckert W R, Barbour A G. The extended promoters for two outer membrane lipoprotein genes of Borrelia spp. uniquely included a T-rich region. Mol Microbiol. 1999;33:41–51. doi: 10.1046/j.1365-2958.1999.01443.x. [DOI] [PubMed] [Google Scholar]
- 16.Stevenson B, Schwan T G, Rosa P. Temperature-related differential expression of antigens in the Lyme disease spirochete, Borrelia burgdorferi. Infect Immun. 1995;63:4535–4539. doi: 10.1128/iai.63.11.4535-4539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stevenson B, Bono J L, Schwan T G, Rosa P. Borrelia burgdorferi Erp proteins are immunogenic in mammals infected by tick bite, and their synthesis is inducible in cultured bacteria. Infect Immun. 1998;66:2648–2654. doi: 10.1128/iai.66.6.2648-2654.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suk K, Das S, Sun W, Jwang B, Barthold S W, Flavell R A, Fikrig E. Borrelia burgdorferi genes selectively expressed in the infected host. Proc Natl Acad Sci USA. 1995;92:4269–4273. doi: 10.1073/pnas.92.10.4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tilly K, Fuhrman J, Campbell J, Samuels D S. Isolation of Borrelia burgdorferi genes encoding homologues of DNA-binding protein HU and ribosomal protein S20. Microbiology. 1996;142:2471–2479. doi: 10.1099/00221287-142-9-2471. [DOI] [PubMed] [Google Scholar]