Abstract
Objective
The clinical cases of patients with multisystem inflammatory syndrome (MIS-C) were analyzed via a systematic review and meta-analysis of the clinical findings, treatments, and possible outcomes of articles retrieved via database searches.
Sources
The authors searched the PubMed, Scielo, Web of Science, Science Direct, EMBASA, EBSCO, and Scopus databases for articles containing the keywords “multisystem inflammatory syndrome in children” or “MIS-C” or “PIMS-TS” or “SIMP” and “COVID-19” or “SARS-CoV-2” published between December 1st, 2019 and July 10th, 2021. Patient characteristics, tissue and organ comorbidities, the incidence of symptoms after COVID-19 infection, treatment, and patient evolution in the articles found were evaluated. The data were abstracted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and Newcastle-Ottawa Scale (NOS).
Findings
In total, 98 articles (2275 patients) were selected for demographics, clinical treatment, and outcomes of patients diagnosed with MIS-C. The average age of children with MIS-C, 56.8% of whom were male, was of nine years. Fever (100%), gastrointestinal (GI) (82%), and abdominal pain (68%) were the decisive symptoms for the diagnosis of MIS-C. Shock and/or hypotension were common in patients with MIS-C. Cardiac symptoms (66%) predominated over respiratory (39%) and neurological (28%) symptoms. MIS-C treatment followed the common guidelines for treating children with septic shock and Kawasaki disease (KD) and proved to be effective.
Conclusions
This meta-analysis highlights the main clinical symptoms used for the diagnosis of MIS-C, the differences between MIS-C and KD, and the severity of the inflammatory process and urgency for hospital care.
Keywords: MIS-C, PIMS-TS, COVID-19, SARS-CoV-2, Children
Introduction
In April 2020, during the peak of the coronavirus disease (COVID-19) pandemic in Europe, reports on children in England with hyperinflammatory shock, the characteristics of which are similar to those of Kawasaki disease (KD) and toxic shock syndrome (TSS), were published. The Royal College of Pediatrics and Child Health referred to this acute condition as pediatric multisystem inflammatory syndrome temporally associated with COVID-19 (PIMS-TS).1 As more cases emerged worldwide, the disease was called multisystem inflammatory syndrome in children (MIS-C) by the U.S. Centers for Disease Control and Prevention (CDC) and the World Health Organization (WHO).2,3
An initial challenge faced by physicians was differentiating patients with MIS-C due to KD and TSS from patients with MIS-C related to COVID-19. Several questions about the symptoms and the possibilities of treatment have been raised.1, 2, 3
At the beginning of the pandemic, children were not at high risk for serious manifestations of COVID-19, such as severe acute respiratory syndrome (SARS). However, as the pandemic evolved, more serious complications, including thrombotic events, myocardial dysfunction, and coronary artery disease or aneurysms, manifested in the pediatric age group with MIS-C.
The aim of this systematic review was to describe the main symptoms of MIS-C and characterize its treatment and possible outcomes.
Methods
Literature search and selection criteria
The authors conducted an online search of the PubMed (http://www.ncbi.nlm.nih.gov/pubmed), Scielo (http://www.scielo.br/), Web of Science (https://clarivate.com/products/web‐of‐science/), Science Direct (https://www.sciencedirect.com/), Embase (www.elsevier.com/embase), EBSCO (https://www.ebscohost.com), and Scopus (https://www.scopus.com/) databases using the keywords “multisystem inflammatory syndrome in children” or “MIS-C” or “PIMS-TS” (pediatric inflammatory multisystem syndrome temporally associated with COVID-19) or “SIMP” (síndrome inflammatory multissistêmica pediátrica) and “COVID-19” or “SARS-CoV-2” to identify relevant studies published between December 1st, 2019 and July 10th, 2021. Before starting our search, the authors searched the Cochrane Library (https://www.cochranelibrary.com) and the National Institute for Health Research database (https://www.crd.york.ac.uk/prospero/) for systematic reviews and meta-analyses on a similar subject, but no articles were found (registration: PROSPERO CRD42020204774).
The risk of bias and the quality of the systematic review was assessed using a quality assessment tool published by the National Institutes of Health. The items included in this systematic review (Supplemental information) were evaluated using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist and the Newcastle-
Ottawa scale (NOS)
To find additional eligible studies, the authors checked the reference lists of the papers found by our search. Additional studies were included in our review if they presented (1) systemic inflammatory syndrome in children or adolescents with COVID-19 and (2) clinical information and outcomes for children and adolescents.
Studies were included in our quantitative analysis if they had a sample size ≥6. The authors did not exclude any article because of language. The series of cases and studies that investigated the pathological characteristics of tissues and organs were evaluated using qualitative analysis.
Some retrieved articles were excluded from this systematic review because (1) the author of the study made the diagnosis of KD and did not consider the possibility of MIS-C related to COVID-19 or PIMS-TS. (2) The study did not present any confirmation that the patient had contact with people infected with COVID-19 or that the RT-PCR test for SARS-CoV-2 and the serological tests were negative. (3) The study was on children who required intensive care before MIS-C and PIMS were identified; however, if the study did not meet the inclusion criteria, it was excluded to avoid bias. (4) The study used the same patient database as another study, so the information overlapped. (5) The article was opinion, editorial, or comment; review article; or health guidelines. These articles were excluded because they did not contain basic patient data.
Data selection was in accordance with the PRISMA and NOS guidelines.
Statistical analysis
The present research is characterized as a systematic review and meta-analysis. Research of this type is carried out by systematically selecting data and later applying statistical tests. The systematic review was carried out in accordance with the PRISMA guidelines.4
Determination of heterogeneity
To assess the heterogeneity of our meta-analysis, the authors used the Higgins and Thompson test (I²), with the following interpretation of the results: 25% = low heterogeneity, 50% = moderate heterogeneity, and ≥ 75% = high heterogeneity. A heterogeneity of ≥ 50% indicates significant differences among the results of the studies used in the meta-analysis; thus, the randomized effect was used. On the other hand, when the heterogeneity was < 50%, the fixed effect was used, which considers the heterogeneity as insignificant. This interpretation and statistical application are extremely important for assertive results.5
Proportion transformation models and methods
When the heterogeneity among the survey data showed results without significance, the inverse model was used, allowing for the return of the transformation of proportions. This model is associated with the Freeman-Tukey double sine transformation (PFT) for the exact probability transformation. However, when the surveys plotted on the graph had several similar, and some discrepant data, the inverse model, associated with the arcsine transformation (PAS) was used for approximate likelihood transformations. When the heterogeneity among the survey data was significant, the mixed generalized linear model (GLMM), associated with the logistic transformation (PLOGIT), was used for the approximate likelihood transformations.
Determination of bias
The bias in our search results was determined by analyzing funnel plot graphs, which was feasible only when the number of plotted surveys was ≥ 10. This takes into account the inefficiency of the graph when the sample size is small.6
Sample significance
For all statistical analyses, an alpha level of 5% was previously defined as significant; thus, P < 0.05 was considered statistically significant. Statistical analyses were performed using the RStudio® version 4.0.2, and STATA® statistical software ver. 16.0 (StataCorp LLC, College Station, TX, USA).
Results
Study selection and characteristics
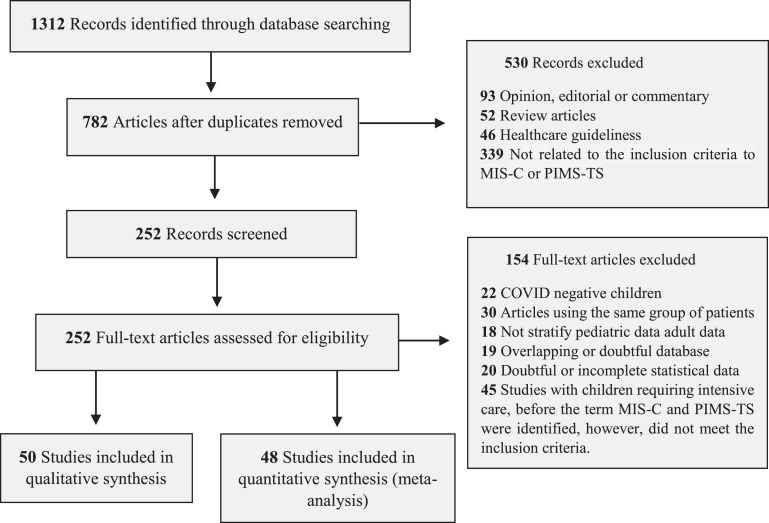
The inclusion and exclusion criteria for articles followed the guidelines of the Royal College of Pediatrics and Child Health (RCPCH), the CDC, and the WHO (Supplementary Table 1). The search of the databases yielded 1312 articles, of which 252 were examined in full, and 98 were selected for systematic review (Figure 1 and Supplementary Tables 2 and 3).
Figure 1.
PRISMA flow diagram of the search of databases. The diagram contains the steps of identification, screening, eligibility, and inclusion.
The articles included in the systematic review included 26 case series, 35 observational cohort studies, and 37 case reports (Table 1). The authors divided the analysis into qualitative studies with five or fewer patients and quantitative studies with six or more patients (Figure 1 and Supplementary Table 2). The number of patients in the quantitative meta-analysis articles was 2197 children, adolescents, and young adults. All data, forest plot graphs, and bias analysis (funnel plot) are provided in the Supplementary Figures.
Table 1.
Characteristics of the studies selected in the systematic review and meta-analysis.
| Articles (2020/2021) | Country | Study | Total cases | Age in years | Total male |
|---|---|---|---|---|---|
| Abdel-Haq et al. 20217 | USA | Observational | 33 | 6 (0.3-17) | 15 |
| Abdel-Mannan et al. 20208 | UK | Case series | 4 | 12 (8-15) | 2 |
| Acharrya et al. 20209 | India | Case report | 1 | 0.3 | 1 |
| Alkan et al. 202110 | Turkey | Observational | 36 | 7.8 (1.7-17) | 19 |
| Bahrami et al. 202011 | Iran | Case report | 1 | 5 | 0 |
| Balasubramanian et al. 202012 | India | Case report | 1 | 8 | 1 |
| Bapst et al. 202013 | Switzerland | Case report | 1 | 13 | 1 |
| Bektaş et al. 202114 | Turkey | Case report | 2 | 10.5 | 1 |
| Belhadjer et al. 202015 | France/Switzerland | Observational | 35 | 10 (2–16) | 18 |
| Belot et al. 202016 | France | Observational | 108 | 8 (5–11) | 53 |
| Blondiaux et al. 202017 | France | Case series | 4 | 9 (6–12) | 1 |
| Blumfield et al. 202118 | USA | Observational | 16 | 10 (1-20) | 10 |
| Buonsenso et al. 202019 | Italy | Case report | 1 | 11 | 0 |
| Capone et al. 202020 | USA | Observational | 33 | 8.6 (5.5–12.6) | 20 |
| Carter et al. 202021 | UK | Observational | 25 | 12,5 (7.7-14.4) | 15 |
| Cattalini et al. 202122 | Italy | Observational | 53 | 7 (4.5-11) | 31 |
| Cheung et al. 202023 | USA | Observational | 17 | 8 (1.8–16) | 8 |
| Chiotos et al. 202024 | USA | Case series | 6 | 7.5 (5–14) | 1 |
| Cogan et al. 202025 | Belgium | Case report | 1 | 19 | 0 |
| Dallan et al. 202026 | Switzerland | Case series | 3 | 11 (10-12) | 2 |
| Dasgupta and Finch 202027 | USA | Case report | 1 | 8 | 0 |
| Davies et al. 202028 | UK | Observational | 78 | 11 (8-14) | 52 |
| De Paulis et al. 202029 | Brazil | Case report | 1 | 4 | 0 |
| Deza Leon et al. 202030 | USA | Case report | 1 | 6 | 0 |
| Dhanalakshmi et al. 202031 | India | Case series | 19 | 6 (1-16) | 8 |
| Dionne et al. 202032 | USA | Observational | 25 | 9.5 (2.7 – 15) | 15 |
| Diorio et al. 202033 | USA | Case series | 6 | 6 (5-7) | 2 |
| Dolhnikoff et al. 202034 | Brazil | Case report | 1 | 11 | 0 |
| Dolinger et al. 202035 | USA | Case report | 1 | 14 | 1 |
| Domico et al. 202036 | USA | Case report | 1 | 11 | 1 |
| Dufort et al. 202037 | USA | Observational | 99 | (0–20) | 53 |
| Farias et al. 202038 | Brazil | Case series | 11 | 4.9 (0.7-11) | 9 |
| Farias et al. 202039 | Brazil | Case report | 1 | 0.7 | 0 |
| Feldstein et al. 202040 | USA | Observational | 186 | 8.3 (3.3–12.5) | 115 |
| Flood et al. 202141 | UK and Ireland | Observational | 268 | 8.2 (4-12.1) | 161 |
| Giannattasio et al. 202142 | Italy | Case report | 1 | 9 | 1 |
| Godfred-Cato et al. 202043 | USA | Observational | 570 | 8 (4-12) | 316 |
| Greene et al. 202044 | USA | Case report | 1 | 11 | 0 |
| Grimaud et al. 202045 | France | Observational | 20 | 10 (2.9–15) | 10 |
| Gruber et al. 202046 | USA | Case series | 8 | 11.5 (3-20) | 4 |
| Gupta et al. 202047 | India | Case report | 1 | 7 | 0 |
| Hameed et al. 202048 | UK | Observational | 35 | 11 | 27 |
| Heidemann et al. 202049 | USA | Case series | 3 | 6 (5-7) | 2 |
| Hutchison et al. 202050 | USA | Case report | 1 | 14 | 1 |
| Jain et al. 202051 | India | Observational | 23 | 7.2 (0.8-14) | 11 |
| Joshi et al. 202052 | USA | Case series | 3 | 10.6 (6-13) | 2 |
| Kashyap et al. 202153 | India | Observational | 12 | 6.5 | 9 |
| Kaushik et al. 202054 | USA | Observational | 33 | 10 (6–13) | 20 |
| Kest et al. 202055 | USA | Case series | 3 | 8 (6-10) | 1 |
| Khesrani et al. 202056 | Algeria | Case report | 1 | 9 | 0 |
| Klocperk et al. 202057 | Czechia | Case report | 1 | 8 | 0 |
| Lang et al. 202058 | Germany | Case report | 2 | (10-13) | 0 |
| Lee and Margolskee 202059 | USA | Case report | 1 | 5 | 0 |
| Lee et al. 202060 | USA | Observational | 28 | 9 (0.1-17) | 15 |
| Lee et al. 202061 | USA | Case report | 1 | 17 | 1 |
| Licciardi et al. 202062 | Italy | Case series | 2 | 12, 7 | 1 |
| Lin et al. 202063 | USA | Case report | 1 | 13 | 0 |
| Mamishi et al. 202064 | Iran | Observational | 45 | 7 (4–9.9) | 24 |
| Mehler et al. 202165 | Germany | Case series | 9 | 12.1 (1-16) | 6 |
| Meredith et al. 202166 | UK | Case report | 1 | 10 | 0 |
| Miller et al. 202067 | USA | Observational | 44 | 7.3 (0.6–20) | 20 |
| Mills et al. 202168 | USA | Case series | 2 | 9.5 | 0 |
| Moghadam et al. 202069 | France | Case report | 1 | 21 | 1 |
| Moraleda et al. 202070 | Spain | Observational | 31 | 7.6 (4.5-11-5) | 18 |
| Nathan et al. 202071 | France | Case series | 2 | 5.5 (5-11) | 0 |
| Ng et al. 202072 | UK | Case series | 3 | 16, 17, 13 | 2 |
| Nguyen et al. 202073 | USA | Case report | 1 | 10 | 0 |
| Okarska-Napierala et al. 202074 | Poland | Case report | 1 | 14 | 1 |
| Paolino and Wlillians 202075 | USA | Case series | 3 | 7.6 (6-9) | 2 |
| Patnaik et al. 202176 | India | Observational | 21 | 8.5 (2-16) | 13 |
| Penner et al. 202177 | UK | Observational | 46 | 10.2 (8.8-13.3) | 30 |
| Pereira et al. 202078 | Brazil | Case series | 6 | 7.78 (0.01-17.6) | 5 |
| Perez-Toledo et al. 202079 | UK | Case series | 8 | 9 (7–14) | 5 |
| Pouletty et al. 202080 | France | Observational | 16 | 10 (4.7–12.5) | 8 |
| Prata-Barbosa et al. 202081 | Brazil | Case series | 10 | 5.2 (1.5−8.4) | 8 |
| Prieto et al. 202182 | Spain | Case series | 5 | 7 (5-12) | 3 |
| Ramcharan et al. 202083 | UK | Observational | 15 | 8.8 (6.4–11.2) | 11 |
| Rauf et al. 202084 | India | Case report | 1 | 5 | 1 |
| Regev et al. 202085 | Israel | Case report | 1 | 16 | 0 |
| Riollano-Cruz et al. 202086 | USA | Observational | 15 | 12 (3–20) | 11 |
| Riphagen et al. 202087 | UK | Case series | 8 | 8.9 (4–14) | 5 |
| Roberts et al. 202188 | USA | Observational | 50 | 9.6 (6.2-14) | 33 |
| Rodriguez-Gonzalez 202089 | Spain | Case report | 1 | 0.6 | 1 |
| Rogo et al. 202090 | USA | Case series | 4 | 11.2 (3-20) | 3 |
| Sadiq et al. 202091 | Pakistan | Case series | 8 | 9.5 (8-10.5) | 7 |
| Saeed and Shorafa 202092 | Iran | Case report | 1 | 3 | 1 |
| Sandoval et al. 202193 | Chile | Case series | 8 | 5.4 (1.5-12) | 3 |
| Schupper et al. 202094 | Germany | Case report | 1 | 5 | 1 |
| Shenker et al. 202095 | USA | Case report | 1 | 12 | 1 |
| Torres et al. 202096 | Chile | Observational | 27 | 6 (0-14) | 14 |
| Toubiana et al. 202097 | France | Observational | 21 | 7.9 (3.7–16.6) | 9 |
| Vari et al. 202098 | USA | Case report | 1 | 14 | 1 |
| Verdoni et al. 202099 | Italy | Case series | 10 | 7.5 (2.9–16) | 7 |
| Verkuil et al. 2020100 | USA | Case report | 1 | 14 | 0 |
| Webb et al. 2020101 | South Africa | Observational | 23 | 6.6 (4.7-8.4) | 17 |
| Whittaker et al. 2020102 | UK | Observational | 58 | 9 (5.7–14) | 25 |
| Yonker et al. 2020103 | USA | Observational | 18 | 7.7 | 14 |
| Yozgat et al. 2020104 | Turkey | Case report | 1 | 3 | 0 |
Demographic characteristics and comorbidities
Meta-analysis showed that 0.58 (0.55 - 0.61) of the children with MIS-C were male, and the median age of all children was 8.9 years (range = 0.1 days to 20 years old).
Only 23 articles included in the meta-analysis reported the race/ethnicity of the patients. Approximately 0.33 (0.26−0.42) of the children were Hispanic, 0.29 (0.24−0.34) were Black, 0.32 (0.24−0.40) were White, 0.05 (0.02−0.13) were Asian, 0.11 (0.07−0.16) were multiracial or other, and 0.13 (0.07−0.21) had no ethnicity specified in the study (Table 2).
Table 2.
Meta-analysis of pooled demographic and clinical characteristics of MIS-C or PIMS-TS patients.
| Characteristics | Total | Events | Pooled mean proportion %(95%CI) | Heterogeneity I2 (%) | Combined |
|---|---|---|---|---|---|
| Demographics | Prop CI95% | ||||
| Sex Male | 2.144 | 1.234 | 0.58 [0.55-0.61] | 31%, p = 0.03 | Random |
| Ethnicity | |||||
| White | 1627 | 338 | 0.19 [0.13-0.26] | 84%, p < 0.01 | Random |
| Multiracial or outhers | 1.514 | 139 | 0.11 [0.07-0.16] | 77%, p < 0.01 | Random |
| Black or Afrodescendents | 1.627 | 477 | 0.32 [0.24-0.40] | 74%, p < 0.01 | Random |
| Asian | 1.627 | 158 | 0.05 [0.02-0.13] | 79%, p < 0.01 | Random |
| Hipanic | 1.043 | 340 | 0.33 [0.26-0.42] | 55%, p < 0.02 | Random |
| Not declared | 1.134 | 175 | 0.13 [0.07-0.21] | 82%, p < 0.01 | Random |
| Clinical features | |||||
| Fever | 2.144 | 2.067 | 1.00 [0.98-1.00] | 78%, p < 0.01 | Random |
| Cough | 1.388 | 535 | 0.41 [0.28-0.55] | 93%, p < 0.01 | Random |
| Headache | 1.173 | 280 | 0.28 [0.21-0.37] | 70%, p < 0.01 | Random |
| Dyspnea | 874 | 235 | 0.29 [0.21-0.38] | 65%, p < 0.01 | Random |
| Conjunctivitis | 978 | 541 | 0.54 [0.47-0.61] | 58%, p < 0.01 | Random |
| Sore throat | 279 | 57 | 0.20 [0.12-0.31] | 71%, p < 0.01 | Random |
| Diarrhoea | 1.542 | 655 | 0.58 [0.49-0.67] | 76%, p < 0.01 | Random |
| Vomiting | 1.541 | 736 | 0.66 [0.56-0.75] | 73%, p < 0.01 | Random |
| Abdominal pain | 1.598 | 763 | 0.68 [0.62-0.74] | 24%, p < 0.12 | Random |
| GI symptoms (not specifics) | 1.228 | 986 | 0.82 [0.71-0.89] | 87%, p < 0.01 | Random |
| Erythema | 1.724 | 814 | 0.59 [0.53-0.65] | 51%, p < 0.01 | Random |
| Shock | 1.544 | 675 | 0.60 [0.51-0.69] | 84%, p < 0.01 | Random |
| Hypotension | 1.697 | 890 | 0.59 [0.53-0.65] | 62%, p < 0.01 | Random |
| Cardiac symptoms | 1.837 | 1.251 | 0.66 [0.58-0.74] | 87%, p < 0.01 | Random |
| Neurologic symptoms | 1.494 | 488 | 0.28 [0.20-0.38] | 83%, p < 0.01 | Random |
| Respiratory symptoms | 1.695 | 869 | 0.39 [0.30-0.49] | 88%, p < 0.01 | Random |
| Comorbidity | 1.805 | 604 | 0.33 [0.27-0.40] | 80%, p < 0.01 | Random |
| Laboratory features | |||||
| Serological test confirmation | 2.044 | 2.102 | 0.69 [0.60-0.77] | 84%, p < 0.01 | Random |
| RT-PCR | 2.102 | 588 | 0.31 [0.24-0.38] | 76%, p < 0.01 | Random |
| Treatment | |||||
| Inotropics | 1.965 | 913 | 0.54 [0.47-0.60] | 77%, p < 0.01 | Random |
| Steroids | 1.973 | 1.145 | 0.64 [0.52-0.74] | 68%, p < 0.01 | Random |
| Antibiotics | 777 | 395 | 0.77 [0.54-0.95] | 97%, p < 0.01 | Random |
| IVIG | 1.963 | 1.501 | 0.84 [0.79-0.88] | 79%, p < 0.01 | Random |
| Antiplatelet | 1.625 | 1.116 | 0.78 [0.63-0.89] | 97%, p < 0.01 | Random |
| Biological Immunodulation | 1.401 | 355 | 0.27 [0.16-0.42] | 77%, p < 0.01 | Random |
| Antiviral therapy | 295 | 45 | 0.16 [0.08-0.29] | 67%, p < 0.01 | Random |
| ICU | 1.973 | 1.294 | 0.76 [0.67-0.84] | 77%, p < 0.01 | Random |
| (MV/NIV/ HFNC) | 1.919 | 731 | 0.50 [0.39-0.62] | 82%, p < 0.01 | Random |
| ECMO | 641 | 36 | 0.06 [0.03-0.10] | 65%, p < 0.01 | Random |
| Outcomes | |||||
| Recoverd | 1.973 | 1.935 | 1.00 [0.99-1.00] | 13%, p < 0.24 | Random |
| Death | 1.973 | 38 | 0.01 [0.01-0.03] | 22%, p = 0.11 | Random |
PICU, pediatric intensive care unit; MV, mechanical ventilation; NIV, noninvasive ventilation; HFNC, high-flow nasal cannula; ECMO, extracorporeal membrane oxygenation.
Only 41 studies reported specific comorbidities and were included in the meta-analysis. Of the 1973 children and adolescents in whom MIS-C was diagnosed, approximately 0.33 (0.27 ± 0.40) had a comorbidity. Several comorbidities were mentioned in the articles evaluated in the qualitative analysis. The most cited comorbidities were asthma, obesity and diabetes. Other less frequent comorbidities were associated with cardiac, renal, neurological, dermatological, and hematological disorders.7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104 The analysis of some comorbidities was discussed in specific studies.105, 106, 107, 108, 109, 110, 111, 112, 113, 114, 115, 116, 117
Clinical manifestations
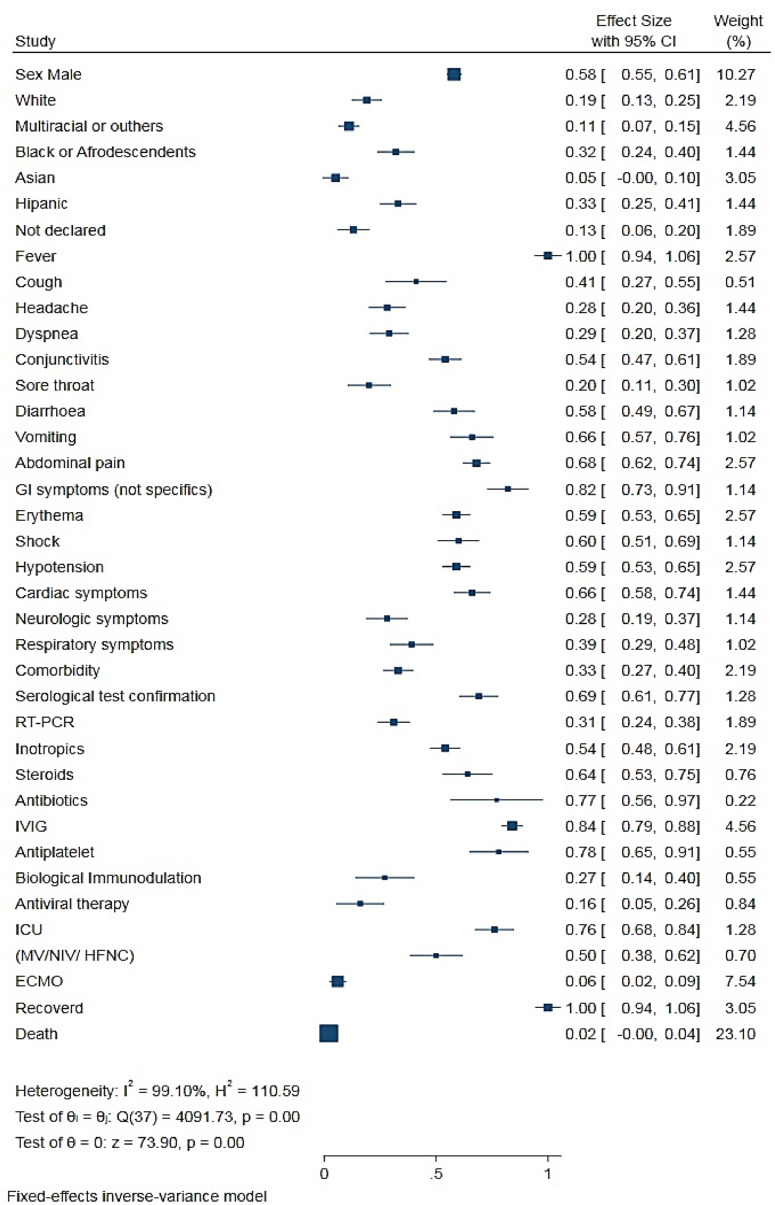
The analysis of the symptom data and clinical characteristics of all patients with MIS-C (Table 2 and Figure 2) showed that the most common symptoms were fever, 1.00 (0.98−1.00); gastrointestinal symptoms, 0.82 (0.71−0.89); abdominal pain, 0.68 (0, 62−0.74); erythema and rash, 0.59 (0.53−0.65); and non-purulent conjunctivitis, 0.54 (0.47−0.61). Cough [0.41 (0.28−0.55)], dyspnea [0.29 (0.21−0.38)], and sore throat [0.20 (0.12−0.31)] also were reported. In contrast with adults, respiratory symptoms in children [0.39 (0.30−0.49)] were less prevalent. Cardiac comorbidities were commonly observed in children with MIS-C [0.66 (0.58−0.74)].
Figure 2.
Summary of the size of the effect of proportions on all the variables studied in the meta-analysis.
Treatment of patients with MIS-C
Thirty-three articles that met the inclusion criteria presented clinical characteristics and the complete outcome of the treatment of patients with MIS-C (Table 2). The treatment offered to these patients involved the WHO protocols for treating patients with septic shock and KD.2
Of the 1294 patients with MIS-C, 0.76 (0.67−0.84) needed intensive hospitalization. Because of the rapid and progressive instability caused by the inflammatory process, 0.54 (0.47−0.60) of the patients needed stabilization and inotropic agents. Shock or hypotension was reported in 0.60 (0.51−0.69) and 0.59 (0.53−0.65) of the patients, respectively.
The authors observed the following variations in the treatment of patients with MIS-C: intravenous immunoglobulin (IVIG), 0.84 (0.79−0.88); antiplatelet or anticoagulant, 0.78 (0.63−0.89); steroid, 0.64 (0.52−0.74); biological immunomodulator, 0.27 (0.16−0.42); and antiviral, 0.16 (0.08−0.29). Approximately 0.50 (0.39−0.62) of the patients with COVID-19-related MIS-C required some respiratory support, and 0.06 (0.03−0.10) eventually needed membrane oxygenation cardiopulmonary bypass (extracorporeal membrane oxygenation [ECMO]).
Some studies reported the use of broad-spectrum antibiotics in the first days of hospitalization; however, once the diagnosis of MIS-C was confirmed, the antibiotics were suspended. Only 0.02 (0.01−0.05) of the patients died despite the severity of the clinical symptoms of MIS-C.
To determine the statistical significance of all the characteristics studied, the authors performed a size test on the effect of proportions on all the variables studied in the meta-analysis (Figure 2).
Discussion
This systematic review analyzed and summarized 98 publications that included case reports, case series, and broader observational studies of patients with MIS-C. All the criteria were followed, and all information was noted for statistical analysis and evaluation. The results of this review confirm that there is a new multisystem inflammatory syndrome related to SARS-CoV-2.
In April 2020, alarming news emerged about children with evidence of recent SARS-CoV-2 infection and who developed a severe multisystem disease with fever, severe abdominal pain, hypotension and/or shock, and myocardial dysfunction with markedly elevated damage markers. This syndrome is called pediatric multisystem inflammatory syndrome temporally associated with COVID-19 (PIMS-TS) in Europe and multisystem inflammatory syndrome in children (MIS-C) by the CDC.2 Although the symptoms and characteristics of MIS-C are similar to those of KD, several studies have presented significant differences that distinguish the two diseases.1,2,3,87,88,95,105 Studies have shown that MIS-C occurs in children and adolescents, where the average age of those studied was 08−11 years.11,14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24,32,36,41,46,52,85, 86, 87 In our systematic review, the mean age of the children with MIS-C was nine years. This contrasts with studies on the incidence of KD in children with an average age of 5 years.8,21,24,61,79,83,96,98,105,107,108,116
Despite the incidence of COVID-19 in Asian countries, the prevalence of MIS-C there is lower, although cases have been registered worldwide according to the WHO (2020). Our systematic review, which included studies from 18 countries, found there was no statistically significant difference in the incidence of MIS-C in Asian children. This contrasts with studies that showed a predominance of KD in children of Asian origin.105, 106, 107, 108 In addition, children with MIS-C had significant abdominal pain that required advanced imaging and surgical consultation, whereas abdominal pain rarely occurs with KD.95, 96, 97, 98,105,108,109
Children with MIS-C have gastrointestinal symptoms more often than do adults with COVID-19.93,108,109 As most children with gastrointestinal symptoms are not severely ill, the authors can conclude that children are more vulnerable to gastrointestinal involvement than to respiratory involvement than are adults.73,93,94,108, 109, 110 Some children had abdominal pain so severe that they underwent surgery for suspected peritonitis or appendicitis that resulted in the diagnosis of MIS-C.50,60,107, 108, 109 The most common conditions associated with abdominal pain include ascites and mesenteric lymphadenitis.13,65,73,107, 108, 109
Cardiac involvement was commonly observed in children with MIS-C (Table 2). Fever, skin rashes, and gastrointestinal symptoms also were common. Case report studies showed that the symptoms of patients hospitalized with MIS-C quickly became acute. Placement in the intensive care unit, treatment for shock and hypotension, fluid resuscitation, and ventilatory support were necessary in most cases. Many patients with MIS-C develop cardiac symptoms, including mild coronary artery dilation or, rarely, aneurysms.11,16, 26,28,32,36,55,81,82,89,111, 112, 113, 114, 115, 116, 117
That mild transient coronary artery dilation can develop as a result of a cytokine storm with high IL-6 levels has been demonstrated in systemic-onset juvenile idiopathic arthritis, and it could result from a similar cytokine storm in MIS-C.86,96,97,111, 112, 113 However, persistent coronary artery aneurysms and their complications have been previously attributed to only KD in pediatric patients.83,98,104, 105, 106, 107, 108, 109, 110,112, 113, 114, 115, 116, 117
Another theory about the cause of cardiac injury is that a direct viral infection causes myocarditis. SARS-CoV-2 may directly cause myocardial damage by entering cardiomyocytes via the angiotensin-converting enzyme 2 (ACE2) receptor. The virus is also capable of activating CD8+ T lymphocyte migration to cardiomyocytes and causing myocardial inflammation through cell-mediated cytotoxicity.113, 114, 115, 116 Endomyocardial biopsies from patients with COVID-19 have shown viral particles, and inflammatory infiltrates in the myocardium.111, 112, 113, 114, 115, 116, 117 All patients in the articles reviewed who had cardiac symptoms were followed up for a longer period, and the total regression of their cardiac symptoms was observed.
Our systematic review found that the immediate medical support offered to patients with MIS-C that was associated with treatment proved effective toward their recovery [1.00 (0.99−1.00)]. In addition, the treatment of patients with MIS-C correlated with that of patients with KD and with the control of the systemic inflammatory process and cardiac injury as reported in other studies.45,100,101,102
The successful use of steroids, in addition to IL-1 receptor antagonists (Anakinra) and IVIG, to control KD has been described. The anti-IL-6 receptor monoclonal antibody tocilizumab has been used successfully in treating chronic inflammatory processes such as juvenile idiopathic arthritis.67 The authors observed the use of preventive treatment that included the use of antiplatelet drugs or anticoagulants as well as broad-spectrum antibiotics initially until severe inflammation was contained, and then the diagnosis of MIS-C was confirmed.
Limitations
This systematic review has some limitations. Because the authors are still working within the situation of a global pandemic, we believe that patient overload and the need for urgent care have prevented hospitals and researchers from providing more detailed information about symptoms, examinations, and outcomes. In addition, several studies included in this review have points of bias resulting from the type of case, the absence of statistical analysis, patient data in more than one article, or difficulty in separating the data of children from that of adults. The authors believe that the inclusion and exclusion criteria used to obtain articles for this review, as well as the attention paid in analyzing the data and statistics, minimized the observed biases.
Conclusions
The results of this systematic review show MIS-C as a severe inflammatory syndrome that affects older children, in contrast to DK. Many organs are affected, and children need hospitalization and fluid and respiratory support. The treatments proposed by the health guidelines (WHO and RCPCH) were followed and proved to be effective in the total recovery of patients.
Funding
Dr. Melissa AG Avelino coordinates the project: "Differential diagnosis and pediatric clinical evolution of COVID-19 in the context of the seasonality of respiratory viruses in a capital of the Midwest Brazil."/CAPES. Dra. Mônica O. Santos, Dr. Paulo A. N Silva, Dr. André L. E Moreira and Dr. Célia RM Ito were supported by grant (CNPJ Capes: 00.889.834 / 0001-08).
Conflicts of interest
The authors declare no conflicts of interest.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jped.2021.08.006.
Appendix. Supplementary materials
References
- 1.RCPCH. Paediatric multisystem inflammatory syndrome temporally associated with COVID- 19 (PIMS) - guidance for clinicians. 2020;178:379-85. [Cited 2020 Nov 23]. Available from:https://www.rcpch.ac.uk/resources/paediatric-multisystem-inflammatory-syndrome-temporally-associated-covid-19-pims-guidance.
- 2.CDC . 2020. Centers for disease control and prevention. Emergency Preparedness and Response: Multisystem inflammatory syndrome in children (MIS-C) associated with COVID-19. May 14. [Google Scholar]
- 3.WHO . WHO; Geneva: 2020. Organization World Health. Multisystem inflammatory syndrome in children and adolescents temporally related to COVID-19. [Google Scholar]
- 4.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 7.Abdel-Haq N, Asmar BI, Deza Leon MP, McGrath EJ, Arora HS, Cashen K, et al. SARS-CoV-2-associated multisystem inflammatory syndrome in children: clinical manifestations and the role of infliximab treatment. Eur J Pediatr. 2021;180:1581–1591. doi: 10.1007/s00431-021-03935-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abdel-Mannan O, Eyre M, Löbel U, Bamford A, Eltze C, Hameed B, et al. Neurologic and radiographic findings associated with COVID-19 infection in children. JAMA Neurol. 2020;77:1440–1445. doi: 10.1001/jamaneurol.2020.2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Acharyya BC, Acharyya S, Das D. Novel Coronavirus mimicking Kawasaki disease in an infant. Indian Pediatr. 2020;57:753–754. doi: 10.1007/s13312-020-1924-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alkan G, Sert A, Oz SK, Emiroglu M, Yılmaz R. Clinical features and outcome of MIS-C patients: an experience from Central Anatolia. Clin Rheumatol. 2021;40:4179–4189. doi: 10.1007/s10067-021-05754-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bahrami A, Vafapour M, Moazzami B, Rezaei N. Hyperinflammatory shock related to COVID-19 in a patient presenting with multisystem inflammatory syndrome in children: First case from Iran. J Paediatr Child Health. 2021;57:922–925. doi: 10.1111/jpc.15048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Balasubramanian S, Nagendran TM, Ramachandran B, Ramanan AV. Hyper-inflammatory syndrome in a child with COVID-19 treated successfully with intravenous Immunoglobulin and Tocilizumab. Indian Pediatr. 2020;57:681–683. doi: 10.1007/s13312-020-1901-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bapst T, Romano F, Muller M, Rohr M. Special dermatological presentation of paediatric multisystem inflammatory syndrome related to COVID-19: erythema multiforme. BMJ Case Rep. 2020;13 doi: 10.1136/bcr-2020-236986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bektaş G, Nihal A, Kübra B, Esra Ş. Reversible splenial lesion syndrome associated with SARS-CoV-2 infection in two children. Brain Dev. 2021;43:230–233. doi: 10.1016/j.braindev.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Belhadjer Z, Meot M, Bajolle F, Khraiche D, Legendre A, Abakka S, et al. Acute heart failure in multisystem inflammatory syndrome in children in the context of global SARS-CoV-2 pandemic. Circulation. 2020;142:429–436. doi: 10.1161/CIRCULATIONAHA.120.048360. [DOI] [PubMed] [Google Scholar]
- 16.Belot A, Antona D, Renolleau S, Javouhey E, Hentgen V, Angoulvant F, et al. SARS-CoV-2-related paediatric inflammatory multisystem syndrome, an epidemiological study, France. Euro Surveill. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.22.2001010. 1 March to 17 May 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blondiaux E, Parisot P, Redheuil A, Tzaroukian L, Levy Y, Sileo C, et al. Cardiac MRI in children with multisystem inflammatory syndrome associated with COVID-19. Radiology. 2020;297:e283–e288. doi: 10.1148/radiol.2020202288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blumfield E, Levin TL, Kurian J, Lee EY, Liszewski MC. Imaging findings in multisystem inflammatory syndrome in children (MIS-C) associated with COVID-19. AJR Am J Roentgenol. 2021;216:507–517. doi: 10.2214/AJR.20.24032. [DOI] [PubMed] [Google Scholar]
- 19.Buonsenso D, Di Sante G, Sali M, Group CC-S. Cytokine profile in an adolescent with pediatric multisystem inflammatory syndrome temporally related to COVID-19. Pediatr Infect Dis J. 2020;39:e213–e215. doi: 10.1097/INF.0000000000002802. [DOI] [PubMed] [Google Scholar]
- 20.Capone CA, Subramony A, Sweberg T, Schneider J, Shah S, Rubin L, et al. Characteristics, cardiac involvement, and outcomes of multisystem inflammatory syndrome of childhood associated with severe acute respiratory syndrome coronavirus 2 infection. J Pediatr. 2020;224:141–145. doi: 10.1016/j.jpeds.2020.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carter MJ, Fish M, Jennings A, Doores KJ, Wellman P, Seow J, et al. Peripheral immunophenotypes in children with multisystem inflammatory syndrome associated with SARS-CoV-2 infection. Nat Med. 2020;26:1701–1717. doi: 10.1038/s41591-020-1054-6. [DOI] [PubMed] [Google Scholar]
- 22.Cattalini M, Della Paolera S, Zunica F, Bracaglia C, Giangreco M, Verdoni L, et al. Rheumatology Study Group of the Italian Pediatric Society. Defining Kawasaki disease and pediatric inflammatory multisystem syndrome-temporally associated to SARS-CoV-2 infection during SARS-CoV-2 epidemic in Italy: results from a national, multicenter survey. Pediatr Rheumatol Online J. 2021;19:e29. doi: 10.1186/s12969-021-00511-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheung EW, Zachariah P, Gorelik M, Boneparth A, Kernie SG, Orange JS, et al. Multisystem inflammatory syndrome related to COVID-19 in previously healthy children and adolescents in New York city. JAMA. 2020;324:294–296. doi: 10.1001/jama.2020.10374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chiotos K, Bassiri H, Behrens EM, Blatz AM, Chang J, Diorio C, et al. Multisystem inflammatory syndrome in children during the Coronavirus 2019 Pandemic: a case series. J Pediatric Infect Dis Soc. 2020;9:393–398. doi: 10.1093/jpids/piaa069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cogan E, Foulon P, Cappeliez O, Dolle N, Vanfraechem G. De Backer D. multisystem inflammatory syndrome with complete Kawasaki disease features associated with SARS-CoV- 2 infection in a young adult. A case report. Front Med. 2020;7:428. doi: 10.3389/fmed.2020.00428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dallan C, Romano F, Siebert J, Politi S, Lacroix L, Sahyoun C. Septic shock presentation in adolescents with COVID-19. Lancet Child Adolesc Health. 2020;4:e21–e23. doi: 10.1016/S2352-4642(20)30164-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dasgupta K, Finch SE. A case of pediatric multisystem inflammatory syndrome temporally associated with COVID-19 in South Dakota. S D Med. 2020;73:246–251. [PubMed] [Google Scholar]
- 28.Davies P, Evans C, Kanthimathinathan HK, Lillie J, Brierley J, Waters G, et al. Intensive care admissions of children with paediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS) in the UK: a multicentre observational study. Lancet Child Adolesc Health. 2020;4:669–677. doi: 10.1016/S2352-4642(20)30215-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Paulis M, Oliveira DB, Vieira RP, Pinto IC, Machado RR, Cavalcanti MP, et al. Multisystem inflammatory syndrome associated with COVID-19 with neurologic manifestations in a child: a brief report. Pediatr Infect Dis J. 2020;39:e321–e324. doi: 10.1097/INF.0000000000002834. [DOI] [PubMed] [Google Scholar]
- 30.Deza Leon MP, Redzepi A, McGrath E, Abdel-Haq N, Shawaqfeh A, Sethuraman U, et al. COVID-19-associated pediatric multisystem inflammatory syndrome. J Pediatric Infect Dis Soc. 2020;9:407–408. doi: 10.1093/jpids/piaa061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dhanalakshmi K, Venkataraman A, Balasubramanian S, Madhusudan M, Amperayani S, Putilibai S, et al. Epidemiological and clinical profile of pediatric inflammatory multisystem syndrome - temporally associated with SARS-CoV-2 (PIMS-TS) in Indian children. Indian Pediatr. 2020;57:1010–1014. doi: 10.1007/s13312-020-2025-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dionne A, Mah DY, Son MBF, Lee PY, Henderson L, Baker AL, et al. atrioventricular block in children with multisystem inflammatory syndrome. Pediatrics. 2020:e146. doi: 10.1542/peds.2020-009704. [DOI] [PubMed] [Google Scholar]
- 33.Diorio C, Henrickson SE, Vella LA, McNerney KO, Chase J, Burudpakdee C, et al. Multisystem inflammatory syndrome in children and COVID-19 are distinct presentations of SARS-CoV-2. J Clin Invest. 2020;130:5967–5975. doi: 10.1172/JCI140970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dolhnikoff M, Ferreira Ferranti J, de Almeida Monteiro RA, Duarte-Neto AN, Soares Gomes-Gouvea M, Viu Degaspare N, et al. SARS-CoV-2 in cardiac tissue of a child with COVID- 19-related multisystem inflammatory syndrome. Lancet Child Adolesc Health. 2020;4:790–794. doi: 10.1016/S2352-4642(20)30257-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dolinger MT, Person H, Smith R, Jarchin L, Pittman N, Dubinsky MC, et al. Pediatric crohn disease and multisystem inflammatory syndrome in children (MIS-C) and COVID-19 treated with infliximab. J Pediatr Gastroenterol Nutr. 2020;71:153–155. doi: 10.1097/MPG.0000000000002809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Domico M, McCanta AC, Hunt JL, Ashouri N, Nugent D, Kelly RB. High-grade heart block requiring transvenous pacing associated with multisystem inflammatory syndrome in children during the COVID-19 pandemic. HeartRhythm Case Rep. 2020;6:811–814. doi: 10.1016/j.hrcr.2020.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dufort EM, Koumans EH, Chow EJ, Rosenthal EM, Muse A, Rowlands J, et al. Multisystem inflammatory syndrome in children in New York State. N Engl J Med. 2020;383:347–358. doi: 10.1056/NEJMoa2021756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Farias EC, Piva JP, de Mello M, do Nascimento L, Costa CC, Machado MM, et al. Multisystem inflammatory syndrome associated with coronavirus disease in children: a multi-centered study in Belem, Para, Brazil. Pediatr Infect Dis J. 2020;39:e374–e376. doi: 10.1097/INF.0000000000002865. [DOI] [PubMed] [Google Scholar]
- 39.Farias EC, Justino MC, Ferraz ML, Mello MF. Multisystem inflammatory syndrome in a child associated with coronavirus disease 19 in the Brazilian Amazon: fatal outcome in an infant. Rev Paul Pediatr. 2020;38 doi: 10.1590/1984-0462/2020/38/2020165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Feldstein LR, Rose EB, Horwitz SM, Collins JP, Newhams MM, Son MB, et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. 2020;383:334–346. doi: 10.1056/NEJMoa2021680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Flood J, Shingleton J, Bennett E, Walker B, Amin-Chowdhury Z, Oligbu G, et al. Paediatric multisystem inflammatory syndrome temporally associated with SARS-CoV-2 (PIMS-TS): prospective, national surveillance, United Kingdom and Ireland, 2020. Lancet Reg Health Eur. 2021;3 doi: 10.1016/j.lanepe.2021.100075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Giannattasio A, Maglione M, Zenzeri L, Mauro A, Di Mita O, Iodice RM, Tipo V. A child with a severe multisystem inflammatory syndrome following an asymptomatic COVID-19 infection: A novel management for a new disease? J Med Virol. 2021;93:112–114. doi: 10.1002/jmv.26189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Godfred-Cato S, Bryant B, Leung J, Oster ME, Conklin L, Abrams J, et al. COVID-19- Associated multisystem inflammatory syndrome in children - United States, March-July 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1074–1080. doi: 10.15585/mmwr.mm6932e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Greene AG, Saleh M, Roseman E, Sinert R. Toxic shock-like syndrome and COVID-19: Multisystem inflammatory syndrome in children (MIS-C) Am J Emerg Med. 2020;38:2492. doi: 10.1016/j.ajem.2020.05.117. e5-2492.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grimaud M, Starck J, Levy M, Marais C, Chareyre J, Khraiche D, et al. Acute myocarditis and multisystem inflammatory emerging disease following SARS-CoV-2 infection in critically ill children. Ann Intensive Care. 2020;10:69. doi: 10.1186/s13613-020-00690-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gruber CN, Patel RS, Trachtman R, Lepow L, Amanat F, Krammer F, et al. Mapping systemic inflammation and antibody responses in multisystem inflammatory syndrome in children (MIS-C) Cell. 2020;183:982–995. doi: 10.1016/j.cell.2020.09.034. e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gupta N, Richter R, Robert S, Kong M. Viral sepsis in children. Front Pediatr. 2018;6:252. doi: 10.3389/fped.2018.00252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hameed S, Elbaaly H, Reid CE, Santos RM, Shivamurthy V, Wong J, et al. Spectrum of imaging findings on chest radiographs, US, CT, and MRI images in multisystem inflammatory syndrome in children (MIS-C) associated with COVID-19. Radiology. 2020 doi: 10.1148/radiol.2020202543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heidemann SM, Tilford B, Bauerfeld C, Martin A, Garcia RU, Yagiela L, et al. three cases of pediatric multisystem inflammatory syndrome associated with COVID-19 due to SARS-CoV-2. Am J Case Repor. 2020;21 doi: 10.12659/AJCR.925779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hutchison L, Plichta AM, Lerea Y, Madora M, Ushay HM. Neuropsychiatric symptoms in an adolescent boy with multisystem inflammatory syndrome in children. Psychosomatics. 2020;61:739–744. doi: 10.1016/j.psym.2020.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jain S, Sen S, Lakshmivenkateshiah S, Bobhate P, Venkatesh S, Udani S, et al. Multisystem inflammatory syndrome in children with COVID-19 in Mumbai, India. Indian Pediatr. 2020;57:1015–1019. doi: 10.1007/s13312-020-2026-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Joshi K, Kaplan D, Bakar A, Jennings JF, Hayes DA, Mahajan S, et al. Cardiac dysfunction and shock in pediatric patients with COVID-19. JACC Case Rep. 2020;2:1267–1270. doi: 10.1016/j.jaccas.2020.05.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kashyap H, Kumar RN, Gautam S, Gupta A, Gupta S, Tiwari PK. Multisystem inflammatory syndrome in children (MIS-C) associated with COVID-19 infection. Indian J Pediatr. 2021;88:e1053. doi: 10.1007/s12098-021-03832-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kaushik S, Aydin SI, Derespina KR, Bansal PB, Kowalsky S, Trachtman R, et al. Multisystem inflammatory syndrome in children associated with severe acute respiratory syndrome Coronavirus 2 infection (MIS-C): a multi-institutional study from New York city. J Pediatr. 2020;224:24–29. doi: 10.1016/j.jpeds.2020.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kest H, Kaushik A, DeBruin W, Colletti M, Goldberg D. Multisystem inflammatory syndrome in children (MIS-C) associated with 2019 novel Coronavirus (SARS-CoV-2) infection. Case Rep Pediatr. 2020;2020 doi: 10.1155/2020/8875987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Khesrani LS, Chana K, Sadar FZ, Dahdouh A, Ladjadj Y, Bouguermouh D. Intestinal ischemia secondary to Covid-19. J Pediatr Surg Case Rep. 2020;61 doi: 10.1016/j.epsc.2020.101604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Klocperk A, Parackova Z, Dissou J, Malcova H, Pavlicek P, Vymazal T, et al. Case report: systemic inflammatory response and fast recovery in a pediatric patient with COVID-19. Front Immunol. 2020;11:1665. doi: 10.3389/fimmu.2020.01665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lang P, Eichholz T, Bakchoul T, Streiter M, Petrasch M, Bosmuller H, et al. Defibrotide for the treatment of pediatric inflammatory multisystem syndrome temporally associated with severe acute respiratory syndrome Coronavirus 2 infection in 2 pediatric patients. J Pediatric Infect Dis Soc. 2020;9:622–625. doi: 10.1093/jpids/piaa117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee WS, Margolskee E. Leukoerythroblastosis and plasmacytoid lymphocytes in a child with SARS-CoV-2-associated multisystem inflammatory syndrome. Blood. 2020;136:914. doi: 10.1182/blood.2020007132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee PY, Platt CD, Weeks S, Grace RF, Maher G, Gauthier K, et al. Immune dysregulation and multisystem inflammatory syndrome in children (MIS-C) in individuals with haploinsufficiency of SOCS1. J Allergy Clin Immunol. 2020;146:1194–1200. doi: 10.1016/j.jaci.2020.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee PY, Day-Lewis M, Henderson LA, Friedman KG, Lo J, Roberts JE, et al. Distinct clinical and immunological features of SARS-CoV-2-induced multisystem inflammatory syndrome in children. J Clin Invest. 2020;130:5942–5950. doi: 10.1172/JCI141113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Licciardi F, Pruccoli G, Denina M, Parodi E, Taglietto M, Rosati S, et al. SARS-CoV-2-Induced Kawasaki-Like hyperinflammatory syndrome: a novel COVID phenotype in children. Pediatrics. 2020;146 doi: 10.1542/peds.2020-1711. [DOI] [PubMed] [Google Scholar]
- 63.Lin J, Lawson EC, Verma S, Peterson RB, Sidhu R. Cytotoxic lesion of the corpus callosum in an adolescent with multisystem inflammatory syndrome and SARS-CoV-2 infection. AJNR Am J Neuroradiol. 2020;41:2017–2019. doi: 10.3174/ajnr.A6755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mamishi S, Movahedi Z, Mohammadi M, Ziaee V, Khodabandeh M, Abdolsalehi MR, et al. Multisystem inflammatory syndrome associated with SARS-CoV-2 infection in 45 children: a first report from Iran. Epidemiol Infect. 2020;148:e196. doi: 10.1017/S095026882000196X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mehler K, Jung N, Oberthuer A. Is it all MIS-C? Unusual findings in a series of nine German patients with multisystem inflammatory syndrome in children after SARS-CoV-2 infection. I J of Infect Dis. 2021;106:405–408. doi: 10.1016/j.ijid.2021.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Meredith J, Khedim CA, Henderson P, Wilson DC, Russell RK. Paediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 [PIMS-TS] in a patient receiving infliximab therapy for inflammatory bowel disease. J Crohns Colitis. 2021;15:687–691. doi: 10.1093/ecco-jcc/jjaa201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Miller J, Cantor A, Zachariah P, Ahn D, Martinez M, Margolis KG. Gastrointestinal symptoms as a major presentation component of a novel multisystem inflammatory syndrome in children that is related to Coronavirus disease 2019: a single center experience of 44 cases. Gastroenterology. 2020;159:1571–1574. doi: 10.1053/j.gastro.2020.05.079. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mills T, Trivedi A, Tremoulet AH, Hershey D, Burns JC. Hyponatremia in patients with multisystem inflammatory syndrome in children. Pediatr Infect Dis J. 2021;40:e344–e346. doi: 10.1097/INF.0000000000003219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Moghadam P, Blum L, Ahouach B, Radjou A, Lambert C, Scanvic A, et al. Multisystem inflammatory syndrome with particular cutaneous lesions related to COVID-19 in a young adult. Am J Med. 2020;134:e36–e37. doi: 10.1016/j.amjmed.2020.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Moraleda C, Serna-Pascual M, Soriano-Arandes A, Simó S, Epalza C, Santos M, et al. Multi- inflammatory syndrome in children related to severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2) in Spain. Clin Infect Dis. 2020;72:e397–e401. doi: 10.1093/cid/ciaa1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nathan N, Prevost B, Sileo C, Richard N, Berdah L, Thouvenin G, et al. The wide spectrum of COVID-19 clinical presentation in children. J Clin Med. 2020;9:e2950. doi: 10.3390/jcm9092950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ng KF, Kothari T, Bandi S, Bird PW, Goyal K, Zoha M, et al. COVID-19 multisystem inflammatory syndrome in three teenagers with confirmed SARS-CoV-2 infection. J Med Virol. 2020;92:2880–2886. doi: 10.1002/jmv.26206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nguyen DC, Haydar H, Pace ER, Zhang XS, Dobbs KR. Pediatric case of severe COVID-19 with shock and multisystem inflammation. Cureus. 2020;12:e8915. doi: 10.7759/cureus.8915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Okarska-Napierala M, Zalewska E, Kuchar E. Fever and diarrhea as the only symptoms of Multisystem Inflammatory Syndrome in Children (MIS-C) Gastroenterology. 2020;160:968–969. doi: 10.1053/j.gastro.2020.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Paolino J, Williams DA. Peripheral blood smears of children with multisystem inflammatory syndrome demonstrate prominence of early myeloid forms with morphologic evidence of toxic change. Pediatr Blood Cancer. 2020;68:e28551. doi: 10.1002/pbc.28551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Patnaik S, Jain MK, Ahmed S, Dash AK, RK P, Sahoo B, et al. Short-term outcomes in children recovered from multisystem inflammatory syndrome associated with SARS-CoV-2 infection. Rheumatol Int. 2021;14:1957–1962. doi: 10.1007/s00296-021-04932-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Penner J, Abdel-Mannan O, Grant K, Maillard S, Kucera F, Hassell J, et al. 6-month multidisciplinary follow-up and outcomes of patients with paediatric inflammatory multisystem syndrome (PIMS-TS) at a UK tertiary paediatric hospital: a retrospective cohort study. Lancet Child Adolesc Health. 2021;5:473–482. doi: 10.1016/S2352-4642(21)00138-3. [DOI] [PubMed] [Google Scholar]
- 78.Pereira MF, Litvinov N, Farhat SC, Eisencraft AP, Gibelli M, Carvalho WB, et al. Severe clinical spectrum with high mortality in pediatric patients with COVID-19 and multisystem inflammatory syndrome. Clinics. 2020;75:e2209. doi: 10.6061/clinics/2020/e2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Perez-Toledo M, Faustini SE, Jossi SE, Shields AM, Kanthimathinathan HK, Allen JD, et al. Serology confirms SARS-CoV-2 infection in PCR-negative children presenting with Paediatric Inflammatory Multi-System Syndrome. medRxiv. 2020 [Google Scholar]
- 80.Pouletty M, Borocco C, Ouldali N, Caseris M, Basmaci R, Lachaume N, et al. Paediatric multisystem inflammatory syndrome temporally associated with SARS-CoV-2 mimicking Kawasaki disease (Kawa-COVID-19): a multicentre cohort. Ann Rheum Dis. 2020;79:999–1006. doi: 10.1136/annrheumdis-2020-217960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Prata-Barbosa A, Lima-Setta F, Santos GRD, Lanziotti VS, de Castro REV, de Souza DC, et al. Pediatric patients with COVID-19 admitted to intensive care units in Brazil: a prospective multicenter study. J Pediatr (Rio J) 2020;96:582–592. doi: 10.1016/j.jped.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Prieto LM, Toral B, LLorente A, Coca D, Blázquez-Gamero D. Cardiovascular magnetic resonance imaging in children with pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 and heart dysfunction. Clin Microbiol Infect. 2021;27:648–650. doi: 10.1016/j.cmi.2020.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ramcharan T, Nolan O, Lai CY, Prabhu N, Krishnamurthy R, Richter AG, et al. Paediatric inflammatory multisystem syndrome: temporally associated with SARS-CoV-2 (PIMS-TS): cardiac features, management and short-term outcomes at a UK tertiary paediatric hospital. Pediatr Cardiol. 2020;41:1391–1401. doi: 10.1007/s00246-020-02391-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rauf A, Vijayan A, John ST, Krishnan R, Latheef A. Multisystem inflammatory syndrome with features of atypical Kawasaki disease during COVID-19 Pandemic. Indian J Pediatr. 2020;87:745–747. doi: 10.1007/s12098-020-03357-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Regev T, Antebi M, Eytan D, Shachor-Meyouhas Y, Ilivitzki A, Aviel YB, et al. Pediatric inflammatory multisystem syndrome with central nervous system involvement and hypocomplementemia following SARS-COV-2 Infection. Pediatr Infect Dis J. 2020;39:e206–e207. doi: 10.1097/INF.0000000000002804. [DOI] [PubMed] [Google Scholar]
- 86.Riollano-Cruz M, Akkoyun E, Briceno-Brito E, Kowalsky S, Reed J, Posada R, et al. Multisystem inflammatory syndrome in children related to COVID-19: a New York city experience. J Med Virol. 2020;93:424–433. doi: 10.1002/jmv.26224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Riphagen S, Gomez X, Gonzalez-Martinez C, Wilkinson N, Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395:1607–1608. doi: 10.1016/S0140-6736(20)31094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Roberts JE, Campbell JI, Gauvreau K, Lamb GS, Newburger J, Son MB, et al. Differentiating multisystem inflammatory syndrome in children: a single-centre retrospective cohort study. Arch Dis Child. 2021;8 doi: 10.1136/archdischild-2021-322290. archdischild-2021-322290. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rodriguez-Gonzalez M, Castellano-Martinez A, Cascales-Poyatos HM, Perez-Reviriego AA. Cardiovascular impact of COVID-19 with a focus on children: a systematic review. World J Clin Cases. 2020;8:5250–5283. doi: 10.12998/wjcc.v8.i21.5250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rogo T, Mathur K, Purswani M. Systemic inflammation with cardiac involvement in pediatric patients with evidence of COVID-19 in a community hospital in the Bronx, New York. J Pediatr Infect Dis Soc. 2020;9:502–503. doi: 10.1093/jpids/piaa087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sadiq M, Aziz OA, Kazmi U, Hyder N, Sarwar M, Sultana N, et al. Multisystem inflammatory syndrome associated with COVID-19 in children in Pakistan. Lancet Child Adolesc Health. 2020;4:e36–e37. doi: 10.1016/S2352-4642(20)30256-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Saeed A, Shorafa E. Status epilepticus as a first presentation of COVID-19 infection in a 3 years old boy; Case report and review the literature. IDCases. 2020;22:e00942. doi: 10.1016/j.idcr.2020.e00942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sandoval F, Julio K, Méndez G, Valderas C, Echeverría AC, Perinette MJ, et al. Neurologic features associated with SARS-CoV-2 infection in children: a case series. Report. J Child Neurol. 2021;36:853–866. doi: 10.1177/0883073821989164. [DOI] [PubMed] [Google Scholar]
- 94.Schupper AJ, Yaeger KA, Morgenstern PF. Neurological manifestations of pediatric multi- system inflammatory syndrome potentially associated with COVID-19. Childs Nerv Syst. 2020;36:1579–1580. doi: 10.1007/s00381-020-04755-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shenker J, Trogen B, Schroeder L, Ratner AJ, Kahn P. Multisystem inflammatory syndrome in children associated with status epilepticus. J Pediatr. 2020;227:300–301. doi: 10.1016/j.jpeds.2020.07.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Torres JP, Izquierdo G, Acuna M, Pavez D, Reyes F, Fritis A, et al. Multisystem inflammatory syndrome in children (MIS-C): report of the clinical and epidemiological characteristics of cases in Santiago de Chile during the SARS-CoV-2 pandemic. Int J Infect Dis. 2020;100:75–81. doi: 10.1016/j.ijid.2020.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Toubiana J, Poirault C, Corsia A, Bajolle F, Fourgeaud J, Angoulvant F, et al. Kawasaki-like multisystem inflammatory syndrome in children during the covid-19 pandemic in Paris, France: prospective observational study. BMJ. 2020;369:m2094. doi: 10.1136/bmj.m2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Vari D, Miller JM, Rellosa N, Srivastava S, Frizzola M, Thacker D. Severe cardiac dysfunction in a patient with multisystem inflammatory syndrome in children associated with COVID-19: Retrospective diagnosis of a puzzling presentation. A case report. Prog Pediatr Cardiol. 2020;58 doi: 10.1016/j.ppedcard.2020.101270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Verdoni L, Mazza A, Gervasoni A, Martelli L, Ruggeri M, Ciuffreda M, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395:1771–1778. doi: 10.1016/S0140-6736(20)31103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Verkuil LD, Liu GT, Brahma VL, Avery RA. Pseudotumor cerebri syndrome associated with MIS-C: a case report. Lancet. 2020;396:532. doi: 10.1016/S0140-6736(20)31725-6. [DOI] [PubMed] [Google Scholar]
- 101.Webb K, Abraham DR, Faleye A, McCulloch M, Rabie H, Scott C. Cape Town MISC-Team. Multisystem inflammatory syndrome in children in South Africa. Lancet Child Adolesc Health. 2020;4:e38. doi: 10.1016/S2352-4642(20)30272-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Whittaker E, Bamford A, Kenny J, Kaforou M, Jones CE, Shah P, et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA. 2020;324:259–269. doi: 10.1001/jama.2020.10369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yonker LM, Neilan AM, Bartsch Y, Patel AB, Regan J, Arya P, et al. Pediatric severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2): clinical presentation, infectivity, and immune responses. J Pediatr. 2020;227:45–52. doi: 10.1016/j.jpeds.2020.08.037. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yozgat CY, Uzuner S, Bursal Duramaz B, Yozgat Y, Erenberk U, Iscan A, et al. Dermatological manifestation of pediatrics multisystem inflammatory syndrome associated with COVID-19 in a 3-year-old girl. Dermatol Ther. 2020;33:e13770. doi: 10.1111/dth.13770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Burns JC. The riddle of Kawasaki disease. N Engl J Med. 2007;356:659–661. doi: 10.1056/NEJMp068268. [DOI] [PubMed] [Google Scholar]
- 106.Jenco M. AAP provides guidance on diagnosing, treating MIS-C. Am Acad Pediatr. 2020:2. https://publications.aap.org/aapnews/news/7007?autologincheck=redirected [Cited Oct 28] Available from. [Google Scholar]
- 107.Makino N, Nakamura Y, Yashiro M, Kosami K, Matsubara Y, Ae R, et al. Nationwide epidemiologic survey of Kawasaki disease in Japan. Pediatr Int. 2019;61:397–403. doi: 10.1111/ped.13809. 2015-2016. [DOI] [PubMed] [Google Scholar]
- 108.Colomba C, La Placa S, Saporito L, Corsello G, Ciccia F, Medaglia A, et al. Intestinal involvement in Kawasaki disease. J Pediatr. 2018;202:186–193. doi: 10.1016/j.jpeds.2018.06.034. [DOI] [PubMed] [Google Scholar]
- 109.Oba J, Carvalho WB, Silva CA, Delgado AF. Gastrointestinal manifestations and nutritional therapy during COVID-19 pandemic: a practical guide for pediatricians. Einstein. 2020;18:eRW5774. doi: 10.31744/einstein_journal/2020RW5774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Xia W, Shao J, Guo Y, Peng X, Li Z, Hu D. Clinical and CT features in pediatric patients with COVID-19 infection: different points from adults. Pediatr Pulmonol. 2020;55:1169–1174. doi: 10.1002/ppul.24718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Binstadt BA, Levine JC, Nigrovic PA, Gauvreau K, Dedeoglu F, Fuhlbrigge RC, et al. Coronary artery dilation among patients presenting with systemic-onset juvenile idiopathic arthritis. Pediatrics. 2005;116:e89–e93. doi: 10.1542/peds.2004-2190. [DOI] [PubMed] [Google Scholar]
- 112.Akhmerov A, Marban E. COVID-19 and the Heart. Circ Res. 2020;126:1443–1455. doi: 10.1161/CIRCRESAHA.120.317055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tissières P, Teboul JL. SARS-CoV-2 post-infective myocarditis: the tip of COVID-19 immune complications? Ann Intensive Care. 2020;10:98. doi: 10.1186/s13613-020-00717-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wei X, Fang Y, Hu H. Immune-mediated mechanism in coronavirus fulminant myocarditis. Eur Heart J. 2020;41:1855–1858. doi: 10.1093/eurheartj/ehaa333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tavazzi G, Pellegrini C, Maurelli M, Belliato M, Sciutti F, Bottazzi A, et al. Myocardial localization of coronavirus in COVID-19 cardiogenic shock. Eur J Heart Fail. 2020;22:911–915. doi: 10.1002/ejhf.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gordon JB, Kahn AM, Burns JC. When children with Kawasaki disease grow up: myocardial and vascular complications in adulthood. J Am Coll Cardiol. 2009;54:1911–1920. doi: 10.1016/j.jacc.2009.04.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Inciardi RM, Lupi L, Zaccone G, Italia L, Raffo M, Tomasoni D, et al. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:819–824. doi: 10.1001/jamacardio.2020.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.