Abstract
The main objective of the study was to determine if rodent housing conditions, specifically housing climate, could impact the in vivo performance of poly(lactide-co-glycolide) (PLGA) microspheres through temperature modification of the subcutaneous space. Vivitrol®, a once monthly naltrexone injectable suspension, was chosen as a model PLGA microparticle formulation for this study. Two lots of Vivitrol were used to ascertain any potential differences that may exist between the batches and if in vitro characterization techniques could delineate any variation(s).
The pharmacokinetics of the naltrexone-PLGA microparticles were determined in the rodent model under two different housing climates (20 vs. 25 °C). The results demonstrate that such difference in housing temperature resulted in a change in subcutaneous temperature but actually within a narrow range (36.31–36.77 °C) and thus minimally influenced the in vivo performance of subcutaneously injected microparticles. The shake-flask method was used to characterize the in vitro release at 35, 36, and 37 °C and demonstrated significant differences in the in vitro release profiles across this range of temperatures. Minimal differences in the in vitro characterization of the two lots were found. While these results did not provide statistical significance, the local in vivo temperature may be a parameter that should be considered when evaluating microparticle performance. The IVIVCs demonstrate that in vitro release at 37 °C may not accurately represent the in vivo conditions (i.e., subcutaneous space in rodents), and in certain instances lower in vitro release temperatures may more accurately represent the in vivo microenvironment and provide better correlations. Future studies will determine the extent temperature and specifically co-housing, may have on the relative impact of the in vivo performance of injectable polymeric microparticles based upon the significant differences observed in the in vitro release profiles across the range of 35–37 °C.
Keywords: PLGA microparticles, Vivitrol, In vivo-in vitro correlation, Naltrexone, Pharmacokinetics
1. Introduction
Poly(lactide-co-glycolide) (PLGA) and PLGA-based polymers are most widely used in controlled drug delivery systems, including nano-and microparticles, solid implants, and in situ forming implants (Makadia and Siegel, 2011). The release kinetics from these systems are controlled by diffusion, erosion, and/or a combination of the two, along with the polymer molecular weight (Mittal et al., 2007; Toshiro et al., 1991; Zolnik et al., 2006), lactide:glycolide (L:G) ratio (Amann et al., 2010), polymer end-cap (Huang et al., 2013), drug physicochemical properties (Miyajima et al., 1999; Sandor et al., 2001), microparticle size (Acharya et al., 2010; Chen et al., 2017), drug loading (Gasmi et al., 2016), and release testing conditions (Garner et al., 2018).
Drug release from PLGA microparticles typically illustrates a triphasic release profile: (Phase 1) an initial burst release due to dissolution of surface and/or pore associated drug, followed by (Phase 2) a lag phase where release is minimal or a relatively slow near constant release, due to drug in regions of the microparticle that have undergone local swelling, and finally, (Phase 3) an enhanced release due to substantial polymer swelling and/or polymer erosion.
Temperature may arguably be one of the biggest influences on the in vitro performance of PLGA microspheres. The effect of temperature on drug release from PLGA-based microparticle and disc systems reported that no significant drug release occurred below the Tg, whereas rates increased with an increase in temperature (Aso et al., 1994). More importantly, release can be accelerated or decelerated simply by raising or lowering the temperature, respectively. Release experiments may be performed with as small as 0.5 °C increments to accurately discriminate formulations. For example, it was reported that Vivitrol® observed slower release for samples at 36.5 than 37 °C, and faster release was observed at 37.5 °C (2006). The difference was described as being particularly noticeable during the sustained release or phase 3, governed primarily by erosion.
While the in vitro release method is primarily utilized for discriminatory analysis between formulations for batch release, ensuring consistent product safety and performance, a major goal is often to develop a correlation between the in vitro and in vivo drug release. With an in vitro-in vivo correlation (IVIVC) established, the in vitro method could be used as substitute for bioequivalence studies that may be required for scale-up and/or post-approval changes (D’Souza, 2019). Furthermore, it may also reduce the need for animal studies and potential clinical trials, ultimately resulting in reduced costs and development timelines for generic long-acting microparticles. While precise control and modification over the in vitro release conditions is readily obtainable, potential variability in the in vivo environment may not be accounted for or accurately controlled.
We hypothesize that variable housing climates, specifically “warm” vs. “cold”, could influence or impart quantifiable differences in the subcutaneous (SQ) temperature in rodents, ultimately producing a measurable change in the pharmacokinetic performance. The Guide for the Care and Use of Laboratory Animals recommends air temperatures of 18–26 °C for mice, rats, hamsters, gerbils, and guinea-pigs (National Research, 1996). Considering 0.5 °C increments were reported to cause a noted difference in the in vitro release of Vivitrol (2006), this housing temperature range may be broad enough to produce SQ temperature differences and as a result, lead to observable pharmacokinetic differences. While thermal regulation through tail-skin vasodilation for heat loss or vasoconstriction for heat retention will occur to account for the environmental differences (Grant, 1963; O’Leary et al., 1985), oscillating temperatures may supply enough energy to the microparticle to result in structural rearrangement, i.e., PLGA annealing and/or healing. Pore healing has been demonstrated to minimize the burst release of large molecules (Mazzara et al., 2013) and encapsulation of protein molecules and antigens through raising the temperature above the Tg (Desai and Schwendeman, 2013; Reinhold et al., 2012).
To test this hypothesis, two batches of Vivitrol were used for the in vivo pharmacokinetic study. The same two batches were extensively characterized in vitro via a sample and separate release method at 35, 36, and 37 °C. The pharmacokinetic profiles were compared to the in vitro release profiles to determine if an IVIVC is present, and subsequently what temperature may be more representative of the in vivo environment. A characterization of Vivitrol’s physicochemical properties was performed to determine if any differences in the in vivo/in vitro performance of the lots could be traced back to the microparticle properties.
As a means to measure the temperature accurately and without further stress unto the animal, SQ temperature probes were injected subcutaneously and monitored throughout the study. While Vivitrol is administered as an intramuscular gluteal injection once a month or every 28 days, in preclinical studies minimal differences were observed in the rodent model between SQ and IM delivery. In humans,Vivitrol is given as a deep intramuscular injection, and after prolonged periods of sitting, sleeping, or exercising, elevated localized temperatures may occur and potentially alter the release kinetics, due to macro and micro-morphological changes in the polymeric matrix. In addition, compressive forces due to sitting may further alter the structural matrix of PLGA and result in modified release kinetics.
Minimal information is available on how the subcutaneous environment, specifically temperature, may impact the in vivo release and subsequent analysis. Few studies have been published on what effect a variable or oscillating temperature profile in vivo may have on microparticle release. The closing or healing of pores in PLGA drug delivery system has been identified, with reproducible healing at temperature above the glass transition temperature (Kang and Schwendeman, 2007; Mazzara et al., 2013). While the temperatures in those studies may be outside typical subcutaneous temperatures, it demonstrates that the molecular mobility of PLGA near the glass transition temperature, coupled with the plasticizing effect of interstitial fluid, can lead to polymer rearrangement and potentially modified release kinetics. Furthermore, it is unknown what effect cyclical opening and closing of the pores, and/or structural polymeric rearrangement, may have on drug release due to the oscillating and variable subcutaneous temperatures. Therefore, the main objective of the study is to determine if a potential difference in subcutaneous temperature imparted due to housing climate differences can impact the pharmacokinetic response of PLGA microspheres.
2. Materials and methods
Two lots of Vivitrol (Alkermes plc, Dublin, Ireland), 2018–1052T and 2017–1037T, were purchased through the Purdue University Pharmacy. Both lots were stored at 4 °C. Phosphate buffered saline with 0.05% Tween 20 and sodium ascorbate were purchased from Sigma Aldrich. Methanol, acetonitrile, potassium phosphate, was purchased from Fisher Scientific. All other chemicals obtained commercially were either ACS or USP grade.
2.1. In vitro release
20 mL of pH 7.4 phosphate buffered saline with 0.05% Tween 20 and 0.0625% (w/v) sodium ascorbate and approximately 5 mg of Vivitrol were placed in a stoppered 50 mL Erlenmeyer flask and placed in 35.0, 36.0, and 37.0 °C (±0.3 °C) glycerol baths at 30 RPM in shaking incubators. 1 mL aliquots were taken at various time points and replaced with fresh release medium. Naltrexone content in buffer was analyzed via high performance liquid chromatography (HPLC). The HPLC had the following conditions: Mobile Phase: 65:35 methanol:potassium phosphate buffer, pH 6.6; flow rate: 1.0 mL/min; autosampler temperature: room temperature; column temperature: 30 °C; detection: 210 nm (UV); total run time: 7 min; injection volume: 10 μL; column: Zorbax SB-C18 150 × 4.6 mm, 5 μm; approximate retention time of naltrexone: 4.8 min.
2.2. Naltrexone loading and benzyl alcohol content
Approximately 5 mg of Vivitrol was accurately weighed, dissolved in 5 mL of acetonitrile, and diluted with mobile phase. 2.5 μL was then injected with the same HPLC conditions to that of the in vitro release samples.
2.3. Thermal analysis
A TA Q2000 differential scanning calorimeter was used for thermal analysis. Samples (~5 mg) were analyzed in hermetically sealed aluminum pans under a dry argon purge at 50 mL/min. Indium was used for temperature and heat of fusion calibration (ΔHf). Samples were heated at 20 °C/min to temperatures approximately 20 °C above the glass transition (Tg).
2.4. Particle size distribution
The particle size distribution was measured using a CILAS 1190 particle size analyzer (Madison, WI). Approximately 50 mg of microspheres were dispersed in 1.5 mL of a 0.1% Tween 80 aqueous solution and subsequently analyzed. Each lot was measured in triplicate.
2.5. Powder X-ray diffraction
Powder diffraction (XRD) data were collected on a Panalytical Empyrean X-ray diffractometer equipped with Bragg-Brentano HD optics, a sealed tube copper X-ray source (λ = 1.54178 Å), soller slits on both the incident and receiving optics sides, and a PixCel3D Medipix detector. Samples were packed in metal sample cups with a sample area 16 mm wide and 2 mm deep. Anti-scatter slits and divergence slits as well as masks were chosen based on sample area and starting θ angle. Data were collected between 4 and 30° in 2θ using the Panalytical Data Collector software.
2.6. Imaging
The morphology of the Vivitrol lots was characterized with a Tescan Vega 3 scanning electron microscope. For the internal morphology assessment, microparticles were placed in a −80 °C freezer under sealed conditions, sectioned with a razor blade, and subsequently placed under vacuum in a desiccator to equilibrate to room temperature to avoid any water induced changes. Microparticles were then mounted onto carbon taped aluminum stubs and sputter coated with a gold–palladium mixture under vacuum in the presence of argon.
2.7. Polymer molecular weight characterization
We dissolved samples in acetone and filtered through a 0.22 μm PTFE filter and collection into an HPLC auto-sampler vial for injection. The samples were analyzed using GPC with quadruple detector (4D). The GPC-4D system consisted of an Agilent 1260 Infinity II HPLC connected to Dawn Heleos II (MALLS) coupled to Dynapro Nanostar DLS via optical cable, Optilab T-rEX (RI detector) and Viscostar III viscometer operated by Astra 7 software. GPC analysis was performed by injecting 50.0 μL of ~ 2.5 mg/ml polymer solution. The separation was performed with a linear gradient column (Tosoh Bioscience LLC, TSKgel GMHHR-L, 7.8 mm × 30 cm) at 0.6 mL/min flow of acetone with a 60-minute run time.
2.8. In vivo pharmacokinetic study
The Purdue University Institutional Animal Care and Use Committee approved all animal procedures. Sprague-Dawley rats from Envigo (Indianapolis, IN.) were used for the study and were acclimated for longer than one month prior to the study in either a “warm” or “cold” room. The warm room rats (n = 8) were housed in a room with a temperature of 20.0 ~ 21.7 °C for the duration of the study and on top of reptile heater pads to give an interior cage temperature of 25.0 ~ 25.6 °C. The cold room (n = 8) temperature was 17.2 ~ 20.0 °C for the duration of the study. Each Vivitrol lot was distributed amongst four rats, injected subcutaneously in the scapular region at 50 mg/kg in an aqueous-based vehicle composed of 0.9% sodium chloride, 0.02% Tween 20, and 0.5% sodium carboxymethylcellulose.
During the course of the study, the animals were observed for overt toxicity and any existing test site abnormalities, including redness, swelling, bleeding, discharge, and bruising at the injection site. Body weights were taken and recorded at administration and at the various blood draw time points. Rats were anesthetized and bled (approximately 250 μL) via the tail or submandibular vein. Blood was collected in labeled potassium ethylenediaminetetraacetic acid tubes. We then centrifuged the blood for 10 min at 5,000 rpm at 4 °C. The plasma fraction was transferred to labeled 1 mL plastic tubes and stored at −80 °C prior to analysis.
2.9. Plasma analysis
Naltrexone was analyzed by liquid chromatography mass spectrometry. A stable labeled deuterated analog of naltrexone was used as an internal standard and for quantitation. D3-Naltrexone was purchased from Sigma Aldrich (St. Louis, MO). All stock solutions were prepared using 100% methanol and stored at −20 °C when not in use. The plasma calibration curve was 1–50 ng/mL final concentration.
Plasma samples were stored at −80 °C until analysis. The plasma was thawed and 0.1 mL aliquoted into a tube for naltrexone extraction. To each sample 5 ng/mL of d3-naltrexone was added just prior to extraction. Each sample was extracted with 5X volume of methyl tert-butyl ether (MtBE). After vortexing for 10 min the samples were centrifuged at 13,000 rpm for 10 min. The supernatant was collected, transferred to a new tube, and dried using a rotary evaporation device. The samples were subsequently reconstituted in 0.1 mL of 5% acetonitrile + 0.1% formic acid just prior to LC/MS/MS analysis.
The analysis was done with an Agilent 1260 Infinity II liquid chromatography system coupled to an Agilent 6470 QQQ mass spectrometer (Santa Clara, CA). Reverse phase chromatography using a Water’s T3 column (2.1 × 50 mm, 3.5 μm) was used for separation (Santa Clara, CA). Buffer A consisted of water + 0.1% formic acid and buffer B was acetonitrile + 0.1% formic acid. The linear LC gradient was as follows: time 0 min, 0% B; time 1 min, 0% B; time 10 min, 95% B; time 10.5 min 95% B; time 11 min, 0% B; time 15 min, 0% B. The flow rate was 0.3 mL/min with a total run time of 10 min. Multiple reaction monitoring was used to analyze each compound (table 1). Positive polarity electrospray ionization was used with the following source conditions: gas temperature 330 °C, gas flow 8 L/minute, nebulizer pressure 45 psi, sheath gas temperature 250 °C, sheath gas flow 7 L/minute, capillary voltage 4000 V, nozzle voltage 1000 V, and an electron multiplier voltage of + 400. Data were processed using Agilent Masshunter Quantitative analysis software (V.B.08).
Table 1.
Drug loading, residual benzyl alcohol content, and particle size of two lots of Vivitrol.
| Drug Loading (%) |
Residual Benzyl Alcohol (%) |
Particle Size (μm) ± SD | |||
|---|---|---|---|---|---|
| d 10 | d 50 | d 90 | |||
| 1037T | 32.87 ± 0.49 | 0.85 ± 0.01 | 47.3 ± 0.2 | 77.4 ± 0.9 | 118.3 ± 1.4 |
| 1052T | 33.14 ± 0.29 | 0.89 ± 0.00* | 45.6 ± 0.4* | 74.4 ± 0.8* | 113.8 ± 1.6* |
statistically significant at P < 0.05
2.10. Subcutaneous temperature monitoring
An IPTT-300 one inch Transponder from BioMedic Data Systems was injected subcutaneously into each rat in the middle portion of the back, slightly below the scruff, for subcutaneous temperature monitoring, and a DAS-8007 Reader was used to capture the subcutaneous temperature various times over the course of the study. The reader was placed within 3–6″ of the rat and moved in a circular motion above the proximity of where the transponder was injected. Rats were not handled prior to temperature readings so as to not induce any stress response when recording the temperature. The IPTT-300 is a glass-encapsulated radio frequency transmitter, preventing interaction between Vivitrol and the transponder. A transponder implanted in a group 1052 T-WR rodent stopped functioning shortly after implantation, therefore, averages in that group consist of only 3 rodents.
2.11. Statistical Data analysis
All data are presented as means with standard error of the mean (SEM) or standard deviation (SD). Statistical analyses were performed using Prism 9.0 (GraphPad Software, La Jolla, CA) using unpaired t-tests at significance levels of P < 0.05.
3. Results and discussion
Core body temperature of the human body is generally regarded as approximately 37 °C. However, the temperature of SC tissue at a comfortable environmental temperature of ~ 20 °C has been shown to average approximately 34 °C (Barcroft and Edholm, 1946; Webb, 1992). Sites closer to the body’s core, such as chest and back, demonstrate slightly higher temperatures than the extremities such as thighs, calves, feet, and arms. Importantly, one of the roles of the SC tissue is systemic thermal regulation. Consequentially, the SC tissue temperature has been shown to average 30 °C on the onset of shivering and 36 °C on the onset of sweating (Webb, 1992). SC tissue temperatures are typically maintained within a range of 30–36 °C, below the typical 37 °C temperature of release characterization. While Vivitrol is to be given as an intramuscular injection, preliminary studies in the rodent model demonstrated the release of naltrexone from the Vivitrol microspheres based on the plasma concentrations was relatively independent of the route of administration (IM vs SQ) (Bartus et al., 2003).
The release of naltrexone from the two lots of microspheres was performed with a sample and separate method at 35, 36, and 37 °C to determine the impact temperature has on release from the Vivitrol microspheres. As previously described, Vivitrol demonstrated a slower release rate for samples at 36.5 than at 37 °C, and faster release is observed at 37.5 °C (2006); although the absolute magnitude of rate differences was not stated. Multiple reports have documented demonstrating the effect of temperature on release (Andhariya et al., 2017a; Shen et al., 2016; Zolnik et al., 2006), although these studies are typically for accelerated release purposes with regards to discriminatory formulation assessment and not within the range of physiological temperatures.
Fig. 1 illustrates the release of Lot 1052T vs 1037T. Lot 1052T has a slightly higher release rate relative to 1037T for the three release temperatures characterized. The profiles show a short lag period of ~ 1 day, followed by relatively zero-order release. Therefore, the release is likely governed by a combination of drug diffusion, polymer rearrangement, and polymer degradation. Since the drug loading is similar between the batches (Table 1), the polymer molecular weight or some other structural difference due to processing may be the cause for the slight difference between the two lots. As a simple means to compare the magnitude of temperature impact on release, one can observe the time to reach ~ 50% release. Batch 1052T takes ~ 10 days to reach 50% release at 37 °C, whereas at 35 °C, slightly longer than ~ 20 days. Small changes in temperature can produce significant differences in the observed release.
Fig. 1.
Naltrexone in vitro release profiles at 35, 36, and 37 °C via sample and separate method.
The route of administration (IM or SQ) was previously shown to have no significant effect on either the plasma naltrexone levels or area under the curves in the rodent model for Vivitrol (Bartus et al., 2003). The plasma naltrexone concentrations increased to approximately one-half the maximum within 24 hrs of injection and the maximum levels were observed by Day 3. The plasma levels did not significantly differ from each other between 3 and 14 days (IM) or 3 and 21 days (SQ) post-injection.
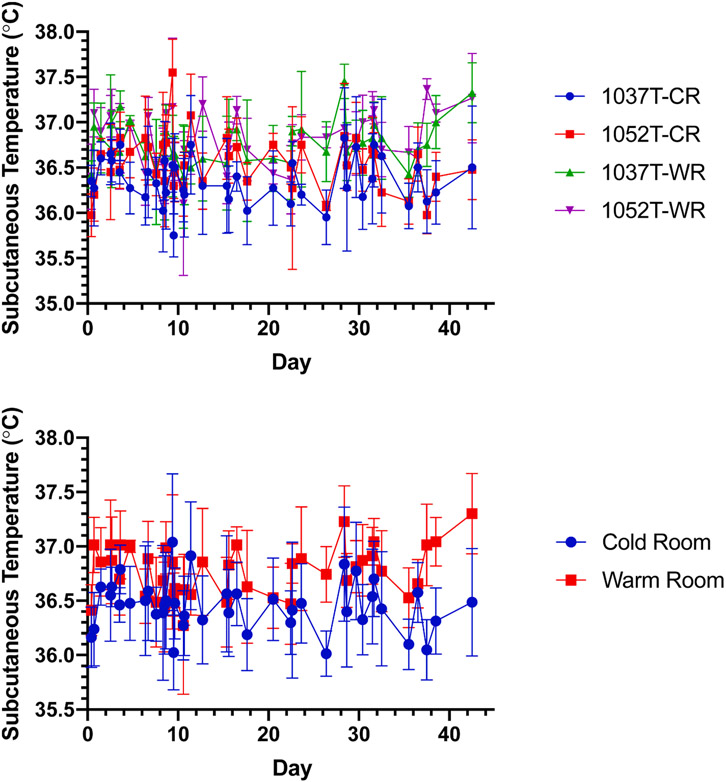
Fig. 2 illustrates comparison plots of in vivo pharmacokinetic profiles with the representative in vivo measured SQ temperatures shown in Fig. 3. All pharmacokinetic profiles appear to show either 2 or 3 ‘burst’ peaks, that occur within the first 24 h, or at Day 3 and/or Day 10. After Day 10, all profiles appear to show steady release with elimination being greater than absorption/release as all profiles illustrate a continuing decrease after Day 10. Table 2 lists the calculated AUCd0-42 values and these align with the in vitro release profiles, in that 1052T-WR has a greater drug exposure (i.e., faster release) and 1037T-CR has a lower drug exposure (i.e., slower release), although statistically significant differences (P < 0.05) of AUCd0-42 were not observed when comparing between the two housing conditions of the same lot of Vivitrol. While significant noise is observed in the recorded subcutaneous temperatures, a statistically significant difference is observed between the overall average of each 1037T-CR and 1037T-WR and 1052T-CR and 1052T-WR. While the rodents were co-housed to provide enrichment due to their highly social nature, this may have resulted in observed subcutaneous temperatures higher had they been individually housed due to them being in close proximity for the duration of the study.
Fig. 2.
Pharmacokinetic profile comparison of 1037T and 1052T in cold room (CR) and warm room (WR).
Fig. 3.
Subcutaneous temperature (TSQ) profile comparison of 1037T and 1052T in cold (CR) and warm room (WR) (top), average of CR compared to WR (middle), and average temperatures of rodents housed in the cold room vs warm room (bottom).
Table 2.
AUCd0-42 values for the two Vivitrol lots and respective room environments.
| Cold Room | Warm Room | |||
|---|---|---|---|---|
| 1037T | 1052T | 1037T | 1052T | |
| AUQd0-42 (ng•d/mL) ± SEM | 277.5 ± 23.5 | 294.2 ± 20.1 | 310.4 ± 36.9 | 332.5 ± 30.6 |
CR vs WR comparison not statistically significant at P < 0.05
Inherent differences in the rate of absorption can result in clinically significant differences in early exposure and drug response (Lee et al., 2016). A transient decrease in the plasma concentration of the active metabolite of risperidone upon the switch from the deltoid to the gluteal muscle has been observed for Risperdal Consta®, a PLGA-based microsphere product (Elliott et al., 2010). This difference was hypothesized to be based upon a number of potential factors, including (i) blood flow differences between the gluteal muscle and deltoid muscle, where the blood flow rate in the gluteal muscle is significantly lower than the deltoid muscle (Evans et al., 1975); and (ii) increased injection into fatty tissue when given as a gluteal injection relative to a deltoid injection, resulting in decreased hydrolytic degradation of the polymeric matrix and or water absorption slowing the release rate. Water content in lean mass (or fat-free mass) is about 70 ~ 75%, whereas in fat tissue it is approximately 10% (Lorenzo et al., 2019). Increased water content in PLGA matrices has demonstrated increased degradation rates (Keles et al., 2015) and increased mobility in the polymeric chains (D’Souza et al., 2014), and both factors could lead to an increase in the drug release rate. While temperature has a significant role in vitro, a myriad of other factors, including temperature, may also influence the drug release kinetics in vivo.
The cumulative AUCd0-42 values for the in vivo tests were compared to the in vitro release rates obtained with the sample and separate method (Fig. 4). Lot 1037T-CR correlates well with the 37 °C in vitro testing early, but then shifts towards the 36 °C in vitro results after approximately 10 days. The 1037T-WR correlates well with the 36 °C in vitro curve for the duration of release. Interestingly, both in vivo tests of lot 1052T are nearly indistinguishable from the 36 °C in vitro release testing for the duration of the release. These results, coupled with the measured subcutaneous temperatures, demonstrate that 37 °C may not accurately represent the in vivo conditions (i.e., subcutaneous space in rodents), and in certain instances lower in vitro release temperatures may more accurately represent and provide better correlation.
Fig. 4.
IVIVC for Vivitrol lots 1037 T (left) and 1052 T (right).
To delineate the physico-chemical properties of the two Vivitrol lots that may have contributed to the slight difference observed in the in vitro release patterns, a further in vitro characterization was performed. With the approval of generic long-acting injectables on the horizon, these proposed generic products should be qualitatively (Q1) and quantitatively (Q2) the same as the reference listed drug to be considered. Comprehensive characterization of PLGA is required for the generic application of polymer-based products. Key properties of PLGA including L:G ratio and L:G ratio distribution, molecular weight distributions, polymer-end group, and polydispersity among other factors that could all impact the release mechanism, release rate, and degradation profile of the microspheres. Furthermore, the synthetic method, catalyst driven method, sterilization method, could also all impact the potential generic product.
The molecular weight of the two lots of Vivitrol were determined (Table 3) with GPC-4D. The Mn, Mw, and Mz for 1037T are all slightly lower (8.2, 5.6, and 6.1%, respectively) than 1052T. An important point to consider is the starting PLGA polymer in these two batches of Vivitrol may or may not be from different PLGA lots. Second, the slight differences noted could be due to small variations in the manufacturing process. Naltrexone is known to cause nucleophilic attack of the PLGAA ester bond, resulting in a decrease in molecular weight (Wright et al., 2003). The drug loading, residual benzyl alcohol, and representative particle sizes in Table 1 illustrate that both batches have similar properties. Of these characterizations thus far, the polymer molecular weight is the only parameter that may have contributed to the difference observed in vitro, although very similar between lots.
Table 3.
Full characterization of two different lots of Vivitrol.
| Vivitrol Lot | Mn (kDa) ± SD | Mw (kDa) ± SD | Mz (kDa) ± SD | MHS slope (a) |
|---|---|---|---|---|
| 1037T | 38.43 ± 0.34 | 49.49 ± 0.24 | 62.50 ± 0.65 | 0.650 |
| 1052T | 41.90 ± 0.22* | 52.43 ± 0.22* | 66.57 ± 0.73* | 0.649 |
statistically significant at P < 0.05 for CR vs WR for each batch
Vivitrol is a unique example of a long-acting polymer microsphere, in that the solid-state form of naltrexone may have an influence on the release rate. According to US Patent 7,279,579 B2, naltrexone exists in four different forms in the microparticle product, and the ratio of these forms influence the in vitro release rate (Brittain et al., 2007). It is unknown whether these 4 forms are uniformly distributed throughout a microparticle or if a form preference may exist as a function of particle size or other microparticle property. The diffraction patterns of the two batches are illustrated in Fig. 5. The peak positions appear to be similar between the two batches with no unique peaks in either batch, therefore the same crystalline forms appear to be present in both batches. Multiple studies have focused on compositionally equivalent microspheres and microsphere manufacturing method variability, producing similar loadings and release profiles (Andhariya et al., 2017b; Garner et al., 2018; Shen et al., 2015). For a true-comparison between batches, the drug solid-state form, its respective size, distribution throughout the polymeric matrix, and the macro- and microscopic morphology should also be considered, as could dictate release.
Fig. 5.
Diffraction patterns of 1037T and 1052T.
While SEM alone likely cannot distinguish between batches, 1037T and 1052T were both imaged to determine if any distinguishing features can be found between the two batches. Fig. 6 illustrates the overall morphology and cross-sectioned microspheres of the two batches. Overall, the surface of the microspheres are relatively smooth, with a number of microspheres exhibiting collapsed or buckled morphologies, likely due to a polymer shell forming during emulsification, followed by collapse during solvent extraction and evaporation (Sah, 1997). Some particles appear to exhibit some small pores on the surface, but the particles appear relatively smooth overall. Naltrexone crystals could not be observed on the surface, whereas crystals have been found on the surface of Risperdal Consta® microspheres [unpublished data]. The interior of the microspheres provides more details relative to the surface in terms of drug distribution and morphology. Spherical pores or cavities varying in size are present throughout the interior of the microspheres, probably due to water uptake during solvent extraction owing to the solubility of ethyl acetate in water and vice versa (Sah, 1997). Upon closer inspection, multiple drug domains, assumed to be individual crystals based on the distinct morphology, appear to be relatively homogenously dispersed throughout the interior matrix of the microsphere (Fig. 7). This homogenous distribution could be an additional reason responsible for the near zero-order release profile shown, in addition to the ratio of polymorphs that has been shown to control the release (Brittain et al., 2007). While a conclusion should not necessarily be drawn based on SEM images alone, vastly different network structures are notably observed in Vivitrol microspheres: thin-skin layer microspheres that are completely hollow, thick-skin particles with a hollow core, particles with relatively homogeneously distributed spherical cavities, and a sponge-like matrix interior morphology (see Fig. 6).
Fig. 6.
Vivitrol morphology of two different lots, 1037T (left) and 1052T (right).
Fig. 7.
Vivitrol internal morphology of lot 1052T illustrating network structure and drug distribution. (Yellow arrows point to suspected crystalline naltrexone dispersed in PLGA matrix).
While the manufacturing process is likely different for all FDA approved microspheres, the polymer chains will become essentially frozen and their segmental mobility reduced at some instance during their production. For the Vivitrol microspheres this may occur during or after the ethanol wash, where additional solvent may be removed and the polymer molecule may undergo further rearrangement in succession, simultaneously, or during the final drying. Between this final rearrangement and characterization/use, additional aging of the microspheres will occur. Vivitrol is to be refrigerated prior to usage and is stable for 7 days at 25 °C. Even if the material is refrigerated, aging will occur, albeit at much slower kinetics. During aging, segmental mobility causes volume relaxation and thus a reduction in free volume (Pan et al., 2007). Volume reduction due to structural relaxation can alter drug diffusivity within the microspheres (Allison, 2008). This structural relaxation can also lead to phase separation between the drug and polymer, resulting in much different drug release kinetics. Structural relaxation could result in a burst release and/or increased kinetics due to free volume decreases. This decrease in free volume may result in phase separation of drug or decreased kinetics if the drug is in crystalline form or domains whereby the free volume change will result in decreased water uptake and subsequent diffusional.
The potential effects of aging and structural relaxation on the drug release were assessed through enthalpic recovery experiments. Ideally, the structural relaxation kinetics of the microspheres are compared to the raw material, i.e., the PLGA polymer. Enthalpic relaxation is observed as an endothermic event that arises as a result of un-aging the material on heating through the Tg. This endothermic event is representative of the change in enthalpy of the glass when the polymer chains relax from higher energetic conformations to lower energetic ones. Aged samples have smaller free volumes and potential energy than unaged ones, therefore more energy is required for the glass transition resulting in an increase in the area of the endothermic peak (Hutchinson et al., 1999).
The representative Tg and ΔHR of the two lots of Vivitrol are summarized in Table 4. No clear statistical difference is observed for the Tg or the ΔHR between the two lots. The extent of structural relaxation or the magnitude of difference that may impact drug release kinetics is relatively unknown, as the impact is likely to depend on the processing, formulation (i.e., residual solvents, drug, and polymer), and storage conditions. The physical aging of a PLGA polymeric matrix, as demonstrated by the amplitude of the endothermic overshoot associated with the Tg, was shown to slow down with crystalline progesterone (Rosilio et al., 1998). Encapsulated proteins (e.g., bovine serum albumin and beta-casein) were shown to have no significant effect on the glass transition of PLGA microparticles, whereas DNA and poly(vinyl alcohol) were shown to have mild anti-plasticizing effects (Rouse et al., 2007). Unfortunately, the effects on drug release were not discussed in either study. A dexamethasone-PLGA microparticle system with a drug loading of ~ 7.5 w/w % and particle size of ~ 7 μm showed an initial enthalpic relaxation of ~ 5 J/g, considered to be due to vacuum drying of the microspheres for 24 hrs at room temperature, and increased to ~ 7 J/g by 3 months at 4 °C storage and ~ 13 J/g by 3 months at 25 °C storage (Rawat and Burgess, 2011). Slightly slower release was noted around day 20 for microparticles annealed at 25 °C, speculated to be due to physical aging. Each system may be unique and the effect of aging and/or free volume may impact each system differently. Further studies are necessary to evaluate the effects of physical aging as a function of formulation, manufacturing process, and storage.
Table 4.
Comparison of Tg and ΔHR of the two Vivitrol lots.
| Vivitrol Lot | Tg,onset (°C) ± SD | ΔHR (J/g) ± SD |
|---|---|---|
| 1037T | 42.2 ± 2.0 | 1.7 ± 0.2 |
| 1052T | 41.4 ± 1.2 | 3.0 ± 0.9 |
not statistically significant at P < 0.05
4. Conclusion
While this was a pilot study, large scale studies should be performed to determine if the in vivo climate, specifically temperature and pressure (e.g. rubbing, exercise, etc.), may induce statistically significant differences in the resultant in vivo performance. The animals were co-housed for their enrichment and social well-being; however, the close proximity in their housing environment may have dampened the intended effects of climate on subcutaneous temperature and the respective pharmacokinetic profiles. Future experiments will look into the effect of singly housed vs co-housed rodents. In vitro release experiments at 37 °C may be insufficient alone to develop an IVIVC, and should be tightly controlled due to the temperature-dependent release. Finally, small physicochemical differences between microparticle lots may have a cumulative effect on their performance, and a single analytical measurement may not be sufficient to delineate between batches. Multiple analytical methods should be pooled and the representative results combined to accurately quantify potential differences in batches. Multiple small differences in physico-chemical parameters may concomitantly impact performance. In conclusion, while a statistical difference was noted in the subcutaneous temperature of the two housing climates, a statistical difference in the pharmacokinetic profile was not observed for the two different lots of Vivitrol administered by subcutaneous dosing.
Acknowledgments
This study was supported by the grant UG3 DA048774 from the National Institute of Drug Abuse (NIDA), grant F30 HL145980 to F.W.D. from the National Heart, Lung, and Blood Institute (NHLBI), the Chong Kun Dang Pharmaceutical Corp., and the Showalter Trust Fund.
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijpharm.2021.120738.
References
- 2006. Clinical Pharmacology and Biopharmaceutics Review. Application Number 21-897; February/2006.
- Acharya G, Shin CS, Vedantham K, McDermott M, Rish T, Hansen K, Fu Y, Park K, 2010. A study of drug release from homogeneous PLGA microstructures. J. Control. Release 146, 201–206. [DOI] [PubMed] [Google Scholar]
- Allison SD, 2008. Effect Of Structural Relaxation On The Preparation And Drug Release Behavior Of Poly(lactic-co-glycolic)acid Microparticle Drug Delivery Systems. J. Pharm. Sci 97, 2022–2035. [DOI] [PubMed] [Google Scholar]
- Amann LC, Gandal MJ, Lin R, Liang Y, Siegel SJ, 2010. In vitro-in vivo correlations of scalable PLGA-risperidone implants for the treatment of schizophrenia. Pharm Res 27, 1730–1737. [DOI] [PubMed] [Google Scholar]
- Andhariya JV, Choi S, Wang Y, Zou Y, Burgess DJ, Shen J, 2017a. Accelerated in vitro release testing method for naltrexone loaded PLGA microspheres. Int. J. Pharm 520, 79–85. [DOI] [PubMed] [Google Scholar]
- Andhariya JV, Shen J, Choi S, Wang Y, Zou Y, Burgess DJ, 2017b. Development of in vitro-in vivo correlation of parenteral naltrexone loaded polymeric microspheres. J. Control. Release 255, 27–35. [DOI] [PubMed] [Google Scholar]
- Aso Y, Yoshioka S, Li Wan Po A, Terao T, 1994. Effect of temperature on mechanisms of drug release and matrix degradation of poly(d, l-lactide) microspheres. J. Control. Release 31, 33–39. [Google Scholar]
- Barcroft H, Edholm OG, 1946. Temperature and blood flow in the human forearm. J Physiol 104, 366–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartus RT, Emerich DF, Hotz J, Blaustein M, Dean RL, Perdomo B, Basile AS, 2003. Vivitrex®, an Injectable, Extended-Release Formulation of Naltrexone, Provides Pharmacokinetic and Pharmacodynamic Evidence of Efficacy for 1 Month in Rats. Neuropsychopharmacology 28, 1973–1982. [DOI] [PubMed] [Google Scholar]
- Brittain HG, Dickason DA, Hotz J, Lyons SL, Ramstack M, Wright SG, 2007. Polymorphic Forms of Naltrexone. Alkermes Inc, USA. [Google Scholar]
- Chen W, Palazzo A, Hennink WE, Kok RJ, 2017. Effect of Particle Size on Drug Loading and Release Kinetics of Gefitinib-Loaded PLGA Microspheres. Mol. Pharm 14, 459–467. [DOI] [PubMed] [Google Scholar]
- D’Souza S, 2019. Injectables. Vitro Drug Release Testing of Special Dosage Forms; 55–85. [Google Scholar]
- D’Souza S, Dorati R, DeLuca PP, 2014. Effect of Hydration on Physicochemical Properties of End-Capped PLGA. Advances in Biomaterials 2014, 834942. [Google Scholar]
- Desai K-GH, Schwendeman SP, 2013. Active self-healing encapsulation of vaccine antigens in PLGA microspheres. J. Control. Release 165, 62–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott ESR, Purvis TL, Nelson LA, Sommi RW, 2010. Inconsistency in Risperidone Long-Acting Injection Steady-State Plasma Levels When Switching From Deltoid to Gluteal Administration. The Journal of Clinical Pharmacology 50, 721–724. [DOI] [PubMed] [Google Scholar]
- Evans EF, Proctor JD, Fratkin MJ, Velandia J, Wasserman AJ, 1975. Blood flow in muscle groups and drug absorption. Clin. Pharmacol. Ther 17, 44–47. [DOI] [PubMed] [Google Scholar]
- Garner J, Skidmore S, Park H, Park K, Choi S, Wang Y, 2018. Beyond Q1/Q2: The Impact of Manufacturing Conditions and Test Methods on Drug Release From PLGA-Based Microparticle Depot Formulations. J. Pharm. Sci 107, 353–361. [DOI] [PubMed] [Google Scholar]
- Gasmi H, Siepmann F, Hamoudi MC, Danede F, Verin J, Willart JF, Siepmann J, 2016. Towards a better understanding of the different release phases from PLGA microparticles: Dexamethasone-loaded systems. Int. J. Pharm 514, 189–199. [DOI] [PubMed] [Google Scholar]
- Grant RT, 1963. Vasodilatation and body warming in the rat. J Physiol 167, 311–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CL, Kumar S, Tan JJZ, Boey FYC, Venkatraman SS, Steele TWJ, Loo JSC, 2013. Modulating drug release from poly(lactic-co-glycolic acid) thin films through terminal end-groups and molecular weight. Polym. Degrad. Stab 98, 619–626. [Google Scholar]
- Hutchinson JM, Smith S, Horne B, Gourlay GM, 1999. Physical Aging of Polycarbonate: Enthalpy Relaxation, Creep Response, and Yielding Behavior. Macromolecules 32, 5046–5061. [Google Scholar]
- Kang J, Schwendeman SP, 2007. Pore Closing and Opening in Biodegradable Polymers and Their Effect on the Controlled Release of Proteins. Mol. Pharm 4, 104–118. [DOI] [PubMed] [Google Scholar]
- Keles H, Naylor A, Clegg F, Sammon C, 2015. Investigation of factors influencing the hydrolytic degradation of single PLGA microparticles. Polym. Degrad. Stab 119, 228–241. [Google Scholar]
- Lee LHN, Choi C, Gershkovich P, Barr AM, Honer WG, Procyshyn RM, 2016. Proposing the Use of Partial AUC as an Adjunctive Measure in Establishing Bioequivalence Between Deltoid and Gluteal Administration of Long-Acting Injectable Antipsychotics. Eur. J. Drug Metab. Pharmacokinet 41, 659–664. [DOI] [PubMed] [Google Scholar]
- Lorenzo I, Serra-Prat M, Yébenes JC, 2019. The Role of Water Homeostasis in Muscle Function and Frailty: A Review. Nutrients 11, 1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makadia HK, Siegel SJ, 2011. Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers (Basel) 3, 1377–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzara JM, Balagna MA, Thouless MD, Schwendeman SP, 2013. Healing kinetics of microneedle-formed pores in PLGA films. J. Control. Release 171, 172–177. [DOI] [PubMed] [Google Scholar]
- Mittal G, Sahana DK, Bhardwaj V, Ravi Kumar MNV, 2007. Estradiol loaded PLGA nanoparticles for oral administration: Effect of polymer molecular weight and copolymer composition on release behavior in vitro and in vivo. J. Control. Release 119, 77–85. [DOI] [PubMed] [Google Scholar]
- Miyajima M, Koshika A, Okada JI, Ikeda M, 1999. Mechanism of drug release from poly(l-lactic acid) matrix containing acidic or neutral drugs. J. Control. Release 60, 199–209. [DOI] [PubMed] [Google Scholar]
- National Research C, 1996. Guide for the Care and Use of Laboratory Animals. The National Academies Press, Washington, DC. [Google Scholar]
- O’Leary DS, Johnson JM, Taylor WF, 1985. Mode of neural control mediating rat tail vasodilation during heating. J. Appl. Physiol 59, 1533–1538. [DOI] [PubMed] [Google Scholar]
- Pan P, Zhu B, Inoue Y, 2007. Enthalpy Relaxation and Embrittlement of Poly(l-lactide) during Physical Aging. Macromolecules 40, 9664–9671. [Google Scholar]
- Rawat A, Burgess DJ, 2011. Effect of physical ageing on the performance of dexamethasone loaded PLGA microspheres. Int. J. Pharm 415, 164–168. [DOI] [PubMed] [Google Scholar]
- Reinhold SE, Desai K-GH, Zhang L, Olsen KF, Schwendeman SP, 2012. Self-Healing Microencapsulation of Biomacromolecules without Organic Solvents. Angewandte Chemie International Edition 51, 10800–10803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosilio V, Deyme M, Benoit JP, Madelmont G, 1998. Physical Aging of Progesterone-Loaded Poly(D, L,-lactide-co-glycolide) Microspheres. Pharm Res 15, 794–798. [DOI] [PubMed] [Google Scholar]
- Rouse JJ, Mohamed F, van der Walle CF, 2007. Physical ageing and thermal analysis of PLGA microspheres encapsulating protein or DNA. Int. J. Pharm 339, 112–120. [DOI] [PubMed] [Google Scholar]
- Sah H, 1997. Microencapsulation techniques using ethyl acetate as a dispersed solvent: effects of its extraction rate on the characteristics of PLGA microspheres. J. Control. Release 47, 233–245. [Google Scholar]
- Sandor M, Enscore D, Weston P, Mathiowitz E, 2001. Effect of protein molecular weight on release from micron-sized PLGA microspheres. J. Control. Release 76, 297–311. [DOI] [PubMed] [Google Scholar]
- Shen J, Choi S, Qu W, Wang Y, Burgess DJ, 2015. In vitro-in vivo correlation of parenteral risperidone polymeric microspheres. J. Control. Release 218, 2–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Lee K, Choi S, Qu W, Wang Y, Burgess DJ, 2016. A reproducible accelerated in vitro release testing method for PLGA microspheres. Int. J. Pharm 498, 274–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toshiro H, Hiroaki O, Yasuaki O, Hajime T, 1991. Factors influencing the profiles of TRH release from copoly(d, l-lactic/glycolic acid) microspheres. Int. J. Pharm 72, 199–205. [Google Scholar]
- Webb P, 1992. Temperatures of skin, subcutaneous tissue, muscle and core in resting men in cold, comfortable and hot conditions. Eur. J. Appl. Physiol 64, 471–476. [DOI] [PubMed] [Google Scholar]
- Wright SG, Rickey ME, Ramstack M, Lyons SL, Hotz J, 2003. Method for preparing microparticles having a selected polymer molecular weight. Alkermes Controlled Therapeutics, Ib. II, USA. [Google Scholar]
- Zolnik BS, Leary PE, Burgess DJ, 2006. Elevated temperature accelerated release testing of PLGA microspheres. J. Control. Release 112, 293–300. [DOI] [PubMed] [Google Scholar]