Abstract
In addition to the ubiquitous mevalonate pathway, Streptomyces sp. strain CL190 utilizes the nonmevalonate pathway for isopentenyl diphosphate biosynthesis. The initial step of this nonmevalonate pathway is the formation of 1-deoxy-d-xylulose 5-phosphate (DXP) by condensation of pyruvate and glyceraldehyde 3-phosphate catalyzed by DXP synthase. The corresponding gene, dxs, was cloned from CL190 by using PCR with two oligonucleotide primers synthesized on the basis of two highly conserved regions among dxs homologs from six genera. The dxs gene of CL190 encodes 631 amino acid residues with a predicted molecular mass of 68 kDa. The recombinant enzyme overexpressed in Escherichia coli was purified as a soluble protein and characterized. The molecular mass of the enzyme was estimated to be 70 kDa by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and 130 kDa by gel filtration chromatography, suggesting that the enzyme is most likely to be a dimer. The enzyme showed a pH optimum of 9.0, with a Vmax of 370 U per mg of protein and Kms of 65 μM for pyruvate and 120 μM for d-glyceraldehyde 3-phosphate. The purified enzyme catalyzed the formation of 1-deoxyxylulose by condensation of pyruvate and glyceraldehyde as well, with a Km value of 35 mM for d-glyceraldehyde. To compare the enzymatic properties of CL190 and E. coli DXP synthases, the latter enzyme was also overexpressed and purified. Although these two enzymes had different origins, they showed the same enzymatic properties.
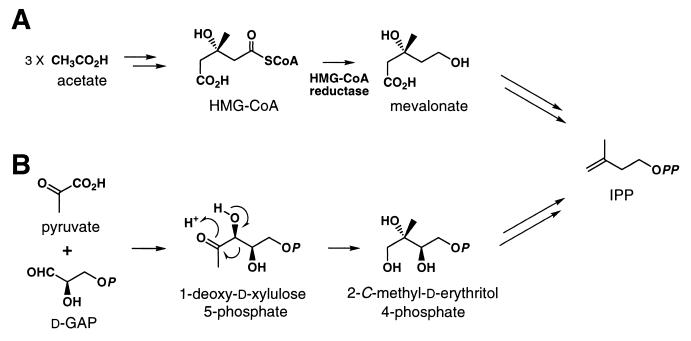
Isoprenoids found in all organisms play important roles, such as steroid hormones in mammals, carotenoids in plants, and ubiquinone or menaquinone in bacteria (18). All isoprenoids are synthesized by consecutive condensations of the five-carbon monomer isopentenyl diphosphate (IPP). It was generally believed that IPP is only synthesized by condensation of three molecules of acetyl coenzyme A through the mevalonate pathway (Fig. 1A). However, it has recently been revealed that not all living organisms possess this ubiquitous pathway and that IPP is synthesized through a mevalonate-independent pathway (nonmevalonate pathway) in many bacteria, green algae, and the chloroplasts of higher plants (Fig. 1B) (4, 13, 14, 17, 21, 22). The initial step of this nonmevalonate pathway is the formation of 1-deoxy-d-xylulose 5-phosphate (DXP) by condensation of pyruvate and glyceraldehyde 3-phosphate catalyzed by DXP synthase. The dxs gene homologs encoding DXP synthase have been cloned from Escherichia coli (12, 25), peppermint (Mentha × piperita) (10), and pepper (Capsicum annuum L.) (1). No detailed studies on the enzymatic properties of the DXP synthases from these organisms, however, have been reported. To our knowledge, only Km values for pyruvate and glyceraldehyde 3-phosphate of the recombinant DXP synthase from pepper were characterized (1).
FIG. 1.
IPP biosynthesis via the mevalonate pathway (A) and via the nonmevalonate pathway (B). HMG-CoA, 3-hydroxy-3-methylglutaryl coenzyme A; d-GAP, d-glyceraldehyde 3-phosphate. The pathway leading to IPP from 2-C-methyl-d-erythritol 4-phosphate is undefined.
Harker et al. and our group have independently found that ubiquinone production in E. coli was enhanced by overexpression of DXP synthase or DXP reductoisomerase, the enzyme catalyzing the second step of the nonmevalonate pathway (7; M. Harker and P. M. Bramley, Abstr. 4th Eur. Symp. Plant Isoprenoids, p. 22, 1999; H. Motoyama, K. Miyake, S. Hashimoto, A. Ozaki, H. Seto, T. Kuzuyama, and S. Takahashi, unpublished data). We have also found that overexpression of DXP synthase was more effective in this enhancement than that of DXP reductoisomerase. Although these data suggested that DXP synthase functioned as the rate-limiting enzyme in the nonmevalonate pathway, theoretical predictions have never been made on the basis of kinetic parameters such as the catalytic efficiency (kcat/Km) for enzymes involved in the pathway. In order to gain insight into the rate-limiting enzyme for the nonmevalonate pathway, it is important to determine the kinetic parameters of the enzymes responsible for this pathway. Therefore, we investigated the enzymatic properties of DXP synthase from E. coli, from which DXP reductoisomerase has also been cloned and characterized in our laboratory (26).
Unlike plants and fungi, Streptomyces spp., which are eubacteria, produce very few isoprenoids as secondary metabolites. Based on the results obtained by feeding experiments using 13C-labeled precursors indicating that some isoprenoids of Streptomyces origin, such as terpentecin (8), naphterpin (23), and napyradiomycin (24), were synthesized by the mevalonate pathway, all Streptomyces species were assumed without doubt to employ the same pathway for isoprenoid biosynthesis. We have recently demonstrated, however, that Streptomyces sp. strain CL190 possesses both the mevalonate and nonmevalonate pathways (21). Interestingly, the organism utilized the nonmevalonate pathway at the early growth stage but replaced it with the mevalonate pathway at the later stage of fermentation. The presence of these two pathways for isoprenoid biosynthesis in this organism raises a question about their roles in primary and secondary metabolite biosynthesis (16, 21).
As the first approach to answer the question by detailed analyses of the enzymes and genes involved in the two metabolic pathways, we have purified and cloned 3-hydroxy-3-methylglutaryl coenzyme A reductase, the rate-limiting enzyme in the mevalonate pathway, from Streptomyces sp. strain CL190 (27). Our attention was then directed to the cloning of genes responsible for the nonmevalonate pathway from CL190, an organism that utilizes both the mevalonate and nonmevalonate pathways for IPP biosynthesis.
In this paper, we report the cloning of the DXP synthase gene, dxs, from Streptomyces sp. strain CL190. The gene was overexpressed in E. coli, and its recombinant DXP synthase was purified to homogeneity and characterized in detail. In addition, the E. coli DXP synthase was overexpressed and purified, and its enzymatic properties were compared with those of CL190 DXP synthase.
MATERIALS AND METHODS
PCR amplification of a dxs gene probe and cloning of the dxs gene from the CL190 genome.
Several homologous regions of DXP synthase homologs were found in E. coli (accession no., AF035440), Haemophilus influenzae (accession no., P45205), Bacillus subtilis (accession no., P54523), Rhodobacter capsulatus (accession no., P26242), Synechocystis sp. strain PCC6803 (accession no., S75175), and Arabidopsis thaliana (accession no., Q38854). Two amino acid sequences, Trp Asp Val Gly His Asn and Ile Ala Glu Asn His Ala, were highly conserved among them, and thus the corresponding forward oligonucleotide primer, pCDXS1 (5′-TGGGACGTSGGSCACCAG), and the reverse primer, pCDXS2 (5′-ACSGCGTGCTGCTCSGCG), were synthesized (Amersham Pharmacia Biotech). The letter S in these primers stands for G or C. PCR was carried out in 20 μl (total volume) of PCR buffer (Boehringer) containing 50 ng of total DNA from Streptomyces sp. strain CL190, a 0.2 mM concentration of each deoxynucleoside triphosphate, 2.5 pmol of each primer, and 1.8 U of Taq polymerase (Boehringer) for 25 cycles (0.5 min at 95°C, 0.5 min at 50°C, and 1 min at 72°C). In this PCR, a DNA fragment of 0.9 kb was amplified and then used as the DNA probe for the colony hybridization method. By using this 0.9-kb DNA fragment, a 2.9-kb SphI-SphI fragment was obtained from the CL190 genome. The sequence of this 2.9-kb DNA fragment was determined as described below.
Construction of the plasmid for overexpression in E. coli of the dxs gene.
On the basis of the total nucleotide sequence of the dxs gene from Streptomyces sp. strain CL190, two oligonucleotide primers, 5′-GGGAAGCTTACGATTCTGGAGAACATCCGG-3′ (5′ of the dxs gene) and 5′-CCCAAGCTTTGCGGGCTGCTCCTCGGCCGG′ (3′ of the dxs gene), including a HindIII restriction site (underlined) were synthesized (Amersham Pharmacia Biotech) and used together with total DNA from CL190 to amplify the dxs gene. PCR was carried out in 20 μl (total volume) of PCR buffer (Boehringer) containing 50 ng of total DNA from CL190, a 0.2 mM concentration of each deoxynucleoside triphosphate, 2.5 pmol of each primer, and 1.8 U of Taq polymerase (Boehringer) for 25 cycles (0.5 min at 95°C, 0.5 min at 60°C, and 1 min at 72°C). In this PCR, a single DNA fragment of 1.9 kb was amplified. The PCR fragment was cleaved with HindIII and cloned into the HindIII site in pUC118 (Takara Shuzo). E. coli JM109 (Takara Shuzo) was used as the recipient in this transformation. DNA sequencing as described above was used to analyze clones for correct insert DNA, and then the correct DNA fragment was cloned into the HindIII site in the multicloning site of the expression vector pQE30 (Qiagen) to give plasmid pQCDXS. pQCDXS was designed to encode a recombinant enzyme with an affinity tag consisting of six consecutive histidine residues at the N-terminal region. Ni-nitrilotriacetic acid agarose resin has a strong affinity for a protein that has such histidine residues.
Expression and purification of the recombinant DXP synthase.
E. coli M15 containing pREP4 (neo lacI) (Qiagen) was used as the host for expression of the Streptomyces sp. strain CL190 dxs gene. M15(pREP4, pQCDXS) was cultured at 18°C in 100 ml of Luria-Bertani medium (19) containing 25 μg of kanamycin (Nacalai, Kyoto, Japan)/ml and 200 μg of ampicillin (Sigma)/ml for 12 h with the addition of 0.1 mM isopropyl-β-d-thiogalactopyranoside upon reaching an optical density at 660 nm of 0.8. Cells were harvested by centrifugation and resuspended in buffer A composed of 100 mM Tris-HCl (pH 8.0), 1 mM MgCl2, 1 mM dl-dithiothreitol, and 0.1 mM thiamine diphosphate. After brief sonication, the lysate was centrifuged at 10,000 × g for 20 min and the supernatant was collected. The crude extract was applied to a Ni-nitrilotriacetic acid agarose column (1.3 by 20 mm) (Qiagen) previously equilibrated with buffer A. The resin was washed with 50 mM imidazole in buffer A, and then the protein that bound to the resin was eluted with 200 mM imidazole in buffer A. The active fractions were combined and used as the purified DXP synthase in the subsequent experiments.
Determination of the molecular mass.
The molecular mass of the recombinant DXP synthase was estimated by gel filtration on a Superdex 200 (1.6- by 60-cm) column (Amersham Pharmacia Biotech) which was equilibrated with 20 mM sodium phosphate buffer (pH 7) containing 0.15 M NaCl. The column was eluted at a flow rate of 0.5 ml/min, and fractions of 2 ml were collected. The molecular mass was estimated by comparing the elution of DXP synthase with that of standard proteins ferritin (440 kDa), catalase (232 kDa), aldolase (158 kDa), and bovine serum albumin (66 kDa).
Assay for DXP synthase.
The standard assay system consisted of 100 mM Tris-HCl (pH 8.0) containing 1 mM MgCl2, 2 mM dl-dithiothreitol, 1 mM sodium pyruvate, 2 mM dl-glyceraldehyde 3-phosphate, and 150 μM thiamine diphosphate in a final volume of 0.5 ml. The reaction was initiated by adding the enzyme solution to the complete assay mixture at 37°C, and after a 10 min-incubation the reaction was halted by incubation at 100°C for 1 min. Next, the reaction mixture was treated with alkaline phosphatase (Sigma) at 56°C for 60 min to dephosphorylate completely the reaction product, DXP. Production of the resulting dephosphorylated compound, 1-deoxyxylulose (DX), was monitored by a refractive index spectrometer (model RI-71; Showa Denko, Tokyo, Japan) with a Shodex KS-801 (8- by 300-mm) column (Showa Denko), eluted with H2O at a flow rate of 1 ml/min at 80°C. DX was eluted at 8.6 min under this condition. The amount of DX production was precisely estimated by using chemically synthesized DX as the standard. One unit of DXP synthase activity was defined as the amount of the enzyme that caused the production of 1 μmol of DXP per min at 37°C. All the assays for the calculation of Km and Vmax values of both Streptomyces and E. coli enzymes were done at 37°C. These values were calculated with Lines&Kinetics software, version 1.0 (3).
Detection of DXP.
Production of DXP by the DXP synthase was monitored at 195 nm by high-performance liquid chromatography with a Senshu Pak NH2-1251-N (4.6- by 250-mm) column (Senshu Science, Tokyo, Japan) eluted with 100 mM KH2PO4 (pH 3.5) at a flow rate of 1 ml/min (9). DXP was eluted at 8.1 min under this condition.
Electrophoresis.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and native PAGE were performed in slab gels having an 8 to 25% polyacrylamide gradient with the PhastSystem (Amersham Pharmacia Biotech). Protein was visualized by Coomassie brilliant blue R-250 staining. The protein concentration was measured by the method of Bradford (2) with a protein assay kit (Bio-Rad Laboratories), using bovine serum albumin as the standard.
DNA sequence analysis.
The DNA sequence was determined by the dideoxy chain termination method (20) with an automated sequencer (model 4000L; Li-cor) and the protocol of the supplier. The FASTA program (11, 15) performed a homology search of the protein databases. Amino acid sequences aligned by the GENETYX program (Software Development, Tokyo, Japan) were then edited visually to align consensus motifs.
Nucleotide sequence accession number.
The nucleotide sequence of the 2,941-bp SphI-SphI fragment including the dxs gene of Streptomyces sp. strain CL190 has been deposited in the DDBJ, EMBL, and GenBank nucleotide sequence databases with accession no. AB026631.
RESULTS
Cloning and DNA sequencing the dxs gene from Streptomyces sp. strain CL190.
DXP synthase genes have been cloned from E. coli, peppermint, and A. thaliana, and at least nine amino acid sequences of DXP synthase homologs were available from the database of DNA Data Bank of Japan (DDBJ). The amino acid sequences of these DXP synthase homologs had significant similarity to one another over the entire sequences. In particular, two amino acid sequences, Trp Asp Val Gly His Asn and Ile Ala Glu Asn His Ala, were highly conserved among these DXP synthase homologs from E. coli, B. subtilis, R. capsulatus, Synechocystis sp. strain PCC6803, and A. thaliana. Thus, we attempted to clone the dxs gene from Streptomyces sp. strain CL190 by colony hybridization with a DNA probe which was generated by PCR with oligonucleotide primers prepared based on the highly conserved amino acid sequences just mentioned. By using these oligonucleotide primers together with CL190 total DNA, a 906-bp fragment was amplified. With this 906-bp DNA fragment as the dxs gene probe, a 2.9-kb SphI-SphI fragment was then obtained and sequenced. Sequence analysis of this 2.9-kb fragment identified one complete open reading frame (ORF) (Fig. 2). The ORF consisted of 1,896 bp starting with initiation codon GTG at position 926 and ending with termination codon TGA at position 2819 (Fig. 2). A putative Shine-Dalgarno sequence, GAAGG, was found 15 bp upstream of the initiation codon. The deduced amino acid sequence corresponding to the ORF showed significant sequence similarity to DXP synthase homologs from E. coli (accession no., AF035440), H. influenzae (accession no., P45205), B. subtilis (accession no., P54523), R. capsulatus (accession no., P26242), Synechocystis sp. strain PCC6803 (accession no., S75175), and A. thaliana (accession no., Q38854) (Fig. 3). The amino acid sequences encoded by the CL190 ORF corresponding to the highly conserved sequences were Trp Asp Thr Gly His Asn and Ile Ala Glu Asn His Ala (Fig. 3). Thus, only Thr was substituted for Val in the highly conserved sequence of the CL190 ORF product. The significant similarity suggested that the ORF encoded DXP synthase in CL190.
FIG. 2.
Nucleotide sequence of the 2.9-kb SphI-SphI DNA fragment including the dxs gene from Streptomyces sp. strain CL190 and deduced amino acid sequence. The dxs gene consists of 1,896 bp starting with initiation codon GTG at position 926 and ending with termination codon TGA at position 2819. A putative Shine-Dalgarno sequence, GAAGG, was found 15 bp upstream of the initiation codon. An incomplete ORF product flanking the N terminus of the dxs gene product showed 24% identity in the 120-amino-acid region of overlap with the Helicobacter pylori putative protein (accession no., P56414) involved in the biosynthesis of the molybdopterin precursor from guanosine.
FIG. 3.
Multiple alignment of the amino acid sequences of the Streptomyces DXP synthase and other DXP synthase homologs. Identical amino acids among the seven proteins are marked by asterisks. Dashes indicate gaps introduced for the optimization of the alignment. The amino acid sequences used for design of the PCR primers are underlined. CL190, Streptomyces sp. strain CL190; ECOLI, E. coli; HAEIN, H. influenzae; BACSU, B. subtilis; RHOCA, R. capsulatus; SYNY3, Synechocystis sp. strain PCC6803; ARATH, A. thaliana. For accession numbers, see Materials and Methods.
Enzymatic function of the ORF product from CL190.
To verify the enzymatic function of the product of the complete ORF to be that of a DXP synthase, the corresponding gene was overexpressed in E. coli. The QIAexpress system was used because of the advantages of high-level expression and easy purification with Ni-nitrilotriacetic acid agarose resin. Incubation of the purified recombinant protein with pyruvate and dl-glyceraldehyde 3-phosphate in the presence of thiamine diphosphate at 30°C for 12 h in the assay system for DXP synthase resulted in the production of DXP, which was detected by chromatography with a Senshu Pak NH2-1251-N column. Omission of thiamine diphosphate from the reaction mixture resulted in failure of DXP production, indicating that the recombinant protein absolutely requires thiamine diphosphate for the enzymatic reaction. The enzymatic function of the product of the ORF of Streptomyces sp. strain CL190 was thus confirmed to be that of a thiamine diphosphate-dependent DXP synthase.
Enzymatic properties for DXP synthase from CL190.
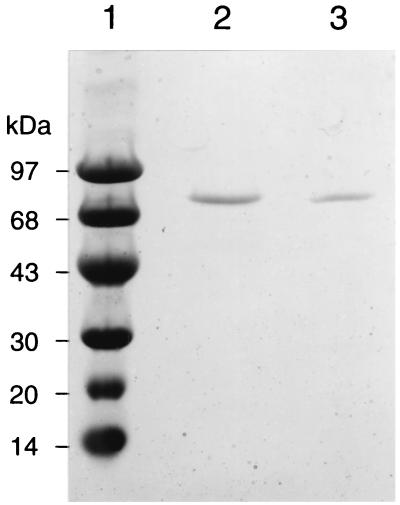
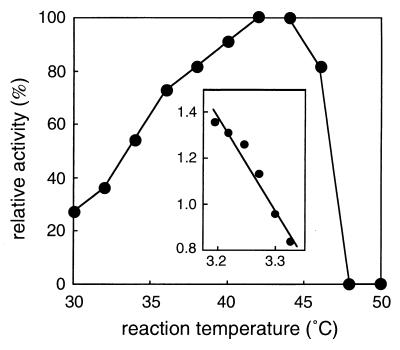
The purified recombinant DXP synthase showed a single band on SDS-PAGE gel and native PAGE gel. SDS-PAGE showed a subunit molecular mass of 70 kDa (Fig. 4). Native PAGE performed with a 8 to 25% polyacrylamide gradient gel gave a protein band with a mobility corresponding to 140 kDa. By gel filtration chromatography, the molecular mass of the enzyme was estimated to be 130 kDa. These results clearly suggested that the enzyme is most likely to be a dimer. The enzyme showed a pH optimum of 9.0. The effect of temperature on the enzyme activity was investigated over the range of 30 to 50°C. The maximum activity was observed at 42 to 44°C. The activation energy was estimated to be 99 kJ per mol by an Arrhenius plot whose curve was straight over the range of 30 to 40°C (Fig. 5). The enzyme required Mg2+ or Mn2+, and the optimum concentration of the divalent cations was 1 mM. The enzyme activity was completely lost by an addition of EDTA. The Km values were calculated as 65 μM for pyruvate and 120 μM for d-glyceraldehyde 3-phosphate, and Vmax was 370 U per mg of protein. The purified enzyme catalyzed the formation of DX by condensation of pyruvate and d-glyceraldehyde as well. However, the Km value for d-glyceraldehyde was 290-fold higher than that for d-glyceraldehyde 3-phosphate. These kinetic parameters are summarized in Table 1.
FIG. 4.
Electrophoresis of the purified CL190 and E. coli DXP synthase overexpressed in E. coli. Purified DXP synthases of CL190 and E. coli obtained by using a Ni-nitrolotriacetic acid agarose column were analyzed by SDS–8 to 25% PAGE. Lanes: 1, molecular mass standard; 2, SDS-treated CL190 enzyme (0.2 μg); 3, SDS-treated E. coli enzyme (0.1 μg). Proteins were stained with Coomassie brilliant blue R-250.
FIG. 5.
Temperature dependence of the CL190 DXP synthase activity and the Arrhenius plot (insert). The DXP synthase activity of Streptomyces sp. strain CL190 was measured in the complete assay mixture as described in Materials and Methods except for the reaction temperature. One hundred percent activity corresponds to 0.42 U. All data are average values for duplicate determinations. The insert shows the Arrhenius plot used to estimate the activation energy of the enzyme.
TABLE 1.
Comparisons of enzymatic properties among CL190, E. coli, and pepper DXP synthases
| DXP synthase source |
Km for:
|
Vmax (U/mg of protein) | Optimum temp (°C) | Optimum pHa | Activation energy (kJ/mol) | Divalent cations | Molecular mass (kDa) by:
|
Multimeric form | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pyruvate (μM) | d-Glyceraldehyde 3-phosphate (μM) | d-Glyceraldehyde (mM) | SDS-PAGE | Gel filtration chromatography | |||||||
| CL190 | 65 | 120 | 35 | 370 | 42–44 | 9.0 | 99 | Mg2+, Mn2+ | 70 | 130 | Dimer |
| E. coli | 96 | 240 | 38 | 300 | 42–44 | 7.5–8.0 | 63 | Mg2+, Mn2+ | 69 | 120 | Dimer |
| Pepperb | 500 | 750 | |||||||||
Assay solutions consisted of 100 mM Tris-HCl at pH 7.0 to 9.5.
From Bouvier et al. (1).
Enzymatic properties of DXP synthase from E. coli.
Although E. coli DXP synthase was cloned by two independent groups (12, 25), no detailed studies of its enzymatic properties have been reported. In order to compare the enzymatic properties of CL190 and E. coli DXP synthases, we overexpressed and purified E. coli DXP synthase (9). The purified E. coli DXP synthase showed a single band with a molecular mass of 69 kDa (Fig. 4). The native molecular mass of the enzyme was estimated to be 117 kDa by gel filtration chromatography, suggesting that the E. coli enzyme is also likely to form a dimer. The enzymatic properties of E. coli DXP synthase were similar to those of the CL190 enzyme (Table 1). A difference between the E. coli and CL190 enzymes was found only in the pH optimum, with the E. coli enzyme showing an optimum activity at pH 7.5 to 8.0.
DISCUSSION
We successfully cloned the dxs gene encoding DXP synthase from Streptomyces sp. strain CL190 by colony hybridization with a DNA probe generated by PCR with oligonucleotide primers prepared on the basis of the highly conserved amino acid sequences among DXP synthase homologs from six genera. The dxs gene from CL190 encoded 631-residue DXP synthase with a predicted molecular mass of 68 kDa. The deduced amino acid sequence showed around 38% identity to the DXP synthase homologs found in the SWISS-PROT database.
In order to characterize CL190 DXP synthase, we overexpressed the CL190 dxs gene in E. coli. Moreover, in order to compare the enzymatic properties of the CL190 DXP synthase with those of the E. coli DXP synthase, which had not been characterized in detail, we overexpressed, purified, and characterized the E. coli enzyme as well. The DXP synthases of both CL190 and E. coli were purified as soluble proteins and showed similar enzymatic properties (Table 1). On the other hand, it has been reported that the CapTKT2 gene was cloned from pepper and that the recombinant CapTKT2 gene product expressed in E. coli catalyzed DXP formation with Km values of 500 μM for pyruvate and 750 μM for d-glyceraldehyde 3-phosphate (Table 1) (1). The values of the kinetic parameters of pepper DXP synthase are much higher than those of CL190 and E. coli enzymes (Table 1).
Recently we cloned and characterized E. coli DXP reductoisomerase, the enzyme for the second step of the nonmevalonate pathway (26). DXP reductoisomerase simultaneously catalyzes intramolecular rearrangement and reduction of DXP to form 2-C-methyl-d-erythritol 4-phosphate (Fig. 1). The catalytic efficiency, kcat/Km, for E. coli DXP reductoisomerase was calculated to be 2.2 × 107 M−1 · s−1 in the presence of Mg2+ (T. Kuzuyama, S. Takahashi, M. Takagi, T. Shimuzu, and H. Seto, unpublished data). On the other hand, the kcat/Km value for E. coli DXP synthase was estimated to be 2.8 × 106 M−1 · s−1 in this study. Thus this value for DXP synthase is lower than that for DXP reductoisomerase by a factor of 8. This difference suggests that DXP synthase is a rate-limiting enzyme in the nonmevalonate pathway, at least in E. coli. This suggestion is also supported by the finding that overexpression of DXP synthase or DXP reductoisomerase in E. coli resulted in an increase of ubiquinone production and that overexpression of DXP synthase was more effective in this increase than that of DXP reductoisomerase (7; Harker and Bramley, Abstr. 4th Eur. Symp. Plant Isoprenoids; Motoyama et al., unpublished data). At present it is difficult to determine the rate-limiting step of the nonmevalonate pathway, because most reaction steps of this pathway remain undefined. However, the results obtained above seem to imply that the DXP synthase reaction is the rate-limiting step of the nonmevalonate pathway.
3-Hydroxy-3-methylglutaryl coenzyme A reductase is the rate-limiting enzyme of the mevalonate pathway in humans (6), and its specific inhibitors, pravastatin and related compounds, are used as cholesterol-lowering agents (28). If DXP synthase were the rate-limiting enzyme of the nonmevalonate pathway, its specific inhibitors would be reasonable antibacterials and herbicides with no toxicity to humans. Screening for DXP synthase inhibitors from natural products is now in progress in our laboratory.
ACKNOWLEDGMENTS
This work was supported in part by a Grant-in-Aid for Encouragement of Young Scientists from the Japan Society for the Promotion of Science (JSPS) (11760086) to T.K., by a grant from the Uehara Memorial Foundation to T.K., by a Research for the Future Program (RFTF) grant from JSPS (JSPS-RFTF96I00301) to H.S., and by a Grant-in-Aid for Scientific Research (B) from the Ministry of Education, Science, Sports and Culture of Japan (10460047) to H.S.
REFERENCES
- 1.Bouvier F, d'Harlingue A, Suire C, Backhous R A, Camara B. Dedicated roles of plastid tranketolases during the early onset of isoprenoid biogenesis in pepper fruits. Plant Physiol. 1998;117:1423–1431. doi: 10.1104/pp.117.4.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 3.Claros M G, Cánovas F M. Lines&Kinetics: a graphic tool to deal with linear regressions and enzyme kinetics. Embnet News. 1998;5:5–7. [Google Scholar]
- 4.Eisenreich W, Schwarz M, Catayrade A, Arigoni D, Zenk M H, Bacher A. The deoxyxylulose phosphate pathway of terpenoid biosynthesis in plants and microorganisms. Chem Biol. 1998;5:R221–R233. doi: 10.1016/s1074-5521(98)90002-3. [DOI] [PubMed] [Google Scholar]
- 5.Funayama S, Ishibashi M, Komiyama K, Omura S. Biosynthesis of furaquinocins A and B. J Org Chem. 1990;55:1132–1133. [Google Scholar]
- 6.Goldstein J L, Brown M S. Regulation of the mevalonate pathway. Nature. 1990;343:425–430. doi: 10.1038/343425a0. [DOI] [PubMed] [Google Scholar]
- 7.Harker M, Bramley P M. Expression of prokaryotic 1-deoxy-d-xylulose-5-phosphatases in Escherichia coli increases carotenoid and ubiquinone biosynthesis. FEBS Lett. 1999;448:115–119. doi: 10.1016/s0014-5793(99)00360-9. [DOI] [PubMed] [Google Scholar]
- 8.Isshiki K, Tamamura T, Sawa T, Naganawa H, Takeuchi T, Umezawa H. Biosynthetic studies of terpentecin. J Antibiot. 1986;39:1634–1635. doi: 10.7164/antibiotics.39.1634. [DOI] [PubMed] [Google Scholar]
- 9.Kuzuyama T, Takahashi S, Watanabe H, Seto H. Direct formation of 2-C-methyl-d-erythritol 4-phosphate from 1-deoxy-d-xylulose 5-phosphate by 1-deoxy-d-xylulose 5-phosphate reductoisomerase, a new enzyme in the non-mevalonate pathway to isopentenyl diphosphate. Tetrahedron Lett. 1998;39:4509–4512. [Google Scholar]
- 10.Lange B M, Wildung M R, McCaskill D, Croteau R. A family of transketolases that directs isoprenoid biosynthesis via a mevalonate-independent pathway. Proc Natl Acad Sci USA. 1998;95:2100–2104. doi: 10.1073/pnas.95.5.2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lipman D J, Pearson W R. Rapid and sensitive similarity protein searches. Science. 1985;227:1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- 12.Lois L M, Campos N, Putra S R, Danielsen K, Rohmer M, Boronat A. Cloning and characterization of a gene from Escherichia coli encoding a transketolase-like enzyme that catalyzes the synthesis of d-1-deoxylylulose 5-phosphate, a common precursor for isoprenoid, thiamine, and pyridoxol biosynthesis. Proc Natl Acad Sci USA. 1998;95:2105–2110. doi: 10.1073/pnas.95.5.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Orihara N, Furihata K, Seto H. Studies on the biosynthesis of terpenoidal compounds produced by Actinomycetes. 2. Biosynthesis of carquinostatin B via the non-mevalonate pathway in Streptomyces exfoliatus. J Antibiot. 1997;50:979–981. doi: 10.7164/antibiotics.50.979. [DOI] [PubMed] [Google Scholar]
- 14.Orihara N, Kuzuyama T, Takahashi S, Furihata K, Seto H. Studies on the biosynthesis of terpenoid compounds produced by Actinomycetes. 3. Biosynthesis of isoprenoid side chain of novobiocin via the non-mevalonate pathway in Streptomyces niveus. J Antibiot. 1998;51:676–678. doi: 10.7164/antibiotics.51.676. [DOI] [PubMed] [Google Scholar]
- 15.Pearson W R, Lipman D J. Improved tools for biological sequence comparison. Proc Natl Acad Sci USA. 1988;85:2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rohmer M. Isoprenoids including carotenoids and steroids. In: Barton D, Nakanishi K, editors. Comprehensive natural products chemistry. Vol. 2. Amsterdam, The Netherlands: Elsevier; 1999. pp. 45–67. [Google Scholar]
- 17.Rohmer M, Seemann M, Horbach S, Bringer-Meyer S, Sahm H. Glyceraldehyde 3-phosphate and pyruvate as precursors of isoprenic units in an alternative non-mevalonate pathway for terpenoid biosynthesis. J Am Chem Soc. 1996;118:2564–2566. [Google Scholar]
- 18.Sacchettini J C, Poulter C D. Creating isoprenoid diversity. Science. 1997;277:1788–1789. doi: 10.1126/science.277.5333.1788. [DOI] [PubMed] [Google Scholar]
- 19.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 20.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seto H, Watanabe H, Furihata K. Simultaneous operation of the mevalonate and non-mevalonate pathways in the biosynthesis of isopentenyl diphosphate in Streptomyces aeriouvifer. Tetrahedron Lett. 1996;37:7979–7982. [Google Scholar]
- 22.Seto H, Orihara N, Furihata K. Studies on the biosynthesis of terpenoids produced by Actinomycetes. Part 4. Formation of BE-40644 by the mevalonate and nonmevalonate pathways. Tetrahedron Lett. 1998;39:9497–9500. [Google Scholar]
- 23.Shin-ya K, Furihata K, Hayakawa Y, Seto H. Biosynthetic studies of naphterpin, a terpenoid metabolite of Streptomyces. Tetrahedron Lett. 1990;31:6025–6026. [Google Scholar]
- 24.Shiomi K, Iinuma H, Naganawa H, Isshiki K, Takeuchi T, Umezawa H. Biosynthesis of napyradiomycins. J Antibiot. 1987;40:1740–1745. doi: 10.7164/antibiotics.40.1740. [DOI] [PubMed] [Google Scholar]
- 25.Sprenger G A, Schorken U, Wiegert T, Grolle S, Graaf A A, Taylar S V, Begley T P, Bringer-Meyer S, Sahm H. Identification of a thiamin-dependent synthase in Escherichia coli required for the formation of the 1-deoxy-d-xylulose 5-phosphate precursor to isoprenoids, thiamin, and pyridoxol. Proc Natl Acad Sci USA. 1997;94:12857–12862. doi: 10.1073/pnas.94.24.12857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takahashi S, Kuzuyama T, Watanabe H, Seto H. A 1-deoxy-d-xylulose 5-phosphate reductoisomerase catalyzing the formation of 2-C-methyl-d-erythritol 4-phosphate in an alternative nonmevalonate pathway for terpenoid biosynthesis. Proc Natl Acad Sci USA. 1998;95:9879–9884. doi: 10.1073/pnas.95.17.9879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takahashi S, Kuzuyama T, Seto H. Purification, characterization, and cloning of a eubacterial 3-hydroxy-3-methylglutaryl coenzyme A reductase, a key enzyme involved in biosynthesis of terpenoids. J Bacteriol. 1999;181:1256–1263. doi: 10.1128/jb.181.4.1256-1263.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Watanabe Y, Ito T, Shiomi M, Tsujita Y, Kuroda M, Arai M, Fukami M, Tamura A. Preventive effect of pravastatin sodium, a potent inhibitor of 3-hydroxy-3-methylglutaryl coenzyme A reductase, on coronary atherosclerosis and xanthoma in WHHL rabbits. Biochim Biophys Acta. 1988;960:294–302. [PubMed] [Google Scholar]