Abstract
Background
The adverse effect of excessive salt intake has been recognized in decades. Researchers have mainly focused on the association between salt intake and hypertension. However, studies in recent years have proposed the existence of extra‐renal sodium storage and provided insight into the immunomodulatory function of sodium.
Objectives
In this review, we discuss the modulatory effects of high salt on various innate and adaptive immune cells and immune‐regulated diseases.
Methods
We identified papers through electronic searches of PubMed database from inception to March 2022.
Results
An increasing body of evidence has demonstrated that high salt can modulate the differentiation, activation and function of multiple immune cells. Furthermore, a high‐salt diet can increase tissue sodium concentrations and influence the immune responses in microenvironments, thereby affecting the development of immune‐regulated diseases, including hypertension, multiple sclerosis, cancer and infections. These findings provide a novel mechanism for the pathology of certain diseases and indicate that salt might serve as a target or potential therapeutic agent in different disease contexts.
Conclusion
High salt has a profound impact on the differentiation, activation and function of multiple immune cells. Additionally, an HSD can modulate the development of various immune‐regulated diseases.
This review describes the modulatory effect of high salt on various immune cells and immune‐regulated diseases. High salt significantly affects the differentiation, activation and function of multiple innate and adaptive immune cells and dominantly induces a pro‐inflammatory state in microenvironments, thus influencing the development of various immune‐regulated diseases and serving as a target or potential therapeutic agent in different disease contexts.
1. INTRODUCTION
Salt (NaCl), common in our daily life, can be found naturally in various foods and is used in food manufacturing, the chemical industry, clinical therapy and so forth. For instance, salty condiments contain plenty of NaCl, whereas saline is the most frequently administered intravenous fluid. 1 Although salt is necessary for the human body, excessive salt intake can be detrimental and increase the risk of diseases such as hypertension, heart failure and renal disease. 2 In addition, the immunomodulatory function of salt has been reported. Early studies found that high salt increased the cytokine production in peripheral blood mononuclear cells (PBMCs). 3 , 4 In the past decades, growing evidence has indicated that high salt can influence various immune cells. Moreover, a high‐salt diet (HSD) has a pronounced effect on immune‐regulated diseases, and salt is potentially applied in immune therapy. This review will summarize some of the recent advances in the immunomodulatory effect of high salt. We will first introduce sodium homeostasis and its physiological functions. Then, we focused on the effect of high salt on various immune cells in microenvironments. Furthermore, the influence of HSD on immune‐regulated diseases and relevant immune responses will be reviewed. Finally, we will briefly discuss the present and potential clinical applications of high salt.
2. SODIUM HOMEOSTASIS AND PHYSIOLOGICAL FUNCTIONS
Dietary salt is an important source of sodium, of which homeostasis is tightly regulated. 5 Sodium homeostasis in the traditional two‐compartment model is regulated by the kidney. 6 However, in recent years, studies have suggested that large amounts of Na+ are stored in extra‐renal tissues, particularly the skin and muscles. 7 , 8 , 9 , 10 Mechanistically, excessive Na+ in bone, cartilage and skin is stored by non‐osmotic binding with polyanionic proteoglycans, generating a hypertonic environment in local tissue and acting as an osmotically inactive Na+ reservoir. 11 , 12 , 13 , 14 Moreover, tissue sodium storage is linked to certain diseases. Increased skin or muscle Na+ storage can be observed in patients with multiple sclerosis (MS), 15 refractory hypertension, 10 lipedema, 16 systemic sclerosis 17 or end‐stage renal disease. 18 , 19 Tissue Na+ storage may reflect the activity and progression of some diseases, such as systemic lupus erythematosus (SLE), MS and psoriasis. 20 , 21 , 22 , 23 , 24 Of note, Na+ tends to accumulate in inflammatory and infection sites. In subtotal‐nephrectomized mice (5/6Nx) fed an HSD, sodium storage in the abdominal wall tissues was increased, 25 and the total sodium concentration of patients with triple‐negative breast cancer was two to threefold higher in the tumour than in normal tissue. 26
This dynamic and tightly regulated sodium homeostasis contributes to critical physiological functions in the body. 27 Specifically, sodium is the major osmotically effective cation that keeps the plasma volume within normal limits and maintains suitable cell volume necessary for cellular survival and function. 28 , 29 , 30 The resting membrane potential of cells largely depends on the transmembrane sodium concentration gradient, whereas the generation of action potential is mediated by a brief sodium influx. 28 , 31 Sodium also participates in various metabolic reactions. Data from humans showed that severe sodium deprivation can negatively affect glucose metabolism. 32 Moreover, an adequate sodium intake is required for mammalian survival and development. 33 , 34 The growth of protoplasm, fat and bone was retarded in sodium‐deprived rats, 35 while gestational sodium restriction can impair foetal growth and nervous system development. 36 , 37
3. THE EFFECT OF HIGH SALT ON IMMUNE CELLS
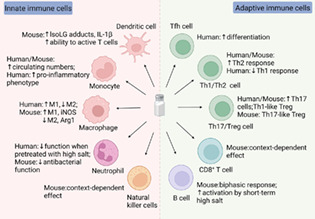
Immune cells comprise innate and adaptive immune cells. Innate immune cells include granulocytes, natural killer (NK) cells, macrophages, monocytes and dendritic cells (DCs). Adaptive immune cells include T lymphocytes and B lymphocytes, which express T cell receptor (TCR) and B cell receptor (BCR), respectively. 38 Extensive studies have suggested the immunomodulatory function of salt, and we particularly focus on the effect of high salt on the differentiation, activation and function of immune cells in microenvironments. Furthermore, considering the difference between mouse and human immune cells, 39 the high salt effects on immune cells showed discrepancies among species (Figure 1).
FIGURE 1.
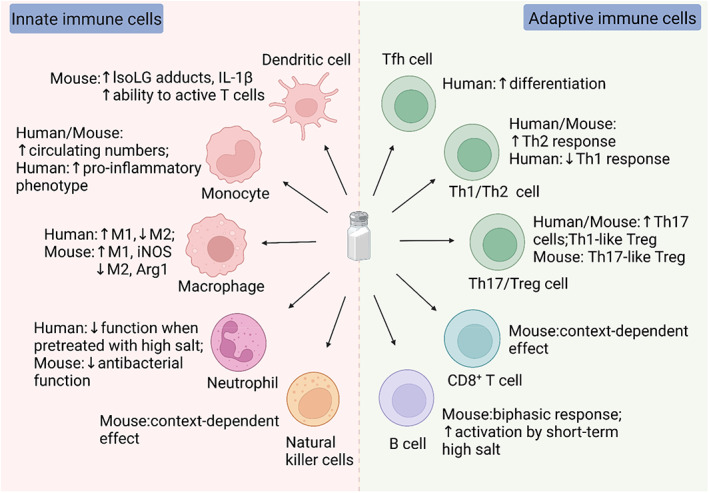
The effect of high salt on innate and adaptive immune cells. High salt enhances the formation of IsoLG‐protein adducts in murine dendritic cells, along with increased IL‐1β secretion and subsequent activation of T cells. An HSD increases circulating monocytes in mice and humans, and high salt can induce pro‐inflammatory human monocytes. Moreover, high salt induces M1 macrophages, Th17 cells and Th2 cells, while suppressing M2 macrophages, Treg cells and Th1 cells. The differentiation of human Tfh cells is increased by high salt. The sequence of high salt exposure and stimulation affects the outcome of human neutrophil activation, whereas the antibacterial functionality of murine neutrophils is suppressed by high salt. The high salt effects on murine NK cells and CD8+ T cells are context‐dependent, while murine B cells respond to high salt in a biphasic manner.
3.1. The effect of high salt on immune cell proliferation and death
In humans and rodents, plasma Na+ concentration is approximately 140 millimolar (mM), while Na+ concentrations in interstitium and lymphoid tissue range between 160 and 250 mM. 40 , 41 Based on these findings, most studies enrich the media with additional 20–100 mM NaCl to investigate the effect of high salt on immune cells. 42 , 43 , 44 , 45 Importantly, adding 40 mM NaCl can simulate the Na+ concentrations found in the skin of high‐salt‐diet rodents. 46 These clinically relevant high salt can trigger osmoprotective responses in immune cells and affect cellular homeostasis.
Studies have demonstrated that immune cells rely on the nuclear factor of activated T cells‐5 (NFAT5 or TonEBP) to adapt to hypertonic stress. 47 The guanine nucleotide exchange factor (GEF) Brx and p38 mitogen‐activated protein kinase (MAPK) can activate NFAT5 and mediate the osmoprotective response in immune cells. 48 , 49 In terms of immune cell proliferation, NFAT5 can induce osmoprotective responses that enable T lymphocytes to resume cyclin expression. 50 Furthermore, CD24 was identified as an NFAT5‐regulated gene involved in the adaptation of murine proliferating T cells to high salt. 51 Additionally, the exposure conditions and cell lines are critical. Human and murine T cell proliferation was suppressed at additional NaCl above 40 mM, but increasing NaCl below 40 mM promoted T cell proliferation. 51 , 52 , 53 , 54 , 55 The augmented T cell proliferation is related to p38/MAPK activation, 52 enhanced monocyte function 53 and altered macrophage function. 45 , 56 Moreover, adding 40 mM NaCl can inhibit B cell proliferation. 57
In addition, high NaCl can damage cytoskeleton, increase DNA breaks, inhibit translation and cause cell cycle arrest. 58 , 59 , 60 Osmoprotective mechanisms enable cells to survive and function. Failure to accommodate leads to cell death. 58 Partial loss of NFAT5 function reportedly led to impaired lymphocyte growth and function. 40 , 61 Additionally, salt exposure conditions and cell lines can influence the outcome of adaptation. In PBMCs, additional NaCl below 80 mM had no significant effect on cell apoptosis, whereas severe cell death was observed at 800 mM. 62 Adding 100 mM NaCl for 24 h potentiated murine macrophage apoptosis, along with inhibited protein kinase B (PKB or Akt) activation. 63 , 64 Moreover, adding 40 mM NaCl had little impact on the growth or apoptosis of CD4+ T cells, but increasing concentrations further led to cell death. 43 , 65
3.2. The effect of high salt on innate immune cells
3.2.1. Neutrophils
Neutrophils, a type of polymorphonuclear leukocyte, are the major effectors of acute inflammation. 66 High‐salt treatment after human neutrophil activation can increase superoxide production and elastase release. 67 , 68 In contrast, high salt pretreatment can suppress diverse human neutrophil functions such as degranulation, 69 superoxide production, 70 , 71 phagocytosis, 72 adhesion 73 , 74 and migration. 42 , 75 These effects are related to adenosine triphosphate (ATP) release, p38/MAPK activation and cytoskeletal remodelling. 76 , 77 , 78 , 79 Moreover, NaCl above 209 mM can significantly suppress neutrophil extracellular trap (NET) formation while promoting apoptosis in human neutrophils. 80 Consistently, high NaCl can impaire the antimicrobial capacities of both human and murine neutrophils. In human neutrophils, the reduced bactericidal activity was due to the decreased reactive oxygen species (ROS) production. 81 Furthermore, an HSD in mice can increase the urea accumulation in the renal medulla and elevate the glucocorticoid level in the blood, resulting in the suppressed neutrophil antibacterial functionality. Healthy humans who accepted an HSD also showed hyperglucocorticoidism and inhibited neutrophil function. 82 In summary, the sequence of high‐salt exposure and stimulation critically affects the outcome of human neutrophil activation, whereas high salt can suppress the antibacterial functionality of murine neutrophils.
3.2.2. Natural killer cells
NK cell is a prototypical member of Group 1 innate lymphoid cells (ILC1) that can produce interferon‐γ (IFN‐γ). 83 , 84 A study demonstrated that HSD suppressed the proliferation, activation and function of NK cells in mice. 85 Mice fed an HSD displayed decreased NK cells in the spleen and lungs, while NK cell maturation in the spleen and bone marrow was blocked. Mechanistically, HSD downregulated CD122 expression in NK cells via ROS signalling and thus reduced the responsiveness to interleukin (IL)‐15 in NK cells and inhibited their function. 85 However, Rizvi et al. 86 found that an HSD in mice enhanced NK cell function in tumour immunity by decreasing PD‐1 expression and increasing IFN‐γ and serum hippurate. These two distinct conclusions indicated the importance of the disease context in the high salt effect on murine NK cells.
3.2.3. Mononuclear phagocyte family
Macrophages, DCs and monocytes form a family of mononuclear myeloid cells that are specialized in antigen presentation. 87 These three myeloid cells also belong to the mononuclear phagocyte system (MPS), a concept encompassing progenitors, macrophages, DCs and monocytes. 88 Tissue‐resident macrophages can be derived from embryonic progenitors or they can be of monocytic origin under inflammatory conditions, the relative proportion of which depends on the tissue. 89 , 90 DCs and monocytes originate from haematopoietic stem cells, but they have distinct precursors. 88 Moreover, DCs comprise conventional DCs (cDCs) and plasmacytoid DCs (pDCs). 91 In this review, we considered cDCs and not pDCs. Monocytes demonstrate plasticity and can be differentiated into macrophages and dendritic‐like cells. 92
Macrophages
Macrophage activation phenotypes have been extended to a spectrum model with two extremes, M1 and M2 macrophages. 93 M1 macrophages are polarized by inflammatory stimuli, mediating the immune response to bacteria and intracellular pathogens. M2 macrophages are anti‐inflammatory macrophages, existing in settings such as helminth immunity, asthma and allergy. 94 , 95
Murine macrophages
Generally, high salt potentiates the activation and function of M1 macrophages, while suppressing the activation and function of M2 macrophages (Figure 2). 45 , 56 , 96 , 97 First, high salt can induce p38/MAPK‐dependent NFAT5 activation in M1 macrophages, resulting in inducible nitric oxide synthase (iNOS or NOS2)‐dependent nitric oxide (NO) production and tumour necrosis factor (TNF) secretion. 41 These responses boosted the function of macrophages, facilitating anti‐leishmanial control and regulating salt‐induced hypertension. 41 , 46 Additionally, the Na+/Ca2+ exchanger 1 (NCX1) expressed on murine macrophages can sense Na+ and contribute to NFAT5 accumulation. 98 , 99 Second, high salt can activate caspase‐1 and trigger IL‐1β release in macrophages. These responses required the activation of the nucleotide‐binding domain and leucine‐rich repeat pyrin domain 3 (NLRP3) and the nucleotide‐binding domain and leucine‐rich repeat caspase recruitment domain 4 (NLRC4) inflammasomes via mitochondrial ROS. These salt‐activated inflammasomes can act as innate sensing components and promote T helper (Th)17 response. 44 Third, the p38/cFos/activator protein 1 (AP1) and extracellular signal‐regulated kinase (Erk)1/2/cFos/AP1 pathways mediated the salt‐driven pro‐inflammatory profile in M1 macrophages, while the Erk1/2/signal transducer and activator of transcription (STAT)6 pathway mediated the salt‐induced suppression of M2 macrophages. 97 Furthermore, high NaCl can activate macrophages without extra activators, leading to a novel activation state named M(Na). 97 Last, high salt can inhibit the mitochondrial respiration in macrophages, resulting in the improved bactericidal function of M1 macrophages and suppressed function of M2 macrophages. 45 , 56 , 99 The inhibited M2 macrophage activation by high salt is associated with blunted Akt/mechanistic target of rapamycin signalling. 56
FIGURE 2.
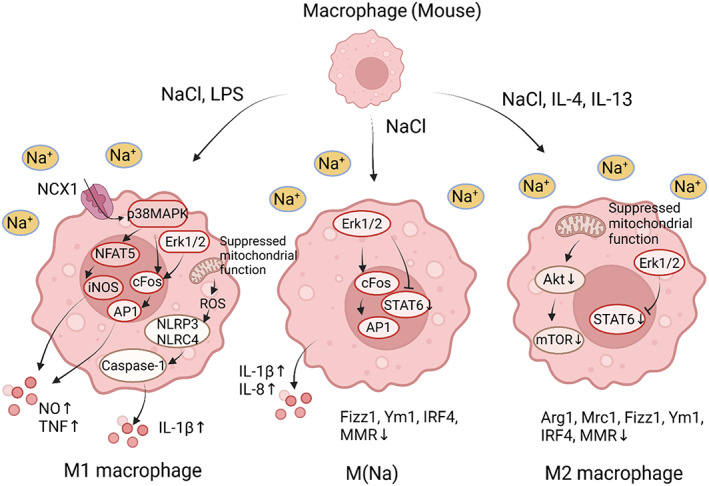
High salt induces a pro‐inflammatory profile in murine macrophages. NCX1 expressed on M1 macrophages can sense extracellular Na+, and high salt increases NO and TNF production by p38/MAPK‐dependent NFAT5 activation and downstream iNOS upregulation. Moreover, high salt activates NLRP3 and NLRC4 inflammasomes via mitochondrial ROS, thereby increasing IL‐1β in a caspase‐1‐dependent manner. Additionally, high salt induces a pro‐inflammatory profile in M1 and M(Na) macrophages through the p38/cFos/AP1 and Erk1/2/cFos/AP1 pathways, whereas the Erk1/2/STAT6 pathway mediates the salt‐driven suppression of M2 and M(Na) macrophages. In M2 macrophages, high salt downregulates the expression of Arg1, mannose receptor, C type 1 (Mrc1), inflammatory zone 1 (Fizz1), chitinase‐like 3 (Chil3 or Ym1), interferon regulatory factor 4 (IRF4) and macrophage mannose receptor (MMR). Furthermore, high salt suppresses mitochondrial metabolism and AKT/mTOR signalling in M2 macrophages. The mitochondrial function of M1 macrophages is also suppressed by high salt.
Apart from the direct activation of pro‐inflammatory macrophages, high salt can induce macrophage migration. In vitro, macrophages can migrate towards NaCl in a dose‐dependent manner. 100 In subtotal‐nephrectomized mice (5/6Nx), an HSD increased macrophage infiltration in the peritoneal wall, heart and para‐aortic tissues. 25 The mechanisms involved the enhanced expression of NFAT5‐dependent monocyte chemotactic protein‐1 (MCP‐1) in mesothelial cells and cardiomyocytes. 25
Human macrophages
High salt can induce M(Na) in human macrophages, characterized by enhanced pro‐inflammatory and suppressed anti‐inflammatory gene expression. 97 Salt‐activated M1 macrophages with increased pro‐inflammatory cytokine production were also observed. 45 , 97 Moreover, high salt inhibited the mitochondrial respiration in M1 and M2 macrophages, resulting in the suppressed function of M2 macrophages. 99 However, studies have identified the discrepancies between mouse and human macrophages. For instance, iNOS and arginase‐1 (Arg1), two enzymes important for murine macrophage arginine metabolism, might not be functional in human macrophages. 39 , 101 Thus, the investigations in murine macrophages cannot be simply extrapolated to human macrophages, and the high salt effect on human macrophages needs to be further explored.
Monocytes
Two studies in healthy subjects have revealed a positive association between salt intake levels and monocyte numbers. 102 , 103 Data showed that minimal changes in plasma sodium concentration caused by HSD can transiently inhibit the mitochondrial function of human circulating monocytes. 99 A recent study found that high salt can drive human monocytes to a DC‐like phenotype, characterized by the formation of isolevuglandin (IsoLG) adducts, expression of CD83 and increased production of IL‐1β. These responses induced subsequent T‐cell activation with increased IL‐17A production. 104 Consistently, monocytes from humans with high skin sodium exhibited enhanced IsoLG adduct accumulation and CD83 expression. 104 Other studies also observed pro‐inflammatory human monocytes induced by high salt in vitro or by an HSD. 45 , 105 In addition, a long‐term HSD remarkably increased the circulating monocytes in mice by driving monocyte mobilization from bone marrow. 106 Taken together, an HSD can increase circulating monocytes in mice and humans, and high salt can induce pro‐inflammatory human monocytes.
Dendritic cells
Current evidence has revealed the impact of high salt on murine DCs. High salt impeded the cross‐priming capacity of murine DCs in a toll/IL1 receptor domain‐containing adapter‐inducing interferon‐beta (TRIF)‐dependent manner. 107 Moreover, murine DCs treated with high salt acquired an M2‐like signature. 108 Nevertheless, the maturation, antigen presentation and inflammatory cytokine expression of murine DCs were reportedly enhanced by high salt. 107 , 109 , 110 Specifically, elevated sodium enters DCs through amiloride‐sensitive channels and consequently increases the formation of IsoLG adducts and the production of IL‐1β. These salt‐activated DCs can promote the secretion of IL‐17A and IFN‐γ in T cells. 110 Data in mice fed an HSD corroborated these findings. 111 In addition, an HSD in SLE mice can activate DCs through the p38/MAPK‐STAT1 pathway. 112 Tubbs et al. 113 found that the increased cytokine production in murine DCs upon high NaCl treatment was mediated by p38/MAPK, serum and glucocorticoid‐inducible kinase 1 (SGK1) and downstream toll‐like receptor 4 (TLR4). Collectively, these findings suggested that high salt can activate murine DCs and increase their production of inflammatory cytokines.
3.3. The effect of high salt on adaptive immune cells
3.3.1. CD4 + T cells
CD4+ T cells are central to the adaptive immune response against pathogens. 114 Naïve CD4+ T cells can differentiate into distinct Th cell subsets, including Th1, Th2, Th17, (regulatory T) Treg and follicular helper T (Tfh) cells. 115 Th1 cells, which produce IFN‐γ, are vital for cellular immunity. Th2 cells, essential for immunoglobulin E (IgE) production, play a major role in allergic and helminth responses. 116 Th17 cells participate in mucosal immunity against pathogens and contribute to autoimmune disorders. 117 Pro‐inflammatory and anti‐inflammatory Th17 phenotypes have been described. 118 , 119 , 120 Treg cells are critical to maintaining immune tolerance, and can be categorized into thymus‐derived Treg (tTreg), peripherally derived Treg (pTreg) and in vitro‐induced Treg (iTreg) cells. 121 , 122 Tfh cells are crucial for IgG‐mediated humoral responses and are required to develop germinal centre responses. 123 , 124 Based on the heterogeneity and plasticity of Th cells, 125 high salt exerts different effects on Th cell subsets.
Th17 cells
High salt can induce distinct phenotypes of Th17 cells in different cellular contexts, and Th17‐polarizing cytokines, such as transforming growth factor‐β (TGF‐β) and IL‐6, strongly influence this process (Figure 3). Moreover, high salt intake can induce pro‐inflammatory Th17 cells by affecting gut microbiota and their metabolites. Notably, these high salt effects on Th17 cells appear similar between mice and humans.
FIGURE 3.
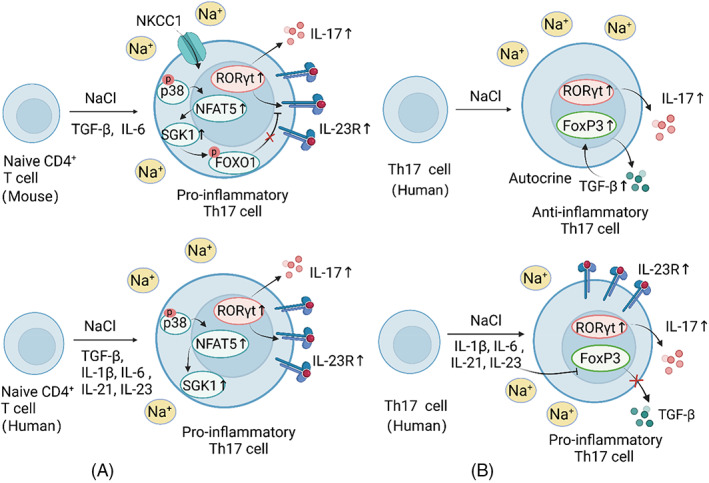
The context‐dependent effect of high salt on Th17 cells. (A) In the presence of Th17‐polarizing cytokines, high salt boosts the induction of pathogenic murine and human Th17 cells. These responses are related to p38/MAPK, NFAT5 and SGK1 activation. (B) In the absence of Th17‐polarizing cytokines, high salt induces anti‐inflammatory human Th17 cells by a significant upregulation of FoxP3 and the autocrine of TGF‐β. However, additional pro‐inflammatory cytokines can block TGF‐β secretion, and therefore, human Th17 cells exhibit a pro‐inflammatory phenotype. Similarly, high salt shows context‐dependent effect on the pathogenicity of murine Th17 cells, but the autocrine TGF‐β production was below the detection level.
In 2013, two seminal papers showed that high salt can induce human and murine naïve CD4+ T cells to differentiate into pathogenic Th17 cells in the presence of Th17‐polarizing cytokines. 43 , 126 In vitro, high salt induced a highly pathogenic and stable phenotype of Th17 cells, characterized by increased expression of IL‐17A and IL‐23R. The mechanisms involved the activation of p38/MAPK, NFAT5 and SGK1. 43 Specifically, SGK1 can stabilize this salt‐induced pathogenic Th17 cell phenotype by deactivating mouse forkhead box protein O (FOXO)1, a repressor of RORγt‐mediated IL‐23R expression. 126 In vivo, an HSD exacerbated experimental autoimmune encephalomyelitis (EAE), along with increased Th17 cells in the gut‐associated lymphoid tissue and the central nervous system (CNS) of mice. These responses can be abrogated by either p38/MAPK inhibitor or SGK1 deficiency. 43 , 126 In addition, Na+‐K+‐2Cl− cotransporter 1 may participate in sensing extracellular NaCl in murine Th17 cells and mediate the salt‐induced increase in SGK1 and IL‐23R. 127
In contrast, Matthias et al. 128 proposed a dual context‐dependent effect of high NaCl on CD4+ T cells. High NaCl without additional polarizing cytokines endowed human Th17 cells with a stable, pathogen‐specific and anti‐inflammatory phenotype, characterized by a significant upregulation of FoxP3 and a moderate increase in IL‐17A. This salt‐induced Th17 phenotype was regulated by p38/MAPK, NFAT5 and SGK1. 128 However, Th17‐polarizing cytokines suppressed the autocrine secretion of TGF‐β in human Th17 cells, which was important for FoxP3 upregulation induced by high salt, and thus switched the Th17 phenotype from anti‐inflammatory to pro‐inflammatory. Similarly, TGF‐β governed the induction of pro‐ versus anti‐inflammatory murine Th17 cells by high salt in vitro and in an EAE mouse model. 128 Cumulatively, the obscuration caused by Th17‐polarizing cytokines in the high‐salt‐induced differentiation of T cells may explain the divergent outcomes compared to previous reports.
In addition, the high salt effect on Th17 cells can be mediated by gut microbiota and their metabolites. 129 Indeed, an HSD in mice significantly reduced several intestinal bacteria, particularly the Lactobacillus murinus. Studies have indicated that increased salt consumption results in the depletion of L. murinus and thereby induces pro‐inflammatory Th17 cells in mice. 130 , 131 Mechanistically, Lactobacilli can metabolize tryptophan to indole metabolites. Faecal indoles, confirmed to be an inhibitory factor of Th17 differentiation, were reportedly decreased by HSD. 130 Further investigations suggested that oral gavage of L. murinus or Lactobacillus reuteri in mice restored the decrease of faecal indoles caused by HSD and blunted high‐salt‐induced Th17 activation. Consistently, a high salt challenge on healthy volunteers increased the peripheral blood Th17 cells, accompanied by reduced intestinal Lactobacillus spp. survival. 130 In summary, these studies linked HSD to the gut‐immune axis and suggested that HSD can induce pro‐inflammatory Th17 cells by decreasing gut Lactobacillus spp. in mice and humans.
Treg cells
High salt enables murine Treg cells to acquire an SGK1‐dependent, pro‐inflammatory phenotype, which has proved to be Th1‐like 132 or Th17‐like 133 in different reports, leading to inhibited suppressive function of Treg cells. By contrast, a salt‐induced Th1‐like phenotype has been observed in human Treg cells (Figure 4).
FIGURE 4.
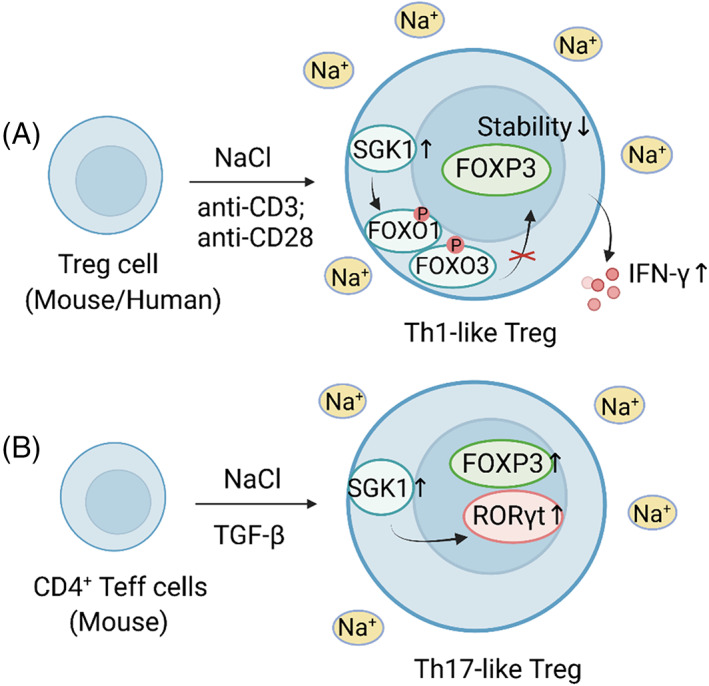
High salt induces pro‐inflammatory Treg cells. (A) High salt enables murine and human Treg cells to acquire a Th1‐like phenotype. High salt enhances the phosphorylation of FOXO1/FOXO3 by activating SGK1, followed by decreased Foxp3 stability and increased IFN‐γ secretion. (B) High salt can induce Th17‐like Treg cells. High salt can enhance TGF‐β‐mediated Treg cell induction while facilitating the co‐expression of RORγt
On the one hand, high NaCl impaired the suppressive capacity of murine and human Treg cells in vitro and in vivo, leading to a Th1‐type phenotype with increased SGK1‐dependent IFN‐γ secretion. 132 Specifically, FOXO1, a downstream target of SGK1, can stabilize the Foxp3 locus alongside FOXO3. Treg cells exposed to high salt displayed enhanced phosphorylation of FOXO1/FOXO3, and the decreased Foxp3 stability may be responsible for Treg suppression. 132 On the other hand, Yang et al. 133 found a Th17‐like phenotype of murine Treg cells with preserved suppressive function upon high salt treatment. These salt‐induced RORγt+Foxp3+ Treg cells were associated with SGK1 activation and were not significant producers of IL‐17A. 133 Consistently, an HSD decreased the Treg proliferation and proportion in a murine transplantation model. 55 Although it has been corroborated that high NaCl can promote RORγt expression in murine tTreg cells, whether high salt affects murine iTreg cells is controversial. 133 , 134 Data also suggested that high salt had little impact on the development and function of human iTreg cells. 134
Other T helper cells
High salt can induce human Th2 but suppress Th1 cell responses on multiple regulatory levels. 65 , 135 IL‐4 and IL‐13, the Th2 signature cytokines, were enhanced in human naïve and memory T cells under high NaCl conditions, whereas the production of IFN‐γ was decreased. These effects were dependent on NFAT5 and SGK1. 65 Consistently, an HSD augmented Th2 responses in food allergy mice, 136 while the high‐salt formulation of Al(OH)3 enhanced the ovalbumin (OVA)‐induced Th2 response in mice. 109 In addition, high NaCl can significantly increase the polarization of human Tfh cells. 62 Mechanistically, high NaCl enhanced the gene expression of ten‐eleven translocation (TET)2 and TET3 in human CD4+ T cells, along with upregulated expression of sialophorin (spn), and then induced the DNA hypomethylation, ultimately contributing to enhanced Tfh cell differentiation. These findings provide a potential epigenetic mechanism for high‐salt‐induced autoimmunity. 62
3.3.2. CD8 + T cells
CD8+ T cells play a vital role in immunity to intracellular pathogens and tumours. 137 HSD increased the renal infiltration of CD8+ T lymphocytes in mice. 138 , 139 In an E.G7‐OVA tumour mouse model, OVA/Al/high salt formulation displayed an enhanced antitumor effect via CD8+ cytotoxic T lymphocytes‐mediated immunity. 109 By contrast, Popovic et al. 107 found that high NaCl inhibited the DC‐dependent activation of murine CD8+ T cells in a TRIF‐dependent manner.
3.3.3. B cells
B lymphocytes are immune cells that express clonally diverse cell surface immunoglobulin receptors. 140 High salt might activate murine splenic B cells and increase immunoglobulin production via the Brx/p38/MAPK/NFAT5 pathway. 49 Additionally, a short‐term increase in NaCl concentration fostered murine B cell activation and differentiation, whereas chronic exposure to high NaCl dampened the differentiation of plasmablasts. Mechanistically, murine B cells treated with high NaCl showed inhibited p38/MAPK pathway activity and delayed NFAT5 response. 57
4. THE EFFECT OF HIGH‐SALT DIET ON IMMUNE‐REGULATED DISEASES
Given the high salt effects on immune cells and the excessive sodium storage in local tissues caused by an HSD, 46 extensive studies have demonstrated the impact of an HSD on various immune‐regulated disorders in mouse models (Table 1) and in humans (Table 2).
TABLE 1.
The effect of high‐salt diet on immune‐regulated diseases in mouse models
| Disease | Effect | Responses | References |
|---|---|---|---|
| Hypertension | Increased | ↑MPS cells | 46 |
| ↑DCs | 110 | ||
| ↑Th17 cells and IL‐17 | 130 | ||
| ↓Lactobacillus spp. | 130 | ||
| Actively induced EAE | Exacerbated | ↑Th17 cells | 130 |
| ↓Lactobacillus spp. | 130 | ||
| ↑Pro‐inflammatory myeloid cells | 45 | ||
| ↑Blood–brain barrier permeability | 141 | ||
| Spontaneous EAE | Suppressed | ↓Blood–brain barrier permeability | 142 |
| Experimental colitis | Exacerbated | ↑Macrophages | 143, 144, 145 |
| ↑Th17 cells and ILC3 | 131, 146, 147 | ||
| ↓Treg cells | 132 | ||
| ↓Lactobacillus spp. and butyrate | 131 | ||
| ↑Intestinal fibroblasts | 148 | ||
| Systemic lupus erythematosus | Exacerbated | ↑Th1 and Th17 cells | 149 |
| ↑Tfh cells | 62 | ||
| ↑DCs | 112 | ||
| Collagen‐induced arthritis | Exacerbated | ↑Th17 cells and IL‐17 | 150 |
| No significant influence | / | 151 | |
| K/BxN serum transfer‐induced arthritis | No significant influence | / | 151 |
| Cancer | Exacerbated | ↑Th17 cells and IL‐17 | 152 |
| ↑Helicobacter pylori infection | 153 | ||
| Inhibited | ↓MDSCs | 154, 155 | |
| ↑T cells | 154 | ||
| ↑Bifidobacterium and NK cross‐talk | 86 | ||
| Infection | Exacerbated | ↓Neutrophils | 82 |
| Prevented | ↑M1 and ↓M2 macrophages | 41, 97, 156, 157 | |
| Ischemic stroke | Aggravated | ↑Blood–brain barrier disruption | 158 |
| ↑M1 and ↓M2 macrophages | 159, 160 | ||
| Cognitive disorders | Impaired | ↑Th17 cells and IL‐17 | 161 |
| ↑M1 microglia | 162 | ||
| Wound healing | Delayed | ↓M2 macrophages | 56 |
| Acute renal failure | Aggravated | ↑Th17 cells | 163, 164 |
| Transplantation rejection | Accelerated | ↓Treg cells | 55, 132 |
| Osteopenia | Increased | ↑Th17 cells and ↓Treg cells | 165 |
↑: increased; ↓: decreased.
TABLE 2.
The effect of high‐salt diet on immune‐regulated diseases in humans
| Disease | Effect | Design of the Study | Salt intake assessment | References |
|---|---|---|---|---|
| Hypertension |
Increased |
Pilot clinical trial | 2‐Week high‐salt diet | 130 |
| Meta‐analysis | 24‐h urine collection/sodium manipulation | 166 | ||
| Cardiovascular disease | Increased risk | Meta‐analysis | Multiple 24‐h urine collection | 167 |
| Multiple sclerosis | Increased disease activity | Cohort | Spot urine collection | 168 |
| No association | Cohort | Spot urine collection | 169 | |
| Cohort | Semiquantitative food frequency questionnaire (FFQ) | 170 | ||
| Case–control | Block Kids Food Screener (NutritionQuest) | 171 | ||
| Case–control | Block Kids Food Screener | 172 | ||
| Ulcerative colitis or Crohn's disease | No association | Nested case–control | Semiquantitative FFQ | 173 |
| Systemic lupus erythematosus | Positive association | Clinical trial | 5‐Week dietary regimen | 174 |
| Rheumatoid arthritis | Positive association | Nested case–control | Semiquantitative FFQ | 175 |
| Case–case | Food Frequency Questionnaires | 176 | ||
| Cross‐sectional and case–control | Semiquantitative FFQ | 177 | ||
| Cross‐sectional |
Spot urine collection (urine Na/K ratio) |
178 | ||
| Case–control | 24‐h urine collection and FFQ | 179 | ||
| Clinical trial | 5‐Week dietary regimen | 174 | ||
| Gastric cancer | Increased risk | Meta‐analysis | FFQ | 180 |
| Oesophageal cancer | Increased risk | Meta‐analysis | Validated questionnaires | 181 |
| Lung, testicular and bladder cancer | Increased risk | Case–control | FFQ | 182 |
| Renal cell cancer | Increased risk | Cohort | FFQ | 183 |
| Pancreatic cancer | Increased risk | Case–control | Questionnaire | 184 |
| Infection | Increased risk | Clinical trial | 1‐Week high‐salt diet | 82 |
| Ischemic stroke | Positive association | Case–control | Spot urine collection | 158 |
| Positive association risk of death; no association risk of onset | Meta‐analysis | Questionnaire/24‐ h urinary sodium excretion/overnight urine sodium/24‐h dietary recall/a self‐monitoring device | 185 | |
| Cognitive disorders | Positive association | Meta‐analysis | 24‐h urine collection/FFQ/food diaries | 186 |
| Renal transplant and post‐transplant hypertension | Positive association | Clinical trial | Strict sodium diet | 187, 188 |
| Cross‐sectional | 24‐h urine collection | 189 | ||
| Comparative | 24‐h urine collection | 190 | ||
| No association | Comparative | 24‐h urine collection | 191 | |
| Osteoporosis | Increased risk | Meta‐analysis | Spot or 24‐h urine collection/FFQ/24‐h dietary recalls | 192 |
4.1. Hypertension and associated cardiovascular disease
It is well recognized that dietary salt intake has a direct causal relationship with blood pressure and can increase the risk of cardiovascular events and death. 166 , 167 , 193 Studies have demonstrated that an HSD can contribute to hypertension via the immune system. First, an HSD in mice can prime hypertension through DC‐dependent T cell activation. 110 , 111 A study on human monocytes implied a similar mechanism. 104 Second, an HSD can induce the generation of Th17 cells by reducing Lactobacillus species, contributing to hypertension in mice and humans. 130 Last, salt‐driven alterations in short‐chain fatty acids (SCFAs), a subset of fatty acids generated by gut microbiota, were observed in hypertensives 194 and mice with salt‐sensitive hypertension. 195 Given the immunomodulatory functions of SCFAs, 196 salt‐induced hypertension might be mediated by SCFAs. In contrast, in response to skin Na+ accumulation caused by HSD, interstitial MPS cells secrete NFAT5‐dependent vascular endothelial growth factor‐C (VEGF‐C), which increases the hyperplasia of lymph capillaries and provides a buffering mechanism for salt‐driven hypertension. 46 , 197 , 198
4.2. Multiple sclerosis
MS is a chronic autoimmune demyelinating disease of the CNS. 199 EAE, the model of MS, can be induced by active or passive immunization. It can also be developed from opticospinal EAE (OSE) spontaneously. 200 Data showed that an HSD can aggravate actively induced EAE. First, exacerbated EAE by an HSD showed enhanced CNS infiltration and peripherally induced pathogenic Th17 cells. 43 , 126 Another study highlighted the role of gut microbiota in this process, as supplementation with L. murinus or L. reuteri blunted salt‐induced pathogenic Th17 cells and ameliorated EAE exacerbation. 130 Second, EAE mice fed an HSD showed augmented macrophage infiltration in the CNS, and enhanced pro‐inflammatory cytokine production in myeloid cells. 45 Last, high salt intake can exacerbate EAE by increasing blood–brain barrier (BBB) permeability. 141 This effect might be related to the decreased tight junction (TJ) proteins in endothelial cells. 158 , 201 However, another study reported that an HSD suppressed spontaneous EAE by upregulating serum corticosterone and tightening BBB. 142 The discrepancy between induced and spontaneous EAE may be the consequence of altered BBB properties by pertussis toxin used in the active immunization.
Data from humans suggested that the high salt effect on MS is controversial. A cohort study reported that high salt intake was associated with increased disease activity in patients with MS. 168 Conversely, four other human studies found no association between HSD and MS progression. 169 , 170 , 171 , 172 However, these clinical studies measured dietary salt intake by spot urine collections or food questionnaires, which may lead to invalid results, 202 , 203 and more accurate measurement methods for salt intake are needed in follow‐up studies.
4.3. Intestinal bowel disease
IBD, encompassing ulcerative colitis and Crohn's disease, is a chronic relapsing inflammatory disorder of the gastrointestinal tract. 204 , 205 Data showed that an HSD can exacerbate DSS‐ and TNBS‐induced colitis, leading to increased mortality in mice. 113 , 146 This exacerbation of colitis is associated with the p38/MAPK‐dependent production of pro‐inflammatory cytokines by intestinal mononuclear cells. 143 , 144 Further investigations corroborated the participation of CD4+ T cells and macrophages. 145 Depleting macrophages reduced the severity of DSS‐induced colitis promoted by high salt intake. 144 Furthermore, an HSD exacerbated TNBS‐induced colitis by enhancing the intestinal Th17 response. 146 The salt‐driven reduction in Lactobacillus and butyrate levels might mediate this salt‐induced Th17 response. 131 In addition, Type 3 innate lymphoid cells ILC3, a kind of IL‐17‐producing cell increased in the colon of high‐salt‐diet mice, may participate in the salt‐driven aggravation of experimental colitis. 147 Moreover, an HSD can aggravate experimental colitis by blocking the suppressive function of Treg cells. 132 Last, data suggested that high dietary salt can promote intestinal fibrosis in TNBS‐induced colitis by activating intestinal fibroblasts. 148 Nevertheless, a nested case–control study in women did not find an association between dietary sodium and the risk of ulcerative colitis or Crohn's disease. 173 More investigations in humans are warranted to clarify the role of HSD in IBD.
4.4. Systemic lupus erythematosus and rheumatoid arthritis
SLE is an autoimmune, connective‐tissue disorder that involves multiple systems. 206 Lupus nephritis is one of the most severe organ manifestations in SLE. 207 Data showed that an HSD accelerated lupus progression and increased the mortality in MRL/lpr mice, a mouse model of SLE. 62 , 149 In MRL/lpr mice fed an HSD, the ratio of Th17/Treg was significantly increased, 149 and a higher proportion of Tfh cells was observed in the spleen. Given the pathogenic role of Tfh cells in lupus, an HSD might accelerate the SLE progression by inducing Tfh cell differentiation. 62 Additionally, an HSD accelerated the progression of murine lupus by activating DCs through the p38/MAPK‐STAT1 pathway. 112
RA is a chronic inflammatory joint disease that can cause cartilage, bone damage and disability. 208 Collagen‐induced arthritis (CIA) and K/BxN serum transfer‐induced arthritis (STIA) are mouse models of RA. CIA depends on adaptive and innate immunity, while STIA predominantly mimics the innate effector phase. 151 CIA mice fed an HSD showed more severe arthritis, higher Th17 cell proportion in splenocytes and increased IL‐17 expression in synovium and intestine. 150 Sehnert et al. 151 did not observe aggravated CIA or STIA in high‐salt‐diet mice, but they found that a low‐salt diet ameliorated the severity of CIA and STIA. Consistently, a study enrolled RA and SLE patients demonstrated that a restricted dietary salt intake can dampen the pro‐inflammatory response in patients with autoimmune diseases. 174 Moreover, high salt consumption among smokers was reportedly associated with increased RA risk. 175 , 176 By contrast, another study confirmed the association between high sodium intake and RA, particularly in nonsmokers. 177 Increased sodium excretion was also observed in patients with RA, 179 and further investigation suggested a correlation between RA disease activity and urinary Na/K ratio. 178
4.5. Cancer
It is known that the Na+ concentration is raised in solid tumours and can affect cell metabolism and immune function. Thus, it seems promising that dietary salt can influence the development of tumours and has the potential to be administered in cancer therapy. 209
Compelling evidence suggests that an HSD can suppress the progression of tumours. High salt intake suppressed the tumour growth and lung metastasis in a breast cancer murine model, 210 while an HSD reduced ETBF‐promoted colon carcinogenesis by decreasing the IL‐17A and iNOS expression. 211 Moreover, an HSD can inhibit the growth of transplanted melanoma, mammary cancer and Lewis lung carcinoma in mice by reducing myeloid‐derived suppressor cells (MDSCs). 154 , 155 Specifically, monocytic MDSCs (M‐MDSCs) differentiated into M1 macrophages, while granulocytic MDSCs (PMN‐MDSCs) converted to a pro‐inflammatory phenotype, therefore reactivating the antitumor actions of T cells. An HSD also enhanced the antitumor activation of PD‐1 inhibitors. 154 Additionally, an HSD induced NK cell‐mediated tumour immunity by augmenting the intratumor localization of Bifidobacterium. 86
However, given that high salt intake is a potent inducer of pro‐inflammatory states, the adverse effect of HSD on cancer has also been reported. 212 In vitro, high salt can synergize with IL‐17 to enhance the proliferation, treatment resistance and Warburg‐like metabolism of breast cancer cells. 213 , 214 , 215 In mice, an HSD accelerated the development and lung metastasis of breast cancer. 152 , 216 Data suggested that the IL‐17F produced by salt‐induced Th17 cells activated the MAPK signalling in breast cancer cells. 152 In humans, high salt intake is a risk factor for lung, testicular, bladder, 182 renal cell, 183 pancreatic, 184 oesophageal 181 and gastric cancer. 180 Particularly, an HSD can promote Helicobacter pylori infection, gastric mucosa damage, hypergastrinemia and cell proliferation, therefore contributing to gastric carcinogenesis. 153 These double‐sided effects indicated that the high salt effect on tumours might change with different tissues and phases of tumours.
4.6. Infections
An HSD might promote the elimination of Escherichia coli (E. coli) and Leishmania major (L. major) infection by increasing skin Na+ concentrations. 41 , 46 , 156 The salt‐driven ameliorated cutaneous L. major infection is related to the enhanced iNOS expression in macrophages, 41 while the salt‐augmented antibacterial activity against E. coli of macrophages hinges on the increased autophagy and autolysosomal targeting. 156 Additionally, an HSD can protect mice from lethal vesicular stomatitis virus (VSV) infection through the macrophage activation via p38/MAPK/activating transcription factor 2ATF2/AP1 pathway. 157 In murine models of acute lung injury induced by LPS, an HSD aggravated lung inflammation by activating macrophages. 97 In contrast, excessive salt intake aggravated uropathogenic E. coli‐induced pyelonephritis and systemic Listeria monocytogenes infection in mice by suppressing the antibacterial function of neutrophils. Healthy volunteers who accepted an HSD also showed impaired neutrophil functions and might be more vulnerable to infection. 82 These discrepancies indicated that the effect of HSD on infections might depend on the tissue sodium distributions and the organ‐specific responses.
4.7. Ischemic stroke
Ischemic stroke is a cerebrovascular disease that causes high mortality worldwide. 217 It has been reported that HSD in mice can exacerbate ischemic stroke. 218 An animal study linked HSD to ischemic brain damage. 219 Mechanistically, an HSD in mice enhanced BBB disruption during ischemia via the p38/MAPK/SGK1 pathway, accompanied by downregulated TJ protein expression in endothelial cells. 158 Additionally, an HSD induced pro‐inflammatory microglia by increasing aldose reductase (AR) protein expression via p38/MAPK and thus exacerbated ischemic stroke. 159 HSD also decreased the expression of the phagocytic molecule triggering receptor expressed on myeloid cells 2 TREM2 and induced a pro‐inflammatory phenotype in macrophages, leading to the postponed recovery of stroke lesions. 160 Moreover, high urinary sodium levels in humans were associated with large ischemic lesions. 158 Data from humans also showed that high salt intake was associated with the risk of ischemic stroke death, but was not associated with the risk of ischemic stroke onset. 185
4.8. Cognitive disorders
Cognitive disorders, common in psychiatric and neurological diseases, are a major societal burden. 220 High salt intake has been reported to impair cognitive functions via the gut‐brain axis. HSD can induce gut Th17 responses and increase circulating IL‐17, which suppresses cerebral endothelial NO production, leading to cerebral hypoperfusion, neurovascular dysregulation and cognitive impairment in mice. 161 Furthermore, an HSD caused cognitive dysfunction in mice by inducing an inflammatory environment and triggering apoptosis in the brain. 162 Several human studies linked HSD to impaired cognitive function, while a clinical trial showed that a low‐salt intake might improve cognition. 186 Nonetheless, most studies employed food questionnaires or diet records, and higher‐quality studies are needed.
4.9. Transplantation rejection
In a humanized xenogeneic graft‐versus‐host disease x‐GvHD murine model, an HSD blocked the immunosuppressive function of Treg cells and consequently worsened the severity and onset of transplantation rejection. 132 Another study demonstrated that mice fed an HSD displayed accelerated cardiac allograft rejection, accompanied by decreased Treg cells. 55 Furthermore, several human studies confirmed that dietary salt can influence the graft failure and mortality of renal transplant recipients by regulating blood pressure, 187 , 188 , 189 , 190 while another publication found no connection between dietary sodium and the prevalence or severity of post‐transplant hypertension. 191
4.10. Osteoporosis
Studies in humans suggested that the calcium loss caused by excessive salt intake might increase the risk of osteoporosis. 192 The enhanced bone loss and impaired bone‐microarchitecture in high‐salt‐diet mice are attributable to the increase in osteoclastogenic Th17 cells and the reduction in anti‐osteoclastogenic Treg cells caused by HSD. 165 , 221
4.11. Others
Wound healing was delayed in high‐salt‐diet mice owing to reduced M2 activation. 56 Additionally, HSD in mice during recovery from acute renal failure accelerated the progress towards chronic kidney disease and interstitial fibrosis. These responses were associated with Th17 cell activation in the kidney. 163 , 164 Moreover, an HSD might exacerbate food allergy in mice, 136 while sodium may participate in the progression of atopic dermatitis by regulating Th2 responses. 65
5. CLINICAL APPLICATION OF HIGH SALT
The clinical applications of salt can date back to the middle ages, when salt was used as a treatment for toothaches, upset stomachs and so forth. 222 Nowadays, high salt in clinical practice is primarily applied in the form of intravenous injection or nebulization. Hypertonic saline resuscitation can promote volume expansion, improve microcirculation and modulate immune responses in certain critically ill patients. 223 , 224 In addition, hypertonic saline has been adopted as an alternative to mannitol in patients with raised intracranial pressure. 225 Nebulized hypertonic saline is already used in the treatment of cystic fibrosis, 226 non‐cystic fibrosis bronchiectasis 227 and viral bronchiolitis. 228 Hypertonic saline inhalation in patients with cystic fibrosis can rehydrate the airway surface liquid, increase mucociliary clearance and improve lung function. 229 , 230 Most guidelines recommend the use of nebulized hypertonic saline in bronchiectasis therapy to facilitate airway clearance. 227 , 231 Nonetheless, studies about the application of nebulized hypertonic saline in patients with viral bronchiolitis demonstrated conflicting results. 228 Additionally, hypertonic saline nasal irrigation might be helpful in patients with chronic rhinosinusitis. 232 Furthermore, an increase in salt concentration in cancer vaccines can significantly change the physicochemical properties of the vaccine formulation and enhance its efficacy, showing invaluable potential in cancer therapy. 109 , 233 Salt‐activated CD4+ T cells that were derived from tumour‐bearing mice and injected into mice with breast cancer also elicited a strong anticancer response. 234 As the effect of high salt on immune cells and immune‐regulated diseases has been studied in recent decades, further investigation on its feasibility and applicability in clinical therapy should be conducted.
6. CONCLUSION AND FUTURE DIRECTION
In recent decades, we have witnessed the immunomodulatory effect of high salt. High salt has a profound impact on the differentiation, activation and function of multiple immune cells. These alterations in immune cells are dependent on the cellular milieu and disease context. Additionally, an HSD can affect the sodium concentration of local tissues, dominantly induce a pro‐inflammatory profile in microenvironments and thereby modulate the development of various immune‐regulated diseases, suggesting salt as a target or potential agent in the immune therapy of different diseases. However, owing to the variable cellular context in local tissues and different types and phases of diseases, the high salt effect on humans is intricate and changeable. The exact impact of HSD on diseases such as cancer needs to be further illustrated. Although some of the current epidemiological evidence has indicated the association between HSD and several diseases, since the sodium measurement methods used in most studies failed to estimate the mean sodium intake of individuals accurately, these conclusions might not be credible and higher‐quality clinical studies are warranted to validate the effect of HSD on diseases in populations. Moreover, novel applications of high salt, such as enhancing the efficacy of cancer vaccines by increasing salt concentrations in vaccine formulations, should be further explored.
AUTHOR CONTRIBUTIONS
Min Luo contributed to the conception and design of the review. The first draft of the manuscript was written by Xian Li. Xian Li and Aqu Alu created all the figures and tables. Yuquan Wei and Xiawei Wei critically revised the manuscript. All authors contributed to the article and approved the submitted version.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
ACKNOWLEDGEMENTS
This work was supported by the National Science Foundation for Excellent Young Scholars (32122052) and the National Natural Science Foundation Regional Innovation and Development (no. U19A2003).
Li X, Alu A, Wei Y, Wei X, Luo M. The modulatory effect of high salt on immune cells and related diseases. Cell Prolif. 2022;55(9):e13250. doi: 10.1111/cpr.13250
Funding information National Natural Science Foundation Regional Innovation and Development; National Science Foundation for Excellent Young Scholars, Grant/Award Numbers: U19A2003, 32122052
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Finfer S, Myburgh J, Bellomo R. Intravenous fluid therapy in critically ill adults. Nat Rev Nephrol. 2018;14(9):541‐557. [DOI] [PubMed] [Google Scholar]
- 2. He FJ, Tan M, Ma Y, MacGregor GA. Salt reduction to prevent hypertension and cardiovascular disease: JACC state‐of‐the‐art review. J Am Coll Cardiol. 2020;75(6):632‐647. [DOI] [PubMed] [Google Scholar]
- 3. Shapiro L, Dinarello CA. Osmotic regulation of cytokine synthesis in vitro. Proc Natl Acad Sci U S A. 1995;92(26):12230‐12234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shapiro L, Dinarello CA. Hyperosmotic stress as a stimulant for proinflammatory cytokine production. Exp Cell Res. 1997;231(2):354‐362. [DOI] [PubMed] [Google Scholar]
- 5. Zeidel ML. Salt and water: not so simple. J Clin Invest. 2017;127(5):1625‐1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Guyton AC. Blood pressure control‐‐special role of the kidneys and body fluids. Science. 1991;252(5014):1813‐1816. [DOI] [PubMed] [Google Scholar]
- 7. Titze J, Lang R, Ilies C, et al. Osmotically inactive skin Na+ storage in rats. Am J Physiol Renal Physiol. 2003;285(6):F1108‐F1117. [DOI] [PubMed] [Google Scholar]
- 8. Titze J, Bauer K, Schafflhuber M, et al. Internal sodium balance in DOCA‐salt rats: a body composition study. Am J Physiol Renal Physiol. 2005;289(4):F793‐F802. [DOI] [PubMed] [Google Scholar]
- 9. Kopp C, Linz P, Wachsmuth L, et al. (23)Na magnetic resonance imaging of tissue sodium. Hypertension. 2012;59(1):167‐172. [DOI] [PubMed] [Google Scholar]
- 10. Kopp C, Linz P, Dahlmann A, et al. 23Na magnetic resonance imaging‐determined tissue sodium in healthy subjects and hypertensive patients. Hypertension. 2013;61(3):635‐640. [DOI] [PubMed] [Google Scholar]
- 11. Nikpey E, Karlsen TV, Rakova N, Titze JM, Tenstad O, Wiig H. High‐salt diet causes osmotic gradients and Hyperosmolality in skin without affecting interstitial fluid and lymph. Hypertension. 2017;69(4):660‐668. [DOI] [PubMed] [Google Scholar]
- 12. Titze J, Shakibaei M, Schafflhuber M, et al. Glycosaminoglycan polymerization may enable osmotically inactive Na+ storage in the skin. Amer J Physiol Heart Circ Physiol. 2004;287(1):H203‐H208. [DOI] [PubMed] [Google Scholar]
- 13. Schafflhuber M, Volpi N, Dahlmann A, et al. Mobilization of osmotically inactive Na+ by growth and by dietary salt restriction in rats. Am J Physiol Renal Physiol. 2007;292(5):F1490‐F1500. [DOI] [PubMed] [Google Scholar]
- 14. Sterns RH. Disorders of plasma sodium‐‐causes, consequences, and correction. New Engl J Med. 2015;372(1):55‐65. [DOI] [PubMed] [Google Scholar]
- 15. Huhn K, Linz P, Pemsel F, et al. Skin sodium is increased in male patients with multiple sclerosis and related animal models. Proc Natl Acad Sci U S A. 2021;118(28):e2102549118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Crescenzi R, Marton A, Donahue PMC, et al. Tissue sodium content is elevated in the skin and subcutaneous adipose tissue in women with lipedema. Obesity. 2018;26(2):310‐317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kopp C, Beyer C, Linz P, et al. Na+ deposition in the fibrotic skin of systemic sclerosis patients detected by 23Na‐magnetic resonance imaging. Rheumatology (Oxford). 2017;56(4):556‐560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dahlmann A, Dörfelt K, Eicher F, et al. Magnetic resonance‐determined sodium removal from tissue stores in hemodialysis patients. Kidney Int. 2015;87(2):434‐441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kopp C, Linz P, Maier C, et al. Elevated tissue sodium deposition in patients with type 2 diabetes on hemodialysis detected by (23)Na magnetic resonance imaging. Kidney Int. 2018;93(5):1191‐1197. [DOI] [PubMed] [Google Scholar]
- 20. Carranza‐León DA, Oeser A, Marton A, et al. Tissue sodium content in patients with systemic lupus erythematosus: association with disease activity and markers of inflammation. Lupus. 2020;29(5):455‐462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Paling D, Solanky BS, Riemer F, et al. Sodium accumulation is associated with disability and a progressive course in multiple sclerosis. Brain. 2013;136(Pt 7):2305‐2317. [DOI] [PubMed] [Google Scholar]
- 22. Brownlee WJ, Solanky B, Prados F, et al. Cortical grey matter sodium accumulation is associated with disability and secondary progressive disease course in relapse‐onset multiple sclerosis. J Neurol Neurosurg Psychiatry. 2019;90(7):755‐760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Collorone S, Prados F, Kanber B, et al. Brain microstructural and metabolic alterations detected in vivo at onset of the first demyelinating event. Brain. 2021;144(5):1409‐1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Maifeld A, Wild J, Karlsen TV, et al. Skin sodium accumulates in psoriasis and reflects disease severity. J Invest Dermatol. 2022;142(1):166‐178.e168. [DOI] [PubMed] [Google Scholar]
- 25. Sakata F, Ito Y, Mizuno M, et al. Sodium chloride promotes tissue inflammation via osmotic stimuli in subtotal‐nephrectomized mice. Lab Invest. 2017;97(4):432‐446. [DOI] [PubMed] [Google Scholar]
- 26. Ianniello C, Moy L, Fogarty J, et al. Multinuclear MRI to disentangle intracellular sodium concentration and extracellular volume fraction in breast cancer. Sci Rep. 2021;11(1):5156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Minegishi S, Luft FC, Titze J, Kitada K. Sodium handling and interaction in numerous organs. Am J Hypertens. 2020;33(8):687‐694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hurley SW, Thunhorst RL, Johnson AK. Frontiers in neuroscience sodium appetite sensitization. In: De Luca LA Jr, Menani JV, Johnson AK, eds. Neurobiology of Body Fluid Homeostasis: Transduction and Integration. CRC Press/Taylor & Francis; 2014. [PubMed] [Google Scholar]
- 29. Delpire E, Gagnon KB. Water homeostasis and cell volume maintenance and regulation. Curr Top Membr. 2018;81:3‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bortner CD, Cidlowski JA. Ions, the movement of water and the apoptotic volume decrease. Front Cell Dev Biol. 2020;8:611211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Raghavan M, Fee D, Barkhaus PE. Generation and propagation of the action potential. Handb Clin Neurol. 2019;160:3‐22. [DOI] [PubMed] [Google Scholar]
- 32. Lind L, Lithell H, Gustafsson IB, Pollare T, Ljunghall S. Metabolic cardiovascular risk factors and sodium sensitivity in hypertensive subjects. Am J Hypertens. 1992;5(8):502‐505. [DOI] [PubMed] [Google Scholar]
- 33. Al‐Dahhan J, Jannoun L, Haycock GB. Effect of salt supplementation of newborn premature infants on neurodevelopmental outcome at 10‐13 years of age. Arch Dis Child Fetal Neonatal Ed. 2002;86(2):F120‐F123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Segar JL, Grobe CC, Balapattabi K, Ritter ML, Reho JJ, Grobe JL. Dissociable effects of dietary sodium in early life upon somatic growth, fluid homeostasis, and spatial memory in mice of both sexes. Am J Physiol Regul Integr Comp Physiol. 2021;320(4):R438‐r451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fine BP, Ty A, Lestrange N, Levine OR. Sodium deprivation growth failure in the rat: alterations in tissue composition and fluid spaces. J Nutr. 1987;117(9):1623‐1628. [DOI] [PubMed] [Google Scholar]
- 36. Bursey RG, Watson ML. The effect of sodium restriction during gestation of offspring brain development in rats. Am J Clin Nutr. 1983;37(1):43‐51. [DOI] [PubMed] [Google Scholar]
- 37. Sakuyama H, Katoh M, Wakabayashi H, Zulli A, Kruzliak P, Uehara Y. Influence of gestational salt restriction in fetal growth and in development of diseases in adulthood. J Biomed Sci. 2016;23:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Parkin J, Cohen B. An overview of the immune system. Lancet. 2001;357(9270):1777‐1789. [DOI] [PubMed] [Google Scholar]
- 39. Mestas J, Hughes CC. Of mice and not men: differences between mouse and human immunology. J Immunol. 2004;172(5):2731‐2738. [DOI] [PubMed] [Google Scholar]
- 40. Go WY, Liu X, Roti MA, Liu F, Ho SN. NFAT5/TonEBP mutant mice define osmotic stress as a critical feature of the lymphoid microenvironment. Proc Natl Acad Sci U S A. 2004;101(29):10673‐10678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jantsch J, Schatz V, Friedrich D, et al. Cutaneous Na+ storage strengthens the antimicrobial barrier function of the skin and boosts macrophage‐driven host defense. Cell Metab. 2015;21(3):493‐501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sheppard FR, Moore EE, McLaughlin N, Kelher M, Johnson JL, Silliman CC. Clinically relevant osmolar stress inhibits priming‐induced PMN NADPH oxidase subunit translocation. J Trauma. 2005;58(4):752‐757. [DOI] [PubMed] [Google Scholar]
- 43. Kleinewietfeld M, Manzel A, Titze J, et al. Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature. 2013;496(7446):518‐522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ip WK, Medzhitov R. Macrophages monitor tissue osmolarity and induce inflammatory response through NLRP3 and NLRC4 inflammasome activation. Nat Commun. 2015;6:6931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hucke S, Eschborn M, Liebmann M, et al. Sodium chloride promotes pro‐inflammatory macrophage polarization thereby aggravating CNS autoimmunity. J Autoimmun. 2016;67:90‐101. [DOI] [PubMed] [Google Scholar]
- 46. Machnik A, Neuhofer W, Jantsch J, et al. Macrophages regulate salt‐dependent volume and blood pressure by a vascular endothelial growth factor‐C‐dependent buffering mechanism. Nat Med. 2009;15(5):545‐552. [DOI] [PubMed] [Google Scholar]
- 47. Aramburu J, López‐Rodríguez C. Regulation of inflammatory functions of macrophages and T lymphocytes by NFAT5. Front Immunol. 2019;10:535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Morancho B, Minguillón J, Molkentin JD, López‐Rodríguez C, Aramburu J. Analysis of the transcriptional activity of endogenous NFAT5 in primary cells using transgenic NFAT‐luciferase reporter mice. BMC Mol Biol. 2008;9:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kino T, Takatori H, Manoli I, et al. Brx mediates the response of lymphocytes to osmotic stress through the activation of NFAT5. Sci Signal. 2009;2(57):ra5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Drews‐Elger K, Ortells MC, Rao A, López‐Rodriguez C, Aramburu J. The transcription factor NFAT5 is required for cyclin expression and cell cycle progression in cells exposed to hypertonic stress. PLoS One. 2009;4(4):e5245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Berga‐Bolaños R, Drews‐Elger K, Aramburu J, López‐Rodríguez C. NFAT5 regulates T lymphocyte homeostasis and CD24‐dependent T cell expansion under pathologic hypernatremia. J Immunol. 2010;185(11):6624‐6635. [DOI] [PubMed] [Google Scholar]
- 52. Loomis WH, Namiki S, Ostrom RS, Insel PA, Junger WG. Hypertonic stress increases T cell interleukin‐2 expression through a mechanism that involves ATP release, P2 receptor, and p38 MAPK activation. J Biol Chem. 2003;278(7):4590‐4596. [DOI] [PubMed] [Google Scholar]
- 53. Junger WG, Liu FC, Loomis WH, Hoyt DB. Hypertonic saline enhances cellular immune function. Circ Shock. 1994;42(4):190‐196. [PubMed] [Google Scholar]
- 54. Coimbra R, Junger WG, Liu FC, Loomis WH, Hoyt DB. Hypertonic/hyperoncotic fluids reverse prostaglandin E2 (PGE2)‐induced T‐cell suppression. Shock. 1995;4(1):45‐49. [DOI] [PubMed] [Google Scholar]
- 55. Safa K, Ohori S, Borges TJ, et al. Salt accelerates allograft rejection through serum‐ and glucocorticoid‐regulated Kinase‐1‐dependent inhibition of regulatory T cells. J Am Soc Nephrol. 2015;26(10):2341‐2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Binger KJ, Gebhardt M, Heinig M, et al. High salt reduces the activation of IL‐4‐ and IL‐13‐stimulated macrophages. J Clin Invest. 2015;125(11):4223‐4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Cvetkovic L, Perisic S, Titze J, Jäck HM, Schuh W. The impact of Hyperosmolality on activation and differentiation of B lymphoid cells. Front Immunol. 2019;10:828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Burg MB, Ferraris JD, Dmitrieva NI. Cellular response to hyperosmotic stresses. Physiol Rev. 2007;87(4):1441‐1474. [DOI] [PubMed] [Google Scholar]
- 59. Jobava R, Mao Y, Guan BJ, et al. Adaptive translational pausing is a hallmark of the cellular response to severe environmental stress. Mol Cell. 2021;81(20):4191‐4208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Dmitrieva NI, Burg MB. Analysis of DNA breaks, DNA damage response, and apoptosis produced by high NaCl. Am J Physiol Renal Physiol. 2008;295(6):F1678‐F1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Trama J, Go WY, Ho SN. The osmoprotective function of the NFAT5 transcription factor in T cell development and activation. J Immunol. 2002;169(10):5477‐5488. [DOI] [PubMed] [Google Scholar]
- 62. Wu H, Huang X, Qiu H, et al. High salt promotes autoimmunity by TET2‐induced DNA demethylation and driving the differentiation of Tfh cells. Sci Rep. 2016;6:28065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kerby GS, Cottin V, Accurso FJ, et al. Impairment of macrophage survival by NaCl: implications for early pulmonary inflammation in cystic fibrosis. Am J Physiol Lung Cell Mol Physiol. 2002;283(1):L188‐L197. [DOI] [PubMed] [Google Scholar]
- 64. Lang KS, Fillon S, Schneider D, Rammensee HG, Lang F. Stimulation of TNF alpha expression by hyperosmotic stress. Pflugers Arch. 2002;443(5–6):798‐803. [DOI] [PubMed] [Google Scholar]
- 65. Matthias J, Maul J, Noster R, et al. Sodium chloride is an ionic checkpoint for human T(H)2 cells and shapes the atopic skin microenvironment. Sci Transl Med. 2019;11(480):eaau0683. [DOI] [PubMed] [Google Scholar]
- 66. Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13(3):159‐175. [DOI] [PubMed] [Google Scholar]
- 67. Ciesla DJ, Moore EE, Zallen G, Biffl WL, Silliman CC. Hypertonic saline attenuation of polymorphonuclear neutrophil cytotoxicity: timing is everything. J Trauma. 2000;48(3):388‐395. [DOI] [PubMed] [Google Scholar]
- 68. Partrick DA, Moore EE, Offner PJ, Johnson JL, Tamura DY, Silliman CC. Hypertonic saline activates lipid‐primed human neutrophils for enhanced elastase release. J Trauma. 1998;44(4):592‐597. [DOI] [PubMed] [Google Scholar]
- 69. Junger WG, Hoyt DB, Davis RE, et al. Hypertonicity regulates the function of human neutrophils by modulating chemoattractant receptor signaling and activating mitogen‐activated protein kinase p38. J Clin Invest. 1998;101(12):2768‐2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hampton MB, Chambers ST, Vissers MC, Winterbourn CC. Bacterial killing by neutrophils in hypertonic environments. J Infect Dis. 1994;169(4):839‐846. [DOI] [PubMed] [Google Scholar]
- 71. Matsumoto T, Kumazawa J, van der Auwera P. Suppression of leukocyte function and intracellular content of ATP in hyperosmotic condition comparable to the renal medulla. J Urol. 1989;142(2 Pt 1):399‐402. [DOI] [PubMed] [Google Scholar]
- 72. Kuroda T, Harada T, Tsutsumi H, Kobayashi M. Hypernatremic suppression of neutrophils. Burns. 1997;23(4):338‐340. [DOI] [PubMed] [Google Scholar]
- 73. Angle N, Hoyt DB, Cabello‐Passini R, Herdon‐Remelius C, Loomis W, Junger WG. Hypertonic saline resuscitation reduces neutrophil margination by suppressing neutrophil L selectin expression. J Trauma. 1998;45(1):7‐12. [DOI] [PubMed] [Google Scholar]
- 74. Thiel M, Buessecker F, Eberhardt K, et al. Effects of hypertonic saline on expression of human polymorphonuclear leukocyte adhesion molecules. J Leukoc Biol. 2001;70(2):261‐273. [PubMed] [Google Scholar]
- 75. Rizoli SB, Kapus A, Parodo J, Rotstein OD. Hypertonicity prevents lipopolysaccharide‐stimulated CD11b/CD18 expression in human neutrophils in vitro: role for p38 inhibition. J Trauma. 1999;46(5):794‐798. [DOI] [PubMed] [Google Scholar]
- 76. Rizoli SB, Rotstein OD, Kapus A. Cell volume‐dependent regulation of L‐selectin shedding in neutrophils. A role for p38 mitogen‐activated protein kinase. J Biol Chem. 1999;274(31):22072‐22080. [DOI] [PubMed] [Google Scholar]
- 77. Rizoli SB, Rotstein OD, Parodo J, Phillips MJ, Kapus A. Hypertonic inhibition of exocytosis in neutrophils: central role for osmotic actin skeleton remodeling. Am J Physiol Cell Physiol. 2000;279(3):C619‐C633. [DOI] [PubMed] [Google Scholar]
- 78. Ciesla DJ, Moore EE, Musters RJ, Biffl WL, Silliman CA. Hypertonic saline alteration of the PMN cytoskeleton: implications for signal transduction and the cytotoxic response. J Trauma. 2001;50(2):206‐212. [DOI] [PubMed] [Google Scholar]
- 79. Chen Y, Shukla A, Namiki S, Insel PA, Junger WG. A putative osmoreceptor system that controls neutrophil function through the release of ATP, its conversion to adenosine, and activation of A2 adenosine and P2 receptors. J Leukoc Biol. 2004;76(1):245‐253. [DOI] [PubMed] [Google Scholar]
- 80. Nadesalingam A, Chen JHK, Farahvash A, Khan MA. Hypertonic saline suppresses NADPH oxidase‐dependent neutrophil extracellular trap formation and promotes apoptosis. Front Immunol. 2018;9:359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Krampert L, Bauer K, Ebner S, et al. High Na(+) environments impair phagocyte oxidase‐dependent antibacterial activity of neutrophils. Front Immunol. 2021;12:712948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Jobin K, Stumpf NE, Schwab S, et al. A high‐salt diet compromises antibacterial neutrophil responses through hormonal perturbation. Sci Transl Med. 2020;12(536):eaay3850. [DOI] [PubMed] [Google Scholar]
- 83. O'Brien KL, Finlay DK. Immunometabolism and natural killer cell responses. Nat Rev Immunol. 2019;19(5):282‐290. [DOI] [PubMed] [Google Scholar]
- 84. Spits H, Artis D, Colonna M, et al. Innate lymphoid cells‐‐a proposal for uniform nomenclature. Nat Rev Immunol. 2013;13(2):145‐149. [DOI] [PubMed] [Google Scholar]
- 85. Zeng X, Li Y, Lv W, et al. A high‐salt diet disturbs the development and function of natural killer cells in mice. J Immunol Res. 2020;2020:6687143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Rizvi ZA, Dalal R, Sadhu S, et al. High‐salt diet mediates interplay between NK cells and gut microbiota to induce potent tumor immunity. Sci Adv. 2021;7(37):eabg5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Haniffa M, Bigley V, Collin M. Human mononuclear phagocyte system reunited. Semin Cell Dev Biol. 2015;41:59‐69. [DOI] [PubMed] [Google Scholar]
- 88. Guilliams M, Ginhoux F, Jakubzick C, et al. Dendritic cells, monocytes and macrophages: a unified nomenclature based on ontogeny. Nat Rev Immunol. 2014;14(8):571‐578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Gentek R, Molawi K, Sieweke MH. Tissue macrophage identity and self‐renewal. Immunol Rev. 2014;262(1):56‐73. [DOI] [PubMed] [Google Scholar]
- 90. Locati M, Curtale G, Mantovani A. Diversity, mechanisms, and significance of macrophage plasticity. Annu Rev Pathol. 2020;15:123‐147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Bassler K, Schulte‐Schrepping J, Warnat‐Herresthal S, Aschenbrenner AC, Schultze JL. The myeloid cell compartment‐cell by cell. Annu Rev Immunol. 2019;37:269‐293. [DOI] [PubMed] [Google Scholar]
- 92. Jakubzick CV, Randolph GJ, Henson PM. Monocyte differentiation and antigen‐presenting functions. Nat Rev Immunol. 2017;17(6):349‐362. [DOI] [PubMed] [Google Scholar]
- 93. Xue J, Schmidt SV, Sander J, et al. Transcriptome‐based network analysis reveals a spectrum model of human macrophage activation. Immunity. 2014;40(2):274‐288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Murray PJ. Macrophage polarization. Annu Rev Physiol. 2017;79:541‐566. [DOI] [PubMed] [Google Scholar]
- 95. Saha S, Shalova IN, Biswas SK. Metabolic regulation of macrophage phenotype and function. Immunol Rev. 2017;280(1):102‐111. [DOI] [PubMed] [Google Scholar]
- 96. Fehrenbach DJ, Abais‐Battad JM, Dasinger JH, Lund H, Mattson DL. Salt‐sensitive increase in macrophages in the kidneys of dahl SS rats. Am J Physiol Renal Physiol. 2019;317(2):F361‐f374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Zhang WC, Zheng XJ, Du LJ, et al. High salt primes a specific activation state of macrophages, M(Na). Cell Res. 2015;25(8):893‐910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Neubert P, Homann A, Wendelborn D, et al. NCX1 represents an ionic Na+ sensing mechanism in macrophages. PLoS Biol. 2020;18(6):e3000722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Geisberger S, Bartolomaeus H, Neubert P, et al. Salt transiently inhibits mitochondrial energetics in mononuclear phagocytes. Circulation. 2021;144(2):144‐158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Müller S, Quast T, Schröder A, et al. Salt‐dependent chemotaxis of macrophages. PLoS One. 2013;8(9):e73439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Saas P, Chagué C, Maraux M, Cherrier T. Toward the characterization of human pro‐resolving macrophages? Front Immunol. 2020;11:593300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Zhou X, Zhang L, Ji WJ, et al. Variation in dietary salt intake induces coordinated dynamics of monocyte subsets and monocyte‐platelet aggregates in humans: implications in end organ inflammation. PLoS One. 2013;8(4):e60332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Yi B, Titze J, Rykova M, et al. Effects of dietary salt levels on monocytic cells and immune responses in healthy human subjects: a longitudinal study. Transl Res. 2015;166(1):103‐110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Ruggeri Barbaro N, Van Beusecum J, Xiao L, et al. Sodium activates human monocytes via the NADPH oxidase and isolevuglandin formation. Cardiovasc Res. 2021;117(5):1358‐1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Wenstedt EF, Verberk SG, Kroon J, et al. Salt increases monocyte CCR2 expression and inflammatory responses in humans. JCI Insight. 2019;4(21):e130508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Fan A, Oladiran O, Shi XQ, Zhang J. High‐salt diet decreases mechanical thresholds in mice that is mediated by a CCR2‐dependent mechanism. J Neuroinflammation. 2020;17(1):179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Popovic ZV, Embgenbroich M, Chessa F, et al. Hyperosmolarity impedes the cross‐priming competence of dendritic cells in a TRIF‐dependent manner. Sci Rep. 2017;7(1):311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Chessa F, Mathow D, Wang S, et al. The renal microenvironment modifies dendritic cell phenotype. Kidney Int. 2016;89(1):82‐94. [DOI] [PubMed] [Google Scholar]
- 109. Luo M, Shao B, Yu JY, et al. Simultaneous enhancement of cellular and humoral immunity by the high salt formulation of Al(OH)(3) adjuvant. Cell Res. 2017;27(4):586‐589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Barbaro NR, Foss JD, Kryshtal DO, et al. Dendritic cell Amiloride‐sensitive channels mediate sodium‐induced inflammation and hypertension. Cell Rep. 2017;21(4):1009‐1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Ferguson JF, Aden LA, Barbaro NR, et al. High dietary salt‐induced dendritic cell activation underlies microbial dysbiosis‐associated hypertension. JCI Insight. 2019;5(13):e126241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Xiao ZX, Hu X, Zhang X, et al. High salt diet accelerates the progression of murine lupus through dendritic cells via the p38 MAPK and STAT1 signaling pathways. Signal Transduct Target Ther. 2020;5(1):34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Tubbs AL, Liu B, Rogers TD, Sartor RB, Miao EA. Dietary salt exacerbates experimental colitis. J Immunol. 2017;199(3):1051‐1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. O'Shea JJ, Paul WE. Mechanisms underlying lineage commitment and plasticity of helper CD4+ T cells. Science. 2010;327(5969):1098‐1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations (*). Annu Rev Immunol. 2010;28:445‐489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Zhu X, Zhu J. CD4 T helper cell subsets and related human immunological disorders. Int J Mol Sci. 2020;21(21):8011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Korn T, Bettelli E, Oukka M, Kuchroo VK. IL‐17 and Th17 cells. Annu Rev Immunol. 2009;27:485‐517. [DOI] [PubMed] [Google Scholar]
- 118. Zielinski CE, Mele F, Aschenbrenner D, et al. Pathogen‐induced human TH17 cells produce IFN‐γ or IL‐10 and are regulated by IL‐1β. Nature. 2012;484(7395):514‐518. [DOI] [PubMed] [Google Scholar]
- 119. Noster R, de Koning HD, Maier E, Prelog M, Lainka E, Zielinski CE. Dysregulation of proinflammatory versus anti‐inflammatory human T(H)17 cell functionalities in the autoinflammatory Schnitzler syndrome. J Allergy Clin Immunol. 2016;138(4):1161‐1169.e1166. [DOI] [PubMed] [Google Scholar]
- 120. Stockinger B, Omenetti S. The dichotomous nature of T helper 17 cells. Nat Rev Immunol. 2017;17(9):535‐544. [DOI] [PubMed] [Google Scholar]
- 121. Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133(5):775‐787. [DOI] [PubMed] [Google Scholar]
- 122. Abbas AK, Benoist C, Bluestone JA, et al. Regulatory T cells: recommendations to simplify the nomenclature. Nat Immunol. 2013;14(4):307‐308. [DOI] [PubMed] [Google Scholar]
- 123. Ueno H, Banchereau J, Vinuesa CG. Pathophysiology of T follicular helper cells in humans and mice. Nat Immunol. 2015;16(2):142‐152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Gowthaman U, Chen JS, Eisenbarth SC. Regulation of IgE by T follicular helper cells. J Leukoc Biol. 2020;107(3):409‐418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Zhu J. T helper cell differentiation, heterogeneity, and plasticity. Cold Spring Harb Perspect Biol. 2018;10(10):a030338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Wu C, Yosef N, Thalhamer T, et al. Induction of pathogenic TH17 cells by inducible salt‐sensing kinase SGK1. Nature. 2013;496(7446):513‐517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Norlander AE, Saleh MA, Pandey AK, et al. A salt‐sensing kinase in T lymphocytes, SGK1, drives hypertension and hypertensive end‐organ damage. JCI Insight. 2017;2(13):e92801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Matthias J, Heink S, Picard F, et al. Salt generates antiinflammatory Th17 cells but amplifies pathogenicity in proinflammatory cytokine microenvironments. J Clin Invest. 2020;130(9):4587‐4600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Müller DN, Wilck N, Haase S, Kleinewietfeld M, Linker RA. Sodium in the microenvironment regulates immune responses and tissue homeostasis. Nat Rev Immunol. 2019;19(4):243‐254. [DOI] [PubMed] [Google Scholar]
- 130. Wilck N, Matus MG, Kearney SM, et al. Salt‐responsive gut commensal modulates T(H)17 axis and disease. Nature. 2017;551(7682):585‐589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Miranda PM, De Palma G, Serkis V, et al. High salt diet exacerbates colitis in mice by decreasing lactobacillus levels and butyrate production. Microbiome. 2018;6(1):57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Hernandez AL, Kitz A, Wu C, et al. Sodium chloride inhibits the suppressive function of FOXP3+ regulatory T cells. J Clin Invest. 2015;125(11):4212‐4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Yang YH, Istomine R, Alvarez F, et al. Salt sensing by serum/glucocorticoid‐regulated kinase 1 promotes Th17‐like inflammatory adaptation of Foxp3(+) regulatory T cells. Cell Rep. 2020;30(5):1515‐1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Luo Y, Xue Y, Wang J, et al. Negligible effect of sodium chloride on the development and function of TGF‐β‐induced CD4(+) Foxp3(+) regulatory T cells. Cell Rep. 2019;26(7):1869‐1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Yao Y, Jiang Q, Jiang L, et al. Lnc‐SGK1 induced by helicobacter pylori infection and highsalt diet promote Th2 and Th17 differentiation in human gastric cancer by SGK1/Jun B signaling. Oncotarget. 2016;7(15):20549‐20560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Liu Z, Li SK, Huang CK, Huang CF. A high‐sodium diet modulates the immune response of food allergy in a murine model. Nutrients. 2021;13(11):3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. St Paul M, Ohashi PS. The roles of CD8(+) T cell subsets in antitumor immunity. Trends Cell Biol. 2020;30(9):695‐704. [DOI] [PubMed] [Google Scholar]
- 138. Bier A, Khashab R, Sharabi Y, Grossman E, Leibowitz A. Melatonin prevents T lymphocyte infiltration to the kidneys of hypertensive rats, induced by a high‐salt diet, by preventing the expression of CXCR3 ligand chemokines. Nutrients. 2021;13(10):3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Jeon J, Lee K, Yang KE, et al. Dietary modification alters the Intrarenal immunologic micromilieu and susceptibility to ischemic acute kidney injury. Front Immunol. 2021;12:621176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. LeBien TW, Tedder TF. B lymphocytes: how they develop and function. Blood. 2008;112(5):1570‐1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Krementsov DN, Case LK, Hickey WF, Teuscher C. Exacerbation of autoimmune neuroinflammation by dietary sodium is genetically controlled and sex specific. FASEB J. 2015;29(8):3446‐3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Na SY, Janakiraman M, Leliavski A, Krishnamoorthy G. High‐salt diet suppresses autoimmune demyelination by regulating the blood‐brain barrier permeability. Proc Natl Acad Sci U S A. 2021;118(12):e2025944118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Monteleone I, Marafini I, Dinallo V, et al. Sodium chloride‐enriched diet enhanced inflammatory cytokine production and exacerbated experimental colitis in mice. J Crohns Colitis. 2017;11(2):237‐245. [DOI] [PubMed] [Google Scholar]
- 144. Guo HX, Ye N, Yan P, et al. Sodium chloride exacerbates dextran sulfate sodium‐induced colitis by tuning proinflammatory and antiinflammatory lamina propria mononuclear cells through p38/MAPK pathway in mice. World J Gastroenterol. 2018;24(16):1779‐1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Vaartjes D, Nandakumar KS, Holmdahl R, Raposo B. Increased salt exposure affects both lymphoid and myeloid effector functions, influencing innate‐associated disease but not T‐cell‐associated autoimmunity. Immunology. 2018;154(4):683‐694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Wei Y, Lu C, Chen J, et al. High salt diet stimulates gut Th17 response and exacerbates TNBS‐induced colitis in mice. Oncotarget. 2017;8(1):70‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Aguiar SLF, Miranda MCG, Guimarães MAF, et al. High‐salt diet induces IL‐17‐dependent gut inflammation and exacerbates colitis in mice. Front Immunol. 2017;8:1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Amamou A, Rouland M, Yaker L, et al. Dietary salt exacerbates intestinal fibrosis in chronic TNBS colitis via fibroblasts activation. Sci Rep. 2021;11(1):15055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Yang X, Yao G, Chen W, Tang X, Feng X, Sun L. Exacerbation of lupus nephritis by high sodium chloride related to activation of SGK1 pathway. Int Immunopharmacol. 2015;29(2):568‐573. [DOI] [PubMed] [Google Scholar]
- 150. Jung SM, Kim Y, Kim J, et al. Sodium chloride aggravates arthritis via Th17 polarization. Yonsei Med J. 2019;60(1):88‐97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Sehnert B, Pohle S, Heuberger C, et al. Low‐salt diet attenuates B‐cell‐ and myeloid‐cell‐driven experimental Arthritides by affecting innate as well as adaptive immune mechanisms. Front Immunol. 2021;12:765741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Chen J, Liu X, Huang H, Zhang F, Lu Y, Hu H. High salt diet may promote progression of breast tumor through eliciting immune response. Int Immunopharmacol. 2020;87:106816. [DOI] [PubMed] [Google Scholar]
- 153. Gaddy JA, Radin JN, Loh JT, et al. High dietary salt intake exacerbates helicobacter pylori‐induced gastric carcinogenesis. Infect Immun. 2013;81(6):2258‐2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. He W, Xu J, Mu R, et al. High‐salt diet inhibits tumour growth in mice via regulating myeloid‐derived suppressor cell differentiation. Nat Commun. 2020;11(1):1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Willebrand R, Hamad I, Van Zeebroeck L, et al. High salt inhibits tumor growth by enhancing anti‐tumor immunity. Front Immunol. 2019;10:1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Neubert P, Weichselbaum A, Reitinger C, et al. HIF1A and NFAT5 coordinate Na(+)‐boosted antibacterial defense via enhanced autophagy and autolysosomal targeting. Autophagy. 2019;15(11):1899‐1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Zhang WC, Du LJ, Zheng XJ, et al. Elevated sodium chloride drives type I interferon signaling in macrophages and increases antiviral resistance. J Biol Chem. 2018;293(3):1030‐1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Zhang T, Fang S, Wan C, et al. Excess salt exacerbates blood‐brain barrier disruption via a p38/MAPK/SGK1‐dependent pathway in permanent cerebral ischemia. Sci Rep. 2015;5:16548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Zhang T, Wang D, Li X, et al. Excess salt intake promotes M1 microglia polarization via a p38/MAPK/AR‐dependent pathway after cerebral ischemia in mice. Int Immunopharmacol. 2020;81:106176. [DOI] [PubMed] [Google Scholar]
- 160. Hu M, Lin Y, Men X, et al. High‐salt diet downregulates TREM2 expression and blunts efferocytosis of macrophages after acute ischemic stroke. J Neuroinflammation. 2021;18(1):90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Faraco G, Brea D, Garcia‐Bonilla L, et al. Dietary salt promotes neurovascular and cognitive dysfunction through a gut‐initiated TH17 response. Nat Neurosci. 2018;21(2):240‐249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Hu L, Zhu S, Peng X, et al. High salt elicits brain inflammation and cognitive dysfunction, accompanied by alternations in the gut microbiota and decreased SCFA production. J Alzheimers Dis. 2020;77(2):629‐640. [DOI] [PubMed] [Google Scholar]
- 163. Mehrotra P, Patel JB, Ivancic CM, Collett JA, Basile DP. Th‐17 cell activation in response to high salt following acute kidney injury is associated with progressive fibrosis and attenuated by AT‐1R antagonism. Kidney Int. 2015;88(4):776‐784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Spurgeon‐Pechman KR, Donohoe DL, Mattson DL, Lund H, James L, Basile DP. Recovery from acute renal failure predisposes hypertension and secondary renal disease in response to elevated sodium. Am J Physiol Renal Physiol. 2007;293(1):F269‐F278. [DOI] [PubMed] [Google Scholar]
- 165. Dar HY, Singh A, Shukla P, et al. High dietary salt intake correlates with modulated Th17‐Treg cell balance resulting in enhanced bone loss and impaired bone‐microarchitecture in male mice. Sci Rep. 2018;8(1):2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Filippini T, Malavolti M, Whelton PK, Naska A, Orsini N, Vinceti M. Blood pressure effects of sodium reduction: dose‐response meta‐analysis of experimental studies. Circulation. 2021;143(16):1542‐1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Ma Y, He FJ, Sun Q, et al. 24‐Hour urinary sodium and potassium excretion and cardiovascular risk. New Engl J Med. 2022;386(3):252‐263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Farez MF, Fiol MP, Gaitán MI, Quintana FJ, Correale J. Sodium intake is associated with increased disease activity in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2015;86(1):26‐31. [DOI] [PubMed] [Google Scholar]
- 169. Fitzgerald KC, Munger KL, Hartung HP, et al. Sodium intake and multiple sclerosis activity and progression in BENEFIT. Ann Neurol. 2017;82(1):20‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Cortese M, Yuan C, Chitnis T, Ascherio A, Munger KL. No association between dietary sodium intake and the risk of multiple sclerosis. Neurology. 2017;89(13):1322‐1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. McDonald J, Graves J, Waldman A, et al. A case‐control study of dietary salt intake in pediatric‐onset multiple sclerosis. Mult Scler Relat Disord. 2016;6:87‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Nourbakhsh B, Graves J, Casper TC, et al. Dietary salt intake and time to relapse in paediatric multiple sclerosis. J Neurol Neurosurg Psychiatry. 2016;87(12):1350‐1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Khalili H, Malik S, Ananthakrishnan AN, et al. Identification and characterization of a novel association between dietary potassium and risk of Crohn's disease and ulcerative colitis. Front Immunol. 2016;7:554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Scrivo R, Massaro L, Barbati C, et al. The role of dietary sodium intake on the modulation of T helper 17 cells and regulatory T cells in patients with rheumatoid arthritis and systemic lupus erythematosus. PLoS One. 2017;12(9):e0184449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Sundström B, Johansson I, Rantapää‐Dahlqvist S. Interaction between dietary sodium and smoking increases the risk for rheumatoid arthritis: results from a nested case‐control study. Rheumatology. 2015;54(3):487‐493. [DOI] [PubMed] [Google Scholar]
- 176. Jiang X, Sundström B, Alfredsson L, Klareskog L, Rantapää‐Dahlqvist S, Bengtsson C. High sodium chloride consumption enhances the effects of smoking but does not interact with SGK1 polymorphisms in the development of ACPA‐positive status in patients with RA. Ann Rheum Dis. 2016;75(5):943‐946. [DOI] [PubMed] [Google Scholar]
- 177. Salgado E, Bes‐Rastrollo M, de Irala J, Carmona L, Gómez‐Reino JJ. High sodium intake is associated with self‐reported rheumatoid arthritis: a cross sectional and Case control analysis within the SUN cohort. Medicine. 2015;94(37):e924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Minamino H, Katsushima M, Hashimoto M, et al. Urinary sodium‐to‐potassium ratio associates with hypertension and current disease activity in patients with rheumatoid arthritis: a cross‐sectional study. Arthrit Res Ther. 2021;23(1):96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Marouen S, du Cailar G, Audo R, et al. Sodium excretion is higher in patients with rheumatoid arthritis than in matched controls. PLoS One. 2017;12(10):e0186157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Wu B, Yang D, Yang S, Zhang G. Dietary salt intake and gastric cancer risk: a systematic review and meta‐analysis. Front Nutr. 2021;8:801228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Banda KJ, Chiu HY, Hu SH, Yeh HC, Lin KC, Huang HC. Associations of dietary carbohydrate and salt consumption with esophageal cancer risk: a systematic review and meta‐analysis of observational studies. Nutr Rev. 2020;78(8):688‐698. [DOI] [PubMed] [Google Scholar]
- 182. Hu J, La Vecchia C, Morrison H, Negri E, Mery L. Salt, processed meat and the risk of cancer. Eur J Cancer Prev. 2011;20(2):132‐139. [DOI] [PubMed] [Google Scholar]
- 183. Deckers IA, van den Brandt PA, van Engeland M, et al. Long‐term dietary sodium, potassium and fluid intake; exploring potential novel risk factors for renal cell cancer in The Netherlands cohort study on diet and cancer. Br J Cancer. 2014;110(3):797‐801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Ghadirian P, Baillargeon J, Simard A, Perret C. Food habits and pancreatic cancer: a case‐control study of the francophone community in Montreal, Canada. Cancer Epidemiol Biomarkers Prev. 1995;4(8):895‐899. [PubMed] [Google Scholar]
- 185. Li XY, Cai XL, Bian PD, Hu LR. High salt intake and stroke: meta‐analysis of the epidemiologic evidence. CNS Neurosci Ther. 2012;18(8):691‐701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Mohan D, Yap KH, Reidpath D, et al. Link between dietary sodium intake, cognitive function, and dementia risk in middle‐aged and older adults: a systematic review. J Alzheimers Dis. 2020;76(4):1347‐1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. de Vries LV, Dobrowolski LC, van den Bosch JJ, et al. Effects of dietary sodium restriction in kidney transplant recipients treated with renin‐angiotensin‐aldosterone system blockade: a randomized clinical trial. Am J Kidney Dis. 2016;67(6):936‐944. [DOI] [PubMed] [Google Scholar]
- 188. Keven K, Yalçin S, Canbakan B, et al. The impact of daily sodium intake on posttransplant hypertension in kidney allograft recipients. Transplant Proc. 2006;38(5):1323‐1326. [DOI] [PubMed] [Google Scholar]
- 189. van den Berg E, Geleijnse JM, Brink EJ, et al. Sodium intake and blood pressure in renal transplant recipients. Nephrol Dial Transplant. 2012;27(8):3352‐3359. [DOI] [PubMed] [Google Scholar]
- 190. Rodrigo E, Monfá E, Albines Z, et al. Sodium excretion pattern at 1 year after kidney transplantation and high blood pressure. Ann Transplant. 2015;20:569‐575. [DOI] [PubMed] [Google Scholar]
- 191. Moeller T, Buhl M, Schorr U, Distler A, Sharma AM. Salt intake and hypertension in renal transplant patients. Clin Nephrol. 2000;53(3):159‐163. [PubMed] [Google Scholar]
- 192. Fatahi S, Namazi N, Larijani B, Azadbakht L. The association of dietary and urinary sodium with bone mineral density and risk of osteoporosis: a systematic review and meta‐analysis. J Am Coll Nutr. 2018;37(6):522‐532. [DOI] [PubMed] [Google Scholar]
- 193. Neal B, Wu Y, Feng X, et al. Effect of salt substitution on cardiovascular events and death. New Engl J Med. 2021;385(12):1067‐1077. [DOI] [PubMed] [Google Scholar]
- 194. Chen L, He FJ, Dong Y, et al. Modest sodium reduction increases circulating short‐chain fatty acids in untreated Hypertensives: a randomized, double‐blind, placebo‐controlled trial. Hypertension. 2020;76(1):73‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Bier A, Braun T, Khasbab R, et al. A high salt diet modulates the gut microbiota and short chain fatty acids production in a salt‐sensitive hypertension rat model. Nutrients. 2018;10(9):1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Luu M, Visekruna A. Short‐chain fatty acids: bacterial messengers modulating the immunometabolism of T cells. Eur J Immunol. 2019;49(6):842‐848. [DOI] [PubMed] [Google Scholar]
- 197. Machnik A, Dahlmann A, Kopp C, et al. Mononuclear phagocyte system depletion blocks interstitial tonicity‐responsive enhancer binding protein/vascular endothelial growth factor C expression and induces salt‐sensitive hypertension in rats. Hypertension. 2010;55(3):755‐761. [DOI] [PubMed] [Google Scholar]
- 198. Wiig H, Schröder A, Neuhofer W, et al. Immune cells control skin lymphatic electrolyte homeostasis and blood pressure. J Clin Invest. 2013;123(7):2803‐2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372(9648):1502‐1517. [DOI] [PubMed] [Google Scholar]
- 200. Faber H, Kurtoic D, Krishnamoorthy G, et al. Gene expression in spontaneous experimental autoimmune encephalomyelitis is linked to human multiple sclerosis risk genes. Front Immunol. 2020;11:2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Deng Z, Zhou L, Wang Y, et al. Astrocyte‐derived VEGF increases cerebral microvascular permeability under high salt conditions. Aging. 2020;12(12):11781‐11793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Appel LJ, Foti K. Extreme variability in urinary sodium excretion: time to stop use of spot urines to predict clinical outcomes. Hypertension. 2021;78(5):1637‐1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Judge C, Narula S, Mente A, Smyth A, Yusuf S, O'Donnell MJ. Measuring sodium intake: research and clinical applications. J Hypertens. 2021;39(12):2344‐2352. [DOI] [PubMed] [Google Scholar]
- 204. Glassner KL, Abraham BP, Quigley EMM. The microbiome and inflammatory bowel disease. J Allergy Clin Immunol. 2020;145(1):16‐27. [DOI] [PubMed] [Google Scholar]
- 205. Ananthakrishnan AN, Bernstein CN, Iliopoulos D, et al. Environmental triggers in IBD: a review of progress and evidence. Nat Rev Gastroenterol Hepatol. 2018;15(1):39‐49. [DOI] [PubMed] [Google Scholar]
- 206. D'Cruz DP, Khamashta MA, Hughes GR. Systemic lupus erythematosus. Lancet. 2007;369(9561):587‐596. [DOI] [PubMed] [Google Scholar]
- 207. Anders HJ, Saxena R, Zhao MH, Parodis I, Salmon JE, Mohan C. Lupus nephritis. Nat Rev Dis Primers. 2020;6(1):7. [DOI] [PubMed] [Google Scholar]
- 208. Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet. 2016;388(10055):2023‐2038. [DOI] [PubMed] [Google Scholar]
- 209. Leslie TK, James AD, Zaccagna F, et al. Sodium homeostasis in the tumour microenvironment. Biochim Biophys Acta Rev Cancer. 2019;1872(2):188304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Xu Y, Wang W, Wang M, et al. High salt intake attenuates breast cancer metastasis to lung. J Agric Food Chem. 2018;66(13):3386‐3392. [DOI] [PubMed] [Google Scholar]
- 211. Hwang S, Yi HC, Hwang S, Jo M, Rhee KJ. Dietary salt administration decreases Enterotoxigenic Bacteroides fragilis (ETBF)‐promoted tumorigenesis via inhibition of colonic inflammation. Int J Mol Sci. 2020;21(21):8034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Allu AS, Tiriveedhi V. Cancer salt nostalgia. Cell. 2021;10(6):1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Amara S, Ivy MT, Myles EL, Tiriveedhi V. Sodium channel γENaC mediates IL‐17 synergized high salt induced inflammatory stress in breast cancer cells. Cell Immunol. 2016;302:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Babaer D, Amara S, Ivy M, et al. High salt induces P‐glycoprotein mediated treatment resistance in breast cancer cells through store operated calcium influx. Oncotarget. 2018;9(38):25193‐25205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Amara S, Zheng M, Tiriveedhi V. Oleanolic acid inhibits high salt‐induced exaggeration of Warburg‐like metabolism in breast cancer cells. Cell Biochem Biophys. 2016;74(3):427‐434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Wang X, Liu X, Jia Z, Zhang Y, Wang S, Zhang H. Evaluation of the effects of different dietary patterns on breast cancer: monitoring circulating tumor cells. Foods. 2021;10(9):2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Barthels D, Das H. Current advances in ischemic stroke research and therapies. Biochim Biophys Acta Mol Basis Dis. 2020;1866(4):165260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Schmidt‐Pogoda A, Strecker JK, Liebmann M, et al. Dietary salt promotes ischemic brain injury and is associated with parenchymal migrasome formation. PLoS One. 2018;13(12):e0209871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Sumiyoshi M, Kitazato KT, Yagi K, et al. The accumulation of brain water‐free sodium is associated with ischemic damage independent of the blood pressure in female rats. Brain Res. 2015;1616:37‐44. [DOI] [PubMed] [Google Scholar]
- 220. Deguil J, Bordet R. Contributions of animal models of cognitive disorders to neuropsychopharmacology. Therapie. 2021;76(2):87‐99. [DOI] [PubMed] [Google Scholar]
- 221. Tiyasatkulkovit W, Aksornthong S, Adulyaritthikul P, et al. Excessive salt consumption causes systemic calcium mishandling and worsens microarchitecture and strength of long bones in rats. Sci Rep. 2021;11(1):1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Morris MJ, Na ES, Johnson AK. Salt craving: the psychobiology of pathogenic sodium intake. Physiol Behav. 2008;94(5):709‐721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Pfortmueller CA, Schefold JC. Hypertonic saline in critical illness ‐ a systematic review. J Crit Care. 2017;42:168‐177. [DOI] [PubMed] [Google Scholar]
- 224. Poli‐de‐Figueiredo LF, Cruz RJ Jr, Sannomiya P, Rocha ESM. Mechanisms of action of hypertonic saline resuscitation in severe sepsis and septic shock. Endocr Metab Immune Disord Drug Targets. 2006;6(2):201‐206. [DOI] [PubMed] [Google Scholar]
- 225. Ropper AH. Hyperosmolar therapy for raised intracranial pressure. New Engl J Med. 2012;367(8):746‐752. [DOI] [PubMed] [Google Scholar]
- 226. Elborn JS. Cystic fibrosis. Lancet. 2016;388(10059):2519‐2531. [DOI] [PubMed] [Google Scholar]
- 227. Máiz Carro L, Martínez‐García MA. Nebulized hypertonic saline in noncystic fibrosis bronchiectasis: a comprehensive review. Ther Adv Respir Dis. 2019;13:1753466619866102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Florin TA, Plint AC, Zorc JJ. Viral bronchiolitis. Lancet. 2017;389(10065):211‐224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Donaldson SH, Bennett WD, Zeman KL, Knowles MR, Tarran R, Boucher RC. Mucus clearance and lung function in cystic fibrosis with hypertonic saline. New Engl J Med. 2006;354(3):241‐250. [DOI] [PubMed] [Google Scholar]
- 230. Elkins MR, Robinson M, Rose BR, et al. A controlled trial of long‐term inhaled hypertonic saline in patients with cystic fibrosis. New Engl J Med. 2006;354(3):229‐240. [DOI] [PubMed] [Google Scholar]
- 231. Anuradha K, Gunathilaka PKG, Wickramasinghe VP. Effectiveness of hypertonic saline nebulization in airway clearance in children with non‐cystic fibrosis bronchiectasis: a randomized control trial. Pediatr Pulmonol. 2021;56(2):509‐515. [DOI] [PubMed] [Google Scholar]
- 232. Chong LY, Head K, Hopkins C, et al. Saline irrigation for chronic rhinosinusitis. Cochrane Database Syst Rev. 2016;4(4):Cd011995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Yan W, Huang L. The effects of salt on the physicochemical properties and immunogenicity of protein based vaccine formulated in cationic liposome. Int J Pharm. 2009;368(1–2):56‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Tiriveedhi V, Ivy MT, Myles EL, Zent R, Rathmell JC, Titze J. Ex vivo high salt activated tumor‐primed CD4+T lymphocytes exert a potent anti‐cancer response. Cancers. 2021;13(7):1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


