Abstract
Acute lung injury (ALI) usually causes acute respiratory distress syndrome (ARDS), or even death in critical ill patients. Immune cell infiltration in inflamed lungs is an important hallmark of ARDS. Macrophages are a type of immune cell that participate in the entire pathogenic trajectory of ARDS and most prominently via their interactions with lung alveolar epithelial cells (AECs). In the early stage of ARDS, classically activated macrophages secrete pro-inflammatory cytokines to clearance of the pathogens which may damage alveolar AECs cell structure and result in cell death. Paradoxically, in late stage of ARDS, anti-inflammatory cytokines secreted by alternatively activated macrophages dampen the inflammation response and promote epithelial regeneration and alveolar structure remodeling. In this review, we discuss the important role of macrophages and AECs in the progression of ARDS.
KEY WORDS: Macrophages, Alveolar epithelial cells, Acute respiratory distress syndrome
Introduction
Acute respiratory distress syndrome (ARDS) is characterized by acute, diffuse, inflammatory lung injury leading to increased alveolar capillary permeability and loss of aerated lung tissue. Hypoxemia is the major judgement criteria for ARDS, with estimated mortality rates of 27%, 32%, and 45% in mild, moderate, and severe hypoxemia cases, respectively [1]. ARDS arises from a variety of direct causes including pneumonia (bacterial and viral), inhalational lung injury, lung contusion, and chest trauma and indirect causes such as sepsis, shock, pancreatitis, cardiopulmonary bypass, and burns [2].
Lung injury and recovery are tightly coordinated processes requiring the orchestration of multiple cellular processes to restore tissue homeostasis. These processes include absorption of alveolar fluid, recovery of vascular permeability, reformation of epithelial monolayers, and maturation of resident macrophages. As alteration of tissue microenvironment in ARDS, alveolar macrophages (AM) can dynamically acquire pro-inflammatory phenotype (M1 type) or anti-inflammatory/tissue remodeling phenotype (M2 type) to meet functional demand [3]. Alveolar epithelial cells (AECs) can be characterized into two subtypes: (i) type I AECs which promote gaseous exchange and (ii) type II AECs which synthesis and secrete surfactant proteins to maintain alveolar tension.
Both macrophages and AECs are highly plastic cells with their function governed by a series of complex signaling networks. Understanding their phenotypic characteristics will produce valuable insights on biological processes of ARDS. Therefore, we’d like to review the role of macrophages and AECs engaged in the inflammatory response and tissue repair processes in ARDS.
The macrophages involved in the ARDS
The immune system monitors the microenvironment and exhibits tolerance to self-tissues while effective against invading pathogens. AMs consist of tissue-resident or recruited cells that form the first line of defense to foreign invaders. Resident AMs are identified by the expression of SiglecF+CD11c+CD64+F4/80+CD11b− are long-lived, self-renewable cells of predominantly embryonic origin. AMs are located at the air-tissue interface and act as an immunosuppressive M2-like phenotype in their normal state [4, 5]. Resident AMs can also originate from fetal monocytes and differentiate into mature AMs under the influence of GM-CSF, TGF-β, and PPARγ after birth [6–8], indicating the vital role of the local microenvironment on the shaping of specific tissue-resident macrophages. A previous study reports sessile AMs communicate with the alveolar epithelium to modulate immunity [9]; notably, not every alveolus contained an AM, only a small percentage of AMs (10%) were completely sessile population that communicated with epithelial via gap junctions, the majority of cells often crawled between alveoli, and these PKH26+ patrolling AMs utilize pores of Kohn to monitor and rapidly scavenge inhaled bacteria [10].
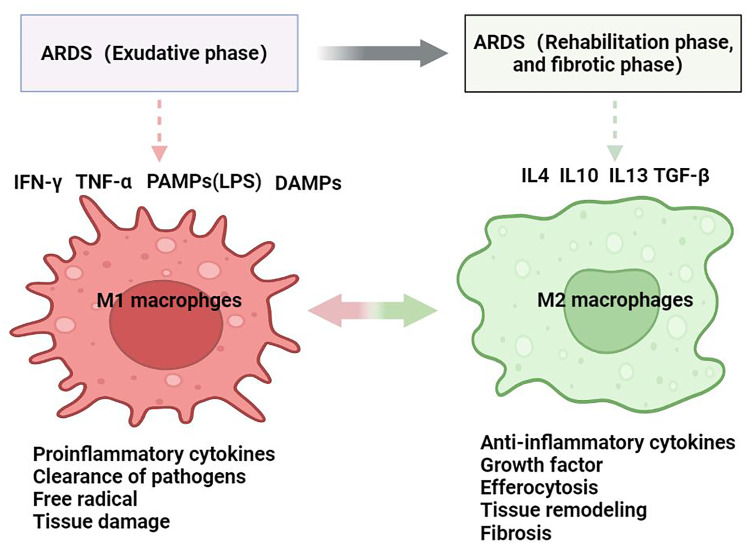
ARDS can be pathologically classified into an exudative phase, rehabilitation phase, and fibrotic phase. In the exudative phase of ARDS, pathogens activated macrophages via pattern recognition receptors (PRRs) including Toll-like receptors (TLRs), nucleotide-binding oligomerization domain Leucine-rich repeats containing receptors (NLRs), transmembrane C-type lectin receptors (CLRs), and retinoic acid-inducible gene-I-like receptors (RLRs), which promote the shift of resident AMs cells to the predominant M1 phenotype [11], and then these resident AMs coordinate with two types of circulating peripheral blood monocytes: GR-1lowCCR2lowCX3CR1high monocytes which participate in resolution of inflammation and tissue repair and GR-1highCCR2highCX3CR1low monocytes which are predominantly inflammatory and migrate to injured and infected sites to clear microorganisms. The signaling transduction pathway proteins NF-κB, JAK/STAT, JNK are likewise critical for M1 macrophage polarization, and production of pro-inflammatory cytokines including TNF-α, IL-1β, IL-6, IL-12, IL-15, IL-23, reactive oxygen species (ROS), reactive nitrogen species (RNS), and proteolytic enzymes, as reviewed previously [12, 13]. Functionally, this population of macrophages recruit neutrophils and lymphocytes to clear of pathogens, but an excessive secretion of pro-inflammatory cytokines can have a detrimental effect on alveoli structure, leading to increased alveolar permeability and pulmonary edema (Fig. 1).
Fig. 1.
With the development of ARDS from exudative phase to rehabilitation and fibrotic phase, the macrophages are shift to classically activated macrophage(M1) with the release of pro-inflammatory cytokines, free radical to clearance of pathogens and induce tissue damage, then shift to the alternative activated macrophages(M2) for which to produce the anti-inflammatory cytokines to dampen the pro-inflammation, and produce the growth factors for promoting the tissue repair function.
The second phase of ARDS is the rehabilitation phase. After clearance of pathogenic factors, inflammation resolution and tissue repair initiate a “pro-resolution” CD11blow macrophages, which are characterized by the enhanced apoptotic leukocyte engulfment, unresponsiveness to TLR ligands, and increased migration to draining lymph nodes following by a reduction of M1 macrophage subtypes and a corresponding increase in M2 phenotype cells. The M2 macrophages are classified into the M2a, CD163+CD200R+CXCX1+Arg1+ wound healing macrophage subtypes, which are induced by IL-4, IL-13, express high levels of mannose receptor, decoy IL-1 receptor and CCL17, and secretion of TGF-β, IGF, and fibronectin to support tissue repair; M2b macrophage subset, namely CD86+IL4Rα+MHCII+ regulatory macrophages, are induced by immune complexes together with IL-1β or LPS, and regulate the immune response by varied expression of both pro- and anti-inflammatory cytokine like IL-1, IL-6, TNF-α, and IL-10; M2c subset, also known as the CD206+CD163+CCR2+CD150+ deactivation macrophages, is induced by IL-10, TGF-β, or glucocorticoids and has strong anti-inflammatory properties and profibrotic activity [13]. M2 macrophages can promote lung tissue repair, alleviate epithelial cell damage, restore pulmonary barrier function, and produce anti-inflammatory response. Phagocytosis also plays an important role in the removal of necrotic cells and detritus, recruitment of apoptotic neutrophils, and enhancement of efferocytosis through release of IL-4, IL-13, and IL-10 [3, 14–16] (Fig. 1). These results suggest that M2 macrophages are key orchestrators in the regulation of immune response to lung injury and repair during the rehabilitation phase.
In the last stage of ARDS, the destroyed pulmonary alveoli attempt to restore tissue homeostasis by supporting alveolar structure reconstruction, with this period being characterized by the expansion of fibroblasts, matrix reformation, and proliferation and differentiation of AEC II cells into AEC I cells. However, extensive basement membrane damage and inadequate or delayed re-epithelialization can still lead to fibrosis formation in this stage [17]. M1 macrophages play an important role in resolving pathological fibroproliferative response by producing of matrix metalloproteinases (MMPs) and a variety of antifibrotic cytokines such as CXCL10. In parallel, M2 macrophages express arginase 1 and resistin-like α to limit fibrosis, but excessive expression of IL-4 and IL-13 can induce collagen deposition via TGF-β pathways [18–20]. A recent study in COVID-19 by using scRNA-seq revealed six populations of monocyte/macrophage with distinct gene expression signatures and identify CD163/LGMN profibrotic macrophage that expression of fibrotic genes SPP1, TGFB1, LGMN, and CCL18 [21]. Taken together, macrophages participate in every single stage of ARDS. In the exudative phase of ARDS, AMs shift into the M1 phenotype to clear microorganisms, induce severe inflammatory responses, and damage lung tissue. Following entry into the rehabilitation stage, M2 macrophages take center task to resolve tissue damage and promote repair processes. However, excessive M2 polarization becomes detrimental in later stage ARDS and contributes to the pulmonary fibrosis processes.
The role of M1/M2 macrophages in specific causes of ARDS
Pneumonia is the most common risk factor for ARDS, a study from Mayo Clinic revealed that bacteria, fungi, and viral infection can lead to pneumonia-associated ARDS. Among these pathogens, respiratory syncytial virus (RSV) pneumonia, pneumocystis jiroveci, blastomyces species, and streptococcus pneumonia are the most common causes of ARDS [22]. The COVID-19 leads to ARDS cases predominately in recent years [23]. While most patients with COVID-19 infection exhibited mild symptoms, some patients developed a severe immune reaction to ARDS known as a cytokine storm or “macrophage activation syndrome,” which is a systemic reaction to massive macrophage activation [24, 25]. This reaction is not due to the viral infection directly, but rather caused by the overreaction of the immune system. In patients with macrophage activation syndrome, increased pro-inflammatory cytokines are correlated with fever, disseminated intravascular coagulation, ARDS, and damaged lung structure [26]. In the severely damaged lung, monocyte-derived macrophages are the predominate macrophage lineages, and infiltrate alveolar cavities along with neutrophils and lymphocytes [27–30]. In the fibrotic phase, SARS-CoV-2 triggers newly recruited monocyte-derived macrophages which adopt a fibrosis-associated phenotype and express genes with well-known pathogenic functions in fibrosis [21]. These provoking studies provide a unique opportunity to explore new therapeutic methods for ARDS that focus on macrophage activation, cytokine storms, and fibrotic formation.
For bacterial infection in ARDS, gram-positive and negative bacterial infections had a similar frequency of ALI (20% versus 15%, respectively) [31, 32]. The resident AMs initiate an immune response against pathogens by release of proinflammatory cytokines, and stimulation of neighboring AECs and resident macrophages to produce chemokine to recruit neutrophils, monocytes, and lymphocyte cells to the site of infection. After clearance of pathogens, the M1 macrophages shift to the M2 macrophages to dampen the inflammatory response and promote tissue remodeling. The reprogrammed macrophages are thus polarized upon specific signals to meet function demands during infection, resolution, and repair, as reviewed by previous studies [3, 33].
RSV infection, another factor that contributes to ARDS, can significantly increase AMs, neutrophil, eosinophil, and lymphocyte infiltration and induce pro-inflammatory factors such as IL-1, IL-6, IL-12, IL-13, TNF-α, IFN-γ, MCP1, and CCL3 [34–36]. Moreover, RSV infection stimulates Gas6/Axl and prompts a shift of M1-like to an M2-like phenotype, suppressing caspase-1 activation, and IL-18 production. The reduced IL-18 levels inhibit NK cell-mediated IFN-γ production, NO, and TNF-α production, impairing the control of bacterial infection [37]. It has been hypothesized that these interlinked processes could be a potential mechanism in a mixed viral and bacterial infection scenario. Indeed, complex macrophage–pathogen interplay in RSV infection is an area of particular interest, with macrophages interacting with the NK cells/T cells for clearance of viruses and bacteria on the one hand, and the virus supporting macrophage function to maintain the pathogen coexistence and corresponding transmission for the host on the other hand.
The opportunistic fungal pathogen pneumocystis is of particular interest to healthcare practitioner due to its association with life-threatening pneumonia or ARDS in immune-compromised individuals, such as AIDS, organ transplants, cancer, and congenital immunodeficiency patients. A study from complicated with positive HIV revealed that the bronchoalveolar lavage fluid cell population comprises of more than 10% lymphocytes and 5% neutrophils in an activated macrophage phenotype setting [38], indicating that CD4+ T cells rather than macrophages play the most important role in the progression of ARDS. Other studies suggest that macrophage phenotype status dictate the outcome of the pneumocystis infection, with M2 macrophages associated with pneumocystis clearance in immunocompetent hosts, while M1 phenotype are associated with pneumocystis infection in susceptible immunosuppressed individuals. The adoptive transfer of both M1 and M2 macrophages has been observed in pneumocystis infected immunosuppressed hosts, with the best outcomes observed following M2 transfer [39]. However, this finding was not supported by another study that found either M1 or M2 polarization is required to initiate pneumocystis host defense [40]. The above findings have prompted many questions on mechanisms by which host immunity governs the outcome of pneumocystis infection. While M1 macrophages are involved in the immune defense via proinflammatory cytokines induction/production by inducing an adaptive immune response, M2 macrophages are engaged in efferocytosis to clear pneumocystis viral particles. However, it is important to note that the M1/M2 classification has only been explored in vitro paradigms that do not reflect the heterogeneity of macrophages in vivo. In fact, different forms of infection by pathogens such as pneumonia, SARS-CoV-2, bacterial infections, and respiratory virus infections may produce a mixed population of macrophages that have dual function in healing processes such as the clearance of microorganisms and resolution of inflammation, as well as pathogenic processes such as fibrosis. Better understanding of the mechanism underlying this delicate/complex balancing act in lung injury and repair will greatly benefit future research efforts in this field.
AECs and ARDS
The hallmark pathogenic event in ARDS is an injury to the AECs, with the recovery and fate of AECs directly determining patient outcomes. There are two types of AECs. Type I AECs are terminally differentiated cells, covering most areas of the alveolar surface. These cells primarily participate in gaseous exchange and help maintain ion and fluid balance at the air–liquid interface. Additionally, AEC I cell express receptor for advanced glycation end products (RAGE) involved in a variety of functions including pro-inflammatory cytokines and apoptosis [41–43].
Type II AECs retain progenitor cell properties, produce surfactant complex, and are responsible for lowering the superficial tension to prevent alveolar collapse and stabilize the airways epithelial barrier [3, 44]. Pulmonary surfactant is a complex substance containing about 90% lipids and 10% surfactant proteins (SP). There are four types of SP including SP-A, SP-B, SP-C, and SP-D. SP-A and SP-D are pulmonary collections that are able to bind to viruses to facilitate pathogen removal [45].
ARDS involves both innate and adaptive host responses that can further damage the alveolar endo/epithelial barrier. Alveolar leakage is associated with AEC I apoptosis through the Fas, P53, Bcl signaling and decline of the AEC I pool [46, 47]. Some studies point to cell necrosis as the dominant pathogenic event in AEC cell death in LPS-induced lung injury [48]. Ferroptosis, a new form of cell death, is also involved in the ARDS [49]. After AEC I cells death, AEC II cells proliferate and differentiate into AEC I cells, and the potential mechanism to initiate the AEC II activation and proliferation may be associated with the injury, inflammatory milieu, hyperoxia, and the oxidants [50, 51]. As such, re-epithelialization is a critical step in restoring normal alveolar structure and re-established gas exchange. Gene profiling shows that proliferating AEC II cells possess high levels of cell cycle markers such as mKi67 and Pcna, with an intermediate cell state characterized by reduced cell cycle genes and downregulation of AEC II cell markers but enhanced levels of AEC I cell markers which are further upregulated in maturation processes [52].
Multiple signaling pathways promote AEC II cell proliferation. Wnt/β-catenin is expressed in a small subset of AEC II cells that function as alveolar stem cells during homeostasis. Following lung injury, these stem cells quickly divide and proliferate via autocratic Wnt signaling [53, 54]. Notch signaling is sequentially upregulated in AEC II cells and downregulated by Dlk1 during the proliferation phase, prompting differentiation of AEC I cells [55]. The TGF-β signaling pathway also undergoes a similar low–high-low expression pattern during AEC II cells proliferation and enters an intermediate state, and during differentiation of AEC I cells [56]. Similarly, bone morphogenetic proteins (BMPs), which belong to the TGF-β superfamily of proteins, maintain active in AEC II cell during homeostasis, while deactivate to promote AEC II cell proliferation during regeneration and then reactivate to promote AEC I cell differentiation [57]. These findings indicate that signaling pathway plays a critical role in determine the cell fate. Finally, the transcription factors YAP/TAZ play a critical role in AEC II-to-AEC I differentiation in alveolar repair, with TAZ nuclear localization being observed in AEC I but not AEC II cells, while study also found that YAP/TAZ-specific knockout AEC II cells exhibit prolonged inflammation and fibrosis processes [58, 59]. The erythroblast transformation-specific (ETS) transcription factor family member ETV5 is additionally necessary for AEC II cell proliferation and maintenance [60]. Though amount of the signaling pathways involved in the epithelial regeneration, how would a better understanding of AEC differentiation and re-epithelialization will lead to an improved outcome for patients with ARDS.
Interaction between AMs and AECs in ARDS
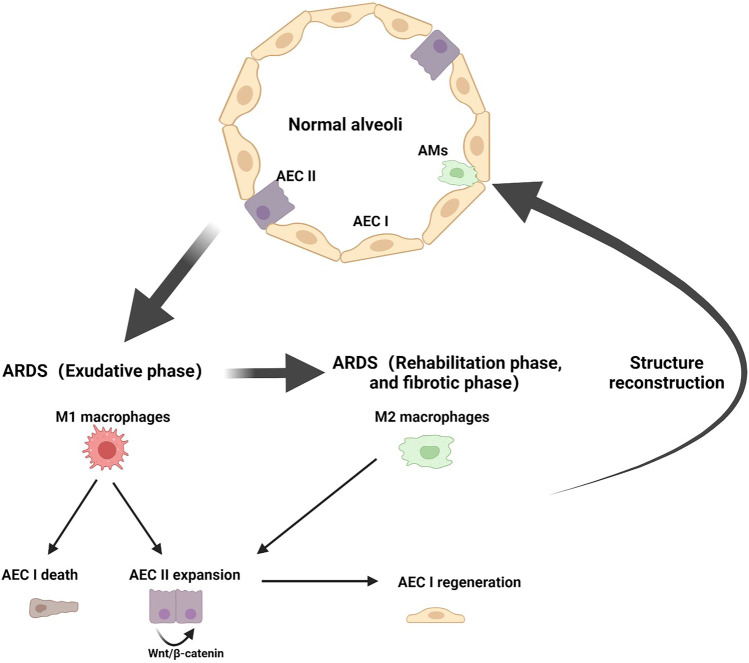
In a steady-state microenvironment setting, AECs and AMs interact with each other to maintain a quiescent state through ligands–receptors interaction, such as CD200R, PD-1, and SIRP1α on AMS and their ligands CD200, PDL-1, and CD47 on AECs [61–63]. While under pathological conditions, macrophages contribute to both epithelial injury and repair. Macrophage-derived TNF-α stimulates the production of GM-CSF in AEC II and promotes AEC II cell proliferation via autocrine signaling [47]. Macrophage TFF2 signaling induces Wnt expression essential for AEC II cell proliferation [64]. Moreover, AMs interact with the alveolar wall through connexin 43 (Cx43)-containing gap junction channels and participate in cell intercommunicate through synchronized Ca2+ wave activity during bacterial pneumonia [9]. Our previous study showed that M1 macrophages-derived amphiregulin (AREG) can protect against AECs injury during ARDS [65] (Fig. 2).
Fig. 2.
The M1 macrophages interact with the AEC I to induce the cell death and the same way contribute to the AEC II expansion, M2 macrophages primary promote the AEC II proliferation as well as induce fibrosis, and the AEC II to AEC I differentiation is an important checkpoint for driving the alveoli structure reconstruction, which is a crucial step for returning structural and functional homeostasis.
In addition to these signaling pathways, metabolites also serve as cell–cell interaction messengers and affect outcomes of ARDS. Study showed that glutamate is increased in ARDS, taurine can lead to the release of free radical products, and arginine upregulation can promote NO production and alleviate the pulmonary hypertension associated with hypoxia, threonine and gluconeogenic amino acid can regulate immune responses by antibody production, and arginine upregulation has also been associated with sepsis [66].
How these metabolites determine the polarization of immune cells infiltrated in the lung tissues react with neighboring cells and finally drive the disease progression remain largely unknown. M1 macrophages express iNOS which catabolized arginine to NO as an inflammation hallmark, while M2 macrophages express Arg-1 which catabolized arginine to produce L-ornithine and additional catabolic processes that produce polyamines and L-proline products for cell proliferation and collagen production [67, 68]. This metabolic reprogramming, on one hand, provides the support for macrophages' proliferation and, on the other hand, promotes the synthesis of the cytokines to support their function. Previous studies have found that healthy AEC II cells are highly oxidative and preferentially utilize lactate as a metabolic substrate for mitochondrial ATP production [69]; moreover, LDH enzyme activity can affect lactate oxidation and promote the formation of pulmonary fibrosis [70]. A recent study explored that in serious deceased COVID-19 patients, an upregulated glycolysis and oxidative phosphorylation are observed in AEC II cells, which may suppress alveolar epithelial differentiation and surfactant production; furthermore, increased glycolysis, oxidative phosphorylation, and inositol phosphate metabolism may also promote macrophages activation and contribute to lung injury [71]. Above all, metabolites can serve as an energy source for the cell survival as well as vital substrates for signal regulation, fine-tuning cell fate via metabolic processes, gene expression, or post-transcriptional modifications.
Future direction
In this study, we reviewed the role of AMs and AECs in the development of ARDS; notably, many other cells like the endothelial cells and adaptive immune cells also engage in the pathological changes in the ARDS. Future studies should explore more mechanisms on cell–cell interactions and phenotypic switching and investigate their impact on pathogens elimination and alveolar structure regeneration. As cell interactions have the potential to influence cell fate through receptors–ligand binding, signaling pathways, energetic demands, and metabolites coordination via juxtacrine, paracrine, or autocrine signaling. Thus, advanced technological methods like RNA-seq, ATAC-seq, mass spectrometry should be applied to investigate genetic, epigenetic, proteomic, and metabolic regulation in ARDS, to explain how these cells undergo activation, cell death, proliferation, and differentiation and finally maintaining structural and functional homeostasis.
Data and materials availability
All data generated are included in this article.
Author contributions
HT wrote the manuscript. YX and SZ revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the National Natural Science Foundation of China (No. 82172143, 81670068).
Declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Younian Xu, Email: xyn0103@hust.edu.cn.
Shihai Zhang, Email: zhangshihai@vip.163.com.
References
- 1.Force ADT, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS. Acute respiratory distress syndrome: The Berlin Definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 2.Fan E, Brodie D, Slutsky AS. Acute Respiratory Distress Syndrome: Advances in Diagnosis and Treatment. JAMA. 2018;319:698–710. doi: 10.1001/jama.2017.21907. [DOI] [PubMed] [Google Scholar]
- 3.Herold S, Mayer K, Lohmeyer J. Acute lung injury: How macrophages orchestrate resolution of inflammation and tissue repair. Frontiers in Immunology. 2011;2:65. doi: 10.3389/fimmu.2011.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duan M, Li WC, Vlahos R, Maxwell MJ, Anderson GP, Hibbs ML. Distinct macrophage subpopulations characterize acute infection and chronic inflammatory lung disease. The Journal of Immunology. 2012;189:946–955. doi: 10.4049/jimmunol.1200660. [DOI] [PubMed] [Google Scholar]
- 5.Aggarwal NR, King LS, D'Alessio FR. Diverse macrophage populations mediate acute lung inflammation and resolution. American Journal of Physiology. Lung Cellular and Molecular Physiology. 2014;306:L709–725. doi: 10.1152/ajplung.00341.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woo YD, Jeong D, Chung DH. Development and Functions of Alveolar Macrophages. Molecules and Cells. 2021;44:292–300. doi: 10.14348/molcells.2021.0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guilliams M, De Kleer I, Henri S, Post S, Vanhoutte L, De Prijck S, Deswarte K, Malissen B, Hammad H, Lambrecht BN. Alveolar macrophages develop from fetal monocytes that differentiate into long-lived cells in the first week of life via GM-CSF. Journal of Experimental Medicine. 2013;210:1977–1992. doi: 10.1084/jem.20131199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schneider C, Nobs SP, Heer AK, Kurrer M, Klinke G, van Rooijen N, Vogel J, Kopf M. Alveolar macrophages are essential for protection from respiratory failure and associated morbidity following influenza virus infection. PLoS Pathogens. 2014;10:e1004053. doi: 10.1371/journal.ppat.1004053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Westphalen K, Gusarova GA, Islam MN, Subramanian M, Cohen TS, Prince AS, Bhattacharya J. Sessile alveolar macrophages communicate with alveolar epithelium to modulate immunity. Nature. 2014;506:503–506. doi: 10.1038/nature12902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neupane AS, Willson M, Chojnacki AK, Vargas ESCF, Morehouse C, Carestia A, Keller AE, Peiseler M, DiGiandomenico A, Kelly MM, et al. Patrolling Alveolar Macrophages Conceal Bacteria from the Immune System to Maintain Homeostasis. Cell. 2020;183(110–125):e111. doi: 10.1016/j.cell.2020.08.020. [DOI] [PubMed] [Google Scholar]
- 11.Li D, Wu M. Pattern recognition receptors in health and diseases. Signal Transduction and Targeted Therapy. 2021;6:291. doi: 10.1038/s41392-021-00687-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen X, Tang J, Shuai W, Meng J, Feng J, Han Z. Macrophage polarization and its role in the pathogenesis of acute lung injury/acute respiratory distress syndrome. Inflammation Research. 2020;69:883–895. doi: 10.1007/s00011-020-01378-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galli G, Saleh M. Immunometabolism of Macrophages in Bacterial Infections. Frontiers in Cellular and Infection Microbiology. 2020;10:607650. doi: 10.3389/fcimb.2020.607650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim S, Elkon KB, Ma X. Transcriptional suppression of interleukin-12 gene expression following phagocytosis of apoptotic cells. Immunity. 2004;21:643–653. doi: 10.1016/j.immuni.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 15.Byrne A, Reen DJ. Lipopolysaccharide induces rapid production of IL-10 by monocytes in the presence of apoptotic neutrophils. The Journal of Immunology. 2002;168:1968–1977. doi: 10.4049/jimmunol.168.4.1968. [DOI] [PubMed] [Google Scholar]
- 16.Huang X, Xiu H, Zhang S, Zhang G. The Role of Macrophages in the Pathogenesis of ALI/ARDS. Mediators of Inflammation. 2018;2018:1264913. doi: 10.1155/2018/1264913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thompson BT, Chambers RC, Liu KD. Acute Respiratory Distress Syndrome. New England Journal of Medicine. 2017;377:562–572. doi: 10.1056/NEJMra1608077. [DOI] [PubMed] [Google Scholar]
- 18.Duru N, Wolfson B, Zhou Q. Mechanisms of the alternative activation of macrophages and non-coding RNAs in the development of radiation-induced lung fibrosis. World Journal of Biological Chemistry. 2016;7:231–239. doi: 10.4331/wjbc.v7.i4.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mora AL, Torres-Gonzalez E, Rojas M, Corredor C, Ritzenthaler J, Xu J, Roman J, Brigham K, Stecenko A. Activation of alveolar macrophages via the alternative pathway in herpesvirus-induced lung fibrosis. American Journal of Respiratory Cell and Molecular Biology. 2006;35:466–473. doi: 10.1165/rcmb.2006-0121OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.D'Alessio FR, Craig JM, Singer BD, Files DC, Mock JR, Garibaldi BT, Fallica J, Tripathi A, Mandke P, Gans JH, et al. Enhanced resolution of experimental ARDS through IL-4-mediated lung macrophage reprogramming. American Journal of Physiology. Lung Cellular and Molecular Physiology. 2016;310:L733–746. doi: 10.1152/ajplung.00419.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wendisch D, Dietrich O, Mari T, von Stillfried S, Ibarra IL, Mittermaier M, Mache C, Chua RL, Knoll R, Timm S, et al. SARS-CoV-2 infection triggers profibrotic macrophage responses and lung fibrosis. Cell. 2021;184(6243–6261):e6227. doi: 10.1016/j.cell.2021.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kojicic M, Li G, Hanson AC, Lee KM, Thakur L, Vedre J, Ahmed A, Baddour LM, Ryu JH, Gajic O. Risk factors for the development of acute lung injury in patients with infectious pneumonia. Critical Care. 2012;16:R46. doi: 10.1186/cc11247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berlin DA, Gulick RM, Martinez FJ. Severe Covid-19. New England Journal of Medicine. 2020;383:2451–2460. doi: 10.1056/NEJMcp2009575. [DOI] [PubMed] [Google Scholar]
- 24.Kosyreva A, Dzhalilova D, Lokhonina A, Vishnyakova P, Fatkhudinov T. The Role of Macrophages in the Pathogenesis of SARS-CoV-2-Associated Acute Respiratory Distress Syndrome. Frontiers in Immunology. 2021;12:682871. doi: 10.3389/fimmu.2021.682871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McGonagle D, Sharif K, O'Regan A, Bridgewood C. The Role of Cytokines including Interleukin-6 in COVID-19 induced Pneumonia and Macrophage Activation Syndrome-Like Disease. Autoimmunity Reviews. 2020;19:102537. doi: 10.1016/j.autrev.2020.102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tay MZ, Poh CM, Renia L, MacAry PA, Ng LFP. The trinity of COVID-19: Immunity, inflammation and intervention. Nature Reviews Immunology. 2020;20:363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liao M, Liu Y, Yuan J, Wen Y, Xu G, Zhao J, Cheng L, Li J, Wang X, Wang F, et al. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nature Medicine. 2020;26:842–844. doi: 10.1038/s41591-020-0901-9. [DOI] [PubMed] [Google Scholar]
- 28.Boumaza A, Gay L, Mezouar S, Bestion E, Diallo AB, Michel M, Desnues B, Raoult D, La Scola B, Halfon P, et al. Monocytes and Macrophages, Targets of Severe Acute Respiratory Syndrome Coronavirus 2: The Clue for Coronavirus Disease 2019 Immunoparalysis. Journal of Infectious Diseases. 2021;224:395–406. doi: 10.1093/infdis/jiab044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang C, Xie J, Zhao L, Fei X, Zhang H, Tan Y, Nie X, Zhou L, Liu Z, Ren Y, et al. Alveolar macrophage dysfunction and cytokine storm in the pathogenesis of two severe COVID-19 patients. eBioMedicine. 2020;57:102833. doi: 10.1016/j.ebiom.2020.102833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoepel, W., H.J. Chen, C.E. Geyer, S. Allahverdiyeva, X.D. Manz, S.W. de Taeye, J. Aman, L. Mes, M. Steenhuis, G.R. Griffith, et al. 2021. High titers and low fucosylation of early human anti-SARS-CoV-2 IgG promote inflammation by alveolar macrophages. Sci Transl Med 13. [DOI] [PMC free article] [PubMed]
- 31.Li G, Malinchoc M, Cartin-Ceba R, Venkata CV, Kor DJ, Peters SG, Hubmayr RD, Gajic O. Eight-year trend of acute respiratory distress syndrome: A population-based study in Olmsted County, Minnesota. American Journal of Respiratory and Critical Care Medicine. 2011;183:59–66. doi: 10.1164/rccm.201003-0436OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He H, Sun B, Liang L, Li Y, Wang H, Wei L, Li G, Guo S, Duan J, Li Y, et al. A multicenter RCT of noninvasive ventilation in pneumonia-induced early mild acute respiratory distress syndrome. Critical Care. 2019;23:300. doi: 10.1186/s13054-019-2575-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Amarante-Mendes GP, Adjemian S, Branco LM, Zanetti LC, Weinlich R, Bortoluci KR. Pattern Recognition Receptors and the Host Cell Death Molecular Machinery. Frontiers in Immunology. 2018;9:2379. doi: 10.3389/fimmu.2018.02379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller AL, Bowlin TL, Lukacs NW. Respiratory syncytial virus-induced chemokine production: Linking viral replication to chemokine production in vitro and in vivo. Journal of Infectious Diseases. 2004;189:1419–1430. doi: 10.1086/382958. [DOI] [PubMed] [Google Scholar]
- 35.Mäkelä MJ, Tripp R, Dakhama A, Park J-W, Ikemura T, Joetham A, Waris M, Anderson LJ, Gelfand EW. Prior airway exposure to allergen increases virus-induced airway hyperresponsiveness. Journal of Allergy and Clinical Immunology. 2003;112:861–869. doi: 10.1016/S0091-6749(03)02020-7. [DOI] [PubMed] [Google Scholar]
- 36.Senft AP, Taylor RH, Lei W, Campbell SA, Tipper JL, Martinez MJ, Witt TL, Clay CC, Harrod KS. Respiratory syncytial virus impairs macrophage IFN-alpha/beta- and IFN-gamma-stimulated transcription by distinct mechanisms. American Journal of Respiratory Cell and Molecular Biology. 2010;42:404–414. doi: 10.1165/rcmb.2008-0229OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shibata T, Makino A, Ogata R, Nakamura S, Ito T, Nagata K, Terauchi Y, Oishi T, Fujieda M, Takahashi Y, Ato M. Respiratory syncytial virus infection exacerbates pneumococcal pneumonia via Gas6/Axl-mediated macrophage polarization. The Journal of Clinical Investigation. 2020;130:3021–3037. doi: 10.1172/JCI125505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gaborit BJ, Tessoulin B, Lavergne RA, Morio F, Sagan C, Canet E, Lecomte R, Leturnier P, Deschanvres C, Khatchatourian L, et al. Outcome and prognostic factors of Pneumocystis jirovecii pneumonia in immunocompromised adults: A prospective observational study. Annals of Intensive Care. 2019;9:131. doi: 10.1186/s13613-019-0604-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nandakumar, V., D. Hebrink, P. Jenson, T. Kottom, and A.H. Limper. 2017. Differential Macrophage Polarization from Pneumocystis in Immunocompetent and Immunosuppressed Hosts: Potential Adjunctive Therapy during Pneumonia. Infect Immun 85. [DOI] [PMC free article] [PubMed]
- 40.Zhang ZQ, Wang J, Hoy Z, Keegan A, Bhagwat S, Gigliotti F, Wright TW. Neither classical nor alternative macrophage activation is required for Pneumocystis clearance during immune reconstitution inflammatory syndrome. Infection and Immunity. 2015;83:4594–4603. doi: 10.1128/IAI.00763-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johnson MD, Bao HF, Helms MN, Chen XJ, Tigue Z, Jain L, Dobbs LG, Eaton DC. Functional ion channels in pulmonary alveolar type I cells support a role for type I cells in lung ion transport. Proc Natl Acad Sci U S A. 2006;103:4964–4969. doi: 10.1073/pnas.0600855103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wong MH, Chapin OC, Johnson MD. LPS-stimulated cytokine production in type I cells is modulated by the renin-angiotensin system. American Journal of Respiratory Cell and Molecular Biology. 2012;46:641–650. doi: 10.1165/rcmb.2011-0289OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Demling N, Ehrhardt C, Kasper M, Laue M, Knels L, Rieber EP. Promotion of cell adherence and spreading: A novel function of RAGE, the highly selective differentiation marker of human alveolar epithelial type I cells. Cell and Tissue Research. 2006;323:475–488. doi: 10.1007/s00441-005-0069-0. [DOI] [PubMed] [Google Scholar]
- 44.Rock JR, Hogan BL. Epithelial progenitor cells in lung development, maintenance, repair, and disease. Annual Review of Cell and Developmental Biology. 2011;27:493–512. doi: 10.1146/annurev-cellbio-100109-104040. [DOI] [PubMed] [Google Scholar]
- 45.Watson A, Madsen J, Clark HW. SP-A and SP-D: Dual Functioning Immune Molecules With Antiviral and Immunomodulatory Properties. Frontiers in Immunology. 2020;11:622598. doi: 10.3389/fimmu.2020.622598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Albertine KH, Soulier MF, Wang Z, Ishizaka A, Hashimoto S, Zimmerman GA, Matthay MA, Ware LB. Fas and Fas Ligand Are Up-Regulated in Pulmonary Edema Fluid and Lung Tissue of Patients with Acute Lung Injury and the Acute Respiratory Distress Syndrome. The American Journal of Pathology. 2002;161:1783–1796. doi: 10.1016/S0002-9440(10)64455-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cakarova L, Marsh LM, Wilhelm J, Mayer K, Grimminger F, Seeger W, Lohmeyer J, Herold S. Macrophage tumor necrosis factor-alpha induces epithelial expression of granulocyte-macrophage colony-stimulating factor: Impact on alveolar epithelial repair. American Journal of Respiratory and Critical Care Medicine. 2009;180:521–532. doi: 10.1164/rccm.200812-1837OC. [DOI] [PubMed] [Google Scholar]
- 48.Tamada N, Tojo K, Yazawa T, Goto T. Necrosis Rather Than Apoptosis is the Dominant form of Alveolar Epithelial Cell Death in Lipopolysaccharide-Induced Experimental Acute Respiratory Distress Syndrome Model. Shock. 2020;54:128–139. doi: 10.1097/SHK.0000000000001425. [DOI] [PubMed] [Google Scholar]
- 49.Qu M, Zhang H, Chen Z, Sun X, Zhu S, Nan K, Chen W, Miao C. The Role of Ferroptosis in Acute Respiratory Distress Syndrome. Front Med (Lausanne) 2021;8:651552. doi: 10.3389/fmed.2021.651552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pociask DA, Scheller EV, Mandalapu S, McHugh KJ, Enelow RI, Fattman CL, Kolls JK, Alcorn JF. IL-22 Is Essential for Lung Epithelial Repair following Influenza Infection. The American Journal of Pathology. 2013;182:1286–1296. doi: 10.1016/j.ajpath.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pogach MS, Cao Y, Millien G, Ramirez MI, Williams MC. Key developmental regulators change during hyperoxia-induced injury and recovery in adult mouse lung. Journal of Cellular Biochemistry. 2007;100:1415–1429. doi: 10.1002/jcb.21142. [DOI] [PubMed] [Google Scholar]
- 52.DePianto, D.J., J.A.V. Heiden, K.B. Morshead, K.H. Sun, Z. Modrusan, G. Teng, P.J. Wolters, and J.R. Arron. 2021. Molecular mapping of interstitial lung disease reveals a phenotypically distinct senescent basal epithelial cell population. JCI Insight 6. [DOI] [PMC free article] [PubMed]
- 53.Nabhan AN, Brownfield DG, Harbury PB, Krasnow MA, Desai TJ. Single-cell Wnt signaling niches maintain stemness of alveolar type 2 cells. Science. 2018;359:1118–1123. doi: 10.1126/science.aam6603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zacharias WJ, Frank DB, Zepp JA, Morley MP, Alkhaleel FA, Kong J, Zhou S, Cantu E, Morrisey EE. Regeneration of the lung alveolus by an evolutionarily conserved epithelial progenitor. Nature. 2018;555:251–255. doi: 10.1038/nature25786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Finn J, Sottoriva K, Pajcini KV, Kitajewski JK, Chen C, Zhang W, Malik AB, Liu Y. Dlk1-Mediated Temporal Regulation of Notch Signaling Is Required for Differentiation of Alveolar Type II to Type I Cells during Repair. Cell Reports. 2019;26(2942–2954):e2945. doi: 10.1016/j.celrep.2019.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jiang P. Gil de Rubio R, Hrycaj SM, Gurczynski SJ, Riemondy KA, Moore BB, Omary MB, Ridge KM, Zemans RL: Ineffectual Type 2-to-Type 1 Alveolar Epithelial Cell Differentiation in Idiopathic Pulmonary Fibrosis: Persistence of the KRT8(hi) Transitional State. American Journal of Respiratory and Critical Care Medicine. 2020;201:1443–1447. doi: 10.1164/rccm.201909-1726LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chung, M.I., M. Bujnis, C.E. Barkauskas, Y. Kobayashi, and B.L.M. Hogan. 2018. Niche-mediated BMP/SMAD signaling regulates lung alveolar stem cell proliferation and differentiation. Development 145. [DOI] [PMC free article] [PubMed]
- 58.Liu Z, Wu H, Jiang K, Wang Y, Zhang W, Chu Q, Li J, Huang H, Cai T, Ji H, et al. MAPK-Mediated YAP Activation Controls Mechanical-Tension-Induced Pulmonary Alveolar Regeneration. Cell Reports. 2016;16:1810–1819. doi: 10.1016/j.celrep.2016.07.020. [DOI] [PubMed] [Google Scholar]
- 59.Sun, T., Z. Huang, H. Zhang, C. Posner, G. Jia, T.R. Ramalingam, M. Xu, H. Brightbill, J.G. Egen, A. Dey, and J.R. Arron. 2019. TAZ is required for lung alveolar epithelial cell differentiation after injury. JCI Insight 5. [DOI] [PMC free article] [PubMed]
- 60.Zhang Z, Newton K, Kummerfeld SK, Webster J, Kirkpatrick DS, Phu L, Eastham-Anderson J, Liu J, Lee WP, Wu J, et al. Transcription factor Etv5 is essential for the maintenance of alveolar type II cells. Proc Natl Acad Sci U S A. 2017;114:3903–3908. doi: 10.1073/pnas.1621177114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jiang-Shieh YF, Chien HF, Chang CY, Wei TS, Chiu MM, Chen HM, Wu CH. Distribution and expression of CD200 in the rat respiratory system under normal and endotoxin-induced pathological conditions. Journal of Anatomy. 2010;216:407–416. doi: 10.1111/j.1469-7580.2009.01190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shinohara M, Ohyama N, Murata Y, Okazawa H, Ohnishi H, Ishikawa O, Matozaki T. CD47 regulation of epithelial cell spreading and migration, and its signal transduction. Cancer Science. 2006;97:889–895. doi: 10.1111/j.1349-7006.2006.00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hu JF, Zhang W, Zuo W, Tan HQ, Bai W. Inhibition of the PD-1/PD-L1 signaling pathway enhances innate immune response of alveolar macrophages to mycobacterium tuberculosis in mice. Pulmonary Pharmacology & Therapeutics. 2020;60:101842. doi: 10.1016/j.pupt.2019.101842. [DOI] [PubMed] [Google Scholar]
- 64.Hung LY, Sen D, Oniskey TK, Katzen J, Cohen NA, Vaughan AE, Nieves W, Urisman A, Beers MF, Krummel MF, Herbert DR. Macrophages promote epithelial proliferation following infectious and non-infectious lung injury through a Trefoil factor 2-dependent mechanism. Mucosal Immunology. 2019;12:64–76. doi: 10.1038/s41385-018-0096-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xu Y, Meng C, Liu G, Yang D, Fu L, Zhang M, Zhang Z, Xia H, Yao S, Zhang S. Classically Activated Macrophages Protect against Lipopolysaccharide-induced Acute Lung Injury by Expressing Amphiregulin in Mice. Anesthesiology. 2016;124:1086–1099. doi: 10.1097/ALN.0000000000001026. [DOI] [PubMed] [Google Scholar]
- 66.Viswan A, Singh C, Rai RK, Azim A, Sinha N, Baronia AK. Metabolomics based predictive biomarker model of ARDS: A systemic measure of clinical hypoxemia. PLoS ONE. 2017;12:e0187545. doi: 10.1371/journal.pone.0187545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Van den Bossche, J., J. Baardman, M.P. de Winther. 2015. Metabolic Characterization of Polarized M1 and M2 Bone Marrow-derived Macrophages Using Real-time Extracellular Flux Analysis. J Vis Exp. [DOI] [PMC free article] [PubMed]
- 68.Jha Abhishek K, Huang Stanley C-C, Sergushichev A, Lampropoulou V, Ivanova Y, Loginicheva E, Chmielewski K, Stewart Kelly M, Ashall J, Everts B, et al. Network Integration of Parallel Metabolic and Transcriptional Data Reveals Metabolic Modules that Regulate Macrophage Polarization. Immunity. 2015;42:419–430. doi: 10.1016/j.immuni.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 69.Lottes RG, Newton DA, Spyropoulos DD, Baatz JE. Lactate as substrate for mitochondrial respiration in alveolar epithelial type II cells. American Journal of Physiology. Lung Cellular and Molecular Physiology. 2015;308:L953–961. doi: 10.1152/ajplung.00335.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Newton DA, Lottes RG, Ryan RM, Spyropoulos DD, Baatz JE. Dysfunctional lactate metabolism in human alveolar type II cells from idiopathic pulmonary fibrosis lung explant tissue. Respiratory Research. 2021;22:278. doi: 10.1186/s12931-021-01866-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li, S., F. Zhao, J. Ye, K. Li, Q. Wang, Z. Du, Q. Yue, S. Wang, Q. Wu, and H. Chen. 2022. Cellular metabolic basis of altered immunity in the lungs of patients with COVID-19. Med Microbiol Immunol. [DOI] [PMC free article] [PubMed]