Abstract
Osteoarthritis (OA) is the most common form of arthritis associated with ageing. Vitamin D has diverse biological effect on bone and cartilage, and observational studies have suggested it potential benefit in OA progression and inflammation process. However, the effect of vitamin D on OA is still contradictory. Here, we investigated the therapeutic potential of vitamin D in OA. Six-week-old male Wistar rats were injected with monosodium iodoacetate (MIA) to induce OA. Pain severity, cartilage destruction, and inflammation were measured in MIA-induced OA rats. Autophagy activity and mitochondrial function were also measured. Vitamin-D (1,25(OH)2D3) and celecoxib were used to treat MIA-induced OA rats and OA chondrocytes. Oral supplementation of vitamin D resulted in significant attenuations in OA pain, inflammation, and cartilage destruction. Interestingly, the expressions of MMP-13, IL-1β, and MCP-1 in synovial tissues were remarkably attenuated by vitamin D treatment, suggesting its potential to attenuate synovitis in OA. Vitamin D treatment in OA chondrocytes resulted in autophagy induction in human OA chondrocytes and increased expression of TFEB, but not LC3B, caspase-1 and -3, in inflamed synovium. Vitamin D and celecoxib showed a synergistic effect on antinociceptive and chondroprotective properties in vivo. Vitamin D showed the chondroprotective and antinociceptive property in OA rats. Autophagy induction by vitamin D treatment may be a promising treatment strategy in OA patients especially presenting vitamin D deficiency. Autophagy promoting strategy may attenuate OA progression through protecting cells from damage and inflammatory cell death.
Keywords: Osteoarthritis, Autophagy, Inflammatory cell death, Vitamin D, Mitochondria
INTRODUCTION
Osteoarthritis (OA) is the most common degenerative joint disorder in aged population, disrupting quality of life and causing disability (1,2,3). OA is characterized by progressive cartilage destruction, joint space narrowing, synovial inflammation, and chronic pain (2,4,5). Well-recognized risk factors for OA development are age, female gender, obesity, and joints injuries (6,7,8). As there is no disease-modifying treatment that can alter OA progression, its treatments mainly focus on alleviating pain. Interestingly, several dietary factors including vitamin D, and C have been suggested to be effective in preventing the development or progression of OA (9,10). Although vitamin D has the most extensive epidemiologic clues in OA development among several nutrients (9,11,12,13), the reason why vitamin D deficiency is associated with OA remains elusive. In addition, there have been still several studies that claim that vitamin D has no benefit in suppressing OA progression in patients (14,15,16,17).
Interestingly, vitamin D deficiency are associated with OA risk factors such as sarcopenia and mitochondrial dysfunction. Vitamin D has potential role in improving muscle strength and function in elderly people (18,19,20) and its biological roles on skeletal muscle have been widely investigated (21). Impaired mitochondrial biogenesis has gained spotlight in OA development (22,23). Mitochondrial function is associated with vitamin D status or vitamin D receptor in various organs (24,25,26,27). Vitamin D deficiency in elderly develops for several reasons, including reduced capacity of human skin to produce vitamin D3 (28). These previous evidences suggest that vitamin D may have a disease-modifying potential in OA, a representative chronic inflammatory joint disease associated with aging. Chondrocytes that is the only cell type in normal articular cartilage is responsible for the production and maintenance of extracellular matrix (ECM) in cartilage. Chondrocytes express vitamin D receptor (29,30), inferring that vitamin D may regulate chondrocyte metabolisms or senescence.
Autophagy is a self-degradative mechanism for maintaining cellular homeostasis and protecting cells through removal of unnecessary and dysfunctional components (31,32,33). Defective autophagy in chondrocytes leads to increased chondrocyte apoptosis and promotes joint aging and OA progression (4,34,35). Therefore, it is important to understand the mechanism of autophagy to prevent and cure OA. Recent studies have demonstrated that autophagy regulation, through use of drugs and molecular modifications, restricts OA development and progression (36,37,38). The PI3K/AKT/mTOR signaling pathway is a known modulator of autophagy (39). It has been reported that the mTOR inhibitor rapamycin decreases matrix metalloproteinase 13 (MMP-13) levels in OA chondrocytes, and protects articular cartilage against oxidative stress and cell death (40,41).
Despite of the potential association between vitamin D deficiency and OA, the role of vitamin D supplement and its molecular roles on OA chondrocytes is currently unclear. In this study, we investigated the effects of vitamin D in an OA animal model. We observed that vitamin D supplementation improved OA pain, cartilage destruction, and joint inflammation. Vitamin D activated autophagy flux through lysosomal biogenesis in OA chondrocytes in vitro. Oral administration of vitamin D also attenuated inflammation cell death in inflamed synovium. Furthermore, co-administration of vitamin D and celecoxib demonstrated a synergistic effect on prevention of progressive joint destruction in OA joints. Our findings suggest the chondroprotective potential of vitamin D, as a nutrient, in OA.
MATERIALS AND METHODS
Animals
Male Wistar rats weighing 140–230 g (6 wk old) at the start of the experiment were purchased from Central Lab Animal Inc. (Seoul, Korea). The animals were housed 2-per-cage in a room with controlled temperature (21°C–22°C) and lighting (12/12 h light/dark cycle), and had access to sterile food and water. The mice were randomized into 3 or 4 groups of 6 mice each. All the procedures were approved by the Animal Research Ethics Committee of The Catholic University of Korea (2019-0302-01).
OA induction and treatment
The animals were randomized to the groups before the study began. Following isoflurane anesthesia, 50 µl of 3-mg monosodium iodoacetate (MIA; Sigma-Aldrich, St. Louis, MO, USA) was injected into the intra-articular space of the right knee through the patellar ligament, using a 26.5-G needle. Control rats were injected with an equivalent volume of saline. Three days after MIA injection, vitamin-D (1,25-dihydroxyvitamin D3 [1,25(OH)2D3]) (Hanlim Pharm. Co., Ltd., Seoul, Korea) and celecoxib (Hanlim Pharm. Co., Ltd.) were orally administered daily in the doses of 100 IU or 500 IU, and 30 mg/kg, respectively. Vehicle-treated animals were administered an equivalent volume of 10% DMSO solution.
Assessment of pain behavior
Mechanical sensitivity was used to assess pain, as previously described (42,43). Following MIA injection, a dynamic plantar aesthesiometer (Ugo Basile, Gemonio, VA, Italy) was used to assess the response. Von Frey hair was used for mechanical sensitivity assessment. Pain was scored based on previously published standards. MIA-treated mice were tested for hind-paw response to mechanical stimulation of the masseter, using rigid von Frey filaments and a force transducer (Electronic von Frey, model 2290; IITC Inc., Woodland Hills, CA, USA). The force required to elicit hind-paw withdrawal was recorded 3 times following stimulations at 1-min intervals. The mean 3 values were used for analysis.
Weight-bearing measurement
Weight bearing was evaluated using an incapacitance tester (Linton Instrumentation, Norfolk, UK) that included a dual-channel weight mean value. The rats were attentively positioned in a plastic chamber. The strength applied by an individual hind limb was averaged over more than a 3-s time. The individual data point was the average of 3 measurements. The percentage of weight divided onto the handled (ipsilateral) hind limb was calculated utilizing the following equation: (Weight on Right Leg/Weight on Right Leg and Left Leg)×100.
Human-chondrocyte separation and differentiation
The study was approved by the Institutional Inspection Board of Uijeongbu St. Mary’s Hospital (UC14CNSI0150). All 5 volunteers were from Uijeongbu St. Mary’s Hospital and were fully informed. Written consent was obtained from all OA patients who fulfilled the American College of Rheumatology criteria. Cartilage samples were obtained during the joint replacement surgery. Chondrocytes were isolated using a previously published method (44). Human chondrocytes were seeded in 24-well plates at a density of 5×104. The chondrocytes were treated with vitamin-D (20 nM), or with celecoxib (1 μM) in the presence of 20 ng/ml of human recombinant IL-1β (R&D Systems, Minneapolis, MN, USA) for 2 days.
Gene-expression analysis using real-time PCR
Whole RNAs were isolated from the chondrocytes using the TRI reagent (Thermo Fisher Scientific, Waltham, MA, USA). The cDNA was arranged by reverse transcription of the single-stranded RNA according to the manufacturer’s instructions using a High-Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific). The mRNA were analyzed using real-time PCR with the LightCycler FastStart DNA Master SYBR Green I kit (Takara Bio Inc., Shiga, Japan) according to the manufacturer’s directions. The following primers were utilized in the sequences: control human gene β-actin, 5′-GGA CTT CGA GCA AGA GAT GG-3′ (sense) and 5′-TGT GTT GGC GTA CAG GTC TTT G-3′ (antisense); human MMP-1, 5′-CTG AAG GTG ATG AAG CAG CC-3′ (sense) and 5′-AGT CCA AGA GAA TGG CCG AG-3′ (antisense); MMP-3, 5′-CTC ACA GAC CTG ACT CGG TT-3′ (sense) and 5′-CAC GCC TGA AGG AAG AGA TG-3′ (antisense); MMP-13, 5′-CTA TGG TCC AGG AGA TGA AG-3′ (sense) and 5′-AGA GTC TTG CCT GTA TCC TC-3′ (antisense). All expression values were normalized to that of β-actin mRNA. PCR amplification and analysis were performed using a LightCycler real-time PCR system (Roche Holding AG, Basel, Switzerland).
Histological and immunohistochemical analyses
Histological changes were assessed to determine the effects of each agent on cartilage degeneration in the knee joints of OA rats. The animals were perfused via the ascending aorta with 10% neutral-buffered formalin (pH 7.4). The knee joints, including the patella and the joint capsule, were resected and fixed for an additional 48 h at 4°C. The fixed specimens were decalcified for 6 days using 5% formic acid at 4°C. The specimens were then embedded in paraffin. Standardized 7-μm serial sagittal sections were obtained from the medial and lateral midcondylar level and were stained with H&E, and safranin O–fast green to evaluate the proteoglycan content. Articular cartilage OA histopathology of Rat was assessed using the Osteoarthritis Research Society International (OARSI) and Mankin scoring methods (45,46). Immunohistochemistry slides were deparaffinized and rehydrated using graded ethanol series. The sections were depleted of endogenous peroxidase activity using methanolic H2O2, and then blocked with normal goat serum for 30 min. The samples were incubated overnight at 4°C with antibodies to IL-1β (Santa Cruz Biotechnology, Dallas, TX, USA), MCP-1 (Abcam, Cambridge, UK), MMP-13 (Abcam), caspase-1 (Abcam), casepase-3 (Abcam), LC3B (Abcam), MCP-1 (Abcam), and transcription factor EB (TFEB; Proteintech, Rosemont, IL, USA). The samples were then incubated with the respective secondary antibodies, biotinylated anti-mouse IgG or rabbit IgG, for 20 min, conjugated to a streptavidin–peroxidase complex (Vector Laboratories, Burlingame, CA, USA) for 1 h, and finally with 3,3′-diaminobenzidine (Agilent Technologies, Santa Clara, CA, USA). The sections were counterstained with Mayer’s hematoxylin and photographed using the Olympus photomicroscope (Olympus, Tokyo, Japan). The all images were obtained from each mouse, and showing representative images. The numbers of positive cells showing in each image were measured.
Oxygen consumption rate (OCR) measurement
An XF24 Extracellular Flux Analyzer (Seahorse Bioscience, North Billerica, MA, USA) was used to measure the cellular OCR. Chondrocytes were plated at 2×104 per well in XF 24-well culture microplates for 24 h. Next day, Cells were incubated with XF assay media supplemented with 1 mM sodium pyruvate, 2.5 mM glucose, and 4 mM GlutaMax in non-CO2 incubator for 30 min. Mitochondrial electron transport was assessed through sequential injections of 4 μM oligomycin, 3 μM carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone (FCCP), and 2 μM rotenone/2 μM antimycin A. Basal respiration was calculated as baseline OCR-rotenone/antimycin A OCR; ATP-linked respiration as basal respiration-oligomycin OCR; maximum respiration rate as FCCP OCR-rotenone/antimycin A OCR; and reserve capacity as maximal respiration-basal respiration.
ELISA
The concentrations of IL-6, MCP-1, and IL-8 in culture supernatants were measured by DuoSet ELISA kit (R&D System). The 96-well plates (Nunc, Roskilde, Denmark) coated with capture antibodies for anti-human IL-6, anti-human MCP-1, or anti-human IL-8 (R&D Systems) and incubated overnight at 4°C. After the overnight incubation, the plates were blocked with phosphate-buffered saline containing 1% bovine serum albumin and 0.05% Tween 20 for 2 h at room temperature. Cell culture supernatants were added to the plates and incubated at room temperature for 2 h. Subsequently, the plates were washed, detection antibodies were then added, and the reaction mixtures were incubated for 2 h at room temperature. The plates were washed again and then incubated with streptavidin-HRP for 20 min. Following an additional wash step, substrate solution was added and incubated for 20 min and then added stop solution. The results were analyzed by determining the absorption at 405 nm (A405).
Immunoblotting
Cells were lysed with RIPA Lysis and Extraction Buffer (Thermo Fisher Scientific) including Halt™ Protease Inhibitor Cocktail (Thermo Fisher Scientific) and protein concentrations were determined using the Bradford method (Molecular Devices, San Jose, CA, USA). Protein samples were separated using sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to Hybond membranes (Amersham Pharmacia Biotech, Piscataway, NJ, USA). Proteins were incubated with antibodies against LC3B (Abcam), LAMP1 (Santa Cruz), and β-actin (Santa Cruz Biotechnology) for 15 min. The membrane was washed and incubated with horseradish peroxidase-conjugated secondary antibody for 10 min at room temperature. Band density was estimated by image-capture densitometry.
Immunofluorescence
Human chondrocytes were cultured with IL-1β (20 ng/ml) in the absence or presence of 1,25(OH)2D3 (20 nM) or metformin (1 mM) for 24 h. To measure autophagy influx, cells were stained with FITC-conjugated anti-LC3B (Santa Cruz Biotechnology), PE-conjugated anti-LAMP1 (Santa Cruz Biotechnology), and DAPI for nucleus. To measure co-localization between LC3B and mitochondria, cells were loaded with 50 nM of MitoTracker Deep Red (MTDR; Thermo Fisher Scientific) and stained with FITC-conjugated anti-LC3B. Fluorescence analysis was performed using the ZEN2012 (blue edition; ZEISS, Oberkochen, Germany) program. The positive color was analyzed against the background value by dividing the sites of the photograph.
Statistical analysis
Data are presented as means ± standard deviation of at least 3 independent experiments or independent samples and for 6 mice in each group. One-way ANOVA followed by Bonferroni post hoc test was used to compare differences between ≥3 groups. The Mann-Whitney U test was used to compare numerical data between 2 groups. To assess the Gaussian distribution and the equality of variance, Shapiro-Wilk test and Levene test were used, respectively. A p-value <0.05 was considered statistically significant. Statistical analysis performed using GraphPad Prism software (version 5.01; GraphPad Software, San Diego, CA, USA).
RESULTS
1,25(OH)2D3 attenuates pain production and cartilage destruction in MIA-induced OA rats
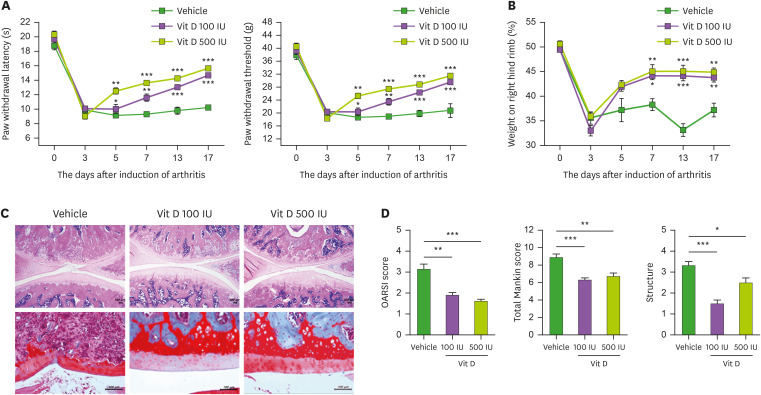
Pain and cartilage destruction are predominant characteristics of OA. Thus, we assessed secondary tactile allodynia in MIA-induced OA rats to determine whether vitamin-D can attenuate pain. In the von Frey hair assessment test, the paw withdrawal latency (PWL) and the paw withdrawal threshold (PWT) were prolonged and increased significantly in the inflamed hind paw of the rats given oral vitamin-D (100 and 500 IU/day) compared with vehicle-treated OA rats (Fig. 1A). Weight bearing was also significantly increased by vitamin-D treatment in OA rats compared with vehicle-treated animals (Fig. 1B). These results demonstrated the significant anti-nociceptive effect of vitamin-D in vivo. Then, to evaluate the chondroprotective property of vitamin-D, the isolated knee joints from 3 groups were analyzed microscopically. Staining with H&E and safranin O–fast green showed the attenuated destruction of articular cartilage in OA rats by vitamin-D treatment (Fig. 1C). OARSI and Mankin scores revealed significantly reduced cartilage destruction in the vitamin-D-treated MIA-induced OA rats (100 or 500 IU/day) compared to the vehicle-treated animals (Fig. 1D). Above results showed the significant anti-nociceptive and chondroprotective properties of vitamin-D in vivo.
Figure 1. The therapeutic effect of vitamin D in MIA-induced-OA model. (A) Pain behavior was analyzed as PWL (left) and PWT (right) in non-induced rats (wild type, n=4), and vehicle-treated (n=4), 100-IU vitamin-D-treated (n=4), and 500-IU vitamin-D-treated (n=4) MIA-induced OA rats. (B) Weight bearing was measured in all groups. (C) H&E (top) and safranin O (bottom) stained joints. (D) Bar graphs show averaged OARSI scores (left), Mankin scores (center), and structures (right). Data are presented as means ± SD.
*p<0.05; **p<0.005; ***p< 0.001.
The effect of vitamin-D on MMP-13, IL-1β, and MCP-1 expression in OA synovium and human OA chondrocytes
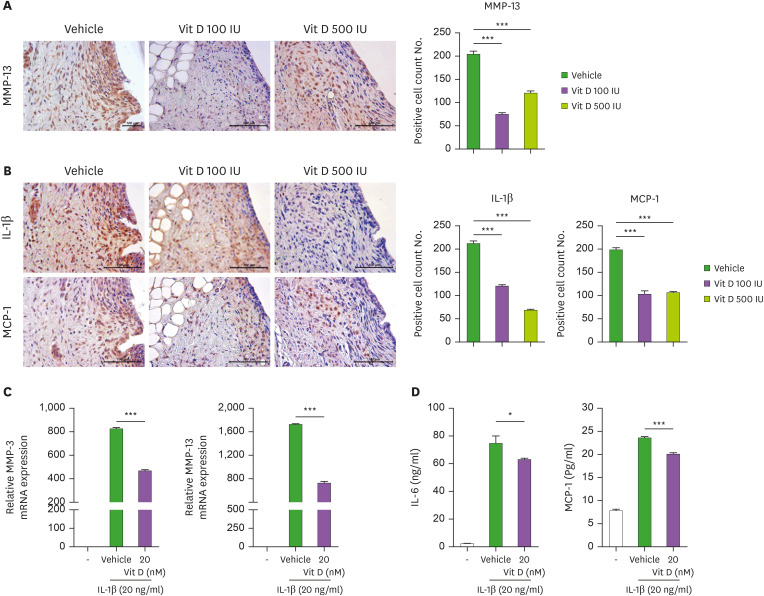
Although cartilage degradation is the characteristic feature of OA, synovitis is frequently observed in OA patients, even before evident cartilage degeneration has occurred. Mounting evidences have suggested that synovitis and resultant inflammatory mediators might contribute to cartilage destruction in OA. MMP-13 plays a pivotal role in OA by degrading type II collagen in articular cartilage. Interestingly, the expression of MMP genes, including MMP-13, were upregulated not only in cartilage but also in OA synovium (47). So, we determined to evaluate the effect of vitamin-D on MMP-13 expression in OA synovium isolated from each group of OA rats. We confirmed that vitamin-D treatment, even in lower dose (100 IU/day), significantly inhibit the expression of MMP-13 in synovium of experimental OA rats (Fig. 2A).
Figure 2. Vitamin D decreases catabolic factors and inflammatory cytokines in the human-OA chondrocytes. (A) MMP-13 immunohistochemistry in synovium. The bar graph shows the mean number of MMP-13-positive cells in the tissue. (B) Immunohistochemistry for IL-1β (top) and MCP-1 (bottom). Bar graphs show the mean number of IL-1β (left) and MCP-1 (right) positive cells in the synovium. (C) mRNA levels of MMP-3 and MMP-13 in human OA chondrocytes were measured by real-time PCR. (D) The concentrations of IL-6 and MCP-1 in culture supernatants were measured by ELISA. Data are presented as means ± SD of 3 independent experiments.
*p<0.05; ***p<0.001.
MCP-1 has pro-arthritic potential through induction of chondrocyte apoptosis (48). It is an important chemokine secreted by synovial fibroblast. Like IL-1β, MCP-1 also propagated synovial inflammation and cartilage damage in OA animals (49). Thus, we next examined the expression of IL-1β and MCP-1 in inflamed synovium. We identified that the expressions of IL-1β, and MCP-1 were significantly attenuated in vitamin-D-treated rats compared to vehicle-treated group (Fig. 2B). In vivo results demonstrated the significantly attenuated expression of MMP-13, IL-1β, and MCP-1 in OA synovium by vitamin-D treatment. Next, to explore the effect of vitamin-D in human OA chondrocytes, human OA chondrocytes were stimulated with 20 ng/ml of IL-1β in the presence or absence of vitamin-D (20 nM). The mRNA expression of MMP-3 and MMP-13 in IL-1β-stimulated OA chondrocytes were significantly decreased by vitamin-D treatment (Fig. 2C). In addition, IL-6 and MCP-1 expressions in culture supernatants were examined by ELISA (Fig. 2D). Vitamin-D treatment decreased the production of these proinflammatory mediators from IL-1β-stimulated OA chondrocytes.
Vitamin-D treatment can enhance autophagic flux and mitochondrial activity in OA chondrocytes
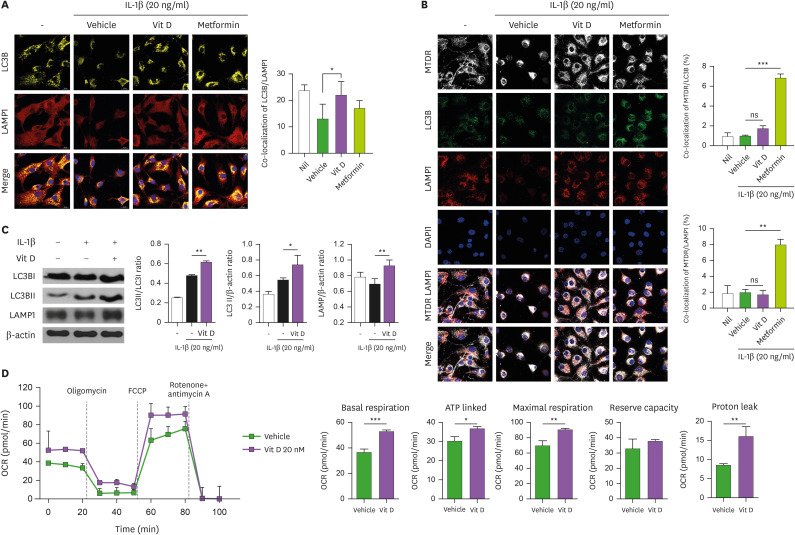
Autophagy is a lysosome-dependent macromolecular cycle. It is characterized by the formation of autophagosomes for the delivery to lysosomes. LC3B is a well-known autophagosomal protein. Immunofluorescent staining showed that 20 nM of vitamin-D treatment in IL-1β-stimulated human OA chondrocytes increased LC3B level (Fig. 3A). Metformin is used as positive control, as an autophagy inducer. Recent study revealed that metformin can activate autophagy in murine chondrocytes (38). Human OA chondrocytes also showed the similar result (Fig. 3A). Lysosomal associated membrane protein 1 (LAMP1) is widely used lysosomal marker. The fusion of the autophagosomes with lysosomes is an important stage of autophagic flux. To determine whether vitamin-D would affect the fusion of autophagosomes with lysosomes, we performed immunostaining for LAMP1 and quantified the colocalization of LAMP1 with LC3 after vitamin-D treatment in IL-1β-stimulated OA chondrocytes (Fig. 3A). We found that chondrocytes treated with 20 nM of vitamin-D significantly increased the colocalization of LC3B with LAMP1 compared with vehicle treated cells (Fig. 3A). Although metformin treatment showed the tendency to increase colocalization of LC3B with LAMP1, the difference did not meet statistical significance. We sought to examine the effect of vitamin-D on mitophagy, confocal microscopy was used to examine the co-localization of MitoTracker with LC3B, as an index of mitophagy. We observed that co-localization between LC3/MTDR (mitochondrial marker) or LAMP1/MTDR in the presence of vitamin-D did not differ with that of vehicle-treated OA chondrocytes (Fig. 3B), indicating the insignificant effect of vitamin-D on mitophagy in vitro.
Figure 3. Vitamin D activates autophagic flux. (A) Confocal microscopy images of LC3B and LAMP1 in untreated OA chondrocytes and vehicle-, 1,25(OH)2D3 (20 nM)-, or metformin (1 mM)-treated OA chondrocytes incubated with 20 ng/ml of IL-1β. The bar graph shows the mean Pearson’s correlation coefficient between LC3B and LAMP1. (B) Representative confocal microscopy images demonstrating co-localization of LC3B and MTDR or LAMP1 and MTDR in untreated or IL-1β (20 ng/ml)-treated human OA chondrocytes in the presence or absence of 1,25(OH)2D3 or metformin (scale bar: 20 µm) (C) Immunoblot showing LC3BI, LC3BII, LAPM1, and β-actin expression in the presence or absence of 1.25(OH)2D3 in IL-1β-stimulated OA chondrocytes. Bar graph shows the LC3BII/LC3BI ratio, LC3B and LAMP1 activities. (E) OCR kinetics in control and vitamin-D-treated chondrocytes. Bar graphs show basal respiration, ATP-linked, maximal respiration, reserve capacity, and proton leak in control and vitamin-D-treated chondrocytes. Data are presented as means ± SD.
ns, not significant.
*p<0.05; **p<0.005; ***p<0.001.
Next, to study the effect of vitamin-D on autophagosome and autolysosome formation, we assessed endogenous LC3-I, LC3-II and LAMP1 by western-blotting. The results demonstrated that the amounts of LC3-II as well as LC3-II/LC3-I ratio are increased by vitamin-D treatment in IL-1β-treated chondrocytes as compared with vehicle-treated cells (Fig. 3C), indicating increased autophagosome. LC3-II is relatively specifically associated with autophagosomes and autolysosomes. At the final stages of autophagy, autophagosomes fuse with lysosomes to form single-membrane-bound autophagolysosome, and subsequently degraded, that is necessary for lysosomal activation. To determine the formation of autophagolysome, LAMP1 expression which is widely used lysosomal marker, was assessed by immunoblotting. We identified that vitamin-D treatment significantly increased LAMP1 level in OA chondrocytes, implying the increase in autophagolysosome formation and lysosomal activity (Fig. 3C). Mitochondrial dysfunction is implicated in both OA onset and its progression (50). As mitochondria plays a pivotal role in autophagy, through autophagy induction and autophagosomal biogenesis from mitochondria (51), the respiratory ability of OA chondrocytes was examined. The results showed that the respiratory ability of mitochondria in IL-1β-stimulated OA chondrocytes was enhanced by vitamin-D treatment compared with vehicle treated cell (Fig. 3D, upper panel). The basal, maximal, ATP-linked mitochondrial respiratory rates, and mitochondrial proton leak were all higher in vitamin-D-treated OA chondrocytes than in vehicle-treated cells (Fig. 3D, lower panel). Taken together, our findings suggest that vitamin-D treatment in OA chondrocytes can enhance autophagic flux and mitochondrial function, implying protective potential of vitamin D on degenerative chondrocytes.
In vivo effect of vitamin-D on autophagic flux and inflammatory cell death in OA synovial tissues
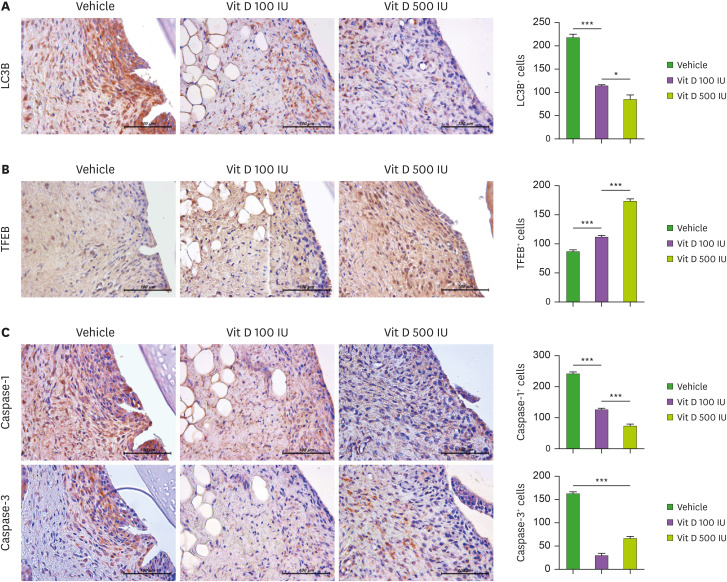
Recent study suggested that autophagic degradation is blocked in OA (52). Lysosomal dysfunction is suggested to be implicated in OA pathogenesis (53). We determined to compare the changes of autophagic flux and inflammatory cell death in synovial tissues among the vehicle- and vitamin-D-treated groups. Autophagic flux and lysosomal activity was analyzed by immunohistochemistry of LC3B and TFEB in synovial tissues of OA rats. TFEB is a master transcriptional factor that mainly regulates autophagy-lysosomal pathway with its own positive feedback loops. TFEB is required for the clearance of damaged lysosomes and is essential for lysosomal homeostasis (54). Our results showed that vitamin-D treatment in OA rats significantly increased TFEB level at synovial tissues in a dose-dependent manner, whether LC3B level was reciprocally decreased (Fig. 4A and B). Interestingly, recent study claimed that LC3 prominence even when LC3-II is not formed may indicate the incomplete inhibition of autophagosome biogenesis and abrogated autophagosome formation (55). Based on that, it is estimated that vitamin-D treatment may enhance autophagic flux via restoring lysosomal dysfunction in OA synovial tissue cells.
Figure 4. Mitochondrial activity and inflammatory cell death. Synovial tissues of OA knees isolated from each group of rats were stained with LC3B (A) and TFEB (B). Immunohistochemically identified LC3B- and TFEB-positive cells were counted. (C) The expression of caspase-1 and -3 by vehicle- or vitamin D-treatment was analyzed by immunohistochemistry in the synovium of rats with MIA-induced OA. The count of positive cells of each antibody is shown on the right. The data are reported as the mean ± SD of 3 independent experiments, with 6 animals per group.
*p<0.05; ***p<0.001.
Next, we examined caspase-1 and -3 level in synovial tissues. Caspase-1 and -3 belongs to the caspase family of proteins responsible for apoptosis and inflammation. The result demonstrated that vitamin-D treatment inhibit caspase-1 and -3 level in synovial tissues in vivo (Fig. 4C and D). Taken together, anti-arthritic effect of vitamin-D in terms of synovitis was associated with significant enhancement of autophagic flux via lysosomal activity and decrease in inflammation cell death among synovial tissues cells.
Synergistic effects of vitamin-D and celecoxib on pain behavior and joint destruction in rats of experimental OA
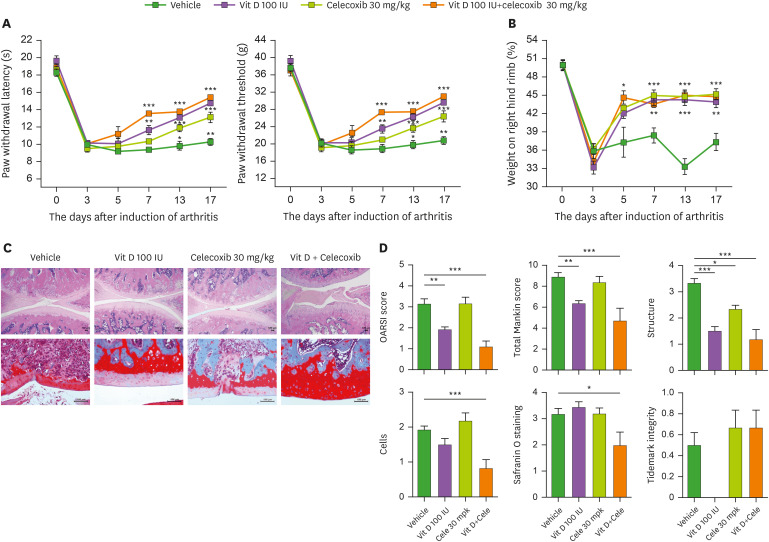
Celecoxib, an anti-inflammatory cyclooxygenase-2 inhibitor, is one of the most commonly prescribed nonsteroidal anti-inflammatory drugs (NSAIDs) to attenuate pain and inflammation in OA patients to lessen the risk of gastrointestinal adverse events and development of cardiovascular diseases that are greater importance in in elderly patients than relatively younger OA patients. So, we determined to study whether vitamin-D may exert synergistic effects with celecoxib regarding pain and cartilage degeneration. The treatment with each agent vitamin-D (100 IU), celecoxib (30 mg/kg), or combinations of these 2 agents) was initiated at 3 days after OA induction through MIA injection. In the von Frey hair assessment test, the PWL and PWT were significantly prolonged in the inflamed hind paw of the rats given oral vitamin-D alone or celecoxib alone compared with vehicle-treated group (Fig. 5A). Although the treatment with vitamin-D alone was superior to celecoxib alone regarding pain behavior, combined administration of vitamin-D showed a superior anti-nociceptive property compared to celecoxib alone (Fig. 5A). In weight bearing data, co-administration of vitamin-D and celecoxib did not show a synergic effect compared to monotherapy (Fig. 5B). Next, we examined whether co-administration with vitamin-D and celecoxib has synergistic effects on cartilage damage and histological changes compared with celecoxib monotherapy. As shown in Fig. 5C, the joints from rats with MIA-induced OA demonstrated that cartilage degeneration and proteoglycan depletion, as demonstrated by H&E and safranin O–fast green stain, were more attenuated in combination of vitamin-D and celecoxib-treated OA group, compared with those of either agent alone. The results demonstrated that combined administration of vitamin-D and celecoxib induces a synergistic effect on histomorphological changes compared to celecoxib monotherapy (Fig. 5D). These results demonstrated the antinociceptive and chondroprotective synergism of vitamin D and celecoxib in an experimental OA animal model.
Figure 5. The effect of vitamin D and celecoxib combination in an OA-animal model. (A) Pain behavior was analyzed as PWL (left) and PWT (right) of vehicle-treated (n=4), 30-mg/kg celecoxib-treated (n=4), 100-IU vitamin-D-treated (n=4), and celecoxib-vitamin-D-combination-treated (n=4) MIA-induced-OA rats. (B) Weight bearing was measured in all groups. (C) H&E (top) and safranin O (bottom) stained joints. (D) Bar graphs show mean OARSI scores (top and left), Mankin scores (top and center), structures (top and right), cells (bottom and left), safranin O staining (bottom and center), and tidemark integrity (bottom and right). Data are presented as means ± SD.
*p<0.05; **p<0.005; ***p<0.001.
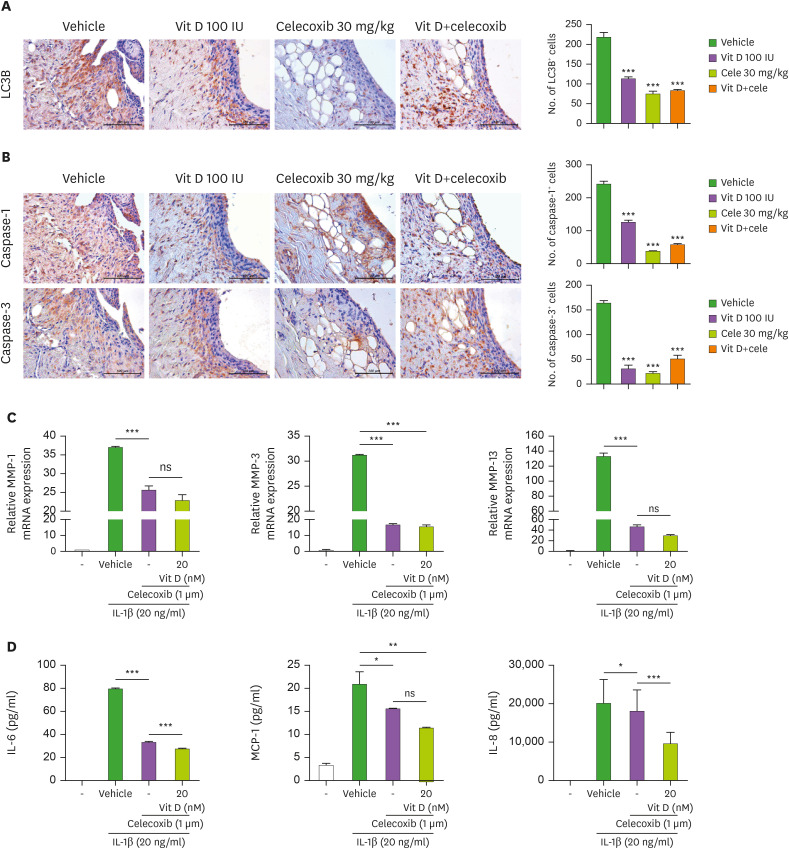
Synergistic effects of vitamin-D and celecoxib on apoptosis
We also investigated the synergistic effects of vitamin-D and celecoxib on inflammation and inflammatory cell death. The number of LC3B (Fig. 6A), and caspase-1 and -3 (Fig. 6B) positive cells were significantly reduced in the joints of 3 groups of OA rats (both in either agent alone and combination therapy) as compared with those of vehicle-treated animals. The mRNA expressions of MMP-1, MMP-3 and MMP-13 in IL-1β-stimulated OA chondrocytes were significantly decreased by celecoxib treatment (1 μM) (Fig. 6C). Our results showed that the treatment with vitamin-D in human chondrocytes did not affect the mRNA expressions of these MMPs in celecoxib-treated cells. Then, the concentrations of IL-6, MCP1 and IL-8 in culture supernatants in these cells were analyzed by ELISA. The results showed a significantly further decreased production of IL-6, MCP-1, and IL-8 by vitamin-D treatment, implying a synergistic effect of vitamin-D in terms of anti-inflammatory properties when co-administered with celecoxib.
Figure 6. The effect of vitamin D and celecoxib combination on inflammatory cell death. (A, B) The expression of LC3B (A) and caspase-1 and -3 (B) in the OA synovium of each group of rats were determined by immunohistochemistry. The count of positive cells for each antibody is shown (right panel). The data are represented as means ± SD of 3 independent experiments, with 6 animals per group. (C) The human OA chondrocytes were cultured with IL-1β (20 ng/ml) in the presence or absence of celecoxib (1 μM) or 1,25(OH)2D3 (20 nM) for 2 days. mRNA levels of MMP-1, -3, and -13 were measured by real-time PCR. (D) The concentration of IL-6, MCP-1, and IL-8 in culture supernatants of human OA chondrocytes were analyzed by enzyme-linked immunosorbent assay. Data are presented as means ± SD of 3 independent experiments.
ns, not significant.
*p<0.05; **p<0.005; ***p<0.001.
DISCUSSION
OA is the most common type of arthritis that occurs in middle age, and has multiple risk factors, including age, obesity, gender, and genetics. There is no cure for OA, but analgesic and anti-inflammatory drugs, such as corticosteroids and NSAIDs, are commonly used (56,57). Although these medicines effectively reduce pain, they are associated with safety issues. Steroids can cause numerous complications, making their long-term use difficult, while NSAIDs can cause gastrointestinal problems and hepatotoxicity. Therefore, it is necessary to develop medications for long-term use without such complications. Vitamin D has proven long-term safety, and may be useful in the treatment of OA patients. In this study, we investigated the effects of vitamin D in OA.
MMPs are enzymes involved in the degradation of ECM proteins. Increased MMP levels were observed in OA patients and animal models (58). MMP-13 is known to cause cartilage degradation. We previously reported that high MMP-13 levels in OA chondrocytes decreased when co-cultured with metformin-treated mesenchymal stem cells (59). MMP-13 expression is affected by several cytokines and growth factors, including IL-1β. IL-1β is a key mediator of inflammatory response, and is regulated by caspase 1. Several studies have demonstrated that IL-1β is involved in autoimmune inflammation (60). We previously demonstrated that MMP-13 and IL-1β levels decreased significantly in OA chondrocytes, and pain severity indices, such as PWL and PWT, and cartilage destruction reduced with metformin.
Age is a major risk factor for OA, as autophagy is decreased with aging. The expression of autophagy-related proteins decreases with age in both human and mouse articular cartilage (4). Therefore, autophagy regulation is a therapeutic strategy for slowing OA progression. Autophagy is related to several signaling pathways. A recent study showed that baicalin protects OA chondrocytes against IL-1β-induced apoptosis and ECM degradation through autophagy activation (61). MMP-13 and caspase expression, and apoptosis rates, decreased, while LC3-II levels increased in baicalin-treated chondrocytes. A recent study demonstrated that vitamin D reduced OA through activation of chondrocyte autophagy by mediating the AMPK-mTOR signaling pathway (62). Vitamin-D downregulates mTOR expression followed by inducing autophagy by inhibiting mTORC1 complex (63,64). On the other hand, Chen et al. (65) reported vitamin-D inhibits mitophagy in TNF-α-induced inflammatory condition and ischemia/reperfusion-induced cardiac injury condition (66). The role of vitamin-D in autophagy and mitophagy needs further studies. Vitamin D is widely considered to be beneficial in several diseases. However, its effects remain controversial (67,68,69). A recent study demonstrated that vitamin-D deficiency is associated with OA onset and progression (70). Clinically, significant improvements in the Manchester Foot Pain and Disability Index have been reported in vitamin-D-treated OA patients compared to the placebo group (71). Vitamin D is commonly used in combination with calcium for the treatment of osteoporosis. The major risk factor for osteoporosis is also aging. Therefore, if the effectiveness of vitamin D in OA is demonstrated, it may prove a valuable treatment option in older patients.
In this study, we demonstrated chondroprotective effects of vitamin-D in OA animals and human OA chondrocytes. Vitamin-D treatment significantly decreased pain severity, cartilage destruction, and inflammation in experimental OA model. MMP-13, IL-1β, and MCP-1 levels were also significantly decreased in OA synovium of vitamin-D-treated group. The increase in TFEB expression while decreasing LC3B level supports the hypothesis that vitamin-D administration showed anti-nociceptive and chondroprotective effects by enhancing autophagic flux in OA synovium. The synergistic effect of vitamin-D and celecoxib shown in our present study may have clinical significance in that it suggests the importance of vitamin-D as a nutrient in treatment strategies for OA patients. Taken together, our findings demonstrated that vitamin-D has chondroprotective and pain-reducing properties by enhancing autophagic flux in chondrocytes as well as in OA synovium.
ACKNOWLEDGEMENTS
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (grant number NRF-2015R1D1A1A01057065 and 2020R1I1A1A01072520).
Abbreviations
- ECM
extracellular matrix
- FCCP
carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone
- LAMP1
lysosomal associated membrane protein 1 MIA, monosodium iodoacetate
- MMP
matrix metalloproteinase
- MTDR
MitoTracker Deep Red
- NSAID
nonsteroidal anti-inflammatory drug
- OA
osteoarthritis
- OARSI
osteoarthritis Research Society International
- OCR
oxygen consumption rate
- PWL
paw withdrawal latency
- PWT
paw withdrawal threshold
- TFEB
transcription factor EB
Footnotes
Conflict of Interest: The authors declare no potential conflicts of interest.
- Conceptualization: Jhun J, Moon SJ, Park SH, Cho ML. Data curation.
- Formal analysis: Jhun J.
- Investigation: Jhun J, Kwon JY, Na HS, Cho KH.
- Methodology: Na HS, Cho KH, Kim SA.
- Project administration: Kim SJ.
- Resources: Kim SJ.
- Supervision: Cho ML.
- Validation: Woo JS.
- Writing - original draft: Woo JS, Cho ML.
- Writing - review & editing: Woo JS, Moon SJ, Cho ML.
References
- 1.Martel-Pelletier J, Barr AJ, Cicuttini FM, Conaghan PG, Cooper C, Goldring MB, Goldring SR, Jones G, Teichtahl AJ, Pelletier JP. Osteoarthritis. Nat Rev Dis Primers. 2016;2:16072. doi: 10.1038/nrdp.2016.72. [DOI] [PubMed] [Google Scholar]
- 2.Bowden JL, Hunter DJ, Deveza LA, Duong V, Dziedzic KS, Allen KD, Chan PK, Eyles JP. Core and adjunctive interventions for osteoarthritis: efficacy and models for implementation. Nat Rev Rheumatol. 2020;16:434–447. doi: 10.1038/s41584-020-0447-8. [DOI] [PubMed] [Google Scholar]
- 3.Moon SJ, Jeong JH, Jhun JY, Yang EJ, Min JK, Choi JY, Cho ML. Ursodeoxycholic acid ameliorates pain severity and cartilage degeneration in monosodium iodoacetate-induced osteoarthritis in rats. Immune Netw. 2014;14:45–53. doi: 10.4110/in.2014.14.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caramés B, Taniguchi N, Otsuki S, Blanco FJ, Lotz M. Autophagy is a protective mechanism in normal cartilage, and its aging-related loss is linked with cell death and osteoarthritis. Arthritis Rheum. 2010;62:791–801. doi: 10.1002/art.27305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sellam J, Berenbaum F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat Rev Rheumatol. 2010;6:625–635. doi: 10.1038/nrrheum.2010.159. [DOI] [PubMed] [Google Scholar]
- 6.Prieto-Alhambra D, Judge A, Javaid MK, Cooper C, Diez-Perez A, Arden NK. Incidence and risk factors for clinically diagnosed knee, hip and hand osteoarthritis: influences of age, gender and osteoarthritis affecting other joints. Ann Rheum Dis. 2014;73:1659–1664. doi: 10.1136/annrheumdis-2013-203355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zheng H, Chen C. Body mass index and risk of knee osteoarthritis: systematic review and meta-analysis of prospective studies. BMJ Open. 2015;5:e007568. doi: 10.1136/bmjopen-2014-007568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Slauterbeck JR, Kousa P, Clifton BC, Naud S, Tourville TW, Johnson RJ, Beynnon BD. Geographic mapping of meniscus and cartilage lesions associated with anterior cruciate ligament injuries. J Bone Joint Surg Am. 2009;91:2094–2103. doi: 10.2106/JBJS.H.00888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lane NE, Gore LR, Cummings SR, Hochberg MC, Scott JC, Williams EN, Nevitt MC. Serum vitamin D levels and incident changes of radiographic hip osteoarthritis: a longitudinal study. Study of osteoporotic fractures research group. Arthritis Rheum. 1999;42:854–860. doi: 10.1002/1529-0131(199905)42:5<854::AID-ANR3>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 10.Chang Z, Huo L, Li P, Wu Y, Zhang P. Ascorbic acid provides protection for human chondrocytes against oxidative stress. Mol Med Rep. 2015;12:7086–7092. doi: 10.3892/mmr.2015.4231. [DOI] [PubMed] [Google Scholar]
- 11.McAlindon TE, Felson DT, Zhang Y, Hannan MT, Aliabadi P, Weissman B, Rush D, Wilson PW, Jacques P. Relation of dietary intake and serum levels of vitamin D to progression of osteoarthritis of the knee among participants in the Framingham Study. Ann Intern Med. 1996;125:353–359. doi: 10.7326/0003-4819-125-5-199609010-00001. [DOI] [PubMed] [Google Scholar]
- 12.Bergink AP, Uitterlinden AG, Van Leeuwen JP, Buurman CJ, Hofman A, Verhaar JA, Pols HA. Vitamin D status, bone mineral density, and the development of radiographic osteoarthritis of the knee: the Rotterdam Study. J Clin Rheumatol. 2009;15:230–237. doi: 10.1097/RHU.0b013e3181b08f20. [DOI] [PubMed] [Google Scholar]
- 13.Heidari B, Heidari P, Hajian-Tilaki K. Association between serum vitamin D deficiency and knee osteoarthritis. Int Orthop. 2011;35:1627–1631. doi: 10.1007/s00264-010-1186-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Felson DT, Niu J, Clancy M, Aliabadi P, Sack B, Guermazi A, Hunter DJ, Amin S, Rogers G, Booth SL. Low levels of vitamin D and worsening of knee osteoarthritis: results of two longitudinal studies. Arthritis Rheum. 2007;56:129–136. doi: 10.1002/art.22292. [DOI] [PubMed] [Google Scholar]
- 15.McAlindon T, LaValley M, Schneider E, Nuite M, Lee JY, Price LL, Lo G, Dawson-Hughes B. Effect of vitamin D supplementation on progression of knee pain and cartilage volume loss in patients with symptomatic osteoarthritis: a randomized controlled trial. JAMA. 2013;309:155–162. doi: 10.1001/jama.2012.164487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jin X, Jones G, Cicuttini F, Wluka A, Zhu Z, Han W, Antony B, Wang X, Winzenberg T, Blizzard L, et al. Effect of vitamin D supplementation on tibial cartilage volume and knee pain among patients with symptomatic knee osteoarthritis: a randomized clinical trial. JAMA. 2016;315:1005–1013. doi: 10.1001/jama.2016.1961. [DOI] [PubMed] [Google Scholar]
- 17.Perry TA, Parkes MJ, Hodgson R, Felson DT, O’Neill TW, Arden NK. Effect of vitamin D supplementation on synovial tissue volume and subchondral bone marrow lesion volume in symptomatic knee osteoarthritis. BMC Musculoskelet Disord. 2019;20:76. doi: 10.1186/s12891-019-2424-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agergaard J, Trøstrup J, Uth J, Iversen JV, Boesen A, Andersen JL, Schjerling P, Langberg H. Does vitamin-D intake during resistance training improve the skeletal muscle hypertrophic and strength response in young and elderly men? - A randomized controlled trial. Nutr Metab (Lond) 2015;12:32. doi: 10.1186/s12986-015-0029-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Granic A, Hill TR, Davies K, Jagger C, Adamson A, Siervo M, Kirkwood TB, Mathers JC, Sayer AA. Vitamin D status, muscle strength and physical performance decline in very old adults: a prospective study. Nutrients. 2017;9:379. doi: 10.3390/nu9040379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chung C, Silwal P, Kim I, Modlin RL, Jo EK. Vitamin D-cathelicidin axis: at the crossroads between protective immunity and pathological inflammation during infection. Immune Netw. 2020;20:e12. doi: 10.4110/in.2020.20.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pojednic RM, Ceglia L. The emerging biomolecular role of vitamin D in skeletal muscle. Exerc Sport Sci Rev. 2014;42:76–81. doi: 10.1249/JES.0000000000000013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruiz-Romero C, Calamia V, Mateos J, Carreira V, Martínez-Gomariz M, Fernández M, Blanco FJ. Mitochondrial dysregulation of osteoarthritic human articular chondrocytes analyzed by proteomics: a decrease in mitochondrial superoxide dismutase points to a redox imbalance. Mol Cell Proteomics. 2009;8:172–189. doi: 10.1074/mcp.M800292-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dave M, Attur M, Palmer G, Al-Mussawir HE, Kennish L, Patel J, Abramson SB. The antioxidant resveratrol protects against chondrocyte apoptosis via effects on mitochondrial polarization and ATP production. Arthritis Rheum. 2008;58:2786–2797. doi: 10.1002/art.23799. [DOI] [PubMed] [Google Scholar]
- 24.Dzik KP, Skrobot W, Kaczor KB, Flis DJ, Karnia MJ, Libionka W, Antosiewicz J, Kloc W, Kaczor JJ. Vitamin D deficiency is associated with muscle atrophy and reduced mitochondrial function in patients with chronic low back pain. Oxid Med Cell Longev. 2019;2019:6835341. doi: 10.1155/2019/6835341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stio M, Lunghi B, Iantomasi T, Vincenzini MT, Treves C. Effect of vitamin D deficiency and 1,25-dihydroxyvitamin D3 on rat heart metabolism. J Mol Cell Cardiol. 1994;26:1421–1428. doi: 10.1006/jmcc.1994.1161. [DOI] [PubMed] [Google Scholar]
- 26.Ashcroft SP, Fletcher G, Philp AM, Jenkinson C, Das S, Hansbro PM, Atherton PJ, Philp A. Diet-induced vitamin D deficiency reduces skeletal muscle mitochondrial respiration. J Endocrinol. 2021;249:113–124. doi: 10.1530/JOE-20-0233. [DOI] [PubMed] [Google Scholar]
- 27.Blajszczak CC, Nonn L. Vitamin D regulates prostate cell metabolism via genomic and non-genomic mitochondrial redox-dependent mechanisms. J Steroid Biochem Mol Biol. 2019;195:105484. doi: 10.1016/j.jsbmb.2019.105484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.MacLaughlin J, Holick MF. Aging decreases the capacity of human skin to produce vitamin D3. J Clin Invest. 1985;76:1536–1538. doi: 10.1172/JCI112134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Y, Zhu J, DeLuca HF. Identification of the vitamin D receptor in osteoblasts and chondrocytes but not osteoclasts in mouse bone. J Bone Miner Res. 2014;29:685–692. doi: 10.1002/jbmr.2081. [DOI] [PubMed] [Google Scholar]
- 30.Tetlow LC, Woolley DE. Expression of vitamin D receptors and matrix metalloproteinases in osteoarthritic cartilage and human articular chondrocytes in vitro . Osteoarthritis Cartilage. 2001;9:423–431. doi: 10.1053/joca.2000.0408. [DOI] [PubMed] [Google Scholar]
- 31.Mizushima N, Komatsu M. Autophagy: renovation of cells and tissues. Cell. 2011;147:728–741. doi: 10.1016/j.cell.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 32.Marycz K, Weiss C, Śmieszek A, Kornicka K. Evaluation of oxidative stress and mitophagy during adipogenic differentiation of adipose-derived stem cells isolated from equine metabolic syndrome (EMS) horses. Stem Cells Int. 2018;2018:5340756. doi: 10.1155/2018/5340756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Son J, Kim MJ, Lee JS, Kim JY, Chun E, Lee KY. Hepatitis B virus X protein promotes liver cancer progression through autophagy induction in response to TLR4 stimulation. Immune Netw. 2021;21:e37. doi: 10.4110/in.2021.21.e37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rockel JS, Kapoor M. Autophagy: controlling cell fate in rheumatic diseases. Nat Rev Rheumatol. 2016;12:517–531. doi: 10.1038/nrrheum.2016.92. [DOI] [PubMed] [Google Scholar]
- 35.López de Figueroa P, Lotz MK, Blanco FJ, Caramés B. Autophagy activation and protection from mitochondrial dysfunction in human chondrocytes. Arthritis Rheumatol. 2015;67:966–976. doi: 10.1002/art.39025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feng K, Chen H, Xu C. Chondro-protective effects of celastrol on osteoarthritis through autophagy activation and NF-κB signaling pathway inhibition. Inflamm Res. 2020;69:385–400. doi: 10.1007/s00011-020-01327-z. [DOI] [PubMed] [Google Scholar]
- 37.Yang H, Wen Y, Zhang M, Liu Q, Zhang H, Zhang J, Lu L, Ye T, Bai X, Xiao G, et al. MTORC1 coordinates the autophagy and apoptosis signaling in articular chondrocytes in osteoarthritic temporomandibular joint. Autophagy. 2020;16:271–288. doi: 10.1080/15548627.2019.1606647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang C, Yao Z, Zhang Y, Yang Y, Liu J, Shi Y, Zhang C. Metformin mitigates cartilage degradation by activating AMPK/SIRT1-mediated autophagy in a mouse osteoarthritis model. Front Pharmacol. 2020;11:1114. doi: 10.3389/fphar.2020.01114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Galluzzi L, Bravo-San Pedro JM, Levine B, Green DR, Kroemer G. Pharmacological modulation of autophagy: therapeutic potential and persisting obstacles. Nat Rev Drug Discov. 2017;16:487–511. doi: 10.1038/nrd.2017.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Caramés B, Taniguchi N, Seino D, Blanco FJ, D’Lima D, Lotz M. Mechanical injury suppresses autophagy regulators and pharmacologic activation of autophagy results in chondroprotection. Arthritis Rheum. 2012;64:1182–1192. doi: 10.1002/art.33444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sasaki H, Takayama K, Matsushita T, Ishida K, Kubo S, Matsumoto T, Fujita N, Oka S, Kurosaka M, Kuroda R. Autophagy modulates osteoarthritis-related gene expression in human chondrocytes. Arthritis Rheum. 2012;64:1920–1928. doi: 10.1002/art.34323. [DOI] [PubMed] [Google Scholar]
- 42.Lee J, Hong YS, Jeong JH, Yang EJ, Jhun JY, Park MK, Jung YO, Min JK, Kim HY, Park SH, et al. Coenzyme Q10 ameliorates pain and cartilage degradation in a rat model of osteoarthritis by regulating nitric oxide and inflammatory cytokines. PLoS One. 2013;8:e69362. doi: 10.1371/journal.pone.0069362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee SY, Lee SH, Na HS, Kwon JY, Kim GY, Jung K, Cho KH, Kim SA, Go EJ, Park MJ, et al. The therapeutic effect of STAT3 signaling-suppressed MSC on pain and articular cartilage damage in a rat model of monosodium iodoacetate-induced osteoarthritis. Front Immunol. 2018;9:2881. doi: 10.3389/fimmu.2018.02881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kwon JY, Lee SH, Na HS, Jung K, Choi J, Cho KH, Lee CY, Kim SJ, Park SH, Shin DY, et al. Kartogenin inhibits pain behavior, chondrocyte inflammation, and attenuates osteoarthritis progression in mice through induction of IL-10. Sci Rep. 2018;8:13832. doi: 10.1038/s41598-018-32206-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pauli C, Whiteside R, Heras FL, Nesic D, Koziol J, Grogan SP, Matyas J, Pritzker KP, D’Lima DD, Lotz MK. Comparison of cartilage histopathology assessment systems on human knee joints at all stages of osteoarthritis development. Osteoarthritis Cartilage. 2012;20:476–485. doi: 10.1016/j.joca.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pritzker KP, Gay S, Jimenez SA, Ostergaard K, Pelletier JP, Revell PA, Salter D, van den Berg WB. Osteoarthritis cartilage histopathology: grading and staging. Osteoarthritis Cartilage. 2006;14:13–29. doi: 10.1016/j.joca.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 47.Davidson RK, Waters JG, Kevorkian L, Darrah C, Cooper A, Donell ST, Clark IM. Expression profiling of metalloproteinases and their inhibitors in synovium and cartilage. Arthritis Res Ther. 2006;8:R124. doi: 10.1186/ar2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu YK, Ke Y, Wang B, Lin JH. The role of MCP-1-CCR2 ligand-receptor axis in chondrocyte degradation and disease progress in knee osteoarthritis. Biol Res. 2015;48:64. doi: 10.1186/s40659-015-0057-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Raghu H, Lepus CM, Wang Q, Wong HH, Lingampalli N, Oliviero F, Punzi L, Giori NJ, Goodman SB, Chu CR, et al. CCL2/CCR2, but not CCL5/CCR5, mediates monocyte recruitment, inflammation and cartilage destruction in osteoarthritis. Ann Rheum Dis. 2017;76:914–922. doi: 10.1136/annrheumdis-2016-210426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Blanco FJ, Rego I, Ruiz-Romero C. The role of mitochondria in osteoarthritis. Nat Rev Rheumatol. 2011;7:161–169. doi: 10.1038/nrrheum.2010.213. [DOI] [PubMed] [Google Scholar]
- 51.Rambold AS, Lippincott-Schwartz J. Mechanisms of mitochondria and autophagy crosstalk. Cell Cycle. 2011;10:4032–4038. doi: 10.4161/cc.10.23.18384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tang Q, Zheng G, Feng Z, Chen Y, Lou Y, Wang C, Zhang X, Zhang Y, Xu H, Shang P, et al. Trehalose ameliorates oxidative stress-mediated mitochondrial dysfunction and ER stress via selective autophagy stimulation and autophagic flux restoration in osteoarthritis development. Cell Death Dis. 2017;8:e3081. doi: 10.1038/cddis.2017.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ansari MY, Ball HC, Wase SJ, Novak K, Haqqi TM. Lysosomal dysfunction in osteoarthritis and aged cartilage triggers apoptosis in chondrocytes through BAX mediated release of Cytochrome c. Osteoarthritis Cartilage. 2021;29:100–112. doi: 10.1016/j.joca.2020.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nakamura S, Shigeyama S, Minami S, Shima T, Akayama S, Matsuda T, Esposito A, Napolitano G, Kuma A, Namba-Hamano T, et al. LC3 lipidation is essential for TFEB activation during the lysosomal damage response to kidney injury. Nat Cell Biol. 2020;22:1252–1263. doi: 10.1038/s41556-020-00583-9. [DOI] [PubMed] [Google Scholar]
- 55.Runwal G, Stamatakou E, Siddiqi FH, Puri C, Zhu Y, Rubinsztein DC. LC3-positive structures are prominent in autophagy-deficient cells. Sci Rep. 2019;9:10147. doi: 10.1038/s41598-019-46657-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Evans CH, Kraus VB, Setton LA. Progress in intra-articular therapy. Nat Rev Rheumatol. 2014;10:11–22. doi: 10.1038/nrrheum.2013.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu Q, Niu J, Li H, Ke Y, Li R, Zhang Y, Lin J. Knee symptomatic osteoarthritis, walking disability, NSAIDs use and all-cause mortality: population-based Wuchuan Osteoarthritis Study. Sci Rep. 2017;7:3309. doi: 10.1038/s41598-017-03110-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fu J, Li S, Feng R, Ma H, Sabeh F, Roodman GD, Wang J, Robinson S, Guo XE, Lund T, et al. Multiple myeloma-derived MMP-13 mediates osteoclast fusogenesis and osteolytic disease. J Clin Invest. 2016;126:1759–1772. doi: 10.1172/JCI80276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Park MJ, Moon SJ, Baek JA, Lee EJ, Jung KA, Kim EK, Kim DS, Lee JH, Kwok SK, Min JK, et al. Metformin augments anti-inflammatory and chondroprotective properties of mesenchymal stem cells in experimental osteoarthritis. J Immunol. 2019;203:127–136. doi: 10.4049/jimmunol.1800006. [DOI] [PubMed] [Google Scholar]
- 60.Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KH. Interleukin-1 and IL-23 induce innate IL-17 production from γδ T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009;31:331–341. doi: 10.1016/j.immuni.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 61.Li Z, Cheng J, Liu J. Baicalin protects human OA chondrocytes against IL-1β-induced apoptosis and ECM degradation by activating autophagy via miR-766-3p/AIFM1 axis. Drug Des Devel Ther. 2020;14:2645–2655. doi: 10.2147/DDDT.S255823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kong C, Wang C, Shi Y, Yan L, Xu J, Qi W. Active vitamin D activates chondrocyte autophagy to reduce osteoarthritis via mediating the AMPK-mTOR signaling pathway. Biochem Cell Biol. 2020;98:434–442. doi: 10.1139/bcb-2019-0333. [DOI] [PubMed] [Google Scholar]
- 63.Wang J, Lian H, Zhao Y, Kauss MA, Spindel S. Vitamin D3 induces autophagy of human myeloid leukemia cells. J Biol Chem. 2008;283:25596–25605. doi: 10.1074/jbc.M801716200. [DOI] [PubMed] [Google Scholar]
- 64.Lisse TS, Hewison M. Vitamin D: a new player in the world of mTOR signaling. Cell Cycle. 2011;10:1888–1889. doi: 10.4161/cc.10.12.15620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen YC, Sung HC, Chuang TY, Lai TC, Lee TL, Lee CW, Lee IT, Chen YL. Vitamin D3 decreases TNF-α-induced inflammation in lung epithelial cells through a reduction in mitochondrial fission and mitophagy. Cell Biol Toxicol. 2021:1–24. doi: 10.1007/s10565-021-09629-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee TL, Lee MH, Chen YC, Lee YC, Lai TC, Lin HY, Hsu LF, Sung HC, Lee CW, Chen YL. Vitamin D attenuates ischemia/reperfusion-induced cardiac injury by reducing mitochondrial fission and mitophagy. Front Pharmacol. 2020;11:604700. doi: 10.3389/fphar.2020.604700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Minisola S, Ferrone F, Danese V, Cecchetti V, Pepe J, Cipriani C, Colangelo L. Controversies surrounding vitamin D: focus on supplementation and cancer. Int J Environ Res Public Health. 2019;16:189. doi: 10.3390/ijerph16020189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lewis JR, Sim M, Daly RM. The vitamin D and calcium controversy: an update. Curr Opin Rheumatol. 2019;31:91–97. doi: 10.1097/BOR.0000000000000584. [DOI] [PubMed] [Google Scholar]
- 69.Glendenning P, Inderjeeth CA. Controversy and consensus regarding vitamin D: recent methodological changes and the risks and benefits of vitamin D supplementation. Crit Rev Clin Lab Sci. 2016;53:13–28. doi: 10.3109/10408363.2015.1074157. [DOI] [PubMed] [Google Scholar]
- 70.Jin X, Antony B, Wang X, Persson MS, McAlindon T, Arden NK, Srivastava S, Srivastava R, Van Middelkoop M, Bierma-Zeinstra SM, et al. Effect of vitamin D supplementation on pain and physical function in patients with knee osteoarthritis (OA): an OA Trial Bank protocol for a systematic review and individual patient data (IPD) meta-analysis. BMJ Open. 2020;10:e035302. doi: 10.1136/bmjopen-2019-035302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tu L, Zheng S, Cicuttini F, Jin X, Han W, Zhu Z, Antony B, Winzenberg T, Jones G, Gu J, et al. Effects of vitamin D supplementation on disabling foot pain in patients with symptomatic knee osteoarthritis. Arthritis Care Res (Hoboken) 2021;73:781–787. doi: 10.1002/acr.24371. [DOI] [PubMed] [Google Scholar]