Abstract
Background
Recent literature suggests wide variations exist in the international management of locally advanced pancreatic cancer. This study sought to evaluate how geography contributes to variations in management of locally advanced pancreatic cancer.
Methods
An electronic survey investigating preferences for the evaluation and management of locally advanced pancreatic cancer was distributed to an international cohort of pancreatic surgeons. Surgeons were classified according to geographic location of practice, and survey responses were compared across locations.
Results
A total of 153 eligible responses were received from 4 continents: North and South America (n = 94, 61.4%), Europe (n = 25, 16.3%), and Asia (n = 34, 22.2%). Preferences for the use and duration of neoadjuvant chemotherapy and radiotherapy varied widely. For example, participants in Asia commonly preferred 2 months of neoadjuvant chemotherapy (61.8%), whereas North and South American participants preferred 4 months (52.1%), and responses in Europe were mixed (P = .006). Participants in Asia were less likely to consider isolated liver or lung metastases contraindications to exploration and consequently had a greater propensity to consider exploration in a vignette of oligometastatic disease (56.7% vs North and South America: 25.6%, Europe: 43.5%; P = .007).
Conclusion
In an international survey of pancreatic surgeons, attitudes regarding locally advanced pancreatic cancer and metastatic disease management varied widely across geographic locations. Better evidence is needed to define optimal management of locally advanced pancreatic cancer.
INTRODUCTION
Pancreatic ductal adenocarcinoma (PDAC) is expected to become the second leading cause of cancer-related mortality by 2030 [1], and therapeutic advances remain slow. This is particularly true for the approximately 30% of patients who present with locally advanced pancreas cancer (LAPC) that extensively involves the surrounding vasculature and makes curative-intent resection more challenging. Although a multitude of retrospective studies report encouraging survival after neoadjuvant therapy and resection in selected patients with LAPC [[2], [3], [4]], there remains a lack of consensus for the optimal multidisciplinary management of this complex patient cohort. Recent studies have highlighted wide variations among institutions and providers regarding critical aspects of LAPC care, including preferences for evaluation and management, attitudes regarding treatment contraindications, classifications of resectability, and eligibility for exploration [[5], [6], [7]]. These inconsistencies in self-reported attitudes and practice persist even among the highest-volume pancreatic surgeons internationally [8].
Although numerous studies have reported wide variations in LAPC management, few studies have investigated provider characteristics associated with the observed variations [8]. Geographic location of practice is one such characteristic that has been identified as a major contributor to wide variations observed in both medical [9] and surgical treatments [10] and a broad range of health care services [[11], [12], [13]]. In addition, geographic variations have been reported to influence cancer care at all levels of analysis, from global variations among continents to local variations at the levels of county, zip code, or hospital referral region [9,[14], [15], [16]]. These regional differences persist across a diverse range of malignancies, including breast [[17], [18], [19], [20]], colon [10], prostate [21,22], endometrial [23], and liver [24] cancers, among others. In some circumstances, geographic variations have been shown to impact health outcomes [25,26]. However, the extent to which geography is associated with management of LAPC is unclear.
In this context, we sought to better understand how geographic location of practice contributes to variations observed in the multidisciplinary management of LAPC. To do this, we leveraged data from a previously published international survey [5] of pancreatic cancer surgeons to examine the association between geographic location of practice (by continent) and self-reported attitudes regarding the evaluation and management of LAPC, contraindications to surgery, and the propensity to consider exploration. Although causes of variation in complex multidisciplinary management are no doubt multifactorial, studies of associated provider characteristics could offer insight into factors contributing to practice variation and may inform the development and implementation of interventions to reduce unwarranted variations in practice and potentially improve outcomes.
METHODS
Study Design and Participants
This study used data from a recently published international survey distributed to pancreatic surgeons via web-based survey platform (Qualtrics, Provo, UT) [5]. Potential respondents were identified using a PubMed literature search and publicly available lists from various pancreatic cancer-focused organizations and were distributed within multiple international surgical societies and the Americas Hepato-Pancreato-Biliary Association. Responses from surgeons that have operated on PDAC within the last 5 years were included for final analysis, whereas surveys with < 50% of items completed or those completed by medical students, surgical residents, or surgeons involved in survey creation were excluded. This study was determined to be exempt by the Johns Hopkins Medicine institutional review board.
Survey Content and Methodology
The survey tool was developed using a previously published tool of similar scope and content [27] and was iteratively refined over 3 months. The full survey is available as an appendix to the original article [5]. The final survey consisted of 5 sections. The first section evaluated practice characteristics such as geographic location, years in practice, clinical focus, and availability of PDAC specialists. The second investigated clinical volumes and preferences for the initial evaluation of LAPC patients. The third section explored preferences for LAPC management, whereas the fourth examined attitudes regarding contraindications to surgery following neoadjuvant therapy. The fifth section investigated the propensity to consider exploration in 6 clinical vignettes of LAPC or oligometastatic disease with good response to neoadjuvant therapy (accompanied by videos of post-neoadjuvant arterial- and venous-phase computed tomography imaging) and the rationale for recommendations chosen [5]. In all clinical vignettes, the post-neoadjuvant imaging showed no evidence of progression or metastases. All 6 cases were based on actual patients that underwent R0 resection, although respondents were unaware of resection status at the time of survey completion.
Study Exposure and Statistical Analysis
To evaluate the relationship between survey responses and geographic location of practice, in this study, surgeons were categorized into 3 groups according to the continent in which they practice: North or South America, Europe, and Asia. For the primary analysis, surgeon characteristics, preferences for the evaluation and management, and the propensity to consider exploration and resection for 6 clinical vignettes were compared across geographic categories. Descriptive statistics are presented as number (percent) or median (interquartile range) for parametric and nonparametric data, respectively. Missing data, when present, are included in the analysis, resulting in some values that equal less than 100%. For all statistical tests, P values are 2 tailed, and α is set at .05. All analyses were performed using STATA version 16.1 (StataCorp, College Station, TX) [5].
RESULTS
Between July and December of 2018, 177 participants completed the survey, yielding an estimated response rate of 10.6% [5]. Nine surgeons reported not having operated on a patient with PDAC within 5 years, 3 participants identified as surgical residents, and 3 identified as medical students. An additional 9 partially completed surveys were excluded, leaving 153 eligible responses.
The characteristics of respondents according to geographic location of practice are shown in Table 1. A majority of respondents reported practicing in North and South America (NSA, n = 94, 61.4%), whereas 16.3% (n = 25) practice in Europe and 22.2% (n = 34) practice in Asia. The self-reported focus of surgical practice for a majority of respondents from the Americas was surgical oncology (40, 42.6%), while a majority of those in Europe (19, 76%) and Asia (27, 79.4%) identified their primary focus as hepato-pancreato-biliary (HPB) surgery (P = .01). Surgeons practicing in the Americas reported fewer years in practice (median 10 years, IQR [[5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17]]) as compared to those in Europe and Asia (14, IQR [[6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25]] and 15, IQR [[9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20]], P = .02). When high volume thresholds were defined as > 10 cases/year for surgeons and > 25 cases/year for hospitals, fewer surgeons in Asia reported practicing in high-volume hospitals (77% vs 92% for NSA and 96% for EUR, P = .03) (Table 1). However, when thresholds > 25 cases/year for surgeons and > 50/year for hospitals were used, no statistically significant differences were identified in respondents' reported case volumes.
Table 1.
Characteristics and evaluation preferences of participating surgeons by geographic location. Numbers represent count (%) unless stated otherwise.
|
Americas |
Europe |
Asia |
P value | |
|---|---|---|---|---|
| n = 94 | n = 25 | n = 34 | ||
| Practice type | NS | |||
| Independently practicing surgeon | 89 (94.7) | 24 (96.0) | 30 (88.2) | |
| Surgical fellow | 5 (5.3) | 1 (4.0) | 4 (11.7) | |
| Focus of surgical practice | .01 | |||
| General surgery | 4 (4.3) | 2 (8.0) | 1 (2.9) | |
| Surgical oncology | 40 (42.6) | 4 (16.0) | 4 (11.8) | |
| HPB surgery | 47 (50.0) | 19 (76.0) | 27 (79.4) | |
| Other⁎ | 3 (3.2) | 0 (0) | 2 (5.9) | |
| Years in practice (median [IQR]) | 10 (5–17) | 14 (6–25) | 15 (9–20) | .02 |
| Practice setting† | ||||
| Community practice | 4 (4.3) | 1 (4.0) | 0 (0) | NS |
| Teaching | 17 (18.1) | 6 (24.0) | 1 (2.9) | NS |
| University | 69 (73.4) | 19 (76.0) | 30 (88.2) | NS |
| Government | 6 (6.4) | 2 (8.0) | 10 (29.4) | .001 |
| Urban | 14 (14.9) | 2 (8.0) | 11 (32.4) | .03 |
| Rural | 1 (1.1) | 0 (0) | 0 (0) | NS |
| Practice in multiple hospitals | 4 (4.3) | 1 (4.0) | 0 (0) | NS |
| Available specialists† | ||||
| Medical oncologist specialized in PDAC | 85 (90.4) | 25 (100.0) | 33 (97.1) | NS |
| Radiation oncologist specialized in PDAC | 77 (81.9) | 23 (92.0) | 25 (73.5) | NS |
| Interventional radiology-biliary procedures | 85 (90.4) | 25 (100.0) | 34 (100.0) | NS |
| Gastroenterology-advanced endoscopy | 87 (92.6) | 25 (100.0) | 30 (88.2) | NS |
| None | 4 (4.3) | 0 (0) | 0 (0) | NS |
| Institution offers PDAC clinical trials | 84 (89.4) | 24 (96.0) | 27 (79.4) | NS |
| Roughly how many pancreatectomies do you typically perform for PDAC annually? | ||||
| > 10 | 83 (88.3) | 23 (92.0) | 26 (76.5) | NS |
| > 25 | 54 (57.5) | 18 (72.0) | 17 (50.0) | NS |
| Roughly how many pancreatectomies are typically performed for PDAC annually at your institution? | ||||
| > 25 | 86 (91.5) | 24 (96.0) | 26 (76.5) | .03 |
| > 50 | 65 (69.2) | 18 (72.0) | 19 (55.9) | NS |
| High-volume surgeon (10/y) at high-volume hospital (25/y) | 79 (84.0) | 22 (88.0) | 21 (61.8) | .01 |
| High-volume surgeon (25/y) at high-volume hospital (50/y) | 48 (51.1) | 15 (60.0) | 11 (32.4) | NS |
| Preferred diagnostic tests for initial staging | ||||
| Pancreatic protocol CT scan | 91 (96.8) | 25 (100.0) | 34 (100.0) | NS |
| CA 19-9 | 91 (96.8) | 23 (92.0) | 29 (85.3) | NS |
| Liver function tests | 71 (75.5) | 14 (56.0) | 15 (44.1) | .002 |
| Endoscopic ultrasound | 60 (63.8) | 11 (44.0) | 23 (67.6) | NS |
| CEA | 33 (35.1) | 8 (32.0) | 18 (52.9) | NS |
| MRI | 22 (23.4) | 8 (32.0) | 23 (67.6) | <.001 |
| Diagnostic laparoscopy | 21 (22.3) | 7 (28.0) | 4 (11.8) | NS |
| PET/CT | 8 (8.5) | 4 (16.0) | 15 (44.1) | <.001 |
| Abdominal ultrasound | 4 (4.3) | 6 (24.0) | 12 (35.3) | <.001 |
| Participate in multidisciplinary conference | 91 (96.8) | 25 (100.0) | 34 (100.0) | NS |
| What criteria do you use to define resectability?† | ||||
| AHPBA | 63 (67.0) | 6 (24.0) | 9 (26.5) | <.001 |
| NCCN | 39 (41.5) | 13 (52.0) | 24 (70.6) | .01 |
| MD Anderson Cancer Center | 25 (26.6) | 3 (12.0) | 3 (8.8) | .046 |
| Intergroup (Alliance) | 10 (10.6) | 0 (0) | 1 (2.9) | NS |
| Japan Pancreas Society | 0 (0) | 0 (0) | 9 (26.5) | <.001 |
| Dutch Pancreatic Cancer Group | 0 (0) | 6 (24.0) | 0 (0) | <.001 |
AHPBA, The Americas Hepato-Pancreato-Biliary Association; CEA, carcinoembryonic antigen; CT, computed tomography; MRI, magnetic resonance imaging; NCCN, National Comprehensive Cancer Network; PET, positron emission tomography.
Pancreas surgery only (3), transplant surgery (2).
Participants could select all that apply.
The preferences for the evaluation of LAPC according to geographic location of practice are also shown in Table 1. Compared to respondents in the Americas and Europe, surgeons in Asia were more likely to use magnetic resonance imaging, positron emission tomography/computed tomography, and abdominal ultrasound in the initial evaluation. The criteria used to define resectability also varied by geography. Although the National Comprehensive Cancer Network (NCCN) criteria were used frequently across all regions, the Americas Hepato-Pancreato-Biliary Foundation (AHPBA) criteria were more often used by respondents in the Americas, the Dutch Pancreatic Cancer Group criteria were more often used by respondents in Europe, and the Japan Pancreas Society criteria were more often used by respondents in Asia.
As shown in Table 2, geographic area of practice influenced neoadjuvant treatment preferences for LAPC in multiple areas. Respondents in the Americas (77, 81.9%) and Europe (17, 68.0%) were more likely to always recommended neoadjuvant therapy to patients with advanced PDAC compared to surgeons in Asia (16, 47.1%; P = .001). In addition, surgeons in both the Americas (65, 69.2%) and Europe (19, 76.0%) were more likely to recommend FOLFIRINOX for neoadjuvant systemic therapy compared to surgeons in Asia (12, 35.3%; P < .001), who instead preferred gemcitabine-based combination chemotherapy regimens (15 [44.1%] vs 6 [6.4%] in the Americas and 1 [4.0%] from Europe, P < .001). The preferred duration of therapy also varied, with surgeons in the Americas more often recommending a longer duration of therapy (4 months: 49, 52.1%) than counterparts in Asia (2 months: 21, 61.8%) (P = .006). Similar wide variations were observed in respondent's perceived utility of radiotherapy. In particular, 49 (52.1%) of surgeons in the Americas always or often recommend neoadjuvant radiation therapy to patients with advanced PDAC compared to 4 (16%) and 10 (29.4%) in Europe and Asia, respectively (P < .001). Although details on specific operative techniques were limited by the nature of the survey, surgeons in Asia (18, 52.9%) were more likely to offer a minimally invasive pancreaticoduodenectomy for PDAC compared to those in the Americas (25, 26.6%) and Europe (8, 32%, P = .02). However, among respondents offering a minimally invasive approach, utilization of a robotic approach was more commonly reported by surgeons in the Americas and Europe compared to surgeons in Asia. Use of diagnostic laparoscopy also differed among the 3 cohorts.
Table 2.
LAPC management preferences of participating surgeons by geographic locations. Numbers represent count (%) unless stated otherwise.⁎
|
Americas |
Europe |
Asia |
P value | |
|---|---|---|---|---|
| n = 94 | n = 25 | n = 34 | ||
| Do you recommend neoadjuvant systemic therapy to patients with advanced PDAC? | .001 | |||
| Always | 77 (81.9) | 17 (68.0) | 16 (47.1) | |
| Often | 12 (12.8) | 4 (16.0) | 7 (20.6) | |
| Sometimes | 5 (5.3) | 4 (16.0) | 11 (32.4) | |
| Never | 0 (0) | 0 (0) | 0 (0) | |
| What neoadjuvant systemic therapy do you prefer or typically recommend? | <.001 | |||
| FOLFIRINOX | 65 (69.2) | 19 (76.0) | 12 (35.3) | |
| Gemcitabine & Abraxane | 6 (6.4) | 1 (4.0) | 10 (29.4) | |
| Gemcitabine & Xeloda | 0 (0) | 0 (0) | 5 (14.7) | |
| Gemcitabine only | 0 (0) | 1 (4.0) | 0 (0) | |
| Defer to medical oncology | 20 (21.3) | 3 (12.0) | 7 (20.6) | |
| What duration of neoadjuvant systemic therapy do you prefer or typically recommend? | .006 | |||
| At least 2 mo | 30 (31.9) | 9 (36.0) | 21 (61.8) | |
| At least 3 mo | 4 (4.3) | 5 (20.0) | 2 (5.9) | |
| At least 4 mo | 49 (52.1) | 9 (36.0) | 8 (23.5) | |
| At least 6 mo | 11 (11.7) | 2 (8.0) | 3 (8.8) | |
| Do you recommend neoadjuvant radiation therapy to patients with advanced PDAC? | <.001 | |||
| Always | 24 (25.5) | 2 (8.0) | 9 (24.5) | |
| Often | 25 (26.6) | 2 (8.0) | 1 (2.9) | |
| Sometimes | 42 (44.7) | 13 (52.0) | 18 (52.9) | |
| Never | 3 (3.2) | 8 (32.0) | 6 (17.6) | |
| When do you typically recommend neoadjuvant radiation therapy? | .005 | |||
| All nonmetastatic PDACs | 13 (13.8) | 3 (12.0) | 1 (2.9) | |
| Borderline resectable and locally advanced PDACs | 41 (43.6) | 10 (40.0) | 14 (41.2) | |
| Locally advanced PDACs only | 14 (14.9) | 2 (8.0) | 8 (23.5) | |
| Any vessel involvement | 9 (9.6) | 1 (4.0) | 3 (8.8) | |
| Any arterial involvement | 14 (14.9) | 1 (4.0) | 2 (5.9) | |
| What neoadjuvant radiation therapy do you prefer or typically recommend? | .002 | |||
| Conventional chemoradiation over 5–6 wk | 54 (57.4) | 8 (32.0) | 19 (55.9) | |
| Stereotactic body radiation therapy over 1–2 wk | 29 (30.8) | 8 (32.0) | 6 (17.6) | |
| Other⁎ | 8 (8.5) | 1 (4.0) | 3 (8.8) | |
| How often do you reevaluate PDAC patients receiving neoadjuvant therapy? | .015 | |||
| I defer to medical/radiation oncology, and I reevaluate after the completion of therapy | 14 (14.9) | 6 (24.0) | 14 (41.2) | |
| I reevaluate the patient with medical/radiation oncology with every occurrence of imaging | 67 (71.3) | 17 (68.0) | 14 (41.2) | |
| I reevaluate the patient during therapy, but less often than medical/radiation oncology | 13 (13.8) | 2 (8.0) | 6 (17.6) | |
| Do you use diagnostic laparoscopy prior to resection? | .001 | |||
| Routinely | 39 (41.5) | 5 (20.0) | 2 (5.9) | |
| Selectively | 46 (48.9) | 13 (52.0) | 24 (70.6) | |
| No | 9 (9.6) | 7 (28.0) | 8 (23.5) | |
| Offers minimally invasive Whipple | 25 (26.6) | 8 (32.0) | 18 (52.9) | .02 |
| Laparoscopic | 13 (52.0) | 2 (25.0) | 12 (66.7) | .01 |
| Robotic | 8 (32.0) | 3 (37.5) | 1 (5.5) | |
| Both | 4 (16.0) | 3 (37.5) | 5 (27.8) | |
| Offers vascular resection and reconstruction to appropriately selected patients undergoing Whipple | ||||
| Venous | 93 (98.9) | 25 (100.0) | 30 (88.2) | .007 |
| Arterial | 54 (57.4) | 8 (32.0) | 14 (41.2) | .04 |
Other responses included "Only in trials," "Depends," "Radiotherapy over 3 weeks," and "36 Gy with Gemcitabine."
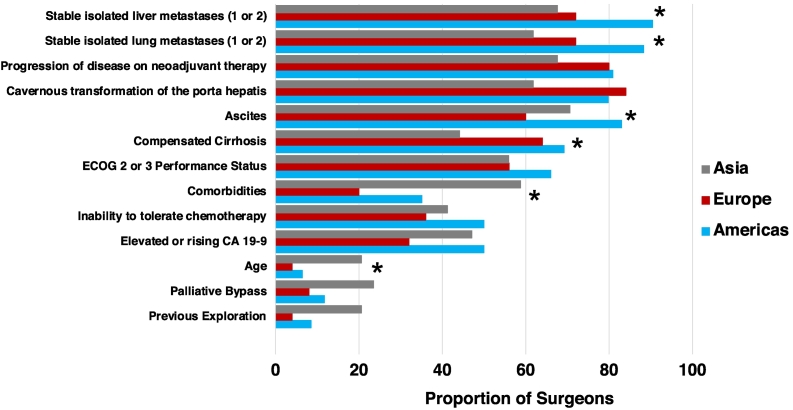
Figure 1 illustrates respondent perceptions regarding the characteristics often or always considered a contraindication to exploration following neoadjuvant therapy, according to geographic location of practice. Compared to surgeons in Europe and Asia, surgeons in the Americas were more likely to consider stable isolated liver metastases (85 [90.4%] vs 18 [72%] and 23 [67.6%], respectively; P = .004), stable isolated lung metastases (83 [88.3%] vs 18 [72%] and 21 [61.8%], P = .002), and ascites (78 [83%] vs 15 [60%] and 24 [76%], P = .04) a contraindication to exploration following neoadjuvant therapy. Significantly more surgeons in both the Americas and Europe considered compensated cirrhosis often or always a contraindication to exploration, whereas surgeons in Asia were more likely to consider comorbidities and older age contraindications to exploration following neoadjuvant therapy.
Fig 1.
Characteristics often or always considered a contraindication to exploration following neoadjuvant therapy, according to geographic location of practice (ECOG: Eastern Oncology Cooperative Group; asterisk indicates significant difference <.05).
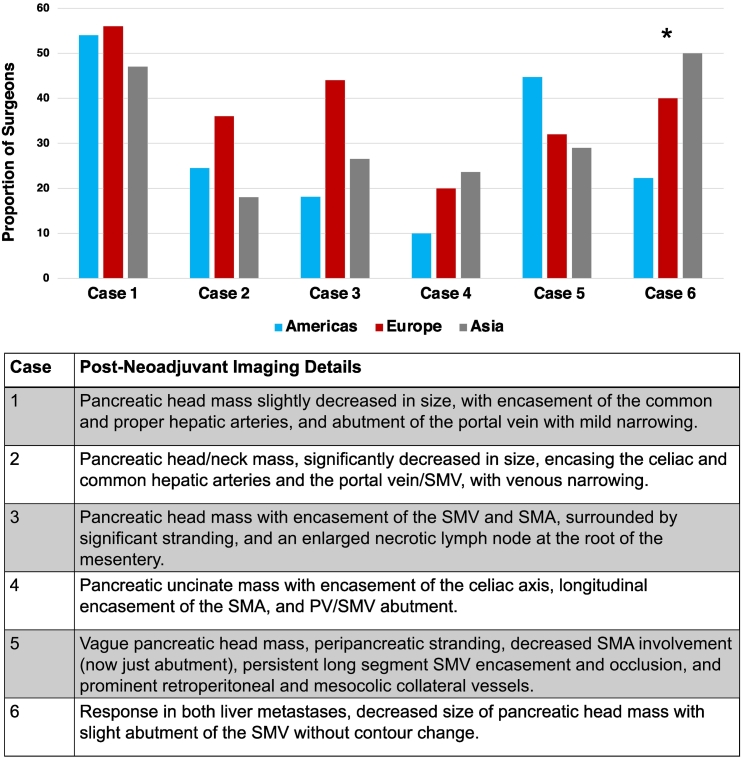
Figure 2 shows the proportion of surgeons in Asia, Europe, and the Americas willing to offer exploration following good response to neoadjuvant therapy for each of the 6 clinical vignettes. A short description of each clinical vignette is provided along with the figure. No significant differences were identified in the propensity to consider exploration in any vignette of LAPC and responses varied widely, whereas surgeons in Asia were significantly more likely to consider exploration (17, 50%) in a patient with oligometastatic liver metastases and a resectable pancreatic head lesion compared to surgeons in Europe (10, 40%) and the Americas (21, 22.3%, P = .034).
Fig 2.
Proportion of surgeons willing to consider exploration after good response to neoadjuvant therapy for each clinical vignette, according to geographic location of practice (asterisk indicates significant difference <.05), along with brief descriptions of neoadjuvant treatment response for each clinical vignette [5].
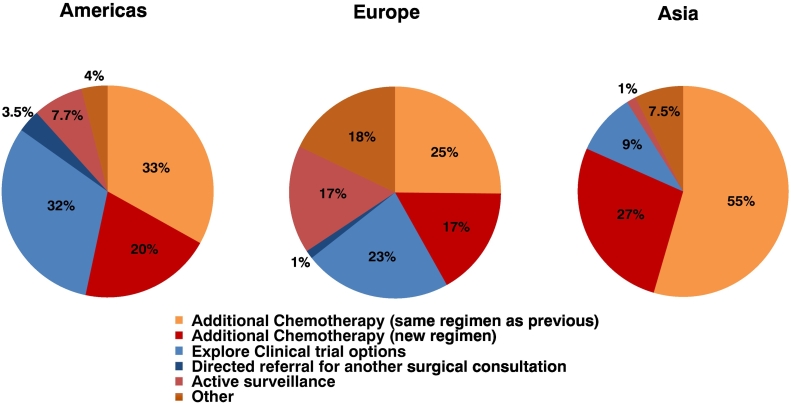
Figure 3 shows the cumulative alternative treatments recommended if exploration was not offered in any of the 6 clinical vignettes according to the geographic location of practice. Overall, surgeons in the Americas equally preferred continuation of the same chemotherapy regimen (33%) and consideration of a clinical trial (32%), whereas 20% recommended consideration of a new chemotherapy regimen. A majority of surgeons in Asia recommended continuation of the same chemotherapy regimen (55%), whereas 27% recommended initiation of a new chemotherapy regimen and few recommended clinical trials (9%). Recommendations of surgeons in Europe were highly variable, as continuation of the same chemotherapy regimen (25%), exploration of clinical trials (23%), initiation of a new chemotherapy regimen (17%), and active surveillance (17%) were all considered. Surgeons in the Americas and Asia infrequently recommended active surveillance, whereas referral for another surgical consultation was rarely chosen by any respondents.
Fig 3.
Cumulative recommendations for additional treatment following good response to neoadjuvant therapy for the 6 clinical vignettes if exploration is not offered, according to geographic location of practice.
DISCUSSION
This analysis of an international survey of current practice found that significant variations exist in attitudes regarding the multidisciplinary management of LAPC according to the geographic location of surgeon practice. These variations included differences in the preferred neoadjuvant systemic therapy recommended, the reasons for use and type of neoadjuvant radiotherapy preferred, approaches to surgical resection, perceived contraindications to exploration, and alternative treatments recommended if exploration was not pursued. Moreover, little consistency was observed across geographic locations of practice in the propensity of surgeons to consider exploration following neoadjuvant therapy for 5 clinical vignettes of LAPC. However, the decreased perception of oligometastatic disease as a contraindication to exploration among surgeons in Asia correlated with an increased propensity to consider exploration after neoadjuvant therapy in a vignette of oligometastatic liver disease with a resectable primary.
The results of this survey suggest that there are important differences in attitudes regarding the chemotherapy regimen preferred and the duration of therapy recommended across geographic locations of practice. Surgeons in both the Americas and Europe generally prefer FOLFIRINOX over gemcitabine-based regimens in the neoadjuvant setting, whereas the preferences of surgeons in Asia are reversed. Recent institutional series from high-volume centers in both the Americas and Europe corroborate these findings [2,3,[28], [29], [30]]. To further complicate the issue, recent LAPC management guidelines differ in preferred neoadjuvant regimens recommended. NCCN recommends either FOLFIRINOX or gemcitabine-nab paclitaxel [31], whereas the Dutch Pancreatic Cancer group guidelines recommend FOLFIRINOX for all patients with good performance status [32] and the Japanese Pancreas Society recommends gemcitabine-based regimens in patients with LAPC [33]. The differential utilization of S-1 across regions may also contribute to the variation observed herein [[34], [35], [36]].
Similarly, these results suggest that there is significant geographical variation in attitudes regarding reasons for use and type of neoadjuvant radiotherapy preferred for LAPC. The overall lack of consensus on neoadjuvant radiotherapy is not surprising, as its utilization in LAPC is debated and evidence evaluating its use is mixed [[37], [38], [39], [40], [41]]. Recent retrospective studies have suggested equivalent safety and outcomes in SBRT versus standard conventional therapy in LAPC patients undergoing resection [42]. However, randomized trials, such as ESPAC-1, and the recent ALLIANCE A021501 trial have failed to show survival benefit. The ALLIANCE A021501 trial, for example, was designed to compare neoadjuvant mFOLFIRINOX with or without radiation in patients with borderline resectable PDAC against historical control. However, it was closed at interim analysis as the neoadjuvant chemoradiation arm had significantly lower R0 resection rates (25% vs 60%) and worse overall survival (OS) (47.3 vs 50% 18-month OS) compared to historical control and fared even worse when compared to the neoadjuvant chemotherapy-only arm (47.3 vs 67.9% 18-month OS) [43]. Nevertheless, among the cohort of surgeons that responded to this survey, those practicing in the Americas were more likely to recommend neoadjuvant radiation therapy than counterparts in Europe or Asia.
Differences in the use of minimally invasive (MIS) approaches among geographic locations of practice identified in this study are also supported by the current literature. Retrospective reviews and meta-analyses support the use of MIS techniques for pancreaticoduodenectomy [[44], [45], [46]]. Although a previously published international survey data of high-volume pancreatic surgeon did not identify significant differences in the rates of minimally invasive pancreaticoduodenectomy, that study did not compare the rates of laparoscopic versus robotic approaches among continents [47]. The present survey identified the highest rates of MIS pancreaticoduodenectomy among surgeons in Asia, yet among those offering a MIS approach, utilization of a robotic platform was more common in the Americas and Europe. These findings likely represent strong evidence supporting a laparoscopic approach within Eastern populations [46] and varying resource and financial considerations between countries. As access and experience to the robotic platform continue to expand globally, the international opinion represented by this small cohort of survey respondents is likely to evolve. An international consensus statement on robotic pancreatic surgery was recently published to inform safe practice and promote program development [48].
No consistency was observed in the propensity to consider exploration for 5 clinical vignettes of LAPC following neoadjuvant therapy across locations of practice. Although the statistical power of these analyses is limited by sample size, these findings are not surprising given the lack of strong evidence or consensus on a preferred management approach for LAPC. Moreover, these findings are corroborated by a similar analysis of the same data evaluating the association between surgeon volume and the propensity to consider exploration [8]. However, the finding of a significant geographical difference in the propensity to consider exploration in a vignette of oligometastatic pancreatic cancer is profound and is strengthened by parallel differences in the evaluation of perceived contraindications to exploration across geography. There have been growing reports of curative-intent approaches in highly selected patients with oligometastatic pancreatic cancer [[49], [50], [51], [52]]. However, most guidelines recommend against exploration in the setting of metastatic disease [53,54], so the lack of consensus among surgeons in this study is anticipated. The increased self-reported propensity of surgeons from Asia to consider exploration in patients with oligometastatic disease is a unique finding and warrants further investigation, as it may represent previously undefined and evolving variations in practice and treatment philosophy. One such clinical trial (CSPAC-1) evaluating this question is actively recruiting patients [55].
This study has several important limitations. First, this study is subject to all the limitations of the original survey including a small sample size, a low survey response rate, unverifiable responses, and response bias, all of which serve to limit the generalizability of the survey findings [5]. In addition, when combined with a small sample size, the multiple geographic categories serve to further limit the sample size and generalizability of results within any individual geographic cohort and limit statistical power. However, given the international nature and the clinical complexity of the study question (evaluating international attitudes regarding multidisciplinary management of LAPC across geographic locations), to our knowledge, these survey results are the only data available to investigate this study question. Second, geographic locations of practice are represented by continents, which themselves represent many diverse countries, cultures, health systems, institutions, and providers. For example, although responses from South America would ideally be analyzed separately, a small sample size (n = 6) limited statistical reliability. Although this may further limit the generalizability of these results, the geographic locations chosen for the survey instrument were left intentionally broad to ensure anonymity of responses. However, the expected heterogeneity within broad geographic categories would be expected to bias any statistical analyses toward the null hypothesis (no variation identified). Third, it remains unclear whether differences in attitudes regarding multidisciplinary management of LAPC translate into differences in outcomes for patients undergoing therapy. However, the purpose of this study is not to make inferences about best practice.
Previous reports have identified geographic variations in the surgical management of both benign [56] and malignant [10] conditions and across a wide array of operations [[57], [58], [59]]. Within the field of oncology, geographic variation has been shown to influence the management of diverse malignancies including breast [[17], [18], [19], [20]], prostate [21,22], colon [10], endometrial [23], and liver [24] cancers. For example, although no international studies have been conducted, previous national studies from the United States [17,18] and Spain [19] suggest that there is substantial geographic variation in the use of breast conservation surgery that cannot be accounted for by differences in hospital characteristics [18] or patient demographics [17] but is more likely due to regional policies and local economic status [19]. Interestingly, a more recent study by the Netherlands on the same subject found that geographic variation has decreased since the publication of detailed evidence-based national guidelines, although the generalizability of this study to countries across the globe remains questionable [20]. Similar examples are found in literature evaluating variations in the management of prostate cancer, as prior research demonstrates geographic variation in treatment patterns at the national [60,61], regional [21], and state [62] levels, again influenced by regional cultures and local income levels.
In this study, we have identified significant geographic variation in surgeon attitudes and preferences for the management of LAPC. A thorough understanding of literature evaluating the impact of geographic variation on treatment and outcomes suggests that these findings may have important implications for improving the care provided to LAPC patients. Although causes of geographic variation are no doubt multifactorial, potential contributors include mixed literature and weak evidence to support current practice; differences in patient characteristics and provider beliefs across locations [63]; national, regional, or institutional cultures; and the availability of specific technologies or therapeutics, among others.
The wide variation observed among this international cohort of high-volume pancreatic surgeons further highlights the need for stronger evidence to define the optimal management of LAPC. Although multi-institutional multimodality randomized trials are difficult to conduct, success is possible. The JCOG1407 trial evaluating use of neoadjuvant FOLFIRINOX versus gemcitabine/nab-paclitaxel in LAPC patients, for example, was recently reported but showed mixed results [64]. Additional clinical trials evaluating treatment sequencing [65], the preferred systemic therapy regimen [66,67], and use of radiotherapy [68,69] are currently under way, and results are eagerly awaited. However, until stronger evidence is available, these findings emphasize the need for improved international collaboration to better understand best practices and define consensus guidelines for the multidisciplinary management of this complex disease.
Author Contribution
BNR, ABB, RWK, AE, VPG, GG, CLW, MJW, and JH were involved in the conception or design of this work. All authors were involved in the distribution of the survey and evaluation of the results. BNR and ABB performed statistical analysis. LRM, ABB, RWK, CZ, and BNR drafted the abstract and manuscript. All authors critically reviewed the manuscript and offered substantial edits. All authors approve the final manuscript for submission.
Conflict of Interest
All authors have no conflicts of interest to declare.
Funding Source
None.
Ethics Approval
This study was ruled exempt by the Johns Hopkins Medicine institutional review board.
Footnotes
Presentations: Presented as a poster presentation at the 2020 AHPBA Annual Meeting in Miami, FL, and as a virtual oral presentation at the 2020 International Hepato-Pancreato-Biliary Association World Congress and the 2020 Pancreas Club Annual Meeting.
References
- 1.Rahib L., Smith B.D., Aizenberg R., Rosenzweig A.B., Fleshman J.M., Matrisian L.M. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74(11):2913–2921. doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 2.Gemenetzis G., Groot V.P., Blair A.B., Laheru D.A., Zheng L., Narang A.K., et al. Survival in locally advanced pancreatic cancer after neoadjuvant therapy and surgical resection. Ann Surg. 2019;270(2):340–347. doi: 10.1097/SLA.0000000000002753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Michelakos T., Pergolini I., Castillo C.F., Honselmann K.C., Cai L., Deshpande V., et al. Predictors of resectability and survival in patients with borderline and locally advanced pancreatic cancer who underwent neoadjuvant treatment with FOLFIRINOX. Ann Surg. 2019;269(4):733–740. doi: 10.1097/SLA.0000000000002600. [DOI] [PubMed] [Google Scholar]
- 4.Truty M.J., Kendrick M.L., Nagorney D.M., Smoot R.L., Cleary S.P., Graham R.P., et al. Factors predicting response, perioperative outcomes, and survival following total neoadjuvant therapy for borderline/locally advanced pancreatic cancer. Ann Surg. 2021;273(2):341–349. doi: 10.1097/SLA.0000000000003284. [DOI] [PubMed] [Google Scholar]
- 5.Reames B.N., Blair A.B., Krell R.W., Groot V.P., Gemenetzis G., Padussis J.C., et al. Management of locally advanced pancreatic cancer: results of an international survey of current practice. Ann Surg. 2021;273(6):1173–1181. doi: 10.1097/SLA.0000000000003568. [DOI] [PubMed] [Google Scholar]
- 6.Heinrich S., Besselink M., Moehler M., Van Laethem J.-L., Ducreux M., Grimminger P., et al. Opinions and use of neoadjuvant therapy for resectable, borderline resectable, and locally advanced pancreatic cancer: international survey and case-vignette study. BMC Cancer. 2019;19(1) doi: 10.1186/s12885-019-5889-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kirkegård J., Aahlin E.K., Al-Saiddi M., Bratlie S.O., Coolsen M., De Haas R.J., et al. Multicentre study of multidisciplinary team assessment of pancreatic cancer resectability and treatment allocation. Br J Surg. 2019;106(6):756–764. doi: 10.1002/bjs.11093. [DOI] [PubMed] [Google Scholar]
- 8.Blair A.B., Krell R.W., Ejaz A., Groot V.P., Gemenetzis G., Padussis J.C., et al. Proclivity to explore locally advanced pancreas cancer is not associated with surgeon volume. J Gastrointest Surg. 2021;25(10):2562–2571. doi: 10.1007/s11605-021-05034-w. [DOI] [PubMed] [Google Scholar]
- 9.Weinstein S.J., House S.A., Chang C.H., Wasserman J.R., Goodman D.C., Morden N.E. Small geographic area variations in prescription drug use. Pediatrics. 2014;134(3):563–570. doi: 10.1542/peds.2013-4250. [DOI] [PubMed] [Google Scholar]
- 10.Reames B.N., Sheetz K.H., Waits S.A., Dimick J.B., Regenbogen S.E. Geographic variation in use of laparoscopic colectomy for colon cancer. J Clin Oncol. 2014;32(32):3667–3672. doi: 10.1200/JCO.2014.57.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keyhani S., Falk R., Bishop T., Howell E., Korenstein D. The relationship between geographic variations and overuse of healthcare services: a systematic review. Med Care. 2012;50(3):257–261. doi: 10.1097/MLR.0b013e3182422b0f. [DOI] [PubMed] [Google Scholar]
- 12.O’Hare A.M. Regional variation in health care intensity and treatment practices for end-stage renal disease in older adults. JAMA. 2010;304(2):180. doi: 10.1001/jama.2010.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shipman S.A., Lan J., Chang C.H., Goodman D.C. Geographic maldistribution of primary care for children. Pediatrics. 2011;127(1):19–27. doi: 10.1542/peds.2010-0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leape L.L., Park R.E., Solomon D.H., Chassin M.R., Kosecoff J., Brook R.H. Does inappropriate use explain small-area variations in the use of health care services? JAMA. 1990;263(5):669–672. [PubMed] [Google Scholar]
- 15.Marino L.V., Bell K.L., Woodgate J., Doolan A., Group BDAPCI An international survey of the nutrition management of chylothorax: a time for change. Cardiol Young. 2019;29(9):1127–1136. doi: 10.1017/S1047951119001525. [DOI] [PubMed] [Google Scholar]
- 16.Naughton C., Bennett K., Feely J. Regional variation in prescribing for chronic conditions among an elderly population using a pharmacy claims database. Ir J Med Sci. 2006;175(3):32–39. doi: 10.1007/BF03169170. [DOI] [PubMed] [Google Scholar]
- 17.Farrow D.C., Hunt W.C., Samet J.M. Geographic variation in the treatment of localized breast cancer. N Engl J Med. 1992;326(17):1097–1101. doi: 10.1056/NEJM199204233261701. [DOI] [PubMed] [Google Scholar]
- 18.Nattinger A.B., Gottlieb M.S., Veum J., Yahnke D., Goodwin J.S. Geographic variation in the use of breast-conserving treatment for breast cancer. N Engl J Med. 1992;326(17):1102–1107. doi: 10.1056/NEJM199204233261702. [DOI] [PubMed] [Google Scholar]
- 19.Ridao-Lopez M., Garcia-Armesto S., Abadia-Taira B., Peiro-Moreno S., Bernal-Delgado E. Income level and regional policies, underlying factors associated with unwarranted variations in conservative breast cancer surgery in Spain. BMC Cancer. 2011;11:145. doi: 10.1186/1471-2407-11-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Steenbergen L.N., van de Poll-Franse L.V., Wouters M.W., Jansen-Landheer M.L., Coebergh J.W., Struikmans H., et al. Variation in management of early breast cancer in the Netherlands, 2003-2006. Eur J Surg Oncol. 2010;36(Suppl. 1):S36–S43. doi: 10.1016/j.ejso.2010.06.021. [DOI] [PubMed] [Google Scholar]
- 21.Caram M.E.V., Estes J.P., Griggs J.J., Lin P., Mukherjee B. Temporal and geographic variation in the systemic treatment of advanced prostate cancer. BMC Cancer. 2018;18(1) doi: 10.1186/s12885-018-4166-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rebbeck T.R. Prostate cancer genetics: variation by race, ethnicity, and geography. Semin Radiat Oncol. 2017;27(1):3–10. doi: 10.1016/j.semradonc.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.White B., Nordin A., Fry A., Ahmad A., McPhail S., Roe C., et al. Geographic variation in the use of lymphadenectomy and external-beam radiotherapy for endometrial cancer: a cross-sectional analysis of population-based data. BJOG. 2019;126(12):1456–1465. doi: 10.1111/1471-0528.15914. [DOI] [PubMed] [Google Scholar]
- 24.Liu C.-Y., Chen K.-F., Chen P.-J. Treatment of liver cancer. Cold Spring Harb Perspect Med. 2015;5(9) doi: 10.1101/cshperspect.a021535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosenberg B.L., Kellar J.A., Labno A., Matheson D.H.M., Ringel M., Vonachen P., et al. Quantifying geographic variation in health care outcomes in the United States before and after risk-adjustment. PLOS ONE. 2016;11(12) doi: 10.1371/journal.pone.0166762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Welch H.G. Geographic variation in diagnosis frequency and risk of death among Medicare beneficiaries. JAMA. 2011;305(11):1113. doi: 10.1001/jama.2011.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krell R.W., Reames B.N., Hendren S., Frankel T.L., Pawlik T.M., Chung M., et al. Surgical referral for colorectal liver metastases: a population-based survey. Ann Surg Oncol. 2015;22(7):2179–2194. doi: 10.1245/s10434-014-4318-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hackert T., Sachsenmaier M., Hinz U., Schneider L., Michalski C.W., Springfeld C., et al. Locally advanced pancreatic cancer: neoadjuvant therapy with folfirinox results in resectability in 60% of the patients. Ann Surg. 2016;264(3):457–463. doi: 10.1097/SLA.0000000000001850. [DOI] [PubMed] [Google Scholar]
- 29.Huang L., Jansen L., Balavarca Y., Van Der Geest L., Lemmens V., Van Eycken L., et al. Nonsurgical therapies for resected and unresected pancreatic cancer in Europe and USA in 2003-2014: a large international population-based study. Int J Cancer. 2018;143(12):3227–3239. doi: 10.1002/ijc.31628. [DOI] [PubMed] [Google Scholar]
- 30.Weniger M., Moir J., Damm M., Maggino L., Kordes M., Rosendahl J., et al. Respect—a multicenter retrospective study on preoperative chemotherapy in locally advanced and borderline resectable pancreatic cancer. Pancreatology. 2020;20(6):1131–1138. doi: 10.1016/j.pan.2020.06.012. [DOI] [PubMed] [Google Scholar]
- 31.Tempero M.A., Malafa M.P., Al-Hawary M., Behrman S.W., Benson A.B., Cardin D.B., et al. Pancreatic adenocarcinoma, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2021;19(4):439–457. doi: 10.6004/jnccn.2021.0017. [DOI] [PubMed] [Google Scholar]
- 32.Van Veldhuisen E., Van Den Oord C., Brada L.J., Walma M.S., Vogel J.A., Wilmink J.W., et al. Locally advanced pancreatic cancer: work-up, staging, and local intervention strategies. Cancer. 2019;11(7):976. doi: 10.3390/cancers11070976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Okusaka T., Nakamura M., Yoshida M., Kitano M., Uesaka K., Ito Y., et al. Clinical practice guidelines for pancreatic cancer 2019 from the Japan Pancreas Society: a synopsis. Pancreas. 2020;49(3):326–335. doi: 10.1097/MPA.0000000000001513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chuah B., Goh B.-C., Lee S.-C., Soong R., Lau F., Mulay M., et al. Comparison of the pharmacokinetics and pharmacodynamics of S-1 between Caucasian and East Asian patients. Cancer Sci. 2011;102(2):478–483. doi: 10.1111/j.1349-7006.2010.01793.x. [DOI] [PubMed] [Google Scholar]
- 35.Kobayakawa M., Kojima Y. Tegafur/gimeracil/oteracil (S-1) approved for the treatment of advanced gastric cancer in adults when given in combination with cisplatin: a review comparing it with other fluoropyrimidine-based therapies. Onco Targets Ther. 2011:193. doi: 10.2147/OTT.S19059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shirao K., Hoff P.M., Ohtsu A., Loehrer P.J., Hyodo I., Wadler S., et al. Comparison of the efficacy, toxicity, and pharmacokinetics of a uracil/tegafur (UFT) plus oral leucovorin (LV) regimen between Japanese and American patients with advanced colorectal cancer: joint United States and Japan study of UFT/LV. J Clin Oncol. 2004;22(17):3466–3474. doi: 10.1200/JCO.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 37.Boone B.A., Steve J., Krasinskas A.M., Zureikat A.H., Lembersky B.C., Gibson M.K., et al. Outcomes with FOLFIRINOX for borderline resectable and locally unresectable pancreatic cancer. J Surg Oncol. 2013;108(4):236–241. doi: 10.1002/jso.23392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eguchi H., Yamada D., Iwagami Y., Gotoh K., Kawamoto K., Wada H., et al. Prolonged neoadjuvant therapy for locally advanced pancreatic cancer. Dig Surg. 2018;35(1):70–76. doi: 10.1159/000475477. [DOI] [PubMed] [Google Scholar]
- 39.Hosein P.J., Macintyre J., Kawamura C., Maldonado J.C., Ernani V., Loaiza-Bonilla A., et al. A retrospective study of neoadjuvant FOLFIRINOX in unresectable or borderline-resectable locally advanced pancreatic adenocarcinoma. BMC Cancer. 2012;12(1):199. doi: 10.1186/1471-2407-12-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marthey L., Sa-Cunha A., Blanc J.F., Gauthier M., Cueff A., Francois E., et al. FOLFIRINOX for locally advanced pancreatic adenocarcinoma: results of an AGEO multicenter prospective observational cohort. Ann Surg Oncol. 2015;22(1):295–301. doi: 10.1245/s10434-014-3898-9. [DOI] [PubMed] [Google Scholar]
- 41.Rangelova E., Wefer A., Persson S., Valente R., Tanaka K., Orsini N., et al. Surgery improves survival after neoadjuvant therapy for borderline and locally advanced pancreatic cancer: a single institution experience. Ann Surg. 2021;273(3):579–586. doi: 10.1097/SLA.0000000000003301. [DOI] [PubMed] [Google Scholar]
- 42.Blair A.B., Rosati L.M., Rezaee N., Gemenetzis G., Zheng L., Hruban R.H., et al. Postoperative complications after resection of borderline resectable and locally advanced pancreatic cancer: the impact of neoadjuvant chemotherapy with conventional radiation or stereotactic body radiation therapy. Surgery. 2018;163(5):1090–1096. doi: 10.1016/j.surg.2017.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Katz M., Shi Q., Meyers J., Herma J., Choung M., Wolpin B., et al. Alliance A021501: preoperative mFOLFIRINOX or mFOLFIRINOX plus hypofractionated radiation therapy (RT) for borderline resectable (BR) adenocarcinoma of the pancreas. J Clin Oncol. 2021;39(3):377. [Google Scholar]
- 44.Da Dong X., Felsenreich D.M., Gogna S., Rojas A., Zhang E., Dong M., et al. Robotic pancreaticoduodenectomy provides better histopathological outcomes as compared to its open counterpart: a meta-analysis. Sci Rep. 2021;11(1) doi: 10.1038/s41598-021-83391-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Hilst J., de Graaf N., Abu Hilal M., Besselink M.G. The landmark series: minimally invasive pancreatic resection. Ann Surg Oncol. 2021;28(3):1447–1456. doi: 10.1245/s10434-020-09335-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang M., Li D., Chen R., Huang X., Li J., Liu Y., et al. Laparoscopic versus open pancreatoduodenectomy for pancreatic or periampullary tumours: a multicentre, open-label, randomised controlled trial. Lancet Gastroenterol Hepatol. 2021;6(6):438–447. doi: 10.1016/S2468-1253(21)00054-6. [DOI] [PubMed] [Google Scholar]
- 47.van Hilst J., de Rooij T., Abu Hilal M., Asbun H.J., Barkun J., Boggi U., et al. Worldwide survey on opinions and use of minimally invasive pancreatic resection. HPB. 2017;19(3):190–204. doi: 10.1016/j.hpb.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 48.Liu R., Wakabayashi G., Palanivelu C., Tsung A., Yang K., Goh B.K.P., et al. International consensus statement on robotic pancreatic surgery. HepatoBiliary Surgery and Nutrition. 2019;8(4):345–360. doi: 10.21037/hbsn.2019.07.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Frigerio I., Regi P., Giardino A., Scopelliti F., Girelli R., Bassi C., et al. Downstaging in stage IV pancreatic cancer: a new population eligible for surgery? Ann Surg Oncol. 2017;24(8):2397–2403. doi: 10.1245/s10434-017-5885-4. [DOI] [PubMed] [Google Scholar]
- 50.Hackert T., Niesen W., Hinz U., Tjaden C., Strobel O., Ulrich A., et al. Radical surgery of oligometastatic pancreatic cancer. Eur J Surg Oncol. 2017;43(2):358–363. doi: 10.1016/j.ejso.2016.10.023. [DOI] [PubMed] [Google Scholar]
- 51.Lu F., Poruk K.E., Weiss M.J. Surgery for oligometastasis of pancreatic cancer. Chin J Cancer Res. 2015;27(4):358–367. doi: 10.3978/j.issn.1000-9604.2015.05.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Crippa S., Bittoni A., Sebastiani E., Partelli S., Zanon S., Lanese A., et al. Is there a role for surgical resection in patients with pancreatic cancer with liver metastases responding to chemotherapy? Eur J Surg Oncol. 2016;42(10):1533–1539. doi: 10.1016/j.ejso.2016.06.398. [DOI] [PubMed] [Google Scholar]
- 53.Tempero M.A. NCCN guidelines updates: pancreatic cancer. J Natl Compr Canc Netw. 2019;17(5.5):603–605. doi: 10.6004/jnccn.2019.5007. [DOI] [PubMed] [Google Scholar]
- 54.Tempero M.A., Malafa M.P., Chiorean E.G., Czito B., Scaife C., Narang A.K., et al. Pancreatic Adenocarcinoma, Version 1.2019. J Natl Compr Canc Netw. 2019;17(3):202–210. doi: 10.6004/jnccn.2019.0014. [DOI] [PubMed] [Google Scholar]
- 55.Wei M., Shi S., Hua J., Xu J., Yu X. Chinese Study Group for Pancreatic Cancer. Simultaneous resection of the primary tumour and liver metastases after conversion chemotherapy versus standard therapy in pancreatic cancer with liver oligometastasis: protocol of a multicentre, prospective, randomised phase III control trial (CSPAC-1) BMJ Open. 2019;9(12) doi: 10.1136/bmjopen-2019-033452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Miriam M., Burger M.J., Timmermans A., Matthé P. Regional and temporal variation in hysterectomy rates and surgical routes for benign diseases in the Netherlands. Acta Obstet Gynecol Scand. 2012;91(2):220–225. doi: 10.1111/j.1600-0412.2011.01309.x. [DOI] [PubMed] [Google Scholar]
- 57.Kauh C.Y., Blachley T.S., Lichter P.R., Lee P.P., Stein J.D. Geographic variation in the rate and timing of cataract surgery among US communities. JAMA Ophthalmology. 2016;134(3):267. doi: 10.1001/jamaophthalmol.2015.5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Maciejewski M.L., Arterburn D.E., Berkowitz T.S.Z., Weidenbacher H.J., Liu C.F., Olsen M.K., et al. Geographic variation in obesity, behavioral treatment, and bariatric surgery for veterans. Obesity. 2019;27(1):161–165. doi: 10.1002/oby.22350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Patel M.R., Greiner M.A., Dimartino L.D., Schulman K.A., Duncan P.W., Matchar D.B., et al. Geographic variation in carotid revascularization among Medicare beneficiaries, 2003–2006. Archives of Internal Medicine. 2010;170(14) doi: 10.1001/archinternmed.2010.194. [DOI] [PubMed] [Google Scholar]
- 60.Cary K.C., Punnen S., Odisho A.Y., Litwin M.S., Saigal C.S., Cooperberg M.R. Nationally representative trends and geographic variation in treatment of localized prostate cancer: the Urologic Diseases in America project. Prostate Cancer Prostatic Dis. 2015;18(2):149–154. doi: 10.1038/pcan.2015.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Krupski T.L., Kwan L., Afifi A.A., Litwin M.S. Geographic and socioeconomic variation in the treatment of prostate cancer. J Clin Oncol. 2005;23(31):7881–7888. doi: 10.1200/JCO.2005.08.755. [DOI] [PubMed] [Google Scholar]
- 62.Polednak A.P. Geographic variation in the treatment of prostate cancer in Connecticut. Int J Technol Assess Health Care. 1993;9(2):304–310. doi: 10.1017/s0266462300004517. [DOI] [PubMed] [Google Scholar]
- 63.Birkmeyer J.D., Reames B.N., McCulloch P., Carr A.J., Campbell W.B., Wennberg J.E. Understanding of regional variation in the use of surgery. Lancet. 2013;382(9898):1121–1129. doi: 10.1016/S0140-6736(13)61215-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ozaka M., Ueno M., Ishii H., Mizusawa J., Katayama H., Kataoka T., et al. Randomized phase II study of modified FOLFIRINOX versus gemcitabine plus nab-paclitaxel combination therapy for locally advanced pancreatic cancer (JCOG1407) 2021;39(15):4017. [Google Scholar]
- 65.Gemcitabine hydrochloride with or without erlotinib hydrochloride followed by the same chemotherapy regimen with or without radiation therapy and capecitabine or fluorouracil in treating patients with pancreatic cancer that has been removed by surgery. https://ClinicalTrials.gov/show/NCT01013649
- 66.A randomized phase III trial comparing folfirinox to gemcitabine in locally advanced pancreatic carcinoma. https://ClinicalTrials.gov/show/NCT02539537
- 67.Nab-paclitaxel and gemcitabine vs gemcitabine alone as adjuvant therapy for patients with resected pancreatic cancer (the "Apact" Study) https://ClinicalTrials.gov/show/NCT01964430
- 68.Phase III FOLFIRINOX (mFFX) +/− SBRT in locally advanced pancreatic cancer. https://ClinicalTrials.gov/show/NCT01926197
- 69.Pancreatic carcinoma: Chemoradiation compared with chemotherapy alone after induction chemotherapy. https://ClinicalTrials.gov/show/NCT01827553