ABSTRACT
The 20th and 21st centuries have witnessed a substantial increase in human life expectancy and in the number of men and women aged 60 years and older. Aging is associated with a large number of health conditions, including sarcopenia, which has been the subject of important research in the past 30 years. Sarcopenia is characterized by an age-related loss of muscle mass, weakness, and impaired physical performance. The condition can be diagnosed with a combination of measurements of these three elements. The precise definition of sarcopenia and the selection of optimal assessment methods have changed significantly in the past 20 years; nonetheless, the prevalence of sarcopenia in the general older population is in the range of 5–15%. Molecular and cellular events at the muscle cell level impact the size and quality of muscles (force adjusted for size). The active and passive mechanical properties of single muscle fibers are altered by changes in the structure and function of various cellular elements. Systemic factors such as inflammation, loss of hormonal influence, and deleterious lifestyle choices also contribute to sarcopenia. The consequences of sarcopenia include many adverse effects such as impairments in activities of daily living, falls, loss of independence, and increased mortality. Several rehabilitative interventions have been tested, and the safest and most effective is the use of progressive resistance exercise. An increase in dietary protein intake has synergistic effects. Future research should focus on a consensus definition of sarcopenia, identification of the best assessment methods, understanding of biological mechanisms, and testing of innovative interventions.
Keywords: aging, skeletal muscle, exercise
INTRODUCTION
The societal and individual importance of addressing sarcopenia is captured by the Portuguese poet Fernando Pessoa (1888–1935): “…the first function of life is action, just as the first property of things is motion”.
The decade of 2021–2030 has been declared the Decade of Healthy Aging by the World Health Organization (WHO) and the General Assembly of the United Nations (UN). A fundamental objective of this declaration is to transform the world into a better place in which to grow older. Healthy aging has been defined as the process of developing and maintaining the functional ability that enables well-being in older age. According to the WHO, functional ability refers to a set of attributes that enable people to be and to do what they have reason to value including to be mobile and move around, build and maintain relationships, meet their own basic needs, learn, grow, make decisions, and contribute to society. To foster healthy aging, strategic objectives, plans, and consequent actions are needed to create age-friendly environments, combat ageism, make integrated care accessible, and develop long-term care services. All of these relate to an important concept in rehabilitation medicine which is to maintain or enhance function/functioning and quality of life independent of chronological age. These actions are particularly relevant to people who require rehabilitation services, including those experiencing impairments and/or disabilities, such as sarcopenia and frailty, and those who are at risk of developing them.
This historic declaration by the WHO and the UN is an explicit recognition of the importance of a dramatic increase in life expectancy and in the number of people in older age groups that characterized the 20th century and the first two decades of the 21st century. These are population groups that require special socioeconomic community infrastructure, services, and organization that takes into account their many needs. In particular, older men and women require accessible health care services appropriate for their age and their multiple health conditions. The utilization of health services by this age group is very high, in part because of the presence of co-morbidities and age-related chronic conditions. This state of affairs must be taken into account by countries and governments when planning the communities of the future.1)
Sarcopenia was first described by Irving Rosenberg in a scientific meeting in 1988, and his comments defining age-related loss of muscle mass as sarcopenia were published in 1989.2,3) Because age-related sarcopenia could become an important contributor to the challenges mentioned in the preceding paragraphs, the purpose of this article is to discuss briefly some of the most relevant issues related to the definition, assessment, prevalence, pathophysiology, and clinical diagnosis of sarcopenia. Brief comments will also be made about the scientific evidence that supports some already available and appropriate therapeutic and rehabilitative interventions. Finally, a few suggestions about future research directions and priorities are made at the end of this review.
AGING AND WORLD DEMOGRAPHICS
The recognition that the human population worldwide is aging is not a 21st-century phenomenon. For example, the biology of senescence was discussed by Comfort in 1956 in a now classic book on the topic.4) Important demographic changes have been recorded in the past two centuries in all continents of the globe, and a few quantitative observations support this statement. According to the WHO, already, there are more than 1 billion people aged 60 years or older, with most living in low- and middle-income countries.5) Life expectancy in many countries has increased dramatically in the past century and it is greater than 80 years in several countries around the world, particularly in Asia and Europe.5) Furthermore, in many countries, the percentage of people older than 60 years is projected to be more than 40% by the year 2050. This is the case, for example, in countries like Japan, Italy, Portugal, and Greece. It has been suggested that, at that time, 15–20% of the total human population will belong to this age group. Although the COVID-19 pandemic has resulted in a decrease in life-expectancy in many countries, this effect is not expected to last, provided that the implementation of public health measures, such as vaccination and therapeutic efforts, can control the virus.6)
It is of special interest to note that the study of extreme longevity has received significant attention in recent decades.7) The number of centenarians, both men and women, is increasing in many countries of the world, including Japan, Sweden, Denmark, France, and Switzerland. Some areas of the world (known as “blue zones”) have been identified as home to a proportionally larger number of centenarians; these include Okinawa, Japan; Ikaria, Greece; Sardinia, Italy; Nicoya, Costa Rica; and Loma Linda, California, USA.8) Furthermore, a subgroup of this population, known as the supercentenarians, has also been identified and studied.9) Although centenarians may have distinctive genotypes,10) to study those who have lived longer than the median age for humans can provide useful insights into the basic mechanisms of aging. Some centenarians have demonstrated unusual physiological capacities and ability to adapt to exercise training.11) However, to the author’s knowledge, only very few studies have addressed the issue of sarcopenia in centenarians.12)
THE IMPORTANCE FOR REHABILITATION OF STUDYING AND UNDERSTANDING SARCOPENIA
A quick look at the number of scientific publications related to sarcopenia in the PubMed database (https://pubmed.ncbi.nlm.nih.gov/?term=sarcopenia) shows that the topic has become a very active area for investigation. For example, in 2000 and 2010, respectively, only 39 and 241 papers were published on the topic. This number had increased to 2567 by 2020. In other words, the number of papers increased by a factor of 65 over the 20-year period from 2000 to 2020. In 2021, the number of papers was 3135, which represents 7% of all papers published under the search term of “aging”.
One of the most important justifications for identifying sarcopenia early in the process is the substantial list of negative consequences and adverse effects associated with this condition. An example of an obvious and perhaps most immediate effect of sarcopenia is a reduction in functional capacity.13) Functional capacity can be assessed with an evaluation of performance of activities of daily living. It has been reported that reductions in handgrip strength are associated with increasing odds of limitations in activities of daily living such as eating, walking, bathing, dressing, and transferring.14) Furthermore, the presence of the reduced capacity to perform any of these activities is associated with a higher hazard for mortality. This is a good example of how a specific diagnostic element (as opposed to a combination) of sarcopenia is associated with negative consequences. Other well-known adverse effects of aging and sarcopenia include falls, fractures, increased risk of hospitalization, and increased mortality (e.g., in cancer patients).14,15)
Although functional loss is characteristic of advanced adult age, we should not assume that this decline is unalterable. Sarcopenia, just like aging, is not necessarily a random process: it is influenced by where we live, our health behaviors, access to health care, and genotype. Furthermore, several trajectories of mobility limitations over time have been identified, and the dynamic nature of some of these trajectories has been demonstrated.16) The reversibility of some of the changes in skeletal muscle function and structure with age has been demonstrated in clinical trials using exercise and other rehabilitative interventions (see below). These observations emphasize the importance of studying potential interventions tailored for this age group.
THE PRINCIPAL CLINICAL MANIFESTATIONS OF SARCOPENIA
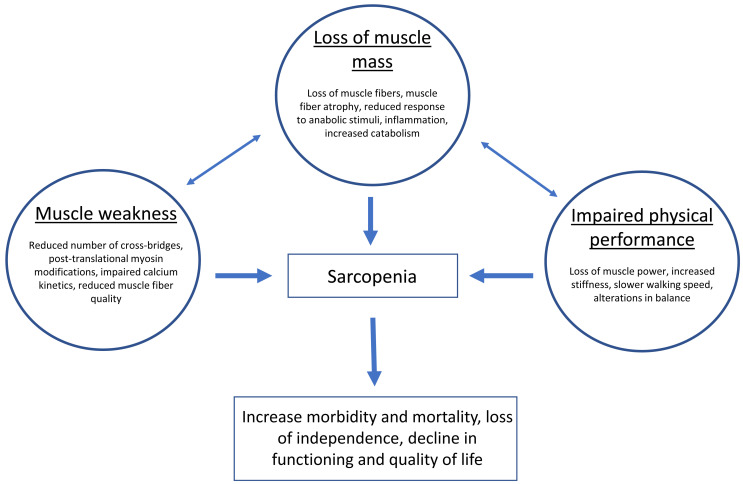
As previously mentioned, current definitions of sarcopenia (Table 1) include three main elements: loss of skeletal muscle mass, loss of skeletal muscle strength, and impaired physical (motor) performance (Fig. 1). Each of these elements can be evaluated using a variety of technologies and tests.17) Both the definition and evaluation strategies have changed in the past 34 years, and it is reasonable to speculate that more changes will occur as continuing research advances our understanding of sarcopenia.
Table 1. Recent definitions of sarcopenia by two international working groups.
| European Working Group on Sarcopenia in Older People 2 (EWGSOP2) | Asian Working Group for Sarcopenia
(AWGS-2019) |
| Sarcopenia is a progressive and generalized skeletal
muscle disorder that is associated with increased likelihood of adverse outcomes
including falls, fractures, physical disability, and mortality |
Age-related loss of skeletal muscle mass plus loss of
muscle strength and/or reduced physical performance |
Fig. 1.
Determinants of changes in the three fundamental elements of sarcopenia.
The loss of skeletal muscle mass with aging in humans was described before the term sarcopenia was proposed.18) Although this loss may happen at different rates in various muscle groups, it appears to be a consistent finding independent of the anatomical area (see below). A substantial contributor to muscle atrophy in older men and women is the loss of motor neurons and associated muscle fibers expressing the type II myosin heavy chain isoform (at the time, no distinction was made between type IIA and IIX fiber types). These losses are accompanied by a reduction in the size of both type I and II muscle fibers. Although changes in the nervous system contribute to the sarcopenic phenotype in vivo, a review of these changes is beyond the scope of this article, the focus of which is on adaptations in skeletal muscle.
Aging is characterized by a loss of sensitivity to anabolic stimuli that leads to impaired protein synthesis.19) Simultaneously, there is an increase in protein catabolism, directly associated with a chronic sub-clinical inflammatory state20) and the accumulation of senescent cells that secrete inflammatory substances.21) A recent systematic review and meta-analysis has shown that high levels of circulating inflammatory markers are associated with significantly lower muscle mass and strength.22) In fact, it has been suggested that inflammation measured by circulating levels of IL-6 is the only known cross-sectional and longitudinal predictor of multimorbidity and one of the strongest predictors of incident mobility loss and disability in activities of daily living.23) It is of interest to note that some authors have argued recently that similar mechanisms are active in patients with human immunodeficiency virus24) or COVID-1925) because both exhibit a sarcopenic phenotype.
These two changes, i.e., impaired protein synthesis and increased catabolism, limit the capacity of aged muscle to replace muscle tissue that has been lost. Furthermore, a reduction in the number26) and function27) of satellite cells, particularly those associated with type II fibers, limits the regenerative potential of muscle and its response to age-related atrophy and injury.28) This reduction in satellite cells may result from the accumulation of somatic mutations.29) Recently, it was also hypothesized that the microvascular network between muscle fibers plays a significant role in satellite cell function.30) According to this hypothesis, the distance between satellite cells and neighboring capillaries is increased in older muscles, a situation that contributes to satellite cell dysfunction. Finally, it must be noted that estimates of skeletal muscle size in the elderly must take into account the presence of fat (myosteatosis) at different levels, including the tissues surrounding the muscle, the space between muscles and muscle fibers, and the intracellular space.31,32) Fatty infiltration of skeletal muscle may lead to an overestimation of muscle size, underestimation of muscle mass loss, impaired energy metabolism, and mitochondrial dysfunction, among other adverse effects.
The capacity to generate force needed to initiate and maintain movement is the fundamental physiological/mechanical property of skeletal muscle. Muscle strength, defined as the maximal force developed by a muscle or muscle group at a specified velocity, is reduced in both older men and women.33,34) This is the reason why an older person requires a higher percentage of his/her strength to perform activities of daily living such as rising from a chair or climbing stairs. The reduction in strength with advanced adult age was reported several decades ago in cross-sectional studies33) in which different age groups were compared. An age-related decline in muscle strength was confirmed in longitudinal studies in which individuals were followed for more than a decade and tested at baseline and follow-up using the same technology and methods.34) Muscle strength loss was observed in muscles of the upper limbs (e.g., elbow flexors and extensors) and lower limbs (knee flexors and extensors).35) The loss of strength in the upper limbs was, on average, approximately 1% per year and that of the lower limbs 1.5% per year. Women demonstrated slower decline in strength than men, particularly in muscles of the upper limbs. Of significance, depending on the muscle group and sex, 7–32% of the participants did not show a decline in strength over the course of a 10-year study.35) Specific factors contributing to the preservation of strength have not been clearly identified and are in need of further research.
It must be noted that, although there is correlation between muscle size (mass, volume, or cross-sectional area) and muscle strength, the relative reduction in strength with aging is larger than the decline in muscle size. This has been explained by a combination of age-related changes in the nervous system leading to the impaired activation of motor units36,37,38) and the presence of multiple molecular and cellular alterations at the level of the individual muscle fiber that are independent of size, i.e., these alterations result in changes in muscle quality. The latter hypothesis is supported by evidence demonstrating reduced force production in segments of isolated muscle fibers activated with calcium in vitro in the absence of nervous influence.39) The underlying mechanism for this reduction in muscle function at the level of individual cells is related to a reduction in the number of actin–myosin cross-bridges and the impaired kinetics of existing bridges. These intracellular changes are discussed in more detail in the next section.
The third manifestation of sarcopenia is a reduction in physical performance and mobility. The multifactorial nature of mobility decline in old age has been reviewed by other investigators.40) The loss of muscle mass and the reduction in muscle strength are important contributors to the functional impairment and mobility problems that are found in most older adults. However, it could be argued that it is the loss of muscle power (strength/time) that impacts functional independence because many activities of daily living and the prevention of falls depend more on the rapid development of force than on the maximal strength.41,42) It is of interest that the underlying physiological mechanisms that contribute to the loss of muscle power in older adults are different in healthy adults vs. mobility-limited older adults.43) Neuromuscular activation declines more significantly in healthy older adults, whereas mobility-limited adults show more significant reductions in muscle size and strength. Another example of the importance of muscle power is the demonstration of a threshold needed to rise from a chair that correlates with mobility limitations and disability.44) Finally, a combination of loss of strength, reduction in muscle shortening velocity, increased tissue (muscle, joint capsule, ligaments, tendons) stiffness, and alterations in balance may all contribute to the slow walking speed that is characteristic of elderly men and women.45) It is noteworthy that the increase in inflammation biomarkers, such as IL-6 and CRP, correlate with the reduction in walking speed in the elderly.46)
MOLECULAR AND CELLULAR CHANGES UNDERLYING SARCOPENIA
During the past three decades, researchers have described many physiological, biochemical, and molecular changes that contribute to the development of sarcopenia with advanced age and have explained the associated loss of muscle mass, weakness, and impaired physical performance (Fig. 1). Some of these changes take place at the level of the individual muscle fibers and recently have been discussed in detail.47) The study of segments of single muscle fibers has been made possible with the use of percutaneous muscle biopsy to obtain tissue samples and the activation of permeabilized individual fibers in vitro using maximal concentrations of calcium. A fundamental observation in these experiments is an age-related reduction in the force-generating capacity of the fiber that is independent of its size.39) In other words, age-related sarcopenia is associated with a loss of muscle quality (defined as force adjusted for cross-sectional area) and not only with the loss of muscle tissue (atrophy). These observations do not negate the contribution to human sarcopenia of impairments in the nervous system but strongly support the idea that muscle weakness and impaired motor performance may exist even in the presence of normal activation of motor units.
Several intracellular changes have been described in skeletal muscle fibers that impair the ability of the fiber to generate force. These changes include mutations in the myosin molecule,48) post-translational modification of myosin and other myoproteins (glycation, oxidation),49) alterations in cytoskeletal proteins such as titin that contribute to muscle stiffness and other important passive mechanical properties,50,51) a reduction in the number of actin–myosin cross-bridges and impaired cross-bridge kinetics leading to longer myosin attachment times and reduced rates of myosin force production,52) mitochondrial dysfunction,53,54) and intracellular accumulation of lipid droplets,55) among others. Furthermore, abnormalities in calcium handling, such as impaired sarcoplasmic reticulum storage, release, re-uptake, and leakage, as well as impaired calcium saturation and binding, have a negative impact on calcium sensitivity and cross-bridge cycling.56) Passive mechanical properties are also affected by aging.47) For example, mechanical properties such as muscle fiber elasticity measured as instantaneous stiffness is higher in older people.57) The combined effect of these alterations is a substantial reduction in the capacity of the fibers to generate the force needed for functional mobility.
A detailed review of extracellular changes that may contribute to muscle weakness is beyond the scope of this brief review. However, it is important to recognize that the force that is generated inside the muscle cells must be transmitted to other tissues to produce joint movement, and the aging process is also known to have a negative effect on the mechanical properties of extra-muscular tissues such as tendons, which are important links between muscle cells and joint motion.
HOW TO DIAGNOSE SARCOPENIA
Several attempts have been made during the past two decades to develop a specific definition of sarcopenia that includes specific diagnostic criteria and algorithms that could help health professionals to identify this health condition. Two of these attempts included groups of experts working together from various countries in Asia58) and in Europe.59) These two groups have published definitions that are presented in Table 1. It is important to note that the definition proposed by the European Working Group on Sarcopenia in Older People 2 (EWGSOP2) does not make direct reference to age or aging. In contrast, the definition proposed by the Asian Working Group for Sarcopenia (AWGS 2019) is age dependent. This means that muscle atrophy associated with other chronic conditions, such as cancer and stroke at any age, is not included in the definition of the AWGS 2019.
Both EWGSOP2 and AWGS 2019 have published algorithms that includes a step-by-step process that can be followed in the clinical setting to identify persons with sarcopenia and to define the degree of severity of the condition. The diagnostic criteria and final diagnoses based on the results of tests for the three elements of sarcopenia are presented in Table 2. There are many similarities and some important differences between these two algorithms. The AWGS 2019 algorithm begins with recommendations to find “cases” based on the presence of conditions such as, among others, functional decline, repeated falls, malnutrition, chronic conditions, cognitive impairments, reduction in calf circumference, or a score of ≥4 in the SARC-F questionnaire (strength, assistance walking, rise from a chair, climb stairs, and falls).60) The diagnostic tests that follow the identification of a case include muscle strength (handgrip), tests of physical performance [6-m walk, five-time chair stand test, short physical performance battery (SPPB)], and an assessment of appendicular skeletal muscle mass (ASM) using dual-energy X-ray absorptiometry (DXA) or bioelectrical impedance analysis (BIA). Sarcopenia is diagnosed in the presence of low ASM and one of the other two elements, low muscle strength or low physical performance. If all three elements are present, sarcopenia is designated as severe. It is important to note that if case finding is done in a primary health care or community services setting, the presence of low muscle strength (handgrip strength) or impaired physical performance (five-time chair stand test) should be interpreted as possible sarcopenia, and the patient should be referred for evaluation using the AWGS 2019 algorithm described above.
Table 2. Criteria and diagnosis based on two definitions published by international working groups.
| European Working Group on Sarcopenia in Older People 2 (EWGSOP2) |
Asian Working Group for Sarcopenia (AWGS 2019) |
||
| Criteria | Diagnosis | Criteria | Diagnosis |
| Low muscle strength | Probable sarcopenia | Low muscle strength or low physical performance | Possible sarcopenia |
| Low muscle strength and low muscle mass | Confirmed sarcopenia | Low muscle mass and
low muscle strength
or low physical performance |
Sarcopenia |
| Low muscle strength, low muscle mass and low physical performance | Severe sarcopenia | Low muscle mass and low muscle strength
and low physical performance |
Severe sarcopenia |
The EWGSOP2 algorithm also begins the diagnostic process with “case” finding using the SARC-F questionnaire or clinical suspicion. This is followed by a muscle strength test. In this algorithm, however, in addition to the handgrip strength test, the chair stand test is considered to be a test of muscle strength and not of physical performance as it is in the AWGS 2019. It could be argued that a chair stand test is more than a strength test and also requires balance and coordination. If muscle strength is low, this is considered probable sarcopenia. A test of muscle quantity (by DXA, BIA, CT, or MRI) follows, and the results are used to measure muscle mass. If both strength and muscle mass are low, sarcopenia is confirmed. A significant difference with the approach taken by the AWGS 2019 is that tests of physical performance (gait speed, SPPB, time up and go test, 400 m walk) are used only to determine if sarcopenia is severe. If this is the case, it is classified separately. Both working groups and algorithms include specific quantitative criteria that have been developed/chosen for their respective regions of the world. A comparison of these values is presented in Table 3. There is a need to confirm that these criteria apply to the population under study in any particular country. The validity and reliability of these measurements must be considered.
Table 3. Comparison of cut-off values for various tests as recommended by EWGSOP2 and AWGS 2019.
| Test | European Working Group on Sarcopenia in Older People 2 (EWGSOP2) |
Asian Working Group for Sarcopenia (AWGS 2019) |
| Handgrip strength (kg) | M: <27; F: <16 | M: <28; F: <18 |
| Physical performance | ||
| 6-m walk speed (m/s) | <1.0 | |
| Gait speed (m/s) | ≤0.8 | |
| Five-time chair stand test (s) | >15 | ≥12 |
| Short Physical Performance Battery (points) | ≤8 | ≤9 |
| 400 m walk (min) | Non-completion or ≥6 | |
| Time up and go (s) | ≥20 | |
| Appendicular skeletal muscle mass (DXA) | ||
| Total (kg) | M: <20; F <15 | |
| Total/height2 (kg/m2) | M: <7.0; F <5.5 | M: <7.0; F: <5.4 |
M, men; F, women.
Finally, a more recent diagnostic algorithm was published by a group of experts of the International Society of Physical and Rehabilitation Medicine.17) That algorithm has several distinctive elements. For example, case finding includes adults with disorders related to the renin angiotensin system and the assessment of muscle mass is made using the sonographic thigh adjustment ratio. Tests of mobility such as gait speed and the ability to rise from a chair are used as the last step in the identification of severe sarcopenia.
PREVALENCE AND INCIDENCE OFSARCOPENIA
Not all older men and women have or will develop sarcopenia. Several studies have determined the prevalence of sarcopenia in various countries and in different patient populations. The results have been variable. It is possible that cultural and biological determinants of the various physiological factors that contribute to sarcopenia differ among populations. Another explanation offered for these discrepancies has been the use of different (or incomplete) definitions of sarcopenia. Furthermore, the use of different assessment techniques and instruments may have contributed to the lack of consistency among studies. In general, in studies using DXA to measure appendicular muscle mass in older community dwelling populations, estimates vary between 5% and 15%.58,61)
The prevalence of sarcopenia is reportedly higher in both acute and rehabilitation geriatric clinical services. For example, in one recent cross-sectional study of 601 patients using the AWGS 2019 criteria,58) the prevalence of probable sarcopenia (low handgrip strength and normal muscle mass) was estimated to be 24.6%, the prevalence of confirmed sarcopenia (low handgrip strength and low muscle mass) was 22.6%, and the prevalence of severe sarcopenia (low handgrip strength, low muscle mass, and impaired physical performance) was 19.7%.62) All of these estimates are higher than those published for the general population and may reflect the summation of acute effects of illness or injury, chronic co-morbidities, malnutrition associated with swallowing difficulties, and deconditioning associated with hospitalization for medical interventions.63)
Because it is of significant public health and clinical interest to be able to identify those at risk of developing the condition, some researchers have looked at predictors of future sarcopenia. In a very recent 2-year longitudinal study of 1636 older participants in South Korea who did not have sarcopenia at baseline, 13.5% of men and 11.7% of women developed the condition during the course of the study.64) The investigators used the AWGS 2019 criteria for diagnosis.58) A higher incidence of sarcopenia was reported when the participants had some of the following conditions at baseline: older age, lower body mass index, more co-morbidities (e.g., hypertension, diabetes, and atherosclerotic heart disease), lower levels of physical activity in women, lower engagement in resistance exercise training in men, or higher glycosylated hemoglobin and insulin resistance. In another cross-sectional study conducted in Japan, exercise habits during middle-age were found to protect against the development of sarcopenia in older age.65) It can be seen that some of these factors are modifiable and justify public health campaigns to identify and offer early rehabilitation to those at risk.
REHABILITATIVE INTERVENTIONS: THE CASE FOR STRENGTHENING EXERCISE
As mentioned in the previous section, observational studies have demonstrated that high levels of physical activity are associated with a lower incidence of sarcopenia.65) This observation was also reported before the current definitions were published and included a lower incidence of sarcopenic obesity in those who engaged in moderate or high physical activity.66) These epidemiological data highlight the importance of lifestyle habits in the development of sarcopenia.
Exercise training studies have shown that many physiological variables respond to the stimulus of frequent and regular exercise even in older men and women. The most effective and safe intervention to increase muscle mass and strength in older men and women is a type of exercise known in clinical rehabilitation as progressive resistance exercise (PRE: strength training, high resistance training, and strength conditioning). This observation was made as early as 1988.67) Interestingly, this type of exercise, not designed to impact aerobic capacity, was also shown to have a positive effect on determinants of oxygen consumption such as muscle capillarization and the concentration of oxidative enzymes.68)
The basic principles of exercise prescription also apply to older age groups and include the selection of exercise that overloads muscle (and other tissues), the frequency of training (2–4 sessions per week), the training sequence (three sets of 10–12 repetitions each), the intensity of training (40–80% of the one repetition maximum depending on the fitness and health condition of the person), and the duration of training (lifelong commitment). In a non-exerciser starting a training program, the intensity should be low during the first few weeks and increased as tolerated by the person. Resting periods between sets of 1–2 min duration are usually included. Similar recommendations were made in a recent systematic review of studies in physically frail elderly people.69) In general, the response of older individuals to this type of training is not uniform and, while some show significant adaptations, others do not.70) The latter group, known as non-responders, may require a more precise and individualized exercise prescription that takes into account specific genetic and environmental factors, among others.71) The optimization of exercise recommendations continues to be a research challenge. Finally, because a reduction in muscle mass occurs frequently together with a decline in bone mineral density, it is relevant to note the results of a recent meta-analysis showing the beneficial effects of resistance training on both skeletal muscle and the skeleton.72)
An interesting variant of PRE training is called power- or velocity-specific training. One key element of this type of training is the inclusion of fast concentric muscle actions combined with slow eccentric components. This type of training is considered to be more functional in nature because it takes into account “time” as a factor, which is an important consideration in the performance of many activities of daily living. In other words, many things in daily life must be done quickly. A series of exercises is performed using simple devices such as a chair and a stepper. Exercises can be done wearing a weighted vest as resistance (in addition to body weight). The benefits of power- or velocity-specific training in older age groups have been demonstrated in several clinical trials.73,74) A recent systematic review and meta-analysis reported evidence that power training has benefits on both physical function and on self-reported function.75)
In this section we have limited our discussion to the use of strengthening exercise in the rehabilitation of sarcopenia because of the strength of the available evidence. A discussion of other types of exercise is beyond the scope of this brief article and could be the topic of another review. However, it is important to recognize that interventions including multiple types of exercise, such as aerobic exercise, have been shown to have significant beneficial effects on mobility, strength, and muscle mass.76) Furthermore, exercise-based interventions are also effective in many chronic conditions frequently present in older men and women. For example, the inclusion of aerobic exercise in the treatment and rehabilitation of older men and women will also help with the management of age-associated health conditions or co-morbidities such as cardiovascular disease, hypertension, and diabetes.
Finally, a detailed discussion of other non-exercise interventions is beyond the scope of this review, but it is important to note that nutritional interventions based on an increase in protein intake, and especially higher leucine intake, have been shown to be synergistic with PRE. If the regular diet does not contain enough protein, a supplement can be considered. The value of vitamin D supplementation remains to be more clearly elucidated.
THE FUTURE OF SARCOPENIA
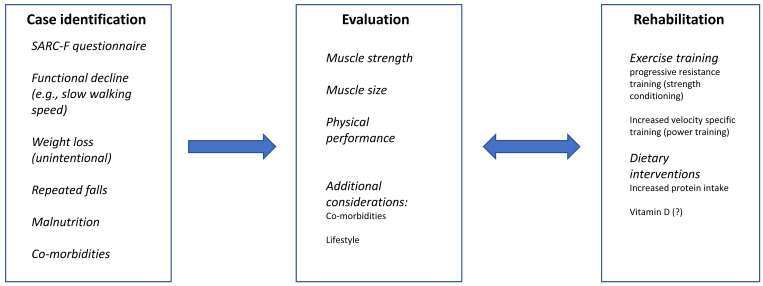
Future research should focus on the three fundamental areas of knowledge in sarcopenia (Fig. 2). Perhaps, one of the most urgent needs in the field of sarcopenia research is to reach consensus and agreement on the best definition for the condition. Since its first description in 1988 (published in 1989),2,3) the definition of sarcopenia has evolved to a description that includes multiple signs (see description of main elements of sarcopenia above) and typical clinical findings. Nonetheless, a comparison between the two very recent published definitions, one from Asia and one from Europe, discussed above, show that we have not reached a universal agreement. It is important to resolve these differences because it would contribute to a better description of the condition for all health professionals working with geriatric populations and a more precise determination of the true incidence and prevalence of this condition in various populations. Agreement would also help to orient research efforts designed to understand the fundamental cellular and physiological events leading to sarcopenia. Furthermore, techniques to evaluate persons with suspected sarcopenia as well as assessment strategies to establish a valid and reliable diagnosis will be influenced by a consensus definition. An excellent effort to achieve this goal is the Global Leadership Initiative in Sarcopenia (https://www.eugms.org/news/read/article/661.html).
Fig. 2.
Three fundamental steps in sarcopenia assessment, treatment, and rehabilitation.
The scientific agenda will continue to evolve as we learn more about the general process of aging as well as the basic molecular and cellular events that control muscle size, muscle activation, and the muscle capacity to generate force. It is worth noting that these findings will have an impact on the care of older men and women that goes beyond the identification and rehabilitation of sarcopenia. Basic research on inflammation, satellite cell biology, and cellular anabolic pathways, among other areas, is needed. Of equal importance is the identification of potential therapeutic targets and interventions that could be used to prevent, minimize, or reverse sarcopenia.
With regards to potential future interventions to rehabilitate sarcopenia, it is interesting to speculate about the potential benefits of important scientific advances of the past few decades. For example, in a recent review, the potential benefits of several pharmacologic agents that may target age-related impairments in basic molecular and cellular processes were discussed.77) One interesting aspect of the aging process mentioned above is the accumulation of senescent cells, which are unique in the sense that they eventually lose their proliferative potential and stop multiplying but become resistant to apoptosis and do not die. Instead, they become the source of proinflammatory cytokines and chemokines that may contribute to sarcopenia.21) Senolytics are a class of drugs that can selectively clear senescent cells.78) Experiments using animal models have shown that transplanting senescent cells into young mice results in physical dysfunction (slow walking speed, reduce grip strength) and spreads senescence to host tissue cells. Treatment of young and aged mice with senolytics increased survival and physical function.79) Furthermore, treatment with senolytics reduced the number of senescent cells and the secretion of pro-inflammatory cytokines in explants of human tissue, and senolytics have been used experimentally in patients with diabetic kidney disease.80) At the time of this writing, three clinical trials are registered (clinicaltrials.gov) using senolytics, one for skeletal health and two for Alzheimer’s disease, but none for the treatment of muscle atrophy and weakness.
The use of stem cells, including induced pluripotent stem cells, has received significant attention as a potential intervention in a variety of conditions where tissue regeneration may have clinical benefits.81) It has been shown that rejuvenation of existing muscle stem cells in animals is possible and may increase the regenerative potential in aged muscle.82) However, at the time of this writing, only one clinical trial was registered (clinicaltrials.gov) with the aim of studying the potential benefit of mesenchymal stem cells in the treatment of human frailty and included outcomes used to diagnose sarcopenia. Practical issues have limited the more extensive use of this approach, but it is reasonable to speculate that these issues will be resolved in the next few years.81)
Finally, the discovery of CRISPR (clustered regularly interspaced short palindromic repeats), a technology that has simplified the process of gene editing, suggests the possibility of altering genes that may be associated with the aging process,83) including those associated with sarcopenia.84) This technology is already being used in human clinical trials of various illnesses (https://www.labiotech.eu/best-biotech/crispr-technology-cure-disease/), including muscular dystrophy. To our knowledge, however, it is not being applied to rejuvenate or regenerate skeletal muscle in older men and women.
CONCLUDING REMARKS
The study of sarcopenia is important because life expectancy and the number of people in older age groups in the world are increasing. Although not every person develops age-related sarcopenia, a significant percentage of them do. Older persons, particularly those with impaired mobility, require a community infrastructure adapted to their needs and accessible health services. Groups of experts in different parts of the world should strive to find consensus on the topic of sarcopenia, agree on a definition that is acceptable and can be uniformly applied in epidemiological studies, and settle on a diagnostic process that will consider basic changes in skeletal muscle, placing particular emphasis on motor performance and functional mobility. The basic science of sarcopenia must continue to advance because this could be the source of innovative therapeutic and rehabilitative interventions. It remains to be seen whether research into personalized exercise prescription, stem cell biology, gene editing, pharmacological and nutritional interventions, and socioeconomic determinants of sarcopenia will have an impact on the incidence, prevalence, and progression of sarcopenia. Nonetheless, the many advances in this field and the increasing interest of the scientific community in this condition are encouraging.
ACKNOWLEDGMENTS
Dr. Frontera’s research is partially funded by Grant S21 MD001830-04, NIMHD, NIH, and Grant RO1 AG046149-07A1, NIMH, NIH. The content of this report is solely the responsibility of the author and does not necessarily represent the official views of the US National Institutes of Health.
Footnotes
CONFLICTS OF INTEREST: The author declares that there are no conflicts of interest.
REFERENCES
- 1.Lopreite M,Mauro M: The effects of population ageing on health care expenditure: a Bayesian VAR analysis using data from Italy. Health Policy 2017;121:663–674. 10.1016/j.healthpol.2017.03.015 [DOI] [PubMed] [Google Scholar]
- 2.Rosenberg IH: Summary comments. Am J Clin Nutr 1989;50:1231–1233. 10.1093/ajcn/50.5.1231 [DOI] [Google Scholar]
- 3.Rosenberg IH: Sarcopenia: origins and clinical relevance. J Nutr 1997;127(Suppl):990S–991S. 10.1093/jn/127.5.990S [DOI] [PubMed] [Google Scholar]
- 4.Comfort A: The Biology of Senescence. Churchill Livingstone, London, 1956. [Google Scholar]
- 5.United Nations: United Nations World Population Prospects 2019. revision. https://population.un.org/wpp/. Accessed June 23, 2022.
- 6.Aburto JM,Schöley J,Kashnitsky I,Zhang L,Rahal C,Missov TI,Mills MC,Dowd JB,Kashyap R: Quantifying impacts of the COVID-19 pandemic through life-expectancy losses: a population-level study of 29 countries. Int J Epidemiol 2022;51:63–74. 10.1093/ije/dyab207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robine J-M,Cubaynes S: Worldwide demography of centenarians. Mech Ageing Dev 2017;165:59–67. 10.1016/j.mad.2017.03.004 [DOI] [PubMed] [Google Scholar]
- 8.Buettner D: The Blue Zones. National Geographic Society, Washington DC, USA, 2012. [Google Scholar]
- 9.Santos-Lozano A,Sanchis-Gomar F,Pareja-Galeano H,Fiuza-Luces C,Emanuele E,Lucia A,Garatachea N: Where are supercentenarians located? A worldwide demographic study. Rejuvenation Res 2015;18:14–19. 10.1089/rej.2014.1609 [DOI] [PubMed] [Google Scholar]
- 10.Yashin AI,Arbeev KG,Wu D,Arbeeva LS,Bagley O,Stallard E,Kulminski AM,Akushevich I,Fang F,Wojczynski MK,Christensen K,Newman AB,Boudreau RM,Province MA,Thielke S,Perls TT,An P,Elo I,Ukraintseva SV: Genetics of human longevity from incomplete data: new findings from the long life family study. J Gerontol A Biol Sci Med Sci 2018;73:1472–1481. 10.1093/gerona/gly057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lepers R,Stapley PJ,Cattagni T: Centenarian athletes: examples of ultimate human performance? Age Ageing 2016;45:729–733. 10.1093/ageing/afw111 [DOI] [PubMed] [Google Scholar]
- 12.da Silva AP,Matos A,Ribeiro R,Gil Â,Valente A,Bicho M,Gorjão-Clara J: Sarcopenia and osteoporosis in Portuguese centenarians. Eur J Clin Nutr 2017;71:56–63. 10.1038/ejcn.2016.174 [DOI] [PubMed] [Google Scholar]
- 13.Alcazar J,Aagaard P,Haddock B,Kamper RS,Hansen SK,Prescott E,Ara I,Alegre LM,Frandsen U,Suetta C: Assessment of functional sit-to-stand muscle power: cross-sectional trajectories across the lifespan. Exp Gerontol 2021;152:111448. . 10.1016/j.exger.2021.111448 [DOI] [PubMed] [Google Scholar]
- 14.McGrath RP,Vincent BM,Lee M,Kraemer WJ,Peterson MD: Handgrip strength, function, and mortality in older adults: a time-varying approach. Med Sci Sports Exerc 2018;50:2259–2266. 10.1249/MSS.0000000000001683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cooper R,Kuh D,Hardy R, Mortality Review Group, FALCon and HALCyon Study Teams: Objectively measured physical capability levels and mortality: systematic review and meta-analysis. BMJ 2010;341:c4467. 10.1136/bmj.c4467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prakash KC,Neupane S,Leino-Arjas P,Härmä M,von Bonsdorff MB,Rantanen T,von Bonsdorff ME,Hinrichs T,Seitsamo J,Ilmarinen J,Nygård CH: Trajectories of mobility limitations over 24 years and their characterization by shift work and leisure-time physical activity in midlife. Eur J Public Health 2019;29:882–888. 10.1093/eurpub/ckz069 [DOI] [PubMed] [Google Scholar]
- 17.Kara M,Kaymak B,Frontera WR,Ata AM,Ricci V,Ekiz T,Chang K-V,Han D-S,Michail X,Quittan M,Lim J-Y,Bean JF,Franchignoni F,Özçakar F: Diagnosing sarcopenia: functional perspectives and a new algorithm from ISarcoPRM. J Rehabil Med 2021;53:jrm00209. 10.2340/16501977-2851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lexell J,Taylor CC,Sjöström M: What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J Neurol Sci 1988;84:275–294. 10.1016/0022-510X(88)90132-3 [DOI] [PubMed] [Google Scholar]
- 19.Dickinson JM,Volpi E,Rasmussen BB: Exercise and nutrition to target protein synthesis impairments in aging skeletal muscle. Exerc Sport Sci Rev 2013;41:216–223. 10.1097/JES.0b013e3182a4e699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferrucci L,Penninx BW,Volpato S,Harris TB,Bandeen-Roche K,Balfour J,Leveille SG,Fried LP,Md JM: Change in muscle strength explains accelerated decline of physical function in older women with high interleukin-6 serum levels. J Am Geriatr Soc 2002;50:1947–1954. 10.1046/j.1532-5415.2002.50605.x [DOI] [PubMed] [Google Scholar]
- 21.Zhang X,Habiballa L,Aversa Z,Ng YE,Sakamoto AE,Englund DA,Pearsall VM,White TA,Robinson MM,Rivas DA,Dasari S,Hruby AJ,Lagnado AB,Jachim SK,Granic A,Sayer AA,Jurk D,Lanza IR,Khosla S,Fielding RA,Sreekumaran Nair K,Schafer MJ,Passos JF,LeBrasseur NK: Characterization of cellular senescence in aging skeletal muscle. Nat Aging 2022;. 2:601–615 10.1038/s43587-022-00250-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tuttle CS,Thang LA,Maier AB: Markers of inflammation and their association with muscle strength and mass: a systematic review and meta-analysis. Ageing Res Rev 2020;64:101185. 10.1016/j.arr.2020.101185 [DOI] [PubMed] [Google Scholar]
- 23.Ferrucci L,Gonzalez-Freire M,Fabbri E,Simonsick E,Tanaka T,Moore Z,Salimi S,Sierra F,Cabo R: Measuring biological aging in humans: a quest. Aging Cell 2020;19:e13080. 10.1111/acel.13080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deminice R,Oliveira VH,Webel AR,Erlandson KM: Sarcopenia related to human immunodeficiency virus: protective effects of exercise. Exerc Sport Sci Rev 2022;50:73–80. 10.1249/JES.0000000000000282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Piotrowicz K,Gąsowski J,Michel JP,Veronese N: Post-COVID-19 acute sarcopenia: physiopathology and management. Aging Clin Exp Res 2021;33:2887–2898. 10.1007/s40520-021-01942-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Verdijk LB,Dirks ML,Snijders T,Prompers JJ,Beelen M,Jonkers RA,Thijssen DH,Hopman MT,Van Loon LJ: Reduced satellite cell numbers with spinal cord injury and aging in humans. Med Sci Sports Exerc 2012;44:2322–2330. 10.1249/MSS.0b013e3182667c2e [DOI] [PubMed] [Google Scholar]
- 27.McKay BR,Ogborn DI,Baker JM,Toth KG,Tarnopolsky MA,Parise G: Elevated SOCS3 and altered IL-6 signaling is associated with age-related human muscle stem cell dysfunction. Am J Physiol Cell Physiol 2013;304:C717–C728. 10.1152/ajpcell.00305.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Borja-Gonzalez M,Casas-Martinez JC,McDonagh B,Goljanek-Whysall K: Inflamma-miR-21 negatively regulates myogenesis during ageing. Antioxidants 2020;9:345. 10.3390/antiox9040345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Franco I,Johansson A,Olsson K,Vrtačnik P,Lundin P,Helgadottir HT,Larsson M,Revêchon G,Bosia C,Pagnani A,Provero P,Gustafsson T,Fischer H,Eriksson M: Somatic mutagenesis in satellite cells associates with human skeletal muscle aging. Nat Commun 2018;9:800. 10.1038/s41467-018-03244-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nederveen JP,Betz MW,Snijders T,Parise G: The importance of muscle capillarization for optimizing satellite cell plasticity. Exerc Sport Sci Rev 2021;49:284–290. 10.1249/JES.0000000000000270 [DOI] [PubMed] [Google Scholar]
- 31.Dahlqvist JR,Vissing CR,Hedermann G,Thomsen C,Vissing J: Fat replacement of paraspinal muscles with aging in healthy adults. Med Sci Sports Exerc 2017;49:595–601. 10.1249/MSS.0000000000001119 [DOI] [PubMed] [Google Scholar]
- 32.Akazawa N,Kishi M,Hino T,Tsuji R,Tamura K,Hioka A,Moriyama H: Relationship between muscle mass and fraction of intramuscular adipose tissue of the quadriceps in older inpatients. PLoS One 2022;17:e0263973. 10.1371/journal.pone.0263973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frontera WR,Hughes VA,Lutz KJ,Evans WJ: A cross-sectional study of muscle strength and mass in 45- to 78-yr-old men and women. J Appl Physiol 1991;71:644–650. 10.1152/jappl.1991.71.2.644 [DOI] [PubMed] [Google Scholar]
- 34.Frontera WR,Hughes VA,Fielding RA,Fiatarone MA,Evans WJ,Roubenoff R: Aging of skeletal muscle: a 12-yr longitudinal study. J Appl Physiol 2000;88:1321–1326. 10.1152/jappl.2000.88.4.1321 [DOI] [PubMed] [Google Scholar]
- 35.Hughes VA,Frontera WR,Wood M,Evans WJ,Dallal GE,Roubenoff R,Singh MA: Longitudinal muscle strength changes in older adults: influence of muscle mass, physical activity, and health. J Gerontol A Biol Sci Med Sci 2001;56:B209–B217. 10.1093/gerona/56.5.B209 [DOI] [PubMed] [Google Scholar]
- 36.Rozand V,Sundberg CW,Hunter SK,Smith A: Age-related deficits in voluntary activation: a systematic review and meta-analysis. Med Sci Sports Exerc 2020;52:549–560. 10.1249/MSS.0000000000002179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kara M,Özçakar L,Kaymak B,Ata A,Frontera W: A “Neuromuscular Look” to sarcopenia: Is it a movement disorder? J Rehabil Med 2020;52:jrm00042. 10.2340/16501977-2672 [DOI] [PubMed] [Google Scholar]
- 38.Venturelli M,Reggiani C,Schena F: Beyond the current knowledge on sarcopenia: new insight on neuromuscular factors. Aging Clin Exp Res 2022;34:1183–1185. 10.1007/s40520-022-02082-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Frontera WR,Suh D,Krivickas LS,Hughes VA,Goldstein R,Roubenoff R: Skeletal muscle fiber quality in older men and women. Am J Physiol Cell Physiol 2000;279:C611–C618. 10.1152/ajpcell.2000.279.3.C611 [DOI] [PubMed] [Google Scholar]
- 40.Rantakokko M,Mänty M,Rantanen T: Mobility decline in old age. Exerc Sport Sci Rev 2013;41:19–25. 10.1097/JES.0b013e3182556f1e [DOI] [PubMed] [Google Scholar]
- 41.Gerstner GR,Thompson BJ,Rosenberg JG,Sobolewski EJ,Scharville MJ,Ryan ED: Neural and muscular contributions to the age-related reductions in rapid strength. Med Sci Sports Exerc 2017;49:1331–1339. 10.1249/MSS.0000000000001231 [DOI] [PubMed] [Google Scholar]
- 42.McKinnon NB,Connelly DM,Rice CL,Hunter SW,Doherty TJ: Neuromuscular contributions to the age-related reduction in muscle power: mechanisms and potential role of high velocity power training. Ageing Res Rev 2017;35:147–154. 10.1016/j.arr.2016.09.003 [DOI] [PubMed] [Google Scholar]
- 43.Reid KF,Pasha E,Doros G,Clark DJ,Patten C,Phillips EM,Frontera WR,Fielding RA: Longitudinal decline of lower extremity muscle power in healthy and mobility-limited older adults: influence of muscle mass, strength, composition, neuromuscular activation and single fiber contractile properties. Eur J Appl Physiol 2014;114:29–39. 10.1007/s00421-013-2728-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alcazar J,Alegre LM,Suetta C,Júdice PB,Van Roie E,González-Gross M,Rodríguez-Mañas L,Casajús JA,Magalhães JO,Nielsen BR,García-García FJ,Delecluse C,Sardinha LB,Ara I: Threshold of relative muscle power required to rise from a chair and mobility limitations and disability in older adults. Med Sci Sports Exerc 2021;53:2217–2224. 10.1249/MSS.0000000000002717 [DOI] [PubMed] [Google Scholar]
- 45.Cummings SR,Studenski S,Ferrucci L: A diagnosis of dismobility – giving mobility clinical visibility: a Mobility Working Group recommendation. JAMA 2014;311:2061–2062. 10.1001/jama.2014.3033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Beavers DP,Kritchevsky SB,Gill TM,Ambrosius WT,Anton SD,Fielding RA,King AC,Rejeski WJ,Lovato L,McDermott MM,Newman AB,Pahor M,Walkup MP,Tracy RP,Manini TM: Elevated IL-6 and CRP levels are associated with incident self-reported major mobility disability: a pooled analysis of older adults with slow gait speed. J Gerontol A Biol Sci Med Sci 2021;76:2293–2299. 10.1093/gerona/glab093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lim JY,Frontera WR: Single skeletal muscle fiber mechanical properties: a muscle quality biomarker of human aging. Eur J Appl Physiol 2022;122:1383–1395. 10.1007/s00421-022-04924-4 [DOI] [PubMed] [Google Scholar]
- 48.Li M,Ogilvie H,Ochala J,Artemenko K,Iwamoto H,Yagi N,Bergquist J,Larsson L: Aberrant post-translational modifications compromise human myosin motor function in old age. Aging Cell 2015;14:228–235. 10.1111/acel.12307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moen RJ,Klein JC,Thomas DD: Electron paramagnetic resonance resolves effects of oxidative stress on muscle proteins. Exerc Sport Sci Rev 2014;42:30–36. 10.1249/JES.0000000000000004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang Y,Chen JS,He Q,He X,Basava RR,Hodgson J,Sinha U,Sinha S: Microstructural analysis of skeletal muscle force generation during aging. Int J Numer Methods Biomed Eng 2020;36:e3295. 10.1002/cnm.3295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nishikawa K: Titin: a tunable spring in active muscle. Physiology (Bethesda) 2020;35:209–217. 10.1152/physiol.00036.2019 [DOI] [PubMed] [Google Scholar]
- 52.Miller MS,Bedrin NG,Callahan DM,Previs MJ,Jennings ME II,Ades PA,Maughan DW,Palmer BM,Toth MJ: Age-related slowing of myosin actin cross-bridge kinetics is sex specific and predicts decrements in whole skeletal muscle performance in humans. J Appl Physiol 2013;115:1004–1014. 10.1152/japplphysiol.00563.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tezze C,Romanello V,Desbats MA,Fadini GP,Albiero M,Favaro G,Ciciliot S,Soriano ME,Morbidoni V,Cerqua C,Loefler S,Kern H,Franceschi C,Salvioli S,Conte M,Blaauw B,Zampieri S,Salviati L,Scorrano L,Sandri M: Age-associated loss of OPA1 in muscle impacts muscle mass, metabolic homeostasis, systemic inflammation, and epithelial senescence. Cell Metab 2017;25:1374–1389.e6. 10.1016/j.cmet.2017.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hood DA,Memme JM,Oliveira AN,Triolo M: Maintenance of skeletal muscle mitochondria in health, exercise, and aging. Annu Rev Physiol 2019;81:19–41. 10.1146/annurev-physiol-020518-114310 [DOI] [PubMed] [Google Scholar]
- 55.Correa-de-Araujo R,Addison O,Miljkovic I,Goodpaster BH,Bergman BC,Clark RV,Elena JW,Esser KA,Ferrucci L,Harris-Love MO,Kritchevsky SB,Lorbergs A,Shepherd JA,Shulman GI,Rosen CJ: Myosteatosis in the context of skeletal muscle function deficit: an interdisciplinary workshop at the National Institute on Aging. Front Physiol 2020;11:963. 10.3389/fphys.2020.00963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mazara N,Zwambag DP,Noonan AM,Weersink E,Brown SH,Power GA: Rate of force development is Ca2+-dependent and influenced by Ca2+-sensitivity in human single muscle fibres from older adults. Exp Gerontol 2021;150:111348. 10.1016/j.exger.2021.111348 [DOI] [PubMed] [Google Scholar]
- 57.Ochala J,Frontera WR,Dorer DJ,Hoecke JV,Krivickas LS: Single skeletal muscle fiber elastic and contractile characteristics in young and older men. J Gerontol A Biol Sci Med Sci 2007;62:375–381. 10.1093/gerona/62.4.375 [DOI] [PubMed] [Google Scholar]
- 58.Chen L-K,Woo J,Assantachai P,Auyeung T-W,Chou M-Y,Iijima K,Jang HC,Kang L,Kim M,Kim S,Kojima T,Kuzuya M,Lee JSW,Lee SY,Lee W-J,Lee Y,Liang C-K,Lim J-Y,Lim WS,Peng L-N,Sugimoto K,Tanaka T,Won CW,Yamada M,Zhang T,Akishita M,Arai H: Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J Am Med Dir Assoc 2020;21:300–307. 10.1016/j.jamda.2019.12.012 [DOI] [PubMed] [Google Scholar]
- 59.Cruz-Jentoft AJ,Bahat G,Bauer J,Boirie Y,Bruyère O,Cederholm T,Cooper C,Landi F,Rolland Y,Sayer AA,Schneider SM,Sieber CC,Topinkova E,Vandewoude M,Visser M,Zamboni M: Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2) and the Extended Group for EWGSOP2: Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019;48:16–31. 10.1093/ageing/afy169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Malmstrom TK,Miller DK,Simonsick EM,Ferrucci L,Morley JE: SARC-F: a symptom score to predict persons with sarcopenia at risk for poor functional outcomes. J Cachexia Sarcopenia Muscle 2016;7:28–36. 10.1002/jcsm.12048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li R,Xia J,Zhang X,Gathirua-Mwangi WG,Guo J,Li Y,McKenzie S,Song Y: Associations of muscle mass and strength with all-cause mortality among US older adults. Med Sci Sports Exerc 2018;50:458–467. 10.1249/MSS.0000000000001448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bertschi D,Kiss CM,Beerli N,Kressig RW: Sarcopenia in hospitalized geriatric patients: insights into prevalence and associated parameters using new EWGSOP2 guidelines. Eur J Clin Nutr 2021;75:653–660. 10.1038/s41430-020-00780-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nishioka S,Matsushita T,Yamanouchi A,Okazaki Y,Oishi K,Nishioka E,Mori N,Tokunaga Y,Onizuka S: Prevalence and associated factors of coexistence of malnutrition and sarcopenia in geriatric rehabilitation. Nutrients 2021;13:3745. 10.3390/nu13113745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Choe HJ,Cho BL,Park YS,Roh E,Kim HJ,Lee SG,Kim BJ,Kim M,Won CW,Park KS,Jang HC: Gender differences in risk factors for the 2 year development of sarcopenia in community‐dwelling older adults. J Cachexia Sarcopenia Muscle 2022;13:1908–1918. 10.1002/jcsm.12993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Akune T,Muraki S,Oka H,Tanaka S,Kawaguchi H,Nakamura K,Yoshimura N: Exercise habits during middle age are associated with lower prevalence of sarcopenia: the ROAD study. Osteoporos Int 2014;25:1081–1088. 10.1007/s00198-013-2550-z [DOI] [PubMed] [Google Scholar]
- 66.Ryu M,Jo J,Lee Y,Chung YS,Kim KM,Baek WC: Association of physical activity with sarcopenia and sarcopenic obesity in community-dwelling older adults: the Fourth Korea National Health and Nutrition Examination Survey. Age Ageing 2013;42:734–740. 10.1093/ageing/aft063 [DOI] [PubMed] [Google Scholar]
- 67.Frontera WR,Meredith CN,O’Reilly KP,Knuttgen HG,Evans WJ: Strength conditioning in older men: skeletal muscle hypertrophy and improved function. J Appl Physiol 1988;64:1038–1044. 10.1152/jappl.1988.64.3.1038 [DOI] [PubMed] [Google Scholar]
- 68.Frontera WR,Meredith CN,O’Reilly KP,Evans WJ: Strength training and determinants of VO2max in older men. J Appl Physiol 1990;68:329–333. 10.1152/jappl.1990.68.1.329 [DOI] [PubMed] [Google Scholar]
- 69.Lopez P,Pinto RS,Radaelli R,Rech A,Grazioli R,Izquierdo M,Cadore EL: Benefits of resistance training in physically frail elderly: a systematic review. Aging Clin Exp Res 2018;30:889–899. 10.1007/s40520-017-0863-z [DOI] [PubMed] [Google Scholar]
- 70.Lavin KM,Roberts BM,Fry CS,Moro T,Rasmussen BB,Bamman MM: The importance of resistance exercise training to combat neuromuscular aging. Physiology (Bethesda) 2019;34:112–122. 10.1152/physiol.00044.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Izquierdo M,Duque G,Morley JE: Physical activity guidelines for older people: knowledge gaps and future directions. Lancet Healthy Longev 2021;2:e380–e383. 10.1016/S2666-7568(21)00079-9 [DOI] [PubMed] [Google Scholar]
- 72.O’Bryan SJ,Giuliano C,Woessner MN,Vogrin S,Smith C,Duque G,Levinger I: Progressive resistance training for concomitant increases in muscle strength and bone mineral density in older adults: a systematic review and meta-analysis. Sports Med 2022;52:1939–1960. 10.1007/s40279-022-01675-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Reid KF,Callahan DM,Carabello RJ,Phillips EM,Frontera WR,Fielding RA: Lower extremity power training in elderly subjects with mobility limitations: a randomized controlled trial. Aging Clin Exp Res 2008;20:337–343. 10.1007/BF03324865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bean JF,Kiely DK,LaRose S,O’Neill E,Goldstein R,Frontera WR: Increased velocity exercise specific to task (InVEST) training vs. the National Institute on Aging’s (NIA) strength training program: changes in limb power and mobility. J Gerontol A Biol Sci Med Sci 2009;64A:983–991. 10.1093/gerona/glp056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Balachandran AT,Steele J,Angielczyk D,Belio M,Schoenfeld BJ,Quiles N,Askin N,Abou-Setta AM: Comparison of power training vs traditional strength training on physical function in older adults: a systematic review and meta-analysis. JAMA Netw Open 2022;5:e2211623. 10.1001/jamanetworkopen.2022.11623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bernabei R,Landi F,Calvani R,Cesari M,Del Signore S,Anker SD,Bejuit R,Bordes P,Cherubini A,Cruz-Jentoft AJ,Di Bari M,Friede T,Gorostiaga Ayestarán C,Goyeau H,Jónsson PV,Kashiwa M,Lattanzio F,Maggio M,Mariotti L,Miller RR,Rodriguez-Mañas L,Roller-Wirnsberger R,Rýznarová I,Scholpp J,Schols AMWJ,Sieber CC,Sinclair AJ,Skalska A,Strandberg T,Tchalla A,Topinková E,Tosato M,Vellas B,von Haehling S,Pahor M,Roubenoff R,Marzetti E, SPRINTT consortium: Multicomponent intervention to prevent mobility disability in frail older adults: randomised controlled trial (SPRINTT project). BMJ 2022;377:e068788. 10.1136/bmj-2021-068788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li Z,Zhang Z,Ren Y,Wang Y,Fang J,Yue H,Ma S,Guan F: Aging and age‐related diseases: from mechanisms to therapeutic strategies. Biogerontology 2021;22:165–187. 10.1007/s10522-021-09910-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Robbins PD,Jurk D,Khosla S,Kirkland JL,LeBrasseur NK,Miller JD,Passos JF,Pignolo RJ,Tchkonia T,Niedernhofer LJ: Senolytic drugs: reducing senescent cell viability to extend health span. Annu Rev Pharmacol Toxicol 2021;61:779–803. 10.1146/annurev-pharmtox-050120-105018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xu M,Pirtskhalava T,Farr JN,Weigand BM,Palmer AK,Weivoda MM,Inman CL,Ogrodnik MB,Hachfeld CM,Fraser DG,Onken JL,Johnson KO,Verzosa GC,Langhi LG,Weigl M,Giorgadze N,LeBrasseur NK,Miller JD,Jurk D,Singh RJ,Allison DB,Ejima K,Hubbard GB,Ikeno Y,Cubro H,Garovic VD,Hou X,Weroha SJ,Robbins PD,Niedernhofer LJ,Khosla S,Tchkonia T,Kirkland JL: Senolytics improve physical function and increase lifespan in old age. Nat Med 2018;24:1246–1256. 10.1038/s41591-018-0092-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hickson LJ,Langhi Prata LG,Bobart SA,Evans TK,Giorgadze N,Hashmi SK,Herrmann SM,Jensen MD,Jia Q,Jordan KL,Kellogg TA,Khosla S,Koerber DM,Lagnado AB,Lawson DK,LeBrasseur NK,Lerman LO,McDonald KM,McKenzie TJ,Passos JF,Pignolo RJ,Pirtskhalava T,Saadiq IM,Schaefer KK,Textor SC,Victorelli SG,Volkman TL,Xue A,Wentworth MA,Wissler Gerdes EO,Zhu Y,Tchkonia T,Kirkland JL: Senolytics decrease senescent cells in humans: preliminary report from a clinical trial of dasatinib plus quercetin in individuals with diabetic kidney disease. EBioMedicine 2019;47:446–456. 10.1016/j.ebiom.2019.08.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yamanaka S: Pluripotent stem cell-based cell therapy – promise and challenges. Cell Stem Cell 2020;27:523–531. 10.1016/j.stem.2020.09.014 [DOI] [PubMed] [Google Scholar]
- 82.Cosgrove BD,Gilbert PM,Porpiglia E,Mourkioti F,Lee SP,Corbel SY,Llewellyn ME,Delp SL,Blau HM: Rejuvenation of the muscle stem cell population restores strength to injured aged muscles. Nat Med 2014;20:255–264. 10.1038/nm.3464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pratt J,Boreham C,Ennis S,Ryan AW,De Vito G: Genetic associations with aging muscle: a systematic review. Cells 2019;9:12. 10.3390/cells9010012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Khanal P,He L,Stebbings G,Onambele-Pearson GL,Degens H,Williams A,Thomis M,Morse CI: Prevalence and association of single nucleotide polymorphisms with sarcopenia in older women depends on definition. Sci Rep 2020;10:2913. 10.1038/s41598-020-59722-9 [DOI] [PMC free article] [PubMed] [Google Scholar]