Abstract
Objectives
Evidence from several lines of research suggests the critical role of neuropeptide oxytocin in social cognition and social behavior. Though a few studies have examined the effect of oxytocin on clinical symptoms of schizophrenia, the underlying neurobiological changes are underexamined. Hence, in this study, we examined the effect of oxytocin on the brain’s effective connectivity in schizophrenia.
Methods
31 male patients with schizophrenia (SCZ) and 21 healthy male volunteers (HV) underwent resting functional magnetic resonance imaging scans with intra-nasal oxytocin (24 IU) and placebo administered in counterbalanced order. We conducted a whole-brain effective connectivity analysis using a multivariate vector autoregressive granger causality model. We performed a conjunction analysis to control for spurious changes and canonical correlation analysis between changes in connectivity and clinical and demographic variables.
Results
Three connections, sourced from the left caudate survived the FDR correction threshold with the conjunction analysis; connections to the left supplementary motor area, left precentral gyrus, and left frontal inferior triangular gyrus. At baseline, SCZ patients had significantly weaker connectivity from caudate to these three regions. Oxytocin, but not placebo, significantly increased the strength of connectivity in these connections. Better cognitive insight and lower negative symptoms were associated with a greater increase in connectivity with oxytocin.
Conclusions
These findings provide a preliminary mechanistic understanding of the effect of oxytocin on brain connectivity in schizophrenia. The study findings provide the rationale to examine the potential utility of oxytocin for social cognitive deficits in schizophrenia.
Keywords: neuropeptide, psychosis, negative symptoms, social cognition, fMRI
Introduction
Emerging evidence from several lines of research suggests the critical role of neuropeptide oxytocin in social cognition and social behavior across species.1 Several studies have examined the potential of intranasal oxytocin as an adjunct treatment for social cognitive deficits in schizophrenia.2 While the effect of oxytocin in improving positive and negative symptoms and neurocognitive deficits is still debated, higher-level social cognition is likely to improve with intranasal oxytocin.3 Though several studies have examined the effect of oxytocin on clinical symptoms of schizophrenia, the underlying neurobiological changes are not well explored. In a first fMRI study in schizophrenia patients, authors examined the effect of intranasal oxytocin on brain activity using implicit facial emotion recognition paradigm and reported attenuated amygdala activity for emotional faces.4 Recent task-based fMRI studies in patients with schizophrenia have reported modulatory effect of oxytocin on social brain regions using theory of mind tasks5 and stochastically rewarded decision-making task.6
Studies in healthy volunteers suggest that intranasal oxytocin has a modulatory effect on large-scale resting-state brain networks; oxytocin enhanced intrinsic cortico-striatal functional connectivity7 and amygdala – prefrontal cortex connectivity.8 The only such study in schizophrenia used a seed-based connectivity analysis with the amygdala as seed. The authors reported increased functional connectivity between the amygdala and left middle temporal gyrus, superior temporal sulcus, and angular gyrus in schizophrenia patients with intranasal oxytocin. The study also reported a correlation between negative symptoms and increased connectivity between the amygdala and temporal lobe structures with oxytocin.9
In the studies outlined above, functional connectivity analysis has been the most prevalent mode of research focusing on the strength of connectivity between regions. On the other hand, effective connectivity examines how one neural system exerts influence on another and gives valuable insights into information flow between different brain regions. In other words, effective connectivity analysis allows inferences on directional causal influence.10 Only a few studies have examined the effective connectivity changes with intranasal oxytocin. A recent study reported intranasal oxytocin to modulate the effective connectivity between the precuneus and the dorsolateral prefrontal cortex in healthy individuals.11 To date, no study has examined the effect of intranasal oxytocin on effective connectivity in schizophrenia using resting-state fMRI.
Hence, in this study, we examined the effect of intranasal oxytocin on effective connectivity in schizophrenia using fMRI. We employed a single-blind placebo-controlled design to investigate the impact of single-dose intranasal oxytocin on the whole-brain effective connectivity. As the scanner environment-related stress may cause sympathetic arousal and affect brain activity during the first scan, we considered a three-scan paradigm.12–15 We conducted a baseline scan without administering any drug, and in the subsequent two scans, we administered placebo and oxytocin in counterbalanced order. Considering the paucity of literature in the patient population, we conducted a whole-brain analysis rather than restricting it to specific regions of interest like the amygdala. We ran a conjunction analysis to identify directional connections in the brain that simultaneously satisfied all the hypotheses and corrected for the whole brain multiple comparisons. Specifically, we tested whether patients with schizophrenia (SCZ) will have significantly weaker connections than healthy volunteers (HV) at baseline and whether oxytocin will significantly strengthen connectivity in SCZ than HV compared to their respective baselines.
Materials and Methods
Overview of the Design
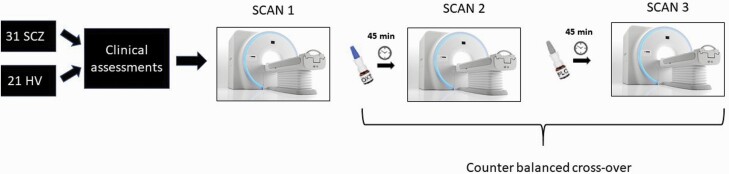
The study involved 31 male patients with schizophrenia (SCZ) and 21 healthy male volunteers (HV). All subjects underwent three resting functional magnetic resonance imaging (rsfMRI) scans. During the first scan (referred to as baseline scan), no drug was administered. Subjects self-administered 24 IU oxytocin or saline nasal spray 45 min before the second and third scan. The Institute’s human ethics committee approved the study. All participants gave written informed consent. A schematic diagram of the study design is presented in figure 1.
Fig. 1.
Overview of the study design.
Subjects
Patients were recruited from the National Institute of Mental Health and Neurosciences (NIMHANS), Bangalore, India, between 2013 and 2018. Healthy controls were recruited from the community using flyers, advertisements, and word of mouth. We interviewed all patients using the structured clinical interview for DSM-IV Axis I disorders (SCID-I)16 and recruited those diagnosed with schizophrenia or schizoaffective disorder. We excluded those with substance dependence/abuse in the past 12 months (except nicotine), concurrent comorbid axis-I disorder, less than ten years of formal education, risk of harm to self or others, history of electroconvulsive therapy in the preceding six months, clinically significant physical illness, presence of metal implants in the body, claustrophobia and current upper respiratory tract infection or nose block. Also, healthy volunteers were excluded if they had a lifetime history of any axis-1 diagnosis or family history of schizophrenia in a first-degree relative. All patients were on treatment with antipsychotic medications with some on a combination of antipsychotics [Risperidone = 14; Clozapine = 3; Haloperidol=2;Fluphenazine = 2; Aripiprazole = 5; Olanzapine = 4; Amisulpiride = 6; Flupenthixol = 2; Ziprasidone = 1; Zuclopenthixol = 1]. The chlorpromazine equivalent17 of the antipsychotics was calculated, and the mean dose was 505.48(375.72).
Assessments
The severity of clinical symptoms was documented using the Positive and Negative Syndrome Scale (PANSS)18 and the Scale for the Assessment of Negative Symptoms (SANS).19 Level of functioning was assessed with the Global Assessment of Function Scale (GAF),20 depressive symptoms with Calgary Depression Scale for schizophrenia (CDSS),21 and severity of nicotine dependence through Fagerstrom Test for Nicotine Dependence (FTND).22
Oxytocin/Placebo Administration
In the second and third scans, subjects self-administered six puffs of oxytocin (24 IU) or saline nasal spray 45min before each scan. The participants administered the drug in the presence of an experimenter as per the established guidelines.23 We used identical and covered spray bottles to blind the participants to the drug administered. We also counterbalanced the order of administration of oxytocin or placebo. Participants were asked not to use alcohol the previous night and not to smoke cigarettes for at least four hours before the scan. Further details of the oxytocin administration are given in the supplementary material.
OXTR Genotyping
OXTR single-nucleotide polymorphism (rs2254298) was examined since it has been reported to influence the response to oxytocin.24 Among the participants, 2 SCZ and 1 HV did not agree to genotyping. The polymorphism was determined by standard allelic discrimination TaqMan assay that uses the 5′ exonuclease activity of Taq DNA polymerase to detect a fluorescent reporter signal generated after PCR amplification.
Image Acquisition and Preprocessing
The blood oxygenation level-dependent (BOLD) T2* weighted echo-planar images (TR = 2500 ms, TE = 30 ms, flip angle = 78 deg, no interslice gap, matrix size = 64 × 64; slice thickness = 3 mm; the number of slices = 46) were acquired using a 3-Tesla MRI scanner (Skyra, Siemens Healthcare, Germany). Participants were scanned for 6 min 22 sec in a single run, providing 153 volumes for analysis. Anatomical T1 reference images were also acquired using 3D MPRAGE structural sequence (TR = 2200, TE = 2.45 ms, flip angle = 8 deg, FOV = 256, matrix size = 256*256; slice thickness = 1 mm; number of slices = 176). We used foam pads to reduce the head motion. During the scan, participants viewed a white fixation “+” on a black background using an MR-compatible visual stimulation system. Participants were instructed to look at the fixation cross and allow their minds to wander.
Standard resting-state fMRI preprocessing was carried out in Data Processing Assistant for Resting-State fMRI (DPARSF) (http://rfmri.org/DPARSF). The first three volumes were discarded from the resting functional data to avoid scanner instability. Preprocessing steps included slice timing, motion correction was done with the help of 24 Friston motion parameters and Framewise Displacement (FD) followed by normalization to MNI space, detrending, and regressing out nuisance covariates such as white matter signal and cerebrospinal fluid signal. Data was spatially smoothed using a 6 mm full width half maximum (FWHM) Gaussian kernel and excluded all participants with more than 3 mm movement. We employed a popular blind deconvolution algorithm to minimize the non-neural variability of the HRF and estimate the latent neuronal time-series.25 The method considers resting-state fMRI data as “spontaneous event-related” with randomly occurring events and estimates voxel-specific HRFs using Weiner deconvolution. The low pass filtering (0.01–0.1 Hz) was run on the deconvolved data. Given the high dimensionality of whole-brain fMRI data, filtered mean deconvolved fMRI time series were obtained from 90 functional brain regions across the entire brain using MarSBar (http://marsbar.sourceforge.net) and WFU-pick-atlas (https://www.nitrc.org/projects/wfu_pickatlas).
The whole-brain effective connectivity analysis was run using a multivariate vector autoregressive (MVAR) model.26,27 Directionality obtained from the MVAR model, such as Granger causality, is a reliable method for making inferences about directional influences between brain regions using fMRI. It is also validated by in-vivo electrophysiological ground truth data as well.28 Details of the model are given in supplementary material. As a data-driven approach, Granger causality analysis does not need the specification of connectivity priors. The model assumes that if past values of a time series T1 (from neural system X) help predict the present and future values of the time series T2 (from neural system Y), directional causal influence from T1 to T2 can be inferred.29 A whole-brain effective connectivity matrix was obtained for each participant (90 × 90, since we had 90 ROIs) following the previous studies’ methods.30,31 A Granger causality value of 0 represents no causal relationship from the source to the destination region, a value of 1 represents strong positive causality, and a value of −1 represents strong negative causality.
Group-level Analysis
We performed a conjunction analysis to control for spurious changes due to within-subject, between scan variability. This would enable us to generalize the results of the study to the population in general.32 Based on the previous literature, the conjunction analysis was conducted with the tests described below (1) Group difference between SCZ and HV in the baseline scan and placebo scan, with the hypothesis that SCZ will have weaker connections compared to HV (independent sample t-test) (2) In both SCZ and HV, comparison of the placebo scans and baseline scans will not show a significant change in connectivity (paired t-test) (3) In SCZ, the oxytocin scan will result in greater connectivity compared to baseline scan (paired t-test) (4) The change in connectivity between the baseline scan and the oxytocin scan will be greater in SCZ than HV (independent sample t-test). We applied FDR correction to control for multiple comparisons.
Correlation with Behavior
We conducted a canonical correlation analysis (CCA) between changes in connectivity on one-side and clinical and demographic variables on the other side. CCA is a method to identify the relation between variables from different modalities. Due to its’ ability to handle large number of variables, CCA is ideal for examining the multi-faceted relation between functional brain connectivity and behavior.33 Further details of CCA are given in the supplementary material. We used permutation testing with 1000,000 iterations to find significant latent variables that correlate with each other between the imaging and non-imaging measures and their loadings.34 To examine the possible confounding effect of antipsychotic medication, we conducted a Pearson’s correlation analysis between CPZ equivalent of antipsychotic dose and change in the effective connectivity (example: connectivity during oxytocin minus connectivity during baseline) in connections that survived the correction threshold with the conjunction analysis.
Results
The socio-demographic and clinical characteristics of the sample are given in table 1. There was a significant difference between the groups in years of education and age, which were used as covariates in the group-level analysis. The number of homozygous GG and heterozygous AG were equally distributed in the patient and control groups (table 1).
Table 1.
Demographic and Clinical Characteristics of Participants
| Schizophrenia (mean ± SD) n = 31 |
Healthy volunteer (mean ± SD) N = 21 |
t/χ 2 | P | |
|---|---|---|---|---|
| Age | 31.87 ± 7.67 | 25.33 ± 3.86 | 3.60 | 0.001 |
| Education | 15.06 ± 2.17 | 16.90 ± 2.68 | 2.72 | 0.009 |
| Global Assessment of functioning | 60.32 ± 15.91 | 93.81 ± 2.48 | 9.54 | <0.001 |
| PANSS -positive | 11.65 ± 5.43 | - | - | - |
| PANSS-negative | 12.39 ± 5.54 | - | - | - |
| PANSS- general psychopathology | 26.03 ± 4.92 | - | - | - |
| PANSS - total | 49.77 ± 12.37 | - | - | - |
| SANS | 24.06 ± 16.97 | - | - | - |
| Clinical Global Impression | 3.48 ± 1.12 | - | - | - |
| CDSS | 2.03 ± 1.66 | - | - | - |
| Age at onset of psychosis in years | 21.57 ± 4.58 | - | - | - |
| Duration of illness in years | 8.8 5 ± 5.75 | - | - | - |
| OXTR polymorphism (G/G:A/G) | 26:5 | 18:3 | 0.03 | 1.00 |
t – Independent t-test; χ2 – Chi-square test; PANSS – Positive and negative syndrome scale; SANS – Scale for assessment of negative symptoms; OXTR – Oxytocin receptor polymorphism (rs2254298)
Group Level Analysis
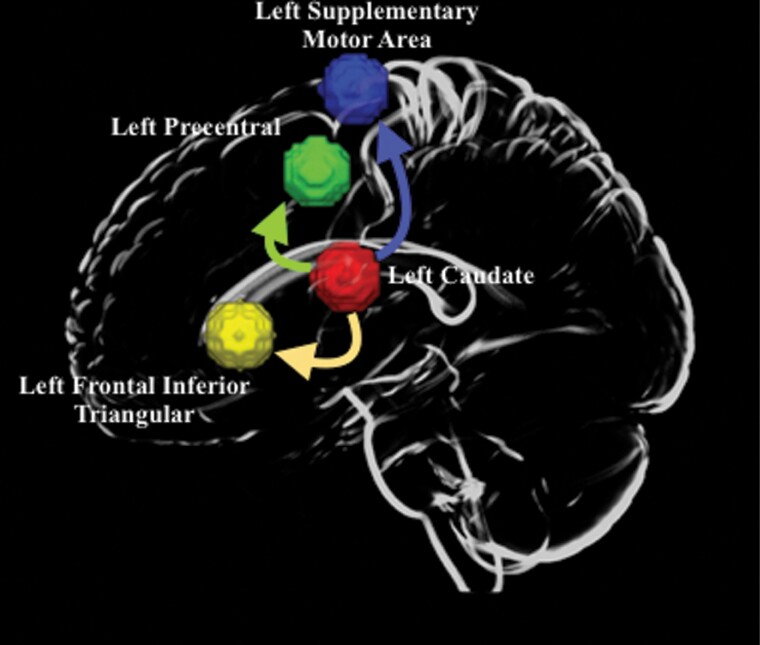
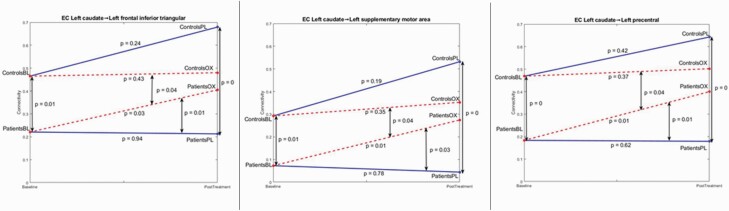
Three connections survived the FDR correction threshold with the conjunction analysis on the whole brain. All three connections were sourced from the left caudate. These connections were from left caudate to (1) left supplementary motor area (MNI coordinates – x = −5,y = 5,z = 6) 2) left precentral gyrus (MNI coordinates –x = −39;y = −6;z = 51) 3) left frontal inferior triangular gyrus (MNI coordinates –x = −46;y = 30;z = 14). None of the other connections survived the stringent threshold that was applied. (figure 2 and Supplementary fig. S2). At baseline, SCZ patients had significantly weaker connectivity from caudate to these three regions (P < .05, FDR corrected); (1) Left caudate and left supplementary motor area (pcorr = 0.01) (2) Left caudate and left precentral gyrus (pcorr = 0.0) (3) Left caudate and left frontal inferior triangular (pcorr = 0.01).
Fig. 2.
Brain regions involved in the affected network. The regions were defined based on the WFU-pickatlas brain atlas. The left caudate, is the source of all three connections.
Neither SCZ nor HV had a significant difference between baseline and placebo conditions in these connections; SCZ: (1) Left caudate and left supplementary motor area (pcorr = 0.78) (2) Left caudate and left precentral gyrus (pcorr = 0.62) (3) Left caudate and left frontal inferior triangular (pcorr = 0.94). HV: (1) Left caudate and left supplementary motor area (pcorr = 0.19) (2) Left caudate and left precentral gyrus (pcorr = 0.42) (3) Left caudate and left frontal inferior triangular (pcorr = 0.24).
There was a significant group X condition effect in all three networks; (1) Left caudate and left supplementary motor area (pcorr = 0.04) (2) Left caudate and left precentral gyrus (pcorr = 0.04) (3) Left caudate and left frontal inferior triangular (pcorr = 0.04). While SCZ had a significant difference between baseline and oxytocin conditions in these connections, there was no significant difference in HV. SCZ: (1) Left caudate and left supplementary motor area (pcorr = 0.01) (2) Left caudate and left precentral gyrus (pcorr = 0.01) iii) Left caudate and left frontal inferior triangular (pcorr = 0.03). HV: (1) Left caudate and left supplementary motor area (pcorr = 0.35) (2) Left caudate and left precentral gyrus (pcorr = 0.37) (3) Left caudate and left frontal inferior triangular (pcorr = 0.43).
SCZ also had significant difference between placebo and oxytocin conditions in (1) Left caudate and left supplementary motor area (pcorrected = 0.03) (2) Left caudate and left precentral gyrus (pcorrected = 0.01) (3) Left caudate and left frontal inferior triangular (pcorrected = 0.01). The results are graphically represented in figure 3 along with connectivity strengths.
Fig. 3.
Graphical representation of the effective connectivity analysis showing the connections from left caudate to (A) left frontal inferior triangular gyrus, (B) left the supplementary motor area, and (C) left precentral gyrus. Solid lines: placebo, dashed lines: Oxytocin, BL: Baseline, PL: Placebo, OX: Oxytocin.
Correlation with Behavior
The CCA algorithm determined four modes. For the connectivity difference between oxytocin and placebo in patients, mode-3 was significant (FDR corrected P-values – mode-1 = 0.35, mode-2 = 0.26, mode-3 = 0.009 and mode-4 = 0.16). For the connectivity difference between oxytocin and placebo in patients, all three aths were positively correlated with SES and BCIS SR and negatively correlated with age of onset, PANSS Negative, and CGI Severity (Supplementary fig. S2). This shows that the better the socioeconomic status and cognitive insight, the earlier the age of onset, and the lesser the severity of negative symptoms, the greater the increase in connectivity with oxytocin than placebo.
There was no significant relationship between the CPZ equivalent of antipsychotics and change in effective connectivity in the three connections that survived the correction threshold in both oxytocin vs baseline comparison and placebo vs baseline comparison. The r and P values for individual connections are given below; (a) connectivity during oxytocin minus connectivity during baseline - left Caudate to left Frontal Inferior Triangular gyrus (r = −.0988; P = .6170), left supplementary motor area (r = 0.2565; P = .1877) and left Precentral gyrus (r = .1160; P = .5566) (b) connectivity during oxytocin minus connectivity during baseline – left Caudate to left frontal inferior triangular gyrus (r = −.0432; P = .8272), left supplementary motor area (r = .1307; P = .5074) and left Precentral gyrus (r = .0506; P = .7983).
Discussion
To the best of our knowledge, this is the first study to examine changes in whole-brain effective connectivity with intranasal oxytocin in patients with schizophrenia. We conducted a whole-brain conjunction analysis with stringent correction for multiple comparisons. We also controlled for potential confounding factors by matching the participants for oxytocin receptor variations, following a three-scan paradigm to avoid scan order-related biases and blinding participants to the drug administered. We found three affected connections, anchored in the left caudate, with connections to the left SMA, left pars triangularis, and left precentral gyrus. The connections were weaker in SCZ at baseline, which significantly improved with OXT. We also found that those with better cognitive insight and lesser negative symptoms had more significant enhancement in connectivity with oxytocin. While an earlier study reported the relation between negative symptoms and oxytocin-induced changes on amygdala functional (non-directional) connectivity,9 we have reported the directional connections with the caudate.
Several lines of research in schizophrenia have consistently reported abnormal structure, function, and dopamine neurotransmission in the caudate nucleus.35–39 Alterations in the reward pathway, which involves the caudate, are implicated in the symptomatology of schizophrenia.40,41 Several studies have also reported abnormalities in left pars triangularis,42,43 dysfunctional motor systems including the motor cortex and supplementary motor area44 and angular gyrus in schizophrenia.45 These innervations are also part of the neural networks implicated in several social cognitive functions. While the caudate has been traditionally implicated in motor functions, emerging literature suggests its role in social cognitive functions such as social decision making.46–49 Caudate, through its connections with the amygdala and cortical regions, could contribute to the social decision-making process by integrating social stimuli, memories, preconceptions of trust, and anticipated reward.50,51 Oxytocin administration increased caudate activity while participants performed tasks measuring trust, face processing, and parental attachment48,51–55 This is also supported by resting fMRI studies in which the modulatory effect of oxytocin on several large-scale networks involving the caudate nucleus have been demonstrated7,56–59 The left pars triangularis is part of the Broca’s area and is proposed as a crucial node of the mirror neuron system (MNS), involved in speech and processing speech associated gestures.60,61 The precentral gyrus containing the motor cortex and the supplementary motor area are core regions of the mirror neuron system. They are involved in action recognition, imitation, and agent representation.62
Despite the increased research interest in oxytocin in the last decade, we do not have a complete understanding of the neurobiology of oxytocin. Available evidence suggests that the oxytocin system is complex and has multifaceted influences on behavior mediated by modulation of oxytocin receptors rich brain regions such as the amygdala, insula, and striatum.3,63 While most of the initial seed-based analysis approach reported oxytocin’s effect on amygdala connections, several whole-brain analyses have suggested the modulatory effect of oxytocin in other brain regions as well. While we did not find a significant effect of oxytocin on amygdala connectivity, this could be due to methodological differences as the apriori conjunction analysis followed in the current study restricted multiple levels. For example, the connectivity will not be considered significant in the conjunction analysis if the effect of OXT on amygdala connectivity was similar across SCZ and HV or in opposite directions across groups as reported in the previous study.9 The correlation with clinical variables suggests higher socioeconomic status, better cognitive insight, lesser the severity of negative symptoms, and earlier the age of onset to be associated with greater response with oxytocin. While the first three variables are good prognostic factors, the earlier age at onset is considered as poor prognostic factor in schizophrenia.64 We do not have a definitive explanation for this association. Pending replication in an independent sample, these correlations need to be considered preliminary and to be examined in future studies.
The study findings have potential implications. How the modulation of resting-state networks with intranasal oxytocin seen in the current study translates to real-life social behavior remains to be examined.2 Interestingly, a few recent studies have reported oxytocin to have a significant influence on the functional brain activity and connectivity in social brain regions such as the amygdala, medial prefrontal cortex, temporoparietal junction, posterior cingulate gyrus, and insula using task-based fMRI.4–6 Given the close correspondence between task-based activation and resting-state activity,65 and resting-state fMRI connectivity and theory of mind task performance in schizophrenia,66 our findings suggest the possibility of oxytocin enhanced functional connectivity translating to better social cognitive function in schizophrenia patients.
The strengths of the study involve using a whole-brain effective connectivity analysis as opposed to the more widely used seed-based and functional connectivity approaches. The subjects were matched on genotype to control for the potential confound. We believe the use of three scans in analysis increased the methodological rigor by controlling the confounding effects of the scan order, such as cortisol release and changes in brain activation.12–15 This is particularly relevant in schizophrenia, as previous studies have shown disrupted stress-related dopamine release in patients with schizophrenia.67
However, our findings need to be considered in the background of a few limitations. First, we included only men as oxytocin’s effect on resting-state connectivity varies by sex.68 The groups were not matched on age and education. While it is ideal for matching the groups on these demographic variables, several studies have reported that patients with schizophrenia tend to have lower academic achievement compared to age-matched healthy volunteers.69,70 Hence, our sample is more representative of a real-world scenario. We statistically controlled for age and education in the analysis. Second, all patients were on treatment with antipsychotic medications with minimal symptoms, which may affect the connectivity. Earlier studies have reported interaction between oxytocin and dopamine in the nucleus accumbens.71 However, there was no relationship between CPZ equivalent of antipsychotic dose and oxytocin response in any of the paths like an earlier study.4 While the inclusion of drug naïve participants with severe symptoms would be ideal for controlling the confounding effect of medication, conducting a three-scan study in drug naïve individuals is practically challenging. Also, as oxytocin is examined as an add-on treatment, the current design has more potential clinical utility as it is likely to be used as an add-on treatment. Third, we administered 24 IU of oxytocin in this study. While this dose of oxytocin elicited effective brain activity54,72 and reached adequate CSF levels in healthy individuals,73 emerging evidence from a few recent studies suggest that patients with schizophrenia may show better response with higher dose of oxytocin >40 IU.74,75 As this evidence was not available at the time of study conduct, we used a lower dose of oxytocin. Future studies may consider using a higher dose of oxytocin. Also, due to ethical and logistic reasons we did not do a CSF analysis after administration of oxytocin to ensure absorption considering three scan design. As intranasal administration of oxytocin is challenging, future studies may consider doing a CSF or plasma levels after administration to ensure adequate absorption, if feasible. Finally, we conducted a single-dose study that provides inadequate information about the safety of oxytocin and its effect on behavior. However, the findings of this proof-of-concept study provide the rationale to do a long-term clinical trial with repeated doses to ascertain the definitive effects of oxytocin on brain connectivity, behavior, and long-term safety.
Conclusion
Findings of the current study suggest a significant modulatory effect of oxytocin on brain networks anchored in the left caudate with directional connections to the left SMA, left pars triangularis, and left precentral gyrus. These connections were significantly weaker in schizophrenia patients and significantly improved with oxytocin. These findings provide a preliminary mechanistic understanding of the effect of oxytocin on brain connectivity in schizophrenia. The study findings provide the rationale to examine the potential utility of oxytocin for social cognitive deficits in schizophrenia.
Supplementary Material
Acknowledgments
The authors have declared that there are no conflicts of interest in relation to the subject of this study. NPR conceptualized and designed the study. VK and AJ were involved in the data collection, data analysis, interpretation of results, and manuscript preparation. GD and NPR were involved in designing the analysis, analysis of the data, interpretation of the data and manuscript preparation. PD, UT, BN, DC, AD, AP were involved in data collection and manuscript preparation. BL was involved in data analysis and interpretation of findings. RDB, VK, SV, GV, were involved in interpretation of results and manuscript preparation. VK, AJ, GD, and NPR wrote the first draft of the manuscript and all authors contributed to revisions. All authors have approved the final manuscript.
Contributor Information
Vittal Korann, Department of Psychiatry, National Institute of Mental Health and Neurosciences, Bangalore, Karnataka, India.
Arpitha Jacob, Department of Psychiatry, National Institute of Mental Health and Neurosciences, Bangalore, Karnataka, India.
Bonian Lu, AU MRI Research Center, Department of Electrical and Computer Engineering, Auburn University, Auburn, AL, USA.
Priyanka Devi, Department of Psychiatry, National Institute of Mental Health and Neurosciences, Bangalore, Karnataka, India.
Umesh Thonse, Department of Psychiatry, National Institute of Mental Health and Neurosciences, Bangalore, Karnataka, India.
Bhargavi Nagendra, Department of Psychiatry, National Institute of Mental Health and Neurosciences, Bangalore, Karnataka, India.
Dona Maria Chacko, Department of Psychiatry, National Institute of Mental Health and Neurosciences, Bangalore, Karnataka, India.
Avyarthana Dey, Department of Psychiatry, National Institute of Mental Health and Neurosciences, Bangalore, Karnataka, India.
Anantha Padmanabha, Department of Psychiatry, National Institute of Mental Health and Neurosciences, Bangalore, Karnataka, India.
Venkataram Shivakumar, Department of Psychiatry, National Institute of Mental Health and Neurosciences, Bangalore, Karnataka, India.
Rose Dawn Bharath, Department of Psychiatry, National Institute of Mental Health and Neurosciences, Bangalore, Karnataka, India.
Vijay Kumar, Department of Psychiatry, National Institute of Mental Health and Neurosciences, Bangalore, Karnataka, India.
Shivarama Varambally, Department of Psychiatry, National Institute of Mental Health and Neurosciences, Bangalore, Karnataka, India.
Ganesan Venkatasubramanian, Department of Psychiatry, National Institute of Mental Health and Neurosciences, Bangalore, Karnataka, India.
Gopikrishna Deshpande, AU MRI Research Center, Department of Electrical and Computer Engineering, Auburn University, Auburn, AL, USA; Department of Psychological Sciences, Auburn University, Auburn, AL, USA; Center for Neuroscience, Auburn University, Auburn, AL, USA.
Naren P Rao, Department of Psychiatry, National Institute of Mental Health and Neurosciences, Bangalore, Karnataka, India.
Funding
Funded by Department of Science and Technology, Government of India - (PI- Dr. Naren P Rao; IFA/12/LSBM/36).
References
- 1. Meyer-Lindenberg A, Domes G, Kirsch P, Heinrichs M. Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nat Rev Neurosci. 2011;12(9):524–538. doi: 10.1038/nrn3044. [DOI] [PubMed] [Google Scholar]
- 2. Zheng W, Xiao-Min Z, Qing-E Z, et al. Adjunctive intranasal oxytocin for schizophrenia: a meta-analysis of randomized, double-blind, placebo-controlled trials. Schizophr Res. 2019;206. doi: 10.1016/j.schres.2018.12.007 [DOI] [PubMed] [Google Scholar]
- 3. Bürkner PC, Williams DR, et al. Institute of Psychology Muenster, Germany U of M. Intranasal oxytocin may improve high-level social cognition in schizophrenia, but not social cognition or neurocognition in general: a multilevel bayesian meta-analysis. Schizophr Bull. 2017;43(6):1291–1303. doi: 10.1093/schbul/sbx053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shin NY, Park HY, Jung WH, et al. Effects of oxytocin on neural response to facial expressions in patients with schizophrenia. Neuropsychopharmacology. 2015;40(8):1919–1927. doi: 10.1038/NPP.2015.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. de Coster L, Lin L, Mathalon DH, Woolley JD. Neural and behavioral effects of oxytocin administration during theory of mind in schizophrenia and controls: a randomized control trial. Neuropsychopharmacology. 2019;44(11):1925–1931. doi: 10.1038/S41386-019-0417-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wigton R, Tracy DK, Verneuil TM, et al. The importance of pro-social processing, and ameliorating dysfunction in schizophrenia. An FMRI study of oxytocin. Schizophr Res Cogn. 2021;27. doi: 10.1016/J.SCOG.2021.100221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bethlehem RAI, Lombardo M, Lai MC, et al. Intranasal oxytocin enhances intrinsic corticostriatal functional connectivity in women. Transl Psychiatry. 2017;7(4). doi: 10.1038/tp.2017.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Eckstein M, Markett S, Keith MK, et al. Oxytocin differentially alters resting state functional connectivity between amygdala subregions and emotional control networks: inverse correlation with depressive traits. Neuroimage 2017;14:9. doi: 10.1016/j.neuroimage.2017.01.078. [DOI] [PubMed] [Google Scholar]
- 9. Abram S, de Coster L, Roach BJ, et al. Oxytocin enhances an amygdala circuit associated with negative symptoms in schizophrenia: a single-dose, placebo-controlled, crossover, randomized control trial. Schizophr Bull. 2020;46(3):661–669. doi: 10.1093/schbul/sbz091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Deshpande G, Santhanam P, Hu X. Instantaneous and causal connectivity in resting state brain networks derived from functional MRI data. Neuroimage 2011;54(2):1043–1052. doi: 10.1016/j.neuroimage.2010.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kumar J, Iwabuchi SJ, Völlm BA, Palaniyappan L. Oxytocin modulates the effective connectivity between the precuneus and the dorsolateral prefrontal cortex. Eur Arch Psychiatry Clin Neurosci. 2020;270(5):567–576. doi: 10.1007/s00406-019-00989-z. [DOI] [PubMed] [Google Scholar]
- 12. Muehlhan M, Lueken U, Siegert J, Hans-Ulrich W, Michael N.S, Kirschbaum C. Enhanced sympathetic arousal in response to FMRI scanning correlates with task induced activations and deactivations. PLoS One. 2013;8(8). doi: 10.1371/journal.pone.0072576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Raz A, Lieber B, Soliman F, et al. Ecological nuances in functional magnetic resonance imaging (fMRI): psychological stressors, posture, and hydrostatics. Neuroimage 2005;25(1):1–7. doi: 10.1016/j.neuroimage.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 14. Tessner KD, Walker EF, Hochman K, Hamann S. Cortisol responses of healthy volunteers undergoing magnetic resonance imaging. Hum Brain Mapp. 2006;27(11):889–895. doi: 10.1002/hbm.20229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zandbelt BB, Gladwin TE, Raemaekers M, et al. Within-subject variation in BOLD-fMRI signal changes across repeated measurements: quantification and implications for sample size. Neuroimage 2008;42(1):196–206. doi: 10.1016/j.neuroimage.2008.04.183. [DOI] [PubMed] [Google Scholar]
- 16. First M, Spitzer R, Gibbon M, Williams J.. Structured clinical interview for DSM-IV-TR axis I disorders, research version, patient edition. 2002. [Google Scholar]
- 17. Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003;64(6):663–667. doi: 10.4088/JCP.v64n0607. [DOI] [PubMed] [Google Scholar]
- 18. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 19. Andreasen NC. Scale for the Assessment of Negative Symptoms (SANS). Iowa city: University of Iowa; 1981. [Google Scholar]
- 20. Endicott J. The global assessment scale. Arch Gen Psychiatry. 2011;33(6):766. doi: 10.1001/archpsyc.1976.01770060086012. [DOI] [PubMed] [Google Scholar]
- 21. Addington J, Shah H, Liu L, Addington D. Reliability and validity of the calgary depression scale for schizophrenia (CDSS) in youth at clinical high risk for psychosis. Schizophr Res. 2014;153(1-3):64–67. doi: 10.1016/j.schres.2013.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fagerstrom KO, Schneider NG. Measuring nicotine dependence: a review of the Fagerstrom Tolerance Questionnaire. J Behav Med. 1989;12(2):159–182. doi: 10.1007/BF00846549. [DOI] [PubMed] [Google Scholar]
- 23. Guastella AJ, Hickie IB, McGuinness MM, et al. Recommendations for the standardisation of oxytocin nasal administration and guidelines for its reporting in human research. Psychoneuroendocrinology 2013;38(5):612–625. doi: 10.1016/j.psyneuen.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 24. Seeley SH, Chou Y, O’Connor MF. Intranasal oxytocin and OXTR genotype effects on resting state functional connectivity: a systematic review. Neurosci Biobehav Rev. 2018;95:17–32. doi: 10.1016/j.neubiorev.2018.09.011. [DOI] [PubMed] [Google Scholar]
- 25. Wu GR, Liao W, Stramaglia S, Ding JR, Chen H, Marinazzo D. A blind deconvolution approach to recover effective connectivity brain networks from resting state fMRI data. Med Image Anal. 2013;17(3):365–374. doi: 10.1016/j.media.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 26. Deshpande G, Sathian K, Hu X. Assessing and compensating for zero-lag correlation effects in time-lagged Granger causality analysis of FMRI. IEEE Trans Biomed Eng. 2010;57(6):1446–1456. doi: 10.1109/TBME.2009.2037808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Deshpande G, Hu X, Lacey S, Stilla R, Sathian K. Object familiarity modulates effective connectivity during haptic shape perception. Neuroimage 2010;49(3):1991–2000. doi: 10.1016/j.neuroimage.2009.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang Y, David O, Hu X, Deshpande G. Can Patel’s τ accurately estimate directionality of connections in brain networks from fMRI? Magn Reson Med. 2017;78(5):2003–2010. doi: 10.1002/mrm.26583. [DOI] [PubMed] [Google Scholar]
- 29. Granger CWJ. Investigating causal relations by econometric models and cross-spectral methods. Econometrica 1969;37(3):424–438. doi: 10.2307/1912791. [DOI] [Google Scholar]
- 30. Grant MM, Wood K, Sreenivasan K, et al. Influence of early life stress on intra- and extra-amygdaloid causal connectivity. Neuropsychopharm Off Publ Am Coll Neuropsychopharm. 2015;40(7):1782–1793. doi: 10.1038/npp.2015.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rangaprakash D, Wu GR, Marinazzo D, Hu X, Deshpande G. Hemodynamic response function (HRF) variability confounds resting-state fMRI functional connectivity. Magn Reson Med. 2018;80(4):1697–1713. doi: 10.1002/mrm.27146. [DOI] [PubMed] [Google Scholar]
- 32. Friston KJ, Holmes AP, Price CJ, Büchel C, Worsley KJ. Multisubject fMRI studies and conjunction analyses. Neuroimage 1999;10(4):385–396. doi: 10.1006/nimg.1999.0484. [DOI] [PubMed] [Google Scholar]
- 33. Wang HT, Smallwood J, Mourao-Miranda J, et al. Finding the needle in a high-dimensional haystack: canonical correlation analysis for neuroscientists. Neuroimage 2020;216:116745. doi: 10.1016/j.neuroimage.2020.116745. [DOI] [PubMed] [Google Scholar]
- 34. Smith SM, Elliott LT, Alfaro-Almagro F, et al. Brain aging comprises many modes of structural and functional change with distinct genetic and biophysical associations. Elife 2020:9. doi: 10.7554/eLife.52677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Weinstein JJ, Chohan MO, Slifstein M, Kegeles LS, Moore H, Abi-Dargham A. Pathway-specific dopamine abnormalities in schizophrenia. Biol Psychiatry. 2017;81(1):31–42. doi: 10.1016/j.biopsych.2016.03.2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chase HW, Loriemi P, Wensing T, Eickhoff SB, Nickl-Jockschat T. Meta-analytic evidence for altered mesolimbic responses to reward in schizophrenia. Hum Brain Mapp. 2018;39(7):2917–2928. doi: 10.1002/hbm.24049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Haijma S, van Haren N, Cahn W, Koolschijn PCMP, Hulshoff Pol HE, Kahn RS. Brain volumes in schizophrenia: a meta-analysis in over 18 000 subjects. Schizophr Bull. 2013;39(5):1129–1138. doi: 10.1093/schbul/sbs118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fusar-Poli P, Meyer-Lindenberg A. Striatal presynaptic dopamine in schizophrenia, part II: meta-analysis of [18F/11C]-DOPA PET studies. Schizophr Bull. 2013;39(1):33–42. doi: 10.1093/schbul/sbr180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Li Y, Li W, Xie D, Wang Y, Cheung EFC, Chan RCK. Grey matter reduction in the caudate nucleus in patients with persistent negative symptoms: an ALE meta-analysis. Schizophr Res. 2018;192:9–15. doi: 10.1016/j.schres.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 40. Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: Version III - The final common pathway. Schizophr Bull. 2009;35(3):549–562. doi: 10.1093/schbul/sbp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kapur S. Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. Am J Psychiatry. 2003;160(1):13–23. doi: 10.1176/appi.ajp.160.1.13. [DOI] [PubMed] [Google Scholar]
- 42. Li T, Wang Q, Zhang J, et al. Brain-wide analysis of functional connectivity in first-episode and chronic stages of schizophrenia. Schizophr Bull. 2017;43(2):436–448. doi: 10.1093/schbul/sbw099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Iwashiro N, Koike S, Satomura Y, et al. Association between impaired brain activity and volume at the sub-region of Broca’s area in ultra-high risk and first-episode schizophrenia: a multi-modal neuroimaging study. Schizophr Res. 2016;172(1-3):9–15. doi: 10.1016/j.schres.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 44. Abboud R, Noronha C, Diwadkar VA. Motor system dysfunction in the schizophrenia diathesis: neural systems to neurotransmitters. Eur Psychiatry. 2017;44:125–133. doi: 10.1016/j.eurpsy.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Torrey EF. Schizophrenia and the inferior parietal lobule. Schizophr Res. 2007;97(1-3):215–225. doi: 10.1016/j.schres.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 46. Crinion J, Turner R, Grogan A, et al. Language control in the bilingual brain. Science (1979) 2006;312(5779):1537–1540. doi: 10.1126/science.1127761. [DOI] [PubMed] [Google Scholar]
- 47. Santos GS, Nagasaka Y, Fujii N, Nakahara H. Encoding of social state information by neuronal activities in the macaque caudate nucleus. Social Neurosci. 2012;7(1):42–58. doi: 10.1080/17470919.2011.578465. [DOI] [PubMed] [Google Scholar]
- 48. Wang D, Yan X, Li M, Ma Y. Neural substrates underlying the effects of oxytocin: A quantitative meta-analysis of pharmaco-imaging studies. Social Cog Aff Neurosci. 2017;12(10):1565–1573. doi: 10.1093/scan/nsx085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kemp J, Berthel MC, Dufour A, et al. Caudate nucleus and social cognition: neuropsychological and SPECT evidence from a patient with focal caudate lesion. Cortex. 2013;49(2):559–571. doi: 10.1016/j.cortex.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 50. Wigton R, Radua J, Allen P, et al. Neurophysiological effects of acute oxytocin administration: Systematic review and meta-analysis of placebo-controlled imaging studies. J Psychiatry Neurosci. 2015;40(1):E1–E22. doi: 10.1503/jpn.130289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Grace SA, Rossell SL, Heinrichs M, Kordsachia C, Labuschagne I. Oxytocin and brain activity in humans: a systematic review and coordinate-based meta-analysis of functional MRI studies. Psychoneuroendocrinology 2018;96:6–24. doi: 10.1016/j.psyneuen.2018.05.031. [DOI] [PubMed] [Google Scholar]
- 52. Rilling JK, DeMarco AC, Hackett PD, et al. Effects of intranasal oxytocin and vasopressin on cooperative behavior and associated brain activity in men. Psychoneuroendocrinology 2012;37(4):447–461. doi: 10.1016/j.psyneuen.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Pincus D, Kose S, Arana A, et al. Inverse effects of oxytocin on attributing mental activity to others in depressed and healthy subjects: a double-blind placebo controlled fMRI study. Front Psychiatry. 2010;1(OCT). doi: 10.3389/fpsyt.2010.00134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Baumgartner T, Heinrichs M, Vonlanthen A, Fischbacher U, Fehr E. Oxytocin shapes the neural circuitry of trust and trust adaptation in humans. Neuron 2008;58(4):639–650. doi: 10.1016/j.neuron.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 55. Wittfoth-Schardt D, Gründing J, Wittfoth M, et al. Oxytocin modulates neural reactivity to children’s faces as a function of social salience. Neuropsychopharmacology. 2012;37(8):1799–1807. doi: 10.1038/npp.2012.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Joseph JE, Vaughan BK, Camp CC, et al. Oxytocin-induced changes in intrinsic network connectivity in cocaine use disorder: modulation by gender, childhood trauma, and years of use. Front Psychiatry. 2019;10. doi: 10.3389/fpsyt.2019.00502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Xin F, Zhou F, Zhou X, et al. Oxytocin modulates the intrinsic dynamics between attention-related large-scale networks. Cereb Cortex. 2018. doi: 10.1093/cercor/bhy295. [DOI] [PubMed] [Google Scholar]
- 58. Zhao Z, Ma X, Geng Y, et al. Oxytocin differentially modulates specific dorsal and ventral striatal functional connections with frontal and cerebellar regions. Neuroimage 2019;184:781–789. doi: 10.1016/j.neuroimage.2018.09.067. [DOI] [PubMed] [Google Scholar]
- 59. Brodmann K, Gruber O, Goya-Maldonado R. Intranasal oxytocin selectively modulates large-scale brain networks in humans. Brain Connect. 2017;7(7):454–463. doi: 10.1089/brain.2017.0528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Skipper JI, Goldin-Meadow S, Nusbaum HC, Small SL. Speech-associated gestures, Broca’s area, and the human mirror system. Brain Lang. 2007;101(3):260–277. doi: 10.1016/j.bandl.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Fazio P, Cantagallo A, Craighero L, et al. Encoding of human action in Broca’s area. Brain 2009;132(7):1980–1988. doi: 10.1093/brain/awp118. [DOI] [PubMed] [Google Scholar]
- 62. Jeon H, Lee SH. From neurons to social beings: short review of the mirror neuron system research and its socio-psychological and psychiatric implications. Clin Psychopharmacol Neurosci. 2018;16(1):18–31. doi: 10.9758/cpn.2018.16.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Quintana DS, Lischke A, Grace S, Scheele D, Ma Y, Becker B. Advances in the field of intranasal oxytocin research: lessons learned and future directions for clinical research. Mol Psychiatry. 2020. doi: 10.1038/s41380-020-00864-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Immonen J, Jääskeläinen E, Korpela H, Miettunen J. Age at onset and the outcomes of schizophrenia: a systematic review and meta-analysis. Early Inter Psychiatry 2017;11(6):453–460. doi: 10.1111/EIP.12412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Nickerson LD. Replication of resting state-task network correspondence and novel findings on brain network activation during task fMRI in the human connectome project study. Sci Rep. 2018;8(1):1–12. doi: 10.1038/s41598-018-35209-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zemánková P, Lošák J, Czekóová K, et al. Theory of mind skills are related to resting-state frontolimbic connectivity in schizophrenia. Brain Connect. 2018;8(6):350–361. doi: 10.1089/brain.2017.0563. [DOI] [PubMed] [Google Scholar]
- 67. Schifani C, Tseng HH, Kenk M, et al. Cortical stress regulation is disrupted in schizophrenia but not in clinical high risk for psychosis. Brain 2018;141(7):2213–2224. doi: 10.1093/brain/awy133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ebner NC, Chen H, Porges E, et al. Oxytocin’s effect on resting-state functional connectivity varies by age and sex. Psychoneuroendocrinology 2016;69:50–59. doi: 10.1016/j.psyneuen.2016.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Frissen A, Lieverse R, Marcelis M, Drukker M, Delespaul P. Psychotic disorder and educational achievement: a family-based analysis. Soc Psychiatry Psychiatr Epidemiol. 2015;50(10):1511–1518. doi: 10.1007/s00127-015-1082-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Welham J, Isohanni M, Jones P, McGrath J. The antecedents of Schizophrenia: a review of birth cohort studies. Schizophr Bull. 2009;35(3):603–623. doi: 10.1093/schbul/sbn084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Liu Y, Wang ZX. Nucleus accumbens oxytocin and dopamine interact to regulate pair bond formation in female prairie voles. Neuroscience 2003;121(3):537–544. doi: 10.1016/S0306-4522(03)00555-4. [DOI] [PubMed] [Google Scholar]
- 72. Sripada CS, Phan KL, Labuschagne I, Welsh R, Nathan PJ, Wood AG. Oxytocin enhances resting-state connectivity between amygdala and medial frontal cortex. Int J Neuropsychopharmacol. 2013;16(2):255–260. doi: 10.1017/S1461145712000533. [DOI] [PubMed] [Google Scholar]
- 73. Striepens N, Kendrick KM, Hanking V, et al. Elevated cerebrospinal fluid and blood concentrations of oxytocin following its intranasal administration in humans. Sci Rep. 2013;3:3440. doi: 10.1038/srep03440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Sabe M, Zhao N, Crippa A, Strauss GP, Kaiser S. Intranasal oxytocin for negative symptoms of schizophrenia: systematic review, meta-analysis, and dose-response meta-analysis of randomized controlled trials. Int J Neuropsychopharmacol. 2021;24(8):601–614. doi: 10.1093/IJNP/PYAB020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Wynn JK, Green MF, Hellemann G, Reavis EA, Marder SR. A dose-finding study of oxytocin using neurophysiological measures of social processing. Neuropsychopharmacology. 2019;44(2):289–294. doi: 10.1038/S41386-018-0165-Y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.