Abstract
Chitinase-3-like protein 1 (CHI3L1/YKL-40) has long been known as a biomarker for early detection of neuroinflammation and disease diagnosis of Alzheimer’s disease (AD). In the brain, CHI3L1 is primarily provided by astrocytes and heralds the reactive, neurotoxic state triggered by inflammation and other stress signals. However, how CHI3L1 acts in neuroinflammation or how it contributes to AD and relevant neurodegenerative conditions remains unknown. In peripheral tissues, our group and others have uncovered that CHI3L1 is a master regulator for a wide range of injury and repair events, including the innate immunity pathway that resembles the neuroinflammation process governed by microglia and astrocytes. Based on assessment of current knowledge regarding CHI3L1 biology, we hypothesize that CHI3L1 functions as a signaling molecule mediating distinct neuroinflammatory responses in brain cells and dysfunctions to precipitate the neurodegeneration. We also recommend future research directions to validate such assertions for better understanding of disease mechanism.
Keywords: CHI3L1/YKL-40, biomarker, neuroinflammation, neurodegeneration, Alzheimer’s disease
1. Narrative
Does the common phrase “use it or lose it” apply to the human brain? The brain is constantly in use to compute a huge amount of information about the body’s condition and surroundings. Such information is processed and stored in the form of electrical signals transmitted through millions of circuits made of neurons. Neurons connect with one another through specialized cellular junctions called synapses in a highly dynamic fashion. New synapses are formed or the connectivity of existing synapses in use gets strengthened upon the acquirement of new memory and experiences. Conversely, loss of synapses underlies cognitive decline along brain aging and is exacerbated in degenerative disorders such as Alzheimer’s disease (AD) – a medical crisis that affects nearly one third of American elders.
Under the pathological examination of donated brain tissues, AD is characterized by the abnormal accumulation of two hallmark proteins: phospho-tau inside the neurons (a.k.a. neurofibrillary tangles) and amyloid-beta (Aβ) between brain cells (a.k.a. amyloid plaques). These hallmark proteins have long been the focus of AD research and believed to cause the loss of synapses and memory impairment. However, after decades of intense investigation, the causative role of these proteins in AD has been contested, with recent studies shifting toward identifying other factors and processes implicated in the development of AD. Recent evidence frames neuroinflammation, the inflammatory processes taking place inside the central nervous system (CNS), as one such process that does not simply respond to hallmark pathologies, but is itself pathogenic. Neuroinflammation is important for neuronal and brain homeostasis in normal, healthy conditions; however, neuroinflammation can go awry, where microglia and astrocytes transform into reactive, toxic states which can release molecules that damage neurons. The combination of glial toxicity, coupled with decreased neuronal support, implicates neuroinflammation in neurodegenerative disorders, including AD. This is further supported by the fact that many AD risk genes are both expressed in glia and are involved in inflammatory mechanisms. Despite the recent focus on the involvement of neuroinflammation in AD pathogenesis, the mechanisms, signaling pathways, and the totality of factors triggered by inflammation leading to massive loss of synapses, neuronal dysfunction, and neurodegeneration in AD are still not well understood.
The challenge in identifying abnormal early mechanisms that trigger neuroinflammation and the neurotoxic background to support neurodegeneration in AD has encouraged the study of biomarkers to monitor AD pathology and progression. The cerebrospinal fluid (CSF) can be analyzed for biomarkers, molecules that may change depending on certain conditions or diseases, which serve as read-outs for the degree of neurodegeneration and disease stage. Here, we focus on a recently characterized biomarker, Chitinase-3-like protein 1 (CHI3L1), a protein mainly expressed by astrocytes in the brain. Elevated levels of CHI3L1 in the CSF are able to be detected in the earliest stages of AD, even before the development of cognitive symptoms. Increasing on a linear trend, CHI3L1 levels predict the rate of cognitive decline and risk of AD development. Beyond AD, elevated CHI3L1 CSF levels are associated with other neurodegenerative disorders in a progression-dependent manner as well.
Most CHI3L1 research has been conducted in peripheral systems outside the brain, particularly in the lungs, where it has been characterized as an inflammatory molecule. There have been several receptors identified in the periphery that CHI3L1 is able to interact with, initiating signaling pathways that can affect a wide variety of functions. CHI3L1 is able to increase cell division and survival, inhibit cell death, stimulate tissue remodeling, and activate immune cells, among other functions. Due to its implication in innate and adaptive immunity, it has been determined that CHI3L1 is an important player in injury and repair responses in the periphery. However, beyond its utility as an AD biomarker and expression by astrocytes, little is known about the role of CHI3L1 in the brain.
Recently, there have been some studies that attempt to determine the cell-specific effect of CHI3L1 in the CNS. Not only is CHI3L1 expressed in astrocytes, but its expression is increased in reactive, neurotoxic astrocytes, which are induced by microglia. Beyond expression, CHI3L1 has been shown to affect individual CNS cells as it is able to modulate microglia secretions and activation states, increase neuronal death, and modify oligodendrocyte precursor cell division. The expression of CHI3L1 and its effect on inflammatory cells in the brain supports an effector role in the neuroinflammatory process. There have also been recent, limited studies regarding the role of CHI3L1 in neuroinflammation and AD which claim that CHI3L1 may contribute to AD progression by altering the neuroinflammatory process and amyloid burden. However, there has been some contention on the effect of CHI3L1 and its mechanism of action pertaining to the CNS as differing studies describe some opposing results. Further study is needed to clarify the effect of CHI3L1 on the individual CNS cells, and the subsequent effects in the context of AD.
Based on the evidence in the periphery and these new findings in the CNS, we hypothesize that CHI3L1 functions as a neuroinflammatory signaling molecule to regulate inflammatory responses in a cell type-specific manner, and that such signaling function can derail to instigate the neurodegeneration process. We propose future research needed to address this hypothesis, with experiments focusing on identifying the functional outputs of CHI3L1 in neuroinflammation and determining the effects of CHI3L1 manipulations in pro-inflammatory context relevant to AD in vitro and in vivo. Future research will advance our knowledge of the role of CHI3L1 in AD pathogenesis and perhaps lead to the development of interventions, prevention strategies, or in other medical advances to combat AD and potentially other neurodegenerative conditions. With these potential advances, ideally, one can continuously “use” their brain and never “lose it.”
2. Introduction
The attention given to hallmark pathologies as causative agents for Alzheimer’s Disease (AD), namely extracellular amyloid plaques and intraneuronal neurofibrillary tangles (NFTs), comprised of amyloid beta (Aβ) and phospho-tau, respectively, has framed the inflammatory response to these molecules as secondary. However, recent evidence suggests otherwise, implicating neuroinflammation as an early factor in AD progression. Neuroinflammation is a process mainly involving microglia and astrocytes and is necessary for healthy brain function. However, in many neurological disorders such as AD there is an uncontrolled immune imbalance leading to abnormalities including increased microglial and astroglial activation, enhanced inflammatory cytokines and oxidative stress, vascular impairment and compromised integrity of blood-brain barrier (BBB), decreased neurogenesis, loss of synapses and eventually neuronal death; these abnormalities have been implicated in AD because they have detrimental effects on neuroprotection and cause neuroinflammation [1-4]. Despite the prevalence of these events in AD, the questions still remain: what causes this “uncontrolled immune imbalance”, is neuroinflammation a direct causative factor in AD pathogenesis, and, if so, what is the precise mechanism underlying neuroinflammation and neurodegeneration in AD?
The inflammatory molecule Chitinase-3-like protein 1 (CHI3L1) has emerged to be a recent focus in the field of AD. CHI3L1, also called YKL-40 in humans and BRP-39 in mice, is a well-characterized inflammatory molecule and well known as a powerful biomarker that can be detected at the earliest stage of pathogenesis and help differentiate AD from other forms of dementia [5-7]. Cerebrospinal fluid (CSF) levels of CHI3L1 predict the severity of clinical symptoms and pathological features and can reliably distinguish AD from other forms of dementia [5-7]. CHI3L1 is a secreted glycoprotein that belongs to the diverse glycoside hydrolase family 18 [8]. While it preserves their chitin binding capacity, CHI3L1 lacks chitinase activity owing to loss-of-function mutations of its chitinase domain [9, 10].
CHI3L1 is indeed most studied in the field of pulmonary inflammation, injury repair and tissue remodeling, e.g. asthma and lung fibrosis, and its multifaceted signaling functions through a specific receptor system have been pioneered by us and documented by others in both innate and adaptive immunity [11-14]. It is now clear that CHI3L1 is a master regulator for a wide range of injury and repair responses, including the innate immunity pathway that resembles the neuroinflammation process initiated by microglia and coordinated by astrocytes (Figure 1) [9, 10, 12-16]. Nevertheless, how CHI3L1 operates in neuroinflammation and AD pathogenesis remains to be elucidated. Based on the known function of CHI3L1 in peripheral tissues and recent findings in the central nervous system (CNS), we propose here the potential role of CHI3L1 in neuroinflammation and neurodegeneration, going beyond merely a biomarker. It is our hypothesis that CHI3L1, secreted by astrocytes upon microglia reactivation, functions as an inflammatory signaling molecule to mediate inflammatory responses most likely in a cell type-specific fashion, and that such signaling function can derail to instigate the neurodegeneration process. Finally, here we propose some actionable approaches to test these claims. We believe an expansion of this research topic will ultimately inspire new therapeutics targeting neuroinflammation for AD and relevant neurodegenerative disorders.
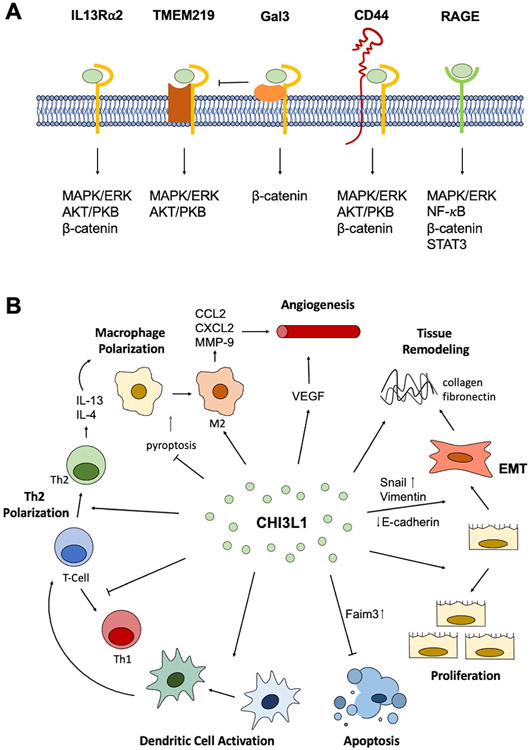
Figure 1. CHI3L1 regulation and function in the periphery.
(A) The known CHI3L1 receptors and the signaling pathways activated by CHI3L1-receptor binding. (B) The main functions of CHI3L1 identified in the periphery include increased proliferation, cell survival, dendritic cell activation, Th2 polarization, M2 macrophage polarization, angiogenesis, tissue remodeling, and epithelial-mesenchymal transition (EMT).
3. CHI3L1 as a Powerful Biomarker in Neurodegenerative Disease
Chitins and chitinase family members have demonstrated utility as biomarkers for variety of inflammation-prone brain diseases, including AD. Not only are chitin levels elevated in the CNS, CSF, and plasma in AD patients, but chitin has also been implicated in influencing Aβ accumulation and AD pathogenesis [17]. Similarly, the levels of chitinase family members, including chitotriosidase, acidic mammalian chitinase, chitinase 3-like protein 2, chitinase domain–containing 1, and CHI3L1, are increased in AD brains versus healthy controls [18]. In AD, these chitinases were increased at both the protein and RNA levels [18]. While increases in the levels of chitins and several chitinase family members have been documented, CHI3L1 has been the most frequently studied one for its reliability and robustness in serving as an AD biomarker. Scores of groups have long found elevated CHI3L1 levels in the CSF of AD patients (Table 1, a summary of most recent results). CHI3L1 is a secreted chitinase-dead, catalytically inactive glycoprotein expressed and secreted mainly by astrocytes in the brain (Figure 2) [8, 14, 19]. Longitudinal studies suggest a correlation between higher levels of CHI3L1 and the increased rate of cognitive decline as well as the increased risk of AD development [6]. CHI3L1’s promise as an AD biomarker relates to its increasing levels in the CSF as a function of AD progression.
Table 1.
A summary of recent studies (not included in previous review articles) consistently showing elevated levels of CHI3L1 in CSF samples from AD patients.
| Study | Healthy Controls (HC) | Alzheimer's Disease (AD) | HC (n) | AD (n) |
|---|---|---|---|---|
| Baldacci et al., 2017 [37] | 98 ng/mL | 146 ng/mL | 21 | 35 |
| Gispert et al., 2017 [38] | 271.36 ± 104.49 ng/mL (e4−); 252.65 ± 32.67 ng/mL (e4+) | 327.79 ± 133.27 ng/mL (e4−); 339.96 ± 107.99 ng/mL (e4+) | 44 (e4−); 5 (e4+) | 8 (e4−); 7 (e4+) |
| Llorens et al., 2017 (Cohort 1) [33] | 250 ± 77 pg/mL | 386 ± 221 pg/mL | 62 | 84 |
| Llorens et al., 2017 (Cohort 2) [33] | 226 ± 82 pg/mL | 311 ± 113 pg/mL | 35 | 28 |
| Muszynski et al.,2017 [39] | 291 ng/mL | 387 ng/mL | 23 | 45 |
| Paterson et al., 2018 [40] | 111 ng/mL | 163 ng/mL | 29 | 114 |
| Sutphen et al., 2018 [41] | 384.1 ± 20.08 ng/mL (Ab−), 399.6 ± 19.4 ng/mL (Ab+) | 471.9 ± 41.86 ng/mL | 35 (Ab−); 21 (Ab+) | 16 |
| Zhang et al., 2018 [22] | 397.2 ± 25.7 ng/mL | 471.9 ± 39.5 ng/mL | 32 | 18 |
| Dai & Gong, 2019 [26] | 87.710 ± 0.928 ng/mL | 382.700 ± 6.267 ng/mL | 184 | 166 |
| Bos et al., 2019 [42] | 127 ± 45.4 ng/mL (Ab−); 175.1 ± 63.6 ng/mL (Ab+) | 184.2 ± 64.6 ng/mL (Ab−); 193.6 ± 68.7 ng/mL (Ab+) | 95 (Ab−); 45 (Ab+) | 23 (Ab−); 157 (Ab+) |
| Morenas-Rodriguez et al., 2019 [43] | 238.8 ± 49.2 ng/mL | 295.3 ± 54.1 ng/mL | 44 | 50 |
| Nordengen et al., 2019 [44] | 145 ± 46 ng/mL | 221 ± 70 ng/mL | 36 | 27 |
| Villar-Pique et al., 2019 [45] | 84 ± 84 ng/mL | 133 ± 110 ng/mL | 70 | 50 |
| Abu-Rumeileh et al., 2020 [46] | 145 ng/mL | 240 ng/mL | 40 | 40 |
| Antonell et al., 2020 [47] | 217.23 ng/mL | 305.12 ng/mL | 50 | 108 |
| Wang et al., 2020 [48] | 335.0 ng/mL | 467.1 ng/mL | 35 | 11 |
| Woollacott et al., 2020 [30] | 108 ± 30 ng/mL | 163 ± 67 ng/mL | 18 | 15 |
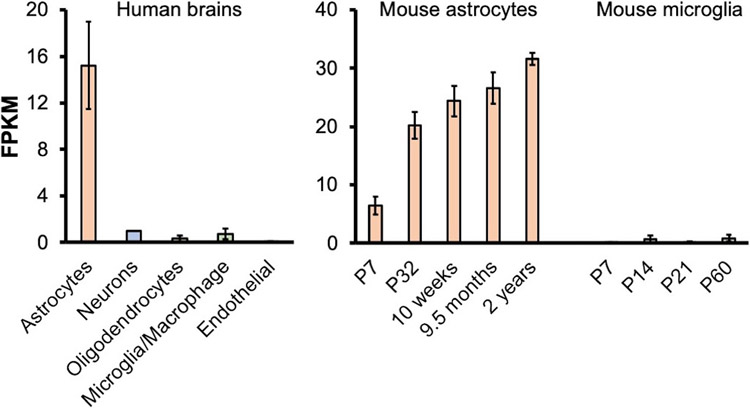
Figure 2. CHI3L1 expression in CNS cells.
CHI3L1 expression in living tissues acutely prepared from human brains (left) [34] and in mouse astrocytes [35] and microglia [36] in vivo at different ages (right). The mean and standard error of the mean (Mean ± SEM) are shown. RNA-Seq datasets from Dr. Ben Barres group, https://www.brainrnaseq.org/.
Increased CHI3L1 levels has been implicated in the reduction of cortical volume in AD, a function of neurodegeneration. High CSF levels of CHI3L1 correlate with cortical thinning in the precuneus and superior parietal cortex, cortical areas involved in episodic and working memory, as well as thinning of the middle and inferior temporal areas [6, 20]. The latter finding is noteworthy, as thinning in these areas occurs first in the spatial distribution of neurodegeneration in AD [21]. Additionally, a longitudinal study showed an association between CHI3L1 expression levels and hippocampal atrophy, an early event in AD progression [22]. Hippocampal CHI3L1 levels were significantly higher in AD brains compared to healthy controls [18]. In addition to the hippocampus, other brain regions vulnerable to AD and aging, including the entorhinal cortex, primary visual cortex, posterior cingulate cortex, superior frontal gyrus, and the middle temporal gyrus, all had significantly increased levels of CHI3L1 in AD compared to age-matched controls [18].
While CHI3L1 levels are increased in a variety of brain regions in AD, CHI3L1-expressing astrocyte number is significantly increased in the cortical layers and white matter in AD [23]. In the frontal cortex, CHI3L1 protein levels showed a positive trend along with disease progression, in line with the enhanced reactivation of astrocytes that express CHI3L1 [23]. Despite this trend, there was a significant increase in CHI3L1-expressing astrocytes in severe AD only [23]. CHI3L1, however, can exist in its secreted form, which is present early in disease state. It may take time for CHI3L1 to accumulate intracellularly, which may explain why this study shows that increased CHI3L1-expressing astrocytes are not apparent until severe stages. The increased CHI3L1-expressing cells in the white matter were also strongly correlated with Iba1-expressing microglia numbers [23]. Both CHI3L1-postive astrocytes and Iba1-positive microglia numbers negatively correlate with perceptual speed and episodic memory in early AD, suggesting an association between white matter degeneration and early memory deficits in AD [23]. Intriguingly, in the same study, researchers also found a significant correlation between CHI3L1 and neuronal pentraxin II (NPTX2) levels. NPTX2 is a member of the pentraxin family and has been well-characterized to govern glutamatergic synaptic formation and plasticity. Recently, NPTX2 has been implicated in neuroinflammation regulation as well as AD pathogenesis [23, 24]. CHI3L1 and NPTX2 expression are not only correlated, but were the only consistently significant biomarkers that best predicted AD neuropathology over 24 months, as determined via memory performance, medial temporal lobe volume, and relation to inflammation [24]. The levels of NPTX2 are correlated with cognitive performance and hippocampal volume, implicating NPTX2 in neurodegeneration [25]. Conversely to CHI3L1, there is a reduction of NPTX2 in AD brains and CSF [24, 25]. This reduction of NPTX2 in AD was witnessed at both protein and RNA levels. Reduced NPTX2 has been further implicated in AD because NPTX2 knock-out mouse models with amyloidosis have disrupted hippocampal rhythmicity and excitatory synapse function on parvalbumin interneurons, which disrupts hippocampal homeostasis resulting in brain activity that is reportedly increased in mild cognitive impairment and AD [25].
CHI3L1 has been studied in the CSF in relation to other hallmark AD biomarkers as well. CHI3L1 CSF levels correlate with early neurodegeneration in individuals with low CSF levels of Aβ, suggesting that CHI3L1 precedes amyloid pathology [6, 20]. While there is a stronger correlation between tau and CHI3L1 in Aβ-positive individuals, Aβ-negative individuals also display a positive correlation between CHI3L1 CSF levels and CSF t-tau [6]. This further suggests that CHI3L1 is elevated in conditions of neuronal loss prior to Aβ deposition, placing CHI3L1, and by extension neuroinflammation, to precede Aβ pathology. Additionally, carriers of the APOE-ε4 allele, the most significant genetic risk allele for late-onset AD, show a correlation between age and CHI3L1 and Aβ42 CSF levels [20]. Taken together, this shows that CHI3L1 CSF levels increase in proportion to AD progression, from neurodegeneration to cognitive decline, making CHI3L1 a powerful AD biomarker.
Beyond expression and CSF levels, CHI3L1 polymorphisms have also been implicated in AD development. In a study of 166 AD patients in the Chinese Han population, two single-nucleotide polymorphisms (SNPs) in the Chi3l1 gene were found to modulate the risk of AD development [26]. Notably, the G allele of rs4950928 C>G appears to be protective of AD pathology while the T allele of rs10399931 C>T in the Chi3l1 gene confers risk. In addition, the protective and risk allele also varied in CHI3L1 plasma concentration, with patients carrying the risk allele showing greater levels of CHI3L1 in the CSF [26]. These recent findings highlight the importance of elucidating the role CHI3L1 and its polymorphisms play in the pathogenesis of AD.
In addition to AD, CHI3L1 expression is associated with other neurodegenerative disorders. Elevated CHI3L1 CSF levels have been found in Amyotrophic Lateral Sclerosis (ALS), Frontotemporal Dementia (FTD), Parkinson’s Disease (PD), and Multiple Sclerosis (MS) in a progression-dependent manner [27-30]. In the early stages of MS, levels of CHI3L1 correlate with worse performance of executive functions [28, 31]. Similarly, in PD, the increase in CHI3L1 CSF levels correlates with faster cognitive decline [27]. In ALS, fold increases in CHI3L1-positive cells, mainly GFAP expressing astrocytes, were observed in motor cortex white matter, with expression being negatively correlated with survival time [29, 32]. CHI3L1 was also detected in the cerebellum and frontal cortex of patients with sporadic Creutzfeldt-Jakob Disease, a neurodegenerative disorder that exhibits a very robust inflammatory response [33]. It is evident that CHI3L1 has utility as a biomarker in neurodegenerative disease, has an emerging role in AD pathogenesis, and is related to inflammation; however, how CHI3L1 functions and dysfunctions in the brain remains to be elucidated.
4. Biological and Pathological Function of CHI3L1
4.1. CHI3L1 Expression & Regulation
While largely uncharacterized in the brain, CHI3L1 expression, regulation, and function have been well defined in the periphery. Beyond CNS expression in astrocytes (Figure 2), CHI3L1 is expressed in a variety of peripheral cells including macrophages, dendritic cells, neutrophils, CD4 and CD8 T cells, chondrocytes, synovial cells, fibroblasts, osteocytes, epithelial cells, endothelial cells, smooth muscle cells, hepatic stellate cells, Kupffer cells, and tumor cells [12, 49-55]. CHI3L1 expression and secretion by these specific cell types is regulated by a variety of factors, of which cytokines comprise the largest group. Through its expression in a variety of cells, particularly immune cells, as well as its regulation by cytokines and other inflammatory factors, it is clear that CHI3L1 plays a role in immune response and inflammation.
4.2. Identified CHI3L1 Receptors
Currently, there have been 6 identified CHI3L1 receptors: interleukin-13 receptor subunit alpha-2 (IL-13Rα2), transmembrane protein 219 (TMEM219), galectin-3 (Gal-3), CD44, chemo-attractant receptor-homologous 2 (CRTH2), and receptor for advanced glycation end product (RAGE) (Figure 1A).
CHI3L1 binds to IL-13Rα2 and, along with IL-13, forms a multimeric complex. There is no competition in binding between CHI3L1 and IL-13 nor is there an abrogative effect on each other’s signaling because they bind different locations on the receptor [9]. Upon binding to IL-13Rα2, CHI3L1 was shown to activate MAPK/ERK, AKT/PKB, and Wnt/β-catenin signaling pathways [9]. The activation of these signaling pathways via CHI3L1-IL-13Rα2 binding resulted in the regulation of oxidant injury, apoptosis, pyroptosis, inflammasome activation, antibacterial responses, melanoma metastasis, and TGF- β1 production [9].
TMEM219 physically interacts with IL-13Rα2-CHI3L1 complex and plays a critical role in subsequent signaling. The complexing of TMEM219 with IL-13Rα2 and CHI3L1 was shown to augment IL-13 binding to IL-13Rα2 [56]. Experimentally silencing TMEM219 decreased CHI3L1-induced MAPK/ERK and AKT/PKB activation, but had no effect on Wnt/β-catenin signaling indicating that TMEM219 interaction with IL-13Rα2 plays a critical role in subsequent MAPK/ERK and AKT/PKB activation but not Wnt/β-catenin signaling [56]. Due to this interaction and activation of similar pathways, TMEM219 also plays a role in the regulation of apoptosis, oxidant injury, melanoma metastasis, and TGF- β1 induction.
The CHI3L1 receptor Gal-3 has also demonstrated to physically interact with IL-13Rα2, where it competes with TMEM219 for IL-13Rα2 binding [55]. Unlike TMEM219 which has an antiapoptotic role, Gal-3 promotes apoptosis when accumulated extracellularly; however, when expressed intracellularly, Gal-3 has been shown to influence macrophage differentiation and fibroproliferative repair [55]. Additionally, it has been demonstrated that CHI3L1 binding to the Gal-3- IL-13Rα2 complex activates Wnt/β-catenin signaling [55].
Another identified CHI3L1 receptor, CD44, also interacts and forms a complex with IL-13Rα2. The physical interaction between CD44 and IL-13Rα2 activates CHI3L1-induced MAPK/ERK, AKT/PKB, and Wnt/β-catenin signaling [57]. CD44 is a cell-surface transmembrane glycoprotein known to play a role in cell growth, survival, and differentiation, which aligns with the aforementioned processes affected by activation of the three signaling cascades [55, 57].
CRTH2 is a CHI3L1 receptor that was identified in Hermansky-Pudlak mutant cells, which have decreased membrane expression of IL-13Rα2 [55]. CHI3L1 binding to CRTH2 enhances collagen accumulation and promotes fibrotic responses [55].
While many of the identified CHI3L1 receptors interact and complex with IL-13Rα2, RAGE does not interact with IL-13Rα2 in CHI3L1 signaling responses. CHI3L1 binds surface RAGE and activates MAPK/ERK, Wnt/β-catenin, NF-κB, and STAT3 signaling which promote cell proliferation, migration, and survival [54, 58, 59]. While not directly attributed to RAGE binding, it has been shown that CHI3L1 is able to increase the production of IL-8 in a NF-κB-dependent manner, whereby CHI3L1 induces IκBα phosphorylation and degradation, which releases NF-κB from complex and allows it to activate the IL-8 gene [60]. IL-8 then stimulates cell proliferation and migration. CHI3L1-induced stimulation of the STAT3 pathway was also identified to be NF-κB-dependent [59, 61]. RAGE is currently the only known receptor to activate NF-κB, which is critical in mediating continued CHI3L1 production, suggesting a positive feedback loop where CH3L1 is able to increase its expression by activating NF-κB via RAGE binding.
4.3. Proliferation & Cell Survival
One of the consequences of CHI3L1 receptor binding, particularly to IL-13Rα2, TMEM219, CD44, and RAGE, is increased proliferation and cell survival (Figure 1B). CHI3L1 treatment or overexpression in multiple cell types, either in vitro or in vivo, has been shown to increase proliferation [52, 62-65]. The increased number of cells witnessed in these cases were in fact due to increased proliferation and not only decreased apoptosis because DNA synthesis was increased by CHI3L1 [62, 65]. The CHI3L1-induced activation of MAPK/ERK and AKT/PKB signaling are mainly responsible for the increases in proliferation. Both pathways are associated with mitogenesis control and inhibition of MAPK/ERK and AKT/PKB signaling significantly reduced proliferation [9, 52, 65]. In addition to increasing proliferation, CHI3L1 also increases cell survival by inhibiting apoptosis (Figure 1B). In vivo studies using CHI3L1 null mice have demonstrated that knocking out CHI3L1 enhances levels of the apoptotic marker annexin V, particularly in macrophages, T cells, and eosinophils [66]. Loss of CHI3L1 increased the expression of the death receptor Fas on these cells [66]. The antiapoptotic function of CHI3L1 is driven by the activation of the AKT/PKB signaling pathways as well as by increased expression of Fas apoptosis-inhibiting molecule (Faim) 3 [9, 66].
4.4. Tissue Remodeling & Angiogenesis
In addition to increased connective tissue cell proliferation, CHI3L1 is also implicated in tissue remodeling as it is expressed in tissues with high levels of remodeling activity and has the ability to interact with and bind numerous extracellular matrix (ECM) components including collagen types I through IV, hyaluronic acid, and fibronectin [51, 67]. CHI3L1 has also been shown to stimulate fibrosis and ECM gene expression (Figure 1B) [64, 68]. The expression of CHI3L1 by chondrocytes, osteocytes, synoviocytes, and fibroblasts can stimulate the synthesis of proteoglycans and increases collagen accumulation, both of which make up a large portion of the ECM [16, 52, 68].
Epithelial-mesenchymal transition (EMT), a biological process that also enhances ECM components, is regulated by CHI3L1 (Figure 1B). CHI3L1 overexpression up-regulates the mesenchymal markers N-cadherin and Vimentin as well as the EMT activators Twist, Snail, and Slug, while the epithelial marker E-cadherin was down-regulated [57, 67]. EMT progression is induced by CHI3L1 and appears to be CD44-dependent because the use of a neutralizing antibody against the CD44 receptor blocked EMT [57].
CHI3L1 promotes migration of human microvascular endothelial cells and increases vascular endothelial growth factor (VEGF), suggesting a role in angiogenesis (Figure 1B). Treatment of cells with CHI3L1 enhanced endothelial cell migration and tube formation, while CHI3L1 knockdown reduced VEGF and suppressed angiogenesis [58, 63, 64, 69]. In addition to enhanced migration of human microvascular endothelial cells to form vasculature, CHI3L1 also enhances the migration of macrophages through the ECM and increases macrophage production of matrix metalloprotinase-9 (MMP9), C-chemokine ligand 2 (CCL2), and CX motif ligand 2 (CXCL2), which are all proinflammatory and pro-invasive factors [58, 63, 70].
4.5. Macrophage Maturation & Activation
CHI3L1 is expressed in macrophages throughout differentiation, maturation, and activation, but is absent in its precursor, monocytes [8, 50, 70-72]. Similarly, CHI3L1 expression is upregulated during dendritic cell maturation and activation as well [50, 66]. While CHI3L1 aids in cell survival via increasing proliferation and decreasing apoptosis, CHI3L1 also further promotes macrophage survival by inhibiting macrophage pyroptosis. Macrophage pyroptosis is caspase-1 dependent and is therefore prevented by CHI3L1 via inhibition of caspase-1 [9, 73]. This anti-pyroptosis function was witnessed after CHI3L1-IL-13Rα2 binding, which also results in the inhibition of Nlrp3 inflammasome activation, a complex that plays a role in inflammation, immune response, and apoptosis [9, 73].
Macrophages can be activated into different polarization states, characterized as either classically activated, M1 macrophages, or alternatively activated, M2 macrophages. Treatment with CHI3L1 either does not augment or reduces the expression of markers associated with classic M1 activation, such as iNOS; however, CHI3L1 expression has still been witnessed in M1 macrophages [16, 71]. Conversely, CHI3L1 causes a significant increase in arginase-1, macrophage mannose receptor, MHC class II, YM-1, CD206, and FIZZ-1, all of which are molecules associated with alternative M2 activation (Figure 1B) [16, 66]. The CHI3L1-induced activation of both macrophages and dendritic cells suggests that CHI3L1 plays an important role in the innate immune response.
4.6. Th2 Polarization
CHI3L1 not only functions in regulation of innate immunity, but its role also extends into and affects the adaptive immune response, specifically by exerting effects on T cells. T cells express CHI3L1, which has been shown to increase T cell proliferation and activation [12, 66]. Specifically, CHI3L1 is highly expressed and activates the T-helper 2-type (Th2) state in CD4+ T cells (Figure 1B) [12, 61, 66, 74]. Similar to macrophages, CD4+ T cells activate into differing polarization phenotypes depending on the signals received. Th2 cells produce cytokines including IL-4 and IL-13, which evoke antibody responses, particularly IgE, and are typically involved in allergies and helminth infection [10, 75]. CHI3L1 levels are positively correlated with IgE levels and CHI3L1 was shown to play a role in antigen and allergen sensitization [60, 66]. IL-4 and IL-13, produced by CHI3L1-induced Th2 cells, are able to activate macrophages to an M2 phenotype, while macrophages are also able to activate CD4+ T cells via antigen presentation. The ability of these cells to be activated by CHI3L1 and each other indicates that CHI3L1 may both directly and indirectly activate these cells, thus bridging the innate and adaptive immune response. CHI3L1 not only activates the Th2 phenotype, but also inhibits Th1 polarization because null mutations of CHI3L1 resulted in Th1 differentiation [12, 66]. CHI3L1 is a negative regulator of Th1 cells and drives Th2 differentiation, making CHI3L1 an important factor in the adaptive immune response. Due to its association in both innate and adaptive immunity, CHI3L1 is thought to play a role in a variety of inflammatory diseases, affecting pathology and progression. While numerous studies support the implication of CHI3L1 in the pathogenesis of various peripheral diseases, there could be a bi-phasic effector function (protective vs provocative) of CHI3L1 depending on the levels of expression, activation status of CHI3L1, the presence of certain receptors, and disease context.
CHI3L1 has clear involvement in peripheral innate and adaptive immunity, where it has been determined that CHI3L1 is an important player in injury and repair responses. This innate immunity in the periphery resembles the neuroinflammatory process initiated by microglia and coordinated by astrocytes in the CNS. Considering the similarities between immunities, the information regarding biological and pathological functions of CHI3L1 in the periphery may be important in understanding the inflammatory mechanism in the CNS, especially in the context of AD.
5. CHI3L1 in Neuroinflammation
5.1. Glial Role in Neuroinflammation
Neuroinflammation was once regarded as merely a secondary response in the neurodegeneration process. Now, after myriad preclinical studies and a number of large genomic data analyses from AD patients and risk carriers, neuroinflammation sits at the center of AD pathogenesis [76, 77]. Neuroinflammation is mediated by microglia and astrocytes, and involves rapid and elaborate intercellular signaling, which is necessary for healthy brain function. Additionally, these inflammatory cells are responsible for synaptic pruning of neurons and neuroprotective support, thereby further maintaining normal brain function [7]. However, the neuroinflammatory process can become imbalanced and derail normal brain functions to cause neurodegeneration. Specifically, neuroinflammation is mainly driven by activated microglia which stimulate astrocytes to trigger the inflammatory cascades eventually leading to synapse loss and neuronal death [78, 79].
Both microglia and astrocyte reactivity have been classified into differing activation polarization states named M1 and M2 and A1 and A2, respectively, in analogy to the M1/M2 macrophage convention nomenclature. M1/A1 are thought to be more pro-inflammatory while M2/A2 are thought to be anti-inflammatory [4, 78-80]. Despite the persistent use of this nomenclature by some researchers, others believe this dichotomy to be an oversimplification and that it is likely intermediate activation states exist [81]. Nonetheless, while microglia have become a major focus in AD research, these A1 reactive astrocytes are also believed to underline neurodegenerative disorders, including AD, and are reported to be enriched in aged mouse brains (Figure 2) [35, 78]. A1 astrocytes have been shown to be destructive to synapses, hence believed to be neurotoxic, while A2 astrocytes seem to be protective [82-84]. Recent data shows that A1 astrocytes are induced by activated microglia, namely via the secretion of three cytokines, IL-1α, TNF-α and C1q [79, 85]. It has been shown that these A1 reactive astrocytes rapidly kill neurons and oligodendrocytes as they release neurotoxic factors and lose neuroprotective abilities, including the promotion of neuronal survival, outgrowth, synaptogenesis, and phagocytosis [35, 79, 85]. Conversely, blocking A1 astrocyte conversion by microglia is shown to be neuroprotective in models of Parkinson’s disease [86]. The rapid and dynamic interactions between microglia and astrocytes, such as the induction of neurotoxic A1 reactive astrocytes by activated microglia, are among the major drivers for pathogenesis of AD and age-related neurodegeneration, but these mechanisms are still not well understood [35, 79, 87]. Remarkably, CHI3L1 expression is robustly induced in reactivated astrocytes in vivo and in vitro by independent inflammatory stimuli, and considered to be part of the neurotoxic gene signature [78, 85].
5.2. Astrocyte Secretion of CHI3L1
In the brain, CHI3L1 is most abundantly and almost exclusively expressed in astrocytes throughout neural development, and its expression peaks in the last stage of lifespan, coinciding with the upsurge of neurotoxic A1 astrocytes and unchecked inflammatory responses in the aged brains [34-36]. Of particular relevance to AD, CHI3L1 expression is highly enriched in astrocytes and significantly elevated in the diseased human brain cortical tissues as compared to healthy controls [23, 88]. Such an expression pattern supports an effector role in the neuroinflammatory axis of microglia–astrocyte–neuron. It has been experimentally shown in vitro that CHI3L1 expression in astrocytes is increased by IL-1, IL-6, and TNF-α, as well as M1 macrophage conditioned media, while M2 macrophage conditioned media inhibited CHI3L1 transcription [89, 90]. These factors, particularly IL-1 and TNF-α, have been shown to induce A1 astrocyte reactivity, which suggests that CHI3L1 is expressed and secreted by A1 astrocytes. Additionally, the combination of A1 astrocyte induction by cytokines secreted mainly by M1 microglia and the increase in CHI3L1 expression in astrocytes by M1 macrophage conditioned media further ties A1 reactivity and CHI3L1 expression together. If CHI3L1 expression and secretion is increased in A1 astrocytes, perhaps CHI3L1 is neurotoxic because A1 astrocytes have been shown to induce neuronal death. In future AD research, it will be important to uncover a regulatory mechanism governing the microglia-induced conversion of A1 reactive astrocytes in neuroinflammation and the sequelae of impaired neuronal integrity, with a specific focus on CHI3L1 and its involvement in this process.
5.3. CHI3L1 Effect on CNS Cells
Beyond its expression by astrocytes, not much is known about CHI3L1 in the CNS. While CHI3L1 has been well-characterized in the periphery, there have been limited studies on the biological functions of CHI3L1 in the CNS or the effect of CHI3L1 on brain cells. Recent studies have emerged that attempt to define the effects of CHI3L1 on microglia, neurons, and oligodendrocytes.
While microglia are thought to be the initiators in the neuroinflammatory axis of microglia–astrocyte–neuron, microglia can be affected by inflammatory factors, including CHI3L1. It has been demonstrated that CHI3L1 is able to modulate the microglial secretome and polarization activation state in vitro and in vivo. One group studied the effects of a CHI3L1 inhibitor they generated and found that CHI3L1 inhibition decreased lipopolysaccharide (LPS)-induced expression of the inflammatory proteins COX-2, iNOS, and Iba-1 in microglial BV-2 cells [91]. Furthermore, in the brain, the same CHI3L1 inhibitor decreased the M1 markers TNF, IL-1β, IL-6, and CD86, while CHI3L1 overexpression increased the expression of these M1 markers [92]. Conversely, M2 markers such as Arg1, Mrc1, TGF-β, and IL-10 were unaffected by the inhibition of CHI3L1 [92]. This indicates that CHI3L1 may drive the activation of microglia to an M1, pro-inflammatory phenotype. However, other research using microglia isolated from middle cerebral artery occlusion-injured CHI3L1 knockout mice describe opposite results where CHI3L1 knockout increased the M1 markers iNOS, CD86, IL-1β, and IL-6, and decreased the M2 markers Arg1, Mrc1, IL-10, and IL-4Rα [93]. These results suggest that CHI3L1 activates microglia toward an M2, anti-inflammatory phenotype. Further study is needed to clarify the effect of CHI3L1 on microglia and the subsequent effects of these microglia on astrocytes and neurons.
There has been very limited research on the direct effect of CHI3L1 on neurons. In one in vitro study, researchers added recombinant CHI3L1 to primary mouse cortical neuron cultures and assessed neuronal survival and function [94]. It was found that CHI3L1 was cytotoxic to the neurons as it shortened total neurite length, induced neurite length retraction, and increased cell death after 48 hours of exposure [94]. The researchers wanted to determine if CHI3L1 was cytotoxic to glia, so they treated independently cultured microglia and astrocytes with CHI3L1 and witnessed no effect on cell death, demonstrating CHI3L1 cytotoxicity is neuron-specific [94].
Many neurodegenerative diseases are associated with the loss of myelin sheath, the cellular structure formed by oligodendrocytes that insulates the axons of CNS neurons to promote rapid and efficient neuronal signal conduction, amongst other metabolic support functions [95, 96]. While demyelination has been well characterized in diseases such as Multiple Sclerosis, this process has become increasingly observed in other common neurodegenerative diseases like AD [97]. In fact, a recent publication claims that myelin damage can precede clinical AD symptoms by 20 years [97]. It has been recently reported that CHI3L1 can stimulate the proliferation of oligodendrocyte progenitor cells (OPCs) as an indirect method for myelin formation [95]. One such publication shows that downregulation of CHI3L1 protein expression in OPCs results in reduced proliferation and that this reduced proliferation phenotype was able to be rescued upon exogenous CHI3L1 introduction [95]. Another group found that increased CHI3L1 secretion can activate an EGFR-dependent MAPK signaling cascade in neural stem cells to induce differentiation into OPCs, and subsequently into myelin-forming oligodendrocytes in vitro and in vivo, further supporting a role of CHI3L1 in oligodendrogenesis [98]. However, agreement on the effect of CHI3L1 and its mechanism of action pertaining to oligodendrogenesis is not without contention. It has been shown using human iPSC models of Alexander Disease (AxD) that astrocytes harboring disease-causing GFAP mutations have increased CHI3L1 expression and can reduce the proliferative capacity of OPCs [99]. Additionally, proliferation was decreased in OPCs cultured in AxD astrocyte conditioned media and this effect was rescued upon treatment with a CHI3L1 neutralizing antibody [99]. This demonstrates that astrocytes can secrete factors which inhibit OPC proliferation, and that CHI3L1 is a confirmed mediator of this process. Further investigation into the role of CHI3L1 on oligodendrogenesis is needed to gain a more complete understanding of this protein’s function in AD and other demyelinating diseases.
Blood brain barrier dysfunction is commonly observed in the early stage of neurodegenerative disorders affected by neuroinflammation, particularly in AD. In AD, BBB homeostasis is disrupted leading to hypoperfusion that prevents appropriate amounts of oxygen, glucose, and other nutrients from reaching the brain [100]. There has been recent evidence that CHI3L1 may physically associate with the BBB and act upon the endothelial cells, possibly contributing to the compromised BBB integrity witnessed in AD. White matter damage has been tied to BBB dysfunction, and as previously discussed, CHI3L1 has been implicated in white matter degeneration in AD brains [23]. Additionally, CHI3L1-expressing astrocytes have been shown to associate with blood vessels, further implicating CHI3L1 in BBB disruption [23]. It has been shown that the increased CSF levels of CHI3L1 in AD patients significantly correlate with increased albumin ratio, a marker for BBB breakdown [39]. This suggests that the increases in CHI3L1 expression in AD lead to decreased stability and increased permeability of BBB. The direct effect of CHI3L1 on BBB integrity is supported by its reported role in angiogenesis and vascular tissue remodeling in the periphery. While CHI3L1 has been shown to promote angiogenesis in the periphery via increasing VEGF, there has also been evidence that VEGF can downregulate the tight junction protein zona occludens-1 which can lead to BBB damage [58, 63, 101]. It has also been detailed in the periphery that CHI3L1 increases macrophage production of MMP9 and CCL2 [58, 63, 70]. MMPs, such as MMP9, have been shown to cause BBB breakdown by disrupting the basal lamina proteins and by degrading tight junction complexes, while CCL2 can recruit peripheral immune cells which further promotes inflammation that can damage the BBB [101]. Along with peripheral immune cells, inflammation produced by glial cells can also negatively affect expression of tight-junction proteins and result in BBB damage [101]. As detailed above, CHI3L1 can modulate the expression and secretion of pro-inflammatory cytokines by glial cells, further implicating CHI3L1 in BBB dysfunction [91, 92]. While CHI3L1 has been implicated in BBB disruption and remodeling of blood vasculature in AD, the precise mechanism is still unknown and further study is required to determine the precise role of CHI3L1 on BBB dysfunction in AD.
5.4. Known Effects of CHI3L1 on Neuroinflammation & AD
In addition to the cell-specific effect of CHI3L1 in the CNS, there are a few studies that have tried to examine the role of CHI3L1 in the context of general neuroinflammation and CNS disease, including AD. In one study, the role of CHI3L1 in AD was assessed via the use of a CHI3L1 inhibitor. The researchers used an Aβ1-42-induced AD mouse model to determine the effect of CHI3L1 inhibition on amyloid burden and memory impairment. The CHI3L1 inhibitor decreased the expression levels of the Aβ1-42-induced inflammatory proteins iNOS, COX-2, GFAP, and Iba-1 through inactivation of NF-κB signaling by reducing translocation of p65, p50, and p-IκBα into the nucleus [91]. The CHI3L1 inhibitor was also shown to decrease the mRNA levels of TNF-α, IL-1β, and IL-6 in the Aβ1-42-induced AD mice brains [91]. In addition to cytokine expression levels, the researchers assessed components involved in β-secretase activity and Aβ generation, namely BACE1 and APP. The CHI3L1 inhibitor decreased the expression of BACE1 and APP in LPS-treated BV-2 cells and cultured astrocytes [91]. Additionally, the CHI3L1 inhibitor decreased the β-secretase activity that was increased in the brains of the Aβ1-42-induced AD mice [91]. The Aβ1-42-induced AD mice had significantly higher Aβ1-42 levels compared to controls and these levels were reduced by the CHI3L1 inhibitor [91]. Overall, this study determined that inhibition of CHI3L1 can reduce amyloidogenesis resulting in a memory recovery effect in this AD mouse model.
A follow-up study by this same group used the same CHI3L1 inhibitor to investigate its effect on neuroinflammation and memory loss in another AD model, Tg2576 transgenic mice. The CHI3L1 inhibitor reduced the number of iNOS, COX-2, GFAP, and Iba-1 positive cells, indicating an inhibition of microglial and astrocytic activation, and also decreased the levels of TNF-α, IL-1β, and IL-6 in the brains of the Tg2576 transgenic mice [92]. These results are consistent with the group’s previous results in the Aβ1-42-induced AD model. Reduced NF-κB signaling by CHI3L1 inhibition was also supported in the Tg2576 transgenic mouse model of AD as it was shown that the CHI3L1 inhibitor reduced the levels of p-IκBα in the brain [92]. However, in this Tg2576 transgenic model, decreased levels of p-ERK1/2 were also witnessed in the CHI3L1 inhibitor-treated brains, thus implicating the ERK signal transduction pathway in the inhibitory effect on neuroinflammation by the CHI3L1 inhibitor [92]. In addition to reduced neuroinflammation and NF-κB and ERK signaling, the CHI3L1 inhibitor reduced the levels of APP and BACE1, β-secretase activity, and Aβ plaque accumulation in the Tg2576 transgenic mice [92]. In accordance with what was observed in the Aβ1-42-induced AD mice, the CHI3L1 inhibitor also alleviated memory impairment and mitigated impaired cognitive function in the Tg2576 AD model [92].
While the previous studies utilized a CHI3L1 inhibitor to assess the role of CHI3L1 in neuroinflammation and AD, a different group aimed to determine this role via CHI3L1 deletion. Our collaborative study used an APP/PS1 model of AD crossed with the Chi3l1 knockout line we generated to analyze the effect of CHI3L1 deletion on AD progression. The deletion of CHI3L1 decreased amyloid plaque burden in the APP/PS1 mice [88]. In the APP/PS1 mouse, CHI3L1 deletion also increased the microglial lysosomal marker CD68, which suggested to the researchers that the decreased amyloid plaque accumulation may be a result of increased phagocytosis by glia [88]. The researchers tested phagocytic capabilities of glial cells in vitro and found that CHI3L1 knockdown increased phagocytosis of zymosan-coated pHrodo-labeled beads as well as Aβ42 peptide in both microglia and astrocytes, but with a more pronounced effect in microglia [88]. In addition to its effect on microglial phagocytosis, CHI3L1 deletion modestly increased microglia activation and LPS-induced inflammatory cytokine expression in astrocytes and microglia [88]. These results disagree with the previous studies that utilize the CHI3L1 inhibitors which witnessed reduced neuroinflammation when CHI3L1 was inhibited. Overall, this study of CHI3L1 deletion in APP/PS1 AD mice demonstrated that CHI3L1 can contribute to AD progression by altering glial function and amyloid burden.
Together these three studies suggest that therapeutic intervention targeting CHI3L1 may perhaps be able to reduce memory impairment by decreasing neuroinflammation and amyloidogenesis alone or in conjunction with each other. However, there have been other studies that have found CHI3L1 to be anti-inflammatory where loss of CHI3L1 can modestly potentiate pro-inflammatory cytokine expression or even exacerbate neuroinflammation [88, 93]. Further study regarding the effect of CHI3L1 on neuroinflammation is needed to reconcile the discrepancies in this limited field of CHI3L1 CNS function.
6. Conclusion
6.1. Knowledge Gaps and Proposed Hypothesis
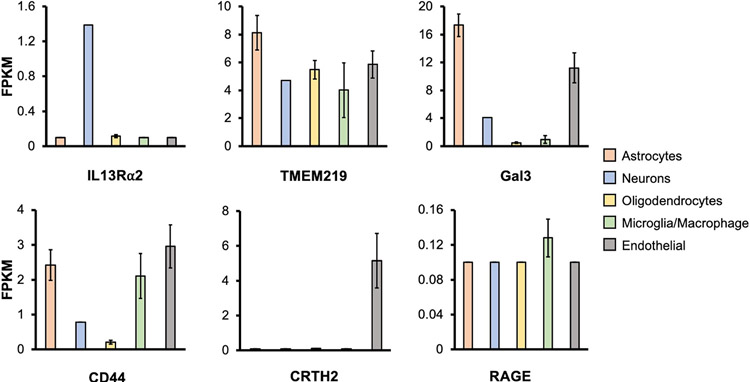
Understanding the mechanisms of CHI3L1 in AD represents a knowledge void to be filled with high priority. A delineation of the key regulatory mechanism for neuroinflammation by CHI3L1 is likely to offer an important scientific framework by which new strategies to anti-neuroinflammation therapy for AD can be developed. Thus, the overall focus in this field should be to i) characterize the signaling mechanism(s) whereby CHI3L1 governs neuroinflammation, both physiologically and pathologically, and ii) to explore the translational potential of CHI3L1 signaling in remedying the pro-inflammatory status in AD brains. In peripheral tissues, it has been well documented that CHI3L1 is a master regulator for a wide range of injury and repair responses, including the innate immunity pathway that resembles the neuroinflammation process initiated by microglia and coordinated by astrocytes [9, 10, 12-16]. Given this similarity, the signaling function of CHI3L1 in microglia and astrocytes may mirror that of the peripheral cells. In fact, all of the known CHI3L1 receptors identified in the periphery are expressed, though some lowly expressed, in major CNS cell types, further supporting the idea that CHI3L1 may signal through similar known pathways in the brain (Figure 3) [34]. These receptors expressed in the brain are capable of engaging the ligand of CHI3L1 and may convey neuroinflammatory signals in individual brain cell types.
Figure 3. CHI3L1 receptor expression in CNS cells.
RNA-Sequencing transcriptome of the known CHI3L1 receptors in cell types isolated from human brain was profiled [34]. The mean and standard error of the mean (Mean ± SEM) are shown. RNA-Seq datasets from Dr. Ben Barres group, https://www.brainrnaseq.org/.
Based on this evidence from the periphery and new, recent evidence in the brain, our central hypothesis is that CHI3L1 functions as a neuroinflammation signaling molecule to trigger the receptor system on brain cells and regulate inflammatory responses in a cell type-specific manner. We propose that CHI3L1 plays an essential role in the intricate cross-talks between microglia and astrocytes in the highly dynamic process of neuroinflammation (Figure 4). Furthermore, on top of the well-characterized regulation of ECM by CHI3L1, the signaling function in endothelial cells and perhaps pericytes may contribute to the vascular impairment and compromised BBB integrity as observed at the early stage of AD pathology preceding cognitive decline. The effects of CHI3L1 on the self-renewal of oligodendrocyte lineage cells and myelination homeostasis have been documented as mentioned above [99], and such oligodendroglial failures can aggravate the dystrophy and degeneration of neurons. Finally, the CHI3L1 effect on neurons can be potentially linked to the loss of synapses as well as the accumulation of misfolded proteins and the appearance of well-defined neurodegenerative features before the overt formation of plaques and tangles. We believe studying the overall underappreciated CHI3L1 function in the brain cells will help identify the pathogenic determinants of neuroinflammation that contribute to AD and other neurodegenerative disorders.
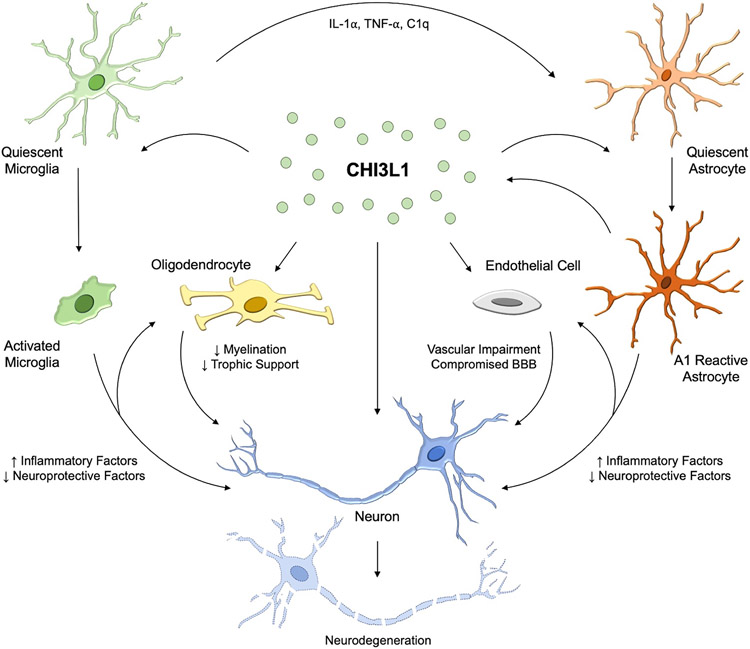
Figure 4. Potential Role of CHI3L1 in Neurodegeneration and AD.
Supported by reported data in the field, our working hypothesis is that microglia-induced CHI3L1 secretion from astrocytes augments inflammation and promotes neural damages. Microglia secrete IL-α, TNF-α and C1q, to activate astrocytes to an A1 reactive phenotype which induces increased CHI3L1 secretion. CHI3L1 can then act upon the glial cells and alter glial activation as well as the expression and secretion of inflammatory or neuroprotective factors. The increased inflammatory factors or direct CHI3L1 action may affect oligodendrocytes and endothelial cells leading to demyelination and reduced trophic support as well as vascular impairment and compromised BBB integrity, respectively. Similarly, CHI3L1 may act directly on the neurons or in combination with the increased inflammation and reduced neuroprotective factors to lead to neural damages. The increased neuroinflammation and resulting neurotoxicity thus contribute to pathology seen in AD.
The major limitation of our conclusion here lies in the complexity of intercellular interactions among brain cell types and the potentially diverse composition of CHI3L1 receptors in distinct inflammatory processes and cellular states. This notion has been complicating the interpretation of findings uncovered in some peripheral diseases, in which the biological function of CHI3L1 can be bi-phasic (protective vs provocative) depending on the levels of expression as well as activation status of CHI3L1 along the transition from acute inflammation to chronic repair. For example, the effects of complete loss of CHI3L1 by knockout can be different from the findings based on partial inhibition of CHI3L1 by an inhibitor compound or neutralizing antibody. In this perspective, future studies need to be directed to determine the importance of homeostatic as well as stimulated regulation of CHI3L1 in various in vivo and in vitro experimental settings as well. We thus outline below a roadmap for continued investigation and future translational work.
6.2. Recommendations for Future Research
As such, recommended forward action would be to i) identify the functional outputs of CHI3L1 expression in neuroinflammation, ii) determine the effects of CHI3L1 manipulations in pro-inflammatory context relevant to AD, and iii) evaluate the in vivo significance of CHI3L1 in neuroinflammation and AD manifestations. First, in single and mixed cultures of microglia, astrocytes, and neurons, cell type-specific CHI3L1 manipulations and the known CHI3L1 receptor manipulations should be conducted. This will allow researchers to dissect out cell type-specific contributions to neuroinflammation and determine if CHI3L1 acts upon the known receptors and signaling pathways in the brain. Alternative receptors should also be probed via an unbiased approach to determine any potential new CHI3L1 receptors outside of the ones already characterized in the periphery. Glial transcriptomics and essential functions altered by the CHI3L1 and receptor modulations should be profiled in order to characterize the CHI3L1 CNS biology.
Secondly, similar experiments in which CHI3L1 expression is modulated should be conducted in cells with relevant AD mutations, including those of amyloid precursor protein, presenilin, and apolipoprotein E. The effects of inhibition or overexpression of CHI3L1 signaling on AD relevant mutation-associated neuroinflammation should be examined. In both cases, the in vitro single or mixed cell cultures can be garnered from animal models, or, alternatively, by cells induced from stem cells. Induced CNS cell models derived from stem cells allow for the use of human cells, which may be able to better recapitulate AD compared to the animal counterparts. Bypassing the limitation of animal models, namely the intrinsic human nature of AD and its associated genetics, data gathered from experiments with induced neurons, astrocytes, and microglia will allow the characterization of the effects of CHI3L1 manipulations in the context of AD [100, 102-104]. These in vitro stem cell models, however, clearly have technical limitations. First, this reductionist approach fails to factor in the functions from diverse cell types and signals present in physiological brain microenvironments, because the iPSC-derived neural cells mostly display features that represent only a particular cell subtype or cellular state. Secondly, the lack of interactions among multiple brain cell types – required to reconstitute a niche for proper neural development – actually retards the maturation and impairs the overall functionality in the cultures. This is of particular importance in the context of aging and neurodegeneration [100]. To address this, the emerging 3D culture models that simulate a higher level of cell type and tissue diversity and thus better recapitulate the in vivo CNS milieu can be considered in conjunction with established animal models to stringently test the role of CHI3L1 in AD.
Third, the in vivo significance of CHI3L1 in neuroinflammation and AD manifestations should be examined by the use of Chi3l1-floxed mice for astrocyte- or microglia-specific conditional CHI3L1 knockout via stereotaxic viral injections as well as established mouse models of AD. Using cell-specific CHI3L1 knockouts in established models of AD can allow for the determination of the CHI3L1 necessity in AD pathogenesis. Once CHI3L1 CNS biology and its role in AD pathogenesis is determined, further tests can be conducted in the context of other neurodegenerative diseases to identify if such neuroinflammatory control can underlie neurodegenerative disease. This information can potentially be used in targeted therapeutics to combat AD and its progression or in other medical advances related to this disease. For example, there have been recent studies in the pulmonary system in which an anti-CHI3L1 antibody was used to neutralize secreted CHI3L1 and decreased lung inflammation [105]. Additionally, as discussed above, a CHI3L1 inhibitor has been used in the context of AD and was shown to reduce amyloid burden and improve impaired memory and cognitive function witnessed in the AD mouse models [91, 92]. Perhaps this antibody or inhibitor could be used to neutralize or inhibit CHI3L1, respectively, in the CNS in humans in clinical investigation settings. This anti-CHI3L1 antibody, CHI3L1 inhibitor, and the development of additional therapeutics may be critical in slowing neurodegeneration and AD progression.
Future research will not only greatly advance our knowledge on the CHI3L1 contributions to AD pathogenesis but also present a proof of principle for forthcoming studies focusing on neuroinflammation. It is our expectation that we will conclude the necessity of CHI3L1 in astrocytes for neuroinflammation and AD development and uncover an in vivo CHI3L1 mechanism that can be targeted to lower disease manifestations. Characterizing this signaling mechanism in depth as well as identifying the functional outputs of CHI3L1 expression in neuroinflammation will ultimately provide new opportunities for the development of much-needed intervention and prevention strategies for AD patients and risk carriers and potentially other neurodegenerative conditions.
Acknowledgements
This work was supported by grants from National Institutes of Health (R00 AG054616 to Y.A.H. and T32 GM136566 to K.C.), the Pilot Project award from the Stem Cells and Aging Center for Biomedical Research Excellence (COBRE) at the Rhode Island Hospital (to Y.A.H.), and the Postdoctoral Fellowship Program in Alzheimer's Disease Research from the BrightFocus Foundation (A2021002F to B.A.). Y.A. H. is a GFL Translational Professor from Center for Translational Neuroscience in the Brown Institute for Translational Sciences.
Footnotes
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Bishop NA, Lu T, and Yankner BA, Neural mechanisms of ageing and cognitive decline. Nature, 2010. 464(7288): p. 529–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frick LR, Williams K, and Pittenger C, Microglial dysregulation in psychiatric disease. Clin Dev Immunol, 2013. 2013: p. 608654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Han X, et al. Astrocyte Senescence and Alzheimer's Disease: A Review. Front Aging Neurosci, 2020. 12: p. 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singhal G and Baune BT, Microglia: An Interface between the Loss of Neuroplasticity and Depression. Front Cell Neurosci, 2017. 11: p. 270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hampel H, et al. Alzheimer's disease biomarker-guided diagnostic workflow using the added value of six combined cerebrospinal fluid candidates: Abeta1-42, total-tau, phosphorylated-tau, NFL, neurogranin, and YKL-40. Alzheimers Dement, 2018. 14(4): p. 492–501. [DOI] [PubMed] [Google Scholar]
- 6.Janelidze S, et al. CSF biomarkers of neuroinflammation and cerebrovascular dysfunction in early Alzheimer disease. Neurology, 2018. 91(9): p. e867–e877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muszynski P, et al. YKL-40 as a Potential Biomarker and a Possible Target in Therapeutic Strategies of Alzheimer's Disease. Curr Neuropharmacol, 2017. 15(6): p. 906–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rehli M, Krause SW, and Andreesen R, Molecular characterization of the gene for human cartilage gp-39 (CHI3L1), a member of the chitinase protein family and marker for late stages of macrophage differentiation. Genomics, 1997. 43(2): p. 221–5. [DOI] [PubMed] [Google Scholar]
- 9.He CH, et al. Chitinase 3-like 1 regulates cellular and tissue responses via IL-13 receptor alpha2. Cell Rep, 2013. 4(4): p. 830–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sutherland TE, Chitinase-like proteins as regulators of innate immunity and tissue repair: helpful lessons for asthma? Biochem Soc Trans, 2018. 46(1): p. 141–151. [DOI] [PubMed] [Google Scholar]
- 11.He CH, et al. Chitinase 3-like 1 regulates cellular and tissue responses via IL-13 receptor α2. Cell Rep, 2013. 4(4): p. 830–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim DH, et al. Regulation of chitinase-3-like-1 in T cell elicits Th1 and cytotoxic responses to inhibit lung metastasis. Nat Commun, 2018. 9(1): p. 503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ober C, et al. Effect of variation in CHI3L1 on serum YKL-40 level, risk of asthma, and lung function. N Engl J Med, 2008. 358(16): p. 1682–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee CG, et al. Role of chitin and chitinase/chitinase-like proteins in inflammation, tissue remodeling, and injury. Annu Rev Physiol, 2011. 73: p. 479–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwak EJ, et al. Chitinase 3-like 1 drives allergic skin inflammation via Th2 immunity and M2 macrophage activation. Clin Exp Allergy, 2019. 49(11): p. 1464–1474. [DOI] [PubMed] [Google Scholar]
- 16.Zhou Y, et al. Chitinase 3-like 1 suppresses injury and promotes fibroproliferative responses in Mammalian lung fibrosis. Sci Transl Med, 2014. 6(240): p. 240ra76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lomiguen C, et al. Possible Role of Chitin-Like Proteins in the Etiology of Alzheimer's Disease. J Alzheimers Dis, 2018. 66(2): p. 439–444. [DOI] [PubMed] [Google Scholar]
- 18.Sanfilippo C, Malaguarnera L, and Di Rosa M, Chitinase expression in Alzheimer's disease and non-demented brains regions. J Neurol Sci, 2016. 369: p. 242–249. [DOI] [PubMed] [Google Scholar]
- 19.Wurm J, et al. Astrogliosis Releases Pro-Oncogenic Chitinase 3-Like 1 Causing MAPK Signaling in Glioblastoma. Cancers (Basel), 2019. 11(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alcolea D, et al. Relationship between cortical thickness and cerebrospinal fluid YKL-40 in predementia stages of Alzheimer's disease. Neurobiol Aging, 2015. 36(6): p. 2018–23. [DOI] [PubMed] [Google Scholar]
- 21.Dickerson BC, et al. The cortical signature of Alzheimer's disease: regionally specific cortical thinning relates to symptom severity in very mild to mild AD dementia and is detectable in asymptomatic amyloid-positive individuals. Cereb Cortex, 2009. 19(3): p. 497–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang H, et al. Cerebrospinal fluid phosphorylated tau, visinin-like protein-1, and chitinase-3-like protein 1 in mild cognitive impairment and Alzheimer's disease. Transl Neurodegener, 2018. 7: p. 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moreno-Rodriguez M, et al. Frontal cortex chitinase and pentraxin neuroinflammatory alterations during the progression of Alzheimer's disease. J Neuroinflammation, 2020. 17(1): p. 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Swanson A, Willette AA, and I. Alzheimer's Disease Neuroimaging, Neuronal Pentraxin 2 predicts medial temporal atrophy and memory decline across the Alzheimer's disease spectrum. Brain Behav Immun, 2016. 58: p. 201–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiao MF, et al. NPTX2 and cognitive dysfunction in Alzheimer's Disease. Elife, 2017. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dai QH and Gong DK, Association of the Polymorphisms and Plasma Level of CHI3L1 with Alzheimer's Disease in the Chinese Han Population: A Case-Control Study. Neuropsychobiology, 2019. 77(1): p. 29–37. [DOI] [PubMed] [Google Scholar]
- 27.Hall S, et al. Longitudinal Measurements of Cerebrospinal Fluid Biomarkers in Parkinson's Disease. Mov Disord, 2016. 31(6): p. 898–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quintana E, et al. Cognitive impairment in early stages of multiple sclerosis is associated with high cerebrospinal fluid levels of chitinase 3-like 1 and neurofilament light chain. Eur J Neurol, 2018. 25(9): p. 1189–1191. [DOI] [PubMed] [Google Scholar]
- 29.Vu L, et al. Cross-sectional and longitudinal measures of chitinase proteins in amyotrophic lateral sclerosis and expression of CHI3L1 in activated astrocytes. J Neurol Neurosurg Psychiatry, 2020. 91(4): p. 350–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Woollacott IOC, et al. Cerebrospinal Fluid YKL-40 and Chitotriosidase Levels in Frontotemporal Dementia Vary by Clinical, Genetic and Pathological Subtype. Dement Geriatr Cogn Disord, 2020. 49(1): p. 56–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hojsgaard Chow H, et al. Progressive multiple sclerosis, cognitive function, and quality of life. Brain Behav, 2018. 8(2): p. e00875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sanfilippo C, et al. CHI3L1 and CHI3L2 overexpression in motor cortex and spinal cord of sALS patients. Mol Cell Neurosci, 2017. 85: p. 162–169. [DOI] [PubMed] [Google Scholar]
- 33.Llorens F, et al. YKL-40 in the brain and cerebrospinal fluid of neurodegenerative dementias. Mol Neurodegener, 2017. 12(1): p. 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Y, et al. Purification and Characterization of Progenitor and Mature Human Astrocytes Reveals Transcriptional and Functional Differences with Mouse. Neuron, 2016. 89(1): p. 37–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clarke LE, et al. Normal aging induces A1-like astrocyte reactivity. Proc Natl Acad Sci U S A, 2018. 115(8): p. E1896–E1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bennett ML, et al. New tools for studying microglia in the mouse and human CNS. Proc Natl Acad Sci U S A, 2016. 113(12): p. E1738–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baldacci F, et al. Two-level diagnostic classification using cerebrospinal fluid YKL-40 in Alzheimer's disease. Alzheimers Dement, 2017. 13(9): p. 993–1003. [DOI] [PubMed] [Google Scholar]
- 38.Gispert JD, et al. The APOE epsilon4 genotype modulates CSF YKL-40 levels and their structural brain correlates in the continuum of Alzheimer's disease but not those of sTREM2. Alzheimers Dement (Amst), 2017. 6: p. 50–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muszynski P, et al. The Relationship between Markers of Inflammation and Degeneration in the Central Nervous System and the Blood-Brain Barrier Impairment in Alzheimer's Disease. J Alzheimers Dis, 2017. 59(3): p. 903–912. [DOI] [PubMed] [Google Scholar]
- 40.Paterson RW, et al. Cerebrospinal fluid in the differential diagnosis of Alzheimer's disease: clinical utility of an extended panel of biomarkers in a specialist cognitive clinic. Alzheimers Res Ther, 2018. 10(1): p. 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sutphen CL, et al. Longitudinal decreases in multiple cerebrospinal fluid biomarkers of neuronal injury in symptomatic late onset Alzheimer's disease. Alzheimers Dement, 2018. 14(7): p. 869–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bos I, et al. Cerebrospinal fluid biomarkers of neurodegeneration, synaptic integrity, and astroglial activation across the clinical Alzheimer's disease spectrum. Alzheimers Dement, 2019. 15(5): p. 644–654. [DOI] [PubMed] [Google Scholar]
- 43.Morenas-Rodriguez E, et al. Different pattern of CSF glial markers between dementia with Lewy bodies and Alzheimer's disease. Sci Rep, 2019. 9(1): p. 7803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nordengen K, et al. Glial activation and inflammation along the Alzheimer's disease continuum. J Neuroinflammation, 2019. 16(1): p. 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Villar-Pique A, et al. Plasma YKL-40 in the spectrum of neurodegenerative dementia. J Neuroinflammation, 2019. 16(1): p. 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abu-Rumeileh S, et al. CSF biomarkers of neuroinflammation in distinct forms and subtypes of neurodegenerative dementia. Alzheimers Res Ther, 2019. 12(1): p. 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Antonell A, et al. Synaptic, axonal damage and inflammatory cerebrospinal fluid biomarkers in neurodegenerative dementias. Alzheimers Dement, 2020. 16(2): p. 262–272. [DOI] [PubMed] [Google Scholar]
- 48.Wang L, et al. Cerebrospinal fluid levels of YKL-40 in prodromal Alzheimer's disease. Neurosci Lett, 2020. 715: p. 134658. [DOI] [PubMed] [Google Scholar]
- 49.Nordenbaek C, et al. YKL-40, a matrix protein of specific granules in neutrophils, is elevated in serum of patients with community-acquired pneumonia requiring hospitalization. J Infect Dis, 1999. 180(5): p. 1722–6. [DOI] [PubMed] [Google Scholar]
- 50.Di Rosa M, et al. CHI3L1 nuclear localization in monocyte derived dendritic cells. Immunobiology, 2016. 221(2): p. 347–56. [DOI] [PubMed] [Google Scholar]
- 51.Connor JR, et al. Human cartilage glycoprotein 39 (HC gp-39) mRNA expression in adult and fetal chondrocytes, osteoblasts and osteocytes by in-situ hybridization. Osteoarthritis Cartilage, 2000. 8(2): p. 87–95. [DOI] [PubMed] [Google Scholar]
- 52.De Ceuninck F, et al. YKL-40 (cartilage gp-39) induces proliferative events in cultured chondrocytes and synoviocytes and increases glycosaminoglycan synthesis in chondrocytes. Biochem Biophys Res Commun, 2001. 285(4): p. 926–31. [DOI] [PubMed] [Google Scholar]
- 53.Hakala BE, White C, and Recklies AD, Human cartilage gp-39, a major secretory product of articular chondrocytes and synovial cells, is a mammalian member of a chitinase protein family. J Biol Chem, 1993. 268(34): p. 25803–10. [PubMed] [Google Scholar]
- 54.Hamilton G and Rath B, Circulating tumor cell interactions with macrophages: implications for biology and treatment. Transl Lung Cancer Res, 2017. 6(4): p. 418–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhao T, et al. Chitinase-3 like-protein-1 function and its role in diseases. Signal Transduct Target Ther, 2020. 5(1): p. 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee CM, et al. IL-13Ralpha2 uses TMEM219 in chitinase 3-like-1-induced signalling and effector responses. Nat Commun, 2016. 7: p. 12752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Geng B, et al. Chitinase 3-like 1-CD44 interaction promotes metastasis and epithelial-to-mesenchymal transition through beta-catenin/Erk/Akt signaling in gastric cancer. J Exp Clin Cancer Res, 2018. 37(1): p. 208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kzhyshkowska J, et al. Role of chitinase-like proteins in cancer. Biol Chem, 2016. 397(3): p. 231–47. [DOI] [PubMed] [Google Scholar]
- 59.Low D, et al. Chitinase 3-like 1 induces survival and proliferation of intestinal epithelial cells during chronic inflammation and colitis-associated cancer by regulating S100A9. Oncotarget, 2015. 6(34): p. 36535–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tang H, et al. YKL-40 induces IL-8 expression from bronchial epithelium via MAPK (JNK and ERK) and NF-kappaB pathways, causing bronchial smooth muscle proliferation and migration. J Immunol, 2013. 190(1): p. 438–46. [DOI] [PubMed] [Google Scholar]
- 61.Tran HT, et al. Chitinase 3-like 1 synergistically activates IL6-mediated STAT3 phosphorylation in intestinal epithelial cells in murine models of infectious colitis. Inflamm Bowel Dis, 2014. 20(5): p. 835–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bara I, et al. Role of YKL-40 in bronchial smooth muscle remodeling in asthma. Am J Respir Crit Care Med, 2012. 185(7): p. 715–22. [DOI] [PubMed] [Google Scholar]
- 63.Kawada M, et al. Chitinase 3-like 1 promotes macrophage recruitment and angiogenesis in colorectal cancer. Oncogene, 2012. 31(26): p. 3111–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Recklies AD, et al. Inflammatory cytokines induce production of CHI3L1 by articular chondrocytes. J Biol Chem, 2005. 280(50): p. 41213–21. [DOI] [PubMed] [Google Scholar]
- 65.Recklies AD, White C, and Ling H, The chitinase 3-like protein human cartilage glycoprotein 39 (HC-gp39) stimulates proliferation of human connective-tissue cells and activates both extracellular signal-regulated kinase- and protein kinase B-mediated signalling pathways. Biochem J, 2002. 365(Pt 1): p. 119–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee CG, et al. Role of breast regression protein 39 (BRP-39)/chitinase 3-like-1 in Th2 and IL-13-induced tissue responses and apoptosis. J Exp Med, 2009. 206(5): p. 1149–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jefri M, et al. YKL-40 regulated epithelial-mesenchymal transition and migration/invasion enhancement in non-small cell lung cancer. BMC Cancer, 2015. 15: p. 590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhou Y, et al. Chitinase 3-like-1 and its receptors in Hermansky-Pudlak syndrome-associated lung disease. J Clin Invest, 2015. 125(8): p. 3178–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Eurich K, et al. Potential role of chitinase 3-like-1 in inflammation-associated carcinogenic changes of epithelial cells. World J Gastroenterol, 2009. 15(42): p. 5249–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Libreros S, et al. Induction of proinflammatory mediators by CHI3L1 is reduced by chitin treatment: decreased tumor metastasis in a breast cancer model. Int J Cancer, 2012. 131(2): p. 377–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Di Rosa M, et al. Evaluation of CHI3L-1 and CHIT-1 expression in differentiated and polarized macrophages. Inflammation, 2013. 36(2): p. 482–92. [DOI] [PubMed] [Google Scholar]
- 72.Rehli M, et al. Transcriptional regulation of CHI3L1, a marker gene for late stages of macrophage differentiation. J Biol Chem, 2003. 278(45): p. 44058–67. [DOI] [PubMed] [Google Scholar]
- 73.Dela Cruz CS, et al. Chitinase 3-like-1 promotes Streptococcus pneumoniae killing and augments host tolerance to lung antibacterial responses. Cell Host Microbe, 2012. 12(1): p. 34–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kang MJ, et al. Role of Chitinase 3-Like-1 in Interleukin-18-Induced Pulmonary Type 1, Type 2, and Type 17 Inflammation; Alveolar Destruction; and Airway Fibrosis in the Murine Lung. Am J Respir Cell Mol Biol, 2015. 53(6): p. 863–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Romagnani S, T-cell subsets (Th1 versus Th2). Ann Allergy Asthma Immunol, 2000. 85(1): p. 9–18; quiz 18, 21. [DOI] [PubMed] [Google Scholar]
- 76.Jansen IE, et al. Genome-wide meta-analysis identifies new loci and functional pathways influencing Alzheimer's disease risk. Nat Genet, 2019. 51(3): p. 404–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mathys H, et al. Single-cell transcriptomic analysis of Alzheimer's disease. Nature, 2019. 570(7761): p. 332–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liddelow SA and Barres BA, Reactive Astrocytes: Production, Function, and Therapeutic Potential. Immunity, 2017. 46(6): p. 957–967. [DOI] [PubMed] [Google Scholar]
- 79.Liddelow SA, et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature, 2017. 541(7638): p. 481–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tang Y and Le W, Differential Roles of M1 and M2 Microglia in Neurodegenerative Diseases. Mol Neurobiol, 2016. 53(2): p. 1181–1194. [DOI] [PubMed] [Google Scholar]
- 81.Escartin C, et al. Reactive astrocyte nomenclature, definitions, and future directions. Nat Neurosci, 2021. 24(3): p. 312–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bush TG, et al. Leukocyte infiltration, neuronal degeneration, and neurite outgrowth after ablation of scar-forming, reactive astrocytes in adult transgenic mice. Neuron, 1999. 23(2): p. 297–308. [DOI] [PubMed] [Google Scholar]
- 83.Zador Z, et al. Role of aquaporin-4 in cerebral edema and stroke. Handb Exp Pharmacol, 2009(190): p. 159–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zamanian JL, et al. Genomic analysis of reactive astrogliosis. J Neurosci, 2012. 32(18): p. 6391–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Barbar L, et al. CD49f Is a Novel Marker of Functional and Reactive Human iPSC-Derived Astrocytes. Neuron, 2020. 107(3): p. 436–453 e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yun SP, et al. Block of A1 astrocyte conversion by microglia is neuroprotective in models of Parkinson's disease. Nat Med, 2018. 24(7): p. 931–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shi Y, et al. ApoE4 markedly exacerbates tau-mediated neurodegeneration in a mouse model of tauopathy. Nature, 2017. 549(7673): p. 523–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lananna BV, et al. Chi3l1/YKL-40 is controlled by the astrocyte circadian clock and regulates neuroinflammation and Alzheimer's disease pathogenesis. Sci Transl Med, 2020. 12(574). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bhardwaj R, et al. RelB/p50 complexes regulate cytokine-induced YKL-40 expression. J Immunol, 2015. 194(6): p. 2862–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bonneh-Barkay D, et al. Astrocyte and macrophage regulation of YKL-40 expression and cellular response in neuroinflammation. Brain Pathol, 2012. 22(4): p. 530–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Choi JY, et al. K284-6111 prevents the amyloid beta-induced neuroinflammation and impairment of recognition memory through inhibition of NF-kappaB-mediated CHI3L1 expression. J Neuroinflammation, 2018. 15(1): p. 224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ham HJ, et al. K284-6111 alleviates memory impairment and neuroinflammation in Tg2576 mice by inhibition of Chitinase-3-like 1 regulating ERK-dependent PTX3 pathway. J Neuroinflammation, 2020. 17(1): p. 350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Im JH, et al. Deletion of Chitinase-3-like 1 accelerates stroke development through enhancement of Neuroinflammation by STAT6-dependent M2 microglial inactivation in Chitinase-3-like 1 knockout mice. Exp Neurol, 2020. 323: p. 113082. [DOI] [PubMed] [Google Scholar]
- 94.Matute-Blanch C, et al. Chitinase 3-like 1 is neurotoxic in primary cultured neurons. Sci Rep, 2020. 10(1): p. 7118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jiang L, et al. Astrocytes induce proliferation of oligodendrocyte progenitor cells via connexin 47-mediated activation of Chi3l1 expression. Eur Rev Med Pharmacol Sci, 2019. 23(7): p. 3012–3020. [DOI] [PubMed] [Google Scholar]
- 96.Stadelmann C, et al. Myelin in the Central Nervous System: Structure, Function, and Pathology. Physiol Rev, 2019. 99(3): p. 1381–1431. [DOI] [PubMed] [Google Scholar]
- 97.Nasrabady SE, et al. White matter changes in Alzheimer's disease: a focus on myelin and oligodendrocytes. Acta Neuropathol Commun, 2018. 6(1): p. 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Starossom SC, et al. Chi3l3 induces oligodendrogenesis in an experimental model of autoimmune neuroinflammation. Nat Commun, 2019. 10(1): p. 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Li L, et al. GFAP Mutations in Astrocytes Impair Oligodendrocyte Progenitor Proliferation and Myelination in an hiPSC Model of Alexander Disease. Cell Stem Cell, 2018. 23(2): p. 239–251 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Essayan-Perez S, et al. Modeling Alzheimer's disease with human iPS cells: advancements, lessons, and applications. Neurobiol Dis, 2019. 130: p. 104503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.da F onseca AC, et al. The impact of microglial activation on blood-brain barrier in brain diseases. Front Cell Neurosci, 2014. 8: p. 362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Assetta B, et al. Generation of Human Neurons and Oligodendrocytes from Pluripotent Stem Cells for Modeling Neuron-Oligodendrocyte Interactions. J Vis Exp, 2020(165). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Huang YA, et al. ApoE2, ApoE3, and ApoE4 Differentially Stimulate APP Transcription and Abeta Secretion. Cell, 2017. 168(3): p. 427–441 e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tcw J, et al. An Efficient Platform for Astrocyte Differentiation from Human Induced Pluripotent Stem Cells. Stem Cell Reports, 2017. 9(2): p. 600–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kim MJ, et al. Chitinase 3-like 1 protein plays a critical role in respiratory syncytial virus-induced airway inflammation. Allergy, 2019. 74(4): p. 685–697. [DOI] [PMC free article] [PubMed] [Google Scholar]