ABSTRACT
Background
Short-term randomized trials suggest that a 500 mg/d vitamin C supplement reduces serum urate, whereas observational studies show vitamin E is inversely associated with gout risk.
Objectives
We evaluated the effect of supplemental vitamin C (prespecified primary exposure) and vitamin E (prespecified secondary exposure) on new diagnoses of gout.
Methods
We performed a post hoc analysis of data from the Physicians’ Health Study II, a randomized, double-blind, placebo-controlled factorial trial of randomized vitamin C (500 mg/d) and vitamin E (400 IU every other day). The primary outcome was new gout diagnoses, self-reported at baseline and throughout the follow-up period of ≤10 y.
Results
Of 14,641 randomly assigned male physicians in our analysis, the mean age was 64 ± 9 y; 1% were Black, and 6.5% had gout prior to randomization. The incidence rate of new gout diagnoses during follow-up was 8.0 per 1000 person-years among those assigned vitamin C compared with 9.1 per 1000 person-years among those assigned placebo. The vitamin C assignment reduced new gout diagnoses by 12% (HR: 0.88; 95% CI: 0.77, 0.99; P = 0.04). These effects were greatest among those with a BMI <25 kg/m 2 (P-interaction = 0.01). Vitamin E was not associated with new gout diagnoses (HR: 1.05; 95% CI: 0.92, 1.19; P = 0.48).
Conclusions
Vitamin C modestly reduced the risk of new gout diagnoses in middle-aged male physicians. Additional research is needed to determine the effects of higher doses of vitamin C supplementation on serum urate and gout flares in adults with established gout.
The Physicians’ Health Study II is registered at clinicaltrials.gov (identifier: NCT00270647).
Keywords: vitamin C, vitamin E, gout, randomized trial, uric acid
Introduction
Gout is a form of painful arthropathy characterized by attacks of severe joint pain that reflect an intense inflammatory response to precipitation of urate crystals in articular tissues (1, 2). Furthermore, gout affects >5% of US males (3), and hospitalization related to gout has been increasing in the United States and Canada (4, 5). Hyperuricemia is a leading risk factor of both incident and recurrent gout (6, 7) as well as the target of preventive treatment strategies, including weight loss and healthy food choices (8). Although urate-lowering pharmaceuticals are the mainstay strategy to prevent gout flares in patients with gout, it has long been hypothesized that vitamin C (ascorbic acid), an essential water-soluble antioxidant, can also be used to prevent gout in adults with and without a history of gout. Moreover, there has been limited improvement in urate-lowering therapies over time, suggesting the importance of population-wide prevention strategies (3).
Dietary and supplemental vitamin C intake is inversely associated with gout in observational studies (9–12), and in molecular studies, vitamin C has been shown to disassemble urate crystals (13). Animal models have shown that vitamin C inhibits urate synthesis (14), whereas human physiology studies have demonstrated that vitamin C increases urinary excretion of urate (15). Furthermore, a meta-analysis of clinical trials demonstrated that vitamin C supplementation lowered urate in adults without gout, particularly at a dose of 500 mg/d (16). However, the extent to which 500 mg/d vitamin C supplementation might prevent gout has never been demonstrated in a long-term randomized clinical trial.
Vitamin E intake has also been inversely associated with hyperuricemia in the US population (17). Moreover, it has been shown to lower urate in select rat models (18) and in human case reports (19). This has led some to speculate whether interventions high in vitamin E might be used for hyperuricemia and gout (20). However, this has never been tested in a randomized trial.
In the present study, we used data from the Physicians’ Health Study II (PHS II) to examine the effect of randomized vitamin C supplementation on new diagnoses of gout and to determine whether the association was modified by cardiovascular disease (CVD)–related subgroups. We hypothesized that by lowering serum urate concentrations, vitamin C would reduce risk of new diagnoses of gout. We secondarily examined the effect of vitamin E on new diagnoses of gout.
Methods
The PHS II was an investigator-initiated, 2 × 2 × 2 × 2 factorial, randomized, double-blind, placebo-controlled trial conducted from July 1997 to August 2007 at Brigham and Women's Hospital in Boston, Massachusetts (21, 22). In brief, the PHS II examined the effects of vitamin C, vitamin E, a multivitamin, and β-carotene on cancer and CVD risk (21, 23, 24). The β-carotene intervention was terminated early at the recommendation of the PHS II Data Safety and Monitoring Board, whereas the multivitamin intervention was continued beyond the vitamin C and vitamin E study period (21, 23, 24). All participants provided written informed consent to participate in the clinical trial. The PHS II was approved by the Institutional Review Board of Brigham and Women's Hospital.
Study population
PHS II enrolled and randomly assigned 14,641 US male physicians aged ≥50 y, excluding those with a history of cirrhosis, active liver disease, taking anticoagulants, or reporting a serious illness that might preclude participation. Participants were required to forego any current use of multivitamin supplements containing >100% of the recommended daily allowance of vitamin C, vitamin E, β-carotene, or vitamin A throughout the study, as well as individual use of vitamin C, vitamin E, or β-carotene supplements.
In PHS II, participants were followed for ≤10 y for the occurrence of a disease end point, death, last known questionnaire return date, or the end of the vitamin C and vitamin E interventions on August 31, 2007. The primary end point of the CVD study was major cardiovascular events, a composite end point that included nonfatal myocardial infarction (MI), nonfatal stroke, and cardiovascular mortality. In the present study, follow-up was also a maximum of 10 y and continued up to the occurrence of gout, death, last known questionnaire return date, or the end of the vitamin C and vitamin E interventions, whichever came first.
Trial interventions and follow-up
Participants were randomly allocated to the following PHS II interventions in a factorial design: vitamin C (500 mg synthetic ascorbic acid or its placebo daily; BASF Corporation) or vitamin E (400 IU synthetic α-tocopherol or its placebo every other day; BASF Corporation). Randomized multivitamin and β-carotene assignments are described elsewhere, but were taken in combination with vitamin C and vitamin E as part of a 2 × 2 × 2 × 2 factorial, placebo-controlled randomized clinical trial (21–23).
During the trial, participants received monthly calendar packs containing vitamin C or placebo (taken daily) and vitamin E or placebo (taken every other day) every 6 mo for the first year and annually thereafter. Treatment and follow-up continued in blinded fashion through August 31, 2007, the scheduled end of the vitamin C and E components of the PHS II.
On each follow-up questionnaire, self-reported adherence was defined as taking at least two-thirds of the study agents. For active vitamin C and its placebo, the proportion of adherent participants at 4 y was 78% and 78% (P = 0.99), and at the end of follow-up, 71% and 71% (P = 0.54) (21, 23). For active vitamin E and its placebo, the proportion of adherent participants at 4 y was 78% and 77% (P = 0.12), and at the end of follow-up (mean of 8 y), the proportion of adherent participants was 72% and 70% (P = 0.004) (21, 23). There were no differences between groups in the average rates of nontrial vitamin C (3.8% active and 4.4% placebo) or vitamin E (3.2% active and 3.1% placebo) supplement use for ≥31 d/y at the end of the trial (each P > 0.05).
Primay study outcome: Gout
The primary outcome of this post hoc analysis was self-reported new diagnosis of gout based on an affirmative response to the following question: “Since you last responded to a questionnaire were you newly diagnosed as having gout? (yes, no).” This self-reported approach has been shown to accurately assess gout and has been used in other studies (25–27). Participants responding “yes” were asked to provide the date of the gout diagnosis. When a date was not reported (27.1% of the time across annual surveys) we used the date of the questionnaire as the diagnosis date. Participants were asked about gout status yearly throughout the trial follow-up period; during the first year of follow-up they were asked twice (separated by a 6-mo interval).
Baseline covariates
We used the following self-reported covariates determined during the PHS II baseline questionnaire: age, race (Black or non-Black), BMI (kg/m2) from self-reported height and weight, history of gout, hypertension, high cholesterol, diabetes, MI or stroke, current alcohol use, aspirin use in the past 12 mo, and history of cancer. Serum creatinine was previously measured at baseline via a modified Jaffe method in a subcohort of 5287 participants to estimate glomerular filtration rate (28).
Statistical analyses
We first compared baseline population characteristics by the randomized vitamin C and vitamin E assignments using means (±SD) and proportions. Data were analyzed separately for randomized vitamin C and vitamin E. Cox proportional hazards models with a multiplicative interaction term between vitamin C and vitamin E were used to determine there was no interaction between these 2 assignments. Our primary comparison was for randomized vitamin C compared with placebo, with a secondary comparison for randomized vitamin E compared with placebo. Time to first gout event after randomization according to randomized vitamin C and vitamin E status was illustrated using cumulative incidence plots. We also determined incidence rates and HRs comparing vitamin C or vitamin E and gout risk using Cox proportional hazards models that included age at randomization, recruitment source (a prior PHS I or newly recruited PHS II participant), β-carotene assignment, multivitamin assignment, and vitamin C or E assignment. These main effects models included stratification by baseline gout status to account for different risk relations conferred by a prior history of gout. We tested the proportional hazards assumption for both vitamin C and E treatment via a time-dependent covariate analysis with models that included age at randomization as well as randomized supplement assignments (vitamin C, vitamin E, β-carotene, and multivitamin). Models were repeated and compared with and without participants with gout at baseline. The test for nonproportionality was nonsignificant for both vitamin C (P = 0.46) and vitamin E (P = 0.38).
We also considered whether the effect of the vitamin C intervention on gout was modified according to the following prespecified subgroups: age (<60, 60–69, ≥70 y), black race (no or yes), gout reported at baseline (no or yes), BMI (<25, 25 to <30, ≥30 kg/m2), alcohol use (rarely/never or any), aspirin use (no or yes), history of hypertension (no or yes), history of high cholesterol (no or yes), history of diabetes (no or yes), history of MI or stroke (no or yes), and estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2 [i.e., stage 3 chronic kidney disease (CKD); no or yes].
All analyses were performed in SAS version 9.4 (SAS Institute). A 2-tailed P value <0.05 was considered statistically significant.
Results
Baseline characteristics
Baseline characteristics of PHS II participants according to vitamin C assignment are shown in Table 1 (see Supplemental Figure 1 for number of participants by assignment). There were nominal differences by intervention assignment.
TABLE 1.
Baseline characteristics of the Physicians’ Health Study II population according to vitamin C assignment
| Placebo, n = 73121 | Active, n = 73291 | |
|---|---|---|
| Mean ± SD or % | Mean ± SD or % | |
| Age, y | 64.3 ± 9.1 | 64.3 ± 9.2 |
| Black, % | 1 | 1 |
| BMI, kg/m2 | 25.9 ± 3.4 | 26.0 ± 3.4 |
| History of hypertension, % | 43 | 41 |
| History of high cholesterol, % | 35 | 36 |
| History of diabetes, % | 6 | 6 |
| History of myocardial infarction or stroke, % | 5 | 5 |
| Serum creatinine, mg/dL | 1.0 ± 0.2 | 1.0 ± 0.3 |
| Estimated glomerular filtration rate <60 mL/min/1.73 m2,% | 5 | 6 |
| Current alcohol use, % | 81 | 81 |
| Aspirin use, % | 76 | 76 |
| Gout, % | 7 | 6 |
| History of cancer, % | 9 | 9 |
| β-Carotene assignment, % | 50 | 50 |
| Vitamin C assignment, % | 0 | 100 |
| Vitamin E assignment, % | 50 | 50 |
| Multivitamin assignment, % | 50 | 50 |
Number reporting race was 7293/7312 for those assigned placebo/active vitamin E; for BMI n = 7310/7328; for hypertension n = 7271/7291; for high cholesterol n = 7038/7118; for diabetes n = 7304/7322; for creatinine and estimated glomerular filtration rate n = 2654/2633; for current alcohol use n = 7262/7284, and for aspirin use n = 7185/7243.
Effects of vitamin C on gout
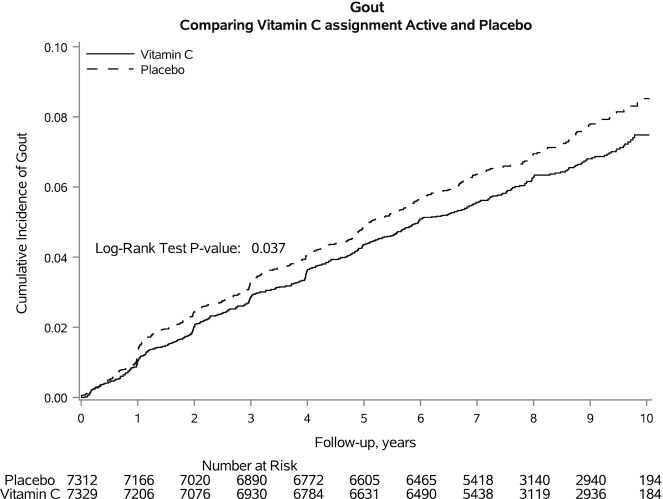
The cumulative incidence of gout is shown in Figure 1. The total incidence rate of gout was 8.6 (95% CI: 8.0, 9.1) per 1000 person-years; the incidence rate was 80.8 (95% CI: 73.1, 88.5) per 1000 person-years among those with gout at baseline and 5.1 (95% CI: 4.7, 5.5) per 1000 person-years among those without gout at baseline. Those assigned to placebo had a higher incidence of gout (P = 0.037). The incidence rate among those assigned to vitamin C was 8.0 cases per 1000 person-years, whereas the incidence rate among those assigned to placebo was 9.1 per 1000 person-years (Table 2). Compared with placebo, the vitamin C assignment lowered the risk of gout with an HR of 0.88 (95% CI: 0.77, 0.99). Excluding adults with prevalent gout attenuated the association between vitamin C and new diagnoses of gout.
FIGURE 1.
Kaplan–Meier cumulative incidence plots of gout during the trial follow-up period according to vitamin C or placebo assignment.
TABLE 2.
Vitamin C (primary exposure) and risk of gout1
| Vitamin C | Placebo | Vitamin C vs. placebo | ||||
|---|---|---|---|---|---|---|
| n Events/n participants | Incidence rate (per 1000 person-years) | n Events/n participants | Incidence rate (per 1000 person-years) | HR (95%CI) | P | |
| Vitamin C vs. placebo | 454/7329 | 8.0 | 517/7312 | 9.1 | 0.88 (0.77, 0.99) | 0.04 |
| Vitamin C vs. placebo, excluding prevalent gout | 262/6865 | 4.8 | 290/6823 | 5.4 | 0.90 (0.76, 1.07) | 0.23 |
HRs were generated using Cox proportional hazards models that included age at randomization, recruitment source (prior PHS I or newly recruited PHS II participant), β-carotene assignment, multivitamin assignment, and vitamin C or E assignment. Models included baseline gout status as a stratification term. PHS, Physicians’ Health Study.
Stratified analyses
There was limited evidence of effect modification across strata of age, Black race, baseline gout diagnosis, alcohol use, aspirin use, hypertension, high cholesterol, diabetes, MI or stroke, and low eGFR (Table 3). Notably, there was evidence that the effect of vitamin C varied by baseline BMI. Incidence rates were 6.6, 8.8, and 15.9 per 1000 person-years for those with a BMI <25, 25 to <30, and ≥30 kg/m2. The HRs of vitamin C compared with placebo with gout for those with a BMI <25, 25 to <30, and ≥30kg/m2 were 0.74 (95% CI: 0.59, 0.92), 0.85 (95% CI: 0.71, 1.01), and 1.29 (95% CI: 0.95, 1.74), respectively (P-interaction = 0.01).
TABLE 3.
Association between vitamin C and gout according to subgroups1
| n Placebo/n active | HR (95% CI) | P-interaction2 | |
|---|---|---|---|
| Age, y | |||
| <60 | 2938/2953 | 0.80 (0.63, 1.01) | 0.66 |
| 60–69 | 2348/2348 | 0.90 (0.73, 1.10) | |
| ≥70 | 2026/2028 | 0.92 (0.75, 1.14) | |
| Black | |||
| No | 7239/7249 | 0.87 (0.77, 0.99) | 0.54 |
| Yes | 73/80 | 1.09 (0.44, 2.70) | |
| Prevalent gout | |||
| No | 6823/6865 | 0.90 (0.76, 1.07) | 0.58 |
| Yes | 489/464 | 0.87 (0.71, 1.05) | |
| BMI, kg/m2 | |||
| <25 | 3080/3060 | 0.74 (0.59, 0.92) | 0.01 |
| 25 to <30 | 3487/3505 | 0.85 (0.71, 1.01) | |
| ≥30 | 743/763 | 1.29 (0.95, 1.74) | |
| Rare/no alcohol use | |||
| No | 5896/5920 | 0.90 (0.79, 1.04) | 0.25 |
| Yes | 1366/1364 | 0.73 (0.52, 1.03) | |
| Aspirin use in the past 12 mo | |||
| No | 1623/1638 | 0.73 (0.54, 0.97) | 0.18 |
| Yes | 5562/5605 | 0.91 (0.79, 1.05) | |
| History of hypertension | |||
| No | 4154/4252 | 0.91 (0.74, 1.13) | 0.76 |
| Yes | 3117/3039 | 0.88 (0.75, 1.03) | |
| History of high cholesterol | |||
| No | 4472/4494 | 0.83 (0.70, 0.99) | 0.41 |
| Yes | 2566/2624 | 0.93 (0.77, 1.12) | |
| History of diabetes | |||
| No | 6841/6880 | 0.86 (0.76, 0.99) | 0.47 |
| Yes | 463/442 | 1.04 (0.68, 1.57) | |
| History of myocardial infarction or stroke | |||
| No | 6942/6945 | 0.89 (0.78, 1.02) | 0.32 |
| Yes | 370/384 | 0.71 (0.46, 1.09) | |
| eGFR <60 mL/min/1.73 m2 | |||
| No | 2258/2215 | 1.04 (0.82, 1.32) | 0.24 |
| Yes | 396/418 | 0.79 (0.52, 1.18) | |
HRs were generated using Cox proportional hazards models that included age at randomization, recruitment source (prior PHS I or newly recruited PHS II participant), β-carotene assignment, multivitamin assignment, and vitamin C or E assignment. Models included baseline gout status as a stratification term. Models were repeated with multiplicative interaction terms to enable comparisons across strata. eGFR, estimated glomerular filtration rate; PHS, Physicians’ Health Study.
Based on a joint test for an effect using Wald statistics that test whether the parameters associated with that effect are zero.
Effects of vitamin E on gout
We secondarily evaluated the effect of vitamin E on new diagnoses of gout. Participant characteristics were balanced by randomized vitamin E assignment (Supplemental Table 1). We found no effect between vitamin E assignment and newly diagnosed gout (HR: 1.05; 95% CI: 0.92, 1.19) (Supplemental Figure 2; Table 4). Excluding participants with gout prerandomization did not alter the lack of effect from vitamin E supplementation on gout risk.
TABLE 4.
Vitamin E (secondary exposure) and risk of gout1
| Vitamin E | Placebo | Vitamin E vs. placebo | ||||
|---|---|---|---|---|---|---|
| n Events/n participants | Incidence rate (per 1000 person-years) | n Events/n participants | Incidence rate (per 1000 person-years) | HR (95%CI) | P | |
| Vitamin E vs. placebo | 498/7315 | 8.8 | 473/7326 | 8.3 | 1.05 (0.92, 1.19) | 0.48 |
| Vitamin E vs. placebo, excluding prevalent gout | 275/6832 | 5.1 | 277/6856 | 5.1 | 1.00 (0.85, 1.19) | 0.96 |
HRs were generated using Cox proportional hazards models that included age at randomization, recruitment source (prior PHS I or newly recruited PHS II participant), β-carotene assignment, multivitamin assignment, and vitamin C or E assignment. Models included baseline gout status as a stratification term. PHS, Physicians’ Health Study.
Discussion
In this population of male physicians, we found that treatment with 500 mg/d supplemental vitamin C was associated with a lower risk of a new gout diagnosis, whereas vitamin E supplementation had no effect on newly diagnosed gout. Overall, the effects of vitamin C on gout were modest. Effects were greater in adults with a normal BMI. Additional trials are needed to examine the effects of higher doses of vitamin C on serum urate and gout in a population with more detailed characterization of gout symptoms and severity.
Elevated serum urate is one of the most important risk factors for gout (6, 7). Observational studies have demonstrated inverse associations of plasma ascorbate (29) or vitamin C intake (10) with serum urate. Animal models have demonstrated that vitamin C lowers serum urate (30, 31) by inhibiting urate synthesis (14). Moreover, in small clinical studies, vitamin C lowered serum urate by increasing the fractional clearance of urate (15, 32) through inhibition of anion exchange at the proximal tubule (33). In a meta-analysis of trials testing vitamin C (median vitamin C dose was 500 mg/d; median duration 30 d) predominantly in adults without gout, despite substantial heterogeneity, vitamin C reduced urate by −0.35 mg/dL (16). In a secondary analysis of a large vitamin C trial of adults without gout, urate-lowering effects were greater in those with higher serum urate at baseline (34). In adults with gout, a small pilot study suggested a mild, pre-post reduction in serum urate from vitamin C of 0.23 mg/dL; however, this was not statistically significant and this study did not examine new gout diagnoses (35). Although our study showed a reduction in gout risk, we did not find any difference in this effect by CKD status. Whether this implies a larger role for the effects of vitamin C on urate synthesis rather than renal clearance should be explored in subsequent research.
The potential for vitamin C to reduce gout risk has been suggested in a large prospective cohort study in which dietary vitamin C intake was associated with a lower incidence of gout (9). However, our study represents the first evidence from a large randomized trial that vitamin C supplementation might reduce new diagnoses of gout. Nevertheless, the effects of vitamin C beyond existing pharmacological treatments cannot be established in our study, and our findings should not be viewed as a justification to forego urate-lowering therapies in adults with prior gout. Research is needed to understand the role of vitamin C in those actively treated with urate-lowering therapy (35). Furthermore, given the small magnitude of effect observed from vitamin C, future work should prospectively examine the impact of vitamin C dose on urate reduction.
In stratified analyses, our data suggested vitamin C could be more efficacious in preventing gout in adults with a normal BMI. Potential mechanisms for this observation are unclear. It is possible that this reflects volume of distribution and dose effects; that is, 500 mg/d is an inadequate dose for adults with higher BMI. Notably, several prospective studies have observed lower gout risk at even higher vitamin C intake, suggesting that 500 mg/d might not be adequate even in a general population (9). However, in one large study of vitamin C and serum urate there was no difference in short-term urate reduction across categories of BMI (<25 compared with ≥25 kg/m2) (34). Our observation could also reflect distinct phenotypes of gout in adults with higher BMI (36, 37) that are less prone to benefit from vitamin C supplementation. Indeed, obesity is a significant risk factor for gout (38–41) that is not directly affected by vitamin C supplementation. This might also be relevant for environmental and lifestyle practices that could differ across categories of BMI. It should also be noted that this analysis is based on self-reported BMI, which could contribute to misclassification of obesity status. Although more research is needed to confirm this finding, BMI could represent an important design consideration in future prospective studies and clinical trials that examine vitamin C and gout prevention.
Observational evidence suggests that vitamin E is inversely associated with hyperuricemia (17), underlying the hypothesis that vitamin E might prevent gout. Our study is among the first to test this hypothesis in the context of a randomized trial. We did not observe an association between vitamin E assignment and gout risk, suggesting that vitamin E supplementation is not an effective strategy to prevent new diagnoses of gout.
Our study has important limitations to consider. First, our findings might not be generalizable to broader populations given the absence of women, limited number of Black adults, and high education levels. Second, although compliance was high over the long-term course of the study intervention, it is possible that the effects would have been different with greater adherence or if participants were asked to refrain from consuming their recommended daily allowance of vitamin C in the form of multivitamins. Nevertheless, our high adherence rates and low out-of-study supplementation use could reflect typical supplement utilization in real life. Third, PHS II did not enroll participants based on gout history or hyperuricemia. Vitamin C might have greater urate-lowering effects in adults with hyperuricemia (34), so future studies should focus specifically on populations with hyperuricemia and gout as well as those taking urate-lowering drugs. Fourth, measures of serum urate at baseline and follow-up were not available, and thus we are unable to quantify the effect of vitamin C on serum urate. Fifth, a single 500-mg/d vitamin C supplement was tested with no safety concerns during the trial (21). However, we could not evaluate whether higher amounts of vitamin C would be more effective or cause more side effects than 500 mg/d, which should be examined in future clinical trials. This is particularly important because we plan to evaluate the role of baseline dietary vitamin C intake as a modifier of the effect of 500 mg/d vitamin C supplementation tested in PHS II in future analyses. Sixth, gout was identified based on self-report and was not among the prespecified outcomes of PHS II that triggered medical record review and adjudication. As a result, some diagnostic misclassification is possible among these physician participants. Moreover, the questionnaire asked about newly diagnosed gout, which could be difficult to interpret in participants with a prior history of gout and does not quantify the severity or number of diagnoses in the preceding year. In addition, gout diagnoses were not confirmed with crystal aspiration. Associations might differ with more rigorous event ascertainment. Seventh, the magnitude of effect was relatively small. Whether this effect would be greater at higher doses of vitamin C should be evaluated in subsequent work. Eighth, our stratified analyses could be particularly susceptible to multiple comparisons and an increased possibility of Type 1 error. Ninth, medical treatment or recommendations after a diagnosis of gout were not characterized by this study. Further, incident cases of gout were censored at the reported date of diagnosis. Last, the magnitude of risk reduction observed for the effect of vitamin C was small. Although vitamin C is generally well-tolerated with limited side effects, this small magnitude of risk reduction and current lack of reproducibility is not sufficient for population-wide recommendations around vitamin C supplementation to reduce gout.
Our study also has notable strengths, including its randomized and masked design, high retention rates, large size, and long-term follow-up with 971 reported gout events. Other studies on vitamin C and gout were substantially smaller and did not look at gout as their clinical outcome (35). Moreover, the inclusion of a population of physicians might improve the diagnostic accuracy of self-reported gout.
In conclusion, in this population of middle-aged male physicians, 500 mg/d vitamin C supplementation could reduce the risk of a new gout diagnosis. This could represent a favorable option for some patients, but should not be viewed as a reason to discontinue or replace urate-lowering therapies. Subsequent research is needed to establish the efficacy of vitamin C to lower both urate and the symptoms, incidence, and severity of gout.
Supplementary Material
ACKNOWLEDGEMENTS
We thank participants of the Physicians’ Health Study II who volunteered in support of this research.
The authors’ responsibilities were as follows—SPJ and HDS: conceptualized and designed the study; SPJ: drafted the manuscript; JMG, RJG, JEB, and HDS: assisted with data collection; NG and VYB: analyzed the data; and all authors: provided critical edits to the manuscript, and read and approved the final version.
The authors report no conflicts of interest.
Notes
SPJ is supported by an NIH/National Heart, Lung, and Blood Institute (NHLBI) grant (7K23HL135273). The Physicians' Health Study II is supported by grants was supported grants CA 097193, CA 34944, CA 40360, HL 26490, and HL 34595 from the National Institutes of Health, Bethesda, CA USA. Study agents and packaging were provided by BASF Corporation, Pfizer (formerly Wyeth, American Home Products, and Lederle) (New York, NY, USA), and DSM Nutritional Products, Inc. (formerly Roche Vitamins) (Parsippany, NJ, USA).
Supplemental Figures 1 and 2 and Supplementa Table 1 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: CKD, chronic kidney disease; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; MI, myocardial infarction; PHS II, Physicians’ Health Study II.
Contributor Information
Stephen P Juraschek, Division of General Medicine, Department of Medicine, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, MA, USA.
J Michael Gaziano, Division of Aging, Brigham and Women's Hospital and Harvard Medical School, Boston, MA, USA; Massachusetts Veterans Epidemiology Research and Information Center (MAVERIC), VA Boston Healthcare System, Boston, MA, USA.
Robert J Glynn, Division of Preventive Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, MA, USA; Department of Biostatistics, Harvard T.H. Chan School of Public Health, Boston, MA, USA.
Natalya Gomelskaya, Division of Preventive Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, MA, USA.
Vadim Y Bubes, Division of Preventive Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, MA, USA.
Julie E Buring, Division of Preventive Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, MA, USA; Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA, USA.
Robert H Shmerling, Division of Rheumatology and Clinical Immunology, Department of Medicine, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, MA, USA.
Howard D Sesso, Division of Aging, Brigham and Women's Hospital and Harvard Medical School, Boston, MA, USA; Division of Preventive Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, MA, USA; Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA, USA.
Data Availability
Data described in the manuscript, code book, and analytic code will be made available upon request pending proposal approval.
References
- 1. Neogi T. Clinical practice. Gout. N Engl J Med. 2011;364(5):443–52. [DOI] [PubMed] [Google Scholar]
- 2. Kim KY, Ralph Schumacher H, Hunsche E, Wertheimer AI, Kong SX. A literature review of the epidemiology and treatment of acute gout. Clin Ther. 2003;25(6):1593–617. [DOI] [PubMed] [Google Scholar]
- 3. Chen-Xu M, Yokose C, Rai SK, Pillinger MH, Choi HK. Contemporary prevalence of gout and hyperuricemia in the United States and decadal trends: the National Health and Nutrition Examination Survey, 2007–2016. Arthritis Rheumatol. 2019;71(6):991–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lim SY, Lu N, Oza A, Fisher M, Rai SK, Menendez MEet al. Trends in gout and rheumatoid arthritis hospitalizations in the United States, 1993–2011. JAMA. 2016;315(21):2345–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rai SK, Aviña-Zubieta JA, McCormick N, De Vera MA, Shojania K, Sayre ECet al. The rising prevalence and incidence of gout in British Columbia, Canada: population-based trends from 2000 to 2012. Semin Arthritis Rheum. 2017;46(4):451–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hall AP, Barry PE, Dawber TR, McNamara PM. Epidemiology of gout and hyperuricemia. A long-term population study. Am J Med. 1967;42(1):27–37. [DOI] [PubMed] [Google Scholar]
- 7. Campion EW, Glynn RJ, DeLabry LO. Asymptomatic hyperuricemia. Risks and consequences in the normative aging study. Am J Med. 1987;82(3):421–6. [DOI] [PubMed] [Google Scholar]
- 8. FitzGerald JD, Dalbeth N, Mikuls T, Brignardello-Petersen R, Guyatt G, Abeles AMet al. American College of Rheumatology guideline for the management of gout. Arthritis Care Res (Hoboken). 2020;72(6):744–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Choi HK, Gao X, Curhan G. Vitamin C intake and the risk of gout in men: a prospective study. Arch Intern Med. 2009;169(5):502–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gao X, Curhan G, Forman JP, Ascherio A, Choi HK. Vitamin C intake and serum uric acid concentration in men. J Rheumatol. 2008;35:1853–8. [PMC free article] [PubMed] [Google Scholar]
- 11. So MW, Lim D-H, Kim S-H, Lee S. Dietary and nutritional factors associated with hyperuricemia: the seventh Korean National Health and Nutrition Examination Survey. Asia Pac J Clin Nutr. 2020;29:609–17. [DOI] [PubMed] [Google Scholar]
- 12. Towiwat P, Li Z-G. The association of vitamin C, alcohol, coffee, tea, milk and yogurt with uric acid and gout. Int J Rheum Dis. 2015;18(5):495–501. [DOI] [PubMed] [Google Scholar]
- 13. Chattaraj KG, Paul S. The miscibility and solubility of uric acid and vitamin C in the solution phase and their structural alignment in the solid-liquid interface. Phys Chem Chem Phys. 2021;23(28):15169–82. [DOI] [PubMed] [Google Scholar]
- 14. Feigelson P. The inhibition of xanthine oxidase in vitro by trace amounts of L-ascorbic acid. J Biol Chem. 1952;197(2):843–50. [PubMed] [Google Scholar]
- 15. Stein HB, Hasan A, Fox IH. Ascorbic acid-induced uricosuria. A consequence of megavitamin therapy. Ann Intern Med. 1976;84(4):385–8. [DOI] [PubMed] [Google Scholar]
- 16. Juraschek SP, Miller ER, Gelber AC. Effect of oral vitamin C supplementation on serum uric acid: a meta-analysis of randomized controlled trials. Arthritis Care Res (Hoboken). 2011;63(9):1295–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang L, Shi X, Yu J, Zhang P, Ma P, Sun Y. Dietary vitamin E intake was inversely associated with hyperuricemia in US adults: NHANES 2009–2014. Ann Nutr Metab. 2020;76(5):354–60. [DOI] [PubMed] [Google Scholar]
- 18. Seifi B, Kadkhodaee M, Zahmatkesh M. Effect of vitamin E therapy on serum uric acid in DOCA-salt-treated rats. Acta Physiol Hung. 2011;98(2):214–20. [DOI] [PubMed] [Google Scholar]
- 19. Bishop C, Rand R, Talbott JH. Rate of conversion of isotopic glycine to uric acid in the normal and gouty human and how this is affected by vitamin E and folic acid. Metabolism. 1955;4:174–82. [PubMed] [Google Scholar]
- 20. Yoon I-S, Park D-H, Kim J-E, Yoo J-C, Bae M-S, Oh D-Set al. Identification of the biologically active constituents of Camellia japonica leaf and anti-hyperuricemic effect in vitro and in vivo. Int J Mol Med. 2017;39(6):1613–20. [DOI] [PubMed] [Google Scholar]
- 21. Sesso HD, Buring JE, Christen WG, Kurth T, Belanger C, MacFadyen Jet al. Vitamins E and C in the prevention of cardiovascular disease in men: the Physicians’ Health Study II randomized controlled trial. JAMA. 2008;300(18):2123–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Christen WG, Gaziano JM, Hennekens CH. Design of Physicians’ Health Study II—a randomized trial of beta-carotene, vitamins E and C, and multivitamins, in prevention of cancer, cardiovascular disease, and eye disease, and review of results of completed trials. Ann Epidemiol. 2000;10(2):125–34. [DOI] [PubMed] [Google Scholar]
- 23. Gaziano JM, Glynn RJ, Christen WG, Kurth T, Belanger C, MacFadyen Jet al. Vitamins E and C in the prevention of prostate and total cancer in men: the Physicians’ Health Study II randomized controlled trial. JAMA. 2009;301(1):52–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gaziano JM, Sesso HD, Christen WG, Bubes V, Smith JP, MacFadyen Jet al. Multivitamins in the prevention of cancer in men: the Physicians’ Health Study II randomized controlled trial. JAMA. 2012;308(18):1871–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McAdams MA, Maynard JW, Baer AN, Köttgen A, Clipp S, Coresh Jet al. Reliability and sensitivity of the self-report of physician-diagnosed gout in the campaign against cancer and heart disease and the atherosclerosis risk in the community cohorts. J Rheumatol. 2011;38(1):135–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cadzow M, Merriman TR, Dalbeth N. Performance of gout definitions for genetic epidemiological studies: analysis of UK biobank. Arthritis Res Ther. 2017;19(1):181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Solomon DH, Glynn RJ, MacFadyen JG, Libby P, Thuren T, Everett BMet al. Relationship of interleukin-1β blockade with incident gout and serum uric acid levels: exploratory analysis of a randomized controlled trial. Ann Intern Med. 2018;169(8):535–42. [DOI] [PubMed] [Google Scholar]
- 28. Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene Tet al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367(1):20–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sinha R, Block G, Taylor PR. Determinants of plasma ascorbic acid in a healthy male population. Cancer Epidemiol Biomarkers Prev. 1992;1:297–302. [PubMed] [Google Scholar]
- 30. Barja G, López-Torres M, Pérez-Campo R, Rojas C, Cadenas S, Prat Jet al. Dietary vitamin C decreases endogenous protein oxidative damage, malondialdehyde, and lipid peroxidation and maintains fatty acid unsaturation in the guinea pig liver. Free Radical Biol Med. 1994;17(2):105–15. [DOI] [PubMed] [Google Scholar]
- 31. Rojas C, Cadenas S, Pérez-Campo R, López-Torres M, Barja G. Effect of vitamin C on antioxidants, lipid peroxidation, and GSH system in the normal guinea pig heart. J Nutr Sci Vitaminol (Tokyo). 1994;40(5):411–20. [DOI] [PubMed] [Google Scholar]
- 32. Sutton JL, Basu TK, Dickerson JW. Effect of large doses of ascorbic acid in man on some nitrogenous components of urine. Hum Nutr Appl Nutr. 1983;37:136–40. [PubMed] [Google Scholar]
- 33. Berger L, Gerson CD, Yü TF. The effect of ascorbic acid on uric acid excretion with a commentary on the renal handling of ascorbic acid. Am J Med. 1977;62(1):71–6. [DOI] [PubMed] [Google Scholar]
- 34. Huang H-Y, Appel LJ, Choi MJ, Gelber AC, Charleston J, Norkus EPet al. The effects of vitamin C supplementation on serum concentrations of uric acid: results of a randomized controlled trial. Arthritis Rheum. 2005;52(6):1843–7. [DOI] [PubMed] [Google Scholar]
- 35. Stamp LK, O'Donnell JL, Frampton C, Drake JM, Zhang M, Chapman PT. Clinically insignificant effect of supplemental vitamin C on serum urate in patients with gout: a pilot randomized controlled trial. Arthritis Rheum. 2013;65(6):1636–42. [DOI] [PubMed] [Google Scholar]
- 36. Fatima T, Nilsson PM, Turesson C, Dehlin M, Dalbeth N, Jacobsson LTHet al. The absolute risk of gout by clusters of gout-associated comorbidities and lifestyle factors—30 years follow-up of the Malmö Preventive Project. Arthritis Res Ther. 2020;22:244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Richette P, Clerson P, Périssin L, Flipo R-M, Bardin T. Revisiting comorbidities in gout: a cluster analysis. Ann Rheum Dis. 2015;74(1):142–7. [DOI] [PubMed] [Google Scholar]
- 38. Juraschek SP, Miller ER, Gelber AC. Body mass index, obesity, and prevalent gout in the United States in 1988–1994 and 2007–2010. Arthritis Care Res (Hoboken). 2013;65(1):127–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Choi HK, Atkinson K, Karlson EW, Curhan G. Obesity, weight change, hypertension, diuretic use, and risk of gout in men: the health professionals follow-up study. Arch Intern Med. 2005;165(7):742–8. [DOI] [PubMed] [Google Scholar]
- 40. Chen J-H, Yeh W-T, Chuang S-Y, Wu Y-Y, Pan W-H. Gender-specific risk factors for incident gout: a prospective cohort study. Clin Rheumatol. 2012;31(2):239–45. [DOI] [PubMed] [Google Scholar]
- 41. Lyngdoh T, Vuistiner P, Marques-Vidal P, Rousson V, Waeber G, Vollenweider Pet al. Serum uric acid and adiposity: deciphering causality using a bidirectional Mendelian randomization approach. PLoS One. 2012;7(6):e39321. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the manuscript, code book, and analytic code will be made available upon request pending proposal approval.